Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea
|
|
|
- Jewel Bryant
- 5 years ago
- Views:
Transcription
1 Original Article Yonsei Med J 2019 Feb;60(2): pissn: eissn: Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee Sohn 1,4, Dong-Yoon Kang 5,6, Han-Ki Park 1,7, Min-Suk Yang 1,8, Ju-Yeun Lee 9, and Hye-Ryun Kang 1,5,6,10 1 Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul; 2 Department of Internal Medicine, Chungbuk National University Hospital, Cheongju; 3 Regional Pharmacovigilance Center, Chungbuk National University Hospital, Cheongju; 4 Department of Internal Medicine, Kyung-Hee University Hospital, Seoul; 5 Regional Pharmacovigilance Center, Seoul National University Hospital, Seoul; 6 Drug Safety Monitoring Center, Seoul National University Hospital, Seoul; 7 Department of Internal Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu; 8 Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul; 9 College of Pharmacy, Seoul National University, Seoul; 10 Division of Allergy and Clinical Immunology, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. Purpose: Despite morbidities and fatalities, nationwide epidemiologic data for severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS), are not widely available. We aimed to investigate SCAR epidemiology over the last two decades in Korea. Materials and Methods: We analyzed individual case safety reports (ICSRs) of SCARs in the Korea Adverse Event Reporting System from 1988 to Administered drugs, demographic profiles, and causality assessment according to the World Health Organization-Uppsala Monitoring Center system were analyzed. Results: A total of 755 SCAR cases (508 SJS/TEN, 247 DRESS) were reported. The number of SCAR ICSRs has been increasing with increasing ICSRs for overall adverse drug events. Since 2010, the number of SCAR ICSRs has increased up to 100 cases/year. Allopurinol was the most common causative drug (SJS/TEN: 10.2%; DRESS: 11.3%; SCAR ICSRs: 10.6%), followed by carbamazepine (SJS/TEN: 8.7%; DRESS: 9.7%; SCAR ICSRs: 8.6%). Regarding drug groups, antiepileptics (19.5%) and antibiotics for systemic use (12.7%) were common causative drug groups. Twenty SCAR-related deaths were recorded. Antibacterials were the most common causes of deaths (8 cases), followed by antiepileptics (5 cases). The potential risk of SCARs was not specified in the drug information leaflet for 40.2% of drugs causing SJS/TEN and 82.5% causing DRESS syndrome in Korea. Conclusion: The number of SCAR ICSRs has increased rapidly with recent active pharmacovigilance programs in Korea. Allopurinol and antiepileptics are the most common individual and categorical causative agents, respectively. Key Words: Pharmacovigilance, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug hypersensitivity syndrome Received: September 19, 2018 Revised: December 21, 2018 Accepted: December 29, 2018 Corresponding author: Hye-Ryun Kang, MD, PhD, Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea. Tel: , Fax: , helenmed@snu.ac.kr The authors have no potential conflicts of interest to disclose. Copyright: Yonsei University College of Medicine 2019 This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Introduction An adverse drug reaction (ADR) is defined as an unintended harmful reaction to a drug. 1 With advances in medicine, people are taking more medications, and adverse drug events (ADEs) are rapidly increasing. Lazzarou, et al. 2 reported that ADRs are the fourth leading cause of death in the US, after heart disease, cancer, and stroke. According to the Global Burden of Disease report of 2013, the number of worldwide ADR-related deaths was in 2013, a 1.5-fold increase over that reported in 208
2 Min-Gyu Kang, et al Thus, ADR is an important medical issue related to patient safety and a heavy socioeconomic burden. Cutaneous ADR is the most common type of ADR and can be caused by many widely used medications. 4 Despite cutaneous ADRs being usually self-limited, some ADEs can manifest as severe cutaneous adverse reactions (SCARs), such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reactions with eosinophilia and systemic symptoms (DRESS) syndrome. Although early diagnosis of SCARs is crucial, it is difficult to differentiate SCARs at initial presentation from other disease conditions with cutaneous manifestations. Therefore, the mortality due to SCARs is considerably high (20 25%), and morbidities causing lasting disabilities are also relatively common. 5 The epidemiology of SCARs seems to be different between countries. SCAR development is known to be significantly influenced by both genetic background and the pattern of drug usage. Previous studies have reported that the epidemiology of SCARs in Korean people seem different from that in Europeans, as well as the Han Chinese in East Asia. 6,7 However, to date, there has been no nationwide epidemiologic study of SCARs in Korea. In this study, we aimed to investigate the epidemiology and common causative drugs of SCARs by reviewing individual case safety reports (ICSRs) of the Korea Adverse Event Reporting System (KAERS) since its introduction in /005/0014/0100 ; and 3) DRESS syndrome, WHO-ART code 2309/002/1810/. ICSRs of drug hypersensitivity syndrome (WHO-ART code 2309/001/1810/ ), which was formerly usually used to describe DRESS-like symptoms, were also extracted. Two allergy specialists with experience in pharmacovigilance independently reviewed the detailed information in each ICSR of suspected SCARs and rechecked the causality assessment and culprit drugs (Fig. 1). Among the IC- SRs of suspected SCARs extracted from the KIDS-KD, only the ICSRs in which two allergy specialists assessed the causality as possible, probable, or certain were included for analysis. Some ICSRs coded for drug hypersensitivity syndrome were re-categorized into ICSRs for DRESS syndrome if they matched the diagnostic criteria for DRESS syndrome. For the assessment of causative drugs, we included ICSRs in which a single drug was listed as the causative drug or in which multiple drugs were listed but co-administration was usually regarded as standard therapy, such as anti-tuberculosis treatments. We did not consider ICSRs of SCARs in which all the co-administered drugs were listed as causative drugs or there was no detailed information to assess the culprit drug based on the history of ICSRs. To minimize the possibility of duplication or overestimation of SCAR ICSRs, reports from pharmaceutical companies based on literature reviews or consumer protection agencies lacking drug information or causality assessments were excluded from analysis. MaterialS and Methods KAERS The KAERS is an ADE reporting system developed by the Korea Institute of Drug Safety and Risk Management (KIDS), an official agency for managing drug safety. It contains ICSRs of any suspected ADEs voluntarily reported by healthcare personnel, consumers, and manufacturers. Each ICSR contains the following information: 1) demographic profile of the patient with the suspected ADE, 2) phenotype coded according to World Health Organization Adverse Reactions Terminology (WHO- ART 092 version), seriousness, and outcome, 3) causative drug or drugs and/or co-administered drugs coded according to the Anatomical Therapeutic Chemical (ATC) classification system and the dose/route/frequency/duration of these medications, and 4) causality assessment performed by specialists of each regional pharmacovigilance center according to the WHO- Uppsala Monitoring Center (UMC) criteria. Selection of SCAR ICSRs This study was approved by the Review Board of Korea Institute of Drug Safety and Risk Management. We extracted ICSRs from 1988 to 2013 that were in line with WHO-ART codes from the KIDS-KAERS database (KIDS-KD): 1) SJS, WHO-ART code 0042/001/0014/0100 ; 2) TEN, WHO-ART code Results Summary of SCAR ICSRs From 1988 to 2013, a total of ADEs were spontaneously included in the KAERS. Of those, 755 cases (0.16%) were of SCARs, comprising 508 cases of SJS/TEN (67.3%) and 247 cases of DRESS syndrome (32.7%). Since the reporting of the first SCAR case in 1991, an increasing tendency has been observed for SCAR ICSRs (Fig. 2). Since 2010, more than 100 SCAR IC- SRs have been submitted annually. Despite the increasing tendency of SCAR ICSRs, the proportion of SCAR ICSRs among all ICSRs did not increase (0.19% in 2010, 0.25% in 2011, 0.17% in 2012, and 0.11% in 2013). The clinical characteristics of SCAR ICSRs are described in Table 1. The mean age of patients with SCARs was 50.3±20.7 years (mean±sd), of which 28.4% were older adults (age: 65 years or more) and 8.2% were children/adolescents (age: 18 years or less). Male patients accounted for 48.7% of the total ICSRs. The mean age and proportion of the older adult patients with SJS/TEN were not significantly different from those with DRESS syndrome (53.1±17.8 years vs. 48.8±22.0 years and 29.7% vs. 27.7%, respectively). Further, the proportion of childhood/ adolescent patients was significantly higher in the SJS/TEN group than in the DRESS group (11.1% vs. 2.6%, respectively, p<0.01). 209
3 Trends in SCAR ICSRs in Korea Total ADE ICSRs in KIDS-KD, (n=478519) WHO-ART code screening Code for SJS: 0042/001/ 0014/0100 Code for TEN: 0013/005/ 0014/0100 Code for DRESS: 2309/002/1810/ Code for DHS: 2309/001/1810/ Review by two allergists separately Review by two allergists separately 1) Reports from pharmaceutical companies 2) Case reports 3) Duplicated cases 4) Follow-up reports Excluded Only included 1) when the ICSRs had clinical information suitable for DRESS and 2) both allergists assessed the causality of ICSR was possible or more. SJS/TEN ICSRs (n=508) DRESS ICSRs (n=247) Total SCAR ICSRs (n=755) Fig. 1. Scheme of the selection process for ICSRs of SCARs in the KIDS-KD. ICSRs, individual case safety reports; SCARs, severe cutaneous adverse reactions; KIDS-KD, Korea Institute of Drug Safety and Risk Management Korea Adverse Event Reporting System database; ADE, adverse drug event; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; DRESS syndrome, drug reaction with eosinophilia and systemic symptoms syndrome; DHS, drug hypersensitivity syndrome; WHO-ART, World Health Organization Adverse Reactions Terminology. The most common drug administration route was oral (72.3%), followed by intravenous injection (25.8%), subcutaneous injection (1.0%), and eye-drop instillation (0.9%). The drug administration route was not different between the DRESS and SJS/TEN groups (p>0.05). The median time for symptom latency (from drug administration to symptom onset) was 19 days, with a mean±sd of 24.6±21.5 days. The median time for symptom latency was longer in the DRESS group than in the SJS/TEN group, by a median of 11 days (31.2±26.0 days vs. 20.4±17.0 days, p<0.05). Causative drugs The most common causative drug in the SCAR ICSRs was allopurinol (10.6%), the proportion of which was the highest in both the DRESS and SJS/TEN groups (Table 2), followed by 210 carbamazepine (8.6%), lamotrigine (4.4%), methazolamide (2.1%), valproic acid (2.1%), vancomycin (1.6%), dapsone (1.5%), paracetamol (1.3%), amoxicillin and/or enzyme inhibitor (1.2%), piperacillin and/or enzyme inhibitor (1.2%), and sulfamethoxazole and trimethoprim (1.2%). Table 2 shows detailed information for causative drugs listed in SCAR ICSRs. According to the ATC subgroups, antiepileptics (ATC code: N03A) were the most common causative drugs (19.5% of total SCAR ICSRs), followed by antibacterials (ATC code: J01A, 12.7%), anti-gout preparations (ATC code: M04A, 10.6%), and non-steroidal anti-inflammatory drugs (NSAIDs, ATC code: M01A, 4.7%). Among antiepileptics, carboxamide derivatives (ATC code N03AF, 9.7% of total SCAR ICSRs) including carbamazepine and oxcarbazepine were the most common SCAR-causing
4 Min-Gyu Kang, et al. 250 DRESS SJS/TEN Total Total ADE SCAR ICSRs (case) Total ICSRs (case) Fig. 2. Trends in annual numbers of ICSRs of SCARs in KAERS. ICSRs, individual case safety reports; SCARS, severe cutaneous adverse reactions; KAERS, Korea Adverse Event Reporting System; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; DRESS syndrome, drug reaction with eosinophilia and systemic symptoms syndrome. Table 1. Baseline Characteristics in Individual Case Safety Reports of SCARs Total DRESS syndrome SJS/TEN Number of self-reports Age (mean±sd, yr) 50.3± ± ±22.0 Elderly ( 65 yrs) (%) Children/adolescents ( 18 yrs) (%) Male sex (%) Number of causative drugs (mean±sd) 1.6± ± ±1.2 Latent period [median (mean±sd), days] 19 (24.6±21.5) 27 (31.2±26.0) 16 (20.4±17.0) Route of administration (%) Oral Intravenous injection Others (subcutaneous or eye-drop instillation) SD, standard deviation; SCARs, severe cutaneous adverse reactions; DRESS syndrome, drug reaction with eosinophilia and systemic symptoms syndrome; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis. drugs, followed by other antiepileptics including lamotrigine (ATC code N03AX, 6.2%), fatty acid derivatives including valproic acid (ATC code: N03AG, 2.1%), and hydantoin derivatives including phenytoin (ATC code: N03AB, 1.1%). Among antibacterials, cephalosporin (ATC code: J01DB, J01DC, J01DD, and J01DE) was the most common SCAR-causing antibacterial (3.6% of total SCAR ICSRs), followed by beta-lactam antibacterials; penicillins (ATC code: J01C, 2.8%) including ampicillin, amoxicillin, and piperacillin; other antibacterials including glycopeptide antibacterials (ATC code: J01X, 1.7%); quinolone antibacterials (ATC code: J01M, 1.5%); and sulfonamides and trimethoprim (ATC code: J01E, 1.2%). Among NSAIDs (ATC code: M01A, 4.4%), propionic acid derivatives (ATC code: M01AE) including ibuprofen and dexibuprofen were the most common SCAR-causing NSAIDs (2.0%), followed by acetic acid derivatives and related substances including diclofenac or aceclofenac (ATC code: M01AB, 0.8%), coxibs (ATC code: M01AH, 0.5%), and fenamates (ATC code: M01AG, 0.5%). Fig. 3 shows the detailed proportions of SCAR-causing drugs according to the pharmacologic main group (level 2) or pharmacologic/therapeutic subgroup (level 3) per the WHO s ATC classification system. Drug labeling information We reviewed the drug labeling information provided by the Korea Pharmaceutical Information Center ( kr) to examine whether the potential to cause SCARs was specified in the drug labeling information (Table 2). The labeling information of 59.8% of the 61 SJS/TEN-causing drugs specified the potential risk for SJS/TEN. In almost all SJS/TEN cases (90.2%), SJS/TEN was caused by drugs whose potential to cause the condition was specified in the drug labeling information. With regard to DRESS, the labeling information of only 14 of 80 (17.5%) causative drugs specified the potential risk for DRESS. Approximately 60% of DRESS cases were attributable to drugs whose labeling information did not specify the potential to cause DRESS. Among antitubercular drugs, which were the culprit drugs in 12 DRESS cases, the drug label 211
5 Trends in SCAR ICSRs in Korea for only ethambutol mentioned its potential to cause DRESS syndrome. The potential risk for the development of DRESS was not specified in the leaflets of other anti-tuberculosis drugs, such as rifampin, isoniazid, and pyrazinamide. Mortality review SCAR-related deaths were reported in 2.6% SCAR ICSRs (20 out of 755 SCAR ICSRs) (Table 3). With regard to DRESS syndrome, the drug-associated mortality rate was 1.2%, with voriconazole, phenytoin, and oxcarbazepine each accounting for Table 2. Common SCAR-Causing Drugs and Drug Labeling Information SCARs n DRESS syndrome n SJS/TEN n Total 755 Total 247 Total 508 Allopurinol 80 Allopurinol 28 Allopurinol 52 Carbamazepine 65 Carbamazepine 24 Carbamazepine 41 Lamotrigine 33 Dapsone* 9 Lamotrigine 25 Methazolamide 16 Lamotrigine* 8 Methazolamide 16 Valproic acid 16 Oxcarbazepine 8 Valproic acid 10 Vancomycin 12 Vancomycin 7 Paracetamol 9 Dapsone 11 Piperacillin and enzyme inhibitor* 7 Amoxicillin and enzyme inhibitor 8 Paracetamol 10 Cefotaxime* 6 TMP/SMX 7 Amoxicillin and enzyme inhibitor 9 Valproic acid 6 Ibuprofen 6 Piperacillin and enzyme inhibitor 9 Ciprofloxacin* 4 Ceftriaxone 5 TMP/SMX 9 Phenytoin* 4 Iloprost* 5 Oxcarbazepine 8 Rifampicin* 3 Vancomycin 5 Phenytoin 8 Others 56 Acetylsalicylic acid 4 Ceftriaxone 7 Undetermined 77 Celecoxib 4 Ibuprofen 7 Doxycycline* 4 Cefotaxime 6 Mefenamic acid 4 Ciprofloxacin 6 Phenytoin 4 Acetylsalicylic acid 5 Amoxicillin and enzyme inhibitor 3 Iloprost 5 Clarithromycin 3 Celecoxib 4 Diclofenac 3 Diclofenac 4 Dorzolamide 3 Doxycycline 4 Enalapril 3 Mefenamic acid 4 Gabapentin 3 Propionic acid derivatives 4 Methotrexate 3 Sulfasalazine 4 Propionic acid derivatives 3 Clarithromycin 3 Tramadol 3 Dorzolamide 3 Zonisamide 3 Enalapril 3 Others 105 Fenofibrate 3 Undetermined 164 Gabapentin 3 Levetiracetam 3 Methotrexate 3 Moxifloxacin 3 Rifampicin 3 Rivaroxaban 3 Sunitinib 3 Topiramate 3 Tramadol 3 Zonisamide 3 Others 127 Undetermined 241 SCARs, severe cutaneous adverse reactions; DRESS syndrome, drug reactions with eosinophilia and systemic symptoms syndrome; SJS/TEN, Stevens-Johnsons syndrome/toxic epidermal necrolysis. Others: drugs for which less than three cases were reported. Undetermined: cases in which two or more drugs were simultaneously reported as a causative agent so that the culprit drug could not be identified. TMP/SMX: trimethoprim and sulfamethoxazole. *Drugs for which the labeling information did not include the risk of SCARs. 212
6 Min-Gyu Kang, et al. M01A, 5.6% M01, 4.7% S01, 2.8% J04, 2.6% B01, 2.3% L01, 2.3% N02, 2.1% S01, 4.1% L01, 2.8% N02, 2.8% B01, 2.6% H02, 2.0% M01, 2.0% B01, 1.6% Others, 6.1% A H02, 1.3% B C Fig. 3. Proportion of causative drugs for SCARs categorized by the main therapeutic group (second level) per ATC classification. (A) Total, (B) SJS/TEN, (C) DRESS syndrome. SCARs, severe cutaneous adverse reactions; ATC, anatomical therapeutic chemical; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; DRESS syndrome, drug reaction with eosinophilia and systemic symptom syndrome. ATC code: B01 (antithrombotic agents), H02 (corticosteroids for systemic use), J01 (antibacterials for systemic use), J04 (antimycobacterials), L01 (antineoplastic agents), M01 (anti-inflammatory and antirheumatic products), M04 (anti-gout preparations), N02 (analgesics), N03 (antiepileptics), S01 (ophthalmologicals), and undetermined (cases in which two or more drugs were simultaneously reported as a causative agent so that the culprit drug could not be identified). one death. The mortality rate in the SJS/TEN group was 3.3%, with two cases attributable to allopurinol, eight to anti-infectives, two to antiepileptics, two to antitubercular drugs, and one case each to fenofibrate, methazolamide, and anti-leukemia drugs. Among the cases in which death occurred, death in two DRESS syndrome cases (voriconazole and phenytoin) and three SJS/TEN cases (cefazedone, doxycycline, and fenofibrate) was attributable to drugs whose potential to cause SCARs was not indicated on the drug label. Discussion In this study, we investigated SCAR ICSRs submitted to the KAERS since Recently, the tendency for SCAR ICSRs has increased, with more than 100 SCAR ICSRs spontaneously submitted annually. Allopurinol and carbamazepine were the most common SCAR-causing drugs. Antiepileptics and antibacterials were the most common SCAR-inducing drug groups. The mortality rates in SCAR ICSRs in Korea were considerably lower (2.6%) than those reported in other countries. The potential risk for SCAR development was not sufficiently specified in the drug labeling information, especially for DRESS syndrome (82.5% of drugs in the DRESS group and 17.5% of drugs in the SJS/TEN group). Since SCARs are rare, their epidemiology has not been sufficiently studied in Korea. Few studies have been published on the epidemiologic nature and causative drugs of SCARs in Korea. Furthermore, these studies were conducted in a single hospital or a limited number of hospitals, making it difficult to draw nationwide assumptions regarding SCAR development. Recently, a multicenter study based on the Korean Pharmacovigilance Research Network database reported that there were 100 SCAR cases from July 2009 to December Yang, et al. 9 evaluated the nationwide claims database in Korea and reported that the incidence of SJS ranged from 3.96 to 5.03 per million and that of TEN ranged from 0.94 to 1.45 per million Table 3. Summary of Cases of SCAR-Related Deaths and Drug Labeling Information for SCARs Diagnosis Causative drug ATC code Number Voriconazole* J 1 DRESS syndrome Oxcarbazepine N 1 Phenytoin* N 1 Fenofibrate* C 1 Cefazedone* J 1 Cefoperazone/cefuroxime/ amikacin J 1 Doxycycline* J 1 Isoniazid J 1 Levofloxacin/ethambutol/ rifampicin/isoniazid J 1 Levofloxacin/meropenem J 1 Tigecycline J 2 SJS/TEN Vancomycin/teicoplanin/ J 1 ceftriaxone Methotrexate L 1 Vincristine/rituximab/ prednisolone/doxorubicin/ L 1 cyclophosphamide Allopurinol M 2 Carbamazepine N 1 Valproic acid N 1 Methazolamide S 1 SCARs, severe cutaneous adverse reactions; DRESS syndrome, drug reactions with eosinophilia and systemic symptom syndrome; SJS/TEN, Stevens- Johnsons syndrome/toxic epidermal necrolysis; ATC, anatomical therapeutic chemical. *Drugs for which the labeling information did not include the risk of SCARs. from 2010 to In our study, 755 SCAR cases were reported since ADE spontaneous reporting began in Korea. The number of ICSRs of SCARs has been increasing in Korea. More than 100 SCAR cases have been spontaneously reported every year since A total of 193 cases was spontaneously re- 213
7 Trends in SCAR ICSRs in Korea ported to KAERS in It remains unclear whether the increasing trend of SCAR ICSRs resulted from an actual increase in the development of SCARs in Korea. Although SCAR ICSRs are steadily increasing, the proportion of SCAR ICSRs among ICSRs of overall adverse events is declining. Thus, the increasing SCAR ICSRs seem to be the result of active pharmacovigilance by regional pharmacovigilance centers rather than being truly an increase in SCAR ICSRs. Although allopurinol-induced and carbamazepine-induced SCARs are both common in Korea and in other countries, 10 the epidemiology of SCARs in Korea might be somewhat different from that in other countries. 11 Allopurinol-induced SCARs are common in Korea and Taiwan. The HLA-B*58:01 genotype is a well-known risk factor for allopurinol-induced SCARs in the Han Chinese in Taiwan (OR 580.3). 12 The allele frequency of HLA-B*58:01 is 12.2% in Koreans 13 and 9 11% in the Han Chinese, 12 such that HLA-B*58:01 screening proved to be effective to prevent allopurinol-induced SCARs in Korea 14 and Taiwan. 15 However, the allele frequency of HLA-B*58:01 is lower in Japanese (1 2%) 16 and Caucasians (1 6%). 17 Carbamazepine-induced SCAR is also common in Korea and Taiwan. In the Han Chinese, the incidence of carbamazepine-induced SCARs was more than 10-fold higher than that in other ethnic groups. 18 The HLA-B*15:02 haplotype is clearly associated with carbamazepine-induced SCARs (OR ). 19 However, the HLA-B15:02 allele frequency was show to be quite low (0.41%) in the Korean population, compared to that in Han Chinese (10 15%). 19,20 Instead, HLA-A3101 is highly associated with carbamazepine-induced SJS in Korean population. 6 Abacavir, which is an anti-retroviral drug used to treat HIV/AIDS, is known to frequently elicit hypersensitivity reactions in individuals of European descent. 21 However, abacavir-induced SCAR ICSRs have not been reported in Korea. Methazolamide was one of the common SCAR-causing drugs in our study. HLA- B*59:01 is a known risk factor for methazolamide-induced SCARs. The HLA-B*59:01 allele is present in 1 2% of Korean population, but in <0.5% of Chinese population, and is extremely rare in Caucasian individuals. 22 To date, methazolamide-related SCARs have been reported only in Koreans, Japanese, and Chinese. The mortality rate in SCAR ICSRs in our study was much lower than rates in other studies. 21,23-25 Mortality due to SJS and TEN has been previously reported to be 1 13% and 30 50%, respectively. In our study, the mortality rates in SJS ICSRs and TEN ICSRs were 2.9% (12 cases among 408 SJS ICSRs) and 5% (5 cases among 100 TEN ISCRs), respectively. The mortality rates in DRESS ICSRs were also quite low in our study (1.2%), compared to those in other studies (approximately 10%). 26,27 It is difficult to generalize whether the mortality rate associated with SCARs in Korea is lower than rates in other countries. We retrospectively reviewed the SCAR ICSRs in the KIDS-KD. Sekula, et al. 24 reported that a considerable number of SCARrelated deaths occurred after successful treatment and following hospital discharge. If the outcomes in SCAR ICSRs are not properly followed up, SCAR-related mortality might be underestimated. However, Yang, et al. 9 analyzed Korean national health insurance data and reported that the mortality associated with TEN was approximately 15%, which was still lower than that in other countries. The labeling information of many drugs in our study did not contain relevant warnings for the possibility of SCAR development. Information about the potential risk for DRESS syndrome was not mentioned in the labeling information for approximately two-thirds of the DRESS-causing drugs in our study. Although approximately 5% of all DRESS syndrome cases were attributable to dapsone and lamotrigine, the relevant labeling information for DRESS syndrome was not included for either drug. Among the 10 common DRESS syndrome-inducing drugs, the risk for DRESS syndrome was mentioned in the labeling information for only five drugs. Considering the severity of SCARs, it will be necessary to mention the potential risk of SCAR development in the labeling information during post-marketing surveillance. This study has some limitations. First, it was based on the ICSRs from the KIDS-KD, which is based on a spontaneous ADE reporting system. Therefore, the numbers may be underestimated due to underreporting. Second, the information in the ICSRs might not be sufficient to fully evaluate the complex epidemiologic nature of SCARs. Important epidemiologic factors were sometimes omitted in the ICSRs. Notably, multiple drugs taken at the time of symptom onset were registered as causative agents in cases in which the SCAR-inducing drug could not be determined. To overcome these limitations, the SCAR Special Interest Group was formed and a web-based SCAR registry was established in Korea. The SCAR registry enrolled patients with SCARs and gathered detailed epidemiologic information on baseline characteristics and various SCAR-associated phenotypes, in addition to collecting blood samples for the evaluation of genetic factors, such as HLA haplotype. We investigated the epidemiologic characteristics and causative drugs of SCARs in Korea using a nationwide ICSR database. Allopurinol was the most common SCAR-inducing individual drug in both the DRESS and SJS/TEN groups. Antiepileptics were the most common SCAR-causing drug group, followed by antibacterials and NSAIDs. We found that, despite the seriousness of SCARs, the potential of a drug to cause SCARs was not mentioned in the labeling information for more than half of the culprit agents studied. ACKNOWLEDGEMENTS This research was supported by a grant from the Ministry of Food and Drug Safety to the regional pharmacovigilance center in
8 Min-Gyu Kang, et al. AUTHOR CONTRIBUTIONS Conceptualization: Min-Gyu Kang, Han-Ki Park, Hye-Ryun Kang. Data curation: Min-Gyu Kang, Han-Ki Park. Formal analysis: Min- Gyu Kang, Han-Ki Park. Funding acquisition: Min-Gyu Kang, Hye- Ryun Kang. Investigation: Min-Gyu Kang, Hani-Ki Park, Hye-Ryun Kang. Methodology: Min-Gyu Kang, Dong-Yoon Kang, Min-Seok Yang, Kyung-Hee Sohn. Project administration: Min-Gyu Kang. Resources: Min-Gyu Kang, Han-Ki Park. Software: Min-Gyu Kang. Supervision: Min-Seok Yang, Hye-Ryun Kang, Ju-Yeun Lee. Validation: Min-Gyu Kang, Kyung-Hee Sohn, Ju-Yeun Lee. Visualization: Min- Gyu Kang. Writing original draft: Min-Gyu Kang, Hye-Ryun Kang. Writing review & editing: Min-Gyu Kang, Dong-Yoon Kang, Min- Seok Yang, Ju-Yeun Lee, Han-Ki Park, Kyung-Hee Sohn, Hye-Ryun Kang. ORCID ids Min-Gyu Kang Kyung-Hee Sohn Dong-Yoon Kang Han-Ki Park Min-Suk Yang Ju-Yeun Lee Hye-Ryun Kang REFERENCES International drug monitoring: the role of national centres. Report of a WHO meeting. World Health Organ Tech Rep Ser 1972;498: Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998;279: GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, : a systematic analysis for the Global Burden of Disease Study Lancet 2015;385: Swanson L, Colven RM. Approach to the patient with a suspected cutaneous adverse drug reaction. Med Clin North Am 2015;99: Yang MS, Kang MG, Jung JW, Song WJ, Kang HR, Cho SH, et al. Clinical features and prognostic factors in severe cutaneous drug reactions. Int Arch Allergy Immunol 2013;162: Kim SH, Lee KW, Song WJ, Kim SH, Jee YK, Lee SM, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res 2011;97: Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, et al. Positive and negative associations of HLA class I alleles with allopurinolinduced SCARs in Koreans. Pharmacogenet Genomics 2011;21: Kim MY, Yang MS, Kang HR, Cho SH, Min KU. Analysis of drugs causing severe cutaneous adverse reactions, based on the Korean Database of spontaneously reported adverse drug reactions. Korean J Med 2014;86: Yang MS, Lee JY, Kim J, Kim GW, Kim BK, Kim JY, et al. Incidence of Stevens-Johnson Syndrome and toxic epidermal necrolysis: a nationwide population-based study using National Health Insurance Database in Korea. PLoS One 2016;11:e Park HJ, Kim YJ, Kim DH, Kim J, Park KH, Park JW, et al. HLA allele frequencies in 5802 Koreans: varied allele types associated with SJS/TEN according to culprit drugs. Yonsei Med J 2016;57: Jung JW, Kim JY, Park IW, Choi BW, Kang HR. Genetic markers of severe cutaneous adverse reactions. Korean J Intern Med 2018;33: Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005; 102: Lee KW, Oh DH, Lee C, Yang SY. Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens 2005;65: Jung JW, Kim DK, Park HW, Oh KH, Joo KW, Kim YS, et al. An effective strategy to prevent allopurinol-induced hypersensitivity by HLA typing. Genet Med 2015;17: Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS, et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ 2015;351:h Niihara H, Kaneko S, Ito T, Sugamori T, Takahashi N, Kohno K, et al. HLA-B*58:01 strongly associates with allopurinol-induced adverse drug reactions in a Japanese sample population. J Dermatol Sci 2013;71: Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics 2008;18: Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med 2011;364: Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics 2006;16: Lee KW, Jeon H, Park JY. HLA-B*15 diversity in the Korean population. Tissue Antigens 2000;56: Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128: Shu C, Shu D, Tie D, Yu M, Zhang R, Wang T, et al. Toxic epidermal necrolysis induced by methazolamide in a Chinese-Korean man carrying HLA-B*59:01. Int J Dermatol 2015;54: Schöpf E, Stühmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Arch Dermatol 1991;127: Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol 2013;133: Sekula P, Liss Y, Davidovici B, Dunant A, Roujeau JC, Kardaun S, et al. Evaluation of SCORTEN on a cohort of patients with Stevens- Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res 2011;32: Eshki M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol 2009;145: Chiou CC, Yang LC, Hung SI, Chang YC, Kuo TT, Ho HC, et al. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: a study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol 2008;22:
The Diabetes Epidemic in Korea
 Review Article Endocrinol Metab 2016;31:349-33 http://dx.doi.org/.3803/enm.2016.31.3.349 pissn 2093-96X eissn 2093-978 The Diabetes Epidemic in Korea Junghyun Noh Department of Internal Medicine, Inje
Review Article Endocrinol Metab 2016;31:349-33 http://dx.doi.org/.3803/enm.2016.31.3.349 pissn 2093-96X eissn 2093-978 The Diabetes Epidemic in Korea Junghyun Noh Department of Internal Medicine, Inje
Epidemiologic characteristics of cervical cancer in Korean women
 Review Article J Gynecol Oncol Vol. 25, No. 1:70-74 pissn 2005-0380 eissn 2005-0399 Epidemiologic characteristics of cervical cancer in Korean women Hyun-Joo Seol, Kyung-Do Ki, Jong-Min Lee Department
Review Article J Gynecol Oncol Vol. 25, No. 1:70-74 pissn 2005-0380 eissn 2005-0399 Epidemiologic characteristics of cervical cancer in Korean women Hyun-Joo Seol, Kyung-Do Ki, Jong-Min Lee Department
Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital
 International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
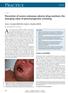 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
Standardized Thyroid Cancer Mortality in Korea between 1985 and 2010
 Original Article Endocrinol Metab 2014;29:530-535 http://dx.doi.org/10.3803/enm.2014.29.4.530 pissn 2093-596X eissn 2093-5978 Standardized Thyroid Cancer Mortality in Korea between 1985 and 2010 Yun Mi
Original Article Endocrinol Metab 2014;29:530-535 http://dx.doi.org/10.3803/enm.2014.29.4.530 pissn 2093-596X eissn 2093-5978 Standardized Thyroid Cancer Mortality in Korea between 1985 and 2010 Yun Mi
1 Introduction. Kenneth Irungu 1 David Nyamu
 Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Relation between the Peripherofacial Psoriasis and Scalp Psoriasis
 pissn 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 28, No. 4, 2016 http://dx.doi.org/10.5021/ad.2016.28.4.422 ORIGINAL ARTICLE Relation between the Peripherofacial Psoriasis and Scalp Psoriasis Kyung Ho
pissn 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 28, No. 4, 2016 http://dx.doi.org/10.5021/ad.2016.28.4.422 ORIGINAL ARTICLE Relation between the Peripherofacial Psoriasis and Scalp Psoriasis Kyung Ho
Multiple Drug Hypersensitivity
 Multiple Drug Hypersensitivity Werner J Pichler ADR-AC GmbH Bern UPDATE ON DRUG HYPERSENSITIVITY, Bern, March 23rd 2017 Definition Multiple drug hypersensitivity (MDH) MDH is a syndrome that develops as
Multiple Drug Hypersensitivity Werner J Pichler ADR-AC GmbH Bern UPDATE ON DRUG HYPERSENSITIVITY, Bern, March 23rd 2017 Definition Multiple drug hypersensitivity (MDH) MDH is a syndrome that develops as
The Epidemiology of Diabetes in Korea
 Review http://dx.doi.org/10.4093/dmj.2011.35.4.303 pissn 2233-6079 eissn 2233-6087 D I A B E T E S & M E T A B O L I S M J O U R N A L The Epidemiology of Diabetes in Korea Dae Jung Kim Department of Endocrinology
Review http://dx.doi.org/10.4093/dmj.2011.35.4.303 pissn 2233-6079 eissn 2233-6087 D I A B E T E S & M E T A B O L I S M J O U R N A L The Epidemiology of Diabetes in Korea Dae Jung Kim Department of Endocrinology
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
Cutaneous drug reactions to antiepileptic drugs and relation with HLA alleles in the Turkish population
 O R I G I N A L A R T I C L E Eur Ann Allergy Clin Immunol Vol 50, N 1, 36-41, 2018 S. Büyüköztürk 1, Ç. Kekik 2, A.Z. Gökyiğit 3, F.İ. Tezer Filik 4i, G. Karakaya 4, S. Saygı 4i, A.B. Dursun 5, S. Kirbaş
O R I G I N A L A R T I C L E Eur Ann Allergy Clin Immunol Vol 50, N 1, 36-41, 2018 S. Büyüköztürk 1, Ç. Kekik 2, A.Z. Gökyiğit 3, F.İ. Tezer Filik 4i, G. Karakaya 4, S. Saygı 4i, A.B. Dursun 5, S. Kirbaş
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW
 PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
Journal of Breast Cancer
 Journal of Breast Cancer ORIGINAL ARTICLE J Breast Cancer 215 March; 18(1): 8-15 http://dx.doi.org/1.448/jbc.215.18.1.8 Survival Improvement in Korean Breast Cancer Patients Due to Increases in Early-Stage
Journal of Breast Cancer ORIGINAL ARTICLE J Breast Cancer 215 March; 18(1): 8-15 http://dx.doi.org/1.448/jbc.215.18.1.8 Survival Improvement in Korean Breast Cancer Patients Due to Increases in Early-Stage
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME
 EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
Changes in the seroprevalence of IgG anti-hepatitis A virus between 2001 and 2013: experience at a single center in Korea
 pissn 2287-2728 eissn 2287-285X Original Article Clinical and Molecular Hepatology 214;2:162-167 Changes in the seroprevalence of IgG anti-hepatitis A virus between 21 and 213: experience at a single center
pissn 2287-2728 eissn 2287-285X Original Article Clinical and Molecular Hepatology 214;2:162-167 Changes in the seroprevalence of IgG anti-hepatitis A virus between 21 and 213: experience at a single center
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review
 Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review
 140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
 Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
 Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Diagnostic Analysis of Patients with Essential Hypertension Using Association Rule Mining
 Original Article Healthc Inform Res. 2010 June;16(2):77-81. pissn 2093-3681 eissn 2093-369X Diagnostic Analysis of Patients with Essential Hypertension Using Association Rule Mining A Mi Shin, RN, MS 1,
Original Article Healthc Inform Res. 2010 June;16(2):77-81. pissn 2093-3681 eissn 2093-369X Diagnostic Analysis of Patients with Essential Hypertension Using Association Rule Mining A Mi Shin, RN, MS 1,
: Ajou University College of Medicine, Suwon, Korea; Ajou University College of Medicine, Graduate
 CURRICULUM VITAE NAME Hyun Woo Lee, M.D. EDUCATION 1991.3.-2001.2 : Ajou University College of Medicine, Suwon, Korea; Doctor of Medicine 2004.3-2006.2 Ajou University College of Medicine, Graduate School,
CURRICULUM VITAE NAME Hyun Woo Lee, M.D. EDUCATION 1991.3.-2001.2 : Ajou University College of Medicine, Suwon, Korea; Doctor of Medicine 2004.3-2006.2 Ajou University College of Medicine, Graduate School,
Christy Pu Institutes Degree Department Period National Yang-Ming University (Taiwan)
 Christy Pu cypu@ym.edu.tw Degree Institutes Degree Department Period National Yang-Ming University (Taiwan) PhD Public Health 09/2005~06/2008 University of Oxford (UK) MSc Economics 08/2002~07/2003 University
Christy Pu cypu@ym.edu.tw Degree Institutes Degree Department Period National Yang-Ming University (Taiwan) PhD Public Health 09/2005~06/2008 University of Oxford (UK) MSc Economics 08/2002~07/2003 University
Health economics and drug safety
 Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
Health economics and drug safety Professor Dyfrig Hughes FFRPS FBPhS FLSW Centre for Health Economics & Medicines Evaluation Bangor University, Wales @HughesDyfrig ADEs that are not reactions to a medicine
Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan Pei Chen, Ph.D., Juei-Jueng Lin, M.D., Chin-Song Lu, M.D.,
T h e n e w e ngl a nd j o u r na l o f m e dic i n e original article Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan Pei Chen, Ph.D., Juei-Jueng Lin, M.D., Chin-Song Lu, M.D.,
Treatment of Right Colonic Diverticulitis: The Role of Nonoperative Treatment
 Original Article Journal of the Korean Society of DOI: 10.3393/jksc.2010.26.6.402 pissn 2093-7822 eissn 2093-7830 Treatment of Right Colonic Diverticulitis: The Role of Nonoperative Treatment Ma Ru Kim,
Original Article Journal of the Korean Society of DOI: 10.3393/jksc.2010.26.6.402 pissn 2093-7822 eissn 2093-7830 Treatment of Right Colonic Diverticulitis: The Role of Nonoperative Treatment Ma Ru Kim,
Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics
 MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
A survey of dental treatment under general anesthesia in a Korean university hospital pediatric dental clinic
 Original Article pissn 2383-9309 eissn 2383-9317 J Dent Anesth Pain Med 2016;16(3):203-208 http://dx.doi.org/10.17245/jdapm.2016.16.3.203 A survey of dental treatment under general anesthesia in a Korean
Original Article pissn 2383-9309 eissn 2383-9317 J Dent Anesth Pain Med 2016;16(3):203-208 http://dx.doi.org/10.17245/jdapm.2016.16.3.203 A survey of dental treatment under general anesthesia in a Korean
A study of the awareness of chronic liver diseases among Korean adults
 The Korean Journal of Hepatology 2011;17:99-105 DOI: 10.3350/kjhep.2011.17.2.99 Original Article A study of the awareness of chronic liver diseases among Korean adults Dae Won Jun 1,6*, Yong Kyun Cho 2,6*,
The Korean Journal of Hepatology 2011;17:99-105 DOI: 10.3350/kjhep.2011.17.2.99 Original Article A study of the awareness of chronic liver diseases among Korean adults Dae Won Jun 1,6*, Yong Kyun Cho 2,6*,
Prediction of Cancer Incidence and Mortality in Korea, 2018
 pissn 1598-2998, eissn 256 Cancer Res Treat. 218;5(2):317-323 Special Article https://doi.org/1.4143/crt.218.142 Open Access Prediction of Cancer Incidence and Mortality in Korea, 218 Kyu-Won Jung, MS
pissn 1598-2998, eissn 256 Cancer Res Treat. 218;5(2):317-323 Special Article https://doi.org/1.4143/crt.218.142 Open Access Prediction of Cancer Incidence and Mortality in Korea, 218 Kyu-Won Jung, MS
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
Department of Pharmacology & NIPER-Guwahati, Gauhati Medical College & Hospital, Guwahati , Assam, INDIA. 3
 J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia
 ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
Preventable Adverse Drug Reaction in pharmacovigilance database
 Preventable Adverse Drug Reaction in pharmacovigilance database Vu Dinh Hoa National Drug Information and Adverse Drug Monitoring Center Hanoi University of Pharmacy Vietnam 1 Introductions Until 2017:
Preventable Adverse Drug Reaction in pharmacovigilance database Vu Dinh Hoa National Drug Information and Adverse Drug Monitoring Center Hanoi University of Pharmacy Vietnam 1 Introductions Until 2017:
Association-heterogeneity mapping identifies an Asian-specific association of the GTF2I locus with rheumatoid arthritis
 Supplementary Material Association-heterogeneity mapping identifies an Asian-specific association of the GTF2I locus with rheumatoid arthritis Kwangwoo Kim 1,, So-Young Bang 1,, Katsunori Ikari 2,3, Dae
Supplementary Material Association-heterogeneity mapping identifies an Asian-specific association of the GTF2I locus with rheumatoid arthritis Kwangwoo Kim 1,, So-Young Bang 1,, Katsunori Ikari 2,3, Dae
Skin Manifestations of Drug Reactions
 Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Recent Trend in the Incidence of Premalignant and Malignant Skin Lesions in Korea between 1991 and 2006
 J Korean Med Sci 2010; 25: 924-9 ISSN 1011-8934 DOI: 10.3346/jkms.2010.25.6.924 Recent Trend in the Incidence of Premalignant and Malignant Skin Lesions in Korea between 1991 and 2006 We evaluated the
J Korean Med Sci 2010; 25: 924-9 ISSN 1011-8934 DOI: 10.3346/jkms.2010.25.6.924 Recent Trend in the Incidence of Premalignant and Malignant Skin Lesions in Korea between 1991 and 2006 We evaluated the
Cutaneous drug reactions
 Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
 BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
두경부영역의악성림프종 태경 1 이형석 1 서인석 1 이용섭 1 조석현 1 최정혜 2 안명주 2. Hodgkin s and Non-Hodgkin s Lymphoma of Head and Neck
 KISEP Head and Neck Korean J Otolaryngol 2003;46:324-30 한양대학교의과대학이비인후과학교실, 1 내과학교실 2 태경 1 이형석 1 서인석 1 이용섭 1 조석현 1 최정혜 2 안명주 2 Hodgkin s and Non-Hodgkin s Lymphoma of Head and Neck Kyung Tae, MD 1, Hyung
KISEP Head and Neck Korean J Otolaryngol 2003;46:324-30 한양대학교의과대학이비인후과학교실, 1 내과학교실 2 태경 1 이형석 1 서인석 1 이용섭 1 조석현 1 최정혜 2 안명주 2 Hodgkin s and Non-Hodgkin s Lymphoma of Head and Neck Kyung Tae, MD 1, Hyung
Original Article. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C
 Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Acne is one of the most common skin diseases. It usually occurs during adolescence, but can
 DOI: 10.5124/jkma.2010.53.7.623 pissn: 1975-8456 eissn: 2093-5951 http://jkma.org Pharmacotherapeutics Pharmacologic Treatment of Acne Dae Hun Suh, MD Department of Dermatology, Seoul National University
DOI: 10.5124/jkma.2010.53.7.623 pissn: 1975-8456 eissn: 2093-5951 http://jkma.org Pharmacotherapeutics Pharmacologic Treatment of Acne Dae Hun Suh, MD Department of Dermatology, Seoul National University
Recent experiences to review data from MRCTs and progress of research on ethnic factors. Dr Yoshiaki Uyama
 Recent experiences to review data from MRCTs and progress of research on ethnic factors Dr Yoshiaki Uyama (PMDA) Visiting Professor, Graduate School of Advanced Clinical Science, Chiba University Visiting
Recent experiences to review data from MRCTs and progress of research on ethnic factors Dr Yoshiaki Uyama (PMDA) Visiting Professor, Graduate School of Advanced Clinical Science, Chiba University Visiting
Original Article General Laboratory Medicine INTRODUCTION
 Original Article General Laboratory Medicine Ann Lab Med 2018;38:249-254 https://doi.org/10.3343/alm.2018.38.3.249 ISSN 2234-3806 eissn 2234-3814 Budget Impact of the Accreditation Program for Clinical
Original Article General Laboratory Medicine Ann Lab Med 2018;38:249-254 https://doi.org/10.3343/alm.2018.38.3.249 ISSN 2234-3806 eissn 2234-3814 Budget Impact of the Accreditation Program for Clinical
Statistical data preparation: management of missing values and outliers
 KJA Korean Journal of Anesthesiology Statistical Round pissn 2005-6419 eissn 2005-7563 Statistical data preparation: management of missing values and outliers Sang Kyu Kwak 1 and Jong Hae Kim 2 Departments
KJA Korean Journal of Anesthesiology Statistical Round pissn 2005-6419 eissn 2005-7563 Statistical data preparation: management of missing values and outliers Sang Kyu Kwak 1 and Jong Hae Kim 2 Departments
Time Series Changes in Cataract Surgery in Korea
 pissn: 111-8942 eissn: 292-9382 Korean J Ophthalmol 218;32(3):182-189 https://doi.org/1.3341/kjo.217.72 Time Series Changes in Cataract Surgery in Korea Original Article Ju Hwan Song 1*, Jung Youb Kang
pissn: 111-8942 eissn: 292-9382 Korean J Ophthalmol 218;32(3):182-189 https://doi.org/1.3341/kjo.217.72 Time Series Changes in Cataract Surgery in Korea Original Article Ju Hwan Song 1*, Jung Youb Kang
Incidence and Mortality after Proximal Humerus Fractures Over 50 Years of Age in South Korea: National Claim Data from 2008 to 2012
 J Bone Metab 2015;22:17-21 http://dx.doi.org/10.11005/jbm.2015.22.1.17 pissn 2287-6375 eissn 2287-7029 Original Article Incidence and Mortality after Proximal Humerus Fractures Over 50 s of Age in South
J Bone Metab 2015;22:17-21 http://dx.doi.org/10.11005/jbm.2015.22.1.17 pissn 2287-6375 eissn 2287-7029 Original Article Incidence and Mortality after Proximal Humerus Fractures Over 50 s of Age in South
Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study
 open access Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study Tai-Ming Ko, 1,2 Chang-Youh Tsai, 3,4 Shih-Yang
open access Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study Tai-Ming Ko, 1,2 Chang-Youh Tsai, 3,4 Shih-Yang
Non-Beta-lactam Antibiotic: Testing and Desensitization
 Non-Beta-lactam Antibiotic: Testing and Desensitization David A. Khan, MD Professor of Medicine Allergy & Immunology Program Director Division of Allergy & Immunology 1 Disclosures n Research Grants n
Non-Beta-lactam Antibiotic: Testing and Desensitization David A. Khan, MD Professor of Medicine Allergy & Immunology Program Director Division of Allergy & Immunology 1 Disclosures n Research Grants n
Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies
 /, 2016, Vol. 7, (No. 49), pp: 81870-81879 Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies Ran Wu 1,*, Yi-ju Cheng 2,3,*,
/, 2016, Vol. 7, (No. 49), pp: 81870-81879 Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies Ran Wu 1,*, Yi-ju Cheng 2,3,*,
Carbamazepine Hypersensitivity: Progress Toward Predicting the Unpredictable
 Carbamazepine Hypersensitivity: Progress Toward Predicting the Unpredictable Current Literature In Clinical Science HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. McCormack
Carbamazepine Hypersensitivity: Progress Toward Predicting the Unpredictable Current Literature In Clinical Science HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. McCormack
Prevalence of Sarcopenia Adjusted Body Mass Index in the Korean Woman Based on the Korean National Health and Nutritional Examination Surveys
 J Bone Metab 2016;23:243-247 https://doi.org/10.11005/jbm.2016.23.4.243 pissn 2287-6375 eissn 2287-7029 Original Article Prevalence of Sarcopenia Adjusted Body Mass Index in the Korean Woman Based on the
J Bone Metab 2016;23:243-247 https://doi.org/10.11005/jbm.2016.23.4.243 pissn 2287-6375 eissn 2287-7029 Original Article Prevalence of Sarcopenia Adjusted Body Mass Index in the Korean Woman Based on the
Insulin Secretory Capacity and Insulin Resistance in Korean Type 2 Diabetes Mellitus Patients
 Review Article Endocrinol Metab 2016;31:354-360 http://dx.doi.org/10.3803/enm.2016.31.3.354 pissn 2093-596X eissn 2093-5978 Insulin Secretory Capacity and Insulin Resistance in Korean Type 2 Diabetes Mellitus
Review Article Endocrinol Metab 2016;31:354-360 http://dx.doi.org/10.3803/enm.2016.31.3.354 pissn 2093-596X eissn 2093-5978 Insulin Secretory Capacity and Insulin Resistance in Korean Type 2 Diabetes Mellitus
Supplementary Appendix
 Supplementary Appendix Increased Risk of Atrial Fibrillation and Thromboembolism in Patients with Severe Psoriasis: a Nationwide Population-based Study Tae-Min Rhee, MD 1, Ji Hyun Lee, MD 2, Eue-Keun Choi,
Supplementary Appendix Increased Risk of Atrial Fibrillation and Thromboembolism in Patients with Severe Psoriasis: a Nationwide Population-based Study Tae-Min Rhee, MD 1, Ji Hyun Lee, MD 2, Eue-Keun Choi,
PedsCases Podcast Scripts
 PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
Is the Initial Size of Tuberculous Lymphadenopathy associated with Lymph Node Enlargement during Treatment?
 Brief Communication https://doi.org/10.3947/ic.2017.49.2.130 Infect Chemother 2017;49(2):130-134 ISSN 2093-2340 (Print) ISSN 2092-6448 (Online) Infection & Chemotherapy Is the Initial Size of Tuberculous
Brief Communication https://doi.org/10.3947/ic.2017.49.2.130 Infect Chemother 2017;49(2):130-134 ISSN 2093-2340 (Print) ISSN 2092-6448 (Online) Infection & Chemotherapy Is the Initial Size of Tuberculous
Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015)
 1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
Bugs and Drugs: What s New in Hypersensitivity Reactions?
 Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Adverse Drug Reactions (ADRs) Outline
 Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Analysis of Chemotherapy Telephone Helpline in the Ambulatory Oncology Unit
 , pp.24-28 http://dx.doi.org/10.14257/astl.2013 Analysis of Chemotherapy Telephone Helpline in the Ambulatory Oncology Unit Hee Jin, Kim 1, Hye Jin, Kim 1 and Kyung Ja, Kang 2 1 Seoul National University
, pp.24-28 http://dx.doi.org/10.14257/astl.2013 Analysis of Chemotherapy Telephone Helpline in the Ambulatory Oncology Unit Hee Jin, Kim 1, Hye Jin, Kim 1 and Kyung Ja, Kang 2 1 Seoul National University
Current status of hepatic surgery in Korea
 Korean J Hepatol. 2009 Dec; 15(Suppl 6):S60 - S64. DOI: 10.3350/kjhep.2009.15.S6.S60 Current status of hepatic surgery in Korea Kyung Sik Kim Department of Surgery, Severance Hospital, Yonsei University
Korean J Hepatol. 2009 Dec; 15(Suppl 6):S60 - S64. DOI: 10.3350/kjhep.2009.15.S6.S60 Current status of hepatic surgery in Korea Kyung Sik Kim Department of Surgery, Severance Hospital, Yonsei University
Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients
 original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
Prediction of Cancer Incidence and Mortality in Korea, 2013
 pissn 1598-2998, eissn 256 Cancer Res Treat. 213;45(1):15-21 Special Article http://dx.doi.org/1.4143/crt.213.45.1.15 Open Access Prediction of Cancer Incidence and Mortality in Korea, 213 Kyu-Won Jung,
pissn 1598-2998, eissn 256 Cancer Res Treat. 213;45(1):15-21 Special Article http://dx.doi.org/1.4143/crt.213.45.1.15 Open Access Prediction of Cancer Incidence and Mortality in Korea, 213 Kyu-Won Jung,
TRANSPARENCY COMMITTEE OPINION. 19 July 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 July 2006 Keppra 250 mg, film-coated tablets Box of 60 tablets (CIP code: 356 013-6) Keppra 500 mg, film-coated
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 July 2006 Keppra 250 mg, film-coated tablets Box of 60 tablets (CIP code: 356 013-6) Keppra 500 mg, film-coated
The Clinical Effects of Carthami-Flos Pharmacopuncture on Posterior Neck pain of Menopausal Women
 ISSN 2093-6966 Journal of Pharmacopuncture 71 http://dx.doi.org/10.3831/kpi.2011.14.4.071 Received : Nov 10, 2011 Revised : Nov 25, 2011 Accepted : Nov 30, 2011 KEY WORDS: Carthami-Flos; Neck pain, Pharmacopuncture;
ISSN 2093-6966 Journal of Pharmacopuncture 71 http://dx.doi.org/10.3831/kpi.2011.14.4.071 Received : Nov 10, 2011 Revised : Nov 25, 2011 Accepted : Nov 30, 2011 KEY WORDS: Carthami-Flos; Neck pain, Pharmacopuncture;
Address for correspondence: Dr. Patel Raksha M. R-3, Doctor s Quarters, Jail Road, Vadodara- 01, Gujarat, India.
 Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Wei-Chung Cheng ( 鄭維中 )
 Wei-Chung Cheng ( 鄭維中 ) Graduate Institute of Biomedical Scienses China Medical University 8F, No. 6, Hsueh-Shih Road, Taichung 404, Taiwan TaiChung 404, Taiwan E-mail: wccheng@mail.cmu.edu.tw Office :
Wei-Chung Cheng ( 鄭維中 ) Graduate Institute of Biomedical Scienses China Medical University 8F, No. 6, Hsueh-Shih Road, Taichung 404, Taiwan TaiChung 404, Taiwan E-mail: wccheng@mail.cmu.edu.tw Office :
Objective research on tongue manifestation of patients with eczema
 Technology and Health Care 25 (2017) S143 S149 DOI 10.3233/THC-171316 IOS Press S143 Objective research on tongue manifestation of patients with eczema Zhifeng Yu, Haifang Zhang, Linjie Fu and Xiaozuo
Technology and Health Care 25 (2017) S143 S149 DOI 10.3233/THC-171316 IOS Press S143 Objective research on tongue manifestation of patients with eczema Zhifeng Yu, Haifang Zhang, Linjie Fu and Xiaozuo
A new classification system of nasal contractures
 Original Article J Cosmet Med 2017;1(2):106-111 https://doi.org/10.25056/jcm.2017.1.2.106 pissn 2508-8831, eissn 2586-0585 A new classification system of nasal contractures Geunuck Chang 1, Donghak Jung
Original Article J Cosmet Med 2017;1(2):106-111 https://doi.org/10.25056/jcm.2017.1.2.106 pissn 2508-8831, eissn 2586-0585 A new classification system of nasal contractures Geunuck Chang 1, Donghak Jung
α chain β chain C N C N β 2 α 1 β 1 α 2
 a α chain c β chain C N C N β 2 m α chain b d N C N C β 1 α 2 Supplemental Figure 1 Major histocompatibility complex (MHC) class I and class II structures. (a, b)representation of the structure of the
a α chain c β chain C N C N β 2 m α chain b d N C N C β 1 α 2 Supplemental Figure 1 Major histocompatibility complex (MHC) class I and class II structures. (a, b)representation of the structure of the
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Drug Allergy A Guide to Diagnosis and Management
 Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Clinical Course of Segmental Vitiligo: A Retrospective Study of Eighty-Seven Patients
 Ann Dermatol Vol. 26, No. 1, 2014 http://dx.doi.org/10.5021/ad.2014.26.1.61 ORIGINAL ARTICLE Clinical Course of Segmental Vitiligo: A Retrospective Study of Eighty-Seven Patients Ji-Hye Park, Mi-Young
Ann Dermatol Vol. 26, No. 1, 2014 http://dx.doi.org/10.5021/ad.2014.26.1.61 ORIGINAL ARTICLE Clinical Course of Segmental Vitiligo: A Retrospective Study of Eighty-Seven Patients Ji-Hye Park, Mi-Young
The Economic Burden of Inflammatory Heart Disease in Korea
 ORIGINAL ARTICLE http://dx.doi.org/10.4070/kcj.2011.41.12.712 Print ISSN 1738-5520 / On-line ISSN 1738-5555 Copyright 2011 The Korean Society of Cardiology Open Access The Economic Burden of Inflammatory
ORIGINAL ARTICLE http://dx.doi.org/10.4070/kcj.2011.41.12.712 Print ISSN 1738-5520 / On-line ISSN 1738-5555 Copyright 2011 The Korean Society of Cardiology Open Access The Economic Burden of Inflammatory
The Pulmonary Embolism Severity Index in Predicting the Prognosis of Patients With Pulmonary Embolism
 ORIGINAL ARTICLE DOI: 10.3904/kjim.2009.24.2.123 The Pulmonary Embolism Severity Index in Predicting the Prognosis of Patients With Pulmonary Embolism Won-Ho Choi 1, Sung Uk Kwon 1,2, Yoon Jung Jwa 1,
ORIGINAL ARTICLE DOI: 10.3904/kjim.2009.24.2.123 The Pulmonary Embolism Severity Index in Predicting the Prognosis of Patients With Pulmonary Embolism Won-Ho Choi 1, Sung Uk Kwon 1,2, Yoon Jung Jwa 1,
Factors that cause influence on the knowledge of oral health of university students.
 Biomedical Research 2017; 28 (12): 5565-5571 ISSN 0970-938X www.biomedres.info Factors that cause influence on the knowledge of oral health of university students. Hye-Young Kim 1, Dong-il Chun 2, Yi-Sub
Biomedical Research 2017; 28 (12): 5565-5571 ISSN 0970-938X www.biomedres.info Factors that cause influence on the knowledge of oral health of university students. Hye-Young Kim 1, Dong-il Chun 2, Yi-Sub
The Symptom Trajectory of Attention-Deficit Hyperactivity Disorder in Korean School-Age Children
 ORIGINAL ARTICLE https://doi.org/10.30773/pi.2017.11.01.1 Print ISSN 1738-3684 / On-line ISSN 1976-3026 OPEN ACCESS The Symptom Trajectory of Attention-Deficit Hyperactivity Disorder in Korean School-Age
ORIGINAL ARTICLE https://doi.org/10.30773/pi.2017.11.01.1 Print ISSN 1738-3684 / On-line ISSN 1976-3026 OPEN ACCESS The Symptom Trajectory of Attention-Deficit Hyperactivity Disorder in Korean School-Age
SAMPLE REPORT MENTAL HEALTH DNA INSIGHT
 PERSONAL DETAILS DOB Jan 1, 19XX ETHNICITY Caucasian ORDERING HEALTHCARE PROFESSIONAL Glenn Braunstein.D. 6777 Nancy Ridge Drive San Diego, CA 92121 US LABORATORY INFO ACCESSION NUBER ACTIVATION CODE SPECIEN
PERSONAL DETAILS DOB Jan 1, 19XX ETHNICITY Caucasian ORDERING HEALTHCARE PROFESSIONAL Glenn Braunstein.D. 6777 Nancy Ridge Drive San Diego, CA 92121 US LABORATORY INFO ACCESSION NUBER ACTIVATION CODE SPECIEN
CURRICULUM VITAE China Medical College, College of Medicine, Taichung, Taiwan, R.O.C.
 CURRICULUM VITAE NAME: Huang, Chung-Ming OFFICE ADDRESS: China Medical University Hospital, No. 2, Yuh-Der Road, Taichung, Taiwan, R.O.C., 北 路 2 EDUCATION: 1978-1985 China Medical College, College of Medicine,
CURRICULUM VITAE NAME: Huang, Chung-Ming OFFICE ADDRESS: China Medical University Hospital, No. 2, Yuh-Der Road, Taichung, Taiwan, R.O.C., 北 路 2 EDUCATION: 1978-1985 China Medical College, College of Medicine,
The detection rate of early gastric cancer has been increasing owing to advances in
 Focused Issue of This Month Sung Hoon Noh, MD, ph.d Department of Surgery, Yonsei University College of Medicine E - mail : sunghoonn@yuhs.ac J Korean Med Assoc 2010; 53(4): 306-310 Abstract The detection
Focused Issue of This Month Sung Hoon Noh, MD, ph.d Department of Surgery, Yonsei University College of Medicine E - mail : sunghoonn@yuhs.ac J Korean Med Assoc 2010; 53(4): 306-310 Abstract The detection
A comparison of statistical methods for adjusting the treatment effects in genetic association studies of quantitative traits
 34 1 Journal of the Korean Society of Health Information and Health Statistics Volume 34, Number 1, 2009, pp. 53 62 53 한경화 1), 임길섭 2), 박성하 3), 장양수 4), 송기준 2) 1), 2), 3), 4) A comparison of statistical
34 1 Journal of the Korean Society of Health Information and Health Statistics Volume 34, Number 1, 2009, pp. 53 62 53 한경화 1), 임길섭 2), 박성하 3), 장양수 4), 송기준 2) 1), 2), 3), 4) A comparison of statistical
Experience of Korea Acute Myocardial Infarction Registry (KAMIR)
 Japan-Korea Joint AMI Symposium Korean Society of Myocardial Infarction Apr 18-19 2014 Experience of Korea Acute Myocardial Infarction Registry (KAMIR) Myung Ho Jeong, MD, PhD, FACC, FAHA, FESC, FSCAI,
Japan-Korea Joint AMI Symposium Korean Society of Myocardial Infarction Apr 18-19 2014 Experience of Korea Acute Myocardial Infarction Registry (KAMIR) Myung Ho Jeong, MD, PhD, FACC, FAHA, FESC, FSCAI,
Past and Current Status of Adult Type 2 Diabetes Mellitus Management in Korea: A National Health Insurance Service Database Analysis
 Review Clinical Diabetes & Therapeutics Diabetes Metab J 218;42:93-1 https://doi.org/1.493/dmj.218.42.2.93 pissn 2233-679 eissn 2233-687 DIABETES & METABOLISM JOURNAL Past and Current Status of Adult Type
Review Clinical Diabetes & Therapeutics Diabetes Metab J 218;42:93-1 https://doi.org/1.493/dmj.218.42.2.93 pissn 2233-679 eissn 2233-687 DIABETES & METABOLISM JOURNAL Past and Current Status of Adult Type
Complications Requiring Hospital Admission and Causes of In-Hospital Death over Time in Alcoholic and Nonalcoholic Cirrhosis Patients
 Gut and Liver, Vol. 10, No. 1, January 2016, pp. 95-100 ORiginal Article Complications Requiring Hospital Admission and Causes of In-Hospital Death over Time in and Cirrhosis Patients Hee Yeon Kim, Chang
Gut and Liver, Vol. 10, No. 1, January 2016, pp. 95-100 ORiginal Article Complications Requiring Hospital Admission and Causes of In-Hospital Death over Time in and Cirrhosis Patients Hee Yeon Kim, Chang
Adverse drug reactions
 Medications William Smith Adverse drug reactions Allergy? Side-effect? Intolerance? Background Adverse drug reactions (ADRs) vary from life-threatening anaphylaxis to minor common side-effects. Objective
Medications William Smith Adverse drug reactions Allergy? Side-effect? Intolerance? Background Adverse drug reactions (ADRs) vary from life-threatening anaphylaxis to minor common side-effects. Objective
Factors Influencing Satisfaction with Life in Female Nursing College Students with Irritable Bowel Syndrome
 Vol.132 (Healthcare and Nursing 2016), pp.198-203 http://dx.doi.org/10.14257/astl.2016. Factors Influencing Satisfaction with Life in Female Nursing College Students with Irritable Bowel Syndrome Sung
Vol.132 (Healthcare and Nursing 2016), pp.198-203 http://dx.doi.org/10.14257/astl.2016. Factors Influencing Satisfaction with Life in Female Nursing College Students with Irritable Bowel Syndrome Sung
University of Groningen
 University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
1. Introduction. Abstract. Young-Ju Kim 1 and Myoungjin Kwon 2 *
 Indian Journal of Science and Technology, Vol 9(41), DOI: 10.17485/ijst/2016/v9i41/103903, November 2016 ISSN (Print) : 0974-6846 ISSN (Online) : 0974-5645 Identification of Factors Influencing Subjective
Indian Journal of Science and Technology, Vol 9(41), DOI: 10.17485/ijst/2016/v9i41/103903, November 2016 ISSN (Print) : 0974-6846 ISSN (Online) : 0974-5645 Identification of Factors Influencing Subjective
Antithrombotic Therapy in Patients with Atrial Fibrillation
 Antithrombotic Therapy in Patients with Atrial Fibrillation June Soo Kim, M.D., Ph.D. Department of Medicine Cardiac & Vascular Center, Samsung Medical Center Sungkyunkwan University School of Medicine
Antithrombotic Therapy in Patients with Atrial Fibrillation June Soo Kim, M.D., Ph.D. Department of Medicine Cardiac & Vascular Center, Samsung Medical Center Sungkyunkwan University School of Medicine
Estimation of Stellate Ganglion Block Injection Point Using the Cricoid Cartilage as Landmark Through X-ray Review
 Original Article Korean J Pain 2011 September; Vol. 24, No. 3: 141-145 pissn 2005-9159 eissn 2093-0569 http://dx.doi.org/10.3344/kjp.2011.24.3.141 Estimation of Stellate Ganglion Block Injection Point
Original Article Korean J Pain 2011 September; Vol. 24, No. 3: 141-145 pissn 2005-9159 eissn 2093-0569 http://dx.doi.org/10.3344/kjp.2011.24.3.141 Estimation of Stellate Ganglion Block Injection Point
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients
 Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Prevalence of Psoriasis in Korea: A Population-Based Epidemiological Study Using the Korean National Health Insurance Database
 pissn 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 29, No. 6, 2017 https://doi.org/10.5021/ad.2017.29.6.761 ORIGINAL ARTICLE Prevalence of Psoriasis in Korea: A Population-Based Epidemiological Study Using
pissn 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 29, No. 6, 2017 https://doi.org/10.5021/ad.2017.29.6.761 ORIGINAL ARTICLE Prevalence of Psoriasis in Korea: A Population-Based Epidemiological Study Using
Endocrine Surgery. Characteristics of the Germline MEN1 Mutations in Korea: A Literature Review ORIGINAL ARTICLE. The Korean Journal of INTRODUCTION
 ORIGINAL ARTICLE ISSN 1598-1703 (Print) ISSN 2287-6782 (Online) Korean J Endocrine Surg 2014;14:7-11 The Korean Journal of Endocrine Surgery Characteristics of the Germline MEN1 Mutations in Korea: A Literature
ORIGINAL ARTICLE ISSN 1598-1703 (Print) ISSN 2287-6782 (Online) Korean J Endocrine Surg 2014;14:7-11 The Korean Journal of Endocrine Surgery Characteristics of the Germline MEN1 Mutations in Korea: A Literature
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Cognitive impairment evaluated with Vascular Cognitive Impairment Harmonization Standards in a multicenter prospective stroke cohort in Korea Supplemental Methods Participants From
SUPPLEMENTAL MATERIAL Cognitive impairment evaluated with Vascular Cognitive Impairment Harmonization Standards in a multicenter prospective stroke cohort in Korea Supplemental Methods Participants From
Analysis of International Journal of Clinical Preventive Dentistry Research Trends Using Word Network Analysis
 Original Article Int J Clin Prev Dent 2018;14(3):184-189 ㆍ https://doi.org/10.15236/ijcpd.2018.14.3.184 ISSN (Print) 1738-8546 ㆍ ISSN (Online) 2287-6197 Analysis of International Journal of Clinical Preventive
Original Article Int J Clin Prev Dent 2018;14(3):184-189 ㆍ https://doi.org/10.15236/ijcpd.2018.14.3.184 ISSN (Print) 1738-8546 ㆍ ISSN (Online) 2287-6197 Analysis of International Journal of Clinical Preventive
Establishment of Korean prostate cancer database by the Korean Urological Oncology Society
 Original Article - Urological Oncology Investig Clin Urol 217;58:434-439. https://doi.org/1.4111/icu.217.58.6.434 pissn 2466-493 eissn 2466-54X Establishment of Korean prostate cancer database by the Korean
Original Article - Urological Oncology Investig Clin Urol 217;58:434-439. https://doi.org/1.4111/icu.217.58.6.434 pissn 2466-493 eissn 2466-54X Establishment of Korean prostate cancer database by the Korean
The liver and skin are two of the commonest
 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
