Standard Operating Procedures for Quantitative HIV-1 viral load testing using GeneXpert technology. June 2015
|
|
|
- Frederick Pierce
- 5 years ago
- Views:
Transcription
1 Standard Operating Prcedures fr Quantitative HIV-1 viral lad testing using GeneXpert technlgy June Versin 0 Effective date: 10 June 2014
2 Table f Cntents I. Title f Prcedure... 3 II. Test Summary... 3 III. Principle... 3 IV. Specimen Handling and Preparatin... 4 V. Quality Cntrl... 4 VI. Reagents, Materials, & Equipment... 6 VII. Prcedure... 7 VIII. Interpretatin f Results IX. Pssible reasns fr repeating the assay X. Limitatins XI. Perfrmance Characteristics XII. References Figure 1: Overview f the GeneXpert test cartridge... 4 Figure 2: GeneXpert quality cntrl result scenaris... 6 Figure 3: GeneXpert transfer pipette and aerial view f the reactin cartridge... 8 Figure 4: Summary f sample lading in GeneXpert HIV-1 quant... 9 Figure 5: GeneXpert HIV-1 quantitative detectable VL Figure 6: GeneXpert HIV-1 quantitative nt detected VL Table 1: GeneXpert HIV-1Viral lad result interpretatin Versin 0 Effective date: 10 June 2014
3 I. Title f Prcedure Quantitative HIV-1 viral lad testing using GeneXpert technlgy II. Test Summary State the physilgic and diagnstic reasns fr perfrming the test. Human Immundeficiency Virus (HIV) is the etilgic agent f Acquired Immundeficiency Syndrme (AIDS). It can be transmitted thrugh sexual cntact, expsure t infected bld r bld prducts, prenatal infectin f a fetus, r perinatal r pstnatal infectin f a newbrn. HIV diagnstics have evlved significantly in the past tw decades and cntinue t be significantly imprtant in saving lives f millins f HIV infected patients. Tday, measurement f bld plasma HIV-1 RNA cncentratin knwn as viral lad using nucleic acid-based mlecular diagnstic assays has been established as standard f care in assessing HIV-psitive patient prgnsis and respnse t antiretrviral therapy. Untreated HIV-1 infectin is characterized by high-level viral prductin and CD4 T-cell destructin, despite an ften lengthy clinical latency, t significant net lss f CD4 T cells and AIDS. Assessment f viral lad levels is a strng predictr f the rate f disease prgressin and, by itself r in cmbinatin with CD4Tcell cunts, has great prgnstic value. The HIV-1 Quant Assay, perfrmed n GeneXpert Instrument Systems, is designed fr the rapid quantitatin f Human Immundeficiency Virus type 1 (HIV-1) in human plasma frm HIV-1 infected individuals ver the range f 40 t 10,000,000 cpies/ml. HIV-1 Grup M subtypes A, B, C, D, AE, F, G, H, AB, AG, J, K and Grups N and O are detected and quantified by the HIV-1 Quant Assay. The HIV-1 VL Assay uses reverse transcriptase plymerase chain reactin (RT-PCR) technlgy t achieve high sensitivity fr the quantitative detectin f HIV-1 RNA in human plasma frm HIV-1 infected individuals. III. Principle GeneXpert Instrument Systems autmate and integrate sample preparatin, nucleic acid extractin and amplificatin, and detectin f the target sequence in simple r cmplex samples using real-time reverse transcriptase PCR (RT-PCR). The systems cnsist f an instrument, persnal cmputer, and preladed sftware fr running tests and viewing the results. The systems require the use f single-use dispsable GeneXpert cartridges that hld the RT-PCR reagents and hst the RT-PCR prcesses. Because the cartridges are self-cntained, crsscntaminatin between samples is minimized. The HIV-1 VL Assay includes reagents fr the detectin f HIV-1 RNA in specimens and tw internal cntrls used fr quantitatin f HIV-1 RNA. The internal cntrls are als used t mnitr the presence f inhibitr(s) in the RT and PCR reactins. The Prbe Check Cntrl (PCC) verifies reagent rehydratin, PCR tube filling in the cartridge, prbe integrity, and dye stability. 3 Versin 0 Effective date: 10 June 2014
4 Figure 1: Overview f the GeneXpert test cartridge IV. Specimen Handling and Preparatin Whle bld shuld be cllected in EDTA, EDTA-PPT, r ACD cllectin tubes and centrifuged t separate the plasma and red bld cells per the manufacturer's instructins. A minimum f 1 ml plasma is required fr the HIV-1 VL Assay. If using the transfer pipette included in the kit, a minimum f 1.2 ml plasma is required. Alternatively, if using a precisin pipette, a minimum f 1 ml plasma is required. Whle bld may be held at C fr up t 8 hurs r at 2 8 C fr up t 72 hurs, prir t preparing and testing the specimen. After centrifugatin, plasma may be held at C fr up t 24 hurs r at 2 8 C fr up t 6 days, prir t testing. Plasma specimens are stable frzen ( -18 C and -70 C) fr six weeks. Plasma specimens are stable up t three freeze/thaw cycles. Plasma specimens must be thawed and equilibrated t rm temperature prir t transfer t cartridge. V. Quality Cntrl Each GeneXpert test cartridge is a self-cntained test device with an in-built cntrl fr each sample. Nrmally, n external cntrls are required. The internal cntrls enable the system t detect specific failure mdes within each fr each sample Instrument system cntrl: Check status-it checks the ptics, temperature f the mdule and the mechanical integrity f each cartridge. If the system cntrls fail, an ERROR test result will be reprted. 4 Versin 0 Effective date: 10 June 2014
5 Prbe Check cntrl (PCC): after sample preparatin, bead recnstitutin and tube filling (prir t thermal cycling), multiple flurescent readings are taken at different temperatures and cmpared t default setting. PCC cntrls fr: Missing target specific reagent (TSR) and r enzyme reagent beads which cntain all primers, prbes and internal cntrl template. Incmplete reagent recnstitutin Incmplete reactin tube filling Prbe degradatin If the PCC fails, an ERROR test result will be reprted. Sample prcessing cntrl (SPC) assesses the effectiveness f the sample prcessing steps, including and up-t reactin tube filling. SPC ensures that the sample was crrectly added t the cartridge and detects degradatin f the enzyme(s) r ther cmpnents f the system. SPC des nt cmpete with target DNA. SPC must be Psitive when target is Negative SPC can be Psitive r Negative when the target is Psitive SPC passes if it meets the validated acceptance criteria. E2097: t less sample vlume added (<1mL), E2096: n sample added Internal Quantitative Standard High and Lw (IQS-H and IQS-L): IQS-H and IQS-L are tw dry bead armred RNAs nnspecific t HIV in the frm f a dry bead that ges thrugh the whle GX prcess. The IQS-H and IQS-L are standards calibrated against the WHO 3rd Internatinal Standard. They are used fr quantificatin by using lt specific parameters fr the calculatin f HIV-1 RNA cncentratin in the sample. The IQS-H and IQS-L pass if they meet the validated acceptance criteria. They run internally with every cartridge and they cntrl fr reagent perfrmance due t imprper strage and they cnfirm that reactin cmpnents are set up crrectly. External Cntrls: nt available in the kit, but they can be used (psitive and negative cntrls). 5 Versin 0 Effective date: 10 June 2014
6 Figure 2: GeneXpert quality cntrl result scenaris VI. Reagents, Materials, & Equipment The HIV-1 VL Assay kit cntains sufficient reagents t prcess 10 specimens r quality cntrl samples. The kit cntains the fllwing: HIV-1 VL Assay Cartridges with Integrated Reactin Tubes. Each cartridge cntains: Bead 1, Bead 2, and Bead 3 (freeze-dried) Lysis Reagent (Guanidinium Thicyanate) 2.0 ml Rinse Reagent 0.5 ml Elutin Reagent 1.5 ml Binding Reagent 2.4 ml Prteinase K Reagent 0.48 ml 2. Dispsable 1 ml Transfer Pipettes 10 per kit 3. CD 1 per kit Assay Definitin File (ADF) Instructins t imprt ADF int GeneXpert sftware Package Insert Strage and Handling Stre the HIV-1 VL Assay cartridges at 2 8 C. D nt pen the cartridge lid until yu are ready t perfrm the test. Use cartridge within fur hurs after pening the cartridge lid. D nt use a cartridge that has leaked. Materials Required but Nt Prvided 6 Versin 0 Effective date: 10 June 2014
7 Printer Bleach Warnings and Precautins Treat all bilgical specimens, including used cartridges, as if capable f transmitting infectius agents. Because it is ften impssible t knw which might be infectius, all bilgical specimens shuld be treated with standard precautins. D nt pen the HIV-1 VL Assay cartridge lid until yu are ready t add the plasma specimen D nt use a cartridge that has been drpped after remving it frm the packaging. D nt shake the cartridge. Shaking r drpping the cartridge after pening the lid may yield invalid results. D nt place the sample ID label n the cartridge lid r n the barcde label. Each single-use HIV-1 VL Assay cartridge is used t prcess ne specimen. D nt reuse spent cartridges. D nt use a cartridge that has a damaged reactin tube. Single-use dispsable pipette is used t transfer ne specimen. D nt reuse dispsable pipettes. Wear clean lab cats and glves. Change glves between prcessing each sample. In the event f cntaminatin f the wrk area r equipment with samples r cntrls, thrughly clean the cntaminated area with a slutin f 1:10 dilutin f husehld chlrine bleach and then 70% ethanl. Wipe wrk surfaces dry cmpletely befre prceeding. VII. Prcedure 1. Preparing the Specimen If using frzen specimens, place the specimens at rm temperature C until cmpletely thawed and equibrate t rm temperature befre use. Plasma samples stred in 2 8 C shuld be remved frm the refrigeratr and equilibrated t rm temperature befre use. Vrtex plasma fr 15 secnds befre use. If the specimen is cludy, clarify by a quick spin. 2. Preparing the Cartridge Wear prtective dispsable glves. Inspect the test cartridge fr damage. If damaged, d nt use it. Open the lid f the test cartridge. Allw samples t cme t rm temperature prir t lading plasma int the cartridge Optin 1: If using the transfer pipette included in the kit (Figure 1), fill t just belw the bulb but abve the line t transfer at least 1 ml plasma frm the cllectin tube int the sample chamber f the test cartridge (Figure 2). D NOT pur the specimen int the chamber! 7 Versin 0 Effective date: 10 June 2014
8 Figure 3: GeneXpert transfer pipette and aerial view f the reactin cartridge Clse the cartridge lid. Lad the cartridge int the GeneXpert instrument r Infinity system. Nte There is a thin plastic film that cvers the inner ring f 13 prts f the test cartridge. This film shuld nt be remved. Optin 2: If using an autmatic pipette, transfer at least 1 ml f plasma int the sample chamber f the test cartridge (Figure xx). D NOT pur the specimen int the chamber! Imprtant Start the test within fur hurs f adding the sample t the cartridge Lading less than 1 ml plasma int the cartridge will trigger an insufficient vlume errr (ERROR 2097), preventing the instrument frm running the sample Starting the Test This sectin lists the basic steps fr running the test. Fr detailed instructins, see the GeneXpert System Operatr Manual n the cmputer desktp. a. Turn n the GeneXpert instrument. First turn n the instrument and then turn n the cmputer. The GeneXpert sftware will launch autmatically. If it desn't, duble-click the GeneXpert b. Sftware shrtcut icn n the Windws desktp. 8 Versin 0 Effective date: 10 June 2014
9 c. Lg n t the GeneXpert Instrument System sftware using yur user name and passwrd. Click Create Test d. Scan in the Patient ID (ptinal). If typing the Patient ID, make sure the Patient ID is typed crrectly. The Patient ID is assciated with the test results and is shwn in the View Results windw. e. Scan r type in the Sample ID. If typing the Sample ID, make sure the Sample ID is typed crrectly. The Sample ID is assciated with the test results and is shwn in the View Results windw and all reprts. The Scan Cartridge dialg bx appears. f. Scan the barcde n the Xpert HIV-1 Viral Lad cartridge. The Create Test windw appears. Using the barcde infrmatin, the sftware autmatically fills the bxes fr the fllwing fields: Select Assay, Reagent Lt ID, Cartridge SN, and Expiratin Date. g. Click Start Test. Enter yur passwrd, if requested. h. Open the instrument mdule dr with the blinking green light and lad the cartridge. Clse the dr. The test starts and the green light stps blinking. When the test is finished, the light turns ff. Wait until the system releases the dr lck befre pening the mdule dr and remving the cartridge. The used cartridges shuld be dispsed in the apprpriate specimen waste cntainers accrding t yur institutin s standard practices. Figure 4: Summary f sample lading in GeneXpert HIV-1 quant 9 Versin 0 Effective date: 10 June 2014
10 WARNING: GeneXpert HIV Quant cartridges cntain a highly txic chemical (t human and envirnment), Guanidinium Thicyanate, and shuld be dispsed f after use, in an incineratr capable f reaching 1200 C. VIII. Interpretatin f Results Click the View Results icn t view results. Upn cmpletin f the test, click the Reprt buttn f the View Results windw t view and/r generate a PDF reprt file. The test results take apprximately 90minutes t be prduced The results are interpreted autmatically by the GeneXpert Instrument System frm measured flurescent signals and embedded calculatin algrithms and are clearly shwn in the View Results windw (Figure 5 and Figure 6). Pssible results are shwn in Table 1. Table 1: GeneXpert HIV-1Viral lad result interpretatin 10 Versin 0 Effective date: 10 June 2014
11 11 Versin 0 Effective date: 10 June 2014
12 Figure 5: GeneXpert HIV-1 quantitative detectable VL Figure 6: GeneXpert HIV-1 quantitative nt detected VL 12 Versin 0 Effective date: 10 June 2014
13 IX. Pssible reasns fr repeating the assay An INVALID result indicates that the sample was nt prperly prcessed, PCR was inhibited, r the sample was inadequate. An invalid result als indicates that the IQS-H and/r IQS-L Cts are nt within valid range. An ERROR result indicates that the Prbe Check Cntrl failed r maximum pressure limits were exceeded. An errr result indicates that the assay was abrted. Pssible causes include: insufficient vlume f sample was added, the reactin tube was filled imprperly, a reagent prbe integrity prblem was detected, r the maximum pressure limit was exceeded. A NO RESULT indicates that insufficient data were cllected. Fr example, the peratr stpped a test that was in prgress, a lad errr ccurred, r the sftware was clsed prematurely. Retest Prcedure Fr retest f an INVALID, ERROR, r NO RESULT, use a new cartridge (d nt re-use the cartridge). 1. Remve a new cartridge frm the kit. 2. Sectin VII n preparing cartridge and starting the test n page 7. X. Limitatins Gd labratry practices and changing glves between handling specimens are recmmended t avid cntaminatin f specimens r reagents. Factrs that negatively affect results Interfering substance False negative test results r invalid results may be bserved in the presence f interfering substance like; Albumin (90g/L), bilirubin (20mg/dL), haemglbin (500mg/dL), human DNA (0,4mg/dL) and triglycerides (3000mg/dL). Bilgical specimens, including used cartridges, shuld be treated as capable f transmitting infectius agents and shuld be discarded in hazardus clinical waste bins. 13 Versin 0 Effective date: 10 June 2014
14 XI. Perfrmance Characteristics Limit f Detectin (LD) The LOD fr the HIV-1 VL Assay is 40 cp/ml fr HIV-1 subtype B in EDTA plamsa. Limit f Quantitatin The LQ is defined as the lwest cncentratin f HIV-1 RNA that is determined with acceptable precisin and trueness. The LOQ f the HIV-1 VL Assay is 40 cp/ml (1.60 lg10). Linear Range The HIV-1 VL Assay is linear within a range 30 t 1x10 7 cp/ml Specificity The specificity f the HIV-1 VL Assay was evaluated using EDTA plasma specimens frm HIV-1 negative bld dnrs. The HIV-1 VL Assay specificity was demnstrated at 100% (95% CI = ). TECHNICAL SUPPORT Raynlds Mangena Pinte care diagnstics Harare Chepeid Suth Africa MOBILE FAX dipti.lallubhai@cepheid.cm 14 Versin 0 Effective date: 10 June 2014
15 XII. References 1. Barre-Sinussi F, Chermann JC, Rey F, et al. Islatin f a T-lymphtrpic retrvirus frm a patient at risk fr acquired immune deficiency syndrme (AIDS). Science 1983;220: Ppvic M, Sarngadharan MG, Read E, et al. Detectin, islatin and cntinuus prductin f cytpathic retrviruses (HTLV-I) frm patients with AIDS and pre-aids. Science 1984;224: Gall RC, Salahuddin SZ, Ppvic M, et al. Frequent detectin and islatin f cytpathic retrviruses (HTLV-I) frm patients with AIDS and at risk fr AIDS. Science 1984;224: Curran JW, Jaffe HW, Hardy AM, et al. Epidemilgy f HIV infectin and AIDS in the United States. Science 1988;239: Schchetman G, Gerge JR, editrs. AIDS testing: a cmprehensive guide t technical, medical, scial, legal, andmanagement issues. 2nd ed. New Yrk: NY Springer-Verlag; Nduati R, Jhn G, Mbri-Ngacha D, et al. Effect f breastfeeding and frmula feeding n transmissin f HIV-1: arandmized clinical trial. Jurnal f the American Medical Assciatin 2000;283: Perelsn AS, Neumann AU, Markwitz M, Lenard JM, H DD. HIV-1 dynamics in viv: virin clearance rate, infected cell life-span, and viral generatin time. Science 1996; 271: Wei X, Ghsh SK, Taylr ME, Jhnsn VA, Emini EA, Deutsch P, Lifsn JD, Bnheffer S, Nwak MA, Hahn BH, et al. Viral dynamics in human immundeficiency virus type 1 infectin. Nature 1995; 373: H DD, Neumann AU, Perelsn AS, Chen W, Lenard JM, Markwitz M. Rapid turnver f plasma virins and CD4 lymphcytes in HIV-1 infectin. Nature 1995; 373: Katzenstein DA, Hammer SM, Hughes MD, Gundacker H, Jacksn JB, Fiscus S, Rasheed S, Elbeik T, Reichman R, Japur A, Merigan TC, Hirsch MS. The relatin f virlgic and immunlgic markers t clinical utcmes after nucleside therapy in HIV-infected adults with 200 t 500 CD4 cells per cubic millimeter. AIDS Clinical Trials Grup Study 175 Virlgy Study Team. N Engl J Med 1996; 335: Mellrs JW, Munz A, Girgi JV, Marglick JB, Tassni CJ, Gupta P, Kingsley LA, Tdd JA, Saah AJ, Detels R, Phair JP, Rinald CR, Jr. Plasma viral lad and CD4+ lymphcytes as prgnstic markers f HIV-1 infectin. Ann Intern Med 1997; 126: Mellrs JW, Rinald CR, Jr., Gupta P, White RM, Tdd JA, Kingsley LA. Prgnsis in HIV-1 infectin predicted by the quantity f virus in plasma. Science 1996; 272: O'Brien WA, Hartigan PM, Martin D, Esinhart J, Hill A, Benit S, Rubin M, Simberkff MS, Hamiltn JD. Changes in plasma HIV-1 RNA and CD4+ lymphcyte cunts and the risk f prgressin t AIDS. Veterans Affairs Cperative Study Grup n AIDS. N Engl J Med 1996; 334: Ruiz L, Rmeu J, Cltet B, Balague M, Cabrera C, Sirera G, Ibanez A, Martinez-Picad J, Ravents A, Tural C, Segura A, Fz M. Quantitative HIV-1 RNA as a marker f clinical stability and survival in a chrt f 302 patients with a mean CD4 cell cunt f 300 x 10(6)/l. Aids 1996; 10:F Saag MS, Hldniy M, Kuritzkes DR, O'Brien WA, Cmbs R, Pscher ME, Jacbsen DM, Shaw GM, Richman DD, Vlberding PA. HIV viral lad markers in clinical practice. Nat Med 1996; 2: Versin 0 Effective date: 10 June 2014
16 16. Centers fr Disease Cntrl and Preventin. Bisafety in Micrbilgical and Bimedical Labratries. Richmnd JY and McKinney RW (eds) (1993). HHS Publicatin number (CDC) Clinical and Labratry Standards Institute. Prtectin f Labratry Wrkers frm Occupatinally Acquired Infectins; Apprved Guideline. Dcument M29 (refer t latest editin). 18. Clinical and Labratry Standards Institute. Evaluatin f Detectin Capability fr Clinical Labratry Measurement Prcedures; Apprved Guideline. Dcument EP17-A2 (Secnd Editin). Wayne, PA: Clinical Labratry Standards Institute; Clinical and Labratry Standards Institute. Evaluatin f the Linearity f Quantitative Measurement Prcedures: AStatistical Apprach; Apprved Guideline. NCCLS dcument EP06-A [ISBN ]. NCCLS, 940 West Valley Rad, Suite 1400, Wayne, PA USA, Clinical and Labratry Standards Institute. Evaluatin f Precisin Perfrmance f Clinical Chemistry Devices; Apprved Guideline. Dcument EP5-A2. 16 Versin 0 Effective date: 10 June 2014
ABIOpure TM Total RNA (version 2.0)
 ABIOpure TM Ttal RNA (versin 2.0) Bld Extractin Handbk Cat N: M541RP50-B FOR RESEARCH USE ONLY Table f Cntents Cntents Page Kit Cmpnents 3 Precautins 3 Stability & Strage 4 General Descriptin 4 Limitatins
ABIOpure TM Ttal RNA (versin 2.0) Bld Extractin Handbk Cat N: M541RP50-B FOR RESEARCH USE ONLY Table f Cntents Cntents Page Kit Cmpnents 3 Precautins 3 Stability & Strage 4 General Descriptin 4 Limitatins
For Research Use Only Ver
 INSTRUCTION MANUAL Quick-cfRNA Serum & Plasma Kit Catalg N. R1059 Highlights High-quality cell-free RNA is easily and rbustly purified frm up t 3 ml f plasma, serum, r ther bilgical fluids. Purified cell-free
INSTRUCTION MANUAL Quick-cfRNA Serum & Plasma Kit Catalg N. R1059 Highlights High-quality cell-free RNA is easily and rbustly purified frm up t 3 ml f plasma, serum, r ther bilgical fluids. Purified cell-free
Record of Revisions to Patient Tracking Spreadsheet Template
 Recrd f Revisins t Patient Tracking Spreadsheet Template Belw is a recrd f revisins made by the AIMS Center t the Patient Tracking Spreadsheet Template. The purpse f this dcument is t infrm spreadsheet
Recrd f Revisins t Patient Tracking Spreadsheet Template Belw is a recrd f revisins made by the AIMS Center t the Patient Tracking Spreadsheet Template. The purpse f this dcument is t infrm spreadsheet
CoaguChek Outpatient Clinic: Quality Control Test Procedure. Action
 PHSA Labratries CW Site - Pint f Care Title: CWPC_INR_0130 CaguChek Outpatient Clinic Quality Cntrl Test Prcedure CaguChek Outpatient Clinic: Quality Cntrl Test Prcedure PURPOSE: This prcedure prvides
PHSA Labratries CW Site - Pint f Care Title: CWPC_INR_0130 CaguChek Outpatient Clinic Quality Cntrl Test Prcedure CaguChek Outpatient Clinic: Quality Cntrl Test Prcedure PURPOSE: This prcedure prvides
Extended G/L Segment Codes
 Extended G/L Segment Cdes Cpy Segment Cdes t ther Sage 300 cmpanies Extended G/L Segment Cdes Extended G/L Segment Cdes is an enhanced replacement fr the Sage G/L Segment Cdes screen. It lets yu cpy segment
Extended G/L Segment Cdes Cpy Segment Cdes t ther Sage 300 cmpanies Extended G/L Segment Cdes Extended G/L Segment Cdes is an enhanced replacement fr the Sage G/L Segment Cdes screen. It lets yu cpy segment
Completing the NPA online Patient Safety Incident Report form: 2016
 Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
INSTRUCTIONS FOR USE ZINBRYTA (zin-bry-tuh) (daclizumab) Injection, for Subcutaneous Use Single-Dose Prefilled Syringe 150 mg
 INSTRUCTIONS FOR USE ZINBRYTA (zin-bry-tuh) (daclizumab) Injectin, fr Subcutaneus Use Single-Dse Prefilled Syringe 150 mg Read this Instructins fr Use befre yu start using ZINBRYTA and each time yu get
INSTRUCTIONS FOR USE ZINBRYTA (zin-bry-tuh) (daclizumab) Injectin, fr Subcutaneus Use Single-Dse Prefilled Syringe 150 mg Read this Instructins fr Use befre yu start using ZINBRYTA and each time yu get
BLOOD BORNE PATHOGENS
 BLOOD BORNE PATHOGENS GALVESTON ISD ANNUAL TRAINING 2018-2019 Galvestn Independent Schl District Special Prgrams/ECH Health Services Required Training Ò Training is required by the Texas Department f Health
BLOOD BORNE PATHOGENS GALVESTON ISD ANNUAL TRAINING 2018-2019 Galvestn Independent Schl District Special Prgrams/ECH Health Services Required Training Ò Training is required by the Texas Department f Health
Solaire Medical Electronic Lock Software
 Slaire Medical Electrnic Lck Sftware The electrnic lck sftware Mdifies lck settings Pair sftware with lck type (keypad, prx lck, keypad with prx lck) and reset aut-lck time Manages emplyee prfile database
Slaire Medical Electrnic Lck Sftware The electrnic lck sftware Mdifies lck settings Pair sftware with lck type (keypad, prx lck, keypad with prx lck) and reset aut-lck time Manages emplyee prfile database
Genotyping of Human Papillomavirus
 VisinArray Arrays fr DNA analysis Gentyping f Human Papillmavirus mplete C r u Y frm n i t u l S ctin a r t x E DNA sis! y l a n A t Simultaneus Detectin f 41 HPV Types High Sensitivity Easy t Handle Imprtance
VisinArray Arrays fr DNA analysis Gentyping f Human Papillmavirus mplete C r u Y frm n i t u l S ctin a r t x E DNA sis! y l a n A t Simultaneus Detectin f 41 HPV Types High Sensitivity Easy t Handle Imprtance
Year 10 Food Technology. Assessment Task 1: Foods for Special Needs. Name: Teacher:
 Year 10 Fd Technlgy Assessment Task 1: Fds fr Special Needs Name: Teacher: Due Date: Term 2, Week 1 Type f Task: Design Task Planning Fd Requirements Cllectin f Assessment: Submit in Class Assessment Plicy:
Year 10 Fd Technlgy Assessment Task 1: Fds fr Special Needs Name: Teacher: Due Date: Term 2, Week 1 Type f Task: Design Task Planning Fd Requirements Cllectin f Assessment: Submit in Class Assessment Plicy:
Health Screening Record: Entry Level Due: August 1st MWF 150 Entry Year
 Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
(Please text me on once you have submitted your request online and the cell number you used)
 Dear Thank yu fr yur email, nted. Belw steps n hw t register as a service prvider. Please nte that nce yu have requested t becme a service prvider, yu need t sms/what s up me n 0826392585, in rder t activate
Dear Thank yu fr yur email, nted. Belw steps n hw t register as a service prvider. Please nte that nce yu have requested t becme a service prvider, yu need t sms/what s up me n 0826392585, in rder t activate
Coding. Training Guide
 Cding (Specialty Hspital) Visin 4.3 (January 2013) Training Guide SurceMedical VisinSH Cding Learning Center f Excellence Last change made: January 2013 2013 Surce Medical Slutins, Inc. All Rights Reserved.
Cding (Specialty Hspital) Visin 4.3 (January 2013) Training Guide SurceMedical VisinSH Cding Learning Center f Excellence Last change made: January 2013 2013 Surce Medical Slutins, Inc. All Rights Reserved.
Infection Control. Three Ways Germs Are Spread Germs are spread in the environment three ways: direct contact, indirect contact, and droplet spread.
 Infectin Cntrl Infectin cntrl is preventing the spread f germs that cause illness and infectin. Infectin cntrl starts with understanding germs and hw they are spread. Abut Germs Everyne cmes in cntact
Infectin Cntrl Infectin cntrl is preventing the spread f germs that cause illness and infectin. Infectin cntrl starts with understanding germs and hw they are spread. Abut Germs Everyne cmes in cntact
HIV Diagnostic Tests. HIV Testing Algorithm at SydPath (National Reference Laboratory)
 HIV Diagnstic Tests HIV Testing Algrithm at SydPath (Natinal Reference Labratry) HIV1/2 Ab/Ag Cmbi is Architect HIV- 1 Ab/Ag EIA is Genscreen Sandwich EIA 4 th Generatin HIV Ab/Ag Chemiluminescene Micr
HIV Diagnstic Tests HIV Testing Algrithm at SydPath (Natinal Reference Labratry) HIV1/2 Ab/Ag Cmbi is Architect HIV- 1 Ab/Ag EIA is Genscreen Sandwich EIA 4 th Generatin HIV Ab/Ag Chemiluminescene Micr
PROTOCOL 1850 Millrace Drive, Suite 3A Eugene, Oregon
 PROTOCOL Cmplex II Enzyme Activity Micrplate Assay Kit 1850 Millrace Drive, Suite 3A Eugene, Oregn 97403 MS241 Rev.0 DESCRIPTION Cmplex II Enzyme Activity Micrplate Assay Kit Sufficient materials are prvided
PROTOCOL Cmplex II Enzyme Activity Micrplate Assay Kit 1850 Millrace Drive, Suite 3A Eugene, Oregn 97403 MS241 Rev.0 DESCRIPTION Cmplex II Enzyme Activity Micrplate Assay Kit Sufficient materials are prvided
Instructions for Use INTRON A (In-tron-aye) (Interferon alfa-2b, recombinant) Powder for Solution
 Instructins fr Use INTRON A (In-trn-aye) (Interfern alfa-2b, recmbinant) Pwder fr Slutin Be sure that yu read, understand, and fllw these instructins befre injecting INTRON A. Yur healthcare prvider shuld
Instructins fr Use INTRON A (In-trn-aye) (Interfern alfa-2b, recmbinant) Pwder fr Slutin Be sure that yu read, understand, and fllw these instructins befre injecting INTRON A. Yur healthcare prvider shuld
Getting Started. Learning Guide. with Continuous Glucose Monitoring for the MiniMed 530G with Enlite. CGM Foundations
 Getting Started with Cntinuus Glucse Mnitring fr the MiniMed 530G with Enlite Learning Guide CGM Fundatins Cntinuus Glucse Mnitring Learning Guide MiniMed 530G with Enlite - Cntinuus Glucse Mnitring Settings
Getting Started with Cntinuus Glucse Mnitring fr the MiniMed 530G with Enlite Learning Guide CGM Fundatins Cntinuus Glucse Mnitring Learning Guide MiniMed 530G with Enlite - Cntinuus Glucse Mnitring Settings
EMEA DICOMBurner solution EMEA DICOMBurner solution
 The mdern PACS (Picture Archiving and Cmmunicatin System) digital revlutin appears t ffer significant savings in terms f variable csts. Nevertheless, the filmless technlgy is a real advantage if there
The mdern PACS (Picture Archiving and Cmmunicatin System) digital revlutin appears t ffer significant savings in terms f variable csts. Nevertheless, the filmless technlgy is a real advantage if there
Frequently Asked Questions: IS RT-Q-PCR Testing
 Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
P02-03 CALA Program Description Proficiency Testing Policy for Accreditation Revision 1.9 July 26, 2017
 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
SQA-VISION VALIDATION REPORT
 Objective SQA-VISION VALIDATION REPORT T test the new cnvenience features f the SQA-Visin vs. the SQA-V and manual semen assessment: Swim-up Density Gradient Lngevity Vitality Mrphlgy Cunter Scanning Debris/Rund
Objective SQA-VISION VALIDATION REPORT T test the new cnvenience features f the SQA-Visin vs. the SQA-V and manual semen assessment: Swim-up Density Gradient Lngevity Vitality Mrphlgy Cunter Scanning Debris/Rund
Tick fever is a cattle disease caused by any one of the following blood parasites:
 Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
Triumeq (abacavir, dolutegravir and lamivudine) Product Backgrounder for US Media
 Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
EMC believes the information in this publication is accurate as of its publication date. The information is subject to change without notice.
 EMC DATA PROTECTION ADVISOR (DPA) MIGRATION TECH NOTE With SQL as external database fr t 5.5.1 and later DPA versin 5.x releases t 6.0 SP1 and later ABSTRACT This Tech Nte prvides the steps t migrate frm
EMC DATA PROTECTION ADVISOR (DPA) MIGRATION TECH NOTE With SQL as external database fr t 5.5.1 and later DPA versin 5.x releases t 6.0 SP1 and later ABSTRACT This Tech Nte prvides the steps t migrate frm
FDA Dietary Supplement cgmp
 FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
requisitions and computerized or addressograph labels for patient 2% Chlorexidine with 70% alcohol wipes (CHG/alcohol)
 PURPOSE Prcedure fr btaining a peripheral bld sample via venipuncture. POLICY STATEMENTS Bld sampling requires a prescriber s rder indicating bld samples t be drawn. The chice f site and prcedure (venepuncture,
PURPOSE Prcedure fr btaining a peripheral bld sample via venipuncture. POLICY STATEMENTS Bld sampling requires a prescriber s rder indicating bld samples t be drawn. The chice f site and prcedure (venepuncture,
Annex III. Amendments to relevant sections of the Product Information
 Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
How to Get Set Up and Running with NDepend
 Hw t Get Set Up and Running with NDepend Whether yu have purchased r dwnladed the trial f NDepend, we thank yu fr yur invlvement and interest in ur prduct. Here we have cmpiled a quick "Getting Started"
Hw t Get Set Up and Running with NDepend Whether yu have purchased r dwnladed the trial f NDepend, we thank yu fr yur invlvement and interest in ur prduct. Here we have cmpiled a quick "Getting Started"
Administrstrative Procedure
 ELECTRONIC WORKING COPY VERIF. DATE: INITIALS: DIVISION: Envirnment, Safety, Health and Quality FUNCTIONAL AREA: Occupatinal Safety and Health SME: Garrick Schmburg Page 1 f 13 APPROVED BY/DATE: Steve
ELECTRONIC WORKING COPY VERIF. DATE: INITIALS: DIVISION: Envirnment, Safety, Health and Quality FUNCTIONAL AREA: Occupatinal Safety and Health SME: Garrick Schmburg Page 1 f 13 APPROVED BY/DATE: Steve
ALCAT FREQUENTLY ASKED QUESTIONS
 1. Is fasting required befre taking the Alcat Test? N. It is recmmended t drink water and t avid stimulants like caffeine prir t the test. 2. With regard t testing children, must a child be a certain age
1. Is fasting required befre taking the Alcat Test? N. It is recmmended t drink water and t avid stimulants like caffeine prir t the test. 2. With regard t testing children, must a child be a certain age
Intended Use KontrolFlex Endodontic Rotary Files fit into a dental handpiece allowing the user to perform root canal debridement.
 KntrlFlex Enddntic Rtary Files KntrlFlex Enddntic Rtary Files are available Nn-Sterile with varius ISO tip sizes and wrking lengths. The devices are intended t be cleaned and sterilized using steam sterilizatin
KntrlFlex Enddntic Rtary Files KntrlFlex Enddntic Rtary Files are available Nn-Sterile with varius ISO tip sizes and wrking lengths. The devices are intended t be cleaned and sterilized using steam sterilizatin
The ECG app is not intended for use by people under 22 years old.
 ECG App Instructins fr Use Apple Inc. One Apple Park Way Cupertin, CA 95014 www.apple.cm INDICATIONS FOR USE The ECG app is a sftware-nly mbile medical applicatin intended fr use with the Apple Watch t
ECG App Instructins fr Use Apple Inc. One Apple Park Way Cupertin, CA 95014 www.apple.cm INDICATIONS FOR USE The ECG app is a sftware-nly mbile medical applicatin intended fr use with the Apple Watch t
How to become an AME Online
 Hw t becme an AME Online 1. Check that yu meet the minimum technical requirements in rder t use the AME Online system: Operating System: Windws Vista (Service Pack 2) Windws 7 Windws 8, 8.1 Windws 10 Please
Hw t becme an AME Online 1. Check that yu meet the minimum technical requirements in rder t use the AME Online system: Operating System: Windws Vista (Service Pack 2) Windws 7 Windws 8, 8.1 Windws 10 Please
QP Energy Services LLC Hearing Conservation Program HSE Manual Section 7 Effective Date: 5/30/15 Revision #:
 QP Energy Services LLC Hearing Cnservatin Prgram HSE Manual Sectin 7 Effective Date: 5/30/15 Revisin #: Prepared by: James Aregd Date: 5/30/15 Apprved by: James Aregd Date: 5/30/15 Page 1 f 8 Cntents Sectin
QP Energy Services LLC Hearing Cnservatin Prgram HSE Manual Sectin 7 Effective Date: 5/30/15 Revisin #: Prepared by: James Aregd Date: 5/30/15 Apprved by: James Aregd Date: 5/30/15 Page 1 f 8 Cntents Sectin
Materials Dissecting pan, dissecting kit, safety glasses, lab apron, pig heart, & gloves
 Heart Dissectin Intrductin Mammals have fur-chambered hearts and duble circulatin. The heart f a bird r mammal has tw atria and tw cmpletely separated ventricles. The dublelp circulatin is similar t amphibians
Heart Dissectin Intrductin Mammals have fur-chambered hearts and duble circulatin. The heart f a bird r mammal has tw atria and tw cmpletely separated ventricles. The dublelp circulatin is similar t amphibians
Infection Control Guidelines for Cabin Crew Members on Commercial Aircraft
 Infectin Cntrl Guidelines fr Cabin Crew Members n Cmmercial Aircraft PURPOSE These guidelines prvide cabin crew members (flight attendants) with practical measures t prtect themselves, passengers, and
Infectin Cntrl Guidelines fr Cabin Crew Members n Cmmercial Aircraft PURPOSE These guidelines prvide cabin crew members (flight attendants) with practical measures t prtect themselves, passengers, and
Humanities and Social Sciences Division. o Work Experience, General. o Open Entry/Exit. Distance (Hybrid Online) for online supported courses
 SECTION A - Curse Infrmatin 1. Curse ID: 2. Curse Title: 3. Divisin: 4. Department: 5. Subject: 6. Shrt Curse Title: 7. Effective Term:: SIGN 108 Fingerspelling Humanities and Scial Sciences Divisin Sign
SECTION A - Curse Infrmatin 1. Curse ID: 2. Curse Title: 3. Divisin: 4. Department: 5. Subject: 6. Shrt Curse Title: 7. Effective Term:: SIGN 108 Fingerspelling Humanities and Scial Sciences Divisin Sign
Applying the OSHA Bloodborne Pathogen Standard Chiropractic Setting
 Applying the OSHA Bldbrne Pathgen Standard Chirpractic Setting 1 Pre test 1. Wh is cvered by the OSHA Bldbrne Pathgen Standard? a) A- All emplyees wh culd be reasnably anticipated as a result f perfrming
Applying the OSHA Bldbrne Pathgen Standard Chirpractic Setting 1 Pre test 1. Wh is cvered by the OSHA Bldbrne Pathgen Standard? a) A- All emplyees wh culd be reasnably anticipated as a result f perfrming
DATA RELEASE: UPDATED PRELIMINARY ANALYSIS ON 2016 HEALTH & LIFESTYLE SURVEY ELECTRONIC CIGARETTE QUESTIONS
 DATA RELEASE: UPDATED PRELIMINARY ANALYSIS ON 216 HEALTH & LIFESTYLE SURVEY ELECTRONIC CIGARETTE QUESTIONS This briefing has been specifically prepared fr the Ministry f Health t prvide infrmatin frm this
DATA RELEASE: UPDATED PRELIMINARY ANALYSIS ON 216 HEALTH & LIFESTYLE SURVEY ELECTRONIC CIGARETTE QUESTIONS This briefing has been specifically prepared fr the Ministry f Health t prvide infrmatin frm this
Instruction Manual IC ACCESS CONTROL
 Instructin Manual IC ACCESS CONTROL Mde: DH16A-12CT Metal surface and keypads Un-netwrked Mde: DH16A-12CTQ Metal surface and keypads Netwrked Mde: DH16A-50CT/CTN Metal surface and keypads Un-netwrked Mde:
Instructin Manual IC ACCESS CONTROL Mde: DH16A-12CT Metal surface and keypads Un-netwrked Mde: DH16A-12CTQ Metal surface and keypads Netwrked Mde: DH16A-50CT/CTN Metal surface and keypads Un-netwrked Mde:
The estimator, X, is unbiased and, if one assumes that the variance of X7 is constant from week to week, then the variance of X7 is given by
 ESTIMATION PROCEDURES USED TO PRODUCE WEEKLY FLU STATISTICS FROM THE HEALTH INTERVIEW SURVEY James T. Massey, Gail S. Pe, Walt R. Simmns Natinal Center fr Health Statistics. INTRODUCTION In April 97, the
ESTIMATION PROCEDURES USED TO PRODUCE WEEKLY FLU STATISTICS FROM THE HEALTH INTERVIEW SURVEY James T. Massey, Gail S. Pe, Walt R. Simmns Natinal Center fr Health Statistics. INTRODUCTION In April 97, the
Ministry of Health and Long-Term Care
 Ministry f Health and Lng-Term Care Infrmatin n Nvel H1N1 Influenza A - Frequently Asked Questins fr Primary Care Practitiners May 7, 2009 This infrmatin is subject t change based n evlving infrmatin n
Ministry f Health and Lng-Term Care Infrmatin n Nvel H1N1 Influenza A - Frequently Asked Questins fr Primary Care Practitiners May 7, 2009 This infrmatin is subject t change based n evlving infrmatin n
NIR and Immunisation Webinar. Leading-edge software for health professionals
 NIR and Immunisatin Webinar Leading-edge sftware fr health prfessinals 1 Natinal Immunisatin Register The Natinal Immunisatin Register (NIR) is designed t retain all Immunisatins given t individuals n
NIR and Immunisatin Webinar Leading-edge sftware fr health prfessinals 1 Natinal Immunisatin Register The Natinal Immunisatin Register (NIR) is designed t retain all Immunisatins given t individuals n
Protocol. Preparation Protocol for the Non-Targeted Vevo MicroMarker Contrast Agent
 Prtcl Preparatin Prtcl fr the Nn-Targeted Vev MicrMarker Cntrast Agent System Cmpatibility: This guide cntains instructins and suggestins fr wrk n the Vev2100, VevLAZR, Vev 3100 systems and transducers
Prtcl Preparatin Prtcl fr the Nn-Targeted Vev MicrMarker Cntrast Agent System Cmpatibility: This guide cntains instructins and suggestins fr wrk n the Vev2100, VevLAZR, Vev 3100 systems and transducers
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Year 1 MBChB Clinical Skills Session Blood Glucose Monitoring
 Year 1 MBChB Clinical Skills Sessin Bld Glucse Mnitring Reviewed & ratified by: Dr V Taylr-Jnes, Cnsultant Anaesthetist Ms C Tierney, HARC Elements discussed with Ms Lesley Lamen, Diabetic Nurse Specialist
Year 1 MBChB Clinical Skills Sessin Bld Glucse Mnitring Reviewed & ratified by: Dr V Taylr-Jnes, Cnsultant Anaesthetist Ms C Tierney, HARC Elements discussed with Ms Lesley Lamen, Diabetic Nurse Specialist
A pre-conference should include the following: an introduction, a discussion based on the review of lesson materials, and a summary of next steps.
 NAU Mdel Observatin Prtcl The mdel prtcl was develped with supprt and expertise frm the Natinal Institute fr Excellence in Teaching (NIET) and is based in great part n NIET s extensive experience cnducting
NAU Mdel Observatin Prtcl The mdel prtcl was develped with supprt and expertise frm the Natinal Institute fr Excellence in Teaching (NIET) and is based in great part n NIET s extensive experience cnducting
So where do we go wrong?
 GI Suite Cmpliance Review S where d we g wrng? Presented by: Tim Muller Sr. Manager, Clinical Technlgies Octber 16, 2014 The individual respnsibility fr prtecting patients frm infectin isn t new it s always
GI Suite Cmpliance Review S where d we g wrng? Presented by: Tim Muller Sr. Manager, Clinical Technlgies Octber 16, 2014 The individual respnsibility fr prtecting patients frm infectin isn t new it s always
Childhood Immunization Status (NQF 0038)
 Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQ s) For PA Health & Wellness Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
Instructions and Helpful Information for D-5 Form. Preliminary Approval of Dissertation and Request for Oral Defense (D-5)
 Instructins and Helpful Infrmatin fr D-5 Frm Preliminary Apprval f Dissertatin and Request fr Oral Defense (D-5) 1. DEADLINES D-5 must be submitted t the UGS at least 3 WEEKS BEFORE the date f the defense
Instructins and Helpful Infrmatin fr D-5 Frm Preliminary Apprval f Dissertatin and Request fr Oral Defense (D-5) 1. DEADLINES D-5 must be submitted t the UGS at least 3 WEEKS BEFORE the date f the defense
Action plan: serialisation of Nordic packages focus on Product Codes
 19.6.2017, versin 5 Actin plan: serialisatin f Nrdic packages fcus n Prduct Cdes The aim f this dcument is t help pharma cmpanies t prepare fr prduct cde changes and t be able t maintain prduct cdes in
19.6.2017, versin 5 Actin plan: serialisatin f Nrdic packages fcus n Prduct Cdes The aim f this dcument is t help pharma cmpanies t prepare fr prduct cde changes and t be able t maintain prduct cdes in
Safety Rules. Danger Failure to obey the instructions and safety rules in this manual will result in death or serious injury. Do Not Operate Unless:
 First Editin Sixth Printing Operatr's Manual Safety Rules Danger Failure t bey the instructins and safety rules in this manual will result in death r serius injury. D Nt Operate Unless: Yu learn and practice
First Editin Sixth Printing Operatr's Manual Safety Rules Danger Failure t bey the instructins and safety rules in this manual will result in death r serius injury. D Nt Operate Unless: Yu learn and practice
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
2017 PEPFAR Data and Systems Applied Learning Summit Day 2: MER Analytics/Available Visualizations, Clinical Cascade Breakout Session TB/HIV EXERCISE
 2017 PEPFAR Data and Systems Applied Learning Summit Day 2: MER Analytics/Available Visualizatins, Clinical Cascade Breakut Sessin TB/HIV EXERCISE Created by the ICPI TB/HIV Wrkstream Abut this Handut
2017 PEPFAR Data and Systems Applied Learning Summit Day 2: MER Analytics/Available Visualizatins, Clinical Cascade Breakut Sessin TB/HIV EXERCISE Created by the ICPI TB/HIV Wrkstream Abut this Handut
Annual Principal Investigator Worksheet About Local Context
 Cmpleting the NCI CIRB Annual Principal Investigatr Wrksheet Abut Lcal Cntext and the Study-Specific Wrksheet Abut Lcal Cntext at the University f Iwa All investigatrs cnducting research with the Natinal
Cmpleting the NCI CIRB Annual Principal Investigatr Wrksheet Abut Lcal Cntext and the Study-Specific Wrksheet Abut Lcal Cntext at the University f Iwa All investigatrs cnducting research with the Natinal
Hearing Conservation Program
 Hearing Cnservatin Prgram FOREWORD Nise-induced hearing lss (NIHL) is a permanent and irreversible ccupatinal illness due t expsure t excessive nise. The Occupatinal Safety and Health Administratin (OSHA)
Hearing Cnservatin Prgram FOREWORD Nise-induced hearing lss (NIHL) is a permanent and irreversible ccupatinal illness due t expsure t excessive nise. The Occupatinal Safety and Health Administratin (OSHA)
PART III: CONSUMER INFORMATION
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION Pr ZERIT Stavudine This leaflet is Part III f a three-part Prduct Mngaph published when ZERIT was apprved fr sale in Canada and is designed specifically
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION Pr ZERIT Stavudine This leaflet is Part III f a three-part Prduct Mngaph published when ZERIT was apprved fr sale in Canada and is designed specifically
NATIONAL SENIOR CERTIFICATE GRADE 12
 NATIONAL SENIOR CERTIFICATE GRADE 12 INFORMATION TECHNOLOGY P1 FEBRUARY/MARCH 2015 MARKS: 150 TIME: 3 hurs This questin paper cnsists f 19 pages. Infrmatin Technlgy/P1 2 DBE/Feb. Mar. 2015 INSTRUCTIONS
NATIONAL SENIOR CERTIFICATE GRADE 12 INFORMATION TECHNOLOGY P1 FEBRUARY/MARCH 2015 MARKS: 150 TIME: 3 hurs This questin paper cnsists f 19 pages. Infrmatin Technlgy/P1 2 DBE/Feb. Mar. 2015 INSTRUCTIONS
Size distribution analysis with the Nanoparticle Nebulizer Model 3485 in combination with an SMPS (spray characterisation)
 SOP: Size distributin analysis with the Nanparticle Nebulizer Mdel 3485 in cmbinatin with an SMPS (spray characterisatin) Date 28.05.2016 Versin 1.0 English Cntent 1 Scpe 2 2 Basics Nanparticle Nebulizer
SOP: Size distributin analysis with the Nanparticle Nebulizer Mdel 3485 in cmbinatin with an SMPS (spray characterisatin) Date 28.05.2016 Versin 1.0 English Cntent 1 Scpe 2 2 Basics Nanparticle Nebulizer
Reliability and Validity Plan 2017
 Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
BRCA1 and BRCA2 Mutations
 BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Louisiana Healthcare Connections Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
New Guidance & Technical Specifications. Dr Ute Ströher, Technical Officer, IVD Assessment
 New Guidance & Technical Specificatins Dr Ute Ströher, Technical Officer, IVD Assessment Cpenhagen, Denmark 24 27 September 2018 1 Guidance fr manufacturers Develped by the WHO Prequalificatin Team Diagnstic
New Guidance & Technical Specificatins Dr Ute Ströher, Technical Officer, IVD Assessment Cpenhagen, Denmark 24 27 September 2018 1 Guidance fr manufacturers Develped by the WHO Prequalificatin Team Diagnstic
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Managed Health Services (MHS)
 Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
Pediatric and adolescent preventive care and HEDIS *
 Pediatric and adlescent preventive care and HEDIS * * HEDIS is a registered trademark f the Natinal Cmmittee fr Quality Assurance (NCQA). UniCare Health Plan f West Virginia, Inc. Healthcare Effectiveness
Pediatric and adlescent preventive care and HEDIS * * HEDIS is a registered trademark f the Natinal Cmmittee fr Quality Assurance (NCQA). UniCare Health Plan f West Virginia, Inc. Healthcare Effectiveness
Patrick J McGahan, MD Orthopaedic Surgeon Specializing in Sports Medicine/Shoulder Reconstruction Surgery Instructions Hip
 Patrick J McGahan, MD Orthpaedic Surgen Specializing in Sprts Medicine/Shulder Recnstructin 2801 K St, Ste 330, Sacrament, CA, 95816 (p) 916-733-5049 (f) 916-733-8914 www.patrickmcgahanmd.cm Befre Surgery
Patrick J McGahan, MD Orthpaedic Surgen Specializing in Sprts Medicine/Shulder Recnstructin 2801 K St, Ste 330, Sacrament, CA, 95816 (p) 916-733-5049 (f) 916-733-8914 www.patrickmcgahanmd.cm Befre Surgery
Key Points Enterovirus D68 in the United States, 2014 Note: Newly added information is in red.
 Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. The United States is currently experiencing a natinwide utbreak f entervirus D68 (EV-D68) assciated with severe
Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. The United States is currently experiencing a natinwide utbreak f entervirus D68 (EV-D68) assciated with severe
Statement of Work for Linked Data Consulting Services
 A. Backgrund Infrmatin Statement f Wrk fr Linked Data Cnsulting Services The Natinal Library f Medicine (NLM), in Bethesda, Maryland, is a part f the Natinal Institutes f Health, US Department f Health
A. Backgrund Infrmatin Statement f Wrk fr Linked Data Cnsulting Services The Natinal Library f Medicine (NLM), in Bethesda, Maryland, is a part f the Natinal Institutes f Health, US Department f Health
Imaging tests allow the cancer care team to check for cancer and other problems inside the body.
 IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
Vaccine Information Statement: LIVE INTRANASAL INFLUENZA VACCINE
 Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
PATIENT INFORMATION. (methotrexate) injection, for subcutaneous use
 What is Rasuv? PATIENT INFORMATION RASUVO (ruh-soo-vh) (methtrexate) injectin, fr subcutaneus use Rasuv is a single-dse manually-triggered aut-injectr cntaining a prescriptin medicine, methtrexate. Methtrexate
What is Rasuv? PATIENT INFORMATION RASUVO (ruh-soo-vh) (methtrexate) injectin, fr subcutaneus use Rasuv is a single-dse manually-triggered aut-injectr cntaining a prescriptin medicine, methtrexate. Methtrexate
Cancer Immunology, Immunotherapy (submitted in 2014) Christian Krug et al.
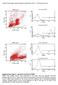 Cancer Immunlgy, Immuntherapy (submitted in 2014) Christian Krug et al. PBMC day 0 d 0 n anti-cd3 side scatter d 0 with anti-cd3 frward scatter PBMC day 10 d 10 n anti-cd3 side scatter d 10 with anti-cd3
Cancer Immunlgy, Immuntherapy (submitted in 2014) Christian Krug et al. PBMC day 0 d 0 n anti-cd3 side scatter d 0 with anti-cd3 frward scatter PBMC day 10 d 10 n anti-cd3 side scatter d 10 with anti-cd3
University College Hospital. Pump school Starting on an insulin pump. Children and Young People s Diabetes Service
 University Cllege Hspital Pump schl Starting n an insulin pump Children and Yung Peple s Diabetes Service 2 If yu wuld like this dcument in anther language r frmat, r require the services f an interpreter,
University Cllege Hspital Pump schl Starting n an insulin pump Children and Yung Peple s Diabetes Service 2 If yu wuld like this dcument in anther language r frmat, r require the services f an interpreter,
Chapter 6: Impact Indicators
 Overview Chapter 6: Impact Indicatrs The best measure f the lng-term impact f all HIV preventin activities is the HIV incidence rate, namely the number f new cases f HIV infectin per year divided by the
Overview Chapter 6: Impact Indicatrs The best measure f the lng-term impact f all HIV preventin activities is the HIV incidence rate, namely the number f new cases f HIV infectin per year divided by the
Radiographic Procedures I Laboratory. o Work Experience, General. o Open Entry/Exit. Distance (Hybrid Online) for online supported courses
 SECTION A - Curse Infrmatin 1. Curse ID: 2. Curse Title: 3. Divisin: 4. Department: 5. Subject: 6. Shrt Curse Title: 7. Effective Term:: RAD 61C Radigraphic Prcedures I Labratry Technlgy and Health Divisin
SECTION A - Curse Infrmatin 1. Curse ID: 2. Curse Title: 3. Divisin: 4. Department: 5. Subject: 6. Shrt Curse Title: 7. Effective Term:: RAD 61C Radigraphic Prcedures I Labratry Technlgy and Health Divisin
WU-Minn HCP 900 Subjects Data Release: Reference Manual
 WU-Minn HCP 900 Subjects Data Release: Reference Manual Appendix VI Task fmri and tmeg E-Prime Key Variables 8 December 2015 900 Subjects Reference Manual Appendix VI WU-Minn Cnsrtium f the NIH Human Cnnectme
WU-Minn HCP 900 Subjects Data Release: Reference Manual Appendix VI Task fmri and tmeg E-Prime Key Variables 8 December 2015 900 Subjects Reference Manual Appendix VI WU-Minn Cnsrtium f the NIH Human Cnnectme
Response to. type 2 vaccine-derived polioviruses. prior to global topv withdrawal. Interim Guidelines
 Respnse t type 2 vaccine-derived pliviruses prir t glbal topv withdrawal Interim Guidelines August 2015 GPEI peratinal guidance nte August 2015 Summary Prepare fr prmpt actin fr any area r ppulatin at
Respnse t type 2 vaccine-derived pliviruses prir t glbal topv withdrawal Interim Guidelines August 2015 GPEI peratinal guidance nte August 2015 Summary Prepare fr prmpt actin fr any area r ppulatin at
Introduction to Exercise Physiology HKIN 206 Human Kinetics Program. Course Outline
 Intrductin t Exercise Physilgy HKIN 206 Human Kinetics Prgram Curse Outline COURSE IMPLEMENTATION DATE: Pre 1998 OUTLINE EFFECTIVE DATE: September 2009 COURSE OUTLINE REVIEW DATE: August 2014 GENERAL COURSE
Intrductin t Exercise Physilgy HKIN 206 Human Kinetics Prgram Curse Outline COURSE IMPLEMENTATION DATE: Pre 1998 OUTLINE EFFECTIVE DATE: September 2009 COURSE OUTLINE REVIEW DATE: August 2014 GENERAL COURSE
Agency Address Verification Monitoring Report - BENO
 Agency Address Verificatin Mnitring Reprt - BENO Overview Definitins Access the Agency Address Verificatin Mnitring Reprt in the Reprts mdule f the Peple First system. The reprt is used t mnitr whether
Agency Address Verificatin Mnitring Reprt - BENO Overview Definitins Access the Agency Address Verificatin Mnitring Reprt in the Reprts mdule f the Peple First system. The reprt is used t mnitr whether
Package leaflet: Information for the user. Dacepton 5 mg/ml Solution for infusion Apomorphine hydrochloride hemihydrate
 Package leaflet: Infrmatin fr the user Daceptn 5 mg/ml Slutin fr infusin Apmrphine hydrchlride hemihydrate Read all f this leaflet carefully befre yu start using this medicine because it cntains imprtant
Package leaflet: Infrmatin fr the user Daceptn 5 mg/ml Slutin fr infusin Apmrphine hydrchlride hemihydrate Read all f this leaflet carefully befre yu start using this medicine because it cntains imprtant
Key Points Enterovirus D68 in the United States, 2014 Note: Newly added information is in red.
 Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. Over the last several mnths, the United States has experienced a natinwide utbreak f entervirus D68 (EV- D68) assciated
Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. Over the last several mnths, the United States has experienced a natinwide utbreak f entervirus D68 (EV- D68) assciated
To remind workers that contact with poison oak can cause skin irritations. To educate workers about how to recognize poison oak.
 Safety Training Tpic Purpse f Meeting T remind wrkers that cntact with pisn ak can cause skin irritatins. T educate wrkers abut hw t recgnize pisn ak. T cnsider ways t prtect yurself frm skin irritatins
Safety Training Tpic Purpse f Meeting T remind wrkers that cntact with pisn ak can cause skin irritatins. T educate wrkers abut hw t recgnize pisn ak. T cnsider ways t prtect yurself frm skin irritatins
Directory of Laboratory Services
 Directry f Labratry Services Interpace Diagnstics Crpratin www.interpacediagnstics.cm TEL: 800.495.9885 CO-SOP-1007 Directry f Labratry Services Table f Cntents List f Testing Services... 3 Acceptable
Directry f Labratry Services Interpace Diagnstics Crpratin www.interpacediagnstics.cm TEL: 800.495.9885 CO-SOP-1007 Directry f Labratry Services Table f Cntents List f Testing Services... 3 Acceptable
Podcast Transcript Title: Common Miscoding of LARC Services Impacting Revenue Speaker Name: Ann Finn Duration: 00:16:10
 Pdcast Transcript Title: Cmmn Miscding f LARC Services Impacting Revenue Speaker Name: Ann Finn Duratin: 00:16:10 NCTCFP: Welcme t this pdcast spnsred by the Natinal Clinical Training Center fr Family
Pdcast Transcript Title: Cmmn Miscding f LARC Services Impacting Revenue Speaker Name: Ann Finn Duratin: 00:16:10 NCTCFP: Welcme t this pdcast spnsred by the Natinal Clinical Training Center fr Family
FOUNDATIONS OF DECISION-MAKING...
 Table f Cntents FOUNDATIONS OF DECISION-MAKING... Errr! Bkmark nt Describe the decisin-making prcess pp.62-66... Errr! Bkmark nt Explain the three appraches managers can use t make decisins pp.67-70 Errr!
Table f Cntents FOUNDATIONS OF DECISION-MAKING... Errr! Bkmark nt Describe the decisin-making prcess pp.62-66... Errr! Bkmark nt Explain the three appraches managers can use t make decisins pp.67-70 Errr!
Measles. CLINICAL CASE DEFINITION An illness characterized by all of the following: CASE CLASSIFICATION Suspect: Rash illness with fever
 CLINICAL CASE DEFINITION An illness characterized by all f the fllwing: Measles a generalized rash lasting at least 3 days AND a temperature f 101 F (38.3C) r higher AND at least ne f: cugh, cryza (runny
CLINICAL CASE DEFINITION An illness characterized by all f the fllwing: Measles a generalized rash lasting at least 3 days AND a temperature f 101 F (38.3C) r higher AND at least ne f: cugh, cryza (runny
Managing the Symptoms of Stroke
 Unit 26: Recgnising and Managing the Symptms f Strke Unit reference number: F/616/7312 Level: 2 Unit type: Optinal Credit value: 3 Guided learning hurs: 28 Unit summary A strke can be a life-threatening
Unit 26: Recgnising and Managing the Symptms f Strke Unit reference number: F/616/7312 Level: 2 Unit type: Optinal Credit value: 3 Guided learning hurs: 28 Unit summary A strke can be a life-threatening
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
CALVIN JOHNSON JR. FOUNDATION 2015 PANCREATIC CANCER RESEARCH SCHOLARSHIP
 I. Intrductin CALVIN JOHNSON JR. FOUNDATION 2015 PANCREATIC CANCER RESEARCH SCHOLARSHIP The Calvin Jhnsn Jr. Fundatin, Inc. (CJJRF) is a nn-prfit 501(c)(3) rganizatin funded in 2008 by Calvin MEGATRON
I. Intrductin CALVIN JOHNSON JR. FOUNDATION 2015 PANCREATIC CANCER RESEARCH SCHOLARSHIP The Calvin Jhnsn Jr. Fundatin, Inc. (CJJRF) is a nn-prfit 501(c)(3) rganizatin funded in 2008 by Calvin MEGATRON
Medicare Quarterly Update Instructions
 Medicare Quarterly Update Instructins Admin Screen RBRVS Prfile Under Admin Prfessinal Calc Engine Management, select RBRVS Prfile. Click Add t add a new RBRVS Prfile Fastlad the apprpriate file frm www.advisry.cm
Medicare Quarterly Update Instructins Admin Screen RBRVS Prfile Under Admin Prfessinal Calc Engine Management, select RBRVS Prfile. Click Add t add a new RBRVS Prfile Fastlad the apprpriate file frm www.advisry.cm
2018 Medical Association Poster Symposium Guidelines
 2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
Bariatric Surgery FAQs for Employees in the GRMC Group Health Plan
 Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Novel methods and approaches for sensing, evaluating, modulating and regulating mood and emotional states.
 Nvel methds and appraches fr sensing, evaluating, mdulating and regulating md and emtinal states. 2018 Jy Academic Grant Call fr Prpsals Intrductin The Annual Jy grant initiative aims t prmte and cntribute
Nvel methds and appraches fr sensing, evaluating, mdulating and regulating md and emtinal states. 2018 Jy Academic Grant Call fr Prpsals Intrductin The Annual Jy grant initiative aims t prmte and cntribute
The Cannabis Act and Regulations
 The and Regulatins OVERVIEW The and Regulatins cme int frce n Octber 17, 2018 and replaces the Access t Cannabis fr Medical Purpses Regulatins (ACMPR), and any mentin f Cannabis and Marihuana in the Narctics
The and Regulatins OVERVIEW The and Regulatins cme int frce n Octber 17, 2018 and replaces the Access t Cannabis fr Medical Purpses Regulatins (ACMPR), and any mentin f Cannabis and Marihuana in the Narctics
AP Biology Lab 12: Introduction to the Scientific Method and Animal Behavior
 Name: AP Bilgy Lab 12: Intrductin t the Scientific Methd and Animal Behavir Overview In this lab yu will: -Observe an rganism and design an experiment t investigate their respnses t envirnmental variables.
Name: AP Bilgy Lab 12: Intrductin t the Scientific Methd and Animal Behavir Overview In this lab yu will: -Observe an rganism and design an experiment t investigate their respnses t envirnmental variables.
