The Pennsylvania State University. The Graduate School. College of Medicine THE REGULATION OF MTORC1 SIGNALING IN IMMOBILIZED RAT
|
|
|
- Marjorie White
- 6 years ago
- Views:
Transcription
1 The Pennsylvania State University The Graduate School College of Medicine THE REGULATION OF MTORC1 SIGNALING IN IMMOBILIZED RAT HINDLIMB SKELETAL MUSCLE A Dissertation in Physiology by Andrew R. Kelleher 2014 Andrew R. Kelleher Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2014
2 The dissertation of Andrew R. Kelleher was reviewed and approved* by the following: Leonard S. Jefferson, Jr. Evan Pugh Professor of Physiology Dissertation Adviser Chair of Committee Scot R. Kimball Professor of Cellular and Molecular Physiology Christopher R. Yengo Associate Professor of Cellular and Molecular Physiology Lisa Shantz Associate Professor of Cellular and Molecular Physiology Ian A. Simpson Professor of Neural and Behavioral Sciences Donald Gill Professor and Chair of Cellular and Molecular Physiology *Signatures are on file in the Graduate School.! ii!
3 ABSTRACT Limb immobilization, limb suspension, and bed rest cause substantial loss of skeletal muscle mass, a phenomenon termed disuse atrophy. Disuse atrophy is attributed to a depression in the rates of protein synthesis in a fasted state, and a resistance to stimulation by nutrients and other anabolic stimuli. Skeletal muscle protein synthesis is modulated by mechanistic target of rapamycin complex 1 (mtorc1) signaling, which is repressed during hindlimb immobilization. The overall goal of this research was to understand the molecular mechanisms responsible for repression of mtorc1 signaling in immobilized rat hindlimb soleus muscle. The overall hypothesis was that mtorc1 signaling is repressed in immobilized rat hindlimb skeletal muscle due to induction in the mrna expression of two repressors of mtorc1 signaling, regulated in development and DNA damage responses (REDD) 1 and REDD2. The studies show that REDD1 and REDD2 mrna expression are induced in association with repression of mtorc1 signaling after 1-3 days of hindlimb immobilization. Hindlimb immobilization repressed mtorc1 signaling in a fasted state and blunted the stimulation of mtorc1 signaling in response to a bolus of leucine, a potent nutrient stimulator of mtorc1 signaling. Fixed muscle length was identified as a physiological trigger for the expression of genes associated with disuse atrophy, particularly REDD1 and REDD2. These genes were induced in soleus muscle immobilized for 3 days in a shortened position, but not in a stretched position. Aging was also associated with repression of mtorc1 signaling and induction in the mrna expression of REDD2, while 7 days of remobilization was associated with augmented mtorc1 signaling and repression in the mrna expression of REDD2. Collectively, these findings indicate that the mrna expression of REDD1, and primarily REDD2 at time points longer than 3 days, is associated with changes in mtorc1 signaling under conditions of hindlimb immobilization, aging, and remobilization. REDD1 and REDD2 act as repressors and governors of the capacity for mtorc1 signaling in fasted and fed states. Furthermore, impaired PDK1 signaling to 70-kDa ribosomal protein S6 kinase 1 (p70s6k1) and induction of REDD1 and REDD2 are associated with resistance to anabolic stimulation of mtorc1 signaling. The results support the overall hypothesis that mtorc1 signaling is repressed in immobilized rat hindlimb skeletal muscle due to induction of REDD1 and REDD2 mrna expression.! iii!
4 TABLE OF CONTENTS List of Tables...vi List of Figures...vii List of Abbreviations...ix Acknowledgements...x Chapter 1. INTRODUCTION...1 Disuse Atrophy...1 Protein Turnover...3 mtorc1 Signaling...6 REDD1 and REDD2...9 Disuse and mtorc1 Signaling...11 Fixed Muscle Length...13 Aging and Remobilization...14 Figures...17 Chapter 2. MATERIALS AND METHODS...20 Animals...20 Hindlimb Immobilization...20 Administration of Puromycin and Sample Collection...21 SDS-PAGE and Immunoblot Procedure...23 Measurement of p70s6k1 and 4E-BP1 Hyperphosphorylation...25 Measurement of Skeletal Muscle Protein Synthesis...25 Measurement of mrna Expression...26 Statistical Analysis...27 Chapter 3. THE REPRESSORS OF MTORC1 SIGNALING REDD1/2 ARE RAPIDLY INDUCED AND ACTIVATION OF P70S6K1 BY A NUTRIENT STIMULUS IS DEFECTIVE IN SKELETAL MUSCLE OF AN IMMOBILIZED RAT HINDLIMB..29 Introduction...29 Methods...30 Results...31 Discussion...36 Figures and Tables...40 Chapter 4. CHANGES IN REDD1, REDD2, AND ATROGENE MRNA EXPRESSION ARE PREVENTED IN SKELETAL MUSCLE FIXED IN A STRETCHED POSITION DURING HINDLIMB IMMOBILIZATION...51 Introduction...51 Methods...51 Results...52 Discussion...55 Figures...59! iv
5 Chapter 5. AGE-RELATED RESPONSES OF MTORC1 SIGNALING IN RAT HINDLIMB SKELETAL MUSCLE TO IMMOBILIZATION AND REMOBILIZATION...64 Introduction...64 Methods...65 Results...66 Discussion...72 Figures...78 Chapter 6. CONCLUSIONS AND FUTURE DIRECTIONS...87 mtorc1 Signaling and the Genesis of Disuse Atrophy...88 Anabolic Resistance...91 Limitations...98 Muscle Length and Disuse Atrophy Conclusion Figure REFERENCES...109! v
6 TABLES Table 3.1 Protein concentration of soleus muscle from immobilized and control hindlimbs after 1-3 days of hindlimb immobilization 44 Table 3.2 End points not associated with immobilization-induced repression of mtorc1 signaling.47! vi
7 FIGURES Figure 1.1. Our current understanding of the genesis of disuse atrophy of skeletal muscle...17 Figure 1.2. mtorc1 signaling modulates the rates of protein synthesis in response to stimulation by growth factors and nutrients...18 Figure 1.3. Growth factors and nutrients are both necessary for full activation of mtorc Figure 3.1. Time course changes in muscle weight following immobilization...43 Figure 3.2. Effect of immobilization on rates of protein synthesis in soleus muscle...45 Figure 3.3. Effect of immobilization on p70s6k1 hyperphosphorylation and 4E-BP1 phosphorylation in the soleus muscle...46 Figure 3.4. Effect of immobilization on site-specific p70s6k1 phosphorylation in the soleus muscle...48 Figure 3.5. Effect of immobilization on REDD mrna expression in soleus muscle...49 Figure 3.6. Regulation of mtorc1 signaling under conditions of 1-3 days of hindlimb immobilization...50 Figure 4.1. Mass and rates of protein synthesis in the soleus are reduced only when the muscle is immobilized in a shortened position...59 Figure 4.2. Phosphorylation of p70s6k1 at Thr389 in the soleus is reduced only when the muscle is immobilized in a shortened position...60 Figure 4.3. REDD1 and REDD2 mrna expression in the soleus is induced only when the muscle is immobilized in a shortened position...61 Figure 4.4. Atrogene expression is induced in the soleus only when the muscle is immobilized in a shortened position...62 Figure 4.5. Phosphorylation of Akt at Ser473 and FoxO3a at Ser253 are reduced only when the soleus muscle is immobilized in a shortened position...63 Figure 5.1. Young rats are more susceptible to hindlimb immobilization-induced loss of muscle mass than older rats...78! vii!
8 Figure 5.2. Nutrient-induced activation of mtorc1 signaling declines dramatically during aging and in older rats the pathway is not responsive to immobilization, but is augmented following remobilization...79 Figure 5.3. Feeding-induced activation of Akt is blunted with age and hindlimb immobilization in skeletal muscle from young vs. older rats, and is augmented following remobilization...80 Figure 5.4. REDD2, but not REDD1, mrna expression increases with age and becomes unresponsive to immobilization, but is repressed following remobilization...81 Figure 5.5. REDD2 mrna expression is inversely proportional to phosphorylation of p70s6k1 at Thr389 in rat soleus muscle...82 Figure 5.6. Nutrient-induced activation of PDK1 signaling declines during 7 days of hindlimb immobilization and aging, but in older rats the pathway is not responsive to immobilization...83 Figure 5.7. MAFbx mrna increases with age and 7 days of immobilization, while MuRF1 increases with immobilization only in 2-month old animals and decreases in response to remobilization...84 Figure 5.8. Gastrocnemius muscle RNA content decreases with aging...85 Figure 5.9. Regulation of mtorc1 signaling under conditions of aging and 7 days of hindlimb immobilization...86 Figure 6.1. Phosphorylation of p70s6k1 at Thr389 was not attenuated despite reduced muscle mass-to-body mass ratio in soleus muscle from the immobilized hindlimb of REDD1 knockout...108! viii!
9 ABBREVIATIONS 4E-BP1, eukaryotic initiation factor 4E-binding protein 1 AMPK, AMP-activated protein kinase ATF4, activating transcription factor 4 Deptor, DEP domain containing mtor-interacting protein eif, eukaryotic initiation factor eif2α, alpha subunit of eukaryotic initiation factor 2 ER, endoplasmic reticulum ERK, extracellular signal-regulated kinase FoxO, forkhead box O Grb10, growth factor receptor-bound protein 10 GAP, GTPase activating protein HIF1-α, hypoxia-inducing factor 1 alpha subunit IGF-1, insulin-like growth factor LKB1, liver kinase B1 MAFbx, Muscle Atrophy F-box MEFs, murine embryonic fibroblasts mtorc1, mechanistic target of rapamycin complex 1 MuRF1, Muscle Ring Finger 1 p70s6k1, 70 kilodalton ribosomal protein S6 kinase 1 PDCD4, programmed cell death 4 PI(3)K, phosphatidylinositol-3-oh kinase PP2A, protein phosphatase 2A Rag, Ras-related GTP binding REDD, regulated in development and DNA damage responses Rheb, Ras-homology enriched in brain SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis TBC1D7, Tre2-Bub2-Cdc16-1 domain family member 7 Tbp, TATA-binding protein TBS-T, Tris-buffered saline + Tween 20 TSC, tuberous sclerosis complex! ix
10 ACKNOWLEDGMENTS I would like to thank my mentors, Dr. Leonard Jefferson and Dr. Scot Kimball, for their guidance, patience, support, generosity, and trust throughout my doctoral training. I would also like to thank my doctoral committee members, Dr. Christopher Yengo, Dr. Lisa Shantz and Dr. Ian Simpson, for their guidance, respect and confidence in my abilities to complete this doctoral dissertation. A special thank you to all members of the Jefferson Laboratory for their time, effort, technical expertise, positive attitude and teamwork during all experiments over the last five years of research. Specifically, I would like to thank Sharon Rannels, Holly Lacko, Dr. Bradley Gordon, Dr. Alex Tuckow, Dr. Michael Dennis, Dr. Ruud Schilder, Lydia Kutzler, Emily Sun, Lauren Luongo, Tony Martin, Chen Yang, Joel Coble, Suhana Ravi and Abid Kazi. In addition, I acknowledge the National Institutes of Health (NIH DK-15658, L.S.J), the Pennsylvania Department of Health (Tobacco Settlement Funds), and the Abbott Nutrition Company (S.R.K.) for their financial support of my doctoral dissertation experiments. I recognize that my accomplishments are products of the love and generous support of friends and family. I would be remiss if I did not thank my wonderful friends for their friendship and sharing in so many joyous occasions. I would especially like to thank friends like Darshan Trivedi, Rachel Drew, Jimmy McGhee, Andrew Winslow, Julian Joseph, Clayton Belcher, Matt and Annie Muller, Mariano Russo, Dan and Lynley Lapp, and Jacob and Katy Serfass. A special thanks to my former biology and exercise physiology mentors who inspired me to pursue a scientific career including Dr. Mary Constant Byrne and Dr. Paul Meier from Muhlenberg College, and Dr. Stefan Keslacy and Dr. Lori Ploutz-Snyder from Syracuse University. Thank you to my loving family, especially Jason, Colin, Eddie, Ave, Mott, and Dr. Stephen and Polly Newsholme. A special thank you to my parents, Cheryl and Kevin, for all their love, faith, support and confidence in me. Sadly, my father passed away while I was writing this doctoral dissertation. Finally, I would like to dedicate this dissertation and all Ph.D. work to my loving wife and mother-to-be, Rosalind, who is my source of inspiration and strength.! x
11 Chapter 1. INTRODUCTION Disuse Atrophy Skeletal muscle is a remarkably plastic tissue that is essential for health and survival. Comprising 40% of body weight, it is important for locomotion, energy consumption, fuel for other tissues and amino acid storage (51). Plasticity allows skeletal muscle to adapt its size as well as metabolic, structural and contractile properties in response to altered activity (24, 34, 60). Increased mechanical loading and workload lead to gains in skeletal muscle mass, (also known as hypertrophy). In contrast, physical inactivity, mechanical unloading, hypodynamia and hypokinesia lead to losses in skeletal muscle mass (also known as atrophy). Disuse is an expansive label for low mechanical load or unloading of muscle with physical inactivity (24). This thesis investigates some of the molecular mechanisms associated with skeletal muscle atrophy in response to disuse. The loss of skeletal muscle mass due to disuse (e.g. limb immobilization, limb unloading/suspension and bed rest) that occurs in the absence of local or systemic inflammation is a phenomenon known as disuse atrophy (143, 162). Disuse atrophy is a major healthcare problem that can lead to physical frailty, loss of independent living, prolonged periods of rehabilitation, increased risks of falls and fractures and reduced overall prognosis (139, 143, 200). Exercise and physical training, nutritional aids, growth factors, ergogenic supplements and drugs have been implemented in an attempt to prevent or reverse disuse atrophy (for review, see (34). Unfortunately, these approaches have not led to effective therapeutic interventions that prevent disuse atrophy in humans. The development of such therapeutic interventions will require an understanding of the 1
12 molecular mechanisms responsible for disuse atrophy. To this end, the objective of my thesis research is to understand the molecular mechanism(s) responsible for disuse atrophy. Rodent models of disuse atrophy are often used in studies aimed at understanding the molecular mechanisms that regulate skeletal muscle mass. Immobilization of a limb (also known as limb immobilization) in humans is equivalent to hindlimb immobilization in rodents (82). Hindlimb immobilization is a procedure that involves fixation of an animal s ankle joint (123) and sometimes knee joint (62) with a cast or material that prevents joint mobility. The prevention of joint mobility reduces mechanical loading on skeletal muscles in an immobilized hindlimb and leads to disuse atrophy, particularly in muscles fixed in a shortened position (21). Unloading of a limb (also known as limb unloading or limb suspension) in humans is used to simulate the effects of extended periods of microgravity and spaceflight on human tissues (85). An equivalent model in rodents is hindlimb unloading; a procedure that involves the fixation of a rodent s tail to a cage ceiling in order to lift the rodent s hindlimbs off the cage floor for extended periods (154). This procedure reduces mechanical loading on the anti-gravity muscles of rodent hindlimbs, which leads to disuse atrophy. Finally, denervation is used to reduce muscle contraction muscles of the lower hindlimbs of rodents by damaging or removing a portion of the sciatic nerve (76). Denervation prevents neuromuscular communication between the spine and hindlimb muscles. Consequently, disuse atrophy results from a lack of muscular activation and loading (178). Compared to humans, rodent models exhibit disuse atrophy quite rapidly. For example, 2 weeks of limb immobilization reduced vastus lateralis muscle fiber diameter 2
13 (cross-sectional area or CSA) 5-20% in human subjects (72, 201). Similarly, 2 weeks of bed rest and limb unloading reduced human leg lean mass 4% and vastus lateralis muscle CSA 5%, respectively (2, 50, 61). In contrast, rat soleus muscle mass was reduced 56% in the immobilized hindlimb after 10 days of hindlimb immobilization (220). Moreover, 2 weeks of hindlimb unloading caused a 37-60% reduction in rat soleus muscle CSA (44, 66). Differences in the extent of disuse atrophy between humans and rodents may be explained by the high rates of metabolism and protein turnover in rodents (162). As a benefit, the molecular mechanisms that cause disuse atrophy may be easier to identify in rapid-atrophying rodents because the processes responsible for atrophy are likely to be amplified. Other benefits of using rodent models to study disuse atrophy include more control over experimental characteristics, short-lifespan, ease of sample collection, and manipulation of gene expression (1). With this in mind, I characterized a rodent model of unilateral hindlimb immobilization and used this model to investigate the molecular mechanisms responsible for disuse atrophy. Protein Turnover Disuse atrophy is the result of an imbalance in skeletal muscle protein turnover. The turnover of skeletal muscle proteins involves the ongoing processes of protein synthesis and protein degradation (51). Skeletal muscle atrophy occurs under conditions wherein the rates of protein degradation exceed the rates of protein synthesis resulting in a negative net protein balance. This can result from either a reduction in the rates of protein synthesis, a rise in the rates of protein degradation, or a combination of both processes (80). In human skeletal muscle, the rates of protein synthesis are depressed 3
14 during limb immobilization (69, 70), unloading (40) and bed rest (191). This depression in the rates of protein synthesis is observed in both fasted (post-absorptive) and fed (postprandial) states (50, 72, 127, 162). In the fed state, the depression in protein synthesis is attributed to a resistance to stimulation by nutrients and other anabolic stimuli termed anabolic resistance ( ). On average, the rates of protein synthesis in fasted and fed states are depressed by ~60% and ~50%, respectively, resulting in a rate of loss of protein equal to ~0.5%/day (162). This calculated rate of loss is in agreement with the observed loss of muscle mass. In addition, biomarkers such as 13 C-labelled amino acid appearance and 3-methylhistidine excretion measured in human blood and urine before and after bed rest (16, 61) and spaceflight (197, 198) indicate that the rates of protein degradation do not increase in response to disuse. Therefore, it would seem that the depressions in rates of protein synthesis in the fasted and fed states are primarily responsible for the observed disuse atrophy in humans. In rodent skeletal muscle, disuse leads to depressed rates of protein synthesis in addition to augmented rates of protein degradation (74-76, 121). The observed changes in rodent skeletal muscle protein turnover occur rapidly with disuse whereby 6 hours of hindlimb immobilization depressed the rates of protein synthesis (22). Similar to humans, rates of protein synthesis are depressed ~50% in rat soleus muscle during hindlimb immobilization (75). Moreover, hindlimb immobilization causes resistance to nutrient-induced stimulation of protein synthesis in rodent gastrocnemius muscle (127). If rodents and humans exhibit the same manifestations of depressed protein synthesis in response to disuse, then rodent models are suitable for studying human-relevant mechanisms of disuse atrophy. Since disuse atrophy in humans is the result of 4
15 depressions in skeletal muscle protein synthesis and not increases in skeletal muscle protein degradation, my thesis research is focused on understanding the molecular mechanisms responsible for depressions in rates of protein synthesis. Unlike humans, the rates of protein degradation increase in rat soleus muscle during hindlimb immobilization (74, 75), hindlimb suspension (137) and denervation (74, 76). Increases in the rates of protein degradation during disuse in rodents have been attributed to increases in skeletal muscle inflammatory signaling (12, 13), metabolic disturbances and mitochondrial dysfunction (48, 164, 165), muscle remodeling, and activation of apoptosis (144, 165). The rates of protein degradation are modulated in part through the ATP-dependent ubiquitin proteasomal pathway (for review see (182)). In response to disuse, the proteasome-mediated degradation of skeletal muscle protein is regulated by the expression of atrogenes ; two muscle-specific E3 ubiquitin ligases known as MAFbx/atrogin-1 (Muscle Atrophy F-box) and MuRF1 (Muscle Ring Finger 1) (18). A critical role for these two ligases in disuse atrophy was demonstrated in atrogene knockout animals that exhibited resistance to denervation-induced atrophy in gastrocnemius muscle (18). Hindlimb immobilization and unloading induce both MAFbx and MuRF1 gene expression in skeletal muscle (11, 189). However, the role of atrogenes and other mediators of protein degradation in human disuse atrophy are unclear, because the rates of protein degradation do not increase during disuse (143, 162, 171). While the precise role of atrogenes in disuse atrophy is unclear, they can be used to assess gene expression patterns associated with disuse atrophy. 5
16 mtorc1 Signaling The rates of protein synthesis in skeletal muscle are modulated, in part, by the mechanistic target of rapamycin complex 1 (mtorc1) signaling pathway. Nutrients, growth factors such as insulin and insulin-like growth factor (IGF-1), and exercise activate mtorc1 signaling, while hypoxia, stress, DNA damage and low energy levels repress it (118, 129). Upon activation, mtorc1 phosphorylates protein substrates at specific residues including p70 ribosomal protein S6 kinase 1 (p70s6k1) at Thr389, eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) at Ser65, and uncoordinated- 51 like autophagy activating kinase 1 (ULK1) at Ser757 (8, 27, 79, 114, 146). When phosphorylated, these substrates promote cap-dependent mrna translation and repress autophagy (114, 118). Phosphorylation of 4E-BP1 prevents its association with eukaryotic initiation factor (eif) 4E and thereby permits eif4e to associate with eif4g and eif4a in the formation of the active mrna cap-binding complex, eif4f (118). Capdependent mrna translation is also bolstered by phosphorylation of p70s6k1, which subsequently phosphorylates two proteins that participate in cap-dependent translation initiation and another that participates in translation elongation. Activated p70s6k1 phosphorylates programmed cell death 4 (PDCD4), which interacts with eif4a and prevents its association with eif4g (218). Once phosphorylated, PDCD4 is released from eif4a, allowing eif4a to interact with eif4g (80). Activated p70s6k1 also phosphorylates eif4b, which enhances the RNA helicase activity of eif4a (176). Finally, activated p70s6k1 phosphorylates and inactivates eukaryotic elongation factor (eef) 2 kinase, which reduces phosphorylation of eef2 and allows eef2 to participate in mrna translation elongation (212). Measuring the phosphorylation state of mtorc1 6
17 substrates provides a readout of mtorc1 signaling and the activation state of critical participants in protein synthesis. Nutrients, particularly amino acids such as leucine, are necessary but not sufficient for full activation of mtorc1 (88, 129, 149). Nutrients induce the movement of mtorc1 to lysosomal membranes where Ras-related GTP binding (Rag) proteins are located (179, 180). On the lysosomal surface, Rag proteins interact with a protein complex called Ragulator that is essential for activation of mtorc1 in response to amino acids (179). Rag proteins form a GTPase heteroduplex consisting of either RagA or RagB in association with either RagC or RagD (115, 180). When RagA or RagB is bound to GTP and RagC or RagD is bound to GDP, the heterodimer promotes mtorc1 signaling. In contrast, when RagA or RagB is bound to GDP and RagC or RagD is bound to GTP, the heterodimer does not promote mtorc1 signaling. Localizing mtorc1 to the lysosome brings it into close proximity with a small GTPase called ras homolog enriched in brain (Rheb) (129). Rheb activates mtorc1 when Rheb is associated with GTP, while Rheb GDP does not (136). Thus, the activation of mtorc1 requires nutrients to signal through the Rag and Ragulator proteins and localizes mtorc1 to the lysosome where it can interact with Rheb GTP (149). Rheb GTP/GDP-loading state is regulated by growth factors such as insulin and IGF-1 (46, 149). Growth factors and exercise promote mtorc1 signaling through activation of Akt and extracellular signal-regulated kinase (ERK) signaling pathways, respectively. These lead to phosphorylation and repression of tuberous sclerosis complex (TSC) function (102, 138, 142). The TSC complex is composed of the TSC tumor suppressors, TSC1 and TSC2, and Tre2-Bub2-Cdc16-1 domain family member 7 7
18 (TBC1D7) (46). TSC2 is a GTPase activating protein (GAP) for Rheb, which promotes GTP hydrolysis by Rheb, while TSC1 and TBC1D7 are required for the stability and full GAP activity of TSC2 towards Rheb (101, 149). The TSC complex negatively regulates mtorc1 signaling through its Rheb-GAP activity whereby mtorc1-activating Rheb GTP gets converted into its inactive Rheb GDP state (129). Like nutrients, growth factors are also necessary but not sufficient for full activation of mtorc1 (88, 129, 149). Perhaps the interdependence of nutrient and growth factor signaling for activation of mtorc1 can be explained by mtorc1 localization and Rheb GTP/GDP-loading state. According to a recent unifying model for integrated coregulation of mtorc1 by nutrients and growth factors, nutrients localize mtorc1 to the lysosome and bring it into close proximity with its essential activator Rheb. At the same time, growth factor signaling represses TSC complex function to promote Rheb-dependent activation of mtorc1 (149). Many repressors of mtorc1 signaling act on the TSC complex (80). Hypoxia, stress, DNA damage and low energy increase TSC2 GAP activity towards Rheb by phosphorylation on alternative residues from Akt and ERK that stabilize the TSC complex (45, 103, 196). Energy stress activates Liver kinase B1 (LKB1), which leads to the phosphorylation and activation of AMP-activated protein kinase (AMPK) (193). Activated AMPK phosphorylates TSC2 on distinct residues that promote TSC2 GAP activity toward Rheb and repression of mtorc1 signaling (20, 192). Genotoxic and oxidative stress repress mtorc1 signaling through the p53-dependent genes, Sestrin 1 and Sestrin2, which activate AMPK (26). Moreover, stress to the endoplasmic reticulum (ER stress) is known to induce activating transcription factor 4 (ATF4) and increase the 8
19 expression of Sestrin 2 and another known repressor of mtorc1 signaling, REDD1 (regulated in development and DNA damage responses) (25). REDD1 and REDD2 Although the molecular mechanisms are still unclear, REDD1 and REDD2 repress mtorc1 signaling through the TSC complex (152, 196). REDD1 and REDD2 share only 34% DNA and 61% amino acid sequence homology, but seem to repress mtorc1 signaling by a similar mechanism upstream of Rheb that requires TSC2 (152). One mechanism that has been accepted by the field involves the release of TSC2 from growth factor-induced association with inhibitory proteins (45, 152). According to a model proposed by DeYoung et al (45), insulin and growth factors lead to Aktmediated phosphorylation of TSC2. This promotes TSC2/ association and thereby inhibits TSC function. In the presence of REDD1 and REDD2, proteins dissociate from TSC2 and bind REDD proteins (45, 152). Knockdown of REDD1 expression in muscle cells leads to disruption of the TSC complex (211). Stabilization of TSC2 in the TSC complex is believed to promote GTP hydrolysis by Rheb, leading to repression of mtorc1 signaling through its Rheb-GAP activity whereby mtorc1- activating Rheb GTP gets converted into its inactive Rheb GDP state (129). In contrast, structural-based docking and functional analyses suggest that REDD1 does not directly bind to proteins (209). Some recent evidence from our lab suggests that REDD1 may act as a targeting protein for the catalytic subunit of protein phosphatase 2 (PP2A). According to this model, REDD1 recruits PP2A to dephosphorylate Akt at Thr308, which reduces phosphorylation of TSC2, Rheb-GTP loading and mtorc1 signaling (42). 9
20 Since REDD1 and REDD2 share a conserved mtorc1-inhibitory motif, it is assumed that they repress mtorc1 signaling by a similar mechanism with few exceptions (150). For example, differences in the N-terminus of REDD1 allow it, and not REDD2, to be sequestered to the plasma membrane where it does not repress mtorc1 signaling in response to G-protein-coupled receptor signaling. Also, REDD2 contains a distinct loop region that allows for REDD2 to interact with interferon-regulatory factor-1, which may allow for cross-talk between mtorc1 signaling and cytokine pathways during the regulation of cell growth (84). REDD1 and REDD2 expression is induced under conditions of stress. In animal models, REDD1 is induced during obesity (217), starvation (145), and conditions of hypercortisolemia including stroke, type 2 diabetes, and stress induced by confinement (125). In vitro, REDD1 is induced in response to DNA damage (58), oxidative stress, energy stress (196), hypoxia (45), hypertonicity (160) glucocorticoids (211) and ER stress (214). The mechanism in which REDD1 is induced during ER stress is wellcharacterized. ER stress activates PKR-like ER kinase (PERK), which phosphorylates eif2alpha and leads to an increases the mrna and protein expression of a REDD1 transcription factor, ATF4 (119, 214). ATF4 induces REDD1 expression in response to both ER stress and growth factor deprivation (i.e. serum starvation) (43, 214). The induction in REDD1 expression seems to be regulated at the level of mrna transcription (i.e. serum starvation-induced ER stress caused an increase in the transcription rate of REDD1 mrna in Rat 2 fibroblasts) (43). REDD2 is induced in response to hypoxia (152, 168), hypertonicity (160), artherosclerotic lesions (100) and hindlimb unloading (163), and it is currently unknown if ER stress, ATF4 or growth factor deprivation are 10
21 also inducers. Growth factor signaling may play a role in the repression of both REDD1 and REDD2 mrna expression. REDD1 mrna and protein expression was induced in rat gastrocnemius muscle under conditions of low serum insulin (e.g. starvation and diabetes) and reduced after refeeding (145). IGF-1 treatment reduced REDD2 mrna expression in myotubes and rat gastrocnemius muscle following IGF-1 treatment (63). Thus, a relationship exists between reduced or impaired growth factor signaling and induction of REDD1 and/or REDD2 mrna expression. However, the precise mechanisms responsible for regulation of REDD1 and REDD2 mrna transcription under such conditions is currently unknown. Disuse and mtorc1 Signaling Depressed rates of protein synthesis during disuse are associated with attenuated mtorc1 signaling. Ten days of hindlimb immobilization reduced p70s6k1 phosphorylation in soleus muscle from immobilized hindlimbs of adult rats (220). Similarly, 7 days of hindlimb immobilization attenuated mtorc1 signaling in gastrocnemius muscle from immobilized hindlimbs of young mice (127) and rats (126). Attenuated mtorc1 signaling has also been observed in other models of disuse. Compared to control conditions, phosphorylation of p70s6k1 was reduced in rat soleus muscle after just 12 hours of hindlimb unloading (10, 94). Moreover, reduced p70s6k1 phosphorylation has consistently been observed in rat soleus, gastrocnemius, and quadriceps muscles after 1 and up to 21 days of hindlimb unloading (10, 86, 94, 135). An important role for mtorc1 signaling in the regulation of skeletal muscle mass during disuse is further supported by gene knockdown studies in mice. Genetic deletion 11
22 of the mtorc1 obligatory component, raptor, inactivated mtorc1, prevented skeletal muscle growth and enhanced atrophy (15). In the same study, deletion of the mtorc1 suppressor, TSC1, induced skeletal muscle hypertrophy and made mouse soleus muscle resistant to denervation-induced atrophy. Furthermore, gene silencing of another mtorc1 inhibitory protein, DEP domain containing mtor-interacting protein (Deptor), rescued rates of protein synthesis and preserved skeletal muscle mass in gastrocnemius muscle during hindlimb immobilization (109). Despite strong evidence implicating attenuated mtorc1 signaling in depressed rates of protein synthesis and disuse atrophy, the molecular mechanism responsible for repression of mtorc1 signaling during disuse is unknown. Moreover, the majority of these studies have assessed mtorc1 signaling in a fasted condition and not in a fed condition. As a result, the role of mtorc1 signaling in anabolic resistance to nutrient stimulation is also unknown. Hindlimb unloading has been found to induce skeletal muscle p53 (194) and REDD2 (163) expression. However, no study has characterized the expression patterns of these repressors with mtorc1 signaling during disuse atrophy. Thus, the first goal of my research was to understand the molecular mechanisms responsible for repression of mtorc1 signaling in skeletal muscle during hindlimb immobilization. Some evidence suggests that ATF4 and impaired growth factor signaling may be responsible for disuse atrophy. A prototypical ER stress response was not exhibited in rat soleus muscle following 7 days of hindlimb unloading (97). However, this does not preclude ATF4 from playing a role in disuse atrophy. Interestingly, mice that had the ER stress-induced protein ATF4 knocked out were resistant to muscle atrophy after 24 hours of starvation and 3 days of hindlimb immobilization (56). Moreover, silencing of growth 12
23 arrest and DNA damage-inducible 45a protein, a gene induced by ATF4, protected mouse tibialis anterior muscle from atrophy induced by 1 day of fasting, and 7 days of denervation and hindlimb immobilization. Physical inactivity and disuse are also associated with insulin resistance and impaired growth factor signaling through the Akt/TSC pathway (159, 187). Impaired growth factor (i.e. insulin) signaling has been attributed to the attenuated phosphorylation of Akt at Ser473 observed in muscles during hindlimb immobilization and unloading (54, 87, 159, 220). As introduced in 'REDD1 and REDD2', ATF4 and reduced or impaired growth factor signaling are associated with induction of REDD1 and REDD2. This has led me to speculate that REDD1 and REDD2 are induced in skeletal muscle during disuse due to induction of ATF4 and impaired growth factor signaling. Fixed Muscle Length Fixed muscle length during hindlimb immobilization plays a key role in the regulation of skeletal muscle mass and protein synthesis. Prior investigations by two separate laboratories show differences in the amount of disuse atrophy when hindlimb muscles were immobilized in either a shortened or stretched position (21, 75). Disuse atrophy of the soleus muscle was observed when a rat ankle joint was immobilized for 7 days in full plantarflexion (soleus muscle placed in a shortened position). In contrast, no disuse atrophy of the soleus muscle was observed when a rat ankle joint was immobilized for 7 days in full dorsiflexion (soleus muscle placed in a stretched position). In association with the observed patterns for muscle mass, rates of protein synthesis were depressed in the soleus muscle immobilized in plantarflexion, but not in soleus 13
24 immobilized in dorsiflexion (75). The same phenomenon has been observed in other disuse models including hindlimb unloading and denervation (76, 137). However, the molecular mechanism by which fixed muscle length regulates skeletal muscle mass and protein synthesis is unknown. Depressed rates of protein synthesis in skeletal muscle fixed in a shortened position may be the result of repressed mtorc1 signaling. Upon close analyses of the studies that have assessed mtorc1 signaling during limb immobilization, there is an association between repression of mtorc1 signaling and fixed limb position that is under-recognized. The evidence that indicates mtorc1 signaling is attenuated during disuse comes from studies where skeletal muscle is placed in a shortened position (94, 126, 127, 220). Interestingly, those studies that have not observed attenuated mtorc1 signaling used immobilization techniques where the muscle of interest was fixed in a neutral (not stretched or shortened) position (40, 72). No study has measured mtorc1 signaling in skeletal muscle maintained in a stretched position during disuse. If skeletal muscle protein synthesis is not depressed when muscles are fixed in stretched positions during disuse, and mtorc1 signaling modulates the rates of protein synthesis, then mtorc1 signaling is not likely to be attenuated in these muscles. Understanding how fixed muscle length influences mtorc1 signaling during disuse will elucidate the mechanism responsible for disuse atrophy during hindlimb immobilization. Therefore, the second goal of my research was to understand the role of fixed muscle length during hindlimb immobilization in the regulation of mtorc1 signaling. 14
25 Aging and Remobilization Aging has a profound influence on skeletal muscle mass. In humans and rodents, skeletal muscle hypertrophies during the juvenile and adolescent stages prior to adulthood, and atrophies during old age. In the fasted state, initial studies observed higher rates of protein synthesis in skeletal muscle from young adults compared to elderly (213, 219). However, more recent studies have not observed differences in the fasted rates of protein synthesis between young and old adults (64, 65, 167, 206). Rather, the negative protein balance that causes age-induced skeletal muscle atrophy likely results from anabolic resistance to exercise (55), amino acid infusion (83) and feeding (38, 39, 174). Such studies have not observed differences in mtorc1 signaling between young and old adult humans (38, 83) or rats (166) in either fasted or fed states. However, some have observed blunted nutrient-induced stimulation of mtorc1 signaling in muscles from old subjects (64, 83, 98). Unlike juveniles and adolescents, the muscles of young adult subjects are not actively growing. As a result, none of these studies have compared the nutrient-induced stimulation of mtorc1 signaling between skeletal muscles from actively growing young subjects and non-growing older subjects. Since disuse- and age-induced skeletal muscle atrophy are linked in the blunted stimulation of protein synthesis and mtorc1 signaling, I speculate that disuse and aging share a common mechanism responsible for regulating mtorc1 signaling. Remobilization following hindlimb immobilization rescues the muscle mass lost during disuse in young (23, 33), but not old (140, 155) subjects. Recovery of lost muscle mass during remobilization involves multiple stress signaling pathways and various transcriptional and hypertrophic mechanisms (33, 82, 139). Among these mechanisms, 15
26 remobilization increases the rates of protein synthesis and activates mtorc1 signaling in skeletal muscle (98). However, the mechanism responsible for remobilization-induced activation of mtorc1 signaling is unknown. It is important that this mechanism be understood because it will help us to understand the regulation of skeletal muscle mass in response to changes in mechanical loading and physical activity. I speculate that the same mechanism responsible for repression of mtorc1 signaling during hindlimb immobilization is involved (only in a reversed manner) in the activation of mtorc1 signaling during remobilization. Therefore, the third goal of my research was to understand and compare the mechanisms responsible for modulation of mtorc1 signaling with aging, hindlimb immobilization and remobilization. The overall objective of my dissertation research project was to understand the molecular mechanism(s) responsible for the repression of mtorc1 signaling in immobilized rat hindlimb skeletal muscle. I hypothesized that the molecular mechanism(s) responsible for the repression of mtorc1 signaling with hindlimb immobilization are also responsible for the modulation of mtorc1 signaling with changes in fixed muscle length, aging, and remobilization. 16
27 17
28 Figure 1.2. mtorc1 signaling modulates the rates of protein synthesis in response to stimulation by growth factors and nutrients. Growth factors activate PI(3)K, which in turn leads to the activation of both PDK1 and Akt. Once activated, PDK1 promotes Akt and p70s6k1 signaling by phosphorylation of these proteins. Akt and activated ERK phosphorylate and inhibit TSC GAP activity towards Rheb. This reduces the conversion of Rheb-GTP into Rheb-GDP. The presence of nutrients stimulates the formation of RagA/B-GTP RagC/D-GDP heterodimers. These heterodimers, along with Rheb-GTP lead to the full activation of mtorc1. When activated, mtorc1 phosphorylates p70s6k1, ULK1, and 4E-BP1. This, in turn, promotes protein synthesis through the activation of eukayotic initiation factors (eifs) and release of eif4e from 4E-BP1. 18
29 A) Growth Factor and Amino Acid Deprivation - mtorc1 OFF mtorc1 TSC Rheb GDP Lysosome GDP RagA RagC GTP Ragulator cytosol lumen B) Growth Factor and Amino Acid Stimulation - mtorc1 ON Growth Factors Nutrients p TSC p p p p Rheb GTP GTP RagA mtorc1 RagC GDP Lysosome Ragulator cytosol lumen Figure 1.3. Growth factors and nutrients are both necessary for full activation of mtorc1. A) In the absence of growth factors, TSC promotes the conversion of mtorc1-activating Rheb GTP into inactive Rheb GDP. In the absence of nutrients (amino acids), mtorc1 does not bind Rag proteins on the lysosomal surface. B) Stimulation by growth factors leads to the phosphorylation of TSC2 on inhibitory residues that reduce TSC2 GAP activity towards Rheb and prevent GTP hydrolysis of Rheb GTP. In the presence of nutrients, RagA/B GDP and RagC/D GTP gets converted into RagA/B GTP and RagC/D GDP that is capable of binding mtorc1 on the lysosomal surface. Recruitment of mtorc1 to the lysosome places it in close proximity to Rheb GTP for activation. 19
30 Chapter 2. Methods Animals Male Sprague-Dawley rats aged 2, 9, and 18 months [Charles River Laboratories, Wilmington, MD; and Harlan Laboratories, Indianapolis, IN] were housed in wire cages in a temperature- (25 C) and light-controlled environment. Rats were provided rodent chow (Harlan-Teklad 8604, Indianapolis, IN; and AIN-93M, Research Diets, New Brunswick, NJ) and water ad libitum. Before hindlimb immobilization, rats were adapted to a reversed 12:12-hr light-dark cycle (lights off at 0700 hrs) for one week. Animal facilities and experimental protocols were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine. Hindlimb Immobilization Rats were anesthetized using isoflurane inhalation (2.5%) and subjected to unilateral hindlimb immobilization as described previously (123). The left hindlimb was shaved, taped (1/2 Curity Kendall Standard Porous Tape, Walpole, MA) and wrapped in four layers of protective cast padding (Specialist brand; Johnson and Johnson, Raynham, MA). Fiberglass casting tape (VetCast Plus veterinary casting tape; 3M, St. Paul, MN) was activated by hot water, and five layers were wrapped over cast padding and allowed to harden. Multiple layers of casting tape were used to wrap around the leg and over the toes in order to construct a strong smooth cast. Except for a subset of rats that were immobilized in ankle dorsiflexion (Chapter 4), all rat hindlimbs were immobilized with the foot positioned in full plantarflexion so as to place the soleus muscle placed in a 20
31 shortened position. When the casting material had hardened, rats were removed from the anesthesia apparatus and allowed to recover in their cages. Rats were anesthetized using isoflurane during cast removal. Casts were removed by making two lateral incisions using a Stryker Cast Cutter (Kalamazoo, MI). Incisions were connected across the boot of the cast and the cast halves were pulled apart by hand. Cast padding and tape were cut using scissors and peeled off the rat leg. Two controls were included in initial hindlimb immobilization experiments (Chapter 3). Controls consisted of non-immobilized rats and the contralateral, nonimmobilized hindlimb of rats subjected to unilateral hindlimb immobilization. Nonimmobilized rats were included to determine if changes in biomechanical gait patterns or circulating stress hormones due to the hindlimb immobilization procedure influenced skeletal muscle in the non-immobilized hindlimb of rats subjected to hindlimb immobilization. As described in the Results section of Chapter 3, no differences in muscle mass-to-body mass ratio, rates of protein synthesis, mtorc1 signaling, or REDD1 and REDD2 gene expression were observed between controls. Since hindlimb immobilization did not influence the variables of interest in skeletal muscle from the nonimmobilized hindlimb, follow-up experiments (Chapters 4 and 5) did not include nonimmobilized rats as additional controls. Administration of Puromycin and Sample Collection On the day of the experiment, rats were anesthetized using isoflurane inhalation (2.5%). After 5 minutes of anesthesia, rats were placed on a heating pad covered with sterile absorbent pads to maintain body temperature for the remainder of the procedure. 21
32 A dose (0.040 µmol/g body wt) of puromycin dihydrochloride (AG Scientific, San Diego, CA) in a solution of 10 mg/ml saline was administered via tail vein injection for the measurement of protein synthesis. Ten minutes following administration of puromycin (30 min post-gavage), muscles were individually excised, cleared of visible fascia, weighed, and either homogenized or frozen using freeze clamps in liquid nitrogen. Soleus muscles were homogenized in 10 volumes of homogenization buffer consisting of 20 mmol/l N-2-hydroxyethylpiperazine-N -2-ethanesulfonic acid (ph 7.4), 100 mmol/l KCl, 0.2 mmol/l EDTA, 2 mmol/l ethylene glycol-bis(β-aminoethyl ether)-n,n,n,n - tetraacetic acid, 1 mmol/l dithiothreitol, 50 mmol/l sodium fluoride, 50 mmol/l β- glycerophosphate, 0.1 mmol/l phenylmethylsulfonyl fluoride, 1 mmol/l benzamidine, and 0.5 mmol/l sodium vanadate on ice using a Polytron homogenizer (37). An aliquot (0.5 ml) of the homogenate was reserved for the measurement of mrna expression as described in Measurement of mrna Expression below. The remainder of the homogenate was immediately centrifuged at 2,000 x g for 3 minutes at 4 C. An aliquot (200 µl) of the supernatant fraction was added to an equal volume of 2X Laemmli buffer consisting of M Tris HCl (ph 6.8), 12.5% (vol/vol) glycerol, 1.25% sodium dodecyl sulfate, 1.25% (vol/vol) β-mercaptoethanol, and 0.1% bromophenol blue and boiled at 100 C for 5 minutes. A separate aliquot (10 µl) of supernatant was used to measure protein concentration by BioRad Protein Assay. While under isoflurane anesthesia, rats were euthanized by opening the chest cavity. 22
33 SDS-PAGE and Immunoblot Procedure Soleus muscle samples in 2X Laemmli sample buffer were diluted with 1X Laemmli sample buffer (2X Laemmli sample buffer diluted with an equal volume of water) to equal protein concentrations as assessed in triplicate by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA) using crystalline BSA as a standard. Equal volumes of soleus muscle sample (20 µg) in sample buffer were loaded onto Criterion precast 4-20% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels (Bio-Rad) (208). Following SDS-PAGE, resolved proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Pall Life Sciences) for 1.5 hours. Membranes were subsequently blocked with 5% nonfat dry milk in Tris-buffered saline + Tween 20 (TBS-T) for 1 hour at room temperature. PVDF membranes were carefully cut into strips using a razor and each strip was incubated overnight at 4 C with one of the following primary antibodies recognizing proteins phosphorylated on specific residues including: p70s6k1 Thr 389 (1:1000 dilution), 4E-BP1 Thr 37/46 (1:1000 dilution), 4E-BP1 Ser 65 (1:1000 dilution), AMPK Thr 172 (1:1000 dilution), eukaryotic initiation factor 2 alpha (eif2α) Ser 51 (1:3000 dilution), p42/44 (ERK 1/2) Thr 202 Tyr 204 (1:1000 dilution), ULK1 Ser 757 (1:1000 dilution), TSC2 Ser 939 (1:1000 dilution), Akt Ser 473 (1:2000 dilution), Akt Thr 308 (1:1000 dilution), or FoxO3a Ser 253 (1:1000 dilution), all of which were purchased from Cell Signaling Technology, Inc (Danvers, MA). Alternatively, blots were probed with antibodies against AMPK (1:1000 dilution), ERK1/2 (1:1000 dilution), ULK1 (1:1000 dilution) or Akt (1:1000 dilution) purchased from Cell Signaling Technology; p70s6k1 (1:10,000 dilution) or 4E-BP1 (1:10,000 dilution) purchased from Bethyl Laboratories, Inc (Montgomery, TX); α-tubulin purchased from Santa Cruz 23
34 Biotechnology (Santa Cruz, CA) (1:1000 dilution); hypoxia-inducing factor 1 alpha subunit (HIF1-α) (1:1000 dilution) purchased from Novus Biologicals (Littleton, CO); or eif2α (mouse monoclonal antibody; 1:5000 dilution; hybridoma cells provided by the late Dr. Edgar Henshaw) which was produced in house. Activating transcription factor 4 (ATF4) antibody (1:200 dilution) was generously provided by Dr. Michael Kilberg (University of Florida). In a similar manner, equal volumes of soleus muscle sample (90 µg) in sample buffer were subjected to SDS-PAGE, transferred to a PVDF membrane, blocked, and probed with an antibody recognizing anti-phospho-p70s6k1 Thr 229 (1 µg/µl dilution) purchased from Abcam (Cambridge, MA). After overnight incubation with primary antibody, membranes were washed with TBST (3 times, 5 minutes each) and probed with appropriate horseradish peroxidase-conjugated secondary antibodies (Bethyl; 1:10,000 dilution in TBS-T with 5% nonfat dry milk) for 1 hour at room temperature. Blots were washed again with TBS-T (2 times, 5 minutes each), and incubated with enhanced chemiluminescence (ECL) (Pierce; Thermo Scientific, Rockford, IL) or ECL Plus (GE Healthcare, Fairfield, CT) for 1 or 5 minutes, respectively. Blots were developed using a FluorChem M Multifluor System (ProteinSimple, San Jose, CA) and band densities were quantified using AlphaView (ProteinSimple) and Genetools (Syngene, Cambridge, MA) software. For determination of protein phosphorylation status, membranes were initially immunoblotted with phosphospecific antibodies and then stripped (using buffer containing 62.5 mm Tris HCl, 69.4 mm SDS, and 18.3 µm β-mercaptoethanol, ph 6.7) and reprobed with antibodies directed against the respective total protein. 24
35 Measurement of p70s6k1 and 4E-BP1 Hyperphosphorylation The phosphorylation of p70s6k1 and 4E-BP1 causes a decrease in the electrophoretic mobility of the proteins during SDS-PAGE (68, 89, 131). As a result, p70s6k1 and 4E-BP1 in tissue extracts gets separated into multiple electrophoretic forms during SDS-PAGE with the more slowly migrating forms representing more highly phosphorylated proteins (5). Phosphorylation of p70s6k1 and 4E-BP1 was assessed by immunoblot analysis using 7.5% and 15% polyacrylamide gels, respectively, with 0.19% bisacrylamide to permit resolution of p70s6k1 and 4E-BP1 into multiple electrophoretic forms (5, 120). Polyclonal antibodies for p70s6k1 (1:10,000 dilution) or 4E-BP1 (1:10,000 dilution) purchased from Bethyl Laboratories, Inc (Montgomery, TX) recognized both phosphorylated and unphosphorylated forms of the proteins. Following SDS-PAGE, p70s6k1 separated into 4 distinct bands, while 4E-BP1 separated into 3 bands. Hyperphosphorylation of p70s6k1 was calculated as percentage of the protein in the top 3 bands relative to all 4 bands. Hyperphosphorylation of 4E-BP1 was calculated as percentage of the protein in the top band (γ-form) relative to all 3 bands (37). Measurement of Skeletal Muscle Protein Synthesis Skeletal muscle protein synthesis was measured by the incorporation of puromycin into peptide chains (78). Equivalent amounts of soleus muscle protein (40 µg) were dissolved in Laemmli buffer and subjected to immunoblot analysis as described in SDS-PAGE and Immunoblot Procedure with a few exceptions. Electrophoresis was terminated when the dye front reached the bottom of the gel. Proteins were transferred to 25
36 a PVDF membrane for 2.5 hours to ensure transfer of all proteins. Membranes were incubated and rocked overnight at 4 C with a mouse monoclonal anti-puromycin antibody generated in-house (1 µg/ml in Tris-buffered saline). Following development of these immunoblots, puromycin incorporation was assessed by summating the immunoblot intensity of all protein bands in a sample lane. Soleus muscle samples from rats not injected with puromycin were included in immunoblot analysis and values obtained using these samples were subtracted from values obtained for the other samples. Measurement of mrna Expression Total RNA was isolated from 500 µl of skeletal muscle homogenate in 1 ml TRIzol reagent (Invitrogen, Grand Island, NY) following a standard TRIzol protocol according to the manufacturer s instructions. The RNA pellet was reconstituted in 40 µl RNase-free water, and the total RNA (1 µl) concentration was determined (NanoDrop 2000; Thermo Fischer Scientific, Waltham, MA). Total RNA was reverse transcribed using an ABI High Capacity cdna reverse transcription kit (Applied Biosystems, Foster City, CA). For quantitation of specific mrna, reverse transcriptase polymerase chain reaction was performed using Taqman gene expression assays and the gene expression master mix (Applied Biosystems, Foster City, CA) (109). Samples were loaded in triplicate on a 384-well plate and 2 µl (100 ng) cdna was placed in each well with 0.5 µl gene expression assay, 5 µl gene expression master mix and 2.5 µl water. The cycling parameters as described (109), were an initial 50 C for 1 min followed by 40 cycles at 95 C for 15 s followed by 60 C for 1 min. Real-time PCR quantitation was calculated on the basis of C t values, where C t is defined as the PCR cycle number that 26
37 crosses an arbitrary signal threshold on the amplification plot. The comparative quantitation method 2 ΔΔCt was used in presenting gene expression of target genes in reference to an endogenous control, and 2 ΔCt was used in presenting expression of each housekeeping gene in validating the use of the endogenous control gene. Δ C t is expressed as the difference between the target and control samples (C t target C t control ). Primers were purchased from Applied Biosystems including: Ddit4 (REDD1) (Assay ID: Rn _g1); Ddit4l (REDD2) (Rn _g1); Sesn1 (Rn _m1); RGD (Sesn2) (Rn _m1); ATF4 (Rn _g1); Trim63 (MuRF1) (Rn _m1); Fbxo32 (MAFbx) (Rn _m1); β-actin (Rn _m1); and Tbp (Rn _m1). β-actin and Tbp mrna expression were compared against the gene expression of other common housekeeping genes including Rpo2-1 (Polr2a) (Rn _m1), Rpl32 (Rn _g1) and Hprt (Rn _g1). β-actin and Tbp mrna expression were used as internal controls as their expression did not change substantially with aging or in response to hindlimb immobilization or remobilization. Statistical Analysis For Chapters 3 and 4, results from individual experiments (n = 5 per group) were replicated in two or more independent experiments. Results are presented as means ± SE calculated from pooled data in Chapters 3 and 5. Results from the immobilized hindlimb soleus are presented as a percentage of the non-immobilized hindlimb soleus ± SEM in Chapter 4. For each of the variables measured, no differences were observed in soleus muscles from the non-immobilized hindlimbs between dorsiflexed and plantarflexed immobilization techniques. Outliers were determined using Grubb s test (alpha level 27
38 0.05) and excluded from further analysis. Paired t tests were used to compare differences between soleus muscles from immobilized and non-immobilized hindlimbs within the same rat. Unpaired Student s t-test was used to compare differences between results obtained from different groups and conditions. All comparisons were analyzed using GraphPad Software. Differences between groups were considered significant at p <
39 Chapter 3. THE REPRESSORS OF MTORC1 SIGNALING REDD1/2 ARE RAPIDLY INDUCED AND ACTIVATION OF P70S6K1 BY A NUTRIENT STIMULUS IS DEFECTIVE IN SKELETAL MUSCLE OF AN IMMOBILIZED RAT HINDLIMB Introduction In humans, disuse atrophy results from a depression in skeletal muscle protein synthesis (80, 162). The depression is observed in both the fasted and fed state (anabolic resistance) (50, 72, 127, 162). The rates of protein synthesis in skeletal muscle are modulated by mtorc1 signaling (118). Despite evidence of attenuated mtorc1 signaling in skeletal muscle during hindlimb immobilization (155, 220), hindlimb unloading (10, 94), and bed rest (50), the mechanism responsible for repression of mtorc1 signaling during disuse is unknown. Furthermore, anabolic resistance to nutrient-induced stimulation of protein synthesis is associated with attenuated nutrientinduced stimulation of mtorc1 signaling during hindlimb immobilization (127, 140) and bed rest (50). However, the molecular mechanism responsible for resistance to nutrient stimulation is unknown. The goal of the research presented in this chapter was to 1) characterize a model of immobilization-induced disuse atrophy that exhibits depressed rates of protein synthesis and attenuated mtorc1 signaling, and 2) assess known repressors of mtorc1 signaling in order to gain a better understanding of the molecular mechanism(s) responsible for immobilization-induced disuse atrophy. I hypothesized that hindlimb immobilization would depress rates of protein synthesis in rat hindlimb skeletal muscle due to increased expression and/or activation of one or more repressors of the mtorc1 signaling pathway. Furthermore, I hypothesized that hindlimb 29
40 immobilization would blunt nutrient-induced stimulation of mtorc1 signaling due to increased expression and/or activation of one or more repressors of the mtorc1 signaling pathway. Methods Experimental design After a 1-week adaptation period, rats (8-9 weeks of age; g) were anesthetized with isoflurane inhalation (2.5%) and subjected to unilateral hindlimb immobilization for 1, 2, 3, or 7 days prior to the day of the experiment. Control rats were prepared for immobilization but were not immobilized. An equal number of rats from each experimental group were processed on experimental days. All rats were fasted overnight (21h), but allowed free access to water. On the day of the experiment, all rats (except for those immobilized for 7 days) were randomly divided into groups that received either saline (0.155 M) or 1.35 g L-leucine/kg body wt by oral gavage as a nutrient stimulus as described previously (6). Rats subjected to 7 days of hindlimb immobilization received saline by oral gavage. Fifteen minutes after oral gavage, the rats were anesthetized using isoflurane and remained anesthetized for the remainder of the experiment. Thirty minutes after oral gavage, puromycin was administered by injection. At 40 minutes post-gavage, the soleus, gastrocnemius, and plantaris muscles were individually excised, weighed, and prepared for analysis as described in Chapter 2, Administration of Puromycin and Sample Collection. The experiment was repeated once and the figures presented include data from both experiments. 30
41 Results Effect of immobilization on muscle mass Initially, experiments were designed to examine the time course of changes in muscle mass and protein synthesis following unilateral hindlimb immobilization. Illustrated in Figure 3.1 is the muscle mass-to-body mass ratio of three muscles of the plantarflexor group, the soleus, gastrocnemius, and plantaris. Following 1 and 2 days of immobilization, there was no detectable change in mass for any of the three muscles. By 3 days, however, the mass for each muscle was significantly (p < 0.05) reduced and the reduction in mass was greater 7 days following immobilization. No differences in muscle mass-to-body mass ratio were observed between control (non-immobilized) rats and the control (contralateral, non-immobilized) hindlimbs of immobilized rats. Because the reduction in mass was greatest for the soleus muscle it was selected for further investigation into the mechanism(s) contributing to disuse atrophy. No differences in protein content (mg/g) were observed in muscles from immobilized compared to control hindlimbs (Table 3.1). Effect of immobilization on muscle protein synthesis To investigate the cause of the observed loss of muscle mass, rates of protein synthesis were measured in the soleus muscle after 1, 2, 3, and 7 days of immobilization. As illustrated in Figure 3.2, hindlimb immobilization depressed rates of protein synthesis approximately 40-50% on each of the days studied compared to contralateral nonimmobilized (control) hindlimbs. The effect was maximal after only a single day of 31
42 immobilization. No differences in rates of protein synthesis were observed between control rats and the control hindlimbs of immobilized rats. Effect of immobilization on mtorc1 signaling To gain an understanding of potential events that cause depressed protein synthesis following hindlimb immobilization, mtorc1 signaling was assessed across the first three days of immobilization in both a fasted condition (saline gavage) and in response to an oral leucine gavage as a nutrient stimulus. An analysis of p70s6k1 (Figure 3.3A) and 4E-BP1 (Figure 3.3B) hyperphosphorylation demonstrated attenuated mtorc1 signaling in soleus muscle of the immobilized compared to the control hindlimb across the 3-day time period. As described in Chapter 2 ( Measurement of p70s6k1 and 4E-BP1 Hyperphosphorylation ), the phosphorylation of p70s6k1 and 4E- BP1 causes a decrease in the electrophoretic mobility of the proteins during SDS-PAGE and the proteins separate into multiple bands. During SDS-PAGE, the more slowly migrating forms of p70s6k1 and 4E-BP1 represent more highly phosphorylated proteins. As depicted in Figures 3.3A and 3.3B, respectively, p70s6k1 and 4E-BP1 migrated more quickly during SDS-PAGE in soleus muscle from an immobilized hindlimb compared to a control hindlimb. This is indicative of a lower phosphorylation state of these proteins during hindlimb immobilization, because phosphorylation retards gel migration. In addition, soleus muscles from saline-gavaged rats exhibited more quickly-migrating proteins during SDS-PAGE compared to leucine-gavaged rats. This analysis showed that mtorc1 signaling was elevated in response to the nutrient stimulus in the soleus muscle of both the immobilized and control hindlimbs. While the relative magnitude of increase 32
43 in the phosphorylation state of p70s6k1 and 4E-BP1 were similar between hindlimbs, the maximal response was attenuated in the soleus muscle from the immobilized hindlimb. Similar results were obtained when the relative phosphorylation of 4E-BP1 at Ser65 (i.e., a site phosphorylated by mtorc1) was assessed. Figure 3.3C shows that relative phosphorylation of 4E-BP1 Ser65 was attenuated in the soleus muscle of the immobilized compared to the control hindlimb across the 3-day time period. While the nutrient stimulus produced an elevation in 4E-BP1 Ser65 phosphorylation in the soleus muscle of both the immobilized and control hindlimbs, the maximal response was attenuated in the immobilized hindlimb. No differences in the phosphorylation patterns for p70s6k1 or 4E-BP1 were observed between control rats and the control hindlimbs of immobilized rats. Overall, these results point to an immobilization-induced attenuation of mtorc1 signaling that is nonetheless responsive to stimulation by leucine administration. Effect of immobilization on repressors of mtorc1 signaling A number of upstream regulatory inputs were investigated as potential mediators of the attenuated mtorc1 signaling observed in soleus muscle of immobilized compared to control hindlimbs (Table 3.2). Sestrin 1 and Sestrin 2, whose expression is regulated by the transcription factor p53, activate LKB1 to phosphorylate AMPK at Thr172 (26, 134). Phosphorylation at this site is required for AMPK activation, which in turn activates TSC2 and represses mtorc1 signaling (20, 134). However, examination of mrna expression for Sestrin 1 and Sestrin 2 revealed no differences in soleus muscle between immobilized and control hindlimbs. Moreover, no differences were observed in 33
44 protein expression of Sestrin 1 or p53 in soleus muscle between immobilized and control hindlimbs. Finally, immobilization did not alter AMPK phosphorylation at Thr172. In contrast, two regulatory inputs were observed to change in parallel with the attenuation of mtorc1 signaling following immobilization. As illustrated in Figure 3.4, expression of mrna for REDD1 and REDD2, both of which repress mtorc1 signaling through activation of TSC1/2 (152, 196), was induced as early as 1 day following immobilization and their expression increased further following 2 and 3 days. REDD1 and REDD2 mrna expression was lowered by leucine gavage in the soleus muscle of rats subjected to hindlimb immobilization for 2 and 3 days, however, with the exception of REDD1 mrna expression in control soleus muscle of rats immobilized for 2 days, the decrease was not statistically significant. No differences in either REDD1 or REDD2 mrna expression were observed between control rats and the control hindlimbs of rats subjected to hindlimb immobilization. Hypoxia was considered as a potential mediator of the induced expression of REDD1 and REDD2 mrna. We next examined protein expression of HIF1-α, a known inducer of REDD1 mrna expression (45, 105), as a potential mediator of the responses of REDD1 and REDD2 mrna to immobilization. However, its expression was not elevated with immobilization (Table 3.2). Stress in the endoplasmic reticulum (ER stress) was also considered as being responsible for the elevated expression of REDD1 and REDD2 (214). In particular, ATF4 has been shown to increase in expression and induce REDD1 mrna expression in response to ER stress. Moreover, ATF4 has been shown to play a critical role in disuse atrophy (56). However, no significant differences were observed in eif2α phosphorylation at Ser51 (a marker of ER stress (122)) or ATF4 34
45 mrna and protein expression (a known inducer of REDD1) (214) in soleus muscle between immobilized and control hindlimbs (Table 3.2). Finally, blood borne regulatory inputs such as glucocorticoids, which are known to induce REDD1 expression in muscle (145, 211), were also considered. However, these were not pursued as a likely cause of REDD1 mrna induction due to the lack of a systemic effect on mtorc1 signaling in the control hindlimb of the same rat. In other words, the induction in REDD1 mrna expression was localized to soleus muscle from the immobilized hindlimb. If circulating glucocorticoids were responsible for the induction in REDD1 mrna expression, then REDD1 would be induced in soleus muscle from both hindlimbs of a rats subjected to hindlimb immobilization when compared to control rats. Thus, glucocorticoids were ruled out as a likely cause of the localized induction in REDD1 mrna expression during hindlimb immobilization. Effect of immobilization on p70s6k1 phosphorylation at Thr389 Intriguingly, although leucine-induced phosphorylation of 4E-BP1 at Ser65 appeared to be unaffected by immobilization, a different pattern of response was observed when relative phosphorylation of p70s6k1 at Thr389 was assessed as a marker of mtorc1 signaling (Figure 3.5A). Thus, although relative phosphorylation at this site was likewise attenuated across the 3-day time period, the response to the leucine-induced stimulus was markedly different between the two conditions. In the soleus muscle of the immobilized hindlimb, phosphorylation at this site was elevated 3-4 fold following leucine administration whereas in the control hindlimb the elevation was 7-14 fold compared to the saline-administered control. Given that phosphorylation of p70s6k at 35
46 Thr229 (a PDK1 targeted site) is a prerequisite for mtorc1-mediated phosphorylation of Thr389 (112), its responses to immobilization and a nutrient stimulus, respectively, were assessed. As illustrated in Figure 3.5B, phosphorylation of p70s6k1 at Thr229 was of similar magnitude in the soleus muscle of both the immobilized and control hindlimb and for the former did not respond to the leucine-induced stimulus. In contrast, leucine administration produced a robust elevation in Thr229 phosphorylation in the soleus of the control hindlimb. Thus, analysis of p70s6k1 phosphorylation sites Thr389 and Thr229 supports an immobilization-induced state of anabolic resistance that has been described in other models of disuse atrophy (50, 72, 172). Discussion The first goal of the studies presented in this chapter was achieved having characterized a model of immobilization-induced disuse atrophy that exhibited depressed rates of protein synthesis and attenuated mtorc1 signaling. Protein synthesis was maximally depressed in the soleus muscle within 24 hours of immobilization and the depression was sustained for at least 7 days compared to the soleus muscle from the contralateral control hindlimb. In contrast, protein synthesis was unaltered in the soleus muscle of the control limb compared to control rats. This finding is in agreement with previous studies showing that immobilization-induced depression of protein synthesis begins to manifest as early as 6 hours after application of a cast, and is maintained throughout a 7-day period (22, 75). The magnitude of depression in response to immobilization observed in the present study (reduction to approximately 50% of either the control hindlimb or control rat) also agrees with previous studies utilizing animal 36
47 models of immobilization [reduction of 30-70% compared to control (22, 75)] as well as in human studies using limb unloading (40) and bed rest (61) in which protein synthesis was reduced to 53% and 50% of control values, respectively. The depression of protein synthesis was accompanied by attenuated signaling through mtorc1 in the soleus muscle of immobilized compared to that of the control hindlimbs. The attenuation of mtorc1 signaling occurred within 24 hours of immobilization, as assessed by a reduction in the proportion of p70s6k1 and 4E-BP1 present in hyperphosphorylated forms, as well as reduced phosphorylation of p70s6k1 at Thr389 and 4E-BP1 at Ser65. Attenuated mtorc1 signaling has been observed in skeletal muscle of rats and mice subjected to hindlimb unloading (19, 94), immobilization (127), or denervation (94), but was not observed in one study in rats following 5 days of unilateral hindlimb immobilization (123). Studies in humans have failed to detect a change in mtorc1 signaling in skeletal muscle following 10 or 21 days of limb unloading, despite significant reductions in the rate of myofibrillar protein synthesis (40). The basis for these disparate results in mtorc1 signaling are not clear, but could be due to different muscles being studied, e.g. unloading attenuated mtorc1 signaling in soleus muscle (19), but not in vastus lateralis (40). Differences in age group might also explain disparate results, e.g. juvenile, rapid-growing rats vs. adult, non-growing humans. Finally, disparate results could be due to the model being employed, e.g. immobilization vs. unloading, species, or to the feeding status of the animals. The second goal of the studies presented in this chapter was also achieved through assessing known repressors of mtorc1 signaling and identifying an induction in the mrna expression of REDD1 and REDD2 with hindlimb immobilization (Figure 3.6). 37
48 The immobilization-induced attenuation of mtorc1 signaling likely involves upstream regulatory inputs to the kinase. The TSC complex acts to integrate signals from several upstream pathways, including those emanating from Akt and ERK (138, 190). Both of these kinases phosphorylate, and thereby inactivate the TSC complex, leading to a stimulation of mtorc1 signaling. In the studies presented here, immobilization did not reduce phosphorylation of Akt at Ser473 or TSC2 at Ser939, a site directly phosphorylated by Akt (177). The possibility that immobilization promoted reduced phosphorylation of TSC2 by ERK1/2 could not be tested because of the lack of an antibody that reliably detects rat TSC2 phosphorylated at Ser644, a site phosphorylated by ERK1/2 (138). However, no difference in ERK1/2 Thr202/Tyr204 phosphorylation was observed, suggesting that ERK1/2 signaling did not mediate the immobilizationinduced attenuation of mtorc1 signaling. In addition to Akt and ERK, the TSC1 TSC2 complex is also regulated by the p53 AMPK signaling pathway. Previous studies have shown that p53 protein expression is induced in gastrocnemius muscle in response to 14 days of hindlimb unloading (194), and chronic activation of p53 in muscle promotes atrophy (186). Moreover, two downstream targets of p53 action, Sestrins 1 and 2, have been shown to repress mtorc1 signaling through activation of AMPK (26). However, neither Sestrin 1 or Sestrin 2 mrna, Sestrin 1 protein expression, nor AMPK phosphorylation on the activating residue, Thr172, were observed, suggesting that the attenuation of mtorc1 signaling was not mediated by the p53 Sestrin AMPK signaling pathway. Like AMPK, REDD1 and REDD2 also act to attenuate mtorc1 signaling through a mechanism involving TSC1/2 (152, 196). REDD1 and REDD2 mrna 38
49 expression was induced (100% and 400%, respectively) after one day of immobilization followed by a larger induction ( % and %) after 2 and 3 days: changes that paralleled the greater attenuation of mtorc1 signaling at the later time points. Enhanced protein expression of either REDD1 or REDD2 could not be assessed because of the lack of antibodies that reliably detect the rat proteins. Nonetheless, previous studies have demonstrated concomitant responses of REDD1 mrna and protein expression to ER stress (214), starvation (145), and dexamethasone treatment (211). Thus, it seems reasonable to conclude that the increases in REDD1 and REDD2 mrna expression observed in the studies presented here are indicative of similar responses in expression of the respective proteins. REDD1 and REDD2 mrna expression are potently induced in response to a variety of stresses. One potential mechanism for the observed induction of REDD1/2 expression with hindlimb immobilization could be development of hypoxia (45) due to a reduction in blood flow to the immobilized hindlimb. However, HIF-1α expression was not elevated in soleus muscle from the immobilized hindlimb compared to the contralateral, control hindlimb. In addition, the lack of change in either eif2α phosphorylation on Ser51 or ATF4 expression suggest that ER stress was not involved in the induction of REDD1 expression (214). REDD1 expression is also upregulated in response to other stresses, e.g. conditions that cause DNA damage (58) or decrease ATP concentrations (196), or in response to oxidative stress (58). However, the mechanism(s) through which such stresses lead to upregulation of REDD1 expression are presently undefined. 39
50 In addition to depressed rates of protein synthesis in the fasted condition, both immobilization (72) and bed rest (16, 50) are associated with development of resistance to nutrient-induced stimulation of skeletal muscle protein synthesis in humans. Moreover, two studies showed that skeletal muscle protein synthesis is resistant to nutrient-induced stimulation during hindlimb immobilization in old rats (140) and in mice (127). The studies showed that global rates of protein synthesis in skeletal muscle were unresponsive to chow feeding or oral leucine administration, respectively, in an immobilized hindlimb compared to fasted controls. In the studies presented here, the leucine-induced stimulus produced an elevation of similar magnitude in mtorc1 signaling in soleus muscle from both the immobilized and control hindlimbs, as assessed by gel-shift (hyperphosphorylation) analysis of the phosphorylation state of p70s6k1 and 4E-BP1. Assessment of the phosphorylation of 4E-BP1 at Ser65, a site of mtorc1- mediated phosphorylation (71), produced results similar to those obtained with the gelshift analysis. Substrates of mtorc1 vary greatly in their response to both pharmacological (e.g. rapamycin and Torin) and natural (e.g. amino acids and growth factors) regulators of mtorc1 signaling (106). For example, the phosphorylation of p70s6k1 at Thr389 is sensitive to inhibition by rapamycin, while the phosphorylation of growth factor receptor-bound protein 10 (Grb10) at Ser150, another mtorc1 phosphorylation site, is resistant to rapamycin. In addition, when murine embryonic fibroblasts (MEFs) were placed in media containing 100, 20, and 0% of normal levels of amino acids, phosphorylation of p70s6k1 at Thr389 was strongly reduced when cells were placed in medium containing 20% amino acids. In contrast, Grb10 phosphorylation at Ser150 was reduced only when placed in medium containing 0% amino acids. Similar 40
51 results were obtained when MEFs were placed in medium containing 10, 2, or 0% fetal bovine serum (a potent growth factor). Interestingly, phosphorylation of p70s6k1 at Thr389 was more resistant to rapamycin treatment and reductions in amino acids when Thr389 was mutated to Ser389. It was concluded from these experiments that the sequence composition of an mtorc1 phosphorylation site, particularly the presence of serine or threonine as the phosphoacceptor, is a mechanism for allowing downstream effectors of mtorc1 to respond differently to regulators of mtorc1 signaling. It is noteworthy that all of the mtorc1 phosphorylation sites on 4E-BP1 as well as the rapamycin-resistant site in the turn motif of p70s6k1 (Ser371) are followed by a Pro residue (112, 156), providing a possible explanation for the coordinated changes in 4E- BP1 phosphorylation on Ser65 and hyperphosphorylation of 4E-BP1 and p70s6k1. In contrast, p70s6k1 Thr389 is not followed by Pro, but instead is followed by a Tyr residue (106, 112), suggesting that its phosphorylation by mtorc1 might be differentially regulated compared to 4E-BP1. Indeed, in contrast to 4E-BP1 phosphorylation on Ser65 or hyperphosphorylation of either 4E-BP1 or p70s6k1, phosphorylation of p70s6k1 on both Thr229 and Thr389 in response to the nutrient stimulus was severely blunted in soleus muscle from immobilized compared to control hindlimbs. Since phosphorylation of Thr229 by PDK1 is a prerequisite for phosphorylation of Thr389 by mtorc1 (112), the results are consistent with a model in which PDK1 activation is rapidly impaired in response to immobilization. In conclusion, the results presented here demonstrate an immobilization-induced attenuation of mtorc1 signaling mediated by REDD1 and REDD2 that is nonetheless responsive to a leucine stimulus. More importantly, they suggest that phosphorylation of p70s6k1 on 41
52 Thr389, which is required for full activation of the kinase and thus signaling to its downstream substrates, fails to respond appropriately to the leucine stimulus. This lack of response is apparently due to a defect in PDK1 signaling and would explain the phenomenon of anabolic resistance referred to in other models of disuse atrophy (50, 72, 172). 42
53 43
54 Table 3.1 Protein concentration of soleus muscle from immobilized and control hindlimbs after 1-3 days of hindlimb immobilization Protein Concentration (µg/ul) Days Immobilized Immobilized Hindlimb Control Hindlimb Control 6.77 ± day 6.65 ± ± days 6.51 ± ± days 6.73 ± ± 0.44 Data are means ± SE, n = muscles/group, and are expressed as the protein concentration (µg/µl). Rats had one hindlimb immobilized in plantarflexion (soleus fixed in a shortened position) for 1-3 days. No significant differences were observed between groups. 44
55 45
56 46
57 Table 3.2 End points not associated with immobilization-induced repression of mtorc1 signaling. End point Compared to Non-Immobilized Limb (%) p53 protein ± 12.8 Sestrin 1 mrna ± 14.9 Sestrin 1 protein 69.5 ± 15.9 Sestrin 2 mrna ± 31.2 AMPK Thr 172 phosphorylation ± 13.6 Akt Ser 473 phosphorylation 94.7 ± 2.7 Akt Thr 308 phosphorylation ± 9.6 TSC2 Ser 939 phosphorylation 61.7 ± 22.0 ERK Thr 202 Tyr 204 phosphorylation 82.3 ± 6.0 eif2α Ser 51 phosphorylation ± 13.6 ATF4 mrna ± 14.5 ATF4 protein 83.9 ± 12.6 HIF1-α protein 75.9 ± 17.7 Data are means ± SE, n = 5-6 rats/group, and are expressed as a percentage of measurements from immobilized hindlimbs relative to measurements from nonimmobilized hindlimbs. No leucine effects were observed. Rats had one hindlimb immobilized for 1 day. * p < 0.05 versus non-immobilized limbs. 47
58 48
59 49
60 Figure
61 Chapter 4. CHANGES IN REDD1, REDD2, AND ATROGENE MRNA EXPRESSION ARE PREVENTED IN SKELETAL MUSCLE FIXED IN A STRETCHED POSITION DURING HINDLIMB IMMOBILIZATION Introduction Disuse atrophy and depressed rates of protein synthesis are observed when skeletal muscle is immobilized in a shortened position, but not in a stretched position (75, 76, 137). Thus, shortened muscle length is likely a key physiological stimulus for disuse atrophy. The mechanism(s) through which immobilization in a stretched position acts to prevent disuse atrophy is currently unknown. The goal of this chapter was to gain an understanding of the role of fixed muscle length in the regulation of protein synthesis and degradation in the soleus muscle of an immobilized rat hindlimb. I hypothesized that the soleus placed in a stretched position would not exhibit disuse atrophy and would be protected from changes in gene expression known to be associated with disuse atrophy. Methods Experimental design Rats (8-9 weeks of age; g) were maintained on a standard 12:12-hr lightdark cycle (lights on at 0700 hours) for one week before the experiment began. They were anesthetized with isoflurane inhalation (2.5%) and subjected to unilateral hindlimb immobilization. One group of rats had the ankle joint of one hindlimb immobilized in full plantarflexion, which placed the soleus in a shortened position, as described in the Methods section. Another group of rats had the ankle joint of one hindlimb immobilized 51
62 in full dorsiflexion, which placed the soleus in a stretched position (75). The hindlimbs were immobilized for 3 days prior to removal of the soleus muscle for subsequent analysis. An equal number of rats from the two experimental groups were processed on the days of tissue harvest. All rats were fasted overnight (18h), but allowed free access to water. On tissue harvest days, rats were anesthetized using isoflurane and remained anesthetized for the remainder of the procedure. Immobilization for 3 days was selected for all analysis based on the results of Chapter 3 showing that disuse atrophy was manifest at this time point in this experimental model. Results from Chapter 3 also showed that the hindlimb immobilization procedure did not affect the variables of interest in the contralateral, non-immobilized hindlimbs of immobilized rats. Therefore, the control hindlimb of immobilized rats was used as the control. Results from the immobilized hindlimb soleus are presented as a percentage of the non-immobilized hindlimb soleus ± SEM. Results Effect of immobilization on muscle mass and protein synthesis As illustrated in Figure 4.1A, immobilization of soleus in a stretched position prevented the atrophy observed in the soleus muscle immobilized in a shortened position, (i.e. muscle mass was significantly reduced (p < 0.05) in soleus immobilized in a shortened position when expressed relative to soleus muscle from the contralateral control hindlimb). To investigate the cause of the observed differences in muscle mass, rates of protein synthesis were measured in the soleus muscle after 3 days of immobilization. As illustrated in Figure 4.1B, immobilization of soleus muscle in a 52
63 stretched position prevented the reduction in rates of protein synthesis observed in the soleus muscle immobilized in a shortened position (i.e. rates of protein synthesis were reduced approximately 70% in soleus muscle immobilized in a shortened position when expressed relative to that of the soleus muscle from the control hindlimb). Effect of immobilization on regulation of mtorc1 signaling To gain an understanding of potential molecular events contributing to the changes in protein synthesis during hindlimb immobilization, mtorc1 signaling was assessed after 3 days of immobilization. An analysis of the phosphorylation state of p70s6k1 at Thr389 (Figure 4.2) demonstrated that immobilization of the soleus muscle in a stretched position prevented the reduction in mtorc1 signaling observed in the soleus muscle immobilized in a shortened position (i.e. phosphorylation of p70s6k1 at Thr389 was reduced approximately 85% in soleus muscle immobilized in a shortened position when expressed relative to that of the soleus muscle from the control hindlimb). Attenuation of mtorc1 signaling following hindlimb immobilization in plantarflexion is associated with induced expression of the genes encoding the mtorc1 repressors REDD1 and REDD2 (111). In the studies presented here, immobilization of the soleus muscle in a stretched position prevented the induction in both REDD1 (Figure 4.3A) and REDD2 (Figure 4.3B) mrna expression that was observed in soleus muscle immobilized in a shortened position (i.e. mrna expression of REDD1 and REDD2, both of which repress mtorc1 signaling through activation of the tuberous sclerosis complex (152, 196), was enhanced approximately 250% and nearly 500%, respectively, in soleus muscle immobilized in a shortened position when expressed relative to that of the soleus 53
64 muscle from the control hindlimb). Interestingly, soleus muscle immobilized in a stretched position displayed a 40% reduction in REDD2 expression compared to the contralateral control hindlimb (Figure 4.3B). Overall, these results are consistent with a model in which immobilization of soleus muscle in a stretched position prevents the induction of REDD1 and REDD2 expression that are responsible for attenuated mtorc1 signaling and protein synthesis in skeletal muscle immobilized in a shortened position. Effect of immobilization on atrogene expression Next, I assessed mrna expression of the E3 ubiquitin ligases, MAFbx and MuRF1, as markers of proteasome-mediated degradation of skeletal muscle proteins during atrophy (17). An analysis of mrna expression of MAFbx (Figure 4.4A) and MuRF1 (Figure 4.4B) demonstrated that immobilization of soleus muscle in a stretched position prevented the induction observed in soleus muscle immobilized in a shortened position (i.e. mrna expression of MAFbx and MuRF1 increased over 150% and 100%, respectively, in soleus muscle immobilized in a shortened position when expressed relative to that of the soleus muscle from the control hindlimb). These results suggest that fixed muscle length regulates atrogene expression whereby soleus muscle immobilized in a stretched position prevents induction of atrogene expression observed in soleus muscle immobilized in a shortened position. 54
65 Effect of immobilization on regulation of atrogene expression To gain an understanding of potential molecular events contributing to the changes in atrogene expression during hindlimb immobilization, Akt and FoxO3a phosphorylation were assessed after 3 days of immobilization. An analysis of the phosphorylation state of Akt at Ser473 (Figure 4.5A) demonstrated that immobilization of the soleus muscle in a stretched position prevented the reduction in Akt phosphorylation observed in the soleus muscle immobilized in a shortened position (i.e. phosphorylation of Akt at Ser473 was reduced approximately 45% in soleus muscle immobilized in a shortened position when expressed relative to that of the soleus muscle from the control hindlimb). Akt suppresses atrogene expression through the phosphorylation of Forkhead box O (FoxO) transcription factors (183). An analysis of the phosphorylation state of FoxO3a at Ser253 (Figure 4.5B) demonstrated that immobilization of the soleus muscle in a stretched position prevented the reduction in FoxO3a phosphorylation observed in the soleus muscle immobilized in a shortened position (i.e. phosphorylation of FoxO3a at Ser253 was reduced approximately 40% in soleus muscle immobilized in a shortened position when expressed relative to that of the soleus muscle from the control hindlimb). However, it should be noted that this reduction in phosphorylation of FoxO3a at Ser253 in the soleus muscle immobilized in a shortened position did not reach statistical significance due to a small number of samples used in this analysis (n = 2-4). 55
66 Discussion The goal of the studies described in the second chapter was achieved by gaining an understanding of the role of fixed muscle length in the regulation of protein turnover in immobilized rat hindlimb skeletal muscle. In support of my hypothesis, soleus muscle placed in a stretched position did not exhibit disuse atrophy and was protected from changes in gene expression known to be associated with disuse atrophy. Though it has been shown that soleus muscle immobilized in a stretched position does not exhibit disuse atrophy or depressed rates of protein synthesis (21, 74, 75, 137, 184), the studies presented here investigated gene expression changes that might contribute to these observations. The results indicate that immobilization of soleus muscle in a stretched as compared to a shortened position prevents induction of gene expression for the mtorc1 repressors, REDD1 and REDD2, which modulate mtorc1 signaling (152, 196). In addition, immobilization of soleus muscle in a stretched as compared to a shortened position prevents induction of expression of atrogenes, which modulate rates of protein degradation (18). Together, these findings help to explain why soleus muscle fixed in a stretched position does not exhibit disuse atrophy. In accordance with other reports (189, 195), the studies presented here confirm that induction of atrogene expression is dependent on fixed muscle length. Senf and colleagues (188, 189) observed induction of atrogene expression in rat soleus muscle immobilized in a shortened position for 3 days. In contrast, Soares and colleagues (195) observed a suppression of atrogene expression in rat soleus muscle immobilized for 1 and 2 days in a stretched position returning to non-stretched expression levels after 4 days. I did not observe a suppression of atrogene expression after 3 days of immobilization of 56
67 the soleus muscle in a stretched position. Perhaps atrogene expression was suppressed at earlier time points and returned to control levels by the third day. Still, the results of the present study agree with both Senf et al. (189) and Soares et al. (195) whereby muscle immobilized in a stretched position prevents the induction of atrogene expression while muscle immobilized in a shorted position leads to the induction of atrogene expression. The induction in atrogene expression is associated with a reduction in the phosphorylation of Akt at Ser473 and FoxO3a at Ser253 in soleus muscle from a hindlimb immobilized in a shortened position. Atrogene expression is modulated by FoxO transcription factors, such as FoxO3a (183). Akt regulates FoxO3a via phosphorylation on residues, such as Ser253. Phosphorylation prevents the translocation of FoxO3a from the cytoplasm to the nucleus. In my model, 3 days of hindlimb immobilization in a shortened position reduced Akt-mediated phosphorylation of FoxO3a. FoxO3a in a less-phosphorylated state is likely responsible for induction of atrogenes at this time point. In contrast, no attenuation in the phosphorylation of Akt at Ser473 and FoxO3a at Ser253 was observed in soleus muscle immobilized in a stretched position. Thus, the regulation of Akt and FoxO3a phosphorylation appears to be dependent on fixed muscle length. Other studies have provided evidence to suggest that mtorc1 signaling is related to the extent to which a muscle is stretched. For example, acute passive stretch of skeletal muscle cells (185) and tissue (3, 95) enhances mtorc1 signaling. While some studies have shown an activation of Akt and signaling to the mtorc1 complex in response to stretch (3, 49, 185), Hornberger et al. (95) suggested that acute passive stretch of skeletal muscle ex vivo stimulates mtorc1 signaling even in the absence of PI3K/Akt 57
68 signaling. The latter suggestion would imply that muscle stretch stimulates mtorc1 signaling by an Akt-independent mechanism (95). Gene expression is also influenced by skeletal muscle stretch whereby muscle immobilized in a stretched position exhibits repression of fast type and activation of slow myosin genes (30, 31, 77). Stretch and other mechanical stimuli can influence muscle gene expression through mechanosensory proteins in the muscle sarcomere (132). Proteins that bind the Z-disk and M-line relay mechanical strain information to cellular systems that control gene expression in the nucleus (67). For example, transcription factors and their interacting proteins bind to the muscle scaffolding protein, titin, and are released in response to increases in sarcomere length (132). Like titin, obscurin also binds numerous sarcomeric proteins and contains a signaling domain near its C-terminus that can be coupled to serine/threonine protein kinase domains (113). Other proteins that link skeletal muscle strain with the regulation of gene expression include nebulin, myopalladin, muscle LIM protein, and nuclear factor of activated T-cells C (132, 207). In conclusion, the results of this chapter support the hypothesis that immobilization of soleus muscle in a stretched position prevents changes associated with disuse atrophy such as induction of REDD1, REDD2 and atrogenes, consequently leading to the attenuation of mtorc1 signaling and rates of protein synthesis as well as accelerated rates of protein degradation. Thus, fixed muscle length plays an important role in the regulation of atrophic gene expression. 58
69 Figure 4.1. Mass and rates of protein synthesis in the soleus are reduced only when the muscle is immobilized in a shortened position. Rates of protein synthesis were measured by the amount of puromycin incorporated into protein as assessed by immunoblot analysis. Rats had one hindlimb immobilized for 3 days in a position to place the soleus muscle in either a stretched or shortened position (Imm: Immobilized; N Leg = Non-Immobilized (control) limb). Bars represent A) the mean mass of the soleus muscle, and B) the mean rates of protein synthesis in the soleus muscle from an immobilized hindlimb expressed as a percentage of the mass of the soleus muscle from the contralateral control hindlimb. Data are mean percentages ± SEM, n = 10 rats/group. * p = < 0.05 versus soleus muscle from the control hindlimb. 59
70 60
71 61
72 62
73 A Phospho Akt S473 / Total Akt (% of Non-Immobilized Leg) * Shortened p = Stretched B Phospho FoxO3a S253 / Total Protein (% of Non-Immobilized Leg) Shortened Stretched Figure 4.5 Phosphorylation of A) Akt at Ser473 and B) FoxO3a at Ser253 are reduced only when the soleus muscle is immobilized in a shortened position. Phosphorylation of Akt at Ser473 and FoxO3a at Ser253 were assessed by protein immunoblot analysis. Rats had one hindlimb immobilized for 3 days in a position to place the soleus in either a shortened or stretched position. Bars represent the mean phosphorylation of Akt at Ser473/total Akt protein or phosphorylation of FoxO3a at Ser253/total protein ratio in the soleus muscle from an immobilized hindlimb expressed as a percentage of that in the soleus muscle from the contralateral control hindlimb. No changes in total Akt expression were observed in any group across 3 days of hindlimb immobilization. Total FoxO3a protein could not be accurately assessed by immunoblot. Data are mean percentages ± SEM, n = 10 rats/group. * p < 0.05 versus soleus muscle from the control hindlimb. 63
74 Chapter 5. AGE-RELATED RESPONSES OF MTORC1 SIGNALING IN RAT HINDLIMB SKELETAL MUSCLE TO IMMOBILIZATION AND REMOBILIZATION Introduction Loss of skeletal muscle mass and function in the elderly leads to physical frailty, increased risk of falls and fractures, and increased morbidity and mortality (35, 139, 200). A period of inactivity (e.g. limb immobilization, bed rest, etc.) worsens the problem since inactivity causes skeletal muscle atrophy (199). Both age- and inactivity-induced muscle atrophy may, in part, be due to resistance to anabolic stimulation (e.g. exercise and amino acids) (55, 72, 170). In contrast, remobilization promotes skeletal muscle hypertrophy due, in part, to enhanced anabolic stimulation (98, 126). If the molecular mechanisms responsible for anabolic stimulation in muscle can be understood, then this will lead to the development of therapeutic interventions aimed at preventing age- and inactivityinduced skeletal muscle atrophy. Based on the results of Chapters 3 and 4, I asked how mtorc1 signaling may be regulated by REDD1 and REDD2 in response to aging and remobilization. I tested the hypothesis that REDD1 and/or REDD2 mrna expression is elevated in muscle from old compared to young rats in association with attenuated mtorc1 signaling in response to nutrient stimulation. Moreover, I hypothesized that the immobilization-induced stimulation of REDD1 and/or REDD2 expression is exacerbated in old compared to young rats. I also tested the hypothesis that remobilization reverses the immobilizationinduced induction of REDD1 and/or REDD2 expression in conjunction with restoration of mtorc1 signaling. 64
75 Methods Experimental Design Rats 2 (juvenile), 9 (young adult), and 18 (old adult) months of age were anesthetized by isoflurane inhalation (2.5%) and subjected to unilateral hindlimb immobilization for 7 days prior to removal of the soleus muscle for subsequent analysis. An additional group of rats aged 9 months was subjected to unilateral hindlimb immobilization for 7 days, and following removal of the cast, were allowed to remobilize for 7 days (remobilization group). All rats were fasted overnight (18h) but allowed free access to water. On tissue harvest days, rats were individually caged and provided with rodent chow (AIN-93M) for 10 min (rats consumed grams). They were subsequently anesthetized using isoflurane and remained anesthetized for the rest of the experiment. The soleus muscle was chosen for analysis in the present study so that the results could be compared to those from Chapters 3 and 4, which investigated the effects of unilateral hindlimb immobilization on mtorc1 signaling in young rats. The soleus muscle was removed from the immobilized and the contralateral non-immobilized (control) hindlimbs 45 min after the start of chow feeding. This time point was selected based on previous studies showing that the peak response of mtorc1 signaling to a nutrient-induced stimulus occurs between 45 and 60 min (6). Ribosome content was calculated by measuring the total RNA concentration in a portion of muscle as described in Measurement of mrna expression and applying this concentration to the whole muscle. 65
76 Statistical analysis In order to assess the effects of remobilization following hindlimb immobilization, the soleus muscle from the remobilized limb of rats in the remobilization group was compared to the soleus muscle from 9-month old immobilized rats. Nonlinear regression (curve fit) analysis was used to assess the relative relationship between p70s6k1 phosphorylation at Thr389 and REDD2 mrna expression for all samples. Results Effect of age and remobilization on immobilization-induced loss of muscle mass As illustrated in Figure 5.1A, the soleus muscle mass-to-body mass ratio declined with age, being significantly lower (p < 0.05) in 18-month old rats compared to both 2- and 9-month old rats. Absolute mass of the soleus (Figure 5.1B) was larger in 9- and 18- month old rats compared to 2-month old rats, but no significant differences in muscle mass were observed between 9- and 18-month old rats. In response to 7 days of hindlimb immobilization, muscle mass-to-body mass ratio was reduced in rats from all age groups. Notably, the loss of muscle mass following hindlimb immobilization was inversely proportional to age, where the muscle mass-to-body mass ratio was reduced 40%, 15%, and 8% in the soleus muscle from the immobilized hindlimb of 2-, 9-, and 18-month old rats, respectively, when compared to the soleus muscle from the contralateral nonimmobilized hindlimb (referred to hereafter as the control hindlimb). A similar relationship between age and disuse atrophy was observed when results were expressed as absolute muscle mass (Figure 5.1B) where muscle mass was reduced 40%, 15%, and 7% in the soleus muscle from the immobilized hindlimb of 2-, 9-, and 18-month old rats. 66
77 No difference in muscle mass-to-body mass ratio was observed between the soleus muscle from the control hindlimb of 9-month old immobilized and remobilized rats. Following 7 days of remobilization, muscle mass-to-body mass ratio and absolute mass were further reduced (p < 0.05) in the soleus muscle from the remobilized hindlimb compared to either the immobilized or the control hindlimb of 9-month old rats. Effect of age, immobilization, and remobilization on mtorc1 signaling In order to gain an understanding of the potential molecular events responsible for age- and immobilization-induced skeletal muscle atrophy, rats were provided with a nutrient stimulus to produce an anabolic response. This was assessed by analysis of the phosphorylation state of residues on two proteins known to be direct targets of mtorc1, p70s6k1 Thr389 (Figure 5.2A) and ULK1 Ser757 (Figure 5.2B). The phosphorylation of ULK1 at Ser757 was included here as a direct readout of mtorc1 signaling that is not influenced by prior PDK1 phosphorylation. The analysis demonstrated that aging was associated with an 83% and 78% attenuation of the nutrient-induced stimulation of p70s6k1 phosphorylation at Thr389 in the soleus muscle from the control hindlimb of 9- and 18-month old rats, respectively, relative to the soleus muscle from the control hindlimb of 2-month old rats. In response to 7 days of hindlimb immobilization, phosphorylation of p70s6k1 at Thr389 was reduced 68% and 48% in the soleus muscle from the immobilized hindlimb relative to the control hindlimb of 2- and 9-month old rats, respectively, whereas it was statistically different from the control value in 18-month old rats. ULK1 phosphorylation at Ser757 also declined with age following the nutrient stimulus. In response to immobilization, it was reduced 48% relative to the control 67
78 hindlimb of 2-month old rats, but no difference was observed in its phosphorylation state between hindlimbs of 9- or 18-month old rats. Following 7 days of remobilization, mtorc1 signaling was augmented in the soleus muscle from the remobilized limb relative to the control hindlimb, i.e. phosphorylation of p70s6k1 at Thr389 and ULK1 at Ser757 was elevated 160% and 42%, respectively, in the soleus muscle from the remobilized hindlimb. Effect of age, immobilization, and remobilization on Akt activation As a biomarker of an upstream signaling input to mtorc1, phosphorylation of Akt at Ser473 was assessed as an index of the activation state of the kinase. The assessment demonstrated that aging was associated with attenuation of the nutrientinduced activation of Akt in soleus muscle from the control hindlimb of 9- and 18-month old rats relative to the soleus muscle from the control hindlimb of 2-month old rats (Figure 5.3). In response to 7 days of hindlimb immobilization, the nutrient-induced phosphorylation of Akt at Ser473 was reduced 30% in the soleus muscle from the immobilized hindlimb relative to the soleus muscle from the control hindlimb of 2-month old rats. No difference in phosphorylation of Akt at Ser473 was observed between the soleus muscle from the immobilized and the control hindlimb of 9- and 18-month old rats. Following 7 days of remobilization, phosphorylation of Akt at Ser473 was augmented 90% in the soleus muscle from the remobilized limb relative to the soleus from the control hindlimb. 68
79 Effect of age, immobilization, and remobilization on REDD mrna expression In order to gain an understanding of the mechanism(s) responsible for the observed responses of mtorc1 signaling to aging, hindlimb immobilization, and remobilization, the mrna expression was assessed of two repressors of the pathway, i.e. REDD1 and REDD2. No change in REDD1 mrna expression was observed in response to aging, hindlimb immobilization, or remobilization (Figure 5.4A). In contrast, aging was associated with increased expression of REDD2 mrna, i.e. REDD2 mrna expression was induced approximately 250% and 300% in the soleus muscle from the control hindlimb of 9- and 18-month old rats, respectively, relative to the control hindlimb of 2-month old rats (Figure 5.4B). In response to 7 days of hindlimb immobilization, REDD2 mrna expression was induced by over 400% in the soleus muscle from the immobilized hindlimb relative to the soleus muscle from the control hindlimb of 2-month old rats. In 9-month old rats, REDD2 mrna expression in the soleus muscle from the immobilized hindlimb relative to the soleus muscle from the control hindlimb was further increased by 77% above the aging-induced response. However, this trend did not reach statistical significance (p = 0.16). Moreover, in 18- month old rats, immobilization did not enhance REDD2 mrna expression above the aging-induced response. Following 7 days of remobilization, REDD2 mrna expression was repressed 38% in the soleus muscle from the remobilized limb relative to the soleus muscle from the control hindlimb, and was significantly lower than the REDD2 mrna expression in the soleus muscle from the immobilized hindlimb of 9-month old rats. 69
80 Relationship between p70s6k1 Thr389 phosphorylation and REDD2 mrna expression Across age groups and conditions, there appeared to be an association between mtorc1 signaling and REDD2 mrna expression in the soleus from rat hindlimbs, i.e. the phosphorylation of p70s6k1 at Thr389 and ULK1 at Ser757 was inversely proportional to REDD2 mrna expression. A scatter plot with the natural logarithm (ln) of REDD2 mrna expression and the phosphorylation of p70s6k1 at Thr389 plotted on the x- and y-axes, respectively, revealed a linear relationship between these two variables. As illustrated in Figure 5.5, low REDD2 mrna expression was associated with high phosphorylation of p70s6k1 at Thr389 and increases in REDD2 mrna expression were associated with reductions in phosphorylation of p70s6k1 at Thr389. Fitting a linear regression to these points reveals a negative correlation (r 2 = ) between REDD2 mrna expression and phosphorylation of p70s6k1 at Thr389 (p < ). Effect of age and immobilization on PDK1 phosphorylation sites on Akt and p70s6k1 In order to gain an understanding of PDK1 signaling in response to aging and 7 days of hindlimb immobilization, I assessed the phosphorylation states of p70s6k1 at Thr229 and Akt at Thr308. Aging was associated with an attenuation of the nutrientinduced stimulation of p70s6k1 phosphorylation at Thr229 in soleus muscle from the control hindlimb (Figure 5.6A). In response to 7 days of hindlimb immobilization, the phosphorylation of p70s6k1 at Thr229 was reduced in 2-month old rats, but not in 9- and 70
81 18-month old rats. I observed similar results for the phosphorylation of Akt at Thr308, whereby aging was associated with an attenuation of the nutrient-induced stimulation of Akt phosphorylation at Thr308 in soleus muscle from the control hindlimb (Figure 5.6B). In response to 7 days of hindlimb immobilization, the phosphorylation of Akt at Thr308 was reduced in 2-month old rats, but not in 9- and 18-month old rats. Effect of age, immobilization, and remobilization on atrogene mrna expression I assessed the mrna expression of the E3 ubiquitin ligases, MAFbx and MuRF1 (also known as 'atrogenes' (18), as biomarkers associated with the proteasome-mediated degradation of skeletal muscle proteins during atrophy. The mrna expression of MAFbx (Figure 5.7A) was elevated 63% and 47% in the non-immobilized hindlimb of 9- and 18-month old rats compared to the non-immobilized hindlimb of 2-month old rats. Moreover, after seven days of immobilization, MAFbx mrna expression was higher in the immobilized compared to the control hindlimb in each age group. Aging had no significant effect on the mrna expression of MuRF1 (Figure 5.7B). Interestingly, although MuRF1 was elevated in the immobilized hindlimb of 2-month old rats, it was not elevated in response to immobilization in 9- and 18-month old rats. In response to 7 days of remobilization, MAFbx expression was lower in the remobilized hindlimb compared to the immobilized hindlimb of 9-month old rats. However, MAFbx mrna expression was not statistically different between the remobilized and non-immobilized hindlimb of the same rat. In contrast, MuRF1 mrna expression was reduced in the remobilized hindlimb compared to both the non-immobilized and immobilized hindlimbs of 9-month old rats. 71
82 Effect of age, immobilization, and remobilization on gastrocnemius muscle mass-tobody mass ratio and RNA content In order to gain an understanding of the translational capacity of skeletal muscle in response to aging, hindlimb immobilization, and remobilization, ribosome biogenesis was assessed (32). Not enough soleus muscle tissue was available for this analysis, so gastrocnemius muscles from the same rats were used to measure ribosome content. Like the soleus muscle, gastrocnemius mass-to-body mass ratio declined with age, being significantly lower (p < 0.05) in 18-month old rats compared to both 2- and 9-month old rats (Figure 5.8A). Additionally, muscle mass-to-body mass ratio was reduced in rats from all age groups in response to 7 days of hindlimb immobilization. Total RNA content was measured as an indicator of ribosome biogenesis (Figure 5.8B). RNA content declined with age, being significantly lower (p < 0.05) in 9- and 18-month old rats compared to both 2-month old rats, but a statistically significant difference in RNA content was not observed between 9- and 18-month old rats. Although not significantly (p < 0.05) different, RNA content declined with hindlimb immobilization and increased with remobilization. Discussion The goal of the studies presented in this third chapter was to assess REDD1 and REDD2 mrna as surrogates for protein expression in skeletal muscle in response to aging and remobilization. This goal was achieved and an association between mtorc1 signaling and REDD2, but not REDD1, mrna expression in soleus muscle across a 72
83 number of variables including aging, immobilization and remobilization was observed. Sarcopenia, defined as an age-related loss of muscle mass and function, is thought to be in large part a consequence of the development of resistance to nutrient (particularly amino acids)-induced stimulation of muscle protein synthesis (144, 170). In young adult humans (e.g. approximately 25 years old), muscle protein synthesis is stimulated in response to consumption of relatively small quantities (e.g g) of essential amino acids, whereas in older adults (e.g. approximately 65 years old) this amount of amino acids is ineffective (107, 108). However, ingesting larger quantities of amino acids (e.g g) effectively stimulates muscle protein synthesis in both young and older individuals (202, 203). Notably, the responsiveness of muscle protein synthesis in the elderly to small quantities of essential amino acids can be overcome by increasing the leucine content of the mixture (73, 108). Similar results have been reported for studies in rats in which the age-related resistance of skeletal muscle protein synthesis to stimulation by a protein diet was overcome by supplementation with leucine (39). Combined, the results from both human and rat studies suggest that the age-related loss of muscle responsiveness to amino acids is largely due to impaired leucine sensitivity. In both animals (5) and humans (47), leucine induces stimulation of protein synthesis through activation of the mtorc1 signaling pathway. In agreement with the impaired responsiveness of muscle protein synthesis to stimulation by leucine, the sensitivity of mtorc1 signaling to activation by the amino acid is also attenuated in muscle from older individuals compared to young adults (83). In this study a similar phenomenon was observed in rats, i.e. aging was associated with a proportional reduction in nutrient-induced activation of mtorc1 signaling. Notably, the decline in sensitivity 73
84 of mtorc1 to activation by nutrients was inversely correlated with changes in expression of REDD2, which in addition to REDD1, is a dominant repressor of the pathway. Many studies have focused on the role of REDD1 and/or REDD2 in repressing mtorc1 signaling under conditions of cell stress (36, 57, 141), with many fewer assessing their role under physiological conditions. However, recent reports have demonstrated an inverse correlation between changes in mtorc1 signaling and REDD1 and/or REDD2 expression in skeletal muscle in response to fasting and re-feeding (145) as well as after a bout of exercise (151, 157). This study extends the observations to show that elevated REDD2 mrna expression in 9- and 18-month old rats is associated with a reduction in mtorc1 signaling. The mechanism through which aging acts to induce REDD2 expression is unknown. However, it is notable that many of the stressors that have been shown to enhance REDD1 and/or REDD2 gene transcription, e.g. endoplasmic reticulum stress (214) or conditions that cause DNA damage (130) or increase production of reactive oxygen species (58), have been reported to be elevated in muscle from older compared to younger individuals (13, 133, 144, 158). Thus, it is tempting to speculate that one, or more, of these stressors is responsible for mediating the induction of REDD2 mrna expression observed in the present study. It should be noted that, in a previous study in humans (53), no difference in expression of either REDD1 or REDD2 RNA was observed in skeletal muscle from young compared to older individuals. However, the apparent discrepancy between the results of that study and this one is likely due to the age of the individuals assessed. In this study, a significant reduction in REDD2 mrna was observed between 2 and 9 months of age, with a further slight, non-significant, reduction occurring between 9 and 18 months. Thus, much of the 74
85 decline occurred during the transition from young, rapidly growing to middle aged rats, thus emphasizing the importance of the age of the subject being studied. Chapters 3 and 4 showed mtorc1 signaling to be inversely proportional to both REDD1 and REDD2 mrna expression in skeletal muscle of an immobilized rat hindlimb (110, 111). In those studies, a rapid induction of both REDD1 and REDD2 mrna expression was observed following hindlimb immobilization of 1 to 3 days duration compared to the values observed in either the contralateral, non-immobilized limb or the limb of a control rat (111). In the present chapter, following 7 days of hindlimb immobilization, REDD1 mrna expression had returned to the control value whereas REDD2 mrna expression remained elevated. Thus, the rapid induction of REDD1 mrna expression observed in the prior studies likely resulted from a stimulus associated with the immobilization procedure. In contrast, based on the results presented here, REDD2 induction would seem to be the more relevant response to immobilization. Such a possibility is consistent with the finding that of the tissues that have been examined REDD2 mrna expression is highest in skeletal muscle (152). Again, it should be noted that the data presented herein are for mrna and not protein expression for REDD2. Presently, assessing changes in REDD2 protein expression is not feasible due to the lack of anti-redd2 antibodies. Another key result of the studies presented in this chapter is that the nutrientinduced activation of mtorc1 signaling in response to remobilization is associated with repression of REDD2 mrna expression. This result agrees with other studies that have also observed an activation of mtorc1 signaling in skeletal muscle following 1-7 days of remobilization (33, 126, 127, 155). Repression of REDD2 mrna expression during 75
86 remobilization would provide a mechanism for relieving the resistance to nutrientinduced activation of mtorc1 signaling following immobilization. Notably, despite the activation of mtorc1 signaling, muscle mass continued to fall during the period of remobilization. A similar phenomenon has been previously reported (126, 140). The molecular basis for the delayed recovery of muscle mass with remobilization is unknown. However, it is tempting to speculate that it may be related to a reduction in RNA content, and thus ribosome abundance. In young, growing animals ribosome number is elevated to allow for higher rates of protein synthesis after an anabolic stimulus, whereas fewer ribosomes would be needed to maintain the relatively static muscle mass in older animals. Thus, an increase in ribosome number in the older animals may be required for recovery of muscle mass following remobilization. Notably, ribosome biogenesis, which is under the control of mtorc1 signaling (99), is reduced during hindlimb suspension and immobilization (9), and enhanced during reloading following hindlimb suspension (90). Due to the relatively slow turnover of ribosomes, it is not surprising to observe a delay in the response of skeletal muscle protein synthesis and growth (i.e. mass) to immobilization and/or remobilization. Models of both aging- and inactivity-induced muscle atrophy are associated with insulin resistance in skeletal muscle (29, 35, 159, 205, 215, 220). The alterations in the nutrient-induced stimulation of phosphorylation of Akt at Ser473 observed in the studies described in this chapter in response to aging and hindlimb immobilization and remobilization are in agreement with other reports involving hindlimb immobilization and remobilization and functional overload of skeletal muscle (33, 98, 155, 220). It remains to be determined whether these changes in the phosphorylation of Akt at Ser473 are 76
87 linked with changes in REDD2 mrna expression. Thus, future studies are needed to determine the contributions of induced REDD2 mrna expression versus attenuated Akt phosphorylation on mtorc1 signaling during aging, hindlimb immobilization, and remobilization. In conclusion, nutrient-induced activation of mtorc1 signaling was enhanced and REDD2 mrna expression was reduced in skeletal muscle from young compared to older rats. Moreover, mtorc1 signaling was activated by 7 days of remobilization in association with reduced REDD2 mrna expression. No differences in REDD1 mrna expression were observed in skeletal muscle due to aging, hindlimb immobilization, or remobilization. Therefore, REDD2 expression appears to play a prominent role in the regulation of mtorc1 signaling in skeletal muscle during aging, and hindlimb immobilization and remobilization, and consequently in modulating skeletal muscle mass (Figure 5.9). 77
88 78
89 79
90 80
91 81
92 82
93 A Phospho p70s6k1 T229 / Total (arbitrary values) # * Immobilized Limb Control Limb Age (months) B Phospho Akt T308 / Total (arbitrary values) # Immobilized Limb Control Limb Age (months) Figure 5.6 Nutrient-induced activation of PDK1 signaling declines during 7 days of hindlimb immobilization and aging, but in older rats the pathway is not responsive to immobilization. Rats 2-, 9-, and 18-months of age had one hindlimb immobilized for 7 days. Relative phosphorylation of A) p70s6k1 at Thr229 and B) Akt at Thr308 were assessed by protein immunoblot analysis. Bars represent the mean phospho/total protein ratio in soleus muscle from control (gray) and immobilized (black) hindlimbs. No differences were observed in total p70s6k1 or Akt protein expression. Data are mean ± SEM, n = 5-6 rats/group. * p < 0.05 compared to control limb; # p < 0.05 compared to equivalent limb in 9-month old rat; + p < 0.05 compared to equivalent limb in 18-month old rat. 83
94 A MAFbx mrna % # # * * # * Non-Immobilized Limb Immobilized Limb Remobilized Limb R 18 Age (months) B MuRF1 mrna % # * # * Non-Immobilized Limb Immobilized Limb Remobilized Limb R 18 Age (months) Figure 5.7 MAFbx mrna increases with age and 7 days of immobilization, while MuRF1 increases with immobilization only in 2-month old animals and decreases in response to remobilization. MAFbx and MuRF1 mrna expression were assessed by Taqman gene expression assay. Rats, 2-, 9-, and 18-months of age, had one hindlimb immobilized for 7 days, and an additional group of 9-month old rats had their casts removed after 7 days and were allowed to remobilize for an additional 7 days (9R). Bars represent the mean A) MAFbx mrna-, or B) MuRF1 mrna-to-tbp mrna ratio in soleus from control (dark gray), immobilized (black), and remobilized (light gray) hindlimbs expressed as a percentage of the same ratio in soleus from control hindlimbs of 9-month old rats. Tbp gene expression was verified against three common loading controls as a reliable housekeeping gene. Data are mean ± SEM, n = 4-6 rats/group. * p < 0.05 compared to control limb. # p < 0.05 compared to equivalent limb in 9-month old rat. + p < 0.05 compared to equivalent limb in 18-month old rat. 84
95 Figure
96 Figure 5.9 Regulation of mtorc1 signaling under conditions of aging and 7 days of hindlimb immobilization. Nutrient-induced stimulation of mtorc1 signaling is attenuated under such conditions in association with induction in REDD2 mrna expression. It is postulated that an induction in REDD2 leads to repression of mtorc1 signaling by promoting TSC GAP activity towards Rheb. In addition, aging and 7 days of hindlimb immobilization were associated with impaired PDK1 signaling to p70s6k1 and Akt. Insulin resistance may play a role in this impairment, but the precise mechanism is unknown. 86
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy se
 A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
FACTORS AFFECTING SKELETAL MUSCLE PROTEIN SYNTHESIS IN THE HORSE
 University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2011 FACTORS AFFECTING SKELETAL MUSCLE PROTEIN SYNTHESIS IN THE HORSE Ashley Leigh Wagner University
University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2011 FACTORS AFFECTING SKELETAL MUSCLE PROTEIN SYNTHESIS IN THE HORSE Ashley Leigh Wagner University
EFFECT OF ENDURANCE EXERCISE ALONE AND IN COMBINATION WITH IGF-1 ADMINISTRATION ON CELLULAR MARKERS INVOLVED IN SARCOPENIA.
 EFFECT OF ENDURANCE EXERCISE ALONE AND IN COMBINATION WITH IGF-1 ADMINISTRATION ON CELLULAR MARKERS INVOLVED IN SARCOPENIA PhD thesis Mohammad Mosaferi Ziaaldini Doctoral School of Sport Sciences University
EFFECT OF ENDURANCE EXERCISE ALONE AND IN COMBINATION WITH IGF-1 ADMINISTRATION ON CELLULAR MARKERS INVOLVED IN SARCOPENIA PhD thesis Mohammad Mosaferi Ziaaldini Doctoral School of Sport Sciences University
The Regulation of Skeletal Muscle Protein Turnover During the Progression of Cancer Cachexia in the Apc Min/+ Mouse
 University of South Carolina Scholar Commons Faculty Publications Chemistry and Biochemistry, Department of 9-19-2011 The Regulation of Skeletal Muscle Protein Turnover During the Progression of Cancer
University of South Carolina Scholar Commons Faculty Publications Chemistry and Biochemistry, Department of 9-19-2011 The Regulation of Skeletal Muscle Protein Turnover During the Progression of Cancer
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus
 Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Antibodies for Unfolded Protein Response
 Novus-lu-2945 Antibodies for Unfolded rotein Response Unfolded roteins ER lumen GR78 IRE-1 GR78 ERK Cytosol GR78 TRAF2 ASK1 JNK Activator Intron RIDD elf2α Degraded mrna XB1 mrna Translation XB1-S (p50)
Novus-lu-2945 Antibodies for Unfolded rotein Response Unfolded roteins ER lumen GR78 IRE-1 GR78 ERK Cytosol GR78 TRAF2 ASK1 JNK Activator Intron RIDD elf2α Degraded mrna XB1 mrna Translation XB1-S (p50)
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-09-1-0279 TITLE: Regulation of mtor by Nutrients PRINCIPAL INVESTIGATOR: Kun-Liang Guan CONTRACTING ORGANIZATION: University of San Diego La Jolla, CA 92093 REPORT DATE: July 2010
AD Award Number: W81XWH-09-1-0279 TITLE: Regulation of mtor by Nutrients PRINCIPAL INVESTIGATOR: Kun-Liang Guan CONTRACTING ORGANIZATION: University of San Diego La Jolla, CA 92093 REPORT DATE: July 2010
Scholar Commons. University of South Carolina. Song Gao University of South Carolina. Theses and Dissertations
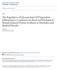 University of South Carolina Scholar Commons Theses and Dissertations 2017 The Regulation of Glycoprotein130 Dependent Inflammatory Cytokines one Basal and Mechanical Stimuli Induced Protein Synthesis
University of South Carolina Scholar Commons Theses and Dissertations 2017 The Regulation of Glycoprotein130 Dependent Inflammatory Cytokines one Basal and Mechanical Stimuli Induced Protein Synthesis
Brief Critical Review
 Brief Critical Review March 2007: 122 129 Leucine and Protein Synthesis: mtor and Beyond Martha H. Stipanuk, PhD The effects of amino acid intake on protein synthesis in the intact rat appear to be mediated
Brief Critical Review March 2007: 122 129 Leucine and Protein Synthesis: mtor and Beyond Martha H. Stipanuk, PhD The effects of amino acid intake on protein synthesis in the intact rat appear to be mediated
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Amino Acids: Regulation of Global and Specific mrna Translation. Dr. Scot R. Kimball
 Amino Acids: Regulation of Global and Specific mrna Translation Scot R. Kimball Department of Cellular and Molecular Physiology, The Pennsylvania State University, College of Medicine, Hershey, PA 17033
Amino Acids: Regulation of Global and Specific mrna Translation Scot R. Kimball Department of Cellular and Molecular Physiology, The Pennsylvania State University, College of Medicine, Hershey, PA 17033
supplementary information
 Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Protein MultiColor Stable, Low Range
 Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Branched Chain Amino Acid, Leucine: The Effects of Leucine on Skeletal Tissue in Relation to Aerobic Exercise. Shea Teresi. For
 Branched Chain Amino Acid, Leucine: The Effects of Leucine on Skeletal Tissue in Relation to Aerobic Exercise By Shea Teresi For Dr. William R. Proulx, RD Associate Professor of Nutrition & Dietetics In
Branched Chain Amino Acid, Leucine: The Effects of Leucine on Skeletal Tissue in Relation to Aerobic Exercise By Shea Teresi For Dr. William R. Proulx, RD Associate Professor of Nutrition & Dietetics In
Receptor mediated Signal Transduction
 Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
G-Protein Signaling. Introduction to intracellular signaling. Dr. SARRAY Sameh, Ph.D
 G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
AMPK Phosphorylation Assay Kit
 AMPK Phosphorylation Assay Kit Catalog Number KA3789 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
AMPK Phosphorylation Assay Kit Catalog Number KA3789 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Phospho-AKT Sampler Kit
 Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Supplemental Information. Increased 4E-BP1 Expression Protects. against Diet-Induced Obesity and Insulin. Resistance in Male Mice
 Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Cell Reports, Volume 16 Supplemental Information Increased 4E-BP1 Expression Protects against Diet-Induced Obesity and Insulin Resistance in Male Mice Shih-Yin Tsai, Ariana A. Rodriguez, Somasish G. Dastidar,
Professor Christopher Proud
 South Australian Health and Medical Research Institute Professor Christopher Proud Cell Signalling & Gene Regulation Professor Christopher G. Proud Nutrition and Metabolism Theme Leader South Australian
South Australian Health and Medical Research Institute Professor Christopher Proud Cell Signalling & Gene Regulation Professor Christopher G. Proud Nutrition and Metabolism Theme Leader South Australian
Optimizing Nutritional Strategies to Promote Growth in Newborns
 Optimizing Nutritional Strategies to Promote Growth in Newborns Teresa A. Davis, Ph.D. Professor of Pediatrics USDA/ARS Children s Nutrition Research Center, Baylor College of Medicine, Houston, TX Disclosure
Optimizing Nutritional Strategies to Promote Growth in Newborns Teresa A. Davis, Ph.D. Professor of Pediatrics USDA/ARS Children s Nutrition Research Center, Baylor College of Medicine, Houston, TX Disclosure
The elements of G protein-coupled receptor systems
 The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
Biol403 MAP kinase signalling
 Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Supplementary Material
 Supplementary Material The Androgen Receptor is a negative regulator of eif4e Phosphorylation at S209: Implications for the use of mtor inhibitors in advanced prostate cancer Supplementary Figures Supplemental
Supplementary Material The Androgen Receptor is a negative regulator of eif4e Phosphorylation at S209: Implications for the use of mtor inhibitors in advanced prostate cancer Supplementary Figures Supplemental
Fridtjof Seeberg Master thesis in Sport Sciences
 Fridtjof Seeberg Native whey- and milk-protein supplementation combined with resistance exercise, induces similar anabolic signaling-responses downstream of mtor in elderly. Master thesis in Sport Sciences
Fridtjof Seeberg Native whey- and milk-protein supplementation combined with resistance exercise, induces similar anabolic signaling-responses downstream of mtor in elderly. Master thesis in Sport Sciences
Insights into the role and regulation of TCTP in skeletal muscle
 /, 2017, Vol. 8, (No. 12), pp: 18754-18772 Insights into the role and regulation of TCTP in skeletal muscle Craig A. Goodman 1,2,3, Allison M. Coenen 1, John W. Frey 1, Jae-Sung You 1, Robert G. Barker
/, 2017, Vol. 8, (No. 12), pp: 18754-18772 Insights into the role and regulation of TCTP in skeletal muscle Craig A. Goodman 1,2,3, Allison M. Coenen 1, John W. Frey 1, Jae-Sung You 1, Robert G. Barker
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
Nutrition & Metabolism Cell Signalling & Gene Regulation PhD & Honours Projects 2018
 Nutrition & Metabolism Cell Signalling & Gene Regulation PhD & Honours Projects 2018 Prof. Proud s laboratory studies the signalling pathways by which hormones, growth factors and nutrients regulate the
Nutrition & Metabolism Cell Signalling & Gene Regulation PhD & Honours Projects 2018 Prof. Proud s laboratory studies the signalling pathways by which hormones, growth factors and nutrients regulate the
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Warm Up! Test review (already! ;))
 Warm Up! Test review (already! ;)) Write a question you might find on the Unit 5 test next week! (Multiple choice, matching, fill in, or short answer!) - challenge yourself and be ready to share!!! PowerPoint
Warm Up! Test review (already! ;)) Write a question you might find on the Unit 5 test next week! (Multiple choice, matching, fill in, or short answer!) - challenge yourself and be ready to share!!! PowerPoint
Protocol for Western Blo
 Protocol for Western Blo ng SDS-PAGE separa on 1. Make appropriate percentage of separa on gel according to the MW of target proteins. Related recommenda ons and rou ne recipes of separa on/stacking gels
Protocol for Western Blo ng SDS-PAGE separa on 1. Make appropriate percentage of separa on gel according to the MW of target proteins. Related recommenda ons and rou ne recipes of separa on/stacking gels
Principles of Genetics and Molecular Biology
 Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Transcriptional Regulation of Skeletal Muscle Atrophy-Induced Gene Expression by Muscle Ring Finger-1 and Myogenic Regulatory Factors
 UNF Digital Commons UNF Theses and Dissertations Student Scholarship 2017 Transcriptional Regulation of Skeletal Muscle Atrophy-Induced Gene Expression by Muscle Ring Finger-1 and Myogenic Regulatory Factors
UNF Digital Commons UNF Theses and Dissertations Student Scholarship 2017 Transcriptional Regulation of Skeletal Muscle Atrophy-Induced Gene Expression by Muscle Ring Finger-1 and Myogenic Regulatory Factors
For the quantitative measurement of ATP Synthase Specific activity in samples from Human, Rat and Cow
 ab109716 ATP Synthase Specific Activity Microplate Assay Kit Instructions for Use For the quantitative measurement of ATP Synthase Specific activity in samples from Human, Rat and Cow This product is for
ab109716 ATP Synthase Specific Activity Microplate Assay Kit Instructions for Use For the quantitative measurement of ATP Synthase Specific activity in samples from Human, Rat and Cow This product is for
Signal Transduction Pathway Smorgasbord
 Molecular Cell Biology Lecture. Oct 28, 2014 Signal Transduction Pathway Smorgasbord Ron Bose, MD PhD Biochemistry and Molecular Cell Biology Programs Washington University School of Medicine Outline 1.
Molecular Cell Biology Lecture. Oct 28, 2014 Signal Transduction Pathway Smorgasbord Ron Bose, MD PhD Biochemistry and Molecular Cell Biology Programs Washington University School of Medicine Outline 1.
EFFECT OF WHEY AND CASEIN ON POST -EXERCISE PROTEIN SYNTHESIS
 EFFECT OF WHEY AND CASEIN ON POST -EXERCISE PROTEIN SYNTHESIS EFFECT OF WHEY AND CASEIN PROTEINS ON MUSCLE PROTEIN SYNTHESIS AFTER RESISTANCE EXERCISE By JASON E. TANG, B.Sc. A Thesis Submitted to the
EFFECT OF WHEY AND CASEIN ON POST -EXERCISE PROTEIN SYNTHESIS EFFECT OF WHEY AND CASEIN PROTEINS ON MUSCLE PROTEIN SYNTHESIS AFTER RESISTANCE EXERCISE By JASON E. TANG, B.Sc. A Thesis Submitted to the
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
K-LISA mtor Activity Kit Cat. No. CBA055
 User Protocol CBA055 Rev. 20 December 2006 JSW Page 1 of 8 K-LISA mtor Activity Kit Cat. No. CBA055 Note that this user protocol is not lot-specific and is representative of the current specifications
User Protocol CBA055 Rev. 20 December 2006 JSW Page 1 of 8 K-LISA mtor Activity Kit Cat. No. CBA055 Note that this user protocol is not lot-specific and is representative of the current specifications
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
ab E3 Ligase Auto- Ubiquitilylation Assay Kit
 ab139469 E3 Ligase Auto- Ubiquitilylation Assay Kit Instructions for Use For testing ubiquitin E3 ligase activity through assessment of their ability to undergo auto-ubiquitinylation This product is for
ab139469 E3 Ligase Auto- Ubiquitilylation Assay Kit Instructions for Use For testing ubiquitin E3 ligase activity through assessment of their ability to undergo auto-ubiquitinylation This product is for
ab Membrane Fractionation Kit Instructions for Use For the rapid and simple separation of membrane, cytosolic and nuclear cellular fractions.
 ab139409 Membrane Fractionation Kit Instructions for Use For the rapid and simple separation of membrane, cytosolic and nuclear cellular fractions. This product is for research use only and is not intended
ab139409 Membrane Fractionation Kit Instructions for Use For the rapid and simple separation of membrane, cytosolic and nuclear cellular fractions. This product is for research use only and is not intended
KEY CONCEPT QUESTIONS IN SIGNAL TRANSDUCTION
 Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
RayBio KinaseSTAR TM Akt Activity Assay Kit
 Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Cell Signaling part 2
 15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
20X Buffer (Tube1) 96-well microplate (12 strips) 1
 PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
Chapter 15: Signal transduction
 Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
Review Article Linking Cancer Cachexia-Induced Anabolic Resistance to Skeletal Muscle Oxidative Metabolism
 Hindawi Oxidative Medicine and Cellular Longevity Volume 2017, Article ID 8018197, 14 pages https://doi.org/10.1155/2017/8018197 Review Article Linking Cancer Cachexia-Induced Anabolic Resistance to Skeletal
Hindawi Oxidative Medicine and Cellular Longevity Volume 2017, Article ID 8018197, 14 pages https://doi.org/10.1155/2017/8018197 Review Article Linking Cancer Cachexia-Induced Anabolic Resistance to Skeletal
Biol220 Cell Signalling Cyclic AMP the classical secondary messenger
 Biol220 Cell Signalling Cyclic AMP the classical secondary messenger The classical secondary messenger model of intracellular signalling A cell surface receptor binds the signal molecule (the primary
Biol220 Cell Signalling Cyclic AMP the classical secondary messenger The classical secondary messenger model of intracellular signalling A cell surface receptor binds the signal molecule (the primary
An oxygen-regulated switch in the protein synthesis machinery
 An oxygen-regulated switch in the protein synthesis machinery 1 Inhibition of translation during acute hypoxia is mediated by eif2α phosphorilation mouse embryo fibroblasts (MEFs) 1 Watson et al., BIOLOGIA
An oxygen-regulated switch in the protein synthesis machinery 1 Inhibition of translation during acute hypoxia is mediated by eif2α phosphorilation mouse embryo fibroblasts (MEFs) 1 Watson et al., BIOLOGIA
PHYSIOLOGY, ENDOCRINOLOGY, AND REPRODUCTION. Research Note. Energy sensing in developing chicken embryos and posthatch chicks from different size eggs
 PHYSIOLOGY, ENDOCRINOLOGY, AND REPRODUCTION Research Note Energy sensing in developing chicken embryos and posthatch chicks from different size eggs Q. Hu, U. Agarwal, and B. J. Bequette 1 Animal and Avian
PHYSIOLOGY, ENDOCRINOLOGY, AND REPRODUCTION Research Note Energy sensing in developing chicken embryos and posthatch chicks from different size eggs Q. Hu, U. Agarwal, and B. J. Bequette 1 Animal and Avian
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL 0.4 micron for Overall Exosome Isolation (Cell Media) NBP2-49826 For research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 303.730.1950 - P:
PRODUCT INFORMATION & MANUAL 0.4 micron for Overall Exosome Isolation (Cell Media) NBP2-49826 For research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 303.730.1950 - P:
CONTRACTING ORGANIZATION: University of California, San Diego La Jolla, CA
 AWARD NUMBER: W81XWH-13-1-0055 TITLE: Crosstalk between mtorc1 and camp Signaling PRINCIPAL INVESTIGATOR: Kun-Liang Guan CONTRACTING ORGANIZATION: University of California, San Diego La Jolla, CA 92093-0621
AWARD NUMBER: W81XWH-13-1-0055 TITLE: Crosstalk between mtorc1 and camp Signaling PRINCIPAL INVESTIGATOR: Kun-Liang Guan CONTRACTING ORGANIZATION: University of California, San Diego La Jolla, CA 92093-0621
Requires Signaling though Akt2 Independent of the. Transcription Factors FoxA2, FoxO1, and SREBP1c
 Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
Cell Metabolism, Volume 14 Supplemental Information Postprandial Hepatic Lipid Metabolism Requires Signaling though Akt2 Independent of the Transcription Factors FoxA2, FoxO1, and SREBP1c Min Wan, Karla
2402 : Anatomy/Physiology
 Dr. Chris Doumen Lecture 2 2402 : Anatomy/Physiology The Endocrine System G proteins and Adenylate Cyclase /camp TextBook Readings Pages 405 and 599 through 603. Make use of the figures in your textbook
Dr. Chris Doumen Lecture 2 2402 : Anatomy/Physiology The Endocrine System G proteins and Adenylate Cyclase /camp TextBook Readings Pages 405 and 599 through 603. Make use of the figures in your textbook
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein
 Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
1. Materials and Methods 1.1 Animals experiments process The experiments were approved by the Institution Animal Ethics Committee of Jilin University
 1. Materials and Methods 1.1 Animals experiments process The experiments were approved by the Institution Animal Ethics Committee of Jilin University (Reference NO. 2015-003). 96 Kunming (KM) mice (8 weeks;
1. Materials and Methods 1.1 Animals experiments process The experiments were approved by the Institution Animal Ethics Committee of Jilin University (Reference NO. 2015-003). 96 Kunming (KM) mice (8 weeks;
Human IL-2. Pre-Coated ELISA Kit
 Human IL-2 (Interleukin 2) Pre-Coated ELISA Kit Catalog No: 90-2083 1 96 well Format (96 tests) Detection Range: 31.2 2000 pg/ml Sensitivity: < 18.75 pg/ml This immunoassay kit allows for the in vitro
Human IL-2 (Interleukin 2) Pre-Coated ELISA Kit Catalog No: 90-2083 1 96 well Format (96 tests) Detection Range: 31.2 2000 pg/ml Sensitivity: < 18.75 pg/ml This immunoassay kit allows for the in vitro
Growth and Differentiation Phosphorylation Sampler Kit
 Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
DISRUPTION OF BRANCHED-CHAIN AMINO ACID CATABOLISM IMPAIRS RAT MYOBLAST SURVIVAL AND DIFFERENTIATION ZAMEER N. DHANANI
 DISRUPTION OF BRANCHED-CHAIN AMINO ACID CATABOLISM IMPAIRS RAT MYOBLAST SURVIVAL AND DIFFERENTIATION ZAMEER N. DHANANI A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the
DISRUPTION OF BRANCHED-CHAIN AMINO ACID CATABOLISM IMPAIRS RAT MYOBLAST SURVIVAL AND DIFFERENTIATION ZAMEER N. DHANANI A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the
Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
 Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Catalog Number KA1538 48 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use...
Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Catalog Number KA1538 48 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use...
REGULATION OF ENZYME ACTIVITY. Medical Biochemistry, Lecture 25
 REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
Effec<ve Use of PI3K and MEK Inhibitors to Treat Mutant K Ras G12D and PIK3CA H1047R Murine Lung Cancers
 Effec
Effec
TITLE: Overcoming Resistance to Inhibitors of the Akt Protein Kinase by Modulation of the Pim Kinase Pathway
 AWARD NUMBER: W81XWH-12-1-0560 TITLE: Overcoming Resistance to Inhibitors of the Akt Protein Kinase by Modulation of the Pim Kinase Pathway PRINCIPAL INVESTIGATOR: Andrew S. Kraft, MD CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-12-1-0560 TITLE: Overcoming Resistance to Inhibitors of the Akt Protein Kinase by Modulation of the Pim Kinase Pathway PRINCIPAL INVESTIGATOR: Andrew S. Kraft, MD CONTRACTING ORGANIZATION:
A Dissertation. entitled. The Impact of FoxO1 on Skeletal Muscle Protein Synthesis. Rachael A. Potter
 A Dissertation entitled The Impact of FoxO1 on Skeletal Muscle Protein Synthesis by Rachael A. Potter Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy
A Dissertation entitled The Impact of FoxO1 on Skeletal Muscle Protein Synthesis by Rachael A. Potter Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy
OxiSelect Malondialdehyde (MDA) Immunoblot Kit
 Product Manual OxiSelect Malondialdehyde (MDA) Immunoblot Kit Catalog Number STA- 331 10 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipid peroxidation is a well-defined
Product Manual OxiSelect Malondialdehyde (MDA) Immunoblot Kit Catalog Number STA- 331 10 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipid peroxidation is a well-defined
Optimizing Protein in a Carbohydrate World
 Optimizing Protein in a Carbohydrate World Donald K. Layman, Ph.D. Professor Emeritus Department of Food Science & Human Nutrition University of Illinois at Urbana Champaign The confused consumer 1 Myth:
Optimizing Protein in a Carbohydrate World Donald K. Layman, Ph.D. Professor Emeritus Department of Food Science & Human Nutrition University of Illinois at Urbana Champaign The confused consumer 1 Myth:
Amersham ECL Prime Western blotting reagent
 GE Healthcare Life Sciences Data file 28-9857-23 AA Western blotting reagents Amersham ECL Prime Western blotting reagent Since its introduction in 199, the enhanced chemiluminescence (ECL) Western Blotting
GE Healthcare Life Sciences Data file 28-9857-23 AA Western blotting reagents Amersham ECL Prime Western blotting reagent Since its introduction in 199, the enhanced chemiluminescence (ECL) Western Blotting
Supplementary Material for
 Supplementary Material for Parathyroid Hormone Signaling through Low-density-lipoprotein-related Protein 6 Mei Wan, Chaozhe Yang, Jun Li, Xiangwei Wu, Hongling Yuan, Hairong Ma, Xi He, Shuyi Nie, Chenbei
Supplementary Material for Parathyroid Hormone Signaling through Low-density-lipoprotein-related Protein 6 Mei Wan, Chaozhe Yang, Jun Li, Xiangwei Wu, Hongling Yuan, Hairong Ma, Xi He, Shuyi Nie, Chenbei
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Table S1. Primers and fluorescent probes used for qrt-pcr analysis of relative expression levels of PPP family phosphatases. gene name forward primer, 5-3 probe, 5-3 reverse primer,
SUPPLEMENTARY MATERIAL Table S1. Primers and fluorescent probes used for qrt-pcr analysis of relative expression levels of PPP family phosphatases. gene name forward primer, 5-3 probe, 5-3 reverse primer,
Convergent and Divergent Mechanisms in Aging and Cancer
 Convergent and Divergent Mechanisms in Aging and Cancer Mariana S. De Lorenzo, PhD Department of Cell Biology & Molecular Medicine delorems@umdnj.edu LEARNING OBJECTIVES 1. To identify convergent and divergent
Convergent and Divergent Mechanisms in Aging and Cancer Mariana S. De Lorenzo, PhD Department of Cell Biology & Molecular Medicine delorems@umdnj.edu LEARNING OBJECTIVES 1. To identify convergent and divergent
Introduction! Introduction! Introduction! Chem Lecture 10 Signal Transduction & Sensory Systems Part 2
 Chem 452 - Lecture 10 Signal Transduction & Sensory Systems Part 2 Questions of the Day: How does the hormone insulin trigger the uptake of glucose in the cells that it targets. Introduction! Signal transduction
Chem 452 - Lecture 10 Signal Transduction & Sensory Systems Part 2 Questions of the Day: How does the hormone insulin trigger the uptake of glucose in the cells that it targets. Introduction! Signal transduction
For the rapid, sensitive and accurate quantification of Ras in various samples
 ab128504 Ras Assay Kit Instructions for Use For the rapid, sensitive and accurate quantification of Ras in various samples This product is for research use only and is not intended for diagnostic use.
ab128504 Ras Assay Kit Instructions for Use For the rapid, sensitive and accurate quantification of Ras in various samples This product is for research use only and is not intended for diagnostic use.
Western Immunoblotting Preparation of Samples:
 Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Chapter 9. Cellular Signaling
 Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Eukaryotic Initiation Factor 2 Phosphorylation and Translational Control in Metabolism 1,2
 Thematic Review Series: Nutrient Control of Metabolism and Cell Signaling Eukaryotic Initiation Factor 2 Phosphorylation and Translational Control in Metabolism 1,2 Thomas D. Baird and Ronald C. Wek* Department
Thematic Review Series: Nutrient Control of Metabolism and Cell Signaling Eukaryotic Initiation Factor 2 Phosphorylation and Translational Control in Metabolism 1,2 Thomas D. Baird and Ronald C. Wek* Department
RalA ACTIVATION ASSAY BIOCHEM KIT
 RalA ACTIVATION ASSAY BIOCHEM KIT Cat. # BK040 ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 Technical assistance: (303) - 322-2254 World Wide Web: Write to
RalA ACTIVATION ASSAY BIOCHEM KIT Cat. # BK040 ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 Technical assistance: (303) - 322-2254 World Wide Web: Write to
Signal Transduction Cascades
 Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Insulin mrna to Protein Kit
 Insulin mrna to Protein Kit A 3DMD Paper BioInformatics and Mini-Toober Folding Activity Student Handout www.3dmoleculardesigns.com Insulin mrna to Protein Kit Contents Becoming Familiar with the Data...
Insulin mrna to Protein Kit A 3DMD Paper BioInformatics and Mini-Toober Folding Activity Student Handout www.3dmoleculardesigns.com Insulin mrna to Protein Kit Contents Becoming Familiar with the Data...
PRMT BIOLOGY DURING SKELETAL MUSCLE DISUSE
 PRMT BIOLOGY DURING SKELETAL MUSCLE DISUSE PROTEIN ARGININE METHYLTRANSFERASE EXPRESSION, LOCALIZATION, AND ACTIVITY DURING DISUSE-INDUCED SKELETAL MUSCLE PLASTICITY By DEREK W. STOUTH, B.Sc. Kin Honours
PRMT BIOLOGY DURING SKELETAL MUSCLE DISUSE PROTEIN ARGININE METHYLTRANSFERASE EXPRESSION, LOCALIZATION, AND ACTIVITY DURING DISUSE-INDUCED SKELETAL MUSCLE PLASTICITY By DEREK W. STOUTH, B.Sc. Kin Honours
EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
 EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
Over-expression of MKP-3 and knockdown of MKP-3 and FOXO1 in primary rat. day, cells were transduced with adenoviruses expressing GFP, MKP-3 or shgfp,
 SUPPLEMENTAL METHODS Over-expression of MKP-3 and knockdown of MKP-3 and FOXO1 in primary rat hepatocytes Primary rat hepatocytes were seeded as described in experimental procedures. The next day, cells
SUPPLEMENTAL METHODS Over-expression of MKP-3 and knockdown of MKP-3 and FOXO1 in primary rat hepatocytes Primary rat hepatocytes were seeded as described in experimental procedures. The next day, cells
Enzymes Part III: regulation II. Dr. Mamoun Ahram Summer, 2017
 Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Lecture #27 Lecturer A. N. Koval
 Lecture #27 Lecturer A. N. Koval Hormones Transduce Signals to Affect Homeostatic Mechanisms Koval A. (C), 2011 2 Lipophilic hormones Classifying hormones into hydrophilic and lipophilic molecules indicates
Lecture #27 Lecturer A. N. Koval Hormones Transduce Signals to Affect Homeostatic Mechanisms Koval A. (C), 2011 2 Lipophilic hormones Classifying hormones into hydrophilic and lipophilic molecules indicates
regulation by amino acids
 Novel modes of mtorc1 regulation by amino acids The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Buel, Gwen. 2016. Novel
Novel modes of mtorc1 regulation by amino acids The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Buel, Gwen. 2016. Novel
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates
 HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
Eukaryotic transcription (III)
 Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Protein Dephosphorylation Methods
 Protein Dephosphorylation Methods Phosphospecific antibodies are designed to differentiate between the phosphorylated and the non-phosphorylated states of a protein. The method to determine if or how well
Protein Dephosphorylation Methods Phosphospecific antibodies are designed to differentiate between the phosphorylated and the non-phosphorylated states of a protein. The method to determine if or how well
STRATEGIES TO IMPROVE SKELETAL MUSCLE PROTEIN TURNOVER DURING DIETARY ENERGY RESTRICTION
 STRATEGIES TO IMPROVE SKELETAL MUSCLE PROTEIN TURNOVER DURING DIETARY ENERGY RESTRICTION PROTEIN AND RESISTANCE EXERCISE STRATEGIES TO IMPROVE SKELETAL MUSCLE PROTEIN TURNOVER DURING DIETARY ENERGY RESTRICTION
STRATEGIES TO IMPROVE SKELETAL MUSCLE PROTEIN TURNOVER DURING DIETARY ENERGY RESTRICTION PROTEIN AND RESISTANCE EXERCISE STRATEGIES TO IMPROVE SKELETAL MUSCLE PROTEIN TURNOVER DURING DIETARY ENERGY RESTRICTION
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
THE IMPACT OF MTOR, TFEB AND BID ON NON-ALCOHOLIC FATTY LIVER DISEASE AND METABOLIC SYNDROME
 THE IMPACT OF MTOR, TFEB AND BID ON NON-ALCOHOLIC FATTY LIVER DISEASE AND METABOLIC SYNDROME Hao Zhang Submitted to the faculty of the University Graduate School in partial fulfillment of the requirements
THE IMPACT OF MTOR, TFEB AND BID ON NON-ALCOHOLIC FATTY LIVER DISEASE AND METABOLIC SYNDROME Hao Zhang Submitted to the faculty of the University Graduate School in partial fulfillment of the requirements
Chem Lecture 10 Signal Transduction
 Chem 452 - Lecture 10 Signal Transduction 111130 Here we look at the movement of a signal from the outside of a cell to its inside, where it elicits changes within the cell. These changes are usually mediated
Chem 452 - Lecture 10 Signal Transduction 111130 Here we look at the movement of a signal from the outside of a cell to its inside, where it elicits changes within the cell. These changes are usually mediated
Post-translational modifications of proteins in gene regulation under hypoxic conditions
 203 Review Article Post-translational modifications of proteins in gene regulation under hypoxic conditions 1, 2) Olga S. Safronova 1) Department of Cellular Physiological Chemistry, Tokyo Medical and
203 Review Article Post-translational modifications of proteins in gene regulation under hypoxic conditions 1, 2) Olga S. Safronova 1) Department of Cellular Physiological Chemistry, Tokyo Medical and
Western Blot Analysis of Rat Pituitar Recognized by Human Antipituitary. y Antigens A. antibodies
 Endocrine Journal 1995, 42(1), 115-119 NOTE Western Blot Analysis of Rat Pituitar Recognized by Human Antipituitary y Antigens A ntibodies SHIGEKI YABE, MASAMI MURAKAMI*, KAYOKO MARUYAMA, HIDEKO MIWA,
Endocrine Journal 1995, 42(1), 115-119 NOTE Western Blot Analysis of Rat Pituitar Recognized by Human Antipituitary y Antigens A ntibodies SHIGEKI YABE, MASAMI MURAKAMI*, KAYOKO MARUYAMA, HIDEKO MIWA,
THE IMPACT OF INSULIN DYSREGULATION ON PROTEIN METABOLISM IN HORSES
 University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2018 THE IMPACT OF INSULIN DYSREGULATION ON PROTEIN METABOLISM IN HORSES Caroline Margot Marcelle
University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2018 THE IMPACT OF INSULIN DYSREGULATION ON PROTEIN METABOLISM IN HORSES Caroline Margot Marcelle
