MYOEPITHELIOMA OF SOFT TISSUE AND BONE CRISTINA ANTONESCU, MD. Memorial Sloan-Kettering Cancer Center, New York, NY
|
|
|
- Egbert Bennett
- 6 years ago
- Views:
Transcription
1 MYOEPITHELIOMA OF SOFT TISSUE AND BONE CRISTINA ANTONESCU, MD Memorial Sloan-Kettering Cancer Center, New York, NY The spectrum of myoepithelial (ME) tumors represents a family of lesions with variable terminology, based on anatomic location: such as pleomorphic adenoma in the salivary gland, benign mixed tumor in the skin, and myoepithelial tumor/parachordoma in the soft tissue. Although genetic studies in pleomorphic adenoma have shown frequent rearrangements of PLAG1 and HMGA2 1-3, similar abnormalities have not been identified in myoepithelial lesions from other tissues 4. Thus it remains unclear if the myoepithelioma subsets described above share a common pathogenesis. Until recently, the genetic hallmark of soft tissue ME tumors was still under investigation, with only two cases analyzed cytogenetically, reporting disparate chromosomal translocations. A t(1;22)(q23;q12) resulting in an EWSR1-PBX1 fusion was first described as a sole cytogenetic event in a soft tissue ME tumor, arising in the foot of a 59-year old female 5, while a 2 nd case, of an occipital soft tissue ME carcinoma, arising in a 40-year old female, showed a t(19;22)(q13;q12), resulting in an EWSR1- ZNF444 fusion 6. In addition, EWSR1 gene rearrangement by FISH has been reported in two ME tumors in the pediatric age group 7. In a recent study we undertook a systematic molecular analysis of a large spectrum of ME tumors, including lesions from a variety of anatomic locations, age groups and lesions with differing biologic potential 8. Minimum criteria for confirming the morphologic diagnosis included the co-reactivity for EMA +/- cytokeratin and S100+/- GFAP. During this talk I will be summarizing our findings. EWSR1 rearrangement is a common event in deep-seated soft tissue and bone ME tumors. Thirty (45%) of the 66 cases tested showed the presence of an EWSR1 gene rearrangement by FISH. More than half of the cases (n=16) occurred in children and young adults. The most common presentation was in the deep-seated soft tissues of the extremities, followed by the head and neck location. Five of the six osseous ME tumors showed an EWSR1 rearrangement. Four of the six lung ME tumors were positive, while only two of the six cutaneous tumors showed an EWSR1 break-apart signal by FISH. Morphologically, in the EWSR1 rearranged tumors there were several different patterns identified: (1) tumors composed mainly of small blue cells with scant cytoplasm, monotonous cytomorphology and ill-defined cell borders, arranged in solid sheets; (2) tumors with a predominantly epithelioid or rhabdoid appearance, with moderate to abundant eosinophilic or clear cytoplasm and eccentric nuclei; or (3) tumors composed of spindle or ovoid cells embedded in a prominent sclerotic stroma. None of the EWSR1 positive tumors showed the presence of ductal or glandular differentiation or cartilage/bone matrix formation. Immunohistochemically, all tumors showed S100 protein staining, typically strong and diffuse, as well EMA and/or cytokeratin AE1/AE3. EWSR1-POU5F1 fusion is identified in a subset of deep soft tissue ME tumors with distinctive clear cell morphology. The five positive tumors had striking similarities, all of them presenting in the deep soft tissues of extremities, in children or young adults, and microscopically composed predominantly of epithelioid cells with abundant clear
2 cytoplasm, arranged in a nested growth pattern. These tumors were strongly and diffusely positive for both EMA and S100 protein, but lacked OCT4 expression, which seems surprising since this is the transcription factor encoded by POU5F1. Interestingly, a similar fusion between EWSR1 and POU5F1 has been reported previously in a case of undifferentiated bone tumor of the pelvis, carrying a t(6;22)(p21;q12) 9. The tumor was composed of both primitive round cells and short spindle cells and reportedly was immunopositive for S100 protein and focally for cytokeratin. These morphologic features raise the possibility of an intra-osseous ME tumor. Subsequently, a similar EWSR1-POU5F1 fusion was identified in 3 hidradenomas of the skin and 1 case of mucoepidermoid carcinoma of salivary gland, which, in contrast to our findings, showed OCT4 expression immunohistochemically 10. Although not identical, the transcript composition of the EWSR1-POU5F1 reported in these cases was quite similar with the one identified in our ME tumor. These two epithelial tumor types have been previously shown to be molecularly related, both entities showing a CRTC1- MAML2 fusion in approximately half of the cases 11, 12. A FISH investigation of 10 additional hidradenomas lacking the CRTC1-MAML2 fusion transcript identified two cases with EWSR1 gene rearrangement 10. However, none of the 5 eccrine hidradenomas and 6 salivary gland mucoepidermoid carcinoma tested in our study showed an EWSR1 rearrangement. The relationship between these EWSR1-fusion positive skin adnexal tumors and tumors reported in this series with features of superficial myoepithelioma and cutaneous benign mixed tumor requires additional investigation. However, most of the cutaneous ME tumors and the lesions displaying ductal/glandular differentiation in this study were negative for EWSR1 gene rearrangement, suggesting an alternative pathogenesis. Furthermore, the presence of EWSR1 gene rearrangement in only one of the three mucoepidermoid carcinoma studied by Moller and coworkers 10 and none of the five in the present series suggests that other mechanisms may be involved in the pathogenesis of that tumor type. In addition, none of the 5 myoepithelial carcinomas of salivary gland studied showed involvement of the EWSR1 gene, suggesting that at least a subset of ME tumors arising in salivary gland may not be related to their soft tissue counterparts. POU5F1 is a transcription factor and its expression is restricted to germ cells in mature adult tissue and in human tumors is typically present in germ cell tumors 13. The multiple cell types seen in the composition of tumors positive for the EWSR1-POU5F1 fusion in our study may be indicative of a multi-phenotypic differentiation. ME tumor is a prototypical example of dual differentiation to both epithelial and mesenchymal lineages. POU5F1 expression as evidenced by OCT4 immunoreactivity was documented in the EWSR1-POU5F1 positive hidradenomas 10 however, it was negative in all 5 ME tumors in the present study, using the same antibody. EWSR1-PBX1 fusion is present in a subset of ME tumors associated with a bland sclerotic appearance or clear cell morphology. Morphologically, 3 of the tumors, located in the foot, hip and pelvic bone, showed a deceptively bland appearance, being composed mainly of spindle cells embedded in a fibrotic stroma, resembling in areas desmoid-type fibromatosis. The other two cases, located in the forearm and lung, were composed of epithelioid or ovoid uniform cells with abundant clear cytoplasm. EWSR1-ZNF444 fusion is a rare occurrence in ME tumors. Only one tumor had this rearrangement, which occurred in the lung of a 64-year old female. The
3 morphologic appearance was quite typical, with predominantly epithelioid cells with scant, clear cytoplasm, arranged in Indian-files or pseudo-rosettes separated by prominent sclerotic stroma. A focal spindle cell component was also noted. Tumor cells were positive for cytokeratin AE1:AE3 and S100 protein, but negative for EMA. Salivary gland myoepithelial carcinomas nor the other related tumors showed an EWSR1 gene rearrangement. None of the tumors included in the control groups, including: 5 salivary gland myoepithelial carcinomas ex-pleomorphic adenoma; 6 salivary gland mucoepidermoid carcinoma; 5 cutaneous eccrine hidradenomas; 3 ossifying fibromyxoid tumors and 2 chordoma periphericum showed an EWSR1 abnormality. Differential Diagnosis. The differential diagnosis of ME tumors is typically quite broad and may vary depending on patient age and anatomic location. Ossifying fibromyxoid tumor (OFMT), an S100 protein-positive soft tissue tumor with an uncertain line of differentiation, shares significant morphologic overlap with ME tumors. Although only 3 OFMTs were included for genetic analysis, the lack of EWSR1 abnormalities suggests that this entity may not be related to ME tumors. The distinction from extraskeletal myxoid chondrosarcoma (EMC) can often be quite difficult, especially in large, deep-seated soft tissue tumors associated with myxoid changes. The presence of EWSR1 gene rearrangement, used previously to support the diagnosis of EMC, is now demonstrated in both tumor entities, in a significant number of cases. In spite of the morphologic overlap and EWSR1 gene abnormality, we do not believe the two tumors are related. The consistently strong EMA and S100 protein coreactivity, the common clear cell changes and nested growth pattern seen in ME tumors, but not in most EMC, should help in the distinction. Furthermore a predominant myxoid stroma throughout the tumor is rarely seen in ME lesions. In EWSR1-positive tumors having a small cell/undifferentiated appearance, an alternative Ewing sarcoma/pnet diagnosis was excluded either by negativity for CD99 immunostaining or by RT-PCR for EWSR1-FLI1 and EWSR1-ERG. The presence of EWSR1 gene rearrangement in about half of ME tumors which can be readily detected by FISH analysis can serve as a powerful diagnostic tool in challenging cases. However, this finding adds ME tumors to an already growing family of EWSR1 gene-rearranged tumors, which are often considered in the differential diagnosis, especially in the pediatric age group 14. ME tumors with EWSR1 gene rearrangement often have uniform rounded cell morphology and clear cytoplasm, presenting in the deep soft tissues of the extremities. These findings pose significant overlap both microscopically and clinically with other pediatric tumors, especially with Ewing sarcoma, also characterized by recurrent translocations involving the EWSR1 gene. In difficult cases which are positive for EWSR1 rearrangement, efforts to identify the translocation partner should be undertaken by RT-PCR methods for a definitive diagnosis and to avoid unnecessary systemic treatment. These findings reinforce the fact that recurrent chromosomal translocations involving EWSR1 do not occur only in aggressive high grade sarcomas, but also in tumors with low or undetermined malignant potential, like ME tumors and so-called angiomatoid fibrous histiocytoma. Furthermore, these results provide solid evidence for a unifying concept of soft tissue ME with similar tumors arising in bone and at visceral
4 locations. However, the data presented here do not support a pathogenetic relationship between soft tissue ME tumors and their salivary gland counterparts. Additional cases will need to be analyzed in order to further investigate the relationship of soft tissue ME lesions and cutaneous benign mixed tumors. References 1. Kas K, Voz ML, Roijer E, Astrom AK, Meyen E, Stenman G, Van de Ven WJ. Promoter swapping between the genes for a novel zinc finger protein and betacatenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat Genet. 1997;15: Martins C, Fonseca I, Roque L, Pereira T, Ribeiro C, Bullerdiek J, Soares J. Plag1 gene alterations in salivary gland pleomorphic adenoma and carcinoma expleomorphic adenoma: A combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol. 2005;18: Persson F, Andren Y, Winnes M, Wedell B, Nordkvist A, Gudnadottir G, Dahlenfors R, Sjogren H, Mark J, Stenman G. High-resolution genomic profiling of adenomas and carcinomas of the salivary glands reveals amplification, rearrangement, and fusion of hmga2. Genes Chromosomes Cancer. 2009;48: Hallor KH, Teixeira MR, Fletcher CD, Bizarro S, Staaf J, Domanski HA, von Steyern FV, Panagopoulos I, Mandahl N, Mertens F. Heterogeneous genetic profiles in soft tissue myoepitheliomas. Mod Pathol. 2008;21: Brandal P, Panagopoulos I, Bjerkehagen B, Gorunova L, Skjeldal S, Micci F, Heim S. Detection of a t(1;22)(q23;q12) translocation leading to an EWSR1- PBX1 fusion gene in a myoepithelioma. Genes Chromosomes Cancer. 2008;47: Brandal P, Panagopoulos I, Bjerkehagen B, Heim S. T(19;22)(q13;q12) translocation leading to the novel fusion gene EWSR1-ZNF444 in soft tissue myoepithelial carcinoma. Genes Chromosomes Cancer Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: An aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31: Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Katabi N, Edelman M, Rosenberg AE, Nielsen GP, Dal Cin P, Fletcher CD. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the ewsr1 gene. Genes Chromosomes Cancer. 2010;49: Yamaguchi S, Yamazaki Y, Ishikawa Y, Kawaguchi N, Mukai H, Nakamura T. Ewsr1 is fused to pou5f1 in a bone tumor with translocation t(6;22)(p21;q12). Genes Chromosomes Cancer. 2005;43: Moller E, Stenman G, Mandahl N, Hamberg H, Molne L, van den Oord JJ, Brosjo O, Mertens F, Panagopoulos I. Pou5f1, encoding a key regulator of stem cell pluripotency, is fused to ewsr1 in hidradenoma of the skin and mucoepidermoid carcinoma of the salivary glands. J Pathol. 2008;215:78-86
5 11. Behboudi A, Enlund F, Winnes M, Andren Y, Nordkvist A, Leivo I, Flaberg E, Szekely L, Makitie A, Grenman R, Mark J, Stenman G. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the mect1-maml2 fusion oncogene. Genes Chromosomes Cancer. 2006;45: Winnes M, Molne L, Suurkula M, Andren Y, Persson F, Enlund F, Stenman G. Frequent fusion of the crtc1 and maml2 genes in clear cell variants of cutaneous hidradenomas. Genes Chromosomes Cancer. 2007;46: Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol. 2007;31: Romeo S, Dei Tos AP. Soft tissue tumors associated with ewsr1 translocation. Virchows Arch. 2010;456:
6 Background Spectrum of ME Tumors Myoepithelial Tumors of Soft Tissue and Bone Cristina Antonescu, MD Department of Pathology Memorial Sloan-Kettering Cancer Center, New York Family of tumors with variable terminology based on anatomic location: pleomorphic adenoma (salivary gland) benign mixed tumor (skin) soft tissue myoepithelioma, parachordoma Definition: immunohistochemical evidence of myoepithelial differentiation (EMA/CK, S100) Uncertain if any/all have a common pathogenesis 2/11/2011 2/11/2011 Soft Tissue ME Tumors Genetics Few reports of EWSR1 gene rearrangement one case report each, the fusion partner was identified as being either PBX1 or ZNF444 Molecular Study of Large Spectrum of ME Tumors/Patients 66 ME tumors with confirmed diagnosis and adequate material for molecular analysis 47 Soft Tissue 7 Cutaneous 6 Bone 6 Visceral (lung) Age distribution: 15 children, 11 young adults (<30 years), 40 adults Gender distribution: 36 females/30 males 2/11/2011 2/11/2011 Antonescu CR Genes Chromosomes Cancer 2010 Control Group Other Related or Morphologically Similar Entities Salivary gland myoepithelial carcinoma (expleomorphic adenoma) (n=5) Salivary gland mucoepidermoid carcinoma (n=6) Cutaneous eccrine hidradenomas (n=5) Ossifying Fibromyxoid Tumor (n=3) Chordoma Periphericum (n=2) Results:EWSR1 Rearrangement in 45% of ME tumors Common presentations: Pediatric: 8/15 Deep soft tissue Bone: 5/6 Visceral: 4/6 Malignant histology ME83-EWSR1 Telomeric- Centromeric 2/11/2011 2/11/2011 #
7 ME Tumors with EWSR1 gene rearrangement Histologic patterns: 1.Small blue cell phenotype 2.Epithelioid or rhabdoid histology with eosinophilic or clear cytoplasm 3.Spindle or ovoid appearance in a prominent sclerotic stroma 4.Lack ductal, squamous, or matrix differentiation ME tumor with EWSR1 rearrangement 1. Small blue cell phenotype 53 yr F, R index finger, superficial IHC: s100 & EMA positive 2/11/2011 2/11/2011 ME tumor with EWSR1 rearrangement ME tumor with EWSR1 rearrangement 2. Epithelioid morphology with clear or eosinophilic cytoplasm 3. Sclerotic background, mixed spindle & epithelioid cells 20y M, Left foot, subcutaneous, benign 32yM, subcutaneous R ankle, benign S100 positive, EMA negative, CK positive S100 & EMA positive 2/11/2011 2/11/2011 EWSR1 -POU5F1 Fusion in ME Tumors POU5F1 (a.k.a OCT3/4) FISH 3 RACE &RT-PCR for EWSR1-POU5F1 1 2 M EWSR1 EXON6 POU5F1 EXON2 encodes a transcription factor which binds to the octamer motif (ATGCAAAT) present in the promoter or enhancer regions of target genes. 1. ME1 2. Negative Contr M. marker ME1-POU5F1 Telomeric/ Centromeric FISH is essential for keeping germ cells and embryonic stem cells in an immature and pluripotent status POU5F1reactivation has been found to be implicated in human cancer: germ cell tumors (OCT3/4 IHC marker), bladder tumors 2/11/2011 2/11/2011 #
8 VIM NSE 39y F, undifferentiated sarcoma of the pubic bone (negative for O13) highly aggressive, DOD in 6 mo close to half of hidradenomas and MECs display the same CRTC1 MAML2 fusion gene HA composed of 3 cell types: clear, cuticular, poroid MEC composed of 3 cell types: mucus-forming, intermediate, squamous EWSR1-POU5F1 (+) in 3 HA and 1 MEC S100 CK 2/11/2011 2/11/2011 OCT4 Hidradenoma with t(6;12)(p21;q12) and EWSR1 POU5F1 gene fusion ME Tumor with EWS-POU5F1(3 RACE, RT-PCR, FISH) Nested epithelioid appearance with clear cytoplasm EWS-POU5F1 fusion positive ME tumor 9 yr Male, deep soft tissue, arm S100 protein EMA 2/11/2011 2/11/2011 EWSR1-POU5F1 fusion positive Nested, diffuse clear cell changes EWSR1-POU5F1 Positive ME Tumors 34 yr F, wrist, deep, malignant N = 5 cases Age: 7-34 yrs (mean 21 yrs), 2 children Location: deep soft tissue extremities (5/5) Morphology: extensive clear cell change (4/5) Malignant potential: 4/5 IHC: positive for S100 & EMA, OCT4 negative (5/5) 2/11/2011 IHC: s100 & EMA positive; OCT4 negative 2/11/2011 #
9 EWSR1-PBX1 positive ME Tumor EWSR1-PBX1 fusion positive ME tumors PBX1 break-apart N=5 (3F, 2 M) Age: mean 45 (range years) Location: 3 soft tissue, 1 bone, 1lung 3 cases with bland spindle cell proliferation in a sclerotic background, resembling fibromatosis 2 cases with abundant clear cell morphology (one spindle, one epithelioid) 3 benign/2 malignant IHC: 5/5 positive S100 & EMA/CK 2/11/2011 2/11/2011 ME tumor with EWSR1- PBX1 fusion Bland, Fibromatosis -like ME Tumor with EWSR1- PBX1 fusion Bland, sclerotic areas 59 yr Female, foot, subcutaneous, benign 2/11/ yr Male, L hip mass S100 & EMA positive 2/11/2011 S100 & EMA positive Summary EWSR1 gene rearrangement is a common genetic event in soft tissue, bone and visceral ME tumors, particularly in the pediatric and young adults. ME tumors should be considered in the differential diagnosis of EWSR1 rearranged tumors. EWSR1-POU5F1fusion was identified in 5 (8%) ME tumors, involving the extremity deep soft tissues, occurring in children or young adults and displaying a prominent clear cell morphology Summary EWSR1-PBX1 and EWSR1-ZNF444 fusions, previously reported in ME tumors, were detected in additional 6 cases of both soft tissue and visceral sites EWSR1fusion negative ME tumors are more often benign, cutaneous or superficially located and display ductal or cartilage differentiation. Additional studies are required to identify other EWSR1 fusion partners involved in ME tumors. 2/11/2011 2/11/2011 #
10 Summary No EWSR1 gene rearrangements were detected in any of the salivary gland tumors, or morphologically similar tumors tested These findings question a pathogenetic link between soft tissue ME tumors and the more common salivary gland or cutaneous counterparts 2/11/2011 #
11 Update on PEComa Cyril Fisher MD DSc FRCPath London UK Keywords: Perivascular epithelioid cell tumor, angiomyolipoma, lymphangioleiomyomatosis, clear cell myomelanocytic tumor, tuberous sclerosis, sirolimus Introduction PEComas are mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells which are immunoreactive for both smooth muscle and melanocytic markers. The nature of this cell is, however, conjectural since no normal counterpart has been described. Bonetti et al (1992) 1 first noted an unusual cell type, which was immunoreactive with melanocytic markers, and had an epithelioid appearance, clear-acidophilic cytoplasm and a perivascular distribution, in both angiomyolipoma (AML) and clear cell sugar tumor of the lung 2. The PEComa family now additionally includes lymphangioleiomyomatosis (LAM), primary extrapulmonary sugar tumor (PEST), 3 clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres (CCMMT), 4 abdominopelvic sarcoma of perivascular epithelioid cells, 5 are simply termed PEComa. and other tumors with similar features at various sites that Some authors include renal capsular leiomyomas (capsulomas), but others consider them as monotypic spindle cell angiomyolipoma.
12 There is an association between PEComas and tuberous sclerosis complex. The cells in PEComas are typically arranged around blood vessels, and appear to form the vessel wall, often infiltrating the smooth muscle of small to medium-sized vessels. Periluminal cells are usually epithelioid and the more peripheral cells spindle-shaped. The cells have small, centrally located round to oval nuclei with inconspicuous nucleoli, although there is sometimes focally marked nuclear atypia. PECs have clear or eosinophilic cytoplasm and sometimes clear cell change with a perinuclear eosinophilic zone. Rarely there is prominent melanin pigmentation. Fatty change can be seen, especially in the peripheral cells which can mimic lipoblasts. The differential diagnosis can include carcinomas, smooth muscle tumors and adipocytic tumors. There have been recent advances in therapy of malignant PECOmas related to increased knowledge of specific genetic changes and their effects on metabolic pathways. Angiomyolipoma (AML) This accounts for less than 1% of renal tumors, but it is the most common type of PEComa. Renal AML are found in approximately 47% of patients known to have tuberous sclerosis complex (TSC.). The presence of multiple AML is diagnostic of TSC. However, approximately 80% of patients with AML do not have TSC. Renal AML have a mean age of diagnosis of years for non-tsc patients and for TSC patients. Females outnumber males by 4:1 in surgical series, although the sex distribution is equal in radiological studies, implying responsiveness to hormones. A small number of apparent AML are described in other locations, notably the liver, 6-13 but also in spleen, 14 heart, 15 vagina, 16, 17 ovary 18, spermatic cord 19, palate, 20 nose, 21 mediastinum, 22
13 retroperitoneum and GI tract. Apparently benign monotypic epithelioid AML-like tumors have been described in nasal cavity, 23 liver, 24 adrenal gland, 25 and tibia. 26 Renal AML are expansile, non-infiltrative masses, which can grow during pregnancy and can present with hemorrhage possibly because the abnormal vessels have reduced elastin. Pain and hematuria are other symptoms. Renal microhamartomas might represent small AML. Radiological detection of fat is useful in diagnosis. The tumors arise in cortex or medulla, or from capsular stromal proliferations, and are sharply demarcated from the adjacent kidney. Nodal involvement is not uncommon. Once considered a malformation, AML is now considered a clonal lesion (as initially demonstrated by non-random X-chromosome inactivation 27, 28 ) representing a neoplastic process involving all components. Classic AML shows tortuous, thick-walled blood vessels, lipid-distended PEC resembling fat, irregularly arranged sheets and bundles of smooth muscle-like spindle cells and a clear-eosinophilic component arranged around blood vessels. Both thickwalled vessels with walls composed of PEC and vessels with normal smooth muscle wall are present in AML. Lymphangiomyomatous areas can be seen. Predominance of the myoid-appearing or lipid-distended PEC may be mistaken for smooth muscle or adipocytic tumors, respectively. 29 About 7% of cases of AML, especially in patients with TSC, can also show almost exclusively epithelioid morphology ( monotypic epithelioid AML ) Epithelioid AML can show striking cytologic atypia, and multinucleation, and frankly malignant examples usually also have mitotic activity and focal necrosis. Epithelioid AML with clear cell change resembles extra-pulmonary CCST. Cystic 35, 36 and intraglomerular 37 variants have been described. Renal cell
14 carcinomas have been described in association with AML, both sporadically and in association with TSC. These are mostly clear cell carcinomas, but also include examples of chromophobe, collecting duct, and papillary carcinomas and oncocytoma, which occurs more commonly in TSC-associated than sporadic AML. 38 Histologically typical renal AML usually behave in a benign fashion, and even tumors which grow into vena cava or spread to regional nodes do not progress. The difficulty in confidently recognizing malignancy in AML is compounded by the wellknown existence of multicentric AML and AML arising in lymph nodes. There are about 32 reported cases of malignant AML in kidney or liver. 29, 32, 33, Tumors with both sarcoma-like 40, 41, 46 and carcinoma-like 57 histology have been reported to arise in preexisting benign AML. However, most malignant AML show epithelioid morphology and epithelioid AML are more frequently malignant, with metastases within abdomen or to lung or liver. 33 Although in ones series all examples of atypical AML had a benign outcome, 34 a larger study showed that 9/CD34 (26%) of epithelioid AML with atypical features behaved in a malignant fashion with local recurrence and metastasis. All epithelioid AML should therefore be followed up closely. Criteria for distinguishing benign from malignant epithelioid angiomyolipoma have been suggested as follows: (1) > or =70% atypical epithelioid cells, (2) > or =2 mitotic figures per 10 HPF, (3) atypical mitotic figures, and (4) necrosis; the presence of 3 or all of the features was highly predictive of malignant behavior. 33 Metastatic AML sometimes displays CK positivity in a proportion of cells. 58 This can be diagnostically misleading since examples of AML mimicking carcinoma have been described in patients without TSC. 32
15 Hepatic AML 59, 60 occur mostly in women and up to 10% are associated with TSC. They are histologically similar to renal AML but are more frequently epithelioid, 61, 62 and many contain foci of extramedullary hemopoiesis. An association with GIST has been reported. 63 Lymphangiomyomatosis (LAM) The incidence and prevalence of LAM are very low, with about 1000 cases in the United States in Between 0.1 and 2.3 % of TSC patients develop LAM which occurs almost exclusively in women, with a mean age at presentation in the early 30 s, although exceptional cases have been documented in men. Approximately half of the patients with LAM will also have renal AML. Most cases occur in the lung but extrapulmonary examples of LAM have been reported in lymph nodes, lymphatics (mediastinum, mesentery, pleura, pelvis) and several other organs including uterus, 65, 66 nearly always in patients with TSC and sometimes associated with pulmonary LAM (appearing later) and renal AML. 67 In extrapulmonary sites there are masses containing chylous fluid filled cysts with spindle or epithelioid smooth muscle cells in fascicles or trabeculae in their walls. 67 The true incidence is probably higher because of misdiagnoses as epithelioid smooth muscle tumor. LAM is an abnormal proliferation of smooth muscle-like PEC around bronchial lymphatics, interlobular septa and pleura. Rare cases show prominent clear cell change, resembling multiple CCST in a LAM-like distribution. LAM of lymph nodes or thoracic duct shows a perilymphatic proliferation of PEC. 68 LAM is a progressive disease destroying lung parenchyma with cyst formation, eventuating in respiratory failure. Using VEGFR-3, a marker for lymphatic endothelial cells,
16 lymphangiogenesis has been demonstrated in LAM and implicated in its progression. 69 Multifocal micronodular pneumocyte hyperplasia has been seen in association with LAM This is another pulmonary lesion occurring with TSC, 70, 71 and has been shown to have LOH of TSC2 (and rarely TSC1). 72 Clear cell ( sugar ) tumor (CCST) First described in 1963 by Liebow and Castleman, CCST is very rare, with only approximately 40 reported cases. CCST has been reported in association with LAM and TSC, although the majority of cases are sporadic. One example has been seen in association with adenomatoid papillary hyperplasia 73 (a lesion strictly related to TSC). CCST show a slight female predominance, with a mean age of 57 years. Pulmonary CCST are peripherally located ( coin lesion ) unencapsulated tumors composed of sheets and cords of polygonal, occasionally spindled, cells around sinusoidal vessels which can be focally hyalinized and display a hemangiopericytomatous pattern. The lesional cells have prominent cytoplasmic borders and clear or granular eosinophilic cytoplasm. Alveoli can be entrapped, and mature adipose tissue has been described. The tumor cells can contain glycogen and melanosomes. Extra-pulmonary CCST resemble their pulmonary counterparts, but often display nuclear atypia, mitotic activity and necrosis. Primary extrapulmonary sugar tumor (PEST) has been described in various sites including pancreas 74, breast 75, uterus 76, bone 26, heart, 3 and vulva, 3 and clinically malignant pulmonary and extra-pulmonary CCST have been reported. 77 Most extrapulmonary CCST are now simply identified as PEComas rather than separately designated.
17 Other PEComas PEComas at other sites are uncommon and are very rarely associated with TSC. They mostly occur in females. Many have epithelioid or clear cell morphology but some are spindled. One group of spindled PEComas comprise an entity termed clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres (CCMMT). The original report of CCMMT 4 described seven cases, 6 in females, with a mean age at diagnosis of 11 years (range 3-21 years). They involved or were adjacent to the falciform ligament or ligamentum teres. In the same issue of AJSP, a similar tumor was reported as a clear cell epithelioid tumor arising in the ligamentum teres. 78 As originally described, CCMMT is predominantly a spindle cell lesion consisting of clear to faintly eosinophilic moderately-sized spindle cells arranged in fascicular and nested patterns, with a rich capillary network. The cells have small but distinct nucleoli and low mitotic activity. CCMMT are positive for HMB45 melan-a, MiTF and SMA, but rarely for S100 protein or desmin. EM reveals hemidesmosomes primitive junctions, glycogen and stage II or III melanosomes. Follow-up data, available in seven of eight cases of CCMT (median duration,18 months, and 22 yrs in the case of Tanaka et al 78 ), showed 6 patients to be free of disease and one to have a radiographically presumed lung metastasis. Two cases of CCMT in soft tissue of thigh, including one with necrosis and atypia was published without follow-up information, 79, 80 but another in abdominal wall recurred after 6 years. 81 PEComas with spindle or epithelioid morphology have subsequently been described mostly in intra-abdominal sites (notably uterus and retroperitoneum) but also
18 others. Reported location include GI tract (jejunum, colon, rectum), 82, 83, 84 pancreas, 85 common bile duct, 86 retroperitoneum, 87 urinary bladder, oral cavity, nose, orbit, laryngopharynx, 91 pericardium, 92 rib, 93 skull base, 94 soft tissue of the thigh 79 (including two with malignant histological features 80, 95 ), upper limb, 90, 96 abdominal wall, 81 skin and bone. 100 A case has been reported in the colon in a patient with prior adrenal neuroblastoma. 101 Uterine PEComas appear to be relatively common, and some have recurred or metastasized. Vang and Kempson 106 described eight cases in females between 40 and 75 years (mean 54), in two morphologic groups. Group A tumors demonstrated a tonguelike growth pattern like that in low-grade endometrial stromal sarcoma and were composed of clear to pale granular cytoplasm, with diffuse HMB-45, and SMA positivity. Group B tumors were composed of epithelioid cells, smaller numbers of which were HMB-45-positive. They also featured extensive SMA and a lesser degree of the endometrial stromal sarcoma growth pattern. Two of the four patients with group B tumors had pelvic lymph nodes involved by lymphangioleiomyomatosis, and one of these patients had TSC. Only one of the eight tumors (in Group A) displayed positivity for melan-a. Group A PEComas, group B PEComas, and epithelioid smooth muscle tumors were all parts of a continuous histologic spectrum. Several other authors have noted the possible morphologic and immunophenotypic overlap between PEComas and uterine epithelioid smooth muscle 102, tumors (ESM) with occasional expression of HMB45. Others, however, regard all such tumors with a myomelanocytic phenotype as PEComas. 5 Clinical features favoring PEComa include an association with TSC (but only in 9% of cases), their
19 occurrence in younger patients, and the occurrence of metastasis in the absence of significant mitotic activity. Histologically, both tumors may display clear cells, epithelioid cells, stromal hyalinization and multinucleated giant cells. However, cells with pale (rather than deeply eosinophilic) granular or clear cytoplasm and ovoid rather than blunt ended nuclei, that are closely related to a delicate vascular network and lack paranuclear vacuoles, are more suggestive of PEComa than of ESM. Also, markers of smooth muscle differentiation (SMA, desmin, h-caldesmon) can be expressed in both, but melan-a is frequently negative in HMB-45 positive uterine tumours. Recent immunohistochemical data (requiring further study) suggest that CD1a can be expressed 112, 113 in PEComas but not smooth muscle tumors. Criteria for malignancy may differ from those proposed for uterine smooth muscle tumors and for other PEComas 114 and it has been suggested that >1/10 HPF and/or coagulative necrosis indicate potential for aggressive behaviour. 108 Renal capsular PEComas ( capsulomas ) are smooth muscle-like tumors (spindle cell PEComas). They are seen in patients with TSC, and are HMB-45 and SMA positive. They might represent early AML. Histologic Variants Some PEComas (19% in one series 115 ) in females display extensive stromal hyalinization (sclerosing PEComa). Reported cases have arisen mostly in retroperitoneum, and they can also be seen in the uterus. They display cords of uniform epithelioid cells, sometimes with mitoses, in a hypocellular, collagenous stroma; some have a spindle cell component or sheet-like pattern. These tumours are immunoreactive for desmin, SMA, h-caldesmon,
20 HMB45, MiTF and SMA, with a minority expressing melan-a. Malignant examples 45, 58, 116 have been described. Myxoid change, stromal microcysts, multinucleated cells and spider cells can occasionally be seen. Atypical and Malignant PEComa A malignant extra-renal/extrapulmonary variant, abdominopelvic sarcoma of PEC, was described in 2001 in four females aged 19 to 41 years, one of whom had features of TSC. Each presented with a tumor mass involving the serosa of the ileum, uterus or pelvis. 117 The tumors had sheets of large polygonal cells with glycogen-rich cytoplasm and moderately pleomorphic nuclei, a delicate vasculature, necrosis and occasional mitotic figures, and displayed overlapping features of clear cell sugar tumor of the lung and epithelioid angiomyolipoma. Intracytoplasmic pigment was present in two cases and tumor cells were positive for HMB45 and melan-a. Lymphovascular invasion was present in all, lymph node metastasis in two cases and metastasis to the ovary in one. Two patients developed widespread metastatic disease at 10 and 28 months. A similar tumor arose in jejunum, then recurred in the pelvis. 82 Malignant PEComas have now also been described at numerous sites including uterus, 102, 104, 105, 107, 114, retroperitoneum, 90, 121, 122 GI tract, 82, 117, 123 bladder, 90 prostate 124 and soft tissue, 4, 90, 95, 96 with about 45 cases in the literature. While focal nuclear atypia, manifest as large, hyperchromatic nuclei, can be seen in some (mostly epithelioid) PEComas, obviously malignant examples are often composed of large epithelioid or clear cells (and less commonly with spindled areas) with marked nuclear
21 pleomorphism, mitoses and necrosis. They retain the typical PEComa immunophenotype. In a study of 24 soft tissue and gynecologic PEComas, 3 recurred locally and 5 metastasized. 114 Two cases in soft tissue of thigh, including one with necrosis and atypia, 79, 80 were published without follow-up information, but another in abdominal wall recurred after 6 years. 81 It has been proposed that PEComas displaying two or more of the features of large size (>5cm), infiltrative growth, high nuclear grade and hypercellularity, mitoses 1 per 50hpf, necrosis or vascular invasion should be regarded as potentially malignant. 114, 125 Small tumors (<5 cm diameter) with atypia but no mitoses appear to be benign. Those with nuclear pleomorphism/multinucleated giant cells only (symplastic PEComas), or size >5cm, can be regarded as of uncertain malignant potential. Large size might also 82, 114 be an adverse prognostic factor. Immunohistochemistry PEComas co-express melanocytic markers, such as gp100 protein (HMB-45) 126, 127 in a granular pattern in cytoplasm, melan-a, tyrosinase, and microphthalmia transcription factor (MiTF); and muscle markers, such as smooth muscle actin, pan-muscle actin, muscle myosin, calponin 72 and sometimes h-caldesmon. Desmin is less often positive though more frequently expressed in cutaneous 98 and sclerosing 115 PEComas. Cytokeratin positivity can occasionally be seen. 128,114 About a third of PEComas, however, also express S100 protein, usually focally, which does not exclude the diagnosis, 114 and CD1a positivity has also been reported in some PEComas, 112 unlike in epithelioid smooth muscle tumors of uterus. 113 The most sensitive melanocytic markers
22 for the diagnosis of PEComa are HMB-45 (in about 80% of AML), Melan-A and (less so) MiTF. 129 Nuclear expression of TFE3 has also been reported in a number of PEComas, though its sensitivity is method-dependent. 134 In AML, co-expression of melanocytic and smooth muscle markers is seen in both myoid-appearing and lipid-distended PEC. Epithelioid AML tend to express melanocytic markers more strongly than myoid markers, with the converse in predominantly spindled PEComas. Immunohistochemically, all cases of CCMT were positive with antibodies to HMB-45 and negative for S-100 protein. In three of the seven cases studied immunohistochemically, the tumors expressed smooth muscle actin, melan-a, microphthalmia transcription factor (MiTF), and myosin, but not desmin. Estrogen receptor positivity has been shown in epithelioid renal AML, 29, 135 and progesterone receptor positivity in a number of AML 29, in CCST in the breast 75 and in jejunal 82 and uterine PEComas. 136 CD117 is expressed in some AML and PEComas, predominantly in the cytoplasm of both spindle and epithelioid cells, with strong perinuclear staining in vacuolated clear epithelioid cells. 137 EMA positivity has been reported in one case in soft tissue. 81 Recently, cathepsin K, a papain-like cysteine protease with high matrix-degrading activity, has been demonstrated by immunohistochemistry in AML and LAM (in which it 138, 139 conceivably causes damage to collagen or elastic fibers in pulmonary parenchyma). Cathepsin K is under the transcriptional control of MiTF, and is overexpressed both in 140, 141 PEComas, and in osteoclasts in which it is involved in bone resorption. Ultrastructure
23 PEComas have cytoplasmic glycogen and lipid droplets. Crystalline structures with various patterns, some resembling renin granules, have been described in AML, as well as rare premelanosomes, 142 and membrane bound granules of uncertain nature. 74 In AML, spindle cells tend to express smooth muscle differentiation, whereas melanocytic differentiation is more frequently found in epithelioid AML. 128 CCST lack junctions, microlumina and microvilli. 74 There are short profiles of RER, and numerous glycogen granules with variable electron density. In CCMMT, as well as melanosomes (see above) filament bundles with dense bodies, and external lamina have been observed, 78 and stage II melanosomes have been found in uterine PECOma. 136 Genetics The genes involved in TSC are TSC1 on chromosome 9q and TSC2 on chromosome 16p13.3. These genes relate to enzymes involved in catecholamine metabolism and melanin formation. TSC1 acts to stabilize TSC2, and loss of TSC1 leads to lack of TSC2 expression. TSC2 encodes tuberin which interacts with hamartin, the product of TSC1, to function together as a heterodimer and affect signalling pathways concerned with growth and proliferation. 144 In particular, tuberin, via Rheb (=Ras homolog enriched in brain protein) GTPase inactivation (conversion to GDP), inhibits the mtorc1 kinase which complexes Rheb-GTP to mtor1 as mtorc1. This complex is composed of mtor (=mammalian target of rapamycin), regulatory associated protein of mtor (Raptor) mammalian LST8/G-protein β-subunit like protein (mlst8/gβl) 145 and the recently identified partners PRAS40 and DEPTOR. 146
24 Activated mtorc1 drives the mtor/p70s6k metabolic pathway, which leads to increased cell growth and inter alia regulates vascular smooth muscle differentiation. 147, 148 Activation of mtor pathway leads to elevated phospho-p706k with reduced phospho-akt, and phosphorylation of ribosomal protein subunit S6 (a target of S6Kinase). Detection of the latter by immunohistochemistry using antibodies to components of S6 can act as an indicator of mtorc1 activation. These ribosomal proteins influence a feedback loop via ATK. Malfunction of the TSC1/TSC2 complex can arise by somatic deletion of TSC1 or inactivating or mis-sense mutations in TSC1 or TSC2. In individuals with TSC this is a second hit. 121 Allelic changes at 16p and LOH mainly at the TSC2-containing region have been found in both sporadic and TSC-associated AML 149 and in PEComas, and a small number of CCMMT have also been shown to lack tuberin expression. 4 These changes, which lead to cell growth and proliferation via constitutive upregulation of the mtorc1 complex as described above, have been reported in TSCassociated renal tumors, 150 sporadic AML, 151, 152 LAM, and other PEComas, and also in multifocal micronodular pneumocyte hyperplasia. 72 Sirolimus (previously called rapamycin) is an mtorc1 inhibitor, 153 and therapy with this and related drugs has so far shown a clinical response in a number of (but not all 55 ) patients with angiomyolipoma or malignant PEComa, 120, 121 as well as LAM 157, 158 and other TSC-related lesions 159, 160 such as subependymal giant-cell astrocytoma. Its occurrence at different sites, consistent morphology and immunophenotype, and absence of normal counterpart cell, suggest that PEComa might be a translocationassociated sarcoma. However, this has not yet been demonstrated. One case studied
25 cytogenetically disclosed a t(3;10) rearrangement, 4 and SFPQ/PSF-TFE3 gene fusion (also reported in a melanotic Xp11 translocation renal carcinoma 161 ) has been described in a single case. 101 TFE3 gene fusions without identified partners have also been demonstrated in a small number of non-renal PEComas in young patients without tuberous sclerosis whose tumors demonstrated predominant alveolar architecture, epithelioid morphology, minimal immunoreactivity for muscle markers, and TFE3 immunoreactivity. 134 TFE3 is a member of the MiT family of basic helix-loophelix leucine zipper transcription factors, and it interacts with Mitf, which is involved in differentiation of melanocytes 133, 162, 163 and also osteoclasts. 164 Differential diagnosis This includes tumors with clear cell morphology, other spindle and epithelioid mesenchymal neoplasms including gastrointestinal stromal tumors, and those with positivity for melanocytic antigens (melanoma, clear cell sarcoma, some smooth muscle tumors, and adrenal cortical carcinoma), rhabdomyosarcoma, myoepithelioma, mature adipose tissue tumors, and alveolar soft part sarcoma. Epithelioid PEComas can also be confused with renal and carcinomas. Most of the tumors in the differential diagnosis can be distinguished by clinical and other morphological features and immunophenotype. Clear cell sarcoma, a tumor with melanocytic differentiation, usually involves soft tissue of extremities but can occur at other sites including the gastrointestinal tract. Although the diffuse S100 protein positivity seen in this tumor argues against PEComa, demonstration of t(12;22)(q13;q12) or of the EWS-ATF fusion gene (or EWS-CREB3L2
26 in the GI tract variant, which often lacks melanocytic markers) can be of diagnostic use. 165 Conclusions PEComas are a family of tumors, some of which are related to abnormalities in the TSC genes, which may arise in almost any location. A corresponding non-neoplastic cell has not been demonstrated. The members of this group are characterized by their distinctive morphology and myomelanocytic immunophenotype. Epithelioid AML, CCMMT, and CCST show considerable morphologic overlap, supporting the view that they are variants of a single entity. Nonetheless, the age, sex, presentation, gross and microscopic appearances are different for each of these lesions. Some cases are not epithelioid, some are not perivascular, some are HMB45 negative, and many not associated with TSC. It seems therefore appropriate for the present to retain some of the individual terms to reflect not only morphologic but significant clinical differences. aggressive. PEComas can be histologically and clinically malignant and can be Cases that have nuclear atypia or exceed 5 cm in diameter but lack significant mitotic activity and necrosis after thorough sampling can be regarded as of uncertain malignant potential and followed up closely. Abnormalities in TSC1/TSC2 genes lead to cell proliferation via activation of mtor pathway, which can in some cases be inhibited by sirolimus and its derivatives.
27 References 1. Bonetti F, Pea M, Martignoni G, et al. PEC and sugar. Am J Surg Pathol. 1992;16: Bonetti F, Pea M, Martignoni G, et al. Clear cell ("sugar") tumor of the lung is a lesion strictly related to angiomyolipoma--the concept of a family of lesions characterized by the presence of the perivascular epithelioid cells (PEC). Pathology. 1994;26: Tazelaar HD, Batts KP, Srigley JR. Primary extrapulmonary sugar tumor (PEST): a report of four cases. Mod Pathol. 2001;14: Folpe AL, Goodman ZD, Ishak KG, et al. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres: a novel member of the perivascular epithelioid clear cell family of tumors with a predilection for children and young adults. Am J Surg Pathol. 2000;24: Martignoni G, Pea M, Reghellin D, et al. PEComas: the past, the present and the future. Virchows Arch. 2008;452: Goodman ZD, Ishak KG. Angiomyolipomas of the liver. Am J Surg Pathol. 1984;8: Kristal H, Sperber F. Hepatic angiomyolipoma in a tuberous sclerosis patient. Isr J Med Sci. 1989;25: Nonomura A, Mizukami Y, Kadoya M. Angiomyolipoma of the liver: a collective review. J Gastroenterol. 1994;29: Tsui WM, Colombari R, Portmann BC, et al. Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol. 1999;23: Dalle I, Sciot R, de Vos R, et al. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36: Nonomura A, Enomoto Y, Takeda M, et al. Invasive growth of hepatic angiomyolipoma; a hitherto unreported ominous histological feature. Histopathology. 2006;48: Deng YF, Lin Q, Zhang SH, et al. Malignant angiomyolipoma in the liver: a case report with pathological and molecular analysis. Pathol Res Pract. 2008;204: Petrolla AA, Xin W. Hepatic angiomyolipoma. Arch Pathol Lab Med. 2008;132: Hulbert JC, Graf R. Involvement of the spleen by renal angiomyolipoma: metastasis or multicentricity? J Urol. 1983;130: Tsai CC, Chou CY, Han SJ, et al. Cardiac angiomyolipoma: radiologic and pathologic correlation. J Formos Med Assoc. 1997;96: Chen KT. Angiomyolipoma of the vagina. Gynecol Oncol. 1990;37: Peh SC, Sivanesaratnam V. Angiomyolipoma of the vagina--an uncommon tumour. Case report. Br J Obstet Gynaecol. 1988;95: Anderson AE, Yang X, Young RH. Epithelioid angiomyolipoma of the ovary: a case report and literature review. Int J Gynecol Pathol. 2002;21: Castillenti TA, Bertin AP. Angiomyolipoma of the spermatic cord: case report and literature review. J Urol. 1989;142: Piattelli A, Fioroni M, Rubini C, et al. Angiomyolipoma of the palate. Report of a case. Oral Oncol. 2001;37:323-5.
28 21. Watanabe K, Suzuki T. Mucocutaneous angiomyolipoma. A report of 2 cases arising in the nasal cavity. Arch Pathol Lab Med. 1999;123: Knight CS, Cerfolio RJ, Winokur TS. Angiomyolipoma of the anterior mediastinum. Ann Diagn Pathol. 2008;12: Banerjee SS, Eyden B, Trenholm PW, et al. Monotypic angiomyolipoma of the nasal cavity: a heretofore undescribed occurrence. Int J Surg Pathol. 2001;9: Yamasaki S, Tanaka S, Fujii H, et al. Monotypic epithelioid angiomyolipoma of the liver. Histopathology. 2000;36: D'Antonio A, Caleo A, Caleo O, et al. Monotypic epithelioid angiomyolipoma of the adrenal gland: an unusual site for a rare extrarenal tumor. Ann Diagn Pathol. 2009;13: Insabato L, De Rosa G, Terracciano LM, et al. Primary monotypic epithelioid angiomyolipoma of bone. Histopathology. 2002;40: Green AJ, Sepp T, Yates JR. Clonality of tuberous sclerosis harmatomas shown by non-random X-chromosome inactivation. Hum Genet. 1996;97: Paradis V, Laurendeau I, Vieillefond A, et al. Clonal analysis of renal sporadic angiomyolipomas. Hum Pathol. 1998;29: L'Hostis H, Deminiere C, Ferriere JM, et al. Renal angiomyolipoma: a clinicopathologic, immunohistochemical, and follow-up study of 46 cases. Am J Surg Pathol. 1999;23: Eble JN, Amin MB, Young RH. Epithelioid angiomyolipoma of the kidney: a report of five cases with a prominent and diagnostically confusing epithelioid smooth muscle component. Am J Surg Pathol. 1997;21: Mai KT, Perkins DG, Collins JP. Epithelioid cell variant of renal angiomyolipoma. Histopathology. 1996;28: Martignoni G, Pea M, Bonetti F, et al. Carcinomalike monotypic epithelioid angiomyolipoma in patients without evidence of tuberous sclerosis: a clinicopathologic and genetic study. Am J Surg Pathol. 1998;22: Brimo F, Robinson B, Guo C, et al. Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol. 2010;34: Aydin H, Magi-Galluzzi C, Lane BR, et al. Renal angiomyolipoma: clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol. 2009;33: Davis CJ, Barton JH, Sesterhenn IA. Cystic angiomyolipoma of the kidney: a clinicopathologic description of 11 cases. Mod Pathol. 2006;19: Fine SW, Reuter VE, Epstein JI, et al. Angiomyolipoma with epithelial cysts (AMLEC): a distinct cystic variant of angiomyolipoma. Am J Surg Pathol. 2006;30: Martignoni G, Bonetti F, Pea M, et al. Renal disease in adults with TSC2/PKD1 contiguous gene syndrome. Am J Surg Pathol. 2002;26: Jimenez RE, Eble JN, Reuter VE, et al. Concurrent angiomyolipoma and renal cell neoplasia: a study of 36 cases. Mod Pathol. 2001;14: Kragel PJ, Toker C. Infiltrating recurrent renal angiomyolipoma with fatal outcome. J Urol. 1985;133:90-1.
29 40. Ferry JA, Malt RA, Young RH. Renal angiomyolipoma with sarcomatous transformation and pulmonary metastases. Am J Surg Pathol. 1991;15: Lowe BA, Brewer J, Houghton DC, et al. Malignant transformation of angiomyolipoma. J Urol. 1992;147: Al-Saleem T, Wessner LL, Scheithauer BW, et al. Malignant tumors of the kidney, brain, and soft tissues in children and young adults with the tuberous sclerosis complex. Cancer. 1998;83: Pea M, Bonetti F, Martignoni G, et al. Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identification of malignant epithelioid angiomyolipoma. Am J Surg Pathol. 1998;22: Christiano AP, Yang X, Gerber GS. Malignant transformation of renal angiomyolipoma. J Urol. 1999;161: Cibas ES, Goss GA, Kulke MH, et al. Malignant epithelioid angiomyolipoma ('sarcoma ex angiomyolipoma') of the kidney: a case report and review of the literature. Am J Surg Pathol. 2001;25: Chandrasoma S, Moatamed N, Chang A, et al. Angiomyolipoma of the kidney: expanding disease spectrum demonstrated by 3 cases. Appl Immunohistochem Mol Morphol. 2004;12: Warakaulle DR, Phillips RR, Turner GD, et al. Malignant monotypic epithelioid angiomyolipoma of the kidney. Clin Radiol. 2004;59: Inci O, Kaplan M, Yalcin O, et al. Renal angiomyolipoma with malignant transformation, simultaneous occurrence with malignity and other complex clinical situations. Int Urol Nephrol. 2006;38: El Jack AK, Tomaszewski JE, Haller DG, et al. Metastatic PEComa arising from renal angiomyolipoma: MRI findings. J Magn Reson Imaging. 2007;26: Huang KH, Huang CY, Chung SD, et al. Malignant epithelioid angiomyolipoma of the kidney. J Formos Med Assoc. 2007;106:S Liu H, Wang HQ, Li X, et al. [Malignant epithelioid angiomyolipoma of the kidney: report of a case]. Zhonghua Bing Li Xue Za Zhi. 2007;36: Moudouni SM, Tligui M, Sibony M, et al. Malignant epithelioid renal angiomyolipoma involving the inferior vena cava in a patient with tuberous sclerosis. Urol Int. 2008;80:102-4; discussion Nguyen TT, Gorman B, Shields D, et al. Malignant hepatic angiomyolipoma: report of a case and review of literature. Am J Surg Pathol. 2008;32: Sato K, Ueda Y, Tachibana H, et al. Malignant epithelioid angiomyolipoma of the kidney in a patient with tuberous sclerosis: an autopsy case report with p53 gene mutation analysis. Pathol Res Pract. 2008;204: Higa F, Uchihara T, Haranaga S, et al. Malignant epithelioid angiomyolipoma in the kidney and liver of a patient with pulmonary lymphangioleiomyomatosis: lack of response to sirolimus. Intern Med. 2009;48: Arrabal-Polo MA, Arrabal-Martin M, Palao-Yago F, et al. Wunderlich syndrome from a malignant epithelioid angiomyolipoma. Urol J. 2009;6: Aoyama T, Fujikawa K, Yoshimura K, et al. Bilateral renal cell carcinoma in a patient with tuberous sclerosis. Int J Urol. 1996;3:150-1.
30 58. Martignoni G, Pea M, Rigaud G, et al. Renal angiomyolipoma with epithelioid sarcomatous transformation and metastases: demonstration of the same genetic defects in the primary and metastatic lesions. Am J Surg Pathol. 2000;24: Fang SH, Zhou LN, Jin M, et al. Perivascular epithelioid cell tumor of the liver: a report of two cases and review of the literature. World J Gastroenterol. 2007;13: Garcia TR, Mestre de Juan MJ. Angiomyolipoma of the liver and lung: a case explained by the presence of perivascular epithelioid cells. Pathol Res Pract. 2002;198: Huang PC, Chen JT, Ho WL. Clinicopathologic analysis of renal and extrarenal angiomyolipomas: report of 44 cases. Zhonghua Yi Xue Za Zhi (Taipei). 2000;63: Lin CN, Chiang HS, Hsu SI, et al. Renal angiomyolipoma with a prominent angiomatous component and extramedullary hematopoiesis: a case report. Zhonghua Yi Xue Za Zhi (Taipei). 1994;53: Paiva CE, Moraes Neto FA, Agaimy A, et al. Perivascular epithelioid cell tumor of the liver coexisting with a gastrointestinal stromal tumor. World J Gastroenterol. 2008;14: Hohman DW, Noghrehkar D, Ratnayake S. Lymphangioleiomyomatosis: A review. Eur J Intern Med. 2008;19: Gyure KA, Hart WR, Kennedy AW. Lymphangiomyomatosis of the uterus associated with tuberous sclerosis and malignant neoplasia of the female genital tract: a report of two cases. Int J Gynecol Pathol. 1995;14: Longacre TA, Hendrickson MR, Kapp DS, et al. Lymphangioleiomyomatosis of the uterus simulating high-stage endometrial stromal sarcoma. Gynecol Oncol. 1996;63: Matsui K, Tatsuguchi A, Valencia J, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases. Hum Pathol. 2000;31: Torres VE, Bjornsson J, King BF, et al. Extrapulmonary lymphangioleiomyomatosis and lymphangiomatous cysts in tuberous sclerosis complex. Mayo Clin Proc. 1995;70: Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis in Lymphangioleiomyomatosis: Its Implication in the Progression of Lymphangioleiomyomatosis. Am J Surg Pathol. 2004;28: Maruyama H, Seyama K, Sobajima J, et al. Multifocal micronodular pneumocyte hyperplasia and lymphangioleiomyomatosis in tuberous sclerosis with a TSC2 gene. Mod Pathol. 2001;14: Wu K, Tazelaar HD. Pulmonary angiomyolipoma and multifocal micronodular pneumocyte hyperplasia associated with tuberous sclerosis. Hum Pathol. 1999;30: Hayashi T, Kumasaka T, Mitani K, et al. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod Pathol. 2010;23: Bonetti F, Pea M, Martignoni G, et al. The perivascular epithelioid cell and related lesions. Adv Anat Pathol. 1997;4: Zamboni G, Pea M, Martignoni G, et al. Clear cell "sugar" tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996;20:
31 75. Govender D, Sabaratnam RM, Essa AS. Clear cell 'sugar' tumor of the breast: another extrapulmonary site and review of the literature. Am J Surg Pathol. 2002;26: Pea M, Martignoni G, Zamboni G, et al. Perivascular epithelioid cell. Am J Surg Pathol. 1996;20: Sale GE, Kulander BG. 'Benign' clear-cell tumor (sugar tumor) of the lung with hepatic metastases ten years after resection of pulmonary primary tumor. Arch Pathol Lab Med. 1988;112: Tanaka Y, Ijiri R, Kato K, et al. HMB-45/melan-A and smooth muscle actinpositive clear-cell epithelioid tumor arising in the ligamentum teres hepatis: additional example of clear cell 'sugar' tumors. Am J Surg Pathol. 2000;24: Folpe AL, McKenney JK, Li Z, et al. Clear cell myomelanocytic tumor of the thigh: report of a unique case. Am J Surg Pathol. 2002;26: Diment J, Colecchia M. Myomelanocytic tumor of the thigh. Am J Surg Pathol. 2003;27: Fukunaga M. Perivascular epithelioid cell tumor (PEComa) of soft tissue: case report with ultrastructural study. Apmis. 2004;112: Yanai H, Matsuura H, Sonobe H, et al. Perivascular epithelioid cell tumor of the jejunum. Pathol Res Pract. 2003;199: Shi HY, Wei LX, Sun L, et al. Clinicopathologic Analysis of 4 Perivascular Epithelioid Cell Tumors (PEComas) of the Gastrointestinal Tract. Int J Surg Pathol Ryan P, Nguyen VH, Gholoum S, et al. Polypoid PEComa in the rectum of a 15- year-old girl: case report and review of PEComa in the gastrointestinal tract. Am J Surg Pathol. 2009;33: Hirabayashi K, Nakamura N, Kajiwara H, et al. Perivascular epithelioid cell tumor (PEComa) of the pancreas: immunoelectron microscopy and review of the literature. Pathol Int. 2009;59: Sadeghi S, Krigman H, Maluf H. Perivascular Epithelioid Clear Cell Tumor of the Common Bile Duct. Am J Surg Pathol. 2004;28: Shin JS, Spillane A, Wills E, et al. PEComa of the retroperitoneum. Pathology. 2008;40: Pan CC, Yu IT, Yang AH, et al. Clear cell myomelanocytic tumor of the urinary bladder. Am J Surg Pathol. 2003;27: Sukov WR, Cheville JC, Amin MB, et al. Perivascular epithelioid cell tumor (PEComa) of the urinary bladder: report of 3 cases and review of the literature. Am J Surg Pathol. 2009;33: Weinreb I, Howarth D, Latta E, et al. Perivascular epithelioid cell neoplasms (PEComas): four malignant cases expanding the histopathological spectrum and a description of a unique finding. Virchows Arch. 2007;450: Huai-yin S, Li-xin W, Lu S, et al. Perivascular epithelioid cell tumors of the laryngopharynx: three case reports and literature review. Pathol Res Pract. 2009;205: Mollazadeh R, Moaref AR, Ghazinoor M, et al. Pericardial PEComa: echocardiographic features. Int J Cardiol. 2009;132:e Torii I, Kondo N, Takuwa T, et al. Perivascular epithelioid cell tumor of the rib. Virchows Arch. 2008;452:
32 94. Lehman NL. Malignant PEComa of the skull base. Am J Surg Pathol. 2004;28: Harris GC, McCulloch TA, Perks G, et al. Malignant perivascular epithelioid cell tumour ("PEComa") of soft tissue: a unique case. Am J Surg Pathol. 2004;28: Osei DA, Alvandi F, Brooks JS, et al. PEComa of the Upper Extremity: A Unique Case and Description of an Initial Response to Neoadjuvant Chemotherapy. Sarcoma. 2007;2007: Mentzel T, Reisshauer S, Rutten A, et al. Cutaneous clear cell myomelanocytic tumour: a new member of the growing family of perivascular epithelioid cell tumours (PEComas). Clinicopathological and immunohistochemical analysis of seven cases. Histopathology. 2005;46: Walsh SN, Sangueza OP. PEComas: a review with emphasis on cutaneous lesions. Semin Diagn Pathol. 2009;26: Chaplin A, Conrad DM, Tatlidil C, et al. Primary cutaneous PEComa. Am J Dermatopathol. 2010;32: Yamashita K, Fletcher CD. PEComa presenting in bone: clinicopathologic analysis of 6 cases and literature review. Am J Surg Pathol.34: Tanaka M, Kato K, Gomi K, et al. Perivascular epithelioid cell tumor with SFPQ/PSF-TFE3 gene fusion in a patient with advanced neuroblastoma. Am J Surg Pathol. 2009;33: Silva EG, Deavers MT, Bodurka DC, et al. Uterine epithelioid leiomyosarcomas with clear cells: reactivity with HMB-45 and the concept of PEComa. Am J Surg Pathol. 2004;28: Fukunaga M. Perivascular epithelioid cell tumor of the uterus: report of four cases. Int J Gynecol Pathol. 2005;24: Greene LA, Mount SL, Schned AR, et al. Recurrent perivascular epithelioid cell tumor of the uterus (PEComa): an immunohistochemical study and review of the literature. Gynecol Oncol. 2003;90: Dimmler A, Seitz G, Hohenberger W, et al. Late pulmonary metastasis in uterine PEComa. J Clin Pathol. 2003;56: Vang R, Kempson RL. Perivascular epithelioid cell tumor ('PEComa') of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol. 2002;26: Armah HB, Parwani AV. Malignant perivascular epithelioid cell tumor (PEComa) of the uterus with late renal and pulmonary metastases: a case report with review of the literature. Diagn Pathol. 2007;2: Fadare O. Perivascular epithelioid cell tumor (PEComa) of the uterus: an outcome-based clinicopathologic analysis of 41 reported cases. Adv Anat Pathol. 2008;15: Fadare O. Uterine PEComa: appraisal of a controversial and increasingly reported mesenchymal neoplasm. Int Semin Surg Oncol. 2008;5: Silva EG, Bodurka DC, Scouros MA, et al. A uterine leiomyosarcoma that became positive for HMB45 in the metastasis. Ann Diagn Pathol. 2005;9: Simpson KW, Albores-Saavedra J. HMB-45 reactivity in conventional uterine leiomyosarcomas. Am J Surg Pathol. 2007;31:95-8.
33 112. Adachi Y, Horie Y, Kitamura Y, et al. CD1a expression in PEComas. Pathol Int. 2008;58: Fadare O, Liang SX. Epithelioid smooth muscle tumors of the uterus do not express CD1a: a potential immunohistochemical adjunct in their distinction from uterine perivascular epithelioid cell tumors. Ann Diagn Pathol. 2008;12: Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29: Hornick JL, Fletcher CD. Sclerosing PEComa: Clinicopathologic Analysis of a Distinctive Variant With a Predilection for the Retroperitoneum. Am J Surg Pathol Ramaiah S, Ganesan R, Mangham DC, et al. Malignant variant of sclerosing perivascular epithelioid cell tumor arising in the adnexa. Int J Gynecol Pathol. 2009;28: Bonetti F, Martignoni G, Colato C, et al. Abdominopelvic sarcoma of perivascular epithelioid cells. Report of four cases in young women, one with tuberous sclerosis. Mod Pathol. 2001;14: Liang SX, Pearl M, Liu J, et al. "Malignant" uterine perivascular epithelioid cell tumor, pelvic lymph node lymphangioleiomyomatosis, and gynecological pecomatosis in a patient with tuberous sclerosis: a case report and review of the literature. Int J Gynecol Pathol. 2008;27: Liu JL, Lin YM, Lin MC, et al. Perivascular epithelioid cell tumor (PEComa) of the uterus with aggressive behavior at presentation. Hematol Oncol Stem Cell Ther. 2009;2: Italiano A, Delcambre C, Hostein I, et al. Treatment with the mtor inhibitor temsirolimus in patients with malignant PEComa. Ann Oncol. 2010;21: Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mtor inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mtorc1 in tumors. J Clin Oncol. 2010;28: Koenig AM, Quaas A, Ries T, et al. Perivascular epitheloid cell tumour (PEComa) of the retroperitoneum - a rare tumor with uncertain malignant behaviour: a case report. J Med Case Reports. 2009;3: Agaimy A, Wunsch PH. Perivascular epithelioid cell sarcoma (malignant PEComa) of the ileum. Pathol Res Pract. 2006;202: Pan CC, Yang AH, Chiang H. Malignant perivascular epithelioid cell tumor involving the prostate. Arch Pathol Lab Med. 2003;127:E Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41: Pea M, Bonetti F, Zamboni G, et al. Melanocyte-marker-HMB-45 is regularly expressed in angiomyolipoma of the kidney. Pathology. 1991;23: Weeks DA, Malott RL, Arnesen M, et al. Hepatic angiomyolipoma with striated granules and positivity with melanoma--specific antibody (HMB-45): a report of two cases. Ultrastruct Pathol. 1991;15: Stone CH, Lee MW, Amin MB, et al. Renal angiomyolipoma: further immunophenotypic characterization of an expanding morphologic spectrum. Arch Pathol Lab Med. 2001;125:751-8.
34 129. Chang KL, Folpe AL. Diagnostic utility of microphthalmia transcription factor in malignant melanoma and other tumors. Adv Anat Pathol. 2001;8: Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms (PEComas) of soft tissue and gynecologic origin: a cliniopathologic study of 24 cases. Mod Pathol. 2005;18:14A Cho HY, Chung DH, Khurana H, et al. The role of TFE3 in PEComa. Histopathology. 2008;53: Kuroda N, Goda M, Kazakov DV, et al. Perivascular epithelioid cell tumor of the nasal cavity with TFE3 expression. Pathol Int. 2009;59: Dickson BC, Brooks JS, Pasha TL, et al. TFE3 Expression in Tumors of the Microphthalmia-Associated Transcription Factor (MiTF) Family. Int J Surg Pathol Argani P, Aulmann S, Illei PB, et al. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010;34: Cho NH, Shim HS, Choi YD, et al. Estrogen receptor is significantly associated with the epithelioid variants of renal angiomyolipoma: A clinicopathological and immunohistochemical study of 67 cases. Pathol Int. 2004;54: Park SH, Ro JY, Kim HS, et al. Perivascular epithelioid cell tumor of the uterus: immunohistochemical, ultrastructural and molecular study. Pathol Int. 2003;53: Makhlouf HR, Remotti HE, Ishak KG. Expression of KIT (CD117) in angiomyolipoma. Am J Surg Pathol. 2002;26: Garnero P, Borel O, Byrjalsen I, et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273: Chilosi M, Pea M, Martignoni G, et al. Cathepsin-k expression in pulmonary lymphangioleiomyomatosis. Mod Pathol. 2009;22: Meadows NA, Sharma SM, Faulkner GJ, et al. The expression of Clcn7 and Ostm1 in osteoclasts is coregulated by microphthalmia transcription factor. J Biol Chem. 2007;282: Drake FH, Dodds RA, James IE, et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271: Kaiserling E, Krober S, Xiao JC, et al. Angiomyolipoma of the kidney. Immunoreactivity with HMB-45. Light- and electron-microscopic findings. Histopathology. 1994;25: van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277: Martignoni G, Pea M, Reghellin D, et al. Molecular pathology of lymphangioleiomyomatosis and other perivascular epithelioid cell tumors. Arch Pathol Lab Med. 2010;134: Kim DH, Sarbassov DD, Ali SM, et al. mtor interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110: Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412: Goncharova EA, Goncharov DA, Eszterhas A, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J Biol Chem. 2002;277:
35 148. Martin KA, Rzucidlo EM, Merenick BL, et al. The mtor/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C Henske EP, Neumann HP, Scheithauer BW, et al. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer. 1995;13: Kenerson HL, Aicher LD, True LD, et al. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62: El-Hashemite N, Zhang H, Henske EP, et al. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet. 2003;361: Kenerson H, Folpe AL, Takayama TK, et al. Activation of the mtor pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol. 2007;38: Yu J, Parkhitko AA, Henske EP. Mammalian target of rapamycin signaling and autophagy: roles in lymphangioleiomyomatosis therapy. Proc Am Thorac Soc. 2010;7: Krischock L, Beach R, Taylor J. Sirolimus and tuberous sclerosis-associated renal angiomyolipomas. Arch Dis Child. 2010;95: Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358: Wolff N, Kabbani W, Bradley T, et al. Sirolimus and temsirolimus for epithelioid angiomyolipoma. J Clin Oncol. 2010;28:e Davies DM, Johnson SR, Tattersfield AE, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med. 2008;358: Taille C, Debray MP, Crestani B. Sirolimus treatment for pulmonary lymphangioleiomyomatosis. Ann Intern Med. 2007;146: Krueger DA, Franz DN. Current management of tuberous sclerosis complex. Paediatr Drugs. 2008;10: Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363: Chang IW, Huang HY, Sung MT. Melanotic Xp11 translocation renal cancer: a case with PSF-TFE3 gene fusion and up-regulation of melanogenetic transcripts. Am J Surg Pathol. 2009;33: Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38: Righi A, Dimosthenous K, Rosai J. PEComa: another member of the MiT tumor family? Int J Surg Pathol. 2008;16: Steingrimsson E, Tessarollo L, Pathak B, et al. Mitf and Tfe3, two members of the Mitf-Tfe family of bhlh-zip transcription factors, have important but functionally redundant roles in osteoclast development. Proc Natl Acad Sci U S A. 2002;99: Zambrano E, Reyes-Mugica M, Franchi A, et al. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol. 2003;11:75-81.
36
37 ISBSTP, San Antonio, February 2011 Update on PEComa Cyril Fisher MD DSc FRCPath London, UK Neoplasms with perivascular epithelioid cell differentiation (PEComas) Mesenchymal tumours composed of histologically and immunohistochemically distinctive perivascular epithelioid cells WHO, 2002 No normal counterpart has been described HMB45 Melan-A SMA h-cal PEComa 1
38 PEComa: General Features Spindle or epithelioid cells Clear or granular cytoplasm Perivascular arrangement Myomelanocytic immunophenotype Melanosomes Relationship to TSC PEComa Renal Other angiomyolipoma extrarenal angiomyolipoma classic extrapulmonary sugar tumor epithelioid, oncocytoma-like CCMMT of FL/LT cystic, intraglomerular abdomino-pelvic sarcoma microhamartoma PEComa NOS capsuloma lymphangiomatosis of sinus Pulmonary lymphangioleiomyomatosis clear cell sugar tumour Angiomyolipoma Angiomyolipoma Commonest PEComa <1% renal tumours 8-20% associated with TSC (47% of TSC) F:M 4:1, mean 50 yrs (30 yrs in TSC) Can be multicentric, bilateral Rarely other sites liver, spleen, lung, heart, vagina, ovary, pancreas, spermatic cord, palate, nose, GI tract, adrenal 2
39 Variants adipocyte -rich spindle cell-rich epithelioid sclerosing atypical malignant Vascular invasion Nodal involvement Assoc renal cancer Angiomyolipoma Angiomyolipoma and Renal Ca Clear Cell 64% Chromophobe 12% Papillary 6% Collecting duct 3% Oncocytoma 15% Jimenez et al, Mod Pathol 2001 Pulmonary PEComas and associated lesions Lymphangioleiomyomatosis Clear cell sugar tumour Multifocal micronodular pneumocyte hyperplasia Lymphangioleiomyomatosis Rare: 0.4 per 100,000 99% females, mean age 38 Dyspnoea, cough, haemoptysis Pneumothorax, chylothorax Progressive pulmonary insufficiency outcome relates to extent 5 yr survival = 50% 10 yr survival = 25% Lymphangioleiomyomatosis Lymphangioleiomyomatosis HMB45 SMA ER 3
40 Lymphangioleiomyomatosis In up to 35% of TSC 47-60% of LAM have AML Multifocal micronodular pneumocyte hyperplasia assoc. LAM and TSC - LOH of TSC2/1 - phospho-p70s6k + Clear Cell Sugar Tumor Mostly sporadic F > M Mean age 57 yrs Peripheral coin lesions Unencapsulated Liebow & Castleman, 1963 Poppet, 1991; Hayashi 2010 Primary Extrapulmonary Sugar Tumors (PEST) 1996 Pea F 57 Uterus 1996 Zamboni F 60 Pancreas 2001 Tazelaar F 9 Rectum F 20 M 29 F 40 Perineum Interatrial septum Rectum 2002 Govender F 16 Breast Clear Cell Myomelanocytic Tumor 7F:1M Ages 3-21 (median 11 yrs) In/adjacent to ligamentum teres or falciform ligament 5 20 cm (median 8cm) 1 had lung metastasis Folpe et al AJSP 2000; Tanaka et al, 2000 Clear Cell Myomelanocytic Tumor Fascicular and nested patterns Mostly spindled, uniform Small nucleolus, few mitoses Clear/eosinophilic cytoplasm Pigment iron, melanin Vascular pattern t(3;10) in one case Folpe et al AJSP 2000; 24:
41 Pecomas: Sites Intra-abdominal falciform ligament/lig teres 9 omentum, mesentery retroperitoneum 86 GI tract GU tract GYN Head & neck stomach, jejunum, ileum, colon liver, common bile duct, pancreas kidney, bladder, prostate, cord uterus, cervix, vagina broad ligament, round ligament 3 larynx/phx, skull base, parotid, oral Skin/ST/bone upper limb, lower limb, trunk 1 Other atrium, mediastinum, adrenal Uterine PEComa TSC (9%), young age Pale cytoplasm, rounded nuclei Like SMT but HMB45 positive SMA, h-caldesmon, desmin in both Melan-A mostly negative?cd1a in PEComa Vang, 2002, Silva 2005; Fadare 2007; Fadare Adachi 2008 Cutaneous PEComa Variants In dermis and subcutis Epithelioid or spindle cells Clear/granular cytoplasm Few mitoses HMB45+, melan A± Des+, SMA± Rarely malignant De Saint Aubain Somerhausen 2005; Mentzel 2005;Tan 2007; Liegl 2008; Calder 2008; Walsh 2009; Chaplin 2010 Sclerosing PEComa 19% of 70 PEComas M = F, yrs Retroperitoneum Two malignant cases Desmin, SMA, h-caldesmon HMB45, melan A (23%) Hornick, 2008; Ramaih
42 PEComa: IHC Positive Positive HMB45 Melan A MiTF SMA Calponin Negative CK EMA Desmin Caldesmon CD117 S100 pr TFE3 Cathepsin K?CD1a PEComa: IHC % 90 TFE HMB45 MelA MiTF SMA Des S100 CK CD117 CD34 TFE3 Malignant Angiomyolipoma Malignant Angiomyolipoma 32 cases Kidney, liver Most epithelioid rarely spindled rarely pleomorphic carcinoma with fat Kragel 1985; Ferry 1991; Lowe 1992; Al-Saleem 1998; Pea 1998; Christiano 1999; L Hostis 1999; Martignoni 2000; Cibas 2001; Chandrasoma 2004; Warakaulle 2004; Inci 2006; El Jack 2007; Huang 2007; Liu 2007; Moudoni 2008; Nguyan 2008; Sato 2008; Higa 2009; Arabel Polo 2009; Higa 2009; Brimo 2010 Renal Epithelioid Angiomyolipoma Abdominopelvic Sarcoma Aydan et al: 7.7% of 194 AML - none malignant F28 Cecum, ileum 9.0 Liver, dod 28 mos Brimo et al: 9/34 atypical EAML (26%) malignant F19 Uterus (LS) 5.5 Lung, bone 10mos F40 Pelvis 2.5 NFU F41 Uterus (myo) ovary 6.0 NED 6 mos 70% atypical epithelioid cells 2 mitotic figures per 10 hpf atypical mitotic figures necrosis TSC, Bi AML 3 or 4 features = highly predictive of malignancy Aydan, 2009; Brimo 2010 Bonetti et al, Mod Pathol
43 Malignant PEComas 44 cases: 36F, 8M Uterus 22 GIT 5 Retro/peritoneum 7 Limbs 3 Head and neck 3 Abdominal wall 2 Prostate 1 Bladder 1 Malignant PEComa Folpe 2000, Bonetti 2001; Dimmler 2003; Greene 2003; Pan 2003; Park 2003, Yanai 2003; Fukunaga 2004; Harris 2004; Lehman 2004; Silva 2004; Evert 2005; FOlpe 2005; Agaimy 2006; Yamamoto 2006; Armah 2007; Osei 2007; Weinreb 2007; Liang 2008; Lian 2008; Calder 2008; Koenig 2009; Huai-yin 2009; Pattampaspong 2009; Ramaih 2009; Wagner 2010; Italiano 2010 Atypical PEComa Criteria for Assessing Malignancy Size > 5 cm Mitoses 1/50hpf Necrosis Marked hypercellularity Nuclear atypia Infiltrative growth absence of features = benign 2 features = malignant Pleomorphism only = symplastic Size >5cm only = UMP Folpe 2005, 2010 Tuberous Sclerosis Complex & PEComa TSC AML LAM TSC % AML LAM Others 6 ut 0 0 Cardiac rhabdomyoma Astrocytic hamartomas Subependymal nodules, SEGA Angiofibromas, periungual fibromas Genetics In TSC, inactivating mutations in TSC1 (9q34) (27%) - hamartin TSC2 (16p13.3)(73%) - tuberin In AML and LAM LOH mainly for TSC2 Allelic changes at 16p One CCMMT had t(3;10) 7
44 AKT p13k IRS1 modified from Huang 2008 TSC1 acts to stabilize TSC2 somatic del/mutation in TSC (2 nd hit) leads to lack of TSC2 expression mtorc1 activation via Rheb phosphorylation of p70s6k and S6 Feedback loop p70s6k S6 Rapamycin (sirolimus) mtorc1 inhibition in AML, LAM, PEComa Targeted Therapy Taille 2007; Bissler, 2008; Davies 2008; Krischock 2010, Wagner 2010; Yu 2010; Krueger 2010 Rapamycin (sirolimus) mtorc1 inhibition in AML, LAM, PEComa In SEGA (everolimus) Targeted Therapy Differential Diagnosis Melanoma Clear cell sarcoma (TA) Clear cell carcinoma Leiomyosarcoma GI stromal tumour Taille 2007; Bissler, 2008; Davies 2008; Krischock 2010, Wagner 2010; Yu 2010; Krueger 2010 Clear Cell Sarcoma (TA) Clear Cell Sarcoma yrs (7 83) F > M Distal extremities, bowel Tendons, aponeuroses Multinodular Long history High grade 8
45 Clear Cell Sarcoma Leiomyosarcoma HMB45 Soft tissue GI tract S100pr + S100pr + HMB45 + HMB45 - Melan A + Melan A - t(12;22)(q13;q12) S100pr EWSR1-ATF1 t(2;22)(q33;q12) EWSR1-CREB1 Leiomyosarcoma RMS MyoFS SMA + DES h-cal ± + Desmin + ++ ± Calponin + H-caldesmon SMM + Myogenin HMB45 - Melan-A Epithelioid Smooth Muscle Tumor PEComa vs Uterine SMT Younger patients TSC association (9%) Paler cytoplasm Ovoid nuclei, no CKvacuoles Vascular network Melan-A positive CD1A positive Silva 2005; Fadare 2008, Adachi 2008 Gastrointestinal Stromal Tumor % GIST Mutational analysis CD117 DOG1 CD34 SMA Des h-cal S100pr HMB MelA 9
46 PEComa: Summary Mesenchymal tumors composed of perivascular epithelioid cells Family including AML, CCST, LAM and others in many sites Some associated with TSC Spindled, epithelioid and clear cells Myomelanocytic and wider phenotype Constitutive activation of mtor susceptible to therapy Spectrum from benign to malignant 10
47 Other New entities in soft tissue tumors. Angelo Paolo Dei Tos MD Departments of Pathology and Oncology General Hospital of Treviso, Italy Introduction During the past decade classification schemes have evolved continuously. The reappraisal of previously described entities have greatly contributed to major conceptual shifts. Pleomorphic malignant fibrous histiocytoma and hemangiopericytoma still represent the best examples, as their decline have allowed proper recognition of specific sarcoma subtypes such as pleomorphic rhabdomyosarcoma, myxofibrosarcoma, giant cell tumor of soft tissues or solitary fibrous tumor. By contrast, many lesions currently reported as new, with time has become part of spectrum of lesions. Examples are so called lipomatous hemangiopericytoma or giant cell angiofibroma (now both part of the morphologic continuum of solitary fibrous tumor) or hyalinizing spindle cell tumor with giant rosettes that has merged with low-grade fibromyxoid sarcoma to form a single tumor entity. Nonetheless, distinctive new lesions continue to be reported. This brief review will focus on a small group of recently reported mesenchymal lesions. Trendy entities like PEComa and myoepithelial lesions of soft tissue and bone are covered by distinct contributions. Myopericytoma Perivascular neoplasms comprise traditionally glomus tumor and so called hemangiopericytoma (HPC). Whereas glomus tumor represents a welldefined entity, the existence of HPC as a separate entity has been questioned. A number of neoplasms of different lines of differentiation are in fact characterized by a HPC-like vascular growth pattern, and the concept that most HPC represents examples of solitary fibrous tumors has eventually
48 reached a broad consensus. However, there exist lesions composed of cells expressing contractile proteins and organized in a perivascular growth pattern, that fulfills criteria set by Stout in his original description of hemangiopericytoma (1). These distinctive lesions have been collectively labeled as myopericytoma (2,3). Myopericytoma tends to occur in the superficial soft tissue of adults (peak incidence is in the fifth decade). Most frequently it arises in the lower limbs, followed by the upper limbs, trunk and head and neck. Microscopically, myopericytoma is composed of a spindle cell, myoid proliferation, organized in a distinctive perivascular pattern of growth, however a broad morphologic spectrum is observed. Immunohistochemically, most cases express smooth muscle actin and h-caldesmon. Desmin immunopositivity is rarely encountered. The clinical behavior is benign in most cases, however prominent cytologic atypia (which is rarely observed) correlates with potential of local recurrence and also metastatic spread (4). Epithelioid angiomatous nodule Cutaneous epithelioid vascular proliferations comprise a morphologic spectrum ranging from benign, reactive to frankly malignant conditions. A group of morphologically distinct cutaneous epithelioid vascular lesions that does not fit in any of the known entities was reported by Brenn in 2004 and labeled as epithelioid angiomatous nodule (EAN) (5). EAN usually arises in the trunk, extremities and, more rarely, on the face of adult patients with no gender predominance. Clinically, EAN presents as single (or multiple) erythematous to bluish nodules (or papules), of small size and short duration. Microscopically, EAN is composed of a circumscribed, unilobular, mostly solid proliferation of large polygonal epithelioid endothelial cells with vesicular nuclei and conspicuous nucleoli. Nuclear atypia is never observed. EAN is generally associated with a lymphoplasmacytic inflammatory infiltrate most prominent at the periphery of the lesion. Varying numbers of eosinophils are found scattered throughout the lesion. Immunohistochemically, neoplastic cells express common endothelial differentiation markers. Epithelioid angiomatous nodule is a benign, cutaneous vascular lesion, the relevance of
49 which resides in the fact it is not infrequently confused with epithelioid angiosarcoma. Reticular microcystic schwannoma Reticular microcystic schwannoma (RMS) is a rare variant of schwannoma with a distinctive predilection for viscera, especially the gastrointestinal tract (6), and mostly occurring in elderly patients (peak incidence is in the 6 th decade). A female predominance is observed. They are usually small, asymptomatic lesions most often measuring less than 3 cm in size. RMS usually occurs in the stomach, small bowel, and proximal large intestine. Microscopically it exhibits a striking microcystic and reticular lesional growth pattern with anastomosing and intersecting strands of spindle cells. Neoplastic cells tend to distribute around islands of myxoid or collagenous/hyalinized stroma. Mitotic activity is generally low and both atypia and necrosis are absent. RMS differs from usual schwannomas of the gastrointestinal tract also by lacking the characteristic peripheral cuff of lymphocytes. Immunohistochemically, strong nuclear and cytoplasmic positivity for S-100 and variably strong glial fibrillary acidic protein staining is generally observed. The histologic appearance of RMS raises a broad differential diagnosis, which includes gastrointestinal stromal tumor, perineurioma, and in more epithelioid examples, even an epithelial malignancy. Awareness of the entity and the use of an immunohistochemical panel of differentiation markers allows proper classification in most cases. RMS is an entirely benign neural neoplasm with no significant recurrent potential. Plexiform angiomyxoid tumor of the stomach Very recently, Takahashi and coworker have reported the occurrence in the gastric antrum of adult patients (peak incidence is in the 4 th decade) of a distinctive mesenchymal lesion characterized by a strikingly plexiform pattern of growth (7) The neoplasm can attain a large size and is composed of a
50 cytologically bland spindle cell proliferation, separated by abundant intercellular myxoid matrix, and associated with a rich capillary size vascular network. Cytologic atypia is generally absent and mitotic count very low. Immunohistochemically, tumor cells express alpha-smooth muscle actin and muscle actin, whereas are consistently negative for KIT, DOG1, CD34, and S- 100 protein. All cases analyzed so far have not shown mutations in the KIT or PDGFRA gene. As preliminary ultrastructural data seemed to point to myofibroblastic differentiation the authors proposed to label the lesion as plexiform angiomyxoid tumor of the stomach (PAMT). PAMT appears to be a benign entity, however, the frequently observed ulceration of the gastric mucosa may lead to potentially fatal gastrointestinal bleeding. A larger confirmatory series has been recently reported suggesting to modify the original label into plexiform fibromyxoma (8). Whatever the name, PAMT should be separated from GIST, nerve sheath tumors, and other fibromyxoid neoplasms Epithelioid myxofibrosarcoma Myxofibrosarcoma (MFS) is one of the most common soft tissue sarcomas of elderly patients and is exhibits a predilection for the superficial soft tissues of the limbs. Classic forms are represented by a multinodular, most often superficial proliferation composed of variably atypical neoplastic cells, mucinladen pseudolipoblasts, set in richly vascularized myxoid stroma. Recently, Nascimento and collaborators reported on a variant of myxofibrosarcoma that, while keeping most of the clinicopathologic features of the classic variant, is characterized by a striking epithelioid morphology, being therefore termed epithelioid myxofibrosarcoma (EMFS) (9). EMFS is typically characterized by a multinodular, infiltrating growth pattern with alternation of hypercellular and hypocellular myxoid areas. The presence of characteristic, prominent curvilinear vessels is almost often seen. Neoplastic cells tend to arrange singly and in small clusters, or sheets, wherein they show epithelioid morphology with round nuclei, vesicular chromatin, prominent nucleoli, and moderate amounts of eosinophilic cytoplasm. The epithelioid areas tend to be
51 multifocal with admixed areas of conventional MFS. Immunostains are most often negative for common differentiation markers. Differential diagnosis tends to be broad and include carcinoma, melanoma, and myoepithelial malignancies. EMFS tends to occur in the limbs of elderly patients (peak incidence is in the 6 th decade). EMFS in most cases is a high-grade sarcoma. A 70% local recurrence rate, associated with a 50% metastatic rate make EMFS a clinically more aggressive than conventional type myxofibrosarcoma. Pseudomyogenic hemangioendothelioma. Pseudomyogenic hemangioendothelioma (PMEHE) accounts as the most recently reported mesenchymal lesions (10). PMEHE represents a low-grade putative vascular malignancy most often occurring in the soft tissues of young adults. A male predominance is observed. Two third of the cases arise in the limbs followed by the trunk and the head and neck region. Multifocality has been reported in more than 60% of cases and half of cases were superficially located. Microscopically most tumor cells exhibit a spindled morphology, and are arranged in fascicles and sheets. A striking cytoplasmic eosinophilia reminiscent of rhabdomyoblastic differentiation is observed, along with vesicular nuclei harboring variably prominent nucleoli. Immunohistochemically, all tumors were positive for cytokeratin AE1/AE3 and FLI1, whereas approximately half of the cases variably expressed CD31. INI1 nuclear expression is preserved in all tumors tested so far. Clinically half of the patients experienced local recurrence within one year from diagnosis. Metastastic spread seems to be fairly rare as only one patient developed lymph node metastasis, and a second one developed widespread tumor dissemination. PMEHE may be closely related to the lesion previously reported under the name epithelioid sarcoma-like hemangioendothelioma (11). The recognition of this entity is crucial, in particular if the discrepancy between morphology and clinical behavior is considered.
52 References. 1. Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring Zimmermann s pericytes. Ann Surg 1942;116: Granter SR, Badizadegan K, Fletcher CD. Myofibromatosis in adults, glomangiopericytoma, and myopericytoma: a spectrum of tumors showing perivascular myoid differentiation. Am J Surg Pathol. 1998; 22: Mentzel T, Dei Tos AP, Sapi Z, Kutzner H. Myopericytoma of skin and soft tissues: clinicopathologic and immunohistochemical study of 54 cases. Am J Surg Pathol 2006; 30: Brenn T, Fletcher CD. Cutaneous epithelioid angiomatous nodule: a distinct lesion in the morphologic spectrum of epithelioid vascular tumors. Am J Dermatopathol 2004; 26: McMenamin ME, Fletcher CD. Malignant myopericytoma: expanding the spectrum of tumours with myopericytic differentiation. Histopathology 2002; 41: Liegl B, Bennett MW, Fletcher CD. Microcystic/reticular schwannoma: a distinct variant with predilection for visceral locations. Am J Surg Pathol 2008; 32: Takahashi Y, Shimizu S, Ishida T, Aita K, Toida S, Fukusato T, Mori S. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol 2007; 31:
53 8. Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol 2009; 33: Nascimento AF, Bertoni F, Fletcher CD. Epithelioid variant of myxofibrosarcoma: expanding the clinicomorphologic spectrum of myxofibrosarcoma in a series of 17 cases. Am J Surg Pathol. 2007; 31: Hornick JL, Flecther CDM. Pseudomyogenic hemangioendothelioma: a distinctive often multicentric tumor with indolent behavior. Am J Surg Pathol 2011; in press. 11. Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003; 27:
54 Newly Recognized Soft Tissue Tumors Angelo Paolo Dei Tos M.D. Departments of Pathology & Oncology Treviso, ITALY New Entity Reappraisal of lesions already described previously MFH, HPC Resuscitation of an entity that had been abandoned Pleomorphic RMS, GCT of Soft Tissues New clinicopathologic entities Clinical History 50 year old male with a 2 cm, subcutaenous lump on thigh #
55 H-Caldesmon SMA Myopericytoma Diagnosis Myopericytoma M > F Peak incidence in 5th decade (age range: ) Lower limbs > upper limbs > head & neck > trunk Subcutaneous, solitary (rarely multiple), most often painless Myopericytoma Oval spindle shaped myoid cell Perivascular growth pattern Glomus type features = glomangiopericytoma SMA + True hemangiopericytomas (?) Classic HPC-like Angioleiomioma-like like Myofibroma-like Cellular Atypical/Malignant Morphology #
56 Classic type HPC-like Angioleiomyoma-likelike Myofibroma-like Hypcdllular Cellular MP #
57 Malignant/atypical myopericytoma Hemangiopericytoma 1942, Stout and Murray Concept of perivascular (pericytic) neoplasm Pericyte Rouget (1873) Zimmermann (1923) Subsequent integration of lesions with HPC-like vascular pattern HPC Diagnosis of exclusion Closely related (synonymous) with SFT Avoid creation of new monster Myopericytoma = true HPC Hemangiopericytoma Spindle cells Perivascular pattern of growth Contractile properties SMA +/h-caldesmon + Clinical History Hemangiopericytoma = Myopericytoma 52 year old male with a 6 cm, mass located in the right thigh #
58 Immunohistochemistry Vimentin + Scattered AE1/AE3 + ve cells Diagnosis Epithelioid Myxofibrosarcoma Myxoid Sarcomas Myxoid DFSP Myxoid Liposarcoma Myxofibrosarcoma Low Grade fibromyxoid sarcoma Myxoid Chondrosarcoma Myxoid Leyomiosarcoma Embryonal Rhabdomyosarcoma Acral myxoid fibrosarcoma Myxofibrosarcoma Angervall, 1977 Myxoid MFH of Enzinger Spectrum of myxoid lesions High grade = myxoid MFH Histologic grade related to clinical outcome #
59 Myxofibrosarcoma Elderly patients Lower limbs >upper limbs > limb girdles 2/3 subcutis, 1/3 deep seated IHC: vimentin, MSA and SMA (focal) Low grade no Mets High grade 30% metastatic rate Lungs > bone > mets Overall 5 years = 60% Epithelioid Myxofibrosarcoma Elderly patients Limbs 50% metastatic rate Lungs > bone 73% recurrence rate Overall 5 years = 37% More aggressive then ordinary myxofibrosarcoma Clinical History 52 year old male with a 6 cm, mass located in stomach Diagnosed as GIST S-100 #
60 Diagnosis Microcystic-reticular reticular schwannoma Microcystic-reticular reticular schwannoma Peak incidence in the sixth decades (any age) GI tract followed by superficial soft tissue of the extremities Striking microcystic and reticular lesional growth pattern S100 +; GFAP +/- #
61 Microcystic-reticular reticular schwannoma Clinical behavior seems benign with no reported recurrences Differential diagnosis mainly with GIST KIT, DOG1 + Clinical History 41 year old female with a 4 cm, ulcerated mass located in the stomach #
62 SMA DES h-cal KIT Diagnosis Plexiform angiomyxoid myofibroblastic tumor of the stomach Plexiform angiomyxoid myofibroblastic tumor of the stomach Few cases reported Elderly patients with gastrointestinal hemorrhage due to mucosal ulceration GI tract Striking plexiform growth pattern Cytologically bland spindle cells set in intercellular myxoid matrix Rich capillary sized vascular network Variable expression of myogenic markers #
63 Plexiform angiomyxoid myofibroblastic tumor of the stomach EM: myofibroblastic differentiation No mutations in the KIT and PDFGRA genes reported Clinical behavior seems to be benign Gastric bleeding potentially represents a life-threatening condition Adults 12 cases Gastric antrum Benign clinicall behavior Clinical History 18 year old male with a 3 cm, subcutaneous lump in the calf Basketball player #
64 Cytokeratin AE1/AE3 CD31 IHC Cytokeratina AE1/AE3 + CD31 + CD34 INI1 + Smooth muscle actin focally + Diagnosis Pseudomyogenic Hemangioendothelioma Pseudomyogenic Hemangioendothelioma 50 cases (Fletcher & Hornick) Male predominance Peak incidence in 3rd decade 54% lower limb, 24% upper limb, 18% trunk, 4% head & neck Local recurrences in 58% LN mets in one case Pseudomyogenic Hemangioendothelioma Plump spindle cells Brightly eosinophilic cytoplasm Immunohistochemical co-expression of epithelial and vascular markers Relationships with epithelioid sarcoma- like hemangioendothelioma #
65 Stout AP Cancer Mar-Apr;15:400-9 Martin JF et al. Ann Anat Path :484 Acknowledgements Pathology Licia Laurino Sabrina Rossi Salvatore Romeo Cytogenetics Lucia Zanatta Laura Valori Eleonora Cappelletto Francesca Pol Molecular Pathology Luisa Toffolatti Marta Campo Dell Orto Giovanna Gallina Elisa Squizzato Irene Carraretto Serena Chinellato #
66 Notochordal Tumors Key words: chordoma, benign notochordal cell tumor Benign Notochordal Cell Tumor Benign notochordal cell tumor (BNCT), also previously known as giant notochordal cell rest is a distinctive slow growing notochordal cell proliferation that appears to behave in an indolent fashion. The vast majority are asymptomatic incidental findings detected during careful dissection at autopsy or in clinical imaging studies of the axial skeleton. At autopsy, approximately 23.5% have been found in the clivus, 29.5% in the sacrum, 17.5% in the coccyx, 17.5% in the cervical spine and 11.5% in the lumbar vertebra. Radiographically, they frequently manifest as an area of sclerosis in the vertebral body with the lesion being bright on T2 weighted MRI images. Although they are usually small (<1cm,) they may a involve substantial portion of a vertebral body, but, do not demonstrate bone destruction. They have been identified adjacent to chordomas and they may represent a benign precursor. Morphologically, BNCT consists of relatively well-delineated sheets of large polyhedral cells that may be focally surrounded by or abut rebuttressed sclerotic bone trabeculae and entrap hematopoietic marrow. The cells have abundant clear to pale pink cytoplasm and mildly pleomorphic round nuclei containing fine or homogeneously dense chromatin. Those with clear cytoplasm resemble adipocytes. Some of the cells with pink cytoplasm contain round hyaline globules of varying size. The globules are PAS positive diastase resistant and are also present in small extracellular cystic spaces. No mitoses are present and there is no myxoid
67 stroma. Immunohistochemically, BNCT has the same profile as chordoma. The only real reliable feature that distinguishes BNCT from chordoma aside from the clinical and radiographic findings is the lack of myxoid matrix in BNCT. Chordoma Chordoma is a rare primary malignant tumor of bone and it is defined by its phenotype, which recapitulates the embryonic notochord. Its purported origin from persistent rests of notochord (or benign notochordal cell tumor) may explain its anatomic distribution, which is virtually restricted to the axial skeleton. It accounts for approximately 5% of primary bone tumors, and its age adjusted incidence is 0.08 per 100,000. A very small percentage of cases are familial, and in at least one instance, the mode of inheritance was probably autosomal dominant. Cytogenetic and genome-wide analyses have revealed loss of genetic material at chromosomes 1p21 and 36, and 3p in sporadic and familial tumors while linkage analysis has shown that familial chordoma is linked to 7q33. Recently familial chordomas have been associated with brachyury gene duplication, something that does not appear to be present in sporadic chordomas. There have also been reports of chordomas arising in patients with tuberous sclerosis (the only syndrome known to be associated with chordoma). Additionally hyperactivation of Akt/mTORC1 has been identified in sporadic chordomas (due to PTEN loss) suggesting that Akt and mtorc1 inhibition may possibly be effective in treating chordoma. Chordoma is usually diagnosed during the fourth to eighth decades of life; only 5% of tumors develop in patients less than 20 years of age. Men are affected more frequently than
68 women. The tumor most commonly develops in the sacrum (50%) followed by the skull base (35%) and the mobile spine (15%). Rare cases have originated in bones of the extremities (chordoma periphericum). Pain and neurologic deficits specific to the site of origin are common symptoms, and some tumors, especially those arising in the sacrum or the mobile spine may be present for years before they are diagnosed. Radiographically, chordoma presents as a destructive lytic tumor that frequently extends into the soft tissues forming a sizable mass. In the sacrum the soft tissue component is characteristically anterior and may displace the rectum and extend along the sacral nerve roots into the sciatic notch. The tumor is either centered in or broaches the midline. Chordoma has high water content, and appears radiolucent on CT and extremely bright on T2 weight MRI images. Foci of calcification are frequently present. On gross inspection, chordoma is a soft, tan-gray, oozing, gelatinous and lobulated mass that is well delineated from surrounding tissues. The tumors vary in size, those in the skull base are the smallest and are usually 2-5 cm in diameter, whereas, those in the sacrum can be very large and are usually greater than 10 cm in size. Pathologically, chordoma is classified into the conventional, chondroid and dedifferentiated variants. A component of the conventional type is virtually always present in the chondroid and dedifferentiated variants. Conventional chordoma has a lobular growth pattern, infiltrates the marrow space, encases preexisting bony trabeculae, and frequently transgresses the cortex forming a welldemarcated soft tissue mass. The tumor is composed of large epithelioid cells arranged in cohesive nests and cords in which one tumor cell may wrap around or hug' another. The nuclei
69 of the neoplastic cells are of moderate size, darkly staining and may contain a small nucleolus or pseudoinclusion. The eosinophilic cytoplasm is abundant, and sometimes contains multiple round clear vacuoles that may contain wisps of mucinous material. The vacuoles impart a bubbly appearance to the cytoplasm and the cells containing them are known as physaliphorous cells. These cells are characteristic but not pathognomonic of chordoma as other types of tumors (carcinoma, chondrosarcoma, epithelioid hemangioendothelioma, liposarcoma) may have similar appearing cells and some chordomas may lack them altogether. Therefore, their diagnostic significance is limited. Additional morphologic features, in otherwise conventional chordoma, include pleomorphism and spindling of the tumor cells and in some tumors the physaliphorous cells have a large single cytoplasmic vacuole that causes them to mimic adipocytes. Mitotic activity is usually limited in conventional chordoma, but foci of necrosis are common, especially in larger tumors. The stroma is myxoid, frothy, basophilic and surrounds the cords and nests of cells. We have noted an aggressive variant chordoma in children in which the tumor cells grow in large solid sheets with little or no stroma, have vesicular nuclei and exhibit numerous mitoses. Ultrastructurally, the neoplastic cells in conventional chordoma have villous-like surface projections, abundant cytoplasmic glycogen, mitochondria-rough endoplasmic reticulum complexes, cytoplasmic processes that wrap around an adjacent cell, and epithelial features, including well developed desmosomes, intracytoplasmic lumina and tonofilament-like bundles of intermediate filaments. The vacuoles in the physaliphorous cells are formed by either dilated rough endoplasmic reticulum, cytoplasmic inclusions of the extracellular space or intracellular lumina.
70 Immunohistochemically, conventional chordoma typically expresses the epithelial markers keratin, including keratins 8 and 19, and epithelial membrane antigen EMA and the vast majority also stain with antibodies to S-100. Chordomas also express vimentin in most cases and variable numbers may also stain with antibodies to carcinoembryonic antigen and glial fibrillary acidic protein. This profile can be very helpful in distinguishing chondrosarcoma from chordoma, in that chondrosarcomas are negative for epithelial markers, especially, keratin. Recently, the nuclear transcription factor brachyury, which is vital to notochord development, has been identified as a relatively specific marker for chordoma, and commercial antibodies are now available. Several recent studies have shown that, other than chordoma, only hemangioblastoma routinely expresses this antigen. Chondroid chordoma was originally defined as a tumor that contains areas of conventional chordoma as well as regions that resemble low-grade hyaline type chondrosarcoma. Its clinical significance was related to its prognosis in that the length of survival for patients with chondroid chordoma was longer than that for patients with conventional chordoma, yet there was no difference in overall survival. Following the initial description controversy developed regarding the existence of this variant of chordoma, as some investigators argued that it merely represented a form of chondrosarcoma. Fortunately, the issue is now resolved, as several studies have convincingly shown that chondroid chordoma represents a distinct morphologic subtype of chordoma and is not a chondrosarcoma. These studies have also shown that chondroid chordoma has no associated survival advantage and has a biologic potential similar to conventional chordoma.
71 Chondroid chordoma most commonly arises in the skull base, and less frequently in the mobile spine and sacrococcygeal region. Histologically, the chondroid regions merge with or abruptly appose the surrounding conventional component. The chondroid areas are composed of neoplastic cells distributed individually in lacunar-like spaces that are surrounded by solid appearing hyalinized matrix similar in appearance to hyaline cartilage. The quantity of the chondroid component in any individual tumor is variable; in some cases it is so abundant that it may be difficult to distinguish the tumor from a bona fide chondrosarcoma. Ultrastructurally, the tumor cells in the chondroid areas exhibit the same epithelial characteristics as those in conventional foci, and the cells also have the same immunohistochemical profile in that they express epithelial markers and brachyury. Accordingly, the chondroid appearance in these tumors represents a morphologic change in the extracellular matrix and cell distribution, and does not represent the presence of hyaline cartilage in a chordoma. Dedifferentiated chordoma is the rarest subtype of chordoma. It is composed of a high grade or poorly differentiated spindle cell sarcoma that arises in the background of conventional chordoma. The dedifferentiation results from ongoing cumulative mutations in conventional chordoma cells and may arise de novo within a primary tumor, as a component of a recurrence or within a tumor that has been previously irradiated. Dedifferentiation develops in less than 5% of conventional chordomas and most frequently complicates sacrococcygeal tumors. Grossly the dedifferentiated component is fish flesh-like in appearance, and morphologically it is usually distinct from the areas of conventional chordoma. The light microscopic features, immunohistochemical phenotype and ultrastructural characteristics of the dedifferentiated component usually are those of a pleomorphic fibroblastic sarcoma,
72 although in some tumors osteosarcomatous differentiation is present. The significance of identifying dedifferentiated chordoma is its aggressive biological behavior. It has the worst prognosis of all chordomas and is usually rapidly fatal with systemic spread occurring in approximately 90% of cases. The prognosis of chordoma is affected by a variety of clinical and pathologic characteristics. Important features include tumor location, size, and resectability, as well as the age and gender of the patient. Chordomas of the sacrum have the best prognosis and the longest overall survival. Local recurrences for sacrococcygeal tumors are commonplace, especially after incomplete excision. The 5 and 10 year modern era survival rates of range from 60-95% and 40-60% respectively, however, long survival does not imply that they are disease or recurrence free. In the mobile spine, the 5 year survival rate is approximately 55% and the mean survival is about 4.7 years with only 15% of patients being alive without tumor after a mean follow-up of almost 2 years. Complete excision of spinal tumors is difficult to accomplish, therefore, local recurrence rates are very high and range from 62-75%. In the skull base, large tumors, female gender, and age greater than 40 years have been associated with a poorer outcome. In a large series in which the patients were treated with surgery and radiation, 46% of patients developed local progression with a median of 69 months follow-up. Other investigators have reported a 5 year local control rate of 59%. In another analysis, the 5 year recurrence free survival was 65% while the 5 and 10 year estimated overall survival rates in another study were 51% and 35%, respectively.
73 The rate of metastatic spread of chordoma varies widely in the literature and ranges from less than 5% to 43%. The metastases tend to occur late in the clinical course and are usually noted after a period of several years or more. Common sites of dissemination include the lung, skin and bone. Tumors in the sacrococcygeal region and mobile spine metastasize more frequently than those originating in the skull base. The low rate of systemic spread from skull base neoplasms, which is reported to be 0 10%, is probably related to the fact that the patients succumb to the local affects of their tumor before metastases develop. There are no effective chemotherapeutic agents for the treatment of this disease. There is some in vitro evidence to suggest that Akt and mtorc1 inhibition might be effective.
74 Fibrous Tumors of Infancy and Childhood: An Update International Society of Bone and Soft Tissue Pathology 2011 Cheryl M. Coffin, M. D. Goodpasture Professor of Pathology Vice Chair and Executive Medical Director of Anatomic Pathology Vanderbilt University, Nashville, TN Key Words: Fibroblastic-myofibroblastic tumors, fibromas, fibromatoses, Gardner fibroma, desmoid, lipofibromatosis, infantile fibrosarcoma, primitive myxoid mesenchymal tumor of infancy, low grade fibromyxoid sarcoma, inflammatory myofibroblastic tumor. Introduction Fibroblastic and myofibroblastic neoplasms account for approximately 12% of soft tissue tumors in the first two decades of life, excluding the pseudosarcomas such as nodular fasciitis and related lesions and inflammatory myofibroblastic tumor (1-8). The histologic similarities, differences in biologic potential, and clinical and molecular genetic variations among this group of lesions create clinical, diagnostic and therapeutic challenges. The histologic spectrum encompasses reactive, malformative, pseudosarcomatous, and neoplastic lesions. In recent decades, the concept of intermediate or borderline fibroblastic-myofibroblastic tumors has been refined for neoplasms with a tendency for local recurrence or rare metastases. This presentation focuses on recently described fibroblastic neoplasms or new advances in the knowledge about these lesions in young patients. Gardner Fibroma and Desmoid Fibromatosis Gardner fibroma is a benign plaque-like mass with a predilection for children and young adults, and a strong association with APC mutation and familial adenomatous polyposis (9 and 10). It is associated with concurrent or subsequent development of desmoids. The mass is composed of coarse collagen fibers separated by clear clefts and interspersed with bland spindle cells and sparse mast cells. Although it is unknown whether Gardner fibroma represents an overgrowth or a neoplasm, nuclear beta-catenin reactivity has been observed in a subset of cases. There is over expression of beta-catenin and other proteins in the APC and WNT pathways. The optimal therapeutic approach to Gardner fibroma is not fully understood, since surgery may promote the growth of a subsequent desmoid in the region of a Gardner fibroma. Desmoid tumors are relatively frequent in children although they are categorized among the so-called adult fibromatoses (11-16). Approximately 15-40% of desmoids occur in patients in the first two decades of life, and 19-60% of pediatric fibroblasticmyofibroblastic tumors are desmoids. Multiple desmoids occur in 3-12% of cases. The trunk and extremities are the most common sites, although desmoids can occur in soft tissue anywhere in the body. The grossly circumscribed mass is composed of sheets and fascicles of fibroblasts and myofibroblasts in a variably collagenized and myxoid background. The microscopic borders are often infiltrative. A pattern of elongated 1
75 slender blood vessels at the periphery of fascicles is distinctive. Nuclear beta-catenin reactivity is frequently demonstrated. The recurrence rate in children and adolescents in 60% overall, and 20% of patients have multiple recurrences. Fewer than 2% die of local complications. Up to 25% of young patients have adenomatous polyposis coli. This may be an underestimate of the frequency of APC mutation since other manifestations of APC may not be obvious in children and adolescents. Other genetic aberrations in desmoids include beta-catenin mutations, chromosome 5q deletion, and trisomies 8 and 20. Desmoid fibromatoses are treated with surgery; chemotherapy may be effective in selected cases. Lipofibromatosis Lipofibromatosis is a recently described intermediate, locally recurrent neoplasm (17-19). It has a predilection for infancy and early childhood, and approximately 25% are congenital. The distal extremities are the favored site. Lipofibromatosis has a male predilection. The poorly circumscribed mass is composed of slender interfacing bundles of mature fibroblasts interspersed with mature adipose tissue. 75% recur locally. Little is known about the cytogenetics or molecular genetic aspects of lipofibromatosis, although a complex translocation involving chromosomes 4, 6, and 9 has been reported in one case. Infantile Fibrosarcoma Infantile fibrosarcoma is an intermediate rarely metastasizing neoplasm that occurs mainly in infancy and very early childhood (20-29). Approximately 50% are congenital and the sex distribution is equal. Infantile fibrosarcoma can arise on the extremities, trunk, or head and neck as a rapidly growing tumor that reaches a very large size in proportion to the size of the child. Histologically, spindle, primitive angulated, and round cell patterns may be seen, and zonal necrosis is frequent. A translocation between chromosomes 12 and 15 with an ETV6-NTRK3 gene fusion is considered characteristic. In addition, gains of chromosomes 8, 11, 17, and 21 may be observed. Surgery and chemotherapy are effective treatments. It is now recognized that infantile fibrosarcoma and cellular congenital mesoblastic nephroma are histologically identical tumors with the same gene fusion and the same response to treatment. Primitive Myxoid Mesenchymal Tumor of Infancy Primitive myxoid mesenchymal tumor of infancy is a rare recently described primitive tumor of infancy that occurs on the trunk, extremities, and head and neck (29). The multinodular growth is composed of primitive cells with a myxoid background and lacks characteristic immunohistochemical features. Recurrence and metastasis occur. No information is yet available about cytogenetic or molecular genetic features. Primitive myxoid mesenchymal tumor has been provisionally included among the intermediate or low grade malignant potential fibroblastic-myofibroblastic tumors because of its tendency for recurrence, rare frequency of metastasis, and ultrastructural features of primitive fibroblasts. Low Grade Fibromyxoid Sarcoma 2
76 Low grade fibromyxoid sarcoma is a specific subtype of fibrosarcoma with a low frequency of metastasis (30-36). Approximately 20% occur in the first two decades of life. Although the proximal extremities and trunk are the most common sites, the head and neck is a preferred site in children. The tumor may be superficial or deep, although pediatric cases are more frequently located in superficial soft tissue. A variety of histologic variants have been reported, including myxoid and cellular variants, hyalinizing spindle cell with giant collagen rosettes, and perhaps sclerosing epithelioid fibrosarcoma. A translocation between chromosomes 7 and 16 with a FUS-CREB3L2 gene fusion has been reported. The recurrence rate is 10%. Approximately 6% of patients have late metastases. Complete surgical excision is effective treatment. Inflammatory Myofibroblastic Tumor Inflammatory myofibroblastic tumor is a distinctive lesion composed of myofibroblastic spindle cells accompanied by an inflammatory infiltrate of plasma cells, lymphocytes and eosinophils, according to the current WHO classification (2, 37-48). It is currently regarded as an intermediate, rarely metastasizing neoplasm. The age distribution is wide, but inflammatory myofibroblastic tumor is most frequent in the first three decades of life. It can arise in the mesentery, omentum, retroperitoneum, lungs, mediastinum, head and neck, live, and genitourinary tract as a solitary or multinodular mass. Up to 25% of patients have a clinical syndrome of fever, weight loss, growth failure, anemia, thrombocytosis, polyclonal hyperglobulinemia, and elevated inflammatory markers. Genetic abnormalities have been reported including chromosome 2p23 abnormalities with ALK gene rearrangements in 50-70% of inflammatory myofibroblastic tumors. The fusion oncogenes are numerous and include tropomyosin, clathrin, RAN binding protein 2, and other oncogenes. Detection of the ALK abnormality with immunohistochemistry has high sensitivity and specificity. The ALK rearrangement can be demonstrated with conventional tumor karyotype, fluorescent in situ hybridization, and RT-PCR. Local recurrence has been reported in up to 25% of extrapulmonary inflammatory myofibroblastic tumors. Metastases are rare. Although surgery is the standard treatment, and chemotherapy may be effective, small molecule inhibitors of ALK are a recent therapeutic option for inflammatory myofibroblastic tumor with an ALK gene rearrangement. Prognostic indicators have been elusive. Recent finding of clinical and prognostic importance is the recognition of the round cell variant of inflammatory myofibroblastic tumor. Round cell inflammatory myofibroblastic tumor is ALK positive with a distinctive membranous and dot-like pattern of ALK reactivity and a gene fusion between ALK and RANBP2, which has a predilection for males, involves intraabdominal sites, and has a mean age of 35 years, with an age range of infancy to the sixth decade. The majority of patients reported thus far with round cell inflammatory myofibroblastic tumor have experienced rapid local recurrence, a metastatic rate of 25%, and a death rate of 38%. One of the most important aspects of the diagnosis of inflammatory myofibroblastic tumor is recognizing it from its many mimics. Many different reactive infections, fibroinflammatory, and neoplastic conditions can simulate inflammatory myofibroblastic tumor. Although the term inflammatory pseudotumor is embedded in the literature, we recommend abandoning this term because of its lack of specificity and 3
77 the significant potential for confusion about pathologic classification. IgG4-related sclerosing disease encompasses a clinical syndrome with multiorgan system involvement by a diffuse fibroinflammatory proliferation and is distinguished by its infiltrative growth pattern, presence of abundant lymphoid aggregates, and obliterative phlebitis. The IgG4/ IgG plasma cell ratio in inflammatory myofibroblastic tumor can overlap with IgGrelated sclerosing disease and is not a reliable test for distinguishing between the two lesions. There are many neoplastic mimics of inflammatory myofibroblastic tumor, such as lymphoma (Hodgkin s disease, anaplastic large cell lymphoma, other lymphomas, and extramedullary myeloid sarcoma), dendritic cell neoplasms (especially follicular dendritic cell tumor arising in the context of Castleman disease), sarcomatoid carcinomas, malignant melanoma, plexiform fibromyxoma, and other sarcomas (inflammatory liposarcoma, inflammatory leiomyosarcoma). At this point in time, understood that inflammatory myofibroblastic tumor is regarded as an intermediate rarely metastasizing neoplasm, and in 50-70% of cases it is an ALKoma with the potential for targeted treatment with small molecule inhibitors. It is also well recognized that inflammatory myofibroblastic tumor has a predilection for younger patients and an origin in body cavities. Ongoing challenges include identification of prognostic markers, optimal treatment, and appropriate classification of ALK-negative inflammatory myofibroblastic tumors. The pathologic paradox is the neoplastic proliferation of spindled myofibroblastic cells combined with the prominent inflammatory infiltrate. Summary and Conclusions Fibroblastic-myofibroblastic tumors in young patients are an important and challenging group of lesions with extensive histologic and immunohistochemical similarities. Cytogenetic and molecular genetic identification of mutations, deletions, gene rearrangements, and alterations in receptor tyrosine kinases has yielded new insights about this group of tumors. Whether non-round cell undifferentiated sarcomas in young patients will eventually be recognized as primitive fibroblastic neoplasms and included among the fibroblastic-myofibroblastic tumors in young patients is an important point for future consideration. It is also evident that many tumors previously classified as fibrohistiocytic tumors could be subsumed into the fibroblastic-myofibroblastic category because of their cellular characteristics and phenotypes. Finally, the extent to which treatment can be tailored to these individual tumors and to the individual patients will be an important area for further investigation. The time will soon come for a revised morphologic-genetic-managerial classification. 4
78 References 1. Coffin CM, Dehner LP, O Shea PA (eds). Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. Williams and Wilkins, Philadelphia, Fletcher CDM, Unni KK, Mertens F (eds). Pathology and Genetics of Tumours of Soft Tissue and Bone. World Health Organization Classification of Tumours. Lyon, France, Rosenberg HS, Stenback WA, Spjut HJ. The fibromatoses of infancy and childhood. Perspect Pediatr Pathol 1978; 4: Allen PW. The fibromatoses: a clinicopathologic classification based on 140 cases. Am J Surg Pathol 1977; 1: and Coffin S, Boccon-Gibod L. Fibroblastic- myofibroblastic proliferations of childhood and adolescence. Ann Pathol 2004; 24: Schmidt D. Fibrous tumors and tumor-like lesions of childhood: diagnosis, differential diagnosis, and prognosis. Curr Top Pathol 1995; 89: Coffin CM, Dehner LP. Fibroblastic- myofibroblastic tumors in children and adolescents: a clinicopathologic study of 108 examples in 103 patients. Pediatr Pathol 1991; 11: Schmidt D, Harms D. Fibromatosis of infancy and childhood. Histology, ultrastructure and clinicopathologic correlation. Z Kinderchir 1985; 40: Wehrli BM, Weiss Sw, Yandow S, Coffin CM. Gardner- associated fibromas (GAF) in young patients: a distinct fibrous lesion that identifies unsuspected Gardner syndrome and risk for fibromatosis. Am J Surg Pathol 2001; 25: Coffin CM, Hornick JL, Zhou H, Fletcher CD. Gardner fibroma: a clinicopathologic and immunohistochemical analysis of 45 patients with 57 fibromas. Am J Surg Pathol 2007; 31: Ayala AG, Ro HY, Goepfert H, et al. Desmoid fibromatosis: a clinicopathologic study of 25 children. Semin Diagn Pathol 1986; 3: Coffin CM, Randall RL, Million L, Zhous H. Desmoid fibromatosis in childhood and adolescence: an analysis of 65 patients in the first two decades of life. Mod Pathol 2010; 23(Suppl 1): 394A. 5
79 13. Meazza C, Bisogno G, Gronchi A, et al. Aggressive fibromatosis in children ad adolescents: the Italian experience. Cancer 2010; 116: Lazar AJ, Tuvin D, Hajibashi S, et al. Specific mutations in the beta- catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 2008; 173: Eccles DM, Lunt PW, Wallis Y, et al. An unusually severe phenotype for familial adenomatous polyposis. Arch Dis Child 1997; 77: Skapek SX, Ferguson WS, Granowetter L, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol 2007; 25: Fetsch JF, Miettinen M, Laskin WB, et al. A clinicopathologic study of 45 pediatric soft tissue tumors with an admixture of adipose tissue and fibroblastic elements, and a proposal for classification as lipofibromatosis. Am J Surg Pathol 2000; 24: Ayadi L, Charfi S, Ben Hamed Y, et al. Pigmented lipofibromatosis in unusual location: case report and review of the literature. Virchows Arch 2008; 452: Kenney B, Richkind KE, Friedlaender G, Zambrano E. Chromosomal rearrangements in lipofibromatosis. Cancer Genet Cytogenet 2007; 179: Chung EB, Enzinger FM. Infantile fibrosarcoma. Cancer 1976; 38: Coffin CM, Jaszcz W, O Shea PA, Dehner LP. So-called congenital-infantile fibrosarcoma: does it exist and what is it? Pediatr Pathol 1994; 14: Knezevich SR, Garnett MJ, Pysher TJ, et al. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res 1998; 58: Knezevich SR, McFadden DE, Tao W, et al. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet 1998; 18: Bourgeois JM, Knezevich SR, Mathers JA, Sorensen PH. Molecular detection of the ETV6- NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol 2000; 24: Sheng WQ, Hisaoka M, Okamoto S, et al. Congenital- infantile fibrosarcoma. A clinicopathologic study of 10 cases and molecular detection of the ETV6-NTRK3 fusion transcripts using paraffin embedded tissues. Am J Clin Pathol 2001; 115:
80 26. Argani P, Fritsch M, Kadkol SS, et al. Detection of the ETV60 NTRK3 chimeric RNA of infantile fibrosarcoma/ cellular congenital mesoblastic nephroma in paraffin- embedded tissue: application to challenging pediatric renal stromal tumors. Mod Pathol 2000; 13: Bernstein R, Zeltzer PM, Lin F, Carpenter PM. Trisomy 11 and other nonrandom trisomies in congenital fibrosarcoma. Cancer Genet Cytogenet 1994; 78: McCahon E, Sorensen PH, Davis JH, et al. Non- resectable congenital tumors with the ETV6-NTRK3 gene fusion are highly responsive to chemotherapy. Med Pediatr Oncol 2003; 40: Alaggio R, Ninfo V, Rosolen A, Coffin CM. Primitive myxoid mesenchymal tumor of infancy: a clinicopathologic report of 6 cases. Am J Surg Pathol 2006; 30: Evans HL. Low-grade fibromyxoid sarcoma. A report of two metastasizing neoplasms having a deceptively benign appearance. Am J Clin Pathol 1987; 88: Folpe AL, Lane KL, Paull G, Weiss SW. Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol 2000; 24: Lane KL, Shannon RJ, Weiss SW. Hyalinizing spindle cell tumor with giant rosettes: a distinctive tumor closely resembling low- grade fibromyxoid sarcoma. Am J Surg Pathol 1997; 21: Evans HL. Low-grade fibromyxoid sarcoma. A report of 12 cases. Am J Surg Pathol 1993; 17: Goodlad JR, Mentzel T, Fletcher CD. Low grade fibromyxoid sarcoma: clinicopathological analysis of eleven new cases in support of a distinct entity. Histopathology 1995; 26: Billings SD, Giblen G, Ganburg- Smith JC. Superficial low-grade fibromyxoid sarcoma (Evans tumor): a clinicopathologic analysis of 19 cases with a unique observation in the pediatric population. Am J Surg Pathol 2005; 29: Guillou L, Benhattar J, Gengler C, et al. Translocation- positive low- grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French Sarcoma Group. Am J Surg Pathol 2007; 31:
81 37. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995; 19: Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res 1999; 59: Hussong JW, Brown M, Perkins SL, et al. Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod Pathol 1999; 12: Biselli R, Ferlini C, Fattorossi A, et al. Inflammatory myofibroblastic tumor (inflammatory pseudotumor): DNA flow cytometric analysis of nine pediatric cases. Cancer 1996; 77: Coffin CM, Patel A, Perkins S, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol 2001; 14: Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol 2001; 25: Cessna MH, Zhou H, Sanger WG, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol 2002; 15: Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007; 31: Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol 2008; 61: Hornick JL, Wang WL, Roy A, et al. Round cell inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK: an aggressive intraabdominal variant. Mod Pathol 2010; 23(Suppl 1): 21A. 47. Dehner LP, Coffin CM. Idiopathic fibrosclerotic disorders and other inflammatory pseudotumors. Semin Diagn Pathol 1998; 15: Saab ST, Hornick JL, Fletcher CD, et al. IgG4 plasma cells in inflammatory myofibroblastic tumor: inflammatory marker or pathogenetic link? Mod Pathol 2010; 23(Suppl 1): 27A. 8
82 Fibrous Tumors of Infancy and Childhood: An Update International Society of Bone and Soft Tissue Pathology 2011 Cheryl M. Coffin, M. D. Goodpasture Professor of Pathology Vice Chair and Executive Medical Director of Anatomic Pathology Vanderbilt University, Nashville, TN Key Facts and Challenges >10% of soft tissue tumors in newborns to 20 year olds have a fibroblastic-myofibroblastic phenotype The clinicopathologic spectrum encompasses reactive, malformative, benign, intermediate, and malignant lesions Morphologic and immunohistochemical similarities Biologic, genetic, and therapeutic diversity Objectives To review the current classification of fibroblastic-myofibroblastic tumors To summarize new information and recently described lesions To discuss evolving knowledge and ongoing challenges of inflammatory myofibroblastic tumor (IMT) Classification Benign Malformations/ overgrowths Pseudosarcomas Fibromas Fibromatoses, juvenile and adult Intermediate fibroblastic-myofibroblastic neoplasms (locally recurrent vs. rarely metastasizing) Fibroblastic-myofibroblastic sarcomas Gardner Fibroma Benign plaquelike mass Predilection for children and young adults Strong association with FAP/APC Overexpression of beta-catenin and other proteins in the APC and Wnt pathways Association with concurrent or subsequent desmoids Surgery vs. no treatment? Overgrowth or neoplasm? Juvenile Desmoid Tumors 15-40% of desmoids occur in NB 20 y.o 3-12% are multiple Trunk and extremities are most common sites 60% recur; 20% have multiple recurrences Death in < 2% APC known in 25% 1
83 Lipofibromatosis Intermediate, locally recurrent neoplasm Infancy and childhood; 25% congenital Distal extremities favored site Male predilection 75% local recurrence rate t(4;6;9) in one case Infantile Fibrosarcoma Intermediate, rarely metastasizing neoplasm Infancy; 50% congenital Extremities, trunk, head/ neck Rapid growth, large size t(12;15) with ETV6-NTRK3 fusion Gains of chromosomes 8,11,17,21 Surgery, chemotherapy effective Primitive Myxoid Mesenchymal Tumor of Infancy Rare primitive tumor of infancy Trunk, extremities, head, and neck Multinodular growth Recurrence, metastasis Intermediate or low grade malignant potential Low Grade Fibromyxoid Sarcoma A specific subtype of fibrosarcoma 20% in first two decades of life Proximal extremities and trunk most common sites, superficial or deep Predilection for head/ neck and superficial sites in children Histologic variants Recurrence in 9%, metastases in 6% (late) t(7;16)(q34;p11) with FUS-CREB3L2 gene fusion Inflammatory Myofibroblastic Tumor Intermediate, rarely metastasizing neoplasm Infancy to adulthood, most frequent in first 3 decades Mesentery, omentum, retroperitoneum, lung, mediastinum, head/neck, liver, GU tract Clinical syndrome: fever, weight loss, growth failure, anemia, thrombocytosis, polyclonal hyperglobulinemia, elevated ESR Local recurrence in 25%; metastasis rare Prognostic indicators elusive Genetic Abnormalities Chromosome 2p23 abnormalities with ALK gene rearrangements in 50-70% of IMTs Fusion oncogenes: tropomyosin (TPM3, TPM4), clathrin (CLTC), Ran-binding protein 2 (RANBP2), CARS, ATIC, SEC31L1 Detection: immunohistochemistry, FISH, RT- PCR Small molecule ALK inhibitors: a new therapeutic option 2
84 Males Round Cell IMT: A Morphologic- Prognostic- Molecular Subtype? Intraabdominal Mean age 35 yr. (7mo.- 59 yr.) Rapid local recurrence in 100% Metastases in 25 % Death in 38% ALK- positive with RAN-BP2 fusion IMT: What It Is Not A pseudotumor (abandon the term!) IgG4- related sclerosing disease Lymphoma, dendritic cell neoplasm, carcinoma, melanoma, other sarcomas IMT: What It Is An intermediate (rarely metastasizing) neoplasm An ALKoma with potential for targeted treatment (sometimes) A tumor with a predilection for body cavities and younger patients A pathologic paradox An ongoing challenge Rethinking the Fibroblastic- Myofibroblastic Tumors Mutations, deletions, rearrangements, receptor tyrosine kinases, and more? Is it time for a revised morphologic- geneticmanagerial classification? Will non-round cell undifferentiated sarcomas in young patients eventually be recognized as primitive fibroblastic neoplasms? To what extent can treatment be tailored to tumors and to individual patients? There is a bright future for complexity, what with one thing leading to another. E.B. White 3
85 Introduction New Therapeutic Targets in Sarcomas Sunday 27 February 2011 USCAP) Alexander Lazar MD/PhD Associate Professor of Pathology & Dermatology Sections of Soft Tissue & Bone/Sarcoma Pathology & Dermatopathology Faculty, Sarcoma Research Center Sarcoma is a complex family of malignancies composed of more than 100 types showing differentiation toward soft tissues or bone. They are rare, accounting for about 1 % of major invasive malignancies. Benign mesenchymal tumors are more than 100-fold more common and sometimes distinguishing these two categories can be challenging. Interestingly, successful therapies for rare cancers seem to be over-represented amongst therapies recently approved in the US by the Food and Drug Administration (FDA). While the Orphan Drug Act (1983) has played an important role, it is also possible that rare cancers may show a more homogenous molecular pathogenesis and thus be more uniquely targetable as a whole than in more common cancers where more widely variable genetic features may complicate therapeutic applications (for example, pulmonary carcinomas with EGFR mutations versus those with ALK translocations versus those with other mechanisms). The rarity of some cancers may be, at least in part, a byproduct of the limitation of the events that can produce them. For instance, in the translocation-associated sarcomas, the production of the fusion gene appears to be necessary (if not always sufficient) for these tumors to arise. The early event of the gene fusion may dramatically limit subsequent genetic variability leading to more uniform oncogenic addiction and therapeutic vulnerability that can be exploited when discovered. The degree to which this is true may vary between sarcoma types for instance simple karyotype versus complex karyotype but may be a feature in a significant proportion of sarcoma types. Indeed, it is possible, that in the end most cancers may need to be broken into smaller more homogeneous molecular subgroups for the purposes of treatment effectively making multiple rare cancers out of the more common types. Clearly, in recent times, rare cancers have been disproportionately represented amongst the initial FDA-approved indications for drugs that are highly efficacious with limited toxicity suggesting that such populations may be uniquely targetable. Sarcoma Genetic Overview Sarcomas can be classified in multiple ways, but they broadly fall into the following genetic types: Simple karyotype: Characteristic chromosomal translocations / gene fusions Characteristic gene mutations
86 Limited chromosomal amplifications and deletions Complex karyotype: Genetic instability (extensive) It is hypothesized that knowledge of the molecular pathogenesis of sarcomas may allow the application of rationally applied precision therapies. In this lecture, the following therapies for sarcoma will be very briefly discussed. The goal is to provide insight into recent therapeutic approaches stressing the pathogenic mechanism of the sarcoma where applicable. Dermatofibrosarcoma protuberans (DFSP) This tumor has a characteristic translocation t(17;22) leading to overproduction of platelet derived growth factor B (PDGFB) that acts as an autocrine tumorigenic factor. Tyrosine kinase inhibitors (such as imatinib mesylate and others) that can antagonize the interaction of PDGFB with its receptor show efficacy in this tumor. Chondrosarcoma Both chondrosarcomas and osteosarcomas can show expression of Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) / apoptosis ligand 2 (Apo2) or Death receptors. Treatment with recombinant TRAIL can show efficacy. Pigmented Villonodular Synovitis (PVNS) A translocation t(1;2) leading to overexpression of CSF1 shows both autocrine effects on clonal tumor cells but also attracts numerous non-neoplastic inflammatory cells that expression the CSF1 receptor. This has been referred to as a paracrine-landscape effect. Treatment with tyrosine kinase inhibitors that can block the CSF1 receptor are useful. Essentially this agent is disrupting the effect of the neoplastic component on the tumor microenvironment as in giant cell tumor of bone below. This is a novel and interesting treatment paradigm that can only be explained based on knowledge of the unique pathogenic mechanism. Giant Cell Tumor of Bone The mononuclear cells in this disease produce receptor activator for nuclear factor κ B ligand (RANK) ligand which attracts giant cells expressing RANK (receptor). Disrupting this interaction with denosumab can show profound inhibitory effects. Essentially this agent is disrupting the effect of the neoplastic component on the tumor microenvironment as in PVNS above. Ewing Sarcoma Ewing sarcoma is most often associated with a translocation involving chromsomes 11 and 22; alternative fusion genes involving EWSR1 (22q12) and other genes are also described. A small subset of Ewing cases recalcitrant to traditional treatments show good responses to insulin-like growth factor receptor 1 (IGFR1) inhibitors. Currently
87 research is focused on determining why only certain cases respond with the goal of using this knowledge to expand therapeutic vulnerability. Clear Cell Sarcoma This sarcoma is most commonly associated with a translocation t(12;22) involving the ATF1 and EWSR1 (or rarely CREB1) genes leading to microphthalmia-associated transcription factor (Mitf) overexpression that subsequently induces expression of cmet. Inhibitors of cmet can be useful and represent blocking of a key effector downstream from a tumor-associated fusion gene. Deep (Plexiform) Neurofibroma Recent results have demonstrated that neurofibromata that evolve in the setting of neurofibromatosis 1 are not only dependent on loss of heterozygosity of the NF1 locus in Schwann cells, but required mast cells with haploinsufficiency at the NF1 locus as well. Treaments that stabilize the overactive mast cells such as inhibiting the KIT receptor with imatinib mesylate or other agents, can cause neurofibroma regression. This is an example directly targeting the non-neoplastic tumor microenvironment to achieve tumor control. Desmoid Fibromatosis Sporadic desmoid fibromatosis is associated with activating mutations in CTNNB1, the gene encoding β-catenin, in greater than 80 % of cases. Anti-extrogen therapy has been effective in a small subset of desmoids which uniformly express nuclear ERβ, but lack ERα. Since ERβ is uniformly present, it does not predict response. More recently, use of sorafenib, a broad tyrosine kinase inhibitor with some anti-vegf activity as well has shown profound effects in this tumor. The underlying mechanism is not clear. Myxoid Liposarcoma This tumor is associated with t(12;16) fusing the FUS and DDIT3 genes (FUS is substituted by EWSR1 on occasion). Trabectedin was initially discovered as a natural product of the sea squirt Ecteinascidia turbinata. In myxoid liposarcoma this drug shows anti-tumor effect often associated with hyalinization and maturation of neoplastic fat cells. Recent data indicates that this drug may specifically interrupt the interaction of the fusion gene with DNA. This would represent a mechanism of action that directly attacks the function of the characteristic fusion protein of this neoplasm. Conclusions This is an exciting time in targeted therapy in mesenchymal neoplasia. While there is still so much to be learned, we are beginning to better understand the molecular pathogenesis of certain types of these tumors and are using this knowledge to more precisely target specific tumor types. This list above is not comprehensive and many other innovative and exciting therapeutic clinical trials and individual creative
88 applications of therapies are currently underway. With time, we should have a greatly improved armamentarium to use against these tumors. Disclaimers & Disclosures: This handout and the associated lecture discuss off-label uses of a number of pharmaceutical agents. The author is solely describing the effects and proposed mechanisms and is not endorsing or promoting the use of these agents in this fashion. In addition, disclosure of the author s participation in the speaker bureau of Novartis is here noted.
89 Road Map Why rare cancers and sarcoma may be uniquely targetable Examples of targeting sarcomas based on molecular mechanisms New Therapeutic Targets in Sarcomas Sunday 27 February 2011 USCAP) Alexander Lazar MD/PhD Associate Professor of Pathology & Dermatology Sections of Soft Tissue & Bone/Sarcoma Pathology & Dermatopathology Faculty, Sarcoma Research Center Background Practice-based observation of an unexpected feature of FDA anti-cancer drug approval: Many groundbreaking therapeutic discoveries (very high response rates with minimal toxicity) in oncology occurred disproportionately in orphan tumors (very rare cancers) Rare disorders are defined as those with a prevalence of < 0.07% in the Unites States and < 0.05% in Europe. Goal Investigate the relationship between FDA approval rateof new drugs and tumor frequency. Braiteh F and Kurzrock R. Mol Cancer Ther 2007; 6(4): Methods Reviewed NEW anti-cancer therapeutic agents approved by the FDA for the period of Identified the cancer type (CAx) for the initial approval Excluded hormonal and supportive therapy drugs and newer indications for previously approved agents Identified 2005 U.S. estimated number (Nx) of new cases (CAx) A cancer type (CAx) may have more than one agent approved (nx 1) Success score (SSx) CAx is the ratio of Number agents approved over cases (SSx=nx / Nxx 1,000) Braiteh F and Kurzrock R. Mol Cancer Ther 2007; 6(4): Results Between 1990 and 2005, the FDA approved 51NEW anticancer agents Agent Cancer Indication RR (%) Date approved Levamisole Colon (in combination with 5-FU) 1 N/A Jun Idarubicin Acute myeloid leukemia (AML) in adults % Sep Altretamine Ovarian cancer 18-20% Dec Fludarabine B-cell lymphocytic leukemia (CLL) % Apr Pentostatin Hairy cell leukemia % Oct Aldesleukin Treatment of adults with metastatic renal cell carcinoma 14-20% May Teniposide, VM-26 Childhood acute lymphoblastic leukemia (ALL) % Jul Paclitaxel Ovarian cancer % Dec Cladribine Hairy cell leukemia % Feb Pegaspargase Acute lymphocytic leukemia 78% Feb Vinorelbine Non-small cell lung cancer (NSCLC) % Dec Doxorubicin liposomal AIDS-related Kaposi's sarcoma % Nov Tretinoin, ATRA Acute promyelocytic leukemia (APL) 86-91% Nov Daunorubicin liposomal HIV related Kaposi's sarcoma 25-32, 73% Apr Docetaxel Breast cancer 53, % May Gemcitabine Pancreatic cancer % May Topotecan Ovarian cancer % May Irinotecan Colon and rectum cancer 18% Jun Rituximab Non-Hodgkin's lymphoma 48-54% Nov Capecitabine Breast cancer 20,30,36,42% Apr BCG Live Bladder cancer 39% Aug Trastuzumab Breast cancer 11.6% Sep Valrubicin Bladder cancer 37-40% Sep Braiteh F and Kurzrock R. Mol Cancer Ther 2007; 6(4):
90 Results Agent Cancer Indication RR (%) Date approved Alitretinoin Topical AIDS-related Kaposi's sarcoma. 35% Feb Results Busulfan intravenous Chronic myelogenous leukemia. 100% 2 Feb Denileukin diftitox Cutaneous T-cell lymphoma 30% Feb Methoxsalen Cutaneous T-cell lymphoma 50% Feb Cytarabine liposomal Intrathecal therapy of lymphomatous meningitis 71-78% Apr Temozolomide Anaplastic astrocytoma 11-35% Aug Epirubicin Breast cancer 28% Sep Exemestane Breast cancer 15% Oct Bexarotene capsules Cutaneous T-cell lymphoma 55% Dec Gemtuzumab ozogamicin CD33 positive acute myeloid leukemia i 30% May Arsenic trioxide Acute promyelocytic leukemia (APL) 85% Sep Alemtuzumab B-cell chronic lymphocytic leukemia (B-CLL) 33% May Imatinib mesylate Chronic myelogenous leukemia 95% May Ibritumomab Tiuxetan Non-Hodgkin's lymphoma 80% Feb Oxaliplatin Colon and rectum 53% Aug Gefitinib Non-small cell lung cancer 12% May Bortezomib Multiple Myeloma 35-38% May Tositumomab/I-131T CD20-positive non-hodgkin's lymphoma 71% 3 Dec Pemetrexed disodium Mesothelioma 41.3% Feb Cetuximab EGFR-expressing metastatic colorectal carcinoma 9-10% to 53% 4 Feb Bevacizumab Colon and rectum (added to IFL) 44.8% Feb Azacitidine Myelodysplastic syndromes 49% 5 May Erlotinib Non Small-Cell Lung Cancer NSCLC 8.9% Nov Clofarabine Acute lymphoblastic leukemia 48% Dec Paclitaxel protein-bound particles Breast 33% Jan Nelarabine T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma 55% Oct Sorafenib Renal cell carcinoma 55% Dec Braiteh F and Kurzrock R. Mol Cancer Ther 2007; 6(4): These 51 agents had an initial indication for 21 different cancers Only one fifth (n=12/51 or 21%) were indicated for any of the four leading cancers (prostate, lung, breast or colon) Braiteh F and Kurzrock R. Mol Cancer Ther 2007; 6(4): Lenalidomide Myelodysplastic syndromes 88% Dec Braiteh F and Kurzrock R. Mol Cancer Ther 2007; 6(4): Agents approved by the FDA between Success Score (SS)= n/n x Estimated new cases in the U.S. in 2005 (x1,000) Estimated new cases in the U.S in 2005 (x 1,000) SS= n/n x 1000 The SS was inversely proportionate to the Ca incidence (Spearman's rank correlation test R= , p=0.005) Ratio (Number of agents approved/incidencex1,000) Incidence/Year 2005 SS= (n/n x 1,000) Number of new agents approved by the FDA between Incidence/Year 2005 Braiteh F and Kurzrock R. Mol Cancer Ther 2007; 6(4): Braiteh F and Kurzrock R. Mol Cancer Ther 2007; 6(4): Response Rate (%) Estimated new cases in the U.S. in 2005 (x1,000) Discussion Role of the Orphan Drug Act? Orphan Drug Act (1982) created government incentives such as seven years of exclusive marketing needed to encourage research efforts in rare diseases Mean Response Rate of the cancer to drugs approved between Incidence/Year 2005 Orphan Drug Act. No Pub. L. 2
91 Discussion Before 1982, some 10orphan drugs had been developed Discussion Drugs Approved by the FDA for the Top 7 Types of Rare Diseases Addressed by Orphan Drug Act Since the enactment of the Orphan Drug Act, approximately 300new orphan drugs have been approved and marketed in the US and more than 800additional drugs are in the research pipeline Haffner ME. N Engl J Med 2006; 354(5): Haffner ME. N Engl J Med 2006; 354(5): Discussion The physiological basis for tumors being raremay be the same reason that they are ultimately so treatable Rare Cancer Common Cancer Cancers are driven by a single or relatively single aberrant mechanism may result in oncogenic addiction and profound therapeutic susceptibility Molecular Sarcomagenesis Point Mutations: GIST: KIT, PDGFR, BRAF Desmoid: CTNNB1 Gene Amplification: Dediff Liposarcoma: MDM2 / CDK4 Neuroblastoma: n-myc Gene Deletion: Osteosarcoma: p53 Retinoblastoma: RB1 Translocation: Ewing Sarcoma: EWSR1-FLI1 Dermatofibrosarcoma: COL1A-PDGFB Protein Overexpression: Desmoid Tumor: ER Chondrosarcoma: Death receptors Tumor type ES/PNET Desmoplastic round cell tumor Extraskeletal myxoid chondrosarcoma Clear cell sarcoma Alveolar rhabdomyosarcoma Myxoid liposarcoma Synovial sarcoma Alveolar soft part sarcoma Dermatofibrosarcoma protuberans (& Giant cell fibroblastoma) Low grade fibromyxoid sarcoma Angiomatoid (malignant) fibrous histiocytoma Congenital fibrosarcoma (& Mesoblastic nephroma) Endometrial stromal sarcoma Inflammatory Myofibroblastic Tumor Cytogenetic Translocation t(11;22)(q24;q12) t(21;22)(q22;q12) t(7;22)(p22;q12) t(2;22)(q33;q12) t(17;22)(q12;q12) inv(22) t(11;22)(p13;q12) t(9;22)(q22;q12) t(9;17)(q22;q11) t(9;15)(q22;q21) t(12;22)(q13;q12) t(2;13)(q35;q14) t(1;13)(p36;q14) t(12;16)(q13;p11) t(12;22)(q13;q12) t(x;18)(p11;q11) t(x;17)(p11.2;q25) t(17;22)(q22;q13) t(7;16)(q32-34;p11) t(12;16)(p11;q13) t(16;22)(q13;q12) t(12;15)(p13;q25) t(7;17)(p15;q21) translocationsat2p23 Genes involved EWSR1-FLI1 EWSR1-ERG EWSR1-ETV1 EWSR1-FEV EWSR1-E1AF EWSR1-ZSG EWSR1-WT1 EWSR1-CHN RBP56-CHN CHN-TCF12 EWSR1-ATF1 PAX3-FKHR PAX7-FKHR FUS-DDIT3 EWSR1-DDIT3 SSX1-SYT SSX2-SYT ASPL-TFE3 COL1A1-PDGFB FUS-CREB3L2 FUS-CREB3L1 FUS-ATF1 EWSR1-ATF1 ETV6-NTRK3 JAZF1-JJAZ1 ALK,multiplefusionpartners 3
92 Hypothesis Dermatofibrosarcoma protuberans Understanding Specific Molecular Etiology Can Lead to Specific Molecular Therapy PDGF in Dermatofibrosarcoma Collagen1A Col-PDGFB PDGFB Ring Chromosome PDGF-B intracellular PDGF Receptor Rutkowski et al. J Clin Oncol 2010; 28(10): Rutkowski et al. J Clin Oncol 2010; 28(10):
93 Chondrosarcoma TRAIL-R in Chondrosarcoma intracellular Procaspase-8 Death Receptor Caspase-8 TRAIL, APO2 ligand Caspase Cascade Pigmented Villonodular Synovitis Baseline 4 years on therapy Autocrine and Paracrine effects of CSF1 Autocrine CSF1R CSF1R t(1;2) COL6A-CSF1 CSF1R CSF1 CSF1 Chemotaxis t(1;2) leads to the overexpression of active CSF1 which in turn attracts non-neoplastic inflammatory cells expressing the CSF1 receptor (CSF1R) through a paracrine-landscape effect. Adapted from West et al. PNAS
94 Imatinib Can Produce a Significant Response as Early as 2 Months Giant Cell Tumor of Bone Baseline 8 weeks This patient underwent margin negative resection and remains free of disease at 22 months off imatinib. Giant Cell Tumor (GCT) of Bone Human RANK Human RANKL courtesy of Martine Roudier, Amgen Radiologic Response to Denosumab Pre-Treatment Week 13 Post-Treatment courtesy of Judith Bovee, LUMC 6
95 Ewing Sarcoma courtesy of Judith Bovee, LUMC courtesy of Bogdan Czerniak, UTMDACC Targeted Therapy IGF1R Targeted Therapy IGF1R Clear Cell Sarcoma Problem Response rates thus far 6-9% 7
96 MET in Clear Cell Sarcoma EWSR1 EWSR1-ATF1 MET Receptor ATF1 HGF courtesy of H-H Homann, U Ruhr, Bochum DE & T Mentzel Transcription Translation MITF intracellular 9/11/06 10/17/06 12/1/06 Deep NF1-Associated Neurofibroma (? MPNST) Katz et al. Expert Rev Mol Med 2009; 11:e30. Katz et al. Expert Rev Mol Med 2009; 11:e30. Staser et al. Blood 2010; 116(2):
97 Desmoid Fibromatosis Yang et al. Cell 2008; 135(3): Estrogen Receptor in Desmoid Estrogen intracellular Estrogen Receptor Transcription Translation ER Response Element Proliferation Survival Desmoid TMA Biomarkers Sorafenib: 21 yo male w/ axillary and neurovascular compromise. Heavily pre-treated. Forequarter amputation only surgical option. 10/28/08: Post Gadolinium(upper extremity) 1/12/10: Post Gadolinium courtsey of Robert Maki, MSKCC 9
98 19 yo woman with popliteal desmoid with progression after surgery (x3), radiation (x2) and chemotherapy (3 lines) Radiological outcome by SIZEand duration of response (months) TTR: 30%: 10 months 10%: 4 months Partial Response: 25% Stable Disease: 70% 7/9/09:Post Gadolinium (popliteal surface) 12/11/09: Post Gadolinium Choi Response: 55% courtsey of Robert Maki, MSKCC courtsey of Robert Maki, MSKCC Specificity Profile: imatinib mesylate & sorafenib Other Sarcomas Fabian et al. Nat. Biotech. 23, 329 (2005) Kinase dendrogram adapted from Manning et al. Science298, 1912 (2002) Other Avenues Myxoid liposarcoma trabectedin Inflamm myofibroblastic tumor crizotinib WD/DD Liposarcoma MDM2 & CDK4 inhibitors ASPS cmet inhibitors & anti-angiogenic agents mtor inhibitors PEComa; multiple types HDAC inhibitors multiple types Target Classification of Sarcomas NGFR MPNST Paraganglioma Neuroblastoma, Neuroepithelioma Schwannoma Granular Cell Tumor Immunotherapy Translocation Sarcomas EWSFT, MLS, ARMS, SS, DSRCT, Gene Mutation TRAIL Receptor Osteosarcoma Chondrosarcoma Transcription Factors ESFT, Myxoid Lipo, ARMS, SS, DSRCT MDM2 / CDK4 Dedifferentiated liposarcoma Vascular Differentiation Angiosarcoma (Hemangio, Lymphangio) Hemangioendothelioma HPC/SFT HDAC, Methylation Synovial sarcoma ALT / Dedifferentiated Liposarcoma Dedifferentiated Chondrosarcoma MPNST Small Cell Sarcomas Kinases EWSFT (IGFs) DFSP (PDGF-B) IMFT (Alk-1) ASPS (met, VEGF) GIST (KIT, PDGFR) RANK GCT of bone Estrogen Receptor Desmoid Tumor (ER-beta) HSP-90 GIST Dedifferentiated Liposarcoma mtor PEComa, Angiomyolipoma, LAM 10
The Genetics of Myoepithelial Tumors: salivary glands, soft tissue and bone
 The Genetics of Myoepithelial Tumors: salivary glands, soft tissue and bone Cristina Antonescu, MD Memorial Sloan-Kettering Cancer Center, New York Nothing to declare Disclosure Spectrum of Myoepithelial
The Genetics of Myoepithelial Tumors: salivary glands, soft tissue and bone Cristina Antonescu, MD Memorial Sloan-Kettering Cancer Center, New York Nothing to declare Disclosure Spectrum of Myoepithelial
Financial disclosures
 Mesenchymal Neoplasms with Melanocytic Differentiation By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchcock Medical Center Geisel
Mesenchymal Neoplasms with Melanocytic Differentiation By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchcock Medical Center Geisel
59 yo male with past medical history of prostate carcinoma, presented with upper abdominal pain
 December 2016 59 yo male with past medical history of prostate carcinoma, presented with upper abdominal pain Contributed by: Divya Sharma, MD. Fellow, Gastrointestinal Pathology, Department of Pathology
December 2016 59 yo male with past medical history of prostate carcinoma, presented with upper abdominal pain Contributed by: Divya Sharma, MD. Fellow, Gastrointestinal Pathology, Department of Pathology
1/10/2018. Soft Tissue Tumors Showing Melanocytic Differentiation. Overview. Desmoplastic/ Spindle Cell Melanoma
 2016 MFMER slide-1 2016 MFMER slide-2 2016 MFMER slide-3 Soft Tissue Tumors Showing Melanocytic Differentiation Andrew L. Folpe, M.D. Professor of Laboratory Medicine and Pathology Mayo Clinic, Rochester,
2016 MFMER slide-1 2016 MFMER slide-2 2016 MFMER slide-3 Soft Tissue Tumors Showing Melanocytic Differentiation Andrew L. Folpe, M.D. Professor of Laboratory Medicine and Pathology Mayo Clinic, Rochester,
A 42-year-old woman with a liver mass
 April 2016 Case of the Month A 42-year-old woman with a liver mass Contributed by: Natalia I. Rush, MD, Resident Physician, Indiana University School of Medicine, Department of Pathology and Laboratory
April 2016 Case of the Month A 42-year-old woman with a liver mass Contributed by: Natalia I. Rush, MD, Resident Physician, Indiana University School of Medicine, Department of Pathology and Laboratory
A 25 year old female with a palpable mass in the right lower quadrant of her abdomen
 May 2016 A 25 year old female with a palpable mass in the right lower quadrant of her abdomen Contributed by: Paul Ndekwe, MD, Resident Physician, Indiana University School of Department of Pathology and
May 2016 A 25 year old female with a palpable mass in the right lower quadrant of her abdomen Contributed by: Paul Ndekwe, MD, Resident Physician, Indiana University School of Department of Pathology and
Myxo-inflammatory Fibroblastic sarcoma
 AKA Myxo-inflammatory Fibroblastic sarcoma Acral Myxoinflammatory fibroblastic sarcomaam.j.surg.path1998; 22; 911-924 Inflammatory myxoid tumour of soft parts with bizarre giant cells [Pathol.Res.Pract.
AKA Myxo-inflammatory Fibroblastic sarcoma Acral Myxoinflammatory fibroblastic sarcomaam.j.surg.path1998; 22; 911-924 Inflammatory myxoid tumour of soft parts with bizarre giant cells [Pathol.Res.Pract.
Newer soft tissue entities
 Newer soft tissue entities Examples among fibroblastic tumors Turku, May 6, 2010 Markku Miettinen, M.D. AFIP, Washington, DC Fibroblastic neoplasms Solitary fibrous tumor /Hemangiopericytoma Low-grade
Newer soft tissue entities Examples among fibroblastic tumors Turku, May 6, 2010 Markku Miettinen, M.D. AFIP, Washington, DC Fibroblastic neoplasms Solitary fibrous tumor /Hemangiopericytoma Low-grade
57th Annual HSCP Spring Symposium 4/16/2016
 An Unusual Malignant Spindle Cell Lesion to Involve the Breast Erinn Downs-Kelly, D.O. Associate Professor of Pathology University of Utah & ARUP Laboratories No disclosures Case 39 y/o female with no
An Unusual Malignant Spindle Cell Lesion to Involve the Breast Erinn Downs-Kelly, D.O. Associate Professor of Pathology University of Utah & ARUP Laboratories No disclosures Case 39 y/o female with no
ACCME/Disclosures ALK FUSION-POSITIVE MESENCHYMAL TUMORS. Tumor types with ALK rearrangements. Anaplastic Lymphoma Kinase. Jason L.
 Companion Meeting of the International Society of Bone and Soft Tissue Pathology The Evolving Concept of Mesenchymal Tumors ALK FUSION-POSITIVE MESENCHYMAL TUMORS Jason L. Hornick, MD, PhD March 13, 2016
Companion Meeting of the International Society of Bone and Soft Tissue Pathology The Evolving Concept of Mesenchymal Tumors ALK FUSION-POSITIVE MESENCHYMAL TUMORS Jason L. Hornick, MD, PhD March 13, 2016
SMOOTH MUSCLE TUMOURS
 SMOOTH MUSCLE TUMOURS NORMAL SMOOTH MUSCLE Cytology Immunohistochemistry Ultrastructure Masson Trichrome Smooth Muscle Ultrastructure Many myofilaments running parallel to the long axis of the smooth
SMOOTH MUSCLE TUMOURS NORMAL SMOOTH MUSCLE Cytology Immunohistochemistry Ultrastructure Masson Trichrome Smooth Muscle Ultrastructure Many myofilaments running parallel to the long axis of the smooth
Case 2. Dr. Sathima Natarajan M.D. Kaiser Permanente Medical Center Sunset
 Case 2 Dr. Sathima Natarajan M.D. Kaiser Permanente Medical Center Sunset History 24 year old male presented with a 3 day history of right flank pain, sharp in nature Denies fever, chills, hematuria or
Case 2 Dr. Sathima Natarajan M.D. Kaiser Permanente Medical Center Sunset History 24 year old male presented with a 3 day history of right flank pain, sharp in nature Denies fever, chills, hematuria or
SOFT TISSUE TUMOR PATHOLOGY: AN UPDATE
 SOFT TISSUE TUMOR PATHOLOGY: AN UPDATE Jason L. Hornick, MD, PhD July 18, 2013 Department of Pathology Brigham and Women s Hospital Harvard Medical School Boston, MA, USA I have no disclosures. New Soft
SOFT TISSUE TUMOR PATHOLOGY: AN UPDATE Jason L. Hornick, MD, PhD July 18, 2013 Department of Pathology Brigham and Women s Hospital Harvard Medical School Boston, MA, USA I have no disclosures. New Soft
Disclosure. Relevant Financial Relationship(s) None. Off Label Usage None MFMER slide-1
 Disclosure Relevant Financial Relationship(s) None Off Label Usage None 2013 MFMER slide-1 Case Presentation A 43 year old male, with partial nephrectomy for a right kidney mass 2013 MFMER slide-2 2013
Disclosure Relevant Financial Relationship(s) None Off Label Usage None 2013 MFMER slide-1 Case Presentation A 43 year old male, with partial nephrectomy for a right kidney mass 2013 MFMER slide-2 2013
Financial disclosures
 Cutaneous Mesenchymal Neoplasms with EWSR1 Rearrangement By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchc Geisel School of
Cutaneous Mesenchymal Neoplasms with EWSR1 Rearrangement By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchc Geisel School of
Cutaneous Mesenchymal Neoplasms with EWSR1 Rearrangement
 Cutaneous Mesenchymal Neoplasms with EWSR1 Rearrangement By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchcock Medical Center
Cutaneous Mesenchymal Neoplasms with EWSR1 Rearrangement By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchcock Medical Center
04/09/2018. Salivary Gland Pathology in the Molecular Era Old Friends, Old Foes, & New Acquaintances
 Salivary Gland Pathology in the Molecular Era Old Friends, Old Foes, & New Acquaintances Jennifer L. Hunt, MD, MEd Aubrey J. Hough Jr, MD, Endowed Professor of Pathology Chair of Pathology and Laboratory
Salivary Gland Pathology in the Molecular Era Old Friends, Old Foes, & New Acquaintances Jennifer L. Hunt, MD, MEd Aubrey J. Hough Jr, MD, Endowed Professor of Pathology Chair of Pathology and Laboratory
Special slide seminar
 Special slide seminar Tomáš Rozkoš The Fingerland Department of Pathology Charles University Medical Faculty and Faculty Hospital in Hradec Králové Czech Republic Case history, 33 years old resistance
Special slide seminar Tomáš Rozkoš The Fingerland Department of Pathology Charles University Medical Faculty and Faculty Hospital in Hradec Králové Czech Republic Case history, 33 years old resistance
Diplomate of the American Board of Pathology in Anatomic and Clinical Pathology
 A 33-year-old male with a left lower leg mass. Contributed by Shaoxiong Chen, MD, PhD Assistant Professor Indiana University School of Medicine/ IU Health Partners Department of Pathology and Laboratory
A 33-year-old male with a left lower leg mass. Contributed by Shaoxiong Chen, MD, PhD Assistant Professor Indiana University School of Medicine/ IU Health Partners Department of Pathology and Laboratory
Enterprise Interest Nothing to declare
 Enterprise Interest Nothing to declare Diagnoses one would not like to miss in soft tissue pathology early in your career Marta Sbaraglia, MD Department of Pathology Hospital of Treviso University of Padua
Enterprise Interest Nothing to declare Diagnoses one would not like to miss in soft tissue pathology early in your career Marta Sbaraglia, MD Department of Pathology Hospital of Treviso University of Padua
Salivary Glands 3/7/2017
 Salivary Glands 3/7/2017 Goals and objectives Focus on the entities unique to H&N Common board type facts Information for your future practice Salivary Glands Salivary Glands Major gland. Paratid. Submandibular.
Salivary Glands 3/7/2017 Goals and objectives Focus on the entities unique to H&N Common board type facts Information for your future practice Salivary Glands Salivary Glands Major gland. Paratid. Submandibular.
Science & Technologies RETROPERITONEAL TUMOR: DIFFERENTIAL DIAGNOSIS BEYOND THE USUALLY SUSPECTED. Medical University Sofia, Bulgaria
 RETROPERITONEAL TUMOR: DIFFERENTIAL DIAGNOSIS BEYOND THE USUALLY SUSPECTED Vesela Ivanova *, Tihomir Dikov *, Goran Derimachkovski **, Petar Panchev ** * Department of General and Clinical Pathology, Medical
RETROPERITONEAL TUMOR: DIFFERENTIAL DIAGNOSIS BEYOND THE USUALLY SUSPECTED Vesela Ivanova *, Tihomir Dikov *, Goran Derimachkovski **, Petar Panchev ** * Department of General and Clinical Pathology, Medical
The World Health Organization defines PEComas as mesenchymal
 ORIGINAL ARTICLE Perivascular Epithelioid Cell Neoplasms of Soft Tissue and Gynecologic Origin A Clinicopathologic Study of 26 Cases and Review of the Literature Andrew L. Folpe, MD,* Thomas Mentzel, MD,
ORIGINAL ARTICLE Perivascular Epithelioid Cell Neoplasms of Soft Tissue and Gynecologic Origin A Clinicopathologic Study of 26 Cases and Review of the Literature Andrew L. Folpe, MD,* Thomas Mentzel, MD,
Part 1. Slides 1-38, Rita Alaggio Soft tissue tumors Trondheim 14. mars 2013
 Part 1 Slides 1-38, Rita Alaggio Soft tissue tumors Trondheim 14. mars 2013 Pediatric Pathology Soft Tissue Tumors AN UPDATE Rita Alaggio Azienda Ospedaliera Università di Padova Soft Tissue Tumors More
Part 1 Slides 1-38, Rita Alaggio Soft tissue tumors Trondheim 14. mars 2013 Pediatric Pathology Soft Tissue Tumors AN UPDATE Rita Alaggio Azienda Ospedaliera Università di Padova Soft Tissue Tumors More
CYSTIC TUMORS OF THE KIDNEY JOHN N. EBLE, M.D. CYSTIC NEPHROMA
 Page 1 CYSTIC TUMORS OF THE KIDNEY JOHN N. EBLE, M.D. Department of Pathology & Laboratory Medicine Phone (317) 274-4806 Medical Science A-128 FAX: (317) 278-2018 635 Barnhill Drive jeble @iupui.edu Indianapolis,
Page 1 CYSTIC TUMORS OF THE KIDNEY JOHN N. EBLE, M.D. Department of Pathology & Laboratory Medicine Phone (317) 274-4806 Medical Science A-128 FAX: (317) 278-2018 635 Barnhill Drive jeble @iupui.edu Indianapolis,
Desmoplastic Melanoma R/O BCC. Clinical Information. 74 y.o. man with lesion on left side of neck r/o BCC
 R/O BCC Sabine Kohler, M.D. Professor of Pathology and Dermatology Dermatopathology Service Stanford University School of Medicine Clinical Information 74 y.o. man with lesion on left side of neck r/o
R/O BCC Sabine Kohler, M.D. Professor of Pathology and Dermatology Dermatopathology Service Stanford University School of Medicine Clinical Information 74 y.o. man with lesion on left side of neck r/o
Tumores de células pequeñas, redondas y azules: diagnóstico diferencial cuando el tiempo apremia
 Tumores de células pequeñas, redondas y azules: diagnóstico diferencial cuando el tiempo apremia Sílvia Bagué Servei de Patologia Hospital de Sant Pau Barcelona Soft tissue sarcomas Heterogeneous group
Tumores de células pequeñas, redondas y azules: diagnóstico diferencial cuando el tiempo apremia Sílvia Bagué Servei de Patologia Hospital de Sant Pau Barcelona Soft tissue sarcomas Heterogeneous group
Case: The patient is a 24 year- old female who was found to have multiple mural nodules within the antrum. Solid and cystic components were noted on
 Case: The patient is a 24 year- old female who was found to have multiple mural nodules within the antrum. Solid and cystic components were noted on imaging. There is no significant past medical history.
Case: The patient is a 24 year- old female who was found to have multiple mural nodules within the antrum. Solid and cystic components were noted on imaging. There is no significant past medical history.
Mody. AIS vs. Invasive Adenocarcinoma of the Cervix
 Common Problems in Gynecologic Pathology Michael T. Deavers, M.D. Houston Methodist Hospital, Houston, Texas Common Problems in Gynecologic Pathology Adenocarcinoma in-situ (AIS) of the Cervix vs. Invasive
Common Problems in Gynecologic Pathology Michael T. Deavers, M.D. Houston Methodist Hospital, Houston, Texas Common Problems in Gynecologic Pathology Adenocarcinoma in-situ (AIS) of the Cervix vs. Invasive
21/07/2017. Hobnail endothelial cells are not the same as epithelioid endothelial cells
 UPDATE IN CUTANEOUS VASCULAR S DERMATOPATHOLOGY SESSION BELFAST PATHOLOGY JUNE 21/2017 Dr E Calonje St John s Institute of Dermatology, London, United Kingdom THE FAMILY OF VASCULAR S WITH EPITHELIOID
UPDATE IN CUTANEOUS VASCULAR S DERMATOPATHOLOGY SESSION BELFAST PATHOLOGY JUNE 21/2017 Dr E Calonje St John s Institute of Dermatology, London, United Kingdom THE FAMILY OF VASCULAR S WITH EPITHELIOID
Update in Salivary Gland Pathology. Benjamin L. Witt University of Utah/ARUP Laboratories February 9, 2016
 Update in Salivary Gland Pathology Benjamin L. Witt University of Utah/ARUP Laboratories February 9, 2016 Objectives Review the different appearances of a selection of salivary gland tumor types Establish
Update in Salivary Gland Pathology Benjamin L. Witt University of Utah/ARUP Laboratories February 9, 2016 Objectives Review the different appearances of a selection of salivary gland tumor types Establish
Various hereditary, acquired and neoplastic conditions can lead to cyst formation in the kidney.
 Dr. Fatima AlAl-Hashimi Hashimi,, MD, FRCPath Salmaniya Medical Complex, Bahrain Various hereditary, acquired and neoplastic conditions can lead to cyst formation in the kidney. The most frequently encountered
Dr. Fatima AlAl-Hashimi Hashimi,, MD, FRCPath Salmaniya Medical Complex, Bahrain Various hereditary, acquired and neoplastic conditions can lead to cyst formation in the kidney. The most frequently encountered
Malignant Peripheral Nerve Sheath Tumor
 C H A P T E R 120 Malignant Peripheral Nerve Sheath Tumor Currently, malignant peripheral nerve sheath tumor (MPNST) is the most commonly used generic name for the neoplasms known in the past as neurosarcoma,
C H A P T E R 120 Malignant Peripheral Nerve Sheath Tumor Currently, malignant peripheral nerve sheath tumor (MPNST) is the most commonly used generic name for the neoplasms known in the past as neurosarcoma,
3/27/2017. Disclosure of Relevant Financial Relationships
 Ophthalmic Pathology Evening Specialty Conference USCAP 2017 5 th March, 2017 Mukul K. Divatia, MD Assistant Professor Department of Pathology & Genomic Medicine Weill Cornell Medical College Houston Methodist
Ophthalmic Pathology Evening Specialty Conference USCAP 2017 5 th March, 2017 Mukul K. Divatia, MD Assistant Professor Department of Pathology & Genomic Medicine Weill Cornell Medical College Houston Methodist
Keywords solitary fibrous tumor, dedifferentiation, dedifferentiated solitary fibrous tumor, STAT6, GRIA2, cytokeratin, rhabdomyosarcomatous
 758452IJSXXX10.1177/1066896918758452International Journal of Surgical PathologyCreytens et al research-article2018 Pitfalls in Pathology Multifocal Cytokeratin Expression in a Dedifferentiated Solitary
758452IJSXXX10.1177/1066896918758452International Journal of Surgical PathologyCreytens et al research-article2018 Pitfalls in Pathology Multifocal Cytokeratin Expression in a Dedifferentiated Solitary
Cellular Neurothekeoma
 Cellular Neurothekeoma Scott W Binder, MD Pritzker Professor of Pathology & Dermatology Sr. Vice Chair Director, Pathology Clinical Services Chief, Dermatopathology Geffen/UCLA School of Medicine Clinical
Cellular Neurothekeoma Scott W Binder, MD Pritzker Professor of Pathology & Dermatology Sr. Vice Chair Director, Pathology Clinical Services Chief, Dermatopathology Geffen/UCLA School of Medicine Clinical
Papillary Lesions of the Breast A Practical Approach to Diagnosis. (Arch Pathol Lab Med. 2016;140: ; doi: /arpa.
 Papillary Lesions of the Breast A Practical Approach to Diagnosis (Arch Pathol Lab Med. 2016;140:1052 1059; doi: 10.5858/arpa.2016-0219-RA) Papillary lesions of the breast Span the spectrum of benign,
Papillary Lesions of the Breast A Practical Approach to Diagnosis (Arch Pathol Lab Med. 2016;140:1052 1059; doi: 10.5858/arpa.2016-0219-RA) Papillary lesions of the breast Span the spectrum of benign,
From Morphology to Molecular Pathology: A Practical Approach for Cytopathologists Part 1-Cytomorphology. Songlin Zhang, MD, PhD LSUHSC-Shreveport
 From Morphology to Molecular Pathology: A Practical Approach for Cytopathologists Part 1-Cytomorphology Songlin Zhang, MD, PhD LSUHSC-Shreveport I have no Conflict of Interest. FNA on Lymphoproliferative
From Morphology to Molecular Pathology: A Practical Approach for Cytopathologists Part 1-Cytomorphology Songlin Zhang, MD, PhD LSUHSC-Shreveport I have no Conflict of Interest. FNA on Lymphoproliferative
Objectives. Salivary Gland FNA: The Milan System. Role of Salivary Gland FNA 04/26/2018
 Salivary Gland FNA: The Milan System Dr. Jennifer Brainard Section Head Cytopathology Cleveland Clinic Objectives Introduce the Milan System for reporting salivary gland cytopathology Define cytologic
Salivary Gland FNA: The Milan System Dr. Jennifer Brainard Section Head Cytopathology Cleveland Clinic Objectives Introduce the Milan System for reporting salivary gland cytopathology Define cytologic
أملس عضلي غرن = Leiomyosarcoma. Leiomyosarcoma 1 / 5
 Leiomyosarcoma 1 / 5 EPIDEMIOLOGY Exact incidence is unknown, but older studies suggest that leiomyosarcomas comprise approximately 3 percent of soft-tissue sarcomas. Superficial leiomyosarcoma occurs
Leiomyosarcoma 1 / 5 EPIDEMIOLOGY Exact incidence is unknown, but older studies suggest that leiomyosarcomas comprise approximately 3 percent of soft-tissue sarcomas. Superficial leiomyosarcoma occurs
What is New in the 2015 WHO Lung Cancer Classification? Zhaolin Xu, MD, FRCPC, FCAP
 What is New in the 2015 WHO Lung Cancer Classification? Zhaolin Xu, MD, FRCPC, FCAP Professor, Dept of Pathology, Dalhousie University, Canada Pulmonary Pathologist and Cytopathologist, QEII HSC Senior
What is New in the 2015 WHO Lung Cancer Classification? Zhaolin Xu, MD, FRCPC, FCAP Professor, Dept of Pathology, Dalhousie University, Canada Pulmonary Pathologist and Cytopathologist, QEII HSC Senior
Neoplasia literally means "new growth.
 NEOPLASIA Neoplasia literally means "new growth. A neoplasm, defined as "an abnormal mass of tissue the growth of which exceeds and is uncoordinated with that of the normal tissues and persists in the
NEOPLASIA Neoplasia literally means "new growth. A neoplasm, defined as "an abnormal mass of tissue the growth of which exceeds and is uncoordinated with that of the normal tissues and persists in the
Disclosures. An update on ancillary techniques in the diagnosis of soft tissue tumors. Ancillary techniques. Introduction
 Disclosures An update on ancillary techniques in the diagnosis of soft tissue tumors. I have nothing to disclose. Andrew Horvai, MD, PhD Clinical Professor, Pathology Introduction Ancillary techniques
Disclosures An update on ancillary techniques in the diagnosis of soft tissue tumors. I have nothing to disclose. Andrew Horvai, MD, PhD Clinical Professor, Pathology Introduction Ancillary techniques
ACCME/Disclosures. Cribriform Lesions of the Prostate. Case
 Cribriform Lesions of the Prostate Ming Zhou, MD, PhD Departments of Pathology and Urology New York University Langone Medical Center New York, NY Ming.Zhou@NYUMC.ORG ACCME/Disclosures The USCAP requires
Cribriform Lesions of the Prostate Ming Zhou, MD, PhD Departments of Pathology and Urology New York University Langone Medical Center New York, NY Ming.Zhou@NYUMC.ORG ACCME/Disclosures The USCAP requires
Surgical Pathology Evening Specialty Conference USCAP 2015
 Surgical Pathology Evening Specialty Conference USCAP 2015 John R. Goldblum, M.D. Chairman, Department of Pathology, Cleveland Clinic Professor of Pathology, Cleveland Clinic Lerner College of Medicine
Surgical Pathology Evening Specialty Conference USCAP 2015 John R. Goldblum, M.D. Chairman, Department of Pathology, Cleveland Clinic Professor of Pathology, Cleveland Clinic Lerner College of Medicine
Disclosure of Relevant Financial Relationships
 Surgical Pathology Companion Meeting Case 5: Locally Recurrent Chest wall Mass Cristina Antonescu, MD Department of Pathology, Memorial Sloan Kettering Cancer Center Disclosure of Relevant Financial Relationships
Surgical Pathology Companion Meeting Case 5: Locally Recurrent Chest wall Mass Cristina Antonescu, MD Department of Pathology, Memorial Sloan Kettering Cancer Center Disclosure of Relevant Financial Relationships
Selected Pseudomalignant Soft Tissue Tumors of the Skin and Subcutis
 Selected Pseudomalignant Soft Tissue Tumors of the Skin and Subcutis Andrew L. Folpe, M.D. Professor of Laboratory Medicine and Pathology Mayo Clinic, Rochester, MN folpe.andrew@mayo.edu 2016 MFMER slide-1
Selected Pseudomalignant Soft Tissue Tumors of the Skin and Subcutis Andrew L. Folpe, M.D. Professor of Laboratory Medicine and Pathology Mayo Clinic, Rochester, MN folpe.andrew@mayo.edu 2016 MFMER slide-1
Uterine mesenchymal tumors: Hereditary perspectives
 Uterine mesenchymal tumors: Hereditary perspectives Two hereditary syndromes are known to be related to uterine mesenchymal tumors: Hereditary Leiomyomatosis and Renal Cell Carcinoma syndrome (HLRCC) and
Uterine mesenchymal tumors: Hereditary perspectives Two hereditary syndromes are known to be related to uterine mesenchymal tumors: Hereditary Leiomyomatosis and Renal Cell Carcinoma syndrome (HLRCC) and
Lung Tumor Cases: Common Problems and Helpful Hints
 Lung Tumor Cases: Common Problems and Helpful Hints Brandon T. Larsen, MD, PhD Senior Associate Consultant Department of Laboratory Medicine and Pathology Mayo Clinic Arizona Arizona Society of Pathologists
Lung Tumor Cases: Common Problems and Helpful Hints Brandon T. Larsen, MD, PhD Senior Associate Consultant Department of Laboratory Medicine and Pathology Mayo Clinic Arizona Arizona Society of Pathologists
Endometrial Stromal Tumors
 Endometrial Stromal Tumors WHO Categories: Endometrial Stromal Nodule (ESN) Endometrial Stromal Sarcoma, low grade (LGESS) Endometrial Stromal Sarcoma, high grade (HGESS) Undifferentiated Uterine Sarcoma
Endometrial Stromal Tumors WHO Categories: Endometrial Stromal Nodule (ESN) Endometrial Stromal Sarcoma, low grade (LGESS) Endometrial Stromal Sarcoma, high grade (HGESS) Undifferentiated Uterine Sarcoma
Oncocytic-Appearing Salivary Gland Tumors. Oncocytic, Cystic, Mucinous, and High Grade Salivary Gland Tumors SALIVARY GLAND FNA: PART II
 William C. Faquin, MD, PhD Professor of Pathology Harvard Medical School Director of Head and Neck Pathology Massachusetts Eye and Ear Massachusetts General Hospital SALIVARY GLAND FNA: PART II Oncocytic,
William C. Faquin, MD, PhD Professor of Pathology Harvard Medical School Director of Head and Neck Pathology Massachusetts Eye and Ear Massachusetts General Hospital SALIVARY GLAND FNA: PART II Oncocytic,
Normal endometrium: A, proliferative. B, secretory.
 Normal endometrium: A, proliferative. B, secretory. Nội mạc tử cung Nội mạc tử cung Cyclic changes in endometrium.. Approximate relationship of useful microscopic changes. Arias-Stella reaction in endometrial
Normal endometrium: A, proliferative. B, secretory. Nội mạc tử cung Nội mạc tử cung Cyclic changes in endometrium.. Approximate relationship of useful microscopic changes. Arias-Stella reaction in endometrial
Case Presentation. Maha Akkawi, MD, Fatima Obeidat, MD, Tariq Aladily, MD. Department of Pathology Jordan University Hospital Amman, Jordan
 Case Presentation Maha Akkawi, MD, Fatima Obeidat, MD, Tariq Aladily, MD Department of Pathology Jordan University Hospital Amman, Jordan The 25th Annual Congress of the ADIAP The 8/11/2013 1 5th International
Case Presentation Maha Akkawi, MD, Fatima Obeidat, MD, Tariq Aladily, MD Department of Pathology Jordan University Hospital Amman, Jordan The 25th Annual Congress of the ADIAP The 8/11/2013 1 5th International
Note: The cause of testicular neoplasms remains unknown
 - In the 15- to 34-year-old age group, they are the most common tumors of men. - Tumors of the testis are a heterogeneous group of neoplasms that include: I. Germ cell tumors : 95%; all are malignant.
- In the 15- to 34-year-old age group, they are the most common tumors of men. - Tumors of the testis are a heterogeneous group of neoplasms that include: I. Germ cell tumors : 95%; all are malignant.
ESS: Pathologic Insights
 GEIS XVI INTERNATIONAL SYMPOSIUM Seville 4th October 2018 ESS: Pathologic Insights Sílvia Bagué The Royal Marsden Hospital London (United Kingdom) I have no conflicts of interest Endometrial stromal sarcoma
GEIS XVI INTERNATIONAL SYMPOSIUM Seville 4th October 2018 ESS: Pathologic Insights Sílvia Bagué The Royal Marsden Hospital London (United Kingdom) I have no conflicts of interest Endometrial stromal sarcoma
Synonyms. Nephrogenic metaplasia Mesonephric adenoma
 Nephrogenic Adenoma Synonyms Nephrogenic metaplasia Mesonephric adenoma Definition Benign epithelial lesion of urinary tract with tubular, glandular, papillary growth pattern Most frequently in the urinary
Nephrogenic Adenoma Synonyms Nephrogenic metaplasia Mesonephric adenoma Definition Benign epithelial lesion of urinary tract with tubular, glandular, papillary growth pattern Most frequently in the urinary
Disclosures. An update on ancillary techniques in the diagnosis of soft tissue tumors. Ancillary techniques. Introduction
 Disclosures An update on ancillary techniques in the diagnosis of soft tissue tumors. I have nothing to disclose. Andrew Horvai, MD, PhD Clinical Professor, Pathology Introduction Ancillary techniques
Disclosures An update on ancillary techniques in the diagnosis of soft tissue tumors. I have nothing to disclose. Andrew Horvai, MD, PhD Clinical Professor, Pathology Introduction Ancillary techniques
4/12/2018. MUSC Pathology Symposium Kiawah Island April 18, Jesse K. McKenney, MD
 MUSC Pathology Symposium Kiawah Island April 18, 2018 Jesse K. McKenney, MD 1 Urothelial Carcinoma with Alternative Differentiation 2 Urothelial Carcinoma with Alternative Differentiation Recognition as
MUSC Pathology Symposium Kiawah Island April 18, 2018 Jesse K. McKenney, MD 1 Urothelial Carcinoma with Alternative Differentiation 2 Urothelial Carcinoma with Alternative Differentiation Recognition as
Case 27 Male 42. Painless, static, well-circumscribed, subcutaneous nodule right lower leg,?lipoma. The best diagnosis is:
 Case 27 Male 42. Painless, static, well-circumscribed, subcutaneous nodule right lower leg,?lipoma. The best diagnosis is: A. Angiosarcoma B. Haemangiopericytoma C.Myopericytoma D.Myofibroma E. Angioleiomyoma
Case 27 Male 42. Painless, static, well-circumscribed, subcutaneous nodule right lower leg,?lipoma. The best diagnosis is: A. Angiosarcoma B. Haemangiopericytoma C.Myopericytoma D.Myofibroma E. Angioleiomyoma
3/24/2017 DENDRITIC CELL NEOPLASMS: HISTOLOGY, IMMUNOHISTOCHEMISTRY, AND MOLECULAR GENETICS. Disclosure of Relevant Financial Relationships
 DENDRITIC CELL NEOPLASMS: HISTOLOGY, IMMUNOHISTOCHEMISTRY, AND MOLECULAR GENETICS Jason L. Hornick, M.D., Ph.D. Director of Surgical Pathology and Immunohistochemistry Brigham and Women s Hospital Professor
DENDRITIC CELL NEOPLASMS: HISTOLOGY, IMMUNOHISTOCHEMISTRY, AND MOLECULAR GENETICS Jason L. Hornick, M.D., Ph.D. Director of Surgical Pathology and Immunohistochemistry Brigham and Women s Hospital Professor
No financial or other disclosures
 Case 2014-5 Esther N. Bit-Ivan, DO Northwestern University Jason Wang, MD Jason Park, MD Korgun Koral, MD Children s Medical Center Charles Timmons, MD Veena Rajaram, MD No financial or other disclosures
Case 2014-5 Esther N. Bit-Ivan, DO Northwestern University Jason Wang, MD Jason Park, MD Korgun Koral, MD Children s Medical Center Charles Timmons, MD Veena Rajaram, MD No financial or other disclosures
Slide seminar. Asist. Prof. Jože Pižem, MD, PhD Institute of Pathology Medical Faculty, University of Ljubljana
 Slide seminar Asist. Prof. Jože Pižem, MD, PhD Institute of Pathology Medical Faculty, University of Ljubljana Case 5 A 57-year-old man with a dermal/subcutaneous lesion on the scalp, which was interpreted
Slide seminar Asist. Prof. Jože Pižem, MD, PhD Institute of Pathology Medical Faculty, University of Ljubljana Case 5 A 57-year-old man with a dermal/subcutaneous lesion on the scalp, which was interpreted
Original Article From angiomyolipoma to malignant epithelioid angiomyolipoma of the kidney, a case report with a history of eight years
 Int J Clin Exp Med 2015;8(11):21252-21256 www.ijcem.com /ISSN:1940-5901/IJCEM0015600 Original Article From angiomyolipoma to malignant epithelioid angiomyolipoma of the kidney, a case report with a history
Int J Clin Exp Med 2015;8(11):21252-21256 www.ijcem.com /ISSN:1940-5901/IJCEM0015600 Original Article From angiomyolipoma to malignant epithelioid angiomyolipoma of the kidney, a case report with a history
Atypical Palisaded Myofibroblastoma of Lymph Node: Report of a rare case.
 ISPUB.COM The Internet Journal of Pathology Volume 10 Number 1 Atypical Palisaded Myofibroblastoma of Lymph Node: Report of a rare case. V Kinnera, R Nandyala, M Yootla, K Mandyam Citation V Kinnera, R
ISPUB.COM The Internet Journal of Pathology Volume 10 Number 1 Atypical Palisaded Myofibroblastoma of Lymph Node: Report of a rare case. V Kinnera, R Nandyala, M Yootla, K Mandyam Citation V Kinnera, R
Immunohistochemical Evaluation of Necrotic Malignant Melanomas
 Anatomic Pathology / EVALUATION OF NECROTIC MALIGNANT MELANOMAS Immunohistochemical Evaluation of Necrotic Malignant Melanomas Daisuke Nonaka, MD, Jordan Laser, MD, Rachel Tucker, HTL(ASCP), and Jonathan
Anatomic Pathology / EVALUATION OF NECROTIC MALIGNANT MELANOMAS Immunohistochemical Evaluation of Necrotic Malignant Melanomas Daisuke Nonaka, MD, Jordan Laser, MD, Rachel Tucker, HTL(ASCP), and Jonathan
An Overview of Genital Stromal Tumors
 An Overview of Genital Stromal Tumors By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchcock Medical Center Geisel School of Medicine
An Overview of Genital Stromal Tumors By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchcock Medical Center Geisel School of Medicine
Gross appearance of nodular hyperplasia in material obtained from suprapubic prostatectomy. Note the multinodular appearance and the admixture of
 Tiền liệt tuyến Tiền liệt tuyến Gross appearance of nodular hyperplasia in material obtained from suprapubic prostatectomy. Note the multinodular appearance and the admixture of solid and microcystic areas.
Tiền liệt tuyến Tiền liệt tuyến Gross appearance of nodular hyperplasia in material obtained from suprapubic prostatectomy. Note the multinodular appearance and the admixture of solid and microcystic areas.
Disclosure of Relevant Financial Relationships
 Ewing and Ewing like sarcomas Using Genetic Signatures in Refining Small Blue Round Cell Tumor Classification Cristina Antonescu, MD Department of Pathology Disclosure of Relevant Financial Relationships
Ewing and Ewing like sarcomas Using Genetic Signatures in Refining Small Blue Round Cell Tumor Classification Cristina Antonescu, MD Department of Pathology Disclosure of Relevant Financial Relationships
An unusual superficial small round cell sarcoma
 An unusual superficial small round cell sarcoma - Diagnostic problems - Differential diagnosis Antonio Llombart Bosch Isidro Machado Dep. Pathology Univ. Medical School Valencia, Institute of Oncology
An unusual superficial small round cell sarcoma - Diagnostic problems - Differential diagnosis Antonio Llombart Bosch Isidro Machado Dep. Pathology Univ. Medical School Valencia, Institute of Oncology
Differential diagnosis of hematolymphoid tumors composed of medium-sized cells. Brian Skinnider B.C. Cancer Agency, Vancouver General Hospital
 Differential diagnosis of hematolymphoid tumors composed of medium-sized cells Brian Skinnider B.C. Cancer Agency, Vancouver General Hospital Lymphoma classification Lymphoma diagnosis starts with morphologic
Differential diagnosis of hematolymphoid tumors composed of medium-sized cells Brian Skinnider B.C. Cancer Agency, Vancouver General Hospital Lymphoma classification Lymphoma diagnosis starts with morphologic
Self assessment case. Dr Saleem Taibjee Dorset County Hospital, Dorchester
 Self assessment case Dr Saleem Taibjee saleemtaibjee@gmail.com Dorset County Hospital, Dorchester Clinical details 34-year-old man: Shave excision Skin tag / papilloma left thigh The best diagnosis is:
Self assessment case Dr Saleem Taibjee saleemtaibjee@gmail.com Dorset County Hospital, Dorchester Clinical details 34-year-old man: Shave excision Skin tag / papilloma left thigh The best diagnosis is:
Gross appearance of peritoneal cysts. They have a thin, translucent wall and contain a clear fluid.
 Gross appearance of peritoneal cysts. They have a thin, translucent wall and contain a clear fluid. So-called multicystic benign mesothelioma. A, Gross appearance. So-called multicystic benign mesothelioma.
Gross appearance of peritoneal cysts. They have a thin, translucent wall and contain a clear fluid. So-called multicystic benign mesothelioma. A, Gross appearance. So-called multicystic benign mesothelioma.
Lesions Mimicking Adenoid Cystic Carcinoma. Diagnostic Problems in Salivary Gland Pathology An Update 5/29/2009
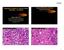 Diagnostic Problems in Salivary Gland Pathology An Update Lesions Mimicking Adenoid Cystic Carcinoma Stacey E. Mills, M.D. W.S. Royster Professor of Pathology Director of Surgical and Cytopathology University
Diagnostic Problems in Salivary Gland Pathology An Update Lesions Mimicking Adenoid Cystic Carcinoma Stacey E. Mills, M.D. W.S. Royster Professor of Pathology Director of Surgical and Cytopathology University
Pathology Mystery and Surprise
 Pathology Mystery and Surprise Tim Smith, MD Director Anatomic Pathology Medical University of South Carolina Disclosures No conflicts to declare Some problem cases Kidney tumor Scalp tumor Bladder tumor
Pathology Mystery and Surprise Tim Smith, MD Director Anatomic Pathology Medical University of South Carolina Disclosures No conflicts to declare Some problem cases Kidney tumor Scalp tumor Bladder tumor
Spinal Cord Compression caused by Metastatic Epithelial Myoepithelial Carcinoma of the Parotid Gland
 Spinal Cord Compression caused by Metastatic Epithelial Myoepithelial Carcinoma of the Parotid Gland Pages with reference to book, From 249 To 250 Irshad N. Soomro,Akber S. Hussainy,Rashida Ahmed,Sheema
Spinal Cord Compression caused by Metastatic Epithelial Myoepithelial Carcinoma of the Parotid Gland Pages with reference to book, From 249 To 250 Irshad N. Soomro,Akber S. Hussainy,Rashida Ahmed,Sheema
EPITHELIOID ANGIOMYOLIPOMA OF THE KIDNEY MIMICKING RENAL CELL CARCINOMA: A CLINICOPATHOLOGIC ANALYSIS OF CASES AND LITERATURE REVIEW
 EPITHELIOID ANGIOMYOLIPOMA OF THE KIDNEY MIMICKING RENAL CELL CARCINOMA: A CLINICOPATHOLOGIC ANALYSIS OF CASES AND LITERATURE REVIEW Chia-Chun Tsai, 1 Wen-Jeng Wu, 1,3 Ching-Chia Li, 1,3 Chii-Jye Wang,
EPITHELIOID ANGIOMYOLIPOMA OF THE KIDNEY MIMICKING RENAL CELL CARCINOMA: A CLINICOPATHOLOGIC ANALYSIS OF CASES AND LITERATURE REVIEW Chia-Chun Tsai, 1 Wen-Jeng Wu, 1,3 Ching-Chia Li, 1,3 Chii-Jye Wang,
Epithelial tumors. Dr. F.F. Khuzin, PhD Dr. M.O. Mavlikeev
 Epithelial tumors Dr. F.F. Khuzin, PhD Dr. M.O. Mavlikeev Epithelial tumors Tumors from the epithelium are the most frequent among tumors. There are 2 group features of these tumors: The presence in most
Epithelial tumors Dr. F.F. Khuzin, PhD Dr. M.O. Mavlikeev Epithelial tumors Tumors from the epithelium are the most frequent among tumors. There are 2 group features of these tumors: The presence in most
Unusual Variants of Bladder Cancer Cristina Magi-Galluzzi, MD, PhD
 Unusual Variants of Bladder Cancer Cristina Magi-Galluzzi, MD, PhD Director of Genitourinary Pathology, Professor of Pathology, Lerner College of Medicine Cleveland Clinic Objectives Update on variants
Unusual Variants of Bladder Cancer Cristina Magi-Galluzzi, MD, PhD Director of Genitourinary Pathology, Professor of Pathology, Lerner College of Medicine Cleveland Clinic Objectives Update on variants
What really matters When and Why. Pathology of Uterine Mesenchymal Lesions. Nafisa Wilkinson London
 What really matters When and Why Pathology of Uterine Mesenchymal Lesions Nafisa Wilkinson London Patient centred approach immunohistochemistry Histological diagnosis Next generation sequencing Genetic
What really matters When and Why Pathology of Uterine Mesenchymal Lesions Nafisa Wilkinson London Patient centred approach immunohistochemistry Histological diagnosis Next generation sequencing Genetic
An Overview of Cutaneous Vascular Neoplasms
 An Overview of Cutaneous Vascular Neoplasms By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchcock Medical Center Geisel School
An Overview of Cutaneous Vascular Neoplasms By Konstantinos Linos MD, FCAP, FASDP Bone, Soft Tissue and Dermatopathology Assistant Professor of Pathology Dartmouth-Hitchcock Medical Center Geisel School
The Relevance of Cytologic Atypia in Cutaneous Neural Tumors
 The Relevance of Cytologic Atypia in Cutaneous Neural Tumors Recent Findings - New Developments New Problems Zsolt B. Argenyi, M.D. Professor of Pathology & Dermatology Director of Dermatopathology Department
The Relevance of Cytologic Atypia in Cutaneous Neural Tumors Recent Findings - New Developments New Problems Zsolt B. Argenyi, M.D. Professor of Pathology & Dermatology Director of Dermatopathology Department
Mesenchymal Tumors. Cavernous Hemangioma (CH) VASCULAR TUMORS MESENCHYMAL TUMORS OF THE LIVER: WHAT S NEW AND UNUSUAL (MY PERSPECTIVE)
 Mesenchymal Tumors MESENCHYMAL TUMORS OF THE LIVER: WHAT S NEW AND UNUSUAL (MY PERSPECTIVE) CURRENT ISSUES IN ANATOMIC PATHOLOGY MAY 23, 2014 Linda Ferrell, MD, UCSF Focus on Vascular Tumors Benign and
Mesenchymal Tumors MESENCHYMAL TUMORS OF THE LIVER: WHAT S NEW AND UNUSUAL (MY PERSPECTIVE) CURRENT ISSUES IN ANATOMIC PATHOLOGY MAY 23, 2014 Linda Ferrell, MD, UCSF Focus on Vascular Tumors Benign and
Tumors of kidney and urinary bladder
 Tumors of kidney and urinary bladder Overview of kidney tumors Benign and malignant Of the benign: papillary adenoma -cortical -small (0.5cm) -in 40% of population -clinically insignificant The most common
Tumors of kidney and urinary bladder Overview of kidney tumors Benign and malignant Of the benign: papillary adenoma -cortical -small (0.5cm) -in 40% of population -clinically insignificant The most common
Molecular Diagnosis of Soft Tissue Tumors: Avoid Pitfalls
 Molecular Diagnosis of Soft Tissue Tumors: Avoid Pitfalls Cristina Antonescu, MD Department of Pathology Memorial Sloan-Kettering Cancer Center, New York Overview I. When should we rely on the help of
Molecular Diagnosis of Soft Tissue Tumors: Avoid Pitfalls Cristina Antonescu, MD Department of Pathology Memorial Sloan-Kettering Cancer Center, New York Overview I. When should we rely on the help of
Urinary Bladder: WHO Classification and AJCC Staging Update 2017
 Urinary Bladder: WHO Classification and AJCC Staging Update 2017 Houston Society of Clinical Pathologists 58 th Annual Spring Symposium Houston, TX April 8, 2017 Jesse K. McKenney, MD Classification
Urinary Bladder: WHO Classification and AJCC Staging Update 2017 Houston Society of Clinical Pathologists 58 th Annual Spring Symposium Houston, TX April 8, 2017 Jesse K. McKenney, MD Classification
Case year old female presented with asymmetric enlargement of the left lobe of the thyroid
 Case 4 22 year old female presented with asymmetric enlargement of the left lobe of the thyroid gland. No information available relative to a prior fine needle aspiration biopsy. A left lobectomy was performed.
Case 4 22 year old female presented with asymmetric enlargement of the left lobe of the thyroid gland. No information available relative to a prior fine needle aspiration biopsy. A left lobectomy was performed.
Case 4 Diagnosis 2/21/2011 TGB
 Case 4 22 year old female presented with asymmetric enlargement of the left lobe of the thyroid gland. No information available relative to a prior fine needle aspiration biopsy. A left lobectomy was performed.
Case 4 22 year old female presented with asymmetric enlargement of the left lobe of the thyroid gland. No information available relative to a prior fine needle aspiration biopsy. A left lobectomy was performed.
1 NORMAL HISTOLOGY AND METAPLASIAS
 1 NORMAL HISTOLOGY AND METAPLASIAS, MD Anatomy and Histology 1 Metaplasias 2 ANATOMY AND HISTOLOGY The female breast is composed of a branching duct system, which begins at the nipple with the major lactiferous
1 NORMAL HISTOLOGY AND METAPLASIAS, MD Anatomy and Histology 1 Metaplasias 2 ANATOMY AND HISTOLOGY The female breast is composed of a branching duct system, which begins at the nipple with the major lactiferous
Evening Specialty Conference Bone and Soft Tissue Pathology. Diagnostic pitfalls in bone and soft tissue pathology
 Evening Specialty Conference Bone and Soft Tissue Pathology. Case 1 Elizabeth G Demicco, MD, PhD Mount Sinai Hospital, New York Disclosure of Relevant Financial Relationships USCAP requires that all planners
Evening Specialty Conference Bone and Soft Tissue Pathology. Case 1 Elizabeth G Demicco, MD, PhD Mount Sinai Hospital, New York Disclosure of Relevant Financial Relationships USCAP requires that all planners
5/21/2018. Prostate Adenocarcinoma vs. Urothelial Carcinoma. Common Differential Diagnoses in Urological Pathology. Jonathan I.
 Common Differential Diagnoses in Urological Pathology Jonathan I. Epstein Prostate Adenocarcinoma vs. Urothelial Carcinoma 1 2 NKX3.1 NKX3.1 3 4 5 6 Proposed ISUP Recommendations Option to use PSA as a
Common Differential Diagnoses in Urological Pathology Jonathan I. Epstein Prostate Adenocarcinoma vs. Urothelial Carcinoma 1 2 NKX3.1 NKX3.1 3 4 5 6 Proposed ISUP Recommendations Option to use PSA as a
Molecular pathology in soft tissue tumors. Sylvia Höller Pathologie
 Molecular pathology in soft tissue tumors Sylvia Höller Pathologie When do we perform molecular testing? Morphology and IHC are not clearly fitting with an entity some translocations are entity specific
Molecular pathology in soft tissue tumors Sylvia Höller Pathologie When do we perform molecular testing? Morphology and IHC are not clearly fitting with an entity some translocations are entity specific
BSD 2015 Case 19. Female 21. Nodule on forehead. The best diagnosis is:
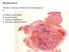 BSD 2015 Case 19 Female 21. Nodule on forehead. The best diagnosis is: A. mixed tumour of skin B. porocarcinoma C. nodular hidradenoma D. metastatic adenocarcinoma BSD 2015 Case 19 Female 21 Nodule on
BSD 2015 Case 19 Female 21. Nodule on forehead. The best diagnosis is: A. mixed tumour of skin B. porocarcinoma C. nodular hidradenoma D. metastatic adenocarcinoma BSD 2015 Case 19 Female 21 Nodule on
Contents Part I Introduction 1 General Description 2 Natural History: Importance of Size, Site, Histopathology
 Contents Part I Introduction 1 General Description... 3 1.1 Introduction... 3 1.2 Incidence and Prevalence... 5 1.3 Predisposing and Genetic Factors... 8 References... 16 2 Natural History: Importance
Contents Part I Introduction 1 General Description... 3 1.1 Introduction... 3 1.2 Incidence and Prevalence... 5 1.3 Predisposing and Genetic Factors... 8 References... 16 2 Natural History: Importance
G3.02 The malignant potential of the neoplasm should be recorded. CG3.02a
 G3.02 The malignant potential of the neoplasm should be recorded. CG3.02a Conventional adrenocortical neoplasm. Each of the below parameters is scored 0 when absent and 1 when present. 3 or more of these
G3.02 The malignant potential of the neoplasm should be recorded. CG3.02a Conventional adrenocortical neoplasm. Each of the below parameters is scored 0 when absent and 1 when present. 3 or more of these
Review of the AP Part II Practical Examination. Dr David Clift Co Chief Examiner
 Review of the AP Part II Practical Examination Dr David Clift Co Chief Examiner General Remarks The part II practical examination involved 15 cases which were presented with sufficient clinical data to
Review of the AP Part II Practical Examination Dr David Clift Co Chief Examiner General Remarks The part II practical examination involved 15 cases which were presented with sufficient clinical data to
Classification (1) Classification (3) Classification (2) Spindle cell lesions. Spindle cell lesions of bladder (Mills et al.
 Non-epithelial tumours and nonepithelial tumour-like lesions of the bladder Dr Jonathan H Shanks The Christie NHS Foundation Trust, Manchester, UK Classification (1) Myofibroblastic proliferations and
Non-epithelial tumours and nonepithelial tumour-like lesions of the bladder Dr Jonathan H Shanks The Christie NHS Foundation Trust, Manchester, UK Classification (1) Myofibroblastic proliferations and
Differential Diagnosis of Oral Masses. Palatal Lesions
 Differential Diagnosis of Oral Masses Palatal Lesions Palatal Masses Periapical Abscess Torus Palatinus Mucocele Lymphoid Hyperplasia Adenomatous Hyperplasia Benign Salivary Neoplasms Malignant Salivary
Differential Diagnosis of Oral Masses Palatal Lesions Palatal Masses Periapical Abscess Torus Palatinus Mucocele Lymphoid Hyperplasia Adenomatous Hyperplasia Benign Salivary Neoplasms Malignant Salivary
University Journal of Pre and Para Clinical Sciences
 ISSN 2455 2879 Volume 2 Issue 1 2016 Metaplastic carcinoma breast a rare case report Abstract : Metaplastic carcinoma of the breast is a rare malignancy with two distinct cell lines described as a breast
ISSN 2455 2879 Volume 2 Issue 1 2016 Metaplastic carcinoma breast a rare case report Abstract : Metaplastic carcinoma of the breast is a rare malignancy with two distinct cell lines described as a breast
the urinary system pathology Dr. Fairoz A Eltorgman
 the urinary system pathology Dr. Fairoz A Eltorgman Tumors of the renal pelvis & kidney Benign tumors of the renal pelvis: Hemangioma Leiomyoma Malignant tumors: Transitional cell carcinoma Squamous cell
the urinary system pathology Dr. Fairoz A Eltorgman Tumors of the renal pelvis & kidney Benign tumors of the renal pelvis: Hemangioma Leiomyoma Malignant tumors: Transitional cell carcinoma Squamous cell
DIAGNOSTIC SLIDE SEMINAR: PART 1 RENAL TUMOUR BIOPSY CASES
 DIAGNOSTIC SLIDE SEMINAR: PART 1 RENAL TUMOUR BIOPSY CASES Dr. Andrew J. Evans MD, PhD, FACP, FRCPC Consultant in Genitourinary Pathology University Health Network, Toronto, ON Case 1 43 year-old female,
DIAGNOSTIC SLIDE SEMINAR: PART 1 RENAL TUMOUR BIOPSY CASES Dr. Andrew J. Evans MD, PhD, FACP, FRCPC Consultant in Genitourinary Pathology University Health Network, Toronto, ON Case 1 43 year-old female,
