MATHEMATICAL MODEL AND VALIDATION OF MICROVASCULAR FUNCTION IN LOW PERFUSION TISSUE
|
|
|
- Leonard Sanders
- 5 years ago
- Views:
Transcription
1 MATHEMATICAL MODEL AND VALIDATION OF MICROVASCULAR FUNCTION IN LOW PERFUSION TISSUE by Eric Zakher A thesis submitted in conformity with the requirements for the degree of Master of Applied Science The Edward S. Rogers Sr. Department of Electrical & Computer Engineering University of Toronto Copyright by Eric Zakher 2018
2 Mathematical Model and Validation of Microvascular Function in Low Perfusion Tissue Abstract Eric Zakher Master of Applied Science The Edward S. Rogers Sr. Department of Electrical & Computer Engineering University of Toronto 2018 Many diseases are caused or triggered following microvascular endothelial dysfunction, such as coronary artery disease, diabetes, and hypertension. Currently, blood flow is used as the main measurement to assess vasomodulation. It has however been proven to not be accurate as many other factors must be considered. This dissertation presents a mathematical model to assess vasomodulation in low perfusion tissue using MRI. It was developed by taking the first-order derivative of the T 1 relaxation times (ΔT 1 ) from a series of T 1 -weighted images of rat s kidneys (high perfusion tissue) and leg muscles (low perfusion tissue). A gas protocol was used to assess vasoconstriction with a severe hypercapnic condition and vasodilation with a mild hypercapnic condition. The gas mixtures were administered using a fixed inspired concentration delivery technique. Perfusion measurements using Doppler flowmetry were obtained as validation to the model. Every vasomodulatory scenario with detailed analysis of the ΔT1 plots are covered. ii
3 Acknowledgments Firstly, I would like to sincerely thank my supervisor Dr. Hai-Ling Margaret Cheng for her constant support throughout the research process. Her knowledge in multiple fields provided me with a unique and valuable outlook, allowing me to achieve the highest standards. She provided me with kind and constructive feedback, pushing me to develop and refine my analytical, technological, and research skills. Further, I would like to thank Tameshwar Ganesh for introducing and familiarizing me with his exemplary research, and always providing me with an open, patient, and reassuring environment to discuss and debate topics and ideas. Additionally, I would like to thank every member of the Cheng lab for contributing their knowledge and skills towards my research, as well as helping in building a competitive, positive, and respectful lab environment. I would also like to thank my partner, Brittany, and my family for their endless love, support, and reassurance throughout my academic experience. I would like to give an honorable mention to my brother, Karl for his artistic eye in helping to produce numerous figures in chapter 1. Lastly, I would like to thank the University of Toronto for providing me with an outstanding environment and experience that helped me build valuable lifelong skills. iii
4 Table of Contents Acknowledgments... iii Table of Contents... iv List of Tables... vii List of Figures... viii Introduction... 1 Chapter Microcirculation Classification and Function Microvascular Endothelial Dysfunction Microvascular Dysfunction Diseases Coronary Artery Disease - CAD Diabetes Mellitus Organ Anatomy and Microcirculation Gas Challenge Hypercapnia Skeletal Muscle and Kidney Responses MRI MRI Signal Transverse Relaxation Time (T2) iv
5 1.5.3 Longitudinal Relaxation Time (T1) K-Space Contrast Agents MRI Artifacts Chapter Introduction to Model Hypothesis and Aims Mathematical Proof Chapter Experiments Gas Challenge Protocol MRI Protocol Optronix Protocol Data Analysis Results and Analysis Chapter Summary of Findings Discussion Challenges Limitations v
6 4.2 Future Works Conclusion References vi
7 List of Tables Table 1: Risks Factors for the Diagnosis of CAD, Adapted from Ref. [10]... 6 Table 2: Types of ECF Contrast Agents (Gadolinium-Based), Adapted from Ref. [44] Table 3: Types of BP Contrast Agents, Adapted from Ref. [44] Table 4: Types of Targeted or Organ-Specific Contrast Agent, Adapted from Ref. [44] Table 5: Characteristics of the Partial Derivative T1/ in Relation to Vasomodulation vii
8 List of Figures Figure 1: Schematic of the microcirculation system, adapted from ref. [2]... 4 Figure 2: Anatomy of the skeletal muscle, adapted from ref. [17]... 7 Figure 3: Anatomy of the vascular system of skeletal muscles, adapted from ref. [15]... 8 Figure 4: Anatomy of the kidney's microcirculation, adapted from ref. [19, 20]... 9 Figure 5: A) Difference in echoe time (TE) results in variation in signal intensity for different tissue types, adapted from ref. [40], B) T2-weighted image of a rat showing the variation in signal intensities between the paraspinal muscles (dark), kidney (gray), and fat (bright) Figure 6: A) Difference in repetition time (TR) results in variation in signal intensity for different tissue types, adapted from ref. [40], B) T1-weighted image of a rat showing the variation in signal intensities between the paraspinal muscles (gray) and kidney (bright) Figure 7: A) K-space and its final image representation, B) Low-Pass filter applied to K-space filtering high frequency components, C) High-pass frequency components applied to K-space filtering low frequency components [42] Figure 8: Motion artifact on cardiac MRI resulting in blurring and ghosting Figure 9: Schematic of the Gas Protocol Figure 10: Inadequate sensitivity of T1 measurements to assess vasomodulation in low perfusion rat skeletal muscle. The highly perfused kidney cortex (red) exhibits large changes in T1 in response to hypercapnia, whereas changes in skeletal muscle is very modest (blue) (A). The small degree of change in muscle can be similarly appreciated on T1-weighted images (B) compared to visible signal changes in the kidney (C) Figure 11: Improved sensitivity to vasomodulation in low perfusion setting via the parameter T1 Vasoconstriction in response to 20% CO2 and vasodilation in response to 5% CO2 are clearly delineated in the leg skeletal muscle of a representative rat using the proposed metric, the partial derivative T viii
9 Figure 12: The ability to tease out vasoconstriction and vasodilation in skeletal muscle using the parameter T1 is illustrated across all animals. A similar color is used in the kidney and leg muscle graphs to indicate data from the same animal Figure 13:Average changes in T1 and heart rate in response to severe and mild hypercapnia. (A) The maximum vasoconstriction in muscle induced by 20% CO2 as measured on ( T1)/ and the maximum vasodilation induced by 5% CO2 are averaged over all animal. (B) The average change in heart rate in response to 20% and to 5% CO2 are also shown Figure 14: Optical perfusion validation. Laser Doppler perfusion measurements over the course of hypercapnic challenge intervals are shown for a representative rat. Blood perfusion units (BPU) are relative quantities and do not indicate absolute blood flow Figure 15: ROI taken for the arm (left bicep) (A), upper paraspinal (B), and lower paraspinal muscles (C) Figure 16: Partial derivative of T1 for the kidney where the magnitude for the hypoxic gas challenge (red) were smaller than the hypercapnic gas challenge (blue) Figure 17: (A) Probe placement for the leg and arm muscles, (B) and paraspinal and kidney for the perfusion analysis during the gas challenge ix
10 Introduction In recent years, studying vasomodulation of the microvascular network has become increasingly important amongst scientists. As a matter of fact, epidemic diseases such as diabetes have shown to directly be associated to functional disturbances in the microcirculation unanimously known as microvascular endothelial dysfunction [1]. Such disruption in the system is said to precede many complications such as neuropathy, heart, and vascular diseases. [1]. As the great physician Hippocrates once said, Declare the past, diagnose the present, foretell the future. With this in mind, early diagnosis of microvascular dysfunction is a must to properly administer treatments to prevent further development of such diseases. So, what methods currently exist to accurately assess microvascular endothelial dysfunction? Tissues characterized to have high perfusion such as kidneys are uncomplicated in asserting the microvascular system as opposed to low perfusion tissues such as skeletal muscles. Physicians are therefore currently adopting techniques that revolve around the estimation of blood flow in larger arteries to determine potential problems in the microvasculature [2]. Additionally, each imaging modality has different purposes; however, none currently stands out when it comes to analyzing vasomodulation in the microvascular system. In light of recent advancements in MRI and to the fact that current MRI techniques are unable to detect microvascular tones [3], a model to further advance its clinical use in the near future is demonstrated. This technique is a mathematical model used to understand and further analyze the microvascular system in low perfusion tissue. This research project was carried to fulfill a problem that arose during the analysis of a study conducted by Ganesh et al. J Magn Reson Imaging 2018 [4]. Firstly, regarding this study, its aim consisted of investigating microvascular dysfunction through different time points to ultimately track the healing process in hindlimb muscles that had been injured through an ischemicreperfusion injury. Microvascular endothelial dysfunction was developed using a leg occlusion pressure cuff to restrict blood flow to muscle tissues, followed with a reperfusion injury by removing the pressure cuff. In fact, the injury is triggered by tissue inflammation, reperfusion injury, microvascular obstruction, and endothelial dysfunction. The main analysis of this disease was carried using a simple T1 analysis using a Gadolinium contrast agent. With this intention, the original T1 analysis offered very little useful information to truly understand if microvascular 1
11 dysfunction was achieved or not. It was by developing the mathematical model presented in this dissertation that the study was successfully achieved. This dissertation is divided in four distinct chapters. In the first place, a background section will be introduced to cover important topics related to the aims in question through multiple literature reviews. A brief overview of the microcirculation will first be covered to then discuss microvascular endothelial dysfunction by presenting two significant diseases that greatly affect today s population worldwide. A description of the anatomy of both organs analyzed in this study consisting of skeletal muscles and kidneys will follow. An introduction to the gas challenge protocol used as stimuli for the microvascular system will also be covered showing different gas delivery techniques which include fixed inspiration concentration, dynamic endtidal forcing, and prospective end-tidal targeting. Responses observed during hypercapnia will also be presented for both organs in question. Chapter 1 will finally close with a brief overview of important MRI concepts including signal detection, transverse and longitudinal relaxation times, K-space, contrast agent, and image artifacts. Furthermore, the next chapter Chapter 2, will introduce the aims and hypothesis with a detailed interpretation of the mathematical model and its proof. Chapter 3 will present the experimental procedures and protocol employed as well as analysis of the experimental results. This dissertation will then be wrapped up in a conclusive chapter 4 where a summary of findings, which includes a brief overview of the study, its challenges, and its limitations will be discussed, to finally present potential future works using the developed model. 2
12 Chapter 1 Background 1.1 Microcirculation Classification and Function The microcirculation comprises a network of arterioles, capillaries, and venules corresponding to approximately only 7% of the body s total blood volume [5, 6]. Arterioles have various resting lumen diameter sizes from which they are classified [7]. Larger arterioles are approximately 100 µm in lumen s diameter in human and precede a network of even smaller arterioles, capillaries, and venules [6, 7]. Indeed, their larger size comes with a complex wall layer comprising, from proximity to the middle, the endothelial layer, the internal elastic lamina and a smooth muscle layer [7]. Its wall structure is essential in controlling blood flow to respective organs by means of wall toning vasomodulation [7]. Arterioles have the ability to dilate by up to 50% and can also exert strong constriction capabilities in response to various stimuli, overall making it essential in blood flow regulation of the microcirculation [7]. As a matter of fact, since arterioles comprises the region with the most pressure drop, it is important to note that constriction is more dominant than its dilatory counterpart [7]. Downstream to arterioles, a series of capillary network is located [7]. They respond to fluxes of blood flow by means of vascular resistance through hydrostatic and oncotic pressure gradients [7]. In fact, the capillary network is in charge to facilitate fluid exchange depending on its permeability and surface area [7]. In summary, its role highly depends on the arterioles response to external stimuli, thus, capillaries will react accordingly [7]. Venules are the last segment of the microcirculation which directly follows the capillaries [7]. Out of the whole microcirculation network, venules have on average larger lumen diameter and are more numerous, arranged in a similar fashion to arterioles [7]. Its main difference compared to arterioles is its lack of vascular smooth muscle in vessels lesser than 300 µm in diameter [7]. Like capillaries, their response to stimuli highly depends on the arterioles, with their vascular resistance being only 10% of the total vascular resistance [7]. In fact, their only role is to balance 3
13 or control blood flow in response to capillary pressure which ultimately affects the fluid exchanges between the vascular network and its tissue [7]. At last, arterioles, capillaries, and venules continuously work together to maintain healthy tissue function [5]. Albeit its minimal blood volume circulation, its task is of upmost importance involving the distribution of vital nutrients and oxygen to the tissue in response to external stimuli [5]. This distribution is achieved thanks to the low velocity observed in microcirculation, facilitating the difficult exchange of diffusible substances between the interstitial space and the plasma [7]. Figure 1: Schematic of the microcirculation system, adapted from ref. [2] Microvascular Endothelial Dysfunction Microvascular dysfunction incorporates many disorders with endothelial dysfunction being the most important as it is characterized by the vessel s inability to constrict or dilate. This loss of vasomodulation is due to a malfunction of the endothelium layer in the vessel walls. Endothelial cells are no longer seen as only serving as barrier, but due to their strategical placement in the vessel s wall, they serve as boundary between the blood and the vascular muscle cells [8]. As a result, it can continuously interact with cells in the blood as well as participate in multiple 4
14 functions with the vascular muscle cells [8]. Intermediaries of multiple chemicals are also heavily involved in maintaining overall homeostasis of the system, again affecting both, cells in the blood and the vascular muscle cells [8]. For this reason, the shifts in chemical changes are directly involved in overall muscle tone vasodilation or vasoconstriction [8]. At last, when factors in charge to preserve endothelial cells ability to relax and constricts under physiological conditions are compromised, endothelial dysfunction arises in the vascular system [8]. 1.2 Microvascular Dysfunction Diseases Coronary Artery Disease - CAD Coronary Artery Disease (CAD) is directly caused by coronary atherosclerosis which is characterized by a build-up of fibrous plaque along the vessel wall made of a mixture of tissue, cells, and lipids [9]. Individuals with CAD are currently being assessed based on a list of risk factors (traditional or non-traditional) as shown in Table 1 [10]. In fact, patients diagnosed for CAD identify with one or none of the traditional risk factors and half of these factors are directly related to pathogens of atherosclerosis disease [10]. For this reason, atherogenesis is said to also have an important role in the development of non-traditional or unknown risk factors [10]. Consequently, most treatments are directly targeted for these risk factors instead of atherosclerosis which is actually stemmed by endothelial dysfunction [10]. For this reason, understanding and developing diagnostic methods for endothelial dysfunction has become the prime subject of research for CAD. Moreover, it has previously been shown that endothelial dysfunction is commonly associated with acute coronary syndromes and that reversing it is indeed possible [11]. The process from which CAD is formed by endothelial dysfunction is simple. Firstly, blood flow in the coronary arteries depend on the level of nitric-oxide (NO) production triggered by responses in myocardial needs [12]. An increase in NO therefore causes vasodilation [12]. This chemical dependency subsequently results in endothelial-dependent vasodilation when there is an increase in oxygen free radicals which depletes NO [12]. This event then causes injury of the epicardial arteries resulting in tissue inflammation and in extreme cases can cause myocardial infarction [12]. 5
15 Table 1: Risks Factors for the Diagnosis of CAD, Adapted from Ref. [10] Diabetes Mellitus Diabetes Mellitus is increasingly becoming an epidemic disease due to the high proportion of today s population being obese or having unhealthy diets [13]. There are currently two major types of diabetes; type 1 and type 2. Type 1 is known as a genetic disease where pancreatic beta cells undergo autoimmune destruction [13]. On the other hand, type 2 diabetes is usually attained during adulthood and is characterized by resistance to insulin [13]. The importance of studying diabetes and its pre-diabetic phase is important because it has been shown to be directly involved with multiple other diseases such as heart failure, CAD, and hypertension [14]. As discussed previously and shown in table 1, endothelial dysfunction is a predecessor of CAD. Similarly, it is also important to highlight that diabetes as well proceeds endothelial dysfunction and precedes CAD [1]. In fact, patients diagnosed with type 2 diabetes have shown to have vascular atherosclerosis injuries and is associated to both; microvascular and macrovascular complications such as neuropathy and CAD, respectively [1]. Diabetes Mellitus, type 2 specifically, shunts the body s insulin absorption process consequently decreasing the production of NO [1]. Additionally, diabetes s insulin resistance characteristic is detriment to the increase in the vasoconstrictive promoter endothelin-1 as well as more efficient cellular proliferation [1]. Indeed, endothelin-1 has shown to be vital in regulating vasoconstriction [1]. For this reason, 6
16 with the decrease of NO and endothelin-1 which subsequently removes the vessel s ability to vasodilate and vasoconstrict, endothelial dysfunction is inevitable [1]. 1.3 Organ Anatomy and Microcirculation Skeletal Muscle Anatomy The skeletal muscle is interesting, in that it is subjected to 25% of the cardiac output at rest [15]. It however only corresponds to 0.43% of the total cardiac output relative to skeletal muscle weight in human [16]. As a result, it is one of the organs with the lowest perfusion, yet it can sustain and anticipate oxidative levels during the most strenuous activities [15]. Skeletal muscles are made of several long muscle fibers (myofibers), bundled inside a thin layer of connective tissue called endomysium [15]. Each bundle of myofibers are arranged in parallel inside a thick envelop of connective tissue called the perimysium [15]. Figure 2: Anatomy of the skeletal muscle, adapted from ref. [17] There are three distinct types of myofibers; Type 1, Type 2a, and Type 2b [15]. Both, Type 1 and Type 2a myofibers rely on oxidative metabolism for energy resources as opposed to Type 2b which rely on elevated profusions of glycogen and phosphocreatine storage [15]. Indeed, Type 2b are known to easily fatigue due to their sparse capillary network [15]. The dense capillary network in Type 1 and Type 2a have increased amounts of oxygen-binding protein myoglobin [15]. As a result, dense capillary networks are observed which is essential for oxidative metabolism [15]. 7
17 As described previously, the vascular network consists of; arteries, arterioles, capillary network, venules, and veins. In skeletal muscles, arteries are situated along the long axis and control the magnitude of blood flow before feeding it into the arterioles which serves in controlling proper distribution of blood to tissues [15, 18]. As shown in figure 3, arterioles are parallel to the muscle fibers and feed perpendicularly into the capillary network which surrounds the myofibrils in parallel [15]. For this reason, inconsistency in circumference sizes as well as the different distributions of the capillary network results in inhomogeneity of O2 distribution in the muscles [15]. Finally, venules and veins are arranged in the same manner as arterioles and arteries [15]. Figure 3: Anatomy of the vascular system of skeletal muscles, adapted from ref. [15] Kidney Anatomy The kidneys are very light, consisting of only 0.4% of the total body weight in human [19]. Its main and most tedious role is to filter the blood plasma to eliminate anything not needed by the body and returning the filtered blood back into the bloodstream [19]. This is achieved using an astonishing 1.2 million nephrons that links with the capillary network [19]. For this reason, they are in charge of constantly regulating blood flow by adjusting changes in pressure and volume [19]. The kidneys are C-shaped and are suspended in place and rest against the dorsal abdominal wall by the lowest vertebrae [19]. The parenchyma is divided in two sections; the outer renal cortex and the renal medulla [19]. In fact, the renal medulla is the most vascularized part of the 8
18 kidney as it contains the nephrons which are responsible in filtering the blood [19]. The aorta sends the blood through each renal artery which divide into interlobar arteries and expand to the cortex around the renal medulla [19]. Interlobar arteries divide again into afferent arterioles supplying one nephron each within a capillary network called the glomerulus [19]. The blood then exits through the efferent arterioles to then divide into the peritubular capillaries around renal tubules [19]. The circulation network then ends with the blood flowing to the veins and leaving the kidney [19]. Figure 4: Anatomy of the kidney's microcirculation, adapted from ref. [19, 20] 9
19 1.4 Gas Challenge Hypercapnia Hypercapnia refers to the increase in carbon dioxide in the bloodstream, generally due to inadequate respiration. This increase in carbon dioxide affects the body in multiple ways through different pathways in the sympathetic nervous system (SNS) [21]. The most obvious changes due to SNS is the massive increase in heart rate during severe hypercapnia [21]. Although respiratory acidosis has shown to be the main cause, an increase in heart rate could also be a result of the sympathetic nervous activation of norepinephrine and epinephrine [21, 22, 23]. Therefore, different levels of hypercapnia lead to different degrees of vasomodulation [21]. For example, when subjected to high levels of hypercapnia, the SNS response will help in the binding of endothelin-1 to alpha-adrenergic receptors causing vasoconstriction in the kidney from baseline conditions [21, 22]. On the other hand, from a severe hypercapnic condition, a mild hypercapnic gas challenge results in increased levels of norepinephrine and epinephrine which releases NO and inhibits vasoconstriction, in turn, vasodilating the kidney [21]. The body s response to hypercapnic conditions is different for each organ. A study by Vantanajal et al. compared the effect of hypercapnia in terms of cerebral blood flow and femoral blood flow [24]. They obtained opposite results in the brain, where during mild hypercapnic conditions, vasoconstriction was mediated by alpha-adrenergic receptors and vasodilation was observed during severe hypercapnia [24]. These results reinforce the idea that the sympathetic nervous activity is depended to each organ [24]. In this case, the higher number of alpha-adrenergic receptors in the skeletal muscles causes vasoconstriction to redirect the blood to vital organ [24]. The ability to control vasomodulation using gas challenges has shown to be accurate and non-invasive compared to the administration of drugs [24] Skeletal Muscle and Kidney Responses Skeletal muscles respond differently to high levels of CO2 and low levels of O2, depending on their location in the body relative to the kidneys [4], and their metabolic activity [25]. In fact, many other factors such as hormonal, and neurogenic responses will determine how blood flow will be distributed in the body [26]. In addition, due to the substantial amount of ventilation 10
20 techniques as well as the fact that different levels of hypercapnia will affect organs differently, it makes it particularly difficult to truly assess vascular changes [27]. For example, hypercapnia has shown to increase blood flow in the brain [28] and previous studies observed decreased perfusion in the femoral artery, leading to the assumption that vasoconstriction is observed in the skeletal muscles [22]. On the contrary, later studies have shown that hypercapnia actually increased blood flow in both peripheral and cerebral circulations [24]. More importantly, perfusion in the cerebral circulation was observed to be eight times higher than perfusion femoral responses, based on pressure measurements [24]. This response is due to the fact that neurons require higher metabolic activity than muscles when homeostasis is altered [24]. Besides, this leads the body to divert blood flow to vital organs following responses from the sympathetic nervous system [24]. At last, each type of skeletal muscle has distinct vascular beds consisting of different endothelium and smooth muscle cells [24]. As a result, there are variations in vasodilatory mediated compounds such as nitric oxide (NO) and prostacyclin (PGI2) in turn, producing different responses to hypercapnia [29]. The kidneys, being highly vascularized as well as heavily being involved in filtering the blood, respond much faster to hypercapnia. Using T1 MRI measurements and a blood pool agent as guideline for microvascular volume, Ganesh et al. (2017) demonstrated that both mild and severe hypercapnia, 2% CO2 and 20% CO2 respectively, resulted in large increase in T1 [21]. Thus, lower volume of blood was observed accompanied by shrinkage of the kidneys [21]. Similarly, Joseph et al. reported that upon the administration of 30% CO2 to a dog, renal blood flow fell to approximately 60% compared to control values even though a steady increase in heart rate and respiration were recorded [30]. In contrast, hypercapnia does not always cause an increase in heart rate, seen in patient with stable chronic obstructive pulmonary disease (COPD) [31]. Indeed, although renal blood flow is significantly reduced in patients with COPD, cardiac and humoral responses have shown to not directly mediate renal vasoconstriction suggesting that many other factors could be contributing [31]. 11
21 1.4.3 Techniques to Administer Hypercapnia Fixed inspired concentration is a widely accessible and inexpensive technique that does not use computerized methods to control levels of gases administered to the patient [32]. The gas mixture constituting of fixed concentrations, is placed in a gas reservoir and inspired using a face mask [32]. Two simple one-way valves, inspiratory valve and expiratory valve, are placed in a tube before the face mask to assure constant and accurate administration of the gas mixture [32]. The main drawback with this technique is that the anticipation of breathing rate during the gas challenge is not considered [32]. In fact, when the subject is exposed to higher levels of CO2, breathing rate increases, changing the end-tidal volume [32]. As a result, there is massive overshoot of CO 2 which consequently affects the accuracy of the administered gases [32]. Dynamic end-tidal forcing is a more advanced gas delivering system where computerized control administration of gas mixture is used [32, 33]. In contrast to the fixed inspired concentration method, this technique counteracts the overshoot of end-tidal volume by setting appropriate partial pressure of end-tidal volumes of O2 and CO2 using a computerized feedback process [33]. Therefore, a computer tracks levels of end-tidal gases inspired throughout an experiment and simultaneously calculates the partial pressures required to be administered, balancing the system [33]. The main drawback with this technique is the inability for clinical usage. This is due to its bulky, expensive, and complex technology being difficult to operate and reproduce, consequently limiting it to its only usage in research settings [34]. Prospective end-tidal targeting is like dynamic end-tidal forcing, a computerized gas delivery system where instead does not use continuous assessment of breathing patterns [35]. As an alternative, end-tidal volume of O2 and CO2 is analyzed after some time during the gas challenge and gases are administered accordingly [35]. This technique administers gas concentrations rapidly and has shown to be more accurate in assessing the changes in end-tidal CO2 [35]. 12
22 1.5 MRI MRI Signal The physics behind MRI was inspired by NMR where nuclei of subatomic particles generate spins [36]. In fact, only particles with odd numbers of neutrons or protons will generate this spin [36]. For example, carbon-13 an isotope of carbon-12, generates spin properties due to its odd number of neutrons (seven) and even number of protons (six) [36]. Compared to NMR, MRI takes the advantage of the abundance of water in the body to take information from the spins of the large amounts of isotopes of hydrogen, sodium, and phosphorous [36]. Scientists previously understood that the momenta of electrons and protons were described as angular momentum, meaning that they rotated about a linear plane unless they were affected by an external torque producing the angular motion about the origin [36]. It was however later proved by the Stern- Gerlash experiment, that particles are actually largely affected by their intrinsic properties of mass and charge resulting in their half-integral measurements of angular momentum called spin [37]. This phenomenon results in the protons having magnetic attributes which when subjected to an applied magnetic field B0, will align and said to precess in the direction of the field [36]. In fact, in tissues, every proton spin in random directions. When the B0-field is applied by the MRI, most protons will align to the field producing a net magnetization field parallel to B0. The frequency at which the protons precesse is called the Larmor frequency and is dependent on the applied magnetic field strength and the gyromagnetic ratio [36]. This frequency is important, because signal detection on MRI is dependent on the RF coils that are tuned to the Larmor frequency which forces the protons to lean toward the transverse plane following a different perpendicular magnetic field called B1-field [36]. The RF coils send radio frequency pulses (RF-pulse), tipping the protons to a specific degree about the B0 direction [36]. The protons then dissipate energy and return to their equilibrium state parallel to the B0-field [36]. The signal contrast is controlled using different echo times (TE) and repetition times (TR), which determine the time difference between excitation and MRI sampling, and successive excitations [36]. These imaging parameters, TE and TR, produce differential tissue contrast dependent on the native transverse relaxation time (T2) and longitudinal relaxation time (T1) [36]. 13
23 1.5.2 Transverse Relaxation Time (T2) Not all protons will precess at the Larmor frequency. Differences in precessional frequencies will arise as a result of intrinsic tissue properties, including water content and ultrastructure. In the presence of a broadening of precessional frequencies, phase incoherence develops and the net magnetization in the transverse plane will decay, creating a drop in signal intensity [36].The transverse relaxation time T2 is the time it takes for the transverse magnetization to decay to approximately 37% of the original equilibrium state in the transverse plane. [36]. In other words, when analyzing different tissues, the difference in echo time for the proton to become out of phase with each other will result in difference in T2-weighted signals [38]. Indeed, each proton s magnetic field will interact with each other resulting in dephasing of their spin [36]. That is, the longer the echo time, the greater the difference between tissues will be observed [38]. Additionally, as mentioned earlier, tissues have different distributions in the magnetic field which is also referred as magnetic field inhomogeneity. An inhomogeneity in magnetic field results in distortion of the original B0-field which leads to discrepancy in the net magnetization field exerted on each proton observed in a voxel [36]. The difference between the original magnetic field and the distorted magnetic field is called T2 [36]. At last, the sum of the rate of T2 and T2 results in the overall relaxation rate of T2 * which represents the interaction between each proton and the inhomogeneity in the magnetic field [39]. 14
24 Figure 5: A) Difference in echoe time (TE) results in variation in signal intensity for different tissue types, adapted from ref. [40], B) T 2 -weighted image of a rat showing the variation in signal intensities between the paraspinal muscles (dark), kidney (gray), and fat (bright). As described in figure 5 above, a longer TE allows observation of larger differences in signal intensity between tissues. Tissues with lower T2 relaxation times have faster exponential decay and darker signal intensities Longitudinal Relaxation Time (T1) After protons are subjected to an RF-pulse and protons align in the transverse plane, there is a loss of magnetization vector in the B0 direction [36]. The time it takes for the net magnetization field of protons to recover along the B0 direction (return to 63% of its equilibrium state) is represented as the T1 relaxation time [36]. Unlike the transverse relaxation time, longitudinal relaxation time involves the changes that occur at the Larmor frequency as well as only being present in the transverse plane of the B0-field [36]. 15
25 Figure 6: A) Difference in repetition time (TR) results in variation in signal intensity for different tissue types, adapted from ref. [40], B) T 1 -weighted image of a rat showing the variation in signal intensities between the paraspinal muscles (gray) and kidney (bright). As observed in figure 6, shorter TR allows greater T1 weighting as opposed to longer TR. Tissues with shorter T1 results in higher relaxation rate and higher signal intensities K-Space MRI is a very interesting imaging modality that encompasses theories from many fields. Image acquisition requires various signal processing techniques to convert frequencies produced by the magnetic fields which are situated in a spatial frame called K-space. K-space includes all the information present in the image sampled and is a result of multiple 2D or 3D Fourier transform computed in a time-varying magnetic field [41]. Each point in K-space relates to the signal intensities and spatial frequencies of multiple pixels in the final image where the kx-axis and kyaxis represent the frequency information in the x and y axis of the original image, respectively [41]. Thus, the final image quality will depend on the frequencies sampled in k-space which represents the borders, contours, and contrast characteristics of the MRI image [42]. Higher spatial frequencies and lower spatial frequencies are in the outer rows and inner rows, respectively [42]. Hence, when higher frequencies are removed using a low-pass filter for instance, there is a loss of information of the borders and contours of the image [42]. Alternatively, a high-pass filter will remove the low frequency component of the image resulting 16
26 in loss of overall contrast in the image and only edges of the images are observed [42]. Both effects in K-space are shown in figure 7 below. Figure 7: A) K-space and its final image representation, B) Low-Pass filter applied to K-space filtering high frequency components, C) High-pass frequency components applied to K-space filtering low frequency components [42] Contrast Agents Contrast agents in MRI are important to analyze specific aspects of a tissue. They must have high relaxivity, high stability, specific biodistribution, rapid clearance, low osmolality and viscosity, and finally low toxicity to be classified as efficient contrast agent [43]. A high relaxivity permits clinicians or researchers to use the contrast agent in lower quantities to lower T1 or T2, reducing overall toxicity [43]. A contrast agent that is unstable results in dissipation of its metal ions to other places in the body, losing control or sight of its location, in turn also increasing toxicity levels [43]. Thus, in the case of the most popular Gadolinium-based contrast agent, a chelate is used to modify its structure to block its binding ability with the highly abundant free cation in the blood, Ca2 + [43]. In term of biodistribution, there are currently three types; extracellular fluid (ECF) agents, blood pool (BP) agents, and targeted and organ specific agents [44]. ECF agents are distributed in the extracellular space and is also known as an extravascular contrast agent [44]. Over time, this type of agent will first be dispersed in the 17
27 intravascular space and then enter the interstitial space after being redistributed by the vascular system [44]. Table 2: Types of ECF Contrast Agents (Gadolinium-Based), Adapted from Ref. [44] BP agents are intravascular contrast agents, meaning that they do not cross the endothelial layer, thus remaining in the vascular system until renal excretion [44]. They are designed in three different categories: noncovalent binding of low-molecule Gd to human albumin, increased size of using liposomes or polymers, or nanoparticles [44]. Table 3: Types of BP Contrast Agents, Adapted from Ref. [44] The final biodistribution is the targeted and organ-specific contrast agent which collects in specific tissue types in high concentration increasing its signal intensity compared to surrounding tissues [44]. 18
28 Table 4: Types of Targeted or Organ-Specific Contrast Agent, Adapted from Ref. [44] A contrast with low osmolality and low viscosity will be more rapidly administered as one bolus and help in having more control over the toxicity levels administered [44]. At last a good contrast agent will rapidly be renally cleared by the body because prolonged stay in the body could result in increased toxicity levels or change in the agent structure [44]. Some contrast agents increase T1 properties of some tissue, in turn increasing the signal intensity [45]. For this reason, this type of contrast agent is also called a positive-contrast agent [45]. In fact, T1-weighted contrast agents are made of exogenous paramagnetic metal ions (e.g. Gadolinium-based contrast agents) that highly react with water protons [46]. On the other hand, some contrast agents increase T2 properties of some tissue, resulting in a decrease in signal intensity [45]. These types of agents are called negative-contrast agents because they lower the overall signal intensity of the affected tissues [45] MRI Artifacts The most common type of artifacts in MRI and the most difficult to correct is the motion artifact, producing ghosting and blurring in the image [47]. Its difficulty to correct is due to the fact that there exists a wide range of methods to account multiple imaging scenarios [47]. For instance, cardiac MRI will be subjected to breathing motion as demonstrated in figure 10, resulting in changes in magnitude and phases in k-space [48]. Compared to heart motion which has been somewhat corrected with ECG-gating, breathing motion is irregular throughout an imaging session and highly varies from person to person, resulting in this difficulty to obtain free-artifact 19
29 images of the heart [48]. The effect of motion artifacts on the final image will depend on the location at which the motion arose during encoding to k-space [49]. When the motion occurred during the encoding to the x-axis of K-space, ghosting of the image will be present in the frequency domain of the image [49]. Alternatively, if motion happens during the encoding to the y-axis of K-space, ghosting will be present in the phase domain of the image [49]. At last, blurring occurs when motion happens during the encoding of the center of K-space, affecting the whole image [49]. As mentioned previously, there exist multiple methods to correct motion artifacts. The most common for cardiac MRI for instance are breath-holding and, respiratory and ECG-gating [49]. Other methods such as faster image acquisitions and adjustments of MRI parameters are also used to reduce blurring for more general body images [49]. Figure 8: Motion artifact on cardiac MRI resulting in blurring and ghosting Since MRI acquisitions use Fourier transform notions to process the image, the Nyquist theorem is of major importance. It states that any continuous-time signals must be sampled at twice its maximal frequency to avoid oversampling or undersampling. Failing to sample appropriately, results in distortion or aliasing of the signal. Hence in MRI, when a signal is not sampled at a correct sample rate, the field-of-view (FOV) is observed to be smaller than the actual size of the body part being imaged, resulting in the excess parts of the image being wrapped-around in the final image [49]. This type of artifact is called aliasing artifact and most of the time happens when reducing the FOV to increase spatial resolution [49]. Current techniques to avoid this type of artifacts involves GRAPPA or SENSE reconstruction methods which adds the missing lines in K-space as a result from undersampled data [49]. 20
30 Chapter 2 Research Hypothesis and Theory Section 2.3 of this chapter is presented in Zakher E et al. Magn Reson Med 2018 (submitted) 2.1 Introduction to Model The mathematical model described next assesses endothelial dysfunction in low perfusion tissue. It was originally developed to analyze the induced microvascular injury in the leg muscle of a rat model. In fact, this technique involved the use of MRI and a series of T 1 -Weighted sequences to track the vascular response of both, control and injured leg over time. The simple T1-relaxation times resulted in clear vascular responses in the kidney, however, not in leg muscles. Hence, the first partial derivative of T1 was calculated to observe small changes that are normally hard to visualise in the microvascular system. Following the definition of the derivative for the explanation of what T 1 illustrates; the derivative of T 1 represents the sensitivity of T1 at small changes in time, allowing distinction of vasomodulation when not visible in regular T1 measurements. 2.2 Hypothesis and Aims The original hypothesis was that when a decrease in ΔT1 was observed, vasoconstriction of the microvascular bed occurred. On the other hand, when an increase in ΔT1 was observed, it resulted in vasodilatory effects. Careful analysis of the derivative however led to new hypothesis, resulting in analyzing the overall vasomodulation response with respect to negative and positive values of ΔT1. Thus, this involves understanding the system behaving as a whole including perfusion responses and blood volume in the circulation as an outcome of vascular changes. The aims of the research are: 1. Creating the model with mathematical proof using MRI theory 2. Assessing true vasomodulation using gas challenge and apply the mathematical model 3. Validating the model using a common perfusion technique 21
31 2.3 Mathematical Proof The intravenous administration of a T1 contrast agent is used to impart higher signal intensity to tissue; this is achieved through the reduction of T1 relaxation times of water protons in close proximity to the agent. Tissues that are more richly vascularized will undergo relatively greater contrast enhancement. If the contrast agent remains intravascular, essentially only the blood-pool water protons will experience enhanced relaxation effects. In this scenario, any signal changes in a voxel can be assumed to arise primarily from changes in the vascular volume fraction in that voxel, because this is the only space where the contrast agent resides. In other words, if the vascular volume increases through vasodilation or decreases through vasoconstriction, the T1 relaxation effect will increase or decrease, respectively the T1 relaxation time is effectively dominated by the blood volume fraction. To appreciate how the blood volume fraction in a voxel is related to the overall T1, let us revisit Equation (1), which states that the longitudinal relaxation rate (1/T1) is proportional to the concentration of contrast agent in a homogeneous system: 1 = 1 + r T 1 T 1 C (1) 10 where 1/T 10 is the relaxation rate in the absence of contrast, C is the time-dependent concentration of the contrast agent, and r 1 is a constant for a given contrast agent known as the relaxivity. Since the contrast agent is confined to the vascular space, its concentration in an imaging voxel is simply the contrast concentration in blood (C b ) weighted by the fractional blood volume (v b ). We can rewrite Equation (1) as follows: 1 = 1 + r T 1 T 1 C b v b (2) 10 The evolution of T1 with time will be influenced by renal excretion of the contrast agent and any vasomodulation (i.e. changes in v b ) that may be present. To understand this temporal evolution, we take the partial derivative of Equation (2) with respect to time: 22
32 ( 1 ) = ( 1 + r T 1 T 1 C b v b ) (3) 10 We solve Equation (3) by taking the quotient rule on the left hand side and the product rule on the right hand side. Since both C b and v b are time-dependent, the result is: T 1 = rt 1 2 ( C b v b C b v b ) (4) Assuming that the renal elimination rate is a constant K = C b following final equation to analyze vasomodulation in low perfusion tissue: in Equation (4), we arrive at the T 1 = rt 1 2 (Kv b C b v b ) (5) Equation (5) enables us to understand what the partial derivative T 1 signifies physiologically. Table 5 describes the main scenarios that may arise in vivo. We consider each scenario in the following. Table 5: Characteristics of the Partial Derivative T 1 in Relation to Vasomodulation Scenario Sign T 1 Temporal characteristics Vasomodulation 1. K = 0, v b < 0 Positive N/A Vasoconstriction 2. K = 0, v b > 0 Negative N/A Vasodilation 3. K = constant, v b = 0 Positive N/A None 4. K = constant, v b > 0 Negative Trends towards an increase Vasodilation 5. K = constant, v b < 0 Positive Trends towards a decrease Vasoconstriction Scenarios 1 and 2 represent the simplest case where the renal elimination rate constant K is zero. Although this situation never occurs in practice, it represents the extreme situation where the contrast agent does not leave the body and the blood concentration remains constant. Under these 23
33 ideal circumstances, positive and negative T 1 respectively, and the magnitude of T 1 values signify vasoconstriction and vasodilation, describes how fast vasomodulation occurs. Scenarios 3 to 5 represent the more realistic situation where renal elimination is present. In the absence of vasomodulation (Scenario 3), T 1 takes on a positive value, and we do not expect this value to vary with time assuming the elimination rate is approximately constant. The partial derivative T 1 takes on a negative value (Scenario 4) if only if vasodilation occurs so rapidly that the second term in Equation (5) dominates the first term. More physiologically relevant, however, is gradual vasodilation, since vessel relaxation is not instantaneous. Here, we should observe an initial decrease in the value of T 1 followed by an increase. The initial decrease arises from a positive v b (i.e. active vasodilation) that reduces the effect of the first term in Equation (5), whereas the subsequent increase in T 1 arises from a dilated blood volume fraction (i.e. a higher v b in the first term now dominates, especially as vasodilation slows down or ceases). Scenario 5 describes vasoconstriction. Since v b is negative, the value of T 1 can only be positive. As the blood volume fraction decreases, the first term in Equation (5) decreases, in opposition to the increase exerted by a negative v b in the second term. The direction in which T 1 changes over this interval depends on the magnitude of the two opposing forces. However, once vasoconstriction has set in and blood vessels are fully constricted, the first term with a reduced v b dominates and there will be net decrease in the value of T 1 compared to baseline. 24
34 Chapter 3 Experiment Protocols and Analysis This chapter is presented wholly in Zakher E et al. Magn Reson Med 2018 (submitted) 3.1 Experiments Gas Challenge Protocol This study was approved by our institutional animal care committee (protocol #36668). All procedures were conducted in accordance with the Canadian Council on Animal Care. Male adult Sprague Dawley rats (N = 6) (Charles River Laboratories), 6 weeks old and weighing g, were used. Rats were anesthetized on 3% isoflurane (Forene, Abbott Labs, Baar, Switzerland) in room air (2 L/min flow rate) prior to intubation with a 14-gauge angiocatheter. A controlled gas mixing circuit described previously [21] was used to deliver graded levels of CO2 and O2. The gas blender consisted of a computer-controlled GSM-3 mixer (CWE Inc., Ardmore, PA, USA) and blended O2, CO2, and N2 at a constant total flow of 2 L/min. The output gas mixture was fed first into an isoflurane vaporizer at a flow of 2 L/min and then into an MRI compatible ventilator (MRI-1 Ventilator; CWE, Ardmore, PA, USA). Mechanical ventilation was employed to allow a controlled rate and depth of breathing. The endotracheal tube was connected to a flexible tubing from the gas delivery system. Ventilation was maintained at 70 breaths per minute. Vital signs (heart rate, blood oxygen saturation) of the rat were monitored and recorded using a rodent oximeter and physiological monitor (MouseOX Plus; STARR Life Sciences) mounted on the hind paw. These physiological measurements were taken in real-time to ensure we could monitor the animal s vital signs dynamically. A 27G angiocath was inserted into the lateral tail vein and connected to a 3-way stopcock for contrast injection during imaging. During MRI, animals were administered normoxia (21% O2 room air) to establish baseline. They were then subjected to severe hypercapnia (20% CO2, 21% O2, N2-balanced) followed by mild hypercapnia (5% CO2, 21% O2, N2-balanced). 25
35 Figure 9: Schematic of the Gas Protocol MRI Protocol Imaging was performed on a 3-Tesla clinical scanner (Achieva 3.0T TX, Philips Medical Systems, best, The Netherlands), with the animal placed prone on a water blanket (HTP-1500, Adroit Medical Systems, Loudon, TN) set at 38 C to maintain core body temperature. An 8-channel wrist coil was used for signal detection. Coronal imaging slices were positioned to include the kidneys and the leg skeletal muscle. Animals were subjected to a 20-minute baseline period of normoxia, and T1-mapping was performed following the method of Cheng and Wright [50] using flip angles (FA) of 2, 10, 25, and 35. Gadofosveset (0.3 mmol/kg) was administered via tail vein injection after T1-mapping was completed. A series of three-dimensional T1-weighted fast field echo sequences were then acquired every two minutes with the following sequence parameters: repetition time = 6.11 ms, echo time = 3.23 ms, FA = 20, number of signal averaging = 2, field of view = 120 mm, thirteen 2-mm thick slices, and mm in-plane resolution. A 10-minute interval of imaging was performed on normoxia after Gadofosveset injection before applying 20% CO2 followed by 5% CO2. 26
36 3.1.3 Optronix Protocol To validate observations on MRI, real-time laser Doppler perfusion measurements were taken using the OxyFlo (Oxford Optronix Ltd, Oxford, UK) fiber-optic system. Measurements were in blood perfusion units (BPU), which is a relative quantity of volumetric flow rate. A fiber-optic probe was placed in the leg muscle between the medial and lateral heads of the gastrocnemius Data Analysis All data analysis was performed using in-house software developed in Matlab (v.8.3) (Mathworks, Natick, MA). Region of interests (ROI) were outlined in the leg muscle and kidney cortex to encompass the tissue at their largest cross-section. To study microvascular response to gas challenge, T1 relaxation times were calculated from the post-contrast signal intensity and precontrast T1 via the spoiled gradient echo signal equation. The mean T1 relaxation time within the ROIs was computed at all time-points 2 minutes apart across different gas challenge episodes. The partial derivative of T1 with respect to time was then calculated at all time-points throughout different gas challenges. 3.2 Results and Analysis Results Figure 10 illustrates the difficulty of discerning vasomodulation in the microvascular bed of tissue with low perfusion such as skeletal muscle. Whereas the highly perfused kidney exhibits large changes in signal intensity on T1-weighted images as a result of gas-induced vasomodulation, the magnitude of signal changes in skeletal muscle is much smaller. Quantitative analysis of T1 relaxation times did not yield a marked improvement in sensitivity to vasomodulation in muscle. 27
37 Figure 10: Inadequate sensitivity of T 1 measurements to assess vasomodulation in low perfusion rat skeletal muscle. The highly perfused kidney cortex (red) exhibits large changes in T 1 in response to hypercapnia, whereas changes in skeletal muscle is very modest (blue) (A). The small degree of change in muscle can be similarly appreciated on T1- weighted images (B) compared to visible signal changes in the kidney (C) The proposed analysis of the partial derivative of T1 dramatically increased the sensitivity to vasomodulation in low perfusion tissues (figure 11). Results in the kidney are also shown to provide a reference for interpreting the evolution of T 1, as renal response to gas challenges is well understood and significant vasoconstriction and vasodilation have been consistently observed in response to 20% CO2 followed by 5% CO2 [21]. This response in the kidney is consistent with our mathematical model, where trends in T 1 toward a decrease or increase represent vasoconstriction and vasodilation, respectively. The trends observed in the leg skeletal muscle suggest immediate vasoconstriction to 20% CO2 followed by vasodilation, and continued vasodilation on 5% CO2. 28
38 Figure 11: Improved sensitivity to vasomodulation in low perfusion setting via the parameter T 1 Vasoconstriction in response to 20% CO2 and vasodilation in response to 5% CO2 are clearly delineated in the leg skeletal muscle of a representative rat using the proposed metric, the partial derivative T 1 Vasomodulation results on MRI for all animals are shown in figure 12. There was substantial subject-to-subject variation in the responses to hypercapnia, which was due in part to individual variability in heart rate elevation. However, the overall trends in T 1 changes remained consistent, and leg muscle response closely followed the renal vasomodulatory patterns in any individual animal. 29
39 Figure 12: The ability to tease out vasoconstriction and vasodilation in skeletal muscle using the parameter T 1 is illustrated across all animals. A similar color is used in the kidney and leg muscle graphs to indicate data from the same animal. Figure 13 summarizes across all animals the percentage changes in T 1 and in heart rate. In leg muscle, there was an overall decrease in T 1 in response to 20% CO 2 and an overall increase upon transitioning to 5% CO2. At the same time, the heart rate doubled during severe hypercapnia but returned to one-third of the maximum upon transition to milder CO2 levels. Figure 13:Average changes in T 1 and heart rate in response to severe and mild hypercapnia. (A) The maximum vasoconstriction in muscle induced by 20% CO2 as measured on ( T 1 )/ and the maximum vasodilation induced by 5% CO2 are averaged over all animal. (B) The average change in heart rate in response to 20% and to 5% CO 2 are also shown. 30
40 Figure 14 illustrates a laser Doppler perfusion recording in a representative animal. During the severe hypercapnia interval, perfusion in leg muscle is seen to decrease initially followed by a gradual increase, which continues upon transition to 5% CO 2. Figure 14: Optical perfusion validation. Laser Doppler perfusion measurements over the course of hypercapnic challenge intervals are shown for a representative rat. Blood perfusion units (BPU) are relative quantities and do not indicate absolute blood flow. 31
41 Chapter 4 General Discussion, Future Work and Conclusion Section of this chapter is presented in Zakher E. et al. Magn Reson Med 2018 (submitted) 4.1 Summary of Findings Discussion The skeletal muscle microvasculature is involved in numerous pathologies, including ischemia and metabolic syndromes such as diabetes. A technology that opens a window on this system would be invaluable for the early detection of various diseases. However, there is currently no technology available to probe the microvasculature non-invasively in deep, low perfused tissue. Specifically, there is no technology to measure vasomodulatory capacity, which is an important index of vascular health and reflects the ability of microvessels to adjust blood flow in response to changing metabolic demands. This paper describes a new analytical method that allows sensitive assessment of microvascular vasomodulation in low perfusion tissue. It was shown that the method was sensitive to both vasoconstriction and vasodilation in the leg skeletal muscle, and MRI results were corroborated by invasive perfusion measurements on laser Doppler. When interpreting the temporal evolution of the new metric T 1, one must bear in mind that two discrete influences determine the value of T 1 : the absolute microvascular blood volume at any point in time and the active vasomodulation at that instant in time. MRI results in the leg muscle across all animals revealed a consistent initial decrease in T 1 32 immediately after exposure to 20% CO2 this decrease is due primarily to an overall reduction in blood volume from vasoconstriction. Halfway through this hypercapnic interval, T 1 gradually increases, which we interpret as vasodilation; optical perfusion measurements confirmed a return to increased perfusion. The most likely explanation for this reversal phenomenon is the expansion of blood vessels due to higher pressure from a greatly elevated heart rate. On transition to 5% CO2, T 1 the vessels continue to dilate to accommodate higher perfusion. continues increasing as
42 In the kidney, vasoconstriction was also observed during 20% CO2 followed by vasodilation during 5% CO2. However, the evolution of T 1 exhibited a few notable differences. First, upon application of 20% CO2, several animals demonstrated an initial hump in T 1. This is most likely a result of active vasoconstriction (second term in Equation (5)) dominating the influence of a total lower blood volume (first term in Equation (5)). Similarly, during the initial interval after the transition to 5% CO2, the values of T 1 were negative, which implies active vasodilation to the extent that, again, the second term in Equation (5) dominates. Another interesting observation is that as renal vasodilation continues during the milder hypercapnic interval, a point is reached where blood is diverted from skeletal muscle downstream from the kidney this is reflected in the downturn of the T 1 graph in leg muscle in Figure 15 and is known as the steal effect. It is worth noting that a truly equivalent validation method does not exist. In this study, we used optical Doppler perfusion measurements as the best alternative, but like most clinical techniques for assessment of vasomodulation, optical Doppler also evaluates volumetric blood flow and not changes in vessel tone. An increase in perfusion measured on optical Doppler can simply result from a higher heart rate without necessarily changes in vessel diameter. However, when we take in account heart rate variations in our interpretation of Doppler perfusion measurements, we can form a more accurate depiction of vasomodulatory responses. For example, during 20% CO2, Doppler measurements indicated decreased perfusion at the same time that heart rate was greatly elevated. In the presence of greater perfusion pressure, decreased perfusion can only arise as a result of vasoconstriction. Our model is a first-order mathematical approximation of the effect of vasomodulation on contrastinduced T1 effects. It assumes that renal elimination of the contrast agent is constant, but this may be true only over a short time interval and not over the entire duration of the imaging experiment. Future work will model more accurately this elimination profile. The model also is not sensitive to second-order effects that may distinguish more clearly rapid versus slow vasomodulation. In its present form, our model can clearly separate vasodilation from vasoconstriction but cannot ascribe the change to hormonal or neuronal factors [51, 52] on the basis of the timing of vasomodulation. Further efforts are required to develop a more complex model that encompasses these elements. 33
43 Another noteworthy fact is that there exists substantial individual variation in response to hypercapnia. A marathon runner, for example, will likely have greater vasodilatory capacity in the leg skeletal muscle compared to a healthy non-athlete. The magnitude of response will, therefore, differ amongst individuals. This is the reason why it is less useful to average the responses across all animals and more useful to present the responses individually as we have done. The ultimate utility of the proposed analytical method will rest on its ability to identify diseased or injured microvessels on the basis of their vasomodulatory capacity. There are a number of models to investigate, including ischemic insult, hypertension, and obesity. In ischemic injury where the endothelium is affected, we can expect a reduced capacity to adjust vessel tone compared to healthy microvessels. A recent report by Ganesh et al [4] demonstrated that it is possible to distinguish injured ischemic skeletal muscle on the basis of a lower vasomodulatory capacity as measured by the proposed analysis. Future work will focus on a range of other microvascular pathologies to validate the general utility of the new method in skeletal muscular disease, cerebrovascular disease, and other pathologies in a low perfusion setting Challenges This research did come with its fair share of challenges. Initially, measurements were taken in the upper paraspinal and lower paraspinal muscle, and arm muscle (bicep). The idea behind this was to look at the difference in vasomodulation and perfusion between muscles upstream and downstream of the kidneys. Even though the data seemed to be following similar trends with the upstream muscles (arm and upper paraspinal) and downstream muscles (leg and lower paraspinal), their responses were deemed inaccurate because of the difficulty in taking clear images of both, the paraspinal muscle and the arm. Additionally, the analysis on the leg muscle and the kidneys were taken over multiple slices as opposed to the paraspinal where only one slice was usable. For the arm muscle, it was hard to make a clear ROI of the bicep without including bones or other tissues. Figure 18 below is a representation of the ROI taken for the arm, upper paraspinal, and lower paraspinal muscle. 34
44 Figure 15: ROI taken for the arm (left bicep) (A), upper paraspinal (B), and lower paraspinal muscles (C) The severe hypercapnic challenge used in this study is hard on the rats. In fact, three rats were lost, one during an MRI session, and 2 during perfusion measurements. For this reason, a hypoxic challenge (12% O2, Nitrogen-balanced) was used resulting in all the rats to survive. Data were however not as clear as the severe hypercapnic challenge resulting in lower magnitudes being observed. Figure 16: Partial derivative of T1 for the kidney where the magnitude for the hypoxic gas challenge (red) were smaller than the hypercapnic gas challenge (blue) 35
45 Finally, as previously discussed, validation methods are difficult to come by. Perfusion measurements do not clearly represent changes in vessel tone. Probe placement was also a tedious process as the smallest incision was required to minimize injury to the rat, resulting in the introduction of a lot of noise in the raw signal. Additionally, larger incision results in inflammation, which leads to ischemia and finally increase pressure resulting in loss of vasomodulation tone because of increased interstitial pressure. Figure 17: (A) Probe placement for the leg and arm muscles, (B) and paraspinal and kidney for the perfusion analysis during the gas challenge Limitations The main obvious limitation is the use of a severe hypercapnic challenge. Albeit the fact that it truly vasoconstricted the microcirculation of the leg muscle, it is uncertain if it was achieved by the normal vasomodulation mechanism of the vascular network. Therefore, in a disease model, the developed technique cannot assess microvascular endothelial dysfunction with accuracy yet. In fact, as discussed earlier, many other factors could play a major role in vessel tone when subjected to severe conditions. For instance, during increased levels of hypercapnic conditions, the CO2 will diffuse from the blood stream to brain tissues, resulting in an increase in acidosis 36
MR Advance Techniques. Vascular Imaging. Class II
 MR Advance Techniques Vascular Imaging Class II 1 Vascular Imaging There are several methods that can be used to evaluate the cardiovascular systems with the use of MRI. MRI will aloud to evaluate morphology
MR Advance Techniques Vascular Imaging Class II 1 Vascular Imaging There are several methods that can be used to evaluate the cardiovascular systems with the use of MRI. MRI will aloud to evaluate morphology
Cardiovascular Physiology
 Cardiovascular Physiology Lecture 1 objectives Explain the basic anatomy of the heart and its arrangement into 4 chambers. Appreciate that blood flows in series through the systemic and pulmonary circulations.
Cardiovascular Physiology Lecture 1 objectives Explain the basic anatomy of the heart and its arrangement into 4 chambers. Appreciate that blood flows in series through the systemic and pulmonary circulations.
Chapter 14 Blood Vessels, Blood Flow and Pressure Exam Study Questions
 Chapter 14 Blood Vessels, Blood Flow and Pressure Exam Study Questions 14.1 Physical Law Governing Blood Flow and Blood Pressure 1. How do you calculate flow rate? 2. What is the driving force of blood
Chapter 14 Blood Vessels, Blood Flow and Pressure Exam Study Questions 14.1 Physical Law Governing Blood Flow and Blood Pressure 1. How do you calculate flow rate? 2. What is the driving force of blood
Physiology of Circulation
 Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1
 BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
BIPN100 F15 Human Physiol I (Kristan) Lecture 14 Cardiovascular control mechanisms p. 1 Terms you should understand: hemorrhage, intrinsic and extrinsic mechanisms, anoxia, myocardial contractility, residual
Structure and organization of blood vessels
 The cardiovascular system Structure of the heart The cardiac cycle Structure and organization of blood vessels What is the cardiovascular system? The heart is a double pump heart arteries arterioles veins
The cardiovascular system Structure of the heart The cardiac cycle Structure and organization of blood vessels What is the cardiovascular system? The heart is a double pump heart arteries arterioles veins
Chapter 9. Body Fluid Compartments. Body Fluid Compartments. Blood Volume. Blood Volume. Viscosity. Circulatory Adaptations to Exercise Part 4
 Body Fluid Compartments Chapter 9 Circulatory Adaptations to Exercise Part 4 Total body fluids (40 L) Intracellular fluid (ICF) 25 L Fluid of each cell (75 trillion) Constituents inside cell vary Extracellular
Body Fluid Compartments Chapter 9 Circulatory Adaptations to Exercise Part 4 Total body fluids (40 L) Intracellular fluid (ICF) 25 L Fluid of each cell (75 trillion) Constituents inside cell vary Extracellular
Syllabus References. Resources. Video: MRI Introduction
 MRI Lesson Outline Syllabus References 9.6.4.2.5 Define precessing and relate the frequency of the precessing to the composition of the nuclei and the strength of the applied external magnetic field 9.6.4.2.6
MRI Lesson Outline Syllabus References 9.6.4.2.5 Define precessing and relate the frequency of the precessing to the composition of the nuclei and the strength of the applied external magnetic field 9.6.4.2.6
PHYSIOLOGY MeQ'S (Morgan) All the following statements related to blood volume are correct except for: 5 A. Blood volume is about 5 litres. B.
 PHYSIOLOGY MeQ'S (Morgan) Chapter 5 All the following statements related to capillary Starling's forces are correct except for: 1 A. Hydrostatic pressure at arterial end is greater than at venous end.
PHYSIOLOGY MeQ'S (Morgan) Chapter 5 All the following statements related to capillary Starling's forces are correct except for: 1 A. Hydrostatic pressure at arterial end is greater than at venous end.
Basics of MRI Part I
 Basics of MRI Part I Mathew J. Dixon, D.O. Chairman Department of Radiology Memorial Health University Medical Center Savannah, GA Objectives Brief History Concept of MRI Creation of a Magnetic Field Concepts
Basics of MRI Part I Mathew J. Dixon, D.O. Chairman Department of Radiology Memorial Health University Medical Center Savannah, GA Objectives Brief History Concept of MRI Creation of a Magnetic Field Concepts
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
d) Cardiovascular System Higher Human Biology
 d) Cardiovascular System Higher Human Biology What can your remember about the heart and blood vessels? What is the Cardiovascular System? The cardiovascular system, also known as the circulatory system,
d) Cardiovascular System Higher Human Biology What can your remember about the heart and blood vessels? What is the Cardiovascular System? The cardiovascular system, also known as the circulatory system,
Chapter 12. Capillaries. Circulation. The circulatory system connects with all body tissues
 Chapter 12 Circulation The circulatory system connects with all body s In many animals, microscopic blood vessels called capillaries Form an intricate network among the Red blood cell song Figure 23.1A
Chapter 12 Circulation The circulatory system connects with all body s In many animals, microscopic blood vessels called capillaries Form an intricate network among the Red blood cell song Figure 23.1A
Cardiovascular system: Blood vessels, blood flow. Latha Rajendra Kumar, MD
 Cardiovascular system: Blood vessels, blood flow Latha Rajendra Kumar, MD Outline 1- Physical laws governing blood flow and blood pressure 2- Overview of vasculature 3- Arteries 4. Capillaries and venules
Cardiovascular system: Blood vessels, blood flow Latha Rajendra Kumar, MD Outline 1- Physical laws governing blood flow and blood pressure 2- Overview of vasculature 3- Arteries 4. Capillaries and venules
UNIT 4: BLOOD VESSELS
 UNIT 4: BLOOD VESSELS Dr. Moattar Raza Rizvi NRS237, Physiology Generalized Structure of Blood Vessels 1 Tunica interna (tunica intima) Endothelial layer that lines the lumen of all vessels In vessels
UNIT 4: BLOOD VESSELS Dr. Moattar Raza Rizvi NRS237, Physiology Generalized Structure of Blood Vessels 1 Tunica interna (tunica intima) Endothelial layer that lines the lumen of all vessels In vessels
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
P215 SPRING 2019: CIRCULATORY SYSTEM Chaps 13, 14 & 15: pp , , , I. Major Functions of the Circulatory System
 P215 SPRING 2019: CIRCULATORY SYSTEM Chaps 13, 14 & 15: pp 360-390, 395-404, 410-428 433-438, 441-445 I. Major Functions of the Circulatory System 1. 2. 3. 4. II. Structure of the Heart 1. atria 2. ventricles
P215 SPRING 2019: CIRCULATORY SYSTEM Chaps 13, 14 & 15: pp 360-390, 395-404, 410-428 433-438, 441-445 I. Major Functions of the Circulatory System 1. 2. 3. 4. II. Structure of the Heart 1. atria 2. ventricles
Control of blood tissue blood flow. Faisal I. Mohammed, MD,PhD
 Control of blood tissue blood flow Faisal I. Mohammed, MD,PhD 1 Objectives List factors that affect tissue blood flow. Describe the vasodilator and oxygen demand theories. Point out the mechanisms of autoregulation.
Control of blood tissue blood flow Faisal I. Mohammed, MD,PhD 1 Objectives List factors that affect tissue blood flow. Describe the vasodilator and oxygen demand theories. Point out the mechanisms of autoregulation.
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
THE DIFINITIVE GUIDE TO HUMAN ANATOMY & PHYSIOLOGY (HAP 2).
 THE DIFINITIVE GUIDE TO HUMAN ANATOMY & PHYSIOLOGY (HAP 2). Pages 2-49 Lecture 1 notes: Cardiovascular 1. Pages 50-97 Lecture 2 notes: Cardiovascular 2. Pages 98-128 Lecture 3 notes: Respiratory 1. Pages
THE DIFINITIVE GUIDE TO HUMAN ANATOMY & PHYSIOLOGY (HAP 2). Pages 2-49 Lecture 1 notes: Cardiovascular 1. Pages 50-97 Lecture 2 notes: Cardiovascular 2. Pages 98-128 Lecture 3 notes: Respiratory 1. Pages
Previous talks. Clinical applications for spiral flow imaging. Clinical applications. Clinical applications. Coronary flow: Motivation
 for spiral flow imaging Joao L. A. Carvalho Previous talks Non-Cartesian reconstruction (2005) Spiral FVE (Spring 2006) Aortic flow Carotid flow Accelerated spiral FVE (Fall 2006) 2007? Department of Electrical
for spiral flow imaging Joao L. A. Carvalho Previous talks Non-Cartesian reconstruction (2005) Spiral FVE (Spring 2006) Aortic flow Carotid flow Accelerated spiral FVE (Fall 2006) 2007? Department of Electrical
Extra notes for lab- 1 histology. Slide 1 : cross section in the elastic artery ( aortic arch, ascending aorta, descending aorta )
 Extra notes for lab- 1 histology Slide 1 : cross section in the elastic artery ( aortic arch, ascending aorta, descending aorta ) - twin of ascending aorta is the pulmonary trunk. Ascending aorta represents
Extra notes for lab- 1 histology Slide 1 : cross section in the elastic artery ( aortic arch, ascending aorta, descending aorta ) - twin of ascending aorta is the pulmonary trunk. Ascending aorta represents
Special circulations, Coronary, Pulmonary. Faisal I. Mohammed, MD,PhD
 Special circulations, Coronary, Pulmonary Faisal I. Mohammed, MD,PhD 1 Objectives Describe the control of blood flow to different circulations (Skeletal muscles, pulmonary and coronary) Point out special
Special circulations, Coronary, Pulmonary Faisal I. Mohammed, MD,PhD 1 Objectives Describe the control of blood flow to different circulations (Skeletal muscles, pulmonary and coronary) Point out special
Ch. 12 The Circulatory System. The heart. The heart is a double pump. A quick note on arteries vs. veins. = the muscular pump of the CV system
 Ch. 12 The Circulatory System The heart A.k.a. the cardiovascular system Blood was discussed in Ch. 11 Focus of Ch. 12: heart and blood vessels = the muscular pump of the CV system ~ 100,000 heartbeats/day!
Ch. 12 The Circulatory System The heart A.k.a. the cardiovascular system Blood was discussed in Ch. 11 Focus of Ch. 12: heart and blood vessels = the muscular pump of the CV system ~ 100,000 heartbeats/day!
The Circulatory System. Lesson Overview. Lesson Overview The Circulatory System
 33.1 THINK ABOUT IT More than one-third of the 1.2 million Americans who suffer a heart attack each year die. This grim evidence shows that the heart and the circulatory system it powers are vital to life.
33.1 THINK ABOUT IT More than one-third of the 1.2 million Americans who suffer a heart attack each year die. This grim evidence shows that the heart and the circulatory system it powers are vital to life.
Collin County Community College
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 6 Blood Vessels 1 Anatomy of Blood Vessels Walls of blood vessels contain 3 distinct layers : Tunica intima innermost layer includes
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 6 Blood Vessels 1 Anatomy of Blood Vessels Walls of blood vessels contain 3 distinct layers : Tunica intima innermost layer includes
Biology. A Guide to the Natural World. Chapter 30 Lecture Outline Transport and Exchange 1: Blood and Breath. Fifth Edition.
 Biology A Guide to the Natural World Chapter 30 Lecture Outline Transport and Exchange 1: Blood and Breath Fifth Edition David Krogh 30.1 The Cardiovascular System The Cardiovascular System The human cardiovascular
Biology A Guide to the Natural World Chapter 30 Lecture Outline Transport and Exchange 1: Blood and Breath Fifth Edition David Krogh 30.1 The Cardiovascular System The Cardiovascular System The human cardiovascular
Cardiovascular System. Blood Vessel anatomy Physiology & regulation
 Cardiovascular System Blood Vessel anatomy Physiology & regulation Path of blood flow Aorta Arteries Arterioles Capillaries Venules Veins Vena cava Vessel anatomy: 3 layers Tunica externa (adventitia):
Cardiovascular System Blood Vessel anatomy Physiology & regulation Path of blood flow Aorta Arteries Arterioles Capillaries Venules Veins Vena cava Vessel anatomy: 3 layers Tunica externa (adventitia):
Human Anatomy and Physiology - Problem Drill 09: The Muscular System
 Human Anatomy and Physiology - Problem Drill 09: The Muscular System Question No. 1 of 10 The muscular system of the human body fulfills many different roles. Which of the following statements about the
Human Anatomy and Physiology - Problem Drill 09: The Muscular System Question No. 1 of 10 The muscular system of the human body fulfills many different roles. Which of the following statements about the
Levels of Organization. Chapter 19 6/11/2012. Homeostasis & Organization of the animal body. 4 Primary Tissues
 Levels of Organization Chapter 19 Homeostasis & Organization of the animal body Chemical Cellular Tissue Organs System Level Organismic 1-2 4 Primary Tissues 1. Epithelial Tissue: covers surfaces lines
Levels of Organization Chapter 19 Homeostasis & Organization of the animal body Chemical Cellular Tissue Organs System Level Organismic 1-2 4 Primary Tissues 1. Epithelial Tissue: covers surfaces lines
1Pulse sequences for non CE MRA
 MRI: Principles and Applications, Friday, 8.30 9.20 am Pulse sequences for non CE MRA S. I. Gonçalves, PhD Radiology Department University Hospital Coimbra Autumn Semester, 2011 1 Magnetic resonance angiography
MRI: Principles and Applications, Friday, 8.30 9.20 am Pulse sequences for non CE MRA S. I. Gonçalves, PhD Radiology Department University Hospital Coimbra Autumn Semester, 2011 1 Magnetic resonance angiography
CIE Biology A-level Topic 14: Homeostasis
 CIE Biology A-level Topic 14: Homeostasis Notes Communication is essential for the survival of organism as all living organisms must be able to detect and respond to changes in both their internal and
CIE Biology A-level Topic 14: Homeostasis Notes Communication is essential for the survival of organism as all living organisms must be able to detect and respond to changes in both their internal and
Blood Flow, Blood Pressure, Cardiac Output. Blood Vessels
 Blood Flow, Blood Pressure, Cardiac Output Blood Vessels Blood Vessels Made of smooth muscle, elastic and fibrous connective tissue Cells are not electrically coupled Blood Vessels Arteries arterioles
Blood Flow, Blood Pressure, Cardiac Output Blood Vessels Blood Vessels Made of smooth muscle, elastic and fibrous connective tissue Cells are not electrically coupled Blood Vessels Arteries arterioles
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D.
 Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Control of blood tissue blood flow. Faisal I. Mohammed, MD,PhD
 Control of blood tissue blood flow Faisal I. Mohammed, MD,PhD 1 Objectives List factors that affect tissue blood flow. Describe the vasodilator and oxygen demand theories. Point out the mechanisms of autoregulation.
Control of blood tissue blood flow Faisal I. Mohammed, MD,PhD 1 Objectives List factors that affect tissue blood flow. Describe the vasodilator and oxygen demand theories. Point out the mechanisms of autoregulation.
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
Topic 6: Human Physiology
 Topic 6: Human Physiology 6.2 The Blood System D.4 The Heart Essential Questions: 6.2 The blood system continuously transports substances to cells and simultaneously collects waste products. D.3 The chemical
Topic 6: Human Physiology 6.2 The Blood System D.4 The Heart Essential Questions: 6.2 The blood system continuously transports substances to cells and simultaneously collects waste products. D.3 The chemical
Microcirculation. Lecture Block 11 (contributions from Brett Burton)
 Lecture Block 11 (contributions from Brett Burton) Elements of Arterioles, capillaries, venules Structure and function: transport Fluid balance Lymph system Vessels of the Circulatory System Diameter Aorta
Lecture Block 11 (contributions from Brett Burton) Elements of Arterioles, capillaries, venules Structure and function: transport Fluid balance Lymph system Vessels of the Circulatory System Diameter Aorta
CIE Biology GCSE. 9: Transport in animals. Notes.
 CIE Biology GCSE 9: Transport in animals Notes The circulatory system acts as the main transport system in animals. It is made up of blood vessels such as arteries, veins and capillaries, in which blood
CIE Biology GCSE 9: Transport in animals Notes The circulatory system acts as the main transport system in animals. It is made up of blood vessels such as arteries, veins and capillaries, in which blood
Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins
 Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away
Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM
 Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM In Physiology Today Hemodynamics F = ΔP/R Blood flow (F) High to low pressure Rate = L/min Pressure (P) Hydrostatic pressure Pressure exerted
Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM In Physiology Today Hemodynamics F = ΔP/R Blood flow (F) High to low pressure Rate = L/min Pressure (P) Hydrostatic pressure Pressure exerted
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Any of these questions could be asked as open question or lab question, thus study them well
 Any of these questions could be asked as open question or lab question, thus study them well describe the factors which regulate cardiac output describe the sympathetic and parasympathetic control of heart
Any of these questions could be asked as open question or lab question, thus study them well describe the factors which regulate cardiac output describe the sympathetic and parasympathetic control of heart
Blood Vessels. Over view. We have about 60,000 miles of blood vessels!
 Blood Vessels Over view 3 types of blood vessels arteries - carry blood away from heart "branch", "diverge", and "fork" veins - carry blood toward heart "join", "merge", and "converge" capillaries - site
Blood Vessels Over view 3 types of blood vessels arteries - carry blood away from heart "branch", "diverge", and "fork" veins - carry blood toward heart "join", "merge", and "converge" capillaries - site
The Cardiovascular and Lymphatic Systems Cardiovascular System Blood Vessels Blood Vessels Arteries Arteries Arteries
 CH 12 The Cardiovascular and s The Cardiovascular and s OUTLINE: Cardiovascular System Blood Vessels Blood Pressure Cardiovascular System The cardiovascular system is composed of Blood vessels This system
CH 12 The Cardiovascular and s The Cardiovascular and s OUTLINE: Cardiovascular System Blood Vessels Blood Pressure Cardiovascular System The cardiovascular system is composed of Blood vessels This system
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Lab Period: Name: Physiology Chapter 14 Blood Flow and Blood Pressure, Plus Fun Review Study Guide
 Lab Period: Name: Physiology Chapter 14 Blood Flow and Blood Pressure, Plus Fun Review Study Guide Main Idea: The function of the circulatory system is to maintain adequate blood flow to all tissues. Clinical
Lab Period: Name: Physiology Chapter 14 Blood Flow and Blood Pressure, Plus Fun Review Study Guide Main Idea: The function of the circulatory system is to maintain adequate blood flow to all tissues. Clinical
The Excretory System. Biology 20
 The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
Non Contrast MRA. Mayil Krishnam. Director, Cardiovascular and Thoracic Imaging University of California, Irvine
 Non Contrast MRA Mayil Krishnam Director, Cardiovascular and Thoracic Imaging University of California, Irvine No disclosures Non contrast MRA-Why? Limitations of CTA Radiation exposure Iodinated contrast
Non Contrast MRA Mayil Krishnam Director, Cardiovascular and Thoracic Imaging University of California, Irvine No disclosures Non contrast MRA-Why? Limitations of CTA Radiation exposure Iodinated contrast
General Anatomy of Urinary System
 General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
Chapter 12. Excretion and the Interaction of Systems
 Chapter 12 Excretion and the Interaction of Systems 1 2 Goals for This Chapter 1. Identify the main structures and functions of the human excretory system 2. Explain the function of the nephron 3. Describe
Chapter 12 Excretion and the Interaction of Systems 1 2 Goals for This Chapter 1. Identify the main structures and functions of the human excretory system 2. Explain the function of the nephron 3. Describe
Magnetic Resonance Angiography
 Magnetic Resonance Angiography 1 Magnetic Resonance Angiography exploits flow enhancement of GR sequences saturation of venous flow allows arterial visualization saturation of arterial flow allows venous
Magnetic Resonance Angiography 1 Magnetic Resonance Angiography exploits flow enhancement of GR sequences saturation of venous flow allows arterial visualization saturation of arterial flow allows venous
The Circulatory System
 The Circulatory System Key Questions What are the functions of the circulatory system? How does the heart pump blood through the body? What are three types of blood vessels? Vocabulary myocardium atrium
The Circulatory System Key Questions What are the functions of the circulatory system? How does the heart pump blood through the body? What are three types of blood vessels? Vocabulary myocardium atrium
The Cardiovascular and Lymphatic Systems
 BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 12 The Cardiovascular and Lymphatic Systems Lecture Presentation Anne Gasc Hawaii Pacific University and
BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 12 The Cardiovascular and Lymphatic Systems Lecture Presentation Anne Gasc Hawaii Pacific University and
Sunday, July 17, 2011 URINARY SYSTEM
 URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
Cardiovascular Responses to Exercise
 CARDIOVASCULAR PHYSIOLOGY 69 Case 13 Cardiovascular Responses to Exercise Cassandra Farias is a 34-year-old dietician at an academic medical center. She believes in the importance of a healthy lifestyle
CARDIOVASCULAR PHYSIOLOGY 69 Case 13 Cardiovascular Responses to Exercise Cassandra Farias is a 34-year-old dietician at an academic medical center. She believes in the importance of a healthy lifestyle
Vascular System Part One
 Vascular System Part One Objectives Trace the route taken by blood as it leaves, and then returns to the heart. Describe the structure of the walls of arteries and veins. Discuss the structure and function
Vascular System Part One Objectives Trace the route taken by blood as it leaves, and then returns to the heart. Describe the structure of the walls of arteries and veins. Discuss the structure and function
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
Oxygen Carbon dioxide Water vapour Nitrogen
 1. The table shows the percentage of various gases in atmospheric air, exhaled air and in air samples collected from the alveoli and the trachea of a healthy human. Gas Atmospheric air(inhaled air) Exhaled
1. The table shows the percentage of various gases in atmospheric air, exhaled air and in air samples collected from the alveoli and the trachea of a healthy human. Gas Atmospheric air(inhaled air) Exhaled
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
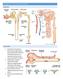 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The Cardiovascular System
 The Cardiovascular System The Cardiovascular System A closed system of the heart and blood vessels The heart pumps blood Blood vessels allow blood to circulate to all parts of the body The function of
The Cardiovascular System The Cardiovascular System A closed system of the heart and blood vessels The heart pumps blood Blood vessels allow blood to circulate to all parts of the body The function of
Essentials of Clinical MR, 2 nd edition. 99. MRA Principles and Carotid MRA
 99. MRA Principles and Carotid MRA As described in Chapter 12, time of flight (TOF) magnetic resonance angiography (MRA) is commonly utilized in the evaluation of the circle of Willis. TOF MRA allows depiction
99. MRA Principles and Carotid MRA As described in Chapter 12, time of flight (TOF) magnetic resonance angiography (MRA) is commonly utilized in the evaluation of the circle of Willis. TOF MRA allows depiction
Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla.
 2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
Clinical Applications
 C H A P T E R 16 Clinical Applications In selecting pulse sequences and measurement parameters for a specific application, MRI allows the user tremendous flexibility to produce variations in contrast between
C H A P T E R 16 Clinical Applications In selecting pulse sequences and measurement parameters for a specific application, MRI allows the user tremendous flexibility to produce variations in contrast between
10. Thick deposits of lipids on the walls of blood vessels, called, can lead to serious circulatory issues. A. aneurysm B. atherosclerosis C.
 Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
Introduction to Emergency Medical Care 1
 Introduction to Emergency Medical Care 1 OBJECTIVES 6.1 Define key terms introduced in this chapter. Slides 11, 15, 17, 26, 27, 31, 33, 37, 40 42, 44, 45, 51, 58 6.2 Describe the basic roles and structures
Introduction to Emergency Medical Care 1 OBJECTIVES 6.1 Define key terms introduced in this chapter. Slides 11, 15, 17, 26, 27, 31, 33, 37, 40 42, 44, 45, 51, 58 6.2 Describe the basic roles and structures
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
PHYSICS OF MRI ACQUISITION. Alternatives to BOLD for fmri
 PHYSICS OF MRI ACQUISITION Quick Review for fmri HST-583, Fall 2002 HST.583: Functional Magnetic Resonance Imaging: Data Acquisition and Analysis Harvard-MIT Division of Health Sciences and Technology
PHYSICS OF MRI ACQUISITION Quick Review for fmri HST-583, Fall 2002 HST.583: Functional Magnetic Resonance Imaging: Data Acquisition and Analysis Harvard-MIT Division of Health Sciences and Technology
Chp. 5 The cardiovascular system. What are the function of the cardiovascular system? Arteries and arterioles:
 5.1 Overview of the cardiovascular system Chp. 5 The cardiovascular system Includes the heart and blood vessels Brings nutrients to cells and helps get rid of wastes Blood is refreshed in the lung, kidneys,
5.1 Overview of the cardiovascular system Chp. 5 The cardiovascular system Includes the heart and blood vessels Brings nutrients to cells and helps get rid of wastes Blood is refreshed in the lung, kidneys,
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
Cardiovascular System: Vessels and Circulation (Chapter 21)
 Cardiovascular System: Vessels and Circulation (Chapter 21) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Sources for figures and content: Marieb,
Cardiovascular System: Vessels and Circulation (Chapter 21) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Sources for figures and content: Marieb,
Body Fluids and Fluid Compartments
 Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
Physiology of Circulation. Dr. Hiwa Shafiq 16/12/2018
 Physiology of Circulation Dr. Hiwa Shafiq 16/12/2018 Overview of the circulation The function of the circulation is to: 1. transport nutrients to the body tissues 2. transport waste products away 3. conduct
Physiology of Circulation Dr. Hiwa Shafiq 16/12/2018 Overview of the circulation The function of the circulation is to: 1. transport nutrients to the body tissues 2. transport waste products away 3. conduct
PART A: MULTIPLE CHOICE (100 questions 65% of exam mark)
 1 PART A: MULTIPLE CHOICE (100 questions 65% of exam mark) I: Wellness and Homeostasis 1. Determine the false statement about homeostasis. A) Homeostasis refers to the body s attempt to adjust to a fluctuating
1 PART A: MULTIPLE CHOICE (100 questions 65% of exam mark) I: Wellness and Homeostasis 1. Determine the false statement about homeostasis. A) Homeostasis refers to the body s attempt to adjust to a fluctuating
1. Label the Diagram using the following terms: artery, arterioles, vein, venules, capillaries, valve, inner wall, middle wall, outer wall
 Bio 20 Ms. Nyboer Arteries, Veins, Capillaries, and the Heart Structure and Function Workbook Use your textbook (Ch. 10) and notes to fill in this workbook Part A: Arteries, Veins, Capillaries 1. Label
Bio 20 Ms. Nyboer Arteries, Veins, Capillaries, and the Heart Structure and Function Workbook Use your textbook (Ch. 10) and notes to fill in this workbook Part A: Arteries, Veins, Capillaries 1. Label
Ch17-18 Urinary System
 Ch17-18 Urinary System Main Function: Filter the blood Other Functions: maintain purity and consistency of internal fluids eliminates nitrogenous wastes, toxins, and drugs from the body regulates blood
Ch17-18 Urinary System Main Function: Filter the blood Other Functions: maintain purity and consistency of internal fluids eliminates nitrogenous wastes, toxins, and drugs from the body regulates blood
CAPILLARY FLUID EXCHANGE
 CAPILLARY FLUID EXCHANGE Aubrey E. Taylor and Timothy M. Moore Department of Physiology, University of South Alabama, College of Medicine, Mobile, Alabama 36688-0002 AM. J. PHYSIOL. 277 (ADV. PHYSIOL.
CAPILLARY FLUID EXCHANGE Aubrey E. Taylor and Timothy M. Moore Department of Physiology, University of South Alabama, College of Medicine, Mobile, Alabama 36688-0002 AM. J. PHYSIOL. 277 (ADV. PHYSIOL.
DRUG DISTRIBUTION. Distribution Blood Brain Barrier Protein Binding
 DRUG DISTRIBUTION Distribution Blood Brain Barrier Protein Binding DRUG DISTRIBUTION Drug distribution is a reversible transport of drug through the body by the systemic circulation The drug molecules
DRUG DISTRIBUTION Distribution Blood Brain Barrier Protein Binding DRUG DISTRIBUTION Drug distribution is a reversible transport of drug through the body by the systemic circulation The drug molecules
BIOL 219 Spring Chapters 14&15 Cardiovascular System
 1 BIOL 219 Spring 2013 Chapters 14&15 Cardiovascular System Outline: Components of the CV system Heart anatomy Layers of the heart wall Pericardium Heart chambers, valves, blood vessels, septum Atrioventricular
1 BIOL 219 Spring 2013 Chapters 14&15 Cardiovascular System Outline: Components of the CV system Heart anatomy Layers of the heart wall Pericardium Heart chambers, valves, blood vessels, septum Atrioventricular
Cardiovascular System. Biology 105 Lecture 15 Chapter 12
 Cardiovascular System Biology 105 Lecture 15 Chapter 12 Outline I. Functions of cardiovascular system II. Components of the cardiovascular system: I. Blood vessels II. Heart III. Regulation of the heartbeat
Cardiovascular System Biology 105 Lecture 15 Chapter 12 Outline I. Functions of cardiovascular system II. Components of the cardiovascular system: I. Blood vessels II. Heart III. Regulation of the heartbeat
The Cardiovascular System: Vessels and Routes. Pulmonary Circulation H E A R T. Systemic Circulation
 The Cardiovascular System: Vessels and Routes 1. Overview of Blood Circulation A. Pulmonary Circulation Lung Arterioles Pulmonary Artery Capillaries Pulmonary Circulation Venules Pulmonary Veins H E A
The Cardiovascular System: Vessels and Routes 1. Overview of Blood Circulation A. Pulmonary Circulation Lung Arterioles Pulmonary Artery Capillaries Pulmonary Circulation Venules Pulmonary Veins H E A
7.L.1.4 Circulatory System Guided Study Notes. Circulation
 1 7.L.1.4 Circulatory System Guided Study Notes Circulation Sect. 1: The Body s Transport System Sect. 2: A Closer Look at Blood Vessels Sect. 3: Blood and Lymph Sect. 4: Cardiovascular Health Sect. 1:
1 7.L.1.4 Circulatory System Guided Study Notes Circulation Sect. 1: The Body s Transport System Sect. 2: A Closer Look at Blood Vessels Sect. 3: Blood and Lymph Sect. 4: Cardiovascular Health Sect. 1:
Cardiovascular System L-5 Special Circulations, hemorrhage and shock. Dr Than Kyaw March 2012
 Cardiovascular System L-5 Special Circulations, hemorrhage and shock Dr Than Kyaw March 2012 Special circulation (Coronary, Pulmonary, and Cerebral circulations) Introduction Special attention to circulation
Cardiovascular System L-5 Special Circulations, hemorrhage and shock Dr Than Kyaw March 2012 Special circulation (Coronary, Pulmonary, and Cerebral circulations) Introduction Special attention to circulation
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Chapter 42: Circulation / Gas Exchange. d = t 2
 Chapter 42: Circulation / Gas Exchange Transport systems connect organs of exchange with body cells Diffusion Lung Blood 100 m 1 s 1 mm 100 s 1 cm 10000 s d = t 2 Bulk Flow (Pressure) Blood Cells Methods
Chapter 42: Circulation / Gas Exchange Transport systems connect organs of exchange with body cells Diffusion Lung Blood 100 m 1 s 1 mm 100 s 1 cm 10000 s d = t 2 Bulk Flow (Pressure) Blood Cells Methods
Bodies and Systems. What is your body made of?
 What is your body made of? You might say that you are made of organs like skin and a heart. You might say that you are made of tissue, cells, or even atoms. All these answers are correct. Multicellular
What is your body made of? You might say that you are made of organs like skin and a heart. You might say that you are made of tissue, cells, or even atoms. All these answers are correct. Multicellular
Chapter 9, Part 2. Cardiocirculatory Adjustments to Exercise
 Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER
 MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
Six main classes of blood vessels (on handout) Wall structure of arteries and veins (on handout) Comparison: Arteries vs. Veins (on handout)
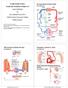 Cardiovascular System: Vessels and Circulation (Chapter 21) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Six main classes of blood vessels Primary Sources
Cardiovascular System: Vessels and Circulation (Chapter 21) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Six main classes of blood vessels Primary Sources
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
The Cardiovascular System. The Structure of Blood Vessels. The Structure of Blood Vessels. The Blood Vessels. Blood Vessel Review
 The Cardiovascular System The Blood Vessels The Structure of Blood Vessels Blood Vessel Review Arteries carry blood away from the heart Pulmonary trunk to lungs Aorta to everything else Microcirculation
The Cardiovascular System The Blood Vessels The Structure of Blood Vessels Blood Vessel Review Arteries carry blood away from the heart Pulmonary trunk to lungs Aorta to everything else Microcirculation
Fifth Year Biology. Excretion. Miss Rochford
 Fifth Year Biology Excretion Miss Rochford In this Topic Excretion in plants Excretion and homeostasis Skin Organs of excretion Urinary system Kidneys Nephron Control of urine volume Characteristics of
Fifth Year Biology Excretion Miss Rochford In this Topic Excretion in plants Excretion and homeostasis Skin Organs of excretion Urinary system Kidneys Nephron Control of urine volume Characteristics of
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
Blood Pressure Fox Chapter 14 part 2
 Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
Chapter 7. The Nervous System: Structure and Control of Movement
 Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
