Structural requirements for 5-HT 2A and 5-HT 1A serotonin receptor potentiation by the biologically active lipid oleamide
|
|
|
- Susan Williamson
- 5 years ago
- Views:
Transcription
1 Proc. Natl. Acad. Sci. USA Vol. 95, pp , April 1998 Chemistry Structural requirements for 5-HT 2A and 5-HT 1A serotonin receptor potentiation by the biologically active lipid oleamide DALE L. BOGER*, JEAN E. PATTERSON, AND QING JIN Department of Chemistry and the Skaggs Institute for Chemical Biology, The Scripps Research Institute, North Torrey Pines Road, La Jolla, CA Communicated by Julius Rebek Jr., The Scripps Research Institute, La Jolla, CA, January 28, 1998 (received for review October 7, 1997) ABSTRACT Oleamide is an endogenous fatty acid primary amide that possesses sleep-inducing properties in animals and that has been shown to effect serotonergic receptor responses and block gap junction communication. Herein, the potentiation of the 5-HT 1A receptor response is disclosed, and a study of the structural features of oleamide required for potentiation of the 5-HT 2A and 5-HT 1A response to serotonin (5-HT) is described. Of the naturally occurring fatty acids, the primary amide of oleic acid (oleamide) is the most effective at potentiating the 5-HT 2A receptor response. The structural features required for activity were found to be highly selective. The presence, position, and stereochemistry of the 9 -cis double bond is required, and even subtle structural variations reduce or eliminate activity. Secondary or tertiary amides may replace the primary amide but follow a well defined relationship requiring small amide substituents, suggesting that the carboxamide serves as a hydrogen bond acceptor but not donor. Alternative modifications at the carboxamide as well as modifications of the methyl terminus or the hydrocarbon region spanning the carboxamide and double bond typically eliminate activity. A less extensive study of the 5-HT 1A potentiation revealed that it is more tolerant and accommodates a wider range of structural modifications. An interesting set of analogs was identified that inhibit rather than potentiate the 5-HT 2A, but not the 5-HT 1A, receptor response, further suggesting that such analogs may permit the selective modulation of serotonin receptor subtypes and even have opposing effects on the different subtypes. Oleamide (1) is an endogenous fatty acid primary amide that accumulates in the cerebrospinal fluid under conditions of sleep deprivation (1 3) and induces physiological sleep in animals (1). Structure 1 Consistent with its role as a prototypical member of a new class of biological signaling molecules, enzymatic regulation of the endogenous concentrations of oleamide has been described (1, 4 7) or proposed (8). Fatty acid amide hydrolase (FAAH) is an integral membrane protein that degrades 1 to oleic acid, and potent inhibitors of the enzyme have been detailed (4, 9 11). The characterization and neuronal distribution of FAAH have been disclosed (5 7), and the enzyme was found to possess the ability to hydrolyze a range of fatty acid amides including anandamide, which serves as an endogenous ligand for the cannabinoid receptor (12, 13). Unlike anandamide, an appealing feature of this new class of biological signaling agents is the The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C solely to indicate this fact by The National Academy of Sciences $ PNAS is available online at http: primary amide, suggesting that their storage and release may be controlled in a manner analogous to that of peptide hormones terminating in a primary amide (8). Recent studies have shown the oleamide modulates serotonergic neurotransmission (14, 15). In the first disclosure of such effects, oleamide was shown to potentiate 5-HT 2C and 5-HT 2A receptor-mediated chloride currents in transfected frog oocytes but not those elicited by the 5-HT 3 ion-gated channel receptor or other G protein-coupled receptors (14). This potentiation was greatest for the 5-HT 2C receptor subtype where the effect was observed at concentrations as low as 1 nm and was maximal at 100 nm oleamide. Oleamide did not alter the serotonin (5-HT) EC 50 but instead increased receptor efficacy. Similarly, oleamide has been reported to potentiate phosphoinositide hydrolysis in rat pituitary P11 cells expressing the 5-HT 2 receptor but to inhibit 5-HT 7 receptor-mediated stimulation of camp levels in HeLa cells transfected with the receptor (15). In these efforts, oleamide was shown to act as a weak agonist at the 5-HT 7 receptor but to behave as an unsurmountable antagonist in the presence of serotonin illustrating that it may act at an allosteric site. Thus, oleamide has been shown to enhance (5-HT 2A, 5-HT 2C ), disrupt (5-HT 7 ), or have no effect (5-HT 3 ) on serotonergic signal transduction at various receptor subtypes. Serotonin receptors have been implicated in anxiety, depression, appetite, and thermoregulation as well as sleep and mood regulation, and strong links between 5-HT 1, 5-HT 2, and 5-HT 7 and the regulation of sleep have been disclosed (16, 17). Herein we describe a study that defines the features of oleamide required for potentiation of the 5-HT 2A receptor response and report the analogous but more tolerant potentiation of the 5-HT 1A receptor, which has not been previously examined. A set of analogs that inhibit rather than potentiate the 5-HT 2A, but not 5-HT 1A, receptor response was identified suggesting such agents may permit selective modulation of serotonin receptor subtypes or even have opposing effects on the different subtypes. MATERIALS AND METHODS Materials. The analogs examined were purchased (Sigma, Pfaltz & Bauer, Aldrich), prepared as described (3, 4, 18), or synthesized following protocols previously detailed (3, 4, 18, 19). Methods. The assays were conducted with R-SAT kits (Receptor Technologies, Winooski, VT) containing NIH 3T3 cells expressing the rat 5-HT 2A receptor (20, 21) or RAT-1 cells expressing the human 5-HT 1A receptor (22, 23) cotransfected with the -galactosidase gene and were performed according to the procedures provided (24, 25). The cells in DMEM containing serotonin (100 nm) and the analogs (500 nm) were incubated in a humidified 5% CO 2 incubator at 37 C for 4 or 5 days for the 5-HT 2A and 5-HT 1A transfected cells, Abbreviations: 5-HT, serotonin (5-hydroxytryptamine); FAAH, fatty acid amide hydrolase. *To whom reprint requests should be addressed at: Department of Chemistry, The Scripps Research Institute, North Torrey Pines Road, BCC-483, La Jolla, CA boger@scripps.edu. 4102
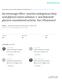
2 Chemistry: Boger et al. Proc. Natl. Acad. Sci. USA 95 (1998) 4103 respectively. Levels of -galactosidase were measured after incubation with the chromogenic substrate o-nitrophenyl- - D-galactopyranoside (26) at 30 C in a humidified incubator for a recommended period of time, and the absorbance was measured at 405 nm. The results were normalized to 100% for oleamide (relative % potentiation) for ease of comparison and are the average of 2 8 determinations. RESULTS The effects of oleamide and its analogs on rat 5-HT 2A and human 5-HT 1A receptors were examined by using R-SATtransfected cellular assays linked to a colorimetric -galactosidase assay (24 26), which provide results identical to those derived from second messenger assays. Activation of the 5-HT 2A or 5-HT 1A receptors in 5-HT-dependent cell lines results in cell proliferation measured by the levels of -galactosidase produced. Analogous to the findings of Huidobro- Toro and Harris (14), treatment with oleamide alone had no effect, but its coadministration with 5-HT provided a significant potentiation of the effect of 5-HT administration alone. A maximal response with the rat 5-HT 2A receptor was observed at 100 nm oleamide when assayed at 100 nm or 1 M 5-HT, the concentrations at which the potentiation response (165 and 170%, respectively) was greatest. At higher concentrations of oleamide (1 M), no additional potentiation was observed with the rat 5-HT 2A receptor (Table 1A). Similarly, the maximal potentiation for human 5-HT 1A was observed at 100 nm 5-HT (Fig. 1), and concentrations as low as 1 10 nm oleamide produced a measurable effect (Table 1B). The maximum effect was observed at concentrations of 100 nm 5-HT, and oleamide treatment provided a 370% (100 nm oleamide) or 560% (1 M oleamide) potentiation approximating the magnitude observed with the 5-HT 2C receptor (14). An extensive series of agents was tested at 500 nm for their ability to potentiate the rat 5-HT 2A or human 5-HT 1A receptor response to 100 nm 5-HT. This spans the concentration range (100 nm 1 M) in which oleamide exhibits its greatest potentiation and employs a 5-HT concentration at which the effect was found to be largest. The concentrations employed in the study are well within physiologically relevant concentrations. Serotonin levels in human cerebrospinal fluid (3.3 ng ml), plasma (3.4 ng ml), and platelets (748 ng 10 9 platelets) translate into nm concentrations (27), which could be much higher at the synapse, and oleamide levels in human plasma (31.7 g ml, 110 M) (28) and mouse neuroblastoma N 18 TG 2 cells (1.5 g 10 9 cells, ca. 100 the concentration of anandamide) typically exceed those examined (29). There was no additional effect when the FAAH enzyme inhibitor phenylmethylsulfonyl fluoride (1) was included in the assay, suggesting that the agents susceptible to protease degradation are stable in the assay. The analog screening was conducted with Table 1. 5-HT 1A and 5-HT 2A potentiation 5-HT conc. Relative % response at oleamide conc.* ( M) 1 M 0.1 M 0.01 M M A. 5-HT 1A potentiation B. 5-HT 2A potentiation * 8%. FIG. 1. Potentiation of the human 5-HT 1A receptor response observed in R-SAT assays in RAT-1 cells transfected with the receptor and linked to a colorimetric -galactosidase assay. the rat 5-HT 2A assay, and a subset of analogs was also examined in the human 5-HT 1A assay. The results reported are normalized to a 100% potentiation by oleamide for the ease of direct comparisons. Fatty Acid Primary Amides. The first series examined was the primary amides (3, 4, 30, 31) of the naturally occurring fatty acids and related synthetic analogs (Table 2). From these studies, important trends in structural requirements of the endogenous agent emerged. The most effective primary amide of the naturally occurring fatty acids was oleamide. In the 5-HT 2A assay and for agents that contain one double bond, the presence, position, and stereochemistry of the olefin as well as the chain length were found to have a pronounced effect. Removal of the 9 double bond (18:0) or its replacement with a trans double bond (18:1 9-trans ) resulted in no observable potentiation. Shortening the chain length to 14 or 16 carbons resulted in the loss of activity, providing weak inhibitors, whereas lengthening the chain substantially diminished the effectiveness. A 9 -cis olefin exhibited the strongest effect, and the potency sharply declined as its position was moved in either direction. This is especially clear in the oleamide series (18:1) where the potency sharply declined as the distance from the 9 position increased. Although this is central to the structure of oleamide and potentially represents a relationship with either the carboxamide or methyl terminus, the inactivity of the primary amides of 20:1 9, 22:1 9, and 24:1 9 and the modest activity of 20:1 13, 22:1 13, and 24:1 15 suggest that it may be the 9 relationship with the methyl terminus that may be most important. Moreover, whereas extending the distance between the carboxamide and the double bond resulted in reduced or inactive compounds, shortening the length typically provided agents that displayed progressively more potent inhibition versus potentiation. Although the significance of this is not yet clear, it suggests the possibility that tightly regulated endogenous agents may serve to both potentiate or inhibit a serotonin receptor response. With the polyunsaturated fatty acid primary amides, those containing two cis double bonds exhibited modest activity, and those containing 3 6 double bonds were typically less active. The exception to this generalization is -linolenamide 18:3 6,9,12, which proved to be a more effective but still less potent agent. Arachidonamide containing four cis double bonds was ineffective. The behavior of 18:0, 18:1 9-trans, 18:1 8, 18:1 12, and 18:2 9,12 proved analogous to the observations detailed by Huidobro- Toro and Harris (14) in studies with the 5-HT 2C receptor. These similar observations not only indicate that the R-SAT assay for assessment of the 5-HT 2A receptor potentiation provides observations analogous to second messenger assays but also implies that the oleamide structural features required for activity may be well conserved throughout the 5-HT 2 receptor subtypes.
3 4104 Chemistry: Boger et al. Proc. Natl. Acad. Sci. USA 95 (1998) Table 2. Fatty acid primary amides Table 3. Carboxamide terminus In contrast, the 5-HT 1A receptor was found to be more tolerant of structural changes. Like the effects at the 5-HT 2A receptor, the saturated or trans fatty acid primary amides 18:0 and 18:1 9-trans as well as 18:1 8 were less effective than oleamide, albeit not inactive, on the 5-HT 1A receptor. Similarly, 14:1 9, 16:1 9, and linoleamide 18:2 9,12 were more effective on the 5-HT 1A receptor than 5-HT 2A with the latter two approaching the potency of oleamide. Carboxamide Terminus. A study of the carboxamide terminus revealed well defined structural requirements. Not only was the primary amide of oleic acid capable of potentiating the 5-HT response, but secondary and tertiary amides also provided a comparable potentiation provided the amide substituents were small (Table 3). The activity smoothly progresses through the series with the maximum effect observed with the NMe 2 tertiary amide. As the amide substituents further increased in size, the effect diminished and ultimately provided inactive derivatives. Thus, the primary carboxamide is not required although its efficacy approximates that of the most potent amide. This suggests that the carboxamide may serve as an H-bond acceptor but need not serve as an H-bond donor. In addition, the cyclopropyl amide was uniquely effective at inhibiting the response to serotonin at the 5-HT 2A receptor. Although endogenous agents that may act similarly have not been identified, such allosteric inhibitors at 5-HT 2A may prove to be useful biochemical tools and potentially interesting therapeutics. In contrast to the well defined effects on the 5-HT 2A receptor, both the isopropyl and cyclopropyl amides as well as the methyl amide were found to be effective at potentiating 5-HT 1A even though the first two were inactive or inhibitory on 5-HT 2A. Such distinctions suggest that derivatives of oleamide may be developed that not only possess greater potentiation effects but that can also selectively modulate the various serotonin receptor subtypes or even have opposing effects (i.e., cyclopropyl amide). Alternative substitutions for the carboxamide including oleic acid itself, oleyl esters, alcohols, amines, aldehydes, acetals, and electrophilic ketones did not provide a comparable potentiation of 5-HT 2A but appear to be better tolerated with 5-HT 1A (Table 4). Of particular interest are oleyl aldehyde and the trifluoromethyl ketone (Table 4, R H and CF 3 ). Both not only possess polarized carbonyls and can serve as H-bond acceptors, but both are potent inhibitors of FAAH, which is responsible for the degradation of oleamide (4). This dual activity suggests that they may not only potentiate the activity of oleamide by inhibiting its degradation but that they may also serve as oleamide agonists at the 5-HT 1A receptor. Oleyl Ethanolamide, Anandamide, and Related Structures. An important subset of modified carboxamides is the ethanolamide derivatives (32 35), which include anandamide (12). Consequently, the ethanolamides and bis(ethanol)amides of oleic and arachidonic acid were examined (Table 5). Both derivatives of oleic acid were inactive, providing no effect on
4 Chemistry: Boger et al. Proc. Natl. Acad. Sci. USA 95 (1998) 4105 Table 4. a Toxic to cells. the 5-HT 2A receptor whereas those of arachidonic acid including anandamide were weakly inhibitory. Similarly, recent studies have implicated 2-arachidonyl glycerol as an endogenous ligand for the cannabinoid receptor (36), and diacylglycerols including 1-oleyl-2-acetylglycerol have been reported as inhibitors of Chinese hamster V79 cell gap junctions (31). This latter compound was examined, and it did not potentiate but rather weakly inhibited the 5-HT 2A receptor response. Both oleyl ethanolamide and anandamide were found to potentiate the 5-HT 1A receptor response to 5-HT, and the former was more potent. Although this might be interpreted to suggest a special significance for the ethanolamides, oleyl Table 5. Carboxamide terminus Ethanolamides propanolamide was equally effective. As such, the results are more consistent with the simpler interpretation that the 5-HT 1A receptor potentiation is more tolerant of modifications in the carboxamide terminus and accommodates a wider range of secondary or tertiary amides (cf. Tables 3 and 4). Putative Precursors and Related Agents. The potential that oleamide may be stored as a N-oleyl glycinamide derivative and released on -hydroxylation of glycine by a peptidylglycine -amidating monooxygenase (8) led to the examination of a set of N-oleyl glycine derivatives (Table 6). None of the derivatives potentiated the 5-HT 2A serotonin receptor response, and most proved to be weak inhibitors. This is consistent with their behavior as large secondary or tertiary amide derivatives, and their activity follows the prior trends (Table 3). In contrast, N-oleyl glycine was a weak potentiator of the 5-HT 1A receptor consistent with its more tolerant accommodation of modifications in the carboxamide terminus. Methyl Terminus. The potentiation of 5-HT 2A or 5-HT 1A was especially sensitive to the structural characteristics at the methyl terminus (Table 7). Extending the length of the methyl terminus chain (cf. Table 2) and the incorporation of polar functional groups resulted in a loss of activity. Modifications in the Double Bond. The agents 2 10 were examined to define the role of the olefin (Table 8). Analogous to the observations made with octadecanamide (18:0) and the trans-9-octadecenamide (18:1 9-trans ), which were ineffective at potentiating 5-HT 2A (cf. Table 2), nearly all agents were ineffective. These include 7 10 for which a benzene ring was incorporated into the structure at a location that mimics the 9 double bond as well as 5 and 6, which mimic a hairpin conformation. Although it is difficult to draw specific conclusions from their inactivity, it highlights that the effects of oleamide at the 5-HT 2A receptor are surprisingly selective for the endogenous lipid. The exceptions include 9-octadecynamide (2), which was nearly equipotent with oleamide, and 4, which was approximately 50% as effective. The observation that the former is so effective suggests that the appropriate presentation of a -system in addition to the conformational effects of the cis double bond may be important. Consistent with this, 4 versus 3 proved surprisingly effective and may benefit from the partial characteristics of the cyclopropane, which would allow it to mimic both the and conformational characteristics of the cis double bond. Similar observations with the 5-HT 1A receptor were made with 2 and 4. In contrast to the 5-HT 2A results, both 9 and 10, but not 8, were effective at potentiating the 5-HT 1A receptor response and imply that an extended versus hairpin conformation of oleamide might be important at the 5-HT 1A receptor. Modifications in the Linking Chain. Modifications in the seven-carbon chain linking the olefin and carboxamide were examined and found to have a detrimental effect with 5-HT 2A (Table 9). Substitution of the -carbon or its replacement with a heteroatom resulted in a loss of activity or provided agents Table 6. N-Oleyl glycine derivatives
5 4106 Chemistry: Boger et al. Proc. Natl. Acad. Sci. USA 95 (1998) Table 7. Methyl terminus Table 9. Linker modifications that inhibited the 5-HT 2A response. Most notable are 2,2- dimethyloleamide as well as the urethane (X NH), which proved to be potent inhibitors. In contrast, most of the linking chain modifications did not adversely affect the 5-HT 1A potentiation, and this is significant in several respects. It is consistent with the greater tolerance for carboxamide modifications observed at the 5-HT 1A receptor and highlights again that many oleamide analogs may have distinguishing effects on the serotonin receptor subtypes. Moreover, the first seven entries in Table 9 constitute analogs that are more resistant to hydrolytic FAAH degradation and suggest an improved duration of effect would accompany their enhanced efficacy in vivo. In addition, the two -ketoamides (X CO, COCH 2 ) in Table 9 are potent inhibitors of FAAH (4) illustrating that they may serve to potentiate the effects of oleamide by inhibiting its hydrolysis and serve as oleamide agonists in their own right at the 5-HT 1A receptor. Table 8. Olefin modifications and substitutions DISCUSSION AND CONCLUSIONS We have shown that the structural features of oleamide required for potentiation of the 5-HT 2A receptor response are well defined, supporting a selective site of action. Of the naturally occurring fatty acids, oleamide is the most effective, and other endogenous fatty acid amides including arachidonamide, anandamide, and oleyl ethanolamide were less active or ineffective. For oleamide, the presence, position, and stereochemistry of 9 -cis double bond is required, and even subtle structural variations reduce or eliminate activity. Secondary or tertiary amides but not acids, esters, aldehydes, alcohols, amines, acetals, or electrophilic or polarized ketones may replace the primary carboxamide. Even the amide substitutions follow a well defined relationship limited to small amide substituents. Modifications of the methyl terminus or in the hydrocarbon chain linking the carboxamide and cis double bond typically eliminate the activity. In contrast, the 5-HT 1A receptor was more tolerant of structural modifications especially at the carboxamide terminus. The well defined structural features of oleamide required for potentiation of the 5-HT 2A or 5-HT 1A receptor response in the presence of serotonin provide the opportunity to correlate the properties with physiological states including sleep (38, 39). Such studies will clarify whether the serotonergic effects of oleamide and related agents may be responsible. Many of the well defined structural features of oleamide are tightly conserved among the 5-HT 2A, 5-HT 1A, and 5-HT 2C (14) receptors suggesting they may be well conserved throughout the 5-HT 1 and 5-HT 2 receptor subtypes. However, distinguishing structural effects were observed where the 5-HT 1A receptor was more tolerant of structural modifications in the carboxamide terminus of oleamide. In addition, several agents including a small set of naturally occurring fatty acid primary amides were identified that inhibited rather than potentiated the 5-HT 2A but not 5-HT 1A receptor response. Although the significance of these observations is not yet clear, it not only suggests the possibility that tightly regulated endogenous agents may serve to both potentiate and inhibit a serotonin response but that analogs of oleamide may permit the selective modulation of serotonin receptor subtypes and, in selected instances, even have opposing effects on the different receptor subtypes. The studies to date have demonstrated that at concentrations of 100 nm serotonin, 100 nm oleamide potentiates 5-HT 2C (365%) (14), 5-HT 1A (370%, results herein), and 5-HT 2A receptors [165% for results herein, 228% (15), and 260% (14)], inhibits 5-HT 7 receptors ( 50%) (15), and has no effect on the
6 Chemistry: Boger et al. Proc. Natl. Acad. Sci. USA 95 (1998) 4107 ion-gated channel 5-HT 3 receptor. The identification of such agents provides new biochemical tools for the study of serotonin receptors and may lead to therapeutic applications involving selective modulation of the serotonin response at the receptor subtypes. We gratefully acknowledge the financial support of The Skaggs Institute for Chemical Biology and the National Institutes of Health (Grant CA42056). 1. Cravatt, B. F., Prospero-Garcia, O., Siuzdak, G., Gilula, N. B., Henriksen, S. J., Boger, D. L. & Lerner, R. A. (1995) Science 268, Lerner, R. A., Siuzdak, G., Prospero-Garcia, O., Henriksen, S. J., Boger, D. L. & Cravatt, B. F. (1994) Proc. Natl. Acad. Sci. USA 91, Cravatt, B. F., Lerner, R. A. & Boger, D. L. (1996) J. Am. Chem. Soc. 118, Patterson, J. E., Ollmann, I. R., Cravatt, B. F., Boger, D. L., Wong, C.-H. & Lerner, R. A. (1996) J. Am. Chem. Soc. 118, Cravatt, B. F., Giang, D. K., Mayfield, S. P., Boger, D. L., Lerner, R. A. & Gilula, N. B. (1996) Nature (London) 384, Giang, D. K. & Cravatt, B. F. (1997) Proc. Natl. Acad. Sci. USA 94, Thomas, E. A., Cravatt, B. F., Danielson, P. E., Gilula, N. B. & Sutcliffe, J. G. (1997) J. Neurosci. Res. 50, Merkler, D. J., Merkler, K. A., Stern, W. & Fleming, F. F. (1996) Arch. Biochem. Biophys. 330, Koutek, B., Prestwich, G. D., Howlett, A. C., Chin, S. A., Salehani, D., Akhavon, N. & Deutsch, D. G. (1994) J. Biol. Chem. 269, De Petrocellis, L., Melck, D., Ueda, N., Maurelli, S., Kurahashi, Y., Yamamoto, S., Marino, G. & Marzo, V. D. (1997) Biochem. Biophys. Res. Commun. 231, Deutsch, D. G., Omeir, R., Arreaza, G., Salehani, D., Prestwich, G. D., Huang, Z. & Howlett, A. (1997) Biochem. Pharmacol. 53, Devane, W. A., Hanus, L., Breuer, A., Pertwee, R. G., Stevenson, L. A., Griffin, G., Gibson, D., Mandelbaum, A., Etinger, A. & Mechoulam, R. (1992) Science 258, Di Marzo, V. & Fontana, A. (1995) Prostaglandins Leukotrienes Essent. Fatty Acids 53, Huidobro-Toro, J. P. & Harris, R. A. (1996) Proc. Natl. Acad. Sci. USA 93, Thomas, E. A., Carson, M. J., Neal, M. J. & Sutcliffe, J. G. (1997) Proc. Natl. Acad. Sci. USA 94, Leonard, B. E. (1996) Psychother. Psychosom. 65, Lovenberg, T. W., Baron, B. M., de Lecea, L., Miller, J. D., Prosser, R. A., Rea, M. A., Foye, P. E., Racke, M., Slone, A. L., Siegel, B. W., Danielson, P. E., Sutcliffe, J. G. & Erlander, M. G. (1993) Neuron 11, Roe, E. T., Miles, T. D. & Swern, D. (1952) J. Am. Chem. Soc. 74, Patterson, J. E. (1997) Ph.D. thesis (The Scripps Research Institute, La Jolla, CA). 20. Suter, S., Trosko, J. E., El-Fouly, M. H., Lockwood, L. R. & Koestner, A. (1987) Fundam. Appl. Toxicol. 9, Pritchett, D. B., Bach, A. W., Wozny, M., Taleb, O., Dal Toso, R., Shih, J. C. & Seeburg, P. H. (1988) EMBO J. 7, Lam, S., Shen, Y., Nguyen, T., Messier, T., Brann, M., Comings, D., George, S. B. & O Dowd, B. (1996) Biochem. Biophys. Res. Commun. 219, Kobilka, B. K., Frielle, T., Collins, S., Yang-Feng, T., Kobilka, T. S., Francke, U., Lefkowitz, R. J. & Caron, M. G. (1987) Nature (London) 329, Messier, T. L., Dorman, C. M., Braüner-Osborne, H., Eubanks, D. & Brann, M. R. (1995) Pharmacol. Toxicol. 76, Brann, M. R., Messior, T., Dorman, C. & Lannigan, D. (1996) J. Biomol. Screening 1, Brauner-Osborne, H. & Brann, M. R. (1996) Eur. J. Pharmacol. 295, Kumar, A. M., Kumar, M., Deepika, K., Fernandez, J. B. & Eisdorfer, C. (1990) Life Sci. 47, Arafat, E. S., Trimble, J. W., Andersen, R. N., Dass, C. & Desiderio, D. M. (1989) Life Sci. 45, Bisogno, T., Sepe, N., De Petrocellis, L., Mechoulam, R. & Di Marzo, V. (1997) Biochem. Biophys. Res. Commun. 239, Wakamatsu, K., Masaki, T., Itoh, F., Kondo, K. & Katsuichi, S. (1990) Biochem. Biophys. Res. Commun. 168, Jain, M. K., Ghomashchi, F., Yu, B.-Z., Bayburt, T., Murphy, D., Houck, D., Brownell, J., Reid, J. C., Solowiej, J. E., Wong, S.-M., Mocek, U., Jarrel, R., Sasser, M. & Gelb, M. H. (1992) J. Med. Chem. 35, Bachur, N. R., Masek, K., Melmon, K. L. & Udenfriend, S. (1965) J. Biol. Chem. 240, Ramachandran, C. K., Murray, D. K. & Nelson, D. H. (1992) Arch. Biochem. Biophys. 8, Schmid, H. H. O., Schmid, P. C. & Natarajan, V. (1990) Prog. Lipid Res. 29, Hanus, L., Gopher, A., Almog, S. & Mechoulam, R. (1993) J. Med. Chem. 36, Mechoulam, R., Ben-Shabat, S., Hanus, L., Ligumsky, M., Kaminski, N. E., Schatz, A. R., Gopher, A., Almog, S., Martin, B. R., Compton, D. R., Pertwee, R. G., Griffin, G., Bayewitch, M., Barg, J. & Vogel, Z. (1995) Biochem. Pharmacol. 50, Aylsworth, C. F., Trosko, J. E. & Welsch, C. W. (1986) Cancer Res. 46, Lerner, R. A. (1997) Proc. Natl. Acad. Sci. USA 94, Guan, X. G., Cravatt, B. F., Ehring, G. R., Hall, J. E., Boger, D. L., Lerner, R. A. & Gilula, N. B. (1997) J. Cell Biol. 139,
Molecular characterization of human and mouse fatty acid amide hydrolases
 Proc. Natl. Acad. Sci. USA Vol. 94, pp. 2238 2242, March 1997 Biochemistry Molecular characterization of human and mouse fatty acid amide hydrolases DAN K. GIANG AND BENJAMIN F. CRAVATT The Skaggs Institute
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 2238 2242, March 1997 Biochemistry Molecular characterization of human and mouse fatty acid amide hydrolases DAN K. GIANG AND BENJAMIN F. CRAVATT The Skaggs Institute
Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide
 Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14115 14119, December 1997 Pharmacology Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide ELIZABETH A. THOMAS,
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 14115 14119, December 1997 Pharmacology Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide ELIZABETH A. THOMAS,
Bioorganic & Medicinal Chemistry Letters 15 (2005)
 Bioorganic & Medicinal Chemistry Letters 15 (2005) 1423 1428 Discovery of an exceptionally potent and selective class of fatty acid amide hydrolase inhibitors enlisting proteome-wide selectivity screening:
Bioorganic & Medicinal Chemistry Letters 15 (2005) 1423 1428 Discovery of an exceptionally potent and selective class of fatty acid amide hydrolase inhibitors enlisting proteome-wide selectivity screening:
Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand
 FEBS 19751 FEBS Letters 422 (1998) 69^73 Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand Sravan Kumar Goparaju, Natsuo Ueda, Hiroko Yamaguchi, Shozo
FEBS 19751 FEBS Letters 422 (1998) 69^73 Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand Sravan Kumar Goparaju, Natsuo Ueda, Hiroko Yamaguchi, Shozo
Factors to Consider in the Study of Biomolecules
 Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General structure and functional groups
Lipids and Membranes
 Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Lipids Lipids are hydrophobic or amphiphilic insoluble in water soluble in organic solvents soluble in lipids Lipids are used as energy storage molecules structural components of membranes protective molecules
Chapter 2: Cellular Mechanisms and Cognition
 Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Medicinal Chemistry I Examination Name December 15, 2009 Med. Chem. #
 Medicinal Chemistry Examination ame December 15, 2009 Med. Chem. # Part. 80 Points (40 Questions; 2 Points Each) Select the BEST answer for each of the following. f none of the answers are correct, do
Medicinal Chemistry Examination ame December 15, 2009 Med. Chem. # Part. 80 Points (40 Questions; 2 Points Each) Select the BEST answer for each of the following. f none of the answers are correct, do
1 C 2 C 3 C 4 C 5 C 6 C 7 C 8 C
 I. Carbon atoms form an enormous variety of structures A. Carbon has 4 valence electrons in the outer shell and therefore may form up to 4 covalent bonds B. Carbon tends to bond to C, H, O, N, S, and P
I. Carbon atoms form an enormous variety of structures A. Carbon has 4 valence electrons in the outer shell and therefore may form up to 4 covalent bonds B. Carbon tends to bond to C, H, O, N, S, and P
Learning Objectives. How do drugs work? Mechanisms of Drug Action. Liam Anderson Dept Pharmacology & Clinical Pharmacology
 How do drugs work? Mechanisms of Drug Action Liam Anderson Dept Pharmacology & Clinical Pharmacology Learning Objectives Describe the potential drug targets within a human body. Describe the role of receptors,
How do drugs work? Mechanisms of Drug Action Liam Anderson Dept Pharmacology & Clinical Pharmacology Learning Objectives Describe the potential drug targets within a human body. Describe the role of receptors,
Cannabinoids 101. Matthew Hill, Ph.D. Hotchkiss Brain Institute University of Calgary
 Cannabinoids 101 Matthew Hill, Ph.D. Hotchkiss Brain Institute University of Calgary Disclosures Have received honoraria for Scientific Consultation: Pfizer International GW Pharmaceuticals Receive operating
Cannabinoids 101 Matthew Hill, Ph.D. Hotchkiss Brain Institute University of Calgary Disclosures Have received honoraria for Scientific Consultation: Pfizer International GW Pharmaceuticals Receive operating
JNJ : selective and slowly reversible FAAH inhibitor. Central and Peripheral PK/PD
 JNJ-42165279: selective and slowly reversible FAAH inhibitor Central and Peripheral PK/PD The Endocannabinoid System Research initiated by efforts to elucidate the active substance of Cannabis (THC in
JNJ-42165279: selective and slowly reversible FAAH inhibitor Central and Peripheral PK/PD The Endocannabinoid System Research initiated by efforts to elucidate the active substance of Cannabis (THC in
Introduction to the Study of Lipids
 Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Introduction to the Study of Lipids Factors to Consider in the Study of Biomolecules What are the features of the basic building blocks? (ex: monosaccharides, alcohols, fatty acids, amino acids) 1) General
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1)
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Lipids are broadly classified in to simple, complex and derived, which are further subdivided into different groups.
 Paper No. 01 Paper Title: Food Chemistry Module -9: Classification of lipids Lipids are organic substances which are insoluble in water and soluble in organic solvents. Lipids are not polymers and exist
Paper No. 01 Paper Title: Food Chemistry Module -9: Classification of lipids Lipids are organic substances which are insoluble in water and soluble in organic solvents. Lipids are not polymers and exist
Biology 12 - Biochemistry Practice Exam
 Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
From Atoms to Cells: Fundamental Building Blocks. Models of atoms. A chemical connection
 From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
where R doesn t have to equal R or R
 hem 263 Nov 24, 2016 arboxylic Acids and Derivatives arboxylic acids are very important compounds in nature and serve as building blocks for preparing related derivatives such as esters and amides. The
hem 263 Nov 24, 2016 arboxylic Acids and Derivatives arboxylic acids are very important compounds in nature and serve as building blocks for preparing related derivatives such as esters and amides. The
Name the ester produced when methanol and pentanoic acid react. methyl pentanoate. Name the type of reaction used to make an ester
 1 Name the ester produced when methanol and pentanoic acid react methyl pentanoate 2 Name the type of reaction used to make an ester condensation reaction 3 Name the by-product of the reaction used to
1 Name the ester produced when methanol and pentanoic acid react methyl pentanoate 2 Name the type of reaction used to make an ester condensation reaction 3 Name the by-product of the reaction used to
Fats, Cholesterol, and Hormones
 Fats, Cholesterol, and Hormones 1 Types of Fats Lipids biological origin sparingly soluble in water Main classes of lipids Fatty Acids long hydrocarbon chains with a carboxylic acid on one end Triacylglycerols
Fats, Cholesterol, and Hormones 1 Types of Fats Lipids biological origin sparingly soluble in water Main classes of lipids Fatty Acids long hydrocarbon chains with a carboxylic acid on one end Triacylglycerols
1. Which of the following contributes to the tertiary structure of proteins?
 Chemistry 11 Spring 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free
Chemistry 11 Spring 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free
OBJECTIVE. Lipids are largely hydrocarbon derivatives and thus represent
 Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
Paper 4. Biomolecules and their interactions Module 20: Saturated and unsaturated fatty acids, Nomenclature of fatty acids and Essential and non-essential fatty acids OBJECTIVE The main aim of this module
Chapter 6 Communication, Integration, and Homeostasis
 Chapter 6 Communication, Integration, and Homeostasis About This Chapter Cell-to-cell communication Signal pathways Novel signal molecules Modulation of signal pathways Homeostatic reflex pathways Cell-to-Cell
Chapter 6 Communication, Integration, and Homeostasis About This Chapter Cell-to-cell communication Signal pathways Novel signal molecules Modulation of signal pathways Homeostatic reflex pathways Cell-to-Cell
MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS
 December 6, 2011 Lecturer: Eileen M. Lafer MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS Reading: Stryer Edition 6: Chapter 26 Images: All images in these notes were taken from Lehninger,
December 6, 2011 Lecturer: Eileen M. Lafer MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS Reading: Stryer Edition 6: Chapter 26 Images: All images in these notes were taken from Lehninger,
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON
 CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
CARBOXYLIC ACIDS AND THEIR DERIVATIVES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON RED ANT WAS SOURCE OF FORMIC ACID (RCOOH) Lecture 8 ORGANIC CHEMISTRY 2 Introduction The carboxyl group (-CO
Details of Organic Chem! Date. Carbon & The Molecular Diversity of Life & The Structure & Function of Macromolecules
 Details of Organic Chem! Date Carbon & The Molecular Diversity of Life & The Structure & Function of Macromolecules Functional Groups, I Attachments that replace one or more of the hydrogens bonded to
Details of Organic Chem! Date Carbon & The Molecular Diversity of Life & The Structure & Function of Macromolecules Functional Groups, I Attachments that replace one or more of the hydrogens bonded to
Received May 26, Figure 1. FAAH substrates /jm049614v CCC: $27.50 xxxx American Chemical Society
 Discovery of a Potent, Selective, and Efficacious Class of Reversible r-ketoheterocycle Inhibitors of Fatty Acid Amide Hydrolase Effective as Analgesics Dale L. Boger,*,, Hiroshi Miyauchi,, Wu Du,, Christophe
Discovery of a Potent, Selective, and Efficacious Class of Reversible r-ketoheterocycle Inhibitors of Fatty Acid Amide Hydrolase Effective as Analgesics Dale L. Boger,*,, Hiroshi Miyauchi,, Wu Du,, Christophe
Drug Receptor Interactions and Pharmacodynamics
 Drug Receptor Interactions and Pharmacodynamics Dr. Raz Mohammed MSc Pharmacology School of Pharmacy 22.10.2017 Lec 6 Pharmacodynamics definition Pharmacodynamics describes the actions of a drug on the
Drug Receptor Interactions and Pharmacodynamics Dr. Raz Mohammed MSc Pharmacology School of Pharmacy 22.10.2017 Lec 6 Pharmacodynamics definition Pharmacodynamics describes the actions of a drug on the
Chapter 10. Carboxylic Acids and Derivatives. Naming Carboxylic Acids and Derivatives. Carboxylic Acids: RCOOH (RCO 2 H)
 Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
By: Dr Hadi Mozafari 1
 Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
Biological lipids are a chemically diverse group of compounds, the common and defining feature of which is their insolubility in water. By: Dr Hadi Mozafari 1 Fats and oils are the principal stored forms
Physiology Unit 1 CELL SIGNALING: CHEMICAL MESSENGERS AND SIGNAL TRANSDUCTION PATHWAYS
 Physiology Unit 1 CELL SIGNALING: CHEMICAL MESSENGERS AND SIGNAL TRANSDUCTION PATHWAYS In Physiology Today Cell Communication Homeostatic mechanisms maintain a normal balance of the body s internal environment
Physiology Unit 1 CELL SIGNALING: CHEMICAL MESSENGERS AND SIGNAL TRANSDUCTION PATHWAYS In Physiology Today Cell Communication Homeostatic mechanisms maintain a normal balance of the body s internal environment
1. Denaturation changes which of the following protein structure(s)?
 Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoylglycerol cannabinoid activity.
 See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/222303665 An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoylglycerol
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/222303665 An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoylglycerol
I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins
 Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Cannabinoid Therapeutics: Marijuana and Beyond Chair: Igor Grant, M.D.
 Cannabinoid Therapeutics: Marijuana and Beyond Chair: Igor Grant, M.D. Advances in understanding the molecular mechanisms underlying cannabinoid effects, coupled with increased public acceptance that cannabinoids
Cannabinoid Therapeutics: Marijuana and Beyond Chair: Igor Grant, M.D. Advances in understanding the molecular mechanisms underlying cannabinoid effects, coupled with increased public acceptance that cannabinoids
Psych 181: Dr. Anagnostaras
 Psych 181: Dr. Anagnostaras Lecture 5 Synaptic Transmission Introduction to synaptic transmission Synapses (Gk., to clasp or join) Site of action of most psychoactive drugs 6.5 1 Synapses Know basic terminology:
Psych 181: Dr. Anagnostaras Lecture 5 Synaptic Transmission Introduction to synaptic transmission Synapses (Gk., to clasp or join) Site of action of most psychoactive drugs 6.5 1 Synapses Know basic terminology:
2. Which of the following is NOT true about carbohydrates
 Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
UC Irvine UC Irvine Previously Published Works
 UC Irvine UC Irvine Previously Published Works Title Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2- arachidonylglycerol Permalink https://escholarship.org/uc/item/92w2j8v2
UC Irvine UC Irvine Previously Published Works Title Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2- arachidonylglycerol Permalink https://escholarship.org/uc/item/92w2j8v2
Basics of Pharmacology
 Basics of Pharmacology Pekka Rauhala Transmed 2013 What is pharmacology? Pharmacology may be defined as the study of the effects of drugs on the function of living systems Pharmacodynamics The mechanism(s)
Basics of Pharmacology Pekka Rauhala Transmed 2013 What is pharmacology? Pharmacology may be defined as the study of the effects of drugs on the function of living systems Pharmacodynamics The mechanism(s)
Chapter 20. Cell - Cell Signaling: Hormones and Receptors. Three general types of extracellular signaling. endocrine signaling. paracrine signaling
 Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
2013 W. H. Freeman and Company. 10 Lipids
 2013 W. H. Freeman and Company 10 Lipids CHAPTER 10 Lipids Key topics: Biological roles of lipids Structure and properties of storage lipids Structure and properties of membrane lipids Structure and properties
2013 W. H. Freeman and Company 10 Lipids CHAPTER 10 Lipids Key topics: Biological roles of lipids Structure and properties of storage lipids Structure and properties of membrane lipids Structure and properties
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond
 Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Chem 1120 Final 210 points Dr. Luther Giddings
 Chem 1120 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Chem 1120 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Lecture Outline. Hormones & Chemical Signaling. Communication Basics: Overview. Communication Basics: Methods. Four methods of cell communication
 Lecture Outline Hormones & Chemical Signaling Communication Basics Communication Overview Communication Methods Signal pathways Regulation (modulation) of signal pathways Homeostasis... again Endocrine
Lecture Outline Hormones & Chemical Signaling Communication Basics Communication Overview Communication Methods Signal pathways Regulation (modulation) of signal pathways Homeostasis... again Endocrine
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 24 Chemical Communications Neurotransmitters & Hormones
 Chapter 24 Chemical Communications Neurotransmitters & Hormones 1 Chemical Communication Terms and definitions: Neuron: A nerve cell. Neurotransmitter: A chemical messenger between a neuron and another
Chapter 24 Chemical Communications Neurotransmitters & Hormones 1 Chemical Communication Terms and definitions: Neuron: A nerve cell. Neurotransmitter: A chemical messenger between a neuron and another
Review of Neurochemistry What are neurotransmitters?
 Review of Neurochemistry What are neurotransmitters? In molecular terms, neurotransmitters are molecules that ( ) and of neurons by, for example, increasing or decreasing enzymatic activity or altering
Review of Neurochemistry What are neurotransmitters? In molecular terms, neurotransmitters are molecules that ( ) and of neurons by, for example, increasing or decreasing enzymatic activity or altering
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Name. The following exam contains 44 questions, valued at 2.6 points/question. 2. Which of the following is not a principal use of proteins?
 Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Section: Chapter 5: Multiple Choice. 1. The structure of synapses is best viewed with a(n):
 Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Endocannabinoids in the central nervous system an overview
 Endocannabinoids in the central nervous system an overview E. Fride Department of Behavioral Sciences,College of Judea and Samaria, Ariel, Israel Prostaglandins, Leukotrienes and Essential FattyAcids (2002)
Endocannabinoids in the central nervous system an overview E. Fride Department of Behavioral Sciences,College of Judea and Samaria, Ariel, Israel Prostaglandins, Leukotrienes and Essential FattyAcids (2002)
15.1 Lipids 15.2 Fatty Acids. Copyright 2009 by Pearson Education, Inc.
 Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
Chapter 15 Lipids 15.1 Lipids 15.2 Fatty Acids Copyright 2009 by Pearson Education, Inc. 1 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but
CELLULAR METABOLISM. Metabolic pathways can be linear, branched, cyclic or spiral
 CHM333 LECTURE 24 & 25: 3/27 29/13 SPRING 2013 Professor Christine Hrycyna CELLULAR METABOLISM What is metabolism? - How cells acquire, transform, store and use energy - Study reactions in a cell and how
CHM333 LECTURE 24 & 25: 3/27 29/13 SPRING 2013 Professor Christine Hrycyna CELLULAR METABOLISM What is metabolism? - How cells acquire, transform, store and use energy - Study reactions in a cell and how
Carboxylic Acids and Their Derivatives. Chapter 17. Carboxylic Acids and Their Derivatives
 Chapter 17 Carboxylic Acids and Their Derivatives Chapter 17 suggested problems: 36, 38, 40, 42, 44, 52, 54, 56, 62, 64, 66, 70 Class Notes I. Carboxylic acids (organic acids) and their derivatives A.
Chapter 17 Carboxylic Acids and Their Derivatives Chapter 17 suggested problems: 36, 38, 40, 42, 44, 52, 54, 56, 62, 64, 66, 70 Class Notes I. Carboxylic acids (organic acids) and their derivatives A.
Biological role of lipids
 Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Lipids Lipids Organic compounds present in living organisms, insoluble in water but able to be extracted by organic solvents such as: chloroform, acetone, benzene. Extraction = the action of taking out
Receptors and Drug Action. Dr. Subasini Pharmacology Department Ishik University, Erbil
 Receptors and Drug Action Dr. Subasini Pharmacology Department Ishik University, Erbil Receptors and Drug Action Receptor Receptor is defined as a macromolecule or binding site located on the surface or
Receptors and Drug Action Dr. Subasini Pharmacology Department Ishik University, Erbil Receptors and Drug Action Receptor Receptor is defined as a macromolecule or binding site located on the surface or
Calpain Assays, Nerve injury and Repair
 Calpain Assays, Nerve injury and Repair Annual Progress Report (G-3 3-Y44) April 2001 James C. Powers School of Chemistry and Biochemistry Georgia Institute of Technology Atlanta, GA 30332-0400 (404) 894-4038
Calpain Assays, Nerve injury and Repair Annual Progress Report (G-3 3-Y44) April 2001 James C. Powers School of Chemistry and Biochemistry Georgia Institute of Technology Atlanta, GA 30332-0400 (404) 894-4038
The Nervous System Mark Stanford, Ph.D.
 The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
PHRM20001: Pharmacology - How Drugs Work!
 PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
Organic & Biochemistry Pacing Guide. Day Date SCS Objectives Essential Questions Content Tasks/Strategies. How are covalent compounds formed?
 Organic & Biochemistry Pacing Guide Course Description: Course Description: This course is designed to provide students with an opportunity to continue their study of the principles of chemistry. The topics
Organic & Biochemistry Pacing Guide Course Description: Course Description: This course is designed to provide students with an opportunity to continue their study of the principles of chemistry. The topics
Essential Biology 3.2 Carbohydrates, Lipids, Proteins. 1. Define organic molecule.
 1. Define organic molecule. An organic molecule is a molecule that contains carbon and is found in living things. There are many organic molecules in living things. The same (or very similar) molecules
1. Define organic molecule. An organic molecule is a molecule that contains carbon and is found in living things. There are many organic molecules in living things. The same (or very similar) molecules
Organic Chemistry 3540
 rganic Chemistry 3540 December 8, 2004 (8 Pages, 13 Parts) ame 1. (8%) Many organic compounds found in living systems are complex molecules which can be characterized, in part, by simply listing the chemical
rganic Chemistry 3540 December 8, 2004 (8 Pages, 13 Parts) ame 1. (8%) Many organic compounds found in living systems are complex molecules which can be characterized, in part, by simply listing the chemical
Neurotransmitters. Chemical transmission of a nerve signal by neurotransmitters at a synapse
 Neurotransmitters A chemical released by one neuron that affects another neuron or an effector organ (e.g., muscle, gland, blood vessel). Neurotransmitters are small molecules that serve as messengers
Neurotransmitters A chemical released by one neuron that affects another neuron or an effector organ (e.g., muscle, gland, blood vessel). Neurotransmitters are small molecules that serve as messengers
Cell Signaling (part 1)
 15 Cell Signaling (part 1) Introduction Bacteria and unicellular eukaryotes respond to environmental signals and to signaling molecules secreted by other cells for mating and other communication. In multicellular
15 Cell Signaling (part 1) Introduction Bacteria and unicellular eukaryotes respond to environmental signals and to signaling molecules secreted by other cells for mating and other communication. In multicellular
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Chapter Sections: 3.1 Carbon s Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins 3.
 Chapter Sections: 3.1 Carbon s Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins 3.6 Nucleic Acids Student Goals: By the end of this lecture series, students should
Chapter Sections: 3.1 Carbon s Place in the Living World 3.2 Functional Groups 3.3 Carbohydrates 3.4 Lipids 3.5 Proteins 3.6 Nucleic Acids Student Goals: By the end of this lecture series, students should
MODULE No.26: Drug Metabolism
 SUBJECT Paper No. and Title Module No. and Title Module Tag PAPER No. 9: Drugs of Abuse MODULE No. 26: Drug Metabolism FSC_P9_M26 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Sites of Drug
SUBJECT Paper No. and Title Module No. and Title Module Tag PAPER No. 9: Drugs of Abuse MODULE No. 26: Drug Metabolism FSC_P9_M26 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Sites of Drug
Chapter 15 An Introduction to Organic Chemistry, Biochemistry, and Synthetic Polymers. An Introduction to Chemistry by Mark Bishop
 Chapter 15 An Introduction to Organic Chemistry, Biochemistry, and Synthetic Polymers An Introduction to Chemistry by Mark Bishop Chapter Map Organic Chemistry Organic chemistry is the chemistry of carbon-based
Chapter 15 An Introduction to Organic Chemistry, Biochemistry, and Synthetic Polymers An Introduction to Chemistry by Mark Bishop Chapter Map Organic Chemistry Organic chemistry is the chemistry of carbon-based
Acid/Base chemistry. NESA Biochemistry Fall 2001 Review problems for the first exam. Complete the following sentences
 1 NESA Biochemistry Fall 2001 eview problems for the first exam Acid/Base chemistry 1. 2 3 is a weak acid. 2. The anion of a weak acid is a weak base 3. p is the measure of a solutions acidity. 4. 3 and
1 NESA Biochemistry Fall 2001 eview problems for the first exam Acid/Base chemistry 1. 2 3 is a weak acid. 2. The anion of a weak acid is a weak base 3. p is the measure of a solutions acidity. 4. 3 and
Receptors Families. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Receptors Families Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Receptor Families 1. Ligand-gated ion channels 2. G protein coupled receptors 3. Enzyme-linked
Receptors Families Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Receptor Families 1. Ligand-gated ion channels 2. G protein coupled receptors 3. Enzyme-linked
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Carboxylic Acids and Their Derivatives
 arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
arboxylic Acids and Their Derivatives Families ontaining the arbonyl Group Family Y Z Y Z aldehyde or ketone carboxylic acid or -- ester or -- acid halide or -F,-l,-Br,-I acid anhydride or amide or -N
Chapters 9 and 10 Lipids and Membranes
 Chapters 9 and 10 Lipids and Membranes Lipids- a class of biological molecules defined by low solubility in water and high solubility in nonpolar solvents. Lipids contain or are derived from fatty acids.
Chapters 9 and 10 Lipids and Membranes Lipids- a class of biological molecules defined by low solubility in water and high solubility in nonpolar solvents. Lipids contain or are derived from fatty acids.
Why Carbon? What does a carbon atom look like?
 Biomolecules Organic Chemistry In the 1800 s it was believed to be impossible to recreate molecules in a lab Thus, the study of organic chemistry was originally the study of molecules in living organisms
Biomolecules Organic Chemistry In the 1800 s it was believed to be impossible to recreate molecules in a lab Thus, the study of organic chemistry was originally the study of molecules in living organisms
1/3/2011. Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl compounds or carboxylic acid derivatives Chapter 17 Carboxylic Acids and Their Derivatives. Nucleophilic
CHAPTER 3. Carbon & the Molecular Diversity of Life
 CHAPTER 3 Carbon & the Molecular Diversity of Life Carbon: The Organic Element Compounds that are synthesized by cells and contain carbon are organic So what is inorganic? Why are carbon compounds so prevalent?
CHAPTER 3 Carbon & the Molecular Diversity of Life Carbon: The Organic Element Compounds that are synthesized by cells and contain carbon are organic So what is inorganic? Why are carbon compounds so prevalent?
Macro molecule = is all the reactions that take place in cells, the sum of all chemical reactions that occur within a living organism Anabolism:
 Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Additional problems: 1. Match and label the conjugate acid and base pairs in the following reactions. Which one of these systems is a good buffer?
 1 ESA Biochemistry Fall 2001 eview problems for the first exam Acid/Base chemistry Sections to review: 10.2,.3,.4,.6.9,.12,.13 omplete the following sentences 1. 2 3 is a acid. 2. The anion of a weak acid
1 ESA Biochemistry Fall 2001 eview problems for the first exam Acid/Base chemistry Sections to review: 10.2,.3,.4,.6.9,.12,.13 omplete the following sentences 1. 2 3 is a acid. 2. The anion of a weak acid
CH 3. Lipids CHAPTER SUMMARY
 H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
H 3 C H 3 C 15 H 3 C H Views of Cholesterol APTER SUMMARY 15.1 The Nature of can best be defined as biomolecules which are soluble to a great extent in solvents. In contrast to carbohydrates, proteins
Patrick, An Introduction to Medicinal Chemistry 5e Chapter 1 Drugs and drug targets: an overview
 Patrick, An Introduction to dicinal hemistry 5e Answers to end-of-chapter questions 1) The ability of a molecule to cross the fatty cell membrane has little to do with its size, but more with its hydrophobic
Patrick, An Introduction to dicinal hemistry 5e Answers to end-of-chapter questions 1) The ability of a molecule to cross the fatty cell membrane has little to do with its size, but more with its hydrophobic
3150:112 SAMPLE TEST 2. Print out a copy Answer the questions on your own. Check the answers at GOBC Ans.pdf. Good Luck!
 SAMPLE TEST 2 3150:112 Print out a copy Answer the questions on your own. Check the answers at GOBC Ans.pdf. Good Luck! QUESTIONS 1-3 REFER TO TE FOLLOWING: A. C 2 O O B. C 2 O O O C 2 O C. O C 2 O 1.
SAMPLE TEST 2 3150:112 Print out a copy Answer the questions on your own. Check the answers at GOBC Ans.pdf. Good Luck! QUESTIONS 1-3 REFER TO TE FOLLOWING: A. C 2 O O B. C 2 O O O C 2 O C. O C 2 O 1.
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Section 1 Lecture 1- Origins of Life Life probably started by Hydrothermal Vents.
 Section 1 Lecture 1- Origins of Life Life probably started by Hydrothermal Vents. Photosynthesis originated around 3GA, as cells figured out how to fix CO2 and release O2. Eukaryotes originates 1.5-2.5
Section 1 Lecture 1- Origins of Life Life probably started by Hydrothermal Vents. Photosynthesis originated around 3GA, as cells figured out how to fix CO2 and release O2. Eukaryotes originates 1.5-2.5
Steroid Hormones Synthesis
 *I ll try my best to incorporate the Slides in this Sheet; you don t need to study the slides if you study this sheet. Steroid Hormones Synthesis - The figure to the right is the Steroid nucleus, it has
*I ll try my best to incorporate the Slides in this Sheet; you don t need to study the slides if you study this sheet. Steroid Hormones Synthesis - The figure to the right is the Steroid nucleus, it has
Membrane associated receptor transfers the information. Second messengers relay information
 Membrane associated receptor transfers the information Most signals are polar and large Few of the signals are nonpolar Receptors are intrinsic membrane proteins Extracellular and intracellular domains
Membrane associated receptor transfers the information Most signals are polar and large Few of the signals are nonpolar Receptors are intrinsic membrane proteins Extracellular and intracellular domains
2013 W. H. Freeman and Company. 10 Lipids
 2013 W. H. Freeman and Company 10 Lipids Storage lipids: TG lipid 의기능 : 1 Energy source 3 Electrical insulator 2 Thermal insulator 4 Membrane 의구성성분, 방수, 부력, cofactor, signaling 등 지방대사이상 : obesity, atherosclerosis,
2013 W. H. Freeman and Company 10 Lipids Storage lipids: TG lipid 의기능 : 1 Energy source 3 Electrical insulator 2 Thermal insulator 4 Membrane 의구성성분, 방수, 부력, cofactor, signaling 등 지방대사이상 : obesity, atherosclerosis,
Carboxylic Acids and their Derivatives I
 2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
CHAPTER 9: CATALYTIC STRATEGIES. Chess vs Enzymes King vs Substrate
 CHAPTER 9: CATALYTIC STRATEGIES Chess vs Enzymes King vs Substrate INTRODUCTION CHAPTER 9 What are the sources of the catalytic power and specificity of enzymes? Problems in reactions in cells Neutral
CHAPTER 9: CATALYTIC STRATEGIES Chess vs Enzymes King vs Substrate INTRODUCTION CHAPTER 9 What are the sources of the catalytic power and specificity of enzymes? Problems in reactions in cells Neutral
Chem 60 Takehome Test 2 Student Section
 Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Multiple choice: 1 point each. Mark only one answer for each question. 1. are composed primarily of carbon and hydrogen, but may also include oxygen, nitrogen, sulfur, phosphorus, and a few other elements.
Chapters 13/14: Carboxylic Acids and Carboxylic Acid Derivatives
 CHM 201 (Elements of Organic Chemistry) Dr. Virgil Lee Cal Poly Pomona Chapters 13/14: Carboxylic Acids and Carboxylic Acid Derivatives resonance stabilized OH group donates electron density to carbonyl
CHM 201 (Elements of Organic Chemistry) Dr. Virgil Lee Cal Poly Pomona Chapters 13/14: Carboxylic Acids and Carboxylic Acid Derivatives resonance stabilized OH group donates electron density to carbonyl
Identifying Functional Groups. (Chapter 2 in the Klein text)
 Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
Receptors. Dr. Sanaa Bardaweel
 Receptors Types and Theories Dr. Sanaa Bardaweel Some terms in receptor-drug interactions Agonists: drugs that mimic the natural messengers and activate receptors. Antagonist: drugs that block receptors.
Receptors Types and Theories Dr. Sanaa Bardaweel Some terms in receptor-drug interactions Agonists: drugs that mimic the natural messengers and activate receptors. Antagonist: drugs that block receptors.
Chem 5 PAL Worksheet Lipids Smith text Chapter 15
 Chem 5 PAL Worksheet Lipids Smith text Chapter 15 Principle: Fatty acids are carboxylic acids with long (usually > 14) carbon chains which can be saturated (no carbon-carbon double bonds) are unsaturated
Chem 5 PAL Worksheet Lipids Smith text Chapter 15 Principle: Fatty acids are carboxylic acids with long (usually > 14) carbon chains which can be saturated (no carbon-carbon double bonds) are unsaturated
GUTS Lecture Syllabus for Lipid Structure and Nomenclature
 GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
GUTS Lecture Syllabus for Lipid Structure and Nomenclature For Questions or Assistance contact: Dr. Gwen Sancar, gsancar@ad.unc.edu Learning bjectives After completing the GUTS lecture and associated self-
Chymotrypsin Lecture. Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin
 Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Chemistry in Everyday life
 Therapeutic Action of drugs Chemistry in Everyday life Question 1 What are competitive inhibitors? Drugs inhibit the attachment of substrate on active site of enzymes by specific ways. When the drugs compete
Therapeutic Action of drugs Chemistry in Everyday life Question 1 What are competitive inhibitors? Drugs inhibit the attachment of substrate on active site of enzymes by specific ways. When the drugs compete
Life History of A Drug
 DRUG ACTION & PHARMACODYNAMIC M. Imad Damaj, Ph.D. Associate Professor Pharmacology and Toxicology Smith 652B, 828-1676, mdamaj@hsc.vcu.edu Life History of A Drug Non-Specific Mechanims Drug-Receptor Interaction
DRUG ACTION & PHARMACODYNAMIC M. Imad Damaj, Ph.D. Associate Professor Pharmacology and Toxicology Smith 652B, 828-1676, mdamaj@hsc.vcu.edu Life History of A Drug Non-Specific Mechanims Drug-Receptor Interaction
Synaptic transmission
 Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Chapter 20 Carboxylic Acids. Introduction
 hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
hapter 20 arboxylic Acids Introduction arbonyl (-=) and hydroxyl (-H) on the same carbon is carboxyl group. arboxyl group is usually written -H or 2 H. Aliphatic acids have an alkyl group bonded to -H.
