Epigenetic Vulnerabilities in Triple-Negative Breast Cancer
|
|
|
- Archibald Stevenson
- 5 years ago
- Views:
Transcription
1 Epigenetic Vulnerabilities in Triple-Negative Breast Cancer By Ingrid Yunchi Kao A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Medical Biophysics University of Toronto Copyright by Ingrid Yunchi Kao 2017
2 Epigenetic Vulnerabilities in Triple-Negative Breast Cancer Abstract Ingrid Yunchi Kao Master of Science Department of Medical Biophysics University of Toronto 2017 Breast cancer is the most commonly diagnosed cancer amongst Canadian women. To date, three main histopathological subtypes based on the expression of estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 with different clinical outcomes dictate the treatment approach. Among these, the Triple-Negative Breast Cancer (TNBC) subtype does not express any of the hormone receptors and has the least survival rate due to lack of targeted therapy and poor prognosis. Recently, a category of highly selective and potent drug-like molecules ideal to assess therapeutic potential of new targets, known as chemical probes, have been designed to target epigenetic enzymes. Here, we determined the impact of 56 epigenetic chemical probes on the proliferation of TNBC cell lines. We identified distinct response across these cell lines after inhibition of the bromodomains, methyltransferases, methyl lysine transferases and lysine demethylases. Our results revealed epigenetic targets that could lead to potential therapeutic opportunities. ii
3 Acknowledgments This project would not have been possible without the support and guidance of my supervisor Dr. Mathieu Lupien. I thank him for giving me this opportunity to study and conduct research under his tutelage. His outstanding knowledge and view in science have been blessings. In addition, his enthusiasm has always been an important driving force for my research. I also want to thank him for teaching me to be proactive, which is an important attitude when learning. Lastly, although there are stressful times in graduate school, Mathieu always encourage me and remind me to have fun with science. He is truly an inspiring mentor. Next, I am extremely grateful to members in my lab for all their help and support. In particularly, I want to thank my mentor Genevieve Deblois, who trained me and allowed me to learn and participate in her project throughout my degree. She is not only an outstanding researcher but also an incredible mentor. I really appreciate how much she taught me. I would also like to thank Qin Wu for her great contribution to this project. She has been a very efficient and lovely person to work with. I also want thank my committee members Dr. Pam Ohashi and Dr. Vuk Stambolic for their input and advices. They have always been very helpful. Lastly, I want to acknowledge my friends and family for their unconditional and neverending support in the pursuit of my graduate education. In particularly, I would like to thank my siblings for being my emotional support throughout my degree. I also want to thank my friend Nina for always being there for me. iii
4 Abstract Acknowledgments Table of contents ii ii Chapter 1 1 Introduction Epigenetics Cancer Epigenetics DNA methylation DNA methyltransferases CpG/non-CpG methylation and gene expression DNA hypo/hypermethylation in cancer DNA demethylation Histone modification Histone lysine acetylation/deacetylation Histone acetylation/deacetylation in cancer Histone methylation/demethylation Histone methylation/demethylation in cancer Histone variants Epigenetic drugs in cancer Drugs targeting DNA methylation Drugs targeting histone modification 15 Histone methyltransferase inhibitors 15 Histone demethylase inhibitors 16 Histone acetyltransferase inhibitors 17 Histone deacetylase inhibitors Chemical probes Bromodomain (BRD) inhibitors 19 BAZ2A/2B inhibitors 19 BRD7/9 inhibitors 19 BRPF inhibitors 20 BET family inhibitors 20 CBP/p300 inhibitors 21 PCAF inhibitors 22 SMARCA/PB1 inhibitors 22 iv
5 Methyltransferase (MT) inhibitors 22 PRMT inhibitors 22 SETD7 inhibitors 24 EZH2/1 inhibitors 24 DOT1L inhibitors 25 SUV420H1/H2 inhibitors 25 SYMD2 inhibitors Methyl Lysine Binders 26 L3MBTL3 26 EED inhibitor Histone lysine demethylases 27 LSD1 inhibitors 27 JMJD3/ KDM6B, UTX/KDM6A, JARID1B/KDM5B Arginine deiminases 29 PAD WD40 29 WDR Combination therapy 29 Chapter 2 31 Exploring epigenetic vulnerabilities of Triple-Negative Breast Cancer Results Bromodomain inhibitors Methyl transferase inhibitors Methyl-lysine binder inhibitors KDM inhibitors Discussion and Future Directions Tumour heterogeneity BET and BRPF2 family inhibitors can be potential treatment for TNBC PRMTs could be a potential target of TNBC Type I PRMT PRMT Some TNBC cell lines are sensitive to EZH1, EZH2 dual inhibition Epigenetic probe toxicity Future directions Materials and Methods Breast cancer cell line classification 55 v
6 2.3.2 Cell culture IncuCyte ZOOM Assay Chemical probe usage Western blot Bioinformatics & Statistical Analysis 58 Supplementary 1 58 Supplementary 2 60 References 69 vi
7 Chapter 1 Introduction 1.1 Epigenetics Epigenetics is the mitotically heritable changes governing DNA template processes without altering the primary nucleotide sequence. These changes mainly include DNA methylation, histone posttranslational modifications and histone variants, allowing formation of different chromatin states that regulate transcription, chromatin interaction, genomic stability, DNA replication and repair. Chromatin state is largely dependent on the location and function of the covalent marks within the genome. These covalent marks are laid by epigenetic enzymes known as writers such as histone methyltransferases, histone acetyltransferases and DNA methyltransferases. Active or inactive promoters, enhancers or gene bodies are all associated with specific combinations of different covalent marks. These marks can instruct the proteins that recognize them, known as the readers such as methyl-cpg binding domain proteins (MBD) and the chromo-, PWWP, tudor-, bromo-, and PHD-finger-domain proteins to identify genomic regions. Lastly, this process is contributed by the erasers including histone demethylases, histone deacetylases and the ten-eleven translocation (TET) family of 5-methylytosine oxidases, which are enzymes that remove these active or repressive marks (Tessarz & Kouzarides, 2014) Cancer Epigenetics Epigenetics regulate numerous essential cellular processes including differentiation, development and homeostasis. Therefore, disruption of the epigenome can give rise to serious human diseases, such as cancer (Tollefsbol, 2016). Alteration of the distribution and abundance of epigenetic modifications has significant implication on the procedure of tumour initiation, progression and therapeutic response. Although it was long known that abnormalities in the DNA sequence such as point mutations, copy number alterations and translocations can lead to downregulation of tumour suppressors and upregulation of oncogenes, promoting tumorigenesis. 1
8 However, genetics has gradually been recognized as an explanation restricted to local DNA changes without considering the global dynamic nature of chromatin structure and interaction. Fortunately, advance in research technology has been allowing more sophisticated research in epigenetics. In the past few years, many national and international mapping projects (Roadmap Epigenomics Mapping Consortium, the International Human Epigenome Consortium, and BLUEPRINT) had been initiated aiming to elucidate the genome-wide distribution of epigenetic marks on normal and cancerous tissues (P. A. Jones, Issa, & Baylin, 2016). In addition, numerous tumour genomes have been sequenced and frequent mutations have been identified in epigenetic enzymes, demonstrating the importance of epigenetics to cancer initiation and development. These projects also uncovered evidence showcasing abnormalities in both genetics, epigenetics and their crosstalk in cancers. Herein, the main epigenetic mechanisms associated with cancer: DNA methylation, covalent histone modification and histone variants will be discussed in this chapter. 1.2 DNA methylation DNA methylation is a tightly regulated and tissue-specific epigenetic mark that alter functional states of DNA regions by the addition or removal of a methyl group at the fifth carbon of cytosine to form a 5-methyl carbon (5mC) (Law & Jacobsen, 2010). It is essential in many cellular processes including embryonic development, chromatin structure, transcription, X chromosome inactivation and genomic imprinting (Deaton & Bird, 2011). Majority (over 80%) of the 5mCs in human somatic cells are located at repetitive sequences, intergenic regions and gene bodies while others are found at promoters. Dysregulation of DNA methylation can lead to broad reprogramming of gene and DNA element expression. This can give rise to diseases such as cancer DNA methyltransferases In mammals, DNA methyltransferases (DNMTs: DNMT1, DNMT3A, DNMT3B, and DNMT3L) are responsible for the deposition and maintenance of DNA methylation patterns. These DNMTs contain a regulatory N-terminal domain and catalytic C-terminal domain and utilize 2
9 the cofactor S-adenosylmethionine (SAM) as a source of methyl group to form 5mC. DNMT1 is responsible to maintain DNA methylation and ensure chromatin structure. It functions in a replication dependent manner, favoring hemimethylated DNA, meaning that it relies on the methylated pattern from the parental DNA as a template to create a newly synthesized daughter strand with the same pattern (Moore, Le, & Fan, 2013). DNMT1 was found highly expressed in post-mitotic neurons in the central nervous system. In fact, it is closely involved with neuronalrelated processes such as neuron differentiation, migration and connections and can cause hereditary sensory neuropathy dementia if mutated (Klein et al., 2011). DNMT3A and DNMT3B are de novo methyltransferases independent of replication, meaning they have the ability to deposit novel DNA methylation patterns on unmodified DNA. This suggest their crucial role in DNA methylation establishment during early embryonic development (Moore et al., 2013). Interestingly, DNMT3L is a catalytically inactive de novo methyltransferase that interacts with DNMT3a and DNMT3b to enhance methylation. It was shown to be highly expressed in embryonic stem cells and is important for genetic imprinting in oocytes (Jurkowska, Jurkowski, & Jeltsch, 2011). In embryonic stem cells, DNMT3L favours methylation at gene bodies of housekeeping genes and antagonize promoters of bivalent genes, which are genes with both a repressive mark H3K27me3 and active mark H3K4me3 (Neri et al., 2013) CpG/non-CpG methylation and gene expression Inhibition of gene expression caused by CpG dinucleotide methylation was first shown in studies focusing on CpG enriched regions. Most genes in vertebrates contain an approximately 1 kilobase (kb) long CpG island. Studies have also identified regions with ~2kb of CpG islands, named CpG shores, that are associated with tissue, aging and cancer related hypomethylation (Shimoda et al., 2014). Methylation at CpGs can lead to both gene silencing or activation depending of the methylation location in the genome. For example, hypermethylation within gene bodies can result in increase of gene expression whereas hypermethylation at gene promoters and enhancers can lead to formation of heterochromatin and transcriptional silencing (Jang, Shin, Lee, & Do, 2017; X. Yang et al., 2014). The methylated cytosine acts as a docking site for various 3
10 methyl binding proteins (MBD1, MBD2, MBD3, MeCP2) that will then be recognized by histone modifying enzymes such as histone deacetylases and cause gene repression (Baylin & Jones, 2011). Methylated DNA are primarily at the cytosine in CpG dinucleotides but also can be at non-cpg sites including CpA, CpT and CpC (adenine, thymine, another cytosine). These non-cpg methylations are often found in human embryonic stem cells and brain tissues catalyzed by DNMT3A and DNMT3B (Jang H.S., 2017). However, the mechanism of non-cpg methylation is not well understood and controversial. Some studies suggested that methylation occur at non-cpg sites is a consequence of the hyperactivity of non-specific de novo methylation by DNMT3a and DNMT3b at CpG sites and others argue that it is correlated with tissue specificity and gene expression (Jang et al., 2017) DNA hypo/hypermethylation in cancer It has been well known that cancer cells have abnormal DNA methylation patterns characterized by promoter hypermethylation and genome-wide hypomethylation. Loss of function mutation in DNA demethylases (TET1, TET2 and TET3) or overexpression of DNA methyltransferases (DNMT1, DNMT3A, DNMT3B) can result in promoter hypermethylation whereas loss of function mutation of DNA methyltransferases can lead to DNA hypomethylation (Pfister & Ashworth, 2017). In cancer, hypomethylation at repetitive sequences is one of the causes of genomic instability as it encourages chromosomal rearrangement and aid in tumorigenesis. Moreover, hypomethylation of retrotransposons lead to their random relocation and insertion and also causes genomic instability. Work from earlier studies have observed hypomethylation mainly at repetitive regions such as satellite repeats and LINE-1 retrotransposons in cancer (Timp & Feinberg, 2013). However, improvement of the whole genome bisulfite sequencing technology revealed that DNA hypomethylation occurs at megabase-scale genomic regions. Although these regions typically contain LINE-1, regions without LINE-1 were also observed to be methylated, suggesting that this hypomethylation is not retrotransposon-specific (Reddington, Sproul, & Meehan, 2014). 4
11 Furthermore, hypomethylation at promoters can lead to activation of proliferation-promoting genes in cancers such as melanoma associated antigen (MAGE) in melanoma, S100 in colon cancer and related-ras (R-Ras) and MAPSIN in gastric carcinoma (Wilson, Power, & Molloy, 2007). Interestingly, recent studies using genetic profiling techniques revealed that tumorigenesis is not always caused by silencing of tumour suppressor genes. Aberrantly hypomethylated genes were found transcriptionally silenced in the corresponding normal cells that give rise to cancer instead, suggesting that these genes might not be the driver in tumorigenesis and not all epigenetic alterations in cancer are detrimental (Sproul & Meehan, 2013). The emerging picture of the role of DNA methylation has gradually shifted from gene expression alone to also genome organization in the nucleus. Much of the hypomethylation in cancer is characterized with lamin associated domains (LADs) that associate with the nuclear lamina at the nucleus periphery (Kulis et al., 2015). Hypomethylation is also associated with partially methylated domains (PMDs), which seems to couple with unorganized nucleus and large organized chromatin lysine K (LOCKs), which are large heterochromatin regions enriched with histone methylation on H3K9 or H3K27 (Madakashira & Sadler, 2017). Similar to hypomethylation, DNA hypermethylation can also promote tumorigenesis by silencing tumour suppressor genes and their transcription factors that regulate essential cellular processes including DNA repair, apoptosis and replication. One of the earliest example was found in retinoblastoma in which the promoter CpG island of the tumour suppressor gene Rb is hypermethylated (Greger, Passarge, Höpping, Messmer, & Horsthemke, 1989). Other tumour suppressor genes such as p16 INK4A, MLH1 and BRCA1 were also found hypermethylated in many cancers (Baylin, 2005; P. A. Jones & Baylin, 2002, 2007). Furthermore, DNA hypermethylation at transcription factors can also silence additional groups of downstream genes. For instance, the transcription factor RUNX3 in esophageal squamous cell carcinoma and GATA4 and GATA5 in both gastric and colorectal cancer respectively, are silenced by DNA hypermethylation (Akiyama et al., 2003; Long et al., 2007). Interestingly, H3K27me3, a PRC2-deposited histone mark has been observed to locate at regions that are DNA hypomethylated in breast cancer cells and oppositely, loss of H3K27me3 is associated with DNA hypermethylation in prostate cancer cell lines (Hon et 5
12 al., 2012). These observations imply that DNA methylation can compensate for PRC-2 deposited histone marks H3K27me3 or vice versa (Reddington et al., 2013) DNA demethylation In addition to DNA methylation, mechanisms involving methyl group removal were also identified. DNA methylation in the form of 5mC can be reversed back to the original unmodified state by either passive or active demethylation. Passive demethylation takes place when the DNMT fails to maintain the methyl group after DNA replication. In comparison, active DNA demethylation is achieved through ten-eleven translocation (TET1/2/3) and activation-induced cytidine deaminase (AID) family of enzymes (Scourzic, Mouly, & Bernard, 2015). Firstly, 5mC can be oxidized by TET enzymes to form 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC). The enzyme thymine-dna-glycosylase (TDG) then removes the carboxyl group from 5caC and 5fC coupled with base excision repair pathways. Though other TET TDG-independent mechanisms have been suggested to mediate active DNA demethylation, the TET TDG pathway has the most support (Xiaoji Wu & Zhang, 2017). 1.3 Histone modification In eukaryotes, DNA is packaged into a string of nucleosomes to stringently regulate gene expression. They are formed by ~147 base pairs of DNA wrapped around four core histones (H2A, H2B, H3 and H4) present as a H2A-H2B dimer and H3-H4 tetramer with a linker histone H1 that connects the nucleosomes together (Luger, Mäder, Richmond, Sargent, & Richmond, 1997). Ordered disassembly of nucleosomes allows access of RNA polymerase II to the DNA for transcription initiation. Increasing posttranslational or covalent histone protein modifications have been revealed to engage in essential cellular processes such as transcription, replication and DNA repair (Bannister & Kouzarides, 2011; Kouzarides, 2007). While earlier studies have observed dysregulation of histone modifications in cancer, more recent studies using genome-wide approaches have found mutations in chromatin modifiers and readers that benefit tumorigenesis as well (Janczar et al., 2017). They found both histone alterations across the genome and on specific gene locus (Bannister & Kouzarides, 2011). The N-terminus of histones can undergo a variety of 6
13 posttranslational modifications such as methylation, acetylation, ubiquitylation, SUMOylation and phosphorylation (Kouzarides, 2007). Here, the two most studied histone modifications relevant to cancer: acetylation and methylation, will be discussed Histone lysine acetylation/deacetylation Histone acetylation is associated with transcriptional activation by relaxing the chromatin compaction. Its level is balanced by two enzymes: the histone lysine acetyltransferases (HATs) and histone deacetylases (HDACs). Acetylation of the ε-amino group on lysine residues removes positive charges of histones using acetyl-coa as the acetyl group donor. There are two major families of HATs: the Gcn5-related N-acetyltransferases (GNAT) that is specific for the deposition of H3K9, H3K14 and H3K36 and the camp response element binding protein (CBP/p300) that is specific for H2AK5, H3K9, H3K23, and H3K56 acetylation. Furthermore, nucleic histones are acetylated by type A HATs and cytoplasmic histones that are newly synthesized are acetylated by type B HAT, which has a more housekeeping role. The acetylated lysine is recognized by the bromodomain which is presented on many transcriptional activators (Biswas & Rao, 2017). In addition, incorporation of an acetyl group on lysine can not only alter chromatin compaction but also intracellular ph. In fact, reduction of histone acetylation level is associated with lower intracellular ph in many tumours discussed below (section 1.2.2) (McBrian et al., 2013). Opposite to HATs, HDACs remove acetyl groups from the ε-n-acetyl lysine amino acid on histones. However, other than histones, deacetylases were also found to target many nonhistone proteins (Ganai, 2016). In human, the HDAC family comprises of 18 proteins classified into four classes (Class I, II, III, IV). Class I, II and IV HDACs have a zinc finger dependent active site whereas class II HDAC activity is dependent on NAD+ (Clawson, 2016) Histone acetylation/deacetylation in cancer Imbalanced activity of HAT and HDAC is often observed in cancer (Biswas & Rao, 2017). Hyperactivity of HAT can lead to hyperacetylation causing broad activation of protooncogenes whereas hypoacetylation can lead to silencing of tumour suppressor genes. The HAT Gcn5 is responsible for a range of essential cellular activities including cell cycle, cell 7
14 proliferation, DNA repair and transcription. Therefore, anomalous activity of this enzyme is associated with malignant growth. Indeed, studies have observed that Gcn5, in couple with the transcriptional adapter protein Ada3, can promote breast cancer proliferation (Germaniuk- Kurowska et al., 2007). Gcn5 alone or with E2F1 and various cell cycle regulatory proteins was also observed to increase non-small cell lung cancer proliferation (L. Chen et al., 2013). The study by Jude et al. proposed the role of the HAT-related enzymes: Tip60, MOZ, MORF in cancer development. Human Tip60 contributes to tumorigenesis through association with the NuA4 complex and connection with other subunits within the complex (Judes et al., 2015). Fusion proteins formed by chromosomal translocation of HAT and its related enzymes such as MOZ, MORF, CBP and p300 was found to alter global histone acetylation in cancer (X.-J. Yang, 2004). For example, fusion of MOZ with transcriptional factors such as MOZ-TIF2 is associated with acute myeloid leukemia (Deguchi et al., 2003; Timmermann, Lehrmann, Polesskaya, & Harel-Bellan, 2001). Missense mutation of the p300 gene was observed in colon and gastric adenocarcinoma with the loss of wild type allele (Iyer, Ozdag, & Caldas, 2004). Furthermore, point and nonsense mutation was found in p300/cbp, leading to the dysfunction of HAT in large B cell lymphoma (Haery, Lugo-Picó, Henry, Andrews, & Gilmore, 2014). In addition to HATs, overexpression of HDACs can reduce acetylation and cause transcriptional repression of tumour suppressive pathways such as the DNA damage/repair pathways and cell cycle regulatory pathways. For example, increase HDAC expression is associated with transcriptional repression at gene locus encoding the tumour suppressor CDKN1A, and of DNA damage repair genes such as BRCA1 and ATR. Furthermore, overexpression of HDACs have been observed in solid tumours, and in some cases, distinct expression have been shown between subtypes. For example, HDAC1 has a higher expression in hormone receptorpositive breast cancer yet HDAC2 and HDAC3 are more expressed in hormone receptor-negative cancers (Müller et al., 2013). Several studies have revealed association of numerous cancers with genome-wide loss of the acetylation of lysine 16 on histone 4 (H4K16ac) and trimethylation of lysine 20 on histone 4 (H4K20me3) through high-throughput sequencing. This could be due to the overexpression of the class III HDAC SIRT1 that is responsible of deacetylating histone H4K16 (Fraga et al., 2005). In addition, HDAC overexpression may also influence non-histone substrates. 8
15 For instance, HDAC1 and HDAC2 can regulate acetylation of the onco-suppressor gene p53 to inhibit its function (Ceccacci & Minucci, 2016) Histone methylation/demethylation Histone methylation is generally found on lysine (mono-, di- tri-methyl) or arginine residues (mono-, symmetrically or unsymmetrically di-methyl). It is regulated by histone lysine methyltransferases (KMTs) and histone lysine demethylases (KDMs). Histone methylation can induce either gene expression or repression. For example, tri-methylation of lysine 27 on histone 3 (H3K27me3) and lysine 9 on histone 3 (H3K9me3) at gene promoters promote transcriptional repression while tri-methylation of lysine 4 on histone 3 (H3K4me3) lead to transcriptional activation (Kouzarides, 2007; Liang et al., 2004). Unlike histone acetylation, methylation does not alter charge of histone proteins (Biswas & Rao, 2017). Histone methylation is regulated by the SET-domain containing protein family, the non- SET domain protein family and the protein arginine methyltransferases 1 (PRMT1) family. Enzymes from the SET domain family tend to methylate lysine of H3 and H4. For instance, MLL1 is the protein complex involved in H3K4 methylation that contains ASH2L, HCFC2/HCF1, WDR5 and RbBP5 (Avdic et al., 2011). The SET and MYND domain containing protein 2 (SMYD2) methylates H3K36 and H3K4 to initiate transcription (Derissen, Beijnen, & Schellens, 2013). The EZH2 HMT uses its SET domain to di- or trimethylate H3K27. SUV39H1 is an HMT that utilize its SET domain to methylate H3K9. Various evidences support the concept that SUV39H1 exists in a megacomplex with demethylase activities (Fritsch et al., 2010). In addition, G9a is another KMT that also methylates H3K9. Interestingly, although SET7/9 containing enzyme normally monomethylate H3K4, the mutation of Tyr 3053 to Phe can transform it into a dimethyltransferase (Biswas & Rao, 2017). Enzymes with non-set domain are also involved in methylation. DOT1L is a wellstudied (KMT) that not only methylates H3K79 but also play important roles in the cell cycle and DNA damage response (McLean, Karemaker, & van Leeuwen, 2014). 9
16 Lastly, the PRMT family is responsible for depositing mono- or dimethyl groups on arginine residues. Studies have further classified PRMT into several classes with different functions that will be discussed later under the chemical probe section (Y. Yang & Bedford, 2013). There are two groups of demethylases: the LSD family and the JMJC domain containing demethylase family. The former consists of LSD1 and LSD2 both characterized by their amine oxidase-like (AOL) domain, a SWIRM (SWI3, RSC8 and Moira) domain that can remove monoand dimethylation. Catalytic activity is carried out only at the AOL domain through oxidation using the cofactor FAD to remove methyl group (Højfeldt, Agger, & Helin, 2013). LSD1 is the first identified KDM that can remove both activation (H3K4 methylation) and repressive marks (H3K9 methylation) depending on the interaction partners (Metzger et al., 2005; Shi et al., 2004). It has also been shown to demethylate non-histone proteins such as p53, E2F1 and DNMT1 (Højfeldt et al., 2013). Structural analysis showed that LSD1 and LSD2 have distinct domain structures and distribution in the genome. SWIRM domain of LSD2 is required for its demethylase activity due to its association with the AOL domain via a coiled loop absent in LSD1. In addition, LSD1 interacts with the RE1-silencing transcription factor complex (corest) through coiled-coil Tower domain. LSD2 is missing this domain but contains an amino-terminal zinc finger domain (Fang et al., 2013). The second family of demethylases is characterized by the JMJC domain, which is present in 31 human proteins in which 17 of them are demethylases (Kooistra & Helin, 2012). Two cofactors, Fe(II) and 2-oxoglutarate can bound in the JMJC domain and regulate their enzymatic activities. Furthermore, these cofactors can react with dioxygen to form a highly active oxoferryl (Fe(IV)=O) intermediate that hydroxylates the ζ-methyl groups of the methylated lysine substrate and induce demethylation of mono-, di- and trimethylated lysines (Højfeldt et al., 2013) Histone methylation/demethylation in cancer Dysregulation of lysine methylation on histones have been extensively studied in the context of cancer development. Disruption of methylation mark deposition cause aberrant silencing of tumour suppressor genes and promote tumorigenesis. For example, the lysine methyltransferase 10
17 SUV39H1 that has a tumour suppressive function can be found to downregulate tumour suppressor genes such as p15 INK4B by laying H3K9me in acute myeloid leukemia (AML) (Lakshmikuttyamma, Scott, DeCoteau, & Geyer, 2010). Increase expression of G9a, the methyltransferase of H3K9, was reported in hepatoma and was suggested to modulate chromatin structure to promote malignancy (Kondo et al., 2007, 2008). EZH2 is the enzymatic subunit of PRC2 that methylates lysine 27 on H3 (H3K27me) and have been observed to extensively link to metastasis. Overexpression of EZH2 was reported in both prostate and breast cancer (Valk-Lingbeek, Bruggeman, & van Lohuizen, 2004). However, it is unclear whether this oncogenic association is due to the methylation of H3K27. This is because many abnormalities of this regulatory axis have been found in cancer that including overexpression of EZH2, loss-of-function or gain-of-function mutations of EZH2, mutations of the demethylase UTX, and mutations of the SWI/SNF chromatin remodeling complex (K. H. Kim & Roberts, 2016). DOT1L catalyzes the methylation at lysine 79 at histone 3 (H3K79) and initiate transcriptional elongation. It was found that mixed lineage leukemia (MLL) related fusion proteins such as AF9 and AF10 can recruit DOT1L to facilitate H3K79 methylation favoring the disease (McLean et al., 2014). In addition, increase level of lysine 36 on histone 3 methylation (H3K36) and along with SMYD2 was found in cancers such as esophageal squamous cell carcinoma and was recently revealed to promote growth in pancreatic cancer (Reynoird et al., 2016). Other than KMTs, abnormal levels of histone lysine demethylases (KDMs) have also been found in cancers (Cloos, Christensen, Agger, & Helin, 2008). Overexpression of LSD1 has been reported in numerous cancers including bladder, colorectal, breast and prostate cancer (Hayami et al., 2011; Kauffman et al., 2011). Furthermore, LSD1 was proposed to maintain AML that has MLL translocations (Harris et al., 2012). Inhibiting LSD1 in AML was later on being showed to reactivate the all-trans retinoic acid (ATRA) differentiation pathway, increasing the interests of developing drugs that target LSD1 (Schenk et al., 2012). Among the JMJC domain, UTX, JMJD2, JARID1B and FBXL10 have been reported to associate with cancer. UTX is the demethylase for H3K27me3 and H3K27me2. Loss-of-function mutation of UTX has been observed in many cancers such as esophageal squamous cell carcinomas, multiple myeloma, colorectal cancer, breast cancer, renal cell carcinomas and 11
18 glioblastoma (Højfeldt et al., 2013). This lead to the increase of H3K27 methylation, which is similar to the outcome of EZH2 hyperactivity observed in various human cancers mentioned before. Members of the JMJD2 family is responsible for the demethylation of the H3K9me3, H3K9me2 H3K36me3 and H3K36me2 marks, which are often found overexpressed in cancers. For example, amplification of the JMJD2C gene was reported in medulloblastoma, squamous cell carcinoma, and breast cancer while reduction of JMJD2B decrease proliferation of various cancer cell lines (Højfeldt et al., 2013). JARID1B, also known as KDM5B or PLU1, is the demethylase of H3K4me2/3 and is required for development (Albert et al., 2013). Overexpression of JARID1B has been observed in cancers including prostate, breast and bladder cancer. It has also been reported as a requirement for the proliferation of breast cancer and melanoma (Roesch et al., 2010; Yamane et al., 2007). Lastly, FBXL10, also known as KDM2B, catalyzes the demethylation of H3K36me2 and H3K36me1. In addition, FBXL10 contains a F-box domain and a CXXC DNA binding domain (Xudong Wu, Johansen, & Helin, 2013). The demethylase activity of FBXL is required for AML and pancreatic cancer growth where it was found overexpressed (J. He, Nguyen, & Zhang, 2011; Tzatsos et al., 2013). 1.4 Histone variants Histone variants are important for the regulation of chromatin structure is suggested in DNA-related processes such as transcription, cell cycle, DNA damage/repair, apoptosis and chromosome stability. Histone variants can differ from their canonical counter proteins from only a few amino acids to addition of large non-histone domains and rely on chaperones/remodelers to exchange them with their counter proteins (Zink & Hake, 2016). These differences allow changes in nucleosome structure, stability and post-translational modifications. Histone variants genes are non-allelic and they express in a replication-independent manner throughout the cell cycle compare to their counter proteins. Variant H3.3 differs from its counter proteins H3.1 and H3.2 by only 4-5 amino acids. It can be found at actively transcribed gene bodies, endogenous retroviral elements, transcription start sites, enhancers, pericentric heterochromatin and telomeres while H3.1 and H3.2 are deposited all over the genome (Elsässer, Noh, Diaz, Allis, & Banaszynski, 2015; Zink & Hake, 2016). Moreover, H3.3 has recently been 12
19 found to maintain silencing of imprinted genes (Voon et al., 2015). Mutation of K37 or G34 in H3.3 have been identified in pediatric brain tumours and is an indicator of poor prognosis in diffuse intrinsic pontine gliomas and adult brainstem gliomas (S. Yang et al., 2016). Mutation of DAXX/ATRX, one of the chaperone complex responsible for H3.3 localization at telomeres and pericentric heterochromatin have also been reported in pancreatic neuroendocrine tumors (Marinoni et al., 2014). The two variants of H3A are H2A.Z and macroh2a. The H2A.Z is 60% identical to its counter protein H2A. It was suggested to take part in DNA-related processes based on its ability to affect gene expression, chromosome stability and DNA repair. H2A.Z was found overexpressed in many cancers such as melanoma, hepatocellular carcinoma and breast cancer and has prognostic values (Zink & Hake, 2016). H2A.Z.1 and H2A.Z.2 have different roles. H2A.Z.1 is more involved in iµmune response genes whereas H2A.Z.2 regulates the S-phase genes of cell cycle. It was reported in hepatocellular carcinoma that H2A.Z.1 increase epithelial-mesenchymal transition (H. D. Yang et al., 2016). Both H2A.Z.1 and H2A.Z.2 are overexpressed in melanoma and H2A.Z.2 have been found to increase transcription of oncogenes (Vardabasso et al., 2015). In addition, p400 and SRCAP, the two chromatin remodelers of H2A.Z are also reported to be overexpressed in some cancers as well. p400 was found to facility WNT signaling and decrease the tumour suppressor function of KAT5, a histone acetyltransferase (Chevillard-Briet et al., 2014). macroh2a contains a H2A-resembling region that is 64% similar to H2A, which is then connected to a non-histone macro domain via a lysine rich linker (Buschbeck & Di Croce, 2010). It resides at repressive chromatin regions such as the inactive X chromosome and the facultative heterochromatin and was suggested to suppress gene expression by interfering with TF binding (Zink & Hake, 2016). Unlike H2A.Z, macroh2a functions as a tumour suppressor and its expression is downregulated in cancers such as metastatic melanoma, teratoma and breast cancer (Buschbeck & Di Croce, 2010; Creppe et al., 2012). 13
20 1.5 Epigenetic drugs in cancer Epigenetic drugs are a relatively new class of drugs that act on epigenetic modifiers and readers which have become an attractive target for cancer therapy. Increasing studies have shown that cancer cells not only utilize genetic alterations to survive conventional tumour therapies and immune surveillance, but also through modifications of epigenetic processes. This is due to the fact that epigenetics are somatically heritable and can induce changes in gene expression and other DNA repetitive elements, allowing tumour cells to evolve more than they could have by genetic mutations alone (P. A. Jones et al., 2016). Because of this, increasing effort has been put into the development of targeting the epigenome to restore causal epigenetic aberrations in cancer cells back into a normal state. In fact, many epigenetic drugs discovered to date can effectively reverse DNA methylation or histone modification Drugs targeting DNA methylation Advancement of the epigenetic field allows development of many DNMT inhibitors (DNMTi) that are tested in clinical trials. DNMTi can be categorized into two general classes: nucleoside analogues and non-nucleoside analogues (Biswas & Rao, 2017). 5-azacytidine (azacutidine) and 5-aza2 deoxycitidine (Decitabine) are two very first and well-known nucleosides analogue. It was found that treatment of cells using these chemicals prevent DNA methylation and induce gene expression, resulting in differentiation of cultured cells (Constantinides, Jones, & Gevers, 1977). Both of these drugs get intercalated with the DNA by DNMTs during S phase of the cell cycle. After intercalation, they form an irreversible covalent complex with the enzyme that leads to proteasomal degradation. Promising results from clinical trials have led to the approval of Azacitidine and Decitabine for the management of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) by the FDA (Derissen et al., 2013). In addition, Zuebularine was developed to improve efficacy and decrease cytotoxicity. However, although it has a better anticancer effect compare to Azacitidine and Decitabine, there is a limitation on oral bioavailability and incompetent metabolism (Holleran et al., 2005). Currently a nucleoside analogue derived from decitabine SGI-110 is undergoing clinical trials for the treatment for MDA and AML (Issa et al., 2015). 14
21 Due to the cytotoxicity of nucleoside analogues, some interest have been drawn to nonnucleoside analogue inhibitors that have been developed to bind to the catalytic site of DNMT without intercalating into the DNA (Song, Han, & Bang, 2011). SGI-1027 is a quinolone derived compounds that can bind to DNMT1, DNMT3A and DNMT3B and also AT-rich regions in the genome (Datta et al., 2009). More recent studies showed that it is more selective towards DNMT3A compare to DNMT1 (Rilova et al., 2014). RG108 is a phthalimido-l-tryptophan derivative DNMTi developed in silico. Studies have demonstrated that treatment of RG108 alone or in combination with HDACi can induce expression of E-cadherin in leukemia cells (Savickiene, Treigyte, Jazdauskaite, Borutinskaite, & Navakauskiene, 2012). Furthermore, studies have shown that treatment using RG108 can upregulate methylated silenced genes involved in antioxidant enzymes and prevent oxidative stress in retinal pigment epithelial cells (Tokarz, Kaarniranta, & Blasiak, 2016) Drugs targeting histone modification Currently, histone deacetylase inhibitors are the only drugs targeting histone modifications approved by the FDA. However, ongoing studies have identified many inhibitors that target other enzymes involved in histone protein alteration. Histone methyltransferase inhibitors EPZ is an inhibitor that targets the S-adenosyl methionine binding domain of DOT1L (Daigle et al., 2011). It was observed to decrease the H3K78 mark in MLL cells. Moreover, a new inhibitor of DOT1L, the EPZ-5676 was found to decrease H3K79 methylation and reduce MLL fusion gene expression. In vivo studies of EPZ-5676 in MLL-translocated xenograft model showed effective suppression of tumour growth without significant adverse effect (Daigle et al., 2013). A recent study has showed that EPZ-5676 have a antiproliferative effect when combining with the stand-of-care drugs cytarabine or daunorubicin in MLL-rearranged leukemia cells (Klaus et al., 2014). Furthermore, many compounds have been developed to target the EZH2 due to its overexpression in numerous cancers. The S-adenosyl-L-homocysteine hydrolase inhibitor DZNep has been shown to induce apoptosis and hinder metastasis by targeting 15
22 the EZH2 in chondrosarcoma. (Girard et al., 2014). EPZ is another EZH2 inhibitor that successfully lower levels of H3K27 methylation in lymphoma cells. A more advanced inhibitor EPZ-6438 can effectively halt tumour cell proliferation in vivo using a Hodgkin lymphoma xenograft model (Knutson et al., 2014). AZ505 is a selective inhibitor of SMYD2 that binds to its peptide binding pocket (Ferguson et al., 2011). A recent study developed A-893 based on the structure of AZ50. It is shown to be more potent due to the additional hydroxyl group that allows hydrogen bond formation with lysine (Sweis et al., 2015). LLY-507 is another SMYD2 inhibitor reported to inhibit growth of several cancer cell lines such as breast and liver cancer cell lines (H. Nguyen et al., 2015). The G9a inhibitors BIX and UNC0638 are both effective in decreasing H3K9 methylation under in vitro settings (Kubicek et al., 2007; Vedadi et al., 2011). Recently, a peptide-competitive inhibitor of G9a, named A-366 was developed. It was shown to be highly effective in leukemic cell lines (Pappano et al., 2015). Lastly, SET7/9 was known to methylate H3K4 and the estrogen receptor. Cyproheptadin was recently identified as an inhibitor of the SET7/9 domain that regulates estrogen dependent transcription in breast cancer (Takemoto et al., 2016). Histone demethylase inhibitors KDM inhibitors can be classified into two classes: the amine oxidases like histone demethylases (Lysine-specific demethylases LSD1/2) and the iron and α-ketoglutarate dependent jumonji C (JmjC) domain containing demethylase (Højfeldt et al., 2013). There is a growing interest in discovery of compounds that inhibits LSD1. Inhibition using Phenelzine, Tranylcypromine and Pargyline was observed to reduce multiplication rate of lung and prostate carcinoma cells (Prusevich et al., 2014). Pargyline has been shown to prevent epithelial to mesenchymal transition of prostate cancer cell lines and the process of becoming androgen resistant (M. Wang et al., 2015). HCI-2509, a LSD1 inhibitor can effectively reduce levels of c- MYC in prostate cancer cell lines and can possibly be used to treat docetaxel resistant prostate cancer (Gupta et al., 2016). Two new inhibitors based on the structure of tranylcypromine ORY and GSK are currently undergoing clinical trials (Zheng et al., 2016). SAHA (vorinostat) is a hydroxamic acid derivative that inhibits KDM4E (Rose et al., 2008). N- oxalylglycine (NOG) is a JmjC KDM inhibitor that relate to α-ketoglutarate and was initially 16
23 found to inhibit prolyl hydroxylase (Hopkinson et al., 2013). Furthermore, screening performed on 8-hydroxyquinoline derivatives led to discovery of IOX1, an inhibitor of many KDMs (Hopkinson et al., 2013). Several flavonoids have been identified to have inhibit JmjC KDMs including myricetin, epigallocatechin gallate and caffeic acid (McAllister et al., 2016). Histone acetyltransferase inhibitors In silico screening have identified the compound C646 as a competitive inhibitor of p300. C646 can cause cell cycle arrest and apoptosis in AML1-ETO-positive AML (X.-N. Gao et al., 2013). The natural compound, anacardic acid, was shown to inhibit a member of the MYST family and therefore, became a structure analogue for many followed up studies (Sun, Jiang, Chen, & Price, 2006). For example, one of the studies identified 6-alkylsalicylates as a selective Tip60 inhibitor (Ghizzoni et al., 2012). Recently, two pyridoisothiazolone derivatives PU139 and PU141 was reported to inhibit Gcn5, PCAF, CBP and p300. Furthermore, efficacy of these drugs was also proven in xenografts models where they were able to decrease acetylation (Gajer et al., 2015). Histone deacetylase inhibitors Currently, the HDACi Vorinostat, Belinostat and Romidepsin have been approved for clinical use by the FDA (P. A. Jones et al., 2016). Other hydroxamic acid derivatives including Abexinostat, Pracinostat, Resminostat, Givinostat, Panobinostat, Quisinostat are still undergoing through developmental phases. Abexinostat is an anticancer compound in preclinical study. Phase 1 clinical trial has demonstrated its benefit to patients with follicular lymphoma and phase 2/3 trail have found that it was well tolerated and effective (Evens et al., 2016; Morschhauser et al., 2015). Pracinostat is another HDACi that was well-tolerated with modest activity in patients with myelofibrosis and has been approved by the FDA in combination with Azacitidine to treat AML (Quintás-Cardama et al., 2012). Furthermore, Resminostat wes also reported to be well tolerated in patients with Hodgkin lymphoma and was recently used in combination with sorafinib to benefit hepatocellular carcinoma patients (Bitzer et al., 2016). Givinostat is used to treat Hodgkin lymphoma and multiple myeloma and can be used in combination with hydroxycarbamide to treat polycythemia vera (Finazzi et al., 2013). Panobinostat is approved by the FDA to treat multiple 17
24 melanoma. It can be taken with bortezomib and dexamethasone to improve melanoma patient outcome. Quisinostat is an oral hydroxamate inhibitor that has anti-tumour activity and has been reported to benefit patients with relapsed or refractory cutaneous T cell lymphoma and is now undergoing phase 2 clinical trial (Child et al., 2016; Venugopal et al., 2013). CUDC-907 is a compound against both HDAC and PI3K and is effective in treating patients with multiple melanoma and relapse or refractory lymphoma (Younes et al., 2016). CUDC-101 is another multiinhibitor again HDAC, HER2 and EGFR has recently been studied in patients with head and neck squamous carcinoma in a phase 1 study (Galloway et al., 2015) Chemical probes In addition to all of the epigenetic drugs mentioned above, we now have access to epigenetic chemical probes with higher efficacy, efficiency and lower toxicity. Chemical probes are small molecules that can penetrate cells and selectively inhibit enzymes responsible for chromatin regulatory marks, allowing users to address mechanistic and phenotypic questions about their targets in biochemical studies (Arrowsmith et al., 2015). More excitingly, chemical probes were designed following the pharmacological proof of concept that is necessary for the translation of these drug-like molecules into cancer therapy. They also complement genetic approaches for target validation such as CRISPR and RNA interference with unique advantages. First of all, they can rapidly and reversibly inhibit protein functions in a time and dose-dependent manner in many different cell types. Since most protein targets function as part of larger a complex, knockdown or knockout experiments can lead to disruption of the entire complex, which does not always phenocopy inhibition of a single enzymatic or biochemical function. Secondly, chemical probes closely resemble actual therapeutic drugs and can provide insight of druggability of the target for future drug development. Lastly, chemical probes can be tested in combination with other agents, which, enables more sophisticated and complex studies to be conducted in search of new therapeutic targeting strategies. Because of all these qualities, chemical probes have the potential as treatment for more aggressive cancers such as triple-negative breast cancer, which will be discussed in the next chapter. Here, several chemical probes used in this study and their targets will be discussed below. 18
25 Bromodomain (BRD) inhibitors Bromodomains are evolutionary conserved modules that enables protein-protein interaction by the recognition of acetylated lysine containing sequences in histone or nuclear proteins. They regulate gene expressions by acting as scaffolds to facilitate protein complexes assembly or as transcription factors and co-regulators. BRD containing proteins can engage in chromatin modifications by operating as methyltransferases, ATP-dependent chromatinremodeling complexes, HATs and helicases. They were also reported to have different expression levels in tissues, suggesting tissue specificity (Fujisawa & Filippakopoulos, 2017). BAZ2A/2B inhibitors The bromodomain adjacent to zinc finger domain (BAZ) is a family of ubiquitously expressed proteins containing BAZ1A, BAZ1B, BAZ2A and BAZ2B (M. H. Jones, Hamana, Nezu, & Shimane, 2000). BAZ2A is a component of the nucleolar remodeling complex (NoRC), a member of the imitation switch chromatin remodeling complexes (ISWI). NoRC is known to regulate noncoding RNA expression and formation of repressive heterochromatin at centromeres and telomeres (Mayer, Neubert, & Grummt, 2008; Strohner et al., 2001). Overexpression of BAZ2A was observed to correlated with prostate cancer recurrence and metastatic potential (Gu et al., 2015). In comparison, BAZ2B is not as well studied but was proposed to regulate nucleosome mobilization through ISWI. Overexpression of BAZ2B can negatively affect pediatric B cell acute lymphoblastic leukemia patient outcome (Drouin et al., 2015). GSK2801 is an acetyl-lysine competitive inhibitor of BAZ2A/2B by direct binding and BAZ2-ICR uses intramolecular stacking interaction to efficiently occupy the shallow BRD pocket of BAZ2A/2B (P. Chen et al., 2016; Drouin et al., 2015). BRD7/9 inhibitors BRD7 and BRD9 are members of the SWI/SNF nucleosome remodeling complex BAF and PBAF (Kadoch et al., 2013; Middeljans et al., 2012). Together, BRD7 and BRD9 are crucial in monitoring gene expression. BRD7 was discovered as a tumour suppressor through regulation of p53 and PI3K and its expression is often downregulated in cancer. BRD7 polymorphism was also 19
26 shown to increase risk of developing pancreatic cancer. Similarly, BRD9 was found either mutated or differentially expressed in various cancers (C.-L. Chen et al., 2016; Cleary et al., 2013; Huang, Chen, Pan, Yao, & Ma, 2015; Liu et al., 2016). LP99 and BI9564 are inhibitors that show high affinity to BRD9 and moderate activity to BRD7 while TP472 has high affinity to both (Clark et al., 2015). A BRD9 specific inhibitor was also developed, known as I-BRD9. BRPF inhibitors The bromodomain-phd finger protein (BRPF) supports complex assembly of the MYST-family histone acetyltransferases, which have essential roles in DNA replication, repair and transcriptional activation (Carlson & Glass, 2014). BRPF1 is a component of the monocytic leukemic zinc finger complex in which its translocation is associated with aggressive myeloid leukemia (T. Brown, Swansbury, & Taj, 2012). BRPF2, also known as BRD1 is important in embryotic development and was reported to play a role in embryonic stem cell differentiation via H3K14ac. Compare to BRPF1/2, function of BRPF3 is not well characterized. It was recently discovered that BRPF3 can form a complex with HBO1 that acetylates H3K14 and activate DNA replication origin. Proper regulation of replication initiation is important to preserve genome integrity and prevent tumorigenesis (Feng et al., 2016). Chemical probes NI-57 and OF-1 target the entire BRPF family (BRAF1/2/3) and probe PFI-4 targets a specific family member, BRPF1B. In addition, BAY-299 is a dual inhibitor of the BRPF family member BRPF2 and the TATA box binding protein-associated factor TAF1 and TAF1L (Bouché et al., 2017). BET family inhibitors There are four proteins in the BET family: BRD2/3/4, which are ubiquitously expressed and BRDT, which is only expressed in testis. BRD2 and BRD4 are responsible for recruiting the positive transcription elongation factor complex (P-TEFb) to acetylated chromatin via its BRDs during transcriptional elongation (Z. Yang et al., 2005). In terms of diseases, BRD2 plays a role in rheumatoid arthritis and BRD4 is associated with immune diseases through the NF-κβ dependent genes (Prinjha, Witherington, & Lee, 2012). Furthermore, the BET family proteins are highly involved in cancer. They have been shown to directly alter expression of cancer related genes such as MYC. They also contribute to pathogenesis of leukemia and virus associated cancers such as 20
27 Kaposi sarcoma and cervical cancers (Barbieri, Cannizzaro, & Dawson, 2013; Belkina & Denis, 2012; Josling, Selvarajah, Petter, & Duffy, 2012; Muller, Filippakopoulos, & Knapp, 2011). Due to this, BET proteins are seen as potential targets to prevent cancerous cell growth and have been studied in several cell lines and animal models (Delmore et al., 2011; Garcia et al., 2016; Mertz et al., 2011; Puissant et al., 2013). JQ1 inhibits BET proteins by displacing them from the acetylated lysine residues. It was shown to inhibit BRD4 function by decreasing expression of translocated c-myc, leading to cell cycle arrest and apoptosis in leukemia, multiple myeloma, lymphoma and neuroblastoma cells (Delmore et al., 2011; Mertz et al., 2011; Puissant et al., 2013). It was also shown that treatment of JQ1 on nuclear protein in testis midline carcinoma that has a translocation of BRD3 and BRD4 resulted in G1 cell cycle arrest, differentiation and apoptosis (Filippakopoulos et al., 2010). Another BET family inhibitor, PFI-1, can also block BET protein activities by binding to the acetylated lysine binding site of BRD2 and BRD4. PFI-1 can hinder proliferation of leukemic cell lines. Exposure of sensitive cell lines to PFI-1 also lead to cell cycle arrest, differentiation, apoptosis and downregulation of MYC expression (Picaud et al., 2013). CBP/p300 inhibitors The lysine acetyltransferases CBP and p300 share high sequence similarity, especially in their bromodomains (Hay DA, 2014). They are transcriptional co-activators essential in numerous biological processes including DNA replication, repair and cell proliferation. CBP and p300 are associated with Rubinstein-Taybi syndrome (RTS) and chromosomal translocation of CBP/p300 with MOZ was observed in acute myeloid leukemia (Kitabayashi et al., 2001; Wincent et al., 2016). CBP has also been associated with neurodegenerative diseases such as Amyotrophic Lateral Sclerosis, Alzheimer's and poly glutamine repeat diseases such as Huntington s disease (Valor, Viosca, Lopez-Atalaya, & Barco, 2013). SGC-CBP30 is an inhibitor of both CBP and BRD4 and I-CBP112 is a CBP/p300 selective inhibitor against their BRDs. 21
28 PCAF inhibitors The P300/CBP-associated factor (PCAF/KAT2B) regulates expression of several essential genes such as INS (Preproinsulin precursor of insulin) and transcription factors such as p53, FOX1 and p27 by acetylation of H3 and H4 (Ge, Jin, Zhang, Yan, & Zhai, 2009; J.-Y. Kim et al., 2012; Pérez-Luna et al., 2012; Schiltz et al., 1999). It is involved in diseases such as glioblastoma, medulloblastoma, neuro-inflaµmation and HIV (Humphreys et al., 2017; Malatesta et al., 2013). In fact, there are PCAF inhibitors developed to prevent interaction of the CAF bromodomain and acetylated HIV Tat protein as HIV treatment (Mujtaba et al., 2002). Several PCAF inhibitors have been developed for cancer but have not yet been clinically tested. L-moses is a new inhibitor of the PCAF by displacing it from H3.3 that requires investigation (Moustakim et al., 2017). SMARCA/PB1 inhibitors The last group of BRD is the Polybromodomian protein 1 (PB1) and a SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A2 and A4 (SMARCA2, SMARCA4). It was shown that the 6 BRDs in PB1 are frequently mutated in cancer. Loss of function of SMARCA4 and other alteration of the SWI/SNF chromatin remodeling complex is also linked to a number of cancers. PFI-3 is developed as a highly selective inhibitor of SMARCA2, SMARCA4 and PB1 over other BRD inhibitors (Brownlee, Chambers, Oliver, & Downs, 2012) Methyltransferase (MT) inhibitors PRMT inhibitors To date, 9 ubiquitously expressed mammalian protein arginine methyltransferases (PRMT1/2/3/4/5/6/7/8/9) in charge of cellular processes such as proliferation and differentiation have been identified. Overexpression of these PRMTs have been reported in a variety of cancers. (Kaniskan, Konze, & Jin, 2015; Wei, Mundade, Lange, & Lu, 2014; Y. Yang & Bedford, 2013). PRMT1 is the major asymmetric methyltransferase that deposit the ω-n G,N G -asymmetric dimethylarginine mark on histone H4 at arginine 3, which is associated with transcriptional 22
29 activation. Upregulation of PRMT1 expression has been observed in breast, prostate, lung, colon, bladder cancer and leukemia (Y. Yang & Bedford, 2013). PRMT2 has less methyltransferase activity on H4 and interacts with numerous nuclear receptors independent of the ligand. It was shown that in tumours, the mrna expression of PRMT2 correlates with the ER positive status (Zhong et al., 2012). PRMT3 has multiple substrates that can be involved in cancer such as the von Hippel Lindau tumour suppressor protein and DAL1 tumour suppressor protein. SGC707 is a noncompetitive (allosteric) inhibitor to both the S-(5 -adenosyl)-l-methionine and peptide substrate of PRMT3 and MX1 is its negative control probe (Y. Yang & Bedford, 2013). PRMT4 (also known as CARM1) is responsible for the asymmetric dimethylation of H3R17 and H3R26 and regulates a number of important cellular processes including DNA damage response, cell cycle and mrna splicing by methylating histones, transcriptional factors, coregulators, splicing factors and RNA polymerase II (Cheng, Côté, Shaaban, & Bedford, 2007; El Messaoudi et al., 2006; Y.-H. Lee & Stallcup, 2011). It can also methylates non-histone proteins involved in cell cycle regulation such as p300 (Y.-H. Lee & Stallcup, 2011). PRMT4 overexpression can activate oncogenic pathways including FOS, E2F1, WNT β-catenin and AIB1 and create a favorable microenvironment for tumour progression (Y. Yang & Bedford, 2013). It is also shown to be elevated in castration-resistant prostate cancer and breast tumours (El Messaoudi et al., 2006; H. Hong et al., 2004). A selective inhibitor of PRMT4, TP064 was developed and TP064N is the negative control but detailed reports have not yet been released. PRMT5 is the major type II syµmetric arginine methyltransferase that can deposit repressive histone marks H3R8me2s and H4R3me2s. It was shown to have the ability to repress tumour suppressors. For example, PRMT5 overexpression was observed to correlate with reduced expression of ST7, NM23 and RB family tumour suppressors. GSK591 is a SAM uncompetitive, peptide competitive inhibitor for the PRMT5-MEP50 complex and SGC2096 is its negative control probe. PRMT6s primary target is H3 (generating H3R2me2a), and DNA polymerase β (Y. Yang & Bedford, 2013). It was observed to be overexpressed in bladder and lung cancer cells (Yoshimatsu et al., 2011). MS049 is a non-competitive inhibitor of both PRMT4 and PRMT6 by occupying their substrate binding sites. 23
30 PRMT7 is responsible for H4R3me2 deposition on male germline imprinted gene via CTCFL interaction. Interestingly, in metastasizing breast cancers, the PRMT7 gene is located genomic regions containing high copy number aberrations. PRMT8 is brain specific and plasma membrane associated. It is the most mutated PRMT out of all in cancer genomes. Lastly, PRMT9 has two putative AdoMet-binding motifs. Currently, there are no probes against PRMT9. The chemical probe MS023 is a type I PRMT inhibitor that targets Type 1 PRMT: PRMT1/2/3/6/8 and CARM1 and MS094 is its negative control (Y. Yang & Bedford, 2013). SETD7 inhibitors SET domain containing 7 lysine methyltransferase (SETD7) was characterized as a mono-methyltransferase of H3K4me1 (J. Lee, Sayegh, Daniel, Clarke, & Bedford, 2005; H. Wang et al., 2001). It was shown to target a range of transcription factors in vitro, such as TAF10, p53, ER, p65, STAT3, Rb, Mypt, Tat, and Foxo3 (Barsyte-Lovejoy et al., 2014). Furthermore, SETD7 interacts with Pdx1 in pancreatic β cells and regulates DNMT1 in a brain tissue specific manner (Deering, Ogihara, Trace, Maier, & Mirmira, 2009; J. Wang et al., 2009). This diversity of SETD7 substrates suggested its importance in metabolism, inflammation and cancer. (R)-PFI-2 is a potent and selective competitive inhibitor of SETD7 by occupying the substrate binding site. It can modulate the Hippo pathway by SETD7 inhibition (Barsyte-Lovejoy et al., 2014). Its negative control probe is (S)-PFI-2. EZH2/1 inhibitors EZH2 is a subunit of the Polycomb repressive complex 2 (PRC2) that methylates H3K27 to induce transcriptional silencing (Di Croce & Helin, 2013). Overexpression of EZH2 has been linked to poor progression in prostate, breast, bladder, endometrial cancer and melanoma (K. H. Kim & Roberts, 2016). EZH2 inhibitors are normally designed to compete with the cofactor S- adenosyl-l-methionine (SAM). UNC1999 was the first orally available EZH2 inhibitor that targets EZH2 and its Y641 mutant, successfully reducing H3K27me3. UNC1999 also targets EZH1, with only 10-fold less potency compared to EZH2 as they share 96% sequence similarity in their catalytic domains 24
31 (Konze et al., 2013). UNC1999 was competitive with the cofactor SAM and non-competitive with histone 3 substrates. Its negative control is UNC2400, which is >1000-fold less active than UNC1999. GSK126, another EZH2 inhibitor, is >1000-fold more selective for EZH2 compare to other methyltransferases and >150-fold compared to EZH1 (McCabe et al., 2012; Verma et al., 2012). GSK343 is also an EZH2 inhibitor that prevents tri-methylation of H3K27 and is >1000- fold more selective towards EZH2 than other methyltransferases and >60-fold compare to for EZH1. DOT1L inhibitors Disruptor of telomeric silencing 1-like protein (DOT1L) is the only histone lysine methyltransferase confirmed lacking a SET domain while most other lysine methyltransferases contain conserved SET domains. DOT1L has a structure more similar to PRMTs. It methylates lysine 79 on histone 3 (H3K79), a mark responsible for active chromatin and transcriptional elongation (A. T. Nguyen & Zhang, 2011). Furthermore, aberrant expression of DOT1L has been associated with mix-lineage leukemia (MLL), an aggressive form of leukemia that accounts for 70% of infant leukemias (Daigle et al., 2013). Therefore, DOT1L inhibitors were initially developed to treat MLL. SGC0946 is a selective probe towards DOT1L and SGC 0649 is the negative control probe (Yu et al., 2012). SUV420H1/H2 inhibitors A-196 is a substrate-competitive inhibitor that inhibits the activity of two highly homologous methyltransferases: SUV420H1 and SUV420H2, targeting H4K20 methylation. Past studies suggested the role of SUV420 in DNA damage response by facilitating non-homologousend-joining through di- and tri-methylation on H4K20 (Bromberg et al., 2017). Loss of SUV420 can result in disruption of genome integrity leading to decrease proliferation, cell cycle dysregulation and telomere elongation (H. Wu et al., 2013). A-197 and SGC2043 are two negative controls for A
32 SYMD2 inhibitors SET and MYND domain containing protein 2 (SMYD2) can methylate both histone and non-histone proteins such as TP53 and RB1. SMYD2 can promote transcriptional activation by methylating histone H3 lysine 4 (H3K4me) and histone H3 lysine 36 (H3K36me2) (M. A. Brown, Sims, Gottlieb, & Tucker, 2006; Cho et al., 2012; Komatsu et al., 2009). SMYD2 has been proposed as a potential target in cancer since it was observed overexpressed in esophageal, bladder, gastric cancers and leukemia (Eggert et al., 2016). Furthermore, these studies found SYMD2 expression also correlate with poor survival rate (Komatsu et al., 2009). BAY598 is a SYMD2 peptide-competitive probe for SMYD2 and BAY369 is its negative inhibitor Methyl Lysine Binders Methyl lysine binders (MLB) are proteins with domains that bind methylation marks and convey the methylation signals downstream (Yun, Wu, Workman, & Li, 2011). These MLBs are either equipped with catalytic functions or the ability to recruit other protein complexes (Kouzarides, 2007; Taverna, Li, Ruthenburg, Allis, & Patel, 2007). Major families of the MLB include the malignant brain tumor (MBT) domains, chromatin organization modifier domains (chromodomains), plant homeodomain (PHD) fingers, Agenet domains, WD40 repeat domains, Tudor domains, and the proline-tryptophan-tryptophan-proline domains (Maurer-Stroh et al., 2003). L3MBTL3 MBT domains recognize mono- and di- methylated lysine at H3 and H4 that is associated with repression of gene expression. Their misregulation can result in diseases. For instance, homologous deletion of SCML2, L3MBTL2 and L3MBTL3 are found in meduloblastoma and MBTD1, L3MBTL1 and L3MBTL3 are involved in hematopoiesis (James et al., 2013; Northcott et al., 2009). UNC1215 is an inhibitor of L3MBT3 that competitively displace mono or dimethylysine peptides by binding to its Kme pocket. The negative control probe UNC1079 is >1000-fold weaker compared to UNC1215 (Milosevich & Hof, 2016). 26
33 EED inhibitor The embryonic ectoderm development (EED) protein is another important core subunit of PRC2 that can bind to H3K27me3 and support EZH2 conformation. EED mediated PRC2 H3K27 trimethylation can stimulate methyltransferase activity of PRC2 and propagate H3K27me3 and gene silencing (Y. He et al., 2017). Moreover, EED expression has been shown to increase TGF-βdependent expression of EMT-associated genes (Oktyabri, Tange, Terashima, Ishimura, & Suzuki, 2014). A-395 is a potent inhibitor of EED by binding to its H3K27me3 binding pocket and therefore, also hinders activity of PRC2. A-395N is the inactive EED analog used as a negative control probe Histone lysine demethylases To date, two families of histone lysine demethylases (KDM): the flavin-dependent lysinespecific demethylases (LSDs or KDM1s) and the larger family of 2-oxoglutarate (2OG)-, ferrous iron-, and oxygen-dependent demethylases (JMJC KDMs) have been identified (Klose RJ, 2006). Both families were shown to catalyze N-methyl-lysine demethylation through oxidative mechanisms (Walport, Hopkinson, & Schofield, 2012). LSD1 inhibitors Lysine-specific histone demethylase 1 (LSD1) is responsible for demethylating H3K4 and was observed to be overexpressed in many cancers such as breast, prostate, lung cancer and leukemia (Mohammad HP, 2015). It is responsible to maintain pluripotency in embryonic stem cells and normal hematopoiesis (Adamo et al., 2011; Sprüssel et al., 2012). Studies that knocking down LSD1 in lung and bladder cancer cells showed decreased cell proliferation (Lv et al., 2012). Furthermore, an upregulation of LSD1 expression lung cancer cells was observed to negatively affect H3K9 acetylation, Twist1 and N-cadherin expression, and positively affect E-cadherin expression, suggesting an increase of invasive and metastasize potential (Lv et al., 2012). In acute myeloid leukemia, LSD1 expression was highest in less differentiated subtypes (Mohammad & Kruger, 2016). GSK-LSD1 is an irreversible and orally available inhibitor of LSD1 that is 27
34 currently in clinical trials as a treatment of refractory small cell lung carcinoma and acute myeloid leukemia (Bennesch, Segala, Wider, & Picard, 2016). JMJD3/ KDM6B, UTX/KDM6A, JARID1B/KDM5B The demethylases subfamily (JMJD3/KDM6B and UTX/KDM6A) can activate transcription by removal of H3K27 di- and tri- methyl groups (Kouzarides, 2007). UTX is ubiquitously expressed while JMJD3 is expressed upon stimulation by viruses, inflaµmation and oncogenes (Arcipowski, Martinez, & Ntziachristos, 2016). They share 84% sequence similarity in their JmjC domain (S. Hong et al., 2007). JMJ3D expression can be altered by Epstein-Barr viruses, which is associated with Burkitt's lymphoma, Hodgkin's lymphoma and post-transplant lymphoma (Geng & Wang, 2015). It is also linked to essential biological processes including stem cell differentiation, macrophage activities and senescence through the INK4A-ARF locus (Williams et al., 2014). UTX is bound to genes involved in cell cycle centered on the tumour suppressor gene RB and RB-binding proteins, therefore, is implicated in cancer when mutated (J. K. Wang et al., 2010). Studies have shown that both JMJD3 and UTX are required development and differentiation and are involved with development regulators such as HOX genes (Agger et al., 2007). Differently, as a demethylase, JARID1B is capable of demethylating H3K4 and plays a role in the transcriptional repression of some tumor suppressor genes and may also be involved in genomic stability and DNA repair. Its upregulated in certain cancer cells. GSKJ4 is a selective chemical probe that targets the jumonji demethylases as a competitive inhibitor of their cofactors α-ketoglutarate and Fe 2+ but not the substrate (Kruidenier et al., 2012). It was proposed as an anti-cancer agent due to its effect against lymphoblastic leukemia and pediatric brainstem glioma (Ntziachristos et al., 2014). More recently, GSKJ4 was reported to also target the JARID1B, which is responsible for the demethylation of H3K4me2 and me3 (Heinemann et al., 2014). 28
35 Arginine deiminases PAD-4 Protein Arginine Deiminase 4 (PAD4) is a calcium dependent enzyme responsible to transform protein arginine into citrulline, a process called citrullination or deamination (Bicker & Thompson, 2013). PAD4 regulates histone arginine methylation at targets various sites on histone H3 and H4 by converting methyl-arg to citrulline. Furthermore, PAD4 is strongly associated with immune and inflammatory responses and therefore, is associated with diseases such as Rheumatoid Arthritis, and multiple sclerosis. In cancer, it was found overexpressed only in malignant tumours and may contribute to tumour progression (Chang et al., 2009). GSK484 is an inhibitor of PAD4 by binding to the low-calcuium form of PAD4 and compete with its substrate. It was shown to inhibit both cellular citrulination in neutrophils and neutrophil extracellular trap formation (Lewis et al., 2015) WD40 WDR5 WD40 repeat-containing protein 5 (WDR5) is a highly-conserved protein that is part of the SET/MLL histone methyltransferase complexes. It was shown that p30, the most coµmon form of CEBPA gene mutation in acute myeloid leukemia, preferentially interacts with WDR5. Past studies have also shown that p30 binds to genomic regions containing MLL dependent H3K4me3 marks. In p30-dependent AML models, down-regulation of WDR5 inhibits proliferation and promote differentiation. OICR-9429 is an antagonist of the WDR5-MLL interaction by competitively binding to WDR5, therefore, was proposed as a treatment for CEBPA gene mutated AML (Grebien et al., 2015) Combination therapy Cancer is a very heterogeneous disease which can potentially be tackled via combination therapy that simultaneously target multiple oncogenic targets is of current interest. Only a few monotherapies such as the tyrosine kinase inhibitors for gastrointestinal stromal tumour (GIST) or 29
36 chronic myeloid leukaemia (CML) patients are effective. Moreover, epidermal growth factor receptor (EGFR) and serine/threonine-protein kinase BRAF inhibitors in cancers harboring specific mutations was also shown to rapidly decrease tumour size. Unfortunately, this response is often followed by the development of drug resistance. Previous work has also introduced the concept that majority of cancers respond better to combination therapy (DeVita & DeVita- Raeburn, 2015). For example, novel combination treatment using epigenetic drugs (primarily HDACi) with other cancer therapeutics such as radiotherapy, hormonal therapy, kinase inhibitors, DNA-damaging chemotherapeutic agents, and DNA methyltransferase inhibitors are under investigation (Thurn KT, 2011). To our knowledge, the low toxicity of chemical probes made them a group of ideal candidates for combination therapy to improve patient outcome (Thurn, Thomas, Moore, & Munster, 2011). In fact, it is very likely that the future of epigenetic therapy, especially in solid tumours, would be dependent on combination therapy utilizing different epigenetic therapy or combination of these treatment with existing cancer treatments such as chemotherapy and immunotherapy. Therefore, more attention has been paid to the potential of chemical probes in the clinic. 30
37 Chapter 2 Exploring epigenetic vulnerabilities of Triple-Negative Breast Cancer TNBC is the most aggressive form of breast cancer that tend to occur in younger and premenopausal women with African American ancestry compared to other subtypes (Morris et al., 2007). Patients diagnosed with TNBC have the poorest survival rate due to the aggressiveness of the disease and the lack of targeted therapy. Furthermore, patients with TNBC have a greater likelihood of distant recurrence and death within five years of diagnosis (Dent et al., 2007). Currently, the primary established treatment for TNBC patients in both early and advanced stages of the disease is chemotherapy such as taxol, doxorubicin and/or platinum agents. Although TNBC have a higher response to chemotherapy in comparison to other breast cancer subtypes, intrinsic or acquired clinical drug resistance often develops, favoring tumour progression and decreasing patient survival. Therefore, major effort was fostered to discover curative therapeutic alternatives against TNBC. Epigenetic modifications is a labile process that provides opportunities for therapeutic intervention to restore cancer epigenomes. Although increasing research has begun to recognize the importance of epigenetics in TNBC progression, majority of these studies have focused on DNA methylation and mainly on other breast cancer subtypes (Clare Stirzaker, 2016). A more recent shrna screen conducted on breast cancer cell lines revealed essential epigenetic pathways specific to TNBC (Marcotte et al., 2016). This suggests that targeting these epigenetic pathways can be a potential treatment for TNBC. Past studies have recognized breast cancer cell lines as reasonable models to identify essential genes by genomic and proteomic analyses (Marcotte et al., 2016). Based on this, we treated 16 breast cancer cell lines with our panel of 51 epigenetic chemical probes targeting domains of epigenetic enzymes to better understand their essentiality in breast cancer, mainly TNBC, cell proliferation. Currently, many epigenetic drugs such as DNMT and HDAC inhibitors 31
38 have been showing promising results in the clinic on other cancers (mentioned in chapter 1). Therefore, this project aims to explore and elucidate altered tumorigenic epigenetic processes that are potentially druggable in TNBC as therapeutic targets to better patient outcome. 2.1 Results In this study, we investigated the proliferation outcome of epigenetic alterations using 51 epigenetic chemical probes on 14 TNBC cell lines (CAL120, HS578t, HCC38, HCC3153, MDA- MB231, BT549, MDA-MB436, MX1, SUM159, MDA-MB157, HCC1143, MDA-MB468 and SUM149 HCC1806). In addition, we also investigated the response of 1 HER2 (OCUBM) and 1 Luminal B (MCF7) cell line to assess epigenetic profiles of non-tnbc cell lines (Figure 1). Effect of probes on proliferation was measured by percentage confluence of cells using the IncuCyte Zoom TM live cell imaging system and normalized to the control vehicle (DMSO). To support an on-target mechanism of action, comparatively inactive enantiomers were used. Possibility of false positives was avoided by considering probes that decreased confluence for over 10% as a real effect. Chemical probes that negatively affected the breast cancer cell lines will be mentioned here. 32
39 Table 1: Epigenetic chemical probes and their targets Probe Protein family target Specific targets BAZ2-ICR Bromodomain BAZ2A/B GSK2801 Bromodomain BAZ2A/B LP99 Bromodomain BRD9/BRD7 BI9564 Bromodomain BRD9/BRD7 TP472 Bromodomain BRD9/BRD7 IBRD9 Bromodomain BRD9 NI57 Bromodomain BRPF1/BRPF2/BRPF3 OF1 Bromodomain BRPF1/BRPF2/BRPF3 PFI-4 Bromodomain BRPF1B, isoform of BRPF1 BAY299 Bromodomain BRPF2 (also known as BRD1) SGC-CBP30 Bromodomain CREBBP/EP300 I-CBP112 Bromodomain CREBBP/EP300 PFI-1 Bromodomain BET family: BRD2/3/4/BRDT JQ1 Bromodomain BET family: BRD2/3/4/BRDT JQ1N neg ctrl for JQ1 PFI-3 Bromodomain SMARCAm PB1 L-moses bromodomain PCAF D-moses neg ctrl for L-moses 33
40 Probe Protein family target Specific targets MS023 Methyltransferase Type 1 PRMT: PRMT1/2/3/6/8, CARM1 MS094 neg ctrl for MS023 SGC707 Methyltransferase PRMT3 XY1 neg ctrl for SGC707 TP064 Methyltransferase PRMT4 TP064N neg ctrl for TP064 GSK591 Methyltransferase PRMT5 SGC2096 neg ctrl for GSK591 MS049 Methyltransferase PRMT4/PRMT6 R-PFI-2 Methyltransferase SETD7 S-PFI-2 neg ctrl for R-PFI-2 UNC1999 Methyltransferase EZH2/1 UNC2400 neg ctrl of UNC1999 GSK126 Methyltransferase EZH2 GSK343 Methyltransferase EZH2 UNC0642 Methyltransferase G9a/GLP A-366 Methyltransferase G9a/GLP SGC0946 Methyltransferase DOT1L SGC0649 neg ctrl for SGC
41 Probe Protein family target Specific targets A196 Methyltransferase SUV420H1/H2 A197 neg ctrl of A196 SGC2043 neg ctrl for A196 BAY598 Methyltransferase SYMD2 BAY369 neg ctrl for BAY598 UNC1215 Methyl Lysine Binder L3MBTL3 UNC1079 neg ctrl of UNC1215 A395 Methyl Lysine Binder EED A395N neg ctrl for A395 GSKLSD1 KDM LSD1 GSKJ4 KDM JMJD3, UTX, JARID1B GSKJ5 neg ctrl of GSKJ4 OICR9429 WD40 WDR5 GSK484 Arginine deiminases PAD-4 35
42 36
43 Figure 1 Effect of 51 epigenetic chemical probes on the confluence of 16 breast cancer cell lines This heatmap shows the hierarchical clustering of the effect of 51 epigenetic probes on the confluence of 16 breast cancer cell lines. All cell confluence in percentage is normalized to the control vehicle (DMSO), which has the value of 1 and has the colour white on the histogram. Growth that was better than DMSO (>1) after probe treatment has the colour blue and growth that is worse than DMSO (<1) has the colour red. Intensity of the colours is associate with strength of the effect. Rows correspond to epigenetic drugs (black: active probes, blue: inactive probes, red: DMSO) and columns correspond to cell lines (black: TNBC, red: luminal). Active probes are in the colour black and their inactive enantiomers are in the colour blue. Both luminal cell lines are highlighted in red Bromodomain inhibitors 16 bromodomain inhibitors were tested on the cells by directly adding them into the media (Figure 2). PFI-1, the inhibitor of the BET family: BRD2/3/4/BRDT decreased confluence of 10 TNBC cell lines HCC1806, SUM149, MDA-MB231, MX1, MDA-MB436, HCC3153, MDA-MB468, HCC38, HS578t, CAL120 for >30% and MDA-MB157 for ~10% at 5µM. It also decreased confluence of luminal B cell line MCF7 for over 30%. 1uM of PFI-1 decreased SUM149 confluence to 20% MDA-MB468 to 65% and MDA-MB436, HS578t, HCC3153 to only ~90%. JQ1 is another BET family inhibitor that showed drastic effect across all breast cancer cell lines. At 5µM, JQ1 decreased all cell line confluence for at least 40% except the HER2 breast cancer cell line OCUBM. At 1µM, JQ1 also notably decreased all cell line confluence for at least 20% and had no effect on OCUBM. The inactive enantiomer of JQ1, JQ1N decreased SUM149 confluence for ~80% at 5µM and 40% at 1µM. BAY299 is an inhibitor for BRPF2 (also known as BRD1) and TAF1. At 5µM it decreased confluence of all of the cell lines for >20% except OCUBM. At 1µM, it negatively affected MDA-MB436, HCC3153, HCC38, MDA-MB231, MX1, SUM149 and MCF7 confluence for >20%. OF1 is a BRPF1/BRPF2/BRPF3 inhibitor that decreased confluence of MDA-MB468, MX1, SUM159 and MCF7 for over 20% at 5µM and over 10% at 1µM. NI57 is also an inhibitor of BRPF1/BRPF2/BRPF3 and at 5µM it decreased confluence of MDA-MB436 and MCF7 for 10%, MX1 for 20% and SUM149 for 30%. It only decreased MX1 for 13% at 1µM. PFI-4 is an inhibitor 37
44 for BRPF1B. It decreased confluence of SUM149 and MCF7 for ~20% at 5µM and did not have any negative effect on cell lines at 1µM. TP472 is an inhibitor of both BRD9 and BRD7. Results showed that it decreased confluence of cell lines MDA-MB436, MDA-MB468, MX1, SUM149 for over 40% at 5µM but only <10% at 1µM. TP472 also decreased MCF7 confluence for 54% at 5µM. BI9564 is another BRD9/BRD7 inhibitor that decrease confluence of cell lines SUM149 for 13% at 5µM and MX1 for 17% at 1µM. LP99 is also a BRD9/7 inhibitor that only decrease MDA-MB436 confluence for 13% at 5µM. None of the luminal cell lines responded to BI9564 and LP99. IBRD9 is an inhibitor of BRD9. At 5µM it decreased confluence for the cell lines MDA-MB436, MDA-MB468 and SUM149 for >40% and MX1 for 16%. IBRD9 also decreased confluence of MCF7 cells for 51% at 5µM. It also decreased SUM149 confluence for 19% at 1µM. PFI-3 is a SMARCAm PB1 inhibitor that only decreased confluence of SUM149 for 70% and 18% at 5µM and 1µM respectively. Both SGC-CBP30 and I-CBP112 inhibit CREBBP/EP300. SGC-CBP30 decreased confluence of MDA-MB436, SUM149 and MCF7 for more than 40% and OCUBM for 10%. At 1µM, it affected MDA-MB436, SUM149 and MCF7. I-CBP112 significantly decreased SUM149 confluence for 65%, MDA-MB436, MCF7 for 15% and MDA- MB468 for 24%. It only decreased confluence for SUM149 for 34% at 1µM. BAZ2-ICR and GSK2801 are inhibitors of BAZ2A/B. They did not affect the majority of cell confluence at both 5µM and 1µM across our cell line panel. The only exception is MX1, where its confluence decreased for 14% under 1µM of BAZ2-ICR treatment. L-moses is the inhibitor for PCAF and it only had a slight effect on HS578 and SUM149 (~10%) at 5µM. D-moses, the inactive enantiomer of L-moses decreased MX1 and SUM149 confluence for 11% and 24% respectively at 5µM and MDA-MB231, SUM149 for 13% and 17% at 1µM. Taken together, this shows that probes such as PFI-1 and JQ1 that inhibit the BET family and BAY299 that inhibits the BRPF2 are most effective against all of the breast cancer cell lines on our panel. Probes that gave dramatic response at 5µM but has little or no response at 1µM such as TP472 and IBRD9 requires further dose and time assessment to ensure specificity. Most bromodomain inhibitors had no effect on the HER2 breast cancer cell line OCUBM. The TNBC 38
45 cell line SUM149 showed the greatest response to our panel of bromodomain inhibitors whereas BT549, SUM159PT, HCC1143 and MDA-MB157 had the least response. Response to PFI-4 (BRPF1B inhibitor) is specific for the luminal B cell line MCF7 only but not the TNBC and HER2. 39
46 40
47 Figure 2 Effect of 16 bromodomain inhibitors on the confluence of 16 breast cancer cell lines This heatmap shows the hierarchical clustering of the effect of 16 bromodomain inhibitors on the confluence of 16 breast cancer cell lines. For description of this heatmap, refer to figure Methyl transferase inhibitors 14 methyltransferase inhibitors and their negative control enantiomers were tested (Figure 3). UNC1999, GSK126 and GSK343 are all EZH2 inhibitors. UNC1999 decreased confluence of the TNBC cell lines BT549, MDA-MB436, MDA-MB468, MDA-MB231, SUM149 for at least 30% and the luminal B cell line MCF7 for 13% at 5µM. At 1µM, UNC1999 decreased confluence of HS578t, HCC3153, SUM149 and OCUBM for ~10%. UNC2400, the inactive negative control of UNC1999, only decreased the growth of SUM149 for 14% at 5µM. In addition, dose and time response was carried out to assess specificity of these probes on BT549, HS578t and MDA-MB436 to UNC1999 using 6 different concentrations (DMSO/0uM, 0.01uM, 0.1uM, 1uM, 5uM, 20uM). Result showed that response of these three cell lines to UNC1999 was dose and time dependent (Figure 4). Western blot was performed to assess efficacy of UNC1999 in decreasing the H3K27me3 mark on a sensitive and non-sensitive cell line, BT549 and HS578 respectively. Result showed a decrease of the H3K27me3 mark for both cell lines around 3 days and a completely lost of the mark on 7 days under 5µM of UNC1999 treatment. In comparison, UN2400, the negative control probe of UNC1999 that is <1000 less potent treated cells did not remove the H3K27me3 mark (Figure 5). GSK126 and GSK343 are also EZH2 inhibitors. At 5µM, GSK126 only decreased the confluence of MDA-MB468 for 72% and did not affect the other cell lines. It also decreased confluence of OCUBM for 13% at 1uM. Response of MDA-MB436, HS578 and BT549 to GSK126 is time and dose dependent (Figure 4). Western blot was also performed on these three cell lines after 5µM of GSK126 treatment. Result showed a decrease of the H3K27me3 mark after treating around 3 days and a complete loss of H3K27me3 mark on 7 days although GSK126 did not decrease cell confluence (Figure 5). GSK343 only decreased SUM149 confluence for ~40% but not the other cell lines at 5µM. It did not affect any of the cell lines at 1µM. Response to GSK343 is also dose and time dependent (Figure 4). Western blot was also performed on MDA- 41
48 MB436, HS578 and BT549 after 5µM of GSK343 treatment. Again, results also showed a decrease of the H3K27me3 mark after treating around 3 days and a complete loss of H3K27me3 mark on 7 days, even though GSK343 did not decrease confluence of cells (Figure 5). GSK591 is a PRMT5 inhibitor. It decreased confluence of cell lines BT549, MDA- MB436, HCC3153, HCC1143, HCC38, HCC1806 for 10~25% and MDA-MB468, MDA-MB231, MX1, SUM149, MCF7 for 40~60%. SUM159, MDA-MB157 and OCUBM did not respond to GSK591. SGC2096 is the negative control probe of GSK591 and it only had a negative effect on SUM149 at 1µM. MS023 is an inhibitor that targets all of the type I PRMTs (PRMT1/2/3/4/6/8). At 5µM, MS023 decreased confluence of cell lines MDA-MB436, MDA-MB468, MDA-MB231 and MX1 for >30% and SUM149 for 21%. It also decreased MDA-MB436, MDA-MB468 MDA- MB231 and MX1 confluence at 1µM. The negative control of MS023, MS094 only lower the confluence of SUM149 for 13% and not the other cell lines. SGC707 only decreased confluence of SUM149 for 10% at 5uM and MDA-MB436 for 14% at 1uM. The negative control probe XY1 only affected SUM149 for 12%. TP064 is a PRMT4 inhibitor. It decreased MDA-MB436 confluence for 18% and SUM149 for 11% at 5µM. It also decreased the confluence of these cell lines and MDA-MB468 for 15~20% after treatment with TP064 at 1uM. The inactive negative probe of TP064 is TP064N. Results showed that TP0064N decreased MDA-MB468 and SUM149 confluence (10~20%) at 5µM and SUM149 for 19% at 1µM. MS049 is inhibits both PRMT4 and PRMT6. It only decreased confluence of SUM149 for ~15% at both 5µM and 1µM. R-PFI-2 inhibits the SETD7 and S-PFI-2 is its negative control probe. Both of them decreased confluence of OCUBM at 5µM and 1µM where R-PFI-2 decreased its confluence for 13% S-PFI-2 decreased its confluence for 50%. UNC0642 is G9a/GLP inhibitors. It only decreased confluence for MDA-MB468 and SUM149 at 5µM for 13% and 17% respectively and did not show any effect at 1µM. A366 is another G9a/GLP inhibitor that only decreased CAL120 and HCC3153 confluence for only 10% and SUM149 confluence for 20%. It has no effect on any cell lines at 1uM. 42
49 SGC0946 targets DOT1L. Results showed that at 5uM, SGC0946 decreased confluence of MDA-MB468 and SUM149 for ~20%. SGC0649 is the negative control probe of SGC0946 and it did not have any effect across our breast cancer cell line panel. A196 inhibits SUV420H1/H2 and it decreased confluence of MDA-MB468 for 11% and SUM149 for 34% at 5µM. It only decreased SUM149 for 24% at 1µM. A197 is the negative control probe for A196 and at 5µM it decreased CAL120, HCC3153MDA-MB157, MX1, OCUBM confluence for 10%~15% and SUM149 for 26%. At 1µM it decreased HS578t confluence for 10%. Another control probe for A196, SGC2043 only negatively affected SUM149 confluence for 18% at 5µM. BAY598 is the inhibitor for SYMD2, it decreased MDA-MB468, SUM149 and OCUBM confluence for ~20% at 5µM and for 10% at 1µM. The negative control BAY369 decreased OCUBM confluence for 50% at 5µM and 20% at 1µM. It also decreased SUM149 confluence for ~20% at both 5µM and 1µM. In addition to direct methyltransferase inhibitors, probes that targets enzymes involved in methyl transferring processes were also included in this category. OICR9429 is not a direct inhibitor of methyltransferases but inhibits the WDR5 domain which takes part of the SET/MLL histone methyltransferase complexes. OICR9429 treatment decreased confluence of MDA-MB436 and SUM149 confluence for 12% and MCF7 for almost 30% at 5µM. At 1µM OICR9429 decreased MDA-MB436 for 10% and SUM149 confluence for 19%. GSK484 is also another chemical probe that does not directly target methyltransferase but PAD4, that regulates histone arginine methylation. It only decreased confluence of SUM149 for 14%. Among all the breast cancer cell lines, SUM159, HS578t, CAL120, HCC1143 and MDA- MB157 did not respond to most probes that target histone methylation. In comparison, SUM149 and MDAMB468 were sensitive to many methyltransferase inhibitors. 43
50 44
51 Figure 3 Effect of 14 methyltransferase inhibitors on the confluence 16 breast cancer cell lines This heatmap shows the hierarchical clustering of the effect of 14 methyltransferase inhibitors, 1 WDR5 and 1PAD4 inhibitor and their corresponding negative control probes on the confluence of 16 BC cell lines. For description of this heatmap, refer to figure 1. Figure 4 Growth effect of EZH2 inhibitors on three TNBC cell lines Time and dose dependent response of three TNBC cell lines: BT549, HS578 and MDA-MB436 treated with EZH2 inhibitors using 5 concentrations (0.01 µm, 0.1µM, 1µM, 5µM, 20µM, and DMSO). Effect of these inhibitors was measured by cell confluence. 45
52 A B Figure 5 Assessment of H3K27me3 mark after UNC1999 treatment on three TNBC cell lines MDA- MB436, HS578t and BT549 (A) Western blot detection of the H3K27 trimethylation levels after treatment of UNC1999 (5µM) for 3, 7 and 10 days performed on one sensitive cell line BT549 and one non-sensitive cell line HS578t at 5µM. Result showed a decrease of H3K27me3 mark around 3 days for cells treated with UNC1999 but not for ones treated with the inactive compound UNC2400. (B) Western blot detection of the H3K27 trimethylation mark on BT549, HS578t and MDA-MB436 after treatment of GSK126 and GSK343 at 5uM. Results showed a decrease of H3K27me3 around 3 days HS578t and around 7 days for BT549, MDA-MB436 after GSK126 treatment. HS578t, GSK343 showed slight decrease of H3K27me3 around 3 days and MDA- MB436 around 7 days. 46
53 2.1.3 Methyl-lysine binder inhibitors UNC1215 is the inhibitor that targets L3MBTL3 (Figure 6). It decreased OCUBM confluence for 31% at 5µM and OCUBM and SUM149 for 20% at 1µM. UNC1079 is the inactive negative control of UNC1215. At 5µM it decreased confluence of OCUBM for 32% and SUM149 for 56%. At 1µM it decreased OCUBM confluence for 28% and SUM149 for 42%. A395 is an EED inhibitor and A395N is its negative control. Treatment of A395 only affected MDA-MB468 but not the other cell lines. Furthermore, treatment of A395N resulted in decreased confluence of SUM149 for 12% at 5µM and 10% at 1µM. Most of the cell lines were insensitive to methyl-lysine binder inhibitors while OCUBM and SUM149 were sensitive to L3MBTL3 inhibition KDM inhibitors GSKLSD1 is an inhibitor of LSD1 (Figure 7). It only decreased confluence of SUM149 for 35% and 22% at 5 µm and 1 µm respectively. GSKJ4 is another lysine demethylase inhibitor that inhibits JMJD3, UTX and JARID1B. Cell lines BT549, CAL120, MDA-MB436, MDA- MB468, HCC3153, HCC38, HCC1806, MDA-MB231, MX1, SUM149 and MCF7 had drastic decrease in confluence to GSKJ4 for over 70%. In comparison, HS578t, HCC1143and MDA- MB157 had less response to GSKJ4, their confluence decreased for 30%~40% after treatment. Two cell lines: SUM159 and OCUBM did not respond to GSKJ4. GSKJ5 is the negative control of GSKJ4 and it only decreased confluence of OCUBM at 5uM for 33%. SUM159 did not respond to any of the KDM inhibitors while SUM149 was sensitive to both GSKJ4 and GSKLSD1. 47
54 Figure 6 Effect of 2 methyl-lysine binder inhibitors on the confluence of 16 breast cancer cell lines. This heatmap shows hierarchical clustering of the confluence after treatment of 2 methyl-lysine binder inhibitors UNC1215 and A395 and their corresponding negative controls UNC1079 and A395N. For description of the heatmap, refer to Figure 1 48
55 Figure 7 Effect of 2 lysine demethylase inhibitors on the confluence of 16 breast cancer cell lines. This heatmap shows hierarchical clustering of the confluence after treatment of 2 KDM inhibitors GSKJ4 and its negative control probe GSKJ5 and GSKLSD1. For description of the heatmap, refer to Figure 1 49
Gene Expression DNA RNA. Protein. Metabolites, stress, environment
 Gene Expression DNA RNA Protein Metabolites, stress, environment 1 EPIGENETICS The study of alterations in gene function that cannot be explained by changes in DNA sequence. Epigenetic gene regulatory
Gene Expression DNA RNA Protein Metabolites, stress, environment 1 EPIGENETICS The study of alterations in gene function that cannot be explained by changes in DNA sequence. Epigenetic gene regulatory
Epigenetics DNA methylation. Biosciences 741: Genomics Fall, 2013 Week 13. DNA Methylation
 Epigenetics DNA methylation Biosciences 741: Genomics Fall, 2013 Week 13 DNA Methylation Most methylated cytosines are found in the dinucleotide sequence CG, denoted mcpg. The restriction enzyme HpaII
Epigenetics DNA methylation Biosciences 741: Genomics Fall, 2013 Week 13 DNA Methylation Most methylated cytosines are found in the dinucleotide sequence CG, denoted mcpg. The restriction enzyme HpaII
Histones modifications and variants
 Histones modifications and variants Dr. Institute of Molecular Biology, Johannes Gutenberg University, Mainz www.imb.de Lecture Objectives 1. Chromatin structure and function Chromatin and cell state Nucleosome
Histones modifications and variants Dr. Institute of Molecular Biology, Johannes Gutenberg University, Mainz www.imb.de Lecture Objectives 1. Chromatin structure and function Chromatin and cell state Nucleosome
Lecture 8. Eukaryotic gene regulation: post translational modifications of histones
 Lecture 8 Eukaryotic gene regulation: post translational modifications of histones Recap.. Eukaryotic RNA polymerases Core promoter elements General transcription factors Enhancers and upstream activation
Lecture 8 Eukaryotic gene regulation: post translational modifications of histones Recap.. Eukaryotic RNA polymerases Core promoter elements General transcription factors Enhancers and upstream activation
Epigenetics. Lyle Armstrong. UJ Taylor & Francis Group. f'ci Garland Science NEW YORK AND LONDON
 ... Epigenetics Lyle Armstrong f'ci Garland Science UJ Taylor & Francis Group NEW YORK AND LONDON Contents CHAPTER 1 INTRODUCTION TO 3.2 CHROMATIN ARCHITECTURE 21 THE STUDY OF EPIGENETICS 1.1 THE CORE
... Epigenetics Lyle Armstrong f'ci Garland Science UJ Taylor & Francis Group NEW YORK AND LONDON Contents CHAPTER 1 INTRODUCTION TO 3.2 CHROMATIN ARCHITECTURE 21 THE STUDY OF EPIGENETICS 1.1 THE CORE
R. Piazza (MD, PhD), Dept. of Medicine and Surgery, University of Milano-Bicocca EPIGENETICS
 R. Piazza (MD, PhD), Dept. of Medicine and Surgery, University of Milano-Bicocca EPIGENETICS EPIGENETICS THE STUDY OF CHANGES IN GENE EXPRESSION THAT ARE POTENTIALLY HERITABLE AND THAT DO NOT ENTAIL A
R. Piazza (MD, PhD), Dept. of Medicine and Surgery, University of Milano-Bicocca EPIGENETICS EPIGENETICS THE STUDY OF CHANGES IN GENE EXPRESSION THAT ARE POTENTIALLY HERITABLE AND THAT DO NOT ENTAIL A
Epigenomics. Ivana de la Serna Block Health Science
 Epigenomics Ivana de la Serna Block Health Science 388 383-4111 ivana.delaserna@utoledo.edu Outline 1. Epigenetics-definition and overview 2. DNA methylation/hydroxymethylation 3. Histone modifications
Epigenomics Ivana de la Serna Block Health Science 388 383-4111 ivana.delaserna@utoledo.edu Outline 1. Epigenetics-definition and overview 2. DNA methylation/hydroxymethylation 3. Histone modifications
Epigenetics q&more 01.11
 Laurie. Knight, istockphoto.com Epigenetics 6 Bookmarks About the reading of genes in the Book of Life Prof. Dr. Manfred Jung, Julia M. Wagner, Institute for Pharmaceutical Sciences, Albert-Ludwig-University
Laurie. Knight, istockphoto.com Epigenetics 6 Bookmarks About the reading of genes in the Book of Life Prof. Dr. Manfred Jung, Julia M. Wagner, Institute for Pharmaceutical Sciences, Albert-Ludwig-University
Eukaryotic transcription (III)
 Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Molecular Cell Biology. Prof. D. Karunagaran. Department of Biotechnology. Indian Institute of Technology Madras
 Molecular Cell Biology Prof. D. Karunagaran Department of Biotechnology Indian Institute of Technology Madras Module-9 Molecular Basis of Cancer, Oncogenes and Tumor Suppressor Genes Lecture 6 Epigenetics
Molecular Cell Biology Prof. D. Karunagaran Department of Biotechnology Indian Institute of Technology Madras Module-9 Molecular Basis of Cancer, Oncogenes and Tumor Suppressor Genes Lecture 6 Epigenetics
Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3 and Hariom Yadav 3
 Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3 and Hariom Yadav 3 1 Department of Biotechnology, JMIT, Radaur, Haryana, India 2 KITM, Kurukshetra, Haryana, India 3 NIDDK, National Institute of Health,
Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3 and Hariom Yadav 3 1 Department of Biotechnology, JMIT, Radaur, Haryana, India 2 KITM, Kurukshetra, Haryana, India 3 NIDDK, National Institute of Health,
Biochemical Determinants Governing Redox Regulated Changes in Gene Expression and Chromatin Structure
 Biochemical Determinants Governing Redox Regulated Changes in Gene Expression and Chromatin Structure Frederick E. Domann, Ph.D. Associate Professor of Radiation Oncology The University of Iowa Iowa City,
Biochemical Determinants Governing Redox Regulated Changes in Gene Expression and Chromatin Structure Frederick E. Domann, Ph.D. Associate Professor of Radiation Oncology The University of Iowa Iowa City,
Expert Intelligence for Better Decisions Epigenetics:
 Expert Intelligence for Better Decisions Epigenetics: Emerging Targets, Available Technologies, Expert Interviews, and an Epigenetic Community Perspective Using This Document Insight Pharma Reports are
Expert Intelligence for Better Decisions Epigenetics: Emerging Targets, Available Technologies, Expert Interviews, and an Epigenetic Community Perspective Using This Document Insight Pharma Reports are
Epigenetics Armstrong_Prelims.indd 1 04/11/2013 3:28 pm
 Epigenetics Epigenetics Lyle Armstrong vi Online resources Accessible from www.garlandscience.com, the Student and Instructor Resource Websites provide learning and teaching tools created for Epigenetics.
Epigenetics Epigenetics Lyle Armstrong vi Online resources Accessible from www.garlandscience.com, the Student and Instructor Resource Websites provide learning and teaching tools created for Epigenetics.
mirna Dr. S Hosseini-Asl
 mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
Stem Cell Epigenetics
 Stem Cell Epigenetics Philippe Collas University of Oslo Institute of Basic Medical Sciences Norwegian Center for Stem Cell Research www.collaslab.com Source of stem cells in the body Somatic ( adult )
Stem Cell Epigenetics Philippe Collas University of Oslo Institute of Basic Medical Sciences Norwegian Center for Stem Cell Research www.collaslab.com Source of stem cells in the body Somatic ( adult )
Genetics and Genomics in Medicine Chapter 6 Questions
 Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Eukaryotic Gene Regulation
 Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
Ch. 18 Regulation of Gene Expression
 Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Chromatin-Based Regulation of Gene Expression
 Chromatin-Based Regulation of Gene Expression.George J. Quellhorst, Jr., PhD.Associate Director, R&D.Biological Content Development Topics to be Discussed Importance of Chromatin-Based Regulation Mechanism
Chromatin-Based Regulation of Gene Expression.George J. Quellhorst, Jr., PhD.Associate Director, R&D.Biological Content Development Topics to be Discussed Importance of Chromatin-Based Regulation Mechanism
Epigenetics and Cancer
 Epigenetics and Cancer Ya-Hui Chi March 24, 2016 1 Institute of Biotechnology and Pharmaceutical Research, NHRI, Zhunan, Taiwan Question 1: What causes cancer? Douglas Hanahan and Robert A. Weinberg 2
Epigenetics and Cancer Ya-Hui Chi March 24, 2016 1 Institute of Biotechnology and Pharmaceutical Research, NHRI, Zhunan, Taiwan Question 1: What causes cancer? Douglas Hanahan and Robert A. Weinberg 2
PRC2 crystal clear. Matthieu Schapira
 PRC2 crystal clear Matthieu Schapira Epigenetic mechanisms control the combination of genes that are switched on and off in any given cell. In turn, this combination, called the transcriptional program,
PRC2 crystal clear Matthieu Schapira Epigenetic mechanisms control the combination of genes that are switched on and off in any given cell. In turn, this combination, called the transcriptional program,
Epigenetics ~from mechanism to therapy~ Literature seminar January 31, 2012 Soichi Ito (B4)
 Epigenetics ~from mechanism to therapy~ Literature seminar January 31, 2012 Soichi Ito (B4) 1 Contents Introduction Central dogma What is the Epigenetics? Topics 1. DNA methylation 2. Histone modification
Epigenetics ~from mechanism to therapy~ Literature seminar January 31, 2012 Soichi Ito (B4) 1 Contents Introduction Central dogma What is the Epigenetics? Topics 1. DNA methylation 2. Histone modification
Are you the way you are because of the
 EPIGENETICS Are you the way you are because of the It s my fault!! Nurture Genes you inherited from your parents? Nature Experiences during your life? Similar DNA Asthma, Autism, TWINS Bipolar Disorders
EPIGENETICS Are you the way you are because of the It s my fault!! Nurture Genes you inherited from your parents? Nature Experiences during your life? Similar DNA Asthma, Autism, TWINS Bipolar Disorders
DNA Methylation and Cancer
 DNA Methylation and Cancer October 25, 2016 Dominic Smiraglia, Ph.D. Department of Cancer Genetics From Alan Wolffe, Science and Medicine, 1999 Vital Statistics Human genome contains 3 billion bp ~ 50,000
DNA Methylation and Cancer October 25, 2016 Dominic Smiraglia, Ph.D. Department of Cancer Genetics From Alan Wolffe, Science and Medicine, 1999 Vital Statistics Human genome contains 3 billion bp ~ 50,000
Not IN Our Genes - A Different Kind of Inheritance.! Christopher Phiel, Ph.D. University of Colorado Denver Mini-STEM School February 4, 2014
 Not IN Our Genes - A Different Kind of Inheritance! Christopher Phiel, Ph.D. University of Colorado Denver Mini-STEM School February 4, 2014 Epigenetics in Mainstream Media Epigenetics *Current definition:
Not IN Our Genes - A Different Kind of Inheritance! Christopher Phiel, Ph.D. University of Colorado Denver Mini-STEM School February 4, 2014 Epigenetics in Mainstream Media Epigenetics *Current definition:
The role of epigenetics in breast. cancer progression
 The role of epigenetics in breast cancer progression Aslı Dilber Yıldırım January 2013 Master thesis, Cancer Genomics & Developmental Biology Under daily supervision of Rick van Nuland, MSc Examiners:
The role of epigenetics in breast cancer progression Aslı Dilber Yıldırım January 2013 Master thesis, Cancer Genomics & Developmental Biology Under daily supervision of Rick van Nuland, MSc Examiners:
Transcription and chromatin. General Transcription Factors + Promoter-specific factors + Co-activators
 Transcription and chromatin General Transcription Factors + Promoter-specific factors + Co-activators Cofactor or Coactivator 1. work with DNA specific transcription factors to make them more effective
Transcription and chromatin General Transcription Factors + Promoter-specific factors + Co-activators Cofactor or Coactivator 1. work with DNA specific transcription factors to make them more effective
Epigenetics in cancer stem cells
 Toh et al. Molecular Cancer (2017) 16:29 DOI 10.1186/s12943-017-0596-9 REVIEW Epigenetics in cancer stem cells Tan Boon Toh 1, Jhin Jieh Lim 1 and Edward Kai-Hua Chow 1,2,3* Open Access Abstract Compelling
Toh et al. Molecular Cancer (2017) 16:29 DOI 10.1186/s12943-017-0596-9 REVIEW Epigenetics in cancer stem cells Tan Boon Toh 1, Jhin Jieh Lim 1 and Edward Kai-Hua Chow 1,2,3* Open Access Abstract Compelling
Identification of a First-In-Class PRMT5 Inhibitor with Potent In Vitro and In Vivo Activity in Preclinical Models of Mantle Cell Lymphoma
 Identification of a First-In-Class PRMT5 Inhibitor with Potent In Vitro and In Vivo Activity in Preclinical Models of Mantle Cell Lymphoma Elayne Penebre, Kristy G Kuplast, Christina R Majer, P Ann Boriak-Sjodin,
Identification of a First-In-Class PRMT5 Inhibitor with Potent In Vitro and In Vivo Activity in Preclinical Models of Mantle Cell Lymphoma Elayne Penebre, Kristy G Kuplast, Christina R Majer, P Ann Boriak-Sjodin,
Unique, expert portfolio
 EPIGENETICS Enzymes Kits Cell Lines Screening Services INNOVATIVE PRODUCTS TO FUEL YOUR EXPERIMENTS Unique, expert portfolio www.bpsbioscience.com HISTONE METHYLTRANSFERASES HISTONE DEMETHYLASES ACETYLTRANSFERASES
EPIGENETICS Enzymes Kits Cell Lines Screening Services INNOVATIVE PRODUCTS TO FUEL YOUR EXPERIMENTS Unique, expert portfolio www.bpsbioscience.com HISTONE METHYLTRANSFERASES HISTONE DEMETHYLASES ACETYLTRANSFERASES
Epigenetics and Colorectal Cancer Pathogenesis
 Cancers 2013, 5, 676-713; doi:10.3390/cancers5020676 Article OPEN ACCESS cancers ISSN 2072-6694 www.mdpi.com/journal/cancers Epigenetics and Colorectal Cancer Pathogenesis Kankana Bardhan and Kebin Liu
Cancers 2013, 5, 676-713; doi:10.3390/cancers5020676 Article OPEN ACCESS cancers ISSN 2072-6694 www.mdpi.com/journal/cancers Epigenetics and Colorectal Cancer Pathogenesis Kankana Bardhan and Kebin Liu
DNA methylation & demethylation
 DNA methylation & demethylation Lars Schomacher (Group Christof Niehrs) What is Epigenetics? Epigenetics is the study of heritable changes in gene expression (active versus inactive genes) that do not
DNA methylation & demethylation Lars Schomacher (Group Christof Niehrs) What is Epigenetics? Epigenetics is the study of heritable changes in gene expression (active versus inactive genes) that do not
Epigenetics & cancer. Present by : Sanaz Zebardast Under supervision : Dr. Gheibi. 31 December 2016
 Epigenetics & cancer Present by : Sanaz Zebardast Under supervision : Dr. Gheibi 31 December 2016 1 contents Introduction Epigenetic & signaling pathways Epigenetic & integral protein Epigenetic & apoptosis
Epigenetics & cancer Present by : Sanaz Zebardast Under supervision : Dr. Gheibi 31 December 2016 1 contents Introduction Epigenetic & signaling pathways Epigenetic & integral protein Epigenetic & apoptosis
NOVEL HISTONE DEMETHYLASE INHIBITORS SYNERGISTICALLY ENHANCE THE EFFECTS OF A DNA HYPOMETHYLATING AGENT IN BREAST CANCER CELLS. Benjamin R.
 NOVEL HISTONE DEMETHYLASE INHIBITORS SYNERGISTICALLY ENHANCE THE EFFECTS OF A DNA HYPOMETHYLATING AGENT IN BREAST CANCER CELLS By Benjamin R. Leadem A dissertation submitted to The Johns Hopkins University
NOVEL HISTONE DEMETHYLASE INHIBITORS SYNERGISTICALLY ENHANCE THE EFFECTS OF A DNA HYPOMETHYLATING AGENT IN BREAST CANCER CELLS By Benjamin R. Leadem A dissertation submitted to The Johns Hopkins University
DNA Methylation and Demethylation as Targets for Anticancer Therapy
 Biochemistry (Moscow), Vol. 70, No. 5, 2005, pp. 533-549. Translated from Biokhimiya, Vol. 70, No. 5, 2005, pp. 651-669. Original Russian Text Copyright 2005 by Szyf. DNA Methylation and Demethylation
Biochemistry (Moscow), Vol. 70, No. 5, 2005, pp. 533-549. Translated from Biokhimiya, Vol. 70, No. 5, 2005, pp. 651-669. Original Russian Text Copyright 2005 by Szyf. DNA Methylation and Demethylation
EPIGENOMICS PROFILING SERVICES
 EPIGENOMICS PROFILING SERVICES Chromatin analysis DNA methylation analysis RNA-seq analysis Diagenode helps you uncover the mysteries of epigenetics PAGE 3 Integrative epigenomics analysis DNA methylation
EPIGENOMICS PROFILING SERVICES Chromatin analysis DNA methylation analysis RNA-seq analysis Diagenode helps you uncover the mysteries of epigenetics PAGE 3 Integrative epigenomics analysis DNA methylation
The silence of the genes: clinical applications of (colorectal) cancer epigenetics
 The silence of the genes: clinical applications of (colorectal) cancer epigenetics Manon van Engeland, PhD Dept. of Pathology GROW - School for Oncology & Developmental Biology Maastricht University Medical
The silence of the genes: clinical applications of (colorectal) cancer epigenetics Manon van Engeland, PhD Dept. of Pathology GROW - School for Oncology & Developmental Biology Maastricht University Medical
Changes in DNA methylation and histone modification during epigenetic transitions
 Oregon Health & Science University OHSU Digital Commons Scholar Archive May 2009 Changes in DNA methylation and histone modification during epigenetic transitions Jon Andrew Oyer Follow this and additional
Oregon Health & Science University OHSU Digital Commons Scholar Archive May 2009 Changes in DNA methylation and histone modification during epigenetic transitions Jon Andrew Oyer Follow this and additional
Epigenetic Mechanisms
 RCPA Lecture Epigenetic chanisms Jeff Craig Early Life Epigenetics Group, MCRI Dept. of Paediatrics Overview What is epigenetics? Chromatin The epigenetic code What is epigenetics? the interactions of
RCPA Lecture Epigenetic chanisms Jeff Craig Early Life Epigenetics Group, MCRI Dept. of Paediatrics Overview What is epigenetics? Chromatin The epigenetic code What is epigenetics? the interactions of
Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P Matthias & RG Clerc
 Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P Matthias & RG Clerc P. Matthias, 16 April 2014 DNA methylation basics Acetylation Acetyltransferases/Deacetylases
Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P Matthias & RG Clerc P. Matthias, 16 April 2014 DNA methylation basics Acetylation Acetyltransferases/Deacetylases
Lecture 10. Eukaryotic gene regulation: chromatin remodelling
 Lecture 10 Eukaryotic gene regulation: chromatin remodelling Recap.. Eukaryotic RNA polymerases Core promoter elements General transcription factors Enhancers and upstream activation sequences Transcriptional
Lecture 10 Eukaryotic gene regulation: chromatin remodelling Recap.. Eukaryotic RNA polymerases Core promoter elements General transcription factors Enhancers and upstream activation sequences Transcriptional
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
The Epigenome Tools 2: ChIP-Seq and Data Analysis
 The Epigenome Tools 2: ChIP-Seq and Data Analysis Chongzhi Zang zang@virginia.edu http://zanglab.com PHS5705: Public Health Genomics March 20, 2017 1 Outline Epigenome: basics review ChIP-seq overview
The Epigenome Tools 2: ChIP-Seq and Data Analysis Chongzhi Zang zang@virginia.edu http://zanglab.com PHS5705: Public Health Genomics March 20, 2017 1 Outline Epigenome: basics review ChIP-seq overview
609G: Concepts of Cancer Genetics and Treatments (3 credits)
 Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
The role of DNMT3A and HOXA9 hypomethylation in acute myeloid leukemia (AML)
 Campbell Drohan BIOL 463 December 2016 The role of DNMT3A and HOXA9 hypomethylation in acute myeloid leukemia (AML) Introduction Epigenetic modifications of DNA and histones are key to gene regulation
Campbell Drohan BIOL 463 December 2016 The role of DNMT3A and HOXA9 hypomethylation in acute myeloid leukemia (AML) Introduction Epigenetic modifications of DNA and histones are key to gene regulation
Epigenetics: The Future of Psychology & Neuroscience. Richard E. Brown Psychology Department Dalhousie University Halifax, NS, B3H 4J1
 Epigenetics: The Future of Psychology & Neuroscience Richard E. Brown Psychology Department Dalhousie University Halifax, NS, B3H 4J1 Nature versus Nurture Despite the belief that the Nature vs. Nurture
Epigenetics: The Future of Psychology & Neuroscience Richard E. Brown Psychology Department Dalhousie University Halifax, NS, B3H 4J1 Nature versus Nurture Despite the belief that the Nature vs. Nurture
Genomic Methods in Cancer Epigenetic Dysregulation
 Genomic Methods in Cancer Epigenetic Dysregulation Clara, Lyon 2018 Jacek Majewski, Associate Professor Department of Human Genetics, McGill University Montreal, Canada A few words about my lab Genomics
Genomic Methods in Cancer Epigenetic Dysregulation Clara, Lyon 2018 Jacek Majewski, Associate Professor Department of Human Genetics, McGill University Montreal, Canada A few words about my lab Genomics
Fragile X Syndrome. Genetics, Epigenetics & the Role of Unprogrammed Events in the expression of a Phenotype
 Fragile X Syndrome Genetics, Epigenetics & the Role of Unprogrammed Events in the expression of a Phenotype A loss of function of the FMR-1 gene results in severe learning problems, intellectual disability
Fragile X Syndrome Genetics, Epigenetics & the Role of Unprogrammed Events in the expression of a Phenotype A loss of function of the FMR-1 gene results in severe learning problems, intellectual disability
Biochimica et Biophysica Acta
 BBAGRM-00286; No. of pages: 19; 4C: 8, 12 Biochimica et Biophysica Acta xxx (2010) xxx xxx Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbagrm
BBAGRM-00286; No. of pages: 19; 4C: 8, 12 Biochimica et Biophysica Acta xxx (2010) xxx xxx Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbagrm
Epigene.cs: What is it and how it effects our health? Overview. Dr. Bill Stanford, PhD OFawa Hospital Research Ins.tute University of OFawa
 Epigene.cs: What is it and how it effects our health? Dr. Bill Stanford, PhD OFawa Hospital Research Ins.tute University of OFawa Overview Basic Background Epigene.cs in general Epigene.cs in cancer Epigene.cs
Epigene.cs: What is it and how it effects our health? Dr. Bill Stanford, PhD OFawa Hospital Research Ins.tute University of OFawa Overview Basic Background Epigene.cs in general Epigene.cs in cancer Epigene.cs
Beads-on-a- The 30nm Fibre Active Chromosome The Metaphase Chromosome. Less active genes During interphase During cell division
 Overview of Epigenetics Figure 2.2. The Increasing Structural Complexity of Genetic Information from the Double-Helical Structure of DNA, through Nucleosome and Chromatin Structures, to the Chromosome
Overview of Epigenetics Figure 2.2. The Increasing Structural Complexity of Genetic Information from the Double-Helical Structure of DNA, through Nucleosome and Chromatin Structures, to the Chromosome
Repressive Transcription
 Repressive Transcription The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Guenther, M. G., and R. A.
Repressive Transcription The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Guenther, M. G., and R. A.
Epigenetic Inheritance
 (2) The role of Epigenetic Inheritance Lamarck Revisited Lamarck was incorrect in thinking that the inheritance of acquired characters is the main mechanism of evolution (Natural Selection more common)
(2) The role of Epigenetic Inheritance Lamarck Revisited Lamarck was incorrect in thinking that the inheritance of acquired characters is the main mechanism of evolution (Natural Selection more common)
DNA methylation: a potential clinical biomarker for the detection of human cancers
 DNA methylation: a potential clinical biomarker for the detection of human cancers Name: Tong Samuel Supervisor: Zigui CHEN Date: 1 st December 2016 Department: Microbiology Source: cited from Jakubowski,
DNA methylation: a potential clinical biomarker for the detection of human cancers Name: Tong Samuel Supervisor: Zigui CHEN Date: 1 st December 2016 Department: Microbiology Source: cited from Jakubowski,
Multistep nature of cancer development. Cancer genes
 Multistep nature of cancer development Phenotypic progression loss of control over cell growth/death (neoplasm) invasiveness (carcinoma) distal spread (metastatic tumor) Genetic progression multiple genetic
Multistep nature of cancer development Phenotypic progression loss of control over cell growth/death (neoplasm) invasiveness (carcinoma) distal spread (metastatic tumor) Genetic progression multiple genetic
RUNX1 and FPD/AML Translational Research. The Leukemia and Lymphoma Society / Babich Family Foundation Partnership. September 2016
 www.lls.org www.runx1.com RUNX1 and FPD/AML Translational Research The Leukemia and Lymphoma Society / Babich Family Foundation Partnership September 2016 Prepared by L. Greenberger, PhD Chief Scientific
www.lls.org www.runx1.com RUNX1 and FPD/AML Translational Research The Leukemia and Lymphoma Society / Babich Family Foundation Partnership September 2016 Prepared by L. Greenberger, PhD Chief Scientific
Dominic J Smiraglia, PhD Department of Cancer Genetics. DNA methylation in prostate cancer
 Dominic J Smiraglia, PhD Department of Cancer Genetics DNA methylation in prostate cancer Overarching theme Epigenetic regulation allows the genome to be responsive to the environment Sets the tone for
Dominic J Smiraglia, PhD Department of Cancer Genetics DNA methylation in prostate cancer Overarching theme Epigenetic regulation allows the genome to be responsive to the environment Sets the tone for
Lecture 27. Epigenetic regulation of gene expression during development
 Lecture 27 Epigenetic regulation of gene expression during development Development of a multicellular organism is not only determined by the DNA sequence but also epigenetically through DNA methylation
Lecture 27 Epigenetic regulation of gene expression during development Development of a multicellular organism is not only determined by the DNA sequence but also epigenetically through DNA methylation
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/32781 holds various files of this Leiden University dissertation. Author: Benard, Anne Title: Epigenetic prognostic biomarkers in colorectal cancer Issue
Cover Page The handle http://hdl.handle.net/1887/32781 holds various files of this Leiden University dissertation. Author: Benard, Anne Title: Epigenetic prognostic biomarkers in colorectal cancer Issue
MicroRNA-29a Reveals Oncogenic Role on Myeloid Malignancies by Regulating DNMT3A
 MicroRNA-29a Reveals Oncogenic Role on Myeloid Malignancies by Regulating DNMT3A Heba Alkhatabi, PhD Assistant Professor Department of Medical Laboratory Collage of Applied Medical science King Abdul Aziz
MicroRNA-29a Reveals Oncogenic Role on Myeloid Malignancies by Regulating DNMT3A Heba Alkhatabi, PhD Assistant Professor Department of Medical Laboratory Collage of Applied Medical science King Abdul Aziz
Oncology 520 Epigene0cs and Cancer. February 16, 2011
 Oncology 520 Epigene0cs and Cancer February 16, 2011 Outline What is epigene0cs? What are examples of epigene0c processes? What is the epigenome and how is it decoded? Are epigene0c mechanisms important
Oncology 520 Epigene0cs and Cancer February 16, 2011 Outline What is epigene0cs? What are examples of epigene0c processes? What is the epigenome and how is it decoded? Are epigene0c mechanisms important
Alpha thalassemia mental retardation X-linked. Acquired alpha-thalassemia myelodysplastic syndrome
 Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
Alpha thalassemia mental retardation X-linked Acquired alpha-thalassemia myelodysplastic syndrome (Alpha thalassemia mental retardation X-linked) Acquired alpha-thalassemia myelodysplastic syndrome Schematic
Epigenetics and Human Disease
 Epigenetics and Human Disease May 28, 2014 1 Angelman Syndrome & Prader-Willi Syndrome Sister Syndromes Angelman Syndrome ~1/20,000 births happy disposition smile often bouts of laughter minimal verbal
Epigenetics and Human Disease May 28, 2014 1 Angelman Syndrome & Prader-Willi Syndrome Sister Syndromes Angelman Syndrome ~1/20,000 births happy disposition smile often bouts of laughter minimal verbal
The Biology and Genetics of Cells and Organisms The Biology of Cancer
 The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Introduction. Cancer Biology. Tumor-suppressor genes. Proto-oncogenes. DNA stability genes. Mechanisms of carcinogenesis.
 Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
Introduction to Cancer Biology
 Introduction to Cancer Biology Robin Hesketh Multiple choice questions (choose the one correct answer from the five choices) Which ONE of the following is a tumour suppressor? a. AKT b. APC c. BCL2 d.
Introduction to Cancer Biology Robin Hesketh Multiple choice questions (choose the one correct answer from the five choices) Which ONE of the following is a tumour suppressor? a. AKT b. APC c. BCL2 d.
FOLLICULAR LYMPHOMA- ILLUMINA METHYLATION. Jude Fitzgibbon
 FOLLICULAR LYMPHOMA- ILLUMINA METHYLATION Jude Fitzgibbon j.fitzgibbon@qmul.ac.uk Molecular Predictors of Clinical outcome Responders Alive Non-responders Dead TUMOUR GENETICS Targeted therapy, Prognostic
FOLLICULAR LYMPHOMA- ILLUMINA METHYLATION Jude Fitzgibbon j.fitzgibbon@qmul.ac.uk Molecular Predictors of Clinical outcome Responders Alive Non-responders Dead TUMOUR GENETICS Targeted therapy, Prognostic
oncogenes-and- tumour-suppressor-genes)
 Special topics in tumor biochemistry oncogenes-and- tumour-suppressor-genes) Speaker: Prof. Jiunn-Jye Chuu E-Mail: jjchuu@mail.stust.edu.tw Genetic Basis of Cancer Cancer-causing mutations Disease of aging
Special topics in tumor biochemistry oncogenes-and- tumour-suppressor-genes) Speaker: Prof. Jiunn-Jye Chuu E-Mail: jjchuu@mail.stust.edu.tw Genetic Basis of Cancer Cancer-causing mutations Disease of aging
Therapeutic and Prognostic Role of Epigenetic Abnormalities in MDS. Stephen D. Nimer, MD Sylvester Comprehensive Cancer Center December 5, 2014
 Therapeutic and Prognostic Role of Epigenetic Abnormalities in MDS Stephen D. Nimer, MD Sylvester Comprehensive Cancer Center December 5, 2014 DISCLOSURE I have no relevant financial relationships to disclose.
Therapeutic and Prognostic Role of Epigenetic Abnormalities in MDS Stephen D. Nimer, MD Sylvester Comprehensive Cancer Center December 5, 2014 DISCLOSURE I have no relevant financial relationships to disclose.
Epigenetics in ovarian cancer: premise, properties, and perspectives
 Yang et al. Molecular Cancer (2018) 17:109 https://doi.org/10.1186/s12943-018-0855-4 REVIEW Epigenetics in ovarian cancer: premise, properties, and perspectives Open Access Qilian Yang 1, Yuqing Yang 2,
Yang et al. Molecular Cancer (2018) 17:109 https://doi.org/10.1186/s12943-018-0855-4 REVIEW Epigenetics in ovarian cancer: premise, properties, and perspectives Open Access Qilian Yang 1, Yuqing Yang 2,
Imprinting. Joyce Ohm Cancer Genetics and Genomics CGP-L2-319 x8821
 Imprinting Joyce Ohm Cancer Genetics and Genomics CGP-L2-319 x8821 Learning Objectives 1. To understand the basic concepts of genomic imprinting Genomic imprinting is an epigenetic phenomenon that causes
Imprinting Joyce Ohm Cancer Genetics and Genomics CGP-L2-319 x8821 Learning Objectives 1. To understand the basic concepts of genomic imprinting Genomic imprinting is an epigenetic phenomenon that causes
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Introduction to Genetics
 Introduction to Genetics Table of contents Chromosome DNA Protein synthesis Mutation Genetic disorder Relationship between genes and cancer Genetic testing Technical concern 2 All living organisms consist
Introduction to Genetics Table of contents Chromosome DNA Protein synthesis Mutation Genetic disorder Relationship between genes and cancer Genetic testing Technical concern 2 All living organisms consist
The interplay of histone modifications writers that read
 Review Histones and Chromatin Review series The interplay of histone modifications writers that read Tianyi Zhang, Sarah Cooper *, & Neil Brockdorff Abstract Histones are subject to a vast array of posttranslational
Review Histones and Chromatin Review series The interplay of histone modifications writers that read Tianyi Zhang, Sarah Cooper *, & Neil Brockdorff Abstract Histones are subject to a vast array of posttranslational
Epigenetics: A historical overview Dr. Robin Holliday
 Epigenetics 1 Rival hypotheses Epigenisis - The embryo is initially undifferentiated. As development proceeds, increasing levels of complexity emerge giving rise to the larval stage or to the adult organism.
Epigenetics 1 Rival hypotheses Epigenisis - The embryo is initially undifferentiated. As development proceeds, increasing levels of complexity emerge giving rise to the larval stage or to the adult organism.
PI3K Background. The SignalRx R & D pipeline is shown below followed by a brief description of each program:
 PI3K Background The phosphatidylinositol 3-kinase (PI3K) pathway is a key cell signaling node whose dysregulation commonly results in the transformation of normal cells into cancer cells. The role of PI3K
PI3K Background The phosphatidylinositol 3-kinase (PI3K) pathway is a key cell signaling node whose dysregulation commonly results in the transformation of normal cells into cancer cells. The role of PI3K
Alternative splicing. Biosciences 741: Genomics Fall, 2013 Week 6
 Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
Control of nuclear receptor function by local chromatin structure
 MINIREVIEW Control of nuclear receptor function by local chromatin structure Malgorzata Wiench, Tina B. Miranda and Gordon L. Hager Laboratory of Receptor Biology and Gene Expression, National Cancer Institute,
MINIREVIEW Control of nuclear receptor function by local chromatin structure Malgorzata Wiench, Tina B. Miranda and Gordon L. Hager Laboratory of Receptor Biology and Gene Expression, National Cancer Institute,
Chapter 9. Cells Grow and Reproduce
 Chapter 9 Cells Grow and Reproduce DNA Replication DNA polymerase Addition of a nucleotide to the 3 end of a growing strand Use dntps as substrate Release of pyrophosphate Initiation of Replication Replication
Chapter 9 Cells Grow and Reproduce DNA Replication DNA polymerase Addition of a nucleotide to the 3 end of a growing strand Use dntps as substrate Release of pyrophosphate Initiation of Replication Replication
Review. Ageing 2: Cancer! Review: Mutations. Mutations 2/14/11. The Raw Material for Evolution. The Double Edged Sword
 Ageing 2: Cancer! Review: The force of natural selection declines with ageing due to increase in extrinsic mortality (= weakening of natural selection) and reduction in reproduction with age (selection
Ageing 2: Cancer! Review: The force of natural selection declines with ageing due to increase in extrinsic mortality (= weakening of natural selection) and reduction in reproduction with age (selection
p53 and Apoptosis: Master Guardian and Executioner Part 2
 p53 and Apoptosis: Master Guardian and Executioner Part 2 p14arf in human cells is a antagonist of Mdm2. The expression of ARF causes a rapid increase in p53 levels, so what would you suggest?.. The enemy
p53 and Apoptosis: Master Guardian and Executioner Part 2 p14arf in human cells is a antagonist of Mdm2. The expression of ARF causes a rapid increase in p53 levels, so what would you suggest?.. The enemy
Karyotype analysis reveals transloction of chromosome 22 to 9 in CML chronic myelogenous leukemia has fusion protein Bcr-Abl
 Chapt. 18 Cancer Molecular Biology of Cancer Student Learning Outcomes: Describe cancer diseases in which cells no longer respond Describe how cancers come from genomic mutations (inherited or somatic)
Chapt. 18 Cancer Molecular Biology of Cancer Student Learning Outcomes: Describe cancer diseases in which cells no longer respond Describe how cancers come from genomic mutations (inherited or somatic)
The mutations that drive cancer. Paul Edwards. Department of Pathology and Cancer Research UK Cambridge Institute, University of Cambridge
 The mutations that drive cancer Paul Edwards Department of Pathology and Cancer Research UK Cambridge Institute, University of Cambridge Previously on Cancer... hereditary predisposition Normal Cell Slightly
The mutations that drive cancer Paul Edwards Department of Pathology and Cancer Research UK Cambridge Institute, University of Cambridge Previously on Cancer... hereditary predisposition Normal Cell Slightly
Chemistry 107 Exam 4 Study Guide
 Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
TMEFF2 is an epigenetic modulator that promotes androgen independent growth in castrationresistant. prostate cancer cells. Joshua Corbin.
 TMEFF2 is an epigenetic modulator that promotes androgen independent growth in castrationresistant prostate cancer cells by Joshua Corbin August, 2014 Director of Thesis: Maria Ruiz-Echevarria, Ph.D. Major
TMEFF2 is an epigenetic modulator that promotes androgen independent growth in castrationresistant prostate cancer cells by Joshua Corbin August, 2014 Director of Thesis: Maria Ruiz-Echevarria, Ph.D. Major
Altered Histone Modifications in Gliomas
 REVIEW ARTICLE Brain Tumor Res Treat 2014;2(1):7-21 / pissn 2288-2405 / eissn 2288-2413 http://dx.doi.org/10.14791/btrt.2014.2.1.7 Altered Histone Modifications in Gliomas Young Zoon Kim Division of Neuro-Oncology,
REVIEW ARTICLE Brain Tumor Res Treat 2014;2(1):7-21 / pissn 2288-2405 / eissn 2288-2413 http://dx.doi.org/10.14791/btrt.2014.2.1.7 Altered Histone Modifications in Gliomas Young Zoon Kim Division of Neuro-Oncology,
Comparative effect of Various HDAC-inhibitors in-vitro on T- Cell Lymphoma cell lines alone and in combination with conventional anti-cancer drugs
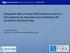 Comparative effect of Various HDAC-inhibitors in-vitro on T- Cell Lymphoma cell lines alone and in combination with conventional anti-cancer drugs Arshad H. Banday Mentor:Dr. Francisco Hernandez-Illizaliturri
Comparative effect of Various HDAC-inhibitors in-vitro on T- Cell Lymphoma cell lines alone and in combination with conventional anti-cancer drugs Arshad H. Banday Mentor:Dr. Francisco Hernandez-Illizaliturri
LESSON 3.2 WORKBOOK. How do normal cells become cancer cells? Workbook Lesson 3.2
 For a complete list of defined terms, see the Glossary. Transformation the process by which a cell acquires characteristics of a tumor cell. LESSON 3.2 WORKBOOK How do normal cells become cancer cells?
For a complete list of defined terms, see the Glossary. Transformation the process by which a cell acquires characteristics of a tumor cell. LESSON 3.2 WORKBOOK How do normal cells become cancer cells?
An Overview of the Molecular Basis of Epigenetics
 C H A P T E R 1 An Overview of the Molecular Basis of Epigenetics J. David Sweatt, 1 Eric J. Nestler, 2 Michael J. Meaney 3 and Schahram Akbarian 4 1 McKnight Brain Institute, Department of Neurobiology,
C H A P T E R 1 An Overview of the Molecular Basis of Epigenetics J. David Sweatt, 1 Eric J. Nestler, 2 Michael J. Meaney 3 and Schahram Akbarian 4 1 McKnight Brain Institute, Department of Neurobiology,
Cancer and Gene Regulation
 OpenStax-CNX module: m44548 1 Cancer and Gene Regulation OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m44548 1 Cancer and Gene Regulation OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
EZH2 Inhibitors as Novel Cancer Therapeutics!!!! Robert A. Copeland, Ph.D.! Epizyme, Inc.!!! 20 November 2014!
 EZH2 Inhibitors as Novel Cancer Therapeutics Robert A. Copeland, Ph.D. Epizyme, Inc. 20 November 2014 2013 Accomplishments Disclosure Information EORTC-NCI-AACR Meeting, 20 November 2014 Robert A. Copeland,
EZH2 Inhibitors as Novel Cancer Therapeutics Robert A. Copeland, Ph.D. Epizyme, Inc. 20 November 2014 2013 Accomplishments Disclosure Information EORTC-NCI-AACR Meeting, 20 November 2014 Robert A. Copeland,
Biochemistry 673 Lecture 2 Jason Kahn, UMCP Introduction to steroid hormone receptor (nuclear receptor) signalling
 Biochemistry 673 Lecture 2 Jason Kahn, UMCP Introduction to steroid hormone receptor (nuclear receptor) signalling Resources: Latchman Lodish chapter 10, 20 Helmreich, chapter 11 http://www.nursa.org,
Biochemistry 673 Lecture 2 Jason Kahn, UMCP Introduction to steroid hormone receptor (nuclear receptor) signalling Resources: Latchman Lodish chapter 10, 20 Helmreich, chapter 11 http://www.nursa.org,
INTERACTION DRUG BODY
 INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
BIO360 Fall 2013 Quiz 1
 BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
NEW DEVELOPMENTS IN EPIGENETICS AND POTENTIAL CLINICAL APPLICATIONS
 NEW DEVELOPMENTS IN EPIGENETICS AND POTENTIAL CLINICAL APPLICATIONS Susan E. Bates, M.D. Columbia University Medical Center, New York, NY and J.J.P Bronx VA Medical Center, Bronx, NY TARGETING THE EPIGENOME
NEW DEVELOPMENTS IN EPIGENETICS AND POTENTIAL CLINICAL APPLICATIONS Susan E. Bates, M.D. Columbia University Medical Center, New York, NY and J.J.P Bronx VA Medical Center, Bronx, NY TARGETING THE EPIGENOME
Chapter 19 Eukaryotic Genomes
 Chapter 19 Eukaryotic Genomes Lecture Outline Overview: How Eukaryotic Genomes Work and Evolve Two features of eukaryotic genomes present a major information-processing challenge. First, the typical multicellular
Chapter 19 Eukaryotic Genomes Lecture Outline Overview: How Eukaryotic Genomes Work and Evolve Two features of eukaryotic genomes present a major information-processing challenge. First, the typical multicellular
HGD 5502 EPIGENETICS. Dr. Abhi Veerakumarasivam (2011)
 HGD 5502 EPIGENETICS Dr. Abhi Veerakumarasivam (2011) Outline History Definition Mechanisms of Epigenetics Epigenetic Processes Review History Conrad Hall Waddington (1905 1975) Developmental biologist,
HGD 5502 EPIGENETICS Dr. Abhi Veerakumarasivam (2011) Outline History Definition Mechanisms of Epigenetics Epigenetic Processes Review History Conrad Hall Waddington (1905 1975) Developmental biologist,
Protein methylation CH 3
 Protein methylation CH 3 methionine S-adenosylmethionine (SAM or adomet) Methyl group of the methionine is activated by the + charge of the adjacent sulfur atom. SAM S-adenosylhomocysteine homocysteine
Protein methylation CH 3 methionine S-adenosylmethionine (SAM or adomet) Methyl group of the methionine is activated by the + charge of the adjacent sulfur atom. SAM S-adenosylhomocysteine homocysteine
