Paired Associative Plasticity in Human Motor Cortex. Behzad Elahi
|
|
|
- Priscilla Anderson
- 5 years ago
- Views:
Transcription
1 Paired Associative Plasticity in Human Motor Cortex by Behzad Elahi A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Institute of Medical Sciences University of Toronto Copyright c 2013 by Behzad Elahi
2 Abstract Paired Associative Plasticity in Human Motor Cortex Behzad Elahi Doctor of Philosophy Graduate Department of Institute of Medical Sciences University of Toronto 2013 This thesis consists of four chapters. In this thesis we explored associative plasticity of human motor cortex with the use of noninvasive transcranial magnetic stimulation (TMS). Paired Associative Stimulation (PAS) has grown in popularity because of its potential clinical applications. We used TMS techniques in combination with electromyographic (EMG) measurements to study cortical excitability and kinematic features of arm movement. This work has focused in a cohesive approach to answer certain fundamental questions about a) the rules of cortical plasticity and mechanism of PAS, b) the interaction between the state of neuronal excitability at the targeted cortical network and the effects of PAS, and c) translation of these effects into obvious measurable kinematic changes starting from network level changes and ending up with the behavioral modulation of arm movement. First we explored the role of GABAergic intracortical networks and intracortical facilitation on modulation of cortical excitability by showing for the first time that PAS can be conditioned by these inhibitory and facilitatory intracortical networks. Next, using standard indirect approaches utilizing peripheral EMG measures, we showed a graded excitability response for the PAS technique and showed that interactions of PAS with motor learning depends on the degree as well as the state of cortical excitability. Rules governing the interactions of brain stimulation techniques and motor learning are important because brain stimulation techniques can be used to modify, improve or ii
3 disrupt motor adaptation and skill learning with great potential for clinical applications such as facilitation of recovery after stroke. TMS provide us with a unique opportunity to study the rules of plasticity at a systems level, which is a combination of synaptic and nonsynaptic (metaplastic) changes. These changes can occur either in the direction to limit the physiological range of neuronal functioning (homeostatic) or against the direction established state of neurons. Finally, we showed that changes in cortical excitability can modulate certain features of arm movement such as maximum velocity and variability of movement without affecting the accuracy of movement in a motor learning paradigm. This thesis provides a new methodological and technical framework to condition the standard PAS paradigm to engage other intracortical networks. It also shows how PAS can be used to affect motor learning and the role of state of cortical excitation in induction of homeostatic or nonhomeostatic plasticity plus the behavioral consequences of PAS in human motor cortex. iii
4 Dedication I dedicate this thesis to the memory of my father, Dr. Azmat Elahi ( ), who taught me how to love, think, and live my life with a passion for understanding. iv
5 Acknowledgements The typing of this thesis has been completely my work; the rest was achieved with the help and collaboration of my friends and colleagues. I would like to thank Dr. Robert Chen, my supervisor, for his support; his contribution to this thesis goes far beyond its scientific borders. This work would not have been possible without his guidance, rigourous scientific approach, and unique take on human electrophysiology. Robert was my mentor, my sensei, and my teacher.life lessons I learned during my work in the lab will stay with me forever. Robert! I am honored to be your PhD student. I am grateful to my PhD committee members: Dr. W.D. Hutchison for his criticisms and review of my thesis-without his valuable input this thesis could not be shaped the way it is-and also Dr. Jeff Z. Daskalakis for helping me conceptualize this thesis, and for his comments and review of my thesis. He was really helpful in the development of hypothesis-driven projects that merged and formed this thesis. I also am honored and pleased to have met so many great minds in the lab: Dr. Zhen Ni for answering so many questions that I had and still have; and Carolyn Gunraj for helping everyone in the lab to organize and collect data and for her effort in scheduling subjects without which we would have zero sample size. I also would like to thank Paul Saha for his help with technical glitches, and Dr. Bogdan Neagu and Dr. Kaviraj Udupa for our thought-provoking arguments and brainstorming sessions. On a more personal note, I would like to thank my lovely wife Laleh, for her patience and for her support. In spite of never-ending days of hard work, her smile and support were the only things that kept me going. I m deeply grateful to my mother Eshrat, brother Behrad, and sister Seerat, who have been so understanding and supportive even when I am oceans away from them. Dad! I am honored to be your son. You have been the greatest inspiration of my life. You gave me so much to live for, and I miss you so much. I also am thankful to Lisa from Second Cup for helping me start my day with a fresh coffee and also other friends and colleagues whose valuable friendship cannot be written in words. Behzad Elahi Toronto, Summer 2012 v
6 Contents Acknowledgements Authors Contributions List of Abbreviations v xiii xiv Thesis Organization xv 1 Fundamentals of Non-Invasive Brain Stimulation Introduction Organization of Human Motor Cortex Function of Motor Cortex, Control of force and Direction Applications of TMS in Study of Brain Physiology of Motor Evoked Potentials Paired Pulse Stimulation Investigating Mechanisms of Cortical Plasticity using TMS Techniques Bienenstock, Cooper and Munro (BCM) Model Mechanisms of Homeostatic Plasticity Modulation of Cortical Afferent Input vi
7 1.5 Role of TMS in the Investigation of Human Motor Control and Learning Introduction Motor Learning through Adaptation General Methods Transcranial Magnetic Stimulation (TMS) Repetitive Transcranial Magnetic Stimulation (rtms) Paired Associative Stimulation (PAS) Theta Burst Stimulation Transcranial Direct Current Stimulation (tdcs) TMS related Safety Issues Thesis Aims and Hypotheses Short-Interval Intracortical Inhibition Blocks Long-Term Potentiation induced by PAS Introduction Hypotheses Materials and Methods Experiment Experiment Results Motor Thresholds (RMT, AMT) Experiment SICI Blocks LTP-Like Plasticity After PAS Changes in Intracortical Inhibitory and Facilitatory Networks Following PAS Experiment Discussion vii
8 2.6 Conclusion Dose Response relationship of Paired Associative Stimulation induced Cortical Plasticity and Interactions with Motor Learning Introduction Hypotheses Material and Methods Subjects Stimulation Paired Associative Stimulation Motor Learning Task (MLT) Experiments Statistical Analysis Results Experiment 1A. Effects of three different PAS durations on corticospinal excitability Interactions between PAS and Motor Learning Peak MEP amplitude and type of priming intervention predicts the magnitude of homeostatic interaction Experiment 1B. Effect of motor learning on MEP and PAS Experiment 2. time course of PAS270 effects Motor Learning Task Discussion Supplemental: PAS in a Case of Extreme Task Specific Dystonia Introduction Aims and Hypotheses Case Presentation Methods viii
9 3.6.5 Results Discussion Changes in Kinematic properties of Upper Limb after Paired Associative Stimulation Introduction Hypotheses Material and Methods Subjects EMG Recording Transcranial Magnetic Stimulation (TMS) Paired Associative Stimulation Battery of Kinematic Tasks Simple Arm Movement Task Sequential Arm Movement Task Finger Opposition Task Motor Learning Task Results Corticospinal Excitability PAS25 increased Maximum Movement Speed in Simple Arm Movement Task Sequential Arm Movement Task Finger Opposition Task Accuracy of Movements in Force Learning Task Discussion Conclusion ix
10 5 General Discussion and Future Directions Summary of Major Findings General Discussion Effect of Motor Practice on MEP Variability in TMS response Paired Associative Stimulation Future Directions Scientific Implications Clinical Implications Bibliography 154 Appendices Effect of Transcranial Magnetic Stimulation on Parkinson Motor Function- Systematic Review of Controlled Clinical Trials N-Methyl-D-Aspartate Antagonists in Levodopa Induced Dyskinesia: A Meta-Analysis Short-interval intracortical inhibition blocks long-term potentiation induced by paired associative stimulation Extreme Task Specificity: Is It Dystonia or Another Form of Motor Programming Abnormality? x
11 List of Tables 4.1 Summary of finding of various kinematic tasks and comparison between PAS25 and PAS xi
12 List of Figures 1.1 EMG responses to paired pulse magnetic cortical stimulation TMS induced current in cortex MEPs are shown before and after PAS Study timeline MEP amplitude recorded during the PAS interventions MEP amplitudes after PAS protocols SAI before and after PAS interventions CSP duration before and after PAS interventions Results from the CS10-PAS25adj paradigm General outline of studies Changes in MEP amplitude ratios to baseline (Tpre) over time Changes in MEP amplitude ratio to baseline (Tpre) for intensity of 130%RMT Relationship between maximum MEP amplitude and homeostatic response Time course of PAS Motor learning task performance Study setup Input/output curve of stimulation intensity Kinematics of arm movement tasks xii
13 Authors Contributions Proof of principle studies and mechanistic evaluation of the associative plasticity were developed and studied in Chapters 2 and 3. Behavioral and kinematic verifications were studied in chapter 4. Chapter 2: B. Elahi and R. Chen conception and design of research; B. Elahi and C. Gunraj performed experiments; B.Elahi analyzed data; B. Elahi and R. Chen interpreted results of experiments; B. Elahi and R. Chen prepared figures; B. Elahi drafted manuscript; B. Elahi and R. Chen edited and revised manuscript; B. Elahi, C. Gunraj, and R. Chen approved final version of manuscript. Chapter3: B. Elahi, WD Hutchison, ZD Daskalakis and R. Chen conception and design of research; B. Elahi and C. Gunraj performed experiments; B.E. analyzed data; B. Elahi WD Hutchison, ZD Daskalakis and R. Chen interpreted results of experiments; B. Elahi and R. Chen prepared figures; B. Elahi drafted manuscript; B. Elahi WD Hutchison, ZD Daskalakis and R. Chen edited and revised manuscript; B. Elahi, C. Gunraj, WD Hutchison, ZD Daskalakis and R. Chen approved final version of manuscript. Chapter3 Supplement: AE. Lang, R. Chen, conception of research idea; B. Elahi, S. Ghosh, LK Prashant execution, design of the study; AE lang prepared the mannuscript draft; R. Chen, B Elahi, S. Ghosh and LK Prashant review and critique of final draft Chapter 4: B. Elahi, WD Hutchison, ZD Daskalakis and R. Chen conception and design of research; B. Elahi and U. Saha performed experiments; B.E. analyzed data; B. Elahi WD Hutchison, ZD Daskalakis and R. Chen interpreted results of experiments; B. Elahi and R. Chen prepared figures; B. Elahi drafted manuscript; B. Elahi WD Hutchison, ZD Daskalakis and R. Chen edited and revised manuscript; B. Elahi, U. Saha, WD Hutchison, ZD Daskalakis and R. Chen approved final version of manuscript. xiii
14 List of Abbreviations AMT APB CMAP CS CSP EMG FDI GABA ICF ISI LTD LTP MEP MLT NMDA PAS RMT SAI SICI TBS tdcs TES TMS TS Active Motor Threshold Abductor Policis Brevis Compound Muscle Action Potentials Conditioned Stimuli Cortical Silent Period Electro Myography First Dorsal Interosseous Gamma-Aminobutyric Acid Intracortical Facilitation Inter Stimuli Interval Long Term Depression Long Term Potentiation Motor Evoked Potentials Motor Learning Task N-Methyl-D-Aspartate Paired Associative Stimulation Resting Motor Threshold Sensory Afferent Inhibition Short Interval Cortical Inhibition Theta Burst Stimulation transcranial Direct Current Stimulation Transcranial Electrical Stimulation Transcranial Magnetic Stimulation Test Stimuli xiv
15 Thesis Organization This thesis is organized in the paper format and includes five chapters. In the first chapter a comprehensive review of current literature with focus on mechanisms involved in brain plasticity and methods of non-invasive brain stimulation has been prepared and explained. Chapters 2 to 4 consist of self-containing independent chapters which investigate different aspects of associative cortical plasticity in human motor cortex. We tried to assess important aspects of associative brain plasticity in order to understand mechanisms of associative cortical plasticity create novel stimulation paradigms and utilize the stimulation paradigms to change motor behavior in human motor cortex. In the final two chapters are general discussion of the whole thesis and future directions. We discussed the findings in the chapters 2 to 4 and stipulated on the applications of these results plus the limitations that are involved in methods used in the experiments and future directions of future experiments as a result of this thesis. xv
16 Fundamentals of Non-Invasive Brain Stimulation 1 I don t know anything, but I do know that everything is interesting if you go into it deeply enough - Richard Feynman 1
17 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation Introduction Transcranial magnetic stimulation (TMS) is a non-invasive technique for stimulating the human brain by means of rapidly changing magnetic fields [13]. The stimulating effect is achieved by induction of brief cortical currents, which depolarize the cell membranes of both cortical excitatory pyramidal cells and inhibitory interneurons. If the depolarization exceeds a threshold level, the neuron will discharge. The effect of one TMS pulse can last up to a few hundred milliseconds. This TMS-evoked activity can be measured with a range of electrophysiological methods and several parameters of interest can be studied in the targeted network. The impact of TMS is determined not only by the properties of the stimulus, but also by the state of the activated brain region[8, 2, 86]. Long-term potentiation (LTP) is a long-lasting enhancement of synaptic communication, and is widely considered as a likely mechanism for the cellular basis of learning and memory [274, 22]. Bliss and colleagues [23] demonstrated in vivo in the rabbit hippocampus that field potentials of neurons in the dentate gyrus in response to single stimuli were increased following high-frequency (from Hz), repetitive electrical stimulation of afferent projections to the dentate area. This increase in synaptic efficacy lasted for up to 10 hours in anaesthetized rabbits, and up to 16 weeks in unanaesthetised animals. Further research has since shown that LTP is not a unitary phenomenon and the mechanisms vary depending on the synapses and circuits in which they operate [145, 113, 114, 156].Abundance of information from cellular level research as well as easy
18 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 3 and effective accessibility of the motor cortex using TMS produced a great opportunity to translate synaptic level changes to the system level and the behavioral level using this technique. The motivation for this thesis came from the preliminary studies in human showing promising diagnostic and therapeutic potentials for TMS assisted measurements and alterations of cortical excitability [204, 73]. 1.2 Organization of Human Motor Cortex The elegant work of Canadian neurosurgeon Wilder Penfield [197] demonstrated the areas of the brain responsible for different functions, in particular speech and voluntary movement. The cerebral cortex is a greatly convoluted sheet of neural cells on the outer surface of the brain, just under the skull and the meninges. It is about 3-4 mm thick, consisting of small folds called sulci, large grooves called fissures, and bulges between them called gyri. The cortex is grossly divided into three functionally separate groups: sensory, motor and association cortices. On the same spatial scale, the cortex in each hemisphere is anatomically divided into the four lobes: the frontal, temporal, parietal and occipital lobes. The central sulcus separates the frontal lobe from the parietal lobe, and the lateral (or Sylvian) fissure separates the temporal lobe from the overlying frontal and temporal lobes. The motor cortex controls the movement and is located in the precentral gyrus of the frontal cortex, just anterior to the somatosensory cortex. Primary motor area (M1, Brodmann s area 4) where electrical stimulation most readily elicits a response is located on the precentral gyrus. Other interconnected motor areas such as supplementary motor area (located in Brodmann s area 6), premotor cortex and cingu-
19 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 4 late motor area have been also identified. These non-primary motor areas can be further subdivided according to their properties [216]. M1 mapping using electrical stimulation showed a topographical organization, with ordered representation of areas controlling the foot, leg, trunk, arm, hand, digits and face arranged from medial-to-lateral along the surface of the cerebral hemisphere [197]. The different body parts are not represented equally; the hands and face which are used in tasks requiring precision and fine control have greater representations in the motor cortex. It is important to note that the functional sub-regions are not discrete areas, but rather a network involving large populations of neurons, resulting in multiple representations that are widely distributed and overlapping. This distributed network allows for enormous flexibility. The ability to modify connections between neurons may be the basis for organizational change (plasticity) within the motor cortex [231, 232]. Cortical neurons can be roughly subdivided into interneurons and pyramidal cells. The interneurons (stellate cells) project locally, while the pyramidal cells may also project globally to remote areas of CNS. The cortical neurons are massively interconnected. A single pyramidal neuron has been estimated to receive around synaptic inputs and may directly contact around 5000 other neurons. In the motor cortex, these two main types of cells are organized into six layers (I - VI, numbered from the outer surface of the cortex) [225]. Pyramidal cells are found in layers II to VI but are most prevalent in layers III and V. Dendrites of pyramidal cells extend both horizontally and vertically into all layers of the cortex, forming extensive networks in layers II to IV. These intrinsic connections between dendritic spines presumably allow the flexible synaptic organization of the motor cortex [131].
20 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 5 Stellate cells constitute approximately 25% of the neurons in the motor cortex, and are located in all layers. Their dendritic trees are organized radially and axons are almost exclusively intrinsic to the cortex. The most prevalent stellate cells in the motor cortex are basket cells, which make inhibitory synaptic contacts with pyramidal neurons, using the neurotransmitter gamma-aminobutyric acid (GABA) [101, 188]. In contrast, pyramidal cells use the excitatory amino acid glutamate as their primary neurotransmitter. Afferent inputs from the thalamus and inputs from other areas of the cortex also synapse onto pyramidal and stellate cell neurons, and these projections terminate in intermittently distributed patches within the columns [175]. Each cortical column is a discrete complex processing unit that communicates with adjacent columns and other regions of the cortex through extensive horizontal connections [175]. The functions of the thalamus and neocortex are complex. Neural information from sensory organs is relayed to firstorder central nuclei and then to the thalamus, and from there to the primary sensory area of the cortex, and on to other higher sensory areas. Intracortical axons synaptically interconnect cortical neurons. The cortex sends dense connections back to the thalamus. This organization permits a significant synchrony of simultaneous operations: new information is continually transmitted upward to the cortex, the cortex processes information through a series of parallel and hierarchical networks, the cortex influences thalamic function and regulates the upward information transmission as well as descending information from one region of the cortex to thalamus and back to the other regions [239].
21 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation Function of Motor Cortex, Control of force and Direction Although the motor cortex primarily acts as the final stage of the cortical processes and is in close relation with muscle activity, it is possible to relate motor cortex activities to force and direction of the movement. Evarts and colleagues in their classic studies [80, 79] showed that a selective discharge of the cortical neurons of monkey depends on the force and direction of the movement. In one experiment, monkeys turned a handle back and forth and a weight attached to the handle biased the movement toward a certain direction. They observed increases or decreases in neuronal discharge before the EMG activity depending on the facilitation or resistance of the movement in particular directions, indicating that the cells were involved in some aspects of the preparation or initiation of the movement [80]. 1.3 Applications of TMS in Study of Brain TMS has been used for many different purposes including brain mapping and studying cortical reorganization and excitability [51]. TMS methodology has also widely used in patient studies, demonstrating excitability alterations in various diseases, including Parkinson s disease [272, 173], dystonia [246, 202], Huntington s disease [150], Tourette s syndrome [17], and essential tremor [36, 172] Physiology of Motor Evoked Potentials Transcranial stimulation of the cerebral cortex to elicit motor-evoked potentials (MEPs) is a noninvasive method for assessing the integrity of the central motor pathway func-
22 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 7 tion. An MEP may be defined as the electrical muscular response elicited by artificially stimulating the motor cortex or motor pathway above the spinal motor neuron [276]. TMS was introduced in 1985 and since then has largely replaced the painful transcranial electrical stimulation (TES) [166] as a diagnostic clinical tool. MEP recordings For routine MEP studies, the magnetic stimulator is connected to a standard EMG machine to synchronize the recording with the TMS pulse. Measuring MEPs from the upper limbs requires post-stimulus analysis time of 50 ms, and 100 ms for the lower limbs. If the CSP following the MEP is also analyzed, the recording time is typically extended to ms [221]. MEPs are usually recorded with bipolar surface electrodes configuration taped to the skin overlying the target muscle. Filter setting should be relatively open ( Hz), and a low-pass filter of <1 Hz is recommended to minimize the duration of the stimulus artifact during magnetic stimulation [221]. The subject should be seated comfortably, with easy access to the subject s head and spine for stimulation of these areas. After localizing the optimal stimulation site, this coil position is usually marked with a pen on the scalp and used for the remainder of the testing for this muscle. The magnetic coil may be fixed with a coil holder or other stabilization device to ensure stable recordings without excessive coil movements. Magnetic stimulators that are commercially available mainly induce two types of pulses 1) monophasic stimulator, with a rapid initial current and slow decays and 2) biphasic or polyphasic stimulator. Direction of induced current in monophasic stimulators depends
23 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 8 on the coil s orientation while the biphasic stimulators are less dependent on the coil s orientation [31]. For most TMS studies and for more focal stimulation, butterfly or figure-8 coils are used that consist of two adjacent round coils with opposite current direction. Mapping studies with a focal coil indicate that the distal upper-limb region on average best stimulated 5 cm lateral and cm anterior to vertex and the proximal upper limb at cm lateral and cm anterior to vertex [277]. In another study the optimal coil position for responses in a particular muscle varied up to 2 cm between individuals [168]. A useful approach in order to find proper stimulation spot is to stimulate at vertex and then 1 cm away in the four quadrants. Optimization of coil positioning is necessary for focal figure-8- coil because the MEP latency varies significantly as the function of coil positioning [91]. In monophasic TMS current direction depends on coil orientation and largest responses are obtained when the coil axis is to the parasagittal plane with a backward-flowing current in the coil so that the induced current in the brain is perpendicular to the precentral gyrus flowing posterior-anteriorly [31, 221]. MEP measurements MEP threshold MEP threshold is the lowest stimulus intensity of TMS that gives a recordable MEP in a target muscle. The motor threshold is usually provides a reference for setting the stimulation intensity for recording other parameters. A common definition of the MEP threshold at rest is the stimulus intensity required to elicit reproducible MEPs of 50 to100 V in 50% of consecutive trials [221]. It is practical to start the stimula-
24 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 9 tion below the expected threshold intensity and increase stimulator output in a step up fashion with larger steps at values significantly lower than motor threshold and smaller steps in values close to the motor threshold until 50% of 10 stimulations produce a measurable response [208]. This method seems arbitrary and other techniques have developed to measure a more physiologically relevant motor threshold by defining two lower and upper thresholds. Lower threshold is the highest intensity evoking responses with a probability of zero and upper threshold is the lowest intensity that can produce MEP 100% of time. This method minimizes the number of stimuli needed. Measures of upper and lower thresholds are are normally distributed and are independent of age, gender, and hemisphere [169]. MEP threshold is generally lower for distal than proximal muscles; lowest threshold values are reported for intrinsic hand muscles and finger extensors, this is consistent with their larger cortical representations of these muscle[221, 169]. Lower-extremity muscles and pelvic muscles have higher thresholds. MEP threshold varies widely in the healthy population, with high correlation between siblings [277]. There is no consistent evidence to support a significant role of gender and age [169, 277]. A lower threshold has been reported for the dominant hemisphere[152, 265].Other factors that have been shown to influence motor threshold are sodium-channel blockers [294] posture (lower when sitting vs. lying supine), mental activity[4] and closing and opening of eyes [223]. An interstimulus interval of 3 s has been recommended for determination of MEP threshold to prevent any facilitatory or inhibitory influence on the subsequent stimulation (Rothwell et al. 1999). MEP latency
25 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 10 MEP latency can be defined as the time between the TMS and start of MEP recordings. MEP latency has been shown to be the most reliable (considering the inherent variability of measurements) of all the different parameters that can be measured by TMS induced MEP. MEP latency in combination with a measure of the peripheral nerve conduction time can produce the central motor conduction time which indicate the duration of central processing of the TMS evoked motor response and is a measure of pyramidal tract function. MEP amplitude MEP amplitude is another marker for the degree of cortical and pyramidal tract activation. Plus MEP amplitude may be a useful parameter of cortical excitability in combination of MEP threshold measurement[276]. Cortical Silent Period (CSP) represents an inhibitory phenomenon whereby a second, much delayed EMG response is seen after stimulation when the muscle is active. The early part (the first ms) of the CSP is likely produced by spinal mechanisms such as Renshaw inhibition; the later part is of cortical origin and likely mediated by cortical inhibitory interneurons that are activated by TMS [41]. The CSP duration thus provides a measure of cortical inhibitory mechanisms including gamma-aminobutyric acid GABA B function, and is influenced by GABAergic medication, dopamine, and ethanol [296]. When recording the CSP, the exact measurement criteria for determining the duration need to be specified. Most commonly, the CSP duration is measured from the beginning of the preceding MEP to the return of voluntary EMG activity. CSP has been shown to have high test-retest reliability intra-individually [266], therefore it may be used as
26 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 11 suitable parameter for monitoring of treatment induced changes over time. MEP size variability MEP size can vary from stimulus to stimulus even when all the other stimulation parameters are kept constant [135]. Fast Fourier transformation and cross-correlation analysis did not identify a consistent dominant frequency for this variability, suggesting that the variability in MEP size could be random and maybe the result of varying synchronization, varying numbers of excited motor neurons, or varying numbers of repetitive discharges however the role of these factors are not clear. In one study [77] wide range of variability in TMS induced compound MEP amplitudes in relaxed muscles was observed (coefficient of variation, range ). In the same study Ellaway and colleagues found a positive correlation for amplitudes of the MEPs in one muscle with those in the others. Clamping the coil relative to the head or altering the orientation of the coil all failed to affect the variability of MEPs [77]. This finding might suggest that variability in the MEP measures could stem from fluctuations in excitability of the corticospinal pathway. It is also possible that variability rise from small variations of facilitation by voluntary contraction or cognitive events. Variation may also stem from inadvertent movements of the coil during stimulation, even though previous studies have shown that the contribution of coil movements does not account for the observed MEP variability [77, 100]. In order to quantify repetitive discharges of motor neurons in response to single Z Graggen et al. [288]used triple stimulation technique with an additional nerve stimulus in the periphery to cancel the first descending action potential from TMS. This study showed a significant variability in repetitive motor neuron discharges after TMS however, further studies are necessary to confirm their findings [288].
27 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 12 Spinal cord also seems to play an important role in this variability. In one experiment epidural recordings of descending volleys after TES were compared to compound muscle action potentials (CMAP). This study showed that CMAPs have a much higher trial-totrial variability than corticospinal volleys recorded from the epidural space, suggesting a substantial contribution from subcortical processes to MEP variability [286]. Effect of practice on MEP amplitudes Transcranial magnetic stimulation allows us to study M1 reorganization in humans. TMS can help us detect changes in MEP amplitudes in response to motor practice. In general, the MEP amplitude recorded from muscles engaged in the training movement increases. Few studies have shown that this effect is focal and did not affect the antagonist and uninvolved muscles in a motor practice task [35, 176]. Additional measurements are necessary to localize the origin of increased MEP amplitudes. It is possible that changes in MEP amplitudes stem from cortical or spinal motor neurons or both cortical and spinal neurons and result in increased excitability measured through MEP amplitudes. There are 3 commonly used methods to differentiate cortical vs. spinal origins of these changes in MEP. 1) Direct recording of the TMS-evoked descending volleys with epidural electrodes [63] 2) or compare TMS induced MEP amplitudes with TES. Study of TMS induced MEP latency and TES induced MEP latency suggests that TMS primarily activates pyramidal tract neurons trans-synaptically [57] while TES stimulates pyramidal tract neurons predominantly at their axon hillock [9]; therefore comparison of TMS and TES induced MEPs can differentiate cortical vs. subcortical origin of training induced changes in MEP amplitudes; finally 3) TMS of the brain
28 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 13 stem is another method to differentiate between changes at the cortical and spinal level. MEPs evoked by TMS of the brain stem should be relatively unaffected by changes in cortical excitability. Training-induced increases in TMS induced MEP amplitudes could reflect increases of excitability of the same number of pyramidal tract neurons (LTP) in the motor cortex or an increase in the number of pyramidal tract neurons of similar excitability activated by the TMS pulse (excitability threshold) or a combination of both Paired Pulse Stimulation Paired pulse paradigm is another valuable technique that investigates the excitability of different cortical circuits. The motor evoked potential amplitude elicited by stimulation of M1 can be modulated by a preceding conditioning pulse delivered either to the same cortical area or elsewhere, allowing the exploration of intra- and inter-regional physiological interactions in real time [210]. Short interval itracortical inhibition (SICI) using paired pulse paradigm, Kujirai et al [144] used stimuli subthreshold for evoking EMG responses in relaxed muscles to condition responses evoked by a later, suprathreshold magnetic or electric test stimuli. Different interstimulus intervals (ISI) between the conditioning and test stimuli will change the net motor output evoked by the suprathreshold test stimuli and the observed effects are related to different intracortical circuits. Intracortical inhibition will occur at ISIs of 1 to 4 ms[144](figure 1.1). Evidence for the cortical origin of this inhibitory interaction comes from direct recordings of descending spinal cord volleys. These recordings showed that conditioning stimulation had suppressed the
29 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 14 size of both the descending spinal cord volleys and the EMG responses evoked by the test stimulus. Inhibition of the descending spinal volleys was most pronounced at ISI 1 ms and had disappeared by ISI 5 ms [66]. SICI can be enhanced pharmacologically using GABA A agonists [121, 296] suggesting possible GABAergic mechanism for this inhibition, however, it has also been suggested that SICI of different ISIs could be mediated by different mechanisms[82] such as refractoriness or changes in axonal excitability of excitatory interneurons [82]. Figure 1.1: Recordings are from relaxed first dorsal interosseous are inhibited by a prior, subthreshold, magnetic conditioning stimulus. (A) shows examples of EMG data from a single subject. The first trace shows absence of any responses to the conditioning stimulus given alone. The lower two records have two superimposed traces, the response to the test stimulus given alone, and the response to the test stimulus when given 3 (middle traces) or 2 ms (lower traces) after a conditioning stimulus. The larger of the two traces (dotted line) is the response to the test stimulus alone. It is dramatically suppressed at these two interstimulus intervals (ISI). (B) shows the mean (/pm SE) time course of suppression in 10 subjects. (Adopted from Kujirai et al. [144]with permission from Wiley publications) Intracortical facilitation using paired pulse paradigm with conditioning and test stimli at ISIs of 6-30 ms it is possible to activate glutamatergic interneurons resulting in intracortical facilitation (ICF) [291]. This effect was first described by Kujirai et al. [144] in showing facilitation
30 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 15 of response of test stimuli at intervals of 10 to 15 ms. Facilitation gets stronger by increasing intensity of conditioning stimuli or reducing intensity of test stimuli [144, 56]. If a conditioning stimulus is given to the brain areas other than motor cortex, area-toarea facilitation and inhibition can be assessed by detecting the changes in the size of conditioned MEPs relative to test MEPs alone [144, 211, 53, 53]. This method allow us to investigate the inter-regional, intrahemispheric [106, 53, 42] or interhemispheric connections[211, 53, 12, 177, 178] by detecting the changes in the motor cortical output due to application of the conditioning pulse. Another interesting application of single or rapid rate TMS is in cognitive neuroscience. Performance of subjects in various cognitive tasks can be modulated by TMS. This technique is often referred to as virtual lesion studies. TMS applied in healthy subjects during a cognitive process most commonly leads to disruptions in task performance [174]. TMS can also facilitate behavior if single TMS pulses are applied just before the onset of a cognitive process [99]. This facilitatory effect can be result of disruption of the function in a brain area which normally has a role in suppression of TMS targeted region [174]. Such functional release suggests that TMS-induced neuronal activity can spread beyond the directly stimulated area to anatomically connected sites [46]. TMS can also be used in combination with other tools in neuroscience to increase spatial or temporal properties of TMS techniques. Such efforts can also open doors to study brain function in various states with higher resolution. For example TMS and EEG [54, 84] or TMS-PET [256] and TMS-fMRI [38, 177]are combined to increase the spatial resolution of TMS to study the effects in longer time scale (hours, days) or to study brain reactivity or pathophysiology of neurological disorders.
31 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation Investigating Mechanisms of Cortical Plasticity using TMS Techniques The human nervous system retains the potential for morphological and functional reorganization throughout life [232]. This potential for change has been termed plasticity. Plasticity of neural connections may occur at both the synaptic level [222] and at the regional level where changes can involve large networks of cells in response to lesions or training [242, 214]. Plastic changes are believed to be the foundation for learning, memory and the repair of damage following brain injury [222]. Plastic changes occur in human motor cortex. Hamdy et al [104] showed that removal of sensory input can induce changes in cortical motor representation that reverse when the sensation was restored. The mechanisms underlying cortical plasticity have been studied. These changes may be due to increased excitatory neurotransmitter release, increased density of postsynaptic receptors or the removal or reduction of tonic inhibition [40]. Reduced inhibitory inputs onto excitatory synapses is the most likely mechanism in short-term plastic changes and is likely due to reduction of GABAergic inhibition [40, 147]. Pharmacological blockade of GABA-mediated inhibition through the application of the GABA antagonist bicuculline into the rodent motor cortex resulted in rapid changes in the size and distribution of cortical representational areas, suggesting that GABAergic neurons play a vital role in cortical map reorganisation due to short term plasticity. [125]. Another important process involved in short-term reorganization is the ability to modulate synaptic efficacy. Increased effectiveness of synaptic transmission was first described
32 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 17 in the rabbit hippocampus [23, 24] where it was noted that stimulation of any of the three major input pathways resulted in increased amplitude of excitatory postsynaptic potentials in the target hippocampal neurons. This was termed long-term potentiation (LTP). It requires high frequency stimulation of excitatory afferents [24]; in contrast, low frequency stimulation can induce long-term depression (LTD) [71]. In general, the induction of LTP has four requirements: cooperativity, associativity, input-specificity and involvement of N-methyl-D-aspartate (NMDA) and GABA receptors [22, 194, 20]. Cooperativity requires synchronous activation of neurons [24]. Associativity refers to convergent activity of pre and postsynaptic stimulation of neurons in a spike timing dependent pattern [20]. This is consistent with Hebb s postulate: When an axon of cell A is near enough to excite cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A s efficiency, as one of the cells firing B, is increased [107]. The ability to form new synapses in the adult cortex is carefully balanced by the retraction of existing but perhaps unused synapses, so that the density of stable synapses remains unchanged [264]. Homeostatic regulation of neural circuits is necessary to prevent them from becoming hyper- or hypo-active [268]. In order to maintain this homeostasis, it is proposed that changes in synaptic weight, rather than wiring, may underlie cortical plasticity [44, 45]. However, a continuous increase in excitability cannot be maintained (limitation of Hebb s rule) within the physiologic range unless other compensatory or homeostatic mechanisms also modulate synaptic activities [268]. Bienenstock, Cooper and Munro in their mathematical model (BCM) proposed that the incoming patterns of impulses and change in
33 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 18 the efficacy of a given synapse depends not only on instantaneous pre- and postsynaptic activities, but also on a slowly varying time-averaged value of the postsynaptic activity [21]. Later, Abraham and Bear introduced the concept of metaplasticity, which refers to a higher-order form of synaptic plasticity where prior synaptic activity leads to a persistent change in the direction or magnitude of subsequent activity-dependent plasticity, without affecting actual synaptic efficacy [3]. Metaplasticity serves as a homeostatic factor because it ensures that plasticity is kept within a working range and away from saturation. TMS can be used to investigate these rules of cortical plasticity in human subjects and in neurological disorders. One of these techniques is called Paired Associative Stimulation (PAS) which is the technique used in most of the experiments in this thesis Bienenstock, Cooper and Munro (BCM) Model TMS techniques can also be used to investigate the rules governing the synaptic plasticity at the system level. Bienenstock, Cooper and Munro (BCM) model is one of the well established models explaining the synaptic modification in response to the history of the activity and timing of the pre and post synaptic neurons. Bienenstock et al. in their study on visual cortex led to formulation of synaptic modification rule [21]. Φ C t [C t θm t ] (1.1) where Φ is a nonlinear function of synaptic modification and C t is post synaptic activity, C t itself is also a scalar function of sum of presynaptic activity x and synaptic efficacy
34 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 19 w or C t = Σx t.w t. θm in this formula determines the direction of synaptic efficacy change, or the threshold where synaptic modification changes the sign from LTP to LTD or vice versa. BCM theory makes several explicit assumptions about synaptic modification. 1) Bidirectional pattern of LTD and LTP induction with an existing threshold level θm t. 2) θm t slides depending on the prior history of synaptic activity. 3) Change of modification threshold occurs for all excitatory synapses terminating on the affected neurons [21]. These rules can be tested at system level using plasticity inducing techniques such as PAS. However, we must note that studying these rules at system level has certain limitations. Namely, the synaptic output measured in TMS studies (mainly MEP in motor cortex) is the summation of neuronal firing in response to the stimulation, therefore neuronal recruitment and population firing and statistics come into play and can increase or decrease the outcome of measured or induced variability. Also, non-synaptic changes occur at system level, such as network reorganization and changes in the remote areas of the cortex other than the targeted area can contribute to the findings Mechanisms of Homeostatic Plasticity Hebbian forms of plasticity operate by positive feedback rules that like any other positive feedback loops tend to destabilize neuronal networks and disrupt neuronal function. Homeostatic plasticity might provide the necessary negative feedback loop to keep balance of synaptic strength and plasticity within a physiologic range. NMDA and AMPA receptors have been shown to play an important role in synaptic modifications and in-
35 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 20 duction of LTP or LTD. Ca2+ influx through NMDA sensitive glutamate receptors can trigger two forms of synaptic plasticity: LTD and LTP. LTD is induced by low levels of postsynaptic NMDA-receptor activation, such as low-frequency stimulation, whereas LTP is induced by the stronger activation that occurs following high-frequency stimulation. However homeostatic response can occur as result of coactivation of AMPA and NMDA receptors and receptor trafficking through changes in the number of postsynaptic receptors [157, 278]. Two possible mechanism for homeostatic plasticity have been suggested [267, 3]: Synaptic Scaling: it is a homeostatic form of plasticity that tends to restore neuronal activity to pre activation levels. It has been shown that chronic blockade of afferent input of cortical or spinal neurons increases the amplitude of miniature excitatory postsynaptic currents [186, 278] and prolonged postsynaptic depolarization or blockade of GABA-mediated inhibition can increase excitatory postsynaptic currents. [148]. This process happens gradually over time (hours, days), affects all neuronal inputs and is multiplicative which means that it scales synapses proportionally to their initial strength [267]. Metaplasticity refers to a higher-order form of synaptic plasticity where prior synaptic activity leads to a persistent change in the direction or magnitude of subsequent Hebbian modifications without affecting actual synaptic efficacy [15]. Experiment in rats visual cortex showed that threshold of LTP or LTD induction in visual cortical slices after exposure to light or raising studied animals in darkness during their early developmental period were significantly different and susceptibility to
36 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 21 LTP induction can be prolonged by rearing animals in darkness. This finding indicates that threshold of LTP or LTD induction depends on sensory experience[136]. Visual cortex of light-deprived rats showed enhancement in LTP and reduction in LTD induction over a range of stimulation frequencies, and that these effects can be reversed by few days of light exposure supporting the hypothesis that a sliding synaptic modification threshold allows synaptic weights in neural networks to achieve a stable equilibrium [137]. Molecular mechanism for this change in synaptic modification threshold is not clearly understood; Number of studies had shown that visual stimuli and olfactory learning can increase proportion of NR2A (subunit with fast kinetics) containing receptors at cortical synapses which can be one possible mechanism of changes in synaptic modification threshold [206, 205]. A switch in NMDA receptors, slow vs. fast decaying subunits composition, could govern the balance between LTP and LTD by controlling biophysical properties of NMDA receptors. Anatomical location, subcellular localization and downstream signaling of these subunits were also shown to be important factors in determining balance between potentiation and depression of synapses. NMDA receptors at mature synapses contain mostly NR2A whereas NR2B containing receptors are mostly extrasynaptic [263, 160]; suggesting that NR2B activation can occur as result of glutamate spillover onto extrasynaptic space. NMDA trafficking have been studied rigorously however, more evidence is needed in order to build a clear picture of events occurring during homeostatic plasticity and high order synaptic modifications. Studies are required to precisely determine receptor trafficking
37 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 22 rates and how they can be modulated by varied patterns of activity or stimulations Modulation of Cortical Afferent Input Changes in afferent input can lead to a reduction of cortical inhibition. For example, withdrawal of sensory inputs has revealed rapid and dramatic alterations in the representational maps of M1 that mimic changes which occur following limb amputation. In particular, temporary ischemic nerve block has been used experimentally to induce motor cortex disinhibition [212, 297]. This is consistent with the view that the pattern of somatosensory input to the central nervous system plays an important role in maintaining cortical representation [32]. Conversely, relevant sensory stimulation can induce plastic changes that increase the representation of target muscles. Prolonged sensory stimulation, designed to mimic repetitive natural stimulation over a large skin surface, applied to adult owl monkeys [126] resulted in significant remodelling of the primary somatosensory cortex, with considerable expansion of the stimulated receptive fields. Godde et al. (1996) extended this work, replacing repetitive nerve or digital stimulation with paired sensory inputs, according to Hebb s postulate. This associative pairing of tactile stimulation involved simultaneous weak electrical stimuli to two non-overlapping receptive fields of the digits of adult rats at random intervals. This resulted in enlargement of the stimulated receptive fields. A control experiment that stimulated only one skin site with the same temporal characteristics induced no change in receptive fields. A similar paradigm was then applied to human subjects and resulted in a significant improvement in spatial discrimination in the stimulated digits only [95]. This work forms
38 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 23 the basis for the associative stimulation technique used by Ridding and co-workers [199]. 1.5 Role of TMS in the Investigation of Human Motor Control and Learning Introduction Aristotle gives us a synopsis of the debate about the mind and hand interaction as far back as 350 BCE by saying:...now it is the opinion of Anaxagoras that the possession of these hands is the cause of man being of all the animals the most intelligent. But it is more rational to suppose that his endowment with hands is the consequence rather than the cause of his superior intelligence [187] The numerous skeletal and muscular degrees of freedom of the hand provide skillful hand movement that is an exception among species of animals that we know today. For example a person can reach to tip of his nose on a straight line as well as number of other possible paths between finger and the tip of the nose. Although all the neuro-musculo-skeletal systems are important and very complex, human hand stands alone for its evolutionary importance and its impact on progress and development of our physical and cognitive abilities [30, 153, 146]. The universal utility of the hand is even more improved by the ability of using tools. The human thumb is much longer, relative to the index finger, than the thumb of other primates and this allows humans to grasp and manipulate objects
39 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 24 between the tips of the thumb and index finger. Humans have more specialized control of their hand and digits. As a result human have developed large cortical representations devoted to control of the hand. In addition to its manipulative function, the hand is a highly sensitive perceptive organ, which enables humans to perceive the environmental sensory stimuli and feedback that information to the motor system [98, 284]. Therefore, we decided in this thesis to focus on the sensory-motor integration in motor cortex. Specifically, we targeted the cortical regions that are responsible for the control of muscles of the thumb Motor Learning through Adaptation The acquisition and long-term preservation of motor skills has a fundamental role in our lives. For example skills such as writing, playing guitar, or riding a bike are all learned through repetitive practice. Motor skill learning refers to the process by which movements are executed more quickly and more accurately with practice [283]. Noninvasive brain stimulation techniques in humans provide us with additional information about the recruitment of specific neuronal circuits during the various stages of motor skill learning [293, 207, 257]. A variety of tasks and experimental paradigms have been used to study motor adaptations. In one set of paradigms including visuomotor tracking, isometric force-production tasks and sequential movement tasks, motor skill learning is required. Another model for studying motor learning, which does not necessarily involve the acquisition of a new skill, is adaptation to externally induced perturbations, such as those induced by a force field
40 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 25 (dynamic adaptation) [236] or by visuomotor rotations (visuomotor adaptation)[139]. In this thesis, we focused on the skill learning through force-production and sequential movement motor skill learning tasks. The process of motor learning can be classified according to the type of the information that the motor system use as learning signal. This process can be classified into: 1. Use-dependent learning. Use-dependent learning refers to the phenomenon that the motor system can adapt to a new state through the pure repetition of movements. For example, the repeated execution of thumb abduction movements biases the direction of the movements elicited by TMS over the thumb area of motor cortex [48]. Further studies showed that use-dependent learning will reduce movement variability [273] and can occur in parallel with error based learning[68]. The effect of this type of learning was reduced substantially by dextromethorphan (an NMDA receptor blocker) and by lorazepam (a GABA A receptor-positive allosteric modulator) [35]. 2. Error based learning. When a movement is made, the sensorimotor system can sense the movement outcome and this knowledge is compared to the desired or predicted outcome. The motor system then adjusts to reduce the error, based on the sensory information received. Error-based learning is the presumed mechanism behind many well-studied adaptation paradigms, including prism adaptation [159], visuomotor adaptation [141], saccade adaptation[196], and grip force adaptation [85].
41 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation Reward based learning. This learning paradigm is least used method for study of human motor learning, because the appropriate reward signal for motor system is yet to be developed. However, the reward or reinforcement based learning tries to address the question of variability even when the error has reached zero in error based learning. Such learning might be important in motor learning through reward signals (dopaminergic signals from ventral tegmental area (VTA) of midbrain). Destroying VTA dopaminergic neurons in rat prevented the improvements in forelimb skill learning seen in controls during daily training, [112] indicating the importance of reward signaling in acquisition of motor skills. Furthermore, motor skill learning can be divided into a fast (early) stage, in which significant improvements can be seen within a single training session, and a later, slow stage, in which further gains are achieved across multiple practice sessions. Skill can be retained after a single or multiple training sessions. The relative duration of fast and slow learning is highly task specific. For example, the fast stage of learning an explicitly known sequence of key- press movements could last minutes, whereas the fast stage of learning in playing a complex musical piece may last months. Performance improvements during skill acquisition can occur not only during training (online learning), but also between sessions, with no further practice (offline learning) [27]. Non-invasive brain stimulation techniques can be used to modulate motor learning in human in both the fast and the late offline stage. Anodal tdcs applied to the M1 during motor practice transiently improves performance within a single session (fast motor learning) [182, 25]. The Same technique was able to augment prolonged skill acquisition across 5 days through an effect on offline
42 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 27 skill gains between sessions (consolidation) [209]. Also PAS (LTP) has been shown to enhance motor learning (measured through increased accelerations) when practice occurred right after the PAS (LTP) [128]. Therefore, it had been suggested that enhancement of cortical excitability can enhance motor learning. However, this is not always true as homeostatic plasticity rules might be engaged in this process. For example, in one study PAS (LTP) protocol resulted in in decreased motor learning when the practice sessions were delayed for 90 minutes after the stimulation [128]. This complex and sometimes paradoxical behavior are the main focus of this thesis to clarify the complex rules that are governing the interactions between enhanced cortical excitability and motor learning. 1.6 General Methods Transcranial Magnetic Stimulation (TMS) The development of TMS [13] has enabled safe and painless investigations of the motor cortex and the integrity of the central motor pathways. The stimulation coil placed over the subjects head consists of tightly wound coils of insulated wire. Although the basic principles of TMS seem simple, the mechanisms by which it works are not. Capacitors from TMS machine discharges high amplitude electric current in the TMS coil in fractions of a millisecond and in turn generates a magnetic field up to times the strength of the earth s magnetic field (2.2 Tesla) (Figure (1.2)). This magnetic field passes unimpeded through the scalp and tissue. The magnetic field induces electrical current in the brain that depolarizes superficial neurons (at a effective depth of 1 cm)
43 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 28 (a) TMS magnetic field (b) 3D coil and brain model Figure 1.2: a) TMS magnetic field, electrical current in the coil (I t ) will generate a strong magnetic field B t ; b) realistic 3D model of brain and TMS coil based on MRI imaging [275, 224]. Currently, TMS coils have two common designs: they can be circular and generate a diffuse ring of magnetic field, or they can be a figure-of-8 coil in which the summation of the two circular coils is greatest at the center. The figure-of-8 coil design allows for more focal stimulation. According to the Lenz s law, the flow of the induced current will be parallel but opposite in direction to the current in the coil. The magnetic field pulse is generated by driving a current pulse I(t) through an induction coil [13]. TMS pulse reaches a peak amplitude of more than 10,000 amperes within less than 100 microseconds. Pulse generator design is very simple and consists of a capacitor (C), a switch, and the stimulating coil (with inductance L). The circuit forms an RLC oscillator with a series resistance (R). The capacitor, charged to several kilovolts, is discharged through the coil by gating the switch usually controlled through a computer interface. Both the electric field E and current density J = ρ E (ρ being conductivity) induced
44 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 29 in the neural tissue are proportional to first derivative of the current d(i) = d(t): E(r) J(t) di dt = U 0 Lω eαt (1.2) Where U 0 is the charging potential of the capacitor, ω discharge frequency, L shows the coil inductance and α is the signal decay coefficient. The generated TMS pulses can produce heat in the coil and cable and electronic components. Most of the circuit s total resistance is in the coil (e.g., 15 out of 20mΩ); therefore most of the heat dissipation happens in the coil. This brings into focus coil warming, which is limited by the Safety Standards for Medical Equipment (IEC-601) to temperatures below 41 elsius. The optimal temperature rise should be limited to about 0.1/ per pulse. Total electric field induced in the tissue, E(r; t), is the sum of two terms: 1) the current integrated over the coil, and 2) the charge integrated over the tissue surface, denoted as primary and secondary electric fields, respectively. The primary electric field is induced directly by the changing magnetic field. In response to this field, charged ions in the tissue move, following the electric field lines until they reach the surface of the tissue or the skull. Thus, E causes a flow of current according to Ohm s law, J = ρ E, with ρ being conductivity [122]. The induced electric field strength for brain stimulation should be in the order of hundreds of mv/mm to elicit sufficient motor-cortex activation that would lead to measurable peripheral EMG responses [122]. This induces electrical currents in the underlying neural tissue. If the stimulus intensity
45 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 30 is sufficient, it will depolarize nearby neurons by opening voltage-gated ion channels. Since the cell membrane behaves as a leaky capacitor, faster and stronger changes in the electromagnetic environment are more effective for excitation [191]. The gradient of a TMS-induced electric field along a distal axon has been considered as the primary mechanism of activation [14]. TMS induced activation of motor cortex results in a number of descending volleys in the corticospinal tract that synapse with the spinal motorneurons and elicit an electromyographic response termed the motor evoked potential (MEP) in the target muscle [34]. TMS is unique in that it offers a non-invasive, painless method for stimulating the brain in human subjects. The stimulating effect depends on several important factors, including the shape of the stimulating coil (circular, figure-of-eight, cone-shaped), the waveform of the current pulse driven through the coil (monophasic/biphasic), or the cytoarchitectonic structure of the stimulated area. With commonly used stimulation parameters and focal figure-of-eight coils, the superficial cortical structures are activated within the cone-shaped volume of a few cubic centimeters, extending approximately 2-3 centimeters in depth from the surface of the human skull [26]. The H coil is another new class of TMS coils, designed to achieve effective stimulation of deep neuronal regions without inducing strong fields superficially, thus broadly expanding the potential use of TMS to stimulate deeper structures. H-coil can reach an effective field at a depth of 3 cm beneath the surface, while the standard figure-8 coil can reach a depth of less than 1 cm. The ability of the H-coils to stimulate effectively deeper neuronal structures is obtained at the cost of a wider electrical field distribution and loss of focality of TMS [224].
46 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation Repetitive Transcranial Magnetic Stimulation (rtms) In the past two decades, noninvasive brain stimulation has become an emerging field in clinical neuroscience due to its capability to transiently modulate cortical excitability. TMS pulses can be applied repetitively to a targeted area of cortex at certain fixed frequency this method of brain stimulation changes cortical excitability and it is called repetitive transcranial Magnetic Stimulation (rtms). It has been shown that rtms at frequencies of 5 Hz and higher can enhance motor cortex excitability[192] whereas lower frequencies rtms (1 Hz and lower) can transiently depress cortical excitability[39]. Modulation of the amplitude of motor evoked potentials (MEPs) produced in the target muscle during rtms showed a pattern of inhibitory and excitatory effects which depended on the rtms frequency and intensity. It has been shown that at higher than 5Hz frequencies magnetic coil situated over the optimal scalp position for activating the small hand muscles, rtms led to spread of excitation, as evident from the induction of progressively larger MEPs in the other muscles [192]. rtms has been studied as a potential treatment in many neurological and psychiatric disorders. TMS and imaging studies suggested that there is decreased cortical excitability in Parkinson s disease [76]. Several randomized controlled trials used rtms to treat the PD motor symptoms. However, the sample size was small in these studies and certain effects may not be detected because of insufficient power. Therefore in a meta-analysis ten randomized, controlled clinical trials were included. Pooling of the results from these trials yielded an effect size of 0.58 in Unified Parkinson Disease Rating Scale (UPDRS) for high-frequency rtms studies and no significant effects for low-frequency rtms studies
47 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 32 [75] indicating the beneficial effect of high-frequency rtms on motor signs in Parkinson s disease (see appendix). High frequency rtms is an FDA approved technique for treatment of depression. A recent meta-analysis of all the rtms clinical trial for depression showed that clinically significant therapeutic benefit from rtms for depression with the rtms monotherapy being more effective than rtms as adjunctive to antidepressant medication [245]. It is important to note that rtms has minimal side effects in comparison to other techniques such as drugs and electroconvulsive treatment Paired Associative Stimulation (PAS) An experimental paradigm widely used to induce plasticity in the human motor cortex is paired associative stimulation (PAS) [251, 213, 250]. This technique uses repetitive median nerve stimulation paired with cortical stimulation. The electrical nerve stimulation and cortical TMS pulses are timed so that the peripheral input and the central stimulus arrive synchronously or near-synchronously at the motor cortex. The time between the two modes of stimulation is critical; initially 25 ms was chosen to allow for peripheral conduction time from the periphery to the somatosensory cortex (20 ms) and from there to the motor cortex (3 ms) (Figure 1.3). The effect of PAS on MEP size was noticeably dependent on the timing of the TMS pulse with respect to the afferent median nerve stimulation. Stefan et al. [251] discovered that interstimulus intervals up to 35 ms were effective in generating LTP-like effect, provided the peripheral volley arrived prior to the cortical stimulus. Reversing the sequence of arrival of the afferent signals so that
48 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 33 Figure 1.3: Motor Evoked Potentials are shown before and after Paired Associative stimulation. Inter stimuli interval of around 25ms resulted in increased MEP amplitude the peripheral volley arrived after the cortical stimulus induced depression of cortical excitability, as proposed by the strict temporal Hebbian rules [285]. This is consistent with the idea that induction of plasticity in this way is similar to LTP and LTD in spike timing dependent paradigm. Repetitive stimulation of either the periphery or the cortex, while not strictly fulfilling the requirements for associative LTP plasticity, may also induce plastic change in the somatosensory cortex. Prolonged peripheral nerve stimulation [130, 37, 138, 287], muscle vibration [218] or high frequency stimulation of the motor cortex with repetitive TMS (rtms) (greater than 5 Hz) [192] also result in enhanced cortical excitability of the target muscles. In contrast, low-frequency rtms (1Hz or less) may depress motor cortical excitability [39]. PAS-induced plasticity occurs at the level of the cortex The following lines of evidence suggest that the site of action of PAS-induced plasticity is at the level of the cortex: PAS25 apart from increasing the size of the MEP amplitude, also led to an increase in the duration of the silent period recorded from the pre-
49 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 34 contracted Abductor pollicis brevis (APB) muscle. This observation points to a cortical site of the PAS-induced plasticity as the silent period is generated cortically[250]. Electrical brainstem stimulation, which excites corticospinal axons directly at the level of the craniocervical junction downstream of the cortex [270], remain unchanged after PAS [251]. Also, the F-wave which is an index of spinal motor neuron excitability, did not change after PAS [251]. Epidural recordings of descending corticospinal activity evoked by TMS demonstrated that PAS-induced changes of later descending volleys [59], which reflect the intracortical trans-synaptic activation of pyramidal neurons by TMS are affected(discussed below in more detail) [62]. Finally, PAS interferes in a highly specific manner with volitional preparatory cortical motor activity, as measured by changes in movement-related cortical potentials (MR- CPs) in EEG recordings. PAS affects MRCPs only of those movements targeted by PAS. PAS induced LTP decreased MRCP negativity during the late Bereitschaftspotential [140](-500 to 0ms before movement onset), only in the abductor pollicis brevis task that involved the APB muscle, and predominantly over the central scalp electrodes contralateral to the thumb movements. This effect correlated negatively with the LTP induced increase in MEP amplitude (APB muscle). Facilitatory PAS (ISI of N20+2ms) correlate with less MRCP amplitudes while inhibitory PAS(ISI of N20-5ms) and PAS(random ISI) did not affect MRCP amplitude. These findings indicate a specific interference of PAS on preparatory volitional motor cortical activity [151].
50 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 35 Features of PAS induced plasticity PAS is capable of producing both LTP and LTD-like plasticity. This bidirectional effect depends on the timing between the pairs of stimuli. Therefore it has been suggested that PAS is a type of spike timing dependent plasticity (STDP) [171]. Among the few properties of PAS technique are its rapid induction (after intervention of only 30 min), long duration, reversibility, and NMDA-receptor activation. After facilitatory PAS (ISI of 25ms) MEP-amplitudes increased for at least 60 min. After inhibitory PAS, MEP amplitudes remained depressed for approximately 120 min. The changes in cortical excitability reversed within 24 hr after PAS25[251]. Both the increase and the decrease of MEP amplitudes following facilitatory PAS or inhibitory PAS were blocked with dextromethorphan, an NMDA receptor antagonist. Moreover, PAS10 failed to induce a decrease in MEP size if the subjects were premedicated by nimodipine, an L-type voltage gated calcium-channel antagonist. These features indicate that the mechanism behind PAS probably occurs through synaptic modification and fits with the spike timing dependent plasticity model. PAS can induce a somatotopically specific plasticity. In one study, both APB and FDI were stimulated by TMS however choosing median nerve for peripheral nerve stimulation (APB is innervated by Median nerve) the amplitudes of TMS-evoked MEP recorded from the first dorsal interosseus muscle (FDI innervated by Ulnar nerve) remained unchanged in the presence of a substantial increase in the MEP amplitude recorded from the APB muscle, which had the central representation stimulated by PAS [213]. Other studies have also found that the effect of the PAS25 was specific to the hand area and recording
51 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 36 from muscles in upper arm and foot did not show any facilitation after the stimulation [251, 279]. Similar techniques can also be applied to other brain networks to study plasticity and integration of sensory stimuli in cortex. For example, the PAS technique was used to induce plasticity in somatosensory cortex. PAS was applied to the primary somatosensory cortex by repetitive stimulation of the median nerve stimulation followed by TMS targeted to the somatosensory cortex. This procedure led to significant enhancement of the amplitude of the P25 of somatosensory evoked potentials (SEP) obtained from median nerve stimulation. Similar to motor cortex, the relative timing of the stimulation modalities was critical for modulation of SEP and plasticity induction were bidirectional in nature[142]. Network specificity and other features of the PAS technique described above made this a suitable technique to investigate features of motor control and motor learning mechanisms in human subjects. I-wave changes after PAS Interneurons play a critical role in cortical facilitation. Sub-threshold cortical stimulation, implicates cortical pathway to be activated for these changes to occur (this sentence is not clear). In monkeys, single electrical stimuli applied to motor cortex produce multiple descending volleys in the pyramidal tracts. The earliest of these descending volleys is termed the direct wave (D wave), which is produced by direct activation of pyramidal tract neurons (PTNs). The later waves are termed indirect waves (I waves), which are produced by indirect activation of PTNs via interneurons [6, 133]. I waves are numbered
52 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 37 according to their latencies. The earliest I wave is termed I1, and the later waves are named I2, I3 etc. The ability to record descending corticospinal activity evoked by TMS in conscious humans provides very useful insight into the after effects of PAS because the synchronous neural volleys provide an indirect measure of the effectiveness of synaptic input to corticospinal neurons [61]. Repetitive discharge of corticospinal neurons are produced by a complex mechanism involving a both intrinsic neuronal properties and interactions between chains of inhibitory and excitatory interneurons in the motor cortex [67]. By studying changes in I-waves generated after the PAS, Di Lazzaro et al shown that the effects of PAS to the motor cortex occurs at the level of cortical interneurons. PAS led to a pronounced increase in the excitability of cortical circuits generating the later I-waves, while the earlier I-waves are unaffected [59]. Although the origin of these I-waves are not completely clear, there is evidence to support that the early and late I-waves are generated by independent cortical mechanisms [62, 67] Clinical applications of PAS PAS can provide a unique perspective to study disorders of plasticity. The capability to produce both LTP and LTD, reproducibility, and network specificity can be used to investigate the pathophysiology of neurological and psychiatric disorders. One disorder in which neuro-plasticity has been suggested to play a pathogenic role is focal dystonia, which occurs in some subjects with repetitive movements. Several studies have revealed that neuronal representations are altered in focal hand dystonia. Digit somatotopy and inter-digit spacing are altered and these changes may be linked to repetitive actions and neuroplasticity [202, 201, 177].
53 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 38 Quartarone et al. [200] first described increased cortical response to PAS in patients with focal hand dystonia, showing that neuroplasticity is disturbed in patients with writers cramp, a form of focal hand dystonia. Other studies then discovered plastic changes in digit representation in cortex as well as abnormal homeostatic mechanisms in this group of patients [202, 177]. Furthermore, patients with focal hand dystonia lacked the normal increase in silent period duration induced by PAS, a physiological measure that has been linked with neuronal inhibition mediated by GABA -receptors. This finding confirms that abnormal neuronal plasticity play a role in pathophysiology of focal hand dystonia. Increased response to PAS also occurred in other conditions such as Costello syndrome, which is rare congenital disease characterized by delayed development and intellectual disability, distinctive facial features and mutation in HRAS gene[69], indicating that multiple and different underlying mechanisms could potentially result in exaggerated cortical plasticity. PAS has also been used to investigate plasticity in other diseases. Levodopa induced dyskinesia, which is related to the drug treatment in Parkinson s disease, has also been associated with aberrant plasticity in the human motor cortex (M1). PAS induced LTP was shown to be deficient in Parkinson s disease off medications and was restored by levodopa in non-dyskinetic subjects. However, a deficient plastic response remained in patients with dyskinesia [173]. Reduced LTP-like effects have also been seen in Huntington s disease [52]. PAS can modulate the human sensorimotor cortex in predictable and bidirectional pattern. This promising protocol may offer a tool to investigate the mechanisms of cortical plasticity in humans. It provides us with a tool to modulate as well as to detect abnormal
54 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 39 cortical plasticity Theta Burst Stimulation Huang [117, 115] described after effects of burst of high frequency (50Hz) low intensity repetitive stimulations as a safe and rapid method of plasticity induction in human motor cortex. The basic all the stimulation pattern in this category are bursts of 3 stimuli at 50Hz frequencies with inter-stimuli interval of 20ms which will be repeated at 5 Hz (theta) frequency. This pattern can be repeated in various paradigms, intermittent (itbs, usually 2s of stimulation and 8s gap), intermediate (imtbs, usually 5s stimulation and 10s gap) and continuous (ctbs, without gap, 300 or 600 continuous bursts of stimulations). Most reports have found that ctbs reduces MEPs, whereas itbs increases MEPs, in both cases for about 30 minutes after the end of stimulation. Recently Gentner [92, 249] emphasized the regulatory effect of prior voluntary contraction of targeted muscles. They showed that without prior voluntary motor activation, application of ctbs for a duration of 20 s (ctbs300) facilitated subsequently evoked motor potentials (MEP) recorded from APB muscle. In contrast, MEP-size was depressed, when ctbs300 was preceded by voluntary activity of sufficient duration. However, when ctbs was extended over 40 s depression will occur regardless of prior muscle activation. These findings provide in vivo evidence for extremely rapid metaplasticity reversing potentiation of corticospinal excitability to depression. Polarity-reversing metaplasticity adds considerable complexity to the brain s response toward new experiences[92]. One study using a motor performance task had shown that right thumb force level control was impaired, post contra lateral
55 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 40 M1LEFT, TBS 600 stimulation only, for a duration of at least 5 min. This is another known remote after effect of TBS [249]. In another study recording electroencephalogram, ctbs applied over the left primary motor cortex (M1), which results in significant decrease in beta-band cortico-muscular coherence for the C3 scalp site, as well as the MEP amplitude in min, and then recovered to the original levels in min. This shows that ctbs-over-m1 can inhibit the cortico-muscular synchronization in parallel with the decline of cortico-spinal excitability [228] Transcranial Direct Current Stimulation (tdcs) In contract to TMS which uses a burst of magnetic pulse to excite neurons tdcs uses a weak direct current through sets of electrodes placed on a subject s scalp. In principle part of the applied current enters the brain. This current flows from anodal to cathodal electrodes. It has been shown that tdcs can modulate neuronal excitability of targeted brain area in a polarity specific manner. In this technique the strength and duration of after effect can be controlled by varying the current intensity and duration of stimulation. Polarity will define the sign of induced plasticity. Anodal stimulation (anode over the targeted brain area) increases the excitability, while cathodal stimulation decreases it [180, 181]. The feasibility of inducing long-lasting excitability modulations in a noninvasive, cheap, painless, and reversible way makes this technique a potentially valuable tool in neuroplasticity modulation. Pharmacological studies showed that NMDA antagonist dextromethorphan can suppress
56 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 41 the post-stimulation effects of both anodal and cathodal tdcs, suggesting the involvement of NMDA receptors in both LTP-like and LTD-like effects of tdcs. However since carbamazepine a Sodium channel blocker selectively eliminated anodal effects. It has been suggested that anodal tdcs require a depolarization of membrane potentials [149]. Transcranial Direct Current Stimulation (tdcs) and motor learning Several studies so far have shown improvement in motor learning after anodal tdcs [182, 161]. This effect was not demonstrated by the cathodal tdcs indicating a polarity dependent effect [182]. It has also been shown that tdcs can enhance motor skill learning both during stimulation (online) and between multiple sessions of stimulation (offline) resulting in a prolonged enhancement in the motor skill learning measures [209]. Simultaneous anodal transcranial direct current stimulation (tdcs) and training promotes improvement in motor performance and motor learning in healthy individuals. Timing between motor learning and the tdcs plays an important role, in one study application of tdcs prior to performance of the sequence-learning task led to slower learning after both anodal and cathodal tdcs [247] which is consistent with the idea that anodal tdcs interacts with subsequent motor learning in a metaplastic manner. Metaplastic effects at the level of M1 have also been investigated using preconditioning tdcs followed by rtms [244]. It has been shown that when two episodes of brain stimulation were applied one after the other immediately or with small pause preconditioning stimulation could change the direction of second stimulation after effect [244, 88]. Following studies in healthy subjects anodal tdcs were used in few clinical trial to improve cognitive [170] and motor functions after stroke [119, 154].
57 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation 42 Precise mechanism of tdcs resulting in improvement of motor skill learning is not clear. Suppression of the inhibitory system after anodal tdcs has been demonstrated by reduction in SICI suggesting a locally reduced activity of the GABAergic intracortical system [183]. Magnitude of this reduction in SICI was correlated with motor learning performance [183].The magnitude of M1 GABA decrease induced by anodal tdcs correlated positively with both the degree of motor learning and the degree of fmri signal change within the left M1 during learning with no significant changes in excitatory transmitters [248]. indicating that GABAergic system might play a role in motor learning alteration following tdcs. Studies in mice brain slices model of DCS showed that DCS can induce a long-lasting synaptic potentiation, which is polarity specific, NMDA receptor dependent, and requires coupling of DCS with repetitive low-frequency synaptic activation (mimicking motor practice) and result in BDNF-secretion and TrkB activation. These findings indicate the importance of combined motor learning and DCS to induce synaptic changes in targeted area of cortex TMS related Safety Issues TMS using single pulses is safe if used with basic precautions. It has become a standard diagnostic tool for neurologists in many countries. Contraindications to TMS are generally related to exposure to the magnetic field. The large magnetic pulse may damage electronic devices, and metal objects will be subject to mechanical forces and induced Eddy current may cause heating. These contraindications are [220]:
58 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation Implanted metal devices such as cardiac pacemakers or defibrillators, intrathecal drug delivery pumps, or spinal cord, vagus nerve, or similar stimulators. The risk of damage to the internal electronics of a programmable device is related to the distance between the stimulating coil and the device 2. Acoustic devices such as cochlear implants. 3. Presence of intracranial metal such as aneurysm clips that might be dislodged by high-intensity TMS. It is prudent to ask about any prior neurosurgical procedure. 4. History of epileptic seizures. There are a few reports of seizures occurring at or shortly after single-pulse magnetic stimulation, mostly in patients with epilepsy [110, 118, 49].There is no report of single-pulse TMS inducing a seizure in a normal subject. A meta-analysis of epilepsy patients estimated that the risk of a TMSassociated seizure ranges from 0.0 to 2.8% for single-pulse TMS, with higher risk for medically intractable epilepsy patients and after recent lowering of antiepileptic drugs [234]. Other safety concerns relate to the high-intensity impulse noise artifact associated with the discharge of the magnetic coil that is often placed in close proximity to the ear and many investigators use earplugs for the subjects [221]. Occasionally subjects report mild headache during or after prolonged stimulation studies that is usually self-limited and can be treated with over-the counter analgesics [220].
59 Chapter 1. Fundamentals of Non-Invasive Brain Stimulation Thesis Aims and Hypotheses Associative plasticity is among the fundamental working blocks of our nervous system. In this thesis, we investigate ways to induce, modulate and alter cortical excitability using rules of associative plasticity. Our primary goal was to find a reproducible, effective, simple and physiologically meaningful method to improve adaptation and motor skill learning in human subjects using associative plasticity rules to non-invasively stimulate the human motor cortex. The specific hypotheses of this thesis include: 1. Co-activating GABAergic short-interval intracortical inhibition (SICI) during PAS (SICI conditioned PAS) will block the induction of associative plasticity in the motor cortex. 2. Co-activation of glutaminergic intracortical facilitation (ICF) network will increase PAS facilitatory effect (ICF conditioned PAS). 3. Intracortical inhibition will increase and intracortical facilitation will reduce after SICI conditioned PAS and opposite effect will be seen after ICF conditioned PAS. 4. Long term potentiation like effect after PAS will increase in a graded fashion by increasing number of PAS pairs. 5. Homeostatic response to PAS depends on the level of the induced plasticity. We expect to see homeostatic interaction after large LTP inductions and no interaction after smaller LTP inductions. 6. PAS improves human motor learning through increasing the weight of synapses in sensory motor network. 7. PAS increase acceleration, rapidity of movement and improve motor learning.
60 Short-Interval Intracortical Inhibition Blocks Long-Term Potentiation induced by PAS 2 Published in Journal of Neurophysiology. January 11, 2012 The mind has exactly the same power as the hands; not merely to grasp the world, but to change it - Colin Wilson 45
61 Chapter 2. SICI blocks LTP induced by PAS Introduction LONG-TERM POTENTIATION (LTP) is hypothesized to play an important role in learning and memory [10, 124]. Paired associative stimulation (PAS) is a widely used experimental paradigm to induce plasticity in the human motor cortex [213, 250]. This technique uses repetitive pairing of nerve stimulation and cortical transcranial magnetic stimulation (TMS) that are timed so that the peripheral input and the central stimulus arrive near synchronously at the motor cortex. Because PAS shares several features of spike timing plasticity, such as associativity, input specificity, and cooperativity [16, 251], it probably represents associative plasticity and LTP in the primary motor cortex (M1). Reduction in cortical excitability is achieved when the peripheral nerve stimulation precedes the cortical stimulus [285]. These studies suggest that PAS can induce LTP and longterm depression (LTD)-like phenomena and further indicate that temporal Hebbian rules are involved in the induction of cortical plasticity. Cortical inhibition is critical for the regulation of neuronal excitability and plasticity [40]. Studies using hippocampal slices revealed that the activation of GABA A receptors blocked LTP induction [78]. In humans, increased inhibition induced by administration of lorazepam, a positive allosteric modulator of the GABA A receptor, reduced practice-dependent LTP-like plasticity in the motor cortex [35, 297]. Diazepam, another benzodiazepine, produced a nonsignificant trend towards reduction in PAS-induced LTP plasticity [108]. The GABA B receptor agonist, baclofen, also decreased PAS-induced LTP plasticity in the human motor cortex [163]. However, modulation of PAS using brain stimulation has not been examined. Abnormal associative LTP-like plasticity measured by PAS may play a critical role in the pathophysiology of neurological and psychiatric disorders, such as primary focal hand dystonia [204], Parkinson s disease [173], Costello syndrome [69], and schizophrenia [87, 253]. Short-interval cortical inhibition (SICI) is a widely studied cortical inhibitory circuit and
62 Chapter 2. SICI blocks LTP induced by PAS 47 is elicited by a subthreshold conditioning stimulus (CS) followed by a suprathreshold test stimulus (TS) at interstimulus intervals (ISI) of 1-6 ms [144]. Pharmacological studies have shown that drugs such as lorazepam that increase GABA A activity enhance SICI [64, 121, 296], which suggests that it is mediated by GABA A receptors. However, SICI has not been investigated as a focal, non-pharmacological means to modulate plasticity. In the present study, we examined the effects of activating SICI during the induction of LTP-like plasticity in the motor cortex. 2.2 Hypotheses We hypothesize that GABA A -mediated cortical inhibition activated by SICI will suppress PAS-induced LTP-like plasticity. Specific hypotheses for this chapter are: 1. Co-activating GABAergic short-interval intracortical inhibition (SICI) during PAS (SICI conditioned PAS) will block the induction of associative plasticity in the motor cortex. 2. Co-activation of glutaminergic Intracortical facilitation (ICF) network will increase PAS facilitatory effect (ICF conditioned PAS). 3. Intracortical inhibition will increase and intracortical facilitation will reduce after SICI conditioned PAS and opposite effect will be seen after ICF conditioned PAS.
63 Chapter 2. SICI blocks LTP induced by PAS Materials and Methods Experiment 1 Subjects We recruited 13 right-handed subjects with no history of neurological or psychiatric disorders and with normal neurological examination results. The University Health Network Research Ethics Board approved the experimental protocol, and all subjects provided written informed consent. Two subjects were excluded due to high resting motor thresholds (RMT), which made it difficult to obtain adequate motor evoked potential (MEP) amplitudes. Therefore, 11 healthy volunteers (7 men and 4 women) aged 35.2±13.4 yr (mean±sd) were studied. Each subject participated in three stimulation sessions, and each session was administered at least 1wk apart. Electromyogram recording Surface electromyograms (EMG) were recorded from the left abductor pollicis brevis (APB) and first dorsal interosseous (FDI) muscles using bipolar Ag-AgCl electrodes. The signals were amplified 1,000 times, filtered (5 to 500 Hz), digitized (Cambridge Electronic Design Micro 1401), and recorded using Signal software (version 3.07). EMG was continuously monitored with visual and auditory feedback to ensure complete muscle relaxation. The stimulation parameters, such as the coil location and stimulation intensities, were optimized based on the APB responses. The FDI measurements were used to confirm the findings in the APB muscle and to determine if the similar effects also occurred in another muscle in the proximity of the APB muscle but innervated by a different nerve (ulnar nerve).
64 Chapter 2. SICI blocks LTP induced by PAS 49 TMS We used two Magstim 200 stimulators (Magstim, Whitland, Dyfed, UK) connected via a Bistim module and an 8-shaped coil (outside diameter of each loop was 9.5 cm) to apply TMS to the right M1. The TMS trigger pulses were delivered from a Micro1401 interface (Cambridge Electronics Design, Cambridge, UK) controlled by Signal Software (3.07). The subjects were seated in a comfortable position, and the coil was held tangentially to the skull with the handle pointing backwards and laterally at 45 to the sagittal plane, at the optimal scalp site to evoke MEP in the relaxed left APB muscle. The motor hot spot was marked on a default image in the Brainsight (Magstim) stereotactic image guidance system to facilitate the positioning of the TMS coils over a subject s scalp. The experimental protocol is shown in Fig 2.1 RMT was defined as the minimum stimulator output that evoked MEPs of > 50µV in at least 5 out of 10 trials when the APB muscle was completely relaxed. Active motor threshold (AMT) was defined as the minimum intensity of stimulation output that elicited APB MEPs of at least 200µV in 5 out of 10 consecutive trials while the participants maintained a force level of 20% of their maximum contraction. MEP amplitude was measured as the average of 10 trials in which TMS generated an EMG response of at least 1 mv peak-to-peak amplitude at the baseline. At each time point (Fig 2.1), RMT, AMT, and MEP amplitudes were measured first. The balance and interactions between inhibitory and facilitatory circuits determine the final output from the M1 [102]. We therefore tested several well-established intracortical inhibitory and facilitatory circuits to explore the possible changes in these networks following each intervention. SICI mostly represent GABA A -mediated inhibition [127, 296], intracortical facilitation (ICF) reflects glutamatergic activities [235, 292], and short latency afferent inhibition (SAI)is related to cholinergic [60] neural networks TS. CS inten-
65 Chapter 2. SICI blocks LTP induced by PAS 50 sity was 90% AMT. ISI of 2 ms was used for SICI and 10 ms for ICF. SAI was studied using a conditioning-test protocol described by Tokimura et al. [262]. The left median nerve was stimulated through bipolar electrodes at the wrist (cathode proximal) using a 200-µs square wave pulse at three times the sensory threshold. The ISI between median nerve stimulation and TMS was set to the latency of the N20 somatosensory evoked potential plus 2 ms. The N20 somatosensory evoked potential was evoked by stimulation of the left median nerve and was recorded with the active electrode, which was placed 3 cm posterior to C4 (according to the International EEG system) and referenced to Fz. Two hundred responses were averaged to identify the N20 peak. SICI, ICF, and SAI were measured in the same experimental session with 10 trials for each ISI and 10 trials of TS alone, delivered in a random order. The TS generated a 1mV MEP amplitude in the left APB muscle at baseline, and the TS intensities were adjusted at each time point after PAS to produce similar test MEP amplitudes. Cortical silent period (CSP) was measured at the end of each assessment. CSP was defined as the time between MEP onset and return of voluntary EMG activity [55] and was assessed from 10 trials during isometric contraction (constant 20% maximum voluntary contraction under visual feedback through an oscilloscope) of the APB muscle with TMS at 130% RMT. PAS PAS was achieved by repetitively pairing stimulation of the left median nerve and TMS of the right M1. The median nerve was stimulated at three times sensory threshold, and TMS intensity was adjusted to produce MEPs with 1 mv peak-to-peak amplitude. The ISI was 25 ms, which was optimal for inducing a sustained increase in motor cortex excitability through PAS [251]. One hundred and eighty paired stimuli were delivered at
66 Chapter 2. SICI blocks LTP induced by PAS Hz during a 30 min period. We refer to this paradigm as PAS25. PAS in the presence of SICI.We combined median nerve stimulation and SICI in two different experimental paradigms. In the first paradigm, the parameters were the same as PAS25 except that a CS at 90% AMT was delivered 2 ms (CS2) before the TS (23 ms after median nerve stimulation). We refer to this paradigm as CS2-PAS25. Because CS2 reduces the MEP amplitude evoked by the TS, in the second paradigm, we increased the TS intensity to produce 1 mv MEPs in the presence of SICI induced by CS2. This paradigm is designed to match the MEP amplitudes produced by the CS2-adjusted TS pulse combination to that produced by TS alone in the PAS25 condition (CS2-PAS25adj). Corticospinal excitability and cortical inhibition were measured before and at different times after PAS, as indicated in Figure 2.1. These three PAS paradigms were performed in separate sessions at least 1wk apart and in random order. MEP amplitudes during PAS. To examine the changes in MEP amplitudes during PAS, MEPs were recorded during the PAS protocols,and the amplitudes of every 10 MEPs were averaged Experiment 2 PAS in the presence of ICF. Four new subjects and two subjects from experiment 1 (3 men and 3 women, aged 39.5 ± 9 yr) participated. Subthreshold conditioning stimuli 10 ms before the test stimuli 1996c). This protocol was designed to condition PAS with a CS 10 ms before the TS to test whether the effects seen with PAS in the presence of SICI are specific to the ISI used. The TS intensity was adjusted to produce 1 mv amplitude in the presence of CS10, which was set at 90% AMT. This protocol is referred to as CS10-PAS25adj. The pre and post-pas measurements are the same as experiment 1.
67 Chapter 2. SICI blocks LTP induced by PAS 52 Figure 2.1: Study timeline. AMT, active motor threshold; CSP, cortical silent period; ICF, intracortical facilitation; MEP, motor evoked potentials; RMT, resting motor threshold; SAI, short latency afferent inhibition; SICI, short-interval cortical inhibition; TS, test stimulus. These measures were taken before the paired associative stimulation (PAS) protocols and immediately (T0), 20 (T20), 40 (T40), and 60 (T60) min after the interventions. The top right corner illustrates the combination of peripheral and transcranial magnetic stimulation (TMS) pulses used in each PAS protocol. Statistical analysis. For RMT, AMT, CSP, SICI, ICF, MEP, and SAI, values after PAS from T0 to T60 were expressed as a ratio of baseline and were used for statistical analysis. In experiment 1, repeated-measures ANOVA with intervention (PAS25, CS2-PAS25, and CS2-PAS25adj) and time (T0, T20, T40, and T60) were used as the within-subject factors. Separate analyses were performed for the APB and FDI muscles because all the adjustments in stimulus intensities were based on the APB muscle, and therefore the MEP amplitudes for different test conditions were not matched for the FDI muscle. Significant ANOVA main effects were further explored using Fisher s post hoc analyses and separate one-way ANOVAs. Peak-to-peak MEP amplitudes during PAS were analyzed using an ANOVA with intervention as the between-subject factors and time as the within-subject factor. MEPs from every 10 consecutive stimuli were averaged and used for analyses. Baseline was defined as the first time bin of 10 MEPs at the beginning of each of the test interventions. For
68 Chapter 2. SICI blocks LTP induced by PAS 53 experiment 2, repeated-measure ANOVA with the factor time (5 levels, baseline-t60) as within-subject factors for APB muscle were tested. Values after intervention from T0 to T60 were expressed as a ratio to baseline. Effect of time was further explored using Fisher s least-significant difference and Fisher s post hoc analysis. P values <0.05 were considered significant. StatView (SAS Institute) software was used for analysis. Values are presented as means ± SE, unless specified otherwise. 2.4 Results None of the subjects reported any adverse effect Motor Thresholds (RMT, AMT) For experiment 1, no significant change in RMT (43 ± 1.4% at baseline) and AMT (36 ± 1.2% at baseline) of the APB muscle was observed after any of the three PAS protocols. There were no significant interactions or main effects for either muscle. In experiment 2, the RMT and AMT were 46 ±3.8 and 36 ± 2.7% at baseline. Similarly, no significant change in RMT and AMT was detected Experiment 1 SICI blocks LTP-like plasticity during PAS. There were significant effects of intervention (P < ), time (P < 0.001), and time intervention (P < 0.001; Figure 2.2) on the MEP amplitude during the 30 min of PAS (median nerve stimulation followed by TMS). Post hoc analysis confirmed significant differences among all three interventions with the highest MEP amplitudes in PAS25, followed by CS2-PASadj, and then CS2-PAS25. Figure 2.2 shows that the significant time intervention is due to a marked increase in
69 Chapter 2. SICI blocks LTP induced by PAS 54 MEP amplitude during PAS25 and CS2-PAS25adj, but not during CS2-PAS25. There was a significant effect of time on MEP amplitudes for the PAS25 session (P < ). Post hoc analysis showed that MEP amplitude was significantly increased compared with the first MEP (baseline) after 4 min and reached a plateau after about 16 min (Figure 2.2). In the CS2-PAS25 session, there was also a significant effect of time on MEP amplitude (P < 0.005). Post hoc analysis showed that MEP amplitude was mostly unchanged except for transient increases at 15 and 25 min (Figure 2.2). For the CS2-PAS25adj session, there was also a significant effect of time after stimulation (P < 0.001). Post hoc analysis showed an increased MEP from 20 min into the stimulation session through the end of the session (P <0.002) (Figure 2.2). Figure 2.2: MEP amplitude recorded during the PAS interventions. Open symbols represent significant difference compared with the first time bin for each intervention. Each point represents averaged MEP amplitudes (mv) from 10 stimuli for standard PAS (PAS25,square), CS2-PAS25 (circle), and the CS2-adjusted TS pulse combination to that produced by TS alone in the PAS25 condition (CS2-PAS25adj, triangle). : P < 0.05 and : P < 0.001; error bars denote ±SE.
70 Chapter 2. SICI blocks LTP induced by PAS SICI Blocks LTP-Like Plasticity After PAS For MEP amplitudes from single-pulse TMS in the APB muscle, ANOVA showed a significant effect of intervention (P < 0.001), but no significant effect for time and time intervention interaction (Figure 2.3). Post hoc analysis confirmed that there were significantly higher MEP amplitudes for PAS25 compared with CS2-PAS25 (P < ) and CS-PAS25adj (P = ). The MEP amplitudes for CS2-PAS25 and CSPAS25adj sessions were not significantly different from each other. One-way ANOVA for PAS25 showed a significant effect of time on MEP amplitude (P = 0.009). Post hoc analysis showed higher MEP amplitudes at all time points measured up to 1 h after the PAS25 compared with baseline (T0: P = , T20: P = 0.02, T40: P = 0.013, T60: P = 0.009). There was no significant effect of time following CS2-PAS25 and CS2-PAS25adj stimulation protocols Changes in Intracortical Inhibitory and Facilitatory Networks Following PAS SICI. There was no significant effect of intervention, time, and time intervention interaction for SICI of the APB muscle. SAI. There was a significant effect of intervention for the APB muscle (P < 0.02), but there was no significant effect of time and time intervention interaction (Figure 2.4A). Post hoc analysis showed SAI was significantly less after CS2-PAS25adj compared with that after CS2-PAS25 (P = 0.008; Figure 2.4B). Separate one-way ANOVAs for each intervention revealed no effect of time with CS2-PAS25, CS2-PAS25adj, and PAS25. ICF. There was no significant effect of intervention, time, and time intervention in-
71 Chapter 2. SICI blocks LTP induced by PAS 56 Figure 2.3: MEP amplitudes after PAS protocols. MEP in APB muscle is shown. MEP amplitudes were normalized to baseline (before interventions) for PAS25 (square), CS2- PAS25 (circle), and CS2-PAS25adj (triangle). Values more than 1 indicate increased MEP amplitude, and values less than 1 indicate decreased MEP amplitude after PAS. teraction for APB muscle. CSP. ANOVA showed a significant effect of time (ANOVA APB P=0.006), but there was no effect of intervention and time intervention interaction (Figure 2.5). Post hoc analysis showed that CSP was increased at 40 and 60 min after interventions compared with that at baseline (T40, P<0.002; T60, P<0.009). Stimulation effects in the FDI muscle. The intensity that generates 1 mv amplitude in APB resulted in 1.7 ± 0.3, 1.4 ± 0.3,and 1.8 ± 0.3 mv MEP amplitude in PAS25, CS2-PAS25, and CS2-PAS25adj protocols, respectively, at baseline. A similar pattern of changes in MEP amplitude was observed after PAS protocols in FDI muscle. ANOVA showed significant effect for intervention (P = 0.006) and no effect for time and time
72 Chapter 2. SICI blocks LTP induced by PAS 57 Figure 2.4: SAI before and after PAS interventions. A: SAI in the APB muscle. Values above 1 indicate facilitation, and values below 1 indicate inhibition. B: the effects of the different interventions are shown as the ratio of SAI averaged for all time points after PAS normalized to the baseline SAI for the three PAS conditions tested (PAS25, CS2-PAS25, and CS2-PAS25adj). Values above 1 indicated reduction in SAI after PAS, and values less than 1 indicate an increase in SAI after PAS compared with baseline values. Error bars represent±se; P < 0.05 Figure 2.5: CSP duration before and after PAS interventions. CSP are shown in ms. Each bar represents 1 intervention protocol. Error bars represent ±SE. : P < 0.05.
73 Chapter 2. SICI blocks LTP induced by PAS 58 intervention on MEP amplitude ratios after PAS. Post hoc analysis showed higher MEP amplitude in PAS25 compared with CS2-PAS25 (P <0.01) and CS2-PAS25adj (P < 0.01). This confirms similar time courses for MEP amplitude after PAS in both ABP and FDI muscles Experiment 2 PAS in the presence of ICF. The MEP amplitude at baseline was 0.94 ± 0.08 mv. Repeated-measure ANOVA showed a nonsignificant trend for the effect of time (P = 0.09) in the APB muscle. No significant effect of time was observed forfdi muscle. Fisher s post hoc analysis showed a significantly higher MEP amplitude at T60 compared with baseline (P <0.01) in APB muscle (Figure 2.6A). One-way ANOVA showed no significant effect of time on normalized values of SICI (53.9± 11% at baseline), ICF (131.2 ± 24% at baseline), SAI (35.9 ± 9.4% at baseline), and CSP (172 ± 11 ms at baseline) for both APB (Figure 2.6B) and FDI muscle groups. Figure 2.6: Results from the CS10-PAS25adj paradigm. A: MEP amplitudes in the APB muscle after the CS10-PAS25adj protocol. MEP amplitudes were normalized to baseline (before interventions). B: results for SICI, SAI, and ICF are shown. Values above 100% indicate facilitation, and values below 100% indicate inhibition. Error bars represent ± SE.
74 Chapter 2. SICI blocks LTP induced by PAS Discussion MEP amplitudes significantly increased following PAS25 compared with baseline, but the MEP amplitudes after CS2-PAS25 and CS2-PAS25adj were unchanged. Thus, applying SICI, which activated GABAergic interneurons, inhibited PAS-induced LTP-like plasticity aftereffect in the motor cortex. This effect was specific to the conditioning-test pulse (CS2) interval that elicited SICI because the same stimuli at a different conditioning pulse interval (CS10) that did not produce SICI showed MEP facilitation after the intervention (Figure 2.6). These findings demonstrate that noninvasive and non-pharmacological TMS techniques can effectively modulate cortical plasticity. Inhibitory interneurons play an important role in the regulation of cortical plasticity. In rat hippocampal slices [78], application of midazolam, a benzodiazepine, markedly inhibited LTP induction and prevented the expected increase in excitatory postsynaptic potentials and population spikes following theta burst stimulation. This effect was reversed by bicuculline, a GABA A receptor antagonist [78], which indicates that benzodiazepines suppress LTP induction through enhancement of GABA A receptor-mediated inhibition. In rat motor cortex [109], bicuculline increased the ability of theta burst stimulation to induce LTP. Several pharmacological studies in humans have shown that benzodiazepines suppressed cortical plasticity [35, 297]. In the present study, we investigated the role of inhibitory circuits in the regulation of plasticity in motor cortex by activating SICI during facilitatory PAS25, and we found disruption of LTP-like plasticity induction. Because SICI decreased the MEP amplitude induced by TS and this may affect LTP-like plasticity induction, we included a control experiment (CS2-PAS25adj) with adjusted TS intensity to generate the same MEP amplitude as in PAS25 as a measure of postsynaptic activity. Because the induction of LTP-like plasticity was suppressed in both CS2-PAS25 and CS2-PAS25adj conditions, the finding cannot be explained by
75 Chapter 2. SICI blocks LTP induced by PAS 60 reduced postsynaptic activity in the CS2-PAS25 condition but is likely the result of activation of inhibitory circuits responsible for SICI. This effect cannot be attributed to nonspecific effects of adding the CS during PAS because the same CS at a different ISI (10 ms) resulted in LTP-like effect. Direct recordings of corticospinal descending volleys showed that SICI reduced late I- waves [65], whereas facilitatory PAS increased later I-waves [59]. The possibility that our results could partially be explained by different I-wave composition in the CS2-PAS25adj condition compared with PAS25 condition cannot be excluded. However, epidural recordings of corticospinal waves [179] showed that adjustment of MEP amplitude in the presence of CS2 also restored the late I-wave amplitude to that of TS alone, suggesting the PAS25 and the CS2-PAS25adj protocols in our study likely had similar late I-wave amplitudes. Another consideration is whether repetitive 0.1 Hz paired pulse stimulation at 2 ms ISI itself depressed MEP amplitude. Further studies are needed to exclude this possibility, but we considered it unlikely because 0.1 Hz single-pulse TMS had no effect [39], whereas 0.2 Hz suprathreshold paired-pulse TMS at 1.5 ms ISI increased in MEP amplitude [259]. LTP-like plasticity is decreased in several neurological and psychiatric disorders with abnormal motor learning, such as Parkinson s disease [173], schizophrenia [54, 87, 253], and Huntington s disease [190]. On the other hand, exaggerated LTP-like and LTD-like plasticity in the motor cortex and a loss of topographic specificity has been observed in writer s cramp [200, 204, 280]. Our study shows that SICI modulates the controlling mechanism of cortical plasticity, which can potentially be useful in the treatment of conditions with abnormal plasticity and disrupted inhibition, such as dystonia. MEP amplitude during PAS. EMG recording during the stimulation phase showed that, at the beginning of PAS, the MEP amplitudes (with median nerve stimulation followed
76 Chapter 2. SICI blocks LTP induced by PAS 61 by TMS) were 1 mv (Figure 2.2). This is likely because the median nerve stimulation 25 ms before TMS produced MEP inhibition, similar to SAI. The MEP amplitudes induced by CS2-PAS25 were smaller than those elicited after PAS25 (Figure 2.2), likely due to further MEP inhibition caused by adding the CS2 pulse. In the CS2-PAS25adj condition, the TMS intensities were adjusted to produce 1 mv MEP in the presence of CS2 (without median nerve stimulation). With the addition of median nerve stimulation, the higher MEP amplitude for CS2-PAS25adj compared with PAS25 at the beginning of PAS suggests that there exists an inhibitory interaction between SAI and SICI. These findings are consistent with those of previous studies, which showed mutual inhibitory interactions between SICI and SAI [7, 250]. The MEP increase during PAS25 and CS2-PAS25adj may be due to an increase in MEP amplitude or a decrease in SAI. After PAS25, there was no change in SAI (Figure 2.4A), but the MEP amplitude was increased (Figure 2.3); it is likely due to an increase in MEP amplitude and corticospinal excitability. In contrast, after CS2-PAS25adj, there was no increase in MEP amplitude compared with baseline (Figure 2.3), but there was reduction in SAI (Figure 2.4B). Therefore, the increase in MEP amplitude during CS2-PAS25adj (Figure 2.2) is likely related to decrease in SAI. The finding that MEP amplitudes did not change during CS2-PAS25 is consistent with the observation that neither MEP amplitudes (Figure 2.4) nor SAI changed after CS2-PAS25 (Figure 2.4A) Intracortical inhibition and facilitation after PAS. Similar to our findings of no overall change in SICI and ICF after PAS25, previous PAS studies did not show significant changes in SICI after excitatory PAS25 [58, 163, 227, 250] although a significant decrease in SICI after inhibitory PAS was observed in one study [227]. The finding that SICI was unchanged after CS2-PAS25 and CS2-PAS25adj conditions is consistent with the blockade of LTP plasticity by SICI. If SICI was diminished during these conditions, it
77 Chapter 2. SICI blocks LTP induced by PAS 62 would not be expected to block PAS-induced plasticity. We found a significant increase in CSP (Figure 2.5 after PAS25 and the conditioned PAS interventions, similar to previous studies [173, 251]. Because administration of the GABA B receptor agonist, baclofen, and GABA reuptake inhibitor, tiagabine, prolonged CSP [241, 281], it is probably mediated through postsynaptic GABAB receptors. CSP is thought to be partially mediated through activity in recurrent collaterals from discharging pyramidal tract neurons [189]. Prolonged CSP after PAS25 is probably due to facilitation of inhibitory interneurons and up-regulation of GABAB receptors in postsynaptic neurons. MEP amplitude reflects the excitability of the corticospinal system, and it increased after PAS25, whereas CSP increased after all three interventions. Both electrophysiological and pharmacological studies suggest that multiple mechanisms are involved in generating the CSP, including the loss of voluntary drive, activation of inhibitory interneurons, activation of corticospinal recurrent collaterals, and after hyperpolarization [90, 258, 295]. The different effects of PAS interventions on MEP and CSP suggest that the same circuits do not mediate them. However, authors cannot precisely explain why CSP is increased in all three protocols. SAI is decreased after the stimulation in CS2-PAS25adj. This observation could suggest decreased SAI itself or an increased tonic inhibitory interaction on SAI from other circuits after CS2-PAS25adj. We showed in a previous study that GABA B -mediated long-interval intracortical inhibition and SAI had mutual inhibitory interactions [269]. We speculate that this inhibitory interaction or interactions with other cortical circuits may be involved, but this needs to be clarified in future studies.
78 Chapter 2. SICI blocks LTP induced by PAS Conclusion Potential implications of the present findings. TMS techniques, such as repetitive TMS, have been used to assess and treat diseases with abnormal cortical excitability and plasticity [43, 73]. Our finding that simultaneously activating SICI during PAS suppresses LTPlike plasticity supports the hypothesis that GABA A -mediated inhibition plays a crucial role in regulating cortical plasticity. This demonstration of a nonpharmacological way of modulating plasticity in humans has several implications. The method can be modified by using different ISI or stimulus intensities to examine the effects of other cortical circuits on cortical plasticity. This may be a method to examine abnormal regulation of plasticity in neurological and psychiatric disorders. Moreover, suppression of LTP-like mechanisms may be tested as a possible treatment for disorders associated with increased LTP-like plasticity such as dystonia. Noninvasive and non-pharmacological modulation of cortical plasticity may be more advantageous than pharmacological intervention because it may have fewer side effects and it can be targeted to specific brain areas.
79 Dose Response relationship of Paired Associative Stimulation induced Cortical Plasticity and Interactions with Motor Learning 3 64
80 Chapter 3. PAS Dose-Response Curve and Motor Learning Introduction Long-term potentiation (LTP) likely plays an important role in motor learning. One method of studying plasticity in human motor cortex is paired associative stimulation (PAS). This method pairs transcranial magnetic stimulation (TMS) and peripheral nerve stimulation to alter cortical excitability. PAS protocol has been shown to result in both LTP and long term depression (LTD) like-plasticity depending on the time interval between TMS and peripheral nerve stimulation [251]. To explain the relationship between pre and post synaptic activity and strength of synaptic transmission, Hebb [107] and later Bienenstock, Cooper and Munro (BCM) had shown in their models that efficacy of a given synapse depends not only on instantaneous pre- and postsynaptic activities, but also on a slowly varying time-averaged value of the postsynaptic activity [21]. One approach to test this model is to pair activation of pre- and post-synaptic at varying intervals and record the synaptic activity after different numbers of stimuli [28]. PAS can be used to test rules governing cortical plasticity in human. It is important to note that BCM model assumes a linear relationship between number of pre and post synaptic stimulation pairs and amount of induced plasticity. In this study, this concept was tested by varying the number of stimulation pairs and motor evoked potentials (MEPs) were used as a measure of motor cortical output. Abraham and Bear introduced the concept of metaplasticity, which refers to a higher-order form of synaptic plasticity where prior synaptic activity leads to a persistent change in the direction or magnitude of subsequent activity-dependent plasticity, without affecting actual synaptic efficacy [3]. Metaplasticity serves as a homeostatic factor because it ensures that plasticity is kept within a working range and away from saturation. Although BCM model of synaptic plasticity explains the synaptic behavior of the neurons in response to their activities, the rules governing these metaplastic interactions have not been clearly defined. In this study,
81 Chapter 3. PAS Dose-Response Curve and Motor Learning 66 we investigated the effects of three different PAS interventions with different numbers of stimulation pairs and their interactions with subsequent motor learning. We hypothesized that PAS effect is dose-dependent. We also examined the metaplastic interactions between PAS effects and motor learning. We tested the hypothesis that homeostatic response occur after a certain threshold of PAS induced LTP has been exceeded. 3.2 Hypotheses In this study, we investigated the effects of three different PAS interventions with different numbers of stimulation pairs and their interaction with subsequent motor learning. We tried to show that the PAS effect follows a dose-dependent pattern. In the next stage we looked into the interactions with motor learning and if these interactions can be modulated depending on the number of pairs in PAS or in other words, levels of LTP-like effect generated from the priming stimulation. The specific hypotheses for this study are: 1. Long term potentiation like effect after PAS will increase in a graded fashion by increasing number of PAS pairs. 2. Homeostatic response to PAS depends on the level of the induced plasticity. We expect to see homeostatic interaction after large LTP inductions and no interaction after smaller LTP inductions. 3. PAS improves human motor learning through increasing the weight of synapses in sensory motor network.
82 Chapter 3. PAS Dose-Response Curve and Motor Learning Material and Methods Subjects Ten healthy subjects (6 men and 4 women) with no history of neurological or psychiatric disorders and with normal neurological examination were recruited. The majority of the participants were right-handed (8 right-handed, 2 left-handed), with a mean age of 39.6 ± 14 years (± SD). The University Health Network Research Ethics Board approved the experimental interventions and all subjects provided written informed consent. The subjects participated in four experimental sessions with each session conducted at least one week apart Stimulation Two Magstim stimulators (Magstim, Whitland, Dyfed, UK), a Bistim module and a figure-of-eight shaped coil (outside diameter of each loop was 9.5 cm) were used to apply TMS to the left M1. The two stimulators were connected via a Bistim module to produce paired stimuli at short intervals. The trigger pulses for TMS was delivered from a Micro1401 interface (Cambridge Electronics Design) controlled by Signal Software (3.07). The coil was held with the handle pointing laterally at 45 angle to the sagittal plane at the optimal scalp site to evoke a MEP in the relaxed abductor pollicis brevis (APB) muscle of the right hand. The motor hot-spot was then marked and was used for the rest of the session. MEP was recorded through surface bipolar Ag-AgCl electrodes from the APB, and first dorsal interosseous (FDI) muscles of the right hand. EMG signals then were amplified 1000 times, filtered (2 Hz - 2KHz) and digitized through a CED 1401 interface.
83 Chapter 3. PAS Dose-Response Curve and Motor Learning Paired Associative Stimulation PAS involves a series of paired peripheral and cortical stimuli. Electrical stimuli were delivered to the median nerve of the right wrist at intensity sufficient to produce a small motor response in the APB muscle. The median nerve stimulus was followed by suprathreshold TMS to the left M1 adjusted to produce 1mV peak-to-peak MEP amplitude in the APB muscle. The interval between median nerve stimulation and TMS was set to 25ms to induce facilitatory PAS. We used 3 different durations of PAS with 90, 180 and 270 pairs of stimuli at 0.25 Hz frequency. These interventions are termed PAS90, PAS180 and PAS270 in the manuscript Motor Learning Task (MLT) The subjects performed brisk isometric abductions with their right thumb to a target force window. The thumb abduction force was recorded by a high accuracy force transducer (Omega Stamford, Connecticut USA, Model LCCA-25). The tactile Go signal was generated by an electrical stimulation of median nerve at the wrist at 200% perceptual threshold. After the subject s maximum force was established, the target force window was defined as between 35 and 45% of the subject s maximum force and was displayed as two horizontal lines on a computer screen. The target window was of the same size (4 cm wide) for all the subjects. The Go signal frequency was 0.5 Hz. The force signal was fed back to the subjects who were informed whether they had reached, undershot or overshot the target through graphic presentation of the signal and target on the monitor. Each MLT included 6 blocks of 50 isometric contractions of the APB muscle with 30s pause in between each block. The trials with undershoot and overshoot force levels from the target window were considered errors. The error rate and the percentage of correct response were used as measures
84 Chapter 3. PAS Dose-Response Curve and Motor Learning 69 of performance of the motor learning task. The percentages of correct responses were compared across the six blocks. Improvement in percentage of correct response between blocks 1 and 6 was considered as a measure of practice dependent motor learning Experiments Experiments 1a and 1b were performed in a random order. In all the experiments, MEP changes were captured using both input-output curve (I/O) and single intensity TMS. I/O curve in APB muscle was measured at four time points: before (Tpre), at 15 and 30 minutes after the first (priming) intervention (T15 and T30), and at 15 minutes after second intervention (T 15)(Figure 3.1). The I/O curves were obtained from four intensities at 100%, 110%, 120% and 130% of baseline RMT with 10 TMS pulses for each intensity. Single intensity TMS at 130% RMT were used to capture changes immediately after the priming intervention (T0) and immediately after the second intervention (T 0). Twenty TMS pulses were used to produce the average MEP for the single intensity measure and the results were compared to 10 TMS pulses at 130%RMT at baseline. Intensities for both I/O curve and single intensity TMS were adjusted to RMT at baseline and it remained constant for the rest of the experiment.
85 Figure 3.1: General outline of studies. Sessions in Experiments 1a (3 sessions) and 2 (1 session) were studied in random order. Chapter 3. PAS Dose-Response Curve and Motor Learning 70
86 Chapter 3. PAS Dose-Response Curve and Motor Learning 71 Experiment 1. PAS and motor learning interactions a) Effect of number of PAS pairs on LTP-like plasticity and interactions with motor learning. The experimental design is outlined in Figure 3.1. Subjects received PAS90, PAS180, or PAS270 in three separate visits at least one week apart in random order in combination with Experiment 1b, followed by the MLT at 1 hour after the end of the PAS protocols. b) The effects of motor learning on PAS90. The design is similar to experiment 1a except that we changed the order of MLT and PAS. The subjects underwent the MLT first followed by PAS90 1 hour later. This study is designed to assess the effect of motor learning on MEP and to evaluate the effect of motor learning on PAS. T0 and T 0 are the immediate measurements post MLT and post PAS. Input output curve (I/O) was measured at four time points, before MLT (Tpre), 15 and 30 minutes after MLT (T15 and T30) and at 15 minutes after PAS90(T 15) (Figure 3.1). This experiment was performed in one visit with same subjects who participated in experiment1a and in random order of with the visits in experiment 1a. Experiment 2. Time course of PAS The effect of PAS270 without the MLT was assessed in 6 subjects who also participated in Experiment 1. After PAS270, the subjects were followed for the 75 minutes and MEP and I/O curve were measured at the same time points as in experiment 1a. (Figure 3.1).
87 Chapter 3. PAS Dose-Response Curve and Motor Learning Statistical Analysis Repeated measures analysis of variance (RMAOVA) was used to compare means in a general linear model using SPSS Sphericity of variance was assumed in RMAOVA when Mauchly s test of sphericity was not significant. If sphericity was violated, Greenhouse and Geisser correction were applied [97]. In all the statistical analysis of cortical excitability ratio of MEP amplitude to baseline (Tpre) was used. The factor Time represents the time point at which measurements was done. For the intensity of 130%RMT, two extra time points (T0, T 0) were measured immediately after first or second intervention (Figure 3.1). Intensity of 130%RMT for the remaining time points were measured in combination with other intensities in I/O curves. In experiment 1A, PAS duration (3 PAS interventions with 90,180 and 270 paired stimuli), Time and Intensity were used as within subjects factors. Post-hoc analyses of significant findings were performed using paired t-test. To investigate the interaction between PAS duration and MLT on MEP amplitudes induced at intensity of 130%RMT, a separate RMANOVA with factors Time and PAS duration was performed. Intensity of 130%RMT was used because the extra time points can show the time course with greater details. For Experiments 1b and 2, two separate RMANOVA, one for the I/O curves with factors Time and Intensity and one for intensity of 130%RMT with factor Time were used. Student s t-test was used for post-hoc comparison of significant effects. To investigate if PAS durations (PAS90, PAS180, PAS270) and the level of LTP-like plasticity after PAS can predict the degree of homeostatic interaction, we used multivariable linear stepwise regression analysis with factors PAS duration (3 levels) and Maximum MEP amplitude (maximum value of the average of 10 stimuli for each intensity between T0-T30) and Maximum MEP amplitude ratio (maximum value of the average of 10 stimuli for each intensity between T0-T30 as a ratio to baseline values
88 Chapter 3. PAS Dose-Response Curve and Motor Learning 73 (Tpre) and homeostatic response as dependent variable. Homeostatic response was calculated by subtracting the average of MEP amplitude ratios to baseline (Tpre) after the second interventions (T 15) from average of MEP amplitude ratios of I/O curves of T15 and T30. RMNOVA with factors Intervention (PAS90, PAS180, and PAS 270 conditioning and no PAS conditioning), and Trial block (blocks of motor practice) as with-in subject variables was used to investigate the performance in MLT with a preceding PAS paradigm (Experiment 1A) or without a conditioning paradigm (Experiment 1B). 3.4 Results RMT at baseline was ± 1.6% (mean±se) of stimulator output. No significant differences was observed among the different study sessions Experiment 1A. Effects of three different PAS durations on corticospinal excitability RMANOVA for the factors PAS duration (3 levels, PAS90, PAS180, PAS270), Time (3 levels, Tpre, T15, T30) and Intensity (4 levels, %RMT) as within subject factors for ratio of MEP to baseline measured in I/O curves of APB muscle after PAS showed significant effect of PAS duration (F(2,4.292), p=0.032), Time (F(2,5.932), p=0.012) and PAS duration Time interaction (F(4,1.631), p=0.016) and no significant effect of Intensity. (Figure 3.2) Post-hoc paired t-test showed significantly higher ABP MEP amplitude ratio after PAS270
89 Chapter 3. PAS Dose-Response Curve and Motor Learning 74 compared to PAS90 (p=0.022), and a trend for higher MEP amplitude ratio compared to PAS180 (p=0.07). No significant difference between PAS90 and PAS180 was detected. This finding support our primary hypothesis that increasing the number of PAS repetition pairs increases the LTP-like effects produced by PAS. Post-hoc analysis for the factor Time showed significant increase in MEP amplitude was observed for T15 (p=0.05) and T30 (p=0.02) when compared to Tpre. No significant differences were detected between T15 and T30. Figure 3.2 showed that the significant PAS duration Time interaction is due to increased effect of PAS270 from T15 to T30, whereas the effect of PAS90 decreased from T15 to T30. This finding indicates that duration of LTP-like effect is also influenced by PAS duration Interactions between PAS and Motor Learning We used MEP amplitudes generated by 130% RMT to investigate PAS-MLT interactions (Figure 3.3A). RMANOVA for MEP ratio to baseline with factors PAS duration (3 levels, PAS90, PAS180, PAS270), Time (5 levels, T0, T15, T30, T 0 and T 15) showed a significant PAS duration Time interaction (F (8, 5.34), p <0.001). No significant effect for factors Time and PAS duration were detected. The significant interaction was because of the significant reduction of MEP amplitude after MLT in the PAS270 group whereas MEP amplitude increased after MLT in the PAS90 and PAS180 groups. These findings indicate homeostatic interaction between PAS270 and MLT and no such interaction was found in PAS90 and PAS180 groups (Figure 3.3A)
90 Chapter 3. PAS Dose-Response Curve and Motor Learning Peak MEP amplitude and type of priming intervention predicts the magnitude of homeostatic interaction When the variables PAS duration, maximum MEP amplitude and MEP amplitude ratio were entered into a multiple linear regression model, 57% of the variation of the Homeostatic response was explained. The correlation with MEP amplitude ratio (p=0.34) was not significant, whereas the correlation with maximum MEP amplitude (p=0.01) and PAS duration (p <0.001) were significant. When MEP amplitude ratio was removed from the model the remaining two variables explained 56% (p <0.001) of the variation of Homeostatic response. PAS duration alone explained 39% and maximum MEP amplitude accounted for 17% of this variation. Because there was a significant effect of PAS duration, we examined each duration separately. The Pearson correlation coefficients were significant only for the PAS270 (R 2 = 0.59, p = 0.006, Figure 3.4) Experiment 1B. Effect of motor learning on MEP and PAS90 RMANOVA with Time (4 levels, Tpre-T 15) and Intensity (4 levels, %RMT) as within subject factor for MEP amplitude ratios in I/O curve of APB showed a significant effect of Time (F (3, 4.227), p=0.016) and no significant effect for Intensity and Intensity Time. Similarly RMANOVA for intensity of 130% RMT with Time (6 levels, Tpre-T 15) showed significant effect of Time (F (5, 4.5), p=0.001) and pairwise comparison of the MEP amplitude at different time points showed a non-significant MEP increase at T15 (p=0.17) compared to Tpre and a significant decline in MEP amplitudes from T15 to T 15 (p =0.02) and T 30(p =0.006).(Figure 3.3B)
91 (a) Experiment 1a (b) Experiment 1b (c) Experiment 2 Figure 3.2: Changes in MEP amplitude ratios to baseline (Tpre) over time in Experiment 1A. Each line represents one intensity in the I/O curves. Each PAS protocol is shown in a separate graph A) PAS 90 B) PAS180 and C) PAS270. Values above 1 indicate an increase in MEP amplitude ratio (LTP-like effect) and below 1 indicates reduction in MEP amplitude ratio (LTD-like effect) compared to baseline. Error bars represent standard error of mean. Chapter 3. PAS Dose-Response Curve and Motor Learning 76
92 Chapter 3. PAS Dose-Response Curve and Motor Learning 77 Figure 3.3: Changes in MEP amplitude ratio to baseline (Tpre) for intensity of 130%RMT with time. A) Each line represents one PAS session in experiment 1A; B) Experiment 1B. With the motor learning task (MLT) followed by PAS90 just before T 0.Error bars represent standard error of mean Experiment 2. time course of PAS270 effects The data are shown in Figure 5. RMANOVA for MEP amplitude ratio to baseline (Tpre) of I/O curves showed a trend for factor Time (4 levels, Tpre, T15, T30 and T 15; F (3, 2.8), p =0.07), no significant effect of Intensity (4 levels, %) but significant for Time Intensity interaction (F (9, 3.2), p =0.004). The significant interaction was due to different modulation of MEP amplitude ratios measured by different intensities. Intensities of 100, 110 and 130%RMT showed reduction while intensity of 120%RMT showed increase after T15. Moreover, the intensity of 130%RMT showed larger increase in MEP amplitude ratios compare to intensities of %RMT after one hour (T 0- T 15) (Figure 3.5). RMANOVA of MEP amplitude ratios to baseline (Tpre) for 130% RMT with factors Time (6 levels, Tpre-T 15) confirmed the significant effect of Time (F (5, 2.8), p =0.03). Posthoc t-tests showed significant increase at T0, T15 and T 15
93 Chapter 3. PAS Dose-Response Curve and Motor Learning 78 compared to baseline and a significant increase from T30 to T 15, indicating an early and a later phase of increase in MEP amplitude ratio after PAS Motor Learning Task RMANOVA with Intervention (4 levels, sessions1-3 of experiment 1A and experiment 1B) and Trial block (6 level) as within subject factors showed a significant effect for the Trial block (F (5, 7.89), p =0.007) on the percentage of correct responses. No significant effect was observed for different Interventions and Trial block Intervention interaction. Pair wise comparisons for the Trial blocks showed a significant increase in the percentages of correct responses between first and second blocks (p =0.033), third and fourth blocks (p =0.037) and fourth and fifth blocks (p =0.035). This comparison indicates similar increase in the percentages of correct responses over the course of the MLT in all Interventions. (Figure3.6)
94 Figure 3.4: Relationship between maximum MEP amplitude and homeostatic response for each PAS duration. The vertical axis represent changes in combined mean MEPs from I/O curves at T15 and T30 minus mean MEPs of I/O curves at T15 (homeostatic response). Larger values indicate stronger homeostatic responses. The horizontal axis represents the maximum MEP amplitudes before MLT. Each PAS session is shown in separate plots. A)PAS90 B)PAS180 and C)PAS270. Error bars represent standard error of mean. Chapter 3. PAS Dose-Response Curve and Motor Learning 79
95 Chapter 3. PAS Dose-Response Curve and Motor Learning Discussion Associative plasticity is thought to be an important neurophysiological correlate of memory formation and learning [50]. In this study, we showed that duration of PAS induction, defined as the number of paired stimuli, influences the plasticity outcome. We also found that the interaction of PAS intervention with a simple motor learning paradigm depends on the state of cortical excitability before the motor learning paradigm and PAS duration. The LTP-like effect observed after PAS270 was reversed by MLT which produced LTD-like effect after PAS270 (homeostatic interaction). This type of interaction was not seen after PAS90 and PAS180. Since the MLT by itself increases MEP amplitudes, the LTD-like effect that we observed is related to the priming effect of PAS on MLT. The time course of PAS270 was also studied with more time points than previous studies for 75 minutes after PAS and we found that LTD-like effect did not occur. In contrast, we found a late phase of increase in cortical excitability after PAS270. This finding is in-line with previous studies that showed gradual increase in MEP after PAS for duration of over one hour [251, 173, 74]. To our knowledge, the role of different levels of LTP-like plasticity on homeostatic and non-homeostatic responses has not been studied in human motor cortex. We showed graded response after PAS and its interactions with motor learning. It has been postulated that PAS LTP-like effect is due to spike-timing dependent plasticity (STDP) [250]. STDP works by correlating pre- and postsynaptic neuronal spikes, and can result in strengthening or weakening of synapses, depending on the temporal order of spiking. Most experimental studies of STDP have focused on the timing of spike pairs [158]. Although the BCM model [21] of STDP explains the main aspects of the synaptic plasticity at microscopic level, certain limitations exist for this model at the networks level. Application of STDP to larger system level scale requires certain considerations. Reorganization of the network, nature of the collective responses
96 Chapter 3. PAS Dose-Response Curve and Motor Learning 81 Figure 3.5: MEP amplitude ratio to baseline (Tpre) for PAS270 alone (Experiment 2). A) I/O curves. Each line shows one intensity. B) Intensity of 130%RMT. Error bars represent standard error of mean. measured through the network output and propagation of effect to other parts of the network are important issues that are not included in the BCM model of STDP. This includes the effect of number of stimulus pairs and its relation to plasticity induction. In this study, we found increasing MEP amplitude in response to longer duration of PAS induction. However, the synaptic or non-synaptic mechanisms of these changes need to be elucidated. MEP amplitude is an established marker for the degree of cortical and pyramidal tract activation [2]. There are at least two possible explanations for training induced increases in MEP amplitudes. MEP amplitudes increase could reflect a) improved connectivity of interneurons to pyramidal tract neurons in the motor cortex or b) an increase in the number of cortical neurons of similar excitability activated by the TMS (excitability threshold), or a combination of both. In a previous study [74] we showed no significant changes in response to PAS in resting and active motor thresholds, and in this study we showed no significant role of Intensity in amount of PAS plasticity effect measured in
97 Chapter 3. PAS Dose-Response Curve and Motor Learning 82 I/O curves therefore it is more probable that the results observed in this study are due to changes in interneuronal connectivity than changes in neuronal threshold. We also observed both homeostatic and non-homeostatic interaction in response to MLT depending on the history of LTP-like effect induced by condition PAS. Jung and Ziemann [128] observed that PAS induced LTP-like effect on motor learning could in part be nonhomeostatic because motor learning could be enhanced after PAS LTP. This effect could be due to non-saturated priming LTP. Studies in mice and rat hippocampus showed that saturated LTP occludes [155] while non-saturated LTP facilitates subsequent learning [18]. However, we did not observe modulation of the MLT by prior PAS as all experimental groups showed similar improvement in MLT. This could be due to different motor training paradigm in our study compared to previous studies. We measured improvement in the accuracy of thumb abduction in a use dependent paradigm and in response to a tactile Go signal whereas a previous study measured increase in the speed and acceleration of the thumb movements [128]. The underlying mechanisms for improvement in motor accuracy and speed are likely different. Further studies to explore modulatory effects of PAS on various components of skill acquisition and adaptation are needed. One such relationship has been explained by Fitt s law indicating a trade-off between motor speed and accuracy [83]. Furthermore, motor skill learning can be divided into a fast (early) stage, in which significant improvements can be seen within a single training session, and a later, slow stage, in which further gains can be achieved across multiple practice sessions or between training sessions (offline learning). In one study [207], improvement in motor accuracy in a rotary pursuit task was observed one week after the facilitatory PAS. In the current study we examined the early stage of use dependent motor learning and therefore it is possible that improvement in movement accuracy occurred later and remained undetected.
98 Chapter 3. PAS Dose-Response Curve and Motor Learning 83 Modulation of PAS by motor learning has also been demonstrated. Several studies showed that prior motor learning produced a homeostatic effect on the subsequent associative plasticity in healthy subjects. Motor learning decreases subsequent PAS induced LTP plasticity but increases LTD plasticity of the trained motor cortex (M1)[293]. This is in-line with our findings. Facilitatory PAS resulted in a paradoxical LTD in a previously trained motor cortex. It is interesting to note that even the shortest PAS duration used in this study (PAS90) resulted in paradoxical LTD-like plasticity in a previously trained subject. However, we did not investigate longer PAS durations after MLT to determine if longer PAS durations can also produce similar interactions. The delay between priming stimulation (PAS) and motor learning seems to play an Figure 3.6: Performances during the motor learning task in experiment 1A (PAS 90-MLT, PAS 180-MLT, and PAS 270-MLT) and 1B (MLT- PAS 90). The x-axis shows the trial blocks for the motor learning task and the y-axis indicates performance as percentages of correct responses for each block. Error bars represent standard error of mean. important role in induction of the homeostatic responses [128]. One study showed that homeostatic and non-homeostatic interaction between PAS and subsequent learning of rapid thumb flexion movements depended on the time delay between priming stimulation (PAS) and motor learning [128]. Facilitatory PAS improved motor acceleration imme-
99 Chapter 3. PAS Dose-Response Curve and Motor Learning 84 diately after the LTP induction (non-homeostatic) and reduced the motor acceleration with 90 minutes delay (homeostatic). Therefore, we delayed the second intervention by one hour to allow sufficient time for the metaplastic mechanism to occur. The longer PAS duration (PAS270) in this experiment showed an early and a late phase of increased cortical excitability (Figure 5B). This late increase in excitability after PAS had also been observed in previous studies [251, 173]. In contrast to PAS270 alone (Figure 5B), PAS270-MLT combination (Figure 3A) resulted in significant reduction of MEP amplitude. This interaction cannot be explained by time course of PAS270 alone or the effect of MLT alone. The mechanism of reversal of synaptic potentiation could be explained by depotentiation or synaptic metaplasticity. The role of depotentiation is not well known. It has been suggested that both depotentiation and synaptic metaplasticity prevent unnecessary information to be stored by keeping neuronal firing rate within a physiological range [3, 289]. Few studies have shown deficits of depotentiation processes in a mouse model of schizophrenia [238] and homeostatic responses in diseases such as dystonia [202]. Understanding homeostatic metaplasticity in both healthy subjects and disorders of cortical plasticity is important because it may lead to development of new electrophysiological biomarkers or diagnostic tools for these conditions. In this study we showed graded LTP-like response to PAS duration and showed that homeostatic interaction between PAS and subsequent motor learning is related to the maximum amount of MEP amplitude achieved before the motor training and duration of PAS intervention. Understanding the rules of synaptic plasticity at the systems level will ultimately help us to develop efficient protocols to modulate the motor cortex or new biomarkers to capture abnormalities of cortical plasticity in patients with neurological and psychiatric disorders.
100 Chapter 3. PAS Dose-Response Curve and Motor Learning Supplemental: PAS in a Case of Extreme Task Specific Dystonia Published in Movement Disorders, 28 June Introduction Focal hand dystonia may be task specific, as is the case with writer s cramp. In early stages, task specificity can be so specific that it may be mistaken for a psychogenic movement disorder [237] Aims and Hypotheses Related to the article by Shamim et al [237] and the accompanying editorial by Edwards and Rothwell [72], we would like to describe our experience with a patient with even greater task-specific motor dysfunction. This is cae report of an extreme task specific dystonia. We hypothesized that Paired Associative Stimulation (PAS) can be used to detect abnormalities that differentiate organic vs. psychogenic dystonia Case Presentation A 71-year-old man had a 15-year progressive history of transiently getting stuck in the second and third parts of his signature. These symptoms were worse with stress, and he felt anxious and tense while struggling with signing. He noted no difficulty in other writing tasks or other manual functions. He had isolated reduction in right arm swing with no features of parkinsonism or dystonia, including mirror dystonia. While attempting to sign his name, he would get stuck for up to several seconds in attempting to initiate each of the three components of the signature that began at the top of the line. Repeated
101 Chapter 3. PAS Dose-Response Curve and Motor Learning 86 signing caused increasing duration of blocks stress, and anxiety with perspiration and the need to remove his sweater. Apart from signing, he had no difficulty writing and could transition from severe blocking in signing to smooth, uninterrupted writing only to have the blocks return when signing his name Methods We assessed cortical inhibition (i.e. short afferent inhibition; SAI), short interval intracortical inhibition (SICI), and plasticity (i.e. paired associated stimulation of peripheral nerve and motor cortex to induce plasticity; PAS25 protocol). Interstimulus interval (ISI) was 20 ms for SAI, 2 ms for SICI, and 25 ms for PAS25 protocol. Median nerve stimulation (3 perceptual threshold) a TMS over the motor cortex (i.e. over the optimal position for evoked abductor pollicis brevis [APB] motor evoked potentials [MEPs]) were used. TMS-induced MEPs were recorded from APB and abductor digiti minimi (ADM) muscles using surface electromyography (EMG) electrodes. MEP intensity curves were measured at baseline and 10, 20 and 30 minutes after the PAS25 protocol Results Before PAS (baseline), SICI (53% control or 47% inhibition) and SAI (50% of control) for the APB muscle were normal. There was a tendency for reduction in MEP amplitude at 10 and 20 minutes after PAS25. However, at 30 minutes after the PAS25, MEP amplitudes increased by a factor of 2.39 in the APB muscle and 2.66 in the ADM muscle at stimulus intensities that produced MEPs of 1mV in the APB at baseline.
102 Chapter 3. PAS Dose-Response Curve and Motor Learning Discussion Clinically, we felt that our patient could have a form of performance anxiety or signing block (akin to some cases of the yips [5]), although it remained possible that he had an extreme form of task-specific dystonia. In contrast to Shamim et al. s patients [237], there were no features supportive of dystonia. Also, against dystonia, SICI and SAI were normal. SICI has been shown to be diminished in both organic and psychogenic dystonia[5]. SAI is normal in both psychogenic and organic dystonia[203]. However, the PAS25 protocol resulted in an excessive increase in cortical excitability (e.g., increase in MEP size) in homotopic (i.e. APB) and heterotopic (i.e. ADM) muscles. A previous study showed abnormal increase in PAS-induced cortical plasticity (leading to excessive, and heterotopic increase in, cortically evoked MEPs) in organic, but not psychogenic, dystonia[203]. This experience fails to provide a definitive answer as to whether our patient and others with such extreme, longstanding, and isolated task-specific motor problems have a form of dystonia or some other type of motor programming disturbance. Edwards and Rothwell [72] postulate that focused attention could cause unwanted involuntary movement, perhaps initially caused by stressful situations during performance, and, eventually, these unwanted movements could be incorporated into the task itself (and, eventually, into other tasks) and appear even in the absence of stress. They speculated that enhanced plasticity could produce vulnerability for patients to develop task-specific dystonia that spreads to involve other tasks. However, our patient maintained his pure isolated signing motor block without progression to clear dystonia or to interference with any other tasks, despite electrophysiological evidence of increased plasticity. His preserved intracortical inhibition might have played a role in limiting the spread of symptoms. This may be similar to professional musicians (without dystonia) who have increased PAS plasticity, but preserved cortical inhibition [219].
103 Changes in Kinematic properties of Upper Limb after Paired Associative Stimulation Introduction Our hands allow us to perform delicate, complex, individuated, fine motor movements [240, 107] and learning motor skills relies on integrated sensory inputs to the cortex. Sensory inputs are also important in accurate movement execution. These ability to perform skillful movements are associated with large, orderly, somatotopic, highly differentiated representations of the hand in the thalamus, basal ganglia, somatosensory cortex and motor cortex [197, 126, 129]. These motor and sensory topographical representations are subject to constant change by physiological and pathological factors such as limb sensory deprivation and dystonia [167, 40, 165]. Paired associative stimulation (PAS) is an experimental paradigm widely used to induce topographically localized plasticity in the human motor cortex [213, 250]. This technique uses repetitive median nerve stimulation paired with cortical stimulation. The electrical nerve stimulation and cortical TMS pulses are timed so that the peripheral input and the central stimulus arrive near-synchronously at the motor cortex. The time between the two modes of stimulation is critical; initially 25ms was chosen to allow for peripheral conduction time from the periphery to the so- 88
104 Chapter 4. Upper Arm Kinematics after PAS 89 matosensory cortex ( 20ms) and from there to the motor cortex ( 3 ms). However, Stefan et al. discovered that inter-stimulus intervals up to 35ms were effective, provided the peripheral volley arrived prior to the cortical stimulus [251]. Reversing the sequence of arrival of the afferent signals so that the peripheral volley arrived after the cortical stimulus induced depression of cortical excitability [50, 251], as proposed by the strict temporal spike timing dependent plasticity (STDP) model [158, 20]. This feature plus some other characteristics of PAS such as NMDA dependency [250] is consistent with the idea that induction of plasticity in this way is similar to LTP and LTD. Patients with focal dystonia had deficient cortical synaptic plasticity [201, 132]. Patients with generalized or segmental dystonia showed changes in the movement kinematics such as slower task execution in sequential movements compared to simple one-step movements [123]. PAS has grown in popularity and its potential for clinical uses. 4.2 Hypotheses In this study we focused on the behavioral consequences of PAS stimulations, specifically the effect of cortical excitability induced by PAS on kinematics of upper limb in simple reaching task, finger opposition, sequential arm movement and motor learning. The specific hypotheses of this study include: 1. PAS improves human motor learning through increasing the weight of synapses in sensory motor network. 2. PAS increase acceleration, rapidity of movement and improve motor learning.
105 Chapter 4. Upper Arm Kinematics after PAS Material and Methods Subjects Nine healthy right handed subjects (5 men and 4 women), aged 29±5 years (mean±sd), participated in the study. They had normal neurological examination, with no history of mental illness or epilepsy and no contraindication for TMS [220]. All the subjects were able to perform the tasks involved in this study. Each subject participated in two study sessions at least one week apart. All the subjects participating in this study confirmed their willingness by both written and oral consent EMG Recording Surface electromyographic (EMG) were recorded from the right abductor pollicis brevis (APB) using bipolar Ag-AgCl electrodes. The signals were amplified 1000 times, and filtered (5 Hz Hz) (Intronix Technologies Corporation Model 2024F, Bolton, Ontario Canada) and digitized CED 1401 interface Transcranial Magnetic Stimulation (TMS) Magstim 200 stimulator (Magstim, Whitland, Dyfed, UK), and a figure-of-eight shaped coil (outside diameter of each loop was 9.5cm) were used to apply TMS to the left M1. The trigger pulses for TMS were delivered from a Micro1401 interface (Cambridge Electronics Design) controlled by Signal Software (3.07). The coil was held tangentially to the skull with the handle pointing backwards and laterally at an angle of 45to the sagittal plane at the optimal scalp site to evoke a MEP in the relaxed APB muscle of the right hand and the motor hot-spot was marked.
106 Chapter 4. Upper Arm Kinematics after PAS Paired Associative Stimulation PAS involves a series of paired peripheral and cortical stimuli. An electrical stimulus was delivered to the median nerve of the right wrist at intensity sufficient to produce a small muscle twitch in the APB muscle. The electrical stimulus was then coupled with a supra-threshold TMS adjusted to produce 1mV peak-to-peak MEP amplitude in the APB muscle. The interval between median nerve stimulation and TMS was 25ms for PAS. One hundred and eighty pairs of stimuli were applied to left M1 at 0.25 Hz frequency. PAS with 100ms interval was used as the sham control. Previous studies have shown that this inter stimuli interval does not affect the cortical excitability. Outcome of interest and evaluation Rest Motor Threshold (RMT) was defined as the minimum stimulator output that is capable of evoking MEPs of more than 50 V in at least 5 out of 10 trials when ABP muscle was completely relaxed. Five intensities were used to plot the Input/output (I/O) curves, 100% RMT, and 100%RMT +10, +20 and +30% of RMT and10 MEPs were averaged for each intensity Battery of Kinematic Tasks Setup A cm table was placed in front of the subject sitting at 10cm away from the margin of the table and in front of a computer screen. The subjects elbow and the table were at the same level. A reference point was marked in the center of the table and 4 metallic disks were placed at 30cm from the center of the table (Figure 4.1). The disks were 1cm in diameter and were fixed to the surface of the table. An electromagnetic
107 Chapter 4. Upper Arm Kinematics after PAS 92 3-dimentional (POLHEMUS FASTRAK, Colchester, Vermont USA) object tracker was used to provide the position of a pen like stylus held in the subjects hand. The sampling rate was 21.33Hz. Metallic disks are connected through a circuit that produces signals to mark the onset and duration of touch for each disk by the subjects. Data from these markers were recorded in Signal software and were used to analysis the movements Simple Arm Movement Task The subjects were seated comfortably in a quiet room in front of the table with their elbow resting on the margin of the table. They held the stylus pointing at the reference point. A visual Ready signal was shown on the computer screen followed by a Go signal. Go signal indicated both the time and direction for their movement to one of the four possible directions on the table in front of them. Each signal was illuminated for 600ms and the interval between Ready and Go signal was set to vary randomly between 2-2.5s. After the Go signal, subjects moved their arm in the direction of the Go signal to reach the assigned metallic disk and moved back to the center position as fast as they could. Forty movements were recorded to each of the 4 possible directions. Direction of the movement to Go signal were varied randomly and produced about 10 movements for each direction Sequential Arm Movement Task For this task, after the Ready signal the subjects received three Go signals and they had to memorize the direction order of each signal. Each visual signal lasted for 600ms followed by 400ms pause. After the 3rd signal, the subjects had to reach the first, second and third metallic disks in order indicated by the Go signals and move back to center position after each reach. The subjects were asked to perform the task as fast as they
108 Chapter 4. Upper Arm Kinematics after PAS 93 could. Twenty sequential arm movements were recorded. Simple and sequential movement tasks were performed in random order. The directions of movement in sequential movement task was also randomized. Task analysis In simple and sequential arm movement tasks, the following properties were measured: (a) trajectory path and the velocity profile; (b) final position error, (c) peak velocity and d) movement duration. Velocity was calculated in 3D space by calculating the changes in distance over time. Movement rapidity index were also calculated by measuring the Touch Duration (TD) and Inter Touch Interval (ITI) of each metallic task by the following formula: 1/(T D + IT I). This parameter shows the number of touches per second and it is expressed in Hz [11, 29]. The Path-Displacement variability was calculated by subtracting the displacement from the total motion path for simple arm movement task. This variable shows the extent of deviation from a direct path toward the goal. These properties were used to compare between simple and sequential movement and between PAS25 and PAS100 (Sham) groups[128] Finger Opposition Task A costume designed glove with conductive tips was used. The subjects wore the glove and were instructed to open up their hand in supination position on the table and perform two different sequences of finger oppositions after a tactile Go signal delivered through a bar electrode located over the median nerve at wrist. The tactile Go signal was adjusted to two times of each subject s perceptual threshold for the duration of 200ms. The contact of the thumb to each digit produced a signal which was used to calculate touch duration and inter touch interval for each digit. The signals were amplified 1000
109 Chapter 4. Upper Arm Kinematics after PAS 94 times, and filtered (5 Hz Hz) (Intronix Technologies Corporation Model 2024F, Bolton, Ontario Canada) and digitized through a CED 1401 interface and were recorded in Signal software. The right hand was studied. In Sequence 1 the subjects to touched digits 2, 3, 4 and 5 sequentially with their thumb as fast as possible. In Sequence 2, the subjects touched digits 4, 2, 5 and 3 with their thumb in this order as fast as possible Motor Learning Task The subjects performed brisk isometric abductions with their right thumb to a target force window. The thumb abduction force was recorded by a small force transducer (Strain Measurement, load cell). The tactile Go signal (200% of perceptual threshold) was electrical stimulation of median nerve at the wrist. The Go Signal frequency was 0.5 Hz. EMG and force signals were fed back to the subject on a computer screen and subjects were informed of correct responses. After the subject s maximum force was established, the target force window was defined as between 35 and 45% of the subject s maximum force and was displayed as two horizontal lines on the computer screen. The target window was the same size (4cm wide) for all the subjects on computer screen. Each motor learning task included 4 blocks of 25 isometric contractions of ABP muscles with 30s pause between blocks. Study Design Each participant was tested in two separate sessions at least one week apart in random order. Each session started with the I/O curve measurement followed by either PAS25 or PAS100 (sham). I/O curve measurement were repeated 4 times during the experiment before and at 5(T5), 60(T60) and 80(T80) minutes after the PAS. Simple and sequential movement tasks were performed at 25 minutes post PAS, followed by finger opposition task and the motor learning task (Figure 4.1).
110 (a) Study setup (b) Study design Figure 4.1: a) Study setup, showing the arm movement task. The subject is sitting in front of computer screen and a Go signal shows direction and time of the movement initiation. the subject should move the stylus from the center position to the shown direction and move back to center position as fast as possible. 2D/3D position of the stylus as well as the Touch duration and Inter touch interval of movement to each corner will be recorded.b) Study design, Subjects are randomized into two groups, one receiving PAS25 and the other group PAS100. A battery of kinematic tests and force learning task were then performed. Input/output (I/O) curves were measured at baseline (T0), and 5 (T5), 60 (T60) and 80 (T80) minutes post PAS Chapter 4. Upper Arm Kinematics after PAS 95
111 Chapter 4. Upper Arm Kinematics after PAS 96 Statistical analyses Repeated measures analysis of variance (RMANOVA) was used to compare means in a general linear model using SPSS Sphericity of variance was assumed when Mauchly s test of sphericity was not significant. If sphericity was violated, Greenhouse and Geisser corrections were applied.[97] Intervention (Sham vs. PAS25), Time and Intensity (of TMS in I/O curve measurements), Trial of practice blocks of motor learning were used as within subject factors. RMANOVA were applied when the number of groups or the number of factors were more than two. MEP amplitudes measured in APB muscle were normalized to their baseline values and normalized values were used in the analyses. Paired t-tests were used to test the differences between PAS25 and PAS100 for the various kinematic tasks. If the main ANOVA effect is significant, post-hoc testing were performed using Bonferroni test if there were more than two group and the paired sample t-test for two groups. P-value of less than 0.05 was considered as statistically significant. 4.4 Results Corticospinal Excitability The RMANOVA for normalized MEP ratios for the APB muscle with Intensity (4 levels, 100%-130%RMT), Time (3 levels, T5-T80) Intervention (2 level, PAS25 and PAS100) as with-in subject factors showed significant effect of intervention (F (1, 6.22), p =0.03), and a trend for Time (F (2, 3.58), p =0.052), and Time intensity interaction (F (6, 2.74), p =0.1). The effect of intervention was due to greater increase in MEP amplitudes in the PAS25 group compared to the PAS100. (Figure 4.2)
112 Chapter 4. Upper Arm Kinematics after PAS 97 Figure 4.2: Input/output curve of stimulation intensity (% resting motor threshold) and Motor Evoked Potentials (MEP) induced in targeted muscle of hand (APB) for A) PAS25 and B)PAS100. Each column shows a particular time point T0: Baseline and T5, T60 and T80: 5, 60 and 80 minutes after the PAS. Error bars indicate standard error of mean PAS25 increased Maximum Movement Speed in Simple Arm Movement Task RMANOVA for the maximum velocity with factors Intervention (PAS25 and PAS100) and Task (sequential vs. simple arm movement task) also showed significant effect of intervention (F (1, 29.02), p <0.001) but no significant changes for the factors Task and Task Intervention interaction. This finding also confirms the effect of PAS25 on the simple arm movement task (Figure 4.3(b)). The maximum velocity of simple arm movement task increased was lower in the PAS100 group (190.1±7.5 cm/s) compared to the PAS25 group (265.0±20 cm/s, P =0.013). However, the difference between the average speed and the rapidity index for the two groups were not significant. The results can be explained by longer path of movement in simple arm movement task after PAS25 as a result of increased movement variability, as demonstrated by longer path-displacement variability in the PAS25 group (p =0.008). After PAS25, subjects traveled on average 4 cm longer distances on a 30 cm displacement track. (Table 4.1)
113 Table 4.1: Summary of finding of various kinematic tasks and comparison between PAS25 and PAS100; * indicated statistically significant value; Mean difference shows average of the values in PAS25 group minus values in PAS100 group Task PAS25 PAS100 Mean Difference SE p-value Simple arm movement task Maximum velocity (cm/s) * Reaction Time Rapidity index Path-Displacement variation * Average speed (cm/s) Sequential arm movement task Maximum velocity (cm/s) Rapidity index (Hz) Opposition task (sequence 1) Rapidity index (Hz) Opposition task (sequence 2) Rapidity index (Hz) Motor learning task (% correct responses) First block Final block Chapter 4. Upper Arm Kinematics after PAS 98
114 Chapter 4. Upper Arm Kinematics after PAS Sequential Arm Movement Task No significant differences were detected for maximum velocity, rapidity index and reaction time between PAS25 and PAS100 for this task (Table 4.1). RMANOVA for comparison of reaction time between PAS groups and simple vs. sequential arm movement task showed a trend for the task used (F (1, 17.6), p=0.052) but no significant effect for factor Intervention and Intervention Task interaction. These findings indicate slight increase in reaction time for the sequential arm movement task compared to simple arm movement task (Figure 4.3(b)) Finger Opposition Task RMNOVA for rapidity index showed a trend for a significant effect of Intervention (PAS25 and PAS100) (F (1, 3.97), p=0.09) which indicated a trend for increased rapidity of movement after PAS25 with no significant effects of task difficulty (Sequence 1 and 2) and Intervention test difficulty interaction (Table 4.1) Accuracy of Movements in Force Learning Task RMANOVA for the percentage of correct responses showed no significant effect of Intervention and intervention trial interaction, but the effect of practice trial was significant (p=0.04). This indicates that in the PAS25 and PAS100 groups had similar improvement in the percentage of correct responses after both PAS protocols and with practice (Figure4.3(c)).
115 (a) Reaction time (b) maximum velocity (c) force learning task Figure 4.3: A) Reaction time for arm movement task, comparing the sequential vs. single arm movement task B) Maximum velocity measured for each simple and sequential movement task C) accuracy of of movement, measured with a force learning task showing the percentages of correct responses for each trial of PAS interventions. Asterisk(*), indicates statistically significant difference (p < 0.05). Error bars represent Standard error of means. Chapter 4. Upper Arm Kinematics after PAS 100
116 Chapter 4. Upper Arm Kinematics after PAS Discussion This study shows that changes in cortical excitability of motor cortex can generate measurable changes in kinematics of movement. PAS25 compared to sham PAS resulted in increased maximum velocity of movement in simple arm movement task and increased variability in motion path. However, these changes were not observed in sequential arm movement or sequential finger opposition tasks. The motor cortex has been classically viewed as a region which encodes information related to the direction and force of movement [80]. The rates of individual neuronal discharge were found to change with direction of movement in the M1 of monkey [93], but none of the cortical neurons responded uniquely to a single direction. Moreover, the amplitude of the movement seems to be unrelated to the discharge rates of these neurons suggesting that the amplitude and direction of movement are encoded separately in the M1 [217, 96]. In this study we provide further information using non-invasive brain stimulation in human to demonstrate that the changes in cortical excitability of M1 can change the maximum of velocity of arm in simple reaching task. However, this increase in velocity did not change the accuracy of movement in the motor learning paradigm. It is interesting to note that increased in maximum velocity did not translated into the faster overall movement of arm measured by rapidity index possibly as a result of increased movement variability. The supplementary motor area (SMA) is known to be involved in high-level planning and production of complex movement sequences [282]. it was shown in a PET imaging study that SMA is strongly activated when people imagine themselves performing complex sequences of finger movements [215]. Both human and animal studies further confirmed these findings by showing that people or animals with damage to SMA have difficulty with high-level control of movement [33, 89, 111]. Transient inactivation of the SMA with
117 Chapter 4. Upper Arm Kinematics after PAS 102 high frequency rtms had also been shown to interrupted movement sequences by inducing accuracy errors only in the complex sequence, while stimulation over the primary motor cortex induced errors in both the complex and scale sequences [94]. TMS applied over SMA seems to be important in bimanual movements as well, few studies have shown disruption of anti-phase index finger movements as well as inter-manual transfer of task improvement from one hand to the other[198]. In this study we used two sequential movement tasks: Sequential arm movement and finger opposition. None of these tasks showed significant changes in our measurements after PAS, suggesting that PAS induced behavioral effects are specific to the targeted cortical region. SMA functioning at least in the measurement we used in this experiment remained consistent between PAS25 and PAS100. Abraham and Bear introduced the concept of metaplasticity, which refers to a higherorder form of synaptic plasticity where prior synaptic activity leads to a persistent change in the direction or magnitude of subsequent activity-dependent plasticity, without affecting actual synaptic efficacy (Abraham 1996). History of cortical activity can modulate motor learning but little is known about the extent of this modulation and rules governing these interactions. Previous studies have shown that facilitatory PAS can alter subsequent motor learning by the changing peak acceleration of the trained movement [128] but we did not find significant differences between facilitatory and sham PAS in the accuracy of the motor learning task. This might due to different outcome measures used. It worth mentioning that peak acceleration of arm movement have increased in this study however the accuracy of the movement is not compromised as a result of speed-accuracy trade off. Furthermore, it is possible that improvement in accuracy measured in motor learning task may occur in a longer time scale. One study has shown that improvement in rotatory pursuit task can occur up to at least one week after brain stimulation [207].
118 Chapter 4. Upper Arm Kinematics after PAS 103 However, the focus of current study was to capture immediate kinematic changes after topographically specific M1 cortical excitability change. Changes in variability of movement are also an interesting finding. It has been suggested that motor variability and noise in the motor control system can actually benefit skill learning and adaptation [193]. In one study, theta burst stimulation was used to modulate thumb acceleration in a learning paradigm and authors observed significant increase in the coefficient of variability after the real TBS stimulation compared to The sham protocol [257]. This may be similar to our findings of increased Path-displacement variability in simple arm movement task after the PAS25 paradigm. 4.6 Conclusion In summary, the result of this study will help the design of future experiments with PAS or other brain stimulation paradigms in the M1 by identifying the features of movement affected by increased M1 excitability. Only some aspects of the movement were affected by the stimulation, namely maximum arm velocity and path-displacement variability but performance in the motor learning task which requires precision and accuracy was not affected. These findings suggest that brain stimulation techniques may be used to preferentially modulate the velocity of movement without compromising accuracy or precision.
119 General Discussion and Future Directions Summary of Major Findings 1. Application of SICI, which activated GABAergic interneurons, inhibited PAS induced LTP-like plasticity in the motor cortex. 2. PAS can be conditioned selectively to engage GABAergic or Glutaminergic interneurons. 3. Increasing number of stimulus pairs in PAS correlates with increasing amount of LTP-like effect. 4. Graded increased in cortical excitability after PAS correlate with induction of homeostatic response after motor training. 5. Motor practice induced a use dependent plastic change in M1 captured using TMS induced MEP amplitudes measurement. 6. preconditioning of PAS with motor practice resulted in homeostatic response in M1 corticospinal excitability even with smaller numbers PAS. 104
120 Chapter 5. General Discussion and Future Directions PAS preconditioning of motor training did not significantly change movement accuracy. 8. PAS increased variability of hand movement in simple arm movements. 9. PAS increased maximum velocity of movement in simple arm movement task however; velocity of movement in complex hand movement was not affected. 5.2 General Discussion Research has made significant progress in the field of non-invasive brain stimulation, starting from the observation that both facilitatory and inhibitory PAS effects may persist after the induction of plasticity. Compared to other stimulation paradigms such as TBS and rtms, PAS seems to be the most efficient protocol [58] and, a logical extension of this will be attempts to use PAS as a therapeutic tool in neurologic and psychiatric disorders characterized by dysfunction of distinct brain networks such as Parkinson disease. In this thesis, we showed that the influence of several major factors such as intracortical facilitatory and inhibitory networks as well as the parameters of stimulation (number of pairs) on the effect of PAS. However, there are other of variables that may affect the PAS response. Attention [252], cortisol level [230], circadian cycle [229], dopamine level [260]and age [81] may influence the PAS effect as well as various PAS parameters such as intensity of median nerve stimulation, repetition rates and ISIs, just to name a few. The finding that PAS response is exaggerated or diminished in certain diseases and that the certain medications for example dopaminergic drugs can modulate the PAS effect all
121 Chapter 5. General Discussion and Future Directions 106 indicate the possibility of clinical application of this technique as a noninvasive predictor of the clinical response after treatment or as a diagnostic tool. Although we answered few of these questions, further studies are required to investigate the complex interactions between brain, PAS and other environmental factors in both healthy and diseases Effect of Motor Practice on MEP In general MEP amplitude recorded from muscle groups involved in training movement increases [35, 176]. Increases in MEP amplitudes are often associated with improved performance or changes in the kinematics of movements elicited by TMS of M1 after training protocols [35, 176]. and may reflect changes in the motor output zone related to motor learning. Muellbacher et al. [176]studied the learning-related changes in M1 excitability with TMS while and found that subjects rapidly learned to optimize ballistic contractions measured via pinch acceleration and peak force and improvement in subjects performance were associated with concomitant increase in MEP amplitudes in targeted muscles. MEPs returned to their baseline amplitude after subjects had acquired the new skill, no practice induced changes in MEP amplitude were observed with task over learning [176]. These findings are consistent with concepts of multiple overlapping motor representations in animal studies of motor cortex [70, 233]. Intracortical microstimulation of macaque monkey motor cortex showed extensive, horizontally oriented, intrinsic axon collaterals that provide inputs to many different forelimb movement repre-
122 Chapter 5. General Discussion and Future Directions 107 sentations these neurons may be recruited during complex movements to coordinate the activity of motor cortical zones during a use dependent plastic change in motor cortex [120]. Use-dependent and skill-dependent plasticity contributes to the recovery of motor function after injury to the brain [184, 185] and this functional plasticity of the motor cortex accompanied by changes in synaptic morphology in animal models [185]. These findings set the stage for development of new, more effective rehabilitation interventions. Cortical stimulation can enhance the beneficial effects of motor training on performance, cortical plasticity and motor cortical excitability [119, 134]. In contrast to the previously described beneficial effect cortical stimulation in recovery of stroke related loss of usedependent plasticity [119, 134] we found that in healthy subject cortical stimulation did not further improve increased MEP amplitudes after use-dependent plasticity and even resulted in homeostatic reduction of MEP amplitudes after increasing the amount cortical stimulation. Cortical stimulation did not affect motor learning task performance either. Possible explanations for this paradoxical findings could be 1) cortical stimulation may improve loss of function in a pathological condition but not necessarily improve MEP amplitudes or motor behavior performance in already optimally functioning healthy subjects; 2) it can also possible that improvement in behavioral effect occur in different time scale (for example weeks or months after cortical stimulation and motor practice) previous studies had shown correlation between MEP amplitude and improvement in kinematics of movement. In our study of healthy subjects preconditioning of use-dependent plasticity
123 Chapter 5. General Discussion and Future Directions 108 with cortical stimulation at higher number of stimulation pairs resulted in reduction in MEP amplitudes. This might indicate that healthy subjects have already reached their best performance and further increase in performance is not possible and increases the possibility of first explanation for our findings Variability in TMS response Inter- and intra- individual variability exists in most TMS measures. Much of the TMS studies assume little difference between individuals in order to compare healthy subjects with groups of patients or the effect of a particular intervention on the MEP. Although age and sex are commonly matched between groups the rest of influencing factors are often being neglected. Intra-individual variability is usually considered as noise which is a naive assumption as critical information might lie within these changes of variability in one subject. This issue recently attracted some attention for example one study it has been showed that itbs increased performance variability, which correlated with learning outcome and suggest that increase motor output variability may have role in improvement of performance after itbs [257]. Age is another important factor for inter-individual variability. Response to cortical stimulation interventions can be affected significantly by age. One study showed that the magnitude of MEP increased by PAS in the young and middle but not in the elderly and its change was negatively correlated with the age. These results suggest that the human M1 shows age dependent reduction of cortical plasticity[81]. Decreased M1 ex-
124 Chapter 5. General Discussion and Future Directions 109 citability maybe caused by reduced intracortical circuits responsiveness or disruption of sensorimotor integration or both. Attenuation of in paired pulse intracortical inhibition or changes with age has not been confirmed yet[277]. In this thesis looking at the PAS responses in groups of subjects in chapter 3 and 4 indicate significant variability between subjects. Part of this difference can be explained by the difference between average age of subjects participated in different experiments. Genetic factors also participate in significant inter-individual variation of responses of the brain to TMS. One study showed that individuals with the val66met polymorphism in the brain-derived neurotrophic factor (BDNF) gene show less increase in the MEP after motor training [164]. Other factor that can participate in the inter-individual variability of brain to TMS are gross anatomy of human scalp, and distance between motor cortex and surface of the head[254, 162] Paired Associative Stimulation In our experiment similar to previous studies we found an increase in the size of the MEP amplitude, as well as an increase in the duration of the CSP recorded from the contracting targeted muscles [200, 251, 252, 74]. Therefore, PAS-induced plasticity, although may influence active neuronal circuits involved in GABA B receptor mediated inhibition. In one study [143] using current direction to preferentially activate early or late I waves after PAS authors found that the increased effectiveness with use of anterior to posterior
125 Chapter 5. General Discussion and Future Directions 110 current direction in PAS over posterior to anterior current direction which suggests I3 input to corticospinal neurons which selectively more active with anterior to posterior current direction has an important role in induction of associative plasticity in the human motor cortex. In this way, PAS-induced plasticity may be different from TBS-induced plasticity which appears to rely on modulation of the early I-waves[116]. Our results add to these findings as we demonstrate selective reduction of PAS effect by engaging GABA A receptors input to pyramidal neurons using SICI. Safety Safety and tolerability are key issues not just for the risk-benefit ratio assessment of novel therapeutics, but also for their impact on patient commitment and compliance with a time consuming brain stimulation paradigm. In order to increase subjects compliance we used 0.2 Hz frequency for PAS paradigms used in this thesis instead of 0.1 Hz used in original PAS study by Stefan et al.[251] It is also important to investigate the effect of single vs. multiple sessions of brain stimulation to understand the magnitude of additional sessions of stimulations on the measure of interest. We strictly followed published safety guidelines [220] for TMS. TMS in general is a very safe and thousands of people have had the experience with no adverse effects although seizures have been reported in few cases. The common side effect is usually limited to local pain as a result of the pressure of the coil, mild headache and possibly transient hearing changes as result of discharge related noise. In this thesis we found no major or minor adverse effects of PAS which increase the favorability of this technique for potential clinical applications.
126 Chapter 5. General Discussion and Future Directions 111 Limitations TMS is a great tool because of its safety record, temporal resolution and because it makes it possible to manipulate brain activity in human non-invasively. However, certain limitations exist for the majority of TMS studies: Poor spatial resolution both as a result of the limited focality of stimulation as well as the conventional localization of the area of interest according to EEG system or based on the motor hotspot. Several streams of research are underway to tackle these issues by improving the focality of TMS coils and also by combining imaging (e.g. MRI) with TMS [243] to improve spatial resolution and use of optically tracked frameless stereotaxic neuronavigation systems, which incorporate individual MRI data to deliver TMS in anatomically precise locations. Cellular mechanisms underlying the TMS induced events are not well understood. Several studies have used receptor agonist and antagonist to derive plausible mechanistic explanations for TMS induced interactions. However, the majority of these studies had significant limitations because of the small number of drugs that are available to be tested safely in human. Simultaneous observation of PAS effect at cellular level may be necessary to provide definitive evidence for the underlying mechanism of actions of this paradigm. The application of TMS to disrupt a cortical process and deducing the relevance of that area in performance of tasks is also a complex issue that needs to be addressed. TMS induced impairment of task performance could be the result of different chains of effects:
127 Chapter 5. General Discussion and Future Directions 112 TMS can increase the function of an area that inhibit the task performance or disrupt the function of an area that facilitate the task performance, or to inhibit or excite an area of the brain that compete or promote with the region of the brain relevant to process under the study. These chains of event are crucial in the interpretation of the results from TMS studies [195]. Metabolic changes measured by PET and blood oxygen level changes using fmri both showed TMS induced changes [226, 19, 255]. There is also possibility of undetected TMS induced changes as results of limited temporal resolution of imaging techniques. It is possible that some of the physiologically relevant changes after TMS were undetected in a millisecond window after the stimulation. One way to deal with this issue is to combine EEG measures with TMS to identify these effects [261]. TMS may be used to manipulate brain function to narrow down brain-behavior relationship to functionally relevant hypotheses. Understanding advantages and disadvantages of this technique are necessary to interpret result of TMS studies and to design new ones. In this thesis, we tried to relate our understanding of the mechanisms of neuronal plasticity at the cellular level to the system and behavior level. The next logical step would be to use findings of this study and apply them to the patient population - from bench to the bedside.
128 Chapter 5. General Discussion and Future Directions Future Directions Scientific Implications In Chapter 2, we showed that intracortical inhibition plays an important role in maintaining cortical excitability. We demonstrated the conditioning effect of SICI and ICF on PAS paradigm. It would be interesting to assess the observed effects using pharmacological manipulations to block the GABAergic interneurons, although currently there is no approved GABA antagonist medication for human use, or similarly benzodiazepine receptors (e.g. flumazenil) and assess the conditioned PAS paradigm to confirm if it is possible to rescue the effect of SICI conditioned PAS. Long interval intracortical inhibition (LICI) is another form of cortical inhibition and is evaluated using a suprathreshold conditioning stimulus and at ISIs between 50 and 200 ms [271]. Pharmacological studies suggested that SICI is mediated by GABA A receptors while LICI is probably mediated by GABA B receptors [296, 47, 290]. Measuring LICI after conditioned PAS paradigm can show the differential effect of PAS on subtypes of GABAergic interneurons. In Chapter 3, we found a graded response to PAS with different numbers of stimuli pairs and also interaction with motor learning. Homeostatic interactions theoretically happen at longer time scales, although we showed that it is possible to induce homeostatic interactions within hours. It would be interesting to follow PAS protocols for a longer period of time to observe such interactions. Improvements in motor learning can also occur on longer time scales (days) [207] The fact that we did not observed immediate changes in motor learning does not exclude the possibility of behavioral effects in longer time frame,
129 Chapter 5. General Discussion and Future Directions 114 therefore studies with longer periods of follow-up are warranted. Studies of LTD inducing PAS protocols are also important to show both sides of cortical plasticity in term of interactions with motor learning and also homeostatic interactions. For example, graded response to PAS protocol at ISI of 10ms to induce LTD and its metaplastic interactions with motor learning or other physiologically relevant paradigms should be conducted for further investigations. Behavioral aspects of PAS have been studied thoroughly in Chapter 4. Increased in peak acceleration and variability of hand movement task were observed after PAS. Increase in variability of motion path could be of physiological importance [103] because learning processes might require increased motor variability as an inherent feature for performance improvement, planning and learning [193]. Similar to our findings in chapter 4, Thumb acceleration and coefficient of variability had been shown to increase after TBS [103, 257]. However we did not find a significant increase in accuracy of movement in experiments done in chapter 3. It is important that both acceleration and accuracy of movement to be measured in single set of experiment for reliable speed-accuracy trade-off analysis. At this point, we can only speculate that PAS may result in improvement of acceleration without changing the accuracy of movement in a learning paradigm. As one study had shown improvement in motor learning behavior after a rotatory pursuit task one week after the plasticity induction [207]. It might as well be of interest to explore accuracy and speed of movement in a motor practice task after PAS for longer time scales. It is possible that accuracy of movement also
130 Chapter 5. General Discussion and Future Directions 115 improve after certain time lag. Another possible explanation for lack of accuracy changes in our study might be related to task sensitivity issues. It is likely that our motor learning task used in chapter 3 was not precise enough to detect minute changes in movement accuracy. Further studies are required to compare different protocols in their behavioral aspects. It will be of interest to investigate LTD inducing protocols in term of their behavioral correlates and motor learning variability. Remote behavioral changes for example in cognition networks involved in motor decision making and regions other than the targeted hand muscles needs further attention to confirm if the PAS behavioral changes are local or a more global phenomenon Clinical Implications Focal noninvasive brain stimulation including PAS provides a flexible tool that can fill the gap between the neuroscience of clinical disorders and the clinical science of their treatment. The ability to stimulate the brain noninvasively has attracted clinical researchers for its therapeutic potential in psychiatry and neurology. While tools like TMS present great therapeutic promise, the realization of that promise requires an in-depth understanding of pathophysiology of illness of interest, and of the mechanisms by which brain stimulation paradigms can induce plastic changes in the functioning of those abnormal circuits. Since TMS is a focal intervention, its clinical utility will ultimately depend upon our knowledge of the intracortical networks in the underlying disorder. Similarly, phasic rather than tonic nature of TMS needs knowledge of the ongoing endogenous processes in the brain and the mechanism and nature of TMS interference with these processes. This thesis helps with understanding of part of these mechanism and cortical
131 Chapter 5. General Discussion and Future Directions 116 processes. Some of the direct implications of current thesis and potential applications are as follows: GABAergic system is impaired in several neurological conditions such as Parkinson s disease [105] and Huntington s chorea [1]. Therefore, conditioned PAS paradigm could potentially have differential response in this population of patients in compared to healthy controls. Less GABAergic activity in the motor cortex could potentially diminish the PAS blockade induced by SICI conditioned PAS paradigm. This method can potentially open a new venue to test and diagnose GABAergic system abnormalities in motor cortex. PAS with longer duration of stimulation could induce homeostatic responses within hours after the stimulation. This is also of clinical relevance because it may provide new diagnostic tools to investigate pathophysiology conditions with impaired homeostatic plasticity interactions, such as in dystonia [202]. It is important that we can test homeostatic interactions reliably within hours and we showed in Chapter 3 that PAS with longer duration of stimulation is able to induce such interactions. Although immediate improvement in motor learning was not observed in healthy subjects, potential implications for rehabilitation medicine cannot be excluded. It might be still possible to improve motor learning in patients with reduced or impaired learning abilities. Improvement after stroke and spinal cord injuries should be studied as potential targets for interventions to improve motor learning especially with longer period of observations and multiple stimulation sessions. Increased motion variability and improved movement acceleration observed after PAS in Chapter 4 also indicates that PAS can produce clinical effects in patients with neurodegenerative disorders and after strokes by network reorganization and boosting the motor output.
132 Bibliography [1] G. Abbruzzese, A. Buccolieri, R. Marchese, C. Trompetto, P. Mandich, and M. Schieppati. Intracortical inhibition and facilitation are abnormal in huntington s disease: a paired magnetic stimulation study. Neurosci Lett, 228(2):87 90, Jun [2] G. Abbruzzese and C. Trompetto. Clinical and research methods for evaluating cortical excitability. J Clin Neurophysiol, 19(4):307 21, [3] W. C. Abraham and M. F. Bear. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci, 19(4):126 30, [4] H. Ackermann, E. Scholz, W. Koehler, and J. Dichgans. Influence of posture and voluntary background contraction upon compound muscle action potentials from anterior tibial and soleus muscle following transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol, 81(1):71 80, Feb [5] Charles H. Adler, Debra Crews, Kanav Kahol, Marco Santello, Brie Noble, Joseph G. Hentz, and John N. Caviness. Are the yips a task-specific dystonia or golfer s cramp? Mov Disord, 26(11): , Sep
133 Bibliography 118 [6] E. D. Adrian and G. Moruzzi. Impulses in the pyramidal tract. J Physiol, 97(2): , Dec [7] H. Alle, T. Heidegger, L. Krivanekova, and U. Ziemann. Interactions between short-interval intracortical inhibition and short-latency afferent inhibition in human motor cortex. J Physiol, 587(Pt 21): , [8] V. E. Amassian, P. J. Maccabee, and R. Q. Cracco. Focal stimulation of human peripheral nerve with the magnetic coil: a comparison with electrical stimulation. Exp Neurol, 103(3):282 9, [9] V. E. Amassian, G. J. Quirk, and M. Stewart. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalogr Clin Neurophysiol, 77(5): , [10] I. Antonov, I. Antonova, E. R. Kandel, and R. D. Hawkins. Activity-dependent presynaptic facilitation and hebbian ltp are both required and interact during classical conditioning in aplysia. Neuron, 37(1):135 47, [11] L. Avanzino, M. Bove, C. Trompetto, A. Tacchino, C. Ogliastro, and G. Abbruzzese. 1-hz repetitive tms over ipsilateral motor cortex influences the performance of sequential finger movements of different complexity. European Journal of Neuroscience, 27(5): , [12] M. Bajbouj, J. Gallinat, L. Niehaus, U. E. Lang, S. Roricht, and B. U. Meyer. Abnormalities of inhibitory neuronal mechanisms in the motor cortex of patients with schizophrenia. Pharmacopsychiatry, 37(2):74 80, [13] A. T. Barker, R. Jalinous, and I. L. Freeston. Non-invasive magnetic stimulation of human motor cortex. Lancet, 1(8437):1106 7, 1985.
134 Bibliography 119 [14] P. J. Basser, R. S. Wijesinghe, and B. J. Roth. The activating function for magnetic stimulation derived from a three-dimensional volume conductor model. IEEE Trans Biomed Eng, 39(11): , [15] M. F. Bear, L. N. Cooper, and F. F. Ebner. A physiological basis for a theory of synapse modification. Science, 237(4810):42 48, Jul [16] M. F. Bear and R. C. Malenka. Synaptic plasticity: Ltp and ltd. Curr Opin Neurobiol, 4(3):389 99, [17] A. Berardelli, A. Curra, G. Fabbrini, F. Gilio, and M. Manfredi. Pathophysiology of tics and tourette syndrome. J Neurol, 250(7):781 7, [18] T. W. Berger. Long-term potentiation of hippocampal synaptic transmission affects rate of behavioral learning. Science, 224(4649): , May [19] Sven Bestmann, Jrgen Baudewig, Hartwig R. Siebner, John C. Rothwell, and Jens Frahm. Functional mri of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci, 19(7): , Apr [20] G. Bi and M. Poo. Synaptic modification by correlated activity: Hebb s postulate revisited. Annual review of neuroscience, 24(1): , [21] E. L. Bienenstock, L. N. Cooper, and P. W. Munro. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci, 2(1):32 48, [22] T. V. Bliss and G. L. Collingridge. A synaptic model of memory: long-term potentiation in the hippocampus. Nature, 361(6407):31 9, 1993.
135 Bibliography 120 [23] T. V. Bliss and A. R. Gardner-Medwin. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol, 232(2):357 74, [24] T. V. Bliss and T. Lomo. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol, 232(2):331 56, [25] P. S. Boggio, L. O. Castro, E. A. Savagim, R. Braite, V. C. Cruz, R. R. Rocha, S. P. Rigonatti, M. T. Silva, and F. Fregni. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett, 404(1-2):232 6, [26] D. E. Bohning, A. P. Pecheny, C. M. Epstein, A. M. Speer, D. J. Vincent, W. Dannels, and M. S. George. Mapping transcranial magnetic stimulation (tms) fields in vivo with mri. Neuroreport, 8(11):2535 8, [27] M. Borich, M. Furlong, D. Holsman, and T. J. Kimberley. Goal-directed visuomotor skill learning: off-line enhancement and the importance of the primary motor cortex. Restor Neurol Neurosci, 29(2):105 13, [28] Zuner A. Bortolotto, Mascia Amici, William W. Anderson, John T R. Isaac, and Graham L. Collingridge. Synaptic plasticity in the hippocampal slice preparation. Curr Protoc Neurosci, Chapter 6:Unit 6.13, Jan [29] M. Bove, A. Tacchino, A. Novellino, C. Trompetto, G. Abbruzzese, and M.F. Ghilardi. The effects of rate and sequence complexity on repetitive finger movements. Brain research, 1153:84 91, [30] PW Brand, AM Hollister, and JM Agee. Transmission. Mosby, 1999.
136 Bibliography 121 [31] J. P. Brasil-Neto, L. G. Cohen, M. Panizza, J. Nilsson, B. J. Roth, and M. Hallett. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol, 9(1): , Jan [32] J. P. Brasil-Neto, J. Valls-Sole, A. Pascual-Leone, A. Cammarota, V. E. Amassian, R. Cracco, P. Maccabee, J. Cracco, M. Hallett, and L. G. Cohen. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain, 116 ( Pt 3):511 25, [33] C. Brinkman. Supplementary motor area of the monkey s cerebral cortex: shortand long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci, 4(4):918 29, [34] D. Burke, R. Hicks, S. C. Gandevia, J. Stephen, I. Woodforth, and M. Crawford. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol, 470:383 93, [35] C. M. Butefisch, B. C. Davis, S. P. Wise, L. Sawaki, L. Kopylev, J. Classen, and L. G. Cohen. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A, 97(7):3661 5, [36] R. Cantello. Applications of transcranial magnetic stimulation in movement disorders. J Clin Neurophysiol, 19(4):272 93, [37] C. S. Charlton, M. C. Ridding, P. D. Thompson, and T. S. Miles. Prolonged peripheral nerve stimulation induces persistent changes in excitability of human motor cortex. J Neurol Sci, 208(1-2):79 85, [38] R. Chen, D. J. Anastakis, C. T. Haywood, D. J. Mikulis, and R. T. Manktelow. Plasticity of the human motor system following muscle reconstruction: a magnetic
137 Bibliography 122 stimulation and functional magnetic resonance imaging study. Clin Neurophysiol, 114(12): , [39] R. Chen, J. Classen, C. Gerloff, P. Celnik, E. M. Wassermann, M. Hallett, and L. G. Cohen. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology, 48(5): , [40] R. Chen, L. G. Cohen, and M. Hallett. Nervous system reorganization following injury. Neuroscience, 111(4):761 73, [41] R. Chen, A. M. Lozano, and P. Ashby. Mechanism of the silent period following transcranial magnetic stimulation. evidence from epidural recordings. Exp Brain Res, 128(4): , Oct [42] R. Chen, A. Tam, C. Butefisch, B. Corwell, U. Ziemann, J. C. Rothwell, and L. G. Cohen. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol, 80(6): , [43] Robert Chen and Kaviraja Udupa. Measurement and modulation of plasticity of the motor system in humans using transcranial magnetic stimulation. Motor Control, 13(4): , Oct [44] D. B. Chklovskii. Synaptic connectivity and neuronal morphology: two sides of the same coin. Neuron, 43(5):609 17, [45] D. B. Chklovskii, B. W. Mel, and K. Svoboda. Cortical rewiring and information storage. Nature, 431(7010):782 8, [46] Philippe A. Chouinard, Gabriel Leonard, and Toms Paus. Changes in effective connectivity of the primary motor cortex in stroke patients after rehabilitative therapy. Exp Neurol, 201(2): , Oct 2006.
138 Bibliography 123 [47] Jason Chu, Carolyn Gunraj, and Robert Chen. Possible differences between the time courses of presynaptic and postsynaptic gabab mediated inhibition in the human motor cortex. Exp Brain Res, 184(4): , Feb [48] J. Classen, J. Liepert, S. P. Wise, M. Hallett, and L. G. Cohen. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol, 79(2): , [49] J. Classen, O. W. Witte, G. Schlaug, R. J. Seitz, H. Holthausen, and R. Benecke. Epileptic seizures triggered directly by focal transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol, 94(1):19 25, Jan [50] J. Classen, A. Wolters, K. Stefan, M. Wycislo, F. Sandbrink, A. Schmidt, and E. Kunesch. Paired associative stimulation. Suppl Clin Neurophysiol, 57:563 9, [51] L. G. Cohen, U. Ziemann, R. Chen, J. Classen, M. Hallett, C. Gerloff, and C. Butefisch. Studies of neuroplasticity with transcranial magnetic stimulation. J Clin Neurophysiol, 15(4):305 24, [52] D. Crupi, M. F. Ghilardi, C. Mosiello, A. Di Rocco, A. Quartarone, and F. Battaglia. Cortical and brainstem ltp-like plasticity in huntington s disease. Brain Res Bull, 75(1):107 14, [53] Z. J. Daskalakis, B. K. Christensen, P. B. Fitzgerald, L. Roshan, and R. Chen. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol, 543(Pt 1):317 26, [54] Z. J. Daskalakis, F. Farzan, M. S. Barr, J. J. Maller, R. Chen, and P. B. Fitzgerald. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a tms-eeg study. Neuropsychopharmacology, 33(12):2860 9, 2008.
139 Bibliography 124 [55] Z. J. Daskalakis, G. F. Molnar, B. K. Christensen, A. Sailer, P. B. Fitzgerald, and R. Chen. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clin Neurophysiol, 114(5):938 44, [56] Zafiris J. Daskalakis, Guillermo O. Paradiso, Bruce K. Christensen, Paul B. Fitzgerald, Carolyn Gunraj, and Robert Chen. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol, 557(Pt 2): , Jun [57] B. L. Day, P. D. Thompson, J. P. Dick, K. Nakashima, and C. D. Marsden. Different sites of action of electrical and magnetic stimulation of the human brain. Neurosci Lett, 75(1): , Mar [58] V. Di Lazzaro, M. Dileone, F. Pilato, F. Capone, G. Musumeci, F. Ranieri, V. Ricci, P. Bria, R. Di Iorio, and C. de Waure. Modulation of motor cortex neuronal networks by rtms: comparison of local and remote effects of six different protocols of stimulation. Journal of Neurophysiology, 105(5):2150, [59] V. Di Lazzaro, M. Dileone, F. Pilato, P. Profice, A. Oliviero, P. Mazzone, A. Insola, F. Capone, F. Ranieri, and P. A. Tonali. Associative motor cortex plasticity: direct evidence in humans. Cereb Cortex, 19(10): , [60] V. Di Lazzaro, A. Oliviero, M. Meglio, B. Cioni, G. Tamburrini, P. Tonali, and J. C. Rothwell. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol, 111(5):794 9, [61] V. Di Lazzaro, A. Oliviero, F. Pilato, E. Saturno, M. Dileone, P. Mazzone, A. Insola, P. A. Tonali, and J. C. Rothwell. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol, 115(2):255 66, [62] V. Di Lazzaro, A. Oliviero, F. Pilato, E. Saturno, M. Dileone, M. Meglio, B. Cioni, C. Colosimo, P. A. Tonali, and J. C. Rothwell. Direct recording of the output of
140 Bibliography 125 the motor cortex produced by transcranial magnetic stimulation in a patient with cerebral cortex atrophy. Clin Neurophysiol, 115(1):112 5, [63] V. Di Lazzaro, A. Oliviero, P. Profice, A. Insola, P. Mazzone, P. Tonali, and J. C. Rothwell. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res, 124(4): , Feb [64] V. Di Lazzaro, A. Oliviero, E. Saturno, M. Dileone, F. Pilato, R. Nardone, F. Ranieri, G. Musumeci, T. Fiorilla, and P. Tonali. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol, 564(Pt 2):661 8, [65] V. Di Lazzaro, D. Restuccia, A. Oliviero, P. Profice, L. Ferrara, A. Insola, P. Mazzone, P. Tonali, and J. C. Rothwell. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol, 508 ( Pt 2):625 33, [66] V. Di Lazzaro, D. Restuccia, A. Oliviero, P. Profice, L. Ferrara, A. Insola, P. Mazzone, P. Tonali, and J. C. Rothwell. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res, 119(2):265 8, [67] Vincenzo Di Lazzaro, Ulf Ziemann, and Roger N. Lemon. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimul, 1(4): , Oct [68] J. Diedrichsen, O. White, D. Newman, and N. Lally. Use-dependent and errorbased learning of motor behaviors. J Neurosci, 30(15): , 2010.
141 Bibliography 126 [69] M. Dileone, P. Profice, F. Pilato, P. Alfieri, L. Cesarini, E. Mercuri, C. Leoni, M. Tartaglia, R. Di Iorio, and G. Zampino. Enhanced human brain associative plasticity in costello syndrome. The Journal of physiology, 588(18):3445, [70] J. P. Donoghue, S. Suner, and J. N. Sanes. Dynamic organization of primary motor cortex output to target muscles in adult rats. ii. rapid reorganization following motor nerve lesions. Exp Brain Res, 79(3): , [71] S. M. Dudek and M. F. Bear. Homosynaptic long-term depression in area ca1 of hippocampus and effects of n-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci U S A, 89(10): , May [72] Mark J. Edwards and John C. Rothwell. Losing focus: How paying attention can be bad for movement. Mov Disord, 26(11): , Sep [73] B. Elahi and R. Chen. Effect of transcranial magnetic stimulation on parkinson motor function systematic review of controlled clinical trials. Mov Disord, 24(3):357 63, [74] B. Elahi, C. Gunraj, and R. Chen. Short-interval intracortical inhibition blocks long-term potentiation induced by paired associative stimulation. J Neurophysiol, 107(7): , April [75] B. Elahi, S. Nikfar, S. Derakhshani, M. Vafaie, and M. Abdollahi. On the benefit of probiotics in the management of pouchitis in patients underwent ileal pouch anal anastomosis: a meta-analysis of controlled clinical trials. Dig Dis Sci, 53(5): , [76] P. H. Ellaway, N. J. Davey, D. W. Maskill, and J. P. Dick. The relation between bradykinesia and excitability of the motor cortex assessed using transcranial magnetic stimulation in normal and parkinsonian subjects. Electroencephalogr Clin Neurophysiol, 97(3): , Jun 1995.
142 Bibliography 127 [77] P. H. Ellaway, N. J. Davey, D. W. Maskill, S. R. Rawlinson, H. S. Lewis, and N. P. Anissimova. Variability in the amplitude of skeletal muscle responses to magnetic stimulation of the motor cortex in man. Electroencephalogr Clin Neurophysiol, 109(2): , Apr [78] M. S. Evans and K. E. Viola-McCabe. Midazolam inhibits long-term potentiation through modulation of gabaa receptors. Neuropharmacology, 35(3):347 57, [79] E. V. Evarts and J. Tanji. Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol, 39(5): , Sep [80] E.V. Evarts. Role of motor cortex in voluntary movements in primates. Comprehensive Physiology, 0:1, [81] Dina Fathi, Yoshino Ueki, Tatsuya Mima, Satoko Koganemaru, Takashi Nagamine, Amal Tawfik, and Hidenao Fukuyama. Effects of aging on the human motor cortical plasticity studied by paired associative stimulation. Clin Neurophysiol, 121(1):90 93, Jan [82] R. J. Fisher, Y. Nakamura, S. Bestmann, J. C. Rothwell, and H. Bostock. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res, 143(2): , Mar [83] P. M. Fitts. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol, 47(6):381 91, [84] P. B. Fitzgerald, Z. J. Daskalakis, K. Hoy, F. Farzan, D. J. Upton, N. R. Cooper, and J. J. Maller. Cortical inhibition in motor and non-motor regions: a combined tms-eeg study. Clin EEG Neurosci, 39(3):112 7, 2008.
143 Bibliography 128 [85] J. R. Flanagan and A. M. Wing. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci, 17(4): , [86] M. D. Fox, M. A. Halko, M. C. Eldaief, and A. Pascual-Leone. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcmri) and transcranial magnetic stimulation (tms). Neuroimage, 1:1, [87] Marina V. Frantseva, Paul B. Fitzgerald, Robert Chen, Bertram Mller, Melissa Daigle, and Zafiris J. Daskalakis. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cereb Cortex, 18(5): , May [88] K. Fricke, A. A. Seeber, N. Thirugnanasambandam, W. Paulus, M. A. Nitsche, and J. C. Rothwell. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol, 105(3): , Mar [89] C. D. Frith, S. J. Blakemore, and D. M. Wolpert. Abnormalities in the awareness and control of action. Philos Trans R Soc Lond B Biol Sci, 355(1404): , [90] P. Fuhr, R. Agostino, and M. Hallett. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol, 81(4): , Aug [91] P. Fuhr, L. G. Cohen, B. J. Roth, and M. Hallett. Latency of motor evoked potentials to focal transcranial stimulation varies as a function of scalp positions stimulated. Electroencephalogr Clin Neurophysiol, 81(2):81 89, Apr 1991.
144 Bibliography 129 [92] R. Gentner, K. Wankerl, C. Reinsberger, D. Zeller, and J. Classen. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex, 18(9): , [93] A. P. Georgopoulos, J. T. Lurito, M. Petrides, A. B. Schwartz, and J. T. Massey. Mental rotation of the neuronal population vector. Science, 243(4888):234 6, [94] C. Gerloff, B. Corwell, R. Chen, M. Hallett, and L. G. Cohen. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain, 120 ( Pt 9): , Sep [95] B. Godde, F. Spengler, and H. R. Dinse. Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. Neuroreport, 8(1):281 5, [96] J. Gordon, M. F. Ghilardi, and C. Ghez. Accuracy of planar reaching movements. i. independence of direction and extent variability. Exp Brain Res, 99(1):97 111, [97] S. W. Greenhouse and S. Geisser. On methods in the analysis of profile data. Psychometrika, 24(2):95 112, [98] C. Grefkes and G.R. Fink. of Book: Sensorimotor control of grasping: physiology and pathophysiology. Cambridge University Press, [99] M. H. Grosbras and T. Paus. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur J Neurosci, 18(11):3121 6, [100] L. D. Gugino, J. R. Romero, L. Aglio, D. Titone, M. Ramirez, A. Pascual-Leone, E. Grimson, N. Weisenfeld, R. Kikinis, and M. E. Shenton. Transcranial magnetic
145 Bibliography 130 stimulation coregistered with mri: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clin Neurophysiol, 112(10): , Oct [101] A. Gupta, Y. Wang, and H. Markram. Organizing principles for a diversity of gabaergic interneurons and synapses in the neocortex. Science, 287(5451):273 8, [102] M. Hallett. Transcranial magnetic stimulation: a primer. Neuron, 55(2):187 99, [103] Masashi Hamada, Nagako Murase, Alkomiet Hasan, Michelle Balaratnam, and John C. Rothwell. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex, Jun [104] S. Hamdy, J. C. Rothwell, Q. Aziz, K. D. Singh, and D. G. Thompson. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci, 1(1):64 8, [105] R. Hanajima, Y. Terao, Y. Shirota, S. Ohminami, S. Nakatani-Enomoto, S. Okabe, H. Matsumoto, R. Tsutsumi, and Y. Ugawa. Short-interval intracortical inhibition in parkinson s disease using anterior-posterior directed currents. Exp Brain Res, 214(2): , Oct [106] R. Hanajima, Y. Ugawa, Y. Terao, K. Ogata, and I. Kanazawa. Ipsilateral corticocortical inhibition of the motor cortex in various neurological disorders. J Neurol Sci, 140(1-2):109 16, [107] C. O. Hebb and H. Konzett. The effect of certain analgesic drugs on synaptic transmission as observed in the perfused superior cervical ganglion of the cat. Q J Exp Physiol Cogn Med Sci, 35(3):213 7, 1949.
146 Bibliography 131 [108] T. Heidegger, K. Krakow, and U. Ziemann. Effects of antiepileptic drugs on associative ltp-like plasticity in human motor cortex. European Journal of Neuroscience, 32(7): , [109] G. Hess and J. P. Donoghue. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol, 71(6):2543 7, [110] V. Hmberg and J. Netz. Generalised seizures induced by transcranial magnetic stimulation of motor cortex. Lancet, 2(8673):1223, Nov [111] N. Hon, R. A. Epstein, A. M. Owen, and J. Duncan. Frontoparietal activity with minimal decision and control. J Neurosci, 26(38):9805 9, [112] J. A. Hosp, A. Pekanovic, M. S. Rioult-Pedotti, and A. R. Luft. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci, 31(7):2481 7, [113] E. P. Huang. Synaptic plasticity: going through phases with ltp. Curr Biol, 8(10):R350 2, [114] E. P. Huang and C. F. Stevens. The matter of mind: molecular control of memory. Essays Biochem, 33:165 78, [115] Y. Y. Huang and E. R. Kandel. Theta frequency stimulation up-regulates the synaptic strength of the pathway from ca1 to subiculum region of hippocampus. Proc Natl Acad Sci U S A, 102(1):232 7, [116] Y. Z. Huang, M. J. Edwards, E. Rounis, K. P. Bhatia, and J. C. Rothwell. Theta burst stimulation of the human motor cortex. Neuron, 45(2):201 6, 2005.
147 Bibliography 132 [117] Y. Z. Huang and J. C. Rothwell. The effect of short-duration bursts of highfrequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clin Neurophysiol, 115(5): , [118] A. Hufnagel and C. E. Elger. Induction of seizures by transcranial magnetic stimulation in epileptic patients. J Neurol, 238(2): , Apr [119] Friedhelm Hummel and Leonardo G. Cohen. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair, 19(1):14 19, Mar [120] G. W. Huntley and E. G. Jones. Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: a correlative anatomic and physiological study. J Neurophysiol, 66(2): , Aug [121] T. V. Ilic, F. Meintzschel, U. Cleff, D. Ruge, K. R. Kessler, and U. Ziemann. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol, 545(Pt 1):153 67, [122] R. J. Ilmoniemi, J. Ruohonen, and J. Karhu. Transcranial magnetic stimulation a new tool for functional imaging of the brain. Crit Rev Biomed Eng, 27(3-5):241 84, [123] R. Inzelberg, T. Flash, E. Schechtman, and A. D. Korczyn. Kinematic properties of upper limb trajectories in idiopathic torsion dystonia. J Neurol Neurosurg Psychiatry, 58(3):312 9, [124] A. Iriki, C. Pavlides, A. Keller, and H. Asanuma. Long-term potentiation in the motor cortex. Science, 245(4924):1385 7, [125] K. M. Jacobs and J. P. Donoghue. Reshaping the cortical motor map by unmasking latent intracortical connections. Science, 251(4996):944 7, 1991.
148 Bibliography 133 [126] W. M. Jenkins, M. M. Merzenich, M. T. Ochs, T. Allard, and E. Guic-Robles. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol, 63(1):82 104, [127] H. Y. Jung, Y. H. Sohn, A. Mason, E. Considine, and M. Hallett. Flumazenil does not affect intracortical motor excitability in humans: a transcranial magnetic stimulation study. Clin Neurophysiol, 115(2):325 9, [128] P. Jung and U. Ziemann. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci, 29(17): , [129] J. H. Kaas. Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci, 14:137 67, [130] A. Kaelin-Lang, A. R. Luft, L. Sawaki, A. H. Burstein, Y. H. Sohn, and L. G. Cohen. Modulation of human corticomotor excitability by somatosensory input. J Physiol, 540(Pt 2):623 33, [131] E. R. Kandel, J. H. Schwartz, and T. M. Jessell. Principles of neural science. Appleton Lange, [132] J. S. Kang, C. Terranova, R. Hilker, A. Quartarone, and U. Ziemann. Deficient homeostatic regulation of practice-dependent plasticity in writer s cramp. Cereb Cortex, 21(5): , [133] D. Kernell and W. Chien-Ping. Post-synaptic effects of cortical stimulation on forelimb motoneurones in the baboon. J Physiol, 191(3): , Aug [134] Eman M. Khedr, Mohamed A. Ahmed, Nehal Fathy, and John C. Rothwell. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology, 65(3): , Aug 2005.
149 Bibliography 134 [135] L. Kiers, D. Cros, K. H. Chiappa, and J. Fang. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol, 89(6): , Dec [136] A. Kirkwood, H. K. Lee, and M. F. Bear. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature, 375(6529): , May [137] A. Kirkwood, M. C. Rioult, and M. F. Bear. Experience-dependent modification of synaptic plasticity in visual cortex. Nature, 381(6582): , Jun [138] M. E. Knash, A. Kido, M. Gorassini, K. M. Chan, and R. B. Stein. Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res, 153(3):366 77, [139] E. I. Knudsen and P. F. Knudsen. Visuomotor adaptation to displacing prisms by adult and baby barn owls. J Neurosci, 9(9): , [140] H. H. KORNHUBER and L. DEECKE. [changes in the brain potential in voluntary movements and passive movements in man: Readiness potential and reafferent potentials]. Pflugers Arch Gesamte Physiol Menschen Tiere, 284:1 17, May [141] J. W. Krakauer, Z. M. Pine, M. F. Ghilardi, and C. Ghez. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci, 20(23): , [142] L. Krivanekova, M. K. Lu, B. Bliem, and U. Ziemann. Modulation of excitability in human primary somatosensory and motor cortex by paired associative stimulation targeting the primary somatosensory cortex. Eur J Neurosci, 34(8): , 2011.
150 Bibliography 135 [143] Kayoko Kujirai, Takashi Kujirai, Thomas Sinkjaer, and John C. Rothwell. Associative plasticity in human motor cortex during voluntary muscle contraction. J Neurophysiol, 96(3): , Sep [144] T. Kujirai, M. D. Caramia, J. C. Rothwell, B. L. Day, P. D. Thompson, A. Ferbert, S. Wroe, P. Asselman, and C. D. Marsden. Corticocortical inhibition in human motor cortex. J Physiol, 471:501 19, [145] A. U. Larkman and J. J. Jack. Synaptic plasticity: hippocampal ltp. Curr Opin Neurobiol, 5(3):324 34, [146] M.L. Latash. Progress in motor control: Bernstein s traditions in movement studies, volume 1. Human Kinetics, [147] L. M. Levy, U. Ziemann, R. Chen, and L. G. Cohen. Rapid modulation of gaba in sensorimotor cortex induced by acute deafferentation. Ann Neurol, 52(6):755 61, [148] D. Liao, X. Zhang, R. O Brien, M. D. Ehlers, and R. L. Huganir. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci, 2(1):37 43, Jan [149] David Liebetanz, Michael A. Nitsche, Frithjof Tergau, and Walter Paulus. Pharmacological approach to the mechanisms of transcranial dc-stimulation-induced after-effects of human motor cortex excitability. Brain, 125(Pt 10): , Oct [150] C. Lorenzano, L. Dinapoli, F. Gilio, A. Suppa, S. Bagnato, A. Curra, M. Inghilleri, and A. Berardelli. Motor cortical excitability studied with repetitive transcranial magnetic stimulation in patients with huntington s disease. Clin Neurophysiol, 117(8): , 2006.
151 Bibliography 136 [151] Ming-Kuei Lu, Barbara Bliem, Patrick Jung, Noritoshi Arai, Chon-Haw Tsai, and Ulf Ziemann. Modulation of preparatory volitional motor cortical activity by paired associative transcranial magnetic stimulation. Hum Brain Mapp, 30(11): , Nov [152] R. A. Macdonell, B. E. Shapiro, K. H. Chiappa, S. L. Helmers, D. Cros, B. J. Day, and B. T. Shahani. Hemispheric threshold differences for motor evoked potentials produced by magnetic coil stimulation. Neurology, 41(9): , Sep [153] C.L. MacKenzie and T. Iberall. The grasping hand, volume 104. North Holland, [154] Sangeetha Madhavan, Kenneth A Weber, 2nd, and James W. Stinear. Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: implications for rehabilitation. Exp Brain Res, 209(1):9 17, Mar [155] Noelia Madronal, Jos M. Delgado-Garca, and Agnes Gruart. Differential effects of long-term potentiation evoked at the ca3 ca1 synapse before, during, and after the acquisition of classical eyeblink conditioning in behaving mice. J Neurosci, 27(45): , Nov [156] R. C. Malenka and M. F. Bear. Ltp and ltd: an embarrassment of riches. Neuron, 44(1):5 21, [157] Roberto Malinow and Robert C. Malenka. Ampa receptor trafficking and synaptic plasticity. Annu Rev Neurosci, 25: , [158] H. Markram, J. Lubke, M. Frotscher, and B. Sakmann. Regulation of synaptic efficacy by coincidence of postsynaptic aps and epsps. Science, 275(5297): , 1997.
152 Bibliography 137 [159] T. A. Martin, J. G. Keating, H. P. Goodkin, A. J. Bastian, and W. T. Thach. Throwing while looking through prisms. i. focal olivocerebellar lesions impair adaptation. Brain, 119 ( Pt 4): , [160] Peter V. Massey, Benjamin E. Johnson, Peter R. Moult, Yves P. Auberson, Malcolm W. Brown, Elek Molnar, Graham L. Collingridge, and Zafar I. Bashir. Differential roles of nr2a and nr2b-containing nmda receptors in cortical long-term potentiation and long-term depression. J Neurosci, 24(36): , Sep [161] Atsushi Matsuo, Hiroshi Maeoka, Makoto Hiyamizu, Koji Shomoto, Shu Morioka, and Keiko Seki. Enhancement of precise hand movement by transcranial direct current stimulation. Neuroreport, 22(2):78 82, Jan [162] K. A. McConnell, Z. Nahas, A. Shastri, J. P. Lorberbaum, F. A. Kozel, D. E. Bohning, and M. S. George. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry, 49(5): , Mar [163] M. N. McDonnell, Y. Orekhov, and U. Ziemann. Suppression of ltp-like plasticity in human motor cortex by the gabab receptor agonist baclofen. Exp Brain Res, 180(1):181 6, [164] Stephanie A. McHughen, Paul F. Rodriguez, Jeffrey A. Kleim, Erin D. Kleim, Laura Marchal Crespo, Vincent Procaccio, and Steven C. Cramer. Bdnf val66met polymorphism influences motor system function in the human brain. Cereb Cortex, 20(5): , May [165] A. L. McKenzie, S. S. Nagarajan, T. P. Roberts, M. M. Merzenich, and N. N. Byl. Somatosensory representation of the digits and clinical performance in patients with focal hand dystonia. Am J Phys Med Rehabil, 82(10):737 49, 2003.
153 Bibliography 138 [166] P. A. Merton and H. B. Morton. Stimulation of the cerebral cortex in the intact human subject. Nature, 285(5762):227, May [167] M. M. Merzenich and W. M. Jenkins. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther, 6(2):89 104, [168] B. U. Meyer, T. C. Britton, H. Kloten, H. Steinmetz, and R. Benecke. Coil placement in magnetic brain stimulation related to skull and brain anatomy. Electroencephalogr Clin Neurophysiol, 81(1):38 46, Feb [169] K. R. Mills and K. A. Nithi. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve, 20(5): , May [170] Carlo Miniussi, Stefano F. Cappa, Leonardo G. Cohen, Agnes Floel, Felipe Fregni, Michael A. Nitsche, Massimiliano Oliveri, Alvaro Pascual-Leone, Walter Paulus, Alberto Priori, and Vincent Walsh. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul, 1(4): , Oct [171] Florian Mller-Dahlhaus, Ulf Ziemann, and Joseph Classen. Plasticity resembling spike-timing dependent synaptic plasticity: the evidence in human cortex. Front Synaptic Neurosci, 2:34, [172] N. Modugno, Y. Nakamura, S. Bestmann, A. Curra, A. Berardelli, and J. Rothwell. Neurophysiological investigations in patients with primary writing tremor. Mov Disord, 17(6): , [173] F. Morgante, A. J. Espay, C. Gunraj, A. E. Lang, and R. Chen. Motor cortex plasticity in parkinson s disease and levodopa-induced dyskinesias. Brain, 129(4):1059, 2006.
154 Bibliography 139 [174] F. M. Mottaghy, A. Pascual-Leone, L. J. Kemna, R. Topper, H. Herzog, H. W. Muller-Gartner, and B. J. Krause. Modulation of a brain-behavior relationship in verbal working memory by rtms. Brain Res Cogn Brain Res, 15(3):241 9, [175] V. B. Mountcastle. The columnar organization of the neocortex. Brain, 120 ( Pt 4):701 22, [176] W. Muellbacher, U. Ziemann, B. Boroojerdi, L. Cohen, and M. Hallett. Role of the human motor cortex in rapid motor learning. Exp Brain Res, 136(4): , Feb [177] A. J. Nelson, T. Hoque, C. Gunraj, Z. Ni, and R. Chen. Bi-directional interhemispheric inhibition during unimanual sustained contractions. BMC Neurosci, 10:31, [178] Z. Ni, C. Gunraj, A. J. Nelson, I. J. Yeh, G. Castillo, T. Hoque, and R. Chen. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex, 19(7): , [179] Z. Ni, C. Gunraj, A. Wagle-Shukla, K. Udupa, F. Mazzella, A. M. Lozano, and R. Chen. Direct demonstration of inhibitory interactions between long interval intracortical inhibition and short interval intracortical inhibition. J Physiol, 589(Pt 12): , [180] M. A. Nitsche and W. Paulus. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol, 527 Pt 3: , Sep [181] M. A. Nitsche and W. Paulus. Sustained excitability elevations induced by transcranial dc motor cortex stimulation in humans. Neurology, 57(10): , Nov 2001.
155 Bibliography 140 [182] M. A. Nitsche, A. Schauenburg, N. Lang, D. Liebetanz, C. Exner, W. Paulus, and F. Tergau. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci, 15(4):619 26, [183] Michael A. Nitsche, Antje Seeber, Kai Frommann, Cornelia Carmen Klein, Christian Rochford, Maren S. Nitsche, Kristina Fricke, David Liebetanz, Nicolas Lang, Andrea Antal, Walter Paulus, and Frithjof Tergau. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol, 568(Pt 1): , Oct [184] R. J. Nudo, G. W. Milliken, W. M. Jenkins, and M. M. Merzenich. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci, 16(2): , Jan [185] Randolph J. Nudo. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin N Am, 14(1 Suppl):S57 S76, Feb [186] R. J. O Brien, S. Kamboj, M. D. Ehlers, K. R. Rosen, G. D. Fischbach, and R. L. Huganir. Activity-dependent modulation of synaptic ampa receptor accumulation. Neuron, 21(5): , Nov [187] W. Ogle. Aristotle: De partibus animalium, [188] V. E. Okhotin and S. G. Kalinichenko. The histophysiology of neocortical basket cells. Neurosci Behav Physiol, 32(5):455 70, [189] M. Orth and J. C. Rothwell. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol, 115(5): , 2004.
156 Bibliography 141 [190] M. Orth, S. Schippling, S. A. Schneider, K. P. Bhatia, P. Talelli, S. J. Tabrizi, and J. C. Rothwell. Abnormal motor cortex plasticity in premanifest and very early manifest huntington disease. J Neurol Neurosurg Psychiatry, 81(3):267 70, [191] M. Panizza, J. Nilsson, B. J. Roth, P. J. Basser, and M. Hallett. Relevance of stimulus duration for activation of motor and sensory fibers: implications for the study of h-reflexes and magnetic stimulation. Electroencephalogr Clin Neurophysiol, 85(1):22 9, [192] A. Pascual-Leone, J. Valls-Sole, E. M. Wassermann, and M. Hallett. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain, 117 ( Pt 4):847 58, [193] J. L. Patton, M. E. Stoykov, M. Kovic, and F. A. Mussa-Ivaldi. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp Brain Res, 168(3):368 83, [194] O. Paulsen and T. J. Sejnowski. Natural patterns of activity and long-term synaptic plasticity. Curr Opin Neurobiol, 10(2):172 9, [195] Toms Paus. Inferring causality in brain images: a perturbation approach. Philos Trans R Soc Lond B Biol Sci, 360(1457): , May [196] D. Pelisson, N. Alahyane, M. Panouilleres, and C. Tilikete. Sensorimotor adaptation of saccadic eye movements. Neurosci Biobehav Rev, 34(8): , [197] W. Penfield and T. Rasmussen. The cerebral cortex of man. Macmillan, [198] Monica A. Perez, Satoshi Tanaka, Steven P. Wise, Daniel T. Willingham, and Leonardo G. Cohen. Time-specific contribution of the supplementary motor area to intermanual transfer of procedural knowledge. J Neurosci, 28(39): , Sep 2008.
157 Bibliography 142 [199] H. S. Pyndt and M. C. Ridding. Modification of the human motor cortex by associative stimulation. Exp Brain Res, 159(1): , Nov [200] A. Quartarone, S. Bagnato, V. Rizzo, H. R. Siebner, V. Dattola, A. Scalfari, F. Morgante, F. Battaglia, M. Romano, and P. Girlanda. Abnormal associative plasticity of the human motor cortex in writer s cramp. Brain, 126(Pt 12): , [201] A. Quartarone, F. Morgante, A. Sant angelo, V. Rizzo, S. Bagnato, C. Terranova, H. R. Siebner, A. Berardelli, and P. Girlanda. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry, 79(9):985 90, [202] A. Quartarone, V. Rizzo, S. Bagnato, F. Morgante, A. Sant Angelo, M. Romano, D. Crupi, P. Girlanda, J. C. Rothwell, and H. R. Siebner. Homeostatic-like plasticity of the primary motor hand area is impaired in focal hand dystonia. Brain, 128(Pt 8): , [203] A. Quartarone, V. Rizzo, C. Terranova, F. Morgante, S. Schneider, N. Ibrahim, P. Girlanda, K. P. Bhatia, and J. C. Rothwell. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain, 132(Pt 10): , Oct [204] A. Quartarone, H. R. Siebner, and J. C. Rothwell. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci, 29(4):192 9, [205] E. M. Quinlan, D. H. Olstein, and M. F. Bear. Bidirectional, experience-dependent regulation of n-methyl-d-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A, 96(22): , Oct [206] E. M. Quinlan, B. D. Philpot, R. L. Huganir, and M. F. Bear. Rapid, experiencedependent expression of synaptic nmda receptors in visual cortex in vivo. Nat Neurosci, 2(4): , Apr 1999.
158 Bibliography 143 [207] T. K. Rajji, S. K. Liu, M. V. Frantseva, B. H. Mulsant, J. Thoma, R. Chen, P. B. Fitzgerald, and Z. J. Daskalakis. Exploring the effect of inducing long-term potentiation in the human motor cortex on motor learning. Brain Stimul, 4(3):137 44, [208] Valerie Reid. Transcranial magnetic stimulation. Phys Med Rehabil Clin N Am, 14(2):307 25, ix, May [209] J. Reis, H. M. Schambra, L. G. Cohen, E. R. Buch, B. Fritsch, E. Zarahn, P. A. Celnik, and J. W. Krakauer. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A, 106(5):1590 5, [210] J. Reis, O. B. Swayne, Y. Vandermeeren, M. Camus, M. A. Dimyan, M. Harris- Love, M. A. Perez, P. Ragert, J. C. Rothwell, and L. G. Cohen. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol, 586(2):325 51, [211] M. C. Ridding, B. Brouwer, and M. A. Nordstrom. Reduced interhemispheric inhibition in musicians. Exp Brain Res, 133(2):249 53, [212] M. C. Ridding and J. C. Rothwell. Reorganisation in human motor cortex. Can J Physiol Pharmacol, 73(2):218 22, [213] M. C. Ridding and J. L. Taylor. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. J Physiol, 537(Pt 2):623 31, [214] V. Rizzo, H. R. Siebner, N. Modugno, A. Pesenti, A. Munchau, W. Gerschlager, R. M. Webb, and J. C. Rothwell. Shaping the excitability of human motor cortex with premotor rtms. J Physiol, 554(Pt 2):483 95, 2004.
159 Bibliography 144 [215] P. E. Roland, B. Larsen, N. A. Lassen, and E. Skinhj. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol, 43(1): , Jan [216] P. E. Roland and K. Zilles. Functions and structures of the motor cortices in humans. Curr Opin Neurobiol, 6(6):773 81, [217] D. A. Rosenbaum. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen, 109(4):444 74, [218] K. Rosenkranz and J. C. Rothwell. The effect of sensory input and attention on the sensorimotor organization of the hand area of the human motor cortex. J Physiol, 561(Pt 1):307 20, [219] K. Rosenkranz, A. Williamon, and J. C. Rothwell. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci, 27(19):5200 6, [220] S. Rossi, M. Hallett, P.M. Rossini, and A. Pascual-Leone. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12): , [221] P. M. Rossini, A. T. Barker, A. Berardelli, M. D. Caramia, G. Caruso, R. Q. Cracco, M. R. Dimitrijevi, M. Hallett, Y. Katayama, and C. H. Lcking. Noninvasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. report of an ifcn committee. Electroencephalogr Clin Neurophysiol, 91(2):79 92, Aug [222] P. M. Rossini and G. Dal Forno. Neuronal post-stroke plasticity in the adult. Restor Neurol Neurosci, 22(3-5): , 2004.
160 Bibliography 145 [223] P. M. Rossini and S. Rossi. Clinical applications of motor evoked potentials. Electroencephalogr Clin Neurophysiol, 106(3): , Mar [224] Yiftach Roth, Alon Amir, Yechiel Levkovitz, and Abraham Zangen. Threedimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep h-coils. J Clin Neurophysiol, 24(1):31 38, Feb [225] J. Rothwell, D. Burke, R. Hicks, J. Stephen, I. Woodforth, and M. Crawford. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol, 481 ( Pt 1):243 50, [226] Elisabeth Rounis, Lucy Lee, Hartwig R. Siebner, James B. Rowe, Karl J. Friston, John C. Rothwell, and Richard S J. Frackowiak. Frequency specific changes in regional cerebral blood flow and motor system connectivity following rtms to the primary motor cortex. Neuroimage, 26(1): , May [227] H. Russmann, J. C. Lamy, E. A. Shamim, S. Meunier, and M. Hallett. Associative plasticity in intracortical inhibitory circuits in human motor cortex. Clin Neurophysiol, 120(6): , [228] M. Saglam, K. Matsunaga, N. Murayama, Y. Hayashida, Y. Z. Huang, and R. Nakanishi. Parallel inhibition of cortico-muscular synchronization and corticospinal excitability by theta burst tms in humans. Clin Neurophysiol, 119(12): , [229] Martin V. Sale, Michael C. Ridding, and Michael A. Nordstrom. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res, 181(4): , Aug 2007.
161 Bibliography 146 [230] Martin V. Sale, Michael C. Ridding, and Michael A. Nordstrom. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci, 28(33): , Aug [231] J. N. Sanes. The relation between human brain activity and hand movements. Neuroimage, 11(5 Pt 1):370 4, [232] J. N. Sanes and J. P. Donoghue. Plasticity and primary motor cortex. Annu Rev Neurosci, 23: , [233] J. N. Sanes, S. Suner, and J. P. Donoghue. Dynamic organization of primary motor cortex output to target muscles in adult rats. i. long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res, 79(3): , [234] Lara M. Schrader, John M. Stern, Lisa Koski, Marc R. Nuwer, and Jerome Engel, Jr. Seizure incidence during single- and paired-pulse transcranial magnetic stimulation (tms) in individuals with epilepsy. Clin Neurophysiol, 115(12): , Dec [235] P. Schwenkreis, K. Witscher, F. Janssen, A. Addo, R. Dertwinkel, M. Zenz, J. P. Malin, and M. Tegenthoff. Influence of the n-methyl-d-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett, 270(3):137 40, [236] R. Shadmehr, M. A. Smith, and J. W. Krakauer. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci, 33:89 108, [237] Ejaz A. Shamim, Jason Chu, Linda H. Scheider, Joseph Savitt, H. A. Jinnah, and Mark Hallett. Extreme task specificity in writer s cramp. Mov Disord, 26(11): , Sep 2011.
162 Bibliography 147 [238] Alon Shamir, Oh-Bin Kwon, Irina Karavanova, Detlef Vullhorst, Elias Leiva- Salcedo, Megan J. Janssen, and Andres Buonanno. The importance of the nrg- 1/erbb4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci, 32(9): , Feb [239] S.M. Sherman and RW Guillery. Functional organization of thalamocortical relays. Journal of Neurophysiology, 76(3): , [240] C. S. Sherrington. Observations on the scratch-reflex in the spinal dog. J Physiol, 34(1-2):1 50, [241] H. R. Siebner, J. Dressnandt, C. Auer, and B. Conrad. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve, 21(9): , [242] H. R. Siebner and J. Rothwell. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res, 148(1):1 16, [243] Hartwig R. Siebner, Til O. Bergmann, Sven Bestmann, Marcello Massimini, Heidi Johansen-Berg, Hitoshi Mochizuki, Daryl E. Bohning, Erie D. Boorman, Sergiu Groppa, Carlo Miniussi, Alvaro Pascual-Leone, Reto Huber, Paul C J. Taylor, Risto J. Ilmoniemi, Luigi De Gennaro, Antonio P. Strafella, Seppo Khknen, Stefan Klppel, Giovanni B. Frisoni, Mark S. George, Mark Hallett, Stephan A. Brandt, Matthew F. Rushworth, Ulf Ziemann, John C. Rothwell, Nick Ward, Leonardo G. Cohen, Jrgen Baudewig, Toms Paus, Yoshikazu Ugawa, and Paolo M. Rossini. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul, 2(2):58 80, Apr [244] Hartwig R. Siebner, Nicolas Lang, Vincenzo Rizzo, Michael A. Nitsche, Walter Paulus, Roger N. Lemon, and John C. Rothwell. Preconditioning of low-frequency
163 Bibliography 148 repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci, 24(13): , Mar [245] Christina W. Slotema, Jan Dirk Blom, Hans W. Hoek, and Iris E C. Sommer. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rtms)? a meta-analysis of the efficacy of rtms in psychiatric disorders. J Clin Psychiatry, 71(7): , Jul [246] Young H. Sohn and Mark Hallett. Surround inhibition in human motor system. Experimental Brain Research, 158(4): , [247] C. J. Stagg, G. Jayaram, D. Pastor, Z. T. Kincses, P. M. Matthews, and H. Johansen-Berg. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia, 49(5): , Apr [248] Charlotte J. Stagg, Velicia Bachtiar, and Heidi Johansen-Berg. The role of gaba in human motor learning. Curr Biol, 21(6): , Mar [249] K. Stefan, R. Gentner, D. Zeller, S. Dang, and J. Classen. Theta-burst stimulation: remote physiological and local behavioral after-effects. Neuroimage, 40(1):265 74, [250] K. Stefan, E. Kunesch, R. Benecke, L. G. Cohen, and J. Classen. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol, 543(Pt 2): , [251] K. Stefan, E. Kunesch, L. G. Cohen, R. Benecke, and J. Classen. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain, 123 Pt 3:572 84, 2000.
164 Bibliography 149 [252] K. Stefan, M. Wycislo, and J. Classen. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol, 92(1):66 72, [253] K. E. Stephan, T. Baldeweg, and K. J. Friston. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry, 59(10):929 39, [254] Mark G. Stokes, Christopher D. Chambers, Ian C. Gould, Tracy R. Henderson, Natasha E. Janko, Nicholas B. Allen, and Jason B. Mattingley. Simple metric for scaling motor threshold based on scalp-cortex distance: application to studies using transcranial magnetic stimulation. J Neurophysiol, 94(6): , Dec [255] A. P. Strafella and T. Paus. Cerebral blood-flow changes induced by paired-pulse transcranial magnetic stimulation of the primary motor cortex. J Neurophysiol, 85(6): , Jun [256] A. P. Strafella, T. Paus, M. Fraraccio, and A. Dagher. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain, 126(Pt 12): , [257] J. T. Teo, O. B. Swayne, B. Cheeran, R. J. Greenwood, and J. C. Rothwell. Human theta burst stimulation enhances subsequent motor learning and increases performance variability. Cereb Cortex, 21(7): , [258] F. Tergau, V. Wanschura, M. Canelo, S. Wischer, E. M. Wassermann, U. Ziemann, and W. Paulus. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res, 124(4): , Feb [259] G. W. Thickbroom, M. L. Byrnes, D. J. Edwards, and F. L. Mastaglia. Repetitive paired-pulse tms at i-wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin Neurophysiol, 117(1):61 6, 2006.
165 Bibliography 150 [260] Nivethida Thirugnanasambandam, Jessica Grundey, Walter Paulus, and Michael A. Nitsche. Dose-dependent nonlinear effect of l-dopa on paired associative stimulation-induced neuroplasticity in humans. J Neurosci, 31(14): , Apr [261] Gregor Thut and Alvaro Pascual-Leone. Integrating tms with eeg: How and what for? Brain Topogr, 22(4): , Jan [262] H. Tokimura, V. Di Lazzaro, Y. Tokimura, A. Oliviero, P. Profice, A. Insola, P. Mazzone, P. Tonali, and J. C. Rothwell. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol, 523 Pt 2:503 13, [263] K. R. Tovar and G. L. Westbrook. The incorporation of nmda receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci, 19(10): , May [264] J. T. Trachtenberg, B. E. Chen, G. W. Knott, G. Feng, J. R. Sanes, E. Welker, and K. Svoboda. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature, 420(6917):788 94, [265] W. J. Triggs, R. Calvanio, R. A. Macdonell, D. Cros, and K. H. Chiappa. Physiological motor asymmetry in human handedness: evidence from transcranial magnetic stimulation. Brain Res, 636(2): , Feb [266] W. J. Triggs, D. Menkes, J. Onorato, R. S. Yan, M. S. Young, K. Newell, H. W. Sander, O. Soto, K. H. Chiappa, and D. Cros. Transcranial magnetic stimulation identifies upper motor neuron involvement in motor neuron disease. Neurology, 53(3): , Aug 1999.
166 Bibliography 151 [267] G. G. Turrigiano, K. R. Leslie, N. S. Desai, L. C. Rutherford, and S. B. Nelson. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature, 391(6670): , Feb [268] G. G. Turrigiano and S. B. Nelson. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci, 5(2):97 107, [269] K. Udupa, Z. Ni, C. Gunraj, and R. Chen. Interactions between short latency afferent inhibition and long interval intracortical inhibition. Exp Brain Res, 199(2):177 83, [270] Y. Ugawa, J. C. Rothwell, B. L. Day, P. D. Thompson, and C. D. Marsden. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol, 29(4): , Apr [271] J. Valls-Sol, A. Pascual-Leone, E. M. Wassermann, and M. Hallett. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol, 85(6): , Dec [272] J. Valls-Sole, A. Pascual-Leone, J. P. Brasil-Neto, A. Cammarota, L. McShane, and M. Hallett. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with parkinson s disease. Neurology, 44(4):735 41, [273] T. Verstynen and P. N. Sabes. How each movement changes the next: an experimental and theoretical study of fast adaptive priors in reaching. J Neurosci, 31(27): , [274] L. L. Voronin. Long-term potentiation in the hippocampus. Neuroscience, 10(4): , 1983.
167 Bibliography 152 [275] Tim Wagner, Jarrett Rushmore, Uri Eden, and Antoni Valero-Cabre. Biophysical foundations underlying tms: setting the stage for an effective use of neurostimulation in the cognitive neurosciences. Cortex, 45(9): , Oct [276] E. Wassermann, C.M. Epstein, and U. Ziemann. The Oxford handbook of transcranial stimulation. Oxford University Press, USA, [277] Eric M. Wassermann. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol, 113(7): , Jul [278] A. J. Watt, M. C. van Rossum, K. M. MacLeod, S. B. Nelson, and G. G. Turrigiano. Activity coregulates quantal ampa and nmda currents at neocortical synapses. Neuron, 26(3): , Jun [279] D. Weise, A. Schramm, M. Beck, K. Reiners, and J. Classen. Loss of topographic specificity of ltd-like plasticity is a trait marker in focal dystonia. Neurobiol Dis, 42(2):171 6, [280] David Weise, Axel Schramm, Katja Stefan, Alexander Wolters, Karlheinz Reiners, Markus Naumann, and Joseph Classen. The two sides of associative plasticity in writer s cramp. Brain, 129(Pt 10): , Oct [281] K. J. Werhahn, E. Kunesch, S. Noachtar, R. Benecke, and J. Classen. Differential effects on motorcortical inhibition induced by blockade of gaba uptake in humans. J Physiol, 517 ( Pt 2):591 7, [282] M. Wiesendanger, H. Hummelsheim, M. Bianchetti, D. F. Chen, B. Hyland, V. Maier, and R. Wiesendanger. Input and output organization of the supplementary motor area. Ciba Found Symp, 132:40 62, 1987.
168 Bibliography 153 [283] D. B. Willingham. A neuropsychological theory of motor skill learning. Psychol Rev, 105(3):558 84, [284] F.R. Wilson. The hand: How its use shapes the brain, language, and human culture. Vintage, [285] A. Wolters, F. Sandbrink, A. Schlottmann, E. Kunesch, K. Stefan, L. G. Cohen, R. Benecke, and J. Classen. A temporally asymmetric hebbian rule governing plasticity in the human motor cortex. J Neurophysiol, 89(5): , [286] I. J. Woodforth, R. G. Hicks, M. R. Crawford, J. P. Stephen, and D. J. Burke. Variability of motor-evoked potentials recorded during nitrous oxide anesthesia from the tibialis anterior muscle after transcranial electrical stimulation. Anesth Analg, 82(4): , Apr [287] C. W. Wu, P. van Gelderen, T. Hanakawa, Z. Yaseen, and L. G. Cohen. Enduring representational plasticity after somatosensory stimulation. Neuroimage, 27(4):872 84, [288] W. J. Z Graggen, A. M. Humm, N. Durisch, M. R. Magistris, and K. M. Rsler. Repetitive spinal motor neuron discharges following single transcranial magnetic stimuli: a quantitative study. Clin Neurophysiol, 116(7): , Jul [289] Ping Zhou and William Zev Rymer. Factors governing the form of the relation between muscle force and the emg: a simulation study. J Neurophysiol, 92(5): , Nov [290] U. Ziemann. Pharmacology of tms. Suppl Clin Neurophysiol, 56:226 31, [291] U. Ziemann, D. Bruns, and W. Paulus. Enhancement of human motor cortex inhibition by the dopamine receptor agonist pergolide: evidence from transcranial magnetic stimulation. Neurosci Lett, 208(3):187 90, 1996.
169 Bibliography 154 [292] U. Ziemann, R. Chen, L. G. Cohen, and M. Hallett. Dextromethorphan decreases the excitability of the human motor cortex. Neurology, 51(5):1320 4, [293] U. Ziemann, T. V. Ilic, C. Pauli, F. Meintzschel, and D. Ruge. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci, 24(7): , [294] U. Ziemann, S. Lnnecker, B. J. Steinhoff, and W. Paulus. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol, 40(3): , Sep [295] U. Ziemann, S. Lonnecker, B. J. Steinhoff, and W. Paulus. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res, 109(1):127 35, [296] U. Ziemann, S. Lonnecker, B. J. Steinhoff, and W. Paulus. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol, 40(3):367 78, [297] U. Ziemann, W. Muellbacher, M. Hallett, and L. G. Cohen. Modulation of practicedependent plasticity in human motor cortex. Brain, 124(Pt 6): , 2001.
170 Appendices 155
171 .1 Effect of Transcranial Magnetic Stimulation on Parkinson Motor Function-Systematic Review of Controlled Clinical Trials 156
172 Movement Disorders Vol. 24, No. 3, 2009, pp Ó 2008 Movement Disorder Society Effect of Transcranial Magnetic Stimulation on Parkinson Motor Function Systematic Review of Controlled Clinical Trials Behzad Elahi, MD, 1 * Behrad Elahi, MD, 2 and Robert Chen, MBBChir, MSc, FRCPC 1 1 Division of Neurology, University of Toronto and Toronto Western Research Institute, Toronto, Ontario, Canada 2 Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran Abstract: The objective of this study was to evaluate the effects of repetitive Transcranial Magnetic Stimulation (rtms) on motor signs in Parkinson s disease (PD). Medline, Embase, CINAHL, Web of Science, Scopus bibliographic, and Google Scholar databases were searched. Relevant controlled clinical trials published between January 1985 and October 2007 were extracted, reviewed, and validated according to the study protocol. The outcome of interest was the motor section of the Unified Parkinson s Disease Rating Scale (UPDRS). We calculated the effect size for the included studies. Sensitivity analysis was performed to further assess factors that may change the results. Ten randomized, controlled clinical trials were included in the metaanalysis. Pooling of the results from these trials yielded an effect size of in UPDRS for high-frequency rtms studies and no significant effects for low-frequency rtms studies. The benefit of high-frequency rtms on motor signs in PD was confirmed by the meta-analysis. Lower frequency rtms had little effect on motor signs in PD. Ó 2008 Movement Disorder Society Key words: Parkinson s disease; meta-analysis; motor function; tremor Since transcranial magnetic stimulation (TMS) was introduced by Barker et al. in 1985, 1 it has become a safe, noninvasive, and painless way to study the central nervous system. Repetitive pulses of TMS (rtms) can modulate the excitability of the targeted brain area. rtms at frequencies of 5 Hz and higher can enhance motor cortex excitability, 2,3 whereas lower frequencies rtms (1 Hz and lower) can transiently depress cortical excitability. 4 rtms has been studied as a potential treatment in many neurological and psychiatric disorders. TMS and imaging studies suggested that there is decreased cortical excitability in Parkinson s disease (PD). 5 Several randomized controlled trials used rtms to treat the PD motor symptoms. However, the sample size was small *Correspondence to: Dr. Behzad Elahi, (Dr Robert Chen s Lab), Toronto Western Hospital, 399 Bathurst Street, Toronto, Ontario, Canada M5T 2S8 behzad.elahi@utoronto.ca Potential conflict of interest: None reported. Received 26 February 2008; Revised 3 July 2008; Accepted 24 September 2008 Published online 29 October 2008 in Wiley InterScience ( DOI: /mds in these studies and certain effects may not be detected because of insufficient power. We therefore conducted a meta-analysis to evaluate the effect of TMS on motor signs in PD. MATERIALS AND METHODS Search Strategy The Medline, Embase, CINAHL, Web of Science, Scopus bibliographic, and Google Scholar databases were searched for studies investigating the effect of TMS in PD. Articles published between January 1985 and October 2007 were retrieved. The search terms were TMS, noninvasive brain stimulation, UPDRS, and PD. The reference lists from retrieved articles were also hand searched for any additional applicable studies. Conference abstracts and unpublished data were not included. Selection Criteria The search strategy outlined above yield 164 relevant articles. Inclusion criteria for this study were as
173 358 B. ELAHI ET AL. FIG. 1. Algorithm of study selection and inclusion in the meta-analysis. follows: (1) prospective clinical studies, (2) must have a control group (3) the motor function was measured with the Motor (Part III) section of the Unified Parkinson s Disease Rating Scale (UPDRS), and (4) the results were reported in the form of mean and standard deviation. A few studies did not report the mean or standard deviation and we contacted the authors for these values. No language limitation was imposed. Two authors independently reviewed the articles for the quality and validity of the trials. Data on the therapeutic regimen, sample size, and trial duration were extracted, and results were summarized in a standard summary data sheet. The selection process is shown in Figure 1. Disagreements were resolved by discussion and consensus between reviewers. Data from the selected studies are shown in Table 1. Analysis All the included studies were pooled and weighted. The data were analyzed using Statsdirect (2.6.1). Effect size and confidence intervals (95% CI) were calculated using the DerSimonian Laird method. Effect size is the magnitude of a treatment effect and was calculated as the difference in scores between treatment and control groups divided by the standard deviation of the scores. The absolute effect size (d) of more than 0.5 was traditionally considered as medium to high effect 6 and is likely to be clinically relevant. The Cochran Q and I square inconsistency tests were used to examine heterogeneity. Funnel-plot analysis was used as bias indicator. Sensitivity analysis was performed to examine the effects of certain methodological variations among studies. Both random and fixed effect model were used to arrive at a conclusion. RESULTS The included trials represented 275 patients from 10 studies Sham treatment was given to 125 patients in the control groups, and 135 patients were in the rtms groups. The treatment regimen, pulse intensity, and concomitant drug intake varied among studies and are summarized in Table 1. All the included studies were randomized controlled clinical trials. In most of the studies, the patients and UPDRS raters were blinded to the treatment assignment. 7,12 15,17 In one study, the patients and raters were not blinded. 16 In two studies, it was not stated whether the raters were blinded. 9,11 We separated studies into two groups, those that used rtms at frequencies higher than 1 Hz and studies that used 1 Hz or lower frequencies. The reason for this classification is the opposite effect of these frequencies on cortical excitability. Low-frequency rtms (1 Hz or less) over the primary motor cortex produce inhibitory effects, 4 whereas high-frequency rtms generally increases cortical excitability. 18,19 There were 152 patients in high-frequency group and 123 patients in low-frequency group. Patients in sham group of Lefaucheur et al. 13 were included in both the lowand high-frequency groups because this study compared both low- and high-frequencies rtms to sham stimulation. Sensitivity Analysis For higher frequency rtms studies, early (same day) versus late UPDRS evaluation did not change the final result. There is also no significant difference between the fixed and random effect models. Cochran Movement Disorders, Vol. 24, No. 3,
174 TABLE 1. Summary of included studies Study Blinding Mean age (yr) PD duration (yr) Men/ women H&Y stage Evaluation time after rtms PD drug status Intensity Pulses per day Days rtms parameters Frequency (Hz) Coil Site Part of UPDRS used UPDRS after sham rtms UPDRS after real TMS Siebner et al., Okabe et al., Shimamoto et al., del Olmo et al., Khedr et al., Fregni et al., Lomarev et al., Boggio et al., Ikeguchi et al., Lefauheur et al., _1 Lefaucheur et al., _2 Nonblinded 57 7/ hr Off 90% MT 2, F8 M1 Part III Blinded rater /37 16 wk Off 110% MT C M1 Part III Blinded rater / mo On 0.31 T C Frontal Total ? / days On 90% MT F8 DLPFC Part III Blinded rater / mo Off 120% MT 2, F8 M1 Part III Blinded rater / wk Off 110% MT F8 Left DLPC Blinded rater / mo On 100% MT 1, F8 Bilateral DLPFC Blinded rater /10 8 wk On 110% MT F8 Left DLPFC Part III Part III Part III ? subjects 1 4 Immediate Off 70% output C Prefrontal Part III a a Blinded rater 64 7/ Immediate Off 80% MT F8 Left M1 Part III a a Blinded rater 64 7/ Immediate Off 80% MT 2, F8 Left M1 Part III a a PD duration and UDPRS scores are expressed as mean 6 standard deviation. a Extracted from the figures. PD, Parkinson s disease; H&Y, Hoehn and Yahr scale; rtms, repetitive transcranial magnetic stimulation; MT, motor threshold; T, tesla; F8, figure of eight; C, circular; M1, motor cortex; DLPFC, dorsolateral prefrontal cortex; UPDRS, Unified Parkinson s Disease Rating Scale. 159
175 360 B. ELAHI ET AL. FIG. 2. Individual and pooled effect size for motor UPDRS in PD patients treated with high-frequency rtms. The size of the squares increases with increasing sample size. Q test for heterogeneity (Cochran Q ; P ) and I square inconsistency (I 2 5 0%) tests indicate that the included studies for high-frequency rtms are sufficiently homogenous for the result to be combined in a fixed effect model (Fig. 2). For lower frequency TMS studies, the results were heterogeneous (Cochran Q ; P < 0.01). I 2,a measure of the inconsistency of the results and is less dependent on the number studies than the Cochran Q, also showed high inconsistency between studies. Sensitivity analysis of the evaluation time (immediately after vs. days to months after) resulted in significant difference in effect sizes when Okabe et al. study was included. The results from remaining studies showed acceptable consistency (I %; Cochran Q ; P ). However, the Okabe et al. study was the largest and had more patients than the other lowfrequency studies combined. High-Frequency rtms Studies The pooled mean effect size estimate (d1) is calculated using direct weights defined as the inverse of the variance of d for each study/stratum, which was (95% CI to 20.27; P ) for the fixed effect model and (95% CI to 20.27) for the random effect model. Therefore, with the random effects model, the true effect size was at least 0.58 lower in the treatment groups compared with the control groups (Fig. 2). This is equivalent to a 6.68 (95% CI to 23.69) point decrease in motor UPDRS score in the random and fixed effect models. Regression of normalized effect versus precision for high-frequency studies is shown in Figure 3. Although we found no significant asymmetry (Egger: bias (95% CI to 4.6) P 5 0.6), because of the small number of studies and different methodologies used, the results should be interpreted with caution and the power of this analysis is low (Fig. 3). Low-Frequency rtms Studies Studies in this category are different both in their design and in the reported outcomes. The largest study by Okabe et al. 15 on 85 patients showed decrease in motor UPDRS from (mean 6 SD) to rtms group but the reduction in motor UPDRS was even greater in the sham rtms group (from to ). Shimamoto et al. 7 reported total UPDRS but not motor UPDRS, which is our outcome of interest. Therefore, we analyzed the two remaining studies with the total of 16 patients in each group and showed no significant reduction in motor UPDRS between control and treatment group. The effect size calculated using the random effect model (DerSimonian Laird method for weighted mean difference) was (P ) (Fig. 4). DISCUSSION This study confirms that high-frequency rtms can significantly reduce motor signs in PD patients and all included trials showed this reduction. On the other hand, our low-frequency rtms studies showed variable Movement Disorders, Vol. 24, No. 3,
176 EFFECT OF TMS ON PARKINSON MOTOR FUNCTION 361 FIG. 3. Bias indicator for high-frequency rtms controlled studies. Each dot represents one study. The horizontal axis shows the effect size. The vertical axis shows the standard error of the effect size, which is an indicator of the sample size. Larger studies have smaller standard errors and they are located in higher part of the graph and smaller studies are in lower part of the graph. The vertical line represents the pooled effect size. The diagonal lines show linear extrapolation of the 95% confidence limit of the effect size. results with no significant overall improvement in UPDRS scores. There are several limitations of our study. First, the study outcomes were not uniformly reported. Second, there are considerable differences in the rtms protocol. Moreover, the analyzed studies also varied in patient selection criteria, demographics, and duration of follow-up and stages of PD. We used sensitivity FIG. 4. Standardized effect sizes for with low-frequency rtms studies. The mean and 95% confidence limits for each study are shown. Movement Disorders, Vol. 24, No. 3,
177 362 B. ELAHI ET AL. analysis to examine some of these sources of the heterogeneity such as time to evaluate motor function after intervention. We also used I 2 as an index of inconsistency; if there was little variation between trials, I 2 would be low and a fixed effects model might be appropriate. An alternative approach, random effects, allows the study outcomes to vary in a normal distribution between studies. Many investigators consider the random effects approach to be a more natural choice than fixed effects model, for example, in the context of medical decision making. 20 We therefore used both random effect and fixed effect models of analysis. The different blinding techniques in rtms studies may also have influenced our results. Several different methods of sham (placebo) stimulation were used. Five trials used a sham coil, 7,8,10,13,15 three studies used changes in coil angle, 9,12,16 one study stimulated the occipital area, 11 and one study flipped the side of the coil applied to the scalp. 14 However, the findings from high-frequency rtms studies are consistent and the effects of this variability are likely to be small. Motor UPDRS, our outcome of interest, is a widely accepted scale. It had been shown to be a reliable and valid, with high internal consistency. 21 Our study supports the hypothesis that high-frequency rtms can modulate underactive brain regions in PD patients 22,23 and produce clinically significant motor improvement. On the other hand, the lower frequency rtms, although potentially safer, do not have such effect. However, low-frequency rtms is a potential treatment for levodopa-induced dyskinesia, which was not analyzed in this study. 24,25 Fregni et al. 26 reviewed the efficacy of rtms and electroconvulsive therapy (ECT) for the treatment of motor dysfunction in PD. They calculated a pooled effect size of 0.62 in a random effects model for TMS treatment and 1.68 for ECT treatment, and from a fixed effects model the effect size was 0.59 for TMS and 1.55 for ECT treatment. Our study included more recently TMS literature, 9,14 and we separated high- and low-frequencies rtms studies. Although high-frequency rtms has potential adverse effects, including induction of seizures, it is generally safe when used within safety guidelines. 27,28 It is well tolerated, easy to apply, and can be used as an adjunct to other treatment modalities in PD patients. Some of the factors that limit wide spread clinical use of therapeutic rtms are the cost and limited availability of the devices to specialized centers, less knowledge of potential long-term side effects compared with drug therapies, and the requirement for skilled personnel. However, our results showed that high-frequency rtms is a promising treatment of motor symptoms in PD. A large, randomized controlled trial with appropriate follow-up will be useful to further define its role in the treatment of PD. Future studies are also needed to clarify the optimal stimulation parameters, how the different stages of PD affect the response to rtms, and the effects of rtms on other aspects of the disease such as gait, cognition, and memory. Acknowledgments: We thank Dr. Shingo Okabe and Dr. Yoshikazu Ugawa for providing extra data from their studies. REFERENCES 1. Barker AT, Jalinous R, Freeston IL. Noninvasive magnetic stimulation of human motor cortex. Lancet 1985;2: Pascual-Leone A, alls-sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994;117: Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cohen LG, Hallett M. Akinesia in Parkinson s disease. I. Shortening of simple reaction time with focal, single-pulse transcranial magnetic stimulation. Neurology 1994;44: Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 1997;48: Ellaway PH, Davey NJ, Maskill DW, Dick JP. The relation between bradykinesia and excitability of the motor cortex assessed using transcranial magnetic stimulation in normal and parkinsonian subjects. Electroencephalogr Clin Neurophysiol 1995;97: Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, New Jersey: Lawrence Erlbaum Associates; Shimamoto H, Takasaki K, Shigemori M, Imaizumi T, Ayabe M, Shoji H. Therapeutic effect and mechanism of repetitive transcranial magnetic stimulation in Parkinson s disease. J Neurol 2001; 248 (Suppl 3):III48 III Boggio PS, Fregni F, Bermpohl F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson s disease and concurrent depression. Mov Disord 2005; 20: del Olmo MF, Bello O, Cudeiro J. Transcranial magnetic stimulation over dorsolateral prefrontal cortex in Parkinson s disease. Clin Neurophysiol 2007;118: Fregni F, Santos CM, Myczkowski ML, et al. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson s disease. J Neurol Neurosurg Psychiatry 2004;75: Ikeguchi M, Touge T, Nishiyama Y, Takeuchi H, Kuriyama S, Ohkawa M. Effects of successive repetitive transcranial magnetic stimulation on motor performances and brain perfusion in idiopathic Parkinson s disease. J Neurol Sci 2003;209: Khedr EM, Farweez HM, Islam H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson s disease patients. Eur J Neurol 2003;10: Lefaucheur JP, Drouot X, Von Raison F, Menard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson s disease. Clin Neurophysiol 2004;115: Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rtms for the treatment of Parkinson s disease. Mov Disord 2006;21: Movement Disorders, Vol. 24, No. 3,
178 EFFECT OF TMS ON PARKINSON MOTOR FUNCTION Okabe S, Ugawa Y, Kanazawa I. 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic sham stimulation in Parkinson s disease. Mov Disord 2003;18: Siebner HR, Rossmeier C, Mentschel C, Peinemann A, Conrad B. Short-term motor improvement after sub-threshold 5-Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson s disease. J Neurol Sci 2000;178: Fregni F, Boggio PS, Bermpohl F, et al. Immediate placebo effect in Parkinson s disease is the subjective relief accompanied by objective improvement? Eur Neurol 2006;56: Fujiki M, Steward O. High frequency transcranial magnetic stimulation mimics the effects of ECS in upregulating astroglial gene expression in the murine CNS. Brain Res Mol Brain Res 1997; 44: Speer AM, Kimbrell TA, Wassermann EM, et al. Opposite effects of high and low frequency rtms on regional brain activity in depressed patients. Biol Psychiatry 2000;48: Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005;25: Martinez-Martin P, Gil-Nagel A, Gracia LM, Gomez JB, Martinez-Sarries J, Bermejo F. Unified Parkinson s Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord 1994;9: Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson s disease subjects. Brain 1995;118: Tada Y. Motor association cortex activity in Parkinson s disease a functional MRI study. Rinsho Shinkeigaku 1998;38: Wagle-Shukla A, Angel MJ, Zadikoff C, et al. Low-frequency repetitive transcranial magnetic stimulation for treatment of levodopa-induced dyskinesias. Neurology 2007;68: Koch G, Brusa L, Caltagirone C, et al. rtms of supplementary motor area modulates therapy-induced dyskinesias in Parkinson disease. Neurology 2005;65: Fregni F, Simon DK, Wu A, Pascual-Leone A. Non-invasive brain stimulation for Parkinson s disease: a systematic review and meta-analysis of the literature. J Neurol Neurosurg Psychiatry 2005;76: Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5 7, Electroencephalogr Clin Neurophysiol 1998;108: Chen R, Gerloff C, Classen J, Wassermann EM, Hallett M, Cohen LG. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 1997;105: Movement Disorders, Vol. 24, No. 3,
179 .2 N-Methyl-D-Aspartate Antagonists in Levodopa Induced Dyskinesia: A Meta-Analysis 164
180 ORIGINAL ARTICLE 1499 N-Methyl-D-Aspartate Antagonists in Levodopa Induced Dyskinesia: A Meta- Analysis Behzad Elahi, Nicolás Phielipp, Robert Chen ABSTRACT: Background: Levodopa-induced dyskinesias (LID) are amongst the most disabling side-effects of levodopa therapy for Parkinson s disease (PD). It has been suggested that that N-Methyl-D-Aspartate (NMDA)-receptor antagonist may reduce peak-dose dyskinesia in PD patients and may lead to motor improvement. In this study, we compared the efficacy of NMDA receptor antagonists versus placebo in the treatment of LID in PD through a meta-analysis of controlled trials. Methods: Electronic search of Pubmed ( ), Medline ( ), EMBASE ( ) and other databases for relevant studies were performed. Controlled clinical trials of the effects of NMDA antagonists on LID that fulfill the study protocol were selected. Pooled data from included studies was then used to perform random and fixed effect models meta-analysis. Results: The search resulted in 11 randomized, placebo controlled clinical trials that involved a total of 253 PD patients with peak-dose LID. The outcome measures were various dyskinesia rating scales and the Unified Parkinson Disease Rating Scale (UPDRS) subscales III and IV. The analysis showed significant reduction in Standard Mean Difference (SMD) for UPDRS IV (SMD -1.45; 95% CI to -0.63) and UPDRS III (SMD -0.41; 95% CI to -0.12) after treatment with amantadine. Other included drugs did not show significant change in the outcomes measured. Conclusion: This metaanalysis provides an update on the clinical trials and confirms the short-term benefits of amantadine therapy in the treatment of dyskinesia. The effects of other NMDA receptor antagonists need to be evaluated further in clinical trials. RÉSUMÉ: Antagonistes de la N-méthyl-D-aspartate dans le traitement des dyskinésies induites par la lévodopa : une méta-analyse. Contexte : Les dyskinésies induites par la lévodopa (DIL) sont parmi les effets secondaires du traitement de la maladie de Parkinson (MP) qui sont les plus invalidants. Selon certaines données, les antagonistes du récepteur de la N-méthyl-D-aspartate (NMDA) pourraient diminuer les dyskinésies lorsque l'effet de la dose de lévodopa est à son maximum chez les patients atteints de la MP et pourraient améliorer la motricité. Dans cette étude, nous avons comparé l'efficacité des antagonistes du récepteur NMDA à celle d'un placebo dans le traitement des DIL dans la MP au moyen d'une méta-analyse portant sur des essais cliniques à répartition aléatoire. Méthode : Nous avons effectué une recherche électronique PUBMED ( ), Medline ( ), EMBASE ( ) ainsi que d'autres banques de données afin d'identifier les études pertinentes. Nous avons sélectionné les essais cliniques contrôlés portant sur les effets d'antagonistes de la NMDA sur les DIL qui rencontraient les critères de sélection de notre protocole. Nous avons effectué une méta-analyse selon un modèle à effets aléatoires et un modèle à effets fixes sur les données regroupées des différentes études. Résultats : Notre recherche a permis d'identifier 11 essais cliniques contrôlés par placebo et à répartition aléatoire, auxquels un total de 253 patients, atteints de la MP et présentant des DIL lorsque l'effet de la dose est à son maximum, ont participé. Différentes échelles d'évaluation et les sous-échelles III et IV de la United Parkinson Disease Rating Scale (UPDRS) ont été utilisées comme mesures des résultats. Cette analyse a montré une diminution significative de la différence moyenne standardisée (DMS) pour l'updrs IV (DMS -1,45; IC à 95% -2,28 à -0,63) et pour UPDRS III (DMS -0,41; IC à 95% -0,69 à -0,12) après le traitement par l'amantadine. Nous n'avons pas observé de changement significatif au niveau de la mesure des résultats en ce qui concerne les autres médicaments utilisés. Conclusion : Cette méta-analyse fournit une mise à jour sur les essais cliniques et confirme les bénéfices à court terme du traitement par l'amantadine dans le traitement des dyskinésies. Les effets d'autres antagonistes des récepteurs NMDA devront faire l'objet d'études cliniques supplémentaires. Can J Neurol Sci. 2012; 39: BACKGROUND Dopaminergic medications are effective treatments for Parkinson's Disease (PD), but over time therapeutic complications such as motor fluctuations and levodopa-induced dyskinesias (LID) frequently develops. Levodopa-induced dyskinesias are abnormal involuntary movements primarily affecting the extremities, trunk, or jaw. The underlying mechanism of LID is not completely known but it is associated with changes in dopamine receptors 1 and in the subunit phosphorylation pattern of co-expressed ionotropic N-Methyl- D-Aspartate (NMDA) glutamatergic receptors. 2 Sensitization of these receptors may augment cortical excitatory input to the spiny efferent striatal neurons, thus altering striatal output and compromise motor functions. 3 N-Methyl-D-Aspartate antagonists monotherapy have been shown to reduce parkinsonian symptoms without induction of dyskinesia in animal models of PD. 4 Current management guidelines for treatment of peak dose dyskinesia in PD encourage use of amantadine as an add-on From Toronto Western Research Institute, Toronto Western Hospital; Division of Neurology, Department of Medicine, University of Toronto, Toronto, Ontario, Canada. RECEIVED AUGUST 29, FINAL REVISIONS SUBMITTED JANUARY 9, Correspondence to: Behzad Elahi, MP13-314, Toronto Western Hospital, 399 Bathurst Street, Toronto, Ontario, M5T 2S8, Canada. THE CANADIAN JOURNAL OF NEUROLOGICAL SCIENCES 1 165
181 THE CANADIAN JOURNAL OF NEUROLOGICAL SCIENCES medication, and reduction in the doses of levodopa and monoamine oxidase B (MAO-B) inhibitors or cathecol-o-methyl transferase (COMT) inhibitors. 5,6 Other approaches including deep brain stimulation, 7 low frequency repetitive transcranial magnetic stimulation, 8 and new anti-dyskinetic drugs targeting non-dopaminergic receptors such as NMDA or metabotropic glutamate receptor (mglur) subtypes 9,10 are promising alternative treatment options. Meta-analysis is being used increasingly to combine results from multiple research studies to produce a summary estimate of the treatment effect. Meta-analysis is particularly useful when small number of subjects were enrolled in each of the trials to improve the analytic power of the studies by evaluating the collective body of evidence. This study evaluates published randomized, placebo controlled clinical trials which used drugs with NMDA receptor antagonistic properties to determine whether this group of medications is effective in management of LID in PD. METHODS Criteria for inclusion in this meta-analysis All randomized, controlled trials comparing NMDA receptor antagonists (mainly amantadine and dextrometorphan) with placebo in the treatment of dyskinesia are included in this study. The patients recruited in these studies must have met standard criteria for the diagnosis of PD 11 and have had experienced LID. Only prospective clinical studies with placebo control group were included in this study. Primary and secondary outcome measures Our primary outcome was changes in dyskinesia rating scales used by the included studies. Secondary outcomes for this study were changes in the motor section (Part III) of the Unified Parkinson Disease Rating Scale (UPDRS) and changes in scale of complications of therapy, duration and severity of dyskinesias in UPDRS part IVa. Search methods for identification of studies Electronic searches Medline ( ), Embase ( ), CINAHL, Web of Science, Scopus bibliographic, and Google Scholar databases were searched for studies investigating the effect of NMDA receptor antagonists in treatment of LID in PD. Articles published between January 1985 and September 2010, were retrieved. Reference lists of the retrieved trials and review articles were manually inspected for cross-references. Conference abstracts and unpublished data were excluded. MeSH terms and text words were searched for NMDA antagonists (amantadine, dextrometorphan, dextrorphan, Table 1: Characteristics of the studies included in the meta-analysis Study Design Sample size Silva-Junior 2005 Age Drug Dosage Duration of PD P Amantadine mg/day H&Y - 2.5± ±2.1 (IVa) UPDRS IV Dyskinesia UPDRS III followup* Drug Placebo Drug Placebo Drug Placebo 3.7± ± ± ± ±5.3 3w (IVa) (CDRS) (CDRS) Del Dotto 2001 P ± 8 Amantadine 200 mg IV 8.4 ± 3 Luginger 2000 CO ± ± 0.5 Amantadine 300mg/day 10.1 ± ± ± 1.7 (AIMS) 7.0 ± 8.2 (IVa) Merello 1999 CO ± 3 Memantine 30 mg /day 14.5 ± ± 6.2 (IVa) 14.5 ± 9.4 (Iva baseline) (IVa) 9.1 ± 9.1 (Marconi) 8.3 ± 1.8 (AIMS) 19.3 ± 13.7 (Marconi) 21.6 ± ± 9.7 ~50 ± 20 ~68 ± 20 2w ± ± hours after infusion 2w Parkinson study group 2001 P ±8.4 Remacemide Hydrochloride mg/day 13.3 ± ± ±0.6 (Goetz) 1.7 ±0.6 (Goetz) 26.7± ±13.4 2w Snow 2000 CO ± 8.90 Amantadine mg/d 10.6 ± ± 1.6(IVa) 4.3± 1.5(IVa) 22 ± 13.2 (Goetz) 29.0 ± 12.6 (Goetz) 22.3 ± ±9.0 3w Thomas 2004 P ±5.2 Amantadine 300 mg/day 7.9 ± ± ± 2.8 (IVa) 6.7± 2.6 (IVa) 10.5 ± 1.3 (DRS) 9.2 ±2.0 (DRS) 48.1 ±7.8 (I-III) 52.9 ± days Verhagen Metman 1998c Verhagen Metman1998b CO ±3.4 Dextromethorphan + quinidine 200 mg/day CO ± 2 Amantadine 350 ± 15 mg/day 180 mg/day 12 ± ± ± 0.3 (off) 3.5 ± ± 0.7 (AIMS) 1 (Items 32, 34, 39) 4 (Items 32, 34, 39) 3.6 ±0.6 (AIMS) 4.2 ±1.1 (AIMS) 7.0 ± 0.9 (AIMS) - - 3w - - 6w Verhagen Metman1998a CO ± 2.3 Dextromethorphan mg/day 15 ± ± ± 0.3 Items 32, 34) Wolf 2010 P 32 67±7.7 Amantadine 100mg/day 16.8± ± 0.4 (items 32, 33) 3.4 ± 0.6 Items 32, 34) 4.4± 0.4 (items 32, 33) AIMS: Abnormal Involuntary Movements Scale; CDRS: Clinical Dyskinesia Rating Scale; CO: Cross-over; DRS: Dyskinesia Rating Scale; H&Y: Hoehn and Yahr Parkinson's disease staging scale; P: Parallel design; PD: Parkinson s disease; RCT: Randomized Clinical Trial; UPDRS: Unified Parkinson Disease Rating Scale; item IVa, *: follow-up indicates the most immediate evaluation time point after the end of treatment for each study. This is different from the maximum follow-up time for each study. 4.6 ± 1.3 (AIMS) 3.5 ± 1.0 (AIMS) ± ± ±2 2 w 27.7± 3.7 3w 2 166
182 LE JOURNAL CANADIEN DES SCIENCES NEUROLOGIQUES ibogaine, riluzole, memantine, remacemide, glycine and glutamate antagonists) with dyskinesia or Parkinson's disease and their related derivatives. Data collection and analysis Three trained individuals (BE, NP, XS) independently reviewed the articles for the quality and validity of the trials. Data on the therapeutic regimen, sample size, trial duration and outcomes were extracted and results were summarized in a standard summary data sheet. Disagreements were resolved by discussion and consensus between reviewers. The characteristics of the included studies are shown in Table 1. All three reviewers also assessed the studies for risk of bias in blinding and allocation, and scored the quality of included studies using a critical appraisal toolkit. 12 Measures of treatment effect Unit of analysis issues The standardized mean difference (SMD) used in this systematic review is the effect size known in social science as Hedges (adjusted) g. SMD is used as a summary statistic in meta-analysis when all studies assessed the same outcome, but measured it in a variety of ways (such as different scales). In this circumstance, it is necessary to standardize the results of the studies to a uniform scale before they can be combined. The standardized mean difference expresses the size of the intervention effect in each study relative to the variability observed in that study. Thus, studies in which the difference in means is the same proportion of the standard deviation will have the same SMD, regardless of the actual scales used to make the measurements. 13 In this study all the included scales point in the same direction; lower scores indicate improvement and higher scores represent deterioration of parkinsonism or dyskinesia. parallel groups design in four studies 16,20,22,24 and cross-over design in the remaining studies. 14,15,17,19,21,23 The participants in all the included studies were diagnosed with PD and had peak dose LID. The majority of the included patients had moderate to advanced PD with Hoehn and Yahr stage ranged from 2 to 4, and the mean age ranged from 59 to 67.7 years old. The route of drug administration was oral tablets or capsules except in one study which used intravenous amantadine. 18 Since the duration of follow-up varied among the included studies (range 0-12 months), we only considered the immediate measurements of LID after the last dose of medication used in each study. Various regimen and dose were used in each study (Table1) and the duration of drug administration ranged from one day to six weeks. In most of the studies, the measurements of LID were performed after an oral or intravenous levodopa challenge test. 15,23 However, in four studies LID measurements were recorded in both "On" and "Off" drug states and a levodopa challenge test was not performed. 14,16,22,24 In these studies, results from On state were used in the analysis. Risk of bias in the included studies Allocation concealment Most studies used computer generated or random number tables to allocate study subjects to treatment and placebo groups. Allocation method is not stated in one study 14 and was done manually by a neurologist not involved in patient evaluations in another study. 15 Assessment of heterogeneity The Cochran Q and I square inconsistency tests were used to examine heterogeneity. A statistically significant Cochran Q may indicate a problem with heterogeneity although heterogeneity cannot be excluded with a non-significant result. Sensitivity analysis, subgroup analysis for the different drugs and assessment methods was performed to examine methodological variations among studies. Both random and fixed effect models were used to arrive at conclusions. RevMan verision (Cochrane Information Management System) was used for analysis. RESULTS Description of included studies Eleven studies were included in analysis with a total of 233 participants (Table 1). Two studies used dextromethorphan, 14,15 one study used remacemide, 16 one study used memantine 17 and amantadine was used in the remaining studies The quality of the studies was assessed by all three reviewers and all the included studies had moderate to high quality scores. 12 We excluded seven studies 12,25-30 due to lack of control group and one study due to comparison of their data with a historical control group. 31 The design of trials varied between randomized, Figure 1: Bias indicator for NMDA antagonist effect on peak dose levodopa induced dyskinesia. The horizontal axis shows the standard mean difference (SMD). The vertical axis shows the standard error of SMD effect size, which is an indicator of the sample size. Larger studies have smaller standard errors and are located in the upper part of the graph; smaller studies are in the lower part of the graph. The vertical line represents the pooled effect size for random-effect model of metaanalysis. Volume 39, No. 4 July
183 THE CANADIAN JOURNAL OF NEUROLOGICAL SCIENCES Table 2: Effects of NMDA antagonists on levodopa-induced dyskinesia measured by various scales and subgroup analysis Outcome or Subgroup No. of No. of Effect size estimate [95% P-value studies participants confidence interval] Dyskinesia various scales* [-1.88, -0.32] Amantadine [-2.28, -0.63] Other drugs [-2.45, 0.74] 0.29 UPDRS IV [-1.66, -0.30] Amantadine [-1.92, -0.28] Other drugs [-4.76, 1.72] 0.39 UPDRS III [-0.60, -0.09] Amantadine [-0.69, -0.12] Other drugs [-0.66, 0.41] 0.64 Random-effect model and inverse variance was used to calculate effect size estimate; * The dyskinesia scales used include the Abnormal Involuntary Movements Scale (AIMS), the Clinical Dyskinesia Rating Scale (CDRS), the Dyskinesia Rating Scale (DRS), the Goetz Dyskinesia Rating Scale and the Marconi Scale. Blinding All the included studies were double blind, although proper blinding of the raters was not clearly stated in one study. 17 Effects of interventions The pooled SMD effect size was calculated by pooled intervention-specific standard deviations for each study/stratum (see Table 2 for estimate of effect size for subgroup analyses). We pooled all the studies with dyskinesia as their primary outcome which used dyskinesia scales other than UPDRS IV (9 studies, 203 participants). This analysis showed a significant improvement in favor of the experimental drug (SMD -1.10; 95% CI to -0.32, P<0.001) in both the random-effect model with the DerSimonian and Laird method and the fixed- Figure 2: Individual and random-effect model of pooled standardized mean difference (SMD) for various dyskinesia rating scales in PD patients treated with amantadine and other drugs. The size of the squares increases with increasing sample size. Significant improvement in dyskinesia rating scales was observed for amantadine but not for other drugs
184 LE JOURNAL CANADIEN DES SCIENCES NEUROLOGIQUES Figure 3: Individual and random-effect model of pooled standardized mean difference (SMD) for UPDRS-IV subscale in PD patients treated with amantadine and other drugs. The size of the squares increases with increasing sample size. Amantadine resulted in significant reduction in UPDRS-IV scores no significant effect was observed for other drugs. effect model. Test of variation, or heterogeneity, among the intervention effects indicates a heterogeneous data with Tau² = 1.11 (P<0.0001) and I square test for inconsistency of 82%. Funnel plot for these studies shows that studies showing greater improvement in dyskinesia scores after NMDA receptor antagonists therapy tend to have larger Standard Error (SE) of SMDs. Visual inspection of the funnel plot shows few studies with negative results and high SE (lower right side of the plot), suggesting that no study has been published with small sample size and negative results, possibly due to a publication bias against negative results. However, firm conclusion cannot be made because of the small number of studies included and the low power for analysis of asymmetry in the funnel plots (Figure 1). In the next step, the same statistical models were used for subgroup analysis of different drugs. There was a significant improvement in dyskinesia scales used in favor of amantadine (SMD -1.45; 95% CI to -0.63) with overall effect of Z = 3.44 (P<0.001) for the random effect model and Z=6.01 (P<0.001) for the fixed-effect model (six studies, 159 participants). Subgroup analysis for amantadine showed significant heterogeneity with I2 =79%. Subgroup analysis for other drugs (dextrometorphan and remacemide, three studies, 44 subjects) did not show significant effect of drug treatment on dyskinesia (Figure 2). UPDRS sub-scale IV for dyskinesia was an outcome measure in eight studies (195 patients). Luginger et al. 19 was excluded from this calculation because UPDRS IV score after placebo therapy was not available. Meta-analysis shows SMD (95% confidence interval) of [-1.66, -0.30] with the random effect model, which indicates a significant improvement in favor of NMDA antagonist (Z = 2.83; P = 0.005) (Figure 3). Test for heterogeneity showed heterogeneous data with Tau² = 0.95 and I 2 = 74%. Subgroup analysis for amantadine showed significant improvement after amantadine therapy compared to the placebo group in both random and fixed-effect models (P<0.01) (Figure 3). These finding translate to approximately 1.33 point reduction in UPDRS-IV sub-scale for amantadine. The findings for memantine and dextromethorphan (other drugs) were not significant for UPDRS IV scale (Table 2 for details). Finally, analysis for changes in motor sub-scale of UPDRS (Part III) from nine studies and 258 patients indicates a significant reduction in UPDRS III after NMDA antagonist therapy in both random and fixed-effect model (P< 0.01) with pooled effect size SMD = [CI: -0.60, -0.09] (Figure 4). This finding indicates an approximately 1.28 point reduction in UPDRS III. Details for subgroup analyses are shown in Table 2. Number of patients in this analysis was larger than total number of participants (233) for all studies. This is mainly because of the crossover design of some of the studies. Patients in each arm were counted separately and therefore the majority of patients in crossover design studies were counted twice. For the fixed effect model, Cochran Q test for non-combinability of studies was not significant (Cochran Q = 7.4 (df = 8) P = 0.49, I² (inconsistency) = 0%). None of the included studies reported any severe adverse Volume 39, No. 4 July
185 THE CANADIAN JOURNAL OF NEUROLOGICAL SCIENCES Figure 4: Individual and fixed-effect model of pooled standardized mean difference (SMD) for UPDRS-III subscale in PD patients treated with amantadine and other drugs. The size of the squares increases with increasing sample size. Treatment with amantadine resulted in mild but significant improvement in UPDRS-III score no such effect was observed for other medications. event. Some of the studies reported side effects such as confusion and worsening of hallucination. Because these minor side effects were not systematically reported in the studies, they were not analyzed further. DISCUSSION Our results indicate amantadine can be effective in treatment of peak dose LID in PD. Treatment with amantadine resulted in large effect size (>0.8) 32 in favor of improvement in UPDRS-IV, other dykinesia rating scales and in UPDRS-III subscale for PD motor symptoms. The effects of other medications such as dextrometorphan and remacemide on LID were not significant. Since the majority of studies used amantadine, the statistical power for the effects of other medications was much less than that for amantadine. There is only one published systematic reviews on treatment of LID and only amantadine was studied 33. The current study has a larger scope and evaluated other NMDA receptor antagonists. This review also includes more studies than the previous one 33 and confirms the efficacy of amantadine as a short-term treatment for LID. Our study also highlights the importance of using validated scales to assess the severity of the dyskinesia, include longer follow-up periods and in case of crossover studies, and sufficient washout period. The effects of novel NMDA receptor antagonists on LID need to be evaluated in proper clinical trials. A major concern in interpretation of results is that several different dyskinesia rating scales were used to assess the primary outcome in the included studies. Several rating scales have been used in clinical studies since the 1970s for the assessment of dyskinesia in PD. Some were specifically developed for dyskinesia in PD, whereas others were part of global scales that measure motor disability in PD such as UPDRS-IV. Some scales were originally developed for use in other syndromes with dyskinesia, but were adapted to score PD dyskinesia 34. An example is abnormal involuntary movement scale (AIMS) which was originally developed for assessment of tardive dyskinesia. 35 In this meta-analysis, the majority of the studies used either UPDRS-IV or AIMS for evaluation of dyskinesia. Although UPDRS-IV is widely being used in practice, the items covering dyskinesia have not been independently studied from a clinimetric perspective. On the other hand, AIMS has high inter and intra rater reliability 36,37 but cannot differentiate between different movement abnormalities. Considering these limitations, both of these tools and especially the AIMS are among the recommended scales for use in assessment of dyskinesia in PD. 36 Another limitation of this study is that no study examined the long-term results of NMDA receptor antagonist treatment on 6 170
186 LE JOURNAL CANADIEN DES SCIENCES NEUROLOGIQUES LID and therefore only short-term results are reported here. However, in one study 24 patients on stable doses of amantadine were randomized to receive placebo or continue taking amantadine. This study reported worsening of symptoms after amantadine cessation and demonstrated longer term effects of amantadine therapy. There may be potential publication bias for negative studies (Figure 1). Registration of future clinical trials may reduce publication bias. The different study regimen, route of drug administration, and different dyskinesia scales used can potentially reduce the precision of our findings. Four of the included studies used a parallel group design and six studies used a cross-over design. Cross-over design offers several advantages over parallel group design. Each participant acts as his or her own control and this reduces variation among participants. It also increases the study power as every participant receives both interventions. However, there is a possibility of inadequate washout and may be more prone to unblinding due to beneficial or adverse effects. Sensitivity analysis between parallel and cross over studies was not significant but we observed a larger effect size for studies with cross-over design. We only analyzed published data and we did not search the unindexed or unpublished data, academic theses and conference abstracts which may result in a publication bias for favorable results. We imposed no language limitation in our search, nevertheless all the included studies were in English. CONCLUSIONS Amantadine appeared to be safe and effective for reducing of LID. However, further studies are needed to examine the longterm effects of NMDA antagonist therapy. ACKNOWLEDGMENT The authors thank Ms. Xing Sun for helping us rate the quality of the included studies. FUNDING This work was supported by the Catherine Manson Chair in Movement Disorders. Dr. Elahi is supported by a CIHR fellowship in field of dystonia and Dr. Chen is supported by a CIHR-Industry Partnered (Medtronic Inc) Investigator Award. REFERENCES 1. Gerfen CR, Engber TM, Mahan LC, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science Dec 7;250(4986): Chase TN. Levodopa therapy: consequences of the nonphysiologic replacement of dopamine. Neurology. 1998;50(5 Suppl 5): S17-S Calon F, Rajput AH, Hornykiewicz O, Bedard PJ, Di Paolo T. Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson's disease. Neurobiol Dis. 2003;14(3): Nash JE, Fox SH, Henry B, et al. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson's disease. Exp Neurol. 2000;165(1): Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology Apr 11;66(7): Horstink M, Tolosa E, Bonuccelli U, et al. Review of the therapeutic management of Parkinson's disease. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: late (complicated) Parkinson's disease. Eur J Neurol Nov;13(11): Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol Jan;8(1): Wagle-Shukla A, Angel MJ, Zadikoff C, et al. Low-frequency repetitive transcranial magnetic stimulation for treatment of levodopa-induced dyskinesias. Neurology Feb 27;68(9): Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM. Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3- thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Pharmacol Exp Ther Jun;333(3): Morissette M, Dridi M, Calon F, et al. Prevention of levodopainduced dyskinesias by a selective NR1A/2B N-methyl-Daspartate receptor antagonist in parkinsonian monkeys: implication of preproenkephalin. Mov Disord Jan;21(1): Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry Mar;55(3): Guyatt GH, Sackett DL, Cook DJ. Users' guides to the medical literature. II. How to use an article about therapy or prevention. B. What were the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA Jan 5;271(1): Higgins JPT e, Green S e. Cochrane Handbook for Systematic Reviews of interventions, handbook/hbook.htm; September Verhagen ML, Blanchet PJ, van den MP, Del Dotto P, Natte R, Chase TN. A trial of dextromethorphan in parkinsonian patients with motor response complications. Mov Disord. 1998;13(3): Verhagen ML, Del Dotto P, Natte R, van den MP, Chase TN. Dextromethorphan improves levodopa-induced dyskinesias in Parkinson's disease. Neurology. 1998;51(1): Parkinson Study Group. Evaluation of dyskinesias in a pilot, randomized, placebo-controlled trial of remacemide in advanced Parkinson disease. Arch Neurol. 2001;58(10): Merello M, Nouzeilles MI, Cammarota A, Leiguarda R. Effect of memantine (NMDA antagonist) on Parkinson's disease: a doubleblind crossover randomized study. Clin Neuropharmacol. 1999; 22(5): Del Dotto P, Pavese N, Gambaccini G, et al. Intravenous amantadine improves levadopa-induced dyskinesias: an acute double-blind placebo-controlled study. Mov Disord. 2001;16(3): Luginger E, Wenning GK, Bosch S, Poewe W. Beneficial effects of amantadine on L-dopa-induced dyskinesias in Parkinson's disease. Mov Disord. 2000;15(5): Silva-Junior FP, Braga-Neto P, Sueli MF, de Bruin VM. Amantadine reduces the duration of levodopa-induced dyskinesia: a randomized, double-blind, placebo-controlled study. Parkinson-ismRelat Disord. 2005;11(7): Snow BJ, Macdonald L, McAuley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson's disease: a double-blind, placebo-controlled study. Clin Neuropharmacol. 2000;23(2): Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75(1): Verhagen ML, Del Dotto P, van den MP, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson's disease. Neurology. 1998;50(5): Volume 39, No. 4 July
187 THE CANADIAN JOURNAL OF NEUROLOGICAL SCIENCES 24. Wolf E, Seppi K, Katzenschlager R, et al. Long-term antidyskinetic efficacy of amantadine in Parkinson's disease. Mov Disord Jul 30;25(10): Evidente VG, Adler CH, Caviness JN, Gwinn-Hardy K. A pilot study on the motor effects of rimantadine in Parkinson's disease. Clin Neuropharmacol Jan-Feb;22(1): Hanagasi HA, Kaptanoglu G, Sahin HA, Emre M. The use of NMDA antagonist memantine in drug-resistant dyskinesias resulting from L-dopa. Mov Disord Sep;15(5): Rajput AH, Uitti RJ, Lang AE, Rajput A, Kumar R, Galvez-Jimenez N. Amantidine ameliorates levodopa-induced dyskinesia. Neurology. 1997;48(suppl 3):A Rajput AH, Rajput A, Lang AE, Kumar R, Uitti RJ, Galvez-Jimenez N. New use for an old drug: amantadine benefits levodopainduced dyskinesia. Mov Disord Sep;13(5): Ruzicka E, Streitova H, Jech R, et al. Amantadine infusion in treatment of motor fluctuations and dyskinesias in Parkinson's disease. J Neural Transm. 2000;107(11): Spieker S, Loschmann PA, Klockgether T. The NMDA antagonist budipine can alleviate levodopa-induced motor fluctuations. Mov Disord May;14(3): Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol. 1999;56(11): Cohen J. Statistical power analysis for the behavioral sciences: Lawrence Erlbaum; Crosby NJ, Deane KH, Clarke CE. Amantadine for dyskinesia in Parkinson's disease. CochraneDatabaseSystRev. 2003(2): CD Martínez-Martín P, Cubo E. Scales to measure parkinsonism. Handbook of Clinical Neurology. 2007;83: Goetz CG. Rating scales for dyskinesias in Parkinson's disease. Mov Disord. 1999;14(Suppl. 1): Colosimo C, Martínez-Martín P, Fabbrini G, et al. Task force report on scales to assess dyskinesia in Parkinson's disease: Critique and recommendations. Mov Disord. 2010;25(9): Sweet RA, DeSensi EG, Zubenko GS. Reliability and applicability of movement disorder rating scales in the elderly. J Neuropsychiatry Clin Neurosci Winter;5(1):
188 .3 Short-interval intracortical inhibition blocks long-term 173 potentiation induced by paired associative stimulation
189 Short-interval intracortical inhibition blocks long-term potentiation induced by paired associative stimulation Behzad Elahi, Carolyn Gunraj and Robert Chen J Neurophysiol 107: , First published 11 January 2012; doi: /jn You might find this additional info useful... This article cites 53 articles, 26 of which can be accessed free at: Updated information and services including high resolution figures, can be found at: Additional material and information about Journal of Neurophysiology can be found at: This information is current as of July 9, Downloaded from jn.physiology.org on July 9, 2012 Journal of Neurophysiology publishes original articles on the function of the nervous system. It is published 12 times a year (monthly) by the American Physiological Society, 9650 Rockville Pike, Bethesda MD Copyright 2012 by the American Physiological Society. ISSN: , ESSN: Visit our website at 174
190 J Neurophysiol 107: , First published January 11, 2012; doi: /jn Short-interval intracortical inhibition blocks long-term potentiation induced by paired associative stimulation Behzad Elahi, 1,2 Carolyn Gunraj, 1 and Robert Chen 1,2 1 Toronto Western Research Institute, University Health Network, Toronto, Ontario; and 2 Division of Neurology, Department of Medicine, University of Toronto, Toronto, Ontario, Canada Submitted 8 March 2011; accepted in final form 6 January 2012 Elahi B, Gunraj C, Chen R. Short-interval intracortical inhibition blocks long-term potentiation induced by paired associative stimulation. J Neurophysiol 107: , First published January 11, 2012; doi: /jn Paired associative stimulation (PAS) of the motor cortex leads to increased motor evoked potential (MEP) amplitudes in the stimulated hand muscles. We hypothesized that evoking GABA A receptor-mediated short-interval intracortical inhibition (SICI) simultaneously with excitatory PAS would depress long-term potentiation plasticity in motor cortex. Four different PAS paradigms were tested, standard PAS (PAS25) and three conditioned PAS protocols (CS2-PAS25, CS2-PAS25adj, and CS10-PAS25adj). A subthreshold conditioning stimulus 2 ms (CS2) or 10 ms (CS10) before the test stimuli was added to the conditioned PAS protocols. Since CS2 has inhibitory and CS10 has facilitatory effect on cortical excitability, in the CS2-PAS25adj and CS10-PAS25adj protocols, TS intensity was adjusted to produce a 1-mV MEP in the presence of CS2 or CS10 to control for the degree of corticospinal excitation. As expected, MEP amplitudes after PAS25 were higher compared with that at baseline, but importantly, MEP amplitudes did not change after PAS was induced in the presence of SICI in either the CS2-PAS25 or CS2- PAS25adj condition. Furthermore, the CS10-PAS25adj protocol showed significantly increased MEP amplitude at 60 min after PAS compared with baseline. These results show that SICI blocked the induction of long-term potentiation-like plasticity in the motor cortex, indicating that GABAergic circuits play an important role in the regulation of cortical plasticity. The study demonstrates a noninvasive and nonpharmacological way to achieve focal modulation of plasticity. cortical plasticity; -aminobutyric acid; associative plasticity; motor cortex; transcranial magnetic stimulation LONG-TERM POTENTIATION (LTP) is hypothesized to play an important role in learning and memory (Antonov et al. 2003; Iriki et al. 1989). Paired associative stimulation (PAS) is a widely used experimental paradigm to induce plasticity in the human motor cortex (Ridding and Taylor 2001; Stefan et al. 2002). This technique uses repetitive pairing of nerve stimulation and cortical transcranial magnetic stimulation (TMS) that are timed so that the peripheral input and the central stimulus arrive near-synchronously at the motor cortex. Because PAS shares several features of spike timing plasticity, such as associativity, input specificity, and cooperativity (Bear and Malenka 1994; Stefan et al. 2000), it probably represents associative plasticity and LTP in the primary motor cortex (M1). Reduction in cortical excitability is achieved when the peripheral nerve stimulation precedes the cortical stimulus (Wolters et al. 2003). These studies suggest that PAS can induce LTP and long-term depression (LTD)-like phenomena and Address for reprint requests and other correspondence: R. Chen, Toronto Western Hospital, 7th Fl., Rm. 7MCL411, 399 Bathurst St., Toronto, Ontario, Canada M5T 2S8 ( robert.chen@uhn.ca). further indicate that temporal Hebbian rules are involved in the induction of cortical plasticity. Cortical inhibition is critical for the regulation of neuronal excitability and plasticity (Chen et al. 2002). Studies using hippocampal slices revealed that the activation of GABA A receptors blocked LTP induction (Evans and Viola-McCabe 1996). In humans, increased inhibition induced by administration of lorazepam, a positive allosteric modulator of the GABA A receptor, reduced practice-dependent LTP-like plasticity in the motor cortex (Butefisch et al. 2000; Ziemann et al. 2001). Diazepam, another benzodiazepine, produced a nonsignificant trend towards reduction in PAS-induced LTP plasticity (Heidegger et al. 2010). The GABA B receptor agonist, baclofen, also decreased PAS-induced LTP plasticity in the human motor cortex (McDonnell et al. 2007). However, modulation of PAS using brain stimulation has not been examined. Abnormal associative LTP-like plasticity measured by PAS may play a critical role in the pathophysiology of neurological and psychiatric disorders, such as primary focal hand dystonia (Quartarone et al. 2006), Parkinson s disease (Morgante et al. 2006), Costello syndrome (Dileone et al. 2010), and schizophrenia (Frantseva et al. 2007; Stephan et al. 2006). Short-interval cortical inhibition (SICI) is a widely studied cortical inhibitory circuit and is elicited by a subthreshold conditioning stimulus (CS) followed by a suprathreshold test stimulus (TS) at interstimulus intervals (ISI) of 1 6 ms (Kujirai et al. 1993). Pharmacological studies have shown that drugs such as lorazepam that increase GABA A activity enhance SICI (Di Lazzaro et al. 2005; Ilic et al. 2002; Ziemann et al. 1996a), which suggests that it is mediated by GABA A receptors. However, SICI has not been investigated as a focal, nonpharmacological means to modulate plasticity. In the present study, we examined the effects of activating SICI during the induction of LTP-like plasticity in the motor cortex. We hypothesize that GABA A -mediated cortical inhibition activated by SICI will suppress PAS-induced LTP-like plasticity. MATERIALS AND METHODS Experiment 1 Subjects. We recruited 13 right-handed subjects with no history of neurological or psychiatric disorders and with normal neurological examination results. The University Health Network Research Ethics Board approved the experimental protocol, and all subjects provided written informed consent. Two subjects were excluded due to high resting motor thresholds (RMT), which made it difficult to obtain adequate motor evoked potential (MEP) amplitudes. Therefore, 11 healthy volunteers (7 men and 4 women) aged yr (mean SD) were studied. Each subject participated in three stimulation sessions, and each session was administered at least 1 wk apart. Downloaded from jn.physiology.org on July 9, /12 Copyright 2012 the American Physiological Society
191 1936 SICI DURING PAS BLOCKS LTP INDUCTION Electromyogram recording. Surface electromyograms (EMG) were recorded from the left abductor pollicis brevis (APB) and first dorsal interosseous (FDI) muscles using bipolar Ag-AgCl electrodes. The signals were amplified 1,000 times, filtered (5 500 Hz), digitized (Cambridge Electronic Design Micro 1401), and recorded using Signal software (version 3.07). EMG was continuously monitored with visual and auditory feedback to ensure complete muscle relaxation. The stimulation parameters, such as the coil location and stimulation intensities, were optimized based on the APB responses. The FDI measurements were used to confirm the findings in the APB muscle and to determine if the similar effects also occurred in another muscle in the proximity of the APB muscle but innervated by a different nerve (ulnar nerve). TMS. We used two Magstim 200 stimulators (Magstim, Whitland, Dyfed, UK) connected via a Bistim module and an 8-shaped coil (outside diameter of each loop was 9.5 cm) to apply TMS to the right M1. The TMS trigger pulses were delivered from a Micro1401 interface (Cambridge Electronics Design, Cambridge, UK) controlled by Signal Software (3.07). The subjects were seated in a comfortable position, and the coil was held tangentially to the skull with the handle pointing backwards and laterally at 45 to the sagittal plane, at the optimal scalp site to evoke MEP in the relaxed left APB muscle. The motor hot spot was marked on a default image in the Brainsight (Magstim) stereotactic image guidance system to facilitate the positioning of the TMS coils over a subject s scalp. The experimental protocol is shown in Fig. 1. RMT was defined as the minimum stimulator output that evoked MEPs of 50 V inat least 5 out of 10 trials when the APB muscle was completely relaxed. Active motor threshold (AMT) was defined as the minimum intensity of stimulation output that elicited APB MEPs of at least 200 V in5 out of 10 consecutive trials while the participants maintained a force level of 20% of their maximum contraction. MEP amplitude was measured as the average of 10 trials in which TMS generated an EMG response of at least 1 mv peak-to-peak amplitude at the baseline. At each time point (Fig. 1), RMT, AMT, and MEP amplitudes were measured first. The balance and interactions between inhibitory and facilitatory circuits determine the final output from the M1 (Hallett 2007). We therefore tested several well-established intracortical inhibitory and facilitatory circuits to explore the possible changes in these networks following each intervention. SICI mostly represent GABA A -mediated inhibition (Jung et al. 2004; Ziemann et al. 1996a), intracortical facilitation (ICF) reflects glutamatergic activities (Schwenkreis et al. 1999; Ziemann et al. 1998), and short latency afferent inhibition (SAI) is related to cholinergic (Di Lazzaro et al. 2000) neural networks. SICI and ICF were evoked with a subthreshold CS followed by a TS. CS intensity was 90% AMT. ISI of 2 ms was used for SICI and 10 ms for ICF. SAI was studied using a conditioning-test protocol described by Tokimura et al. (Tokimura et al. 2000). The left median nerve was stimulated through bipolar electrodes at the wrist (cathode proximal) using a 200- s square wave pulse at three times the sensory threshold. The ISI between median nerve stimulation and TMS was set to the latency of the N20 somatosensory evoked potential plus 2 ms. The N20 somatosensory evoked potential was evoked by stimulation of the left median nerve and was recorded with the active electrode, which was placed 3 cm posterior to C4 (according to the International EEG system) and referenced to Fz. Two hundred responses were averaged to identify the N20 peak. SICI, ICF, and SAI were measured in the same experimental session with 10 trials for each ISI and 10 trials of TS alone, delivered in a random order. The TS generated a 1-mV MEP amplitude in the left APB muscle at baseline, and the TS intensities were adjusted at each time point after PAS to produce similar test MEP amplitudes. Cortical silent period (CSP) was measured at the end of each assessment. CSP was defined as the time between MEP onset and return of voluntary EMG activity (Daskalakis et al. 2003) and was assessed from 10 trials during isometric contraction (constant 20% maximum voluntary contraction under visual feedback through an oscilloscope) of the APB muscle with TMS at 130% RMT. PAS. PAS was achieved by repetitively pairing stimulation of the left median nerve and TMS of the right M1. The median nerve was stimulated at three times sensory threshold, and TMS intensity was adjusted to produce MEPs with 1 mv peak-to-peak amplitude. The ISI was 25 ms, which was optimal for inducing a sustained increase in motor cortex excitability through PAS (Stefan et al. 2000). One hundred and eighty paired stimuli were delivered at 0.1 Hz during a 30-min period. We refer to this paradigm as PAS25. PAS in the presence of SICI. We combined median nerve stimulation and SICI in two different experimental paradigms. In the first paradigm, the parameters were the same as PAS25 except that a CS at 90% AMT was delivered 2 ms (CS2) before the TS (23 ms after median nerve stimulation). We refer to this paradigm as CS2-PAS25. Because CS2 reduces the MEP amplitude evoked by the TS, in the second paradigm, we increased the TS intensity to produce 1-mV MEPs in the presence of SICI induced by CS2. This paradigm is designed to match the MEP amplitudes produced by the CS2-adjusted TS pulse combination to that produced by TS alone in the PAS25 condition (CS2-PAS25adj). Corticospinal excitability and cortical inhibition were measured before and at different times after PAS, as indicated in Fig. 1. These three PAS paradigms were performed in separate sessions at least 1 wk apart and in random order. MEP amplitudes during PAS. To examine the changes in MEP amplitudes during PAS, MEPs were recorded during the PAS protocols, and the amplitudes of every 10 MEPs were averaged. Experiment 2 PAS in the presence of ICF. Four new subjects and two subjects from experiment 1 (3 men and 3 women, aged yr) participated. Subthreshold conditioning stimuli 10 ms before the test stimuli Downloaded from jn.physiology.org on July 9, 2012 Fig. 1. Study timeline. AMT, active motor threshold; CSP, cortical silent period; ICF, intracortical facilitation; MEP, motor evoked potentials; RMT, resting motor threshold; SAI, short latency afferent inhibition; SICI, short-interval cortical inhibition; TS, test stimulus. These measures were taken before the paired associative stimulation (PAS) protocols and immediately (T0), 20 (T20), 40 (T40), and 60 (T60) min after the interventions. The top right corner illustrates the combination of peripheral and transcranial magnetic stimulation (TMS) pulses used in each PAS protocol. J Neurophysiol doi: /jn
192 SICI DURING PAS BLOCKS LTP INDUCTION 1937 have been shown to cause ICF (Berardelli et al. 2008; Ziemann et al. 1996c). This protocol was designed to condition PAS with a CS 10 ms before the TS to test whether the effects seen with PAS in the presence of SICI are specific to the ISI used. The TS intensity was adjusted to produce 1 mv amplitude in the presence of CS10, which was set at 90% AMT. This protocol is referred to as CS10-PAS25adj. The preand post-pas measurements are the same as experiment 1. Statistical analysis. For RMT, AMT, CSP, SICI, ICF, MEP, and SAI, values after PAS from T0 to T60 were expressed as a ratio of baseline and were used for statistical analysis. In experiment 1, repeated-measures ANOVA with intervention (PAS25, CS2-PAS25, and CS2-PAS25adj) and time (T0, T20, T40, and T60) were used as the within-subject factors. Separate analyses were performed for the APB and FDI muscles because all the adjustments in stimulus intensities were based on the APB muscle, and therefore the MEP amplitudes for different test conditions were not matched for the FDI muscle. Significant ANOVA main effects were further explored using Fisher s post hoc analyses and separate one-way ANOVAs. Peak-to-peak MEP amplitudes during PAS were analyzed using an ANOVA with intervention as the between-subject factors and time as the within-subject factor. MEPs from every 10 consecutive stimuli were averaged and used for analyses. Baseline was defined as the first time bin of 10 MEPs at the beginning of each of the test interventions. For experiment 2, repeated-measure ANOVA with the factor time (5 levels, baseline-t60) as within-subject factors for APB muscle were tested. Values after intervention from T0 to T60 were expressed as a ratio to baseline. Effect of time was further explored using Fisher s least-significant difference and Fisher s post hoc analysis. P values 0.05 were considered significant. StatView (SAS Institute) software was used for analysis. Values are presented as means SE, unless specified otherwise. RESULTS None of the subjects reported any adverse effect. Motor Thresholds (RMT, AMT) For experiment 1, no significant change in RMT (43 1.4% at baseline) and AMT (36 1.2% at baseline) of the APB muscle was observed after any of the three PAS protocols. There were no significant interactions or main effects for either muscle. In experiment 2, the RMT and AMT were Fig. 2. MEP amplitude recorded during the PAS interventions. Open symbols represent significant difference compared with the first time bin for each intervention. Each point represents averaged MEP amplitudes (mv) from 10 stimuli for standard PAS (PAS25, ), CS2-PAS25 ( ), and the CS2-adjusted TS pulse combination to that produced by TS alone in the PAS25 condition (CS2-PAS25adj, Œ). *P 0.05 and **P 0.001; error bars denote SE. Fig. 3. MEP amplitudes after PAS protocols. MEP in APB muscle is shown. MEP amplitudes were normalized to baseline (before interventions) for PAS25 ( ), CS2-PAS25 ( ), and CS2-PAS25adj (Œ). Values more than 1 indicate increased MEP amplitude, and values less than 1 indicate decreased MEP amplitude after PAS. and % at baseline. Similarly, no significant change in RMT and AMT was detected. Experiment 1 SICI blocks LTP-like plasticity during PAS. There were significant effects of intervention (P ), time (P 0.001), and time intervention (P 0.001; Fig. 2) on the MEP amplitude during the 30 min of PAS (median nerve stimulation followed by TMS). Post hoc analysis confirmed significant differences among all three interventions with the highest MEP amplitudes in PAS25, followed by CS2-PASadj, and then CS2-PAS25. Figure 2 shows that the significant time intervention is due to a marked increase in MEP amplitude during PAS25 and CS2-PAS25adj, but not during CS2-PAS25. There was a significant effect of time on MEP amplitudes for the PAS25 session (P ). Post hoc analysis showed that MEP amplitude was significantly increased compared with the first MEP (baseline) after 4 min and reached a plateau after about 16 min (Fig. 2). In the CS2-PAS25 session, there was also a significant effect of time on MEP amplitude (P 0.005). Post hoc analysis showed that MEP amplitude was mostly unchanged except for transient increases at 15 and 25 min (Fig. 2). For the CS2-PAS25adj session, there was also a significant effect of time after stimulation (P 0.001). Post hoc analysis showed an increased MEP from 20 min into the stimulation session through the end of the session (P 0.002) (Fig. 2). SICI Blocks LTP-Like Plasticity After PAS For MEP amplitudes from single-pulse TMS in the APB muscle, ANOVA showed a significant effect of intervention (P 0.001), but no significant effect for time and time intervention interaction (Fig. 3). Post hoc analysis confirmed that there were significantly higher MEP amplitudes for PAS25 compared with CS2-PAS25 (P ) and CS-PAS25adj (P ). The MEP amplitudes for CS2-PAS25 and CS- PAS25adj sessions were not significantly different from each other. One-way ANOVA for PAS25 showed a significant effect of time on MEP amplitude (P 0.009). Post hoc analysis showed higher MEP amplitudes at all time points measured up to 1 h after the PAS25 compared with baseline Downloaded from jn.physiology.org on July 9, 2012 J Neurophysiol doi: /jn
193 1938 SICI DURING PAS BLOCKS LTP INDUCTION Fig. 4. SAI before and after PAS interventions. A: SAI in the APB muscle. Values above 1 indicate facilitation, and values below 1 indicate inhibition. B: the effects of the different interventions are shown as the ratio of SAI averaged for all time points after PAS normalized to the baseline SAI for the three PAS conditions tested (PAS25, CS2-PAS25, and CS2-PAS25adj). Values above 1 indicated reduction in SAI after PAS, and values less than 1 indicate an increase in SAI after PAS compared with baseline values. Error bars represent SE; *P (T0: P , T20: P 0.02, T40: P 0.013, T60: P 0.009). There was no significant effect of time following CS2- PAS25 and CS2-PAS25adj stimulation protocols. Changes in Intracortical Inhibitory and Facilitatory Networks Following PAS SICI. There was no significant effect of intervention, time, and time intervention interaction for SICI of the APB muscle. SAI. There was a significant effect of intervention for the APB muscle (P 0.02), but there was no significant effect of time and time intervention interaction (Fig. 4A). Post hoc analysis showed SAI was significantly less after CS2- PAS25adj compared with that after CS2-PAS25 (P 0.008; Fig. 4B). Separate one-way ANOVAs for each intervention revealed no effect of time with CS2-PAS25, CS2-PAS25adj, and PAS25. ICF. There was no significant effect of intervention, time, and time intervention interaction for APB muscle. CSP. ANOVA showed a significant effect of time (ANOVA APB P 0.006), but there was no effect of intervention and time intervention interaction (Fig. 5). Post hoc analysis showed that CSP was increased at 40 and 60 min after interventions compared with that at baseline (T40, P 0.002; T60, P 0.009). Stimulation effects in the FDI muscle. The intensity that generates 1 mv amplitude in APB resulted in , , and mv MEP amplitude in PAS25, CS2-PAS25, and CS2-PAS25adj protocols, respectively, at baseline. A similar pattern of changes in MEP amplitude was observed after PAS protocols in FDI muscle. ANOVA showed significant effect for intervention (P 0.006) and no effect for time and time intervention on MEP amplitude ratios after PAS. Post hoc analysis showed higher MEP amplitude in PAS25 compared with CS2- PAS25 (P 0.01) and CS2-PAS25adj (P 0.01). This confirms similar time courses for MEP amplitude after PAS in both ABP and FDI muscles. Experiment 2 PAS in the presence of ICF. The MEP amplitude at baseline was mv. Repeated-measure ANOVA showed a nonsignificant trend for the effect of time (P 0.09) in the APB muscle. No significant effect of time was observed for FDI muscle. Fisher s post hoc analysis showed a significantly higher MEP amplitude at T60 compared with baseline (P 0.01) in APB muscle (Fig. 6A). One-way ANOVA showed no significant effect of time on normalized values of SICI ( % at baseline), ICF ( % at baseline), SAI ( % at baseline), and CSP ( ms at baseline) for both APB (Fig. 6B) and FDI muscle groups. Downloaded from jn.physiology.org on July 9, 2012 Fig. 5. CSP duration before and after PAS interventions. CSP are shown in ms. Each bar represents 1 intervention protocol. Error bars represent SE. *P DISCUSSION MEP amplitudes significantly increased following PAS25 compared with baseline, but the MEP amplitudes after CS2- PAS25 and CS2-PAS25adj were unchanged. Thus, applying SICI, which activated GABAergic interneurons, inhibited PAS-induced LTP-like plasticity aftereffect in the motor cortex. This effect was specific to the conditioning-test pulse (CS2) interval that elicited SICI because the same stimuli at a different conditioning pulse interval (CS10) that did not produce SICI showed MEP facilitation after the intervention (Fig. 6). These findings demonstrate that noninvasive and nonpharmacological TMS techniques can effectively modulate cortical plasticity. Inhibitory interneurons play an important role in the regulation of cortical plasticity. In rat hippocampal slices (Evans and Viola-McCabe 1996), application of midazolam, a benzo- J Neurophysiol doi: /jn
194 SICI DURING PAS BLOCKS LTP INDUCTION 1939 Fig. 6. Results from the CS10-PAS25adj paradigm. A: MEP amplitudes in the APB muscle after the CS10-PAS25adj protocol. MEP amplitudes were normalized to baseline (before interventions). B: results for SICI, SAI, and ICF are shown. Values above 100% indicate facilitation, and values below 100% indicate inhibition. Error bars represent SE. diazepine, markedly inhibited LTP induction and prevented the expected increase in excitatory postsynaptic potentials and population spikes following theta burst stimulation. This effect was reversed by bicuculline, a GABA A receptor antagonist (Evans and Viola-McCabe 1996), which indicates that benzodiazepines suppress LTP induction through enhancement of GABA A receptor-mediated inhibition. In rat motor cortex (Hess and Donoghue 1994), bicuculline increased the ability of theta burst stimulation to induce LTP. Several pharmacological studies in humans have shown that benzodiazepines suppressed cortical plasticity (Butefisch et al. 2000; Ziemann et al. 2001). In the present study, we investigated the role of inhibitory circuits in the regulation of plasticity in motor cortex by activating SICI during facilitatory PAS25, and we found disruption of LTP-like plasticity induction. Because SICI decreased the MEP amplitude induced by TS and this may affect LTP-like plasticity induction, we included a control experiment (CS2-PAS25adj) with adjusted TS intensity to generate the same MEP amplitude as in PAS25 as a measure of postsynaptic activity. Because the induction of LTP-like plasticity was suppressed in both CS2-PAS25 and CS2-PAS25adj conditions, the finding cannot be explained by reduced postsynaptic activity in the CS2-PAS25 condition but is likely the result of activation of inhibitory circuits responsible for SICI. This effect cannot be attributed to nonspecific effects of adding the CS during PAS because the same CS at a different ISI (10 ms) resulted in LTP-like effect. Direct recordings of corticospinal descending volleys showed that SICI reduced late I-waves (Di Lazzaro et al. 1998), whereas facilitatory PAS increased later I-waves (Di Lazzaro et al. 2009). The possibility that our results could partially be explained by different I-wave composition in the CS2-PAS25adj condition compared with PAS25 condition cannot be excluded. However, epidural recordings of corticospinal waves (Ni et al. 2011) showed that adjustment of MEP amplitude in the presence of CS2 also restored the late I-wave amplitude to that of TS alone, suggesting the PAS25 and the CS2-PAS25adj protocols in our study likely had similar late I-wave amplitudes. Another consideration is whether repetitive 0.1 Hz pairedpulse stimulation at 2 ms ISI itself depressed MEP amplitude. Further studies are needed to exclude this possibility, but we considered it unlikely because 0.1 Hz single-pulse TMS had no effect (Chen et al. 1997), whereas 0.2 Hz suprathreshold paired-pulse TMS at 1.5 ms ISI increased in MEP amplitude (Thickbroom et al. 2006). LTP-like plasticity is decreased in several neurological and psychiatric disorders with abnormal motor learning, such as Parkinson s disease (Morgante et al. 2006), schizophrenia (Daskalakis et al. 2008; Frantseva et al. 2007; Stephan et al. 2006), and Huntington s disease (Orth et al. 2010). On the other hand, exaggerated LTP-like and LTD-like plasticity in the motor cortex and a loss of topographic specificity has been observed in writer s cramp (Quartarone et al. 2003; Quartarone et al. 2006; Weise et al. 2006). Our study shows that SICI modulates the controlling mechanism of cortical plasticity, which can potentially be useful in the treatment of conditions with abnormal plasticity and disrupted inhibition, such as dystonia. MEP amplitude during PAS. EMG recording during the stimulation phase showed that, at the beginning of PAS, the MEP amplitudes (with median nerve stimulation followed by TMS) were 1 mv (Fig. 2). This is likely because the median nerve stimulation 25 ms before TMS produced MEP inhibition, similar to SAI. The MEP amplitudes induced by CS2-PAS25 were smaller than those elicited after PAS25 (Fig. 2), likely due to further MEP inhibition caused by adding the CS2 pulse. In the CS2-PAS25adj condition, the TMS intensities were adjusted to produce 1 mv MEP in the presence of CS2 (without median nerve stimulation). With the addition of median nerve stimulation, the higher MEP amplitude for CS2- PAS25adj compared with PAS25 at the beginning of PAS suggests that there exists an inhibitory interaction between SAI and SICI. These findings are consistent with those of previous studies, which showed mutual inhibitory interactions between SICI and SAI (Alle et al. 2009; Stefan et al. 2002). The MEP increase during PAS25 and CS2-PAS25adj may be due to an increase in MEP amplitude or a decrease in SAI. Downloaded from jn.physiology.org on July 9, 2012 J Neurophysiol doi: /jn
195 1940 SICI DURING PAS BLOCKS LTP INDUCTION After PAS25, there was no change in SAI (Fig. 4A), but the MEP amplitude was increased (Fig. 3); it is likely due to an increase in MEP amplitude and corticospinal excitability. In contrast, after CS2-PAS25adj, there was no increase in MEP amplitude compared with baseline (Fig. 3), but there was reduction in SAI (Fig. 4B). Therefore, the increase in MEP amplitude during CS2-PAS25adj (Fig. 2) is likely related to decrease in SAI. The finding that MEP amplitudes did not change during CS2-PAS25 is consistent with the observation that neither MEP amplitudes (Fig. 3) nor SAI changed after CS2-PAS25 (Fig. 4A). Intracortical inhibition and facilitation after PAS. Similar to our findings of no overall change in SICI and ICF after PAS25, previous PAS studies did not show significant changes in SICI after excitatory PAS25 (Di Lazzaro et al. 2011; McDonnell et al. 2007; Russmann et al. 2009; Stefan et al. 2002) although a significant decrease in SICI after inhibitory PAS was observed in one study (Russmann et al. 2009). The finding that SICI was unchanged after CS2-PAS25 and CS2-PAS25adj conditions is consistent with the blockade of LTP plasticity by SICI. If SICI was diminished during these conditions, it would not be expected to block PAS-induced plasticity. We found a significant increase in CSP (Fig. 5) after PAS25 and the conditioned PAS interventions, similar to previous studies (Morgante et al. 2006; Stefan et al. 2000). Because administration of the GABA B receptor agonist, baclofen, and GABA reuptake inhibitor, tiagabine, prolonged CSP (Siebner et al. 1998; Werhahn et al. 1999), it is probably mediated through postsynaptic GABA B receptors. CSP is thought to be partially mediated through activity in recurrent collaterals from discharging pyramidal tract neurons (Orth and Rothwell 2004). Prolonged CSP after PAS25 is probably due to facilitation of inhibitory interneurons and upregulation of GABA B receptors in postsynaptic neurons. MEP amplitude reflects the excitability of the corticospinal system, and it increased after PAS25, whereas CSP increased after all three interventions. Both electrophysiological and pharmacological studies suggest that multiple mechanisms are involved in generating the CSP, including the loss of voluntary drive, activation of inhibitory interneurons, activation of corticospinal recurrent collaterals, and afterhyperpolarization (Fuhr et al. 1991; Tergau et al. 1999; Ziemann et al. 1996b). The different effects of PAS interventions on MEP and CSP suggest that the same circuits do not mediate them. However, authors cannot precisely explain why CSP is increased in all three protocols. SAI is decreased after the stimulation in CS2-PAS25adj. This observation could suggest decreased SAI itself or an increased tonic inhibitory interaction on SAI from other circuits after CS2-PAS25adj. We showed in a previous study that GABA B -mediated long-interval intracortical inhibition and SAI had mutual inhibitory interactions (Udupa et al. 2009). We speculate that this inhibitory interaction or interactions with other cortical circuits may be involved, but this needs to be clarified in future studies. Potential implications of the present findings. TMS techniques, such as repetitive TMS, have been used to assess and treat diseases with abnormal cortical excitability and plasticity (Chen and Udupa 2009; Elahi and Chen 2009). Our finding that simultaneously activating SICI during PAS suppresses LTPlike plasticity supports the hypothesis that GABA A -mediated inhibition plays a crucial role in regulating cortical plasticity. This demonstration of a nonpharmacological way of modulating plasticity in humans has several implications. The method can be modified by using different ISI or stimulus intensities to examine the effects of other cortical circuits on cortical plasticity. This may be a method to examine abnormal regulation of plasticity in neurological and psychiatric disorders. Moreover, suppression of LTP-like mechanisms may be tested as a possible treatment for disorders associated with increased LTPlike plasticity such as dystonia. Noninvasive and nonpharmacological modulation of cortical plasticity may be more advantageous than pharmacological intervention because it may have fewer side effects and it can be targeted to specific brain areas. GRANTS The study was supported by the Canadian Institutes of Health Research (CIHR, Grant MOP 62917). R. Chen is supported by a CIHR Industry- Partnered Investigator Award and Catherine Manson Chair in Movement Disorders. B. Elahi is supported by a CIHR-Dystonia Medical Research of Canada fellowship in the field of dystonia. DISCLOSURES No conflicts of interest, financial or otherwise, are declared by the authors. AUTHOR CONTRIBUTIONS Author contributions: B. Elahi and R. Chen conception and design of research; B. Elahi and C. Gunraj performed experiments; B.E. analyzed data; B. Elahi and R. Chen interpreted results of experiments; B. Elahi and R. Chen prepared figures; B. Elahi drafted manuscript; B. Elahi and R. Chen edited and revised manuscript; B. Elahi, C. Gunraj, and R. Chen approved final version of manuscript. REFERENCES Alle H, Heidegger T, Krivanekova L, Ziemann U. Interactions between short-interval intracortical inhibition and short-latency afferent inhibition in human motor cortex. J Physiol 587: , Antonov I, Antonova I, Kandel ER, Hawkins RD. Activity-dependent presynaptic facilitation and hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron 37: , Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4: , Berardelli A, Abbruzzese G, Chen R, Orth M, Ridding MC, Stinear C, Suppa A, Trompetto C, Thompson PD. Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul 1: , Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA 97: , Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: , Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience 111: , Chen R, Udupa K. Measurement and modulation of plasticity of the motor system in humans using transcranial magnetic stimulation. Motor Control 13: , Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Arch Gen Psychiatry 65: , Daskalakis ZJ, Molnar GF, Christensen BK, Sailer A, Fitzgerald PB, Chen R. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clin Neurophysiol 114: , Di Lazzaro V, Dileone M, Pilato F, Capone F, Musumeci G, Ranieri F, Ricci V, Bria P, Di Iorio R, de Waure C. Modulation of motor cortex neuronal networks by rtms: comparison of local and remote effects of six different protocols of stimulation (Abstract). J Neurophysiol 105: 2150, Downloaded from jn.physiology.org on July 9, 2012 J Neurophysiol doi: /jn
196 SICI DURING PAS BLOCKS LTP INDUCTION 1941 Di Lazzaro V, Dileone M, Pilato F, Profice P, Oliviero A, Mazzone P, Insola A, Capone F, Ranieri F, Tonali PA. Associative motor cortex plasticity: direct evidence in humans. Cereb Cortex 19: , Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in human motor cortex. Exp Brain Res 135: , Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol 569: , Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119: , Dileone M, Profice P, Pilato F, Alfieri P, Cesarini L, Mercuri E, Leoni C, Tartaglia M, Di Iorio R, Zampino G. Enhanced human brain associative plasticity in Costello syndrome. J Physiol 588: 3445, Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function-systematic review of controlled clinical trials. Movement Disorders 24: , Evans MS, Viola-McCabe KE. Midazolam inhibits long-term potentiation through modulation of GABAA receptors. Neuropharmacology 35: , Frantseva MV, Fitzgerald PB, Chen R, Moller B, Daigle M, Daskalakis ZJ. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill leaning. Cerebral Cortex 18: , Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol 81: , Hallett M. Transcranial magnetic stimulation: a primer. Neuron 55: , Heidegger T, Krakow K, Ziemann U. Effects of antiepileptic drugs on associative LTPâ like plasticity in human motor cortex. Eu J Neurosci 32: , Hess G, Donoghue JP. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol 71: , Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 545: , Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation in the motor cortex. Science 245: , Jung HY, Sohn YH, Mason A, Considine E, Hallett M. Flumazenil does not affect intracortical motor excitability in humans: a transcranial magnetic stimulation study. Clin Neurophysiol 115: , Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: , McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res 180: , Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson s disease and levodopa-induced dyskinesias (Abstract). Brain 129: 1059, Ni Z, Gunraj C, Wagle-Shukla A, Udupa K, Mazzella F, Lozano AM, Chen R. Direct demonstration of inhibitory interactions between long interval intracortical inhibition and short interval intracortical inhibition. J Physiol 589: , Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol 115: , Orth M, Schippling S, Schneider SA, Bhatia KP, Talelli P, Tabrizi SJ, Rothwell JC. Abnormal motor cortex plasticity in premanifest and very early manifest Huntington disease. J Neurol Neurosurg Psychiatry 81: , Quartarone A, Bagnato S, Rizzo V, Siebner H, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P. Abnormal associative plasticity of the human motor cortex in writer s cramp. Brain 126: , Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci 29: , Ridding MC, Taylor JL. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. J Physiol 537: , Russmann H, Lamy JC, Shamim EA, Meunier S, Hallett M. Associative plasticity in intracortical inhibitory circuits in human motor cortex. Clin Neurophysiol 120: , Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, Malin JP, Tegenthoff M. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett 270: , Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve 21: , Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol 543: , Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123: , Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry 59: , Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U, Paulus W. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res 124: , Thickbroom GW, Byrnes ML, Edwards DJ, Mastaglia FL. Repetitive paired-pulse TMS at I-wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin Neurophysiol 117: 61 66, Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 523: , Udupa K, Ni Z, Gunraj C, Chen R. Interactions between short latency afferent inhibition and long interval intracortical inhibition. Exp Brain Res 199: , Weise D, Schramm A, Stefan K, Wolters A, Reiners K, Naumann M, Classen J. The two sides of associative plasticity in writer s cramp. Brain 129: , Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 517: , Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol 89: , Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology 51: , Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res 109: , 1996a. Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 40: , 1996b. Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practicedependent plasticity in human motor cortex. Brain 124: , Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 496: , 1996c. Downloaded from jn.physiology.org on July 9, 2012 J Neurophysiol doi: /jn
197 .4 Extreme Task Specificity: Is It Dystonia or Another Form of Motor Programming Abnormality? 182
198 LETTERS: PUBLISHED ARTICLES Extreme Task Specificity: Is It Dystonia or Another Form of Motor Programming Abnormality? Related to the article by Shamim et al. 1 and the accompanying editorial by Edwards and Rothwell, 2 we would like to describe our experience with a patient with even greater task-specific motor dysfunction. A 71-year-old man had a 15-year progressive history of transiently getting stuck in the second and third parts of his signature. These symptoms were worse with stress, and he felt anxious and tense while struggling with signing. He noted no difficulty in other writing tasks or other manual functions. He had isolated reduction in right arm swing with no features of parkinsonism or dystonia, including mirror dystonia. While attempting to sign his name, he would get stuck for up to several seconds in attempting to initiate each of the three components of the signature that began at the top of the line. Repeated signing caused increasing duration of blocks, stress, and anxiety with perspiration and the need to remove his sweater. Apart from signing, he had no difficulty writing and could transition from severe blocking in signing to smooth, uninterrupted writing only to have the blocks return when signing his name (see Video). We assessed cortical inhibition (i.e., short afferent inhibition; SAI), short interval intracortical inhibition (SICI), and plasticity (i.e., paired associated stimulation of peripheral nerve and motor cortex to induce plasticity; PAS25 protocol). Interstimulus interval (ISI) was 20 ms for SAI, 2 ms for SICI, and 25 ms for PAS25 protocol. 3 Median nerve stimulation (3 perceptual threshold) and transcranial magnetic stimulation (TMS) over the motor cortex (i.e., over the optimal position for evoked abductor pollicis brevis [APB] motor evoked potentials [MEPs]) were used. TMS-induced MEPs were recorded from APB and abductor digiti minimi (ADM) muscles using surface electromyography (EMG) electrodes. MEP intensity curves were measured at baseline and 10, 20, and 30 minutes after the PAS25 protocol Additional Supporting Information may be found in the online version of this article. * Correspondence to: Anthony E. Lang, Movement Disorders Center and the Edmond J. Safra Program in Parkinson s Disease, Toronto Western Hospital, Toronto, Ontario, Canada; lang@uhres.utoronto.ca Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article. Published online in Wiley Online Library (wileyonlinelibrary.com). DOI: /mds Before PAS (baseline), SICI (53% control or 47% inhibition) and SAI (50% of control) for the APB muscle were normal. There was a tendency for reduction in MEP amplitude at 10 and 20 minutes after PAS25. However, at 30 minutes after the PAS25, MEP amplitudes increased by a factor of 2.39 in the APB muscle and 2.66 in the ADM muscle at stimulus intensities that produced MEPs of 1 mv in the APB at baseline. Clinically, we felt that our patient could have a form of performance anxiety or signing block (akin to some cases of the yips 4 ), although it remained possible that he had an extreme form of task-specific dystonia. In contrast to Shamim et al. s patients, 1 there were no features supportive of dystonia. Also, against dystonia, SICI and SAI were normal. SICI has been shown to be diminished in both organic and psychogenic dystonia. 4 SAI is normal in both psychogenic and organic dystonia. 5 However, the PAS25 protocol resulted in an excessive increase in cortical excitability (e.g., increase in MEP size) in homotopic (i.e., APB) and heterotopic (i.e., ADM) muscles. A previous study showed abnormal increase in PAS-induced cortical plasticity (leading to excessive, and heterotopic increase in, cortically evoked MEPs) in organic, but not psychogenic, dystonia. 5 This experience fails to provide a definitive answer as to whether our patient and others with such extreme, longstanding, and isolated task-specific motor problems have a form of dystonia or some other type of motor programming disturbance. Edwards and Rothwell 2 postulate that focused attention could cause unwanted involuntary movement, perhaps initially caused by stressful situations during performance, and, eventually, these unwanted movements could be incorporated into the task itself (and, eventually, into other tasks) and appear even in the absence of stress. They speculated that enhanced plasticity could produce vulnerability for patients to develop task-specific dystonia that spreads to involve other tasks. However, our patient maintained his pure isolated signing motor block without progression to clear dystonia or to interference with any other tasks, despite electrophysiological evidence of increased plasticity. His preserved intracortical inhibition might have played a role in limiting the spread of symptoms. This may be similar to professional musicians (without dystonia) who have increased PAS plasticity, but preserved cortical inhibition. 6 Further studies are required in larger numbers of similar patients to advance our understanding of task-specific motor disorders. Legend to the Video Video 1. The patient is variably asked to repeatedly sign his name or write a sentence. He demonstrates blocking at three different components of his signature with progressive increases in the duration of these events as he experiences more stress and anxiety in performing the task. When he switches to writing other words or a sentence, he has no similar trouble and does not experience the stress he feels when signing. There is no overt dystonic posture or tremor observed on writing or signing. Movement Disorders, Vol. 000, No. 000,
Paired-Pulse TMS to one Brain Region. Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation LEASE DO NOT COPY
 Paired-Pulse TMS to one Brain Region Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation Paired-Pulse Paradigms Sequential pulses applied to the same cortical region Variable
Paired-Pulse TMS to one Brain Region Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation Paired-Pulse Paradigms Sequential pulses applied to the same cortical region Variable
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
The Physiology of the Senses Chapter 8 - Muscle Sense
 The Physiology of the Senses Chapter 8 - Muscle Sense www.tutis.ca/senses/ Contents Objectives... 1 Introduction... 2 Muscle Spindles and Golgi Tendon Organs... 3 Gamma Drive... 5 Three Spinal Reflexes...
The Physiology of the Senses Chapter 8 - Muscle Sense www.tutis.ca/senses/ Contents Objectives... 1 Introduction... 2 Muscle Spindles and Golgi Tendon Organs... 3 Gamma Drive... 5 Three Spinal Reflexes...
P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center. Wednesday, 16 March 2009, 1:00p.m. 2:00p.m.
 Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon
 Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Voluntary Movements. Lu Chen, Ph.D. MCB, UC Berkeley. Outline. Organization of the motor cortex (somatotopic) Corticospinal projection
 Voluntary Movements Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Organization of the motor cortex (somatotopic) Corticospinal projection Physiology of motor neurons Direction representation, population coding
Voluntary Movements Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Organization of the motor cortex (somatotopic) Corticospinal projection Physiology of motor neurons Direction representation, population coding
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Acetylcholine (ACh) Action potential. Agonists. Drugs that enhance the actions of neurotransmitters.
 Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Neurophysiology of systems
 Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
Neural Basis of Motor Control. Chapter 4
 Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Memory Systems II How Stored: Engram and LTP. Reading: BCP Chapter 25
 Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
biological psychology, p. 40 The study of the nervous system, especially the brain. neuroscience, p. 40
 biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
Lesson 14. The Nervous System. Introduction to Life Processes - SCI 102 1
 Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Neural Basis of Motor Control
 Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Exam 1 PSYC Fall 1998
 Exam 1 PSYC 2022 Fall 1998 (2 points) Briefly describe the difference between a dualistic and a materialistic explanation of brain-mind relationships. (1 point) True or False. George Berkely was a monist.
Exam 1 PSYC 2022 Fall 1998 (2 points) Briefly describe the difference between a dualistic and a materialistic explanation of brain-mind relationships. (1 point) True or False. George Berkely was a monist.
PHY3111 Mid-Semester Test Study. Lecture 2: The hierarchical organisation of vision
 PHY3111 Mid-Semester Test Study Lecture 2: The hierarchical organisation of vision 1. Explain what a hierarchically organised neural system is, in terms of physiological response properties of its neurones.
PHY3111 Mid-Semester Test Study Lecture 2: The hierarchical organisation of vision 1. Explain what a hierarchically organised neural system is, in terms of physiological response properties of its neurones.
The Cerebellum. Outline. Lu Chen, Ph.D. MCB, UC Berkeley. Overview Structure Micro-circuitry of the cerebellum The cerebellum and motor learning
 The Cerebellum Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Overview Structure Micro-circuitry of the cerebellum The cerebellum and motor learning 2 Overview Little brain 10% of the total volume of the brain,
The Cerebellum Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Overview Structure Micro-circuitry of the cerebellum The cerebellum and motor learning 2 Overview Little brain 10% of the total volume of the brain,
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE
 TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
Introduction to Computational Neuroscience
 Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
Introduction to Computational Neuroscience Lecture 7: Network models Lesson Title 1 Introduction 2 Structure and Function of the NS 3 Windows to the Brain 4 Data analysis 5 Data analysis II 6 Single neuron
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM
 STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
Water immersion modulates sensory and motor cortical excitability
 Water immersion modulates sensory and motor cortical excitability Daisuke Sato, PhD Department of Health and Sports Niigata University of Health and Welfare Topics Neurophysiological changes during water
Water immersion modulates sensory and motor cortical excitability Daisuke Sato, PhD Department of Health and Sports Niigata University of Health and Welfare Topics Neurophysiological changes during water
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
Physiology of synapses and receptors
 Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
The Three Pearls DOSE FUNCTION MOTIVATION
 The Three Pearls DOSE FUNCTION MOTIVATION Barriers to Evidence-Based Neurorehabilitation No placebo pill for training therapy Blinded studies often impossible Outcome measures for movement, language, and
The Three Pearls DOSE FUNCTION MOTIVATION Barriers to Evidence-Based Neurorehabilitation No placebo pill for training therapy Blinded studies often impossible Outcome measures for movement, language, and
Synaptic Plasticity and the NMDA Receptor
 Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
The Nervous System. Nerves, nerves everywhere!
 The Nervous System Nerves, nerves everywhere! Purpose of the Nervous System The information intake and response system of the body. Coordinates all body functions, voluntary and involuntary! Responds to
The Nervous System Nerves, nerves everywhere! Purpose of the Nervous System The information intake and response system of the body. Coordinates all body functions, voluntary and involuntary! Responds to
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
CISC 3250 Systems Neuroscience
 CISC 3250 Systems Neuroscience Levels of organization Central Nervous System 1m 10 11 neurons Neural systems and neuroanatomy Systems 10cm Networks 1mm Neurons 100μm 10 8 neurons Professor Daniel Leeds
CISC 3250 Systems Neuroscience Levels of organization Central Nervous System 1m 10 11 neurons Neural systems and neuroanatomy Systems 10cm Networks 1mm Neurons 100μm 10 8 neurons Professor Daniel Leeds
Motor Functions of Cerebral Cortex
 Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Psychology in Your Life
 Sarah Grison Todd Heatherton Michael Gazzaniga Psychology in Your Life SECOND EDITION Chapter 2 The Role of Biology in Psychology 1 2016 W. W. Norton & Company, Inc. 2.1 How Do Our Nervous Systems Affect
Sarah Grison Todd Heatherton Michael Gazzaniga Psychology in Your Life SECOND EDITION Chapter 2 The Role of Biology in Psychology 1 2016 W. W. Norton & Company, Inc. 2.1 How Do Our Nervous Systems Affect
Psychology 320: Topics in Physiological Psychology Lecture Exam 2: March 19th, 2003
 Psychology 320: Topics in Physiological Psychology Lecture Exam 2: March 19th, 2003 Name: Student #: BEFORE YOU BEGIN!!! 1) Count the number of pages in your exam. The exam is 8 pages long; if you do not
Psychology 320: Topics in Physiological Psychology Lecture Exam 2: March 19th, 2003 Name: Student #: BEFORE YOU BEGIN!!! 1) Count the number of pages in your exam. The exam is 8 pages long; if you do not
-Ensherah Mokheemer. -Amani Nofal. -Loai Alzghoul
 -1 -Ensherah Mokheemer -Amani Nofal -Loai Alzghoul 1 P a g e Today we will start talking about the physiology of the nervous system and we will mainly focus on the Central Nervous System. Introduction:
-1 -Ensherah Mokheemer -Amani Nofal -Loai Alzghoul 1 P a g e Today we will start talking about the physiology of the nervous system and we will mainly focus on the Central Nervous System. Introduction:
CASE 48. What part of the cerebellum is responsible for planning and initiation of movement?
 CASE 48 A 34-year-old woman with a long-standing history of seizure disorder presents to her neurologist with difficulty walking and coordination. She has been on phenytoin for several days after having
CASE 48 A 34-year-old woman with a long-standing history of seizure disorder presents to her neurologist with difficulty walking and coordination. She has been on phenytoin for several days after having
CASE 49. What type of memory is available for conscious retrieval? Which part of the brain stores semantic (factual) memories?
 CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
Motor systems.... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington
 Motor systems... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington 1 Descending pathways: CS corticospinal; TS tectospinal; RS reticulospinal; VS
Motor systems... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington 1 Descending pathways: CS corticospinal; TS tectospinal; RS reticulospinal; VS
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Non-therapeutic and investigational uses of non-invasive brain stimulation
 Non-therapeutic and investigational uses of non-invasive brain stimulation Robert Chen, MA, MBBChir, MSc, FRCPC Catherine Manson Chair in Movement Disorders Professor of Medicine (Neurology), University
Non-therapeutic and investigational uses of non-invasive brain stimulation Robert Chen, MA, MBBChir, MSc, FRCPC Catherine Manson Chair in Movement Disorders Professor of Medicine (Neurology), University
Chapter 12 Nervous Tissue
 9/12/11 Chapter 12 Nervous Tissue Overview of the nervous system Cells of the nervous system Electrophysiology of neurons Synapses Neural integration Subdivisions of the Nervous System 1 Subdivisions of
9/12/11 Chapter 12 Nervous Tissue Overview of the nervous system Cells of the nervous system Electrophysiology of neurons Synapses Neural integration Subdivisions of the Nervous System 1 Subdivisions of
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Cerebral Cortex 1. Sarah Heilbronner
 Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Implantable Microelectronic Devices
 ECE 8803/4803 Implantable Microelectronic Devices Fall - 2015 Maysam Ghovanloo (mgh@gatech.edu) School of Electrical and Computer Engineering Georgia Institute of Technology 2015 Maysam Ghovanloo 1 Outline
ECE 8803/4803 Implantable Microelectronic Devices Fall - 2015 Maysam Ghovanloo (mgh@gatech.edu) School of Electrical and Computer Engineering Georgia Institute of Technology 2015 Maysam Ghovanloo 1 Outline
The Nervous System. Biological School. Neuroanatomy. How does a Neuron fire? Acetylcholine (ACH) TYPES OF NEUROTRANSMITTERS
 Biological School The Nervous System It is all about the body!!!! It starts with an individual nerve cell called a NEURON. Synapse Neuroanatomy Neurotransmitters (chemicals held in terminal buttons that
Biological School The Nervous System It is all about the body!!!! It starts with an individual nerve cell called a NEURON. Synapse Neuroanatomy Neurotransmitters (chemicals held in terminal buttons that
The Brain & Homeostasis. The Brain & Technology. CAT, PET, and MRI Scans
 The Brain & Homeostasis Today, scientists have a lot of information about what happens in the different parts of the brain; however they are still trying to understand how the brain functions. We know
The Brain & Homeostasis Today, scientists have a lot of information about what happens in the different parts of the brain; however they are still trying to understand how the brain functions. We know
Neuromorphic computing
 Neuromorphic computing Robotics M.Sc. programme in Computer Science lorenzo.vannucci@santannapisa.it April 19th, 2018 Outline 1. Introduction 2. Fundamentals of neuroscience 3. Simulating the brain 4.
Neuromorphic computing Robotics M.Sc. programme in Computer Science lorenzo.vannucci@santannapisa.it April 19th, 2018 Outline 1. Introduction 2. Fundamentals of neuroscience 3. Simulating the brain 4.
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle
 J Physiol (2003), 550.3, pp. 933 946 DOI: 10.1113/jphysiol.2003.042606 The Physiological Society 2003 www.jphysiol.org Differential modulation of intracortical inhibition in human motor cortex during selective
J Physiol (2003), 550.3, pp. 933 946 DOI: 10.1113/jphysiol.2003.042606 The Physiological Society 2003 www.jphysiol.org Differential modulation of intracortical inhibition in human motor cortex during selective
Applied Neuroscience. Conclusion of Science Honors Program Spring 2017
 Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Introduction to Physiological Psychology
 Introduction to Physiological Psychology Review Kim Sweeney ksweeney@cogsci.ucsd.edu www.cogsci.ucsd.edu/~ksweeney/psy260.html Today n Discuss Final Paper Proposal (due 3/10) n General Review 1 The article
Introduction to Physiological Psychology Review Kim Sweeney ksweeney@cogsci.ucsd.edu www.cogsci.ucsd.edu/~ksweeney/psy260.html Today n Discuss Final Paper Proposal (due 3/10) n General Review 1 The article
PSYC& 100: Biological Psychology (Lilienfeld Chap 3) 1
 PSYC& 100: Biological Psychology (Lilienfeld Chap 3) 1 1 What is a neuron? 2 Name and describe the functions of the three main parts of the neuron. 3 What do glial cells do? 4 Describe the three basic
PSYC& 100: Biological Psychology (Lilienfeld Chap 3) 1 1 What is a neuron? 2 Name and describe the functions of the three main parts of the neuron. 3 What do glial cells do? 4 Describe the three basic
Brain and behaviour (Wk 6 + 7)
 Brain and behaviour (Wk 6 + 7) What is a neuron? What is the cell body? What is the axon? The basic building block of the nervous system, the individual nerve cell that receives, processes and transmits
Brain and behaviour (Wk 6 + 7) What is a neuron? What is the cell body? What is the axon? The basic building block of the nervous system, the individual nerve cell that receives, processes and transmits
Cerebrum-Cerebral Hemispheres. Cuneyt Mirzanli Istanbul Gelisim University
 Cerebrum-Cerebral Hemispheres Cuneyt Mirzanli Istanbul Gelisim University The largest part of the brain. Ovoid shape. Two incompletely separated cerebral hemispheres. The outer surface of the cerebral
Cerebrum-Cerebral Hemispheres Cuneyt Mirzanli Istanbul Gelisim University The largest part of the brain. Ovoid shape. Two incompletely separated cerebral hemispheres. The outer surface of the cerebral
Neurophysiological Basis of TMS Workshop
 Neurophysiological Basis of TMS Workshop Programme 31st March - 3rd April 2017 Sobell Department Institute of Neurology University College London 33 Queen Square London WC1N 3BG Brought to you by 31 March
Neurophysiological Basis of TMS Workshop Programme 31st March - 3rd April 2017 Sobell Department Institute of Neurology University College London 33 Queen Square London WC1N 3BG Brought to you by 31 March
Homework Week 2. PreLab 2 HW #2 Synapses (Page 1 in the HW Section)
 Homework Week 2 Due in Lab PreLab 2 HW #2 Synapses (Page 1 in the HW Section) Reminders No class next Monday Quiz 1 is @ 5:30pm on Tuesday, 1/22/13 Study guide posted under Study Aids section of website
Homework Week 2 Due in Lab PreLab 2 HW #2 Synapses (Page 1 in the HW Section) Reminders No class next Monday Quiz 1 is @ 5:30pm on Tuesday, 1/22/13 Study guide posted under Study Aids section of website
Practical. Paired-pulse on two brain regions
 Practical Paired-pulse on two brain regions Paula Davila Pérez, MD Berenson-Allen Center for Noninvasive Brain Stimulation Beth Israel Deaconess Medical Center Harvard Medical School Plans for the afternoon
Practical Paired-pulse on two brain regions Paula Davila Pérez, MD Berenson-Allen Center for Noninvasive Brain Stimulation Beth Israel Deaconess Medical Center Harvard Medical School Plans for the afternoon
EEG in the ICU: Part I
 EEG in the ICU: Part I Teneille E. Gofton July 2012 Objectives To outline the importance of EEG monitoring in the ICU To briefly review the neurophysiological basis of EEG To introduce formal EEG and subhairline
EEG in the ICU: Part I Teneille E. Gofton July 2012 Objectives To outline the importance of EEG monitoring in the ICU To briefly review the neurophysiological basis of EEG To introduce formal EEG and subhairline
skilled pathways: distal somatic muscles (fingers, hands) (brainstem, cortex) are giving excitatory signals to the descending pathway
 L15 - Motor Cortex General - descending pathways: how we control our body - motor = somatic muscles and movement (it is a descending motor output pathway) - two types of movement: goal-driven/voluntary
L15 - Motor Cortex General - descending pathways: how we control our body - motor = somatic muscles and movement (it is a descending motor output pathway) - two types of movement: goal-driven/voluntary
The Nervous System. Neuron 01/12/2011. The Synapse: The Processor
 The Nervous System Neuron Nucleus Cell body Dendrites they are part of the cell body of a neuron that collect chemical and electrical signals from other neurons at synapses and convert them into electrical
The Nervous System Neuron Nucleus Cell body Dendrites they are part of the cell body of a neuron that collect chemical and electrical signals from other neurons at synapses and convert them into electrical
Ameen Alsaras. Ameen Alsaras. Mohd.Khatatbeh
 9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
The Brain and Behavior
 PNS Chapter 1 The Brain and Behavior 18-698 / 42-632 Neural Signal Processing Spring 2017 Prof. Byron Yu Roadmap Introduction to neuroscience Chapter 1 The brain and behavior Chapter 2 Nerve cells and
PNS Chapter 1 The Brain and Behavior 18-698 / 42-632 Neural Signal Processing Spring 2017 Prof. Byron Yu Roadmap Introduction to neuroscience Chapter 1 The brain and behavior Chapter 2 Nerve cells and
Body control systems. Nervous system. Organization of Nervous Systems. The Nervous System. Two types of cells. Organization of Nervous System
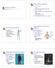 Body control systems Nervous system Nervous system Quick Sends message directly to target organ Endocrine system Sends a hormone as a messenger to the target organ Slower acting Longer lasting response
Body control systems Nervous system Nervous system Quick Sends message directly to target organ Endocrine system Sends a hormone as a messenger to the target organ Slower acting Longer lasting response
Neurobiology: The nerve cell. Principle and task To use a nerve function model to study the following aspects of a nerve cell:
 Principle and task To use a nerve function model to study the following aspects of a nerve cell: INTRACELLULAR POTENTIAL AND ACTION POTENTIAL Comparison between low and high threshold levels Comparison
Principle and task To use a nerve function model to study the following aspects of a nerve cell: INTRACELLULAR POTENTIAL AND ACTION POTENTIAL Comparison between low and high threshold levels Comparison
Activity-Dependent Development II April 25, 2007 Mu-ming Poo
 Activity-Dependent Development II April 25, 2007 Mu-ming Poo 1. The neurotrophin hypothesis 2. Maps in somatic sensory and motor cortices 3. Development of retinotopic map 4. Reorganization of cortical
Activity-Dependent Development II April 25, 2007 Mu-ming Poo 1. The neurotrophin hypothesis 2. Maps in somatic sensory and motor cortices 3. Development of retinotopic map 4. Reorganization of cortical
Cellular Bioelectricity
 ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture 24 Thursday, March 6, 2014 8. NEURAL ELECTROPHYSIOLOGY We will look at: Structure of the nervous system Sensory transducers and neurons Neural coding
ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture 24 Thursday, March 6, 2014 8. NEURAL ELECTROPHYSIOLOGY We will look at: Structure of the nervous system Sensory transducers and neurons Neural coding
Guided Reading Activities
 Name Period Chapter 28: Nervous Systems Guided Reading Activities Big idea: Nervous system structure and function Answer the following questions as you read modules 28.1 28.2: 1. Your taste receptors for
Name Period Chapter 28: Nervous Systems Guided Reading Activities Big idea: Nervous system structure and function Answer the following questions as you read modules 28.1 28.2: 1. Your taste receptors for
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Primary motor cortical metaplasticity induced by priming over the supplementary motor area
 J Physiol 587.20 (2009) pp 4845 4862 4845 Primary motor cortical metaplasticity induced by priming over the supplementary motor area Masashi Hamada 1, Ritsuko Hanajima 1, Yasuo Terao 1,ShingoOkabe 1, Setsu
J Physiol 587.20 (2009) pp 4845 4862 4845 Primary motor cortical metaplasticity induced by priming over the supplementary motor area Masashi Hamada 1, Ritsuko Hanajima 1, Yasuo Terao 1,ShingoOkabe 1, Setsu
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR
 Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR In Physiology Today What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR In Physiology Today What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may
Neurons. Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons.
 Neurons Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons. MBL, Woods Hole R Cheung MSc Bioelectronics: PGEE11106 1 Neuron
Neurons Pyramidal neurons in mouse cerebral cortex expressing green fluorescent protein. The red staining indicates GABAergic interneurons. MBL, Woods Hole R Cheung MSc Bioelectronics: PGEE11106 1 Neuron
Introduction to TMS Transcranial Magnetic Stimulation
 Introduction to TMS Transcranial Magnetic Stimulation Lisa Koski, PhD, Clin Psy TMS Neurorehabilitation Lab Royal Victoria Hospital 2009-12-14 BIC Seminar, MNI Overview History, basic principles, instrumentation
Introduction to TMS Transcranial Magnetic Stimulation Lisa Koski, PhD, Clin Psy TMS Neurorehabilitation Lab Royal Victoria Hospital 2009-12-14 BIC Seminar, MNI Overview History, basic principles, instrumentation
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Mechanosensation. Central Representation of Touch. Wilder Penfield. Somatotopic Organization
 Mechanosensation Central Representation of Touch Touch and tactile exploration Vibration and pressure sensations; important for clinical testing Limb position sense John H. Martin, Ph.D. Center for Neurobiology
Mechanosensation Central Representation of Touch Touch and tactile exploration Vibration and pressure sensations; important for clinical testing Limb position sense John H. Martin, Ph.D. Center for Neurobiology
Strick Lecture 3 March 22, 2017 Page 1
 Strick Lecture 3 March 22, 2017 Page 1 Cerebellum OUTLINE I. External structure- Inputs and Outputs Cerebellum - (summary diagram) 2 components (cortex and deep nuclei)- (diagram) 3 Sagittal zones (vermal,
Strick Lecture 3 March 22, 2017 Page 1 Cerebellum OUTLINE I. External structure- Inputs and Outputs Cerebellum - (summary diagram) 2 components (cortex and deep nuclei)- (diagram) 3 Sagittal zones (vermal,
HUMAN MOTOR CONTROL. Emmanuel Guigon
 HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
The Nervous System. Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes =
 The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
Motor Systems I Cortex. Reading: BCP Chapter 14
 Motor Systems I Cortex Reading: BCP Chapter 14 Principles of Sensorimotor Function Hierarchical Organization association cortex at the highest level, muscles at the lowest signals flow between levels over
Motor Systems I Cortex Reading: BCP Chapter 14 Principles of Sensorimotor Function Hierarchical Organization association cortex at the highest level, muscles at the lowest signals flow between levels over
BENG 260 Supplementary neurophysiology slides
 BENG 260 Supplementary neurophysiology slides Fall 2013 Slides are taken from Vander s Human Physiology, 11 th edition, McGraw Hill (ISBN 0077216091)" These slides cover:" Chapter 6, Neuronal Signaling
BENG 260 Supplementary neurophysiology slides Fall 2013 Slides are taken from Vander s Human Physiology, 11 th edition, McGraw Hill (ISBN 0077216091)" These slides cover:" Chapter 6, Neuronal Signaling
The Nervous System. B. The Components: 1) Nerve Cells Neurons are the cells of the body and are specialized to carry messages through an process.
 The Nervous System A. The Divisions: 1) The Central Nervous System includes the and. The brain contains billions of nerve cells called, and trillions of support cells called. 2) The Peripheral Nervous
The Nervous System A. The Divisions: 1) The Central Nervous System includes the and. The brain contains billions of nerve cells called, and trillions of support cells called. 2) The Peripheral Nervous
Neural Communication. Central Nervous System Peripheral Nervous System. Communication in the Nervous System. 4 Common Components of a Neuron
 Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Biomarkers in Schizophrenia
 Biomarkers in Schizophrenia David A. Lewis, MD Translational Neuroscience Program Department of Psychiatry NIMH Conte Center for the Neuroscience of Mental Disorders University of Pittsburgh Disease Process
Biomarkers in Schizophrenia David A. Lewis, MD Translational Neuroscience Program Department of Psychiatry NIMH Conte Center for the Neuroscience of Mental Disorders University of Pittsburgh Disease Process
Biological Process 9/7/10. (a) Anatomy: Neurons have three basic parts. 1. The Nervous System: The communication system of your body and brain
 Biological Process Overview 1. The Nervous System: s (a) Anatomy, (b) Communication, (c) Networks 2. CNS/PNS 3. The Brain (a) Anatomy, (b) Localization of function 4. Methods to study the brain (Dr. Heidenreich)
Biological Process Overview 1. The Nervous System: s (a) Anatomy, (b) Communication, (c) Networks 2. CNS/PNS 3. The Brain (a) Anatomy, (b) Localization of function 4. Methods to study the brain (Dr. Heidenreich)
Omar Sami. Muhammad Abid. Muhammad khatatbeh
 10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
AdvAnced TMS. Research with PowerMAG Products and Application Booklet
 AdvAnced TMS Research with PowerMAG Products and Application Booklet Table of ConTenTs Introduction p. 04 Legend p. 06 Applications» navigated TMS p. 08» clinical Research p. 10» Multi-Modal TMS p. 12»
AdvAnced TMS Research with PowerMAG Products and Application Booklet Table of ConTenTs Introduction p. 04 Legend p. 06 Applications» navigated TMS p. 08» clinical Research p. 10» Multi-Modal TMS p. 12»
Announcement. Danny to schedule a time if you are interested.
 Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
Physiology. D. Gordon E. Robertson, PhD, FCSB. Biomechanics Laboratory, School of Human Kinetics, University of Ottawa, Ottawa, Canada
 Electromyography: Physiology D. Gordon E. Robertson, PhD, FCSB Biomechanics Laboratory, School of Human Kinetics, University of Ottawa, Ottawa, Canada Nervous System Central Nervous System (cerebellum,
Electromyography: Physiology D. Gordon E. Robertson, PhD, FCSB Biomechanics Laboratory, School of Human Kinetics, University of Ottawa, Ottawa, Canada Nervous System Central Nervous System (cerebellum,
Ch 8. Learning and Memory
 Ch 8. Learning and Memory Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by H.-S. Seok, K. Kim, and B.-T. Zhang Biointelligence
Ch 8. Learning and Memory Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by H.-S. Seok, K. Kim, and B.-T. Zhang Biointelligence
CYTOARCHITECTURE OF CEREBRAL CORTEX
 BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
Ch 8. Learning and Memory
 Ch 8. Learning and Memory Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by H.-S. Seok, K. Kim, and B.-T. Zhang Biointelligence
Ch 8. Learning and Memory Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by H.-S. Seok, K. Kim, and B.-T. Zhang Biointelligence
Synapses and synaptic plasticity. Lubica Benuskova Lecture 8 How neurons communicate How do we learn and remember
 Synapses and synaptic plasticity Lubica Benuskova Lecture 8 How neurons communicate How do we learn and remember 1 Brain is comprised of networks of neurons connected and communicating via synapses ~10
Synapses and synaptic plasticity Lubica Benuskova Lecture 8 How neurons communicate How do we learn and remember 1 Brain is comprised of networks of neurons connected and communicating via synapses ~10
Nervous System, Neuroanatomy, Neurotransmitters
 Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Portions from Chapter 6 CHAPTER 7. The Nervous System: Neurons and Synapses. Chapter 7 Outline. and Supporting Cells
 CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
Bursting dynamics in the brain. Jaeseung Jeong, Department of Biosystems, KAIST
 Bursting dynamics in the brain Jaeseung Jeong, Department of Biosystems, KAIST Tonic and phasic activity A neuron is said to exhibit a tonic activity when it fires a series of single action potentials
Bursting dynamics in the brain Jaeseung Jeong, Department of Biosystems, KAIST Tonic and phasic activity A neuron is said to exhibit a tonic activity when it fires a series of single action potentials
Anatomy of the basal ganglia. Dana Cohen Gonda Brain Research Center, room 410
 Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
Primary Functions. Monitor changes. Integrate input. Initiate a response. External / internal. Process, interpret, make decisions, store information
 NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
LESSON 3.3 WORKBOOK. Why does applying pressure relieve pain?
 Postsynaptic potentials small changes in voltage (membrane potential) due to the binding of neurotransmitter. Receptor-gated ion channels ion channels that open or close in response to the binding of a
Postsynaptic potentials small changes in voltage (membrane potential) due to the binding of neurotransmitter. Receptor-gated ion channels ion channels that open or close in response to the binding of a
All questions below pertain to mandatory material: all slides, and mandatory homework (if any).
 ECOL 182 Spring 2008 Dr. Ferriere s lectures Lecture 6: Nervous system and brain Quiz Book reference: LIFE-The Science of Biology, 8 th Edition. http://bcs.whfreeman.com/thelifewire8e/ All questions below
ECOL 182 Spring 2008 Dr. Ferriere s lectures Lecture 6: Nervous system and brain Quiz Book reference: LIFE-The Science of Biology, 8 th Edition. http://bcs.whfreeman.com/thelifewire8e/ All questions below
Physiology of Tactile Sensation
 Physiology of Tactile Sensation Objectives: 1. Describe the general structural features of tactile sensory receptors how are first order nerve fibers specialized to receive tactile stimuli? 2. Understand
Physiology of Tactile Sensation Objectives: 1. Describe the general structural features of tactile sensory receptors how are first order nerve fibers specialized to receive tactile stimuli? 2. Understand
COGNITIVE SCIENCE 107A. Motor Systems: Basal Ganglia. Jaime A. Pineda, Ph.D.
 COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
Version A. AP* Biology: Nervous System. Questions 1 and 2. Name: Period
 Name: Period Version A AP* Biology: Nervous System Directions: Each of the questions or incomplete statements below is followed by four suggested answers or completions. Select the one that is best in
Name: Period Version A AP* Biology: Nervous System Directions: Each of the questions or incomplete statements below is followed by four suggested answers or completions. Select the one that is best in
