Link to publication in the UWA Research Repository
|
|
|
- Eugenia Rose
- 5 years ago
- Views:
Transcription
1 Age estimation using the hand-wrist: morphological assessment of skeletal development in Western Australia Maggio, A. (2014). Age estimation using the hand-wrist: morphological assessment of skeletal development in Western Australia Link to publication in the UWA Research Repository Rights statement This work is protected by Copyright. You may print or download ONE copy of this document for the purpose of your own non-commercial research or study. Any other use requires permission from the copyright owner. The Copyright Act requires you to attribute any copyright works you quote or paraphrase. General rights Copyright owners retain the copyright for their material stored in the UWA Research Repository. The University grants no end-user rights beyond those which are provided by the Australian Copyright Act Users may make use of the material in the Repository providing due attribution is given and the use is in accordance with the Copyright Act Take down policy If you believe this document infringes copyright, raise a complaint by contacting repository-lib@uwa.edu.au. The document will be immediately withdrawn from public access while the complaint is being investigated. Download date: 02. Jul. 2018
2 Age estimation using the hand-wrist: morphological assessment of skeletal development in Western Australia. Ariane Maggio (BA, BSc, GDipForSci) This thesis is presented for the degree of Master of Forensic Science University of Western Australia Centre for Forensic Science 2014
3 DECLARATION I declare that the research presented in this thesis, for the Master of Forensic Science at the University of Western Australia, is my own work. The results of the work have not been submitted for assessment, in full or part, within any other tertiary institute, except where due acknowledgement has been made in the text. Ariane Maggio ii
4 ABSTRACT With increasing global migration the importance of estimating age for living individuals has increased due to the need to assess legal culpability and/or legal and social characterisation in the absence of documentation. Age estimates in living sub-adult individuals rely on the analysis of radiographs or CT scans of skeletal morphology in order to evaluate the skeletal development based on morphoscopic or morphometric analyses. In Australia, various age estimation techniques have been utilised in order to evaluate the age of individuals who do not have documentation to determine legal minority/culpability. The latter estimation techniques are not population specific and are thus known to be less accurate than population specific standards for the estimation of age. The study aimed to produce sex specific age estimation standards for a contemporary Western Australian population from the morphoscopic evaluation of the skeletal development of the hand and wrist. The study sample comprised 360 individuals (180 male, 180 female) between birth and 25 years of age. Age assessment was performed following the established Greulich & Pyle and Tanner-Whitehouse morphoscopic methods. Prior to data collection a precision test was performed to evaluate the level of intraobserver agreement. After ensuring normal distribution, data was analysed using a range of parametric statistical approaches. A paired sample t-test was performed to compare mean chronological and mean estimated skeletal age. A one-way repeated measures ANOVA was performed to assess if the difference in mean estimated skeletal age was significant between age estimation methods. The linear relationship between stated chronological and estimated skeletal age was first established using Pearson s correlation; thereafter age estimation models were developed using simple linear and polynomial regression. Sex specific differences in the timing of skeletal maturity were assessed using a two-way repeated measures ANOVA and by graphical comparison. iii
5 Estimated skeletal age for all methods was significantly correlated with stated chronological age (p< 0.001), and age prediction accuracy ranging from ±0.400 to ± years. Linear age estimation models were formulated, with prediction accuracy ranging from ±0.005 to ± years. Polynomial age estimation models were formulated, with prediction accuracy ranging from ±0.600 to ±1.700 years. The Greulich & Pyle method was the most accurate in simple linear regression (male: SEE ±0.005 years; female: ±0.412 years). The TW2 Carpal and 20-bone methods were the most accurate polynomial models for males (SEE ±1.200 years) and females (±0.600 years) respectively. This study provides viable contemporary alternatives for estimating the age in a sub-adult Western Australian population that are equivalent to established standards developed from hand-wrist bones. Furthermore, the study showed that the Greulich & Pyle and Tanner-Whitehouse methods are not reliable for the determination of legal minority and/or culpability in Western Australia. iv
6 TABLE OF CONTENTS DECLARATION... II ABSTRACT... III TABLE OF CONTENTS... V FIGURES... X TABLES... XIII ACKNOWLEDGEMENTS... XVI Professional... xvi Personal... xvi Dedication...xvii CHAPTER ONE INTRODUCTION Introduction Aims and Objectives Sources of Data Thesis Outline Limitations of this Study Discussion... 6 CHAPTER TWO HAND-WRIST SKELETAL ANATOMY AND DEVELOPMENT Introduction Skeletal Anatomy of the Hand-Wrist Skeletal Development The Radius and Ulna Development of the Radius and Ulna The Carpals Development of the Carpals v
7 2.6 The Metacarpals Development of the Metacarpals The Phalanges Development of the Phalanges Sesamoid Bones CHAPTER THREE AN OVERVIEW OF AGE ESTIMATION IN THE SUB-ADULT SKELETON Introduction Forensic Age Estimation and Legal Culpability Skeletal vs. Chronological Age Legal Culpability Casework Involving Skeletonised Remains Casework Involving Age Estimation in the Living Skeletal Visualisation Radiography Multi-Detector Computed Tomography Magnetic Resonance Imaging Age Estimation Techniques Molecular Morphometric Morphoscopic CHAPTER FOUR HAND-WRIST AGE ESTIMATION METHODOLOGIES AND STUDIES Introduction Medical Application of Hand-Wrist Age Estimation Methods Inter- and Intra-observer Agreement Atlas Methods Greulich & Pyle (1959) Validation studies vi
8 4.4.2 Thiemann-Nitz (Thiemann & Nitz 1991; Thiemann et al. 2006) Gilsanz & Ratib Atlas (2005; 2011) Mathematical/Scoring Methods Tanner-Whitehouse Method (Tanner et al. 1962; 1983; 2001) Validation studies The FELS Method (Roche et al. 1988) The Cameriere Method (Cameriere et al. 2005) The Contribution of the Present Research Thesis CHAPTER FIVE MATERIALS AND METHODS Introduction Materials Morphoscopic Assessment Visualisation Greulich & Pyle (1959) Tanner-Whitehouse (1983; 2001) Statistical Analyses Intra-observer Precision Descriptive Statistics Comparison of Means Univariate Significance Tests Assessment of Age Estimation Accuracy Regression Analyses CHAPTER SIX RESULTS Introduction Intra-observer Precision Greulich & Pyle Tanner-Whitehouse Descriptive Statistics vii
9 6.3.1 Normality Range, Mean, and Standard Deviation of Estimated Skeletal Age Statistical Evaluation of the Greulich & Pyle Age Estimation Method Correlation Age Prediction Accuracy Statistical Evaluation of the Tanner-Whitehouse Age Estimation Methods Correlation Age Prediction Accuracy Formulation of Age Estimation Standards Linear Regression Polynomial Regression Skeletal Age Tables and Skeletal Maturity Curves Sex specific Differences in Skeletal Maturity Two-way repeated measures ANOVA Skeletal Maturity Curves Population Specificity of Age Estimation Standards Skeletal Maturity Scores (SMS) Maturity Curves CHAPTER SEVEN DISCUSSION AND CONCLUSION Introduction Measurement Precision Age Prediction Accuracy Tanner-Whitehouse Systems Greulich & Pyle System Timing of Skeletal Maturity Sex specific Differences in Skeletal Maturity Population trends Factors that Influence Skeletal Maturation Socioeconomic status viii
10 7.5.2 Genetics Other Environmental Factors Forensic Applications Limitations Future Research Conclusion REFERENCES APPENDICES APPENDIX I I.1 Precision Test Data Greulich & Pyle I.2 Precision Test Data Tanner-Whitehouse APPENDIX II II.1 Age Estimates Existing Standards II.2 Age Estimates Linear Regression II.3 Age Estimates Polynomial Regression APPENDIX III III.1 Correlation Graphs Chronological vs. Skeletal Age APPENDIX IV IV.1 Standard Error and Skeletal Age Differences Existing Standards IV.2 Standard Error and Skeletal Age Differences W.A Standards APPENDIX V V.1 Comparison of WA and foreign RUS standards Repeated Measures ANOVA. 162 ix
11 FIGURES FIGURE 2.1: ADULT RIGHT HAND-WRIST... 8 FIGURE 2.2: UNFUSED EPIPHYSES OF THE HAND-WRIST... 9 FIGURE 2.3: STAGES OF INTRAMEMBRANOUS OSSIFICATION FIGURE 2.4: STAGES OF ENDOCHONDRAL OSSIFICATION FIGURE 2.5: LEFT CARPALS AND METACARPALS FIGURE 2.6: RIGHT METACARPALS, DORSAL VIEW FIGURE 2.7: METACARPAL GROWTH FIGURE 2.8: RIGHT HAND PHALANGES FIGURE 5.1: COMPARISON OF EXAMPLE RADIOGRAPH TO GREULICH & PYLE STANDARDS FIGURE 5.2: TANNER-WHITEHOUSE SKELETAL DEVELOPMENT STAGES OF THE RADIUS FIGURE 5.3: COMPARISON OF AN EXAMPLE DISTAL RADIUS RADIOGRAPH TO TANNER- WHITEHOUSE RADIUS DIAGRAM FIGURE 5.4: SKELETAL ELEMENTS UTILIZED BY TANNER-WHITEHOUSE METHODS FIGURE 6.1: SCATTER PLOT WITH ASSOCIATED REGRESSION LINES SHOWING THE RELATIONSHIP BETWEEN STATED CHRONOLOGICAL AND GREULICH-PYLE ESTIMATED SKELETAL AGE FIGURE 6.2: SCATTER PLOT WITH ASSOCIATED REGRESSION LINES SHOWING THE RELATIONSHIP BETWEEN STATED CHRONOLOGICAL AND TW2 RUS ESTIMATED SKELETAL AGE x
12 FIGURE 6.3: SMOOTHED TW-WA RUS MATURITY STANDARDS FOR FEMALES FIGURE 6.4: SMOOTHED TW-WA CARPAL MATURITY STANDARDS FOR FEMALES FIGURE 6.5: SMOOTHED TW-WA 20-BONE MATURITY STANDARDS FOR FEMALES FIGURE 6.6: SMOOTHED TW-WA RUS MATURITY STANDARDS FOR MALES FIGURE 6.7: SMOOTHED TW-WA CARPAL MATURITY STANDARDS FOR MALES FIGURE 6.8: SMOOTHED TW-WA 20-BONE MATURITY STANDARDS FOR MALES FIGURE 6.9: THE RELATIONSHIP BETWEEN TW-WA RUS SKELETAL MATURITY SCORE AND ESTIMATED SKELETAL AGE FOR MALES AND FEMALES FIGURE 6.10: THE RELATIONSHIP BETWEEN TW-WA CARPAL SKELETAL MATURITY SCORE AND ESTIMATED SKELETAL AGE FOR MALES AND FEMALES FIGURE 6.11: MALE TW-WA RUS SKELETAL MATURITY CURVE COMPARED TO SIX FOREIGN POPULATION STANDARDS FIGURE 6.12: FEMALE TW-WA RUS SKELETAL MATURITY CURVE COMPARED TO SIX FOREIGN POPULATION STANDARDS FIGURE 6.13: MALE TW-WA CARPAL SKELETAL MATURITY CURVE COMPARED TO THREE FOREIGN POPULATION STANDARDS FIGURE 6.14: FEMALE TW-WA CARPAL SKELETAL MATURITY CURVE COMPARED TO THREE FOREIGN POPULATION STANDARDS FIGURE 7.1: COMPARISON OF AN EXAMPLE DISTAL PHALANX I RADIOGRAPH TO TANNER-WHITEHOUSE STAGE F DISTAL PHALANX I STANDARD FIGURE AIII.1: SCATTERPLOT SHOWING THE RELATIONSHIP BETWEEN STATED CHRONOLOGICAL AND TW2 CARPAL ESTIMATED SKELETAL AGE FOR MALES AND FEMALES WITH REGRESSION LINES xi
13 FIGURE AIII.2: SCATTERPLOT SHOWING THE RELATIONSHIP BETWEEN STATED CHRONOLOGICAL AND TW2 20-BONE ESTIMATED SKELETAL AGE FOR MALES AND FEMALES WITH REGRESSION LINES FIGURE AIII.3: SCATTERPLOT SHOWING THE RELATIONSHIP BETWEEN STATED CHRONOLOGICAL AND TW3 RUS ESTIMATED SKELETAL AGE FOR MALES AND FEMALES WITH REGRESSION LINES xii
14 TABLES TABLE 2.1: APPROXIMATE AGE RANGE FOR DEVELOPMENTAL MILESTONES OF THE CARPAL BONES TABLE 2.2: APPROXIMATE AGE RANGE FOR DEVELOPMENTAL MILESTONES OF THE METACARPALS TABLE 2.3: APPROXIMATE AGE RANGE FOR DEVELOPMENTAL MILESTONES OF THE PHALANGES TABLE 5.1: AGE DISTRIBUTION FOR THE TOTAL SAMPLE TABLE 5.2: RANGE OF TANNER-WHITEHOUSE ESTIMABLE SKELETAL AGES TABLE 5.3: KAPPA STATISTIC RANGES AND CORRESPONDING LEVEL OF AGREEMENT. 48 TABLE 6.1: TANNER-WHITEHOUSE INTRA-OBSERVER AGREEMENT TABLE 6.2: KOLMOGOROV-SMIRNOV TEST FOR NORMALITY OF ESTIMATED SKELETAL AGE TABLE 6.3: RANGE, MEAN AND STANDARD DEVIATION OF ESTIMATED AGE FOR THE FIVE METHODS ANALYSED TABLE 6.4: CALCULATED STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE FOR THE GREULICH & PYLE METHOD TABLE 6.5: CALCULATED STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE FOR EACH OF THE TANNER-WHITEHOUSE AGE ESTIMATION METHODS TABLE 6.6: LINEAR REGRESSION EQUATIONS FROM THE GREULICH & PYLE AND FOUR TANNER-WHITEHOUSE AGE ESTIMATION METHODS EXAMINED TABLE 6.7: POLYNOMIAL REGRESSION EQUATIONS FROM THE 50 TH CENTILE TANNER- WHITEHOUSE MATURITY SCORES xiii
15 TABLE 6.8: FEMALE TW-WA RUS SKELETAL AGE FOR A GIVEN SKELETAL MATURITY SCORE TABLE 6.9: FEMALE TW-WA CARPAL AGE FOR A GIVEN SKELETAL MATURITY SCORE.. 67 TABLE 6.10: FEMALE TW-WA 20-BONE AGE FOR A GIVEN SKELETAL MATURITY SCORE TABLE 6.11: MALE TW-WA RUS SKELETAL AGE FOR A GIVEN SKELETAL MATURITY SCORE TABLE 6.12: MALE TW-WA CARPAL AGE FOR A GIVEN SKELETAL MATURITY SCORE (SMS) TABLE 6.13: MALE TW-WA 20-BONE AGE FOR A GIVEN SKELETAL MATURITY SCORE.. 75 TABLE 6.14: TWO-WAY REPEATED MEASURES ANOVA TABLE 6.15: MEAN AGE IN MALES FOR ATTAINMENT OF SUCCESSIVE TW RUS SKELETAL MATURITY SCORES FOR VARIOUS POPULATIONS TABLE 6.16: MEAN AGE IN FEMALES FOR ATTAINMENT OF SUCCESSIVE TW RUS SKELETAL MATURITY SCORES FOR VARIOUS POPULATIONS TABLE 7.1: COMPARISON OF MEAN SKELETAL AGE DIFFERENCE FOR GREULICH & PYLE SKELETAL AGE ESTIMATES FROM DIFFERENT POPULATIONS TABLE AI.1: GREULICH & PYLE PRECISION TEST DATA TABLE AI.2: FEMALE TANNER-WHITEHOUSE MATURITY SCORES BY ELEMENT FOR THE PRECISION TEST TABLE AI.3: MALE TANNER-WHITEHOUSE MATURITY SCORES BY ELEMENT FOR THE PRECISION TEST TABLE AII.1: AGE ESTIMATION DATA FOR FEMALES: STATED AGE; SKELETAL MATURITY SCORES AND SKELETAL AGE ESTIMATES xiv
16 TABLE AII.2: AGE ESTIMATION DATA FOR MALES: STATED AGE; SKELETAL MATURITY SCORES AND SKELETAL AGE ESTIMATES TABLE AII.5: AGE ESTIMATES FOR FEMALES USING LINEAR REGRESSION TABLE AII.6: AGE ESTIMATES FOR MALES USING LINEAR REGRESSION TABLE AII.7: AGE ESTIMATES FOR FEMALES USING POLYNOMIAL REGRESSION TABLE AII.8: AGE ESTIMATES FOR MALES USING POLYNOMIAL REGRESSION TABLE AIV.1: TW2 RUS STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE BY 12 MONTH AGE GROUP TABLE AIV.2: TW2 CARPAL STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE BY 12 MONTH AGE GROUP TABLE AIV.3: TW2 20-BONE STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE BY 12 MONTH AGE GROUP TABLE AIV.4: TW3 STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE BY 12 MONTH AGE GROUP TABLE AIV.5: GREULICH & PYLE STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE BY 12 MONTH AGE GROUP, INDIVIDUAL BY SEX TABLE AIV.6: TW-WA RUS STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE BY 12 MONTH AGE GROUP TABLE AIV.7: TW-WA CARPAL STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE BY 12 MONTH AGE GROUP TABLE AIV.8: TW-WA 20-BONE STANDARD ERROR AND MEAN SKELETAL AGE DIFFERENCE BY 12 MONTH AGE GROUP TABLE AV.1: RESULTS OF PAIRWISE COMPARISONS: WESTERN AUSTRALIAN STANDARD VS. FOREIGN STANDARDS xv
17 ACKNOWLEDGEMENTS This research project has been a year long journey, which would not have been possible without the support of a number of people. I take this opportunity to offer my sincere appreciation to the many people who have made this project possible, and without whom I would not have been able to complete this research. Professional First, I must thank my co-ordinating supervisor, Professor Daniel Franklin. I am extremely grateful for his seemingly never-ending supply of patience and support, as well as his thoughtful and detailed feedback on this thesis. Thanks are also due to my co-supervisor Ms Ambika Flavel, without whom I would have floundered in a pit of formatting related despair! I would also like to thank the staff and my fellow research students at the Centre for Forensic Science, firstly for providing me with the resources to facilitate this research project, but also for their support and feedback. Special thanks are also due to Adj. A/Prof. Rob Hart from Frontier Medical Imaging International for providing the radiographs used in this research project, without which this project would not exist. Personal I would not have been able to do this without the support of my family and friends. I especially wish to thank my mother, Maureen Maggio, who has been a bastion of loving support and continual encouragement throughout my studies, and indeed for my entire life. Thanks must also go to my lovely sister Emma Claudius, the best and most supportive sister a girl could have! I am also extremely grateful to my stepfather, Lindsay Claudius, for his own special brand of support and reassurance. xvi
18 I want to thank my close friend, Timo Ernst, for his support, guidance, reassurance and for reminding me of what I am capable of. Thanks are also due to the family cat Osiris, who closely supervised the writing of the majority of this thesis, and continually grounded me with the reminder that there were other important things in life outside of my research (such as feeding him). Lastly, to all of my family and friends, thank you for all of your guidance, patience and support. Dedication I would like to dedicate this thesis in memory of my grandmother, Artemisia Maggio (nee Gatti), who encouraged and supported my studies, but unfortunately passed away before she could see me reach this milestone. I hope she would have been proud. xvii
19
20 CHAPTER ONE INTRODUCTION 1.1 Introduction Forensic anthropology is the application of the theory and method of the discipline of physical anthropology in a medico-legal context (Cattaneo 2007). This typically involves the analysis of skeletal remains towards establishing personal identity. To achieve this, and only once the remains have been identified as human, a forensic anthropologist will aim to generate a biological profile (estimating age, sex, stature, and ancestry) in addition to assessing the remains for any pathology or trauma (Steadman 2012). Generally, standards derived from documented skeletal collections of known sex and age are utilised to predict these indicators of identity (Dirkmaat & Cabo 2012). Standards for the estimation of biological attributes (e.g. age, sex, and stature) derived from one population are generally less accurate when applied to a foreign population (Santoro et al. 2012). In addition, the skeletal collections studied in the formation of standards often comprise historic individuals, which do not always accurately represent modern populations, due to temporal variance of genetic and environmental factors that influence skeletal development. This loss of accuracy, along with the lack of documented skeletal collections in some countries, has led to the increased use of radiographs and computer-tomographic (CT) scans as a data source from which standards representing modern populations are formulated (Franklin 2010; Franklin et al CT scans and radiographs are a demonstrated and appropriate source of biological data, as well as a proxy for physical skeletal collections (Acheson 1966; Dedouit et al. 2012; Franklin et al. 2013). Furthermore, the use of scans and radiographs as a proxy for skeletal collections also has the benefit of including areas of the skeleton that may not be present in traditional skeletal collections (such as epiphyses) due to differential 1
21 preservation and taphonomic processes associated with death and post mortem environment (Hackman et al. 2010). With increasing global migration there is a growing role for forensic anthropologists to provide age estimates for living individuals who cannot provide documentary evidence of their date of birth, and therefore their chronological age (Schmeling et al. 2006). Age estimates in living individuals rely on the analysis of radiographs or CT scans of skeletal morphology, such as the epiphyseal regions of bones, in order to evaluate the skeletal development of the individual based on morphology and/or metrics. The degree of skeletal development is then used to assess (or infer- see below) the age of the individual (Hackman et al. 2010). Chronological age estimates are most frequently derived from the quantitative analysis of skeletal morphology; however these analyses are in actuality only an estimate of skeletal age. The latter, however, does not necessarily accurately represent chronological age, as individual growth and development varies in relation to a variety of complex factors, such as nutrition, environment, exposure to pathogens, and genetics (The Royal Australian College of Physicians 2012). Irrespective, the assessed skeletal age is often used to infer a chronological age estimate where no other documentation is available (Schmeling et al. 2006). There are several anthropological based techniques for estimating age in living individuals (Schmeling et al. 2006). These techniques include dental analysis, radiographic analysis of the clavicle, elbow, knee, foot and hand-wrist (Franklin 2010). In the absence of the dentition, the most widely used techniques for age estimation rely on the morphological assessment of skeletal growth and development (Franklin 2010). Sex specific age estimation standards are known to be more accurate due to the presence of sex differences in the timing of skeletal development and maturation (Clarke & Hayman 1962); it has been demonstrated that females on average physically develop earlier than males (Tanner 1971). For this reason, it is important to develop 2
22 population and sex-specific forensic standards for estimating age in order to improve the accuracy of the age final estimate (Santoro et al. 2012). 1.2 Aims and Objectives The purpose of the present study is to apply radiographic hand-wrist age estimation techniques to individuals from a Western Australian population in order to assess their accuracy and thereafter derive population specific age estimation standards. The importance of this study is that it has the potential to contribute to forensic standards for Western Australians, as well as assessing the reliability of using established handwrist age estimation techniques on foreign populations. The specific aims of this thesis are: i) To assess the relationship between skeletal and chronological age Although dental age estimation is generally considered the most accurate approach in juveniles, the assessment of skeletal development and epiphyseal fusion are also used to estimate age (Franklin 2010). The most widely accepted methods for age estimation using hand-wrist radiographs are those of Greulich and Pyle (1959) and Tanner et al. (1983; 2001). The former method involves the visual assessment of the development of the bones of the hand-wrist from an anterior-posterior radiograph, using reference atlas images and written descriptions (Greulich & Pyle 1959). There are several studies concerning the applicability of the Greulich-Pyle method (e.g. Büken et al. 2007; Santoro et al. 2012) that have demonstrated that the method is less accurate when applied to a foreign population. However, due to its relative ease of use, it is still widely used to estimate age in living individuals. In comparison, the Tanner- Whitehouse methods (TW2; TW3) utilize a cumulative scoring system. Studies by Schmidt et al. (2008), Bull et al. (1999) and King et al. (1994) suggest that the TW methods are more accurate than the Greulich-Pyle method. To this end, the present study will assess the accuracy of the Greulich-Pyle, TW2 and TW3 methods for age estimation using hand-wrist radiographs in a Western Australian population. The primary aim is to determine which of the aforementioned hand-wrist methods are the most accurate for forensic application in Western Australia. 3
23 ii) To assess sex-specific differences in the timing of skeletal maturation in the hand-wrist bones It is generally accepted that females physically mature on average two years earlier than males (Tanner 1971); this is believed to be related to an earlier onset of puberty in females. Greulich and Pyle (1959:125) state that the rate of skeletal development of the bones of the hand and wrist differs between males and females from as early as three months of age. This difference in the rate of development increases, especially around the early teen years and the onset of puberty (Hewitt & Acheson 1961; Tanner 1971). As a result of this differential rate of development between the sexes, the most accurate skeletal development age estimation standards are sex-specific. This study will evaluate the average ages that males and females meet the developmental stages outlined by Tanner et al. (2001) and quantify the difference in the rate of hand-wrist skeletal development between males and females in a Western Australian population. iii) To develop population specific hand-wrist age estimation standards for a Western Australian population As noted above, it has been demonstrated that the Greulich & Pyle and Tanner- Whitehouse methods are less accurate when applied to foreign populations (e.g. Andersen 1971; Schmidt et al. 2008). It is important, therefore, to develop population specific forensic age estimation standards in order to improve the accuracy of the final estimate (Santoro et al. 2012). The present study will, therefore, develop hand-wrist age estimation standards for forensic application in Western Australia. 1.3 Sources of Data A total of 360 anterior-posterior hand-wrist radiographs (180 male; 180 female) from birth through to 25 years of age, were acquired from the Western Australian Department of Health, and represent patients presenting to the major hospitals in Western Australia (e.g. Royal Perth, Sir Charles Gairdner). These scans, acquired from the Picture Archiving and Communication Systems (PACS) database, were anonymised prior to receipt in order to maintain patient confidentiality, with only the sex of the individual, their date of birth and the date of exam provided. Radiographs that 4
24 displayed major anomalies (e.g. polydactyly) or considerable skeletal trauma (e.g. fractures) of the bones of the hand and wrist were excluded. 1.4 Thesis Outline Chapter Two will discuss the anatomy and development of the skeletal elements of the hand-wrist. Chapter Three will present a general overview of the main methods of estimating age from the human skeleton, with a specific focus on forensic applications. The methodology of morphological age estimation techniques using radiographic images will be examined in detail. In Chapter Four the literature pertinent to the present project is reviewed by discussing previous relevant hand-wrist age estimation studies. Chapter Five will outline the materials and methods employed in this research project. The results of this study are presented in Chapter Six. A critical discussion of the results of the study, including interpretations of the results, is presented in Chapter Seven. This final chapter concludes with a brief discussion of the limitations of this study and potential directions for further research. 1.5 Limitations of this Study Data was collected from anonymised clinical scans obtained from a PACS database, and as such there is an obvious sampling bias towards those individuals who have attended a hospital clinic for treatment. This is an inherent issue of all medical modalities and as such scans displaying major anomalies or considerable skeletal trauma were excluded. It is assumed that the analysed scans are representative of a Western Australian population. Ongoing research by the Centre for Forensic Science (Franklin & Flavel, 2013, unpublished data) suggests that cranial scans from the same PACS database contain an ethnic mix consistent with population census data (see also Chapter Seven). Finally, this study has assessed a mixture of left and right hand-wrist radiographs and has not accounted for potential bilateral asymmetry. This is because it is practically 5
25 impossible to obtain a large sample of bilateral clinical hand radiographs as they are not routinely done in Western Australian hospitals. 1.6 Discussion It is known that applying age estimation standards to foreign populations decreases the accuracy of an age estimate (Santoro et al. 2012). There are currently no handwrist age estimation standards specific to a Western Australian population. That population consists of individuals from a variety of diverse ethnic backgrounds, some of which are not adequately represented in current foreign standards. Western Australian population specific age estimation standards have significant forensic relevance as they will provide information that can be used to aid in the formation of a biological profile, or for use in providing an age estimate for living individuals within Australia, who in rare instances, do not have appropriate age documentation. Furthermore, the creation of standards for a Western Australian population would contribute to the pool of knowledge on human variation (in regards to skeletal morphology and development), as well as the applicability of forensic standards to foreign populations which could guide forensic practice in other jurisdictions. 6
26 CHAPTER TWO HAND-WRIST SKELETAL ANATOMY AND DEVELOPMENT 2.1 Introduction The specific aim of this thesis is to assess the accuracy of analysing the skeletal development of the hand-wrist for the purpose of estimating the age of sub-adults in Western Australia. This necessitates an understanding of skeletal growth, anatomy and development, which is accordingly discussed in this chapter. Specific emphasis is placed on defining the approximate ages at which the various elements first appear and subsequently develop into an adult shape in dry bone and fuse to form the adult hand-wrist. 2.2 Skeletal Anatomy of the Hand-Wrist The skeletal structure of the human hand-wrist is homologous to the limb structures of other species, including; the paw of a bear, the wing of a bat or bird and the flipper of a whale (Saladin 2007). The human hand, however, underwent significant developmental changes as hominids evolved to a fully bipedal locomotion (Jones & Lederman 2006). This change in locomotion was highly significant to the evolution of the human hand-wrist as it facilitated the use of the upper limbs for non-locomotion related tasks, such as the construction and use of tools (Marzke 1997). The underlying architecture of the hand-wrist thus reflects functional adaptations for precision movements (Landsmeer 1962; Marzke 1997). The latter adaptations include the radial orientation of the head of the third metacarpal, the asymmetry of the heads of the second and fifth metacarpals, and a change in the orientation away from the sagittal plane of the proximal joint of the second metacarpal (Marzke 1997). A longer thumb relative to finger length, with increased movement at the proximal joint, is yet another adaptation that allows the human hand to form a precision grip (Mackenzie & Iberall 1994; Marzke 1997). 7
27 The normal human adult hand-wrist (Figure 2.1) contains 29 separate bones; five metacarpals, eight carpals, 14 phalanges and the distal ends of the radius and ulna (White et al. 2011). These bones are assigned to three anatomical regions; the wrist, the palm and the digits (Saladin 2007). Sesamoid bones are also typically present at the heads of metacarpals I and V (White & Folkens 2005). Figure 2.1: Adult right hand-wrist, ventral view (adapted from Drake et al. 2009). The eight carpal bones are tightly articulated in two rows to form the wrist (White et al. 2011). The proximal row of the carpals articulate with the distal ends of the radius and ulna to form the wrist joint (White & Folkens 2005). The wrist articulates distally to the palm of the hand which is formed by the five metacarpals (White et al. 2011). These in turn articulate with the phalanges to form the digits; the four fingers and thumb (Saladin 2007). The fingers have three phalanges each (proximal, middle and distal), whereas the thumb has only proximal and distal phalanges (White et al. 2011). The juvenile hand-wrist skeleton initially contains more than 29 separate bones (see Figure 2.2). During development the secondary ossification centres (epiphyses) of the 8
28 radius, ulna, metacarpals and phalanges ossify and develop separate to their primary centres (diaphyses) (Scheuer & Black 2000). Thereafter, during skeletal development, fusion of the primary and secondary centres occurs. The radius, ulna, metacarpals and phalanges are normally present at birth (Scheuer & Black 2004), although in a less developed form, whilst the carpals develop postnatally (Scheuer & Black 2000). Specific development of the bones of the hand and wrist is described below. Figure 2.2: Unfused epiphyses of the hand-wrist, left dorsal view (from Tanner et al. 2001). 2.3 Skeletal Development There are two processes responsible for the formation of bone tissue; intramembranous and endochondral ossification (see Figures 2.3 and 2.4 below). Intramembranous ossification occurs within a fibrous embryonic connective tissue membrane, where mesenchymal cells cluster and differentiate into osteoblasts to form a primary ossification centre (Burr & Allen 2013). In comparison, during endochondral ossification, a hyaline cartilage model is first formed by chondrocytes, which then acts as a precursor for bone formation (Burr & Allen 2013). Secondary ossification centres, such as the epiphyses of the radius and ulna, are typically formed through endochondral ossification. 9
29 Figure 2.3: Stages of intramembranous ossification: A) Differentiation of mesenchymal cells; B) Calcification of bone matrix and formation of osteocytes; C) Formation of trabecular bone and formation of the periosteum; D) Formation of compact bone and cavities for blood vessels (from Marieb & Hoehn 2006). Figure 2.4: Stages of endochondral ossification: A) Hyaline cartilage precursor; B) Deterioration of cartilage matrix; C) Formation of trabecular bone; D) Formation of the medullary cavity; E) Completed development (from Marieb & Hoehn 2006). 2.4 The Radius and Ulna The radius and the ulna form the distal portion of the upper limb (White et al. 2011). The distal end of the radius articulates with the lunate on the medial side and the scaphoid on the lateral side (White & Folkens 2005; see also Figure 2.1 and 2.2). 10
30 2.4.1 Development of the Radius and Ulna It is important to note that the following are generalised developmental milestones and the timing of skeletal maturation can vary (see below). The primary ossification centre (diaphysis) of the radius develops by endochondral ossification at approximately week 7 of fetal development (Scheuer & Black 2000). The diaphysis is present at birth (excluding developmental retardation), with the secondary ossification centre for the distal epiphysis developing between 1 and 2 years of age (Scheuer & Black 2004). The secondary centre for the proximal epiphysis of the radius appears at approximately 5 years of age, with the styloid process of the distal epiphysis forming by 8 years of age (Schaefer et al. 2010). The proximal epiphysis of the radius fuses between the ages of 12 and 16 in females, and 14 to 18 in males (Scheuer & Black 2004). The distal epiphysis of the radius fuses between 14 to 19 in females and 16 to 20 in males (Greulich & Pyle 1959). The primary ossification centre (diaphysis) of the ulna similarly starts to develop prenatally at week 7 (Scheuer & Black 2000). At birth the ulnar diaphysis is normally present, with the secondary ossification centre (distal epiphysis) appearing between 5 to 7 years of age (Schaefer et al. 2010). The styloid process forms at approximately 8 to 10 years of age, when the secondary centres for the proximal epiphysis develop (Scheuer & Black 2004). In females the proximal epiphysis fuses between the ages of 12 and 15, while in males it fuses between the ages of 14 and 18 (Schaefer et al. 2010). The distal epiphysis of the ulna typically fuses between the ages of 15 and 19 for both sexes (Baumann et al. 2009; Greulich & Pyle 1959). 2.5 The Carpals There are eight carpal bones in the adult human wrist, arranged in two rows between the metacarpals and the distal radius and ulna (Figure 2.5; White et al. 2011). The proximal row includes the scaphoid, lunate, triquetral and pisiform. The trapezium, trapezoid, capitate and hamate form the distal row (White & Folkens 2005). 11
31 Figure 2.5: Left carpals and metacarpals, ventral view. Metacarpals shown in green, distal carpals in blue, proximal carpals in red (adapted from Standrig 2008). The scaphoid is positioned between the radius and trapezium at the base of the thumb; it is the most lateral and proximal carpal (see Figure 2.5). The scaphoid is characterised by a major concave surface for the head of the capitate, adjacent to the blunt tail-like tubercule, as well as a major convex surface for articulation with the distal end of the radius (White & Folkens 2005). Medial to the scaphoid is the lunate (Figure 2.5), which on its proximal surface has a convex facet that articulates with the radius, and on the distal surface has a concave surface for articulation with the capitate (White & Folkens 2005). The triquetral has three distinct articular surfaces for the hamate, lunate and pisiform (White et al. 2011). The articular surface for the pisiform is a single circular elevated facet, on the medial surface of the bone. The hamate facet is distal to the lateral lunate facet, although the two are continuous (White & Folkens 2005). The pisiform is the smallest of the carpals; it is technically a sesamoid bone because it develops within a tendon (White et al. 2011). The pisiform is pea-shaped with a flattened articular surface for the triquetral (White & Folkens 2005). The trapezium is the most lateral carpal of the distal row, situated at the base of the metacarpal I (Figure 2.5). It is irregularly shaped with a large saddle shaped articular surface for metacarpal I (White & Folkens 2005). The trapezium also articulates with metacarpal II and the scaphoid 12
32 (White & Folkens 2005). The trapezoid is the smallest carpal of the distal row and is roughly shaped like a boot. It articulates with the trapezium, the scaphoid, the capitate and metacarpal II (Figure 2.5). The capitate has a rounded proximal head and a square shaped distal end which articulates with metacarpals II and III (and occasionally IV; Figure 2.5; White et al. 2011). The hamate is the large triangular shaped carpal that articulates with the capitate, the triquetral and the lunate, and the bases of the metacarpals IV and V (White & Folkens 2005; Figure 2.5). The hamate has a hook-shaped projection (hamulus) on the palmar surface which is an attachment point for a fibrous band though which the flexor tendons of the wrist pass (White et al. 2011) Development of the Carpals Unlike other bones of the hand-wrist, the carpals do not develop epiphyses (Scheuer & Black 2004; Burr & Allen 2013). Scheuer and Black (2004) note that with the exception of the pisiform, the sequence of appearance of the carpals is circular, starting with the capitate, proceeding counter-clockwise (in anatomical position) and ending with the trapezium and trapezoid. Table 2.1: Approximate age range for developmental milestones of the carpal bones (adapted from Scheuer & Black 2004). Bone Ossified centre present Developmental Milestone Adult morphology reached Capitate 2-4 months years Hamate 3-5 months years Triquetral 1-3 years years Lunate 3-4 years years Scaphoid 5-6 years years Trapezium 4-5 years years Trapezoid 4-5 years years Pisiform 8-10 years years 13
33 The capitate is the first carpal to ossify, typically appearing between 2 to 3 months postnatal in females, and 3 to 4 months in males (Table 2.1). The primary centre for the capitate can sometimes be present at birth (Scheuer & Black 2000). The hamate ossifies next; it develops articular surfaces as it changes in size and shape from a small roughened nodule to an inverted triangle (Scheuer & Black 2000). By 11 to 14 years of age the hamulus is radiographically identifiable (Greulich & Pyle 1959). The triquetral has a highly variable onset of ossification, developing in the first year for some individuals, and the second or third for others (Johnston et al. 1968). The lunate develops recognisable articular surfaces for the scaphoid and the triquetral after 4 to 5 years of age (Scheuer & Black 2000). The ossification centres appear concurrently for the trapezium and trapezoid at approximately 4 to 5 years of age (Scheuer & Black 2004). The scaphoid forms a distinct tear-drop shape at 7 to 8 years of age (Scheuer & Black 2004). The pisiform is the last carpal to commence ossification at approximately 8 to 10 years of age (Scheuer & Black 2004). 2.6 The Metacarpals There are five metacarpals in the normal human hand (Figure 2.6), which form the palm and articulate at their proximal ends with the distal row of the carpals (White et al. 2011). The distal ends of the metacarpals articulate with the proximal phalanges (Figures 2.1 and 2.2). The metacarpals are numbered MCI (lateral) through to MCV (medial); MCI articulates with the phalanges that form the thumb, whilst MCV is the most medial, and articulates with the phalanges that form the 5 th digit (White et al. 2011). The metacarpals are all tubular bones that are longer than they are wide, with a distal articular surface (head) and more rectangular proximal ends (base) (White & Folkens 2005). The first metacarpal (MCI) is the shortest; it is broad and robust with a single articular surface on the proximal end (White & Folkens 2005). The second metacarpal (MCII) is the longest metacarpal and articulates with the base of the index finger. The base of MCII is long, curved and has a wedge shaped proximal surface that articulates with the 14
34 trapezoid, capitate and trapezium, and laterally to the sharp styloid process on the base of MCIII (White et al. 2011). The third metacarpal (MCIII) also articulates with the capitate and MCIV. The latter metacarpal is shorter and more gracile than MCII and MCIII, with a square shaped base and between 3 and 4 proximal facets that articulate with the hamate, MCIII and MCV (White & Folkens 2005). It is also sometimes articulated with the capitate (White et al. 2011). The fifth metacarpal (MCV) has two proximal facets and is the thinnest and shortest of all of the metacarpals; it articulates with the hamate and MCIV (White & Folkens 2005). Figure 2.6: Right metacarpals, dorsal view (adapted from White et al. 2011) Development of the Metacarpals The second to fifth metacarpals ossify from two centres. The primary ossification centre forms the shaft and base of the metacarpal prenatally (intramembranous ossification) whilst the secondary ossification centre forms the distal ends postnatally (endochondral ossification) (Scheuer & Black 2004). The primary ossification centre of MCI forms the shaft and head of the metacarpal prenatally, and the base develops from a secondary ossification centre postnatally (Scheuer & Black 2000). 15
35 Table 2.2: Approximate age range for developmental milestones of the metacarpals (adapted from Scheuer & Black 2004). Bone Developmental Milestone Primary centre ossifies Secondary centre ossifies Fusion MCI 9-11 weeks prenatal months postnatal years MCII 8-10 weeks prenatal months postnatal years MCIII 8-10 weeks prenatal months postnatal years MCIV 8-10 weeks prenatal months postnatal years MCV 8-10 weeks prenatal months postnatal years The primary centres of the metacarpals ossify prenatally between approximately 8 and 10 weeks (Table 2.2). Ossification commences in MCII and MCIII, followed by MCIV and MCV, with MCI the last to ossify at 9 to 11 weeks (Scheuer & Black 2004). The heads of MCII-V develop from secondary ossification centres between 16 and 29 months of age, whilst the base of MCI begins to ossify at between 25 and 37 months of age (Scheuer & Black 2000). The metacarpal heads appear as circular undifferentiated ossified centres until approximately 5 or 6 years of age (Figure 2.7), at which stage they begin to develop distal articular surfaces (Tanner et al. 2001). Complete fusion of the metacarpals occurs between 14 and 16 years of age (Greulich & Pyle 1959). Figure 2.7: Metacarpal growth. Pairs of immature carpals (at 1 and 6 years of age) are shown in ventral view, with MCI on the left and MCV on the right (White et al. 2011). 16
36 2.7 The Phalanges There are 14 phalanges that form the digits of a normal human hand (Saladin 2007). Like the metacarpals the phalanges are numbered from I through V, according to digit of origination. Phalanx I is digit I the thumb (Tanner et al. 2001). The five proximal phalanges articulate with the distal ends of the metacarpals (Figure 2.8). The four middle (or intermediate) phalanges articulate with the distal ends of the proximal phalanges, with the exception of the thumb, which does not have a middle phalanx. The five distal phalanges, which form the tips of the digits, articulate with the distal ends of the middle phalanges for digits II through V, and directly with the distal end of the proximal phalanx of digit I. In general, the phalanges of the hand are all shorter than the metacarpals (White & Folkens 2005). The dorsal surfaces of the phalanges are more smooth and rounded than the ventral surfaces (White et al. 2011). Each phalanx has raised ridges that extend laterally; these are the soft tissue attachment sites (see Figure 2.8 and Jones & Lederman 2006). Figure 2.8: Dorsal view of the right hand phalanges (adapted from White et al. 2011). Proximal phalanges shown in red, middle phalanges in blue, distal phalanges in green. 17
37 The proximal phalanges have a single proximal concave facet for articulation with the heads of the metacarpals (White & Folkens 2005). There are two projections of their distal ends (the ulnar and radial condyles) that articulate with the two proximal facets of the bases of the middle phalanges (II to V) or the base of distal phalanx I (Figure 2.8; Mackenzie & Iberall 1994). Proximal phalanx III is the longest, followed by the IV, II, and V (White et al. 2011). Proximal phalanx I is the shortest and V is the narrowest. Proximal phalanx III has the largest maximum width at the head (Garrido Varas & Thompson 2011). The middle phalanges have a distal facet which articulates with the proximal end of distal phalanges II through V. The distal phalanges have two proximal facets that articulate with the heads of the middle phalanges and proximal phalanx I (White & Folkens 2005). The distal ends of the distal phalanges have a non-articular pad called the distal phalangeal tuberosity (White et al. 2011) Development of the Phalanges Hand phalanges ossify from a primary centre (intramembranous) for the shaft and a secondary centre (endochondral) for the bases (Scheuer & Black 2004). As with the metacarpals, the shafts of the phalanges develop in utero and are present at birth, whilst the epiphyses that form the bases of the phalanges form postnatally (Scheuer & Black 2000). Table 2.3 summarises the approximate age at which the primary centres (diaphyses) and secondary centres (distal epiphyses) commence ossification, in addition to the age at which fusion typically occurs. Table 2.3: Approximate age range for developmental milestones of the phalanges (adapted from Scheuer & Black 2004). Bone Primary centre ossifies Developmental Milestone Secondary centre ossifies Fusion Distal phalanges 7-9 weeks prenatal 2-3 years years Middle phalanges weeks prenatal 2-3 years years Proximal phalanges 9-11 weeks prenatal 1-2 years years 18
38 2.8 Sesamoid Bones There are typically two sesamoid bones in the hand (White & Folkens 2005), not including the pisiform, which for the purpose of this chapter was discussed with the carpals. The sesamoids develop within the tendons of the hand. They protect those tendons from entering the metacarpophalangeal joint space, thus resulting in greater movement to the associated digit (Doyle & Botte 2003: 65). Hand sesamoids are commonly located at the base of the 1 st and 5 th digits, appearing as small rounded nodules of bone (see distal end of MCI in Figure 2.1). The sesamoids are the last bones in the hand to commence ossification (Scheuer & Black 2004) between the age of 11 to 15 years in females, and 13 to 18 years in males (Scheuer & Black 2000). 19
39 THIS PAGE HAS BEEN INTENTIONALLY LEFT BLANK 20
40 CHAPTER THREE AN OVERVIEW OF AGE ESTIMATION IN THE SUB-ADULT SKELETON 3.1 Introduction The purpose of this chapter is to discuss age estimation in a forensic context, with specific reference to cases of legal minority and/or culpability. The chapter also presents an introduction to the available methods for estimating age in sub-adult individuals. The medical modalities for visualisation of skeletal structures in living individuals are also accordingly reviewed. This leads into the next chapter that provides a review of sub-adult age estimation studies that focus specifically on the growth and development of the hand-wrist. 3.2 Forensic Age Estimation and Legal Culpability Age is one of a number of biological attributes a forensic anthropologist will assess in the skeleton towards providing an osteobiography; the remaining elements are sex, stature, ancestry, as well as an assessment of trauma and pathology (Cattaneo 2007). An osteobiography provides important information to investigative authorities towards identifying the individual being assessed (Steadman 2012). A forensic anthropologist will also frequently employ medical modalities (such as radiography) to visualise skeletal structures, especially in living individuals for the specific purpose of assessing legal minority and/or culpability (Schmeling & Black 2010) Skeletal vs. Chronological Age Age estimation methods employed by forensic anthropologists derive an estimate of skeletal age; however, this is not the same as actual chronological age. The level of variation involved in the development of the skeleton reflects the difference (or error) involved in estimating skeletal age. For adult (skeletally mature) individuals, age estimation methods typically assess the skeletal elements that fuse later in life (e.g. medial clavicle), as well as the degeneration of the skeleton (e.g. auricular surface; Black et al. 2010). For sub-adult individuals, age estimates involve the assessment of 21
41 skeletal development and growth relative to full maturity (Roche & Sun 2005). The skeletal and chronological ages of an individual may differ considerably depending on factors such as different growth patterns, the effects of illness and nutrition, socioeconomic status, as well as occupational stresses (Schmeling et al. 2000; The Royal Australian College of Physicians 2012). Furthermore, the standards used to calculate an age estimate reflect the average skeletal age for a given chronological age for a particular reference sample (e.g. Greulich & Pyle 1959; Tanner et al. 2001). It is important therefore, to use population specific standards to maximise the accuracy of the estimate (Schmeling et al. 2007) Legal Culpability The age of legal culpability refers to the age at which an individual is deemed to be an adult and therefore can be held legally responsible for their actions (Schmeling et al. 2004). Legal culpability for most jurisdictions is 18 years of age (Schmeling et al. 2004) and under the United Nations Convention on the Rights of the Child (1989) and the United Nations High Commissioner for Refugees Guidelines on Policies and Procedures in Dealing with Unaccompanied Children Seeking Asylum (1997), there is a statutory obligation for countries that recognise these documents to grant asylum to individuals under that age. Eighteen years of age, therefore, is a significant developmental milestone that needs to be clearly defined from 17 years of age and younger. For example, if an undocumented individual commits a crime and is assessed to be older than 18 years of age, they may face a harsher penalty than if they were under the age of legal culpability. In Australia any individual involved in people smuggling assessed as being older than 18 years of age faces a mandatory minimum sentence of five years imprisonment. Individuals under 18 years of age are typically deported back to their country of origin without conviction (Australian Human Rights Commission 2012) Casework Involving Skeletonised Remains For skeletal material referred to the forensic anthropologist the techniques used to estimate age are not that dissimilar to those used in age assessment of living individuals (Milner & Boldsen 2012 and see below). Estimating age in the sub-adult 22
42 skeleton relies on the assessment of skeletal and/or dental material in order to evaluate growth and development (Komar & Buikstra 2008). The presence (or absence) of particular bones, the size of bones relative to full maturity, and the degree of epiphyseal development and fusion are all assessed in order to derive a statistically quantified estimation of age (Roche & Sun 2005). In assessing the dentition, crown and root development, and the positioning and eruption of the teeth in the alveolus and oral cavity, are used to estimate age (Hillson 2002) Casework Involving Age Estimation in the Living The importance of estimating age for living individuals has increased due to the need to assess legal culpability and/or legal and social characterisation (Schmeling & Black 2010). While there are many areas where age estimation techniques can be applied to living individuals, the most prevalent requirement is to estimate the age of individuals without identity documentation, such as; refugees and asylum seekers (Schmeling et al. 2006); perpetrators and victims of human trafficking (Australian Human Rights Commission 2012); and in the investigation of crimes against (or involving) juvenile individuals (such as child pornography Cattaneo et al. 2009). Assessments of the skeletal age of living individuals are also often requested in cases involving the adoption of an individual that does not have adequate age documentation (Melsen et al. 1986). Another application is in competitive sports to ensure that an individual is competing in the appropriate age group (Malina 2011). The assessment of age in living individuals by a forensic anthropologist will by necessity involve radiographic imaging (Black et al. 2010). There is a preference for age estimation techniques that subject the individual being assessed to the least amount of radiation, while maintaining accuracy and minimizing invasiveness (Schmeling et al and see below). While the methods for assessing age in living individuals vary depending on the region of the skeleton being assessed, they can also be applied to skeletal remains, as they are based on the assessment of skeletal growth and development as described in published age estimation standards (Milner & Boldsen 2012). 23
43 3.3 Skeletal Visualisation There are a variety of visualisation methods that afford the opportunity to assess skeletal development (or degeneration) and thus derive an estimation of age. These are briefly discussed below Radiography Radiographs are two dimensional images produced by passing an x-ray beam through tissue (Conaghan et al. 2010). The absorption of the x-ray beam by the tissue is dependent on its density (Dhawan 2011). Radiographs display four different tissue densities: air; fat; soft tissue or fluid; and bone or calcification (Conaghan et al. 2010). Transmitted x-rays interact with a detection device, which is either a photographic film or a digital detector (Dhawan 2011). Radiographs superimpose body structures (Conaghan et al. 2010) and as such they are of limited value in visualising complex bony anatomy, such as the base of the skull (Dhawan 2011). Radiographic resolution is dependent on the resolving power of the detection device; a higher resolution requires a higher dose of ionising radiation that can be damaging to living tissue (Fazel et al. 2009) Multi-Detector Computed Tomography Multi-Detector Computed Tomography (MDCT) is an x-ray technique that produces cross-sectional images (Dhawan 2011). These cross-sectional images provide greater detail than radiographs because the anatomical structures are not superimposed (Conaghan et al. 2010). Furthermore, MDCT images can be visualised as two dimensional images in any orthogonal or multiple oblique planes (Conaghan et al. 2010). Three dimensional surface rendered images can also be reconstructed that show surface features meeting a predetermined density threshold. Multi-planar rendering allows the reconstruction of non-orthogonal planes, which assists in the visualisation of structures not visible in orthogonal reconstructions (Conaghan et al. 2010). 24
44 3.3.3 Magnetic Resonance Imaging Magnetic resonance imaging (MRI) involves the realignment of the magnetisation of specific atomic nuclei within the body (Dhawan 2011). Radio-frequency pulses are applied to systematically alter this alignment, which results in a rotating magnetic field that is detectable by a scanner, and is recorded as a digital reconstruction of the scanned region (Conaghan et al. 2010). An MRI provides higher contrast between different tissues in comparison to radiography and MDCT scans, however it is not as effective at visualising bone (Dhawan 2011). An MRI does not using ionising radiation and is thus considered a safer alternative to radiography and MDCT (Kuperman 2000). 3.4 Age Estimation Techniques There are three broad methodological categories that can be used to estimate age in the human skeleton (molecular, metric and morphological) with each having inherent advantages and disadvantages dependent on specific case requirements and the skeletal structures available for analysis. These main methodological approaches are discussed below, and a review of published hand-wrist age estimation methods (and standards) follows in Chapter Four Molecular These age estimation methods are based on the analysis of molecules within skeletal or dental tissues (Yekkala et al. 2006); one example is amino acid racemization. Amino acids are the principal components of proteins and are normally composed of the L- amino acids (Yekkala et al. 2006). Racemization is a chemical process where L- amino acids are transformed into D- amino acids; this occurs throughout life and continues after death (Csapó et al. 1994). The ratio of D- and L- amino acids is measured using chromatography and is then plotted against a standard calibration curve to derive an estimate of age. The process of racemization is affected by changes in ph and temperature, which generally need to be known in order to provide an accurate estimation (Arany & Ohtani 2011). The latter method is destructive (requiring tissue extraction) and because human remains are forensic evidence, such sampling is frequently not permissible (Ohtani & Yamamoto 2010). 25
45 3.4.2 Morphometric Morphometric methods typically rely on the acquisition of linear measurements that are statistically analysed to derive an age estimate. Morphometric methods are less subjective than morphological methods (see below) as they involve measurements from defined points on the bone, as opposed to visual assessment of developmental phases (McKeown & Schmidt 2012). While morphometric analyses typically involve several measurements from defined landmarks (which can be time consuming to measure), their accuracy and reliability are readily quantified (Slice 2005) Morphoscopic Morphoscopic age estimation methods are based on quantifying the morphology of skeletal ossification centres; from appearance through to subsequent fusion (Franklin 2010). Morphoscopic methods can be used to estimate age in skeletal remains and for living individuals (Ritz-Timme et al. 2000). It is well known that these methods can be rapidly applied, although this is dependent on the skill and experience of the assessor and the number of skeletal elements to be assessed (Greulich & Pyle 1959). Morphoscopic methods, however, are inherently subjective (Gonsior et al. 2013) and are therefore more prone to human error than morphometric based assessments. This subjectivity (high inter- and intra- observer error) results from the difficulty of the assessment of skeletal elements that fall in between the defined discrete stages or categories that are utilised during morphoscopic assessments (Tanner et al. 1983; 2001). 26
46 CHAPTER FOUR HAND-WRIST AGE ESTIMATION METHODOLOGIES AND STUDIES 4.1 Introduction This chapter provides a review of literature specific to sub-adult age estimation based on the morphoscopic analysis of the hand and wrist. Prior to skeletal maturation the development of the hand and wrist bones are known to have a positive correlation with skeletal age. There are three broad categories of methods used to assess skeletal development: atlas; mathematical/scoring; and computer assisted. As the latter involves a toolset that is not available for this research thesis, only the atlas and mathematical/scoring methods are discussed below. Six specific hand-wrist age estimation methods are discussed in detail, including the two main methods utilised in the present research thesis. Method validation studies are also considered, as they assess the accuracy those approaches when applied to various populations that are geographically or temporally removed. The validation studies considered emphasise the importance of the present thesis and assist in justifying its important contribution to the existing body of research. 4.2 Medical Application of Hand-Wrist Age Estimation Methods Of the six methods discussed in this chapter, only one was initially developed for forensic use (Cameriere et al. 2005). The remaining methods were initially developed to measure the level of skeletal development relative to population norms for medical application. Delayed skeletal development can be the result of malnutrition or pathology (e.g., Ferguson et al. 1982; Walther et al. 1981), while advanced skeletal development can be indicative of hormonal imbalance, which can lead to precocious and early onset puberty or growth disorders (De Donno et al. 2013; Hewitt & Acheson 1961; Tanner et al. 2001). 27
47 4.3 Inter- and Intra-observer Agreement Inter- and intra-observer agreement refers to the level of consensus between repeated assessments, either by multiple, or a single, assessor (Landis & Koch 1977). This is important, as the methods discussed below are morphoscopic and therefore inherently subjective (see Chapter 3). Not all of the studies discussed in this chapter have published a quantified observer error/agreement value, and this must be taken into consideration when evaluating the accuracy of their results. 4.4 Atlas Methods Atlas based methods are based on the radiographic assessment of hand-wrist skeletal development through comparison to standard images of known age. Most existing atlases typically comprise sex-specific series of radiographic images that represent stated age intervals. Three atlases are discussed below: Greulich & Pyle (1959); Thiemann-Nitz (1991; Thiemann et al 2006); and Gilsanz & Ratib (2005; 2011). The present thesis only utilised one of the aforementioned methods (Greulich & Pyle) to the Western Australian population because it appears to be the most widely applied and has been used to determine legal minority/culpability in Australia (Australian Human Rights Commission 2012). Validation studies using this method are also accordingly reviewed Greulich & Pyle (1959) Greulich and Pyle s Radiographic Atlas of Skeletal Development of the Hand and Wrist is the recommended method in all international protocols for hand-wrist sub-adult age estimation for forensic application (Cameriere et al. 2008). The atlas is based on the system established by Todd (1937) who developed a radiographic atlas of hand skeletal maturation based on data from the Brush Foundation between 1931 and The latter research series consists of longitudinal data of above average economic and/or educational status individuals born in the United States, who were Caucasian and of Northern European ancestry (Simmons & Greulich 1944). Greulich & Pyle utilised both Todd s data, and data subsequently obtained from the series between 1936 and
48 Greulich & Pyle assessed the skeletal development of a total of 6,879 hand-wrist radiographs (3,458 males, 3,421 females). From these radiographs a series of sex specific standards were selected, developmentally spanning birth to 19 years of age. The interval between age standards is 3 months up until 1 year of age, 6 months until 5 years of age, and annually thereafter (with an additional standard at 13.5 years for females, and 15.5 years for males). The radiograph selected to be the age standard was deemed to be the most representative (e.g., the anatomical mode) of development within the specific age group. The standards could then be directly compared to a hand-wrist radiograph of an individual of unknown age in order to estimate their age, although this was not the original intent of the authors; it is important to note that the atlas was not designed for forensic age estimation, but rather to provide an estimate of skeletal status for paediatric application (Greulich & Pyle 1959:44). A written description of the development of each bone (or significant developmental milestones) visible in the standard image was also developed to aid the use of the standards for future skeletal age assessments. The authors state that the accuracy of the atlas standards is between 0.6 to 1.1 years, but did note that applying the standards to foreign populations could reduce their accuracy Validation studies It has been established that population specific age estimation standards are the most accurate (Matsuoka et al. 1999; Schmeling et al. 2006). Validation studies assess the accuracy of existing published methods/standards when applied to populations that are geographically and/or temporally removed from the reference sample of the original sample. Six validation studies that have utilised the Greulich & Pyle atlas are reviewed below. i) Brown & Grave (1976) The aim of this study was to evaluate the accuracy of the Greulich & Pyle method as applied to an Indigenous Australian population. Between 1961 and 1971 the authors evaluated 123 right hand-wrist radiographs (73 male, 50 female). The sample comprised Aboriginal individuals between 5 and 20 years of age from Yuendumu (near 29
49 Alice Springs), and consisted of 90% Walbiri and 10% Pintubi individuals with no traceable European ancestry. The authors found that, compared to the atlas standards, there was a statistically significant developmental delay of the hand skeleton of up to 0.8 years in the Aboriginal males. This delay decreased with age, with a difference of 1-3 months in late adolescence, compared to 6-10 months between the ages of 7 and 14. For females the difference did not exceed 6 months and was not statistically significant. The authors concluded that the standards were applicable to females within the population. ii) Büken et al. (2007) The aim of this study was to examine the accuracy of the Greulich & Pyle atlas when applied to a Turkish population, with a specific focus on the accuracy of the technique around the age of legal minority and culpability (16 and 18 years of age respectively). A total 492 (257 male, 241 female) left hand-wrist radiographs from individuals between 11 and 19 years of age were obtained from schools in the Central Düzce region in Turkey. The sample population was Caucasian in origin and of a low to middle socioeconomic status. The intra-class correlation (ICC) was calculated as a measure of the inter-observer agreement (r = to r = 0.895). The radiographs were evaluated and the estimated skeletal age was subtracted from chronological age to give a mean difference. This mean difference was statistically significant for females, but not for males. Estimated skeletal age was on average advanced in females in comparison to their stated chronological age (by 0.17 to 1.1 years). However, for males the skeletal ages were delayed for the and year age groups by 0.01 to 0.58 years, but significantly advanced (by 0.88 to 0.98 years) for the year age group. The authors cautioned against using this method for evaluating legal age because of the significant difference in skeletal and chronological age present around the time of legal minority and culpability (15 to 19 years). iii) Moradi et al. (2012) The aim of this study was to assess the accuracy of the Greulich & Pyle atlas when applied to an Iranian population. A total of 425 (303 male, 122 female) hand-wrist 30
50 radiographs were evaluated; the subjects were between 6 to 18 years of age and from Isfahan, Iran. The inter- and intra-observer variation (the level of agreement between assessors and assessments) for the skeletal age estimates was not statistically significant. Mean skeletal age difference was calculated by subtracting the skeletal age estimate from the known chronological age of the subject. For the whole population (both sexes) there was a mean difference between actual and estimated age of 0.25 ± 0.95 years. Males had a mean difference of 0.37 ± 0.98 years, whilst females had a mean difference of ± 0.78 years. The mean difference for both males and females was not statistically significant. On the basis of their findings the authors concluded that the Greulich & Pyle atlas is suitable for application in an Iranian population. iv) Santos et al. (2011) The aim of this study was to test the accuracy of the Greulich & Pyle atlas when applied to a Portuguese population, with specific attention to the ages of legal minority (16 years) and culpability (18 years). The authors evaluated 230 (136 male, 94 female) hand-wrist radiographs from individuals between 12 to 20 years of age. The level of intra-observer agreement was assessed using Pearson s correlation coefficient and indicated a strong positive relationship (r = 0.99). The overall mean difference between estimated skeletal and actual chronological age was approximately 1 month and not statistically significant. They did state, however, that using the Greulich & Pyle method to estimate age at the Portuguese ages of minority and legal culpability resulted in 48.4% false positives, therefore limiting the reliability of the method within the 15 to 18 year age range. v) Patil et al. (2012) The aim of this study was to determine if the Greulich & Pyle atlas could be accurately applied to an Indian population. The authors examined hand-wrist radiographs of 375 sub-adults (194 male, 181 female) from Nagpur, India. The age range of the sample was birth through to 19 years of age. Left hand-wrist radiographs were taken where possible, although the right was used if necessary. No intra- or inter-observer agreement values were given by the authors. Overall, males had a mean difference 31
51 between estimated skeletal and actual chronological age of -0.7 years, with the largest difference being 2.11 years in the 8 to 9 year subgroup. In comparison, females had an overall difference of between -0.2 to -0.8 years, with the 4 to 5 year subgroup having the largest mean overestimation at years. The authors concluded that the Greulich & Pyle standards are not applicable to their sample population. They proposed adjusting the Greulich & Pyle atlas standards by summing the skeletal age estimate with their calculated mean difference in order to improve the accuracy of the method. vi) Paxton et al. (2013) The purpose of this study was to determine if the Greulich & Pyle atlas standards could be used to accurately estimate age in an undifferentiated Australian population. The authors examined hand-wrist radiographs of 406 individuals (276 males, 130 females) between 12 months and 18 years of age. The sample comprised individuals who had presented with suspected hand trauma to Townsville Hospital, Queensland in Of the 406 radiographs, 241 were of the right hand and 162 of the left. Inter- and intraobserver differences were found to be non-significant. The authors found that estimated skeletal age was greater than actual chronological age for 44% of the sample, with a statistically significant mean skeletal age difference of -2.2 months. They noted that age prediction error increased with advancing age; the mean difference of the adolescent (13 to 18 years) group was -3.8 months, in comparison to 0.81 months for the early childhood group (1 to 4 years). The authors also found an overall smaller mean difference for males compared to females (-1.5 and -3.7 months respectively). The authors determined that the Greulich & Pyle atlas was applicable to an undifferentiated Northern Queensland population based on their observation that the mean difference for their sample lay within the published standard deviations of the atlas. However, the authors also cited the need for a customised set of Australian standards in order to provide a greater level of accuracy. 32
52 4.4.2 Thiemann-Nitz (Thiemann & Nitz 1991; Thiemann et al. 2006) The aim of this study was to produce an atlas of hand-wrist skeletal development (similar to that of Greulich & Pyle) for paediatric use in Germany. The authors of this atlas analysed 5,200 hand-wrist radiographs of individuals from birth to 18 years of age. The radiographs were obtained from 20 medical institutions in the former German Democratic Republic between 1977 and Each standard is separated by an age interval: 3 months between standards from birth until 1 year of age; 6 month intervals from 1 to 14 years of age; and yearly intervals up to 18 years of age. The standard images are accompanied by diagrams and written descriptions. The atlas was revised in 2006 to include an extra 300 individuals who fell outside of the double height and weight standard deviations, and had therefore had been excluded from the original atlas. In the most recent revision the authors state that the standard deviation of the skeletal age estimates is between 0.2 and 1.2 years. There is a lack of foreign validation studies for this atlas as it has only generally been applied to German populations (due to the lack of an available English translation). The atlas was not designed for forensic application, but rather to assess where an individual child lies within the population norm for growth so that medical treatment can be accordingly arranged Gilsanz & Ratib Atlas (2005; 2011) This atlas was produced to provide a more contemporary and readily accessible atlas than that of Greulich & Pyle. The authors assessed left hand-wrist digital radiographs of 550 individuals (225 male, 225 female) recruited from schools and activity clubs in Los Angeles. All subjects were Caucasian with European ancestry. The radiographs were grouped into 29 sex-specific age groups, from birth to 18 years of age. Each reference age standard in the atlas was chosen from a set of 9 digital radiographs per age group and sex, with a composite image created where necessary to produce an idealised average. The authors have also produced an application compatible with smartphones and tablets. No specific value is given for the accuracy of the atlas, however, Schmidt et al. (2009) applied these standards to a German sample of 180 individuals (90 male, 90 female) between 10 to 18 years of age. They found that the 33
53 atlas overestimated chronological age by up to 7.2 months between the ages of 14 to 18, which they suggested was the result of population variation. Furthermore, as with the Greulich & Pyle (1959) and the Thiemann-Nitz atlases, the Gilsanz & Ratib method was not intended specifically for forensic application, but rather for use by paediatricians to assess growth. 4.5 Mathematical/Scoring Methods In addition to existing atlas approaches for the estimation of age, there are other established methods based on the statistical analysis of hand-wrist skeletal development. The Tanner-Whitehouse (Tanner et al. 1962; 1983; 2001) and FELS methods (Roche et al. 1988) are both based on scoring skeletal development milestones and then comparing them to derived population growth standards. Cameriere et al. (2005) has also developed a ratio method that involves measuring the area of particular bones of the wrist, which are then analysed using linear regression. The aforementioned methods are all discussed in greater detail below. As the present thesis utilises the Tanner-Whitehouse method, a series of selected validation studies using that method are also accordingly reviewed Tanner-Whitehouse Method (Tanner et al. 1962; 1983; 2001) In 1962 Tanner et al. formulated a method for the assessment of skeletal maturity based on the growth and development of the hand-wrist. This method is known as the Tanner-Whitehouse (TW1) method. There have been two major revisions to the method since its initial publication; these are referred to as TW2 and TW3 respectively. Originally based on a reference sample of British children of average socioeconomic status, the reference sample was further supplemented in subsequent revisions, and thus estimated skeletal age varies depending on which revision is utilised. The earlier versions (TW1 and TW2) are based on a British reference sample consisting of individuals between 1 to 21 years of age. The sample was a mix of cross-sectional and longitudinal data taken from schools, health studies and from male navy cadets at Ganges, between 1946 to The TW3 method does not utilise the British reference sample from the earlier versions, and instead uses more contemporary data obtained 34
54 by Beunen et al. (1990; 1994) for Belgian youths, Hernandez et al. (1991) for Spanish children, in addition to a North American (Texan) sample (Tanner et al. 1997). Tanner et al. defined successive stages of skeletal development for 20 of the bones in the hand-wrist complex and each stage was assigned a weighted biological sex-specific score. After each bone is scored, the sum of the scores is used to derive a skeletal maturity score, which can be then used to estimate skeletal age. A skeletal maturity score of 1000 is considered full skeletal maturity. The authors do not state a specific accuracy value as such, other than stating that the standard deviation from the 50 th centile score is approximately one year between 5 years of age and full skeletal maturity. They have, however, provided sex specific graphs of the 97 th, 90 th, 75 th, 50 th, 25 th, 10 th and 3 rd centile curves for each maturity scoring method (RUS, Carpal and 20 bone). It is important to note that the method was not intended for forensic application, but rather for use by paediatricians to aid in the diagnosis of endocrine and growth disorders (Tanner et al. 1983: v) Validation studies The Tanner-Whitehouse method has been the subject of the most number of validation studies of the three mathematical/scoring methods discussed in this chapter. Six selected validation studies that have utilised the Tanner-Whitehouse method are reviewed below. i) Marshall et al. (1970) The aim of this study was to compare skeletal development in a Jamaican population (near Kingston) to the Tanner-Whitehouse reference sample. In this study hand-wrist radiographs from 812 (397 males, 415 females) individuals between 6 months to 16 years of age were examined. The sample comprised individuals predominantly of West African ancestry, although some had a variable European ancestry. The 20- bone score following the TW1 method was used to estimate age; it was found that up until 2.5 years of age the Jamaican population showed advanced skeletal maturity in comparison to the TW1 standards (by as much as 0.91 years for males and 0.75 years 35
55 for females). Between 2.5 to 10 years of age there was no statistically significant difference between the Jamaican sample and the TW standards. After 10 years of age the Jamaican sample fell behind the standard, with a mean difference of years for males and years for females. The authors conclude that rapid skeletal maturity in early childhood is likely the result of genetic variance; however, a more recent paper by Schmeling et al. (2000) contradicts this statement, by arguing that any differences in the timing of skeletal maturation between populations are the result of socioeconomic factors, rather than genetic variance (i.e. ethnicity). ii) Schmidt et al. (2008) The aim of this study was to evaluate the accuracy of the Tanner-Whitehouse method (using TW2 and TW3) in a German population. The authors examined 92 hand-wrist radiographs (48 male, 44 female) obtained from individuals residing in Papenburg between 1986 and The individuals studied were 12 to 16 years of age. Each radiograph was assessed using the TW2 and TW3 RUS scoring methods. Inter- and intra-observer accuracy was not given. The authors found the mean difference between estimated skeletal and actual chronological age for both TW2 and TW3 ranged between -0.1 to +1.4 years, and -0.4 to +0.2 years, respectively. The authors concluded that the TW3 standard is more accurate than TW2 and thus more applicable for forensic application. iii) Zhang et al. (2008) The objective of this study was to evaluate the accuracy of the TW3 standard as applied to a Chinese population and to formulate population specific standards. The authors examined left hand-wrist radiographs of a massive sample comprising 17,401 individuals (8,685 males, 8,716 females) between 1 to 20 years of age, living in Shanghai, Guangzhou, Wenzhou, Dalian and Shijiazhuang in All subjects in the study were from an above-average socioeconomic environment. The radiographs were assessed using the TW3 Carpal and RUS systems. Intra-observer agreement was calculated, with 90.1% to 93.6% agreement for the RUS system, and 89.4% to 90.1% agreement for the carpal system. The maturity scores obtained were smoothed using 36
56 the LMS method defined by Cole (1990). In order to obtain the standard centile curve for the population, the data was plotted in a five-point polynomial regression model against known chronological age. The authors determined that skeletal maturity after 6 years of age in males, and 10 years of age in females, was advanced by as much as 2 years over the TW3 standards. iv) Ashizawa et al. (1996) The aim of this study was to evaluate the accuracy of the TW2 standards in a Japanese (Tokyo) population and to produce population specific age estimation standards. In this study the authors analysed a total of 1,457 left hand-wrist radiographs (753 male, 704 female) from individuals between 3 and 18 years of age. They calculated that there was between 70.7% (radius and ulna) to 77.4% (metacarpals and phalanges) agreement between three repeated assessments, with >90% agreement between two assessments. The authors assessed the radiographs using the TW2 RUS system; 50 th centile scores were calculated and smoothed using a cubic spline function to produce skeletal maturity curves. Their skeletal maturity curves were compared to the existing TW2 50 th centile maturity curve standards; a statistically significant difference was found in skeletal maturity at ages 9 and 11 (for girls and boys respectively). They also concluded that Japanese children reached adult maturity up to 2 years earlier than the TW2 standards, which they suggest is due to ecological and socioeconomic factors. v) Van Lethe et al. (1998) The aim of this study was to evaluate the accuracy of the TW method for age estimation in a Danish (Amsterdam) population. This study analysed 208 (114 male, 94 female) left hand-wrist radiographs taken from 60 subjects (30 male, 30 female) annually between the ages of 12 and 16 years of age. The radiographs obtained were evaluated using the TW2 method (although it is not clear if they used the RUS, Carpal or 20-bone score). The intra-class correlation (measurement of intra-observer agreement) was calculated as r = The authors found that there was a statistically significant mean skeletal age difference of to years for males between 13 and 15 years of age. In comparison, females had a statistically significant mean skeletal 37
57 age difference of to years between 13 and 14 years of age. The authors concluded that the mean skeletal age differences indicate that the TW2 method was applicable to the Danish population. vi) Powell et al. (2008) The aim of the study was to describe factors associated with skeletal age deviation (the difference between assessed skeletal age and chronological age) by assessing the accuracy of the TW method in a Tasmanian population. The authors examined left hand-wrist radiographs of 640 sub-adults (435 males, 205 females) between 7 and 17 years of age. The sample consisted predominantly of Caucasian individuals who had previously sustained an upper-limb fracture and had attended hospital in Hobart, Tasmania between 1998 and Skeletal age was estimated using the TW2 RUS system. Intra-observer agreement was assessed using 31 radiographs with a month between assessments; the intra-observer coefficient of variation was 1.1%. Skeletal age minus actual chronological age was used to calculate the skeletal age deviation (SAD). For individuals with a TW2 maturity score that indicated skeletal maturity had been reached, the SAD was regarded as 0. The authors found that there was a mean SAD of +0.5 years for the whole sample, with males having a more advanced bone age than females (SAD +0.6 years and +0.3 years respectively). There was no significant difference in mean SAD according to age group and so the authors concluded that the TW2 method was applicable to the study population The FELS Method (Roche et al. 1988) The FELS method is an alternative to that of Tanner-Whitehouse. A total of 13,823 left hand-wrist radiographs were assessed, taken from 677 individuals (355 males, 322 females) from Yellow Springs, Ohio. The radiographs were taken between 1932 and 1977 as part of the Fels Longitudinal Study of Growth and Development. Radiographs were taken at the following age intervals: three months after 1 month of age until 1 year of age; and 6 month intervals until 18 years of age. The sample comprised predominantly Caucasian individuals, with data from 15 African-American individuals 38
58 excluded from the standards. Each radiograph was assessed for a portion of 85 graded maturity indicators and 13 metric indicators. These indicators include (but are not limited to) the presence or absence of an epiphysis, the ratio of epiphyseal to metaphyseal width, and the presence or absence of bony projections (such as the hamulus and the styloid process). The specific indicators used in an assessment are dependent on the level of development visible within the radiograph, the average being around 50. Each indicator is then assigned a numerical grade, with 61 presence/absence (two-grade) indicators, 17 three-grade, 5 four-grade and two fivegrade indicators. The authors developed a computer program that allows the calculation of skeletal age and the standard error of the estimate once the assessed grades have been entered. Each grade has an allocated age range based on the mean age at which it was first visible. The authors graphically demonstrated a strong linear correlation between mean chronological and skeletal ages for both males and females, except at 1, 3 and 6 months of age, which they attribute to advanced skeletal development within the reference sample at these ages. They state that the 90 th percentile of the standard error was 0.3 years. This method is not widely used, however, as it involves the grading of an average of 50 indicators, in comparison to the Tanner-Whitehouse method that utilises 13 to The Cameriere Method (Cameriere et al. 2005) The purpose of this study was to produce an alternative hand-wrist age estimation method specific for forensic application. An initial sample of 150 (89 male, 61 female) digital hand-wrist radiographs from a Caucasian Italian population were examined. The sample consists of individuals between 5 to 17 years of age. A method was developed based on the measurement of the total area of the carpal bones (Bo). This was then expressed as a ratio against the full area from the proximal ends of the epiphyses of the radius and ulna to the distal ends of the distal carpals (Ca). These measurements were obtained using the lasso tool in Adobe Photoshop, with the ratios of Bo/Ca plotted against chronological age to obtain a sex-specific linear regression formula. The 39
59 regression formula for the Italian population accounted for over 80% of the variance and could be used to predict age with a calculated standard error of ± 1.19 years. The authors also tested this method on a Slovenian sample and found that the regression model obtained explained 86% of the variance and had a standard error of ± 0.96 years (Cameriere et al. 2008). This method has yet to be widely validated for use in foreign populations. 4.6 The Contribution of the Present Research Thesis As discussed above, the accuracy of the existing hand-wrist age estimation standards differ when they are applied to foreign populations. Schmeling et al. (2000) has proposed that any difference in skeletal maturation rate is due to socioeconomic factors, such as availability of medical care and nutritional intake, as opposed purely to ethnic variation. This is supported by variability in skeletal maturation rates between predominantly Caucasian populations (e.g. Tanner et al. 1997; Büken et al. 2007). Brown & Grave (1976), Powell et al. (2008) and Paxton et al. (2013) have evaluated the accuracy of hand-wrist age estimation techniques in Australian populations and demonstrated the need for population specific standards for Australia in order to improve on the accuracy of the methods. As hand-wrist age estimation standards are lacking in this region, the present study aims to fill this gap, as well as assess the accuracy and reliability of the existing foreign standards in a Western Australian population. 40
60 CHAPTER FIVE MATERIALS AND METHODS 5.1 Introduction As the overall objective of the present research thesis was to evaluate the accuracy of hand-wrist skeletal age estimation, the present chapter describes the application of the morphoscopic age estimation methods utilised in the present study. The statistical methods applied for the analysis and interpretation of the data are also outlined, including the rationale for selecting specific statistical tests. These include a precision test to assess the level of observer agreement, tests for normality, and an array of parametric tests for assessing mean differences and standard error of the age estimates, in addition to constructing sex-specific skeletal age estimation standards. 5.2 Materials The study sample comprises hand-wrist radiographs from 360 individuals (180 male; 180 female) from birth to 24 years of age (see Table 5.1 for age distribution). The radiographs were acquired from a Picture Archiving and Communication Systems (PACS) database that contains medical scans from various Western Australian hospitals. As the majority of usable scans are those that involve the clinical diagnosis of strains, sprains, and soft tissue injuries, any radiographs that showed major anomalies (e.g. polydactyly) or considerable skeletal trauma (e.g. fractures) of the bones of the hand and wrist were excluded. Table 5.1: Age distribution (in years) for the total sample (individual sex). Sex n Min. Max. Mean SD Male Female It is a requirement in Australia that clinical scans are anonymized prior to receipt for use in research, with only sex and age information retained (National Statement on 41
61 Ethical Conduct in Human Research NHMRC, 2013). Data relating to the ancestry of each individual is generally not recorded in clinical evaluation as it is not deemed to be medically relevant. As a result, the ancestry of the individuals of the study sample is unknown, although from recent census data it is known that the Western Australian population is predominantly Caucasian (ABS, 2012). Ethics approval to undertake this project was granted by the University of Western Australia s Human Research Ethics Committee (Project No RA/4/1/4362). 5.3 Morphoscopic Assessment The quantification of hand-wrist skeletal development was performed using two published standards: the atlas based method of Greulich & Pyle (1959) and the scoring based method of Tanner et al. (1983; 2001). These methodological approaches are described below Visualisation The digital hand-wrist radiographs were first imported into DICOM viewing software (OsiriX for Macintosh or RadiAnt for PC) to facilitate visual assessment using the Greulich & Pyle (1959) and Tanner-Whitehouse (1983; 2001) methods Greulich & Pyle (1959) Following the specific directions of Greulich & Pyle, the state of development for each bone is assessed, commencing at the distal epiphyses of the radius and ulna, and thereafter proceeding distally to conclude at the distal phalanges. The stage of handwrist development present in the radiograph referred for assessment is compared using written descriptions keyed to visual standards. Following Greulich & Pyle (1959: 36), if the radiograph being assessed is developmentally intermediate to the standards (as shown in Figure 5.1), it is assigned the age of whichever standard it most closely resembles. 42
62 a) G/P Female Standard 15 6 years 10 months b) Example WA Female 7 years 10 months c) G/P Female Standard 16 7 years 10 months Figure 5.1: Comparison of an example radiograph to Greulich & Pyle standards. Standard images (left hand) adapted from Greulich & Pyle (1959). The Western Australian female radiograph (MAN000736) is more similar to G/P 15 than G/P 16 based on the state of development shown by the carpals and the phalangeal epiphyses. Note however that the ulnar epiphysis shows advanced development similar to G/P 16, while the remaining epiphyses are similar in size and developmental state between the two Greulich & Pyle reference radiographs Tanner-Whitehouse (1983; 2001) In the Tanner-Whitehouse methods each skeletal region of interest (e.g., the distal end of the radius) is assigned an alphabetical stage of development, from A to either H or I (as shown in Figures 5.2 to 5.3). Stage A (not shown) represents no development, where the epiphysis (or bone) is not visible; this is equivalent to the Newborn standards of the Greulich & Pyle atlas. Stage I, therefore, represents full skeletal development (fusion completed), although some specific elements (such as the ulna) are only scored up to Stage H (fusion commencing) due to their variable morphology during this period of development. 43
63 Figure 5.2: Tanner-Whitehouse skeletal development stages of the radius. Images adapted from Tanner et al Each radiographic image corresponds to the pictorial representation and associated alphabetical stage below it. Each alphabetical stage has an associated numerical weighted score which is summed to produce a skeletal maturity score; a score of 1000 indicates full skeletal maturity. Individuals younger than the minimum estimable age (Table 5.2) are unable to be assessed using the Tanner-Whitehouse methods. Individuals older than the maximum estimable age (equivalent to a score of 1000; Table 5.2) will have their skeletal age underestimated. 44
64 a) Example WA Female Radius Radiograph b) Tanner-Whitehouse Radius Stage F Diagram Figure 5.3: Comparison of an example distal radius radiograph to Tanner-Whitehouse radius diagram. Stage F diagram (b) adapted from Tanner et al. (2001) and a detailed view of the left distal radius (a) taken from the example WA female hand radiograph (MAN000736). The radial epiphysis was assessed as corresponding to Tanner-Whitehouse Radius Stage F. In the TW3 method this stage gives a score of 78 (female radius stage F) which contributes to the total RUS skeletal maturity score. Table 5.2: Range of Tanner-Whitehouse estimable skeletal ages (years), by method and sex. Method Male Female Range (years) Range (years) TW2 RUS TW2 Carpal TW2 20-bone TW3 RUS In order to derive an estimate of skeletal age the skeletal maturity score is compared to the centile curves and age estimation tables defined by Tanner et al. (1983; 2001). The skeletal elements assessed are dependent on which TW scoring method is applied. All three scoring methods; RUS (Figure 5.4a), Carpal (Figure 5.4b) and 20-bone (both RUS and carpal) are utilised in the present study. The radius, ulna and the metacarpals 45
65 and phalanges of the 1 st, 3 rd and 5 th digits are assessed using the RUS method (Figure 5.4a). All carpal bones (except the pisiform) are assessed using the Carpal method (Figure 5.4b). The 20-bone method is a combination of the RUS and Carpal methods; it assesses all of the skeletal elements used by those methods. The stages and weighted biological scores are the same for the TW2 and TW3 methods; the only difference is that the age estimation tables and centile curves have been updated in the latter method (see Chapter Four). Figure 5.4: Skeletal elements utilized by the Tanner-Whitehouse methods. Images adapted from Tanner et al. (2001). The radius, ulna and the metacarpals and phalanges of digits I, III and V are assessed using the Tanner-Whitehouse RUS method (a). RUS is short for Radius, Ulna and Selected Short Bones, referring to the elements used to produce the skeletal maturity score. All of the carpal bones, with except the pisiform, are used to produce skeletal maturity score for the TW carpal method. The TW 20-bone method (not shown) utilizes both the RUS and Carpal elements. 46
66 5.4 Statistical Analyses The following sections describe the statistical analyses performed in the present study. Intra-observer precision is evaluated prior to applying parametric tests that statistically quantify differences between assessed skeletal maturity score (and estimated skeletal age) and stated chronological age. The standard error of each estimation method, and the strength of the linear association (Pearson s correlation) between stated chronological age and estimated skeletal age, is also calculated. The resultant skeletal age estimation formulae are derived based from polynomial modelling of the relationship between stated chronological age and the assigned Tanner-Whitehouse skeletal maturity score (least-squares regression). The statistical methods discussed in the following sections are constrained by some provisional assumptions, including the normal distribution of data, equal variances (homoscedasticity and sphericity), and independent random samples (Currell & Dowman 2009). Normality was assessed using the Kolmogorov-Smirnov test. If the Kolmogorov-Smirnov statistic is significant, it can be then concluded that the data violates assumptions of normality (Currell & Dowman 2009). Homoscedasticity is tested (Levene s test) as a part of the SPSS output for ANOVA and t-tests. If the ratio for the Levene s test is non-significant, equal variances (and therefore homoscedasticity) are assumed (Allen & Bennett 2010). Sphericity is tested by Mauchly s test as part of the repeated measures ANOVA and occurs when the F ratio is non-significant. If sphericity is not present, the F ratio can be corrected using the Greenhouse-Geisser correction factor (Greenhouse & Geisser 1959). All statistical analyses are performed using the statistical software Statistical Package for the Social Sciences, version 19 (IBM SPSS 19) and Microsoft Excel 2010 (Microsoft 2010). The specific statistics applied are described below Intra-observer Precision Prior to any data collection a precision test is conducted to assess the magnitude of intra-observer error. Precision refers to the consistency of repeated assessments using 47
67 the same standardised method. The precision of stage and age estimate assignment is assessed for the Greulich & Pyle and TW methods. The skeletal development of the same 20 individuals was assessed on three separate occasions, with a week between repeat assessments to prevent recall of figures. Intra-observer error is then quantified for the second and third repeat assessments using the Cohen Kappa statistic, with the first assessment omitted as a trial run. Cohen s Kappa is used to measure the level of agreement between repeat assessments; the stronger the level of agreement, the more precise the estimate (see Table 5.3; Peat 2001). Table 5.3: Kappa statistic ranges and corresponding level of agreement (adapted from Landis & Koch 1977). Kappa Statistic (κ) Strength of Agreement κ < 0.0 Poor 0.0 κ < 0.2 Slight 0.2 κ < 0.4 Fair 0.4 κ < 0.6 Moderate 0.6 κ < 0.8 Substantial 0.8 κ 1.0 Almost perfect agreement Kappa is defined by the following formula: ( ) ( ) ( ) Pr (a) = relative observed agreement between ratings (or raters) Pr (e) = hypothetical probability of random agreement Descriptive Statistics Prior to any analyses assessing the relationships between stated chronological age and assessed skeletal age, descriptive statistics, including the mean, maximum and minimum values, and standard deviations, are calculated. 48
68 5.4.3 Comparison of Means Univariate Significance Tests i) Paired Sample t-test A paired sample t-test is used to compare mean chronological and estimated skeletal age. For this particular test, the null hypothesis is that the mean difference between the two samples is equal to 0 (Townsend 2012). If the t score is greater than the p- value (< 0.05) relative to the degrees of freedom (df) of the sample, then the null hypothesis is rejected (Madrigal 1998). ii) Analysis of Variance (ANOVA) A one-way repeated measures ANOVA is used to compare the differences present in a single group for which a given variable has been repeatedly measured. A one-way repeated measures ANOVA is used to assess if there are any statistically significant differences in estimated skeletal age between the various skeletal age estimation methods applied to the Western Australian sample. It is also used to evaluate if the skeletal ages and associated RUS skeletal maturity scores are significantly different to comparable population studies. A two-way repeated measures ANOVA is used to assess if there is a significant interaction between sex and the overall accuracy of the skeletal age estimation method applied. With repeated-measures ANOVAs, there are three sources of variability: between repeats, between individuals, and random (Girden 1992). The repeated measures ANOVA produces two F ratios: one tests the null hypothesis that the repeat means are identical, whilst the other tests the null hypothesis that the individual means are identical (Girden 1992). Assuming sphericity, if the null hypothesis is true, then the F ratio is expected to be near Assessment of Age Estimation Accuracy One of the primary objectives of the present study is to statistically quantify the relationship between chronological and skeletal age; therefore, it is necessary to analyse the accuracy of the various age estimation methods applied. The mean skeletal age difference (SAD), and the standard error of the estimate (SEE) are both calculated for the full sample (individual by sex) in addition to the 17 age groups (e.g. Group 1: 1 to 1.9 years old through to Group 17: 17 to 17.9 years old). 49
69 i) Mean Skeletal Age Difference (SAD) The skeletal age difference refers to the variation between stated chronological and estimated skeletal age; this is typically presented as a mean value. While it is not a true measure of accuracy, since negative and positive values can factor each other out, it has been used in similar studies (e.g. Patil et al. 2012; Paxton et al. 2013) in place of a standard error value. It is therefore, necessary for comparative purposes. The SAD is calculated by subtracting estimated skeletal age from stated chronological age. ii) Standard Error of the Estimate (SEE) The standard error of the estimate (SEE) is a measure of the prediction accuracy of a given estimation method or regression formula (Dytham 2010). The formula used for the calculation of the SEE is as follows: ( ) x i = chronological age x ii = estimated skeletal age n = number of individuals Regression Analyses Correlation and regression share a similar mathematical basis, as well as the premise of assessing linear relationships between variables (Townsend 2012). In addition to the assumptions outlined in Section 5.4, regression analysis also requires that the dependent variable be continuous and non-categorical (Freund et al. 2006). In regards to correlation, the assumption of normality broadens to include a bivariate normal distribution. Regression is different from correlation because there is an inherent presumption of prediction between the covariates (Freund et al. 2006). i) Correlation Pearson s correlation is where a coefficient (r) describes a linear relationship between two continuous variables (covariates) (Townsend 2012:131; Dytham 2010). Prior to performing the correlation analysis, linearity is evaluated by plotting estimated skeletal 50
70 age against stated chronological age. The r value (-1 to +1) produced in the analysis is a measure of the strength and direction of the linear relationship (Townsend 2012); a positive value indicates a positive relationship, whereas a negative value indicates the opposite. The closer the absolute value is to 1, the stronger the relationship is deemed to be (Currell & Dowman 2009). The linearity of the relationship between stated chronological and estimated skeletal age is evaluated; the significance of the correlation is compared to the critical value of r appropriate to the degrees of freedom (df = n-2). ii) Calculation of Centile Scores The calculation of centile scores is an approach used in various Tanner-Whitehouse validation studies to derive sex and population specific standards (e.g. Zhang et al. 2008; Ashizawa et al. 1996). These centile scores are used in conjunction with regression analysis to produce smoothed maturity scores. The smoothing of the maturity scores minimises sample bias due to individuals within the population who display significantly advanced or delayed skeletal development (Tanner et al. 1997). The 97 th, 90 th, 75 th, 50 th, 25 th, 10 th and 3 rd centile maturity scores were calculated from the age estimation data, for each year age group, using Microsoft Excel iii) Polynomial Regression Polynomial regression is a form of linear regression where the relationship between the independent and dependent variables is modelled as an n th order polynomial (Freund et al. 2006). It can be used to describe non-linear trends such as growth. The predictive equation takes the form: is the estimate of the dependent variable (maturity score) is the intercept (constant), where the line intersects the y axis - are the slopes (regression coefficients) of the independent variable is the value of the independent variable (estimated skeletal age) 51
71 is the random error The calculated 50 th centile maturity scores are plotted against the stated chronological age to assess the overall shape of the curve, which is subsequently smoothed using a 4 to 5 (or 6 where possible) polynomial least-squares regression line (Microsoft Excel 2010). The determination of when to use a 6-point regression line over a 4 or 5-point line is dependent on whether the trend line intersects the maximum skeletal maturity score of 1000 defined by Tanner et al. (2001). If it does not, the lower point regression formula is selected for that scoring method. The remaining centile scores are also smoothed to produce a maturity curve graph; however, following Tanner et al. (2001), only the equation for the 50 th centile smoothed curve is used to derive sex-specific age estimation tables. These tables are then used calculate the standard error and mean SAD to assess the accuracy of the derived standards in the Western Australian population. 52
72 CHAPTER SIX RESULTS 6.1 Introduction The overall objective of the present study was to evaluate the accuracy of morphoscopic hand-wrist age estimation methods as applied to a Western Australian population. In order to do this, it was necessary to assess the relationship between stated chronological and estimated skeletal age, and to assess any sex specific differences in the timing of skeletal maturity. A variety of statistical analyses were accordingly performed to evaluate the Greulich & Pyle and Tanner-Whitehouse age estimation methods. The present chapter outlines the results of the precision test for the skeletal age estimation methods analysed. The descriptive statistics for each of the age estimation methods examined are presented, including the mean, standard deviation, maximum and minimum values, and normality tests to assess the validity of using parametric tests to analyse the data. The results of the statistical comparisons of stated chronological and estimated skeletal age are also presented. Thereafter, the analysis of each of the age estimation methods is presented, including the standard error and the mean skeletal age difference. The results of the assessments of sex specific differences in the timing of skeletal maturation are also provided. Consequently, sex and population specific age estimation standards developed from the analysis of the data are outlined. The chapter concludes with a series of comparisons to results of previous research performed in other populations. 6.2 Intra-observer Precision The following section presents the results of the statistical analysis of intra-observer accordance for the Greulich & Pyle and Tanner-Whitehouse age estimation methods. The raw data for those analyses is presented in Appendix I. Intra-observer accordance was evaluated using the Cohen s Kappa, statistic (Cohen 1968). The Greulich & Pyle 53
73 skeletal age estimates and the raw Tanner-Whitehouse RUS skeletal element scores each had Kappa values Greulich & Pyle Kappa was calculated based on the repeat assessment of 20 hand-wrist radiographs (10 male, 10 female) over three separate occasions, with a week between each reassessment. The derived Kappa value (κ) was calculated from the second and third repeats. Kappa was calculated as (p < 0.001) which indicates near perfect agreement (Koch & Landis 1977) Tanner-Whitehouse Kappa values were also calculated from the same 20 radiographs (see above) for each of the 20 individual skeletal elements required for the Tanner-Whitehouse method. All elements evaluated had a Kappa value of > 0.80 (all p <0.001) as shown in Table 6.1. Table 6.1: Tanner-Whitehouse intra-observer agreement. Bone Kappa (κ) Bone Kappa (κ) Radius *** Distal Phalanx I *** Ulna *** III *** Metacarpal I *** V *** III *** Capitate *** V *** Hamate *** Proximal Phalanx I *** Triquetral *** III *** Lunate *** V *** Scaphoid *** Middle Phalanx III *** Trapezium *** Key: κ = Kappa V *** Trapezoid *** Significance: NS = Non significant, * p < 0.05, ** p < 0.01, *** p < In considering the individual bones assessed, the lowest intra-observer accordance was for proximal phalanx I and middle phalanx V (both κ = 0.869); the ulna, metacarpal I, 54
74 proximal phalanges III and V, distal phalanx III, the capitate and hamate had the highest level of intra-observer accordance (all κ = 1.00). 6.3 Descriptive Statistics Normality The Kolmogorov-Smirnov normality test was applied to assess the distribution of estimated skeletal and stated chronological age using each estimation method; this was performed prior to the application of various parametric tests based on assumptions of normality. The results of this test are presented below. i) Estimated Skeletal Age The Kolmogorov-Smirnov values for estimated skeletal age were significant for all estimation methods for both sexes (p < 0.001; see Table 6.2), indicating that estimated skeletal age is not normally distributed. However, according to Altman & Bland (1995), the sample examined in the present study is large enough such that the apparent nonnormality of the data does not preclude parametric statistical testing (see also Elliott & Woodward 2007). Table 6.2: Kolmogorov-Smirnov test for normality of estimated skeletal age. Sex Age Estimation Method n K-S Statistic Male TW2 RUS *** TW2 Carpal *** TW2 20-bone *** TW3 RUS *** Greulich-Pyle *** Female TW2 RUS *** TW2 Carpal *** TW2 20-bone *** TW3 RUS *** Greulich-Pyle *** Significance: NS = Non-significant, * p < 0.05, ** p < 0.01, *** p <
75 iii) Stated Chronological Age Male and female stated age is normally distributed; the Kolmogorov-Smirnov value (both 0.06) was non-significant Range, Mean, and Standard Deviation of Estimated Skeletal Age Descriptive statistics, including the range and standard deviation for each of the age estimation methods (in years) applied to the Western Australian sample, are outlined in Table 6.3. Table 6.3: Range, mean and standard deviation (in years) of estimated age for the five methods analysed. Method Male Female n Range Mean SD n Range Mean SD TW2 RUS TW2 Carpal TW2 20-bone TW3 RUS Greulich & Pyle The Greulich & Pyle method had the widest age estimation range (male: 0 to 19 years, SD = ±6.0 years; female: 0 to 18 years, SD = ±5.8 years). The TW3 RUS method had the narrowest range for females (2.3 to 15.0 years, SD = ±4.5 years), while the TW2 Carpal method had the narrowest range for males (2.4 to 15.0 years, SD = ±4.4 years). 6.4 Statistical Evaluation of the Greulich & Pyle Age Estimation Method Correlation There was a strong positive linear correlation (see Figure 6.1) between stated and estimated age for the Greulich & Pyle method (male: r = 0.970; female: r = 0.972). 56
76 18 16 Stated Chronological Age (Years) SEX Male R 2 =0.941 Female R 2 = Estimated Skeletal Age (Years) Figure 6.1: Scatter plot with associated regression lines showing the relationship between stated chronological age and Greulich-Pyle estimated skeletal age. 57
77 6.4.2 Age Prediction Accuracy The SEE and SAD values for the Greulich & Pyle method as applied to the Western Australian sample, are presented in Table 6.4. The Greulich & Pyle method was more accurate for females (SEE ± 1.8 years) than males (SEE ± 3.0 years). As both the SEE and SAD are calculated in years, large SEE and SAD values indicate low accuracy. The mean skeletal age difference can be positive or negative; the former equates to an underestimation, and the latter an overestimation, of skeletal age. The mean SAD values indicate an average overestimation of 0.24 years in males and an underestimation of 0.14 years in females. Table 6.4: Calculated standard error and mean skeletal age difference (chronological skeletal age) for the Greulich & Pyle method for males and females. n SEE Mean SAD (± years) (years) Male *** Female NS Significance: NS = Non-significant, * p < 0.05, ** p < 0.01, *** p < Statistical Evaluation of the Tanner-Whitehouse Age Estimation Methods Correlation Estimated skeletal age (see Appendix II) was significantly correlated with actual chronological age (p < 0.001); the strongest correlation was for the TW2 20-bone method (male: r = 0.954; female: r = 0.942), followed by the TW2 RUS (male: r = 0.950; female: r = 0.938) and the TW3 RUS methods (male: r = 0.943; female: r = 0.937). The TW2 Carpal method had the lowest correlation for both sexes (male: 0.910; female: 0.934). Overall, a consistently positive linear correlation between stated chronological and estimated skeletal age was evident (see Figure 6.2). 58
78 Stated Chronological Age (years) SEX Male R 2 =0.903 Female R 2 = Estimated Skeletal Age (years) Figure 6.2: Scatter plot with associated regression lines showing the relationship between stated chronological age and TW2 RUS estimated skeletal age (see Appendix III for plots of other TW methods examined). 59
79 6.5.2 Age Prediction Accuracy The overall standard error of the estimate (SEE) and mean skeletal age difference (SAD) values for each Tanner-Whitehouse age estimation method, as applied to the Western Australian population are presented in Table 6.5. The TW2 Carpal method was the most accurate for males (SEE ± 0.9 years) and the TW3 RUS method was the most accurate for females (SEE ± 0.4 years). In contrast, the TW2 RUS and carpal methods were the least accurate for males (SEE ± 10.1 years) and females (SEE ± 11.4 years) respectively. Overall the age estimation methods were more accurate for females than males, with the exception of the TW2 Carpal method. Table 6.5: Calculated standard error and mean skeletal age difference (chronological skeletal age) for each of the Tanner-Whitehouse age estimation methods for males and females. Method n SEE (± years) Mean SAD (years) Male TW2 RUS *** TW2 Carpal *** TW2 20-bone NS TW3 RUS NS Female TW2 RUS NS TW2 Carpal *** TW2 20-bone NS TW3 RUS *** Significance: NS = Non-significant, * p < 0.05, ** p < 0.01, *** p < The SAD values showed the same overall pattern to the SEE values; the TW2 Carpal and TW3 RUS methods had the smallest SAD values for males (-0.08) and females (- 0.04) respectively. The highest SAD values (either positive or negative) were for the TW2 RUS and TW2 Carpal methods for males (-0.81) and females (+1.09) respectively. The pattern of large (positive or negative) SAD values, as compared to the equivalent 60
80 SEE values, appears to suggest that the SAD can be used as a proxy for SEE. The SEE and mean SAD values for each 12 month age range are presented in Appendix IV. 6.6 Formulation of Age Estimation Standards As one of the main aims of the present research thesis was to formulate population specific age estimation standards for Western Australia, a series of linear and polynomial regression analyses were accordingly performed Linear Regression Growth is generally non-linear, however, given the strong positive linear correlation for all the applied age estimation methods, the prediction accuracy of linear models were explored (see Table 6.6). i) Greulich & Pyle In addition to having being the most strongly correlated to stated chronological age, the Greulich-Pyle age estimation method also displayed the lowest standard error of the estimate (SEE) in the linear regression equations (see Table 6.6) of the applied age estimation methods (male: ± years; female: ± years). This method also explained the highest proportion of variation (R 2 ) across all groupings (males: 94.1%, females: 94.5%). ii) Tanner-Whitehouse For males the linear regression equation for the TW2 Carpal method was the least accurate (SEE ± years), while the TW2 20 bone equation was the most accurate (SEE ± years). Conversely, for females the TW2 Carpal equation was the most accurate (SEE ± years), and the TW3 RUS equation was the least accurate (SEE ± years). Interestingly, the linear regression equation improved the accuracy of the TW2 Carpal method for females from ±11.4 years to approximately ±5.3 years. In contrast, it significantly decreased the accuracy of the TW2 Carpal method for males from ±0.9 years to approximately ±32.9 years. The overall accuracy of the derived 61
81 linear regression equations was less than the accuracy of the unaltered TW2 RUS, TW2 20-bone and TW3 RUS methods for both males and females by 8.7 to 21.8 years. Table 6.6: Linear regression equations from the Greulich & Pyle and four Tanner- Whitehouse age estimation methods examined. Sex Method n # Equation SEE (±years) r R 2 Male TW2 RUS 167 CA es = (SA) TW2 Carpal 157 CA es = (SA) TW2 20-bone 170 CA es = (SA) TW3 RUS 166 CA es = (SA) Greulich-Pyle 180 CA es = 0.959(SA) Female TW2 RUS 170 CA es = (SA) TW2 Carpal 177 CA es = (SA) TW2 20-bone 170 CA es = (SA) TW3 RUS 166 CA es = (SA) Greulich-Pyle 180 CA es = (SA) Key: CA es = Estimated Chronological Age, SA = Estimated Skeletal Age. # All equations significant at p < Polynomial Regression Due to the relatively low accuracy values of the linear regression equations for the Tanner-Whitehouse methods, and the fact that growth is a non-linear phenomenon, a polynomial regression trend line was fitted to the plot of stated chronological age against 50 th centile maturity scores (Table 6.7). As the TW2 and TW3 RUS maturity scores are identical, they are referred to as TW RUS in Table 6.7. Polynomial regression analysis was not performed on the Greulich & Pyle age estimates as this method does not utilize a skeletal maturity score. The accuracy of the Tanner-Whitehouse age estimation methods was improved in comparison to the linear regression equations by an average of years for males 62
82 and years for females. The TW2 20-bone method was most accurate for females (SEE ± 0.6 years) and the TW2 Carpal method was most accurate for males (SEE ± 1.2 years). The accuracy of the TW RUS derived polynomial formula (SEE 2.0 years) was lower in comparison to the calculated SEE value for the unaltered TW3 RUS method (SEE 0.4 years) for females. In contrast, the accuracy of the TW RUS polynomial formula (SEE 1.5 years) increased markedly for males in comparison to the unaltered TW2 RUS method (SEE 10.1 years). The explanatory power of the regression equations (R 2 adj) improved for all of the Tanner-Whitehouse methods and accounted for approximately 99% of sample variance Skeletal Age Tables and Skeletal Maturity Curves The smoothed population specific curves for 97 th, 90 th, 75 th, 50 th, 25 th, 10 th and 3 rd centile maturity scores for each of the three established Tanner-Whitehouse methods (RUS, Carpal and 20-bone) are shown in Figures 6.3 to 6.8. The 50 th centile curve represents the population maturity mean with the range of population variation represented by the remaining centiles. The formulae of the 50 th centile curves were used to produce the skeletal age tables. These population and sex-specific reference curves and tables have been designed to be used in conjunction with the established methods of Tanner et al. (1983; 2001) to estimate age. 63
83 Table 6.7: Polynomial regression equations from the 50 th centile Tanner-Whitehouse maturity scores for age estimation (years) for males and females. Sex Method # Equation SEE (± years) Mean SAD (years) r R 2 adj Male TW RUS SMS = (SA) (SA) (SA) (SA) (SA) (SA) TW2 Carpal SMS= (SA) (SA) (SA) (SA) (SA) TW2 20-bone SMS= (SA) (SA) (SA) (SA) (SA) Female TW RUS SMS = (SA) (SA) (SA) (SA) (SA) (SA) TW2 Carpal SMS= (SA) (SA) (SA) (SA) TW2 20-bone SMS= (SA) (SA) (SA) (SA) (SA) Key: SMS = Tanner-Whitehouse skeletal maturity score, SA = Estimated skeletal age # All significant at p <
84 Table 6.8: Female TW-WA RUS skeletal age for a given skeletal maturity score (SMS). Skeletal Age (years) SMS Skeletal Age (years) SMS Skeletal Age (years) SMS Skeletal Age (years) SMS
85 Skeletal Maturity Score Skeletal Age (years) Figure 6.3: Smoothed TW-WA RUS maturity standards for females. 66
86 Table 6.9: Female TW-WA Carpal age for a given skeletal maturity score (SMS). Skeletal Age (years) SMS Skeletal Age (years) SMS Skeletal Age (years) SMS
87 Skeletal Maturity Score Skeletal Age (years) Figure 6.4: Smoothed TW-WA Carpal maturity standards for females. 68
88 Table 6.10: Female TW-WA 20-bone age for a given skeletal maturity score (SMS). Skeletal Age (years) SMS Skeletal Age (years) SMS Skeletal Age (years) SMS
89 Skeletal Maturity Score Skeletal Age (years) Figure 6.5: Smoothed TW-WA 20-bone maturity standards for females. 70
90 Table 6.11: Male TW-WA RUS skeletal age for a given skeletal maturity score (SMS). Skeletal Age (years) SMS Skeletal Age (years) SMS Skeletal Age (years) SMS Skeletal Age (years) SMS
91 Skeletal Maturity Score Skeletal Age (years) Figure 6.6: Smoothed TW-WA RUS maturity standards for males. 72
92 Table 6.12: Male TW-WA Carpal age for a given skeletal maturity score (SMS). Skeletal Age (years) SMS Skeletal Age (years) SMS 73 Skeletal Age (years) SMS Skeletal Age (years) SMS
93 Skeletal Maturity Score Skeletal Age (years) Figure 6.7: Smoothed TW-WA Carpal maturity standards for males. 74
94 Table 6.13: Male TW-WA 20-bone age for a given skeletal maturity score (SMS). Skeletal Age (years) SMS Skeletal Age (years) SMS 75 Skeletal Age (years) SMS Skeletal Age (years) SMS
95 Skeletal Maturity Score Skeletal Age (years) Figure 6.8: Smoothed TW-WA 20-bone maturity standards for males. 76
96 6.7 Sex specific Differences in Skeletal Maturity Another aim of the present project was to assess the magnitude and timing of any sex specific differences in skeletal maturity. This was quantified using a two-way repeated measures ANOVA of stated chronological and estimated skeletal age, in addition to graphical comparison of the Western Australian RUS and Carpal skeletal maturity curves obtained for both sexes Two-way repeated measures ANOVA A two-way repeated measures ANOVA was performed to evaluate if sex has a statistical influence on the accuracy of the five hand-wrist age estimation methods. Mauchly s test indicated that the assumption of sphericity is violated (χ 2 (14) = , p < 0.001), therefore the degrees of freedom (df) was corrected using the Greenhouse-Geisser correction factor (ε = 0.474). A significant interaction between age estimation method and sex was evident (F 0 = 2.372, F 1 = ), indicating that sex has a significant influence on age prediction accuracy (Table 6.14). Table 6.14: Two-way repeated measures ANOVA: Comparison of chronological and estimated skeletal ages by sex. F-ratio Mean χ 2 Mauchly s W ε F Difference 0 F 1 (years) *** *** *** ±0.56 *** Key: ε = Greenhouse-Geisser correction factor. Significance: NS = Non significant, * p < 0.05, ** p < 0.01, *** p < Skeletal Maturity Curves It was evident in the Western Australian population that RUS skeletal maturation occurred earlier in females (14.4 years) than males (15.2 years); this trend of advanced development in females was evident from as early as 2 years of age, where the mean skeletal maturity score for females was 173, in comparison to a mean skeletal maturity score of 13 for males (Figure 6.9). The rate of skeletal maturation increases in females 77
97 after 9 years of age; the slope of the graph (m) increases from 36 (before 9 years) to 106 (between 9 and 14.4 years of age). In comparison, the rate of skeletal maturity increases after 11 years of age in males; the slope increases from 41 (before 9) to 147 (between 9 and 15.2 years of age) Skeletal Maturity Score (SMS) Estimated Skeletal Age (Years) Figure 6.9: The relationship between RUS skeletal maturity score and estimated skeletal age for males and females. Estimated skeletal ages were obtained using the TW RUS polynomial regression formulae from Table 6.7 and plotted against the corresponding skeletal maturity score. 78
98 Carpal skeletal maturation also occurred earlier on average in females (12.1 years) than males (14.3 years). In contrast to RUS skeletal maturity, the carpal scores were similar for males and females at 2 years of age (172 and 184 respectively), however, female carpal maturity accelerates relative to males (by as much as 2 years) after approximately 8 years of age (Figure 6.10) Skeletal Maturity Score (SMS) Estimated Skeletal Age (Years) Figure 6.10: The relationship between TW-WA Carpal skeletal maturity score and estimated skeletal age for males and females. Estimated skeletal ages were obtained using the TW Carpal polynomial regression formulae from Table 6.7 and plotted against the corresponding skeletal maturity score. 79
99 6.8 Population Specificity of Age Estimation Standards It has been stated throughout the present thesis that population specific age estimation standards are the most accurate (e.g. Tanner et al. 1997; Patil et al. 2012). As such, it is necessary to quantify differences in the timing of hand-wrist maturity between the Western Australian and other populations. The Western Australian skeletal maturity scores were compared to published data from foreign populations using repeated measures ANOVA and through graphical comparison (see below) Skeletal Maturity Scores (SMS) To assess if the timing of skeletal development in the Western Australian population was significantly different from foreign populations a one way repeated measures ANOVA was performed on the average age of attaining a selection of Tanner- Whitehouse RUS skeletal maturity scores (Table 6.15 and 6.16). Table 6.15: Mean age (in years) in males for attainment of successive TW RUS skeletal maturity scores for various populations (adapted from Tanner et al. 1997). Population SMS WA TW2 1 TW3 2 Spain 3 USA 4 Belgium 5 Japan 6 China 7 *** ** *** * *** *** NS Significance: NS = Non-significant, * p < 0.05, ** p < 0.01, *** p < Tanner et al. (1983); 2 Tanner et al. (2001); 3 Hernández et al. (1991); 4 Tanner et al. (1997); 5 Beunen et al. (1990); 6 Ashizawa et al. (1996); 7 Zhang et al. (2008). 80
100 The results of the ANOVA (see Appendix V for detailed results) indicate that the mean age of Western Australians in attaining various Tanner-Whitehouse skeletal maturity scores is significantly different for males (Table 6.15) for all populations except the Chinese. The mean age of attaining various Tanner-Whitehouse skeletal maturity scores for Western Australian females was not significantly different for all populations, with the only significant difference being that of the original TW2 Standard population (see Table 6.16). Table 6.16: Mean age (in years) in females for attainment of successive TW RUS skeletal maturity scores for various populations (adapted from Tanner et al. 1997). Population SMS WA TW2 1 TW3 2 Spain 3 USA 4 Belgium 5 Japan 6 China 7 ** NS NS NS NS NS NS Significance: NS = Non-significant, * p < 0.05, ** p < 0.01, *** p < Tanner et al. (1983); 2 Tanner et al. (2001); 3 Hernández et al. (1991); 4 Tanner et al. (1997); 5 Beunen et al. (1990); 6 Ashizawa et al. (1996); 7 Zhang et al. (2008) Skeletal Maturity Curves To compare the specific timing of hand-wrist skeletal development across populations, the Western Australian skeletal maturity curves derived for the RUS and Carpal methods are plotted against similar foreign studies. It should be noted that the minimum ages for the Belgian population study are 12 and 6 years for males and females respectively (Beunen et al. 1990). 81
101 i) TW RUS RUS standards from this study (Western Australia) and six foreign populations are plotted in Figures 6.11 and The Western Australian RUS maturity scores are comparatively delayed for males until approximately 9 years of age, where they advance over the other populations, until skeletal maturity is reached at 15.2 years. This is also evident in Table 6.15, where the skeletal maturity scores for males are advanced on the European, Japanese and American populations after 12.3 years of age TW2 Standard 900 TW3 Standard Japanese 800 Chinese Belgian Skeletal Maturity Score (SMS) European-American Western Australian Estimated Skeletal Age (Years) Figure 6.11: Male TW-WA RUS skeletal maturity curve compared to six foreign population standards (Tanner et al. 1983; 1997; 2001; Ashizawa et al. 1996; Zhang et al. 2008; Beunen et al. 1990). 82
102 The Western Australian RUS skeletal maturity scores are similarly delayed between the ages of 3.5 and 6 years for females. Skeletal development (indicated by SMS) is delayed behind the TW3 standard and the other European populations (see Table 6.16) at 9.8 years of age, before advancing over all the European and American standards at approximately 13 years of age. Thereafter, it shows further advancement, and skeletal maturity is attained by 14.4 years, in advance of the Japanese and Chinese populations Skeletal Maturity Score (SMS) TW2 Standard TW3 Standard Japanese Chinese Belgian European-American Western Australian Estimated Skeletal Age (years) Figure 6.12: Female TW-WA RUS skeletal maturity curve compared to six foreign population standards (Tanner et al. 1983; 1997; 2001; Ashizawa et al. 1996; Zhang et al. 2008; Beunen et al. 1990). 83
103 RUS skeletal maturity was reached on average by 15.2 years in males, and 14.4 years in females in Western Australia; consistently earlier than the TW2 and TW3 standards. For males the difference in estimated age at maturity is 3 years for TW2, and 1.3 years for TW3 compared to the Western Australian population. The difference between skeletal ages at maturity for the published TW standards and the Western Australian population is less for females (1.6 and 0.6 years for TW2 and TW3 respectively). The Asian populations reached skeletal maturity at a similar time (at 15.0 to 15.5 years of age) to the Western Australian population (14.4 years) for females (see Figure 6.12). ii) TW Carpal Carpal standards from this study and three comparative foreign populations are plotted in Figures 6.13 and Male carpal development is advanced over the Chinese population until 4 years of age, where thereafter it is comparatively delayed; reaching skeletal maturity 0.8 years after the Chinese population (Figure 6.13) Skeletal Maturity Score (SMS) Western Australia United Kingdom China Belgium Estimated Skeletal Age (Years) Figure 6.13: Male TW-WA Carpal skeletal maturity curve compared to three foreign population standards (Tanner et al. 1983; Zhang et al. 2008; Beunen et al. 1990). 84
104 The Western Australian male standard was delayed in comparison to the UK standard (TW2) for males until 12 years of age; however, it reaches carpal skeletal maturity 0.7 years before the UK standard (Figure 6.13). The Belgian population (Beunen et al. 1990) only comprises data from after 12 years of age, and is comparatively delayed in comparison to the Western Australian population for males, which reaches carpal maturity approximately 0.4 years earlier Skeletal Maturity Score (SMS) Western Australia United Kingdom China Belgium Estimated Skeletal Age (Years) Figure 6.14: Female TW-WA Carpal skeletal maturity curve compared to three foreign population standards (Tanner et al. 1983; Zhang et al. 2008; Beunen et al. 1990). Females showed a similar pattern to males, with delayed carpal development in comparison to the UK and Belgian populations up until 12 years of age (Figure 6.14). After 12 years of age, carpal development accelerates over the European populations. The Western Australian sample reaches skeletal maturity approximately 0.4 years before the Belgian population and 0.9 years before the UK standard (TW2). The Western Australian standard is developmentally delayed, with skeletal maturity reached approximately 0.6 years after the Chinese population. 85
105 CHAPTER SEVEN DISCUSSION AND CONCLUSION 7.1 Introduction The primary aim of this project was to develop sex-specific age estimation standards for a contemporary Western Australian population based on the analysis of the growth and development of hand and wrist bones. A necessary prerequisite to data collection and analysis was ensuring that intra-observer error was within acceptable limits; the results of those analyses are briefly discussed in this chapter. The accuracies of the derived age estimation standards are also considered and the statistical analysis of hand-wrist growth and development is discussed. Factors that influence the timing of skeletal maturation are also considered. The final conclusions of the thesis are presented, including limitations and suggestions for further research. 7.2 Measurement Precision As morphoscopic methods of age estimation are known to be inherently subjective (Gonsior et al. 2013), a precision test was undertaken prior to primary data collection to statistically quantify intra-observer agreement. All of the calculated intra-observer (Kappa) values indicated near perfect agreement, as defined by Landis & Koch (1977). The lowest observer agreement was for the assessment of proximal phalanx I and middle phalanx V using the Tanner-Whitehouse method (κ = 0.869). This is possibly related to the partial flexion of the 1 st and 5 th digits in some radiographs, which obscured the epiphyseal-metaphyseal junction. The most accurately assessed bones were proximal phalanges III and V, distal phalanx V, the capitate, and the hamate (κ = 1.000). The high Kappa values for the latter elements was likely due to the fact they displayed development that was indicative of only one of the discrete stages defined by Tanner et al. (2001); an example of this is shown in Figure 7.1. The results of the precision test are in line with published intra-class correlations (ICC) of r= to r = 86
106 0.99 (Büken et al. 2007; Santos et al. 2011) and percentage identities of 70.7% and above (Ashizawa et al. 1996) of similar validation studies. a) b) a) Example WA Male Distal Phalanx I Radiograph. b) Tanner-Whitehouse Distal Phalanx I Stage F Standard. Figure 7.1: Comparison of an example distal phalanx I radiograph to Tanner- Whitehouse Stage F Distal Phalanx I standard. Stage F Distal Phalanx I Standard (b) adapted from Tanner et al. (2001).The Western Australian male distal phalanx I radiograph (MAN000145) closely resembles the Tanner-Whitehouse stage F standard. This is especially evident at the dorsal surface of the epiphyseal base, where the articular surface for Proximal Phalanx I has resulted in distinct curvature. 7.3 Age Prediction Accuracy The first aim of the present research thesis was to assess the accuracy of selected established hand-wrist age estimation methods as applied to a contemporary Western Australian population. This initial assessment was necessary to determine which method would be the most suitable basis for formulating population specific age estimation standards; however, it was found that no single age estimation method was the most accurate across the sexes. 87
107 7.3.1 Greulich & Pyle System The Greulich & Pyle atlas has previously been applied in Australia for the estimation of the age of legal minority and the legal culpability of alleged people smugglers (Australian Human Rights Commission 2012). It was important to determine the accuracy of the method when applied to a Western Australian population, as the reference sample that the standard is derived from comprises European-American individuals from the 1930 s and 1940 s. Due to the standard being temporally removed from a contemporary sample, it can be expected that there will be some error present (see below). The unaltered Greulich & Pyle method showed a positive and linear relationship between stated chronological and estimated skeletal age when applied to the Western Australian population. The Greulich & Pyle atlas was found to be more accurate than the TW2 Carpal method for females and the TW2 RUS and TW2 20-bone methods for both males and females (Table 6.4). It was less accurate than the TW2 Carpal method for males and TW3 RUS method for males and females (Table 6.4). The mean SAD values for the Greulich & Pyle method, when corrected with the Western Australian specific linear regression formulae (Table 6.5), were found to be relatively low for both males and females. Those accuracy values were within published range of the original standard (±0.6 to 1.1 years; Greulich & Pyle 1959). In comparison to SAD values from similar studies, the Western Australian population was the most similar to the Eastern Australian population (Table 7.1), with males having a mean SAD value closer to 0 than females. The Portuguese population had the closest mean SAD value for males, while the Iranian population had the closest mean SAD value for females (Table 7.1). Overall, there was no apparent trend as to which sex had the lowest SAD value across the populations studied. The mean SAD is a popular measure in comparative studies, being used as a proxy for SEE. It must be noted, however, that it is an artificially low value; because it is an average of both positive and negative values it is not a true indicator of accuracy. 88
108 Table 7.1: Comparison of mean skeletal age difference (SAD) for Greulich & Pyle skeletal age estimates from different populations. Population n Male Mean SAD (years) n Female Mean SAD (years) Western Australian (this study) Indigenous Australian Eastern Australian 2 (Queensland) Turkish Iranian Portuguese Indian Brown & Grave (1976); 2 Paxton et al. (2013); 3 Büken et al. (2007); 4 Moradi et al. (2012); 5 Santos et al. (2011); 6 Patil et al While the overall accuracy of the Greulich & Pyle method increased with the application of the population specific linear regression formulae, it is useful only until the age of 16 years. These results once again show that population specific standards are the most reliable and accurate for age estimation, however, use of this standard may be inappropriate for the determination of legal majority and culpability (see below) Tanner-Whitehouse Systems The most accurate age estimation method for males was the TW2 Carpal method (SEE ±0.9 years), while the TW3 RUS method was the most accurate for females (SEE ±0.4 years; see Table 6.5). The TW2 RUS method was the least accurate for males, while the TW2 Carpal method was the least accurate for females. The overall accuracy of the RUS and 20-bone methods for males, and the carpal and 20-bone methods for females, was improved in the Western Australian population specific standards (derived from the polynomial regression formulae; Table 6.7) in comparison to the original unaltered methods. The increased accuracy of the Western Australian 89
109 standards, compared to the TW2 and TW3 methods, supports the suggestion that population specific standards are the most reliable and accurate for age estimation (e.g. Patil et al. 2012, Schmeling et al. 2000). The Western Australian specific carpal standard was less accurate for males than the original TW2 Carpal method. For females, the Western Australian specific RUS standard was less accurate than the original TW3 RUS method, but more accurate than the original TW2 RUS method. The loss of accuracy for the male carpal and female RUS standards was likely the result of sample size, as individuals with advanced or significantly delayed skeletal development have a greater effect within smaller samples (Tanner et al. 1997). The mean skeletal age differences (SAD) for the nonspecific (range: -0.8 to +1.1 years) and population specific standards (range: -0.2 to +0.1 years) were all within the accuracy of similar published studies (e.g. range:-2.0 to +1.1 years; see Schmidt et al and Zhang et al. 2008). As mentioned previously, the SAD is not truly representative of prediction accuracy. 7.4 Timing of Skeletal Maturity Sex specific Differences in Skeletal Maturity RUS skeletal maturation occurred earlier for females than males (14.4 and 15.2 years respectively) in the Western Australian population. The female sample showed an increased rate of skeletal maturation after 9 years of age (see Figure 6.2), in line with the reported average onset of female puberty (as early as 8 years of age) by The Longitudinal Study of Australian Children (LSAC 2012). Males showed this increased rate of skeletal maturity after 11 years of age (Figure 6.2), in accordance with the reported average onset of puberty (between years of age) in Australian males (LSAC 2012). Carpal skeletal maturation occurred approximately 2 years earlier in females in the Western Australian population ( 12.1 years; 14.3 years). The carpal scores were similar for both sexes at 2 years of age at which point only the capitate was present; thereafter female carpal maturity accelerated to be advanced over that of the males by 2 years on average, after approximately 8 years of age (as shown in 90
110 Figure 6.3). The earlier attainment of skeletal maturity in females in comparison to males is consistent with the results of similar population studies (see below for further discussion) Population trends Census data from Western Australia indicates that this population is predominantly Caucasian (79.51%) with the next largest percentage of the population identifying as being of Asian descent (10.38%; ABS 2012). It was necessary, therefore, to compare the Western Australian population standards to similar population groups. The RUS skeletal maturity curves derived for the Western Australian population were compared to the maturity curves for the TW2 and TW3 standards, in addition to population specific maturity curves for Belgian, Spanish, European-American, Japanese and Chinese populations. The European and European-American populations share a similar ancestral background to Western Australia, comprising of predominantly Caucasian individuals, while the Japanese and Chinese population studies share a similar geographic location. The Western Australian population generally attained skeletal maturity (a score of 1000) earlier than the other populations (Figures 6.10 and 6.11), with the exception of carpal maturity for males and females (Figures 6.12 and 6.13), which was delayed in comparison to the Chinese sample. Given the predominantly Caucasian ancestry of the population, it could be logically assumed that the rate of skeletal maturity in Western Australia would be most similar to the European or European-American populations. This is clearly not the case, however, as the difference in the approximate average age of attainment of specific successive RUS skeletal maturity scores (Tables 6.14 to 6.15) was significant for all but the Chinese population for males, in comparison to the Western Australian population. Aside from ancestry, the Chinese population study was most contemporary to the Western Australian population, with the data collected primarily in 2005, in contrast to the other population studies (comprising of data from the 1960 s to 1990 s). 91
111 Furthermore, the male Western Australian maturity curve most closely resembles the Chinese maturity curve from 13 years of age onwards, and the female maturity curve most closely resembles that of the Japanese population. For females only the TW2 standard (a population from the United Kingdom) was significantly different to the Western Australian population. The factors that can influence the rate of skeletal maturation, and therefore provide an explanation for the pattern of development present in Western Australia, are discussed in the following sections. 7.5 Factors that Influence Skeletal Maturation Skeletal maturation is influenced by a variety of different factors that are typically inter-related. Puberty is one of the main factors that have been shown to influence the rate and timing of skeletal maturation, due to the increased secretion of sex steroids, growth hormones and insulin-like growth factors (Saggese et al. 2002). While skeletal maturation does not necessarily influence pubertal onset (Flor-Cisneros et al. 2006), individuals with precocious puberty display advanced skeletal development in comparison to their chronological age (De Donno et al. 2013; Greulich & Pyle 1959). Socioeconomic status, genetics, and other environmental factors are discussed below in regard to their influence on the timing of the onset of puberty, and therefore their effect on skeletal maturation. As the Western Australian population displayed an increased rate of skeletal maturation at the approximate average onset of puberty, between 8 to 11 and 10 to 12 years of age (for females and males respectively; LSAC 2012), each of these factors that can influence pubertal onset is discussed in relation to the Western Australian population where possible Socioeconomic status Socioeconomic status is suggested to be a major influence in skeletal development (Schmeling et al. 2005) although there are conflicting factors involved. Individuals of a lower socioeconomic status are generally more prone to poor nutrition, pathology and trauma through lack of access to adequate medical care, thereby delaying skeletal development (Bradley & Corwyn 2002). Conversely, in low socioeconomic environments with high mortality rates, skeletal development can be advanced, due to 92
112 early onset puberty as a result of evolutionary pressure to reproduce (Gillette & Folinsbee 2012). The effects of nutrition, access to medical care, population mortality, and stress on the onset of puberty (and therefore skeletal development) are discussed in further detail below. i) Nutrition It has been shown that well-nourished individuals progress through puberty earlier than under-nourished individuals (Simondon et al. 1997). There has been an (approximately) four year decrease in the average age at pubertal onset over the last 200 years, which has occurred parallel to improvements in nutrition (Cameron 1996). The National Children s Nutritional Activity Survey (DoHA 2008) found that while the nutritional intake of children in Australia has generally improved, and despite access to a varied diet, not all children are getting adequate nutrition for optimal development. As a result those individuals with poorer nutrition could be expected to show delayed skeletal development, barring any other influencing factors. ii) Access to Medical Care Pathology is another factor that can influence the onset of puberty and therefore skeletal development (Ballinger et al. 2003). Growth processes utilize much of the body s resources, and if an individual is fighting a pathogen, these growth processes can be interrupted (Garn et al. 1968). If an individual is of a lower socioeconomic status, without access to adequate medical attention, they may show delayed development as a result of pathogenic events (Zemel et al. 2007). In Australia however, even low socioeconomic status individuals have access to medical care through Medicare (DoHS 2014) and so it is expected that this will limit the impact on skeletal growth and development would be less likely to be delayed. iii) Population Mortality As mentioned previously, in some societies early onset puberty has developed as an evolutionary adaptation to high population mortality. Historically in a population with a high mortality rate and a short lifespan, an earlier onset of puberty would be 93
113 necessary for procreation to occur (Gillette & Folinsbee 2012). It would be logical to assume that the skeletal development of such a population would be advanced as a result of the earlier onset of puberty. In Australia, population mortality is relatively low and the average lifespan is approximately 67.2 to 79.7 years for males, and 72.9 to 84.2 years for females (AIHW, 2013). Following this logic, skeletal maturation and pubertal onset should be delayed. However, the average onset of puberty in Australia, as published by LSAC (2012), could be considered early onset for females, as it is occurs as early as 8 years of age (Kaplowitz & Oberfield 1999). While there is a noted evolutionary adaptation for earlier development in some populations, this is unlikely to be an influencing factor on the skeletal development of a contemporary Western Australian sample due to a lack of evolutionary pressure (i.e. high mortality) necessitating earlier reproduction. iv) Stress Stress can influence the timing of the onset of puberty and, therefore, the timing of skeletal development and maturation of an individual. Stress can occur as a result of family antecedents, prolonged familial distress (e.g. socio-emotional stressor), economic anxiety, bullying, and pathology. Arım et al. (2011) demonstrated that girls whose fathers were unemployed, and boys living with fathers who had not finished secondary school, were more likely to experience early puberty. The study linked family socioeconomic status to psychosocial stress in earlier onset puberty. Incidences of acute stress increase the secretion of growth hormone, thereby resulting in rapid growth and potentially an earlier pubertal onset (Delemarre-van de Waal 1993). Conversely, chronic stress has an inhibitory effect on growth hormone secretion, suppressing growth and delaying puberty (Delemarre-van de Waal 1993). While stress could be an influencing factor on the earlier development of the Western Australian population, the biological and psychological stress levels of the present sample are unknown. 94
114 Genetics Socioeconomic factors are suggested as having a significant influence on skeletal maturation (Schmeling et al. 2005); however, there is evidence that genetics can also have a significant effect on the timing of the onset of puberty within an individual. This is supported by research that has demonstrated a familial inheritance pattern to precocious and delayed puberty (de Vries et al. 2004; Sedlmeyer et al. 2002). However, no medical or family history is available for the Western Australian sample examined in this thesis, and as such the contribution of genetics to the rate of skeletal maturation is unknown Other Environmental Factors There are a variety of other environmental factors that can influence the onset of puberty in a given population (and therefore the rate of skeletal maturation), these include: exposure to endocrine disruptors, synthetic and naturally occurring hormones and body weight (Fisher & Eugster 2012). Natural endocrine disruptors (such as phytoestrogens) can mimic growth and sexual hormones which can stimulate or inhibit puberty (Fisher & Eugster 2012). Artificial endocrine disruptors such as phthalates (a chemical component of most plastics) have also been linked to early onset puberty (Durmaz et al. 2010; Chou et al. 2009). Furthermore, exposure to naturally occurring sex and growth hormones in some medications and food can also advance or delay puberty. In girls, oestrogen is one of the major sex hormones that influence puberty and an excess can lead to an early onset, while conversely in boys, excess oestrogen can delay puberty. Studies have also provided evidence that suggest a child s body weight can also influence the rate of sexual and skeletal maturation, with obesity linked to an earlier onset of puberty in girls (Aksglaede et al. 2009). Given the current childhood obesity trend in Australia (DoHA 2008), body weight could be an influencing factor on earlier pubertal onset in Australian children, and therefore have a flow-on effect on skeletal maturation. All of the factors mentioned above could have the potential to contribute to the earlier skeletal development in the Western Australian population in comparison to the other population studies. 95
115 7.6 Forensic Applications This project has demonstrated that it is possible to estimate age in sub-adult Western Australians, based on a morphoscopic analysis of hand-wrist skeletal development, with a relatively high degree of expected accuracy. Throughout the project it has been noted that the rate of skeletal growth and development varies between population groups (e.g. Schmeling et al. 2000, Schmeling et al. 2005; Patil et al. 2012, Tanner et al. 1997). As a result, the most reliable and accurate standards for developing an osteobiography (or biological profile) are those that are population specific. This has been shown in the present study through the comparison of the accuracy of existing standards to the accuracy of the derived Western Australian age estimation models. Anthropological skeletal standards for Australia are relatively scarce (Donlon 2009), particularly for Western Australia. As discussed in detail in Chapter Four, there have been a number of studies that have investigated the potential of estimating age using the hand-wrist skeleton. The Greulich & Pyle atlas has been in use since its publication in 1959, and there have been a few validation studies that have attempted to adjust the method for use in a specific population. There are validation studies using the Tanner-Whitehouse method, originally published in 1962, which have developed their own population specific standards. However, these are not suited for application to an Australian population (e.g. Ashizawa et al. 1996; Beunen et al. 1990; Tanner et al. 1997; Zhang et al. 2008). In this regard, therefore, the present study has made novel contributions to the forensic anthropology discipline. Sex-specific age estimation standards developed for a contemporary Western Australian population also have the potential to contribute to developing a biological profile of an unknown victim, or estimating age for undocumented sub-adult individuals, provided they are under the maximum skeletal ages of 14.4 and 15.2 years (females and males respectively). The age of legal majority and culpability in Australia is 18 years of age (Age of Majority Act (WA) s 5); the present research project has demonstrated that the derived Western Australian standards are not applicable to determine legal majority or culpability, as RUS skeletal maturity was attained at 14.4 and 15.2 years of age (for 96
116 females and males respectively). Furthermore, caution must be taken when applying the Greulich & Pyle correction factor to estimate skeletal age, as it has a maximum overestimation of 2.5 years at the ages of 16 and 18, and may only be applicable to the reference sample studied. 7.7 Limitations Resource constraints (time, availability of scans, budget) limited the total sample to 360 individuals. Tanner et al. (2001) has stated that to derive age estimation standards a minimum of 1000 scans for each sex is required, evenly distributed by age group, although less can be used if the scans are from a longitudinal study. As such, with a small sample size obtained from a clinical database, the sample analysed in the present study may not be truly representative of the Western Australian population at large. The socioeconomic status and ancestry of the individuals in the sample are unknown, although prior research using MDCT scans of skulls, obtained from the same PACS database accessed in the present study, demonstrated that the assessed percentages of each ancestral group (e.g. Caucasian) mirrored current census data (Franklin & Flavel, 2013, unpublished data). However, individuals of a lower socioeconomic status could be more likely to attend a hospital for diagnosis, as opposed to a private clinic. As discussed previously (see above) socioeconomic status is suggested as a major factor that influences the timing of skeletal development, as those of a lower status are more prone to poor nutrition and pathology, and therefore delayed skeletal development (Schmeling et al. 2005). Australia has an advanced socioeconomic standing, so this effect should theoretically be minimal (OECD, 2013). For this reason further testing in the wider Western Australian population is recommended (see below). Another potential limitation of this study is the lack of assessment of bilateral asymmetry. The radiographs used in this study were a mixture of left and right hand wrists, while the age estimation methods applied were designed for the left hand wrist. There was a lack of radiographs of both hands obtained from the same individual 97
117 at the same time for an assessment of asymmetry to be performed. Although any asymmetry in skeletal development should be minor (Baer & Durkatz 1957), its effect on the derived Western Australian age estimation standards cannot be evaluated at this stage. 7.8 Future Research The replication of this research on a larger Western Australian sample of known and diverse ancestry and socioeconomic backgrounds, may overcome any unintentional bias due to sampling. Any future research would also benefit from an assessment of bilateral asymmetry in the timing of hand and wrist skeletal development. Additional data collection might be facilitated by obtaining radiographs from private radiology clinics, or by obtaining radiographs from schools within Western Australia, as was performed by Tanner et al. (1983) in the United Kingdom. For this to occur, a new method of low-dose radiography that does not expose the participants to high levels of ionizing radiation would be a necessity for ethics approval (Ramsthaler et al. 2009). Alternatively, post-mortem sources, or an expanded sample from a greater Australian population, could be utilised. Ideally, a large scale project should incorporate a mixture of cross-sectional and longitudinal studies to give the most information about skeletal development within the population (Tanner et al. 2001). Furthermore, a larger scale project could involve assessment of radiographs by multiple assessors, in order to quantify inter-observer accordance, and therefore further evaluate the subjectivity of the age estimation methods. To this end, it may be expedient to work collaboratively with paediatricians, radiologists and hand surgeons, whose specialist knowledge would be a distinct advantage to such a project. Future collaborations with physical and forensic anthropologists would be mutually beneficial and would have the potential to contribute to their respective fields. Other technological advances that could benefit such a study include the use of 3D morphometric imaging methods to enable high resolution imaging of hand-wrist morphology, as has already been applied to the clavicle (e.g. Schulze et al. 2006). This 98
118 would allow for the formation of morphometric hand-wrist age estimation methods, which have the potential to be considerably less subjective than the morphological methods already in use. Using such imaging methods, further research could expand into shape or ratio based analyses, such as that proposed by Cameriere et al (2005). Multivariate statistical techniques could also accompany these shape analyses to interpret landmarks and coordinate data (Ross & Kimmerle 2009). Such techniques are already in use for craniofacial interpretation, but there is the potential for use in other anatomical regions. 7.9 Conclusion Age estimation is one of the main components of biological profiling for the purpose of identification (Franklin 2010) and as such it is important to ensure that any age estimates are as accurate as possible. The present research project has demonstrated the utility and limitations of two widely applied and established morphoscopic methods for estimating age based on the analysis of skeletal growth and development of the hand-wrist in a Western Australian population. Intra-observer accordance showed near perfect agreement (range: κ=0.869 to κ=1.000). The project demonstrated that females mature physically up to 2 years earlier than males. A series of novel statistically quantified population and sex-specific hand-wrist age estimation standards were also outlined with accuracies ranging from ± years to ±2.0 years. 99
119 REFERENCES Acheson, RM 1966, Studies in the Reliability of Assessing Skeletal Maturity from X- rays. Part III. Greulich-Pyle Atlas and Tanner-Whitehouse Method Contrasted, Human Biology, vol. 38, no. 3, pp Age of Majority Act 1972 (WA) Aksglaede, L, Juul, A, Olsen, LW & Sørensen, TIA 2009, Age at Puberty and the Emerging Obesity Epidemic, PLoS ONE, vol. 4, no. 12, pp. e8450. Allen, P & Bennet, K 2010, PASW Statistics by SPSS: a practical guide, version 18.0, Cengage Learning Australia, South Melbourne. Altman, DG & Bland, JM 1995, Statistics notes: the normal distribution, British Medical Journal, vol. 310, no. 6975, pp Andersen, E 1971, Comparison of Tanner-Whitehouse and Greulich-Pyle methods in a large scale Danish survey, American Journal of Physical Anthropology, vol. 35, no.3, pp Arany, S & Ohtani, S 2011, Age estimation of bloodstains: A preliminary report based on aspartic acid racemization rate, Forensic Science International, vol. 212, no. 1, pp. e36-e39. Arım, RG, Tramonte, L, Shapka, JD, Dahinten, VS & Willms, JD 2011, The Family Antecedents and the Subsequent Outcomes of Early Puberty, Journal of Youth and Adolescence, vol. 40, pp Ashizawa, K, Asami, T, Anzo, M, Matsuo, N, Matsuoka, H, Murata, M, Ohtsuki, F, Satoh, M, Tanaka, T, Tatara, H & Tsukagoshi, K 1996, Standard RUS skeletal maturation of Tokyo children, Annals of Human Biology, vol. 23, no. 6, pp
120 Australian Bureau of Statistics (ABS) 2012, Ancestry. Available from: < me/factsheetsa?opendocument&navpos=450>. [16 December 2013]. Australian Department of Health (DoHA) 2008, 2007 Australian National Children's Nutrition and Physical Activity Survey - Main Findings. Available from: < [8 January 2014]. Australian Institute of Health and Welfare (AIHW) 2013, Life Expectancy. Available from: < [8 January 2014]. Baer, MJ & Durkatz J 1957, Bilateral asymmetry in skeletal maturation of the hand and wrist: a roentgenographic analysis, American Journal of Physical Anthropology, vol. 15, no. 2, pp Ballinger, AB, Savage MO & Sanderson IR 2003, Delayed Puberty Associated with Inflammatory Bowel Disease, Pediatric Research, vol. 53, pp Baumann, U, Schulz, R, Reisinger, W, Heinecke, A, Schmeling, A & Schmidt, S 2009, Reference study on the time frame for ossification of the distal radius and ulnar epiphyses on the hand radiograph, Forensic Science International, vol. 191, no. 1, pp Beunen, GP, Lefevre, J, Ostyn, M, Renson, R, Simons, J & Van Gerven, D 1990, Skeletal maturity in Belgian youths assessed by the Tanner-Whitehouse method (TW2), Annals of Human Biology, vol. 17, no. 5, pp Beunen, GP, Malina, RM, Lefevre, J, Claessens, AL, Renson, R, Simons, J, Maes, H, Vanreusel, B & Lysens, R 1994, Size, fatness and relative fat distribution of males of contrasting maturity status during adolescence and as adults, International Journal of Obesity, vol. 18, pp Black, S, Aggrawal, A & Payne-James, J (eds) 2010, Age Estimation in the Living: The Practitioner s Guide, John Wiley & Sons Ltd, Hoboken. 101
121 Bradley, RH & Corwyn, RF 2002, Socioeconomic Status and Child Development, Annual Review of Psychology, vol. 53, pp Brown, T, & Grave, KC 1976, Skeletal maturation in Australian Aborigines, Australian Pediatric Journal, vol. 12, pp Büken, B, Şafak, AA, Yazıcı, B, Büken, E & Mayda AS 2007, Is the assessment of bone age by the Greulich-Pyle method reliable at forensic age estimation for Turkish children?, Forensic Science International, vol. 173, pp Bull, RK, Edwards, PD, Kemp, PM, Fry, S & Hughes, IA 1999, Bone age assessment: A large scale comparison of the Greulich and Pyle, and Tanner and Whitehouse (TW2) methods, Archives of Disease in Childhood, vol. 81, no. 2, pp Burr, DB & Allen, MR (eds) 2013, Basic and Applied Bone Biology, Elsevier, London. Cameriere, R, Ferrante, L, Mirtella, D & Cingolani, M 2005, Carpals and epiphyses of radius and ulna as age indicators, International Journal of Legal Medicine, vol. 120, pp Cameriere, R, Ferrante, L, Ermenc, B, Mirtella, D & Štrus, K 2008, Age estimation using carpals: Study of a Slovenian sample to test Cameriere's method, Forensic Science International, vol.174, no. 2, pp Cameron, J 1996, Nutritional determinants of puberty, Nutritional Reviews, vol. 54, no. 2, pp. S17. Cattaneo, C 2007, 'Forensic anthropology: developments of a classical discipline in the new millennium', Forensic Science International, vol. 165, no. 2-3, pp Cattaneo, C, Ritz-Timme, S, Gabriel, P, Gibelli, D, Giudici, E, Poppa, P, Nohrden, D, Assmann, S, Schmitt, R & Grandi, M 2009, The difficult issue of age assessment on pedo-pornographic material, Forensic Science International, vol. 183, no. 1, pp. e21- e
122 Chou, YY, Huang, PC, Lee, CC, Wu, MH & Lin, SJ 2009, Phthalate exposure in girls during early puberty, Journal of Pediatric Endocrinology and Metabolism, vol. 22, pp Clarke, HH & Hayman, NR 1962, Reduction of Bone Assessments Necessary for the Skeletal Age Determination of Boys, Research Quarterly. American Association for Health, Physical Education and Recreation, vol. 33, no. 2, pp Cohen, J 1968, Weighted kappa: nominal scale agreement provision for scaled disagreement or partial credit, Psychological Bulletin, vol. 70, pp Conaghan, PG, O Connor, P & Isenberg, DA 2010, Musculoskeletal Imaging, Oxford University Press, Oxford. Csapó, J, Csapó-Kiss, Z, Némethy, S, Folestad, S, Tivesten, A & Martin, T 1994, Age determination based on amino acid racemization: A new possibility, Amino Acids, vol. 7, no. 3, pp Currell, G & Dowman, A 2009, Essential Mathematics and Statistics for Science, John Wiley & Sons Ltd., Chicester. De Donno, A, Roca, R, Introna, F & Santoro V 2013, A case of an adoptive girl with precocious puberty: The problem of age estimation, Forensic Science International, vol. 231, no. 1-3, pp. 400.e1-400.e4. de Vries, L, Kauschansky, A, Shohat, M & Phillip M 2004, Familial Central Precocious Puberty Suggests Autosomal Dominant Inheritance, The Journal of Clinical Endocrinology & Metabolism, vol. 89, no. 4, pp Dedouit, F, Auriol, J, Rosseau, H, Rouge, D, Crubèzy, E & Telmon, N 2012, Age assessment by magnetic resonance imaging of the knee: A preliminary study, Forensic Science International, vol. 217, pp. 232.e1-232.e7. 103
123 Delemarre-van de Waal, HA 1993, Environmental Factors Influencing Growth and Pubertal Development, Environmental Health Perspectives Supplements, vol. 101, no. 2, pp Department of Human Services (DoHS) 2014, Medicare Services. Available from: < [8 January 2014]. Dhawan, AP 2011, Medical Image Analysis, John Wiley & Sons Ltd., New Jersey. Dirkmaat, DC & Cabo, LL 2012, Forensic anthropology: embracing the new paradigm, in A Companion to Forensic Anthropology, ed DC Dirkmaat, Blackwell, Oxford, pp Donlon, D 2009, The development and current state of forensic anthropology: an Australian perspective in Handbook of Forensic Anthropology and Archaeology, eds S Blau & DH Ubelaker, Left Cross Press Inc., California. Doyle, JR & Botte, MJ 2003, Surgical Anatomy of the Hand and Upper Extremity, Lippincott Williams & Wilkins, Philadelphia, pp Drake, R, Vogl, W & Mitchell, AWM 2009, Gray s Anatomy for Students, 2nd edn, Elsevier Health Sciences, Philadelphia. Durmaz, E, Özmert, EN, Erkekoğlu, P, Giray, B, Derman, O, Hıncal, F & Yurdakök, K 2010, Plasma Phthlate Levels in Pubertal Gynecomastia, Pediatrics, vol. 125, no. 1, pp. e.122-e.129. Dytham, C 2010, Choosing and Using Statistics: A Biologist s Guide, John Wiley & Sons Ltd., Chicester. Elliott, AC & Woodward, WA 2007, Statistical analysis quick reference guidebook with SPSS examples, 1st edn, Sage Publications, London. 104
124 Fazel, R, Krumholz, HM, Wang, Y, Ross, JS, Chen, J, Ting, HH, Shah, ND, Nasir, K, Einstein, AJ & Nallamothu, BK 2009, Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures, The New England Journal of Medicine, vol. 361, no. 9, pp Ferguson, AC, Murray, AB & Tze, W 1982, Short stature and delayed skeletal maturation in children with allergic disease, Journal of Allergy and Clinical Immunology, vol. 69, no. 5, pp Fisher, M.M & Eugster, EA 2012, What is in our environment that effects puberty?, Reproductive Toxicology, In Press. Available from: < [6 January 2014] Flor-Cisneros, A, Roemmich, JN, Rogol, AD & Baron, J 2006, Bone age and onset of puberty in normal boys, Molecular and Cellular Endocrinology, vol , pp Franklin, D 2010, Forensic age estimation in human skeletal remains: Current concepts and future directions, Legal Medicine, vol. 12, pp Franklin, D, Cardini, A, Flavel, A, Kuliukas, A, Marks, MK, Hart, R & O'Higgins, P 2013, Concordance of traditional osteometric and volume-rendered MSCT interlandmark cranial measurements, International Journal of Legal Medicine, vol. 127, pp Freund, RJ, Wilson, WJ & Sa, P 2006, Regression Analysis, 2nd edn, Elsevier Science, San Diego. Garn, SM, Silverman, FN, Hertzog, JP & Rohmann, VM 1968, Lines and bands of increased density: their implication to growth and development, Medical Radiology and Photography, vol. 44, pp Garrido Varas, CE & Thompson, TJU 2011, Metric dimensions of the proximal phalanges of the human hand and their relationship to side, position, and asymmetry, Journal of Comparative Human Biology, vol. 62, no. 2, pp
125 Gillette, MT & Folinsbee, KE 2012, Early Menarche as an Alternative Reproductive Tactic in Human Females: An Evolutionary Approach to Reproductive Health Issues, Evolutionary Psychology, vol. 10, no. 5, pp Gilsanz, V & Ratib, O 2005, Hand Bone Age: A Digital Atlas of Skeletal Maturity, Springer-Verlag, Berlin. Gilsanz, V & Ratib, O 2011, Hand Bone Age: A Digital Atlas of Skeletal Maturity, 2nd edn, Springer-Verlag, Berlin. Girden, E 1992, ANOVA : Repeated Measures, Sage Publications, California. Gonsior, M, Ramsthaler, F, Gehl, A & Verhoff, M 2013, Morphology as a cause for different classification of the ossification stage of the medial clavicular epiphysis by ultrasound, computed tomography, and macroscopy, International Journal of Legal Medicine, vol. 127, no. 5, pp Greenhouse, SW & Geisser, S 1959, On Methods in the Analysis of Profile Data, Psychometrika, vol. 24, no. 2, Greulich, WW & Pyle, SI 1959, Radiographic Atlas of Skeletal Development of the Hand and Wrist, 2 nd edn, Stanford University Press, Stanford. Hackman, SL, Buck, A & Black, S 2010, Age Evaluation from the Skeleton, in Age Estimation in the Living: The Practitioner s Guide, eds S Black, A Aggrawal & J Payne- James, John Wiley & Sons Ltd, Hoboken, pp Hernández, M, Sánchez, E, Sobradillo, B & Rincón, JM 1991, Skeletal maturation and height prediction: atlas and scoring methods, Diaz de Santos, Madrid. Hewitt, D & Acheson, RM 1961, Some aspects of skeletal development through adolescence. I. Variations in the rate and pattern of skeletal maturation at puberty, American Journal of Physical Anthropology, vol. 19, pp Hillson, S 2002, Dental Anthropology, 2nd edn, Cambridge University Press, Cambridge. 106
126 Johnston, FE, Whitehouse, RH & Hertzog, KP 1968, Normal variability in the age and first onset of ossification of the triquetral, American Journal of Physical Anthropology, vol. 28, no. 1, pp Jones, LA & Lederman, SJ 2006, Human hand function, Oxford University Press, Oxford. Kaplowitz, PB & Oberfield, SE 1999, Re-examination of the Age Limit for Defining When Puberty Is Precocious in Girls in the United States: Implications for Evaluation and Treatment, Pediatrics, vol. 104, no. 4, pp King, DG, Steventon DM, O Sullivan, MP, Cook, AM, Hornsby, VP & Jefferson IJ et al. 1994, Reproducibility of bone ages when performed by radiology registrars: an audit of Tanner and Whitehouse II versus Greulich and Pyle methods, British Journal of Radiology, vol. 64, pp Komar, DA & Buikstra, JE 2008, Forensic Anthropology: Contemporary Theory and Practice, Oxford University Press, Oxford. Kuperman, V 2000, Magnetic Resonance Imaging: Physical Principles and Applications, Elsevier Science, San Diego. Landis, JR & Koch GG 1977, An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers, Biometrics, vol. 33, no. 2, pp Landsmeer, JM 1962, Power grip and precision handling, Annals of the Rheumatic Diseases, vol. 21, pp Mackenzie, CL & Iberall, T 1994, The Grasping Hand, Elsevier, Burlington. Madrigal, L 1998, Statistics in Anthropology, Cambridge University Press, Cambridge. Malina, RM 2011, Skeletal age and age verification in youth sport, Sports Medicine, vol. 41, no. 11, pp
127 Marieb, EN & Hoehn, K 2006, Human Anatomy & Physiology, 7th edn, Pearson Benjamin Cummings, San Francisco. Marshall, WA, Ashcroft, MT & Bryan, G 1970, Skeletal maturation of the hand and wrist in Jamaican children, Human Biology, vol. 42, no. 3, pp Marzke, MW 1997, Precision grips, hand morphology, and tools, American Journal of Physical Anthropology, vol. 102, no. 1, pp Matsuoka, H, Sato, K, Sugihara, S & Murata, M 1999, Bone maturation reflects the secular change in growth, Hormone Research, vol. 52, pp McKeown, AH & Schmidt, RW 2012, Geometric morphometrics, in Research Methods in Human Biology, eds EA Di Gangi & MK Moore, Elsevier Science, Oxford. Melsen, B, Wenzel, A, Miletic, T, Andreasen, J, Vagn-hansen, PL & Terp, S 1986, Dental and skeletal maturity in adoptive children: assessments at arrival and after one year in the admitting country, Annals of Human Biology, vol. 13, no. 2, pp Milner, GR & Boldsen, JL 2012, Transition analysis: A validation study with known-age modern American skeletons, American Journal of Physical Anthropology, vol. 148, no. 1, pp Moradi, M, Sirous, M & Morovatti, P 2012, The reliability of skeletal age determination in an Iranian sample using Greulich and Pyle method, Forensic Science International, vol. 223, pp.372.e1-372.e4 National Health and Medical Research Council (NHMRC) 2013, National Statement on Ethical Conduct in Human Research updated Available from < [17 March 2013]. Ohtani, S & Yamamoto, T 2010, Age Estimation by Amino Acid Racemization in Human Teeth, Journal of Forensic Sciences, vol. 55, no. 6, pp
128 Organisation for Economic Co-operation and Development (OECD) 2013, Country Snapshot Australia. Available from: < Note-AUSTRALIA.pdf>. [9 January 2014]. Patil, ST, Parchand, MP, Meshram, MM & Kamdi, NY 2012, Applicability of Greulich and Pyle skeletal age standards to Indian children, Forensic Science International, vol. 216, pp.200.e1-200.e4. Paxton, M, Lamont, A & Sitwell, A 2013, The reliability of the Greulich-Pyle method in bone age determination among Australian children, Journal of Medical Imaging and Radiation Oncology, vol. 57, pp Peat, J 2001, Health Science Research: A Handbook of Quantitative Methods, Allen & Unwin, Sydney. Powell, S, Ma, D & Jones, G 2008, Determinants of Skeletal Age Deviation in a Cross- Sectional Study, Journal of Clinical Endocrinology and Metabolism, vol. 93, no. 2, pp Ramsthaler, F, Proschek, P, Betz, W & Verhoff, M 2009, How reliable are the risk estimates for X-ray examinations in forensic age estimations? A safety update, International Journal of Legal Medicine, vol.123, no. 3, pp Ritz-Timme, S, Cattaneo, C, Collins, MJ, Waite, ER, Schütz, HW, Kaatsch, HJ & Borrman, HIM 2000, Age estimation: The state of the art in relation to the specific demands of forensic practise, International Journal of Legal Medicine, vol. 113, pp Roche, AF & Sun, SS 2005, Human Growth: Assessment and Interpretation, Cambridge University Press, Cambridge. Roche, AF, Cameron Chumlea, W, & Thissen, D 1988, Assessing the Skeletal Maturity of the Hand-wrist: Fels Method, Charles C. Thomas, Springfield. 109
129 Ross, AH & Kimmerle, EH 2009, Contribution of quantitative methods in forensic anthropology: a new era, in Handbook of Forensic Anthropology and Archaeology, eds S Blau & DH Ubelaker, Left Cross Press Inc., California. Saggese, G, Baroncelli GI & Bertelloni, S 2002, Puberty and bone development, Best Practice & Research Clinical Endocrinology and Metabolism, vol. 16, no. 1, pp Saladin, KS 2007, Anatomy & Physiology: the Unity of Form and Function, McGraw Hill, New York. Santoro, V, Roca, R, De Donno, A, Fiandaca, C, Pinto, G, Tafuri, S & Introna, F 2012, Applicability of Greulich and Pyle and Demirijan aging methods to a sample of Italian population, Forensic Science International, vol. 221, pp. 153.e1-153.e5. Santos, C, Ferreira, M, Alves, FC & Cunha, E 2011, Comparative study of Greulich and Pyle Atlas and Maturos 4.0 program for age estimation in a Portuguese sample, Forensic Science International, vol. 212, no. 1, pp.276.e1-276.e7. Schaefer, M, Scheuer, L & Black, S 2010, Juvenile Osteology: A Laboratory and Field Manual, Elsevier, Burlington. Scheuer, L & Black, S 2000, Developmental Juvenile Osteology, Elsevier, Burlington. Scheuer, L & Black, S 2004, The Juvenile Skeleton, Elsevier, Burlington. Schmeling, A & Black, S 2010, An introduction to the history of age estimation in the living, in Age Estimation in the Living: The Practitioner s Guide, eds S Black, A Aggrawal & J Payne-James, John Wiley & Sons Ltd, Hoboken, pp Schmeling, A, Reisinger, W, Loreck, D, Vendura, K, Markus, W & Geserick, G 2000, Effects of ethnicity on skeletal maturation: consequences for forensic age estimations, International Journal of Legal Medicine, vol. 113, pp
130 Schmeling, A, Olze, A, Reisinger, W & Geserick, G 2004, Forensic age diagnostics of living people undergoing criminal proceedings, Forensic Science International, vol. 144, pp Schmeling, A, Olze, A, Reisinger, W & Geserick, G 2005, Forensic age estimation and ethnicity, Legal Medicine, vol. 7, pp Schmeling, A, Reisinger, W, Geserick, G & Olze, A 2006, Age estimation of unaccompanied minors. Part I. General considerations, Forensic Science International, vol. 159S, pp. S61-S64. Schmeling, A, Geserick, G, Reisinger, W & Olze, A 2007 Age estimation, Forensic Science International, vol. 165, pp Schmidt, S, Nitz, I, Schulz, R & Schmeling, A 2008, Applicability of the skeletal age determination method of Tanner and Whitehouse for forensic age diagnostics, International Journal of Legal Medicine, vol. 122, pp Schulze, D, Rother, U, Fuhrmann, A, Richel, S, Faulmann, G & Heiland M 2006, Correlation of age and ossification of the medial clavicular epiphysis using computed tomography, Forensic Science International, vol. 158, pp Sedlmeyer, IL, Hirschhorn, JN & Palmert, MR 2002, Pedigree analysis of constitutional delay of growth and maturation: determination of familial aggregation and inheritance patterns, The Journal of Clinical Endocrinology & Metabolism, vol. 87, no. 12, pp Simmons, K & Greulich, WW 1944, The Brush Foundation study of child growth and development: II. Physical growth and development, Monographs of the Society for Research in Child Development, vol.9, no.1, pp.i-87. Simondon, KB, Simon, I & Simondon, F 1997, Nutritional status and age at menarche of Sengalese adolescents, Annals of Human Biology, vol. 24, no. 6, pp
131 Slice, D (ed.) 2005, Modern Morphometrics in Physical Anthropology, Plenum Publishers, New York. Standrig, S (ed.) 2008, Gray s Anatomy, 40th edn, Elsevier, London. Steadman, DW 2012, The places we will go: paths forward in forensic anthropology in Forensic Science : Current Issues, Future Directions, ed. DH Ubelaker, John Wiley & Sons Ltd, West Sussex, pp Tanner, JM 1971, Sequence, tempo and individual variation in the growth and development of boys and girls aged twelve to sixteen, Daedalus, vol. 100, no. 4, pp Tanner, JM, Whitehouse, RH & Healy MJR 1962, A New System for Estimating Skeletal Maturity from the Hand and Wrist with Standards Derived from a Study of 2600 Healthy Children. Part II. The Scoring System, International Child Centre, Paris. Tanner, JM, Whitehouse, RH, Cameron, N, Marshall, WA, Healy, MJR & Goldstein, H 1983, Assessment of Skeletal Maturity and Prediction of Adult Height, 2 nd edn, Academic Press, London. Tanner, JM, Oshman, D, Babbage, F & Healy, MJR 1997, Tanner-Whitehouse bone age reference values for North-American children, The Journal of Pediatrics, vol. 131, no. 1, pp Tanner, JM, Healy, MJR, Goldstein, H & Cameron, N 2001, Assessment of Skeletal Maturity and Prediction of Adult Height (TW3 Method), W.B Saunders, London. The Longitudinal Study of Australian Children (LSAC) 2012, The Longitudinal Study of Australian Children: Annual Report. Available from: < [6 January 2014] 112
132 The Royal Australian College of Physicians 2012, The Australian Human Rights Commission s inquiry into the treatment of individuals suspected of people smuggling offenses who say they are children. Available from: < [9 March 2013] Thiemann, HH & Nitz, l 1991, Röntgenatlas der normalen Hand im Kindesalter, Thieme- Verlag, Stuttgart. Thiemann, HH, Nitz, l & Schmeling, A 2006, Röntgenatlas der normalen Hand im Kindesalter, Thieme-Verlag, Stuttgart. Todd, TW 1937, Atlas of Skeletal Maturation, C.V. Mosby, St. Louis. Townsend, J 2012, Practical Statistics for Environmental and Biological Scientists, John Wiley & Sons Ltd., Chicester. UN General Assembly, Convention on the Rights of the Child, 20 November 1989, United Nations, Treaty Series, vol. 1577, pp. 3. Available from: < [1 September 2013]. United Nations High Commissioner for Refugees (UNHCR) 1997, Guidelines on Policies and Procedures in Dealing with Unaccompanied Children Seeking Asylum. Available from: < [27 July 2013]. van Lethe, FJ, Kemper, HCG & van Mechelen, W 1998, Skeletal maturation in adolescence: a comparison between the Tanner-Whitehouse II and Fels method, European Journal of Pediatrics, vol. 157, pp Walther, FJ, Ramaekers, LHJ & van Engelshoven JMA 1981, Skeletal maturity at birth and at the age of 3 years of infants malnourished in utero, Early Human Development, vol. 5, no. 2, pp White, TD & Folkens, PA 2005, The Human Bone Manual, Elsevier, Boston. 113
133 White, TD, Black M & Folkens, PA 2011, Human Osteology, Elsevier, Boston. Yekkala, R, Meers, C, Van Shepdael, A, Hoogmartens, J, Lambrichts, I & Willems, G 2006, Racemization of aspartic acid from human dentin in the estimation of chronological age, Forensic Science International, vol. 159, pp. S89-S94. Zemel, BE, Kawchak, DA, Ohene-Frempong, K, Schall, JI & Stallings, VA 2007 Effects of Delayed Pubertal Development, Nutritional Status, and Disease Severity on Longitudinal Patterns of Growth Failure in Children With Sickle Cell Disease, Pediatric Research, vol. 61, no. 5, Zhang, S, Liu, L, Wu, Z, Liu, G, Ma, Z, Shen, X, & Xu, R 2008, Standards of TW3 skeletal maturity for Chinese children, Annals of Human Biology, vol. 35, no. 3, pp
134 APPENDIX I I.1 Precision Test Data Greulich & Pyle Table AI.1: Greulich & Pyle precision test data (estimated age in years). MAN Accession No. Sex Attempt 1 Attempt 2 Attempt 3 MAN F MAN F MAN F MAN F MAN F MAN F MAN F MAN F MAN F MAN F MAN M MAN M MAN M MAN M MAN M MAN M MAN M MAN M MAN M MAN M I.2 Precision Test Data Tanner-Whitehouse Key for tables: RAD = radius; ULN= Ulna; MC = Metacarpal; P = Proximal phalanx, M = Middle phalanx; D = Distal phalanx; CAP = Capitate; HAM = Hamate; TRI = Triquetral; LUN = Lunate; SCA = Scaphoid; TZM = Trapezium; TZD = Trapezoid. 115
135 116 Table AI.2: Female Tanner-Whitehouse maturity scores by element for the precision test.
136 Table AI.3: Male Tanner-Whitehouse maturity scores by element for the precision test. 117
Introduction to Human Osteology Chapter 3: Hands and Feet
 Introduction to Human Osteology Chapter 3: Hands and Feet Roberta Hall Kenneth Beals Holm Neumann Georg Neumann Gwyn Madden Revised in 1978, 1984, and 2008 Bones of the Hand Eight carpal bones, in two
Introduction to Human Osteology Chapter 3: Hands and Feet Roberta Hall Kenneth Beals Holm Neumann Georg Neumann Gwyn Madden Revised in 1978, 1984, and 2008 Bones of the Hand Eight carpal bones, in two
The skeleton consists of: Bones: special connective tissue, hard. Cartilage: special connective tissue, less hard than bones. Joints: joint is the
 The skeleton consists of: Bones: special connective tissue, hard. Cartilage: special connective tissue, less hard than bones. Joints: joint is the location at witch two bones make contact, whereas ligaments
The skeleton consists of: Bones: special connective tissue, hard. Cartilage: special connective tissue, less hard than bones. Joints: joint is the location at witch two bones make contact, whereas ligaments
Lecture 04 Osteology Of Hand
 Lecture 04 Osteology Of Hand By: A. Prof. Dr Farooq A. Khan PMC Date: 09 th Jan. 2018 HAND Distal to wrist joint Divided into three parts: Carpus Metacarpus Digits Bones Carpal bones o Prox row S L Tq
Lecture 04 Osteology Of Hand By: A. Prof. Dr Farooq A. Khan PMC Date: 09 th Jan. 2018 HAND Distal to wrist joint Divided into three parts: Carpus Metacarpus Digits Bones Carpal bones o Prox row S L Tq
Chapter 8. The Pectoral Girdle & Upper Limb
 Chapter 8 The Pectoral Girdle & Upper Limb Pectoral Girdle pectoral girdle (shoulder girdle) supports the arm consists of two on each side of the body // clavicle (collarbone) and scapula (shoulder blade)
Chapter 8 The Pectoral Girdle & Upper Limb Pectoral Girdle pectoral girdle (shoulder girdle) supports the arm consists of two on each side of the body // clavicle (collarbone) and scapula (shoulder blade)
Figure 1: Bones of the upper limb
 BONES OF THE APPENDICULAR SKELETON The appendicular skeleton is composed of the 126 bones of the appendages and the pectoral and pelvic girdles, which attach the limbs to the axial skeleton. Although the
BONES OF THE APPENDICULAR SKELETON The appendicular skeleton is composed of the 126 bones of the appendages and the pectoral and pelvic girdles, which attach the limbs to the axial skeleton. Although the
Skeletal System. Supplementary Information
 Skeletal System Supplementary Information COMMON ANATOMICAL TERMS Planes run through the body side to side and front to back eg. median plane Surfaces of the body are also named eg. anterior surface This
Skeletal System Supplementary Information COMMON ANATOMICAL TERMS Planes run through the body side to side and front to back eg. median plane Surfaces of the body are also named eg. anterior surface This
Chapter 8 The Skeletal System: The Appendicular Skeleton. Copyright 2009 John Wiley & Sons, Inc.
 Chapter 8 The Skeletal System: The Appendicular Skeleton Appendicular Skeleton It includes bones of the upper and lower limbs Girdles attach the limbs to the axial skeleton The pectoral girdle consists
Chapter 8 The Skeletal System: The Appendicular Skeleton Appendicular Skeleton It includes bones of the upper and lower limbs Girdles attach the limbs to the axial skeleton The pectoral girdle consists
A&P 1 Skeletal Lab Guide Week 2 - Appendicular Skeleton and Joints Lab Exercises: Pectoral Girdle
 A&P 1 Skeletal Lab Guide Week 2 - Appendicular Skeleton and Joints Lab Exercises: Pectoral Girdle PLEASE NOTE: Your group will need an articulated skeleton, a disarticulated skeleton, and the joint models
A&P 1 Skeletal Lab Guide Week 2 - Appendicular Skeleton and Joints Lab Exercises: Pectoral Girdle PLEASE NOTE: Your group will need an articulated skeleton, a disarticulated skeleton, and the joint models
Bone Development. V. Gilsanz and O. Ratib, Hand Bone Age, DOI / _2, Springer-Verlag Berlin Heidelberg 2012
 Bone Development 2 Skeletal maturity is a measure of development incorporating the size, shape and degree of mineralization of bone to define its proximity to full maturity. The assessment of skeletal
Bone Development 2 Skeletal maturity is a measure of development incorporating the size, shape and degree of mineralization of bone to define its proximity to full maturity. The assessment of skeletal
Vicente Gilsanz Osman Ratib. Hand Bone Age. A Digital Atlas of Skeletal Maturity. Second Edition
 Hand Bone Age Vicente Gilsanz Osman Ratib Hand Bone Age A Digital Atlas of Skeletal Maturity Second Edition Prof. Vicente Gilsanz Department of Radiology Childrens Hospital Los Angeles 4650 Sunset Blvd.,
Hand Bone Age Vicente Gilsanz Osman Ratib Hand Bone Age A Digital Atlas of Skeletal Maturity Second Edition Prof. Vicente Gilsanz Department of Radiology Childrens Hospital Los Angeles 4650 Sunset Blvd.,
Pectoral (Shoulder) Girdle
 Chapter 8 Skeletal System: Appendicular Skeleton Pectoral girdle Pelvic girdle Upper limbs Lower limbs 8-1 Pectoral (Shoulder) Girdle Consists of scapula and clavicle Clavicle articulates with sternum
Chapter 8 Skeletal System: Appendicular Skeleton Pectoral girdle Pelvic girdle Upper limbs Lower limbs 8-1 Pectoral (Shoulder) Girdle Consists of scapula and clavicle Clavicle articulates with sternum
Introduction. The wrist contains eight small carpal bones, which as a group act as a flexible spacer between the forearm and hand.
 Wrist Introduction The wrist contains eight small carpal bones, which as a group act as a flexible spacer between the forearm and hand. Distal forearm Distal forearm 4 Distal end of the radius A. anterior
Wrist Introduction The wrist contains eight small carpal bones, which as a group act as a flexible spacer between the forearm and hand. Distal forearm Distal forearm 4 Distal end of the radius A. anterior
COURSE TITLE: Skeletal Anatomy and Fractures of the Lower Arm, Wrist, and Hand
 COURSE DESCRIPTION Few parts of the human body are required to pivot, rotate, abduct, and adduct like the wrist and hand. The intricate and complicated movements of the arm, wrist, and hand exist partly
COURSE DESCRIPTION Few parts of the human body are required to pivot, rotate, abduct, and adduct like the wrist and hand. The intricate and complicated movements of the arm, wrist, and hand exist partly
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 8 The Skeletal System: The Appendicular Skeleton The Appendicular Skeleton The 126 bones of the appendicular skeleton are primarily concerned
Principles of Anatomy and Physiology 14 th Edition CHAPTER 8 The Skeletal System: The Appendicular Skeleton The Appendicular Skeleton The 126 bones of the appendicular skeleton are primarily concerned
Sports Medicine Part I : ANATOMY OF THE SPINE, ABDOMEN AND SHOULDER COMPLEX
 Sports Medicine 25 1.1 Part I : ANATOMY OF THE SPINE, ABDOMEN AND SHOULDER COMPLEX c.w.p. Wagner High School, Sports Medicine, A. Morgan, T. Morgan 2008 Anatomy of the Upper Body In this section of the
Sports Medicine 25 1.1 Part I : ANATOMY OF THE SPINE, ABDOMEN AND SHOULDER COMPLEX c.w.p. Wagner High School, Sports Medicine, A. Morgan, T. Morgan 2008 Anatomy of the Upper Body In this section of the
Exercise 11. The Appendicular Skeleton
 Exercise 11 The Appendicular Skeleton The Appendicular Skeleton The appendicular skeleton contains 126 bones. Consists of the upper and lower limbs, the pectoral girdles, and the pelvic girdles. The pectoral
Exercise 11 The Appendicular Skeleton The Appendicular Skeleton The appendicular skeleton contains 126 bones. Consists of the upper and lower limbs, the pectoral girdles, and the pelvic girdles. The pectoral
Ch. 5 - Skeletal System
 Ch. 5 - Skeletal System Bones are living, ever-changing structures. This allows them grow and adapt to new situations that the body encounters. The functions of the skeletal system: 1) support bones are
Ch. 5 - Skeletal System Bones are living, ever-changing structures. This allows them grow and adapt to new situations that the body encounters. The functions of the skeletal system: 1) support bones are
An Introduction to the Appendicular Skeleton
 An Introduction to the Appendicular Skeleton The Appendicular Skeleton is composed of the 126 bones of the appendages (limbs) and the pectoral and pelvic girdles, which attach to the axial skeleton. Each
An Introduction to the Appendicular Skeleton The Appendicular Skeleton is composed of the 126 bones of the appendages (limbs) and the pectoral and pelvic girdles, which attach to the axial skeleton. Each
PRE-LAB EXERCISES. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure:
 1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the appendicular skeleton,
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the appendicular skeleton,
Dr Nabil khouri MD. MSc. Ph.D
 Dr Nabil khouri MD. MSc. Ph.D Foot Anatomy The foot consists of 26 bones: 14 phalangeal, 5 metatarsal, and 7 tarsal. Toes are used to balance the body. Metatarsal Bones gives elasticity to the foot in
Dr Nabil khouri MD. MSc. Ph.D Foot Anatomy The foot consists of 26 bones: 14 phalangeal, 5 metatarsal, and 7 tarsal. Toes are used to balance the body. Metatarsal Bones gives elasticity to the foot in
Bio 103 Skeletal System 45
 45 Lecture Outline: SKELETAL SYSTEM [Chapters 7, 8] Introduction A. Components B. Functions 1. 2. 3. 4. Classification and Parts A. Bone Shapes 1. Long: 2. Short: 3. Flat: 4. Irregular: 5. Sesamoid: B.
45 Lecture Outline: SKELETAL SYSTEM [Chapters 7, 8] Introduction A. Components B. Functions 1. 2. 3. 4. Classification and Parts A. Bone Shapes 1. Long: 2. Short: 3. Flat: 4. Irregular: 5. Sesamoid: B.
ANATOMICAL DISPOSITION OF CARPAL BONES OF GOLDEN RETRIEVER DOG BY X-RAY EXPOSURE
 Explor. Anim. Exploratory Med. Res., Vol.2, Animal Issue and - 1, Medical 2012, p. Research, 76-80 Vol.2, Issue -1, July, 2012 ISSN 2277-470X ANATOMICAL DISPOSITION OF CARPAL BONES OF GOLDEN RETRIEVER
Explor. Anim. Exploratory Med. Res., Vol.2, Animal Issue and - 1, Medical 2012, p. Research, 76-80 Vol.2, Issue -1, July, 2012 ISSN 2277-470X ANATOMICAL DISPOSITION OF CARPAL BONES OF GOLDEN RETRIEVER
Hand Anatomy A Patient's Guide to Hand Anatomy
 Hand Anatomy A Patient's Guide to Hand Anatomy Introduction Few structures of the human anatomy are as unique as the hand. The hand needs to be mobile in order to position the fingers and thumb. Adequate
Hand Anatomy A Patient's Guide to Hand Anatomy Introduction Few structures of the human anatomy are as unique as the hand. The hand needs to be mobile in order to position the fingers and thumb. Adequate
8/25/2014. Radiocarpal Joint. Midcarpal Joint. Osteology of the Wrist
 Structure and Function of the Wrist 2 joints and 10 different bones Combine to create wrist motion Anatomical Terms: Wrist/Hand Palmar = anterior aspect of the wrist and hand Dorsal = posterior aspect
Structure and Function of the Wrist 2 joints and 10 different bones Combine to create wrist motion Anatomical Terms: Wrist/Hand Palmar = anterior aspect of the wrist and hand Dorsal = posterior aspect
CHAPTER 8 LECTURE OUTLINE
 CHAPTER 8 LECTURE OUTLINE I. INTRODUCTION A. The appendicular skeleton includes the bones of the upper and lower extremities and the shoulder and hip girdles. B. The appendicular skeleton functions primarily
CHAPTER 8 LECTURE OUTLINE I. INTRODUCTION A. The appendicular skeleton includes the bones of the upper and lower extremities and the shoulder and hip girdles. B. The appendicular skeleton functions primarily
10/12/2010. Upper Extremity. Pectoral (Shoulder) Girdle. Clavicle (collarbone) Skeletal System: Appendicular Skeleton
 Skeletal System: Appendicular Skeleton Pectoral girdle Pelvic girdle Upper limbs Lower limbs 8-1 Pectoral (Shoulder) Girdle Consists of scapula and clavicle Clavicle articulates with sternum (Sternoclavicular
Skeletal System: Appendicular Skeleton Pectoral girdle Pelvic girdle Upper limbs Lower limbs 8-1 Pectoral (Shoulder) Girdle Consists of scapula and clavicle Clavicle articulates with sternum (Sternoclavicular
Hole s Human Anatomy and Physiology Eleventh Edition. Mrs. Hummer. Chapter 7 Skeletal System
 Hole s Human Anatomy and Physiology Eleventh Edition Mrs. Hummer Chapter 7 Skeletal System 1 Chapter 7 Skeletal System Bone Classification Long Bones Short Bones Flat Bones Irregular Bones Sesamoid (Round)
Hole s Human Anatomy and Physiology Eleventh Edition Mrs. Hummer Chapter 7 Skeletal System 1 Chapter 7 Skeletal System Bone Classification Long Bones Short Bones Flat Bones Irregular Bones Sesamoid (Round)
A. Incorrect! The appendicular skeleton includes bones of the shoulder, arm, hand, pelvis, leg and foot.
 Anatomy and Physiology - Problem Drill 08: The Skeletal System III No. 1 of 10 1. Which of the following statements about the appendicular skeleton is correct? A. The appendicular skeleton includes bones
Anatomy and Physiology - Problem Drill 08: The Skeletal System III No. 1 of 10 1. Which of the following statements about the appendicular skeleton is correct? A. The appendicular skeleton includes bones
GENERAL SCOPE AND USES OF PHYSICAL/BIOLOGICAL ANTHROPOLOGY. Paper No. & Title: B.A./B.Sc. (Honours) 2 dn semester. (Practical)
 GENERAL SCOPE AND USES OF PHYSICAL/BIOLOGICAL ANTHROPOLOGY Course name: Physical Anthropology Paper No. & Title: B.A./B.Sc. (Honours) 2 dn semester (Practical) Topic No. & Title: 5/12 (Part-I) Drawing
GENERAL SCOPE AND USES OF PHYSICAL/BIOLOGICAL ANTHROPOLOGY Course name: Physical Anthropology Paper No. & Title: B.A./B.Sc. (Honours) 2 dn semester (Practical) Topic No. & Title: 5/12 (Part-I) Drawing
Exercise Science Section 2: The Skeletal System
 Exercise Science Section 2: The Skeletal System An Introduction to Health and Physical Education Ted Temertzoglou Paul Challen ISBN 1-55077-132-9 Role of the Skeleton Protection Framework Attachments for
Exercise Science Section 2: The Skeletal System An Introduction to Health and Physical Education Ted Temertzoglou Paul Challen ISBN 1-55077-132-9 Role of the Skeleton Protection Framework Attachments for
Main Menu. Wrist and Hand Joints click here. The Power is in Your Hands
 1 The Wrist and Hand Joints click here Main Menu K.5 http://www.handsonlineeducation.com/classes/k5/k5entry.htm[3/23/18, 1:40:40 PM] Bones 29 bones, including radius and ulna 8 carpal bones in 2 rows of
1 The Wrist and Hand Joints click here Main Menu K.5 http://www.handsonlineeducation.com/classes/k5/k5entry.htm[3/23/18, 1:40:40 PM] Bones 29 bones, including radius and ulna 8 carpal bones in 2 rows of
Chapter 8B. The Skeletal System: Appendicular Skeleton. The Appendicular Skeleton. Clavicle. Pectoral (Shoulder) Girdle
 The Appendicular Skeleton Chapter 8B The Skeletal System: Appendicular Skeleton 126 bones Pectoral (shoulder) girdle Pelvic (hip) girdle Upper limbs Lower limbs Functions primarily to facilitate movement
The Appendicular Skeleton Chapter 8B The Skeletal System: Appendicular Skeleton 126 bones Pectoral (shoulder) girdle Pelvic (hip) girdle Upper limbs Lower limbs Functions primarily to facilitate movement
Radiological Estimation of Age from Hand Bone in Sudanese Infants and Toddlers
 Open Journal of Internal Medicine, 2014, 4, 13-21 Published Online March 2014 in SciRes. http://www.scirp.org/journal/ojim http://dx.doi.org/10.4236/ojim.2014.41003 Radiological Estimation of Age from
Open Journal of Internal Medicine, 2014, 4, 13-21 Published Online March 2014 in SciRes. http://www.scirp.org/journal/ojim http://dx.doi.org/10.4236/ojim.2014.41003 Radiological Estimation of Age from
Forearm and Wrist Regions Neumann Chapter 7
 Forearm and Wrist Regions Neumann Chapter 7 REVIEW AND HIGHLIGHTS OF OSTEOLOGY & ARTHROLOGY Radius dorsal radial tubercle radial styloid process Ulna ulnar styloid process ulnar head Carpals Proximal Row
Forearm and Wrist Regions Neumann Chapter 7 REVIEW AND HIGHLIGHTS OF OSTEOLOGY & ARTHROLOGY Radius dorsal radial tubercle radial styloid process Ulna ulnar styloid process ulnar head Carpals Proximal Row
Physical therapy of the wrist and hand
 Physical therapy of the wrist and hand Functional anatomy wrist and hand The wrist includes distal radius, scaphoid, lunate, triquetrum, pisiform, trapezium, trapezoid, capitate, and hamate. The hand includes
Physical therapy of the wrist and hand Functional anatomy wrist and hand The wrist includes distal radius, scaphoid, lunate, triquetrum, pisiform, trapezium, trapezoid, capitate, and hamate. The hand includes
The Appendicular Skeleton
 8 The Appendicular Skeleton PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris 8-1 The Pectoral Girdle The Pectoral Girdle Also called shoulder girdle Connects the
8 The Appendicular Skeleton PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris 8-1 The Pectoral Girdle The Pectoral Girdle Also called shoulder girdle Connects the
Chapter 7: Skeletal System
 Chapter 7: Skeletal System The Skeletal System Introduction P. 182 Bone is an organ made up of tissues: It is made up of the following components. Cartilage Blood Nerves Bone Connective Bone Classification
Chapter 7: Skeletal System The Skeletal System Introduction P. 182 Bone is an organ made up of tissues: It is made up of the following components. Cartilage Blood Nerves Bone Connective Bone Classification
Chapter 7 Skeletal System. Skeletal System: Bone Functions: Describe the role the skeletal system plays in each of the following functions.
 Chapter 7 Skeletal System Skeletal System: Bone Functions: Describe the role the skeletal system plays in each of the following functions. support protection muscle attachment - movement blood production
Chapter 7 Skeletal System Skeletal System: Bone Functions: Describe the role the skeletal system plays in each of the following functions. support protection muscle attachment - movement blood production
10/15/2014. Wrist. Clarification of Terms. Clarification of Terms cont
 Wrist Clarification of Terms Palmar is synonymous with anterior aspect of the wrist and hand Ventral is also synonymous with anterior aspect of the wrist and hand Dorsal refers to the posterior aspect
Wrist Clarification of Terms Palmar is synonymous with anterior aspect of the wrist and hand Ventral is also synonymous with anterior aspect of the wrist and hand Dorsal refers to the posterior aspect
Biology 218 Human Anatomy
 Chapter 8 Adapted from Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 203) 1. The appendicular skeleton contains 126 bones that form: i. two pectoral (shoulder) girdles two upper limbs i one pelvic
Chapter 8 Adapted from Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 203) 1. The appendicular skeleton contains 126 bones that form: i. two pectoral (shoulder) girdles two upper limbs i one pelvic
THE SKELETAL SYSTEM. Focus on the Pectoral Girdle
 THE SKELETAL SYSTEM Focus on the Pectoral Girdle Appendicular Skeleton 126 bones Includes bones of the limbs (arms and legs) Pectoral girdle (shoulder) Pelvic girdle (hip) Pectoral Girdle (the shoulder)
THE SKELETAL SYSTEM Focus on the Pectoral Girdle Appendicular Skeleton 126 bones Includes bones of the limbs (arms and legs) Pectoral girdle (shoulder) Pelvic girdle (hip) Pectoral Girdle (the shoulder)
Wrist and Hand Anatomy
 Wrist and Hand Anatomy Bone Anatomy Scapoid Lunate Triquetrium Pisiform Trapeziod Trapezium Capitate Hamate Wrist Articulations Radiocarpal Joint Proximal portion Distal portion Most surface contact found
Wrist and Hand Anatomy Bone Anatomy Scapoid Lunate Triquetrium Pisiform Trapeziod Trapezium Capitate Hamate Wrist Articulations Radiocarpal Joint Proximal portion Distal portion Most surface contact found
UNIT 4 - SKELETAL SYSTEM LECTURE NOTES
 UNIT 4 - SKELETAL SYSTEM LECTURE NOTES 4.01 FUNCTIONS OF THE SKELETAL SYSTEM A. Support 1. Provides a framework for the body 2. Supports soft tissue 3. Serves as a point of attachment for ligaments, tendons,
UNIT 4 - SKELETAL SYSTEM LECTURE NOTES 4.01 FUNCTIONS OF THE SKELETAL SYSTEM A. Support 1. Provides a framework for the body 2. Supports soft tissue 3. Serves as a point of attachment for ligaments, tendons,
Biology 218 Human Anatomy. Adapted from Martini Human Anatomy 7th ed. Chapter 7 The Skeletal System Appendicular Division
 Adapted from Martini Human Anatomy 7th ed. Chapter 7 The Skeletal System Appendicular Division Introduction The appendicular skeleton includes: Pectoral girdle Shoulder bones Upper limbs Pelvic girdle
Adapted from Martini Human Anatomy 7th ed. Chapter 7 The Skeletal System Appendicular Division Introduction The appendicular skeleton includes: Pectoral girdle Shoulder bones Upper limbs Pelvic girdle
Lab Exercise #04 The Skeletal System Student Performance Objectives
 Lab Exercise #04 The Skeletal System Student Performance Objectives The material that you are required to learn in this exercise can be found in either the lecture text or the supplemental materials provided
Lab Exercise #04 The Skeletal System Student Performance Objectives The material that you are required to learn in this exercise can be found in either the lecture text or the supplemental materials provided
Chapter 6 & 7 The Skeleton
 Chapter 6 & 7 The Skeleton Try this Make clockwise circles with your RIGHT foot, while doing this, draw the number 6 in the air with you RIGHT hand what happens to your foot???? Bony Background Adult body
Chapter 6 & 7 The Skeleton Try this Make clockwise circles with your RIGHT foot, while doing this, draw the number 6 in the air with you RIGHT hand what happens to your foot???? Bony Background Adult body
Dr.Israa H. Mohsen. Lecture 5. The vertebral column
 Anatomy Lecture 5 Dr.Israa H. Mohsen The vertebral column The vertebral column a flexible structure consisting of 33 vertebrae holds the head and torso upright, serves as an attachment point for the legs,
Anatomy Lecture 5 Dr.Israa H. Mohsen The vertebral column The vertebral column a flexible structure consisting of 33 vertebrae holds the head and torso upright, serves as an attachment point for the legs,
Appendicular Skeleton. Dr. Carmen E. Rexach Anatomy 35 Mt. San Antonio College
 Appendicular Skeleton Dr. Carmen E. Rexach Anatomy 35 Mt. San Antonio College Pectoral girdle clavicle scapula Upper limb brachium antebrachium carpus manus Pelvic girdle oscoxae Lower limb femoral region
Appendicular Skeleton Dr. Carmen E. Rexach Anatomy 35 Mt. San Antonio College Pectoral girdle clavicle scapula Upper limb brachium antebrachium carpus manus Pelvic girdle oscoxae Lower limb femoral region
Ossification = Osteogenesis
 Ossification = Osteogenesis Ossification = Osteogenesis Parts of the fetal skeleton form during the first few weeks after conception By the end of the 8 th week, the skeletal pattern is formed : cartilage
Ossification = Osteogenesis Ossification = Osteogenesis Parts of the fetal skeleton form during the first few weeks after conception By the end of the 8 th week, the skeletal pattern is formed : cartilage
SKELETAL SYSTEM 206. AXIAL SKELETON 80 APPENDICULAR SKELETON 126 (see Figure 6.1) Clavicle. Clavicle. Pectoral girdles. Scapula. Scapula.
 SKELETAL SYSTEM 206 AXIAL SKELETON 80 APPENDICULAR SKELETON 126 (see Figure 6.1) Pectoral girdles 4 Clavicle Scapula 2 2 Clavicle Scapula Humerus 2 Humerus Upper limbs 60 Radius 2 Ulna Carpal bones Metacarpal
SKELETAL SYSTEM 206 AXIAL SKELETON 80 APPENDICULAR SKELETON 126 (see Figure 6.1) Pectoral girdles 4 Clavicle Scapula 2 2 Clavicle Scapula Humerus 2 Humerus Upper limbs 60 Radius 2 Ulna Carpal bones Metacarpal
The Skeletal System. Chapter 7a. Skeletal System Introduction Functions of the skeleton Framework of bones The skeleton through life
 The Skeletal System Skeletal System Introduction Functions of the skeleton Framework of bones The skeleton through life Chapter 7a Support Protection Movement Storage areas Minerals Lipids Hemopoiesis
The Skeletal System Skeletal System Introduction Functions of the skeleton Framework of bones The skeleton through life Chapter 7a Support Protection Movement Storage areas Minerals Lipids Hemopoiesis
Hands PA; Obl. Lat.; Norgaard s Thumb AP; Lat. PA. PA; Lat.: Obls.; Elongated PA with ulnar deviation
 Projections Region Basic projections Additional / Modified projections Upper Limbs Hands PA; Obl. Lat.; Norgaard s Thumb ; Lat. PA Fingers PA; Lat. Wrist PA; Lat. Obls. Scaphoid Lunate Trapezium Triquetral
Projections Region Basic projections Additional / Modified projections Upper Limbs Hands PA; Obl. Lat.; Norgaard s Thumb ; Lat. PA Fingers PA; Lat. Wrist PA; Lat. Obls. Scaphoid Lunate Trapezium Triquetral
Chapter 5 The Skeletal System
 Chapter 5 The Skeletal System The Skeletal System Parts of the skeletal system Bones (skeleton) Joints Cartilages Ligaments (bone to bone)(tendon=bone to muscle) Divided into two divisions Axial skeleton:
Chapter 5 The Skeletal System The Skeletal System Parts of the skeletal system Bones (skeleton) Joints Cartilages Ligaments (bone to bone)(tendon=bone to muscle) Divided into two divisions Axial skeleton:
Wrist and Hand Anatomy/Biomechanics
 Wrist and Hand Anatomy/Biomechanics Kristin Kelley, DPT, OCS, FAAOMPT Orthopaedic Manual Physical Therapy Series Charlottesville 2017-2018 Orthopaedic Manual Physical Therapy Series 2017-2018 Anatomy -
Wrist and Hand Anatomy/Biomechanics Kristin Kelley, DPT, OCS, FAAOMPT Orthopaedic Manual Physical Therapy Series Charlottesville 2017-2018 Orthopaedic Manual Physical Therapy Series 2017-2018 Anatomy -
Anatomy - Hand. Wrist and Hand Anatomy/Biomechanics. Osteology. Carpal Arch. Property of VOMPTI, LLC
 Wrist and Hand Anatomy/Biomechanics Kristin Kelley, DPT, OCS, FAAOMPT The wrist The metacarpals The Phalanges Digit 1 thumb Digit 5 digiti minimi Anatomy - Hand Orthopaedic Manual Physical Therapy Series
Wrist and Hand Anatomy/Biomechanics Kristin Kelley, DPT, OCS, FAAOMPT The wrist The metacarpals The Phalanges Digit 1 thumb Digit 5 digiti minimi Anatomy - Hand Orthopaedic Manual Physical Therapy Series
SKELETAL SYSTEM. Introduction Notes (pt 1)
 SKELETAL SYSTEM Introduction Notes (pt 1) I. INTRODUCTION 1. Bones include active, living tissues: bone tissue, cartilage, dense connective tissue, blood, and nervous tissue. 2. Bones: support and protect
SKELETAL SYSTEM Introduction Notes (pt 1) I. INTRODUCTION 1. Bones include active, living tissues: bone tissue, cartilage, dense connective tissue, blood, and nervous tissue. 2. Bones: support and protect
Bones of the Upper Limb *
 OpenStax-CNX module: m46368 1 Bones of the Upper Limb * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will
OpenStax-CNX module: m46368 1 Bones of the Upper Limb * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will
10/10/2014. Structure and Function of the Hand. The Hand. Osteology of the Hand
 Structure and Function of the Hand 19 bones and 19 joints are necessary to produce all the motions of the hand The Hand Dorsal aspect Palmar aspect The digits are numbered 1-5 Thumb = #1 Little finger
Structure and Function of the Hand 19 bones and 19 joints are necessary to produce all the motions of the hand The Hand Dorsal aspect Palmar aspect The digits are numbered 1-5 Thumb = #1 Little finger
Kinesiology of The Wrist and Hand. Cuneyt Mirzanli Istanbul Gelisim University
 Kinesiology of The Wrist and Hand Cuneyt Mirzanli Istanbul Gelisim University Bones The wrist and hand contain 29 bones including the radius and ulna. There are eight carpal bones in two rows of four to
Kinesiology of The Wrist and Hand Cuneyt Mirzanli Istanbul Gelisim University Bones The wrist and hand contain 29 bones including the radius and ulna. There are eight carpal bones in two rows of four to
The Skeletal System THE APPENDICULAR SKELETON
 The Skeletal System THE APPENDICULAR SKELETON The appendicular skeleton consists of the girdles and the skeleton of the limbs. The upper (anterior) limbs are attached to the pectoral (shoulder) girdle
The Skeletal System THE APPENDICULAR SKELETON The appendicular skeleton consists of the girdles and the skeleton of the limbs. The upper (anterior) limbs are attached to the pectoral (shoulder) girdle
Magnetic Resonance Atlas of Skeletal Development of the Knee
 Magnetic Resonance Atlas of Skeletal Development of the Knee A Standard of Reference Andrew T. Pennock, MD James D. Bomar, MPH Rady Children s Hospital, San Diego University of California, San Diego 2
Magnetic Resonance Atlas of Skeletal Development of the Knee A Standard of Reference Andrew T. Pennock, MD James D. Bomar, MPH Rady Children s Hospital, San Diego University of California, San Diego 2
Microanatomy, Physiology of Bone & Joints
 Microanatomy, Physiology of Bone & Joints The Skeleton There are 206 bones in the human body. The bones that are required in this syllabus are the cranium, mandible, clavicle, sternum, scapula, ribs, humerous,
Microanatomy, Physiology of Bone & Joints The Skeleton There are 206 bones in the human body. The bones that are required in this syllabus are the cranium, mandible, clavicle, sternum, scapula, ribs, humerous,
V. Gilsanz/O. Ratib Hand Bone Age
 V. Gilsanz/O. Ratib Hand Bone Age Vicente Gilsanz Osman Ratib Hand Bone Age A Digital Atlas of Skeletal Maturity With 88 Figures Vicente Gilsanz, M.D., Ph.D. Department of Radiology Childrens Hospital
V. Gilsanz/O. Ratib Hand Bone Age Vicente Gilsanz Osman Ratib Hand Bone Age A Digital Atlas of Skeletal Maturity With 88 Figures Vicente Gilsanz, M.D., Ph.D. Department of Radiology Childrens Hospital
Lab Activity 11: Group II
 Lab Activity 11: Group II Muscles Martini Chapter 11 Portland Community College BI 231 Origin and Insertion Origin: The place where the fixed end attaches to a bone, cartilage, or connective tissue. Insertion:
Lab Activity 11: Group II Muscles Martini Chapter 11 Portland Community College BI 231 Origin and Insertion Origin: The place where the fixed end attaches to a bone, cartilage, or connective tissue. Insertion:
BLUE SKY SCHOOL OF PROFESSIONAL MASSAGE AND THERAPEUTIC BODYWORK. Musculoskeletal Anatomy & Kinesiology I TERMINOLOGY, STRUCTURES, & SKELETAL OVERVIEW
 BLUE SKY SCHOOL OF PROFESSIONAL MASSAGE AND THERAPEUTIC BODYWORK Musculoskeletal Anatomy & Kinesiology I TERMINOLOGY, STRUCTURES, & SKELETAL OVERVIEW MSAK101-I Session 1 Learning Objectives: 1. Define
BLUE SKY SCHOOL OF PROFESSIONAL MASSAGE AND THERAPEUTIC BODYWORK Musculoskeletal Anatomy & Kinesiology I TERMINOLOGY, STRUCTURES, & SKELETAL OVERVIEW MSAK101-I Session 1 Learning Objectives: 1. Define
Magnitude of Sex Differences in Dichotomous Ossification Sequences of the Hand and Wrist
 Magnitude of Sex Differences in Dichotomous Ossification Sequences of the Hand and Wrist STANLEY M. GARN, ANDREW K. POZNANSKI AND KAY E. LARSON Cpnter for Human Growth and Development, and Department of
Magnitude of Sex Differences in Dichotomous Ossification Sequences of the Hand and Wrist STANLEY M. GARN, ANDREW K. POZNANSKI AND KAY E. LARSON Cpnter for Human Growth and Development, and Department of
ARM Brachium Musculature
 ARM Brachium Musculature Coracobrachialis coracoid process of the scapula medial shaft of the humerus at about its middle 1. flexes the humerus 2. assists to adduct the humerus Blood: muscular branches
ARM Brachium Musculature Coracobrachialis coracoid process of the scapula medial shaft of the humerus at about its middle 1. flexes the humerus 2. assists to adduct the humerus Blood: muscular branches
Amy Warenda Czura, Ph.D. 1 SCCC BIO130 Lab 7 Appendicular Skeleton & Articulations
 The Skeletal System II: Appendicular Skeleton and Articulations Exercises 11, 13 (begins: page 145 in 9 th and 10 th editions) Exercises 10, 11 (begins: page 147 in 11 th edition, page 149 in 12 th edition)
The Skeletal System II: Appendicular Skeleton and Articulations Exercises 11, 13 (begins: page 145 in 9 th and 10 th editions) Exercises 10, 11 (begins: page 147 in 11 th edition, page 149 in 12 th edition)
RADIOGRAPHY OF THE WRIST
 RADIOGRAPHY OF THE WRIST Patient Position: WRIST PA Projection, elbow in same plane Part Position: Hand ; fingers centered to IR Central Ray: Structures Shown: NOTE: Optional AP projection best demonstrates
RADIOGRAPHY OF THE WRIST Patient Position: WRIST PA Projection, elbow in same plane Part Position: Hand ; fingers centered to IR Central Ray: Structures Shown: NOTE: Optional AP projection best demonstrates
Osteology. Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College
 Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
SUPERIEUR ARM AND HAND
 Pectoral girdle, SUPERIEUR ARM AND HAND Danil Hammoudi.MD The pectoral girdle is the set of bones which connect the upper limb to the axial skeleton on each side. It consists of the clavicle scapula in
Pectoral girdle, SUPERIEUR ARM AND HAND Danil Hammoudi.MD The pectoral girdle is the set of bones which connect the upper limb to the axial skeleton on each side. It consists of the clavicle scapula in
International Society for Magnetic Resonance in Medicine (ISMRM), The 24th Annual Meeting and Exhibition, Singapore, 7-13 May 2016.
 Development of an Automated Shape and Textural Software Model of the Paediatric Knee for Estimation of Skeletal Age. Caron Parsons 1,2, Charles Hutchinson 1,2, Emma Helm 2, Alexander Clarke 3, Asfand Baig
Development of an Automated Shape and Textural Software Model of the Paediatric Knee for Estimation of Skeletal Age. Caron Parsons 1,2, Charles Hutchinson 1,2, Emma Helm 2, Alexander Clarke 3, Asfand Baig
NOTES: Skeletal System (Ch 5, part 1)
 NOTES: Skeletal System (Ch 5, part 1) Individual bones are the organs of the skeletal system. A bone contains very active tissues. BONE STRUCTURE: *Bone structure reflects its function. Parts of a long
NOTES: Skeletal System (Ch 5, part 1) Individual bones are the organs of the skeletal system. A bone contains very active tissues. BONE STRUCTURE: *Bone structure reflects its function. Parts of a long
Chapter 8. The Appendicular Skeleton. Lecture Presentation by Lee Ann Frederick University of Texas at Arlington Pearson Education, Inc.
 Chapter 8 The Appendicular Skeleton Lecture Presentation by Lee Ann Frederick University of Texas at Arlington An Introduction to the Appendicular Skeleton The Appendicular Skeleton 126 bones Allows us
Chapter 8 The Appendicular Skeleton Lecture Presentation by Lee Ann Frederick University of Texas at Arlington An Introduction to the Appendicular Skeleton The Appendicular Skeleton 126 bones Allows us
Anatomy. Anatomy deals with the structure of the human body, and includes a precise language on body positions and relationships between body parts.
 Anatomy deals with the structure of the human body, and includes a precise language on body positions and relationships between body parts. Proper instruction on safe and efficient exercise technique requires
Anatomy deals with the structure of the human body, and includes a precise language on body positions and relationships between body parts. Proper instruction on safe and efficient exercise technique requires
Pectoral girdle, SUPERIEUR ARM AND HAND. Danil Hammoudi.MD
 Pectoral girdle, SUPERIEUR ARM AND HAND Danil Hammoudi.MD The pectoral girdle is the set of bones which connect the upper limb to the axial skeleton on each side. It consists of the clavicle scapula in
Pectoral girdle, SUPERIEUR ARM AND HAND Danil Hammoudi.MD The pectoral girdle is the set of bones which connect the upper limb to the axial skeleton on each side. It consists of the clavicle scapula in
RADIOGRAPHY OF THE HAND, FINGERS & THUMB
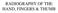 RADIOGRAPHY OF THE HAND, FINGERS & THUMB FINGERS (2nd 5th) - PA Projection Patient Position: Seated; hand ; elbow on IR table top Part Position: Fingers centered to IR unless protocol is Central Ray: Perpendicular
RADIOGRAPHY OF THE HAND, FINGERS & THUMB FINGERS (2nd 5th) - PA Projection Patient Position: Seated; hand ; elbow on IR table top Part Position: Fingers centered to IR unless protocol is Central Ray: Perpendicular
Dr. Mahir Alhadidi Anatomy Lecture #9 Feb,28 th 2012
 Quick Revision: Upper arm is divided into two compartments: 1. Anterior Compartment: Contains three muscles (Biceps brachii, Coracobrachialis, Brachialis). Innervated by Musculocutaneous nerve. 2. Posterior
Quick Revision: Upper arm is divided into two compartments: 1. Anterior Compartment: Contains three muscles (Biceps brachii, Coracobrachialis, Brachialis). Innervated by Musculocutaneous nerve. 2. Posterior
Chapter 6: Skeletal System: Bones and Bone Tissue
 Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
[[Sally Leaning Towards Peter To Take Cold Hand]]
![[[Sally Leaning Towards Peter To Take Cold Hand]] [[Sally Leaning Towards Peter To Take Cold Hand]]](/thumbs/84/91174469.jpg) In this lecture we will talk about the bones of the hand, and the muscles and contents of the forearm. *The hand bones are: - Carpal bones. -Metacarpals. -Phalanges. *The carpal bones (wrist bones): They
In this lecture we will talk about the bones of the hand, and the muscles and contents of the forearm. *The hand bones are: - Carpal bones. -Metacarpals. -Phalanges. *The carpal bones (wrist bones): They
Cross border migration has increased on a. Value of the appearance of left hand wrist ossification centres to age estimation in Roma population
 Rom J Leg Med [21] 299-304 [2013] DOI: 10.4323/rjlm.2013.299 2013 Romanian Society of Legal Medicine Value of the appearance of left hand wrist ossification centres to age estimation in Roma population
Rom J Leg Med [21] 299-304 [2013] DOI: 10.4323/rjlm.2013.299 2013 Romanian Society of Legal Medicine Value of the appearance of left hand wrist ossification centres to age estimation in Roma population
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
The Skeletal System. Mosby items and derived items 2010, 2006, 2002, 1997, 1992 by Mosby, Inc., an affiliate of Elsevier Inc.
 The Skeletal System Functions of Skeletal System Provides internal framework that supports the body Protects internal organs Helps fight disease by producing white blood cells 2 Functions of Skeletal System
The Skeletal System Functions of Skeletal System Provides internal framework that supports the body Protects internal organs Helps fight disease by producing white blood cells 2 Functions of Skeletal System
Biology 152 Appendicular Skeleton Anatomy Objectives
 Biology 152 Appendicular Skeleton Anatomy Objectives We will learn proper bone names, left/right/medial, and the parts of bones in this exercise. Start by learning the names of the bones. As you gain comfort
Biology 152 Appendicular Skeleton Anatomy Objectives We will learn proper bone names, left/right/medial, and the parts of bones in this exercise. Start by learning the names of the bones. As you gain comfort
Bone Flashcards for 10a
 Bone Flashcards for 0a CLAVICLE (collar bone). Sternal extremity (end) flat end. Acromial extremity (end) rounded end. SCAPULA (shoulder blade). Right or left scapula?. Superior border (superior margin).
Bone Flashcards for 0a CLAVICLE (collar bone). Sternal extremity (end) flat end. Acromial extremity (end) rounded end. SCAPULA (shoulder blade). Right or left scapula?. Superior border (superior margin).
CHAPTER 6: THE UPPER EXTREMITY: THE ELBOW, FOREARM, WRIST, AND HAND
 CHAPTER 6: THE UPPER EXTREMITY: THE ELBOW, FOREARM, WRIST, AND HAND KINESIOLOGY Scientific Basis of Human Motion, 12 th edition Hamilton, Weimar & Luttgens Presentation Created by TK Koesterer, Ph.D.,
CHAPTER 6: THE UPPER EXTREMITY: THE ELBOW, FOREARM, WRIST, AND HAND KINESIOLOGY Scientific Basis of Human Motion, 12 th edition Hamilton, Weimar & Luttgens Presentation Created by TK Koesterer, Ph.D.,
Muscular Nomenclature and Kinesiology - One
 Chapter 16 Muscular Nomenclature and Kinesiology - One Lessons 1-3 (with lesson 4) 1 Introduction 122 major muscles covered in this chapter Chapter divided into nine lessons Kinesiology study of human
Chapter 16 Muscular Nomenclature and Kinesiology - One Lessons 1-3 (with lesson 4) 1 Introduction 122 major muscles covered in this chapter Chapter divided into nine lessons Kinesiology study of human
Classification of bones
 Classification of bones compact intramembranous axial histology development regional spongy Intra cartilaginous appendicular flat Irregular shape Sesamoid Long Short Wormian pneumatic Classification
Classification of bones compact intramembranous axial histology development regional spongy Intra cartilaginous appendicular flat Irregular shape Sesamoid Long Short Wormian pneumatic Classification
Unit 5 Skeletal System
 Unit 5 Skeletal System Nov 21 10:24 PM I. Functions A. Support: > internal framework, structure, anchors & supports soft tissue organs B. Protection: > protects vital organs C. Movement: > provides attach
Unit 5 Skeletal System Nov 21 10:24 PM I. Functions A. Support: > internal framework, structure, anchors & supports soft tissue organs B. Protection: > protects vital organs C. Movement: > provides attach
Ligaments of Elbow hinge: sagittal plane so need lateral and medial ligaments
 Ligaments of Elbow hinge: sagittal plane so need lateral and medial ligaments Ulnar Collateral ligament on medial side; arising from medial epicondyle and stops excess valgus movement (lateral movement)
Ligaments of Elbow hinge: sagittal plane so need lateral and medial ligaments Ulnar Collateral ligament on medial side; arising from medial epicondyle and stops excess valgus movement (lateral movement)
Bones are made of OSSEOUS TISSUE
 SKELETAL SYSTEM Functions of the Skeletal System Bones are made of OSSEOUS TISSUE Support and Protection Body movement Blood cell formation (bone marrow) Storage of inorganic materials (salt, calcium,
SKELETAL SYSTEM Functions of the Skeletal System Bones are made of OSSEOUS TISSUE Support and Protection Body movement Blood cell formation (bone marrow) Storage of inorganic materials (salt, calcium,
Wrist & Hand Assessment and General View
 Wrist & Hand Assessment and General View Done by; Mshari S. Alghadier BSc Physical Therapy RHPT 366 m.alghadier@sau.edu.sa http://faculty.sau.edu.sa/m.alghadier/ Functional anatomy The hand can be divided
Wrist & Hand Assessment and General View Done by; Mshari S. Alghadier BSc Physical Therapy RHPT 366 m.alghadier@sau.edu.sa http://faculty.sau.edu.sa/m.alghadier/ Functional anatomy The hand can be divided
Chapter 8 The Skeletal System: The Appendicular Skeleton. Copyright 2009 John Wiley & Sons, Inc.
 Chapter 8 The Skeletal System: The Appendicular Skeleton Appendicular Skeleton The primary function is movement It includes bones of the upper and lower limbs Girdles attach the limbs to the axial skeleton
Chapter 8 The Skeletal System: The Appendicular Skeleton Appendicular Skeleton The primary function is movement It includes bones of the upper and lower limbs Girdles attach the limbs to the axial skeleton
Important Parts of Bones
 Important Parts of Bones For 2015 Know: Humerus (posterior) Clavical Femur (Anterior) Foot Hand Mandible Os Coxa Scapula Skull (Anterior, Inferior, Lateral) Sternum Humerus (posterior) A. olecranon fossa
Important Parts of Bones For 2015 Know: Humerus (posterior) Clavical Femur (Anterior) Foot Hand Mandible Os Coxa Scapula Skull (Anterior, Inferior, Lateral) Sternum Humerus (posterior) A. olecranon fossa
Skeletal Maturity Assessment: Calcaneal Apophyseal Ossification
 Yale University EliScholar A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine January 2015 Skeletal Maturity Assessment: Calcaneal Apophyseal Ossification
Yale University EliScholar A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine January 2015 Skeletal Maturity Assessment: Calcaneal Apophyseal Ossification
The Great Orion Nebula in the Constellation of Orion, (NASA, Hubble Space Telescope)
 TRAPEZIUM FRACTURE The Great Orion Nebula in the Constellation of Orion, (NASA, Hubble Space Telescope) One thousand, five hundred light years away, in the constellation of Orion lies the Great Nebula
TRAPEZIUM FRACTURE The Great Orion Nebula in the Constellation of Orion, (NASA, Hubble Space Telescope) One thousand, five hundred light years away, in the constellation of Orion lies the Great Nebula
Characteristics. Bones. Functions of the Skeleton
 Characteristics Bones The Introduction 206 bones hard, rigid bones cells (osteocyctes) are a mixture of a ground substance, collagen fibres, P, Ca highly resistant to compression and tension also somewhat
Characteristics Bones The Introduction 206 bones hard, rigid bones cells (osteocyctes) are a mixture of a ground substance, collagen fibres, P, Ca highly resistant to compression and tension also somewhat
Bone. Development. Tim Arnett. University College London. Department of Anatomy and Developmental Biology
 Bone Development Tim Arnett Department of Anatomy and Developmental Biology University College London Bone development Outline Bone composition matrix + mineral Bone formation - intramembranous & endochondral
Bone Development Tim Arnett Department of Anatomy and Developmental Biology University College London Bone development Outline Bone composition matrix + mineral Bone formation - intramembranous & endochondral
The Skeletal System PART A
 5 The Skeletal System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Skeletal System
5 The Skeletal System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Skeletal System
