Cross regulatory interactions between Fgf8 and Shh in the avian frontonasal prominence
|
|
|
- Daisy Harper
- 5 years ago
- Views:
Transcription
1 136 Congenital Anomalies 2007; 47, doi: /j x ORIGINAL ARTICLE Cross regulatory interactions between Fgf8 and Shh in the avian frontonasal prominence Arhat Abzhanov 1 *, Dwight R. Cordero 2, Jonaki Sen 1, Clifford J. Tabin 1, and Jill A. Helms 3 1 Department of Genetics, Harvard Medical School, 2 Department of Obstetrics, Gynecology and Reproductive Biology, Brigham and Women s Hospital and Harvard Medical School, Boston, Massachusetts, and 3 Department of Plastic and Reconstructive Surgery, Stanford University, Stanford, California, USA ABSTRACT The frontonasal prominence of the developing avian embryo contains an organizing center, defined by juxtaposition of the Sonic hedgehog (Shh) and Fibroblast growth factor 8 (Fgf8) expression domains. This molecular interface presages any detectable growth of the frontonasal prominence, and experiments involving transplantation of this boundary epithelium have demonstrated it is a source of dorsalventral and rostral-caudal patterning information for the neural crest-derived mesenchyme of the upper beak. We explored the ontogeny of this organizing center by mapping the expression domains of both genes and their receptors and downstream targets. We tested the extent to which Shh and Fgf8 regulate each other s expression in this frontonasal organizer by either blocking or ectopically activating these pathways. Our experiments revealed mutual antagonism between the two molecules, which aids in establishing and maintaining a molecular boundary that subsequently influences patterning and growth of the middle and upper face. Key Words: cerebral cortex, cranial neural crest, craniofacial, differentiation, Fgf8, forebrain, Shh, signaling INTRODUCTION A discrete region of facial ectoderm regulates dorsal-ventral patterning and proximal-distal growth of the frontonasal prominence (FNP) based on the fact that when ectopically transplanted, the ectodermal graft causes a molecular re-patterning of Hox-free neural crest (Hu et al. 2003). The end result is a duplication of distal FNP structures, including the premaxilla, and an epithelial specialization on the avian beak called the egg tooth (Hu et al. 2003). In this regard, a discrete region of facial ectoderm, which we call the frontonasal ectodermal zone (FEZ), exhibits features that are shared by other embryonic organizing centers (Spemann & Mangold 1924). The juxtaposition of Shh and Fgf8 expression domains in the FEZ suggest that these two molecules play a role in mediating FEZ organizing activity. In other regions of the craniofacial complex, Fgf8 and Shh provide critical instructions that direct the patterning and growth of underlying cranial neural crest cells (Couly et al. 1993; Francis-West et al. 1998; Trainor et al. 2002; Cordero et al. Correspondence: Jill A. Helms DDS, PhD, Department of Plastic and Reconstructive Surgery, Stanford University, Stanford, CA 94305, USA. jhelms@stanford.edu Received June ; revised and accepted August *Present address: Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA. 2004; Creuzet et al. 2004; Jeong et al. 2004). These data indicate that independently, Fgf8 and Shh are important for proper patterning of the FNP; they do not, however, test the functional significance of a molecular boundary between the two molecules. In previous experiments, we transplanted dorsal Fgf8-positive ectoderm to a more ventral region of the FNP, and positioned ventral Shh-positive ectoderm in a more dorsal location; consequently, the resulting upper beak has an inverted dorsoventral polarity (Hu et al. 2003). While this result indicates that the relative positions of Shh and Fgf8 are important for proper craniofacial morphogenesis, it does not exclude the possibility that other molecules expressed in that region of the frontonasal ectoderm are responsible for the shift in facial patterning. In an effort to clarify the importance of a molecular boundary between these two signaling molecules we identified where and when this boundary is established in the brain and in the face, and how long it persists. We then performed a series of gain- and loss-of-function experiments within the avian FNP to test how Shh and Fgf8 affected each other s expression, and to determine the morphological effects resulting from perturbations in the molecular boundary. Taken together, these findings illustrate that the maintenance of a boundary between Fgf8 and Shh in the FNP depends on reciprocal inhibitory interactions between the two molecules, not only in the frontonasal prominence, but also in the underlying forebrain neuroectoderm. MATERIALS AND METHODS Viral infections of early embryos Fertilized eggs were obtained from SPAFAS (Norwich, CT, USA), incubated at 100 F, and the embryos were staged according to Hamburger and Hamilton (Hamburger & Hamilton 1951). The RCAS::Fgf8 and RCAS::Shh constructs have been described in detail elsewhere (Riddle et al. 1993; Abzhanov & Tabin 2004). To infect embryos for in vivo studies we pooled high-titer concentrated virus into the peri-facial region of stage 15 embryos. A control virus of a similar titer, RCAS(B)::AP (alkaline phosphatase), was used to display the extent of epithelial infection. After 36 h of infection (i.e. stage 20) and 48 h (i.e. stage 23; not shown) the extent of infection ranged from patchy (10 20% of head surface) to consistent (60 70% of head surface) staining, indicating that there was an inherent variability in the extent of protein production within the frontonasal prominence. RNAi construct preparation and testing The RNAi construct targeting chick Fgf8 was generated using pcs/u6 vector (Harpavat and Cepko, pers. comm.), which is a modified version of pbs/u6 (Sui et al. 2002). The following annealed oligonucleotides were digested with ApaI (blunted) and
2 Cross-regulation of Fgf8 and Shh 137 EcoRI and inserted in the vector: 5 -GGAGCAGAGCCTGGTGA CAGA-3, 5 -AGCTTCTGTCACCAGGCTCTGCTCC-3, 5 -AG CTTCTGTCACCAGGCTCTGCTCCCTTTTTG-3 and 5 -AATT CAAAAAGGGAGCAGAGCCTGGTGACAGA-3. These oligonucleotides correspond to Fgf8 coding region The U6-hairpin construct was then cloned into the RCAS viral system using Gateway cloning (Invitrogen, Carlsbad, CA, USA). These viruses are effective at knocking down targeted transcripts and protein in vivo (Harpavat and Cepko, pers. comm.). The RNAi constructs were tested in vitro and in vivo as previously described (Matsuda & Cepko 2004). Cyclopamine treatment Cyclopamine was obtained from Toronto Research Chemicals (Toronto, Canada) and diluted in 95% ethanol to a stock solution of 10 mm. This stock was used to make a 50 mm cyclopamine solution in 1% ethanol/phosphate buffered saline (PBS). We injected ml of this cyclopamine stock solution or 1% ethanol/pbs controls into the peri-facial region of stage 15 chick embryos. Embryos were evaluated and collected 36 and 48 h following exposure. Immunohistochemical procedures and in situ hybridization In situ hybridization analyzes were performed on whole chick embryos as well as paraffin wax-embedded sections as described (Albrecht et al. 1997). Subclones of the various cdnas were linearized to transcribe digoxygenin-labeled antisense riboprobes, and hybridizations and washes were performed at high stringency as described elsewhere (Albrecht et al. 1997). Morphologic evaluation The embryonic heads were photographed and measured in NIH Image 1.62 (National Institutes of Health, Bethesda, MD, USA). Arbitrary measurements were used for the Analysis of Variance function (anova toolbox; t-test) in Excel X to calculate standard deviations for the data. RESULTS Ontogeny of the Shh and Fgf8 boundary in facial ectoderm correlates with FEZ activity We examined the expression patterns of Fgf8 and Shh at stage 15 to determine when a molecular boundary is first established between these two molecules and how long such a molecular boundary persists. By whole-mount in situ hybridization Shh is faintly visible in the neuroectoderm, but is conspicuously absent from the surface (facial) ectoderm (Fig. 1A); at the same stage, Fgf8 is expressed in a dorsal region of facial ectoderm (Fig. 1B). By sectioned in situ hybridization, Shh expression in the neuroectoderm can be localized to a ventral region (Fig. 1C,E), while Fgf8 is expressed in a complementary, more dorsal domain (Fig. 1D,F). Both molecules are expressed in overlapping regions of the pharyngeal endoderm as well (Fig. 1E,F). Based on the expression of their receptors, and in the case of Shh, a downstream target gene Gli1, the Shh and Fgf8 pathways are potentially acting in the neuroectoderm, the facial ectoderm, and in the facial neural crest at stage 15 (Fig. 1G J). Ptc1 expression is noted the neuroectoderm which corresponds to Shh expression (Fig. 1E), whereas Gli1expression is broader, involving the neuroectoderm, mesenchyme and not head ectoderm (Fig. 1G,I). The expression patterns of (Fig. 1H) Fgfr1 and (Fig. 1J) Fgfr2 are broad (Fig. 1H), involving the neuro and facial ectoderm, facial mesenchyme (Fig. 1J) dorsal neural and facial ectoderm, respectively. Since Fgf8 was expressed in facial ectoderm at stage 15, we examined older embryos to determine when a molecular boundary between Shh and Fgf8 was established in facial ectoderm. By whole-mount in situ hybridization Shh expression is first evident at stage 20 in the ventral facial ectoderm (Fig. 2A); sectioned in situ hybridization analyzes confirmed this induction in facial ectoderm (Fig. 2G, arrowhead), and also illustrated another new domain of Shh in the ventral telencephalon (Fig. 2G, asterisk) not present at stage 15 (Fig. 1E). Shh continued to be expressed in the diencephalon and in pharyngeal endoderm at stage 20. Ptc1 and Gli1 transcripts were detected throughout the neuroectoderm, facial ectoderm, and facial neural crest, indicating that all three tissues were targets of Shh activity at stage 20 (Fig. 2J,M). Shh persisted in ventral facial ectoderm, as well as the other neuroectodermal and pharyngeal endodermal domains from stage 22 through 25 (Fig. 2B,C,H,I) as did Ptc1 and Gli1 (Fig. 2K,L,N,O), which indicated the pathway was likely to be active even at these later stages. The expression domains of Ptc1 and Gli1 overlap in the ventral neuroectoderm and facial ectoderm. Ptc1 expression appears less prominent in the more dorsal neuroectodermal domains of Ptc1 appear less than that observed in the same domains of Gli1. This may reflect the decrease in negative regulation from Shh, since Ptc1 expression is increased by Shh. We next examined Fgf8 expression at the same time points as we had used in our Shh analysis. By whole-mount in situ hybridization we found that Fgf8 persisted in dorsal facial ectoderm at stage 20 (Fig. 3A). Sectioned in situ hybridization analyzes bore this out and also revealed Fgf8 expression in the dorsal telencephalon and the optic recess (Fig. 3C). Fgf receptors 1, 2 and 3 were widely expressed in the neuroectoderm, facial ectoderm, and facial neural crest at this stage as well (Fig. 3D F). By stage 22, however, there was a notable change in the facial Fgf8 domain: by both wholemount and section analyzes we found that Fgf8 expression was barely detectable in facial ectoderm (Fig. 3G,I, arrowheads); expression persisted, however, in the optic recess, the isthmus, the mandible, and elsewhere in the craniofacial complex (Fig. 3I). Likewise, Fgf receptors I, 2, and 3 continued to be strongly expressed throughout the craniofacial tissues (Fig. 3J L). By stage 25, Fgf8 expression was not detected in the frontonasal prominence ectoderm, but was retained in the nasal ectoderm, the optical recess, and pharyngeal endoderm (Fig. 3M,O). The three Fgf receptors continued to be widely expressed in the craniofacial tissues as they were at earlier stages (Fig. 3P R). Double in situ hybridization demonstrated the relative positions of the Shh and Fgf8 expression domains at two key time points, stage 15 and stage 20 (Figs 4A,B; 8A). (i) At stage 15, Fgf8 (black) is expressed in the rostro-dorsal domain in the forebrain and facial ectoderm. Shh(brown) is expressed in the ventral diencepahlon. (ii) In stage 20 embryos, the Fgf8 facial domain does not change, however, two new domains of Shh are observed; one in the telencephalon and the second in the facial ectoderm. (iii) The juxtaposed domains of Fgf8 and Shh in the facial ectoderm formed a molecular boundary, the FEZ. Our previous transplantation studies indicated that the FEZ had organizing activity at stage 20 but lost this ability by stage 25 (Hu et al. 2003). We therefore wondered if the transient molecular boundary of Fgf8 and Shh, which only existed from stage 20 22, was responsible for FEZ activity. We explored this issue by manipulating Fgf8 and Shh signaling, independent of one another, after first assessing the morphological consequences of these assays. Morphological effects of Fgf8 disruption To understand the morphological significance of the Fgf8/Shh boundary, we started by modifying the normal expression pattern of
3 138 A. Abzhanov et al.
4 Cross-regulation of Fgf8 and Shh 139 Fig. 1 Fgf8 expression in dorsal facial ectoderm is established before Shh induction in ventral facial ectoderm.. (A) At stage 15, whole-mount in situ hybridization shows that Shh is not expressed in facial ectoderm whereas (B) Fgf8 shows a definitive dorsal expression domain in this tissue. (C,D) Hoescht nuclear staining is used to show the anatomy of the craniofacial tissues on sagittal sections at stage 15. The dorsal and ventral neuroectoderm (d ne and v ne, respectively) closely approximates the facial ectoderm (fe). The pharyngeal endoderm (pe) and Rathke s pouch (rp) are nearly contiguous at this stage because the buccopharyngeal membrane has yet to rupture. (E) In situ hybridization for Shh shows that its expression is restricted to the prosencephalon and the buccophyaryneal membrane at this stage. (F) At this developmental stage Fgf8 transcripts are evident in both dorsal neuroectoderm and dorsal facial ectoderm, as well as in pharyngeal endoderm. At the same stage Shh transcripts are limited to ventral diencephalon. (G) Ptc1, which is a direct target of Shh signaling and (H) Fgf receptor 1 (Fgfr1) is expressed throughout the neuroectoderm, facial ectoderm and frontonasal neural crest. (I) Gli1, another target of Shh signaling, is expressed in a similar wide domain as Ptc1 as widespread is strongly expressed in a dorsal the neural and facial ectoderm. In the neuroectoderm. (J) Fgf receptor 2 (Fgfr2) is expressed in the craniofacial and brain regions. D, dorsal; di, diencephalon; epi, surface epithelium; fnp, fronto-nasal primordium; mn, mandible; ncm, neural-crest derived mesenchyme; neu, neuroepithelium; or, optic recess; pe, pharyngeal endoderm; rp, Rathke s pouch; V, ventral. Bars (A) 100 mm; (B) 200 mm. Fgf8 by ectopically and broadly misexpressing it in facial ectoderm. We achieved this by delivering RCAS::Fgf8 to head ectoderm at stage 15. The virus produced widespread infection by stage 18. The most notable facial phenotypic alteration caused by Fgf8 misexpression in head ectoderm was a dramatic increase in head size, along with a concomitant broadening of the FNP (n = 14/19); these changes were apparent within 36 h of infection (Fig. 5A,B,F). Conversely, inhibiting Fgf signaling by infection with RCAS::Fgf8 RNAi caused a slight decrease in the width of the FNP relative to control embryos, but no apparent change in the size of the head (n = 11/14; Fig. 5A,C,F). These morphological effects became more pronounced with time. Five days after infection (at stage 31, E7) we compared control and treated embryos. By this stage in normal development the FNP has fused with the maxillary prominences (MXP) and the characteristic shape of the upper beak is established (Fig. 5G). In addition, an ectodermal specialization, the egg tooth, appears at the distal tip of the upper beak (Fig. 5G, arrow), which acts as a landmark of distal FNP polarity. Compared to untreated controls, RCAS::Fgf8-treated embryos had grossly malformed FNP, in that the FNP was wider at its distal tip, abnormally constricted in its proximal region, and foreshortened in its proximodistal growth (n = 12/17; Fig. 5H). Embryos treated with RCAS::Fgf8 that did not show gross facial abnormalities lacked an egg tooth on the distal FNP (14/17), suggesting that dorsal-ventral patterning of the upper beak was disrupted. Five days after inhibiting Fgf8 signaling by RCAS::Fgf8 RNAi, the facial form was disrupted in a more subtle manner than observed in embryos over-expressing Fgf8. For example, the upper beaks were subjectively smaller than in controls (n = 7/10; Fig. 5I). Careful measurements of the beak width, height and length were taken from both control and RCAS::Fgf8 RNAi treated embryos at E7. Width and height were not statistically different from E7 control embryos. The beak length was approximately 17% shorter and had a P-value of as a result of the inherent variability associated with viral infections. Inhibiting Fgf signaling caused the elimination of the egg tooth, as observed with over-expression of Fgf8 (Fig. 5H). Morphological effects resulting from manipulating Shh signaling We employed a parallel approach to test the consequences of disrupting Shh signaling in the face. First, we used RCAS::Shh to ectopically express Shh in head ectoderm at stage 15. As with the RCAS::Fgf8, ectopic expression of Shh also led to an increase in head size (Fig. 5D, compared to control (Fig. 5A). In addition, we noted a mediolateral expansion of the FNP (n = 17/17; Fig. 5D,F), similar to our previous results using cells expressing Shh (Hu & Helms 1999). In contrast, inhibiting Shh signaling with cyclopamine led to narrowing of the mediolateral axis of the FNP and in some cases, decreased outgrowth of the MXP (n = 9/13; Fig. 5E,F; also see Chen et al. 2002; Cordero et al. 2004). Over-expression of Shh led to a broader upper beak that was covered with multiple egg teeth (n = 11/12; arrows, Fig. 5J). This latter finding suggested that Shh signaling, but not Fgf8, was involved in the induction of this ectodermal thickening on the dorsal surface of the upper beak. As with the Fgf-treated embryos, these morphological effects worsened over time. Five days after treatment (at stage 31, E7) the outgrowth of the palatal shelves from the MXP was insufficient to cover the nasal tubercules, which resulted in palatal insufficiency and an exposure of the nasal tubercules (data not shown). This defect is analogous to cleft palate in mammals. In addition, the FNP failed to fuse with the MXP and lateral nasal prominence, which caused clefting in the external nares. When we compared the morphological consequences of Shh inhibition we were surprised to find that embryos treated with cyclopamine (Fig. 5K) looked remarkably similar to those treated with RCAS- ::Fgf8 RNAi (Fig. 5I). Both groups of embryos lacked egg teeth and exhibited a characteristic deformation of the FNP where the proximal region was constricted and the distal region widened (n = 7/15). We drew three conclusions from these Fgf8 and Shh gain- and loss-of-function studies. First, we found that separately inhibiting either pathway caused truncations in the outgrowth of the FNP, which indicates that proper development of the FNP requires precise coordination of both signaling pathways. Second, disrupting Fgf8 activity blocked formation of the egg tooth, which can be viewed as a defect in specification of dorsal surface ectoderm. This result suggests that an Fgf8 boundary is necessary for patterning dorsal surface ectoderm. Third, over-expression of Shh disrupted this same patterning of frontonasal ectoderm, but in an opposite manner in which it induced ectopic teeth. The implication from this latter result is that Shh, acting in conjunction with endogenously expressed genes, is sufficient to induce dorsal patterning within the facial ectoderm. We wondered whether ectopic Shh cooperated with endogenous Fgf8 in the frontonasal ectoderm to create multiple FEZ domains, the morphological consequence of which was multiple egg teeth. Therefore, we set out to determine how perturbations in Shh affected the endogenous gene expression in the frontonasal prominence, and conversely, whether disruptions in Fgf affected these same expression domains in different or similar ways. Perturbing Shh signaling disrupts endogenous Fgf8 expression To determine the molecular consequences of the Shh gain- and loss-of-function experiments, embryos were collected 36 and 48 h after treatment (corresponding to stages 20 and 23, respectively).
5 140 A. Abzhanov et al. st.20 st.22 st.25 Shh Shh Ptc1 Gli1
6 Cross-regulation of Fgf8 and Shh 141 Fig. 2 Expression of Shh and its signaling pathway members in stage 20, 22 and 25 embryonic heads (A O). Shh expression, as revealed by whole-mount in situ hybridization, was localized to a broad domain in the ventral surface of the head. (A C) Hoechst staining reveals tissue morphology, which correlates with the in situ hybridization analysis shown in (D F). (G) At stage 20 Shh expression was found in three distinct domains in the ventral facial ectoderm (white arrowhead), ventral diencephalon (di) and ventral telencephalon (te). These expression domains were maintained essentially unaltered through (H) stage 22 and (M) stage 25 (I). Ptc1 was expressed abundantly throughout the facial ectoderm and brain neuroepithelium at stages 20 (J), 22 (K) and 25 (L). In contrast, expression of Gli1 was increased in areas adjacent to, but not expressing Shh, such as optic recess (or) and dorsal-rostral telencephalon at stages 20 (M) and 22 (N). By stage 25, expression of Gli1 was up-regulated in the FNP mesenchyme (white arrow) and in most of the brain neuroepithelium (O). An RCAS-specific probe (RSCH) was first used to identify sites of infection and the extent of viral spread (Fig. 6G,J), which was necessary in order to determine which endogenous gene expression patterns overlapped with ectopic Shh. We carefully evaluated all tissues sections through the head regions of the control and treated embryos. Because facial morphology was abnormal as a consequence of the treatments, the morphological appearances of the tissue sections differed between the treatment groups. Wherever possible, we presented tissue sections in the midline of the embryos, as indicated by the presence of Rathke s pouch in the majority of samples (data not shown). Thirty-six hours after RCAS::Shh infection at stage 15, the retrovirus was restricted to the facial ectoderm (Fig. 6G). The forced expression of Shh resulted in a continuous domain from dorsal facial ectoderm into its normal domain in the ventral region (Fig. 6H compared to control, 6B). Thus, Shh was ectopically expressed in Fgf8-positive facial ectoderm (Fig. 6C,H). The expression domains of Shh and Fgf8 were examined on adjacent tissue sections, and from these analyzes it appeared that multiple Fgf8/Shh boundaries were created as a result of the ectopic Shh expression. Alternatively, the multiple egg teeth phenotype could be caused by a prolonged period of the Shh and Fgf8 coexposure. This prolonged interaction might have led to the formation of multiple egg teeth, thus suggesting that the exact timing of up- or down-regulation is important for the development of the frontonasal prominence. After 48 h, Shh continued to be expressed ectopically in dorsal facial ectoderm (Fig. 6K compared to control, 6E). At this time point in control embryos Fgf8 has been down-regulated in dorsal facial ectoderm (Fig. 6F), and the forced expression of Shh did not alter this down-regulation (Fig. 6L). One important change we did observe was that the ectopic expression of Shh in facial ectoderm correlated with a loss of Fgf8 expression in the optic recess (asterisks in Fig. 6L compared to control, Fig. 6F). Examination of all tissue sections revealed that while the characteristic morphology of the optic recess remained, Fgf8 expression was lost from this region (Fig. 6L). The optic recess (recessus opticus), a structure of unclear function, is defined as a small angular recess or diverticulum at the junction of the floor and anterior wall of the third brain ventricle. It appears that Fgf8 expression was closely associated with this structure. Although our experiments did not directly address the regulatory relationship between Shh and Fgf8 during the neuroectodermal development at the optic recess but their mutually exclusive expression domains suggests negative interactions. Our results suggest a certain degree of the regulatory influence of the developing FNP on the neuroectoderm. Next, we evaluated the molecular consequences of Shh signal inhibition. Thirty-six hours (stage 20) following cyclopamine exposure at stage 15, Shh expression was not induced in the telencephalic neuroectoderm (Fig. 6N compared with control, 6B). Shh transcripts were also lost in ventral facial ectoderm (Fig. 6N), indicating that both the brain and facial domain of Shh are induced in response to a Shh-dependent signal (see also Cordero et al. 2004). The loss of Shh in ventral facial ectoderm was correlated by an expansion of Fgf8 into this ventral domain (Fig. 6O compared with control, Fig. 6C). The telencephalic domain of Fgf8 was unaffected by cyclopamine exposure (Fig. 6O) but the distinctive morphology of the optic recess was lost (Fig. 6O compared with control, Fig. 6C). Forty-eight hours later, Shh transcripts were once again detectable in the ventral-most surface epithelium, and in Rathke s pouch (Fig. 6Q). Fgf8 transcripts were detectable only in the maxillary prominences which, due to the collapse of the frontonasal prominence, were now located nearer to the midline (Fig. 6R). Fgf8 over-expression represses Shh Since Fgf8 is induced in facial ectoderm long before Shh is expressed in this tissue, we tested whether Fgf8 played a role in establishing Shh expression. We ectopically drove Fgf8 expression using RCAS::Fgf8 and found that 36 h after infection the retrovirus was largely restricted to facial ectoderm (Fig. 7G). This resulted in ectopic Fgf8 in ventral facial ectoderm, which effectively created an uninterrupted band of Fgf8 in facial ectoderm (Fig. 7I compared to control, Fig. 7C). Concomitant with the gain of ventral Fgf8, Shh was lost in ventral facial ectoderm (Fig. 7H, compared with control, Fig. 7B). Neither the Fgf8 nor the Shh expression domains in the forebrain were adversely affected by ectopic Fgf8 expression in stage 15 facial ectoderm. These same molecular relationships were maintained at the 48 h time point; RCAS::Fgf8 infection continued in facial ectoderm (Fig. 7J compared to control, Fig. 7D), which temporally prolonged and spatially extended the Fgf8 domain (Fig. 7L compared to control, Fig. 7F). The persistent Fgf8 domain correlated with the loss of endogenous Shh in ventral facial ectoderm (Fig. 7K compared with Fig. 7E). Once again, the expression of Shh in the brain was unaffected by ectopic Fgf8 in facial ectoderm (Fig. 7K compared to control, Fig. 7E). We next evaluated the molecular consequences of inhibiting Fgf8 activity in the facial ectoderm. We evaluated embryos 36 h after infection with RCAS::Fgf8 RNAi and found viral RNA in facial ectoderm and to a lesser degree, in underlying mesenchyme (Fig. 7M compared with control, Fig. 7A). The RNAi infection decreased, but did not completely eliminate Fgf8 expression in facial ectoderm (Fig. 7O compared with control, Fig. 7C). In about half of the embryos treated, we also noted that the telencephalic domain of Fgf8 was abolished by RCAS::Fgf8 RNAi (Fig. 7O) and was probably due to the ectopic effect of RNAi on neuroectoderm. Fgf8 inhibition had no discernable effect on Shh expression in either the brain or face (compare 36 h time point, Fig. 7N with control, Fig. 7B; and 48 h time point, Fig. 7Q with control, Fig. 7E).
7 142 A. Abzhanov et al. stage 20 stage 22 stage 25 Fgf8 Hoechst Fgf8 FgfR1 FgfR2 FgfR3
8 Cross-regulation of Fgf8 and Shh 143 Fig. 3 Expression of Fgf8 and Fgf receptors in stage 20, 22 and 25 embryonic heads (A R). (A) Fgf8 expression, by whole-mount in situ hybridization was localized to a broad domain in the dorsal surface of the head as well as in the maxillae, mandibles and hyoid arches. The midline area is indicated with brackets. (G) By stage 22, Fgf8 expression in the FNP was mostly limited to the cells around nasal pits (np) with weaker expression detectable in cells along the dorsal ventral boundary and the midline. (M) By stage 25, in the FNP Fgf8 expression was only detectable in cells around the nasal pits. (B,H,N) Hoechst staining reveals tissue contour for analyzing section in situ hybridization results. (C) By stage 20 the expression of Fgf8 was found in three distinct domains in the dorsal-rostral facial ectoderm (white arrowhead), dorsal-rostral telencephalon (te) and the optic recess (or). (I) By stage 22, Fgf8 expression was down-regulated in the dorsal ectoderm and was not detected by (D) stage 25. Note that the optic recess and telencephalon expression domains persist through stage 25 (D,J,P) FgfRI, one of the Fgf receptors, was expressed abundantly throughout the facial ectoderm and brain neuroepithelium at stages (D) 20 (J) 22 and (P)25. In contrast, expression of other receptors (E,K,Q) FgfR2 and (F,L,R) FgfR3, was strongest in the areas of neuroepithelium adjacent to but not expressing Fgf8, such as ventral telencephalon (te) and ventral diencephalon (di) at stages 20 and 22. (O). By stage 25, expression of FgfR2 was also strongly up-regulated in the FNP mesenchyme (E,K,Q). However, FgfR2was expressed at similar levels throughout facial ectoderm at stages 20, 22 and 25. Abbreviations are the same as in Figure 2. d, dorsal; di, diencephalon; hy, hyoid arch; mn, mandible; mx, maxilla; np, nasal pit; or, optic recess; pe, pharyngeal endoderm; rp, Rathke s pouch; v, ventral. Fig. 4 Expression patterns of Fgf8 and Shh in stages 15 and 20 embryonic chick heads. (A) By stage 15 Fgf8 was expressed in the rostrodorsal domain in both facial ectoderm and brain neuroepithelium whereas Shh expression was found only in the neuroepithelium of the diencephalon. (B) By stage 20 the expression of Shh (red) was found in three distinct domains in the ventral facial epithelium (epi), ventral diencephalon (di) and ventral telencephalon (te). Fgf8 expression (black), as revealed by whole-mount in situ hybridization, was localized to a broad domain in the dorsal surface of the head as well as in the telencephalic neuroepithelium (neu). The dorsal ventral boundary of the FNP and the boundary of the Shh and Fgf8 expression domains in the FEZ are indicated with a concave arrowhead. D, dorsal; di, diencephalon; epi, surface epithelium; FNP, fronto-nasal primordium; mn, mandible; ncm, neural-crest derived mesenchyme; neu, neuroepithelium; or, optic recess; pe, pharyngeal endoderm; rp, Rathke s pouch; V, ventral. Bars, (A) 100 mm; (B) 200 mm. DISCUSSION A hallmark of embryogenesis is that reciprocal signaling between epithelia and mesenchyme regulate the development of tissues and organs. In the case of craniofacial development, multiple epithelia provide instructional cues that direct morphogenesis (reviewed in Le Douarin et al. 2004; Helms et al. 2005). For example, signals emanating from the anterior neural ridge and prechordal plate are important for establishing distinctive territories within the future forebrain and frontonasal prominence (Etchevers et al. 1999; Kuschel et al. 2003; Petryk et al. 2004). In turn, signals emanating from forebrain neuroectoderm are essential for the survival of frontonasal neural crest cells, and for the formation of parts of the craniofacial skeleton. Some of these signals have been identified and include Fgf (Trumpp et al. 1999; Abu-Issa et al. 2002; Walshe & Mason 2003; Creuzet et al. 2004), retinoids (Schneider et al. 2001; Marklund et al. 2004; Song et al. 2004), Wnts (Kapsimali et al. 2004), Bmps (Petryk et al. 2004), and Shh (Chiang et al. 1996; Cordero et al. 2004; Jeong et al. 2004). Signals from the pharyngeal endoderm also regulate patterning the maxillary and mandibular prominences (Graham & Smith 2001; Trokovic et al. 2003; Crump et al. 2004; Haworth et al. 2004; Knight et al. 2004), from which anatomic structures of the upper and lower jaws develop. In the present study we focused on the roles of two signals emanating from surface ectoderm, which covers the frontonasal prominence. In previous experiments we found that a discrete region of facial ectoderm expressed Fgf8 and Shh and when this ectoderm was transplanted into ectopic positions in the face, the dorsoventral and proximodistal patterning of the frontonasal prominence was altered (Hu et al. 2003). While those experiments established the importance of a discrete region of surface ectoderm in patterning the middle and upper face, they did not pinpoint the molecular machinery responsible for eliciting these morphological changes. We were particularly interested in the roles that Fgf8 and Shh played in controlling craniofacial patterning and set out to determine when the molecular boundary between these two molecules was established, and how the individual pathways affected frontonasal patterning and growth. Our experimental approach was predicated on an ability to manipulate gene expression exclusively in the facial ectoderm, while simultaneously maintaining normal expression domains for Fgf8 and Shh in other head epithelia. In that way we hoped to pinpoint the aspects of frontonasal development, which were mediated by Fgf8 and Shh originating from a single region of head epithelium. We focused our studies on disrupting Fgf and Shh signaling after neural crest cells had migrated into the facial prominences. Since this migration is dependent upon signals from neuroectoderm and surface ectoderm (Noden 1983; Couly et al. 1993; Le Douarin & Dupin 1993), we timed our infections and drug delivery to take place after the exodus of cells was complete. In that way we could ascribe any craniofacial anomalies to defects in neural crest cell proliferation or differentiation, rather than to disruptions in migration. Ontogeny of the FEZ One of the first observations we made from our in situ hybridization analyzes was that the juxtaposition of Fgf8 and Shh expression domains, and thus the creation of the FEZ, only exists for a brief period. Before stage 19 Fgf8 is expressed in dorsal facial ectoderm, but Shh is not; after stage 22, Shh is expressed in ventral facial ectoderm but Fgf8 is lost (Figs 2,3). Therefore, Fgf8 and Shh constitute a molecular border for less than less than 16 h, between stages (Fig. 8A). Our previous study showed that FEZ activity roughly correlated with this Fgf8/Shh boundary, as we showed that stage 20 facial ectoderm possessed organizer activity, but stage 25 facial ectoderm did not (Hu & Helms 1999; Hu et al. 2003). We wondered whether
9 144 A. Abzhanov et al. Fig. 5 Morphological consequences of altering the Fgf8 and Shh signaling at stage 15. (A E) Embryos after 36 h and (G K) 5 days following either infection or exposure to cyclopamine. (A) Wild-type embryo at stage 20 showing well-defined primordia, frontonasal (fnp), maxillary (mx) and mandibular (mn). (B) 36 h after RCAS::Fgf8 infection an increase in head size and significant broadening of the frontonasal primordium was noted. (C) Infection with RCAS::Fgf8 RNAi decreased the size and length of the frontonasal primordium. (D) Ectopic Shh expression led to an increase in embryonic head size and broadening of the frontonasal primordium. Note the differential effects of ectopic Shh and Fgf8 on the morphology of the maxillae. The maxillary processes in the RCAS::Fgf8 treated heads are less morphologically delineated whereas exposure to RCAS::Shh lead to subjectively larger well defined maxillary processes as compared to controls. (E) Inhibition of Shh signal transduction with cyclopamine led to a dramatically narrower frontonasal primordium. (F) Diagram showing changes in the FNP width as compared in the infected embryos. (G K) On the 7th day of development (5 days after treatment) the heads were collected and photographed. (G) An upper beak of a control embryonic head with a well-developed egg tooth (short white arrow). (H) RCAS::Fgf8 infection led to development of a shorter but broader upper beak and a complete loss of an egg tooth. (I) RCAS::Fgf8 RNAi treatment caused a similar but less severe phenotype. These embryos exhibited a smaller upper beak than observed in wild-type embryos. (J) Ectopic Shh expression led to formation of ectopic egg teeth on the dorsal surface of a shorter but wider upper beak. (K) Cyclopamine exposure reduced the size of the beak and a loss of the dorsal egg tooth structure. Abbreviations: ey, eye; fnp, frontonasal primordium; mn, mandibular primordium; mx, maxillary primordium; rp, Rathke s pouch; te, telencephalon. Bar, 0.5 mm. FEZ ectoderm lost organizer activity around stage 22, when Fgf8 is down-regulated, or whether the FEZ was able to exhibit organizer activity prior to stage 20, when Shh has yet to be expressed. Definitive answers to these questions, however, could not be gained using a transplantation strategy. Because the readout of our FEZ transplantations was whether the grafted tissue altered craniofacial patterning, we had to consider the possibility that the developmental age of the host was an important variable in this response. In other words, the host facial primordia might have a window of responsiveness that was unrelated to the graft age. Also, the location of the recipient bed may affect the ability of the FEZ to alter craniofacial patterning. Given these experimental constraints we took an alternative tact and instead explored the roles of Shh and Fgf8 using a gain- and loss-of-function approach, and focused on their ability to direct patterning and growth of the frontonasal prominence. Fgf8 negatively regulates Shh expression in the FEZ We found that in facial ectoderm, forced expression of Fgf8 repressed the induction of Shh (Fig. 7H,B,K,E). Likewise, blocking Shh induction allowed the expansion of Fgf8 (Fig. 6C,O). These two results suggest that both Fgf8 and Shh are responsible for maintaining a boundary in facial ectoderm. The absence of Fgf8, however, does not result in an expansion of Shh, which indicates that additional players might be involved: another factor may be
10 RSCH Shh Fgf8 RSCH Shh Fgf8 control RCAS-Shh Cross-regulation of Fgf8 and Shh 145 cyclopamine Fig. 6 Effects of RCAS::Shh infections and cyclopamine treatment on expression of Fgf8 at stages 20 and 23 (36 and 48 h after infection, respectively). The normal anatomic location of Shh and Fgf8 expression interface is shown with a red arrowhead. The optic recess is indicated with a red star. (A C) The expression pattern of RCAS, Shh and Fgf8 in control embryos. (A) Utilizing in situ hybridization, no signal was detected by the RSCH probe in the control embryos. (B) In situ analysis performed with a Shh probe detected three endogenous Shh expression domains: in the diencephalon, ventral telencephalon and ventral surface ectoderm of the FNP. (C) Fgf8 was normally expressed in the optic recess (red star), rostral telencephalon and dorsal surface ectoderm of the FNP. (A F) In situ hybridization results with the RCAS-specific probe (RSCH), Shh and Fgf8 probes on uninfected control embryos. (D) No signal was detected by the RSCH probe in the wild-type embryos. (E) Shh in situ probe again detected three endogenous Shh expression domains: in the diencephalon, ventral telencephalon and ventral surface ectoderm of the FNP. (F) By stage 23 Fgf8 was normally expressed in the optic recess (red star) and weaker in the rostral telencephalon, but was no longer detectable in the dorsal surface ectoderm of the FNP. (G I) Effects of RCAS::Shh infection on Shh and Fgf8 expression. (G) RSCH probe detected signal only in the surface ectoderm. (H) In situ hybridization with Shh probe detected ectopic expression in the dorsal facial epithelium. (I) Expression of Fgf8 in the optic recess was inhibited by the ectopic Shh expression. (J L) Effects of RCAS::Shh infection on Shh and Fgf8 expression. (J) RSCH probe detected signal only in the surface ectoderm. (K) In situ hybridization with Shh probe detected ectopic expression in the facial ectoderm. (L) Expression of Fgf8 in the optic recess was inhibited by the ectopic Shh expression. (M O) In situ hybridizations on cyclopamine treated embryos. (M) No signal was detected with RSCH probe. (N) Shh expression was altered dramatically as its expression in the surface ectoderm was inhibited and only a single expression domain was detected in the forebrain neuroepithelium. (O) Fgf8 was ectopically activated in the facial ectoderm. The expression of Fgf8 appeared to be down-regulated in the rostral telencephalon. (P R) Analysis of gene expression following cyclopamine exposure. Note the absence of the optic recess. The red star represents the presumptive location. (P) No signal was detected with RSCH probe. (Q) Shh expression was altered dramatically as its expression in the surface ectoderm was limited to the ventral-most aspects of the FNP surface and only a single expression domain was detected in the forebrain neuroepithelium. (R) Some ectopic expression of Fgf8 persisted 48 h after the cyclopamine treatment in the facial ectoderm. No expression of Fgf8 coinciding with the location of the optic recess was observed. ey, eye; di, diencephalons; fnp, frotonasal primordium; mn, mandibular primordium; mx, maxillary primordium; rp, Rathke s pouch; te, telencephalon. Bar, 100 mm. present in the dorsal facial ectoderm that is redundant with Fgf8 to suppress Shh expression, and yet another factor may be present in the ventral facial ectoderm that is required to act in addition to Shh to suppress induction of Fgf8 in the dorsal facial ectoderm. This regulation is also unidirectional (i.e. Shh appears to influence the expression of Fgf8 in the facial ectoderm, but Fgf8 does not appear to effect Shh expression). This observation is in keeping with the fact that Fgf8 is expressed in facial ectoderm much earlier than Shh (Fig. 1). One morphological consequence of a ventral shift in Fgf8 is that dorsal skeletal elements, such as the prenasal process of the premaxilla, shift into more ventral positions (Cordero et al. 2004). What is not immediately clear, however, is why at earlier stages the frontonasal prominence is considerably wider as a result of ectopic Fgf8 (Fig. 5B). One explanation may simply be that Fgf8 acts as a trophic factor and induces global neural crest cell proliferation, which ultimately translates into increased chondrogenesis (Abzhanov & Tabin 2004). Certainly, down-regulation of Fgf8 by RNAi indicates that misappropriation of Fgf signaling is detrimental to facial morphogenesis (Fig, 5C,I). Another possible explanation for the craniofacial malformations is that Fgf8 may be a critical determinant of dorsal frontonasal fate, and the defects are a consequence of disrupting dorsal specification. The only indicator that this hypothesis is correct, however, is indirect: the egg tooth, an ectodermal specialization that only appears on the dorsal surface of the frontonasal prominence, does not form when Fgf signaling is perturbed (Fig. 5H,I). We cannot conclude that dorsal fate has been entirely lost since embryos did not typically survive to later stages where the dorsal or ventral character of the skeletal elements could be readily assessed. Also, we lack definitive molecular markers of dorsal versus ventral cell fate in the frontonasal prominence and can therefore only speculate that molecules such as AP2 and Msx1, which are predominantly induced in dorsal mesenchyme underlying Fgf8 positive ectoderm (Hu et al. 2003), are required for dorsal cell fate specification. As we develop a better understanding of the facial axes, and the molecules that establish and maintain those axes, we will be able to revisit this question of how Fgf8 is patterning the frontonasal prominence. Fgf8 expands when Shh expression is inhibited in the FEZ As mentioned previously, blocking Shh signaling with cyclopamine inhibited Shh induction and extended Fgf8 expression into ventral facial ectoderm. The loss of an egg tooth provides some indirect
11 146 A. Abzhanov et al. RSCH Shh Fgf8 RSCH Shh Fgf8 control RCAS-Shh evidence that dorsoventral polarity was disrupted by this loss of Shh and expansion of Fgf8, but this result also raises a perplexing question: what restricts Fgf8 to dorsal facial ectoderm? Shh is not expressed at the time when this Fgf8 domain is established so Shh cannot be the place-holder, even though the cyclopamine results suggest that it is involved. We have examined a number of other genes including members of the Bmp (Bmp2, 4, 7) and Wnt (Wnt 3a, Wnt 4, Wnt 5a, Wnt 7a, Wnt 7b, Wnt 8b, Wnt 9A, Wnt 11, Wnt 13) families but have yet to identify one whose expression pattern would suggest it is involved in restricting Fgf8 to dorsal facial ectoderm (data not shown). If the craniofacial primordia are patterned in a manner similar to other embryonic tissues and organs then it may be the case that Fgf8 expression is restricted by signals from the underlying mesenchyme; in this case, a host of transcription and growth factors become possible candidates for regulating the Fgf8 domain in the frontonasal prominence (Fig. 8B). Shh in the face regulates Fgf8 in the brain Perhaps one of the unexpected findings of our study was that misexpressing Shh throughout facial ectoderm alters Fgf8 expression in the optic recess. We were surprised by this finding and confirmed that the characteristic dip in neuroectoderm, which corresponds to the optic recess, was still present, but that Fgf8 expression was absent. These results suggest that a signal from facial ectoderm cyclopamine Fig. 7 Effects of RCAS::Fgf8 and RCAS::Fgf8-RNAi infections on expression of Shh at stages 20 and 23 (36 and 48 h after infection, respectively). The normal anatomic location of Shh and Fgf8 expression interface is shown with a red arrowhead. The optic recess is indicated with a red star. (A C) In situ hybridization results with the RCAS-specific probe (RSCH), Shh and Fgf8 probes on uninfected control embryos. (A) No signal was detected by RSCH probe in the wild-type embryos. (B) Shh in situ probe detected three domains of endogenous Shh expression in the diencephalon, ventral telencephalon and ventral surface ectoderm of the FNP. (C) Fgf8 was normally expressed in the optic recess (red star), rostral telencephalon and dorsal surface ectoderm of the FNP. (D F) In situ hybridization results with the RCAS-specific probe (RSCH), Shh and Fgf8 probes on uninfected control embryos. (D) No signal was detected by RSCH probe in the wild-type embryos. (E) Shh in situ probe detected three domains of endogenous Shh expression in the diencephalon, ventral telencephalon and ventral surface ectoderm of the FNP. (F) By stage 23 Fgf8 was normally expressed in the optic recess (red star) and weaker in the rostral telencephalon, but was no longer detectable in the dorsal surface ectoderm of the FNP. (G I) In situ hybridization signals from RSCH, Shh and Fgf8 probes following infection with RCAS::Fgf8. (G) RCAS infection with Fgf8 was limited to the surface ectoderm. (H) No or very little expression of Shh was detected in the facial surface ectoderm of the infected embryo. (I) Fgf8 probe detected ectopic expression in the ventral surface ectoderm. (J L) In situ hybridization signals from RSCH, Shh and Fgf8 probes following infection with RCAS::Fgf8. (J) RCAS infection was limited to the surface ectoderm. (K) No or very little expression of Shh was detected in the surface ectoderm of the infected embryo. (L) Fgf8 probe detected ectopic expression in the ventral surface ectoderm. (M O) Molecular effects of RCAS::Fgf8 RNAi infection. (M) Viral infection was limited to the surface ectoderm. (N) Expression domains of Shh appeared to be normal. (O) Expression of Fgf8 in the facial ectoderm and neuroectoderm of the telencephalon was significantly down-regulated in the infected embryos. (P R) Molecular effects of RCAS::Fgf8 RNAi infection. (P) Viral infection was primarily limited to the surface ectoderm with a small area of infection involving the mesenchymal cells that do not express Fgf8 endogenously. (Q) Expression domains of Shh appeared to be normal. (R) No expression of Fgf8 was detected in the surface epithelium and telencephalon in the infected embryos. ey, eye; di, diencephalon; fnp, frontonasal primordium; mn, mandibular primordium; mx, maxillary primordium; rp, Rathke s pouch; te, telencephalon. Bar, 100 mm. influenced gene expression in the underlying forebrain neuroectoderm, which raises the question of how this reciprocal signaling between face and brain actually functions. In our experimental system Shh was forcibly expressed earlier than normal (stage 15) when the forebrain and facial ectoderm are nearly juxtaposed. Therefore, it is feasible that ectopic Shh in facial ectoderm impinged upon Fgf8 in the nearby optic recess. Because signals from the neuroectoderm clearly influence gene expression in facial ectoderm (Cordero et al. 2004), it is not unexpected that signals from facial ectoderm might be able to act back upon the neuroectoderm and influence its early regionalization. Thus, the optic recess, an important feature of the developing brain, can be regulated by Shh expressed in the facial epithelium so that ectopic ventral Shh inhibits Fgf8 expression in the optic recess (but not in the telencephon) without affecting its morphology. It is also clear that overall Shh activity is required both for Fgf8 expression in the optic recess and formation of the structure (Fig. 6A O). CONCLUSION Our primary goal in the present work was to understand the roles for Fgf8 and Shh in establishing and maintaining an organizing center
12 Cross-regulation of Fgf8 and Shh 147 Fig. 8 Sonic hedgehog Fibroblast growth factor 8 in the frontonasal prominence. We found that a mutually inhibitory interaction exists between Fgf8 and Shh whereby Shh is required but not sufficient to restrict Fgf8 expression dorsally, and Fgf8 is sufficient but not required to restrict Shh expression ventrally. Our experiments leave open the question of how this boundary is initially established, but do uncover a mechanism by which the boundary is maintained (Fig. 8). Our results also hint at the possibility that the Fgf8 and Shh expression domains are merely respecting a molecular boundary that is established by other, as yet unknown, molecules. ACKNOWLEDGMENTS We thank Dr A. Dudley and Dr R. Pierce for thoughtful comments on the manuscript. Work on this project in the C.J.T. Laboratory was funded by a program project grant PO1 DK56246 from the NIH. A.A. was supported by the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation Fellowship, DRG1618. J.A.H. is supported by NIH R01 DE A1, and by the Oak Foundation. D.C.R. is supported by the NIH Women s Reproductive Health Research grant K12HD REFERENCES Neural crest-derived mesenchyme Shh and Fgf8 cross regulatory interactions in the developing avian frontonasal prominence. (A) Summary of the Fgf8 and Shh expression domains at stage 20. (B) Multiple expression domains of both Shh (Shh-A, Shh-B and Shh-C) and Fgf8 (Fgf-A, Fgf-B and Fgf-C) in the facial epithelium and neuroectoderm. Our experiments suggest that the two molecules inhibit expression of each other in the facial epithelium that is important for proper boundary formation and/or maintenance. Continued activity of Shh is required for both formation of the optic recess expressing Fgf8 (dotted arrows). Multiple additional unknown players w suggested (question marks) by the fact that expression of Shh and Fgf8 is sometimes required but not sufficient or vice versa. D, dorsal; di, diencephalon; epi, surface epithelium; FNP, fronto-nasal primordium; mn, mandible; ncm, neural-crest derived mesenchyme; neu, neuroepithelium; or, optic recess; pe, pharyngeal endoderm; rp, Rathke s pouch; V, ventral. Bars (A) 100 mm; (B) 200 mm. Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN (2002) Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 129: Abzhanov A, Tabin CJ (2004) Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol 273: Albrecht UEG, Helms JA, Lin H (1997) Visualization of gene expression patterns by in situ hybridization. In: Daston GP (ed.). Molecular and Cellular Methods in Developmental Toxicology. CRC Press, Boca Raton, FL, pp Chen JK, Taipale J, Cooper MK, Beachy PA (2002) Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev 16: Chiang C, Litingtung Y, Lee E et al. (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383: Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA (2004) Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest 114: Couly GF, Coltey PM, Le Douarin NM (1993) The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development 117: Creuzet S, Schuler B, Couly G, Le Douarin NM (2004) Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc Natl Acad Sci USA 101: Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB (2004) An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development 131: Etchevers HC, Couly G, Vincent C, Le Douarin NM (1999) Anterior cephalic neural crest is required for forebrain viability. Development 126: Francis-West P, Ladher R, Barlow A, Graveson A (1998) Signalling interactions during facial development. Mech Dev 75: Graham A, Smith A (2001) Patterning the pharyngeal arches. Bioessays 23: Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88: Haworth KE, Healy C, Morgan P, Sharpe PT (2004) Regionalisation of early head ectoderm is regulated by endoderm and prepatterns the orofacial epithelium. Development 131: Helms JA, Cordero D, Tapadia MD (2005) New insights into craniofacial morphogenesis. Development 132: Hu D, Helms JA (1999) The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development 126: Hu D, Marcucio RS, Helms JA (2003) A zone of frontonasal ectoderm regulates patterning and growth in the face. Development 130: Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP (2004) Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev 18: Kapsimali M, Caneparo L, Houart C, Wilson SW (2004) Inhibition of Wnt/Axin/beta-catenin pathway activity promotes ventral CNS midline tissue to adopt hypothalamic rather than floorplate identity. Development 131: Knight RD, Javidan Y, Nelson S, Zhang T, Schilling T (2004) Skeletal and pigment cell defects in the lockjaw mutant reveal multiple roles for zebrafish tfap2a in neural crest development. Dev Dyn 229: Kuschel S, Ruther U, Theil T (2003) A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of the Gli3 mutant telencephalon. Dev Biol 260: Le Douarin NM, Creuzet S, Couly G, Dupin E (2004) Neural crest cell plasticity and its limits. Development 131: Le Douarin NM, Dupin E (1993) Cell lineage analysis in neural crest ontogeny. J Neurobiol 24: Marklund M, Sjodal M, Beehler BC, Jessell TM, Edlund T, Gunhaga L (2004) Retinoic acid signalling specifies intermediate character in the developing telencephalon. Development 131: Matsuda T, Cepko CL (2004) Electroporation and RNA interference in the rodent retina in vivo and in vitro. PNAS 101: Noden DM (1983) The role of the neural crest in patterning of avian cranial skeletal, connective and muscle tissues. Dev Biol 96: Petryk A, Anderson RM, Jarcho MP et al. (2004) The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev Biol 267: Riddle RD, Johnson RL, Laufer E, Tabin C (1993) Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75: Schneider RA, Hu D, Rubenstein JL, Maden M, Helms JA (2001) Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development 128:
Supporting Information
 Supporting Information Creuzet 10.1073/pnas.0906072106 Fig. S1. Migrating CNC cells express Bmp inhibitors. (A) HNK1 immunolabeling of migrating CNC cells at 7 ss. (B) At this stage, Fgf8 is strongly expressed
Supporting Information Creuzet 10.1073/pnas.0906072106 Fig. S1. Migrating CNC cells express Bmp inhibitors. (A) HNK1 immunolabeling of migrating CNC cells at 7 ss. (B) At this stage, Fgf8 is strongly expressed
The role of Sonic hedgehog in normal and abnormal craniofacial morphogenesis
 Development 126, 4873-4884 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9636 4873 The role of Sonic hedgehog in normal and abnormal craniofacial morphogenesis Diane Hu and
Development 126, 4873-4884 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9636 4873 The role of Sonic hedgehog in normal and abnormal craniofacial morphogenesis Diane Hu and
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE. Thomas Marino, Ph.D.
 BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
Patterning the Embryo
 Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Pharyngeal Apparatus. Pouches Endoderm Grooves Ectoderm Arch Neural Crest Somitomeres Aortic Arch - Vessel
 Pharyngeal Apparatus Pouches Endoderm Grooves Ectoderm Arch Neural Crest Somitomeres Aortic Arch - Vessel Segmental Organization Humans: Arch 1-4 prominent Arch 5 absent Arch 6 - transient First Arch Face
Pharyngeal Apparatus Pouches Endoderm Grooves Ectoderm Arch Neural Crest Somitomeres Aortic Arch - Vessel Segmental Organization Humans: Arch 1-4 prominent Arch 5 absent Arch 6 - transient First Arch Face
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Head and Neck Development and Malformations
 Head and Neck Development and Malformations Yang Chai, DDS, PhD Professor George and MaryLou Boone Chair Ostrow School of Dentistry of USC ychai@usc.edu C D E A. B Learning Objectives - Learn cranial neural
Head and Neck Development and Malformations Yang Chai, DDS, PhD Professor George and MaryLou Boone Chair Ostrow School of Dentistry of USC ychai@usc.edu C D E A. B Learning Objectives - Learn cranial neural
NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM
 LECTURE 4 SKULL NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM CRANIUM NEUROCRANIUM (protective case around brain) VISCEROCRANIUM (skeleton of face) NASOMAXILLARY COMPLEX MANDIBLE (DESMOCRANIUM)
LECTURE 4 SKULL NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM CRANIUM NEUROCRANIUM (protective case around brain) VISCEROCRANIUM (skeleton of face) NASOMAXILLARY COMPLEX MANDIBLE (DESMOCRANIUM)
Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes
 Research article Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes Dwight Cordero, 1 Ralph Marcucio, 1 Diane Hu, 1 William Gaffield, 2 Minal Tapadia,
Research article Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes Dwight Cordero, 1 Ralph Marcucio, 1 Diane Hu, 1 William Gaffield, 2 Minal Tapadia,
Remember from the first year embryology Trilaminar disc has 3 layers: ectoderm, mesoderm, and endoderm
 Development of face Remember from the first year embryology Trilaminar disc has 3 layers: ectoderm, mesoderm, and endoderm The ectoderm forms the neural groove, then tube The neural tube lies in the mesoderm
Development of face Remember from the first year embryology Trilaminar disc has 3 layers: ectoderm, mesoderm, and endoderm The ectoderm forms the neural groove, then tube The neural tube lies in the mesoderm
Polarity and Segmentation. Chapter Two
 Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons.
 Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Regionalization of the nervous system. Paul Garrity 7.68J/9.013J February 25, 2004
 Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Development of the Pharyngeal Arches
 Development of the Pharyngeal Arches Thomas A. Marino, Ph.D. Temple University School of Medicine Competencies: Upon completion of this section of the course, the student must be able to: 1. Recall the
Development of the Pharyngeal Arches Thomas A. Marino, Ph.D. Temple University School of Medicine Competencies: Upon completion of this section of the course, the student must be able to: 1. Recall the
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Embryo#1. Mohammad Hisham Al-Mohtaseb باشق جهاد. 0 P a g e
 Embryo#1 Mohammad Hisham Al-Mohtaseb باشق جهاد 0 P a g e Before you start, it is important to link what you learn in gross anatomy with developmental stages discussed in embryology. Cells that form organs
Embryo#1 Mohammad Hisham Al-Mohtaseb باشق جهاد 0 P a g e Before you start, it is important to link what you learn in gross anatomy with developmental stages discussed in embryology. Cells that form organs
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Chapter 2. Genetic interaction between Gli3 and Alx4 during limb and craniofacial development CH Utrecht, The Netherlands
 Genetic interaction between Gli3 and Alx4 during limb and craniofacial development Lia Panman 1, Thijs Drenth 1, Pascal te Welscher 1,3, Aimee Zuniga 1,2, Rolf Zeller 1,2 1 Department of Developmental
Genetic interaction between Gli3 and Alx4 during limb and craniofacial development Lia Panman 1, Thijs Drenth 1, Pascal te Welscher 1,3, Aimee Zuniga 1,2, Rolf Zeller 1,2 1 Department of Developmental
Development of the Axial Skeleton and Limbs. Professor Alfred Cuschieri Department of Anatomy University of Malta
 Development of the Axial Skeleton and Limbs Professor Alfred Cuschieri Department of Anatomy University of Malta During the Fourth Week the Embryo Is Segmented. Each segment consists of: a segment of neural
Development of the Axial Skeleton and Limbs Professor Alfred Cuschieri Department of Anatomy University of Malta During the Fourth Week the Embryo Is Segmented. Each segment consists of: a segment of neural
Emx2 patterns the neocortex by regulating FGF positional signaling
 Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Tetrapod Limb Development
 Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Tetrapod Limb Development
 Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Developing Molecularly Targeted Therapies for Basal Cell Carcinoma. Ivor Caro, MD, FAAD
 Developing Molecularly Targeted Therapies for Basal Cell Carcinoma Ivor Caro, MD, FAAD Disclosures Genentech, Inc Medical Director, Dermatology (employee) Stock holder Hedgehog Signaling Pathway Fundamental
Developing Molecularly Targeted Therapies for Basal Cell Carcinoma Ivor Caro, MD, FAAD Disclosures Genentech, Inc Medical Director, Dermatology (employee) Stock holder Hedgehog Signaling Pathway Fundamental
SONIC HEDGEHOG (Shh) is a secreted glycoprotein
 Role of Sonic Hedgehog in the Development of the Trachea and Oesophagus By Adonis S. Ioannides, Deborah J. Henderson, Lewis Spitz, and Andrew J. Copp London, England and Newcastle, England Backround/Purpose:
Role of Sonic Hedgehog in the Development of the Trachea and Oesophagus By Adonis S. Ioannides, Deborah J. Henderson, Lewis Spitz, and Andrew J. Copp London, England and Newcastle, England Backround/Purpose:
Tetrapod Limb Development
 IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
04 Development of the Face and Neck. Development of the Face Development of the neck
 04 Development of the Face and Neck Development of the Face Development of the neck Development of the face Overview of facial development The fourth week ~ the twelfth week of prenatal development Between
04 Development of the Face and Neck Development of the Face Development of the neck Development of the face Overview of facial development The fourth week ~ the twelfth week of prenatal development Between
Epithelial-mesenchymal interactions in the development of chick facial primordia and the target of retinoid action
 Development 99, 341-351 (1987) Printed in Great Britain The Company of Biologists Limited 1987 341 Epithelial-mesenchymal interactions in the development of chick facial primordia and the target of retinoid
Development 99, 341-351 (1987) Printed in Great Britain The Company of Biologists Limited 1987 341 Epithelial-mesenchymal interactions in the development of chick facial primordia and the target of retinoid
a) They are the most common cause of pediatric kidney failure. b) They are always symptomatic. c) They can be asymmetric.
 Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
REVIEW OF CLINICAL EMBRYOLOGY OF HEAD AND NECK
 REVIEW OF CLINICAL EMBRYOLOGY OF HEAD AND NECK OUTLINE - EMBRYOLOGY UNDERLYING CLINICAL CONDITIONS I. EARLY DEVELOPMENT OF FACE: CLEFT LIP, CLEFT PALATE, OBSTRUCTED NASOLACRIMAL DUCT II. BRANCHIAL ARCHES
REVIEW OF CLINICAL EMBRYOLOGY OF HEAD AND NECK OUTLINE - EMBRYOLOGY UNDERLYING CLINICAL CONDITIONS I. EARLY DEVELOPMENT OF FACE: CLEFT LIP, CLEFT PALATE, OBSTRUCTED NASOLACRIMAL DUCT II. BRANCHIAL ARCHES
ORTHODONTIC INITIAL ASSESSMENT FORM (OIAF) w/ INSTRUCTIONS
 Use the accompanying Tip Sheet and How to Score the Orthodontic Initial Assessment Form for guidance in completion of the assessment form. You will need this score sheet and a disposable ruler (or a Boley
Use the accompanying Tip Sheet and How to Score the Orthodontic Initial Assessment Form for guidance in completion of the assessment form. You will need this score sheet and a disposable ruler (or a Boley
/06/ PEDIATRIC RESEARCH Vol. 59, No. 3, 2006 Copyright 2006 International Pediatric Research Foundation, Inc.
 0031-3998/06/5903-0349 PEDIATRIC RESEARCH Vol. 59, No. 3, 2006 Copyright 2006 International Pediatric Research Foundation, Inc. Printed in U.S.A. ARTICLES Sonic Hedgehog Is Essential for First Pharyngeal
0031-3998/06/5903-0349 PEDIATRIC RESEARCH Vol. 59, No. 3, 2006 Copyright 2006 International Pediatric Research Foundation, Inc. Printed in U.S.A. ARTICLES Sonic Hedgehog Is Essential for First Pharyngeal
Developmental Biology
 Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
APPENDIX A. MEDICAID ORTHODONTIC INITIAL ASSESSMENT FORM (IAF) You will need this scoresheet and a disposable ruler (or a Boley Gauge)
 APPENDIX A MEDICAID ORTHODONTIC INITIAL ASSESSMENT FORM (IAF) You will need this scoresheet and a disposable ruler (or a Boley Gauge) Name: _ I. D. Number: Conditions: 1. Cleft palate deformities 2. Deep
APPENDIX A MEDICAID ORTHODONTIC INITIAL ASSESSMENT FORM (IAF) You will need this scoresheet and a disposable ruler (or a Boley Gauge) Name: _ I. D. Number: Conditions: 1. Cleft palate deformities 2. Deep
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
 Drawings illustrating the human pharyngeal apparatus. Drawings illustrating the human pharyngeal apparatus. Drawings illustrating the human pharyngeal apparatus. Drawings illustrating the human pharyngeal
Drawings illustrating the human pharyngeal apparatus. Drawings illustrating the human pharyngeal apparatus. Drawings illustrating the human pharyngeal apparatus. Drawings illustrating the human pharyngeal
Class II. Bilateral Cleft Lip and Palate. Clinician: Dr. Mike Mayhew, Boone, NC Patient: R.S. Cleft Lip and Palate.
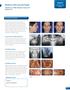 Bilateral Cleft Lip and Palate Clinician: Dr. Mike Mayhew, Boone, NC Patient: R.S. Class II Cleft Lip and Palate Pretreatment Diagnosis Class II dolichofacial female, age 22 years 11 months, presented
Bilateral Cleft Lip and Palate Clinician: Dr. Mike Mayhew, Boone, NC Patient: R.S. Class II Cleft Lip and Palate Pretreatment Diagnosis Class II dolichofacial female, age 22 years 11 months, presented
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Periodontal Disease. Radiology of Periodontal Disease. Periodontal Disease. The Role of Radiology in Assessment of Periodontal Disease
 Radiology of Periodontal Disease Steven R. Singer, DDS srs2@columbia.edu 212.305.5674 Periodontal Disease! Includes several disorders of the periodontium! Gingivitis! Marginal Periodontitis! Localized
Radiology of Periodontal Disease Steven R. Singer, DDS srs2@columbia.edu 212.305.5674 Periodontal Disease! Includes several disorders of the periodontium! Gingivitis! Marginal Periodontitis! Localized
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and
 Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Axis Formation and Mesoderm Induction
 Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
Positional signalling and the development of the humerus in the chick limb bud
 Development 100, 333-338 (1987) Printed in Great Britain The Company of Biologists Limited 1987 333 Positional signalling and the development of the humerus in the chick limb bud L. WOLPERT and AMATA HORNBRUCH
Development 100, 333-338 (1987) Printed in Great Britain The Company of Biologists Limited 1987 333 Positional signalling and the development of the humerus in the chick limb bud L. WOLPERT and AMATA HORNBRUCH
Essentials in Head and Neck Embryology. Part 3 Development of the head, face, and oral cavity
 Essentials in Head and Neck Embryology Part 3 Development of the head, face, and oral cavity Outline General overview of prenatal development Embryonic period phase 1 Formation of bilaminar disk Formation
Essentials in Head and Neck Embryology Part 3 Development of the head, face, and oral cavity Outline General overview of prenatal development Embryonic period phase 1 Formation of bilaminar disk Formation
Skeletal System. Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section
 Skeletal System Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section Learning objectives At the end of this lecture, the medical student will be able to: State the embryonic origin of skeletal
Skeletal System Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section Learning objectives At the end of this lecture, the medical student will be able to: State the embryonic origin of skeletal
Ahtiainen et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
evolution and development of primate teeth
 evolution and development of primate teeth diversity of mammalian teeth upper left molars buccal mesial distal lingual Jernvall & Salazar-Ciudad 07 trends in dental evolution many similar single-cusped
evolution and development of primate teeth diversity of mammalian teeth upper left molars buccal mesial distal lingual Jernvall & Salazar-Ciudad 07 trends in dental evolution many similar single-cusped
DBS: Project Seminar Guidelines
 DBS: Project Seminar Guidelines Updated on 19 January 2011 Project Area Review Seminar ( Project I ) Content: This is a comprehensive review of the literature in the area the student has selected for their
DBS: Project Seminar Guidelines Updated on 19 January 2011 Project Area Review Seminar ( Project I ) Content: This is a comprehensive review of the literature in the area the student has selected for their
Branching morphogenesis of the lung: new molecular insights into an old problem
 86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang 1 and Andrew P. McMahon 2 1 Cardiovascular Research
86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang 1 and Andrew P. McMahon 2 1 Cardiovascular Research
Development of Brain Stem, Cerebellum and Cerebrum
 Development of Brain Stem, Cerebellum and Cerebrum The neural tube cranial to the 4th pair of somites develop into the brain. 3 dilatations and 2 flexures form at the cephalic end of the neural tube during
Development of Brain Stem, Cerebellum and Cerebrum The neural tube cranial to the 4th pair of somites develop into the brain. 3 dilatations and 2 flexures form at the cephalic end of the neural tube during
ODONTOGENESIS- A HIGHLY COMPLEX CELL-CELL INTERACTION PROCESS
 ODONTOGENESIS- A HIGHLY COMPLEX CELL-CELL INTERACTION PROCESS AMBRISH KAUSHAL, MALA KAMBOJ Department of Oral and Maxillofacial Pathology Career Post Graduate Institute of Dental Sciences and Hospital
ODONTOGENESIS- A HIGHLY COMPLEX CELL-CELL INTERACTION PROCESS AMBRISH KAUSHAL, MALA KAMBOJ Department of Oral and Maxillofacial Pathology Career Post Graduate Institute of Dental Sciences and Hospital
Review of Nervous System Anatomy
 For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
Supplementary Figure 1. A microarray screen of organizers compared to non-organizer tissue reveals a putative organizer gene set.
 Supplementary Figure 1. A microarray screen of organizers compared to non-organizer tissue reveals a putative organizer gene set. (a, b) Venn diagrams of 31 enriched (a) and 17 depleted (b) genes significantly
Supplementary Figure 1. A microarray screen of organizers compared to non-organizer tissue reveals a putative organizer gene set. (a, b) Venn diagrams of 31 enriched (a) and 17 depleted (b) genes significantly
European Veterinary Dental College
 European Veterinary Dental College EVDC Training Support Document Preparation of Radiograph Sets (Cat and Dog) Document version : evdc-tsd-radiograph_positioning_(dog_and_cat)-20120121.docx page 1 of 13
European Veterinary Dental College EVDC Training Support Document Preparation of Radiograph Sets (Cat and Dog) Document version : evdc-tsd-radiograph_positioning_(dog_and_cat)-20120121.docx page 1 of 13
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval
 When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Neuroanatomy. Assistant Professor of Anatomy Faculty of Medicine The University of Jordan Dr Maha ELBeltagy
 Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest
 Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest Ankita Das, J. Gage Crump* Broad CIRM Center, University of Southern California Keck School of Medicine,
Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest Ankita Das, J. Gage Crump* Broad CIRM Center, University of Southern California Keck School of Medicine,
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Supplemental Information. Ciliary Beating Compartmentalizes. Cerebrospinal Fluid Flow in the Brain. and Regulates Ventricular Development
 Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
Week 14. Development of the Musculoskeletal System
 Week 14 Development of the Musculoskeletal System Skeletal System Derived from: paraxial mesoderm somites and somitomeres sclerotome sclerotome differentiation induced by SHH from notochord and floor plate
Week 14 Development of the Musculoskeletal System Skeletal System Derived from: paraxial mesoderm somites and somitomeres sclerotome sclerotome differentiation induced by SHH from notochord and floor plate
Gli2 is required for induction of floor plate and adjacent cells, but not most
 Development 125, 2759-2770 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV9597 2759 Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons
Development 125, 2759-2770 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV9597 2759 Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Disrupting hedgehog and WNT signaling interactions promotes cleft lip pathogenesis
 Research article Disrupting hedgehog and WNT signaling interactions promotes cleft lip pathogenesis Hiroshi Kurosaka, 1 Angelo Iulianella, 2 Trevor Williams, 3 and Paul A. Trainor 1,4 1 Stowers Institute
Research article Disrupting hedgehog and WNT signaling interactions promotes cleft lip pathogenesis Hiroshi Kurosaka, 1 Angelo Iulianella, 2 Trevor Williams, 3 and Paul A. Trainor 1,4 1 Stowers Institute
Subject Index. AXIN2, cleft defects 24, 26
 Subject Index ADAMTS, mouse mutants and palate development 37, 38 Africa, cleft lip and palate prevalence 6, 7 Alcohol dependence, pregnancy risks for cleft 25, 61 Altitude, pregnancy risks for cleft 25,
Subject Index ADAMTS, mouse mutants and palate development 37, 38 Africa, cleft lip and palate prevalence 6, 7 Alcohol dependence, pregnancy risks for cleft 25, 61 Altitude, pregnancy risks for cleft 25,
Hepatogenesis I Liver development
 Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
#45 Ortho-Tain, Inc PREVENTIVE ERUPTION GUIDANCE -- PREVENTIVE OCCLUSAL DEVELOPMENT
 #45 Ortho-Tain, Inc. 1-800-541-6612 PREVENTIVE ERUPTION GUIDANCE -- PREVENTIVE OCCLUSAL DEVELOPMENT Analysis and Diagnosis of Occlusion: The ideal child of 5 y ears of age that probably has the best chance
#45 Ortho-Tain, Inc. 1-800-541-6612 PREVENTIVE ERUPTION GUIDANCE -- PREVENTIVE OCCLUSAL DEVELOPMENT Analysis and Diagnosis of Occlusion: The ideal child of 5 y ears of age that probably has the best chance
JOURNAL OF EXPERIMENTAL ZOOLOGY (MOL DEV EVOL) 306B: (2006)
 JOURNAL OF EXPERIMENTAL ZOOLOGY (MOL DEV EVOL) 306B:183 203 (2006) Developmental and Evolutionary Origins of the Vertebrate Dentition: Molecular Controls for Spatio-temporal Organisation of Tooth Sites
JOURNAL OF EXPERIMENTAL ZOOLOGY (MOL DEV EVOL) 306B:183 203 (2006) Developmental and Evolutionary Origins of the Vertebrate Dentition: Molecular Controls for Spatio-temporal Organisation of Tooth Sites
Arrangement of the artificial teeth:
 Lecture Prosthodontic Dr. Osama Arrangement of the artificial teeth: It s the placement of the teeth on a denture with definite objective in mind or it s the setting of teeth on temporary bases. Rules
Lecture Prosthodontic Dr. Osama Arrangement of the artificial teeth: It s the placement of the teeth on a denture with definite objective in mind or it s the setting of teeth on temporary bases. Rules
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Early Embryonic Development
 Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Development of teeth. 5.DM - Pedo
 Development of teeth 5.DM - Pedo Tooth development process of continuous changes in predetermined order starts from dental lamina A band of ectodermal cells growing from the epithelium of the embryonic
Development of teeth 5.DM - Pedo Tooth development process of continuous changes in predetermined order starts from dental lamina A band of ectodermal cells growing from the epithelium of the embryonic
م.م. طارق جاسم حممد REMOVABLE PARTIAL DENTURE INTRODUCTION
 Lec.1 م.م. طارق جاسم حممد REMOVABLE PARTIAL DENTURE INTRODUCTION االسنان طب Prosthodontics is the branch of dentistry pertaining to the restoration and maintenance of oral function, comfort, appearance,
Lec.1 م.م. طارق جاسم حممد REMOVABLE PARTIAL DENTURE INTRODUCTION االسنان طب Prosthodontics is the branch of dentistry pertaining to the restoration and maintenance of oral function, comfort, appearance,
Midgut. Over its entire length the midgut is supplied by the superior mesenteric artery
 Gi Embryology 3 Midgut the midgut is suspended from the dorsal abdominal wall by a short mesentery and communicates with the yolk sac by way of the vitelline duct or yolk stalk Over its entire length the
Gi Embryology 3 Midgut the midgut is suspended from the dorsal abdominal wall by a short mesentery and communicates with the yolk sac by way of the vitelline duct or yolk stalk Over its entire length the
SURGICAL MODEL ACCURACY DEVICE. 25 years - manufacturing and distribution - around the globe research - design - manufacturing - distribution
 SURGICAL MODEL ACCURACY DEVICE 25 years - manufacturing and distribution - around the globe research - design - manufacturing - distribution 2 SMAD - SURGICAL MODEL ACCURACY DEVICE SMAD has be designed
SURGICAL MODEL ACCURACY DEVICE 25 years - manufacturing and distribution - around the globe research - design - manufacturing - distribution 2 SMAD - SURGICAL MODEL ACCURACY DEVICE SMAD has be designed
Prosthetic Options in Implant Dentistry. Hakimeh Siadat, DDS, MSc Associate Professor
 Prosthetic Options in Dentistry Hakimeh Siadat, DDS, MSc Associate Professor Dental Research Center, Department of Prosthodontics & Dental s Faculty of Dentistry, Tehran University of Medical Sciences
Prosthetic Options in Dentistry Hakimeh Siadat, DDS, MSc Associate Professor Dental Research Center, Department of Prosthodontics & Dental s Faculty of Dentistry, Tehran University of Medical Sciences
Mesial Step Class I or Class III Dependent upon extent of step seen clinically and patient s growth pattern Refer for early evaluation (by 8 years)
 Orthodontics and Dentofacial Development Overview Development of Dentition Treatment Retention and Relapse Growth of Naso-Maxillary Complex Develops postnatally entirely by intramenbranous ossification
Orthodontics and Dentofacial Development Overview Development of Dentition Treatment Retention and Relapse Growth of Naso-Maxillary Complex Develops postnatally entirely by intramenbranous ossification
Inner ear development Nervous system development
 Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Pharyngeal apparatus. - At the third week, it is a 3 layered structure: ectoderm, mesoderm and endoderm. This is called trilaminar disc
 Pharyngeal apparatus Remember from the first year embryology - The embryo was disc shaped in the second week of development (this is called embryonic disc) and it is a 2 layered disc (composed of two layers)---bilaminar
Pharyngeal apparatus Remember from the first year embryology - The embryo was disc shaped in the second week of development (this is called embryonic disc) and it is a 2 layered disc (composed of two layers)---bilaminar
Rehabilitating a Compromised Site for Restoring Form, Function and Esthetics- A Case Report
 Research & Reviews: Journal of Dental Sciences Rehabilitating a Compromised Site for Restoring Form, Function and Esthetics- A Case Report Priyanka Prakash* Division of Periodontology, Department of Dental
Research & Reviews: Journal of Dental Sciences Rehabilitating a Compromised Site for Restoring Form, Function and Esthetics- A Case Report Priyanka Prakash* Division of Periodontology, Department of Dental
Development of the Digestive System. W.S. O School of Biomedical Sciences, University of Hong Kong.
 Development of the Digestive System W.S. O School of Biomedical Sciences, University of Hong Kong. Organization of the GI tract: Foregut (abdominal part) supplied by coeliac trunk; derivatives include
Development of the Digestive System W.S. O School of Biomedical Sciences, University of Hong Kong. Organization of the GI tract: Foregut (abdominal part) supplied by coeliac trunk; derivatives include
Differentiation of the facial-vestibulocochlear ganglionic complex in human embryos of developmental stages 13 15
 O R I G I N A L A R T I C L E Folia Morphol. Vol. 68, No. 3, pp. 167 173 Copyright 2009 Via Medica ISSN 0015 5659 www.fm.viamedica.pl Differentiation of the facial-vestibulocochlear ganglionic complex
O R I G I N A L A R T I C L E Folia Morphol. Vol. 68, No. 3, pp. 167 173 Copyright 2009 Via Medica ISSN 0015 5659 www.fm.viamedica.pl Differentiation of the facial-vestibulocochlear ganglionic complex
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Volume 22 No. 14 September Dentists, Federally Qualified Health Centers and Health Maintenance Organizations For Action
 State of New Jersey Department of Human Services Division of Medical Assistance & Health Services Volume 22 No. 14 September 2012 TO: Dentists, Federally Qualified Health Centers and Health Maintenance
State of New Jersey Department of Human Services Division of Medical Assistance & Health Services Volume 22 No. 14 September 2012 TO: Dentists, Federally Qualified Health Centers and Health Maintenance
Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification
 Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification Marcus K. Gustafsson, 1,3,4 Hua Pan, 1,3,4 Deborah F. Pinney, 1,3 Yongliang Liu, 1,3 Anna Lewandowski, 1,3
Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification Marcus K. Gustafsson, 1,3,4 Hua Pan, 1,3,4 Deborah F. Pinney, 1,3 Yongliang Liu, 1,3 Anna Lewandowski, 1,3
EMBO REPORT SUPPLEMENTARY SECTION. Quantitation of mitotic cells after perturbation of Notch signalling.
 EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
BCL11B Regulates Epithelial Proliferation and Asymmetric Development of the Mouse Mandibular Incisor
 BCL11B Regulates Epithelial Proliferation and Asymmetric Development of the Mouse Mandibular Incisor Kateryna Kyrylkova 1, Sergiy Kyryachenko 1, Brian Biehs 2 *, Ophir Klein 2, Chrissa Kioussi 1 *, Mark
BCL11B Regulates Epithelial Proliferation and Asymmetric Development of the Mouse Mandibular Incisor Kateryna Kyrylkova 1, Sergiy Kyryachenko 1, Brian Biehs 2 *, Ophir Klein 2, Chrissa Kioussi 1 *, Mark
Component parts of Chrome Cobalt Removable Partial Denture
 Lec. 5 د.بسام الطريحي Component parts of Chrome Cobalt Removable Partial Denture Major connectors: Are either bars or plates, the difference between them is in the amount of tissue covers. Plates are broad
Lec. 5 د.بسام الطريحي Component parts of Chrome Cobalt Removable Partial Denture Major connectors: Are either bars or plates, the difference between them is in the amount of tissue covers. Plates are broad
Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape
 Access Development the most First recent posted epress version online at online on http://dev.biologists.org/lookup/doi/10.1242/dev.052340 8 September publication 2010 as date 10.1242/dev.052340 8 September
Access Development the most First recent posted epress version online at online on http://dev.biologists.org/lookup/doi/10.1242/dev.052340 8 September publication 2010 as date 10.1242/dev.052340 8 September
Independent regulation of Dlx2 expression in the epithelium and mesenchyme of the first branchial arch
 Development 127, 217-224 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV2450 217 Independent regulation of Dlx2 expression in the epithelium and mesenchyme of the first branchial
Development 127, 217-224 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV2450 217 Independent regulation of Dlx2 expression in the epithelium and mesenchyme of the first branchial
Chapter 5 A Dose Dependent Screen for Modifiers of Kek5
 Chapter 5 A Dose Dependent Screen for Modifiers of Kek5 "#$ ABSTRACT Modifier screens in Drosophila have proven to be a powerful tool for uncovering gene interaction and elucidating molecular pathways.
Chapter 5 A Dose Dependent Screen for Modifiers of Kek5 "#$ ABSTRACT Modifier screens in Drosophila have proven to be a powerful tool for uncovering gene interaction and elucidating molecular pathways.
Fundamental & Preventive Curvatures of Teeth and Tooth Development. Lecture Three Chapter 15 Continued; Chapter 6 (parts) Dr. Margaret L.
 Fundamental & Preventive Curvatures of Teeth and Tooth Development Lecture Three Chapter 15 Continued; Chapter 6 (parts) Dr. Margaret L. Dennis Proximal contact areas Contact areas are on the mesial and
Fundamental & Preventive Curvatures of Teeth and Tooth Development Lecture Three Chapter 15 Continued; Chapter 6 (parts) Dr. Margaret L. Dennis Proximal contact areas Contact areas are on the mesial and
Teeth, orofacial development and
 Teeth, orofacial development and cleft anomalies Miroslav Peterka Variability of jaws in vertebrates. (A) cartilaginous fish shark; (B) an example of a bone fish; (C ) amphibian frog; (D) reptile - turtle;
Teeth, orofacial development and cleft anomalies Miroslav Peterka Variability of jaws in vertebrates. (A) cartilaginous fish shark; (B) an example of a bone fish; (C ) amphibian frog; (D) reptile - turtle;
The cephalic neural crest exerts a critical effect on forebrain and midbrain development
 The cephalic neural crest exerts a critical effect on forebrain and midbrain development Sophie E. Creuzet*, Salvador Martinez, and Nicole M. Le Douarin* *Laboratoire de Développement, Evolution, et Plasticité
The cephalic neural crest exerts a critical effect on forebrain and midbrain development Sophie E. Creuzet*, Salvador Martinez, and Nicole M. Le Douarin* *Laboratoire de Développement, Evolution, et Plasticité
An Anterior Tooth Size Comparison in Unilateral and Bilateral Congenitally Absent Maxillary Lateral Incisors
 An Anterior Tooth Size Comparison in Unilateral and Bilateral Congenitally Absent Maxillary Lateral Incisors Abstract The purpose of this study is to compare the anterior tooth size width in patients with
An Anterior Tooth Size Comparison in Unilateral and Bilateral Congenitally Absent Maxillary Lateral Incisors Abstract The purpose of this study is to compare the anterior tooth size width in patients with
Developing Facial Symmetry Using an Intraoral Device: A Case Report
 Developing Facial Symmetry Using an Intraoral Device: A Case Report by Theodore R. Belfor, D.D.S.; and G. Dave Singh, D.D.Sc., Ph.D., B.D.S. Dr. Theodore Belfor graduated from New York University College
Developing Facial Symmetry Using an Intraoral Device: A Case Report by Theodore R. Belfor, D.D.S.; and G. Dave Singh, D.D.Sc., Ph.D., B.D.S. Dr. Theodore Belfor graduated from New York University College
Embryology of the Nervous System. Steven McLoon Department of Neuroscience University of Minnesota
 Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
