Percutaneous Leads. Directions for Use Content: REV A
|
|
|
- Randolf Fowler
- 6 years ago
- Views:
Transcription
1 Percutaneous Leads Directions for Use CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician Content: REV A
2 Guarantees Boston Scientific Corporation reserves the right to modify, without prior notice, information relating to its products in order to improve their reliability or operating capacity. Drawings are for illustration purposes only. Trademarks All trademarks are the property of their respective holders. Additional Information For Indications and related information, see the Indications DFU or Clinician Manual for your spinal cord stimulator system. For contraindications, warnings, precautions, adverse events summary, physician instructions, sterilization, component disposal, and contact information for Boston Scientific, refer to the Information for Prescribers DFU or Clinician Manual for your spinal cord stimulator system. For other device-specific information not included in this manual, labeling symbols, and warranty, refer to the appropriate DFU as listed on your Reference Guide. For clinical studies supporting the clinical use of the neurostimulation system, refer to the Information for Prescribers DFU or Clinician Manual for your spinal cord stimulator system. Product Model Numbers Model Description SC-2016-xx Infinion 16 Lead and Splitter 2x8 Kit SC-2016-xxE Infinion 16 Lead and Splitter 2x8 Trial Kit SC-2138-xx Linear xxcm 8 Contact Lead SC-2158-xx Linear xxcm 8 Contact Lead SC-2158-xxE Linear xxcm 8 Contact Lead SC-2208-xx Linear ST xxcm 8 Contact Lead SC-2218-xx Linear ST xxcm 8 Contact Lead SC-2218-xxE Linear ST xxcm 8 Contact Lead SC-2352-xx Linear 3-4 xxcm 8 Contact Lead SC-2352-xxE Linear 3-4 xxcm 8 Contact Lead SC-2366-xx Linear 3-6 xxcm 8 Contact Lead SC-2366-xxE Linear 3-6 xxcm 8 Contact Lead SC-3138-xx xxcm 8 Contact Extension SC-3304-xx D4 Splitter 2x4 SC-3354-xx W4 Splitter 2x4 SC-2316-xx Infinion 16 xxcm 16 Contact Lead Kit SC-2316-xxE Infinion 16 xxcm 16 Contact Trial Lead Kit SC-3400-xx xxcm Splitter 2x8 Kit SC-2317-xx Infinion CX xxcm 16 Contact Lead Kit SC-2317-xxE Infinion CX xxcm 16 Contact Trial Lead Kit Note: xx = length (cm), xxe = length (cm) Trial Lead ii of iv
3 Table of Contents Description...1 Percutaneous Leads...1 Trial Leads...1 Lead Extension...1 Infinion 16 Lead...1 Infinion CX Lead...1 Lead Splitters...2 Package Contents...3 Percutaneous Permanent Lead Kits...3 Percutaneous Trial Lead Kit...3 Lead Extension Kit...3 2x4 Splitter Kit...3 Splitter 2x8 Kit...3 Infinion 16 Lead Kit...4 Infinion 16 Trial Lead Kit...4 Infinion 16 Trial Configuration Kit...4 Infinion 16 Configuration Kit...4 Infinion CX Lead Kit...4 Infinion CX Trial Lead Kit...4 Specifications and Technical Data...5 Linear Lead...5 Infinion 16 Lead...5 Infinion CX Lead...6 Lead Extension...6 2x4 Splitter...6 Splitter 2x8...7 Registering the Leads...8 Instructions for Use...9 Lead, Lead Extension, and Splitter Handling and Storage...9 Percutaneous Lead Placement in the Epidural Space...9 Infinion CX Lead Placement in the Epidural Space with Entrada Needle Lead Connection to Splitter...14 Intraoperative Stimulation Testing...16 Securing the Trial Lead...17 Securing the Trial Infinion CX Lead...18 Permanent Lead Anchoring and Tunneling...18 Tunneling the Lead or Lead Extension...20 Connecting the Lead Extension...22 Lead/Extension/Splitter Removal...23 Programming with the Infinion 16 Lead and Infinion CX Lead iii of iv
4 This page intentionally left blank iv of iv
5 Description Description Leads function as a component of Boston Scientific s Spinal Cord Stimulation (SCS) systems by delivering electrical stimulation to the nerve structures in the dorsal aspect of the spinal cord, resulting in an inhibition of pain sensation. The Entrada Needle (Model SC-4220-XX) functions as a component of Boston Scientific s Spinal Cord Stimulation (SCS) systems. Percutaneous Leads The eight-contact percutaneous leads are available in lengths of 30 cm, 50 cm, and 70 cm. Each Linear lead has eight electrode contacts located near the distal end. Each contact is 3 mm in length and is spaced 1 mm, 4 mm, or 6 mm from the adjacent contact. Trial Leads The Trial Lead Kit, containing the Linear Lead and the associated components, is intended to be used for the temporary trial phase. Lead Extension Lead Extensions are designed to connect the percutaneous leads to the IPG for spinal cord stimulation. The extension may be added to a lead to externalize the lead for a trial procedure or to extend the lead when a permanent IPG is implanted. Lead extensions are available in lengths of 25 cm, 35 cm, and 55 cm. Each extension has eight electrode contacts located near the distal end. Each contact is 3 mm in length and is spaced 1 mm from the adjacent contact. The extension can be connected to either the Trial Stimulator (via an OR Cable Assembly included in the leads kit) or directly to the IPG. Infinion 16 Lead The Infinion 16 percutaneous lead is available in lengths of 50 cm and 70 cm. Each lead has 16 contacts located near the distal end. The Infinion 16 lead can be connected directly to a 1x16 OR Cable when using the Precision Spectra or Spectra WaveWriter Trial Stimulator. When connecting to an IPG or a 1x8 or 2x8 OR Cable, a single Infinion 16 lead must be inserted into a Splitter 2x8, which then connects to the IPG s 8 contact ports or the 1x8 or 2x8 OR Cable. Infinion CX Lead The Infinion CX percutaneous lead is available in lengths of 50 cm and 70 cm. Each lead has 16 contacts located near the distal end and two tails with 8 proximal contacts. The Infinion CX lead can be connected directly to a 2x8 OR Cable when using the Precision Spectra or Spectra WaveWriter Trial Stimulator or two 1x8 OR Cables when using the Precision Trial Stimulator. The Infinion CX Lead may be connected directly to the IPG s 8 contact ports of 27
6 Lead Splitters The 2x4 Lead Splitters are designed to connect multiple percutaneous leads to the IPG. The Linear leads may be inserted into a splitter for a maximum of four Linear leads per 2-port IPG and a maximum of eight leads per 4-port IPG. Four of the eight contacts of each Linear lead will be activated. Two configurations of Splitter 2x4 are available: Distal 4 (D4) and Wide 4 (W4). The two versions offer different contact configurations. The Lead Splitter 2x8 is required to connect the Infinion 16 contact lead to the IPG. Each Infinion 1x16 lead requires a Splitter 2x8 to be used with two IPG ports of 27
7 Package Contents Package Contents Percutaneous Permanent Lead Kits (1) Percutaneous Lead with pre-loaded Curved Stylet (1) Stylet Ring with a Curved and a Straight Stylet (4) Suture Sleeves (1) Insertion Needle with Stylet (1) Lead Blank (1) Steering Cap (1) OR Cable Assembly (2) Lead Position Labels left and right (non-sterile) (1) Device Registration Form/Temporary Patient Identification Card (1) Manual Percutaneous Trial Lead Kit (1) Percutaneous Lead with pre-loaded Curved Stylet (1) Suture Sleeve (1) Insertion Needle with Stylet (1) Steering Cap (1) OR Cable Assembly (2) Lead Position Labels left and right (non-sterile) (1) Device Registration Form/Temporary Patient Identification Card (1) Manual Lead Extension Kit (1) Lead Extension (1) Hex Wrench (1) Tunneling Tool Assembly (1) Device Registration Form/Temporary Patient Identification Card (1) Manual 2x4 Splitter Kit (1) Splitter (1) Hex wrench (1) Manual (1) Device Registration Form/Temporary Patient Identification Card Splitter 2x8 Kit (1) Splitter 2x8 (1) Torque Wrench (1) Manual (1) Device Registration Form/Temporary Patient Identification Card of 27
8 Infinion 16 Lead Kit (1) 16 Contact Lead with pre-loaded Curved Stylet (1) Stylet Ring with a Curved and a Straight Stylet (1) Straight Stylet (4) Suture Sleeves (1) Insertion Needle (1) Lead blank (1) Steering Cap (2) Lead Position Labels left and right (non-sterile) (1) Device Registration Form/Temporary Patient Identification Card (1) Manual Infinion 16 Trial Lead Kit (1) 16 Contact Percutaneous Lead with pre-loaded Curved Stylet (1) Suture Sleeve (1) Insertion Needle (1) Steering Cap (2) Lead Position Labels left and right (non-sterile) (1) Device Registration Form/Temporary Patient identification Card (1) Manual Infinion 16 Trial Configuration Kit (1) Infinion 16 Trial Lead Kit (1) 2x8 Splitter Kit Infinion 16 Configuration Kit (1) Infinion 16 Lead Kit (1) 2x8 Splitter Kit Infinion CX Lead Kit (1) 16 Contact Percutaneous Infinion CX Lead with pre-loaded Curved Stylet (2) Suture Sleeves (2) Lead Position Labels left and right (non-sterile) (1) Manual (1) Product Registration Form/Temporary Patient Identification Card (1) Steering Cap Infinion CX Trial Lead Kit (1) 16 Contact Percutaneous Infinion CX Lead with pre-loaded Curved Stylet (2) Suture Sleeves (2) Lead Position Labels left and right (non-sterile) (1) Manual (1) Product Registration Form/Temporary Patient Identification Card (1) Steering Cap of 27
9 Specifications and Technical Data Specifications and Technical Data Linear Lead Part Specifications Lead Lengths 30, 50, 70 cm Lead shape In-line Lead Diameter 1.3 mm Number of Electrode Contacts 8 Electrode Length 3 mm Electrode Spacing 1, 4, or 6 mm Contact Material Platinum/Iridium Insulation Material Polyurethane Conductor Material MP35N-DFT-28% Ag Infinion 16 Lead Part Specifications Lead Lengths 50, 70 cm Lead shape In-line Lead Diameter 1.3 mm Number of Contacts 16 Contact Length 3 mm Contact Spacing 1 mm Contact Material Platinum/Iridium Insulation Material Polyurethane Conductor Material 35N LT-DFT-28% Ag of 27
10 Infinion CX Lead Part Specifications Lead Lengths 50, 70 cm Lead shape In-line Lead Diameter 1.3 mm (each segment) Number of Contacts 16 Contact Length 3 mm Contact Spacing 1 mm Contact Material Platinum/Iridium Insulation Material Polyurethane Conductor Material 35N LT-DFT-28% Ag Lead Extension Part Specifications Extension Lengths 25, 35, 55 cm Extension Diameter 1.3 mm Number of Electrode Contacts 8 Contact Material Platinum/Iridium, Stainless Steel Insulation Material Silicone, Polyurethane Conductor Material MP35N-DFT-28% Ag 2x4 Splitter Part Splitter Length Splitter Diameter Number of Electrode Contacts Contact Material Insulation Material Conductor Material Specifications 25 cm 1.3 mm (each segment) 8 (4 per receptacle) Platinum/Iridium, Stainless Steel Silicone, Polyurethane MP35N-DFT-28% Ag of 27
11 Splitter 2x8 Part Splitter Length Splitter Diameter Number of Electrode Contacts Contact Material Insulation Material Conductor Material Specifications and Technical Data Specifications 30 cm 1.3 mm (each segment) 16 (8 per tail) Platinum/Iridium, Stainless Steel Silicone, Polyurethane MP35N-DFT-28% Ag of 27
12 Registering the Leads In accordance with international practice and regulatory legislation in some countries, a registration form is packed with each Boston Scientific lead/lead extension/splitter. The purpose of this form is to maintain traceability of all products and to secure warranty rights. It also allows the institution involved in the evaluation or replacement of a specific implanted lead, accessory or device to gain quick access to pertinent data from the manufacturer. Fill out the registration form included in the package contents. Return one copy to Boston Scientific, keep one copy for patient records, provide one copy to the patient, and one copy to the physician. Boston Scientific Neuromodulation Corporation Rye Canyon Loop Valencia, California 91355, USA Attention: Customer Service Department of 27
13 Instructions for Use Instructions for Use Physician training is required. Boston Scientific also recommends that implanting physicians read all product labeling prior to using our devices. Lead, Lead Extension, and Splitter Handling and Storage Avoid damaging the lead with sharp instruments or excessive force during surgery. Do not sharply bend or kink the lead, extension, or splitter. Do not tie suture(s) directly to the lead, extension, or splitter body; use the provided suture sleeves. For the Percutaneous Leads avoid forcing the lead into the epidural space by carefully clearing a path using the lead blank. Avoid pulling an implanted lead taut; provide a stress relief loop at the insertion site to minimize tension on the lead. Avoid handling the lead with sharp instruments; use only rubber-tipped forceps. Take care when using sharp instruments such as hemostats or scalpels to prevent damaging the lead. Wipe off any body fluids from the lead connector end before connecting it to any other component. Fluid contamination of these connections could compromise the integrity of the stimulation circuit. Wipe off any body fluids from the lead stylet before inserting or reinserting it into the lead. Store components between 0 C and 45 C (32 F and 113 F) in an area where they are not exposed to liquids or excessive moisture. Temperatures outside of the stated range can cause damage. Percutaneous Lead Placement in the Epidural Space Note: If using an Infinion CX Lead, proceed to Infinion CX Lead Placement in the Epidural Space with Entrada Needle on page Position, prep and drape the patient in the usual accepted manner. Inject a local anesthetic at the needle insertion site. 2. Under fluoroscopic guidance, place the insertion needle into the epidural space with the bevel facing up using an angle of 45 or less. CAUTION: Use only an insertion needle provided by Boston Scientific. Other needles may damage the lead. The stamped number 14 on the needle hub (or the triangle on the hub of the curved Epimed needle, sold separately) corresponds to the orientation of the bevel, which must face up. Turning the bevel ventral (down) may result in lead damage. An angle of more than 45 increases the risk of lead damage of 27
14 WARNING: The angle of the insertion needle should be 45 or less. Steep angles increase the insertion force of the stylet and also present more of an opportunity for the stylet to pierce the lead and cause tissue damage. 3. Remove the needle stylet from the insertion needle and verify entry into the epidural space using the standard technique. 4. OPTIONAL. Under fluoroscopic guidance, insert the lead blank through the insertion needle and into the epidural space. Advance the lead blank to verify entry into the epidural space, then withdraw the blank. 5. While holding the lead stylet handle, place the steering cap over the proximal end of the stylet handle with moderate force until it is held in place. Then slowly insert the lead, with stylet, through the insertion needle. The lead stylet should extend to the tip of the lead. 6. OPTIONAL. If exchange of the lead stylet is desired, carefully pull out the existing stylet and insert the preferred stylet. While inserting the stylet into the lead, if resistance is encountered, withdraw the stylet approximately 3 cm, rotate the lead and/or stylet, and gently advance the stylet. If resistance is still encountered, repeat the above procedure until the stylet can be fully inserted. WARNING: Do not exchange the lead stylet while the electrode array of the lead is in the bevel of the insertion needle. If the electrode array is in the bevel area, remove the lead from the insertion needle before exchanging the stylet. Inserting the lead stylet in the lead while the electrode array is in the bevel of the insertion needle increases the risk of lead and tissue damage. WARNING: If the lead stylet is removed and reinserted, do not use excessive force when inserting the stylet into the lead. The use of instruments, such as forceps, to grasp the stylet during insertion is not recommended as this could result in applying excessive force and could increase the risk of lead and tissue damage. 7. Advance the lead to the appropriate vertebral level under fluoroscopic guidance. A sufficient length of lead (for example, at least 10 cm, or approximately three vertebrae) should reside in the epidural space to aid in lead stabilization. 8. If using a splitter, proceed to Lead Connection to Splitter in this manual. or If not using a splitter, proceed to the instructions for connecting leads or extensions to the OR Cable assembly in the appropriate DFU for your SCS System, as listed in your Reference Guide of 27
15 5 Instructions for Use Infinion CX Lead Placement in the Epidural Space with Entrada Needle Infinion CX Lead 5 5 The Infinion CX Lead has two tails to enable insertion into 8-contact IPG ports without the use of the Splitter 2x8. The two tails will not fit through a standard 14 gauge insertion needle or Epimed needle, therefore these needles are not compatible with the Infinion CX Lead. Do not use a standard 14 gauge introducer or Epimed needle to introduce the Infinion CX Lead. Use only the Entrada Needle with peelable sheath to introduce the Infinion CX Lead into the epidural space. 5 5 An anchor must be pre-loaded on the distal end of the Infinion CX Lead prior to inserting the lead into the Entrada Needle. When using an anchor or suture sleeve with the Infinion CX Lead, the stylet may be in place within the lead when the anchor is loaded on the lead. Ensure that stylet is removed from the lead prior to securing the anchor. Entrada Needle The Entrada Needle with peelable sheath is required to insert the Infinion CX Lead into the epidural space. The Entrada Needle may also be used with all percutaneous leads listed under Product Model Numbers on page ii. Reusing the same Entrada Needle to insert a second lead is not recommended. Fully Assembled Entrada Needle Components Components are marked with dots to indicate use order, and the dots should be in the same direction when assembling the needle. Needle Stylet, Loss of Resistance (LOR) adaptor, Slotted Needle, Peelable Sheath Note: Do not mix components between Entrada 4.5 in and 6.0 in needles. Needles are clearly labeled with needle lengths and are also color coded (as they appear on the product, green=6.0 in and blue=4.5 in) of 27
16 Assembling and Reassembling the Entrada Needle CAUTION: Do not insert or reinsert the needle stylet, LOR adaptor, or slotted needle into the sheath while the sheath is inserted within the patient. 1. Insert the slotted needle into the sheath. 2. Insert the LOR adaptor into the slotted needle. 3. Insert the needle stylet into LOR adaptor and advance forward until fully seated into needle assembly. Infinion CX Lead Placement in the Epidural Space with Entrada Needle 1. Position, prep and drape the patient in the usual accepted manner. Inject a local anesthetic at the needle insertion site. 2. Verify that the Entrada needle is fully assembled by holding onto the sheath hub and applying forward pressure on the stylet cap cover. CAUTION: Do not bend the Entrada needle. Bending the Entrada needle may cause the stylet or LOR adapter to become jammed in the needle assembly and difficult to remove. 3. Recommended for permanent or permanent-trial procedures: Cut down prior to inserting the Entrada Needle and insert needle into incision. Creating the incision before inserting the Entrada Needle provides a clear path for sliding the anchor into the incision. If cutting down after inserting the Entrada Needle, ensure that the sheath is in place and do not damage the sheath. 4. Under fluoroscopic guidance, place the Entrada Needle into the epidural space with the 14G marking facing up using an angle of 45 or less. CAUTION: Use only an Entrada Needle provided by Boston Scientific. Other needles may damage the lead. Turning the bevel ventral (down) may result in lead damage. An angle of more than 45 increases the risk of lead damage of 27
17 Instructions for Use WARNING: The angle of the insertion needle should be 45 or less. Steep angles increase the insertion force of the stylet and also present more of an opportunity for the stylet to pierce the lead and cause tissue damage. Note: If the needle must be repositioned during this procedure, or if the sheath becomes damaged, reassemble the needle outside of the body with a new sheath, see Assembling and Reassembling the Entrada Needle on page Remove the needle stylet from the insertion needle and verify entry into the epidural space using the standard technique. Remove needle stylet Verify entry into the epidural space. 6. OPTIONAL. Under fluoroscopic guidance, insert the lead blank through the LOR adaptor and into the epidural space. Advance the lead blank to verify entry into the epidural space, then withdraw the blank. 7. The LOR adaptor must be removed prior to inserting the lead. Pull the LOR adaptor straight out from the slotted needle without twisting or bending. Recommended: Hold the slotted needle in place while removing the LOR adaptor. Note: An anchor must be loaded over the distal end of the Infinion CX Lead prior to inserting the lead into the Entrada Needle. 8. While holding the lead stylet handle, place the steering cap over the proximal end of the stylet handle with moderate force until it is held in place. The lead stylet should be fully inserted into the lead. Slowly insert the Infinion CX Lead into the slotted needle, directing the distal lead tip into the needle lumen. OPTIONAL. Cover slotted needle with finger to aid in insertion of Infinion CX lead into slotted needle lumen of 27
18 OPTIONAL. If needle steering within the slotted needle is not needed, remove slotted needle and insert lead directly into sheath. 9. OPTIONAL. If exchange of the lead stylet is desired, carefully pull out the existing stylet and insert the preferred stylet. The stylet must be inserted into the Infinion CX Lead tail with one marker band. While inserting the stylet into the lead, if resistance is encountered, withdraw the stylet approximately 3 cm, rotate the lead and/or stylet, and gently advance the stylet. If resistance is still encountered, repeat the above procedure until the stylet can be fully inserted. The stylet must be inserted into the Infinion CX Lead tail with one marker band WARNING: Do not exchange the lead stylet while the electrode array of the lead is in the bevel of the insertion needle. If the electrode array is in the bevel area, remove the lead from the insertion needle before exchanging the stylet. Inserting the lead stylet in the lead while the electrode array is in the bevel of the insertion needle increases the risk of lead and tissue damage. WARNING: If the lead stylet is removed and reinserted, do not use excessive force when inserting the stylet into the lead. The use of instruments, such as forceps, to grasp the stylet during insertion is not recommended as this could result in applying excessive force and could increase the risk of lead and tissue damage. 10. Advance the lead to the appropriate vertebral level under fluoroscopic guidance. A sufficient length of lead (at least 10 cm, or approximately three vertebrae) should reside in the epidural space to aid in lead stabilization. 11. Proceed to the instructions for connecting to the OR Cable assembly in the appropriate DFU for your SCS System, as listed in your Reference Guide. Lead Connection to Splitter 1. Carefully withdraw the stylets from the leads to be inserted into the splitter. 2. Wipe clean the proximal connector ends of the leads. 3. Select the desired splitter model of 27
19 Note: A Splitter 2x8 must be used when implanting the Infinion 16 lead. Instructions for Use 4. Check that the lead connector end can be easily inserted into the splitter without obstruction. If obstruction is encountered, loosen the set screws of the splitter by using the hex wrench provided, turning counterclockwise. Note: The set screw should only be loosened to an amount sufficient to insert a lead. Do not excessively loosen the set screw. This may cause the set screw to dislodge rendering the splitter unusable. 5. Insert proximal connector ends of desired leads into splitter receptacles until they are fully seated - each lead bottoms out in receptacles and retention sleeves (long ring) are under the set screw blocks of the splitter receptacles. Do not tighten set screw at this time. 6. Proceed to the instructions for connecting to the OR Cable assembly in appropriate DFU for your SCS System, as listed in your Reference Guide. 7. Check connections with an impedance measurement. If the impedance is satisfactory proceed to Intraoperative Stimulation Testing on page 16 to confirm proper lead location. Note: Do not tighten set screw mechanical lock before intraoperative stimulation testing. Note: On the 2x4 Splitter, the shorter splitter receptacle corresponds to contacts 1-4, while the longer receptacle corresponds to contacts 5-8. Make note of which lead is connected to each splitter receptacle. Note: On the Splitter 2x8, one tail is laser-marked with bands, and corresponds to contacts 1-8 on the Infinion 16 percutaneous lead; the unmarked tail corresponds to contacts If lead repositioning is required, disconnect the splitter and reinsert the stylet before advancing the lead. Repeat steps 5 to 7 until satisfactory lead position is achieved. 9. Use the hex wrench supplied to tighten down the setscrews until the wrench clicks of 27
20 Medical Adhesive 10. If using the Splitter 2x8, prior to closing the wound, clean the top of the splitter set-screw seal plug and use silicone medical adhesive (e.g. Dow Corning Silastic Medical Adhesive Silicone, Type A - Sterile, as available from Boston Scientific, part number SC-4320) to coat and seal the top of the seal plug that has been penetrated by the hex wrench. Note: Inadvertent damage to the septum seal may lead to unintended stimulation at the Splitter 2x8 if the Medical Adhesive is not used as intended. 11. For intraoperative testing, proceed to the instructions for connecting to the Trial Stimulator in the appropriate DFU for your SCS System as listed in your Reference Guide. or To connect to an IPG, refer to the IPG directions in the appropriate DFU for your SCS System, as listed on your Reference Guide. Intraoperative Stimulation Testing Note: The following steps are for procedural reference only. For detailed stimulation testing procedures and guidelines refer to the appropriate programming manual for your SCS system as listed in the Reference Guide. 1. If using a splitter Visually check splitter to leads connection Check impedance Note: If using the 2x4 Splitter, make note of splitter configuration in the Programming software. Refer to the appropriate programming manual for your SCS System as listed in your Reference Guide. 2. After linking the Clinician Programmer to the Trial Stimulator, check impedances to verify that components are properly connected. Lead impedance is measured and displayed for each of the IPG s contacts. Impedances, indicated by an x or an orange circle in your Programming Software, are considered to be resultant from open or unconnected wires. Refer to your Programming Manual for additional information. 3. Using test stimulation, enlist patient feedback to verify lead placement and pain coverage. Note: If lead repositioning is necessary, turn stimulation off before proceeding. 4. Reposition leads as necessary. If using a splitter, gently pull on lead attached to splitter to reposition caudad or disconnect splitter from leads, re-insert stylet, and advance leads to reposition cephalad. CAUTION: Do not force stylet into lead. 5. Steer lead to new position. 6. Remove stylet, wipe proximal ends of leads, and reconnect splitter. 7. Check impedances of 27
21 8. Repeat steps 1-3 if lead has been repositioned. 9. When the desired paresthesia is achieved: Instructions for Use a) Turn the Trial Stimulator off. b) Unlock each OR Cable Connector and disconnect from the lead(s). c) For percutaneous lead(s) - Slowly withdraw the stylet(s). 10. Record the lead position by capturing a fluoroscopic image to be sure the leads have not moved. Retest if necessary. 11. If using a splitter disconnect splitter from leads. Insert the hex wrench and turn the set screw counter clockwise to loosen. Note: The set screw should only be loosened to an amount sufficient to insert a lead. Do not excessively loosen the set screw. This may cause the set screw to dislodge, rendering the splitter unusable. Option A: For a Temporary Trial, proceed to Securing the Trial Lead on page 17. Option B: For a Permanent Trial, proceed to Permanent Lead Anchoring and Tunneling on page 18. Option C: For a Permanent IPG Implantation using percutaneous leads, proceed to Permanent Lead Anchoring and Tunneling on page 18. Option D: For a Permanent IPG Implantation using paddle leads, proceed to Permanent Lead Anchoring and Tunneling in the Surgical Leads DFU. Before receiving a permanent SCS system, it is recommended that patients undergo a trial procedure so that they can experience stimulation to evaluate if SCS is effective at treating their chronic pain. Securing the Trial Lead Note: If using an Infinion CX Trial Lead, proceed to Securing the Trial Infinion CX Lead. 1. Carefully withdraw the insertion needle from the epidural space by slowly pulling the needle up towards the proximal end of the lead while holding the lead in place. 2. Once the insertion needle tip is exposed, hold the lead as close to the percutaneous exit site as possible, then carefully pull the needle completely from the lead. 3. If desired, a suture may be used to close the wound and stabilize the lead. 4. Place and tape a stress relief loop and dress the wound. 5. If using a splitter, refer to Lead Connection to Splitter on page For temporary lead trials, refer to the instructions for connecting to the OR Cable assembly and External Trial Stimulator in the appropriate DFU for your SCS System as listed in your Reference Guide of 27
22 Securing the Trial Infinion CX Lead 1. Carefully withdraw the slotted needle by slowly pulling the needle towards the proximal end of the lead while stabilizing lead in line with the slot to keep lead in place. Note: Do not reinsert the slotted needle into the sheath while the sheath is within the body. 2. Snap sheath wings apart and peel sheath out of insertion site. 3. Remove the stylet from the lead. 4. If desired, a suture may be used to close the wound and stabilize the lead. 5. Place and tape a stress relief loop and dress the wound. 6. For temporary lead trials, refer to the instructions for connecting to the OR Cable assembly and External Trial Stimulator in the appropriate DFU for your SCS System as listed in your Reference Guide. Permanent Lead Anchoring and Tunneling Removing the Insertion Needle Note: If using an Entrada Needle, proceed to Removing the Entrada Needle. 1. Cut down around the insertion needle to provide access for anchoring the lead. 2. Carefully withdraw the insertion needle from the epidural space by slowly pulling the needle up towards the proximal end of the lead while holding the lead in place. 3. Once the insertion needle tip is exposed, hold the lead as close to the exit site as possible, then carefully pull the needle completely from the lead. Removing the Entrada Needle 1. Carefully withdraw the slotted needle by slowly pulling the needle towards the proximal end of the lead while stabilizing lead in line with the slot to keep lead in place. Note: Do not reinsert the slotted needle into the sheath while the sheath is within the body of 27
23 Instructions for Use 15a Stabilize lead in line with the slot. Slowly remove slotted needle. 2. Snap sheath wings apart and peel sheath out of insertion site. Snap sheath wings apart and peel sheath 3. Remove the stylet from the lead. Anchoring the Lead Leads can be permanently anchored with a suture sleeve or with an anchor. Note: When using the Infinion CX Lead, the anchor or suture sleeve must be loaded over the distal end of the lead prior to inserting the Infinion CX Lead into the Entrada Needle. For the Infinion CX Lead, the stylet may be in place within the lead when the anchor is loaded on the lead, however, the stylet must be removed before the anchor is locked down. Refer to the Directions for Use for your Boston Scientific anchor as listed in your Reference Guide, or continue with the following steps to anchor using a suture sleeve. 1. For percutaneous leads, carefully remove the lead stylet using fluoroscopy to ensure the lead position does not change. 2. Place a suture sleeve over the lead and down to the supraspinous ligament or deep fascial tissue. 3. Ligate the suture sleeve onto the lead by tying a 2-0 silk or other nonabsorbable suture around the center groove of the sleeve to prevent sliding. Circumferential stitches may be tied at the compression slots. CAUTION: Do not use polypropylene sutures as they may damage the suture sleeve. Do not suture directly onto the lead, splitter, or use a hemostat on the lead body. This may damage the lead insulation of 27
24 Note: The 4 cm and 2.3 cm Suture Sleeves each have three (3) compression slots, which are designed to reduce slippage. 4. Suture the sleeve to the supraspinous ligament or deep fascia through the suture sleeve holes. 5. Tie multiple sutures as tightly as possible around the suture sleeve to secure it to the lead. CAUTION: Tightening sutures directly on the lead can damage the lead. 6. For Permanent Trials, proceed to Tunneling the Lead or Lead Extension on page For permanent IPG implantation, refer to IPG instructions in the appropriate DFU for your SCS system as listed in your Reference Guide. Tunneling the Lead or Lead Extension Note: If tunneling Infinion CX Lead(s), it is recommended to use the Long Tunneling Tool (35 cm). 1. Attach the tunneling tool handle to the shaft by turning the locking mechanism clockwise. Tool Handle Locking Mechanism Shaft 2. Mark the desired route of the tunnel. 3. Administer the appropriate local anesthetic along the tunneling path. 4. OPTIONAL. If necessary, bend the tool shaft to conform to the patient s body of 27
25 Instructions for Use 5. Make a small incision at the desired exit site. 6. Create a subcutaneous tunnel between the midline incision and the exit site until the straw is visible and accessible at the exit point. 7. Unscrew and remove the tunneling tool handle. 8. Grasp the tip of the tool with one hand while holding the straw in place with the other hand. Pull the tunneling tool shaft out through the straw. 9. Push the lead or extension proximal ends through the straw, then withdraw the straw. CAUTION: Do not tunnel splitter. Note: If using the 2x8 Splitter and performing a permanent trial, the splitter tails may be tunneled to the exit site. 10. For Permanent Trials: If using the Infinion 16 lead, proceed to Lead Connection to Splitter on page 14. Otherwise, proceed to Connecting the Lead Extension on page For Permanent IPG Implantation, if extensions are used, proceed to the instructions for Connecting the Lead Extension on page For Permanent IPG Implantation, if splitters are used, proceed to the instructions for Lead Connection to Splitter on page For Permanent IPG Implantation, refer to the IPG instructions in the appropriate DFU for your SCS system as listed in your Reference Guide of 27
26 Note: The following Codman Disposable Catheter Passers may be used in place of the Boston Scientific tunneling tool except for the Infinion CX Lead: REF (36 cm); REF (55 cm); REF (65 cm) Note: When using a Codman Disposable Catheter Passer, tunnel from the IPG pocket to the midline incision using the standard technique. Connecting the Lead Extension 1. Wipe clean the proximal end of the lead, then insert the proximal end into the lead extension connector or splitter until it stops and the retention ring (long ring) is under the setscrew. Note: If there appears to be an obstruction when inserting the lead into the lead extension connector, use the hex wrench to loosen (counterclockwise) the setscrew and/or gently rotate the lead to help advance the proximal end. 2. Ensure that the lead is fully inserted before tightening the set screw to prevent lead damage. 3. Using the hex wrench supplied, turn the extension connector setscrew clockwise until it clicks, indicating lock. Note: Ensure that the hex wrench is fully seated in the setscrew before tightening. Note: The hex wrench is torque-limited and cannot be overtightened. 4. Form an appropriately-sized pocket using blunt dissection on either side of midline for coiled excess lead and extension connectors. 5. Place a small loop at the lead for slack. If necessary, loosely tie a suture around the lead-loop, but do not tighten onto the lead. CAUTION: Tightening sutures directly on the lead can damage the lead. 6. Carefully remove excess slack by gently pulling the extensions from the exit wound of 27
27 Instructions for Use 7. For Permanent Trials, if desired, a small suture may be used to close the exit wound of the extension. Place and tape a stress relief loop and dress the wound. Proceed to the instructions for connecting to the OR Cable Connector or External Trial Stimulator in the appropriate DFU for your SCS System, as listed in your Reference Guide. 8. For Permanent Implant, close the midline incision, and proceed to instructions for connecting to the IPG in the appropriate DFU for your SCS System, as listed in your Reference Guide. Lead/Extension/Splitter Removal Remove bandages and properly cleanse the exit site. The method of removal depends upon whether a temporary trial or permanent trial was performed. Option A: Percutaneous Lead Removal after Temporary Trial 1. Clip sutures if used to secure the trial lead(s) in place. 2. Remove the lead(s) and discard. 3. To replace the trial lead(s) with permanent percutaneous lead(s), proceed to the instructions for Percutaneous Lead Placement in the Epidural Space on page 9 or Infinion CX Lead Placement in the Epidural Space with Entrada Needle on page To replace the trial lead(s) with a paddle lead proceed to instructions for Surgical Paddle Lead Placement in the Epidural Space in the Surgical Leads DFU. Option B: Lead Extension Removal After Permanent Trial 1. Open the midline incision to expose the lead extension and connector. 2. Cut the lead extension at the connector. Do not cut the implanted lead. 3. Remove the extension, being careful not to contact non-sterile portions to the patient s body. 4. Loosen the extension connector setscrew using the hex wrench. Disconnect and remove the connector without moving the implanted lead of 27
28 5. Proceed to the IPG Implantation instructions in the appropriate DFU for your SCS system as listed in your Reference Guide. Option C: Splitter Removal 1. If the splitter has been implanted, open the incision to expose the lead and splitter junction. 2. Loosen the connector set screw on the splitter receptacles using the hex wrench provided. 3. Disconnect and discard the splitter components of 27
29 Programming with the Infinion 16 Lead and Infinion CX Lead Programming with the Infinion 16 Lead and Infinion CX Lead For detailed programming instructions, refer to the appropriate Bionic Navigator Software Guide or the Bionic Navigator 3D System Programming Manual. If using the Bionic Navigator 3D Software: When the Infinion 16 lead is used with the Splitter 2x8 and placed properly into the Precision Spectra or Spectra WaveWriter IPG ports (the splitter tail with laser etched bands to the left ports A or C and the unmarked splitter tail to the right ports B or D) the laser marked splitter tail corresponds to contacts 1-8 on the distal end of the Infinion 16 lead and the unmarked splitter tail corresponds to contacts 9-16 of the Infinion 16 Lead. When the Infinion CX Lead is placed properly in the Precision Spectra or Spectra WaveWriter IPG ports (tail with one marker band to the left ports A or C and tail with two marker bands to the right ports B or D) the tail with one marker band corresponds to contacts 1-8 on the distal end of the Infinion CX Lead and the tail with two marker bands corresponds to contacts 9-16 of the Infinion CX Lead. If using Bionic Navigator Software: When the Infinion 16 lead is used with the Splitter 2x8 and placed properly into the Precision IPG ports (laser-marked tail of Splitter 2x8 connecting to IPG port 1-L ), the distal 8 contacts of the Infinion 16 lead (contacts 1-8) will correspond with contacts 1-8 on the left side of the Bionic Navigator software display. Likewise, the proximal 8 contacts of the Infinion 16 lead (contacts 9-16) will correspond with contact 9-16 on the right side of the Bionic Navigator software display. When the Infinion CX Lead is placed properly into the Precision IPG ports (tail with one marker band connecting to IPG port 1-L ), the distal 8 contacts of the Infinion CX Lead (contacts 1-8) will correspond with contacts 1-8 on the left side of the Bionic Navigator software display. Likewise, the proximal 8 contacts of the Infinion CX Lead (contacts 9-16) will correspond with contact 9-16 on the right side of the Bionic Navigator software display. The diagram below depicts this wiring. Using test stimulation, enlist patient feedback to verify lead placement and pain coverage. The user may program with the Infinion 16 lead and Infinion CX Lead using all the functions available in Bionic Navigator for the Linear family of leads (E-Troll, Navigator, etc.). For complete instructions on programming, see the Bionic Navigator Software Guide of 27
30 This page intentionally left blank of 27
31 Programming with the Infinion 16 Lead and Infinion CX Lead This page intentionally left blank of 27
32 Legal Manufacturer Boston Scientific Neuromodulation Corporation Rye Canyon Loop Valencia, CA USA (866) in US and Canada (661) , (661) Fax (866) TTY AUSAustralian Sponsor Address Boston Scientific (Australia) Pty Ltd PO Box 332 BOTANY NSW 1455 Australia Free Phone Free Fax EU Authorized Representative Boston Scientific Limited Ballybrit Business Park Galway, Ireland T: +33 (0) F: +33 (0) Boston Scientific Corporation or its affiliates. All rights reserved
Surgical Leads. Directions for Use. CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician.
 Surgical Leads Directions for Use CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91078747-08 Content: 92190987 REV A Guarantees Boston Scientific
Surgical Leads Directions for Use CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91078747-08 Content: 92190987 REV A Guarantees Boston Scientific
Precision Montage MRI 16 Contact Implantable Pulse Generator
 Precision Montage MRI 16 Contact Implantable Pulse Generator Directions for Use CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91053657-02 REV
Precision Montage MRI 16 Contact Implantable Pulse Generator Directions for Use CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91053657-02 REV
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
ImageReady MRI Head Only Guidelines for Spectra WaveWriter Spinal Cord Stimulator System
 ImageReady MRI Head Only Guidelines for Spectra WaveWriter Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91171762-01
ImageReady MRI Head Only Guidelines for Spectra WaveWriter Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91171762-01
Surgical Lead Implantation Guide
 Surgical Lead Implantation Guide Neurostimulation Therapy for Chronic Pain Innovating for life. Medtronic, PrimeAdvanced, Restore, RestoreAdvanced, RestoreUltra, and Specify are registered trademarks of
Surgical Lead Implantation Guide Neurostimulation Therapy for Chronic Pain Innovating for life. Medtronic, PrimeAdvanced, Restore, RestoreAdvanced, RestoreUltra, and Specify are registered trademarks of
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Implantation Procedure
 VNS Therapy System For Healthcare Professionals October 2010 Worldwide Version Caution: U.S. federal law restricts this device to sale by or on the order of a physician. 2011 0344 Note: This is one part
VNS Therapy System For Healthcare Professionals October 2010 Worldwide Version Caution: U.S. federal law restricts this device to sale by or on the order of a physician. 2011 0344 Note: This is one part
ImageReady MRI Full Body Guidelines for Precision Montage MRI Spinal Cord Stimulator System
 ImageReady MRI Full Body Guidelines for Precision Montage MRI Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91035972-02
ImageReady MRI Full Body Guidelines for Precision Montage MRI Spinal Cord Stimulator System CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91035972-02
MEDICAL CORPORATION Asbury Rd., P.O. Box 758, Farmingdale, NJ USA Fax
 MEDICAL CORPORATION 5206 Asbury Rd., P.O. Box 758, Farmingdale, NJ 07727 USA 732-938-2266 800-323-4035 Fax 732-938-2399 MYO/WIRE Temporary Cardiac Pacing Wire System MYO/WIRE temporary cardiac pacing wires.
MEDICAL CORPORATION 5206 Asbury Rd., P.O. Box 758, Farmingdale, NJ 07727 USA 732-938-2266 800-323-4035 Fax 732-938-2399 MYO/WIRE Temporary Cardiac Pacing Wire System MYO/WIRE temporary cardiac pacing wires.
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS
 DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Percutaneous Lead Kit. Models 3143, 3146, 3149, 3153, 3156, 3159, 3183, 3186, Clinician's Manual
 Percutaneous Lead Kit Models 3143, 3146, 3149, 3153, 3156, 3159, 3183, 3186, 3189 Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical
Percutaneous Lead Kit Models 3143, 3146, 3149, 3153, 3156, 3159, 3183, 3186, 3189 Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical
Lead and Extension Insertion Tool. Model Clinician's Manual
 Lead and Extension Insertion Tool Model 1803 Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE
Lead and Extension Insertion Tool Model 1803 Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE
Valencia Pedicle Screw Surgical Technique
 Valencia Pedicle Screw Surgical Technique VALENCIA CIRCUIT TABLE OF CONTENTS Design Rationale Indications for Use Surgical Technique 1. Pedicle Preparation 2. Screw Insertion 3. Rod Placement 4. Locking
Valencia Pedicle Screw Surgical Technique VALENCIA CIRCUIT TABLE OF CONTENTS Design Rationale Indications for Use Surgical Technique 1. Pedicle Preparation 2. Screw Insertion 3. Rod Placement 4. Locking
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use
 Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Algovita Spinal Cord Stimulation System
 Algovita Spinal Cord Stimulation System Stimulator Models 2408 and 2412 For IPG replacement Implant Manual for Stimulator IPG implant.indb 1 Algovita is a trademark of QIG Group, LLC CoreGuard is a trademark
Algovita Spinal Cord Stimulation System Stimulator Models 2408 and 2412 For IPG replacement Implant Manual for Stimulator IPG implant.indb 1 Algovita is a trademark of QIG Group, LLC CoreGuard is a trademark
Surgical Technique Guide
 Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
TABLE OF CONTENTS. 2 (8144 Rev 2)
 1 (8144 Rev 2) TABLE OF CONTENTS Introduction Conventus CAGE TM - Proximal Humerus...3 Indications and Contraindications...4 Surgical Summary...5 Patient Positioning & Approach...6 Surgical Technique Plate
1 (8144 Rev 2) TABLE OF CONTENTS Introduction Conventus CAGE TM - Proximal Humerus...3 Indications and Contraindications...4 Surgical Summary...5 Patient Positioning & Approach...6 Surgical Technique Plate
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation.
 LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
Fibrlok II 2529 Universal Optical Fiber Splice
 Fibrlok II 2529 Universal Optical Fiber Splice Instructions January 2007 78-8128-0163-3-E Contents: 1.0 General... 3 2.0 Splicing Set-up... 4 3.0 Fiber Preparation... 5 4.0 Splice Assembly... 6 5.0 For
Fibrlok II 2529 Universal Optical Fiber Splice Instructions January 2007 78-8128-0163-3-E Contents: 1.0 General... 3 2.0 Splicing Set-up... 4 3.0 Fiber Preparation... 5 4.0 Splice Assembly... 6 5.0 For
Low Bend Distal Tibia Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
BP and Heart Rate by Telemetry
 BP and Heart Rate by Telemetry Version: 1 Modified from: Butz et al. Physiol Genomics. 2001 Mar 8;5(2):89-97. Edited by: Dr. Lynette Bower, UC Davis Summary Reagents and Materials Protocol Reagent Preparation
BP and Heart Rate by Telemetry Version: 1 Modified from: Butz et al. Physiol Genomics. 2001 Mar 8;5(2):89-97. Edited by: Dr. Lynette Bower, UC Davis Summary Reagents and Materials Protocol Reagent Preparation
Technique Guide. *smith&nephew N8TIVE ACL Anatomic ACL Reconstruction System
 Technique Guide *smith&nephew N8TIVE ACL Anatomic ACL Reconstruction System N8TIVE ACL System The N8TIVE ACL Anatomic Reconstruction System provides a novel and simple approach to ACL repair. The N8TIVE
Technique Guide *smith&nephew N8TIVE ACL Anatomic ACL Reconstruction System N8TIVE ACL System The N8TIVE ACL Anatomic Reconstruction System provides a novel and simple approach to ACL repair. The N8TIVE
Integra B: Camino OLM Intracranial Pressure Monitoring Kit SURGICAL TECHNIQUE
 Integra 110-4B: Camino OLM Intracranial Pressure Monitoring Kit SURGICAL TECHNIQUE Surgical Technique Th OLM ICP Kit was developed in cooperation with Richard C. Ostrup, M.D., Thomas G. Luerssen, M.D.
Integra 110-4B: Camino OLM Intracranial Pressure Monitoring Kit SURGICAL TECHNIQUE Surgical Technique Th OLM ICP Kit was developed in cooperation with Richard C. Ostrup, M.D., Thomas G. Luerssen, M.D.
Visit Linvatec.com today to learn more. Surgical Technique: Sequential Meniscal Running Stitch
 ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
Procedure Manual. Indications For Use. Component Description. Device Description. Contents
 Procedure Manual This manual provides basic information needed for the implantation and testing of the Bioness StimRouter Lead included in the StimRouter Implantable Lead and Lead Introducer Kit (model
Procedure Manual This manual provides basic information needed for the implantation and testing of the Bioness StimRouter Lead included in the StimRouter Implantable Lead and Lead Introducer Kit (model
3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments
 Part of the 3.5 mm LCP 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments Surgical Technique TABLE OF CONTENTS INTRODUCTION 3.5 mm LCP Low Bend Medial Distal Tibia Plate 2 Aiming Instruments
Part of the 3.5 mm LCP 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments Surgical Technique TABLE OF CONTENTS INTRODUCTION 3.5 mm LCP Low Bend Medial Distal Tibia Plate 2 Aiming Instruments
Surgical Technique & Product Catalogue. Guide for Open & MIS Procedures
 Surgical Technique & Product Catalogue Guide for Open & MIS Procedures INTRODUCTION The VIPER Cortical Fix Fenestrated Screw System is the first pedicle screw implant to offer enhanced fixation in both
Surgical Technique & Product Catalogue Guide for Open & MIS Procedures INTRODUCTION The VIPER Cortical Fix Fenestrated Screw System is the first pedicle screw implant to offer enhanced fixation in both
Nit-Occlud. Coil System for PDA Closure IMPLANTATION POCKET GUIDE. Rx only CV / B. Braun Interventional Systems Inc.
 Refer to the Nit-Occlud PDA Instructions for Use for relevant warnings, precautions, complications and contraindications. This device has been designed for single use only. Nit-Occlud Coil System for PDA
Refer to the Nit-Occlud PDA Instructions for Use for relevant warnings, precautions, complications and contraindications. This device has been designed for single use only. Nit-Occlud Coil System for PDA
Algovita. MRI Procedure Guidelines. Spinal Cord Stimulation System. 201x
 Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 201x Algovita
Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 201x Algovita
Conventus CAGE PH Surgical Techniques
 Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
System. Humeral Nail. Surgical Technique
 System Humeral Nail Surgical Technique Contents IMPLANT FEATURES 2 1. INDICATIONS 3 2. PRE-OPERATIVE PLANNING 3 3. PATIENT POSITIONING & FRACTURE REDUCTION 3 4. INCISION 4 5. ENTRY POINT 4-6 6. PROXIMAL
System Humeral Nail Surgical Technique Contents IMPLANT FEATURES 2 1. INDICATIONS 3 2. PRE-OPERATIVE PLANNING 3 3. PATIENT POSITIONING & FRACTURE REDUCTION 3 4. INCISION 4 5. ENTRY POINT 4-6 6. PROXIMAL
The Ceterix NovoStitch Disposable Suture Passer. Do Not Reuse
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
fassier DUVal TelescoPIc IM system s RESCUE SYSTEM 2.0
 fassier DUVal TelescoPIc IM system s RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
fassier DUVal TelescoPIc IM system s RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
A-dec 372L or 572L Dental Light on an A-dec Performer Dental Chair INSTALLATION GUIDE
 A-dec 37L or 57L Dental Light on an A-dec Performer Dental Chair INSTALLATION GUIDE Contents Before You Begin.......... Remove the Covers........ Choose the Procedure...... 3 Install the Rigid Arm.......
A-dec 37L or 57L Dental Light on an A-dec Performer Dental Chair INSTALLATION GUIDE Contents Before You Begin.......... Remove the Covers........ Choose the Procedure...... 3 Install the Rigid Arm.......
Arthroscopic Shoulder Repair Using the Smith & Nephew KINSA Suture Anchor
 Shoulder Series Technique Guide *smith&nephew KINSA Suture Anchor Arthroscopic Shoulder Repair Using the Smith & Nephew KINSA Suture Anchor Reviewed by: Larry D. Field, MD Mississippi Sports Medicine and
Shoulder Series Technique Guide *smith&nephew KINSA Suture Anchor Arthroscopic Shoulder Repair Using the Smith & Nephew KINSA Suture Anchor Reviewed by: Larry D. Field, MD Mississippi Sports Medicine and
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Better Post-Op Pain Control Starts Here
 Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
mild Devices Kit - Instructions for Use
 INDICATION FOR USE The Vertos mild Devices are specialized surgical instruments intended to be used to perform lumbar decompressive procedures for the treatment of various spinal conditions. CONTENTS AND
INDICATION FOR USE The Vertos mild Devices are specialized surgical instruments intended to be used to perform lumbar decompressive procedures for the treatment of various spinal conditions. CONTENTS AND
ompanionport Speciality Medical Devices For The Veterinary Community Surgical Suggestions
 Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
Procedure Manual. Neuromodulation System. Device Description. Contents. Component Description
 Neuromodulation System Procedure Manual This manual provides basic information needed for the implantation and testing of the Bioness StimRouter Lead included in the StimRouter Implantable Lead and Lead
Neuromodulation System Procedure Manual This manual provides basic information needed for the implantation and testing of the Bioness StimRouter Lead included in the StimRouter Implantable Lead and Lead
Correction System. Surgical Technique
 Nextra Hammertoe Correction System Surgical Technique Maximized Bone Purchase* Stable and Secure Phalanx Optimized Screw Design Adjustable Bone-to-Bone Apposition Progressive Ratchet Tightening Mechanism
Nextra Hammertoe Correction System Surgical Technique Maximized Bone Purchase* Stable and Secure Phalanx Optimized Screw Design Adjustable Bone-to-Bone Apposition Progressive Ratchet Tightening Mechanism
M.I.S. MAKE IT SMART IN ONE SYSTEM. Surgical Technique. Hip Knee Spine Navigation
 M.I.S. MAKE IT SMART IN ONE SYSTEM Surgical Technique Hip Knee Spine Navigation M.U.S.T. Mini Open Surgical Technique Hip Knee Spine Navigation 2 C O N T E N T S 1 INTRODUCTION 4 2 SURGICAL TECHNIQUE 5
M.I.S. MAKE IT SMART IN ONE SYSTEM Surgical Technique Hip Knee Spine Navigation M.U.S.T. Mini Open Surgical Technique Hip Knee Spine Navigation 2 C O N T E N T S 1 INTRODUCTION 4 2 SURGICAL TECHNIQUE 5
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Imbibe Bone marrow aspiration needle. Operative technique
 Imbibe Bone marrow aspiration needle Operative technique Imbibe Bone Marrow Aspiration Needle Imbibe Bone marrow aspiration needle Contents 1. Smart design... 3 2. Operative technique... 4 Posterior iliac
Imbibe Bone marrow aspiration needle Operative technique Imbibe Bone Marrow Aspiration Needle Imbibe Bone marrow aspiration needle Contents 1. Smart design... 3 2. Operative technique... 4 Posterior iliac
Electrodes (for TransAeris System)
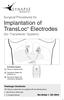 Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
3. PATIENT POSITIONING & FRACTURE REDUCTION 3 8. DISTAL GUIDED LOCKING FOR PROXIMAL NAIL PROXIMAL LOCKING FOR LONG NAIL 13
 Contents IMPLANT FEATURES 2 1. INDICATIONS 3 2. PRE-OPERATIVE PLANNING 3 3. PATIENT POSITIONING & FRACTURE REDUCTION 3 4. INCISION 4 5. ENTRY POINT 4-6 6. PROXIMAL NAIL INSERTION 6-7 7. PROXIMAL LOCKING
Contents IMPLANT FEATURES 2 1. INDICATIONS 3 2. PRE-OPERATIVE PLANNING 3 3. PATIENT POSITIONING & FRACTURE REDUCTION 3 4. INCISION 4 5. ENTRY POINT 4-6 6. PROXIMAL NAIL INSERTION 6-7 7. PROXIMAL LOCKING
Paddle Lead Kit. Models 3214, 3219, 3224, 3228, 3240, 3243, 3244, 3245, 3246, 3262, 3266, 3268, 3283, 3286, Clinician's Manual
 Paddle Lead Kit Models 3214, 3219, 3224, 3228, 3240, 3243, 3244, 3245, 3246, 3262, 3266, 3268, 3283, 3286, 3288 Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the
Paddle Lead Kit Models 3214, 3219, 3224, 3228, 3240, 3243, 3244, 3245, 3246, 3262, 3266, 3268, 3283, 3286, 3288 Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the
Phoenix Atherectomy System Product Overview /LB
 Phoenix Atherectomy System Product Overview Phoenix Atherectomy System- The next generation of atherectomy The first hybrid atherectomy system available Combines the benefits of existing atherectomy systems
Phoenix Atherectomy System Product Overview Phoenix Atherectomy System- The next generation of atherectomy The first hybrid atherectomy system available Combines the benefits of existing atherectomy systems
Fibrlok II 2529 Universal Optical Fiber Splice
 Fibrlok II 2529 Universal Optical Fiber Splice Instructions October 2002 34-7035-4458-4-D Contents: 1.0 General...3 2.0 Splicing Set-up...4 3.0 Fiber Preparation...5 4.0 Splice Assembly...6 5.0 Fiber Repositioning...8
Fibrlok II 2529 Universal Optical Fiber Splice Instructions October 2002 34-7035-4458-4-D Contents: 1.0 General...3 2.0 Splicing Set-up...4 3.0 Fiber Preparation...5 4.0 Splice Assembly...6 5.0 Fiber Repositioning...8
Galt Medical Corp. A leader in vascular and interventional medical devices. part of the GaltNeedleTech TM Family
 Galt Medical Corp. A leader in vascular and interventional medical devices. part of the GaltNeedleTech TM Family MICRO-INTRODUCERS Micro-Introducer Kits Galt s Micro-Introducer Kits with smooth transition,
Galt Medical Corp. A leader in vascular and interventional medical devices. part of the GaltNeedleTech TM Family MICRO-INTRODUCERS Micro-Introducer Kits Galt s Micro-Introducer Kits with smooth transition,
FASSIER DUVAL TELESCOPIC IM SYSTEM S RESCUE SYSTEM 2.0
 FASSIER DUVAL TELESCOPIC IM SYSTEM S RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
FASSIER DUVAL TELESCOPIC IM SYSTEM S RESCUE SYSTEM 2.0 The Rescue System was conceived to retrieve components of the Fassier- Duval telescopic IM System after the treatment has been completed or in case
Cervical Solutions. Optio-C Anterior Cervical Plate. with Allograft/Autograft. Surgical Technique Guide
 Cervical Solutions Optio-C Anterior Cervical Plate with Allograft/Autograft Surgical Technique Guide 2 Optio-C Anterior Cervical Plate with Allograft/Autograft Surgical Technique Guide The Optio-C System
Cervical Solutions Optio-C Anterior Cervical Plate with Allograft/Autograft Surgical Technique Guide 2 Optio-C Anterior Cervical Plate with Allograft/Autograft Surgical Technique Guide The Optio-C System
3. Insert Tocar Sleeves Insert the NCB tissue protection sleeve assembly 1.6 to 10mm through a skin incision (Fig. 38).
 NCB Proximal Humerus Plating System Surgical Technique 19 2. Temporary Plate Fixation The plate can be temporary fixed to the bone with 1.6mm K-wire through the proximal cannulated fixation screw of the
NCB Proximal Humerus Plating System Surgical Technique 19 2. Temporary Plate Fixation The plate can be temporary fixed to the bone with 1.6mm K-wire through the proximal cannulated fixation screw of the
Zimmer MIS Periarticular 3.5mm Proximal Tibial Locking Plate
 Zimmer MIS Periarticular 3.5mm Proximal Tibial Locking Plate Surgical Technique The Science of the Landscape Zimmer MIS Periarticular 3.5mm Proximal Tibial Locking Plate Surgical Technique 1 Zimmer MIS
Zimmer MIS Periarticular 3.5mm Proximal Tibial Locking Plate Surgical Technique The Science of the Landscape Zimmer MIS Periarticular 3.5mm Proximal Tibial Locking Plate Surgical Technique 1 Zimmer MIS
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
FAST1 Intraosseous Infusion System. Training Session
 FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
SYNFLATE SYSTEM SURGICAL TECHNIQUE
 SYNFLATE SYSTEM Vertebral augmentation system for the reduction of fractures and/or creation of a void in cancellous bone in the spine SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SynFlate 2 AO Principles
SYNFLATE SYSTEM Vertebral augmentation system for the reduction of fractures and/or creation of a void in cancellous bone in the spine SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SynFlate 2 AO Principles
DePuy International Ltd St Anthony s Road Leeds LS11 8DT England Tel: +44 (0) Fax: +44 (0)
 Reference: 1. Data on file at DePuy Spine TM 2008 DePuy Spine International is a joint venture with Biedermann Motech, GmbH. This publication is not intended for distribution in the USA. X-ray on front
Reference: 1. Data on file at DePuy Spine TM 2008 DePuy Spine International is a joint venture with Biedermann Motech, GmbH. This publication is not intended for distribution in the USA. X-ray on front
AXIOS Stent and Electrocautery Enhanced Delivery System. Quick Reference Guide
 AXIOS Stent and Electrocautery Enhanced Delivery System Quick Reference Guide AXIOS Stent (front and side views) UPN Codes Flange Diameter (mm) Lumen Diamter (mm) Saddle Length (mm) Catheter OD (Fr) Catheter
AXIOS Stent and Electrocautery Enhanced Delivery System Quick Reference Guide AXIOS Stent (front and side views) UPN Codes Flange Diameter (mm) Lumen Diamter (mm) Saddle Length (mm) Catheter OD (Fr) Catheter
Peritoneal Drainage System
 ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE
 RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
FINELINE II STEROX EZ Models 4469/4470/4471/4472/4473/4474 Guidant Implantable Pacing Lead
 FINELINE II STEROX EZ Models 4469/4470/4471/4472/4473/4474 Guidant Implantable Pacing Lead CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in
FINELINE II STEROX EZ Models 4469/4470/4471/4472/4473/4474 Guidant Implantable Pacing Lead CAUTION: Federal Law restricts this device to sale by or on the order of a physician trained or experienced in
How to use your gonal-f pre-filled pen
 How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
100 Interpace Parkway Parsippany, NJ
 100 Interpace Parkway Parsippany, NJ 07054 www.biometspine.com 800-526-2579 All trademarks are the property of Biomet, Inc. or one of its subsidiaries, unless otherwise indicated. Rx Only. 2009 EBI, LLC.
100 Interpace Parkway Parsippany, NJ 07054 www.biometspine.com 800-526-2579 All trademarks are the property of Biomet, Inc. or one of its subsidiaries, unless otherwise indicated. Rx Only. 2009 EBI, LLC.
Technique Guide. *smith&nephew SPEEDSCREW Fully Threaded Knotless Implant
 Technique Guide *smith&nephew SPEEDSCREW Fully Threaded Knotless Implant SPEEDSCREW system Fully threaded knotless implant system The SPEEDSCREW system is specifically designed for rotator cuff repair
Technique Guide *smith&nephew SPEEDSCREW Fully Threaded Knotless Implant SPEEDSCREW system Fully threaded knotless implant system The SPEEDSCREW system is specifically designed for rotator cuff repair
Surgical Technique. Proximal Humerus Locking Plate
 Surgical Technique Proximal Humerus Locking Plate PERI-LOC Upper Extremity Locked Plating System 3.5mm & 4.5mm Proximal Humerus Locking PlatesCatalog Infor Table of Contents Introduction.........................................................2
Surgical Technique Proximal Humerus Locking Plate PERI-LOC Upper Extremity Locked Plating System 3.5mm & 4.5mm Proximal Humerus Locking PlatesCatalog Infor Table of Contents Introduction.........................................................2
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
ASEPT Pleural Drainage System
 ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
WayPoint Implant and Surgical Kits
 WayPoint Implant and Surgical Kits Directions For Use L011-68 (Rev C0, 2017-03-20) Contains directions for the following products: 66-WP-BKS, 66-WP-IKS, 66-WP-SKS www.fh-co.com FHC, Inc. 1201 Main Street
WayPoint Implant and Surgical Kits Directions For Use L011-68 (Rev C0, 2017-03-20) Contains directions for the following products: 66-WP-BKS, 66-WP-IKS, 66-WP-SKS www.fh-co.com FHC, Inc. 1201 Main Street
Physician s Manual FLEXTEND. Steroid-Eluting Extendable/Retractable Helix Pace/Sense Leads MODELS: 4086/4087/4088
 Physician s Manual FLEXTEND Steroid-Eluting Extendable/Retractable Helix Pace/Sense Leads MODELS: 4086/4087/4088 CONTENTS DEVICE DESCRIPTION... 1 Indications... 1 Contraindications... 1 Warnings and Precautions...
Physician s Manual FLEXTEND Steroid-Eluting Extendable/Retractable Helix Pace/Sense Leads MODELS: 4086/4087/4088 CONTENTS DEVICE DESCRIPTION... 1 Indications... 1 Contraindications... 1 Warnings and Precautions...
Xcela Hybrid PICC with PASV Valve Technology
 Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Instructions. Fibrlok Optical Fiber Splicing System. Issue 6, August 1991
 Instructions TM Fibrlok Optical Fiber Splicing System Issue 6, August 1991 1.0 General The 3M brand Fibrlok TM Optical Fiber Splicing System provides permanent mechanical splices for single or multi mode
Instructions TM Fibrlok Optical Fiber Splicing System Issue 6, August 1991 1.0 General The 3M brand Fibrlok TM Optical Fiber Splicing System provides permanent mechanical splices for single or multi mode
Instructions. Fibrlok II Optical Fiber Splicing System. Issue 1, June 1992
 Instructions TM Fibrlok II Optical Fiber Splicing System Issue 1, June 1992 Contents: 1.0 General... 1 2.0 Splicing Set-up... 3 3.0 Fiber Preparation... 4 4.0 Splice Assembly... 4 5.0 Fiber Repositioning...
Instructions TM Fibrlok II Optical Fiber Splicing System Issue 1, June 1992 Contents: 1.0 General... 1 2.0 Splicing Set-up... 3 3.0 Fiber Preparation... 4 4.0 Splice Assembly... 4 5.0 Fiber Repositioning...
Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System.
 Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 0300-000175-001
Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 0300-000175-001
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
EXCELLA MIS. Spinal System
 EXCELLA MIS Spinal System Excella MIS Spinal System INDICATIONS FOR USE The Innovasis Excella MIS Spinal System is intended for use in the non-cervical area of the spine. WARNING: The safety and effectiveness
EXCELLA MIS Spinal System Excella MIS Spinal System INDICATIONS FOR USE The Innovasis Excella MIS Spinal System is intended for use in the non-cervical area of the spine. WARNING: The safety and effectiveness
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
Eldor Epidural Kit (CSEN 68) Epidural catheter technique
 Eldor Epidural Kit (CSEN 68) Epidural catheter technique Using the epidural needle the epidural space is reached by the loss of resistance technique or the hanging drop technique, while the proximal opening
Eldor Epidural Kit (CSEN 68) Epidural catheter technique Using the epidural needle the epidural space is reached by the loss of resistance technique or the hanging drop technique, while the proximal opening
Aus Artificial Uretheral Sphincter Port System
 NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
