Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson Gilford progeria syndrome mutation
|
|
|
- Curtis Bryant
- 5 years ago
- Views:
Transcription
1 Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson Gilford progeria syndrome mutation Shao H. Yang*, Martin O. Bergo, Julia I. Toth*, Xin Qiao*, Yan Hu*, Salemiz Sandoval*, Margarita Meta, Pravin Bendale, Michael H. Gelb, Stephen G. Young*, and Loren G. Fong* *Division of Cardiology, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, CA 90095; Department of Internal Medicine, Bruna Stråket 16, Third Floor, Sahlgrenska University Hospital, SE Göteborg, Sweden; Musculoskeletal and Quantitative Research Group, Department of Radiology, University of California, San Francisco, CA 94107; and Departments of Chemistry and Biochemistry, University of Washington, Seattle, WA Communicated by Daniel Steinberg, University of California at San Diego, La Jolla, CA, June 6, 2005 (received for review April 20, 2005) Hutchinson Gilford progeria syndrome (HGPS), a progeroid syndrome in children, is caused by mutations in LMNA (the gene for prelamin A and lamin C) that result in the deletion of 50 aa within prelamin A. In normal cells, prelamin A is a CAAX protein that is farnesylated and then processed further to generate mature lamin A, which is a structural protein of the nuclear lamina. The mutant prelamin A in HGPS, which is commonly called progerin, retains the CAAX motif that triggers farnesylation, but the 50-aa deletion prevents the subsequent processing to mature lamin A. The presence of progerin adversely affects the integrity of the nuclear lamina, resulting in misshapen nuclei and nuclear blebs. We hypothesized that interfering with protein farnesylation would block the targeting of progerin to the nuclear envelope, and we further hypothesized that the mislocalization of progerin away from the nuclear envelope would improve the nuclear blebbing phenotype. To approach this hypothesis, we created a gene-targeted mouse model of HGPS, generated genetically identical primary mouse embryonic fibroblasts, and we then examined the effect of a farnesyltransferase inhibitor on nuclear blebbing. The farnesyltransferase inhibitor mislocalized progerin away from the nuclear envelope to the nucleoplasm, as determined by immunofluoresence microscopy, and resulted in a striking improvement in nuclear blebbing (P < by 2 statistic). These studies suggest a possible treatment strategy for HGPS. aging lamin A C laminopathy Hutchinson Gilford progeria syndrome (HGPS) is a progeroid syndrome characterized by a host of aging-like phenotypes, including a wizened appearance of the skin, osteoporosis, alopecia, and premature atherosclerosis (1). Children with HGPS die at the mean age of 13, generally from myocardial infarctions or strokes (1). This disease is caused by the accumulation of a mutant form of prelamin A that cannot be processed to mature lamin A (1). In normal cells, wild-type prelamin A is virtually undetectable because it is fully converted to mature lamin A, a structural protein of the nuclear lamina (2, 3). The nuclear lamina is an intermediate filament meshwork adjacent to the inner nuclear membrane that provides structural support for the nucleus (2, 3). Prelamin A contains a nuclear localization signal and terminates with a CAAX motif (2), in which C is a cysteine, A residues are usually aliphatic amino acids, and X can be one of many different residues. CAAX motifs are also found on lamin B1, lamin B2, the Ras family of proteins, and many other cellular proteins. The CAAX motif triggers three sequential enzymatic posttranslational modifications, beginning with protein prenylation. In the case of prelamin A, the first processing step is carried out by protein farnesyltransferase (FTase) and involves the addition of a 15-carbon farnesyl lipid to the thiol group of the cysteine within the CAAX motif. Second, the last 3 aa of the protein (i.e., AAX) are removed by a prenylprotein-specific endoprotease. For prelamin A, this step is likely to be a redundant function of two endoplasmic reticulum membrane endoproteases, Zmpste24 and Rce1 (4). Third, the newly exposed farnesylcysteine is methylated by Icmt, a prenylprotein-specific membrane methyltransferase of the endoplasmic reticulum (5). After these CAAX-box modifications have been completed, prelamin A (in contrast to other CAAX proteins) undergoes an additional processing step. The last 15 aa of the protein (including the farnesylcysteine methyl ester) are clipped off by Zmpste24 and then degraded, leaving behind mature lamin A (4, 6, 7). The farnesylation of prelamin A is important for its targeting to the nuclear envelope (8 10). Each of the three CAAX motif modifications of prelamin A render the C terminus of the protein more hydrophobic, facilitating its association with the inner nuclear membrane, where the protein is cleaved, releasing mature lamin A (9, 11). In the absence of farnesylation (for example, in mevinolin-treated cells), prelamin A accumulates in the nucleoplasm and does not reach the nuclear envelope (9, 11). In the setting of Zmpste24 deficiency, farnesyl prelamin A accumulates at the nuclear envelope (6, 12) and adversely affects the integrity of the nuclear envelope. The nuclei of Zmpste24- deficient fibroblasts are misshapen, containing numerous nuclear blebs (6, 12). HGPS is most commonly caused by a de novo point mutation in exon 11 of LMNA (1). This mutation, which occurs in codon 608, activates a cryptic splice site and leads to the in-frame deletion of 50 aa within prelamin A. This deletion leaves the CAAX motif intact; hence, the mutant prelamin A (progerin) is predicted to undergo farnesylation, release of the AAX, and carboxyl methylation. However, the site for the second endoproteolytic cleavage step is eliminated by the deletion (1). Thus, progerin cannot be processed to lamin A and likely retains a farnesylcysteine methyl ester at its C terminus. Like Zmpste24- deficient cells, HGPS fibroblasts contain misshapen nuclei with numerous blebs of the nuclear envelope (1). In human HGPS cells, the severity of nuclear blebbing is variable, depending in part on the number of times the cells have been passaged (13). A GFP-tagged progerin has been reported to accumulate along the nuclear envelope of HeLa cells and cause nuclear shape abnormalities (13). We hypothesized that the farnesylation of progerin targets the protein to the nuclear envelope, where it might weaken the Abbreviations: HGPS, Hutchinson Gilford progeria syndrome; FTase, protein farnesyltransferase; FTI, FTase inhibitor; MEF, mouse embryonic fibroblast. To whom correspondence should be addressed at: University of California, 675 Charles East Young Drive South, MacDonald Medical Research Laboratory Building, Room 4770, Los Angeles, CA lfong@mednet.ucla.edu by The National Academy of Sciences of the USA MEDICAL SCIENCES cgi doi pnas PNAS July 19, 2005 vol. 102 no
2 nuclear lamina and cause nuclear blebbing. We further hypothesized that blocking farnesylation with an FTase inhibitor (FTI) would mislocalize progerin away from the nuclear envelope and reduce nuclear blebbing. Some researchers might argue that the latter hypothesis is unattractive, given that the FTI would also block the posttranslational processing of lamin B1 and lamin B2, potentially weakening the lamina further. However, we hypothesized that the salutary effects of blocking farnesylation of progerin would trump any deleterious effects of the FTI on the lamina and lead to an overall improvement in nuclear blebbing. To test the impact of blocking the farnesylation of progerin on nuclear shape, we reasoned that it would be helpful to create gene-targeted HGPS mice that express progerin at levels sufficient to cause nuclear blebbing. The existence of a gene-targeted mouse model would make it possible to study the impact of an FTI on nuclear shape in independent lines of low-passage, genetically identical mouse embryonic fibroblasts (MEFs). In this study, we adopted exactly this strategy, first by generating a mouse model of HGPS and then by using primary MEFs to define the impact of an FTI on the nuclear blebbing phenotype. Materials and Methods Gene-Targeted Mouse Model of HGPS. The DNA fragments for the arms of the gene-targeting vector were generated by long-range PCR of genomic DNA from 129 Ola ES cells. A 6-kb 5 fragment, which spanned from the end of intron 5 to sequences 1.9 kb downstream from the 3 UTR (encoded by exon 12) was amplified with 5 -GGCTTCCTGGTCACTGGATA-3 and 5 - GATCTGCCTGGAAGCTGAGT-3 and cloned into pcr2.1- TOPO-XL (Invitrogen). Next, a 5-kb EcoRI fragment (spanning from a polylinker EcoRI site in pcr2.1-topo-xl to an EcoRI site 0.9 kb downstream from the 3 UTR) was cleaved from the vector and cloned into pbsk (Stratagene). To create the 5 arm of a sequence-replacement vector, this EcoRI fragment was subjected to two sequential site-directed mutagenesis reactions (QuikChange, Stratagene). First, intron 10 was deleted with the primer 5 -GATGGAGAAGAGCTCCTCCATCACCAC- CGTGGTTCCCACTGCAGCGGCTCGGGGGACCCC-3 and the reversed and complemented primer. Second, the last 150 nt of exon 11 and intron 11 were deleted with the primer 5 -GACAAGGCTGCCGGTGGAGCGGGAGCCCAGA- GCTCCCAGAACTGCAGCATCATGTAATCT-3 and the reversed and complemented primer. To create the gene-targeting vector, the mutant EcoRI fragment was cloned into the polylinker EcoRI site of pksloxpntmod. The 3 arm, consisting of sequences immediately downstream of those in the 5 arm, was amplified with the primers 5 -GACAGCCACCTGGTCAGTTT-3 and 5 -GTAACTCTG- GCTGCCCTCAA-3 and then cloned into pcr2.1-topo-xl. To complete the gene-targeting vector, this fragment was cloned into the polylinker AscI site of pksloxpntmod. The integrity of the vector ( 17 kb in length) was verified by DNA sequencing and restriction mapping. The vector was linearized with NotI and electroporated into strain 129 Ola ES cells. To identify clones carrying the targeted Lmna HG allele, we performed Southern blot analyses of EcoRIdigested genomic DNA with a 348-bp 5 -flanking probe. The probe was generated by PCR from mouse genomic DNA with the following primers: 5 -CAAGGAGCTCGGATTCTGTC-3 and 5 -GTCAGGGAAGAGTGCAGAGG-3. The probe detected a 10.4-kb band in the wild-type Lmna allele and a 9.3-kb band in the Lmna HG allele. Genotyping was also performed on genomic DNA by PCR with the following primers: 5 - TGAGTACAACCTGCGCTCAC-3 and 5 -CAGACAGGAG- GTGGCATGT-3. The PCR fragment, spanning from exon 11 to exon 12, was 582 bp in the wild-type Lmna allele and 186 bp in the Lmna HG allele. Creating Lmna HG MEFs. Targeted 129 Ola ES cells were microinjected into C57BL 6 blastocysts to produce chimeric mice. To generate MEFs, chimeras were bred with C57BL 6 females. Wild-type primary MEFs (Lmna / ) and Lmna HG/ MEFs were prepared from the same litter of day 13.5 embryos and genotyped by PCR. Because the MEFs were generated from embryos of chimera matings, they were genetically identical (one 129 Ola chromosome and one C57BL 6 chromosome). Cells homozygous for the Lmna HG mutation were generated by subjecting Lmna HG/ cells to several months of selection in increasing concentrations of G418 (14). The Lmna HG/HG cells were euploid, but many of the cells were differentiated, as determined by morphology. FTIs. For all experiments, we used PB-43, which is a member of the tetrahydroquinoline family of protein FTIs (15) that displays potent inhibition of rat FTase in vitro (IC nm). PB-43 readily crosses cell membranes, as demonstrated by its ability to kill Plasmodium falciparum in human red blood cells (M.H.G., unpublished data). PB-43 was synthesized by described methods (15) and shown to be pure by HPLC on a reverse-phase column. The compound was dissolved in DMSO at a concentration of 10 mm and stored in aliquots at 80 C. Treatment of Cells with the FTI and Western Blot Analyses. Adherent early-passage MEFs in six-well tissue culture plates were incubated with the vehicle control (DMSO) or the indicated concentrations of PB-43 diluted in culture medium at 37 C for 48 h. The cells were washed with PBS, and urea-soluble extracts were prepared as described in ref. 11. Cell pellets solubilized with SDS-containing buffers were also prepared and yielded results indistinguishable from those with urea extraction. Proteins were size-separated on 4 12% gradient polyacrylamide Bis-Tris gels (Invitrogen) and then electrophoretically transferred to nitrocellulose membranes for Western blotting. The following antibody dilutions were used: 1:400 anti-lamin A C goat IgG (sc-6215, Santa Cruz Biotechnology), 1:400 anti-lamin B (sc-6217, Santa Cruz Biotechnology), 1:6,000 anti-mouse prelamin A rabbit antiserum (12, 13), 1:500 anti-hdj-2 mouse IgG (LabVision, Fremont, CA), 1:1,000 anti-actin goat IgG (sc-1616, Santa Cruz Biotechnology), 1:6,000 horseradish peroxidase (HRP)-labeled anti-goat IgG (sc-2020, Santa Cruz Biotechnology), 1:4,000 HRP-labeled anti-mouse IgG (Amersham Biosciences), and 1:6,000 HRP-labeled anti-rabbit IgG (Amersham Biosciences). Antibody binding was detected with the ECL Plus chemiluminescence system (Amersham Biosciences) and exposure to x-ray film. Immunofluoresence Microscopy. Primary cells of different genotypes were grown on coverslips, fixed in 3% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with BSA (12). Cells were incubated for 60 min with antibodies against lamin A (sc-20680) or lamin B (sc-6217) (Santa Cruz Biotechnology). After washing, cells were stained with species-specific Cy3-conjugated secondary antibodies (Jackson Immuno- Research) and DAPI to visualize DNA. Images were obtained on an Axiovert 40CFL microscope (Zeiss) with a oil-immersion objective and processed with AXOVISION software (version 4.2; Zeiss). Nuclear shape abnormalities were scored by two independent observers, who were blinded to genotype or treatment group cgi doi pnas Yang et al.
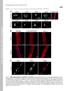
3 Fig. 2. Immunofluorescence microscopy of wild-type and Lmna HG/ fibroblasts. (A E) Immunostaining showing nuclear blebs in Lmna HG/ MEFs. Blebs are indicated by white arrows. (F) Lmna / MEFs. In this experiment, cells were stained for lamin B1 (red). Fig. 1. Production of a mutant Lmna allele, Lmna HG, that yields progerin. (A) Gene-targeting strategy, which involves deleting intron 10, intron 11, and the last 150 nt of exon 11 of Lmna.(B) Southern blot analysis detecting the Lmna HG allele in mouse ES cells, with EcoRI-cleaved genomic DNA and the indicated 5 flanking probe. (C) PCR identification of the Lmna HG allele. Results with wild-type MEFs (WT), heterozygous MEFs (Lmna HG/ ), homozygous ES cells (Lmna HG/HG ), and heterozygous MEFs (Lmna HG/ ) are shown. (D) Western blotting identification of progerin with a lamin A C-specific monoclonal antibody. Wild-type cells, Lmna HG/ MEFs, and Lmna HG/HG cells are shown. On SDS PAGE gels, the electrophoretic migration of progerin in mouse Lmna HG/ MEFs and human HGPS fibroblasts was identical (data not shown). Results We created a mouse model of HGPS that expressed large amounts of progerin. To accomplish that goal, we created a mutant Lmna allele, Lmna HG, that exclusively yields progerin (Fig. 1A). We deleted intron 10 of Lmna, thereby eliminating lamin C synthesis; we also deleted the last 150 nt of exon 11 and intron 11, which results in the synthesis of progerin (and precludes wild-type prelamin A synthesis). Two targeted ES cell clones (from 192 G418- and FIAU-resistant clones) were identified by Southern blotting (Fig. 1B) and used to create 22 high-percentage chimeric mice. Those mice were bred with C57BL 6 mice to produce the Lmna HG/ mice for this study (Fig. 1C). In addition, Lmna HG/HG cell lines were created from Lmna HG/ ES cells by high-g418 selection (Fig. 1D). As predicted, primary MEFs from Lmna HG/ embryos produced progerin, along with lamin A and lamin C from the wild-type Lmna allele (Figs. 1D and 3), whereas the Lmna HG/HG cells yielded only progerin (Fig. 1D). Lmna expression in wild-type ES cells is low (2, 3, 16). However, the Lmna HG/HG cells, which had undergone 2 months of high G418 selection and appeared to be differentiated, expressed progerin at levels comparable with those in MEFs (Fig. 1D). Not surprisingly, the nuclei of many Lmna HG/ MEFs were misshapen and contained large blebs (Fig. 2). We sought to define the impact of an FTI, PB-43, on nuclear shape. To document that this compound was effective in blocking farnesylation, we treated Lmna HG/ MEFs with the PB-43 FTI and then examined the electrophoretic migration of Hdj-2, a farnesylated protein, with Western blot analyses of SDS PAGE gels (Fig. 3). In FTI-treated MEFs, nearly all of the Hdj-2 migrated slowly and almost none migrated normally, indicating that the FTI had been effective in blocking protein farnesylation. We also performed Western blot analyses of wild-type and Lmna HG/ cell extracts with an antibody specific for the C terminus of prelamin A (Fig. 3). The prelamin A-specific antibody does not normally detect prelamin A in cells because prelamin A is farnesylated and rapidly converted to mature lamin A. However, in the presence of the FTI, prelamin A processing is blocked, and prelamin A is easily detectable in both wild-type and Lmna HG/ MEFs (Fig. 3). Western blot analyses with an antibody against the N-terminal portion of lamin A detected mature lamin A in wild-type MEFs and in Lmna HG/ MEFs but detected the slightly larger prelamin A (and virtually no mature lamin A) in MEFs that had been treated with the FTI. The FTI did not yield clear-cut or consistent alterations in the amount of either progerin or lamin B1 in Lmna HG/ MEFs (Fig. 3). To judge the impact of the FTI on nuclear blebbing in Lmna HG/ MEFs, we examined both Lmna / and Lmna HG/ MEFs in a blinded fashion by immunofluorescence microscopy. Lmna HG/ MEFs had more nuclear blebs than Lmna / cells (P , 2 test) (Fig. 4). The FTI did not affect nuclear shape in Lmna / MEFs. However, FTI treatment of the Lmna HG/ MEFs reduced the frequency of nuclear blebbing (P ). This result was consistent in several independently isolated Lmna HG/ cell lines and in several independent experiments (Fig. 4). Of note, the frequency of blebbing in FTI-treated Lmna HG/ MEFs was not different, as determined by the 2 Fig. 3. Western blot analysis of wild-type, Lmna HG/, and Zmpste24 / MEFs. Cells were grown in the presence and absence of an FTI (10 M PB-43), and Western blot analyses of cell extracts were performed with antibodies specific for prelamin A (specific for the extreme C terminus), lamin A C (binds to both lamin A and lamin C), lamin B1, Hdj-2, and actin. MEDICAL SCIENCES Yang et al. PNAS July 19, 2005 vol. 102 no
4 Fig. 4. Bar graph showing increased frequency of nuclear blebbing in Lmna HG/ MEFs and a reduction in nuclear blebbing in Lmna HG/ MEFs treated with an FTI (10 M PB-43). A and B represent two independent experiments. Each black circle shows the frequency of nuclear blebbing with an independently isolated fibroblast cell line. Bars indicate the mean frequency of blebbing. The number of cells with nuclear blebs and the total number of cells examined are recorded within each bar. In both experiments, the FTI did not change the frequency of blebbing in Lmna / MEFs, as determined by the 2 test. Lmna HG/ MEFs exhibited more blebbing than Lmna / cells (P , 2 test). An FTI reduced the frequency of blebbing in Lmna HG/ MEFs (P , 2 test). The frequency of blebbing in FTI-treated Lmna HG/ MEFs was not different from the frequency of blebbing in the treated or untreated Lmna / MEFs. Very similar results, with identical levels of statistical significance, were obtained when the microscopic slides were scored by a second blinded observer. statistic, from the frequency of blebbing in FTI-treated or untreated Lmna / MEFs. Because Lmna HG/ MEFs synthesize lamin A and lamin C in addition to progerin, it was impossible to use immunofluorescence microscopy of Lmna HG/ MEFs to define the location of progerin within those cells. However, the intracellular location of progerin could be examined in experiments with Lmna HG/HG cells, which synthesize exclusively progerin. In those cells, progerin was located mainly at the nuclear envelope (Fig. 5 A and B). However, in the presence of the FTI, virtually all of the progerin was mislocalized to intensely staining aggregates within the nucleoplasm, and none of it was detectable at the nuclear envelope (Fig. 5 C and D). Discussion FTIs were developed initially as anticancer agents (17). The concept was straightforward: to eliminate the farnesyl lipid Fig. 5. Immunofluoresence images showing the distribution of progerin in untreated and FTI-treated Lmna HG/HG cells. DNA was visualized with DAPI (blue), and progerin was visualized with an antibody against lamin A (red). (A and B) Untreated Lmna HG/HG cells, showing progerin along the nuclear envelope. Misshapen nuclei were common (arrow). (C and D) FTI-treated Lmna HG/HG cells, revealing intensely staining progerin aggregates (arrowheads) in the nucleoplasm. from mutationally activated Ras proteins, thereby mislocalizing these signaling proteins away from the plasma membrane, where they cause trouble by stimulating uncontrolled cell division. In this study, we assessed an analogous concept with HGPS, which is a disease in which another farnesylated protein causes trouble in the cell. The synthesis of progerin leads to misshapen nuclei and frequent nuclear blebs (1). We hypothesized that blocking farnesylation would mislocalize progerin away from the nuclear envelope and ameliorate the nuclear blebbing phenotype. We generated a gene-targeted mouse model of HGPS, created genetically identical primary Lmna HG/ MEFs as well as Lmna HG/HG cells, and tested the impact of an FTI on both nuclear blebbing and progerin localization. In untreated cells, progerin was located mainly along the nuclear envelope, whereas in FTI-treated cells the protein was mislocalized to the nucleoplasm. In the Lmna HG/ MEFs, the FTI reduced nuclear blebbing to a baseline level observed in untreated wild-type cells. These studies visualize progerin localization independently of lamin A and lamin C, and they show that mislocalization of progerin is associated with a change in nuclear shape. Interestingly, the FTI did not adversely affect nuclear shape in wild-type fibroblasts. Nuclear blebbing is the principal cellular phenotype in HGPS (13, 18), and the FTI clearly ameliorates this phenotype. The next obvious questions are as follows. Will the nuclear shape abnormalities in Lmna HG/ cells be accompanied by any disease phenotypes at the whole-animal level? And, if so, would an FTI also ameliorate those disease phenotypes, including perhaps the atherosclerotic disease that claims the lives of most humans with the disease? With regard to the first question, the Lmna HG/ mice clearly exhibit unequivocal disease phenotypes (S.H.Y., L.G.F., and S.G.Y., unpublished data). By 4 months of age, all of the Lmna HG/ mice (n 22) exhibit growth retardation and or bone disease, akin to homozygous Zmpste24-deficient mice (4). Detailed radiographic and pathologic analyses (e.g., assessing bone density and defining atherosclerosis susceptibility in inbred backgrounds) will take many months to complete. However, the existence of tractable phenotypes in our heterozygous gene-targeted mice makes us confident that this model could be very useful for assessing therapeutic strategies. To properly assess the impact of FTIs on disease phenotypes in mice, we first need to identify methods for the long-term cgi doi pnas Yang et al.
5 oral delivery of the drugs and document that the drug is efficacious in blocking protein farnesylation in multiple tissues. Again, we anticipate that optimization of FTI delivery schemes will require considerable experimentation. In the meantime, we can only speculate about whether FTIs might be useful for treating the disease phenotypes in the Lmna HG/ mice. Optimists would contend that it makes intuitive sense (i.e., in line with Occam s razor) that improvements in wholeanimal disease phenotypes would parallel improvements in cellular phenotypes. Optimists would also point out that the nuclear blebbing phenotypes in cultured fibroblasts and the whole-animal disease phenotypes were clearly associated in a recent study of Zmpste24-deficient mice (12). Zmpste24- deficient fibroblasts, which accumulate wild-type farnesyl prelamin A, manifest nuclear blebbing (6, 12), and Zmpste24- deficient mice develop a variety of progeria-like phenotypes (4, 12). When prelamin A synthesis was reduced by 50% (by introducing a single copy of a Lmna knockout allele), the nuclear blebbing in fibroblasts and the disease phenotypes in mice were eliminated (12). That study was also intriguing because it proved that dramatic improvements in progeria-like disease phenotypes can occur with a 50% reduction in the amount of farnesyl prelamin A in the cell. Thus, one might easily imagine that an FTI could ameliorate the disease phenotypes even with incomplete inhibition of farnesylation and incomplete mislocalization of progerin (i.e., without pushing FTI doses to levels associated with side effects). However, drug toxicity may not be much of an issue because FTIs have been generally well tolerated in humans and in mice (19, 20). Genetic studies also support the idea that it should be possible to give FTIs safely on a long-term basis (21). Completely inactivating FTase in adult mice by using Cre loxp approaches did not cause histopathological abnormalities or any noticeable disease phenotypes (21). There are also reasons to be pessimistic about the proposition that an FTI would be a panacea for HGPS. Although an FTI mislocalizes progerin and improves nuclear blebbing, one could imagine that nonfarnesylated progerin could still be toxic in vivo. Nonfarnesylated progerin is still a structurally abnormal protein, and it is sobering to remember that even single amino acid substitutions in mature lamin A and lamin C (neither of which is farnesylated) can cause a host of different genetic diseases (e.g., several forms of muscular dystrophy, cardiomyopathy, partial lipodystrophy, and mandibuloacral dysplasia) (2, 3). Note in particular that some mutations in the carboxyl-terminal region of lamin A (i.e., the region not shared with lamin C) cause human genetic diseases (22, 23). Moreover, one could argue that improved nuclear blebbing in cultured cells might not be an accurate indicator of disease phenotypes at the whole-animal level, for the simple reason that some LMNA missense mutations have been reported to cause human disease without affecting nuclear shape in cultured fibroblasts (22). Last, a pessimist would certainly point out that HGPS is a dominant disease, caused by a single mutant chromosome. Treatment with an FTI interferes with the biogenesis of mature lamin A from the normal LMNA allele. To our knowledge, the short or long-term consequences of reducing lamin A biogenesis in humans are not known, although a single LMNA null allele, causing a 50% reduction in both lamin A and lamin C synthesis, causes muscular dystrophy (24). In this study, we sought to create a HGPS allele that yielded large amounts of progerin, and our strategy was successful. Although it may have been technically simpler to introduce the codon 608 point mutation identified in humans with HGPS (24), we did not follow this approach because we worried that that the point mutation approach would not yield sufficient amounts of progerin to elicit phenotypes in the mouse. In humans with HGPS, most of the transcripts from the mutant LMNA allele actually yield wild-type lamin C and wild-type lamin A rather than progerin (1). We worried about this type of an allele for mouse experimentation because experience with mouse Lmna mutations has suggested that mice may be tougher than humans when it comes to the dose of mutant lamin proteins required to elicit disease phenotypes. For example, humans carrying a single LMNA H222P mutation develop muscular dystrophy, whereas two copies of the H222P allele are required to elicit a muscle phenotype in mice (25). Similarly, humans heterozygous for a LMNA nonsense mutation develop muscular dystrophy (24), whereas mice with a single Lmna knockout allele are normal (26). Thus, we worried that the point mutation approach in the mouse might not yield sufficient levels of progerin to elicit phenotypes. Accordingly, we chose a gene-targeting strategy that would guarantee high levels of progerin expression. As it turned out, we generated the first Lmna mutant mice that exhibit a phenotype with a single copy of the mutant allele. Progerin is clearly the culprit molecule in HGPS (1, 13, 18), and studies raise the hope that FTIs might ultimately prove to be useful for treating HGPS. The FTI strategy appears to be well suited for testing in children because the drugs have been studied extensively, are generally well tolerated, and can be given orally. However, FTIs are not the only potential hope for HGPS. Recent studies have indicated that the nuclear blebbing phenotype in HGPS fibroblasts can be ameliorated with morpholino antisense reagents (18) or by expressing short hairpin RNA constructs (RNA interference) (Junko Oshima, personal communication). These RNAi and antisense methods directly reduce the production of progerin transcripts, so the finding of reduced nuclear blebbing is probably not particularly surprising. Nevertheless, those studies were very important because they provided hope to patients affected by HGPS and because they will stimulate interest in overcoming the practical and pharmacological obstacles to delivering RNAi and morpholino oligonucleotides to humans. We thank Dr. Luanne Peters for counting chromosomes in the Lmna HG/HG ES cells. This work was supported in part by National Institutes of Health Grants AI (to M.H.G.), R01 CA099506, and R01 AR and a grant from the Progeria Research Foundation (to S.G.Y.). MEDICAL SCIENCES 1. Eriksson, M., Brown, W. T., Gordon, L. B., Glynn, M. W., Singer, J., Scott, L., Erdos, M. R., Robbins, C. M., Moses, T. Y., Berglund, P., et al. (2003) Nature 423, Mounkes, L. C., Burke, B. & Stewart, C. L. (2001) Trends Cardiovasc. Med. 11, Burke, B. & Stewart, C. L. (2002) Nat. Rev. Mol. Cell Biol. 3, Bergo, M. O., Gavino, B., Ross, J., Schmidt, W. K., Hong, C., Kendall, L. V., Mohr, A., Meta, M., Genant, H., Jiang, Y., et al. (2002) Proc. Natl. Acad. Sci. USA 99, Dai, Q., Choy, E., Chiu, V., Romano, J., Slivka, S. R., Steitz, S. A., Michaelis, S. & Philips, M. R. (1998) J. Biol. Chem. 273, Pendás, A. M., Zhou, Z., Cadiñanos, J., Freije, J. M. P., Wang, J., Hultenby, K., Astudillo, A., Wernerson, A., Rodríguez, F., Tryggvason, K. & Lopéz-Otín, C. (2002) Nat. Genet. 31, Corrigan, D. P., Kuszczak, D., Rusinol, A. E., Thewke, D. P., Hrycyna, C. A., Michaelis, S. & Sinensky, M. S. (2005) Biochem. J. 387, Hennekes, H. & Nigg, E. A. (1994) J. Cell Sci. 107, Lutz, R. J., Trujillo, M. A., Denham, K. S., Wenger, L. & Sinensky, M. (1992) Proc. Natl. Acad. Sci. USA 89, Izumi, M., Vaughan, O. A., Hutchison, C. J. & Gilbert, D. M. (2000) Mol. Biol. Cell 11, Dalton, M. & Sinensky, M. (1995) Methods Enzymol. 250, Fong, L. G., Ng, J. K., Meta, M., Cote, N., Yang, S. H., Stewart, C. L., Sullivan, T., Burghardt, A., Majumdar, S., Reue, K., et al. (2004) Proc. Natl. Acad. Sci. USA 101, Goldman, R. D., Shumaker, D. K., Erdos, M. R., Eriksson, M., Goldman, A. E., Gordon, L. B., Gruenbaum, Y., Khuon, S., Mendez, M., Varga, R. & Collins, F. S. (2004) Proc. Natl. Acad. Sci. USA 101, Yang et al. PNAS July 19, 2005 vol. 102 no
6 14. Mortensen, R. M., Conner, D. A., Chao, S., Geisterfer-Lowrance, A. A. T. & Seidman, J. G. (1992) Mol. Cell. Biol. 12, Nallan, L., Bauer, K. D., Bendale, P., Rivas, K., Yokoyama, K., Hornéy,C.P., Pendyala, P. R., Floyd, D., Lombardo, L. J., Williams, D. K., et al. (2005) J. Med. Chem. 48, Raharjo, W. H., Enarson, P., Sullivan, T., Stewart, C. L. & Burke, B. (2001) J. Cell Sci. 114, Reiss, Y., Goldstein, J. L., Seabra, M. C., Casey, P. J. & Brown, M. S. (1990) Cell 62, Scaffidi, P. & Misteli, T. (2005) Nat. Med. 11, Caraglia, M., D Alessandro, A. M., Marra, M., Giuberti, G., Vitale, G., Viscomi, C., Colao, A., Prete, S. D., Tagliaferri, P., Tassone, P., et al. (2004) Oncogene 23, Rogers, M. J. (2003) Curr. Pharm. Des. 9, Mijimolle, N., Velasco, J., Dubus, P., Guerra, C., Weinbaum, C. A., Casey, P. J., Campuzano, V. & Barbacid, M. (2005) Cancer Cell 7, Muchir, A., Medioni, J., Laluc, M., Massart, C., Arimura, T., van der Kooi, A. J., Desguerre, I., Mayer, M., Ferrer, X., Briault, S., et al. (2004) Muscle Nerve 30, Csoka, A. B., Cao, H., Sammak, P. J., Constantinescu, D., Schatten, G. P. & Hegele, R. A. (2004) J. Med. Genet. 41, Bonne, G., Di Barletta, M. R., Varnous, S., Bécane, H.-M., Hammouda, E.-H., Merlini, L., Muntoni, F., Greenberg, C. R., Gary, F., Urtizberea, J.-A., et al. (1999) Nat. Genet. 21, Arimura, T., Helbling-Leclerc, A., Massart, C., Varnous, S., Niel, F., Lacene, E., Fromes, Y., Toussaint, M., Mura, A. M., Keller, D. I., et al. (2005) Hum. Mol. Genet. 14, Sullivan, T., Escalante-Alcalde, D., Bhatt, H., Anver, M., Bhat, N., Nagashima, K., Stewart, C. L. & Burke, B. (1999) J. Cell Biol. 147, cgi doi pnas Yang et al.
Prelamin A and lamin A appear to be dispensable in the nuclear lamina
 Related Commentary, page 632 Research article Prelamin A and lamin A appear to be dispensable in the nuclear lamina Loren G. Fong, 1 Jennifer K. Ng, 2 Jan Lammerding, 3 Timothy A. Vickers, 4 Margarita
Related Commentary, page 632 Research article Prelamin A and lamin A appear to be dispensable in the nuclear lamina Loren G. Fong, 1 Jennifer K. Ng, 2 Jan Lammerding, 3 Timothy A. Vickers, 4 Margarita
Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson Gilford progeria syndrome
 Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson Gilford progeria syndrome Brian C. Capell*, Michael R. Erdos*, James P. Madigan, James J. Fiordalisi, Renee
Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson Gilford progeria syndrome Brian C. Capell*, Michael R. Erdos*, James P. Madigan, James J. Fiordalisi, Renee
Inhibitors of protein geranylgeranyltransferase-i lead to prelamin A accumulation in cells by inhibiting ZMPSTE24
 Inhibitors of protein geranylgeranyltransferase-i lead to prelamin A accumulation in cells by inhibiting ZMPSTE24 Sandy Y. Chang 1, Sarah E. Hudon-Miller 3, Shaohong Yang 1, Hea-Jin Jung 4, John M. Lee
Inhibitors of protein geranylgeranyltransferase-i lead to prelamin A accumulation in cells by inhibiting ZMPSTE24 Sandy Y. Chang 1, Sarah E. Hudon-Miller 3, Shaohong Yang 1, Hea-Jin Jung 4, John M. Lee
Inhibitors of protein geranylgeranyltransferase-i lead to. prelamin A accumulation in cells by inhibiting ZMPSTE24
 Inhibitors of protein geranylgeranyltransferase-i lead to prelamin A accumulation in cells by inhibiting ZMPSTE24 Sandy Y. Chang, * Sarah E. Hudon-Miller, Shao H. Yang, * Hea-Jin Jung, John M. Lee,* Emily
Inhibitors of protein geranylgeranyltransferase-i lead to prelamin A accumulation in cells by inhibiting ZMPSTE24 Sandy Y. Chang, * Sarah E. Hudon-Miller, Shao H. Yang, * Hea-Jin Jung, John M. Lee,* Emily
Laminopathies and the long strange trip from basic cell biology to therapy
 Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
HMG Advance Access published August 26, 2005
 HMG Advance Access published August 26, 2005 Glynn & Glover 1 Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase
HMG Advance Access published August 26, 2005 Glynn & Glover 1 Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase
Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24
 Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24 Jemima Barrowman, Corinne Hamblet, Megan S. Kane, Susan Michaelis* Department of Cell Biology, The Johns Hopkins School of Medicine,
Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24 Jemima Barrowman, Corinne Hamblet, Megan S. Kane, Susan Michaelis* Department of Cell Biology, The Johns Hopkins School of Medicine,
HYBRID GENE THERAPY FOR AD-EDMD
 HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells
 HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells Catherine Coffinier*, Sarah E. Hudon, Emily A. Farber*, Sandy Y. Chang*, Christine A.
HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells Catherine Coffinier*, Sarah E. Hudon, Emily A. Farber*, Sandy Y. Chang*, Christine A.
Progeria. Premature human aging: t he progerias. Progerias as models for aging. Hutchinson-Gilford syndrome. Definition:
 Premature human aging: t he progerias Reading: Genetic alterations in accelerated ageing syndromes Do they play a role in natural ageing? Monika Puzianowska- Kuznicka. Jacek Kuznicki. 2005. IJBCB, 37;
Premature human aging: t he progerias Reading: Genetic alterations in accelerated ageing syndromes Do they play a role in natural ageing? Monika Puzianowska- Kuznicka. Jacek Kuznicki. 2005. IJBCB, 37;
CORRECTIONS. PNAS August 4, 2009 vol. 106 no
 MEDICAL SCIENCES Correction for A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model, by Brian C. Capell, Michelle Olive, Michael
MEDICAL SCIENCES Correction for A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model, by Brian C. Capell, Michelle Olive, Michael
Progeria, the nucleolus and farnesyltransferase inhibitors
 Nuclear Envelope Disease and Chromatin Organization 2009 287 Progeria, the nucleolus and farnesyltransferase inhibitors Ishita S. Mehta*, Joanna M. Bridger* and Ian R. Kill 1 *Laboratory of Nuclear and
Nuclear Envelope Disease and Chromatin Organization 2009 287 Progeria, the nucleolus and farnesyltransferase inhibitors Ishita S. Mehta*, Joanna M. Bridger* and Ian R. Kill 1 *Laboratory of Nuclear and
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria syndrome
 JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
Importance of molecular cell biology investigations in human medicine in the story of the Hutchinson-Gilford progeria syndrome
 Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Rescue of heterochromatin organization in Hutchinson- Gilford progeria by drug treatment
 Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
Mouse Models of K-RAS- and B-RAFinduced
 Mouse Models of K-RAS- and B-RAFinduced Cancer Martin O. Bergö, Professor Sahlgrenska Cancer Center University of Gothenburg www.gu.se Why models? Why mice? Critical to understanding pathogenesis and identifying
Mouse Models of K-RAS- and B-RAFinduced Cancer Martin O. Bergö, Professor Sahlgrenska Cancer Center University of Gothenburg www.gu.se Why models? Why mice? Critical to understanding pathogenesis and identifying
The LMNA gene encodes the A-type lamins, including lamin A
 A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
Nuclear Envelope and Muscular Dystrophy
 Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson- Gilford progeria fibroblasts
 /, 2017, Vol. 8, (No. 39), pp: 64809-64826 Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson- Gilford progeria fibroblasts Diana Gabriel
/, 2017, Vol. 8, (No. 39), pp: 64809-64826 Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson- Gilford progeria fibroblasts Diana Gabriel
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Hutchinson-Gilford Progeria Syndrome. Mohamed Ibrahim
 Hutchinson-Gilford Progeria Syndrome A new treatment strategy and the role of prelamin A in oncogenesis Mohamed Ibrahim Institute of Medicine Department of Molecular and Clinical Medicine Sahlgrenska Academy
Hutchinson-Gilford Progeria Syndrome A new treatment strategy and the role of prelamin A in oncogenesis Mohamed Ibrahim Institute of Medicine Department of Molecular and Clinical Medicine Sahlgrenska Academy
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Luminescent platforms for monitoring changes in the solubility of amylin and huntingtin in living cells
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Pelagia Research Library. Hutchinson Gilford Progeria Syndrome: A Review
 Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2011, 2 (1): 110-117 ISSN: 0976-8688 CODEN (USA): PSHIBD Hutchinson Gilford Progeria Syndrome: A Review Pankaj Rakha* 1, 2, Arun
Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2011, 2 (1): 110-117 ISSN: 0976-8688 CODEN (USA): PSHIBD Hutchinson Gilford Progeria Syndrome: A Review Pankaj Rakha* 1, 2, Arun
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling
 4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Hutchinson-Gilford Progeria Syndrome and its Relevance to Cardiovascular Diseases and Normal Aging
 382 Biomed Environ Sci, 2013; 26(5): 382-389 Review Hutchinson-Gilford Progeria Syndrome and its Relevance to Cardiovascular Diseases and Normal Aging QI Ying Chun and XIE Xiao Hua # First Department of
382 Biomed Environ Sci, 2013; 26(5): 382-389 Review Hutchinson-Gilford Progeria Syndrome and its Relevance to Cardiovascular Diseases and Normal Aging QI Ying Chun and XIE Xiao Hua # First Department of
Clinical Cell Biology Organelles in Health and Disease
 Department of Ophthalmology University of Kiel, University Medical Center Director: Prof. Dr. Johann Roider Clinical Cell Biology Organelles in Health and Disease Prof. Dr. Alexa Klettner Clinical cell
Department of Ophthalmology University of Kiel, University Medical Center Director: Prof. Dr. Johann Roider Clinical Cell Biology Organelles in Health and Disease Prof. Dr. Alexa Klettner Clinical cell
Nature Medicine: doi: /nm.4322
 1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. Predicted RNA structure of 3 UTR and sequence alignment of deleted nucleotides. (a) Predicted RNA secondary structure of ZIKV 3 UTR. The stem-loop structure
Eiger BioPharmaceuticals Reports on 2018 R&D Day
 Eiger BioPharmaceuticals Reports on 2018 R&D Day Late Stage Rare and Ultra-Rare Disease Pipeline Advancing >$100M in Cash Available to Achieve Key Milestones PALO ALTO, Calif., December 11, 2018 Eiger
Eiger BioPharmaceuticals Reports on 2018 R&D Day Late Stage Rare and Ultra-Rare Disease Pipeline Advancing >$100M in Cash Available to Achieve Key Milestones PALO ALTO, Calif., December 11, 2018 Eiger
The Biology and Genetics of Cells and Organisms The Biology of Cancer
 The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Amersham ECL Prime Western blotting reagent
 GE Healthcare Life Sciences Data file 28-9857-23 AA Western blotting reagents Amersham ECL Prime Western blotting reagent Since its introduction in 199, the enhanced chemiluminescence (ECL) Western Blotting
GE Healthcare Life Sciences Data file 28-9857-23 AA Western blotting reagents Amersham ECL Prime Western blotting reagent Since its introduction in 199, the enhanced chemiluminescence (ECL) Western Blotting
The subcortical maternal complex controls symmetric division of mouse zygotes by
 The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics Xing-Jiang Yu 1,2, Zhaohong Yi 1, Zheng Gao 1,2, Dan-dan Qin 1,2, Yanhua Zhai 1, Xue Chen 1,
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
100 mm Sucrose. +Berberine +Quinine
 8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep
 SUPPLEMENTARY INFORMATION The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness Jinyi Zhang, Naima
SUPPLEMENTARY INFORMATION The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness Jinyi Zhang, Naima
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome
 Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
Reviews. Mechanisms Underlying Caloric Restriction, Lipid Metabolism, and Life Span Regulation Issei Komuro, Guest Editor
 Reviews This Review is part of a thematic series on Biological Role of Senescence in Cardiovascular Disease, which includes the following articles: Telomere Biology and Cardiovascular Disease Vascular
Reviews This Review is part of a thematic series on Biological Role of Senescence in Cardiovascular Disease, which includes the following articles: Telomere Biology and Cardiovascular Disease Vascular
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity
 Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity Jemima Barrowman 1, Patricia A. Wiley 2, Sarah E. Hudon-Miller 2, Christine A. Hrycyna 2 and Susan Michaelis
Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity Jemima Barrowman 1, Patricia A. Wiley 2, Sarah E. Hudon-Miller 2, Christine A. Hrycyna 2 and Susan Michaelis
Embryonic Senescence and Laminopathies in a Progeroid Zebrafish Model
 Embryonic Senescence and Laminopathies in a Progeroid Zebrafish Model Eriko Koshimizu 1,2, Shintaro Imamura 1 a, Jie Qi 1,5 b, Jamal Toure 1, Delgado M. Valdez Jr. 5, Christopher E. Carr 3, Jun-ichi Hanai
Embryonic Senescence and Laminopathies in a Progeroid Zebrafish Model Eriko Koshimizu 1,2, Shintaro Imamura 1 a, Jie Qi 1,5 b, Jamal Toure 1, Delgado M. Valdez Jr. 5, Christopher E. Carr 3, Jun-ichi Hanai
AP VP DLP H&E. p-akt DLP
 A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Automated image analysis of nuclear shape: What can we learn from a prematurely aged cell?
 www.impactaging.com AGING, February 2012, Vol. 4, No 2 Research Paper Automated image analysis of nuclear shape: What can we learn from a prematurely aged cell? Meghan K. Driscoll 1, Jason L. Albanese
www.impactaging.com AGING, February 2012, Vol. 4, No 2 Research Paper Automated image analysis of nuclear shape: What can we learn from a prematurely aged cell? Meghan K. Driscoll 1, Jason L. Albanese
Cell Membranes. Dr. Diala Abu-Hassan School of Medicine Cell and Molecular Biology
 Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
TITLE: The Role of hcdc4 as a Tumor Suppressor Gene in Genomic Instability Underlying Prostate Cancer
 AD Award Number: TITLE: The Role of hcdc4 as a Tumor Suppressor Gene in Genomic Instability Underlying Prostate Cancer PRINCIPAL INVESTIGATOR: Audrey van Drogen, Ph.D. CONTRACTING ORGANIZATION: Sidney
AD Award Number: TITLE: The Role of hcdc4 as a Tumor Suppressor Gene in Genomic Instability Underlying Prostate Cancer PRINCIPAL INVESTIGATOR: Audrey van Drogen, Ph.D. CONTRACTING ORGANIZATION: Sidney
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Problem Set 8 Key 1 of 8
 7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
MRC-Holland MLPA. Description version 12; 13 January 2017
 SALSA MLPA probemix P219-B3 PAX6 Lot B3-0915: Compared to version B2 (lot B2-1111) two reference probes have been replaced and one additional reference probe has been added. In addition, one flanking probe
SALSA MLPA probemix P219-B3 PAX6 Lot B3-0915: Compared to version B2 (lot B2-1111) two reference probes have been replaced and one additional reference probe has been added. In addition, one flanking probe
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
s u p p l e m e n ta ry i n f o r m at i o n
 Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1
 Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
IN the spring of 2007, a 2-year clinical drug trial began at
 Journal of Gerontology: BIOLOGICAL SCIENCES 2008, Vol. 63A, No. 8, 777 787 Copyright 2008 by The Gerontological Society of America Meeting Report Highlights of the 2007 Progeria Research Foundation Scientific
Journal of Gerontology: BIOLOGICAL SCIENCES 2008, Vol. 63A, No. 8, 777 787 Copyright 2008 by The Gerontological Society of America Meeting Report Highlights of the 2007 Progeria Research Foundation Scientific
Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy
 Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Generation of a conditional allele of the Kindlin-2 gene. (A) A restriction map of the relevant genomic region of Kindlin-2 (top), the targeting construct
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Generation of a conditional allele of the Kindlin-2 gene. (A) A restriction map of the relevant genomic region of Kindlin-2 (top), the targeting construct
Probe. Hind III Q,!?R'!! /0!!!!D1"?R'! vector. Homologous recombination
 Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
Postn MCM Smad2 fl/fl Postn MCM Smad3 fl/fl Postn MCM Smad2/3 fl/fl. Postn MCM. Tgfbr1/2 fl/fl TAC
 A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
Supplemental Materials. STK16 regulates actin dynamics to control Golgi organization and cell cycle
 Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Bio 111 Study Guide Chapter 17 From Gene to Protein
 Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
p = formed with HCI-001 p = Relative # of blood vessels that formed with HCI-002 Control Bevacizumab + 17AAG Bevacizumab 17AAG
 A.. Relative # of ECs associated with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 ol b p < 0.001 Relative # of blood vessels that formed with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 l b p = 0.002 Control IHC:
A.. Relative # of ECs associated with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 ol b p < 0.001 Relative # of blood vessels that formed with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 l b p = 0.002 Control IHC:
Supplementary Figure 1. Generation of knockin mice expressing L-selectinN138G. (a) Schematics of the Sellg allele (top), the targeting vector, the
 Supplementary Figure 1. Generation of knockin mice expressing L-selectinN138G. (a) Schematics of the Sellg allele (top), the targeting vector, the targeted allele in ES cells, and the mutant allele in
Supplementary Figure 1. Generation of knockin mice expressing L-selectinN138G. (a) Schematics of the Sellg allele (top), the targeting vector, the targeted allele in ES cells, and the mutant allele in
Structural vs. nonstructural proteins
 Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts
 Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts (TIG-3 cells) were rendered senescent by either serial passage
Supplementary Figure 1 Induction of cellular senescence and isolation of exosome. a to c, Pre-senescent primary normal human diploid fibroblasts (TIG-3 cells) were rendered senescent by either serial passage
Electron micrograph of phosphotungstanic acid-stained exosomes derived from murine
 1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
1 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Physical properties of murine DC-derived exosomes. a, Electron micrograph of phosphotungstanic acid-stained exosomes derived from
Renata Schipp Medical Biology Department
 Renata Schipp Medical Biology Department Deffinition of cell The cell is the smallest structural and functional unit of all known living organisms The cell was discovered by Robert Hooke in 1665 and also
Renata Schipp Medical Biology Department Deffinition of cell The cell is the smallest structural and functional unit of all known living organisms The cell was discovered by Robert Hooke in 1665 and also
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
BCH Graduate Survey of Biochemistry
 BCH 5045 Graduate Survey of Biochemistry Instructor: Charles Guy Producer: Ron Thomas Director: Glen Graham Lecture 7 Slide sets available at: http://hort.ifas.ufl.edu/teach/guyweb/bch5045/index.html David
BCH 5045 Graduate Survey of Biochemistry Instructor: Charles Guy Producer: Ron Thomas Director: Glen Graham Lecture 7 Slide sets available at: http://hort.ifas.ufl.edu/teach/guyweb/bch5045/index.html David
Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene
 Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene YUELIN ZHANG, WEIHUA FAN, MARK KINKEMA, XIN LI, AND
Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene YUELIN ZHANG, WEIHUA FAN, MARK KINKEMA, XIN LI, AND
Cerebral Small Vessel Disease and HAND in ARV-treated Subjects
 Cerebral Small Vessel Disease and HAND in ARV-treated Subjects Cristian L. Achim, MD, PhD Ronald J. Ellis, MD, PhD Virawudh Soontornniyomkij, MD ARROW, Bucharest, Romania October 5-6, 2015 Rationale and
Cerebral Small Vessel Disease and HAND in ARV-treated Subjects Cristian L. Achim, MD, PhD Ronald J. Ellis, MD, PhD Virawudh Soontornniyomkij, MD ARROW, Bucharest, Romania October 5-6, 2015 Rationale and
Differentiation-induced Changes of Mediterranean Fever Gene (MEFV) Expression in HL-60 Cell
 Differentiation-induced Changes of Mediterranean Fever Gene (MEFV) Expression in HL-60 Cell Wenxin Li Department of Biological Sciences Fordham University Abstract MEFV is a human gene that codes for an
Differentiation-induced Changes of Mediterranean Fever Gene (MEFV) Expression in HL-60 Cell Wenxin Li Department of Biological Sciences Fordham University Abstract MEFV is a human gene that codes for an
Genome-editing via Oviductal Nucleic Acids Delivery (GONAD) system: a novel microinjection-independent genome engineering method in mice
 Supplementary Information Genome-editing via Oviductal Nucleic Acids Delivery (GONAD) system: a novel microinjection-independent genome engineering method in mice Gou Takahashi, Channabasavaiah B Gurumurthy,
Supplementary Information Genome-editing via Oviductal Nucleic Acids Delivery (GONAD) system: a novel microinjection-independent genome engineering method in mice Gou Takahashi, Channabasavaiah B Gurumurthy,
Life Sciences 1A Midterm Exam 2. November 13, 2006
 Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Generation and validation of mtef4-knockout mice.
 Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Spherical Nucleic Acids For Advanced Wound Healing Applications Chad A. Mirkin
 Spherical Nucleic Acids For Advanced Wound Healing Applications Chad A. Mirkin Departments of Chemistry, Infectious Disease, Materials Science & Engineering, Chemical & Biological Engineering, and Biomedical
Spherical Nucleic Acids For Advanced Wound Healing Applications Chad A. Mirkin Departments of Chemistry, Infectious Disease, Materials Science & Engineering, Chemical & Biological Engineering, and Biomedical
Supplementary methods:
 Supplementary methods: Primers sequences used in real-time PCR analyses: β-actin F: GACCTCTATGCCAACACAGT β-actin [11] R: AGTACTTGCGCTCAGGAGGA MMP13 F: TTCTGGTCTTCTGGCACACGCTTT MMP13 R: CCAAGCTCATGGGCAGCAACAATA
Supplementary methods: Primers sequences used in real-time PCR analyses: β-actin F: GACCTCTATGCCAACACAGT β-actin [11] R: AGTACTTGCGCTCAGGAGGA MMP13 F: TTCTGGTCTTCTGGCACACGCTTT MMP13 R: CCAAGCTCATGGGCAGCAACAATA
