Radicals. Structure and Stability of Radicals. Radicals are formed from covalent bonds by adding energy in the form of heat (Δ) or light (hν).
|
|
|
- Ursula Fowler
- 5 years ago
- Views:
Transcription
1 Radicals Chapter 15 A small but significant group of reactions involve radical intermediates. A radical is a reactive intermediate with a single unpaired electron, formed by homolysis of a covalent bond. Half-headed arrows are used to show the movement of electrons in radical processes. General Homolysis Reaction Allyl Radical Structure and Stability of Radicals General Features of Radical Reactions Reaction of a Radical X with C-H & C=C Bonds Radicals are formed from covalent bonds by adding energy in the form of heat (Δ) or light (hν). Some radical reactions are carried out in the presence of a radical initiator. Radical initiators, such as peroxides of general structure, RO OR, contain an especially weak bond that serves as a source of radicals. Heating a peroxide readily causes homolysis of the weak O O bond, forming two RO radicals. Radicals undergo two main types of reactions they react with σ bonds, and they add to π bonds. Radical Inhibition 3
2 Radical Halogenation of Alkanes Halogenation of alkanes is only useful with Cl2 or Br2. Reaction with F2 is too violent, and reaction with I2 is too slow to be useful. Common Steps of Radical Reactions Radical halogenation has three distinct steps: When a single hydrogen atom on a carbon has been replaced by a halogen atom, monohalogenation has taken place. When excess halogen is used, it is possible to replace more than one hydrogen atom on a single carbon with halogen atoms. These are the chain reaction steps of a radical mechanism. monohalogenations 1:1 ratio multiple halogenations Chlorination vs Bromination 1. Chlorination is faster than bromination. 2. Chlorination is unselective, yielding a mixture of products 3. Bromination is more selective, often yielding one major product.
3 Stereochemistry from Achiral Starting Material CFCs and the Destruction of the Ozone Layer Halogenation of an achiral starting material such as CH3CH2CH2CH3 forms two constitutional isomers by replacement of either a 1 or 2 hydrogen. CFC s have been used as refrigerants and propellants. formation of the racemic mixture Alternative to CFC s and its less harmful decomposition: Radical Halogenation at an Allylic Carbon An allylic carbon is a carbon adjacent to a double bond. Homolysis of the allylic C H bond in propene generates an allylic radical which has an unpaired electron on the carbon adjacent to the double bond. NBS a Radical Bromination Reagent Note: The reaction and conditions are very important. However, you will not be responsible for this mechanism. The allyl radical is more stable than other radicals because the π bond and the unpaired electron are delocalized. The HBr formed in Step [2] reacts with NBS to form a low concentration of Br2. This is then used for halogenation in Step [3] of the mechanism.
4 Regiochemistry & Stereochemistry of Allylic Halogenation Halogenation at an allylic carbon often results in a mixture of products. Oxidation of Unsaturated Lipids Oils are susceptible to allylic free radical oxidation. (both enantiomers in a 1:1 ratio) A mixture results because the reaction proceeds by way of a resonance-stabilized radical. Antioxidants An antioxidant is a compound that stops an oxidation reaction from occurring. Naturally occurring antioxidants such as vitamin E prevent radical reactions that can cause cell damage. Synthetic antioxidants such as BHT butylated hydroxy toluene are added to packaged and prepared foods to prevent oxidation and spoilage. Vitamin E and BHT are radical inhibitors, which terminate radical chain mechanisms by reacting with the radical. Mechanism of Antioxidant Behavior To trap free radicals, both vitamin E and BHT use a hydroxy group bonded to a benzene ring a general structure called a phenol. Radicals (R ) abstract a hydrogen atom from the OH group of an antioxidant, forming a new resonance-stabilized radical. This new radical does not participate in chain propagation, but rather terminates the chain and halts the oxidation process. Because oxidative damage to lipids in cells is thought to play a role in the aging process, many antiaging formulations contain antioxidants.
5 Radical Additions to Alkenes Electron rich alkenes react with electron deficient radicals. Radicals react with alkenes via radical chain mechanisms consisting of: Initiation, propagation and termination steps HBr adds to alkenes to form alkyl bromides in the presence of heat, light, or peroxides. Radical Additions to Alkenes The regioselectivity of the addition to unsymmetrical alkenes is different from that for addition of HBr in the absence of heat, light, or peroxides. The addition of HBr to alkenes in the presence of heat, light, or peroxides proceeds via a radical mechanism. Regiochemistry of Radical Addition to Alkenes In the first propagation step, the addition of Br to the double bond, there are two possible paths: 1. Path [A] forms the less stable 1 radical. 2. Path [B] forms the more stable 2 radical. (The more stable 2 radical forms faster, so Path [B] is preferred.)
6 Radical vs Ionic Addition of HBr Depending on the reaction conditions, a different species initially reacts with the p bond accounting for the difference in regioselectivity. Radical addition involves initial attack by a bromine radical. Ionic addition involves initial attack on a proton. Polymers and Polymerization Polymers are large molecules made up of repeating units of smaller molecules called monomers. They include biologically important compounds such as proteins and carbohydrates, as well as synthetic plastics such as polyethylene, polyvinyl chloride (PVC) and polystyrene. Polymerization is the process of joining together of monomers to make polymers. For example, joining ethylene monomers together forms the polymer polyethylene, a plastic used in milk containers and plastic bags. Polymers from Ethylene Derivatives Many ethylene derivatives having the general structure CH2=CHZ are also used as monomers for polymerization. The identity of Z affects the physical properties of the resulting polymer. Polymerization of CH2=CHZ usually affords polymers with Z groups on every other carbon atom in the chain. The new radical is always located on the C bonded to Z. This process called head-to-tail polymerization.
Suggested homework problems: 8.8, 8.13, ,
 Chapter 8 Outline: Haloalakanes, Halogenation, & Radical reactions Cover 8.1-8.3 on your own 1. Halogenation of Alkanes A. Mechanism of Halogenation B. Regioselectivity & Stereoselectivity of Halogenation
Chapter 8 Outline: Haloalakanes, Halogenation, & Radical reactions Cover 8.1-8.3 on your own 1. Halogenation of Alkanes A. Mechanism of Halogenation B. Regioselectivity & Stereoselectivity of Halogenation
4 Types of Organic Polar Reactions
 Objective 12 Apply Reactivity Principles to Electrophilic Addition Reactions 1: Alkenes Identify structural features (pi bond) and electrophiles Use curved arrows to predict product 4 Types of Organic
Objective 12 Apply Reactivity Principles to Electrophilic Addition Reactions 1: Alkenes Identify structural features (pi bond) and electrophiles Use curved arrows to predict product 4 Types of Organic
Alkenes. IB Chemistry Topic 10.2
 Alkenes IB Chemistry Topic 10.2 What is the difference between alkanes and alkenes? Which do you think would be more reactive? The relationship between the number of bonds, bond length and bond strength
Alkenes IB Chemistry Topic 10.2 What is the difference between alkanes and alkenes? Which do you think would be more reactive? The relationship between the number of bonds, bond length and bond strength
13. ORGANIC CHEMISTRY
 1. ORGANIC EMISTRY III) ALKENES SYNOPSIS Alkenes are unsaturated hydrocarbons. These contain a C =C. They contain two hydrogens less than corresponding alkanes. Double bonded carbon undergoes hybridisation.
1. ORGANIC EMISTRY III) ALKENES SYNOPSIS Alkenes are unsaturated hydrocarbons. These contain a C =C. They contain two hydrogens less than corresponding alkanes. Double bonded carbon undergoes hybridisation.
Organohalides and Applications of Free Radical Reactions. Dr. Sapna Gupta
 Organohalides and Applications of Free Radical Reactions Dr. Sapna Gupta Applications of Radical Reactions Since these reactions are hard to control they have few practical applications. This does not
Organohalides and Applications of Free Radical Reactions Dr. Sapna Gupta Applications of Radical Reactions Since these reactions are hard to control they have few practical applications. This does not
11/5/ Oxidation of Alkenes: Cleavage to Carbonyl Compounds. Oxidation of Alkenes: Cleavage to Carbonyl Compounds
 8.8 Oxidation of Alkenes: Cleavage to Carbonyl Compounds Ozone (O 3 ) is useful double-bond cleavage reagent Ozone is generated by passing a stream of oxygen through a highvoltage electrical discharge
8.8 Oxidation of Alkenes: Cleavage to Carbonyl Compounds Ozone (O 3 ) is useful double-bond cleavage reagent Ozone is generated by passing a stream of oxygen through a highvoltage electrical discharge
Nature s Chemistry. Fragrances and Skin Care
 St Andrew s and St Bride s High School CfE Higher Chemistry Nature s Chemistry Page 1 of 12 Essential Oils Essential oils are concentrated extracts of the volatile, non-water soluble (hydrophobic) aroma
St Andrew s and St Bride s High School CfE Higher Chemistry Nature s Chemistry Page 1 of 12 Essential Oils Essential oils are concentrated extracts of the volatile, non-water soluble (hydrophobic) aroma
Worksheet Chapter 17: Food chemistry glossary
 Worksheet 17.1 Chapter 17: Food chemistry glossary Aldehydes (alkanals) A homologous series of compounds with the general formula RCHO, where the CHO group (the aldehyde group) consists of a carbonyl group
Worksheet 17.1 Chapter 17: Food chemistry glossary Aldehydes (alkanals) A homologous series of compounds with the general formula RCHO, where the CHO group (the aldehyde group) consists of a carbonyl group
Alkenes. Isomerism in the alkenes
 Alkenes Alkenes are a family of hydrocarbons (compounds containing carbon and hydrogen only) containing a carbon-carbon double bond. The first two are: ethene 2 4 propene 3 6 You can work out the formula
Alkenes Alkenes are a family of hydrocarbons (compounds containing carbon and hydrogen only) containing a carbon-carbon double bond. The first two are: ethene 2 4 propene 3 6 You can work out the formula
Chapter 7- Alkenes: Structure and Reactivity. Ashley Piekarski, Ph.D. Alkene
 Chapter 7- Alkenes: Structure and Reactivity Ashley Piekarski, Ph.D. Alkene What is an alkene func
Chapter 7- Alkenes: Structure and Reactivity Ashley Piekarski, Ph.D. Alkene What is an alkene func
Chapter 23. Functional Groups. Halogen Side Chains What is a halocarbon? How are organic compounds classified?
 23.1 Chapter 23 From a distance, the musicians in an orchestra may look alike, but each musician contributes a unique sound. In a similar way, one hydrocarbon is nearly identical to another until it picks
23.1 Chapter 23 From a distance, the musicians in an orchestra may look alike, but each musician contributes a unique sound. In a similar way, one hydrocarbon is nearly identical to another until it picks
This is an addition reaction. (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic.
 Reactions of Alkenes Since bonds are stronger than bonds, double bonds tend to react to convert the double bond into bonds + X-Y X Y This is an addition reaction. (Other types of reaction have been substitution
Reactions of Alkenes Since bonds are stronger than bonds, double bonds tend to react to convert the double bond into bonds + X-Y X Y This is an addition reaction. (Other types of reaction have been substitution
Diverse Reactions of Alkenes
 Chapter 8- Alkenes: Reactions and Synthesis Ashley Piekarski, Ph.D. Diverse Reactions of Alkenes Alkenes react with many electrophiles to give useful products by addiaon (ocen through special reagents)
Chapter 8- Alkenes: Reactions and Synthesis Ashley Piekarski, Ph.D. Diverse Reactions of Alkenes Alkenes react with many electrophiles to give useful products by addiaon (ocen through special reagents)
Oregon State University
 H 223 Worksheet 9 Notes Oregon State University 1. Draw a primary alcohol and name it. OH 1-propanol Note: A primary alcohol has the form RH 2 OH; a secondary alcohol has the form R 2 H OH; and a tertiary
H 223 Worksheet 9 Notes Oregon State University 1. Draw a primary alcohol and name it. OH 1-propanol Note: A primary alcohol has the form RH 2 OH; a secondary alcohol has the form R 2 H OH; and a tertiary
Alkenes. 1. Isoprene is an alkene that can be tapped from some trees. It is the monomer in natural rubber.
 Alkenes 1. Isoprene is an alkene that can be tapped from some trees. It is the monomer in natural rubber. Limonene is a natural oil found in the rind of oranges and lemons. Both isoprene and limonene contain
Alkenes 1. Isoprene is an alkene that can be tapped from some trees. It is the monomer in natural rubber. Limonene is a natural oil found in the rind of oranges and lemons. Both isoprene and limonene contain
3/27/2011. Chapter 8 Reactions of Alkenes and Alkynes. Alkene Addition Reactions. 8.1 Preparing Alkenes: A Preview of Elimination Reactions
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 8 Reactions of Alkenes and Alkynes Richard Morrison University of Georgia, Athens Alkene Addition Reactions Alkene addition reactions Addition
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 8 Reactions of Alkenes and Alkynes Richard Morrison University of Georgia, Athens Alkene Addition Reactions Alkene addition reactions Addition
Unsaturated Hydrocarbons
 Interchapter G Unsaturated ydrocarbons FPO aption TK. G. unsaturated ydrocarbons G1 In this Interchapter, we shall continue our introduction to organic chemistry by discussing unsaturated hydrocarbons.
Interchapter G Unsaturated ydrocarbons FPO aption TK. G. unsaturated ydrocarbons G1 In this Interchapter, we shall continue our introduction to organic chemistry by discussing unsaturated hydrocarbons.
Alkane C-C single bond (propane) Alkene C=C double bond (propene) Alcohol - OH group (1-propanol) major. minor
 Functional group* and name? Alkane - single bond (propane) *alkanes not really regarded as a functional group Alkene = double bond (propene) Addition of an unsymmetrical reagent to unsymmetrical alkene
Functional group* and name? Alkane - single bond (propane) *alkanes not really regarded as a functional group Alkene = double bond (propene) Addition of an unsymmetrical reagent to unsymmetrical alkene
Chapter 8 Lecture Reactions of Alkenes
 Organic Chemistry, 9 th Edition L. G. Wade, Jr. Chapter 8 Lecture Reactions of Alkenes 2017 Pearson Education, Inc. Catalytic Hydrogenation of Alkenes Hydrogen (H 2 ) can be added across the double bond
Organic Chemistry, 9 th Edition L. G. Wade, Jr. Chapter 8 Lecture Reactions of Alkenes 2017 Pearson Education, Inc. Catalytic Hydrogenation of Alkenes Hydrogen (H 2 ) can be added across the double bond
3/14/2011. Worked Example Stability of Alkenes. 7.4 Alkene Stereochemistry and the E,Z Designation
 7.4 Alkene Stereochemistry and the E,Z Designation E,Z system Sequence rules used to assign priorities to the substituent groups on the double-bond carbons (alkenes) E double bond For German entgegen meaning
7.4 Alkene Stereochemistry and the E,Z Designation E,Z system Sequence rules used to assign priorities to the substituent groups on the double-bond carbons (alkenes) E double bond For German entgegen meaning
SY 2017/ nd Final Term Revision. Student s Name: Grade: 12 B & C. Subject: Chemistry. Teacher Signature
 SY 2017/2018 2 nd Final Term Revision Student s Name: Grade: 12 B & C Subject: Chemistry Teacher Signature Revision Sheet Chemistry Gr 12B Ch-22-23 Organic reaction 1-Choose correct answer. 1) Cellulose
SY 2017/2018 2 nd Final Term Revision Student s Name: Grade: 12 B & C Subject: Chemistry Teacher Signature Revision Sheet Chemistry Gr 12B Ch-22-23 Organic reaction 1-Choose correct answer. 1) Cellulose
Revision Sheet Final Exam Term
 Revision Sheet Final Exam Term-1 2018-2019 Name: Subject: Chemistry Grade: 12 A, B, C Required Materials: Chapter: 22 Section: 1,2,3,4 (Textbook pg. 669-697) Chapter: 23 Section: 1,2 (Textbook pg. 707-715)
Revision Sheet Final Exam Term-1 2018-2019 Name: Subject: Chemistry Grade: 12 A, B, C Required Materials: Chapter: 22 Section: 1,2,3,4 (Textbook pg. 669-697) Chapter: 23 Section: 1,2 (Textbook pg. 707-715)
Chapter 12 Alkenes & Alkynes. Organic and BioChem
 hapter 12 Alkenes & Alkynes Organic and Biohem Section 12.1 Introduction Unsaturated ydrocarbons ontain one or more carbon-carbon double or triple bonds 3 6? 2 2? Three lasses of Unsaturated ydrocarbons
hapter 12 Alkenes & Alkynes Organic and Biohem Section 12.1 Introduction Unsaturated ydrocarbons ontain one or more carbon-carbon double or triple bonds 3 6? 2 2? Three lasses of Unsaturated ydrocarbons
Structure of Alkenes In ethene (ethylene) each carbon is bonded to 3 other atoms, with zero nonbonding electrons => sp 2 hybridization.
 Structure and Synthesis of Alkenes Alkenes (olefins) are hydrocarbons which have carbon carbon double bonds. A double bond is a bond and a bond. Double bond B.D.E. bond B.D.E. = 146 kcal/mol = 83 kcal/mol
Structure and Synthesis of Alkenes Alkenes (olefins) are hydrocarbons which have carbon carbon double bonds. A double bond is a bond and a bond. Double bond B.D.E. bond B.D.E. = 146 kcal/mol = 83 kcal/mol
Chemicals Based on Ethylene
 Chemicals Based on Ethylene Ethylene is sometimes known as the king of petrochemicals because more commercial chemicals are produced from ethylene than from any other intermediate. This unique position
Chemicals Based on Ethylene Ethylene is sometimes known as the king of petrochemicals because more commercial chemicals are produced from ethylene than from any other intermediate. This unique position
Organic Chemistry. AQA Chemistry topic 7
 rganic hemistry AQA hemistry topic 7 7.1 arbon ompounds as fuels and feedstock rude il rude oil is a finite resource found in rocks. It s the remains of an ancient biomass consisting mainly of plankton
rganic hemistry AQA hemistry topic 7 7.1 arbon ompounds as fuels and feedstock rude il rude oil is a finite resource found in rocks. It s the remains of an ancient biomass consisting mainly of plankton
Organic Chemistry. Carey/Giuliano 10th edition. Chapter 10
 Organic Chemistry Carey/Giuliano 10th edition Chapter 10 Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education.
Organic Chemistry Carey/Giuliano 10th edition Chapter 10 Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education.
Chapter 1-2 Review Assignment
 Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
Carbon s unique bonding pattern arises from the hybridization of the electrons.
 Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
Unit 8 Neptune, the 8 th planet of our solar system Organic Chemistry Organic: compound containing carbon, excluding oxides and carbonates Carbon is an allotrope, meaning it has different bonding patterns.
CH [2] (ii) Give the structural formula of another hydrocarbon which is isomeric with the above.
![CH [2] (ii) Give the structural formula of another hydrocarbon which is isomeric with the above. CH [2] (ii) Give the structural formula of another hydrocarbon which is isomeric with the above.](/thumbs/75/72821632.jpg) 1 The alkenes are unsaturated hydrocarbons. They form a homologous series, the members of which have the same chemical properties. They undergo addition reactions and are easily oxidised. (a) The following
1 The alkenes are unsaturated hydrocarbons. They form a homologous series, the members of which have the same chemical properties. They undergo addition reactions and are easily oxidised. (a) The following
Chapter 05 Structure and Preparation of Alkenes: Elimination Reactions part 01
 hapter 05 Structure and Preparation of Alkenes: Elimination Reactions part 01 EM 341: Spring 2012 Prof. Greg ook 2012 Gregory R ook Alkenes ompounds that contain double bonds. Ethylene (Ethene) 2 2012
hapter 05 Structure and Preparation of Alkenes: Elimination Reactions part 01 EM 341: Spring 2012 Prof. Greg ook 2012 Gregory R ook Alkenes ompounds that contain double bonds. Ethylene (Ethene) 2 2012
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
CHEM 261 Mar 9, same
 93 EM 261 Mar 9, 2017 Review: ydrogenation The addition of 2 to an alkene Example: cyclohexene 2 Pd same or stereochemistry: 3 4 2 1 S R Aside: = deuterium = 2 (1 p + and 1 e - ) Product is a meso compound
93 EM 261 Mar 9, 2017 Review: ydrogenation The addition of 2 to an alkene Example: cyclohexene 2 Pd same or stereochemistry: 3 4 2 1 S R Aside: = deuterium = 2 (1 p + and 1 e - ) Product is a meso compound
UNSATURATED HYDROCARBONS
 hemistry 52 hapter 20 UNSATURATED YDROARBONS The unsaturated hydrocarbons consist of three families of homologous compounds that contain multiple bonds between carbon atoms. Alkenes contain carbon carbon
hemistry 52 hapter 20 UNSATURATED YDROARBONS The unsaturated hydrocarbons consist of three families of homologous compounds that contain multiple bonds between carbon atoms. Alkenes contain carbon carbon
2 3 Carbon Compounds Slide 1 of 37
 1 of 37 The Chemistry of Carbon The Chemistry of Carbon Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Carbon atoms have four valence electrons that can join with
1 of 37 The Chemistry of Carbon The Chemistry of Carbon Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Carbon atoms have four valence electrons that can join with
Chapter 10. Carboxylic Acids and Derivatives. Naming Carboxylic Acids and Derivatives. Carboxylic Acids: RCOOH (RCO 2 H)
 Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
Chapter 10 Carboxylic Acids and Derivatives Naming Carboxylic Acids and Derivatives Carboxylic Acids: RCH (RC 2 H) The functional group of a carboxylic acid is a carboxyl group (carbonyl & hydroxyl group)
10/29/ Stability of Alkenes. Stability of Alkenes. Stability of Alkenes
 7.5 Stability of cis and trans isomers Interconversion does not occur spontaneously Cis isomers are less stable than trans isomers because of the steric strain between the two larger substituents on the
7.5 Stability of cis and trans isomers Interconversion does not occur spontaneously Cis isomers are less stable than trans isomers because of the steric strain between the two larger substituents on the
Paper 9: ORGANIC CHEMISTRY-III (Reaction Mechanism-2) Module17: Reduction by Metal hydrides Part-II CHEMISTRY
 Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 17: Reduction by Metal hydrides Part-1I CHE_P9_M17 Table of Contents 1. Learning Outcomes 2. Introduction
Subject Chemistry Paper No and Title Module No and Title Module Tag 9: ORGANIC -III (Reaction Mechanism-2) 17: Reduction by Metal hydrides Part-1I CHE_P9_M17 Table of Contents 1. Learning Outcomes 2. Introduction
Alkenes are very useful in syntheses -they allow us to convert into many of the other types of functional groups.
 Chapter 7: Alkenes: reactions and synthesis Alkenes are very useful in syntheses -they allow us to convert into many of the other types of functional groups. 7.1 Preparation of alkenes: preview Addition
Chapter 7: Alkenes: reactions and synthesis Alkenes are very useful in syntheses -they allow us to convert into many of the other types of functional groups. 7.1 Preparation of alkenes: preview Addition
Identifying Functional Groups. (Chapter 2 in the Klein text)
 Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
Identifying Functional Groups (Chapter 2 in the Klein text) Basic Ideas A functional group is a substructure within a molecule that will have the potential to undergo chemical change, i.e. the group has
Chapter 11 Nutrition: Food for Thought
 Chapter 11 Nutrition: Food for Thought Do you think about the food that goes into your body and how it affects you? How can you interpret the various nutrition information found in the press? What are
Chapter 11 Nutrition: Food for Thought Do you think about the food that goes into your body and how it affects you? How can you interpret the various nutrition information found in the press? What are
Chapter 2 MASS SPECTROMETRY
 Chapter 2 MASS SPECTMETY The mass spectrometer is an instrument that utilizes a variety of methods to ionize organic and biological compounds into simple cations and cationic species containing one unpaired
Chapter 2 MASS SPECTMETY The mass spectrometer is an instrument that utilizes a variety of methods to ionize organic and biological compounds into simple cations and cationic species containing one unpaired
Organic Chemistry Diversity of Carbon Compounds
 Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Organic Chemistry Diversity of Carbon Compounds Hydrocarbons The Alkanes The Alkenes The Alkynes Naming Hydrocarbons Cyclic Hydrocarbons Alkyl Groups Aromatic Hydrocarbons Naming Complex Hydrocarbons Chemical
Life s molecular diversity is based on the. properties of carbon. Chain Ring Branching chain
 Carbon Compounds Life s molecular diversity is based on the properties of carbon Chain Ring Branching chain The Chemistry of Carbon : carbon based Carbon can make 4 covalent bonds The foundation of organic
Carbon Compounds Life s molecular diversity is based on the properties of carbon Chain Ring Branching chain The Chemistry of Carbon : carbon based Carbon can make 4 covalent bonds The foundation of organic
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at Alkenes.
 Save My Exams! The ome of Revision For more awesome GSE and A level resources, visit us at www.savemyexams.co.uk/ Alkenes Question Paper 3 Level IGSE Subject hemistry ExamBoard IE Topic Organic hemistry
Save My Exams! The ome of Revision For more awesome GSE and A level resources, visit us at www.savemyexams.co.uk/ Alkenes Question Paper 3 Level IGSE Subject hemistry ExamBoard IE Topic Organic hemistry
4/7/2011. Chapter 13 Organic Chemistry. Structural Formulas. 3. Petroleum Products
 Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-9. 13-10. Polymers 13-11. Carbohydrates 13-12. Photosynthesis
Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-9. 13-10. Polymers 13-11. Carbohydrates 13-12. Photosynthesis
CHEM 203 HOMEWORK 4 Chemistry of Alkenes - II. Answer the above questions by writing a detailed mechanism for the conversion of A into lanosterol.
 EM 203 MEWK 4 hemistry of Alkenes - II 1. The following questions may have occurred to you: (i) do carbocations occur in living systems? (ii) an an olefin (a Lewis base) react with a carbocation (a Lewis
EM 203 MEWK 4 hemistry of Alkenes - II 1. The following questions may have occurred to you: (i) do carbocations occur in living systems? (ii) an an olefin (a Lewis base) react with a carbocation (a Lewis
Macromolecules. Small molecules that join together to form one large polymer molecules.
 Macromolecules Polymerisation: Polymerisation is the joining of small molecules (monomers), into chains of a very large molecule (polymer). The monomers can be as atoms, simple molecules of ethen as in
Macromolecules Polymerisation: Polymerisation is the joining of small molecules (monomers), into chains of a very large molecule (polymer). The monomers can be as atoms, simple molecules of ethen as in
Chemical Basis For Life Open Ended Questions:
 Chemical Basis For Life Open Ended Questions: Answer the following questions to the best of your ability: Make sure you read each question carefully and provide answers to all of the parts of the question.
Chemical Basis For Life Open Ended Questions: Answer the following questions to the best of your ability: Make sure you read each question carefully and provide answers to all of the parts of the question.
Chemistry 1050 Exam 3 Study Guide
 Chapter 12 Chemistry 1050 Exam 3 Study Guide 12.1 a) Identify alkenes, alkynes and aromatics as unsaturated hydrocarbons. Determine the number of hydrogen atoms needed to complete an alkene structure.
Chapter 12 Chemistry 1050 Exam 3 Study Guide 12.1 a) Identify alkenes, alkynes and aromatics as unsaturated hydrocarbons. Determine the number of hydrogen atoms needed to complete an alkene structure.
Organic & Biochemistry Pacing Guide. Day Date SCS Objectives Essential Questions Content Tasks/Strategies. How are covalent compounds formed?
 Organic & Biochemistry Pacing Guide Course Description: Course Description: This course is designed to provide students with an opportunity to continue their study of the principles of chemistry. The topics
Organic & Biochemistry Pacing Guide Course Description: Course Description: This course is designed to provide students with an opportunity to continue their study of the principles of chemistry. The topics
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chap 7: Alcohols, Phenols, & Thiols
 Chap 7: Alcohols, Phenols, & Thiols Objectives: Chap 7: Alcohols, Phenols, & Thiols (Chapter 7 and pages 283-285 & 296-297, A-1 & A-2 in lab manual) 1. Identify molecules as an alcohol, phenol, glycol,
Chap 7: Alcohols, Phenols, & Thiols Objectives: Chap 7: Alcohols, Phenols, & Thiols (Chapter 7 and pages 283-285 & 296-297, A-1 & A-2 in lab manual) 1. Identify molecules as an alcohol, phenol, glycol,
Organic Chemistry Part 2
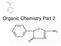 Organic Chemistry Part 2 Benzene Benzene is a special structure C 6 H 6 The carbon-carbon bonds aren t a single or double bond but something in-between Resonance bond CYCLIC HYDROCARBONS Carbon chains
Organic Chemistry Part 2 Benzene Benzene is a special structure C 6 H 6 The carbon-carbon bonds aren t a single or double bond but something in-between Resonance bond CYCLIC HYDROCARBONS Carbon chains
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1)
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Chapter 7-2 Hydrocarbons
 Chapter 7-1 Carbon C atom - atomic # is 6; it has 6 protons and therefore 6 electrons - is in group 14; it has 4 valence electrons - atomic mass is 12; it has 6 neutrons - shares electrons when forming
Chapter 7-1 Carbon C atom - atomic # is 6; it has 6 protons and therefore 6 electrons - is in group 14; it has 4 valence electrons - atomic mass is 12; it has 6 neutrons - shares electrons when forming
Prelab 6: Carboxylic Acids
 The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
The Structure of Carboxylic Acids Prelab 6: Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group,
C. Correct! A compound that consists only of hydrogen and carbon is termed a hydrocarbon.
 Organic Chemistry - Problem Drill 06: Alkanes and Cycloalkanes No. 1 of 10 1. What is a hydrocarbon? (A) A compound that consists of only carbon. (B) A compound that consists of nonmetals. (C) A compound
Organic Chemistry - Problem Drill 06: Alkanes and Cycloalkanes No. 1 of 10 1. What is a hydrocarbon? (A) A compound that consists of only carbon. (B) A compound that consists of nonmetals. (C) A compound
Carbon. Has four valence electrons Can bond with many elements. Can bond to other carbon atoms. Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
 Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Chapter 13: Alcohols, Phenols, and Ethers
 Chapter 13: Alcohols, Phenols, and Ethers ALCOHOLS, PHENOLS, AND ETHERS Hydroxy group the OH functional group An alcohol has an OH group attached to an aliphatic carbon. General formula: R-OH A phenol
Chapter 13: Alcohols, Phenols, and Ethers ALCOHOLS, PHENOLS, AND ETHERS Hydroxy group the OH functional group An alcohol has an OH group attached to an aliphatic carbon. General formula: R-OH A phenol
Name a property of. water why is it necessary for life?
 02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
A general depiction of olefin metathesis is shown below, where you have two olefins that literally switch partners:
 Class 6 This starts the second 1/3 of our semester, where we talk in detail about a few select reactions. We will spend two classes talking about OLEFIN METATHESIS, mostly because it is a fairly important
Class 6 This starts the second 1/3 of our semester, where we talk in detail about a few select reactions. We will spend two classes talking about OLEFIN METATHESIS, mostly because it is a fairly important
Guided Inquiry Skills Lab. Additional Lab 1 Making Models of Macromolecules. Problem. Introduction. Skills Focus. Materials.
 Additional Lab 1 Making Models of Macromolecules Guided Inquiry Skills Lab Problem How do monomers join together to form polymers? Introduction A small number of elements make up most of the mass of your
Additional Lab 1 Making Models of Macromolecules Guided Inquiry Skills Lab Problem How do monomers join together to form polymers? Introduction A small number of elements make up most of the mass of your
2.2 Properties of Water
 2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank.
 Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. 1. (3 points each) First set functional groups A. ether D. amine B.
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. 1. (3 points each) First set functional groups A. ether D. amine B.
Catalysts of Lipid Oxidation
 Catalysts of Lipid Oxidation Iron The most important nonenzymic catalyst for initiation of lipid peroxidation The most abundant transitional metal in biological systems Possibility of various oxidation
Catalysts of Lipid Oxidation Iron The most important nonenzymic catalyst for initiation of lipid peroxidation The most abundant transitional metal in biological systems Possibility of various oxidation
Alcohols and Ethers. Alcohols
 Alcohols and Ethers A patient does not experience pain during surgery when given a general anesthetic. The earliest anesthetics, used during the Civil War, belonged to a class of chemical compounds called
Alcohols and Ethers A patient does not experience pain during surgery when given a general anesthetic. The earliest anesthetics, used during the Civil War, belonged to a class of chemical compounds called
OCR A GCSE Chemistry. Topic 6: Global challenges. Organic chemistry. Notes.
 OCR A GCSE Chemistry Topic 6: Global challenges Organic chemistry Notes C6.2a recognise functional groups and identify members of the same homologous series Prefixes (beginning of the name) o remember
OCR A GCSE Chemistry Topic 6: Global challenges Organic chemistry Notes C6.2a recognise functional groups and identify members of the same homologous series Prefixes (beginning of the name) o remember
CfE Higher Chemistry Homework. Unit 2: Natures Chemistry. The Chemistry of Cooking and Oxidation of Food. 1. Which of the following is an aldehyde?
 CfE Higher Chemistry Homework Unit 2: Natures Chemistry The Chemistry of Cooking and Oxidation of Food 1. Which of the following is an aldehyde? 2. Which is true of a compound with the following formula?
CfE Higher Chemistry Homework Unit 2: Natures Chemistry The Chemistry of Cooking and Oxidation of Food 1. Which of the following is an aldehyde? 2. Which is true of a compound with the following formula?
Properties of Alcohols and Phenols Experiment #3
 Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
National 5 Unit Two : Nature s Chemistry
 National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
Organic photosynthetic reactions
 rganic photosynthetic reactions Singlet oxygen 2 " excited state! excited state 3 2 ground state atomic orbitals molecular orbitals atomic orbitals 5 rganic photosynthetic reactions Singlet oxygen lifetime
rganic photosynthetic reactions Singlet oxygen 2 " excited state! excited state 3 2 ground state atomic orbitals molecular orbitals atomic orbitals 5 rganic photosynthetic reactions Singlet oxygen lifetime
From Atoms to Cells: Fundamental Building Blocks. Models of atoms. A chemical connection
 From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
From Atoms to Cells: A chemical connection Fundamental Building Blocks Matter - all materials that occupy space & have mass Matter is composed of atoms Atom simplest form of matter not divisible into simpler
Copyright 2014 Edmentum - All rights reserved.
 Study Island Copyright 2014 Edmentum - All rights reserved. Generation Date: 04/01/2014 Generated By: Cheryl Shelton Title: Science- biology Cells 1. Below is an image of a plant cell. What processes require
Study Island Copyright 2014 Edmentum - All rights reserved. Generation Date: 04/01/2014 Generated By: Cheryl Shelton Title: Science- biology Cells 1. Below is an image of a plant cell. What processes require
The. Crash Course. Basically, almost all living things are made up of these 4 Elements: - Carbon (C) - Nitrogen (N) - Hydrogen (H) - Oxygen (O)
 The Biochemistry Crash Course Basically, almost all living things are made up of these 4 Elements: - Carbon (C) - Nitrogen (N) - Hydrogen (H) - Oxygen (O) This exercise is designed to familiarize you with
The Biochemistry Crash Course Basically, almost all living things are made up of these 4 Elements: - Carbon (C) - Nitrogen (N) - Hydrogen (H) - Oxygen (O) This exercise is designed to familiarize you with
S4 Chemistry National 5
 S4 hemistry National 5 Nature s hemistry Unit ourse Notes- ydrocarbons and onsumer Products Name lass 1 ydrocarbons ydrocarbons can be categorised into family groups known as OMOLOGOUS SERIES. A homologous
S4 hemistry National 5 Nature s hemistry Unit ourse Notes- ydrocarbons and onsumer Products Name lass 1 ydrocarbons ydrocarbons can be categorised into family groups known as OMOLOGOUS SERIES. A homologous
Biology 12 - Biochemistry Practice Exam
 Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Chapter 7 Structure and Synthesis of Alkenes. Introduction
 Chapter 7 Structure and Synthesis of Alkenes Introduction ydrocarbon with carbon-carbon double bonds Sometimes called olefins, oil-forming gas Planar Pi bond is the functional group. More reactive than
Chapter 7 Structure and Synthesis of Alkenes Introduction ydrocarbon with carbon-carbon double bonds Sometimes called olefins, oil-forming gas Planar Pi bond is the functional group. More reactive than
Name the ester produced when methanol and pentanoic acid react. methyl pentanoate. Name the type of reaction used to make an ester
 1 Name the ester produced when methanol and pentanoic acid react methyl pentanoate 2 Name the type of reaction used to make an ester condensation reaction 3 Name the by-product of the reaction used to
1 Name the ester produced when methanol and pentanoic acid react methyl pentanoate 2 Name the type of reaction used to make an ester condensation reaction 3 Name the by-product of the reaction used to
BIOCHEMISTRY NOTES PT. 3 FOUR MAIN TYPES OF ORGANIC MOLECULES THAT MAKE UP LIVING THINGS
 BIOCHEMISTRY NOTES PT. 3 FOUR MAIN TYPES OF ORGANIC MOLECULES THAT MAKE UP LIVING THINGS 1. 2. 3. 4. CARBOHYDRATES LIPIDS (fats) PROTEINS NUCLEIC ACIDS We call these four main types of carbon- based molecules
BIOCHEMISTRY NOTES PT. 3 FOUR MAIN TYPES OF ORGANIC MOLECULES THAT MAKE UP LIVING THINGS 1. 2. 3. 4. CARBOHYDRATES LIPIDS (fats) PROTEINS NUCLEIC ACIDS We call these four main types of carbon- based molecules
Loose Ends. Reactions. Polymers
 Today Loose Ends Reactions Polymers Names for isolated groups -OH Hydroxyl -NH2 Amino O -C- Carbonyl O -C-O Carboxyl Vitamin D4 Our friend the benzene ring Another important Functional Group Phenol Nomenclature
Today Loose Ends Reactions Polymers Names for isolated groups -OH Hydroxyl -NH2 Amino O -C- Carbonyl O -C-O Carboxyl Vitamin D4 Our friend the benzene ring Another important Functional Group Phenol Nomenclature
Chapter 17 Carboxylic Acids, Esters, and Amides Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 17 Carboxylic Acids, Esters, and Amides Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 17 Carboxylic Acids, Esters, and Amides Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 15 Alcohols, Diols, and Thiols
 Chapter 15 Alcohols, Diols, and Thiols 15.1 Sources of Alcohols Methanol Methanol is an industrial chemical end uses: solvent, antifreeze, fuel principal use: preparation of formaldehyde Methanol Methanol
Chapter 15 Alcohols, Diols, and Thiols 15.1 Sources of Alcohols Methanol Methanol is an industrial chemical end uses: solvent, antifreeze, fuel principal use: preparation of formaldehyde Methanol Methanol
the properties of carbon
 Carbon Compounds Learning Objectives Describe the unique qualities of carbon. Describe the structures and functions of each of the four groups of macromolecules. For each macromolecule you will need to
Carbon Compounds Learning Objectives Describe the unique qualities of carbon. Describe the structures and functions of each of the four groups of macromolecules. For each macromolecule you will need to
Carbon. Isomers. The Chemical Building Blocks of Life
 The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
The Chemical Building Blocks of Life Carbon Chapter 3 Framework of biological molecules consists primarily of carbon bonded to Carbon O, N, S, P or H Can form up to 4 covalent bonds Hydrocarbons molecule
Carbon. p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms
 Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Chemistry 1120 Exam 1 Study Guide
 Chemistry 1120 Exam 1 Study Guide Chapter 3 3.1 a) Know that alcohols contain a hydroxy (-OH) group. Determine the IUPAC name for a given structure by determining the longest chain. b) Determine the number
Chemistry 1120 Exam 1 Study Guide Chapter 3 3.1 a) Know that alcohols contain a hydroxy (-OH) group. Determine the IUPAC name for a given structure by determining the longest chain. b) Determine the number
The Carbon Atom (cont.)
 Organic Molecules Organic Chemistry The chemistry of the living world. Organic Molecule a molecule containing carbon and hydrogen Carbon has 4 electrons in its outer shell and can share electrons with
Organic Molecules Organic Chemistry The chemistry of the living world. Organic Molecule a molecule containing carbon and hydrogen Carbon has 4 electrons in its outer shell and can share electrons with
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n
 Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Ch 21 Carboxylic Acid Derivatives and Nu Acyl Subst n Acid Derivatives and their Names - Acid Halides have a Cl or Br instead of OH. Replace ic acid with yl halide, such as propionyl chloride (a common
Chapter 24. The Chemistry of Life: Organic and Biological Chemistry. Lecture Presentation. James F. Kirby Quinnipiac University Hamden, CT
 Lecture Presentation Chapter 24 The of Life: James F. Kirby Quinnipiac University Hamden, CT Organic and Biochemistry Chapter focus: the molecules that bridge chemistry & biology Most common elements:
Lecture Presentation Chapter 24 The of Life: James F. Kirby Quinnipiac University Hamden, CT Organic and Biochemistry Chapter focus: the molecules that bridge chemistry & biology Most common elements:
The Structure and Function of Biomolecules
 The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition- Elimination at the Acyl Carbon Introduction The carboxyl group (-CO 2 H) is the parent group of a family of compounds called acyl
Lesson Overview. Carbon Compounds. Lesson Overview. 2.3 Carbon Compounds
 Lesson Overview 2.3 The Chemistry of Carbon What elements does carbon bond with to make up life s molecules? Carbon can bond with many elements, including Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
Lesson Overview 2.3 The Chemistry of Carbon What elements does carbon bond with to make up life s molecules? Carbon can bond with many elements, including Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
2.3 Carbon-Based Molecules CARBON BASED MOLECULES
 CARBON BASED MOLECULES KEY CONCEPTS Carbon-based molecules are the foundation of life. Lipids are one class of organic molecules. This group includes fats, oils, waxes, and steroids. Lipids are made of
CARBON BASED MOLECULES KEY CONCEPTS Carbon-based molecules are the foundation of life. Lipids are one class of organic molecules. This group includes fats, oils, waxes, and steroids. Lipids are made of
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
INTRODUCTION TO BIOCHEMISTRY/POLYMERS. 3. With respect to amino acids, polypeptides, and proteins, know:
 INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
Biology: Life on Earth Chapter 3 Molecules of life
 Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
Biology: Life on Earth Chapter 3 Molecules of life Chapter 3 Outline 3.1 Why Is Carbon So Important in Biological Molecules? p. 38 3.2 How Are Organic Molecules Synthesized? p. 38 3.3 What Are Carbohydrates?
Chapterwise Previous year Qs
 221 ALCOHAL PHENOLS AND ETHERS 1. Which one is formed when sodium phenoxide is heated with ethyl iodide? [1988] Phenetole Ethyl phenyl alcohol Phenol None of these Ans: 2. Lucas reagent is [1988] Conc.
221 ALCOHAL PHENOLS AND ETHERS 1. Which one is formed when sodium phenoxide is heated with ethyl iodide? [1988] Phenetole Ethyl phenyl alcohol Phenol None of these Ans: 2. Lucas reagent is [1988] Conc.
Free Radicals in Biology and Medicine
 Free Radicals in Biology and Medicine 0 \ Second Edition BARRY HALLIWELL Professor of Medical Biochemistry, University of London King's College and JOHN M.C. GUTTERIDGE Senior Scientist, National Institute
Free Radicals in Biology and Medicine 0 \ Second Edition BARRY HALLIWELL Professor of Medical Biochemistry, University of London King's College and JOHN M.C. GUTTERIDGE Senior Scientist, National Institute
