Methodological Development of a Clonogenic Assay to Determine Endothelial Progenitor Cell Potential
|
|
|
- Mervin Booth
- 5 years ago
- Views:
Transcription
1 Methodological Development of a Clonogenic Assay to Determine Endothelial Progenitor Cell Potential Haruchika Masuda, Cantas Alev, Hiroshi Akimaru, Rie Ito, Tomoko Shizuno, Michiru Kobori, Miki Horii, Toshiya Ishihara, Kazuya Isobe, Mitsuhiro Isozaki, Johbu Itoh, Yoshiko Itoh, Yoshinori Okada, Brendan A.S. McIntyre, Shunichi Kato, Takayuki Asahara Abstract: The precise and conceptual insight of circulating endothelial progenitor cell (EPC) kinetics is hampered by the absence of an assay system capable of evaluating the EPC differentiation cascade. An assay system for EPC colony formation was developed to delineate circulating EPC differentiation. EPC colony-forming assay using semisolid medium and single or bulk CD133 cells from umbilical cord blood exhibited the formation of two types of attaching cell colonies made of small or large cells featuring endothelial lineage potential and properties, termed small EPC colony-forming units and large EPC colony-forming units, respectively. In vitro and in vivo assays of each EPC colony-forming unit cell revealed a differentiation hierarchy from small EPC to large EPC colonies, indicating a primitive EPC stage with highly proliferative activity and a definitive EPC stage with vasculogenic properties, respectively. Experimental comparison with a conventional EPC culture assay system disclosed EPC colony-forming unit cells differentiate into noncolony-forming early EPC. The fate analysis of single CD133 cells into the endothelial and hematopoietic lineage was achieved by combining this assay system with a hematopoietic progenitor assay and demonstrated the development of colony-forming EPC and hematopoietic progenitor cells from a single hematopoietic stem cell. EPC colony-forming assay permits the determination of circulating EPC kinetics from single or bulk cells, based on the evaluation of hierarchical EPC colony formation. This assay further enables a proper exploration of possible links between the origin of EPC and hematopoietic stem cells, representing a novel and powerful tool to investigate the molecular signaling pathways involved in EPC biology. (Circ Res. 2011;109:20-37.) Key Words: clonogenic assay differentiation endothelial progenitor cell vasculogenesis Despite significant efforts in research and development with respect to endothelial progenitor cell (EPC) biology during the past 10 years after their initial isolation, 1 EPC remain a controversial topic among researchers because there is no definitive delineation of EPC, no clear differentiation hierarchy, or any unambiguously defined isolation protocol. EPC have been quantified and qualified either as cell populations identified by cell surface markers such as CD34, CD133, vascular endothelial growth factor receptor-2 (VEGFR-2), 1 8 or as adhesive cells 6,9,10 and colonies 11 using conventional EPC culture methods to produce spindle-shape adherent cells from peripheral blood (PB), bone marrow (BM), or umbilical cord blood (UCB) mononuclear cells (MNC) with endothelial growth factors and cytokines. These assays using conventional EPC culture protocols were simple and satisfactory to speculate on the vasculogenic properties of EPC-enriched fractions but have recently been criticized. These assays further group heterogeneous EPC into one qualitative category: adhesive cultured EPC without any hierarchical discrimination and proper characterization of contaminating cell populations, consisting mainly of hematopoietic cells. 12,13 Later, Ingram and Yoder et al demonstrated endothelial differential stages, high-proliferative-potential endothelial colonyforming cells (ECFC) and low-proliferative-potential ECFC and, using their original culture parameters, demonstrated clonal colony-forming units (CFU) in outgrowth EPC, cultured adhesive EPC, or differentiated endothelial cells (EC) This carefully conceived culture assay system, demonstrating thorough insight into stem cell biology, contributes significantly to the development of EPC biology via the introduction of a differential hierarchy system for EC. It, Original received September 8, 2010; revision received April 29, 2011; accepted May 3, In April 2011, the average time from submission to first decision for all original research papers submitted to Circulation Research was 15 days. From the Department of Regenerative Medicine Science, Division of Basic Clinical Science (H.M., R.I., T.S., M.K., T.I., K.I., T.A.), Department of Clinical Pharmacology, Division of Basic Clinical Science (M.I.), and Departments of Cell Transplantation & Regenerative Medicine (S.K.), the Teaching and Research Support Center (J.I., Y.I., Y.O.), Tokai University School of Medicine, Isehara, Kanagawa, Japan; the Laboratory for Early Embryogenesis (C.A., B.A.M.), RIKEN Center for Developmental Biology CDB, and the Vascular Regeneration Research Group (C.A., H.A., M.H., T.A.), Institute of Biomedical Research and Innovation IBRI, Kobe, Hyogo, Japan. Correspondence to Takayuki Asahara, Department of Regenerative Medicine Science, Division of Basic Clinical Science, Tokai University School of Medicine, Shimokasuya, Isehara, Kanagawa , Japan. asa777@is.icc.u-tokai.ac.jp 2011 American Heart Association, Inc. Circulation Research is available at DOI: /CIRCRESAHA
2 Masuda et al Endothelial Progenitor Cell Differentiation Assay 21 however, does not likely address the identification of primary circulating EPC from PB, BM, or UCB, but rather the isolation of CFU from cultured adhesive cells of tissuederived EC or EPC (Supplemental Figure I available online at Considering the necessity to create a defined assay to detect EPC qualitatively and quantitatively from primary blood samples, we have developed a new colony assay system. Our method utilizes the conventional methylcellulose assay for stem/progenitor cell identification and is modified to resemble the previously developed semisolid colony assay system for the determination of EPC-CFU The advantage of this semisolid colony assay lies in its ability to estimate the number of immature progenitors, which will go on to form colony units from a single cell, and its possibility to assess the differential quality of colonies without any contamination of differentiated cells. Furthermore, we devised a semisolid media condition capable of evaluating the adhesive potential of endothelial lineage colonies. We performed EPC colony-forming assay (CFA) similar to the one described here using c-kit /Sca-1 /lineage-negative cells as a murine EPC-enriched population and identified two distinct colony types (small and large colonies) that likely represent two distinct EPC populations, primitive (small) and definitive (large) EPC, respectively By taking the useful aspects of these former animal studies, we have established a novel adhesive clonogenic assay system for the quantitative and qualitative analysis of human EPC based on the EPC differentiation hierarchy. The methodological concepts of EPC biology were examined using EPC-CFU analysis of single cells or bulk cells from EPCenriched arbitrary fractions or nonselected populations and cell fate analysis of single cells or bulk cells after suspension conditions. Our newly introduced analytic system clarifies the cell fate of each sample cell, reviews the differentiation hierarchy of EPC, and identifies the determinants of EPC proliferation, commitment, and differentiation in vitro and in vivo. Materials and Methods Methods for each assay or analysis described are detailed in the Supplemental Materials (available online at (1) preparation of MNC and CD133 cells from UCB, PB, PB with granulocyte-colony stimulating factor mobilization (mpb), or BM; (2) protocol for EPC-CFA, bulk cell expansion (EX) culture of UCB-133, and EPC culture assay; (3) endothelial characterization of EPC-CFU by fluorescent staining; (4) harvest of colony cells from EPC-CFU; (5) FACS analysis; (6) real-time polymerase chain reaction for quantification of endothelial marker gene expression of colony cells derived from single cells of UCB-133; (7) in vitro vasculogenic assay of colony cells; (8) in vivo vasculogenic assay of colony cells in murine ischemic hind limb model; (9) single-cellbased hemato-endothelial lineage commitment assay of UCB-133, mpb-133, and BM-133; and (10) statistical analysis. Results Identification of Single-Cell-Derived EPC Colonies To evaluate and qualify single-cell-derived human EPC colonies, we used single cells from UCB-133 that were sorted into individual wells of a 96-well plate (Figure 1A). Among 60 single cells per UCB from 19 different UCB samples (1140 single cells in total), certain single UCB-133 cells Non-standard Abbreviations and Acronyms Ang 1 Ang 2 bfgf CFA CFU EC ECFC EPC HGF HPC HSC IL-10 mpb angiopoietin-1 angiopoietin-2 basic fibroblast growth factor colony forming assay colony forming unit endothelial cell endothelial colony forming cell endothelial progenitor cell hepatocyte growth factor hematopoietic progenitor cell hematopoietic stem cell interleukin-10 peripheral blood with granulocyte-colony stimulating factor mobilization PB peripheral blood PIGF placental growth factor rh-tpo recombinant human thrombopoietin rh-scf recombinant human stem cell factor SOD1 superoxide dismutase 1 UCB umbilical cord blood UCB-MNC umbilical cord blood mononuclear cell UCB-133 primary CD133 cells isolated from UCB UCB-34 primary CD34 cells isolated from UCB UCB-133EX expansion-cultured CD133 cells isolated from UCB, VEGFR-2 vascular endothelial growth factor receptor-2 developed adherent cell colonies (Figure 1B). At day 18 after plating of single UCB-133 cells, two types of colonies could be defined via light microscopy. The first type consisted of small EPC with a diameter of 10 mto20 m. The second type consisted of large EPC with a longitudinal length of 50 m to200 m, forming mainly a monolayer structure (Figure 1B). We termed the former colony a small EPC-CFU and the latter a large EPC-CFU. Both colonies took up acetylated low-density lipoprotein conjugated 1,1 -dioctadecyl- 3,3,3,3 -tetramethylindocarbocyanine perchlorate and were positive for Ulex europaeus agglutinin I conjugated FITC binding, which characterizes endothelial and monocyte lineages (Figure 1B). The expressions of the endothelial-specific genes VEGFR-2, vascular endothelial cadherin, endothelial nitric oxide synthase, endothelial-specific receptor tyrosine kinase, von Willebrand factor, and platelet/ec adhesion molecule (CD31) were detected by quantitative reverse-transcriptase polymerase chain reaction for both analyzed colony types, with large EPC-CFU showing overall higher expression levels (Figure 1C). Both colony types also expressed leukocyte/monocyte lineage marker genes CD45 and CD14, with large EPC-CFU showing lower levels of expression when compared to small EPC-CFU. The average frequencies of small EPC-CFU and large EPC-CFU were 16.8% 1.8% and 5.2% 1.0% out of single UCB-133 cells, respectively. No single UCB-133 cell producing both types of EPC colonies was detected (Figure 1C).
3 22 Circulation Research June 24, 2011 Figure 1. Endothelial progenitor cell colony-forming assay (EPC-CFA) profile derived from single cells of umbilical cord blood (UCB) A, Representative scheme of EPC-CFA derived from single cells of UCB-133. B, Left panel shows representative pictures of each EPC-CFU from single cells at different magnifications. The top, middle, and bottom pictures indicate the features of small EPC colonyforming unit (CFU) and large EPC-CFU in primary EPC-CFA at 2 high-power field (HPF; indicated by white arrowheads), 4 HPF (scale bar 250 m), and 10 HPF (scale bar 100 m). Right panel shows endothelial features of each EPC-CFU by Ulex europaeus agglutinin I conjugated FITC binding and acetylated low-density lipoprotein conjugated 1,1 -dioctadecyl- 3,3,3,3 -tetramethylindocarbocyanine perchlorate uptake (scale bar 100 m). C, Left panel shows relative values of endothelial-specific gene expressions in small EPC-CFU and large EPC-CFU vs GAPDH by quantitative reverse-transcriptase polymerase chain reaction. The bar graph shows the mean SD values (1.8 Ct (Ct1 Ct2) 10 6 ) for two independent single-cell-derived small EPC-CFU and large EPC-CFU. The bar graphs show mean SD. Right panel shows the averaged percentage frequencies of small EPC-CFU and large EPC-CFU from 60 single cells per group. Nineteen UCB samples were investigated at 60 single cells per group per UCB sample. The bar graphs show mean SEM. D, The morphological transition of single UCB-133 derived small EPC-CFU in first EPC-CFA at day 18 (top left) to second EPC derived from small EPC (small EPC32 nd EPC) reseeded at cells/35-mm dish in second EPC-CFA at day 10 (top right; scale bar 100 m). The FACS profiles show the scatter diagrams of small EPC (bottom left) and small EPC3second EPC (bottom right). To investigate the relationship between small EPC-CFU and large EPC-CFU with regard to their differentiation hierarchy, the cells harvested from small EPC-CFU (small EPC) in primary CFA were reseeded and subsequently revealed large EPC-CFU in secondary CFA after 10 days (Figure 1D). This was also confirmed via cell size transition from small to large in the forward scatter plot using FACS analysis (Figure 1D). Single-cell EPC-CFA of CD133 cells revealed differences in the overall EPC commitment frequency between the analyzed samples, with mpb showing higher frequencies of EPC colonies than BM-derived samples (Supplemental Figure II available online at albeit the distribution of each EPC-CFU type remained the same. Our findings indicate that single-cell EPC-CFA enables the
4 Masuda et al Endothelial Progenitor Cell Differentiation Assay 23 assessment of differences in endothelial commitment of single CD133 cells among hematopoietic tissues. Characterization of EPC-CFU After proving that we could generate cells of the endothelial lineage from a single cell, we further developed the methodology to quantify and qualify EPC-CFU from bulk cell populations using MNC and primary or EX-cultured CD133 cells isolated from UCB (UCB-MNC, UCB-133, and UCB-133EX, respectively) (Figure 2A, B). At day 18 after plating of UCB-MNC, UCB-133, or UCB-133EX cells, the features and numbers of adherent colonies were examined via light microscopy. As observed in single-cell EPC-CFA, two types of colonies, small EPC-CFU and large EPC-CFU, were identified and quantified (Figure 2C). Examination of the forward scatter and side scatter patterns obtained by FACS analysis of respective colony cells harvested from small EPC-CFU and large EPC-CFU (small EPC and large EPC) revealed distinct Figure 1 (Continued). differences. Small EPC harvested from primary EPC-CFA converted their morphological features to large EPC in a secondary EPC-CFA. However, large EPC from primary EPC-CFA retained their morphology. When harvesting small EPC, the frequency of small cells in UCB-133 was predominantly higher than in UCB-133EX. However, when large EPC were selectively harvested, the large cell number compared to the small cell number was enriched, especially for UCB-133EX, with the latter originally showing a higher frequency in UCB-133 (Figure 2C). Furthermore, in the secondary EPC-CFA of small EPC, the small cell number decreased and inversely the large cell number increased. In the secondary EPC-CFA of large EPC using UCB-133 or UCB-133EX, the ratio between small and large cell numbers remained similar to the number obtained in the primary EPC-CFA. To elucidate the different ratios between the two types of colonies, we performed secondary EPC-CFA of both small EPC and large EPC from UCB-133 or UCB-133EX, reseed-
5 24 Circulation Research June 24, 2011 ing the cells at escalating low cellular densities (Supplemental Figure III, Supplemental Table I, available online at These findings allow the conclusion that small EPC can functionally differentiate into large EPC, indicating the presence of a differentiation hierarchy between both cell and corresponding colony types. When examining EPC-CFA from UCB-133EX, both colony types took up acetylated low-density lipoprotein conjugated 1,1 -dioctadecyl- 3,3,3,3 -tetramethylindo-carbocyanine perchlorate, were positive for Ulex europaeus agglutinin I conjugated FITC binding, and could be stained with specific antibodies against the endothelial markers VEGFR-2, endothelial nitric oxide synthase, and vascular endothelial cadherin (Figure 2D). Figure 2. Endothelial progenitor cell colony-forming assay (EPC-CFA) to investigate the vasculogenic potential of primary or cell expansion (EX)-cultured cell populations from umbilical cord blood (UCB). A, The schematic protocol of EPC- CFA of each cell population, heterogeneous UCB mononuclear cells (MNC), isolated primary cells (UCB-133 or UCB- 34) or EX-cultured cells. Small EPC colony-forming units (CFU) and large EPC-CFU in first EPC-CFA; small EPC and large EPC, respectively, isolated from small EPC-CFU and large EPC-CFU; second EPC or second EPC-CFU from small EPC or large EPC reseeded at higher cell or lower cell density in second EPC-CFA. B, The positivity of each cell population in UCB stained with mouse antihuman antibodies for CD133 and CD34 by FACS. The upper panels show isotype controls. C, The representative features at day 18 of first or second EPC-CFA using UCB- 133 or UCB-133EX. Photos show small EPC-CFU or large EPC-CFU or both in first EPC-CFA, and then second EPC from small EPC (small EPC-CFU) or large EPC (large EPC-CFU) in second EPC- CFA (scale bars 500 m). The FACS profiles below each photo exhibit the corresponding scatter diagrams gated for the R1 and R2 areas of small and large cells. Percent values indicate the frequency of the cell populations in the adjacent gates. The diagrams of (B, C) are representative of three independent experiments. D, Endothelial characteristics of small EPC- CFU and large EPC-CFU. The left two panels show Ulex europaeus agglutinin I conjugated FITC binding and acetylated low-density lipoprotein conjugated 1,1 dioctadecyl- 3,3,3,3 -tetramethylindocarbocyanine perchlorate uptake of each EPC-CFU (scale bar 50 m). The right two panels show immunocytochemistry of the endothelial-specific antigens vascular endothelial growth factor-2, endothelial nitric oxide synthase, and vascular endothelial cadherin (scale bar 50 m). To discriminate the differential phenotype between small EPC- CFU and large EPC-CFU, functional assays examining EPC in vitro and in vivo were performed. Regarding the cell cycle status of the respective colonies, small EPC revealed high proliferative activity as compared to large EPC in the applied BrdUrd uptake assay. In UCB-133EX, the percentage of small EPC in the S-phase was 11.3% 0.2% (R2), whereas this value for large EPC was only 2.2% 0.3% (R5), as shown by FACS analysis with BrdUrd-FITC and 7-amino-actinomycin D (Figure 3A). By day 18 in semisolid culture with 500 EX-cultured cells per dish, the cell number of small EPC per colony ( ) was 9.6-fold higher than that of large EPC ( ; Figure 3A). Similar results were also ob-
6 Masuda et al Endothelial Progenitor Cell Differentiation Assay 25 Figure 2 (Continued).
7 26 Circulation Research June 24, 2011 Figure 3. Functional characteristics of small endothelial progenitor cell colonyforming units (EPC-CFU) and large EPC- CFU in vitro. Small EPC and large EPC were harvested from each CFU in first EPC colony-forming assay (CFA) using umbilical cord blood (UCB)-133EX. A, Top panel shows proliferative activity of each type of EPC by BrdUrd uptake assay for 16 hours. The scatter diagrams of each type of EPC stained by BrdUrd-FITC and 7-amino-actinomycin D show the corresponding cell cycle patterns: gated in R1/R4 for G0/G1 phase, R 2 /R5 for S-phase, and R3/R6 for G2 M-phase. Bottom panel shows percentage of S-phase of each type of EPC in top panel (n 4 UCB samples). B, Adhesion assay of each type of EPC. The top pictures are representative adhesive EPC. The adhesive cell numbers of each EPC at 10 magnification in high power field (HPF; n 6/group). C, Left panel shows in vitro tube formation assay of human umbilical vein endothelial cell cocultured with each type of EPC in Matrigel. The top picture series shows the representative features of formed tubes in each group at 4 HPF. The bottom bar graphs show the number of closed tubes in each group (n 10/group). Right panel shows incorporation assay of each type of EPC into forming tubes of human umbilical vein endothelial cells in left panel. The top pictures show the features of each type of EPC prelabeled with acetylated lowdensity lipoprotein conjugated 1,1 dioctadecyl- 3,3,3,3 -tetramethylindocarbocyanine perchlorate incorporated into and participating in the tube formation by human umbilical vein endothelial cell at 10 HPF. The arrowheads indicate incorporated EPC. The bottom graphs indicate the numbers of each incorporated EPC at 10 HPF (n 10/each group). D, Sprouting assay of each type of EPC in Matrigel. The features (top) and numbers (bottom) of sprouts by each type of EPC in 4-day long-term Matrigel assay at 10 HPF (n 10/each group). Each arrowhead indicates one sprout from the starting point of each cell or cell mass. B, C, and D, Each assay was performed in triplicate and data are shown in the representative experiment. Data are mean standard error of mean. tained for UCB-133 samples, as shown in Supplemental Figure IV(available online at These findings suggest that small EPC possess a higher proliferative activity than large EPC. In addition, the vasculogenic activities of the two colony types were evaluated using adhesion, sprouting, and tube formation assays. In adhesion assay, large EPC demonstrated 1.6-fold higher adhesive potential than small EPC ( / 10 high-power field [HPF] for small EPC vs / 10 HPF for large EPC; Figure 3B). Tube formation assay using either type of colony cells cocultured with human umbilical vein EC demonstrated that large EPC promoted significantly more tube formation at an approximate rate of 1.3-fold, along with a higher incorporation rate into tube-like structures at 16-fold compared to small EPC (Figure 3C). EPC Matrigel culture assay revealed the intensive sprouting activity of large EPC at 8.5-fold compared to small EPC ( / 10 HPF for small EPC vs / 10 HPF for large EPC; Figure 3D). These findings suggest that colonized large EPC may contribute more actively to neovascular formation by direct incorporation into vascular structures. The functional activity of each colony-forming EPC was assessed by an in vivo neovascularization assay using a murine hind limb ischemic model. The transplantation of large EPC augmented the capillary density two-fold compared to the control media group and 1.5-fold compared to small EPC administration (Figure 4A). Laser Doppler measurement demonstrated large EPC improved blood perfusion in ischemic hind limbs compared with media controls at 3 to 4 weeks, whereas small EPC did not significantly improve perfusion until 4 weeks (Figure 4B). Limb salvage recovery
8 Masuda et al Endothelial Progenitor Cell Differentiation Assay 27 Figure 3 (Continued). could be observed more frequently in the large EPC than the small EPC transplantation group (Figure 4C). Gene expression analysis using human-specific primers 2 days after EPC transplantation revealed that Ki67 is predominantly expressed in small EPC transplants. The expression of VEGF was increased in the small EPC transplanted samples, with large EPC showing an increase in the expression of the proangiogenic growth factor PlGF. Thymosin 4 was expressed at similar levels in both sample types. Endothelial-specific maker expression, including von Willebrand factor, endothelial nitric oxide synthase, and CD31, was higher in large EPC when compared with small EPC (Figure 4D, Supplemental Figure V, available online at By immunohistochemistry, small EPC were preferentially found to be close to but outside of host capillaries (Supplemental Figure VIA available online at and thus appeared to be involved in an indirect interaction with host EC leading to cooperative neovascular formation (Supplemental Figure VIB). However, large EPC were frequently found in close proximity to and rarely outside of the host vasculature possibly mediating direct cooperative vasculogenesis (Supplemental Figure VIC and D, Supplemental Video, available online at As also indicated by the results summarized in Figure 3C, small EPC may assert their proangiogenic properties via supporting paracrine effects, whereas large EPC may be characterized by their ability to contribute directly to vascular structure formation during postnatal vasculogenesis. Taken together, these findings indicate that there are at least two kinds of CFU of EPC, small EPC-CFU and large EPC-CFU. Small EPC are proliferative and likely immature, whereas large EPC are more differentiated and have greater vasculogenic potential. Methodological Development of an EPC-CFA for Bulk Cell Samples To verify the methodological reliability of the herein described EPC-CFA, colony-forming abilities of primary (UCB-133) and cultured (UCB-133EX) samples were further evaluated and compared. As demonstrated in Figure 5A and Supplemental Table II (available online at UCB- 133 developed predominantly small EPC-CFU ( in UCB-133 vs in UCB-133EX), whereas UCB-133EX inversely promoted the formation of large EPC-CFU rather than small EPC-CFU ( in UCB-133EX vs in UCB-133), with the total CFU number staying similar between UCB-133 and UCB-133EX. The CFU number count of EX-cultured cells revealed an expansion of both small EPC- and large EPC-CFU, forming cell types during the conditioning period (20.2-times and
9 28 Circulation Research June 24, times, respectively). These findings indicate that the described EPC-CFA not only enables the detection of differential phenotype changes between CFU but also allows the measurement of the expansion range as well as colony bioactivity for each CFU forming cell population during EX-culture from UCB-133 to UCB-133EX. To verify the accuracy of our applied EPC-CFA and to optimize the cell number necessary for proper evaluation, CD133 cells and MNC isolated from PB, mpb, BM, or UCB were examined dose-dependently (Figure 5B, Online Table III, available online at The colony numbers obtained in EPC-CFA revealed cell dose-dependent occurrence of colonies for each sample, despite a saturation effect when the total colony number exceeded 30 in a 35-mm dish. Further, the colony number of each sample was evaluated by Figure 4. In vivo neovascularization potential of small endothelial progenitor cells (EPC) and large EPC in a mouse ischemic hind limb model. A, The representative feature of each group (top) and the bar graph of microvascular density/mm 2 (bottom), six tissue sections/ mouse (n 10 mice/each group; scale bar 200 m). B, Blood flow perfusion analysis of ischemic hind limbs transplanted with EPC group. The representative features of ischemic hind limb in each group at 4 weeks after transplantation. The line graph represents the ratio of readable units (RU) in ischemic hind limb vs ipsilateral control (n 16/each group). Data are mean standard error of mean. C, Limb salvage score of each EPC transplantation. The upper pictures show the representative features of ischemic hind limbs. The lower graph indicates percent distribution of each limb feature per group. D, Differences between small EPC and large EPC transplants assessed by quantitative reverse-transcriptase polymerase chain reaction of angiogenic factors and endothelial lineage (end lin) markers (n 8 per group). optimizing a cell dose that resulted in an average colony number of 15 to 30. Of note, in BM-MNC and mesenchymal and stromal cells started growing at 2 weeks after culture, interfering with EPC colony formation, indicating that BM-MNC as a cell source is not applicable to EPC-CFA (Supplemental Figure VII available online at despite the fact that EPC- CFU could still be observed. There are also slight differences in the colony phenotype ratio for small EPC-CFU and large EPC-CFU between MNC and CD133 cells for each analyzed tissue or among the applied cellular doses within a cell type (Figure 5C). We speculate that these differences may be attributable to alterations in the level and type of secreted growth factors by selected vs nonselected cell populations. The aforementioned results suggest that our EPC-CFA is precise and
10 Masuda et al Endothelial Progenitor Cell Differentiation Assay 29 Figure 4 (Continued). representative in various sample measurements and can even be used to evaluate nonselected samples when the methodology is properly optimized. Interestingly, the average square area unit values of each EPC-CFU derived from UCB-MNC or UCB-133 cells were significantly higher than the values found for the other analyzed tissue types (PB, BM), indicating superior colony formation potential of UCB-derived EPC (Figure 5D, Supplemental Figure VIII, Supplemental Figure IXA, available online at Furthermore, differences between the number of large EPC-CFU of mpb-133 and PB-133, as well as differences between the square area unit values of small EPC-CFU from MNC of mpb and PB (Supplemental Figure IXB), indicate that granulocyte colony-stimulating factor may have a positive effect on the bioactivity of EPC in vivo. As shown in Supplemental Table IV (available online at each EPC-CFU of MNC and CD133 cells isolated from primary or expanded samples expressed surface markers of the endothelial and monocytic lineages.
11 30 Circulation Research June 24, 2011 Figure 5. Application of endothelial progenitor cell colony-forming assay (EPC-CFA) to quantify vasculogenic potential of stem cell populations. A, Assessment of EPC differentiation/expansion potential from stem cell populations. Left graph shows the number of EPC colony-forming units (CFU) per 250 cells of umbilical cord blood (UCB)-133 and UCB-133EX. Right graph shows the estimated number of EPC-CFU in cells of UCB-133 or whole cells of UCB-133EX from the cell number of UCB-133. B and C, Methodological evaluation of EPC-CFA in primary cell populations of hematopoietic samples (peripheral blood, peripheral blood with granulocyte colonystimulating factor mobilization, bone marrow, UCB). The top and bottom bar graphs represent values for each EPC-CFU number and percent distribution in mononuclear cells (MNC) and CD133 cells with the corresponding seeded primary cell number indicated on the x-axis, respectively. Data are means standard error of mean (n 9 per each cell population). NA indicates not available. D, EPC-CFU number per cells (per cells 5 in UCB) of MNC (left) or 500 cells of CD133 cell (right) among tissues. Evaluation of Growth Factor-Mediated Signaling for EPC Development To demonstrate the significance of the described EPC-CFA and its potential use in EPC biology, the effect of VEGF was examined using our single-cell culture conditions. Furthermore, EPC-CFA was evaluated along with and combined with a hematopoietic progenitor cell (HPC)-CFA to determine the endothelial and hematopoietic differentiation capability of a single stem cell in a hemato-endothelial lineage commitment assay. A total of 720 single cells (60 cells/group/ucb 6UCB samples) sorted from UCB-133 into each well of 96-well
12 Masuda et al Endothelial Progenitor Cell Differentiation Assay 31 Figure 5 (Continued). plates were cultured for 7 days in EX-culture medium, supplemented with rh-tpo, rh-scf, rh-il6, and rh-flt-3 ligand, with or without rh-vegf (Figure 6A, B). EX-culture media allowed single UCB-133 cells to attain similar total cell numbers irrespective of VEGF supplementation (Figure 6C). Although 32% of single UCB-133 cells from both groups did not grow, 68% of UCB-133 cells increased in number to an average rate of 120 cells over 7 days, irrespective of VEGF supplementation (Figure 6C). After these culture conditions, half of the single-cell-derived expanded cells in each well were applied to EPC-CFA and the other half were applied to HPC-CFA, respectively. Among the single-cell-derived cells cultured without VEGF, the frequency of colony development was 0.4% 0.4% for EPC only, 25.4% 3.8% for HPC only, and 24.2% 2.8% for EPC plus HPC. However, after conditioning with VEGF, the frequency of EPC only and EPC plus HPC increased (1.7% 1.4% and 36.3% 3.4%, respectively), whereas the frequency for HPC only decreased (11.7% 3.3%; Figure 6D). Notably, VEGF increased the cellular commitment to large EPC-CFU but not small EPC-CFU or mixed (small and large) EPC-CFU (Figure 6D). Correlative analysis between the cells forming EPC colo-
13 32 Circulation Research June 24, 2011 Figure 6. The application of endothelial progenitor cell colony-forming assay (EPC-CFA) for single-cell fate determination of the hemato-endothelial lineage. A, The schematic protocol of the hemato-endothelial lineage commitment assay combining EPC-CFA with hematopoietic progenitor cell (HPC) CFA for each half of the single cell expansion (EX)-cultured cells. Data are mean standard error of mean (SEM). B, The pictures represent the cells and colonies at each step of the hemato-endothelial lineage commitment assay ( 10 high-power field). C, Left graph shows representative cell numbers per EX-cultured single CD133 cell. Right graph shows percent frequencies of EX-cultured single cells to seeded single CD133 cell. D, Left graph shows percent commitment ratios of single CD133 cells into hematopoietic (Hemato Lin), endothelial (Endo Lin), and both lineages (Hemato-Endo Lin). Percent commitment frequency into Hemato Lin was estimated by counting single umbilical cord blood (UCB)-133 cells producing any colonies of burst forming uniterythroid, CFU-granulocyte macrophage, CFU-macrophage, or CFU-granulocyte erythrocyte monocyte macrophage. Right graph shows percent frequencies of small EPC colony-froming units (CFU) or large EPC-CFU in single CD133 cells. Data are mean SEM (C, left) or mean alone (C right, D; n 4 to6).
14 Masuda et al Endothelial Progenitor Cell Differentiation Assay 33 nies and HPC colonies represented an antagonistic decrease in pro-hpc expanding populations when conditioned with VEGF (Figure 6D). These single-cell culture results indicated the presence of populations that can give rise to both endothelial and hematopoietic progenitors in vitro at a ratio of 24% without VEGF or 36% with VEGF. Furthermore, it demonstrated that VEGF enhanced EPC differentiation and interfered with HPC differentiation. Our EPC-CFA thus can function as a suitable assay to define and test factors and signaling molecules (Supplemental Figure XA, B available online at possibly involved in EPC proliferation, commitment, and differentiation. The hemato-endothelial lineage commitment assay was also effectively used to analyze the differences in hematoendothelial lineage commitment among different hematopoietic cell sources (Supplemental Figure XIA, B, available online at Comparison of EPC-CFA With Conventional EPC Culture Assay We compared and analyzed the relationship of EPC-CFU in EPC-CFA with early EPC, the equivalent phenotype of EC-like cells, circulating angiogenic cells, or CFU-EC detected in conventional EPC culture assay. First, we evaluated the differences in the cell populations giving rise to the aforementioned cells or colonies. Cells isolated from UCB-MNC using antibodies against CD14 and CD133 were examined by EPC-CFA and EPC culture assay. As shown in Supplemental Figure XII (available online at there was almost no double positivity of CD133 and CD14 in UCB- MNC. Early EPC were produced only from CD14 /CD133 (CD14 ) fractions, but not from CD14 /CD133 or CD14 /CD133 (CD133 ) in EPC culture assay. Conversely, the CD14 /CD133 (CD133 ) fraction produced small and large EPC-CFU in EPC-CFA, and the other fractions did not. These findings correspond to the reports by Rhode et al, 24 in which cultured CD14 fractions produce EC-like cells as early EPC, and CFU-EC are formed of CD14 cells derived from circulating angiogenic cells aggregating with CD14 cells in culture. We further followed the changes in positivity of CD133/ CD14 in small EPC-CFU cells (described in Supplemental Methods available online at during the period of EPC-CFA. Positivity for CD133 declined exponentially and reached almost zero at day18 of EPC-CFA; inversely, CD14 levels increased dramatically after 7 days and reached their maximum at day 18 (Supplemental Figure XIIIA available online at Conventional EPC culture assays using small EPC-CFU cells isolated at the differential time points during EPC-CFA disclosed an increase in early EPC productivity along the applied time course. This finding suggests that differentiating initial CD133 cells, possible CD14 cells, in small EPC-CFU may give rise to early EPC (Supplemental Figure XIIIB). We then investigated the FACS profiles of subsequent cultured EPC from small EPC at day 18 in EPC-CFA, exhibiting the presence of primary markers labeling both EC and monocyte lineage cells similar to early EPC or circulating angiogenic cells found in EPC culture assays from UCB-MNC (Supplemental Figure XIV available online at which was similar to the FACS profiles of small EPC and large EPC in selectively harvested EPC (Supplemental Figure XV available online at Additionally, the morphological features among cultured EPC of primary UCB- MNC, noncolonizing EPC from large EPC in second EPC-CFA, and cultured EPC in secondary EPC culture assay from small EPC were quite similar (Supplemental Figure XVI available online at In this regard, we define that colony-forming EPC are derived from CD133 cells and give rise to small EPC-CFU and large EPC-CFU, whereas noncolony-forming EPC express CD14 and have an early EPC phenotype. These findings indicate that the EPC colony-forming potential declines along with the decrease in CD133 positivity, whereas inversely the producing activity of early EPC augments along with the increase in CD14 positivity because of the progress of EPC differentiation from CD133 cells in EPC-CFA. In this regard, an early EPC arising during EPC culture assay likely reflects a differentiating phenotype of an EPC-CFU cell, which it derives from, and thus is not likely related to, the original cell population giving rise to EPC-CFU. Discussion Establishment of a Novel Clonogenic Assay System for Immature EPC The study of EPC has been obscured by the lack of an authoritative and clear definition of EPC. This, in turn, was caused by the lack of a proper and unambiguous EPC assay. Among the EPC assays used over the years, the most reliable assay was the colony assay for endothelial outgrowth cell (EOC) culture, developed by Ingram and Yoder 15 (Supplemental Figure I). The EOC assay demonstrated adhesive endothelial potential at different stages, high-proliferative-potential ECFC and low-proliferative-potential ECFC, revealing clonal CFU in outgrowth EPC, cultured adhesive EPC, or differentiated EC. The EOC is convenient for basic research applications because of its stable profile and reproducibility. However, it does not likely relate to the identification of a primary EPC phenotype from PB, BM, or UCB, but rather to the isolation of CFU from cultured attached cells, which in turn are based on tissue-derived EC or EPC. In this regard, we have established a novel clonogenic assay system for the quantitative and qualitative analysis of primary EPC of the endothelial lineage (Supplemental Figure I). First, the assay was designed to identify EPC-CFU from single cells. After the described isolation and culture conditions, we succeeded in the generation and characterization of EPC colonies from single UCB-133 cells. Furthermore, EPC-CFU could be detected not only from single cells but also from bulk cells of EPC-enriched populations (ie, CD34 or CD133 cells), arbitrary fractions (ie, lineage-negative cells), or nonselected populations (ie, total MNC of PB, or UCB). The capability to measure the EPC-CFU number from bulk cells opens the practical opportunity to quantify reproducibly EPC-CFU from experimental and clinical samples using this assay system. As mentioned, many laboratories have tried conventional EPC assays for clinical samples and
15 34 Circulation Research June 24, 2011 Figure 7. The classification of endothelial progenitor cell (EPC) differentiation detected by EPC colony-forming assay (CFA). EPC-CFA identifies the EPC differentiation hierarchy by the difference of morphological and functional properties after the transition from a nonadhesive to an adhesive phenotype. basic research but found them to be rather incomplete and insufficient for a fundamental and proper quantification and qualification of EPC. In this respect, the methodological applicability and usefulness of the introduced assay could be further proven by its capability for dose-dependent CFU isolation using dose-escalated cell samples of total MNC form PB and UCB. Two Types of EPC-CFU Most importantly, using our described approach, two different types of EPC-CFU derived from a single CD133 cell could be identified, termed small EPC and large EPC, respectively (Figure 7). These progenitor cell-derived populations can form colonies, which are positive for endothelial markers such as VEGFR-2, endothelial nitric oxide synthase, vascular endothelial cadherin, von Willebrand factor, endothelial-specific receptor tyrosine kinase, and CD31. Small EPC reveal a predominant potential for proliferation, showing an overall higher frequency of the S-phase cell-cycle state. Small EPC also give rise to secondary large EPC colonies when reseeded. Large EPC demonstrate a predominantly vasculogenic potential including cell adhesion and tube-like structure formation in vitro, as well as a high in vivo activity toward de novo blood vessel formation after transplantation into a murine ischemic hind limb model, as compared to small EPC. Large EPC did not form secondary colonies but gave rise to isolated endothelial lineage cells when reseeded. Given these findings, small EPC colony-forming cells can be seen as primitive EPC, which are possibly derived from further immature and proliferative EPC, and large EPC colony-forming cells can be seen as definitive-epc, which are more prone to differentiate and promote EPC-mediated cell functions required for vasculogenesis (Figure 7, Supplemental Table V available online at The definitive large EPC are capable to differentiate into a noncolonizing large EPC phenotype, similar to early EPC or circulating angiogenic cells derived via conventional EPC culture and are speculated to represent further differentiated EPC. Approximately 6% of primitive small EPC colony-forming cells and 3% of definitive large EPC colonyforming cells among enriched UCB-EPC populations (UCB-34 or UCB-133) could be observed, whereas only 0.048% of primitive small EPC colony-forming cells and 0.042% of definitive large EPC colony-forming cells among nonenriched UCB-MNC were detected. Being able to detect these two distinct types of EPC enables us to realize our initial objective of a precise and unbiased quantification and qualification of circulating EPC from clinical and biological samples. HSC-Derived EPC and HPC Based on the evidence that hematopoietic stem cells (HSC) and EPC 29,30 are derived from a common precursor (hemangioblast) during embryogenesis, 31,32 the existence of a hemangioblast in the adult has been suggested using in vitro single-cell culture systems utilizing common surface markers shared between EPC and HSC, such as CD34, VEGFR-2, or CD133, as shown for human PB-CD133 or UCB-CD34 / VEGFR-2 and BM-CD34 /VEGFR-2 subsets. 3 5,33 36 However, the specific isolation of a postnatal hemangioblast and the characterization of its origin and biological properties have not been successful thus far. In this article, we have demonstrated that our newly introduced EPC-CFA, in combination with an HPC-CFA, allows for further insights into the origin and properties of hematopoiesis and vasculogenesis in adults. A single UCB-CD133 cell could give rise to simultaneous EPC and HPC colonies at the frequency of 36.3% using specific culture conditions. This finding regarding the frequency of single cells to commit into both EPC and HPC is somewhat surprising. The fact that the majority of the HSC population committed mainly into both HPC and EPC, or HPC alone, but not into EPC alone, indicates that the origin of EPC may be represented by a subset of immature hemangioblastic HSC and that EPC commitment from these cells is closely related to the emergence and formation of HPC. Of note, each CD133 cell exhibited different colony-
16 Masuda et al Endothelial Progenitor Cell Differentiation Assay 35 forming patterns of no EPC-CFU, only small EPC-CFU, or only large EPC-CFU. These findings suggest that even among a scarce cell population like the one expressing the CD133 surface antigen, heterogeneity regarding the ability of EPC commitment exists. The regulatory link between EPC and HPC needs to be further investigated to be able to generate a complete hierarchy illustrating the origin and emergence of these cells, potentially resulting in novel insights not only for vascular biology but also for the hematopoietic system. Colony-Forming and Noncolony-Forming Hematopoietic EPC Because large EPC demonstrate a predominantly vasculogenic potential and do not form secondary colonies but rather give rise to isolated EC-like cells when reseeded, the definitive EPC are capable of differentiating into a noncolonizing large EPC phenotype, similar to early EPC derived via conventional EPC culture methods, and could possibly represent further differentiating fledged EPC departed from the nest of colony-forming EPC. 26,27,37 The widely used EPC culture assays are characterized by the appearance of attached endothelial lineage cells after the conditioning of PB or BM mononuclear cells with endothelial growth factor-supplemented media. 26,27,38 These cultured EPC assay systems have been frequently used by researches as cell sources for cultured EPC, 10,26,27,37,39 EC-like cells, 40 early EPC, 39,4113 and circulating angiogenic cells. 39,42 Therefore, experiments to elucidate the relationship of EPC-CFU in EPC-CFA with early EPC, equivalent phenotype of EC-like cells, circulating angiogenic cells, or CFU-EC detected in conventional EPC culture assay have been performed. The comparative experiments indicate that: colony-forming EPC that give rise to small EPC-CFU and large EPC-CFU are enriched in CD133 (CD133 /CD14 ) cells, whereas early EPC are derived from CD14 (CD133 / CD14 ) cells; EPC colony-forming potential declines along with the decrease in CD133 positivity and, inversely, the producing activity of early EPC augments along with the increase in CD14 during the progress of EPC differentiation from CD133 stem cells in EPC-CFA; and noncolonyforming EPC derived from EPC-CFU express the equivalent phenotype with early EPC. In this regard, we define that colony-forming EPC are derived from CD133 cells and give rise to small EPC-CFU and large EPC-CFU, whereas noncolony-forming EPC express CD14 cells and are identified as early EPC phenotype. These findings indicate that the EPC colony-forming potential declines along with decrease in CD133 positivity, inversely producing activity of early EPC augments along with increase in CD14 during the progress of EPC differentiation from CD133 stem cells in EPC-CFA. These findings also recall that myeloid EPC, suggested by Bailey et al, 43 may be equivalent to the early EPC phenotype derived from EPC-CFU primarily expressing CD45 and CD14, although the relationship between colony-forming EPC and myeloid progenitor cells needs to be clarified. Regarding the relationship of early EPC with EOC, equivalent to ECFC or late EPC, both are considered to be generated from different stem cell fractions as previously reported. 44 Later studies by Case et al 45 and Timmermans et al 40 demonstrated that CD34 CD133 VEGFR-2 or CD133 cells are hematopoietic progenitor cells that do not yield EOC. These findings support the idea that EOC are not likely to derive from HSC or HPC originating from the BM. To address any relationship between EPC-CFU cells and EOC, we performed ECFC culture known to give rise to EOC colonies according to the protocol by Ingram et al; 46 however, EOC colonies could not be detected from three samples of CD34 cell-depleted or CD133 cell-depleted UCB-MNC, whereas total UCB-MNC did. Although it cannot be conclusively ruled out for the moment, the primary and original phenotypes of EPC-CFU and early EPC seem to differ from EOC. Quest for Molecular and Signaling Mechanisms in EPC Biology EPC-CFA, alone or combined with HPC-CFA, encourages the quest for the identification of novel factors and signaling molecules involved in EPC and HSC biology. A single UCB-133 cell developed a cell population that gave rise to both EPC and HPC colonies at a frequency of 36.3% after culture with VEGF and of 24.2% without VEGF. Furthermore, VEGF enhanced EPC commitment with an increase in definitive large EPC-CFU (18.3% vs 7.5%) and enhanced an inhibition in HPC commitment (11.7% vs 25.4%), suggesting that VEGF is a determining factor involved in EPC commitment and is capable of changing the balanced cell fate between EPC and HPC present in immature HSC populations. In this regard, we speculate that the herein described EPC-CFA will function simply and precisely as a valuable assay to detect further determining factors that may regulate EPC and HPC proliferation or commitment, advancing vascular and hematopoietic biology. Standardization of EPC-CFA Although our EPC-CFA was demonstrated to be simple and representative, one still needs to carefully examine and consider several points to achieve a proper quantification and qualification of EPC-CFU. First, the cell number to be examined should be standardized before the main test. A saturation effect was achieved when the total colony number exceeded 30 in a 35-mm dish. Therefore, the optimal sample cell number for EPC-CFA needs to be determined and set to an average colony number of 15 to 30. This has to be performed not only for the reflection of the individual variations between biological and clinical samples but also for the simple consideration of the diversity of mutual interactions in mixed cell populations used for analysis. As an example, gross differences in the colony phenotype ratio for small and large EPC-CFU between primary UCB-133 cells and UCB-MNC make such an adjustment indispensable (Figure 5C). Furthermore, EPC-CFA worked flawlessly for UCB-MNC and PB-MNC, albeit differences in the optimal cell number between the samples could be observed. The observed variability can be accounted for by the overall sample-specific frequency of EPC-CFU and the alterations in the secretion and presence of certain growth factors in the analyzed mixed cell populations. Taken together, the herein introduced EPC-CFA is precise and representative of a variety of
CD34 + VEGFR-3 + progenitor cells have a potential to differentiate towards lymphatic endothelial cells
 CD34 + VEGFR-3 + progenitor cells have a potential to differentiate towards lymphatic endothelial cells Tan YZ et al. J Cell Mol Med. (2014 Mar;18(3):422-33) Denise Traxler-Weidenauer April 2014 Introduction
CD34 + VEGFR-3 + progenitor cells have a potential to differentiate towards lymphatic endothelial cells Tan YZ et al. J Cell Mol Med. (2014 Mar;18(3):422-33) Denise Traxler-Weidenauer April 2014 Introduction
CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow
 White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
DISCOVERING ATCC IMMUNOLOGICAL CELLS - MODEL SYSTEMS TO STUDY THE IMMUNE AND CARDIOVASCULAR SYSTEMS
 DISCOVERING ATCC IMMUNOLOGICAL CELLS - MODEL SYSTEMS TO STUDY THE IMMUNE AND CARDIOVASCULAR SYSTEMS James Clinton, Ph.D. Scientist, ATCC February 19, 2015 About ATCC Founded in 1925, ATCC is a non-profit
DISCOVERING ATCC IMMUNOLOGICAL CELLS - MODEL SYSTEMS TO STUDY THE IMMUNE AND CARDIOVASCULAR SYSTEMS James Clinton, Ph.D. Scientist, ATCC February 19, 2015 About ATCC Founded in 1925, ATCC is a non-profit
PROTOCOLS AND MANUFACTURING FOR CELL-BASED THERAPIES
 SM PROTOCOLS AND MANUFACTURING FOR CELL-BASED THERAPIES Departments of a Regenerative Medicine Science, f Physiology, and g Cell Transplantation and Regenerative Medicine, Tokai University School of Medicine,
SM PROTOCOLS AND MANUFACTURING FOR CELL-BASED THERAPIES Departments of a Regenerative Medicine Science, f Physiology, and g Cell Transplantation and Regenerative Medicine, Tokai University School of Medicine,
Chronic variable stress activates hematopoietic stem cells
 SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
CD34 Hybrid Cells Promote Endothelial Colony-Forming Cell Bioactivity and Therapeutic Potential for Ischemic Diseases
 CD34 Hybrid Cells Promote Endothelial Colony-Forming Cell Bioactivity and Therapeutic Potential for Ischemic Diseases Jun Hee Lee, Sang Hun Lee, So Young Yoo, Takayuki Asahara, Sang Mo Kwon Downloaded
CD34 Hybrid Cells Promote Endothelial Colony-Forming Cell Bioactivity and Therapeutic Potential for Ischemic Diseases Jun Hee Lee, Sang Hun Lee, So Young Yoo, Takayuki Asahara, Sang Mo Kwon Downloaded
Focus on biological identity of endothelial progenitors cells
 European Review for Medical and Pharmacological Sciences Focus on biological identity of endothelial progenitors cells 2015; 19: 4047-4063 V. ZACCONE 1, R. FLORE 1, L. SANTORO 1, G. DE MATTEIS 1, B. GIUPPONI
European Review for Medical and Pharmacological Sciences Focus on biological identity of endothelial progenitors cells 2015; 19: 4047-4063 V. ZACCONE 1, R. FLORE 1, L. SANTORO 1, G. DE MATTEIS 1, B. GIUPPONI
Meeting Report. From December 8 to 11, 2012 at Atlanta, GA, U.S.A
 Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
SDF-1/CXCR4 Axis on Endothelial Progenitor Cells Regulates Bone Fracture Healing
 SDF-1/CXCR4 Axis on Endothelial Progenitor Cells Regulates Bone Fracture Healing Yohei Kawakami, M.D., Ph.D. 1,2, Masaaki Ii 3, Tomoyuki Matsumoto, M.D., Ph.D. 1, Astuhiko Kawamoto, M.D., Ph.D. 2, Yutaka
SDF-1/CXCR4 Axis on Endothelial Progenitor Cells Regulates Bone Fracture Healing Yohei Kawakami, M.D., Ph.D. 1,2, Masaaki Ii 3, Tomoyuki Matsumoto, M.D., Ph.D. 1, Astuhiko Kawamoto, M.D., Ph.D. 2, Yutaka
Stem cell related, postnatal neovascularization requires
 Pivotal Role of Lnk Adaptor Protein in Endothelial Progenitor Cell Biology for Vascular Regeneration Sang-Mo Kwon,* Takahiro Suzuki,* Atsuhiko Kawamoto, Masaaki Ii, Masamichi Eguchi, Hiroshi Akimaru, Mika
Pivotal Role of Lnk Adaptor Protein in Endothelial Progenitor Cell Biology for Vascular Regeneration Sang-Mo Kwon,* Takahiro Suzuki,* Atsuhiko Kawamoto, Masaaki Ii, Masamichi Eguchi, Hiroshi Akimaru, Mika
Human Peripheral Blood-Derived CD31 Cells Have Robust Angiogenic and Vasculogenic Properties and Are Effective for Treating Ischemic Vascular Disease
 Journal of the American College of Cardiology Vol. 56, No. 7, 2010 2010 by the American College of Cardiology Foundation ISSN 0735-1097/$36.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2010.01.070
Journal of the American College of Cardiology Vol. 56, No. 7, 2010 2010 by the American College of Cardiology Foundation ISSN 0735-1097/$36.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2010.01.070
Nature Immunology: doi: /ni.3412
 Supplementary Figure 1 Gata1 expression in heamatopoietic stem and progenitor populations. (a) Unsupervised clustering according to 100 top variable genes across single pre-gm cells. The two main cell
Supplementary Figure 1 Gata1 expression in heamatopoietic stem and progenitor populations. (a) Unsupervised clustering according to 100 top variable genes across single pre-gm cells. The two main cell
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Haematopoietic stem cells
 Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
Getting to the root of Cancer
 Cancer Stem Cells: Getting to the root of Cancer Dominique Bonnet, Ph.D Senior Group Leader, Haematopoietic Stem Cell Laboratory Cancer Research UK, London Research Institute Venice, Sept 2009 Overview
Cancer Stem Cells: Getting to the root of Cancer Dominique Bonnet, Ph.D Senior Group Leader, Haematopoietic Stem Cell Laboratory Cancer Research UK, London Research Institute Venice, Sept 2009 Overview
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
PhD THESIS Epigenetic mechanisms involved in stem cell differentiation
 Romanian Academy Institute of Cellular Biology and Pathology "Nicolae Simionescu" PhD THESIS Epigenetic mechanisms involved in stem cell differentiation Coordinator: Acad. Maya Simionescu PhD Student:
Romanian Academy Institute of Cellular Biology and Pathology "Nicolae Simionescu" PhD THESIS Epigenetic mechanisms involved in stem cell differentiation Coordinator: Acad. Maya Simionescu PhD Student:
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features
 Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Nasser Aghdami MD., PhD
 CONTRIBUTON OF HUMAN INDUCED PLURIPOTENT STEM CELL DERIVED ENDOTHELIAL CELLS IN VASCULAR REGENERATION OF BLEOMYCIN-INDUCED SCLERODERMA MOUSE MODEL Nasser Aghdami MD., PhD Department of Regenerative Medicine
CONTRIBUTON OF HUMAN INDUCED PLURIPOTENT STEM CELL DERIVED ENDOTHELIAL CELLS IN VASCULAR REGENERATION OF BLEOMYCIN-INDUCED SCLERODERMA MOUSE MODEL Nasser Aghdami MD., PhD Department of Regenerative Medicine
BCR-ABL - LSK BCR-ABL + LKS - (%)
 Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Stem cells: units of development and regeneration. Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research.
 Stem cells: units of development and regeneration Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research Concepts 1. Embryonic vs. adult stem cells 2. Hematopoietic stem
Stem cells: units of development and regeneration Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research Concepts 1. Embryonic vs. adult stem cells 2. Hematopoietic stem
Research Article An In Vitro Study of Differentiation of Hematopoietic Cells to Endothelial Cells
 Bone Marrow Research Volume 2011, Article ID 846096, 9 pages doi:10.1155/2011/846096 Research Article An In Vitro Study of Differentiation of Hematopoietic Cells to Endothelial Cells Qi Ru Wang, 1 Bao
Bone Marrow Research Volume 2011, Article ID 846096, 9 pages doi:10.1155/2011/846096 Research Article An In Vitro Study of Differentiation of Hematopoietic Cells to Endothelial Cells Qi Ru Wang, 1 Bao
Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice
 Research article Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice Eric J. Marrotte, 1 Dan-Dan Chen, 1,2 Jeffrey S. Hakim, 1 and Alex
Research article Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice Eric J. Marrotte, 1 Dan-Dan Chen, 1,2 Jeffrey S. Hakim, 1 and Alex
Nature Medicine: doi: /nm.2109
 HIV 1 Infects Multipotent Progenitor Cells Causing Cell Death and Establishing Latent Cellular Reservoirs Christoph C. Carter, Adewunmi Onafuwa Nuga, Lucy A. M c Namara, James Riddell IV, Dale Bixby, Michael
HIV 1 Infects Multipotent Progenitor Cells Causing Cell Death and Establishing Latent Cellular Reservoirs Christoph C. Carter, Adewunmi Onafuwa Nuga, Lucy A. M c Namara, James Riddell IV, Dale Bixby, Michael
Financial Disclosures. Bone Marrow Mononuclear Cells. Cell Based Therapies: What Do They Do and Will They Work in CLI?
 Cell Based Therapies: What Do They Do and Will They Work in CLI? None Financial Disclosures Michael P. Murphy, MD The Vascular and Cardiac Adult Stem Cell Therapy Center Indiana University School of Medicine
Cell Based Therapies: What Do They Do and Will They Work in CLI? None Financial Disclosures Michael P. Murphy, MD The Vascular and Cardiac Adult Stem Cell Therapy Center Indiana University School of Medicine
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb3399 a b c d FSP DAPI 5mm mm 5mm 5mm e Correspond to melanoma in-situ Figure a DCT FSP- f MITF mm mm MlanaA melanoma in-situ DCT 5mm FSP- mm mm mm mm mm g melanoma in-situ MITF MlanaA mm mm
DOI:.38/ncb3399 a b c d FSP DAPI 5mm mm 5mm 5mm e Correspond to melanoma in-situ Figure a DCT FSP- f MITF mm mm MlanaA melanoma in-situ DCT 5mm FSP- mm mm mm mm mm g melanoma in-situ MITF MlanaA mm mm
Hematopoiesis. - Process of generation of mature blood cells. - Daily turnover of blood cells (70 kg human)
 Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
Cytokines, adhesion molecules and apoptosis markers. A comprehensive product line for human and veterinary ELISAs
 Cytokines, adhesion molecules and apoptosis markers A comprehensive product line for human and veterinary ELISAs IBL International s cytokine product line... is extremely comprehensive. The assays are
Cytokines, adhesion molecules and apoptosis markers A comprehensive product line for human and veterinary ELISAs IBL International s cytokine product line... is extremely comprehensive. The assays are
sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5
 sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels
 Manuscript EMBO-2011-78371 Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels Hisamichi Naito, Hiroyasu Kidoya, Susumu Sakimoto, Taku
Manuscript EMBO-2011-78371 Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels Hisamichi Naito, Hiroyasu Kidoya, Susumu Sakimoto, Taku
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
Automated and Standardized Counting of Mouse Bone Marrow CFU Assays
 Automated and Standardized Counting of Mouse Bone Marrow CFU Assays 2 Automated and Standardized Colony Counting Table of Contents 4 Colony-Forming Unit (CFU) Assays for Mouse Bone Marrow 5 Automated Assay
Automated and Standardized Counting of Mouse Bone Marrow CFU Assays 2 Automated and Standardized Colony Counting Table of Contents 4 Colony-Forming Unit (CFU) Assays for Mouse Bone Marrow 5 Automated Assay
Supplemental Information. Granulocyte-Monocyte Progenitors and. Monocyte-Dendritic Cell Progenitors Independently
 Immunity, Volume 47 Supplemental Information Granulocyte-Monocyte Progenitors and Monocyte-endritic ell Progenitors Independently Produce Functionally istinct Monocytes lberto Yáñez, Simon G. oetzee, ndre
Immunity, Volume 47 Supplemental Information Granulocyte-Monocyte Progenitors and Monocyte-endritic ell Progenitors Independently Produce Functionally istinct Monocytes lberto Yáñez, Simon G. oetzee, ndre
Bone marrow derived cells in the tumour microenvironment contain cells with primitive haematopoietic phenotype
 J. Cell. Mol. Med. Vol 14, No 7, 2010 pp. 1946-1952 Bone marrow derived cells in the tumour microenvironment contain cells with primitive haematopoietic phenotype Erika Deak a, b, Stephan Göttig a, Brigitte
J. Cell. Mol. Med. Vol 14, No 7, 2010 pp. 1946-1952 Bone marrow derived cells in the tumour microenvironment contain cells with primitive haematopoietic phenotype Erika Deak a, b, Stephan Göttig a, Brigitte
Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity
 Research article Related Commentary, page 22 Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity Amie S. Corbin, 1,2 Anupriya Agarwal, 1 Marc Loriaux,
Research article Related Commentary, page 22 Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity Amie S. Corbin, 1,2 Anupriya Agarwal, 1 Marc Loriaux,
Hematopoiesis. BHS Liège 27/1/2012. Dr Sonet Anne UCL Mont-Godinne
 Hematopoiesis BHS Liège 27/1/2012 Dr Sonet Anne UCL Mont-Godinne Hematopoiesis: definition = all the phenomenons to produce blood cells Leukocytes = White Blood Cells Polynuclear = Granulocytes Platelet
Hematopoiesis BHS Liège 27/1/2012 Dr Sonet Anne UCL Mont-Godinne Hematopoiesis: definition = all the phenomenons to produce blood cells Leukocytes = White Blood Cells Polynuclear = Granulocytes Platelet
Nature Immunology: doi: /ni Supplementary Figure 1. Huwe1 has high expression in HSCs and is necessary for quiescence.
 Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Review. Critical Reevaluation of Endothelial Progenitor Cell Phenotypes for Therapeutic and Diagnostic Use
 Review This Review is part of a thematic series on Stem Cells, which includes the following articles: Stem Cells Review Series: An Introduction [Circ Res. 2011;109:907 909] Biomaterials to Enhance Stem
Review This Review is part of a thematic series on Stem Cells, which includes the following articles: Stem Cells Review Series: An Introduction [Circ Res. 2011;109:907 909] Biomaterials to Enhance Stem
Flow Cytometry. What is flow cytometry?
 Flow Cytometry Flow Cytometry What is flow cytometry? Flow cytometry is a popular laser-based technology to analyze the characteristics of cells or particles. It is predominantly used to measure fluorescence
Flow Cytometry Flow Cytometry What is flow cytometry? Flow cytometry is a popular laser-based technology to analyze the characteristics of cells or particles. It is predominantly used to measure fluorescence
Chapter 6. Villous Growth
 Core Curriculum in Perinatal Pathology Chapter 6 Villous Growth Overview of vasculogenesis and angiogenesis Vasculogenesis Extraembryonic Vasculogenesis Angiogenesis Branching angiogenesis Sprouting angiogenesis
Core Curriculum in Perinatal Pathology Chapter 6 Villous Growth Overview of vasculogenesis and angiogenesis Vasculogenesis Extraembryonic Vasculogenesis Angiogenesis Branching angiogenesis Sprouting angiogenesis
Bone Marrow Stroma in Myelodysplastic Syndromes
 Bone Marrow Stroma in Myelodysplastic Syndromes Universidad de Salamanca Prof Mª M Consuelo del Cañizo Hematology Dept. University Hospital, Salamanca SPAIN Bone marrow stroma in MDS Introduction Mesenchymal
Bone Marrow Stroma in Myelodysplastic Syndromes Universidad de Salamanca Prof Mª M Consuelo del Cañizo Hematology Dept. University Hospital, Salamanca SPAIN Bone marrow stroma in MDS Introduction Mesenchymal
The Role of Microenvironment in the Control of Tumor Angiogenesis
 The Role of Microenvironment in the Control of Tumor Angiogenesis Domenico Ribatti The Role of Microenvironment in the Control of Tumor Angiogenesis Domenico Ribatti Department of Basic Medical Sciences,
The Role of Microenvironment in the Control of Tumor Angiogenesis Domenico Ribatti The Role of Microenvironment in the Control of Tumor Angiogenesis Domenico Ribatti Department of Basic Medical Sciences,
SUPPLEMENTARY INFORMATION
 a. Smo+/+ b. Smo+/+ 5.63 5.48 c. Lin- d. e. 6 5 4 3 Ter119 Mac B T Sca1 Smo+/+ 25 15 2 o BMT 2 1 5 * Supplementary Figure 1: Deletion of Smoothened does not alter the frequency of hematopoietic lineages
a. Smo+/+ b. Smo+/+ 5.63 5.48 c. Lin- d. e. 6 5 4 3 Ter119 Mac B T Sca1 Smo+/+ 25 15 2 o BMT 2 1 5 * Supplementary Figure 1: Deletion of Smoothened does not alter the frequency of hematopoietic lineages
REGENERATIVE MEDICINE
 REGENERATIVE MEDICINE Concise Review: Circulating Endothelial Progenitor Cells for Vascular Medicine TAKAYUKI ASAHARA, a,b ATSUHIKO KAWAMOTO, b HARUCHIKA MASUDA a a Department of Regenerative Medicine
REGENERATIVE MEDICINE Concise Review: Circulating Endothelial Progenitor Cells for Vascular Medicine TAKAYUKI ASAHARA, a,b ATSUHIKO KAWAMOTO, b HARUCHIKA MASUDA a a Department of Regenerative Medicine
Hematopoiesis. Hematopoiesis. Hematopoiesis
 Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Supplement Material. Spleen weight (mg) LN cells (X106) Acat1-/- Acat1-/- Mouse weight (g)
 Supplement Material A Spleen weight (mg) C Mouse weight (g) 1 5 1 2 9 6 3 2 5 2 1 5 Male LN cells (X16) 4 ** ** Female B 3 2 1 Supplemental Figure I. Spleen weight (A), Inguinal lymph node (LN) cell number
Supplement Material A Spleen weight (mg) C Mouse weight (g) 1 5 1 2 9 6 3 2 5 2 1 5 Male LN cells (X16) 4 ** ** Female B 3 2 1 Supplemental Figure I. Spleen weight (A), Inguinal lymph node (LN) cell number
CD34 + Cells Represent Highly Functional Endothelial Progenitor Cells in Murine Bone Marrow
 CD34 + Cells Represent Highly Functional Endothelial Progenitor Cells in Murine Bone Marrow Junjie Yang 1,2, Masaaki Ii 3, Naosuke Kamei 1,4, Cantas Alev 1,5, Sang-Mo Kwon 6, Atsuhiko Kawamoto 1, Hiroshi
CD34 + Cells Represent Highly Functional Endothelial Progenitor Cells in Murine Bone Marrow Junjie Yang 1,2, Masaaki Ii 3, Naosuke Kamei 1,4, Cantas Alev 1,5, Sang-Mo Kwon 6, Atsuhiko Kawamoto 1, Hiroshi
Diabetes mellitus is associated with both an increased risk
 Human Endothelial Progenitor Cells From Type II Diabetics Exhibit Impaired Proliferation, Adhesion, and Incorporation Into Vascular Structures Oren M. Tepper, BA; Robert D. Galiano, MD; Jennifer M. Capla,
Human Endothelial Progenitor Cells From Type II Diabetics Exhibit Impaired Proliferation, Adhesion, and Incorporation Into Vascular Structures Oren M. Tepper, BA; Robert D. Galiano, MD; Jennifer M. Capla,
Supplemental Figures Supplemental Figure 1:
 Supplemental Figures Supplemental Figure 1: Representative FACS data showing Concurrent Brain cell type Acquisition using either Percoll PLUS (top row) or myelin removal beads (bottom two rows). Debris
Supplemental Figures Supplemental Figure 1: Representative FACS data showing Concurrent Brain cell type Acquisition using either Percoll PLUS (top row) or myelin removal beads (bottom two rows). Debris
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
Supplementary Figure 1. Characterization of basophils after reconstitution of SCID mice
 Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Strikingly Different Angiogenic Properties of Endothelial Progenitor Cell Subpopulations
 Journal of the American College of Cardiology Vol. 51,. 6, 2008 2008 by the American College of Cardiology Foundation ISSN 0735-1097/08/$34.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2007.09.059
Journal of the American College of Cardiology Vol. 51,. 6, 2008 2008 by the American College of Cardiology Foundation ISSN 0735-1097/08/$34.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2007.09.059
THE INFLUENCE OF SODIUM FLUORIDE ON THE CLONOGENECITY OF HUMAN HEMATOPOIETIC PROGENITOR CELLS: PRELIMINARY REPORT
 168 Fluoride Vol. 33 No. 4 168-173 2 Research Report THE INFLUENCE OF SODIUM FLUORIDE ON THE CLONOGENECITY OF HUMAN HEMATOPOIETIC PROGENITOR CELLS: PRELIMINARY REPORT Boguslaw Machaliński, a Maria Zejmo,
168 Fluoride Vol. 33 No. 4 168-173 2 Research Report THE INFLUENCE OF SODIUM FLUORIDE ON THE CLONOGENECITY OF HUMAN HEMATOPOIETIC PROGENITOR CELLS: PRELIMINARY REPORT Boguslaw Machaliński, a Maria Zejmo,
Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic
 Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic differentiation of acute myeogenouse leukemia cells that is enhanced by 1,25-dihydroxyviatmin D 3. To the Editor: Sunitinib,
Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic differentiation of acute myeogenouse leukemia cells that is enhanced by 1,25-dihydroxyviatmin D 3. To the Editor: Sunitinib,
CHAPTER 3 LABORATORY PROCEDURES
 CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
Supplementary. limb. bars
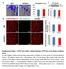 Figure 1. CD163 -/- mice exhibit a similar phenotype ass WT mice in the absence of ischemic injury. a, Laser Doppler analysiss with perfusion quantitation at baseline (n= =10 per group). b, Immunostaining
Figure 1. CD163 -/- mice exhibit a similar phenotype ass WT mice in the absence of ischemic injury. a, Laser Doppler analysiss with perfusion quantitation at baseline (n= =10 per group). b, Immunostaining
CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells
 CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells Karin Pfisterer, Karoline M Lipnik, Erhard Hofer and Adelheid Elbe-Bürger Journal of Investigative Dermatology (2015)
CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells Karin Pfisterer, Karoline M Lipnik, Erhard Hofer and Adelheid Elbe-Bürger Journal of Investigative Dermatology (2015)
TITLE: Properties of Leukemia Stem Cells in a Novel Model of CML Progression to Lymphoid Blast Crisis
 AD Award Number: W81XWH-05-1-0608 TITLE: Properties of Leukemia Stem Cells in a Novel Model of CML Progression to Lymphoid Blast Crisis PRINCIPAL INVESTIGATOR: Craig T. Jordan, Ph.D. CONTRACTING ORGANIZATION:
AD Award Number: W81XWH-05-1-0608 TITLE: Properties of Leukemia Stem Cells in a Novel Model of CML Progression to Lymphoid Blast Crisis PRINCIPAL INVESTIGATOR: Craig T. Jordan, Ph.D. CONTRACTING ORGANIZATION:
SUPPLEMENTARY INFORMATION
 1. Supplementary Figures and Legends Supplementary Fig. 1. S1P-mediated transcriptional regulation of integrins expressed in OP/monocytoid cells. Real-time quantitative PCR analyses of mrna for two integrins,
1. Supplementary Figures and Legends Supplementary Fig. 1. S1P-mediated transcriptional regulation of integrins expressed in OP/monocytoid cells. Real-time quantitative PCR analyses of mrna for two integrins,
ABSTRACT. Professor James M. Hagberg, Ph.D. Department of Kinesiology
 ABSTRACT Title of Document: EFFECTS OF CARDIOVASCULAR DISEASE AND PHYSICAL INACTIVITY ON THE PARACRINE FUNCTION OF CIRCULATING ANGIOGENIC CELLS. Rian Q. Landers-Ramos, Doctor of Philosophy, 2015 Directed
ABSTRACT Title of Document: EFFECTS OF CARDIOVASCULAR DISEASE AND PHYSICAL INACTIVITY ON THE PARACRINE FUNCTION OF CIRCULATING ANGIOGENIC CELLS. Rian Q. Landers-Ramos, Doctor of Philosophy, 2015 Directed
Frontiers of Science Foundation Scholar. Toby Bothwell. Freshman, University of Arkansas. final research manuscripts
 Frontiers of Science Foundation Scholar Toby Bothwell Oklahoma city, OKlahoma Freshman, University of Arkansas final research manuscripts The Effect of Estradiol and Estrogen Receptors α and β on Bone
Frontiers of Science Foundation Scholar Toby Bothwell Oklahoma city, OKlahoma Freshman, University of Arkansas final research manuscripts The Effect of Estradiol and Estrogen Receptors α and β on Bone
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS. Choompone Sakonwasun, MD (Hons), FRCPT
 ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
Supplemental Material
 Supplemental Material Supplementary Fig. 1. EETs stimulate primary tumor growth. a) Schematic presentation of genetic and pharmacological tools used to manipulate endogenous EET levels. b) Endothelial
Supplemental Material Supplementary Fig. 1. EETs stimulate primary tumor growth. a) Schematic presentation of genetic and pharmacological tools used to manipulate endogenous EET levels. b) Endothelial
Production of Exosomes in a Hollow Fiber Bioreactor
 Production of Exosomes in a Hollow Fiber Bioreactor John J S Cadwell, President and CEO, FiberCell Systems Inc INTRODUCTION Exosomes are small lipid membrane vesicles (80-120 nm) of endocytic origin generated
Production of Exosomes in a Hollow Fiber Bioreactor John J S Cadwell, President and CEO, FiberCell Systems Inc INTRODUCTION Exosomes are small lipid membrane vesicles (80-120 nm) of endocytic origin generated
VUmc Basispresentatie
 Clinical diagnostic cytometry Gerrit J Schuurhuis Dept of Hematology VU University Medical Center Amsterdam, Netherlands Use of immunophenotyping at diagnosis to trace residual disease after therapy 1.
Clinical diagnostic cytometry Gerrit J Schuurhuis Dept of Hematology VU University Medical Center Amsterdam, Netherlands Use of immunophenotyping at diagnosis to trace residual disease after therapy 1.
ENDOTHELIAL PROGENITOR CELLS AS A NOVEL BIOMARKER OF VASCULAR HEALTH
 ENDOTHELIAL PROGENITOR CELLS AS A NOVEL BIOMARKER OF VASCULAR HEALTH I Jialal, MD, PhD. FRCPath.DABCC Robert E. Stowell Chair in Experimental Pathology Professor of Pathology and Medicine Director, Laboratory
ENDOTHELIAL PROGENITOR CELLS AS A NOVEL BIOMARKER OF VASCULAR HEALTH I Jialal, MD, PhD. FRCPath.DABCC Robert E. Stowell Chair in Experimental Pathology Professor of Pathology and Medicine Director, Laboratory
Iliac Crest: The Gold Standard
 Iliac Crest: The Gold Standard Iliac crest is often considered the gold standard for harvesting. The iliac crest contains bone marrow which is a rich source of regenerative cells, including: Endothelial
Iliac Crest: The Gold Standard Iliac crest is often considered the gold standard for harvesting. The iliac crest contains bone marrow which is a rich source of regenerative cells, including: Endothelial
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2638 Figure S1 Morphological characteristics of fetal testes and ovaries from 6.5-20 developmental weeks. Representative images of Hematoxylin and Eosin staining of testes and ovaries over
DOI: 10.1038/ncb2638 Figure S1 Morphological characteristics of fetal testes and ovaries from 6.5-20 developmental weeks. Representative images of Hematoxylin and Eosin staining of testes and ovaries over
Chapter 7 Conclusions
 VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
pro-b large pre-b small pre-b CCCP (µm) Rag1 -/- ;33.C9HCki
 a TMRM FI (Median) b TMRM FI (Median) c 20 15 10 5 0 8 6 4 2 0 pro-b large pre-b small pre-b 0 10 20 30 40 50 60 70 80 90 100 TMRM (nm) pro-b large pre-b small pre-b 0 1 2 4 8 16 32 64 128 256 CCCP (mm)
a TMRM FI (Median) b TMRM FI (Median) c 20 15 10 5 0 8 6 4 2 0 pro-b large pre-b small pre-b 0 10 20 30 40 50 60 70 80 90 100 TMRM (nm) pro-b large pre-b small pre-b 0 1 2 4 8 16 32 64 128 256 CCCP (mm)
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
Tissue renewal and Repair. Nisamanee Charoenchon, PhD Department of Pathobiology, Faculty of Science
 Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
PBS Class #2 Introduction to the Immune System part II Suggested reading: Abbas, pgs , 27-30
 PBS 803 - Class #2 Introduction to the Immune System part II Suggested reading: Abbas, pgs. 15-25, 27-30 Learning Objectives Compare and contrast the maturation of B and T lymphocytes Compare and contrast
PBS 803 - Class #2 Introduction to the Immune System part II Suggested reading: Abbas, pgs. 15-25, 27-30 Learning Objectives Compare and contrast the maturation of B and T lymphocytes Compare and contrast
Mesenchymal Stem Cells to Repair Vascular Damage after Chemotherapy: Past, Present and Future
 Mesenchymal Stem Cells to Repair Vascular Damage after Chemotherapy: Past, Present and Future Cell Therapy 2014 Las Vegas, NV, USA Sulaiman Al-Hashmi, PhD Sultan Qaboos University Oman What are MSCs? Stem
Mesenchymal Stem Cells to Repair Vascular Damage after Chemotherapy: Past, Present and Future Cell Therapy 2014 Las Vegas, NV, USA Sulaiman Al-Hashmi, PhD Sultan Qaboos University Oman What are MSCs? Stem
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
Additional file 6. Summary of experiments characterizing development of adaptive immune response
 Additional file 6. Summary of experiments characterizing development of adaptive immune response Tests performed to evaluate the development of adaptive immunity in patients are summarized in Table 1.
Additional file 6. Summary of experiments characterizing development of adaptive immune response Tests performed to evaluate the development of adaptive immunity in patients are summarized in Table 1.
Culture and Characterization of Endothelial Colony Forming Cells from Peripheral Blood, Bone Marrow, and Umbilical Cord Blood of Horses
 Culture and Characterization of Endothelial Colony Forming Cells from Peripheral Blood, Bone Marrow, and Umbilical Cord Blood of Horses by Margaret Marie Salter A Thesis submitted to the Graduate Faculty
Culture and Characterization of Endothelial Colony Forming Cells from Peripheral Blood, Bone Marrow, and Umbilical Cord Blood of Horses by Margaret Marie Salter A Thesis submitted to the Graduate Faculty
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature11463 %Sox17(+) 9 8 7 6 5 4 3 2 1 %Sox17(+) #Sox17(+) d2 d4 d6 d8 d1 d12 d14 d18 25 2 15 1 5 Number of Sox17(+) cells X 1 Supplementary Figure 1: Expression of
SUPPLEMENTARY INFORMATION doi:1.138/nature11463 %Sox17(+) 9 8 7 6 5 4 3 2 1 %Sox17(+) #Sox17(+) d2 d4 d6 d8 d1 d12 d14 d18 25 2 15 1 5 Number of Sox17(+) cells X 1 Supplementary Figure 1: Expression of
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 Award Number: W81XWH-15-1-0314 TITLE: Osteoimmune Mechanisms of Segmental Bone Fracture Healing and Therapy PRINCIPAL INVESTIGATOR: Selvarangan Ponnazhagan, Ph.D. CONTRACTING ORGANIZATION: The University
Award Number: W81XWH-15-1-0314 TITLE: Osteoimmune Mechanisms of Segmental Bone Fracture Healing and Therapy PRINCIPAL INVESTIGATOR: Selvarangan Ponnazhagan, Ph.D. CONTRACTING ORGANIZATION: The University
Quantification of early stage lesions for loss of p53 should be shown in the main figures.
 Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Reviews. This Review is part of a thematic series on Cellular Therapy, which includes the following articles:
 Reviews This Review is part of a thematic series on Cellular Therapy, which includes the following articles: The Stem Cell Movement Genetic Enhancement of Stem Cell Engraftment, Survival, and Efficacy
Reviews This Review is part of a thematic series on Cellular Therapy, which includes the following articles: The Stem Cell Movement Genetic Enhancement of Stem Cell Engraftment, Survival, and Efficacy
Endothelial PGC 1 - α 1 mediates vascular dysfunction in diabetes
 Endothelial PGC-1α mediates vascular dysfunction in diabetes Reporter: Yaqi Zhou Date: 04/14/2014 Outline I. Introduction II. Research route & Results III. Summary Diabetes the Epidemic of the 21st Century
Endothelial PGC-1α mediates vascular dysfunction in diabetes Reporter: Yaqi Zhou Date: 04/14/2014 Outline I. Introduction II. Research route & Results III. Summary Diabetes the Epidemic of the 21st Century
VWF other roles than hemostasis. Summary 1: VWF & hemostasis synthesis 11/4/16. Structure/function relationship & functions kDa.
 VWF other roles than hemostasis Len$ng PJ, Casari C et al JTH 2012 Summary 1: VWF & hemostasis synthesis Structure/function relationship & functions (HMWM) 20.000kDa multimerization propeptide FVIII GPIb
VWF other roles than hemostasis Len$ng PJ, Casari C et al JTH 2012 Summary 1: VWF & hemostasis synthesis Structure/function relationship & functions (HMWM) 20.000kDa multimerization propeptide FVIII GPIb
Supplementary Figures
 Supplementary Figures Supplementary Fig. 1. Galectin-3 is present within tumors. (A) mrna expression levels of Lgals3 (galectin-3) and Lgals8 (galectin-8) in the four classes of cell lines as determined
Supplementary Figures Supplementary Fig. 1. Galectin-3 is present within tumors. (A) mrna expression levels of Lgals3 (galectin-3) and Lgals8 (galectin-8) in the four classes of cell lines as determined
Accelerate Your Research with Conversant Bio
 Accelerate Your Research with Conversant Bio 400+ Participating MDs 50+ Partner sites for tissue procurement Continuous expansion of sourcing capabilities Closely monitored chain of custody Full regulatory
Accelerate Your Research with Conversant Bio 400+ Participating MDs 50+ Partner sites for tissue procurement Continuous expansion of sourcing capabilities Closely monitored chain of custody Full regulatory
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Pleiotrophin Regulates the Expansion and Regeneration of Hematopoietic Stem Cells Heather A Himburg 1, Garrett G Muramoto 1 *, Pamela Daher 1*, Sarah K Meadows 1, J. Lauren Russell
SUPPLEMENTARY INFORMATION Pleiotrophin Regulates the Expansion and Regeneration of Hematopoietic Stem Cells Heather A Himburg 1, Garrett G Muramoto 1 *, Pamela Daher 1*, Sarah K Meadows 1, J. Lauren Russell
Assigning B cell Maturity in Pediatric Leukemia Gabi Fragiadakis 1, Jamie Irvine 2 1 Microbiology and Immunology, 2 Computer Science
 Assigning B cell Maturity in Pediatric Leukemia Gabi Fragiadakis 1, Jamie Irvine 2 1 Microbiology and Immunology, 2 Computer Science Abstract One method for analyzing pediatric B cell leukemia is to categorize
Assigning B cell Maturity in Pediatric Leukemia Gabi Fragiadakis 1, Jamie Irvine 2 1 Microbiology and Immunology, 2 Computer Science Abstract One method for analyzing pediatric B cell leukemia is to categorize
No option-patients : Is angiogenesis with gene or cell therapy still an option?
 No option-patients : Is angiogenesis with gene or cell therapy still an option? Professor Sigrid Nikol Clinical and Interventional Angiology Asklepios-Klinik St. Georg Hamburg, Germany Angiogenic gene
No option-patients : Is angiogenesis with gene or cell therapy still an option? Professor Sigrid Nikol Clinical and Interventional Angiology Asklepios-Klinik St. Georg Hamburg, Germany Angiogenic gene
SUPPLEMENTARY INFORMATION doi: /nature12026
 doi:1.138/nature1226 a 4 35 3 MCSF level (pg/ml) 25 2 15 1 5 1h3 3h 5h 7h 15h 24h b MPP (CD135 KSL) HSC (CD34 CD15 KSLF) c % 4 ** LPS 3 GFP pos cells 2 PU.1 GFP LPS 1 FSCA Ctl NI 24h LPS Sup.Fig.1 Effect
doi:1.138/nature1226 a 4 35 3 MCSF level (pg/ml) 25 2 15 1 5 1h3 3h 5h 7h 15h 24h b MPP (CD135 KSL) HSC (CD34 CD15 KSLF) c % 4 ** LPS 3 GFP pos cells 2 PU.1 GFP LPS 1 FSCA Ctl NI 24h LPS Sup.Fig.1 Effect
Supplemental Information. Tissue Myeloid Progenitors Differentiate. into Pericytes through TGF-b Signaling. in Developing Skin Vasculature
 Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
There was a report about endothelial progenitor cells
 Characterization of Two Types of Endothelial Progenitor Cells and Their Different Contributions to Neovasculogenesis Jin Hur, Chang-Hwan Yoon, Hyo-Soo Kim, Jin-Ho Choi, Hyun-Jae Kang, Kyung-Kook Hwang,
Characterization of Two Types of Endothelial Progenitor Cells and Their Different Contributions to Neovasculogenesis Jin Hur, Chang-Hwan Yoon, Hyo-Soo Kim, Jin-Ho Choi, Hyun-Jae Kang, Kyung-Kook Hwang,
Targeting tumour associated macrophages in anti-cancer therapies. Annamaria Gal Seminar Series on Drug Discovery Budapest 5 January 2018
 Targeting tumour associated macrophages in anti-cancer therapies Annamaria Gal Seminar Series on Drug Discovery Budapest 5 January 2018 Macrophages: Professional phagocytes of the myeloid lineage APC,
Targeting tumour associated macrophages in anti-cancer therapies Annamaria Gal Seminar Series on Drug Discovery Budapest 5 January 2018 Macrophages: Professional phagocytes of the myeloid lineage APC,
115 Endothelial Progenitor Cells: A Novel Laboratory-Based Biomarker of Vascular Health. Ishwarlal Jialal
 115 Endothelial Progenitor Cells: A Novel Laboratory-Based Biomarker of Vascular Health Ishwarlal Jialal 211 Annual Meeting Las Vegas, NV AMERICAN SOCIETY FOR CLINICAL PATHOLOGY 33 W. Monroe, Ste. 16 Chicago,
115 Endothelial Progenitor Cells: A Novel Laboratory-Based Biomarker of Vascular Health Ishwarlal Jialal 211 Annual Meeting Las Vegas, NV AMERICAN SOCIETY FOR CLINICAL PATHOLOGY 33 W. Monroe, Ste. 16 Chicago,
Extended Mammosphere Culture of Human Breast Cancer Cells
 Extended Mammosphere Culture of Human Breast Cancer Cells Application Note The PromoCell 3D Tumorsphere Medium XF The PromoCell 3D Tumorsphere Medium XF has been designed to meet your requirements for
Extended Mammosphere Culture of Human Breast Cancer Cells Application Note The PromoCell 3D Tumorsphere Medium XF The PromoCell 3D Tumorsphere Medium XF has been designed to meet your requirements for
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
CLINICAL USE OF CELLULAR SUBPOPULATION ANALYSIS IN BM
 CLINICAL USE OF CELLULAR SUBPOPULATION ANALYSIS IN BM CANCER RESEARCH CENTRE, UNIVERSITY AND UNIVERSITY HOSPITAL OF SALAMANCA (SPAIN)( Sao Paulo, 18th of April, 2009 IDENTIFICATION OF HPC (I) 1.- In vivo
CLINICAL USE OF CELLULAR SUBPOPULATION ANALYSIS IN BM CANCER RESEARCH CENTRE, UNIVERSITY AND UNIVERSITY HOSPITAL OF SALAMANCA (SPAIN)( Sao Paulo, 18th of April, 2009 IDENTIFICATION OF HPC (I) 1.- In vivo
well for 2 h at rt. Each dot represents an individual mouse and bar is the mean ±
 Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
Review Article Introduction to next generation of endothelial progenitor cell therapy: a promise in vascular medicine
 Am J Transl Res 2015;7(3):411-421 www.ajtr.org /ISSN:1943-8141/AJTR0004722 Review Article Introduction to next generation of endothelial progenitor cell therapy: a promise in vascular medicine Dewi Sukmawati
Am J Transl Res 2015;7(3):411-421 www.ajtr.org /ISSN:1943-8141/AJTR0004722 Review Article Introduction to next generation of endothelial progenitor cell therapy: a promise in vascular medicine Dewi Sukmawati
Eosinophils are required. for the maintenance of plasma cells in the bone marrow
 Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
