Water movement across nephron segments involved with the countercurrent multiplication system
|
|
|
- Arleen Gwendolyn Adams
- 5 years ago
- Views:
Transcription
1 Kidnel' International, Vol. 10 (/976). p Water movement across nephron segments involved with the countercurrent multiplication system JUHA P. KOKKO and C. CRAIG TISHER Department of Internal Medicine, The University of Texas Southwestern Medical School, Dallas, Texas and Departments of Medicine and Pathology, Duke University Medical Center Durham, North Carolina The ability of the kidney to serve as the primary excretory organ for solute and water is its main function, During hydropenia the kidney is able to conserve the bulk of the water that is presented to it, while in clinical states characterized by water excess, the kidney is capable of excreting large amounts of dilute urine. This regulatory capacity for water excretion is the consequence of the countercurrent multiplication system. Most epithelia are indiscriminately permeant to water; however, the countercurrent multiplication system, composed of several nephron segments and capillary endothelia, is unique in that each of its major components possesses a different and well-defined set of characteristics for water permeation. During the past decade considerable progress has been made toward advancing our understanding of those specific properties of the individual segments of the nephron which dictate their water permeability characteristics. The primary purpose of the present paper is to discuss the various mechanisms for water permeation which are operative in those nephron segments which participate in the overall operation of the countercurrent multiplication system. Molecular pathways of water permeation In the absence of coupling of water movement to either active or passive transport of solute, the two primary mechanisms for transepithelial movement of water that have been classically accepted are filtration and diffusion. The magnitude of water movement via these two mechanisms can be described by the filtration coefficient (Pr) and the diffusional coefficient (P) of water. The P1. in turn, is composed of an osmotic and a hydrostatic component: by the International Society of Nephrology. i filtration = L(osmotic) sir; + L0(hydrostatic) P (I) where i, filtration is the volume flux; L (osmotic) is the osmotic water permeability coefficient; ir is the effective osmotic pressure gradient and = where a1 is the reflection coefficient of various substances i, and C1 is the concentration gradient of those substances (a completely impermeant substance has a 01 = I, and therefore, ir1 = RTC1, whereas a partially permeant substance has a 01 < 1 and therefore 7r1 = a,rtc1); L (hydrostatic) is the hydrostatic water permeability coefficient; and zp is the hydrostatic pressure gradient. Though many workers have assumed that L (osmotic) is equal to L (hydrostatic) with a conversion equivalent of I mosm/liter equal to 19 mm Hg, there is no a priori reason that these two terms are necessarily equivalent across complex biological membranes. For example, consider a heteroporous membrane containing small and large pores. The osmotic forces would exert their complete effect across the small pores with o = I, whereas there would be no osmotic force exerted across the larger pores (Fig. 1). On the other hand, the hydrostatic force would be exerted across both large and small pores; however, it would be quantitatively more important across the large pores since the magnitude of the hydrostatic filtration is proportional to the fourth power of the radius (r) of the pore according to the Poiseuille equation: z Pr4t where I is the length of the pore, j is the viscosity of the fluid and t is the time of the experiment. Thus, where a substance cannot exert its full osmotic gradient because of pore size, i.e. where 01 = <I, a given hydrostatic pressure will drive greater amounts 64
2 Water movement in the countercurrent system 65 Fig. 1. Schematics of a heteroporous membrane to illustrate that there may exist pathways of water permeation which are only responsive to hydrostatic forces (large pores with reflection coefficient for all solutes = 0) and pores which are responsive to both hydrostatic and osmotic forces (small pores with reflection coefficient of solutes = I). of water across a membrane than a substance with an equivalent osmotic gradient as determined by freezing point depression. It is also important to recognize that L (osmotic) is not a single term, but may, in turn, be subdivided into L (noncolloid osmotic) and L (colloid osmotic). The difference between these two terms can be classically illustrated in the proximal convoluted tubule where the noncolloidal L across the rabbit proximal convoluted tubule [1, 2] has been found to be equal to approximately 0.03 to 0.05 ni mm' min' mosm'; yet a 2 mosm/liter gradient of protein (roughly 7 g/l00 ml) influences net transport an order of magnitude greater than would be predicted from the L (noncolloidal osmotic) [3 5]. The difference between L (noncolloidal osmotic) and L (colloidal osmotic) is due, most likely, to the fact that colloidal osmotic forces probably affect the anatomical relationships of the epithelium differently than do equivalent noncolloid osmotic forces. A hyperoncotic peritubular environment widens the lateral and basilar intercellular spaces of an isolated perfused proximal convoluted tubule, and autoradiographic studies have demonstrated the presence of protein in the dilated intercellular spaces [61. It was thus argued that the oncotic effect of protein in the intercellular space was, in part, responsible for widening the spaces and, therefore, changing the morphology of the proximal tubule epithelium. No such morphometric studies have been conducted with noncolloidal osmotic forces, but since the osmotic effects are nearly symmetrical [I] while the protein effects are not [5 9], it is reasonable to argue that colloidal and noncolloidal gradients have different morphologic effects on the proximal tubule epithelium. Thus, when comparing induced water fluxes in response to colloid and noncolloid osmotic force, the design of the experiment necessarily constrains one to examine the characteristics of water transport in morphologically different membranes even though the membranes during control conditions are, in fact, the same. It, therefore, is not surprising that the L (noncolloid osmotic) is not equivalent to L(colloid osmotic). At present it is not clear how the magnitude of these two subphenomenological coefficients are related to one another, but it would not be surprising if changes in the epithelium induced by changes in colloid osmotic forces would, in fact, affect the L (noncolloid osmotic). The second primary mode of transepithelial water movement is diffusion. The magnitude of the diffusional water permeation as determined by isotopic means is directly proportional to the area (r2) of the membrane in question. Therefore, a doubling of the membrane area would double the magnitude of diffusional water permeation. A number of studies have attempted to equate P and P1 but, again, there is no reason that the two should necessarily be equal. A number of examples could be cited. First, the P1 may be greater than P. This could happen, for example, if large unstirred layers were to exist at either the luminal or antiluminal side of the epithelium. Unstirred layers do not influence P1, but would act as a diffusion barrier (in series to the epithelium) for P. In
3 66 Kokko and Tisher most epithelia there has been a large discrepancy between P and P with P1 being much greater than P. Part of the explanation for a disproportionately greater P1 may be the presence of a disproportionately smaller P,,, since in some systems these two values have approached each other where vigorous stirring has been undertaken to decrease the thickness of the unstirred layers. The presence of an unstirred layer may not completely explain all of the high P1/P ratios that have been observed and, therefore, other mechanisms must be postulated. Second, there are several mechanisms whereby P may be greater than P1. In the absence of significant hydrostatic pressure gradients, a condition which is approximated across most segments of the nephron, it is quite possible that diffusion of water takes place across large pores where no osmotic flow of water can take place. Also, in the complete absence of pores, a condition where P1 would be zero, diffusion of water could still take place by the kink hypothesis [10], a process in which small pockets of water move en mass across the membrane by virtue of small deformations in membrane structure proteins which are transported across the membrane, but at no time form complete transepithelial channels. This postulated mechanism has been shown to be a kinetically rapid process across epithelia [10]. Methods to measure water movement Phenomenological coefficients. There are a number of different techniques whereby net as well as trace movement of water can be measured across various epithelia. However, in the context of the present discussion, the major technique by which P and P values can be estimated is by the method of microperfusion of isolated segments of renal tubule. Since the segments of the nephron that are involved in the countercurrent multiplication system are not all accessible to in vivo micropuncture techniques, the in vitro microperfusion technique must be utilized to measure P1 and P. The schematics of this method are depicted in Fig. 2. The other major advantage of the in vitro microperfusion technique is that the composition of the luminal and peritubular (bath) fluid can be precisely controlled. The measurement of P, and P requires different experimental conditions, but each of these conditions can be easily approximated using this technique. I. Diffusional water permeability coefficient (Pm). P., is a measure of the velocity at which a water molecule diffuses passively across a given epithelium. It is measured most accurately under the conditions of zero net water flow. In isolated tubules, P.,, can be estimated from the disappearance rate of 3H20 added Fig. 2. Schematics of isolated nephron perfusion technique. Reprinted [31] with permission from the Journal of Clinical Investigation. to the perfusate. The perfusion rate must be rapid enough to insure that complete exchange of 3H20 does not occur, yet slow enough that an adequate number of counts disappear from the perfusate so that an accurate estimate of the permeability coefficient can be made. In addition to the perfusion rate, the contact time of the perfusate with the tubule is important and may be varied by using differing lengths of tubule. A long segment of tubule would increase the time that the isotope has to permeate the tubular epithelium, whereas a short segment of tubule would decrease the time that the isotope has to permeate the tubular epithelium. It is ideal to use the longest segment possible so that errors encountered in the estimation of the length of the tubule are minimized. When all these procedures are followed, the P..., may be calculated according to the following equation [11]: V [3H2OJ1 P,. - In [3H2O]0, To perfusion pump where V1 is the perfusion rate, A is the area of tubule, 3H2OI is the counts per nanoliter of tritiated water in the perfusate and 3H2OOU is the counts per nanoliter of tritiated water in the collected fluid. The units of P., will be cm sec'. The P.., may also be measured from the influx rate of the labeled water when 3H20 is added to the bath. The same general principles are applicable as when measuring P..., from the disappearance rate of the label from the perfusate; however, now P,,, is calculated according to the following equation [3]: V,,[ H20],, A([3H20]1, [3H20].,) where V0 is the collected rate of fluid; [3H20]0 is the concentration of 3H20 in collected fluid; [3H2O]b is the concentration of 3H20 in the bath; ([3H20J1. [3H20]0) is the logarithmic mean concentration gradient of the bath and collected fluid 3H20 concentration; and A is the area of the tubule. 2. Filtration coefficient (P,). The P1 of water across
4 Water movement in the countercurrent system 67 a nephron segment involved in the countercurrent multiplication system is a much more important coefficient, physiologically, than is the diffusional permeability coefficient for water. The reason for this is that osmotic gradients exist across the various nephron segments which provide large driving forces for the net movement of water. The component of P representing the osmotic permeability coefficient may be calculated according to the following equation: z L (noncolloid osmotic) = (2) where z J, is net water movement induced by zir, the logarithmic mean of the osmotic pressure difference between the bath and collected fluid. Operationally, a control i., is first obtained using perfusate which is an isosmolal ultrafiltrate of the same fluid as used for the bath. An osmotic gradient is then induced by addition of a relatively impermeant substance to the bath. Net water movement and osmolality of the collected fluid are then measured. Care must be exercised to prevent osmotic equilibration in these experiments. This may be accomplished by using short segments of nephrons and rapid perfusion rates. Technically it is difficult to measure L (hydrostatic) since with increasing luminal pressures, the tubules tend to come loose from the perfusion pipet. The hydrostatic pressure gradients which exist across the segments of the nephron in the medulla are small but, nevertheless, they cannot be discounted until techniques are developed by which to measure net water flow in response to measured increases in hydrostatic pressure gradients. Morphological techniques. Several morphological techniques have been successfully applied to the study of water transport across biological membranes. Through the use of light and transmission electron microscopy (TEM) and, more recently, scanning electron microscopy (SEM) coupled with ultrastructural tracer techniques and improved methods of tissue preservation, a number of well-defined alterations in cell configuration resulting primarily from the transepithelial movement of water and solutes have been observed. The toad urinary bladder represents one of the first epithelia in which such changes were described in the presence of vasopressin-induced osmotic water flow [12 15]. The major changes that were noted included cell swelling, reflecting an increase in cell volume due to entry of water into the cell, and dilatation of the lateral and basilar intercellular spaces (Fig. 3, a and b). Previous studies have demonstrated that these alterations in cell configuration correlate well with the increase in water movement that has been measured just prior to tissue fixation in these preparations [12 14]. The morphological data also provide at least indirect evidence that the lateral and basilar intercellular spaces represent a partial pathway for ion and water movement. In later studies performed in the toad urinary bladder [16], ultrastructural tracer techniques employing horseradish peroxidase (HRP) provided more direct evidence that the dilated intercellular spaces were the result, at least in part, of increased water flow. HRP, which filled the intercellular spaces in the absence of an osmotic gradient, was quickly washed free of the dilated spaces in the presence of vasopressin and a favorable osmotic gradient. While such techniques provide largely qualitative information, they can and have been used to investigate certain physiological questions concerning pathways of water and solute movement across various transporting epithelia. For instance, Woodhall and Tisher [17], utilizing the morphological changes known to be associated with transepithelial water flow, were able to demonstrate that initial collecting tubules and cortical collecting ducts, but not distal convoluted tubules, were responsive to vasopressin in the rat, contrary to the interpretation of data obtained in several earlier micropuncture studies [18 21,]. The results have been confirmed recently in isolated segments of distal convoluted tubules and cortical collecting ducts in the rabbit [22]. Ultrastructural tracer techniques employing lanthanum have also been utilized to examine pathways of solute and water movement across many transporting epithelia. A generally excellent correlation has been found between electrophysiological evidence and ultrastructural tracer data in support of the presence of paracellular shunt pathways for solute and water movement. For instance, in the mammalian proximal convoluted tubule, where physiological as well as electrophysiological data provide evidence of a functional paracellular shunt pathway, both colloidal lanthanum and ionic lanthanum have been found to easily penetrate the region of the tight junction or zonula occludens (Fig. 4) [23, 24]. On the other hand, in epithelia with high values for transepithelial electrical resistance indicative of the probable absence of a functional paracellular shunt pathway, the tight junction resists lanthanum penetration (Fig. 5). Interestingly, introduction of a gradient across a high resistance membrane such as toad urinary bladder through the addition of urea to the mucosal bathing solution causes the tight junction to become nonoccluding in character [25]. In this situation, the transepithelial electrical resistance falls and electron-dense tracers are capable of penetrating the previously occluding type of tight junction. Thus, via
5 68 Kokko and Tither A rc'. '_ ' : :- :; ::..' -t.'. B Fig. 3. a, Electron micrograph of toad urinary bladder bathed in hypotonic Ringer's solution (112 to 1/8 m Osm/kg H20) on the mucosal surface and isotonic Ringer's solution (225 to 235 mosm/kg H20) on the serosal surface in the absence of vasopressin. Typical ultrastructure of granulated epithelial cells is depicted. M, mucosal surface; S. serosal surface; arrow, intercellular space. Magnification, X3000. Reprinted [14] with permission from the American Journal of Pathology. b, Electron micrograph of toad bladder bathed in the same solutions as those bathing tissue depicted in Fig. 3a, but fixed after addition of vasopressin (100 mu/mi). Cell swelling and dilatation of intercellular spaces (IS) is now quite evident. Magnification, x3000. Reprinted [14] with permission from the American Journal of Pathology.
6 is Water,nove,nent in the countercurrent st'stem r CT' - La- 4j c.. -J ' -t I , - - I e_3' _. --.-ç':1 - k-; - _r r' C k Fig. 4. Electron micrograph of an oblique section through the apical region of epithelium in a proximal convoluted tubule from a normal rat, Individual microvilli (Mv) in oblique and cross-section on the left are surrounded by lanthanum. The electron-dense tracer has penetrated the entire zonula oceludens (ZO) from the luminal surface to the region of the zonula adherens (ZA). LIS, lateral intercellular space. Magnification, X90,000. Reprinted from [221. the use of light microscopy, TEM and SEM coupled with ultrastructural tracer techniques and improved methods of tissue preservation, it has been possible to provide qualitative information regarding both extracellular and transcellular solute and fluid movement in a variety of transporting epithelia. Characteristics of water transport across various nephron segments Descending limb of Henle (DLH). 1. Water permeability characteristics. There are no published in viva micropuncture studies containing data which provide a quantitative evaluation of the water permeability coefficient of the DLH. However, many previous in viva micropuncture studies have found a much higher tubular fluid to plasma (TF/P) inulin concentration at the bend of Henle's loop than can be reasonably expected to exist at the end of the proximal tubule [19, 26 29]. These indirect results, therefore, have been interpreted to suggest that the DLH is quite permeant to the osmotic flow of water. Two studies exist in which the characteristics of water transport across the DLH have been measured directly. First, Morgan and Berliner [30], using freeflow micropuncture techniques in an in vitro isolated papillary tissue slice preparation obtained from rat, estimated an isotopic water permeability of the DLH of 119 X 10 cm sec'. Administration of antidiuretic hormone (ADH) did not change this value. This P is a high value when compared to other segments examined in a similar manner. Morgan and Berliner [30] also measured the L (noncolloid osmotic) by perfusing the thin DLH with a 500 mosm/kg H20 solution while maintaining a 200 mosm/kg H20 gradient favoring the bath by the addition of mannitol, sodium chloride or urea. From the induced net water movement per unit of difference in osmolality, it is possible to calculate an L0 equal to 3.75 X l0 nl cm-2 sec-1 atm-'. Second, Kokko [31], using the in vitro isolated perfused tubule technique and osmotic gradients of 100 mosm/kg H20, estimated that the L of rabbit thin DLH was 1.7 X l0 nl cm-2 sec' atm'. The units of L may be converted from ml cm-2 sec-1 atm-' to cm sec' according to the following equation [321: RT Pj = cr1lp Vw (3) where P, is expressed in cm see' obtained only from noncolloidal osmotic gradients; L is in ml cm2 sec' atm-'; a', is the reflection coefficient of the molecule by which the osmotic gradient was induced; and Vw is the partial molar volume of water. Using this approach, and using the data of Morgan and Berliner [30], the L calculated from their data of 3.75 X 10 ml cm2 sec' atm' is equivalent to a P1 of 5.1 X
7 70 Kokko and Tisher t) )sf.1 p.-.. 't'. I T4 i I-.-. p V H Fig. 5. Electron micrograph depicting the apical region of the outer medullary segment of a collecting duct from a rat with hypothalamic diabetes insipidu.c preserved for histological examination during vasopressin-induced antidiuresis. The tight junction or zonula oceludens (arrow) resists penetration by lanthanum. US, lateral intercellular space. Magnification, X91,200. Reprinted from [54]. 10 cm sec'. Their P is approximately 23 times as great as P. The reasons for this large discrepancy for P and P are not known. It is doubtful that P is decreased due to unstirred layers on the luminal side for reasons which will be covered in greater detail in the discussion of the collecting duct. However, it is quite possible that significant unstirred layers did exist on the blood side of their preparation. Significant unstirred layers do not exist on either side of the in vitro perfused tubule [32], but, unfortunately, the P has not been determined across the DLH perfused lfl Vitro, 2. Structural-functional relationships. In the earliest light microscopic studies of Zimmerman [25] in which the thin limbs of several laboratory animals were evaluated, it was noted that cells of the early descending thin limb were rather complex in configuration and interdigitated freely with one another. More distally in the loop, the cells became less elaborate, especially near the transition from the thin to the thick segment of the ascending limb of Henle. Early ultrastructural studies confirmed the light microscopic findings of Zimmerman [33 36]. Since all thin limb cells were of the simple type in the deeper regions of the inner medulla, it was assumed that the transition from the more complex to the simple type of epithehum occurred somewhere along the DLH [351. Bulger and Trump [36] identified two types of thin limbs in the inner medulla of the rat, but could not distinguish descending from ascending segments. Similar findings have been reported in the human kidney [37]. Schwartz and Venkatachalam [38], using serial section techniques with TEM, believe they have been able to define more precisely the structural differ-
8 Water movement in the countercurrent system 71 ences between DLH from short-looped and longlooped nephrons in the outer medulla and to discern between DLH and thin ascending limbs of Henle (talh) in the inner medulla. Two types of thin limbs were found, type I and type II. Type I thin limbs were composed of cells with complex lateral interdigitations and shallow tight junctions (Fig. 6), while type II thin limbs were generally less complex in configuration, but possessed much deeper tight junctions. All short-looped nephrons which possess only DLH segments were composed of type II epithelium. The DLH's of long-looped nephrons were of type I configuration in the outer medulla, but assumed type II characteristics in the inner medulla. Shortly before the hairpin turn, deep in the inner medulla, the loop of Henle again acquired a more simple but typical type I configuration which persisted throughout the talh to the junction with the thick ascending limb of Henle (TALH). The functional implications of these apparent differences in morphology between the descending and ascending limbs of long loops of Henle, and between descending limbs of short- and long-looped nephrons, remain unclear at present. However, since type I epithelium represents the major portion of both limbs of long-looped nephrons, it would appear difficult to ascribe the differences in water permeation characteristics of the two limbs to differences in the structure of the tight junctions. Recent freeze-fracture studies of thin limbs within the inner medulla also provide evidence for this conchision. No difference in the structural configuration ft I Ii Fig. 6. Electron micrograph of a type! descending thin limb of Henle from the outer medulla of a rat kidney. Lateral interdigitations are extensive and the tight junctions (arrows) are very shallow. Magnification, X 12, NaCI H20 0,..: Fig. 7. Schematics to illustrate the generation of NaCI concentration gradients by the descending limb of Henle. Note that by virtue of water abstraction the DLH fluid remains isosmotic to the papillary interstitium; however, the luminal NaCI concentration rises higher than the interstitial concentration of NaC1 since the total interstitial solute concentration is in large part made up of urea (see text). of tight junctions was observed between descending and ascending thin limbs in rat kidney [39]. 3. Physiological significance. It is of great physiological significance that direct measurements have indicated a high osmotic water permeability across the DLH. Recently developed models of the countercurrent multiplication system which do not require active transport processes in the talh do require, however, that the fluid flowing through the talh has a higher sodium chloride concentration than does the adjacent papillary interstitium. The mechanism by which the DLH can generate an osmotic gradient is schematically shown in Fig. 7. In Fig. 7 it is assumed that approximately 50% of the papillary solute is urea while the remaining 50% is NaCI. This assumption is only a first order approximation, however, since it is based solely on evidence derived from tissue slice analysis [40 42]. As the fluid within the tubule courses from the cortex to the papillary tip, it is continuously equilibrating with a progressively more hypertonic environment. Thus, the intraluminal fluid remains isosmotic to its ambient interstitium, but the luminal sodium concentration would rise higher than 0,
9 72 Kokko and Tisher that of the interstitium. The generated sodium concentration would be a function of the fraction of urea that makes up the total papillary osmolality, and the relative permeability of the DLH to salt, urea and water. Why the in vivo micropuncture studies have not disclosed a sharper sodium concentration gradient betwen the DLH and the adjacent vasa recta than would be predicted on theoretical grounds remains an unanswered question. Perhaps some species variations do exist and a greater fraction of osmotic equilibration within the DLH occurs by solute addition than would be predicted from the direct in vitro microperfusion studies. There exists some indirect evidence that this, in fact, may be the case [26, 28]. Thin ascending limb of Hente (talh). I. Water permeability characteristics. The talh, like the DLH, is relatively inaccessible to micropuncture techniques which allow a quantitative description of phenomenological coefficients which regulate water transport. However, a number of micropuncture papers have noted that the fluid in the talh is less concentrated osmotically than the fluid in the descending limb of Henle or the blood in the adjacent vasa recta [43 45]. Therefore, it is not surprising that direct in vitro studies have noted that the permeability of the tall-i to osmotic water flow is very low [46]. Morgan and Berliner [30] found that a 200 mosm/kg HO gradient of mannitol across the talh in an isolated papillary preparation induced a net water flux of 4.4 ni cm-2 mosm' min'. Imai and Kokko 146] were unable to determine any statistically significant increase in net transport of water when an osmotic gradient of 300 mosm/kg H20 was imposed across the talh of the rabbit by addition of raffinose to the bath. It was concluded that the L is not statistically different from zero. The diffusional water permeability across the talh was also measured by Morgan and Berliner [30] and by Imai and Kokko [46] in the presence and the absence of ADH in the bath. Both sets of investigators found a P between 40 and 50 X 10-6 cm sec' without any increase after the addition of ADH to the bath. Is there a difference between the magnitude of a finite, albeit low P, and the apparent L of zero? The lowest net water flux that can be reasonably determined by the available microperfusion technique is 0.20 ni. If this much water flux was induced by a gradient of 300 mosm/kg H20 in a 1 mm segment of talh, then the L would equal 8.7 X l0- ml cm-2 sec' atm-' or 1.1 X l0- cm sec'. Thus, due to the constraints imposed by the technical difficulties in the accurate measurement of water flows, the lowest level at which a reliable estimation of L can be expressed in units of cm sec-1 is about 1 X l0. It, therefore, becomes clear that if there were a finite L roughly equivalent to 40 to 50 X l0 cm sec', this order of osmotic water permeability could never be measured using net water fluxes. Thus, we are in a position of not being able to compare the magnitude of P1 vs. P. 2. Structural-functional relationships. As noted in the preceding section on "structural-functional relationships" in the DLH, there appears to be very little morphological data to support the concept that differences in the water permeability characteristics between the DLH and the talh are due to differences in the structural configuration of the tight junction. The results of recent freeze-fracture studies, however, have refocused the attention of morphologists on the plasma membrane as a likely site of water permeation in the DLH and talh, for it is in this region where marked differences in structural configuration have been noted between descending and ascending thin limb segments. Humbert et al [39] have reported that intramembranous particles, believed to represent proteins, were much more abundant in plasma membranes of descending than ascending thin limbs. The possible relationship, if any, of this morphological finding to water permeability of the plasma membrane is unknown at this time but, as pointed out by these authors, the asymmetry of the morphological findings clearly parallels the asymmetry of the water permeation characteristics in the two limbs. 3. Physiological significance. The physiological significance of having a talh which is impermeant to water both in the presence and in the absence of ADH is that solute, without water, can be added to the papillary interstitium by the talh. In the context of the present symposium, it is irrelevant whether this addition of solute to the interstitium occurs by active or passive mechanisms; however, it is important that the movement of water out of the talh is not coupled to the movement of solute. If water were to move with solute, then it would be difficult to maintain a progressive rise in the osmolality of the medullary interstitium from the corticomedullary junction to the papillary tip. Thick ascending limb of Henle (TALH). I. Waler permeability characteristics. No isotopic water permeability coefficients have been measured across the TALH. However, Rocha and Kokko [47] and Burg and Green [48] have measured the L across the TALH. Rocha and Kokko [47], using isolated segments of rabbit medullary TALH and osmotic gradients of raffinose and NaCl of approximately 230 mosm/kg H20, were unable to see any statistically significant net flux of water. Burg and Green [48]
10 Waler movement in the countercurrent system 73 5W. Fig. 8. Electron micrograph illustrating lanthanum penetration of the zonula occiudens (arrow) in a TALH from a rat with hypothalamic diabetes insipidus. Magnification, X56,000. F} conducted similar studies using the cortical segment of the rabbit TALH. The osmolality of the perfusate was 180 to 185 mosm/kg H20 less than that of the bath. Under those conditions a very low net movement of water amounting to 0.13 ni mm min1 was measured. Thus, taken together the results of the two investigations suggest that both the cortical and medullary segments of the TALH are essentially impermeant to osmotic water flow. 2. Structural-functional relationships. Generally speaking, the TALH' has not received the same attention from morphologists as most of the other segments of the mammalian nephron. At present, morphological data which may relate to the water permeation characteristics in this region are not extensive. With TEM it has been observed that the tight junction or zonula occludens in this portion of the renal tubule is quite deep and is typical of the occluding type or "tight" tight junctions usually found in epithelia which possess a relatively large transepithelial electrical resistance. However, lanthanum tracer studies (Tisher CC, Yarger WE: unpublished observations) do reveal penetration of the tracer into the tight junction (Fig. 8) in a pattern identical to that which has been observed in the distal convoluted tubule [23]. If the ability of lanthanum to penetrate a 'The distal tubule of the rat kidney, as defined by the morphologist, is composed of three segments that are histologically distinct. These include the thick ascending limb of Henle (pars recta), the macula densa (pars maculata) and the distal convoluted tubule (pars convoluta). Thus, the thick ascending limb of Henle represents the initial segment of the distal tubule. tight junction is sufficient evidence to establish the presence of a potential paracellular shunt pathway, the presence of such a pathway in the TALH would not appear to be of major importance for transepithelial water flow in light of the water permeability characteristics that are ascribed to this segment of the nephron. Data derived from freeze-fracture studies concerning intramembranous particle configuration in plasma membranes of the TALH which might provide information of a structural nature to relate to the known water permeation characteristics are not currently available. 3. Physiological signficance. The TALH subserves a critically important role in the overall operation of the countercurrent multiplication system (CCMS). It is the major energy source, or single effect of the CCMS. It is able to accomplish this by virtue of being water impermeant. Thus, the powerful chloride pump of the TALH [47, 48] is able to separate solute and water and maintain a hypertonic outer medullary interstitium. If water were to follow sodium chloride freely, the transport would then be isotonic in nature and no medullary concentration gradient could be developed and maintained. Distal convoluted tubule (DCT). 1. Water permeability characteristics. The information concerning water transport across the DCT is rather limited. A number of micropuncture studies have noted, however, that the fluid in the distal tubule is hypotonic with respect to plasma. Ullrich, Rumrich and Fuchs [21] have measured the L of the distal tubule of an albino rat and reported that the L increased with the
11 74 Kokko and Tisher administration of ADH; however, Woodhall and usher [17] have suggested, on the basis of morphological data, that the earlier measurements of L may, in part, have been a composite of measurements obtained from DCI and cortical collecting duct epithelia.2 In more recent studies, Gross, Imai and Kokko [22] have directly examined the osmotic water permeability of the rabbit DCI perfused in vitro. Their studies measured net fluid movement in response to either a 150 to 200mOsm/kg H20 gradient induced with raffinose or a 60 mosm/kg H20 gradient induced with NaCl. There was no statistically significant net water flux with either of the imposed gradients. Change in net water flux was then measured after the addition of 200 tu/ml of ADH to the bath. Again, there was no net movement of water. It was concluded that the L of the DCT is not different from zero and that net water flux in this segment of the nephron does not increase in response to ADH administration, thus providing direct evidence to support the earlier morphological observations of Woodhall and Tisher [17] derived from the rat. 2. Structural-functional relationships. An excellent correlation would appear to exist between structural and functional data in this region of the renal tubule. In a combined morphological-physiological study using rats with hereditary hypothalamic diabetes insipidus (Dl), Woodhall and Tisher [17] were unable to demonstrate morphological evidence of transepithelial water flow in the form of cell swelling and intercellular space dilatation in the DCI. The average width of the lateral intercellular spaces of the distal convoluted tubules in the two physiological conditions was not statistically different, measuring 178 SD 70 A (N 144) in four rats in water diuresis and 182 SD 82 A (N = 108) in three vasopressin-treated antidiuretic animals (P > 0.5). 2 The collecting duct is lined by epithelium that is morphologically distinct from that forming the distal convoluted tubule. The first portion of the cortical collecting duct is usually referred to as the initial collecting tubule or connecting portion. In most mammalian kidneys the transition from the distal convoluted tubule to the cortical collecting duct (i.e., the initial collecting tubule) occurs well before the first junction of one renal tubule with another. Therefore, the first junction of two renal tubules is formed by two initial collecting tubules [17]. It has been common practice at the micropuncture table to define the "distal tubule" or "distal convolution" as that segment of the renal tubule that extends between the macula densa and the site of the first junction with another distal tubule or distal convolution. When defined in this manner, it can be seen that this segment of the renal tubule is structurally heterogeneous in character. Thus, the "early" distal tubule of the renal micropuncturist is lined by epithelium characteristic of the distal convoluted tubule, whereas the "late" distal tubule is formed by epithelium characteristic of the initial collecting tubule. The junctional complex in this segment of the nephron is composed of a tight junction, or zonula occiudens, which is approximately 0.3 u in depth, and an intermediate junction, or zonula adherens, thus closely paralleling the structure of the junctional complex in the TALH. Recent studies have demonstrated that the tight junction in the DCI is freely permeable to both colloidal lanthanum and ionic lanthanum [23, 24], suggesting the presence of a potential paracellular shunt pathway for water and solute movement. However, since this segment, like the TALH, is relatively impermeable to water [22], it would appear that such a potential pathway is of little consequence under steady state conditions, at least with respect to water flow. Finally, the general configuration of the luminal surface and apical region of the cells of the TALH and DCI would appear to bear little importance in determining their permeability to water. Although cells from both regions possess numerous short blunt microvilli on their luminal surface, cells forming the DCT are relatively free of apical lateral interdigitations, while those forming the cortical segment of the TALH exhibit complex lateral interdigitations with neighboring cells [49]. This difference in cell configuration can be best appreciated with SEM (Figs. 9 and 10). 3. Physiological significance. The physiological significance of the water impermeability per se of the DCT is simply to allow passage of a dilute tubular fluid to the collecting duct which, in turn, can modulate the osmolality of the fluid according to the physiological needs of the animal. Collecting duct system (CD). I. Waterpermeability characteristics, The CD system is of pivotal importance in regulating the state of water balance in most mammals. This segment of the renal tubule possesses the unique characteristics of having the capacity to vary its permeability to water. Because the water permeability characteristics of the CD are so important, these segments have been studied extensively both with respect to the measurement of the phenomenological coefficients describing water transport and with respect to the description of the paths of transtubular water flow. Though various functional and anatomical differences exist along the length of the CD (i.e., cortical CD vs. outer medullary CD vs. papillary CD), the water permeability characteristics of these segments will be covered together in this symposium since the available water permeation data are so strikingly similar along the entire length of this region of the renal tubule. However, it must be remembered that a number of important physiological differences do exist between these segments, such as
12 Water movement in the countercurrent system 75 * a ' aa 'Il._t tt;k* - ) ' "'a, J -t- a - 1.,', Fig. 9. Scanning electron micrograph illustrating the appearance of a TA LH from the outer cortex near the parent renal corpuscle. Cells in this region of the distal tubule are covered with an extensive population of microvilli and possess extensive apical lateral cell processes. (arrows) Magnification, x4400. Reprinted from [48]. the difference in urea permeability characteristics [50], but the discussion of such differences is beyond the scope of the present text. The morphological differences between these segments will be discussed separately. The water permeability characteristics of the cortical and outer medullary CD have been evaluated solely by the in vitro microperfusion technique [11, 22, 32, 51], whereas the characteristics of the papillary CD have been examined both by in vitro perfusion of isolated rabbit tubules [501 and by free-flow perfusion of collecting ducts in an in vitro preparation employing longitudinal slices of rat kidney [30]. All of these studies indicate that these segments are relatively impermeant to both diffusional and osmotic water flow. However, with the addition of ADH to the ambient bath, both the and Pr increase significantly. Thus, it is clear that net transport of water across the CD system is minimal in the absence of ADH, while significant net quantities of water may be transported across these segments in the presence of ADH. Schafer and Andreoli [32] have provided the most detailed measurements of P and P across cortical collecting tubules isolated from rabbit kidney. They found that ADH increases both the P and P1 of these segments when perfused in vitro. The measurements of P. were performed both with and without an imposed osmotic gradient, whereas the measurements of P were performed with an osmotic gradient. Without ADH, the P1 was approximately 27% greater than P when expressed in comparable units. This is a relatively small difference and may not represent any real difference in P1 and P in view of the sources of error inherent with these types of measurements. However, when ADH was added to the bath, the ratio of P1 to P., rose impressively to approximately 13 (186 and 14.2 cm sec', respectively).
13 76 Kokko and Tisher -S p.' "w ks-''- S- 1 -C, flit A Li - p : " 'tn -r ', s Fig. 10. Scanning electron micrograph of a DCT. The luminal surface of these cells is also covered with an extensive population of microvilli, but apical lateral cell processes are conspicuously absent in this region of the distal tubule as evidenced by the relatively simple contour of the lateral cell borders (arrows). Magnification, X3,000. This large difference clearly cannot be accounted for by experimental variation in the data, The authors advanced several possibilities to explain their data. Using both theoretical and experimental arguments, they ruled out the possibility that laminar flow through double barrier porous membranes or difl'usion resistances imposed by unstirred layers could be the explanation [32]. They felt that the discrepancy between P and P in the presence of ADH most likely reflected cellular constraints to diffusion [32]. 2. Structural-functional relationships. Both light and electron microscopic techniques have been utilized effectively to establish functional-morphological correlations related to fluid reabsorption in the CD. Initial in vitro studies by Ganote et al [52] and Grantham et al [53] using isolated segments of rabbit cortical collecting tubule demonstrated the presence of cell swelling, increased numbers of intracellular vacuoles and dilatation of intercellular spaces during vasopressin-induced osmotic fluid reabsorption. The findings were virtually identical to those described some years earlier in the toad urinary bladder after vasopressin stimulation in the presence of an osmotic gradient [12 14]. In vivo studies by Woodhall and
14 Water movement in the countercurrent srstem 77 TL Fig. 11. Low magnification electron micrograph depicting a portion of cortical CD from an untreated DI rat during water diuresis. No enlargement of intercellular spaces (arrow) or evidence of cell swelling is present. TI, tubular lumen. Magnification, X 7,500. Reprinted [161 with permission from the Journal of Clinical Investigation. Tisher [17] and Tisher, Bulger and Valtin [54] in rats with DI revealed similar findings in the CD. Qua!- itatively, cell swelling and dilatation of intercellular spaces were observed along the total length of the CD (Figs. 11 and 12). Morphometric data obtained in cortical CD segments which included the initial collecting tubule revealed that the outside diamenter of the tubules was not significantly different in vasopressin-treated vs. untreated diuretic DI rats (36.1 SD 5.8 s vs. 38 SD 5.8 t, P > 0.10); however, a significant reduction in the luminal diameter was observed in the vasopressin-treated antidiuretic group (22.7 SD 4.7 z) versus the untreated diuretic group (26.1 SD 5.1 t, P < 0.Ol)due to the presence of cell swelling. Cell height of both the light and dark cells of the cortical CD was significantly greater in vaso- 22 I'. TL s.,;._..i : :t:',;jt '\ 4 Fig. 12. Low magnification electron micrograph depicting a portion of a cortical CD from a DI rat in which the water diuresis was converted to antidiuresis with exogenous vasopressin. Lateral and basilar intercellular spaces (IS) are widely dilated and the light or principal cells (PC) appear swollen. TL, tubular lumen. Magnification, X7,500. Reprinted [l6] with permission from the Journal of Clinical Investigation.
15 78 Kokko and Tisher pressin-treated animals. The average height of the light cells in four untreated DI rats undergoing water diuresis was 5.7 sn 1.3.t vs. 6.7 SD 1.0 t in three vasopressin-treated animals (P < 0.001). The average height of the dark or intercalated cells in the untreated diuretic animals was 5.3 SD 1.1 z vs. 6.8 SD 1.0 t in the treated animals (P < 0.001). Taken together the findings suggested that in response to vasopressin and a favorable osmotic gradient, tubular fluid crossed the luminal cell membranes to enter the cell and exited the cell, at least in part, via the lateral cell membranes, thereby entering the lateral intercellular space. At present there appears to be very little morphological evidence that paracellular shunt pathways play any major role in vasopressin-induced osmotic water flow in the cortical and outer medullary segments of the CD, although potential paracellular pathways may exist within the inner medullary and papillary segments of the CD. Tisher and Yarger [55], employing lanthanum tracer techniques in Dl rats, observed that tight junctions of the cortical and outer medullary segments of the CD resisted penetration by the tracer (Fig. 5), while tight junctions of the inner medullary and papillary segments were freely permeable to lanthanum (Fig. 13). The results were not influenced by the presence or absence of vasopressin. In the cortical segment of the CD, the results correlate well with measurements of transepithelial electrical resistance that have been reported previously. Helman, Grantham and Burg [56], working with isolated segments of rabbit cortical collecting tubules, reported a relatively high transepithelial electrical resistance of approximately 867 I cm2, a finding which appears to be incompatible with the presence of a major paracellular shunt pathway for ion and water movement in this region of the CD. On the other hand, the demonstration of the presence of a nonoccluding type of tight junction in the inner medullary and papillary segments of the CD of the rat does raise the possibility that a functional paracellular shunt pathway, possibly involved in the passive movement of nonelectrolytes such as urea, may exist in this region of the nephron [55]. 3. Physiological significance. The difference in water permeability of the CD in the presence and absence of ADH is of tremendous physiological significance. In the absence of ADH, the kidney can form a max- Fig. 13. Electron micrograph demonstrating lanthanum penetration of the tight junction (zonula occiudens) in the papillary segment of a CD. Tissue preservation was accomplished by microperfusion during water diuresis in the absence of vasopressin. Lanthanum entering from the luminal side has extended beyond the region of the tight junction down to the level of the intermediate junction (zonula adherens) (arrow). Magnification, X86,000. Reprinted from [54].
Functions of Proximal Convoluted Tubules
 1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
Diagram of the inner portions of the kidney
 Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
11/05/1431. Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate
 Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Physiology (6) 2/4/2018. Rahmeh Alsukkar
 Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY
 URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
Kidney Structure. Renal Lobe = renal pyramid & overlying cortex. Renal Lobule = medullary ray & surrounding cortical labryinth.
 Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Transport characteristics of the thin limbs of Henle
 Kidney International, Vol. 22 (1982), pp. 449 453 Transport characteristics of the thin limbs of Henle JUHA P. KOKKO Department of Internal Medicine, University of Texas Health Science Center, Southwestern
Kidney International, Vol. 22 (1982), pp. 449 453 Transport characteristics of the thin limbs of Henle JUHA P. KOKKO Department of Internal Medicine, University of Texas Health Science Center, Southwestern
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi
 Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi At the end of this lecture student should be able to describe: The loop of Henle is referred to as countercurrent
Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi At the end of this lecture student should be able to describe: The loop of Henle is referred to as countercurrent
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Chapter 25: Urinary System
 Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
Renal Physiology II Tubular functions
 Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Normal Renal Function
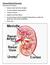 Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Urine Formation. Urinary Physiology Urinary Section pages Urine Formation. Glomerular Filtration 4/24/2016
 Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
Osmoregulation and Renal Function
 1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
Urinary System Review Questions:
 Urinary System Review Questions: 1. This system would be lined with what type of membrane? 2. What type of epithelial tissue would line the opening of the urethra (the exit of the tract)? 3. What type
Urinary System Review Questions: 1. This system would be lined with what type of membrane? 2. What type of epithelial tissue would line the opening of the urethra (the exit of the tract)? 3. What type
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
URINARY SYSTEM. Primary functions. Major organs & structures
 URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
Faculty version with model answers
 Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
Other Factors Affecting GFR. Chapter 25. After Filtration. Reabsorption and Secretion. 5 Functions of the PCT
 Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Homeostasis. Thermoregulation. Osmoregulation. Excretion. how organisms regulate their body temperature
 Homeostasis the steady-state physiological condition of the body Ability to regulate the internal environment important for proper functioning of cells Thermoregulation Homeostasis how organisms regulate
Homeostasis the steady-state physiological condition of the body Ability to regulate the internal environment important for proper functioning of cells Thermoregulation Homeostasis how organisms regulate
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Chapter 24: The Urinary System
 Chapter 24: The Urinary System Overview of kidney functions n Regulation of blood ionic composition n Regulation of blood ph n Regulation of blood volume n Regulation of blood pressure n Maintenance of
Chapter 24: The Urinary System Overview of kidney functions n Regulation of blood ionic composition n Regulation of blood ph n Regulation of blood volume n Regulation of blood pressure n Maintenance of
Identify and describe. mechanism involved in Glucose reabsorption
 Define tubular reabsorption, tubular secretion, transcellular and paracellular transport. Identify and describe mechanism involved in Glucose reabsorption Describe tubular secretion with PAH transport
Define tubular reabsorption, tubular secretion, transcellular and paracellular transport. Identify and describe mechanism involved in Glucose reabsorption Describe tubular secretion with PAH transport
I. Metabolic Wastes Metabolic Waste:
 I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
** TMP mean page 340 in 12 th edition. Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2:
 QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
Basic mechanisms of Kidney function
 Excretion Basic mechanisms of Kidney function Urine formation in Amphibians Urine formation in Mammals Urine formation in Insects Nitrogen balance Kidneys The most fundamental function of kidneys) is to
Excretion Basic mechanisms of Kidney function Urine formation in Amphibians Urine formation in Mammals Urine formation in Insects Nitrogen balance Kidneys The most fundamental function of kidneys) is to
Sodium and chlorine transport
 Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
After studying this lecture, you should be able to...
 Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX
 One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
5.Which part of the nephron removes water, ions and nutrients from the blood?
 Uro question 1.While reading a blood test I notice a high level of creatinine, I could assume from this that A) There is a possibility of a UTI B) There is a possibility of diabetes C) There is a possibility
Uro question 1.While reading a blood test I notice a high level of creatinine, I could assume from this that A) There is a possibility of a UTI B) There is a possibility of diabetes C) There is a possibility
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY
 RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
NORMAL POTASSIUM DISTRIBUTION AND BALANCE
 NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
November 30, 2016 & URINE FORMATION
 & URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
& URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (4) Dr. Attila Nagy 2018
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (4) Dr. Attila Nagy 2018 Intercalated cells Intercalated cells secrete either H + (Typ A) or HCO 3- (Typ B). In intercalated cells Typ A can be observed
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (4) Dr. Attila Nagy 2018 Intercalated cells Intercalated cells secrete either H + (Typ A) or HCO 3- (Typ B). In intercalated cells Typ A can be observed
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Physiology (5) 2/4/2018. Mohammad jaber
 Physiology (5) 2/4/2018 Mohammad jaber Slides are embedded in the sheet in bold, some figure are not included sorry for any mistake,, lets go.. There are two types of urine : 1-diluted urine ( Excess fluid
Physiology (5) 2/4/2018 Mohammad jaber Slides are embedded in the sheet in bold, some figure are not included sorry for any mistake,, lets go.. There are two types of urine : 1-diluted urine ( Excess fluid
QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19
![QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19](/thumbs/80/80449526.jpg) 1 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion, excretion
1 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion, excretion
Salt and Water Balance and Nitrogen Excretion
 Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Histology / First stage The Urinary System: Introduction. Kidneys
 The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
Urinary System. Dr. Ahmed Maher Dr. Ahmed Manhal
 Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
mid ihsan (Physiology ) GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B **
 (Physiology ) mid ihsan GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B ** Colloid pressure in the efferent arteriole is: A- More than that leaving
(Physiology ) mid ihsan GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B ** Colloid pressure in the efferent arteriole is: A- More than that leaving
Sodium Chloride, Urea, and Water Transport
 Sodium Chloride, Urea, and Water Transport in the Thin Ascending Limb of Henle GENERATION OF OSMOTIC GRADIENTS BY PASSIVE DIFFUSION OF SOLUTES MASASHI IMAI and JUHA P. KOKKO From the Department of Internal
Sodium Chloride, Urea, and Water Transport in the Thin Ascending Limb of Henle GENERATION OF OSMOTIC GRADIENTS BY PASSIVE DIFFUSION OF SOLUTES MASASHI IMAI and JUHA P. KOKKO From the Department of Internal
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Urinary System BIO 250. Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat. Routes of Waste Elimination
 Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Intrinsic Differences in Various Segments
 Intrinsic Differences in Various Segments of the Proximal Convoluted Tubule HARRY R. JACOBSON and JuHA P. KoKKo From the Department of Internal Medicine, The University of Texas Southwestern Medical School,
Intrinsic Differences in Various Segments of the Proximal Convoluted Tubule HARRY R. JACOBSON and JuHA P. KoKKo From the Department of Internal Medicine, The University of Texas Southwestern Medical School,
RENAL. 6. Renal acid secretion is affected by all of the following except a. pco 2 b. K c. Carbonic anhydrase d. Aldosterone e. Ca
 RENAL 1. The reabsorption of Na in the proximal tubules a. Reabsorbs 80% of the filtered sodium load b. Causes increased hypertonicity c. Is powered by N/H ATPase d. Shares a common carrier with glucose
RENAL 1. The reabsorption of Na in the proximal tubules a. Reabsorbs 80% of the filtered sodium load b. Causes increased hypertonicity c. Is powered by N/H ATPase d. Shares a common carrier with glucose
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Osmoregulation regulates solute concentrations and balances the gain and loss of water
 Ch 44 Osmoregulation & Excretion Osmoregulation regulates solute concentrations and balances the gain and loss of water Freshwater animals show adaptations that reduce water uptake and conserve solutes
Ch 44 Osmoregulation & Excretion Osmoregulation regulates solute concentrations and balances the gain and loss of water Freshwater animals show adaptations that reduce water uptake and conserve solutes
Renal System and Excretion
 Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
Renal System and Excretion Biology 105 Lecture 19 Chapter 16 Outline Renal System I. Functions II. Organs of the renal system III. Kidneys 1. Structure 2. Function IV. Nephron 1. Structure 2. Function
PARTS OF THE URINARY SYSTEM
 EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Chapter 26 The Urinary System. Overview of Kidney Functions. External Anatomy of Kidney. External Anatomy of Kidney
 Chapter 26 The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the blood and return most
Chapter 26 The Urinary System Kidneys, ureters, urinary bladder & urethra Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra Filter the blood and return most
