Genotype phenotype correlations in laminopathies: how does fate translate?
|
|
|
- Jared Hensley
- 6 years ago
- Views:
Transcription
1 Nuclear Envelope Disease and Chromatin Organization Genotype phenotype correlations in laminopathies: how does fate translate? Juergen Scharner, Viola F. Gnocchi, Juliet A. Ellis 1 and Peter S. Zammit 1 King s College London, Randall Division of Cell and Molecular Biophysics, New Hunt s House, Guy s Campus, London SE1 1UL, U.K. Abstract A-type laminopathies are a group of diseases resulting from mutations in the intermediate filament proteins lamin A and C (both encoded by the LMNA gene), but for which the pathogenic mechanisms are little understood. In some laminopathies, there is a good correlation between the presence of a specific LMNA mutation and the disease diagnosed. In others however, many different mutations can give rise to the same clinical condition, even though the mutations may be distributed throughout one, or more, of the three functionally distinct protein domains of lamin A/C. Conversely, certain mutations can cause multiple laminopathies, with related patients carrying an identical mutation even having separate diseases, often affecting different tissues. Therefore clarifying genotype phenotype links may provide important insights into both disease penetrance and mechanism. In the present paper, we review recent developments in genotype phenotype correlations in laminopathies and discuss the factors that could influence pathology. Introduction Mutations in the LMNA gene cause a variety of diseases collectively called laminopathies. To date, more than 340 unique LMNA mutations ( [1] are known that cause 16 different diseases including A-EDMD (autosomal Emery Dreifuss muscular dystrophy), DCM (dilated cardiomyopathy), LGMD1B (limb-girdle muscular dystrophy 1B), L-CMD (LMNArelated congenital muscular dystrophy), FPLD2 (familial partial lipodystrophy 2), HGPS (Hutchinson Gilford progeria syndrome), atypical WRN (Werner syndrome), MAD (mandibuloacral dysplasia) and CMT2B (Charcot Marie Tooth disorder type 2B). The LMNA gene is alternatively spliced to produce the type V intermediate filament proteins lamin A, C, C2 and del10. Together with B-type lamins, lamin A and C are the major components of the nuclear lamina (a fibrous proteinaceous meshwork underlying the nuclear envelope). The nuclear lamina functions to maintain both nuclear and cellular architecture and plays a role in chromatin organization and gene expression [2]. The presence of mutant forms of lamin A/C is thought to perturb some, or all, of these functions of the nuclear lamina, but it is generally unclear how specific mutations result in a particular laminopathy. Phenotypic clustering, the Key words: autosomal Emery Dreifuss muscular dystrophy (A-EDMD), dilated cardiomyopathy (DCM), familial partial lipodystrophy 2 (FPLD2), Hutchinson Gilford progeria syndrome (HGPS), lamin, laminopathy, LMNA, limb-girdle muscular dystrophy 1B (LGMD1B). Abbreviations used: A-EDMD, autosomal Emery Dreifuss muscular dystrophy; CMT2B, Charcot Marie Tooth disorder type 2B; DCM, dilated cardiomyopathy; FPLD2, familial partial lipodystrophy 2; HGPS, Hutchinson Gilford progeria syndrome; L-CMD, LMNA-related congenital muscular dystrophy; LGMD1B, limb-girdle muscular dystrophy 1B; MAD, mandibuloacral dysplasia; SNP, single nucleotide polymorphism; SREBP, sterol-regulatory-element-binding protein; WRN, Werner syndrome. 1 Correspondence may be addressed to either of these authors ( juliet.ellis@kcl.ac.uk or peter.zammit@kcl.ac.uk). systematic correlation of phenotype with genotype, is becoming increasingly important to understand underlying mechanisms of monogenic diseases [3 5]. Here, we review the correlation of LMNA mutation with clinical diagnosis, to explore genotype phenotype links within laminopathies (Figure 1). Laminopathies with a more consistent genotype phenotype link To date, mutations distributed throughout the gene and affecting almost 20% of the coding sequence of LMNA have been reported from more than 1000 patients, and arise mainly from missense or frameshift mutations ( [1]. In some laminopathies such as HGPS, hot-spot or founder mutations result in a similar phenotype. HGPS is almost always caused by the de novo base mutation c.1824c>t/p.g608g, which results in a cryptic splice donor site in the lamin A-specific exon 11, and deletion of the remaining 50 amino acids of the C-terminus [6]. The resulting truncated protein (termed progerin) retains the CAAX box but lacks the endoproteolytic cleavage site, so it cannot be processed by Zmpste24, and is incorporated into the nuclear lamina carrying the C-terminal farnesyl group. Two unrelated patients with severe forms of HGPS have recently been reported carrying two new mutations (c g>a and c.1821g>a), but both cause a frequent use of the splice donor site that is activated in typical HGPS patients [7]. Other laminopathies also appear to arise from a common LMNA mutation, such as WRN (R133L), CMT2B (R298C) and MAD (R527H) (although R133L also causes FPLD2, and R527H additionally results in A-EDMD) (Figure 1) [8]. Although the number of patients developing HGPS, WRN, CMT2B or MAD is small, it is likely that the presence of a particular mutation will have a predictive value on the Biochem. Soc. Trans. (2010) 38, ; doi: /bst
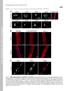
2 258 Biochemical Society Transactions (2010) Volume 38, part 1 Figure 1 Distribution of lamin A mutations and their related laminopathies (a) Schematic diagram of the LMNA gene, with exons encoding their respective protein domains colour coded and the lamin A splice site indicated in red above. HGPS mutations causing a new splice donor site are indicated in black boxes. (b) The lamin A protein with mutations indicated that result in A-EDMD (blue), DCM (green), LGMD1B (black), L-CMD (gold), FPLD2 (red) and CMT2B (plum). indicates that the same amino acid change causes different laminopathies. *R133L also causes WRN, *S143F additionally results in HGPS, *R527H causes MAD too and *R644C also gives rise to a range of other disorders [26 29]. Patients carrying R527C develop either a severe form of MAD and/or progeria [52,53]. Mutation/laminopathy correlations are from [1,9]. Interacting proteins and their corresponding binding regions on lamin A are indicated below [54]. clinical diagnosis and that the pathogenic mechanism will be similar for a given disorder (Figure 2a). Importantly, these disorders are characterized by systemic effects, with many tissues affected, unlike the more common laminopathies. Laminopathies with an inconsistent genotype phenotype link By contrast, laminopathies with striated muscle involvement (A-EDMD, LGMD1B, L-CMD and DCM), together accounting for 60% of all laminopathies, along with those affecting adipose tissue (FPLD2), are caused by a large number of different mutations (Figure 1). A-EDMD (blue in Figure 1) and L-CMD (gold in Figure 1) mutations are distributed throughout the gene, whereas LGMD1B (black in Figure 1) mutations tend to cluster in both the Ig-like fold and coil 2 [1,9]. DCM (green in Figure 1) patients tend to carry mutations throughout the rod domain and can share clinical characteristics with A-EDMD patients, where an isolated cardiac involvement has been described. For FPLD2, there is an apparent hot spot for mutations in the Ig-like fold where 75% of all mutations occur. The remaining 25% are distributed throughout the protein and are distinct from those causing A-EDMD (Figure 1). Interestingly, muscle involvement has not been observed in FPLD2 patients. The C-terminal domain of lamin A binds SREBP1 (sterol-regulatoryelement-binding protein 1) and SREBP2, which play a key role in adipocyte differentiation and cholesterol biosynthesis respectively [10]. Thus certain mutations may disrupt lamin A SREBP1/2 interactions to specifically affect adipocyte function. Phenotypic clustering using mutation type Bonne et al. [11] first attempted a genotype phenotype correlation by mutation type in LMNA. Of the 53 patients analysed, all 12 with isolated heart involvement carried a nonsense mutation in Q6X of the head domain. In the remaining 41 patients with muscle weakness however, attempts to correlate disease severity with the protein domain affected by the missense mutations proved inconclusive. Our own analysis of LMNA patients (
3 Nuclear Envelope Disease and Chromatin Organization Figure 2 Modes of LMNA genotype phenotype correlation (a) A consistent genotype phenotype correlation indicates a likely common disease mechanism. (b) LMNA mutations distributed throughout the protein can each cause a particular laminopathy. Some groups of mutations probably have a common disease mechanism, while other mutations appear to operate by separate pathways to cause the same laminopathy. (c)specificlmna mutations combined with LMNA SNPs, or mutations/snps in other proteins, act through combined mechanisms to cause modified phenotypes, such as atypical laminopathies or different laminopathies in individual patients carrying identical mutations. the domain surface without affecting structural integrity: with R482W, for example, not changing the crystal structure of the mutant protein [13 15]. However, tetrameric aggregates of mutant molecules were found that did not occur in the original structure, such that R482W or R482Q might cause an allosteric effect, allowing the repositioning of the C-terminal β-strand g, leading to a novel aggregation state and so possibly contributing to disease [13]. A similar phenomenon occurs with the F12L mutation in porphobilinogen synthase, which initiates a transition of the aggregation state, significantly changing the enzyme kinetics [16]. Certain mutations, therefore, could have a common mechanism to cause a particular laminopathy, but it is less clear how mutations located in functionally distinct protein domains could operate by the same disease mechanism (Figures 1 and 2b). [1] with frameshift mutations found that 62% (18/29) developed DCM, again associating DCM with truncated protein species (J. Scharner, V. Gnocchi, J.A. Ellis and P.S. Zammit, unpublished work). Benedetti et al. [12] separated neuromuscular patients according to the age of disease onset. ThetypeofLMNA mutation differed, such that 89% (17/19) early-onset patients carried missense mutations and in-frame deletions, while only 63% (5/8) of late-onset patients did. The remaining three late-onset patients had frameshift mutations, presumably resulting in a truncated protein. Interestingly, variants associated with early onset were primarily found in the Ig-like fold (35%) and in coil 2A (24%), while variants associated with late onset mainly occurred in coil 2B (60%) [12]. These results indicate that the underlying disease mechanism may be associated with the functional properties of the affected protein domain rather than with the type of mutation. Structural changes of mutant lamin A in A-EDMD,butnotinFPLD2 Mutations in the Ig-like fold provide evidence of a genotype phenotype correlation [13,14]. This globular region in the C-terminal domain harbours both A-EDMD (e.g. R453W and R572P) and FPLD2 (e.g. R482W, R482Q and K486N), causing mutations in close proximity (Figure 1). Mutations causing laminopathies with striated muscle involvement occur at positions that play a critical role in the structural stability of the C-terminal domain. Conversely, those that lead to FPLD2 result from a lost positive charge on Differential effects of LMNA mutations in post-mitotic cells compared with dividing cells An LMNA mutation causing A-EDMD (R453W) perturbs myogenic differentiation, while an FPLD2-causing mutation (R482W) does not, again suggesting that certain mutations can have tissue-specific effects [17]. Many LMNA mutations probably have common effects, including compromised nuclear integrity and transcriptional regulation, but some may also have additional actions. Particular mutant lamin A variants delay cell cycle progression by prolonging S-phase [18], and some also hinder the exit from cell cycle and/or the nuclear rearrangements, required for myogenic differentiation in immortalized cell lines [17,19]. The functional cells of cardiac muscle, skeletal muscle and adipose tissue are post-mitotic, but skeletal muscle retains a well-characterized stem cell compartment, responsible for homoeostatic myonuclear turnover, hypertrophy and repair [20]. Thus skeletal muscle may additionally be vulnerable to mutations that also affect cell cycle and/or differentiation of myogenic stem cells. Such a mutation, therefore, may not only influence the onset and severity of A-EDMD [21] or produce L-CMD [9], but even influence which tissue is affected and so the type of laminopathy caused. Lamin A or lamin C or both? Since LMNA is alternatively spliced [22], another way to classify mutations is whether they affect both lamin A and lamin C or just one protein. Of the 12 exons in LMNA, exons 11 and 12 are specific to lamin A, while alternative splicing at codon 566 in exon 10 gives lamin C, a unique exon coding for six amino acid residues (Figure 1). As expected given its size, only two mutations, R571S causing DCM [23] and R571C causing muscular dystrophy with axonal neuropathy [24], have been associated with the lamin C-specific tail. Exon 12 encodes eight amino acids that are cleaved during posttranslational modification of prelamin A, so unless mutations affect prelamin A processing, they would not be expected to affect lamin A function. Interestingly, few mutations occur in lamin A-specific exon 11 and those that do are less
4 260 Biochemical Society Transactions (2010) Volume 38, part 1 associated with skeletal muscle involvement: for example, of 18 mutations in exon 11 [1], 56% (10/18) cause DCM and 33% (6/18) result in FPLD2, with only one each causing A-EDMD or LGMD1B (J. Scharner, V. Gnocchi, J.A. Ellis and P.S. Zammit, unpublished work). It is worth noting that as HGPS predominately, but not exclusively, targets lamin A and generates the most severe laminopathy, it has been interpreted as being the more important protein. However, the apparent normal phenotype of the lmna LCO/LCO mouse, where lamin C is the only A-type lamin present, suggests that both lamin A and the other isoforms are dispensable [25]. Effect of modifying genes and SNPs (single nucleotide polymorphisms) on phenotypic variability in laminopathies Patients carrying, for example, the R644C mutation have a range of clinical conditions including DCM, LGMD2B, atypical HGPS, lipodystrophy etc. [26 29]. This extreme phenotypic variability indicates that genetic background contributes to the disease diagnosed [29]. Furthermore, a single mutation can result in DCM either with or without A-EDMD. This provides evidence of epistasis, i.e. effects of the mutated LMNA gene are altered by one or more other genes, the so-called modifying genes (Figure 2c). There are, indeed, rare examples of where an additional mutation in either desmin or emerin results in a more severe disease than might be expected from the LMNA mutation alone [30]. Where patients carry two different pathogenic LMNA mutations, it is not unexpected that they develop a more severe phenotype [31]. However, LMNA also contains SNPs with no apparent pathological phenotype: of 40 on the Leiden Open Variation Database ( 75% (30/40) are silent mutations, and the rest are missense mutations affecting the head (one), central rod (three) or tail (six) domains (J. Scharner, V. Gnocchi, J.A. Ellis and P.S. Zammit, unpublished work). Depending on the context however, these mutations can cause disease: T528M or M540T alone appear non-pathogenic, but when inherited together, they result in an apparently typical HGPS but without prelamin A accumulation [32]. Certainly, a particular LMNA pathogenic mutation/snp combination can increase the penetrance of a phenotype, which offers an explanation for intra- and inter-familiar variations [33], but may also alter the clinical condition diagnosed: while the S583L mutation normally causes FPLD2, when present with T528M it results in FPLD1 [34]. Modifying genes/snps are likely to explain inconclusive attempts to associate mutation with laminopathy (Figure 2c), where, for example, a single mutation has been reported to express phenotypic variability, such as with R60G, Y267C, R377H/L and R644C (Figure 1). Mouse models of laminopathies Modelling diseases in mice is a powerful technology to explore genotype phenotype correlations, disease mechanisms and potential therapies. The majority of laminopathies arise from missense and frameshift mutations, but two nonsense mutations have been reported: patients heterozygous for Y259X have LGMD1B [35], but those heterozygous for Q6X develop DCM [36,37] and mice heterozygous for the targeted lmna null allele also develop a DCM phenotype [38]. The only LMNA-null homozygous subject identified carried Y259X and died at birth, and lmna / mice are characterized by postnatal growth retardation, muscular dystrophy, rapidly progressive DCM and death by 4 8 weeks of age [39,40]. In humans, both the L530P and H222P mutations cause A-EDMD. The H222P mouse recapitulates features of A-EDMD [41], while L530P causes a phenotype akin to HGPS, possibly due to the unintentional inclusion of an additional splicing defect in the C-terminus [42]. The N195K mutation causes DCM in humans and a DCM-like phenotype in mice [43]. Importantly, the L530P, H222P and N195K mouse models only show a phenotype when homozygous for the mutant alides, in contrast with the heterozygous state in patients. DCM with A-EDMD resulting from the M371K mutation also causes a heart phenotype in transgenic mice when under a heart-specific promoter [44]. Collectively, these mutant mice illustrate that aspects of human laminopathies can be successfully modelled in mice, with all the attendant advantages over experimenting on rare patient samples, but again emphasizes that genetic background exerts an influence on the penetrance of the condition, whether in mice or humans. Conclusions and perspectives It must be remembered that laminopathies are rare diseases, and in many cases there are very few patients with a particular mutation(s). That said, what can be concluded about genotype phenotype correlations? In laminopathies such as HGPS, there is a high degree of consistency in the underlying mutation between patients, meaning that the disease mechanism is likely to be common to that particular disorder. There is also a degree of genotype phenotype correlation in FPLD2 for example, where many mutations are in the Ig-like fold and so could have a common disease mechanism, although the rest give little clue as to why they cause this condition. For others however, including DCM, A-EDMD and LGMD1B, there is a much weaker correlation between mutation and disease (Figure 2). A-EDMD and FPLD2 may differ owing to the degree to which a particular mutation affects lamin A/C structure, but this does not, for example, explain how different amino acid substitutions lead to a specific phenotype. It should also be remembered that lamins A and C are distinct proteins and mutations in the common region of LMNA would generate two distinct mutated proteins that could function differently to cause disease and so need to be treated as individual entities [45]. Where a more severe or unexpected phenotype is found with a particular LMNA mutation, a number of factors may be contributing. Whether a mutation also affects cell cycle and differentiation, in addition to nuclear structure, chromosome
5 Nuclear Envelope Disease and Chromatin Organization organization or transcriptional regulation, could certainly contribute to disease onset and severity, but maybe even the laminopathy diagnosed. The emerging evidence of modifying genes and SNPs affecting laminopathies means that, in future, it would be helpful if clinical data were to include the LMNAspecific SNPs, to determine if disease severity/diagnosis can be linked to certain mutation/snp combinations. Screening LMNA for SNPs is also being used in non-laminopathies with overlapping phenotypes, to determine whether there is any correlation with disease severity [46]. Ultimately, genomewide sequencing would be needed to check for mutations in all regulatory regions of LMNA and fully explore the correlation with modifying genes. Finally, EDMD is divided into A-EDMD, and X-linked EDMD caused by mutations in EMD, but together these account for only 50% [47] of clinically diagnosed EDMD. Assuming that LMNA mutations/snps have not been overlooked, mutations in other genes may also result in disorders with a similar phenotype [48]. Indeed, it was recently shown that mutations in LAP2α can cause a condition similar to DCM [49], whereas mutations in SYNE1 or FHL1 produce an EDMD-like phenotype [50,51]. Funding J.S. is supported by a Ph.D. studentship funded by the Biomedical and Health School, King s College London, and V.F.G. is funded by The Medical Research Council [grant number G ]. The P.S.Z. laboratory is supported by The Muscular Dystrophy Campaign, The Wellcome Trust and OPTISTEM (contract ), through the European Union 7th Framework Programme. References 1 Szeverenyi, I., Cassidy, A.J., Chung, C.W., Lee, B.T., Common, J.E., Ogg, S.C., Chen, H., Sim, S.Y., Goh, W.L., Ng, K.W. et al. (2008) The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum. Mutat. 29, Broers, J.L., Ramaekers, F.C., Bonne, G., Yaou, R.B. and Hutchison, C.J. (2006) Nuclear lamins: laminopathies and their role in premature ageing. Physiol. Rev. 86, Freimer, N. and Sabatti, C. (2003) The human phenome project. Nat. Genet. 34, Hegele, R. (2005) LMNA mutation position predicts organ system involvement in laminopathies. Clin. Genet. 68, Scriver, C.R. (2004) After the genome the phenome? J. Inherit. Metab. Dis. 27, Eriksson, M., Brown, W.T., Gordon, L.B., Glynn, M.W., Singer, J., Scott, L., Erdos, M.R., Robbins, C.M., Moses, T.Y., Berglund, P. et al. (2003) Recurrent de novo point mutations in lamin A cause Hutchinson Gilford progeria syndrome. Nature 423, Moulson, C.L., Fong, L.G., Gardner, J.M., Farber, E.A., Go, G., Passariello, A., Grange, D.K., Young, S.G. and Miner, J.H. (2007) Increased progerin expression associated with unusual LMNA mutations causes severe progeroid syndromes. Hum. Mutat. 28, Ben Yaou, R., Muchir, A., Arimura, T., Massart, C., Demay, L., Richard, P. and Bonne, G. (2005) Genetics of laminopathies. Novartis Found. Symp. 264, Quijano-Roy, S., Mbieleu, B., Bonnemann, C.G., Jeannet, P.Y., Colomer, J., Clarke, N.F., Cuisset, J.M., Roper, H., De Meirleir, L., D Amico, A. et al. (2008) De novo LMNA mutations cause a new form of congenital muscular dystrophy. Ann. Neurol. 64, Lloyd, D.J., Trembath, R.C. and Shackleton, S. (2002) A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum. Mol. Genet. 11, Bonne, G., Mercuri, E., Muchir, A., Urtizberea, A., Becane, H.M., Recan, D., Merlini, L., Wehnert, M., Boor, R., Reuner, U. et al. (2000) Clinical and molecular genetic spectrum of autosomal dominant Emery Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann. Neurol. 48, Benedetti, S., Menditto, I., Degano, M., Rodolico, C., Merlini, L., D Amico, A., Palmucci, L., Berardinelli, A., Pegoraro, E., Trevisan, C.P. et al. (2007) Phenotypic clustering of lamin A/C mutations in neuromuscular patients. Neurology 69, Dhe-Paganon, S., Werner, E.D., Chi, Y.I. and Shoelson, S.E. (2002) Structure of the globular tail of nuclear lamin. J. Biol. Chem. 277, Krimm, I., Ostlund, C., Gilquin, B., Couprie, J., Hossenlopp, P., Mornon, J.P., Bonne, G., Courvalin, J.C., Worman, H.J. and Zinn-Justin, S. (2002) The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure 10, Magracheva, E., Kozlov, S., Stewart, C.L., Wlodawer, A. and Zdanov, A. (2009) Structure of the lamin A/C R482W mutant responsible for dominant familial partial lipodystrophy (FPLD). Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65, Breinig, S., Kervinen, J., Stith, L., Wasson, A.S., Fairman, R., Wlodawer, A., Zdanov, A. and Jaffe, E.K. (2003) Control of tetrapyrrole biosynthesis by alternate quaternary forms of porphobilinogen synthase. Nat. Struct. Biol. 10, Favreau, C., Higuet, D., Courvalin, J.C. and Buendia, B. (2004) Expression of a mutant lamin A that causes Emery Dreifuss muscular dystrophy inhibits in vitro differentiation of C2C12 myoblasts. Mol. Cell. Biol. 24, Emerson, L.J., Holt, M.R., Wheeler, M.A., Wehnert, M., Parsons, M. and Ellis, J.A. (2009) Defects in cell spreading and ERK1/2 activation in fibroblasts with lamin A/C mutations. Biochim. Biophys. Acta 1792, Markiewicz, E., Ledran, M. and Hutchison, C.J. (2005) Remodelling of the nuclear lamina and nucleoskeleton is required for skeletal muscle differentiation in vitro. J. Cell Sci. 118, Zammit, P.S. (2008) All muscle satellite cells are equal, but are some more equal than others? J. Cell Sci. 121, Gnocchi, V.F., Ellis, J.A. and Zammit, P.S. (2008) Does satellite cell dysfunction contribute to disease progression in Emery Dreifuss muscular dystrophy? Biochem. Soc. Trans. 36, Lin, F. and Worman, H.J. (1993) Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 268, Fatkin, D., MacRae, C., Sasaki, T., Wolff, M.R., Porcu, M., Frenneaux, M., Atherton, J., Vidaillet, Jr, H.J., Spudich, S. et al. (1999) Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 341, Benedetti, S., Bertini, E., Iannaccone, S., Angelini, C., Trisciani, M., Toniolo, D., Sferrazza, B., Carrera, P., Comi, G., Ferrari, M. et al. (2005) Dominant LMNA mutations can cause combined muscular dystrophy and peripheral neuropathy. J. Neurol. Neurosurg. Psychiatry 76, Fong, L.G., Ng, J.K., Lammerding, J., Vickers, T.A., Meta, M., Cote, N., Gavino, B., Qiao, X., Chang, S.Y., Young, S.R. et al. (2006) Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J. Clin. Invest. 116, Brown, C.A., Lanning, R.W., McKinney, K.Q., Salvino, A.R., Cherniske, E., Crowe, C.A., Darras, B.T., Gominak, S., Greenberg, C.R., Grosmann, C. et al. (2001) Novel and recurrent mutations in lamin A/C in patients with Emery Dreifuss muscular dystrophy. Am. J. Med. Genet. 102, Csoka, A.B., Cao, H., Sammak, P.J., Constantinescu, D., Schatten, G.P. and Hegele, R.A. (2004) Novel lamin A/C gene (LMNA) mutations in atypical progeroid syndromes. J. Med. Genet. 41, Mercuri, E., Brown, S.C., Nihoyannopoulos, P., Poulton, J., Kinali, M., Richard, P., Piercy, R.J., Messina, S., Sewry, C., Burke, M.M. et al. (2005) Extreme variability of skeletal and cardiac muscle involvement in patients with mutations in exon 11 of the lamin A/C gene. Muscle Nerve 31, Rankin, J., Auer-Grumbach, M., Bagg, W., Colclough, K., Nguyen, T.D., Fenton-May, J., Hattersley, A., Hudson, J., Jardine, P., Josifova, D. et al. (2008) Extreme phenotypic diversity and nonpenetrance in families with the LMNA gene mutation R644C. Am. J. Med. Genet. A 146,
6 262 Biochemical Society Transactions (2010) Volume 38, part 1 30 Muntoni, F., Bonne, G., Goldfarb, L.G., Mercuri, E., Piercy, R.J., Burke, M., Yaou, R.B., Richard, P., Recan, D., Shatunov, A. et al. (2006) Disease severity in dominant Emery Dreifuss is increased by mutations in both emerin and desmin proteins. Brain 129, Hegele, R.A., Cao, H., Anderson, C.M. and Hramiak, I.M. (2000) Heterogeneity of nuclear lamin A mutations in Dunnigan-type familial partial lipodystrophy. J. Clin. Endocrinol. Metab. 85, Verstraeten, V.L., Broers, J.L., van Steensel, M.A., Zinn-Justin, S., Ramaekers, F.C., Steijlen, P.M., Kamps, M., Kuijpers, H.J., Merckx, D., Smeets, H.J. et al. (2006) Compound heterozygosity for mutations in LMNA causes a progeria syndrome without prelamin A accumulation. Hum. Mol. Genet. 15, Ellis, J.A. (2006) Emery Dreifuss muscular dystrophy at the nuclear envelope: 10 years on. Cell. Mol. Life Sci. 63, Savage, D.B., Soos, M.A., Powlson, A., O Rahilly, S., McFarlane, I., Halsall, D.J., Barroso, I., Thomas, E.L., Bell, J.D., Scobie, I. et al. (2004) Familial partial lipodystrophy associated with compound heterozygosity for novel mutations in the LMNA gene. Diabetologia 47, van Engelen, B.G., Muchir, A., Hutchison, C.J., van der Kooi, A.J., Bonne, G. and Lammens, M. (2005) The lethal phenotype of a homozygous nonsense mutation in the lamin A/C gene. Neurology 64, Bonne, G., Di Barletta, M.R., Varnous, S., Becane, H.M., Hammouda, E.H., Merlini, L., Muntoni, F., Greenberg, C.R., Gary, F., Urtizberea, J.A. et al. (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery Dreifuss muscular dystrophy. Nat. Genet. 21, Becane, H.M., Bonne, G., Varnous, S., Muchir, A., Ortega, V., Hammouda, E.H., Urtizberea, J.A., Lavergne, T., Fardeau, M., Eymard, B. et al. (2000) High incidence of sudden death with conduction system and myocardial disease due to lamins A and C gene mutation. Pacing Clin. Electrophysiol. 23, Wolf, C.M., Wang, L., Alcalai, R., Pizard, A., Burgon, P.G., Ahmad, F., Sherwood, M., Branco, D.M., Wakimoto, H., Fishman, G.I. et al. (2008) Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J. Mol. Cell. Cardiol. 44, Sullivan, T., Escalante-Alcalde, D., Bhatt, H., Anver, M., Bhat, N., Nagashima, K., Stewart, C.L. and Burke, B. (1999) Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147, Nikolova, V., Leimena, C., McMahon, A.C., Tan, J.C., Chandar, S., Jogia, D., Kesteven, S.H., Michalicek, J., Otway, R., Verheyen, F. et al. (2004) Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J. Clin. Invest. 113, Arimura, T., Helbling-Leclerc, A., Massart, C., Varnous, S., Niel, F., Lacene, E., Fromes, Y., Toussaint, M., Mura, A.M., Keller, D.I. et al. (2005) Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 14, Mounkes, L.C., Kozlov, S., Hernandez, L., Sullivan, T. and Stewart, C.L. (2003) A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 423, Mounkes, L.C., Kozlov, S.V., Rottman, J.N. and Stewart, C.L. (2005) Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum. Mol. Genet. 14, Wang, Y., Herron, A.J. and Worman, H.J. (2006) Pathology and nuclear abnormalities in hearts of transgenic mice expressing M371K lamin A encoded by an LMNA mutation causing Emery Dreifuss muscular dystrophy. Hum. Mol. Genet. 15, Motsch, I., Kaluarachchi, M., Emerson, L.J., Brown, C.A., Brown, S.C., Dabauvalle, M.C. and Ellis, J.A. (2005) Lamins A and C are differentially dysfunctional in autosomal dominant Emery Dreifuss muscular dystrophy. Eur. J. Cell Biol. 84, Gaudy-Marqueste, C., Boyer, A., Navarro, C., Rouzier, C., Harley, J.R., Weiller, P.J., Grob, J.J., Levy, N. and De Sandre-Giovannoli, A. (2009) LMNA, ZMPSTE24, and LBR are not mutated in Scleroderma. Genet. Test. Mol. Biomarkers 13, Cohen, T.V. and Stewart, C.L. (2008) Fraying at the edge: mouse models of diseases resulting from defects at the nuclear periphery. Curr. Top. Dev. Biol. 84, Stewart, C.L., Kozlov, S., Fong, L.G. and Young, S.G. (2007) Mouse models of the laminopathies. Exp. Cell Res. 313, Taylor, M.R., Slavov, D., Gajewski, A., Vlcek, S., Ku, L., Fain, P.R., Carniel, E., Di Lenarda, A., Sinagra, G., Boucek, M.M. et al. (2005) Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum. Mutat. 26, Zhang, Q., Bethmann, C., Worth, N.F., Davies, J.D., Wasner, C., Feuer, A., Ragnauth, C.D., Yi, Q., Mellad, J.A., Warren, D.T. et al. (2007) Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 16, Gueneau, L., Bertrand, A.T., Jais, J.P., Salih, M.A., Stojkovic, T., Wehnert, M., Hoeltzenbein, M., Spuler, S., Saitoh, S., Verschueren, A. et al. (2009) Mutations of the FHL1 gene cause Emery Dreifuss muscular dystrophy. Am. J. Hum. Genet. 85, Cao, H. and Hegele, R.A. (2003) LMNA is mutated in Hutchinson Gilford progeria (MIM ) but not in Wiedemann Rautenstrauch progeroid syndrome (MIM ). J. Hum. Genet. 48, Agarwal, A.K., Kazachkova, I., Ten, S. and Garg, A. (2008) Severe mandibuloacral dysplasia-associated lipodystrophy and progeria in a young girl with a novel homozygous Arg527Cys LMNA mutation. J. Clin. Endocrinol. Metab. 93, Schirmer, E.C. and Foisner, R. (2007) Proteins that associate with lamins: many faces, many functions. Exp. Cell Res. 313, Received 28 August 2009 doi: /bst
Nuclear Envelope and Muscular Dystrophy
 Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
HYBRID GENE THERAPY FOR AD-EDMD
 HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling
 4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
L aminopathies represent a heterogeneous group of genetic
 1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
1of5 ONLINE MUTATION REPORT Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes M Vytopil, S Benedetti, E Ricci, G Galluzzi, A Dello Russo, L Merlini,
THURSDAY, JANUARY
 THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
The laminopathies: a clinical review
 Clin Genet 2006: 70: 261 274 Printed in Singapore. All rights reserved Review # 2006 The Authors Journal compilation # 2006 Blackwell Munksgaard CLINICAL GENETICS doi: 10.1111/j.1399-0004.2006.00677.x
Clin Genet 2006: 70: 261 274 Printed in Singapore. All rights reserved Review # 2006 The Authors Journal compilation # 2006 Blackwell Munksgaard CLINICAL GENETICS doi: 10.1111/j.1399-0004.2006.00677.x
Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C
 Am. J. Hum. Genet. 71:426 431, 2002 Report Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C Giuseppe Novelli, 1 Antoine Muchir, 6 Federica Sangiuolo, 1 Anne Helbling-Leclerc,
Am. J. Hum. Genet. 71:426 431, 2002 Report Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C Giuseppe Novelli, 1 Antoine Muchir, 6 Federica Sangiuolo, 1 Anne Helbling-Leclerc,
H utchinson-gilford progeria syndrome (HGPS; MIM
 609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
609 LETTER TO JMG Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome M Plasilova*, C Chattopadhyay*, P Pal, N A Schaub, S A Buechner, Hj
Laminopathies and the long strange trip from basic cell biology to therapy
 Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
When Lamins Go Bad: Nuclear Structure and Disease
 Leading Edge Review When Lamins Go Bad: Nuclear Structure and Disease Katherine H. Schreiber 1 and Brian K. Kennedy 1,2, * 1 Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945,
Leading Edge Review When Lamins Go Bad: Nuclear Structure and Disease Katherine H. Schreiber 1 and Brian K. Kennedy 1,2, * 1 Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945,
T he LMNA gene encodes two nuclear envelope proteins,
 1of5 ELECTRONIC LETTER A new mutation of the lamin A/C gene leading to autosomal dominant axonal neuropathy, muscular dystrophy, cardiac disease, and leuconychia C Goizet, R Ben aou, L Demay, P Richard,
1of5 ELECTRONIC LETTER A new mutation of the lamin A/C gene leading to autosomal dominant axonal neuropathy, muscular dystrophy, cardiac disease, and leuconychia C Goizet, R Ben aou, L Demay, P Richard,
Novel LMNA mutations in patients with Emery-Dreifuss muscular dystrophy and functional characterization of four LMNA mutations
 Novel LMNA mutations in patients with Emery-Dreifuss muscular dystrophy and functional characterization of four LMNA mutations Juergen Scharner, Charlotte A Brown, Matthew Bower, Susan T Iannaccone, Ismail
Novel LMNA mutations in patients with Emery-Dreifuss muscular dystrophy and functional characterization of four LMNA mutations Juergen Scharner, Charlotte A Brown, Matthew Bower, Susan T Iannaccone, Ismail
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins
 Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
Lamins, laminopathies and disease mechanisms: Possible role for proteasomal degradation of key regulatory proteins VEENA KPARNAIK*, PANKAJ CHATURVEDI and BH MURALIKRISHNA Centre for Cellular and Molecular
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy
 The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
LMNA mutations in atypical Werner s syndrome
 Mechanisms of disease LMNA mutations in atypical Werner s syndrome Lishan Chen, Lin Lee, Brian A Kudlow, Heloisa G Dos Santos, Olav Sletvold, Yousef Shafeghati, Eleanor G Botha, Abhimanyu Garg, Nancy B
Mechanisms of disease LMNA mutations in atypical Werner s syndrome Lishan Chen, Lin Lee, Brian A Kudlow, Heloisa G Dos Santos, Olav Sletvold, Yousef Shafeghati, Eleanor G Botha, Abhimanyu Garg, Nancy B
Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two Chinese siblings: two case reports
 Xiong et al. Journal of Medical Case Reports 2013, 7:63 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two
Xiong et al. Journal of Medical Case Reports 2013, 7:63 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Hutchinson-Gilford progeria syndrome accompanied by severe skeletal abnormalities in two
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,100 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,100 116,000 120M Open access books available International authors and editors Downloads Our
Early-Onset LMNA-Associated Muscular Dystrophy with Later Involvement of Contracture
 JCN Open Access pissn 1738-6586 / eissn 2005-5013 / J Clin Neurol 2017;13(4):405-410 / https://doi.org/10.3988/jcn.2017.13.4.405 ORIGINAL ARTICLE Early-Onset LMNA-Associated Muscular Dystrophy with Later
JCN Open Access pissn 1738-6586 / eissn 2005-5013 / J Clin Neurol 2017;13(4):405-410 / https://doi.org/10.3988/jcn.2017.13.4.405 ORIGINAL ARTICLE Early-Onset LMNA-Associated Muscular Dystrophy with Later
Original Articles. Jason Cowan, MS; Duanxiang Li, MD; Jorge Gonzalez-Quintana, BS; Ana Morales, MS, CGC; Ray E. Hershberger, MD
 Original Articles Morphological Analysis of 13 LMNA Variants Identified in a Cohort of 324 Unrelated Patients With Idiopathic or Familial Dilated Cardiomyopathy Jason Cowan, MS; Duanxiang Li, MD; Jorge
Original Articles Morphological Analysis of 13 LMNA Variants Identified in a Cohort of 324 Unrelated Patients With Idiopathic or Familial Dilated Cardiomyopathy Jason Cowan, MS; Duanxiang Li, MD; Jorge
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1
 Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
LMNA cardiomyopathy: cell biology and genetics meet clinical medicine
 Disease Models & Mechanisms 4, 562-568 (2011) doi:10.1242/dmm.006346 : cell biology and genetics meet clinical medicine Jonathan T. Lu 1, Antoine Muchir 1,2, Peter L. Nagy 2 and Howard J. Worman 1,2, *
Disease Models & Mechanisms 4, 562-568 (2011) doi:10.1242/dmm.006346 : cell biology and genetics meet clinical medicine Jonathan T. Lu 1, Antoine Muchir 1,2, Peter L. Nagy 2 and Howard J. Worman 1,2, *
Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization
 Experimental Cell Research 304 (2005) 582 592 www.elsevier.com/locate/yexcr Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization Jos L.V. Broers
Experimental Cell Research 304 (2005) 582 592 www.elsevier.com/locate/yexcr Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization Jos L.V. Broers
Cardiac effects of the c.1583 C G LMNA mutation in two families with Emery Dreifuss muscular dystrophy
 MOLECULAR MEDICINE REPORTS 12: 5065-5071, 2015 Cardiac effects of the c.1583 C G LMNA mutation in two families with Emery Dreifuss muscular dystrophy LI ZHANG 1, HONGRUI SHEN 2, ZHE ZHAO 2, QI BING 2 and
MOLECULAR MEDICINE REPORTS 12: 5065-5071, 2015 Cardiac effects of the c.1583 C G LMNA mutation in two families with Emery Dreifuss muscular dystrophy LI ZHANG 1, HONGRUI SHEN 2, ZHE ZHAO 2, QI BING 2 and
Progeria. Premature human aging: t he progerias. Progerias as models for aging. Hutchinson-Gilford syndrome. Definition:
 Premature human aging: t he progerias Reading: Genetic alterations in accelerated ageing syndromes Do they play a role in natural ageing? Monika Puzianowska- Kuznicka. Jacek Kuznicki. 2005. IJBCB, 37;
Premature human aging: t he progerias Reading: Genetic alterations in accelerated ageing syndromes Do they play a role in natural ageing? Monika Puzianowska- Kuznicka. Jacek Kuznicki. 2005. IJBCB, 37;
Natural History of Dilated Cardiomyopathy Due to Lamin A/C Gene Mutations
 Journal of the American College of Cardiology Vol. 41, No. 5, 2003 2003 by the American College of Cardiology Foundation ISSN 0735-1097/03/$30.00 Published by Elsevier Science Inc. doi:10.1016/s0735-1097(02)02954-6
Journal of the American College of Cardiology Vol. 41, No. 5, 2003 2003 by the American College of Cardiology Foundation ISSN 0735-1097/03/$30.00 Published by Elsevier Science Inc. doi:10.1016/s0735-1097(02)02954-6
The Nuclear Envelope as a Signaling Node in Development and Disease
 The Nuclear Envelope as a Signaling Node in Development and Disease William T. Dauer 1,2, * and Howard J. Worman 3,4, * 1 Department of Neurology 2 Department of Cell and Developmental Biology University
The Nuclear Envelope as a Signaling Node in Development and Disease William T. Dauer 1,2, * and Howard J. Worman 3,4, * 1 Department of Neurology 2 Department of Cell and Developmental Biology University
Reviews. Mechanisms Underlying Caloric Restriction, Lipid Metabolism, and Life Span Regulation Issei Komuro, Guest Editor
 Reviews This Review is part of a thematic series on Biological Role of Senescence in Cardiovascular Disease, which includes the following articles: Telomere Biology and Cardiovascular Disease Vascular
Reviews This Review is part of a thematic series on Biological Role of Senescence in Cardiovascular Disease, which includes the following articles: Telomere Biology and Cardiovascular Disease Vascular
Nuclear envelopathies: a complex LINC between nuclear envelope and pathology
 Janin et al. Orphanet Journal of Rare Diseases (2017) 12:147 DOI 10.1186/s13023-017-0698-x REVIEW Nuclear envelopathies: a complex LINC between nuclear envelope and pathology Alexandre Janin 1,2,3,4, Delphine
Janin et al. Orphanet Journal of Rare Diseases (2017) 12:147 DOI 10.1186/s13023-017-0698-x REVIEW Nuclear envelopathies: a complex LINC between nuclear envelope and pathology Alexandre Janin 1,2,3,4, Delphine
Workshop report. 1. Introduction
 Neuromuscular Disorders 12 (2002) 187 194 Workshop report www.elsevier.com/locate/nmd 82nd ENMC international workshop, 5th international Emery Dreifuss muscular dystrophy (EDMD) workshop, 1st Workshop
Neuromuscular Disorders 12 (2002) 187 194 Workshop report www.elsevier.com/locate/nmd 82nd ENMC international workshop, 5th international Emery Dreifuss muscular dystrophy (EDMD) workshop, 1st Workshop
Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson Gilford progeria syndrome mutation
 Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson Gilford progeria syndrome mutation Shao H. Yang*, Martin O. Bergo, Julia I. Toth*, Xin Qiao*,
Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson Gilford progeria syndrome mutation Shao H. Yang*, Martin O. Bergo, Julia I. Toth*, Xin Qiao*,
Clinical Spectrum and Genetic Mechanism of GLUT1-DS. Yasushi ITO (Tokyo Women s Medical University, Japan)
 Clinical Spectrum and Genetic Mechanism of GLUT1-DS Yasushi ITO (Tokyo Women s Medical University, Japan) Glucose transporter type 1 (GLUT1) deficiency syndrome Mutation in the SLC2A1 / GLUT1 gene Deficiency
Clinical Spectrum and Genetic Mechanism of GLUT1-DS Yasushi ITO (Tokyo Women s Medical University, Japan) Glucose transporter type 1 (GLUT1) deficiency syndrome Mutation in the SLC2A1 / GLUT1 gene Deficiency
Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients
 Research Article 3893 Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients Hiroaki Mitsuhashi 1, Yukiko K. Hayashi 1, *, Chie Matsuda 2, Satoru Noguchi 1, Shuji Wakatsuki
Research Article 3893 Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients Hiroaki Mitsuhashi 1, Yukiko K. Hayashi 1, *, Chie Matsuda 2, Satoru Noguchi 1, Shuji Wakatsuki
Available online at
 Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 122 134 www.elsevier.com/locate/yexcr Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy
Available online at www.sciencedirect.com R Experimental Cell Research 291 (2003) 122 134 www.elsevier.com/locate/yexcr Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy
Advances in genetic diagnosis of neurological disorders
 Acta Neurol Scand 2014: 129 (Suppl. 198): 20 25 DOI: 10.1111/ane.12232 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd ACTA NEUROLOGICA SCANDINAVICA Review Article Advances in genetic diagnosis
Acta Neurol Scand 2014: 129 (Suppl. 198): 20 25 DOI: 10.1111/ane.12232 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd ACTA NEUROLOGICA SCANDINAVICA Review Article Advances in genetic diagnosis
Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24
 Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24 Jemima Barrowman, Corinne Hamblet, Megan S. Kane, Susan Michaelis* Department of Cell Biology, The Johns Hopkins School of Medicine,
Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24 Jemima Barrowman, Corinne Hamblet, Megan S. Kane, Susan Michaelis* Department of Cell Biology, The Johns Hopkins School of Medicine,
ggcatgcattattcgacgtcgtatcgatgcaaagtggctaggacgtcgatgctagtcgatcga tgactcgatcgatcgatcgagtcgtactgggaacgtctccgccgctctacgtcagcgtctggt
 ggcgcgtcgagatgatcacgacggcatgctagctagccatacgcgtcaatcgtagctagct actctagtacgatgctagctacgtacgtcatgatcgatcgatcgtagctagctagctagctaga gggcgctgctcttcgttgtgcacacttacgtagcatgctagctagctagctgtcagtcagtacga tac Cell
ggcgcgtcgagatgatcacgacggcatgctagctagccatacgcgtcaatcgtagctagct actctagtacgatgctagctacgtacgtcatgatcgatcgatcgtagctagctagctagctaga gggcgctgctcttcgttgtgcacacttacgtagcatgctagctagctagctgtcagtcagtacga tac Cell
Novel LMNA Mutation in a Taiwanese Family with Autosomal Dominant Emery-Dreifuss Muscular Dystrophy
 ASE REPORT Novel LMNA Mutation in a Taiwanese Family with Autosomal Dominant Emery-Dreifuss Muscular Dystrophy Wen-hen Liang, 1 hung-yee Yuo, 2 hun-ya Liu, 3 hee-siong Lee, 4 Kanako Goto, 5 Yukiko K. Hayashi,
ASE REPORT Novel LMNA Mutation in a Taiwanese Family with Autosomal Dominant Emery-Dreifuss Muscular Dystrophy Wen-hen Liang, 1 hung-yee Yuo, 2 hun-ya Liu, 3 hee-siong Lee, 4 Kanako Goto, 5 Yukiko K. Hayashi,
Emery-Dreifuss muscular dystrophy: the most recognizable laminopathy
 Review paper Emery-Dreifuss muscular dystrophy: the most recognizable laminopathy Agnieszka Madej-Pilarczyk, Andrzej Kochański Neuromuscular Unit, Mossakowski Medical Research Centre, Polish Academy of
Review paper Emery-Dreifuss muscular dystrophy: the most recognizable laminopathy Agnieszka Madej-Pilarczyk, Andrzej Kochański Neuromuscular Unit, Mossakowski Medical Research Centre, Polish Academy of
Clinical Genetics in Cardiomyopathies
 Clinical Genetics in Cardiomyopathies Γεώργιος Κ Ευθυμιάδης Αναπληρωτής Καθηγητής Καρδιολογίας ΑΠΘ No conflict of interest Genetic terms Proband: The first individual diagnosed in a family Mutation: A
Clinical Genetics in Cardiomyopathies Γεώργιος Κ Ευθυμιάδης Αναπληρωτής Καθηγητής Καρδιολογίας ΑΠΘ No conflict of interest Genetic terms Proband: The first individual diagnosed in a family Mutation: A
Disruption of spermatogenesis in mice lacking A-type lamins
 Research Article 1173 Disruption of spermatogenesis in mice lacking A-type lamins Manfred Alsheimer 1, *, Bodo Liebe 2, *, Lori Sewell 3, Colin L. Stewart 3, Harry Scherthan 2, and Ricardo Benavente 1,
Research Article 1173 Disruption of spermatogenesis in mice lacking A-type lamins Manfred Alsheimer 1, *, Bodo Liebe 2, *, Lori Sewell 3, Colin L. Stewart 3, Harry Scherthan 2, and Ricardo Benavente 1,
A Comprehensive Study of TP53 Mutations in Chronic Lymphocytic Leukemia: Analysis of 1,287 Diagnostic CLL Samples
 A Comprehensive Study of TP53 Mutations in Chronic Lymphocytic Leukemia: Analysis of 1,287 Diagnostic CLL Samples Sona Pekova, MD., PhD. Chambon Ltd., Laboratory for molecular diagnostics, Prague, Czech
A Comprehensive Study of TP53 Mutations in Chronic Lymphocytic Leukemia: Analysis of 1,287 Diagnostic CLL Samples Sona Pekova, MD., PhD. Chambon Ltd., Laboratory for molecular diagnostics, Prague, Czech
Familial DilatedCardiomyopathy Georgios K Efthimiadis, MD
 Familial DilatedCardiomyopathy Georgios K Efthimiadis, MD Dilated Cardiomyopathy Dilated LV/RV, reduced EF, in the absence of CAD valvulopathy pericardial disease Prevalence:40/100.000 persons Natural
Familial DilatedCardiomyopathy Georgios K Efthimiadis, MD Dilated Cardiomyopathy Dilated LV/RV, reduced EF, in the absence of CAD valvulopathy pericardial disease Prevalence:40/100.000 persons Natural
Importance of molecular cell biology investigations in human medicine in the story of the Hutchinson-Gilford progeria syndrome
 Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
HUTCHINSON-GILFORD progeria syndrome (HGPS)
 Journal of Gerontology: BIOLOGICAL SCIENCES 2007, Vol. 62A, No. 1, 3 8 Copyright 2007 by The Gerontological Society of America Perspectives Article Progeria of Stem Cells: Stem Cell Exhaustion in Hutchinson-Gilford
Journal of Gerontology: BIOLOGICAL SCIENCES 2007, Vol. 62A, No. 1, 3 8 Copyright 2007 by The Gerontological Society of America Perspectives Article Progeria of Stem Cells: Stem Cell Exhaustion in Hutchinson-Gilford
Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells
 1698 Biochemical Society Transactions (2011) Volume 39, part 6 Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells Giovanna Lattanzi 1 National Research Council of Italy, Institute
1698 Biochemical Society Transactions (2011) Volume 39, part 6 Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells Giovanna Lattanzi 1 National Research Council of Italy, Institute
Skeletal myopathy in a family with lamin A/C cardiac disease
 Original Article Skeletal myopathy in a family with lamin A/C cardiac disease Subha Ghosh 1, Rahul Renapurkar 1, Subha V. Raman 2 1 Cleveland Clinic Foundation, Cleveland, OH, USA; 2 The Ohio State University
Original Article Skeletal myopathy in a family with lamin A/C cardiac disease Subha Ghosh 1, Rahul Renapurkar 1, Subha V. Raman 2 1 Cleveland Clinic Foundation, Cleveland, OH, USA; 2 The Ohio State University
Association of the LMNA gene single nucleotide polymorphism rs4641 with dilated cardiomyopathy
 Association of the LMNA gene single nucleotide polymorphism rs4641 with dilated cardiomyopathy J. Yin 1,2, J. Yang 3, F.X. Ren 3, C.M. Sun 4, L.D. Li 3, L.Y. Han 5, S.L. Cai 1, C.H. Zhang 3, Z.Q. Zhang
Association of the LMNA gene single nucleotide polymorphism rs4641 with dilated cardiomyopathy J. Yin 1,2, J. Yang 3, F.X. Ren 3, C.M. Sun 4, L.D. Li 3, L.Y. Han 5, S.L. Cai 1, C.H. Zhang 3, Z.Q. Zhang
Mutations and Disease Mutations in the Myosin Gene
 Biological Sciences Initiative HHMI Mutations and Disease Mutations in the Myosin Gene Goals Explore how mutations can lead to disease using the myosin gene as a model system. Explore how changes in the
Biological Sciences Initiative HHMI Mutations and Disease Mutations in the Myosin Gene Goals Explore how mutations can lead to disease using the myosin gene as a model system. Explore how changes in the
SMA IS A SEVERE NEUROLOGICAL DISORDER [1]
![SMA IS A SEVERE NEUROLOGICAL DISORDER [1] SMA IS A SEVERE NEUROLOGICAL DISORDER [1]](/thumbs/72/67086153.jpg) SMA OVERVIEW SMA IS A SEVERE NEUROLOGICAL DISORDER [1] Autosomal recessive genetic inheritance 1 in 50 people (approximately 6 million Americans) are carriers [2] 1 in 6,000 to 1 in 10,000 children born
SMA OVERVIEW SMA IS A SEVERE NEUROLOGICAL DISORDER [1] Autosomal recessive genetic inheritance 1 in 50 people (approximately 6 million Americans) are carriers [2] 1 in 6,000 to 1 in 10,000 children born
Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies
 Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
2015 PhD proposal. CIFRE Grant SANOFI Centre de Recherche en Myologie Altered calcium homeostasis and LMNA-cardiomyopathy
 2015 PhD proposal Project / Research program: Project Leader: Department/Unit: CIFRE Grant SANOFI Centre de Recherche en Myologie Altered calcium homeostasis and LMNA-cardiomyopathy Antoine Muchir, PhD,
2015 PhD proposal Project / Research program: Project Leader: Department/Unit: CIFRE Grant SANOFI Centre de Recherche en Myologie Altered calcium homeostasis and LMNA-cardiomyopathy Antoine Muchir, PhD,
DEVELOPMENT OF A MOUSE MODEL FOR HUTCHINSON-GILFORD PROGERIA SYNDROME REVEAL DEFECTS IN ADULT STEM CELL MAINTENANCE
 From THE DEPARTMENT OF BIOSCIENCES AND NUTRITION Karolinska Institutet, Stockholm, Sweden DEVELOPMENT OF A MOUSE MODEL FOR HUTCHINSON-GILFORD PROGERIA SYNDROME REVEAL DEFECTS IN ADULT STEM CELL MAINTENANCE
From THE DEPARTMENT OF BIOSCIENCES AND NUTRITION Karolinska Institutet, Stockholm, Sweden DEVELOPMENT OF A MOUSE MODEL FOR HUTCHINSON-GILFORD PROGERIA SYNDROME REVEAL DEFECTS IN ADULT STEM CELL MAINTENANCE
Tumor suppressor genes D R. S H O S S E I N I - A S L
 Tumor suppressor genes 1 D R. S H O S S E I N I - A S L What is a Tumor Suppressor Gene? 2 A tumor suppressor gene is a type of cancer gene that is created by loss-of function mutations. In contrast to
Tumor suppressor genes 1 D R. S H O S S E I N I - A S L What is a Tumor Suppressor Gene? 2 A tumor suppressor gene is a type of cancer gene that is created by loss-of function mutations. In contrast to
Genetic diagnosis of limb girdle muscular dystrophy type 2A, A Case Report
 Genetic diagnosis of limb girdle muscular dystrophy type 2A, A Case Report Roshanak Jazayeri, MD, PhD Assistant Professor of Medical Genetics Faculty of Medicine, Alborz University of Medical Sciences
Genetic diagnosis of limb girdle muscular dystrophy type 2A, A Case Report Roshanak Jazayeri, MD, PhD Assistant Professor of Medical Genetics Faculty of Medicine, Alborz University of Medical Sciences
Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy
 RESEARCH ARTICLE 4435 Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy Cecilia Östlund 1, Gisèle Bonne 2, Ketty Schwartz 2
RESEARCH ARTICLE 4435 Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigantype partial lipodystrophy Cecilia Östlund 1, Gisèle Bonne 2, Ketty Schwartz 2
Bio 111 Study Guide Chapter 17 From Gene to Protein
 Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
Rescue of heterochromatin organization in Hutchinson- Gilford progeria by drug treatment
 Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells
 JCB: ARTICLE Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells Jan Lammerding, 1 Janet Hsiao, 2 P. Christian Schulze, 1 Serguei Kozlov, 3 Colin L. Stewart, 3 and Richard
JCB: ARTICLE Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells Jan Lammerding, 1 Janet Hsiao, 2 P. Christian Schulze, 1 Serguei Kozlov, 3 Colin L. Stewart, 3 and Richard
variant led to a premature stop codon p.k316* which resulted in nonsense-mediated mrna decay. Although the exact function of the C19L1 is still
 157 Neurological disorders primarily affect and impair the functioning of the brain and/or neurological system. Structural, electrical or metabolic abnormalities in the brain or neurological system can
157 Neurological disorders primarily affect and impair the functioning of the brain and/or neurological system. Structural, electrical or metabolic abnormalities in the brain or neurological system can
Laminopathies: A consequence of mechanical stress or gene expression disturbances?
 BIOLOGY AND HEALTH Journal website: www.biologyandhealth.com Narrative review Laminopathies: A consequence of mechanical stress or gene expression disturbances? Alicia Jonker Alicia Jonker I6109457 Maastricht
BIOLOGY AND HEALTH Journal website: www.biologyandhealth.com Narrative review Laminopathies: A consequence of mechanical stress or gene expression disturbances? Alicia Jonker Alicia Jonker I6109457 Maastricht
Proteins that bind A-type lamins: integrating isolated clues
 Commentary 979 Proteins that bind A-type lamins: integrating isolated clues Michael S. Zastrow, Sylvia Vlcek and Katherine L. Wilson* Department of Cell Biology, Johns Hopkins University School of Medicine,
Commentary 979 Proteins that bind A-type lamins: integrating isolated clues Michael S. Zastrow, Sylvia Vlcek and Katherine L. Wilson* Department of Cell Biology, Johns Hopkins University School of Medicine,
A novel lamin A/C gene missense mutation (445 V > E) in immunoglobulin-like fold associated with left ventricular non-compaction
 Europace (2016) 18, 617 622 doi:10.1093/europace/euv044 CLINICAL RESEARCH Channelopathies A novel lamin A/C gene missense mutation (445 V > E) in immunoglobulin-like fold associated with left ventricular
Europace (2016) 18, 617 622 doi:10.1093/europace/euv044 CLINICAL RESEARCH Channelopathies A novel lamin A/C gene missense mutation (445 V > E) in immunoglobulin-like fold associated with left ventricular
Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin A
 available at www.sciencedirect.com www.elsevier.com/locate/yexcr Research Article Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin
available at www.sciencedirect.com www.elsevier.com/locate/yexcr Research Article Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin
RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria syndrome
 JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
JCS epress online publication date 25 January 2005 Research Article 689 RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin A: implications for Hutchinson-Gilford progeria
A guide to understanding variant classification
 White paper A guide to understanding variant classification In a diagnostic setting, variant classification forms the basis for clinical judgment, making proper classification of variants critical to your
White paper A guide to understanding variant classification In a diagnostic setting, variant classification forms the basis for clinical judgment, making proper classification of variants critical to your
Lipodystrophies represent a heterogeneous group
 Brief Genetics Report Lamin A/C Gene Sex-Determined Expression of Mutations in Dunnigan-Type Familial Partial Lipodystrophy and Absence of Coding Mutations in Congenital and Acquired Generalized Lipoatrophy
Brief Genetics Report Lamin A/C Gene Sex-Determined Expression of Mutations in Dunnigan-Type Familial Partial Lipodystrophy and Absence of Coding Mutations in Congenital and Acquired Generalized Lipoatrophy
Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy
 RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
RESEARCH ARTICLE 4447 Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery- Dreifuss muscular dystrophy Wahyu Hendrati Raharjo 1, Paul Enarson 1, Teresa Sullivan
Mandibuloacral dysplasia type A-associated progeria caused by homozygous LMNA mutation in a family from Southern China
 Luo et al. BMC Pediatrics 2014, 14:256 CASE REPORT Open Access Mandibuloacral dysplasia type A-associated progeria caused by homozygous LMNA mutation in a family from Southern China Di-Qing Luo 1*, Xiao-Zhu
Luo et al. BMC Pediatrics 2014, 14:256 CASE REPORT Open Access Mandibuloacral dysplasia type A-associated progeria caused by homozygous LMNA mutation in a family from Southern China Di-Qing Luo 1*, Xiao-Zhu
Pre-implantation genetic diagnosis (pgd) for heart disease determined by genetic factors
 Interventional Cardiology Pre-implantation genetic diagnosis (pgd) for heart disease determined by genetic factors The application of PGD has currently been extended to an increasing number of common disorders
Interventional Cardiology Pre-implantation genetic diagnosis (pgd) for heart disease determined by genetic factors The application of PGD has currently been extended to an increasing number of common disorders
Clinical Cell Biology Organelles in Health and Disease
 Department of Ophthalmology University of Kiel, University Medical Center Director: Prof. Dr. Johann Roider Clinical Cell Biology Organelles in Health and Disease Prof. Dr. Alexa Klettner Clinical cell
Department of Ophthalmology University of Kiel, University Medical Center Director: Prof. Dr. Johann Roider Clinical Cell Biology Organelles in Health and Disease Prof. Dr. Alexa Klettner Clinical cell
MRC-Holland MLPA. Description version 29; 31 July 2015
 SALSA MLPA probemix P081-C1/P082-C1 NF1 P081 Lot C1-0114. As compared to the previous B2 version (lot 0813 and 0912), 11 target probes are replaced or added, and 10 new reference probes are included. P082
SALSA MLPA probemix P081-C1/P082-C1 NF1 P081 Lot C1-0114. As compared to the previous B2 version (lot 0813 and 0912), 11 target probes are replaced or added, and 10 new reference probes are included. P082
Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity
 Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity Jemima Barrowman 1, Patricia A. Wiley 2, Sarah E. Hudon-Miller 2, Christine A. Hrycyna 2 and Susan Michaelis
Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity Jemima Barrowman 1, Patricia A. Wiley 2, Sarah E. Hudon-Miller 2, Christine A. Hrycyna 2 and Susan Michaelis
Hutchinson-Gilford Progeria Syndrome and its Relevance to Cardiovascular Diseases and Normal Aging
 382 Biomed Environ Sci, 2013; 26(5): 382-389 Review Hutchinson-Gilford Progeria Syndrome and its Relevance to Cardiovascular Diseases and Normal Aging QI Ying Chun and XIE Xiao Hua # First Department of
382 Biomed Environ Sci, 2013; 26(5): 382-389 Review Hutchinson-Gilford Progeria Syndrome and its Relevance to Cardiovascular Diseases and Normal Aging QI Ying Chun and XIE Xiao Hua # First Department of
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Accepted Preprint first posted on 14 June 2012 as Manuscript EJE
 Page 1 of 27 Accepted Preprint first posted on 14 June 2012 as Manuscript EJE-12-0268 1 Phenotypic diversity in patients with lipodystrophy associated with LMNA mutations Patricia B. Mory 1, Felipe Crispim
Page 1 of 27 Accepted Preprint first posted on 14 June 2012 as Manuscript EJE-12-0268 1 Phenotypic diversity in patients with lipodystrophy associated with LMNA mutations Patricia B. Mory 1, Felipe Crispim
CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE. Dr. Bahar Naghavi
 2 CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE Dr. Bahar Naghavi Assistant professor of Basic Science Department, Shahid Beheshti University of Medical Sciences, Tehran,Iran 3 Introduction Over 4000
2 CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE Dr. Bahar Naghavi Assistant professor of Basic Science Department, Shahid Beheshti University of Medical Sciences, Tehran,Iran 3 Introduction Over 4000
READ ORPHA.NET WEBSITE ABOUT BETA-SARCOGLYOCANOPATHY LIMB-GIRDLE MUSCULAR DYSTROPHIES
 READ ORPHA.NET WEBSITE ABOUT BETA-SARCOGLYOCANOPATHY LIMB-GIRDLE MUSCULAR DYSTROPHIES (LGMD) Limb-girdle muscular dystrophies (LGMD) are a heterogeneous group of genetically determined disorders with a
READ ORPHA.NET WEBSITE ABOUT BETA-SARCOGLYOCANOPATHY LIMB-GIRDLE MUSCULAR DYSTROPHIES (LGMD) Limb-girdle muscular dystrophies (LGMD) are a heterogeneous group of genetically determined disorders with a
Supplementary Figure 1
 Count Count Supplementary Figure 1 Coverage per amplicon for error-corrected sequencing experiments. Errorcorrected consensus sequence (ECCS) coverage was calculated for each of the 568 amplicons in the
Count Count Supplementary Figure 1 Coverage per amplicon for error-corrected sequencing experiments. Errorcorrected consensus sequence (ECCS) coverage was calculated for each of the 568 amplicons in the
Neuromuscular Disorders 13 (2003) Workshop report
 Neuromuscular Disorders 13 (2003) 508 515 Workshop report www.elsevier.com/locate/nmd 108th ENMC International Workshop, 3rd Workshop of the MYO-CLUSTER project: EUROMEN, 7th International Emery-Dreifuss
Neuromuscular Disorders 13 (2003) 508 515 Workshop report www.elsevier.com/locate/nmd 108th ENMC International Workshop, 3rd Workshop of the MYO-CLUSTER project: EUROMEN, 7th International Emery-Dreifuss
JULY 21, Genetics 101: SCN1A. Katie Angione, MS CGC Certified Genetic Counselor CHCO Neurology
 JULY 21, 2018 Genetics 101: SCN1A Katie Angione, MS CGC Certified Genetic Counselor CHCO Neurology Disclosures: I have no financial interests or relationships to disclose. Objectives 1. Review genetic
JULY 21, 2018 Genetics 101: SCN1A Katie Angione, MS CGC Certified Genetic Counselor CHCO Neurology Disclosures: I have no financial interests or relationships to disclose. Objectives 1. Review genetic
Three new cases of dilated cardiomyopathy caused by mutations in LMNA gene
 Acta Myologica 2017; XXXVI: p. 207-212 Three new cases of dilated cardiomyopathy caused by mutations in LMNA gene Larysa N. Sivitskaya 1, Nina G. Danilenko 1, Tatiyana G. Vaikhanskaya 2, Tatsiyana V. Kurushka
Acta Myologica 2017; XXXVI: p. 207-212 Three new cases of dilated cardiomyopathy caused by mutations in LMNA gene Larysa N. Sivitskaya 1, Nina G. Danilenko 1, Tatiyana G. Vaikhanskaya 2, Tatsiyana V. Kurushka
Title:A novel lamin A/C gene mutation causing spinal muscular atrophy-like phenotype with arrhythmia and cardiac hypofunction: report of one case
 Author's response to reviews Title:A novel lamin A/C gene mutation causing spinal muscular atrophy-like phenotype with arrhythmia and cardiac hypofunction: report of one case Authors: Naotoshi Iwahara
Author's response to reviews Title:A novel lamin A/C gene mutation causing spinal muscular atrophy-like phenotype with arrhythmia and cardiac hypofunction: report of one case Authors: Naotoshi Iwahara
Inauguraldissertation
 CHARACTERISATION OF THE MOLECULAR LINKS BETWEEN THE NUCLEAR PORE COMPLEX AND THE NUCLEAR LAMINS AND RECONSTITUTION OF THE XENOPUS OOCYTE LAMIN ASSEMBLY IN VITRO Inauguraldissertation zur Erlangung der
CHARACTERISATION OF THE MOLECULAR LINKS BETWEEN THE NUCLEAR PORE COMPLEX AND THE NUCLEAR LAMINS AND RECONSTITUTION OF THE XENOPUS OOCYTE LAMIN ASSEMBLY IN VITRO Inauguraldissertation zur Erlangung der
Proteins. Length of protein varies from thousands of amino acids to only a few insulin only 51 amino acids
 Proteins Protein carbon, hydrogen, oxygen, nitrogen and often sulphur Length of protein varies from thousands of amino acids to only a few insulin only 51 amino acids During protein synthesis, amino acids
Proteins Protein carbon, hydrogen, oxygen, nitrogen and often sulphur Length of protein varies from thousands of amino acids to only a few insulin only 51 amino acids During protein synthesis, amino acids
Mutations in the LMNA gene that encodes the nuclear. Effects of Mechanical Stress and Carvedilol in Lamin A/C Deficient Dilated Cardiomyopathy
 Effects of Mechanical Stress and Carvedilol in Lamin A/C Deficient Dilated Cardiomyopathy Suchitra Chandar,* Li Sze Yeo,* Christiana Leimena, Ju-Chiat Tan, Xiao-Hui Xiao, Vesna Nikolova-Krstevski, Yoshinori
Effects of Mechanical Stress and Carvedilol in Lamin A/C Deficient Dilated Cardiomyopathy Suchitra Chandar,* Li Sze Yeo,* Christiana Leimena, Ju-Chiat Tan, Xiao-Hui Xiao, Vesna Nikolova-Krstevski, Yoshinori
Supplementary Online Content
 Supplementary Online Content Ku CA, Hull S, Arno G, et al. Detailed clinical phenotype and molecular genetic findings in CLN3-associated isolated retinal degeneration. JAMA Ophthalmol. Published online
Supplementary Online Content Ku CA, Hull S, Arno G, et al. Detailed clinical phenotype and molecular genetic findings in CLN3-associated isolated retinal degeneration. JAMA Ophthalmol. Published online
Concurrent Practical Session ACMG Classification
 Variant Effect Prediction Training Course 6-8 November 2017 Prague, Czech Republic Concurrent Practical Session ACMG Classification Andreas Laner / Anna Benet-Pagès 1 Content 1. Background... 3 2. Aim
Variant Effect Prediction Training Course 6-8 November 2017 Prague, Czech Republic Concurrent Practical Session ACMG Classification Andreas Laner / Anna Benet-Pagès 1 Content 1. Background... 3 2. Aim
Single Gene (Monogenic) Disorders. Mendelian Inheritance: Definitions. Mendelian Inheritance: Definitions
 Single Gene (Monogenic) Disorders Mendelian Inheritance: Definitions A genetic locus is a specific position or location on a chromosome. Frequently, locus is used to refer to a specific gene. Alleles are
Single Gene (Monogenic) Disorders Mendelian Inheritance: Definitions A genetic locus is a specific position or location on a chromosome. Frequently, locus is used to refer to a specific gene. Alleles are
Downloaded on T20:37:32Z
 Title Author(s) Altered regulation of adipogenesis with respect to disease processes Davies, Stephanie Jane Publication date 2017 Original citation Type of publication Rights Davies, S. J. 2017. Altered
Title Author(s) Altered regulation of adipogenesis with respect to disease processes Davies, Stephanie Jane Publication date 2017 Original citation Type of publication Rights Davies, S. J. 2017. Altered
Identification of a novel duplication mutation in the VHL gene in a large Chinese family with Von Hippel-Lindau (VHL) syndrome
 Identification of a novel duplication mutation in the VHL gene in a large Chinese family with Von Hippel-Lindau (VHL) syndrome L.H. Cao 1, B.H. Kuang 2, C. Chen 1, C. Hu 2, Z. Sun 1, H. Chen 2, S.S. Wang
Identification of a novel duplication mutation in the VHL gene in a large Chinese family with Von Hippel-Lindau (VHL) syndrome L.H. Cao 1, B.H. Kuang 2, C. Chen 1, C. Hu 2, Z. Sun 1, H. Chen 2, S.S. Wang
Progeria, the nucleolus and farnesyltransferase inhibitors
 Nuclear Envelope Disease and Chromatin Organization 2009 287 Progeria, the nucleolus and farnesyltransferase inhibitors Ishita S. Mehta*, Joanna M. Bridger* and Ian R. Kill 1 *Laboratory of Nuclear and
Nuclear Envelope Disease and Chromatin Organization 2009 287 Progeria, the nucleolus and farnesyltransferase inhibitors Ishita S. Mehta*, Joanna M. Bridger* and Ian R. Kill 1 *Laboratory of Nuclear and
Homozygous and Compound Heterozygous Mutations in ZMPSTE24 Cause the Laminopathy Restrictive Dermopathy
 See related Commentary on page xii Homozygous and Compound Heterozygous Mutations in ZMPSTE24 Cause the Laminopathy Restrictive Dermopathy Casey L. Moulson, Gloriosa Go, Jennifer M. Gardner,w Allard C.
See related Commentary on page xii Homozygous and Compound Heterozygous Mutations in ZMPSTE24 Cause the Laminopathy Restrictive Dermopathy Casey L. Moulson, Gloriosa Go, Jennifer M. Gardner,w Allard C.
MRC-Holland MLPA. Description version 30; 06 June 2017
 SALSA MLPA probemix P081-C1/P082-C1 NF1 P081 Lot C1-0517, C1-0114. As compared to the previous B2 version (lot B2-0813, B2-0912), 11 target probes are replaced or added, and 10 new reference probes are
SALSA MLPA probemix P081-C1/P082-C1 NF1 P081 Lot C1-0517, C1-0114. As compared to the previous B2 version (lot B2-0813, B2-0912), 11 target probes are replaced or added, and 10 new reference probes are
SALSA MLPA KIT P060-B2 SMA
 SALSA MLPA KIT P6-B2 SMA Lot 111, 511: As compared to the previous version B1 (lot 11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). Please note that, in contrast to the
SALSA MLPA KIT P6-B2 SMA Lot 111, 511: As compared to the previous version B1 (lot 11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). Please note that, in contrast to the
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Cardiac follow-up in patients with hereditary muscular diseases
 Cardiac follow-up in patients with hereditary muscular diseases Tromsø 7. september 2018 Eystein Theodor Ek Skjølsvik, MD Professor Kristina H. Haugaa, MD, PhD Unit for genetic cardiac diseases, OUS Rikshospitalet
Cardiac follow-up in patients with hereditary muscular diseases Tromsø 7. september 2018 Eystein Theodor Ek Skjølsvik, MD Professor Kristina H. Haugaa, MD, PhD Unit for genetic cardiac diseases, OUS Rikshospitalet
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome
 Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
International Journal of Pharma and Bio Sciences
 International Journal of Pharma and Bio Sciences HUTCHINSON-GILFORD SYNDROME (PROGERIA): A REVIEW SACHIN KUMAR 1 *, AMIT KUMAR 2, MOHIT SINGLA 1 AND ABHISHEK SINGH 3 1. K.N.G.D Modi Institute of Pharmaceutical
International Journal of Pharma and Bio Sciences HUTCHINSON-GILFORD SYNDROME (PROGERIA): A REVIEW SACHIN KUMAR 1 *, AMIT KUMAR 2, MOHIT SINGLA 1 AND ABHISHEK SINGH 3 1. K.N.G.D Modi Institute of Pharmaceutical
