Chemistry Regents Review A. A B. B C. C D. D
|
|
|
- Claribel Anderson
- 6 years ago
- Views:
Transcription
1 hemistry Regents Review Name: ate: 1. Nitrogenous wastes result from the metabolism of 4. Which sugar solution was the first to liberate a measurable volume of O 2?. amino acids. glucose molecules. fatty acids. water molecules 2. Glucose and maltose are classified as organic compounds because they are both. carbon-containing substances. composed of simple elements. waste products. artificial sugars Which substances are most commonly used as building blocks in the synthesis of some lipids?. sugars and starches. amino acids and nucleotides. starches and enzymes 5. Which process is most directly responsible for the production of O 2 in these sugar solutions?. respiration. transpiration. translocation. photosynthesis. glycerol and fatty acids page 1
2 6. Which element is present in living cells and in all organic compounds? 10. Which process requires TP molecules as a source of energy?. potassium. sulfur. nitrogen. carbon. active transport. diffusion. osmosis. passive transport 7. Which gas became more abundant in the Earth s primitive atmosphere as a result of long periods of fermentation by anaerobic organisms?. hydrogen. carbon dioxide. ammonia. methane 11. Which term best describes a solution with a ph of 5?. acidic. neutral. basic. colorless 8. Which is an example of an inorganic compound? 12. Which is an example of an organic substance?. glucose. maltase. water. starch. 6 H 12 O 6. NH 3. H 2 O. O 2 9. biochemical compound that readily combines with oxygen and distributes it throughout the human body is 13. The process in which the net movement of molecules is from a region of lower concentration to a region of higher concentration is known as. urea. water. acetylcholine. hemoglobin. active transport. diffusion. osmosis. passive transport page 2 hemistry Regents Review
3 14. Which structure produces a substance that aids in the mechanical breakdown of fats? 17. The diagram shows some of the events that take place in a plant cell. Use the diagram to answer the following question(s).. liver. thyroid gland. testis. pituitary gland 15. Which elements are present in all organic compounds?. hydrogen and oxygen. nitrogen and oxygen. nitrogen and carbon. hydrogen and carbon The letters X, Y, and Z most likely represent. N 2, 0 2, and H 2 O. O 2, light, and H 2 O. light, ammonia, and H 2 O. light, O 2, and methane 16. oth human insulin and insulin extracted from sheep lower blood sugar levels in humans. This fact is used as supporting evidence for evolution based on comparative. anatomy. embryology. biochemistry. cytology page 3 hemistry Regents Review
4 18. The diagram shows an investigation performed over a period of 6 hours. The gas bubbles produced in this investigation most likely contained molecules of 20. In the diagram, which substance belongs in area Z?. water. oxygen. nitrogen. carbon. oxygen. sulfur dioxide. uric acid. ammonia 21. This consists of chains of nucleotides.. N molecules, only. RN molecules, only. oth N and RN molecules 19. Which substances synthesized by the body enable humans to go for a long period of time without eating?. Neither N nor RN molecules. proteins and mucus. enzymes and hormones. vitamins and lipids. fats and glycogen 22. fter a cookie has been digested, glucose molecules enter the bloodstream by the process of. phagocytosis. diffusion. ingestion. pinocytosis page 4 hemistry Regents Review
5 23. N is a polymer consisting of repeating units known as. dipeptides. nucleotides. amino acids. organic salts 27. The distortion (change in shape) of enzyme molecules which occurs at high temperatures is known as. synthesis. specificity. replication. denaturation 24. Which term is defined as all the chemical reactions that are required to sustain life?. metabolism. regulation. nutrition. synthesis 28. Enzymes for both photochemical and carbonfixation reactions are found within organelles known as. chloroplasts. Golgi complexes. lysosomes. ribosomes 25. The raw materials used by green plants for photosynthesis are. oxygen and water. oxygen and glucose. carbon dioxide and water 29. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin.. carbon dioxide and glucose 26. Enzymes are similar to antibodies in that both. are produced by neurohormones. slow the rate of chemical reactions. are highly specific in their action Urease is most effective at a ph of are involved in hydrolysis reactions page 5 hemistry Regents Review
6 30. Which conclusion can be drawn concerning the enzymes whose activity is represented in the graph?. t a ph of 2.5, the rate of activity of urease is decreasing. 32. Which substances may function as coenzymes?. vitamins. fats. carbohydrates. proteins. t a ph of 4.0, the rate of activity of pepsin is the same as that of urease.. t a ph of 12.0, the rate of activity of trypsin is increasing.. t a ph of 7.5, the rate of activity of urease is the same as that of trypsin. 33. lcohol fermentation and aerobic respiration are similar in that both processes. utilize light. produce ethyl alcohol. require free oxygen. release carbon dioxide 31. In a living system, the structure of an enzyme is determined by the sequence of. phosphate groups in RN. ribose molecules in RN. nitrogen bases in N. deoxyribose molecules in N 34. In the respiratory pathway of some organisms, when oxygen is unavailable, pyruvic acid is converted to. peptide chains. hydrolytic enzymes. lactic acid. nucleic acid 35. This equation represents a process that occurs in both plants and animals. glucose + oxygen enzymes water + carbondioxide + 36 TP In animal cells, much of the carbon dioxide produced is. used for energy. converted to sugar. excreted as waste. stored in vacuoles page 6 hemistry Regents Review
7 36. uring the process of aerobic respiration, energy stored in food is transferred to molecules of 38. Organisms release stored chemical energy from nutrients by the process of. TP. N. glucose. enzymes. assimilation. transportation. respiration. ingestion 39. compound that is synthesized by both humans and geranium plants is 37. Which condition would most directly result in the production of lactic acid by some cells of the human body?. cellulose. TP. ethyl alcohol. chlorophyll. an excess of nitrogen in the atmosphere. an insufficient amount of nitrogen in the atmosphere. an excess of oxygen reaching the cells. an insufficient amount of oxygen reaching the cells 40. Fatigue in muscle tissue is chiefly associated with the production of. carbon dioxide. urea. lactic acid. glycerol 41. Which substance is represented by X in the word equation shown? glucose + X enzymes water + carbon dioxide + TP. alcohol. chlorophyll. oxygen. lactic acid page 7 hemistry Regents Review
8 42. One factor that contributes to the fatigue of a long-distance runner is the accumulation of lactic acid molecules in muscle cells. This lactic acid s produced most directly as a result of. digestion. aerobic respiration. photosynthesis 44. Within which structures does the aerobic phase of aerobic respiration take place?. grana. Golgi bodies. endoplasmic reticula. mitochondria. anaerobic respiration 43. Two species of bacteria produce different respiratory end products. Species always produces TP, O 2, and H 2 O; species always produces TP, ethyl alcohol, and O 2. Which conclusion can correctly be drawn from this information?. Only species is aerobic.. Only species is aerobic. 45. Small molecules combine chemically and form large, complex molecules by a process known as. hydrolysis. digestion. synthesis. nutrition. Species and species are both anaerobic.. Species and species are both aerobic. page 8 hemistry Regents Review
9 46. The structural formulas shown represent certain organic compounds found in living cells. (1) (2) (3) The hydrolysis of a protein would produce molecules represented by (4) (5) page 9 hemistry Regents Review
10 47. Which pair of nucleotides can be held together by weak hydrogen bonds? 48. The diagram illustrates some of the steps in the synthesis of proteins. Which structure is indicated by letter?. 1 and 3. 2 and 3. 3 and 4. 4 and 2. ribosome. mitochondrion. lysosome. Golgi body 49. The diagram illustrates some of the steps in the synthesis of proteins. Structural components of molecule include. ribose, phosphate, adenine, and uracil. ribose, thymine, adenine, and guanine. deoxyribose, ribose, phosphate, and thymine. deoxyribose, uracil, phosphate, and cytosine 50. Which base is normally used in the synthesis of RN but not in the synthesis of N?. adenine. uracil. cytosine. guanine page 10 hemistry Regents Review
11 51. Vegetable oils, such as corn oil, belong to which general class of organic substances?. lipids. proteins. carbohydrates. salts 54. Select the nutrient, chosen from the list below, that is best described by the statement shown. This nutrient can serve as an energy source or provide nondigestible materials that increase the amount of roughage in the human diet.. Lipids. arbohydrates. Proteins. Vitamins 52. The molecular formula for a particular compound is 12 H 22 O 11. This compound is classified as a. carbohydrate. lipid. protein. nucleic acid 55. Which organic molecule is correctly paired with an end product of its digestion?. nucleic acid glycerol. carbohydrate fatty acid. protein amino acid. lipid nucleotide 53. Not all human cells are capable of synthesizing all the compounds required for life. Those compounds that can not be synthesized must be ingested. These ingested compounds include. mutagenic agents. neurotransmitters. essential amino acids. digestive enzymes 56. The term that represents all the chemical activities that occur in an organism is. synthesis. regulation. metabolism. homeostasis page 11 hemistry Regents Review
12 57. Which life function provides substances that may be used by an organism for its growth and for the repair of its tissues?. excretion. reproduction. nutrition. regulation 59. When a person s level of physical activity changes, the circulatory system supplies body cells with amounts of nutrients and oxygen that are appropriate to sustain the new level of activity. This statement best illustrates the concept of. homeostasis. pinocytosis. synthesis. cyclosis 58. For survival, a hummingbird uses a considerable amount of energy. The energy most directly results from the life activity of. transport. excretion. regulation. respiration 60. Normally, when the concentration of glucose in the blood falls below a certain level, stored glucose reenters the blood until the original concentration is reached again. This regulation of the concentration of blood glucose is part of the process known as. synthesis. respiration. pinocytosis. homeostasis page 12 hemistry Regents Review
13 Problem-ttic format version c Educide Software Licensed for use by rooke Kneller Terms of Use at hemistry Regents Review 05/17/
14 Teacher s Key Page
D. glycerol and fatty acids 4. Which is an example of an inorganic compound?
 Name: ate: 1. Glucose and maltose are classified as organic compounds because they are both 3. Which process is most directly responsible for the production of O 2 in these sugar solutions?. carbon-containing
Name: ate: 1. Glucose and maltose are classified as organic compounds because they are both 3. Which process is most directly responsible for the production of O 2 in these sugar solutions?. carbon-containing
Review for Regular Test - H2O, ph, Macromolecules, Enzymes, ATP, Photo, CR A. A B. F C. C D. D
 Macromolecules, Enzymes, TP, Photo, R Name: ate: 1. The accompanying diagram represents some chemical events that take place in one type of autotrophic nutrition. 3. The dark reactions in the stroma are
Macromolecules, Enzymes, TP, Photo, R Name: ate: 1. The accompanying diagram represents some chemical events that take place in one type of autotrophic nutrition. 3. The dark reactions in the stroma are
Properties of Water. 1. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin.
 Name: ate: 1. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin. 1. Which of these enzymes function in the most similar ph range?.
Name: ate: 1. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin. 1. Which of these enzymes function in the most similar ph range?.
Organic Molecules. 1. The structural formulas shown represent certain organic compounds found in living cells.
 Name: ate: 1. The structural formulas shown represent certain organic compounds found in living cells. 1. (1) () (3) Which formula represents a monosaccharide? (4) (5). 1.. 3. 5. Which formula represents
Name: ate: 1. The structural formulas shown represent certain organic compounds found in living cells. 1. (1) () (3) Which formula represents a monosaccharide? (4) (5). 1.. 3. 5. Which formula represents
The building blocks for this molecule are A) amino acids B) simple sugars C) fats D) molecular bases
 1. Base your answer to the following question on the diagram below and on your knowledge of biology. The diagram represents a portion of a starch molecule. The building blocks for this molecule are A)
1. Base your answer to the following question on the diagram below and on your knowledge of biology. The diagram represents a portion of a starch molecule. The building blocks for this molecule are A)
Do Now Makeups. 4. In which organelle would water and dissolved materials be stored? A. 1 B. 2 C. 3 D. 5. A. mitochondria B.
 Do Now Makeups Name: Date: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell? A. mitochondria B. centrosomes
Do Now Makeups Name: Date: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell? A. mitochondria B. centrosomes
Name: Date: Block: Biology 12
 Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
1. What substance could be represented by the letter X in the diagram below?
 1. What substance could be represented by the letter X in the diagram below? A) carbohydrates B) ozone C) carbon dioxide D) water 2. Base your answer to the following question on the diagram below. For
1. What substance could be represented by the letter X in the diagram below? A) carbohydrates B) ozone C) carbon dioxide D) water 2. Base your answer to the following question on the diagram below. For
Biochemistry Name: Practice Questions
 Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
5. Groups A and B in the table below contain molecular formulas of compounds.
 1. Which group consists entirely of organic molecules? A) protein, oxygen, fat B) protein, starch, fat C) water, carbon dioxide, oxygen D) water, starch, protein 2. Which statement describes starches,
1. Which group consists entirely of organic molecules? A) protein, oxygen, fat B) protein, starch, fat C) water, carbon dioxide, oxygen D) water, starch, protein 2. Which statement describes starches,
C) amount of carbon dioxide absorbed by the animal B) rate of respiration of the animal
 Name: 1) A model of a section of a cell membrane is represented below. 4034-1 - Page 1 Which type of molecule is indicated by the arrow? A) carbohydrate B) protein C) lipid D) nucleotide 2) The movement
Name: 1) A model of a section of a cell membrane is represented below. 4034-1 - Page 1 Which type of molecule is indicated by the arrow? A) carbohydrate B) protein C) lipid D) nucleotide 2) The movement
Unit 2 - Characteristics of Living Things
 Living Environment Answer Key to Practice Exam- Parts A and B-1 1. A fully functioning enzyme molecule is arranged in a complex three-dimensional shape. This shape determines the A) specific type of molecule
Living Environment Answer Key to Practice Exam- Parts A and B-1 1. A fully functioning enzyme molecule is arranged in a complex three-dimensional shape. This shape determines the A) specific type of molecule
10. The diagram below shows two different kinds of substances, A and B, entering a cell.
 1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and species
1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and species
Macromolecule Practice Test
 Name: ate: 1. ll living things contain which element?. helium. sodium. copper. carbon 4. Which of the following compounds is most likely to be part of living organisms?. 6 H 12 O 6. F 3. Mol 2. si 2. Plants
Name: ate: 1. ll living things contain which element?. helium. sodium. copper. carbon 4. Which of the following compounds is most likely to be part of living organisms?. 6 H 12 O 6. F 3. Mol 2. si 2. Plants
Chemical Formulas. Chemical Formula CH 3 COCHCHOCHClCHNH Lewis Dot Structure
 Biochemistry . Chemical Formulas A chemical formula represents the chemical makeup of a compound. It shows the numbers and kinds of atoms present in a compound. It is a kind of shorthand that scientists
Biochemistry . Chemical Formulas A chemical formula represents the chemical makeup of a compound. It shows the numbers and kinds of atoms present in a compound. It is a kind of shorthand that scientists
Living Environment. Scientific Inquiry Exam
 Name: Class: 1. Which elements are present in all organic compounds? 1) nitrogen and carbon 3) hydrogen and oxygen 2) nitrogen and oxygen 4) hydrogen and carbon 2. Which substances are inorganic compounds?
Name: Class: 1. Which elements are present in all organic compounds? 1) nitrogen and carbon 3) hydrogen and oxygen 2) nitrogen and oxygen 4) hydrogen and carbon 2. Which substances are inorganic compounds?
Biology I Honors EOC Exam Review: metabolism
 Biology I Honors EOC Exam Review: metabolism 1. One type of anaerobic respiration results in the production of a. water and oxygen c. nitrogen gas and ammonia b. pyruvic acid and glycerol d. alcohol and
Biology I Honors EOC Exam Review: metabolism 1. One type of anaerobic respiration results in the production of a. water and oxygen c. nitrogen gas and ammonia b. pyruvic acid and glycerol d. alcohol and
Cell Organelles, Communication and Transport. A. A gametes; B zygote. B. A zygote; B gametes. C. A organs; B organelles. D. A organelles; B organs
 ell Organelles, ommunication and Transport Name: ate: 1. The diagram below represents a cell of a green plant. 3. Some levels of organization in a multicellular organism are shown in the sequence below.
ell Organelles, ommunication and Transport Name: ate: 1. The diagram below represents a cell of a green plant. 3. Some levels of organization in a multicellular organism are shown in the sequence below.
9. At about 0 C., most enzymes are (1.) inactive (2.) active (3.) destroyed (4.) replicated
 Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
1. Structure A is the a. Cell wall b. Cell membrane c. Vacuole d. Lysosome
 Figure 1 Use Figure 1 to answer the following questions: 1. Structure A is the a. Cell wall b. Cell membrane c. Vacuole d. Lysosome 2. Structure E controls cellular functions. It is the a. Nucleolus b.
Figure 1 Use Figure 1 to answer the following questions: 1. Structure A is the a. Cell wall b. Cell membrane c. Vacuole d. Lysosome 2. Structure E controls cellular functions. It is the a. Nucleolus b.
Mid Term Review. 1. step 1, only 3. both step 1 and step 2 2. step 2, only 4. neither step 1 nor step 2
 Name Mid Term Review 1. Diagrams, tables, and graphs are used by scientists mainly to 1. design a research plan for an experiment 3. organize data 2. test a hypothesis 4. predict the independent variable
Name Mid Term Review 1. Diagrams, tables, and graphs are used by scientists mainly to 1. design a research plan for an experiment 3. organize data 2. test a hypothesis 4. predict the independent variable
Macromolcules, Enzymes, & Cells Intro
 Name: Date: 1. The distortion (change in shape) of enzyme molecules which occurs at high temperatures is known as 5. A characteristic shared by all enzymes, hormones, and antibodies is that their function
Name: Date: 1. The distortion (change in shape) of enzyme molecules which occurs at high temperatures is known as 5. A characteristic shared by all enzymes, hormones, and antibodies is that their function
Renaissance Biology Midterm Study Guide Answers
 Renaissance Biology Midterm Study Guide Answers 2016-2017 LEARNING TARGET 1: List the characteristics of life Made of one or more cells Organization cells -> tissues -> organs -> organ systems -> organisms
Renaissance Biology Midterm Study Guide Answers 2016-2017 LEARNING TARGET 1: List the characteristics of life Made of one or more cells Organization cells -> tissues -> organs -> organ systems -> organisms
Ms. Golub & Ms. Sahar Date: Unit 2- Test #1
 Name Ms. Golub & Ms. Sahar Date: Unit 2- Test #1 1. The interaction between guard cells and a leaf opening would not be involved in A) diffusion of carbon dioxide B) maintaining homeostasis C) heterotrophic
Name Ms. Golub & Ms. Sahar Date: Unit 2- Test #1 1. The interaction between guard cells and a leaf opening would not be involved in A) diffusion of carbon dioxide B) maintaining homeostasis C) heterotrophic
Name # Class Regents Review: Characteristics of Life and Biochemistry
 Name # Class Regents Review: Characteristics of Life and Biochemistry 6. Some processes that occur in a cell are listed below. A. utilize energy B. detect changes in the environment C. rearrange and synthesize
Name # Class Regents Review: Characteristics of Life and Biochemistry 6. Some processes that occur in a cell are listed below. A. utilize energy B. detect changes in the environment C. rearrange and synthesize
Cell Processes Review
 1. Most green algae are able to obtain carbon dioxide from the environment and use it to synthesize organic compounds. This activity is an example of 1) hydrolysis 2) saprophytism 3) cellular respiration
1. Most green algae are able to obtain carbon dioxide from the environment and use it to synthesize organic compounds. This activity is an example of 1) hydrolysis 2) saprophytism 3) cellular respiration
1. Arrows A, B, and C in the diagram below represent the processes necessary to make the energy stored in food available for muscle activity.
 1. Arrows A, B, and C in the diagram below represent the processes necessary to make the energy stored in food available for muscle activity. The correct sequence of processes represented by A, B, and
1. Arrows A, B, and C in the diagram below represent the processes necessary to make the energy stored in food available for muscle activity. The correct sequence of processes represented by A, B, and
Copyright 2014 Edmentum - All rights reserved.
 Study Island Copyright 2014 Edmentum - All rights reserved. Generation Date: 04/01/2014 Generated By: Cheryl Shelton Title: Science- biology Cells 1. Below is an image of a plant cell. What processes require
Study Island Copyright 2014 Edmentum - All rights reserved. Generation Date: 04/01/2014 Generated By: Cheryl Shelton Title: Science- biology Cells 1. Below is an image of a plant cell. What processes require
Biological Molecules
 Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
Chapter 1-2 Review Assignment
 Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
Molecules of Life. Carbohydrates Lipids Proteins Nucleic Acids
 Molecules of Life Carbohydrates Lipids Proteins Nucleic Acids Molecules of Life All living things are composed of the following basic elements: Carbon Hydrogen Oxygen Nitrogen Phosphorous Sulfur Remember
Molecules of Life Carbohydrates Lipids Proteins Nucleic Acids Molecules of Life All living things are composed of the following basic elements: Carbon Hydrogen Oxygen Nitrogen Phosphorous Sulfur Remember
Chapter 2 Part 3: Organic and Inorganic Compounds
 Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Cellular Respiration. Release of Energy From Food (glucose)!
 Cellular Respiration Release of Energy From Food (glucose)! Energy needs of life Animals are energy consumers What do we need energy for? synthesis (building for growth) reproduction active transport movement
Cellular Respiration Release of Energy From Food (glucose)! Energy needs of life Animals are energy consumers What do we need energy for? synthesis (building for growth) reproduction active transport movement
An example of a carbohydrate A) 1 B) 2 C) 3 D) 4
 1. Which chemical formula represents a carbohydrate? A) CH4 B) C3H7O2N C) Cl2H22O11 D) CO2 2. Base your answer to the following question on the diagram below. For each of the following phrases, select
1. Which chemical formula represents a carbohydrate? A) CH4 B) C3H7O2N C) Cl2H22O11 D) CO2 2. Base your answer to the following question on the diagram below. For each of the following phrases, select
Standard B-3: The student will demonstrate an understanding of the flow of energy within and between living systems.
 B-3.1 Summarize the overall process by which photosynthesis converts solar energy into chemical energy and interpret the chemical equation for the process. Taxonomy Level: 2.4-B and 2.1-B Understand Conceptual
B-3.1 Summarize the overall process by which photosynthesis converts solar energy into chemical energy and interpret the chemical equation for the process. Taxonomy Level: 2.4-B and 2.1-B Understand Conceptual
Topic 1: Chemistry of Living Things
 1. Some processes that occur in a cell are listed below.1 utilize energy 2 detect changes in the environment 3 rearrange and synthesize chemical compounds 4. The diagram below represents a sequence of
1. Some processes that occur in a cell are listed below.1 utilize energy 2 detect changes in the environment 3 rearrange and synthesize chemical compounds 4. The diagram below represents a sequence of
Standard 2 Exam Biology. 2. This macromolecule is responsible for short term energy storage and structural support in plants
 1. This macromolecule is responsible for structural support, movement, enzymatic activity, cell communication, and is made of amino acids. a. Lipids b. Carbohydrates c. Proteins d. Nucleic Acids e. ATP
1. This macromolecule is responsible for structural support, movement, enzymatic activity, cell communication, and is made of amino acids. a. Lipids b. Carbohydrates c. Proteins d. Nucleic Acids e. ATP
BIOCHEMISTRY. There are 4 major types of organic compounds each with unique characteristics: A. CARBOHYDRATES Contain,, and. Ratio of H:O is always
 BIOCHEMISTRY All organic compounds must contain and Are the following organic? Why or why not? H2O CO2 CH4 There are 4 major types of organic compounds each with unique characteristics: A. CARBOHYDRATES
BIOCHEMISTRY All organic compounds must contain and Are the following organic? Why or why not? H2O CO2 CH4 There are 4 major types of organic compounds each with unique characteristics: A. CARBOHYDRATES
6. The diagram below represents an interaction between parts of an organism.
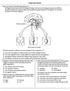 Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Most life processes are a series of chemical reactions influenced by environmental and genetic factors.
 Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
Activity: Biologically Important Molecules
 Activity: Biologically Important Molecules AP Biology Introduction We have already seen in our study of biochemistry that the molecules that comprise living things are carbon-based, and that they are thought
Activity: Biologically Important Molecules AP Biology Introduction We have already seen in our study of biochemistry that the molecules that comprise living things are carbon-based, and that they are thought
Organic Molecules. 8/27/2004 Mr. Davenport 1
 Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Biochemistry Regents Practice
 iochemistry Regents Practice Name: Date: 1. The diagram below represents a sequence of events that occurs in living things. 3. Which statement describes a similarity between all enzymes, antibodies, and
iochemistry Regents Practice Name: Date: 1. The diagram below represents a sequence of events that occurs in living things. 3. Which statement describes a similarity between all enzymes, antibodies, and
Hillcrest High School 2010 Living Environment Regents Review Lesson 5 Life Functions Day 2
 Hillcrest High School 2010 Living Environment Regents Review Lesson 5 Life Functions Day 2 1. Which set of terms best identifies the letters in the diagram below? (1) 1 (2) 2 (3) 3 (4) 4 2. Which process
Hillcrest High School 2010 Living Environment Regents Review Lesson 5 Life Functions Day 2 1. Which set of terms best identifies the letters in the diagram below? (1) 1 (2) 2 (3) 3 (4) 4 2. Which process
Biological Molecules. Carbohydrates, Proteins, Lipids, and Nucleic Acids
 Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
The Building blocks of life. Macromolecules
 The Building blocks of life Macromolecules 1 copyright cmassengale 2 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 3 LIFE ON EARTH IS CARBON-BASED
The Building blocks of life Macromolecules 1 copyright cmassengale 2 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 3 LIFE ON EARTH IS CARBON-BASED
Unit 2 Warm Ups. Equilibrium
 Unit 2 Warm Ups Equilibrium 1. Cell wall 2. Mitochondria 3. Chloroplast 4. Vesicle 5. Vacuole 6. Rough Endoplasmic Reticulum 7. Smooth Endoplasmic Reticulum 8. Cytoskeleton 9. Lysosomes 10.Cell Membrane
Unit 2 Warm Ups Equilibrium 1. Cell wall 2. Mitochondria 3. Chloroplast 4. Vesicle 5. Vacuole 6. Rough Endoplasmic Reticulum 7. Smooth Endoplasmic Reticulum 8. Cytoskeleton 9. Lysosomes 10.Cell Membrane
1. Which of the following structures is not found in bacteria?
 Untitled Document EOC Macromolecules 1. Which of the following structures is not found in bacteria? A. ribosome B. cytoplasm C. cell membrane D. nuclear membrane 4. Plants and animals are composed of organic
Untitled Document EOC Macromolecules 1. Which of the following structures is not found in bacteria? A. ribosome B. cytoplasm C. cell membrane D. nuclear membrane 4. Plants and animals are composed of organic
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
The Atoms of Life. What are other elements would you expect to be on this list? Carbon Hydrogen Nitrogen Oxygen Phosphorous Sulfur (sometimes)
 Macromolecules The Atoms of Life The most frequently found atoms in the body are Carbon Hydrogen Nitrogen Oxygen Phosphorous Sulfur (sometimes) What are other elements would you expect to be on this list?
Macromolecules The Atoms of Life The most frequently found atoms in the body are Carbon Hydrogen Nitrogen Oxygen Phosphorous Sulfur (sometimes) What are other elements would you expect to be on this list?
Essential Components of Food
 Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
***Non-living things may show one or more of these Characteristics, but NEVER ALL of them
 -Living things are highly ORGANIZED -Living things are made up of one or more CELLS -Living things use ENERGY -Living things GROW and develop -Living things RESPOND to changes in the environment -Living
-Living things are highly ORGANIZED -Living things are made up of one or more CELLS -Living things use ENERGY -Living things GROW and develop -Living things RESPOND to changes in the environment -Living
Cellular Transport. 1. A potato core was placed in a beaker of water as shown in the figure below.
 Name: Date: 1. potato core was placed in a beaker of water as shown in the figure below. Which diagram best represents the net movement of molecules?.. C. D. page 1 2. The following question(s) is/are
Name: Date: 1. potato core was placed in a beaker of water as shown in the figure below. Which diagram best represents the net movement of molecules?.. C. D. page 1 2. The following question(s) is/are
Org Biochem Final Test Student Section Ch Samples Page 1 of 5
 Ch 31-35 Samples Page 1 of 5 13. Which of the following is a purine? a) guanine b) cytosine c) thymine d) uracil 20. The three components of a nucleotide are a) glucose, a phosphate group, and choline.
Ch 31-35 Samples Page 1 of 5 13. Which of the following is a purine? a) guanine b) cytosine c) thymine d) uracil 20. The three components of a nucleotide are a) glucose, a phosphate group, and choline.
9.A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids
 9.A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids o o o Food is a good source of one or more of the following: protein,
9.A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids o o o Food is a good source of one or more of the following: protein,
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
A. There are about 100 elements; 25 of them are necessary for life. B. Carbon atoms can form long chains, leading to a huge number of possible
 Ch. 2 How Cells Function 2.1 Chemical reactions take place inside cells. 1. All cells are made of the same elements. A. There are about 100 elements; 25 of them are necessary for life. B. The smallest
Ch. 2 How Cells Function 2.1 Chemical reactions take place inside cells. 1. All cells are made of the same elements. A. There are about 100 elements; 25 of them are necessary for life. B. The smallest
Sample Questions BSC1010C Chapters 5-7
 Sample Questions BSC1010C Chapters 5-7 1. Which type of lipid is most important in biological membranes? a. oils b. fats c. wax d. phospholipids e. triglycerides 2. Which type of interaction stabilizes
Sample Questions BSC1010C Chapters 5-7 1. Which type of lipid is most important in biological membranes? a. oils b. fats c. wax d. phospholipids e. triglycerides 2. Which type of interaction stabilizes
Biology Teach Yourself Series Topic 3: Chemical Nature of the Cell
 Biology Teach Yourself Series Topic 3: Chemical Nature of the Cell A: Level 14, 474 Flinders Street Melbourne VIC 3000 T: 1300 134 518 W: tssm.com.au E: info@tssm.com.au TSSM 2013 Page 1 of 19 Contents
Biology Teach Yourself Series Topic 3: Chemical Nature of the Cell A: Level 14, 474 Flinders Street Melbourne VIC 3000 T: 1300 134 518 W: tssm.com.au E: info@tssm.com.au TSSM 2013 Page 1 of 19 Contents
The Cell and Its Chemical Compounds
 Cell Theory Cell - The basic unit of structure and function in living things. All of an organism s process or functions are carried out in the cell. Robert Hooke - One of the first people to observe cells
Cell Theory Cell - The basic unit of structure and function in living things. All of an organism s process or functions are carried out in the cell. Robert Hooke - One of the first people to observe cells
4. The diagram below represents a cell structure involved in converting energy stored in organic molecules into a form used by animal cells.
 1. All of the following are true regarding cells except? 1) All cells have genetic material 2) All cells have cell walls 3) All cells have plasma membranes 4) All cells can divide to form new cells 2.
1. All of the following are true regarding cells except? 1) All cells have genetic material 2) All cells have cell walls 3) All cells have plasma membranes 4) All cells can divide to form new cells 2.
Organic Molecules Worksheet: Read through each section and answer the following questions.
 Name: Date: Period: Organic Molecules Worksheet: Read through each section and answer the following questions. Organic molecules are the molecules that exist in all living things. They are life s building
Name: Date: Period: Organic Molecules Worksheet: Read through each section and answer the following questions. Organic molecules are the molecules that exist in all living things. They are life s building
Macromolecules. copyright cmassengale
 Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
To be able to answer a question
 1. State Problem or question 2. Gather information 3. State a hypothesis 4. Conduct Experiment 5. Observe, collect, & analyze data 6. State a conclusion7. Repeat many times Observation =Recognizing a FACT
1. State Problem or question 2. Gather information 3. State a hypothesis 4. Conduct Experiment 5. Observe, collect, & analyze data 6. State a conclusion7. Repeat many times Observation =Recognizing a FACT
6/15/2015. Biological Molecules. Outline. Organic Compounds. Organic Compounds - definition Functional Groups Biological Molecules. What is organic?
 Biological Molecules Biology 105 Lecture 3 Reading: Chapter 2 (pages 29 39) Outline Organic Compounds - definition Functional Groups Biological Molecules Carbohydrates Lipids Amino Acids and Proteins Nucleotides
Biological Molecules Biology 105 Lecture 3 Reading: Chapter 2 (pages 29 39) Outline Organic Compounds - definition Functional Groups Biological Molecules Carbohydrates Lipids Amino Acids and Proteins Nucleotides
Biology Midterm Review Date
 Name Period Biology Midterm Review Date 1. One characteristic of all living things is that they A) develop organ systems B) produce identical offspring C) maintain internal stability D) synthesize only
Name Period Biology Midterm Review Date 1. One characteristic of all living things is that they A) develop organ systems B) produce identical offspring C) maintain internal stability D) synthesize only
Organic Compounds. Compounds that contain CARBON are called organic. Macromolecules are large organic molecules.
 Macromolecules Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Macromolecules Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Unit 1: Biochemistry
 Name: Date: Carbohydrates, lipids, proteins, and enzymes 1. All living things contain which element? A. helium B. sodium C. copper D. carbon 4. Which of the following elements is best able to combine with
Name: Date: Carbohydrates, lipids, proteins, and enzymes 1. All living things contain which element? A. helium B. sodium C. copper D. carbon 4. Which of the following elements is best able to combine with
Chapter 2. Chemical Composition of the Body
 Chapter 2 Chemical Composition of the Body Carbohydrates Organic molecules that contain carbon, hydrogen and oxygen General formula C n H 2n O n -ose denotes a sugar molecule Supply energy Glucose Complex
Chapter 2 Chemical Composition of the Body Carbohydrates Organic molecules that contain carbon, hydrogen and oxygen General formula C n H 2n O n -ose denotes a sugar molecule Supply energy Glucose Complex
Life Science Unit I. 4. Use the diagram to answer the question. 1. Eukaryotic cells are differentiated from prokaryotic cells because eukaryotic cells
 Name: ate: 1. Eukaryotic cells are differentiated from prokaryotic cells because eukaryotic cells. are much smaller. 4. Use the diagram to answer the question. ell iagram. have permeable membranes.. have
Name: ate: 1. Eukaryotic cells are differentiated from prokaryotic cells because eukaryotic cells. are much smaller. 4. Use the diagram to answer the question. ell iagram. have permeable membranes.. have
Chapter 3- Organic Molecules
 Chapter 3- Organic Molecules CHNOPS Six of the most abundant elements of life (make up 95% of the weight of all living things)! What are they used for? Structures, enzymes, energy, hormones, DNA How do
Chapter 3- Organic Molecules CHNOPS Six of the most abundant elements of life (make up 95% of the weight of all living things)! What are they used for? Structures, enzymes, energy, hormones, DNA How do
Dalkeith High School Higher Human Biology Homework 3
 Dalkeith High School Higher Human Biology Homework 3 1. During which of the following chemical conversions is A T P produced? A B C Amino acids protein Glucose pyruvic acid Haemoglobin oxyhaemoglobin energy
Dalkeith High School Higher Human Biology Homework 3 1. During which of the following chemical conversions is A T P produced? A B C Amino acids protein Glucose pyruvic acid Haemoglobin oxyhaemoglobin energy
Macromolecules. Honors Biology
 Macromolecules onors Biology 1 The building materials of the body are known as macromolecules because they can be very large There are four types of macromolecules: 1. Proteins 2. Nucleic acids 3. arbohydrates
Macromolecules onors Biology 1 The building materials of the body are known as macromolecules because they can be very large There are four types of macromolecules: 1. Proteins 2. Nucleic acids 3. arbohydrates
Large Biological Molecules Multiple Choice Review
 New Jersey enter for Teaching and Learning Slide 1 / 43 Progressive Science Initiative This material is made freely available at www.njctl.org and is intended for the non-commercial use of students and
New Jersey enter for Teaching and Learning Slide 1 / 43 Progressive Science Initiative This material is made freely available at www.njctl.org and is intended for the non-commercial use of students and
All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds:
 Biochemistry Organic Chemistry All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds: Do not contain carbon Organic
Biochemistry Organic Chemistry All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds: Do not contain carbon Organic
The Chemical Building Blocks of Life. Chapter 3
 The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
9.1 Chemical Pathways ATP
 9.1 Chemical Pathways ATP 2009-2010 Objectives Explain cellular respiration. Describe what happens during glycolysis. Describe what happens during fermentation. Where do we get energy? Energy is stored
9.1 Chemical Pathways ATP 2009-2010 Objectives Explain cellular respiration. Describe what happens during glycolysis. Describe what happens during fermentation. Where do we get energy? Energy is stored
Biological Molecules
 The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
The Structure and Function of Biomolecules
 The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
Organic Chemistry. Organic chemistry is the chemistry of carbon compounds. Biochemistry is the study of carbon compounds that crawl.
 Organic Chemistry Organic chemistry is the chemistry of carbon compounds. Biochemistry is the study of carbon compounds that crawl. Organic Compounds - have carbon bonded to other atoms and determine structure/function
Organic Chemistry Organic chemistry is the chemistry of carbon compounds. Biochemistry is the study of carbon compounds that crawl. Organic Compounds - have carbon bonded to other atoms and determine structure/function
General Biology 1004 Chapter 3 Lecture Handout, Summer 2005 Dr. Frisby
 Slide 1 CHAPTER 3 The Molecules of Life PowerPoint Lecture Slides for Essential Biology, Second Edition & Essential Biology with Physiology Presentation prepared by Chris C. Romero Copyright 2004 Pearson
Slide 1 CHAPTER 3 The Molecules of Life PowerPoint Lecture Slides for Essential Biology, Second Edition & Essential Biology with Physiology Presentation prepared by Chris C. Romero Copyright 2004 Pearson
MY BIOLOGY FINAL EXAM WORKBOOK
 NAME PER DATE MY BIOLOGY FINAL EXAM WORKBOOK DIRECTIONS: This study work book is due on the day of your final exam. Start now! After you have completed this study guide, you need to memorize it! 1. Look
NAME PER DATE MY BIOLOGY FINAL EXAM WORKBOOK DIRECTIONS: This study work book is due on the day of your final exam. Start now! After you have completed this study guide, you need to memorize it! 1. Look
Carbon. Isomers. The Chemical Building Blocks of Life
 The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
Cells and Osmosis BCT Questions. Questions taken from 2-12 to 2-18
 Cells and Osmosis BCT Questions Questions taken from 2-12 to 2-18 1. Which of the following would be least affected by defective receptor proteins on a cell membrane? a. Homeostasis b. Muscle activity
Cells and Osmosis BCT Questions Questions taken from 2-12 to 2-18 1. Which of the following would be least affected by defective receptor proteins on a cell membrane? a. Homeostasis b. Muscle activity
Chapter 3 CELL PROCESSES AND ENERGY
 Chapter 3 CELL PROCESSES AND ENERGY Section 1: Chemical Compounds in Cells Elements= Any substance that cannot be broken down into a simpler form Made up of only one kind of atom Found in the body Carbon
Chapter 3 CELL PROCESSES AND ENERGY Section 1: Chemical Compounds in Cells Elements= Any substance that cannot be broken down into a simpler form Made up of only one kind of atom Found in the body Carbon
Test Review Worksheet 1 Name: Per:
 Test Review Worksheet 1 Name: Per: 1. Put the following in order according to blood flow through the body, starting with the lungs: Lungs, right atrium, left atrium, right ventricle, left ventricle, aorta,
Test Review Worksheet 1 Name: Per: 1. Put the following in order according to blood flow through the body, starting with the lungs: Lungs, right atrium, left atrium, right ventricle, left ventricle, aorta,
Releasing Food Energy
 Releasing Food Energy All food is broken down by the body into small molecules through digestion. By the time food reaches your, bloodstream it has been broken down into nutrient rich molecules that can
Releasing Food Energy All food is broken down by the body into small molecules through digestion. By the time food reaches your, bloodstream it has been broken down into nutrient rich molecules that can
Biomolecule: Carbohydrate
 Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Ch 2 Molecules of life
 Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
Compounds of Life Biological Molecules
 Compounds of Life Biological Molecules By Joseph A. Castellano, Ph.D. RESEED Silicon Valley Reference: Focus on Physical Science, Glencoe/McGraw-Hill, Columbus, Ohio, 2007, Pages 438-442. This textbook
Compounds of Life Biological Molecules By Joseph A. Castellano, Ph.D. RESEED Silicon Valley Reference: Focus on Physical Science, Glencoe/McGraw-Hill, Columbus, Ohio, 2007, Pages 438-442. This textbook
Bio 12 Important Organic Compounds: Biological Molecules NOTES Name:
 Bio 12 Important Organic Compounds: Biological Molecules NOTES Name: Many molecules of life are.(means many molecules joined together) Monomers: that exist individually Polymers: Large organic molecules
Bio 12 Important Organic Compounds: Biological Molecules NOTES Name: Many molecules of life are.(means many molecules joined together) Monomers: that exist individually Polymers: Large organic molecules
Cellular Respiration: Obtaining Energy from Food
 Chapter 6 Cellular Respiration: Obtaining Energy from Food Lectures by Chris C. Romero, updated by Edward J. Zalisko PowerPoint Lectures for Campbell Essential Biology, Fourth Edition Eric Simon, Jane
Chapter 6 Cellular Respiration: Obtaining Energy from Food Lectures by Chris C. Romero, updated by Edward J. Zalisko PowerPoint Lectures for Campbell Essential Biology, Fourth Edition Eric Simon, Jane
Unit 1: Science of Life 1. Define the following terms: Hypothesis: Testable explanation for a phenomenon
 UCS BIOLOGY STUDY GUIDE FOR 1 ST SEMESTER MIDTERM EXAM 2014-2015 Unit 1: Science of Life 1. Define the following terms: Hypothesis: Testable explanation for a phenomenon Experiment: an orderly procedure
UCS BIOLOGY STUDY GUIDE FOR 1 ST SEMESTER MIDTERM EXAM 2014-2015 Unit 1: Science of Life 1. Define the following terms: Hypothesis: Testable explanation for a phenomenon Experiment: an orderly procedure
Chapter 3 Review Assignment
 Class: Date: Chapter 3 Review Assignment Multiple Choice 40 MC = 40 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following organelles produces transport
Class: Date: Chapter 3 Review Assignment Multiple Choice 40 MC = 40 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following organelles produces transport
BIOCHEMISTRY. How Are Macromolecules Formed? Dehydration Synthesis or condensation reaction Polymers formed by combining monomers and removing water.
 BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
BIOCHEMISTRY Organic compounds Compounds that contain carbon are called organic. Inorganic compounds do not contain carbon. Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as
Carbohydrates, Lipids, Proteins, and Nucleic Acids
 Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Cells. Prokaryotic vs. Eukaryotic Euakryotic cells are generally one to one hundred times bigger than prokaryotic cells
 Cell Theory Cells 1. All living things are composed of one or more cell 2. Cell is the basic unit of life 3. All cells come from the division of pre-existing cells Cells are divided into 2 categories:
Cell Theory Cells 1. All living things are composed of one or more cell 2. Cell is the basic unit of life 3. All cells come from the division of pre-existing cells Cells are divided into 2 categories:
Chapter 5 THE STRUCTURE AND FUNCTION OF LARGE BIOLOGICAL MOLECULES
 Chapter 5 THE STRUCTURE AND FUNCTION OF LARGE BIOLOGICAL MOLECULES You Must Know The role of dehydration synthesis in the formation of organic compounds and hydrolysis in the digestion of organic compounds.
Chapter 5 THE STRUCTURE AND FUNCTION OF LARGE BIOLOGICAL MOLECULES You Must Know The role of dehydration synthesis in the formation of organic compounds and hydrolysis in the digestion of organic compounds.
I. ATP: Energy In A Molecule
 I. ATP: Energy In A Molecule All food is broken down by the body into small molecules through digestion By the time food reaches your bloodstream, it has been broken down into nutrient molecules that can
I. ATP: Energy In A Molecule All food is broken down by the body into small molecules through digestion By the time food reaches your bloodstream, it has been broken down into nutrient molecules that can
