Despite significant advances in the treatment of acute
|
|
|
- Shauna Lambert
- 5 years ago
- Views:
Transcription
1 Postinfarct Cytokine Therapy Regenerates Cardiac Tissue and Improves Left Ventricular Function Buddhadeb Dawn, Yiru Guo, Arash Rezazadeh, Yiming Huang, Adam B. Stein, Greg Hunt, Sumit Tiwari, Jai Varma, Yan Gu, Sumanth D. Prabhu, Jan Kajstura, Piero Anversa, Suzanne T. Ildstad, Roberto Bolli Abstract We systematically investigated the comparative efficacy of three different cytokine regimens, administered after a reperfused myocardial infarction, in regenerating cardiac tissue and improving left ventricular (LV) function. Wild-type (WT) mice underwent a 30-minute coronary occlusion followed by reperfusion and received vehicle, granulocyte colony-stimulating factor (G-CSF) Flt-3 ligand (FL), G-CSF stem cell factor (SCF), or G-CSF alone starting 4 hours after reperfusion. In separate experiments, chimeric mice generated by reconstitution of radioablated WT mice with bone marrow from enhanced green fluorescent protein (EGFP) transgenic mice underwent identical protocols. Mice were euthanized 5 weeks later. Echocardiographically, LV function was improved in G-CSF FL and G-CSF SCF treated but not in G-CSF treated mice, whereas LV end-diastolic dimensions were smaller in all three groups. Morphometrically, cytokine-treated hearts had smaller LV diameter and volume. Numerous EGFP-positive cardiomyocytes, capillaries, and arterioles were noted in the infarcted region in cytokine-treated chimeric mice treated with G-CSF FL or G-CSF SCF, but the numbers were much smaller in G-CSF treated mice. G-CSF FL therapy mobilized bone marrow derived cells exhibiting increased expression of surface antigens (CD62L and CD11a) that facilitate homing. We conclude that postinfarct cytokine therapy with G-CSF FL or G-CSF SCF limits adverse LV remodeling and improves LV performance by promoting cardiac regeneration and probably also by exerting other beneficial actions unrelated to regeneration, and that G-CSF alone is less effective. (Circ Res. 2006;98: ) Key Words: myocardial regeneration cytokine stem cell ischemia/reperfusion bone marrow left ventricular function Despite significant advances in the treatment of acute myocardial infarction (MI), no therapies are currently available to restore dead myocardium. Although mobilization of bone marrow cells (BMCs) by cytokines has been suggested to regenerate cardiac tissue after MI and to produce functional improvement, the reports on the effects of cytokine treatment are conflicting. 1 6 Furthermore, previous studies of cytokines have used models of permanent coronary ligation 1 3,5 that do not reflect the fact that most patients with acute MI undergo coronary reperfusion. In addition, these studies have administered cytokines as a pretreatment 1,2 or performed splenectomy 1,2,5 or both, 1,2 neither of which would be clinically feasible. Although various cytokines have been tested, the relative efficacy of different treatments or combinations of treatments has not been elucidated. Finally, the mechanism whereby cytokines improve left ventricular (LV) function and structure after MI remains obscure. It has been proposed that cytokines mobilize BMCs with subsequent homing to the infarcted tissue and transdifferentiation into cardiac lineage, 1 3 but this hypothesis remains unproven. Thus, numerous unresolved issues persist regarding the use of cytokines as a strategy to achieve cardiac repair after MI. Accordingly, in the present study, we examined the effect of three cytokines (granulocyte colony-stimulating factor [G-CSF], stem cell factor [SCF], and Flt-3 ligand [FL]) on cardiac repair. The specific goals were to determine: (1) whether cytokine therapy is efficacious in the setting of a reperfused MI; (2) whether it is efficacious when started after the MI and in the presence of an intact spleen; (3) which cytokine or cytokine combination, among G-CSF, FL, and SCF, is more effective in improving LV remodeling and function; (4) whether cytokine-mobilized BMCs home to the heart and transdifferentiate into the cardiac lineage; and (5) whether cytokines facilitate BMC homing to the injured myocardium by altering the expression profile of adhesion molecules in mobilized BMCs. A well-characterized murine model of temporary coronary occlusion followed by reperfusion (a setting designed to mimic the most common clinical Original received October 21, 2005; revision received February 27, 2006; accepted March 9, From the Institute of Molecular Cardiology (B.D., Y. Guo, A.R., A.B.S., G.H., S.T., J.V., Y. Gu, S.D.P., R.B.) and Institute of Cellular Therapeutics (Y.H., S.T.I.), University of Louisville, Kentucky; and Cardiovascular Research Institute (J.K., P.A.), Department of Medicine, New York Medical College, Valhalla. This manuscript was sent to Stephen F. Vatner, Consulting Editor, for review by expert referees, editorial decision, and final disposition. Correspondence to Roberto Bolli, MD, Division of Cardiology, University of Louisville, Louisville, KY rbolli@louisville.edu 2006 American Heart Association, Inc. Circulation Research is available at DOI: /01.RES
2 Dawn et al Cytokine Therapy for Myocardial Regeneration 1099 setting of acute MI in humans) was used. 7 A thorough assessment of LV function and anatomy was performed in conjunction with careful measurements of cardiac regeneration by confocal microscopy. Materials and Methods A detailed description of all materials and methods is provided in the online supplement, available at Generation and Characterization of EGFP Chimeric Mice EGFP chimeric mice were generated 8 by reconstituting radioablated adult male mice (B6,129 strain) with BMCs from syngeneic enhanced green fluorescent protein (EGFP) transgenic mice. On average, 85% of the peripheral blood nucleated cells were positive for EGFP in the recipients. Experimental Protocol Adult male wild-type (WT; B6,129 strain, 10 to 18 weeks of age) and EGFP chimeric mice were used. The overall experimental design is summarized in supplemental Figure I. Myocardial Infarction Anesthetized mice underwent a 30-minute occlusion of the left anterior descending coronary artery followed by reperfusion. Starting 4 hours after reperfusion, mice received daily subcutaneous injections of vehicle or cytokine as detailed in supplemental Figure I. Echocardiography Serial echocardiograms were obtained at baseline (2 days before coronary occlusion) and 2 and 35 days after reperfusion. Systolic and diastolic anatomical and functional parameters were calculated using standard methods. 1 Morphometry and Histology At the end of the follow-up period, the heart was perfusion fixed in 10% neutral buffered formalin, 1,9 and the left ventricle was sectioned serially perpendicular to its longitudinal axis, processed, and embedded in paraffin. The infarct volume fraction was calculated by computerized planimetry (Image-Pro Plus; Media Cybernetics) of digital images of three Masson s trichrome-stained serial LV sections taken at 0.5- to 1.0-mm intervals along the longitudinal axis. 10 The midsection was used to measure LV diameter and volume. 11,12 All anatomical parameters were corrected according to a uniform sarcomere length. 13 Immunohistochemistry was performed in formalin-fixed 4- mthick histological sections. Cardiomyocytes were recognized by the presence of -sarcomeric actin and troponin T, endothelial cells by plaletet/endothelial cell adhesion molecule-1 (PECAM-1) and von Willebrand factor, and smooth muscle cells by -smooth muscle actin. Colocalization of cell-specific markers with EGFP was used to identify cells that originated from BMCs. All of the primary and secondary antibodies used in these studies are specified in supplemental Table I. A quantitative analysis of cardiomyocyte regeneration was performed in 3 to 7 EGFP chimeric mouse hearts in each of groups V through VIII. Assessment of Cardiomyocyte Proliferation The percentage of cardiomyocyte nuclei positive for 5-bromodeoxyuridine (BrdU) and Ki67 1 was determined at 14 days after reperfusion in 4 to 6 mice in each of groups IX through XII. In addition, the percentage of cardiomyocyte nuclei positive for Ki67 at 35 days after MI was determined in 6 mice in each of groups I through IV. Flow Cytometry After vehicle or cytokine treatment, peripheral blood was harvested from mice in groups XIII through XVI at serial time points, stained with antibodies, and lin - /Sca-1 /c-kit cells were counted from the lymphoid gate with a multiparameter, live sterile cell sorter. 14,15 The flow cytometric procedure is summarized in supplemental Figure II. The expression of adhesion molecules on lin - /Sca-1 /c-kit cells from untreated murine bone marrow and peripheral blood of mice (groups XVII through XX) treated with identical dosages of vehicle or cytokines as groups I through IV, respectively, was also assessed by flow cytometry. Statistical Analysis Data are reported as mean SEM. Morphometric and histologic data were analyzed with one-way ANOVA. Serial echocardiographic parameters were measured with a two-way (time and group) ANOVA followed by Student t tests with the Bonferroni correction. Results Exclusions A total of 188 mice (162 WT and 26 EGFP chimeric) were used. One hundred fifty-six mice were assigned to the MI studies (groups I through XII) and 32 mice to the cellular mobilization (groups XIII through XX) studies. Twenty-nine mice died in the perioperative period. Nine mice were excluded from the study because of failure of the coronary occluder, leaving a total of 14, 19, 20, and 18 WT mice in groups I through IV, respectively, and 3, 7, 6, and 5 EGFP chimeric mice in groups V through VIII, respectively. No mouse was excluded from the cellular mobilization studies (groups XIII through XX; n 4 per group). Myocardial Infarct Size The impact of cytokine therapy on LV structural and functional parameters was assessed in groups I through IV. The infarct volume fraction was similar in the four groups (supplemental Figure III), indicating that the extent of myocardial cell death produced by the coronary occlusion was comparable. The infarct volume fraction measures the volume of scarred tissue expressed as a fraction of the entire left ventricle Because the small islands of regenerated cardiomyocytes scattered throughout the scar area were included in the infarct region, in cytokine-treated hearts, the calculation of the infarct volume fraction was not affected by myocardial regeneration. G-CSF FL and G-CSF SCF Therapy Improves LV Systolic Function Before coronary occlusion (baseline), all parameters of LV function, measured by echocardiography, were similar in groups I through IV (Figure 1). At 48 hours after reperfusion, the degree of LV systolic functional impairment did not differ among groups (Figure 1), indicating that the injury sustained during ischemia/reperfusion was comparable. In vehicletreated mice, there was further functional deterioration between 48 hours and 35 days after reperfusion (Figure 1). In contrast, in G-CSF FL-treated (group II) and G-CSF SCFtreated (group III) mice, LV systolic function remained unchanged or improved at 35 days compared with 48 hours (Figure 1). Mice in groups II and III exhibited significantly higher LV ejection fraction, fractional area change, and fractional shortening and lower LV end-systolic diameter compared with vehicle-treated mice (group I) (Figure 1). Compared with group I, myocardial function in the infarct region was greater in groups II and III (systolic thickening
3 1100 Circulation Research April 28, 2006 Figure 1. A through D, Representative 2D (A and C) and M-mode (B and D) images from a vehicle-treated (A and B) and a G-CSF FL treated (C and D) mouse 35 days after coronary occlusion/ reperfusion. Compared with the vehicletreated heart, the G-CSF FL treated heart exhibits a smaller LV cavity and thicker infarct wall. Contractile activity in the infarct area is present in the G-CSF FL treated mouse (arrowheads) and absent in the vehicle-treated mouse. E through G, Echocardiographic assessment of LV systolic function. Echocardiographic measurements in control and cytokine-treated mice (groups I through IV) are illustrated. The administration of G-CSF FL and G-CSF SCF improved LV systolic function 35 days after MI. Data are mean SEM. n 14 to 19 mice per group. *P 0.05 vs group I. fraction in the infarct wall, %, %, %, and % in groups I, II, III, and IV, respectively), but the differences were not statistically significant. Compared with group I, myocardial function was also enhanced in the nonischemic LV region in groups II and III (systolic thickening fraction, %, %, and % [P 0.05 versus group I] and % in groups I, II, III, and IV, respectively). In mice treated with G-CSF alone (group IV), there was only a marginal improvement in parameters of LV systolic function (Figure 1E through 1G). Cytokine Therapy Halts LV Remodeling Both morphometric and echocardiographic analyses demonstrated that administration of cytokines attenuated adverse LV remodeling 5 weeks after infarction. Morphometrically, LV chamber diameter and LV chamber volume were lower in groups II, III, and IV compared with group I (Figure 2A and 2B; supplemental Figure IV). Echocardiographically, LV anatomical parameters were similar in groups I through IV, both before coronary occlusion (baseline) and 48 hours after reperfusion (Figure 2C and 2D). In vehicle-treated mice (group I), there was significant LV dilatation between 48 hours and 35 days after reperfusion (Figure 2), indicative of adverse LV remodeling. Compared with group I, both echocardiographic (Figure 2C and 2D) and morphometric (supplemental Figure IV) analyses showed smaller LV diameter and LV end-diastolic volume in cytokine-treated mice (groups II through IV). However, the reduction in LV dilatation in group IV was not statistically significant by echocardiography (Figure 2C and 2D), and the reduction in LV volume in group II was not statistically significant by morphometry (supplemental Figure IV). By morphometry, the infarct wall thickness improved in cytokine-treated (groups III and IV) mice (Figure 2E). Interestingly, mice that received G-CSF alone (group IV) also demonstrated a comparable improvement in morphometric parameters of LV remodeling (supplemental Figure IV) despite borderline functional improvement (Figure 1). Cytokine Therapy Promotes Regeneration of Cardiomyocytes In EGFP chimeric mice, the effects of cytokines on LV function and anatomy after infarction were directionally concordant with those in WT mice (supplemental Figures V and VI, respectively), despite the fact that LV size was smaller in the chimeric mice (supplemental Figure VI) compared with WT mice (Figure 2). Histologic quantitation of myocardial regeneration was performed in hearts (n 3 to 7 per group) from EGFP chimeric mice (groups V through VIII). Cardiomyocytes derived from BMCs were identified by concomitant positivity for -sarcomeric actin and EGFP (Figure 3). The extent of regeneration was assessed by measuring the area occupied by EGFP myocytes rather than
4 Dawn et al Cytokine Therapy for Myocardial Regeneration 1101 Figure 2. Cytokine therapy and postinfarct LV remodeling. Representative Masson s trichrome-stained myocardial sections from a control (A) and a G-CSF FL treated (B) WT mouse. Scar tissue and viable myocardium are identified in blue and purple, respectively. The LV cavity is smaller, and the infarcted as well as the noninfarcted walls are thicker in the G-CSF FL treated mouse compared with the control mouse. Panels C through E illustrate measurements of LV structural parameters in diastole by echocardiography (C and D) and morphometry (E) in groups I through IV. Administration of G-CSF FL and G-CSF SCF attenuated adverse LV remodeling and improved cardiac anatomy, whereas G-CSF alone had marginal effects. Data are mean SEM. n 14 to 19 mice per group. *P 0.05 vs group I. their number; the former parameter more accurately reflects the functional impact of regeneration. In the infarcted region, EGFP regenerated cardiomyocytes occupied only % of the myocardial area in group I; in contrast, this fraction of reconstituted myocardium was much higher in groups II, III, and IV ( %, %, and % of the myocardial area, respectively). The area occupied by regenerated cardiomyocytes was similar in the infarct and in the border zone. In contrast, in the noninfarcted region, the area occupied by EGFP myocytes was uniformly low in all four groups ( %, %, %, and % of myocardial area in groups I, II, III, and IV, respectively; Figure 4). Newly formed myocytes expressed connexin 43 (Figure 5), indicating functional integration with adjacent myocytes. EGFP cells that were negative for cardiomyocyte markers constituted %, %, %, and Figure 3. Postinfarct cytokine therapy induces cardiomyocyte regeneration in EGFP chimeric mice. BMCs and myocytes are identified by EGFP (green) and -sarcomeric actin (red), respectively. A through C, Low-magnification images of the infarct area in a vehicle-treated mouse show a small number of EGFP BMCs and rare transdifferentiation into myocytes (C, yellow, arrowhead). D through F, In contrast, images of the infarct area in a G-CSF FL treated mouse show numerous small myocytes (F, yellow) that are positive for both EGFP and -sarcomeric actin. Arrowheads indicate small myocytes that are EGFP, possibly derived from resident stem cells. G through I, Higher magnification images of the infarct area in a G-CSF FL treated mouse show several newly formed myocytes (I, yellow, arrowheads) positive for EGFP and -sarcomeric actin. Nuclei are stained with 4,6-diamidino-2-phenylindole (blue). Bar 20 m.
5 1102 Circulation Research April 28, 2006 Figure 4. Quantitative measurement of myocyte regeneration in the infarct area and in the nonischemic area in EGFP chimeric mice (groups V through VIII). Solid bars show EGFP myocytes (identified by the colocalization of EGFP and -sarcomeric actin) and hatched bars EGFP nonmyocytes (cells positive for EGFP but not for -sarcomeric actin). In the ischemic zone, the majority of EGFP cells were myocytes; in the nonischemic zone, the reverse was observed. Data are mean SEM. n 3 to 7 mice per group. *P 0.05 vs group V; # P 0.05 vs group VIII % of the infarcted myocardial area and %, %, %, and % of the viable myocardial area in groups I, II, III, and IV, respectively (Figure 4). Thus, a fraction of the BMCs that homed to the infarcted myocardium differentiated into noncardiomyocyte cell types. In the ischemic zone, most EGFP cells were cardiomyocytes; in the nonischemic zone, the reverse was observed. Cardiomyocyte Proliferation The effect of cytokine administration on cardiomyocyte proliferation at 14 days after MI was assessed in groups IX through XII by examining BrdU incorporation (Figure 6A and 6C) in cardiomyocyte nuclei. In addition, 6 hearts from each of groups I through IV were examined for Ki67 expression at 35 days after MI (Figure 6B and 6D). Treatment with G-CSF FL, G-CSF SCF, or G-CSF resulted in a significant increase in BrdU and Ki67 cardiomyocytes in the infarcted region, indicating increased numbers of cycling myocytes (Figure 6). The increase observed with G-CSF alone was significantly less than that seen with G-CSF FL and G-CSF SCF. In contrast, the number of cycling myocytes positive for Ki67 in the nonischemic zone at 35 days after MI was comparable in all groups (data not shown). Regeneration of the Vasculature Colocalization of EGFP positivity with -smooth muscle actin positivity in arteriolar smooth muscle cells (Figure 7A) and PECAM-1 positivity in endothelial cells (Figure 7B) were noted predominantly in the infarct area and, to a lesser extent, in the viable myocardium. Thus, EGFP BMCs Figure 6. Assessment of cardiomyocyte proliferation after MI. Representative images of BrdU (A; bright blue) and Ki67 (B; yellow) cardiomyocyte nuclei. Positivity for -sarcomeric actin (red) identifies cardiomyocytes. C and D, Quantitative measurement of proliferating myocytes positive for BrdU (C) and Ki67 (F) at 14 days and 35 days after MI. Nuclei are stained with propidium iodide (dark blue). A and B, bar 10 m. C and D, data are mean SEM. n 3 to 6 mice per group. *P 0.05 vs group I; # P 0.05 vs group IV. differentiated not only into cardiomyocytes but also into vascular structures necessary to supply blood to the newly formed myocardium in the infarcted region. Mobilization of Lin - /Sca-1 /C-kit BMCs The efficacy of different cytokine regimens in mobilizing lin - /Sca-1 /c-kit BMCs was examined by flow cytometry in groups XIII through XVI. Compared with groups XIII, XV, and XVI, G-CSF FL (group XIV) therapy resulted in mobilization of a significantly higher number of lin - /Sca-1 / c-kit BMCs ( , , , and cells/ L at 5 days in groups XIII, XIV, XV, and XVI, respectively). The increase in lin - /Sca-1 /c-kit BMC levels in group XIV was apparent at 3 days of therapy and became progressively more pronounced at 5 and 10 days. Figure 5. EGFP (A and B, green) and -sarcomeric actin (B, red) small newly formed myocyte in the peri-infarct region expresses connexin 43 (A and B, magenta). Bar 10 m. Figure 7. Postinfarct cytokine therapy induces regeneration of vascular structures. A, A newly formed coronary arteriole within the infarcted myocardium is identified by the expression of EGFP (green) and -smooth muscle actin (red) in smooth muscle cells (yellow-green). B, A newly formed capillary profile (arrowhead) within infarcted myocardium is identified by the expression of EGFP (green) and PECAM-1 (red) in capillary endothelial cells (yellow-green). A pre-existing capillary is identified by the arrow. Myocytes are stained in purple ( -sarcomeric actin). Nuclei are stained with 4,6-diamidino-2-phenylindole (blue). Bar 10 m.
6 Dawn et al Cytokine Therapy for Myocardial Regeneration 1103 Figure 8. Flow cytometric assessment of adhesion molecule expression in lin - /Sca-1 /c-kit cells from untreated bone marrow and peripheral blood of mice treated with G-CSF FL, G-CSF SCF, or G-CSF alone. Shown is the overlay of the adhesion molecules (red) and the isotype control (green). PB indicates peripheral blood. G-CSF SCF (group XV) and G-CSF alone (group XVI) resulted in minimal changes in peripheral blood levels of lin - /Sca-1 /c-kit cells. Cytokine Therapy Enhances Expression of Surface Antigens That Favor Homing In groups XVII through XX, peripheral blood cells were analyzed by flow cytometry for expression of adhesion molecules on mobilized lin - /Sca-1 /c-kit BMCs. As shown in supplemental Figure 7, all three treatments (G-CSF FL, G-CSF SCF, and G-CSF alone) resulted in increased expression of CD62P (P-selectin), an adhesion molecule that favors attachment and extravasation of BMCs, 16 and decreased expression of CD49d, an adhesion molecule that plays a critical role in retaining cells within the bone marrow. 17 Both of these changes would be expected to promote release of BMCs from bone marrow and their migration into the cardiac interstitium. However, there were significant differences in adhesion molecule profile between G-CSF FL treated mice on the one hand and G-CSF SCF and G-CSF treated mice on the other hand. Specifically, G-CSF SCF and G-CSF treatment reduced whereas G-CSF FL treatment increased the expression of CD62L (L-selectin) and CD11a (Figure 8), two adhesion molecules that favor extravasation and homing of BMCs Furthermore, the reduction in expression of CD49e, an adhesion molecule that promotes BMC attachment, 17 was comparatively less with G-CSF FL and G-CSF SCF treatment vis-à-vis G-CSF alone (supplemental Figure VII). In conjunction with the quantitative analysis of the number of circulating lin - /Sca-1 /c-kit cells reported above, these results demonstrate significant differences among different cytokine regimens with respect to the modulation of the adhesion molecules responsible for homing. Discussion Most basic and clinical studies of cardiac regeneration have focused on cell-based therapies, 4 and relatively less is known regarding the role of cytokines in this process. The present investigation provides a number of important new findings. Our results demonstrate that: (1) cytokine therapy with a combination of G-CSF FL or G-CSF SCF started after acute MI, halts or attenuates adverse LV remodeling, and improves LV systolic function; (2) cytokine therapy results in mobilization of bone marrow stem cells (lin - /Sca-1 /c-kit ), which home to the injured myocardium and transdifferentiate into the cardiac lineage (cardiomyocytes, vascular smooth muscle cells, endothelial cells); (3) the combination of G-CSF and FL is superior to G-CSF SCF or G-CSF alone with respect to its effects on LV remodeling, LV function, and mobilization of lin - /Sca-1 /c-kit BMCs into the peripheral blood; (4) all three cytokine regimens upregulated the expression of CD62P and downregulated the expression of CD49d on BMCs (CD62P [P-selectin] promotes the extravasation of BMCs, whereas CD49d is important for the retention of these cells in the marrow 16,17 ); and (5) in contrast to G-CSF SCF and G-CSF alone, G-CSF FL treatment increases the expression of adhesion molecules (CD62L and CD11a) that favor homing of BMCs CD62L (L-selectin) and the 2 integrin leukocyte function antigen-1 (CD11a) initiate and facilitate the attachment of peripheral blood cells to the endothelium, thereby enhancing the extravasation of these cells. 17,18 Thus, the present investigation not only establishes the usefulness of cytokine therapy for therapeutic cardiac regeneration but also provides mechanistic insights into this phenomenon and identifies the superiority of a specific cytokine combination (G-CSF FL). These results have important implications for the role of cytokine-based strategies in effecting cardiac repair after acute MI. Previous studies have shown that cytokine therapies initiated before 1,2 or after 3 MI result in improved LV remodeling and function. However, to our knowledge, this is the first study to demonstrate that cytokine administration results in transdifferentiation of mobilized BMCs into the cardiac lineage and in expression of adhesion molecules that favor homing of BMCs to the injured myocardium. Furthermore, this is the first investigation to systematically compare the efficacy of G-CSF, FL, and SCF and their combination on postinfarct LV remodeling and function, and to identify the combination of G-CSF and FL as the most effective treatment. A number of methodological considerations are in order. In contrast to previous studies, which examined models of permanent coronary occlusion, 1 we elected to use a transient
7 1104 Circulation Research April 28, 2006 (30-minute) coronary occlusion followed by reperfusion. This model has greater clinical relevance because most patients with acute MI undergo either spontaneous or therapeutic coronary recanalization. We chose to initiate cytokine treatment after the MI because pretreatment (as used in previous studies 1,2 ) would not be clinically feasible. For similar reasons, we elected not to perform a splenectomy (which was performed in previous investigations 1,2 ). The doses of cytokines used in this study were selected because they are clinically relevant. The large group sizes (14 to 20 mice per group in groups I through IV) were deemed to be important to achieve robust conclusions. In an effort to perform a rigorous assessment of global as well as regional LV function, we used multiple echocardiographic parameters, including fractional shortening (parasternal short-axis M-mode images), fractional area change (parasternal short-axis 2-D images), ejection fraction, and systolic wall thickening. Treatment with G-CSF FL or G-CSF SCF consistently improved all of these parameters, providing solid evidence for the functional benefit of these therapies. This concept is further corroborated by the fact that two independent techniques (echocardiography and morphometry, performed by blinded investigators who were unaware of the data obtained with the other technique) demonstrated that G-CSF FL and G-CSF SCF were effective in mitigating the progressive LV chamber dilatation that is characteristic of adverse LV remodeling and ischemic cardiomyopathy. 19 The data obtained by these two methods were generally concordant. However, in Figure 2E, we present the morphometric infarct wall thickness data because the measurement of the average integrated wall thickness by morphometry may be inherently more accurate. We chose to examine G-CSF, SCF, and FL because these cytokines are used clinically (G-CSF) or have been well characterized with respect to BMC mobilization. 15,20 Comparison of different cytokines or combinations thereof is important because these proteins are known to preferentially mobilize different subsets of BMCs. 15,21 FL was not studied alone because, unlike G-CSF, it does not expand lin - /Sca-1 / c-kit cells. 15 SCF was not studied as single therapy because it has low efficacy in mobilizing BMCs when used alone. 20 Our results indicate that the combination of G-CSF and FL produced the most robust results with respect to myocyte regeneration, LV remodeling, LV function, and mobilization of lin - /Sca-1 /c-kit cells. G-CSF SCF also resulted in significant beneficial effects, albeit to a lesser extent. The most plausible explanation for the superiority of G-CSF FL is that this combination resulted in mobilization of much higher numbers of lin - /Sca-1 /c-kit cells into the peripheral blood, which would be expected to enhance the homing and engraftment of BMCs in the damaged myocardium. In addition to these quantitative differences, our flow cytometric analysis reveals qualitative differences; that is, the lin - /Sca- 1 /c-kit cells mobilized by G-CSF FL expressed higher levels of CD62L and CD11a vis-à-vis the cells mobilized by G-CSF SCF (Figure 8). The expression of these adhesion molecules may facilitate the egress of primitive BMCs from the circulation into injured tissues that express chemoattractants. 22 Having established the functional and structural benefits of cytokine therapy, we used EGFP chimeric mice to investigate the mechanism for these salubrious actions. Our results demonstrate that after administration of G-CSF FL or G-CSF SCF, mobilized BMCs home to the infarcted myocardium and differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells. We found that cardiomyocyte regeneration was considerably greater with G-CSF FL or G-CSF SCF versus G-CSF alone (Figure 4). These findings were independently validated by the measurement of cycling (ie, BrdU and Ki67 ) cardiomyocytes in non-egfp chimeric mice (Figure 6). The mechanism(s) whereby cytokines produce beneficial effects on LV dimensions and function after MI is likely complex. Although the association of greater mobilization of lin - /Sca-1 /c-kit cells with greater improvement in LV remodeling and function after G-CSF FL treatment suggests that these cells may play an important role in cardiac regeneration, our results do not prove that the beneficial effects of cytokines were attributable to this subset of BMCs. The BMCs responsible for cardiac regeneration may be cell types other than the lin-/sca-1 /c-kit cells (eg, multipotent progenitors, endothelial progenitors, etc.). It is also unknown whether, within the lin-/sca-1 /c-kit subset of BMCs, the transdifferentiation was attributable to hematopoietic or nonhematopoietic cells. We recently identified a CXCR4 /Sca- 1 /lin - /CD45 - nonhematopoietic subfraction of BMCs that appear to be committed to differentiation into cardiac lineages and are mobilized after MI in mice as well as in humans. 22 It is possible that these cells may account for the salubrious actions of G-CSF FL and G-CSF SCF noted in this study. Finally, mobilization of resident cardiac stem cells by cytokines could be an additional or alternative mechanism. 23 In support of this hypothesis, we noted small cardiomyocytes in the infarct area of cytokine-treated hearts that were not positive for EGFP (Figure 3F, arrowheads), suggesting that cytokine treatment may have induced migration, differentiation, and possibly proliferation of resident cardiac progenitor cells, which may have contributed to cardiac repair. Harada et al 6 recently concluded that administration of G-CSF ameliorates LV remodeling and dysfunction by inhibiting apoptosis, not by promoting cardiac regeneration. Our results differ because they document transdifferentiation of BMCs into cardiac lineage (Figures 3, 4, and 7; predominantly in the infarct area). Nevertheless, we also noted functional improvement in the noninfarcted myocardium, where the extent of myocyte regeneration was minimal (Figure 4). This enhancement in contractility in the noninfarcted region is likely attributable to nonregenerative actions of cytokines (eg, inhibition of apoptosis, favorable changes in the interstitium, etc.), suggesting that multiple mechanisms contributed to the overall improvement in LV function after G-SCF FL and G-SCG SCF therapy. Although G-CSF alone induced little cardiomyocyte regeneration, it had favorable effects on LV remodeling (Figure 2C and 2D; supplemental Figure IV); however, G-CSF alone did not improve LV function significantly (Figure 1). As shown in Figure 1, measurements of LV systolic function were greater in group
8 Dawn et al Cytokine Therapy for Myocardial Regeneration 1105 IV than in vehicle-treated mice, but the differences were not statistically significant. Despite the large sample sizes (n 15 to 19 per group), this could be attributable to a type II error. Alternatively, it is possible that other nonregenerative beneficial effects of G-CSF on the myocardium resulted in an improvement in LV anatomy which, however, did not result in improved function because of the less efficient regeneration of cardiomyocytes with G-CSF therapy. In conclusion, we demonstrated that a clinically relevant protocol for cytokine therapy exerts profound beneficial effects on postinfarction cardiomyopathy. Specifically, administration of G-CSF FL or G-CSF SCF, started after a reperfused acute MI, results in regeneration of cardiomyocytes and coronary vessels by mobilized BMCs and in improvement in LV remodeling and function via myocyte regeneration and probably via other beneficial actions of cytokines. We also demonstrated that the combination of G-CSF FL is superior to G-CSF SCF or G-CSF alone. These results have significant therapeutic implications. Recent studies with G-CSF have concluded that this cytokine improves LV function and structure in patients with acute MI. 24,25 Our findings suggest that the combination of G-CSF and FL should have even greater efficacy in the clinical arena, providing a rationale for testing G-CSF FL in larger species and in patients. Acknowledgments This study was supported in part by National Institutes of Health grants HL-72410, HL-55757, HL-68088, HL-70897, HL-76794, HL-78825, HL-63442, and DK , national American Heart Association (AHA) scientist development grant N, and AHA fellowship grants B and B. References 1. Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal- Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98: Fukuhara S, Tomita S, Nakatani T, Ohtsu Y, Ishida M, Yutani C, Kitamura S. G-CSF promotes bone marrow cells to migrate into infarcted mice heart, and differentiate into cardiomyocytes. Cell Transplant. 2004; 13: Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, Ogawa S, Okano H, Hotta T, Ando K, Fukuda K. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104: Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96: Deten A, Volz HC, Clamors S, Leiblein S, Briest W, Marx G, Zimmer HG. Hematopoietic stem cells do not repair the infarcted mouse heart. Cardiovasc Res. 2005;65: Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi JI, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11: Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375 H Colson YL, Wren SM, Schuchert MJ, Patrene KD, Johnson PC, Boggs SS, Ildstad ST. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J Immunol. 1995;155: Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410: Anversa P, Beghi C, Kikkawa Y, Olivetti G. Myocardial infarction in rats. Infarct size, myocyte hypertrophy, and capillary growth. Circ Res. 1986; 58: Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100: Li B, Setoguchi M, Wang X, Andreoli AM, Leri A, Malhotra A, Kajstura J, Anversa P. Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circ Res. 1999;84: Anversa P, Olivetti G. The cardiovascular system. In: Page E, Fozzard HA, Solaro RJ, eds. The Heart. 1st ed. New York, NY: Oxford University Press; 2002: Orfao A, Ruiz-Arguelles A. General concepts about cell sorting techniques. Clin Biochem. 1996;29: Neipp M, Zorina T, Domenick MA, Exner BG, Ildstad ST. Effect of FLT3 ligand and granulocyte colony-stimulating factor on expansion and mobilization of facilitating cells and hematopoietic stem cells in mice: kinetics and repopulating potential. Blood. 1998;92: Hope SA, Meredith IT. Cellular adhesion molecules and cardiovascular disease. Part I. Their expression and role in atherogenesis. Intern Med J. 2003;33: Kronenwett R, Martin S, Haas R. The role of cytokines and adhesion molecules for mobilization of peripheral blood stem cells. Stem Cells. 2000;18: Weber C. Novel mechanistic concepts for the control of leukocyte transmigration: specialization of integrins, chemokines, and junctional molecules. J Mol Med. 2003;81: Pfeffer MA, Pfeffer JM, Lamas GA. Development and prevention of congestive heart failure following myocardial infarction. Circulation. 1993;87:IV Molineux G, Migdalska A, Szmitkowski M, Zsebo K, Dexter TM. The effects on hematopoiesis of recombinant stem cell factor (ligand for c-kit) administered in vivo to mice either alone or in combination with granulocyte colony-stimulating factor. Blood. 1991;78: Hess DA, Levac KD, Karanu FN, Rosu-Myles M, White MJ, Gallacher L, Murdoch B, Keeney M, Ottowski P, Foley R, Chin-Yee I, Bhatia M. Functional analysis of human hematopoietic repopulating cells mobilized with granulocyte colony-stimulating factor alone versus granulocyte colony-stimulating factor in combination with stem cell factor. Blood. 2002;100: Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood following myocardial infarction. Circ Res. 2004;95: Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102: Na S, Cho H, Chung J, Hahn J, Lee H, Kang H, Cho Y, Koo B, Kim Y, Youn T, Sohn D, Oh B, Lee M, Park Y, Choi Y, Kim H. Comparison of two methods of peripheral blood stem cell transplantation in patients with myocardial infarction: intracoronary infusion of mobilized peripheral blood stem cell vs. mobilization only [Magic cell investigator: myocardial regeneration and angiogenesis in MI with G-CSF mobilization and intracoronary cell infusion]. J Am Coll Cardiol. 2005;45(suppl A):4A (abstract). 25. Leone A, Galiuto L, Liuzzo G, Giordano A, Calcagni L, Garramone B, Giannico M, Cirillo F, Biasucci LM, Rebuzzi A, Crea F. Granulocytecolony stimulating factor in the acute myocardial infarction (the Rigenera Study). J Am Coll Cardiol. 2005;45(suppl A):238A (abstract).
Resident cardiac stem cells: how to find and use them
 Resident cardiac stem cells: how to find and use them G. Hasenfuß Cardiology and Pneumology Heart Research Center Göttingen Georg-August-University Göttingen Definition: Stem cell Selfrenewal Stem cell
Resident cardiac stem cells: how to find and use them G. Hasenfuß Cardiology and Pneumology Heart Research Center Göttingen Georg-August-University Göttingen Definition: Stem cell Selfrenewal Stem cell
Supporting Information. Calculation of the relative contributions of myocyte proliferation, stem cell. Supporting Information Fig 1 (page 9)
 Supporting Information Table of contents Calculation of the relative contributions of myocyte proliferation, stem cell differentiation and cardioprotection (page 2) Supporting Information Fig 1 (page 9)
Supporting Information Table of contents Calculation of the relative contributions of myocyte proliferation, stem cell differentiation and cardioprotection (page 2) Supporting Information Fig 1 (page 9)
NIH Public Access Author Manuscript J Cell Mol Med. Author manuscript; available in PMC 2012 June 1.
 NIH Public Access Author Manuscript Published in final edited form as: J Cell Mol Med. 2011 June ; 15(6): 1319 1328. doi:10.1111/j.1582-4934.2010.01126.x. TRANSPLANTATION OF EXPANDED BONE MARROW-DERIVED
NIH Public Access Author Manuscript Published in final edited form as: J Cell Mol Med. 2011 June ; 15(6): 1319 1328. doi:10.1111/j.1582-4934.2010.01126.x. TRANSPLANTATION OF EXPANDED BONE MARROW-DERIVED
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
ENDOGENOUS CARDIAC STEM CELLS IN THE REGENERATION OF ACUTE AND CHRONIC ISCHEMIC MYOCARDIUM
 ENDOGENOUS CARDIAC STEM CELLS IN THE REGENERATION OF ACUTE AND CHRONIC ISCHEMIC MYOCARDIUM Bernardo Nadal-Ginard, M.D., Ph.D. New York Medical College Angioplasty Summit 2004, Seoul 04/29/04 MYOCARDIAL
ENDOGENOUS CARDIAC STEM CELLS IN THE REGENERATION OF ACUTE AND CHRONIC ISCHEMIC MYOCARDIUM Bernardo Nadal-Ginard, M.D., Ph.D. New York Medical College Angioplasty Summit 2004, Seoul 04/29/04 MYOCARDIAL
Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function
 Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function Axel Linke*, Patrick Müller*, Daria Nurzynska*, Claudia Casarsa*,
Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function Axel Linke*, Patrick Müller*, Daria Nurzynska*, Claudia Casarsa*,
Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair
 Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair Mouse Model of Myocardial Infarction (MI) All animal work was compliant with the Institutional Animal
Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair Mouse Model of Myocardial Infarction (MI) All animal work was compliant with the Institutional Animal
Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy
 Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy Massimiliano Gnecchi, Zhiping Zhang, Aiguo Ni, Victor J. Dzau Circulation Research 2008 Nov 21;103(11):1204-19 Introduction(1) After AMI all
Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy Massimiliano Gnecchi, Zhiping Zhang, Aiguo Ni, Victor J. Dzau Circulation Research 2008 Nov 21;103(11):1204-19 Introduction(1) After AMI all
Mesenchymal Stem Cells to Repair Vascular Damage after Chemotherapy: Past, Present and Future
 Mesenchymal Stem Cells to Repair Vascular Damage after Chemotherapy: Past, Present and Future Cell Therapy 2014 Las Vegas, NV, USA Sulaiman Al-Hashmi, PhD Sultan Qaboos University Oman What are MSCs? Stem
Mesenchymal Stem Cells to Repair Vascular Damage after Chemotherapy: Past, Present and Future Cell Therapy 2014 Las Vegas, NV, USA Sulaiman Al-Hashmi, PhD Sultan Qaboos University Oman What are MSCs? Stem
Hematopoiesis. - Process of generation of mature blood cells. - Daily turnover of blood cells (70 kg human)
 Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
Stem Cells. Keith Channon. Department of Cardiovascular Medicine University of Oxford John Radcliffe Hospital, Oxford
 Stem Cells Keith Channon Department of Cardiovascular Medicine University of Oxford John Radcliffe Hospital, Oxford Adult Stem Cells Unique cells that are capable of self-renewal Have the ability to differentiate
Stem Cells Keith Channon Department of Cardiovascular Medicine University of Oxford John Radcliffe Hospital, Oxford Adult Stem Cells Unique cells that are capable of self-renewal Have the ability to differentiate
Cardiac Myocytes are Recruited by Bone Marrow-Derived Cells in Intact Murine Heart
 Kobe J. Med. Sci., Vol. 48, No. 6, pp. 161-166, 2002 Cardiac Myocytes are Recruited by Bone Marrow-Derived Cells in Intact Murine Heart SEIMI SATOMI-KOBAYASHI 1, SEINOSUKE KAWASHIMA 1*, TSUYOSHI SAKODA
Kobe J. Med. Sci., Vol. 48, No. 6, pp. 161-166, 2002 Cardiac Myocytes are Recruited by Bone Marrow-Derived Cells in Intact Murine Heart SEIMI SATOMI-KOBAYASHI 1, SEINOSUKE KAWASHIMA 1*, TSUYOSHI SAKODA
Vascular Biology. Rapid Communication
 Vascular Biology Rapid Communication Granulocyte Colony Stimulating Factor Directly Inhibits Myocardial Ischemia-Reperfusion Injury Through Akt Endothelial NO Synthase Pathway Kazutaka Ueda, Hiroyuki Takano,
Vascular Biology Rapid Communication Granulocyte Colony Stimulating Factor Directly Inhibits Myocardial Ischemia-Reperfusion Injury Through Akt Endothelial NO Synthase Pathway Kazutaka Ueda, Hiroyuki Takano,
NIH Public Access Author Manuscript Circulation. Author manuscript; available in PMC 2011 January 19.
 NIH Public Access Author Manuscript Published in final edited form as: Circulation. 2010 January 19; 121(2): 293. doi:10.1161/circulationaha.109.871905. INTRACORONARY ADMINISTRATION OF CARDIAC PROGENITOR
NIH Public Access Author Manuscript Published in final edited form as: Circulation. 2010 January 19; 121(2): 293. doi:10.1161/circulationaha.109.871905. INTRACORONARY ADMINISTRATION OF CARDIAC PROGENITOR
c-kit Stem Cell Therapy of Ischemic Cardiomyopathy
 American Heart Association Scientific Sessions American Heart Association Scientific Sessions c-kit Stem Cell Therapy of Ischemic Cardiomyopathy Roberto Bolli, M.D. Institute of Molecular Cardiology University
American Heart Association Scientific Sessions American Heart Association Scientific Sessions c-kit Stem Cell Therapy of Ischemic Cardiomyopathy Roberto Bolli, M.D. Institute of Molecular Cardiology University
Cardiac Regeneration. Piero Anversa, MD, Annarosa Leri, MD, Jan Kajstura, PHD Valhalla, New York THE HEART AS A POST-MITOTIC ORGAN: THE CONTROVERSY
 Journal of the American College of Cardiology Vol. 47, No. 9, 2006 2006 by the American College of Cardiology Foundation ISSN 0735-1097/06/$32.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2006.02.003
Journal of the American College of Cardiology Vol. 47, No. 9, 2006 2006 by the American College of Cardiology Foundation ISSN 0735-1097/06/$32.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2006.02.003
Cell therapy: enhancing the therapeutic potential of cardiac progenitors for delivery post myocardial infarction. Rita Alonaizan
 Cell therapy: enhancing the therapeutic potential of cardiac progenitors for delivery post myocardial infarction Rita Alonaizan Department of Physiology, Anatomy & Genetics St Catherine s College Supervisor:
Cell therapy: enhancing the therapeutic potential of cardiac progenitors for delivery post myocardial infarction Rita Alonaizan Department of Physiology, Anatomy & Genetics St Catherine s College Supervisor:
TISSUE-SPECIFIC STEM CELLS
 TISSUE-SPECIFIC STEM CELLS Complement Component 3 is Necessary to Preserve Myocardium and Myocardial Function in Chronic Myocardial Infarction MARCIN WYSOCZYNSKI, a MITESH SOLANKI, a SYLWIA BORKOWSKA,
TISSUE-SPECIFIC STEM CELLS Complement Component 3 is Necessary to Preserve Myocardium and Myocardial Function in Chronic Myocardial Infarction MARCIN WYSOCZYNSKI, a MITESH SOLANKI, a SYLWIA BORKOWSKA,
The recognition that myocyte mitosis occurs in the fetal,
 Basic Science for Clinicians Life and Death of Cardiac Stem Cells A Paradigm Shift in Cardiac Biology Piero Anversa, MD; Jan Kajstura, PhD; Annarosa Leri, MD; Roberto Bolli, MD The recognition that myocyte
Basic Science for Clinicians Life and Death of Cardiac Stem Cells A Paradigm Shift in Cardiac Biology Piero Anversa, MD; Jan Kajstura, PhD; Annarosa Leri, MD; Roberto Bolli, MD The recognition that myocyte
Mass Histology Service
 Mass Histology Service A complete anatomical pathology laboratory www.masshistology.com Telephone: (877) 286-6004 Report on Pathology A Time Course Study of the Local Effects of Intramuscular XXXXXXX Injection
Mass Histology Service A complete anatomical pathology laboratory www.masshistology.com Telephone: (877) 286-6004 Report on Pathology A Time Course Study of the Local Effects of Intramuscular XXXXXXX Injection
Postn MCM Smad2 fl/fl Postn MCM Smad3 fl/fl Postn MCM Smad2/3 fl/fl. Postn MCM. Tgfbr1/2 fl/fl TAC
 A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
Chronic variable stress activates hematopoietic stem cells
 SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
Effective Targeting of Quiescent Chronic Myelogenous
 Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
In vivo and in vitro studies have strengthened the notion that
 bcl-2 overexpression promotes myocyte proliferation Federica Limana*, Konrad Urbanek*, Stefano Chimenti*, Federico Quaini*, Annarosa Leri*, Jan Kajstura*, Bernardo Nadal-Ginard*, Seigo Izumo, and Piero
bcl-2 overexpression promotes myocyte proliferation Federica Limana*, Konrad Urbanek*, Stefano Chimenti*, Federico Quaini*, Annarosa Leri*, Jan Kajstura*, Bernardo Nadal-Ginard*, Seigo Izumo, and Piero
Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation
 SUPPLEMENTARY INFORMATION Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation Samantha Arokiasamy 1,2, Christian Zakian 1, Jessica Dilliway
SUPPLEMENTARY INFORMATION Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation Samantha Arokiasamy 1,2, Christian Zakian 1, Jessica Dilliway
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Myocardial infarction
 NEW CARDIAC MARKERS AND CARDIAC REGENERATION Päivi Lakkisto, MD, PhD Specialist in Clinical Chemistry Clinical lecturer University of Helsinki and HUSLAB Minerva Institute for Medical Research Myocardial
NEW CARDIAC MARKERS AND CARDIAC REGENERATION Päivi Lakkisto, MD, PhD Specialist in Clinical Chemistry Clinical lecturer University of Helsinki and HUSLAB Minerva Institute for Medical Research Myocardial
DOWNLOAD PDF CARDIAC REMODELING AND CELL DEATH IN HEART FAILURE
 Chapter 1 : The fibrosis-cell death axis in heart failure Remodeling may be defined as changes in the morphology, structure, and function of the heart related to alterations in loading conditions and/or
Chapter 1 : The fibrosis-cell death axis in heart failure Remodeling may be defined as changes in the morphology, structure, and function of the heart related to alterations in loading conditions and/or
Supplemental Figure I
 Supplemental Figure I Kl ( mmol/l)-induced Force orta M (mn) 1 (mn) 1 Supplemental Figure I. Kl-induced contractions. and, Kl ( mmol/l)-induced contractions of the aorta () and those of mesenteric arteries
Supplemental Figure I Kl ( mmol/l)-induced Force orta M (mn) 1 (mn) 1 Supplemental Figure I. Kl-induced contractions. and, Kl ( mmol/l)-induced contractions of the aorta () and those of mesenteric arteries
1. Cardiomyocytes and nonmyocyte. 2. Extracellular Matrix 3. Vessels שאלה 1. Pathobiology of Heart Failure Molecular and Cellular Mechanism
 Pathobiology of Heart Failure Molecular and Cellular Mechanism Jonathan Leor Neufeld Cardiac Research Institute Tel-Aviv University Sheba Medical Center, Tel-Hashomer שאלה 1 התא הנפוץ ביותר (75%~) בלב
Pathobiology of Heart Failure Molecular and Cellular Mechanism Jonathan Leor Neufeld Cardiac Research Institute Tel-Aviv University Sheba Medical Center, Tel-Hashomer שאלה 1 התא הנפוץ ביותר (75%~) בלב
C57BL/6 Mice are More Appropriate. than BALB/C Mice in Inducing Dilated Cardiomyopathy with Short-Term Doxorubicin Treatment
 Original Article C57BL/6 Mice are More Appropriate Acta Cardiol Sin 2012;28:236 240 Heart Failure & Cardiomyopathy C57BL/6 Mice are More Appropriate than BALB/C Mice in Inducing Dilated Cardiomyopathy
Original Article C57BL/6 Mice are More Appropriate Acta Cardiol Sin 2012;28:236 240 Heart Failure & Cardiomyopathy C57BL/6 Mice are More Appropriate than BALB/C Mice in Inducing Dilated Cardiomyopathy
CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow
 White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
Myocardial Regeneration and Stem Cell Repair
 Myocardial Regeneration and Stem Cell Repair Annarosa Leri, MD, Jan Kajstura, MD, Piero Anversa, MD, and William H. Frishman, MD Abstract: Recent evidence would suggest that the heart is not a terminally
Myocardial Regeneration and Stem Cell Repair Annarosa Leri, MD, Jan Kajstura, MD, Piero Anversa, MD, and William H. Frishman, MD Abstract: Recent evidence would suggest that the heart is not a terminally
Role of Inflammation in Pulmonary Hypertension
 Role of Inflammation in Pulmonary Hypertension K. R. Stenmark University of Colorado Denver, USA Prominent Fibroproliferative Changes are Observed in the Lung Vasculature of Infants With Pulmonary Arterial
Role of Inflammation in Pulmonary Hypertension K. R. Stenmark University of Colorado Denver, USA Prominent Fibroproliferative Changes are Observed in the Lung Vasculature of Infants With Pulmonary Arterial
TISSUE-SPECIFIC STEM CELLS
 TISSUE-SPECIFIC STEM CELLS Concise Review: Stem Cells, Myocardial Regeneration, and Methodological Artifacts PIERO ANVERSA, a ANNAROSA LERI, a MARCELLO ROTA, a TORU HOSODA, a CLAUDIA BEARZI, a KONRAD URBANEK,
TISSUE-SPECIFIC STEM CELLS Concise Review: Stem Cells, Myocardial Regeneration, and Methodological Artifacts PIERO ANVERSA, a ANNAROSA LERI, a MARCELLO ROTA, a TORU HOSODA, a CLAUDIA BEARZI, a KONRAD URBANEK,
c Ischemia (30 min) Reperfusion (8 w) Supplementary Figure bp 300 bp Ischemia (30 min) Reperfusion (4 h) Dox 20 mg/kg i.p.
 a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
Exercise in Adverse Cardiac Remodeling: of Mice and Men
 Exercise in Adverse Cardiac Remodeling: of Mice and Men 17-01-2013 Dirk J Duncker Experimental Cardiology, Cardiology, Thoraxcenter Cardiovascular Research Institute COEUR Erasmus MC, University Medical
Exercise in Adverse Cardiac Remodeling: of Mice and Men 17-01-2013 Dirk J Duncker Experimental Cardiology, Cardiology, Thoraxcenter Cardiovascular Research Institute COEUR Erasmus MC, University Medical
Nature Genetics: doi: /ng Supplementary Figure 1. Parameters and consequences of mononuclear cardiomyocyte frequency.
 Supplementary Figure 1 Parameters and consequences of mononuclear cardiomyocyte frequency. (a) Correlation of the frequency of mononuclear cardiomyocytes to the frequency of cardiomyocytes with three or
Supplementary Figure 1 Parameters and consequences of mononuclear cardiomyocyte frequency. (a) Correlation of the frequency of mononuclear cardiomyocytes to the frequency of cardiomyocytes with three or
International Graduate Research Programme in Cardiovascular Science
 1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
Therapeutic Potential of Human Umbilical Cord Derived Stem Cells in a Rat Myocardial Infarction Model
 Therapeutic Potential of Human Umbilical Cord Derived Stem Cells in a Rat Myocardial Infarction Model Kai Hong Wu, MD, PhD,* Bin Zhou, PhD,* Cun Tao Yu, MD, Bin Cui, MD, Shi Hong Lu, BS, Zhong Chao Han,
Therapeutic Potential of Human Umbilical Cord Derived Stem Cells in a Rat Myocardial Infarction Model Kai Hong Wu, MD, PhD,* Bin Zhou, PhD,* Cun Tao Yu, MD, Bin Cui, MD, Shi Hong Lu, BS, Zhong Chao Han,
Citation for published version (APA): Velde, S. V. D. (2006). Stem cell-mediated regeneration of the infarcted heart: inflammation rules? s.n.
 University of Groningen Stem cell-mediated regeneration of the infarcted heart Velde, Susanne van der IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to
University of Groningen Stem cell-mediated regeneration of the infarcted heart Velde, Susanne van der IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to
Supplemental Table 1. Echocardiography Control (n=4)
 Supplemental Table 1. Echocardiography (n=4) Mlc2v cre/+ ; DNMAML (n=4) LVIDd, mm 3.9±0.3 4.3±0.3 LVIDs, mm 2.6±0.4 2.9±0.2 d, mm 0.72±0.06 0.75±0.1 LVPWd, mm 0.72±0.06 0.77±0.11 FS, % 33±6 33±1 EF, %
Supplemental Table 1. Echocardiography (n=4) Mlc2v cre/+ ; DNMAML (n=4) LVIDd, mm 3.9±0.3 4.3±0.3 LVIDs, mm 2.6±0.4 2.9±0.2 d, mm 0.72±0.06 0.75±0.1 LVPWd, mm 0.72±0.06 0.77±0.11 FS, % 33±6 33±1 EF, %
Journal Club WS 2012/13 Stefanie Nickl
 Journal Club WS 2012/13 Stefanie Nickl Background Mesenchymal Stem Cells First isolation from bone marrow 30 ys ago Isolation from: spleen, heart, skeletal muscle, synovium, amniotic fluid, dental pulp,
Journal Club WS 2012/13 Stefanie Nickl Background Mesenchymal Stem Cells First isolation from bone marrow 30 ys ago Isolation from: spleen, heart, skeletal muscle, synovium, amniotic fluid, dental pulp,
A Comparative Analysis of Capillary Density in the Myocardium of Normotensive and Spontaneously Hypertensive Rats
 Institute of Experimental Morphology, Pathology and Anthropology with Museum Bulgarian Anatomical Society Acta morphologica et anthropologica, 24 (1-2) Sofia 2017 A Comparative Analysis of Capillary Density
Institute of Experimental Morphology, Pathology and Anthropology with Museum Bulgarian Anatomical Society Acta morphologica et anthropologica, 24 (1-2) Sofia 2017 A Comparative Analysis of Capillary Density
Cell Combination Therapy. Disclosures
 Cell Combination Therapy Joshua M. Hare, M.D. Louis Lemberg Professor Senior Associate Dean Chief Science Officer Interdisciplinary Stem Cell Institute The Miller School of Medicine, University of Miami
Cell Combination Therapy Joshua M. Hare, M.D. Louis Lemberg Professor Senior Associate Dean Chief Science Officer Interdisciplinary Stem Cell Institute The Miller School of Medicine, University of Miami
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and
 Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Supplementary Figure S1. Flow cytometric analysis of the expression of Thy1 in NH cells. Flow cytometric analysis of the expression of T1/ST2 and Thy1 in NH cells derived from the lungs of naïve mice.
Multimodality Imaging in Cardiac Stem Cell Research
 Multimodality Imaging in Cardiac Stem Cell Research IL SUK SOHN, MD, PhD Department of Cardiology Kyung Hee University Hospital at Gangdong Kyung Hee University School of Medicine, Seoul, Korea Stem Cell
Multimodality Imaging in Cardiac Stem Cell Research IL SUK SOHN, MD, PhD Department of Cardiology Kyung Hee University Hospital at Gangdong Kyung Hee University School of Medicine, Seoul, Korea Stem Cell
Uncovering the mechanisms of wound healing and fibrosis
 Any Questions??? Ask now or contact support support@sabiosciences.com 1-888-503-3187 International customers: SABio@Qiagen.com Uncovering the mechanisms of wound healing and fibrosis Webinar related questions:
Any Questions??? Ask now or contact support support@sabiosciences.com 1-888-503-3187 International customers: SABio@Qiagen.com Uncovering the mechanisms of wound healing and fibrosis Webinar related questions:
Stem Cell Mobilization by Granulocyte Colony-Stimulating Factor for Myocardial Recovery After Acute Myocardial Infarction
 Journal of the American College of Cardiology Vol. 51, No. 15, 2008 2008 by the American College of Cardiology Foundation ISSN 0735-1097/08/$34.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2007.11.073
Journal of the American College of Cardiology Vol. 51, No. 15, 2008 2008 by the American College of Cardiology Foundation ISSN 0735-1097/08/$34.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2007.11.073
B220 CD4 CD8. Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN
 B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Growth-factor-induced mobilisation of stem cells after acute infarction: which growth factors and when?
 Growth-factor-induced mobilisation of stem cells after acute infarction: which growth factors and when? Giulio Pompilio MD PhD DEPT. OF CARDIOVASCULAR SURGERY LABORATORY OF VASCULAR BIOLOGY AND REGENERATIVE
Growth-factor-induced mobilisation of stem cells after acute infarction: which growth factors and when? Giulio Pompilio MD PhD DEPT. OF CARDIOVASCULAR SURGERY LABORATORY OF VASCULAR BIOLOGY AND REGENERATIVE
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Myocyte turnover occurs in the human heart, and myocyte
 Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy Konrad Urbanek*, Federico Quaini*, Giordano Tasca, Daniele Torella*, Clotilde Castaldo*, Bernardo Nadal-Ginard*, Annarosa
Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy Konrad Urbanek*, Federico Quaini*, Giordano Tasca, Daniele Torella*, Clotilde Castaldo*, Bernardo Nadal-Ginard*, Annarosa
Stem cell homing and engraftment to the heart and new
 Review: Current Perspective Molecular Genetic Advances in Cardiovascular Medicine Focus on the Myocyte Piero Anversa, MD; Mark A. Sussman, PhD; Roberto Bolli, MD Stem cell homing and engraftment to the
Review: Current Perspective Molecular Genetic Advances in Cardiovascular Medicine Focus on the Myocyte Piero Anversa, MD; Mark A. Sussman, PhD; Roberto Bolli, MD Stem cell homing and engraftment to the
Surgery for Acquired Cardiovascular Disease
 Suzuki et al Surgery for Acquired Cardiovascular Disease The reduction of hemodynamic loading assists self-regeneration of the injured heart by increasing cell proliferation, inhibiting cell apoptosis,
Suzuki et al Surgery for Acquired Cardiovascular Disease The reduction of hemodynamic loading assists self-regeneration of the injured heart by increasing cell proliferation, inhibiting cell apoptosis,
Myocardial viability testing. What we knew and what is new
 Myocardial viability testing. What we knew and what is new Dr B K S Sastry, MD, DM. CARE Hospitals, Hyderabad What is Viability Viability Dysfunctional myocardium subtended by diseased coronary arteries
Myocardial viability testing. What we knew and what is new Dr B K S Sastry, MD, DM. CARE Hospitals, Hyderabad What is Viability Viability Dysfunctional myocardium subtended by diseased coronary arteries
Cardioprotective c-kit + cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines
 Related Commentary, page 1838 Research article Cardioprotective c-kit + cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines Shafie Fazel, 1 Massimo Cimini, 1 Liwen
Related Commentary, page 1838 Research article Cardioprotective c-kit + cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines Shafie Fazel, 1 Massimo Cimini, 1 Liwen
Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic
 Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic B cells from WT Nfat2 +/+, TCL1 Nfat2 +/+ and TCL1 Nfat2
Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic B cells from WT Nfat2 +/+, TCL1 Nfat2 +/+ and TCL1 Nfat2
ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1
 ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
The New England Journal of Medicine EVIDENCE THAT HUMAN CARDIAC MYOCYTES DIVIDE AFTER MYOCARDIAL INFARCTION
 EVIDENCE THAT HUMAN CARDIAC MYOCYTES DIVIDE AFTER MYOCARDIAL INFARCTION ANTONIO P. BELTRAMI, M.D., KONRAD URBANEK, M.D., JAN KAJSTURA, PH.D., SHAO-MIN YAN, M.D., NICOLETTA FINATO, M.D., ROSSANA BUSSANI,
EVIDENCE THAT HUMAN CARDIAC MYOCYTES DIVIDE AFTER MYOCARDIAL INFARCTION ANTONIO P. BELTRAMI, M.D., KONRAD URBANEK, M.D., JAN KAJSTURA, PH.D., SHAO-MIN YAN, M.D., NICOLETTA FINATO, M.D., ROSSANA BUSSANI,
Supplementary material page 1/10
 Supplementary Figure 1. Metoprolol administration during ongoing AMI reduces MVO in STEMI patients (a, b) Complete representative CMR exams (short-axis covering the entire left ventricle (LV) from base
Supplementary Figure 1. Metoprolol administration during ongoing AMI reduces MVO in STEMI patients (a, b) Complete representative CMR exams (short-axis covering the entire left ventricle (LV) from base
BCR-ABL - LSK BCR-ABL + LKS - (%)
 Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Patterns of Left Ventricular Remodeling in Chronic Heart Failure: The Role of Inadequate Ventricular Hypertrophy
 Abstract ESC 82445 Patterns of Left Ventricular Remodeling in Chronic Heart Failure: The Role of Inadequate Ventricular Hypertrophy FL. Dini 1, P. Capozza 1, P. Fontanive 2, MG. Delle Donne 1, V. Santonato
Abstract ESC 82445 Patterns of Left Ventricular Remodeling in Chronic Heart Failure: The Role of Inadequate Ventricular Hypertrophy FL. Dini 1, P. Capozza 1, P. Fontanive 2, MG. Delle Donne 1, V. Santonato
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin
 Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
Supplementary Figure 1. Nature Neuroscience: doi: /nn.4547
 Supplementary Figure 1 Characterization of the Microfetti mouse model. (a) Gating strategy for 8-color flow analysis of peripheral Ly-6C + monocytes from Microfetti mice 5-7 days after TAM treatment. Living
Supplementary Figure 1 Characterization of the Microfetti mouse model. (a) Gating strategy for 8-color flow analysis of peripheral Ly-6C + monocytes from Microfetti mice 5-7 days after TAM treatment. Living
Case Report. Case Report. Ana Lúcia Martins Arruda, Altamiro Ozório, Eloisa Mattos, José Lázaro de Andrade, Thomas Porter, Wilson Mathias Jr
 Case Report Hypoperfusion of the Left Ventricle in the Absence of Changes in Segmental Contractility as Observed through Echocardiography by Using Microbubbles During Dobutamine Infusion Ana Lúcia Martins
Case Report Hypoperfusion of the Left Ventricle in the Absence of Changes in Segmental Contractility as Observed through Echocardiography by Using Microbubbles During Dobutamine Infusion Ana Lúcia Martins
Supplementary Material
 Supplementary Material Induction of myocardial infarction Mice were anesthetized by intraperitoneal injection of pentobarbital (7 mg/kg). In the supine position, endotracheal intubation was performed.
Supplementary Material Induction of myocardial infarction Mice were anesthetized by intraperitoneal injection of pentobarbital (7 mg/kg). In the supine position, endotracheal intubation was performed.
Research progress of adult stem cells and clinical applications
 18 4 2006 8 Chinese Bulletin of Life Sciences Vol. 18, No. 4 Aug., 2006 1004-0374(2006)04-0328-05 100850 Q831 A Research progress of adult stem cells and clinical applications XI Jia-Fei, WANG Yun-Fang,
18 4 2006 8 Chinese Bulletin of Life Sciences Vol. 18, No. 4 Aug., 2006 1004-0374(2006)04-0328-05 100850 Q831 A Research progress of adult stem cells and clinical applications XI Jia-Fei, WANG Yun-Fang,
Histopathology: healing
 Histopathology: healing These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information that
Histopathology: healing These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information that
CT for Myocardial Characterization of Cardiomyopathy. Byoung Wook Choi, Yonsei University Severance Hospital, Seoul, Korea
 CT for Myocardial Characterization of Cardiomyopathy Byoung Wook Choi, Yonsei University Severance Hospital, Seoul, Korea Cardiomyopathy Elliott P et al. Eur Heart J 2008;29:270-276 The European Society
CT for Myocardial Characterization of Cardiomyopathy Byoung Wook Choi, Yonsei University Severance Hospital, Seoul, Korea Cardiomyopathy Elliott P et al. Eur Heart J 2008;29:270-276 The European Society
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features
 Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Role of Inflammatory and Progenitor Cells in Pulmonary Vascular Remodeling: Potential Role for Targeted Therapies. Traditional Hypothesis Stress
 3/1/212 Role of Inflammatory and Progenitor Cells in Pulmonary Vascular Remodeling: Potential Role for Targeted Therapies K.R. Stenmark University of Colorado Denver, CO 845 Prominent Fibroproliferative
3/1/212 Role of Inflammatory and Progenitor Cells in Pulmonary Vascular Remodeling: Potential Role for Targeted Therapies K.R. Stenmark University of Colorado Denver, CO 845 Prominent Fibroproliferative
Myocardial Infarction
 Myocardial Infarction MI = heart attack Defined as necrosis of heart muscle resulting from ischemia. A very significant cause of death worldwide. of these deaths, 33% -50% die before they can reach the
Myocardial Infarction MI = heart attack Defined as necrosis of heart muscle resulting from ischemia. A very significant cause of death worldwide. of these deaths, 33% -50% die before they can reach the
Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart
 OPEN ACCESS Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart Konstantinos Malliaras, Yiqiang Zhang, Jeffrey
OPEN ACCESS Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart Konstantinos Malliaras, Yiqiang Zhang, Jeffrey
The New England Journal of Medicine
 The New England Journal of Medicine Copyright 2002 by the Massachusetts Medical Society VOLUME 346 J ANUARY 3, 2002 NUMBER 1 CHIMERISM OF THE TRANSPLANTED HEART FEDERICO QUAINI, M.D., KONRAD URBANEK, M.D.,
The New England Journal of Medicine Copyright 2002 by the Massachusetts Medical Society VOLUME 346 J ANUARY 3, 2002 NUMBER 1 CHIMERISM OF THE TRANSPLANTED HEART FEDERICO QUAINI, M.D., KONRAD URBANEK, M.D.,
Radiologic Assessment of Myocardial Viability
 November 2001 Radiologic Assessment of Myocardial Viability Joshua Moss, Harvard Medical School Year III Patient EF 66yo female with a 3-year history of intermittent chest pain previously relieved by sublingual
November 2001 Radiologic Assessment of Myocardial Viability Joshua Moss, Harvard Medical School Year III Patient EF 66yo female with a 3-year history of intermittent chest pain previously relieved by sublingual
Title: Second-Opinion Stress Tele-Echocardiography for Aged Donor Heart Selection
 Author's response to reviews Title: Second-Opinion Stress Tele-Echocardiography for Aged Donor Heart Selection Authors: Daniele Franchi (franchid@ifc.cnr.it) Davide Cini (davide.cini@cnr.it) Giorgio Arpesella
Author's response to reviews Title: Second-Opinion Stress Tele-Echocardiography for Aged Donor Heart Selection Authors: Daniele Franchi (franchid@ifc.cnr.it) Davide Cini (davide.cini@cnr.it) Giorgio Arpesella
Supplementary Materials. for Garmy-Susini, et al, Integrin 4 1 signaling is required for lymphangiogenesis and tumor metastasis
 Supplementary Materials for Garmy-Susini, et al, Integrin 4 1 signaling is required for lymphangiogenesis and tumor metastasis 1 Supplementary Figure Legends Supplementary Figure 1: Integrin expression
Supplementary Materials for Garmy-Susini, et al, Integrin 4 1 signaling is required for lymphangiogenesis and tumor metastasis 1 Supplementary Figure Legends Supplementary Figure 1: Integrin expression
Cardiac Stem Cells and Mechanisms of Myocardial Regeneration
 Physiol Rev 85: 1373 1416, 2005; doi:10.1152/physrev.00013.2005. Cardiac Stem Cells and Mechanisms of Myocardial Regeneration ANNAROSA LERI, JAN KAJSTURA, AND PIERO ANVERSA Cardiovascular Research Institute,
Physiol Rev 85: 1373 1416, 2005; doi:10.1152/physrev.00013.2005. Cardiac Stem Cells and Mechanisms of Myocardial Regeneration ANNAROSA LERI, JAN KAJSTURA, AND PIERO ANVERSA Cardiovascular Research Institute,
Haematopoietic stem cells
 Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
Detection and Assessment of MI: Use of Imaging Methods. Robert O. Bonow, M.D.
 Detection and Assessment of MI: Use of Imaging Methods Robert O. Bonow, M.D. Detection and Assessment of MI: Use of Imaging Methods Robert O. Bonow, M.D. No Relationships to Disclose Expert Consensus Document
Detection and Assessment of MI: Use of Imaging Methods Robert O. Bonow, M.D. Detection and Assessment of MI: Use of Imaging Methods Robert O. Bonow, M.D. No Relationships to Disclose Expert Consensus Document
Highly Efficient CRISPR/Cas9 Gene Editing and Long-Term Engraftment of Human Hematopoietic Stem and Progenitor Cells
 Highly Efficient CRISPR/Cas9 Gene Editing and Long-Term Engraftment of Human Hematopoietic Stem and Progenitor Cells J. M. Heath, A. Chalishazar, C.S. Lee, W. Selleck, C. Cotta-Ramusino, D. Bumcrot, J.L.
Highly Efficient CRISPR/Cas9 Gene Editing and Long-Term Engraftment of Human Hematopoietic Stem and Progenitor Cells J. M. Heath, A. Chalishazar, C.S. Lee, W. Selleck, C. Cotta-Ramusino, D. Bumcrot, J.L.
BNP mrna expression in DR and DS rat left ventricles (n = 5). (C) Plasma norepinephrine
 Kanazawa, et al. Supplementary figure legends Supplementary Figure 1 DS rats had congestive heart failure. (A) DR and DS rat hearts. (B) QRT-PCR analysis of BNP mrna expression in DR and DS rat left ventricles
Kanazawa, et al. Supplementary figure legends Supplementary Figure 1 DS rats had congestive heart failure. (A) DR and DS rat hearts. (B) QRT-PCR analysis of BNP mrna expression in DR and DS rat left ventricles
Domenico D Amario MD, PhD Università Cattolica del Sacro Cuore Rome:
 Domenico D Amario MD, PhD Università Cattolica del Sacro Cuore Rome: 31.01.2014 (Rosamund et al, Circulation 2007) Discharges in Thousands Hospital discharges for heart failure by sex (United States: 1979-2004).
Domenico D Amario MD, PhD Università Cattolica del Sacro Cuore Rome: 31.01.2014 (Rosamund et al, Circulation 2007) Discharges in Thousands Hospital discharges for heart failure by sex (United States: 1979-2004).
Imaging of Coronary Artery Disease: II
 Acta Radiológica Portuguesa, Vol.XIX, nº 74, pág. 45-51, Abr.-Jun., 2007 Imaging of Coronary Artery Disease: II Jean Jeudy University of Maryland School of Medicine Department of Diagnostic Radiology Armed
Acta Radiológica Portuguesa, Vol.XIX, nº 74, pág. 45-51, Abr.-Jun., 2007 Imaging of Coronary Artery Disease: II Jean Jeudy University of Maryland School of Medicine Department of Diagnostic Radiology Armed
Serum cytokine levels in control and tumor-bearing male and female mice at day 15.
 Supplementary Table 1. Serum cytokine levels in control and tumor-bearing male and female mice at day 15. Male Female Cytokine Control C-26 Control C-26 IL-1β 2.0 ± 0.8 9.6 ± 1.5* 1.8 ± 0.2 6.8 ± 1.4*
Supplementary Table 1. Serum cytokine levels in control and tumor-bearing male and female mice at day 15. Male Female Cytokine Control C-26 Control C-26 IL-1β 2.0 ± 0.8 9.6 ± 1.5* 1.8 ± 0.2 6.8 ± 1.4*
Cardiac Gene Therapy: Beyond the Mouse. David M Kaye Heart Failure Research Group Baker IDI, Melbourne, AUSTRALIA
 Cardiac Gene Therapy: Beyond the Mouse David M Kaye Heart Failure Research Group Baker IDI, Melbourne, AUSTRALIA Presenter Disclosure Information FINANCIAL DISCLOSURE: Equity: Osprey Medical Grants/Research
Cardiac Gene Therapy: Beyond the Mouse David M Kaye Heart Failure Research Group Baker IDI, Melbourne, AUSTRALIA Presenter Disclosure Information FINANCIAL DISCLOSURE: Equity: Osprey Medical Grants/Research
In the name of GOD. Animal models of cardiovascular diseases: myocardial infarction & hypertension
 In the name of GOD Animal models of cardiovascular diseases: myocardial infarction & hypertension 44 Presentation outline: Cardiovascular diseases Acute myocardial infarction Animal models for myocardial
In the name of GOD Animal models of cardiovascular diseases: myocardial infarction & hypertension 44 Presentation outline: Cardiovascular diseases Acute myocardial infarction Animal models for myocardial
Case Report Multimodality Imaging of Chronic Ischemia
 SAGE-Hindawi Access to Research Volume 2011, Article ID 739702, 4 pages doi:10.4061/2011/739702 Case Report Multimodality Imaging of Chronic Ischemia Kiyotake Ishikawa, Dennis Ladage, Kleopatra Rapti,
SAGE-Hindawi Access to Research Volume 2011, Article ID 739702, 4 pages doi:10.4061/2011/739702 Case Report Multimodality Imaging of Chronic Ischemia Kiyotake Ishikawa, Dennis Ladage, Kleopatra Rapti,
Journal Club Semmler Lorenz
 Beer et al. 2015 - Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration Journal Club 13.11.2017 1 Introduction to
Beer et al. 2015 - Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration Journal Club 13.11.2017 1 Introduction to
Dr. Alexander Lyon Senior Lecturer and Consultant Cardiologist Clinical Lead in Cardio-Oncology Royal Brompton Hospital, London UK
 Advanced heart failure - devices, mechanical circulatory support and cardiac transplantation Monday 30 January 2017 Stem cell and gene therapies for heart failure Dr. Alexander Lyon Senior Lecturer and
Advanced heart failure - devices, mechanical circulatory support and cardiac transplantation Monday 30 January 2017 Stem cell and gene therapies for heart failure Dr. Alexander Lyon Senior Lecturer and
Lentiviral vector carrying the murin Apelin precursor gene, 234-bp cdna, (Lenti-Apelin) was
 SUPPLEMENTAL METHODS Construction of recombinant lentiviral vectors Lentiviral vector carrying the murin Apelin precursor gene, 234-bp cdna, (Lenti-Apelin) was constructed. The cdna was inserted into the
SUPPLEMENTAL METHODS Construction of recombinant lentiviral vectors Lentiviral vector carrying the murin Apelin precursor gene, 234-bp cdna, (Lenti-Apelin) was constructed. The cdna was inserted into the
Pearson r = P (one-tailed) = n = 9
 8F4-Specific Lysis, % 1 UPN1 UPN3 8 UPN7 6 Pearson r =.69 UPN2 UPN5 P (one-tailed) =.192 4 UPN8 n = 9 2 UPN9 UPN4 UPN6 5 1 15 2 25 8 8F4, % Max MFI Supplementary Figure S1. AML samples UPN1-UPN9 show variable
8F4-Specific Lysis, % 1 UPN1 UPN3 8 UPN7 6 Pearson r =.69 UPN2 UPN5 P (one-tailed) =.192 4 UPN8 n = 9 2 UPN9 UPN4 UPN6 5 1 15 2 25 8 8F4, % Max MFI Supplementary Figure S1. AML samples UPN1-UPN9 show variable
Cardioprotection by endogenous fibroblast growth factor 2 in cardiac ischemia-reperfusion injury in vivo
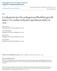 Washington University School of Medicine Digital Commons@Becker Conference Abstracts and Posters Division of Emergency Medicine/Emergency Care Research Section 2011 Cardioprotection by endogenous fibroblast
Washington University School of Medicine Digital Commons@Becker Conference Abstracts and Posters Division of Emergency Medicine/Emergency Care Research Section 2011 Cardioprotection by endogenous fibroblast
Tissue renewal and Repair. Nisamanee Charoenchon, PhD Department of Pathobiology, Faculty of Science
 Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Stem cells: units of development and regeneration. Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research.
 Stem cells: units of development and regeneration Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research Concepts 1. Embryonic vs. adult stem cells 2. Hematopoietic stem
Stem cells: units of development and regeneration Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research Concepts 1. Embryonic vs. adult stem cells 2. Hematopoietic stem
WIEF-AFF ROUNDTABLE Tokyo, Japan 26 May 2015
 WIEF-AFF ROUNDTABLE 2015 Tokyo, Japan 26 May 2015 Regenerative Medicine Goal: - To restore organ +/- tissue function - For pts with serious injuries or chronic disease where the body unable to heal & restore
WIEF-AFF ROUNDTABLE 2015 Tokyo, Japan 26 May 2015 Regenerative Medicine Goal: - To restore organ +/- tissue function - For pts with serious injuries or chronic disease where the body unable to heal & restore
