Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy
|
|
|
- Barnaby Fletcher
- 6 years ago
- Views:
Transcription
1 RESEARCH ARTICLE 4435 Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy Cecilia Östlund 1, Gisèle Bonne 2, Ketty Schwartz 2 and Howard J. Worman 1, * 1 Departments of Medicine and of Anatomy and Cell Biology, College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA 2 INSERM UR523, Institut de Myologie, GH Pitié-Salpétrière, Paris, France *Author for correspondence ( hjw14@columbia.edu) Accepted 6 September 2001 Journal of Cell Science 114, (2001) The Company of Biologists Ltd SUMMARY Autosomal dominant Emery-Dreifuss muscular dystrophy is caused by mutations in the LMNA gene, which encodes lamin A and lamin C. Mutations in this gene also give rise to limb girdle muscular dystrophy type 1B, dilated cardiomyopathy with atrioventricular conduction defect and Dunnigan-type partial lipodystrophy. The properties of the mutant lamins that cause muscular dystrophy, lipodystrophy and dilated cardiomyopathy are not known. We transfected C2C12 myoblasts with cdna encoding wild-type lamin A and 15 mutant forms found in patients affected by these diseases. Immunofluorescence microscopy showed that four mutants, N195K, E358K, M371K and R386K, could have a dramatically aberrant localization, with decreased nuclear rim staining and formation of intranuclear foci. The distributions of endogenous lamin A/C, lamin B1 and lamin B2 were also altered in cells expressing these four mutants and three of them caused a loss of emerin from the nuclear envelope. In the yeast twohybrid assay, the 15 lamin A mutants studied interacted with themselves and with wild-type lamin A and lamin B1. Pulse-chase experiments showed no decrease in the stability of several representative lamin A mutants compared with wild-type. These results indicate that some lamin A mutants causing disease can be aberrantly localized, partially disrupt the endogenous lamina and alter emerin localization, whereas others localize normally in transfected cells. Key words: Nuclear envelope, Lamins, Intermediate filaments, Muscular dystrophy, Lipodystrophy, Cardiomyopathy INTRODUCTION Emery-Dreifuss muscular dystrophy (EDMD) is characterized by contractures of the elbows, Achilles tendons and posterior neck, slow progressive muscle wasting and cardiomyopathy with atrioventricular conduction block (Emery and Dreifuss, 1966; Rowland et al., 1979; Emery, 1989). Emery-Dreifuss muscular dystrophy is inherited in both an autosomal dominant (AD-EDMD, OMIM ) and an X-linked, recessive (OMIM ) manner. In 1994, mutations in emerin, an integral protein of the nuclear envelope inner membrane, were shown to be responsible for X-linked EDMD (Bione et al., 1994; Manilal et al., 1996; Nagano et al., 1996). The connection between muscle disease and mutations in a nuclear envelope protein was unexpected, but was further established when Bonne et al. (Bonne et al., 1999) showed that AD- EDMD, a phenotypically indistinguishable syndrome, is caused by mutations in the LMNA gene, which encodes the nuclear envelope proteins lamins A and C. Mutations in LMNA have also been shown to cause limb girdle muscular dystrophy 1B (LGMD1B, OMIM ), a disease that differs from AD-EDMD by displaying a different distribution of affected skeletal muscle, a later occurrence of cardiac problems and an absence of early contractures (Muchir et al., 2000), and dilated cardiomyopathy without skeletal muscle pathology (OMIM ) (Fatkin et al., 1999; Bécane et al., 2000). LMNA mutations that cause cardiac and skeletal muscle disease are found throughout the gene, most being point mutations resulting in amino acid substitutions. There appears to be no clear correlation between the site of mutation and the severity of disease (Bonne et al., 2000). By contrast, mutations in LMNA clustering only in exons 8 and 11 have been shown to cause Dunnigan-type familial partial lipodystrophy (FPLD, OMIM ) (Cao and Hegele, 2000; Shackleton et al., 2000; Speckman et al., 2000; Vigouroux et al., 2000). FPLD is a rare, autosomal dominant disease characterized by the loss of subcutaneous adipose tissue from the extremities and trunk after the onset of puberty (Köbberling and Dunnigan, 1986). Lamins are intermediate filament proteins that form the nuclear lamina, a meshwork on the nucleoplasmic side of the inner nuclear membrane (Aebi et al., 1986; Fisher et al., 1986; McKeon et al., 1986; Stuurman et al., 1998; Worman and Courvalin, 2000). Two general types of lamins have been identified in somatic cells, A-type and B-type. The somatic cell A-type lamins, lamin A, lamin C and lamin A 10, are alternative splice isoforms encoded by the LMNA gene (Lin and Worman, 1993; Machiels et al., 1996). Two different genes encode the somatic cell B-type lamins, lamin B1 and lamin B2 (Biamonti et al., 1992; Lin and Worman, 1995). Like all intermediate filament proteins, lamins have a conserved central rod-domain containing three α-helical segments, which is responsible for the formation of coiled-coil dimers. The rod-
2 4436 JOURNAL OF CELL SCIENCE 114 (24) domain is flanked by N-terminal head and C-terminal taildomains, which vary significantly between different proteins (Steinert and Roop, 1988). The lamins bind to some of the integral membrane proteins of the inner nuclear envelope (Worman and Courvalin, 2000). Lamin A interacts with emerin and lamina associated polypeptide 1 (LAP1) in vitro (Foisner and Gerace, 1993; Clements et al., 2000; Sakaki et al., 2001). Because the functions of lamins A and C are poorly understood, the mechanisms by which mutations cause different inherited diseases are not clear (Worman and Courvalin, 2000; Hutchison et al., 2001). Currently, it is not known if any of the disease-causing mutations of lamin A alter protein localization, lamina structure, protein-protein interactions or protein stability. We therefore investigated these properties of mutant forms of lamin A found in patients with inherited diseases. MATERIALS AND METHODS Plasmid construction All cloning procedures were performed according to standard methods (Sambrook et al., 1989). For expression in mammalian cells, constructs that expressed FLAG-tagged polypeptides were made in psvk3 (Amersham Pharmacia Biotech, Inc., Piscataway, NJ), which contains a multiple cloning site downstream from the SV-40 early promoter. cdna encoding prelamin A, cloned into the BamHI and SalI restriction sites of plasmid pgbt9 (Ye and Worman, 1995), was excised by restriction endonuclease digestion with SmaI and SalI and ligated into pbft4, digested with the same restriction enzymes. pbft4 is a pbluescript II KS- (Stratagene, La Jolla, CA) based plasmid containing a Kozak sequence, ATG and the FLAG-tag coding sequence 5 to the multiple cloning site. The resulting plasmid was digested with SpeI and XhoI to excise a cdna encoding ATG-FLAGprelamin A that was ligated into psvk3, digested with XbaI (an isoschizomer of SpeI) and XhoI. cdnas encoding mutant forms of prelamin A, except the NLA mutant, which lacked amino acids 1-33, were made using the Transformer TM Site-Directed Mutagenesis Kit (CLONTECH Laboratories, Inc., Palo Alto, CA), following the manufacturer s instructions. NLA was generated by polymerase chain reaction (Saiki et al., 1987), using the Gene Amp PCR System 2400 (Applied Biosystems, Foster City, CA), with a SmaI restriction site engineered at the 5 end of the sense primer and an antisense primer spanning the ApaI site found at nucleotide 746 of prelamin A cdna. Reaction products were digested with SmaI and ApaI and ligated into prelamin A-pBGT9 digested with the same enzymes. The resulting plasmid was digested with SmaI and SalI and ligated into the SmaI and SalI restriction sites of psvf, a vector constructed by insertion of the FLAG-epitope between the EcoRI and KpnI restriction sites of psvk3. For studies with the yeast two-hybrid system, wild-type prelamin A cdna in vectors pgad424 and pgbt9 and lamin B1 cdna in vector pgbt9 were those previously described (Ye and Worman, 1995). cdnas obtained by site-directed mutagenesis were excised from vector psvk3 using the restriction endonucleases SmaI and SalI and ligated into pgbt9 and pgad424 digested with the same enzymes. NLA was inserted into pgbt9 as described above and then digested with SmaI and SalI and ligated into the SmaI and SalI sites of pgad424. All cdnas were sequenced using an ABI Prism 377 automated sequencer (Applied Biosystems). Cell culture, transfection and immunofluorescence microscopy C2C12 cells (a gift from Hal Skopicki, Columbia University, New York, NY) were grown in Dulbecco s modified Eagle medium (D-MEM) containing 10% fetal bovine serum (Life Technologies, Gaithersburg, MD) at 37 C and 10% CO 2. Cells were transfected in chamber slides using Lipofectamine PLUS TM (Life Technologies), following the manufacturer s instructions. The cells were overlaid with the lipid-dna complexes for approximately 23 hours, the first five of which were in serum-free medium. The cells were then allowed to grow in fresh medium for 24 hours post-transfection before preparation for immunofluorescence microscopy. Fixation and labeling of cells for immunofluorescence microscopy were performed as described previously (Östlund et al., 1999). The monoclonal primary antibodies used were anti-flag M5 (Sigma, St Louis, MO) diluted 1:200, anti-pcna (proliferating cell nuclear antigen) P10 (Roche Molecular Biochemicals, Indianapolis, IN) diluted 1:20, anti-nuclear pore complex MAb414 (a gift from Tarik Soliman, Laboratory of Cell Biology, Rockefeller University, New York, NY) diluted 1:5,000 and anti-lamin B2 X223 (a gift from Georg Krohne, University of Würzburg, Würzburg, Germany) diluted 1:400. Polyclonal primary antibodies used were anti-lamin B1 (Cance et al., 1992) at a dilution of 1:2,000, anti-lamin A/C (Cance et al., 1992) at a dilution of 1:1,000, anti-emerin (a gift from Glenn Morris, North East Wales Institute, Wrexham, UK) at a dilution of 1:3,000 and anti- LAP2 (a gift from Katherine Wilson, Johns Hopkins University, Baltimore, MD) at a dilution of 1:500. Secondary antibodies used were Rhodamine Red TM -X-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), Alexa TM 568- conjugated goat anti-rabbit IgG (Molecular Probes, Inc., Eugene, OR) and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.). For DNAstaining, 20 µg/ml propidium iodide (Sigma) was added to the secondary antibody incubation. Labeled and washed slides were dipped in methanol, air-dried, and coverslips were mounted using ProLong Antifade Kit (Molecular Probes, Inc.) or SlowFade Light Antifade Kit (Molecular Probes, Inc.). Immunofluorescence microscopy was performed on a Zeiss LSM 410 confocal laser scanning system attached to a Zeiss Axiovert 100TV inverted microscope (Carl Zeiss, Inc., Thornwood, NY). Images were processed using Photoshop software (Adobe Systems, Inc., San Jose, CA) on a Macintosh G3 computer (Apple Computer, Inc., Cupertino, CA). Yeast two-hybrid assay Saccharomyces cerevisiae strain Y187 (Clontech Laboratories, Inc.) was transformed with plasmids encoding lamins fused to the DNA binding domain of the S. cerevisiae GAL4 protein (pgbt9) or to the GAL4 transcriptional activation domain (pgad424). Transformations and β-galactosidase assays were done according to the Matchmaker Two-Hybrid System manual (Clontech Laboratories, Inc.). Pulse-chase experiment COS-7 cells were grown in D-MEM containing 10% fetal bovine serum (Life Technologies) at 37 C and 5% CO 2. The cells were transfected using Lipofectamine PLUS TM (Life Technologies), following the manufacturer s instructions. The cells were overlaid with the lipid-dna complexes for approximately 23 hours, the first five of which were in serum-free medium, and were then grown in fresh medium for 24 hours before being subcultured at a 1:5 dilution into 60 mm tissue culture dishes. After another 24 hours, cells were washed with D-MEM without methionine and then overlaid with 1 ml D-MEM without methionine, containing 28 µci Pro-mix L-[ 35 S] in vitro cell labeling mix (Amersham Pharmacia Biotech, Inc.). After 1 hour, cells were washed twice with phosphate-buffered saline (PBS) and either incubated in non-radioactive D-MEM with 10% fetal bovine serum for an additional 3 or 25 hours or lysed immediately. For cell lysis, cells were washed three times in PBS and incubated for 40 minutes at 4 C in 440 µl lysis buffer (50 mm Tris-HCl, ph 8, 5 mm ethylenediaminetetraacetic acid, 0.1% sodium dodecyl sulfate
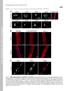
3 Lamin A mutations in disease 4437 (SDS), 1% Triton X-100) with 2% bovine serum albumin (BSA), 0.5 mm phenylmethylsulfonyl fluoride and 4.4 µl protease cocktail inhibitor (Sigma). The cells were then scraped off with a rubber policeman and sheared six times through a 21-gauge needle. After centrifugation for 10 minutes at 10,000 g, the supernatant was incubated overnight at 4 C with 30 µl anti-flag M2-agarose affinity gel (Sigma). The affinity gel was collected by centrifugation for 2 minutes at 325 g, washed three times in lysis buffer with 2% BSA, once with lysis buffer without BSA, once with 100 mm Tris-HCl (ph 6.8) and 0.5 M NaCl, and once with 100 mm Tris-HCl (ph 6.8). For the first, fourth and fifth washes, the gel was incubated for 5 minutes on a rotating wheel at 4 C. Twenty µl SDSsample buffer (Laemmli, 1970) was added to the affinity gel and the samples were incubated for 15 minutes at 70 C and centrifuged for 2 minutes at 325 g. The proteins in the supernatants were separated by SDS-polyacrylamide gel electrophoresis (PAGE). Gels were fixed for 30 minutes in 35% methanol, 10% acetic acid, incubated 30 minutes with Amplify (Amersham Pharmacia Biotech, Inc.), dried and exposed to Hyperfilm ECL (Amersham Pharmacia Biotech, Inc.) at 70 C. Other chemicals Unless otherwise indicated, routine chemicals were obtained from either Fisher Scientific Co. (Pittsburgh, PA) or Sigma. Enzymes for DNA cloning were obtained from either Fisher Scientific Co. or New England Biolabs (Beverly, MA). RESULTS Some lamin A mutants have a dramatically abnormal intranuclear localization To determine if the intracellular localizations of mutant forms of lamin A differ from that of the wild-type, we transiently transfected C2C12 mouse myoblasts with plasmids expressing wildtype and mutated forms of prelamin A. Prelamin A is a precursor form of mature lamin A, which is processed by endoproteolytic cleavage of the final 18 amino acids (Weber et al., 1989; Sinensky et al., 1994). For detection of expressed proteins, FLAG-epitopes were fused to the N- termini of the constructs. The mutations studied were: R60G, L85R, N195K and E203G, found in patients with dilated cardiomyopathy (Fatkin et al., 1999); E358K, M371K, R386K, R453W, W520S, R527P, T528K and L530P, identified in patients with AD-EDMD (Bonne et al., 1999; Bonne et al., 2000); and R482Q, R482W and K486N, found in patients with FPLD (Cao and Hegele, 2000; Shackleton et al., 2000) (Fig. 1A). In transfected cells, FLAG-tagged wild-type lamin A localized to the nuclear periphery, showing colocalization with lamin B1, a marker for the lamina and nuclear envelope (Fig. 1B). A majority of the FLAG-tagged lamin A mutants showed no gross abnormalities in cellular localization; however, four of the mutants, N195K, E358K, Fig. 1. (A) Lamin A and the localization of mutations. The N-terminus is on the left. Domains of lamin A are represented by: white, globular head and tail regions and linker regions in the rod-domain; stripes, α-helical rod-domain regions 1a, 1b and 2; black, the most highly conserved regions of the rod-domain. Underlined mutations are found in dilated cardiomyopathy, mutations in italics in FPLD and others in AD- EDMD. Mutations shown in red are proteins that form aberrant intranuclear foci when expressed in C2C12 cells (Fig. 1B; Table 1). (B) Cellular localization of FLAG-tagged wild-type and mutant forms of lamin A. The panels show laser scanning confocal immunofluorescence microscopy images of C2C12 cells transfected with wild-type prelamin A (WT) or prelamin A with missense mutations as indicated. Arrows show cells with nuclear foci, insets show nucleoplasmic staining in cells expressing mutant protein but lacking foci. Antibodies used were mouse monoclonal antibodies against FLAG recognized by FITC-conjugated secondary antibodies and rabbit polyclonal antibodies against lamin B1 recognized by Alexa TM -conjugated secondary antibodies. The pictures show an overlay of the FITC (green) and Alexa TM (red) channels where areas of colocalization appear yellow. Bar, 10 µm. M371K and R386K, showed a dramatically aberrant localization in many cells, accumulating in large intranuclear foci (Fig. 1B). These four mutants often showed a decrease in nuclear rim localization, giving a more diffuse, nucleoplasmic staining, which was also seen in cells lacking intranuclear foci
4 4438 JOURNAL OF CELL SCIENCE 114 (24) Table 1. Statistical analysis of nuclear foci in cells expressing lamin A mutants Cells with Cells without foci Cells with foci foci (%) WT ± ± R60G ± ± L85R ± ± N195K* ± ± E203G ± ± E358K* ± ± M371K* ± ± R386K* ± ± R453W ± ± R482Q ± ± R482W ± ± K486N ± ± W520S ± ± R527P ± ± T528K ± ± L530P ± ± NLA* ± ± Numbers shown are means from three experiments±s.e. unless otherwise stated. 200 transfected cells of each type were studied in each experiment. Nuclear foci smaller than 0.7 µm were not counted, as lamin foci smaller than this size frequently can be seen in all cells, including untransfected cells. Asterisks (*) denote statistically significant differences between mutant and wild-type (WT) cells in the number of cells with nuclear foci as determined by χ 2 -test (P<0.001). In all other cases, there were no significant differences (P>0.05). (Fig. 1B, insets). The diffuse, nucleoplasmic staining was particularly common for mutant R386K, with approximately 95% of transfected cells with or without large intranuclear foci showing this pattern. Mutants N195K, E358K and M371K showed a mostly diffuse, nucleoplasmic localization in 50-70% of transfected cells. Not all cells expressing the four foci-forming lamin A mutants contained large intranuclear foci. We counted 200 transfected cells expressing wild-type lamin A and each of the 15 mutants for nuclear foci. The results of these studies showed that there were significant numbers of cells with large intranuclear foci only in cells expressing the N195K, E358K, M371K and R386K mutants (Table 1). Only foci larger than 0.7 µm were counted, as many cells, including untransfected controls, frequently contained smaller foci of lamins in their nuclei. A localization of wild-type lamin A in small intranuclear foci has been reported previously (Moir et al., 1994; Broers et al., 1999). Immunofluorescence analysis of cells expressing foci-forming lamin A mutants To investigate further the composition of the abnormal nuclear foci formed by four of the lamin A mutants and their downstream effects on the nuclear envelope, we used several different markers to perform a detailed immunofluorescence microscopy analysis. Mislocalization of the lamin A mutants was often accompanied by disturbances in the localization of endogenous lamin B1. In many cells, lamin B1 was found in the interior of the intranuclear foci, with the mutant lamin A being more concentrated at the edges (Fig. 2). This phenotype was seen to a variable degree, being more frequent with mutants E358K and R386K (approximately 90% of cells with Fig. 2. Aberrant cellular localization of lamin A mutants is often accompanied by a change in lamin B1 localization. The panels show laser scanning confocal immunofluorescence microscopy images of C2C12 cells expressing either wild-type lamin A (WT) or lamin A with missense mutations as indicated, fused to a FLAG-epitope. The bottom panels show the NLA mutant, which lacks the N-terminal head-domain, fused to a FLAG-epitope. Arrows show lamin B1 localized to the nuclear envelope. Antibodies used were monoclonal antibodies against FLAG recognized by FITC-conjugated secondary antibodies (left) and polyclonal antibodies against lamin B1 recognized by Alexa TM -conjugated secondary antibodies (middle). The pictures to the right show an overlay of the FITC (green) and Alexa TM (red) channels. Bars, 10 µm. foci), where the foci often were large, than with mutants N195K (20-50%) and M371K (10-50%). The mislocalization of lamin B1 was, however, not complete, and in virtually all cells, some lamin B1 could still be detected at the nuclear periphery (Fig. 2, arrows). In occasional cells, lamin B1 was missing from one pole of the nucleus (Fig. 2, mutant
5 Lamin A mutations in disease 4439 N195K), or from buds protruding out from the nucleus. This phenotype has been described in cells from mice lacking A-type lamins (Sullivan et al., 1999) and fibroblasts from patients with FPLD (Vigouroux et al., 2001). A similar pattern of nuclear foci was observed previously when a mutant form of lamin A ( NLA), lacking the nonhelical headdomain (amino acids 1-33), was microinjected into mammalian cells or added to Xenopus laevis interphase egg extracts (Spann et al., 1997). When FLAG-tagged NLA was expressed in our system, the same pattern was seen in many cells (Fig. 2) (Table 1). Lamin B2 was also partly mislocalized in some cells with mutant lamin A-containing nuclear foci. Representative results for the lamin A E358K mutant and NLA are shown in Fig. 3A. Although apparently slightly less affected than lamin B1, lamin B2 was sometimes missing from one pole of the nucleus or concentrated near the nuclear foci (Fig. 3A, arrows). However, the peripheral distribution of the integral inner nuclear membrane protein LAP2β appeared normal in all cells (Fig. 3B). We also examined the distribution of nuclear pore complexes in cells containing aberrant lamin foci. Nuclear pore complexes showed a normal localization, even in cells with large intranuclear lamin foci (Fig. 4). They were not present in the foci, even when the foci were situated near the nuclear periphery (Fig. 4, N195K). As for lamin B1, there were occasional cells where the nuclear pore complexes were excluded from one pole of the cell (Fig. 4, M371K), as has also been reported in mouse cells lacking A-type lamins and fibroblasts from patients with FPLD (Sullivan et al., 1999; Vigouroux et al., 2001). To obtain additional information about the effects of foci-forming lamin A mutants on the nuclear lamina, we performed double labeling with anti- FLAG antibodies, recognizing the exogenous lamin A, and anti-lamin A/C antibodies, recognizing the exogenous protein as well as endogenous lamins A and C. This analysis showed an accumulation of endogenous lamins A and C inside the foci, similar to the localization frequently seen with endogenous lamin B1 (Fig. 5). As with lamin B1, however, some lamin A/C still remained at the nuclear periphery in the majority of the cells. In two separate experiments with 100 transfected cells counted, peripheral lamin A/C was present in % of cells expressing mutants N195K, E358K and M371K, and 70-90% of cells expressing mutants R386K and NLA (Fig. 5, arrows). Lamin A has been shown to bind to emerin in vitro (Clements et al., 2000; Sakaki et al., 2001) and, in cells from mice lacking A-type lamins, emerin is partly mislocalized to the cytoplasm (Sullivan et al., 1999). To investigate whether mutant forms of lamin A caused a mislocalization of emerin in the presence of wild-type lamin A, the situation in heterozygous patients, we stained C2C12 cells expressing the foci-forming lamin A mutants with antibodies against emerin (Fig. 6A). A loss of emerin from the nuclear envelope was Fig. 3. Analysis of lamin B2 and LAP2 localization in cells expressing lamin A mutants. The panels show laser scanning confocal immunofluorescence microscopy images of C2C12 cells transfected with the prelamin A E358K or NLA mutants fused to a FLAG-epitope. (A) Staining with monoclonal antibodies against lamin B2 (left panel) and polyclonal antibodies against lamin A/C (middle panel). Arrows indicate concentration of lamin B2 by nuclear foci and absence of lamin B2 from one pole of some nuclei. (B) Staining with monoclonal antibodies against FLAG (left panel) and polyclonal antibodies against LAP2 (middle panel). The monoclonal antibodies were recognized by FITC-conjugated secondary antibodies and the polyclonal antibodies were recognized by rhodamine-conjugated secondary antibodies. The panels to the right show an overlay of the FITC (green) and rhodamine (red) channels. Bars, 10 µm. seen in a subset of all transfected cells, also some cells transfected with wild-type lamin A. Similar results have been reported in transfected HeLa cells by Raharjo et al. (Raharjo et al., 2001). All foci-forming mutants except E358K led to a loss of emerin from the nuclear envelope in a significantly greater number of cells than wild-type lamin A (Fig. 6B). We also investigated loss of emerin from the nuclear envelope in cells expressing three mutants that do not form foci (R60G, E203G and K486N). None of these mutants led to an increased loss of emerin from the nuclear envelope, compared to wild-type (data not shown). Emerin, similar to nuclear pore complex proteins and LAP2, did not localize to the intranuclear lamin foci, further indicating that the foci are not
6 4440 JOURNAL OF CELL SCIENCE 114 (24) Fig. 4. Nuclear pore complexes are not present in nuclear foci. The panels show laser scanning confocal immunofluorescence microscopy images of C2C12 cells transfected with prelamin A with missense mutations as indicated. Short arrows indicate lack of pore complexes in the nuclear foci and long arrows their absence from one pole of some nuclei. Antibodies used were monoclonal antibodies against nuclear pore complex protein p62 (NPC) (left panel) and polyclonal antibodies against lamin A/C (middle panel). The monoclonal antibodies were recognized by FITCconjugated secondary antibodies and the polyclonal antibodies were recognized by rhodamine-conjugated secondary antibodies. The panels to the right show an overlay of the FITC (green) and rhodamine (red) channels. Bars, 10 µm. membrane invaginations of the nuclear envelope but separate structures inside the nucleus. Finally, we examined the association of PCNA and DNA with the aberrant lamin foci. PCNA, a cofactor of DNA polymerase δ, which was shown previously to localize inside intranuclear foci containing NLA in Xenopus egg extract experiments (Spann et al., 1997), very rarely accumulated inside the lamin A foci in our experiments (data not shown). DNA appeared to be mostly excluded from the foci (data not shown). Lamin A mutants interact with other lamins in the yeast two-hybrid assay Lamins, like other intermediate filaments, form parallel dimers through coiled-coil interactions between their α-helical rod-domains (Stuurman et al., 1998). To investigate if the ability of mutant lamin A molecules to interact was impaired, we performed a two-hybrid assay. Lamin A, prelamin A, lamin B1 and lamin C have previously been shown to interact with themselves and with each other in the yeast two-hybrid system (Ye and Worman, 1995). In this system, an interaction between a protein fused to the GAL4 activation domain and a protein fused to the GAL4 DNA-binding domain causes the expression of β-galactosidase, which can be measured using a colorimetric assay (Fields and Song, 1989). We expressed the 15 mutant forms of prelamin A described above as fusion proteins with the GAL4 activation domain, or the GAL4 DNA-binding domain, in S. cerevisiae. All of the mutant proteins interacted with themselves, wild-type prelamin A and wild-type lamin B1 (data not shown). No differences were seen between the mutant and wild-type lamins in the two-hybrid interaction assay. Although it is not possible using this assay to determine whether the proteinprotein interactions formed are the coil-coiled ones in the normal lamina, these results suggest that the primary laminlamin dimerization is not disturbed in these mutants. Fig. 5. Endogenous lamin A/C is localized both to the nuclear foci and to the nuclear rim. Staining with monoclonal antibodies against FLAG, recognizing exogenous protein, and polyclonal anti-lamin A/C antibodies, recognizing both exogenous and endogenous A-type lamins. The monoclonal antibodies were recognized by FITC-conjugated secondary antibodies and the polyclonal antibodies were recognized by rhodamine-conjugated secondary antibodies. The panels show an overlay of the FITC (green) and rhodamine (red) channels. Areas of colocalization appear yellow. Arrows show lamin A/C localized to the nuclear envelope. Bars, 10 µm.
7 Lamin A mutations in disease 4441 Fig. 6. Some lamin A mutations increase the loss of emerin from the nuclear envelope in transfected cells. (A) Laser scanning confocal immunofluorescence microscopy images of C2C12 cells transfected with wild-type (WT) or indicated mutated prelamin A, fused to a FLAG-epitope. In cells expressing mutants causing an increase in emerin loss from the nuclear envelope, examples are shown both of cells with correctly localized and mislocalized emerin. Antibodies used were anti-flag monoclonal antibodies recognized by FITCconjugated secondary antibodies (left) and polyclonal antibodies against emerin recognized by rhodamine-conjugated secondary antibodies (middle). The panels to the right show an overlay of the FITC (green) and rhodamine (red) channels. Bars, 10 µm. (B) Percentage of cells with lack of emerin in the nuclear envelope in cells expressing wild-type (WT) or mutant lamin A. One hundred cells each in two separate experiments were studied. P values denote a statistically significant difference between the mutant and wild-type as determined by χ 2 -test. NS denotes no significant difference. Several lamin A mutants are as stable as wild-type lamin A To investigate whether representative mutant lamin A proteins were less stable than wild-type lamin A, we performed pulsechase analyses. COS-7 cells were transfected with cdnas encoding wild-type or mutant prelamin A with FLAG-epitopes fused to their N-termini. The cells were pulsed with [ 35 S]- methionine for 1 hour and then harvested or grown in nonradioactive media for 3 or 25 hours. Cell lysates were incubated with anti-flag M2-agarose affinity gel and the immunoprecipitates were separated by SDS-PAGE and exposed to Hyperfilm ECL. Representative results are shown in Fig. 7. At time-point zero (no chase), both prelamin A and the slightly smaller mature lamin A could be seen in all samples from transfected cells. After 3 hours of growth in nonradioactive media, no prelamin A could be seen and the total levels of radiolabeled FLAG-tagged lamin A had decreased; it could, however, still be detected in all cases. After 25 hours of incubation, very small amounts of radiolabeled lamin A could still be detected in all samples. These results showed that the representative lamin A mutants N195K, M371K, R386K, R453W, R482W and K486N were as stable
8 4442 JOURNAL OF CELL SCIENCE 114 (24) Fig. 7. Pulse-chase analysis of wild-type and mutant forms of lamin A. COS-7 cells were transiently transfected with wild-type (WT) or mutant forms of prelamin A containing FLAGepitopes, as indicated above the panels. The cells were pulse-labeled with [ 35 S]-methionine for 1 hour. After 0, 3 or 25 hours (indicated above the panels) of incubation in non-radioactive media (chase), cell lysates were prepared and immunoprecipitated using M2 anti-flag agarose. The panels show autoradiograms of precipitated proteins separated by SDS-PAGE. Migration of molecular mass markers is indicated on the left. Plus signs (+) show prelamin A; asterisks (*) show mature lamin A. All experiments were performed in triplicate; representative samples are shown. Owing to the large number of samples and the importance of rapid handling, not all samples could be prepared at once. The results from different experiments varied slightly in labeling efficiency and level of background. The first five panels and the last three panels are taken from two separate experiments. Immunoprecipitates from transfected cells also contained a protein with an apparent molecular mass of 100 kda. The identity of this protein is not clear but it was absent in untransfected cells and recognized by anti-flag and anti-lamin A/C antibodies on western blots (data not shown), suggesting that it is an aberrantly expressed form of lamin A. as wild-type lamin A. Mutant R386K seemed slightly more stable than the others after 3 hours of chase. Wild-type lamin A and these lamin A mutants were also processed normally from prelamin A to the mature protein. DISCUSSION We have investigated 15 lamin A mutants in patients with dilated cardiomyopathy, AD-EDMD and FPLD. These mutations are dominant and patients carry one mutant and one wild-type copy of the LMNA gene. There are many possible mechanisms for how these mutations may cause disease, such as changes in the stability, localization and interactions of the mutant proteins. Mutant lamins are as stable as wild-type lamins Disease phenotypes may be caused by haploinsufficiency of lamins A and C due to low levels of expression or rapid degradation of the mutant lamin protein. One mutation causing AD-EDMD is a nonsense mutation at the codon for amino acid six (Bonne et al., 1999; Bécane et al., 2000); in this case a reduction of lamins A and C most likely caused the disease. In patients with this mutation, both lamin A/C and emerin were correctly localized to the nuclear envelope as shown by immunolabeling of a cardiac biopsy (Bonne et al., 1999), although there was a decrease in the amount of lamin A/C in cardiac tissue, as shown by western blot (Bécane et al., 2000). Our studies indicate no decrease in the stability of several mutant lamins, including those in AD-EDMD, cardiomyopathy and FPLD. Therefore, a simple haploinsufficiency of lamins A can not be the only pathophysiological mechanism. Studies of mutant lamins A and C in fibroblasts from patients carrying the LMNA mutations causing R482Q and R482W substitutions showed these proteins to be present at similar levels to the wildtype proteins in control cells (Vigouroux et al., 2001). Further studies of the levels of mutant lamins A and C in cells from patients carrying other LMNA mutations would provide important confirmation of our results on the stability of the mutant proteins. Mutations in the lamin A rod-domain can cause an abnormal intranuclear localization of the protein Mutant proteins may be unable to carry out their normal functions, or disrupt the functions of associated proteins, because of mislocalization in cells. We examined the intracellular localization of 15 mutant lamin A proteins by immunofluorescence microscopy. The most striking feature in these studies was the formation of intranuclear foci by four of the lamin A mutants (N195K, E358K, M371K and R386K), which was often accompanied by a mislocalization of some of the endogenous lamins. These cells also had a decrease in the localization of the mutant lamins to the nuclear periphery. A nucleoplasmic localization and formation of intranuclear foci have also been reported when the N195K lamin A mutant was expressed in HeLa cells (Raharjo et al., 2001). The four lamin proteins that formed intranuclear foci all had mutations introducing a lysine into the rod-domain. Three out of the four proteins had mutations at the end of the central, α-helical rod-domain, which is evolutionary well conserved between all intermediate filaments (Stuurman et al., 1998). A lamin A mutation (R377H) causing LGMD1B has also recently been mapped to this region (Muchir et al., 2000). The conserved segments of the rod-domain have been shown to play crucial roles in the assembly of intermediate filament dimers into higher order oligomers (Stuurman et al., 1998). Mutations in the corresponding regions of keratin K5 and K14 are responsible for the majority of skin diseases caused by keratin mutations, for example the Dowling-Meara type of epidermolysis bullosa simplex (McLean and Lane, 1995). This disease and others caused by keratin mutations are blistering skin
9 Lamin A mutations in disease 4443 disorders resulting from epithelial fragility. It is conceivable that mutations in lamins A and C cause nuclear fragility. Nuclei of embryonic fibroblasts from mice lacking A-type lamins and Xenopus nuclei assembled in egg extracts with the NLA mutant have been shown to be more fragile than wildtype nuclei (Spann et al., 1997; Sullivan et al., 1999). One hypothesis is that an increased fragility of nuclei can be particularly harmful to muscle cells, where they are subjected to mechanical stress (Worman and Courvalin, 2000; Hutchison et al., 2001). This could explain the musclespecific nature of AD-EDMD and cardiomyopathy but does not explain why the specialized cardiomyocytes involved in atrioventricular conduction may be more readily affected than contractile cardiomyocytes (Morris, 2000). The nuclear stress-hypothesis as a pathophysiological mechanism of disease is less likely in the case of FPLD. The restriction of missense mutations causing FPLD to two regions in exon 8 and 11 implies that they encode a domain important for a specific lamin A function, for example interaction with a protein important for adipocyte survival. This is further suggested by the concentration of the majority of FPLD mutations to codon 482 (Cao and Hegele, 2000; Shackleton et al., 2000; Speckman et al., 2000; Vigouroux et al., 2000). In our studies, as well as in the studies by others (Holt et al., 2001; Raharjo et al., 2001), all using transiently transfected cells, there were no gross abnormalities in the localization or stability of lamin A-containing mutations found in FPLD. However, fibroblasts from FPLD patients with the R482Q and R482W lamin A mutations, and some transfected fibroblasts overexpressing the R482W mutant, show more subtle nuclear abnormalities (Vigouroux et al., 2001). Effects of lamin mutations on protein-protein interactions To examine the ability of mutant lamins to self-interact and to interact with wild-type lamins, we used the yeast two-hybrid assay. Our results showed no indication of impaired laminlamin interactions at this level. However, the two-hybrid assay does not show whether the complexes formed are the usual coiled-coil dimers. Neither would it detect disturbances of higher order lamin interactions, such as filament formation. Subtle defects of lamin filament formation are at this time difficult to detect because lamins, as opposed to cytoplasmic intermediate filaments, do not form stable filaments in vitro. Lamin A binds to LAP1 and emerin (Foisner and Gerace, 1993; Clements et al., 2000; Sakaki et al., 2001). The interaction between lamin A and emerin is especially intriguing because mutations in these two proteins both cause the EDMD phenotype. In cells from mice lacking A-type lamins, emerin is partially mislocalized to the cytoplasm (Sullivan et al., 1999). These mice show typical signs of EDMD. Heterozygous mice, having only one wild-type copy of the LMNA gene, have a slight mislocalization of emerin but are healthy (Sullivan et al., 1999). We have studied the localization of emerin in C2C12 cells expressing mutant forms of lamin A. Our studies showed an increased loss of endogenous emerin from the nuclear envelope when C2C12 cells, which have wild-type lamin A in addition to the mutant forms, were transfected with mutants N195K, M371K, R386K or NLA. However, as cells transfected with wild-type lamin A also exhibited a loss of emerin from the nuclear envelope, although to a significantly lower extent, it is not clear whether the emerin loss was due to specific disturbances in the interactions between emerin and mutant lamins or to a loss of peripheral lamins caused by the mutants. Alternatively, other effects of lamin A overexpression in cells could lead to emerin mislocalization, but whatever these effects may be, certain lamin A mutants enhance them. An increased loss of emerin from the nuclear envelope has also been reported in HeLa cells expressing the lamin A mutants L85R, N195K and L530P, compared with wild-type (Raharjo et al., 2001). Do lamins have a role in gene expression and DNA replication? Alternative hypotheses, which could explain the tissuespecific nature of the diseases caused by mutations in the A- type lamins, are that these proteins are involved in specific gene expression or DNA replication events, or both. A-type lamins have been shown to bind proteins involved in transcriptional regulation such as the retinoblastoma protein (Mancini et al., 1994), and ectopic expression of lamin A has also been shown to induce muscle-specific genes in undifferentiated cells (Lourim and Lin, 1992). A role for the inner nuclear membrane in the regulation of gene expression has previously been suggested by the interaction between the lamin B receptor, an integral protein of the inner nuclear membrane, and human orthologues of Drosophila HP1 (Ye and Worman, 1996; Ye et al., 1997). In Drosophila, HP1 suppresses the expression of normally active euchromatic genes translocated near heterochromatin (Eissenberg et al., 1990). Several other nuclear proteins involved in neuromuscular disease are involved in gene expression, either at the level of transcription, splicing or mrna transport (Morris, 2000). The nuclear lamins are also required for DNA replication during the S-phase and the lamin foci formed in Xenopus egg extracts containing the NLA mutant were previously shown to contain the DNA polymerase δ cofactor PCNA (Spann et al., 1997). PCNA, however, did not accumulate in the foci formed by lamin mutants in C2C12 cells in our studies. The reason for this discrepancy is not clear; one possibility is that PCNA localization is dependent on cell-cycle phase. Although in vitro assembled Xenopus nuclei are in S-phase, it is not clear whether cells containing nuclear foci can enter S-phase. It may also be due to different fixation techniques; our cells were fixed with methanol, which yields a staining pattern where only PCNA bound to specific nuclear structures is recognized by the antibody (Bravo and Macdonald-Bravo, 1987). There may also be differences in lamina structure between mammalian cells versus Xenopus egg extracts, which has been suggested by Izumi et al. (Izumi et al., 2000); they showed that PCNA is not colocalized with NLA foci in CHO cells. The recent results of others (Raharjo et al., 2001; Vigouroux et al., 2001) and those reported in this study provide a starting point towards understanding the properties of disease-causing lamin A/C mutants. They also exclude some possible pathogenetic mechanisms, such as haploinsufficiency of lamin A, absence of prelamin A processing or cytoplasmic sequestration, as the cause of disease in most instances. Further work is necessary to connect the behavior of mutant nuclear lamins and their resulting effects on nuclear envelope structure to muscular dystrophy, cardiomyopathy and lipodystrophy.
10 4444 JOURNAL OF CELL SCIENCE 114 (24) This work was supported by grants from the Muscular Dystrophy Association (MDA) to H.J.W. and the Human Frontiers Science Program to G.B. and H.J.W. Also, C.Ö. received support from the Sweden-America Foundation/Thanks to Scandinavia, Inc. and Karolina Widerström s Foundation, and G.B. and K.S. received support from Association Française contre les Myopathies (AFM) grant H.J.W. is also an Irma T. Hirschl Scholar. The confocal microscopy facility used for part of this project was established by National Institutes of Health grant 1S10-RR10506 and is supported by National Institutes of Health Grant 5 P30-CA13696 as part of the Herbert Irving Cancer Center at Columbia University. We thank Sudhindra Swamy and Theresa Swayne (Columbia University) for help with the confocal microscopy; Jean-Claude Courvalin and Brigitte Buendia (Institut Jacques Monod) and Brian Burke (University of Calgary) for helpful discussions; Glenn Morris (North East Wales Institute), Tarik Soliman (Rockefeller University), Georg Krohne (University of Würzburg) and Katherine Wilson (Johns Hopkins University) for antibodies; and Hal Skopicki (Columbia University) for C2C12 cells. REFERENCES Aebi, U., Cohn, J., Buhle, L. and Gerace, L. (1986). The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323, Bécane, H.-M., Bonne, G., Varnous, S., Muchir, A., Ortega, V., Hammouda, E. H., Urtizberea, J.-A., Lavergne, T., Fardeau, M., Eymard, B. et al. (2000). High incidence of sudden death with conduction system and myocardial disease due to lamins A and C gene mutation. Pacing Clin. Electrophysiol. 23, Biamonti, G., Giacca, M., Perini, G., Contreas, G., Zentilin, L., Weighardt, F., Guerra, M., Della Valle, G., Saccone, S., Riva, S. et al. (1992). The gene for a novel human lamin maps at a highly transcribed locus of chromosome 19 which replicates at the onset of S-phase. Mol. Cell. Biol. 12, Bione, S., Maestrini, E., Rivella, S., Mancini, M., Regis, S., Romeo, G. and Toniolo, D. (1994). Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 8, Bonne, G., Raffaele di Barletta, M., Varnous, S., Bécane, H.-M., Hammouda, E.-H., Merlini, L., Muntoni, F., Greenberg, C. R., Gary, F., Urtizberea, J.-A. et al. (1999). Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 21, Bonne, G., Mercuri, E., Muchir, A., Urtizberea, A., Bécane, H.-M., Recan, D., Merlini, L., Wehnert, M., Boor, R., Reuner, U. et al. (2000). Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann. Neurol. 48, Bravo, R. and Macdonald-Bravo, H. (1987). Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J. Cell Biol. 105, Broers, J. L. V., Machiels, B. M., van Eys, G. J. J. M., Kuijpers, H. J. H., Manders, E. M. M., van Driel, R. and Ramaekers, F. C. S. (1999). Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J. Cell Sci. 112, Cance, W. G., Chaudhary, N., Worman, H. J., Blobel, G. and Cordon- Cardo, C. (1992). Expression of the nuclear lamins in normal and neoplastic human tissue. J. Exp. Clin. Cancer Res. 11, Cao, H. and Hegele, R. A. (2000). Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet. 9, Clements, L., Manilal, S., Love, D. R. and Morris, G. E. (2000). Direct interaction between emerin and lamin A. Biochem. Biophys. Res. Commun. 267, Eissenberg, J. C., James, T. C., Foster-Hartnett, D. M., Hartnett, T., Ngan, V. and Elgin, S. C. (1990). Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 87, Emery, A. E. H. (1989). Emery-Dreifuss syndrome. J. Med. Genet. 26, Emery, A. E. H. and Dreifuss, F. E. (1966). Unusual type of benign X-linked muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 29, Fatkin, D., MacRae, C., Sasaki, T., Wolff, M. R., Porcu, M., Frenneaux, M., Atherton, J., Vidaillet, H. J., Jr, Spudich, S., De Girolami, U. et al. (1999). Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. New. Engl. J. Med. 341, Fields, S. and Song, O. (1989). A novel genetic system to detect proteinprotein interactions. Nature 340, Fisher, D. Z., Chaudhary, N. and Blobel, G. (1986). cdna sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc. Natl. Acad. Sci. USA 83, Foisner, R. and Gerace, L. (1993). Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 73, Holt, I., Clements, L., Manilal, S., Brown, S. C. and Morris, G. E. (2001). The R482Q lamin A/C mutation that causes lipodystrophy does not prevent nuclear targeting of lamin A in adipocytes or its interaction with emerin. Eur. J. Hum. Genet. 9, Hutchison, C. J., Alvarez-Reyes, M. and Vaughan, O. A. (2001). Lamins in disease: why do ubiquitously expressed nuclear envelope proteins give rise to tissue-specific disease phenotypes? J. Cell Sci. 114, Izumi, M., Vaughan, O. A., Hutchison, C. J. and Gilbert, D. M. (2000). Head and/or CaaX domain deletions of lamin proteins disrupt preformed lamin A and C but not lamin B structure in mammalian cells. Mol. Biol. Cell 11, Köbberling, J. and Dunnigan, M. (1986). Familial partial lipodystrophy: two types of an X-linked dominant syndrome, lethal in the hemizygous state. J. Med. Genet. 23, Laemmli, U. K. (1970). Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227, Lin, F. and Worman, H. J. (1993). Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 268, Lin, F. and Worman, H. J. (1995). Structural organization of the human gene (LMNB1) encoding nuclear lamin B1. Genomics 27, Lourim, D. and Lin, J. J. (1992). Expression of wild-type and nuclear localization-deficient human lamin A in chick myogenic cells. J. Cell Sci. 103, Machiels, B. M., Zorenc, A. H. G., Endert, J. M., Kuijpers, H. J. H., van Eys, G. J. J. M., Ramaekers, F. C. S. and Broers, J. L. V. (1996). An alternative splicing product of the lamin A/C gene lacks exon 10. J. Biol. Chem. 271, Mancini, M. A., Shan, B., Nickerson, J. A., Penman, S. and Lee, W.-H. (1994). The retinoblastoma gene product is a cell cycle-dependent, nuclear matrix-associated protein. Proc. Natl. Acad. Sci. USA 91, Manilal, S., Nguyen T. M., Sewry, C. A. and Morris, G. E. (1996). The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum. Mol. Genet. 5, McKeon, F. D., Kirschner, M. W. and Caput, D. (1986). Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 319, McLean, W. H. I. and Lane, E. B. (1995). Intermediate filaments in disease. Curr. Opin. Cell Biol. 7, Moir, R. D., Montag-Lowy, M. and Goldman, R. D. (1994). Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J. Cell Biol. 125, Morris, G. E. (2000). Nuclear proteins and cell death in inherited neuromuscular disease. Neuromusc. Disord. 10, Muchir, A., Bonne, G., van der Kooi, A. J., van Meegen, M., Baas, F., Bolhuis, P. A., de Visser, M. and Schwartz, K. (2000). Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum. Mol. Genet. 9, Nagano, A., Koga, R., Ogawa, M., Kurano, Y., Kawada, J., Okada, R., Hayashi, Y. K., Tsukahara, T. and Arahata, K. (1996). Emerin deficiency at the nuclear membrane in patients with Emery-Dreifuss muscular dystrophy. Nat. Genet. 12, Östlund, C., Ellenberg, J., Hallberg, E., Lippincott-Schwartz, J. and Worman, H. J. (1999). Intracellular trafficking of emerin, the Emery- Dreifuss muscular dystrophy protein. J. Cell Sci. 112, Raharjo, W. H., Enarson, P., Sullivan, T., Stewart, C. and Burke, B. (2001). Nuclear envelope defects associated with LMNA mutations causing dilated
Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy
 RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling
 4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
Nuclear Envelope and Muscular Dystrophy
 Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
7.012 Quiz 3 Answers
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Expression of a Mutant Lamin A That Causes Emery-Dreifuss Muscular Dystrophy Inhibits In Vitro Differentiation of C2C12 Myoblasts
 REFERENCES CONTENT ALERTS Expression of a Mutant Lamin A That Causes Emery-Dreifuss Muscular Dystrophy Inhibits In Vitro Differentiation of C2C12 Myoblasts Catherine Favreau, Dominique Higuet, Jean-Claude
REFERENCES CONTENT ALERTS Expression of a Mutant Lamin A That Causes Emery-Dreifuss Muscular Dystrophy Inhibits In Vitro Differentiation of C2C12 Myoblasts Catherine Favreau, Dominique Higuet, Jean-Claude
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Problem Set #5 4/3/ Spring 02
 Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
HYBRID GENE THERAPY FOR AD-EDMD
 HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy
 Human Molecular Genetics, 2006, Vol. 15, No. 4 653 663 doi:10.1093/hmg/ddi480 Advance Access published on January 13, 2006 Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type
Human Molecular Genetics, 2006, Vol. 15, No. 4 653 663 doi:10.1093/hmg/ddi480 Advance Access published on January 13, 2006 Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type
The cell cycle dependent mislocalisation of emerin may contribute to the Emery-Dreifuss muscular dystrophy phenotype
 Research Article 341 The cell cycle dependent mislocalisation of emerin may contribute to the Emery-Dreifuss muscular dystrophy phenotype Elizabeth A. L. Fairley 1, *, Andrew Riddell 2, Juliet A. Ellis
Research Article 341 The cell cycle dependent mislocalisation of emerin may contribute to the Emery-Dreifuss muscular dystrophy phenotype Elizabeth A. L. Fairley 1, *, Andrew Riddell 2, Juliet A. Ellis
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Bio 401 Sp2014. Multiple Choice (Circle the letter corresponding to the correct answer)
 MIDTERM EXAM KEY (60 pts) You should have 5 pages. Please put your name on every page. You will have 70 minutes to complete the exam. You may begin working as soon as you receive the exam. NOTE: the RED
MIDTERM EXAM KEY (60 pts) You should have 5 pages. Please put your name on every page. You will have 70 minutes to complete the exam. You may begin working as soon as you receive the exam. NOTE: the RED
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Luminescent platforms for monitoring changes in the solubility of amylin and huntingtin in living cells
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
s u p p l e m e n ta ry i n f o r m at i o n
 Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation
 Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
Construction of a hepatocellular carcinoma cell line that stably expresses stathmin with a Ser25 phosphorylation site mutation J. Du 1, Z.H. Tao 2, J. Li 2, Y.K. Liu 3 and L. Gan 2 1 Department of Chemistry,
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Workshop report. 1. Introduction
 Neuromuscular Disorders 12 (2002) 187 194 Workshop report www.elsevier.com/locate/nmd 82nd ENMC international workshop, 5th international Emery Dreifuss muscular dystrophy (EDMD) workshop, 1st Workshop
Neuromuscular Disorders 12 (2002) 187 194 Workshop report www.elsevier.com/locate/nmd 82nd ENMC international workshop, 5th international Emery Dreifuss muscular dystrophy (EDMD) workshop, 1st Workshop
L aminopathies represent a heterogeneous group of genetic
 1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Jewell et al., http://www.jcb.org/cgi/content/full/jcb.201007176/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. IR Munc18c association is independent of IRS-1. (A and
Supplemental material Jewell et al., http://www.jcb.org/cgi/content/full/jcb.201007176/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. IR Munc18c association is independent of IRS-1. (A and
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Anti-Lamin B1/LMNB1 Picoband Antibody
 Anti-Lamin B1/LMNB1 Picoband Antibody Catalog Number:PB9611 About LMNB1 Lamin-B1 is a protein that in humans is encoded by the LMNB1 gene. The nuclear lamina consists of a two-dimensional matrix of proteins
Anti-Lamin B1/LMNB1 Picoband Antibody Catalog Number:PB9611 About LMNB1 Lamin-B1 is a protein that in humans is encoded by the LMNB1 gene. The nuclear lamina consists of a two-dimensional matrix of proteins
WDR62 is associated with the spindle pole and mutated in human microcephaly
 WDR62 is associated with the spindle pole and mutated in human microcephaly Adeline K. Nicholas, Maryam Khurshid, Julie Désir, Ofélia P. Carvalho, James J. Cox, Gemma Thornton, Rizwana Kausar, Muhammad
WDR62 is associated with the spindle pole and mutated in human microcephaly Adeline K. Nicholas, Maryam Khurshid, Julie Désir, Ofélia P. Carvalho, James J. Cox, Gemma Thornton, Rizwana Kausar, Muhammad
TITLE: The Role of hcdc4 as a Tumor Suppressor Gene in Genomic Instability Underlying Prostate Cancer
 AD Award Number: TITLE: The Role of hcdc4 as a Tumor Suppressor Gene in Genomic Instability Underlying Prostate Cancer PRINCIPAL INVESTIGATOR: Audrey van Drogen, Ph.D. CONTRACTING ORGANIZATION: Sidney
AD Award Number: TITLE: The Role of hcdc4 as a Tumor Suppressor Gene in Genomic Instability Underlying Prostate Cancer PRINCIPAL INVESTIGATOR: Audrey van Drogen, Ph.D. CONTRACTING ORGANIZATION: Sidney
Alpha thalassemia mental retardation X-linked. Acquired alpha-thalassemia myelodysplastic syndrome
 Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
Structural vs. nonstructural proteins
 Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
100 mm Sucrose. +Berberine +Quinine
 8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus
 Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Distinct changes in intranuclear lamin A/C organization during myoblast differentiation
 RESEARCH ARTICLE 4001 Distinct changes in intranuclear lamin A/C organization during myoblast differentiation Bh. Muralikrishna, Jyotsna Dhawan, Nandini Rangaraj and Veena K. Parnaik* Centre for Cellular
RESEARCH ARTICLE 4001 Distinct changes in intranuclear lamin A/C organization during myoblast differentiation Bh. Muralikrishna, Jyotsna Dhawan, Nandini Rangaraj and Veena K. Parnaik* Centre for Cellular
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
Supplementary Figure 1. The CagA-dependent wound healing or transwell migration of gastric cancer cell. AGS cells transfected with vector control or
 Supplementary Figure 1. The CagA-dependent wound healing or transwell migration of gastric cancer cell. AGS cells transfected with vector control or 3xflag-CagA expression vector were wounded using a pipette
Supplementary Figure 1. The CagA-dependent wound healing or transwell migration of gastric cancer cell. AGS cells transfected with vector control or 3xflag-CagA expression vector were wounded using a pipette
Laminopathies and the long strange trip from basic cell biology to therapy
 Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
Lamina-associated polypeptide 2α binds intranuclear A-type lamins
 Journal of Cell Science 113, 3473-3484 (2000) Printed in Great Britain The Company of Biologists Limited 2000 JCS1581 3473 Lamina-associated polypeptide 2α binds intranuclear A-type lamins Thomas Dechat
Journal of Cell Science 113, 3473-3484 (2000) Printed in Great Britain The Company of Biologists Limited 2000 JCS1581 3473 Lamina-associated polypeptide 2α binds intranuclear A-type lamins Thomas Dechat
Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid.
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
The nuclear lamina is a proteinaceous component of the
 Meiotic lamin C2: The unique amino-terminal hexapeptide GNAEGR is essential for nuclear envelope association Manfred Alsheimer*, Elisabeth von Glasenapp*, Martina Schnölzer, Hans Heid, and Ricardo Benavente*
Meiotic lamin C2: The unique amino-terminal hexapeptide GNAEGR is essential for nuclear envelope association Manfred Alsheimer*, Elisabeth von Glasenapp*, Martina Schnölzer, Hans Heid, and Ricardo Benavente*
supplementary information
 Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS
 Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Lipodystrophies represent a heterogeneous group
 Brief Genetics Report Lamin A/C Gene Sex-Determined Expression of Mutations in Dunnigan-Type Familial Partial Lipodystrophy and Absence of Coding Mutations in Congenital and Acquired Generalized Lipoatrophy
Brief Genetics Report Lamin A/C Gene Sex-Determined Expression of Mutations in Dunnigan-Type Familial Partial Lipodystrophy and Absence of Coding Mutations in Congenital and Acquired Generalized Lipoatrophy
Nuclear Titin interacts with A- and B-type lamins in vitro and in vivo
 Research Article 239 Nuclear Titin interacts with A- and B-type lamins in vitro and in vivo Michael S. Zastrow 1, *, Denise B. Flaherty 2, Guy M. Benian 2 and Katherine L. Wilson 1, 1 Department of Cell
Research Article 239 Nuclear Titin interacts with A- and B-type lamins in vitro and in vivo Michael S. Zastrow 1, *, Denise B. Flaherty 2, Guy M. Benian 2 and Katherine L. Wilson 1, 1 Department of Cell
3 The abbreviations used are: NE, nuclear envelope; LB, lamin B; LA, lamin A; Ig,
 crossmark THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 290, NO. 46, pp. 27557 27571, November 13, 2015 2015 by The American Society for Biochemistry and Molecular Biology, Inc. Published in the U.S.A. Concentration-dependent
crossmark THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 290, NO. 46, pp. 27557 27571, November 13, 2015 2015 by The American Society for Biochemistry and Molecular Biology, Inc. Published in the U.S.A. Concentration-dependent
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Edens and Levy, http://www.jcb.org/cgi/content/full/jcb.201406004/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Nuclear shrinking does not depend on the cytoskeleton
Supplemental material Edens and Levy, http://www.jcb.org/cgi/content/full/jcb.201406004/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Nuclear shrinking does not depend on the cytoskeleton
The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans
 Research Article 923 The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans Yosef Gruenbaum 1, *, Kenneth K. Lee 2, *, Jun Liu 3, Merav Cohen 1 and Katherine
Research Article 923 The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans Yosef Gruenbaum 1, *, Kenneth K. Lee 2, *, Jun Liu 3, Merav Cohen 1 and Katherine
Recombinant Protein Expression Retroviral system
 Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
The role of the scaffolding protein Tks5 in EGF signaling
 The role of the scaffolding protein Tks5 in EGF signaling PhD Thesis Anna Fekete Doctoral School in Biology Head of the School: Dr. Anna Erdei Structural Biochemistry Doctoral Program Head of the Program:
The role of the scaffolding protein Tks5 in EGF signaling PhD Thesis Anna Fekete Doctoral School in Biology Head of the School: Dr. Anna Erdei Structural Biochemistry Doctoral Program Head of the Program:
Supplementary Figure 1. MAT IIα is Acetylated at Lysine 81.
 IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
IP: Flag a Mascot PTM Modified Mass Error Position Gene Names Score Score Sequence m/z [ppm] 81 MAT2A;AMS2;MATA2 35.6 137.28 _AAVDYQK(ac)VVR_ 595.83-2.28 b Pre-immu After-immu Flag- WT K81R WT K81R / Flag
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Supplementary Material and Methods
 Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Supplementary Information
 Supplementary Information Supplementary Figure 1. EBV-gB 23-431 mainly exists as trimer in HEK 293FT cells. (a) Western blotting analysis for DSS crosslinked FLAG-gB 23-431. HEK 293FT cells transfected
Supplementary Information Supplementary Figure 1. EBV-gB 23-431 mainly exists as trimer in HEK 293FT cells. (a) Western blotting analysis for DSS crosslinked FLAG-gB 23-431. HEK 293FT cells transfected
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis
 Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Supplemental Figure 1
 Supplemental Figure 1 A S100A4: SFLGKRTDEAAFQKLMSNLDSNRDNEVDFQEYCVFLSCIAMMCNEFFEGFPDK Overlap: SF G DE KLM LD N D VDFQEY VFL I M N FF G PD S100A2: SFVGEKVDEEGLKKLMGSLDENSDQQVDFQEYAVFLALITVMCNDFFQGCPDR
Supplemental Figure 1 A S100A4: SFLGKRTDEAAFQKLMSNLDSNRDNEVDFQEYCVFLSCIAMMCNEFFEGFPDK Overlap: SF G DE KLM LD N D VDFQEY VFL I M N FF G PD S100A2: SFVGEKVDEEGLKKLMGSLDENSDQQVDFQEYAVFLALITVMCNDFFQGCPDR
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
SUPPLEMENTARY FIGURES AND TABLE
 SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
293T cells were transfected with indicated expression vectors and the whole-cell extracts were subjected
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization
 Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Supplementary Materials and Methods
 Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
Supplementary Materials and Methods Reagents and antibodies was purchased from iaffin GmbH & Co KG. Cisplatin (ristol-myers Squibb Co.) and etoposide (Sandoz Pharma Ltd.) were used. Antibodies recognizing
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Material
 Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator. of the Interaction with Macrophages
 Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTARY INFORMATION
 a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
Received 26 January 1996/Returned for modification 28 February 1996/Accepted 15 March 1996
 MOLECULAR AND CELLULAR BIOLOGY, June 1996, p. 3012 3022 Vol. 16, No. 6 0270-7306/96/$04.00 0 Copyright 1996, American Society for Microbiology Base Pairing at the 5 Splice Site with U1 Small Nuclear RNA
MOLECULAR AND CELLULAR BIOLOGY, June 1996, p. 3012 3022 Vol. 16, No. 6 0270-7306/96/$04.00 0 Copyright 1996, American Society for Microbiology Base Pairing at the 5 Splice Site with U1 Small Nuclear RNA
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Myne-1, a spectrin repeat transmembrane protein of the myocyte inner nuclear membrane, interacts with lamin A/C
 Research Article 61 Myne-1, a spectrin repeat transmembrane protein of the myocyte inner nuclear membrane, interacts with lamin A/C John M. K. Mislow 1, Marian S. Kim 2, Dawn Belt Davis 1 and Elizabeth
Research Article 61 Myne-1, a spectrin repeat transmembrane protein of the myocyte inner nuclear membrane, interacts with lamin A/C John M. K. Mislow 1, Marian S. Kim 2, Dawn Belt Davis 1 and Elizabeth
7.06 Cell Biology Exam #3 April 23, 2002
 RECITATION TA: NAME: 7.06 Cell Biology Exam #3 April 23, 2002 This is an open book exam and you are allowed access to books, notes, and calculators. Please limit your answers to the spaces allotted after
RECITATION TA: NAME: 7.06 Cell Biology Exam #3 April 23, 2002 This is an open book exam and you are allowed access to books, notes, and calculators. Please limit your answers to the spaces allotted after
International Graduate Research Programme in Cardiovascular Science
 1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
