Keywords: BAF, BANF1, prelamin A, lamin A/C, laminopathies, emerin, EDMD1. Introduction
|
|
|
- Candice Deirdre Gallagher
- 5 years ago
- Views:
Transcription
1 Report Cell Cycle 11:19, ; October 1, 2012; 2012 Landes Bioscience Familial partial lipodystrophy, mandibuloacral dysplasia and restrictive dermopathy feature barrier-to-autointegration factor (BAF) nuclear redistribution Cristina Capanni, 1, * Stefano Squarzoni, 1 Vittoria Cenni, 1 Maria Rosaria D Apice, 2 Alessandra Gambineri, 3 Giuseppe Novelli, 4 Manfred Wehnert, 5 Renato Pasquali, 3 Nadir M. Maraldi 1,6 and Giovanna Lattanzi 1 1 CNR-National Research Council of Italy; Institute of Molecular Genetics; Unit of Bologna-IOR; Bologna, Italy; 2 Laboratory of Medical Genetics; University of Rome Tor Vergata; Rome, Italy; 3 Division of Endocrinology; Department of Clinical Medicine; S. Orsola-Malpighi Hospital; University of Bologna; Bologna, Italy; 4 National Agency for the Evaluation of Universities and Research (ANVUR) and San Pietro Fatebenefratelli Hospital; Rome, Italy; 5 Institute of Human Genetics; University of Greifswald; Greifswald, Germany; 6 Laboratory of Musculoskeletal Cell Biology; IOR; Bologna, Italy Keywords: BAF, BANF1, prelamin A, lamin A/C, laminopathies, emerin, EDMD1 Prelamin A processing impairment is a common feature of a restricted group of rare genetic alterations/disorders associated with a wide range of clinical phenotypes. Changes in histone posttranslational modifications, alterations in non-histone chromatin proteins and chromatin disorganization have been specifically linked to impairment of specific, distinct prelamin A processing steps, but the molecular mechanism involved in these processes is not yet understood. In this study, we show that the accumulation of wild-type prelamin A detected in restrictive dermopathy (RD), as well as the accumulation of mutated forms of prelamin A identified in familial partial lipodystrophy (FPLD) and mandibuloacral dysplasia (MADA), affect the nuclear localization of barrier-to-autointegration factor (BAF), a protein able to link lamin A precursor to chromatin remodeling functions. Our findings, in accordance with previously described results, support the hypothesis of a prelamin A involvement in BAF nuclear recruitment and suggest BAF-prelamin A complex as a protein platform usually activated in prelamin A-accumulating diseases. Finally, we demonstrate the involvement of the inner nuclear membrane protein emerin in the proper localization of BAF-prelamin A complex. Introduction The ability to store and translate instructions from the genetic code is essential to maintain life in all cells. Since the human genetic code is composed of over 3 billion nucleotides, tight packaging and an accurate spatial organization is necessary in order to fit it within each micron-sized nuclei. Packaging of the human genome includes folding the DNA into chromatin fibers, chromosome domains and ultimately chromosomes. This higher-order organization is known to contribute to gene regulation, and, therefore, it is not surprising that defects in chromatin and chromosome organization cause different diseases, including cancer and aging. 1 Recently, chromatin alterations and epigenetic changes have been identified as common features in a group of rare genetic disorders (laminopathies) due to mutations in LMNA gene, a DNA sequence codifying for two of major nuclear lamina components: lamin A and lamin C. 2 In accordance with the nuclear lamina, proposed functions including supporting transcription, replication, genome organization, development and DNA repair; the nuclear lamina defects cause a wide range of clinical phenotypes that can be associated to a specific tissue failure (muscular dystrophy, lipodystrophy, cerebellar disorders) or to multisystem disorders (mandibuloacral dysplasia, premature aging). 3 Among laminopathies themselves, chromatin and nuclear structure alterations appear to be more severe in the prelamin A-accumulating forms of these diseases. 4 Prelamin A is a protein precursor of lamina A, which is posttranslationally modified at its C terminal region, where the CaaX motif triggers a sequence of modifications, including farnesylation, carboxymethylation and proteolytic cleavage by ZMPSTE 24 metalloproteinase. 5,6 The prelamin A maturation pathway has been found altered in Hutchinson-Gilford progeria syndrome (HGPS), 7,8 Mandibuloacral dysplasia (MAD), 9 Dunnigan-type familial partial lipodystrophy (FPLD), atypical Werner syndrome (WS) 10 and restrictive dermopathy (RD). 11 These diseases are characterized by loss of the key architectural chromatin protein HP1 and alterations in the amount of heterochromatin-associated histones H3K9me3 and H3K27me3. 4 Interestingly, similar effects have been obtained in human control fibroblasts induced to accumulate different prelamin A forms through the exogenous expression *Correspondence to: Cristina Capanni; ccapanni@area.bo.cnr.it Submitted: 04/30/12; Revised: 08/11/12; Accepted: 08/16/ Cell Cycle Volume 11 Issue 19
2 Report Report of uncleavable prelamin A mutant constructs or by treatments with prelamin A interfering drugs. 12,13 How can prelamin A affect chromatin organization is unclear, but we recently described its interaction with barrier-to-autointegration factor (BAF), a DNA-binding protein involved in both chromatin dynamics and epigenetic modifications. 14 BAF is a soluble protein localized in both cytoplasm and nucleus, where it interacts with LEM-domain proteins (LAP2, emerin, MAN1, Lem2/NET25) and affects chromatin silencing. BAF can condense DNA in vitro; 15 it influences higher-order chromatin structure and represses transcription at specific promoters. 16,17 In addition, the previously described BAF interactions with histone H3 and retinoblastoma binding protein 4 (RBBP4) suggest its association with nucleosomes and nucleosome remodeling mechanisms. 18 Interestingly, BAF has been identified as a protein responsible for a progeroid syndrome featuring dysmorphic cell nuclei, atrophic skin, generalized lipoatrophy, severe osteoporosis and marked osteolysis features that overlap with those described in prelamin A-accumulating diseases. 19,20 Since experimentally accumulated prelamin A varieties, as well as progerin (a mutated prelamin A form lacking 50 aa), accumulated in HGPS cells, affect BAF distribution, 21 we wondered if a similar event occurs in FPLD, MADA and RD cells which are known to accumulate point-mutated or wild-type prelamin A forms, respectively. In this study, we show that in human skin fibroblasts from RD, MADA and FPLD patients, BAF is prevalently located in the nucleus, where it colocalizes with accumulated prelamin A. Moreover, we demonstrate that LMNA gene mutations occurring in MADA and FPLD cells do not interfere with in vivo BAF-prelamin A interaction. Finally we show that emerin, a BAF and prelamin A binding partners, is involved in BAF-prelamin A complex localization in the nucleus. Our observations strongly suggest that BAF-prelamin A complex could be considered as a chromatin-regulator element involved in the pathophysiological mechanism of prelamin A-accumulating diseases. Results Barrier-to-autointegration factor localization and expression in FPLD, MADA and RD cells. BAF cellular localization was evaluated in control and laminopathic cells. Since FPLD and MADA cells were obtained from adult patients (aged 20 to 30 y) and RD cells were obtained from neonatal patients, we used two different age matched groups of healthy donors referred to in the Figure 1 as control 1 (cont.1, adult healthy donor) and control 2 (cont.2, neonatal healthy donor). BAF immunofluorescence evaluation performed in control cells bearing undetectable levels of prelamin A showed BAF ubiquitously distributed between cytoplasm and nucleus in 80% of the cells, while in less than 15% of the cells, BAF were prevalently located in the nucleus (Fig. 1A and C). In FPLD, MADA and RD cells, the distribution of BAF changed, all prelamin A-positive nuclei showing BAF nuclear recruitment (Fig. 1A). Prelamin A accumulation at the nuclear rim or at the intranuclear aggregates was observed in 45% of FPLD cells. 22 In these cells, BAF was predominantly located in the nucleus, where it was distributed in the nuclear lamina and nucleoplasm as well as at prelamin A-positive structures (Fig. 1A and B). Similar results were obtained in two different MADA cell lines in which a previously described R527H LMNA mutation 9 leads to prelamin A accumulation in 55% of the cells (Fig. 1C); this was detected at the rim of normally shaped nuclei or, alternatively, as intranuclear aggregates (Fig. 1A). BAF nuclear localization was observed in the MADA prelamin A-positive cells, where it perfectly colocalized with prelamin A-labeled structures (Fig. 1A and B). Finally, prelamin A and BAF localization were evaluated in two RD cell lines bearing the same ZMPSTE24 gene mutation (c.1085_1086inst) (Fig. 1A), which causes complete elimination of the last proteolytic cleavage step of prelamin A processing and accumulation of different non-mutated prelamin A intermediates. 23 Using an anti-prelamin A antibody, we detected prelamin A in the nucleus (Fig. 1A and C). BAF nuclear labeling was observed in 90% of RD cells (Fig. 1A and C), where it localized in the nucleoplasm of normally shaped nuclei or colocalized with prelamin A at the nuclear lamina of dysmorphic nuclei (Fig. 1A and B, asterisk). These findings agree with our previous results reporting different BAF nuclear distribution following experimental accumulation of full-length or farnesylated-carboxymethylated prelamin A. 21 Western blotting analysis confirmed that the total amount of BAF was not altered (Fig. 1D) by the high levels of prelamin A, which is accumulated and clearly detectable in FPLD, MADA and RD cells. Different prelamin A mutated forms interact with BAF and affect its cellular distribution. The above described results were confirmed in HEK293 cells expressing processable prelamin A (LA-WT), laminopathic, uncleaved prelamin A (LA-L647R) or laminopathic processable prelamin A mutants (LA-R482Q and LA-R527H) (Fig. 2). FLAG-tagged prelamin A forms were efficiently expressed in HEK293 cells, and the nuclear distribution of each pathological mutant resembled that observed respectively in human fibroblasts from laminopathic patients. Moreover, in accordance with our previously described results, 21,24 we observed that all FLAG-tagged prelamin A constructs (including LA-WT) were able to induce accumulation of prelamin A (Fig. S1). FLAG immunological detection showed that LA-WT and uncleaved prelamin A localized at the nuclear lamina and, in addition, nucleoplasmic distribution of LA-L647R was also detected. 21 LA-R527H localized both at the nuclear lamina and at the intranuclear foci (Fig. 2, arrowhead), while LA-R482Q was observed over the aggregates located at the nuclear lamina (Fig. 2, arrow). BAF nuclear staining was observed in all transfected cells where it colocalized with FLAG-tagged prelamin A forms (Fig. 2A); on the contrary, a prevalent cytoplasmic BAF localization was observed in untransfected cells (Fig. 2A, asterisk). Western blotting analysis confirmed the expression of FLAG-tagged proteins and showed the same BAF protein amount in untransfected and transfected cells (Fig. 2B). In order to evaluate BAF nuclear translocation in response to prelamin A accumulation, HEK293 cells were transfected with GFP-BAF construct alone or in combination with LA-WT or each prelamin A mutant (LA-R527H, LA-R482Q, Cell Cycle 3569
3 Figure 1. Barrier-to-autointegration factor nuclear localization in prelamin A-accumulating diseases. (A) BAF and prelamin A distribution in control (cont.1 adult healthy donor, cont.2 neonatal healthy donor), familial partial lipodystrophy (FPLD, LMNA-R482Q), mandibuloacral dysplasia (MADA, LMNA-R527H) and restrictive dermopathy (RD, ZMPSTE24 c.1085_1086inst). Control and pathological cells at early passage (passage 5 10) were used for each experiment. BAF was evaluated on methanol-fixed cells by a rabbit-polyclonal anti-baf antibody visualized by FITC-conjugated secondary antibody (green). Prelamin A was evaluated using a goat-polyclonal anti-prelamin A antibody visualized by TRITC-conjugated secondary antibody (red). In merge, arrow, arrowhead and asterisk indicate double-stained nuclei. DNA was detected using DAPI. Bar, 10 μm. (B) Higher magnification of prelamin A-BAF-positive nuclei indicated by arrow, arrowhead and asterisk in (A). Prelamin A (red), BAF (green) merge (merge) and merge plus DAPI staining (merge + DAPI) are shown. (C) The percentage of nuclei showing prelamin A staining is reported in the graph as means ± SD of three different counts (100 nuclei per count). Examined samples are control fibroblast from adult healthy donors (cont.1), familial partial lipodystrophy fibroblasts (FPLD), mandibuloacral dysplasia fibroblasts (MADA), control fibroblast from neonate healthy donors (cont.2) and restrictive dermopathy fibroblasts (RD). Asterisks indicate statistically significant differences at the Student s t-test (p < 0.05), with respect to control cells values. (D) Western blotting evaluation of BAF protein levels in control (cont.1 and cont.2), FPLD, MADA and RD cells. Eighty micrograms of total cell lysates were subjected to western blot detection of prelamin A (prelamin A), lamin A/C (lamin A/C), BAF (BAF) and actin (actin). Immunolabeled bands visualized by ECL chemiluminescence are shown. LA-L647R). Total extracts, cytoplasmic fractions and isolated nuclei from single and cotransfected cells were subjected to FLAG and GFP western blotting evaluation. Immunolabeled bands showed that nuclear GFP-BAF level was definitely higher in GFP- BAF/FLAG-prelamin A-expressing cells than in GFP-BAF single transfected cells (Fig. 3). GFP-BAF bands staining was decreased in cytosolic fractions from cotransfected cells (Fig. 3). Nuclear fraction purity and equal protein loading was demonstrated by the exclusive detection of caveolin 1 in whole lysates and cytosolic fractions. On the contrary, lamin B1 staining was used as a nuclear fractions marker and nuclear loading control (Fig. 3). Since BAF nuclear localization in response to prelamin A (WT or mutated forms) accumulation suggested a possible reciprocal protein interaction, 21 a coimmunoprecipitation study was performed (Fig. 4). Total lysates from HEK293 cells expressing GFP- BAF in combination with FLAG-tagged prelamin A constructs (LA-WT, LA-R527H, LA-R482Q, LA-L647R) were subjected to anti-flag immunoprecipitation. Western blotting detection showed FLAG and GFP immunolabeled bands in both total cellular extracts and in FLAG-IP samples (Fig. 4A) while no immunolabeled bands were observed in protein A/G lanes. In the same transfected cells, the interaction of prelamin A mutated or WT 3570 Cell Cycle Volume 11 Issue 19
4 Figure 2. Barrier-to-autointegration factor localization and expression in prelamin A mutants HEK293 transfected cells. (A) BAF localization in HEK293 cells expressing prelamin A mutants. Cells were transfected with wild-type processable prelamin A (LA-WT), MADA processable prelamin A mutant (LA-R527H), FPLD processable prelamin A mutant (LA-R482Q) or uncleavable farnesylated-carboxymethylated prelamin A mutant (LA-L647R). Immunofluorescence detection of overexpressed proteins was performed using a mouse monoclonal anti FLAG-Cy3 conjugated antibody (red). Arrowhead and arrow indicate LA-R527H and LA-R482Q distribution, respectively. Endogenous BAF localization was evaluated by a rabbit polyclonal anti-baf antibody visualized by FITC-conjugated secondary antibody (green). Asterisk indicates BAF distribution in untransfected cells. Nuclei were stained with DAPI. Bar, 10 μm. (B) BAF protein levels in prelamin A mutants expressing cells. BAF protein levels were determined in HEK293 cells expressing FLAG-tagged prelamin A constructs (LA-WT, LA-R527H, LA-R482Q, LA-L647R) as well as in untransfected cells (unt.). Thirty micrograms of total cell lysates were separated by SDS-PAGE (5 20%) and subjected to western blot. FLAG-tagged protein and endogenous BAF detection was performed using a mouse-monoclonal and a rabbit-polyclonal, respectively. Actin (Actin) was detected as a protein-loading control. forms with BAF was also evaluated. GFP-coimmunoprecipitation experiment was performed, and the obtained protein complexes were subject to GFP, FLAG, prelamin A and lamin A western blotting detection (Fig. 4B). GFP, FLAG, prelamin A and lamin A immunolabeled bands were observed in all total lysates as well as in all GFP-IP samples (Fig. 4B), demonstrating that LMNA gene mutations occurring in MADA and FPLD cells do not interfere, per se, with BAF-prelamin A interaction. In addition, comparison of 70 kd lamin A bands with the 74 kd prelamin A bands suggested that BAF interacts preferentially with prelamin A rather than with mature lamin A. Emerin affects BAF-prelamin A complex localization. Finally, we wondered if the BAF and prelamin A-binding protein emerin, involved in the proper localization of lamin A precursor, 24,25 could affect BAF nuclear localization during prelamin A accumulation. Thus, we evaluated prelamin A and BAF localization in mevinolin-treated skin fibroblasts obtained from control and emerin-null patients affected by Emery Dreifuss Muscular Dystrophy type 1 (EDMD1) 26 (Fig. 5). Mevinolin is a prelamin A-interfering drug able to induce the accumulation of non-farnesylated prelamin A through the inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the enzyme responsible for the farnesyl-moiety biosynthesis. After 18 h of pharmacological treatment, BAF and prelamin A were evaluated. In control and EDMD1 untreated cells, prelamin A was undetectable and BAF localized normally throughout the cell compartments (Fig. 5A and B). After mevinolin treatment, both control and EDMD1 cells showed non-farnesylated prelamin A accumulation and BAF nuclear recruitment (Fig. 5B): control cells showed non-farnesylated prelamin A accumulation at intranuclear aggregates, which colocalized with BAF fluorescence (Fig. 5A, arrow and square), while EDMD1 cells showed an altered nuclear distribution of non-farnesylated prelamin A and, unexpectedly, of BAF (Fig. 5A). In accordance with our previously described results, we observed that non-farnesylated prelamin A aggregates were retained at the nuclear periphery in emerin-null cells 24 (Fig. 5A, square). Interestingly, BAF colocalized with these prelamin A-containing delocalized structures (Fig. 5A, arrowhead), suggesting that emerin does not affect BAF cellular distribution or its prelamin A-dependent nuclear translocation, but it could be necessary for the proper localization of the whole prelamin A-BAF protein complex inside the nucleus. Western blotting analysis confirmed a quantitatively comparable BAF protein amount in all samples (Fig. 5C). In order to confirm Cell Cycle 3571
5 these results, we evaluated whether the restoration of emerin expression in EDMD1 cells was able to induce the proper localization of BAF. In this regard, GFP-emerin fusion protein was expressed in emerin-null EDMD1 fibroblasts, and prelamin A was accumulated by mevinolin treatment. We observed that emerin expression in EDMD1 did not affect prelamin A processing or BAF localization (Fig. 6A). On the contrary, mevinolin treatment on GFP-emerin transfected cells showed that the restoring of emerin expression was able to recover prelamin A and BAF nuclear aggregates distribution (Fig. 6B, arrow). Discussion Nuclear structure alteration and chromatin disorganization are considered common features of prelamin A accumulating diseases, whose mechanism and precise molecular processes remain largely unknown. Expanding on our previous work that demonstrated in vivo BAF-prelamin A interaction, this study provides further insights into a possible BAF-prelamin A interaction also in all known diseases involving an accumulation of lamin A precursor. In particular, we demonstrate that prelamin A pathogenic forms identified in FPLD, MADA and RD cells interact with BAF and affect its intracellular distribution. As previously described in HGPS cells, we observe an evident BAF nuclear localization and its colocalization with prelamin A in FPLD, MADA and RD nuclei. Finally, a possible implication of the inner nuclear membrane protein emerin in the proper localization of the BAF-prelamin A complex is proposed. Since BAF has been recently identified as a protein that interacts with mononucleosomes and is also able to affect the epigenetic organization of chromatin, 14 the prelamin A interaction with this DNA-binding protein we describe here may suggest through what kind of molecular platforms the known lamin A precursors could affect chromatin organization. Indeed, nuclear structure alteration and chromatin disorganization are considered common features of different prelamin A-accumulating diseases. 27 In FPLD cells, where farnesylated lamin A precursor is accumulated, nuclei show an altered heterochromatin arrangement with areas characterized by heterochromatin loss. 10 In MADA, nuclei heterochromatin domains are disorganized or completely lost, in accordance with the delocalization of both heterochromatin-associated protein (HP1β) and histone H3 methylated at lysine 9 (Tri-H3K9) and with a lamin B receptor increased solubility. 9 In RD cells, in which the impairment of ZMPSTE24 function profoundly affects the prelamin A maturation pathway at different steps, preventing the production of mature lamina A, heterochromatin is lost, and a generalized nuclear enlargement is detected. Interestingly, the severity of such alterations has been recently linked to the combination of different prelamin A forms accumulated in the nucleus. 23 Thus, in RD normally shaped nuclei, where both unprocessed prelamin A and farnesylated prelamin A are detected, an evident clustering of tri-h3k9 and tri-h4k20 is observed. On the contrary, in highly dysmorphic RD nuclei, where a combination of Figure 3. Barrier-to-autointegration factor nuclear translocation in HEK293 cells transfected with prelamin A mutants. Western blotting analysis of whole cellular lysates, cytosol and isolated nuclei from HEK293 cells transfected with GFP-BAF (GFP-BAF), alone or in combination with: wild-type processable prelamin A (LA-WT + GFP-BAF), MADA processable prelamin A mutant (LA-R527H + GFP-BAF), FPLD processable prelamin A mutant (LA-R482Q+GFP- BAF) or uncleavable prelamin A mutant (LA-L647R+GFP-BAF). Immunolabeled bands of FLAG, GFP, lamin B1 and caveolin 1 are shown. different prelamin A forms (unprocessed and uncleaved-prelamin A) is detected, a disorganization of tri-h4k20 and a decrease of tri-h3k9 are observed. 23 In this regard, a BAF direct interaction with histones H3 and H4 has been recently demonstrated, and also it has been described how posttranslational modifications of such nucleosome components may change in BAF-overexpressing cells. 14 Thus, it is conceivable that BAF nuclear recruitment observed in prelamin A-accumulating diseases could resemble, in some aspects, a BAF-overexpression condition. In accordance with these data, a significant decrease in H4K16 acetylation has been recently described in BAF-overexpressing HEK293 cells and in Zmpste24 / mouse embryonic fibroblasts. 14,28 The acetylation of H4 plays an important role in several processes such as transcriptional activation, chromatin architecture maintenance and DNA repair. 29 Interestingly, it has been demonstrated that prelamin A accumulation inhibits H4 acetylation by interfering with the nuclear retention of histone acetyltransferase Mof. 28 In addition, BAF nuclear accumulation and its direct interaction with histones could prevent epigenetic changes by interfering with the histone-modifying enzymes that bind to the nucleosomes. 14 Finally, it must be considered that the chromatin disorganization observed in prelamin A-accumulating disease could also be due to an inhibition of specific BAF physiological interactions caused by its actual prelamin A binding. This hypothesis is enforced by the decreased level of retinoblastoma binding protein 4 (RBBP4) observed in HGPS cells. 30 RBBP4 is a BAF-binding protein with a role in chromatin organization whose downregulation in HGPS cells may appear in contrast with BAF nuclear recruitment in progerin-accumulating 3572 Cell Cycle Volume 11 Issue 19
6 Figure 4. Barrier-to-autointegration factor interaction with laminopathic forms of prelamin A. HEK293 cells were transfected with FLAGtagged prelamin A constructs (LA-WT, LA-R527H, LA-R482Q, LA-L647R) in combination with GFP-BAF (GFP-BAF) construct. Cotransfected cells were lysed and subjected to coimmunoprecipitation experiments. Total lysates (whole lysate) and immunoprecipitated proteins, FLAG-IP in panel (A) and GFP-IP in panel (B), were subjected to western blotting analysis. FLAG and GFP immunolabeled bands were observed in whole lysates as well as in immunoprecipitated samples, while no protein staining was observed in coimmunoprecipitation samples obtained in absence of anti-flag antibody or anti GFP- antibody (A/G) (A and B). In (B), prelamin A staining was observed in whole lysates and in GFP-IP lanes subjected to specific anti-prelamin A detection (prelamin A) and to anti-lamin A detection performed with Abcam antibody, which detects both mature lamin A (70kDa) and prelamin A (74kDa) (prelamin A, lamin A). cells. 21 Nevertheless, its downregulation is consistent with our hypothesis, which suggests that the persistence of progerin, as well as of other prelamin A forms in a BAF containing complex, could compete with, reduce or eventually abolish BAF interactions with other binding partners. Thus, prelamin A interaction with BAF could block specific BAF protein domains necessary for interaction with DNA remodeling enzymes, like the recently identified endonuclease Ankle1, or with inner nuclear membrane proteins (lem3 in C. elegans) involved in the BAF-mediate DNA damage response. 31,32 In accordance, prelamin A-accumulating diseases are characterized by DNA damage increase. 33,34 Among the described BAF-binding partners, some proteins have been recently identified that not only may help to understand prelamin A chromatin remodeling effects, but may also suggest how prelamin A accumulation causes tissue-specific symptoms. 14 In this regard, an in vivo BAF interaction has been detected with the histone methyltransferase G9a, a chromatin remodeling enzyme involved in the transition of preadipocytes in adipocytes. 14,35 Lipodystrophy is a common phenotypic sign observed in FPLD, MADA and HGPS patients, and we previously demonstrated that prelamin A accumulation interferes with the adipogenic program affecting SREBP1 nuclear translocation. 10 The identification of G9a methyltransferase as a BAFbinding protein suggests an alternative pathological mechanism that could be involved in prelamin A-mediated lipodystrophy. 14 It has been demonstrated that G9a methyltransferase inhibits adipogenic differentiation silencing PPAR-gamma gene expression. In preadipocytes, G9a activity increases the methylation status of the PPAR-gamma gene promoter, reducing its accessibility at transcription machinery. 35 Thus, it is conceivable that BAF-G9a interaction could interfere with the dissociation of the repressor-complexes at the PPAR-gamma regulatory site, leading to the suppression of the late-stage adipogenesis. In accordance, in prelamin A-accumulating mouse preadipocytes induced to differentiate PPAR-gamma, expression is impaired and the adipogenic differentiation can t occur. 10 In MADA and HGPS patients, the accumulation of prelamin A causes premature aging. It has been proposed that premature aging may be a consequence of premature stem cell exhaustion. 36 Mutations in A-type lamins could perturb the balance between proliferation and differentiation in adult stem cells, leading to less efficient tissue regeneration 37,38 and differentiation. 39 Interestingly, BAF interacts with, and seems to regulate the function of, Sox2, a transcription factor involved in maintaining the pluripotency in embryonic stem cells. 14 In accordance, BAF depletion decreases cell survival and cloning efficiency in mouse and human stem cells and promotes the differentiation of both cellular types. We propose that the accumulation of prelamin A could interfere with BAF-mediated gene activation/inactivation function by affecting BAF exchange between cytosol and nucleus. 40 Since BAF downregulation increased the expression of specific cell lineage genes, the forced BAF nuclear retention could abolish BAF nuclear quantitative changes necessary for chromatin remodeling events, which, in turn, should actuate gene expression. 14 Interestingly, a new progeroid syndrome has been recently identified, which is due to a point mutation in BAF encoding gene (BANF1). 19 Néstor-Guillermo progeria syndrome (NGPS) is a chronic progeria syndrome with early onset and slow clinical course. As observed in HGPS, NGPS patients are characterized by aged appearance, growth retardation, decreased subcutaneous fat, thin limbs and stiff joints, but they have no signs of cardiovascular impairment, diabetes mellitus or hypertriglyceridemia in early adulthood. 41 In addition, NGPS shows a severe osteolysis and osteoporosis. At the cellular level, the BAF p.ala12thr mutation reduces BAF protein amount, probably affecting the protein stability and induces nuclear abnormalities. 19 Interestingly, NGPS and HGPS cells show similar nuclear morphological defects, suggesting that BAF downregulation, as well as progerin interaction, affects nuclear structure in the same way. Thus, it is conceivable that BAF interaction with progerin could abolish BAF binding with the nuclear envelope and/or the nuclear lamina proteins involved in BAF structural functions. 14,19 Since prelamin A expression is linked to chromatin remodeling, it is not surprising that this protein precursor is accumulated Cell Cycle 3573
7 Figure 5. Localization and expression of barrier-to-autointegration factor and prelamin A in EDMD1 cells. (A) BAF and prelamin A were localized in control cells (control) treated with 10 μm mevinolin (+mevinolin) and in Emery Dreifuss Muscular Dystrophy type 1 (EDMD1) cells untreated (untreated) or treated (+mevinolin) with 10 μm mevinolin. After pharmacological treatment, BAF (green) and prelamin A staining (red) were performed. BAF and prelamin A were detected using a rabbit-polyclonal antibody and a goat-polyclonal antibody, respectively. Prelamin A and BAF colocalization is indicated by arrow and arrowhead in merge (merge). Squares indicate BAF/prelamin A aggregates localization. In the lower panels, BAF/prelamin A aggregates localization is reported at higher magnification, omitting DAPI staining. DNA was stained by DAPI. Bar, 10 μm. (B) Proportions of cell populations with different BAF localization: Nuc > Cyto, Nuc = Cyto and Nuc < Cyto represent cells with prevailing nuclear staining, uniform or mostly cytoplasmic staining, respectively. The proportion of cell populations was determined in control and EDMD1 cells at the same population doubling untreated (untreated) or mevinolin-treated cells (mevinolin). Data are means ± s.d. of at least 100 cells for each of three independent experiments. Asterisks indicate statistically significant differences at the Student s t-test (p < 0.05), with respect to control and EDMD1 mevinolin untreated cells values. (C) Western blotting evaluation of BAF in prelamin A EDMD1-accumulating cells. Western blotting evaluation of prelamin A, emerin, BAF and actin were performed in total lysates from control cells (control) or Emery Dreifuss Muscular Dystrophy type 1 cells (EDMD1) untreated (unt.) or treated with mevinolin (+mev.). Immunolabeled bands are shown. during skeletal muscle differentiation, which is characterized by a large reorganization of chromatin packaging. 42 We demonstrated that prelamin A governs nuclear positioning in human skeletal muscle cells, and that LMNA gene mutations causing EDMD2 reduce prelamin A protein level and induce nuclear clustering. 43 Interestingly, LMNA gene mutations leading to EDMD2 can impair the lamin A/C-emerin interaction, which has a role in prelamin A localization as underscored by prelamin A delocalization observed in EDMD1 cells. 24 Since prelamin A, emerin and BAF may be considered as components 3574 Cell Cycle Volume 11 Issue 19
8 Figure 6. Emerin exogenous expression rescues BAF intranuclear distribution in EDMD1 cells accumulating prelamin A. Prelamin A and BAF evaluation in EDMD1 GFP-emerin transfected cells with or without mevinolin treatment. In panel A, untreated EDMD1 GFP-emerin transfected cells (green) were subjected to prelamin A (prelamin A) and BAF (BAF) detection, both protein were visualized by TRITC-conjugated secondary antibody (red). In merge (merge+dapi) DNA was detected using DAPI. Bar, 10 μm. In panel B, mevinolin-treated EDMD1 GFP-emerin transfected cells (green). Prelamin A (prelamin A) and BAF (BAF) localization are shown. Arrow indicates recover of intranuclear BAF distribution in GFP-emerin transfected cells. Arrowhead indicates the altered intranuclear BAF distribution in the absence of emerin. of the same protein complexes, our finding on BAF-prelamin A delocalization in emerin-null cells is not surprising and suggests the involvement of this molecular platform in other muscular laminopathies. In particular, the impairment of BAF-prelamin A complex functions could be influenced by disruption of the known structural link between nucleus and cytoplasm (LINC complex). 44 In fact, mutations in LINC complex proteins nesprin 1 and nesprin 2 affect lamin A/C and emerin-localization and cause EDMD4. 45 Unfortunately, in order to better define prelamin A-BAF complex role in muscular dystrophy pathogenesis, important elements are still lacking. In particular, BAF expression, localization and protein interactions during skeletal muscle differentiation have never been described. In conclusion, in this study we demonstrate that prelamin A accumulated in FPLD, MADA and RD cells interacts with BAF and affects its cellular distribution. This finding is in accordance with the rescue of BAF cellular distribution following lowering of progerin amount in HGPS cells treated with rapamycin. 46 Also, our results strongly suggest prelamin A as a BAF-binding protein is able to recruit BAF in the nucleus. Moreover, our findings suggest that prelamin A requires specific protein-binding partners, like BAF, to affect nuclear function not only during disease, but probably also during physiological events requiring important reorganization of genome. Materials and Methods Cell cultures and transfection. Skin fibroblast cultures were obtained from skin biopsies of healthy, FPLD, MADA, RD and EDMD1 patients following a written consent. Cultures were established and cultured in Dulbecco s modified Eagle s medium supplemented with 10% fetal calf serum (FCS) and antibiotics. Control and pathological cells at early-passage (passage 5 10) were used for each experiment. For immunoprecipitation assays, HEK293 cells were used. GFP-BAF, full-length FLAG-tagged rat prelamin A (LA-WT, pci mammalian expression vector) and the mutated constructs LA-R482Q, LA-R527H and LA-L647R were transiently transfected into HEK293 cells using FuGene reagent (Roche). 21 GFP-emerin was transiently transfected in human skin fibroblast as previously described. 24 Biochemical and immunofluorescence analyses were performed 24 h after transfection. In human skin fibroblast cells, the accumulation of prelamin A intermediate was obtained using 25 μm mevinolin (Sigma) in growth medium for 18 h. 24 Western blotting and immunoprecipitation. Human fibroblasts were lysed in lysis buffer containing 20 mm Tris HCl, ph 7.5, 1% SDS, 1 mm Na 3 VO 4, 1 mm PMSF, 5% β-mercaptoethanol and protease inhibitors. Proteins were subjected to SDS gradient gel (5 20%) electrophoresis and transferred to nitrocellulose membrane overnight at 4 C. Incubation with primary antibodies was performed for the indicated time. Bands were revealed by the Amersham ECL detection system. To isolate cytoplasms and nuclei of transfected cells, HEK293 cells pellets were resuspended in a lysis buffer containing 10 mm Tris ph 7.8, 1% Nonidet P-40 (NP-40), 2 mm MgCl2 and protease inhibitors. The separation was obtained by hypotonic shock; nuclei and cytoplasms were obtained by centrifugation at 1,000 g at 4 C. Cytoplasmic fractions and isolated nuclei were subject to SDS gradient gel (5 20%). Cell Cycle 3575
9 For immunoprecipitation experiments, HEK293 transfected cells were lysed in a buffer containing 50 mm TRIS-HCl, ph 8.0, 150 mm NaCl, 1% NP40, 0.1% SDS, 1 mm DTT and protease inhibitors (IP-buffer). 21 Five hundred micrograms of total cell lysates were incubated with specific antibodies overnight at 4 C. Control immunoprecipitations were performed in the presence of aspecific immunoglobulins. After the addition of 30 μl of protein A/G (Santa Cruz Biotechnology) for 60 min at 4 C, immunoprecipitated protein complexes were washed, added to Laemmli s buffer, boiled and subjected to western blot analysis. Immunofluorescence. Human fibroblasts and HEK293 transfected cells grown on coverslips were fixed in methanol at 20 C for 7 min. Samples were incubated with PBS containing 4% BSA to saturate non-specific binding and incubated with primary antibodies and secondary antibodies. The nuclei were then counterstained with 4,6-diamino-2-phenylindole (DAPI). The slides were mounted with an anti-fade reagent in glycerol and observed. Immunofluorescence microscopy was performed using a Nikon E600 epifluorescence microscope and a Nikon oil-immersion objective [100x magnification, 1,3 NA (numerical aperture)]. Photographs were taken using a Nikon digital camera (DXm) and NIS-Element BR2.20 software. All images were taken at similar exposures within an experiment for each antibody. Images were processed using Adobe Photoshop (Adobe Systems). Antibodies. The antibodies employed for western blot analysis or immunofluorescence labeling were: anti-flag, mouse monoclonal (Sigma M2, diluted 1:300, 1 h, for the immunofluorescence analysis and 1:1,000, 1 h, for the western blot analysis); anti-baf, rabbit polyclonal (Santa Cruz Biotechnology FL-89, diluted 1:10, overnight at 4 C for immunofluorescence analysis and 1:100 overnight at 4 C, for the western blot analysis); anti- GFP rabbit polyclonal (Santa Cruz Biotechnology FL, diluted 1:1,000 for 1 h, for the western blot analysis) anti-emerin, mouse monoclonal (Monosan, diluted 1:200 for 1 h, for the western blot analysis); anti-prelamin A, goat polyclonal (Santa Cruz Biotechnology SC-6214, used 1:100 for 1 h at room temperature for the immunofluorescence analysis and 1:500 for 1 h, for the western blot analysis); anti-lamin A/C, goat polyclonal (Santa Cruz Biotechnology N-18, used 1:100 for 1 h for the western blot analysis); anti-lamin A rabbit polyclonal (Abcam ab diluted 1:500 for 1 h, for western blot analysis) anti-actin, goat polyclonal (Santa Cruz Biotechnology I-19, diluted 1:1,000 for 1 h, for the western blot analysis). Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed. Acknowledgments We thank A. Valmori, S. Grasso and D. Zini for the technical support. This work was supported by Italian Ministry for Education, University and Research Prin 2008 to G.L., FIRB 2010 to N.M.M., the Fondazione Carisbo, Italy and A.I.Pro. Sa.B., Italy. The authors are grateful to colleagues of Italian Network for Laminopathies for the helpful discussion. Supplemental Materials Supplemental materials may be found here: References 1. Misteli T. Higher-order genome organization in human disease. Cold Spring Harb Perspect Biol 2010; 2:a000794; PMID: ; org/ /cshperspect.a Worman HJ. Nuclear lamins and laminopathies. J Pathol 2012; 226:316-25; PMID: ; dx.doi.org/ /path Maraldi NM, Lattanzi G, Capanni C, Columbaro M, Merlini L, Mattioli E, et al. Nuclear envelope proteins and chromatin arrangement: a pathogenic mechanism for laminopathies. Eur J Histochem 2006; 50:1-8; PMID: Maraldi NM, Lattanzi G. Involvement of prelamin A in laminopathies. Crit Rev Eukaryot Gene Expr 2007; 17:317-34; PMID: ; org/ /critreveukargeneexpr.v17.i Sinensky M, Fantle K, Trujillo M, McLain T, Kupfer A, Dalton M. The processing pathway of prelamin A. J Cell Sci 1994; 107:61-7; PMID: Corrigan DP, Kuszczak D, Rusinol AE, Thewke DP, Hrycyna CA, Michaelis S, et al. Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24. Biochem J 2005; 387:129-38; PMID: ; 7. De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science 2003; 300:2055; PMID: ; 8. Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 2003; 423:293-8; PMID: ; 9. Filesi I, Gullotta F, Lattanzi G, D Apice MR, Capanni C, Nardone AM, et al. Alterations of nuclear envelope and chromatin organization in mandibuloacral dysplasia, a rare form of laminopathy. Physiol Genomics 2005; 23:150-8; PMID: ; org/ /physiolgenomics Capanni C, Mattioli E, Columbaro M, Lucarelli E, Parnaik VK, Novelli G, et al. Altered pre-lamin A processing is a common mechanism leading to lipodystrophy. Hum Mol Genet 2005; 14: ; PMID: ; ddi Navarro CL, De Sandre-Giovannoli A, Bernard R, Boccaccio I, Boyer A, Geneviève D, et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet 2004; 13: ; PMID: ; org/ /hmg/ddh Lattanzi G, Columbaro M, Mattioli E, Cenni V, Camozzi D, Wehnert M, et al. Pre-Lamin A processing is linked to heterochromatin organization. J Cell Biochem 2007; 102: ; PMID: ; Mattioli E, Columbaro M, Capanni C, Santi S, Maraldi NM, D Apice MR, et al. Drugs affecting prelamin A processing: effects on heterochromatin organization. Exp Cell Res 2008; 314:453-62; PMID: ; Montes de Oca R, Andreassen PR, Wilson KL. Barrier-to-Autointegration Factor influences specific histone modifications. Nucleus 2011; 2:580-90; PMID: ; nucl Skoko D, Li M, Huang Y, Mizuuchi M, Cai M, Bradley CM, et al. Barrier-to-autointegration factor (BAF) condenses DNA by looping. Proc Natl Acad Sci USA 2009; 106: ; PMID: ; org/ /pnas Margalit A, Neufeld E, Feinstein N, Wilson KL, Podbilewicz B, Gruenbaum Y. Barrier to autointegration factor blocks premature cell fusion and maintains adult muscle integrity in C. elegans. J Cell Biol 2007; 178:661-73; PMID: ; org/ /jcb Wang X, Xu S, Rivolta C, Li LY, Peng GH, Swain PK, et al. Barrier to autointegration factor interacts with the cone-rod homeobox and represses its transactivation function. J Biol Chem 2002; 277: ; PMID: ; M Montes de Oca R, Shoemaker CJ, Gucek M, Cole RN, Wilson KL. Barrier-to-autointegration factor proteome reveals chromatin-regulatory partners. PLoS One 2009; 4:e7050; PMID: ; org/ /journal.pone Puente XS, Quesada V, Osorio FG, Cabanillas R, Cadiñanos J, Fraile JM, et al. Exome sequencing and functional analysis identifies BANF1 mutation as the cause of a hereditary progeroid syndrome. Am J Hum Genet 2011; 88:650-6; PMID: ; dx.doi.org/ /j.ajhg Cabanillas R, Cadiñanos J, Villameytide JA, Pérez M, Longo J, Richard JM, et al. Néstor-Guillermo progeria syndrome: a novel premature aging condition with early onset and chronic development caused by BANF1 mutations. Am J Med Genet A 2011; 155A: ; PMID: ; ajmg.a Cell Cycle Volume 11 Issue 19
10 21. Capanni C, Cenni V, Haraguchi T, Squarzoni S, Schüchner S, Ogris E, et al. Lamin A precursor induces barrier-to-autointegration factor nuclear localization. Cell Cycle 2010; 9: ; PMID: ; dx.doi.org/ /cc Gambineri A, Semple RK, Forlani G, Genghini S, Grassi I, Hyden CS, et al. Monogenic polycystic ovary syndrome due to a mutation in the lamin A/C gene is sensitive to thiazolidinediones but not to metformin. Eur J Endocrinol 2008; 159:347-53; PMID: ; Columbaro M, Mattioli E, Schena E, Capanni C, Cenni V, Levy N, et al. Prelamin A processing and functional effects in restrictive dermopathy. Cell Cycle 2010; 9:4766-8; PMID: ; org/ /cc Capanni C, Del Coco R, Mattioli E, Camozzi D, Columbaro M, Schena E, et al. Emerin-prelamin A interplay in human fibroblasts. Biol Cell 2009; 101:541-54; PMID: ; org/ /bc Bengtsson L, Wilson KL. Barrier-to-autointegration factor phosphorylation on Ser-4 regulates emerin binding to lamin A in vitro and emerin localization in vivo. Mol Biol Cell 2006; 17: ; PMID: ; Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet 1994; 8:323-7; PMID: ; dx.doi.org/ /ng Maraldi NM, Lattanzi G, Capanni C, Columbaro M, Mattioli E, Sabatelli P, et al. Laminopathies: a chromatin affair. Adv Enzyme Regul 2006; 46:33-49; PMID: ; Krishnan V, Chow MZ, Wang Z, Zhang L, Liu B, Liu X, et al. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice. Proc Natl Acad Sci USA 2011; 108: ; PMID: ; Li X, Corsa CA, Pan PW, Wu L, Ferguson D, Yu X, et al. MOF and H4 K16 acetylation play important roles in DNA damage repair by modulating recruitment of DNA damage repair protein Mdc1. Mol Cell Biol 2010; 30: ; PMID: ; org/ /mcb Pegoraro G, Kubben N, Wickert U, Göhler H, Hoffmann K, Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol 2009; 11:1261-7; PMID: ; dx.doi.org/ /ncb Brachner A, Braun J, Ghodgaonkar M, Castor D, Zlopasa L, Ehrlich V, et al. The endonuclease Ankle1 requires its LEM and GIY-YIG motifs for DNA cleavage in vivo. J Cell Sci 2012; 125: ; PMID: ; jcs Dittrich CM, Kratz K, Sendoel A, Gruenbaum Y, Jiricny J, Hengartner MO. LEM-3 - A LEM domain containing nuclease involved in the DNA damage response in C. elegans. PLoS One 2012; 7:e24555; PMID: ; pone Musich PR, Zou Y. Genomic instability and DNA damage responses in progeria arising from defective maturation of prelamin A. Aging (Albany NY) 2009; 1:28-37; PMID: Musich PR, Zou Y. DNA-damage accumulation and replicative arrest in Hutchinson-Gilford progeria syndrome. Biochem Soc Trans 2011; 39:1764-9; PMID: ; BST Gulbagci NT, Li L, Ling B, Gopinadhan S, Walsh M, Rossner M, et al. SHARP1/DEC2 inhibits adipogenic differentiation by regulating the activity of C/EBP. EMBO Rep 2009; 10:79-86; PMID: ; dx.doi.org/ /embor Halaschek-Wiener J, Brooks-Wilson A. Progeria of stem cells: stem cell exhaustion in Hutchinson- Gilford progeria syndrome. J Gerontol A Biol Sci Med Sci 2007; 62:3-8; PMID: ; org/ /gerona/ Gotzmann J, Foisner R. A-type lamin complexes and regenerative potential: a step towards understanding laminopathic diseases? Histochem Cell Biol 2006; 125:33-41; PMID: ; org/ /s Vlcek S, Foisner R. Lamins and lamin-associated proteins in aging and disease. Curr Opin Cell Biol 2007; 19: ; PMID: ; org/ /j.ceb Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol 2008; 10:452-9; PMID: ; Haraguchi T, Koujin T, Osakada H, Kojidani T, Mori C, Masuda H, et al. Nuclear localization of barrier-toautointegration factor is correlated with progression of S phase in human cells. J Cell Sci 2007; 120: ; PMID: ; jcs Cabanillas R, Cadiñanos J, Villameytide JA, Pérez M, Longo J, Richard JM, et al. Néstor-Guillermo progeria syndrome: a novel premature aging condition with early onset and chronic development caused by BANF1 mutations. Am J Med Genet A 2011; 155A: ; PMID: ; ajmg.a Brero A, Easwaran HP, Nowak D, Grunewald I, Cremer T, Leonhardt H, et al. Methyl CpG-binding proteins induce large-scale chromatin reorganization during terminal differentiation. J Cell Biol 2005; 169:733-43; PMID: ; org/ /jcb Mattioli E, Columbaro M, Capanni C, Maraldi NM, Cenni V, Scotlandi K, et al. Prelamin A-mediated recruitment of SUN1 to the nuclear envelope directs nuclear positioning in human muscle. Cell Death Differ 2011; 18: ; PMID: ; dx.doi.org/ /cdd Méjat A, Misteli T. LINC complexes in health and disease. Nucleus 2010; 1:40-52; PMID: Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet 2007; 16: ; PMID: ; Cenni V, Bavelloni A, Beretti F, Tagliavini F, Manzoli L, Lattanzi G, et al. Ankrd2/ARPP is a novel Akt2 specific substrate and regulates myogenic differentiation upon cellular exposure to H(2)O(2). Mol Biol Cell 2011; 22: ; PMID: ; org/ /mbc.e Cell Cycle 3577
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Non-commercial use only
 European Journal of Histochemistry 2011; volume 55:e36 Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria V. Cenni, 1 C. Capanni, 1 M. Columbaro, 2 M.
European Journal of Histochemistry 2011; volume 55:e36 Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria V. Cenni, 1 C. Capanni, 1 M. Columbaro, 2 M.
Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling
 4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
4th International Melorheostosis Association Conference Inner Nuclear Membrane Protein MAN1 and Regulation of R-Smad Signaling Howard J. Worman Columbia University New York, NY The Nuclear Envelope By
Rescue of heterochromatin organization in Hutchinson- Gilford progeria by drug treatment
 Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
Cell. Mol. Life Sci. 62 (2005) 2669 2678 1420-682X/05/222669-10 DOI 10.1007/s00018-005-5318-6 Birkhäuser Verlag, Basel, 2005 Cellular and Molecular Life Sciences Research Article Rescue of heterochromatin
HYBRID GENE THERAPY FOR AD-EDMD
 HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
HYBRID GENE THERAPY FOR AD-EDMD Gene Therapy Prof. Isabella Saggio 2017/2018 Bertani Camilla Dezi Clara Difeo Giorgia di Palma Carmen AUTOSOMAL DOMINANT EMERY-DREIFUSS MUSCULAR DYSTROPHY Fig.1 Adapted
Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells
 1698 Biochemical Society Transactions (2011) Volume 39, part 6 Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells Giovanna Lattanzi 1 National Research Council of Italy, Institute
1698 Biochemical Society Transactions (2011) Volume 39, part 6 Prelamin A-mediated nuclear envelope dynamics in normal and laminopathic cells Giovanna Lattanzi 1 National Research Council of Italy, Institute
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies
 Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
Acta Myologica 2011; XXX: p. 138-143 workshop report Laminopathies: many diseases, one gene. Report of the first Italian Meeting Course on Laminopathies G. Lattanzi, S. Benedetti, E. Bertini, G. Boriani,
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
s u p p l e m e n ta ry i n f o r m at i o n
 Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
THURSDAY, JANUARY
 THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
THURSDAY, JANUARY 15 2015 08.30-09.00 WELCOME COFFEE 09.00-09.15 Welcome and opening comments. A. De Sandre-Giovannoli, N. Lévy Presentation of the French Network on EDMD and other nucleopathies. G. Bonne,
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Eukaryotic transcription (III)
 Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
All-trans retinoic acid and rapamycin normalize Hutchinson Gilford progeria fibroblast phenotype
 /, Vol. 6, No. 30 All-trans retinoic acid and rapamycin normalize Hutchinson Gilford progeria fibroblast phenotype Camilla Pellegrini 1,2, Marta Columbaro 2,3, Cristina Capanni 1,2, Maria Rosaria D Apice
/, Vol. 6, No. 30 All-trans retinoic acid and rapamycin normalize Hutchinson Gilford progeria fibroblast phenotype Camilla Pellegrini 1,2, Marta Columbaro 2,3, Cristina Capanni 1,2, Maria Rosaria D Apice
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Histones modifications and variants
 Histones modifications and variants Dr. Institute of Molecular Biology, Johannes Gutenberg University, Mainz www.imb.de Lecture Objectives 1. Chromatin structure and function Chromatin and cell state Nucleosome
Histones modifications and variants Dr. Institute of Molecular Biology, Johannes Gutenberg University, Mainz www.imb.de Lecture Objectives 1. Chromatin structure and function Chromatin and cell state Nucleosome
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1
 Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
Nuclear lamins, diseases and aging Anna Mattout 1, Thomas Dechat 2, Stephen A Adam 2, Robert D Goldman 2 and Yosef Gruenbaum 1 Nuclear lamins are type V intermediate filament proteins. They are the major
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
Supplemental Materials. STK16 regulates actin dynamics to control Golgi organization and cell cycle
 Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome
 Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome Paola Scaffidi & Tom Misteli Hutchinson-Gilford progeria syndrome (HGPS) is a childhood premature
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Nuclear Envelope and Muscular Dystrophy
 Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
Nationwide Children s Hospital August 25, 2015 Nuclear Envelope and Muscular Dystrophy Howard J. Worman, M.D. Columbia University Columbia University Medical Center The Nuclear Envelope By D. W. Fawcett
ab SREBP-2 Translocation Assay Kit (Cell-Based)
 ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
ab133114 SREBP-2 Translocation Assay Kit (Cell-Based) Instructions for Use For analysis of translocation of SREBP-2 into nuclei. This product is for research use only and is not intended for diagnostic
Study of different types of ubiquitination
 Study of different types of ubiquitination Rudi Beyaert (rudi.beyaert@irc.vib-ugent.be) VIB UGent Center for Inflammation Research Ghent, Belgium VIB Training Novel Proteomics Tools: Identifying PTMs October
Study of different types of ubiquitination Rudi Beyaert (rudi.beyaert@irc.vib-ugent.be) VIB UGent Center for Inflammation Research Ghent, Belgium VIB Training Novel Proteomics Tools: Identifying PTMs October
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Dissecting the role of H3K64me3 in mouse pericentromeric heterochromatin.
 Supplementary Information Dissecting the role of H3K64me3 in mouse pericentromeric heterochromatin. Ulrike C. Lange 1, Stéphanie Siebert 2, Mark Wossidlo 3,4, Thomas Weiss 1, Céline Ziegler- Birling 2,
Supplementary Information Dissecting the role of H3K64me3 in mouse pericentromeric heterochromatin. Ulrike C. Lange 1, Stéphanie Siebert 2, Mark Wossidlo 3,4, Thomas Weiss 1, Céline Ziegler- Birling 2,
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death
 www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
Genetics and Genomics in Medicine Chapter 6 Questions
 Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Biochemical Determinants Governing Redox Regulated Changes in Gene Expression and Chromatin Structure
 Biochemical Determinants Governing Redox Regulated Changes in Gene Expression and Chromatin Structure Frederick E. Domann, Ph.D. Associate Professor of Radiation Oncology The University of Iowa Iowa City,
Biochemical Determinants Governing Redox Regulated Changes in Gene Expression and Chromatin Structure Frederick E. Domann, Ph.D. Associate Professor of Radiation Oncology The University of Iowa Iowa City,
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Chairoungdua et al., http://www.jcb.org/cgi/content/full/jcb.201002049/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Expression of CD9 and CD82 inhibits Wnt/ -catenin
Supplemental material Chairoungdua et al., http://www.jcb.org/cgi/content/full/jcb.201002049/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Expression of CD9 and CD82 inhibits Wnt/ -catenin
Alpha thalassemia mental retardation X-linked. Acquired alpha-thalassemia myelodysplastic syndrome
 Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12
 SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
ab Adipogenesis Assay Kit (Cell-Based)
 ab133102 Adipogenesis Assay Kit (Cell-Based) Instructions for Use For the study of induction and inhibition of adipogenesis in adherent cells. This product is for research use only and is not intended
ab133102 Adipogenesis Assay Kit (Cell-Based) Instructions for Use For the study of induction and inhibition of adipogenesis in adherent cells. This product is for research use only and is not intended
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
SUPPLEMENTARY LEGENDS...
 TABLE OF CONTENTS SUPPLEMENTARY LEGENDS... 2 11 MOVIE S1... 2 FIGURE S1 LEGEND... 3 FIGURE S2 LEGEND... 4 FIGURE S3 LEGEND... 5 FIGURE S4 LEGEND... 6 FIGURE S5 LEGEND... 7 FIGURE S6 LEGEND... 8 FIGURE
TABLE OF CONTENTS SUPPLEMENTARY LEGENDS... 2 11 MOVIE S1... 2 FIGURE S1 LEGEND... 3 FIGURE S2 LEGEND... 4 FIGURE S3 LEGEND... 5 FIGURE S4 LEGEND... 6 FIGURE S5 LEGEND... 7 FIGURE S6 LEGEND... 8 FIGURE
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
A. Generation and characterization of Ras-expressing autophagycompetent
 Supplemental Material Supplemental Figure Legends Fig. S1 A. Generation and characterization of Ras-expressing autophagycompetent and -deficient cell lines. HA-tagged H-ras V12 was stably expressed in
Supplemental Material Supplemental Figure Legends Fig. S1 A. Generation and characterization of Ras-expressing autophagycompetent and -deficient cell lines. HA-tagged H-ras V12 was stably expressed in
SUPPLEMENTARY FIGURES AND TABLE
 SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
SUPPLEMENTARY FIGURES AND TABLE Supplementary Figure S1: Characterization of IRE1α mutants. A. U87-LUC cells were transduced with the lentiviral vector containing the GFP sequence (U87-LUC Tet-ON GFP).
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Modulation of TGFbeta 2 levels by lamin A in U2-OS osteoblast-like cells: understanding the osteolytic process triggered by altered lamins
 /, Vol. 6, No. 10 Modulation of TGFbeta 2 levels by lamin A in U2-OS osteoblast-like cells: understanding the osteolytic process triggered by altered lamins Camilla Evangelisti 1, Pia Bernasconi 2, Paola
/, Vol. 6, No. 10 Modulation of TGFbeta 2 levels by lamin A in U2-OS osteoblast-like cells: understanding the osteolytic process triggered by altered lamins Camilla Evangelisti 1, Pia Bernasconi 2, Paola
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
(A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939 (5 µm)
 Supplementary Figure Legends Figure S1. Tankyrase inhibition suppresses cell proliferation in an axin/β-catenin independent manner. (A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939
Supplementary Figure Legends Figure S1. Tankyrase inhibition suppresses cell proliferation in an axin/β-catenin independent manner. (A) SW480, DLD1, RKO and HCT116 cells were treated with DMSO or XAV939
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human
 Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24
 Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24 Jemima Barrowman, Corinne Hamblet, Megan S. Kane, Susan Michaelis* Department of Cell Biology, The Johns Hopkins School of Medicine,
Requirements for Efficient Proteolytic Cleavage of Prelamin A by ZMPSTE24 Jemima Barrowman, Corinne Hamblet, Megan S. Kane, Susan Michaelis* Department of Cell Biology, The Johns Hopkins School of Medicine,
Laminopathies and the long strange trip from basic cell biology to therapy
 Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
Review series Laminopathies and the long strange trip from basic cell biology to therapy Howard J. Worman, 1,2 Loren G. Fong, 3 Antoine Muchir, 1,2 and Stephen G. Young 3,4 1 Department of Medicine and
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy
 The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
The Genes, Proteins, and the Cell Biological Processes Underlying Emery-Dreifuss Muscular Dystrophy Megan E. Mastey a and Elise A. Kikis a Emery-Dreifuss Muscular Dystrophy (EDMD) is a type of muscular
SUPPLEMENTARY INFORMATION
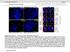 doi: 10.1038/nature05473 SUPPLEMENTARY FIGURES SUPPLEMENTARY INFORMATION Supplementary Figure 1: Association of Runx2 with mitotic chromosomes Mitotic chromosome spreads were prepared for a. Human Saos-2
doi: 10.1038/nature05473 SUPPLEMENTARY FIGURES SUPPLEMENTARY INFORMATION Supplementary Figure 1: Association of Runx2 with mitotic chromosomes Mitotic chromosome spreads were prepared for a. Human Saos-2
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression. Chromatin Array of nuc
 Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb2822 a MTC02 FAO cells EEA1 b +/+ MEFs /DAPI -/- MEFs /DAPI -/- MEFs //DAPI c HEK 293 cells WCE N M C P AKT TBC1D7 Lamin A/C EEA1 VDAC d HeLa cells WCE N M C P AKT Lamin A/C EEA1 VDAC Figure
DOI:.38/ncb2822 a MTC02 FAO cells EEA1 b +/+ MEFs /DAPI -/- MEFs /DAPI -/- MEFs //DAPI c HEK 293 cells WCE N M C P AKT TBC1D7 Lamin A/C EEA1 VDAC d HeLa cells WCE N M C P AKT Lamin A/C EEA1 VDAC Figure
The LMNA gene encodes the A-type lamins, including lamin A
 A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
A lamin A protein isoform overexpressed in Hutchinson Gilford progeria syndrome interferes with mitosis in progeria and normal cells Kan Cao*, Brian C. Capell*, Michael R. Erdos*, Karima Djabali, and Francis
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplemental Figure 1
 Supplemental Figure 1 A S100A4: SFLGKRTDEAAFQKLMSNLDSNRDNEVDFQEYCVFLSCIAMMCNEFFEGFPDK Overlap: SF G DE KLM LD N D VDFQEY VFL I M N FF G PD S100A2: SFVGEKVDEEGLKKLMGSLDENSDQQVDFQEYAVFLALITVMCNDFFQGCPDR
Supplemental Figure 1 A S100A4: SFLGKRTDEAAFQKLMSNLDSNRDNEVDFQEYCVFLSCIAMMCNEFFEGFPDK Overlap: SF G DE KLM LD N D VDFQEY VFL I M N FF G PD S100A2: SFVGEKVDEEGLKKLMGSLDENSDQQVDFQEYAVFLALITVMCNDFFQGCPDR
Article. An EDMD Mutation in C. elegans Lamin Blocks Muscle-Specific Gene Relocation and Compromises Muscle Integrity
 Current Biology 21, 163 1614, October 11, 211 ª211 Elsevier Ltd All rights reserved DOI 1.116/j.cub.211.8.3 An EDMD Mutation in C. elegans Lamin Blocks Muscle-Specific Gene Relocation and Compromises Muscle
Current Biology 21, 163 1614, October 11, 211 ª211 Elsevier Ltd All rights reserved DOI 1.116/j.cub.211.8.3 An EDMD Mutation in C. elegans Lamin Blocks Muscle-Specific Gene Relocation and Compromises Muscle
Tel: ; Fax: ;
 Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Importance of molecular cell biology investigations in human medicine in the story of the Hutchinson-Gilford progeria syndrome
 Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
Interdisc Toxicol. 2010; Vol. 3(3): 89 93. doi: 10.2478/v10102-010-0018-y Published online in: www.intertox.sav.sk & www.versita.com/science/medicine/it/ This is an Open Access article distributed under
Nature Genetics: doi: /ng Supplementary Figure 1. Assessment of sample purity and quality.
 Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Jewell et al., http://www.jcb.org/cgi/content/full/jcb.201007176/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. IR Munc18c association is independent of IRS-1. (A and
Supplemental material Jewell et al., http://www.jcb.org/cgi/content/full/jcb.201007176/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. IR Munc18c association is independent of IRS-1. (A and
* This work was supported, in whole or in part, by National Institutes of Health Grants HL076684, HL62984 (to N. L. W.), and ES (to S. H.
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 286, NO. 31, pp. 27836 27847, August 5, 2011 Printed in the U.S.A. Histone Deacetylase 9 Is a Negative Regulator of Adipogenic Differentiation * S Received for
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 286, NO. 31, pp. 27836 27847, August 5, 2011 Printed in the U.S.A. Histone Deacetylase 9 Is a Negative Regulator of Adipogenic Differentiation * S Received for
Mesenchymal progenitor cells in intramuscular connective tissue development
 Mesenchymal progenitor cells in intramuscular connective tissue development Min Du, Ph.D. Professor and Endowed Chair Department of Animal Sciences Washington State University Pullman, WA Beef Quality:
Mesenchymal progenitor cells in intramuscular connective tissue development Min Du, Ph.D. Professor and Endowed Chair Department of Animal Sciences Washington State University Pullman, WA Beef Quality:
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
THE JOURNAL OF CELL BIOLOGY
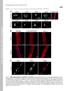 Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
Supplemental Material Mejat et al., http://www.jcb.org/cgi/content/full/jcb.200811035/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. SUN1, SUN2, and Nesprin-1 localization in muscle fibers. (A) SUN1 and SUN2
MCB130 Midterm. GSI s Name:
 1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
supplementary information
 Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
SUPPLEMENTAL FIGURE LEGENDS
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3 and Hariom Yadav 3
 Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3 and Hariom Yadav 3 1 Department of Biotechnology, JMIT, Radaur, Haryana, India 2 KITM, Kurukshetra, Haryana, India 3 NIDDK, National Institute of Health,
Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3 and Hariom Yadav 3 1 Department of Biotechnology, JMIT, Radaur, Haryana, India 2 KITM, Kurukshetra, Haryana, India 3 NIDDK, National Institute of Health,
Reviews. Mechanisms Underlying Caloric Restriction, Lipid Metabolism, and Life Span Regulation Issei Komuro, Guest Editor
 Reviews This Review is part of a thematic series on Biological Role of Senescence in Cardiovascular Disease, which includes the following articles: Telomere Biology and Cardiovascular Disease Vascular
Reviews This Review is part of a thematic series on Biological Role of Senescence in Cardiovascular Disease, which includes the following articles: Telomere Biology and Cardiovascular Disease Vascular
supplementary information
 DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
IP: anti-gfp VPS29-GFP. IP: anti-vps26. IP: anti-gfp - + +
 FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
Emerin inhibits Lmo7 binding to the Pax3 and MyoD promoters and expression of myoblast proliferation genes
 Research Article 1691 Emerin inhibits Lmo7 binding to the Pax3 and MyoD promoters and expression of myoblast proliferation genes Zinaida Dedeic 1, Maureen Cetera 1, Tatiana V. Cohen 2 and James M. Holaska
Research Article 1691 Emerin inhibits Lmo7 binding to the Pax3 and MyoD promoters and expression of myoblast proliferation genes Zinaida Dedeic 1, Maureen Cetera 1, Tatiana V. Cohen 2 and James M. Holaska
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
RayBio KinaseSTAR TM Akt Activity Assay Kit
 Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Epigenetics: The Future of Psychology & Neuroscience. Richard E. Brown Psychology Department Dalhousie University Halifax, NS, B3H 4J1
 Epigenetics: The Future of Psychology & Neuroscience Richard E. Brown Psychology Department Dalhousie University Halifax, NS, B3H 4J1 Nature versus Nurture Despite the belief that the Nature vs. Nurture
Epigenetics: The Future of Psychology & Neuroscience Richard E. Brown Psychology Department Dalhousie University Halifax, NS, B3H 4J1 Nature versus Nurture Despite the belief that the Nature vs. Nurture
