Clinical management of non-occupational and occupational exposure to blood borne pathogens. - A Pocket Reference -
|
|
|
- Penelope Murphy
- 5 years ago
- Views:
Transcription
1 Pocket P.E.P. Clinical management of non-occupational and occupational eposure to blood borne pathogens Development Team: Deborah Yoong, PharmD 1 1, 2, 3 Kevin Gough, MD, MEd 1 Positive Care Clinic, Toronto, ON 2 Department of Medicine, St. Michael s Hospital 3 Faculty of Medicine, University of Toronto Reviewers: Mark Naccarato, BScPharm 1 1, 2, 3 Darrell H.S. Tan, MD, PhD Copyright A Pocket Reference - March 2019 (net review: March 2020)
2 STEP 1 TREAT EXPOSURE SITE & REPORT FOR ASSESSMENT An individual who eperiences an occupational or non-occupational eposure to blood borne pathogens needs to have immediate first aid treatment for any wound and a risk assessment for the likelihood of transmission of a pathogen. SMH staff with occupational eposures should immediately: Remove any contaminated clothing Allow wound to bleed freely. Needlestick injuries should not be squeezed. Flush thoroughly with water If eposed area involves the eyes, nose or mouth, thoroughly flush well with water Report the incident to his/her immediate supervisor and complete the Blood Borne Pathogen Eposure Report. If the source patient is known, it is important to record the source patient s full name and hospital number in the eposure report. Proceed immediately for risk assessment: During Business Hours (Monday to Friday, h): Corporate Health and Safety Services (3 Shuter, Et 5013) After hours, weekends, holidays: Emergency Department non-smh staff with occupational eposures and all non-occupational eposures: should proceed immediately to the ED for assessment. Algorithm for Presentation Following Eposure to a Blood Borne Pathogen Eposure to Blood Borne Pathogen SMH staff OCCUPATIONAL EXPOSURE (e.g. needlestick injury in SMH Staff) Non-SMH staff OCCUPATIONAL OR NON-OCCUPATIONAL (e.g. injured police officer, percutaneous injuries from discarded needles outside of the hospital, seual eposure, including seual assault survivors) SMH staff NON-OCCUPATIONAL (e.g. seual eposure) CHSS (Monday-Friday, h) Emergency Department (after hours, weekends, holidays) Emergency Department STEP 2 ASSESS THE EXPOSURE RISK Many factors contribute to the risk of transmission of a blood borne pathogen, including the type of body fluid involved, the type of injury that occurred, the size of the inoculum, and the attributes of the source and eposed patient. All of the following information should be obtained and recorded.
3 a. Body fluid: body fluids considered potentially infectious for hepatitis B, hepatitis C and HIV include blood, serum, plasma, visibly bloody fluids, semen, vaginal secretions, rectal secretions, cerebrospinal, synovial, pleural, peritoneal, pericardial and amniotic fluid body fluids NOT considered potentially infectious for a blood borne pathogen include feces, nasal secretions, saliva, sputum, sweat, tears, urine and vomitus, unless they are visibly bloody b. Type of Injury/Eposure Occupational eposure: percutaneous - skin puncture or laceration by needle or sharp object mucosal - splash to mucous membranes (e.g. eyes, nose, mouth) cutaneous - contact through nonintact skin (e.g. cuts, dermatitis) Non-occupational eposure: seual activity (eg. insertive or receptive, anal or vaginal intercourse) needle sharing c. Inoculum size volume of infectious fluid involved (e.g. hollow bore vs. solid needle; large volume vs. small volume splash) viral titre in the infectious fluid if known (e.g. well controlled disease vs. poorly controlled) d. Source patient unknown for hepatitis B, hepatitis C, or HIV positive for hepatitis B, hepatitis C, or HIV stage of disease risk of HIV transmission is 8-12X higher during the acute stage of HIV (first 6 months) most recent viral load current and past antiretroviral/antiviral use known or suspected drug resistance negative with no risk factors negative with risk factors (e.g. men who have se with men, multiple seual partners, people who inject drugs) e. Presence of seually transmitted infections (in cases of seual eposures) in both the source and eposed patient Estimated risk of transmission: Following percutaneous eposure to blood or potentially infectious fluid: hepatitis B: 6-30% hepatitis C: 3-10% HIV: 0.23% (0.1% for mucous membrane eposure) Table 1. Estimated Per-Act Risk for Acquisition of HIV by Eposure Route Eposure route Probability of transmission by an infected source Point estimate 95% confidence interval Parenteral Eposure Blood transfusion 92. 5% 89-96% Needle-sharing injection-drug use 0.63% % Percutaneous needle stick 0.23% % Seual Eposure Receptive anal intercourse 1.38% % Insertive anal intercourse 0.11% % Receptive vaginal intercourse 0.08% % Insertive vaginal intercourse 0.04% % Receptive oral intercourse etremely low % Insertive oral intercourse etremely low % The risk of transmission to receptive partner increases with ejaculation, presence of ulcers and seually transmitted infections in the mucous membrane (oral, genital, rectal), and high viral load in the source (e.g. acute or late-stage disease).
4 STEP 3 PERFORM BASELINE TESTING Since serologic testing of the source patient is the most reliable method to assess the risk of eposure, this is strongly recommended. Ascertain if the eposed individual is willing to be tested for antibody to hepatitis B, hepatitis C, and HIV. If the eposed individual is not willing to be tested, do not test the source patient unless they have a separate indication for testing. Source patient (if available): The source patient should be informed of the incident, and asked to undergo testing. Informed consent must be obtained (and for source patients admitted within the hospital, this must be documented in the chart by the attending medical staff or alternate medical delegate). If the source patient is a neonate, testing should be done on the mother. If the source patient does not consent to testing, an application can be made to the medical officer of health requiring mandatory blood testing to protect victims of crime, emergency service workers, and other persons. The application process can be found at: health.gov.on.ca/en/common/legislation/bill105/ Tests: HIV antibody (HIV Ab) using a fourth generation combination p24 antigen-hiv antibody assay or rapid HIV test as per SMH ED PEP protocol; if suspect acute HIV, consider HIV RNA Hepatitis B surface antigen (HBsAg) Hepatitis C antibody (HCV Ab); if HCV Ab positive, test for hepatitis C RNA Source patient (if not available): If the source patient is unknown or cannot be tested, consider their likelihood of having a blood borne pathogen based on local epidemiology. e.g. what is the prevalence of infection in this population? Does the source have risk factors for infection? Eposed individual: If the eposed individual is not willing to be tested for HIV, HIV PEP should not be initiated Tests: HIV antibody (HIV Ab) using a fourth generation combination p24 antigen-hiv antibody test or rapid HIV test as per SMH ED PEP protocol; if suspect acute HIV, consider HIV RNA Hepatitis B surface antigen (HBsAg) Hepatitis B surface antibody (anti-hbs) Hepatitis B core antibody (anti-hbc) Consider testing only if both anti-hbs and HBsAg are negative and there is the possibility of previous eposure. The presence of isolated anti-hbc can be as high as 10-20% in endemic countries. Isolated anti-hbc can be due to a) acute hepatitis B, b) a decline of anti-hbs to undetectable titers years after clearance of acute hepatitis, c) chronic infection with decline of HBsAg to undetectable titers, or rarely, d) false positive test. Hepatitis C antibody (HCV Ab); if positive, test for HCV RNA If starting on HIV PEP: complete blood count, serum creatinine, alanine aminotransferase Pregnancy test (ß-HCG) if appropriate STI testing (for seual eposures): syphilis serology, gonorrhea and chlamydia culture/nucleic acid amplification test (throat, vaginal, rectal, urine) as indicated. Consider incubation periods to distinguish prevalent from incident infection: syphilis (2-12 weeks), gonorrhea (2-7 days), chlamydia (2-3 weeks).
5 Results of Baseline Testing and Action to be taken for Eposed patient Baseline test result Eposed Source Action HBsAg Positive positive, negative, unknown Provide medical referral for hepatitis B management Negative positive, unknown* See Step 4a negative Vaccinate for hepatitis B if not immune (see table 2) HCV Ab and HCV RNA Positive positive, negative, unknown Provide medical referral for hepatitis C management Negative positive, unknown See Step 4b negative No further testing required HIV Ab Positive positive, negative, unknown Provide medical referral for HIV management Negative positive, unknown* See Step 4c Negative No further testing required and PEP can be discontinued in the eposed unless there is strong suspicion of recent HIV infection in the source* *initiation of post-eposure prophylais for hepatitis B or HIV eposure should not be delayed pending source test results STEP 4a PEP MANAGEMENT - HBV EXPOSURE Management of potential hepatitis B eposure is dependent on the vaccination and antibody status of the eposed individual, in addition to the serologic status of the source (Table 2). For the eposed individual who is IMMUNE (e.g. has anti-hbs > 10mIU/mL after completing the 3-dose hepatitis vaccination or natural immunity from previous eposure): no further action is required. For the eposed individual who is NOT IMMUNE (never been vaccinated, anti-hbs < 10mIU/mL after one or two complete series of the hepatitis B vaccine, or has not completed their vaccination series): hepatitis B prophylais and vaccination may be required. For the eposed individual whose antibody status is UNKNOWN: immediately draw blood for anti-hbs (and label as needlestick injury ) prior to giving hepatitis B immune globulin or vaccine. Proceed as if non-immune. Initiation of posteposure prophylais should not be delayed pending test result The management of an individual who is not immune or unknown may include the following: a. Hepatitis B immune Globulin (HBIG) When indicated, HBIG should be given as soon as possible, preferably within 24 hours after the eposure. Efficacy decreases substantially when it is given >48 hours posteposure, and effectiveness when administered after 7 days is unknown. Dosage is 0.06 ml/kg body weight, total dose to be divided into two separate intramuscular administration sites. Dose should be repeated in one month in a known non-responder (e.g. anti-hbs < 10 miu/ml after two complete hepatitis B vaccination series). b. Hepatitis B vaccine series A 3-dose series is indicated in the individual who has not been immunized. Doses should be administered at a site separate from HBIG if co-administered. A second 3-dose series of the hepatitis B vaccination is indicated in an individual who is non-immune (anti-hbs<10miu/ml) after the first course. Completion of the vaccination is indicated in individuals who started the vaccination series, but did not complete all three doses. Pregnant or lactating women can receive the hepatitis B vaccine and the hepatitis B immune globulin
6 Counseling and Follow-up: In addition to receiving general counseling, the non-immune individual should be: counseled on the signs and symptoms of hepatitis that may occur within 6 weeks to 6 months after eposure (e.g. fatigue, loss of appetite, abdominal discomfort, jaundice, change in colour of urine and stool, rash, sore joints) Follow-up Tests: Hepatitis B surface antigen (at 6 months) for possible seroconversion in the non-immune patient Hepatitis B surface antibody (1-2 months after completion of the vaccine series); if HBIG was given at the same time, testing should occur at least 6 month after the HBIG dose Table 2 - Management of hepatitis B eposure Vaccination Status of Eposed HBV Status of Source HBsAg Negative HBsAg Positive or Unknown Unvaccinated Anti-HBs 10mIU/mL No further action required No further action required Anti-HBs < 10mIU/mL Vaccinated Initiate vaccine series HBIG 1* Initiate vaccine series Anti-HBs 10mIU/mL No further action required No further action required Anti-HBs < 10mIU/mL Anti-HBs level unknown Incompletely vaccinated Completed 2 vaccine series No further action required Completed 1 vaccine series initiate second vaccine series Measure Anti-HBs level in eposed, and if: Anti-HBs 10mIU/mL No further action required Anti-HBs < 10mIU/mL a. Completed 2 vaccine series No further action required b. Completed 1 vaccine series Initiate second vaccine series Completed 2 vaccine series HBIG 2, separated by 1 month* Completed 1 vaccine series HBIG 1* initiate second vaccine series Measure Anti-HBs level in eposed, and if: Anti-HBs 10mIU/mL No further action required Anti-HBs < 10mIU/mL a. Completed 2 vaccine series HBIG 2, separated by 1 month* b. Completed 1 vaccine series HBIG 1* initiate second vaccine series Received less than 3 doses Complete vaccine series If anti-hbs < 10 miu/ml after first series, a second vaccine series should be completed HBIG 1* Complete vaccine series (at regular schedule) If anti-hbs < 10 miu/ml after first series, a second vaccine series should be completed * Follow-up testing should be done at 6 months to assess for hepatitis B seroconversion If test results on the source patient reveal the source is not infected, the only required action is to ensure that the eposed individual complete the course of hepatitis B vaccination series and antibody testing. Do not delay management to wait for results of anti-hbs
7 STEP 4b PEP MANAGEMENT - HCV EXPOSURE There is no prophylactic treatment currently available for a person eposed to hepatitis C. Data do not support the use of immune globulin (IG) or antiviral agents, and thus these agents cannot be recommended. Counselling and Follow-up: In addition to receiving general counselling, the individual should be: counseled on the signs and symptoms of hepatitis that may occur within 6 weeks to 6 months after eposure (eg. fatigue, loss of appetite, abdominal discomfort, jaundice, change in colour of urine and stool, rash, sore joints) Follow-up Tests: HCV antibody at 3 and 6 months if source known or unknown to be HCV positive STEP 4c Characteristic of source PEP MANAGEMENT - HIV EXPOSURE Management of a potential HIV eposure is dependent on the risk for HIV transmission which varies with 1) the likelihood the source has transmissible HIV and 2) the nature of the eposure. In the occupational setting: PEP is indicated in the following eposures if the source is known to be HIV-positive with a detectable viral load or unknown status with significant HIV risk factors (e.g. men who have se with men, people who inject drugs): Percutaneous eposure to potentially infectious body fluid (e.g. needlestick injury including hollow-bore or solid needle) Mucous membrane eposure to potentially infectious body fluid (e.g. blood splash to eye) In the non-occupational setting: Eposure type HIV Positive HIV status unknown Viremic or unknown viral load levels unconfirmed VL<40 confirmed VL<40 and no STIs at time of eposure From high risk population (eg. MSM, PWID) From low risk population Needle sharing Initiate PEP Initiate PEP Consider PEP Initiate PEP Consider PEP Anal intercourse (receptive or insertive) Initiate PEP Consider PEP PEP not required Initiate PEP Consider PEP Vaginal intercourse (receptive or insertive) Initiate PEP Consider PEP PEP not required Initiate PEP PEP not required Human bite Consider PEP Consider PEP PEP not required Consider PEP PEP not required Oral contact (oral-penile/ oral-vaginal/ oral-anal; receiving or giving) PEP not required PEP not required PEP not required PEP not required PEP not required Risk of transmission to receptive partner increases with ejaculation, the presence of ulcers, and STIs in the mucous membrane MSM = men who have se with men, PWID = people who inject drugs, STIs = seually transmitted infections, VL = viral load PEP is NOT needed: eposure to stool, urine, tears, saliva, nasal secretions, vomitus, sweat, unless bloody. oral-to-oral contact without mucosal damage contact with intact skin if source was taking PrEP
8 HIV Post Eposure Prophylais Drug Regimens Treatment should be initiated within hours of eposure, ideally within 72 hours; however, the interval after which there is no benefit from PEP is undefined There are no human prospective randomized trials to establish the optimal number of HIV medications for PEP and no data showing superiority of any single 3-drug regimen to prevent HIV infection. If available, the source patient should be interviewed for his/her history of antiretroviral medication use to help avoid prescribing medications to which the virus may be resistant. The recommended HIV PEP regimen is: Truvada (tenofovir DF 300mg/emtricitabine 200mg) 1 tablet po once daily 28 days Dose based on adequate renal function (CrCl 50ml/min) Side effects include nausea, headache, + diarrhea, fatigue, renal failure (rare) *dolutegravir 50mg 1 tablet po once daily 28 days Side effects include headache, diarrhea, nausea, fatigue, insomnia * Based on an interim analysis of an ongoing study which has reported an increased risk of neural tube defects among infants of women who became pregnant while taking dolutegravir, until more information is available, dolutegravir should be avoided in Non-pregnant women of childbearing potential who are seually active or have been seually assaulted and who are not using an effective birth control method AND Women in early pregnancy If a dolutegravir-free regimen is required in these situations, consider using Truvada + darunavir + ritonavir OR Truvada + raltegravir as listed below Alternate antiretrovirals may be required if the source virus is known or suspected to be resistant to the above HIV PEP regimen, or based on availability, convenience or drug interactions. Other regimens that could be considered include the following: Truvada 1 tablet daily + raltegravir 400mg twice daily 28 days Truvada 1 tablet daily + darunavir 800mg daily + ritonavir 100mg daily 28 days Truvada 1 tablet daily + Prezcobi (darunavir/cobicistat 800/150mg) 1 tablet daily 28 days Stribild 1 tablet daily with food 28 days Genvoya 1 tablet daily with food 28 days (epert opinion) If the source patient s HIV serology is subsequently found to be negative and has no symptoms of acute infection, post-eposure prophylais can be discontinued and no further follow up for HIV testing is necessary. The likelihood of the source person being in the window period of HIV infection in the absence of symptoms of acute retroviral syndrome is low. The maimum window period with the 4 th generation tests is as low as 42 days. Consider consulting the Infectious Disease or HIV service for selection of optimal PEP regimen in the following scenarios: source patient is known or suspected to harbour drug resistant HIV eposed individual is known or suspected to be pregnant eposed individual is breast-feeding eposed individual with renal or liver disease which may require antiretroviral dosing adjustment eposed individual on multiple medications that may increase risk of drug interactions delayed evaluation (greater than 72 hours since eposure) Initiation of PEP should not be delayed pending this consultation. Modifications can be made later.
9 Cautions and Contraindications for Using Truvada and dolutegravir Truvada - Emtricitabine and tenofovir are primarily ecreted by the kidneys by a combination of glomerular filtration and active tubular secretion; thus, coadministration of Truvada with drugs that reduce renal function or compete for active tubular secretion may increase serum concentrations of emtricitabine, tenofovir, or other renally eliminated drugs. Some eamples include, but are not limited to NSAIDs, acyclovir, valacyclovir, and valganciclovir. Patients should use other nephrotoic drugs with caution while taking Truvada. Cautions, Contraindications and Drug Interactions with Truvada Contraindicated Patients with severe renal insufficiency (creatinine clearance of <30mL/min, including patients requiring hemodialysis) Use with Caution Patients with renal insufficiency (dose adjustment required) Creatinine Clearance: 50mL/min: 1 tablet daily ml/min: 1 tablet every 48 hours dolutegravir - dolutegravir is metabolized by UGT1A1 with some contribution from CYP3A. Dolutegravir is also a substrate of UGT1A3, UGT1A9, Pgp, and BCRP in vitro; therefore, drugs that induce those enzymes and transporters, may decrease dolutegravir plasma concentration and reduce the therapeutic effect of dolutegravir. Dolutegravir inhibits the renal organic cation transporter (OCT2) and may increase plasma concentrations of drugs that depend on OCT2 for elimination (eg. dofetilide, metformin). Cautions, Contraindications and Drug Interactions with dolutegravir The chart below lists some of the major drug interactions identified; other drug interactions may eist. Contraindicated Patients with severe hepatic impairment (Child Pugh class C) Antiarrhythmics dofetilide Use with caution dose adjustment may be required Increased dolutegravir dosing may be required in setting of severe renal impairment and possible integrase resistance Antacids, laatives, or mineral supplements containing polyvalent cations Anticonvulsants Antibiotics Antiretrovirals Herbal products aluminum (Maalo ), calcium (Tums ), magnesium (Maalo ), iron (dolutegravir should be administered 2 hours before or 6 hours after medications containing polyvalent cations) carbamazepine, ocarbazepine, phenytoin, phenobarbital rifampin efavirenz, etravirine, nevirapine, tipranavir, fosamprenavir St. John s Wort (hypericum perforatum) Oral hypoglycemics metformin Counseling and Follow-up: In addition to receiving general counseling, the eposed individual should be: counseled on the signs and symptoms of HIV infection that may occur within 2-4 weeks after eposure (e.g. flu-like symptoms, weight loss, skin rash, fever, lymphadenopathy, fatigue) counseled on the benefits and side effects of antiretroviral PEP, including the importance of adherence to prevent PEP failure counseled on the importance of safer se practices pending negative serology in follow-up Follow up HIV-associated Tests: 2 week: Serum creatinine and ALT if abnormal at baseline 12 week: HIV Ab using a fourth generation combination p24 antigen-hiv antibody test; consider repeat at 6 months if hepatitis C virus acquired from eposure A confirmatory assay should be done to confirm a diagnosis of HIV infection if a test result is positive
10 GENERAL COUNSELLING AND SUMMARY OF ALL TESTS General Counseling Following a Significant Eposure The individual should receive counseling regarding the risk of transmission following eposure to blood or potentially infectious fluid (see Risk of Transmission) To minimize secondary transmission during the first 12 weeks post-eposure, the individual should be counseled to: not donate blood, semen, tissues, or organs prevent seual transmission (e.g. barrier protection) avoid needle-sharing not share razors or toothbrushes If the source is known or suspected to be positive for HIV, the eposed individual should be counseled to avoid pregnancy and breast-feeding. If hepatitis B, hepatitis C, or HIV seroconversion occurs after a documented eposure and the eposure occurred in an occupational setting, this must be reported to the Joint Health & Safety Committee, Workplace Safety and Insurance Board (WSIB) and the Medical Officer of Health. A referral should be made for medical evaluation and consideration of antiviral therapy. Testing in the Source (if available) HIV (HIV antibody using fourth generation antigen-antibody test OR rapid HIV test per SMH ED protocol) Hepatitis B (surface antigen) Hepatitis C (hepatitis C antibody) Baseline a e Testing in the Eposed Baseline Week 2 Week 12 other HIV (HIV antibody using fourth generation antigen-antibody test OR rapid HIV test per SMH ED protocol) Hepatitis A (hepatitis A antibody) a a b Hepatitis B Surface antigen Surface antibody Core antibody (if required) c d Hepatitis C (hepatitis C antibody) e e ef Gonorrhea and chlamydia screen (urine NAAT, throat and rectal swabs for culture depending on type of seual eposure) Syphilis serology Complete blood count ALT g Serum creatinine g ß-hcg (if applicable) a Consider HIV RNA testing if there are signs/symptoms of acute HIV b Consider repeating HIV serology at 6 months after eposure if hepatitis C infection was acquired from the eposure c Repeat at 6 months if hepatitis B non-immune at time of eposure to eclude hepatitis B transmission d Hepatitis B surface antibody should be repeated 1-2 months after completion of the vaccine series; if HBIG was given at the same time, testing should occur at least 6 month after the HBIG dose e If HCV antibody is positive, follow-up with HCV RNA testing f Consider repeating at 6 months. Anti-HCV can be detected in 80% of patients within 15 weeks after eposure, in >90% within 5 months, and in >97% within 6 months (ref: MMWR 2001;50(RR05;1-43)) g Recommended if abnormal at baseline If testing in the source reveals the source is HIV negative, hepatitis B negative, or hepatitis C negative, follow-up testing for corresponding tests are not necessary in the eposed. References: See full document at Form No Dev. 06/2011 Rev. 03/2017
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
One of your office personnel
 Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
EXPOSURE (HIV/HEPATITIS) BLOOD & BODY FLUIDS
 Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
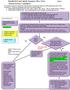 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Clinical Education Initiative OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS. Antonio E. Urbina, MD
 Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Blood-Borne Pathogens and Post-Exposure Prophylaxis
 Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
An International Antiviral Society-USA
 Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
Blood/Body Fluid Exposure Option
 Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
HIV and PEP. LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED
 HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison
 SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Exposures at Non-MUSC Clinical Sites
 BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet. Instructions for Employees/Students:
 MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
Post-Sexual Exposure Prophylaxis (npep)
 Projeto Praça Onze Universidade Federal do Rio de Janeiro Post-Sexual Exposure Prophylaxis (npep) Mauro Schechter Principal Investigator, Projeto Praça Onze Professor of Infectious Diseases Universidade
Projeto Praça Onze Universidade Federal do Rio de Janeiro Post-Sexual Exposure Prophylaxis (npep) Mauro Schechter Principal Investigator, Projeto Praça Onze Professor of Infectious Diseases Universidade
Guidelines for Implementing Pre-Exposure Prophylaxis For The Prevention of HIV in Youth Peter Havens, MD MS Draft:
 Guidelines for Implementing Pre-Exposure Prophylaxis For The Prevention of HIV in Youth Peter Havens, MD MS Draft: 10-2-2015 Clinical studies demonstrate that when a person without HIV infection takes
Guidelines for Implementing Pre-Exposure Prophylaxis For The Prevention of HIV in Youth Peter Havens, MD MS Draft: 10-2-2015 Clinical studies demonstrate that when a person without HIV infection takes
Management of Workplace Exposure to Blood-borne Pathogens
 Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Management
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP)
 Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) For Healthcare Providers About Emtricitabine/Tenofovir Disoproxil
Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) For Healthcare Providers About Emtricitabine/Tenofovir Disoproxil
MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE
 Sandyford Protocols MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE www.hiv-druginteractions.org If you require information on occupational exposure to blood borne viruses, including HIV, please refer to the
Sandyford Protocols MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE www.hiv-druginteractions.org If you require information on occupational exposure to blood borne viruses, including HIV, please refer to the
PrEP for HIV Prevention. Adult Clinical Guideline from the New York State Department of Health AIDS Institute
 PrEP for HIV Prevention Adult Clinical Guideline from the New York State Department of Health AIDS Institute www.hivguidelines.org Purpose of the PrEP Guideline Raise awareness of PrEP among healthcare
PrEP for HIV Prevention Adult Clinical Guideline from the New York State Department of Health AIDS Institute www.hivguidelines.org Purpose of the PrEP Guideline Raise awareness of PrEP among healthcare
Revised April WGH ER #5 Hospital Rd. Whitehorse Phone: (867) Outside of Whitehorse: Local Community Health Centre/Clinic/ER
 Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
Pre and Post Exposure Prophylaxis. Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018
 Pre and Post Exposure Prophylaxis Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018 Indications for Pre and Post Exposure Prophylaxis: occupational and non-occupational
Pre and Post Exposure Prophylaxis Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018 Indications for Pre and Post Exposure Prophylaxis: occupational and non-occupational
Bloodbourne Pathogens (BBP) Occupational Post-Exposure Chemophrophylaxis
 Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Hepatitis B Control. Table of Contents
 Control Table of Contents 1.0 INTRODUCTION... 1 2.0 GOALS... 1 3.0 DEFINITIONS... 1 4.0 CASE MANAGEMENT... 3 5.0 HEPATITIS B POST- EXPOSURE MANAGEMENT... 4 TABLE 1: HEPATITIS B POST EXPOSURE PROPHYLAXIS...
Control Table of Contents 1.0 INTRODUCTION... 1 2.0 GOALS... 1 3.0 DEFINITIONS... 1 4.0 CASE MANAGEMENT... 3 5.0 HEPATITIS B POST- EXPOSURE MANAGEMENT... 4 TABLE 1: HEPATITIS B POST EXPOSURE PROPHYLAXIS...
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS
 STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses
 Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
Confirmed (Laboratory Tests) Serum positive for IgM anti-hbc or, hepatitis B surface antigen (HbsAg).
 Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Chapter 2 Hepatitis B Overview
 Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
Chapter 2 Hepatitis B Overview 23 24 This page intentionally left blank. HEPATITIS B OVERVIEW Hepatitis B Virus The hepatitis B virus (HBV) belongs to the Hepadnaviridae family and is known to cause both
Exposure. Blood. Department of Health & Human Services
 Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
TO DECREASE THE CHANCE OF GETTING
 POST ASSAULT MEDICATIONS Terri Stewart RN Naomi Sugar MD TO DECREASE THE CHANCE OF GETTING Pregnant STDs Chlamydia Gonorrhea Trichomonas Hep B HIV EMERGENCY CONTRACEPTION Plan B Levonorgestrel PLAN B Plan
POST ASSAULT MEDICATIONS Terri Stewart RN Naomi Sugar MD TO DECREASE THE CHANCE OF GETTING Pregnant STDs Chlamydia Gonorrhea Trichomonas Hep B HIV EMERGENCY CONTRACEPTION Plan B Levonorgestrel PLAN B Plan
determine need but regimen does not change based on risk factor ED medicine attending meets
 HIV PEP Guidelines after Sexual Assault Harborview Medical Center January 2015 Initial risk assessment and discussion with patient about risk and PEP is by clinician who does exam (SANE, Ob-Gyn resident,
HIV PEP Guidelines after Sexual Assault Harborview Medical Center January 2015 Initial risk assessment and discussion with patient about risk and PEP is by clinician who does exam (SANE, Ob-Gyn resident,
Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication. For Healthcare Providers
 Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication For Healthcare Providers About TRUVADA for a PrEP Indication INDICATION AND PRESCRIBING CONSIDERATIONS TRUVADA,
Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication For Healthcare Providers About TRUVADA for a PrEP Indication INDICATION AND PRESCRIBING CONSIDERATIONS TRUVADA,
Safety Tips from the WorkSafe People
 Blood Borne Pathogens Training HIV/AIDS Hepatitis B Determining Exposure Protecting Yourself Preventing Exposure during an Emergency HIV/AIDS Definition: AIDS stands for Acquired Immune Deficiency Syndrome.
Blood Borne Pathogens Training HIV/AIDS Hepatitis B Determining Exposure Protecting Yourself Preventing Exposure during an Emergency HIV/AIDS Definition: AIDS stands for Acquired Immune Deficiency Syndrome.
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Prophylaxis
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
Definitions. Appendix A
 Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
El Paso - Ambulatory Clinic Policy and Procedure
 Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
PREP IN PRIMARY CARE TRACY SALAMEH RN, BSN, ACRN HIV CLINICAL SPECIALIST DAKOTA AIDS EDUCATION AND TRAINING CENTER
 PREP IN PRIMARY CARE TRACY SALAMEH RN, BSN, ACRN HIV CLINICAL SPECIALIST DAKOTA AIDS EDUCATION AND TRAINING CENTER THE NEED FOR CONTINUED HIV PREVENTION Estimated new HIV infections in the US for the most
PREP IN PRIMARY CARE TRACY SALAMEH RN, BSN, ACRN HIV CLINICAL SPECIALIST DAKOTA AIDS EDUCATION AND TRAINING CENTER THE NEED FOR CONTINUED HIV PREVENTION Estimated new HIV infections in the US for the most
PrEP and npep for HIV Prevention. Harry Rosado-Santos MD, FACP Associate Professor UU School of Medicine
 PrEP and npep for HIV Prevention Harry Rosado-Santos MD, FACP Associate Professor UU School of Medicine Case Study A 26 y/o M presents for routine asymptomatic screening for sexually transmitted infections
PrEP and npep for HIV Prevention Harry Rosado-Santos MD, FACP Associate Professor UU School of Medicine Case Study A 26 y/o M presents for routine asymptomatic screening for sexually transmitted infections
HIV Management Update 2015
 9/30/15 HIV Management Update 2015 Larry Pineda, PharmD, PhC, BCPS Visiting Assistant Professor Pharmacy Practice and Administrative Science ljpineda@salud.unm.edu Pharmacist Learning Objectives Describe
9/30/15 HIV Management Update 2015 Larry Pineda, PharmD, PhC, BCPS Visiting Assistant Professor Pharmacy Practice and Administrative Science ljpineda@salud.unm.edu Pharmacist Learning Objectives Describe
Bloodborne Pathogens and Exposure Control
 Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV
 Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
43. Guidelines on Needle stick Injury
 43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers
 Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers About emtricitabine/tenofovir disoproxil fumarate for HIV-1 PrEP
Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers About emtricitabine/tenofovir disoproxil fumarate for HIV-1 PrEP
Antonio E. Urbina, M.D. Associate Medical Director Center for Comprehensive Care, West 17 th Street Clinic St. Luke s Roosevelt Hospital DISCLOSURES
 PROPHYLAXISFOLLOWINGHIV, HEPB ANDC EXPOSURES: WHAT SNEW Antonio E. Urbina, M.D. Associate Medical Director Center for Comprehensive Care, West 17 th Street Clinic St. Luke s Roosevelt Hospital DISCLOSURES
PROPHYLAXISFOLLOWINGHIV, HEPB ANDC EXPOSURES: WHAT SNEW Antonio E. Urbina, M.D. Associate Medical Director Center for Comprehensive Care, West 17 th Street Clinic St. Luke s Roosevelt Hospital DISCLOSURES
Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Bloodborne Pathogens Exposure Procedure
 Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY
 CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital
 HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital HIV continues to be a major global public health issue, Approximately
HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital HIV continues to be a major global public health issue, Approximately
FAMILY HIV CENTER NJ CARES SANE
 HIV, Hepatitis B & C Postexposure Prophylaxis for Sexual Assault Victims Update for Case Managers Susan Burrows-Clark, RN, MSN, CNS, C. 10/17/11 Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug
HIV, Hepatitis B & C Postexposure Prophylaxis for Sexual Assault Victims Update for Case Managers Susan Burrows-Clark, RN, MSN, CNS, C. 10/17/11 Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug
Professional Practice Meeting February Hepatitis C
 Professional Practice Meeting February 2018 Hepatitis C Hepatitis C Overview Hepatitis C Virus Enveloped, single-stranded RNA virus 6 main genotypes: Rapidly mutating virus makes vaccination design difficult
Professional Practice Meeting February 2018 Hepatitis C Hepatitis C Overview Hepatitis C Virus Enveloped, single-stranded RNA virus 6 main genotypes: Rapidly mutating virus makes vaccination design difficult
Hepatitis C Best Practice Guidelines For Local Health Departments
 Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication. Training Guide for Healthcare Providers
 TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication Training Guide for Healthcare Providers About TRUVADA for a PrEP indication to reduce the risk of sexually acquired HIV-1 infection in high-risk
TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication Training Guide for Healthcare Providers About TRUVADA for a PrEP indication to reduce the risk of sexually acquired HIV-1 infection in high-risk
PEDIATRIC EMERGENCY DEPARTMENT CLINICAL GUIDELINE: NON- OCCUPATIONAL EXPOSURE TO BLOOD-BORNE PATHOGENS (HIV, Hepatitis B, AND Hepatitis C)
 General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
HIV, STI AND OTHER BLOOD- BORNE DISEASES. Kolářová M., EPI Autumn 2015
 HIV, STI AND OTHER BLOOD- BORNE DISEASES Kolářová M., EPI Autumn 2015 Chlamydia infection Genital warts Gonorrhoea Hepatitis B Hepatitis C HIV infection and AIDS Human papillomavirus infection Sexually
HIV, STI AND OTHER BLOOD- BORNE DISEASES Kolářová M., EPI Autumn 2015 Chlamydia infection Genital warts Gonorrhoea Hepatitis B Hepatitis C HIV infection and AIDS Human papillomavirus infection Sexually
Non-occupational HIV Post Exposure Prophylaxis (npep)
 Mountain West AIDS Education and Training Center Non-occupational HIV Post Exposure Prophylaxis (npep) Robert Harrington, M.D. This presentation is intended for educational use only, and does not in any
Mountain West AIDS Education and Training Center Non-occupational HIV Post Exposure Prophylaxis (npep) Robert Harrington, M.D. This presentation is intended for educational use only, and does not in any
BLOODBORNE PATHOGENS Online Training for Buncombe County Public School Employees
 BLOODBORNE PATHOGENS Online Training for Buncombe County Public School Employees Buncombe County Public Schools require employees to receive annual training for Bloodborne Pathogens. This online training
BLOODBORNE PATHOGENS Online Training for Buncombe County Public School Employees Buncombe County Public Schools require employees to receive annual training for Bloodborne Pathogens. This online training
Commonly Asked Questions About Chronic Hepatitis C
 Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
ACS BLOOD BORNE PATHOGEN TRAINING
 ACS BLOOD BORNE PATHOGEN TRAINING OBJECTIVE Define Blood borne pathogens Instruct how to recognize exposure to BBP Prevent or reduce risk of BBP exposure Identify high risk groups Review ACS exposure protocol
ACS BLOOD BORNE PATHOGEN TRAINING OBJECTIVE Define Blood borne pathogens Instruct how to recognize exposure to BBP Prevent or reduce risk of BBP exposure Identify high risk groups Review ACS exposure protocol
STI s. (Sexually Transmitted Infections)
 STI s (Sexually Transmitted Infections) Build Awareness In Canada and around the world, the trend is clear: sexually transmitted infections (STIs) are on the rise. One of the primary defenses in the fight
STI s (Sexually Transmitted Infections) Build Awareness In Canada and around the world, the trend is clear: sexually transmitted infections (STIs) are on the rise. One of the primary defenses in the fight
HIV Update Objectives. Epidemiology. Epidemiology, Transmission and Natural History. Transmission Risk by Exposure. Transmission 9/29/2014
 Objectives HIV Update 2014 Jay Sizemore, MD, MPH Medical Director Chattanooga CARES Assistant Professor UTCOM Chattanooga 2October 2014 Review HIV epidemiology and screening/testing guidelines Discuss
Objectives HIV Update 2014 Jay Sizemore, MD, MPH Medical Director Chattanooga CARES Assistant Professor UTCOM Chattanooga 2October 2014 Review HIV epidemiology and screening/testing guidelines Discuss
Postexposure prophylaxis (PEP)
 Postexposure Prophylaxis Against Human Immunodeficiency Virus MICHAEL A. TOLLE, MD, MPH, and HEIDI L. SCHWARZWALD, MD, MPH, Baylor College of Medicine, Houston, Texas Family physicians often encounter
Postexposure Prophylaxis Against Human Immunodeficiency Virus MICHAEL A. TOLLE, MD, MPH, and HEIDI L. SCHWARZWALD, MD, MPH, Baylor College of Medicine, Houston, Texas Family physicians often encounter
Introduction to Blood Borne Pathogens
 Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Non-Occupational Post-Exposure HIV Prophylaxis npep
 Non-Occupational Post-Exposure HIV Prophylaxis npep Peter Meacher MD (Chief Medical Officer) Anthony Vavasis MD (Director of Medicine) Callen-Lorde Community Health Center Objectives - at the end of the
Non-Occupational Post-Exposure HIV Prophylaxis npep Peter Meacher MD (Chief Medical Officer) Anthony Vavasis MD (Director of Medicine) Callen-Lorde Community Health Center Objectives - at the end of the
Bloodborne Pathogens. By Deborah Massarella RN, MSN
 Bloodborne Pathogens By Deborah Massarella RN, MSN 2010, 2014 Objectives At conclusion of in-service learners will: 1. Identify two types of personal protective equipment. 2. Identify two prevention measures
Bloodborne Pathogens By Deborah Massarella RN, MSN 2010, 2014 Objectives At conclusion of in-service learners will: 1. Identify two types of personal protective equipment. 2. Identify two prevention measures
Hepatitis B and C Overview, Outbreaks, and Recommendations. Viral Hepatitis Language. Types of Viral Hepatitis 7/1/2013
 Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
Bloodborne Pathogen Exposure STUDENT. Blood Borne Pathogen Exposure. Student. Rev:
 Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Post Exposure Prophylaxis (PEP)
 Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance
Post Exposure Prophylaxis (PEP) Occupational exposure Occupational exposure refers to exposure to potential blood-borne infections (HIV, HBV and HCV) that may occur in healthcare settings during performance
SFAF CLINICAL PROTOCOLS
 SFAF CLINICAL PROTOCOLS Page 1 of Supersedes Date: December 31, 2016 Original Date: August 20, 2014 Version: 03 Policy Section: Patient Care Non-Occupational Post Exposure Prophylaxis Program Back ground:
SFAF CLINICAL PROTOCOLS Page 1 of Supersedes Date: December 31, 2016 Original Date: August 20, 2014 Version: 03 Policy Section: Patient Care Non-Occupational Post Exposure Prophylaxis Program Back ground:
HEPATITIS B INFECTION and Pregnancy. Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011
 HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
HEPATITIS B INFECTION and Pregnancy Caesar Mensah Communicable Diseases & Infection Control Specialist, UK June 2011 HEPATITIS B 26/07/2011 What is Hepatitis B? It is inflammation (infection) of the liver
Post-Exposure Prophylaxis Review for International Visitors
 Post-Exposure Prophylaxis Review for International Visitors Case Presentation 27 yo nurse presents to Urgent Care for a needlestick 2 days ago from a diabetic lancet. Source patient (SP): 35 yo male known
Post-Exposure Prophylaxis Review for International Visitors Case Presentation 27 yo nurse presents to Urgent Care for a needlestick 2 days ago from a diabetic lancet. Source patient (SP): 35 yo male known
Infection/Disease Control HEPATITIS CONTROL PROGRAM
 OPERATING PROCEDURE NO. 153-29 Florida State Hospital Chattahoochee, Florida July 8, 2009 Infection/Disease Control HEPATITIS CONTROL PROGRAM 1. Purpose: To establish consistent surveillance, reporting,
OPERATING PROCEDURE NO. 153-29 Florida State Hospital Chattahoochee, Florida July 8, 2009 Infection/Disease Control HEPATITIS CONTROL PROGRAM 1. Purpose: To establish consistent surveillance, reporting,
patients with blood borne viruses Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled Document Lead:
 CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
Obstetric Complications in HIV-Infected Women. Jeanne S. Sheffield, MD Maternal-Fetal Medicine UT Southwestern Medical School
 Obstetric Complications in HIV-Infected Women Jeanne S. Sheffield, MD Maternal-Fetal Medicine UT Southwestern Medical School Obstetric Complications and HIV Obstetric complications are not increased in
Obstetric Complications in HIV-Infected Women Jeanne S. Sheffield, MD Maternal-Fetal Medicine UT Southwestern Medical School Obstetric Complications and HIV Obstetric complications are not increased in
Training for Employees of Taylor Special Care Services, Inc.
 Training for Employees of Taylor Special Care Services, Inc. TSCS Taylor Special Care Services housing staffing counseling on-going support Simon Pop, MBA Chief Operating Officer 2015 2016 Guidelines:
Training for Employees of Taylor Special Care Services, Inc. TSCS Taylor Special Care Services housing staffing counseling on-going support Simon Pop, MBA Chief Operating Officer 2015 2016 Guidelines:
C 肝職業暴露後之處置 衛福部疾病管制署 中區傳染病防治醫療網 王任賢指揮官
 C 肝職業暴露後之處置 衛福部疾病管制署 中區傳染病防治醫療網 王任賢指揮官 HCV:Structure and Classification Unclassified virus, Member of the flavivirus family (other members yellow fever and dengue) Enveloped single stranded RNA virus Humans
C 肝職業暴露後之處置 衛福部疾病管制署 中區傳染病防治醫療網 王任賢指揮官 HCV:Structure and Classification Unclassified virus, Member of the flavivirus family (other members yellow fever and dengue) Enveloped single stranded RNA virus Humans
How is it transferred?
 STI s What is a STI? It is a contagious infection that is transferred from one person to another through sexual intercourse or other sexually- related behaviors. How is it transferred? The organisms live
STI s What is a STI? It is a contagious infection that is transferred from one person to another through sexual intercourse or other sexually- related behaviors. How is it transferred? The organisms live
Bloodborne Pathogen Refresher Training
 Bloodborne Pathogen Refresher Training This program will review your occupational risks and the steps that you and the County must take to reduce your risks of exposure. Employees must report any occupational
Bloodborne Pathogen Refresher Training This program will review your occupational risks and the steps that you and the County must take to reduce your risks of exposure. Employees must report any occupational
PrEP in the Real World: Clinical Case Studies
 PrEP in the Real World: Clinical Case Studies Kevin L. Ard, MD, MPH April 30, 2015 Massachusetts General Hospital, National LGBT Health Education Center Continuing Medical Education Disclosure Program
PrEP in the Real World: Clinical Case Studies Kevin L. Ard, MD, MPH April 30, 2015 Massachusetts General Hospital, National LGBT Health Education Center Continuing Medical Education Disclosure Program
Management of sharps injuries in the healthcare setting
 Link to this article online for CPD/CME credits Management of sharps injuries in the healthcare setting Anna Riddell, 1 Ioana Kennedy, 2 C Y William Tong 1 3 1 Department of Infection, Barts Health NHS
Link to this article online for CPD/CME credits Management of sharps injuries in the healthcare setting Anna Riddell, 1 Ioana Kennedy, 2 C Y William Tong 1 3 1 Department of Infection, Barts Health NHS
Hepatitis B is a virus that attacks the liver. It is highly infectious. Hepatitis B is transmitted primarily
 BLOOD BORNE PATHOGENS TRAINING FOR SCHOOL STAFF Blood Borne Pathogen (BBP): A blood borne pathogen is defined as an organism found in human blood or other infected body fluids that may cause disease in
BLOOD BORNE PATHOGENS TRAINING FOR SCHOOL STAFF Blood Borne Pathogen (BBP): A blood borne pathogen is defined as an organism found in human blood or other infected body fluids that may cause disease in
You will now begin the Bloodborne Pathogen Refresher Training.
 You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
Viral hepatitis. Supervised by: Dr.Gaith. presented by: Shaima a & Anas & Ala a
 Viral hepatitis Supervised by: Dr.Gaith presented by: Shaima a & Anas & Ala a Etiology Common: Hepatitis A Hepatitis B Hepatitis C Hepatitis D Hepatitis E Less common: Cytomegalovirus EBV Rare: Herpes
Viral hepatitis Supervised by: Dr.Gaith presented by: Shaima a & Anas & Ala a Etiology Common: Hepatitis A Hepatitis B Hepatitis C Hepatitis D Hepatitis E Less common: Cytomegalovirus EBV Rare: Herpes
Blood-borne Pathogens Exposure Policy
 Blood-borne Pathogens Exposure Policy Contents Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis... 1 STUDENT CHECKLIST... 3 PROGRAM CHECKLIST... 4 PROVIDER CHECKLIST... 5 POST-EXPOSURE
Blood-borne Pathogens Exposure Policy Contents Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis... 1 STUDENT CHECKLIST... 3 PROGRAM CHECKLIST... 4 PROVIDER CHECKLIST... 5 POST-EXPOSURE
Blood Borne Pathogens. Becky Walch, R.N. Micheel Valdez, L.V.N.
 Blood Borne Pathogens Becky Walch, R.N. Micheel Valdez, L.V.N. Examples of Blood Borne Pathogens Hepatitis B Hepatitis C Other Hepatitis HIV Hepatitis Hepatitis means inflammation of the liver. Hepatitis
Blood Borne Pathogens Becky Walch, R.N. Micheel Valdez, L.V.N. Examples of Blood Borne Pathogens Hepatitis B Hepatitis C Other Hepatitis HIV Hepatitis Hepatitis means inflammation of the liver. Hepatitis
Case Studies in PrEP Management. Kevin L. Ard, MD, MPH Massachusetts General Hospital, National LGBT Health Education Center April 15, 2016
 Case Studies in PrEP Management Kevin L. Ard, MD, MPH Massachusetts General Hospital, National LGBT Health Education Center April 15, 2016 Continuing Medical Education Disclosure Program Faculty: Kevin
Case Studies in PrEP Management Kevin L. Ard, MD, MPH Massachusetts General Hospital, National LGBT Health Education Center April 15, 2016 Continuing Medical Education Disclosure Program Faculty: Kevin
What is the most important information I should know about tenofovir? What should I discuss with my healthcare provider before taking tenofovir?
 1 of 6 6/10/2016 4:33 PM Generic Name: tenofovir (ten OF oh vir) Brand Name: Viread What is tenofovir? Tenofovir is an antiviral medicine that prevents human immunodeficiency virus (HIV) or hepatitis B
1 of 6 6/10/2016 4:33 PM Generic Name: tenofovir (ten OF oh vir) Brand Name: Viread What is tenofovir? Tenofovir is an antiviral medicine that prevents human immunodeficiency virus (HIV) or hepatitis B
Infectious Disease and Bloodborne Pathogens Training St. Michael-Albertville Public Schools
 Infectious Disease and Bloodborne Pathogens Training St. Michael-Albertville Public Schools Questions? Any time throughout the slide show or throughout the school year: Contact Jake Baxter at IEA Phone:
Infectious Disease and Bloodborne Pathogens Training St. Michael-Albertville Public Schools Questions? Any time throughout the slide show or throughout the school year: Contact Jake Baxter at IEA Phone:
Bloodborne Pathogens Across the Continuum of Care Sue Sebazco, Arlington, Texas A Webber Training Teleclass
 Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV
 POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV DISCLOSURE Relevant relationships with commercial entities none Potential for conflicts of interest within this presentation none
POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV DISCLOSURE Relevant relationships with commercial entities none Potential for conflicts of interest within this presentation none
Bloodborne Infectious Diseases
 Bloodborne Infectious Diseases Dr. Kaya Süer Near East University Faculty of Medicine Infectious Diseases and Clinical Microbiology Bloodborne Pathogens Bloodborne pathogens Pathogenic organisms present
Bloodborne Infectious Diseases Dr. Kaya Süer Near East University Faculty of Medicine Infectious Diseases and Clinical Microbiology Bloodborne Pathogens Bloodborne pathogens Pathogenic organisms present
BLOODBORNE PATHOGENS FOR HEALTH CARE WORKERS CURRICULUM
 Page 1 of 13 BLOODBORNE PATHOGENS FOR HEALTH CARE WORKERS CURRICULUM INTRODUCTION The attached materials will assist in teaching the information about bloodborne pathogens for health care workers as required
Page 1 of 13 BLOODBORNE PATHOGENS FOR HEALTH CARE WORKERS CURRICULUM INTRODUCTION The attached materials will assist in teaching the information about bloodborne pathogens for health care workers as required
Bloodborne Pathogens Training For School Personnel
 Bloodborne Pathogens Training For School Personnel OSHA Defined: Occupational Safety and Health Administration Published a standard to reduce or eliminate health risk, resulting in: Annual training of
Bloodborne Pathogens Training For School Personnel OSHA Defined: Occupational Safety and Health Administration Published a standard to reduce or eliminate health risk, resulting in: Annual training of
Gilead Announces Data Demonstrating Non-Inferiority of Once-Daily Descovy vs. Once-Daily Truvada for Prevention of HIV Infection
 Gilead Announces Data Demonstrating Non-Inferiority of Once-Daily Descovy vs. Once-Daily Truvada for Prevention of HIV Infection March 6, 2019 DISCOVER Trial Meets Primary and Secondary Endpoints and Will
Gilead Announces Data Demonstrating Non-Inferiority of Once-Daily Descovy vs. Once-Daily Truvada for Prevention of HIV Infection March 6, 2019 DISCOVER Trial Meets Primary and Secondary Endpoints and Will
Environmental Health and Safety Offices BLOODBORNE PATHOGENS
 Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
Environmental Health and Safety Offices BLOODBORNE PATHOGENS Purpose! Reduce / eliminate exposure potential Comply with Ohio s Public Employment Risk Reduction Act (reference OSHA) 2! Exposure Determination!
Hepatitis STARS Program. Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003
 Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Infection Control Standard Precautions. CDC Recommendations: Application of Standard Precautions for All Patients
 Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Contamination Incidents Frequently Asked Questions
 Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
