BLIZARD INSTITUTE CORE PATHOLOGY ATLAS OF TINCTORIAL STAINS
|
|
|
- Felicia Chapman
- 6 years ago
- Views:
Transcription
1 BLIZARD INSTITUTE CORE PATHOLOGY ATLAS OF TINCTORIAL STAINS
2 Contents Introduction... 3 Background to Tinctorial Stains... 3 Haematoxylin and Eosin Stain (H&E)... 3 Connective Tissue Stains... 4 Nucleic Acids... 5 Amyloid... 6 Lipids... 6 Carbohydrates (Glycogen and Mucins)... 7 Pigments and Minerals... 8 Artefact pigments... 8 Endogenous Pigments... 8 Exogenous pigments Micro Organisms and Fungi Other P a g e
3 Introduction This atlas is a guide to the Tinctorial Stains being carried out at the Blizard Institute Core Pathology Facility. You will find the stains grouped according to tissue components. Please note if there are stains that you require and are not on the list then please contact the department on, core-pathology@qmul.ac.uk as this list covers the most common stains. Background to Tinctorial Stains Biological tissue has little inherent contrast in either the light or electron microscopy. Staining is employed to give both contrasts to the tissue as well as highlighted particular features of interest. Tinctorial staining (or more commonly known as special staining) has been used to selectively stain cells and cellular components. A variety of compounds used to colour tissue sections include, Safranin, Oil Red O, Congo Red, Fast Green FCF, Silver Salts and numerous natural and artificial dyes that were usually originated from the development of dyes in the textile industry. Histochemistry refers to the science of using chemical reactions between laboratory chemicals and components within tissue. Below is a list of the most commonly used stains in a laboratory s repertoire. If you do not see what you require please contact a member of staff as many of the stains can be adapted and there are more available. Haematoxylin and Eosin Stain (H&E) The H&E stain is probably the most widely used histological stain. Its popularity is based on its comparative simplicity and ability to demonstrate clearly an enormous number of different tissues. Gills haematoxylin and Eosin is the standard method I the lab but other stains; like Ehirlichs and Weigert s haematoxylin for nuclei are also available. Nuclei stain blue, other elements shades of pink/red. 3 P a g e
4 Connective Tissue Stains Connective tissue s major function is to connect together and support the other tissue of the body. It usually consists of a cellular portion in an enveloping framework of non-cellular substances; there are many techniques available for the demonstration of the different connective tissues. Masson Trichrome Nuclei: Black Collagen: Green / Blue Muscle: Red Fibrin: Red Reticulin: Green Elastic: Red Van Gieson Nuclei: Black Collagen: Red Muscle: Yellow MSB Fibrin: Red Collagen: Blue Muscle: Pale Red Erythrocytes: Yellow Nuclei: Blue Elastic Van Gieson (EVG) Elastic: Blue/Black Nuclei: Blue Collagen: Red Muscle: Yellow 4 P a g e
5 Gordon & Sweet s Stain for Reticulin Fibres Reticulin: Black Collagen: Brown (if untoned) Safranin O Nuclei: Black Cytoplasm: Grey/Green Mucus, cartilage, mast cell granules: Red Muscle: Green Nucleic Acids Nucleoproteins are combinations of basic proteins (protamines and histones) and nucleic acids. The two nucleic acids are DNA which is found in the nucleus of the cell and RNA which is located in the cytoplasm of cells, mainly in ribosomes. Both DNA and RNA molecules consist of alternate sugar and phosphate groups with a nitrogenous base being attached to each sugar group. The sugar in DNA is the 5- carbon sugar deoxyribose; in RNA it is ribose. Feulgen Stain DNA: Red-Purple Cytoplasm: Green Methyl Green Pyronin DNA: Green-Blue RNA: Red 5 P a g e
6 Amyloid The classical, Histopathological definition of amyloid is an extracellular proteinaceous deposist exbiting beta sheet strcture. Common to most cross beta type structures they are generally identified by apple-green birefringence when stained with Congo red and seen under polarised light. These deposists often recruit various sugars and other components such as serum amyloid P component, resuting in complex and sometimes ihhomogenous structures. Congo Red Amyloid: Red Nuclei: Blue Eosinophils, Elastin and Keratin: Red Lipids Lipids have been defined, Baker 1946, as naturally occurring fat-like substances that are soluble in organic solvents but not in water, but any no means all lipids resemble fats. Free cholesterol for example, is crystalline and some of the phospholipids are even water soluble. The definition now which is more preferred is, Lovern 1955, lipids are actual potential derivatives of free fatty acids and their metabolites. It is now possible with Tinctorial staining to identify individual each groups of lipids. Please note lipid stains must be carried out on unfixed frozen sections as processing the samples will dissolve out majority of lipids. Oil Red O Unsaturated hydrophobic lipids: Red Phospholipids: Pink Nuclei: Blue Nile Blue Neutral Lipids: Red-Pink Acidic Lipids: Blue 6 P a g e
7 Carbohydrates (Glycogen and Mucins) The two main entities to be considered in tissue carbohydrate demonstration are glycogen and mucins, the latter of which incorporates mucopolysaccharides, mucosunstances and glycoconjugates. Glycogen is a simple polysaccharide which consists of branched or straight chain D- glucose units and can be found in two main forms alpha and beta. A third form has also been described and is a non-particulate glycogen found between beta particles in the alpha rosettes. Mucins cover a variety of substances as mentioned but to try and simply put it; these are hexosamine-containing polysaccharides covalently bound to varying amounts of protein. Free hexose groups are often available, together with certain acidic moieties, the presence of which influences the histochemical reactivity. The different mucins may be present as a single type or more commonly as a mixture. Periodic Acid Schiff (PAS) Glycogen and other periodate-reactive carbohydrates : Mangenta Nuclei: Blue *Glycogen can be digested out with pretreating slide with diastase* Alcian Blue for Acid Mucins Acid Mucopolysaccharides: Blue Nuclei: Red *The ph of the solution can be changed according to the type of mucins you wish to demonstrate* ph0.2: Strongly sulphated mucins. ph1.0: Weakly and strongly sulphated mucins. ph2.5: Most acid mucins except some strongly sulphated mucins. 7 P a g e
8 Pigments and Minerals It is tradition to consider pigments under the following headings Artefact pigments Deposits of pigments which are artefactually produced as a result of the action of some chemical substance, such as the fixative formalin. Formalin pigment this is present as a brown or brownish black deposist in some tissues. The deposit is likely to occur in blood-rich tissues such as spleen, areas of haemorrhage, or tissue which are heavily congested with blood. The pigment is birefringent in polarise light. It can be removed by treating the slide with alcoholic picric acid for a few hours followed by washing in running water. Mercury pigment some fixatives such as Zenker s and Helly s fluid, Heidenhain s Susa and formal-sublimate contain mercuric chloride. Tissues fixed with these can develop a uniform granular black deposits. This can be easily removed by Lugol s iodine and subsequently in sodium thiosulphate. Dichromate deposits tissues fixed in potassium dichromate containing fixatives like Zenker s must be washed in running tap water for some hours, because if the tissue is transferred directly to alcohol an insoluble yellowbrown oxide is precipitated within it. It is possible to remove some of it but not all in a 1% HCL in 70% IMS. Endogenous Pigments Pigments which are produced within the tissue and which serve a physiological function, or are by-products of normal metabolic processes. Bile it is important to identify bile pigmented in the examination of liver, where it is important to distinguish from lipofuschin as both appear yellow/brown on H&E. Fouchet Bile: Green Other Elements: As counterstain 8 P a g e
9 Melanin is a group of brown-black pigmented whose exact chemical structure is mot know. The positive identification of melanin and melanin producing cells can be done by reducing methods like Masson Fontana, enzyme methods and fluorescent methods. The reducing methods demonstrate both formed melanin and also melanin precursors. Masson Fontana Melanin, Argentaffin, Chromaffin, Some Lipofuscins: Black Nuclei: Red Chromaffin, argentaffin and argyrophil granules it is traditional to incorporate these into the heading of endogenous pigments, although the granules are completely lacking in pigment. The names were given to these granules as a result of their staining reactions with chrome and silver salts, resulting in brown and black coloration respectively. Chromaffin cells contain granules which have an affinity for chrome salts. Argentaffin cells have the ability to directly reduce silver solutions with the production of insoluble black metallic silver without the assistance of an external reducing agent. Argyrophil cells need the assistance of an external reducing agent to reduce the silver solution. Grimelius Agryrophil Granules: Black Pancreatic α2 Cells: Black Background: Yellow 9 P a g e
10 Haemosiderin and Iron iron is an important component of the human body, being vital constituent if the oxygen carrying haemoglobin, but also occurring in myoglobin and certain enzymes. Bit all tissue iron is histologically demonstrable; iron which is very tightly complexed protein, as in haemoglobin or myoglobin, cannot be demonstrated unless the iron is released by pretreatment by as strong agent like hydrogen peroxide. Particulate metallic iron, or inert iron oxide, sometimes introduced into tissues by industrial exposure, cannot be demonstrated in that form but various mechanisms within tissues release some of the iron in a demonstrable form, such as deposits surrounded by hemosiderin. The iron can be easily stained when it exists in the ferric form, as in hemosiderin. Perls Prussian Blue Ferric Iron: Blue Nuclei: Red Calcium calcium is present in the body in large quantities, a proportion of which is circulating in the blood in free ionic form. This proportion is not demonstrable histochemically. The most important site of bound (demonstrable) calcium is in the bone where it exists combined with phosphates, carbonates and other anions. Calcium appears blue-black on H&E stained sections, like many metallic cations, it produces a blue black lake with haematoxylin. The von Kossa method is a silver reduction technique and in fact demonstrates phosphates and carbonates but since the demonstrable forms of these anions are almost always found combined with calcium it can be regarded as a stain for calcium. Von Kossa Calcium Deposits: Black Other Elements: As counterstain 10 P a g e
11 Copper is a normal constituent of many tissues and is an important structural component of some of the oxidase enzymes. It is usually present in small amounts and cannot be demonstrated histochemically. In some diseases copper slowly accumulates in certain organs until sufficient amounts are present to permit histochemical demonstration. Rhodanine Copper: Red Nuclei: Light Blue Exogenous pigments These are pigments which gain access to the body accidentally and serve no physiological function. They usually enter by inhalation into lings or implantation into the skin during industrial exposure; most exogenous pigments are minerals. Asbestos is the name given to a special form of silica which exists in the form of long, thin, crystalline fibres. Perls Prussian Blue Ferric Iron: Asbestos bodies Nuclei: Red 11 P a g e
12 Micro Organisms and Fungi The detection and identification of microorganisms on histological sections can be of diagnostic importance, although it is rarely as satisfactory or sensitive as microbiological culture of the tissue. The detection of particular infecting organism in a tissue section is a difficult problem due to the fact that many infecting organism are too small to be seen by light microscopy, those that are large enough cannot be seen on a H&E so special stains have to be used and sometimes the numbers of the organisms are so small that even with the special stains they are sometimes not noted. Gram Stain Gram Positive Organisms: Blue Gram Negative Organisms: Red *some fungi, keratohyalin and keratin may also stain blue* Ziehl-Nelson Tubercle Bacilli: Red Background: Pale Blue Wade-Fite Leprosy and Other Mycobacteria: Red Background: Light Blue Alcian Yellow Toludine Blue Mucin: Yellow Helicobacter Pylori: Blue Background: Pale Blue 12 P a g e
13 Grocott Fungi: Black Pneumocystis: Black Other Elements: As counterstain *Cellulose, chitin, amoeboe, some mucins, melanin, glycogen and starch may also be balck* PAS Fungi: Mangenta Nuclei: Light blue Other Apart from the categories mentioned above, there are many other special stains that are used in both the diagnostic and research world. Please contact us if you cannot see what you are looking for in this atlas as we may be able to suggest an appropriate stain. Hexamine Silver Basement Membrane: Black Other Elements: As counterstain Luxol Fast Blue Cresyl Fast Violet Myelin: Deep Bright Blue Red Blood Cells: Turquoise Lipofuscin: Bright Blue Nissl Granules: Purple Background: Colourless 13 P a g e
The Oral Histology Series Series 5 Special Stains
 The Oral Histology Series Series 5 Special Stains DAVID E. KLINGMAN, Lt Col, USAF, DC Views and opinions expressed are those of the author(s) and do not reflect official policy or position of the United
The Oral Histology Series Series 5 Special Stains DAVID E. KLINGMAN, Lt Col, USAF, DC Views and opinions expressed are those of the author(s) and do not reflect official policy or position of the United
Atlas of Stains. Special Stains on Artisan Link Pro
 Atlas of Stains Special Stains on Artisan Link Pro Intended use Routinely processed samples (paraffin-embedded) may be used. The preferred fixative is neutral buffered formalin. The clinical interpretation
Atlas of Stains Special Stains on Artisan Link Pro Intended use Routinely processed samples (paraffin-embedded) may be used. The preferred fixative is neutral buffered formalin. The clinical interpretation
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Cellular Pathology Department University College London Hospital Rockefeller Building University Street London WC1E 6JJ Contact: Gavyn Barrett
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Cellular Pathology Department University College London Hospital Rockefeller Building University Street London WC1E 6JJ Contact: Gavyn Barrett
Special Staining (I)
 Special Staining (I) Carbohydrates 1- PERIODIC ACID SCHIFF'S (PAS ) Purpose: Glycogen is present in liver, kidney, skeletal and cardiac muscle. The PAS stain is used to demonstrate neutral polysaccharides
Special Staining (I) Carbohydrates 1- PERIODIC ACID SCHIFF'S (PAS ) Purpose: Glycogen is present in liver, kidney, skeletal and cardiac muscle. The PAS stain is used to demonstrate neutral polysaccharides
Preface 1. Fixation and Processing 1
 Contents Preface xi 1. Fixation and Processing 1 Fixation 1 Processing 2 What Should Be Seen in a Well-Fixed, Well-Processed Specimen Stained with Hematoxylin and Eosin 3 Problems Encountered With Fixation
Contents Preface xi 1. Fixation and Processing 1 Fixation 1 Processing 2 What Should Be Seen in a Well-Fixed, Well-Processed Specimen Stained with Hematoxylin and Eosin 3 Problems Encountered With Fixation
B i o c h e m i s t r y N o t e s
 14 P a g e Carbon Hydrogen Nitrogen Oxygen Phosphorus Sulfur ~Major ~Found in all ~Found in most ~Found in all component of all organic organic molecules. molecules. ~Major structural atom in all organic
14 P a g e Carbon Hydrogen Nitrogen Oxygen Phosphorus Sulfur ~Major ~Found in all ~Found in most ~Found in all component of all organic organic molecules. molecules. ~Major structural atom in all organic
Bio 12 Chapter 2 Test Review
 Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
Bio 12 Chapter 2 Test Review 1.Know the difference between ionic and covalent bonds In order to complete outer shells in electrons bonds can be Ionic; one atom donates or receives electrons Covalent; atoms
Chapter 2 Part 3: Organic and Inorganic Compounds
 Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Blizard Institute Core Pathology Pathology and Pharmacy Building Second Floor 80 Newark Street London E1 2ES Contact: Pauline Levey Tel: +44
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Blizard Institute Core Pathology Pathology and Pharmacy Building Second Floor 80 Newark Street London E1 2ES Contact: Pauline Levey Tel: +44
Fixation... Questions 1 Answers 16. Processing... Questions 25 Answers 36. Safety... Questions 67 Answers 73
 Table of Contents Fixation... Questions 1 Answers 16 Processing... Questions 25 Answers 36 Instrumentation... Questions 43 Answers 58 Safety... Questions 67 Answers 73 Laboratory Mathematics & Solution
Table of Contents Fixation... Questions 1 Answers 16 Processing... Questions 25 Answers 36 Instrumentation... Questions 43 Answers 58 Safety... Questions 67 Answers 73 Laboratory Mathematics & Solution
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
the nature and importance of biomacromolecules in the chemistry of the cell: synthesis of biomacromolecules through the condensation reaction lipids
 the nature and importance of biomacromolecules in the chemistry of the cell: synthesis of biomacromolecules through the condensation reaction lipids and their sub-units; the role of lipids in the plasma
the nature and importance of biomacromolecules in the chemistry of the cell: synthesis of biomacromolecules through the condensation reaction lipids and their sub-units; the role of lipids in the plasma
2.3 Carbon-Based Molecules. KEY CONCEPT Carbon-based molecules are the foundation of life.
 KEY CONCEPT Carbon-based molecules are the foundation of life. Carbon atoms have unique bonding properties. Carbon forms covalent bonds with up to four other atoms, including other carbon atoms. Carbon-based
KEY CONCEPT Carbon-based molecules are the foundation of life. Carbon atoms have unique bonding properties. Carbon forms covalent bonds with up to four other atoms, including other carbon atoms. Carbon-based
Biology Chapter 2 Review
 Biology Chapter 2 Review Vocabulary: Define the following words on a separate piece of paper. Element Compound Ion Ionic Bond Covalent Bond Molecule Hydrogen Bon Cohesion Adhesion Solution Solute Solvent
Biology Chapter 2 Review Vocabulary: Define the following words on a separate piece of paper. Element Compound Ion Ionic Bond Covalent Bond Molecule Hydrogen Bon Cohesion Adhesion Solution Solute Solvent
Carbohydrates and mucins. Dr Phil Bryant, Wales, UK
 Carbohydrates and mucins Dr Phil Bryant, Wales, UK Carbohydrates Provide cells with energy by converting to glucose Excess glucose stored in liver and muscle as glycogen Residual unstored glycogen is turned
Carbohydrates and mucins Dr Phil Bryant, Wales, UK Carbohydrates Provide cells with energy by converting to glucose Excess glucose stored in liver and muscle as glycogen Residual unstored glycogen is turned
NOTE: For studying for the final, you only have to worry about those with an asterix (*)
 NOTE: For studying for the final, you only have to worry about those with an asterix (*) (*)1. An organic compound is one that: a. contains carbon b. is slightly acidic c. forms long chains d. is soluble
NOTE: For studying for the final, you only have to worry about those with an asterix (*) (*)1. An organic compound is one that: a. contains carbon b. is slightly acidic c. forms long chains d. is soluble
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
The Structure and Function of Biomolecules
 The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
ABOUT US WORLDWIDE DISTRIBUTION ICON KEY STAIN LINE PRODUCT LIST
 ABOUT US WORLDWIDE DISTRIBUTION ICON KEY STAIN LINE PRODUCT LIST BQCKit Company is one of the three differentiated business lines of Bioquochem S.L. We are a Spanish biotechnology company specialized
ABOUT US WORLDWIDE DISTRIBUTION ICON KEY STAIN LINE PRODUCT LIST BQCKit Company is one of the three differentiated business lines of Bioquochem S.L. We are a Spanish biotechnology company specialized
2.2 Properties of Water
 2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
I. Polymers & Macromolecules Figure 1: Polymers. Polymer: Macromolecule: Figure 2: Polymerization via Dehydration Synthesis
 I. Polymers & Macromolecules Figure 1: Polymers Polymer: Macromolecule: Figure 2: Polymerization via Dehydration Synthesis 1 Dehydration Synthesis: Figure 3: Depolymerization via Hydrolysis Hydrolysis:
I. Polymers & Macromolecules Figure 1: Polymers Polymer: Macromolecule: Figure 2: Polymerization via Dehydration Synthesis 1 Dehydration Synthesis: Figure 3: Depolymerization via Hydrolysis Hydrolysis:
Biological Molecules. Carbohydrates, Proteins, Lipids, and Nucleic Acids
 Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK 142-144 New Cavendish Street W1W 6YF Contact: Mr Tel: +44 (0) 20 7299 4490 Fax: +44 (0)20 7299 4491 E-Mail: Neil.Barrett@unilabs.com Website:
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK 142-144 New Cavendish Street W1W 6YF Contact: Mr Tel: +44 (0) 20 7299 4490 Fax: +44 (0)20 7299 4491 E-Mail: Neil.Barrett@unilabs.com Website:
FOOD. Why do we need food? What's in our food? There are 3 trace elements, Iron (Fe), Copper (Cu) and Zinc (Zn).
 Why do we need food? FOOD 1. As a source of energy keeps our cells and us alive. 2. To make chemicals for our metabolic reactions. 3. As raw materials for growth and repair of our cells and body. What's
Why do we need food? FOOD 1. As a source of energy keeps our cells and us alive. 2. To make chemicals for our metabolic reactions. 3. As raw materials for growth and repair of our cells and body. What's
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
Biology 5A Fall 2010 Macromolecules Chapter 5
 Learning Outcomes: Macromolecules List and describe the four major classes of molecules Describe the formation of a glycosidic linkage and distinguish between monosaccharides, disaccharides, and polysaccharides
Learning Outcomes: Macromolecules List and describe the four major classes of molecules Describe the formation of a glycosidic linkage and distinguish between monosaccharides, disaccharides, and polysaccharides
Review for Test #1: Biochemistry
 Review for Test #1: Biochemistry 1. Know and understand the definitions and meanings of the following terms. Be able to write complete definitions for the terms in BOLD: Biology triglyceride metabolism
Review for Test #1: Biochemistry 1. Know and understand the definitions and meanings of the following terms. Be able to write complete definitions for the terms in BOLD: Biology triglyceride metabolism
Non-hematogenous endogenous pigments
 Non-hematogenous endogenous pigments 0 This group contains the following : 1. Melanins. 2. Lipofuscins. 3. Chromaffin. 4. Pseudomelanosis. 5. Dubin-Johnson pigments. 6. Ceroid-type lipofuscins. 7. Hamazaki-Weisenberg
Non-hematogenous endogenous pigments 0 This group contains the following : 1. Melanins. 2. Lipofuscins. 3. Chromaffin. 4. Pseudomelanosis. 5. Dubin-Johnson pigments. 6. Ceroid-type lipofuscins. 7. Hamazaki-Weisenberg
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 Schedule of Accreditation 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Department of Eye Pathology 1 st Floor, Cayton Street Building UCL Institute of Ophthalmology 11-43 Bath Street
Schedule of Accreditation 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Department of Eye Pathology 1 st Floor, Cayton Street Building UCL Institute of Ophthalmology 11-43 Bath Street
Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE
 Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE Name: Per: Date: All plants, animals and microorganisms use carbohydrates as sources of energy. Carbohydrates are also used as structural building
Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE Name: Per: Date: All plants, animals and microorganisms use carbohydrates as sources of energy. Carbohydrates are also used as structural building
not to be republished NCERT BIOMOLECULES CHAPTER 9 BIOMOLECULES 43 MULTIPLE CHOICE QUESTIONS
 BIOMOLECULES 43 43 CHAPTER 9 BIOMOLECULES MULTIPLE CHOICE QUESTIONS 1. It is said that elemental composition of living organisms and that of inanimate objects (like earth s crust) are similar in the sense
BIOMOLECULES 43 43 CHAPTER 9 BIOMOLECULES MULTIPLE CHOICE QUESTIONS 1. It is said that elemental composition of living organisms and that of inanimate objects (like earth s crust) are similar in the sense
Essential Components of Food
 Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
UNDERSTANDING SPECIAL STAINS
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk UNDERSTANDING SPECIAL STAINS Author : MELANIE DOBROMYLSKYJ Categories : Vets Date : April 14, 2014 MELANIE DOBROMYLSKYJ explains
Vet Times The website for the veterinary profession https://www.vettimes.co.uk UNDERSTANDING SPECIAL STAINS Author : MELANIE DOBROMYLSKYJ Categories : Vets Date : April 14, 2014 MELANIE DOBROMYLSKYJ explains
Organic Compounds: Carbohydrates
 Organic Compounds: Carbohydrates Carbohydrates include sugars and starches Contain the elements C,H,O (H & O ratio like water, 2 H s to 1O), ex. glucose C 6 H 12 O 6 Word means hydrated carbon Classified
Organic Compounds: Carbohydrates Carbohydrates include sugars and starches Contain the elements C,H,O (H & O ratio like water, 2 H s to 1O), ex. glucose C 6 H 12 O 6 Word means hydrated carbon Classified
Macromolecules Cut & Paste
 Macromolecules Cut & Paste Adapted from http://mrswords.weebly.com/uploads/1/5/2/4/15244382/ch_6-3_life_molecules_cut-out_lab.pdf INTRODUCTION Many of the molecules in living cells are so large that they
Macromolecules Cut & Paste Adapted from http://mrswords.weebly.com/uploads/1/5/2/4/15244382/ch_6-3_life_molecules_cut-out_lab.pdf INTRODUCTION Many of the molecules in living cells are so large that they
Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond
 Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Carbohydrates, Lipids, Proteins, and Nucleic Acids
 Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Cell Physiology
 Cell Physiology 21-10-2018 1 The two major parts of a typical cell are the nucleus and the cytoplasm. The nucleus is separated from the cytoplasm by a nuclear membrane, and the cytoplasm is separated from
Cell Physiology 21-10-2018 1 The two major parts of a typical cell are the nucleus and the cytoplasm. The nucleus is separated from the cytoplasm by a nuclear membrane, and the cytoplasm is separated from
Biological molecules
 Biological molecules 04-04-16 Announcements Your lab report 1 is due now Quiz 1 is on Wednesday at the beginning of class, so don t be late Review Macromolecues are large molecules necessary for life made
Biological molecules 04-04-16 Announcements Your lab report 1 is due now Quiz 1 is on Wednesday at the beginning of class, so don t be late Review Macromolecues are large molecules necessary for life made
2014 CURRENT ISSUES IN PATHOLOGY
 2014 CURRENT ISSUES IN PATHOLOGY SPECIAL STAINS IN LIVER BIOPSY PATHOLOGY Sanjay Kakar, MD University of California, San Francisco Trichrome stain : (1) Assess degree of fibrosis. H&E stain is not reliable
2014 CURRENT ISSUES IN PATHOLOGY SPECIAL STAINS IN LIVER BIOPSY PATHOLOGY Sanjay Kakar, MD University of California, San Francisco Trichrome stain : (1) Assess degree of fibrosis. H&E stain is not reliable
The Star of The Show (Ch. 3)
 The Star of The Show (Ch. 3) Why study Carbon? All of life is built on carbon Cells ~72% 2 O ~25% carbon compounds carbohydrates lipids proteins nucleic acids ~3% salts Na, Cl, K Chemistry of Life Organic
The Star of The Show (Ch. 3) Why study Carbon? All of life is built on carbon Cells ~72% 2 O ~25% carbon compounds carbohydrates lipids proteins nucleic acids ~3% salts Na, Cl, K Chemistry of Life Organic
FIXATION OF TISSUES MODULE 5.1 INTRODUCTION OBJECTIVES 5.2 AIMS OF FIXATION 5.3 PRINCIPLE OF FIXATION. Notes
 MODULE Fixation of Tissues 5 FIXATION OF TISSUES 5.1 INTRODUCTION It is a process by which the cells or tissues are fixed in chemical and partly physical state so that they can withstand subsequent treatment
MODULE Fixation of Tissues 5 FIXATION OF TISSUES 5.1 INTRODUCTION It is a process by which the cells or tissues are fixed in chemical and partly physical state so that they can withstand subsequent treatment
Biology Teach Yourself Series Topic 3: Chemical Nature of the Cell
 Biology Teach Yourself Series Topic 3: Chemical Nature of the Cell A: Level 14, 474 Flinders Street Melbourne VIC 3000 T: 1300 134 518 W: tssm.com.au E: info@tssm.com.au TSSM 2013 Page 1 of 19 Contents
Biology Teach Yourself Series Topic 3: Chemical Nature of the Cell A: Level 14, 474 Flinders Street Melbourne VIC 3000 T: 1300 134 518 W: tssm.com.au E: info@tssm.com.au TSSM 2013 Page 1 of 19 Contents
For example, monosaccharides such as glucose are polar and soluble in water, whereas lipids are nonpolar and insoluble in water.
 Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Elements & Macromolecules in Organisms
 Name: Period: Date: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Period: Date: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Biological Chemistry. Is biochemistry fun? - Find it out!
 Biological Chemistry Is biochemistry fun? - Find it out! 1. Key concepts Outline 2. Condensation and Hydrolysis Reactions 3. Carbohydrates 4. Lipids 5. Proteins 6. Nucleic Acids Key Concepts: 1. Organic
Biological Chemistry Is biochemistry fun? - Find it out! 1. Key concepts Outline 2. Condensation and Hydrolysis Reactions 3. Carbohydrates 4. Lipids 5. Proteins 6. Nucleic Acids Key Concepts: 1. Organic
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules I. Polymers What is a polymer? Poly = many; mer = part. A polymer is a large molecule consisting of many smaller sub-units bonded together. What is a monomer?
The Structure and Function of Macromolecules I. Polymers What is a polymer? Poly = many; mer = part. A polymer is a large molecule consisting of many smaller sub-units bonded together. What is a monomer?
Organic Chemistry Worksheet
 Organic Chemistry Worksheet Name Section A: Intro to Organic Compounds 1. Organic molecules exist in all living cells. In terms of biochemistry, what does the term organic mean? 2. Identify the monomer
Organic Chemistry Worksheet Name Section A: Intro to Organic Compounds 1. Organic molecules exist in all living cells. In terms of biochemistry, what does the term organic mean? 2. Identify the monomer
6/15/2015. Biological Molecules. Outline. Organic Compounds. Organic Compounds - definition Functional Groups Biological Molecules. What is organic?
 Biological Molecules Biology 105 Lecture 3 Reading: Chapter 2 (pages 29 39) Outline Organic Compounds - definition Functional Groups Biological Molecules Carbohydrates Lipids Amino Acids and Proteins Nucleotides
Biological Molecules Biology 105 Lecture 3 Reading: Chapter 2 (pages 29 39) Outline Organic Compounds - definition Functional Groups Biological Molecules Carbohydrates Lipids Amino Acids and Proteins Nucleotides
EXERCISE 3 Carbon Compounds
 LEARNING OBJECTIVES EXERCISE 3 Carbon Compounds Perform diagnostic tests to detect the presence of reducing sugars (Benedict s), starch (Lugol s), protein (Biuret), lipid (SudanIV) and sodium chloride
LEARNING OBJECTIVES EXERCISE 3 Carbon Compounds Perform diagnostic tests to detect the presence of reducing sugars (Benedict s), starch (Lugol s), protein (Biuret), lipid (SudanIV) and sodium chloride
WHY IS THIS IMPORTANT?
 CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
Macro molecule = is all the reactions that take place in cells, the sum of all chemical reactions that occur within a living organism Anabolism:
 Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
Macromolecule Macro molecule = molecule that is built up from smaller units The smaller single subunits that make up macromolecules are known as Joining two or more single units together form a M is all
9.A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids
 9.A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids o o o Food is a good source of one or more of the following: protein,
9.A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids o o o Food is a good source of one or more of the following: protein,
Biomolecule: Carbohydrate
 Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
CHEMISTRY OF LIFE 30 JANUARY 2013
 CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
Biological Molecules Ch 2: Chemistry Comes to Life
 Outline Biological Molecules Ch 2: Chemistry Comes to Life Biol 105 Lecture 3 Reading Chapter 2 (pages 31 39) Biological Molecules Carbohydrates Lipids Amino acids and Proteins Nucleotides and Nucleic
Outline Biological Molecules Ch 2: Chemistry Comes to Life Biol 105 Lecture 3 Reading Chapter 2 (pages 31 39) Biological Molecules Carbohydrates Lipids Amino acids and Proteins Nucleotides and Nucleic
Elements & Macromolecules in Organisms
 Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be
Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be
Biology Unit 2 Elements & Macromolecules in Organisms Date/Hour
 Biology Unit 2 Name Elements & Macromolecules in rganisms Date/our Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body
Biology Unit 2 Name Elements & Macromolecules in rganisms Date/our Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body
Figure 2. Figure 1. Name: Bio AP Lab Organic Molecules
 Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
Ch 2 Molecules of life
 Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
Organic compounds. Lipids, Carbohydrates, Proteins, and Nucleic Acids
 Organic compounds Lipids, Carbohydrates, Proteins, and Nucleic Acids Essential for life Organic compounds: Contain carbon Most are covalently bonded Example: C 6 H 12 O 6 (Glucose) Inorganic Compounds:
Organic compounds Lipids, Carbohydrates, Proteins, and Nucleic Acids Essential for life Organic compounds: Contain carbon Most are covalently bonded Example: C 6 H 12 O 6 (Glucose) Inorganic Compounds:
Biology. Slide 1 of 37. End Show. Copyright Pearson Prentice Hall
 Biology 1 of 37 2 of 37 The Chemistry of Carbon The Chemistry of Carbon Organic chemistry is the study of all compounds that contain bonds between carbon atoms. 3 of 37 Macromolecules Macromolecules Macromolecules
Biology 1 of 37 2 of 37 The Chemistry of Carbon The Chemistry of Carbon Organic chemistry is the study of all compounds that contain bonds between carbon atoms. 3 of 37 Macromolecules Macromolecules Macromolecules
½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = X X= 325 g
 BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
Macromolecules. copyright cmassengale
 Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Carbon. Isomers. The Chemical Building Blocks of Life
 The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
Biology 12. Biochemistry. Water - a polar molecule Water (H 2 O) is held together by covalent bonds.
 Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Organic Compounds. Compounds that contain CARBON are called organic. Macromolecules are large organic molecules.
 Macromolecules Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Macromolecules Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Organic Compounds. Compounds that contain CARBON are called organic. Macromolecules are large organic molecules.
 Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Macromolecules 1 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. 2 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent
Most life processes are a series of chemical reactions influenced by environmental and genetic factors.
 Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
Biology 12 - Biochemistry Practice Exam
 Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Chapter 5 THE STRUCTURE AND FUNCTION OF LARGE BIOLOGICAL MOLECULES
 Chapter 5 THE STRUCTURE AND FUNCTION OF LARGE BIOLOGICAL MOLECULES You Must Know The role of dehydration synthesis in the formation of organic compounds and hydrolysis in the digestion of organic compounds.
Chapter 5 THE STRUCTURE AND FUNCTION OF LARGE BIOLOGICAL MOLECULES You Must Know The role of dehydration synthesis in the formation of organic compounds and hydrolysis in the digestion of organic compounds.
Macromolecules Chapter 2.3
 Macromolecules Chapter 2.3 E.Q. What are the 4 main macromolecues found in living things and what are their functions? Carbon-Based Molecules Why is carbon called the building block of life? Carbon atoms
Macromolecules Chapter 2.3 E.Q. What are the 4 main macromolecues found in living things and what are their functions? Carbon-Based Molecules Why is carbon called the building block of life? Carbon atoms
Elements & Macromolecules in Organisms
 Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be
Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be
Lysosomes. Gr: lysis solution, soma body. Membrane bounded vesicles. Usually round ovoid or irregular electron dense bodies m.
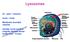 Lysosomes Gr: lysis solution, soma body Membrane bounded vesicles Usually round ovoid or irregular electron dense bodies 0.05 0.5 m. Lysosomes No. varies from a few to several hundred per cell, in different
Lysosomes Gr: lysis solution, soma body Membrane bounded vesicles Usually round ovoid or irregular electron dense bodies 0.05 0.5 m. Lysosomes No. varies from a few to several hundred per cell, in different
Macromolecules. Honors Biology
 Macromolecules onors Biology 1 The building materials of the body are known as macromolecules because they can be very large There are four types of macromolecules: 1. Proteins 2. Nucleic acids 3. arbohydrates
Macromolecules onors Biology 1 The building materials of the body are known as macromolecules because they can be very large There are four types of macromolecules: 1. Proteins 2. Nucleic acids 3. arbohydrates
6 The chemistry of living organisms
 Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Biomolecules. Biomolecules. Carbohydrates. Biol 219 Lec 3 Fall Polysaccharides. Function: Glucose storage Fig. 2.2
 Biomolecules Biomolecules Monomers Polymers Carbohydrates monosaccharides polysaccharides fatty acids triglycerides Proteins amino acids polypeptides Nucleic Acids nucleotides DNA, RNA Carbohydrates Carbohydrates
Biomolecules Biomolecules Monomers Polymers Carbohydrates monosaccharides polysaccharides fatty acids triglycerides Proteins amino acids polypeptides Nucleic Acids nucleotides DNA, RNA Carbohydrates Carbohydrates
Bridging task for 2016 entry. AS/A Level Biology. Why do I need to complete a bridging task?
 Bridging task for 2016 entry AS/A Level Biology Why do I need to complete a bridging task? The task serves two purposes. Firstly, it allows you to carry out a little bit of preparation before starting
Bridging task for 2016 entry AS/A Level Biology Why do I need to complete a bridging task? The task serves two purposes. Firstly, it allows you to carry out a little bit of preparation before starting
Organic Molecules Worksheet: Read through each section and answer the following questions.
 Name: Date: Period: Organic Molecules Worksheet: Read through each section and answer the following questions. Organic molecules are the molecules that exist in all living things. They are life s building
Name: Date: Period: Organic Molecules Worksheet: Read through each section and answer the following questions. Organic molecules are the molecules that exist in all living things. They are life s building
2.1.1 Biological Molecules
 2.1.1 Biological Molecules Relevant Past Paper Questions Paper Question Specification point(s) tested 2013 January 4 parts c and d p r 2013 January 6 except part c j k m n o 2012 June 1 part ci d e f g
2.1.1 Biological Molecules Relevant Past Paper Questions Paper Question Specification point(s) tested 2013 January 4 parts c and d p r 2013 January 6 except part c j k m n o 2012 June 1 part ci d e f g
Chapter 2 The Chemistry of Life Part 2
 Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
Topic 3: Molecular Biology
 Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
PATHOPHYSIOLOGY. DEFINED Involves the study of function that results from disease processes.
 DEFINED Involves the study of function that results from disease processes. What is pathology? Pathology is the branch of medical sciences that treats the essential nature of disease, especially the changes
DEFINED Involves the study of function that results from disease processes. What is pathology? Pathology is the branch of medical sciences that treats the essential nature of disease, especially the changes
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
2 3 Carbon Compounds (Macromolecules)
 2 3 Carbon Compounds (Macromolecules) Slide 1 of 37 Organic Chemistry Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Slide 2 of 37 Carbon Living organisms are
2 3 Carbon Compounds (Macromolecules) Slide 1 of 37 Organic Chemistry Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Slide 2 of 37 Carbon Living organisms are
Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction
 Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction Most biological molecules fall into one of four varieties: proteins, carbohydrates, lipids and nucleic acids. These are sometimes
Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction Most biological molecules fall into one of four varieties: proteins, carbohydrates, lipids and nucleic acids. These are sometimes
Chemical Basis of Life 2.3
 Chemical Basis of Life 2.3 August 13, 212 Agenda General Housekeeping 2.3 Review Terminology Quiz Chapter 2 Assignments Stations Reading Building Molecules Review What is the significance of the valence
Chemical Basis of Life 2.3 August 13, 212 Agenda General Housekeeping 2.3 Review Terminology Quiz Chapter 2 Assignments Stations Reading Building Molecules Review What is the significance of the valence
Lecture Series 2 Macromolecules: Their Structure and Function
 Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Lecture Series 2 Macromolecules: Their Structure and Function Reading Assignments Read Chapter 4 (Protein structure & Function) Biological Substances found in Living Tissues The big four in terms of macromolecules
Macromolecules. Note: If you have not taken Chemistry 11 (or if you ve forgotten some of it), read the Chemistry Review Notes on your own.
 Macromolecules Note: If you have not taken Chemistry 11 (or if you ve forgotten some of it), read the Chemistry Review Notes on your own. Macromolecules are giant molecules made up of thousands or hundreds
Macromolecules Note: If you have not taken Chemistry 11 (or if you ve forgotten some of it), read the Chemistry Review Notes on your own. Macromolecules are giant molecules made up of thousands or hundreds
The Chemical Building Blocks of Life. Chapter 3
 The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
2-2 Properties of Water
 2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
Macromolecules. You are what you eat! Chapter 5. AP Biology
 Macromolecules You are what you eat! Chapter 5 AP Biology Organic Compounds Contain bonds between CARBON glycosidic bond AP Biology Carbohydrates Structure / monomer u monosaccharide Function u energy
Macromolecules You are what you eat! Chapter 5 AP Biology Organic Compounds Contain bonds between CARBON glycosidic bond AP Biology Carbohydrates Structure / monomer u monosaccharide Function u energy
Unit #2: Biochemistry
 Unit #2: Biochemistry STRUCTURE & FUNCTION OF FOUR MACROMOLECULES What are the four main biomolecules? How is each biomolecule structured? What are their roles in life? Where do we find them in our body?
Unit #2: Biochemistry STRUCTURE & FUNCTION OF FOUR MACROMOLECULES What are the four main biomolecules? How is each biomolecule structured? What are their roles in life? Where do we find them in our body?
McMush Lab Testing for the Presence of Biomolecules
 Biology McMush Lab Testing for the Presence of Biomolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These biomolecules are large carbon-based
Biology McMush Lab Testing for the Presence of Biomolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These biomolecules are large carbon-based
What are the most common elements in living organisms? What is the difference between monomers, dimers and polymers?
 What do each of these terms mean? Atom Molecule Element Compound Organic Inorganic What are the most common elements in living organisms? What are the roles of magnesium, iron, phosphate and calcium in
What do each of these terms mean? Atom Molecule Element Compound Organic Inorganic What are the most common elements in living organisms? What are the roles of magnesium, iron, phosphate and calcium in
3. Staining solutions for histology and cytology Mounting and embedding media... 60
 INDEX 1. Hematoxylin stains... 4 2. Eosin... 6 3. Staining solutions for histology and cytology... 8 4. Reagents I... 48 5. Reagents II... 55 6. Decalcifying solutions... 58 7. Fixatives... 59 8. Formalins...
INDEX 1. Hematoxylin stains... 4 2. Eosin... 6 3. Staining solutions for histology and cytology... 8 4. Reagents I... 48 5. Reagents II... 55 6. Decalcifying solutions... 58 7. Fixatives... 59 8. Formalins...
Biological Molecules
 The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
