Stevens-Johnson syndrome (SJS) and toxic epidermal. necrolysis in Asian children
|
|
|
- Frederica Freeman
- 6 years ago
- Views:
Transcription
1 Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare but severe drug reactions. There have been few reviews of SJS and TEN in children. Objectives: To evaluate the clinical profile and treatment outcomes of 15 pediatric patients with SJS or TEN. Methods: We retrospectively reviewed the case notes of all patients diagnosed with SJS or TEN admitted to a tertiary care pediatric hospital from 2001 to Results: We identified 13 cases of SJS, 1 case of SJS/TEN overlap and 1 case of TEN. Four patients were treated with intravenous immunoglobulin (IVIg), 5 patients were treated with systemic corticosteroids, and 6 patients were treated with supportive therapy only. The time to cessation of progression of disease was not significantly different in these 3 groups of patients. The duration of hospital stay was longer for patients treated with IVIG compared with those treated with systemic corticosteroids or supportive therapy. The only death was the patient with TEN treated with IVIG. Limitations: This was a retrospective study with a very small number of patients. Conclusion: The use of intravenous immunoglobulins or systemic corticosteroids did not improve the outcome of SJS and TEN. ( J Am Acad Dermatol 2010;62:54-60.) Key words: corticosteroids; intravenous immunoglobulins; Stevens-Johnson syndrome; toxic epidermal necrolysis. Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), conditions characterized by extensive epidermal cell death, are among the most severe mucocutaneous reactions. They are considered variants of the same disorder with epidermal detachment of less than 10% of total body surface area (BSA) classified as SJS, more than 30% detachment as TEN, and between 10% and 30% as transitional/overlap SJS/TEN. 1 Although SJS may be caused by drugs or infectious agents (eg, Mycoplasma pneumoniae), 2 TEN is almost exclusively attributed to drugs. More than 200 From the Department of Dermatology, Changi General Hospital. Funding sources: None. Conflicts of interest: None declared. Accepted for publication June 28, Reprint requests: Mark Jean-Aan Koh, Department of Dermatology, Changi General Hospital, 2 Simei Street 3, Singapore docmark@pacific.net.sg. Published online October 8, /$36.00 ª 2009 by the American Academy of Dermatology, Inc. doi: /j.jaad Abbreviations used: ALT: alanine transferase BSA: body surface area CRP: C-reactive protein ESR: erythrocyte sedimentation rate IVIG: intravenous immunoglobulin SJS: Stevens-Johnson syndrome TEN: toxic epidermal necrolysis medications have been implicated, most commonly anticonvulsants, sulfonamide antibiotics, penicillins, allopurinol, and oxicam nonsteroidal anti-inflammatory drugs. 3-5 Mortality rates of approximately 5% are reported in SJS, with TEN fatal in up to 30% of cases. 6 Mortality rates in children have been much lower. 7-9 A validated composite score (SCORTEN) has been used to predict mortality in patients with SJS/TEN. 10,11 Treatment of SJS and TEN includes the prompt discontinuation of any inciting drug and good supportive care. The use of systemic corticosteroids in TEN remains controversial and associated with 54
2 JAM ACAD DERMATOL VOLUME 62, NUMBER 1 Kob and Tay 55 complications such as sepsis and gastrointestinal hemorrhage. However, high-dose systemic corticosteroid therapy (eg, dexamethasone pulse therapy) may play a role in the treatment of early TEN before widespread skin loss occurs. 12 A role for systemic corticosteroid therapy in SJS is less controversial with several papers recommending its use to reduce morbidity and improve patient outcome. 13,14 Treatment of SJS and TEN with intrave- CAPSULE SUMMARY nous immunoglobulin (IVIG) has been reported in several case series, but with conflicting results. There are only a few reports of its use in pediatric patients. 9,15-17 We undertook a retrospective analysis of patients with SJS and TEN admitted to the Department of Paediatric Medicine, Kandang Kerbau Hospital, a tertiary care pediatric referral centre in Singapore, over a 5-year period, and report the clinical findings, investigations, treatment, and outcome in these patients. METHODS This retrospective study was approved by the Institutional Review Board Committee of Kandang Kerbau Women s and Children s Hospital. Patients admitted between January 2001 and December 2006, with a diagnosis of SJS, SJS/TEN overlap, or TEN, were identified from the hospital s computer database. A diagnosis of SJS was based on the finding of two or more mucosal sites of involvement, with or without skin involvement and epidermal sloughing involving less than 10% BSA. A diagnosis of TEN and SJS/TEN overlap was based on the presence of epidermal sloughing involving more than 30% and between 10% and 30% BSA, respectively. We reviewed the case notes, charts, investigation results, and treatment records of these patients. Epidemiological data obtained included the age, gender, ethnic group, and medical history. Clinical information obtained included presenting complaints, inciting drug(s), and the duration between the initial consumption of the drug and the onset of symptoms. Investigation results included complete blood cell count, renal panel, liver enzymes, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels, bacteriological cultures, Mycoplasma serology by enzyme-linked immunosorbent essay, viral isolation and histopathology of skin punch d d d Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare but severe drug reactions. There have been only a few reviews of SJS and TEN in children. We performed a retrospective evaluation of the clinical profile and treatment outcomes of 15 pediatric patients with a diagnosis of SJS or TEN. The use of intravenous immunoglobulins and systemic corticosteroids did not improve the outcome of our patients with SJS and TEN. biopsy specimens. Treatment regimens, duration of stay in the intensive care unit, duration of hospitalization, complications and mortality were also recorded. Significance tests to compare the results of treatment regimens were performed by means of Student t tests. RESULTS Patient characteristics We identified 15 patients. Table I summarizes patients characteristics, causes, treatment regimens, complications, and outcomes of these patients. Patients were 3 to 14 years of age, with a mean of 9 years. There was a predominance of male patients, with 11 boys and 4 girls. There were 9 Chinese patients, 5 Malay patients, and 1 Indian patient. Thirteen had a diagnosis of SJS, 1 patient had a diagnosis of SJS/TEN, and 1 patient was diagnosed with TEN. Causes Drugs were the most common cause of SJS (11 of 15 patients). Three patients had confirmed Mycoplasma infection. No cause was ascertained in one patient with SJS. The most common drugs implicated were anticonvulsants and the beta-lactam antibiotics. Three patients reacted to carbamazepine, one patient each to valproate and lamotrigine, two patients to amoxicillin, one to amoxicillin/clavulanate, one to cloxacillin, one to penicillin, and one to trimethoprim/sulfamethoxazole (cotrimoxazole). The mean number of days between ingestion of the drug and onset of symptoms was 9 days, with a range of 2 to 18 days. Patients who had earlier onset (2-3 days) of symptoms gave a history of ingestion of the same drug. Conversely, patients who had a later onset of symptoms (10-18 days) were prescribed the drug for the first time. Clinical presentation Except for one child who presented with mucosal lesions only, the patients had both mucous membrane and cutaneous involvement. The most common skin manifestations were an exanthematous eruption (7 patients) and atypical target lesions (7 patients). Six patients developed blisters and bullae with erosions, and 3 patients had purpuric lesions. All patients had at least two mucosal surface
3 56 Koh and Tay JAM ACAD DERMATOL JANUARY 2010 Table I. Characteristics of patients admitted for SJS or TEN Onset (days after starting drug) Hospital stay (days) Complications Outcome Patient No. Diagnosis Sex Age (y) Drug/Cause Treatment 1 SJS M 9 Valproate 14 Supportive only 6 None Survived 2 SJS/ M 12 Cloxacillin 3 Supportive only 9 None Survived TEN 3 SJS M 12 Carbamazepine 12 Supportive only 9 Hypotension Survived 4 SJS M 10 Trimethoprimsulfamethoxazole 3 Supportive only 3 None Survived 5 SJS M 3 Amoxicillin/ Unknown Supportive only 5 None Survived clavulanate 6 SJS M 5 Unknown NA Supportive only 6 Hyponatremia Survived 5 None Survived 7 SJS F 12 Carbamazepine 18 Prednisolone 1.5 mg/kg/d with tailing dose over 1 mo 8 SJS M 14 Carbamazepine 10 IV hydrocortisone 10 mg/kg/d for 3 days, followed by oral prednisolone 1 mg/kg/d for 1 wk 9 SJS M 12 Mycoplasma NA Prednisolone 1 mg/kg for 5 d, clarithromycin 10 SJS M 9 Mycoplasma NA Azithromycin, hydrocortisone 15 mg/kg/d for 4 d, followed by oral prednisolone 1.5 mg/kg/d, with tailing dose over 1mo 11 SJS M 6 Mycoplasma NA Prednisolone 0.5 mg/kg for 2 wk, clarithromycin 9 Hypotension Survived 3 None Survived 9 None Survived 8 None Survived 12 SJS M 14 Amoxicillin 2 IVIG 1 g/kg/d for 2 45 None Survived days 13 SJS F 5 Amoxicillin 2 IVIG 1 g/kg/d for 2 d 13 UTI, GE Survived 14 SJS F 10 Lamotrigine 10 IVIG 0.5 g/kg/d 15 Cellulitis from Survived for 4 d IV site 15 TEN F 4 Penicillin 14 IVIG 1 g/kg/d for 2 d 18 MOF, neutropenic sepsis, peritonitis Died d, Day(s); GE, gastroenteritis; IV, intravenous; IVIG, intravenous immunoglobulin; MOF, multiple organ failure; NA, not available; SJS, Stevens- Johnson syndrome; TEN, toxic epidermal necrolysis; UTI, urinary tract infection. sites of involvement, with oral mucosal involvement in 15, eye involvement in 11, and genital lesions in 10 patients. Fever higher than 388C was seen in 12 of the 15 patients, and 5 had upper respiratory tract symptoms (eg, cough and blocked nose). Laboratory investigations The mean white blood cell count was /L (range /L), mean hemoglobin level was 13.4 g/dl (range g/dl) and mean platelet count was /L (normal: /L). Only the patient with TEN had abnormal white blood cell count ( /L), hemoglobin (10.7 g/dl), and platelet ( /L) levels on admission. CRP levels ranged from 5.0 to mg/l with a mean of 50.3 mg/l (normal: 1-10 mg/l). ESR ranged from 8 to 130 mm/h, with a mean of 46.2 mm/h (normal: 0-10 mm/h). CRP levels and ESR
4 JAM ACAD DERMATOL VOLUME 62, NUMBER 1 Kob and Tay 57 were normal in the two patients with SJS/TEN overlap and TEN. Liver enzymes were overtly abnormal in two patients. Patient 14, with SJS caused by lamotrigine, had increased serum alanine transaminase (ALT), 247 U/L (normal: 7-31 U/L), and aspartate transaminase, 560 U/L (normal: U/L) levels. The patient with TEN (patient 15), who succumbed to multi-organ failure, had increased serum ALT 270 U/L, aspartate transaminase 418 U/L, alkaline phosphatase 858 U/L (normal: U/L), and total bilirubin 166 mol/l (normal: 2-18 mol/l) levels. Three other patients had marginally raised serum ALT levels of U/L. Except for the patient with TEN, all patients had normal renal function. Three patients had positive mycoplasma serology. Herpes simplex virus isolation from mouth erosions was negative in 9 patients tested. Other viral studies (EBV serology, immunofluorescence for respiratory viruses) performed in a small number of patients were negative. Three patients had a skin biopsy performed to confirm the diagnosis. All 3 biopsy specimens showed an interface dermatitis with apoptotic bodies in the epidermis and a superficial perivascular infiltrate of mononuclear cells with some polymorphonuclear leukocytes. Treatment and outcome Decisions on treatment were based on the severity of disease, as well as on the preference of the treating physician. Three patients with SJS and the only patient with TEN were treated with IVIG, 2 g/kg, administered in divided doses over 2 or 4 days. The patients with SJS were started on a regimen of IVIG 6 to 7 days after onset of the rash. The average time to cessation of progression of disease, defined as cessation of progression of rash and blistering, was 2.7 days (range: 2-3 days). The patient with TEN received IVIG 34 days after the onset of skin disease. This patient was transferred to our institution after 2 weeks of inpatient treatment at a hospital in a neighboring country and died as a result of multiple-organ failure and neutropenic sepsis. Five patients with SJS received systemic corticosteroids. Three patients received oral prednisolone mg/kg per day for a period of 5 days to a month (with tapering doses). Two patients were commenced on a regimen of intravenous hydrocortisone, mg/kg per day, with subsequent conversion to oral prednisolone. These patients were started on a regimen of systemic corticosteroids for an average of 4 days (range: 2-6 days) after onset of the rash. Three also received a macrolide antibiotic (two receiving clarithromycin, one azithromycin) for treatment of mycoplasma infection. The average time to cessation of progression of disease in this group was 1.5 days (range: 1-2 days). The remaining 6 patients received supportive therapy only. From the time the inciting medication was stopped to cessation of progression of disease in this group was 4.2 days (range: 2-6 days). The time to cessation of progression of disease in all 3 groups was not significantly different (P [.05). Apart from the patient who died, complications were few and minor. Two patients had mild hypotension that responded to intravenous fluid therapy, one patient had hyponatremia, and one patient developed cellulitis at the intravenous site. One patient (patient 11) developed corneal ulceration a few days after diagnosis of SJS. However, this resolved after 3 weeks of topical antibiotic eye-drops. The average length of hospital stay for the IVIGtreated group was 23 days (range: days), the systemic corticosteroid-treated group 6.8 days (range: 3-9 days) and the supportive therapy group 6.3 days (range: 3-9 days). DISCUSSION The incidence of SJS and TEN is low, with an estimate of between 1.5 to 2 cases per million per year in the general population, and 0.07 to 0.2 per 1000 hospitalized patients. 6,18,19 In our series, there were 15 cases of SJS and TEN over a 5-year period, giving an estimated incidence of about 2 to 2.5 cases per million per year in the pediatric population. Similar to previous reports, the incidence of SJS far exceeds that of TEN. Contrary to reports of a higher incidence in women, 2 our series had more boys than girls with a ratio of about 2.5:1. The most common drugs implicated were the anticonvulsants and betalactam antibiotics. There was only one case of sulfonamide causation probably because this class of drug is now uncommonly used as first-line antibiotic therapy for children in our country. Mycoplasma infection was a cause of SJS in 3 children. This association appears to be more common in the pediatric population compared to adult patients. 2 All our patients were of Asian descent, with the majority being Chinese. It has been shown that genetic susceptibility plays a strong role in the pathogenesis of SJS and TEN. A recent study showed that HLA-B*1502 is strongly associated with carbamazepine-induced SJS in a Han Chinese population. 20 Another study showed a strong association for HLA- B*5801 with allopurinol-induced SJS/TEN in the Japanese population. 21 In the future, the understanding of HLA associations with drug reactions may be useful to predict a patient s susceptibility to a medication before treatment with the drug is instituted.
5 58 Koh and Tay JAM ACAD DERMATOL JANUARY 2010 Table II. Studies of SJS/TEN in pediatric patients References No. of patients Treatment Outcome Morici et al 15 (2000) 12 (SJS) 7 pts, IVIG (single dose mg/kg) 2 pts, corticosteroids 3 pts, supportive care Duration of fever: 8 d (IVIG group) 14 d (non-ivig group) Mean hospital stay: 12 d (IVIG group) 15 d (non-ivig group) Tristani-Firouzi et al 16 (2002) 8 (TEN) IVIG ( mg/kg/d for 4 d) Arrest of progression: 2.1 d Complete re-epithelialization: 9.1 d Metry, Jung, and Levy 9 (2003) 7 (SJS) IVIG (average dose 2.0 g/kg distributed evenly over 4 d) (5 patients received systemic corticosteroids before IVIG was administered) Cessation of blistering: 2 d Mangla et al 17 (2005) 10 (TEN) IVIG ( mg/kg/d for 5 d) Arrest of progression: 2.1 d Complete re-epithelialization: 8.1 d Leaute-Labreze et al 8 (2000) 10 (SJS) Prednisolone (1 mg/kg/d for 1 wk) Mean duration of disease similar to that of non-steroidetreated group Forman et al 7 (2002) 10 (SJS or TEN) Systemic corticosteroids (no mention of dosage or duration) Lam et al 33 (2004) 10 (SJS or TEN) Prednisolone 1-2 mg/kg/d for 3-5 d (IVIG given for those poorly responsive to prednisolone) pts, Patients; for other abbreviations, see legend for Table I. All 11 pts survived (no comparison group)? Satisfactory results The US Food and Drug Administration has recommended genotyping all Asian patients for HLA- B*1502 before commencing treatment with carbamazepine. 22 SCORTEN, a TEN-specific severity of illness score was first proposed by Bastuji-Garin et al 10 in 2000; it is based on 7 prognostic factors of age, malignancy, surface area involved, heart rate, serum urea, bicarbonate, and glucose. When calculated within 24 hours after admission, SCORTEN has been shown to accurately predict the risk of mortality. As its use in the pediatric population had not been fully validated, it was not used in our center during the period of our study. Treatment of SJS and TEN requires prompt diagnosis with immediate cessation of the causative drug. It has been shown that the earlier the causative drug is withdrawn, the better the prognosis. 23 Optimal supportive therapy is still of utmost importance in the treatment of patients with SJS and TEN. Patients should ideally be managed in a specialized intensive care or burns unit. Essential aspects of supportive therapy include maintenance of fluid and electrolyte homeostasis; environmental temperature control; prevention and treatment of infections; respiratory and nutritional support, and adequate analgesia. Energy requirements of pediatric patients with SJS and TEN are increased, and the application of a 30% factor to resting energy requirements should be made when calculating nutritional support. 24 Early ophthalmologic consultation is essential in evaluating eye involvement and preventing ocular complications. 25 Because of the low incidence of the disease, randomized controlled trials comparing immunosuppressive therapies for SJS and TEN are rare. There has been only one prospective, randomized-controlled trial in the treatment of TEN, involving the use of thalidomide, which was terminated early because of an increased mortality rate in the treatment arm. The majority of reports in the literature involve single-case observations, case series, or small, uncontrolled studies. The largest series to date, arising from the EuroSCAR study, involved a retrospective analysis of 281 patients from France and Germany. This study compared the use of supportive therapy, IVIG, corticosteroids, and IVIG plus corticosteroids. The authors observed no significant benefit from
6 JAM ACAD DERMATOL VOLUME 62, NUMBER 1 Kob and Tay 59 treatment and concluded that evidence is insufficient to recommend any specific treatment. 26 In our study, 4 patients were treated with IVIG (total dose 2 g/kg), 5 patients were treated with systemic corticosteroids (IV hydrocortisone or oral prednisolone) and 6 patients were treated with supportive therapy only. There was no significant difference in the time to cessation of disease between the 3 groups of patients. The duration of hospital stay for the IVIG group was longer than the other two groups of patients. However, we cannot exclude bias toward administering IVIG for more severely affected patients. In 1998, Viard et al 27 first reported that the interaction between Fas and FasL might play a role in apoptosis in TEN and showed that IVIG inhibited Fas-mediated apoptosis in sensitive cell lines by blocking Fas receptors. This article also showed a positive outcome in a series of 10 patients with SJS or TEN treated with IVIG. Since then, there have been several studies evaluating its use in SJS and TEN. Most of these studies have shown a trend toward improved mortality, 28,29 whereas 4 studies did not show any benefit on mortality rates. 26,30-32 Patients who received IVIG within the first 4 days of onset of the skin eruption appeared to have a shorter time to cessation of disease progression and complete reepithelialization. Total doses of 3 to 4 g/kg appeared to be more effective than doses of 2 g/kg. Studies documenting the use of IVIG in children with SJS and TEN appear to show a favorable outcome. However, this may be due to the disease being relatively milder in children compared to adults. Table II summarizes these articles. The outcome of pediatric patients treated with systemic corticosteroids is also unclear. Léauté- Labrèze et al 8 treated 10 children with SJS with prednisolone, 1 mg/kg per day for 1 week, but found that the mean duration of disease was similar to patients in the non-steroid group. Forman, Koren, and Shear 7 described 11 children with SJS or TEN who survived after treatment with oral corticosteroids, but there was no mention of dosage or duration of steroid used. Lam et al 33 treated 10 cases of SJS or TEN with 1 to 2 mg/kg per day of prednisolone for 3 to 5 days. Those patients with poor responsiveness to steroids were treated with additional IVIG at 1 mg/kg per day with satisfactory results. More recently, the use of cyclosporine, 3 mg/kg per day, has been reported in a small case series and several case reports. 34 In addition to its anti-apoptotic activity, cyclosporine inhibits CD8 activation, leading to inhibition of cytotoxic activity. None of our patients was treated with cyclosporine. In conclusion, early withdrawal of a causative drug and optimal supportive therapy are still the most important aspects of management of SJS and TEN. In our pediatric patients, treatment with IVIG or systemic corticosteroids did not appear to improve the outcome of the disease. With a better understanding of the pathogenesis of the condition, more specific therapies may emerge that can target specific mediators of apoptosis. REFERENCES 1. Prendiville J. Stevens-Johnson syndrome and toxic epidermal necrolysis. Adv Dermatol 2002;18: Tay YK, Huff C, Weston WL. Mycoplasma pneumoniae infection in associated with Stevens-Johnson syndrome, not erythema multiforme (von Hebra). J Am Acad Dermatol 1996;35: Schöpf E, Stuhmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome: an epidemiologic study from West Germany. Arch Dermatol 1991;127: Roujeau JC, Guillaume JC, Febre JP, Penso D, Fléchet ML, Girre JP. Toxic epidermal necrolysis (Lyell s syndrome) incidence and drug etiology in France, Arch Dermatol 1990;126: Guillaume JC, Roujeau JC, Revuz J, Penso D, Touraine R. The culprit drugs in 87 cases of toxic epidermal necrolysis (Lyell s syndrome). Arch Dermatol 1987;123: Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331: Forman R, Koren G, Shear NH. Erythema multiforme, Stevens- Johnson syndrome and toxic epidermal necrolysis in children e a review of 10 years experience. Drug Safety 2002;25: Leaute-Labreze C, Lamireau T, Chawki D, Maleville J, Taieb A. Diagnosis, classification, and management of erythema multiforme and Stevens-Johnson syndrome. Arch Dis Child 2000;83: Metry DW, Jung P, Levy ML. Use of intravenous immunoglobulin in children with Stevens-Johnson syndrome and toxic epidermal necrolysis: seven cases and review of the literature. Pediatrics 2003;112: Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol 2000;115: Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, Roujeau JC, Revuz J. Performance of SCORTEN during the first five days of hospitalization to predict the prognosis of toxic epidermal necrolysis. J Invest Dermatol 2006;126: Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol 2007;87: Yamane Y, Aihara M, Ikezawa Z. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to Allergol Int 2007;56: Tripathi A, Ditto AM, Grammer LC, Greenberger PA, McGrath KG, Zeiss CR, et al. Corticosteroid therapy in an additional 13 cases of Stevens-Johnson syndrome: a total series of 67 cases. Allergy Asthma Proc 2000;21: Morici MV, Galen WK, Shetty AK, Lebouef RP, Gouri TP, Cowan GS, et al. Intravenous immunoglobulin therapy for children with Stevens-Johnson syndrome. J Rheumatol 2000; 27: Tristani-Firouzi P, Petersen MJ, Saffle JR, Morris SE, Zone JJ. Treatment of toxic epidermal necrolysis with intravenous
7 60 Koh and Tay JAM ACAD DERMATOL JANUARY 2010 immunoglobulin in children. J Am Acad Dermatol 2002;47: Mangla K, Rastogi S, Goyal P, Solanki RB, Rawal RC. Efficacy of low dose intravenous immunoglobulins in children with toxic epidermal necrolysis: an open uncontrolled study. Indian J Dermatol Venereol Leprol 2005;71: Fiszenson-Albala F, Auzerie V, Mahe E, Farinotti R, Durand- Stocco C, Crickx B, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol 2003;149: Li LF, Ma C. Epidemiological study of severe cutaneous adverse drug reactions in a city district of China. Clin Exp Dermatol 2006;31: Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature 2004;428: Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics 2008;9: Ferrell PB Jr, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics 2008;9: Paquet P, Jacob E, Quatresooz P, Jacquemin D, Piérard GE. Delayed reepithelialization and scarring deregulation following drug-induced toxic epidermal necrolysis. Burns 2007;33: Mayes T, Gottschlich M, Khoury J, Warner P, Kagan R. Energy requirements of pediatric patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Nutr Clin Pract 2008;23: Yip LW, Thong BY, Lim J, Tan AW, Wong HB, Handa S, et al. Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy 2007;62: Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatment on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR study. J Am Acad Dermatol 2008;58: Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998; 282: French LE, Trent JT, Kerdel FA. Use of intravenous immunoglobulin in toxic epidermal necrolysis and Stevens-Johnson syndrome: our current understanding. Int Immunopharmacol 2006;6: Mittmann N, Chan B, Knowles S, Cosentino L, Shear N. Intravenous immunoglobulin use in patients with toxic epidermal necrolysis and Stevens-Johnson syndrome. Am J Clin Dermatol 2006;7: Bachot N, Revuz J, Roujeau JC. Intravenous immunoglobulin for Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol 2003; 139: Shortt R, Gomez M, Mittman N, Cartotto R. Intravenous immunoglobulin does not improve outcome in toxic epidermal necrolysis. J Burn Care Rehabil 2004;25: Brown JM, Silver GM, Halerz M, Walaszek P, Sandroni A, Gamelli RL. Toxic epidermal necrolysis: does immunoglobulin make a difference? J Burn Care Rehabil 2004;25: Lam NS, Yang YH, Wang LC, Lin YT, Chiang BL. Clinical characteristics of childhood erythema multiforme, Stevens- Johnson syndrome and toxic epidermal necrolysis in Taiwanese children. J Microbiol Immunol Infect 2004;37: Arevalo JM, Lorente JA, Gonzalez-Herrada C, Jimenez-Reyes J. Treatment of toxic epidermal necrolysis with cyclosporin A. J Trauma 2000;48:473-8.
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
 Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
 Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
 Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review
 140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients
 original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital
 Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis
 Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update
 Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection
 Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Skin Manifestations of Drug Reactions
 Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Scratching the Surface: A Review of SJS/TEN
 Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
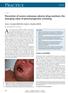 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from 2000 to 2006 Yumiko Yamane 1, Michiko Aihara 1 and Zenro Ikezawa 1
 Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Pediatric Dermatology
 Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
1 Introduction. Kenneth Irungu 1 David Nyamu
 Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Future of Pediatrics: Blisters, Hives and Other Tales from the Emergency Room June 14 th, 2016
 A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash
 CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
Severe Desquamating Disorder After Liver Transplant: Toxic Epidermal Necrolyis or Graft Versus Host Disease?
 Severe Desquamating Disorder After Liver Transplant: Toxic Epidermal Necrolyis or Graft Versus Host Disease? John T. Schulz III, MD, PhD, FACS, and Robert L. Sheridan, MD, FACS Bridgeport Hospital, Bridgeport,
Severe Desquamating Disorder After Liver Transplant: Toxic Epidermal Necrolyis or Graft Versus Host Disease? John T. Schulz III, MD, PhD, FACS, and Robert L. Sheridan, MD, FACS Bridgeport Hospital, Bridgeport,
To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving
 Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC
 SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
Bugs and Drugs: What s New in Hypersensitivity Reactions?
 Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS
 PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME
 EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive Patients
 Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit
 J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
PedsCases Podcast Scripts
 PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
Cutaneous Conditions Associated with Systemic Disease
 Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Emergency Dermatology Dr Melissa Barkham
 Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea 2
 pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2013;27(5):331-340 http://dx.doi.org/10.3341/kjo.2013.27.5.331 Original Article Effect of Age and Early Intervention with a Systemic Steroid, Intravenous
pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2013;27(5):331-340 http://dx.doi.org/10.3341/kjo.2013.27.5.331 Original Article Effect of Age and Early Intervention with a Systemic Steroid, Intravenous
Original Article. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C
 Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Department of Pharmacology & NIPER-Guwahati, Gauhati Medical College & Hospital, Guwahati , Assam, INDIA. 3
 J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
In the past few years, beta-lactam-resistant gram-positive
 Early Diagnosis Is Key in Vancomycin-Induced Linear IgA Bullous Dermatosis and Stevens-Johnson Syndrome Douglas H. Jones, MS Michael Todd, MD Timothy J. Craig, DO Background: With the emergence of highly
Early Diagnosis Is Key in Vancomycin-Induced Linear IgA Bullous Dermatosis and Stevens-Johnson Syndrome Douglas H. Jones, MS Michael Todd, MD Timothy J. Craig, DO Background: With the emergence of highly
Toxic epidermal necrolysis
 REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
Severe cutaneous adverse reactions (SCAR) are life-threatening
 Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS
 STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS *Blanca R. Del Pozzo-Magaña, 1 Alejandro Lazo-Langner 2,3 1. Department of Pediatrics, University
STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS *Blanca R. Del Pozzo-Magaña, 1 Alejandro Lazo-Langner 2,3 1. Department of Pediatrics, University
Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics
 MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW
 STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review
 Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Endocarditis. By : Mehrnoush. dianatkhah
 Endocarditis By : Mehrnoush. dianatkhah Case 5.31, 31 years old woman CC : Fever, dyspnea, 3 days postpartum PMH : Mitral prolapse Fever 38.5 WBC : 8900 ESR : 84 CRP : 10.4 Cr : 0.6 NT Pro BNP: 5469 Physical
Endocarditis By : Mehrnoush. dianatkhah Case 5.31, 31 years old woman CC : Fever, dyspnea, 3 days postpartum PMH : Mitral prolapse Fever 38.5 WBC : 8900 ESR : 84 CRP : 10.4 Cr : 0.6 NT Pro BNP: 5469 Physical
Drug Allergy A Guide to Diagnosis and Management
 Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Several different manifestations
 Toxic epidermal necrolysis and Stevens-Johnson syndrome: A review* Roland Gerull, MD; Mathias Nelle, MD; Thomas Schaible, MD Objectives: The aims of this review are to summarize the definitions, causes,
Toxic epidermal necrolysis and Stevens-Johnson syndrome: A review* Roland Gerull, MD; Mathias Nelle, MD; Thomas Schaible, MD Objectives: The aims of this review are to summarize the definitions, causes,
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions
 Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review
 HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HEMORRHAGIC BULLOUS HENOCH- SCHONLEIN PURPURA: A CASE REPORT
 HEMORRHAGIC BULLOUS HENOCH- SCHONLEIN PURPURA: A CASE REPORT Nirmala Ponnuthurai, Sabeera Begum, Lee Bang Rom Paediatric Dermatology Unit, Institute of Paediatric, Hospital Kuala Lumpur, Malaysia Abstract
HEMORRHAGIC BULLOUS HENOCH- SCHONLEIN PURPURA: A CASE REPORT Nirmala Ponnuthurai, Sabeera Begum, Lee Bang Rom Paediatric Dermatology Unit, Institute of Paediatric, Hospital Kuala Lumpur, Malaysia Abstract
Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
 BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
Severe cutaneous reactions caused by barbiturates in seven Iranian children
 Pharmacology and therapeutics Severe cutaneous reactions caused by barbiturates in seven Iranian children Setareh Mamishi, MD, Fatemeh Fattahi, MD, Zahra Pourpak, MD, PhD, Farzaneh Mirza Aghaee, MD, Zeinab
Pharmacology and therapeutics Severe cutaneous reactions caused by barbiturates in seven Iranian children Setareh Mamishi, MD, Fatemeh Fattahi, MD, Zahra Pourpak, MD, PhD, Farzaneh Mirza Aghaee, MD, Zeinab
Case Rep Dermatol 2009;1:66 70 DOI: / Key Words Coma Blister Barbiturate Overdose Meningoencephalitis
 66 Coma Blisters Joana Rocha a Teresa Pereira a Filipa Ventura a Fernando Pardal b Celeste Brito a Departments of a Dermatology and b Pathology, Hospital de São Marcos, Braga, Portugal Key Words Coma Blister
66 Coma Blisters Joana Rocha a Teresa Pereira a Filipa Ventura a Fernando Pardal b Celeste Brito a Departments of a Dermatology and b Pathology, Hospital de São Marcos, Braga, Portugal Key Words Coma Blister
A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia
 ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
Stevens-Johnson Syndrome as an Unusual Adverse Effect of Azithromycin
 2006;14(1):40-45 CASE REPORT Stevens-Johnson Syndrome as an Unusual Adverse Effect of Azithromycin Neva Brkljačić, Sonja Gracin, Ingrid Prkačin, Mirjana Sabljar-Matovinović, Anna Mrzljak, Zlatka Nemet
2006;14(1):40-45 CASE REPORT Stevens-Johnson Syndrome as an Unusual Adverse Effect of Azithromycin Neva Brkljačić, Sonja Gracin, Ingrid Prkačin, Mirjana Sabljar-Matovinović, Anna Mrzljak, Zlatka Nemet
Diagnosis and Management of Drug-induced Stevens-Johnson Syndrome: Report of Two Cases
 10.5005/jp-journals-10011-1189 CASE REPORT JIAOMR Diagnosis and Management of Drug-induced Stevens-Johnson Syndrome: Report of Two Cases 1 M Venkateshwarlu, 2 B Radhika 1 Professor and Head, Department
10.5005/jp-journals-10011-1189 CASE REPORT JIAOMR Diagnosis and Management of Drug-induced Stevens-Johnson Syndrome: Report of Two Cases 1 M Venkateshwarlu, 2 B Radhika 1 Professor and Head, Department
Emergency Dermatology. Emergency Dermatology
 Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Toxic Epidermal Necrolysis - A Case Report
 The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study
 Q J Med 2012; 105:839 846 doi:10.1093/qjmed/hcs078 Advance Access Publication 28 April 2012 Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study S.M.H. KANNENBERG
Q J Med 2012; 105:839 846 doi:10.1093/qjmed/hcs078 Advance Access Publication 28 April 2012 Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study S.M.H. KANNENBERG
Stevens-Johnson Syndrome : A Case Report
 https://doi.org/10.5933/jkapd.2017.44.4.455 J Korean Acad Pediatr Dent 44(4) 2017 ISSN (print) 1226-8496 ISSN (online) 2288-3819 Stevens-Johnson Syndrome : A Case Report Yongho Song, Nanyoung Lee, Sangho
https://doi.org/10.5933/jkapd.2017.44.4.455 J Korean Acad Pediatr Dent 44(4) 2017 ISSN (print) 1226-8496 ISSN (online) 2288-3819 Stevens-Johnson Syndrome : A Case Report Yongho Song, Nanyoung Lee, Sangho
ACUTE ANNULAR URTICARIA IN A CHILD
 The West London Medical Journal 2013 Vol 5 No 2 pp 1-5 ACUTE ANNULAR URTICARIA IN A CHILD Aaron Yon Dayse Fernandes Michelle Pike Jodi Newcombe Colin Michie 1. ABSTRACT Urticarial skin rashes have a range
The West London Medical Journal 2013 Vol 5 No 2 pp 1-5 ACUTE ANNULAR URTICARIA IN A CHILD Aaron Yon Dayse Fernandes Michelle Pike Jodi Newcombe Colin Michie 1. ABSTRACT Urticarial skin rashes have a range
BJD. Summary. British Journal of Dermatology THERAPEUTICS
 THERAPEUTICS BJD British Journal of Dermatology Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of
THERAPEUTICS BJD British Journal of Dermatology Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of
TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN
 TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN Nadia Khaldi 1, Alain Miras 1, Sophie Gromb 1,2 1 Forensic Science Laboratory, Pellegrin Hospital, University Teaching Hospital Bordeaux, France, 2 Forensic
TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN Nadia Khaldi 1, Alain Miras 1, Sophie Gromb 1,2 1 Forensic Science Laboratory, Pellegrin Hospital, University Teaching Hospital Bordeaux, France, 2 Forensic
Big rashes in little patients:
 ! Big rashes in little patients: Severe drug eruptions and cutaneous infections!! Marcia Hogeling, MD, FAAD Assistant Clinical Professor Director, Pediatric Dermatology Division of Dermatology David Geffen
! Big rashes in little patients: Severe drug eruptions and cutaneous infections!! Marcia Hogeling, MD, FAAD Assistant Clinical Professor Director, Pediatric Dermatology Division of Dermatology David Geffen
AOU Ospedali Riuniti - Ancona
 AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
Interesting Case Series. Linear IgA Bullous Dermatosis
 Interesting Case Series Linear IgA Bullous Dermatosis Sean Chen, BA, a Peter Mattei, MD, a Max Fischer, MD, MPH, a Joshua D. Gay, PA-C, b Stephen M. Milner, MBBS, BDS, FRCS (Ed), FACS, b and Leigh Ann
Interesting Case Series Linear IgA Bullous Dermatosis Sean Chen, BA, a Peter Mattei, MD, a Max Fischer, MD, MPH, a Joshua D. Gay, PA-C, b Stephen M. Milner, MBBS, BDS, FRCS (Ed), FACS, b and Leigh Ann
DERMATOLOGICAL EMERGENCIES. DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE
 DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW
 SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
DOSING GUIDE. Indications. Important Safety Information. Enable the immune system. RECOGNIZE. RESPOND.
 DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
Erythema Multiforme with Reference to Atypical Presentation in an HIV-Positive Patient Following Antiretroviral Therapy Discontinuation
 2009;17(1):9-15 CLINICAL ARTICLE Erythema Multiforme with Reference to Atypical Presentation in an HIV-Positive Patient Following Antiretroviral Therapy Discontinuation Liborija Lugović Mihić, Marija Buljan,
2009;17(1):9-15 CLINICAL ARTICLE Erythema Multiforme with Reference to Atypical Presentation in an HIV-Positive Patient Following Antiretroviral Therapy Discontinuation Liborija Lugović Mihić, Marija Buljan,
Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin
 Turkish Journal of Cancer Vol. 32/ No. 4/2002 Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin ERKAN TOPKAN, FARUK ZORLU, MURAT GÜRKAYNAK Hacettepe University
Turkish Journal of Cancer Vol. 32/ No. 4/2002 Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin ERKAN TOPKAN, FARUK ZORLU, MURAT GÜRKAYNAK Hacettepe University
PAEDIATRIC FEBRILE NEUTROPENIA CARE PATHWAY
 PAEDIATRIC FEBRILE NEUTROPENIA CARE PATHWAY Purpose: This document is intended as a guide to the investigation and management of children presenting in Salisbury District Hospital with suspected neutropenic
PAEDIATRIC FEBRILE NEUTROPENIA CARE PATHWAY Purpose: This document is intended as a guide to the investigation and management of children presenting in Salisbury District Hospital with suspected neutropenic
Intravenous immunoglobulins and plasmapheresis combined treatment in patients with severe toxic epidermal necrolysis: preliminary report *
 British Journal of Plastic Surgery (2005) 58, 504 510 Intravenous immunoglobulins and plasmapheresis combined treatment in patients with severe toxic epidermal necrolysis: preliminary report * M. Lissia*,
British Journal of Plastic Surgery (2005) 58, 504 510 Intravenous immunoglobulins and plasmapheresis combined treatment in patients with severe toxic epidermal necrolysis: preliminary report * M. Lissia*,
Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015)
 1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Purpuric drug eruption without leukocytoclastic vasculitis associated with vancomycin
 Asian Pacific Journal of Allergy and Immunology CASE REPORT Purpuric drug eruption without leukocytoclastic vasculitis associated with vancomycin Tamaki Nakamura, 1 Hiroyuki Wakiguchi, 1 Fumiko Okazaki,
Asian Pacific Journal of Allergy and Immunology CASE REPORT Purpuric drug eruption without leukocytoclastic vasculitis associated with vancomycin Tamaki Nakamura, 1 Hiroyuki Wakiguchi, 1 Fumiko Okazaki,
Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate
 Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
A. Erythema multiforme and related diseases
 Go Back to the Top To Order, Visit the Purchasing Page for Details Chapter Erythema, Erythroderma (Exfoliative Dermatitis) Erythema is caused by telangiectasia or hyperemia in the papillary and reticular
Go Back to the Top To Order, Visit the Purchasing Page for Details Chapter Erythema, Erythroderma (Exfoliative Dermatitis) Erythema is caused by telangiectasia or hyperemia in the papillary and reticular
Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics
 Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics Maja Mockenhaupt, MD; John Messenheimer, MD; Pat Tennis, PhD; and Juergen Schlingmann Abstract Background:
Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics Maja Mockenhaupt, MD; John Messenheimer, MD; Pat Tennis, PhD; and Juergen Schlingmann Abstract Background:
Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital
 International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
Accepted Manuscript. Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need
 Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Severe cutaneous adverse reactions: an evidence based approach
 Original Article 21 Severe cutaneous adverse reactions: an evidence based approach D.P. Shrestha, D. Gurung, A. Kumar Department of Dermatology and Venereology, Maharagunj Campus, Institute of Medicine,
Original Article 21 Severe cutaneous adverse reactions: an evidence based approach D.P. Shrestha, D. Gurung, A. Kumar Department of Dermatology and Venereology, Maharagunj Campus, Institute of Medicine,
CUTANEOUS DRUG REACTIONS OR I WOULDN T HAVE SEEN IT, IF I HADN T BELIEVED IT Edmund J. Rosser Jr., DVM, DACVD
 CUTANEOUS DRUG REACTIONS OR I WOULDN T HAVE SEEN IT, IF I HADN T BELIEVED IT Edmund J. Rosser Jr., DVM, DACVD DERMATOLOGY Pathogenesis Immunologic: can involve Type I, II, III, IV hypersensitivity reactions.
CUTANEOUS DRUG REACTIONS OR I WOULDN T HAVE SEEN IT, IF I HADN T BELIEVED IT Edmund J. Rosser Jr., DVM, DACVD DERMATOLOGY Pathogenesis Immunologic: can involve Type I, II, III, IV hypersensitivity reactions.
Loraine L. W. Chow 1, Kendrick C. Shih 2, Johnny C. Y. Chan 3, Jimmy S. M. Lai 2 and Alex L. K. Ng 2*
 Chow et al. BMC Ophthalmology (2017) 17:65 DOI 10.1186/s12886-017-0464-9 RESEARCH ARTICLE Open Access Comparison of the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis
Chow et al. BMC Ophthalmology (2017) 17:65 DOI 10.1186/s12886-017-0464-9 RESEARCH ARTICLE Open Access Comparison of the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis
Corticosteroids in Severe CAP. Mervyn Mer Department of Medicine & ICU Johannesburg Hospital University of the Witwatersrand
 Corticosteroids in Severe CAP Mervyn Mer Department of Medicine & ICU Johannesburg Hospital University of the Witwatersrand Introduction Much controversy and debate regarding the use of corticosteroids
Corticosteroids in Severe CAP Mervyn Mer Department of Medicine & ICU Johannesburg Hospital University of the Witwatersrand Introduction Much controversy and debate regarding the use of corticosteroids
Mark A. Bechtel, MD Clinical Associate Professor Division Director, Dermatology Ohio State University Medical Center
 Dermatologic Emergencies Mark A. Bechtel, MD Clinical Associate Professor Division Director, Dermatology Ohio State University Medical Center Clinical Features of SJS/TEN Initial symptoms Fever, stinging
Dermatologic Emergencies Mark A. Bechtel, MD Clinical Associate Professor Division Director, Dermatology Ohio State University Medical Center Clinical Features of SJS/TEN Initial symptoms Fever, stinging
ABSTRACT. Keywords: Corticosteroids; Cyclosporine; Dermatology; Etanercept; Immunoglobulin; Necrolysis; Johnson; Stevens; Syndrome REVIEW
 Adv Ther (2017) 34:1235 1244 DOI 10.1007/s12325-017-0530-y REVIEW Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing
Adv Ther (2017) 34:1235 1244 DOI 10.1007/s12325-017-0530-y REVIEW Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing
Cutaneous Drug Reactions
 Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Oral Acetazolamide after Boston Keratoprosthesis in Stevens Johnson Syndrome
 Oral Acetazolamide after Boston Keratoprosthesis in Stevens Johnson Syndrome The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
Oral Acetazolamide after Boston Keratoprosthesis in Stevens Johnson Syndrome The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
Five things not to miss in Dermatology. Dr Judy Wismer Associate Clinical Professor Michael G DeGroote School of Medicine
 Five things not to miss in Dermatology Dr Judy Wismer Associate Clinical Professor Michael G DeGroote School of Medicine Key Descriptives Fever, skin pain Purpura, necrosis Bullae, Mucosal, Skin sloughing
Five things not to miss in Dermatology Dr Judy Wismer Associate Clinical Professor Michael G DeGroote School of Medicine Key Descriptives Fever, skin pain Purpura, necrosis Bullae, Mucosal, Skin sloughing
Health economics and drug safety
 Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
PHM142 Autoimmune Disorders + Idiosyncratic Drug Reactions
 PHM142 Autoimmune Disorders + Idiosyncratic Drug Reactions 1 Autoimmune Disorders Auto-reactivity: low physiological levels (e.g. tolerance) vs. pathogenic levels 80+ types of autoimmune diseases affect
PHM142 Autoimmune Disorders + Idiosyncratic Drug Reactions 1 Autoimmune Disorders Auto-reactivity: low physiological levels (e.g. tolerance) vs. pathogenic levels 80+ types of autoimmune diseases affect
The liver and skin are two of the commonest
 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center
 Management of the Burn Patient Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center American Burn Association Transfer Criteria Burn > 10%
Management of the Burn Patient Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center American Burn Association Transfer Criteria Burn > 10%
- Mycoplasma pneumoniae (MP): important respiratory pathogen in children that cause many upper and lower respiratory tract diseases, including
 KHOA DICH VU HO HAP - Mycoplasma pneumoniae (MP): important respiratory pathogen in children that cause many upper and lower respiratory tract diseases, including wheezing, coryza, bronchopneumonia. -
KHOA DICH VU HO HAP - Mycoplasma pneumoniae (MP): important respiratory pathogen in children that cause many upper and lower respiratory tract diseases, including wheezing, coryza, bronchopneumonia. -
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
GOOD MORNING! AUGUST 5, 2014
 GOOD MORNING! AUGUST 5, 2014 PREP QUESTION During the health supervision visit of a term newborn boy, his mother relates that a cousins child died at age 4 months from sudden infant death syndrome. She
GOOD MORNING! AUGUST 5, 2014 PREP QUESTION During the health supervision visit of a term newborn boy, his mother relates that a cousins child died at age 4 months from sudden infant death syndrome. She
