Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
|
|
|
- Nelson Asher Alexander
- 6 years ago
- Views:
Transcription
1 Acta Derm Venereol 2012; 92: CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and Yong-Kwang Tay Department of Dermatology, Changi General Hospital, Singapore Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, but potentially life-threatening, reactions to medications. Both conditions have significant morbidity and mortality. The aim of this study was to document the epidemiological features, aetiologies, treatment and clinical outcomes of retrospectively reviewed data of all patients with SJS or TEN treated from January 2004 to November 2010 in a general hospital. There were 18 cases of SJS, seven cases of SJS/TEN overlap and three cases of TEN. Mean age was 50.6 years, with a range of years. The male/female ratio was 1. Drugs accounted for 26 cases; one case was caused by Neisseria gonorrhoea infection. Anti-convulsants (35.7%) were the most common implicated drugs followed by antibiotics (28.5%), non-steroidal anti-inflammatory drugs (NSAIDS) (14.3%), allopurinol (7.1%) and traditional Chinese medication (7.1%). In seven cases, multiple drugs were implicated. Most SJS cases (88%) were treated with corticosteroids, of which 61% were given highdose systemic corticosteroids. No infective complications were observed. Six out of the seven SJS/TEN overlap syndrome and all three TEN cases were given intravenous immunoglobulins. One patient with TEN died. In conclusion, anti-convulsants, especially carbamazepine, were the most frequently implicated drugs, followed by antibiotics and NSAIDS. High-dose corticosteroids were effective in SJS, whereas intra venous immunoglobulin were useful in TEN and SJS/TEN overlap syndrome. Key words: Stevens-Johnson syndrome; toxic epidermal necrolysis; Singapore. (Accepted April 15, 2011.) Acta Derm Venereol 2012; 92: Siew-Kiang Tan, Department of Dermatology, Changi General Hospital, 2 Simei Street 3, Singapore, Singapore. Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, but potentially fatal, disorders. They are considered to be clinical entities within a spectrum of adverse cutaneous drug reactions of increasing severity based on their surface of skin detachment (1). Bastuji-Garin et al. (2) proposed the most widely accepted consensus definition, which for SJS consists of epidermal detachment of less than 10% body surface area, for TEN epidermal detachment of more than 30% detachment, and for SJS/TEN overlap syndrome epidermal detachment of between 10% and 30%. The reported incidence varies from 1.2 to 6 per million patient-years for SJS, and from 0.4 to 1.2 per million patient-years for TEN (3). The incidence rises with increasing age and is at least a 1,000-fold higher in patients with human immunodeficiency virus (HIV)/ acquired immunodeficiency syndrome (AIDS) (4). The majority of cases of SJS and TEN are druginduced. Other possible causes include infections, immunizations, environment chemicals and radiation therapy. The most commonly implicated drugs consist of antibiotics, anti-convulsants, non-steroidal antiinflammatory drugs (NSAIDS) and allopurinol. With the increasing number of HIV/AIDS patients, nevirapine, a non-nucleoside reverse transcriptase inhibitor (NNRTI) used to treat HIV-1 infection and AIDS, is the most commonly implicated drug in these patients (5). Although rare, SJS and TEN have a significant impact on public health because of high morbidity and mortality. Mortality in SJS is generally below 5%, whereas TEN has a mortality rate of up to 30% (6). The aim of this study is to present the epidemiological features, aetiologies, treatment and clinical outcomes of SJS and TEN in a general hospital in Singapore during a 7-year-period. METHODS Patients admitted between January 2004 and November 2010 with a diagnosis of SJS, SJS/TEN overlap or TEN were identified from the hospital s computer database. The case notes, charts, investigation results and treatment records of these patients were retrospectively reviewed. Data obtained included the age, gender, ethnic group, medical history, presenting complaints, inciting drugs, duration between the initial consumption of the drug and the onset of symptoms, SCORTEN score and the HLA genotyping, if it was sent. Treatment regimens, duration of hospitalization and mortality were also recorded. There were 18 cases of SJS, seven of SJS/TEN overlap, and three of TEN, giving a total of 28 patients (Table I). Patients were in the age range years. Mean age was 50.6 years, with a range of years. The majority of the patients were in the age range years, and there were an equal number of male and female patients. There were 18 Chinese patients, 9 Malay patients and one Filipino patient The Authors. doi: / Journal Compilation 2012 Acta Dermato-Venereologica. ISSN
2 SJS and TEN in Singapore 63 Table I. Patient characteristics Pat. No. Diagnosis Sex/age, years Cause Onset, days Treatment Hospital stay, days Complications SCORTEN 1 SJS F/13 Paracetamol 2 Prednisolone 8 None 0 2 SJS F/32 Naproxen 12 Prednisolone 5 None 0 3 a TEN M/85 Alfuzosin 7 IVIG 11 Pneumonia, bacteraemia, acute 5 renal failure 4 SJS F/81 Ciprofloxacin 2 Prednisolone 4 Mild bilateral conjunctivitis 1 5 TEN F/47 Carbamazepine 15 IVIG 20 Transaminitis 2 6 TEN F/31 Cephalexin 4 IVIG 18 None 2 7 SJS M/39 Unknown 5 Supportive 6 None 0 8 SJS M/43 Gonorrhoea 7 Supportive 7 Transaminitis 1 9 SJS/TEN F/47 Carbamazepine 14 Prednisolone 21 None 2 10 SJS/TEN M/73 Carbamazepine, diclofenac, 7 IVIG 9 None 3 amoxicillin 11 SJS M/37 Aspirin 2 IV hydrocortisone 8 None 0 12 SJS M/37 Carbamazepine 7 IV hydrocortisone 8 None 0 13 SJS M/84 Amoxicillin/clavulanate, 42 IV hydrocortisone 7 None 1 ceftriaxone, metronidazole, alfuzosin 14 SJS M/79 Erythromycin, Allopurinol 60 IV hydrocortisone 8 Transminitis 1 15 SJS/TEN M/50 TCM that contained 9 IVIG 17 Transminitis 3 phenylbutazone (NSAID) 16 SJS F/35 Carbamazepine 15 IV hydrocortisone 21 Symblepharon, infective keratitis 0 17 SJS F/74 Carbamazepine 11 IV hydrocortisone 11 Transminitis 1 18 SJS M/61 TCM 4 IV hydrocortisone 17 Reactive depression 2 19 SJS/TEN M/55 Amoxicillin/clavulanate, 3 IVIG 20 Acute renal impairment 2 Cloxacillin secondary to dehydration 20 SJS/TEN M/15 Carbamazepine 15 IVIG 19 Neutropaenia, transaminitis 1 21 SJS/TEN F/18 Trimethoprim-sulphamethoxazole 2 IVIG 18 Bicytopaenia, transaminitis 3 22 SJS F/63 Trimethoprim-sulphamethoxazole, 17 IV hydrocortisone 6 None 1 amoxicillin 23 SJS/TEN F/25 Carbamazepine 11 IVIG 12 Transaminitis, subtarsal fibrosis, 1 symblepharon, haemolytic anaemia 24 SJS F/56 Allopurinol 24 IV hydrocortisone 8 Pseudomembranous 1 conjunctivitis 25 SJS M/50 Carbamazepine 16 Prednisolone 5 None 2 26 SJS F/76 Etoricoxib 5 Prednisolone 2 None 1 27 SJS F/76 Ceftriaxone 3 IV hydrocortisone 12 None 1 28 SJS M/33 Carbamazepine 15 IV hydrocortisone 15 None 0 a This patient died. SJS: Stevens-Johnson syndrome; TEN: toxic epidermal necrolysis; IVIG: intravenous immunoglobulins; IV: intravenous; TCM: traditional Chinese medicine. RESULTS Aetiologies One patient with SJS (No. 8) had confirmed gonorrhoea infection; this 43-year-old man presented with a one-week history of targetoid rash associated with oral erosions, reactive arthritis of the left ankle and penile discharge. Genital swab isolated Gram-negative diplococci suggestive of Neisseria gonorrhoeae. He was treated with intramuscular penicillin. No cause was ascertained in one patient with SJS (No. 7). Drugs caused the rest of the cases. Anti-convulsants (35.7%) were the most common implicated drugs, followed by antibiotics (28.5%), NSAIDS (14.3%), allopurinol (7.1%) and traditional Chinese medication (7.1%). In seven cases, multiple drugs were implicated. Carbamazepine (CBZ) was the only anti-convulsant implicated. Beta-lactam antibiotics (5 cases) were the most frequent causative drugs among the antibiotics, followed by sulphonamides (2 cases) and fluroquinolones (1 case). The beta-lactam antibiotics implicated include amoxicillin and cloxacillin from the penicillin group, cefalexin and ceftriaxone from the cephalosporin group and co-amoxiclav (amoxicillin with clavulanic acid) from the combination group. HLA genotyping was carried out for two of our patients with carbamazepine-induced SJS/TEN. Both were positive for the HLA-B*1502 allele. Interval between the first drug intake and onset of symptoms The mean time-period between ingestion of the drug and onset of symptoms was 15.3 days, with a range of 2 days to 2 months. More than half (60.7%) of the patients developed symptoms within 2 weeks. Antibiotics had the shortest interval between ingestion time and onset of symptoms, of which fluoroquinolones with a mean interval of 2 days, beta-lactams with a mean interval of 4 days and sulphonamides with a mean interval of
3 64 S.-K. Tan and Y.-K. Tay 10 days. CBZ had a mean interval of 15 days and allopurinol had a longer interval, of 42 days. Treatment For the SJS cases, only two cases were treated with supportive treatment without corticosteroids, five were given oral corticosteroids (prednisolone mg/kg/ day, which was tailed off over 2 4 weeks) and 11 were given intravenous corticosteroids (hydrocortisone mg/day for 7 10 days). For the SJS/TEN overlap syndrome, one patient was given oral corticosteroids and six were given intravenous immunoglobulins (IVIG). All the three TEN cases were given a total of 3 g/kg IVIG over 3 days. SCORTEN and mortality In order to evaluate prognosis in patients with SJS/ TEN, the validated SCORTEN disease severity scoring system (7) (Table II) was calculated. Seven patients had SCORTEN of 0, 11 patients had SCORTEN of 1, six patients had SCORTEN of 2, three patients had SCORTEN of 3 and one patient with a SCORTEN of 5. The latter was an 85-year-old man who received alfuzosin for his benign prostate hypertrophy. He developed pneumonia, pseudomonas bacteraemia, which was complicated by acute on chronic renal failure. He died 7 days after onset of TEN. DISCUSSION SJS/TEN is a rare disease. In our series, there were only 28 cases of SJS and TEN over a 7-year period. In contrast to earlier studies showing that females are affected with SJS/TEN twice as often as males (8, 9), our series had equal numbers of males and females. The demographics may have changed, however, in recent years, as HIV/ AIDS patients who develop SJS/TEN are primarily men. In a recent study in a teaching hospital in Lomé, Africa, 54.6% of SJS/TEN patients were HIV-positive (10). The most common drugs implicated in our hospital were anti-convulsants. Interestingly, CBZ is the only anti-convulsant implicated. CBZ has been reported as the most common culprit drug for SJS and TEN in several Asian countries. A strong association between Table II. SCORTEN Risk factor 0 1 Age (years) < 40 > 40 Associated malignancy No Yes Heart rate (beats/min) < 120 > 120 Serum blood urea nitrogen (BUN) (mg/dl) < 27 > 27 Detached or compromised body surface (%) < 10 > 10 Serum bicarbonate (meq/l) > 20 < 20 Serum glucose (mg/dl) < 250 > 250 HLA-B*1502 and CBZ-induced SJS/TEN has been reported in Han Chinese (11), Thai (12), Indian (13) and Malay (14) patients. The US FDA and similar regulatory agencies in Canada and Taiwan have updated the CBZ drug label to include the genetic information. Even so, Singapore doctors do not routinely screen patients for HLA-B*1502 allele before starting treatment with CBZ. We want to highlight this finding so that doctors who frequently prescribe CBZ preferably screen their patients for HLA-B*1502 allele. Hung et al. (15) carried out a case-control study and concluded that phenytoin, lamotrigine and oxcarbazepine, which possess an aromatic ring like that of CBZ, share a common risk allele. They suggest that aromatic anti-epileptic drugs should be avoided in the B*1502 carrier and caution should also be exercised for lamotrigine. In Europe and Israel, allopurinol is the most common cause of SJS and TEN. Daily doses equal to or greater than 200 mg were associated with a higher risk. The risk was restricted to short-term use ( 8 weeks) (16). While the HLA-B*1502 allele is absent in the Caucasian (17) and Japanese population (18), HLA-B*5801 is strongly associated with allopurinol-induced SJS/TEN in the European (19), Japanese (18), Han Chinese (20) and Thai populations (21). The mean number of days between ingestion of the drug and onset of symptoms for our patients was 15 days. This is consistent with the typical interval, which is between 1 and 3 weeks. For many drugs, the risk of SJS and TEN was highest in the first weeks of use, particularly for antibiotics, as shown in our study. For most high-risk drugs that are intended for long-term use, the risk of developing SJS or TEN is elevated during the initial 2 months of use (6, 22). To date, the pathogenesis of SJS and TEN is still not fully understood. SJS and TEN are characterized by massive keratinocyte apoptosis. Fas-Fas ligand (FasL)-induced apoptosis in keratinocytes is one of the most thoroughly studied immune mechanisms in SJS/TEN. It was first proposed in 1998 that the death receptor Fas/CD95 plays a key role in the apoptosis of keratinocytes that leads to epidermal necrolysis (23). However, inconsistent findings of Fas, FasL and soluble FasL (sfasl) brought into question the original hypothesis from Viard et al. (23). Subsequently, numerous possible mediators of keratinocyte apoptosis have been suggested, such as peripheral cytotoxic T cells (18), inflammatory cytokines (24), nitric oxide (25), granzyme B (26), perforin (27) and granulysin (28). The strong associations of HLA-B*1502 with CBZ-induced SJS/ TEN and HLA-B*5801 with allopurinol-induced SJS/ TEN also suggested that genetic predisposition plays a role in the pathogenesis of SJS/TEN. Currently, no treatment modality has been established as standard for these patients. Due to the rarity of these disorders, there are no randomized controlled
4 SJS and TEN in Singapore 65 trials of pharmacological agents in the treatment for SJS and TEN. However, there are case reports of successful treatment using IVIG, systemic corticosteroids, plasmapheresis, cyclosporine, cyclophosphamide, antitumour necrosis factor-α (TNF-α) and haemodialysis (29). The controversy over whether systemic corticosteroids should be used to curtail progression remains unresolved. For example, in Germany, corticosteroids are used in 80% of cases of SJS, whereas in France, systemic corticosteroids are used in only 20% of such patients (30). The corticosteroids therapies that were reported to reduce morbidity and improve outcome of SJS/TEN patients includes oral prednisolone, intravenous hydrocortisone, pulsed intravenous methylprednisolone (31) and pulsed intravenous dexamethasone therapy (32). Out of our 18 patients with SJS, 16 were given corticosteroids, of which 11 were given intravenous hydrocortisone. No complication of sepsis was observed in these patients. It appears reasonable to initiate corticosteroids as acutely as possible before significant tissue damage has occurred, or later if the disease is still in its progressive stage. They should be used in combination with antiviral or antimicrobial agents in case of an underlying infectious aetiology. IVIG contains anti-fas antibodies that can abrogate the Fas-mediated keratinocyte apoptosis (23). Most studies on IVIG in SJS and TEN reported improvement in arresting disease progression and reduction in time to skin healing (33 35). In general, mortality varied from 0% to 12% in studies that supported the use of IVIG, and 25% to 41.7% in those that did not demonstrate a beneficial effect (36 38). Mortality was associated with a lower dose of IVIG, longer time of onset before IVIG use, co-existing underlying chronic conditions, older age and greater body surface area involved. In our case series, six out of the seven cases with SJS/TEN overlap syndrome and all three TEN cases were given IVIG. They were given a total of 3 g/kg IVIG over 3 days. Previous studies that used lower dose of IVIG (2 g/kg total dose) reported an increase in mortality (37, 38). All our patients who were given IVIG were admitted to the intensive care unit, where they were monitored closely. The mean length of stay for these patients was 16.9 days. Only one patient with TEN died of pneumonia, pseudomonas bacteraemia and acute on chronic renal failure. None of the patients in our series experienced adverse events related to IVIG, such as anaphylaxis and acute renal failure. We recommend a total dose of 3 g/kg IVIG. Our experience leads us to conclude that steroid therapy with high-dose corticosteroids is effective in SJS, whereas IVIG is effective in TEN. However, multicentre, randomized, placebo-controlled trials using a standardized design are needed to validate these findings. The authors declare no conflict of interest. REFERENCES 1. French LE. Toxic epidermal necrolysis and Stevens Johnson syndrome: our current understanding. Allergol Int 2006; 55: Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993; 129: Wolkenstein P, Revuz J. Drug-induced severe skin reactions. Incidence, management and prevention. Drug Saf 1995; 13: Rzany B, Mockenhaupt M, Stocker U, Hamouda O, Schöpf E. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis in patients with the acquired immunodeficiency syndrome in Germany. Arch Dermatol 1993; 129: Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, Naldi L, Viboud C, Roujeau JC; EuroSCAR Study Group. Nevirapine and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. AIDS 2001; 15: Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994; 331: Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol 2000; 115: Fritsch P. European Dermatology Forum: skin diseases in Europe. Skin diseases with a high public health impact: toxic epidermal necrolysis and Stevens-Johnson syndrome. Eur J Dermatol 2008; 18: Yamane Y, Aihara M, Ikezawa Z. Analysis of Stevens- Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to Allergol Int 2007; 56: Saka B, Kombate K, Mouhari-Toure A, Akakpo S, Tchangaï-Walla K, Pitché P. Stevens-Johnson syndrome and toxic epidermal necrolysis in a teaching hospital in Lomé, Togo: retrospective study of 89 cases. Med Trop (Mars) 2010; 70: Man CB, Kwan P, Baum L, Yu E, Lau KM, Cheng AS, et al. Association between HLA-B*1502 Allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia 2007; 48: Tassaneeyakul W, Tiamkao S, Jantararoungtong T, Chen P, Lin SY, Chen WH, et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia 2010; 51: Mehta TY, Prajapati LM, Mittal B, Joshi CG, Sheth JJ, Patel DB, et al. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereal Leprol 2009; 75: Chang CC, Too CL, Murad S, Hussein S. Association of HLA-B*1502 with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in Malaysian population. Proceeding in 7th Asian-Oceanian Epilepsy Congress, Xiamen Hung SI, Chung WH, Liu ZS, Chen CH, Hsih MS, Hui RC, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics 2010; 11: Halevy S, Ghislain PD, Mockenhaupt M, Fagot JP, Bouwes Bavinck JN, Sidoroff A, et al. Allopurinol is the most com-
5 66 S.-K. Tan and Y.-K. Tay mon cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol 2008; 58: Lonjou C, Thomas L, Borot N, Ledger N, de Toma C, LeLouet H, et al. A marker for Stevens-Johnson syndrome: ethnicity matters. Pharmacogenomics J 2006; 6: Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Steven-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenetics 2008; 9: Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics 2008; 18: Hung SI, Chung WH, Liou LB, Chu CC, Lim M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005; 102: Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY Tiamkao S, Khunarkornsiri U, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens- Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics 2009; 19: Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCARstudy. J Invest Dermatol 2008; 128: Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998; 282: Chave TA, Mortimer NJ, Sladden MJ, Hall AP, Hutchinson PE. Toxic epidermal necrolysis: current evidence, practical management and future directions. Br J Dermatol 2005; 153: Wolkenstein P, Latarjet J, Roujeau J-C, Duguet C, Boudeau S, Vaillant L, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet 1998; 352: Lerner LH, Qureshi AA, Reddy BV, Lerner EA. Nitric oxide synthase in toxic epidermal necrolysis and Stevens-Johnson syndrome. J Invest Dermatol 2000; 114: Posadas SJ, Padial A, Torres MJ, Mayorga C, Leyva L, Sanchez E, et al. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol 2002; 118: Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Garnulysin is a key mediator for disseminated keratinocyte death in Stevens Johnson syndrome and toxic epidermal necrolysis. Nat Med 2008; 14: Mukasa Y, Craven N. Management of toxic epidermal necrolysis and related syndromes. Postgrad Med J 2008; 84: Hynes AY, Kafkala C, Daoud YJ, Foster CS. Controversy in the use of high-dose systemic steroids in the acute care of patients with Stevens-Johnson syndrome. Int Ophthalmol Clin 2005; 45: Yamane Y, Aihara M, Tatewaki S, Matsukura S, Kanbara T, Yamakawa Y, et al. Analysis of treatments and deceased cases of severe adverse drug reactions analysis of 46 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis. Aerugi 2009; 58: Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol 2007; 87: French LE, Trent JT, Kerdel FA. Use of intravenous immunoglobulin in toxic epidermal necrolysis and Stevens- Johnson syndrome: our current understanding. Int Immunopharmacol 2006; 6: Mittmann N, Chan B, Knowles S, Cosentino L, Shear N. Intravenous immunoglobulin use in patients with toxic epidermal necrolysis and Stevens-Johnson syndrome. Am J Clin Dermatol 2006; 7: Chen J, Wang B, Zeng Y, Xu H. High-dose intravenous immunoglobulins in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis in Chinese patients: a retrospective study of 82 cases. Eur J Dermatol 2010; 20: Khalili B, Bahna SL. Pathogenesis and recent therapeutic trends in Stevens-Johnson syndrome and toxic epidermal necrolysis. Ann Allergy Asthma Immunol 2006; 97: Bachot N, Reviz J, Roujeau JC. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol 2003; 139: Brown KM, Silver GM, Halerz M, Walaszek P, Sandroni A, Gamelli RL. Toxic epidermal necrolysis: does immunoglobulin make a difference? J Burn Care Rehabil 2004; 25:
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
 Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Stevens-Johnson syndrome (SJS) and toxic epidermal. necrolysis in Asian children
 Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis
 Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
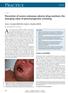 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
 Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review
 140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
1 Introduction. Kenneth Irungu 1 David Nyamu
 Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia
 ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection
 Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update
 Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Department of Pharmacology & NIPER-Guwahati, Gauhati Medical College & Hospital, Guwahati , Assam, INDIA. 3
 J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
Scratching the Surface: A Review of SJS/TEN
 Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients
 original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
Original Article. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C
 Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital
 Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME
 EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from 2000 to 2006 Yumiko Yamane 1, Michiko Aihara 1 and Zenro Ikezawa 1
 Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan Pei Chen, Ph.D., Juei-Jueng Lin, M.D., Chin-Song Lu, M.D.,
T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan Pei Chen, Ph.D., Juei-Jueng Lin, M.D., Chin-Song Lu, M.D.,
Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital
 International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea 2
 pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2013;27(5):331-340 http://dx.doi.org/10.3341/kjo.2013.27.5.331 Original Article Effect of Age and Early Intervention with a Systemic Steroid, Intravenous
pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2013;27(5):331-340 http://dx.doi.org/10.3341/kjo.2013.27.5.331 Original Article Effect of Age and Early Intervention with a Systemic Steroid, Intravenous
Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics
 MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash
 CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit
 J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review
 HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
International Journal of Basic & Clinical Pharmacology. Profile of cutaneous adverse drug reactions of carbamazepine
 Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20175211 Original Research Article Profile of cutaneous
Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20175211 Original Research Article Profile of cutaneous
Severe cutaneous adverse reactions (SCAR) are life-threatening
 Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea
 Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015)
 1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
HLA-A*24:02 as a common risk factor for antiepileptic drug induced cutaneous adverse reactions
 Published Ahead of Print on May 5, 2017 as 10.1212/WNL.0000000000004008 HLA-A*24:02 as a common risk factor for antiepileptic drug induced cutaneous adverse reactions Yi-Wu Shi, PhD* Fu-Li Min, MD* Dong
Published Ahead of Print on May 5, 2017 as 10.1212/WNL.0000000000004008 HLA-A*24:02 as a common risk factor for antiepileptic drug induced cutaneous adverse reactions Yi-Wu Shi, PhD* Fu-Li Min, MD* Dong
A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive Patients
 Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Toxic Epidermal Necrolysis - A Case Report
 The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
Toxic epidermal necrolysis
 REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS
 STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS *Blanca R. Del Pozzo-Magaña, 1 Alejandro Lazo-Langner 2,3 1. Department of Pediatrics, University
STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS *Blanca R. Del Pozzo-Magaña, 1 Alejandro Lazo-Langner 2,3 1. Department of Pediatrics, University
Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
 BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
The liver and skin are two of the commonest
 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
Recent experiences to review data from MRCTs and progress of research on ethnic factors. Dr Yoshiaki Uyama
 Recent experiences to review data from MRCTs and progress of research on ethnic factors Dr Yoshiaki Uyama (PMDA) Visiting Professor, Graduate School of Advanced Clinical Science, Chiba University Visiting
Recent experiences to review data from MRCTs and progress of research on ethnic factors Dr Yoshiaki Uyama (PMDA) Visiting Professor, Graduate School of Advanced Clinical Science, Chiba University Visiting
Tumor Necrosis Factor α Inhibitors in the Treatment of Toxic Epidermal Necrolysis
 ORIGINAL RESEARCH Tumor Necrosis Factor α Inhibitors in the Treatment of Toxic Epidermal Necrolysis Katelyn F. Woolridge, MD; Patrick L. Boler, MD, PharmD; Brian D. Lee, MD PRACTICE POINTS Controversy
ORIGINAL RESEARCH Tumor Necrosis Factor α Inhibitors in the Treatment of Toxic Epidermal Necrolysis Katelyn F. Woolridge, MD; Patrick L. Boler, MD, PharmD; Brian D. Lee, MD PRACTICE POINTS Controversy
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC
 SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW
 SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study
 Q J Med 2012; 105:839 846 doi:10.1093/qjmed/hcs078 Advance Access Publication 28 April 2012 Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study S.M.H. KANNENBERG
Q J Med 2012; 105:839 846 doi:10.1093/qjmed/hcs078 Advance Access Publication 28 April 2012 Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study S.M.H. KANNENBERG
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW
 STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
Cutaneous drug reactions to antiepileptic drugs and relation with HLA alleles in the Turkish population
 O R I G I N A L A R T I C L E Eur Ann Allergy Clin Immunol Vol 50, N 1, 36-41, 2018 S. Büyüköztürk 1, Ç. Kekik 2, A.Z. Gökyiğit 3, F.İ. Tezer Filik 4i, G. Karakaya 4, S. Saygı 4i, A.B. Dursun 5, S. Kirbaş
O R I G I N A L A R T I C L E Eur Ann Allergy Clin Immunol Vol 50, N 1, 36-41, 2018 S. Büyüköztürk 1, Ç. Kekik 2, A.Z. Gökyiğit 3, F.İ. Tezer Filik 4i, G. Karakaya 4, S. Saygı 4i, A.B. Dursun 5, S. Kirbaş
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW
 PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
Oral Acetazolamide after Boston Keratoprosthesis in Stevens Johnson Syndrome
 Oral Acetazolamide after Boston Keratoprosthesis in Stevens Johnson Syndrome The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
Oral Acetazolamide after Boston Keratoprosthesis in Stevens Johnson Syndrome The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
Stevens-Johnson Syndrome : A Case Report
 https://doi.org/10.5933/jkapd.2017.44.4.455 J Korean Acad Pediatr Dent 44(4) 2017 ISSN (print) 1226-8496 ISSN (online) 2288-3819 Stevens-Johnson Syndrome : A Case Report Yongho Song, Nanyoung Lee, Sangho
https://doi.org/10.5933/jkapd.2017.44.4.455 J Korean Acad Pediatr Dent 44(4) 2017 ISSN (print) 1226-8496 ISSN (online) 2288-3819 Stevens-Johnson Syndrome : A Case Report Yongho Song, Nanyoung Lee, Sangho
Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies
 /, 2016, Vol. 7, (No. 49), pp: 81870-81879 Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies Ran Wu 1,*, Yi-ju Cheng 2,3,*,
/, 2016, Vol. 7, (No. 49), pp: 81870-81879 Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies Ran Wu 1,*, Yi-ju Cheng 2,3,*,
Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review
 Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Review Article Treatments for Severe Cutaneous Adverse Reactions
 Hindawi Journal of Immunology Research Volume 2017, Article ID 1503709, 9 pages https://doi.org/10.1155/2017/1503709 Review Article Treatments for Severe Cutaneous Adverse Reactions Yung-Tsu Cho and Chia-Yu
Hindawi Journal of Immunology Research Volume 2017, Article ID 1503709, 9 pages https://doi.org/10.1155/2017/1503709 Review Article Treatments for Severe Cutaneous Adverse Reactions Yung-Tsu Cho and Chia-Yu
Carbamazepine Hypersensitivity: Progress Toward Predicting the Unpredictable
 Carbamazepine Hypersensitivity: Progress Toward Predicting the Unpredictable Current Literature In Clinical Science HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. McCormack
Carbamazepine Hypersensitivity: Progress Toward Predicting the Unpredictable Current Literature In Clinical Science HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. McCormack
Cutaneous Conditions Associated with Systemic Disease
 Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
ABSTRACT. Keywords: Corticosteroids; Cyclosporine; Dermatology; Etanercept; Immunoglobulin; Necrolysis; Johnson; Stevens; Syndrome REVIEW
 Adv Ther (2017) 34:1235 1244 DOI 10.1007/s12325-017-0530-y REVIEW Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing
Adv Ther (2017) 34:1235 1244 DOI 10.1007/s12325-017-0530-y REVIEW Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing
AOU Ospedali Riuniti - Ancona
 AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
cipro loxacin; stevens-johnson syndrome; urinary tract infection; adverse drug reaction
 Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Bibliometric analysis of literature on toxic epidermal necrolysis and Stevens-Johnson syndrome:
 Sweileh Orphanet Journal of Rare Diseases (2017) 12:14 DOI 10.1186/s13023-017-0566-8 RESEARCH Open Access Bibliometric analysis of literature on toxic epidermal necrolysis and Stevens-Johnson syndrome:
Sweileh Orphanet Journal of Rare Diseases (2017) 12:14 DOI 10.1186/s13023-017-0566-8 RESEARCH Open Access Bibliometric analysis of literature on toxic epidermal necrolysis and Stevens-Johnson syndrome:
Genomics in patients with Japanese Ancestry
 Genomics in patients with Japanese Ancestry Yoshiaki Uyama, Ph.D. (PMDA) Visiting Professor, Graduate School of Medicine, Chiba University Visiting Professor, Graduate School of Medicine, Nagoya University
Genomics in patients with Japanese Ancestry Yoshiaki Uyama, Ph.D. (PMDA) Visiting Professor, Graduate School of Medicine, Chiba University Visiting Professor, Graduate School of Medicine, Nagoya University
Bugs and Drugs: What s New in Hypersensitivity Reactions?
 Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
2/28/2010. Pharmacogenomics and the Asian Population. Limited efficacy/response to drugs already on the market
 Pharmacogenomics and the Asian Population Majority are medication related Alan H.B. Wu, Ph.D. Professor, Laboratory Medicine, UCSF Section Chief, Clinical Chemistry, February 27, 20 Limited efficacy/response
Pharmacogenomics and the Asian Population Majority are medication related Alan H.B. Wu, Ph.D. Professor, Laboratory Medicine, UCSF Section Chief, Clinical Chemistry, February 27, 20 Limited efficacy/response
Cutaneous drug reactions
 Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Adverse Drug Reactions (ADRs) Outline
 Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Skin Manifestations of Drug Reactions
 Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study
 open access Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study Tai-Ming Ko, 1,2 Chang-Youh Tsai, 3,4 Shih-Yang
open access Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study Tai-Ming Ko, 1,2 Chang-Youh Tsai, 3,4 Shih-Yang
Cutaneous drug eruptions are seen commonly
 REVIEW ARTICLE Cutaneous drug reactions in children Sandipan Dhar, Raghubir Banerjee, Rajib Malakar Department of Pediatric Dermatology, Institute of Child Health, Kolkata, West Bengal, India ABSTRACT
REVIEW ARTICLE Cutaneous drug reactions in children Sandipan Dhar, Raghubir Banerjee, Rajib Malakar Department of Pediatric Dermatology, Institute of Child Health, Kolkata, West Bengal, India ABSTRACT
Emergency Dermatology Dr Melissa Barkham
 Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN
 TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN Nadia Khaldi 1, Alain Miras 1, Sophie Gromb 1,2 1 Forensic Science Laboratory, Pellegrin Hospital, University Teaching Hospital Bordeaux, France, 2 Forensic
TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN Nadia Khaldi 1, Alain Miras 1, Sophie Gromb 1,2 1 Forensic Science Laboratory, Pellegrin Hospital, University Teaching Hospital Bordeaux, France, 2 Forensic
Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate
 Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs
 European Review for Medical and Pharmacological Sciences Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs L. ORDOÑEZ, E. SALGUEIRO, F.J.
European Review for Medical and Pharmacological Sciences Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs L. ORDOÑEZ, E. SALGUEIRO, F.J.
Big rashes in little patients:
 ! Big rashes in little patients: Severe drug eruptions and cutaneous infections!! Marcia Hogeling, MD, FAAD Assistant Clinical Professor Director, Pediatric Dermatology Division of Dermatology David Geffen
! Big rashes in little patients: Severe drug eruptions and cutaneous infections!! Marcia Hogeling, MD, FAAD Assistant Clinical Professor Director, Pediatric Dermatology Division of Dermatology David Geffen
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients
 Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Study of Stevens - Johnson syndrome
 International Journal of Advances in Scientific Research and Engineering (ijasre) E-ISSN : 2454-8006 DOI: http://dx.doi.org/10.7324/ijasre.2018.32597 Volume 4, Issue 1 January-2018 Study of Stevens - Johnson
International Journal of Advances in Scientific Research and Engineering (ijasre) E-ISSN : 2454-8006 DOI: http://dx.doi.org/10.7324/ijasre.2018.32597 Volume 4, Issue 1 January-2018 Study of Stevens - Johnson
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions
 Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
Intravenous immunoglobulins and plasmapheresis combined treatment in patients with severe toxic epidermal necrolysis: preliminary report *
 British Journal of Plastic Surgery (2005) 58, 504 510 Intravenous immunoglobulins and plasmapheresis combined treatment in patients with severe toxic epidermal necrolysis: preliminary report * M. Lissia*,
British Journal of Plastic Surgery (2005) 58, 504 510 Intravenous immunoglobulins and plasmapheresis combined treatment in patients with severe toxic epidermal necrolysis: preliminary report * M. Lissia*,
To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving
 Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS
 PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
Research. Original Investigation
 Research Original Investigation Relationship Between the HLA-B*1502 Allele and Carbamazepine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis A Systematic Review and Meta-analysis Wimonchat
Research Original Investigation Relationship Between the HLA-B*1502 Allele and Carbamazepine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis A Systematic Review and Meta-analysis Wimonchat
STEVENS-JOHNSON SYNDROME: A CASE REPORT
 STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
a Department of Pharmacology, b Pharmacy Unit, c Department of Medicine, Faculty Thailand Received 24 January 2015 Accepted 18 May 2015
 402 Original article Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions Thachanan Kongpan a, Surakameth Mahasirimongkol e, Parinya Konyoung g, Sirimas Kanjanawart a,
402 Original article Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions Thachanan Kongpan a, Surakameth Mahasirimongkol e, Parinya Konyoung g, Sirimas Kanjanawart a,
Several different manifestations
 Toxic epidermal necrolysis and Stevens-Johnson syndrome: A review* Roland Gerull, MD; Mathias Nelle, MD; Thomas Schaible, MD Objectives: The aims of this review are to summarize the definitions, causes,
Toxic epidermal necrolysis and Stevens-Johnson syndrome: A review* Roland Gerull, MD; Mathias Nelle, MD; Thomas Schaible, MD Objectives: The aims of this review are to summarize the definitions, causes,
Skin testing with β-lactam antibiotics for diagnosis of β-lactam hypersensitivity in children
 Asian Pacific Journal of Allergy and Immunology ORIGINAL ARTICLE Skin testing with β-lactam antibiotics for diagnosis of β-lactam hypersensitivity in children Wiparat Manuyakorn, 1 Prapasiri Singvijarn,
Asian Pacific Journal of Allergy and Immunology ORIGINAL ARTICLE Skin testing with β-lactam antibiotics for diagnosis of β-lactam hypersensitivity in children Wiparat Manuyakorn, 1 Prapasiri Singvijarn,
Severe Desquamating Disorder After Liver Transplant: Toxic Epidermal Necrolyis or Graft Versus Host Disease?
 Severe Desquamating Disorder After Liver Transplant: Toxic Epidermal Necrolyis or Graft Versus Host Disease? John T. Schulz III, MD, PhD, FACS, and Robert L. Sheridan, MD, FACS Bridgeport Hospital, Bridgeport,
Severe Desquamating Disorder After Liver Transplant: Toxic Epidermal Necrolyis or Graft Versus Host Disease? John T. Schulz III, MD, PhD, FACS, and Robert L. Sheridan, MD, FACS Bridgeport Hospital, Bridgeport,
Multiple Drug Hypersensitivity
 Multiple Drug Hypersensitivity Werner J Pichler ADR-AC GmbH Bern UPDATE ON DRUG HYPERSENSITIVITY, Bern, March 23rd 2017 Definition Multiple drug hypersensitivity (MDH) MDH is a syndrome that develops as
Multiple Drug Hypersensitivity Werner J Pichler ADR-AC GmbH Bern UPDATE ON DRUG HYPERSENSITIVITY, Bern, March 23rd 2017 Definition Multiple drug hypersensitivity (MDH) MDH is a syndrome that develops as
Objectives Making CYP450, Drug Interactions, & Pharmacogenetics Easy
 Objectives Making, Drug Interactions, & Pharmacogenetics Easy Anthony J. Busti, MD, PharmD, FNLA, FAHA Describe the differences between phase I and phase II metabolic pathways. Identify the most common
Objectives Making, Drug Interactions, & Pharmacogenetics Easy Anthony J. Busti, MD, PharmD, FNLA, FAHA Describe the differences between phase I and phase II metabolic pathways. Identify the most common
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research J. Chem. Pharm. Res., 2010, 2(2): 618-626 ISSN No: 0975-7384 Stevens-Johnson Syndrome-A life threatening skin disorder: A
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research J. Chem. Pharm. Res., 2010, 2(2): 618-626 ISSN No: 0975-7384 Stevens-Johnson Syndrome-A life threatening skin disorder: A
ORIGINAL ARTICLE. Siew Eng Choon, FRCP 1,2, Yi Shan Der 1, Nai Liang Joel Lai 1, Evelyn Sing Ee Yu 1, Xiao Ling Yap 1, Nalini Nanu Madhavan, MBBS 2
 ORIGINAL ARTICLE Clinical characteristics, culprit drugs and outcome of patients with Acute Generalised Exanthematous Pustulosis seen in Hospital Sultanah Aminah, Johor Bahru Siew Eng Choon, FRCP 1,2,
ORIGINAL ARTICLE Clinical characteristics, culprit drugs and outcome of patients with Acute Generalised Exanthematous Pustulosis seen in Hospital Sultanah Aminah, Johor Bahru Siew Eng Choon, FRCP 1,2,
Address for correspondence: Dr. Patel Raksha M. R-3, Doctor s Quarters, Jail Road, Vadodara- 01, Gujarat, India.
 Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Mechanisms of Drug Hypersensitivity Reactions
 18/5/213 Mechanisms of Drug Hypersensitivity Reactions Munir Pirmohamed NHS Chair of Pharmacogenetics Department of Molecular and Clinical Pharmacology Institute of Translational Medicine University of
18/5/213 Mechanisms of Drug Hypersensitivity Reactions Munir Pirmohamed NHS Chair of Pharmacogenetics Department of Molecular and Clinical Pharmacology Institute of Translational Medicine University of
Health economics and drug safety
 Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
Multiple Drug Allergies
 Management of Patients with Multiple Drug Allergies Roland Solensky, MD, FAAAAI The Corvallis Clinic Corvallis, OR roland.solensky@corvallisclinic.com Conflict of Interest Financial: None Research: Merck,
Management of Patients with Multiple Drug Allergies Roland Solensky, MD, FAAAAI The Corvallis Clinic Corvallis, OR roland.solensky@corvallisclinic.com Conflict of Interest Financial: None Research: Merck,
New Medicines Profile
 New Medicines Profile February 2010 Issue No. 10/02 Eslicarbazepine Concise evaluated information to support the managed entry of new medicines in the NHS Brand Name, (Manufacturer): Zebinix (Eisai Limited)
New Medicines Profile February 2010 Issue No. 10/02 Eslicarbazepine Concise evaluated information to support the managed entry of new medicines in the NHS Brand Name, (Manufacturer): Zebinix (Eisai Limited)
Defaulter rate of follow-up of patients with gonorrhoea at the Genitourinary Medicine Clinic
 Original Article Defaulter rate of follow-up of patients with gonorrhoea at the Genitourinary Medicine Clinic CC Chang, MBBS, MRCP, K Akbal, MBBS, HB Gangaram, MBBS, FRCP and Suraiya H Hussein MBBS, FRCP
Original Article Defaulter rate of follow-up of patients with gonorrhoea at the Genitourinary Medicine Clinic CC Chang, MBBS, MRCP, K Akbal, MBBS, HB Gangaram, MBBS, FRCP and Suraiya H Hussein MBBS, FRCP
Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center
 Management of the Burn Patient Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center American Burn Association Transfer Criteria Burn > 10%
Management of the Burn Patient Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center American Burn Association Transfer Criteria Burn > 10%
Accepted Manuscript. Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need
 Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
