A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia
|
|
|
- Irene Collins
- 5 years ago
- Views:
Transcription
1 ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm, Lee Chin Chan, MMed Dermatology Department, Hospital Pulau Pinang, Jalan Residensi, Georgetown, Penang, Malaysia. ABSTRACT Introduction: Severe cutaneous adverse drug reactions (SCARs) are not uncommon and potentially life-threatening. Our objective is to study the patient characteristics, the pattern of implicated drugs and treatment outcome among patients with SCARs. Methods: A 10-year retrospective analysis of SCARs cases in Penang General Hospital was carried out from January 2006 to December Data collection is based on the Malaysian Adverse Drug Reactions Advisory Committee registry and dermatology clinic records. Results: A total of 189 cases of SCARs were encountered (F:M ratio; 1.2:1.0; mean age of 45 year). The commonest manifestation was Stevens-Johnson Syndrome [SJS] (55.0%), followed by toxic epidermal necrolysis [TEN] (23.8%), drug rash with eosinophilia and systemic symptoms [DRESS] (12.7%), acute generalised exanthematous pustulosis [AGEP] (4.8%), SJS/TEN overlap syndrome (2.6%) and generalised bullous fixed drug eruptions [GBFDE] (1.1%). Mean time to onset for TEN/SJS/Overlap syndrome was 10.5±13 days; AGEP, three days; GBFDE, 2.5±0.7 days, and DRESS, 29.4±5.7 days. The most common drugs implicated were antibiotics (33.3%), followed by allopurinol (18.9%) and anticonvulsant (18.4%). Out of 154 cases of SJS/TEN/overlap syndrome, allopurinol was the commonest causative agents (20.1%). In DRESS, allopurinol accounts for 45.8% of the cases. The mortality rate in SJS, TEN and DRESS were 1.9%, 13.3% and 12.5% respectively. No mortality was observed in AGEP and GBFDE. Conclusion: The commonest manifestations of SCARs in our setting were SJS, TEN and DRESS. Allopurinol was the most common culprit. Thus, judicious allopurinol use is advocated and pre-emptive genetic screening for HLA-B *5801 should be considered. KEY WORDS: Stevens-Johnson Syndrome, Toxic epidermal necrolysis, DRESS, GBFDE, AGEP INTRODUCTION Cutaneous adverse drug reactions (CADRs) is one of the more common adverse drug reactions seen in hospitalised patients. It constitutes 10 to 30% of all reported adverse drug reactions. 1 Clinical presentation ranges from mild reactions like a maculopapular rash to severe, life-threatening conditions such as toxic epidermal necrolysis (TEN). Hospitalisations from drug reactions are as high as 5 to 8%, with CADRs accounting for 2% of these admissions. 2 According to the World Health Organization (WHO), severe cutaneous adverse reactions (SCARs) refers to those requiring hospitalisation, with significant morbidity and mortality or are life-threatening. 3 These encompass Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalised exanthematous pustulosis (AGEP), generalised bullous fixed drug eruptions (GBFDE) and drug rash with eosinophilia and systemic symptoms (DRESS). In Singapore, SCARs constitute 5 to14% of CADR. 4 International studies have shown that the overall mortality rate among patients with SJS and TEN are 10% and 30% respectively, 5 while delayed mortality is seen in 5 to 10% in the following weeks. 6 Mortality rate for DRESS is estimated at 10%. 7 The prevalence and patterns of SCARs differ greatly between different populations and geographic distribution. The incidence of TEN in Singapore is estimated to be at least 1.4 cases/million population, 8 while the incidence in United State is 0.5 cases/million population. 9 Other authors revealed that SJS had an annual incidence of 1.2 to 6 cases per million people which is a 4-fold higher than the incidence of TEN, 0.4 to 1.2 cases per million people. 9,10 DRESS, the other severe idiosyncratic drug reaction associated with multi-organ involvement has an incidence of 1/1000 to 1/10,000 drug exposures. 11 EuroSCAR studies have reported that the majority of the reactions are attributed to a group of high-risk drugs such as allopurinol, carbamazepine, phenytoin, lamotrigine, oxicam nonsteroidal anti-inflammatory drugs, sulfonamide antibiotics and nevirapine. South-East Asian data also showed similar causative agents. 12 Unfortunately, local Malaysian data is lacking. The objective of this study is to study the patient characteristics, implicated drug patterns and treatment outcome among patients with SCARs. This would enable us to better understand the disease pattern, guide us in giving better patient care as well as the judicious medication prescription in the future. This article was accepted: 3 January 2018 Corresponding Author: Loo Chai Har chaihar@gmail.com Med J Malaysia Vol 73 No 2 April
2 Original Article Table I: Drugs implicated in SCAR Drug TOTAL Percentage (%) ANTIBIOTICS Penicillin group (Amoxycillin 9; Ampicillin 3; cloxacillin 3; penicillin 3; co-amoxiclav 5, ampicillin/sulbactam 4) 27 Sulphonamides (Bactrim 21; Sulfadoxine/pyrimethamine 5; Dapsone 1) 27 Cephalosporins (Ceftriazone 4; Cefazolin 1; cefoperazone 1; ceftazidime 1; cephalexin 1) 8 Quinolone (Ciprofloxacin 2, moxifloxacin 2) 4 Tetracyclines (tetracycline 2; Doxycycline 1) 3 Anti-tuberculosis (ethambutol 1; isoniazid 1; rifampicin 1; pyrazinamide 1) 4 Others (Erythromycin 1; Clindamycin 2; Gentamicin 1) 4 ANTICONVULSANTS Phenytoin 22 Carbamazepine 17 Lamotrigine 3 ANTIGOUT Allopurinol 43 NSAIDS/ANALGESIC NSAIDS (Diclofenac 5; Etoricoxib 1; Ibuprofen 2; Mefenamic acid 8; Aspirin 1; Piroxicam 1; NSAIDS 5) 23 Paracetamol 10 Tramadol 1 ANTIRHEUMATIC Leflunamide 1 Sulfasalazine 1 ANTIVIRAL Efavirenz 3; Lamivudine 2; Nevirapine 4; Stavudine 3 12 ANTIHYPERTENSIVES Amlodipine 1 Diltiazem 2 Frusemide 2 Others Carbimazole 1; Cetirizine 2; Omeprazole 4; Ranitidine 2; Recormon 1; Traditional medication 3) TOTAL Table II: Interval between drug administration and onset of symptoms Epidermal Necrolysis AGEP DRESS GBFDE Mean Time to Onset (Days) MATERIALS AND METHODS Data collection This is a 10-year retrospective analysis of SCARs cases seen in Penang Hospital, Malaysia from January 2006 to December Only severe types of CADR, such as SJS, TEN, SJS/TEN overlap syndrome, AGEP, DRESS and GBFDE were included. SJS is defined as an epidermal detachment of <10% body surface area (BSA), while SJS/TEN overlap syndrome involved epidermal detachment of 10 to 30%. A detachment of more than 30% BSA is considered as TEN. 3 AGEP is an acute febrile eruption characterised by numerous small, nonfollicular pustules, arising from large areas of the edematous erythematous base. 3 There are three proposed diagnostic criteria for DRESS, we opted for the RegiSCAR criteria which include hospitalised patients with reaction suspected to be drug-related with at least three out of these findings: fever >38 o C, lymphadenopathy involved at least two sites, internal organ and haematological abnormalities. GBFDE is defined as typically fixed drug eruption (FDE) lesions with widespread blisters and erosions involving more than 10% BSA in at least three out of six sites (head and neck, anterior trunk, back, upper limbs, lower limbs and genitalia). 13 We collected our data from MADRAC (Malaysian Adverse Drug Reactions Advisory Committee) registry and dermatology clinic records ( ). Information on patient demography, type of adverse reactions, causative agents, time to onset, treatment modalities and disease outcome was obtained and analysed. Statistical analysis A descriptive statistical analysis was conducted using Statistical Package for the Social Sciences (SPSS 22.0, IBM, USA). The normally distributed continuous variables are summarized in mean and standard deviation (SD) while the non-normally distributed are expressed as median and inter quartile range (IQR). For categorical variables, frequencies and percentages were tabulated. The significance level was set at P<0.05. Approval from Ministry of Health Malaysia Research Ethics Committee (MREC) was obtained. RESULTS Demographics A total of 189 cases of SCARs were identified over the past 10 years. With a total of 614,747 hospital admissions and 46,708 clinic consultations, SCARs account for 0.03% (0.3/1000) of total hospital admission and 0.42% of dermatology clinic attendance respectively. The majority of patients were Malays (49%, n=93) followed by Chinese (38%, n=71) and Indian (11%, n=20). There was a female preponderance with a female to male ratio of 1.2:1.0. However, a male preponderance was seen in DRESS and AGEP, with a male to female ratio of 1:0.6 and 1: Med J Malaysia Vol 73 No 2 April 2018
3 A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Fig. 1: Interval between drug administration and inset if symptoms. respectively. (p=0.065). Patient s age ranged from 2 to 87 years old, with the mean at 45 years old. There was an increase in SCARs with age. Reaction Pattern of SCARs The commonest reaction pattern of SCARs in our cohort was epidermal necrolysis (81.4%, n=154), followed by DRESS (12.7%, n=24), AGEP (4.8%, n=9) and GBFDE (1.1%, n=2). Epidermal necrolysis included SJS, 55.0% (n=104), SJS/TEN overlap syndrome, 2.6% (n=5) and TEN 23.8% (n=45). Implicated Drugs A total of 228 drugs were identified. They were classified broadly into eight groups (Table I). Antibiotics were the most common at 33.7%, followed by anti-gout (Allopurinol) and anticonvulsant at 18.9% and 18.4% respectively. NSAIDS contributed 10% of SCARs. Cotrimoxazole (n=21), amoxicillin (n=9) and augmentin (n=5) were the commonest implicated antibiotics. Aromatic anticonvulsants like phenytoin (n=22) and carbamazepine (n=17) remained the most common anticonvulsants causing SCARs. Individually, allopurinol was the most prevalent drug implicated (18.9%, n=43), responsible for 20.8% of SJS/TEN (31 out of 149), 46% of DRESS (11 out of 24) and 11% of AGEP (1 out of 9). Interval between the drug administration and onset of symptoms The time frame between drug administration and symptom onset was highly variable. The latency periods based on different types of SCARs are summarised in Tables II. Almost 99% of SCARs occurred within the first eight weeks of drug administration with half of them developing adverse reactions within the first week of drug administration. (Figure 1). DRESS had the longest interval, at 29.4 days. Treatment All patients received symptomatic treatment and this included withholding the offending drugs and prescription of topical medication as well as an oral antihistamine. Approximately 41.3% (n=78) required systemic treatment of either systemic corticosteroid or intravenous immunoglobulin. Of these, systemic corticosteroid was prescribed in six cases of SJS (5.8%), four TEN (8.9%), 21 DRESS (87.5%), five AGEP (55.6%) and two cases of GBFDE (100%). On the other hand, intravenous immunoglobulin was only used for epidermal necrolysis [6 SJS (5.8%); one SJS/TEN (0.5%); 33 TEN (73.3%)]. Fig. 2: Percentage of SCARs in dermatology clinic attendance. Mortality The total mortality of SCARs in this study was 5.8% (n=11), contributed by both epidermal necrolysis and DRESS. TEN had the highest mortality rate at 13.3% (6 out of 45), followed by DRESS 12.5% (3 out of 24) and SJS 1.9% (2 out of 104). Allopurinol was responsible for a total of 5 mortalities (45.5%), of which 3 were attributed to TEN, 1 to SJS and 1 to DRESS. Most of them succumbed due to a combination of factors like sepsis, pneumonia, and multi-organ failure. DISCUSSION From our 10-year review, the rate of hospital admission due to SCARs was 0.3/1000, similar to other international papers by Lee HY et al. (Singapore) 4, Grando LR et al. (Brazil) 14 and Li LF et al. (China). 15 However, this was only half the rate reported by Fiscenson-Albala F et al. (France). 16 SCARs contributed 0.42% of our new dermatology clinic attendance which was much lower than the other Malaysian cohort reported by Choon et al. (0.86%) in There was also a marked decline in SCARs cases seen in our department since the year 2011 as shown in Figure 2. This improvement may be attributed to increased doctor awareness, which translated to a more judicious prescribing habit. In the year 2011, active measures were implemented by the Ministry of Health of Malaysia to curb the alarming issue of rampant allopurinol use among medical practitioners. The prescribing category for allopurinol was raised from Category B (can be prescribed by medical officers) to Category A/KK (only can be prescribed by consultants/specialists/family physician specialists). Reminders were issued to doctors who used allopurinol without justified indication. Uric acid analysis, which was once part of the routine renal function profile was also removed. This is to curb the unnecessary treatment of asymptomatic hyperuricemia without associated complications. The magnitude of adverse reactions caused by allopurinol was detected due to the collaborative efforts of all health care providers in reporting all the reactions to MADRAC. This enabled remedial actions to be implemented. In terms of demographics, the ethnic distribution of SCARs corresponded to both the hospital and clinic patient ethnic composition (Malay 45%, Chinese 33% and Indian 17%), except that in DRESS, there were more Chinese (12 out of 24 cases). However, due to the small sample size, this was statistically insignificant (p=0.054). More male patients were seen in DRESS and AGEP although the overall SCARs have a female predominance. The discordance of gender discrepancy is also found in various other literature reviews. Previously, SJS/TEN affected females more frequently with Med J Malaysia Vol 73 No 2 April
4 Original Article some data even showing the prevalence were high as twice than of males. 18,19 However, several studies reported equal gender involvement, 20,21 while others showed a male preponderance. 15,17,22 SCARs are more commonly seen with increasing age due to the associated co-morbidities requiring polypharmacy. The definition of SCARs is ever evolving as we understand the disease more. This is evidenced by the recent inclusion of a new clinical entity such as GBFDE by RegiSCAR study. The diagnosis of DRESS in my study is based on RegiSCAR criteria, which was implemented later in Thus, with a more standardised diagnostic criterion, more cases of DRESS were accurately recognised. In our setting, only two out of nine cases of FDE fulfilled the diagnostic criteria of GBFDE based on clinical presentation and BSA involvement. This is one of the limitations of a retrospective study where the diagnosis is made based on findings recorded. From this study, we learned that a more structured and detailed information is crucial in adverse drug reaction reporting in order to capture the true number of cases. We believed that with the inclusion of GBFDE in the RegiSCAR study, more GBFDE cases will be reported. Allopurinol being the most common agent contributing to SCARs, is consistent with studies from Asia, Europe and Israel. 17,22,24 However, this does not hold true in the city districts of China, 15 India 25 and Jamaica 21 where antibiotics and anticonvulsants were the main culprits. This discrepancy might be due to the different prescribing practices in different countries. A nationwide population-based study in Taiwan concluded that the risk factors for allopurinol hypersensitivity included female sex, age 60 years or older, initial allopurinol dosage exceeding 100mg/d, associated renal or cardiovascular comorbidities and treatment of asymptomatic hyperuricemia. 26 Many studies have elucidated the strong genetic predisposition of allopurinol-induced SJS/TEN with HLA-B *5801, as seen in Han Chinese in Taiwan, 27 Japanese, 28 Koreans, 29 European 30 and Malaysia. 31 Somkrua et al. s metaanalysis on allopurinol-induced SJS/TEN concluded that the risk was significantly increased by 80 to 97 times among HLA-B* 5801 patients compared to those without the gene. This association was consistent in both Asian and Non-Asian populations. 32 Moreover, we also noticed that allopurinol is responsible for most of the DRESS cases (46%), which is similar to Taiwan studies 7 and other local studies such as Ding WY et al. 33 (52.6%), Choon SE et al. 17 (44%) and Tee SH et al. 22 (40%). The role of genetic testing for HLA-B*5801 prior to allopurinol administration should be considered as it may be potentially cost-effective as well as life-saving if we were to take into consideration the high morbidity and mortality imposed by allopurinol-induced SJS/TEN. Ko TM et al. 34 supported the use of genetic screening of HLA-B*58:01 before initiating allopurinol therapy. There was a significant reduction of incidence of allopurinol-induced SCARs from seven expected cases to none in their cohort study. Other common culprits include over-the-counter drugs such as paracetamol, cetirizine, ranitidine, omeprazole and antihypertensives (amlodipine, diltiazem and frusemide). Hence, we should always bear in mind the risk of drug adverse reactions when prescribing medications and at the same time share this knowledge and awareness not just among doctors but also other healthcare workers like pharmacists and paramedics. In this study, 99% of the administrated drugs showed the typical interval period of two months from drug administration to the onset of the symptoms. With this crucial information, it is important to remind and educate our patients to observe the signs and symptoms of CADR especially during the first two months as prompt withdrawal of the offending medication, especially on the first appearance of skin lesions can improve the outcome tremendously. Gracia-Doval I et al. 35 concluded that the earlier the withdrawal of the causative agent, the better the prognosis with an odds ratio of 0.69 for each day (95% confidence interval, ) as well as lowering the mortality rate by 30% per day. This is especially so with the shorter half-life drugs (<24 hours). One should always anticipate the possibility of SCARs and an earlier follow-up appointment (within the first two months) is crucial for patients treated with high-risk medications for the first time. The time frame between the onset of DRESS and drug administration is longer, at 29.4 days which corresponds to RegiSCAR definition of three weeks or more. The main treatment for SCARs includes immediate withdrawal of the offending drugs with adequate supportive care. To date, the use of systemic corticosteroid in SJS/TEN remains highly controversial. This is due to the lack of prospective, randomized controlled trials in this group of patients. From our observation and experience of local experts, the use of corticosteroid in SJS may provide symptomatic relief, although the mortality rate, unfortunately, remains unchanged. From our study cohort, systemic corticosteroid (5.8%) was used based on a case-tocase basis depending on the cause, complications and patients' comorbidities. It is not a routine practice, and can only be given to patients with a progressive disease when the benefits outweigh their potential harms like sepsis, gastrointestinal bleeding, stress ulcer and poor wound healing. Contrary, several studies do not recommend the use of corticosteroid due to the increased risk of infection and thus prolonging hospitalisation. 36,37 On the other hand, some studies have shown improved outcome with reduced mortality. 6,38 A retrospective study by Yang et al. highlighted the role of combination therapy between systemic corticosteroid and intravenous immunoglobulin (IVIg) in SJS/TEN in order to shorten the hospitalisation duration and arrest the disease progression compared to systemic corticosteroid monotherapy. 39 Intravenous immunoglobulin (IVIG) was only used in SJS (6 out of 104 cases) and TEN (33 out of 45 cases). The use of IVIG in the management of TEN is controversial. Some studies using high-dose IVIg have shown a trend towards improved mortality and children treated with IVIg had a good prognosis. A recent meta-analysis by Huang et al. 40 (2016) did not support the clinical benefits of high dose IVIg for TEN. In order to reduce the incidence of adverse drug reactions, we do recommend a thorough history taking especially a detail drug history with an equal importance on family history of adverse drug reactions. This is because some forms of CADRs have a genetic predisposition. Secondly, genetic testing for carbamazepine and allopurinol should be considered prior to prescribing the drugs. Thirdly, a checklist system can be started for both medical practitioners and pharmacists to counter-check the medication indication to avoid 76 Med J Malaysia Vol 73 No 2 April 2018
5 A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia unnecessary prescription, previous drug adverse reaction and family history prior to dispensing the high risks drugs. A warning pictorial pamphlet with types of CADRs and possible lists of high-risk drugs should be given to patients to increase the awareness so that they are able to recognise earlier signs of cutaneous drug reactions and sought medical attention promptly. Last but not least, there should be equal emphasis on the importance of notification of CADRs to drug authority among healthcare workers. Therefore, the role of MADRAC to educate and share the updated drug information among healthcare workers is crucial. CONCLUSION The commonest manifestations of SCARs in our setting are SJS, TEN and DRESS. Allopurinol remains the most common culprit. Thus, judicious allopurinol use is advocated and pre-emptive genetic screening for HLA-B *5801 should be considered. ACKNOWLEDGEMENT We would like to acknowledge the contribution of National Adverse Drug Reactions Monitoring Centre (esp. Pn Noraisyah) and Pharmacy Department, Hospital Pulau Pinang in providing some of the data. More importantly, we would like to thank the Director-General of Health of Malaysia for his permission to publish this article. REFERENCES 1. Arulmani R, Rajendran AD, Suresh B. Adverse drug reaction monitoring in a secondary care hospital in South India. Br J Clin Pharmacol 2007; 65: Valeyrie-Allanore L, Sassolas B, Roujeau JC. Drug-induced skin, nail and hair disorders. Drug Saf 2007; 30: Goldsmith, Lowell A., Fitzpatrick, Thomas B. (Eds.) (2012) Fitzpatrick's dermatology in general medicine : McGraw-Hill Medical, New York, Lee HY, Tay LK, Thirumoorthy T, Pang SM. Cutaneous adverse drug reactions in hospitalized patients. Singapore Med J 2010; 51: Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S et al. Comprehensive survival analysis of a cohort of patients with Stevens- Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol 2013; 133: Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC., Mockenhaupt M. Effects of treatments on the mortality of Stevens Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol 2008; 58: Chen YC, Chiu HC, Chu CY. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol 2010; 146: Chan HL. Toxic epidermal necrolysis in Singapore 1989 through 1993: incidence and antecedent drug exposure. Arch Dermatol 1995; 131: Chan HL, Stern RS, Arndt KA, Lnaglois J, Jick SS, Jick H et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: A population based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol 1990; 126: Rzany B, Mockenhaupt M, Baur S, Schroder W, Stocker U, Mueller J et al. Epidemiology of erythema exudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany ( ): Structure and results of a population-based registry. J Clin Epidemiol 1996; 49: Knowles SR, Shear NH. Recognition and management of severe cutaneous drug reactions. Dermatol Clin 2007; 25: Mockenhaupt M, Viboud C, Dunnant A, Naldi L, Bouwes Bavinck JN, Halevy S et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR study. J Invest Dermatol 2008; 128: Mizukawa Y, Shiohara T. Non pigmenting fixed drug eruption as a possible abortive variant of toxic epidermal necrolysis: immunohistochemical and serum cytokine analyses. Clin Exp Dermatol 2010; 35: Grando LR, Schmitt TAB, Bakos RM. Severe cutaneous reactions to drugs in the setting of a general hospital. An Bras Dermatol 2014; 89(5): Li LF, Ma C. Epidemiological study of severe cutaneous adverse drug reactions in a city district of China. Clin Exp Dermatol 2006; 31(5): Fiscenson-Albala F, Auzerie V, Mahe E, Farinotti R, Durand-Stocco C, Crickx B et al. Epidemiology and health services research. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol 2003; 149: Choon SE, Lai NM. An epidemiological and clinical analysis of cutaneous adverse drug reactions seen in a tertiary hospital in Johor, Malaysia. Indian J Dermatol Venereol Leprol 2012; 78(6): Fritsch P. European Dermatology Forum: skin diseases in Europe. Skin diseases with a high public health impact: toxic epidermal necrolysis and Stevens-Johnson syndrome. Eur J Dermatol 2008; 18: Yamane Y, Aihara M, Ikezawa Z. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to Allergol Int 2007; 56: Tan SK, Tay YK. Profile and pattern of Stevens-Johnson syndrome and toxic epidermal necrolysis in a general hospital in Singapore: treatment outcomes. Acta Derm Venereol 2012; 92: East-Innis AD, Thompson DS. Stevens-Johnson syndrome and toxic epidermal necrolysis at the University Hospital of the West Indies, Jamaica. West Indian Med J 2013; 62(7): Tee SH, Ng TG. A 5-year retrospective study on the clinical patterns and treatment outcomes of severe cutaneous adverse drug reactions (SCARS) in Hospital Tengku Ampuan Rahimah, Malaysia. Malaysian Journal of Dermatology 2014; 33: Eshki M, Auanore L, Musette P, Milpied B, Grange A, Guillaume JC et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol 2009; 145: Halevy S, Ghislain PD, Mockenhaupt M, Fagot JP, Bouwes Bavinck JN, Sidoroff A et al. Allopurinol is the most common cause of Stevens-Johnson Syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol 2008; 58(1): Mahatme N, Narasimharao R. A study of clinical patterns and causative agents of adverse cutaneous drug reactions. Indian J Drugs Dermatol 2016; 2(1): Yang CY, Chen CH, Deng ST, Huang CS, Lin YJ, Chen YJ et al. Allopurinol use and risk of fatal hypersensitivity reactions: a Nationwide Population- Based Study in Taiwan. JAMA Intern Med 2015; 175: Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA 2005; 102(11): Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K. et al. HLA- B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics 2008; 9(11): Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH et al: Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics 2011; 21(5): Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics 2008; 18: Chang CC, Too CL, Murad S, Hussein SH. Association of HLA-B*5801, A33 and DR17 with allopurinol-induced toxic epidermal necrolysis and Stevens- Johnson syndrome in a multiethnic Malaysian population. Proceeding in 7th Asian-Oceania Epilepsy Congress, Xiamen May Somkrua R, Eickman EE, Saokaew S, Lohitnavy M, Chaiyakunapruk N. Association of HLA-B*5801 allele and allopurinol-induced Stevens Johson syndrome and toxic epidermal necrolysis: a systematic review and metaanalysis. BMC Med Genet 2011; 12: Ding WY, Lee CK, Choon SE. Cutaneous adverse drug reactions seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol 2010; 49(7): Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS et al. Use of HLA-B*5801 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ 2015; 351: h Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol 2000; 136: Hynes AY, Kafkala C, Daoud YJ, Foster CS. Controversy in the use of highdose systemic steroids in the acute care of patients with Stevens-Johnson syndrome. Int Ophthalmol Clin 2005; 45: Ginsburg CM. Stevens-Johnson syndrome in children. Pediatr Infect Dis 1982; 1: Yamane Y, Aihara M, Tatewaki S, Matsukura S, Kanbara T, Yamakawa Y et al. Analysis of treatments and deceased cases of severe adverse drug reactions-analysis of 46 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis. Aerugi 2009; 58: Yang Y, Xu J, Li F, Zhu X. Combination therapy of intra-venous immunoglobulin and corticosteroid in the treatment of toxic epidermal necrolysis and Stevens-Johnson syndrome: a retrospective comparative study in China. Int J Dermatol 2009; 48: Huang YC, Chien YN, Chen YT, Li YC, Chen TJ. Intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systemic review and meta-analysis. G Ital Dermatol Venereol 2016; 151(5): Med J Malaysia Vol 73 No 2 April
Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital
 International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
 Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
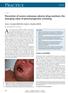 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea
 Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
 Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
1 Introduction. Kenneth Irungu 1 David Nyamu
 Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Severe cutaneous reactions to drugs in the setting of a general hospital *
 758 INVESTIGATION s Severe cutaneous reactions to drugs in the setting of a general hospital * Luciana Rosa Grando 1 Tatiana Aline Berger Schmitt 1 Renato Marchiori Bakos 1 DOI: http://dx.doi.org/10.1590/abd1806-4841.20142997
758 INVESTIGATION s Severe cutaneous reactions to drugs in the setting of a general hospital * Luciana Rosa Grando 1 Tatiana Aline Berger Schmitt 1 Renato Marchiori Bakos 1 DOI: http://dx.doi.org/10.1590/abd1806-4841.20142997
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
Drug Allergy A Guide to Diagnosis and Management
 Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
ORIGINAL ARTICLE. Siew Eng Choon, FRCP 1,2, Yi Shan Der 1, Nai Liang Joel Lai 1, Evelyn Sing Ee Yu 1, Xiao Ling Yap 1, Nalini Nanu Madhavan, MBBS 2
 ORIGINAL ARTICLE Clinical characteristics, culprit drugs and outcome of patients with Acute Generalised Exanthematous Pustulosis seen in Hospital Sultanah Aminah, Johor Bahru Siew Eng Choon, FRCP 1,2,
ORIGINAL ARTICLE Clinical characteristics, culprit drugs and outcome of patients with Acute Generalised Exanthematous Pustulosis seen in Hospital Sultanah Aminah, Johor Bahru Siew Eng Choon, FRCP 1,2,
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC
 SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME
 EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
Skin Manifestations of Drug Reactions
 Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Address for correspondence: Dr. Patel Raksha M. R-3, Doctor s Quarters, Jail Road, Vadodara- 01, Gujarat, India.
 Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Emergency Dermatology Dr Melissa Barkham
 Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics
 MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection
 Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review
 140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
Cutaneous drug reactions
 Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Severe cutaneous adverse reactions (SCAR) are life-threatening
 Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Adverse Drug Reactions (ADRs) Outline
 Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Stevens-Johnson syndrome (SJS) and toxic epidermal. necrolysis in Asian children
 Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital
 Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review
 Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
University of Groningen
 University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
International Journal of Basic & Clinical Pharmacology. Profile of cutaneous adverse drug reactions of carbamazepine
 Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20175211 Original Research Article Profile of cutaneous
Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20175211 Original Research Article Profile of cutaneous
Department of Pharmacology & NIPER-Guwahati, Gauhati Medical College & Hospital, Guwahati , Assam, INDIA. 3
 J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
Pediatric Dermatology
 Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Cutaneous Drug Reactions
 Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
New product information wording Extracts from PRAC recommendations on signals
 12 October 2017 EMA/PRAC/610988/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 25-29 September 2017
12 October 2017 EMA/PRAC/610988/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 25-29 September 2017
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
Original Article. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C
 Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive Patients
 Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan Pei Chen, Ph.D., Juei-Jueng Lin, M.D., Chin-Song Lu, M.D.,
T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan Pei Chen, Ph.D., Juei-Jueng Lin, M.D., Chin-Song Lu, M.D.,
Defaulter rate of follow-up of patients with gonorrhoea at the Genitourinary Medicine Clinic
 Original Article Defaulter rate of follow-up of patients with gonorrhoea at the Genitourinary Medicine Clinic CC Chang, MBBS, MRCP, K Akbal, MBBS, HB Gangaram, MBBS, FRCP and Suraiya H Hussein MBBS, FRCP
Original Article Defaulter rate of follow-up of patients with gonorrhoea at the Genitourinary Medicine Clinic CC Chang, MBBS, MRCP, K Akbal, MBBS, HB Gangaram, MBBS, FRCP and Suraiya H Hussein MBBS, FRCP
Cutaneous drug reactions to antiepileptic drugs and relation with HLA alleles in the Turkish population
 O R I G I N A L A R T I C L E Eur Ann Allergy Clin Immunol Vol 50, N 1, 36-41, 2018 S. Büyüköztürk 1, Ç. Kekik 2, A.Z. Gökyiğit 3, F.İ. Tezer Filik 4i, G. Karakaya 4, S. Saygı 4i, A.B. Dursun 5, S. Kirbaş
O R I G I N A L A R T I C L E Eur Ann Allergy Clin Immunol Vol 50, N 1, 36-41, 2018 S. Büyüköztürk 1, Ç. Kekik 2, A.Z. Gökyiğit 3, F.İ. Tezer Filik 4i, G. Karakaya 4, S. Saygı 4i, A.B. Dursun 5, S. Kirbaş
HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review
 HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
An unpredictable, dose-independent adverse drug reaction which is immunologically or IgEmediated.
 R H E U M A T I S M D I S O R D E R S A N D A L L E R G I E S APPROACH TO DRUG ALLERGY Dr Bernard Thong DEFINITION OF DRUG ALLERGY An unpredictable, dose-independent adverse drug reaction which is immunologically
R H E U M A T I S M D I S O R D E R S A N D A L L E R G I E S APPROACH TO DRUG ALLERGY Dr Bernard Thong DEFINITION OF DRUG ALLERGY An unpredictable, dose-independent adverse drug reaction which is immunologically
ABACAVIR HYPERSENSITIVITY REACTION
 ABACAVIR HYPERSENSITIVITY REACTION Key Risk Minimisation Points: Abacavir Hypersensitivity Reaction (HSR) Abacavir is associated with a risk for hypersensitivity reactions (HSR) characterised by fever
ABACAVIR HYPERSENSITIVITY REACTION Key Risk Minimisation Points: Abacavir Hypersensitivity Reaction (HSR) Abacavir is associated with a risk for hypersensitivity reactions (HSR) characterised by fever
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS
 PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
Big rashes in little patients:
 ! Big rashes in little patients: Severe drug eruptions and cutaneous infections!! Marcia Hogeling, MD, FAAD Assistant Clinical Professor Director, Pediatric Dermatology Division of Dermatology David Geffen
! Big rashes in little patients: Severe drug eruptions and cutaneous infections!! Marcia Hogeling, MD, FAAD Assistant Clinical Professor Director, Pediatric Dermatology Division of Dermatology David Geffen
Bugs and Drugs: What s New in Hypersensitivity Reactions?
 Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015)
 1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
The liver and skin are two of the commonest
 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
Cutaneous drug eruptions are seen commonly
 REVIEW ARTICLE Cutaneous drug reactions in children Sandipan Dhar, Raghubir Banerjee, Rajib Malakar Department of Pediatric Dermatology, Institute of Child Health, Kolkata, West Bengal, India ABSTRACT
REVIEW ARTICLE Cutaneous drug reactions in children Sandipan Dhar, Raghubir Banerjee, Rajib Malakar Department of Pediatric Dermatology, Institute of Child Health, Kolkata, West Bengal, India ABSTRACT
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update
 Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study
 open access Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study Tai-Ming Ko, 1,2 Chang-Youh Tsai, 3,4 Shih-Yang
open access Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study Tai-Ming Ko, 1,2 Chang-Youh Tsai, 3,4 Shih-Yang
Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India
 Study Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India David Pudukadan, Devinder Mohan Thappa Department of Dermatology and STD, Jawaharlal
Study Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India David Pudukadan, Devinder Mohan Thappa Department of Dermatology and STD, Jawaharlal
Endocarditis. By : Mehrnoush. dianatkhah
 Endocarditis By : Mehrnoush. dianatkhah Case 5.31, 31 years old woman CC : Fever, dyspnea, 3 days postpartum PMH : Mitral prolapse Fever 38.5 WBC : 8900 ESR : 84 CRP : 10.4 Cr : 0.6 NT Pro BNP: 5469 Physical
Endocarditis By : Mehrnoush. dianatkhah Case 5.31, 31 years old woman CC : Fever, dyspnea, 3 days postpartum PMH : Mitral prolapse Fever 38.5 WBC : 8900 ESR : 84 CRP : 10.4 Cr : 0.6 NT Pro BNP: 5469 Physical
cipro loxacin; stevens-johnson syndrome; urinary tract infection; adverse drug reaction
 Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW
 STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
 BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
Herbal and homeopathic products, often considered natural and non-toxic, can also cause adverse drug reactions.
 Idiosyncratic and potentially serious cutaneous adverse drug reactions (CADRs), although relatively rare, account for significant morbidity and mortality. RANNAKOE J LEHLOENYA, BSc, MB ChB, FCDerm (SA)
Idiosyncratic and potentially serious cutaneous adverse drug reactions (CADRs), although relatively rare, account for significant morbidity and mortality. RANNAKOE J LEHLOENYA, BSc, MB ChB, FCDerm (SA)
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients
 Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
AOU Ospedali Riuniti - Ancona
 AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
Personalized Medical Care:Recognition, Management, and Maybe Prevention of Cutaneous Hypersensitivity Reactions
 Personalized Medical Care:Recognition, Management, and Maybe Prevention of Cutaneous Hypersensitivity Reactions Bernard A. Cohen, M.D. Johns Hopkins Children s Center Baltimore, Maryland (NO disclosures)
Personalized Medical Care:Recognition, Management, and Maybe Prevention of Cutaneous Hypersensitivity Reactions Bernard A. Cohen, M.D. Johns Hopkins Children s Center Baltimore, Maryland (NO disclosures)
Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital
 Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital Acknowledgements: KKCTH Dr. Ramkumar Consultant Dermatologist Dr. Ramprakash Consultant Ophthalmologist Dr. Prasad Manne
Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital Acknowledgements: KKCTH Dr. Ramkumar Consultant Dermatologist Dr. Ramprakash Consultant Ophthalmologist Dr. Prasad Manne
HLA-A*24:02 as a common risk factor for antiepileptic drug induced cutaneous adverse reactions
 Published Ahead of Print on May 5, 2017 as 10.1212/WNL.0000000000004008 HLA-A*24:02 as a common risk factor for antiepileptic drug induced cutaneous adverse reactions Yi-Wu Shi, PhD* Fu-Li Min, MD* Dong
Published Ahead of Print on May 5, 2017 as 10.1212/WNL.0000000000004008 HLA-A*24:02 as a common risk factor for antiepileptic drug induced cutaneous adverse reactions Yi-Wu Shi, PhD* Fu-Li Min, MD* Dong
Emergency Dermatology. Emergency Dermatology
 Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from 2000 to 2006 Yumiko Yamane 1, Michiko Aihara 1 and Zenro Ikezawa 1
 Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients
 original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
Multiple Drug Hypersensitivity
 Multiple Drug Hypersensitivity Werner J Pichler ADR-AC GmbH Bern UPDATE ON DRUG HYPERSENSITIVITY, Bern, March 23rd 2017 Definition Multiple drug hypersensitivity (MDH) MDH is a syndrome that develops as
Multiple Drug Hypersensitivity Werner J Pichler ADR-AC GmbH Bern UPDATE ON DRUG HYPERSENSITIVITY, Bern, March 23rd 2017 Definition Multiple drug hypersensitivity (MDH) MDH is a syndrome that develops as
Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies
 /, 2016, Vol. 7, (No. 49), pp: 81870-81879 Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies Ran Wu 1,*, Yi-ju Cheng 2,3,*,
/, 2016, Vol. 7, (No. 49), pp: 81870-81879 Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies Ran Wu 1,*, Yi-ju Cheng 2,3,*,
A Case Report on Paracetamol Induced Generalized Fixed Drug Eruptions
 Human Journals Case report November 2016 Vol.:7, Issue:4 All rights are reserved by Dr. C Sugunakar Raju et al. A Case Report on Paracetamol Induced Generalized Fixed Drug Eruptions Keywords: Skin Eruptions,
Human Journals Case report November 2016 Vol.:7, Issue:4 All rights are reserved by Dr. C Sugunakar Raju et al. A Case Report on Paracetamol Induced Generalized Fixed Drug Eruptions Keywords: Skin Eruptions,
Health economics and drug safety
 Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
Carbamazepine Hypersensitivity: Progress Toward Predicting the Unpredictable
 Carbamazepine Hypersensitivity: Progress Toward Predicting the Unpredictable Current Literature In Clinical Science HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. McCormack
Carbamazepine Hypersensitivity: Progress Toward Predicting the Unpredictable Current Literature In Clinical Science HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. McCormack
Analysis of Cutaneous Adverse Drug Reactions at a Tertiary Care Hospital a Prospective Study
 Tropical Journal of Pharmaceutical Research August 2011; 10 (4): 517-522 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online
Tropical Journal of Pharmaceutical Research August 2011; 10 (4): 517-522 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online
Drug eruptions and hepatic involvement: a study
 International Journal of Research in Dermatology http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183819 Drug eruptions and hepatic involvement:
International Journal of Research in Dermatology http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183819 Drug eruptions and hepatic involvement:
New product information wording Extracts from PRAC recommendations on signals
 20 July 2017 EMA/PRAC/406976/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 3-6 July 2017 PRAC The
20 July 2017 EMA/PRAC/406976/2017 Pharmacovigilance Risk Assessment Committee (PRAC) New product information wording Extracts from PRAC recommendations on signals Adopted at the 3-6 July 2017 PRAC The
PedsCases Podcast Scripts
 PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
University Journal of Pre and Para Clinical Sciences
 ISSN 2455 2879 Volume 2 Issue 4 2016 CASE REPORT- A RARE CASE OF DAPSONE HYPERSENSITIVITY SYNDROME VINOTH KUMAR CHITTARANJAN Department of Pharmacology, MADRAS MEDICAL COLLEGE AND GOVERNMENT GENERAL HOSPITAL
ISSN 2455 2879 Volume 2 Issue 4 2016 CASE REPORT- A RARE CASE OF DAPSONE HYPERSENSITIVITY SYNDROME VINOTH KUMAR CHITTARANJAN Department of Pharmacology, MADRAS MEDICAL COLLEGE AND GOVERNMENT GENERAL HOSPITAL
Scratching the Surface: A Review of SJS/TEN
 Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
The prevalence of Stevens Johnson Syndrome caused by antiretroviral in hospitalized patients at Dr. Hasan Sadikin General Hospital Bandung
 The prevalence of Stevens Johnson Syndrome caused by antiretroviral in hospitalized patients at Dr. Hasan Sadikin General Hospital Bandung Nurmilah Maelani*, Irna Sufiawati*, Hartati Purbo Darmadji** *Department
The prevalence of Stevens Johnson Syndrome caused by antiretroviral in hospitalized patients at Dr. Hasan Sadikin General Hospital Bandung Nurmilah Maelani*, Irna Sufiawati*, Hartati Purbo Darmadji** *Department
Mechanisms of Drug Hypersensitivity Reactions
 18/5/213 Mechanisms of Drug Hypersensitivity Reactions Munir Pirmohamed NHS Chair of Pharmacogenetics Department of Molecular and Clinical Pharmacology Institute of Translational Medicine University of
18/5/213 Mechanisms of Drug Hypersensitivity Reactions Munir Pirmohamed NHS Chair of Pharmacogenetics Department of Molecular and Clinical Pharmacology Institute of Translational Medicine University of
An Evidenced-Based Approach to the Adult with a Morbilliform Eruption
 Relatively An Evidenced-Based Approach to the Adult with a Morbilliform Eruption Ben Kaffenberger, MD Assistant Professor, Dermatology Director, Inpatient Dermatology Consult Service Ohio State University
Relatively An Evidenced-Based Approach to the Adult with a Morbilliform Eruption Ben Kaffenberger, MD Assistant Professor, Dermatology Director, Inpatient Dermatology Consult Service Ohio State University
PACKAGE LEAFLET: INFORMATION FOR THE USER
 PACKAGE LEAFLET: INFORMATION FOR THE USER Fucidin 250 mg Tablets sodium fusidate Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
PACKAGE LEAFLET: INFORMATION FOR THE USER Fucidin 250 mg Tablets sodium fusidate Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Department of Allergy and Immunology. Drug allergy. Dean Tey Paediatric Allergist & Immunologist FRACP LECTURE 2014
 Drug allergy Dean Tey Paediatric Allergist & Immunologist FRACP LECTURE 2014 Outline 1. Background 2. SCAR 3. Mechanism of action 4. Assessment on history 5. Investigations 6. Drug desensitisation 7. Specific
Drug allergy Dean Tey Paediatric Allergist & Immunologist FRACP LECTURE 2014 Outline 1. Background 2. SCAR 3. Mechanism of action 4. Assessment on history 5. Investigations 6. Drug desensitisation 7. Specific
BJD. Summary. British Journal of Dermatology THERAPEUTICS
 THERAPEUTICS BJD British Journal of Dermatology Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of
THERAPEUTICS BJD British Journal of Dermatology Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW
 SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
Management of carbamazepine induced drug reaction with eosinophlia and systemic symptoms in Mediheal hospital- Nakuru, Kenya: a case report
 International Journal of Research in Dermatology http://www.ijord.com Case Report DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20184469 Management of carbamazepine induced drug reaction
International Journal of Research in Dermatology http://www.ijord.com Case Report DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20184469 Management of carbamazepine induced drug reaction
CASE REPORT. Narin Sriratanaviriyakul 1,2,3*, Lam-Phuong Nguyen 1,2,3, Mark C Henderson 2 and Timothy E Albertson 1,2,3
 Sriratanaviriyakul et al. Journal of Medical Case Reports 2014, 8:332 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Drug reaction with eosinophilia and systemic symptoms syndrome (DRESS) syndrome
Sriratanaviriyakul et al. Journal of Medical Case Reports 2014, 8:332 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Drug reaction with eosinophilia and systemic symptoms syndrome (DRESS) syndrome
Recent experiences to review data from MRCTs and progress of research on ethnic factors. Dr Yoshiaki Uyama
 Recent experiences to review data from MRCTs and progress of research on ethnic factors Dr Yoshiaki Uyama (PMDA) Visiting Professor, Graduate School of Advanced Clinical Science, Chiba University Visiting
Recent experiences to review data from MRCTs and progress of research on ethnic factors Dr Yoshiaki Uyama (PMDA) Visiting Professor, Graduate School of Advanced Clinical Science, Chiba University Visiting
Review Article Treatments for Severe Cutaneous Adverse Reactions
 Hindawi Journal of Immunology Research Volume 2017, Article ID 1503709, 9 pages https://doi.org/10.1155/2017/1503709 Review Article Treatments for Severe Cutaneous Adverse Reactions Yung-Tsu Cho and Chia-Yu
Hindawi Journal of Immunology Research Volume 2017, Article ID 1503709, 9 pages https://doi.org/10.1155/2017/1503709 Review Article Treatments for Severe Cutaneous Adverse Reactions Yung-Tsu Cho and Chia-Yu
Clinical profile of skin diseases in accident and emergency department attenders
 Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
Accepted Manuscript. Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need
 Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Vancomycin-induced acute generalized exanthematous pustulosis (AGEP) masquerading septic shock an unusual presentation of a rare disease
 Mawri et al. Journal of Intensive Care (2015) 3:47 DOI 10.1186/s40560-015-0114-3 CASE REPORT Vancomycin-induced acute generalized exanthematous pustulosis (AGEP) masquerading septic shock an unusual presentation
Mawri et al. Journal of Intensive Care (2015) 3:47 DOI 10.1186/s40560-015-0114-3 CASE REPORT Vancomycin-induced acute generalized exanthematous pustulosis (AGEP) masquerading septic shock an unusual presentation
Epidemiology of Venous Thromboembolism in the East. Heng Joo NG Department of Haematology Singapore General Hospital Singapore
 Epidemiology of Venous Thromboembolism in the East Heng Joo NG Department of Haematology Singapore General Hospital Singapore Early Literature Tinckler LF Br Med J 1964;1:502 Propagating a notion.. Hwang
Epidemiology of Venous Thromboembolism in the East Heng Joo NG Department of Haematology Singapore General Hospital Singapore Early Literature Tinckler LF Br Med J 1964;1:502 Propagating a notion.. Hwang
IJBCP International Journal of Basic & Clinical Pharmacology
 Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20171661 Original Research Article A study of cutaneous
Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20171661 Original Research Article A study of cutaneous
Drug Utilization Pattern in Rhinittis in Outpatient Department of Government Medical College and C.P.R. Hospital, Kolhapur
 Original article: Drug Utilization Pattern in Rhinittis in Outpatient Department of Government Medical College and C.P.R. Hospital, Kolhapur Dr. Ajit B. Lokare, Dr. Jaiprakash B. Ramanand, Dr. Rama R.
Original article: Drug Utilization Pattern in Rhinittis in Outpatient Department of Government Medical College and C.P.R. Hospital, Kolhapur Dr. Ajit B. Lokare, Dr. Jaiprakash B. Ramanand, Dr. Rama R.
nevirapine, stavudine and lamivudine
 nevirapine, stavudine and lamivudine Information on starting treatment with: nevirapine (NVP) + stavudine (d4t) + lamivudine (3TC) Nevirapine, stavudine and lamivudine (non-fixed dose) 2 Lead in dosing
nevirapine, stavudine and lamivudine Information on starting treatment with: nevirapine (NVP) + stavudine (d4t) + lamivudine (3TC) Nevirapine, stavudine and lamivudine (non-fixed dose) 2 Lead in dosing
Stevens Johnson syndrome/toxic epidermal necrolysis and erythema multiforme drug related hospitalisations in a national administrative database
 https://doi.org/10.1186/s13601-017-0188-1 Clinical and Translational Allergy RESEARCH Open Access Stevens Johnson syndrome/toxic epidermal necrolysis and erythema multiforme drug related hospitalisations
https://doi.org/10.1186/s13601-017-0188-1 Clinical and Translational Allergy RESEARCH Open Access Stevens Johnson syndrome/toxic epidermal necrolysis and erythema multiforme drug related hospitalisations
Research. Original Investigation
 Research Original Investigation Relationship Between the HLA-B*1502 Allele and Carbamazepine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis A Systematic Review and Meta-analysis Wimonchat
Research Original Investigation Relationship Between the HLA-B*1502 Allele and Carbamazepine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis A Systematic Review and Meta-analysis Wimonchat
