Representation of the Body Surface in Somatic Koniocortex in the Prosimian Galago
|
|
|
- Lindsey Flowers
- 5 years ago
- Views:
Transcription
1 JOURNAL OF COMPARATIVE NEUROLOGY 189: (1980) Representation of the Body Surface in Somatic Koniocortex in the Prosimian Galago MRIGANKA SUR, R.J. NELSON, AND J.H. KAAS Departments of Psychology (M.S., J.H.U.) and Anatomy fr.jn., J.H.K.), Vandcrbilt University, Nashville, Tennessee ABSTRACT Microelectrode mapping methods were used to determine the organization of somatosensory cortex in galagos, a prosimian primate. A systematic representation of the controlateral body surface was found within somatic koniocortex with an organization comparable to that of Area 3b ofparietal cortex of monkeys (S-I proper) and primary somatosensory cortex (S-I) of other mammals. Limited studies of the response properties of single neurons within the representation revealed further similarities with the 3b field of monkeys. We conclude that somatic koniocortex of galagos and Area 3b of monkeys are homologous, and suggest the term S-I proper for the representation in both prosimians and monkeys. Differences in the details of S-I proper in galagos and monkeys suggest a more primitive organization in galagos. It is generally recognized that somatosensory cortex of monkeys consists of four distinct architectonic zones: Areas 3a, 3b, 1, and 2. Until recently, these four Areas have been considered to be a single representation of the body and a single functional division of cortex. Microelectrode mapping studies on owl, squirrel and macaque monkeys (Paul et al., 72; Kaas et al., 76, 79 Merzenich et al., 78; Nelson et al., 78b; Sur et al., 78b) have shown that each of Areas 3b and 1 contain a separate and complete map of the body surface, have revealed much of a third representation of deep and cutaneous receptors in Area 2, and have provided evidence for a fourth representation of predominantly deep receptor input in Area 3a. The conclusion of four separate representations within primary somatosensory cortex or S-I of monkeys is compatible with single unit studies showing a differential distribution of receptor types in the four architectonic zones, anatomical studies of the patterns of connections of each of the zones, and the behavioral deficits following lesions in separate zones (for review see Merzenich et al., 78). While multiple representations have been found in S-I of monkeys, only a single representation has been found in the S-I region of other mammals (for review see Sur et al., 78a). The possibility of major differences in the organization of parietal cortex of monkeys and various non-primates suggested that we should investigate the organization of parietal cortex in prosimian primates to see if they reflect the pattern found in non-primates or that in monkeys. However, establishing species differences is a difficult problem, and it might be first useful to simply review the contributions that might result from mapping studies of somatosensory cortex. Perhaps the most important contribution of mapping studies is that they can be a critical first step in establishing the subdivisions of the brain of functional significance. Comparative architectonic studies have long suggested that the cortex of mammals is divided into a number of functionally distinct areas, but architectonic studies by themselves cannot establish the significance of perceived distinctions, and opinions on how to divide the cortex have varied greatly. However, careful microelectrode studies can clearly identify systematic sensory representations, and these representations can then be related to cortical architecture, patterns of connections, properties of neurons, and the behavioral effects of restricted lesions. The evidence to date overwhelmingly supports the view that each sensory representation is distinct in architecture, connections, overall neural response properties, and role in behavior. A second contribution of mapping studies is that they can help identify homologous subdivisions of the brains of separate species with /80/ $ ALAN R. LISS, INC.
2 382 M. SUR, R.J. NELSON AND J.H. KAAS relative ease and a high degree of certainty. If two representations in similar locations in two brains resemble each other in a vast amount of detail, it is reasonable to hypothesize that this detail reflects inheritance from a common ancestor, and that the areas are homologous. This hypothesis can then be supported by other similarities revealed by other types of investigation. For example, the similarities between S-I of the grey squirrel (Sur et al., 78a) and S-I proper (Area 3b) of monkeys (Merzenich et al., 78) led to the conclusion that the two representations are homologous. This contention is supported by similarities in relative position, connections with other parts of the brain, and cortical architecture (see Merzenich et al., 78), and it is open to further test. A third and related contribution of mapping studies is that by defining homologous subdivisions that are responsive to sensory stimuli, a start can be made towards resolving the intriguing issue of how or if species differ in number of cortical areas. It is tempting to suppose that behaviorally advanced mammals have more cortical subdivisions than primitive mammals, but it can be difficult to establish the absence or presence of any cortical subdivision. While mapping studies are limited to cortex responsive to sensory stimuli, the identity of poorly responsive and unresponsive areas bordering sensory representations can subsequently be established by less powerful methods once the problem of identification is restricted. In parietal cortex of the grey squirrel (Sur et al., 78a), for example, the identification of only one subdivision, S-I, with certainty, does not necessarily mean that the other subdivisions of somatosensory cortex of monkeys do not exist in squirrels, but it does clearly limit the quest. Specifically, there is a suggestion of a narrow intermediate architectonic zone between somatic koniocortex (S-I) and the presumptive motor region. Anatomical, electrophysiological, and other approaches can now be directed toward the very limited question of whether or not cortex on the rostral border of S-I contains the homologue of Area 3a in monkeys. Likewise, these approaches can be used to determine if the %on-responsive cortex immediately on the caudal border of S-I in squirrels is the probable homologue of the Area 1 (posterior cutaneous field) representation, or some other subdivision of cortex. Thus, mapping studies can be a critical step in attempts to identify species differences in numbers of areas. The parietal cortex of prosimians invites all these applications of the mapping method. We were first interested in establishing systematic representations of the body and relating these to cortical architecture on the assumption that such representations would be the functionally important subdivisions of parietal cortex. Secondly, it seemed possible that any or all of the representations of monkeys could be identified in prosimians by the detailed organization of the representations. Brodmann ( 09) described only an Area 1 in prosimians so one could argue that the Areas 3a, 3b and 2 representations might be missing. However, Sanides and Krishnamurti ( 67) identified the same subdivision of cortex as somatic koniocortex and as the homologue of Area 3b in monkeys. They also considered a narrow bordering zone of cortex rostral to koniocortex as an intermediate sensori-motor area homologous to Area 3a. They were uncertain about the cortex caudal to koniocortex, although the possible presence of Areas 1 or 2 or both were discussed. Because we are greatly interested in identifying homologous areas in prosimians and simians, and in attempting to determine if differences in number of cortical areas relate to the behavioral advances seen in simians, we investigated the organization of somatosensory cortex with microelectrode mapping methods in two species of galagos, and related our results to the results of a similar study on the slow loris (Krishnamurti et al., 76), and a more limited mapping study of the hand representation in galagos (Carlson and Welt, 77). We conclude that the principal responsive zone, somatic koniocortex, is the homologue of Area 3b or S-I proper of monkeys. Furthermore, there is evidence for bordering zones rostral and caudal to the S-I representation that require more intense stimuli for activation than cells or cell groups in S-I. Some of these results have been brefly described elsewhere (Nelson et al., 78a). METHODS The organization of anterior parietal cortex was determined by microelectrode mapping experiments conducted on 10 adult galagos (8 Galago senegalensis and 2 Galago crassicaudatus). The mapping results were later related to cortical architecture by examining sections from the experimental brains in which small electrolytic lesions had been made to mark significant recording sites, especially at the borders of cortex responsive to very light cutaneous stimulation. The procedures were basically the same as in our earlier studies (Merzenich et
3 BODY SURFACE IN SOMATIC KONIOCORTEX IN GALAW 383 al., '78; Sur et al., '78a) and will only be briefly stated here. Galagos were anesthetized with ketamine hydrochloride (50mg/kg, ZM) and maintained with supplementary injections at a surgical level of anesthesia throughout recording. Parietal and adjoining cortex was exposed and protected by a pool of silicone fluid retained in an acrylic dam constructed around the skull opening. Penetrations were made with glasscoated platinum-iridium microelectrodes with relatively low impedances so that recordings were most commonly from several neurons rather than a single neuron. In mapping studies, data from a large amount of cortex has to be collected in a relatively short time, requiring a large number of penetrations per animal. Multi-unit electrodes facilitate data collection because responses can be obtained immediately in each penetration in the vicinity of layer IV, rather than having to search for maximum amplitude spikes as is often the case with single-unit electrodes. The surface vasculature of the exposed brain and electrode penetrations were observed with a dissecting microscope and penetrations were sited on a high resolution photograph of parietal cortex. Minimum cutaneous receptive fields were defined with light tactile stimulation with fine glass probes, and responses to other stimuli such as intense taps, pressure and joint movement were noted. Receptive field locations were outlined on drawings of galago body parts. Penetrations were usually made 20@300 p apart. On the medial wall, receptive fields were recorded every 25@300 p in depth penetrations. These data were reconstructed by "unfolding" such penetrations. Almost the entire body surface representation within s-i was mapped in two animals. In all other animals, representations of one or more body parts were mapped completely. Our results are based on 1929 recording sites in the 10 mapped animals. Single unit experiments were conducted on two galagos (Galago senegalensis). Controlled stimuli were provided with an electromechanical stimulator (Chubbuck, '66). A PDP-8/a minicomputer was used for data collection and analysis. After recording, the animals were perhsed with saline followed by 10% formalin. The brain was later sectioned on a freezing microtome in the sagittal plane at 50 p. Sections were stained with cresylviolet for cell bodies, or with hematoxylin (Lin and Kaas, '77) for fibers. Experimetal brains were reconstructed from these sections so that mapping results could be related to cortical cytoarchitecture with the aid of the marker lesions. Almost all lesions in all brains were identified later. RESULTS Most of the results are from the smaller galago species, Galago senegalensis and unless otherwise stated, results are reported for Galago senegalensis. However, more limited experiments on the larger Galago crassicaudatus revealed no significant differences in the organization of somatosensory cortex in the two prosimians. The major result is that there is a single systematic representation of the body surface within somatic koniocortex in galagos. Because of its similar somatotopic organization, this field is judged to be homologous to the 3b field (S-I proper) of monkeys. Therefore, the term S-I proper (Merzenich et al., '78) will be used to refer to the representation. Location and overall organization of S-I proper The location of S-I proper on the cerebral hemisphere is shown in Figure 1, and the overall organization of the representation is shown in Figure 2. The representation is just rostral to the caudal tip of the Sylvian fissure and extends from the medial wall to near the rhinal fissure. Parts of the representation on the medial wall were not explored because of limited accessibility, and the most lateral boundary of the representation was not determined rostrally because of difficulties in stimulating within the oral cavity, and caudally because the representation entered the Sylvian fissure. However, the representation as mapped is nearly complete and the medial and lateral ends of the summary diagram probably are very close to the actual borders of the area. Many features of the overall organization of S-I proper are apparent in Figure 2. These features are comparable to S-I proper of monkeys (Merzenich et al., '78; Sur et al., '78b; Nelson et al., '78b; Kaas et al., '79). Thegenitalia, tail and posterior part of the leg are represented on the medial wall, along with the lateral edge of the glabrous foot (parts of digits and the hypothenar pad). The rest of the foot occupies the most medial cortex of the dorsal surface. The glabrous digit tips of the toes are most rostral in this region, followed by the rest of the glabrous digits, the foot pads, and the hairy heel. (The sole of the hind foot of galagos consists of the glabrous pads rostrally and a caudal hairy heel.) The dorsum of the toes and foot is lateral to the glabrous foot representation, as is much
4 384 M. SUR, R.J. NELSON AND J.H. KAAS Fig. 1 The location ofthe body surface representation, S-I proper, on a dorsolateral view ofthe brainofagalago. See Figure 2 for details. of the leg. The trunk is represented with the dorsum rostral and the ventrum caudal, as it is in Area 3b in owl and macaque monkeys. Most of the arm, forearm and wrist falls between the trunk and hand. Like the foot, the glabrous digit tips of the hand (as well as skin extending somewhat onto the dorsum of the digit tips) are most rostral in the representation, followed caudally by the rest of the glabrous digits, the pads of the palm and then the dorsal surfaces of the digits. This location of the dorsal digits differs from that in Area 3b in monkeys where the comparable skin surface is split laterally and medially to the glabrous digits in the representations. Another difference is that the dorsoradial edges of the wrist, forearm and arm are lateral to the hand in galagos, but not in monkeys. More laterally, the chin and neck representations are followed by the lower and upper lip regions and the oral cavity rostrally. The nose and orbital skin is caudal and lateral extending somewhat into the Sylvian fissure. Receptive fields for selected recording sites from extensive experiments in single galagos will be used to illustrate conclusions. These conclusions are based on consistencies in organization revealed by many more recording sites. In addition, observations made in one animal were repeated in two or more other animals. Representation of the head The locations of electrode penetrations for receptive fields on the neck, the chin, the upper and lower lips, the nose and the orbital skin are shown in Figure 3. The rostral to caudal row of recording sites, A through F, illustrates receptive fields that start out on the ventral midline of the chin, move laterally and caudally on the chin and then dorsally on the neck to end near the dorsal midline of the neck. The representation of the lower and upper lips is illustrated by recording sites Sites at the rostral margin of the lower lip representation have receptive fields on the midline of the lower lip. As recording sites move caudally on cortex, receptive fields move laterally on the lower lip with the corner of the mouth being represented at the border of the lower and upper lip representations (penetration 6). Receptive fields then move from lateral to medial on the upper lip, with the midline of the upper lip being represented at the caudal margin of the representation (penetration 10).
5 BODY SURFACE IN SOMATIC KONIOCORTEX IN GALAW mrn Fig. 2 A summary of the somatotopic organization of S-I proper of the galago. The digits (D,-DJ of the foot (upper) and hand (lower) and the interdigital pads (P,-P,) are numbered in the standard manner (see Merzenich et al., '78). Thenar (PTh) and hypothenar (P,) pads are also indicated. The representations of the dorsal hairy surfaces of the digits are shaded. DR, dorso-radial. See Figures 3-8 for documentation. The cheek and the proximal facial skin are represented between the neck and the upper lip representations. Receptive fields here are large and often extend onto the neck and the glabrous upper lip. Penetrations in Figure 3 illustrate the representation of the nose and orbital skin. Distal parts of the nose are represented medially and rostrally within the nose representation while the bridge of the nose is represented laterally and caudally, adjacent to the representation of the orbital skin. In two experiments, the cranial skin was found represented further laterally, bordering cortex judged to be S-I1 (the second somatosensory area). Experiments indicate that S-I1 lies largely caudal and lateral to the cranial and orbital skin representation in S-I and abuts S-I along this common border of skin surface, in a similar manner to that in the squirrel (Sur et al., '77, Nelson et al., '79a). Representation of the wrist, forearm and upper arm One of the ways in which the S-I proper of galagos differs from that in monkeys is that in galagos, a narrow strip of dorso-radial forelimb is represented lateral to the hand, between the hand and head representations, while the rest of the forelimb is represented medial to the hand, between the hand and trunk representations. In monkeys, the entire forelimb representation lies medial to the hand, and the head is represented immediately lateral to the hand. This conclusion is supported by the recording sites and receptive fields on the wrist, forearm and arm from one mapped galago which are illustrated in Figure 4. The dorso-radial wrist and forearm lie immediately lateral to the hand representation (Figure 4, left; points 2 and 5). The dorsal radial hand lies adjacent to the dorsum of digit 1 (point 1). The dorso-radial upper arm lies lateral within the strip (points 3 and 61, adjacent to the chin and neck representation (point 4). Receptive fields 1-4 show a narrow zone of tissue representing a region of skin that bridges what would otherwise be a major discontinuity between the hand and head representations. The main representation of the forelimb is medial to the hand. In this division of S-I proper, rostral to caudal rows of recording sites result in simple progressions of receptive fields around the circumference of the wrist, forearm and arm. Two such progressions are shown in Figure 4 (right). The receptive fields start on the dorsum of the arm, proceed to the ulnar side and then progress radially across the ventrum. The wrist and forearm are most laterally located in the representation, while the upper arm is most medial. Representation of the hand As in Area 3b in monkeys, the glabrous digits are successively represented, thumb to little finger, from lateral to medial in S-I proper of galagos. Likewise, the distal digits are rostral to the proximal digits, and the volar pads are caudal to the digits in both groups of primates (Fig. 5). In galagos, the glabrous ulnar aspect of the hand, including pad 4 and the hypothenar pad, is represented medial to the representation of digit 5 (Fig. 5). Pad 4 is central, the hypothenar
6 386 M. SUR, R.J. NELSON AND J.H. KAAS Galago Fig. 3 Receptive field for recording sites in the head and neck regions of S-I proper. Note that the midline of the upper lip and the dorsal neck are at the caudal extreme while the midline of the lower lip and chin are at the rostral extreme of responsive cortex A pad rostral, and the ulnar margin of the hand caudal within the representation, The radial dorsum of the hand is represented largely lateral to the dorsum of digit 1 (point 1, Figure 4, left) while the central and ulnar dorsum of the hand is represented medial to the hypothenar pad (points 1 and 2, Figure 4, right). The hand representation in galagos has several distinct particularities. First, receptive fields at the rostral margin of the representation are found to be on the glabrous dorsum, around the nail bed of the distal phalanx of the digits. Second, the hairy dorsum of each digit is represented caudal to the digit s glabrous skin representation. Thus, rostral to caudal progressions of recording sites within a digit rep- resentation lead to shifts of receptive field from distal to proximal along a digit ventrum and from proximal to distal on the digit dorsum. For digits 1,2 and 3, the adjoining representation of pads 1-3 lead to a largely continuous transition from the representation of the ventrum to that of the dorsum. These pads at the bases of digits 1,2 and 3 are fairly large and protrude between the digits (Fig. 5). Receptive fields on these pads, for recording sites caudal to the proximal digits, are large and include both ventral and dorsal aspects of the glabrous pads (sites 5 each for rows D, and DJ. Receptive fields for recording sites further caudal in the representation move from the dorsal pads to the proximal digit dorsums. For digits 4 and 5, the
7 Fig. 4 Receptive fields for recording sites in the separate medial and lateral forelimb and limb representations in S-I proper. The lateral representation of the forelimb (left) includes only the dorsoradial skin and serves to join the hand and head representations. Most of the forelimb is represented medial to the hand (right). Note that the wrist and forearm adjoin the hand representation in both representations.
8 388 M. SUR, R.J. NELSON AND J.H. KAAS transition is from the ventral proximal phalanx to the adjoining dorsal proximal phalanx (Fig. 5, row D5). A corollary to the fact that the glabrous dorsum of the distal phalanx of each digit is represented rostrally while the hairy dorsum of the proximal and middle phalanges is represented caudally in the hand representation is that adjacent receptive fields on the dorsum of the distal and middle phalanges are found at opposite ends of the representation. Such is the case with fields 1 and 7 in row D,, and fields 1 and 8 in row D,. Representation of the trunk The dorsal surface of the trunk is represented rostrally and the ventral surface caudally within the trunk representation. A typical row of recording sites in Figure 6 illustrates the progression of receptive fields from the dorsal midline of the back to the ventral midline of the rib-cage in a rostral to caudal sequence on cortex. Rostra1 parts of the trunk are represented laterally on cortex, adjacent to the arm representation, while caudal parts are represented medially, adjacent to the representation of the leg. Representation of the leg The representation of the trunk merges medially with the representation of the hindlimb, which is wrapped around the representation of the foot (Fig. 7). Most of the upper part of the leg that adjoins the trunk is found laterally in the leg region and receptive fields extend from the leg onto the trunk so that the dividing line, in Figure 7, indicates only where receptive fields are mainly on the leg rather than on the trunk. Both the dorsal midline of the trunk, and the lateral proximal leg are represented rostrally thus preserving the continuity of the trunk and the leg in the cortical map. Likewise, the skin under the proximal inner white fur of the leg is represented caudally, adjacent to the representation of the abdomen. Most of the lateral, anterior and medial surfaces of the thigh, knee and shin are represented lateral to the foot. Within this portion of cortex, medial recording sites adjacent to the foot representation have receptive fields on the distal leg while preserving the basic lateral to medial progression of receptive fields in rostral to caudal recording sites. The posterior leg is represented medial and somewhat caudal to the foot representation (receptive fields and recording sites 1G22, Fig. 7). Thus, the hindlimb representation is split around the foot representation. Recording sites lateral and medial to the foot representation correspond to adjacent receptive fields on the leg (e.g., points 8 and 21 or 18; 10 and 19). While there were only a few recording sites in cortex on the medial wall, these recording sites had receptive fields on the genitalia, gluteal region and tail. We propose that the medial representation of the posterior thigh serves to bridge an otherwise major discontinuity between the representations of the glabrous foot and extreme caudal body regions. Representation of the foot The basic organization of the cortex representing the foot is illustrated in Figure 8. The glabrous digits of the foot from the great toe or hallux to the little toe are represented in order from lateral to medial in the rostral half of the foot region. The digit tips are along the rostral border of S-I proper while more proximal parts of the digits are successively represented more caudally. Pads 1-4, which adjoin the digits of the foot, are represented in order from lateral to medial, and the thenar pad is lateral to the hypothenar pad. The hairy heel has a relatively large representation caudal to the pads. The dorsum of the foot is split off to a band of tissue lateral to the representations of digit 1, pad 1, and the thenar pad (Fig. 7 and 8). Individual and species variations in the S-I proper map As in all animals in which we have studied the organization of somatosensory cortex, there were minor differences in galagos in detail in the maps obtained from different individuals. For example, we can compare the hand representations in the two animals shown in Figures 4 and 5. The overall features of the two maps are very similar. However, the digit 5 representation in galago (Fig. 4) extends up to the rostral margin of S-I proper but it does not in galago (Fig. 5). The representation of the hypothenar pad is centrally located within the representation in galago and pad 4 is represented almost entirely between glabrous and hairy digit 5. In galago 77-53, the hypothenar pad is represented rostrally and pad 4 medial to digit 5 centrally within the representation. Similar differences in detail within the foot representation in two galagos can be seen from Figures 6 (galago 77-12) and 7 (galago 77-38). In the two experiments on greater galagos (Galago crassicaudatus) we concentrated on mapping the hand region in great detail. The features of the hand representation in these two Galago crassicaudatus were very similar to
9 HAND Fig. 5 Receptive fields for recording sites in cortex representing the digits of the hand in S-I proper. The hairless dorsum of the digit tips around the nail bed is most rostra1 followed by the distal to proximal glabrous digit surfaces followed by the proximal to distal hairy digit surfaces. Pads partially separate the representations of the hairy and glabrous surfaces of the digits. m x
10 390 M. SUR, R.J. NELSON AND J.H. KAAS Galago Fig, 6 Receptive fields for recording sites in the representation of the trunk in S-I proper. The dorsum is represented rostrally and the ventrum caudally. those found in Galago senegalensis. Differences in these maps and those obtained from senegalensis were comparable to those noted between individual maps for senegalensis. Thus, as in senegalensis, the glabrous digit tips and distal phalanges were located rostrally within S-I proper of crassicaudatus, the glabrous and hairy proximal phalanges were represented centrally, and the distal hairy dorsum of the phalanges were represented caudally. No
11 BODY SURFACE IN SOMATIC KONIOCORTEX IN GALAW 391 obvious species differences were noted in the hand representation in S-I proper of galagos. Response characteristics of neurons in S-I proper In the mapping studies, receptive fields were determined for small clusters of neurons or sometimes single neurons with light tactile stimuli. Neurons in all parts of S-I proper were sensitive to such cutaneous stimuli, and recording sites activated by only the stimulation of deep body tissues and joints were not found. Because of the sensitivity of neurons to light touch and hair movements, and because it was difficult to stimulate exclusively joints and deep body tissues, these studies did not determine if non-cutaneous receptor inputs activate neurons in S-I proper. However, limited quantitative studies of the responses of single neurons to tactile stimuli were made in two galagos to see if two obvious classes of neurons present in S-I proper of monkeys are also found in S-I proper of galagos. The responses of a total of 37 neurons to a steady skin indentation were carefully studied. An electromechanical stimulator (Chubbuck, '66) produced a one second skin indentation in the center of the receptive fields of the studied neurons. Two types of responses to skin indentation were observed. These same two have been found in S-I proper of macaque monkeys (Sur, '79). The two types of neurons are termed slowly adapting (SA) and rapidly adapting (RA) because these two types of cortical neurons appear to receive inputs from slowly adapting and rapidly adapting peripheral receptors, respectively, and because our use of the terminology appears to be consistent with that previously used for classifying neurons in somatosensory cortex of macaque monkeys (Powell and Mountcastle, '59; Mountcastle et al., '69; Paul et al., '72). Typical examples of the two types of responses of neurons in S-I proper of galagos are shown in Figure 9. Both types of neurons exhibited high rates of discharge during stimulus onset and usually during stimulus offset. The offset discharge was usually less than the onset discharge. Both types of neurons showed brief ( msec) periods of response suppression following peak discharge rates. However, the SA and the RA neurons differed in their responses to maintained skin indentations. The SA neurons fired at rates higher than the spontaneous rate during a maintained skin indentation while the RA neurons did not. As in macaque monkeys (Sur, '79), the SA neurons in galagos were much less numerous than the RA neurons, and the SA neurons were largely within the region of layer IV of cortex. Of the 37 cells carefully studied for adaptation properties, only 7 were classified as SA. The recording depths of five of these neurons were between 800 and 900 p, while one neuron was less deep (683 p) and one more deep (1201 p). The F U neurons were found over a wide range of depths of cortical recording, although the 30 carefully studied cells were obtained between the depths of 430 p and 1654 p. Responses outside S-I proper Responses within S-I proper were obtained by light tactile stimulation of clearly defined regions of the skin surface. At the first penetration outside S-I proper, either rostrally or caudally and usually within 250 p from the last penetration, cell groups could no longer be driven by cutaneous stimuli. Vigorous stroking and brisk taps were required on the appropriate body surface, which was always the same as that represented in adjacent S-I proper, to elicit a response. Such responses were usually weak and often barely discernible over the background. "Receptive fields" here were large and diffuse, often covering the entire body part. For example, if receptive fields within S-I proper were confined to portions of a digit, responses outside S-I proper would be obtained by moderate or hard taps over the entire hand. At levels corresponding to the arm, trunk and leg representations in S-I proper, responses outside S-I proper were often elicited by vigorous brushing of hairs on the trunk or limb. The rostro-caudal extent of these bordering zones from which weak responses were obtained was of the order of 0.5mm. The longest extent of the rostral bordering field, of all mapped galagos, was 0.75 mm. The longest extent of the caudal bordering field was 1.25 mm. Medio-laterally, these zones bordered all of S-I proper except at the very lateral extent. Architectonic characteristics of S-I proper In all experiments, small electrolytic lesions were made with the recording electrode at the rostral and caudal margins of the cortex responsive to light cutaneous stimulation (S-I proper). Such marking lesions are shown in Figure 10. These and other marking lesions clearly demonstrate that S-I proper is coextensive with an architectonic field, somatic koniocortex, as defined in prosimians by Sanides and Krishnamurti ('67). The architectonic features of S-I proper are the usual distinguishing features of konio-
12 392 M. SUR. R.J. NELSON AND J.H. KAAS I Trunk - 1 mm Galago Fig. 7 Receptive fields for recording sites in the representation of the leg in S-I proper. The posterior hindlimb is represented caudal and medial to the foot while the rest of the hindlimb is lateral and caudal to the foot. Open circles indicate the locations of recording sites on the medial wall of the cerebral hemisphere folded out to join the dorsolateral view. The recording sites were reached by electrode penetrations from the dorsal surface.
13 BODY SURFACE IN SOMATIC KONIOCORTEX IN GALAW 393 D4 Digits R Galago Fig. 8 Receptive fields for recording sites in the representation of the glabrous foot in S-I proper. Digit tips are rostra1 in the representation. cortex in general and somatic koniocortex in particular (Fig. 10). Layer IV is densely packed with granule cells, and layer VI shows increased packing density with respect to surrounding cortex. One feature of somatic koniocortex more noticeable in galagos than in Area 3b of monkeys is the presence of medium and occasionally large sized pyramidal cells in layer V. However, as noted by Sanides and Krishnamurti ('67) within somatic koniocortex in the slow loris, the number and size of pyramidal cells in layer V varies across the mediolateral extent of the field. As shown in Figure 10, pyramidal cells are more noticeable medially (Fig. 10A) than laterally (Fig. 1OC) in somatic koniocortex. Also, the size of granule
14 394 M. SUR, R.J. NELSON AND J.H. KAAS C SEC. 2.o o SEC. I 8 Fig. 9 Post-stimulus time histograms of the responses of two neurons in S-I proper. Above: Typical slowly adapting neuron (SA) isolated 683 p below the cortical surface with a receptive field on the distal phalanx of digit 3. Note a maintained discharge during skin indentation. Middle: Typical rapidly adapting neuron (RA) isolated 796 p below the cortical surface with a receptive field on the ulnar side of the distal phalanx of digit 3. Note the lack of a maintained discharge. Below: The stimulus waveform which was repeated for 50 cycles. A 1 mm diameter probe first indented skin for 1 sec and then was removed for 1 sec. Rise and fall times, 25.5 msec; indentation depth, 700 p; bin width, 25 msec.
15 BODY SURFACE IN SOMATIC KONIOCORTEX IN GALAW 395 cells in layer IV is not as different from the sizes of other cells as in Area 3b of monkeys. The result of these two aspects is that somatic koniocortex in galagos does not stand out as dramatically from the adjacent cortex as Area 3b of monkeys does. Rostrally, between koniocortex and motor cortex, a narrow zone of cortex with intermediate architectonic characteristics is often apparent. In this strip of cortex, approximately 1 mm wide or less, both layer IV and layer VI are less densely packed with cells than in koniocortex, while layer V has intermediate and large pyramidal cells that are not quite as dense as in motor cortex. Caudal to somatic koniocortex, layers Tv and VI are also less densely packed with neurons. Furthermore, layer I11 also has fewer cells and can be easily distinguished from layer Tv. Some medium sized pyramidal cells appear in layer 111. These bordering zones of cortex are probably included in the areas Ism and park as defined by Sanides and Krishnamurti ( 67) in the slow loris. Because we have not fully defined the extents of the bordering zones of cortex for somatic koniocortex in galagos, we are uncertain if the rostral and caudal bordering zones in galago fully correspond with areas Ism and park of Sanides and Krishnamurti ( 67). DISCUSSION The present study provides a detailed microelectrode map of a single systematic representation of the body surface in rostral parietal cortex of a prosimian, galago. This representation was found to be coextensive with somatic koniocortex, and single neuron recordings have revealed two classes of neurons also present in S-I proper (Area 3b) of monkeys. The results support the.view that somatic koniocortex in galagos is the homologue of Area 3b in monkeys, reveal a specialization of S-I proper in galagos, and suggest a theory of how the basic organization of S-I proper is specified. Topography of the body surface representation in S-I proper of galagos a. S-I proper is a composite of somatotopic regions. In previous discussions of the organization of S-I proper (Area 3b) of monkeys (Merzenich et al., 78; Kaas et al., 79) and of S-I in the grey squirrel (Sur et al., 78a), we have argued that the organizations of the representations are not adequately described by a homunculus or by a simple sequence or unfolding of dermatomes. Rather, the adjacent representations of non-adjacent body parts and the non-adjacent representations of adjacent body parts are, in our view, best described by considering the cortical map as a composite of somatotopic regions. This concept also applies well to S-I proper of galagos where regions of continuous somatotopic representation are joined to form a complete representation with many contained lines of discontinuity. The following are examples of this concept: (1) the glabrous surface of digit 1 of the hand is represented next to part of the forearm; (2) the hairy dorsum of at least digits 1-3 of the hand does not adjoin the glabrous surface of these digits, (3) adjoining parts of the arm are completely separated by the hand representation, and (4) the dorsum of all the digits of the foot is represented lateral to the glabrous surface representation of digit 1. b. S-I proper is more continuous in galagos than in monkeys. While the body surface map in S-I proper of galagos is a composite, it is less disconnected than in monkeys, i.e., there are fewer major discontinuities across the representations of adjacent body parts. Most notably (1) the dorsal hairy surface of the digits is represented in a continuous band caudal to the glabrous digits of the hand and medial to those of the foot in galagos (Fig. 2); in Area 3b of monkeys these hairy surfaces are split so that some of the hairy digits are lateral and some medial to the glabrous digits; (2) the narrow strip of wrist, forearm and upper arm joining digit 1 with the neck and chin as seen in galagos is not found in monkeys; (3) lateral and medial representations of parts of the leg are joined caudal to the representation of the foot in galagos but are separated in monkeys and; (4) in some monkeys part of the head is represented medial to the hand while other parts are represented lateral to the hand ( the splitting of the occiput of classical descriptions) while this is not seen in galagos. It seems likely that topological organization in somatic representations has been sacrificed in various ways as lines of descent evolve specialized sensory requirements, and body parts come to differ in their functional roles as sensory surfaces. Thus, the contention that the less distorted and disrupted representation in galagos reflects a generally more primitive organization than the representation in monkeys is a valid possibility. All of the above differences in S-I proper of galagos and monkeys appear to be consistent with the concept that features of a more primitive organization have been preserved in galagos. In the regions of the foot and hand, major discontinuities are
16 396 M. SUR, R.J. NELSON AND J.H. KAAS avoided in galagos by the representations of appropriate strips of skin. The lateral band of cortex representing a strip of the arm connecting the hand with the shoulder and neck representations perhaps is a remnant of forearm representation that was once joined caudally with the medial forearm representation but was subsequently isolated by the expansion of the hand representation. In S-I of the grey squirrel (Sur et al., '78a) and a number of other mammals, the representation of the hand is not so enlarged and it does not extend caudally across the complete representation. Thus, it is possible for a basically medial forearm representation to adjoin caudally and laterally with the representation of the neck and head. The representation of the foot in galagos does not extend to the caudal border of S-I proper as it does in monkeys. Therefore medial and lateral representations of parts of the leg, which serve to join the trunk with the foot laterally and the gluteal region and foot medially, are joined caudally behind the foot in galagos. This is similar to the organization found in S-I of squirrels (Sur et al., '78a) but not in S-I proper of monkeys where the representation of the foot is presumably so enlarged that it separates lateral and medial parts of the leg (Merzenich et al., '78). Thus, in several ways, the organization of S-I proper of galagos appears to be intermediate between that found in S-I of squirrels and other mammals and S-I proper of monkeys. c. S-I proper in galagos differs from that in monkeys in at least one respect suggesting a specialization in galagos. In monkeys the most rostral recording sites in the hand representation corresponds to receptive fields on the glabrous finger tips. In galagos, receptive fields for the most rostral recording sites are found around the nail bed and often include the dorsal as well as the ventral tip of the finger (Fig. 5). This dorsal skin of the distal phalanx is hairless, and the inclusion of dorsal skin as a continuation of the map of the ventral digits suggests that these surfaces on the distal digits are used together as an important sensory surface in galagos but not in monkeys. Properties of neurons in S-Iproper of galagos In our limited studies of the response characteristics of neurons in S-I proper of galagos, two basic types of neurons were described and termed slowly adapting (SA) and rapidly adapting (RA) neurons (see Fig. 9). Most neurons were of the RA type. Similar classes of neurons, and similar proportions of the two classes were noted in S-I proper (Area 3b) of macaque mon- keys (Sur, '79), suggesting that S-I proper in monkeys and galagos has basically similar inputs and functions. Elsewhere, the existence of SA neurons in Area 3b of monkeys has been noted (Powell and Mountcastle, '59; Mountcastle et al., '69; Paul et al., '72) but their response properties, laminar locations, and numbers have been inadequately described and documented. In particular, the brief and complete suppression of activity following the high frequency onset and offset discharges have not been described. In this way, the SA (as well as RA) cortical neurons differ from the peripheral SA (and RA) neurons which show a sustained but adapting rate of discharge to a steady skin indentation (Burgess et al., '68; Pubols and Pubols, '76). The response profile of the cortical SA neuron suggests a strong recurrent inhibition that quickly disappears and is followed by a return to the adapting "envelope" rate of discharge of the SA receptor input (Sur, '78). Such inhibitory mechanisms also may account for the observation that most SA neurons were in the region of layer IV of cortex. Neurons in layer IV would be subject to the maintained activity of thalamic SA neurons, while cortical neurons in other layers would not be influenced by SA receptor activity unaltered by the powerful suppression of maintained activity. Suppression phenomenon such as that observed in both the SA and RA cortical neurons in galagos after onset and offset bursts has also been described for neurons in somatosensory cortex of cats (Hellweg et al., '77) and raccoons (Pubols and Leroy, '77). Homology of S-I proper in prosimians and monkeys We postulate that the mapped representation in galagos and the representation in Area 3b, S-I proper, are homologous, i.e., that the representation was present in a common ancestor. Furthermore, we believe that this representation evolved early in the phylogeny of mammals or even in reptiles, and that it is present in most, if not all mammals, where it has been commonly identified as S-I (for review see Sur et al., '78a). However, it is possible that in some mammals, such as the cat where separate architectonic fields have been described within S-I (Hassler and Muhs-Clement, '64), additional representations have been confounded with the s-i representation as they have been in monkeys (see Merzenich et al., '78). In such cases, a term such as S-I proper can be used to distinguish the S-I homologue from an older established, more inclusive, and we
17 BODY SURFACE IN SOMATIC KONIOCORTEX IN GALAW 397 would argue, incorrect application of the term, S-I. In galagos, we use the term S-I proper rather than S-I, not because other representations have been confounded in S-I as they have in monkeys, but because we want to both stress the homology of the representation across primates, and we want to allow for the possibility that one or more of the additional S-I representations of monkeys will be revealed in prosimians. The homology of the representation in galagos with S-I proper of monkeys might seem so obvious that there is no need to belabor the issue. However, at least two other possibilities need to be considered. One possibility is that the mapped representation in galagos is the homologue of one of the other representations in monkeys, i.e., the Area 1 posterior cutaneous field, the Area 2 deep body field, or even Area 3a with the deep receptor input. The other possibility is that the single representation in galagos is a composite of the several S-I representations in monkeys. Both possibilities, as well as the possibility that the region in prosimians corresponds to region 3b of monkeys, have been suggested by previous investigators (see below). Until recently, conclusions regarding the identity of subdivisions of cortex in prosimians have been based on architectonic studies. Because motor cortex (Area 4) is characterized by large pyramidal cells, it has been easy to identify in various mammals, and it serves as a useful reference subdivision of cortex. Brodmann ( 09) identified the cortical strip immediately caudal to Area 4 as Area 3 in most primates, and Area 3 was later subdivided into Area 3a and 3b (Vogt, 19). Area 3 (3b) of monkeys was described by Brodmann as having a well developed layer IV of small tightly packed granule cells merging with the densely packed layer 111, layer V with relatively few cells, and a layer VI of densely packed cells. These characteristics were also apparent in Area 1, but they were less pronounced. In appearance, the cortex corresponding to the S-I proper of the present study looks more like Area 1 of monkeys than Area 3b. However, in position relative to motor cortex (Area 4), it corresponds to Area 3b. Brodmann ( 09) apparently favored the similarities in appearance in identifying the cortical strip in prosimians and labeled it Area 1 (while omitting Areas 3 and 2 from the subdivisions of parietal cortex in prosimians). Brodmann also considered the possibility that Area 1 of prosimians was a composite of Areas 3,l and 2 of monkeys, and this alterna- tive was clearly preferred by Clark ( 31). More recently, in a detailed and extensive examination of the architectonics of parietal cortex in the prosimian slow loris, Sanides and Krishnamurti ( 67) renamed the Area 1 field of Brodmann (the Area 1-3 field of Le Gros Clark) in prosimians as somatic koniocortex and suggested that the probable homologue in monkeys was field 3b of the Vogts. In reaching this conclusion, Sanides and Krishnamurti apparently were more influenced by the position of somatic koniocortex relative to motor cortex, and the finding of an intermediate sensorimotor Area (which they homologized with Area 3a) between koniocortex and motor cortex, rather than the appearance of koniocortex in prosimians. They remind us that J. Rose ( 49) pointed out that sensory fields differ greatly in appearance from lower mammals to humans, and note that there is an overall more homogenous cytoarchitectonic picture in slow loris than in humans. The structural similarities of koniocortex in prosimians with Area 1 in monkeys may be because both are less specialized sensory areas than koniocortex (Area 3b) in monkeys, rather than the similarities in appearance being a consequence of homology. Our argument that the koniocortical field of prosimians and Area 3b of monkeys are homologous is based most directly on the similarities in the body representations in these two cortical subdivisions. Most importantly, the glabrous digit tips of the foot and hand point rostrally in the representations in both S-I proper of monkeys (Merzenich et al., 78; Nelson et al., 78b; Sur et al., 78b; Kaas et al., 79) and in koniocortex of prosimians. This feature of S-I proper of prosimians is apparent from results presented here on two species of galagos, which agree with the conclusions of a detailed microelectrode investigation of the hand representation in Galago crassicaudatus (Carlson and Welt, 77) and those of a pioneering microelectrode mapping study on slow loris first reported in 1965 (Krishnamurti and Welker, 65) and more fully in 1976 (Krishnamurti et al., 76). Other features such as the representations of the back rostral to the abdomen and the lower lip rostral to the upper lip in respective positions in the maps also correspond between the Area 3b representation in owl and macaque monkeys and the koniocortex representation in galagos. In contrast, the koniocortical representation of galagos and the Area 1 representation of monkeys differs in all these features, since the Area 1 representation
18 398 M. SUR, R.J. NELSON AND J.H. KAAS
19 ~ BODY SURFACE IN SOMATIC KONIOCORTEX IN GALAGO 399 approximates a mirror reversal of the Area 3b representation. Thus, on the bases of somatotopic organization as well as relative position, the representation mapped in galagos must be considered the homologue of the Area 3b representation of monkeys. This conclusion is also supported by what is known about the connections and response characteristics of S-I proper in prosimians. Our unpublished studies in galagos and the retrograde degeneration study of Krishnamurti et al. ( 72) in the slow loris indicate that somatic koniocortex is connected with the ventroposterior nucleus as are Area 3b and Area 1 of monkeys. Furthermore, both somatic koniocortex in galagos and Area 3b of monkeys have about the same proportions of slowly adapting (SA) and rapidly adapting (RA) neurons (present study and Sur, 78, 79). The conclusion that Area 3b of monkeys is homologous to somatic koniocortex of prosimians does not rule out the possibility that both Areas 3b and 1 of monkeys are homologous with somatic koniocortex of galagos in the special sense of serial homology. A viewpoint that is often seen in comparative studies, including our own, is that single subdivisions of the brain somehow give rise to two or more subdivisions in the course of evolution. When a part existing at one point in the course of evolution replicates so that two parts exist where there was one, both new parts can be considered serial homologues of the original part (Gregory, 35). It is uncertain how useful the concept of serial homologues is when applied to brain parts, but we have previously suggested that the sudden replication of brain structures followed by gradual divergences in organization and functions may have been a common method of brain evolution (Allman and Kaas, 71; Kaas 77). The replicated parts might have, for unknown reasons, taken the form of mirror images as representations of sensory surfaces, thus accounting for the prevalence of approximate mirror image organizations in adjoining sensory representations (Kaas, 77). While such possibilities deserve consideration, we do not now propose that a single somatosensory representation was retained in the primates leading to present day prosimians, while this Area was replicated one or more times in the primates giving rise to monkeys. The negative evidence for the Area 1 and Area 2 representations of monkeys in prosimians from three electrophysiological studies should not be considered conclusive. Many features of homologous cortical areas might be quite variable across different mammalian species. As a particularly relevant example, we found the Area 2 deep body representation of owl monkeys to be almost completely insensitive to light cutaneous stimulation under our recording conditions (Merzenich et al., 78), while the Area 2 representation was easily activated by such stimulations in macaque monkeys(suret al., 78b;Nelsoneta1., 79b, Kaaset al., 79). Our findings that cortex caudal to koniocortex of galagos, together with the similar findings in slow loris by Krishnamurti et al. ( 761, is responsive to more intense somatic stimuli, suggests the possibility of a less responsive Area 1 homologue of monkeys in prosimians. However, it is important to remember that given the clear uncertainty of conclusions based solely on cortical architecture, there is no compelling evidence for or against the existence of Areas 1 and 2 (as well as many other areas) in prosimians. Given the failure of electrophysiological mapping methods to reveal the organization of cortex immediately caudal to %-I proper of prosimians, the most logical next step would seem to be to examine the efferent and afferent connections of this cortex and compare the results with those from Area 1 of monkeys. Such studies could help establish the existence or absence of Area 1 in prosimians. The cortical block as an organizing feature of S-I proper Details of the map in S-I proper of the galago can be used to support the argument that the representation consists of several discrete, separately specified, cortical blocks. As mentioned above, the trunk, forelimb, and hindlimb are represented in register so that continuities are maintained. The dorsal trunk or back is represented rostrally in S-I proper and is adjacent to the rostral representations of the outer or dorsal leg and upper arm; the ventral trunk or belly is represented caudally next to the caudal representation of the ventral arm and inner thigh (Fig. 4, 6, 7, 11). We consider the cortex representing the leg, trunk, and arm between the foot and hand as one of the blocks Fig. 10 Parasagittal brain sections showing cytoarchitectonic features of somatosensory cortex in galagos. In A and C, small electrolytic lesions mark recording sites just rostral (left) and caudal (right) to cortex responsive to light cutaneous stimulation but within cortex responsive to moderate taps. The low threshold responsive zone is in somatic koniocortex or K, (after Sanides and Krishnamurti, 67). In B, cortical regions between (middle), rostral to (left), and caudal to (right) the marker lesions are shown at greater magnification (taken from A).
20 400 M. SUR, R.J. NELSON AND J.H. KAAS A. w 6. Foot B. Orientations of Individual rl Cortical Regions 6. Foot c. Orientations of Cortical Blocks 6 Foot I 4. Trunk 3. Arrn,Forearrn 1. Face u 3. Leg 4. Trunk 5. Arm,Forearrn 2. Hand 1. Face Fig. 11 A. Arrows depict the orientations in which different regions of the body surface are represented in S-I proper, as shown in B. C. Adjoining sectors of cortex with similar orientations. The leg, the trunk, the arm and the forearm can be combined into a single block within which topography is maintained. The foot, hand and face blocks are also internally somatotopic. Orientations of representational surfaces need not be maintained across blocks. of somatotopic regions of S-I proper. The greatly enlarged hand and foot representations are considered as two separate blocks of cortex. Finally, the head representation including the neck, chin, lips, and orbital skin forms another block (Fig. 11). There is somatotopy within this block and contiguous skin regions are largely in register. Thus, the dorsal skin surfaces (the dorsal neck, the midline of the upper lip, the bridge of the nose, the orbital and cranial skin) are represented caudally. Ventral regions of the head (the chin and midline of the lower lip) are represented rostrally. While somatotopic contiguity is largely preserved within blocks, the separate blocks need not be aligned with each other, as seen in Figure 11. Thus, a single body surface orientation, such as in a homunculus, does not describe the organization of S-I proper. The central trunk, arm, and leg block is in a reversed orientation
21 BODY SURFACE IN SOMATIC KONIOCORTEX IN GALAW from that in the foot, hand, and head blocks. In addition, major discontinuities occur across blocks. For example, the neck and chin, which adjoin the trunk on the body surface, are represented in a block lateral to the hand representation and in opposite orientation to the trunk block which is medial to the hand. Thus, the back of the neck is caudal and the adjoining back of the trunk is rostral. Perhaps less obviously, the hand block and the block representing the trunk and limbs are also reversed in orientation with respect to each other. The wrist and forearm are represented medial to the hand with the same orientation as the trunk, that is with the dorsum rostrally and the ventrum caudally. In laterally adjacent cortex, the hairy dorsum of the hand digits is caudal and the glabrous ventrum of the pads and digits is rostral. In the foot block, the orientation is rotated somewhat so that the ventrum is largely medial to the dorsum. Yet the basic orientations of the glabrous surfaces are similar in the hand and foot blocks so that the digit tips are rostral. In S-I proper of galagos, then, there are hand and foot blocks within which the digits point rostral, the trunk block with one orientation and the head block with the opposite orientation. Possibly there is some intrinsic functional advantage in the glabrous digit tips being represented closer to motor cortex. While the foot, hand and head blocks have essentially a similar orientation in the cortical representation, it is not clear why the trunk block in galagos is reversed in orientation. Each of the blocks, however, seems to be a combination of one to several individual regions which are internally somatotopic and are aligned in somatotopic orientation. ACKNOWLEDGMENTS Histological materials were prepared by E. Park. We thank Dr. V. A. Casagrande for the use of the two greater galagos employed in this study. This study was supported by National Science Foundation grant BNS R.J. Nelson was supported by NIH Training grant 1T32 MH LITERATURE CITED Allman,J.M., and J.H. Kaas (1971) Representationofthe visual field in striate and adjoining cortex of the owl monkey (Aotus triuirgatus). Brain Res., 35:8%106. Brcdmann, K. (1909) Vergleischende Lokalisationslehre der Grosshirnrinde. Barth, Leipzig. Burgess, P.R., D. Petit, andr.m. Warren (1968) Receptor types in cat hairy skin supplied by myelinated fibers. J. Neurophysiol., 31 :83%848. Carlson, M., and C. Welt (1977) Single representation of the hand in somatic sensory cortex (SI) of prosimian, Galago cmsicaudatus. Neuroscience Abstr., 3:478. Chubbuck, T.G. (1966) Small-motion biological stimulator. APL Tech. Digest, May-June, Clark, W.E. Le Gros (1931) The brain of microcebus murinus. Proc. Zool. Sac., l01:46%486. Gregoq, W.K. (1935) Reduplication in evolution. Q. Rev. Biol., 10: Hassler. R.. and K. Muhs-Clement (1964) Architetonischer aufbaudes sensomotorischen und parietalen cortex des katze. J. Hirnforsch. 6: Hellweg,F.C., W. Schultz, and O.D. Creutzfeldt (1977) Extracellular and intracellular recodings from cat s whisker projection area: Thalamo-cortical response transfomation. J. Neurophysiol., 40:46%479. Kaas, J.H. (1977) Sensory representations in mammals. In: Function and Formation ofneural Systems, G.S. Stent, ed. Dahlem Konferenzen, Berlin, pp. 6%30. Kaas, J.H., M.M. Merzenich, C.-S. Lin, and M. Sur (1976) A double representation of the body in primary somatosensory cortex ( SI ) of primates. Neurosci. Abstr., 2:914. Kaas, J.H., R.J. Nelson, M. Sur., C.-S. Lin, and M.M. Merzenich (1979) Multiple representations of the body within SI of primates: A redefinition of primary somatosensory cortex. Science, 204: Krishnamurti, A,, R. Kanagsuntheram, and W.C. Wong (1972) Functional significance of the fibrous laminae in the ventrobasal complex of the thalamus of slow loris. J. Comp. Neurol., 145: Krishnamurti, A,, F. Sanides, and W.I. Welker (1976) Microelectrode mapping of modality-specific somatic sensory cerebral neocortex in slow loris. Brain, Behav. Evol., Krishnamurti, A., and W.I. Welker (1965) Somatic sensory area in the cerebral neocortex of slow loris (Nycticebus coucang coucangl. Fed. Proc., 24:140. Lin, C.-S., and J.H. Kaas (1977) Projections from cortical visual areas 17,18 and MT onto the dorsol lateral geniculate nucleus in owl monkeys. J. Comp. Neurol., 173: Merzenich, M.M., J.H. Kaas, M. Sur, and C.3. Lin (1978) Double representation of the body surface within cytoarchitectonic Areas 3b and 1 in SI in the owl monkey (Aotus triuirgatus). J. Cornp. Neurol., 181 : Mountcastle, V.B., W.H. Talbot, H. Sakata, and J. Hyvarinen (1969) Cortical neuronal mechanisms in fluttervibration studies unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J. Neurophysiol., 32: Nelson, R.J., M. Sur., and J.H. Kaas (1978a) The representation of the body surface in somatic koniocortex of a prosimian primate, Galago senegalensis. Anat. Rec., 190: Nelson, R.H., M. Sur, and J.H. Kaas (1978b) Multiple representations of the body surface in postcentral parietal cortex ( SI ) of the squirrel monkey. Neurosci. Abstr., Nelson, R.J., M. Sur, and J.H. Kaas (1979a) The organization of the second somatosensory area (SmII) of the grey squirrel. J. Comp. Neurol., 184 (3):47%490. Nelson, R.J., M. Sur, D.J. Fellernan, and J.H. Kaas (1979b) Representations of the body surface in postcentral somatosensory cortex of Mucaca fascicularis. In preparation. Paul, R.L., M.M. Merzenich, and H. Goodman (1972) Representation of slowly and rapidly adapting cutaneous mechanoreceptors of the hand in Brodmann s areas 3 and 1 of Macaca rnulatta. Brain Res., 36:22%249.
22 402 M. SUR, R.J. NELSON AND J.H. KAAS Powell, T.P.S., and V.B. Mountcastle (1959) Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey. A correlation of findings obtained in a single unit analysis with cytoarchitecture. Bull. Johns Hopkins Hosp., 105:13%162. Pubols, L.M., and R.F. Leroy (1977) Orientation detectors in the primary somatosensory neocortex of the raccoon. Brain Res., 129: Pubols, B.H., andpubols, L.M. (1976) Codingof mechanical stimulus velocity and indentation depth by squirrel monkey and racoon glabrous skin mechanorecepton. J. Neurophysiol.,39: Rose, J.E. (1949) The cellular structure of the auditory region of the cat. J. Comp. Neurol., 91: Sanides, F., and A. Krishnamurti (1967) Cytoarchitectonic subdivisions of sensorimotor and prefrontal regions and of bordering insular and limbic fields in slow loris (Nycticebus coucang coucang). J. Hirnforschung, 9t Sur, M. (1978) Some principles of organization of somatosensory cortex. Ph.D. Thesis, Vanderbilt University, Nashville, Tennessee. Sur. M. (1979) Somatosensory cortex in macaque monkeys: Columnar organization of slowly and rapidly adapting neurons and receptive field organization. Fed. Roc. 38r898. Sur, M., R.J. Nelson, and J.H. Kaas (1977) The representation of the body surface in SmII of the grey squirrel. Neumsci. Abstr., 3t492. Sur, M., R.J. Nelson, and J.H. Kaas (1978a) The representation of the body surface in somatosensory Area I of the grey squirrel. J. Comp. Neurol., Sur, M., R.J. Nelson, and J.H. Kaas (1978b) Postcentral somatosensory cortex in macaque monkeys: multiple body representations and neuronal properties. Neurosci. Abstr., 4:559. Vogt, 0. (1919) Allgeimenere Ergebnisse unserer Hirnforschung. J. Psychol. Neurol. (Lpz.), 25:
Representations of the Body Surface in Postcentral Parietal Cortex of Macaca fascicularis
 THE JOURNAL OF COMPARATIVE NEUROLOGY 192~611-643 (1980) Representations of the Body Surface in Postcentral Parietal Cortex of Macaca fascicularis R.J. NELSON, M. SUR, D.J. FELLEMAN, AND J.H. KAAS Departments
THE JOURNAL OF COMPARATIVE NEUROLOGY 192~611-643 (1980) Representations of the Body Surface in Postcentral Parietal Cortex of Macaca fascicularis R.J. NELSON, M. SUR, D.J. FELLEMAN, AND J.H. KAAS Departments
Representations of the Body Surface. Monkeys: Comparisons With Other Primates
 THE JOURNAL OF COMPARATIVE NEUROLOGY 211:177-192 (1982) Representations of the Body Surface in Cortical Areas 3b and 1 of Squirrel Monkeys: Comparisons With Other Primates MRIGANKA SUR, R. J. NELSON, AND
THE JOURNAL OF COMPARATIVE NEUROLOGY 211:177-192 (1982) Representations of the Body Surface in Cortical Areas 3b and 1 of Squirrel Monkeys: Comparisons With Other Primates MRIGANKA SUR, R. J. NELSON, AND
Double Representation of the Body Surface within Cytoarchitectonic Areas 3b and 1 in SI in the Owl Monkey (Aotus trivirgatus)
 Double Representation of the Body Surface within Cytoarchitectonic Areas 3b and 1 in SI in the Owl Monkey (Aotus trivirgatus) MICHAEL M. MERZENICH,I JON H. KAAS, MRIGANKA SUR AND CHIA-SHENG LIN Coleman
Double Representation of the Body Surface within Cytoarchitectonic Areas 3b and 1 in SI in the Owl Monkey (Aotus trivirgatus) MICHAEL M. MERZENICH,I JON H. KAAS, MRIGANKA SUR AND CHIA-SHENG LIN Coleman
SOMATOSENSORY SYSTEMS
 SOMATOSENSORY SYSTEMS Schematic diagram illustrating the neural pathways that convey somatosensory information to the cortex and, subsequently, to the motor system. Double arrows show reciprocal connections.
SOMATOSENSORY SYSTEMS Schematic diagram illustrating the neural pathways that convey somatosensory information to the cortex and, subsequently, to the motor system. Double arrows show reciprocal connections.
THE JOURNAL OF COMPARATIVE NEUROLOGY 250~ (1986)
 THE JOURNAL OF COMPARATIVE NEUROLOGY 250~403-430 (1986) Microelectrode Maps, Myeloarchitecture, and Cortical Connections of Three Somatotopically Organized Representations of the Body Surface in the Parietal
THE JOURNAL OF COMPARATIVE NEUROLOGY 250~403-430 (1986) Microelectrode Maps, Myeloarchitecture, and Cortical Connections of Three Somatotopically Organized Representations of the Body Surface in the Parietal
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
POSTERIOR 1. situated behind: situated at or toward the hind part of the body :
 ANATOMICAL LOCATION Anatomy is a difficult subject with a large component of memorization. There is just no way around that, but we have made every effort to make this course diverse and fun. The first
ANATOMICAL LOCATION Anatomy is a difficult subject with a large component of memorization. There is just no way around that, but we have made every effort to make this course diverse and fun. The first
The Language of Anatomy. (Anatomical Terminology)
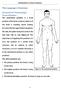 The Language of Anatomy (Anatomical Terminology) Terms of Position The anatomical position is a fixed position of the body (cadaver) taken as if the body is standing (erect) looking forward with the upper
The Language of Anatomy (Anatomical Terminology) Terms of Position The anatomical position is a fixed position of the body (cadaver) taken as if the body is standing (erect) looking forward with the upper
Connections of Areas 3b and 1 of the Parietal Somatosensory Strip with the Ventroposterior Nucleus in the Owl Monkey (Aotus trivirga tus)
 Connections of Areas 3b and 1 of the Parietal Somatosensory Strip with the Ventroposterior Nucleus in the Owl Monkey (Aotus trivirga tus) CHIA-SHENG LIN, MICHAEL M. MERZENICH, MRIGANKA SUR a AND JON H.
Connections of Areas 3b and 1 of the Parietal Somatosensory Strip with the Ventroposterior Nucleus in the Owl Monkey (Aotus trivirga tus) CHIA-SHENG LIN, MICHAEL M. MERZENICH, MRIGANKA SUR a AND JON H.
Neurophysiology of systems
 Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
A Redefinition of Somatosensory Areas in the Lateral Sulcus of Macaque Monkeys
 The Journal of Neuroscience, May 1995, 15(5): 3821-3839 A Redefinition of Somatosensory Areas in the Lateral Sulcus of Macaque Monkeys Leah Krubitzer, Janine Clarey, Rowan Tweedale, Guy Elston, and Mike
The Journal of Neuroscience, May 1995, 15(5): 3821-3839 A Redefinition of Somatosensory Areas in the Lateral Sulcus of Macaque Monkeys Leah Krubitzer, Janine Clarey, Rowan Tweedale, Guy Elston, and Mike
Skin types: hairy and glabrous (e.g. back vs. palm of hand)
 Lecture 19 revised 03/10 The Somatic Sensory System Skin- the largest sensory organ we have Also protects from evaporation, infection. Skin types: hairy and glabrous (e.g. back vs. palm of hand) 2 major
Lecture 19 revised 03/10 The Somatic Sensory System Skin- the largest sensory organ we have Also protects from evaporation, infection. Skin types: hairy and glabrous (e.g. back vs. palm of hand) 2 major
Body Organizations Flashcards
 1. What are the two main regions of the body? 2. What three structures are in the Axial Region? 1. Axial Region (Goes down midline of the body) 2. Appendicular Region (limbs) 3. Axial Region (Goes down
1. What are the two main regions of the body? 2. What three structures are in the Axial Region? 1. Axial Region (Goes down midline of the body) 2. Appendicular Region (limbs) 3. Axial Region (Goes down
The Physiology of the Senses Chapter 8 - Muscle Sense
 The Physiology of the Senses Chapter 8 - Muscle Sense www.tutis.ca/senses/ Contents Objectives... 1 Introduction... 2 Muscle Spindles and Golgi Tendon Organs... 3 Gamma Drive... 5 Three Spinal Reflexes...
The Physiology of the Senses Chapter 8 - Muscle Sense www.tutis.ca/senses/ Contents Objectives... 1 Introduction... 2 Muscle Spindles and Golgi Tendon Organs... 3 Gamma Drive... 5 Three Spinal Reflexes...
Large-Scale Reorganization in the Somatosensory Cortex and Thalamus after Sensory Loss in Macaque Monkeys
 11042 The Journal of Neuroscience, October 22, 2008 28(43):11042 11060 Behavioral/Systems/Cognitive Large-Scale Reorganization in the Somatosensory Cortex and Thalamus after Sensory Loss in Macaque Monkeys
11042 The Journal of Neuroscience, October 22, 2008 28(43):11042 11060 Behavioral/Systems/Cognitive Large-Scale Reorganization in the Somatosensory Cortex and Thalamus after Sensory Loss in Macaque Monkeys
Somatosensation. Recording somatosensory responses. Receptive field response to pressure
 Somatosensation Mechanoreceptors that respond to touch/pressure on the surface of the body. Sensory nerve responds propotional to pressure 4 types of mechanoreceptors: Meissner corpuscles & Merkel discs
Somatosensation Mechanoreceptors that respond to touch/pressure on the surface of the body. Sensory nerve responds propotional to pressure 4 types of mechanoreceptors: Meissner corpuscles & Merkel discs
Anatomical Substrates of Somatic Sensation
 Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Mechanosensation. Central Representation of Touch. Wilder Penfield. Somatotopic Organization
 Mechanosensation Central Representation of Touch Touch and tactile exploration Vibration and pressure sensations; important for clinical testing Limb position sense John H. Martin, Ph.D. Center for Neurobiology
Mechanosensation Central Representation of Touch Touch and tactile exploration Vibration and pressure sensations; important for clinical testing Limb position sense John H. Martin, Ph.D. Center for Neurobiology
 Peripheral facial paralysis (right side). The patient is asked to close her eyes and to retract their mouth (From Heimer) Hemiplegia of the left side. Note the characteristic position of the arm with
Peripheral facial paralysis (right side). The patient is asked to close her eyes and to retract their mouth (From Heimer) Hemiplegia of the left side. Note the characteristic position of the arm with
Chapter 1: Introduction to the Human Body Test Bank
 Chapter 1: Introduction to the Human Body Test Bank MULTIPLE CHOICE 1. What is the branch of science that studies how the body functions? a. Anatomy b. Histology c. Pathology d. Physiology 2. Which word
Chapter 1: Introduction to the Human Body Test Bank MULTIPLE CHOICE 1. What is the branch of science that studies how the body functions? a. Anatomy b. Histology c. Pathology d. Physiology 2. Which word
The Human Body: An Orientation
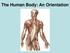 The Human Body: An Orientation Body standing upright Anatomical Position feet slightly apart palms facing forward thumbs point away from body Directional Terms Superior and inferior toward and away from
The Human Body: An Orientation Body standing upright Anatomical Position feet slightly apart palms facing forward thumbs point away from body Directional Terms Superior and inferior toward and away from
Homework Packet. The branch of biological science that studies and describes how body parts. The study of the shape and structure of body parts
 Anatomy & Physiology Chap. 1: The Human Body Name Block: P/W Homework Packet ANATOMY & PHYSIOLOGY DISTINCTIONS 1. Match the term on the right to the appropriate description on the left. Enter the correct
Anatomy & Physiology Chap. 1: The Human Body Name Block: P/W Homework Packet ANATOMY & PHYSIOLOGY DISTINCTIONS 1. Match the term on the right to the appropriate description on the left. Enter the correct
Sensory information processing, somato-sensory systems
 mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
Physiology of Tactile Sensation
 Physiology of Tactile Sensation Objectives: 1. Describe the general structural features of tactile sensory receptors how are first order nerve fibers specialized to receive tactile stimuli? 2. Understand
Physiology of Tactile Sensation Objectives: 1. Describe the general structural features of tactile sensory receptors how are first order nerve fibers specialized to receive tactile stimuli? 2. Understand
Terms of Movements by Prof. Dr. Muhammad Imran Qureshi
 Terms of Movements by Prof. Dr. Muhammad Imran Qureshi Three systems of the body work in coordination to perform various movements of the body. These are: A System of Bones (Osteology), A System of Muscles
Terms of Movements by Prof. Dr. Muhammad Imran Qureshi Three systems of the body work in coordination to perform various movements of the body. These are: A System of Bones (Osteology), A System of Muscles
Anatomy and Physiology Unit 1 Review Sheet
 Anatomy and Physiology Unit 1 Review Sheet Chapter 1 Name Date Hour 1. investigates the body's structure, whereas investigates the processes or functions of living things. A. Physiology, cytology B. Physiology,
Anatomy and Physiology Unit 1 Review Sheet Chapter 1 Name Date Hour 1. investigates the body's structure, whereas investigates the processes or functions of living things. A. Physiology, cytology B. Physiology,
Body Planes & Positions
 Learning Objectives Objective 1: Identify and utilize anatomical positions, planes, and directional terms. Demonstrate what anatomical position is and how it is used to reference the body. Distinguish
Learning Objectives Objective 1: Identify and utilize anatomical positions, planes, and directional terms. Demonstrate what anatomical position is and how it is used to reference the body. Distinguish
Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile
 Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile and position sensations (2) Thermoreceptive senses:
Ch. 47 Somatic Sensations: Tactile and Position Senses (Reading Homework) - Somatic senses: three types (1) Mechanoreceptive somatic senses: tactile and position sensations (2) Thermoreceptive senses:
Lab no 1 Structural organization of the human body
 Physiology Lab Manual Page 1 of 6 Lab no 1 Structural organization of the human body Physiology is the science which deals with functions of the body parts, and how they work. Since function cannot be
Physiology Lab Manual Page 1 of 6 Lab no 1 Structural organization of the human body Physiology is the science which deals with functions of the body parts, and how they work. Since function cannot be
Anatomy The study of the body's structure.
 Anatomy The study of the body's structure. * 1. Systemic- Study of each of the body's systems. 2. Regional- Study of a specific area of the body 3. Surface- Study of external features. Physiology The study
Anatomy The study of the body's structure. * 1. Systemic- Study of each of the body's systems. 2. Regional- Study of a specific area of the body 3. Surface- Study of external features. Physiology The study
Cerebral Cortex 1. Sarah Heilbronner
 Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
TERMINOLOGY AS IT APPLIES TO TICA BREED STANDARDS. Interpretation by Marge Hanna
 TERMINOLOGY AS IT APPLIES TO TICA BREED STANDARDS Interpretation by Marge Hanna 1. Nose: The area, with its underlying cartilage, from the top edge of the nose leather up to the bottom of the bridge of
TERMINOLOGY AS IT APPLIES TO TICA BREED STANDARDS Interpretation by Marge Hanna 1. Nose: The area, with its underlying cartilage, from the top edge of the nose leather up to the bottom of the bridge of
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
The Representation of Peripheral Nerve Inputs in the S-I Hindpaw Cortex of Rats Raised with Incompletely Innervated Hindpaws
 The Journal of Neuroscience April 1986, 6(4): 1129-l 147 The Representation of Peripheral Nerve Inputs in the S-I Hindpaw Cortex of Rats Raised with Incompletely Innervated Hindpaws J. T. Wall and C. G.
The Journal of Neuroscience April 1986, 6(4): 1129-l 147 The Representation of Peripheral Nerve Inputs in the S-I Hindpaw Cortex of Rats Raised with Incompletely Innervated Hindpaws J. T. Wall and C. G.
CHAPTER 10 THE SOMATOSENSORY SYSTEM
 CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Pain and Temperature Objectives
 Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Lateral view of human brain! Cortical processing of touch!
 Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
Fetal Pig Dissection:
 Fetal Pig Dissection: REMEMBER: Dissection involves disassembling and observing something to determine its internal structure and develop an understanding of the relationship of those structures to function.
Fetal Pig Dissection: REMEMBER: Dissection involves disassembling and observing something to determine its internal structure and develop an understanding of the relationship of those structures to function.
How strong is it? What is it? Where is it? What must sensory systems encode? 9/8/2010. Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
The Language of Anatomy
 1 E x e r c i s e The Language of Anatomy If time is a problem, most of this exercise can be done as an out-of-class assignment. Time Allotment: 1/2 hour (in lab). Laboratory Materials Ordering information
1 E x e r c i s e The Language of Anatomy If time is a problem, most of this exercise can be done as an out-of-class assignment. Time Allotment: 1/2 hour (in lab). Laboratory Materials Ordering information
SECTION 1 ANATOMY L A TERI A M TED H G PYRI CO
 COPYRIGHTED MATERIAL A veterinary technician comes in for work in the morning to discover that Fluffy has come in overnight after having been hit by a car. The chart note indicates that there is a cut
COPYRIGHTED MATERIAL A veterinary technician comes in for work in the morning to discover that Fluffy has come in overnight after having been hit by a car. The chart note indicates that there is a cut
Hand Anatomy A Patient's Guide to Hand Anatomy
 Hand Anatomy A Patient's Guide to Hand Anatomy Introduction Few structures of the human anatomy are as unique as the hand. The hand needs to be mobile in order to position the fingers and thumb. Adequate
Hand Anatomy A Patient's Guide to Hand Anatomy Introduction Few structures of the human anatomy are as unique as the hand. The hand needs to be mobile in order to position the fingers and thumb. Adequate
Note: Waxman is very sketchy on today s pathways and nonexistent on the Trigeminal.
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne Stensaas, PhD Note: Waxman is very sketchy on today s pathways and nonexistent on the Trigeminal. Resources: Pathway Quiz for HyperBrain Ch. 5 and
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne Stensaas, PhD Note: Waxman is very sketchy on today s pathways and nonexistent on the Trigeminal. Resources: Pathway Quiz for HyperBrain Ch. 5 and
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
SWGFAST Glossary - Anatomy
 SWGFAST Glossary - Anatomy BALL AREA The large cushion area below the base of the big toe. BRACHYDACTYLY Abnormal shortness of fingers or toes. BULB OF FINGERS (THUMBS, TOES) The portion of the friction
SWGFAST Glossary - Anatomy BALL AREA The large cushion area below the base of the big toe. BRACHYDACTYLY Abnormal shortness of fingers or toes. BULB OF FINGERS (THUMBS, TOES) The portion of the friction
Exam 1 PSYC Fall 1998
 Exam 1 PSYC 2022 Fall 1998 (2 points) Briefly describe the difference between a dualistic and a materialistic explanation of brain-mind relationships. (1 point) True or False. George Berkely was a monist.
Exam 1 PSYC 2022 Fall 1998 (2 points) Briefly describe the difference between a dualistic and a materialistic explanation of brain-mind relationships. (1 point) True or False. George Berkely was a monist.
Anatomy & Physiology Ch 1: The Human Body Worksheet
 Anatomy & Physiology Ch 1: The Human Body Worksheet 1. The structures of the body are organized in successively larger and more complex structures. Fill in the blanks with the correct terms for these increasingly
Anatomy & Physiology Ch 1: The Human Body Worksheet 1. The structures of the body are organized in successively larger and more complex structures. Fill in the blanks with the correct terms for these increasingly
Key Relationships in the Upper Limb
 Key Relationships in the Upper Limb This list contains some of the key relationships that will help you identify structures in the lab. They are organized by dissection assignment as defined in the syllabus.
Key Relationships in the Upper Limb This list contains some of the key relationships that will help you identify structures in the lab. They are organized by dissection assignment as defined in the syllabus.
Strick Lecture 3 March 22, 2017 Page 1
 Strick Lecture 3 March 22, 2017 Page 1 Cerebellum OUTLINE I. External structure- Inputs and Outputs Cerebellum - (summary diagram) 2 components (cortex and deep nuclei)- (diagram) 3 Sagittal zones (vermal,
Strick Lecture 3 March 22, 2017 Page 1 Cerebellum OUTLINE I. External structure- Inputs and Outputs Cerebellum - (summary diagram) 2 components (cortex and deep nuclei)- (diagram) 3 Sagittal zones (vermal,
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Coding of Sensory Information
 Coding of Sensory Information 22 November, 2016 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Sensory Systems Mediate Four Attributes of
Coding of Sensory Information 22 November, 2016 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Sensory Systems Mediate Four Attributes of
https://testbanksolution.net/
 Chapter 1: An Introduction to the Structure and Function of the Body Test Bank MULTIPLE CHOICE 1. Which word is derived from the Greek word meaning cutting up? A. dissection C. pathology B. physiology
Chapter 1: An Introduction to the Structure and Function of the Body Test Bank MULTIPLE CHOICE 1. Which word is derived from the Greek word meaning cutting up? A. dissection C. pathology B. physiology
Cerebrum-Cerebral Hemispheres. Cuneyt Mirzanli Istanbul Gelisim University
 Cerebrum-Cerebral Hemispheres Cuneyt Mirzanli Istanbul Gelisim University The largest part of the brain. Ovoid shape. Two incompletely separated cerebral hemispheres. The outer surface of the cerebral
Cerebrum-Cerebral Hemispheres Cuneyt Mirzanli Istanbul Gelisim University The largest part of the brain. Ovoid shape. Two incompletely separated cerebral hemispheres. The outer surface of the cerebral
9.14 Classes #21-23: Visual systems
 9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
Medical Neuroscience Tutorial Notes
 Medical Neuroscience Tutorial Notes Finding the Central Sulcus MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Medical Neuroscience Tutorial Notes Finding the Central Sulcus MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
First stage Lec.1 : Introduction. Asst.Lec.Dr.ABDULRIDHA ALASADY
 First stage 2018-2019 Lec.1 : Introduction Asst.Lec.Dr.ABDULRIDHA ALASADY Anatomy the study of the structure and shape of the body and body parts & their relationships to one another aided by dissection
First stage 2018-2019 Lec.1 : Introduction Asst.Lec.Dr.ABDULRIDHA ALASADY Anatomy the study of the structure and shape of the body and body parts & their relationships to one another aided by dissection
Chapter 1 An Introduction to the Human Body
 1-1 Chapter 1 An Introduction to the Human Body Anatomy science of structure relationships revealed by dissection (cutting apart) Physiology science of body functions Levels of Organization Chemical Cellular
1-1 Chapter 1 An Introduction to the Human Body Anatomy science of structure relationships revealed by dissection (cutting apart) Physiology science of body functions Levels of Organization Chemical Cellular
The How of Tactile Sensation
 The How of Tactile Sensation http://neuroscience.uth.tmc.edu/s2/chapter02.html Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo Objectives 1. Understand how sensory stimuli are encoded
The How of Tactile Sensation http://neuroscience.uth.tmc.edu/s2/chapter02.html Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo Objectives 1. Understand how sensory stimuli are encoded
Nervous system Reflexes and Senses
 Nervous system Reflexes and Senses Physiology Lab-4 Wrood Slaim, MSc Department of Pharmacology and Toxicology University of Al-Mustansyria 2017-2018 Nervous System The nervous system is the part of an
Nervous system Reflexes and Senses Physiology Lab-4 Wrood Slaim, MSc Department of Pharmacology and Toxicology University of Al-Mustansyria 2017-2018 Nervous System The nervous system is the part of an
topographical anatomy
 Kaan Yücel M.D., Ph.D. 30. September 2014 Tuesday topographical anatomy organization of the human body as major parts or segments Head Neck Trunk thorax, abdomen, back, & pelvis/perineum Upper limbs &
Kaan Yücel M.D., Ph.D. 30. September 2014 Tuesday topographical anatomy organization of the human body as major parts or segments Head Neck Trunk thorax, abdomen, back, & pelvis/perineum Upper limbs &
Anatomical Terminology
 Anatomical Terminology Dr. A. Ebneshahidi Anatomy Anatomy : is the study of structures or body parts and their relationships to on another. Anatomy : Gross anatomy - macroscopic. Histology - microscopic.
Anatomical Terminology Dr. A. Ebneshahidi Anatomy Anatomy : is the study of structures or body parts and their relationships to on another. Anatomy : Gross anatomy - macroscopic. Histology - microscopic.
If time is limited, most of this exercise can be done as an out-of-class assignment.
 EXERCISE 1 Download FULL Solution Manual for Human Anatomy Laboratory Manual with Cat Dissections 8th Edition by Marieb & Smith https://getbooksolutions.com/download/test-bank-for-legal-environment-of-business-7e-nancy-k-kubase-bartley-abrennan-m-neil-browne
EXERCISE 1 Download FULL Solution Manual for Human Anatomy Laboratory Manual with Cat Dissections 8th Edition by Marieb & Smith https://getbooksolutions.com/download/test-bank-for-legal-environment-of-business-7e-nancy-k-kubase-bartley-abrennan-m-neil-browne
Retinotopy & Phase Mapping
 Retinotopy & Phase Mapping Fani Deligianni B. A. Wandell, et al. Visual Field Maps in Human Cortex, Neuron, 56(2):366-383, 2007 Retinotopy Visual Cortex organised in visual field maps: Nearby neurons have
Retinotopy & Phase Mapping Fani Deligianni B. A. Wandell, et al. Visual Field Maps in Human Cortex, Neuron, 56(2):366-383, 2007 Retinotopy Visual Cortex organised in visual field maps: Nearby neurons have
Calisthenic Guidelines
 8 Calisthenics In this chapter you will learn about: Proper form and guidelines for performing calisthenics. Designing a calisthenic exercise program. Abdominal exercise techniques. Calisthenics require
8 Calisthenics In this chapter you will learn about: Proper form and guidelines for performing calisthenics. Designing a calisthenic exercise program. Abdominal exercise techniques. Calisthenics require
COGS 107B Week 1. Hyun Ji Friday 4:00-4:50pm
 COGS 107B Week 1 Hyun Ji Friday 4:00-4:50pm Before We Begin... Hyun Ji 4th year Cognitive Behavioral Neuroscience Email: hji@ucsd.edu In subject, always add [COGS107B] Office hours: Wednesdays, 3-4pm in
COGS 107B Week 1 Hyun Ji Friday 4:00-4:50pm Before We Begin... Hyun Ji 4th year Cognitive Behavioral Neuroscience Email: hji@ucsd.edu In subject, always add [COGS107B] Office hours: Wednesdays, 3-4pm in
Somatic Sensory System I. Background
 Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Motor Systems I Cortex. Reading: BCP Chapter 14
 Motor Systems I Cortex Reading: BCP Chapter 14 Principles of Sensorimotor Function Hierarchical Organization association cortex at the highest level, muscles at the lowest signals flow between levels over
Motor Systems I Cortex Reading: BCP Chapter 14 Principles of Sensorimotor Function Hierarchical Organization association cortex at the highest level, muscles at the lowest signals flow between levels over
Introduction to The Human Body
 1 Introduction to The Human Body FOCUS: The human organism is often examined at seven structural levels: chemical, organelle, cell, tissue, organ, organ system, and the organism. Anatomy examines the structure
1 Introduction to The Human Body FOCUS: The human organism is often examined at seven structural levels: chemical, organelle, cell, tissue, organ, organ system, and the organism. Anatomy examines the structure
Neurological Examination
 Neurological Examination Charles University in Prague 1st Medical Faculty and General University Hospital Neurological examination: Why important? clinical history taking and bedside examination: classical
Neurological Examination Charles University in Prague 1st Medical Faculty and General University Hospital Neurological examination: Why important? clinical history taking and bedside examination: classical
The Somatosensory System
 The Somatosensory System Reading: BCP Chapter 12 cerebrovortex.com Divisions of the Somatosensory System Somatosensory System Exteroceptive External stimuli Proprioceptive Body position Interoceptive Body
The Somatosensory System Reading: BCP Chapter 12 cerebrovortex.com Divisions of the Somatosensory System Somatosensory System Exteroceptive External stimuli Proprioceptive Body position Interoceptive Body
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Overview of Questions
 Overview of Questions What are the sensors in the skin, what do they respond to and how is this transmitted to the brain? How does the brain represent touch information? What is the system for sensing
Overview of Questions What are the sensors in the skin, what do they respond to and how is this transmitted to the brain? How does the brain represent touch information? What is the system for sensing
PHY3111 Mid-Semester Test Study. Lecture 2: The hierarchical organisation of vision
 PHY3111 Mid-Semester Test Study Lecture 2: The hierarchical organisation of vision 1. Explain what a hierarchically organised neural system is, in terms of physiological response properties of its neurones.
PHY3111 Mid-Semester Test Study Lecture 2: The hierarchical organisation of vision 1. Explain what a hierarchically organised neural system is, in terms of physiological response properties of its neurones.
A bilateral cortico-striate projection
 J. Neurol. Neurosurg. Psychiat., 1965, 28, 71 J. B. CARMAN, W. M. COWAN, T. P. S. POWELL, AND K. E. WEBSTER From the Departments of Anatomy, University of Oxford, and University College, London During
J. Neurol. Neurosurg. Psychiat., 1965, 28, 71 J. B. CARMAN, W. M. COWAN, T. P. S. POWELL, AND K. E. WEBSTER From the Departments of Anatomy, University of Oxford, and University College, London During
Functional Reorganization of Primary Somatosensory Cortex in Adult Owl Monkeys After Behaviorally Controlled Tactile Stimulation
 JOURNAL OF NEUROPHYSIOLOGY Vol. 63. No. 1. January 1990. Yrinlcd in C..S..d Functional Reorganization of Primary Somatosensory Cortex in Adult Owl Monkeys After Behaviorally Controlled Tactile Stimulation
JOURNAL OF NEUROPHYSIOLOGY Vol. 63. No. 1. January 1990. Yrinlcd in C..S..d Functional Reorganization of Primary Somatosensory Cortex in Adult Owl Monkeys After Behaviorally Controlled Tactile Stimulation
Nature Neuroscience doi: /nn Supplementary Figure 1. Characterization of viral injections.
 Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Barbell Squat. Gluteals. Quadriceps. Hamstrings
 Gluteals Barbell Squat Quadriceps Hamstrings Select weight. The Olympic bar in the picture weighs 45 pounds. Use safety collars when adding additional plates. Place the bar on your upper back k( (not your
Gluteals Barbell Squat Quadriceps Hamstrings Select weight. The Olympic bar in the picture weighs 45 pounds. Use safety collars when adding additional plates. Place the bar on your upper back k( (not your
Neurobiology Biomed 509 Sensory transduction References: Luo , ( ), , M4.1, M6.2
 Neurobiology Biomed 509 Sensory transduction References: Luo 4.1 4.8, (4.9 4.23), 6.22 6.24, M4.1, M6.2 I. Transduction The role of sensory systems is to convert external energy into electrical signals
Neurobiology Biomed 509 Sensory transduction References: Luo 4.1 4.8, (4.9 4.23), 6.22 6.24, M4.1, M6.2 I. Transduction The role of sensory systems is to convert external energy into electrical signals
Arterial Blood Supply
 Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
LEAH KRUBITZER RESEARCH GROUP LAB PUBLICATIONS WHAT WE DO LINKS CONTACTS
 LEAH KRUBITZER RESEARCH GROUP LAB PUBLICATIONS WHAT WE DO LINKS CONTACTS WHAT WE DO Present studies and future directions Our laboratory is currently involved in two major areas of research. The first
LEAH KRUBITZER RESEARCH GROUP LAB PUBLICATIONS WHAT WE DO LINKS CONTACTS WHAT WE DO Present studies and future directions Our laboratory is currently involved in two major areas of research. The first
Medical Terminology. Anatomical Position, Directional Terms and Movements
 Medical Terminology Anatomical Position, Directional Terms and Movements What we will cover... Content Objectives Students will be able to gain a better understanding and application of medical terminology
Medical Terminology Anatomical Position, Directional Terms and Movements What we will cover... Content Objectives Students will be able to gain a better understanding and application of medical terminology
Anatomy 25 KEY ANATOMICAL TERMINOLOGY Guthrie
 THE FOLLOWING TERMS ARE COMMONLY USED IN ANATOMY. YOU MUST KNOW THEM IN ORDER TO FIND YOUR WAY AROUND THE BODY. CADAVER : A dead human body A NATOMICAL POSITION : The standard reference position of the
THE FOLLOWING TERMS ARE COMMONLY USED IN ANATOMY. YOU MUST KNOW THEM IN ORDER TO FIND YOUR WAY AROUND THE BODY. CADAVER : A dead human body A NATOMICAL POSITION : The standard reference position of the
TRAINING HIPS & THIGHS WORKOUT 50 MINUTES
 HIPS & THIGHS WORKOUT 50 MINUTES INCREASE MUSCLE STRENGTH, TONE AND ENDURANCE IN THE HIPS, THIGHS AND BUTTOCKS LEADING TO LONGER MORE POWERFUL SKATING. ROLLERBLADE HIPS & THIGHS WORKOUT 50 MINUTES SKILLS
HIPS & THIGHS WORKOUT 50 MINUTES INCREASE MUSCLE STRENGTH, TONE AND ENDURANCE IN THE HIPS, THIGHS AND BUTTOCKS LEADING TO LONGER MORE POWERFUL SKATING. ROLLERBLADE HIPS & THIGHS WORKOUT 50 MINUTES SKILLS
Connections of Somatosensory Cortex in Megachiropteran Bats: The Evolution of Cortical Fields in Mammals
 THE JOURNAL OF COMPARATIVE NEUROLOGY 327~473-506 (1993) Connections of Somatosensory Cortex in Megachiropteran Bats: The Evolution of Cortical Fields in Mammals LEAH A. KRUBITZER, MIKE B. CALFORD, AND
THE JOURNAL OF COMPARATIVE NEUROLOGY 327~473-506 (1993) Connections of Somatosensory Cortex in Megachiropteran Bats: The Evolution of Cortical Fields in Mammals LEAH A. KRUBITZER, MIKE B. CALFORD, AND
1.45_Internet Assignment #1: The Human Body: An Orientation
 1.45_Internet Assignment #1: The Human Body: An Orientation Go to the following website to complete the following Activities, Quizzes, and Reading: http://wps.aw.com/bc_marieb_hap_9_oa/218/55856/14299219.cw/index.html
1.45_Internet Assignment #1: The Human Body: An Orientation Go to the following website to complete the following Activities, Quizzes, and Reading: http://wps.aw.com/bc_marieb_hap_9_oa/218/55856/14299219.cw/index.html
راما ندى أسامة الخضر. Faisal Muhammad
 22 راما ندى أسامة الخضر Faisal Muhammad Revision Last time we started talking about sensory receptors, we defined them and talked about the mechanism of their reaction. Now we will talk about sensory receptors,
22 راما ندى أسامة الخضر Faisal Muhammad Revision Last time we started talking about sensory receptors, we defined them and talked about the mechanism of their reaction. Now we will talk about sensory receptors,
YOU MUST BRING GLOVES FOR THIS ACTIVITY
 ACTIVITY 10: VESSELS AND CIRCULATION OBJECTIVES: 1) How to get ready: Read Chapter 23, McKinley et al., Human Anatomy, 5e. All text references are for this textbook. 2) Observe and sketch histology slide
ACTIVITY 10: VESSELS AND CIRCULATION OBJECTIVES: 1) How to get ready: Read Chapter 23, McKinley et al., Human Anatomy, 5e. All text references are for this textbook. 2) Observe and sketch histology slide
Chapter 14: The Cutaneous Senses
 Chapter 14: The Cutaneous Senses Somatosensory System There are three parts Cutaneous senses - perception of touch and pain from stimulation of the skin Proprioception - ability to sense position of the
Chapter 14: The Cutaneous Senses Somatosensory System There are three parts Cutaneous senses - perception of touch and pain from stimulation of the skin Proprioception - ability to sense position of the
VESSELS: GROSS ANATOMY
 ACTIVITY 10: VESSELS AND CIRCULATION OBJECTIVES: 1) How to get ready: Read Chapter 23, McKinley et al., Human Anatomy, 4e. All text references are for this textbook. 2) Observe and sketch histology slide
ACTIVITY 10: VESSELS AND CIRCULATION OBJECTIVES: 1) How to get ready: Read Chapter 23, McKinley et al., Human Anatomy, 4e. All text references are for this textbook. 2) Observe and sketch histology slide
SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Cortical Organization. Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:.
 Cortical Organization Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:. 2. Secondary cortex: located immediately adjacent to primary cortical areas,
Cortical Organization Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:. 2. Secondary cortex: located immediately adjacent to primary cortical areas,
Dr.Israa H. Mohsen. Lecture 5. The vertebral column
 Anatomy Lecture 5 Dr.Israa H. Mohsen The vertebral column The vertebral column a flexible structure consisting of 33 vertebrae holds the head and torso upright, serves as an attachment point for the legs,
Anatomy Lecture 5 Dr.Israa H. Mohsen The vertebral column The vertebral column a flexible structure consisting of 33 vertebrae holds the head and torso upright, serves as an attachment point for the legs,
Year 2004 Paper one: Questions supplied by Megan
 QUESTION 47 A 58yo man is noted to have a right foot drop three days following a right total hip replacement. On examination there is weakness of right ankle dorsiflexion and toe extension (grade 4/5).
QUESTION 47 A 58yo man is noted to have a right foot drop three days following a right total hip replacement. On examination there is weakness of right ankle dorsiflexion and toe extension (grade 4/5).
RECONSTRUCTION OF SUBTOTAL DEFECTS OF THE NOSE BY ABDOMINAL TUBE FLAP. By MICHAL KRAUSS. Plastic Surgery Hospital, Polanica-Zdroj, Poland
 RECONSTRUCTION OF SUBTOTAL DEFECTS OF THE NOSE BY ABDOMINAL TUBE FLAP By MICHAL KRAUSS Plastic Surgery Hospital, Polanica-Zdroj, Poland RECONSTRUCTION of the nose is one of the composite procedures in
RECONSTRUCTION OF SUBTOTAL DEFECTS OF THE NOSE BY ABDOMINAL TUBE FLAP By MICHAL KRAUSS Plastic Surgery Hospital, Polanica-Zdroj, Poland RECONSTRUCTION of the nose is one of the composite procedures in
Anatomical Language. Human Anatomy & Physiology Honors Ms. Chase
 Anatomical Language Human Anatomy & Physiology Honors 2014 2015 Ms. Chase Anatomical Position Body erect, feet slightly apart, palms facing forward, thumbs point away from the body DIRECTIONAL TERMS Allow
Anatomical Language Human Anatomy & Physiology Honors 2014 2015 Ms. Chase Anatomical Position Body erect, feet slightly apart, palms facing forward, thumbs point away from the body DIRECTIONAL TERMS Allow
Medical Terminology. Anatomical Position, Directional Terms and Movements
 Medical Terminology Anatomical Position, Directional Terms and Movements What we will cover... Content Objectives Students will be able to gain a better understanding and application of medical terminology
Medical Terminology Anatomical Position, Directional Terms and Movements What we will cover... Content Objectives Students will be able to gain a better understanding and application of medical terminology
Human Anatomy and Physiology I Laboratory Spinal and Peripheral Nerves and Reflexes
 Human Anatomy and Physiology I Laboratory Spinal and Peripheral Nerves and Reflexes 1 This lab involves the second section of the exercise Spinal Cord, Spinal Nerves, and the Autonomic Nervous System,
Human Anatomy and Physiology I Laboratory Spinal and Peripheral Nerves and Reflexes 1 This lab involves the second section of the exercise Spinal Cord, Spinal Nerves, and the Autonomic Nervous System,
1. Which part of the brain is responsible for planning and initiating movements?
 Section: Chapter 10: Multiple Choice 1. Which part of the brain is responsible for planning and initiating movements? p.358 frontal lobe hippocampus basal ganglia cerebellum 2. The prefrontal cortex is
Section: Chapter 10: Multiple Choice 1. Which part of the brain is responsible for planning and initiating movements? p.358 frontal lobe hippocampus basal ganglia cerebellum 2. The prefrontal cortex is
