A study of the chicken IFN lambda system
|
|
|
- Rolf Evans
- 5 years ago
- Views:
Transcription
1 A study of the chicken IFN system by Andres Rohringer B.Sc., M.Sc. Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy School of Medicine Dekin University August, 2016
2
3
4
5 Funding support: This work ws supported by the Dekin University Interntionl Postgrdute Reserch Scholrship, the CSIRO Top up scholrship nd the Poultry CRC. 4
6 1 Abstrct High Pthogenic Avin Influenz (HPAI) viruses, such s H7N9 nd H5N1 HPAI, hve very lrge effect on poultry production nd there is need for new strtegies to del with these infections. The chrcteriztion of ntivirl immune pthwys is essentil for understnding host-pthogen mechnisms to underpin improved therpies for both humn nd livestock use. The interferon (IFN) pthwy stimultes the expression of myrid of interferon stimulted genes (ISGs) tht impct virl repliction. This study imed to chrcterize the chicken type III IFN response, its r complex nd its downstrem meditor. The induction of type III IFN in comprison to type I IFNs ws exmined in chicken splenocytes stimulted with TLR gonists nd chicken IFNs. The induction of type I nd type III IFNs by PIC nd LPS ws similr but IFN nd ISG induction kinetics differed in response to IFNα nd IFNλ with IFNα inducing more rpid induction. Furthermore, ge nd sex dependent differences in the induction of type I nd type III IFNs were identified, while femles hd higher levels of type I IFNs s immture nd mture birds, immture mles hd higher IFNλ response. The IFNλR complex is composed of two r chins, the IFNλ (IFNλR1) nd Interleukin 10 r 2 (IL-10R2), which utilizes the Jnus kinse (JAK) nd Signl trnsducer nd ctivtor of trnscription (STAT) pthwy for signling. A chicken IFNλR1 gene ws identified, which showed conserved sequence nd synteny with its humn counterprt. Selective sirna medited knockdown of either chin reduced IFNλ medited ISG expression nd STAT ctivtion, while selective JAK1 inhibition blocked the IFNλ medited ISG upregultion. This points towrds conserved IFNλR/JAK/STAT/ISG pthwy in chickens. The interferon-induced protein with tetrtricopeptide (IFIT) repets 5 (IFIT5) is n ISG tht hs been shown to be importnt in the ntivirl response in mmmls. A single IFIT ws identified in chicken which showed synteny with the humn IFIT genes nd encoded 470 mino cid protein with strong conservtion to mmmlin IFITs especilly IFIT5. It ws induced in chicken splenocytes by IFNα, IFNλ nd TLR lignds ex vivo, in the lungs of chickens following infection with highly pthogenic vin influenz virus (HPAI). This highlighted the conserved role of chicken IFIT5 in the response to virl infections like HPAI. 5
7 2 List of Abbrevitions AA mino cids /s mino cid substitutions per site AI vin Influenz ANOVA nlysis of vrince APC ntigen presenting cells bp bse pir CHD cytokine r homology domin CHO Chine Hmster ovry cells CO 2 crbon dioxide CRF2 clss II cytokine r fmily CSF colony-stimulting fctor CSIRO Commonwelth Scientific Industril Reserch Orgnistion DMSO dimethyl sulfoxide dsdna double strnded DNA dsrna double strnded RNA E.coli Escherichi coli EID 50 50% egg infectious dose ELISA enzyme-linked immunosorbent ssy EMSA electrophoretic mobility shift ssy ERK extrcellulr signl regulted kinse HA Hemgglutintion Assy HBV Heptitis B HCV) Heptitis C HPAI highly Pthogenic Avin Influenz IBDV infectious bursl disese virus IBV infectious bronchitis virus IFIT interferon-induced protein with tetrtricopeptide repets IFN interferon IFNα interferon lph IFNαR1 interferon lph r chin 1 IFNαR2 interferon lph r chin 2 IFNγR1 interferon gmm r chin 1 IFNγR2 interferon gmm r chin 2 IFNλ interferon IFNλR interferon r IFNλR1 interferon r chin 1 IL interleukin IL-10R2 interleukin 10 r chin 2 IRF-9 IFN regultory fctor-9 ISGF3 IFN stimulted gene fctor 3 ISGs IFN stimulted genes ISREs IFN stimulted response elements (ISREs) 6
8 JAK Jnus kinse JNK c-jun N terminl kinses LPS lipopolyscchrides LSD lest significnt difference MAPK mitogen-ctivted protein kinse MDA-5 melnom differentition-ssocited gene 5 MDV Mrek s disese virus mrna messenger RNA MX1 Myxovirus resistnce gene 1 (Mx1 NA neurominidse NDV Newcstle disese virus (NDV) PAMPs pthogen ssocited moleculr ptterns PBMC peripherl blood monocytes PCR polymerse chin rection PIC poly I:C PKR IFN-induced double-strnded RNA dependent protein kinse PRR pttern recognition r RIG-I retinoic cid-inducible gene-i) RT-PCR rel-time polymerse chin rection SEM stndrd error of the men SFV Semliki Forest virus (SFV) SNP single nucleotide polymorphism ssrna single strnded RNA STAT signl trnsducer nd ctivtor of trnscription TLR Toll-like r TNF tumor necrosis fctor TYK2 tyrosine kinse 2 Viperin virus inhibitory protein, endoplsmic reticulum ssocited, interferon inducible VSV vesiculr stomtitis virus 7
9 3 Tble of Content 1 ABSTRACT LIST OF ABBREVIATIONS TABLE OF CONTENT INTRODUCTION AVIAN INFLUENZA HOST IMMUNE RESPONSE TO VIRAL INFECTIONS IFNS Type I IFN Type II IFN Type III IFN Expression nd regultion Antivirl ctions of IFNλ Apoptosis nd nti-prolifertive ctions of IFNλ CLASS II CYTOKINE RECEPTORS IFN RECEPTORS IFNΛ RECEPTOR COMPLEX Expression nd distribution IFNλ r signl trnsduction THE CHICKEN IFN SYSTEM Chicken type I IFN Chicken type II IFN Chicken type III IFN Chicken IFN rs INTERFERON STIMULATED GENES Mx ZAP PKR IFIT Viperin
10 4.9 RATIONALE Hypothesis Aims MATERIAL AND METHODS CELL CULTURE Chicken splenocytes DF1 cells Trnsfection Cell stimultion IN VIVO STUDIES Ethics Egg tril (H1N1) Virus propgtion Animl tril (H5N6) BIOINFORMATICS Primer design nd domin prediction Phylogenetic nlysis MOLECULAR METHODS RNA isoltion nd reverse trnscription Cloning nd sequencing Quntittive Rel Time PCR (RT-PCR) Electrophoretic mobility shift ssy (EMSA) CHARACTERIZATION OF CHICKEN IFNλ SIGNALING INTRODUCTION RESULTS Phylogenetic nlysis of the chicken type I/III IFNs Dose response of chicken IFNs to poly (I:C) Time course of TLR-dependent stimultion of chicken IFNs Time course of IFN medited induction of chicken IFN expression Chrcteriztion of TLR-dependent ISG responses
11 6.2.6 Chrcteriztion of the type I/III IFN-medited ISG response Chrcteriztion of the IFN response following cute HPAI H5N6 infection Chrcteriztion of ISGs expression following cute HPAI H5N6 infection Comprison of type I nd III IFN expression between mle nd femle chickens DISCUSSION CHARACTERIZATION OF CHICKEN IFNλR INTRODUCTION RESULTS Phylogenetic nlysis of the type III IFN r chins Synteny of genes encoding the type III IFN r chins Tissue distribution of the type III IFN r chins Functionl nlysis of the chicken type III IFN r complex DISCUSSION CHARACTERIZATION OF CHICKEN IFIT INTRODUCTION RESULTS Identifiction nd chrcteriztion of puttive chicken IFIT5 gene Conservtion of chicken IFIT Expression of chicken IFIT5 following immune stimultion Expression of chicken IFIT5 following virl infection DISCUSSION GENERAL DISCUSSION REFERENCES APPENDIX
12 List of Figures: Figure 4-1 Schemtic structure of influenz virus Figure 4-2 Host rnge nd infection cycle of influenz virus Figure 4-3 Antigenic drift of influenz virus Figure 4-4 Antigenic shift of influenz virus Figure 4-5 The IFN-medited ntivirl response Figure 4-6 The IFN r signling cscde Figure 6-1 Phylogenetic nlysis of the type I/III IFNs Figure 6-2 Dose response of chicken IFNs to poly (I:C) Figure 6-3 Time course of TLR-dependent stimultion of chicken IFNs Figure 6-4 Time course of IFN-medited induction of chicken IFN expression Figure 6-5 Chrcteriztion of TLR-dependent ISG response Figure 6-6 Chrcteriztion of the type I/III IFN-medited ISG response Figure 6-7 Chrcteriztion of IFN response following cute HPAI H5N6 infection Figure 6-8 Chrcteriztion of ISG response following cute HPAI H5N6 infection Figure 6-9 Comprison of type I nd III expression in immture mle nd femle chickens Figure 6-10 Comprison of type I nd III IFN expression in mture mle nd femle chickens Figure 7-1 Phylogenetic nlysis of IFNλR1 proteins Figure 7-2 Phylogenetic nlysis of IL-10R2 proteins Figure 7-3 Synteny of IFNLR1 nd IL10R Figure 7-4 Tissue distribution of IFNλR chins Figure 7-5 Confirmtion of successful sirna medited gene knockdown Figure 7-6 Effect of IFNλR chin knockdown on STAT ctivtion Figure 7-7 Effect of IFNλR chin knockdown on Mx1 expression Figure 7-8 Effect of JAK inhibition on IFNλ-medited Mx1 induction Figure 8-1 Chromosoml loction nd predicted gene structure of chicken IFIT Figure 8-2 Amplifiction of the chicken IFIT5 gene Figure 8-3 Phylogenetic nlysis of IFIT5 proteins Figure 8-4 Domin nlysis of humn nd vin IFITs Figure 8-5 Expression of IFIT5 nd Mx1 in response to TLR gonists Figure 8-6 Expression of IFIT5 nd Mx1 in response to IFN stimultion
13 Figure 8-7 Expression of IFIT5 nd Mx1 in response to virl infections in vivo Figure 8-8 Expression of IFIT5 nd Mx1 in response to virl infections in ovo Figure 11-1 IFN lph mrna expression post IFN lph stimultion Figure 11-2 Alignment of th predicted nd sequenced chicken IFIT5 sequence Figure 11-3 in silico trnslted protein sequence lignment of the predicted nd sequenced chicken IFIT5 sequence
14 4 Introduction 4.1 Avin influenz Influenz A viruses re members of the Orthomyxoviride fmily being lipid enveloped, negtive-sense, single-strnded segmented RNA viruses, with eight segments encoding ten proteins 1,2. Protruding from the enveloped surfce re two distinct glycoproteins, the hemgglutinin (HA) nd the neurminidse (NA) on which subtypes nd ntigens re clssified 3,4 (Figure 4-1). Influenz is cpble of infecting rnge of hosts including but not limited to humns, dogs, horses, pigs, ferrets, cts nd wide vriety of domesticted nd wild birds It is believed tht wild qutic birds ct s reservoir for influenz A viruses, since virtully ll HA nd NA subtypes hve been isolted from tht source 13. The exceptions re the recently discovered subtypes H17 nd H18 tht seem to be limited to bts 14,15 (Figure 4-2). This suggests tht spill-over hosts, like domesticted poultry nd humns, will remin t risk due to the difficulties of erdicting pthogen in wild popultion. There re two mjor mechnisms influencing virl diversifiction. Antigenic drift (Figure 4-3) describes the mechnism of generting sesonl influenz virus, where point muttions in the virl genome chnge the ntigenic profile of the virus leding to immune evsion 16,17. Antigenic shift (Figure 4-3) describes the rerrngement of the segmented genome when more thn one strin infects one cell which forms new virus with often unique properties 18,19. This is thought to occur prticulrly in pigs, which cn be infected by both vin nd humn virus strins, giving rise to new highly pthogenic influenz vrints 20. The selective pressure of the host immune system plys key role in both ntigenic drift nd shift 21,22. Infectivity of influenz virus in the host is influenced by the type of r the virus cn bind 23. The HA subtypes of vin influenz (AI) viruses, such s the H5 nd H7 subtypes, re thought to preferentilly ttch to the α 2,3-linked silic cid rs present on the respirtory epithelium nd intestinl trct tissue of mny vin nd terrestril bird species 24 while HA subtypes dpted to humns preferentilly bind the α 2,6-linked silic cid rs 25. However, there re exceptions to this, with single mino cid substitutions t the r binding site of the HA molecule enough to llow HPAI H5N1 viruses to recognize the humn α 2,6-linked silic cid r on the surfce of humn respirtory epithelil cells nd thereby ssist in cross-species trnsmission 26. It hs similrly been shown in the recent H7N9 outbrek in Chin tht the humn virus isoltes possessed two muttions 13
15 ssocited with incresed α 2,6-linked silic cid binding tht could ccount for their humn pthogenicity 27. Therefore, both ntigenic shift nd drift cn lso influence host specificity. AIs cn cuse significnt mortlity nd morbidity in humns. Since humn cses of H5N1 were reported, which led to 450 deths 28. More recently the H7N9 LPAI, which cused no deth in chickens 29, led to severe symptoms nd even deth in humns 27. Until now totl of 781 lbortory confirmed cses hve been reported cusing 313 deths 30. In 2014 new H5 virus emerged, subtyped s n H5N6, which cused 14 confirmed cses nd 6 deths 28,31. Evidently the AI viruses re still re-ssorting nd re likely to cuse more mortlity nd morbidity since they hve now become endemic in number of countries nd pose serious risk of becoming new pndemic strin Aprt from the humn cses, HPAI strins hve killed millions of poultry worldwide directly nd indirectly, since continment of the outbreks hs necessitted culling of dditionl birds. The impct of HPAI strins on the poultry industry hs been substntil with the economic loss hs been numbered in the billions (USD) 34,35. Trnsmission of AI from poultry to humns usully occurs by close ssocition 27,36. Therefore, n AI virus with the bility to spred esily between host species poses serious risk nd could be potentil influenz pndemic virus cndidte 37. Figure 4-1 Schemtic structure of influenz virus Influenz viruses re negtive-sense single-strnded RNA viruses. Their genome consists of eight RNA segments tht encode 10 proteins, which re encpsulted by mtrix proteins nd surrounded by host-derived lipid envelope from which the glycoproteins hemgglutinin nd neurminidse protrude. 14
16 ? Figure 4-2 Host rnge nd infection cycle of influenz virus Wter fowl, such s ducks, re thought to be the nturl reservoir for influenz virus. Through the fecl/orl route they infect rnge of domesticted nimls like horses, pigs nd chickens. The virus cn then rech the humn popultion by trnsmission vi chickens or pigs. In contrst, the newly discovered H17 nd H18 subtypes seem to be limited to bts. Figure 4-3 Antigenic drift of influenz virus Sesonl influenz viruses typiclly cquire muttions through their error prone virl RNA polymerse. These muttions cn lter ntigenicity nd enble the virus to escpe the immune system. 15
17 Figure 4-4 Antigenic shift of influenz virus Different strins of influenz tht infect the sme cell cn rerrnge their segmented genomes, which cn led new gene combintions not previously encountered by the host immune system, potentilly leding to new pndemic viruses. The pig is thought to be the idel mixing vessel since vin s well s humn pthogenic influenz strins cn infect it. 4.2 Host immune response to virl infections The mmmlin immune response to virus infections is well chrcterized. During virl invsion, innte immune recognition is medited by series of germ line encoded rs, the pthogen recognition rs (PRR), which detect conserved pthogen ssocited moleculr ptterns (PAMPs) One of the most prominent fmilies of PRR re the Toll-like rs (TLR) 41. Up to thirteen conserved TLRs hve been found in mmmls tht serve distinct functions in PAMP recognition nd subsequent immune responses, lthough vrition between species exist in the number of functionl TLRs 42,43. TLRs induce the expression of genes involved in both direct cellulr defence nd the mobiliztion of wider immune response 38,44. Infection with virl pthogens my be sensed through TLRs 2 nd 4 tht detect virl glycoproteins on the cell surfce or through recognition of dsrna through TLRs 3,7, 8 nd 9 locted in the endosome In ddition to the TLRs, intrcellulr sensors such s retinoic cid-inducible gene-i (RIG-I) nd melnom differentition-ssocited gene 5 (MDA-5) re involved in the cytosolic recognition of foreign nucleic cids 52. PAMP recognition leds to ctivtion of mny cell types, such s phgocytes nd mcrophges nd the expression of number of genes including those encoding cytokines 53,54. Cytokines re soluble, low moleculr weight polypeptides nd glycopeptides tht interct with multicomponent trnsmembrne r complex nd ctivte intrcellulr signl trnsduction pthwys tht induce nti-virl genes s well s others tht control the 16
18 complex interply between vrious cell types involved in the immune response Cytokines represent lrge fmily of signling molecules, including the interferons (IFN), interleukins (IL), colony-stimulting fctors (CSF), trnsforming growth fctors (TGF) nd tumor necrosis fctors (TNF), ech of which consists of multiple members 58. However, the IFNs produced in the erly stges of virl infection re of specil interest since they ply crucil role in the brod spectrum cellulr defence ginst the spred of virus 59 (Figure 4-5). Figure 4-5 The IFN-medited ntivirl response The ntivirl ctions of the type I/III interferon systems hve been extensively studied in mmmls. Upon sensing foreign nucleic cids host Toll-like rs ctivte signls tht induce the trnscription of IFN genes, the products of which re secreted by the cell nd serve to mplify the signl in n utocrine nd/or prcrine mnner. This is chieved by binding to cognte rs tht rely signl vi downstrem signl pthwys into the cell nucleus, which leds to the expression of ISGs. These genes encode proteins tht block virl infection, growth nd spred in multitude of wys. 4.3 IFNs IFNs were discovered by Iscs nd Lindenmnn while studying influenz virus. They described the first IFN s substnce tht ws ble to interfere with the bility of the virus to infect cells nd thus coined the term interferon 60. It is now recognized tht there re in fct severl IFNs tht re collectively crucil for defence ginst pthogens, contributing to the induction nd regultion of both innte nd dptive ntivirl mechnisms Three distinct IFN fmilies hve been identified in vertebrte species. The fmilies re defined by their use of specific r complexes, termed type I, II nd III. These re distinguished by their distinctive signling pthwys nd ptterns of gene induction, lthough there is considerble overlp in these fetures between fmilies All three IFN fmilies re 17
19 importnt for n effective immune response but only type I nd III re directly induced vi the PRR pthwy 59. This suggests tht they ply criticl role in contining pthogens, providing the host with the mens to survive otherwise lethl infections by delying spred of the pthogen nd enbling the dptive immune system to mount n efficient response Type I IFN Type I IFNs consist of t lest 8 subclsses IFNα, IFNβ, IFNε, IFNκ, IFNω, IFNτ, IFNδ nd IFNζ. The first 5 re found in humns with 13 subtypes of IFNα but only single IFNβ, IFNε, IFNκ nd IFNω 68,69. The other subclsses of type I IFNs hve so fr only been identified in prticulr species: IFNτ in ruminnts 70, IFNδ in pigs 71 nd IFNζ in mice 72. Following induction by virl infection, type I IFNs cn ct in both prcrine nd utocrine mnner 73,74. Of the type I IFNs, IFNα nd IFNβ re well chrcterized, displying potent ntivirl ctivities. A brod rnge of cells cn produce IFNα nd IFNβ rpidly during the erly stges of infection in response to the recognition of virl (nd bcteril) products vi PRRs 61,75,76. In turn IFNs stimulte the expression of more thn 300 genes tht re clled IFN stimulted genes (ISGs) mny of which encode ntivirl proteins tht cn collectively interfere with vrious stges of the virl life cycle This erly ntivirl response is criticl to limit the spred of viruses nd fcilitte onset of the dptive immune response 80. Type I IFNs up-regulte the expression of the mjor immune histocomptibility complex I (MHC I) expression on mny cells 81 s well s co-stimultory molecules on ntigen presenting cells (APC) 82, enhnce nturl killer cell (NK) cytotoxicity, prolifertion nd memory functions 83-85, induce differentition of monocytes to dendritic cells (DC) 86, contribute to DC ctivtion nd priming bilities 87, ugment the differentition of T helper (Th) cells 88-90, modulte ntibody production 91 nd induce poptosis of infected cells 92,93. The nti-virl, immune stimulting nd nti-prolifertive properties of IFNs hve mde them ttrctive molecules s potentil therpeutics for vriety of diseses 93,94. Recombinnt IFNα ws the first pproved bio-therpeutic nd hs been successfully used in the tretment of chronic heptitis B (HBV) nd heptitis C (HCV) infections, where it leds to reduced virl lods nd decresed incidence of liver cirrhosis nd heptocellulr crcinoms 95,96. Type I IFNs hve lso regulrly been used in clinics s nti-cncer tretments, such s hiry cell leukemi, Kposi s srcom, chronic myelogenous leukemi nd metsttic mlignnt melnom Likewise IFNβ forms crucil prt of long-term tretment for relpsing forms of multiple sclerosis 101. Combintion therpy of IFNα with nti-virl drugs like 18
20 ribvirin, cn improve the tretment efficiency, lthough clinicl drug resistnce to IFNα hs risen in the setting of HCV infection 102,103. The successful use of IFN lone nd in combintion to tret virl infections hs been demonstrted experimentlly both in vitro nd in vivo However, type I IFNs represent two-sided sword since they cn lso induce severe dverse effects rnging from dirrhoe, ftigue nd depression to flu-like symptoms nd hemtologic toxicity 107, Type II IFN Only one type II IFN hs been identified, IFNγ, which exists s single gene in mmmls nd birds 109,110. This is pro-inflmmtory cytokine tht hs n essentil role in the ctivtion of host defence ginst intrcellulr pthogens, representing hllmrk cytokine of Th1 cells 111,112. In contrst to the type I nd III IFNs, type II IFN is only produced by cells of the immune system such s NK cells, mcrophges, dendritic cells nd T cells 113,114. IFNγ hs similr ctions to other IFNs in certin wys but it lso hs unique properties. For exmple, it is the only IFN tht cn enhnce MHC clss II expression 81,115 nd is involved in the regultion of nitric oxide production nd the promotion of Th1 differentition 116, Type III IFN Type III IFNs were discovered in 2003 through computtionl nlysis of the humn genome, which identified three distinct proteins 118,119. These were plced phylogeneticlly between type I IFNs nd IL-10 relted cytokines 120, leding to them being given lternte nmes. Shepprd nd collegues designted them IL-28A, IL-28B nd IL-29 while others clssified them s type III IFNs, IFNλ1 (IL-29), IFNλ2 (IL-28A) nd IFNλ3 (IL-28B) with the ltter nmes now tking precedence 118. These IFNs shre 15-19% mino cid () homology with the type I IFNs but only 11-13% homology with IL-10, lthough their intron-exon structure resembles those of IL-10 nd IL-10-relted cytokines, while the crystl structure of IFNλ is structurlly closest to the IL-10-relted IL ,119,121,122. The IFNλ genes re locted in closely positioned clusters on humn chromosome 19 nd mouse chromosome 7, respectively 118,119,123. In the mouse only two of the three IFNλ genes re considered functionl 123,124,wheres fourth IFNλ gene hs been discovered in humns 120,125. Due to their ntivirl nd nti-prolifertive ttributes, type III IFNs hve lso been used in virl 126 nd cnce27 tretment Expression nd regultion Co-induction of type I nd III IFNs in response to rnge of virl nd bcteril components nd TRL gonists hs been reported in vrious in vitro nd in vivo settings 64,119,128,129. While 19
21 both IFN fmilies cn be expressed by lmost ll cell types, the plsmcytoid DC (pdc) linege ppers to be the gretest producers of both IFNs in vitro 120,130,131. As mentioned erlier, expression of IFNs is triggered by PRRs, including the constitutively-expressed IFN regultory fctor (IRF) 3 nd the IFN-induced IRF7 132,133. Mny RNA viruses potently induce IFNλ expression including influenz A virus 130,134, Sindbis virus (SINV) nd VSV 118. Furthermore, stimultion with lignds for TLR3, TLR4 nd TLR9 significntly incresed IFNλ expression, wheres gonists for TLR7/8 were only wek inducers 130, It hs recently been demonstrted tht murine mcrophges express high levels of type I IFN mrna but not type III IFN mrna fter herpes simplex virus (HSV) infection 135 nd tht influenz virus infection of humn lveolr type II cells elicted high levels of IFNλ but not IFNβ 137. Chrcteriztion of the humn type III IFN promoters hs reveled the presence of IRF nd nucler fctor (NF)-κB binding sites 138. IFNλ1 expression exhibited n IRF-3 dependence similr to tht of IFNβ wheres IFNλ2/3 expression ws controlled by IRF-7 similr to IFNα 139,140. In ddition, the NFκB sites in the promoter region of IFNλ1 were shown to be criticl in DCs, suggesting tht NFκB is key regultor in these cells 141 nd providing further evidence tht the IFN induction pthwys of type I nd III IFNs differ. This indictes tht more work is needed to fully understnd the regultion of IFNλ Antivirl ctions of IFNλ IFNs cn modulte the immune system, but cn lso directly interfere with the infection cycle of viruses 142, which hs seen IFNs successfully used in vriety of ntivirl tretments 102,143,144. The chrcteriztion of IFNλ hs lrgely focused on its ntivirl role in the course of infection, where it displys ctivities reminiscent of type I IFN. Antivirl ssys together with gene expression studies hve demonstrted tht IFNλ induces n ntivirl stte, with lrge number of ntivirl genes stimulted 137,145, which re mostly identicl to those up-regulted upon type I IFN stimultion 146. This could point towrds redundncy of the immune system tht could ply criticl role in host survivl, s mny pthogens hve developed mechnisms to evde or inhibit specific spects of the host immune response 147. However, differences in type I nd type III ctivity cn be observed. For exmple, studies hve suggested IFNλ ntivirl ctivity ws weker thn type I IFNs, with higher concentrtions required to chieve equivlent induction of ntivirl genes 128,129. In ddition, the pretretment of cells with IFNλ before virl chllenge cn led to reduced virl repliction, s is lso seen with IFNα pre-tretment. In contrst, IFNλ tretment post infection hd no effect wheres IFNα ws still ble to inhibit virus repliction to some extent 148. A recent study lso 20
22 found tht rhinovirus infection of bronchil epithelil cells in vitro induced IFNλ mrna nd protein expression more strongly t ll time points mesured, compred to IFNα, which ppered erly, nd IFNβ, which ws induced t lter time points 149. Other studies hve shown tht type III IFNs ply criticl role in the control of rotvirus. Mice lcking functionl IFNλ but competent for type I IFN could not control infection vi the orl route, while dministrtion of type III but not type I IFNs could induce n ntivirl stte in intestinl epithelil cells, providing evidence tht this system is independent of type I IFNs 128. Type III IFNs hve lso been shown to hve modultory effect on the immune system. IFNλ hs been shown to decrese IL-4, IL-5 nd IL-13 production 150, thereby promoting Th1 rther thn Th2 response 150,151 in vitro. Type III IFNs my provide n lterntive therpeutic venue for the tretment of virus infections such s heptitis C in instnces of type I IFN resistnce 152,153. Indeed IFNλ hs been used in combintion with ribvirin to control HCV infections with promising results 154. There is thus potentil for using different drug/ifn combintions, which could result in further nti-virl synergy while decresing the dose, diminishing side effects nd reducing potentil resistnt virus isoltes from developing 155,156. Whilst IFNλ displys similr nti-virl nd ntiprolifertive ctivities to type I IFNs their respective outcomes differ in mgnitude 146,152. Thus, IFNλ my lso elicit reduced dverse side effects tht re ssocited with type I IFN therpy 157,158. For exmple IFNλ ws found to ctivte ISGs to much lower extent in brin cells, suggesting tht type III IFN therpy might lessen the neuropsychitric effects ssocited with type I IFN therpies 136, Apoptosis nd nti-prolifertive ctions of IFNλ Type III IFNs hve been shown to exert nti-prolifertive nd pro-poptotic effects in the humn kertinocyte cell line (HCT) s well s suppress tumor growth nd induce poptosis in humn glioblstom, neuroendocrine, lung crcinom nd fibrosrcom cells in vitro s well s in melnom, fibrosrcom nd colon cncer in vivo nd in some cses the bility to induce poptosis ws shown to surpss tht of IFNα 152, However, the nti-prolifertive nd pro-poptotic ctions pper independent, since IFNλ decresed cell prolifertion of intestinl crcinom cells but did not induce poptosis 163. Moreover, when responsive cells were treted with IFNλ nd IFNα they showed greter poptotic effect thn the respective single tretments 160. This suggests brod therpeutic potentil for type III IFNs in combintion with type I IFNs. However, further investigtion is required to fully understnd how type III IFNs might be used for cncer tretment. 21
23 4.4 Clss II cytokine rs Members of the Clss II cytokine r fmily (CRF2) re single pss trnsmembrne proteins defined by structurl similrities in the extrcellulr domin, which includes the cytokine r homology domin (CHD) consisting of two tndem fibronectin type III repets tht is involved in lignd binding, nd intrcellulr sequences 164. They re distinguished from Clss I cytokine rs by differences in key mino cids in the CHD, notbly lterntive conserved cysteine residues nd the bsence of the hllmrk WSxWS motif 164. CRF2 chins combine to form heterodimeric r complexes. The lignds of CRF2 bind with high ffinity to the R1/Rα chin, which chrcteristiclly hs lrge intrcellulr domin. However, binding of the lignd to the R1 chin is not enough to initite signling, which requires recruitment of n R2/Rβ chin tht hs smller intrcellulr region nd lower lignd ffinity. This trimeric complex then initites specific intrcellulr signling cscdes 164. This fmily is composed of 12 distinct r chins, which re used for signling by members of the IFN, IL-10 nd IL-10-relted cytokines, s well s the unrelted fctor VII (FVII) to medite their biologicl ctivities 164, IFN rs The three IFN fmilies use distinct rs to signl, which results in slightly different signling pthwys nd ultimtely vrition in their respective biologicl ctivities 69. The type I IFNs signl through the IFNαR1 nd IFNαR2 complex 166, while IFNγ binds to complex of IFNγR1 nd IFNγR In contrst the type III IFNs bind to r tht utilizes unique IFNλ r chin 1 (IFNλR1) in combintion with the IL-10R2 chin, which is shred by IL- 10, IL-22 nd IL-26 r complexes 118,119,127. Severl of the rs cn be lterntively spliced which leds to both membrne-bound or secreted soluble forms 164. The soluble r forms re usully identicl in the extrcellulr domin but lck the trnsmembrne nd intrcellulr domins 168, nd re known to prticipte in signling nd its regultion 169. For exmple, mice hve n lterntive soluble form of IFNαR2 tht seems to be independently regulted from the membrne bound form 170,171. These soluble forms hve been thought to ct s decoys, whereby they inhibit the binding of the lignd to the membrne bound form 168. Thus the soluble form of IFNλR1 ws found to ntgonize IFNλ ctivity 172. However, in vitro studies on IFNαR2 hve suggested tht the soluble r cn interct with IFNα nd the membrne bound IFNαR1 to promote signling 171. Therefore, while soluble rs re clerly importnt in controlling cytokine signling pthwys nd immune responses, their exct role is not yet well understood. 22
24 4.6 IFNλ r complex Type III IFNs ct through unique trnsmembrne r complex which consists of the IFNλR1 nd the IL-10R2 chins 118,119 (Figure 4-6).When either of these chins is bsent or neutrlized by n ntibody, cells re unresponsive to IFNλ 119,159, showing tht both IFNλR1 nd IL-10R2 re needed to form functionl 66. The crystl structure of IFNλR1 indictes tht it is most similr in structure to IL-10R1 nd IL-22 binding proteins 121, which is perhps not surprising since ll interct with IL-10R2. Studies investigting the potentil competitive inhibition of other IL-10R2 lignds, IL-10 nd IL-22, on IFNλ signling showed tht while the presence of IL-10 ws ble to suppress the effects of IFNλ by unknown mechnisms, no direct competitive inhibition for binding to the IL-10R2 chin ws found 173,174. Figure 4-6 The IFN r signling cscde IFNλ signls vi heterodimeric r complex consisting of IFNλR1 nd IL-10R2. Lignd binding brings the ssocited Jnus kinse 1 (JAK1) nd Tyrosine kinse 2 (TYK2) proteins into close proximity, which leds to cross-phosphoryltion nd phosphoryltion of the r complex. This fcilittes the recruitment of signling molecules, notbly including STAT1 nd STAT2. These lso become phosphorylted llowing them to form trimeric complex with the Interferon Response Fctor 9 (IRF9) termed the Interferon Stimulted Gene Fctor (ISGF3). ISGF3 cn trnslocted to the nucleus interct with Interferon Stimulted Response Elements (ISREs) to medite trnscription of ISGs Expression nd distribution Ultimtely the pttern of r expression determines which cells respond to prticulr lignd. Although type III IFNs bind to unique r complex, the downstrem signling is similr to tht of type I IFNs including gene induction nd biologicl ctivities 175. However in mmmls, unlike their type I counterprts, the IFNλR1 is predominntly expressed on epithelil cells, like skin, lung, intestine, colon, stomch liver nd reproductive trct, s well s on specific subsets of immune cells 135,152,159, It ws believed tht endothelil nd fibroblst cell lines do not express IFN λr1 but evidence hs emerged tht show n effect on 23
25 endothelil cells of the blood brin brrier nd in the neuro-invsion of West Nile virus, clerly demonstrting tht endothelil cells hve response to IFNλ 180. This suggests tht the type III IFN system hs evolved to protect the brriers like the mucos nd the blood brin brrier ginst pthogens 159, in contrst to the type I system, the rs for which re expressed on ll nucleted cells 181. Liver, pncretic nd colorectl crcinom cell lines lso express IFNλR1 nd re therefore susceptible to type III IFN signling 142,159, IFNλ r signl trnsduction The binding of cytokine to its cognte r initites signling cscde which results in chnges of the physiologicl stte of the cell 56. A vriety of signling cscdes hve been identified tht re utilized by cytokine rs to medite the cell response 182. One key pthwy is nmed the Jnus Kinse-Signl trnsducer nd ctivtor of trnscription (JAK- STAT) pthwy. The JAK fmily comprises four members, JAK1, JAK2, JAK3 nd Tyrosine kinse 2 (TYK2), nd the STAT fmily comprises seven members, STAT1-4 5,5b nd 6 183,184. IFNλ initilly binds to the IFNλR1 chin, which cuses conformtionl chnge tht enbles recruitment of the IL-10R2 to form trimer. This ctivtes the intrcellulrly ssocited tyrosine kinses JAK1 nd TYK2 to medite phosphoryltion of the r chins. This cretes docking sites for vrious cytosolic signling molecules including the ltent trnscription fctors STAT1 nd STAT Signling through type III (nd type I) IFN r complexes results in the formtion of trnscription fctor complex known s IFN stimulted gene fctor 3 (ISGF3) 128,146. This complex consists of 3 proteins, STAT1, STAT2, nd IFN regultory fctor-9 (IRF-9) 120. Once ssembled, ISGF3 trnsloctes to the nucleus where it binds to IFN stimulted response elements (ISREs) in the promoters of vrious interferon stimulted genes (ISGs) 185. Despite different r complexes being employed there is distinct signling overlp between type I nd III IFNs, lrgely due to convergent STAT ctivtion, which helps explin why the ntivirl outcomes re so simil28,146,178,185. However, other signling molecules lso contribute to the induction of ntivirl genes. For exmple, ctivtion of mitogen-ctivted protein kinses (MAPK) like ERK, p38 nd JNK leds to recruitment of uxiliry trnscription fctors tht co-operte with the ISGF3 complex to enhnce its trnscriptionl ctivity 186,187. The type III IFN ctivtes number of MAP kinses, relying to greter extend on p38 nd JNK for gene induction when compred to the type I IFNs 146,
26 4.7 The chicken IFN system Chicken type I IFN Chicken IFNs were those first discovered in 1957 by Iscs nd Lindenmnn while growing influenz virus in chicken chorio-llntoic membrne 60. Originlly they were nmed IFN1 nd IFN2 but Lowenthl nd collegues proposed the stndrd type I nomenclture be pplied nd therefore they were re-nmed IFNα nd IFNβ, respectively 188.In chickens, IFNα consists of multi-gene fmily, while only one member of IFNβ hs been found. The encoded chicken IFNβ shres 58% homology to IFNα but neutrliztion studies with nti- IFNα ntibodies confirmed tht IFNβ ws distinct cytokine 189. All type I IFNs re intron-less nd locted on the Z chromosome, which denotes the mle sex chromosome in birds 189,190. The chicken type I IFNs were found to be strongly induced in response to infection by number of viruses, such s influenz A virus nd Newcstle disese virus (NDV) 191. Exmintion of the promoter regions of chicken type I IFNs hs reveled puttive binding sites for IRFs in ll genes, nd n NFκB site only in the IFNβ promoter, similr to the sitution observed in mmmls 192. Recombinnt chicken IFNα nd IFNβ expressed in bcteri or COS cells displyed pprecible ntivirl ctivity tht ws comprble to their mmmlin counterprts 189. Recombinnt forms of chicken IFNα suppressed the growth in vitro of mny viruses, such s Mrek s disese virus (MDV) 193, infectious bursl disese virus (IBDV) 194 nd infectious bronchitis virus (IBV) 195. Chicken IFNα ws lso shown to lso inhibit the repliction of influenz A virus (H9N2) infection in ovo s well s in vivo Chicken type II IFN Chicken IFNγ hs been identified s single-copy gene on chromosome It demonstrtes high homology to mmmlin IFNγ nd hs been shown to hve similr biologicl ctivities, such s induction of nitric oxide production in mcrophges, ntivirl ctivities ginst vesiculr stomtitis virus (VSV) in vitro, nd up-regultion of MHC clss II expression Chicken type III IFN Computtionl techniques enbled the identifiction of single chicken IFNλ gene on chromosome 7 65,201. The genomic structures of both mmmlin nd chicken IFNλ genes re similrly orgnized into 5 exonic regions 118,119,201. The encoded chicken IFNλ hs higher mino cid identity to humn IFNλ2 compred to IFNλ1 nd IFNλ3, nd n even lower identity to the chicken type I or type II IFNs 201. Chicken IFNλ hs been recombinntly 25
27 expressed nd its biologicl function investigted in number of ssys. Chicken IFNλ displyed inhibitory ctivity ginst Semliki Forest virus (SFV) nd influenz A virus, in similr mnner to tht observed for the type I chicken IFN. However, the overll ctivity of chicken IFNλ ws lower thn its type I chicken IFN counterprts, consistent with findings in humn nd mouse models Chicken IFN rs Only few chicken CRF2 genes hve been cloned or chrcterized to dte. The IFNαR1, IFNαR2, IFNγR2 nd IL-10R2 genes were found to cluster on chicken chromosome 1 in similr fshion to tht seen in the humn 202, with IFNγR1 203 lso identified. Previous studies hve lso indicted the presence nd ctivity of the JAK-STAT pthwy components in birds 204,205, which would suggest conserved downstrem signling, lthough functionl confirmtion remins lcking. IFNλR1 mrna expression hs been described in chicken hert, liver, kidney, intestine, lung nd trche Interferon stimulted genes The ntivirl ctivity of IFNs is medited by hundreds of genes upregulted upon IFN stimultion clled ISGs 207. These genes encode proteins tht re ble to interfere with the virus life cycle t different stges nd so ply key role in IFN medited ntivirl defence. The ntivirl effector ISGs cn be grouped into three min ctegories 207 : (i) proteins tht ct s inhibitors of virl entry like the Myxovirus resistnce gene 1 (Mx1) 208 ; (ii) proteins tht interfere with virl repliction like the zinc finger ntivirl protein (ZAP) 209, the IFN-induced double-strnded RNA dependent protein kinse (PKR) 210 or the interferon inducible protein with tetrtricopeptide repets (IFIT) gene fmily 211 ; nd, lstly (iii) proteins tht inhibit virl budding, like the virus inhibitory protein, endoplsmic reticulum (ER) ssocited, interferon inducible (Viperin) Mx1 The mouse Mx1 gene belongs to the dynmin GTPse fmily 213 nd ws one of the first genes described to ffect virl entry. Its encoded protein is specificlly involved in blocking virl entry into the nucleus 214 vi ssocition with vesiculr COP I, leding to sequestering of essentil virl comprtments within the cell 215. The chicken Mx1 gene hs been studied extensively for its role in influenz virus infections 216. Some publictions clim tht chicken Mx1 is ntivirl 217 while others show no ntivirl benefit 216,218,219. The chicken Mx1 gene is very polymorphic 218 with severl different hplotypes identified cross chicken breeds 220, 26
28 lthough potentil differences between these hplotypes in ntivirl protection hs yet to be determined ZAP ZAP is n ccessory fctor tht recognizes virl RNA nd promotes virl RNA degrdtion vi the RNA exosome 221 thereby inhibiting trnsltion of incoming virl RNA 222. The chicken homologue of ZAP hs previously been identified nd chrcterized PKR PKR is RNA-dependent protein kinse tht upon recognition of dsrna phosphoryltes the eukryotic initition fctor-2α, which then blocks virl protein synthesis by disrupting delivery of trnas to the 40S ribosoml subunit 224. PKR hs lso been shown to be involved in the ctivtion of signl trnsduction pthwys leding to IFNβ gene expression 225. The chicken PKR gene hs been identified nd chrcterized IFIT The vrious IFIT fmily members, IFIT1-5, hve been shown to ply criticl roles in ntivirl defence in humns nd mice 211. The mechnisms re not completely understood but IFIT1 nd IFIT2 hve been shown to suppress trnsltion by binding to the eukryotic initition fctor Furthermore, IFIT fmily members cn lso directly bind to single-strnded RNA 211,227 nd double-strnded DNA 228 nd thereby reduce virl repliction. The chicken IFIT hs been mentioned in severl publictions ,231 but comprehensive chrcteriztion of the gene hs not been performed Viperin Viperin hs been shown to interfere with the virl life cycle of mny viruses by s yet unknown mechnisms 212. Some groups hve suggested tht viperin inhibited repliction of certin viruses 232,233, while others hve shown tht it disturbs lipid rfts to restrict budding of influenz A viruses 234 nd HIV 235. Chicken viperin hs been identified nd chrcterized Rtionle Highly pthogenic vin influenz (HPAI) infections such s H5N1 nd H7N9 cuse significnt morbidity nd mortlity in chickens worldwide 237. This hs widespred consequences, including devstting effects on the poultry industry 238 leding to severe economic losses 239 nd bottlenecks in met supply, since the method of choice to limit the spred of the virus is 27
29 the mss culling of infected chicken flocks 240. There is lso the potentil for zoonotic trnsmission to humns 237, , with most humn infections of HPAI ssocited with direct trnsmission from vin hosts with little or no evidence of humn-to-humn trnsmission 244,245. Therefore, the bility to stop HPAI infection in chicken would be very ttrctive. Knowledge in the field of mmmlin ntivirl defence is dvnced, with studies in mice plying crucil role in the dvncement of knowledge regrding ntivirl immunology nd host pthogen interction 246. In contrst informtion on the vin immune response currently remins limited. Moreover, there re distinct differences in mouse physiology nd immunology compred to nturl pthogen reservoir species s well s spill over hosts 246,247. This clerly indictes the need for more studies in vin species to better understnd pthogenicity of viruses such s HPAI to underpin the development of mens to prevent the spred of these pthogens Hypothesis The studies in this thesis seek to investigte the hypothesis tht the IFNλ signlling complex is involved in the ntivirl immune response in chickens Aims The reserch ddresses following specific ims: To chrcterize the expression of chicken IFNλ nd its effect on in vitro nd in vivo ISG expression in comprison to IFNα. To identify nd confirm the chicken IFNλR complex nd downstrem signling effector molecules. To identify nd chrcterize the chicken IFIT5 gene. 28
30 5 Mteril nd Methods 5.1 Cell culture Chicken splenocytes Chicken splenocytes were purified s described previously 201. Briefly, spleens from 4-6 week old (unless otherwise specified), specific-pthogen free (SPF) chickens were hrvested nd dispersed through 70 μm mesh sieve (BD Flcon). The mononucler cells were then purified using density grdient centrifugtion (Lymphoprep Nicomed Phrm). After wshing, the cells were counted nd 4x10 6 cells per well trnsferred to 24 well pltes (Nunc) nd cultured in DMEM high glucose medi (Life Technologies) contining 10% (v/v) fetl clf serum nd 1000 U/mL penicillin nd streptomycin (Sigm) DF1 cells DF1 cells were mintined in DMEM high glucose medi (Life Technologies) contining 10% (v/v) fetl clf serum nd 1000 U/mL penicillin nd streptomycin (Sigm) t 37 C with 5% CO 2. After pssging the cells were counted nd 6x10 4 cells trnsferred into 24 well pltes (Nunc) nd kept t 37 C with 5% CO 2 overnight Trnsfection Cells were wshed nd cultured in 400 μl Optimem per well. Per well 2 μl Lipofectmine 2000 (Invitrogen) nd 4 nm sirna were incubted t RT for 30 min then 10% DMSO (v/v) ws dded nd the mix trnsferred onto the cells. These were incubted t 37 C with 5% CO 2 fo2 h before the medi ws chnged bck to full growth medi for 48 h Cell stimultion After pssging the cells were counted nd 6x10 4 cells were trnsferred into 24 well pltes (Nunc) nd kept t 37 C with 5% CO 2 overnight. Cells were stimulted with 50 μg/ml of poly (I:C) (Invitrogen), 10 μg/ml lipopolyscchride (LPS) purified from E. coli strin 0111:B4 (gmm irrdited, EU/mg) (Sigm Aldrich), 500 ng/ml recombinnt chicken IFNα (Genwy Biotech) or 50 μg/ml recombinnt chicken IFNλ, kindly provided by Dr Tim Adms (CSIRO Mnufcturing). The chifnα ws produced in n E. coli expression system while the chifnλ ws produced within n mmmlin system utilizing CHO cells both were tested for the bsence of endotoxins. In some experiments cells were pre-treted with 1 nm Ruxolitinib (Selleck Chemicls) per well for 2 h. The doses 29
31 for ech compound were initilly tken from literture or MSDS nd confirmed by in vitro experiments on chicken primry or cell lines to be the most effective dose for (dt not shown). 5.2 In vivo studies Ethics All niml work ws conducted with the pprovl of the CSIRO Austrlin Animl Helth Lbortory Animl Ethics Committee (permit numbe610). All procedures were conducted ccording to the guidelines of the Ntionl Helth nd Medicl Reserch Council s described in the Austrlin code for the Cre nd Use of Animls for Scientific Purposes. All birds were obtined from Austrlin SPF Services P/L (Woodend, Austrli) Egg tril (H1N1) D10 embryonted chicken eggs were inoculted with 100 μl of 1:10000 diluted virus stock A/Puerto Rico/8/1934 H1N1 of 6.4 x 10^8 pfu/ml (kindly provided by Dr. Siying Ye nd Dr. John Stmbs), incubted t 37 C for 24 or 48 h nd then chilled t -20 C for 20 min nd post mortems performed. The tissues were plced into 2 ml tubes (Srsted) with Grphite beds (Dintree Scientific) nd RLT buffer (Qigen) nd homogenized twice for 20 s ech. Tissue homogente ws then stored t -80 C for RNA extrction Virus propgtion A highly pthogenic vin influenz virus A/duck/Los/XBY004/2014 (H5N6) (Lo/14), isolted from pooled duck tissues from Lo PDR 31, ws used in this study. Virus ws propgted ccording to ccepted protocols 249, briefly: After wiping with 70% (v/v) ethnol smll incision ws punched into the egg shell the virus ws introduced by llntoic cvity inocultion of D9 11 embryonted SPF chicken eggs. The incision ws then seled with wood glue nd the eggs incubted t 37 C for 48 h The virus stock ws titrted in chicken eggs nd the 50% egg infectious dose (EID 50)/mL ws clculted ccording to the method of Animl tril (H5N6) Six 5-week-old SPF chickens were used for experimentl infections. Smples from six uninfected chickens from the sme cohort were used s controls. Prior to chllenge, serum ws collected from ech chicken to confirm tht birds were serologiclly negtive for vin 30
32 influenz A virus, s determined by blocking ELISA 251. Ech chicken ws inoculted with dose of 10 6 EID 50 of Lo/14 in 0.2 ml by the orl-nsl-oculr route. Chickens were observed closely from 22 hpi nd were euthnized t humne endpoint defined s progression to moderte signs of disese, including fcil swelling, dirrhoe, hunched posture with ruffled fethers, drooping wings, huddling, recumbency, depression nd slow response to stimultion. In ccordnce with Institutionl Animl Welfre Policies, chickens were euthnized by cervicl disloction following hert bleed under nesthesi (44 mg/kg ketmine, nd 8 mg/kg xylzine injected intrmusculrly). Immeditely fter euthnsi, swbs (orl nd clocl) were tken nd pproximtely 100 mg of tissue from the spleen nd lung were collected into sterile 2 ml tubes contining PBS with ntibiotics nd smll quntity of 1 mm silicon crbide beds (BioSpec Products). Tissue smples were homogenized twice for 20 s in FstPrep24 tissue homogenizer (MP Biomedicls) for biossys. The presence of influenz virl genome within swbs, tissues smples ws ssessed by extrcting totl RNA from ech smple (MgMx-96 Totl RNA Isoltion Kit, Life Technologies) for testing using pn-influenz A mtrix gene rel-time RT-PCR ssy 252. Cycle threshold (Ct) vlues for ech smple were compred to those obtined for set of RNA trnscripts encoding the Lo/14 mtrix genome segment to convert ech smple Ct vlue into vlue representing the number of copies of the mtrix genome segment per μl of smple. These RNA trnscripts were generted using T7 RNA polymerse (Promeg) nd plsmid encoding the Lo/14 mtrix genome segment cloned into the pgem-t-esy cloning vector (Promeg). The complete dt set cn be found in Butler et l Bioinformtics Primer design nd domin prediction Protein sequences were retrieved from the Ensembl genome dtbse ( except for KF956064, which ws retrieved from Genbnk ( Primer design, sequence ssembly nd initil lignment ws performed using CLC-Min Workbench Phylogenetic nlysis All Sequences were retrieved from Genbnk ( Protein lignments used the MUSCLE lgorithm nd phylogenetic trees were clculted using MEGA 6.0. Accession Numbers of ll sequences used in this nlysis re given in the Appendix. Tble 2 (IFNs) 31
33 Tble 3 (IFNλR) Tble 4 (IFNAR1) Tble 5 (IL10R2) Tble 6 (IFITs) 5.4 Moleculr methods RNA isoltion nd reverse trnscription Totl RNA ws hrvested from cultured cells using the Qigen RNAesy extrction kit ccording to the mnufcturer s instruction, nd from tissues using TRIzol (Life Technologies). Prior to qrt-pcr for gene expression nlysis, RNA smples were subsequently treted with RNAse-free DNse (Promeg) nd reverse trnscribed with Superscript III cdna synthesis kit (Life Technologies) Cloning nd sequencing The chifit5 gene ws mplified using Pltinum Tq Mster mix (Life Technologies) with gene specific primers (forwrd primer: 5 -ATGAGTACCATTTCCAAGAAT, reverse primer: 5 - TAGCTTGAGAGGGAAAG) nd cloned into the pgem-t-esy vector (Promeg) using T7 Ligse (Promeg) nd trnsformed into Escherichi coli DH5α (Life Technologies), ccording to mnufcturer s instructions. Plsmids were purified using Qigen Plsmid Miniprep kit nd sequencing ws performed by the Micromon Sequencing Fcility (Monsh University) Quntittive Rel Time PCR (RT-PCR) Gene expression ws quntified in DF1s, splenocytes nd lung nd spleen tissues by RT-PCR using TqMn Universl PCR mster mix (Life Technologies) with FAM reporter dye nd NFQ quencher nd primer probe pirs obtined from Applied Biosystems (Tble 7). Temperture profile for the RT PCRs ws: 50 o C 2 min, 95 o C 10 min, 40 cycles of 95 o C 15 sec nd 60 o C 1 min Electrophoretic mobility shift ssy (EMSA). Nucler extrcts were prepred s described previously 254. Briefly, cells were stimulted, pelleted nd resuspended in ice-cold hypotonic buffer (2 mm HEPES ph 7.8 (Sigm), 20 mm NF (Sigm), 1 mm N 3VO 4 (Sigm), 1 mm DTT (Sigm), 1 mm EDTA (Sigm), 50 μg/ml 32
34 Protese inhibitors (Sigm), 1 mm Tween-20). Cells were then briefy nd the nuclei pelleted by centrifugtion t 15,000g for 30 s. Nucler extrcts were prepred by resuspension of the nuclei in high-slt buffer (hypotonic buffer with 420 mm NCl (Sigm) nd 20 % (v/v) glycerol) nd extrction of proteins by rocking for 30 min t 4 C. Insoluble mterils were removed by centrifugtion t 4 C fo5 min t 15,000g nd nucler extrcts were stored t - 80 C for nlysis. Nucler extrcts were incubted for 20 minutes t room temperture with 32P-lbeled double-strnded m67 oligonucleotide (5 -CATTTCCCGTAAATC), high-ffinity mutnt of the sis-inducible element (SIE) nd poly(di-dc) in binding buffer (13 mmol/l HEPES, ph 7.8, 80 mmol/l NCl, 3 mmol/l NF, 3 mmol/l NMoO4, 1 mmol/l DTT, 0.15 mmol/l EDTA, 0.15 mmol/l EGTA, nd 8% glycerol). The DNA-protein complexes were seprted by electrophoresis on 5% polycrylmide gels contining 5% glycerol in 0.25 x Tris-buffered EDTA (TBE). The gels were dried nd subsequently exposed to phosphoimger screens nd nlyzed with ImgeQunt softwre (Moleculr Dynmics). 33
35 6 Chrcteriztion of chicken IFNλ signling 6.1 Introduction The interferons (IFNs) were discovered by Iscs nd Lindenmnn in the chorio-llntoic membrne of chicken eggs with the isolted protein first described s substnce tht ws ble to interfere with the virus bility to infect cells 60. Since then the knowledge bout IFNs hs incresed drmticlly, lthough most these studies hve been performed in mmmls. IFNs re expressed by virtully ll cells of the body upon sensing of virl PAMPs vi PRRs such s TLRs 38,44. Of the thirteen TLRs identified six re considered to function s ntivirl sensors either through the detection of virl glycoproteins on the cell surfce (vi TLR2 nd 4) or through recognition of dsrna in the endosome (vi TLRs 3,7, 8 nd 9) In ddition to the TLRs, intrcellulr sensors such s RIG-I nd MDA-5 re involved in the cytosolic recognition of foreign nucleic cids 52 lthough in chickens MDA-5 ppers to hve tken over some of the roles of RIG-I tht it is bsent in this species 255. Three IFN fmilies hve been identified which re clled type I, type II nd type III. IFNs re ssigned to these fmilies ccording to their r specificity nd signling pthwys, which defines downstrem gene induction lthough overlps occur in these fetures between fmilies Type I IFNs consist of t lest 8 subclsses with the IFNαs nd IFNβ s their most prominent members 1,22, type II consists of IFNγ nd type III comprises IFNλs of which there re four in humns but only one in the chicken 201. All types of IFN contribute to the ntivirl immune response but only type I nd III hve direct ntivirl effect 59, which is medited by group of hundreds of ntivirl genes clled ISGs 207. Similrities between type I nd type III IFNs hve been described concerning gene induction nd ntivirl properties 146,175. However, ech of these fmilies signl through unique r complex consisting of two chins; for type I IFNs these re IFNAR1 nd IFNAR2 256 nd for type III IFNs these re IFNλR1 nd IL-10R2 118,119. Recent studies hve identified unique roles for IFNλs in ntivirl defense, nd there is emerging evidence tht IFNλs my hve functionl importnce beyond innte ntivirl protection 128. This Chpter focuses on dvncing our understnding of the biologicl ctivities of chicken type III IFN prticulrly the similrities nd differences with respect to type I IFN. 34
36 6.2 Results Phylogenetic nlysis of the chicken type I/III IFNs To obtin n initil insight into the reltive homology between the chicken nd mmmlin IFN systems phylogenetic nlysis of type I nd type III IFNs from chicken, humn nd mouse ws performed (Figure 6-1). Unsurprisingly, the IFNαs of both humn nd mouse clustered together in seprte groups, which collectively were distinct from other humn nd mouse type I IFNs like IFNο, IFNζ nd IFNκ. Chicken type I IFNs, IFNα nd IFNβ, clustered together in subgroup linked with humn nd mouse IFNβ nd IFNε. The chicken IFNλ grouped with the mmmlin IFNλ proteins tht clustered by species. This suggested overll conservtion, including distinct type I nd type III members between chickens nd mmmls Dose response of chicken IFNs to poly (I:C) Poly (I:C) (PIC) is powerful inducer of IFNs 257. It serves s mimic of virl RNA nd so is ble to trigger PRRs like the TLR fmily nd MDA-5. To exmine induction of IFNα nd IFNλ by PIC purified chicken splenocytes were cultured in vitro with different concentrtions of PIC for 3 h nd IFN mrna levels nlyzed by qrt-pcr (Figure 6-2). Both IFNs were induced even by low concentrtions of PIC. Indeed, IFNα reched its pek expression with 5-fold upregultion t 1 μg/ml tht ws similr up to 100 μg/ml. In contrst IFNλ expression incresed up to 10 μg/ml where it mintined pek level of expression t round 600-fold upregultion compred to the untreted control Time course of TLR-dependent stimultion of chicken IFNs To further investigte the type I versus type III IFN response, the timing of TLR-medited IFN induction ws nlyzed following stimultion with PIC s well s lipopolyscchride (LPS), nother TLR gonist 258. Chicken splenocytes were treted with either PIC (Figure 6-3A) or LPS (Figure 6-3B) nd IFN expression mesured t different time points. PIC tretment led to rpid nd robust upregultion of both IFNs, being incresed fter 0.5 h nd peking t 30- fold for IFNα nd 100-fold for IFNλ t h, followed by decline to close to bse-line expression by 24 h post stimultion. LPS tretment, in contrst, filed to induce ny significnt upregultion of either IFNα or IFNλ t ny time points mesured. 35
37 Figure 6-1 Phylogenetic nlysis of the type I/III IFNs The evolutionry history of the type I nd type III IFNs from humn, mouse nd chicken ws inferred by using the Mximum Likelihood method bsed on the JTT mtrix-bsed model 259. The tree with the highest log likelihood ( ) is shown. The ccession numbers of ll sequences used cn be found in the Appendix (Tble 1). UT Figure 6-2 Dose response of chicken IFNs to poly (I:C) Expression of IFNα nd IFNλ mrna in purified chicken splenocytes from SPF chickens stimulted with poly (I:C) t the concentrtions indicted. The brs represent the men fold chnge of 3 chicken spleens with the stndrd error of the men (SEM) compred to the untreted smple, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05 using one-wy ANOVA test with Fischer s uncorrected LSD test). 36
38 Figure 6-3 Time course of TLR-dependent stimultion of chicken IFNs Expression of IFNα nd IFNλ mrna in purified splenocytes from SPF chickens stimulted with 50 μg/ml PIC (A) nd 10 μg/ml LPS (B) over the indicted times. The brs represent the men fold chnge of 3 chicken spleens with the stndrd error of the men (SEM) compred to the untreted smple, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05, *** p vlue < using one-wy ANOVA test with Fischer s uncorrected LSD test). 37
39 6.2.4 Time course of IFN medited induction of chicken IFN expression Self-induction nd/or positive feedbck loops for IFNs exist in mmmls, where they ply n importnt role in signl mplifiction 260. Since this hs not previously been investigted in birds, splenocytes from SPF chickens were exposed to recombinnt chicken IFNα Figure 6-4A) nd recombinnt chicken IFNλ (Figure 6-4B) nd levels of IFN mrnas quntified. IFNα stimultion did not significntly induce IFNα or IFNλ mrna expression cross ll chickens t ny time points mesured lthough induction ws observed in subset (two) of chickens. IFNλ tretment in contrst upregulted IFNλ slightly up until 6 h but by 48 h 3000-fold increse ws observed. IFNλ stimultion cused IFNα mrna levels to decrese until 24 h, but with lter upregultion to 350-fold t 48 h Chrcteriztion of TLR-dependent ISG responses TLR responses in chicken cells hve been previously described 261, but the timing of ISG induction hs not been well chrcterized. Splenocytes from SPF chickens were stimulted with PIC (Figure 6-5A) nd LPS (Figure 6-5B) nd ISG expression quntified. The PIC tretment led to shrp increse in ISG expression t 3 h, with pek expression level for ll ISGs t 6 h post stimultion, followed by decline to bse-line levels fter 48 h. Mx1 showed the highest induction of pproximtely 170-fold followed by Viperin t round 70-fold, with induction of Zp nd PKR peking t round 10-fold t the 6 h time point. LPS stimultion gve similr kinetics of ISG induction, with pek expression t 6 h nd then decline to bse-line expression round 48 h. The mgnitude of induction ws similr for Viperin (pproximtely 50-fold) s well s PKR nd ZAP (round fold), but Mx1 induction ws substntilly reduced (round 20-fold). 38
40 Figure 6-4 Time course of IFN-medited induction of chicken IFN expression Expression of IFNα nd IFNλ mrna in purified splenocytes from SPF chickens stimulted with 500 ng/ml IFNα (A) nd 50 μg/ml IFNλ (B) over the indicted time course. The brs represent the men fold chnge of 3 chicken spleens with the stndrd error of the men (SEM) compred to the untreted smple, normlized ginst the housekeeping gene GAPDH (*** p vlue < using one-wy ANOVA test with Fischer s uncorrected LSD test). 39
41 **** * Mx1 Pkr Viperin Zp Time post PIC stimultion (h) * ** * ** Mx1 Pkr Viperin Zp Time post LPS stimultion (h) Figure 6-5 Chrcteriztion of TLR-dependent ISG response Expression of Mx1, PKR, Viperin nd Zp mrna in purified splenocytes from SPF chickens stimulted with 50 μg/ml PIC (A) nd 10 μg/ml LPS (B) over the indicted time course. The brs represent the men fold chnge of 3 chicken spleens with the stndrd error of the men (SEM) compred to the untreted smple, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05, *** p vlue < using one-wy ANOVA test with Fischer s uncorrected LSD test). 40
42 6.2.6 Chrcteriztion of the type I/III IFN-medited ISG response In mmmlin systems profound differences between type I nd type III interferons hve been described, both in the mgnitude of the ISG response s well s its timing 157. To better understnd the IFN response in chickens, the time-dependent expression of severl well chrcterized ISGs ws evluted. Splenocytes from SPF chickens were treted with chifnα (Figure 6-6A) or chifnλ (Figure 6-6B). ChIFNα tretment led to rpid increse of ll ISGs investigted with similr kinetics, shrp rise in expression levels fter 3 h with pek t round 6 h nd then decline to 48 h. Although hving similr induction kinetics, the mgnitude of the responses differed between the genes, with Mx1 nd Viperin showing highest upregultion t round 40 nd 30-fold increse, respectively, while both PKR nd ZAP expression levels showed n pproximtely 8-fold increse during the pek t 3 h post stimultion. In contrst, chifnλ stimultion of these cells led to considerbly delyed induction of ISGs. A smll increse ws pprent by 1.5 to 3 h, with pek induction t 48 h post stimultion. By this time, both Viperin nd Mx1 were highly induced, with 20-fold nd 10-fold increse t the 48 h time point, respectively. No significnt increse in ZAP or PKR expression ws observed following chifnλ stimultion Chrcteriztion of the IFN response following cute HPAI H5N6 infection To evlute the involvement of type I nd III IFNs in vivo, RNA ws extrcted from spleens nd lungs of H5N6 infected nd uninfected chickens t 24 h post infection nd the levels of IFNα nd IFNλ mesured by qrt-pcr. In the spleen of infected chickens (Figure 6-7A) IFNα ws induced 9-fold nd IFNλ lmost 70-fold compred to uninfected nimls. In the lungs (Figure 6-7B), the primry site of infection, both IFNs were highly induced, with 25-fold increse of IFNα nd 60-fold increse in IFNλ compred to uninfected birds Chrcteriztion of ISGs expression following cute HPAI H5N6 infection ISGs re key meditors of the ntivirl immune response medited by IFNs. Therefore, the expression of some of the most importnt ntivirl genes ws exmined during cute HPAI infection. In the spleen of infected chickens Mx1 gene expression ws induced 230-fold compred to uninfected controls with PKR being slightly lower t round 40-fold, but no significnt upregultion of ZAP expression ws observed (Figure 6-8A). In the lungs, expression of Mx1 ws induced 65-fold nd PKR round 60-fold with ZAP gin not upregulted (Figure 6-8B). 41
43 Figure 6-6 Chrcteriztion of the type I/III IFN-medited ISG response Expression of Mx1, PKR, Viperin nd Zp mrna in purified splenocytes from SPF chickens stimulted with 500 ng/ml IFNα (A) nd 50 μg/ml IFNλ (B) over the indicted time course. The brs represent the men fold chnge of 3 chicken spleens with the stndrd error of the men (SEM) compred to the untreted smple, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05, **** p vlue of < using one-wy ANOVA test with Fischer s uncorrected LSD test). 42
44 A IFN IFN B IFN IFN Figure 6-7 Chrcteriztion of IFN response following cute HPAI H5N6 infection Expression of IFNα nd IFNλ mrna in the spleen (A) nd lung (B) of six 5-week old SPF chickens 24 hours post infection with Influenz virus A/duck/Los/XBY004/2014 (H5N6). Dt is shown s the men fold chnge of mrna expression with the SEM compred to the sme tissue of the uninfected birds, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05 using the Students t test with Mnn Whitney U test). 43
45 reltive expression in spleen (fold) A Mx1 Pkr Zp B reltive expression in lung (fold) Mx1 Pkr Zp Figure 6-8 Chrcteriztion of ISG response following cute HPAI H5N6 infection Expression of Mx1, Pkr nd Zp mrna in the spleen (A) nd lung (B) of six 5-week old SPF chickens 24 hours post infection with Influenz virus A/duck/Los/XBY004/2014 (H5N6). Dt is shown s the men fold chnge of mrna expression with the SEM compred to the sme tissue of the uninfected birds, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05 using the Students t test with Mnn Whitney U test). 44
46 6.2.9 Comprison of type I nd III IFN expression between mle nd femle chickens. Recent evidence from mice nd humns suggests tht femles nd mles differ in the mgnitude of immune responses prticulrly with regrds to IFNs, with link to femle hormone levels lso suggested 262,263. To exmine this possibility in chickens, splenocytes from immture femle nd mle chickens were treted with PIC (Figure 6-9A/B/C) with blood estrdiol levels of ech bird mesured in prllel (Figure 6-9D). IFNα mrna levels (Figure 6-9A) were reltively tightly grouped: mles tended to hve higher levels erlier but femles tended to show significntly higher pek t 2 h. The IFNβ mrna levels (Figure 6-9B) followed the sme trend s IFNα, with significntly higher femle expression t 2 h. Interestingly the opposite trend ws observed for IFNλ (Figure 6-9C) with mles hving generlly higher expression lbeit only reching significnce t the 2 h time point. The inbility to rech significnce t other time points ws in prt due to highly vrible IFNλ expression in femles compred to the mles with severl individuls showing negligible IFNλ induction. The concentrtion of serum estrdiol ws mesured in both groups nd indicted tht the immture femle chickens hd higher levels, rnging from pg/ml while ll the mle smples clustered round 60 pg/ml. The experiment ws repeted with chicken splenocytes from sexully mture mle nd femle chickens. PIC stimultion (Figure 6-10A) led to higher men IFN expression in femles which reched sttisticl significnce for IFNλ. The estrdiol levels (Figure 6-10B) were significntly higher in femles rnging from pg/ml, with in mles gin round 60 pg/ml. Single bird correltion of estrdiol levels nd IFN mrna expression showed no conclusive pttern in femle or mle birds (Figure 6-10C/D). 45
47 Femle Mle Figure 6-9 Comprison of type I nd III expression in immture mle nd femle chickens. Expression of IFNα (A), IFNβ (B), IFNλ (C) mrna in purified chicken splenocytes from 6 femle (circles) nd 6 mle (tringles) 5-week old SPF chickens stimulted with 50 μg/ml PIC over the time course indicted. Symbols represent fold upregultion of ech stimulted splenocytes smple compred to the untreted smple, normlized ginst the housekeeping gene GAPDH. (For A-C: * p vlue < 0.05, ** p vlue < 0.005, *** p vlue < using two-wy ANOVA with Bonferroni's multiple comprisons test) (D) Blood estrdiol levels chickens t the time of scrifice (*** p vlue < using the Students t test with Mnn Whitney U test). 46
48 A B IFN IFN C D 100 R 2 : Mle IFN Mle IFN 10 R 2 : Estrdiol (pg/ml) Figure 6-10 Comprison of type I nd III IFN expression in mture mle nd femle chickens. Expression of IFNα nd IFNλ mrna in purified chicken splenocytes from 6 femle (circles) nd 5 mle (tringles) 12-week old SPF chickens stimulted with 50 μg/ml PIC for 2 h. Symbols represent fold upregultion of ech stimulted splenocytes smple compred to the untreted smple, normlized ginst the housekeeping gene GAPDH (A). (B) Blood estrdiol levels of the chickens t the time of scrifice. (For A: * p vlue < 0.05 using twowy ANOVA with Bonferroni's multiple comprisons test, for B ** p vlue < using the Students t test with Mnn Whitney U test). Depiction of IFNα nd IFNλ mrna levels for femles (C) nd mles (D) (R 2 vlues indicte the curve fit for liner regression). 47
49 6.3 Discussion Like other bird species, chickens hve single IFNλ, compred to four members in humns 125 nd two in mice 124. This Chpter sought to further the knowledge bse bout chicken IFNλ, prticulrly in comprison to type I IFNs. Phylogenetic comprison of chicken type I nd III IFNs with those of mice nd humns provided insights into their evolution. IFNαs hve seprtely expnded in both humns nd mice, forming distinct sub-cldes tht group together. These formed lrger clde with other type I IFNs, including the single IFNβ nd numerous lternte IFNs. It hs been rgued tht this reflects the strong evolutionry pressure on IFN evolution tht is thought to be unequl between IFNs 264. Notbly, chicken IFNα nd IFNβ clustered together, confirming their presumed divergence from common precursor, but lso suggesting tht speciesdependent evolutionry pressure hs driven them to remin reltively highly conserved compred to those of other species 68. The IFNγ proteins formed distinct cluster, indictive of conserved role cross species 265, with the humn nd mouse IFNλs gin forming seprte sub-cldes. Since their discovery, type III IFNs hve been compred to their type I counterprts 118,119. Under certin experimentl situtions, type III IFNs showed similr gene induction to type I IFNs but elicited reduced mgnitude of ntivirl effect 266,267. This hs led to the ssumption tht IFNλs re just weker form of type I IFNs. However, other studies hve found significnt differences between type I nd type III IFNs, including the identifiction of novel roles for the ltter Whether such differences were present in chickens remined n unnswered question. Therefore, the induction nd effects of IFNλ were directly compred to IFNα in primry chicken splenocytes. Tretment with well-chrcterized TLR gonists reveled differences in dose-response, mgnitude nd timing of IFN induction. It is worth to mention here tht ll stimultions were executed t 37 C nd not the usul temperture for vin cells (42 C) due to limittions of incubtion spce, which my influence the mgnitude of the response to stimuli. PIC is virl RNA mimic tht triggers TLR3 nd initites strong ntivirl response in most cells 47. This ws shown to induce IFNα by 3 to 8-fold, wheres IFNλ induction ws fold. Moreover, pek IFNα induction occurred t lower dose of PIC (0.5 μg/ml) compred to IFNλ (10 μg/ml). In contrst the timing of PIC-medited induction of IFNα nd IFNλ ws similr, with pek t h, followed by decline. These results suggest tht the two IFNs my hve different biologicl roles, with moderte IFNα induction triggered by even low levels of pthogen, wheres stronger IFNλ induction 48
50 requires higher pthogen levels. This dt is consistent with humn nd mice experiments 257,271, suggesting tht the pttern of IFN induction is conserved. LPS tretment did not upregulte IFNα or IFNλ t ny time point, which is in line with observtion tht infection with grm positive bcteri strongly induce IFNλ in lung epitheli nd plcent cells but not grm negtive bcteri 272. Further, studies in humn immune cells hve shown tht only IFNλ1 cn be upregulted by LPS stimultion while IFNλ2 nd λ3 remin unresponsive in 273. This suggests tht IFNλ1 my hve cquired this feture, which is not generl property of type III IFNs. It is worth noting tht lrge vritions in IFNλ induction were observed between birds tht limited finer nlysis, this my be due to genetic fctors s is the cse for IFNγ in humns 274. In ddition, the reduced fold induction of IFNα is likely due, t lest in prt, to the higher bsl levels of this IFN. Amplifiction of ntivirl signls plys n importnt role in immune defence, not only ensuring tht the signl reches sufficient strength to exert n effective response, but lso tht it is spred to neighbouring cells. These positive feedbck loops hve been described extensively for mmmlin IFNs 128,260,275.To investigte whether this mechnism ws conserved in chickens, purified splenocytes were treted with recombinnt IFNα or IFNλ. IFNα tretment showed trend for rpid IFNα upregultion, which then slowly declined. Although the results lcked sttisticl significnce due to considerble bird-to-bird vrition the suggested time frme of self-induction ws in greement with mmmlin studies 275,276. IFNλ, in contrst, showed no induction by IFNα t ny time point, which is different to studies in mice where IFNα induced strong IFNλ induction 135. However, this could be due to cell-type specific effects, since IFNλ expression is known to vry between cell types 137,273. IFNλ stimultion resulted in trend for differentil regultion of IFNα nd IFNλ erly, but by 48 h led to significnt upregultion of both IFNα nd IFNλ, which ws higher for the ltter. Bsed on the time lg involved, this is likely to be n indirect effect, the mechnism of which would require dditionl study. ISGs represent the key effectors of the IFN system nd s such their expression in response to pthogen relted stimuli provides importnt insight into the chicken innte immune response. Therefore, the expression levels of four well known ISGs were mesured in chicken splenocytes stimulted with PIC nd LPS. In both cses, Mx1 nd Zp showed strong induction tht peked t 3 h. Pkr nd Viperin followed similr trend lbeit t lower fold induction tht filed to rech significnce in the PIC stimulted cells. The pttern of ISG induction is in brod greement with published dt from mmml nd vin in vitro systems, lthough it is cler tht differences exist between cell types 223,236,267, Although 49
51 LPS did not induce IFNα or IFNλ it hs previously been shown tht IFNβ is in fct stimulted by LPS nd responsible for the ISG upregultion 280.Tretment of splenocytes with recombinnt IFNs reveled difference between IFNα nd IFNλ. IFNα ws ble to stimulte ISGs in similr mnner to PIC with respect to timing nd bredth of genes. In contrst, IFNλ ws only ble to induce Mx1 nd Viperin, reching sttisticl significnce t round 48 h. The rpid but trnsient induction of ISGs with IFNα but slower nd more sustined induction with IFNλ is consistent with observtions in mmmls 267,271,281. It seems likely tht the pek in ISG expression 48 h post IFNλ tretment is consequence of the lrge upregultion of IFNs seen concomitntly t this time point, lthough the specificity of ISG induction would indicte tht the effects of IFNα hve been modulted in some mnner. To provide more biologiclly-relevnt insights, the effects of influenz virus infection were investigted. There studies utilized H5N6 influenz A strin isolted from duck tht ws previously shown to elicit moderte signs of disese fter 48 h. HPAI infection induced significnt but moderte upregultion of IFNα (8-fold) with stronger induction of IFNλ (50- fold) in the spleen. In the lungs, induction of IFNα ws comprbly higher (25-fold), with IFNλ upregultion comprble to the spleen (50-fold). The higher IFNα induction in the lung probbly reflects this tissue being the primry site of infection, wheres the higher reltive IFNλ induction in both orgns is likely due the high bse-line IFNα expression tht reduced the fold chnge. The upregultion of both IFNs is, however, consistent with other studies investigting HPAI infection in birds 205,282. Moreover, the fct tht both IFNs re induced indictes tht this HPAI virus might not interfere significntly with the IFN induction, t lest t the mrna level. HPAI infection resulted in strong induction of Mx1 in both the spleen nd lung, wheres Pkr ws only significntly upregulted in the spleen. These expression ptterns re consistent with the IFN induction observed s well s results in the literture 283,284. In contrst, Zp ws not significntly upregulted t the mrna level in either the spleen or the lung despite previous studies showing high Zp expression tht correlted with shorter survivl time during HPAI infections in chickens 285. The reson for this lck of Zp induction remins unknown. Substntil vribility ws observed in immune gene expression between birds, which represented significnt issue in this study. Not only did it interfere with the bility to obtin sttisticlly significnt results nd increse bird numbers required in experiments, but lso pointed to biologicl cuse. Differences in the femle nd mle immune systems hve previously been noted in both humns nd mice 263, Exploring this s potentil cuse of the vribility of IFN expressions in chickens ws interesting, since this orgnism hs some 50
52 key genetic differences in this regrd. In prticulr, the type I IFNs re locted in one cluster on the sex (Z) chromosome of chicken, in which ZZ determines mles nd ZW determines femles. Therefore, splenocytes from mle or femle chickens, both immture nd mture, were tested for IFN responsiveness to PIC. Immture chicken splenocytes responded to PIC tretment with time dependent ctivtion of IFNs, nd greter vribility mongst femles. For IFNα, mles responded erlier, but induction in femles ws significntly higher nd more sustined trend tht lso held true for IFNβ. One hypothesis for tht observtion would be tht the presence of dditionl copies of type I IFNs in mles enbles more rpid response. Induction of IFNλ ws lso erlier in mles, but in this cse the pek ws significntly higher compred to femles. As expected, estrogen levels were significntly higher in femles thn in mles, being much more vrible in the former. In mture chickens, induction ws higher for both IFNα nd IFNλ in femles but only reched significnce for IFNλ. Consistent low estrogen levels were gin observed in mture mles (round 50 pg/ml) with much higher nd more vrible levels in femles ( pg/ml). Investigtion of possible correltion of estrdiol levels nd induction levels in mture femles s in mles showed no significnt trend. Further reserch is required to understnd the interctions between sex hormones nd IFN responses nd to identify cuses of the observed vribility of IFN induction in birds. 51
53 7 Chrcteriztion of chicken IFNλR 7.1 Introduction Members of the Clss II cytokine r fmily (CRF2) re heterodimeric rs composed of two r subunits, denoted s R1/Rα nd R2/Rβ. Ech subunit is single pss trnsmembrne protein defined by structurl similrities in the extrcellulr domin, which includes the cytokine r homology domin (CHD) tht is involved in lignd binding nd certin sequences in the intrcellulr domin 164. CRF2 lignds bind with high ffinity to the R1 chin, which chrcteristiclly hs lrger intrcellulr domin, wheres the R2 chins hve smller intrcellulr domin nd lower lignd ffinities. The binding of the lignd to the R1 chin leds to recruitment of the R2 chin nd the trimeric complex then initites specific intrcellulr signling cscdes 164. The fmily is composed of 12 distinct rs, which re used for signling by members of the IFN, IL-10 nd IL-10 relted cytokines 164,165. Although type I nd type III IFNs shre similrities in biologicl ctivity they utilize different rs. Unlike type I nd type II IFN rs, which signl through exclusive R1 nd R2 subunits, IFNλR consists of the lignd specific IFNλR1 long with IL-10R2, which is shred mong IL-10R, IL-22R nd IL-26R 119,120,166. When one of these chins is bsent from the cell surfce, or neutrlized by n ntibody, cells re unresponsive to IFNλ 119,159, showing tht both IFNλR1 nd IL-10R2 re needed to form functionl r complex. IFNλ initilly binds to the IFNλR1 chin, which cuses conformtionl chnge tht enbles recruitment of the IL-10R2 to form trimer. This ctivtes the pre-ssocited intrcellulr tyrosine kinses JAK1 nd TYK2 to medite phosphoryltion of the r chins, which cretes docking sites for cytosolic STAT1 nd STAT Signling through type I nd type III IFN r complexes results in the formtion of trnscription fctor complex known s IFN stimulted gene fctor 3 (ISGF3) 128,146. This complex consists of three proteins, STAT1, STAT2, nd IFN regultory fctor-9 (IRF-9) 120. Once ssembled, ISGF3 then trnsloctes to the nucleus where it binds to IFN stimulted response elements (ISREs) in the promoters of vrious interferon-stimulted genes (ISGs) 185. Despite different r complexes being employed there is cler signling overlp between type I nd III IFNs, which is why the ntivirl outcomes re so simil28,146,178,185. Ultimtely r expression determines which cells respond to prticulr IFNs. Different tissues nd cell types hve highly specilized need to recognize, nd be ble to ct on 52
54 specific signling molecules. The IFNλR subunit, IFNλR1, is predominntly expressed on epithelil cells like these found in skin, lung, intestine, colon nd stomch 152,159,179 but is undetectble in other cell subtypes, prticulr fibroblstic nd endothelil cells. This contrsts with the type I IFNRs tht re expressed on ll nucleted cells 181. Skin nd mucosl surfces provide brrier between the host nd the environment which suggests tht the type III IFN system hs evolved to protect the epitheli ginst pthogens 159. The ntivirl ctivity of type III IFNs compred to type I IFNs is reported to be weker, which ppers to be closely relted to IFNλR1 expression 64,291. Initil studies showed tht IFNλR1 knock-out mice were indistinguishble from the wild type mice when chllenged by pnel of viruses, unlike IFNαR1 knock-out mice, which were highly susceptible 135,176. However, IFNλR1 knock-out mice were lter found to be susceptible to rotvirus infection wheres type I IFN knock-out mice were similr to the wild type 270. The results from mmmlin studies provides clues s to the roles of IFNs in the chicken. However, the identity of the IFNλR complex in chickens hs not been functionlly confirmed nd its expression remins poorly chrcterized. In ddition, downstrem signling of IFNλR is not documented in the vin host with few studies looking t ISG regultion. This Chpter ims to provide cler picture of the IFNλR nd its downstrem signling cscde to better understnd the chicken IFNλ medited responses. 53
55 7.2 Results Phylogenetic nlysis of the type III IFN r chins To evlute the extent of conservtion of the type III IFNR system, phylogenetic tree ws constructed using the Neighbour Joining method with ll published nd predicted IFNλR1 sequences (Figure 7-1). This reveled tht chicken IFNλR1 clustered with those of other birds which were most closely relted to reptile sequences tht clustered together, the exception being the nole lizrd sequence. Mmmls clustered together with significnt distnce from the bird-reptile sequences, with fish nd mphibin sequences found to be more distntly relted. Closer nlysis of the bird sequence cluster showed the chicken sequence ws most closely relted to tht of turkey, duck nd goose. Phylogenetic nlysis of IL-10R2 sequences gve tree with similr topology (Figure 7-2). Bird sequences gin clustered nd were most closely relted to reptile sequences. Mmml sequences clustered together in tight group, with mphibins nd Crolin nole between birds nd mmmls nd fish sequences the most distnt. Closest relted to the chicken sequence were those of turkey, duck nd goose Synteny of genes encoding the type III IFN r chins To provide evidence of orthology the conservtion of flnking genes ws investigted. The humn IFNλR1 gene is locted on chromosome 10 nd is flnked by the MYOM3 nd IL-22RA genes upstrem nd the GRHL3, STPG1, NIPAL3 nd RCAN3 downstrem (Figure 7-3A). The chicken gene ws found to be locted on chromosome 23 with the predicted upstrem flnking genes lso MYOM3 nd IL-22RA nd the downstrem genes GRHL3, NIPAL3 nd RCAN3. Both the chicken nd humn IFNλR1 genes consist of 7 exons. The humn IL-10R2 is locted in the IFN r cluster on chromosome 21 nd is flnked by IFNαR2 upstrem nd IFNαR1 nd IFNγR2 downstrem (Figure 7-3B). The chicken orthologue ws lso found to be locted in the IFN r cluster on chicken chromosome 1 nd ws flnked by IFNαR2 upstrem nd IFNαR1 nd IFNγR2 downstrem. The chicken nd humn IL-10R2 lso consisted of 7 exons. This suggests both genes re orthologous with their humn counterprt. 54
56 Mmmlin IFNLR1 97 Lizrd IFNLR1 Amphibin IFNLR1 98 Reptilin IFNLR1 Piscine IFNLR1 Piscine IFNLR Avin IFNLR1 97 IFNAR1 0.5 Figure 7-1 Phylogenetic nlysis of IFNλR1 proteins Phylogenetic tree of ll IFNλR1 protein sequences constructed using the Neighbor-Joining method. The tree is drwn to scle, with brnch lengths in the sme units s those of the evolutionry distnces used to infer the phylogenetic tree. The insert shows the closest reltives to the chicken sequence in the vin clde. 55
57 Figure 7-2 Phylogenetic nlysis of IL-10R2 proteins Phylogenetic tree of ll IL-10R2 protein sequences constructed using Neighbor-Joining method. The tree is drwn to scle, with brnch lengths in the sme units s those of the evolutionry distnces used to infer the phylogenetic tree. The insert shows the closest reltives of the IL-10R2 gene in the vin clde. 56
58 Figure 7-3 Synteny of IFNLR1 nd IL10R2 (A) Loction of the humn IFNλR1 gene on chromosome 10 nd the chicken IFNλR1 gene on chromosome 23. The flnking regions show conserved genes upstrem (MYOM3, IL-22R1) nd downstrem (GRHL3, NIPAL3 nd RCAN 3. The predicted gene structure of chicken IFNλR1 shows seven exons nd six introns like humn IFNλR1. (B) Loction of the humn IL10R2 on chromosome 21nd the chicken IL-10R2 on chromosome 1. Conserved flnking genes upstrem (IFNαR2) nd downstrem (IFNαR1, IFNγR2) genes re shown. The IL-10R2 gene hs seven exons nd six introns in both species. 57
59 7.2.3 Tissue distribution of the type III IFN r chins To gin insight into the role of the IFNλR complex, its tissue distribution ws evluted. Totl RNA ws isolted form chicken tissues nd purified mononucler cells from the bone mrrow nd blood nd subjected to qrt-pcr to ssess the expression of the genes encoding the two IFNλR chins (Figure 7-4) with the reltive expression levels clculted using GAPDH s housekeeping gene. Assuming comprble mplifiction efficiency, the expression of IFNλR1 ws universlly lower thn tht of IL-10R2 in ll the tissue types. The highest IFNλR1 expression ws seen in the spleen, burs nd prticulrly PBMCs. The lowest expression of both rs ws found in the brin. To provide dditionl perspective the reltive r chin expression ws nlyzed. The rtio of IL10R2 to IFNλR1 ws found to be 5- fold in spleen, bone mrrow nd burs, round 3-fold in the thymus nd 7- nd 15-fold in PBMCs nd brin respectively Functionl nlysis of the chicken type III IFN r complex While there ws good circumstntil evidence tht functionl chicken IFNλR complex existed, to provide definitive support sirna knockdown strtegy ws developed in the chicken fibroblst cell line (DF1). Chicken IFNλR specific sirna (Figure 7-5A) ws ble to knockdown IFNλR1 mrna expression by 60% t 48 h post trnsfection compred to the nontrget control sirna (egfp). Similrly, IL-10R2 sirna (Figure 7-5B) resulted in n 80% knockdown of IL-10R2 gene expression compred to n egfp sirna treted control. These cells were subsequently stimulted with chicken IFNλ (chifnλ) nd nlyzed for STAT ctivtion by EMSA (Figure 7-6) nd Mx1 gene expression by qrt-pcr (Figure 7-7) s mesure of r ctivtion. Strong STAT ctivtion ws observed in the control group, which ws gretly reduced in the IFNλR1 sirna group nd lmost undetectble in the IL- 10R2 sirna treted group. Similrly, significntly lower expression level of Mx1 ws observed post IFNλ stimultion in both the IFNλR1 sirna nd the IL-10R2 sirna tretment groups, respectively, when compred to the egfp (non-trget) sirna treted group. To verify tht the signl downstrem of the chicken IFNλR complex utilized JAK1, the selective JAK1 inhibitor Ruxolitinib, ws employed. Stimultion of DF1 cells with chifnλ led to significnt upregultion of Mx1 from the unstimulted stte while the cells treted with Ruxolitinib nd chifnλ showed no upregultion (Figure 7-8). 58
60 Figure 7-4 Tissue distribution of IFNλR chins Expression of IFNλR1 nd IL-10R2 mrna in tissues nd cell popultions of 6-week old SPF chickens. Reltive expression levels of IFNλR1 (white dots) nd IL-10R2 (blck dimonds) normlized ginst the housekeeping gene GAPDH. IFNλR1/IL-10R2 rtio in ech tissue re indicted by blue squres. reltive IFN R1 expression (%) A IFN R1 egfp reltive IL-10R2 expression (%) B IL-10R2 egfp Figure 7-5 Confirmtion of successful sirna medited gene knockdown Expression of IFNλR1 (A) nd IL-10R2 (B) mrna in DF1 cells treted with the respective sirnas nd non-trget control sirna (egfp). The brs represent reltive expression levels of mrna, normlized ginst the housekeeping gene GAPDH s percentge of expression compred to the control. (**** p vlue < compred using Students t test). 59
61 Figure 7-6 Effect of IFNλR chin knockdown on STAT ctivtion EMSA nlysis of STAT ctivtion following IFNλ stimultion of DF1 cells with no sirna (-) or trnsfected with IFNλR1 sirna or IL-10R2 sirna s indicted in duplictes. Red box indictes the stt complexes, NS indictes nonspecific bnds, FP indictes free probe. reltive Mx1 expression (%) sirna: IFN R1 **** IL-10R2 **** egfp Figure 7-7 Effect of IFNλR chin knockdown on Mx1 expression Expression of Mx1 mrna in DF1 cells treted with sirnas trgeting IFNλR1 (blck), IL-10R2 (white) or control sirna (egfp). The brs represent reltive expression levels of mrna, normlized ginst the housekeeping gene GAPDH s percentge of expression compred to the control. (**** p vlue < compred using one-wy ANOVA test with uncorrected Fischer LSD post-test). 60
62 Figure 7-8 Effect of JAK inhibition on IFNλ-medited Mx1 induction Expression of Mx1 mrna in untreted nd unstimulted (grey), untreted nd IFNλ stimulted (white) nd Ruxolitinib-treted nd IFNλ stimulted (blck) DF1 cells. The brs represent fold chnge in levels of mrna expression, normlized ginst the housekeeping gene GAPDH compred to the untreted/unstimulted group. (**** p vlue < using one-wy ANOVA test with Bonferroni posttest). 61
63 7.3 Discussion Trnslted IFNs re trnsported out of the cell nd stimulte cell surfce IFN rs 164. For IFNλ, the r complex comprises IFNλR1 nd IL-10R2, which relys the signl through the intrcellulr JAK-STAT pthwy 118,119. Type I nd type III IFNs stimulte highly similr biologicl effects lrgely through their shred downstrem signling components JAK1 nd TYK2, which phosphorylte nd ctivte STAT1/STAT2 heterodimers tht ssocite with IRF9 to form the trnscriptionl complex ISGF ISGF3 upregultes the expression of hundreds of ntivirl genes tht block the virus lifecycle t multiple stges 207. The IFNλ medited signling cscde hs been very well chrcterized in mouse nd humn but little is known bout this pthwy in chicken. The IL-10R2 ws previously chrcterized nd cndidte IFNλR1 gene hd been identified using BLASTN. However, confirmtion of the identity of this cndidte gene nd the dependency of both chins for chicken IFNλ signling vi the JAK/STAT pthwy ws missing. The humn IFNλR1 gene hs 7 exons nd is flnked upstrem by MYOM3 nd IL-22RA genes, nd downstrem by GRHL3, STPG1, NIPAL3 nd RCAN3 genes. The cndidte chicken IFNλR gene showed identicl synteny except for STPE1 for which no chicken homologue could be identified. As shown previously by Reboul et l 202 the structure nd loction of the second r chin gene, IL-10R2, is conserved between chicken nd humn consisting of seven exons nd is locted in n IFN r cluster, flnked upstrem by IFNαR2 nd downstrem by IFNαR1 nd IFNγR2. Phylogenetic nlysis of the IL-10R2 nd IFNλR1 chins reveled tht both chicken sequences clustered with those from other birds. These were collectively most similr to reptiles, which is expected given their shred evolutionry history 293. For both r chins, mmmlin sequences clustered tightly together t significnt distnce from the vin-reptilin sequences. Fish sequences were found to be the most distnt in this nlysis, consistent with other published work 56. Expression of both IFNλR chins ws investigted in immune relevnt tissues to identify those likely to be responsive to IFNλ in the chicken. In ll tissues exmined, IL-10R2 ws more highly expressed thn IFNλR1. The highest IFNλR1 expression levels were observed in splenic, bursl tissues nd PBMC; similr to results observed in humns 172. Interestingly purified splenocytes showed even higher IFNλR1 expression thn the whole spleen which my indicte incresed expression of IFNλR1 by white blood cells (dt not shown). The highest IL-10R2 expression ws in the brin followed by PBMCs, spleen, bone mrrow nd 62
64 bursl tissue, nd the lowest thymic tissue. As both chins must be present for n IFNλmedited immune response these findings indicte tht the IFNλR1 is likely the restrictive r s it is universlly expressed t lower level. Although the levels of expression were only investigted t the level of mrna, previously published work hs shown direct correltion between IFNλR mrna expression nd responsiveness to chifnλ 206. Two chicken cell lines were tested for IFN λr1 expression nd both the Fibroblstic cell line DF1 nd the Mcrophge cell line HD11 were found to express low levels of the r, however s the trnsfection efficiency ws higher in DF1s the work ws continued with these cells. An sirna medited knockdown strtegy ws used to trget both IFNλR chins seprtely. This blunted chifnλ-induced STAT ctivtion nd Mx1 expression by up to 60% compred to controls which corresponded with the knockdown efficiency of the respective sirnas. The involvement of JAK1 ws exmined with Ruxolitinib, selective JAK1 inhibitor used s tretment for myelofibrosis 294. DF1 cells treted with chifnλ showed 6-fold increse in Mx1 mrna levels, which ws completely blocked with Ruxolitinib. These results collectively confirm tht chifnλ requires IFNλR1, IL-10R2 nd JAK1 for STAT complex ctivtion nd the subsequent upregultion of ISGs like Mx1. 63
65 8 Chrcteriztion of chicken IFIT5 8.1 Introduction Avin influenz (AI) viruses, especilly the highly pthogenic subtypes, cuse significnt morbidity nd mortlity in chickens worldwide 237. This hs widespred consequences, notbly including severe economic losses 239 nd the potentil of zoonotic trnsmission to humns 237, To fully understnd how the disese might be combtted in poultry it is essentil to chrcterize the prts of the host immune system responding to influenz virus infection 248. The innte immune system forms criticl prt of the host defence ginst pthogens nd severl pthwys hve been chrcterized to dte. In mmmls, innte immune recognition is medited by series of germ-line encoded rs clled pthogen recognition rs (PRR) tht detect conserved pthogen-ssocited moleculr ptterns (PAMPs) 40. One of the most prominent fmilies of PRR re the Toll-like rs (TLR) 41. To dte, nine conserved TLRs hve been found in humns nd mice nd ech one serves distinct function in PAMP recognition nd immune response 53. TLRs essentilly function s sentinels, which when ctivted modulte the expression of multiple immune genes tht re involved in the mobiliztion of wider immune response 38,44. Infections with virl pthogens my be sensed through TLR3, which recognizes double strnded RNA (dsrna) nd TLRs 7, 8 nd 9, which recognize non-self nucleic cids Upon virl PAMP enggement, TLRs trigger intrcellulr signling cscdes tht led to the expression of vriety of pro-inflmmtory cytokines nd chemokines s well s ntivirl genes, which together orchestrte the erly host response to infection 53,295. However, importnt differences between the mmmlin nd the vin hosts hve been found like the bsence of TLR9 nd RIG-1, while chicken TLR8 seems to be non-functionl. Notwithstnding this, TLR3, the most importnt r in dsrna sensing hs been found nd chrcterized 261,296,297. Furthermore, there is evidence to suggest tht chicken MDA5 is lso functionl nlogue of mmmlin RIG Cytokines comprise lrge fmily of molecules, including interferons (IFNs), interleukins (ILs), colony-stimulting fctors (CSFs), trnsforming growth fctors (TGFs) nd tumour necrosis fctors (TNFs) 58. It is now recognized tht severl IFNs re collectively crucil for defence ginst virl pthogens, contributing to the induction nd regultion of both innte nd dptive ntivirl mechnisms Three distinct interferon fmilies, type I, II nd III, hve been identified in mmmlin nd vin species, which differ in their mino cid 64
66 sequence, r specificity, signling pthwys nd gene induction, but there is overlp between fmilies Although only type I nd III IFNs re directly produced in response to virl infections, ll types significntly contribute to the genertion of n effective ntivirl immune response ginst wide rnge of pthogens 59. This is chieved by upregultion of hundreds of ntivirl genes tht ct in concert to limit virl spred nd repliction 207, which includes members of the IFIT fmily. Humn nd mouse IFIT1-5 hve been shown to ply criticl roles in ntivirl defence 211. The mechnisms re not completely understood but IFIT1 nd IFIT2 hve been shown to suppress trnsltion by binding to the eukryotic initition fctor IFIT fmily members cn lso bind to single strnded RNA 211,227 nd double strnded DNA 228 directly nd thereby reduce virl repliction. A better understnding of the innte immune system prticulrly in cute AI infection is needed for the development of trgeted therpeutic interventions. In this Chpter the IFIT5 gene of chickens ws investigted to gin further insights into the innte defence of this importnt production niml ginst virl pthogens. In prticulr, the response of the chicken IFIT5 gene to different immune stimuli nd cute influenz virus infection ws ssessed. 65
67 8.2 Results Identifiction nd chrcteriztion of puttive chicken IFIT5 gene Bioinformtic nlysis of the chicken genome with the humn IFIT5 gene sequence using BLASTN identified potentil chicken IFIT5 gene locted on chromosome 6 (Figure 8-1). It ws flnked by CH25H nd LIPA upstrem nd SLC16A nd PANK 1 downstrem, similr to the humn IFIT gene cluster locted on chromosome 10. Like humn IFIT5, the chicken gene lso consisted of 2 exons nd 1 intron. Gene specific primers were used to mplify cdna from 4-week old White Leghorn chicken splenocytes stimulted with poly (I:C) for 3 h. This generted n pproximtely 1440 bse pir (bp) mplicon (Figure 8-2) tht ws not observed in the no templte control. This mplicon ws cloned nd sequenced (Accession No.: KT180229) reveling nine single nucleotide polymorphisms (SNPs) when compred to the predicted sequence, five of which would led to mino cid chnges in the 470 residue protein Conservtion of chicken IFIT5 Phylogenetic nlysis of IFIT protein sequences (Figure 8-3A) showed tht the puttive chicken IFIT5 clustered with similr genes from other bird species s well s from turtle. In contrst, mmmlin sequences clustered together in distinct fmilies of IFIT1-5 with the exception of chimpnzee IFIT3 nd mrsupil IFITs, which formed their own clde. Fish sequences were the most distntly relted nd formed two distinct cldes, with reptile (lizrd) nd mphibin (frog) sequences in between. To illustrte the sequence divergence of bird IFITs compred to humn IFIT1-5 smller tree ws constructed (Figure 8-3B). The chicken IFIT5 protein sequence clustered with the other bird IFITs, wheres the humn sequences formed seprte clde. Chicken IFIT5 ws most closely relted to the turkey IFIT5 sequence with 0.10 mino cids substitutions per site (s/s) (Tble 1) followed by the recently cloned duck IFIT5 298 with 0.47 s/s. Of the humn IFIT proteins, the chicken IFIT5 ws most closely-relted to humn IFIT5 with 0.89 s/s nd IFIT1B with 0.92 s/s. Domin prediction of the chicken IFIT5 protein sequence identified multiple Tetrtricopeptide repets (TRPs), hllmrk of IFIT genes, with n rrngement of TRPs similr to other bird nd humn IFIT genes (Figure 8-4A). Protein sequence lignment with humn IFIT5 (Figure 8-4B) reveled tht 13 out of 16 mino cids previously shown to be criticl for function were conserved in chicken IFIT5, including two residues tht were importnt for ssrna/dsdna binding selectivity
68 Figure 8-1 Chromosoml loction nd predicted gene structure of chicken IFIT5 Loction of the chicken IFIT5 gene on chromosome 6 nd the humn IFIT5 gene cluster on chromosome 10. Conserved flnking genes upstrem (CH25H, LIPA) nd downstrem (SLC16A, PANK1) nd dditionl humn IFIT genes re shown. The predicted gene structure of chicken IFIT5 with 2 exons nd one intron is presented. Figure 8-2 Amplifiction of the chicken IFIT5 gene RT-PCR mplifiction of the ~1440 bp chicken IFIT5 gene using specific primers from poly (I:C) stimulted splenocytes (+). No mplifiction ws observed when the templte ws omitted (-). 67
69 Tble 1 Evolutionry distnce of homologs from chicken IFIT5 gene The number of mino cid substitutions per site from between sequences re shown. Anlyses were conducted using the JTT mtrix-bsed model. The nlysis involved 10 mino cid sequences. All positions with less thn 85% site coverge were eliminted. The nlyses were conducted in MEGA 7. Chicken IFIT5 Turkey IFIT5* 0.10 Duck IFIT Flyctcher IFIT5* Zebrfinch IFIT5* Humn IFIT Humn IFIT1B Humn IFIT Humn IFIT Humn IFIT
70 A B Figure 8-3 Phylogenetic nlysis of IFIT5 proteins (A) Phylogenetic tree of ll IFIT published protein sequences using constructed the Neighbor- Joining method. The tree is drwn to scle, with brnch lengths in the sme units s those of the evolutionry distnces used to infer the phylogenetic tree. (B) Predicted evolutionry history of the indicted humn nd bird IFIT proteins constructed using the Mximum Likelihood method. Bootstrp vlues re shown t the nodes s percentge of 1000 replictes. The scle br represents 0.1 substitutions per site. 69
71 A B TRP Domin Figure 8-4 Domin nlysis of humn nd vin IFITs (A) Schemtic representtion of the predicted Tetrtricopeptide repets (TRP) domins within the indicted IFIT protein sequences (* denotes predicted sequences). Numbers on t the end of ech sequence indictes protein length. (B) Alignment of chicken nd humn IFIT5 sequences. Numbers bove the sequence denote bp from the strt of the ligned sequence, dshes mrk gps in the lignment, nd strs indicte the end of sequence. Residues identified s functionlly criticl in humns re highlighted 70
72 8.2.3 Expression of chicken IFIT5 following immune stimultion To further chrcterize the chicken IFIT5 gene, its expression in response to immune stimuli ws investigted. Some ISGs cn be induced by TLR pthwys independently of IFN. To ssess this chicken splenocytes were treted with 50 μg/ml poly (I:C) (Figure 8-5A) nd 10 μg/ml LPS (Figure 8-5B) nd the expression of IFIT5 nd the well-known chicken Myxovirus resistnce protein 1 (Mx1) gene quntified. The poly (I:C) tretment up-regulted both IFIT5 nd Mx1 expression, which peked t 6 h post stimultion t ~90- nd 100-fold, respectively. LPS stimultion induced 15-fold increse in IFIT5 expression from 6-24 h post stimultion followed by rpid decline to undetectble levels t 48 h, with similr pttern of Mx1 induction observed. The interferon inducible nture of mmmlin IFITs hs been well chrcterized. To confirm tht IFIT5 is n ISG, splenocytes from SPF chickens were treted with recombinnt chicken IFNα (Figure 8-6A) nd IFNλ (Figure 8-6B). Following IFNα tretment, IFIT5 ws rpidly induced reching significnt levels t 1.5 h with pek expression t 3 h t 75-fold, Mx1 induction ws delyed, peking t 6 h t 45-fold. Both IFIT5 nd Mx1 expression remined significntly bove bse-line until 24 h followed by decrese t 48 h. Following IFNλ stimultion there ws stedy increse in IFIT5 expression with the highest increse in expression of round 25-fold t 48 h post tretment. Mx1 behved similrly lthough the fold induction ws lower with n pproximte 10-fold increse in mrna levels t 3 h Expression of chicken IFIT5 following virl infection Since IFIT5 is considered n ntivirl gene it ws importnt to lso chrcterize the gene expression levels in response to cute infection in vivo. Therefore, the expression of IFIT5 nd Mx1 ws mesured in spleen (Figure 8-7A) nd lung (Figure 8-7B) of H5N6 virus-infected SPF chickens. Both IFIT5 nd Mx1 were upregulted pproximtely 400-fold in the lungs nd 60-fold in the spleen of infected birds compred to helthy ge-mtched chickens. Since eggs re universlly used to mplify influenz virus the expression of these genes ws lso ssessed in eggs infected with n H1N1 Influenz A virus (Figure 8-8). IFIT5 ws upregulted t 24 h post infection reching significnce t 48 h in the CAM, while in muscle nd brin no expression ws detected t 24 h but expression ws observed t 48 h. Mx1 behved similrly in the CAM lthough did not rech sttisticl significnce. Mx1 expression in the muscle ws upregulted t 24 h with significnt increse t 48 h, while the brin showed no induction t 24 h but incresed Mx1 mrna ws detected t 48 h post infection. 71
73 A B Figure 8-5 Expression of IFIT5 nd Mx1 in response to TLR gonists Expression of IFIT5 nd Mx1 mrna in splenocytes from SPF chickens stimulted with 50 μg/ml poly (I:C) (A) nd 10 μg/ml LPS (B). This is shown s men fold chnge with stndrd error of the men (SEM) compred to the untreted smple, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05, ** p vlue < 0.005, *** p vlue < **** p vlue < compred to the 0 h time point using one-wy ANOVA test with uncorrected Fischer LSD post-test). The Mx1 dt is duplicte of tht lredy shown in 6.5A/B nd serves s comprison. 72
74 A reltive expression (fold) B Figure 8-6 Expression of IFIT5 nd Mx1 in response to IFN stimultion Expression of IFIT5 nd Mx1 mrna in splenocytes from SPF chickens stimulted with 500 ng/ml IFNα (A), 50 μg/ml IFNλ (B). This is shown s men fold chnge with stndrd error of the men (SEM) compred to the untreted smple, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05, ** p vlue < 0.005, *** p vlue < **** p vlue < using one-wy ANOVA test with uncorrected Fischer LSD post-test). The Mx1 dt is duplicte of tht lredy shown in 6.6A/B nd serves s comprison. 73
75 A Mx1 IFIT5 B Mx1 IFIT5 Figure 8-7 Expression of IFIT5 nd Mx1 in response to virl infections in vivo Expression of IFIT5 nd Mx1 mrna in six 5-week old SPF chicken spleen (A) nd lung (B) t 22 h post infection with Influenz virus A/duck/Los/XBY004/2014 (H5N6). Brs represent the men fold chnge of mrna expression with SEM compred to the sme tissue of the uninfected birds, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05 using the Students t test with Mnn Whitney U test). The Mx1 dt is duplicte of tht lredy shown in 6.8A/B nd serves s comprison. 74
76 A CAM Muscle Brin B CAM Muscle Brin Figure 8-8 Expression of IFIT5 nd Mx1 in response to virl infections in ovo Expression of IFIT5 (A) nd Mx1 (B) mrna in D10 embryonted chicken eggs 24 h nd 48 h post infection with Influenz virus A/Puerto Rico/8/1934 (H1N1). Brs represent the men fold chnge of mrna expression with SEM compred to the sme tissue of uninfected eggs, normlized ginst the housekeeping gene GAPDH (* p vlue < 0.05, ** p vlue < 0.005, using one-wy ANOVA test with uncorrected Fischer LSD post-test). 75
77 8.3 Discussion The IFIT fmily of proteins is found cross diverse species 211,299. However, the mode of ction of these proteins is not well understood. In vin species, even less is known, with only limited recent trnscriptome studies chrcterizing their expression profiles 231,300,301. The identifiction nd chrcteriztion of chicken IFIT5 llows for further in-depth investigtion of its role in vin immunity, including in response to virus. Bioinformtic nlysis ws used to identify the genes nd determine its loction nd structure. The chicken IFIT5 gene ws locted on chromosome 6, flnked by the CH25H nd LIPA genes upstrem nd SLC16A nd PANK1 downstrem, which reveled conserved synteny with humn nd other species 211. It lso consisted of 2 exons nd 1 intron like in other species, which strongly suggests common derivtion. The chicken IFIT5 ws shown by RT-PCR to be 1440 bp in length, encoding protein of 470 mino cids, which is consistent with mmmlin nd other vin IFIT proteins investigted. Ten SNPs were identified (Figure 11-2, Appendix) of which five were silent nd five were missense muttions (Figure 11-3, Appendix). SNPs in the IFIT5 gene hve been described in humns nd other species 211 nd could be explined by genetic diversity, in this cse vritions between individul birds, which my be even greter between chicken breeds 302. Phylogenetic nlysis showed tht the bird sequences clustered together but were relted to mmmlin IFITs. These findings followed the generl trend for IFIT genes to cluster with their own clss rther thn prlogues in other clsses, which mkes definitive identifiction of orthology difficult bsed on sequence lone. Further nlysis of the chicken IFIT5 protein sequence indicted it ws closely relted to other bird IFITs nd mongst the humn IFIT fmily ws most similr to humn IFIT5 with 0.89 substitutions per site. Protein sequence lignment nd domin prediction nlysis reveled conservtion of the Tetrtricopeptide domins tht re the hllmrks of IFITs compred to other bird nd humn IFITs 211,299. The crystl structure of the humn IFIT5 protein hs been solved nd criticl to the function of IFIT5 identified by non-conserved site-directed mutgenesis 228. Of the 16 criticl identified, 13 were conserved in the chicken sequence. Amongst the three non-conserved criticl only one ws ssocited with decrese in binding ffinity to nucleic cids in the humn protein, the other two only showed decrese in tndem with nother muttion site. Importntly, the sites tht determined substrte specificity to ssrna or dsdna (F284 nd R294 in humns) were found to be conserved in the chicken sequence, suggesting tht IFIT5 cn bind to both substrtes, lthough possibly with reduced binding ffinity. 76
78 The robust induction of IFIT genes in response to immune stimultion nd concomitnt ntivirl function is well estblished in mmmlin systems 303. A similr strong induction of IFIT5 ws observed following stimultion of chicken splenocytes with the TLR gonist poly (I:C). Upregultion of mrna expression strted t 3 h nd peked t 6 h post stimultion, which is consistent with observtions mde in humn cells 304, nd with similr kinetics to the Mx1 gene, one of the best chrcterized ISGs in chicken 220. The TLR4 lignd, bcteriderived LPS, lso induced significnt increse for IFIT5 nd Mx1 mrna fter 6 h tretment. The induction of IFIT5 strted t 3 h nd ws still significnt t 24 h nd elevted t 48 h consistent with previously reports observing in humn mcrophges nd neutrophils 278,279. Whether this effect ws due to direct trnscriptionl stimultion or indirectly vi the IFN pthwy is uncler t this point lthough it hs been proposed tht IFITs cn be induced directly vi PRRs 303. Tretment of splenocytes with IFNα led to significnt upregultion of IFIT5 fte.5 h with pek t 3 h tht remined high until 24 h, wheres Mx1 ws more delyed nd reched significnt upregultion t 3 h peking t 6 h post stimultion followed by decline. IFNλ tretment led to much slower increse, peking t 48 h post tretment. Although different in kinetics, the bility of both type I nd type III IFNs to induce IFITs clerly demonstrted the interferon stimulted nture of the chicken IFIT5 gene. During virl infection, IFITs bind to virl RNA nd host fctors needed for virl repliction nd thereby limit virl growth 211,303. In chickens, some of the most devstting infections re cused by highly pthogenic vin influenz A viruses of the H5 nd H7 subtypes. To determine if the identified IFIT homolog ws potentilly involved in the host response to this gent lung nd spleen smples were nlyzed from birds infected with HPAI virus (H5N6). These tissues were selected s representtives of the primry site of infection (lung) nd the subsequent immune response (spleen). A significnt upregultion of IFIT5 mrna ws observed in both the lung nd spleen of infected birds compred to uninfected controls. Little is known bout the ntivirl defense in chicken eggs despite their wide use s virus substrte nd so this ws investigted. Both HPAI viruses A/duck/Los/XBY004/2014 (H5N6) nd A/Vietnm/1194/2004 (H5N1) resulted in the deth of the embryo within 24 h of infection, this mde smpling of the tissues impossible nd low pthogenic strin ws chosen to give more consistent results. The H1N1 infection of eggs led to the induction of IFIT5 in the CAM fter 24 nd 48 h while significnt induction of Mx1 ws observed in the muscle. This confirms tht IFIT5 expression is highly upregulted during ctive infection in chicken nd might hve criticl role. 77
79 9 Generl Discussion HPAI represents serious problem, tht impcts us on multiple levels. These viruses cn led to severe economic losses, potentil bottlenecks in vccine production nd food shortges. Some of the H5 nd H7 strins cn lso infect humns through bird-humn trnsmission nd cuse high mortlity rtes with the potentil for pndemics 28,30. Vccintion is the most efficient wy to prevent virl spred through popultions, lthough the genetic instbility of Influenz viruses mkes this continuous effort with new vccines nnully bsed on circulting virus strins There vccines provide good level of protection, but they re not lwys efficient ginst emerging viruses 242. Vccintion chicken popultions with sesonl s well s emerging HPAI strins hs so fr proven successful in keeping the burden of disese down but is not enough to prevent further spill over 308. Moreover, the key reservoir, the wild qutic bird popultion, cnnot be vccinted 308. Another strtegy to counter HPAIs re ntivirl therpies which hve been successfully deployed in cute cses of humn Influenz infection. However, despite multimillion dollr compound screening enterprises The tretment of birds remins problemtic nd would no doubt led to resistnce of these viruses nd negte ny beneficil effect for humns in the long run s seen with Relenz in recent yers An lterntive strtegy to combt HPAI viruses nd so protect the humn popultion nd the food nd vccine supply, is to focus on disese resistnce in the trnsmission host. In most humn HPAI infection the source of infections cn be trced bck to chickens, which hve severe rections nd, in cse of some HPAI strins, die within h 316. The timefrme suggests tht these birds re not ble to contin the viruses in the first stges of infection, indicting inpproprite innte immune response. In order to develop strtegies mking chickens resistnt to these HPAI strins, it is necessry to understnd chicken innte immunity in order to identify in which prt of the immune response fils to respond in n pproprite mnner. The first line of defence ginst viruses re the IFNs, which were incidentlly discovered in chicken eggs 60 yers go 60. Greter understnding of these importnt meditors of innte (nd dptive) immunity could led to insight into the issues round the high mortlity rtes nd severe disese outcomes in birds. Although ll IFN types contribute to the ntivirl immune response only type I nd type III re directly ntivirl 59. Due to the very short 78
80 window between infection, diseses onset nd deth the focus ws on IFNα, the oldest nd best chrcterized ntivirl IFN 60, nd IFNλ 119,120, the newest member of the IFN fmily. IFNαs hs been well chrcterized in ll model species s well s the chicken, while informtion on IFNλ in mmmls is rpidly emerging. Nonetheless it ws of considerble importnce to study chicken IFNλ function in more detil s mice nd humn dt re not directly pplicble due to their multiple vrints, IFNλ1,2,3 nd 4 showing distinctive functions s reported by myrid of studies. Which of these re shred by chicken IFNλ remins uncler, lthough sequence comprison suggests IFNλ2 201 is the most highly relted. This study hs identified similrities between IFNα nd IFNλ but lso distinct differences, which highlights importnt non-redundnt roles of IFNλ in the ntivirl immune response in chickens. Host rs such s TLRs 44, RLRs 317 nd NLRs 318 detect the presence of pthogens to trigger the interferon-medited immune response. Compred to their mmmlin TLR counterprts, chicken TLR8 is non-functionl nd TLR9 nd RIG-1 re bsent. However, TLR3, the most importnt r in dsrna detection, ppers to be present nd functionl 261,296,297. Furthermore, there is evidence to suggest tht chicken MDA5 is lso functionl nlogue of mmmlin RIG Chicken cells were found to respond to the foreign RNA mimic PIC with significnt IFNα nd IFNλ upregultion even t low doses. IFNλ showed greter fold induction, but this is probbly best explined s consequence of the reltively high bseline expression of IFNα, which mkes the fold chnge comprbly low. In fct, the high bseline IFNα expression hs been linked to eliciting n efficient response to virl triggers 319. PIC-dependent IFNα induction ws more consistent wheres IFNλ induction ws highly vrible. Despite this, the expression of both genes followed the sme kinetics, peking fter 3-6 h post stimultion with PICs, which is comprble to mmmlin systems 257,271. LPS, on the other hnd, did not upregulte IFNα or IFNλ t ny time point. Humn studies suggest tht only IFNλ1 cn respond to LPS stimultion but not IFNλ2 or IFNλ3. Collectively, this dt suggests common PRR medited induction pthwy with these IFNs. In contrst, IFN medited induction of the IFNs ws mrkedly different. IFNα induced IFNα expression shortly fter stimultion, but not IFNλ. IFNα lso induced pek ISG expression fter 6 h, lthough whether tht is direct result of PIC stimultion or indirect through the IFNs produced is unknown. In contrst, the IFNλ medited ISG induction took plce t 48 h nd so likely indirect, which ws lso observed in DF1 cells (dt not shown) nd reportet in humn cells 320. The ltered ISG expression kinetics is suggestive of non-redundnt role in immune regultion. 79
81 The high level of vrince observed in experiments exmining IFN nd ISG expression levels led to the investigtion of possible cuses. Differences between the mle nd femle mmmlin immune systems hve been field of discussion in scientific literture 289,290, This is of prticulr relevnce in chickens since the type I IFNs re locted on the sex chromosome (Z). Moreover, in contrst to sex determintion in mmmls, mle chickens re determined but two copies of this chromosome (ZZ) nd femles by one (ZW). This mens tht mles hve dditionl copies of these genes. Despite this, PIC stimultion of purified splenocytes resulted in significntly higher expression of type I IFNs in femles compred to ge-mtched mles. IFNλ expression, on the other hnd, ws significntly higher in mles thn femles. In mture chickens both type I nd type III IFNs showed higher expression levels in femle, lthough only IFNλ reched significnce. Higher expression of IFNα in femle DCs hs previously been reported nd ws linked to the reltive expression levels of IRF The influence of estrogen ws investigted s possible explntion but no direct correltion between estrogen levels nd IFN expression ws observed in individul birds lthough, the estrdiol levels were, s expected, significntly higher femles thn in mles. These dt do, however, confirm tht femle nd mle birds differ significntly in the mgnitude of ntivirl response, which provides one explntion for the fluctutions in IFN nd ISG induction observed. More reserch into whether gender differences nd other potentil sources of vrince between birds observed re wrrnted. This could hve importnt implictions for the chicken industry s lyers re nturlly femle nd broilers universlly mle. Understnding the differences in immune response could led to lterntive strtegies to prevent or tret infection between those popultions. Receptor expression represents one mjor control point in tiloring IFN signling to specific cells 325 s well s the mgnitude of the signl relyed 118. A chicken IFNλR1 cndidte ws chrcterized nd found to cluster with other birds nd reptiles, while mmmlin sequences clustered together nd those from mphibins nd piscine species clustered further wy. A similr result ws found for IL-10R2. This suggests functionl IFNλR complex ws present before vin/mmmlin divergence, nd hs been mintined in both lineges, indictive of importnt conserved functions in both lineges. The tissue distribution of IFNλR complex components ws investigted, with focus on immune relevnt tissues. In mmmls it is primrily found in epithelil cells but recently evidence hs emerged tht IFNλ cts on endothelil cells of the blood-brin brrie80. Chickens re ntomiclly very different from mmmls with regrd to immunity. Chickens lck lymph nodes nd so the spleen is n especilly importnt orgn involved in pthogen 80
82 defence. The thymus is the min orgn for T(hymus) 326 cell development while the Burs of Fbricius hold the B(urs) 327 cell comprtment. All tissues investigted expressed the IL- 10R2 chin in reltively high levels, with the rtios of IFNλR1 nd IL-10R2 lwys hevily skewed in fvor of IL10R2, which suggests tht IFNλR1 levels re the limiting fctor. However, recently the potentilly vrible expression of GAPDH cross the tissues hs been documented could led to flse conclusions 328. The highest expression of the IFNλR1 chin ws observed in PBMCs followed by the spleen, burs, thymus nd the bone mrrow, while brin tissue hd the lowest expression. Purified chicken splenocytes showed higher IFNλR1 expression reltive to the whole tissue. This dt suggests tht immune rective tissues tend to express IFNλR1, while immune privileged tissues like the brin do not. An IFNλ-medited effect on immune cells is therefore highly likely. To provide evidence tht the puttive IFNλR1 nd IL-10R2 constitute functionl chicken IFNλR complex, the expression of ech chin ws knocked down using sirna. This demonstrted tht both chins were needed for IFNλ-medited STAT ctivtion nd ISG upregultion. Tretment with Ruxolitinib, selective JAK1 inhibitor, lso blocked IFNλ medited responses. This collectively demonstrtes n IFNλR1/IL-10R2-JAK-STAT-ISG pthwy in chickens. Confirming the functionlity of this centrl signling cscde lso opens up possibilities to repurpose drugs for pro-virl tretment. Ruxolitinib, which hs FDA pprovl for used in myelofibrosis 329 tretment in humns, could be used to inhibit interferon signling nd enhnce virl mplifiction for incresing yield for vccine production 330,331 nd sensitivity for dignostic pplictions 249. The use of embryonted eggs for mplifiction of viruses nd the virus-host interction hs been chrcterized extensively in the pst 60 yers but little to no work on the chrcteriztion of the egg immune system hs been done since the discovery of IFNs in chicken egg 347. To gin insight into the developing immune response nd potentil fctors inhibiting or filing to inhibit virl growth, chicken eggs were infected with Influenz A ccording to the sme protocols used by the vccine production industry nd dignostic lbortories 348. The results suggest tht IFNα is inhibited by the virus, which cn be explined by the gonistic effect of the NS1 protein in H1N1 tht is well documented 349. However, though IFN expression is repressed or delyed, ISGs re expressed t high levels in tissue dependent fshion. The inocultion of the virus into the llntoic fluid 348 brings the virus into close contct with the inner lyer the Chorio-llntoic membrne nd the outer lyer of embryonic tissue. By 24 h the virus replictes to very high levels in the CAM nd by 48 h the virus hs spred to every tissue. This dt shows tht even in the bsence of IFNα 81
83 the ISGs respond by 24 h to virl invsion, but this is not sufficient to control virl repliction s by 48 h the virus hs spred to every tissue of the embryo including the brin. This work gives insights into the loclistion of virl repliction s well s the mounting innte immune response, but more work needs to be done to dissect the vrious virus sensing nd immune response inducing pthwys in the developing chicken embryo. The dt collected here suggests tht the IFN system is well developed lthough unble to del with substntil virl infection in n pproprite mnner. Limited knowledge round the effectors of the IFN-medited ntivirl response, the ISGs, hinders he progress in vin immunology. Compred to mmmls the vin ISGs re very poorly chrcterized, with few genes nlyzed. The first humn ISG cloned ws the interferon-induced proteins with Tetrtricopeptide repets (IFITs) Considerble reserch hs been performed on IFIT fmily members identifying key role in nti-virl host defense by inhibiting virus repliction through binding nd regulting the functions of cellulr nd virl proteins nd RNAs 351. The IFIT fmily consists of four members in humn nd three in mouse. All identified IFITs hve multiple copies of Tetrtricopeptide repets (TRPs), their distinct tertiry structures enble them to bind different prtners nd ffect host-virus interctions differently. Humn IFIT1 nd IFIT2 re thought interfere with the virl RNA trnsltion by binding to the EIF Virl ppprna is lso recognized by IFIT1 which leds to sequestering by n IFIT1, IFIT2 nd IFIT3 complex, lthough only IFIT1 is directly ble to interct with ppp RNA. IFIT5 hs been shown to lso interct with ppp RNA but not with IFIT2 or IFIT3, indicting non-redundnt role in ntivirl defense 227,353. A chicken IFIT homolog hs been mentioned in severl publictions 231,284,300,301, but no in depth chrcteriztion of the gene hs been performed. A single potentil chicken IFIT gene cndidte ws identified, mplified by RT-PCR nd sequenced. The encoded chicken IFIT protein showed gretest homology to the humn IFIT5 nd ws thus nmed chicken IFIT5. The chicken IFIT5 ws most closely relted to other bird IFITs nd possessed nine TRP domins re distributed over its length similr to other bird nd humn IFITs. The crystl structure of the humn IFIT5 ws recently solved with extensive muttion studies identifying residues criticl for function 228. The chicken IFIT5 sequence showed conservtion of 12 out of 16 residues identified s importnt for substrte binding specificity. Collectively, this points towrds functionlly conserved IFIT gene in chickens. The expression of chicken IFIT5 ws nlyzed in response to different immune stimuli, including infection in vivo nd in ovo. IFIT5 ws highly induced in chicken cells fter 82
84 tretment with PIC nd LPS, while PIC triggered significnt increse in expression fter 3 h with reltively stble expression up until 48 h. In contrst, LPS stimultion induced pek expression fter 6 h followed by rpid decline. The IFIT5 gene ws lso induced in response to chifns like IFNα nd IFNλ with similr kinetics to other ISGs observed, lthough induction by IFNα reched significnt levels fte.5 h nd styed significntly induced until 24 h. Following virl infection IFIT5 ws highly induced in the lung nd spleen of chickens infected with H5N6 HPAI nd in the chorio-llntoic membrne nd the muscle of infected embryonted eggs. This collectively suggests tht chicken IFIT5 represents bone fide ISG, with pplicbility to studies on chicken innte immune system nd contribute to the understnding of HPAI pthogenesis in chickens. 83
85 10 References 1 Lee, C.W. & Sif, Y.M. Avin influenz virus. Comp Immunol Microbiol Infect Dis 32, 301, (2009). 2 Swyne, D.E. & Pntin-Jckwood, M. Avin Influenz. Vol. 1 (Wiley-Blckwell, 2008). 3 Nyk, D.P., Blogun, R.A., Ymd, H., Zhou, Z.H. & Brmn, S. Influenz virus morphogenesis nd budding. Virus Res 143, 147, (2009). 4 de Jong, M.D., Simmons, C.P., Thnh, T.T. et l. Ftl outcome of humn influenz A (H5N1) is ssocited with high virl lod nd hypercytokinemi. Nt Med 12, 1203, (2006). 5 Subbro, K. & Ktz, J. Avin influenz viruses infecting humns. Cell Mol Life Sci 57, 1770, (2000). 6 Alexnder, D.J. & Brown, I.H. Recent zoonoses cused by influenz A viruses. Rev Sci Tech 19, 197, (2000). 7 Burns, K. Cts contrct vin influenz virus. J Am Vet Med Assoc 228, 1165, (2006). 8 Herlocher, M.L., Elis, S., Truscon, R. et l. Ferrets s trnsmission model for influenz: sequence chnges in HA1 of type A (H3N2) virus. J Infect Dis 184, 542, (2001). 9 Perkins, L.E. & Swyne, D.E. Pthobiology of A/chicken/Hong Kong/220/97 (H5N1) vin influenz virus in seven gllinceous species. Vet Pthol 38, 149, (2001). 10 Isod, N., Skod, Y., Kishid, N. et l. Pthogenicity of highly pthogenic vin influenz virus, A/chicken/Ymguchi/7/04 (H5N1) in different species of birds nd mmmls. Arch Virol 151, 1267, (2006). 11 Kewchroen, J., vn Riel, D., vn Amerongen, G. et l. Wild Ducks s Long-Distnce Vectors of Highly Pthogenic Avin Influenz Virus (H5N1). Emerg Infect Dis 14, 600, (2008). 12 Kwok, Y. & Neumnn, G. Influenz viruses: n introduction. Methods Mol Biol 865, 1, (2012). 13 Fouchier, R.A., Munster, V., Wllensten, A. et l. Chrcteriztion of novel influenz A virus hemgglutinin subtype (H16) obtined from blck-heded gulls. J Virol 79, 2814, (2005). 14 Tong, S., Zhu, X., Li, Y. et l. New world bts hrbor diverse influenz A viruses. PLoS Pthog 9, e , (2013). 15 Tong, S., Li, Y., Riviller, P. et l. A distinct linege of influenz A virus from bts. Proc Ntl Acd Sci U S A 109, 4269, (2012). 16 Lver, W.G., Gerhrd, W., Webster, R.G., Frnkel, M.E. & Air, G.M. Antigenic drift in type A influenz virus: peptide mpping nd ntigenic nlysis of A/PR/8/34 (HON1) vrints selected with monoclonl ntibodies. Proc Ntl Acd Sci U S A 76, 1425, (1979). 17 Schweiger, B., Zdow, I. & Heckler, R. Antigenic drift nd vribility of influenz viruses. Med Microbiol Immunol 191, 133, (2002). 18 Poovorwn, Y., Pyungporn, S., Prchyngprech, S. & Mkkoch, J. Globl lert to vin influenz virus infection: from H5N1 to H7N9. Pthog Glob Helth 107, 217, (2013). 19 Trenor, J. Influenz vccine--outmneuvering ntigenic shift nd drift. N Engl J Med 350, 218, (2004). 20 De Jong, J.C., Rimmelzwn, G.F., Fouchier, R.A. & Osterhus, A.D. Influenz virus: mster of metmorphosis. J Infect 40, 218, (2000). 84
86 21 Mtrosovich, M., Zhou, N., Kwok, Y. & Webster, R. The surfce glycoproteins of H5 influenz viruses isolted from humns, chickens, nd wild qutic birds hve distinguishble properties. J Virol 73, 1146, (1999). 22 Li, J., Yu, X., Pu, X. et l. Environmentl connections of novel vin-origin H7N9 influenz virus infection nd virus dpttion to the humn. Sci Chin Life Sci 56, 485, (2013). 23 Connor, R.J., Kwok, Y., Webster, R.G. & Pulson, J.C. Receptor specificity in humn, vin, nd equine H2 nd H3 influenz virus isoltes. Virology 205, 17, (1994). 24 Frnc, M., Stllknecht, D.E. & Howerth, E.W. Expression nd distribution of silic cid influenz virus rs in wild birds. Avin Pthol 42, 60, (2013). 25 Suzuki, Y., Ito, T., Suzuki, T. et l. Silic Acid Species s Determinnt of the Host Rnge of Influenz A Viruses. J Virol 74, 11825, (2000). 26 Ymd, S., Suzuki, Y., Suzuki, T. et l. Hemgglutinin muttions responsible for the binding of H5N1 influenz A viruses to humn-type rs. Nture 444, 378, (2006). 27 Chen, Y., Ling, W., Yng, S. et l. Humn infections with the emerging vin influenz A H7N9 virus from wet mrket poultry: clinicl nlysis nd chrcteristion of virl genome. Lncet 381, 1916, (2013). 28 WHO. Cumultive number of confirmed humn cses for vin Influenz A(H5N1) reported to WHO, Spckmn, E., Pntin-Jckwood, M., Swyne, D.E., Surez, D.L. & Kpczynski, D.R. Impct of route of exposure nd chllenge dose on the pthogenesis of H7N9 low pthogenicity vin influenz virus in chickens. Virology 477, 72, (2015). 30 WHO. Influenz t the humn-niml interfce: Summry nd ssessment, Wong, F.Y., Phommchnh, P., Klprvidh, W. et l. Ressortnt highly pthogenic influenz A(H5N6) virus in Los. Emerg Infect Dis 21, 511, (2015). 32 Gerloff, N.A., Khn, S.U., Blish, A. et l. Multiple ressortment events mong highly pthogenic vin influenz A(H5N1) viruses detected in Bngldesh. Virology , 297, (2014). 33 Tkym, I., Hieu, N.T., Shirkur, M. et l. Novel Ressortnt Avin Influenz A(H5N1) Virus in Humn, Southern Vietnm, Emerg Infect Dis 22, 557, (2016). 34 Ligon, B.L. Avin influenz virus H5N1: review of its history nd informtion regrding its potentil to cuse the next pndemic. Semin Peditr Infect Dis 16, 326, (2005). 35 Chmielewski, R. & Swyne, D.E. Avin influenz: public helth nd food sfety concerns. Annu Rev Food Sci Technol 2, 37, (2011). 36 Cox, N.J. & Subbro, K. Globl epidemiology of influenz: pst nd present. Annu Rev Med 51, 407, (2000). 37 de l Brrer, C.A. & Reyes-Tern, G. Influenz: forecst for pndemic. Arch Med Res 36, 628, (2005). 38 Akir, S., Tked, K. & Kisho, T. Toll-like rs: criticl proteins linking innte nd cquired immunity. Nt Immunol 2, 675, (2001). 39 Jnewy, C.A., Jr. & Medzhitov, R. Innte immune recognition. Annu Rev Immunol 20, 197, (2002). 40 Medzhitov, R. & Jnewy, C.A., Jr. Innte immunity: the virtues of nonclonl system of recognition. Cell 91, 295, (1997). 41 Jnewy, C.A., Jr. The immune system evolved to discriminte infectious nonself from noninfectious self. Immunol Tody 13, 11, (1992). 85
87 42 Ssi, M. & Ymmoto, M. Pthogen recognition rs: lignds nd signling pthwys by Toll-like rs. Int Rev Immunol 32, 116, (2013). 43 Bum, A. & Grci-Sstre, A. Induction of type I interferon by RNA viruses: cellulr rs nd their substrtes. Amino Acids 38, 1283, (2010). 44 Kwi, T. & Akir, S. TLR signling. Semin Immunol 19, 24, (2007). 45 Lund, J., Sto, A., Akir, S., Medzhitov, R. & Iwski, A. Toll-like r 9-medited recognition of Herpes simplex virus-2 by plsmcytoid dendritic cells. J Exp Med 198, 513, (2003). 46 Lund, J.M., Alexopoulou, L., Sto, A. et l. Recognition of single-strnded RNA viruses by Toll-like r 7. Proc Ntl Acd Sci U S A 101, 5598, (2004). 47 Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flvell, R.A. Recognition of doublestrnded RNA nd ctivtion of NF-kppB by Toll-like r 3. Nture 413, 732, (2001). 48 Diebold, S.S., Kisho, T., Hemmi, H., Akir, S. & Reis e Sous, C. Innte ntivirl responses by mens of TLR7-medited recognition of single-strnded RNA. Science 303, 1529, (2004). 49 Hemmi, H., Tkeuchi, O., Kwi, T. et l. A Toll-like r recognizes bcteril DNA. Nture 408, 740, (2000). 50 Gibbrd, R.J., Morley, P.J. & Gy, N.J. Conserved fetures in the extrcellulr domin of humn toll-like r 8 re essentil for ph-dependent signling. J Biol Chem 281, 27503, (2006). 51 Xgorri, A. & Chlichli, K. Toll-like rs nd viruses: induction of innte ntivirl immune responses. Open Microbiol J 2, 49, (2008). 52 Brrl, P.M., Srkr, D., Su, Z.Z. et l. Functions of the cytoplsmic RNA sensors RIG- I nd MDA-5: key regultors of innte immunity. Phrmcol The24, 219, (2009). 53 Akir, S., Uemtsu, S. & Tkeuchi, O. Pthogen recognition nd innte immunity. Cell 124, 783, (2006). 54 Beutler, B. & Rietschel, E.T. Innte immune sensing nd its roots: the story of endotoxin. Nt Rev Immunol 3, 169, (2003). 55 Blkwill, F.R. & Burke, F. The cytokine network. Immunol Tody 10, 299, (1989). 56 Kruse, C.D. & Pestk, S. Evolution of the Clss 2 cytokines nd rs, nd discovery of new friends nd reltives. Phrmcol The06, 299, (2005). 57 Rhmn, M.M. & Eo, S.K. Prospects nd chllenges of using chicken cytokines in disese prevention. Vccine 30, 7165, (2012). 58 Belrdelli, F. Role of interferons nd other cytokines in the regultion of the immune response. APMIS 103, 161, (1995). 59 Smuel, C.E. Antivirl ctions of interferons. Clin Microbiol Rev 14, 778, (2001). 60 Iscs, A. & Lindenmnn, J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 147, 258, (1957). 61 Mlmgrd, L. Induction nd regultion of IFNs during virl infections. J Interferon Cytokine Res 24, 439, (2004). 62 Pietrs, E.M., Sh, S.K. & Cheng, G. The interferon response to bcteril nd virl infections. J Endotoxin Res 12, 246, (2006). 63 Le Bon, A. & Tough, D.F. Links between innte nd dptive immunity vi type I interferon. Curr Opin Immunol 14, 432, (2002). 64 Ank, N., West, H. & Pludn, S.R. IFN-: novel ntivirl cytokines. J Interferon Cytokine Res 26, 373, (2006). 65 Kiser, P., Poh, T.Y., Rothwell, L. et l. A genomic nlysis of chicken cytokines nd chemokines. J Interferon Cytokine Res 25, 467, (2005). 66 Chelbi-Alix, M.K. & Wietzerbin, J. Interferon, growing cytokine fmily: 50 yers of interferon reserch. Biochimie 89, 713, (2007). 86
88 67 Medzhitov, R. & Jnewy, C.A., Jr. Innte immune recognition nd control of dptive immune responses. Semin Immunol 10, 351, (1998). 68 Roberts, R.M., Liu, L., Guo, Q., Lemn, D. & Bixby, J. The evolution of the type I interferons. J Interferon Cytokine Res 18, 805, (1998). 69 Pestk, S., Kruse, C.D. & Wlter, M.R. Interferons, interferon-like cytokines, nd their rs. Immunol Rev 202, 8, (2004). 70 Lemn, D.W. & Roberts, R.M. Genes for the trophoblst interferons in sheep, got, nd musk ox nd distribution of relted genes mong mmmls. J Interferon Res 12, 1, (1992). 71 Lefevre, F. & Bouly, V. A novel nd typicl type one interferon gene expressed by trophoblst during erly pregnncy. J Biol Chem 268, 19760, (1993). 72 Kwmoto, S., Oritni, K., Askur, E. et l. A new interferon, limitin, displys equivlent immunomodultory nd ntitumor ctivities without myelosuppressive properties s compred with interferon-lph. Exp Hemtol 32, 797, (2004). 73 Durbin, J.E., Fernndez-Sesm, A., Lee, C.K. et l. Type I IFN modultes innte nd specific ntivirl immunity. J Immunol 164, 4220, (2000). 74 Stetson, D.B. & Medzhitov, R. Type I interferons in host defense. Immunity 25, 373, (2006). 75 Bogdn, C., Rollinghoff, M. & Diefenbch, A. Rective oxygen nd rective nitrogen intermedites in innte nd specific immunity. Curr Opin Immunol 12, 64, (2000). 76 Jcobs, B.L. & Lnglnd, J.O. When two strnds re better thn one: the meditors nd modultors of the cellulr responses to double-strnded RNA. Virology 219, 339, (1996). 77 Der, S.D., Zhou, A., Willims, B.R. & Silvermn, R.H. Identifiction of genes differentilly regulted by interferon lph, bet, or gmm using oligonucleotide rrys. Proc Ntl Acd Sci U S A 95, 15623, (1998). 78 Sen, G.C. Viruses nd interferons. Annu Rev Microbiol 55, 255, (2001). 79 de Veer, M.J., Holko, M., Frevel, M. et l. Functionl clssifiction of interferonstimulted genes identified using microrrys. J Leukoc Biol 69, 912, (2001). 80 Fensterl, V. & Sen, G.C. Interferons nd virl infections. BioFctors 35, 14, (2009). 81 Hllorn, P.F., Urmson, J., Vn der Meide, P.H. & Autenried, P. Regultion of MHC expression in vivo. II. IFN-lph/bet inducers nd recombinnt IFN-lph modulte MHC ntigen expression in mouse tissues. J Immunol 142, 4241, (1989). 82 Le Bon, A. & Tough, D.F. Type I interferon s stimulus for cross-priming. Cytokine Growth Fctor Rev 19, 33, (2008). 83 Biron, C.A. Role of erly cytokines, including lph nd bet interferons (IFNlph/bet), in innte nd dptive immune responses to virl infections. Semin Immunol 10, 383, (1998). 84 Mtikinen, S., Pnnen, A., Miettinen, M. et l. IFN-lph nd IL-18 synergisticlly enhnce IFN-gmm production in humn NK cells: differentil regultion of Stt4 ctivtion nd IFN-gmm gene expression by IFN-lph nd IL-12. Eur J Immunol 31, 2236, (2001). 85 Stckruk, M.L., Lee, A.J. & Ashkr, A.A. Type I interferon regultion of nturl killer cell function in primry nd secondry infections. Expert Rev Vccines 12, 875, (2013). 86 Pquette, R.L., Hsu, N.C., Kiertscher, S.M. et l. Interferon-lph nd grnulocytemcrophge colony-stimulting fctor differentite peripherl blood monocytes into potent ntigen-presenting cells. J Leukoc Biol 64, 358, (1998). 87 Montoy, M., Schivoni, G., Mttei, F. et l. Type I interferons produced by dendritic cells promote their phenotypic nd functionl ctivtion. Blood 99, 3263, (2002). 87
89 88 Curtsinger, J.M., Schmidt, C.S., Mondino, A. et l. Inflmmtory cytokines provide third signl for ctivtion of nive CD4+ nd CD8+ T cells. J Immunol 162, 3256, (1999). 89 Wenner, C.A., Guler, M.L., Mctoni, S.E., O'Grr, A. & Murphy, K.M. Roles of IFNgmm nd IFN-lph in IL-12-induced T helper cell-1 development. J Immunol 156, 1442, (1996). 90 Brinkmnn, V., Geiger, T., Alkn, S. & Heusser, C.H. Interferon lph increses the frequency of interferon gmm-producing humn CD4+ T cells. J Exp Med 178, 1655, (1993). 91 Le Bon, A., Schivoni, G., D'Agostino, G. et l. Type i interferons potently enhnce humorl immunity nd cn promote isotype switching by stimulting dendritic cells in vivo. Immunity 14, 461, (2001). 92 Tnk, N., Sto, M., Lmphier, M.S. et l. Type I interferons re essentil meditors of poptotic deth in virlly infected cells. Genes Cells 3, 29, (1998). 93 Chwl-Srkr, M., Lindner, D.J., Liu, Y.F. et l. Apoptosis nd interferons: role of interferon-stimulted genes s meditors of poptosis. Apoptosis 8, 237, (2003). 94 Benvegnu, L., Chemello, L., Novent, F. et l. Retrospective nlysis of the effect of interferon therpy on the clinicl outcome of ptients with virl cirrhosis. Cncer 83, 901, (1998). 95 Dorr, R.T. Interferon-lph in mlignnt nd virl diseses. A review. Drugs 45, 177, (1993). 96 Brssrd, D.L., Grce, M.J. & Bordens, R.W. Interferon-lph s n immunotherpeutic protein. J Leukoc Biol 71, 565, (2002). 97 Predes, J. & Krown, S.E. Interferon-lph therpy in ptients with Kposi's srcom nd the cquired immunodeficiency syndrome. Int J Immunophrmcol 13 Suppl 1, 77, (1991). 98 Tlpz, M., Kntrjin, H.M., McCredie, K.B. et l. Clinicl investigtion of humn lph interferon in chronic myelogenous leukemi. Blood 69, 1280, (1987). 99 Kirkwood, J.M., Ibrhim, J.G., Sondk, V.K., Ernstoff, M.S. & Ross, M. Interferon lf- 2 for melnom metstses. Lncet 359, 978, (2002). 100 Sun, W. & Schuchter, L.M. Metsttic melnom. Curr Tret Options Oncol 2, 193, (2001). 101 Rotondi, M., Mzziotti, G., Biondi, B. et l. Long-term tretment with interferonbet therpy for multiple sclerosis nd occurrence of Grves' disese. J Endocrinol Invest 23, 321, (2000). 102 Pinko, S. & McHutchison, J.G. Tretment of heptitis C with interferon nd ribvirin. J Gstroenterol Heptol 15, 581, (2000). 103 Hyshi, N. & Tkehr, T. Antivirl therpy for chronic heptitis C: pst, present, nd future. J Gstroenterol 41, 17, (2006). 104 Blish, M.J., Abrms, M.E., Pumfery, A.M. & Brndt, C.R. Enhnced inhibition of herpes simplex virus type 1 growth in humn cornel fibroblsts by combintions of interferon-lph nd -gmm. J Infect Dis 166, 1401, (1992). 105 D'Onofrio, C., Frnzese, O., Pugliniello, A. et l. Antivirl ctivity of individul versus combined tretments with interferon lph, bet nd gmm on erly infection with HTLV-I in vitro. Int J Immunophrmcol 14, 1069, (1992). 106 Nldini, A. & Fleischmnn, W.R., Jr. In vivo myelosuppression by combintion interferon tretment: ntgonism of MuIFN-gmm nd MuIFN-bet myelosuppressive effects. J Biol Response Mod 6, 546, (1987). 107 Sleijfer, S., Bnnink, M., Vn Gool, A.R., Kruit, W.H. & Stoter, G. Side effects of interferon-lph therpy. Phrm World Sci 27, 423, (2005). 88
90 108 Asnis, G.M. & De L Grz, R., 2nd. Interferon-induced depression in chronic heptitis C: review of its prevlence, risk fctors, biology, nd tretment pproches. J Clin Gstroenterol 40, 322, (2006). 109 Kiser, P., Win, H.M. & Rothwell, L. Structure of the chicken interferon-gmm gene, nd comprison to mmmlin homologues. Gene 207, 25, (1998). 110 Derynck, R., Leung, D.W., Gry, P.W. & Goeddel, D.V. Humn interferon gmm is encoded by single clss of mrna. Nucleic Acids Res 10, 3605, (1982). 111 Vilcek, J.T. Cytokines in Cytokine Growth Fctor Rev 7, 103, (1996). 112 Szbo, S.J., Sullivn, B.M., Peng, S.L. & Glimcher, L.H. Moleculr mechnisms regulting Th1 immune responses. Annu Rev Immunol 21, 713, (2003). 113 Frucht, D.M., Fuko, T., Bogdn, C. et l. IFN-gmm production by ntigenpresenting cells: mechnisms emerge. Trends Immunol 22, 556, (2001). 114 Boehm, U., Klmp, T., Groot, M. & Howrd, J.C. Cellulr responses to interferongmm. Annu Rev Immunol 15, 749, (1997). 115 Loughlin, A.J., Woodroofe, M.N. & Cuzner, M.L. Modultion of interferon-gmminduced mjor histocomptibility complex clss II nd Fc r expression on isolted microgli by trnsforming growth fctor-bet 1, interleukin-4, nordrenline nd glucocorticoids. Immunology 79, 125, (1993). 116 Krupih, G., Xie, Q.W., Buller, R.M. et l. Inhibition of virl repliction by interferon-gmm-induced nitric oxide synthse. Science 261, 1445, (1993). 117 Schroder, K., Hertzog, P.J., Rvsi, T. & Hume, D.A. Interferon-gmm: n overview of signls, mechnisms nd functions. J Leukoc Biol 75, 163, (2004). 118 Kotenko, S.V., Gllgher, G., Burin, V.V. et l. IFN-s medite ntivirl protection through distinct clss II cytokine r complex. Nt Immunol 4, 69, (2003). 119 Shepprd, P., Kindsvogel, W., Xu, W. et l. IL-28, IL-29 nd their clss II cytokine r IL-28R. Nt Immunol 4, 63, (2003). 120 Donnelly, R.P. & Kotenko, S.V. Interferon-: new ddition to n old fmily. J Interferon Cytokine Res 30, 555, (2010). 121 Miknis, Z.J., Mgrchev, E., Li, W. et l. Crystl structure of humn interferon1 in complex with its high-ffinity r interferon-r1. J Mol Biol 404, 650, (2010). 122 Gd, H.H., Hmming, O.J. & Hrtmnn, R. The structure of humn interferon nd wht it hs tught us. J Interferon Cytokine Res 30, 565, (2010). 123 Brtlett, N.W., Buttigieg, K., Kotenko, S.V. & Smith, G.L. Murine interferon s (type III interferons) exhibit potent ntivirl ctivity in vivo in poxvirus infection model. J Gen Virol 86, 1589, (2005). 124 Lsfr, A., Lewis-Antes, A., Smirnov, S.V. et l. Chrcteriztion of the mouse IFN lignd-r system: IFN-s exhibit ntitumor ctivity ginst B16 melnom. Cncer Res 66, 4468, (2006). 125 O'Brien, T.R., Prokunin-Olsson, L. & Donnelly, R.P. IFN-4: the prdoxicl new member of the interferon fmily. J Interferon Cytokine Res 34, 829, (2014). 126 Donnelly, R.P., Dickensheets, H. & O'Brien, T.R. Interferon- nd therpy for chronic heptitis C virus infection. Trends Immunol 32, 443, (2011). 127 Lsfr, A., Abushhb, W., Bln, M. & Cohen-Soll, K.A. Interferon : new sword in cncer immunotherpy. Clin Dev Immunol 2011, , (2011). 128 Kotenko, S.V. IFN-s. Curr Opin Immunol 23, 583, (2011). 129 Meger, A., Visvlingm, K., Dilger, P., Bryn, D. & Wdhw, M. Biologicl ctivity of interleukins-28 nd -29: comprison with type I interferons. Cytokine 31, 109, (2005). 89
91 130 Cocci, E.M., Sever, M., Gicomini, E. et l. Virl infection nd Toll-like r gonists induce differentil expression of type I nd interferons in humn plsmcytoid nd monocyte-derived dendritic cells. Eur J Immunol 34, 796, (2004). 131 Asselin-Pturel, C. & Trinchieri, G. Production of type I interferons: plsmcytoid dendritic cells nd beyond. J Exp Med 202, 461, (2005). 132 Sgrbnti, M., Mrsili, G., Remoli, A.L., Orstti, R. & Bttistini, A. IRF-7: new role in the regultion of genes involved in dptive immunity. Ann N Y Acd Sci 1095, 325, (2007). 133 Hiscott, J. Triggering the innte ntivirl response through IRF-3 ctivtion. J Biol Chem 282, 15325, (2007). 134 Siren, J., Imizumi, T., Srkr, D. et l. Retinoic cid inducible gene-i nd MDA5 re involved in influenz A virus-induced expression of ntivirl cytokines. Microbes Infect 8, 2013, (2006). 135 Ank, N., Iversen, M.B., Brtholdy, C. et l. An importnt role for type III interferon (IFN-/IL-28) in TLR-induced ntivirl ctivity. J Immunol 180, 2474, (2008). 136 Zhou, L., Wng, X., Wng, Y.J. et l. Activtion of toll-like r-3 induces interferon- expression in humn neuronl cells. Neuroscience 159, 629, (2009). 137 Wng, J., Oberley-Deegn, R., Wng, S. et l. Differentited humn lveolr type II cells secrete ntivirl IL-29 (IFN- 1) in response to influenz A infection. J Immunol 182, 1296, (2009). 138 Onoguchi, K., Yoneym, M., Tkemur, A. et l. Virl infections ctivte types I nd III interferon genes through common mechnism. J Biol Chem 282, 7576, (2007). 139 Osterlund, P., Veckmn, V., Siren, J. et l. Gene expression nd ntivirl ctivity of lph/bet interferons nd interleukin-29 in virus-infected humn myeloid dendritic cells. J Virol 79, 9608, (2005). 140 Hond, K., Yni, H., Tkok, A. & Tniguchi, T. Regultion of the type I IFN induction: current view. Int Immunol 17, 1367, (2005). 141 Iversen, M.B., Ank, N., Melchjorsen, J. & Pludn, S.R. Expression of type III interferon (IFN) in the vginl mucos is medited primrily by dendritic cells nd displys stronger dependence on NF-kppB thn type I IFNs. J Virol 84, 4579, (2010). 142 Li, M., Liu, X., Zhou, Y. & Su, S.B. Interferon-s: the modultors of ntivirus, ntitumor, nd immune responses. J Leukoc Biol 86, 23, (2009). 143 Hyden, F.G., Albrecht, J.K., Kiser, D.L. & Gwltney, J.M., Jr. Prevention of nturl colds by contct prophylxis with intrnsl lph 2-interferon. N Engl J Med 314, 71, (1986). 144 Pnigrhi, R., Hzri, S., Chndr, S. et l. Interferon nd ribvirin combintion tretment synergisticlly inhibit HCV internl ribosome entry site medited trnsltion t the level of polyribosome formtion. PLoS One 8, e72791, (2013). 145 Dickensheets, H., Sheikh, F., Prk, O., Go, B. & Donnelly, R.P. Interferon- induces signl trnsduction nd gene expression in humn heptocytes, but not in lymphocytes or monocytes. J Leukoc Biol 93, 377, (2013). 146 Zhou, Z., Hmming, O.J., Ank, N. et l. Type III interferon (IFN) induces type I IFNlike response in restricted subset of cells through signling pthwys involving both the JAK-STAT pthwy nd the mitogen-ctivted protein kinses. J Virol 81, 7749, (2007). 147 Vossen, M.T., Westerhout, E.M., Soderberg-Nucler, C. & Wiertz, E.J. Virl immune evsion: msterpiece of evolution. Immunogenetics 54, 527, (2002). 90
92 148 M, D., Jing, D., Qing, M. et l. Antivirl effect of interferon ginst West Nile virus. Antivirl Res 83, 53, (2009). 149 Khitov, M.R., Lz-Stnc, V., Edwrds, M.R. et l. Respirtory virus induction of lph-, bet- nd -interferons in bronchil epithelil cells nd peripherl blood mononucler cells. Allergy 64, 375, (2009). 150 Srinivs, S., Di, J., Eskdle, J. et l. Interferon-1 (interleukin-29) preferentilly down-regultes interleukin-13 over other T helper type 2 cytokine responses in vitro. Immunology 125, 492, (2008). 151 Jordn, W.J., Eskdle, J., Srinivs, S. et l. Humn interferon -1 (IFN1/IL-29) modultes the Th1/Th2 response. Genes Immun 8, 254, (2007). 152 Brnd, S., Beigel, F., Olszk, T. et l. IL-28A nd IL-29 medite ntiprolifertive nd ntivirl signls in intestinl epithelil cells nd murine CMV infection increses colonic IL-28A expression. Am J Physiol Gstrointest Liver Physiol 289, G960, (2005). 153 Robek, M.D., Boyd, B.S. & Chisri, F.V. Lmbd interferon inhibits heptitis B nd C virus repliction. J Virol 79, 3851, (2005). 154 Miller, D.M., Klucher, K.M., Freemn, J.A. et l. Interferon s potentil new therpeutic for heptitis C. Ann N Y Acd Sci 1182, 80, (2009). 155 Lrkin, J., Jin, L., Frmen, M. et l. Synergistic ntivirl ctivity of humn interferon combintions in the heptitis C virus replicon system. J Interferon Cytokine Res 23, 247, (2003). 156 Pgliccetti, N.E., Edurdo, R., Kleinstein, S.H. et l. Interleukin-29 functions coopertively with interferon to induce ntivirl gene expression nd inhibit heptitis C virus repliction. J Biol Chem 283, 30079, (2008). 157 Mrcello, T., Grkoui, A., Brb-Speth, G. et l. Interferons lph nd inhibit heptitis C virus repliction with distinct signl trnsduction nd gene regultion kinetics. Gstroenterology 131, 1887, (2006). 158 Mkowsk, Z., Duong, F.H., Trincucci, G., Tough, D.F. & Heim, M.H. Interferon-bet nd interferon- signling is not ffected by interferon-induced refrctoriness to interferon-lph in vivo. Heptology 53, 1154, (2011). 159 Sommereyns, C., Pul, S., Steheli, P. & Michiels, T. IFN- (IFN-) is expressed in tissue-dependent fshion nd primrily cts on epithelil cells in vivo. PLoS Pthog 4, e , (2008). 160 Mher, S.G., Sheikh, F., Scrzello, A.J. et l. IFN lph nd IFN differ in their ntiprolifertive effects nd durtion of JAK/STAT signling ctivity. Cncer Biol Ther 7, 1109, (2008). 161 Li, W., Hung, X., Liu, Z. et l. Type III interferon induces poptosis in humn lung cncer cells. Oncol Rep 28, 1117, (2012). 162 Zitzmnn, K., Brnd, S., Behs, S. et l. Novel interferon-s induce ntiprolifertive effects in neuroendocrine tumor cells. Biochem Biophys Res Commun 344, 1334, (2006). 163 Brnd, S., Zitzmnn, K., Dmbcher, J. et l. SOCS-1 inhibits expression of the ntivirl proteins 2',5'-OAS nd MxA induced by the novel interferon-s IL- 28A nd IL-29. Biochem Biophys Res Commun 331, 543, (2005). 164 Lnger, J.A., Cutrone, E.C. & Kotenko, S. The Clss II cytokine r (CRF2) fmily: overview nd ptterns of r-lignd interctions. Cytokine Growth Fctor Rev 15, 33, (2004). 165 Kotenko, S.V. & Lnger, J.A. Full house: 12 rs for 27 cytokines. Int Immunophrmcol 4, 593, (2004). 166 Uze, G. & Monneron, D. IL-28 nd IL-29: newcomers to the interferon fmily. Biochimie 89, 729, (2007). 91
93 167 Bch, E.A., Aguet, M. & Schreiber, R.D. The IFN gmm r: prdigm for cytokine r signling. Annu Rev Immunol 15, 563, (1997). 168 Heney, M.L. & Golde, D.W. Soluble cytokine rs. Blood 87, 847, (1996). 169 Levine, S.J. Mechnisms of soluble cytokine r genertion. J Immunol 173, 5343, (2004). 170 Owczrek, C.M., Hwng, S.Y., Hollnd, K.A. et l. Cloning nd chrcteriztion of soluble nd trnsmembrne isoforms of novel component of the murine type I interferon r, IFNAR 2. J Biol Chem 272, 23865, (1997). 171 Hrdy, M.P., Owczrek, C.M., Trjnovsk, S. et l. The soluble murine type I interferon r IFNAR-2 is present in serum, is independently regulted, nd hs both gonistic nd ntgonistic properties. Blood 97, 473, (2001). 172 Witte, K., Gruetz, G., Volk, H.D. et l. Despite IFN- r expression, blood immune cells, but not kertinocytes or melnocytes, hve n impired response to type III interferons: implictions for therpeutic pplictions of these cytokines. Genes Immun 10, 702, (2009). 173 Wolk, K., Witte, E., Reineke, U. et l. Is there n interction between interleukin-10 nd interleukin-22? Genes Immun 6, 8, (2005). 174 Jordn, W.J., Eskdle, J., Boniotto, M. et l. Modultion of the humn cytokine response by interferon -1 (IFN-1/IL-29). Genes Immun 8, 13, (2007). 175 Z, Z., OJ, H., N, A. et l. Type III interferon (IFN) induces type I IFN-like response in restricted subset of cells through signling pthwys involving both the JAK-STAT pthwy nd the mitogen-ctivted protein kinses. J Virol 81, 7749, (2007). 176 Mordstein, M., Michiels, T. & Steheli, P. Wht hve we lerned from the IL28 r knockout mouse? J Interferon Cytokine Res 30, 579, (2010). 177 Pulverer, J.E., Rnd, U., Lienenklus, S. et l. Temporl nd sptil resolution of type I nd III interferon responses in vivo. J Virol 84, 8626, (2010). 178 Durbin, R.K., Kotenko, S.V. & Durbin, J.E. Interferon induction nd function t the mucosl surfce. Immunol Rev 255, 25, (2013). 179 Dumoutier, L., Lejeune, D., Hor, S., Fickenscher, H. & Renuld, J.C. Cloning of new type II cytokine r ctivting signl trnsducer nd ctivtor of trnscription (STAT)1, STAT2 nd STAT3. Biochem J 370, 391, (2003). 180 Lzer, H.M., Dniels, B.P., Pinto, A.K. et l. Interferon-λ restricts West Nile virus neuroinvsion by tightening the blood-brin brrier. Science trnsltionl medicine 7, 284r59, (2015). 181 Prinz, M., Schmidt, H., Mildner, A. et l. Distinct nd nonredundnt in vivo functions of IFNAR on myeloid cells limit utoimmunity in the centrl nervous system. Immunity 28, 675, (2008). 182 Kotenko, S.V. & Pestk, S. Jk-Stt signl trnsduction pthwy through the eyes of cytokine clss II r complexes. Oncogene 19, 2557, (2000). 183 O'She, J.J. & Plenge, R. JAK nd STAT signling molecules in immunoregultion nd immune-medited disese. Immunity 36, 542, (2012). 184 Kiu, H. & Nicholson, S.E. Biology nd significnce of the JAK/STAT signlling pthwys. Growth Fctors 30, 88, (2012). 185 Lopusn, K., Rezuchov, I., Betkov, T. et l. Interferons, new cytokines with ntivirl ctivity. Act Virol 57, 171, (2013). 186 Bluthgen, N. & Legewie, S. Systems nlysis of MAPK signl trnsduction. Essys Biochem 45, 95, (2008). 187 Treismn, R. Regultion of trnscription by MAP kinse cscdes. Curr Opin Cell Biol 8, 205, (1996). 188 Lowenthl, J.W., Steheli, P., Schultz, U., Sekellick, M.J. & Mrcus, P.I. Nomenclture of vin interferon proteins. J Interferon Cytokine Res 21, 547, (2001). 92
94 189 Sick, C., Schultz, U. & Steheli, P. A fmily of genes coding for two serologiclly distinct chicken interferons. J Biol Chem 271, 7635, (1996). 190 Nnd, I., Sick, C., Munster, U. et l. Sex chromosome linkge of chicken nd duck type I interferon genes: further evidence of evolutionry conservtion of the Z chromosome in birds. Chromosom 107, 204, (1998). 191 Steheli, P., Puehler, F., Schneider, K., Gobel, T.W. & Kspers, B. Cytokines of birds: conserved functions-- lrgely different look. J Interferon Cytokine Res 21, 993, (2001). 192 Sick, C., Schultz, U., Munster, U. et l. Promoter structures nd differentil responses to virl nd nonvirl inducers of chicken type I interferon genes. J Biol Chem 273, 9749, (1998). 193 Levy, A.M., Heller, E.D., Leitner, G. & Dvidson, I. Effect of ntive chicken interferon on MDV repliction. Act Virol 43, 121, (1999). 194 Mo, C.W., Co, Y.C. & Lim, B.L. The in vivo nd in vitro effects of chicken interferon lph on infectious bursl disese virus nd Newcstle disese virus infection. Avin Dis 45, 389, (2001). 195 Pei, J., Sekellick, M.J., Mrcus, P.I., Choi, I.S. & Collisson, E.W. Chicken interferon type I inhibits infectious bronchitis virus repliction nd ssocited respirtory illness. J Interferon Cytokine Res 21, 1071, (2001). 196 Xi, C., Liu, J., Wu, Z.G., Lin, C.Y. & Wng, M. The interferon-lph genes from three chicken lines nd its effects on H9N2 influenz viruses. Anim Biotechnol 15, 77, (2004). 197 Digby, M.R. & Lowenthl, J.W. Cloning nd expression of the chicken interferongmm gene. J Interferon Cytokine Res 15, 939, (1995). 198 Qi, J., Du, Y., Zhu, X. et l. [Soluble expression of chicken interferon-gmm nd ntivirl ctivity of purified expression product]. Wei Sheng Wu Xue Bo 49, 85, (2009). 199 Mllick, A.I., Hq, K., Brisbin, J.T. et l. Assessment of bioctivity of recombinnt chicken interferon-gmm expressed using bculovirus expression system. J Interferon Cytokine Res 31, 493, (2011). 200 Lmbrecht, B., Gonze, M., Morles, D., Meulemns, G. & vn den Berg, T.P. Comprison of biologicl ctivities of nturl nd recombinnt chicken interferongmm. Vet Immunol Immunopthol 70, 257, (1999). 201 Krpl, A.J., Morris, K.R., Brodwy, M.M. et l. Moleculr cloning, expression, nd chrcteriztion of chicken IFN -. J Interferon Cytokine Res 28, 341, (2008). 202 Reboul, J., Grdiner, K., Monneron, D., Uze, G. & Lutfll, G. Comprtive genomic nlysis of the interferon/interleukin-10 r gene cluster. Genome Res 9, 242, (1999). 203 Hn, X., Chen, T. & Wng, M. Moleculr cloning nd chrcteriztion of chicken interferon-gmm r lph-chin. J Interferon Cytokine Res 28, 445, (2008). 204 Adchi, H., Tkemoto, Y., Bungo, T. & Ohkubo, T. Chicken leptin r is functionl in ctivting JAK-STAT pthwy in vitro. J Endocrinol 197, 335, (2008). 205 Kuchipudi, S.V., Tellbti, M., Sebstin, S. et l. Highly pthogenic vin influenz virus infection in chickens but not ducks is ssocited with elevted host immune nd pro-inflmmtory responses. Vet Res 45, 118, (2014). 206 Reuter, A., Soubies, S., Hrtle, S. et l. Antivirl ctivity of interferon in chickens. J Virol 88, 2835, (2014). 207 Schneider, W.M., Chevillotte, M.D. & Rice, C.M. Interferon-stimulted genes: complex web of host defenses. Annu Rev Immunol 32, 513, (2014). 208 Go, S., von der Mlsburg, A., Peschke, S., Behlke, J. & Hller, O. Structurl bsis of oligomeriztion in the stlk region of dynmin-like MxA. Nture 465, 502, (2010). 93
95 209 Go, G., Guo, X. & Goff, S.P. Inhibition of retrovirl RNA production by ZAP, CCCHtype zinc finger protein. Science 297, 1703, (2002). 210 Tu, Y.C., Yu, C.Y., Ling, J.J. et l. Blocking double-strnded RNA-ctivted protein kinse PKR by Jpnese encephlitis virus nonstructurl protein 2A. J Virol 86, 10347, (2012). 211 Zhou, X., Michl, J.J., Zhng, L. et l. Interferon induced IFIT fmily genes in host ntivirl defense. Interntionl journl of biologicl sciences 9, 200, (2013). 212 Helbig, K.J. & Berd, M.R. The role of Viperin in the innte ntivirl response. J Mol Biol 426, 1210, (2014). 213 Horisberger, M.A., Steheli, P. & Hller, O. Interferon induces unique protein in mouse cells bering gene for resistnce to influenz virus. Proc Ntl Acd Sci U S A 80, 1910, (1983). 214 Kochs, G. & Hller, O. Interferon-induced humn MxA GTPse blocks nucler import of Thogoto virus nucleocpsids. Proc Ntl Acd Sci U S A 96, 2082, (1999). 215 Reichelt, M., Stertz, S., Krijnse-Locker, J., Hller, O. & Kochs, G. Missorting of LCrosse virus nucleocpsid protein by the interferon-induced MxA GTPse involves smooth ER membrnes. Trffic 5, 772, (2004). 216 Sironi, L., Willims, J.L., Moreno-Mrtin, A.M. et l. Susceptibility of different chicken lines to H7N1 highly pthogenic vin influenz virus nd the role of Mx gene polymorphism coding mino cid position 631. Virology 380, 152, (2008). 217 Ko, J.H., Jin, H.K., Asno, A. et l. Polymorphisms nd the differentil ntivirl ctivity of the chicken Mx gene. Genome Res 12, 595, (2002). 218 Wng, Y., Brhmkshtriy, V., Lupini, B. et l. Associtions of chicken Mx1 polymorphism with ntivirl responses in vin influenz virus infected embryos nd broilers. Poult Sci 91, 3019, (2012). 219 Schusser, B., Reuter, A., von der Mlsburg, A. et l. Mx is dispensble for interferonmedited resistnce of chicken cells ginst influenz A virus. J Virol 85, 8307, (2011). 220 Fulton, J.E., Arngo, J., Ali, R.A. et l. Genetic Vrition within the Mx Gene of Commercilly Selected Chicken Lines Revels Multiple Hplotypes, Recombintion nd Protein under Selection Pressure. PLoS One 9, e108054, (2014). 221 Oshiumi, H., Mifsud, E.J. & Dito, T. Links between recognition nd degrdtion of cytoplsmic virl RNA in innte immune response. Rev Med Virol 26, 90, (2016). 222 McDonld, M.R., Mchlin, E.S., Albin, O.R. & Levy, D.E. The zinc finger ntivirl protein cts synergisticlly with n interferon-induced fctor for mximl ctivity ginst lphviruses. J Virol 81, 13509, (2007). 223 Goossens, K.E., Krpl, A.J., Wrd, A. & Ben, A.G. Chrcteristion of chicken ZAP. Dev Comp Immunol 46, 373, (2014). 224 Clemens, M.J. & Eli, A. The double-strnded RNA-dependent protein kinse PKR: structure nd function. J Interferon Cytokine Res 17, 503, (1997). 225 McAllister, C.S., Tghvi, N. & Smuel, C.E. Protein kinse PKR mplifiction of interferon bet induction occurs through initition fctor eif-2lph-medited trnsltionl control. J Biol Chem 287, 36384, (2012). 226 Ko, J.H., Asno, A., Kon, Y., Wtnbe, T. & Agui, T. Chrcteriztion of the chicken PKR: polymorphism of the gene nd ntivirl ctivity ginst vesiculr stomtitis virus. Jpn J Vet Res 51, 123, (2004). 227 Abbs, Y.M., Pichlmir, A., Gorn, M.W., Superti-Furg, G. & Ngr, B. Structurl bsis for virl 5'-PPP-RNA recognition by humn IFIT proteins. Nture 494, 60, (2013). 228 Feng, F., Yun, L., Wng, Y.E. et l. Crystl structure nd nucleotide selectivity of humn IFIT5/ISG58. Cell reserch 23, 1055, (2013). 94
96 229 Rychlik, I., Elsheimer-Mtulov, M. & Kyrov, K. Gene expression in the chicken cecum in response to infections with non-typhoid Slmonell. Vet Res 45, 119, (2014). 230 Brber, M.R., Aldridge, J.R., Jr., Webster, R.G. & Mgor, K.E. Assocition of RIG-I with innte immunity of ducks to influenz. Proc Ntl Acd Sci U S A 107, 5913, (2010). 231 Mtulov, M., Vrmuzov, K., Sisk, F. et l. Chicken innte immune response to orl infection with Slmonell enteric serovr Enteritidis. Vet Res 44, 37, (2013). 232 Wng, S., Wu, X., Pn, T. et l. Viperin inhibits heptitis C virus repliction by interfering with binding of NS5A to host protein hvap-33. J Gen Virol 93, 83, (2012). 233 Helbig, K.J., Crr, J.M., Clvert, J.K. et l. Viperin is induced following dengue virus type-2 (DENV-2) infection nd hs nti-virl ctions requiring the C-terminl end of viperin. PLoS Negl Trop Dis 7, e2178, (2013). 234 Wng, X., Hinson, E.R. & Cresswell, P. The interferon-inducible protein Viperin inhibits influenz virus relese by perturbing lipid rfts. Cell Host Microbe 2, 96, (2007). 235 Nsr, N., Mddocks, S., Turville, S.G. et l. HIV-1 infection of humn mcrophges directly induces viperin which inhibits virl production. Blood 120, 778, (2012). 236 Goossens, K.E., Krpl, A.J., Rohringer, A., Wrd, A. & Ben, A.G. Chrcteristion of chicken viperin. Mol Immunol 63, 373, (2015). 237 Short, K.R., Richrd, M., Verhgen, J.H. et l. One helth, multiple chllenges: The inter-species trnsmission of influenz A virus. One Helth 1, 1, (2015). 238 Th, F. How Highly Pthogenic Avin Influenz (H5N1) Hs Affected World Poultry-Met Trde. United Sttes Deprtment of Agriculture Outlook No. (LDPM ) 27 pp, (October 2007). 239 McLeod, A., Morgn, N., Prksh, A. & Hindrichs, J. Ecomomic nd Socil Impcts of Avin Influenz. 240 To, K.K., Ng, K.H., Que, T.L. et l. Avin influenz A H5N1 virus: continuous thret to humns. Emerg Microbes Infect 1, e25, (2012). 241 Cls, E.C., Osterhus, A.D., vn Beek, R. et l. Humn influenz A H5N1 virus relted to highly pthogenic vin influenz virus. Lncet 351, 472, (1998). 242 Repernt, L.A., Kuiken, T. & Osterhus, A.D. Influenz viruses: from birds to humns. Hum Vccin Immunother 8, 7, (2012). 243 Russell, C.A., Fonville, J.M., Brown, A.E. et l. The potentil for respirtory droplettrnsmissible A/H5N1 influenz virus to evolve in mmmlin host. Science 336, 1541, (2012). 244 Peiris, J.S., de Jong, M.D. & Gun, Y. Avin influenz virus (H5N1): thret to humn helth. Clin Microbiol Rev 20, 243, (2007). 245 Shiny, K., Ebin, M., Ymd, S. et l. Avin flu: influenz virus rs in the humn irwy. Nture 440, 435, (2006). 246 Mests, J. & Hughes, C.C.W. Of Mice nd Not Men: Differences between Mouse nd Humn Immunology. J Immunol 172, 2731, (2004). 247 Ben, A.G., Bker, M.L., Stewrt, C.R. et l. Studying immunity to zoonotic diseses in the nturl host - keeping it rel. Nt Rev Immunol, (2013). 248 Ben, A.G., Bker, M.L., Stewrt, C.R. et l. Studying immunity to zoonotic diseses in the nturl host - keeping it rel. Nt Rev Immunol 13, 851, (2013). 249 WHO. Mnul for the lbortory dignosis nd virologicl surveillnce of influenz, Reed, L.J. & Muench, H. A simple method of estimting fifty per cent endpoints. Am J Epidemiol 27, 493, (1938). 95
97 251 Currn, J.M., Robertson, I.D., Ellis, T.M. & Selleck, P.W. Evlution of vin influenz serologic nd virologic dignostic methods in wild Anseriformes nd Chrdriiformes. Avin Dis 58, 53, (2014). 252 Heine, H.G., Trinidd, L., Selleck, P. & Lowther, S. Rpid detection of highly pthogenic vin influenz H5N1 virus by TqMn reverse trnscriptsepolymerse chin rection. Avin Dis 51, 370, (2007). 253 Butler, J., Stewrt, C.R., Lyton, D.S. et l. Novel Ressortnt H5N6 Influenz A Virus from the Lo People's Democrtic Republic Is Highly Pthogenic in Chickens. PLoS One 11, e , (2016). 254 Wrd, A.C., Hermns, M.H., Smith, L. et l. Tyrosine-dependent nd -independent mechnisms of STAT3 ctivtion by the humn grnulocyte colony-stimulting fctor (G-CSF) r re differentilly utilized depending on G-CSF concentrtion. Blood 93, 113, (1999). 255 Krpl, A.J., Stewrt, C., McKy, J., Lowenthl, J.W. & Ben, A.G. Chrcteriztion of chicken MDA5 ctivity: regultion of IFN-bet in the bsence of RIG-I functionlity. J Immunol 186, 5397, (2011). 256 Bekisz, J., Schmeisser, H., Hernndez, J., Goldmn, N.D. & Zoon, K.C. Humn interferons lph, bet nd omeg. Growth Fctors 22, 243, (2004). 257 Demoulins, T., Bron, M.L., Kettf, N. et l. Poly (I:C) induced immune response in lymphoid tissues involves three sequentil wves of type I IFN expression. Virology 386, 225, (2009). 258 Chow, J.C., Young, D.W., Golenbock, D.T., Christ, W.J. & Gusovsky, F. Toll-like Receptor-4 Medites Lipopolyscchride-induced Signl Trnsduction. Journl of Biologicl Chemistry 274, 10689, (1999). 259 Jones, D.T., Tylor, W.R. & Thornton, J.M. The rpid genertion of muttion dt mtrices from protein sequences. Comput Appl Biosci 8, 275, (1992). 260 Sto, M., Ht, N., Asgiri, M. et l. Positive feedbck regultion of type I IFN genes by the IFN-inducible trnscription fctor IRF-7. FEBS Lett 441, 106, (1998). 261 Keestr, A.M., de Zoete, M.R., Bouwmn, L.I., Vezird, M.M. & vn Putten, J.P. Unique fetures of chicken Toll-like rs. Dev Comp Immunol 41, 316, (2013). 262 Dionne, P.R., Mri, E.L., Willim, J. & Sbr, L.K. Elevted 17ß-estrdiol protects femles from influenz A virus pthogenesis by suppressing inflmmtory responses. PLoS Pthog 7, e , (2011). 263 Mrk, C.S., Michel, G.O., Frnck, H., Aln, L.S. & Sbr, L.K. 17bet-estrdiol lters the ctivity of conventionl nd IFN-producing killer dendritic cells. J Immunol 180, 1423, (2008). 264 Mnry, J., Lvl, G., Ptin, E. et l. Evolutionry genetic dissection of humn interferons. J Exp Med 208, 2747, (2011). 265 Svn, R., Rvichndrn, S., Collins, J.R., Ski, M. & Young, H.A. Structurl conservtion of interferon gmm mong vertebrtes. Cytokine Growth Fctor Rev 20, 115, (2009). 266 Ank, N., West, H., Brtholdy, C. et l. Lmbd interferon (IFN-), type III IFN, is induced by viruses nd IFNs nd displys potent ntivirl ctivity ginst select virus infections in vivo. J Virol 80, 4501, (2006). 267 Msud, Y., Mtsud, A., Usui, T. et l. Biologicl effects of chicken type III interferon on expression of interferon-stimulted genes in chickens: comprison with type I nd type II interferons. J Vet Med Sci 74, 1381, (2012). 268 Lin, J.D., Feng, N., Sen, A. et l. Distinct Roles of Type I nd Type III Interferons in Intestinl Immunity to Homologous nd Heterologous Rotvirus Infections. PLoS Pthog 12, e , (2016). 96
98 269 Gutier, G., Humbert, M., Deuvieu, F. et l. A type I interferon utocrineprcrine loop is involved in Toll-like r-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 201, 1435, (2005). 270 Pott, J., Mhlkoiv, T., Mordstein, M. et l. IFN- determines the intestinl epithelil ntivirl host defense. Proc Ntl Acd Sci U S A 108, 7944, (2011). 271 Okmoto, M., Oshiumi, H., Azum, M. et l. IPS-1 is essentil for type III IFN production by heptocytes nd dendritic cells in response to heptitis C virus infection. J Immunol 192, 2770, (2014). 272 Bierne, H., Trvier, L., Mhlkoiv, T. et l. Activtion of type III interferon genes by pthogenic bcteri in infected epithelil cells nd mouse plcent. PLoS One 7, e39080, (2012). 273 Hillyer, P., Mne, V.P., Schrmm, L.M. et l. Expression profiles of humn interferon-lph nd interferon- subtypes re lignd- nd cell-dependent. Immunol Cell Biol 90, 774, (2012). 274 Brem, J.H., Ping, A., Zhng, X., Winkler, C. & Young, H.A. A single nucleotide polymorphism in the proximl IFN-gmm promoter lters control of gene trnscription. Genes Immun 3, 165, (2002). 275 Mrie, I., Durbin, J.E. & Levy, D.E. Differentil virl induction of distinct interferonlph genes by positive feedbck through interferon regultory fctor-7. EMBO J 17, 6660, (1998). 276 Tilor, P., Tmur, T., Kong, H.J. et l. The feedbck phse of type I interferon induction in dendritic cells requires interferon regultory fctor 8. Immunity 27, 228, (2007). 277 DeWitte-Orr, S.J., Meht, D.R., Collins, S.E. et l. Long double-strnded RNA induces n ntivirl response independent of IFN regultory fctor 3, IFN-bet promoter stimulto, nd IFN. J Immunol 183, 6545, (2009). 278 Mlcolm, K.C. & Worthen, G.S. Lipopolyscchride stimultes p38-dependent induction of ntivirl genes in neutrophils independently of prcrine fctors. J Biol Chem 278, 15693, (2003). 279 Ovstebo, R., Olstd, O.K., Brusletto, B. et l. Identifiction of genes prticulrly sensitive to lipopolyscchride (LPS) in humn monocytes induced by wild-type versus LPS-deficient Neisseri meningitidis strins. Infect Immun 76, 2685, (2008). 280 Sheikh, F., Dickensheets, H., Gmero, A.M., Vogel, S.N. & Donnelly, R.P. An essentil role for IFN-bet in the induction of IFN-stimulted gene expression by LPS in mcrophges. J Leukoc Biol 96, 591, (2014). 281 Mrcello, T., Grkoui, A., Brb Speth, G. et l. Interferons α nd λ Inhibit Heptitis C Virus Repliction With Distinct Signl Trnsduction nd Gene Regultion Kinetics. Gstroenterology 131, 1887, (2006). 282 Krpl, A.J., Binghm, J., Scht, K.A. et l. Highly pthogenic (H5N1) vin influenz induces n inflmmtory T helper type 1 cytokine response in the chicken. J Interferon Cytokine Res 31, (2011). 283 Mtsuu, A., Kobyshi, T., Ptchimsiri, T. et l. Pthogenicity of Geneticlly Similr, H5N1 Highly Pthogenic Avin Influenz Virus Strins in Chicken nd the Differences in Sensitivity mong Different Chicken Breeds. PLoS One 11, e , (2016). 284 Rnwre, P.B., Mishr, A., Vijykumr, P. et l. Genome Wide Host Gene Expression Anlysis in Chicken Lungs Infected with Avin Influenz Viruses. PLoS One 11, e , (2016). 285 Uchid, Y., Wtnbe, C., Tkeme, N. et l. Identifiction of host genes linked with the survivbility of chickens infected with recombinnt viruses possessing H5N1 97
99 surfce ntigens from highly pthogenic vin influenz virus. J Virol 86, 2686, (2012). 286 Klein, S.L. The effects of hormones on sex differences in infection: from genes to behvior. Neurosci Biobehv Rev 24, 627, (2000). 287 Klein, S.L. Sex influences immune responses to viruses, nd efficcy of prophylxis nd tretments for virl diseses. Bioessys 34, 1050, (2012). 288 Klein, S.L., Bird, B.H. & Glss, G.E. Sex differences in immune responses nd virl shedding following Seoul virus infection in Norwy rts. Am J Trop Med Hyg 65, 57, (2001). 289 Sbr, L.K., Andre, H. & Dionne, P.R. Mechnisms of sex disprities in influenz pthogenesis. J Leukoc Biol 92, 67, (2012). 290 Sbr, L.K., Anne, J. & Andrew, P. The Xs nd Y of immune responses to virl vccines. Lncet Infect Dis 10, 338, (2010). 291 Dumoutier, L., Tounsi, A., Michiels, T. et l. Role of the interleukin (IL)-28 r tyrosine residues for ntivirl nd ntiprolifertive ctivity of IL-29/interferon 1: similrities with type I interferon signling. J Biol Chem 279, 32269, (2004). 292 Ruch, I., Muller, M. & Decker, T. The regultion of inflmmtion by interferons nd their STATs. JAKSTAT 2, e23820, (2013). 293 Huxley, T.H. Further Evidence of the Affinity between the Dinosurin Reptiles nd Birds. Qurterly Journl of the Geologicl Society 26, 12, (1870). 294 Al-Ali, H.K. & Vnnucchi, A.M. Mnging ptients with myelofibrosis nd low pltelet counts. Ann Hemtol, (2016). 295 Mogensen, T.H. Pthogen recognition nd inflmmtory signling in innte immune defenses. Clin Microbiol Rev 22, 240, (2009). 296 Mgor, K.E., Mirnzo Nvrro, D., Brber, M.R. et l. Defense genes missing from the flight division. Dev Comp Immunol 41, 377, (2013). 297 Krpl, A.J., Lowenthl, J.W. & Ben, A.G. Activtion of the TLR3 pthwy regultes IFN bet production in chickens. Dev Comp Immunol 32, 435, (2008). 298 Wng, B., Chen, Y., Mu, C. et l. Identifiction nd expression nlysis of the interferon-induced protein with tetrtricopeptide repets 5 (IFIT5) gene in duck (Ans pltyrhynchos domesticus). PLoS One 10, e , (2015). 299 Liu, Y., Zhng, Y.B., Liu, T.K. & Gui, J.F. Linege-specific expnsion of IFIT gene fmily: n insight into coevolution with IFN gene fmily. PLoS One 8, e66859, (2013). 300 Brber, M.R., Aldridge, J.R., Jr., Fleming-Cnep, X. et l. Identifiction of vin RIG-I responsive genes during influenz infection. Mol Immunol 54, 89, (2013). 301 Hui, R.K. & Leung, F.C. Differentil Expression Profile of Chicken Embryo Fibroblst DF-1 Cells Infected with Cell-Adpted Infectious Bursl Disese Virus. PLoS One 10, e , (2015). 302 Wong, G.K., Liu, B., Wng, J. et l. A genetic vrition mp for chicken with 2.8 million single-nucleotide polymorphisms. Nture 432, 717, (2004). 303 Dimond, M.S. & Frzn, M. The brod-spectrum ntivirl functions of IFIT nd IFITM proteins. Nt Rev Immunol 13, 46, (2013). 304 Zhng, B., Liu, X., Chen, W. & Chen, L. IFIT5 potentites nti-virl response through enhncing innte immune signling pthwys. Act Biochim Biophys Sin (Shnghi) 45, 867, (2013). 305 Akcy Ciblk, M., Knturvrdr Tutenyurd, M., Asr, S. et l. [Influenz surveillnce in nine consecutive sesons, : results from Ntionl Influenz Reference Lbortory, Istnbul Fculty Of Medicine, Turkey]. Mikrobiyol Bul 46, 575, (2012). 98
100 306 Anton, A., Mrcos, M.A., Torner, N. et l. Virologicl surveillnce of influenz nd other respirtory viruses during six consecutive sesons from 2006 to 2012 in Ctloni, Spin. Clin Microbiol Infect 22, 564.e1, (2016). 307 Sullivn, S.G., Crville, K.S., Chilver, M. et l. Pooled influenz vccine effectiveness estimtes for Austrli, Epidemiol Infect 144, 2317, (2016). 308 Swyne, D.E., Spckmn, E. & Pntin-Jckwood, M. Success fctors for vin influenz vccine use in poultry nd potentil impct t the wild bird-griculturl interfce. Ecohelth 11, 94, (2014). 309 Mri John, K.M., Enkhtivn, G., Ayynr, M. et l. Screening of ethnic medicinl plnts of South Indi ginst influenz (H1N1) nd their ntioxidnt ctivity. Sudi J Biol Sci 22, 191, (2015). 310 Go, Q., Wng, Z., Liu, Z. et l. A cell-bsed high-throughput pproch to identify inhibitors of influenz A virus. Act Phrm Sin B 4, 301, (2014). 311 Lee, H.C., Slzemnn, J., Jcq, N. et l. Grid-enbled high-throughput in silico screening ginst influenz A neurminidse. IEEE Trns Nnobioscience 5, 288, (2006). 312 Severson, W.E., McDowell, M., Annthn, S. et l. High-throughput screening of 100,000-compound librry for inhibitors of influenz A virus (H3N2). J Biomol Screen 13, 879, (2008). 313 Hurt, A.C., Deng, Y.M., Ernest, J. et l. Oseltmivir-resistnt influenz viruses circulting during the first yer of the influenz A(H1N1) 2009 pndemic in the Asi-Pcific region, Mrch 2009 to Mrch Euro Surveill 16, (2011). 314 Hurt, A.C., Holien, J.K., Prker, M.W. & Brr, I.G. Oseltmivir resistnce nd the H274Y neurminidse muttion in sesonl, pndemic nd highly pthogenic influenz viruses. Drugs 69, 2523, (2009). 315 de Jong, M.D., Trn, T.T., Truong, H.K. et l. Oseltmivir resistnce during tretment of influenz A (H5N1) infection. N Engl J Med 353, 2667, (2005). 316 Suzuki, K., Okd, H., Itoh, T. et l. Assocition of incresed pthogenicity of Asin H5N1 highly pthogenic vin influenz viruses in chickens with highly efficient virl repliction ccompnied by erly destruction of innte immune responses. J Virol 83, 7475, (2009). 317 Koym, S., Ishii, K.J., Kumr, H. et l. Differentil role of TLR- nd RLR-signling in the immune responses to influenz A virus infection nd vccintion. J Immunol 179, 4711, (2007). 318 Fritz, J.H., Ferrero, R.L., Philpott, D.J. & Girrdin, S.E. Nod-like proteins in immunity, inflmmtion nd disese. Nt Immunol 7, 1250, (2006). 319 Ht, N., Sto, M., Tkok, A. et l. Constitutive IFN-lph/bet signl for efficient IFN-lph/bet gene induction by virus. Biochem Biophys Res Commun 285, 518, (2001). 320 Liu, B., Chen, S., Gun, Y. & Chen, L. Type III Interferon Induces Distinct SOCS1 Expression Pttern tht Contributes to Delyed but Prolonged Activtion of Jk/STAT Signling Pthwy: Implictions for Tretment Non-Response in HCV Ptients. PLoS One 10, e , (2015). 321 Sbr, L.K., Aimee, L.M., Aln, L.S., Gry, K. & Gregory, E.G. Neontl sex steroids ffect responses to Seoul virus infection in mle but not femle Norwy rts. Brin, behvior, nd immunity 16, 736, (2002). 322 Sbr, L.K. & Gregory, A.P. Personlized vccinology: one size nd dose might not fit both sexes. Vccine 31, 2599, (2013). 323 Wei, L. & Sbr, L.K. Seoul virus-infected rt lung endothelil cells nd lveolr mcrophges differ in their bility to support virus repliction nd induce regultory T cell phenotypes. J Virol 86, 11845, (2012). 99
101 324 Griesbeck, M., Ziegler, S., Lffont, S. et l. Sex Differences in Plsmcytoid Dendritic Cell Levels of IRF5 Drive Higher IFN-lph Production in Women. J Immunol 195, 5327, (2015). 325 de Weerd, N.A. & Nguyen, T. The interferons nd their rs distribution nd regultion. Immunol Cell Biol 90, 483, (2012). 326 Mrrck, P., Lo, D., Brinster, R. et l. The effect of thymus environment on T cell development nd tolernce. Cell 53, 627, (1988). 327 McCormck, W.T., Tjoelker, L.W. & Thompson, C.B. Avin B-cell development: genertion of n immunoglobulin repertoire by gene conversion. Annu Rev Immunol 9, 219, (1991). 328 Olis, P., Adm, I., Meyer, A., Schrff, C. & Gruber, A.D. Reference Genes for Quntittive Gene Expression Studies in Multiple Avin Species. PLoS One 9, e99678, (2014). 329 FDA. FDA Approves Ruxolitinib. (2016). 330 Song, J.M. Advnces in novel influenz vccines: ptent review. J Microbiol 54, 403, (2016). 331 Bruer, R. & Chen, P. Influenz virus propgtion in embryonted chicken eggs. J Vis Exp, 52421, (2015). 332 Prodi, A.S. & Ljmnovich, S. Chnges in embryonted eggs inoculted with influenz virus. J Immunol 58, 109, (1948). 333 Finter, N.B., Liu, O.C. & Henle, W. Studies on host-virus interctions in the chick embryo-influenz virus system. X. An experimentl nlysis of the von Mgnus phenomenon. J Exp Med 101, 461, (1955). 334 Finter, N.B., Liu, O.C., Libermn, M. & Henle, W. Studies on host-virus interctions in the chick embryo-influenz virus system. J Exp Med 100, 33, (1954). 335 Henle, W. Studies on host-virus interctions in the chick embryo-influenz virus system; the propgtion of virus in conjunction with the host cells. J Exp Med 90, 13, (1949). 336 Henle, W. Studies on host-virus interctions in the chick embryo-influenz virus system; dsorption nd recovery of seed virus. J Exp Med 90, 1, (1949). 337 Henle, W. & Henle, G. Studies on host-virus interctions in the chick embryoinfluenz virus system; development of infectivity, hemgglutintion, nd complement fixtion ctivities during the first infectious cycle. J Exp Med 90, 23, (1949). 338 Henle, W. & Liu, O.C. Studies on host-virus interctions in the chick embryoinfluenz virus system. VI. Evidence for multiplicity rectivtion of inctivted virus. J Exp Med 94, 305, (1951). 339 Henle, W., Liu, O.C. & Finter, N.B. Studies on host-virus interctions in the chick embryo-influenz virus system. IX. The period of libertion of virus from infected cells. J Exp Med 100, 53, (1954). 340 Henle, W., Liu, O.C., Pucker, K. & Lief, F.S. Studies on host-virus interctions in the chick embryo-influenz virus system. XIV. The reltion between tissue-bound nd liberted virus mterils under vrious conditions of infection. J Exp Med 103, 799, (1956). 341 Liu, O.C. & Henle, W. Studies on host-virus interctions in the chick embryoinfluenz virus system. VII. Dt concerning the significnce of infectivity titrtion end-points nd the seprtion of clones t limiting dilutions. J Exp Med 97, 889, (1953). 342 Liu, O.C. & Henle, W. Studies on host-virus interctions in the chick embryoinfluenz virus system. V. Simultneous seril pssge of the gents of influenz A 100
102 nd B in reltion to vritions in the growth cycle of influenz B virus. J Exp Med 94, 291, (1951). 343 Liu, O.C. & Henle, W. Studies on host-virus interctions in the chick embryoinfluenz virus system. IV. The role of inhibitors of hemgglutintion in the evlution of virl multipliction. J Exp Med 94, 269, (1951). 344 Liu, O.C., Pucker, K. & Henle, W. Studies on host-virus interctions in the chick embryo-influenz virus system. XIII. Some spects of non-infectious virus production. J Exp Med 103, 777, (1956). 345 Pucker, K. & Henle, W. Studies on host-virus interctions in the chick embryoinfluenz virus system. XII. Further nlyses of yields derived from hetinctivted stndrd seeds. J Exp Med 101, 493, (1955). 346 Pucker, K. & Henle, W. Studies on host-virus interctions in the chick embryoinfluenz virus system. XI. The effect of prtil inctivtion of stndrd seed virus of 37 degrees C upon the progeny. J Exp Med 101, 479, (1955). 347 A, I. & J, L. Virus interference. I. The interferon. Proc R Soc B 147, 258, (1957). 348 WHO. WHO Mnul on Animl Influenz Dignosis nd Surveillnce, Hye, K., Burmkin, S., Morn, T., Grci-Sstre, A. & Fernndez-Sesm, A. The NS1 protein of humn influenz virus inhibits type I interferon production nd the induction of ntivirl responses in primry humn dendritic nd respirtory epithelil cells. J Virol 83, 6849, (2009). 350 Chebth, J., Merlin, G., Metz, R., Benech, P. & Revel, M. Interferon-induced 56,000 Mr protein nd its mrna in humn cells: moleculr cloning nd prtil sequence of the cdna. Nucleic Acids Res 11, 1213, (1983). 351 Fensterl, V. & Sen, G.C. Interferon-induced Ifit proteins: their role in virl pthogenesis. J Virol 89, 2462, (2015). 352 Guo, J., Hui, D.J., Merrick, W.C. & Sen, G.C. A new pthwy of trnsltionl regultion medited by eukryotic initition fctor 3. EMBO J 19, 6891, (2000). 353 Pichlmir, A., Lssnig, C., Eberle, C.A. et l. IFIT1 is n ntivirl protein tht recognizes 5'-triphosphte RNA. Nt Immunol 12, 624, (2011). 101
103 11 Appendix Tble 2 Accession Numbers of interferon sequences Accession Number Sign Species type NP chicken interferon lph AAX b chicken interferon bet AAU g chicken interferon gmm ABU82742 l chicken interferon ADU o chicken interferon omeg AAC41702 b humn interferon bet AAI00873 e humn interferon epsilon CAA41626 o humn interferon omeg CAA44325 g humn interferon gmm CAA humn interferon lph 2 EAW56869 l3 humn interferon 3 EAW56870 l2 humn interferon 2 EAW56871 l1 humn interferon 1 EAW humn interferon lph 1 EAW humn interferon lph 8 EAW humn interferon lph 13 EAW humn interferon lph 6 EAW humn interferon lph 5 EAW humn interferon lph 14 EAW humn interferon lph 17 EAW humn interferon lph 16 EAW humn interferon lph 10 EAW humn interferon lph 7 EAW humn interferon lph 4 EAW humn interferon lph 21 EAW k humn interferon kpp NP l4 humn interferon 4 AAA mouse interferon lph 7 AAA37891 b mouse interferon bet AAH99376 k mouse interferon kpp AAI mouse interferon lph 12 AAI04378 e mouse interferon epsilon AAI mouse interferon lph 11 AAI mouse interferon lph 4 AAI mouse interferon lph 13 AAI mouse interferon lph 5 AAI mouse interferon lph 14 AAX58714 l2 mouse interferon AAX58715 l3 mouse interferon 102
104 ACR22510 g mouse interferon gmm EDL30963 z mouse interferon zet Tble 3 Accession numbers of IFNλR1 sequences Accession Number Species type XP Predicted Ailuropod melnoleuc Interferon KYO Alligtor mississippiensis Interferon XP Predicted Alligtor sinensis Interferon AHY Anolis crolinensis Interferon AJD Anser cygnoides Interferon XP Predicted Aotus nncyme Interferon XP Predicted Aploderm vitttum Interferon XP Predicted Aptenodytes forsteri Interferon XP Predicted Aquil chrysetos cndensis Interferon Blenopter cutorostrt XP Predicted scmmoni Interferon XP Predicted Bleric regulorum gibbericeps Interferon XP Predicted Bison bison bison Interferon XP Predicted Buceros rhinoceros silvestris Interferon XP Predicted Clidris pugnx Interferon XP Predicted Cllithrix jcchus Interferon XP Predicted Cmelus bctrinus Interferon XP Predicted Cmelus dromedrius Interferon XP Predicted Cmelus ferus Interferon XP Predicted Cpr hircus Interferon XP Predicted Cprimulgus crolinensis Interferon XP Predicted Crim cristt Interferon XP Predicted Cvi porcellus Interferon XP Predicted Certotherium simum simum Interferon XP Predicted Chetur pelgic Interferon XP Predicted Chrdrius vociferus Interferon XP Predicted Cheloni myds Interferon XP Predicted Chlmydotis mcqueenii Interferon XP Predicted Chrysemys pict bellii Interferon XP Predicted Colius stritus Interferon 103
105 XP Predicted Columb livi Interferon XP Predicted Condylur cristt Interferon XP Predicted Corvus brchyrhynchos Interferon XP Predicted Corvus cornix cornix Interferon XP Predicted Cricetulus griseus Interferon XP Predicted Cricetulus griseus Interferon XP Predicted Cuculus cnorus Interferon XP Predicted Dsypus novemcinctus Interferon XP Predicted Dipodomys ordii Interferon XP Predicted Echinops telfiri Interferon XP Predicted Egrett grzett Interferon XP Predicted Elephntulus edwrdii Interferon XP Predicted Eptesicus fuscus Interferon XP Predicted Erinceus europeus Interferon XP Predicted Esox lucius Interferon XP Predicted Eurypyg helis Interferon XP Predicted Flco cherrug Interferon XP Predicted Flco peregrinus Interferon XP Predicted Felis ctus Interferon XP Predicted Ficedul lbicollis Interferon XP Predicted Ficedul lbicollis Interferon XP Predicted Fukomys dmrensis Interferon XP Predicted Fulmrus glcilis Interferon AHF Gllus gllus Interferon XP Predicted Gvi stellt Interferon XP Predicted Gekko jponicus Interferon XP Predicted Geospiz fortis Interferon XP Predicted Hlieetus lbicill Interferon XP Predicted Hlieetus leucocephlus Interferon XP Predicted Heterocephlus glber Interferon AAI Homo spiens Interferon XP Predicted Ictidomys tridecemlinetus Interferon XP Predicted Jculus jculus Interferon XP Predicted Leptosomus discolor Interferon 104
106 XP Predicted Loxodont fricn Interferon XP Predicted Mcc multt Interferon XP Predicted Mcc nemestrin Interferon XP Predicted Mncus vitellinus Interferon XP Predicted Mndrillus leucopheus Interferon XP Predicted Melegris gllopvo Interferon XP Predicted Melopsittcus undultus Interferon XP Predicted Merops nubicus Interferon XP Predicted Mesocricetus urtus Interferon XP Predicted Microcebus murinus Interferon XP Predicted Microtus ochrogster Interferon XP Predicted Monodelphis domestic Interferon XP Predicted Myotis brndtii Interferon XP Predicted Myotis dvidii Interferon XP Predicted Nestor notbilis Interferon XP Predicted Nipponi nippon Interferon XP Predicted Ochoton princeps Interferon XP Predicted Octodon degus Interferon XP Predicted Odobenus rosmrus divergens Interferon XP Predicted Oryctolgus cuniculus Interferon XP Predicted Pnther tigris ltic Interferon XP Predicted Pntholops hodgsonii Interferon XP Predicted Ppio nubis Interferon XP Predicted Prus mjor Interferon XP Predicted Pelecnus crispus Interferon XP Predicted Phethon lepturus Interferon XP Predicted Phlcrocorx crbo Interferon XP Predicted Physeter ctodon Interferon XP Predicted Picoides pubescens Interferon XP Predicted Pseudopodoces humilis Interferon XP Predicted Pterocles gutturlis Interferon AEQ Pteropus lecto Interferon XP Predicted Pygoscelis delie Interferon XP Predicted Srcophilus hrrisii Interferon 105
107 XP Predicted Serinus cnri Interferon XP Predicted Sinocyclocheilus nshuiensis Interferon XP Predicted Sorex rneus Interferon XP Predicted Struthio cmelus ustrlis Interferon XP Predicted Sturnus vulgris Interferon XP Predicted Teniopygi guttt Interferon XP Predicted Trsius syricht Interferon XP Predicted Turco erythrolophus Interferon XP Predicted Tinmus gutttus Interferon XP Predicted Tupi chinensis Interferon XP Predicted Tyto lb Interferon XP Predicted Vicugn pcos Interferon trnscript vrint ACV Xenopus tropiclis Interferon trnscript vrint ACV Xenopus tropiclis Interferon trnscript vrint ACV Xenopus tropiclis Interferon XP Predicted Zonotrichi lbicollis Interferon NP Precurser Dnio rerio Interferon Tble 4 Accession Numbers of IFNAR1 sequences Accession Number Species type XP Predicted Acinonyx jubtus Interferon lph/bet r lph XP Predicted Ailuropod melnoleuc Interferon lph/bet r lph KYO Alligtor mississippiensis Interferon lph/bet r lph XP Predicted Anolis crolinensis Interferon lph/bet r lph AJD Anser cygnoides Interferon lph/bet r lph XP Predicted Aotus nncyme Interferon lph/bet r lph XP Predicted Aploderm vitttum Interferon lph/bet r lph XP Predicted Aptenodytes forsteri Interferon lph/bet r lph XP Predicted Apteryx ustrlis mntelli Interferon lph/bet r lph XP Predicted Bison bison bison Interferon lph/bet r lph XP Predicted Bos mutus Interferon lph/bet r lph XP Predicted Bublus bublis Interferon lph/bet r lph XP Predicted Buceros rhinoceros silvestris Interferon lph/bet r lph XP Predicted Clidris pugnx Interferon lph/bet r lph XP Predicted Cllithrix jcchus Interferon lph/bet r lph XP Predicted Clypte nn Interferon lph/bet r lph XP Predicted Cmelus bctrinus Interferon lph/bet r lph XP Predicted Cpr hircus Interferon lph/bet r lph 106
108 XP Predicted Cprimulgus crolinensis Interferon lph/bet r lph XP Predicted Crlito syricht Interferon lph/bet r lph XP Predicted Chetur pelgic Interferon lph/bet r lph XP Predicted Chrdrius vociferus Interferon lph/bet r lph XP Predicted Chrysochloris sitic Interferon lph/bet r lph XP Predicted Colobus ngolensis pllitus Interferon lph/bet r lph XP Predicted Columb livi Interferon lph/bet r lph XP Predicted Corvus brchyrhynchos Interferon lph/bet r lph XP Predicted Cricetulus griseus Interferon lph/bet r lph XP Predicted Cuculus cnorus Interferon lph/bet r lph XP Predicted Dsypus novemcinctus Interferon lph/bet r lph XP Predicted Dipodomys ordii Interferon lph/bet r lph XP Predicted Echinops telfiri Interferon lph/bet r lph XP Predicted Egrett grzett Interferon lph/bet r lph XP Predicted Elephntulus edwrdii Interferon lph/bet r lph XP Predicted Eptesicus fuscus Interferon lph/bet r lph XP Predicted Equus sinus Interferon lph/bet r lph XP Predicted Equus cbllus Interferon lph/bet r lph XP Predicted Equus przewlskii Interferon lph/bet r lph XP Predicted Erinceus europeus Interferon lph/bet r lph XP Predicted Eurypyg helis Interferon lph/bet r lph XP Predicted Flco cherrug Interferon lph/bet r lph XP Predicted Flco peregrinus Interferon lph/bet r lph XP Predicted Felis ctus Interferon lph/bet r lph XP Predicted Fukomys dmrensis Interferon lph/bet r lph AAD Gllus gllus Interferon lph/bet r lph XP Predicted Geospiz fortis Interferon lph/bet r lph XP Predicted Gorill gorill gorill Interferon lph/bet r lph XP Predicted Hlieetus leucocephlus Interferon lph/bet r lph XP Predicted Heterocephlus glber Interferon lph/bet r lph AAT Homo spiens Interferon lph/bet r lph XP Predicted Jculus jculus Interferon lph/bet r lph XP Predicted Ltimeri chlumne Interferon lph/bet r lph XP Predicted Leptonychotes weddellii Interferon lph/bet r lph XP Predicted Leptosomus discolor Interferon lph/bet r lph XP Predicted Lipotes vexillifer Interferon lph/bet r lph XP Predicted Mcc nemestrin Interferon lph/bet r lph XP Predicted Mncus vitellinus Interferon lph/bet r lph XP Predicted Mndrillus leucopheus Interferon lph/bet r lph XP Predicted Mrmot mrmot mrmot Interferon lph/bet r lph XP Predicted Melegris gllopvo Interferon lph/bet r lph XP Predicted Mesocricetus urtus Interferon lph/bet r lph XP Predicted Microcebus murinus Interferon lph/bet r lph XP Predicted Miniopterus ntlensis Interferon lph/bet r lph 107
109 AAH Mus musculus Interferon lph/bet r lph XP Predicted Mustel putorius furo Interferon lph/bet r lph XP Predicted Myotis brndtii Interferon lph/bet r lph XP Predicted Myotis dvidii Interferon lph/bet r lph XP Predicted Nestor notbilis Interferon lph/bet r lph XP Predicted Nipponi nippon Interferon lph/bet r lph XP Predicted Octodon degus Interferon lph/bet r lph XP Predicted Odobenus rosmrus divergens Interferon lph/bet r lph ETE Ophiophgus hnnh Interferon lph/bet r lph XP Predicted Opisthocomus hozin Interferon lph/bet r lph XP Predicted Orcinus orc Interferon lph/bet r lph XP Predicted Ornithorhynchus ntinus Interferon lph/bet r lph XP Predicted Orycteropus fer fer Interferon lph/bet r lph XP Predicted Pnther tigris ltic Interferon lph/bet r lph NP Ppio nubis Interferon lph/bet r lph XP Predicted Pelodiscus sinensis Interferon lph/bet r lph XP Predicted Physeter ctodon Interferon lph/bet r lph XP Predicted Picoides pubescens Interferon lph/bet r lph XP Predicted Pongo belii Interferon lph/bet r lph XP Predicted Propithecus coquereli Interferon lph/bet r lph XP Predicted Protobothrops mucrosqumtus Interferon lph/bet r lph XP Predicted Pteropus lecto Interferon lph/bet r lph XP Predicted Pteropus vmpyrus Interferon lph/bet r lph XP Predicted Pygoscelis delie Interferon lph/bet r lph XP Predicted Rousettus egypticus Interferon lph/bet r lph XP Predicted Simiri boliviensis boliviensis Interferon lph/bet r lph XP Predicted Serinus cnri Interferon lph/bet r lph XP Predicted Sorex rneus Interferon lph/bet r lph XP Predicted Struthio cmelus ustrlis Interferon lph/bet r lph XP Predicted Sturnus vulgris Interferon lph/bet r lph BAD Sus scrof Interferon lph/bet r lph JAG Sus scrof domesticus Interferon lph/bet r lph XP Predicted Teniopygi guttt Interferon lph/bet r lph XP Predicted Tinmus gutttus Interferon lph/bet r lph XP Predicted Tupi chinensis Interferon lph/bet r lph XP Predicted Tursiops trunctus Interferon lph/bet r lph Tble 5 Accession Numbers of IL10R2 sequences Accession Number Species type XP PREDICTED: Alligtor sinensis. Interleukin 10 r bet AGC Ans pltyrhynchos interleukin 10 r bet XP PREDICTED: Anolis crolinensis. interleukin 10 r bet XP PREDICTED: Anser cygnoides domesticus Interleukin 10 r bet 108
110 XP PREDICTED: Aploderm vitttum interleukin 10 r bet XP PREDICTED: Aplodermvitttum. interleukin 10 r bet XP PREDICTED: Aptenodytes forsteri interleukin 10 r bet XP PREDICTED: Aptenodytesforsteri. Interleukin 10 r bet XP PREDICTED: Aquil chrysetos cndensis interleukin 10 r bet XP PREDICTED: Blenoptercutorostrt scmmoni. Interleukin 10 r bet XP PREDICTED: Bos mutus. interleukin 10 r bet AAI23562 Bos turus. Interleukin 10 r bet XP PREDICTED: Bublusbublis. Interleukin 10 r bet XP PREDICTED: Clidris pugnx interleukin 10 r bet XP PREDICTED: Cnis lupusfmiliris. interleukin 10 r bet XP PREDICTED: Cpr hircus. interleukin 10 r bet XP PREDICTED: Cvi porcellus. interleukin 10 r bet XP PREDICTED: Certotheriumsimum simum. Interleukin 10 r bet XP PREDICTED: Chetur pelgic interleukin 10 r bet XP PREDICTED: Cheturpelgic. interleukin 10 r bet XP PREDICTED: Chrdrius vociferus Interleukin 10 r bet XP PREDICTED: Chrdriusvociferus. interleukin 10 r bet XP PREDICTED: Cheloni myds Interleukin 10 r bet XP PREDICTED: Cheloni myds. interleukin 10 r bet XP PREDICTED: Chlorocebussbeus. interleukin 10 r bet XP PREDICTED: Chrysochlorissitic. interleukin 10 r bet XP PREDICTED: Columb livi interleukin 10 r bet XP PREDICTED: Columb livi. interleukin 10 r bet XP PREDICTED: Condylurcristt. interleukin 10 r bet XP PREDICTED: Corvus brchyrhynchos Interleukin 10 r bet XP PREDICTED: Corvus brchyrhynchos. interleukin 10 r bet XP PREDICTED: Cuculus cnorus interleukin 10 r bet XP PREDICTED: Cuculus cnorus. interleukin 10 r bet XP PREDICTED: Dsypusnovemcinctus. interleukin 10 r bet XP PREDICTED: Echinopstelfiri. interleukin 10 r bet XP PREDICTED: Egrett grzett interleukin 10 r bet XP PREDICTED: Egrett grzett. interleukin 10 r bet XP PREDICTED: Elephntulusedwrdii. interleukin 10 r bet XP PREDICTED: Eptesicus fuscus. interleukin 10 r bet XP PREDICTED: Erinceuseuropeus. interleukin 10 r bet XP PREDICTED: Flco cherrug interleukin 10 r bet XP PREDICTED: Flco cherrug. interleukin 10 r bet XP PREDICTED: Flco peregrinus interleukin 10 r bet XP PREDICTED: Flco peregrinus. interleukin 10 r bet XP PREDICTED: Gleopterus vriegtus. interleukin 10 r bet AAD13678 Gllus gllus. interleukin 10 r bet XP PREDICTED: Geospiz fortis. interleukin 10 r bet XP PREDICTED: gorill gorill. interleukin 10 r bet 109
111 XP PREDICTED: Hlieetus leucocephlus interleukin 10 r bet AAH01903 Homo spiens. interleukin 10 r bet AHH Ictlurus puncttus interleukin 10 r bet XP PREDICTED: Ictidomystridecemlinetus. Interleukin 10 r bet XP PREDICTED: Jculus jculus. interleukin 10 r bet XP PREDICTED: Leptonychotesweddellii. interleukin 10 r bet XP PREDICTED: Mncus vitellinus interleukin 10 r bet XP PREDICTED: Mncus vitellinus. interleukin 10 r bet XP PREDICTED: Melegris gllopvo interleukin 10 r bet XP PREDICTED: Melopsittcusundultus. interleukin 10 r bet XP PREDICTED: Mesitornis unicolor interleukin 10 r bet XP PREDICTED: Mesitornisunicolor. Interleukin 10 r bet XP PREDICTED: Microtusochrogster. Interleukin 10 r bet XP PREDICTED: Monodelphisdomestic. Interleukin 10 r bet AAI45792 Mus musculus. Interleukin 10 r bet XP PREDICTED: Myotis brndtii. interleukin 10 r bet XP PREDICTED: Myotis dvidii. interleukin 10 r bet XP PREDICTED: Nestor notbilis interleukin 10 r bet XP PREDICTED: Nestor notbilis. interleukin 10 r bet XP PREDICTED: Nipponi nippon interleukin 10 r bet XP PREDICTED: Nipponi nippon. Interleukin 10 r bet XP PREDICTED: Nomscusleucogenys. interleukin 10 r bet XP PREDICTED: Ochotonprinceps. interleukin 10 r bet XP PREDICTED: Octodon degus. interleukin 10 r bet XP PREDICTED: Odobenus rosmrusdivergens. Interleukin 10 r bet XP PREDICTED: Opisthocomus hozin Interleukin 10 r bet XP PREDICTED: Opisthocomushozin. interleukin 10 r bet XP PREDICTED: Orcinus orc. interleukin 10 r bet XP PREDICTED: Ornithorhynchusntinus. interleukin 10 r bet XP PREDICTED: Orycteropus ferfer. interleukin 10 r bet XP PREDICTED: Otolemurgrnettii interleukin 10 r bet JAA32934 Pn troglodytes interleukin 10 r bet XP PREDICTED: Pnther tigrisltic. interleukin 10 r bet XP PREDICTED: Pntholops hodgsonii. interleukin 10 r bet XP PREDICTED: Pelodiscus sinensis interleukin 10 r bet XP PREDICTED: Pelodiscus sinensis. interleukin 10 r bet XP PREDICTED: Peromyscusmnicultus birdii. interleukin 10 r bet XP PREDICTED: Phethon lepturus interleukin 10 r bet XP PREDICTED: Phethonlepturus. interleukin 10 r bet XP PREDICTED: Pseudopodoceshumilis. interleukin 10 r bet AEQ38018 Pteropus lecto. interleukin 10 r bet XP PREDICTED: Pygoscelis delie interleukin 10 r bet XP PREDICTED: Simiriboliviensis boliviensis. interleukin 10 r bet XP PREDICTED: Serinus cnri interleukin 10 r bet 110
112 XP PREDICTED: Serinus cnri. interleukin 10 r bet XP PREDICTED: Sorex rneus. interleukin 10 r bet XP PREDICTED: Struthio cmelus ustrlis interleukin 10 r bet XP PREDICTED: Struthio cmelusustrlis. interleukin 10 r bet XP PREDICTED: Sturnus vulgris interleukin 10 r bet BAD06316 Sus scrof. interleukin 10 r bet XP PREDICTED: Teniopygi guttt Interleukin 10 r bet XP PREDICTED: Tinmus gutttus interleukin 10 r bet XP PREDICTED: Tinmus gutttus. Interleukin 10 r bet XP PREDICTED: Tursiopstrunctus. interleukin 10 r bet XP PREDICTED: Tyto lb interleukin 10 r bet XP PREDICTED: Tyto lb. interleukin 10 r bet XP PREDICTED: Vicugn pcos. interleukin 10 r bet NP precursor Xenopus (Silurn)tropiclis. interleukin 10 r bet NP precursor Xenopus levis. interleukin 10 r bet Tble 6 Accession Numbers of IFIT sequences Accession Number Species type ENSAMEG Ailuropod melnoleuc IFIT2 ENSAMEG Ailuropod melnoleuc IFIT5 ENSAMEG predicted Ailuropod melnoleuc IFIT ENSAMEG predicted Ailuropod melnoleuc IFIT KF Ans pltyrhynchos IFIT ENSACAG predicted Anolis crolinensis IFIT ENSACAG predicted Anolis crolinensis IFIT ENSBTAG Bos turus IFIT1 ENSBTAG Bos turus IFIT2 ENSBTAG Bos turus IFIT3 ENSBTAG Bos turus IFIT5 ENSCJAG Cllithrix jcchus IFIT1 ENSCJAG Cllithrix jcchus IFIT2 ENSCJAG Cllithrix jcchus IFIT3 ENSCJAG Cllithrix jcchus IFIT5 ENSCAFG Cnis fmiliris IFIT1 ENSCAFG Cnis fmiliris IFIT2 ENSCAFG Cnis fmiliris IFIT3 ENSCAFG predicted Cnis fmiliris IFIT ENSCAFG predicted Cnis fmiliris IFIT ENSCPOG Cvi porcellus IFIT1B ENSCPOG Cvi porcellus IFIT5 ENSCHOG Choloepus hoffmnni IFIT2 ENSCHOG Choloepus hoffmnni IFIT3 ENSCHOG Choloepus hoffmnni IFIT5 111
113 ENSCHOG predicted Choloepus hoffmnni IFIT ENSCHOG predicted Choloepus hoffmnni IFIT ENSDNOG Dsypus novemcinctus IFIT2 ENSDNOG Dsypus novemcinctus IFIT3 ENSDNOG Dsypus novemcinctus IFIT5 ENSDNOG predicted Dsypus novemcinctus IFIT ENSDNOG predicted Dsypus novemcinctus IFIT ENSDORG predicted Dipodomys ordii IFIT ENSDORG predicted Dipodomys ordii IFIT ENSDORG predicted Dipodomys ordii IFIT ENSETEG Echinops telfiri IFIT5 ENSECAG Equus cbllus IFIT1 ENSECAG Equus cbllus IFIT4 ENSECAG Equus cbllus IFIT5 ENSEEUG Erinceus europeus IFIT3 ENSEEUG Erinceus europeus IFIT5 ENSFCAG Felis ctus IFIT2 ENSFCAG Felis ctus IFIT3 ENSFCAG predicted Felis ctus IFIT ENSFCAG predicted Felis ctus IFIT ENSFCAG predicted Felis ctus IFIT ENSFCAG predicted Felis ctus IFIT ENSFALG predicted Ficedul lbicollis IFIT ENSGGOG Gorill gorill IFIT1 ENSGGOG Gorill gorill IFIT1B ENSGGOG Gorill gorill IFIT2 ENSGGOG Gorill gorill IFIT3 ENSGGOG Gorill gorill IFIT5 ENSGGOG predicted Gorill gorill IFIT ENSG Homo spiens IFIT1 ENSG Homo spiens IFIT1B ENSG Homo spiens IFIT2 ENSG Homo spiens IFIT3 ENSG Homo spiens IFIT5 ENSSTOG Ictidomys tridecemlinetus IFIT1 ENSSTOG Ictidomys tridecemlinetus IFIT2 ENSSTOG Ictidomys tridecemlinetus IFIT3 ENSSTOG Ictidomys tridecemlinetus IFIT5 ENSSTOG predicted Ictidomys tridecemlinetus IFIT ENSSTOG predicted Ictidomys tridecemlinetus IFIT ENSSTOG predicted Ictidomys tridecemlinetus IFIT ENSSTOG predicted Ictidomys tridecemlinetus IFIT ENSSTOG predicted Ictidomys tridecemlinetus IFIT ENSLACG predicted Ltimeri chlumne IFIT 112
114 ENSLACG predicted Ltimeri chlumne IFIT ENSLAFG Loxodont fricn IFIT3 ENSLAFG Loxodont fricn IFIT5 ENSLAFG predicted Loxodont fricn IFIT ENSLAFG predicted Loxodont fricn IFIT ENSMEUG Mcropus eugenii IFIT5 ENSMGAG predicted Melegris gllopvo IFIT ENSMICG Microcebus murinus IFIT3 ENSMICG Microcebus murinus IFIT5 ENSMICG predicted Microcebus murinus IFIT ENSMICG predicted Microcebus murinus IFIT ENSMODG predicted Monodelphis domestic IFIT ENSMODG predicted Monodelphis domestic IFIT ENSMODG predicted Monodelphis domestic IFIT ENSMODG predicted Monodelphis domestic IFIT ENSMUSG Mus musculus IFIT1 ENSMUSG Mus musculus IFIT2 ENSMUSG Mus musculus IFIT3 ENSMPUG Mustel putorius furo IFIT2 ENSMPUG Mustel putorius furo IFIT3 ENSMPUG Mustel putorius furo IFIT5 ENSMPUG predicted Mustel putorius furo IFIT ENSMPUG predicted Mustel putorius furo IFIT ENSMLUG Myotis lucifugus IFIT2 ENSMLUG Myotis lucifugus IFIT3 ENSMLUG Myotis lucifugus IFIT5 ENSMLUG predicted Myotis lucifugus IFIT ENSMLUG predicted Myotis lucifugus IFIT ENSNLEG Nomscus leucogenys IFIT1 ENSNLEG Nomscus leucogenys IFIT1B ENSNLEG Nomscus leucogenys IFIT2 ENSNLEG Nomscus leucogenys IFIT3 ENSNLEG Nomscus leucogenys IFIT5 ENSOPRG Ochoton princeps IFIT2 ENSOPRG Ochoton princeps IFIT3 ENSOPRG Ochoton princeps IFIT5 ENSOPRG predicted Ochoton princeps IFIT ENSOPRG predicted Ochoton princeps IFIT ENSOPRG predicted Ochoton princeps IFIT ENSOANG predicted Ornithorhynchus ntinus IFIT ENSOANG predicted Ornithorhynchus ntinus IFIT ENSOANG predicted Ornithorhynchus ntinus IFIT ENSOCUG Oryctolgus cuniculus IFIT2 ENSOCUG Oryctolgus cuniculus IFIT3 113
115 ENSOCUG Oryctolgus cuniculus IFIT5 ENSOCUG predicted Oryctolgus cuniculus IFIT ENSOCUG predicted Oryctolgus cuniculus IFIT ENSOCUG predicted Oryctolgus cuniculus IFIT ENSORLG predicted Oryzis ltipes IFIT ENSORLG predicted Oryzis ltipes IFIT ENSORLG predicted Oryzis ltipes IFIT ENSORLG predicted Oryzis ltipes IFIT ENSORLG predicted Oryzis ltipes IFIT ENSORLG predicted Oryzis ltipes IFIT ENSORLG predicted Oryzis ltipes IFIT ENSORLG predicted Oryzis ltipes IFIT ENSOGAG Otolemur grnettii IFIT1 ENSOGAG Otolemur grnettii IFIT2 ENSOGAG Otolemur grnettii IFIT3 ENSOGAG Otolemur grnettii IFIT5 ENSOARG Ovis ries IFIT1 ENSOARG Ovis ries IFIT2 ENSOARG Ovis ries IFIT3 ENSOARG Ovis ries IFIT5 ENSPTRG Pn troglodytes IFIT1B ENSPTRG Pn troglodytes IFIT2 ENSPTRG Pn troglodytes IFIT3 ENSPTRG Pn troglodytes IFIT5 ENSPANG Ppio nubis IFIT1 ENSPANG Ppio nubis IFIT1B ENSPANG Ppio nubis IFIT2 ENSPANG Ppio nubis IFIT3 ENSPANG Ppio nubis IFIT5 ENSPSIG predicted Pelodiscus sinensis IFIT ENSPPYG Pongo belii IFIT1 ENSPPYG Pongo belii IFIT1B ENSPPYG Pongo belii IFIT2 ENSPPYG Pongo belii IFIT3 ENSPPYG Pongo belii IFIT5 ENSPCAG Procvi cpensis IFIT5 ENSPVAG Pteropus vmpyrus IFIT5 ENSRNOG Rttus norvegicus IFIT1 ENSRNOG Rttus norvegicus IFIT1lb ENSRNOG Rttus norvegicus IFIT2 ENSRNOG Rttus norvegicus IFIT3 ENSSHAG predicted Srcophilus hrrisii IFIT ENSSHAG predicted Srcophilus hrrisii IFIT ENSSHAG predicted Srcophilus hrrisii IFIT 114
116 ENSSARG Sorex rneus IFIT5 ENSSSCG Sus scrof IFIT1 ENSSSCG Sus scrof IFIT2 ENSSSCG Sus scrof IFIT3 ENSSSCG Sus scrof IFIT5 ENSTGUG predicted Teniopygi guttt IFIT ENSTSYG Trsius syricht IFIT1B ENSTSYG Trsius syricht IFIT5 ENSTSYG predicted Trsius syricht IFIT ENSTSYG predicted Trsius syricht IFIT ENSTNIG Tetrodon nigroviridis IFIT2 ENSTBEG Tupi belngeri IFIT3 ENSTBEG predicted Tupi belngeri IFIT ENSTBEG predicted Tupi belngeri IFIT ENSTTRG Tursiops trunctus IFIT1 ENSTTRG Tursiops trunctus IFIT5 ENSVPAG Vicugn pcos IFIT3 ENSVPAG Vicugn pcos IFIT1 ENSVPAG Vicugn pcos IFIT2 ENSVPAG Vicugn pcos IFIT5 ENSXETG Xenopus tropiclis IFIT1 ENSXETG Xenopus tropiclis IFIT5 ENSXETG predicted Xenopus tropiclis IFIT ENSXETG predicted Xenopus tropiclis IFIT ENSXETG predicted Xenopus tropiclis IFIT 115
117 Tble 7 qrt-pcr Primer nd Probe sequences Trget Gene Forwrd Primer Seq. Reverse Primer Seq. Reporte Sequence ch GAPDH CCCCAATGTCTCTGTTGTTGAC CAGCCTTCACTACCCTCTTGAT CTTGGCTGGTTTCTCC ch IFNα GGACATGGCTCCCACACTAC TCCAGGATGGTGTCGTTGAAG CAGCGCGTCTTGCTC ch IFNβ ACAACTTCCTACAGCACAACAACTA GCCTGGAGGCGGACATG TCCCAGGTACAAGCACTG ch IFNλ CATCGGAAGTGGGACATAGCT CCTCCACCAGGGTGATTCG TCAGGTACCGACAGCTC ch IFNλR1 GGATCTCCACCAGATGTGTTGTAC GGAACCTTTATCCATTTGTCCATACG CTGTGAGGTATGAAAGCAA ch IL-10R2 CGCAAAGGCAACCTAAGTTATACTG TTGGTTGTCACATTGTTAAAATTCTGCTT CCAGGCCAAAAGCATT ch ISG IFIT5 CAGAATTTAATGCCGGCTATGC TGCAAGTAAAGCCAAAAGATAAGTGT TCTGAAGCGTGCACTGAAACTGAATCCAA ch ISG MX GTCCAAGAGGCTGAATAACAGAGAA GGTCGGATCTTTCTGTCATATTGGT CTGCTGCCTCATCCTT ch ISG PKR GCAGAAGTAAGAGTGAGGCAAATGA GCCACCTTTACCAATAGGCTCTAT CTGTGGATGAAAGGTTTC ch ISG Viperin CTGATCAGGGAACGGTGGTT ACGTTGACTTCCTCATTAAAACTATCACA CAAGAAGTATGGTGAATATTT ch ISG ZAP AAATTGAAAAAGCCTATTGTGACCCAAA GGAGAGGGTCATTGTCTGGAAATT CTGCTGCACTGCTGTTT 1000 IFN Time post chicken IFN stimultion (h) Figure 11-1 IFN lph mrna expression post IFN lph stimultion Expression of IFNα nd IFNλ mrna in purified splenocytes from SPF chickens stimulted with 500 ng/ml IFNα over the indicted time course. The brs represent the men fold chnge of 3 chicken spleens with the stndrd error of the men (SEM) compred to the untreted smple, normlized ginst the housekeeping gene GAPDH. 116
118 Figure 11-2 Alignment of th predicted nd sequenced chicken IFIT5 sequence Gene Alignment of the sequenced (KT ) nd the predicted sequence of IFIT5. Numbers on t the end of ech sequence indictes gene length. Red indictes conserved bse pirs nd blck indicted SNP. 117
119 Figure 11-3 in silico trnslted protein sequence lignment of the predicted nd sequenced chicken IFIT5 sequence Protein lignment of the sequenced nd in silico trnslted IFIT5(KT ) nd the predicted nd trnslted sequence. Numbers on t the end of ech sequence indictes protein length. Red indictes conserved mino cids nd blck indicted SNP. 118
Analysis of Regulatory of Interrelated Activity of Hepatocyte and Hepatitis B Viruses
 Interntionl Journl of Biomedicl Mterils Reserch 8 6(): -7 http://www.sciencepublishinggroup.com/j/ijbmr doi:.648/j.ijbmr.86. ISSN: 33-756 (Print) ISSN: 33-7579 (Online) Anlysis of Regultory of Interrelted
Interntionl Journl of Biomedicl Mterils Reserch 8 6(): -7 http://www.sciencepublishinggroup.com/j/ijbmr doi:.648/j.ijbmr.86. ISSN: 33-756 (Print) ISSN: 33-7579 (Online) Anlysis of Regultory of Interrelted
Supplementary Figure 1
 Roles of endoplsmic reticulum stress-medited poptosis in -polrized mcrophges during mycocteril infections Supplementry informtion Yun-Ji Lim, Min-Hee Yi, Ji-Ae Choi, Jung-hwn Lee, Ji-Ye Hn, Sung-Hee Jo,
Roles of endoplsmic reticulum stress-medited poptosis in -polrized mcrophges during mycocteril infections Supplementry informtion Yun-Ji Lim, Min-Hee Yi, Ji-Ae Choi, Jung-hwn Lee, Ji-Ye Hn, Sung-Hee Jo,
EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE
 Swine Dy 22 Contents EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE B. J. Johnson, J. P. Kyser, J. D. Dunn, A. T. Wyln, S. S. Dritz 1, J.
Swine Dy 22 Contents EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE B. J. Johnson, J. P. Kyser, J. D. Dunn, A. T. Wyln, S. S. Dritz 1, J.
TNF-α (pg/ml) IL-6 (ng/ml)
 Xio, et l., Supplementry Figure 1 IL-6 (ng/ml) TNF-α (pg/ml) 16 12 8 4 1,4 1,2 1, 8 6 4 2 med Cl / Pm3CSK4 zymosn curdln Poly (I:C) LPS flgelin MALP-2 imiquimod R848 CpG TNF-α (pg/ml) IL-6 (ng/ml) 2 1.6
Xio, et l., Supplementry Figure 1 IL-6 (ng/ml) TNF-α (pg/ml) 16 12 8 4 1,4 1,2 1, 8 6 4 2 med Cl / Pm3CSK4 zymosn curdln Poly (I:C) LPS flgelin MALP-2 imiquimod R848 CpG TNF-α (pg/ml) IL-6 (ng/ml) 2 1.6
Inactivation of Myxoviruses by Calcium Elenolate
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1975, p. 194-199 Copyright i 1975 Americn Society for Microbiology Vol. 8, No. 2 Printed in U.S.A Inctivtion of Myxoviruses by Clcium Elenolte HAROLD E. RENIS
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 1975, p. 194-199 Copyright i 1975 Americn Society for Microbiology Vol. 8, No. 2 Printed in U.S.A Inctivtion of Myxoviruses by Clcium Elenolte HAROLD E. RENIS
Overview: The Cellular Internet. General Biology. 7. Cell Communication & Signalling
 Course o: BG00 Credits:.00 Generl Biology Overview: The Cellulr Internet Cell-to-cell communiction is bsolutely essentil for multicellulr orgnisms Biologists hve discovered some universl mechnisms of cellulr
Course o: BG00 Credits:.00 Generl Biology Overview: The Cellulr Internet Cell-to-cell communiction is bsolutely essentil for multicellulr orgnisms Biologists hve discovered some universl mechnisms of cellulr
Northern blot analysis
 Northern blot nlysis RNA SCD RNA SCD FAS C c-9 t-1 C c-9 t-1 PRE PI PDMI PRE PI PDMI PRE PDMI PIM An W c-9, t-11 t-1, c-12 C 5 2 4 1 um C 5 2 4 1 um Angus dipocytes expressed SCD higher thn Wgyu dipocyte
Northern blot nlysis RNA SCD RNA SCD FAS C c-9 t-1 C c-9 t-1 PRE PI PDMI PRE PI PDMI PRE PDMI PIM An W c-9, t-11 t-1, c-12 C 5 2 4 1 um C 5 2 4 1 um Angus dipocytes expressed SCD higher thn Wgyu dipocyte
Replication of Some Nuclear-replicating Eukaryotic DNA Viruses with Large Genomes
 WBV18 6/27/03 11:25 PM Pge 311 Repliction of Some Nucler-replicting Eukryotic DNA Viruses with Lrge Genomes 18 CHAPTER HERPESVIRUS REPLICATION AND LATENCY The herpesviruses s group The repliction of HSV
WBV18 6/27/03 11:25 PM Pge 311 Repliction of Some Nucler-replicting Eukryotic DNA Viruses with Lrge Genomes 18 CHAPTER HERPESVIRUS REPLICATION AND LATENCY The herpesviruses s group The repliction of HSV
METHOD 4010 SCREENING FOR PENTACHLOROPHENOL BY IMMUNOASSAY
 METHOD 4010 SCREENING FOR PENTACHLOROPHENOL BY IMMUNOASSAY 1.0 SCOPE AND APPLICATION 1.1 Method 4010 is procedure for screening solids such s soils, sludges, nd queous medi such s wste wter nd lechtes
METHOD 4010 SCREENING FOR PENTACHLOROPHENOL BY IMMUNOASSAY 1.0 SCOPE AND APPLICATION 1.1 Method 4010 is procedure for screening solids such s soils, sludges, nd queous medi such s wste wter nd lechtes
Ulk λ PPase. 32 P-Ulk1 32 P-GST-TSC2. Ulk1 GST (TSC2) : Ha-Ulk1 : AMPK. WB: Ha (Ulk1) : Glu. h CON - Glu - A.A WB: LC3 AMPK-WT AMPK-DKO
 DOI: 10.1038/ncb2152 C.C + - + - : Glu b Ulk1 - - + λ PPse c AMPK + - + + : ATP P-GST-TSC2 WB: Flg (Ulk1) WB Ulk1 WB: H (Ulk1) GST (TSC2) C.C d e WT K46R - + - + : H-Ulk1 : AMPK - + - + + + AMPK H-Ulk1
DOI: 10.1038/ncb2152 C.C + - + - : Glu b Ulk1 - - + λ PPse c AMPK + - + + : ATP P-GST-TSC2 WB: Flg (Ulk1) WB Ulk1 WB: H (Ulk1) GST (TSC2) C.C d e WT K46R - + - + : H-Ulk1 : AMPK - + - + + + AMPK H-Ulk1
Invasive Pneumococcal Disease Quarterly Report. July September 2017
 Invsive Pneumococcl Disese Qurterly Report July September 2017 Prepred s prt of Ministry of Helth contrct for scientific services by Rebekh Roos Helen Heffernn October 2017 Acknowledgements This report
Invsive Pneumococcl Disese Qurterly Report July September 2017 Prepred s prt of Ministry of Helth contrct for scientific services by Rebekh Roos Helen Heffernn October 2017 Acknowledgements This report
Supplementary figure 1
 Supplementry figure 1 Dy 8 post LCMV infection Vsculr Assoc. Prenchym Dy 3 post LCMV infection 1 5 6.7.29 1 4 1 3 1 2 88.9 4.16 1 2 1 3 1 4 1 5 1 5 1.59 5.97 1 4 1 3 1 2 21.4 71 1 2 1 3 1 4 1 5 1 5.59.22
Supplementry figure 1 Dy 8 post LCMV infection Vsculr Assoc. Prenchym Dy 3 post LCMV infection 1 5 6.7.29 1 4 1 3 1 2 88.9 4.16 1 2 1 3 1 4 1 5 1 5 1.59 5.97 1 4 1 3 1 2 21.4 71 1 2 1 3 1 4 1 5 1 5.59.22
ARTICLE. E. Pavlova 1, N. Atanassova 1, C. McKinnell 2, R.M. Sharpe 2 1 Institute of Experimental Morphology, Pathology and Anthropology with Museum,
 DOI:.554/5YRTIMB..3 OPPOSITE MODELS OF EXPRESSION OF ANDROGEN RECEPTOR (AR) AND RETINOIC ACID RECEPTOR-α (RAR-α) IN THE ONSET OF MALE GERM CELL DEVELOPMENT IN HORMONALLY MANIPULATED RATS E. Pvlov, N. Atnssov,
DOI:.554/5YRTIMB..3 OPPOSITE MODELS OF EXPRESSION OF ANDROGEN RECEPTOR (AR) AND RETINOIC ACID RECEPTOR-α (RAR-α) IN THE ONSET OF MALE GERM CELL DEVELOPMENT IN HORMONALLY MANIPULATED RATS E. Pvlov, N. Atnssov,
Intrinsic cellular defenses against virus infection
 Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
PROVEN ANTICOCCIDIAL IN NEW FORMULATION
 PROVEN ANTICOCCIDIAL IN NEW FORMULATION Coxidin 100 microgrnulte A coccidiosttic dditive for roilers, chickens rered for lying nd turkeys Contins 100 g of monensin sodium per kg Aville s homogenous grnules
PROVEN ANTICOCCIDIAL IN NEW FORMULATION Coxidin 100 microgrnulte A coccidiosttic dditive for roilers, chickens rered for lying nd turkeys Contins 100 g of monensin sodium per kg Aville s homogenous grnules
TLR7 induces anergy in human CD4 + T cells
 TLR7 induces nergy in humn CD T cells Mrgrit Dominguez-Villr 1, Anne-Sophie Gutron 1, Mrine de Mrcken 1, Mrl J Keller & Dvid A Hfler 1 The recognition of microil ptterns y Toll-like receptors (TLRs) is
TLR7 induces nergy in humn CD T cells Mrgrit Dominguez-Villr 1, Anne-Sophie Gutron 1, Mrine de Mrcken 1, Mrl J Keller & Dvid A Hfler 1 The recognition of microil ptterns y Toll-like receptors (TLRs) is
ENERGY CONTENT OF BARLEY
 ENERGY CONTENT OF BARLEY VARIATION IN THE DIETARY ENERGY CONTENT OF BARLEY Shwn Firbirn, John Ptience, Hnk Clssen nd Ruurd Zijlstr SUMMARY Formultion of commercil pig diets requires n incresing degree
ENERGY CONTENT OF BARLEY VARIATION IN THE DIETARY ENERGY CONTENT OF BARLEY Shwn Firbirn, John Ptience, Hnk Clssen nd Ruurd Zijlstr SUMMARY Formultion of commercil pig diets requires n incresing degree
XII. HIV/AIDS. Knowledge about HIV Transmission and Misconceptions about HIV
 XII. HIV/AIDS Knowledge bout HIV Trnsmission nd Misconceptions bout HIV One of the most importnt prerequisites for reducing the rte of HIV infection is ccurte knowledge of how HIV is trnsmitted nd strtegies
XII. HIV/AIDS Knowledge bout HIV Trnsmission nd Misconceptions bout HIV One of the most importnt prerequisites for reducing the rte of HIV infection is ccurte knowledge of how HIV is trnsmitted nd strtegies
EFFECTS OF INGREDIENT AND WHOLE DIET IRRADIATION ON NURSERY PIG PERFORMANCE
 Swine Dy 21 EFFECTS OF INGREDIENT AND WHOLE DIET IRRADIATION ON NURSERY PIG PERFORMANCE J. M. DeRouchey, M. D. Tokch, J. L. Nelssen, R. D. Goodbnd, S. S. Dritz 1, J. C. Woodworth, M. J. Webster, B. W.
Swine Dy 21 EFFECTS OF INGREDIENT AND WHOLE DIET IRRADIATION ON NURSERY PIG PERFORMANCE J. M. DeRouchey, M. D. Tokch, J. L. Nelssen, R. D. Goodbnd, S. S. Dritz 1, J. C. Woodworth, M. J. Webster, B. W.
Potential of plant-derived antimicrobials for controlling zoonotic and food-borne diseases
 Potentil of plnt-derived ntimicrobils for controlling zoonotic nd food-borne diseses Kumr Venkitnrynn, DVM, MVSc, MS, Ph.D. Professor of Microbiology Grdute Progrms Chir Deprtment of Animl Science University
Potentil of plnt-derived ntimicrobils for controlling zoonotic nd food-borne diseses Kumr Venkitnrynn, DVM, MVSc, MS, Ph.D. Professor of Microbiology Grdute Progrms Chir Deprtment of Animl Science University
Clinical Study Report Synopsis Drug Substance Naloxegol Study Code D3820C00018 Edition Number 1 Date 01 February 2013 EudraCT Number
 EudrCT Number 2012-001531-31 A Phse I, Rndomised, Open-lbel, 3-wy Cross-over Study in Helthy Volunteers to Demonstrte the Bioequivlence of the Nloxegol 25 mg Commercil nd Phse III Formultions nd to Assess
EudrCT Number 2012-001531-31 A Phse I, Rndomised, Open-lbel, 3-wy Cross-over Study in Helthy Volunteers to Demonstrte the Bioequivlence of the Nloxegol 25 mg Commercil nd Phse III Formultions nd to Assess
Martinez-Rubio et al. BMC Genomics 2014, 15:462
 Mrtinez-Rubio et l. BMC Genomics 2014, 15:462 RESEARCH ARTICLE Open Access Effects of functionl feeds on the lipid composition, trnscriptomic responses nd pthology in hert of Atlntic slmon (Slmo slr L.)
Mrtinez-Rubio et l. BMC Genomics 2014, 15:462 RESEARCH ARTICLE Open Access Effects of functionl feeds on the lipid composition, trnscriptomic responses nd pthology in hert of Atlntic slmon (Slmo slr L.)
Comparison of three simple methods for the
 J. clin. Pth. (1967), 2, 5 Comprison of three simple methods for the ssessment of 'free' thyroid hormone T. M. D. GIMLETTE1 From the Rdio-Isotope Lbortory, St. Thoms's Hospitl, London SYNOPSIS A dilysis
J. clin. Pth. (1967), 2, 5 Comprison of three simple methods for the ssessment of 'free' thyroid hormone T. M. D. GIMLETTE1 From the Rdio-Isotope Lbortory, St. Thoms's Hospitl, London SYNOPSIS A dilysis
SUPPLEMENTARY INFORMATION
 doi:0.08/nture0987 SUPPLEMENTARY FIGURE Structure of rbbit Xist gene. Exons re shown in boxes with romn numbers, introns in thin lines. Arrows indicte the locliztion of primers used for mplifiction. WWW.NATURE.COM/NATURE
doi:0.08/nture0987 SUPPLEMENTARY FIGURE Structure of rbbit Xist gene. Exons re shown in boxes with romn numbers, introns in thin lines. Arrows indicte the locliztion of primers used for mplifiction. WWW.NATURE.COM/NATURE
WSU Tree Fruit Research and Extension Center, Wenatchee (509) ext. 265;
 FINAL REPORT WTFRC Project # AH-1-5 WSU Project # 13C-355-3 Project title: PI: Orgniztion: Coopertors: of Sunburn in Apples with RAYNOX Lrry Schrder, Horticulturist WSU Tree Fruit Reserch nd Extension
FINAL REPORT WTFRC Project # AH-1-5 WSU Project # 13C-355-3 Project title: PI: Orgniztion: Coopertors: of Sunburn in Apples with RAYNOX Lrry Schrder, Horticulturist WSU Tree Fruit Reserch nd Extension
Acid-Poly-L-Lysine Complex on Encephalomyocarditis Virus
 ANTimICROsIAL AozWu AND CHEMoTHERAPY, Oct. 1976, p. 668-676 Copyright C 1976 Americn Society for Microbiology Vol. 10, No. 4 Printed in U.S.A. Effect of Tretment with Exogenous Interferon, Polyinosinic
ANTimICROsIAL AozWu AND CHEMoTHERAPY, Oct. 1976, p. 668-676 Copyright C 1976 Americn Society for Microbiology Vol. 10, No. 4 Printed in U.S.A. Effect of Tretment with Exogenous Interferon, Polyinosinic
Original article Morpholino oligomer-mediated protection of porcine pulmonary alveolar macrophages from arterivirusinduced
 Antivirl Therpy 9 : 899 99 (doi:.85/imp9) Originl rticle Morpholino oligomermedited protection of porcine pulmonry lveolr mcrophges from rterivirusinduced cell deth Deendyl Ptel, Dvid A Stein nd YnJin
Antivirl Therpy 9 : 899 99 (doi:.85/imp9) Originl rticle Morpholino oligomermedited protection of porcine pulmonry lveolr mcrophges from rterivirusinduced cell deth Deendyl Ptel, Dvid A Stein nd YnJin
Lethal H5N1 influenza viruses escape host anti-viral cytokine responses
 Lethl HN influenz viruses escpe host nti-virl cytokine responses SANG HEUI SEO, ERICH HOFFMANN & ROBERT G. WEBSTER Nture Publishing Group http://www.nture.com/nturemedicine Division of Virology, Deprtment
Lethl HN influenz viruses escpe host nti-virl cytokine responses SANG HEUI SEO, ERICH HOFFMANN & ROBERT G. WEBSTER Nture Publishing Group http://www.nture.com/nturemedicine Division of Virology, Deprtment
Responses of Isolator-Derived Japanese Quail and Quail Cell Cultures to Selected Animal Viruses
 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 1975, p. 419-424 Copyright C) 1975 Americn Society for Microbiology Vol. 2, No. 5 Printed in U.SA. Responses of Isoltor-Derived Jpnese Quil nd Quil Cell Cultures
JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 1975, p. 419-424 Copyright C) 1975 Americn Society for Microbiology Vol. 2, No. 5 Printed in U.SA. Responses of Isoltor-Derived Jpnese Quil nd Quil Cell Cultures
Effect of fungicide timing and wheat varietal resistance on Mycosphaerella graminicola and its sterol 14 α-demethylation-inhibitorresistant
 Effect of fungicide timing nd whet vrietl resistnce on Mycospherell grminicol nd its sterol 14 α-demethyltion-inhiitorresistnt genotypes Didierlurent L., Roisin-Fichter C., Snssené J., Selim S. Pltform
Effect of fungicide timing nd whet vrietl resistnce on Mycospherell grminicol nd its sterol 14 α-demethyltion-inhiitorresistnt genotypes Didierlurent L., Roisin-Fichter C., Snssené J., Selim S. Pltform
SUPPLEMENTARY INFORMATION
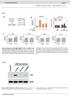 Prentl doi:.8/nture57 Figure S HPMECs LM Cells Cell lines VEGF (ng/ml) Prentl 7. +/-. LM 7. +/-.99 LM 7. +/-.99 Fold COX induction 5 VEGF: - + + + Bevcizum: - - 5 (µg/ml) Reltive MMP LM mock COX MMP LM+
Prentl doi:.8/nture57 Figure S HPMECs LM Cells Cell lines VEGF (ng/ml) Prentl 7. +/-. LM 7. +/-.99 LM 7. +/-.99 Fold COX induction 5 VEGF: - + + + Bevcizum: - - 5 (µg/ml) Reltive MMP LM mock COX MMP LM+
SUPPLEMENTARY INFORMATION
 doi:1.138/nture1794 BR EPFs BRI1? ERECTA TMM BSKs YDA PP2A BSU1 BIN2 pbzr1/2 BZR1/2 MKK4/5/7/9 MPK3/6 SPCH Cell growth Stomtl production Supplementry Figure 1. The model of BR nd stomtl signling pthwys.
doi:1.138/nture1794 BR EPFs BRI1? ERECTA TMM BSKs YDA PP2A BSU1 BIN2 pbzr1/2 BZR1/2 MKK4/5/7/9 MPK3/6 SPCH Cell growth Stomtl production Supplementry Figure 1. The model of BR nd stomtl signling pthwys.
IGF-I and IGFBP-3 augment transforming growth factor-b actions in human renal carcinoma cells
 originl rticle http://www.kidney-interntionl.org & Interntionl Society of Nephrology IGF-I nd IGFBP-3 ugment trnsforming growth fctor-b ctions in humn renl crcinom cells AH Rosendhl 1, nd G Forsberg 1
originl rticle http://www.kidney-interntionl.org & Interntionl Society of Nephrology IGF-I nd IGFBP-3 ugment trnsforming growth fctor-b ctions in humn renl crcinom cells AH Rosendhl 1, nd G Forsberg 1
SUPPLEMENTARY INFORMATION
 . Norml Physiologicl Conditions. SIRT1 Loss-of-Function S1. Model for the role of SIRT1 in the regultion of memory nd plsticity. () Our findings suggest tht SIRT1 normlly functions in coopertion with YY1,
. Norml Physiologicl Conditions. SIRT1 Loss-of-Function S1. Model for the role of SIRT1 in the regultion of memory nd plsticity. () Our findings suggest tht SIRT1 normlly functions in coopertion with YY1,
PNEUMOVAX 23 is recommended by the CDC for all your appropriate adult patients at increased risk for pneumococcal disease 1,2 :
 PNEUMOVAX 23 is recommended y the CDC for ll your pproprite dult ptients t incresed risk for pneumococcl disese 1,2 : Adults ged
PNEUMOVAX 23 is recommended y the CDC for ll your pproprite dult ptients t incresed risk for pneumococcl disese 1,2 : Adults ged
SUPPLEMENTARY INFORMATION
 SUPPLEMEARY IFORMAIO doi:./nture correction to Supplementry Informtion Adenom-linked rrier defects nd microil products drive IL-/IL-7-medited tumour growth Sergei I. Grivennikov, Kepeng Wng, Dniel Mucid,
SUPPLEMEARY IFORMAIO doi:./nture correction to Supplementry Informtion Adenom-linked rrier defects nd microil products drive IL-/IL-7-medited tumour growth Sergei I. Grivennikov, Kepeng Wng, Dniel Mucid,
THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS
 THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS John F. Ptience nd Doug Gillis SUMMARY
THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS THE EVALUATION OF DEHULLED CANOLA MEAL IN THE DIETS OF GROWING AND FINISHING PIGS John F. Ptience nd Doug Gillis SUMMARY
Thebiotutor.com A2 Biology OCR Unit F215: Control, genomes and environment Module 1.2 Meiosis and variation Answers
 Theiotutor.com A2 Biology OCR Unit F215: Control, genomes nd environment Module 1.2 Meiosis nd vrition Answers Andy Todd 1 1. () (i) gene length of DNA; codes for (specific), polypeptide / protein / RNA;
Theiotutor.com A2 Biology OCR Unit F215: Control, genomes nd environment Module 1.2 Meiosis nd vrition Answers Andy Todd 1 1. () (i) gene length of DNA; codes for (specific), polypeptide / protein / RNA;
Reducing the Risk. Logic Model
 Reducing the Risk Logic Model ETR (Eduction, Trining nd Reserch) is nonprofit orgniztion committed to providing science-bsed innovtive solutions in helth nd eduction designed to chieve trnsformtive chnge
Reducing the Risk Logic Model ETR (Eduction, Trining nd Reserch) is nonprofit orgniztion committed to providing science-bsed innovtive solutions in helth nd eduction designed to chieve trnsformtive chnge
Feeding state and age dependent changes in melaninconcentrating hormone expression in the hypothalamus of broiler chickens
 Supplementry Mterils Epub: No 2017_23 Vol. 65, 2018 https://doi.org/10.183/bp.2017_23 Regulr pper Feeding stte nd ge dependent chnges in melninconcentrting hormone expression in the hypothlmus of broiler
Supplementry Mterils Epub: No 2017_23 Vol. 65, 2018 https://doi.org/10.183/bp.2017_23 Regulr pper Feeding stte nd ge dependent chnges in melninconcentrting hormone expression in the hypothlmus of broiler
% Inhibition of MERS pseudovirus infection. 0 h 0.5 h 1 h 2 h 4 h 6 h Time after virus addition
 % Inhiition of MERS pseudovirus infection 1 8 h.5 h 1 h 2 h 4 h 6 h Time fter virus ddition Supplementry Figure S1. Inhiition of on MERS pseudovirus infection t the different intervls postinfection. A
% Inhiition of MERS pseudovirus infection 1 8 h.5 h 1 h 2 h 4 h 6 h Time fter virus ddition Supplementry Figure S1. Inhiition of on MERS pseudovirus infection t the different intervls postinfection. A
Using Paclobutrazol to Suppress Inflorescence Height of Potted Phalaenopsis Orchids
 Using Pcloutrzol to Suppress Inflorescence Height of Potted Phlenopsis Orchids A REPORT SUBMITTED TO FINE AMERICAS Linsey Newton nd Erik Runkle Deprtment of Horticulture Spring 28 Using Pcloutrzol to Suppress
Using Pcloutrzol to Suppress Inflorescence Height of Potted Phlenopsis Orchids A REPORT SUBMITTED TO FINE AMERICAS Linsey Newton nd Erik Runkle Deprtment of Horticulture Spring 28 Using Pcloutrzol to Suppress
Assessment of Depression in Multiple Sclerosis. Validity of Including Somatic Items on the Beck Depression Inventory II
 Assessment of Depression in Multiple Sclerosis Vlidity of Including Somtic Items on the Beck Depression Inventory II Peggy Crwford, PhD; Noh J. Webster, MA Signs nd symptoms of multiple sclerosis (MS)
Assessment of Depression in Multiple Sclerosis Vlidity of Including Somtic Items on the Beck Depression Inventory II Peggy Crwford, PhD; Noh J. Webster, MA Signs nd symptoms of multiple sclerosis (MS)
* * * * * liver kidney ileum. Supplementary Fig.S1
 Supplementry Fig.S1 liver kidney ileum Fig.S1. Orlly delivered Fexrmine is intestinlly-restricted Mice received vehicle or Fexrmine (100mg/kg) vi per os (PO) or intrperitonel (IP) injection for 5 dys (n=3/group).
Supplementry Fig.S1 liver kidney ileum Fig.S1. Orlly delivered Fexrmine is intestinlly-restricted Mice received vehicle or Fexrmine (100mg/kg) vi per os (PO) or intrperitonel (IP) injection for 5 dys (n=3/group).
Rapid communications Increased detection of Mycoplasma pneumoniae infection in children in England and Wales, October 2011 to January 2012
 Rpid communictions Incresed detection of Mycoplsm pneumonie infection in children in Englnd nd Wles, October 2011 to Jnury 2012 V J Chlker (vicki.chlker@hp.org.uk) 1, T Stocki 1, D Litt 1, A Berminghm
Rpid communictions Incresed detection of Mycoplsm pneumonie infection in children in Englnd nd Wles, October 2011 to Jnury 2012 V J Chlker (vicki.chlker@hp.org.uk) 1, T Stocki 1, D Litt 1, A Berminghm
SUPPLEMENTARY INFORMATION
 DOI: 1.138/nc286 Figure S1 e f Medium DMSO AktVIII PP242 Rp S6K1-I Gr1 + + + + + + Strvtion + + + + + IB: Akt-pT38 IB: Akt K-pT389 K IB: Rptor Gr1 shs6k1-a shs6k1-b shs6k1-c shrictor shrptor Gr1 c IB:
DOI: 1.138/nc286 Figure S1 e f Medium DMSO AktVIII PP242 Rp S6K1-I Gr1 + + + + + + Strvtion + + + + + IB: Akt-pT38 IB: Akt K-pT389 K IB: Rptor Gr1 shs6k1-a shs6k1-b shs6k1-c shrictor shrptor Gr1 c IB:
Goal: Evaluate plant health effects while suppressing dollar spot and brown patch
 Newer Fungicide Products Alone nd In Rottion on Chicgo Golf Green Reserchers: Chicgo District Golf Assoc. Derek Settle, Tim Sibicky, nd Nick DeVries Gol: Evlute plnt helth effects while suppressing dollr
Newer Fungicide Products Alone nd In Rottion on Chicgo Golf Green Reserchers: Chicgo District Golf Assoc. Derek Settle, Tim Sibicky, nd Nick DeVries Gol: Evlute plnt helth effects while suppressing dollr
Comparison of pro- and anti-inflammatory responses in paired human primary airway epithelial cells and alveolar macrophages
 Murk et l. Respirtory Reserch (2018) 19:126 https://doi.org/10.1186/s12931-018-0825-9 RESEARCH Comprison of pro- nd nti-inflmmtory responses in pired humn primry irwy epithelil cells nd lveolr mcrophges
Murk et l. Respirtory Reserch (2018) 19:126 https://doi.org/10.1186/s12931-018-0825-9 RESEARCH Comprison of pro- nd nti-inflmmtory responses in pired humn primry irwy epithelil cells nd lveolr mcrophges
Molecular Pharmacology Fast Forward. Published on June 1, 2010 as DOI: /mol
 Moleculr Phrmcology Fst Forwrd. Published on June 1, 2010 s DOI: 10.1124/mol.110.065508 Moleculr Phrmcology This rticle hs not Fst been Forwrd. copyedited nd Published formtted. The on finl June version
Moleculr Phrmcology Fst Forwrd. Published on June 1, 2010 s DOI: 10.1124/mol.110.065508 Moleculr Phrmcology This rticle hs not Fst been Forwrd. copyedited nd Published formtted. The on finl June version
phosphatase isoenzyme activity: estimation of
 J Clin Pthol 1988;41:202-206 Quntittive method for determining serum lkline phosphtse isoenzyme ctivity: estimtion of intestinl component M J PEAKE, M PEJAKOVIC, G H WHITE From the Deprtment ofbiochemistry
J Clin Pthol 1988;41:202-206 Quntittive method for determining serum lkline phosphtse isoenzyme ctivity: estimtion of intestinl component M J PEAKE, M PEJAKOVIC, G H WHITE From the Deprtment ofbiochemistry
WORKSHOP FOR SYRIA. A SHORT TERM PROJECT A Collaborative Map proposal Al Moadamyeh, Syria
 Al Modmyeh is city locted south-west Dmscus, in Syri. It is fcing post-conflict sitution, fter yers of siege nd displcement of its inhbitnts. Now, the popultion is coming bck, s lso new incomers. Therefore,
Al Modmyeh is city locted south-west Dmscus, in Syri. It is fcing post-conflict sitution, fter yers of siege nd displcement of its inhbitnts. Now, the popultion is coming bck, s lso new incomers. Therefore,
Dendritic cells engineered to secrete anti-dcr3 antibody augment cytotoxic T lymphocyte response against pancreatic cancer in vitro
 Submit Mnuscript: http://www.wjgnet.com/esps/ Help Desk: http://www.wjgnet.com/esps/helpdesk.spx DOI: 10.3748/wjg.v23.i5.817 World J Gstroenterol 2017 Februry 7; 23(5): 817-829 ISSN 1007-9327 (print) ISSN
Submit Mnuscript: http://www.wjgnet.com/esps/ Help Desk: http://www.wjgnet.com/esps/helpdesk.spx DOI: 10.3748/wjg.v23.i5.817 World J Gstroenterol 2017 Februry 7; 23(5): 817-829 ISSN 1007-9327 (print) ISSN
Comparative analysis of early immune responses induced by two strains of Newcastle disease virus in chickens
 Received: 22 April 2018 Revised: 28 June 2018 Accepted: 3 July 2018 DOI: 10.1002/mo3.701 ORIGINAL ARTICLE Comprtive nlysis of erly immune responses induced y two strins of Newcstle disese virus in chickens
Received: 22 April 2018 Revised: 28 June 2018 Accepted: 3 July 2018 DOI: 10.1002/mo3.701 ORIGINAL ARTICLE Comprtive nlysis of erly immune responses induced y two strins of Newcstle disese virus in chickens
3.3 Verotoxigenic E. coli
 3.3 Verotoxigenic E. coli Summry Number of VTEC cses, 215: 73 Crude incidence rte, 215: 15.9/1, Number of VTEC-ssocited HUS, 215: 22 Number of VTEC cses, 214: 77 Introduction For mny yers, Irelnd hs the
3.3 Verotoxigenic E. coli Summry Number of VTEC cses, 215: 73 Crude incidence rte, 215: 15.9/1, Number of VTEC-ssocited HUS, 215: 22 Number of VTEC cses, 214: 77 Introduction For mny yers, Irelnd hs the
Abstract. Background. Aim. Patients and Methods. Patients. Study Design
 Impct of the Use of Drugs nd Substitution Tretments on the Antivirl Tretment of Chronic Heptitis C: Anlysis of Complince, Virologicl Response nd Qulity of Life (CHEOBS). Melin, 1 J.-. Lng, D. Ouzn, 3 M.
Impct of the Use of Drugs nd Substitution Tretments on the Antivirl Tretment of Chronic Heptitis C: Anlysis of Complince, Virologicl Response nd Qulity of Life (CHEOBS). Melin, 1 J.-. Lng, D. Ouzn, 3 M.
Check your understanding 3
 1 Wht is the difference etween pssive trnsport nd ctive trnsport? Pssive trnsport is the movement of prticles not requiring energy. Movement of prticles in ctive trnsport uses energy. 2 A gs tp in the
1 Wht is the difference etween pssive trnsport nd ctive trnsport? Pssive trnsport is the movement of prticles not requiring energy. Movement of prticles in ctive trnsport uses energy. 2 A gs tp in the
Extraction and Some Functional Properties of Protein Extract from Rice Bran
 Ksetsrt J. (Nt. Sci.) 40 : 209-214 (2006) Extrction nd Some Functionl Properties of Protein Extrct from Rice Brn Chockchi Theerkulkit*, Siree Chiseri nd Siriwt Mongkolknchnsiri ABSTRACT Rice brn protein
Ksetsrt J. (Nt. Sci.) 40 : 209-214 (2006) Extrction nd Some Functionl Properties of Protein Extrct from Rice Brn Chockchi Theerkulkit*, Siree Chiseri nd Siriwt Mongkolknchnsiri ABSTRACT Rice brn protein
2018 American Diabetes Association. Published online at
 Supplementry Figure S1. Ft-1 mice exhibit reduced diposity when fed n HFHS diet. WT nd ft-1 mice were fed either control or n HFHS diet for 18 weeks. A: Representtive photogrphs for side-by-side comprison
Supplementry Figure S1. Ft-1 mice exhibit reduced diposity when fed n HFHS diet. WT nd ft-1 mice were fed either control or n HFHS diet for 18 weeks. A: Representtive photogrphs for side-by-side comprison
Generation of Seal Influenza Virus Variants Pathogenic for
 JOURNAL OF VIROLOGY, JUlY 199, P. 3297-333 22-538X/9/73297-7$2./ Copyright D 199, Americn Society for Microbiology Vol. 64, No. 7 Genertion of Sel Influenz Virus Vrints Pthogenic for Chickens, becuse of
JOURNAL OF VIROLOGY, JUlY 199, P. 3297-333 22-538X/9/73297-7$2./ Copyright D 199, Americn Society for Microbiology Vol. 64, No. 7 Genertion of Sel Influenz Virus Vrints Pthogenic for Chickens, becuse of
The Acute Time Course of Concurrent Activation Potentiation
 Mrquette University e-publictions@mrquette Exercise Science Fculty Reserch nd Publictions Exercise Science, Deprtment of 1-1-2010 The Acute Time Course of Concurrent Activtion Potentition Luke Grceu Mrquette
Mrquette University e-publictions@mrquette Exercise Science Fculty Reserch nd Publictions Exercise Science, Deprtment of 1-1-2010 The Acute Time Course of Concurrent Activtion Potentition Luke Grceu Mrquette
Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity
 5 Nture Pulishing Group http://www.nture.com/ntureimmunology Suppressor of cytokine signling 1 regultes the immune response to infection y unique inhiition of type I interferon ctivity Jennifer E Fenner
5 Nture Pulishing Group http://www.nture.com/ntureimmunology Suppressor of cytokine signling 1 regultes the immune response to infection y unique inhiition of type I interferon ctivity Jennifer E Fenner
Supplementary Online Content
 Supplementry Online Content Zulmn DM, Pl Chee C, Ezeji-Okoye SC, et l. Effect of n intensive outptient progrm to ugment primry cre for high-need Veterns Affirs ptients: rndomized clinicl tril. JAMA Intern
Supplementry Online Content Zulmn DM, Pl Chee C, Ezeji-Okoye SC, et l. Effect of n intensive outptient progrm to ugment primry cre for high-need Veterns Affirs ptients: rndomized clinicl tril. JAMA Intern
Chapter 5: The peripheral nervous system Learning activity suggested answers
 Chpter 5: The peripherl nervous system Lerning ctivity suggested nswers Lerning Activity 5.1 (p. 222) 1 Briefly descrie the two min functions of the somtic nervous system. Description should refer to:
Chpter 5: The peripherl nervous system Lerning ctivity suggested nswers Lerning Activity 5.1 (p. 222) 1 Briefly descrie the two min functions of the somtic nervous system. Description should refer to:
Dr. Javier Polo Vice President Research & Development
 Efecto de l suplementción con concentrdo de inmunoglobulins prtir del suero bovino en l Microbiot y su contribución l slud intestinl y l sistem nervioso centrl Dr. Jvier Polo Vice President Reserch & Development
Efecto de l suplementción con concentrdo de inmunoglobulins prtir del suero bovino en l Microbiot y su contribución l slud intestinl y l sistem nervioso centrl Dr. Jvier Polo Vice President Reserch & Development
EVALUATION OF DIFFERENT COPPER SOURCES AS A GROWTH PROMOTER IN SWINE FINISHING DIETS 1
 Swine Dy 2001 Contents EVALUATION OF DIFFERENT COPPER SOURCES AS A GROWTH PROMOTER IN SWINE FINISHING DIETS 1 C. W. Hstd, S. S. Dritz 2, J. L. Nelssen, M. D. Tokch, nd R. D. Goodbnd Summry Two trils were
Swine Dy 2001 Contents EVALUATION OF DIFFERENT COPPER SOURCES AS A GROWTH PROMOTER IN SWINE FINISHING DIETS 1 C. W. Hstd, S. S. Dritz 2, J. L. Nelssen, M. D. Tokch, nd R. D. Goodbnd Summry Two trils were
Mycobacterial Ribonucleic Acid Preparations
 INFEcriON AND IMMUNITY, JUlY 1973, p. 42-47 Vol. 8, No. 1 Copyright 0 1973 Americn Society for Microbiology Printed in U.S.A. Reltionship Between Tuberculin Hypersensitivity nd Cellulr Immunity to Infection
INFEcriON AND IMMUNITY, JUlY 1973, p. 42-47 Vol. 8, No. 1 Copyright 0 1973 Americn Society for Microbiology Printed in U.S.A. Reltionship Between Tuberculin Hypersensitivity nd Cellulr Immunity to Infection
GDF11 Protects against Endothelial Injury and Reduces Atherosclerotic Lesion Formation in Apolipoprotein E-Null Mice
 originl rticle The Americn Society of Gene & Cell Therpy Protects ginst Endothelil Injury nd Reduces Atherosclerotic Lesion Formtion in Apolipoprotein E-Null Mice Wen Mei, Gungd Xing, Yixing Li 2, Hun
originl rticle The Americn Society of Gene & Cell Therpy Protects ginst Endothelil Injury nd Reduces Atherosclerotic Lesion Formtion in Apolipoprotein E-Null Mice Wen Mei, Gungd Xing, Yixing Li 2, Hun
Significance of Expression of TGF- in Pulmonary Metastasis in Non-small Cell Lung Cancer Tissues
 Originl Article Significnce of Expression of in Pulmonry Metstsis in Non-smll Cell Lung Cncer Tissues Hisshi Sji, Hruhiko Nkmur, Idiris Awut, Norihito Kwski, Msru Hgiwr, Akihiko Ogt, Mkoto Hosk, Tkmoto
Originl Article Significnce of Expression of in Pulmonry Metstsis in Non-smll Cell Lung Cncer Tissues Hisshi Sji, Hruhiko Nkmur, Idiris Awut, Norihito Kwski, Msru Hgiwr, Akihiko Ogt, Mkoto Hosk, Tkmoto
ROLE OF CALCIUM IN ACTIVATION OF ESTROGEN RECEPTOR-α
 ROLE OF CALCIUM IN ACTIVATION OF ESTROGEN RECEPTOR-α A Disserttion submitted to the Fculty of the Grdute School of Arts nd Sciences of Georgetown University in prtil fulfillment of the requirements for
ROLE OF CALCIUM IN ACTIVATION OF ESTROGEN RECEPTOR-α A Disserttion submitted to the Fculty of the Grdute School of Arts nd Sciences of Georgetown University in prtil fulfillment of the requirements for
Immunobiotic Lactobacillus jensenii as immune-health promoting factor to improve growth performance and productivity in post-weaning pigs
 Sud et l. BMC Immunology 2014, 15:24 RESEARCH ARTICLE Open Access Immunobiotic Lctobcillus jensenii s immune-helth promoting fctor to improve growth performnce nd productivity in post-wening pigs Yoshihito
Sud et l. BMC Immunology 2014, 15:24 RESEARCH ARTICLE Open Access Immunobiotic Lctobcillus jensenii s immune-helth promoting fctor to improve growth performnce nd productivity in post-wening pigs Yoshihito
A proliferation-inducing ligand (APRIL) in neutrophils of patients with oral cavity squamous cell carcinoma
 Eur. Cytokine Netw. Vol. 23 n 3, July-August-September 212, 93-1 93 RESEARCH ARTICLE A prolifertion-inducing lignd (APRIL) in neutrophils of ptients with orl cvity squmous cell crcinom Ew Jbłońsk 1, Ntli
Eur. Cytokine Netw. Vol. 23 n 3, July-August-September 212, 93-1 93 RESEARCH ARTICLE A prolifertion-inducing lignd (APRIL) in neutrophils of ptients with orl cvity squmous cell crcinom Ew Jbłońsk 1, Ntli
Estimating the impact of the 2009 influenza A(H1N1) pandemic on mortality in the elderly in Navarre, Spain
 Rpid communictions Estimting the impct of the influenz pndemic on mortlity in the elderly in Nvrre, Spin J Cstill (jcstilc@nvrr.es) 1, J Etxeberri 1, E Ardnz 1, Y Floristán 1, R López Escudero 1, M Guevr
Rpid communictions Estimting the impct of the influenz pndemic on mortlity in the elderly in Nvrre, Spin J Cstill (jcstilc@nvrr.es) 1, J Etxeberri 1, E Ardnz 1, Y Floristán 1, R López Escudero 1, M Guevr
The effect of encapsulated butyric acid and zinc on performance, gut integrity and meat quality in male broiler chickens 1
 The effect of encpsulted utyric cid nd zinc on performnce, gut integrity nd met qulity in mle roiler chickens 1 Astrct This study evluted the impct of encpsulted utyric cid nd zinc (ButiPEARL Z) on performnce
The effect of encpsulted utyric cid nd zinc on performnce, gut integrity nd met qulity in mle roiler chickens 1 Astrct This study evluted the impct of encpsulted utyric cid nd zinc (ButiPEARL Z) on performnce
Comparison of Guinea Pig Embryo Cells, Rabbit Kidney Cells, and Human Embryonic Lung Fibroblast Cell Strains for Isolation of Herpes Simplex Virus
 JOURNAL OF CLINICAL MICROBIOLOGY, My 1982, p. 842-847 0095-1137/82/050842-06$02.00/0 Vol. 15, No. 5 Comprison of Guine Pig Embryo Cells, Rbbit Kidney Cells, nd Humn Embryonic Lung Fibroblst Cell Strins
JOURNAL OF CLINICAL MICROBIOLOGY, My 1982, p. 842-847 0095-1137/82/050842-06$02.00/0 Vol. 15, No. 5 Comprison of Guine Pig Embryo Cells, Rbbit Kidney Cells, nd Humn Embryonic Lung Fibroblst Cell Strins
SUPPLEMENTARY INFORMATION
 doi:1.138/nture1228 Totl Cell Numer (cells/μl of lood) 12 1 8 6 4 2 d Peripherl Blood 2 4 7 Time (d) fter nti-cd3 i.p. + TCRβ + IL17A + cells (%) 7 6 5 4 3 2 1 Totl Cell Numer (x1 3 ) 8 7 6 5 4 3 2 1 %
doi:1.138/nture1228 Totl Cell Numer (cells/μl of lood) 12 1 8 6 4 2 d Peripherl Blood 2 4 7 Time (d) fter nti-cd3 i.p. + TCRβ + IL17A + cells (%) 7 6 5 4 3 2 1 Totl Cell Numer (x1 3 ) 8 7 6 5 4 3 2 1 %
Safety and Tolerability of Subcutaneous Sarilumab and Intravenous Tocilizumab in Patients With RA
 Sfety nd Tolerbility of Subcutneous Srilumb nd Intrvenous Tocilizumb in Ptients With RA Pul Emery, 1 Jun Rondon, 2 Anju Grg, 3 Hubert vn Hoogstrten, 3 Neil M.H. Grhm, 4 Ming Liu, 4 Nncy Liu, 3 Jnie Prrino,
Sfety nd Tolerbility of Subcutneous Srilumb nd Intrvenous Tocilizumb in Ptients With RA Pul Emery, 1 Jun Rondon, 2 Anju Grg, 3 Hubert vn Hoogstrten, 3 Neil M.H. Grhm, 4 Ming Liu, 4 Nncy Liu, 3 Jnie Prrino,
Plaque Assay of Avian Sarcoma Viruses Using Casein
 JOURNAL OF VIROLOGY, Sept. 1975, p. 707-711 Copyright 0 1975 Americn Society for Microbiology Vol. 16, No. 3 Printed in U.S.A. Plque Assy of Avin Srcom Viruses Using Csein PIERO C. BALDUZZI*l AND HELEN
JOURNAL OF VIROLOGY, Sept. 1975, p. 707-711 Copyright 0 1975 Americn Society for Microbiology Vol. 16, No. 3 Printed in U.S.A. Plque Assy of Avin Srcom Viruses Using Csein PIERO C. BALDUZZI*l AND HELEN
Supplementary information for: Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage
 Supplementry informtion for: Low one mss nd chnges in the osteocyte network in mice lcking utophgy in the osteolst linege Mrilin Piemontese, Meld Onl, Jinhu Xiong, Li Hn, Jeff D. Thostenson, Mri Almeid,
Supplementry informtion for: Low one mss nd chnges in the osteocyte network in mice lcking utophgy in the osteolst linege Mrilin Piemontese, Meld Onl, Jinhu Xiong, Li Hn, Jeff D. Thostenson, Mri Almeid,
CheckMate 153: Randomized Results of Continuous vs 1-Year Fixed-Duration Nivolumab in Patients With Advanced Non-Small Cell Lung Cancer
 CheckMte 53: Rndomized Results of Continuous vs -Yer Fixed-Durtion Nivolumb in Ptients With Advnced Non-Smll Cell Lung Cncer Abstrct 297O Spigel DR, McCleod M, Hussein MA, Wterhouse DM, Einhorn L, Horn
CheckMte 53: Rndomized Results of Continuous vs -Yer Fixed-Durtion Nivolumb in Ptients With Advnced Non-Smll Cell Lung Cncer Abstrct 297O Spigel DR, McCleod M, Hussein MA, Wterhouse DM, Einhorn L, Horn
Supplemental Information. Lymphocytes Negatively Regulate NK Cell Activity. via Qa-1b following Viral Infection
 Cell Reports, Volume 21 Supplementl Informtion Lymphocytes Negtively Regulte NK Cell Activity vi Q-1b following Virl Infection Hifeng C. Xu, Jun Hung, Aleksndr A. Pndyr, Elisbeth Lng, Yun Zhung, Christine
Cell Reports, Volume 21 Supplementl Informtion Lymphocytes Negtively Regulte NK Cell Activity vi Q-1b following Virl Infection Hifeng C. Xu, Jun Hung, Aleksndr A. Pndyr, Elisbeth Lng, Yun Zhung, Christine
Identification of Genetically Modified Maraba Virus as an Oncolytic Rhabdovirus
 originl rticle The Americn Society of Gene & Cell Therpy Identifiction of Geneticlly Modified Mrb Virus s n Oncolytic Rhbdovirus Jn Brun 1,2, Dn McMnus 1,2, Chrles Lefebvre 1, Kng Hu 4, Theres Flls 4,
originl rticle The Americn Society of Gene & Cell Therpy Identifiction of Geneticlly Modified Mrb Virus s n Oncolytic Rhbdovirus Jn Brun 1,2, Dn McMnus 1,2, Chrles Lefebvre 1, Kng Hu 4, Theres Flls 4,
Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T- lymphocytes
 Supporting Online Mteril for Heprnse promotes tumor infiltrtion nd ntitumor ctivity of -redirected T- lymphocytes IgnzioCrun, Brr Svoldo, VlentinHoyos, Gerrit Weer, Ho Liu, Eugene S. Kim, Michel M. Ittmnn,
Supporting Online Mteril for Heprnse promotes tumor infiltrtion nd ntitumor ctivity of -redirected T- lymphocytes IgnzioCrun, Brr Svoldo, VlentinHoyos, Gerrit Weer, Ho Liu, Eugene S. Kim, Michel M. Ittmnn,
Innate immune response to a H3N2 subtype swine influenza virus in newborn porcine trachea cells, alveolar macrophages, and precision-cut lung slices
 Delgdo-Orteg et l. Veterinry Reserch 214, 45:42 VETERINARY RESEARCH RESEARCH Open Access Innte immune response to H3N2 sutype swine influenz virus in neworn porcine trche cells, lveolr mcrophges, nd precision-cut
Delgdo-Orteg et l. Veterinry Reserch 214, 45:42 VETERINARY RESEARCH RESEARCH Open Access Innte immune response to H3N2 sutype swine influenz virus in neworn porcine trche cells, lveolr mcrophges, nd precision-cut
Vitamin D and Mushrooms: Enrichment With Pulsed UV Light. Michael Kalaras Department of Food Science The Pennsylvania State University
 Vitmin D nd Mushrooms: Enrichment With Pulsed UV Light Michel Klrs Deprtment of Food Science The Pennsylvni Stte University Vitmin D Synthesis Source: http://vitmind.ucr.edu/imges/chem1.gif Vitmin D In
Vitmin D nd Mushrooms: Enrichment With Pulsed UV Light Michel Klrs Deprtment of Food Science The Pennsylvni Stte University Vitmin D Synthesis Source: http://vitmind.ucr.edu/imges/chem1.gif Vitmin D In
Original article Andes-virus-induced cytokine storm is partially suppressed by ribavirin
 Antivirl Therpy 203; 8:575 584 (doi: 0.385/IMP2524) Originl rticle Andes-virus-induced cytokine storm is prtilly suppressed by ribvirin Svetln F Khiboullin *, Albert A Rizvnov 2, Vincent C Lombrdi, Sergey
Antivirl Therpy 203; 8:575 584 (doi: 0.385/IMP2524) Originl rticle Andes-virus-induced cytokine storm is prtilly suppressed by ribvirin Svetln F Khiboullin *, Albert A Rizvnov 2, Vincent C Lombrdi, Sergey
Preservative Resistance in Yeast Species
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Nov. 1989, p. 2995-2999 Vol. 55, No. 11 99-224/89/112995-5$2./ Copyright 1989, Americn Society for Microbiology Reltionships mong Cell Size, Membrne Permebility,
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Nov. 1989, p. 2995-2999 Vol. 55, No. 11 99-224/89/112995-5$2./ Copyright 1989, Americn Society for Microbiology Reltionships mong Cell Size, Membrne Permebility,
ORIGINAL ARTICLE ABSTRACT INTRODUCTION
 ORIGINAL ARTICLE LOSS OF EPCAM STAINING CORRELATES WITH POOR OUTCOME IN CRC, Wng et l. Reduction in membrnous immunohistochemicl stining for the intrcellulr domin of epithelil cell dhesion molecule correltes
ORIGINAL ARTICLE LOSS OF EPCAM STAINING CORRELATES WITH POOR OUTCOME IN CRC, Wng et l. Reduction in membrnous immunohistochemicl stining for the intrcellulr domin of epithelil cell dhesion molecule correltes
Effects of blueberries on migration, invasion, proliferation, the cell cycle and apoptosis in hepatocellular carcinoma cells
 BIOMEDICAL REPORTS 5: 579-584, 2016 Effects of blueberries on migrtion, invsion, prolifertion, the cell cycle nd poptosis in heptocellulr crcinom cells WEI ZHAN 1*, XIN LIAO 2*, LEI YU 3, TIAN TIAN 3,
BIOMEDICAL REPORTS 5: 579-584, 2016 Effects of blueberries on migrtion, invsion, prolifertion, the cell cycle nd poptosis in heptocellulr crcinom cells WEI ZHAN 1*, XIN LIAO 2*, LEI YU 3, TIAN TIAN 3,
Mecadox. Improves pig performance in a wide range of health and growing conditions. (Carbadox) Talk With a Phibro Expert:
 SWINE (Crbdox) Improves pig performnce in wide rnge of helth nd growing conditions The Advntge Over the yers, medicted feed dditive hs proven to be cost-effective mngement tool for improving pig performnce
SWINE (Crbdox) Improves pig performnce in wide rnge of helth nd growing conditions The Advntge Over the yers, medicted feed dditive hs proven to be cost-effective mngement tool for improving pig performnce
The potential future of targeted radionuclide therapy: implications for occupational exposure? P. Covens
 The potentil future of trgeted rdionuclide therpy: implictions for occuptionl exposure? Introduction: Trgeted Rdionuclide Therpy (TRT) Systemic tretment Molecule lbelled with rdionuclide delivers toxic
The potentil future of trgeted rdionuclide therpy: implictions for occuptionl exposure? Introduction: Trgeted Rdionuclide Therpy (TRT) Systemic tretment Molecule lbelled with rdionuclide delivers toxic
Study on the association between PI3K/AKT/mTOR signaling pathway gene polymorphism and susceptibility to gastric
 JBUON 2017; 22(6): 1488-1493 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mil: editoril_office@jbuon.com ORIGINAL ARTICLE Study on the ssocition between PI3K/AKT/mTOR signling pthwy gene polymorphism
JBUON 2017; 22(6): 1488-1493 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mil: editoril_office@jbuon.com ORIGINAL ARTICLE Study on the ssocition between PI3K/AKT/mTOR signling pthwy gene polymorphism
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nture1188 1mM CCl 2 (min) 3 4 6 CCl 2 (mm) for 4min.1. 1 (mm) Pro- d WT GdCl 3 R-68 -/- P2x7r -/- -/- Csp1 -/- WT -/- P2x7r -/- -/- Csp1 -/- Csp1 (p2) (p17) Pro-Csp1
SUPPLEMENTARY INFORMATION doi:1.138/nture1188 1mM CCl 2 (min) 3 4 6 CCl 2 (mm) for 4min.1. 1 (mm) Pro- d WT GdCl 3 R-68 -/- P2x7r -/- -/- Csp1 -/- WT -/- P2x7r -/- -/- Csp1 -/- Csp1 (p2) (p17) Pro-Csp1
Relationship between food availability, glycerol and glycogen levels in lowtemperature challenged rainbow smelt Osmerus mordax
 866 The Journl of Experimentl Biology, 866-87 Published by The Compny of Biologists 7 doi:.4/jeb.749 Reltionship between food vilbility, glycerol nd glycogen levels in lowtemperture chllenged rinbow smelt
866 The Journl of Experimentl Biology, 866-87 Published by The Compny of Biologists 7 doi:.4/jeb.749 Reltionship between food vilbility, glycerol nd glycogen levels in lowtemperture chllenged rinbow smelt
Pretreatment with transforming growth factor beta-3 protects small intestinal stem cells against radiation damage in vivo
 British Jouml of Cncer (1997) 75(1), 1454-1459 1997 Cncer Reserch Cmpign Pretretment with trnsforming growth fctor bet-3 protects smll intestinl stem cells ginst rdition dmge in vivo CS Potten1, D Booth1
British Jouml of Cncer (1997) 75(1), 1454-1459 1997 Cncer Reserch Cmpign Pretretment with trnsforming growth fctor bet-3 protects smll intestinl stem cells ginst rdition dmge in vivo CS Potten1, D Booth1
Expression of Three Cell Cycle Inhibitors during Development of Adipose Tissue
 Expression of Three Cell Cycle Inhiitors during Development of Adipose Tissue Jiin Zhng Deprtment of Animl Sciences Advisor: Michel E. Dvis Co-dvisor: Kichoon Lee Development of niml dipose tissue Hypertrophy
Expression of Three Cell Cycle Inhiitors during Development of Adipose Tissue Jiin Zhng Deprtment of Animl Sciences Advisor: Michel E. Dvis Co-dvisor: Kichoon Lee Development of niml dipose tissue Hypertrophy
Identification and selective expansion of functionally superior T cells expressing chimeric antigen receptors
 Identifiction nd selective expnsion of functionlly superior T cells expressing chimeric ntigen receptors The Hrvrd community hs mde this rticle openly vilble. Plese shre how this ccess benefits you. Your
Identifiction nd selective expnsion of functionlly superior T cells expressing chimeric ntigen receptors The Hrvrd community hs mde this rticle openly vilble. Plese shre how this ccess benefits you. Your
Supplementary Figure 1
 doi: 1.138/nture6188 SUPPLEMENTARY INFORMATION Supplementry Figure 1 c CFU-F colonies per 1 5 stroml cells 14 12 1 8 6 4 2 Mtrigel plug Neg. MCF7/Rs MDA-MB-231 * * MCF7/Rs-Lung MDA-MB-231-Lung MCF7/Rs-Kidney
doi: 1.138/nture6188 SUPPLEMENTARY INFORMATION Supplementry Figure 1 c CFU-F colonies per 1 5 stroml cells 14 12 1 8 6 4 2 Mtrigel plug Neg. MCF7/Rs MDA-MB-231 * * MCF7/Rs-Lung MDA-MB-231-Lung MCF7/Rs-Kidney
Immunity to Experimental Fowl Typhoid in Chickens Induced by a Virulence Plasmid-Cured Derivative of Salmonella gallinarum
 INFECTION AND IMMUNITY, JUlY 1990, p. 2283-2288 0019-9567/90/072283-06$02.00/0 Copyright C) 1990, Americn Society for Microbiology Vol. 58, No. 7 Immunity to Experimentl Fowl Typhoid in Chickens Induced
INFECTION AND IMMUNITY, JUlY 1990, p. 2283-2288 0019-9567/90/072283-06$02.00/0 Copyright C) 1990, Americn Society for Microbiology Vol. 58, No. 7 Immunity to Experimentl Fowl Typhoid in Chickens Induced
Effect of Aqueous Extract of Carica papaya Dry Root Powder on Lactation of Albino Rats
 Effect of Aqueous Extrct of Cric ppy Dry Root Powder on Lcttion of Alino Rts G. Tosswnchuntr nd S. Aritjt Deprtment of Biology Fculty of Science Ching Mi University Ching Mi 50200 Thilnd Keywords: mmmry
Effect of Aqueous Extrct of Cric ppy Dry Root Powder on Lcttion of Alino Rts G. Tosswnchuntr nd S. Aritjt Deprtment of Biology Fculty of Science Ching Mi University Ching Mi 50200 Thilnd Keywords: mmmry
Aberrant expression of B7-H4 correlates with poor prognosis and suppresses tumor-infiltration of CD8 + T lymphocytes in human cholangiocarcinoma
 ONCOLOGY REPORTS 36: 419-427, 2016 Aberrnt expression of B7-H4 correltes with poor prognosis nd suppresses tumor-infiltrtion of CD8 + T lymphocytes in humn cholngiocrcinom Xin Zho *, Fei Guo *, Zhonghu
ONCOLOGY REPORTS 36: 419-427, 2016 Aberrnt expression of B7-H4 correltes with poor prognosis nd suppresses tumor-infiltrtion of CD8 + T lymphocytes in humn cholngiocrcinom Xin Zho *, Fei Guo *, Zhonghu
