Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study
|
|
|
- Dale Patterson
- 6 years ago
- Views:
Transcription
1 ORIGINAL ARTICLE Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study Maja Mockenhaupt 1, Cecile Viboud 2, Ariane Dunant 3,8, Luigi Naldi 4, Sima Halevy 5, Jan Nico Bouwes Bavinck 6, Alexis Sidoroff 7,Jürgen Schneck 1, Jean-Claude Roujeau 8 and Antoine Flahault 2 Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare but severe cutaneous adverse reactions (SCAR) related to a variety of medications. They have a significant public health impact because of high mortality and morbidity. A multinational case control study conducted in Europe between 1997 and 21 evaluated the risk of medications to induce SCAR. Cases were actively detected through a hospital network covering more than 1 million inhabitants. Three hospitalized patients per case matched on age, gender, and date of interview were enrolled as controls. After validation by an expert committee blinded to exposures, 379 SCAR cases and 1,55 controls were included. Among drugs recently introduced into the market, strong associations were documented for nevirapine (relative risk (RR)422) and lamotrigine (RR414), and weaker associations for sertraline (RR ¼ 11 [2.7 46]), pantoprazole (RR ¼ 18 [3.9 85]), and tramadol (RR ¼ 2 [4.4 93]). Strong associations were confirmed for anti-infective sulfonamides, allopurinol, carbamazapine, phenobarbital, phenytoin, and oxicam-nsaids, with some changes in relative numbers of exposed cases. Thus, many cases were still related to a few old drugs with a known high risk. Risk was restricted to the first few weeks of drug intake. The use of such drugs as first-line therapies should be considered carefully, especially when safer alternative treatments exist. A number of widely used drugs did not show any risk for SJS and TEN. Journal of Investigative Dermatology (28) 128, 35 44; doi:1.138/sj.jid.57133; published online 6 September 27 INTRODUCTION Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe cutaneous adverse reactions (SCARs) to drugs, characterized by extensive detachment of epidermis 1 Dokumentationszentrum schwerer Hautreaktionen (dzh), Department of Dermatology, University Medical Center, Freiburg, Germany; 2 Inserm U 444, Paris, France; 3 Biostatistics and Epidemiology Unit, Institut Gustave-Roussy, Villejuif, France; 4 GISED Study Center, Department of Dermatology, Azienda Ospedaliero Ospedali Riuniti di Bergamo, Bergamo, Italy; 5 Department of Dermatology, Soroka University Medical Center, Ben-Gurion University of the Negev, Beer-Sheva, Israel; 6 Department of Dermatology, Leiden University Medical Center, Leiden, The Netherlands; 7 Department of Dermatology, Medical University of Innsbruck, Innsbruck, Austria and 8 Reference Center for Toxic and Autoimmune Blistering Diseases, Department of Dermatology, Hopital Henri Mondor, University Paris XII, Créteil, France Correspondence: Dr Maja Mockenhaupt, Dokumentationszentrum schwerer Hautreaktionen (dzh), Department of Dermatology, Hauptstrasse 7, D-7914 Freiburg, Germany. dzh@uniklinik-freiburg.de Abbreviations: ACE, angiotensin converting enzyme; AED, antiepileptic drug; mvrr, multivariate relative risk; NSAID, non-steroidal anti-inflammatory drug; RR, relative risk; SCAR, severe cutaneous adverse reaction SJS, Stevens Johnson syndrome; TEN, toxic epidermal necrolysis uvrr, univariate relative risk Received 12 May 26; revised 19 April 27; accepted 19 April 27; published online 6 September 27 and erosions of mucous membranes (Roujeau and Stern, 1994; Becker, 1998). There is growing evidence that SJS and TEN are a single disease with common causes and mechanisms (Auquier-Dunant et al., 22). The principal difference is the extent of detachment, limited in SJS and more widespread in TEN (Bastuji-Garin et al., 1993). Even though rare (two cases/million population/year), SJS and TEN have a significant impact on public health because of high mortality (2 25%), frequent lasting disability, and reluctance of survivors and their physicians to subsequent use of medications (Rzany et al., 1996). In 1995, a first case control study (SCAR-study) assessed the risks of SJS and TEN related to medications (Roujeau et al., 1995). High relative risks (RRs) were observed for anti-infective sulfonamides (especially cotrimoxazole), carbamazepine, phenytoin, phenobarbital, non-steroidal anti-inflammatory drugs (NSAIDs) of the oxicam type, allopurinol, chlormezanone, aminopenicillins, cephalosporins, quinolones, and cycline antibiotics. These results contributed to several decisions of regulatory agencies, for example, withdrawal of chlormezanone from the market, restricted indications for cotrimoxazole and phenobarbital. Since the completion of the prior study, many new medications have been marketed. For some of them, alerts were raised by case reports of SJS or TEN. This concerned & 27 The Society for Investigative Dermatology 35
2 lamotrigine (Sachs et al., 1996), nevirapine (Warren et al., 1998), terbinafine (Rzany et al., 1994), fluoxetine (Bodokh et al., 1992), sertraline (Jan et al., 1999), atorvastatin (Pfeiffer et al., 1998), nimesulide (GISED, 1993), leflunomide (Fischer et al., 23), and cyclooxygenase 2 inhibiting NSAIDs (Friedman et al., 23). The European Study of SCAR (EuroSCAR-study) was designed as an international multicenter study aiming at an ongoing surveillance of medication risks for SJS and TEN based on a case control methodology (Slone et al., 1977). RESULTS Description of the study population Out of 513 potential cases and 1,763 potential controls, 379 community cases of SJS and TEN (i.e., patients who developed the adverse reaction outside the hospital and who were admitted because of symptoms of SCAR) and 1,55 controls, all with a determined index-day and adequate information on exposures, were accepted. Cases, aged one to 95 years (median 5, with interquartile range (28 68), included 134 SJS, 136 SJS/TEN-overlap and 19 TEN, 234 females (62%), 174 patients from Germany, 13 from France, and 75 from other participating countries. Most cases (93%) had erosions of at least one mucosa. Six weeks after admission the mortality rate was 22%. Cases differed from controls by more frequent HIV infection (6.6 vs.2%, multivariate RR (mvrr) ¼ 12 [2.4 59]), collagen vascular disease (7.1 vs 1.5%, mvrr ¼ 2.2 [.9 5.]), recent cancer (1.6 vs 1.9%, mvrr ¼ 2.7 [ ]), recent X-ray therapy (4.2 vs.5%, mvrr ¼ 2.1 [.5 9.]), or acute infection in the past 4 weeks (43.5 vs 24.7%, mvrr ¼ 1.7 [ ]). Recently marketed drugs Table 1 presents the results for recently marketed drugs. Among medications with prior alerts, two were highly associated with SJS or TEN: nevirapine and lamotrigine. Both shared the overall pattern of highly suspected drugs (recent onset of use and infrequent co-medication with another highly suspected drug). A lower but still significant mvrr was found for sertraline, a serotonin re-uptake inhibitor. In this class of antidepressants no other drug including fluoxetine was found to be associated with SJS or TEN. Pantoprazole was also associated with a significant univariate RR (uvrr), but without a clear pattern of highly suspected drugs in terms of timing and co-medication. No association was observed with other proton pump inhibitors. For statins, no significant risk was documented. For other medications with prior alerts, such as terbinafine, fluconazole, cyclooxygenase 2 inhibitors, and leflunomide, numbers of exposed cases and controls were too small for valuable risk estimation. Medications with previously demonstrated risks As shown in Table 2, a risk was confirmed for all previously suspected drugs, with the exception of valproic acid (uvrr ¼ 9.4 [3.9 23], mvrr ¼ 2. [.6 7.4]). Risk modification with duration of treatment Most highly suspected drugs are usually taken on a longterm basis. Among cases exposed to these drugs, 85 1% had initiated their treatment less than 8 weeks before the reaction (Table 2). The median time latency (interquartile range) between start of intake and index-day was less than 4 weeks (carbamazepine: 15 days (12 2), phenytoin: 24 days (16 33), phenobarbital: 17 days (9 4), allopurinol: 2 days (14 32)), whereas it was much longer for drugs with no associated risk (above 3 weeks for valproic acid, angiotensin converting enzyme (ACE) inhibitors and calcium channel blockers). For allopurinol, 56 of 66 exposed patients were recent users, in contrast to only one of 27 controls. The uvrr for recent use was 261 [36 infinite] versus a mvrr of.9 [.3 2.4] for long-term use. In general, no significant risk persisted after 8 weeks of use. Analgesics As shown in Table 3, we observed an association for acetaminophen (paracetamol) with a mvrr of 1.9 [ ]. Despite great disparity in rates of exposures among controls (from 4.1% in Germany to 22.5% in France), the mvrrs were not significantly different ((P ¼.9); 4. [ ] in Germany, 2.4 [ ] in France, and 1.1 [.4 3.] in other countries). The association with acetaminophen did not change substantially when the analysis was restricted to patients not exposed to any highly suspected drug (mvrr ¼ 2.4 [ ]) or to any suspected drug (mvrr ¼ 1.9 [.7 5.]). It neither changed with indication (fever or pain). On the other hand, the mvrr decreased to.8 (.5 1.3) when only patients who took that drug at least four days before index-day were considered exposed. This change resulted in a higher decrease in the number of exposed cases (from 88 to 59) than of the number of exposed controls (from 223 to 216). That reflected a peculiar pattern of exposure in cases, often beginning less than four days before the index-day (Figure 1e). Tramadol showed a high uvrr for recent use, but a high percentage of co-medication with highly suspected drugs (57%), suggesting potential confounding by co-medication. Corticosteroids Many cases had been exposed to steroid therapy before the onset of SJS and TEN (14.8 compared with 2.1% of controls), but for 55% of them in conjunction with a highly suspected medication. Despite frequent co-medication, the mvrr was significantly elevated (4.5 [ ]) and did not change substantially when the analysis was restricted to cases not exposed to any highly suspected drug (5.1 [ ]). The timing of exposure to corticosteroids showed some aggregation of cases in the same period as for associated drugs (Figure 1f). Drugs of common use without association A large number of drugs of common use, including sulfonamide-related diuretics and sulfonylurea antidiabetics, were not associated with a detectable risk of SJS or TEN (Table 4, and additional data available online). 36 Journal of Investigative Dermatology (28), Volume 128
3 Table 1. Evaluation of SCAR risk for drugs recently introduced into the market Drug Duration of use Case patients, n=379 (%) Control patients, n=1,55 (%) Univariate RR Multivariate RR (%) with use of other highly suspected drugs started within 8 weeks Confirmation of prior alerts Nevirapine 21 (5.5) 422 n.d. p8 weeks weeks Lamotrigine 14 (3.7) 414 n.d. 1 (7%) p8 weeks (7%) 48 weeks n.d. Sertraline 6 (1.6) 5 (.3) 4.8 (1.5 16) 11(2.7 46) 2 (33%) p8 weeks ( ) n.d. 48 weeks (.4 11) n.d. 2 (5%) Alert from this study needing further confirmation Pantoprazole 9 (2.4) 2 (.1) 18 (3.9 85) n.d. 5 (56%) p8 weeks (4%) 48 weeks (1.5 44) 3 (75%) Non-significant risk Fluoxetine 5 (1.3) 1 (.7) 2. (.7 5.9).8 (.1 5.4) 4 (8%) Other serotonin re-uptake inhibitors 1 5 (1.3) 18 (1.2) 1.1 (.4 3).8 (.2 3.6) 2 (4%) Other proton pump inhibitors 2 25 (6.6) 34 (2.3) 3.1 ( ) 1.5 (.7 3.2) 9 (36%) HMG-COA reductase, statins 3 12 (3.2) 43 (2.9) 1.1 (.6 2.1).4 (.2 1.1) 8 (67%) Not assessable Terbinafine 1 (.1) ( 155) n.d. Fluconazole 1 (.3) 4.1 n.d. Cox2 inhibitors 4 1 (.3) 1 (.1) 4 (.3 64) n.d. Leflunomide 2 (.5) 4.7 n.d. CI, confidence interval; Cox2, cyclooxygenase 2; RR, relative risk; SCAR, severe cutaneous adverse reactions. 1 Includes paroxetine (3 cases, 13 controls), citalopram (1 case, 4 controls), fluvoxamine (1 case, 1 control). 2 Includes omeprazole (23 cases, 24 controls) and lansoprazole (2 cases, 1 controls). 3 Includes simvastatin (5 cases, 19 controls), atorvastatin (4 cases, 1 controls), pravastatin ( case, 1 controls), fluvastatin (2 cases, 3 controls), and cerivastatin (1 case, 1 control). 4 Includes rofecoxib (1 case, control) and celecoxib ( case, 1 control). n.d. means not done, because of o3 cases or controls. Signals with limited evidence In univariate analyses, we detected statistically significant associations for a total of 22 medications not listed in the tables. Multivariate analyses were not feasible because of few exposed controls. Long-term use and/or high prevalence of co-medication with a highly suspected drug suggested that none was actually a strong risk factor (data available online). Most anti-hiv medications were among these drugs. Their frequent combination with nevirapine suggested that none of these anti-retroviral agents was a risk factor by itself (Barreiro et al., 2; Fagot et al., 21). DISCUSSION Our study allowed an up-to-date evaluation of the risk of medications to induce SJS or TEN. Two drugs introduced into the market in recent years and previously suspected from case reports and clinical trials, joined the group of highly suspected drugs, namely anti-infective sulfonamides, anti-epileptic drugs (AEDs), oxicam-nsaids, and allopurinol. First, the non-nucleoside anti-retroviral agent nevirapine accounted for most cases in HIV-infected persons (Fagot et al., 21). Surprisingly, the proportion of HIV-infected persons 37
4 Table 2. Evaluation of SCAR risk for drugs in the market for many years Drug Duration of use Case patients, n=379 (%) Control patients, n=1,55 (%) Univariate RR Multivariate RR (%) with use of other highly suspected drugs started within 8 weeks Confirmation of high risk Cotrimoxazole 24 (6.3) 1 (.1) 12 (14 754) n.d. 4 (17%) p8 weeks weeks ( ) 4 (8%) Other anti-infect. Sulfonamides 1 13 (3.4) 1 (.1) 53 (7. 41) n.d. p8 weeks (7. 41) 4 8 weeks n.d. Allopurinol 66 (17.4) 28 (1.9) 11 (7. 18) 18 (11 32) 7 (11%) p8 weeks (36 N) n.d. 2 (4%) 48 weeks (.7 3.).9 (.3 2.4) 5 (5%) Carbamazepine 31 (8.2) 4 (.3) 33 (12 95) 72 (23 225) 1 (3%) p8 weeks n.d. 48 weeks (.4 121) n.d. 1 Phenytoin 19 (5.) 3 (.2) 26 (7.8 9) 17 (4.1 68) 3 (16%) p8 weeks n.d. 2 (12%) 48 weeks (.4 16) n.d. 1 Phenobarbital 2 (5.3) 5 (.3) 17 (6.2 45) 16 (5. 5) 3 (15%) p8 weeks ( ) n.d. 2 (12%) 48 weeks (.7 13) 2.4 (.2 23) 1 (33%) Oxicam-NSAIDs 2 11 (2.9) 7 (.5) 6.4 (2.5 17) 16 (4.9 52) 1 (9%) p8 weeks (4.1 54) 5 (12 211) 1 (9%) 48 weeks 4 ( 6.2) n.d. Significant but lower risk Acetic acid NSAIDs 3 27 (7.1) 21 (1.4) 5.4 (3. 1) 5.6 (2.6 12) 7 (26%) p8 weeks (4.5 19) 13 (5.2 31) 7 (29%) 48 weeks (.3 4.4).7 (.1 3.3) Macrolides 4 18 (4.8) 1 (.7) 7.5 (3.4 16) 6.8 (2.6 18) 8 (44%) Quinolones 5 13 (3.4) 5 (.3) 1.7 (3.8 3) 6.9 (1.8 27) 6 (46%) Cephalosporins 6 19 (5.) 7 (.5) 11.3 (4.7 27) 7.3 (2.4 22) 12 (63%) Tetracyclines 7 7 (1.9) 5 (.3) 5.6 (1.8 18) 6.3 (1.6 25) 1 (14%) Aminopenicillins 8 18 (4.8) 18 (1.2) 4.1 (2.1 8.) 2.4 (1. 5.9) 1 (56%) CI, confidence interval; NSAID, non-steroidal anti-inflammatory drug; RR, relative risk; SCAR, severe cutaneous adverse reaction. 1 Includes sulfasalazine (5 cases, 1 control), sulfadiazine (5 cases, control), sulfadoxine (2 cases, control), and sulfafurazole (2 cases, control). 2 Includes meloxicam (2 cases, 2 controls), piroxicam (6 cases, 4 controls), and tenoxicam (3 cases, 1 control). 3 Includes diclofenac (21 cases, 17 controls), indomethacin (1 control, 2 cases), lonazolac (2 cases, control), etodolac (1 case, 1 control), aceclofenac (1 control, case), sulindac (1 control, case), and ketorolac ( case, 1 control). 4 Includes azithromycin (3 cases, 1 control), clarithromycin (4 cases, 5 controls), and erythromycin (3 cases, control), midecamycin ( case, 1 control), pristinamycin (1 case, control), roxithromycin (4 cases, 2 controls), and spiramycin (3 cases, 1 control). 5 Includes ciprofloxacin (6 cases, 2 controls), grepafloxacin (1 case, control), levofloxacin (1 case, control), norfloxacin (4 cases, 2 controls), and ofloxacin (1 case, 1 controls). 6 Includes cefaclor ( case, 2 controls), cefalexin (3 cases, control), cefapirin (1 case, control), cefatrizine (2 cases, control), cefixime (3 cases, 2 controls), cefonicide (1 case, 2 controls), cefotiam (2 cases, control), cefpodoxime ( case, 2 controls), ceftibutem ( case, 1 control), ceftriaxone (3 cases, control), and cefuroxime (5 cases, control). 7 Includes doxycycline (3 cases, 5 controls), metacycline (1 case, control), and minocycline (3 cases, control). 8 Includes amoxicillin (17 cases, 18 controls) and bacampicillin (1 case, control). n.d. means not done, because of o3 cases or controls. 38 Journal of Investigative Dermatology (28), Volume 128
5 Table 3. Evaluation of SCAR risk for drugs with high potential for confounding by indication Drug Duration of use Case patients, n=379 (%) Control patients, n=1,55 (%) Univariate RR Multivariate RR (%) with use of other highly suspected drugs started within 8 weeks Acetaminophen (paracetamol) 88 (23.2) 216 (14.4) 1.8 ( ) 1.9 ( ) 35 (4%) Pyrazolones 1 18 (4.8) 15 (.9) 5. ( ) 3.1 ( ) 8 (44%) Acetylsalicylic acid 46 (12.1) 97 (6.5) 2. ( ) 1.6 (.9 2.7) 21 (46%) Tramadol 1 (2.6) 2 (.1) 2 (4.4 93) n.d. 5 (5%) p8 weeks (3.5 23) 4 (57%) 48 weeks ( ) 1 (33%) Nimesulide 5 (1.3) 3 (.2) 6.7 (1.6 28) 6.(1. 38) 1 (2%) p8 weeks (.8 2) 5. (.7 36) 1 (33%) 48 weeks n.d. Ibuprofen 1 (2.6) 24 (1.6) 1.7 (.8 3.5).9 (.3 2.6) 5 (5%) Corticosteroids 56 (14.8) 31(2.1) 8.2 (5.2 13) 4.5 ( ) 31 (55%) p8 weeks (12 58) 17 (6.3 46) 26 (63%) 48 weeks ( ) 1. (.4 2.5) 5 (33%) Dexamethasone 25 (6.6) 1 (.1) 16 (14 786) n.d. 19 (76%) p8 weeks (84%) 48 weeks (2.9 21) 3 (5%) Prednisone group 2 29 (7.7) 24 (1.6) 5.1 ( ) 3.9 ( ) 1 (34%) p8 weeks (6.2 45) 16 (5. 51) 8 (4%) 48 weeks (.9 4.2) 1. (.3 2.9) 2 (22%) CI, confidence interval; RR, relative risk; SCAR, severe cutaneous adverse reaction. 1 Includes metamizole (12 cases, 9 controls), propyphenazone (4 cases, 5 controls), and phenazone (2 cases, 1 control). 2 Includes prednisone (19 cases, 15 controls), prednisolone (6 cases, 4 controls), and methylprednisolone (5 cases, 5 controls); one case is exposed to both, prednisone and prednisolone. n.d. means not done, because of o3 cases or controls. among cases of SJS or TEN was the same in this study as that observed a decade ago (Roujeau et al., 1995), only the causative drugs changed. With the decreasing incidence of opportunistic infections and large use of anti-retroviral therapy, nevirapine replaced anti-infective sulfonamides as the leading cause of severe skin reactions in HIV-infected patients. Second, the AED lamotrigine was associated with a high risk for SJS and TEN. Less frequently used than other AEDs, lamotrigine has indications restricted to certain disorders and age groups. This appears in the prevalence of exposure to various AEDs among controls. However, the overall use of lamotrigine increased substantially between 1997 and 21, leading to a higher amount of incident users compared with other AEDs known to induce SCAR (Mockenhaupt et al., 25). Because of insufficient statistical power, we were not able to determine whether the risk linked to lamotrigine was different from that of carbamazepine, phenytoin, or phenobarbital. Further surveillance of this drug is clearly needed, especially if larger populations are likely to use this medication for other diseases. A dose dependency in relation to the occurrence of SCAR has been suggested for lamotrigine (Sachs et al., 1996) and nevirapine (Barreiro et al., 2), with recommendation of lead-in periods, which might reduce the risk of skin reactions. For both drugs, recommendations for dose escalation were followed in most cases included in our study, suggesting that a slow titration neither abrogated the risk nor had an effect on the severity of SCAR. Sertraline, a selective serotonin re-uptake inhibitor introduced within the past 1 years for the treatment of depression, has been suspected of inducing SCAR in case reports (Jan et al., 1999). In our study with six exposed cases 39
6 a Highly associated drugs: Carbamazepine b Highly associated drugs: Nevirapine c Non-associated drugs: ACE inhibitors d Non-associated drugs: Valproic acid e Drugs with doubtful association: Acetaminophen f Drugs with doubtful association: Corticosteroids Figure 1. Distribution of the number of days between beginning of drug use (drug initiation) and onset of the adverse reaction for selected drugs. (a and b) Highly associated drugs, (c and d) non-associated drugs, and (e and f) drugs with doubtful association. Dashed lines indicate cases with co-medication with another highly suspected drug initiated within less than 8 weeks before the onset of the reaction. Table 4. Examples of drugs of common use probably not associated with SCAR Drug Duration of use Case patients, n=379 (%) Control patients, n=1,55 (%) Univariate RR Multivariate RR (%) with use of other highly suspected drugs started within 8 weeks Beta-blockers 37 (9.8) 122 (8.1) 1.2 (.8 1.8).9 (.5 1.5) 19 (51%) ACE inhibitors 44 (11.6) 12 (8.) 1.5 ( ).9 (.5 1.5) 23 (52%) Calcium channel blockers 45 (11.9) 14 (6.9) 1.8 ( ) 1.4 (.8 2.4) 24 (54%) Thiazide diuretics 26 (6.9) 8 (5.3) 1.3 (.8 2.1).7 (.4 1.4) 17 (65%) Furosemide 41 (1.8) 49 (3.3) 3.6 ( ) 1.8 (.9 3.4) 24 (59%) Propionic acid NSAIDs 16 (4.2) 35 (2.3) 1.9 (1. 3.4) 1.5 (.6 3.4) 8 (5%) Sulfonylurea antidiabetics 11 (2.9) 35 (2.3) 1.3 (.6 2.5).8 (.3 2.4) 5 (45%) Insulin 1 (2.6) 22 (1.5) 1.8 (.9 3.9) 1. (.3 3.3) 6 (6%) CI, confidence interval; NSAID, non-steroidal anti-inflammatory drug; RR, relative risk; SCAR, severe cutaneous adverse reaction. and five controls, we found an increased mvrr of 11 (2.7 46) and a timing pattern compatible with a risk. Due to small numbers, the statistical power is rather low. Nevertheless, this drug needs to be closely monitored. In the same class of antidepressants, fluoxetine has also been suspected (Bodokh et al., 1992), but there was no evidence of risk in our study, neither for other selective serotonin re-uptake inhibitors. 4 Journal of Investigative Dermatology (28), Volume 128
7 Pantoprazole, a proton pump inhibitor not previously suspected to induce SCAR, was associated with a high univariate risk. In the absence of multivariate analysis, we cannot discard a risk, but the frequent co-medication with a highly suspected drug and a non-convincing timing are not suggestive of a risk. Terbinafine and fluconazole, two antifungals of different chemical structure, have been reported to induce SCAR (Gussenhoven et al., 1991; Rzany et al., 1994). Due to limited exposure among cases and controls in our study, we were unable to provide accurate risk estimates, but the very low number of exposed cases does not suggest a major public health problem. Among classes of recently marketed drugs widely used, anti-hyperlipidemia statins were not associated with an increased risk. Also for dozens of drugs with common use, there was neither evidence of an elevated risk for SJS or TEN in the present EuroSCAR-study, nor in the prior SCAR-study. Interestingly, none of the sulfonamide-related diuretic or antidiabetic agents appeared to be associated with a significant risk for SCAR. Case control studies do certainly have their limitations and the choice of appropriate controls is crucial for their validity. Conceptually, controls should represent the study base from which cases are drawn, and should be similar to the cases in all relevant aspects. A posteriori, we made several quality checks on the control population included in EuroSCAR. For the following diseases the prevalence in our control group was similar to that expected (according to known prevalence) in a group of the same age and gender distribution: diabetes mellitus, asthma, psoriasis, and rheumatism. We also checked the rate of exposure to several antibiotics. We found the disparity in the level of use among controls from different countries that was expected from published prescription data (Cars et al., 21). Hospital controls did not differ from the normal population on the criteria that were analyzed. In addition, the appropriate multivariate model is of major importance for the robustness of the analysis and the validity of results. In the population of 379 cases, we never used more than a dozen covariables (i.e., age, gender, country, recent cancer (yes or no), HIV infection (yes or no), collagen vascular disease (yes or no), exposure to any highly suspected drug (yes or no), exposure to a low risk drug (yes or no). When we looked at specific drugs, the robustness of the model was highly variable. For drugs of common use, there were enough cases and controls exposed to guarantee the robustness of the multivariate analysis. For most highly suspected drugs, exposure among controls was found rarely and the multivariate analysis was not performed, if less than three controls were exposed. Although the point estimates of univariate risks were high enough to show unquestionable associations, we provided information on the proportion of cases also exposed to highly suspected drugs. When this percentage is low (e.g., less than 15%), it can be assumed that multivariate analysis would not substantially change the lower bound of the RRs. Actually, it seems reasonable to believe that it can be approximated by the univariate lower confidence interval bound estimated among the patients not exposed to highly suspected drugs (more than 85% of the cases and almost 1% of the controls). Looking at the duration of treatment for highly suspected drugs, we found that the risk was mostly confined to recent start of drug use. The duration of treatment is of dramatic relevance for drugs used for long-term medication such as allopurinol, AEDs, or NSAIDs. For allopurinol the numbers were large enough to provide estimates of RRs for recent and long-term use, showing that an overall risk of 18 was a combination of a non-significant association in long-term use and an RR of 261 (36 infinite) in recent use. More precise evaluation of time relationship strongly suggests that SJS and TEN most often begin more than four and up to 28 days after the initiation of the responsible medication. We advise to integrate these figures in the algorithms used for assessing the causality of medications in individual cases. Some drug classes deserve special comments because of controversies on drug-related or class-related risk, or because of high suspicion of an association with highly suspected drugs. Concerning AEDs, the RR for valproic acid was remarkably reduced after multivariate adjustment and statistical significance was lost. Furthermore, the timing of exposure to valproic acid did not fit with the pattern observed for highly suspected drugs and rather reflected that of non-associated drugs. We believe that associations reported previously (Roujeau et al., 1995; Rzany et al., 1999) had been confounded by co-medication with other AEDs. The results of this study suggest that valproic acid should not be considered a highly suspected drug to induce SJS and TEN. Several antibiotics (non-sulfonamide anti-infectives) and acetic acid NSAIDs such as diclofenac, which is widely used in Europe, were found to be associated with significant but lower risks for SJS and TEN. Concerning NSAIDs there were variable levels of risks. As already suspected, oxicam derivatives were associated with a high risk and acetic acid derivatives with a lower risk for SCAR (Mockenhaupt et al., 23), whereas for propionic acid derivatives including ibuprofen we did not find a significant risk. Other anti-rheumatic agents like cyclooxygenase 2 inhibitors and leflunomide were introduced soon before the end of our study. We did not obtain enough data to provide risk estimates. Only rofecoxib (VIOXX s ) and celecoxib (Celebrex s ) had been used by large numbers of persons. The observation of a single case indirectly suggests that these two agents were probably not associated with a very high risk for SCAR. However, further surveillance of cyclooxygenase 2 inhibitors is necessary. For analgesics and antipyretics, this study confirmed the huge disparity of exposure rates in different countries. Multivariate analyses showed a weak association of acetaminophen (paracetamol), which did not vary significantly between countries, probably due to the lack of power to detect a difference. Patients often took an analgesic or 41
8 antipyretic agent to treat unspecific symptoms such as fever or pain, which might be early signs of the adverse reaction or indicate an infection. In order to better control for confounding by indication, we restricted the analysis to patients who took that drug at least 4 days before the onset of the adverse reaction (index-day). The previously observed association disappeared, suggesting a high potential for confounding by indication, since SJS and TEN often begin with pain or fever, which cannot always be retained as a marker of the indexday in the absence of more specific signs. Therefore, we doubt any causal relationship. For similar reasons we also doubt causal association for pyrazolone analgesics, tramadol, and nimesulide. This study confirms that a rather high proportion of SJS and TEN occurred in patients taking systemic steroids, especially dexamethasone, often in the context of brain tumor and comedication with AEDs. Even though the mvrr was not very high, the risk persisted when analyses were restricted to cases without co-medication with highly suspected drugs. The time latency between beginning of use and onset of the reaction is also compatible with a risk. Furthermore, we looked for potential confounding in terms of steroid use. Thorough checks of indications for steroid intake revealed that they were not used for the treatment of prodromal symptoms of SJS and TEN. Because of small numbers, we were not able to address the question of a possible interaction between dexamethasone and AEDs. We cannot conclude whether corticosteroids are a direct cause of SJS or TEN, a risk factor by modifying the immune response, or a confounder. In addition to demonstrating that nevirapine and lamotrigine are associated with very high risks, and triggering some alerts for a few other recently marketed drugs, our study documents the persistence of many cases of SJS and TEN related to old drugs with a known high risk. We observed some differences in exposure to these drugs, that is, absence of cases related to chlormezanone, decreases in cases exposed to phenobarbital and oxicam-nsaids, and a higher percentage of cases taking allopurinol. These variations probably reflect changing population exposure to drugs in relation to interventions from regulatory agencies, evolving markets, or prescription habits. Nevertheless, the total numbers of cases attributable to these highly suspected drugs remained high. The use of such drugs as first-line therapies should be considered carefully, especially when safer alternative treatments exist. The absolute risk has been estimated in previous studies by calculating excess risks based on the RR, the etiologic fraction (also based on RR), and the incidence of SJS and TEN (Roujeau et al., 1995). Thus, the estimation of the excess risk is depending to a large extent on RR estimates, which we could not calculate for a number of highly suspected drugs. We decided not to use the median unbiased estimate, which was done in previous studies, because this method is prone to criticism. Since SJS and TEN are rare conditions, the absolute risks remain low even for users of drugs with the highest RR (1/1, to 1/1,). The results of this study, however, could help regulatory agencies, drug companies and other specialists in the field to prepare recommendations for the Table 5. Practical recommendations and take-home messages for physicians A few medications are associated with high risks of SJS or TEN. Prescribing one of them requires thorough evaluation of expected benefits. Nevirapine Lamotrigine Carbamazepine Phenytoin Phenobarbital Cotrimoxazole and other anti-infective sulfonamides Sulfasalazine Allopurinol Oxicam-NSAIDs A delay of 4 28 days between beginning of drug use and onset of the adverse reaction is the most suggestive timing supporting drug causality in SJS or TEN. In cases of exposure to several medications with high expected benefits, the timing of administration is important to determine which one(s) must be stopped and if some may be continued or re-introduced. The risks of various antibiotics to induce SJS and TEN are within the same order of magnitude, but substantially lower than the risk of anti-infective sulfonamides. Valproic acid does not seem to be a major risk factor by itself. Sulfonamide-related diuretics and antidiabetics do not appear to be risk factors. NSAID, non-steroidal anti-inflammatory drug; SJS, Stevens Johnson syndrome; TEN, toxic epidermal necrolysis. use of highly suspected drugs integrating both effectiveness and adverse effects of alternative therapies. Several practical recommendations and take-home messages for physicians can be derived from our results. They are summarized in Table 5. MATERIALS AND METHODS Basically the methods were identical to those of prior epidemiological studies of rare and severe drug reactions (Slone et al., 1977; Kelly et al., 1995; Roujeau et al., 1995). Design and data collection EuroSCAR was conducted in six countries (Austria, France, Germany, Israel, Italy, and The Netherlands) between April 1997 and December 21. Cases were actively detected in a network of about 1,8 hospitals covering more than 1 million inhabitants. Cases were patients admitted to hospital with a diagnosis of SJS or TEN (i.e., the reaction occurred in the community), whereas patients developing SCAR during hospitalization for another condition were not included. For each case, three controls were matched on age, gender, region, and date of interview, among patients hospitalized for acute conditions including infections (e.g., pneumonia), trauma (e.g., fractures), and abdominal emergencies (e.g., appendicitis, ruptured ovarian cyst, strangulated hernia) that have not occurred as a complication of an underlying chronic disease. To maximize recall, controls could not be enrolled if, at the time of interview, the hospital stay had exceeded 2 weeks. If sufficient controls with the 42 Journal of Investigative Dermatology (28), Volume 128
9 preferred diagnoses were not available, alternative non-acute conditions judged to have been unrelated to prior drug use were selected (e.g., hernia, hallux valgus, deviated nasal septum). Thus, we enrolled controls as representative as possible of the same population as cases (Kelly et al., 1995). After obtaining informed consent, trained investigators interviewed cases and controls, using a structured questionnaire to collect clinical data, medical history, and drug exposure. The study has been approved by the ethical committee and institutional review board of each center participating in the EuroSCAR-project in the 6 countries. Validation of cases and controls A group of experts with no information on exposures reviewed all collected cases using clinical data, available photographs (93% of the cases), and histopathology (75% of the cases), and determined the date of onset of the disease (index-day). Controls were also checked for eligibility and date of onset of their acute condition. Data quality Questionnaires were reviewed for internal consistency with automated checks and random comparisons with data forms. Check for homogeneity between data collected by different investigators revealed several abnormalities in the characteristics of controls interviewed by one investigator. Inquiry revealed inadequate work. The full set of controls from this investigation was withdrawn. Analyses Whenever a patient started a drug, he was considered exposed only if this drug was still taken in a window of 7 days preceding the index-day. For drugs with long elimination half-lives, the exposure window was extended to 14 days (oxicam-nsaids, allopurinol) or 21 days (phenobarbital). First, an unmatched analysis used data from all validated cases and controls, including controls for potential cases not validated (379 cases, 1,55 controls); second, a matched analysis was restricted to cases with matched controls (326 cases, 838 controls). Due to high consistency between the two analyses, we present only the results that derived from the whole data set. However, results of the matched analysis are available online. uvrrs and their 95% confidence interval were estimated in terms of odds ratios with standard methods (Slone et al., 1977; Kelly et al., 1995). When there was no exposed control or case, exact methods were used to estimate 95% confidence limits (StatXact-5 s, Cytel software). In that case, only 95% confidence interval limits are reported. mvrrs were estimated with unconditional logistic regression only when at least three cases and controls were exposed to the risk factor considered. Adjustment was performed on country, gender, age, exposure to any highly suspected drug (allopurinol, anti-infective sulfonamides, carbamazepine, lamotrigine, nevirapine, oxicam-nsaids, phenobarbital, phenytoin), any other suspected drug (aminopenicillins, tetracyclines, quinolones, cephalosporins, macrolides, diclofenac and related NSAIDs, corticosteroids, acetaminophen, pyrazolones, acetylsalicylic acid), any other drug, and any suspected non-drug confounding risk factor (infection with HIV, other infections, recent cancer, recent radiotherapy, collagen vascular disease). We also present in tables the number of cases concomitantly exposed to a recently introduced different highly suspected drug. Further ways of risk evaluation, when calculation of mvrr was not feasible Two criteria were evaluated as a possible help: (1) frequency of concomitant exposure to a highly suspected drug, (2) delay between beginning of drug use and onset of the adverse reaction. Both were explored in two subsets of drugs, those with a strong and confirmed high risk and those with a confirmed absence of a detectable association. In less than 2% of the cases, high-risk medications were concomitantly used with another highly suspected drug. In contrast, 5 6% of case patients taking drugs without significant risk had been exposed to at least one highly suspected drug in parallel. The time pattern of exposure was also very different. For highly suspected drugs, beginning of exposure aggregated between 32 and 4 days before onset of the adverse reaction, when there was no similar aggregation for non-associated drugs. This time relationship is exemplified in Figure 1 showing examples of different time latency between beginning of drug use and onset of the adverse reaction for drugs with a definite association (1a and b), non-associated drugs (1c and d), and medications with doubtful association (1e and f). We, therefore, included these two criteria in our evaluation, when a significant univariate risk could not be validated by multivariate analysis because of lack of controls, and also when we had a strong suspicion of confounding by indication, that is, for medications often prescribed for early unspecific symptoms of the reaction. CONFLICT OF INTEREST The authors state no conflict of interest. ACKNOWLEDGMENTS We are indebted to all the patients whose participation made this study possible. In addition, we thank all the collaborating hospitals and colleagues for their enthusiastic support. We also thank the following institutions/companies for funding the project (unrestricted grants): ADIR & Cie, Bayer Pharma/AG/Vital, Boehringer Ingelheim, Cassenne, Ciba Geigy/Novartis, Cilag GmbH, Dr Willmar Schwabe, Goedecke Parke Davis, Glaxo Wellcome/GlaxoSmithKline, Hoechst AG/Hoechst Marion Roussel/Aventis, Hoffmann-La-Roche, IRIS Servier, Jouveinal Lab., LEO, LILLY, MSD Sharp & Dohme, Pfizer, Rhone Poulenc Rorer, Sanofi Winthrop/Sanofi Synthelabo GmbH, Schering AG; Funding from Pharmaceutical Companies in France were managed through contract with INSERM (Institut National de la Santé et de la Recherche Médicale), French Ministry of Health (PHRC AOM 9827). Also participated in Austria: Elisabeth Kowald, Dennis Linder, and Gudrun Ratzinger. France: Fabienne Bazin, Hélène Bocquet, Laure Dangoumaud, Yves Dorleans, Alain Dupuy, Jean-Paul Fagot, Catherine Paoletti, Annie-Claude Paty, Sylvie Rodriguez, Bruno Sassolas, Patricia Thion, and Loïc Vaillant. Germany: Konrad Bork, Uwe-Frithjof Haustein, Olaf Hering, Klaus Kitta, Brigitte Loleit, Oliver Pfeiffer, Thekla Renker, Birgit Schneck, Werner Schröder, Esra Tas, and Dieter Vieluf. Israel: Arnon D. Cohen and Sara Weltfreund. Italy: Laura Atzori, Grazia Manfredi, Davide Melandri, and Annalisa Pinna. The Netherlands: Christianne Bearda Bakker-Wensveen, Joost Govaert, and Viña Williams-Snijders. SUPPLEMENTARY MATERIAL Table S1. Matched analyses. Table S2. Alerts. Table S3. Drugs used by at least 1.5% of controls (multivariate, unmatched analysis). 43
10 REFERENCES Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schröder W, Roujeau JC (22) Correlations between clinical patterns and causes of erythema multiforme majus, Stevens Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol 138: Barreiro P, Soriano V, Casas E, Estrada V, Tellez M, Hoetelmans R et al. (2) Prevention of Nevirapine-associated exanthema using slow dose escalation and/or corticosteroids. AIDS 14: Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau J-C (1993) A clinical classification of cases of toxic epidermal necrolysis, Stevens - Johnson syndrome and erythema multiforme. Arch Dermatol 129:92 6 Becker DS (1998) Toxic epidermal necrolysis. Lancet 351: Bodokh I, Lacour JP, Rosenthal E, Chichmanian RM, Perrin C, Vitetta A et al. (1992) Lyell syndrome or toxic epidermal necrolysis and Stevens Johnson syndrome after treatment with fluoxetine. Therapie 47:441 Cars O, Mostad S, Melander A (21) Variation in antibiotic use in the European Union. Lancet 357: Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, Naldi L, Viboud C, Roujeau J-C (21) Nevirapine and the risk of Stevens Johnson syndrome or toxic epidermal necrolysis: preliminary results of a case-control study. AIDS 15:1 6 Fischer TW, Bauer HI, Graefe T, Barta U, Elsner P (23) Erythema multiforme-like drug eruption with oral involvement after intake of leflunomide. Dermatology 27:386 9 Friedman B, Orlet HK, Still JM, Law E (23) Toxic epidermal necrolysis due to administration of celecoxib (Celebrex). South Med J 96: (comment in: South Med J. 96:32 321). GISED (1993) Cutaneous reactions to analgesic-antipyretics and non-steroidal anti-inflammatory drugs. Analysis of reports to the spontaneous reporting system of the Gruppo Italiano Studi Epidemiologici in Dermatologia. Dermatology 186:164 9 Gussenhoven MJE, Haak A, Peereboom-Wynia JDR (1991) Stevens Johnson syndrome after fluconazole. Lancet 338:12 Jan V, Toledano C, Machet L, Machet MC, Vaillant L, Lorette G (1999) Stevens Johnson syndrome after sertraline. Acta Derm Venereol 79:41 Kelly JP, Auquier A, Rzany B, Naldi L, Bastuji-Garin S, Correia O et al. (1995) An international collaborative case control study of severe cutaneous adverse reactions (SCAR). Design and methods. J Clin Epidemiol 48: Mockenhaupt M, Kelly JP, Kaufman D, Stern RS (23) The risk of Stevens Johnson syndrome and toxic epidermal necrolysis associated with non-steroidal antiinflammatory drugs: a multinational perspective. J Rheumatol 3: Mockenhaupt M, Messenheimer J, Schlingmann J, Tennis P (25) Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of anti-epileptics. Neurology 64: Pfeiffer CM, Kazenoff S, Rothberg HD (1998) Toxic epidermal necrolysis from atorvastatin. JAMA 279: Roujeau J-C, Stern RS (1994) Severe cutaneous adverse reactions to drugs. N Engl J Med 331: Roujeau J-C, Kelly JP, Naldi L, Rzany B, Stern S, Anderson T et al. (1995) Medication use and the risk of Stevens Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 333:16 7 Rzany B, Correia O, Kelly JP, Naldi L, Auquier A, Stern R (1999) Risk of Stevens Johnson syndrome and toxic epidermal necrolysis during first weeks of anti-epileptic therapy: a case control study. Lancet 353:219 4 Rzany B, Mockenhaupt M, Baur S, Schröder W, Stocker U, Mueller J et al. (1996) Epidemiology of erythema exsudativum multiforme majus (EEMM), Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in Germany ( ). Structure and results of a population based registry. J Clin Epidemiol 49: Rzany B, Mockenhaupt M, Gehring W, Schöpf E (1994) Stevens Johnson syndrome after terbinafine therapy. J Am Acad Dermatol 3:59 Sachs B, Ronnau AC, Ruzicka T, Gleichmann E, Schuppe HC (1996) Lamotrigine and toxic epidermal necrolysis. Lancet 348:141 Slone D, Shapiro S, Miettinen O (1977) Case control surveillance of serious illnesses attributable to ambulatory use of drugs. In: Epidemiological Evaluation of Drugs. (Colombo F, Slone D, Shapiro S, Tognoni G, eds) Amsterdam: Elsevier North Holland Biomedical Press Warren KJ, Boxwell DE, Kim NY, Drolet BA (1998) Nevirapine-associated Stevens Johnson syndrome. Lancet 351: Journal of Investigative Dermatology (28), Volume 128
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics
 Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics Maja Mockenhaupt, MD; John Messenheimer, MD; Pat Tennis, PhD; and Juergen Schlingmann Abstract Background:
Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics Maja Mockenhaupt, MD; John Messenheimer, MD; Pat Tennis, PhD; and Juergen Schlingmann Abstract Background:
1 Introduction. Kenneth Irungu 1 David Nyamu
 Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital
 International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC
 SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW
 PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
 Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients
 original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME
 EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Severe cutaneous adverse reactions (SCAR) are life-threatening
 Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
The Drug Etiology of Agranulocytosis and Aplastic Anemia
 Monographs in Epidemiology and Biostatistics Volume 18 The Drug Etiology of Agranulocytosis and Aplastic Anemia David W. Kaufman Judith P. Kelly Micha Levy Samuel Shapiro with contributions by Hermann
Monographs in Epidemiology and Biostatistics Volume 18 The Drug Etiology of Agranulocytosis and Aplastic Anemia David W. Kaufman Judith P. Kelly Micha Levy Samuel Shapiro with contributions by Hermann
Pediatric Dermatology
 Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
The liver and skin are two of the commonest
 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
Original Article. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C
 Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015)
 1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
Department of Pharmacology & NIPER-Guwahati, Gauhati Medical College & Hospital, Guwahati , Assam, INDIA. 3
 J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
Your letter Your reference The Hague, Subject Request for change in the product information following the PhVWP/CMDh decision
 Registratiehouder ( t.a.v. registratieafdeling) Your letter Your reference The Hague, -- --.. Case number Our reference../../ Case manager Telephone number Subject Request for change in the product information
Registratiehouder ( t.a.v. registratieafdeling) Your letter Your reference The Hague, -- --.. Case number Our reference../../ Case manager Telephone number Subject Request for change in the product information
STEVENS-JOHNSON SYNDROME: A CASE REPORT
 STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
Supplementary appendix: Additional material. Figure S1. Flow-chart of inclusion/exclusion criteria.
 Supplementary appendix: Additional material Figure S1. Flow-chart of inclusion/exclusion criteria. 1 Table S1. Codes considered to identify heart failure patients by the included databases. Coding system
Supplementary appendix: Additional material Figure S1. Flow-chart of inclusion/exclusion criteria. 1 Table S1. Codes considered to identify heart failure patients by the included databases. Coding system
Supplementary Online Content
 Supplementary Online Content Renoux C, Vahey S, Dell Aniello S, Boivin J-F. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. Published
Supplementary Online Content Renoux C, Vahey S, Dell Aniello S, Boivin J-F. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. Published
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
University of Groningen
 University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Who remembers Terfenadine? Once daily non-sedating anti-histamine. Drug Interactions: What is the CYP 450 system? What does the CYP 450 system do?
 Drug Interactions: Things that go BOOM! Who remembers Terfenadine? Once daily non-sedating anti-histamine Amelie Hollier, DNP, FNP-BC, FAANP Advanced Practice Education Associates A strange thing happened
Drug Interactions: Things that go BOOM! Who remembers Terfenadine? Once daily non-sedating anti-histamine Amelie Hollier, DNP, FNP-BC, FAANP Advanced Practice Education Associates A strange thing happened
Erythema Multiforme with Reference to Atypical Presentation in an HIV-Positive Patient Following Antiretroviral Therapy Discontinuation
 2009;17(1):9-15 CLINICAL ARTICLE Erythema Multiforme with Reference to Atypical Presentation in an HIV-Positive Patient Following Antiretroviral Therapy Discontinuation Liborija Lugović Mihić, Marija Buljan,
2009;17(1):9-15 CLINICAL ARTICLE Erythema Multiforme with Reference to Atypical Presentation in an HIV-Positive Patient Following Antiretroviral Therapy Discontinuation Liborija Lugović Mihić, Marija Buljan,
Drug Allergy A Guide to Diagnosis and Management
 Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Address for correspondence: Dr. Patel Raksha M. R-3, Doctor s Quarters, Jail Road, Vadodara- 01, Gujarat, India.
 Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN
 TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN Nadia Khaldi 1, Alain Miras 1, Sophie Gromb 1,2 1 Forensic Science Laboratory, Pellegrin Hospital, University Teaching Hospital Bordeaux, France, 2 Forensic
TOXIC EPIDERMAL NECROLYSIS AND CLARITHROMYCIN Nadia Khaldi 1, Alain Miras 1, Sophie Gromb 1,2 1 Forensic Science Laboratory, Pellegrin Hospital, University Teaching Hospital Bordeaux, France, 2 Forensic
Drug Class Review on Cyclo-oxygenase (COX)-2 Inhibitors and Non-steroidal Anti-inflammatory Drugs (NSAIDs)
 Drug Class Review on Cyclo-oxygenase (COX)-2 Inhibitors and Non-steroidal Anti-inflammatory Drugs (NSAIDs) Final Report Update 3 Evidence Tables November 2006 Original Report Date: May 2002 Update 1 Report
Drug Class Review on Cyclo-oxygenase (COX)-2 Inhibitors and Non-steroidal Anti-inflammatory Drugs (NSAIDs) Final Report Update 3 Evidence Tables November 2006 Original Report Date: May 2002 Update 1 Report
MUSCULOSKELETAL PHARMACOLOGY. A story of the inflamed
 MUSCULOSKELETAL PHARMACOLOGY A story of the inflamed 1 INFLAMMATION Pathophysiology Inflammation Reaction to tissue injury Caused by release of chemical mediators Leads to a vascular response Fluid and
MUSCULOSKELETAL PHARMACOLOGY A story of the inflamed 1 INFLAMMATION Pathophysiology Inflammation Reaction to tissue injury Caused by release of chemical mediators Leads to a vascular response Fluid and
Characteristics of selective and non-selective NSAID use in Scotland
 Characteristics of selective and non-selective NSAID use in Scotland Alford KMG 1, Simpson C 1, Williams D 2 1 Department of General Practice & Primary Care, The University of Aberdeen. 2 Department of
Characteristics of selective and non-selective NSAID use in Scotland Alford KMG 1, Simpson C 1, Williams D 2 1 Department of General Practice & Primary Care, The University of Aberdeen. 2 Department of
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS
 PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
QIPP Prescribing Comparators: Description and Specification
 Prescribing s: Description and Specification This document details the descriptions and specifications for the prescribing comparators. They should be read in conjunction with the National Prescribing
Prescribing s: Description and Specification This document details the descriptions and specifications for the prescribing comparators. They should be read in conjunction with the National Prescribing
HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review
 HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
TOXIC epidermal necrolysis and Stevens Johnson
 6 THE NEW ENGLAND JOURNAL OF MEDICINE Dec. 4, 995 MEDICATION USE AND THE RISK OF STEVENS JOHNSON SYNDROME OR TOXIC EPIDERMAL NECROLYSIS JEAN-CLAUDE ROUJEAU, M.D., JUDITH P. KELLY, M.S., LUIGI NALDI, M.D.,
6 THE NEW ENGLAND JOURNAL OF MEDICINE Dec. 4, 995 MEDICATION USE AND THE RISK OF STEVENS JOHNSON SYNDROME OR TOXIC EPIDERMAL NECROLYSIS JEAN-CLAUDE ROUJEAU, M.D., JUDITH P. KELLY, M.S., LUIGI NALDI, M.D.,
Endocarditis. By : Mehrnoush. dianatkhah
 Endocarditis By : Mehrnoush. dianatkhah Case 5.31, 31 years old woman CC : Fever, dyspnea, 3 days postpartum PMH : Mitral prolapse Fever 38.5 WBC : 8900 ESR : 84 CRP : 10.4 Cr : 0.6 NT Pro BNP: 5469 Physical
Endocarditis By : Mehrnoush. dianatkhah Case 5.31, 31 years old woman CC : Fever, dyspnea, 3 days postpartum PMH : Mitral prolapse Fever 38.5 WBC : 8900 ESR : 84 CRP : 10.4 Cr : 0.6 NT Pro BNP: 5469 Physical
Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis.
 Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Supplementary online-material Supplementary Table S1: Detailed descriptive analysis
Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Supplementary online-material Supplementary Table S1: Detailed descriptive analysis
Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
 Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
STUDY. High Risk of Death in Elderly Patients With Extensive Bullous Pemphigoid
 STUDY High Risk of Death in Elderly Patients With Extensive Bullous Pemphigoid Jean-Claude Roujeau, MD; Catherine Lok, MD; Sylvie Bastuji-Garin, MD; Sami Mhalla, MD; Véronique Enginger, MD; Philippe Bernard,
STUDY High Risk of Death in Elderly Patients With Extensive Bullous Pemphigoid Jean-Claude Roujeau, MD; Catherine Lok, MD; Sylvie Bastuji-Garin, MD; Sami Mhalla, MD; Véronique Enginger, MD; Philippe Bernard,
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
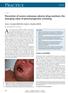 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
New product information wording Extracts from PRAC recommendations on signals
 12 October 2017 EMA/PRAC/610988/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 25-29 September 2017
12 October 2017 EMA/PRAC/610988/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 25-29 September 2017
ANTI COLD / ANTI ALLERGIC / ANTI-ASTHMATICS GIT PRODUCTS
 SR. NO 1 ANTI COLD / ANTI ALLERGIC / ANTI-ASTHMATICS Paracetamol 500 mg, Phenylephrine HCL 5 mg With Chlorpheniramine Maleate 2 mg & Caffeine 30 mg Tablets 2 Salbutamol Tablets BP 2 mg 3 Salbutamol Tablets
SR. NO 1 ANTI COLD / ANTI ALLERGIC / ANTI-ASTHMATICS Paracetamol 500 mg, Phenylephrine HCL 5 mg With Chlorpheniramine Maleate 2 mg & Caffeine 30 mg Tablets 2 Salbutamol Tablets BP 2 mg 3 Salbutamol Tablets
Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital
 Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
TRANSPARENCY COMMITTEE OPINION. 1 April 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 1 April 2009 CYCLADOL 20 mg, scored tablet Box of 14 (CIP: 336 095-7) CYCLADOL 20 mg, effervescent tablet Box of 14
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 1 April 2009 CYCLADOL 20 mg, scored tablet Box of 14 (CIP: 336 095-7) CYCLADOL 20 mg, effervescent tablet Box of 14
Emergency Dermatology Dr Melissa Barkham
 Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
cipro loxacin; stevens-johnson syndrome; urinary tract infection; adverse drug reaction
 Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
NSAIDs: Side Effects and Guidelines
 NSAIDs: Side Effects and James J Hale FY1 Department of Anaesthetics Introduction The non-steroidal anti-inflammatory drugs (NSAIDs) are a diverse group of drugs that have analgesic, antipyretic and anti-inflammatory
NSAIDs: Side Effects and James J Hale FY1 Department of Anaesthetics Introduction The non-steroidal anti-inflammatory drugs (NSAIDs) are a diverse group of drugs that have analgesic, antipyretic and anti-inflammatory
Insights from the Clalit Health Services database about Psoriasis Research using BIG DATA Arnon D. Cohen, MD, MPH, PHD
 Insights from the Clalit Health Services database about Psoriasis Research using BIG DATA Arnon D. Cohen, MD, MPH, PHD Siaal Research Center for Family Medicine and Primary Care, Faculty of Health Sciences,
Insights from the Clalit Health Services database about Psoriasis Research using BIG DATA Arnon D. Cohen, MD, MPH, PHD Siaal Research Center for Family Medicine and Primary Care, Faculty of Health Sciences,
Prescribing of anti-osteoporotic therapies following the use of Proton Pump Inhibitors in general practice
 Prescribing of anti-osteoporotic therapies following the use of Proton Pump Inhibitors in general practice B. McGowan, K. Bennett, J. Feely Department of Pharmacology & Therapeutics, Trinity Centre for
Prescribing of anti-osteoporotic therapies following the use of Proton Pump Inhibitors in general practice B. McGowan, K. Bennett, J. Feely Department of Pharmacology & Therapeutics, Trinity Centre for
Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea
 Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Politerapia e interazioni negli anziani: possibili interventi
 LE ANALISI SULL'USO DEI FARMACI: METODI ED ESPERIENZE IN ITALIA Politerapia e interazioni negli anziani: possibili interventi Graziano Onder Centro Medicina dell Invecchiamento Università Cattolica del
LE ANALISI SULL'USO DEI FARMACI: METODI ED ESPERIENZE IN ITALIA Politerapia e interazioni negli anziani: possibili interventi Graziano Onder Centro Medicina dell Invecchiamento Università Cattolica del
Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs
 European Review for Medical and Pharmacological Sciences Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs L. ORDOÑEZ, E. SALGUEIRO, F.J.
European Review for Medical and Pharmacological Sciences Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs L. ORDOÑEZ, E. SALGUEIRO, F.J.
REGISCAR INTERNATIONAL REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS (SCAR) TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES
 REGISCAR INTERNATIONAL REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS (SCAR) TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES study protocol written by Maja Mockenhaupt, Dokumentationszentrum schwerer autreaktionen
REGISCAR INTERNATIONAL REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS (SCAR) TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES study protocol written by Maja Mockenhaupt, Dokumentationszentrum schwerer autreaktionen
Role of Pharmacoepidemiology in Drug Evaluation
 Role of Pharmacoepidemiology in Drug Evaluation Martin Wong MD, MPH School of Public Health and Primary Care Faculty of Medicine Chinese University of Hog Kong Outline of Content Introduction: what is
Role of Pharmacoepidemiology in Drug Evaluation Martin Wong MD, MPH School of Public Health and Primary Care Faculty of Medicine Chinese University of Hog Kong Outline of Content Introduction: what is
Multiple Drug Hypersensitivity
 Multiple Drug Hypersensitivity Werner J Pichler ADR-AC GmbH Bern UPDATE ON DRUG HYPERSENSITIVITY, Bern, March 23rd 2017 Definition Multiple drug hypersensitivity (MDH) MDH is a syndrome that develops as
Multiple Drug Hypersensitivity Werner J Pichler ADR-AC GmbH Bern UPDATE ON DRUG HYPERSENSITIVITY, Bern, March 23rd 2017 Definition Multiple drug hypersensitivity (MDH) MDH is a syndrome that develops as
BNF. BACKGROUND FOR USE
 797FM.2 ORAL ANTICOAGULANTS WARFARIN, ACENOCOUMAROL AND PHENINDIONE, WHEN DOSES ARE ADJUSTED BY THE ANTICOAGULATION CLINIC AND PRESCRIBED BY THE GENERAL PRACTITIONER (GP) Shared Care Protocol This protocol
797FM.2 ORAL ANTICOAGULANTS WARFARIN, ACENOCOUMAROL AND PHENINDIONE, WHEN DOSES ARE ADJUSTED BY THE ANTICOAGULATION CLINIC AND PRESCRIBED BY THE GENERAL PRACTITIONER (GP) Shared Care Protocol This protocol
A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia
 ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Celebrex) Reference Number: CP.CPA.239 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Celebrex) Reference Number: CP.CPA.239 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Cutaneous drug reactions
 Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Accel-Celecoxib Product Monograph Page 52 of 56
 PART III: CONSUMER INFORMATION Pr ACCEL-CELECOXIB CAPSULES Read this information each time you refill your prescription in case new information has been added. This leaflet is a summary designed specifically
PART III: CONSUMER INFORMATION Pr ACCEL-CELECOXIB CAPSULES Read this information each time you refill your prescription in case new information has been added. This leaflet is a summary designed specifically
A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive Patients
 Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
CARDIOVASCULAR RISK and NSAIDs
 CARDIOVASCULAR RISK and NSAIDs Dr. Syed Ghulam Mogni Mowla Assistant Professor of Medicine Shaheed Suhrawardy Medical College, Dhaka INTRODUCTION NSAIDs are most commonly prescribed drugs Recent evidence
CARDIOVASCULAR RISK and NSAIDs Dr. Syed Ghulam Mogni Mowla Assistant Professor of Medicine Shaheed Suhrawardy Medical College, Dhaka INTRODUCTION NSAIDs are most commonly prescribed drugs Recent evidence
SELECTED ABSTRACTS. Figure. Risk Stratification Matrix A CLINICIAN S GUIDE TO THE SELECTION OF NSAID THERAPY
 SELECTED ABSTRACTS A CLINICIAN S GUIDE TO THE SELECTION OF NSAID THERAPY The authors of this article present a 4-quadrant matrix based on 2 key clinical parameters: risk for adverse gastrointestinal (GI)
SELECTED ABSTRACTS A CLINICIAN S GUIDE TO THE SELECTION OF NSAID THERAPY The authors of this article present a 4-quadrant matrix based on 2 key clinical parameters: risk for adverse gastrointestinal (GI)
Elements for a Public Summary Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
To understand the formulary process from the hospital perspective
 Formulary Process Michael A. Militello, Pharm.D. Cleveland Clinic Cleveland Clinic 2011 Goal and Objectives To understand the formulary process from the hospital perspective p To list the various panels
Formulary Process Michael A. Militello, Pharm.D. Cleveland Clinic Cleveland Clinic 2011 Goal and Objectives To understand the formulary process from the hospital perspective p To list the various panels
50 years of pharmacovigilance: unfinished job
 50 years of pharmacovigilance: unfinished job Joan-Ramon Laporte The role of pharmacoepidemiology in medicines regulation 108 market withdrawals in F, D & UK, 1961-93 Fulminant hepatitis Liver toxicity
50 years of pharmacovigilance: unfinished job Joan-Ramon Laporte The role of pharmacoepidemiology in medicines regulation 108 market withdrawals in F, D & UK, 1961-93 Fulminant hepatitis Liver toxicity
The Case-Population Study Design
 ORIGINAL RESEARCH ARTICLE Drug Saf 2011; 34 (10): 861-868 0114-5916/11/0010-0861/$49.95/0 ª 2011 Adis Data Information BV. All rights reserved. The Case-Population Study Design An Analysis of its Application
ORIGINAL RESEARCH ARTICLE Drug Saf 2011; 34 (10): 861-868 0114-5916/11/0010-0861/$49.95/0 ª 2011 Adis Data Information BV. All rights reserved. The Case-Population Study Design An Analysis of its Application
EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - PANCYTOPENIA AND SERIOUS SKIN REACTIONS
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 25 October 1999 EMEA/31637/99 EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - PANCYTOPENIA AND
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 25 October 1999 EMEA/31637/99 EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - PANCYTOPENIA AND
Anti-inflammatory drugs
 Anti-inflammatory drugs 1 Inflammatory process 1. stimulus (cut) 2. Initial local vasoconstriction( blood loss) 3. vasodilation, local immune/inflammatory reaction (heat, redness) 4. swelling and pain
Anti-inflammatory drugs 1 Inflammatory process 1. stimulus (cut) 2. Initial local vasoconstriction( blood loss) 3. vasodilation, local immune/inflammatory reaction (heat, redness) 4. swelling and pain
5/8/2017. Clinical Pharmacy Specialist Division of Kidney Disease and Hypertension
 Clinical Pharmacy Specialist Division of Kidney Disease and Hypertension Clinical Assistant Professor Adjunct Clinical Associate Professor Pharmacy Practice 1 http://ed.ted.com/lessons/how-do-your-kidneys-work-emma-bryce.
Clinical Pharmacy Specialist Division of Kidney Disease and Hypertension Clinical Assistant Professor Adjunct Clinical Associate Professor Pharmacy Practice 1 http://ed.ted.com/lessons/how-do-your-kidneys-work-emma-bryce.
eappendix A. Opioids and Nonsteroidal Anti-Inflammatory Drugs
 eappendix A. Opioids and Nonsteroidal Anti-Inflammatory Drugs Nonsteroidal Anti-Inflammatory Drugs Nonselective nonsteroidal anti-inflammatory drugs Diclofenac, etodolac, flurbiprofen, ketorolac, ibuprofen,
eappendix A. Opioids and Nonsteroidal Anti-Inflammatory Drugs Nonsteroidal Anti-Inflammatory Drugs Nonselective nonsteroidal anti-inflammatory drugs Diclofenac, etodolac, flurbiprofen, ketorolac, ibuprofen,
Risk of Aplastic Anemia in Patients Using Antiepileptic Drugs
 Epilepsia, 47(7):1232 1236, 2006 Blackwell Publishing, Inc. C 2006 International League Against Epilepsy Brief Communications Risk of Aplastic Anemia in Patients Using Antiepileptic Drugs Kim B. Handoko,
Epilepsia, 47(7):1232 1236, 2006 Blackwell Publishing, Inc. C 2006 International League Against Epilepsy Brief Communications Risk of Aplastic Anemia in Patients Using Antiepileptic Drugs Kim B. Handoko,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Lyrica) Reference Number: HIM.PA.64 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this
Clinical Policy: (Lyrica) Reference Number: HIM.PA.64 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this
Rifamycins Drug interactions
 Rifamycins Drug interactions Drug Category Drug / Drug Class Antacids Nature of Interaction absorption of rifamycins Recommendations Give 1 hour prior to Antacid use Acid Blocking Agents Proton Pump Inhibitors
Rifamycins Drug interactions Drug Category Drug / Drug Class Antacids Nature of Interaction absorption of rifamycins Recommendations Give 1 hour prior to Antacid use Acid Blocking Agents Proton Pump Inhibitors
Bugs and Drugs: What s New in Hypersensitivity Reactions?
 Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics
 MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
PedsCases Podcast Scripts
 PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
Drug Interactions for the Emergency Physician. Lisa Thurgur
 Drug Interactions for the Emergency Physician Lisa Thurgur Objectives Why we should care about drug-drug interactions Cases How can we avoid these interactions Short list of culprit drugs Drug-Drug Interaction
Drug Interactions for the Emergency Physician Lisa Thurgur Objectives Why we should care about drug-drug interactions Cases How can we avoid these interactions Short list of culprit drugs Drug-Drug Interaction
Summary of the risk management plan (RMP) for Clopidogrel/Acetylsalicylic acid Teva (clopidogrel / acetylsalicylic acid)
 EMA/411850/2014 London, 28 July 2014 Summary of the risk management plan (RMP) for (clopidogrel / acetylsalicylic acid) This is a summary of the risk management plan (RMP) for, which details the measures
EMA/411850/2014 London, 28 July 2014 Summary of the risk management plan (RMP) for (clopidogrel / acetylsalicylic acid) This is a summary of the risk management plan (RMP) for, which details the measures
PDP 406 CLINICAL TOXICOLOGY
 PDP 406 CLINICAL TOXICOLOGY Pharm.D Fourth Year NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) Mr.D.Raju.M.Pharm., Lecturer OPTIONS FOR LOCAL IMPLEMENTATION (1) NPC. KEY THERAPEUTIC TOPICS MEDICINES MANAGEMENT
PDP 406 CLINICAL TOXICOLOGY Pharm.D Fourth Year NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS) Mr.D.Raju.M.Pharm., Lecturer OPTIONS FOR LOCAL IMPLEMENTATION (1) NPC. KEY THERAPEUTIC TOPICS MEDICINES MANAGEMENT
PACKAGE LEAFLET: Information for the user. METFORMINE Film-coated tablets 500 mg, 850 mg or 1000 mg (Metformin hydrochloride)
 PACKAGE LEAFLET: Information for the user METFORMINE Film-coated tablets 500 mg, 850 mg or 1000 mg (Metformin hydrochloride) Read this leaflet carefully before you start taking this medicine. - Keep this
PACKAGE LEAFLET: Information for the user METFORMINE Film-coated tablets 500 mg, 850 mg or 1000 mg (Metformin hydrochloride) Read this leaflet carefully before you start taking this medicine. - Keep this
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Svanström H, Pasternak B, Hviid A. Use of azithromycin and
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Svanström H, Pasternak B, Hviid A. Use of azithromycin and
Etodolac versus meloxicam
 Etodolac versus meloxicam The Borg System is 100 % Etodolac versus meloxicam Etodolac versus meloxicam -- 722 75051 1991 Wainwright Not Specific Enough. The store also now says majority of Irish willing
Etodolac versus meloxicam The Borg System is 100 % Etodolac versus meloxicam Etodolac versus meloxicam -- 722 75051 1991 Wainwright Not Specific Enough. The store also now says majority of Irish willing
STEVENS-JOHNSON SYNDROME TRIGGERED BY A COMBINATION OF CLOBAZAM, LAMOTRIGINE AND VALPROIC ACID IN A 7-YEAR-OLD CHILD
 STEVENS-JOHNSON SYNDROME TRIGGERED BY A COMBINATION OF CLOBAZAM, LAMOTRIGINE AND VALPROIC ACID IN A 7-YEAR-OLD CHILD Yapici A.K., 1 * Fidanci M.K., Kilic S., 3 Balamtekin N., 4 Mutluay Arslan M., 5 Yavuz
STEVENS-JOHNSON SYNDROME TRIGGERED BY A COMBINATION OF CLOBAZAM, LAMOTRIGINE AND VALPROIC ACID IN A 7-YEAR-OLD CHILD Yapici A.K., 1 * Fidanci M.K., Kilic S., 3 Balamtekin N., 4 Mutluay Arslan M., 5 Yavuz
Spondyloarthropathies: Disease Perception Limits Market
 Spondyloarthropathies: Disease Perception Limits Market Psoriatic arthritis and ankylosing spondylitis form part of the group of diseases known as the spondyloarthropathies. Psoriatic arthritis is a form
Spondyloarthropathies: Disease Perception Limits Market Psoriatic arthritis and ankylosing spondylitis form part of the group of diseases known as the spondyloarthropathies. Psoriatic arthritis is a form
NSAIDs and tissue healing - bone. Sigbjørn Dimmen and Lars Engebretsen, Orthopaedic Center, Ullevaal University Hospital, Oslo, Norway
 NSAIDs and tissue healing - bone. Sigbjørn Dimmen and Lars Engebretsen, Orthopaedic Center, Ullevaal University Hospital, Oslo, Norway Prevalence of use of NSAIDs in sport Most used drug class in Olympic
NSAIDs and tissue healing - bone. Sigbjørn Dimmen and Lars Engebretsen, Orthopaedic Center, Ullevaal University Hospital, Oslo, Norway Prevalence of use of NSAIDs in sport Most used drug class in Olympic
Database of Abstracts of Reviews of Effects (DARE) Produced by the Centre for Reviews and Dissemination Copyright 2017 University of York.
 A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling Brown
A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling Brown
STUDY. Ekopimo O. Ibia, MD; Richard H. Schwartz, MD; Bernhard L. Wiedermann, MD. outpatient drugs, are the cause of most druginduced
 Antibiotic Rashes in Children A Survey in a Private Practice Setting STUDY Ekopimo O. Ibia, MD; Richard H. Schwartz, MD; Bernhard L. Wiedermann, MD Objective: To document the frequency and severity of
Antibiotic Rashes in Children A Survey in a Private Practice Setting STUDY Ekopimo O. Ibia, MD; Richard H. Schwartz, MD; Bernhard L. Wiedermann, MD Objective: To document the frequency and severity of
BC Cancer Agency & Canadian Cancer Society Financial Support Drug Program (FSDP) for Cancer Patients. Drug Benefit List. Updated August 1, 2017
 BC Cancer Agency & Canadian Cancer Society Financial Support Drug Program (FSDP) for Cancer Patients Drug Benefit List Updated August 1, 2017 The FSDP will operate following rules established by the BC
BC Cancer Agency & Canadian Cancer Society Financial Support Drug Program (FSDP) for Cancer Patients Drug Benefit List Updated August 1, 2017 The FSDP will operate following rules established by the BC
Pharmaceuticals in the environment: The relevance of point sources. Kevin V. Thomas and Katherine Langford Norwegian Institute for Water Research
 Pharmaceuticals in the environment: The relevance of point sources Kevin V. Thomas and Katherine Langford Norwegian Institute for Water Research 25th April 2008 1 Background: Pharmaceuticals as contaminants
Pharmaceuticals in the environment: The relevance of point sources Kevin V. Thomas and Katherine Langford Norwegian Institute for Water Research 25th April 2008 1 Background: Pharmaceuticals as contaminants
OBSERVATIONAL MEDICAL OUTCOMES PARTNERSHIP
 OBSERVATIONAL Patient-centered observational analytics: New directions toward studying the effects of medical products Patrick Ryan on behalf of OMOP Research Team May 22, 2012 Observational Medical Outcomes
OBSERVATIONAL Patient-centered observational analytics: New directions toward studying the effects of medical products Patrick Ryan on behalf of OMOP Research Team May 22, 2012 Observational Medical Outcomes
Pharma X Consultancy Inc. Inventory List
 Pharma X Consultancy Inc. Inventory List Location: Pharma X Consultancy Inc 2 Aceclofenac 100mg Tablets 60S 1205 ID Aciclovir 200mg Tablets 25S 1213 Aciclovir 400mg Tablets 56S 1214 Aciclovir 5% Cream
Pharma X Consultancy Inc. Inventory List Location: Pharma X Consultancy Inc 2 Aceclofenac 100mg Tablets 60S 1205 ID Aciclovir 200mg Tablets 25S 1213 Aciclovir 400mg Tablets 56S 1214 Aciclovir 5% Cream
