Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms
|
|
|
- Katherine Atkins
- 6 years ago
- Views:
Transcription
1 a r t i c l e s Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms Heather A Himburg 1,7, Phuong L Doan 2,7, Mamle Quarmyne 1,3, Xiao Yan 1,3, Joshua Sasine 1, Liman Zhao 1, Grace V Hancock 4, Jenny Kan 1, Katherine A Pohl 1, Evelyn Tran 1, Nelson J Chao 2, Jeffrey R Harris 2 & John P Chute 1,,6 217 Nature America, Inc., part of Springer Nature. All rights reserved. The role of osteolineage cells in regulating hematopoietic stem cell (HSC) regeneration following myelosuppression is not well understood. Here we show that deletion of the pro-apoptotic genes Bak and Bax in osterix (Osx, also known as Sp7 transcription factor 7)-expressing cells in mice promotes HSC regeneration and hematopoietic radioprotection following total body irradiation. These mice showed increased bone marrow (BM) levels of the protein dickkopf-1 (), which was produced in Osx-expressing BM cells. Treatment of irradiated HSCs with in vitro increased the recovery of both long-term repopulating HSCs and progenitor cells, and systemic administration of to irradiated mice increased hematopoietic recovery and improved survival. Conversely, inducible deletion of one allele of in Osx-expressing cells in adult mice inhibited the recovery of BM stem and progenitor cells and of complete blood counts following irradiation. promoted hematopoietic regeneration via both direct effects on HSCs, in which treatment with decreased the levels of mitochondrial reactive oxygen species and suppressed senescence, and indirect effects on BM endothelial cells, in which treatment with induced epidermal growth factor (EGF) secretion. Accordingly, blockade of the EGF receptor partially abrogated -mediated hematopoietic recovery. These data identify as a regulator of hematopoietic regeneration and demonstrate paracrine cross-talk between BM osteolineage cells and endothelial cells in regulating hematopoietic reconstitution following injury. Perivascular stromal cells and vascular endothelial cells (ECs) regulate HSC maintenance in the BM of mice 1 3. Deletion of nestin-expressing mesenchymal stromal cells (MSCs) has also been shown to decrease HSC content in the BM, which is associated with HSC mobilization 4. Leptin receptor (Lepr)- and paired related homeobox 1 (Prx1)-expressing perivascular cells and nestin-expressing stromal cells have been postulated to represent overlapping perivascular populations which regulate HSC maintenance in vivo,6. Recently, maintenance of quiescent, long-term repopulating HSCs was suggested to be dependent on chondroitin sulfate proteoglycan 4 (Cspg4 + or NG2 + ) pericytes localized to the arteriolar vascular niche, which are distinct from Lepr-expressing peri-sinusoidal cells 7. These studies have confirmed the importance of perivascular cells and vascular ECs in regulating HSC maintenance. Expansion of the BM osteoblast population has been shown to amplify the HSC pool in vivo 8,9, and ganciclovir-inducible depletion of BM osteoblasts decreases phenotypic hematopoietic stem and progenitor cell content 1. However, deletion of the genes encoding stem cell factor (SCF), the chemokine CXCL12 or N-cadherin in BM osteoblasts does not have effects on HSC content during homeostasis 1 3,11. Sp7 (Osx) is a transcription factor expressed by mesenchymal progenitor cells, which have been shown via lineage tracing to be osteolineage-restricted in the adult mouse 6,12,13. In the fetal BM, Osx-expressing cells contribute to nascent bone tissues and transient stromal cells, whereas perinatally, Osx-expressing cells contribute to long-lived MSCs and osteolineage cells 6,13. Notably, deletion of Dicer1, which encodes an RNaseIII endonuclease, in Osx-expressing cells promotes myelodysplasia in mice, suggesting that aberrant function of Osx-expressing cells may contribute to the development of hematologic malignancy 14. Deletion of CXCL12 from Osx-expressing cells was also shown to deplete B lymphoid progenitors in homeostasis, but no effect on HSC function was observed 3. Although genetic studies have provided insight into the function of BM niche cells in regulating hematopoiesis during homeostasis, important questions remain regarding the contributions of niche cells during stress or injury, as well as the effects of injury on nichemediated regulation of HSCs. We and others have recently demonstrated the essential role of BM ECs in regulating HSC regeneration following myelotoxicity 1 17, and we identified two BM EC-derived paracrine factors, pleiotrophin (PTN) and EGF, as regulators of HSC regeneration in vivo 18,19. However, the functions of BM mesenchymal and osteolineage cells in regulating HSC regeneration remain less well understood. Here we directly tested the function of Osx-expressing BM cells in regulating hematopoietic regeneration following myelosuppression and discovered that Osx-expressing BM cells promote hematopoietic regeneration via secretion of. 1 Division of Hematology and Oncology, Department of Medicine, University of California, Los Angeles (UCLA), Los Angeles, California, USA. 2 Division of Hematologic Malignancies and Cellular Therapy, Duke University, Durham, North Carolina, USA. 3 Department of Pharmacology and Cancer Biology, Duke University, Durham, North Carolina, USA. 4 Department of Cell and Developmental Biology, University of California, Los Angeles, Los Angeles, California, USA. Broad Center for Regenerative Medicine and Stem Cell Research, University of California, Los Angeles, Los Angeles, California, USA. 6 Jonsson Comprehensive Cancer Center, University of California, Los Angeles, Los Angeles, California, USA. 7 These authors contributed equally to this work. Correspondence should be addressed to J.P.C. (jchute@mednet.ucla.edu). Received 7 July; accepted 11 November; published online December 216; doi:1.138/nm.421 nature medicine VOLUME 23 NUMBER 1 JANUARY
2 A r t i c l e s 217 Nature America, Inc., part of Springer Nature. All rights reserved. RESULTS Deletion of Bak and Bax in Osx-expressing cells radioprotects the hematopoietic system To test whether radioprotection of these cells would alter the hematopoietic response to irradiation, we used Cre-loxP technology to delete loxp-flanked (floxed; FL) genes encoding the pro-apoptotic factors Bak and Bax in Osx-expressing cells (from Sp7 Cre;Bak1 / ;Bax FL/ mice, hereafter referred to as Osx Cre;Bak1 / ;Bax FL/ mice) 1,2. To determine the proportion of Osx-labeled cells that expressed Osx in 8-week-old mice, we used Sp7 Cherry (hereafter referred to as Osx Cherry) reporter mice, because these mice have a stronger reporter signal than mice with a GFP reporter driven by Osx Cre. In 8-week-old Osx Cherry reporter mice, approximately 6% of Cherrylabeled BM cells expressed Osx, as measured by flow cytometry, whereas in -d-old mice, 83% of Cherry-labeled BM cells expressed Osx (Fig. 1a). These results indicate that a subset of Osx-labeled BM cells lose Osx protein expression between birth and adulthood. Adult Osx Cre;Bak1 / ;Bax FL/ mice showed no baseline differences in the frequency of Osx + BM cells, BM trabecular bone content, complete blood counts, HSC content or repopulating HSC function, as compared to those in Osx Cre;Bak1 / ;Bax FL/+ control mice, which retain one wild-type allele of Bax (Supplementary Fig. 1a g). Next we irradiated both strains of mice with cgy total body irradiation (TBI) to assess the response of Osx-expressing BM cells and of hematopoietic stem and progenitor cells to injury. Osx Cre;Bak1 / ;Bax FL/ mice maintained Osx + BM cells at day 3 after irradiation as compared to Osx Cre;Bak1 / ;Bax FL/+ mice, which showed depletion of this population (Fig. 1b and Supplementary Fig. 1h). As compared to Osx Cre;Bak1 / ;Bax FL/+ mice at day 7 after TBI, Osx Cre;Bak1 / ; Bax FL/ mice displayed increased BM cellularity, increased numbers of c-kit + Sca-1 + lineage (KSL) stem progenitors, SLAM + KSL HSCs, colony-forming cells (CFCs), and peripheral blood (PB) white blood cells (WBCs), neutrophils and lymphocytes (Fig. 1c f). In competitive transplantation experiments, congenic Bl6.SJL mice transplanted with BM cells from Osx Cre;Bak1 / ;Bax FL/ mice showed a significant increase in multilineage hematopoietic cell reconstitution in both primary and secondary transplanted mice, as compared to mice that were transplanted with BM from Osx Cre;Bak1 / ;Bax FL/+ mice (Fig. 1g,h). Taken together, these data suggest that the hematopoietic response to radiation injury is regulated by Osx-expressing BM cells and that deletion of the intrinsic pathway of apoptosis in these cells promotes radioprotection of the hematopoietic system. is differentially expressed by Osx + BM cells Because Osx Cre;Bak1 / ;Bax FL/ mice showed enhanced hematopoietic recovery following TBI, we hypothesized that Osx-expressing BM cells might produce factors that promote hematopoietic regeneration. Analysis of BM supernatants from Osx Cre;Bak1 / ;Bax FL/ mice revealed enrichment for several candidate proteins as compared to Osx Cre;Bak1 / ;Bax FL/+ mice (Supplementary Fig. 2a). Among these candidates, we focused on, a Wnt inhibitor, because was previously shown to be expressed and secreted by osteolineage cells under the control of osterix 21, although it has no established role in regulating hematopoietic regeneration. Furthermore, in preliminary screening experiments we found that promoted CFC recovery from BM KSL cells that were irradiated with 3 cgy in vitro, whereas two other candidates, tissue inhibitor of metalloproteinase 2 (Timp2) and the chemokine Ccl2 (also known as MIP-3a), had no effect (data not shown). ELISA confirmed that Osx Cre;Bak1 / ; Bax FL/ mice contained significantly higher levels of protein in the BM than Osx Cre;Bak1 / ;Bax FL/+ mice (Fig. 1i). Expression of mrna was also significantly increased in BM osteolineage cells from Osx Cre;Bak1 / ;Bax FL/ mice, as compared to that in Osx Cre;Bak1 / ;Bax FL/+ mice; in both mouse strains, nearly all of the detectable protein was found in Osx + BM cells, as assessed by flow cytometry (Fig. 1j and Supplementary Fig. 2b). Moreover, we found that Osx + BM cells expressed significantly more than BM ECs in C7BL/6 mice, both at steady state and following TBI (Fig. 1k). These results indicate that Osx + BM cells are the primary source of in both Osx Cre;Bak1 / ;Bax FL/ and wild-type mice. treatment accelerates hematopoietic regeneration in vitro and in vivo We next sought to evaluate whether regulates hematopoietic regeneration following irradiation. First, we assessed the effect of on the function of non-irradiated HSCs in vitro and in vivo. Treatment of non-irradiated BM KSL cells with ng/ml in medium supplemented with thrombopoietin (TPO), SCF and Flt-3 ligand had no effect on total cell expansion, KSL expansion or CFC content, as compared to cells treated with medium alone (Supplementary Fig. 3a,b). However, systemic treatment of C7BL/6 mice with 1 µg subcutaneously every other day for 4 weeks caused a significant increase in the numbers of BM KSL cells, BM c-kit + lin cells and BM CFCs, as well as in the PB neutrophil frequency, compared to saline-treated mice (Supplementary Fig. 3c e). These data suggest that during homeostasis, promotes the in vivo expansion of the myeloid progenitor cell pool. To evaluate the function of in regulating hematopoietic regeneration following irradiation, we irradiated BM KSL cells with 3 cgy and cultured them in cytokine-containing medium with or without ng/ml. treatment significantly increased the recovery of CFCs at day 7 of culture, as compared to treatment with cytokines alone (Fig. 2a). Furthermore, mice that had been competitively transplanted with the progeny of irradiated, - treated KSL cells showed a significant increase in multilineage engraftment of donor CD4.2 + cells over the course of 16 weeks after transplantation (Fig. 2b). These results suggest that directly promotes the regeneration of HSCs capable of primary competitive repopulation following irradiation. Because promotes the regeneration of irradiated hematopoietic stem and progenitor cells (HSPCs) in vitro, we next tested whether systemic administration of could accelerate hematopoietic regeneration in vivo following TBI. Adult C7BL/6 mice were irradiated with 8 cgy TBI and treated subcutaneously with 1 µg or saline every other day for 21 d, beginning at day 1 after TBI. Systemic administration of caused a greater than eightfold increase in levels in the BM of C7BL/6 mice within 1 h, as compared to untreated mice (Fig. 2c). treatment increased the recovery of WBCs, neutrophil counts, platelets and BM cell counts at day 21 as compared to saline-treated mice (Fig. 2d,e). Furthermore, the numbers of BM KSL cells and CFCs were significantly increased in - treated mice as compared to those in saline-treated controls (Fig. 2f,g). Notably, following 8 cgy TBI, only 4 of 1 (27%) C7BL/6 mice that had been treated with saline survived to day 3, as compared to 13 of 14 mice (93%) that had been treated with, a nearly 3% increase in survival in response to treatment (P =.4) (Fig. 2h). We also evaluated whether treatment could affect long-term hematopoiesis in mice following radiation exposure. Adult C7BL/6 mice were irradiated with 6 cgy TBI, treated with 1 µg every other day for 21 d and evaluated at 12 weeks after irradiation. As compared 92 VOLUME 23 NUMBER 1 JANUARY 217 nature medicine
3 a r t i c l e s to saline-treated controls, -treated mice showed no differences in complete blood counts and BM cell counts, percentages of SLAM + KSL or KSL cells, or frequencies of BM myeloid cells, B cells or T cells (Supplementary Fig. 4a c). However, at 12 weeks following 6-cGy irradiation, saline-treated mice had substantially reduced numbers of BM CFCs as compared to irradiated, -treated mice (Fig. 2i). These data suggest that TBI causes a long-term decline in myeloid progenitor cell function and that short-term treatment with abrogates radiation-induced damage to this progenitor cell population. Inhibition of abrogates hematopoietic regeneration following TBI Because systemic administration of augments hematopoietic regeneration and survival of mice following TBI, we next tested 217 Nature America, Inc., part of Springer Nature. All rights reserved. a 1 b 3 c CFU-GEMM d e BFU-E f g KSL cells ( 1 3 /femur) % CD4.2 + % Osx d Osx-cherry d Osx-cherry+ 8 wks Osx-cherry 8 wks Osx-cherry+ SLAM + KSL cells per femur ( 1 2 ) % Mac1 + Gr Osterix CFCs per 1 4 WBM % B CFU-GM WBC ( 1 3 /µl) CD % CD Neutrophils ( 1 3 /µl) (pg/ml) Lymphocytes ( 1 3 /µl) 1, % Osx Non-irradiated % CD4.2 cgy Time after transplant (weeks) mrna expression % Mac1 + Gr h i j k mrna expression % B VE-cadherin + cgy BM cells ( 1 6 /femur) VE-cadherin + cgy 48 h % CD3 + Osx + cgy 1 1 Osx + vgy 48 h Figure 1 Deletion of Bak and Bax in Osx + BM cells radioprotects hematopoietic stem and progenitor cells. (a) Mean percentages of Osx + cells, as measured by flow cytometry, within Osx-labeled BM cells (Osx cherry + ) and Osx-unlabeled cells (Osx cherry ) from -d-old (n = 4 mice/group) and 8-week-old (n = 8 mice/group) Osx Cherry reporter mice. P <.1, P <.1. (b) Left, representative FACS plots showing the percentage of Osx + cells in CD4 BM cells in Osx Cre;Bak1 / ;Bax FL/+ () and Osx Cre;Bak1 / ;Bax FL/ (BAX FL/ ) mice at day +3 following cgy TBI. Right, the mean percentage of Osx + CD4 BM cells (n = 4 mice/group). P =.4. (c) Representative images of H&E-stained femurs from (left) and BAX FL/ (middle) mice at day +7 following cgy TBI (4 ; scale bars, 1 µm) and scatter plot of BM cell counts for mice in each group (n = 11 mice/group) (right). Horizontal lines represent means. P =.9. (d) Mean numbers of BM KSL cells (P =.3) (left) and SLAM + KSL cells (P =.4) (right) in and BAX FL/ mice at day +7 following cgy TBI (n = 11 mice/group). (e) Mean numbers of BM CFCs at day +7 (n = 21 assays/group). WBM, whole bone marrow; CFU-GM, colony-forming unit granulocyte monocyte; BFU-E, burst-forming unit erythroid; CFU-GEMM, colony-forming unit mix. P <.1. (f) Mean PB WBC (P =.1) (left), neutrophil (P =.2) (middle) and lymphocyte (P =.2) (right) counts at day +7 (FL/+, n = 14 mice; FL/, n = 13 mice). (g) Donor (CD4.2 + ) cell engraftment over time in recipient CD4.1 + mice that were transplanted with BM cells from or BAX FL/ mice and 1 1 competing, non-irradiated host BM cells. P =.3, P =.3, P =.2 and P =.4 for 4, 8, 12 and 2 weeks, respectively. Myeloid (Mac1/Gr1; P =.4), B cell (B22; P =.4) and T cell (CD3; P =.3) engraftment levels at 2 weeks are shown (4, 8 and 12 weeks: FL/+, n = 14 mice; FL/, n = 1 mice; 2 weeks: FL/+, n = 9 mice; FL/, n = 13 mice). (h) Mean levels of donor CD4.2 + cell and lineage engraftment in secondary recipient CD4.1 + mice at 2 weeks following competitive transplantation with BM cells from primary mice (FL/+, n = 7 mice; FL/, n = 9 mice). CD4.2 +, P =.4; B22 +, P =.3; CD3 +, P =.4. (i) Mean levels in the BM of non-irradiated or irradiated ( cgy) and BAX FL/- mice. P <.1 and P <.1 (NI FL/+, n = mice; NI FL/-, n = 9 mice; IRR FL/+, n = 8 mice; IRR FL/-, n = 7 mice). (j) Mean expression of in BM osteolineage cells in BAX FL/ mice relative to that in mice (n = 6 mice/group). P =.2. (k) expression in VE-cadherin + BM ECs and Osx + BM cells in mice before ( cgy; VE-cadherin +, n = 3 mice; Osx +, n = 4 mice) and 48 h after cgy TBI (n = 6 mice/group). expression levels in all groups were normalized to that in VE-cadherin + BM ECs at 48 h. P =.3 and P =.3. Throughout, P values were derived by using a two-tailed Student s t-test. nature medicine VOLUME 23 NUMBER 1 JANUARY
4 A r t i c l e s 217 Nature America, Inc., part of Springer Nature. All rights reserved. a CFCs per KSL cells d WBC ( 1 3 /µl) CFU-GM CFU-GEMM % CD4.2 % KSL Medium Time after transplant (weeks) Platelets ( 1 3 /µl) CFCs/1 3 WBM cells % Mac1 + Gr1 + f g h 1 CFU-GM CFU-GEMM Time after irradiation (d) Sca-1 c-kit Medium b Neutrophils ( 1 3 /µl) KSL cells ( 1 3 /femur) 1 1 Medium e % B22 + Survival (%) Medium % CD Medium c (pg ml 1 ) 2, 1, 1, BM cells ( 1 6 /femur) CFCs/1 3 WBM cells cgy BL6 NI BL6 (1 h) (24 h) CFU-GM CFU-GEMM BFU-E Figure 2 promotes hematopoietic regeneration in vitro and in vivo. (a) Mean numbers of CFCs recovered from 7-d cultures of irradiated (3 cgy) BM KSL cells that were treated with TPO, SCF and Flt-3 ligand (TSF)-containing medium (medium), with or without ng/ml (n = 3 assays/ group). P =.3. (b) Left, mean percentage of donor CD4.2 + cell engraftment levels in recipient CD4.1 + mice over time following competitive transplantation with the progeny of irradiated (3 cgy) BM KSL cells that were cultured with or without ng/ml for 7 d. P =.1, P =.3, P =.3, P =.1 for 4, 8, 12 and 16 weeks, respectively. Right, mean percentages of donor myeloid (P =.2), B cell (P =.1) and T cell (P =.1) engraftment at 16 weeks in recipient mice (n = 17 mice/group). (c) concentrations in the BM of mice at baseline, following cgy treatment, and at 1 h and 24 h following administration of 1 µg administration (BL6 non-irradiated (NI), n = mice; BL6, n = mice; (1 h), n = 6 mice; (24 h), n = mice). P <.1, P <.1. (d) Scatter plots of PB WBCs (left), neutrophils (middle) and platelet (right) counts at day +21 from mice irradiated with 8 cgy and then treated with saline or (saline, n = mice;, n = 12 mice). P =.8, P =.2, P =.1; by Mann Whitney U test. (e) Left, representative images of H&E-stained femurs from saline-treated (left) and -treated (middle) mice at day +21 following treatment with 8 cgy (4 ; scale bars, 1 µm). Right, mean BM cell counts (n = mice/group). P =.2. (f) Left, representative FACS plots showing the percentage of BM KSL cells in saline-treated and -treated mice at day +21 following treatment with 8 cgy TBI. Middle and right, the mean percentage of KSL cells (P =.2) (middle) and KSL cell numbers (P =.8) (right) (n = mice/group). P values determined by Mann Whitney U test. (g) Mean numbers of BM CFCs at day +21 following treatment with 8 cgy TBI (n = 6 assays/group). P =.4. (h) Survival of saline-treated (4/1) or -treated (13/14) mice following treatment with 8 cgy TBI (27% versus 93%; P =.4 by log-rank test). (i) Mean numbers of CFCs in C7BL/6 mice at 12 weeks after 6 cgy TBI and treatment with 1 µg or saline, subcutaneously, every other day through day +21 (n = assays/group). P =. 1. Throughout, unless otherwise noted, P values were derived by using a two-tailed Student s t-test. i whether endogenous is necessary for normal hematopoietic regeneration following TBI. We irradiated adult C7BL/6 mice with a sublethal TBI dose of cgy and then administered 1 µg of a blocking -specific antibody or an IgG control antibody every other day for 14 d. Anti- treatment significantly lowered levels in the BM at 24 h after treatment (Supplementary Fig. a). At day 1 following irradiation, mice that were treated with anti- had a significant decrease in BM cell counts and in the numbers of PB WBCs, neutrophils, lymphocytes, KSL cells and CFCs, as compared to those in irradiated, IgG-treated mice (Fig. 3a c and Supplementary Fig. b). Furthermore, treatment with anti- caused a significant decrease in the 3-d survival of irradiated mice (6 of 12 alive, %) as compared to irradiated controls (12 of 12, 1%; P =.6) (Fig. 3d). Taken together, these results suggest that endogenous plays an important role in regulating hematopoietic regeneration and survival following myelosuppression. Deletion of in Osx-expressing BM cells inhibits hematopoietic regeneration We next tested whether deletion of a single allele of in Osx-labeled cells (Osx Cre; FL/+ mice) would affect the hematopoietic response to TBI as compared to +/+ mice. 8-week-old Osx Cre; FL/+ 94 VOLUME 23 NUMBER 1 JANUARY 217 nature medicine
5 a r t i c l e s 217 Nature America, Inc., part of Springer Nature. All rights reserved. a b c d e f Cells ( 1 3 /µl) WBC Neutrophils IgG Anti- Lymphocytes CFU-GEMM BFU-E CFU-GM mice showed no significant differences in hematologic profile or BM stem and progenitor cell content at baseline compared to that in +/+ mice (Supplementary Fig. 6a d). However, at day 1 following -cgy TBI, Osx Cre; FL/+ mice showed a significant decrease in the numbers of WBCs, neutrophils and lymphocytes, as compared to those in +/+ mice (Fig. 3e). BM cell counts and the number of BM KSL cells, CFCs, BM myeloid cells, T cells and B cells were also decreased significantly in Osx Cre; FL/+ mice as compared to those in +/+ mice (Fig. 3f h and Supplementary Fig. 6e). These data suggest that endogenous, produced by Osxlabeled BM cells, has a necessary role in regulating the hematopoietic response to irradiation. Next we treated -cgy-irradiated Osx Cre; FL/+ mice with 1 µg of every other day for 1 d to determine whether treatment could rescue the hematopoietic phenotype of these mice. Although treatment did not alter the complete blood counts or BM cell counts in Osx Cre; FL/+ mice at day 1 after TBI, this treatment did significantly increase the numbers of BM KSL cells and BM CFCs, as compared to those in untreated mice (Fig. 3e h). These results provide evidence that deletion is responsible for the BM stem and progenitor cell abnormalities observed early after radiation injury in Osx Cre; FL/+ mice. Cells ( 1 3 /µl) BM cells ( 1 6 /femur) +/+ 8-week- FL/+ WBC 2 1 Neutrophils KSL cells ( 1 2 /femur) Lymphocytes 1 1 Survival (%) 1 IgG control Anti Time after irradiation (d) BM cells ( 1 6 /femur) Cells ( 1 3 /µl) +/+ 4. +/+ FL/ KSL cells ( 1 2 /femur) WBC week- FL/+ Our initial analysis of Osx Cre; FL/+ mice, in which the Cre protein was expressed from inception, did not allow for discrimination between the effects of deletion in Osx-expressing osteoprogenitors and those in Osx-expressing long-lived MSCs in the BM, which can persist into adulthood 6. In Osx Cre; FL/+ mice, expression of a tetracycline transactivator under the regulation of the osterix (Sp7) promoter controls the expression of a tetracycline response element (TRE)-controlled Cre fusion protein 22, such that doxycycline (Dox) administration prevents Cre-mediated recombination in Osx-expressing cells (Dox-Off). To study the effects of deletion starting at 8 weeks of age and older, we treated Osx Cre; FL/+ mice with Dox from inception to 8 weeks of age (hereafter referred to as 8-week-Osx Cre; FL/+ mice). Irradiation of 8-week-Osx Cre; FL/+ mice with cgy TBI at 1 weeks of age caused a significant decrease in the numbers of PB WBCs, neutrophils and lymphocytes, as well as in BM cell counts and in the numbers of BM KSL cells and BM CFCs at day 1 following TBI, as compared to those in Osx Cre; +/+ mice (Fig. 3i l). The enhanced hematopoietic toxicity following TBI that was observed in 8-week-Osx Cre; FL/+ mice was comparable to that observed in Osx Cre; FL/+ mice bearing a deletion from inception, suggesting that BM osteoprogenitor cells are the primary source of in regulating hematopoietic regeneration. Neutrophils Lymphocytes g h i j k l KSL cells ( 1 2 /femur) +/+ FL/+ FL/+ + CFCs/WBM +/+ FL/+ FL/+ + CFCs/WBM FL/+ + 6 BM cells ( 1 6 /femur) /+ CFU-GEMM BFU-E CFU-GM 8-week- FL/+ Figure 3 Inhibition or deficiency of in Osx-expressing BM cells suppresses hematopoietic regeneration. (a) PB WBCs, neutrophils and lymphocytes in mice treated with anti- or control IgG at day +1 following cgy TBI. P =.4, P <.1, P =.2, respectively (n = 1 mice/ group). (b) Left, representative images of H&E-stained femurs stained with IgG (top) or anti- (bottom) at day +1 (4 ; scale bars, µm). Right, BM cell counts at day +1 (n = mice/group). P <.1. (c) Mean KSL cell numbers at day +1 (n = mice/group). P =.7. (d) Survival analysis of mice irradiated with 7 cgy TBI and treated with anti- or IgG (n = 12 mice/group). P =.6. (e) The hematopoietic profiles (PB WBCs (P <.1), neutrophils (P =.1) and lymphocytes (P =.7)) of Osx Cre; FL/+ ( FL/+ ) and Osx Cre; +/+ ( +/+ ) mice at day 1 after irradiation with cgy TBI and of irradiated FL/+ mice that were treated every other day with through day +1 ( FL/+ + ) and evaluated at day +1 ( +/+, n = 9 mice; FL/+, n = 1 mice; FL/+ +, n = 1 mice). (f) BM cell counts at day +1 ( +/+, n = 9 mice; FL/+, n = 1 mice; FL/+ +, n = 1 mice). P <.1. (g) BM KSL cell numbers at day +1 ( +/+, n = 7 mice; FL/+, n = 1 mice; FL/+ +, n = 1 mice). P =.2, P =.3. (h) Mean numbers of BM CFCs at day +1 ( +/+, n = 6 assays; FL/+, n = 6 assays; FL/+ +, n = 3 assays). P =.8, P =.. (i) Mean concentrations of PB WBCs (P =.1), neutrophils (P <.1) and lymphocytes (P <.1) in Osx Cre; +/+ mice ( +/+ ) and 8-week-Osx Cre; FL/+ mice (8-week- FL/+ ) at day +1 following cgy TBI ( +/+, n = 11 mice; 8-week- FL/+, n = 7 mice). (j) Left, representative images of H&E-stained femurs from +/+ (top) and 8-week- FL/+ (bottom) mice at day +1 (4 ; scale bars, µm). Right, mean BM cell counts per femur ( +/+, n = 11 mice; 8-week- FL/+, n = 7 mice). P <.1. (k) Mean BM KSL cells at day +1 ( +/+, n = 7 mice; 8-week- FL/+, n = 6 mice). P =.1. (l) Mean BM CFCs at day +1 ( +/+, n = 6 assays; 8-week- FL/+, n = 3 assays). P =.. Throughout, P values were derived by using a two-tailed Student s t-test. nature medicine VOLUME 23 NUMBER 1 JANUARY 217 9
6 A r t i c l e s suppresses reactive oxygen species generation and p38-mediated senescence in irradiated HSCs Ionizing radiation can cause hematopoietic injury via generation of reactive oxygen species (ROS) and induction of HSC senescence 23. At 24 h following 3-cGy irradiation of cells in vitro, the levels of mitochondrial ROS increased substantially in BM CD34 KSL cells, KSL cells and lineage-negative (lin ) cells (Fig. 4a and Supplementary Fig. 7a). Treatment with ng/ml suppressed radiationinduced mitochondrial ROS generation in each cell population (Fig. 4a and Supplementary Fig. 7a). In vivo, systemic treatment of C7BL/6 mice irradiated with cgy TBI resulted in a significant decrease in the levels of mitochondrial ROS in BM CD34 KSL cells 217 Nature America, Inc., part of Springer Nature. All rights reserved. a b 2 c % MitoSOX + e % SA-β-Gal g PTN (pg/ml) NI NI Medium h 3 cgy BMEC Wnt3a Wnta f Count Erlotinib % p-p erlotinib NI BMEC + WBC ( 1 3 /ml) Medium 3 cgy Wnt3a Wnta EGF (pg/ml) 1, 1, % SA-β-Gal NEU ( 1 3 /ml) LacZ (Axin2) 3 cgy CD34 KSL KSL Lin NI 8 Irr/ 1 6 Irr/ BMEC NI Medium Wnt3a Wnta p16 mrna BMEC + d BM cells ( 1 6 /femur) Time after irradiation (d) NI Medium Wnt3a Wnta 3 cgy % Axin2 + EGF (pg/ml) NI 1 1 Time after irradiation (d) KSL cells ( 1 2 /femur) p16 mrna NI NI cgy (24 h) cgy (48 h) CD34 KSL KSL Lin NI NI Figure 4 suppresses HSC senescence following irradiation and induces EGF secretion by BM ECs. (a) The percentage of MitoSOX + cells in nonirradiated CD34 KSL cells (NI) and at 24 h after 3 cgy TBI without treatment (medium) or with treatment with ng/ml, 1 ng/ml Wnt3a or 2 ng/ml Wnta; treatment was initiated 1 min before irradiation (n = 4 cell cultures/group). Medium included TPO, SCF and Flt-3 ligand. P <.1, P <.1. (b) The percentage of phospho-p38-positve cells in CD34 KSL cells at 24 h after 3 cgy irradiation (NI, n = 8 cultures; medium, n = 7 cultures;, n = 8 cultures; Wnt3a, n = 8 cultures; Wnta, n = 7 cultures). P <.1, P <.1. (c) The percentage of SA βgal + CD34 KSL cells at 24 h after 3 cgy irradiation (n = 4 cultures/group). P <.1, P <.1. (d) Left, p16 gene expression in BM CD34 KSL cells at 24 h after 3 cgy irradiation in the indicated treatment groups. P =., P =.2 (n = 4 cultures/group). Right, p16 expression in BM KSL cells at 24 and 48 h after cgy TBI in mice treated without (saline) or with 1 µg (n = 4 cultures/group). 24 h, P <.1, P <.1; 48 h, P =.2, P =.2. (e) Mean percentages of SA β-gal + BM KSL cells in non-irradiated mice and in mice at 12 weeks following 6 cgy TBI and treated with saline or 1 µg every other day through day +21 (NI, n = 4 mice; saline, n = 7 mice;, n = 7 mice). P =.4, P =.2. (f) Left, representative histograms of Axin2 LacZ + cells within BM CD34 KSL cells, KSL cells and lin cells in mice at 24 h after cgy TBI in the treatment groups shown. Right, the mean percentage of Axin2 + cells in each cell population at 24 h following cgy TBI with and without treatment (n = 4 mice/group). CD34 KSL cells, P =.9, P =.1; KSL cells, P <.1, P =.4; lin cells, P <.1, P =.2. (g) Left, mean levels of PTN in cultures of BM ECs (n = cultures/group). Middle, mean levels of EGF in BM ECs in response to ng/nl treatment (P <.1; BMEC, n = cultures; BMEC +, n = 1 cultures). Right, mean levels of EGF in C7BL/6 mice over time following cgy TBI and treatment with saline or 1 µg every other day through day +14 (n = mice/group) (day +1, P =.3; day +14, P =.2). (h) Mean values for PB WBCs, neutrophils, BM cell counts and BM KSL cells at days +1 and +14 following cgy TBI and treatment with saline or 1 µg, with and without 1 µg/g erlotinib (n = mice/group). WBCs: day +1, P <.1;day +14, P =.2; neutrophils: day +1, P <.1; day +14, P =.1; BM cell counts: day +14, P =.2; BM KSL cells: day +14, P =.1. Throughout, P values were derived by using a two-tailed Student s t-test. 96 VOLUME 23 NUMBER 1 JANUARY 217 nature medicine
7 a r t i c l e s 217 Nature America, Inc., part of Springer Nature. All rights reserved. at 24 h after irradiation (Supplementary Fig. 7b). In vitro treatment with Wnt3a (a canonical Wnt agonist) or Wnta (a noncanonical Wnt ligand) did not significantly alter mitochondrial ROS levels in irradiated CD34 KSL cells, KSL cells or lin cells beyond the effects observed with radiation alone (Fig. 4a and Supplementary Fig. 7a). Consistent with its effects on decreasing ROS levels in irradiated HSCs, treatment of BM CD34 KSL cells increased CFC recovery in vitro (Supplementary Fig. 7c). However, in the presence of a ROS scavenger, N-acetylcysteine (NAC), treatment did not increase CFC recovery from irradiated KSL cells, suggesting that -mediated hematopoietic regeneration is dependent, at least in part, on suppression of ROS (Supplementary Fig. 7c). Elevated ROS levels drive activation of p38 mitogen-activated protein kinase (MAPK), which mediates a cytokine release phenomenon associated with senescence 23,24. Irradiation significantly increased p38 phosphorylation in BM CD34 KSL cells at 24 h after exposure, and this increase was significantly attenuated by treatment (Fig. 4b). Of note, treatment had more modest effects in reducing p38 phosphorylation in irradiated BM KSL and lin cells (Supplementary Fig. 7e). treatment also significantly reduced senescenceassociated β-galactosidase (SA β-gal) levels in irradiated BM CD34 KSL cells, KSL cells and lin cells (Fig. 4c and Supplementary Fig. 7f). In contrast, treatment with Wnt3a or Wnta had no significant effects on either p38 phosphorylation or SA β-gal levels in irradiated BM CD34 KSL cells (Fig. 4b,c). In keeping with its observed effects on SA β-gal levels, suppressed radiation-induced expression of the p16-encoding isoform of cyclin-dependent kinase inhibitor 2a (Cdkn2a; hereafter referred to as p16) in BM CD34 KSL cells in vitro and attenuated p16 expression in BM KSL cells in mice that were irradiated with cgy (Fig. 4d). treatment also suppressed HSC apoptosis in irradiated BM KSL cells, as measured by caspase-3 caspase-7 activation (Supplementary Fig. 7g). Next we extended our analysis of the effects of on radiationinduced senescence of BM HSC and progenitor cell populations by evaluating the long-term effects of systemic treatment on irradiated mice. At 12 weeks following 6 cgy TBI, C7BL/6 mice showed an increased frequency of SA β-gal + KSL cells, consistent with the idea that senescent BM stem and progenitor cells persist following radiation injury (Fig. 4e). This observation is in accord with a recent study that demonstrated that TBI induces persistent senescence of HSCs and premature aging of the hematopoietic system 2. Notably, mice that were treated with every other day through day 21 following TBI showed a significantly lower percentage of SA β-gal + KSL cells at 12 weeks, as compared to untreated, irradiated mice (Fig. 4e). Coupled with our observation that treatment led to an increase in BM CFCs at 12 weeks following irradiation (Fig. 2i), these data suggest that short-term treatment with promotes longer-term recovery of hematopoietic progenitor cells following radiation injury. inhibits Wnt pathway activation in irradiated HSCs is a member of the Dkk family of secreted proteins that can inhibit Wnt signaling via binding and sequestration of the Wnt coreceptors, LRP and LRP6 26. Using Axin2 LacZ reporter mice as previously described 27,28, we evaluated Wnt pathway activation in BM HSC and progenitor cell subsets in response to irradiation and treatment. Exposure of mice to cgy TBI increased Wnt signaling in BM CD34 KSL cells, KSL cells and lin cells at 24 h after exposure (Fig. 4f). This activation of Wnt signaling was suppressed by treatment in these cell populations at 24 h after irradiation, as measured by the percentage of Axin2 + cells and the mean fluorescence intensity (MFI) of the Axin2 lacz signal (Fig. 4f and Supplementary Fig. 8a). Of note, Wnt pathway activation in response to irradiation seemed to be stronger in the BM CD34 KSL cells and the KSL cells as compared to that in BM lin cells (Fig. 4f). As assessed by the percentage of Axin2 + cells, -mediated suppression of Wnt signaling was comparable across the three cell populations, although MFI analysis suggested that had the strongest suppressive effect in BM KSL cells (Supplementary Fig. 8a). Axin2 mrna levels increased concordantly in BM KSL cells in mice at 24 h after cgy TBI, and this increase in Axin2 mrna was abrogated by treatment (Supplementary Fig. 8b). To determine the functional relevance of Wnt inhibition to - mediated hematopoietic regeneration, we used a viral Cre based approach to delete Ctnnb1, the gene encoding β-catenin, in BM KSL cells from Ctnnb1 FL/FL mice (Supplementary Fig. 9a) 29. At 96 h following 3 cgy irradiation in vitro, treatment decreased ROS generation in Ctnnb1 +/+ BM KSL cells but not in Ctnnb1 / KSL cells (Supplementary Fig. 9b). Similarly, treatment increased CFC recovery at 96 h after irradiation from Ctnnb1 +/+ KSL cells but not from Ctnnb1 / KSL cells (Supplementary Fig. 9c). These data suggest that Wnt pathway inhibition contributes, at least in part, to the early effects of on ROS generation and hematopoietic progenitor recovery. promotes hematopoietic regeneration via induction of EGF secretion by BM ECs Next we tested the possibility that there might be cross-talk between and pleiotrophin (PTN) or EGF, two vascular-niche-derived proteins that we have previously shown to be HSC regenerative factors 16,18,19. We first tested whether treatment of primary BM osteolineage cells (Osx + osteocalcin + cells cultured from femur bone explants) with PTN or EGF could induce secretion. However, this was not observed in 7-d culture; indeed, EGF treatment significantly reduced secretion (Supplementary Fig. 1). Next we asked whether could induce the secretion of these paracrine factors by primary BM ECs, as BM ECs are known to regulate HSC regeneration 1,17,3,31. Although treatment did not induce the secretion of PTN by primary BM ECs, it induced a greater than,- fold increase in EGF levels after 7 d, as compared to that in untreated BM ECs (Fig. 4g). Systemic administration of to mice irradiated with cgy caused a marked increase in EGF levels in the BM at days 1 and 14 following radiation (Fig. 4g). To test the functional relevance of -mediated induction of EGF secretion to hematopoietic regeneration, we orally administered 1 µg per g body weight of erlotinib, an EGF receptor antagonist, daily for 1 d to -cgyirradiated, -treated mice. Erlotinib treatment abrogated - mediated recovery of PB WBCs, neutrophils, BM cell counts and BM KSL cells at day 1 and day 14, as compared to that in mice treated with alone (Fig. 4h). These results suggest that, in addition to its direct effects on hematopoietic cells, promotes hematopoietic regeneration via induction of EGF secretion by BM ECs. DISCUSSION The functions of BM osteolineage cells and MSCs in regulating HSC regeneration are incompletely understood 32,33. Here we demonstrate that deletion of a single allele of in Osx-expressing BM cells abrogates hematopoietic regeneration following irradiation. Inducible deletion of in Osx-expressing cells in >8-week-old mice caused a comparable defect in hematopoietic regeneration in response to TBI as observed in mice bearing deletion of from inception nature medicine VOLUME 23 NUMBER 1 JANUARY
8 A r t i c l e s 217 Nature America, Inc., part of Springer Nature. All rights reserved. in Osx-expressing cells. These data suggest that BM osteoprogenitor cells, rather than long-lived MSCs, are the primary source of that regulates hematopoietic regeneration in adult mice. In gain-of-function studies, systemic treatment accelerated hematopoietic regeneration and improved survival following lethal dose TBI. This marked improvement in survival may have been related to the augmented recovery of myeloid progenitor cells, which have been shown to mediate survival following lethal dose TBI 34. treatment also directly promoted the regeneration of HSCs with competitive repopulating capacity following irradiation in vitro. In a prior study of transgenic mice by Fleming et al., overexpression of at steady state was associated with an increased frequency of HSCs capable of primary competitive repopulation, coupled with a deficiency in HSCs capable of serial transplantation 3. In the current study, we examined the effects of treatment or deletion on the hematopoietic regenerative response to myelotoxic injury, whereas Fleming et al. described the hematopoietic effects of overexpression during homeostasis 3. It is possible that -mediated signaling has distinct effects on HSCs in the setting of stress versus homeostasis, as has been demonstrated for Notch signaling 36. Furthermore, we found that treatment induces BM ECs to secrete EGF, a regenerative factor for HSCs 19, an effect that likely also contributes to the differences in the results of our study as compared to the prior study by Fleming et al. 3. Ionizing radiation causes the generation of ROS in HSCs, and ROS accumulation contributes to radiation-induced HSC apoptosis and senescence 23,37,38. ROS are also deleterious to short- and long-term HSC function and HSC self-renewal capacity 39,4. ROS promote HSC senescence via activation of p38 MAPK and p16 23,24. Conversely, FoxO proteins are essential for downregulating ROS levels in HSCs, and transfer of ROS via gap junctions containing gap junction protein alpha 1 (Gja1; also known as connexin-43) to BM stromal cells can mitigate ROS-mediated HSC senescence 39,4. Notably, ROS generation occurs during BM or cord blood collection in ambient air, and this was recently shown to be highly damaging to HSC yield and function 41. Furthermore, high-dose irradiation has been shown to markedly increase po 2 concentrations in HSC niches via BM vascular disruption 42. These findings underscore the therapeutic importance of suppressing ROS after myelosuppressive injury and may explain, in part, the potency of administration in promoting the survival of lethally irradiated mice. Canonical Wnt signaling has been shown to regulate the fate of HSCs, myeloid progenitors and T cell subsets in a dosage-dependent manner 26 28,43. Using Axin2 lacz reporter mice, we observed that TBI rapidly induced Wnt pathway activation in HSCs and progenitor cells and that systemic treatment with suppressed this response. also suppressed ROS generation and HSC senescence in irradiated mice, concordant with inhibitory effects on Wnt signaling. These findings are consistent with recent studies suggesting that treatment suppresses ROS generation in nonhematopoietic tissues, as well as a report demonstrating that Wnt pathway activation promotes mitochondrial ROS generation and senescence in myoblasts and fibroblasts 44,4. However, in the hematopoietic system, several studies have suggested that activation of canonical Wnt signaling promotes HSC self-renewal A recent study by Lento et al. suggested that mice with deletion of Ctnnb1 in hematopoietic Vav + cells showed reduced hematopoietic progenitor cell recovery at day 14 following TBI 49. Several factors may explain the different observations between our study and that of Lento et al. First, acute inhibition of Wnt signaling with in the first 72 h after irradiation may have different effects on HSC regeneration as compared to that in mice with deletion of Ctnnb1 from inception. Second, it is possible that mediates effects on hematopoietic regeneration via mechanisms that are independent from its inhibitory effects on canonical Wnt signaling, as observed in models of kidney epithelial repair and cardiogenesis,1. As we discovered, acts indirectly on BM ECs, yielding a marked increase in EGF secretion, which contributed to hematopoietic regeneration. In this regard, a recent study demonstrated that ECs express LRP6, a receptor, and that and Dkk2 may regulate angiogenesis 2. Third, the dosage of canonical Wnt pathway activation has been demonstrated to substantially impact HSCs, progenitor cells and HSC differentiation 28,3, and high dose levels of Wnt pathway activation can result in decreased HSC repopulating capacity 4,. Our data suggest that during the early period following radiation injury, strong induction of Wnt signaling in HSCs may be deleterious, whereas early inhibition of Wnt signaling is beneficial. Canonical and noncanonical Wnt signaling have been shown to have discrete effects on hematopoiesis 6. Wnta expression in HSCs increases with aging, causing a shift to noncanonical Wnt signaling and a decline in HSC function in older mice 7. HSC senescence occurs over time in aged mice and is exacerbated by stress, including radiation exposure 23,37. Here we observed that acute radiation injury increased canonical Wnt pathway activation in HSCs and that treatment with suppressed this signaling, in association with repression of ROS generation and HSC senescence. However, treatment with Wnta did not exacerbate radiation-induced ROS generation or HSC senescence. We postulate that the effects of Wnta and noncanonical Wnt signaling during hematopoietic aging may be distinct from the effects of noncanonical or canonical Wnt signaling in the setting of acute injury. Over the past decade, as the cellular composition of the HSC niche has been elucidated, important questions have emerged regarding the hierarchy of cellular niche components and the manner through which niche cells communicate during their orchestration of HSC maintenance and regeneration 8,9. Recent studies suggest that distinct BM ECs within arterial and sinusoidal blood vessels regulate HSC maintenance and activation 6. Our results demonstrate that BM osteoprogenitors regulate hematopoietic regeneration via secretion of, which promotes hematopoietic regeneration directly via inhibition of HSC senescence and indirectly via induction of EGF secretion by BM ECs. This latter mechanism provides a unique example of cross-talk between distinct niche cell types and highlights the importance of cooperative niche cell interactions in hematopoietic regeneration. Methods Methods, including statements of data availability and any associated accession codes and references, are available in the online version of the paper. Note: Any Supplementary Information and Source Data files are available in the online version of the paper. Acknowledgments We thank S. Vainio (University of Oulu) for the FL/+ mice. This work was supported, in part, by NHLBI grant HL (J.P.C.), NIAID grants AI (J.P.C.) and AI (J.P.C.), the California Institute for Regenerative Medicine Leadership Award LA1-814 (J.P.C.) and the NIAID Centers for Medical Countermeasures Against Radiation (CMCR) Pilot Award 2U19AI (H.A.H.). 98 VOLUME 23 NUMBER 1 JANUARY 217 nature medicine
9 a r t i c l e s 217 Nature America, Inc., part of Springer Nature. All rights reserved. AUTHOR CONTRIBUTIONS J.P.C. conceived of and designed the study; H.A.H. designed and performed the majority of the experiments and analyzed data; P.L.D. and J.R.H. performed experiments and analyzed data; M.Q., X.Y., J.S., L.Z., G.V.H., J.K., K.A.P. and E.T. performed experiments; N.J.C. contributed to the design and interpretation of the study; and H.A.H. and J.P.C. wrote the paper. COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests. Reprints and permissions information is available online at reprints/index.html. 1. Ding, L., Saunders, T.L., Enikolopov, G. & Morrison, S.J. Endothelial and perivascular cells maintain hematopoietic stem cells. Nature 481, (212). 2. Ding, L. & Morrison, S.J. Hematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 49, (213). 3. Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for hematopoietic stem cell maintenance. Nature 49, (213). 4. Méndez-Ferrer, S. et al. Mesenchymal and hematopoietic stem cells form a unique bone marrow niche. Nature 466, (21).. Pinho, S. et al. PDGFR-α and CD1 mark human nestin + sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 21, (213). 6. Mizoguchi, T. et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 29, (214). 7. Kunisaki, Y. et al. Arteriolar niches maintain hematopoietic stem cell quiescence. Nature 2, (213). 8. Calvi, L.M. et al. Osteoblastic cells regulate the hematopoietic stem cell niche. Nature 42, (23). 9. Zhang, J. et al. Identification of the hematopoietic stem cell niche and control of the niche size. Nature 42, (23). 1. Visnjic, D. et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 13, (24). 11. Kiel, M.J., Acar, M., Radice, G.L. & Morrison, S.J. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell 4, (29). 12. Park, D. et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 1, (212). 13. Maes, C. et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, (21). 14. Raaijmakers, M.H. et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukemia. Nature 464, (21). 1. Doan, P.L. et al. Tie2 + bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells 31, (213). 16. Himburg, H.A. et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2, (212). 17. Hooper, A.T. et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, (29). 18. Himburg, H.A. et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat. Med. 16, (21). 19. Doan, P.L. et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat. Med. 19, (213). 2. Kirsch, D.G. et al. p3 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 327, (21). 21. Zhang, C. et al. Inhibition of Wnt signaling by the osteoblast-specific transcription factor osterix. Proc. Natl. Acad. Sci. USA 1, (28). 22. Rodda, S.J. & McMahon, A.P. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133, (26). 23. Cho, J. et al. Purinergic P2Y receptor modulates stress-induced hematopoietic stem and progenitor cell senescence. J. Clin. Invest. 124, (214). 24. Freund, A., Patil, C.K. & Campisi, J. p38 MAPK is a novel DNA damage responseindependent regulator of the senescence-associated secretory phenotype. EMBO J. 3, (211). 2. Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, (216). 26. Mao, B. et al. Kremen proteins are dickkopf receptors that regulate Wnt β-catenin signaling. Nature 417, (22). 27. Boudousquié, C. et al. Differences in the transduction of canonical Wnt signals demarcate effector and memory CD8 T cells with distinct recall proliferation capacity. J. Immunol. 193, (214). 28. Luis, T.C. et al. Canonical Wnt signaling regulates hematopoiesis in a dosagedependent fashion. Cell Stem Cell 9, (211). 29. Brault, V. et al. Inactivation of the β-catenin gene by Wnt1 Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, (21). 3. Poulos, M.G. et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 4, (213). 31. Salter, A.B. et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood 113, (29). 32. Olson, T.S. et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 121, (213). 33. Dominici, M. et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood 114, (29). 34. Na Nakorn, T., Traver, D., Weissman, I.L. & Akashi, K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J. Clin. Invest. 19, (22). 3. Fleming, H.E. et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2, (28). 36. Varnum-Finney, B. et al. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J. Clin. Invest. 121, (211). 37. Shao, L. et al. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood 123, (214). 38. Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of hematopoietic stem cells. Nature 431, (24). 39. Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, (27). 4. Taniguchi Ishikawa, E. et al. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc. Natl. Acad. Sci. USA 19, (212). 41. Mantel, C.R. et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell 161, (21). 42. Spencer, J.A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 8, (214). 43. Luis, T.C., Ichii, M., Brugman, M.H., Kincade, P. & Staal, F.J. Wnt signaling strength regulates normal hematopoiesis, and its de-regulation is involved in leukemia development. Leukemia 26, (212). 44. Kim, I.G. et al. Disturbance of DKK1 level is partly involved in survival of lung cancer cells via regulation of ROMO1 and γ-radiation sensitivity. Biochem. Biophys. Res. Commun. 443, 49 (214). 4. Yoon, J.C. et al. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 24, (21). 46. Huang, J. et al. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J. Clin. Invest. 119, (29). 47. Li, W. et al. Apc regulates the function of hematopoietic stem cells largely through β-catenin-dependent mechanisms. Blood 121, (213). 48. Huang, J., Nguyen-McCarty, M., Hexner, E.O., Danet-Desnoyers, G. & Klein, P.S. Maintenance of hematopoietic stem cells through regulation of Wnt and mtor pathways. Nat. Med. 18, (212). 49. Lento, W. et al. Loss of β-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes Dev. 28, (214).. Lin, S.L. et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. USA 17, (21). 1. Foley, A.C. & Mercola, M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 19, (2). 2. Min, J.K. et al. The WNT antagonist dickkopf-2 promotes angiogenesis in rodent and human endothelial cells. J. Clin. Invest. 121, (211). 3. Famili, F. et al. High levels of canonical Wnt signaling lead to loss of stemness and increased differentiation in hematopoietic stem cells. Stem Cell Rep. 6, (216). 4. Kirstetter, P., Anderson, K., Porse, B.T., Jacobsen, S.E. & Nerlov, C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 7, (26).. Scheller, M. et al. Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nat. Immunol. 7, (26). 6. Malhotra, S. et al. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J. Immunol. 181, (28). 7. Florian, M.C. et al. A canonical to noncanonical Wnt signaling switch in hematopoietic stem cell aging. Nature 3, (213). 8. Mendelson, A. & Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2, (214). 9. Morrison, S.J. & Scadden, D.T. The bone marrow niche for hematopoietic stem cells. Nature, (214). 6. Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate hematopoiesis. Nature 32, (216). nature medicine VOLUME 23 NUMBER 1 JANUARY
Meeting Report. From December 8 to 11, 2012 at Atlanta, GA, U.S.A
 Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Pleiotrophin Regulates the Expansion and Regeneration of Hematopoietic Stem Cells Heather A Himburg 1, Garrett G Muramoto 1 *, Pamela Daher 1*, Sarah K Meadows 1, J. Lauren Russell
SUPPLEMENTARY INFORMATION Pleiotrophin Regulates the Expansion and Regeneration of Hematopoietic Stem Cells Heather A Himburg 1, Garrett G Muramoto 1 *, Pamela Daher 1*, Sarah K Meadows 1, J. Lauren Russell
Hematopoiesis. - Process of generation of mature blood cells. - Daily turnover of blood cells (70 kg human)
 Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
Hematopoiesis - Process of generation of mature blood cells - Daily turnover of blood cells (70 kg human) 1,000,000,000,000 total cells 200,000,000,000 red blood cells 70,000,000,000 neutrophils Hematopoiesis
The Role of Rac Signaling in The Perivascular Niche
 The Role of Rac Signaling in The Perivascular Niche Felicia Ciuculescu Diaspora and Higher Education and Research Perspectives in Personalized Medicine- from Concept to Clinical Application Center for
The Role of Rac Signaling in The Perivascular Niche Felicia Ciuculescu Diaspora and Higher Education and Research Perspectives in Personalized Medicine- from Concept to Clinical Application Center for
CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow
 White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
Haematopoietic stem cells
 Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
Haematopoietic stem cells Neil P. Rodrigues, DPhil NIH Centre for Biomedical Research Excellence in Stem Cell Biology Boston University School of Medicine neil.rodrigues@imm.ox.ac.uk Haematopoiesis: An
TISSUE-SPECIFIC STEM CELLS
 TISSUE-SPECIFIC STEM CELLS Inhibition of Aldehyde Dehydrogenase Expands Hematopoietic Stem Cells with Radioprotective Capacity GARRETT G. MURAMOTO, a J. LAUREN RUSSELL, a RACHID SAFI, b ALICE B. SALTER,
TISSUE-SPECIFIC STEM CELLS Inhibition of Aldehyde Dehydrogenase Expands Hematopoietic Stem Cells with Radioprotective Capacity GARRETT G. MURAMOTO, a J. LAUREN RUSSELL, a RACHID SAFI, b ALICE B. SALTER,
Nature Genetics: doi: /ng Supplementary Figure 1
 Supplementary Figure 1 MSI2 interactors are associated with the riboproteome and are functionally relevant. (a) Coomassie blue staining of FLAG-MSI2 immunoprecipitated complexes. (b) GO analysis of MSI2-interacting
Supplementary Figure 1 MSI2 interactors are associated with the riboproteome and are functionally relevant. (a) Coomassie blue staining of FLAG-MSI2 immunoprecipitated complexes. (b) GO analysis of MSI2-interacting
SUPPLEMENTARY INFORMATION doi: /nature12026
 doi:1.138/nature1226 a 4 35 3 MCSF level (pg/ml) 25 2 15 1 5 1h3 3h 5h 7h 15h 24h b MPP (CD135 KSL) HSC (CD34 CD15 KSLF) c % 4 ** LPS 3 GFP pos cells 2 PU.1 GFP LPS 1 FSCA Ctl NI 24h LPS Sup.Fig.1 Effect
doi:1.138/nature1226 a 4 35 3 MCSF level (pg/ml) 25 2 15 1 5 1h3 3h 5h 7h 15h 24h b MPP (CD135 KSL) HSC (CD34 CD15 KSLF) c % 4 ** LPS 3 GFP pos cells 2 PU.1 GFP LPS 1 FSCA Ctl NI 24h LPS Sup.Fig.1 Effect
Chronic variable stress activates hematopoietic stem cells
 SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
SUPPLEMENTARY INFORMATION Chronic variable stress activates hematopoietic stem cells Timo Heidt *, Hendrik B. Sager *, Gabriel Courties, Partha Dutta, Yoshiko Iwamoto, Alex Zaltsman, Constantin von zur
Stem cells: units of development and regeneration. Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research.
 Stem cells: units of development and regeneration Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research Concepts 1. Embryonic vs. adult stem cells 2. Hematopoietic stem
Stem cells: units of development and regeneration Fernando D. Camargo Ph.D. Whitehead Fellow Whitehead Institute for Biomedical Research Concepts 1. Embryonic vs. adult stem cells 2. Hematopoietic stem
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
Nature Immunology: doi: /ni Supplementary Figure 1. Huwe1 has high expression in HSCs and is necessary for quiescence.
 Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
SUPPLEMENTARY INFORMATION
 a. Smo+/+ b. Smo+/+ 5.63 5.48 c. Lin- d. e. 6 5 4 3 Ter119 Mac B T Sca1 Smo+/+ 25 15 2 o BMT 2 1 5 * Supplementary Figure 1: Deletion of Smoothened does not alter the frequency of hematopoietic lineages
a. Smo+/+ b. Smo+/+ 5.63 5.48 c. Lin- d. e. 6 5 4 3 Ter119 Mac B T Sca1 Smo+/+ 25 15 2 o BMT 2 1 5 * Supplementary Figure 1: Deletion of Smoothened does not alter the frequency of hematopoietic lineages
DISCLOSURE. I have the following financial relationships:
 DISCLOSURE I have the following financial relationships: Consultant for: Fate Therapeutics, GlaxoSmithKline, Bone Therapeutics, G1 Therapeutics Contracted Research for: GlaxoSmithKline Royalties from:
DISCLOSURE I have the following financial relationships: Consultant for: Fate Therapeutics, GlaxoSmithKline, Bone Therapeutics, G1 Therapeutics Contracted Research for: GlaxoSmithKline Royalties from:
Protein tyrosine phosphatase σ regulates hematopoietic stem cell repopulating capacity
 The Journal of Clinical Investigation Brief Report Protein tyrosine phosphatase σ regulates hematopoietic stem cell repopulating capacity Mamle Quarmyne, 1,2 Phuong L. Doan, 3 Heather A. Himburg, 1 Xiao
The Journal of Clinical Investigation Brief Report Protein tyrosine phosphatase σ regulates hematopoietic stem cell repopulating capacity Mamle Quarmyne, 1,2 Phuong L. Doan, 3 Heather A. Himburg, 1 Xiao
Getting to the root of Cancer
 Cancer Stem Cells: Getting to the root of Cancer Dominique Bonnet, Ph.D Senior Group Leader, Haematopoietic Stem Cell Laboratory Cancer Research UK, London Research Institute Venice, Sept 2009 Overview
Cancer Stem Cells: Getting to the root of Cancer Dominique Bonnet, Ph.D Senior Group Leader, Haematopoietic Stem Cell Laboratory Cancer Research UK, London Research Institute Venice, Sept 2009 Overview
DISCOVERING ATCC IMMUNOLOGICAL CELLS - MODEL SYSTEMS TO STUDY THE IMMUNE AND CARDIOVASCULAR SYSTEMS
 DISCOVERING ATCC IMMUNOLOGICAL CELLS - MODEL SYSTEMS TO STUDY THE IMMUNE AND CARDIOVASCULAR SYSTEMS James Clinton, Ph.D. Scientist, ATCC February 19, 2015 About ATCC Founded in 1925, ATCC is a non-profit
DISCOVERING ATCC IMMUNOLOGICAL CELLS - MODEL SYSTEMS TO STUDY THE IMMUNE AND CARDIOVASCULAR SYSTEMS James Clinton, Ph.D. Scientist, ATCC February 19, 2015 About ATCC Founded in 1925, ATCC is a non-profit
Hematopoiesis. BHS Liège 27/1/2012. Dr Sonet Anne UCL Mont-Godinne
 Hematopoiesis BHS Liège 27/1/2012 Dr Sonet Anne UCL Mont-Godinne Hematopoiesis: definition = all the phenomenons to produce blood cells Leukocytes = White Blood Cells Polynuclear = Granulocytes Platelet
Hematopoiesis BHS Liège 27/1/2012 Dr Sonet Anne UCL Mont-Godinne Hematopoiesis: definition = all the phenomenons to produce blood cells Leukocytes = White Blood Cells Polynuclear = Granulocytes Platelet
The nucleotide sugar UDP-glucose mobilizes long-term repopulating primitive hematopoietic cells
 Research article The nucleotide sugar UDP-glucose mobilizes long-term repopulating primitive hematopoietic cells Sungho Kook, 1 Joonseok Cho, 1 Sean Bong Lee, 2 and Byeong-Chel Lee 1 1 University of Pittsburgh
Research article The nucleotide sugar UDP-glucose mobilizes long-term repopulating primitive hematopoietic cells Sungho Kook, 1 Joonseok Cho, 1 Sean Bong Lee, 2 and Byeong-Chel Lee 1 1 University of Pittsburgh
BCR-ABL - LSK BCR-ABL + LKS - (%)
 Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Stem cells are undifferentiated cells which are maintained within a specific niche. A stem cell
 Abstract Stem cells are undifferentiated cells which are maintained within a specific niche. A stem cell niche is a microenvironment of cells that maintain stem cell functionality, and one example is the
Abstract Stem cells are undifferentiated cells which are maintained within a specific niche. A stem cell niche is a microenvironment of cells that maintain stem cell functionality, and one example is the
Protein Tyrosine Phosphatase Receptor Type S (PTPRS) Regulates Hematopoietic Stem. Cell Self-Renewal. Mamle Quarmyne
 Protein Tyrosine Phosphatase Receptor Type S (PTPRS) Regulates Hematopoietic Stem Cell Self-Renewal by Mamle Quarmyne Department of Pharmacology and Cancer Biology Duke University Date: Approved: John
Protein Tyrosine Phosphatase Receptor Type S (PTPRS) Regulates Hematopoietic Stem Cell Self-Renewal by Mamle Quarmyne Department of Pharmacology and Cancer Biology Duke University Date: Approved: John
Cytokines, adhesion molecules and apoptosis markers. A comprehensive product line for human and veterinary ELISAs
 Cytokines, adhesion molecules and apoptosis markers A comprehensive product line for human and veterinary ELISAs IBL International s cytokine product line... is extremely comprehensive. The assays are
Cytokines, adhesion molecules and apoptosis markers A comprehensive product line for human and veterinary ELISAs IBL International s cytokine product line... is extremely comprehensive. The assays are
% of live splenocytes. STAT5 deletion. (open shapes) % ROSA + % floxed
 Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
X P. Supplementary Figure 1. Nature Medicine: doi: /nm Nilotinib LSK LT-HSC. Cytoplasm. Cytoplasm. Nucleus. Nucleus
 a b c Supplementary Figure 1 c-kit-apc-eflu780 Lin-FITC Flt3-Linc-Kit-APC-eflu780 LSK Sca-1-PE-Cy7 d e f CD48-APC LT-HSC CD150-PerCP-cy5.5 g h i j Cytoplasm RCC1 X Exp 5 mir 126 SPRED1 SPRED1 RAN P SPRED1
a b c Supplementary Figure 1 c-kit-apc-eflu780 Lin-FITC Flt3-Linc-Kit-APC-eflu780 LSK Sca-1-PE-Cy7 d e f CD48-APC LT-HSC CD150-PerCP-cy5.5 g h i j Cytoplasm RCC1 X Exp 5 mir 126 SPRED1 SPRED1 RAN P SPRED1
Bone Cell Precursors and the Pathophysiology of Bone Loss
 Bone Cell Precursors and the Pathophysiology of Bone Loss HARRY C. BLAIR, a AND JILL L. CARRINGTON b a Departments of Pathology and Cell Biology, University of Pittsburgh, and Pittsburgh VA Medical Center,
Bone Cell Precursors and the Pathophysiology of Bone Loss HARRY C. BLAIR, a AND JILL L. CARRINGTON b a Departments of Pathology and Cell Biology, University of Pittsburgh, and Pittsburgh VA Medical Center,
Supplementary Figure 1. Successful excision of genes from WBM lysates and
 Supplementary Information: Supplementary Figure 1. Successful excision of genes from WBM lysates and survival of mice with different genotypes. (a) The proper excision of Pten, p110α, p110α and p110δ was
Supplementary Information: Supplementary Figure 1. Successful excision of genes from WBM lysates and survival of mice with different genotypes. (a) The proper excision of Pten, p110α, p110α and p110δ was
Nature Immunology: doi: /ni.3412
 Supplementary Figure 1 Gata1 expression in heamatopoietic stem and progenitor populations. (a) Unsupervised clustering according to 100 top variable genes across single pre-gm cells. The two main cell
Supplementary Figure 1 Gata1 expression in heamatopoietic stem and progenitor populations. (a) Unsupervised clustering according to 100 top variable genes across single pre-gm cells. The two main cell
SUPPLEMENTARY INFORMATION
 Haematopoietic stem cell release is regulated by circadian oscillations Simón Méndez-Ferrer *, Daniel Lucas *, Michela Battista * and Paul S. Frenette * Mount Sinai School of Medicine, *Departments of
Haematopoietic stem cell release is regulated by circadian oscillations Simón Méndez-Ferrer *, Daniel Lucas *, Michela Battista * and Paul S. Frenette * Mount Sinai School of Medicine, *Departments of
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
Scientific report: Delineating cellular stages and regulation of human NK cell development to improve NK cell-based therapy for cancer (Dnr )
 Scientific report: Delineating cellular stages and regulation of human NK cell development to improve NK cell-based therapy for cancer (Dnr 130259) The main goal of this project focuses on establishing
Scientific report: Delineating cellular stages and regulation of human NK cell development to improve NK cell-based therapy for cancer (Dnr 130259) The main goal of this project focuses on establishing
Nature Immunology: doi: /ni Supplementary Figure 1. Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice.
 Supplementary Figure 1 Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice. (a, b) Gating strategies for differentiated cells including PMN (CD11b + Ly6G hi and CD11b + Ly6G
Supplementary Figure 1 Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice. (a, b) Gating strategies for differentiated cells including PMN (CD11b + Ly6G hi and CD11b + Ly6G
Molecular Characterization of Leukemia Stem Cell Development. Scott A. Armstrong MD, Ph.D.
 Molecular Characterization of Leukemia Stem Cell Development Scott A. Armstrong MD, Ph.D. Normal and Leukemic Hierarchies NORMAL HSC (SRC) Myeloid progenitor LTC-IC CFU AML LSC (SL-IC) Leukemic LTC-IC
Molecular Characterization of Leukemia Stem Cell Development Scott A. Armstrong MD, Ph.D. Normal and Leukemic Hierarchies NORMAL HSC (SRC) Myeloid progenitor LTC-IC CFU AML LSC (SL-IC) Leukemic LTC-IC
Journal Club Semmler Lorenz
 Beer et al. 2015 - Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration Journal Club 13.11.2017 1 Introduction to
Beer et al. 2015 - Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration Journal Club 13.11.2017 1 Introduction to
HSC Niche Biology and HSC Expansion Ex Vivo
 Feature Review HSC Niche Biology and HSC Expansion Ex Vivo Sachin Kumar 1, * and Hartmut Geiger 1,2,3, * Hematopoietic stem cell (HSC) transplantation can restore a new functional hematopoietic system
Feature Review HSC Niche Biology and HSC Expansion Ex Vivo Sachin Kumar 1, * and Hartmut Geiger 1,2,3, * Hematopoietic stem cell (HSC) transplantation can restore a new functional hematopoietic system
Natural Killer Cells: Development, Diversity, and Applications to Human Disease Dr. Michael A. Caligiuri
 Natural Killer Cells: Development, Diversity, November 26, 2008 The Ohio State University Comprehensive Cancer Center The James Cancer Hospital and Solove Research Institute Columbus, Ohio, USA 1 Human
Natural Killer Cells: Development, Diversity, November 26, 2008 The Ohio State University Comprehensive Cancer Center The James Cancer Hospital and Solove Research Institute Columbus, Ohio, USA 1 Human
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Supplemental Experimental Procedures
 Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Pearson r = P (one-tailed) = n = 9
 8F4-Specific Lysis, % 1 UPN1 UPN3 8 UPN7 6 Pearson r =.69 UPN2 UPN5 P (one-tailed) =.192 4 UPN8 n = 9 2 UPN9 UPN4 UPN6 5 1 15 2 25 8 8F4, % Max MFI Supplementary Figure S1. AML samples UPN1-UPN9 show variable
8F4-Specific Lysis, % 1 UPN1 UPN3 8 UPN7 6 Pearson r =.69 UPN2 UPN5 P (one-tailed) =.192 4 UPN8 n = 9 2 UPN9 UPN4 UPN6 5 1 15 2 25 8 8F4, % Max MFI Supplementary Figure S1. AML samples UPN1-UPN9 show variable
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES. Overview and Mechanism of Action Dr.
 BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
Selective Enhancement of Donor Hematopoietic Cell Engraftment by the CXCR4 Antagonist AMD3100 in a Mouse Transplantation Model
 Selective Enhancement of Donor Hematopoietic Cell Engraftment by the CXCR4 Antagonist AMD3100 in a Mouse Transplantation Model Yubin Kang 1, Benny J. Chen 2, Divino DeOliveira 2, Jeffrey Mito 2, Nelson
Selective Enhancement of Donor Hematopoietic Cell Engraftment by the CXCR4 Antagonist AMD3100 in a Mouse Transplantation Model Yubin Kang 1, Benny J. Chen 2, Divino DeOliveira 2, Jeffrey Mito 2, Nelson
UMBILICAL CORD BLOOD STEM CELLS EXPANDED IN THE PRESENCE OF NICOTINAMIDE (NICORD) PROVIDE LONG TERM MULITI-LINEAGE ENGRAFTMENT
 UMBILICAL CORD BLOOD STEM CELLS EXPANDED IN THE PRESENCE OF NICOTINAMIDE (NICORD) PROVIDE LONG TERM MULITI-LINEAGE ENGRAFTMENT Mitchell E. Horwitz, MD Duke University Medical Center Duke Cancer Institute
UMBILICAL CORD BLOOD STEM CELLS EXPANDED IN THE PRESENCE OF NICOTINAMIDE (NICORD) PROVIDE LONG TERM MULITI-LINEAGE ENGRAFTMENT Mitchell E. Horwitz, MD Duke University Medical Center Duke Cancer Institute
Supplemental Information. Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection. Cell Host & Microbe, Volume 15
 Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
Muscle Stem Cells in Regeneration
 Muscle Stem Cells in Regeneration Dr. F Jeffrey Dilworth BIM6028/SMC6052 Lecture (February 11, 2016) Duchenne Muscular Dystrophy is an X-linked genetic disorder that affects 1 in 3500 males Wells et al,
Muscle Stem Cells in Regeneration Dr. F Jeffrey Dilworth BIM6028/SMC6052 Lecture (February 11, 2016) Duchenne Muscular Dystrophy is an X-linked genetic disorder that affects 1 in 3500 males Wells et al,
Haematopoietic stem cell (HSC) niches are present in diverse tissues
 REVIEW doi:10.1038/nature12984 The bone marrow niche for haematopoietic stem cells Sean J. Morrison 1 & David T. Scadden 2 Niches are local tissue microenvironments that maintain and regulate stem cells.
REVIEW doi:10.1038/nature12984 The bone marrow niche for haematopoietic stem cells Sean J. Morrison 1 & David T. Scadden 2 Niches are local tissue microenvironments that maintain and regulate stem cells.
Blood 101 Introduction Blood and Marrow & Overview of Bone Marrow Failure Diseases. Dr. M. Sabloff October 16 th 2010
 Blood 101 Introduction Blood and Marrow & Overview of Bone Marrow Failure Diseases Dr. M. Sabloff October 16 th 2010 Normal Marrow knee joint white is articular cartilage Adjacent to this is the red marrow
Blood 101 Introduction Blood and Marrow & Overview of Bone Marrow Failure Diseases Dr. M. Sabloff October 16 th 2010 Normal Marrow knee joint white is articular cartilage Adjacent to this is the red marrow
Cell signalling pathways in the HSC niche. Dr. Abdullah Aljedai
 Cell signalling pathways in the HSC niche Dr. Abdullah Aljedai 31-10-2009 Learning objectives & resources 1- To introduce the concept of cell signaling process in the haemopoietic system. 2- To outline
Cell signalling pathways in the HSC niche Dr. Abdullah Aljedai 31-10-2009 Learning objectives & resources 1- To introduce the concept of cell signaling process in the haemopoietic system. 2- To outline
Supplementary Figure 1. Generation of knockin mice expressing L-selectinN138G. (a) Schematics of the Sellg allele (top), the targeting vector, the
 Supplementary Figure 1. Generation of knockin mice expressing L-selectinN138G. (a) Schematics of the Sellg allele (top), the targeting vector, the targeted allele in ES cells, and the mutant allele in
Supplementary Figure 1. Generation of knockin mice expressing L-selectinN138G. (a) Schematics of the Sellg allele (top), the targeting vector, the targeted allele in ES cells, and the mutant allele in
Supplementary Figure S1. PTPN2 levels are not altered in proliferating CD8+ T cells. Lymph node (LN) CD8+ T cells from C57BL/6 mice were stained with
 Supplementary Figure S1. PTPN2 levels are not altered in proliferating CD8+ T cells. Lymph node (LN) CD8+ T cells from C57BL/6 mice were stained with CFSE and stimulated with plate-bound α-cd3ε (10µg/ml)
Supplementary Figure S1. PTPN2 levels are not altered in proliferating CD8+ T cells. Lymph node (LN) CD8+ T cells from C57BL/6 mice were stained with CFSE and stimulated with plate-bound α-cd3ε (10µg/ml)
Supplementary Figure 1
 Supplementary Figure 1 Expression of apoptosis-related genes in tumor T reg cells. (a) Identification of FOXP3 T reg cells by FACS. CD45 + cells were gated as enriched lymphoid cell populations with low-granularity.
Supplementary Figure 1 Expression of apoptosis-related genes in tumor T reg cells. (a) Identification of FOXP3 T reg cells by FACS. CD45 + cells were gated as enriched lymphoid cell populations with low-granularity.
Basis of Immunology and
 Basis of Immunology and Immunophysiopathology of Infectious Diseases Jointly organized by Institut Pasteur in Ho Chi Minh City and Institut Pasteur with kind support from ANRS & Université Pierre et Marie
Basis of Immunology and Immunophysiopathology of Infectious Diseases Jointly organized by Institut Pasteur in Ho Chi Minh City and Institut Pasteur with kind support from ANRS & Université Pierre et Marie
Radiation-induced induced Genomic Instability and Bystander Effects: implications for radiation leukaemogenesis
 Radiation-induced induced Genomic Instability and Bystander Effects: implications for radiation leukaemogenesis University of Dundee Medical School Eric G Wright Professor of Experimental Haematology The
Radiation-induced induced Genomic Instability and Bystander Effects: implications for radiation leukaemogenesis University of Dundee Medical School Eric G Wright Professor of Experimental Haematology The
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Highly Efficient CRISPR/Cas9 Gene Editing and Long-Term Engraftment of Human Hematopoietic Stem and Progenitor Cells
 Highly Efficient CRISPR/Cas9 Gene Editing and Long-Term Engraftment of Human Hematopoietic Stem and Progenitor Cells J. M. Heath, A. Chalishazar, C.S. Lee, W. Selleck, C. Cotta-Ramusino, D. Bumcrot, J.L.
Highly Efficient CRISPR/Cas9 Gene Editing and Long-Term Engraftment of Human Hematopoietic Stem and Progenitor Cells J. M. Heath, A. Chalishazar, C.S. Lee, W. Selleck, C. Cotta-Ramusino, D. Bumcrot, J.L.
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05883 SUPPLEMENTARY INFORMATION Supplemental Figure 1 Prostaglandin agonists and antagonists alter runx1/cmyb expression. a-e, Embryos were exposed to (b) PGE2 and (c) PGI2 (20μM) and
doi: 10.1038/nature05883 SUPPLEMENTARY INFORMATION Supplemental Figure 1 Prostaglandin agonists and antagonists alter runx1/cmyb expression. a-e, Embryos were exposed to (b) PGE2 and (c) PGI2 (20μM) and
Hematopoiesis. Hematopoiesis. Hematopoiesis
 Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Overview of Use of G-CSF in the Treatment of Acute Radiation Injury
 Overview of Use of G-CSF in the Treatment of Acute Radiation Injury Applied Research Associates, Inc. Glen Reeves Distribution Statement A: Approved for public release; distribution is unlimited. This
Overview of Use of G-CSF in the Treatment of Acute Radiation Injury Applied Research Associates, Inc. Glen Reeves Distribution Statement A: Approved for public release; distribution is unlimited. This
TISSUE-SPECIFIC STEM CELLS
 TISSUE-SPECIFIC STEM CELLS a Division of Stem Cell Therapy, b Stem Cell Bank, Center for Stem Cell Biology and Regenerative Medicine, f Laboratory of Molecular Pathogenesis, Center for Experimental Medicine
TISSUE-SPECIFIC STEM CELLS a Division of Stem Cell Therapy, b Stem Cell Bank, Center for Stem Cell Biology and Regenerative Medicine, f Laboratory of Molecular Pathogenesis, Center for Experimental Medicine
CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells
 CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells Karin Pfisterer, Karoline M Lipnik, Erhard Hofer and Adelheid Elbe-Bürger Journal of Investigative Dermatology (2015)
CD90 + Human Dermal Stromal Cells Are Potent Inducers of FoxP3 + Regulatory T Cells Karin Pfisterer, Karoline M Lipnik, Erhard Hofer and Adelheid Elbe-Bürger Journal of Investigative Dermatology (2015)
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Supplemental Information. Granulocyte-Monocyte Progenitors and. Monocyte-Dendritic Cell Progenitors Independently
 Immunity, Volume 47 Supplemental Information Granulocyte-Monocyte Progenitors and Monocyte-endritic ell Progenitors Independently Produce Functionally istinct Monocytes lberto Yáñez, Simon G. oetzee, ndre
Immunity, Volume 47 Supplemental Information Granulocyte-Monocyte Progenitors and Monocyte-endritic ell Progenitors Independently Produce Functionally istinct Monocytes lberto Yáñez, Simon G. oetzee, ndre
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS. Choompone Sakonwasun, MD (Hons), FRCPT
 ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
ACTIVATION AND EFFECTOR FUNCTIONS OF CELL-MEDIATED IMMUNITY AND NK CELLS Choompone Sakonwasun, MD (Hons), FRCPT Types of Adaptive Immunity Types of T Cell-mediated Immune Reactions CTLs = cytotoxic T lymphocytes
Bone Marrow Stroma in Myelodysplastic Syndromes
 Bone Marrow Stroma in Myelodysplastic Syndromes Universidad de Salamanca Prof Mª M Consuelo del Cañizo Hematology Dept. University Hospital, Salamanca SPAIN Bone marrow stroma in MDS Introduction Mesenchymal
Bone Marrow Stroma in Myelodysplastic Syndromes Universidad de Salamanca Prof Mª M Consuelo del Cañizo Hematology Dept. University Hospital, Salamanca SPAIN Bone marrow stroma in MDS Introduction Mesenchymal
CRISPR-mediated Editing of Hematopoietic Stem Cells for the Treatment of β-hemoglobinopathies
 CRISPR-mediated Editing of Hematopoietic Stem Cells for the Treatment of β-hemoglobinopathies Jennifer Gori American Society of Gene & Cell Therapy May 11, 2017 editasmedicine.com 1 Highlights Developed
CRISPR-mediated Editing of Hematopoietic Stem Cells for the Treatment of β-hemoglobinopathies Jennifer Gori American Society of Gene & Cell Therapy May 11, 2017 editasmedicine.com 1 Highlights Developed
Nature Medicine doi: /nm.3150
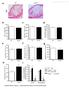 Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Accelerate Your Research with Conversant Bio
 Accelerate Your Research with Conversant Bio 400+ Participating MDs 50+ Partner sites for tissue procurement Continuous expansion of sourcing capabilities Closely monitored chain of custody Full regulatory
Accelerate Your Research with Conversant Bio 400+ Participating MDs 50+ Partner sites for tissue procurement Continuous expansion of sourcing capabilities Closely monitored chain of custody Full regulatory
Foxm1 Transcription Factor is Required for Lung Fibrosis and Epithelial to Mesenchymal Transition.
 Manuscript EMBO-2012-82682 Foxm1 Transcription Factor is Required for Lung Fibrosis and Epithelial to Mesenchymal Transition. David Balli, Vladimir Ustiyan, Yufang Zhang, I-Ching Wang, Alex J. Masino,
Manuscript EMBO-2012-82682 Foxm1 Transcription Factor is Required for Lung Fibrosis and Epithelial to Mesenchymal Transition. David Balli, Vladimir Ustiyan, Yufang Zhang, I-Ching Wang, Alex J. Masino,
CHAPTER 3 LABORATORY PROCEDURES
 CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
Targeting tumour associated macrophages in anti-cancer therapies. Annamaria Gal Seminar Series on Drug Discovery Budapest 5 January 2018
 Targeting tumour associated macrophages in anti-cancer therapies Annamaria Gal Seminar Series on Drug Discovery Budapest 5 January 2018 Macrophages: Professional phagocytes of the myeloid lineage APC,
Targeting tumour associated macrophages in anti-cancer therapies Annamaria Gal Seminar Series on Drug Discovery Budapest 5 January 2018 Macrophages: Professional phagocytes of the myeloid lineage APC,
Supplement Material. Spleen weight (mg) LN cells (X106) Acat1-/- Acat1-/- Mouse weight (g)
 Supplement Material A Spleen weight (mg) C Mouse weight (g) 1 5 1 2 9 6 3 2 5 2 1 5 Male LN cells (X16) 4 ** ** Female B 3 2 1 Supplemental Figure I. Spleen weight (A), Inguinal lymph node (LN) cell number
Supplement Material A Spleen weight (mg) C Mouse weight (g) 1 5 1 2 9 6 3 2 5 2 1 5 Male LN cells (X16) 4 ** ** Female B 3 2 1 Supplemental Figure I. Spleen weight (A), Inguinal lymph node (LN) cell number
ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1
 ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
T cell manipulation of the graft: Yes
 T cell manipulation of the graft: Yes J.H. Frederik Falkenburg Department of Hematology L M U C Allogeneic Hematopoietic Stem Cell Transplantation (SCT) for non-malignant disorders: no need for anti-tumor
T cell manipulation of the graft: Yes J.H. Frederik Falkenburg Department of Hematology L M U C Allogeneic Hematopoietic Stem Cell Transplantation (SCT) for non-malignant disorders: no need for anti-tumor
DECLARATION OF CONFLICT OF INTEREST. No disclosures
 DECLARATION OF CONFLICT OF INTEREST No disclosures micrornas: Role in progression of atherosclerosis Christian Weber Institute for Cardiovascular Prevention (IPEK) Ludwig-Maximilians-University Munich
DECLARATION OF CONFLICT OF INTEREST No disclosures micrornas: Role in progression of atherosclerosis Christian Weber Institute for Cardiovascular Prevention (IPEK) Ludwig-Maximilians-University Munich
Cytokines modulate the functional activities of individual cells and tissues both under normal and pathologic conditions Interleukins,
 Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Cytokines http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter22/animation the_immune_response.html Cytokines modulate the functional activities of individual cells and tissues both under
Normal & Leukaemic haematopoiesis. Dr. Liu Te Chih Dept of Haematology / Oncology National University Health Services Singapore
 Normal & Leukaemic haematopoiesis 2010 Dr. Liu Te Chih Dept of Haematology / Oncology National University Health Services Singapore Use of Immunophenotyping today Lineage assignment Differentiation of
Normal & Leukaemic haematopoiesis 2010 Dr. Liu Te Chih Dept of Haematology / Oncology National University Health Services Singapore Use of Immunophenotyping today Lineage assignment Differentiation of
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
T Cell Development. Xuefang Cao, MD, PhD. November 3, 2015
 T Cell Development Xuefang Cao, MD, PhD November 3, 2015 Thymocytes in the cortex of the thymus Early thymocytes development Positive and negative selection Lineage commitment Exit from the thymus and
T Cell Development Xuefang Cao, MD, PhD November 3, 2015 Thymocytes in the cortex of the thymus Early thymocytes development Positive and negative selection Lineage commitment Exit from the thymus and
sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5
 sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
One Day BMT Course by Thai Society of Hematology. Management of Graft Failure and Relapsed Diseases
 One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
Nature Immunology doi: /ni.3268
 Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Hematopoietic Growth Factors Colony Stimulating Factors. Erythropoietin (Epoetin alfa). Granulocyte-macrophage colonystimulating factor (G-CSF).
 Hematopoietic Growth Factors Colony Stimulating Factors. Erythropoietin (Epoetin alfa). Granulocyte colony-stimulating factor(g-csf). Granulocyte-macrophage colonystimulating factor (G-CSF). Interleukin-11
Hematopoietic Growth Factors Colony Stimulating Factors. Erythropoietin (Epoetin alfa). Granulocyte colony-stimulating factor(g-csf). Granulocyte-macrophage colonystimulating factor (G-CSF). Interleukin-11
Hematopoiesis/Hematopoiesis Physiology
 Hematopoiesis/Hematopoiesis Physiology Definitions Hematopoiesis is the process of continuous generation of mature blood cells in the bone marrow (Figure 1). Blood cells represent different kinds of mature
Hematopoiesis/Hematopoiesis Physiology Definitions Hematopoiesis is the process of continuous generation of mature blood cells in the bone marrow (Figure 1). Blood cells represent different kinds of mature
Transfer protocol of human HSC into NOG mice
 Transfer protocol of human HSC into NOG mice Mice: Adult NOG mice are aged 8-12 weeks. Newborn mice are 1 2 days old. 8-12 week old NOG mice irradiated with 2.5 Gy Intravenous transfer of 1-0.5 x 10 5
Transfer protocol of human HSC into NOG mice Mice: Adult NOG mice are aged 8-12 weeks. Newborn mice are 1 2 days old. 8-12 week old NOG mice irradiated with 2.5 Gy Intravenous transfer of 1-0.5 x 10 5
Supporting Information Table of Contents
 Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Supporting Information Table of Contents Supporting Information Figure 1 Page 2 Supporting Information Figure 2 Page 4 Supporting Information Figure 3 Page 5 Supporting Information Figure 4 Page 6 Supporting
Long-term innate immune memory via effects on bone marrow progenitors
 Long-term innate immune memory via effects on bone marrow progenitors Helen S Goodridge, PhD helen.goodridge@csmc.edu Regenerative Medicine Institute, Cedars-Sinai Medical Center, Los Angeles, USA Fondation
Long-term innate immune memory via effects on bone marrow progenitors Helen S Goodridge, PhD helen.goodridge@csmc.edu Regenerative Medicine Institute, Cedars-Sinai Medical Center, Los Angeles, USA Fondation
Recommended reading: Abbas et al. 5th edition, chapters 7 and 10; Janeway and Travers, 5th edition, chapter 7.
 Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai 10/05/05; 11 AM Shiv Pillai T Lymphocyte Development Recommended reading:
Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai 10/05/05; 11 AM Shiv Pillai T Lymphocyte Development Recommended reading:
Eosinophils are required. for the maintenance of plasma cells in the bone marrow
 Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
Stem Cells and The Endometrium. Director, Division of Reproductive Endocrinology and infertility
 Stem Cells and The Endometrium Hugh S. Taylor, M.D. Director, Division of Reproductive Endocrinology and infertility Nothing to disclose Stem Cells Cells that are capable of both self-renewal and have
Stem Cells and The Endometrium Hugh S. Taylor, M.D. Director, Division of Reproductive Endocrinology and infertility Nothing to disclose Stem Cells Cells that are capable of both self-renewal and have
T cell maturation. T-cell Maturation. What allows T cell maturation?
 T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
Oncolytic Virotherapy: Targeting Cancer Stem Cells
 Oncolytic Virotherapy: Targeting Cancer Stem Cells Cancer Stem Cells (CSCs) or Cancer Initiating Cells (CICs) A consensus of five defining criteria has been established to affirm the existence of CICs:
Oncolytic Virotherapy: Targeting Cancer Stem Cells Cancer Stem Cells (CSCs) or Cancer Initiating Cells (CICs) A consensus of five defining criteria has been established to affirm the existence of CICs:
THE HYPOXIC HEMATOPOIETIC STEM CELL NICHE Consequences of Hypoxiainduced Transcription on Stem Cell Fate
 THE HYPOXIC HEMATOPOIETIC STEM CELL NICHE Consequences of Hypoxiainduced Transcription on Stem Cell Fate Rehn, Matilda Published: 2011-01-01 Link to publication Citation for published version (APA): Rehn,
THE HYPOXIC HEMATOPOIETIC STEM CELL NICHE Consequences of Hypoxiainduced Transcription on Stem Cell Fate Rehn, Matilda Published: 2011-01-01 Link to publication Citation for published version (APA): Rehn,
Identification of a Stroma-Mediated Wnt/b-Catenin Signal Promoting Self-Renewal of Hematopoietic Stem Cells in the Stem Cell Niche
 THE STEM CELL NICHE Identification of a Stroma-Mediated Wnt/b-Catenin Signal Promoting Self-Renewal of Hematopoietic Stem Cells in the Stem Cell Niche JIN-A KIM, a YOUNG-JU KANG, a GYEONGSIN PARK, a MYUNGSHIN
THE STEM CELL NICHE Identification of a Stroma-Mediated Wnt/b-Catenin Signal Promoting Self-Renewal of Hematopoietic Stem Cells in the Stem Cell Niche JIN-A KIM, a YOUNG-JU KANG, a GYEONGSIN PARK, a MYUNGSHIN
T cell development October 28, Dan Stetson
 T cell development October 28, 2016 Dan Stetson stetson@uw.edu 441 Lecture #13 Slide 1 of 29 Three lectures on T cells (Chapters 8, 9) Part 1 (Today): T cell development in the thymus Chapter 8, pages
T cell development October 28, 2016 Dan Stetson stetson@uw.edu 441 Lecture #13 Slide 1 of 29 Three lectures on T cells (Chapters 8, 9) Part 1 (Today): T cell development in the thymus Chapter 8, pages
Supplementary Data. Supplementary Methods:
 Supplementary Data Supplementary Methods: Release kinetics of from collagen sponges in vivo. 2μg of human recombinant 165 was labeled with lexafluor 555 (Microscale Protein Labeling Kit; Invitrogen) as
Supplementary Data Supplementary Methods: Release kinetics of from collagen sponges in vivo. 2μg of human recombinant 165 was labeled with lexafluor 555 (Microscale Protein Labeling Kit; Invitrogen) as
CHAPTER 6 SUMMARIZING DISCUSSION
 CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
Mesenchymal Stem Cells to Repair Vascular Damage after Chemotherapy: Past, Present and Future
 Mesenchymal Stem Cells to Repair Vascular Damage after Chemotherapy: Past, Present and Future Cell Therapy 2014 Las Vegas, NV, USA Sulaiman Al-Hashmi, PhD Sultan Qaboos University Oman What are MSCs? Stem
Mesenchymal Stem Cells to Repair Vascular Damage after Chemotherapy: Past, Present and Future Cell Therapy 2014 Las Vegas, NV, USA Sulaiman Al-Hashmi, PhD Sultan Qaboos University Oman What are MSCs? Stem
Early cell death (FGF) B No RunX transcription factor produced Yes No differentiation
 Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
