SIRT1 Activation by a c-myc Oncogenic Network Promotes the Maintenance and Drug Resistance of Human FLT3-ITD Acute Myeloid Leukemia Stem Cells
|
|
|
- Arthur Atkins
- 5 years ago
- Views:
Transcription
1 Cell Stem Cell Article SIRT1 Activation by a c-myc Oncogenic Network Promotes the Maintenance and Drug Resistance of Human FLT3-ITD Acute Myeloid Leukemia Stem Cells Ling Li, 1 Tereza Osdal, 2 Yinwei Ho, 1 Sookhee Chun, 1 Tinisha McDonald, 1 Puneet Agarwal, 1 Allen Lin, 1 Su Chu, 1 Jing Qi, 1 Liang Li, 1 Yao-Te Hsieh, 1 Cedric Dos Santos, 1 Hongfeng Yuan, 5 Trung-Quang Ha, 2 Mihaela Popa, 3 Randi Hovland, 7 Øystein Bruserud, 2,4 Bjørn Tore Gjertsen, 2,4 Ya-Huei Kuo, 1 Wenyong Chen, 5 Sonia Lain, 6 Emmet McCormack, 2,4, and Ravi Bhatia 1, 1 Division of Hematopoietic Stem Cell and Leukemia Research, City of Hope National Medical Center, Duarte, CA 91010, USA 2 Department of Clinical Science, Hematology Section, University of Bergen, Bergen 5021, Norway 3 KinN Therapeutics AS, Bergen 5008, Norway 4 Department of Internal Medicine, Hematology Section, Haukeland University Hospital, Bergen 5021, Norway 5 Department of Cancer Biology, City of Hope National Medical Center, Duarte, CA 91010, USA 6 Karolinska Institutet, Stockholm 17177, Sweden 7 Center for Medical Genetics and Molecular Medicine, Haukeland University Hospital, Bergen 5021, Norway Correspondence: emmet.mc.cormack@k2.uib.no (E.M.), rbhatia@coh.org (R.B.) SUMMARY The FLT3-ITD mutation is frequently observed in acute myeloid leukemia (AML) and is associated with poor prognosis. In such patients, FLT3 tyrosine kinase inhibitors (TKIs) are only partially effective and do not eliminate the leukemia stem cells (LSCs) that are assumed to be the source of treatment failure. Here, we show that the NAD-dependent SIRT1 deacetylase is selectively overexpressed in primary human FLT3-ITD AML LSCs. This SIRT1 overexpression is related to enhanced expression of the USP22 deubiquitinase induced by c-myc, leading to reduced SIRT1 ubiquitination and enhanced stability. Inhibition of SIRT1 expression or activity reduced the growth of FLT3-ITD AML LSCs and significantly enhanced TKI-mediated killing of the cells. Therefore, these results identify a c-myc-related network that enhances SIRT1 protein expression in human FLT3-ITD AML LSCs and contributes to their maintenance. Inhibition of this oncogenic network could be an attractive approach for targeting FLT3-ITD AML LSCs to improve treatment outcomes. INTRODUCTION Acute myeloid leukemia (AML) is organized as a hierarchy with small populations of self-renewing leukemic stem cells (LSCs) generating the bulk of leukemic cells (Patel et al., 2012). LSCs can resist elimination by conventional therapy and persist as potential sources of relapse. Several studies indicate that LSC gene expression signatures are correlated with poor prognosis in AML patients (Eppert et al., 2011). Better understanding of LSC regulation is critical for developing improved therapies against AML. Internal tandem duplications (ITDs) in the Fms-like tyrosine kinase (FLT3) are seen in 25% 30% of AML patients, constituting the most commonly observed mutation in AML (Kindler et al., 2010). FLT3-ITD is associated with reduced length of remission and survival, consistent with lack of elimination of LSC (Kindler et al., 2010; Horton and Huntly, 2012). The ITD mutation results in constitutive FLT3 activation and altered downstream signaling compared to wild-type (WT) FLT3 (Nakao et al., 1996). In animal models, expression of FLT3-ITD alone results in a myeloproliferative disorder, and cooperating mutations are required for AML development (Chu et al., 2012). Several small molecule FLT3 tyrosine kinase inhibitors (TKIs), such as quizartinib (AC220), are being examined (Levis, 2011; Smith et al., 2012). However, FLT3-TKIs only partially inhibit human FLT3-ITD AML LSCs and demonstrate modest clinical activity (Horton and Huntly, 2012; Levis, 2011; Smith et al., 2012). Resistance can emerge during treatment through point mutations that interfere with drug binding (Smith et al., 2012). Better understanding of molecular events contributing to the drug resistance of FLT3-ITD LSC would aid development of approaches to achieve sustained remissions. The NAD-dependent deacetylase sirtuin 1 (SIRT1) modulates the activity of several intracellular proteins, including p53 (Vaziri et al., 2001). SIRT1 regulates numerous cellular processes including aging, DNA repair, cell cycle, metabolism, and survival (Brooks and Gu, 2009). SIRT1 plays an important role in maintaining self-renewal and differentiation of murine embryonic stem cells and hematopoietic stem cells (HSCs), especially under conditions of stress (Han et al., 2008; Ou et al., 2011). Several studies indicate a pathogenic role for SIRT1 in solid tumors and leukemias (Brooks and Gu, 2009). However, other studies suggest tumor-suppressive functions (Wang et al., 2008a, 2008b), implying that the role of SIRT1 in cancer may be context dependent, varying by the tumor type, specific oncogenes present, and mutation status of p53 or other target proteins (Brooks and Gu, 2009). We have reported that SIRT1 is overexpressed in chronic myeloid leukemia (CML) LSCs and that SIRT1 inhibition selectively eliminates CML LSCs by Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. 431
2 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells A CD34 + CD38 - B CD34 + CD Normal AML Better prognosis AML Intermediate prognosis AML Poor prognosis 0 Normal AML Better prognosis AML Intermediate prognosis AML Poor prognosis C % Viability of Ctrl (Hoechst 33342) FLT3-WT FLT3-ITD D E F % AnnexinV + Cells MV4-11 Molm-13 OCI-AML3 ^ ^ ^ 1 ^^ 2 ^ ^^ 4 5 TV6 concentration (µm) % Viability of Ctrl (Hoechst 33342) FLT3-ITD FLT3-TKD Figure 1. SIRT1 Expression and Sensitivity to TV6 in AML Compared with Normal Stem/Progenitor Cells (A and B) SIRT1 protein expression in CD34 + CD38 (A) and CD34 + CD38 + (B) cells from better risk (n = 10), intermediate-risk (n = 17) and poor-risk (n = 17) AML and normal CB and PBSC samples was analyzed by flow cytometry. Median fluorescence intensity of SIRT1 was expressed relative to that of immunoglobulin G (IgG) control. FLT3-ITD AML samples are indicated in red. (C) Sensitivity of AML samples (n = 20) to TV6 (10 mm) and nutlin-3 (10 mm) treatment for 24 hr. Viability was tested using Hoechst staining. Clinical and genetic parameters for individual samples are shown. Flt3 mutation indicates FLT3-ITD mutation. (D) Effect of 48 hr TV6 treatment on apoptosis of WT p53 FLT3-ITD MV4-11 and Molm-13 AML cells, and on FLT3-WT OCI-AML3 cells, using annexin V/DAPI staining. Results represent mean ± SEM of three separate experiments. p < 0.05 and p < 0.01 for MV4-11 versus OCI-AML3; ^p < 0.05 and ^^p < 0.01 for Molm-13 versus OCI-AML Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. (legend continued on next page)
3 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells increasing p53 acetylation and activity (Li et al., 2012). Although the role of SIRT1 in murine adult HSCs is controversial (Leko et al., 2012; Singh et al., 2013), SIRT1 inhibition has only a minor impact on normal human CD34 + hematopoietic cells (Li et al., 2012; MacCallum et al., 2013). Given the association of SIRT1 activation with BCR-ABL (Yuan et al., 2012) and the reported sensitivity of FLT3-ITD AML samples to p53-activating drugs (Long et al., 2010; McCormack et al., 2012), we were interested in evaluating whether the FLT3-ITD kinase was also associated with increased SIRT1 expression and activity. We studied SIRT1 expression and effects of SIRT1 inhibition in a large group of human AML samples from two centers. We evaluated the association between FLT3-ITD and increased SIRT1 activity, as well as the contribution of SIRT1 to survival, growth, and TKI response of FLT3- ITD AML LSC. Finally, we investigated mechanisms contributing to SIRT1 activation in FLT3-ITD AML. RESULTS SIRT1 Overexpression and Sensitivity to SIRT1 Inhibition in AML CD34 + Cells We measured SIRT1 protein levels in AML and normal cord blood (CB) and PB stem cell (PBSC) CD34 + CD38 + committed progenitors and CD34 + CD38 primitive progenitors by labeling with anti-sirt1 antibody and flow cytometry (Li et al., 2012). The majority of AML CD34 + CD38 cells (n = 44) showed increased SIRT1 expression compared to normal samples (Figure 1A). SIRT1 expression was also increased in AML compared to normal CD34 + CD38 + cells (Figure 1B). SIRT1 levels were higher in cells from patients with poor and intermediate, compared with better risk, genetic lesions based on National Cancer Center Network (NCCN) criteria (O Donnell et al., 2012) (Figure 1A). Since SIRT1 can deacetylate and inhibit p53 activity (Vaziri et al., 2001; Li et al., 2012), we evaluated the effect of the SIRT1 inhibitor Tenovin-6 (TV6; 10 mm) and the MDM2 antagonist nutlin-3 (10 mm) on the viability of AML cells. TV6 and nutlin-3 demonstrated similar efficacy, on average, in inhibiting the viability of AML blasts (Figure S1A available online). As expected, moderate sensitivity to TV6 was seen in p53 wild-type (WT) samples (n = 25) but not p53 mutant samples (n = 5) (Figure S1B). On the other hand, individual AML samples showed discrepancies between TV6 and nutlin-3 sensitivity, suggesting that additional factors may influence sensitivity. In samples showing >20% difference in viability after TV6 and nutlin-3 treatment, the FLT3-ITD mutation was associated with increased TV6 sensitivity (p < 0.001) (Figure S1D). We also observed increased representation of FLT3-ITD in AML samples with the highest sensitivity to TV6 (7 of 20 samples, p < 0.001) (Figure 1C), and in AML CD34 + CD38 and CD34 + CD38 + cells with the highest SIRT1 expression (Figures 1A and 1B). The Molm-13 and MV4-11 AML cell lines (p53 WT and FLT3-ITD + ) showed significantly increased sensitivity to TV6 compared with FLT3 WT (FLT3-WT) and p53 WT OCI-AML3 cells; FLT3-WT and p53 null HL-60 cells; and FLT3-WT and p53 mutant NB4 cells (Figures 1D and S1C). TV6 treatment significantly decreased leukemic burden and prolonged survival of mice xenografted with luciferase expressing Molm-13 cells (McCormack et al., 2012) (Figures 1E, S1E, and S1F). Moreover, TV6 significantly decreased the leukemic burden in mice engrafted with control small hairpin RNA (shrna) but not p53 shrna-expressing Molm13 cells (Figure S1G). FLT3 tyrosine kinase domain (TKD) mutations (D835Y) are also seen in 5% of AML cases (Kindler et al., 2010). It is interesting that FLT3- TKD samples were significantly less sensitive to TV6 compared to FLT3-ITD samples (Figure 1F). Cumulatively, these data suggest that the FLT3-ITD mutation identifies AML cells with increased sensitivity to SIRT1 inhibition. Increased SIRT1 Expression and Sensitivity to SIRT1 Knockdown in FLT-ITD + AML CD34 + cells We further demonstrated that SIRT1 protein levels were significantly increased in FLT3-ITD (n = 8) compared to FLT3-WT (n = 9) or FLT3-TKD (n = 5) AML CD34 + cells (Figure 2A). SIRT1 expression was increased in FLT3-ITD compared to FLT3-WT AML CD34 + CD38 cells (n = 4, each group) coexpressing two well-described LSC markers, CD47 (Majeti et al., 2009) and CD123 (Jordan et al., 2000) (Figure S2A). SIRT1 expression in FLT3-ITD AML samples was highest in S/G2/M, followed by G1 and then G0 phase (data not shown). CB CD34 + cells transduced with FLT3-ITD-expressing lentivirus vectors showed increased SIRT1 and reduced acetylated p53 levels compared to FLT3-WT or control vectors (Figures 2B and S2B). On the other hand, significant differences in SIRT1 messenger RNA (mrna) levels were not seen between FLT3-ITD and FLT3-WT AML CD34 + cells (Figure 2C), or between CB CD34 + cells expressing FLT3-ITD and FLT3-WT genes (data not shown). SIRT1 protein half-life was increased in FLT3-ITD MV4-11 cells treated with cyclohexamide (CHX) to inhibit translation, compared to FLT3-WT OCI-AML3 cells (Figure S2C), and in CD34 + cells ectopically expressing FLT3-ITD compared to FLT3-WT (Figure 2D). These results suggest that increased SIRT1 expression in FLT3-ITD AML cells is related to increased protein stability. Since SIRT1-mediated deacetylation can inhibit p53 activity, we analyzed p53 target gene induction after g-irradiation (3 Gy, 6 hr) in FLT3-ITD and FLT3-WT AML CD34 + cells (all with WT p53 by sequencing). We observed reduced induction of mrna for p53 target genes, especially BAX and NOXA, in FLT3-ITD compared to FLT3-WT cells (Figure 2E). FLT3-ITD MV4-11 cells showed reduced radiation-induced increase in p21, BAX, NOXA, PUMA, and DR5 protein levels compared to FLT3-WT OCI- AML3 cells (Figure S2D). Knockdown of BAX using small interfering RNA (sirna) significantly reduced radiation-induced apoptosis in MV4-11 cells and OCI-AML3 (Figures S2E and (E) Survival of NSG mice transplanted with MOLM-13luc cells and treated starting 5 days later with TV6 intraperitoneally (125 mg/kg daily or 50 mg/kg twice a day) for 12 days. Survival of mice treated with both TV6 regimens was significantly longer than controls (p < 0.05 for 125 mg/kg once daily; p < for 50 mg/kg twice daily). (F) Sensitivity of AML samples with WT p53, FLT3-ITD (n = 5), or FLT3-TKD (n = 5) mutations to TV6 (10 mm) for 24 hr. Viability was tested using Hoechst staining. p < 0.05, p < 0.01, and p < See also Figure S1 and Tables S1, S2, and S3. Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. 433
4 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells A Relative expression (SIRT1/IgG) B Mock CD34 + FLT3-ITDCD34 + FLT3-WT CD34 + SIRT1 pflt3 (Y589/Y591) C FLT3-ITD AML FLT3-WT AML FLT3-TKD AML Normal FLT3 pstat5(y694) D +CHX FLT3-ITD FLT3-WT 0hr 3hr 6hr 9hr 12hr 0hr 3hr 6hr 9hr12hr FLT3 pflt3 (Y589/Y591) SIRT1 (long exp.) SIRT1 (short exp.) % SIRT1 level at 0hr E 32 FLT3-WT AML CD FLT3-ITD AML CD34 + F G Ctrl ShRNA ShSIRT1 SIRT1 Ac-p p21 BAX PUMA DR5 NOXA p53 H % AnnexinV + Cells FLT3-WT Ctrl ShRNA ShSIRT1 ns FLT3-ITD I DAPI CtrlShRNA AV+ 15% FLT3-WT AML ShSIRT1 AV+ 20% Annexin V FLT3-ITD AML CtrlShRNA AV+ 15% ShSIRT1 AV+ 44% Figure 2. Increased SIRT1 Expression and Sensitivity to SIRT1 Knockdown in FLT-ITD + AML CD34 + Cells (A) SIRT1 protein expression in CD34 + cells from FLT3-ITD AML (n = 8), FLT3-WT AML (n = 9), FLT-TKD AML (n = 5), and normal CB or PBSC (n = 9) samples analyzed by intracellular flow cytometry. IgG, immunoglobulin G. (B) Western blotting for SIRT1, FLT3, p-flt3 (Y589/Y591), p-stat5 (Y694), and b-actin in CB CD34 + cells transduced with vectors expressing green fluorescent protein (GFP) alone (Mock), FLT3-ITD, or FLT3-WT. Results are representative of three experiments. (C) SIRT1 mrna expression in CD34 + cells from FLT3-ITD (n = 11), FLT3-WT AML (n = 11), CB or normal PBSC (n = 8) samples analyzed by quantitative PCR. (D) Western blotting for SIRT1, FLT3, pflt3 (Y589/Y591), and b-actin in FLT3-ITD or FLT3-WT expressing CD34 + cells treated with CHX. The right panel indicates the relative amount of SIRT1 analyzed by densitometry (n = 3). exp., exposure. (legend continued on next page) 434 Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc.
5 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells S2F), indicating its importance as an effector of p53 response. Radiation-induced apoptosis of MV4-11, but not OCI-AML3, cells was significantly increased by TV6 pretreatment, and this effect was also prevented by BAX knockdown (Figure S2F). To further evaluate the functional role of SIRT1 in modulating p53 function in FLT3-ITD AML CD34 + cells, we inhibited SIRT1 expression using lentivirus vectors expressing SIRT1 shrna (ShSIRT1) (Figures 2F and 2G) (Li et al., 2012). SIRT1 knockdown increased acetylated p53 levels (Figure 2G) and significantly increased apoptosis of FLT3-ITD cells (p < 0.05) but not FLT3- WT AML CD34 + cells (Figures 2H and 2I) or normal CD34 + cells (Figure S2G). These results suggest that SIRT1 deacetylates p53 and inhibits p53 signaling to maintain survival of FLT3-ITD AML progenitors. Since SIRT2 is also targeted by TV6 and could also regulate AML cell growth (Dan et al., 2012), we compared effects of anti-sirt1 and anti-sirt2 shrna in FLT3-ITD (MV4-11, Molm-13) and FLT3-WT (OCI-AML3) AML cells (Figures S2H S2J). Whereas SIRT1 knockdown significantly reduced survival and proliferation of the two FLT3-ITD cell lines but not the FLT3-WT line, SIRT2 knockdown did not affect survival and proliferation of FLT3-ITD AML cells, suggesting a less critical role. To further exclude off-target effects of ShSIRT1, we expressed a ShSIRT1-resistant SIRT1 construct (PITA-SIRT1-R) containing five silent mutations in the shrna-targeted region in MV4-11 cells (Li et al., 2012). Expression of SIRT1-R maintained SIRT1 protein levels after ShSIRT1 transduction (Figure S2K) and abrogated the ability of ShSIRT1 to inhibit growth (Figure S2L), suggesting that ShSIRT1 effects are indeed related to SIRT1 knockdown. Pharmacological Inhibition of SIRT1 Inhibits AML Progenitors TV6 significantly enhanced acetylated and total p53 levels in FLT3-ITD AML CD34 + cells (Figure 3A, left panel; Figure S3A). Cells pretreated with nutlin-3 to stabilize p53 levels showed a dose-dependent increase in p53 acetylation after TV6 treatment, with minimal change in total p53 levels, indicating that increased p53 acetylation was independent of increase in total p53 (Figure 4C and Figure S3C). TV6 did not increase acetylated or total p53 in FLT3-WT AML CD34 + cells, whereas nutlin-3 increased total p53 levels (Figure 3A, right panel; Figure S3B). In p53 null K562 cells ectopically expressing WT p53 protein, TV6 increased p53 acetylation without changing total p53 levels (Figure S3D). In contrast, in K562 cells ectopically expressing a p53 mutant with eight potential acetylation sites mutated (p53-8kr), TV6 failed to increase p53 acetylation and resulted in significantly less apoptosis (p < 0.01) (Figure S3E), confirming the importance of p53 acetylation for inhibitory effects of TV6. The proliferation inhibitory effects of TV6 were significantly reduced following SIRT1 knockdown in MV4-11 cells, further indicating the importance of SIRT1 expression for TV6 effects (Figure S3F). TV6 also increased expression of mrna for the p53 target genes p21, BAX, PUMA, NOXA, and DR5 (p < 0.05) in FLT3-ITD AML CD34 + cells (Figure 3B). shrna-mediated knockdown of p53 significantly reduced TV6 or SIRT1 knockdown-induced growth inhibition in FLT3-ITD cells (Figures 3C, 3D, S3G, and S3H), supporting an important role for p53 in SIRT1-mediated effects. TV6 treatment resulted in increased inhibition of survival (Figure 3E) and proliferation (Figure 3F) of FLT3-ITD compared to FLT3- WT AML CD34 + cells and normal CD34 + cells (p < 0.05). Similarly, CB CD34 + cells ectopically expressing FLT3-ITD showed increased sensitivity to TV6-mediated apoptosis (Figure 3G) and growth inhibition (Figure 3H), compared to FLT3-WT- or vector-transduced CD34 + hematopoietic cells. SIRT1 Inhibition Enhances AC220-Mediated Inhibition on FLT3-ITD AML Progenitors Knockdown of FLT3 expression in AML CD34 + cells only modestly decreased SIRT1 expression (Figure 4A). Similarly, the FLT3 TKI AC220, despite effectively inhibiting FLT3 kinase activity in FLT3-ITD AML CD34 + cells (Figures 4B and S4A), only partially decreased SIRT1 and p53 levels without significantly affecting p53 acetylation (Figures 4C and S4A) or p53 target gene expression (Figure S4B). These results indicate that FLT3 inhibition only marginally affects SIRT1-p53 signaling in FLT3-ITD AML cells. TV6 alone did not significantly affect FLT3 signaling, as evidenced by pstat5 expression (Figures 4B and 4C). TV6 increased acetylated p53 expression (Figure 4C) and enhanced p53 target gene expression in AC220-treated FLT3-ITD AML CD34 + cells (Figure 4D). SIRT1 knockdown using shrna also increased acetylated p53 expression in AC220- treated FLT3-ITD AML cells (Figure S4C). AC220-induced apoptosis and growth inhibition of MV4-11 cells was significantly enhanced by combination of AC220 with TV6 (Figures S4D and S4E). Consistent with previous reports (Levis, 2011), AC220 only modestly inhibited survival of primary human FLT3-ITD AML CD34 + cells (Figure 4E). The combination of TV6 (1 mm) with AC220 (20nM) significantly reduced the survival, growth, and colony-forming capacity of FLT3-ITD AML CD34 + cells compared to AC220 or TV6 alone (Figures 4E 4G). ShSIRT1 in combination with AC220 also enhanced inhibition of AML cell survival, growth, and colony formation compared to AC220 alone (Figures 4H 4J). These findings indicate that SIRT1 inhibition enhances targeting of FLT3-ITD AML progenitors in combination with TKI. TV6 Enhances Targeting of FLT3-ITD LSCs In Vivo in Combination with TKI We tested the in vivo effect of TV6, AC220, and the combination of both on primary human cells from three FLT3-ITD AML samples engrafted in NOD scid gamma (NSG) mice (Figure 5A). Following confirmation of engraftment in peripheral blood (PB) (>5% human CD45 + cells), mice were treated with either vehicle (control), AC220 (10 mg/kg, gavage), TV6 (100 mg/kg, (E) Expression of p53 target genes in FLT3-ITD (n = 11) and FLT3-WT (n = 9) AML CD34 + cells 6 hr after irradiation (3 Gy) compared with nonirradiated control cells. (F I) ShSIRT1 and control shrna (Ctrl ShRNA) were expressed in FLT3-ITD AML cells. (F) SIRT1 mrna expression in ShSIRT1- and control-shrna-expressing AML CD34 + cells (n = 3 per group). (G) SIRT1, acetylated p53 (Ac-p53), total p53, and b-actin levels in SIRT1- or control-shrna-transduced MV4-11 AML cells analyzed by western blotting. (H) Apoptosis of FLT3-ITD (n = 5) and FLT3-WT (n = 5) AML CD34 + cells expressing control and SIRT1 shrna. Representative fluorescence-activated cell sorting plots are shown in (I). Results represent mean ± SEM. p < 0.05, p < 0.01, and p < See also Figure S2. Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. 435
6 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells A FLT3-ITD FLT3-WT AML578 Vehicle TV 0.5 TV 1.0 TV 2.0 AML752 BV173 Vehicle TV 1.0 TV 2.0 Ac-p53 p53 AML020 BV173 Vehicle TV 1.0 TV 2.0 Nut 5.0 AML488 BV173 Vehicle TV 1.0 TV 2.0 Nut 5.0 Ac-p53 p53 B C Ctrl ShRNA Sh p53 p53 D Cell growth (% of Vehicle ctrl) CtrlShRNA Shp Tenovin-6 (µm) E ^ ^ Normal FLT3-ITD AML FLT3-WT AML ^ ^^ ^ Tenovin-6 ( M) F Cell growth (% of Vehicle ctrl) Normal FLT3-ITD AML FLT3-WT AML ^^ ^ ^ ^ Tenovin-6 (µm) G GFP FLT3-ITD FLT3-WT H Cell growth (% of Vehicle ctrl) Tenovin-6 ( M) Figure 3. Inhibition of SIRT1 by TV6 Reduces FLT3-ITD AML CD34 + Cell Growth and Survival by Activating p53 Signaling (A) Western blotting for acetylated p53 (K382), total p53, and b-actin in FLT3-ITD (left panel) or FLT3-WT (right panel) AML CD34 + cells treated with TV6 or nutlin-3 at indicated doses (mm) for 8 hr. Results from two representative FLT3-ITD samples (out of five) and two representative FLT3-WT samples (out of five) are shown. BV173 cells were included to identify p53 bands. (B) p53 target gene expression in TV6 compared with vehicle-treated FLT3-ITD AML CD34 + cells analyzed by real-time quantitative RT-PCR (n = 7). (C and D) p53 shrna (Sh p53) or control shrna (Ctrl ShRNA) were expressed in MV4-11 cells. (C) Western blotting for p53. (D) Growth of cells exposed to TV6 for 72 hr analyzed by CellTiter-Glo. p < 0.05, p < 0.01, p < 0.001, compared with control shrna. (legend continued on next page) 436 Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc.
7 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells intraperitoneal), or the combination for 4 weeks, after which human leukemia cell engraftment in BM and spleen was analyzed by flow cytometry. Engrafted leukemic cells were CD45 + and CD33 + with variable CD14 and CD15 expression and expressed the FLT3-ITD gene (data not shown). TV6 or AC220 treatment reduced the percentage and total number of human leukemic cells in murine BM (Figures 5B, 5C, and S5A). The combination of TV6 and AC220 significantly reduced AML cell engraftment compared with AC220 alone (Figures 5B and S5A). Human CD34 + cells in BM were significantly reduced in mice treated with TV6 or AC220 alone, with further reduction seen in the combination (Figure S5B). We observed treatment effects with the combination on the percentage and number of AML cell in the spleen and PB that were similar to those in BM cells of NSG mice (Figures S5C and S5D; data not shown). Secondary transplantation of BM cells from mice receiving combination treatment resulted in significantly reduced engraftment in PB at 8 and 16 weeks (Figure 5D) and in BM at 16 weeks (Figures 5E and S5E) compared to AC220 treatment alone, indicating reduced LSC capacity of residual cells. We also studied another sample from a chemotherapy-refractory AML patient using molecular imaging of primary patient xenografts with fluorescently labeled monoclonal antibodies, as described previously (McCormack et al., 2013). We observed significant differences between combination and single-arm groups after 4 weeks of therapy (combination versus TV6, p = ; combination versus AC220, p = 0.015) (Figures 5F and 5G). AC220 or combination treatment also resulted in significant reduction in splenomegaly (Figure S5F). Mice treated with TV6 demonstrated significantly improved survival after discontinuation of treatment compared to control mice (p = 0.02), and the combination of TV6 and AC220 further increased survival compared to AC220 alone (p = ) (Figure 5H). We also observed significantly enhanced survival after secondary transplantation of BM from mice receiving combination treatment (versus AC220, p = 0.03, n = 3) (Figure 5I). The combination of AC220 and TV6 significantly improved survival compared with AC220 (p = ) and standard chemotherapy (p = 0.006) (Figure S5G). These results show that the combination of AC220 and TV6 enhances targeting of primitive human AML LSC in vivo. Cross-Regulation of SIRT1 and MYC in FLT3-ITD AML Cells Since previous studies have shown an association between c-myc and increased SIRT1 protein expression (Menssen et al., 2012), we evaluated the role of c-myc in increased SIRT1 expression in FLT3-ITD AML. Analysis of two large AML gene expression data sets available through the Gene Expression Omnibus (GSE1159: FLT3-ITD AML [n = 73] versus FLT3- WT AML [n = 178] [Valk et al., 2004] and GSE8043: FLT3-ITD AML [n = 120] versus FLT3-WT AML [n = 291] [Bullinger et al., 2008]) showed significant enrichment of a c-myc-related gene set in FLT3-ITD compared to FLT3-WT AML samples (Figure 6A). c-myc activity in AML cells was measured using a lentivirusexpressed c-myc reporter expressing firefly luciferase under control of a minimal CMV promoter and tandem repeats of the E box transcriptional response element. We confirmed that shrna-mediated inhibition of c-myc expression reduced luciferase activity in MV4-11 cells (Figure S6A). AC220 treatment inhibited reporter activity (Figure 6B), suggesting that c-myc is regulated, at least in part, by FLT3-ITD kinase activity, which is consistent with previous reports (Kim et al., 2007). FLT3-ITD kinase may regulate c-myc through STAT5-induced enhancement of PIM kinases (Choudhary et al., 2009), which can modulate c-myc stability and activity via phosphorylation (van der Lugt et al., 1995). This is supported by the observation that FLT3-ITD CD34 + cells showed higher PIM activity compared to cells expressing FLT3-WT, indicated by increased expression of the PIM targets including p-bad (Ser112), p-4ebp1 (Thr37/46), and p-c-myc (Ser62) (Figure 6C); and by the observation that sirna-mediated inhibition of PIM1, but not PIM2, expression resulted in significantly decreased p-c-myc (Ser62), c-myc, and SIRT1 expression in MV4-11 cells (Figure 6D). AC220 treatment reduced c-myc Ser62 phosphorylation and total c-myc levels in MV4-11 cells (Figure S6B) and also reduced c-myc levels in FLT3-ITD AML CD34 + cells (Figures 6E and S6B). To evaluate the role of c-myc in regulating SIRT1 expression, we inhibited c-myc expression in FLT3-ITD AML cells using c-myc shrnas (Sh c-myc-1 and Sh c-myc-2). c-myc knockdown reduced SIRT1 protein expression in MV4-11 cells (Figure 6F). Similarly c-myc sirna reduced SIRT1 protein levels in FLT3-ITD AML CD34 + cells (Figure 6G). Since SIRT1 protein half-life is increased in FLT3-ITD cells, we evaluated whether c-myc regulated SIRT1 protein stability. We observed that shrna-mediated inhibition of c-myc expression led to accelerated reduction in SIRT1 levels in AML cells following CHX treatment (Figure 6H). Proteasome inhibition by PS341 enhanced SIRT1 expression in c-myc knockdown cells (Figure S6C). We next evaluated the role of c-myc in regulating SIRT1 ubiquitination by cotransfecting hemagglutinin (HA)-ubiquitin, Flag-SIRT1, and c-myc into human embryonic kidney 293 (HEK293) cells; immunoprecipitating SIRT1 using an anti-flag antibody; and detecting ubiquitination by western blotting with an anti-ha antibody (Menssen et al., 2012). Coexpression of c-myc significantly inhibited polyubiquitination of SIRT1 (Figure 6I). These findings indicate that c-myc enhances SIRT1 expression by reducing ubiquitination and increasing protein stability. We further assessed the reciprocal role of SIRT1 in regulating c-myc in FLT3-ITD AML cells. SIRT1-mediated acetylation of c-myc has been associated with increased stability and activity via altered proteasomal degradation or indirectly by altered (E and F) FLT3-ITD AML (n = 11), FLT3-WT AML (n = 11), and normal PBSC (n = 4) CD34 + cells were exposed to TV6 for 48 hr. (E) Cell survival was analyzed by annexin V labeling. (F) Cell growth was evaluated using the CellTiter Glo assay. p < 0.01, and p < 0.001, compared with untreated controls; ^p < 0.05, and ^^p < 0.01, compared with normal PBSCs. (G and H) CB CD34 + cells transduced with vectors expressing GFP alone, FLT3-ITD, or FLT3-WT (n = 5 each) were exposed to TV6 for 48 hr. (G) Cell survival was analyzed by annexin V labeling. (H) Cell growth was evaluated using the CellTiter Glo assay. p < 0.05, p < 0.01, p < 0.001, compared with GFP-transduced cells. Error bars represent mean ± SEM. See also Figure S3. Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. 437
8 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells A AML866 Ctrl SiRNA SiFLT3-1 SiFLT3-2 FLT3 AML1074 Ctrl SiRNA SiFLT3-1 FLT3 B IP: FLT3 AML 866 Vehicle AC220 TV6 AC220+TV6 py FLT3 SIRT1 SIRT1 Input IB pstat5 STAT5 C Vehicle AC220 TV6 AC220+TV6 AML 1074 Nutlin-3 Vehicle AC220 TV6 AC220+TV6 Ac-p53 p53 D SIRT1 pstat5 STAT5 E Cell survival (% of Vehicle ctrl) Vehicle Control TV 1µM TV 2µM AC220 Cell growth (% of Vehicle ctrl) F Vehicle AC220 Control TV 1µM TV 2µM Colonies/ cells G Vehicle AC220 Control TV 1µM TV 2µM H I J Cell survival (% of Vehicle ctrl) Vehicle Ctrl ShRNA ShSIRT1 AC Vehicle AC220 Ctrl ShRNA ShSIRT1 Figure 4. SIRT1 Inhibition Enhances AC220-Mediated Inhibition of FLT3-ITD AML CD34+ Cells (A) Western blotting for FLT3, SIRT1, and b-actin in FLT3-ITD AML cells transfected with FLT3 sirna and control sirna (Ctrl SiRNA). (B) FLT3 immunoprecipitates from FLT3-ITD AML CD34 + cells exposed to TV6 (1 mm), AC220 (20nM), or the combination for 8 hr were analyzed for FLT3 and phosphotyrosine (py) by western blotting. Input lysates were analyzed for p-stat5, STAT5, and b-actin. (C) FLT-ITD AML CD34 + cells with or without prior nutlin-3 (5 mm) exposure for 2 hr were exposed to TV6 (1 mm), AC220 (20nM), or the combination for 8 hr and analyzed for acetylated p53 (K382), total p53, SIRT1, p-stat5, STAT5, and b-actin by western blotting. (D) p53 target gene expression in FLT3-ITD AML CD34 + cells treated with TV6 and AC220 compared with AC220 alone (n = 7) (E and F) FLT3-ITD AML CD34 + (n = 11) cells were treated with TV6, AC220, or the combination for 48 hr. (E) Cell survival was analyzed by annexin V labeling. (F) Cell growth was evaluated using the CellTiter-Glo assay. 438 Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. (legend continued on next page)
9 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells protein phosphorylation (Marshall et al., 2011; Menssen et al., 2012). SIRT1 knockdown or TV6 treatment increased c-myc acetylation in FLT3-ITD AML cells, more readily detectable after inhibition of class I and II histone deacetylase activity by Trichostatin A (Figures S6D and S6E) (Vaziri et al., 2001). SIRT1 knockdown or TV6 also reduced c-myc Ser62 phosphorylation in FLT3-ITD AML CD34 + cells, with further reduction seen in combination with AC220 (Figures S6B and S6F). Both ShSIRT1 and TV6 reduced c-myc protein levels (Figures 6E, S6B, S6D, and S6F) and c-myc transcriptional activity (Figure S6G; data not shown). Consistent with a potential role for SIRT1 in regulation of c-myc stability, TV6 reduced the half-life of c-myc protein in CHX-treated cells while increasing the half-life of p53 (Figure S6H). The combination of AC220 with ShSIRT1 or TV6 further reduced c-myc expression and activity (Figures 6E, S6B, S6F, and S6G). These observations suggest that SIRT1-mediated deacetylation leads to enhanced c-myc protein stability, expression, and activity. Our results support the existence of a positive feedback loop in which c-myc and SIRT1 increase each other s expression and activity in FLT3-ITD AML cells, which could contribute to partial maintenance of MYC activity after AC220 treatment. USP22 Interacts with SIRT1 and Maintains Its Protein Expression The USP22 deubiquitinase has been reported to stabilize SIRT1 by removing polyubiquitin chains conjugated on to SIRT1 (Lin et al., 2012). USP22 mrna expression was significantly higher in FLT3-ITD compared to FLT3-WT AML CD34 + cells (Figure 7A). Lentiviral expression of FLT3-ITD in CB CD34 + cells resulted in increased USP22 expression compared to FLT3-WT or vector controls (Figure 7B). Knockdown of endogenous USP22 in MV4-11 cells decreased SIRT1 protein levels (Figure 7C) and significantly reduced cell growth (Figure S7A). The proteasome inhibitor MG132 or PS341 restored SIRT1 expression in USP22 knockdown cells (Figure 7C). Ectopic USP22 expression increased SIRT1 protein levels (Figure 7D) without significantly affecting SIRT1 mrna level (data not shown). The half-life of SIRT1 protein was increased in USP22-overexpressing cells compared to control cells (Figure 7E). AC220 treatment only marginally affected USP22 protein expression in MV4-11 cells (Figures S7B and S7C). USP22 coimmunoprecipitated with SIRT1 (Figure S7B), and this association was not significantly affected by AC220 treatment (Figure S7B). These results indicate that USP22 directly interacts with SIRT1 and contributes to stabilization of SIRT1 protein in FLT3-ITD AML cells. We further evaluated the contribution of USP22 to c-mycmediated reduction in SIRT1 ubiquitination and degradation. Knockdown of c-myc expression reduced USP22 protein levels in MV4-11 cells (Figure 7F), whereas ectopic expression of c-myc in CD34 + or HEK293 cells increased USP22 protein levels (Figures 7G and S7D). It is interesting that c-myc overexpression or knockdown did not affect USP22 mrna levels (Figures S7E and S7F), indicating that MYC may regulate USP22 posttranscriptionally. It is also interesting that downregulation of SIRT1 expression following c-myc knockdown was prevented, at least in part, by USP22 overexpression (Figure 7H). In addition, USP22 knockdown prevented c-myc-mediated reduction of SIRT1 ubiquitination (Figure 7I) and increase in SIRT1 expression (Figure S7G). These results support a role for USP22 in MYC-mediated increase in SIRT1 protein stabilization. DISCUSSION We have found that AML stem/progenitor cells demonstrate varying levels of SIRT1 overexpression and sensitivity to SIRT1 inhibitor treatment. The FLT3-ITD mutation was a major determinant of sensitivity to SIRT1 inhibition. FLT3-ITD AML stem/ progenitor cells demonstrated high SIRT1 protein levels and sensitivity to SIRT1 inhibition both in vitro and in vivo. The effects of SIRT1 inhibition on FLT3-ITD AML cells were related to increased p53 acetylation and transcriptional activity. These results indicate an important role for SIRT1-mediated downregulation of p53 in growth and maintenance of FLT3-ITD AML LSCs. SIRT1 inhibition only modestly inhibited proliferation of normal CD34 + cells without significantly altering their survival, indicating relative selectivity for AML LSCs. The combination of SIRT1 inhibition with the FLT3 TKI AC220 significantly enhanced targeting of FLT3-ITD AML progenitors in vitro and FLT3-ITD AML LSCs in vivo compared to AC220 alone, indicating that SIRT1 activation contributes to therapeutic resistance of FLT3- ITD AML LSCs. Increased SIRT1 protein expression in FLT3-ITD AML stem/ progenitor cells was related to increased protein half-life. AC220 treatment partially inhibited SIRT1 expression in FLT3- ITD + cells. Our studies indicate a potential role for c-myc in SIRT1 regulation in FLT3-ITD cells. Our analysis of public microarray data sets show significant enrichment of c-myc-related genes in FLT3-ITD compared to FLT3-WT AML cells, confirming previous reports of increased c-myc in FLT3-ITD AML cells (Kim et al., 2007). c-myc can potentially modulate SIRT1 activity by increasing NAD+ levels through NAMPT gene induction or by sequestering the SIRT1 inhibitor DBC-1 (Menssen et al., 2012). We identified a mechanism of c-myc regulation of SIRT1 protein expression by reduction of SIRT1 polyubiquitination and proteasomal degradation. AC220 reduced c-myc Ser62 phosphorylation and expression in FLT3-ITD cells, indicating that increased c-myc was at least partially dependent on FLT3 kinase activity. Our results suggest that enhanced c-myc phosphorylation and expression is related to activation of the PIM-1 kinase downstream of FLT3-ITD (Choudhary et al., 2009; Yang et al., 2012). SIRT1 is known to interact with and deacetylate c-myc, but the functional consequences of c-myc deacetylation are unclear. Several groups have reported that c-myc or N-MYC (G) FLT3-ITD AML CD34 + cells (n = 4) exposed to TV-6 or AC220 and combination were plated in methylcellulose progenitor culture, and erythrocytic and granulocytic colonies were enumerated after 14 days. (H J) FLT3-ITD AML (n = 4) CD34 + cells transduced with control shrna (Ctrl ShRNA) and ShSIRT1 vectors were cultured with or without AC220 (20 nm) for 72 hr. (H) Cell survival was analyzed by annexin V labeling. (I) Cell growth was evaluated using the CellTiter-Glo assay. (J) Clonogenic assays were performed, and erythrocytic and granulocytic colonies were enumerated after 14 days. Cumulative results represent the mean ± SEM. p < 0.05 and p < 0.01, compared with indicated groups. See also Figure S4. Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. 439
10 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells A FLT3/ITD+ AML cells Transplant to NSG mice Detectable AML cells in PB Control, AC220, TV6, Combination or Chemotherapy In vivo administrate 4wks Human cell engraftment Imaging Survival Second transplant B C Control TV-6 D F %human CD45+ cells in PB % human CD45+ cell in BM weeks AML 866 E AML 866 AML 866 Control AC220 TV-6 AC220+TV-6 Control AC220 AC220+TV6 16 weeks Control AC220 AC220+TV6 %human CD45+ cells in BM Control AC220 AC220+TV Control AC220 TV6 AC220+TV6 CD33 No. of Human CD45+ cells in BM G AC Control CD45 AC220 AC220+TV6 TV6 AC220+TV6 H I (legend on next page) 440 Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc.
11 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells deacetylation by SIRT1 leads to increased stability and activity through multiple mechanisms, including altered ubiquitination, altered phosphorylation via reduced MKP3 expression (Marshall et al., 2011), and increased c-myc-max heterodimerization (Menssen et al., 2012), whereas one study found that SIRT1 reduced c-myc stability and function (Yuan et al., 2009). We found that SIRT1 inhibition resulted in increased c-myc acetylation, reduced c-myc phosphorylation, enhanced c-myc degradation, and reduced c-myc expression and activity in FLT3-ITD cells. Our results suggest that a feed-forward SIRT1-c-MYC activation loop in FLT3-ITD AML, with reciprocal activation of SIRT1 and c-myc by each other, may contribute to the maintenance of c-myc activity in TKI-treated cells. The USP22 deubiquitinase is reported to complex with SIRT1 and act as a SIRT1 deubiquitinase. USP22 is part of a deathfrom-cancer gene signature (Zhang et al., 2008) associated with poor prognosis in a variety of cancers (Schrecengost et al., 2014). To date, it is known to be part of the SAGA complex required for c-myc-related transformation (Zhang et al., 2008). USP22 expression was significantly increased in FLT3-ITD AML CD34 + cells and was marginally affected by blocking FLT3 kinase activity. We show an important role for c-myc in upregulation of USP22 protein expression in FLT3-ITD cells. However, although USP22 mrna expression was increased in FLT3-ITD AML cells, it was not increased following MYC overexpression, indicating that additional MYC-independent mechanisms may contribute to increased USP22 transcription. Previous reports showed divergent effects of USP22 on SIRT1 levels (Lin et al., 2012; Armour et al., 2013). We show that USP22 directly interacts with SIRT1 in FLT3-ITD AML cells in a FLT3-kinase-independent manner. USP22 knockdown reduced SIRT1 expression in FLT3-ITD AML cells, whereas USP22 overexpression increased SIRT1 levels by increasing protein stability, indicating that USP22 is an important positive regulator of SIRT1 in FLT3-ITD cells. We further show that USP22 plays an important role in mediating c-myc-driven inhibition of SIRT1 ubiquitination and degradation. These findings elucidate a mechanistic connection between FLT3, c-myc, and USP22 in the regulation of SIRT1 in FLT3-ITD AML cells. SIRT1 overexpression was associated with down-modulation of p53 activity in FLT3-ITD AML CD34 + cells. SIRT1 can negatively regulate p53 by deacetylating several lysine sites (Vaziri et al., 2001; Brooks and Gu, 2009). Acetylation of p53 modulates protein stability and transcriptional activity independent of phosphorylation status (Tang et al., 2008). It is interesting that SIRT1 inhibition did not effectively induce p53 activation or inhibit FLT3- WT AML cells, despite their having nonmutated p53, whereas MDM2 inhibitors such as nutlin-3 could activate p53 in FLT3- WT AML cells and even normal cells (Shangary et al., 2008). SIRT1 overexpression may be necessary to inhibit p53 activity and allow maintenance of AML cells in the setting of FLT3-ITDinduced oncogenic stress, similar to its potential role as an adaptive response to BCR-ABL-related oncogenic stress in CML cells (Li et al., 2012). In contrast SIRT1 activation was not a feature of AML cells with FLT3-TKD mutations, which may have different signaling and transformative properties compared to FLT3-ITD mutations (Kindler et al., 2010). Further studies are required to determine the spectrum and nature of oncogenic stimuli that induce SIRT1 activation and sensitivity to SIRT1 inhibition. In addition to p53, SIRT1 can also deacetylate several other proteins that regulate cell growth and survival (Brooks and Gu, 2009). Indeed, our studies show the importance of SIRT1 regulation of c-myc in FLT3-ITD AML cells. The role of additional SIRT1 targets such as Ku70 (Yuan et al., 2012; Wang et al., 2013) in LSC transformation and drug resistance requires further investigation. Our results show the importance of identifying subgroups of patients that could benefit from SIRT1 inhibitor treatment, given the heterogeneous genetic background of leukemias such as AML. Our results are consistent with those of Bradbury et al. (2005), who observed increased SIRT1 expression in most AML samples. In contrast, Dan et al. (2012) found that SIRT1 mrna was unchanged or even slightly diminished in AML blasts, compared with normal CD34 +, cells but did not determine association with AML subtypes. In addition, these studies did not evaluate protein levels, which we show here to be critically important. Another study from Sasca et al. (2014), published while this paper was under review, also reported SIRT1 protein overexpression in FLT3-ITD AML samples. Leukemic blasts from murine AML models based on coexpression of MLL-AF9 or AML1-ETO with FLT3-ITD showed dependence on SIRT1 activity. Although in vivo analysis of primary human AML LSC was not performed, the results of this study are consistent with our own and support SIRT1 inhibition as an attractive therapeutic Figure 5. SIRT1 Inhibition Reduces In Vivo Growth of Primary AML Stem Cells in Immunodeficient Mice (A) T-depleted primary human AML cells were injected into NSG mice ( cells per mouse). After engraftment was confirmed, mice were treated for 4 weeks with AC220 (10 mg/kg/day, oral gavage), TV6 (100 mg/kg/day intraperitoneally), the combination of TV6 and AC220, or vehicle (controls) (n = 7 or 8 mice per group). Engraftment of human cells was analyzed by flow cytometry. Imaging, secondary transplantation, and survival analysis were also performed. (B) The percentage of human AML CD45 + cells from three different samples (AML 866, AML 1074, and AML 1107) in the BM of treated mice. (C) Representative results for CD45 and CD33 expression from AML 866. (D) The percentage of human AML CD45 + cells (AML866) in blood of secondary recipients of BM cells from treated mice ( cells per mouse, n = 6 per group) at 8 and 16 weeks. (E) The percentage and number of human AML CD45 + cells (AML 866) in the BM of secondary recipient mice at 16 weeks. (F) CD34 + cells from a chemotherapy-resistant FLT3-ITD AML patient were injected into NSG mice and treated for 4 weeks (n = 10 per group) with vehicle, AC220, TV6, or the combination. Subsequently, three mice from each group were injected with fluorescently labeled monoclonal antibodies and imaged by time domain optical imaging. PC, photon counts. (G) Quantitative results from bioimaging studies analyzed by Optiview software (Version 3.03, ART). Results represent mean ± SEM for three mice. p < 0.05, p < 0.01, and p < 0.001, compared with combination. (H) Survival of mice from (F) after discontinuation of treatment. PC, photon counts. (I) Survival after transplantation of BM cells from treated mice (F) into secondary recipients. For (B), (D), and (E), results represent the mean ± SEM. p < 0.05, p < 0.01, and p < 0.001; comparison has been indicated in the graph. See also Figure S5. Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. 441
12 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells A Valk et al. NES=2.04; FDR q-val=0.006 Bullinger et al. NES=1.75. FDR q-val =0.096 B Luciferase expression Normalized to Renilla AC220 (nm) C Mock CD34 + FLT3-ITDCD34 + FLT3-WT CD34 + p-flt3 (Y589/Y591) FLT3 SIRT1 p-bad (Ser112) Bad c-myc p-c-myc (Ser62) p-4ebp1 (Thr37/46) 4EBP1 D SIRT1 p-c-myc (Ser62) c-myc PIM-1 PIM-2 E AML 866 c-myc p-c-myc (Ser62) p-stat5 STAT5 SIRT1 G F AML 865 Ctrl ShRNA Sh c-myc-1 Sh c-myc-2 Ctrl SiRNA Si c-myc c-myc SIRT1 c-myc SIRT1 H SIRT1 Ctrl ShRNA % SIRT1 level at 0hr +CHX Sh c-myc I MYC Flag-SIRT IP Flag IB HA IP Flag IB Flag IP MYC Flag-SIRT HA-Ub SIRT1 Input c-myc Flag- SIRT1 Figure 6. Cross-Regulation of SIRT1 and MYC in FLT3-ITD AML Cells (A) Gene set enrichment analysis showing enrichment of a MYC gene set (Schuhmacher et al., 2001) in FLT3-ITD versus FLT3-WT AML cells in two large microarray data sets. Normalized enrichment score (NES) and statistical significance/false discovery rate q value (FDR q val) are indicated. (B) MV4-11 cells cotransfected with a c-myc firefly luciferase reporter and a Renilla luciferase plasmid were exposed with AC220 or vehicle control for 24 hr and assayed for luciferase normalized to Renilla activity. The mean value of triplicate experiments is shown. (C) Western blotting for SIRT1, FLT3, pflt3 (Y589/Y591), p-bad (ser112), Bad, p-4ebp1 (Thr37/46), 4EBP1, p-c-myc (ser62), c-myc, and b-actin in CB CD34 + cells transduced with FUGW, FUGW/FLT3-ITD, or FUGW/FLT3-WT vectors. (legend continued on next page) 442 Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc.
13 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells strategy in FLT3-ITD AML. The selectivity of SIRT1 inhibition toward FLT3-ITD AML cells is expected to yield a high therapeutic index toward leukemic cells. Small molecule SIRT1 inhibitors are being investigated as potential anticancer treatments (Alcaín and Villalba, 2009). Although the pharmacological properties of TV6 may not allow it to be a suitable candidate for drug development, it is a useful compound to study potential therapeutic effects of SIRT1 inhibition in cancer. Our observations support further investigation of Tenovin derivatives and other SIRT1 inhibitors to target FLT3-ITD AML, in combination with TKI treatment. In conclusion, this study improves our understanding of mechanisms underlying the function of SIRT1 as an oncogene versus tumor suppressor gene in specific genetic contexts; elucidates a mechanistic connection between FLT3, c-myc, and USP22 in regulation of SIRT1 and p53, and supports further development of SIRT1 inhibition as a potential approach for more selective targeting of malignant stem cells in selected cancers. EXPERIMENTAL PROCEDURES Samples and Materials PB or BM samples were obtained from AML patients with active disease at diagnosis or at relapse in two centers. Patient characteristics are shown in Tables S1 and S2. Risk groups were based on NCCN criteria (Table S3) (O Donnell et al., 2012). Normal PBSCs were obtained from allogeneic transplant donors. CB was obtained from StemCyte. All subjects signed informed consent forms. Sample acquisition was approved by the City of Hope (COH) Institutional Review Board or the regional Ethics Committee (REK Vest; Norwegian Ministry of Education and Research) in accordance with the Declaration of Helsinki. Mononuclear cells were isolated by Ficoll-Hypaque (Sigma Diagnostics) centrifugation. CD34 + cells or CD3 cells were selected using immunomagnetic columns (Miltenyi Biotec). Details of cell lines, drugs, and DNA constructs are provided in the Supplemental Experimental Procedures. Cell Transduction and Transfection CD34 + cells were transduced with lentivirus vectors expressing shrna or FLT3 constructs (provided by Dr. Linzhao Cheng, Johns Hopkins University). MV4-11 cells were transduced with PITA-SIRT1-R vectors. Human AML CD34 + cells or MV4-11 cells were transfected with sirnas to FLT3, c-myc, BAX, PIM-1, PIM-2, and control sirnas (Applied BioSystems/Ambion) using the Amaxa Nucleofector. Details are provided in the Supplemental Experimental Procedures. Intracellular Staining for SIRT1 CD34 + cells were labeled with CD34-PeCy7, Lin-APC-Cy7 (including CD2, CD3, CD7, CD10, CD19, and CD235a), and CD38-APC or CD38-PE, CD90- PercpCy5.5, CD123-APC, or CD47-APC (ebioscience) antibodies; fixed and permeabilized (Cytofix/Cytoperm Kit, Beckman Coulter); labeled with rabbit anti-human SIRT1 (E1104-1, Millipore) and Alexa Fluor 488-conjugated goat anti-rabbit antibodies (Invitrogen); and analyzed by flow cytometry. Data were analyzed using FlowJo software (version 8.5.2; TreeStar). Analysis of Viability and Growth Cells were labeled with annexin V/DAPI, and the percentage of surviving cells (Annexin V DAPI ) was analyzed. Cell viability was also assessed by Hoechst staining (Invitrogen). Cell growth was evaluated using the CellTiter-Glo assay (Promega). Details are provided in the Supplemental Experimental Procedures. In Vivo Treatment of AML-Engrafted Immunodeficient Mice T-cell-depleted or CD34 + selected human AML cells were transplanted into sublethally irradiated (300 cgy) 8-week-old NOD.Cg-Prkdcscid IL2rgtm1Wjl/ SzJ mice (NSG mice, Jackson Laboratory). After engraftment was confirmed, mice were treated with AC220, TV6, the combination, or vehicle for 4 weeks or with chemotherapy as described elsewhere (McCormack et al., 2013). BM, PB, and spleen cells were analyzed for engraftment by flow cytometry analysis of human CD45 + cells. A group of mice was followed for survival after discontinuation of treatment. BM cells from primary recipients were used for secondary transplantation into irradiated NSG mice ( cells per mouse), and engraftment was analyzed after 16 weeks. The procedure for evaluation of engraftment, the luciferase-expressing MOLM-13 xenograft model, and optical imaging are described elsewhere (McCormack et al., 2012) details of which can also be found in the Supplemental Experimental Procedures. Mouse care and experimental procedures were performed in accordance with approved protocols from the Institutional Animal Care and Use Committee at the COH National Medical Center and Norwegian Animal Research Authority in accordance with The European Convention for the Protection of Vertebrates Used for Scientific Purposes. Luciferase Reporter Assays MV4-11 cells were transduced with c-myc reporter (Cignal Myc Lentivirus Reporter, QIAGEN) and selected for Puro resistance. Reporter activity was measured by the Dual-Glo Luciferase Assay System (Promega) and normalized to EF1a-renilla luciferase. Real-Time Quantitative PCR Analysis Real-time quantitative PCR analysis was performed with primers and probes for p21, BAX, PUMA, NOXA, DR5, USP22, and SIRT1. Details are provided in the Supplemental Experimental Procedures. Immunoprecipitation and Western Blotting Antibodies used for immunoprecipitation were conjugated with Flag/M2 beads (Sigma-Aldrich) or protein A/G beads using an antibody crosslinking kit (Pierce Biotechnology). Western blotting was performed as described in the Supplemental Experimental Procedures. Statistics Data obtained from independent experiments were reported as the mean ± SEM. Student s t test, Mann-Whitney test, and two-way ANOVA with multiple testing were performed to determine statistical significance as appropriate. p < 0.05 was considered statistically significant. (D) Western blotting for PIM-1, PIM-2, SIRT, p-c-myc (Ser62), c-myc, and b-actin in control sirna (Ctrl SiRNA)-, PIM-1- or PIM-2-siRNA-transfected MV4-11 cells. (E) Cells cultured with TV6 (1 mm), AC220 (20 nm), or the combination for 8 hr were analyzed by western blotting for SIRT1, c-myc, p-c-myc (Ser62), b-actin, p-stat5, and STAT5. (F) Western blotting for SIRT1 and c-myc levels in c-myc-shrna-expressing MV4-11 cells. (G) Western blotting for SIRT1 and c-myc levels following sirna-mediated c-myc knockdown in FLT3-ITD AML CD34 + cells. (H) Western blotting for SIRT1 following CHX treatment of c-myc knockdown or control MV4-11 cells (upper panel). The lower panel shows results of densitometry quantitation (n = 3). (I) HEK293 cells were cotransfected with Flag-SIRT1 or vector control, c-myc or empty vector control, and HA-tagged ubiquitin (Ub) as indicated; then lysates were immunoprecipitated (IP) for Flag and western blotted for SIRT1, c-myc, and HA. The right panel shows input levels based on western blotting of lysates for SIRT1, c-myc, and b-actin. IB, immunoblotting. For (B) and (H), results represent mean ± SEM from three independent experiments. p < 0.05, p < 0.01, and p < 0.001; comparison has been made to no drug control (B) or control shrna (H). See also Figure S6. Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. 443
14 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells A USP22 mrna expression (Relative to B2M) FLT3-ITD AML FLT3-WT AML B Mock CD34 + FLT3-ITD CD34 + FLT3-WT CD34 + pflt3 FLT3 SIRT1 USP22 C Ctrl ShRNA ShUSP22-1 ShUSP22-2 Ctrl ShRNA ShUSP22-1 ShUSP22-1 PS431 ShUSP22-1 MG132 USP22 SIRT1 D Flag-GFP Flag-USP22 SIRT1 USP22 FLAG-USP22 E Flag-GFP 0hr 2hr 4hr 8hr +CHX Flag-USP22 10hr 0hr 2hr 4hr 8hr 10hr FLAG-USP22 FLAG-GFP F FLAG-GFP c-myc USP22 G c-myc USP22 SIRT1 SIRT1 H Flag-GFP Flag-USP22 c-myc USP22 SIRT1 I MYC ShUSP22 Flag-SIRT1 IP MYC ShUSP22 Flag-SIRT1 Input IP Flag IB HA IP Flag IB Flag HA-Ub SIRT1 c-myc USP22 Flag-SIRT1 Figure 7. USP22 Interacts with SIRT1 and Maintains Its Protein Expression (A) USP22 mrna levels in FLT3-ITD (n = 12) and FLT3-WT (n = 12) AML CD34 + cells. (B) Western blotting for SIRT1, FLT3, pflt3 (Y589/Y591), USP22, and b-actin in CB CD34 + cells transduced with FUGW, FUGW/FLT3-ITD, or FUGW/FLT3-WT vectors. (C) Western blotting for SIRT1, USP22, and b-actin expression in MV4-11 cells expressing shrna to USP22 or control shrna (Ctrl ShRNA) (left panel). Western blotting for SIRT1, USP22, and b-actin was also performed on shrna-expressing cells incubated with MG132 (10 mm) or PS341 (50 nm) for 4 hr (right panel). (D) Western blotting for SIRT1, USP22, Flag, and b-actin in MV4-11 cells transfected with Flag-GFP or Flag-USP22. (legend continued on next page) 444 Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc.
15 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells SUPPLEMENTAL INFORMATION Supplemental Information for this article includes Supplemental Experimental Procedures, seven figures, and three tables and can be found with this article online at ACKNOWLEDGMENTS This work was supported by National Cancer Institute grants R01 CA95684, P30CA033572, and K99CA184411; the Leukemia and Lymphoma Society; the Bergen Research Foundation; the Norwegian Cancer Society; and the Western Norway Regional Health Authority. We thank StemCyte for CB samples. We thank Linda Seymour and Vincent Paterno for sample acquisition; Xiwei Wu for microarray analysis; the COH Analytical Cytometry Core Facility; and Line Wergeland, Michael Sæterhaug, Sahba Shafiee, Zina Fandalyuk, and Ingvild Haaland for assistance in cell viability assays and preclinical studies. All imaging was conducted at the Molecular Imaging Center, the Department of Biomedicine, University of Bergen. Received: February 4, 2014 Revised: July 11, 2014 Accepted: August 8, 2014 Published: October 2, 2014 REFERENCES Alcaín, F.J., and Villalba, J.M. (2009). Sirtuin inhibitors. Expert Opin. Ther. Pat. 19, Armour, S.M., Bennett, E.J., Braun, C.R., Zhang, X.Y., McMahon, S.B., Gygi, S.P., Harper, J.W., and Sinclair, D.A. (2013). A high-confidence interaction map identifies SIRT1 as a mediator of acetylation of USP22 and the SAGA coactivator complex. Mol. Cell. Biol. 33, Bradbury, C.A., Khanim, F.L., Hayden, R., Bunce, C.M., White, D.A., Drayson, M.T., Craddock, C., and Turner, B.M. (2005). Histone deacetylases in acute myeloid leukaemia show a distinctive pattern of expression that changes selectively in response to deacetylase inhibitors. Leukemia 19, Brooks, C.L., and Gu, W. (2009). How does SIRT1 affect metabolism, senescence and cancer? Nat. Rev. Cancer 9, Bullinger, L., Döhner, K., Kranz, R., Stirner, C., Fröhling, S., Scholl, C., Kim, Y.H., Schlenk, R.F., Tibshirani, R., Döhner, H., and Pollack, J.R. (2008). An FLT3 gene-expression signature predicts clinical outcome in normal karyotype AML. Blood 111, Choudhary, C., Olsen, J.V., Brandts, C., Cox, J., Reddy, P.N., Böhmer, F.D., Gerke, V., Schmidt-Arras, D.E., Berdel, W.E., Müller-Tidow, C., et al. (2009). Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol. Cell 36, Chu, S.H., Heiser, D., Li, L., Kaplan, I., Collector, M., Huso, D., Sharkis, S.J., Civin, C., and Small, D. (2012). FLT3-ITD knockin impairs hematopoietic stem cell quiescence/homeostasis, leading to myeloproliferative neoplasm. Cell Stem Cell 11, Dan, L., Klimenkova, O., Klimiankou, M., Klusman, J.H., van den Heuvel- Eibrink, M.M., Reinhardt, D., Welte, K., and Skokowa, J. (2012). The role of sirtuin 2 activation by nicotinamide phosphoribosyltransferase in the aberrant proliferation and survival of myeloid leukemia cells. Haematologica 97, Eppert, K., Takenaka, K., Lechman, E.R., Waldron, L., Nilsson, B., van Galen, P., Metzeler, K.H., Poeppl, A., Ling, V., Beyene, J., et al. (2011). Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 17, Han, M.K., Song, E.K., Guo, Y., Ou, X., Mantel, C., and Broxmeyer, H.E. (2008). SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2, Horton, S.J., and Huntly, B.J. (2012). Recent advances in acute myeloid leukemia stem cell biology. Haematologica 97, Jordan, C.T., Upchurch, D., Szilvassy, S.J., Guzman, M.L., Howard, D.S., Pettigrew, A.L., Meyerrose, T., Rossi, R., Grimes, B., Rizzieri, D.A., et al. (2000). The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 14, Kim, K.T., Baird, K., Davis, S., Piloto, O., Levis, M., Li, L., Chen, P., Meltzer, P., and Small, D. (2007). Constitutive Fms-like tyrosine kinase 3 activation results in specific changes in gene expression in myeloid leukaemic cells. Br. J. Haematol. 138, Kindler, T., Lipka, D.B., and Fischer, T. (2010). FLT3 as a therapeutic target in AML: still challenging after all these years. Blood 116, Leko, V., Varnum-Finney, B., Li, H., Gu, Y., Flowers, D., Nourigat, C., Bernstein, I.D., and Bedalov, A. (2012). SIRT1 is dispensable for function of hematopoietic stem cells in adult mice. Blood 119, Levis, M. (2011). FLT3/ITD AML and the law of unintended consequences. Blood 117, Li, L., Wang, L., Li, L., Wang, Z., Ho, Y., McDonald, T., Holyoake, T.L., Chen, W., and Bhatia, R. (2012). Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell 21, Lin, Z., Yang, H., Kong, Q., Li, J., Lee, S.M., Gao, B., Dong, H., Wei, J., Song, J., Zhang, D.D., and Fang, D. (2012). USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol. Cell 46, Long, J., Parkin, B., Ouillette, P., Bixby, D., Shedden, K., Erba, H., Wang, S., and Malek, S.N. (2010). Multiple distinct molecular mechanisms influence sensitivity and resistance to MDM2 inhibitors in adult acute myelogenous leukemia. Blood 116, MacCallum, S.F., Groves, M.J., James, J., Murray, K., Appleyard, V., Prescott, A.R., Drbal, A.A., Nicolaou, A., Cunningham, J., Haydock, S., et al. (2013). Dysregulation of autophagy in chronic lymphocytic leukemia with the smallmolecule Sirtuin inhibitor Tenovin-6. Sci. Rep. 3, Majeti, R., Chao, M.P., Alizadeh, A.A., Pang, W.W., Jaiswal, S., Gibbs, K.D., Jr., van Rooijen, N., and Weissman, I.L. (2009). CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138, Marshall, G.M., Liu, P.Y., Gherardi, S., Scarlett, C.J., Bedalov, A., Xu, N., Iraci, N., Valli, E., Ling, D., Thomas, W., et al. (2011). SIRT1 promotes N-Myc oncogenesis through a positive feedback loop involving the effects of MKP3 and ERK on N-Myc protein stability. PLoS Genet. 7, e McCormack, E., Haaland, I., Venås, G., Forthun, R.B., Huseby, S., Gausdal, G., Knappskog, S., Micklem, D.R., Lorens, J.B., Bruserud, O., and Gjertsen, B.T. (2012). Synergistic induction of p53 mediated apoptosis by valproic acid and nutlin-3 in acute myeloid leukemia. Leukemia 26, (E) Flag-USP22-expressing or control MV4-11 cells were incubated with CHX and analyzed by western blotting for Flag, SIRT1, and b-actin. The right panel indicates the relative amount of SIRT1 analyzed by densitometry from three experiments. (F) Western blotting for c-myc, USP22, and b-actin in MV4-11 cells expressing shrna to c-myc or Ctrl ShRNA. (G) Western blotting for SIRT1, c-myc, USP22, and b-actin in CB CD34 + cells transduced with lentivirus vectors expressing c-myc or vector control. (H) Western blotting for SIRT1, c-myc, USP22, and b-actin in Flag-USP22 expressing or control MV4-11 cells expressing c-myc or Ctrl ShRNA. (I) HEK293 cells transduced with lentivirus vectors expressing USP22 or Ctrl ShRNA were cotransfected with Flag-SIRT1 or vector control, c-myc or empty vector control, and HA-tagged ubiquitin (Ub), as indicated, and lysates were immunoprecipitated (IP) for Flag and western blotted for SIRT1, c-myc, and HA. The right panel shows input lysates for western blotting of SIRT1, c-myc, USP22, and b-actin. IB, immunoblotting. p < 0.05, p < 0.01, compared with controls. Results represent mean ± SEM and are representative of three independent experiments. See also Figure S7. Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc. 445
16 Cell Stem Cell SIRT1 in FLT3-ITD AML Stem Cells McCormack, E., Mujic, M., Osdal, T., Bruserud, Ø., and Gjertsen, B.T. (2013). Multiplexed mabs: a new strategy in preclinical time-domain imaging of acute myeloid leukemia. Blood 121, e34 e42. Menssen, A., Hydbring, P., Kapelle, K., Vervoorts, J., Diebold, J., Lüscher, B., Larsson, L.G., and Hermeking, H. (2012). The c-myc oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc. Natl. Acad. Sci. USA 109, E187 E196. Nakao, M., Yokota, S., Iwai, T., Kaneko, H., Horiike, S., Kashima, K., Sonoda, Y., Fujimoto, T., and Misawa, S. (1996). Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 10, O Donnell, M.R., Abboud, C.N., Altman, J., Appelbaum, F.R., Arber, D.A., Attar, E., Borate, U., Coutre, S.E., Damon, L.E., Goorha, S., et al. (2012). Acute myeloid leukemia. J. Natl. Compr. Canc. Netw. 10, Ou, X., Chae, H.D., Wang, R.H., Shelley, W.C., Cooper, S., Taylor, T., Kim, Y.J., Deng, C.X., Yoder, M.C., and Broxmeyer, H.E. (2011). SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood 117, Patel, J.P., Gönen, M., Figueroa, M.E., Fernandez, H., Sun, Z., Racevskis, J., Van Vlierberghe, P., Dolgalev, I., Thomas, S., Aminova, O., et al. (2012). Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 366, Sasca, D., Hähnel, P.S., Szybinski, J., Khawaja, K., Kriege, O., Pante, S.V., Bullinger, L., Strand, S., Strand, D., Theobald, M., and Kindler, T. (2014). SIRT1 prevents genotoxic stress-induced p53 activation in acute myeloid leukemia. Blood 124, Schrecengost, R.S., Dean, J.L., Goodwin, J.F., Schiewer, M.J., Urban, M.W., Stanek, T.J., Sussman, R.T., Hicks, J.L., Birbe, R.C., Draganova-Tacheva, R.A., et al. (2014). USP22 regulates oncogenic signaling pathways to drive lethal cancer progression. Cancer Res. 74, Schuhmacher, M., Kohlhuber, F., Hölzel, M., Kaiser, C., Burtscher, H., Jarsch, M., Bornkamm, G.W., Laux, G., Polack, A., Weidle, U.H., and Eick, D. (2001). The transcriptional program of a human B cell line in response to Myc. Nucleic Acids Res. 29, Shangary, S., Qin, D., McEachern, D., Liu, M., Miller, R.S., Qiu, S., Nikolovska- Coleska, Z., Ding, K., Wang, G., Chen, J., et al. (2008). Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc. Natl. Acad. Sci. USA 105, Singh, S.K., Williams, C.A., Klarmann, K., Burkett, S.S., Keller, J.R., and Oberdoerffer, P. (2013). Sirt1 ablation promotes stress-induced loss of epigenetic and genomic hematopoietic stem and progenitor cell maintenance. J. Exp. Med. 210, Smith, C.C., Wang, Q., Chin, C.S., Salerno, S., Damon, L.E., Levis, M.J., Perl, A.E., Travers, K.J., Wang, S., Hunt, J.P., et al. (2012). Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 485, Tang, Y., Zhao, W., Chen, Y., Zhao, Y., and Gu, W. (2008). Acetylation is indispensable for p53 activation. Cell 133, Valk, P.J., Verhaak, R.G., Beijen, M.A., Erpelinck, C.A., Barjesteh van Waalwijk van Doorn-Khosrovani, S., Boer, J.M., Beverloo, H.B., Moorhouse, M.J., van der Spek, P.J., Löwenberg, B., and Delwel, R. (2004). Prognostically useful gene-expression profiles in acute myeloid leukemia. N. Engl. J. Med. 350, van der Lugt, N.M., Domen, J., Verhoeven, E., Linders, K., van der Gulden, H., Allen, J., and Berns, A. (1995). Proviral tagging in E mu-myc transgenic mice lacking the Pim-1 proto-oncogene leads to compensatory activation of Pim-2. EMBO J. 14, Vaziri, H., Dessain, S.K., Ng Eaton, E., Imai, S.I., Frye, R.A., Pandita, T.K., Guarente, L., and Weinberg, R.A. (2001). hsir2(sirt1) functions as an NADdependent p53 deacetylase. Cell 107, Wang, R.H., Sengupta, K., Li, C., Kim, H.S., Cao, L., Xiao, C., Kim, S., Xu, X., Zheng, Y., Chilton, B., et al. (2008a). Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14, Wang, R.H., Zheng, Y., Kim, H.S., Xu, X., Cao, L., Luhasen, T., Lee, M.H., Xiao, C., Vassilopoulos, A., Chen, W., et al. (2008b). Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol. Cell 32, Wang, Z., Yuan, H., Roth, M., Stark, J.M., Bhatia, R., and Chen, W.Y. (2013). SIRT1 deacetylase promotes acquisition of genetic mutations for drug resistance in CML cells. Oncogene 32, Yang, Q., Chen, L.S., Neelapu, S.S., Miranda, R.N., Medeiros, L.J., and Gandhi, V. (2012). Transcription and translation are primary targets of Pim kinase inhibitor SGI-1776 in mantle cell lymphoma. Blood 120, Yuan, J., Minter-Dykhouse, K., and Lou, Z. (2009). A c-myc-sirt1 feedback loop regulates cell growth and transformation. J. Cell Biol. 185, Yuan, H., Wang, Z., Li, L., Zhang, H., Modi, H., Horne, D., Bhatia, R., and Chen, W. (2012). Activation of stress response gene SIRT1 by BCR-ABL promotes leukemogenesis. Blood 119, Zhang, X.Y., Varthi, M., Sykes, S.M., Phillips, C., Warzecha, C., Zhu, W., Wyce, A., Thorne, A.W., Berger, S.L., and McMahon, S.B. (2008). The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol. Cell 29, Cell Stem Cell 15, , October 2, 2014 ª2014 Elsevier Inc.
17 Cell Stem Cell, Volume 15 Supplemental Information SIRT1 Activation by a c-myc Oncogenic Network Promotes the Maintenance and Drug Resistance of Human FLT3-ITD Acute Myeloid Leukemia Stem Cells Ling Li, Tereza Osdal, Yinwei Ho, Sookhee Chun, Tinisha McDonald, Puneet Agarwal, Allen Lin, Su Chu, Jing Qi, Liang Li, Yao-Te Hsieh, Cedric Dos Santos, Hongfeng Yuan, Trung-Quang Ha, Mihaela Popa, Randi Hovland, Øystein Bruserud, Bjørn Tore Gjertsen, Ya-Huei Kuo, Wenyong Chen, Sonia Lain, Emmet McCormack, and Ravi Bhatia
18 Supplemental figures and legends Supplemental tables Supplemental experimental procedures Supplemental references
19
20 Figure S1. SIRT1 expression and sensitivity to Tenovin-6 in AML compared with normal stem/progenitor cells. (Related to Figure 1) (A) Comparison of TV6 and nutlin-3 efficacy in primary AML cells. CD34 + cells from AML patients (n=34) were cultured with or without TV6 or Nutlin3 (10μM) for 24 hours and cell viability was checked using Hoechst staining. (B) Comparison of TV6 efficacy in AML samples classified by p53 status. 25 samples had wild-type p53, and 5 samples had mutant p53. (C) Comparison of TV6 efficacy in AML cell lines based on p53 status. Cell viability was evaluated after 24 hour drug treatment by Hoechst staining. An increased proportion of apoptotic bodies (arrow F for fragments or C for crimpled nuclei) compared to viable cells (arrow V) were seen in the FLT3-ITD cell line Molm-13 after TV6 treatment. All scale bars represent a size of 10μm. (D) Relationship of TV6 and nutlin-3 sensitivity to FLT3 mutation status in the 10 AML samples with the greatest discrepancy in efficacy between TV6 and Nutlin-3 (>20% difference in viability). Clinical and genetic parameters for individual samples are shown. Flt3 mutation represents FLT3-ITD mutation. (E) NSG mice were transplanted with MOLM-13luc cells and treated with TV6 (50 mg/kg intraperitoneally twice a day) for 12 days (n=6 per group) and engraftment analyzed indicated time by Optix MX2 (ART Inc.) bioluminescence imaging (raster scan points 1 mm apart). Bioluminescent imaging was performed 10 minutes after administration of luciferin (150 mg/kg). Three representative mice from each group are shown. (F) Quantitative results from bioimaging studies. All images and fluorescence lifetime gating was analyzed using Optiview software (Version 2.02, ART Inc.). Results represent Mean±SEM, n=6 per group. Significance: p<0.05, p<0.01, compared with controls. (G) Xenografts of with Molm-13 cells expressing p53 shrna (Molm-13 shp53) (right panel) and control shrna (Molm-13 shctrl) (left panel), were established in NSG mice by subcutaneous injection and treated with TV6 (125 mg/kg intraperitoneally daily) for 12 days (n=5 per group). Tumor volume was analyzed every 3 days as indicated. Results represent Mean±SEM, n=6 per group. Significance: p<0.05, p<0.01, compared with controls.
21
22 Figure S2. Increased SIRT1 expression and sensitivity to SIRT1 knockdown in FLT3-ITD+ AML CD34 + cells. (Related to Figure 2) (A) SIRT1 protein expression in Lin - CD34 + CD38 - CD90 - CD47 + and Lin - CD34 + CD38 - CD90 - CD47 -, as well as in lin - CD34 + CD38 - CD123 + and lin - CD34 + CD38 - CD123 - cells from FLT3-ITD AML samples (n=4) and FLT3-WT AML samples (n=4), analyzed by flow cytometry. Median fluorescence intensity (MFI) of SIRT1 was expressed relative to MFI of IgG control. (B) Western blotting of SIRT1, p53, Ac-p53 (K382) and β-actin expression in CB CD34 + cells transduced with FLT3-ITD or FLT3-WT expressing vectors. Cells were pretreated with TSA (0.1μM) for 2 hrs. (C) MV4-11 [FLT3-ITD] or OCI-AML3 [FLT3-WT] cells were incubated with CHX and analyzed by Western blotting for SIRT1 and β-actin. The bottom panel indicates the relative amount of SIRT1 analyzed by densitometry (n=3). (D) Western blotting to analyze expression of p53 target genes in MV4-11 and OCI-AML3 cells, 16 hrs after irradiation (3Gy or 6Gy) compared with non-irradiated control cells. (E-F) MV4-11 and OCI-AML3 were transfected with Bax SiRNA or Ctrl SiRNA by nucleofection 40 hr before irradiation (3Gy or 6Gy). TV-6 (2μM) was added into culture medium 2 hr before the irradiation. Cells were analyzed 16 hr after irradiation. Results of Western blotting for Bax and β-actin in SiRNA transfected cells are shown in (E), and for apoptosis of MV4-11 (left panel) and OCI- AML3 (right panel) evaluated by Annexin V/DAPI staining in (F). (G) Effect of anti-sirt1 shrna on apoptosis of normal CD34 + progenitors (n=3). Results represent the mean ± SEM. (H-I) Three p53 wild type cell lines (MV4-11[FLT3-ITD], Molm-13 [FLT3-ITD], and OCI-AML3 [FLT3- WT]) were transduced with lentivirus vectors expressing anti-sirt1, anti-sirt2 and control shrnas. (H) Western blotting for SIRT1, SIRT2 and β-actin in ShSIRT1 or Ctrl ShRNA (upper panels), and ShSIRT2 or Ctrl ShRNA (lower panels) expressing cells. (I) Apoptosis was evaluated by Annexin V/DAPI staining. (J) Growth over 72 hours was evaluated using Cell Titer Glo. (K-L) MV4-11 cells were first transduced with lentivirus vectors expressing GFP or a shrna-resistant SIRT1 with five silent mutations in the region targeted by ShSIRT1 (SIRT1-R) and then transduced with ShSIRT1 or Ctrl ShRNA expressing lentivirus vectors. (K) Western blotting for SIRT1 and β-actin expression. (L) Cell growth over 72 hours was evaluated by the Cell Titer Glo assay. Results were normalized to GFP and Ctrl ShRNA co-expressing cells, and represent the mean ± SEM of triplicates.
23
24 Figure S3. Pharmacological inhibition of SIRT1 by Tenovin-6 reduces FLT3-ITD AML CD34 + cell growth and survival by activating p53 signaling. (Related to Figure 3) (A-B) Western blotting for acetylated p53 (K382), total p53, and β-actin of FLT3-ITD (A) or FLT3-WT (B) AML CD34 + cells were cultured with TV6 as indicated dose (μm) for 8 hr. Results from three FLT3-ITD samples and three FLT3-WT samples are shown. (C) MV4-11 cells with or without prior nutlin-3 (5μM) exposure for 2 hrs, were exposed to TV6 for 8 hrs and analyzed by Western blotting for acetylated p53 (K382), total p53 and β-actin. (D-E) K562 cells expressing a tet transactivator gene (K562-TTA) were generated and transfected with acetylation-defective (p53-8kr) and wt p53 constructs by nucleofection. 24 hr after transfection, cells were exposed to TV- 6 at indicated doses. After 8 hours of drug exposure, cell lysates were prepared and analyzed by Western blotting for acetylated p53 (K382), total p53, SIRT1 and β-actin (D). Apoptosis was evaluated after 24h of drug treatment by Annexin V labeling (E). Results represent Mean±SEM for three experiments. Significance: p<0.05, p<0.01. (F) MV4-11 cells were transduced with SIRT1 ShRNA (Sh SIRT1) or control ShRNA (Ctrl ShRNA) expressing lentivirus vectors. Growth of SIRT1 knockdown or control MV4-11 cells exposed to TV6 for 72 hrs was analyzed using Cell Titer Glow. Results were normalized to vehicle treated Ctrl ShRNA transduced cells. Results represent Mean±SEM. Significance values: p<0.05, p<0.01, p<0.001, compared with Ctrl ShRNA group treated with vehicle; ^p<0.05, compared with Sh SIRT1 group treated with vehicle. (G-H) MV4-11 or Molm-13 cells were co-transduced with PLKO-GFP vectors expressing anti-p53 or control shrna and PHIV7-RFP vectors expressing ShSIRT1 or control shrna. Double positive cells were selected for further analysis. Results are shown of Western blotting for SIRT1, p53 and β-actin of MV4-11 (G), and growth of double transduced cells over 72 hrs analyzed using Cell Titer Glow (H). Results represent the Mean±SEM for three experiments. Ratios were normalized to ctrl ShRNA expressing cells.
25
26 Figure S4. SIRT1 inhibition enhances AC220 mediated inhibition of FLT3-ITD AML CD34 + cells. (Related to Figure 4) (A) FLT3-ITD AML CD34 + (left panel) or MV4-11 (right panel) cells were exposed to 20 nm (AML CD34 + ) or 2 nm (MV4-11) AC220 for 8 hours and acetylated p53, total p53, SIRT1, β-actin, p-stat5, and STAT5 were analyzed by Western blotting. (B) Q-PCR analysis of p53 target gene expression in FLT3-ITD AML CD34 + cells treated with AC220 compared with vehicle treated controls (n=7). β-actin served as internal control. Results represent the mean ± SEM. (C) Western blotting for SIRT1, p-stat5, STAT5, acetylated p53, total p53 and β-actin protein levels in SIRT1 or Ctrl ShRNA transduced MV4-11 AML cells with or without AC220 treatment. (D-E) MV4-11 cells were exposed to TV6 (2μM), AC220 at indicated dose (nm) or the combination for 48 hrs. (D) Apoptosis was analyzed by Annexin V/DAPI labeling. (E) Cell growth was evaluated using the Cell Titer Glo assay. Results represent Mean±SEM for three experiments. Significance: p<0.05, p<0.01, p<0.001, compared with vehicle controls; ^p<0.05, ^^p<0.01, compared with AC220 treatment alone.
27
SIRT1 Activation by a c-myc Oncogenic Network Promotes the Maintenance and Drug Resistance of Human FLT3-ITD Acute Myeloid Leukemia Stem Cells
 Article Activation by a c-myc Oncogenic Network Promotes the Maintenance and Drug Resistance of Human FLT3-ITD Acute Myeloid Leukemia Stem Cells Ling Li, 1 Tereza Osdal, 2 Yinwei Ho, 1 Sookhee Chun, 1
Article Activation by a c-myc Oncogenic Network Promotes the Maintenance and Drug Resistance of Human FLT3-ITD Acute Myeloid Leukemia Stem Cells Ling Li, 1 Tereza Osdal, 2 Yinwei Ho, 1 Sookhee Chun, 1
Activation of p53 by SIRT1 Inhibition Enhances Elimination of CML Leukemia Stem Cells in Combination with Imatinib
 Article Activation of p53 by SIRT1 Inhibition Enhances Elimination of CML Leukemia Stem Cells in Combination with Imatinib Ling Li, 1 Lisheng Wang, 1 Liang Li, 1 Zhiqiang Wang, 2 Yinwei Ho, 1 Tinisha McDonald,
Article Activation of p53 by SIRT1 Inhibition Enhances Elimination of CML Leukemia Stem Cells in Combination with Imatinib Ling Li, 1 Lisheng Wang, 1 Liang Li, 1 Zhiqiang Wang, 2 Yinwei Ho, 1 Tinisha McDonald,
Effective Targeting of Quiescent Chronic Myelogenous
 Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
Nature Genetics: doi: /ng Supplementary Figure 1
 Supplementary Figure 1 MSI2 interactors are associated with the riboproteome and are functionally relevant. (a) Coomassie blue staining of FLAG-MSI2 immunoprecipitated complexes. (b) GO analysis of MSI2-interacting
Supplementary Figure 1 MSI2 interactors are associated with the riboproteome and are functionally relevant. (a) Coomassie blue staining of FLAG-MSI2 immunoprecipitated complexes. (b) GO analysis of MSI2-interacting
X P. Supplementary Figure 1. Nature Medicine: doi: /nm Nilotinib LSK LT-HSC. Cytoplasm. Cytoplasm. Nucleus. Nucleus
 a b c Supplementary Figure 1 c-kit-apc-eflu780 Lin-FITC Flt3-Linc-Kit-APC-eflu780 LSK Sca-1-PE-Cy7 d e f CD48-APC LT-HSC CD150-PerCP-cy5.5 g h i j Cytoplasm RCC1 X Exp 5 mir 126 SPRED1 SPRED1 RAN P SPRED1
a b c Supplementary Figure 1 c-kit-apc-eflu780 Lin-FITC Flt3-Linc-Kit-APC-eflu780 LSK Sca-1-PE-Cy7 d e f CD48-APC LT-HSC CD150-PerCP-cy5.5 g h i j Cytoplasm RCC1 X Exp 5 mir 126 SPRED1 SPRED1 RAN P SPRED1
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
supplementary information
 DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Nature Immunology: doi: /ni Supplementary Figure 1. Huwe1 has high expression in HSCs and is necessary for quiescence.
 Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplement Figure S1. Real Time PCR analysis of mrna levels of C/EBPα and PU.1 in wild type (WT) and NQO1-null (NQO1-/-) mice.
 competes with 20S proteasome for binding with C/EBP leading to its stabilization and Relative mrna levels Supplement Figure S1. Real Time PCR analysis of mrna levels of C/EBPα and PU.1 in wild type (WT)
competes with 20S proteasome for binding with C/EBP leading to its stabilization and Relative mrna levels Supplement Figure S1. Real Time PCR analysis of mrna levels of C/EBPα and PU.1 in wild type (WT)
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary Materials and Methods
 Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5
 sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
Sirt1 Hmg20b Gm (0.17) 24 (17.3) 877 (857)
 3 (0.17) 24 (17.3) Sirt1 Hmg20 Gm4763 877 (857) c d Suppl. Figure 1. Screen validation for top candidate antagonists of Dot1L (a) Numer of genes with one (gray), two (cyan) or three (red) shrna scored
3 (0.17) 24 (17.3) Sirt1 Hmg20 Gm4763 877 (857) c d Suppl. Figure 1. Screen validation for top candidate antagonists of Dot1L (a) Numer of genes with one (gray), two (cyan) or three (red) shrna scored
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Control shrna#9 shrna#12. shrna#12 CD14-PE CD14-PE
 a Control shrna#9 shrna#12 c Control shrna#9 shrna#12 e Control shrna#9 shrna#12 h 14 12 CFU-E BFU-E GEMM GM b Colony number 7 6 5 4 3 2 1 6 pm A pa pc CFU-E BFU-E GEMM GM pu pgm A p pg B d f CD11b-APC
a Control shrna#9 shrna#12 c Control shrna#9 shrna#12 e Control shrna#9 shrna#12 h 14 12 CFU-E BFU-E GEMM GM b Colony number 7 6 5 4 3 2 1 6 pm A pa pc CFU-E BFU-E GEMM GM pu pgm A p pg B d f CD11b-APC
SUPPLEMENTARY FIGURES AND TABLE
 SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
Supplementary Information
 Supplementary Information mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis Zhang et al. a b d e g h Rel. Luc. Act. Rel. mrna Rel. mrna
Supplementary Information mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis Zhang et al. a b d e g h Rel. Luc. Act. Rel. mrna Rel. mrna
Supplementary Figure 1 IL-27 IL
 Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
S1a S1b S1c. S1d. S1f S1g S1h SUPPLEMENTARY FIGURE 1. - si sc Il17rd Il17ra bp. rig/s IL-17RD (ng) -100 IL-17RD
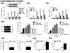 SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
(A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939 (5 µm)
 Supplementary Figure Legends Figure S1. Tankyrase inhibition suppresses cell proliferation in an axin/β-catenin independent manner. (A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939
Supplementary Figure Legends Figure S1. Tankyrase inhibition suppresses cell proliferation in an axin/β-catenin independent manner. (A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939
SUPPLEMENTAL FIGURE LEGENDS
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11700 Figure 1: RIP3 as a potential Sirt2 interacting protein. Transfected Flag-tagged Sirt2 was immunoprecipitated from cells and eluted from the Sepharose beads using Flag peptide.
doi:10.1038/nature11700 Figure 1: RIP3 as a potential Sirt2 interacting protein. Transfected Flag-tagged Sirt2 was immunoprecipitated from cells and eluted from the Sepharose beads using Flag peptide.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic
 Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic differentiation of acute myeogenouse leukemia cells that is enhanced by 1,25-dihydroxyviatmin D 3. To the Editor: Sunitinib,
Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic differentiation of acute myeogenouse leukemia cells that is enhanced by 1,25-dihydroxyviatmin D 3. To the Editor: Sunitinib,
(A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and a
 Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Supplementary Figure 1. MAT IIα is Acetylated at Lysine 81.
 IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
Supplementary Material
 Supplementary Material Summary: The supplementary information includes 1 table (Table S1) and 4 figures (Figure S1 to S4). Supplementary Figure Legends Figure S1 RTL-bearing nude mouse model. (A) Tumor
Supplementary Material Summary: The supplementary information includes 1 table (Table S1) and 4 figures (Figure S1 to S4). Supplementary Figure Legends Figure S1 RTL-bearing nude mouse model. (A) Tumor
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
AHI-1 interacts with BCR-ABL and modulates BCR-ABL transforming activity and imatinib response of CML stem/progenitor cells
 AHI-1 interacts with BCR-ABL and modulates BCR-ABL transforming activity and imatinib response of CML stem/progenitor cells Liang L. Zhou, 1 Yun Zhao, 1 Ashley Ringrose, 1 Donna DeGeer, 1 Erin Kennah,
AHI-1 interacts with BCR-ABL and modulates BCR-ABL transforming activity and imatinib response of CML stem/progenitor cells Liang L. Zhou, 1 Yun Zhao, 1 Ashley Ringrose, 1 Donna DeGeer, 1 Erin Kennah,
Pearson r = P (one-tailed) = n = 9
 8F4-Specific Lysis, % 1 UPN1 UPN3 8 UPN7 6 Pearson r =.69 UPN2 UPN5 P (one-tailed) =.192 4 UPN8 n = 9 2 UPN9 UPN4 UPN6 5 1 15 2 25 8 8F4, % Max MFI Supplementary Figure S1. AML samples UPN1-UPN9 show variable
8F4-Specific Lysis, % 1 UPN1 UPN3 8 UPN7 6 Pearson r =.69 UPN2 UPN5 P (one-tailed) =.192 4 UPN8 n = 9 2 UPN9 UPN4 UPN6 5 1 15 2 25 8 8F4, % Max MFI Supplementary Figure S1. AML samples UPN1-UPN9 show variable
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
Corporate Medical Policy. Policy Effective February 23, 2018
 Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
Kidney. Heart. Lung. Sirt1. Gapdh. Mouse IgG DAPI. Rabbit IgG DAPI
 a e Na V 1.5 Ad-LacZ Ad- 110KD b Scn5a/ (relative to Ad-LacZ) f 150 100 50 0 p = 0.65 Ad-LacZ Ad- c Heart Lung Kidney Spleen 110KD d fl/fl c -/- DAPI 20 µm Na v 1.5 250KD fl/fl Rabbit IgG DAPI fl/fl Mouse
a e Na V 1.5 Ad-LacZ Ad- 110KD b Scn5a/ (relative to Ad-LacZ) f 150 100 50 0 p = 0.65 Ad-LacZ Ad- c Heart Lung Kidney Spleen 110KD d fl/fl c -/- DAPI 20 µm Na v 1.5 250KD fl/fl Rabbit IgG DAPI fl/fl Mouse
Figure S1. Reduction in glomerular mir-146a levels correlate with progression to higher albuminuria in diabetic patients.
 Supplementary Materials Supplementary Figures Figure S1. Reduction in glomerular mir-146a levels correlate with progression to higher albuminuria in diabetic patients. Figure S2. Expression level of podocyte
Supplementary Materials Supplementary Figures Figure S1. Reduction in glomerular mir-146a levels correlate with progression to higher albuminuria in diabetic patients. Figure S2. Expression level of podocyte
Supplementary Information Supplementary Fig. 1. Elevated Usp9x in melanoma and NRAS mutant melanoma cells are dependent on NRAS for 3D growth.
 Supplementary Information Supplementary Fig. 1. Elevated Usp9x in melanoma and NRAS mutant melanoma cells are dependent on NRAS for 3D growth. a. Immunoblot for Usp9x protein in NRAS mutant melanoma cells
Supplementary Information Supplementary Fig. 1. Elevated Usp9x in melanoma and NRAS mutant melanoma cells are dependent on NRAS for 3D growth. a. Immunoblot for Usp9x protein in NRAS mutant melanoma cells
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/8/339/339ra69/dc1 Supplementary Materials for The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and
www.sciencetranslationalmedicine.org/cgi/content/full/8/339/339ra69/dc1 Supplementary Materials for The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and
SUPPLEMENTARY INFORMATION
 Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice
 Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Nature Genetics: doi: /ng Supplementary Figure 1. HOX fusions enhance self-renewal capacity.
 Supplementary Figure 1 HOX fusions enhance self-renewal capacity. Mouse bone marrow was transduced with a retrovirus carrying one of three HOX fusion genes or the empty mcherry reporter construct as described
Supplementary Figure 1 HOX fusions enhance self-renewal capacity. Mouse bone marrow was transduced with a retrovirus carrying one of three HOX fusion genes or the empty mcherry reporter construct as described
Supplementary Information
 Supplementary Information Supplementary Figure 1. Effect of mir mimics and anti-mirs on DTPs a, Representative fluorescence microscopy images of GFP vector control or mir mimicexpressing parental and DTP
Supplementary Information Supplementary Figure 1. Effect of mir mimics and anti-mirs on DTPs a, Representative fluorescence microscopy images of GFP vector control or mir mimicexpressing parental and DTP
Supplementary Figure 1
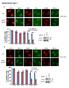 Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Personalized Therapy for Acute Myeloid Leukemia. Patrick Stiff MD Loyola University Medical Center
 Personalized Therapy for Acute Myeloid Leukemia Patrick Stiff MD Loyola University Medical Center 708-327-3216 Major groups of Mutations in AML Targets for AML: Is this Achievable? Chronic Myeloid Leukemia:
Personalized Therapy for Acute Myeloid Leukemia Patrick Stiff MD Loyola University Medical Center 708-327-3216 Major groups of Mutations in AML Targets for AML: Is this Achievable? Chronic Myeloid Leukemia:
Supplemental information
 Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Supplementary Information and Figure legends
 Supplementary Information and Figure legends Table S1. Primers for quantitative RT-PCR Target Sequence (5 -> 3 ) Target Sequence (5 -> 3 ) DAB2IP F:TGGACGATGTGCTCTATGCC R:GGATGGTGATGGTTTGGTAG Snail F:CCTCCCTGTCAGATGAGGAC
Supplementary Information and Figure legends Table S1. Primers for quantitative RT-PCR Target Sequence (5 -> 3 ) Target Sequence (5 -> 3 ) DAB2IP F:TGGACGATGTGCTCTATGCC R:GGATGGTGATGGTTTGGTAG Snail F:CCTCCCTGTCAGATGAGGAC
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
supplementary information
 DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma
 Supplementary information for: Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma Rintaro Hashizume 1, Noemi Andor 2, Yuichiro Ihara 2, Robin Lerner 2, Haiyun
Supplementary information for: Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma Rintaro Hashizume 1, Noemi Andor 2, Yuichiro Ihara 2, Robin Lerner 2, Haiyun
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Predictive PP1Ca binding region in BIG3 : 1,228 1,232aa (-KAVSF-) HEK293T cells *** *** *** KPL-3C cells - E E2 treatment time (h)
 Relative expression ERE-luciferase activity activity (pmole/min) activity (pmole/min) activity (pmole/min) activity (pmole/min) MCF-7 KPL-3C ZR--1 BT-474 T47D HCC15 KPL-1 HBC4 activity (pmole/min) a d
Relative expression ERE-luciferase activity activity (pmole/min) activity (pmole/min) activity (pmole/min) activity (pmole/min) MCF-7 KPL-3C ZR--1 BT-474 T47D HCC15 KPL-1 HBC4 activity (pmole/min) a d
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Peli1 negatively regulates T-cell activation and prevents autoimmunity
 Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
Peli1 negatively regulates T-cell activation and prevents autoimmunity Mikyoung Chang 1,*, Wei Jin 1,5,*, Jae-Hoon Chang 1, Yi-chuan Xiao 1, George Brittain 1, Jiayi Yu 1, Xiaofei Zhou 1, Yi-Hong Wang
condition. Left panel, the HCT-116 cells were lysed with RIPA buffer containing 0.1%
 FIGURE LEGENDS Supplementary Fig 1 (A) sumoylation pattern detected under denaturing condition. Left panel, the HCT-116 cells were lysed with RIPA buffer containing 0.1% SDS in the presence and absence
FIGURE LEGENDS Supplementary Fig 1 (A) sumoylation pattern detected under denaturing condition. Left panel, the HCT-116 cells were lysed with RIPA buffer containing 0.1% SDS in the presence and absence
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb3355 a S1A8 + cells/ total.1.8.6.4.2 b S1A8/?-Actin c % T-cell proliferation 3 25 2 15 1 5 T cells Supplementary Figure 1 Inter-tumoral heterogeneity of MDSC accumulation in mammary tumor
DOI: 1.138/ncb3355 a S1A8 + cells/ total.1.8.6.4.2 b S1A8/?-Actin c % T-cell proliferation 3 25 2 15 1 5 T cells Supplementary Figure 1 Inter-tumoral heterogeneity of MDSC accumulation in mammary tumor
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Supplementary Figure 1. Repression of hepcidin expression in the liver of mice treated with
 Supplementary Figure 1. Repression of hepcidin expression in the liver of mice treated with DMN Immunohistochemistry for hepcidin and H&E staining (left). qrt-pcr assays for hepcidin in the liver (right).
Supplementary Figure 1. Repression of hepcidin expression in the liver of mice treated with DMN Immunohistochemistry for hepcidin and H&E staining (left). qrt-pcr assays for hepcidin in the liver (right).
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Appendix. Table of Contents
 Appendix Table of Contents Appendix Figures Figure S1: Gp78 is not required for the degradation of mcherry-cl1 in Hela Cells. Figure S2: Indel formation in the MARCH6 sgrna targeted HeLa clones. Figure
Appendix Table of Contents Appendix Figures Figure S1: Gp78 is not required for the degradation of mcherry-cl1 in Hela Cells. Figure S2: Indel formation in the MARCH6 sgrna targeted HeLa clones. Figure
SUPPLEMENTARY INFORMATION
 Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
SUPPLEMENTARY INFORMATION
 a. Smo+/+ b. Smo+/+ 5.63 5.48 c. Lin- d. e. 6 5 4 3 Ter119 Mac B T Sca1 Smo+/+ 25 15 2 o BMT 2 1 5 * Supplementary Figure 1: Deletion of Smoothened does not alter the frequency of hematopoietic lineages
a. Smo+/+ b. Smo+/+ 5.63 5.48 c. Lin- d. e. 6 5 4 3 Ter119 Mac B T Sca1 Smo+/+ 25 15 2 o BMT 2 1 5 * Supplementary Figure 1: Deletion of Smoothened does not alter the frequency of hematopoietic lineages
Supplementary Figure 1. Successful excision of genes from WBM lysates and
 Supplementary Information: Supplementary Figure 1. Successful excision of genes from WBM lysates and survival of mice with different genotypes. (a) The proper excision of Pten, p110α, p110α and p110δ was
Supplementary Information: Supplementary Figure 1. Successful excision of genes from WBM lysates and survival of mice with different genotypes. (a) The proper excision of Pten, p110α, p110α and p110δ was
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES. Overview and Mechanism of Action Dr.
 BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 EGFR inhibition activates signaling pathways (a-b) EGFR inhibition activates signaling pathways (a) U251EGFR cells were treated with erlotinib (1µM) for the indicated times followed
Supplementary Figure 1 EGFR inhibition activates signaling pathways (a-b) EGFR inhibition activates signaling pathways (a) U251EGFR cells were treated with erlotinib (1µM) for the indicated times followed
BCR-ABL - LSK BCR-ABL + LKS - (%)
 Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the
 Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
CD14 + S100A9 + Monocytic Myeloid-Derived Suppressor Cells and Their Clinical Relevance in Non-Small Cell Lung Cancer
 CD14 + S1A9 + Monocytic Myeloid-Derived Suppressor Cells and Their Clinical Relevance in Non-Small Cell Lung Cancer Po-Hao, Feng M.D., Kang-Yun, Lee, M.D. Ph.D., Ya-Ling Chang, Yao-Fei Chan, Lu- Wei, Kuo,Ting-Yu
CD14 + S1A9 + Monocytic Myeloid-Derived Suppressor Cells and Their Clinical Relevance in Non-Small Cell Lung Cancer Po-Hao, Feng M.D., Kang-Yun, Lee, M.D. Ph.D., Ya-Ling Chang, Yao-Fei Chan, Lu- Wei, Kuo,Ting-Yu
Supplemental Figure 1
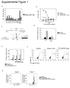 Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplementary Figure 1. Characterization of NMuMG-ErbB2 and NIC breast cancer cells expressing shrnas targeting LPP. NMuMG-ErbB2 cells (a) and NIC
 Supplementary Figure 1. Characterization of NMuMG-ErbB2 and NIC breast cancer cells expressing shrnas targeting LPP. NMuMG-ErbB2 cells (a) and NIC cells (b) were engineered to stably express either a LucA-shRNA
Supplementary Figure 1. Characterization of NMuMG-ErbB2 and NIC breast cancer cells expressing shrnas targeting LPP. NMuMG-ErbB2 cells (a) and NIC cells (b) were engineered to stably express either a LucA-shRNA
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Information
 Supplementary Information Targeted Disruption of the EZH2/EED Complex Inhibits EZH2- dependent Cancer Woojin Kim 1,2,3, Gregory H. Bird 2,3,4, Tobias Neff 5, Guoji Guo 1,2,3, Marc A. Kerenyi 1,2,3, Loren
Supplementary Information Targeted Disruption of the EZH2/EED Complex Inhibits EZH2- dependent Cancer Woojin Kim 1,2,3, Gregory H. Bird 2,3,4, Tobias Neff 5, Guoji Guo 1,2,3, Marc A. Kerenyi 1,2,3, Loren
Targeting methyltransferase PRMT5 eliminates leukemia stem cells in chronic myelogenous leukemia
 Targeting methyltransferase PRMT5 eliminates leukemia stem cells in chronic myelogenous leukemia Yanli Jin,, Ruibao Ren, Jingxuan Pan J Clin Invest. 2016;126(10):3961-3980. https://doi.org/10.1172/jci85239.
Targeting methyltransferase PRMT5 eliminates leukemia stem cells in chronic myelogenous leukemia Yanli Jin,, Ruibao Ren, Jingxuan Pan J Clin Invest. 2016;126(10):3961-3980. https://doi.org/10.1172/jci85239.
Supporting Information. FADD regulates NF-кB activation and promotes ubiquitination of cflip L to induce. apoptosis
 1 2 Supporting Information 3 4 5 FADD regulates NF-кB activation and promotes ubiquitination of cflip L to induce apoptosis 6 7 Kishu Ranjan and Chandramani Pathak* 8 9 Department of Cell Biology, School
1 2 Supporting Information 3 4 5 FADD regulates NF-кB activation and promotes ubiquitination of cflip L to induce apoptosis 6 7 Kishu Ranjan and Chandramani Pathak* 8 9 Department of Cell Biology, School
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
www.sciencesignaling.org/cgi/content/full/9/439/ra78/dc1 Supplementary Materials for Small heterodimer partner mediates liver X receptor (LXR) dependent suppression of inflammatory signaling by promoting
Plasma exposure levels from individual mice 4 hours post IP administration at the
 Supplemental Figure Legends Figure S1. Plasma exposure levels of MKC-3946 in mice. Plasma exposure levels from individual mice 4 hours post IP administration at the indicated dose mg/kg. Data represent
Supplemental Figure Legends Figure S1. Plasma exposure levels of MKC-3946 in mice. Plasma exposure levels from individual mice 4 hours post IP administration at the indicated dose mg/kg. Data represent
Supplementary Figure 1. Deletion of Smad3 prevents B16F10 melanoma invasion and metastasis in a mouse s.c. tumor model.
 A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Figures
 Supplementary Figures Supplementary Figure 1 DOT1L regulates the expression of epithelial and mesenchymal markers. (a) The expression levels and cellular localizations of EMT markers were confirmed by
Supplementary Figures Supplementary Figure 1 DOT1L regulates the expression of epithelial and mesenchymal markers. (a) The expression levels and cellular localizations of EMT markers were confirmed by
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis
 Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure S1 Supplementary Figure S2
 Supplementary Figure S A) The blots shown in Figure B were qualified by using Gel-Pro analyzer software (Rockville, MD, USA). The ratio of LC3II/LC3I to actin was then calculated. The data are represented
Supplementary Figure S A) The blots shown in Figure B were qualified by using Gel-Pro analyzer software (Rockville, MD, USA). The ratio of LC3II/LC3I to actin was then calculated. The data are represented
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Pro-apoptotic signalling through Toll-like receptor 3 involves TRIF-dependent
 Pro-apoptotic signalling through Toll-like receptor 3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells Arnim Weber, Zofia Kirejczyk,
Pro-apoptotic signalling through Toll-like receptor 3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells Arnim Weber, Zofia Kirejczyk,
SUPPLEMENTARY FIGURES AND TABLES
 SUPPLEMENTARY FIGURES AND TABLES Supplementary Figure S1: CaSR expression in neuroblastoma models. A. Proteins were isolated from three neuroblastoma cell lines and from the liver metastasis of a MYCN-non
SUPPLEMENTARY FIGURES AND TABLES Supplementary Figure S1: CaSR expression in neuroblastoma models. A. Proteins were isolated from three neuroblastoma cell lines and from the liver metastasis of a MYCN-non
293T cells were transfected with indicated expression vectors and the whole-cell extracts were subjected
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables. File Name: Peer Review File Description:
 File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables File Name: Peer Review File Description: Primer Name Sequence (5'-3') AT ( C) RT-PCR USP21 F 5'-TTCCCATGGCTCCTTCCACATGAT-3'
File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables File Name: Peer Review File Description: Primer Name Sequence (5'-3') AT ( C) RT-PCR USP21 F 5'-TTCCCATGGCTCCTTCCACATGAT-3'
SHREE ET AL, SUPPLEMENTAL MATERIALS. (A) Workflow for tumor cell line derivation and orthotopic implantation.
 SHREE ET AL, SUPPLEMENTAL MATERIALS SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure 1. Derivation and characterization of TS1-TGL and TS2-TGL PyMT cell lines and development of an orthotopic
SHREE ET AL, SUPPLEMENTAL MATERIALS SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure 1. Derivation and characterization of TS1-TGL and TS2-TGL PyMT cell lines and development of an orthotopic
Nature Immunology doi: /ni.3268
 Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
