Macaque Ventral Premotor Cortex Exerts Powerful Facilitation of Motor Cortex Outputs to Upper Limb Motoneurons
|
|
|
- Cornelius Horton
- 6 years ago
- Views:
Transcription
1 1200 The Journal of Neuroscience, February 4, (5): Behavioral/Systems/Cognitive Macaque Ventral Premotor Cortex Exerts Powerful Facilitation of Motor Cortex Outputs to Upper Limb Motoneurons H. Shimazu, 1 M. A. Maier, 2 G. Cerri, 1 P. A. Kirkwood, 1 and R. N. Lemon 1 1 Sobell Department of Motor Neuroscience and Movement Disorders, Institute of Neurology, University College London, London WC1N 3BG, United Kingdom, and 2 University Paris-VI and Paris-VII and Institut National de la Santé et de la Recherche Médicale U483, Paris, France The ventral premotor area (F5) is part of the cortical circuit controlling visuomotor grasp. F5 could influence hand motor function through at least two pathways: corticospinal projections and corticocortical projections to primary motor cortex (M1). We found that stimulation of macaque F5, which by itself evoked little or no detectable corticospinal output, could produce a robust modulation of motor outputs from M1. Arrays of fine microwires were implanted in F5 and M1. During terminal experiments under chloralose anesthesia, single stimuli delivered to M1 electrodes evoked direct (D) and indirect (I 1,I 2, and I 3 ) corticospinal volleys. In contrast, single F5 shocks were ineffective; double shocks (3 msec separation) evoked small I waves but no D wave. However, when the test (T) M1 shock was conditioned (C) by single or double F5 shocks, there was strong facilitation of I 2 and I 3 waves from M1, with C T intervals of 1 msec. Intracellular recordings from 79 arm and hand motoneurons (MNs) revealed no postsynaptic effects from single F5 shocks. In contrast, these stimuli produced a robust facilitation of I 2 and I 3 EPSPs evoked from M1 (60% of MNs); this was particularly marked in hand muscle MNs (92%). Muscimol injection in M1 reduced I waves from F5 and abolished the F5-induced facilitation of late I waves from M1, and of EPSPs associated with them. Thus, some motor effects evoked from F5 may be mediated by corticocortical inputs to M1 impinging on interneurons generating late corticospinal I waves. Similar mechanisms may allow F5 to modulate grasp-related outputs from M1. Key words: motor cortex; movement (motion; motor activity); premotor; stimulation; corticospinal; monkey Introduction Area F5 in the ventral premotor cortex (PMv) is part of a corticocortical circuit involved in visuomotor control of the hand and in the elaboration of hand shapes appropriate for grasping visible objects. PMv is thought to be involved in transforming information about the location of an object in a visual frame of reference to one in a motor reference frame (Jeannerod et al., 1995; Kakei et al., 1999, 2001, 2003). F5 neurons show object-specific activity during grasp and muscimol injection into area F5 interferes with visually guided grasp (Murata et al., 1997; Rizzolatti et al., 1998, 2002; Fogassi et al., 2001). If area F5 transforms visual information to motor outputs controlling hand shape, how does it influence the hand muscles that shape grasp? PMv, like other premotor areas, is characterized both by having separate corticospinal projections to the spinal cord and by reciprocal corticocortical connections with primary Received Oct. 20, 2003; revised Dec. 3, 2003; accepted Dec. 3, This work was supported in part by the Wellcome Trust and by a European Union project (QLRT ; Corticospinal Modeling). We thank Thomas Brochier, Rachel Spinks, Chris Seers, Helen Lewis, and Sam Shepherd for assistance. Correspondence should be addressed to Professor Roger Lemon, Sobell Department of Motor Neuroscience and Movement Disorders, Institute of Neurology, University College London, Queen Square, London WC1N 3BG, UK. rlemon@ion.ucl.ac.uk. H. Shimazu s present address: Department of Neurology, Tokushima University School of Medicine, Kuramotocho , Tokushima City , Japan. DOI: /JNEUROSCI Copyright 2004 Society for Neuroscience /04/ $15.00/0 motor cortex (M1) (Dum and Strick, 1991, 2002). PMv is electrically excitable, and ricms in F5 evokes hand and digit movements (Hepp-Reymond et al., 1994; Godschalk et al., 1995; Dum and Strick, 2002). However, it is not known which pathways mediate these motor effects. Compared with other premotor areas, corticospinal outputs from F5 are few in number and do not project as far as the cervical enlargement, where motor nuclei supplying hand muscles are located, but terminate at midcervical levels (He et al., 1993; Galea and Darian-Smith, 1994). F5 could also influence hand muscles via its dense corticocortical projections to M1 (Muakkassa and Strick, 1979; Godschalk et al., 1984; Matelli et al., 1986; Ghosh et al., 1987; Tokuno and Nambu, 2000; Dum and Strick, 2002). M1 is known to play a major role in hand control (Porter and Lemon 1993; Lemon et al., 2004). In a previous study, Cerri et al. (2003) showed that although stimulation of F5 through chronically implanted microwires was ineffective in producing EMG responses in contralateral hand muscles when delivered alone, these same stimuli could produce a robust facilitation of responses evoked from M1. EMG responses could be up to four times larger when conditioned by single shocks to F5, and up to 12 times larger for double F5 shocks. The earliest facilitation from F5 occurred at short intervals ( 1 msec), indicating a local site of interaction of unknown location. We have investigated the mechanisms underlying F5 M1 excitatory interactions in terminal experiments under general an-
2 Shimazu et al. Premotor Motor Cortex Interactions in Macaque J. Neurosci., February 4, (5): Table 1. Electrode locations Case Species Weight (kg) Electrode type Location of M1 electrodes Location of F5 electrodes Effect of M1 ricms and threshold CS12 M. fascicularis 3.2 Chronic ve (3) 5.5 mm, ant bank CS, wm ve (1) 3.0 mm, inf bank AS, V II V extension ve (2) 2.0 mm, ant bank CS, V ve (2) 2.0 mm, inf bank AS, V 55 A CS13 M. fascicularis 3.1 Chronic ve (2) 4.5 mm, ant bank CS, VI ve (6) 2.8 mm, inf bank AS, V Thumb flexion ve (1) 2.0 mm, ant bank CS, V ve (5) 6.0 mm, inf bank AS, VI 35 A CS14 M. fascicularis 4.4 Chronic ve (4) 4.0 mm, ant bank CS, V ve (10) 2.2 mm, inf bank AS, V/VI Thumb abduction ve (3) 2.2 mm, ant bank CS, V ve (7) 4.5 mm, inf bank AS, II/III 50 A CS16 M. mulatta 6.9 Acute ve (2) 2.8 mm, convx PG, V/VI ve (4) 1.2 mm, inf bank AS, III Not tested ve (1) 2.8 mm, ant bank CS, V ve (3) 1.5 mm, inf bank AS, II/III CS17 M. mulatta 5.9 Acute ve (2) 1.5 mm, ant bank CS, V ve (4) 1.2 mm, inf bank AS, III Not tested ve (1) 2.0 mm, ant bank CS, V ve (3) 1.2 mm, inf bank AS, II Data are presented as polarity of electrode ( ve/ ve), electrode number (number in parentheses), depth of tip from surface, location, and lamina. CS, Central sulcus; AS, arcuate sulcus; convx, convexity of precentral gyrus (PG); ant, anterior; inf, inferior; wm, white matter. esthesia. We show that conditioning F5 stimulation produces a strong facilitation of the late corticospinal volleys evoked from M1, referred to as I 2 and I 3 waves. Postsynaptic responses to these later waves in hand motoneurons were also enhanced. The detailed time course of the facilitation from F5 suggested that peak effects were observed at the times at which I waves were generated in M1. Finally, we demonstrated that the facilitation from F5 of corticospinal activity and the associated motoneuronal responses were abolished by local microinjection of the GABA A agonist muscimol into the hand area of M1. These facilitatory interactions between F5 and M1 may also be involved in visuomotor transformations during grasp. Materials and Methods The study was performed on five adult, purpose-bred monkeys (Macaca fascicularis and Macaca mulatta; cases CS12, CS13, CS14, CS16, and CS17) (Table 1). Results of related experiments in two of these cases (CS13 and CS14) were reported by Cerri et al. (2003). All procedures were in accordance with the United Kingdom Animals (Scientific Procedures) Act The overall design of the approach is indicated in Figure 1. Under general anesthesia, single bipolar stimuli were delivered to the hand area of M1, using an array of intracortical microwire electrodes (Fig. 1, open circles). The corticospinal volleys generated by these stimuli were recorded from the corticospinal tract using surface electrodes on the contralateral DLF at both a rostral site [rostral volley (Rv), C3 segment] and a caudal site [caudal volley (Cv), C7 or C8]. Postsynaptic potentials evoked in motoneurons by these volleys were recorded intracellularly using glass micropipettes. Motoneurons were identified antidromically from electrodes mounted on upper limb nerve trunks. The M1 stimuli were conditioned with either single or paired shocks to area F5 using another microwire array. The effects of interactions between the condition (F5) and test (M1) shocks were analyzed both in terms of the corticospinal volleys and motoneuron postsynaptic potentials. Identification and chronic implantation of hand representations in F5 and M1. In three of the monkeys (CS12, CS13, and CS14) (Table 1), the hand/digit motor representations in F5 and M1 were identified physiologically, as described fully in our previous study (Cerri et al., 2003). A chamber was first implanted over the precentral gyrus under deep general anesthesia and aseptic precautions, with a full program of postoperative analgesic and antibiotic treatment. Chamber location was guided by an initial structural MRI scan. Subsequently, the motor representations in F5 and M1 were defined using repetitive intracortical microstimulation (ricms) under light sedation with ketamine and medetomidine HCl (Dormitor; Ramsgate, Kent, UK). The doses were 3.6 mg/kg 1 ketamine and mg/kg 1 Dormitor, both given intramuscularly. When ricms mapping was complete, small arrays of fine lowimpedance ( 20 k ) elgiloy microwire electrodes were implanted chronically at the center of the hand and digit representations under full anesthesia; four to five electrodes were implanted in M1 and in F5. Tips of microwires were targeted at the inferior bank of the arcuate sulcus (F5) and rostral bank of the central sulcus (M1) and were between 3 and 6 mm from the pial surface. The electrodes were mounted in a single flat array, with an interelectrode distance of mm. Electrodes were connected to a small miniature D-connector mounted on the skull. Terminal experiment. Terminal experiments were performed as described by Maier et al. (1998, 2002). In brief, all preparatory surgery was performed under deep isoflurane anesthesia. Cuff electrodes were mounted on the median, ulnar, and radial nerves at the axilla (Ma, Ua, and Ra, respectively); median and ulnar nerves at the wrist (Mw and Uw); and the deep radial nerve (DR). A laminectomy over spinal segments C3 to Th1 and an occipital craniotomy were performed. When surgery was complete, isoflurane was discontinued and -chloralose was given (50 80 mg/kg 1, i.v.). The animal was mounted in a spinal frame and headholder, with clamps on the vertebral column at Th3 and in the lumbar region, and then paralyzed with pancuronium bromide (Pavulon; OR-Technika, Cambridge, UK) at a dose of 0.3 mg kg 1 hr 1 intravenously and artificially ventilated at a rate of 45 cycles/min 1. Adequacy of the anesthesia was assessed continuously by reference to the blood pressure, heart rate, and pupillary reflexes. Small doses (2 4 mg/ kg 1, i.v.) of pentobarbitone (Sagatal; Rhone Merieux, Harlow, UK) were administered when necessary. Body temperature was carefully maintained between 37 and 39 C. Fluid balance and blood gases were monitored and maintained; each animal remained in good physiological condition throughout the recording. Mean blood pressure was maintained above 80 mmhg. Location of cortical-stimulating electrodes in terminal experiments. The surface location of intracortical microwire electrodes in the five different cases is shown in Figure 2: the cathode (negative) (Fig. 2 A E, ) and anode (positive) (Fig. 2 A-E, ) that were used in the terminal experiment have been marked in each case. M1 electrodes were located in the anterior bank of the central sulcus, and F5 electrodes were located in the bank of the inferior limb of the arcuate sulcus several millimeters lateral to the spur region. In the final two monkeys in this series (CS16 and CS17), the pairs of intracortical-stimulating electrodes were implanted acutely in the terminal experiment. Electrode placement was guided by magnetic resonance imaging (MRI) and by the results from the three monkeys with chronic implants. Intracortical stimulation of areas F5 and M1. In both chronically and acutely implanted monkeys, cortical areas F5 and M1 were stimulated using monophasic current pulses applied to pairs of intracortical electrodes. The duty cycle was 3 Hz. The intensities ranged from 50 to 400 A, although in the majority of cases they were 200 A. These currents were used to activate a significant proportion of the cortical output to hand and arm motoneurons (Maier et al., 2002). The physical spread of such currents is probably 1 mm (Ranck, 1975; Lemon, 1984; Tokuno and Nambu, 2000). At the start of each experiment, every combination of electrode was tested and the size and number of descending volleys evoked were compared. The final combination and polarity of electrodes used for bipolar stimulation were selected for optimal descending activity with low threshold. In the case of chronic implants (CS12, CS13, and CS14), the same electrode combinations found to be effective for facilitation of hand muscle EMG (Cerri et al., 2003) were used.
3 1202 J. Neurosci., February 4, (5): Shimazu et al. Premotor Motor Cortex Interactions in Macaque Figure 1. Schematic diagram of the experiment. The inset in the top part of the figure indicates sites within the primary motor cortex (M1) and PMv (F5) that were each chronically implanted with five microwires in one of the five monkeys investigated (CS14; see Materials and Methods). Each open circle indicates a microwire electrode. The M1 electrodes were located in the anterior bank of the central sulcus (CS), and the F5 electrodes were located in the inferior bank of the arcuate sulcus (AS). Sp, Spur; PS, principal sulcus. The bottom part of the figure indicates that descending volleys evoked from M1 and F5 were recorded from the lateral corticospinal tract (LCST) at two spinal levels: a rostral level (Rv) at C3 and a caudal level (Cv), usually at C7 or C8. EPSPs and IPSPs [the latter mediated by segmental inhibitory interneurons (Inh IN)] were recorded intracellularly from arm and hand muscle motoneurons in the cervical enlargement, and these were antidromically identified from peripheral nerves. Volleys and postsynaptic potentials evoked from M1 and F5 were compared with those from stimulation of the medullary PT. Condition (C), test (T), and combined (C T) stimuli were interleaved and delivered as follows. Conditioning stimuli were single or double shocks to F5 (duration, 0.2 msec; up to 400 A). Test stimuli were single shocks to M1 (duration, 0.2 msec; up to 400 A). Combined condition test stimuli were delivered either together (simultaneous delivery; C T 0), positive (F5 up to 30 msec before M1) or negative (M1 up to 3.6 msec before F5). Stimulation of the pyramidal tract. An electrode (varnish-insulated tungsten; tip impedance, k at 1 khz) was inserted just rostral to obex and mm lateral to the midline, ipsilateral to the stimulated hemisphere. Conventional electrophysiological criteria were used for final pyramidal tract (PT) electrode positioning (Maier et al., 1998). Stimuli were A (0.1 msec). In CS14, stimuli were delivered through implanted PT electrodes (Cerri et al., 2003). Recording and analysis of corticospinal volleys. Corticospinal volleys excited from the PT, from M1 and from F5, were recorded from the surface of the contralateral dorsolateral funiculus (DLF) at a rostral site (usually C3) and at a caudal site close to the region from which motoneuron recordings were made (C7 or C8) and were each referenced to an electrode located on nearby muscle tissue. Corticospinal volleys and stimulus trigger signals were acquired using a CED 1401plus interface (CED, Cambridge, UK), with a sampling rate of 25 khz. Volleys were averaged in relation to stimulus triggers (C, T, or C T), with 100 shocks per condition, and the peak-to-peak amplitude and latency of each component (D, I 1,I 2,I 3 ) was measured from these averages. The effect of conditioning stimulation on each component was analyzed on a sweepby-sweep basis (see Results) (see Fig. 10). Intracellular recordings from motoneurons were made with glass microelectrodes filled with 3 M potassium acetate and having a DC resistance of 2 5M. A small pressure foot was used to reduce movement of the spinal cord. All motoneurons were identified antidromically from the forelimb nerves. Intracellular and cord surface recordings were digitized directly at 10 khz using a 1401plus interface (CED). Membrane potential was monitored throughout the recording, and only data from stable periods of recording were used for analysis (membrane potential, 50 mv). Latency and amplitude measurements were made from a number of single traces. Intracortical injection of muscimol. In cases CS12, CS13, and CS14, a 0.5% solution of the GABA A agonist muscimol (catalog number M-1523; Sigma, St. Louis, MO) was injected into M1 as close as possible ( 2.0 mm) to the M1 microwire array. A small hole was made in the dura with a 27 gauge needle, and a 29 gauge Hamilton syringe needle advanced to a depth of 4 mm below the pia. Over a 3 5 min period, l ofthe muscimol solution was injected. After a delay of 2 4 min, the needle was raised by 1 mm and an additional injection was made, and this was repeated at depths of 2 mm and then 1 mm below the pia. One (CS13) or two (CS12 and CS14) tracks were made within 2 mm of the M1 array, and the total volume of muscimol injected was 6, 3, and 1.6 l in CS12, CS13, and CS14, respectively. Histology. At the end of the experiment, small electrolytic lesions were placed at the cortical stimulation sites by passing DC (20 A for 20 sec; tip positive). The animal was given an overdose of barbiturate and perfused through the heart with formal saline. Entry points of microwire arrays and the Hamilton microinjection needle were confirmed by photography of the fixed cortical tissue. All sites of stimulating electrodes were confirmed histologically (Suzuki and Azuma, 1976). Frozen sections (50 m) were cut, mounted, and Nissl stained. Each section was inspected carefully for electrode tracks, and sample sections were digitized. Results Location of stimulating sites in M1 and F5 M1 electrodes In every case, the M1 electrodes were located in the anterior bank of the central sulcus: in four of five cases, the tip of the effective M1 cathode was in the deep layers (lamina V/VI) (Table 1). M1 anodes all lay in lamina V. In the three chronic cases, these electrodes were located in the M1 hand area, as confirmed by previous ricms mapping. ricms through the implanted microwires yielded digit movements with currents between 35 and 55 in all cases (Table 1) [histology indicating the location of electrode tips in case CS14 is presented in Fig. 1D of Cerri et al. (2003)], and single shocks delivered to M1 electrode pairs evoked monosynaptic EPSPs in hand and finger muscle motoneurons (see below). F5 electrodes F5 electrodes were located in the bank region of the inferior limb of the arcuate sulcus several millimeters lateral to the spur region. The tips of effective cathodes (Table 1) were either in deep layers (CS12, CS13, CS14) or in lamina III (CS16, CS17), whereas anodes were located in deep layers (V/VI in CS12 and CS13) or superficial layers (II/III).
4 Shimazu et al. Premotor Motor Cortex Interactions in Macaque J. Neurosci., February 4, (5): Figure 2. Location of implanted microwire electrodes. Surface cortical maps of five macaque monkeys (CS13, CS14, CS16, CS17, and CS12) showing the location of stimulating electrodes in the primary motor cortex (M1) and PMv (F5). Stereotaxic coordinates in the rostral (R) caudal and mediolateral (L) directions are indicated on the ordinate and abscissa, respectively. In all cases, the cathodal electrode is shown as and anodal as. In CS13, CS14, and CS12, circled numbers indicate points of penetration of chronically implanted microwires in M1 and F5, which were centered on the respective hand/digit representations in these areas. In CS13 and CS14, these were defined by prior mapping with ricms. Mapping penetrations that yielded low-threshold (M1, 8 10 ; F5, ) movements of digits (F) or face (Œ) are shown. No effects were obtained in penetrations marked with a small open circle. In monkey CS13 ( A), the microwire implant in M1 carried four electrodes (1 4), varying in length from 3 to 5 mm. In monkey CS14 ( B), both the M1 and F5 implants had five electrodes each (M1, 1 5; F5, 6 10), and all electrodes were located in the anterior bank of the CS and inferior bank of the AS, respectively. In CS16 and CS17, pairs of electrodes were introduced into F5 and M1 in the terminal experiment, with locations guided by the results from CS13 and 14 and by recording of corticospinal volleys. The location of the tips of the most effective electrode pairs in each case are listed in Table1. Corticospinal volleys evoked from M1 and F5 M1 stimulation A single stimulus applied to M1 evoked a series of D and I waves in the corticospinal tract (Patton and Amassian, 1954; Kernell and Wu, 1967; Maier et al., 2002). Figure 3A gives examples of averaged volleys recorded at the C8 spinal level; the threshold for these volleys was 25. At100 A, a small early (1.4 msec) D wave and a later and larger I 1 wave were seen. As the stimulus intensity was raised, later I waves occurring at regular intervals of msec were recruited: these were the I 2, I 3, I 4, and I 5 waves. The conduction velocity (CV) of the different waves was deduced from simultaneous recordings at C3 and C7. As expected from previous studies (Kernell and Wu, 1967; Maier et al., 2002), the D and succeeding I waves had a very similar CV: in CS17, for example, the D, I 1, I 2, and I 3 waves all had a CV of 83 m sec 1. F5 stimulation There were three differences in responses from F5 compared with those from M1 (Fig. 3B). First, no D waves were seen in any of the five cases; second, I wave responses were generally smaller and later than those from M1; and finally, single shocks evoked relatively little activity. With double shocks (interstimulus interval, 3 msec), some activity was seen, with I 3 being the clearest component. In three cases in which volley CV was determined (CS14, CS16, and CS17), there was no significant difference between the CV of volleys from M1 (mean of 12 separate estimates, 81 7 m sec 1 ) and that of F5 volleys (mean of 8 estimates, 81 7 m sec 1 )(t test; p 0.97). Effects of F5 M1 interactions on corticospinal volleys These interactions were tested by interleaving C (F5), T (M1), and C T (F5 M1) stimuli (see Materials and Methods). The intensity of the test M1 shock was clearly submaximal for evoking a volley, whereas that of the conditioning F5 shock was generally just above or just below threshold for evoking any detectable descending volleys. The basic finding is presented in Figure 4: when F5 stimulation preceded the M1 test shock by 3 msec, there was a clear facilitation of the later components of the corticospinal response. The examples given in Figure 4 are from CS14 (Fig. 4A D) and CS17 (Fig. 4E H). In both cases, M1 stimulation, given alone, evoked a small D wave and larger I 1, I 2, and I 3 waves (Fig. 4B,F). Conditioning F5 stimulation [just suprathreshold for detectable descending activity in CS14 (Fig. 4A) or just subthreshold in CS17 (Fig. 4E)] produced a marked increase in the amplitude of both the I 2 and I 3 waves but not of the earlier D or I 1 waves (Fig. 4C,G). This is best seen in Figure 4, D and H, where any effects attributable to the F5 shock alone have been subtracted from the conditioned response [i.e., (F5 M1) F5]. Any differences in
5 1204 J. Neurosci., February 4, (5): Shimazu et al. Premotor Motor Cortex Interactions in Macaque Figure 3. Corticospinal volleys evoked from M1 and F5. Surface recordings from C8 (monkey CS16) are shown. Stimulus intensities are as indicated (duration, 0.2 msec). Shown are the averages of 100 sweeps. A, Single shocks to M1 (arrowhead)-evoked responses consisting of a direct wave (D) and a succession of indirect (I) waves (I 2,I 3, etc.). The threshold for volleys was 25. B, No clear responses were obtained to single shocks to F5, except at 400 A. Double shocks (interstimulus interval, 3 msec) evoked a clear I 3 wave; no D wave was observed. In this and all subsequent figures, volley recordings are shown with positivity down. this response (solid line) from the response to M1 alone (dashed line) represents the conditioning effect of the F5 stimulus: the facilitation of the I 2 and I 3 is clear in both cases, with no effect on either the D or I 1 waves. Facilitatory effects were usually obtained from only one or two pairs of F5 electrodes; in CS13 and CS14, the effective electrodes were the same ones found to produce facilitation of EMG in our previous study (Cerri et al., 2003). Figure 5 shows the results obtained for a series of different C T intervals in CS14 (Fig. 5A D) and CS17 (Fig. 5E H): note that while facilitation of the I 3 was present at C T intervals of 0 and 1.6 msec, the augmentation of the I 2 was not seen until longer intervals (3.2 and 4 msec) (Fig. 5 C,D,G,H). Clear and significant increases in I 2 or I 3 waves as a result of F5 conditioning are marked by asterisks (*p 0.05; **p 0.01; paired t test on volley amplitudes). Clear effects on D and I 1 waves were not seen at any of the intervals tested. There was no sign of suppression of any component by F5 stimulation. Broadly similar results were obtained with double F5 shocks (interval, 3 msec) (Fig. 6A, B). The findings were replicated in the other three cases. A more detailed description of the time course of F5 M1 interactions is presented below (see Fig. 10). Postsynaptic responses in hand and forearm motoneurons Intracellular recordings were made from a total of 79 antidromically identified motoneurons (CS12:23, CS13:19, CS14:15, CS16: Figure 4. Facilitation of late I waves evoked from M1 by conditioning stimulus to F5. Surface recordings from C3 segment in two monkeys CS14 ( A D) and CS17 ( E H). The averages of 100 sweeps are shown. A, E, Response to conditioning F5 shock alone; subthreshold for any response in E. B, F, Response to test M1 alone. C, G, Condition and test (F5 M1) produced a clear facilitation of the later I waves (I 2 and I 3 ). In D and H, the amount of facilitation is shown by plotting the test (M1) response alone ( ) with superimposed the conditioned response ( ), after any effects from the conditioning (F5) shock alone had been subtracted. Stimulation: A D: F5, A; M1, A; E H: F5, A; M1, A. Calibration, 10 V. 15, and CS17:7) recorded in spinal segments C7, C8, and Th1. Some of these motoneurons innervated wrist or finger flexors: 21 and 19 were identified from the ulnar and median nerves at the axilla (Ua and Ma), respectively. Others innervated wrist or finger extensors (22, deep radial (DR) nerve). An additional four motoneurons were innervated from radial nerve at the axilla (Ra). Finally, 13 were identified as supplying intrinsic hand muscles [ulnar (Uw; 11) or median nerve (Mw; 2) at the wrist]. Response to PT stimulation Response to PT stimulation was tested in 75 motoneurons: most (95%) gave a monosynaptic EPSP, which in many cases (45%) was followed by a disynaptic IPSP; 20% of motoneurons responded with an oligosynaptic EPSP (Maier et al., 1998). Responses to M1 and F5 stimulation M1 and F5 stimulation responses were tested in all 79 motoneurons, using a single shock of 200 A (M1) in all experiments. For F5, a single 200 A shock was used in CS14 and CS17, and double 200 A shocks (3 msec intershock interval) in the other cases. The short duration of stable intracellular recording prevented the investigation of a detailed F5 M1 interaction time course, and one C T interval was tested (3.0 msec between F5 and M1). A variety of postsynaptic potentials were observed (Figs. 6, 7). The categorization of EPSPs was made according to their association with a particular component of the descending volley, recorded from the surface of the same spinal segment in which the motoneuron was sampled. Maier et al. (2002) argued that the tight coupling between each volley component and the succeed-
6 Shimazu et al. Premotor Motor Cortex Interactions in Macaque J. Neurosci., February 4, (5): Figure 5. Time course of facilitation from F5. Surface recordings from the same cases as Figure 4, with identical stimulus intensities. Data are plotted as in Figure 4, D and H: test (M1) alone, ---;conditioned response (F5 M1) minus condition (F5) alone (F5),. Data in A D and E H are plotted for different C T intervals (0, 2, 3.2, and 4 msec). Note that at short C T intervals (A, B, E, F, 0 and 2 msec), facilitation of I 3 was observed, whereas both I 2 and I 3 were facilitated at longer intervals (C, D, G, H ). *p 0.05; **p 0.01; t test comparison of (F5 M1) F5 with M1 alone. ing EPSP, plus the short segmental delays, was consistent with these EPSPs representing monosynaptic action of impulses grouped within that particular component. Thus, D-EPSPs occurred with a segmental delay after arrival of the D wave that was in the monosynaptic range ( msec) (Maier et al., 2002). Similar criteria were adopted for I 1 -EPSPs; all other EPSPs, occurring in relation to the I 2 and I 3 waves, were classified as late EPSPs. Similarly, IPSPs were classified according to a disynaptic delay after the D wave ( msec) (Maier et al., 2002) as D-IPSPs, I 1 -IPSPs, or late IPSPs. Figure 6A shows typical intracellular recordings from an ulnar hand motoneuron (MN; bottom traces), together with the cord surface recording from the same (C8) segment (top traces). M1 stimulation alone produced a typical sequence of D, I 1, and I 2 waves (dashed lines). The motoneuron response to this activity consisted of a series of small EPSPs (dashed line), with segmental delays ranging from 0.9 to 1.2 msec after the relevant waves in the surface volley. There was no sign of any clear volley from F5 stimulation (two shocks) given alone, and no response was present in the motoneuron (gray lines). The conditioned responses (F5 M1; continuous black lines) showed clear increases in both the I 2 and I 3 waves in the surface recording, and the late EPSPs associated with these components were also enhanced, in particular the EPSP occurring after the I 3 wave. Neither the D-EPSP nor the I 1 -EPSP was changed. An example of facilitation of an IPSP is shown in Figure 6B.In this case, test M1 stimulation (dashed lines) evoked small EPSPs in association with both the D and I 2 waves. Both EPSPs were Figure 6. Facilitation of late EPSPs and IPSPs evoked from M1 by conditioning F5 stimulation. The top traces show surface recordings from C8; the bottom traces show intracellular recording from identified motoneuron. A, Ulnar hand motoneuron. After a single M1 shock ( ), each D and I wave in the surface volley was followed at monosynaptic latency by a small EPSP in the motoneuron: segmental latencies between positivity of surface volley and EPSP onset are indicated by vertical staggered lines. F5 alone produced no responses. Combined F5 M1 stimulation ( ) showed facilitation of I 2 and I 3 waves from M1 by conditioning F5 stimulation (C T interval, 3 msec between second F5 and M1 shocks). This also augmented the later EPSPs associated with the I 2 and I 3 waves. Stimuli: F5, A; M1, A. The averages of 73 sweeps per condition are shown. B, Deep radial motoneuron. After M1 alone, small EPSPs after the D and I 2 waves were followed by large IPSPs. The I 2 -IPSP was facilitated by conditioning F5 stimulation Stimuli: F5, A; M1, A. The averages of 113 sweeps per condition are shown. immediately followed by a sharp IPSP; segmental latency to IPSP onset was 1.5 msec in both cases. The later I 2 -IPSP was facilitated by conditioning F5 stimulation (continuous black line); none of the other features was affected. Figure 7 shows responses from an ulnar motoneuron in CS13 to test stimulation of two different pairs of M1 electrodes, shown in the inset above. The effects from the first pair are shown in Figure 7A: a single shock to M1 electrodes 2 (cathode) and 1 (anode) evoked a D-EPSP and a second later EPSP after the I 3 wave (dashed lines). F5 stimulation (two shocks) given alone evoked no visible descending volley or postsynaptic response (gray lines). However, when F5 stimulation preceded the M1 shock by 3 msec, there was a clear facilitation of the I 3 wave and a marked increase in the amplitude of the late I 3 -EPSP (continuous black lines). When M1 electrodes 3 (cathode) and 4 (anode) were
7 1206 J. Neurosci., February 4, (5): Shimazu et al. Premotor Motor Cortex Interactions in Macaque Summary of motoneuron responses M1 stimulation A total of 50 of 79 (63%) motoneurons showed an early D-EPSP, whereas 42 (53%) showed an I 1 -EPSP and 54 (68%) responded with later EPSPs in association with I 2 or I 3 waves (Fig. 8A). IPSPs were observed in relation to the D wave (22 of 79; 28%), I 1 wave (15 of 79; 19%), or later waves (11 of 79; 15%) (Fig. 8C). F5 stimulation Only late EPSPs, beginning at least 3 msec after the F5 shock(s), were observed after F5 stimulation (Fig. 8D). These late EPSPs were observed in 44 of 79 (56%) motoneurons; late IPSPs were seen in 16 of 79 (20%) cases. In general, late EPSPs from F5 were always smaller than those from M1. Effects of interactions between F5 and M1 stimulation We did not observe any facilitation of the early EPSPs associated with either the D wave (Figs. 6A,B, 7B) or the I 1 wave (Figs. 6A, 7C). In contrast, facilitation of later EPSPs (Figs. 6A, 7B,C) was observed in 47 of 79 motoneurons (60%) (Fig. 8A, black bar). In most cases, a late EPSP present after M1 stimulation given alone was facilitated; however, in nine motoneurons, a late EPSP appeared after conditioning stimulation that was not present in the test M1 condition. The facilitation of late EPSPs was particularly common among intrinsic hand motoneurons (12 of 13 or 92%) (Fig. 8B, black bar), significantly higher than for forearm flexor or extensor motoneurons (35 of 66 or 53%; p 0.05, 2 test). In no case were EPSPs evoked from M1 suppressed by prior F5 stimulation. We observed that the early IPSPs evoked from M1 and associated with the D and I 1 waves were not affected by F5 stimulation, whereas later IPSPs were augmented in 10 of 79 motoneurons (Fig. 8C, black bar). Figure 7. Facilitation of late EPSPs from F5 ulnar motoneuron, case CS13. A, Stimulation of M1 between electrodes 2 (cathode) and 1 (anode) evoked a D-EPSP (0.8 msec after the D wave) and an I 3 -EPSP (0.9 msec after the I 3 wave). The location of electrodes are shown in the inset. F5 stimulation alone (electrode 6 as cathode, electrode 5 as anode) produced no clear responses but, when combined with M1 (C T interval, 3 msec), gave a pronounced facilitation of the I 3 EPSP but did not affect the D-EPSP. Stimuli: F5, A; M1, A. The averages of 100 sweeps per condition are shown. B, The same F5 conditioning stimulus had a different effect on the responses evoked from a different M1 test stimulus, now using electrode 3 as cathode and electrode 4 as anode. The test M1 shock gave I 1 - and I 3 -EPSPs; only the latter was facilitated by conditioning F5 stimulation. Stimuli are the same as in B. The averages of 100 sweeps per condition are shown. used (Fig. 7B), a very different pattern of descending activity and motoneuronal responses was observed: no D wave was present, and the motoneuron exhibited a large I 1 -EPSP, followed by an I 3 -EPSP (segmental latency, 0.9 msec in both cases). Conditioning with F5 facilitated the later I 3 -EPSP, whereas the I 1 -EPSP was unchanged. Effects of muscimol microinjection in M1 hand area We reasoned that if M1 itself was the main site of facilitation from F5, then synaptic inactivation in the vicinity of the M1 array should abolish it. Accordingly, in three monkeys, small volumes of muscimol (1.6 6 l) were injected close to the M1 electrode array. The results shown in Figure 9 are from CS14, in which the smallest volume of muscimol was injected (see Materials and Methods). The later I 2 and I 3 waves evoked from M1 were completely abolished 40 min after muscimol injection, although there were only small decreases in the I 1 wave and no change in D wave amplitude. Importantly, late I-waves from F5 were also substantially decreased (Fig. 9B). Finally, Figure 9C shows that the facilitation of the I 2 and particularly the I 3 waves by F5 conditioning stimulation was decreased 20 min after muscimol injection and completely abolished after 40 min. Similar results were obtained in all three cases tested. Figure 8, E and F, summarizes the results obtained from motoneuron recording before and after muscimol injection (CS14). Before muscimol (Fig. 8E), it was common to find motoneurons yielding late EPSPs, in association with the I 2 and I 3 waves, and these late EPSPs were facilitated from F5 (Fig. 8E, black bar). In the 3 4 hr after the injection, most motoneurons showed only early EPSPs (D- or I 1 -EPSPs) (Fig. 8F). None of these was facilitated by F5 conditioning. Only two motoneurons showed late EPSPs; neither of these was facilitated from F5. Investigation into the detailed time course of F5-evoked facilitation of M1 volleys Because the effects of interactions between F5 and M1 were to facilitate specific components of the descending corticospinal
8 Shimazu et al. Premotor Motor Cortex Interactions in Macaque J. Neurosci., February 4, (5): Figure 8. Summary of F5 facilitatory effects on motoneuronal EPSPs and IPSPs and effects of microinjection of muscimol in M1 on motoneuron responses. A D, Bars chart the number of arm/hand motoneurons that showed postsynaptic responses to stimulation of M1 or F5. M1 stimulation evoked EPSPs ( A) associated with the D wave, I 1 wave, or the late (I 2 and I 3 ) waves; EPSPs facilitated by conditioning F5 stimulation are indicated by the black bar: only late EPSPs from M1 were facilitated. This effect was particularly striking for the subset of motoneurons identified as supplying intrinsic hand muscles ( B). C, M1-evoked IPSPs in a small proportion of motoneurons; again, only late IPSPs from M1 were facilitated by F5 stimulation (black bar). F5 stimulation alone gave rise to late, but not early, EPSPs ( D). Data are from 79 motoneurons in five monkeys. The condition (F5) test (M1) interval was either 3 or 3.6 msec. Test stimuli were single shocks to M1. For F5, either single (one monkey) or double (four monkeys) shocks were used. Stimulus intensity varied from 70 to 200 A. E, F, Effects of microinjection of muscimol on EPSPs from M1 and their facilitation from F5. All data from case CS14. Motoneurons were recorded before ( E) and up to 4 hr after muscimol microinjection in M1 ( F) (see Materials and Methods and the legend to Fig. 9). Before the injection, M1 stimulation yielded EPSPs in association with D, I 1, and I 2 /I 3 waves; these late EPSPs were common, and only these EPSPs were facilitated by conditioning F5 stimulation (E, black bar). After muscimol injection, late EPSPs from M1 were found in only two motoneurons; neither of these was facilitated from F5. M1: single shock, A; F5: two shocks, A. C T interval, 3.0 msec. Figure 9. Effects of microinjection of muscimol into M1 on descending volleys from M1 and F5. All data are from case CS14. A, M1 volleys: a single 400 A shock to M1 evoked D and I waves. After control recordings (before), a 0.5% solution of muscimol was injected close ( 2 mm) to the microwire electrodes from which these effects were obtained; the total volume injected was 1.6 l in two tracks. Forty minutes later, the I 2 and I 3 waves were largely abolished; there was a small reduction in the I 1 wave and no obvious effect on the D wave. The average of 150 sweeps is shown. B, A single 400 A shock to F5 evoked a small I 1 and larger I 3 wave; 40 min after the muscimol injection in M1, there was a marked reduction in both waves. The average of 150 sweeps is shown. C, Effects of muscimol on F5 M1 interaction. A single test M1 shock (180 A) was conditioned by a single F5 shock (150 A), and the C T interval was 0 msec. Control recordings showed a marked facilitation of the I 3 wave; 20 min after muscimol injection, this facilitation was considerably reduced, and it was abolished after 40 min. The average of 100 sweeps is shown. volleys from M1, we investigated the detailed time course of this facilitation to see whether peaks of interaction corresponded to the times at which I waves were generated in M1 (cf. Tokimura et al., 1996; Ziemann et al., 1998). This was investigated in two cases (CS14 and CS17), with recording from the rostral spinal cord electrode (C3). F5 M1 interaction was tested in 0.4 msec steps for C T intervals ranging from 3.6 msec (M1 stimulus precedes F5) to 6.0 msec (F5 precedes M1). Single shocks were used. Additional intervals (10,15, 20, and 30 msec) were also tested. The duty cycle was 3 Hz, and different C T intervals were tested in a block design. Stimuli were given in a sequence of three: F5 alone, then M1 alone, and then F5 M1. For each conditioned response sweep (F5 M1), we measured the peak voltage of a given component (D, I 1, I 2,orI 3 ) and subtracted from it the algebraic sum of the same component evoked by M1 alone and by F5 alone. These values were derived from the sweeps given immediately before the F5 M1 sweep; this approach minimized any effects attributable to slow changes in cortical excitability. This analysis was per-
9 1208 J. Neurosci., February 4, (5): Shimazu et al. Premotor Motor Cortex Interactions in Macaque formed for each of 100 sets of stimuli at the interval being tested, and the mean amplitude of the volley facilitation ( SE) was calculated. This is the amplitude of the component present in the conditioned response over and above the background value, and it is plotted against the C T interval in Figure 10B E. For statistical analysis, a paired t test was performed for 100 pairs of test (M1 alone) versus conditioned [(F5 M1) F5 alone] responses at each C T interval investigated, with results being Bonferroni corrected for multiple comparisons. Finally, to compare effects on the different components, the amount of facilitation was normalized as a percentage of the component recorded in response to M1 alone (Fig. 10A). The results confirmed marked facilitation of the later I waves: up to 300% for I 3 and 175% for I 2 (Fig. 10A C), with little or no effect on the D or I 1 waves (Fig. 10D,E). Figure 10B shows that a significant ( p 0.05) increase in the amplitude of I 3 was first observed at the 0.8 msec interval. There followed a marked modulation in the degree of facilitation with the C T interval, with peaks of facilitation occurring at 0, 1.6, 3.2, 4.4, and 5.6 msec (Fig. 10A, arrows). These intervals correspond closely to the well known I wave periodicity at around 1.2 msec (Patton and Amassian, 1954; Kernell and Wu, 1967; Amassian et al., 1987; Edgley et al., 1997). The facilitation of the I 3 wave declined slowly from 6 to 20 msec, returning to baseline by 30 msec. Sustained facilitation of the I 2 wave was not observed until 1.6 msec (Fig. 10C), but this did not achieve significance ( p 0.05) until 3.2 msec, considerably later than the sustained increase in the I 3 wave. Increased I 2 activity lasted until 15 msec. The first peak in the I 2 facilitation matched precisely that of the second I 3 peak at 1.6 msec (Fig. 10A, second arrow). There was also coincidence in later peaks (e.g., the second I 2 peak with the third I 3 peak at 3.2 msec) (Fig. 10A, third arrow). We did not observe any facilitation of the I 1 wave (Fig. 10D), except at 10 msec, where it was small but significant. There was no significant facilitation of the D wave (Fig. 10E). No significant suppression of any component was seen. Discussion We have demonstrated that premotor cortex can facilitate corticospinal motor outputs from macaque M1 to arm and particularly hand motoneurons. The main facilitatory effects were observed on the later I 2 and I 3 indirect corticospinal volleys evoked from M1 and on responses of motoneurons to these volleys. The results suggest that F5 can facilitate neuronal circuits in M1 that generate these later volleys, and this would explain why F5 M1 facilitation was abolished by injection of muscimol in the hand area of M1. Corticospinal volleys evoked from M1 and F5 Single bipolar stimuli of up to 200 A delivered to the M1 hand region evoked a characteristic pattern of a small D wave and a complex series of larger I waves (cf. Maier et al., 2002). In contrast, we saw no evidence for any D wave activation from F5. These findings are consistent with the relatively large corticospinal projection from M1 (around half of the total projection from the frontal lobe) (Dum and Strick, 1991) compared with the small projection from PMv (4%). This small projection may have gone undetected in surface recordings, especially if the volley was slow and temporally dispersed. Corticospinal neurons in PMv do not project as far as lower cervical segments but terminate at more rostral levels (He et al., 1993; Galea and Darian-Smith, 1994). In keeping with these findings, we did not observe any short-latency EPSPs in motoneurons that could have been evoked by a D wave from F5. Indirect or I waves were evoked from F5; they were small and had higher thresholds than from M1 (Fig. 3). The most prominent feature was the I 3 wave. Two observations hint that at least part of the I wave activity evoked by stimulation in F5 actually arose from within M1. First, local injection of muscimol close to the M1 microwire array greatly reduced the amplitude of I waves evoked from F5 (Fig. 9B). The volume injected in CS14 (1.6 lin two tracks) would not be expected to spread 1 2 mm (Martin and Ghez, 1999), so it is unlikely to have been attributable to a direct GABAergic blockade of F5. Second, I waves evoked from F5 had fast conduction velocities ( 80 m sec 1 ) and were identical to that of D and I waves evoked from M1. Corticospinal neurons in premotor cortex are smaller and more slowly conducting than in M1 (Murray and Coulter, 1981) (T. Brochier and R. N. Lemon, unpublished observations), making it unlikely that these fast I waves arose within F5 itself. Stimulation of premotor areas can evoke indirect responses from M1 corticospinal neurons (CSNs) (Amassian et al., 1987; Ghosh and Porter, 1988; Tokunu and Nambu, 2000). Facilitation of M1 outputs from F5 Stimulation of F5 evoked a two- to fourfold increase in the later I 2 and I 3 corticospinal volleys evoked from the M1 hand area (Fig. 10A). This facilitation occurred at short C T intervals and could be evoked by F5 conditioning shocks, which were themselves subthreshold for any corticospinal response (Fig. 4E H). This facilitation showed evidence of periodicity, with peaks at msec apart (Fig. 10A C); it was completely abolished by local muscimol injection in M1 (Fig. 9C). The facilitation of late corticospinal volleys was reflected in robust increases in the later EPSPs associated with these volleys (Figs. 6 8); this was particularly striking for intrinsic hand muscle motoneurons (Fig. 8B). Site of interaction The facilitation by F5 of late EPSPs from M1 was often present without any detectable response in the motoneuron to F5 stimulation alone (Figs. 6A, 7), indicating a premotoneuronal site of interaction. Because of the divergent projections from the two cortical areas stimulated, interaction could occur at a number of different cortical and subcortical sites (cf. Tanaka and Lisberger, 2002). Is M1 the site of interaction? The facilitation we observed in motoneurons was restricted to the late EPSPs that were time locked to the later I 2 and I 3 waves evoked from M1, and these EPSPs probably represent corticomotoneuronal action of corticospinal impulses in the I 2 and I 3 waves (Kernell and Wu 1967; Day et al., 1989; Edgley et al., 1997; Maier et al., 2002). Thus, the mechanisms in M1 generating the later I waves could have been facilitated from F5. Three arguments support M1 as the interaction site. First, the earliest latency at which facilitation occurred was brief [C T 0 msec (Fig. 5) and C T 0.8 msec (Fig. 10)]. Because conduction time from F5 to M1 is short ( 1.0 msec) (Godschalk et al., 1984), this would allow ample time for activity generated in F5 to facilitate interneuronal circuits generating the I 2 and I 3 waves. Second, injection of muscimol in M1 not only abolished the facilitation from F5 of the later I 2 and I 3 waves evoked from M1 but also abolished late EPSPs in motoneurons and their facilitation from F5 (Figs. 8, 9). Finally, the detailed time course of the F5 M1 interaction showed a series of peaks with a periodicity of 1.2
10 Shimazu et al. Premotor Motor Cortex Interactions in Macaque J. Neurosci., February 4, (5): Figure 11. Possible mechanism explaining facilitation of late I waves Elements in black, on the left of the diagram, represent the three classes of excitatory inputs to CSNs: the D wave, the I 1 wave, and the later (I 2 and I 3 ) waves. A low-threshold inhibitory input pathway is also shown. These four inputs are all excited by stimulation within M1. A similar set of inputs would be excited to CSNs in the premotor cortex (F5), but these are omitted for the sake of clarity. The model proposes that the main excitatory input from F5 to M1 converges on the interneuronal mechanisms in M1 giving rise to the I 2 and I 3 waves, thereby allowing F5 to influence I wave generation in M1 CSNs. Note that although M1 CSNs project directly to hand motoneurons, this is not the case for those located in F5. For detailes, see Discussion and Ilic et al. (2002). 1.6 msec (Fig. 10), and this is to be expected if facilitation acts on the cortical circuits generating the I 2 and I 3 waves. If most of the descending corticospinal activity evoked from M1 actually arose within M1, then the waxing and waning of the facilitatory drive from F5 would be best explained by an interaction within M1 itself. Proposed cortical mechanism The model in Figure 11 is based on that of Ilic et al. (2002), who studied the interaction of pairs of transcranial magnetic stimulation (TMS) pulses delivered to the human motor cortex. Four separate inputs to an M1 CSN are shown: D responses are attributable to direct activation at the axon hillock or main axon (Amassian et al., 1987). Indirect waves arise either through stimulation of presynaptic inputs to the CSN, producing the I 1 response, or through a late I wave interneuronal pathway giving rise to the I 2 and I 3 components (Ziemann and Rothwell, 2000). This latter pathway is more complex and less secure than the I 1 synaptic pathway (di Lazzaro et al., 1999). Finally, inhibitory inputs 4 Figure 10. Detailed time course of effect of F5 M1 interaction on corticospinal volleys. The effect of interacting a single F5 stimulus (200 A) with a single M1 shock (180 A) was tested at a wide range of condition (F5) and test (M1) intervals, performed in a pseudorandom order from 3.6 msec (M1 before F5) to 6.0 msec in 0.4 msec steps and then at longer intervals out to 30 msec. The facilitation of a given component of the recorded descending activity (D wave and different I wave) was determined by subtracting from the conditioned response [(F5 M1), the algebraic sum of the response to test and condition stimuli, given alone (i.e., (F5 M1) ((F5) (M1))]. In B E, the absolute value of this volley facilitation ( SE) has been plotted for the I 3,I 2,I 1, and D waves, respectively. A significant difference between conditioned and test values was estimated with paired t tests: Bonferroni-corrected significant differences ( p 0.05) are indicated by filled circles; open circles indicate not significant differences. In A, the amplitude changes of the different components have been plotted on the same scale, normalized to the percentage of the test alone response.
Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans
 11911 Journal of Physiology (2001), 533.3, pp.903 919 903 Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans Guillaume Nicolas, Véronique Marchand-Pauvert,
11911 Journal of Physiology (2001), 533.3, pp.903 919 903 Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans Guillaume Nicolas, Véronique Marchand-Pauvert,
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Synaptic influences exerted by corticocortical fibres on neurones of the primary
 Journal of Physiology (1988), 400, pp. 617-629 617 With 1 plate and 6 text-figure8 Printed in Great Britain CORTICOCORTICAL SYNAPTIC INFLUENCES ON MORPHOLOGICALLY IDENTIFIED PYRAMIDAL NEURONES IN THE MOTOR
Journal of Physiology (1988), 400, pp. 617-629 617 With 1 plate and 6 text-figure8 Printed in Great Britain CORTICOCORTICAL SYNAPTIC INFLUENCES ON MORPHOLOGICALLY IDENTIFIED PYRAMIDAL NEURONES IN THE MOTOR
Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones
 J Physiol 575.2 (2006) pp 527 541 527 Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones E. Jankowska 1, K. Stecina 1, A. Cabaj 1, L.-G. Pettersson
J Physiol 575.2 (2006) pp 527 541 527 Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones E. Jankowska 1, K. Stecina 1, A. Cabaj 1, L.-G. Pettersson
Bilateral Postsynaptic Actions of Pyramidal Tract and Reticulospinal Neurons on Feline Erector Spinae Motoneurons
 858 The Journal of Neuroscience, January 20, 2010 30(3):858 869 Behavioral/Systems/Cognitive Bilateral Postsynaptic Actions of Pyramidal Tract and Reticulospinal Neurons on Feline Erector Spinae Motoneurons
858 The Journal of Neuroscience, January 20, 2010 30(3):858 869 Behavioral/Systems/Cognitive Bilateral Postsynaptic Actions of Pyramidal Tract and Reticulospinal Neurons on Feline Erector Spinae Motoneurons
Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways
 J Physiol 580.1 (2007) pp 133 147 133 Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways E. Jankowska and K. Stecina Department
J Physiol 580.1 (2007) pp 133 147 133 Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways E. Jankowska and K. Stecina Department
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM
 STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
 Peripheral facial paralysis (right side). The patient is asked to close her eyes and to retract their mouth (From Heimer) Hemiplegia of the left side. Note the characteristic position of the arm with
Peripheral facial paralysis (right side). The patient is asked to close her eyes and to retract their mouth (From Heimer) Hemiplegia of the left side. Note the characteristic position of the arm with
Differential presynaptic inhibition of actions of group II afferents in di- and polysynaptic pathways to feline motoneurones
 Journal of Physiology (2002), 542.1, pp. 287 299 DOI: 10.1113/jphysiol.2001.014068 The Physiological Society 2002 www.jphysiol.org Differential presynaptic inhibition of actions of group II afferents in
Journal of Physiology (2002), 542.1, pp. 287 299 DOI: 10.1113/jphysiol.2001.014068 The Physiological Society 2002 www.jphysiol.org Differential presynaptic inhibition of actions of group II afferents in
Nature Methods: doi: /nmeth Supplementary Figure 1. Activity in turtle dorsal cortex is sparse.
 Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements
 Y. Isomura et al. 1 Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements Yoshikazu Isomura, Rie Harukuni, Takashi Takekawa, Hidenori Aizawa & Tomoki Fukai
Y. Isomura et al. 1 Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements Yoshikazu Isomura, Rie Harukuni, Takashi Takekawa, Hidenori Aizawa & Tomoki Fukai
The Physiology of the Senses Chapter 8 - Muscle Sense
 The Physiology of the Senses Chapter 8 - Muscle Sense www.tutis.ca/senses/ Contents Objectives... 1 Introduction... 2 Muscle Spindles and Golgi Tendon Organs... 3 Gamma Drive... 5 Three Spinal Reflexes...
The Physiology of the Senses Chapter 8 - Muscle Sense www.tutis.ca/senses/ Contents Objectives... 1 Introduction... 2 Muscle Spindles and Golgi Tendon Organs... 3 Gamma Drive... 5 Three Spinal Reflexes...
Chapter 13: The Spinal Cord and Spinal Nerves
 Chapter 13: The Spinal Cord and Spinal Nerves Spinal Cord Anatomy Protective structures: Vertebral column and the meninges protect the spinal cord and provide physical stability. a. Dura mater, b. Arachnoid,
Chapter 13: The Spinal Cord and Spinal Nerves Spinal Cord Anatomy Protective structures: Vertebral column and the meninges protect the spinal cord and provide physical stability. a. Dura mater, b. Arachnoid,
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
Neurophysiology of systems
 Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS
 J. exp. Biol. (1980), 85, 343-347 343 With a figures Printed in Great Britain POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS BY J. Y. KUWADA, G. HAGIWARA AND J. J. WINE
J. exp. Biol. (1980), 85, 343-347 343 With a figures Printed in Great Britain POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS BY J. Y. KUWADA, G. HAGIWARA AND J. J. WINE
(Received 10 April 1956)
 446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC
 1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle
 J Physiol (2003), 550.3, pp. 933 946 DOI: 10.1113/jphysiol.2003.042606 The Physiological Society 2003 www.jphysiol.org Differential modulation of intracortical inhibition in human motor cortex during selective
J Physiol (2003), 550.3, pp. 933 946 DOI: 10.1113/jphysiol.2003.042606 The Physiological Society 2003 www.jphysiol.org Differential modulation of intracortical inhibition in human motor cortex during selective
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM
 STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
The vast majority of our physical interactions with the world
 A cortico-cortical mechanism mediating object-driven grasp in humans L. Cattaneo*, M. Voss*, T. Brochier*, G. Prabhu*, D. M. Wolpert*, and R. N. Lemon* *Sobell Department of Motor Neuroscience and Movement
A cortico-cortical mechanism mediating object-driven grasp in humans L. Cattaneo*, M. Voss*, T. Brochier*, G. Prabhu*, D. M. Wolpert*, and R. N. Lemon* *Sobell Department of Motor Neuroscience and Movement
Cutaneomuscular reflexes recorded from the lower limb
 Journal of Physiology (1995), 487.1, pp.237-242 376 237 Cutaneomuscular reflexes recorded from the lower limb in man during different tasks J. Gibbs, Linda M. Harrison * and J. A. Stephens Department of
Journal of Physiology (1995), 487.1, pp.237-242 376 237 Cutaneomuscular reflexes recorded from the lower limb in man during different tasks J. Gibbs, Linda M. Harrison * and J. A. Stephens Department of
Changes in intracortical excitability induced by stimulation of wrist afferents in man
 12359 Journal of Physiology (2001), 534.3, pp.891 902 891 Changes in intracortical excitability induced by stimulation of wrist afferents in man Jean-Marc Aimonetti and Jens Bo Nielsen * Laboratoire Développement
12359 Journal of Physiology (2001), 534.3, pp.891 902 891 Changes in intracortical excitability induced by stimulation of wrist afferents in man Jean-Marc Aimonetti and Jens Bo Nielsen * Laboratoire Développement
Lecture VIII. The Spinal Cord, Reflexes and Brain Pathways!
 Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit. Supplementary Information. Adam W. Hantman and Thomas M.
 Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Motor Functions of Cerebral Cortex
 Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Research, Australian National University, Canberra, A.C.T. 2601, Australia
 Journal of Physiology (1988), 400, pp. 593-615 593 With 1 plate and 9 text-figures Printed in Great Britain MORPHOLOGY OF PYRAMIDAL NEURONES IN MONKEY MOTOR CORTEX AND THE SYNAPTIC ACTIONS OF THEIR INTRACORTICAL
Journal of Physiology (1988), 400, pp. 593-615 593 With 1 plate and 9 text-figures Printed in Great Britain MORPHOLOGY OF PYRAMIDAL NEURONES IN MONKEY MOTOR CORTEX AND THE SYNAPTIC ACTIONS OF THEIR INTRACORTICAL
The Journal of Physiology
 J Physiol 593.4 (2015) pp 947 966 947 Presynaptic and postsynaptic effects of local cathodal DC polarization within the spinal cord in anaesthetized animal preparations F. Bolzoni 1,2 and E. Jankowska
J Physiol 593.4 (2015) pp 947 966 947 Presynaptic and postsynaptic effects of local cathodal DC polarization within the spinal cord in anaesthetized animal preparations F. Bolzoni 1,2 and E. Jankowska
MOTOR EVOKED POTENTIALS AND TRANSCUTANEOUS MAGNETO-ELECTRICAL NERVE STIMULATION
 MOTOR EVOKED POTENTIAS AND TRANSCUTANEOUS MAGNETO-EECTRICA NERVE STIMUATION Hongguang iu, in Zhou 1 and Dazong Jiang Xian Jiaotong University, Xian, People s Republic of China 1 Shanxi Normal University,
MOTOR EVOKED POTENTIAS AND TRANSCUTANEOUS MAGNETO-EECTRICA NERVE STIMUATION Hongguang iu, in Zhou 1 and Dazong Jiang Xian Jiaotong University, Xian, People s Republic of China 1 Shanxi Normal University,
susceptibility of either the axons in the dorsal and ventral roots, or the intramedullary
 213 J. Physiol. (31958) I40, 2I3-2I9 THE SITE OF ACTION OF PROCAINE ON THE ISOLATED SPINAL CORD OF THE FROG BY M. HARMEL AND J. L. MALCOLM From the Department of Physiology, State University of New York,
213 J. Physiol. (31958) I40, 2I3-2I9 THE SITE OF ACTION OF PROCAINE ON THE ISOLATED SPINAL CORD OF THE FROG BY M. HARMEL AND J. L. MALCOLM From the Department of Physiology, State University of New York,
Rewiring of hindlimb corticospinal neurons after spinal cord injury
 Rewiring of hindlimb corticospinal neurons after spinal cord injury Arko Ghosh, Florent Haiss, Esther Sydekum, Regula Schneider, Miriam Gullo, Matthias T. Wyss, Thomas Mueggler, Christof Baltes, Markus
Rewiring of hindlimb corticospinal neurons after spinal cord injury Arko Ghosh, Florent Haiss, Esther Sydekum, Regula Schneider, Miriam Gullo, Matthias T. Wyss, Thomas Mueggler, Christof Baltes, Markus
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal:
 Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Applied Neuroscience. Conclusion of Science Honors Program Spring 2017
 Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
EPSPs was demonstrated by a double shock test (interstimulus interval < 5 ms) and. (spike-ta) method.
 Journal of Physiology (1991), 435, pp. 243-256 243 With 6 figures Printed in Great Britain DIFFERENTIAL CONNECTIONS BY INTRACORTICAL AXON COLLATERALS AMONG PYRAMIDAL TRACT CELLS IN THE CAT MOTOR CORTEX
Journal of Physiology (1991), 435, pp. 243-256 243 With 6 figures Printed in Great Britain DIFFERENTIAL CONNECTIONS BY INTRACORTICAL AXON COLLATERALS AMONG PYRAMIDAL TRACT CELLS IN THE CAT MOTOR CORTEX
 Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Fig Cervical spinal nerves. Cervical enlargement C7. Dural sheath. Subarachnoid space. Thoracic. Spinal cord Vertebra (cut) spinal nerves
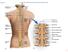 Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Physiology and Plasticity of Morphologically Identified Cells in the Mormyrid Electrosensory Lobe
 The Journal of Neuroscience, August 15, 1997, 17(16):6409 6423 Physiology and Plasticity of Morphologically Identified Cells in the Mormyrid Electrosensory Lobe Curtis C. Bell, 1 Angel Caputi, 2 and Kirsty
The Journal of Neuroscience, August 15, 1997, 17(16):6409 6423 Physiology and Plasticity of Morphologically Identified Cells in the Mormyrid Electrosensory Lobe Curtis C. Bell, 1 Angel Caputi, 2 and Kirsty
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Integrative Synaptic Mechanisms in the Caudal Ganglion of the Crayfish
 Integrative Synaptic Mechanisms in the Caudal Ganglion of the Crayfish JAMES B. PRESTON and DONALD KENNEDY ABSTRACT A study of activity recorded with intracellular micropipettes was undertaken in the caudal
Integrative Synaptic Mechanisms in the Caudal Ganglion of the Crayfish JAMES B. PRESTON and DONALD KENNEDY ABSTRACT A study of activity recorded with intracellular micropipettes was undertaken in the caudal
Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley
 (2002), 542.3, pp. 963 976 DOI: 10.1113/jphysiol.2002.021683 The Physiological Society 2002 www.jphysiol.org Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated
(2002), 542.3, pp. 963 976 DOI: 10.1113/jphysiol.2002.021683 The Physiological Society 2002 www.jphysiol.org Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated
The Journal of Physiology
 J Physiol 595.5 (2017) pp 1743 1761 1743 Does trans-spinal and local DC polarization affect presynaptic inhibition and post-activation depression? D. Kaczmarek 1,2,3, J. Ristikankare 1 and E. Jankowska
J Physiol 595.5 (2017) pp 1743 1761 1743 Does trans-spinal and local DC polarization affect presynaptic inhibition and post-activation depression? D. Kaczmarek 1,2,3, J. Ristikankare 1 and E. Jankowska
Nerve Conduction Studies NCS
 Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nerve Conduction Studies NCS
 Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Tests for presynaptic modulation of corticospinal terminals from peripheral afferents and pyramidal tract in the macaque
 J Physiol 573.1 (2006) pp 107 120 107 Tests for presynaptic modulation of corticospinal terminals from peripheral afferents and pyramidal tract in the macaque A. Jackson 1,S.N.Baker 2 and E. E. Fetz 1
J Physiol 573.1 (2006) pp 107 120 107 Tests for presynaptic modulation of corticospinal terminals from peripheral afferents and pyramidal tract in the macaque A. Jackson 1,S.N.Baker 2 and E. E. Fetz 1
Compound Action Potential, CAP
 Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
A Dynamic Neural Network Model of Sensorimotor Transformations in the Leech
 Communicated by Richard Andersen 1 A Dynamic Neural Network Model of Sensorimotor Transformations in the Leech Shawn R. Lockery Yan Fang Terrence J. Sejnowski Computational Neurobiological Laboratory,
Communicated by Richard Andersen 1 A Dynamic Neural Network Model of Sensorimotor Transformations in the Leech Shawn R. Lockery Yan Fang Terrence J. Sejnowski Computational Neurobiological Laboratory,
Neurophysiological Basis of TMS Workshop
 Neurophysiological Basis of TMS Workshop Programme 31st March - 3rd April 2017 Sobell Department Institute of Neurology University College London 33 Queen Square London WC1N 3BG Brought to you by 31 March
Neurophysiological Basis of TMS Workshop Programme 31st March - 3rd April 2017 Sobell Department Institute of Neurology University College London 33 Queen Square London WC1N 3BG Brought to you by 31 March
Supplemental Information. A Visual-Cue-Dependent Memory Circuit. for Place Navigation
 Neuron, Volume 99 Supplemental Information A Visual-Cue-Dependent Memory Circuit for Place Navigation Han Qin, Ling Fu, Bo Hu, Xiang Liao, Jian Lu, Wenjing He, Shanshan Liang, Kuan Zhang, Ruijie Li, Jiwei
Neuron, Volume 99 Supplemental Information A Visual-Cue-Dependent Memory Circuit for Place Navigation Han Qin, Ling Fu, Bo Hu, Xiang Liao, Jian Lu, Wenjing He, Shanshan Liang, Kuan Zhang, Ruijie Li, Jiwei
(Received 14 January 1954)
 278 J3 Physiol. (I954) I25, 278-29I ELECTRICAL STIMULATION OF THE UNEXPOSED CEREBRAL CORTEX BY T. GUALTIEROTTI* AND A. SPENCER PATERSON From the West London Hospital Medical School, Dan Mason Research
278 J3 Physiol. (I954) I25, 278-29I ELECTRICAL STIMULATION OF THE UNEXPOSED CEREBRAL CORTEX BY T. GUALTIEROTTI* AND A. SPENCER PATERSON From the West London Hospital Medical School, Dan Mason Research
Chapter 4 Neuronal Physiology
 Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
University, Newcastle upon Tyne NE2 4HH
 Journal of Physiology (199), 425, pp. 31-32 31 With 8 figures Printed in Great Britain XCITATION OF TH CORTICOSPINAL TRACT BY LCTROMAGNTIC AND LCTRICAL STIMULATION OF TH SCALP IN TH MACAQU MONKY BY S.
Journal of Physiology (199), 425, pp. 31-32 31 With 8 figures Printed in Great Britain XCITATION OF TH CORTICOSPINAL TRACT BY LCTROMAGNTIC AND LCTRICAL STIMULATION OF TH SCALP IN TH MACAQU MONKY BY S.
Nature Neuroscience: doi: /nn Supplementary Figure 1. Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons
 Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells
 J Physiol 587.2 (2009) pp 401 418 401 Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells E. Jankowska 2,
J Physiol 587.2 (2009) pp 401 418 401 Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells E. Jankowska 2,
The Journal of Physiology Neuroscience
 J Physiol 591.13 (2013) pp 3381 3399 3381 The Journal of Physiology Neuroscience Evidence for long-lasting subcortical facilitation by transcranial direct current stimulation in the cat Francesco Bolzoni
J Physiol 591.13 (2013) pp 3381 3399 3381 The Journal of Physiology Neuroscience Evidence for long-lasting subcortical facilitation by transcranial direct current stimulation in the cat Francesco Bolzoni
J. Physiol. (I955) I30, 396-4I3
 396 J. Physiol. (I955) I30, 396-4I3 THE INHIBITORY SUPPRESSIO1N OF REFLEX DISCHARGES FROM MOTONEURONES By J. S. COOMBS, J. C. ECCLES AND P. FATT From the Department of Physiology, The Australian National
396 J. Physiol. (I955) I30, 396-4I3 THE INHIBITORY SUPPRESSIO1N OF REFLEX DISCHARGES FROM MOTONEURONES By J. S. COOMBS, J. C. ECCLES AND P. FATT From the Department of Physiology, The Australian National
Neurons. Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons.
 Neurons Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons. MBL, Woods Hole R Cheung MSc Bioelectronics: PGEE11106 1 Neuron
Neurons Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons. MBL, Woods Hole R Cheung MSc Bioelectronics: PGEE11106 1 Neuron
Objectives Neurophysiology of brain area related to movement and motor control
 Objectives Neurophysiology of brain area related to movement and motor control 1. Ascending pathways (sensory input) 2. Sensory input treatment, and thalamo-cortical & cortico-thalamic filter 3. Sensory
Objectives Neurophysiology of brain area related to movement and motor control 1. Ascending pathways (sensory input) 2. Sensory input treatment, and thalamo-cortical & cortico-thalamic filter 3. Sensory
Neuroscience with Pharmacology 2 Functions and Mechanisms of Reflexes. Prof Richard Ribchester
 Neuroscience with Pharmacology 2 Functions and Mechanisms of Reflexes Prof Richard Ribchester René Descartes Cogito, ergo sum The 21st century still holds many challenges to Neuroscience and Pharmacology
Neuroscience with Pharmacology 2 Functions and Mechanisms of Reflexes Prof Richard Ribchester René Descartes Cogito, ergo sum The 21st century still holds many challenges to Neuroscience and Pharmacology
Arterial Blood Supply
 Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Variety of muscle responses to tactile stimuli
 Variety of muscle responses to tactile stimuli Julita Czarkowska-Bauch Department of Neurophysiology, Nencki Institute of Experimental Biology, 3 Pasteur St., 02-093 Warsaw, Poland Abstract. Influences
Variety of muscle responses to tactile stimuli Julita Czarkowska-Bauch Department of Neurophysiology, Nencki Institute of Experimental Biology, 3 Pasteur St., 02-093 Warsaw, Poland Abstract. Influences
Physiology of synapses and receptors
 Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Modulation of single motor unit discharges using magnetic stimulation of the motor cortex in incomplete spinal cord injury
 1 SHORT REPORT Division of Neuroscience and Psychological Medicine, Imperial College School of Medicine, Charing Cross Hospital, London W 8RF, UK H C Smith NJDavey D W Maskill P H Ellaway National Spinal
1 SHORT REPORT Division of Neuroscience and Psychological Medicine, Imperial College School of Medicine, Charing Cross Hospital, London W 8RF, UK H C Smith NJDavey D W Maskill P H Ellaway National Spinal
Spinal Interneurons. Control of Movement
 Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Sum of Neurally Distinct Stimulus- and Task-Related Components.
 SUPPLEMENTARY MATERIAL for Cardoso et al. 22 The Neuroimaging Signal is a Linear Sum of Neurally Distinct Stimulus- and Task-Related Components. : Appendix: Homogeneous Linear ( Null ) and Modified Linear
SUPPLEMENTARY MATERIAL for Cardoso et al. 22 The Neuroimaging Signal is a Linear Sum of Neurally Distinct Stimulus- and Task-Related Components. : Appendix: Homogeneous Linear ( Null ) and Modified Linear
Neuroscience 201A (2016) - Problems in Synaptic Physiology
 Question 1: The record below in A shows an EPSC recorded from a cerebellar granule cell following stimulation (at the gap in the record) of a mossy fiber input. These responses are, then, evoked by stimulation.
Question 1: The record below in A shows an EPSC recorded from a cerebellar granule cell following stimulation (at the gap in the record) of a mossy fiber input. These responses are, then, evoked by stimulation.
συν together απτειν to clasp 2h Neuroscience with Pharmacology Functions and Mechanisms of Reflexes Cogito, ergo sum ( I think therefore I am ) Down
 2h Neuroscience with Pharmacology Functions and Mechanisms of Reflexes Neuroscience is studied at many different levels: from brain, to system, network, neurone, synapse, and molecule... Top Up Down René
2h Neuroscience with Pharmacology Functions and Mechanisms of Reflexes Neuroscience is studied at many different levels: from brain, to system, network, neurone, synapse, and molecule... Top Up Down René
Australian National University, Canberra, Australia
 430 J. Phy8iol. (1965), 179, pp. 430-441 With 6 text-figures Printed in Great Britain MUSCLE STRETCH AND THE PRESYNAPTIC INHIBITION OF THE GROUP Ia PATHWAY TO MOTONEURONES BY M. S. DEVANANDAN, ROSAMOND
430 J. Phy8iol. (1965), 179, pp. 430-441 With 6 text-figures Printed in Great Britain MUSCLE STRETCH AND THE PRESYNAPTIC INHIBITION OF THE GROUP Ia PATHWAY TO MOTONEURONES BY M. S. DEVANANDAN, ROSAMOND
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10776 Supplementary Information 1: Influence of inhibition among blns on STDP of KC-bLN synapses (simulations and schematics). Unconstrained STDP drives network activity to saturation
doi:10.1038/nature10776 Supplementary Information 1: Influence of inhibition among blns on STDP of KC-bLN synapses (simulations and schematics). Unconstrained STDP drives network activity to saturation
Paired-Pulse TMS to one Brain Region. Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation LEASE DO NOT COPY
 Paired-Pulse TMS to one Brain Region Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation Paired-Pulse Paradigms Sequential pulses applied to the same cortical region Variable
Paired-Pulse TMS to one Brain Region Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation Paired-Pulse Paradigms Sequential pulses applied to the same cortical region Variable
Exam 1 PSYC Fall 1998
 Exam 1 PSYC 2022 Fall 1998 (2 points) Briefly describe the difference between a dualistic and a materialistic explanation of brain-mind relationships. (1 point) True or False. George Berkely was a monist.
Exam 1 PSYC 2022 Fall 1998 (2 points) Briefly describe the difference between a dualistic and a materialistic explanation of brain-mind relationships. (1 point) True or False. George Berkely was a monist.
Postnatal Development of the Motor Representation in Primary Motor Cortex
 Postnatal Development of the Motor Representation in Primary Motor Cortex SAMIT CHAKRABARTY AND JOHN H. MARTIN Center for Neurobiology and Behavior, Columbia University; and the New York State Psychiatric
Postnatal Development of the Motor Representation in Primary Motor Cortex SAMIT CHAKRABARTY AND JOHN H. MARTIN Center for Neurobiology and Behavior, Columbia University; and the New York State Psychiatric
Interactions between rostral and caudal cortical motor areas in the rat
 J Neurophysiol 113: 3893 3904, 2015. First published April 8, 2015; doi:10.1152/jn.00760.2014. Interactions between rostral and caudal cortical motor areas in the rat J. E. Deffeyes, 1 B. Touvykine, 1
J Neurophysiol 113: 3893 3904, 2015. First published April 8, 2015; doi:10.1152/jn.00760.2014. Interactions between rostral and caudal cortical motor areas in the rat J. E. Deffeyes, 1 B. Touvykine, 1
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #10 Wednesday, November 22, 2006 14. FUNDAMENTALS OF FUNCTIONAL ELECTRICAL STIMULATION (FES) We will look at: Design issues for FES Subthreshold
ECE 795: Quantitative Electrophysiology Notes for Lecture #10 Wednesday, November 22, 2006 14. FUNDAMENTALS OF FUNCTIONAL ELECTRICAL STIMULATION (FES) We will look at: Design issues for FES Subthreshold
Cellular Bioelectricity
 ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture 24 Thursday, March 6, 2014 8. NEURAL ELECTROPHYSIOLOGY We will look at: Structure of the nervous system Sensory transducers and neurons Neural coding
ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture 24 Thursday, March 6, 2014 8. NEURAL ELECTROPHYSIOLOGY We will look at: Structure of the nervous system Sensory transducers and neurons Neural coding
Chapter 11: Nervous System and Nervous Tissue
 Chapter 11: Nervous System and Nervous Tissue I. Functions and divisions of the nervous system A. Sensory input: monitor changes in internal and external environment B. Integrations: make decisions about
Chapter 11: Nervous System and Nervous Tissue I. Functions and divisions of the nervous system A. Sensory input: monitor changes in internal and external environment B. Integrations: make decisions about
Chapter 11: Functional Organization of Nervous Tissue
 Chapter 11: Functional Organization of Nervous Tissue I. Functions of the Nervous System A. List and describe the five major nervous system functions: 1. 2. 3. 4. 5. II. Divisions of the Nervous System
Chapter 11: Functional Organization of Nervous Tissue I. Functions of the Nervous System A. List and describe the five major nervous system functions: 1. 2. 3. 4. 5. II. Divisions of the Nervous System
CALLOSAL RESPONSES OF FAST-RHYTHMIC-BURSTING NEURONS DURING SLOW OSCILLATION IN CATS
 Neuroscience 147 (2007) 272 276 RAPID REPORT CALLOSAL RESPONSES OF FAST-RHYTHMIC-BURSTING NEURONS DURING SLOW OSCILLATION IN CATS Y. CISSÉ, 1,2 D. A. NITA, 2 M. STERIADE AND I. TIMOFEEV* Department of
Neuroscience 147 (2007) 272 276 RAPID REPORT CALLOSAL RESPONSES OF FAST-RHYTHMIC-BURSTING NEURONS DURING SLOW OSCILLATION IN CATS Y. CISSÉ, 1,2 D. A. NITA, 2 M. STERIADE AND I. TIMOFEEV* Department of
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Alterations in Synaptic Strength Preceding Axon Withdrawal
 Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Motor and sensory nerve conduction studies
 3 rd Congress of the European Academy of Neurology Amsterdam, The Netherlands, June 24 27, 2017 Hands-on Course 2 Assessment of peripheral nerves function and structure in suspected peripheral neuropathies
3 rd Congress of the European Academy of Neurology Amsterdam, The Netherlands, June 24 27, 2017 Hands-on Course 2 Assessment of peripheral nerves function and structure in suspected peripheral neuropathies
Introduction to Electrophysiology
 Introduction to Electrophysiology Dr. Kwangyeol Baek Martinos Center for Biomedical Imaging Massachusetts General Hospital Harvard Medical School 2018-05-31s Contents Principles in Electrophysiology Techniques
Introduction to Electrophysiology Dr. Kwangyeol Baek Martinos Center for Biomedical Imaging Massachusetts General Hospital Harvard Medical School 2018-05-31s Contents Principles in Electrophysiology Techniques
At the highest levels of motor control, the brain represents actions as desired trajectories of end-effector
 At the highest levels of motor control, the brain represents actions as desired trajectories of end-effector Normal condition, using fingers and wrist Using elbow as folcrum Using shoulder as folcrum (outstretched
At the highest levels of motor control, the brain represents actions as desired trajectories of end-effector Normal condition, using fingers and wrist Using elbow as folcrum Using shoulder as folcrum (outstretched
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon
 Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Introduction to Computational Neuroscience
 Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
Biological Bases of Behavior. 8: Control of Movement
 Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
Outline. Neuron Structure. Week 4 - Nervous System. The Nervous System: Neurons and Synapses
 Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
Voluntary Movements. Lu Chen, Ph.D. MCB, UC Berkeley. Outline. Organization of the motor cortex (somatotopic) Corticospinal projection
 Voluntary Movements Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Organization of the motor cortex (somatotopic) Corticospinal projection Physiology of motor neurons Direction representation, population coding
Voluntary Movements Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Organization of the motor cortex (somatotopic) Corticospinal projection Physiology of motor neurons Direction representation, population coding
ANSWERS TO PRE- LAB ASSIGNMENTS
 Lab 14 Introduction to Nervous System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. orbicularis oculi b. sternocleidomastoid c. deltoid d. pectoralis major e. biceps brachii f. rectus
Lab 14 Introduction to Nervous System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. orbicularis oculi b. sternocleidomastoid c. deltoid d. pectoralis major e. biceps brachii f. rectus
Circuits & Behavior. Daniel Huber
 Circuits & Behavior Daniel Huber How to study circuits? Anatomy (boundaries, tracers, viral tools) Inactivations (lesions, optogenetic, pharma, accidents) Activations (electrodes, magnets, optogenetic)
Circuits & Behavior Daniel Huber How to study circuits? Anatomy (boundaries, tracers, viral tools) Inactivations (lesions, optogenetic, pharma, accidents) Activations (electrodes, magnets, optogenetic)
What is Anatomy and Physiology?
 Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex
 Archives of Disease in Childhood, 1988, 63, 1347-1352 Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex T H H G KOH AND J A EYRE Department of Child Health,
Archives of Disease in Childhood, 1988, 63, 1347-1352 Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex T H H G KOH AND J A EYRE Department of Child Health,
Paired Associative Transspinal and Transcortical Stimulation Produces Bidirectional Plasticity of Human Cortical and Spinal Motor Pathways
 City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 6-2016 Paired Associative Transspinal and Transcortical Stimulation Produces Bidirectional
City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 6-2016 Paired Associative Transspinal and Transcortical Stimulation Produces Bidirectional
Ube3a is required for experience-dependent maturation of the neocortex
 Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Lesson 14. The Nervous System. Introduction to Life Processes - SCI 102 1
 Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
