Adaptation in auditory hair cells Robert Fettiplace and Anthony J Ricci y
|
|
|
- Peregrine Strickland
- 6 years ago
- Views:
Transcription
1 446 Adaptation in auditory hair cells Robert Fettiplace and Anthony J Ricci y The narrow stimulus limits of hair cell transduction, equivalent to a total excursion of about 100 nm at the tip of the hair bundle, demand tight regulation of the mechanical input to ensure that the mechanoelectrical transducer (MET) channels operate in their linear range. This control is provided by multiple components of Ca 2þ dependent adaptation. A slow mechanism limits the mechanical stimulus through the action of one or more unconventional myosins. There is also a fast, sub-millisecond, Ca 2þ regulation of the MET channel, which can generate resonance and confer tuning on transduction. Changing the conductance or kinetics of the MET channels can vary their resonant frequency. The tuning information conveyed in transduction may combine with the somatic motility of outer hair cells to produce an active process that supplies amplification and augments frequency selectivity in the mammalian cochlea. Addresses Department of Physiology, University of Wisconsin Medical School, Madison, WI 53706, USA fettiplace@physiology.wisc.edu y Neuroscience Center, Louisiana State University Health Sciences Center, New Orleans, LA 70112, USA This review comes from a themed issue on Sensory systems Edited by Clay Reid and King-Wai Yau /$ see front matter ß 2003 Elsevier Ltd. All rights reserved. DOI /S (03) Abbreviations camp cyclic adenosine monophosphate CF characteristic frequency EP endocochlear potential INAD Inactivation No After-potential D MET mechanoelectrical transducer OHC outer hair cell p o probability of opening of mechanoelectrical transducer channels p o -x relationship between p o and hair bundle displacement s A adaptation time constant x hair bundle displacement Introduction Hair cells are the sensory receptors of the inner ear that convert sound-induced vibrations of the cochlear partition into electrical signals. Mechanoelectrical transduction occurs in the hair bundle, which when moved towards its tallest edge opens mechanically gated ion channels near the tips of the component stereocilia [1]. This allows an influx of K þ and Ca 2þ ions that depolarize the hair cell. The current hypothesis regarding transduction is that deflection of the stereocilia exerts tension on the tip links (Figure 1) that transmit force to the mechanoelectrical transducer (MET) channel through elastic elements that are referred to as gating springs [2]. The double-helical structure of the tip link suggests that it is not the gating spring [3], and the molecular identity of the hair cell MET channel is currently unknown [4]. The probability of opening (p o ) of the MET channels is modulated by hair bundle displacement over a maximal excursion of nm, less than the diameter of a stereocilium. To keep them within a narrow operating range and preserve high sensitivity for extrinsic stimuli, the hair cell MET channels are subject to multiple Ca 2þ -controlled mechanisms of adaptation [5]. This review summarizes recent work on adaptation, including its connection to active hair bundle motion, the contribution of unconventional myosins, and a possible role in cochlear frequency selectivity. Multiple mechanisms of adaptation During a maintained displacement of the hair bundle (x), adaptation appears as a decline in the MET channel p o. This decline reflects a translation of the p o -x relationship along the displacement axis in the direction of the stimulus. At least two adaptation mechanisms can be distinguished on the basis of their different kinetics and mechanical correlates. Fast adaptation in turtle cochlear hair cells has a time constant (t A ) of ms [6,7]. Slow adaptation, first reported in frog saccular cells, has a t A of ms [8,9]. However, both fast and slow mechanisms are now known to coexist in the same hair cell [10,11,12]. The different balance of the two components in turtles and frogs may be due to the fact that turtle hair cells, being auditory, are exposed to higher stimulation frequencies than frog vestibular hair cells. The speed of adaptation may therefore be matched to the frequencies to which the cell is exposed. Consistent with this idea, fast adaptation is most conspicuous in mammalian cochlear hair cells with a t A ¼ 4ms[13]. Moreover, t A in the turtle varies with hair cell characteristic frequency (CF), and is faster in the cells tuned to higher frequencies: the corner frequency (1/2pt A ) of the high-pass filter imposed by fast adaptation is approximately two-thirds of the CF [14]. This suggests that fast adaptation may play some part in hair cell frequency selectivity (the ability of the cell to distinguish different frequency components in the stimulus) [7,14]. Further support for this notion comes from the observation that in physiological Ca 2þ concentrations, fast adaptation can display resonance at frequencies in the turtle s auditory range (Figure 1; [14]). Both types of adaptation are regulated by Ca 2þ that enters the stereocilia through highly Ca 2þ -permeable MET
2 Adaptation in auditory hair cells Fettiplace and Ricci 447 Figure 1 (a) (b) T Myosin Tip link Ca 2+ Out MET channels Myosin Closed C Open O Adapted Ca 2+ C In (c) 2.8 mm Ca 2+ Rootlet P o 0.07 mm Ca ms Current Opinion in Neurobiology Sites and action of hair cell adaptation (a) Structural components of the stereocilia associated with transduction and adaptation, showing the electron dense plaques that represent sub-membranous protein complexes. Rotation towards the taller stereocilium exerts force on the tip link and opens MET channels at the stereociliary tip. Tension in the tip link may be adjusted adaptively by myosin arrays (1c, 7a or 15) at either end of the tip link; for example, myosin-1c in the upper plaque tensions the link by climbing up towards the barbed end of the actin filaments. Stereociliary position could also be influenced by the stiffness of the rootlets into the hair cell apex. (b) Hair bundle displacement tensions the tip link (T) which extends the internal and external gating springs causing the channel to go from the closed (C) to the open (O) configuration. Ca 2þ entering the stereocilium through the open channel binds at the inner face of the channel and shuts it. This generates force by increasing tension in the gating springs. (c) Following a bundle deflection (top), the channel opens rapidly then recloses adaptively in a high concentration of 2.8 mm Ca 2þ. Change in p o is plotted against time. When the extracellular Ca 2þ concentration is reduced to 0.07 mm Ca 2þ, a more realistic physiological value closer to that in cochlear endolymph, the adaptation becomes oscillatory at 77 Hz. The resonant frequency of the MET channel varies with hair cell CF. channels [8,15,16]. Fast adaptation probably requires a direct interaction of Ca 2þ with the MET channels to modulate their probability of opening [14,15,17].Onthe basis of the effects of intracellular calcium buffers, the distance Ca 2þ diffuses to its target is estimated as short, nm from the mouth of the channel [14]. Its resulting action occurs in well under a millisecond [16]. Furthermore, Ca 2þ can alter the time constant of channel activation as well as adaptation [18], arguing that it is intimately linked with channel gating. In contrast, slow adaptation is regarded as an input control, in which a Ca 2þ dependent motor controls tension in the elastic elements in series with the channel [9,19].Thereisgood evidence to implicate myosin-1c as the motor that drives slow adaptation in vestibular hair cells [11 ]. One or both phases of adaptation could be mediated through an interaction with calmodulin [20], which is present at the tips of the stereocilia [21] where it interacts with myosin-1c [22]. Besides the two Ca 2þ -driven mechanisms, other pathways may modulate the MET channel s operating range. For example, cyclic adenosine monophosphate (camp) shifts the p o -x relation along the displacement axis in the positive direction, with no affect on fast adaptation [6].ThecAMPeffectmaybemediated through phosphorylation of the MET channel or the myosin motor by protein kinase A.
3 448 Sensory systems Mechanical correlates of adaptation The distinction between fast (channel) adaptation and slow (myosin motor) adaptation is supported by measurements of hair bundle motion during electrical or mechanical hair cell stimulation. The connection between these two types of stimuli is their effect on the intracellular Ca 2þ concentration. Both displacement of the hair bundle towards its shorter edge (closing the MET channels) and depolarization towards the Ca 2þ equilibrium potential (lowering the electrical driving force on Ca 2þ ) reduce Ca 2þ influx. The evoked movements can be classified on the basis of their kinetics, which match those of fast and slow adaptation. The fast active response is complete in a few milliseconds [23 25], but the slow response can extend from 10 to 100 ms [9,19]. The time constant of the fast mechanical response, like that of fast adaptation, varies with hair cell CF and is faster in cells tuned to higher frequencies [23]. It is also possible to distinguish the two mechanisms by their polarity. For large depolarizations that take the membrane potential near the Ca 2þ equilibrium potential, the fast movement is in the positive direction (towards the taller edge of the bundle), whereas the slow movement is in the negative direction. As with adaptation, both components are observable in the same cell [26 ]. An interesting but unexplained observation is that the speed and polarity of the movement depend on the absolute bundle position: steady displacement of the bundle towards its taller edge transforms a fast positive motion into a slower negative one [26 ]. A prediction of the gating spring model of transduction is that as the channel opens there is a decrease in hair bundle stiffness [2], and this is confirmed experimentally [2,26,27, 28]. Ca 2þ interaction with the channel to modulate its p o will therefore cause the bundle to move, connecting fast adaptation to bundle motion [23,28]. In terms of polarity, a positive bundle deflection opens the MET channels and increases Ca 2þ concentration, which recloses the channels and causes negative recoil. In contrast, the increase in Ca 2þ produced by a positive bundle deflection detaches the myosin from the actin core of the stereocilium. This allows the myosin and its attachment to the tip link to slip down the stereocilium, which leads to further positive displacement of the bundle [9]. Evidence for the range of movement of the tip-link s upper attachment point comes from the observation that if the tip links are severed with BAPTA (1,2-bis[o-Aminophenoxy]ethane-N,N,N,N -tetraacetic acid), the electron dense plaque (Figure 1) climbs nm closer to the stereociliary tip [29]. An unconventional myosin as the adaptation motor Five unconventional non-muscle myosins have been localized to hair cells: myosin-1c, 3, 6, 7a and 15 [30,31,32].Of these, the prime contenders for the motor that powers slow adaptation are either myosin-1c [11 ] or myosin 7a [33 ]. Myosin-1c is concentrated in frog hair bundles at the two ends of the tip link: not only in the electron-dense plaque marking its upper attachment point but also most conspicuously at the tip of the stereocilium [34,35]. Convincing evidence for the role of myosin-1c in slow adaptation has come from modifying the ATP-binding site to confer susceptibility to inhibition by certain ADP analogs [36]. Expression of the mutated myosin-1c in mouse utricular hair cells was then shown to render slow adaptation sensitive to blockade by the ADP analogs introduced through the recording pipette, whereas it did not affect fast adaptation [11 ]. Although myosin-7a is distributed along the entire length of the stereocilia [30], its mutation in Shaker1 mice causes a substantial positive shift of the p o -x relation along the displacement axis so that the MET channels are no longer poised to open at the bundle s resting position [33 ]. Thus, myosin-7a may also contribute to adaptation, but how it collaborates with myosin-1c in optimizing transduction is not well understood. Do the two myosin isoforms generate force in the same direction or do they operate in an opposable push-pull manner? How are they controlled by changes in intracellular Ca 2þ concentration? To relieve tip link tension Ca 2þ must cause detachment of the myosin head from actin and its slippage down the stereocilia [5,37], rather than the usual initiation of the power stroke, which would drive the myosin up the stereocilia and therefore increase tip link tension. Myosin-6 is anomalous in tracking backwards along the actin filament [38], and would be more suited as the motor at the top of the tip link. At this point less is known about myosin-3 and myosin-15 so their roles in transduction cannot be properly assessed. Myosin-3 is especially interesting because its homolog is present in Drosophila photoreceptors where it associates with the PDZ scaffolding protein INAD (Inactivation No Afterpotential D) that also binds to the photoreceptor transducer channel [39]. INAD is also present in the vertebrate cochlea [31 ]. The unconventional myosins may perform other cellular functions besides adaptation, including ferrying components of the transduction machinery to their appropriate location [40 ]. Furthermore, mutations in several of the myosins are accompanied by loss of the hair bundle s structural integrity that results in deafness [41,42]. The reverse motor action of myosin-6 [38] may enable it to retrieve proteins transported to the stereociliary tips by other forward-acting myosins. Roles of adaptation mechanisms A reason for having multiple adaptation mechanisms is that the slower mechanism has a wider dynamic range to orient the bundle to a location where fast feedback control of the channels is effective. Thus, fast adaptation may tune the channel for small displacements around a resting position that is continually readjusted by slow adaptation. Fast adaptation could theoretically participate in auditory frequency selectivity by filtering the MET current [14] or by generating fast hair bundle movements
4 Adaptation in auditory hair cells Fettiplace and Ricci 449 that amplify the mechanical input. Hair bundles in the frog saccule have the ability to mechanically amplify a signal leading to spontaneous oscillations. The oscillations occur at low frequencies (5 50 Hz) and it has been hypothesized that they are driven by the slow myosin motor, biasing the displacement-force relation of the hair bundle into a region of negative slope [43 ]. In contrast, active bundle movements produced by Ca 2þ binding to the MET channels can in principle occur at kilohertz frequencies, within the mammalian auditory range [17]. Furthermore, trans-epithelial electrical stimulation of the isolated frog saccule experimentally evokes hair bundle oscillations at frequencies of up to 1 khz [44 ]. There is also in vivo evidence for active hair bundle motion at more than 1 khz in the lizard hearing organ [45 ]. However, it remains to be seen whether or not active motion of the outer hair-cell bundles can generate sufficient force to produce amplification in the intact mammalian cochlea [7]. Tuning of the MET current in turtle auditory hair cells does not require concomitant active bundle motion because it occurs when the bundle is displaced with a rigid stimulating probe (Figure 1; [14]). This suggests that the energy associated with channel gating does not need to move the hair bundle in order to elicit oscillations in the current. The variation in resonant frequency ( Hz), which is within the turtle s auditory range, may therefore involve differences in the MET channel. Several mechanisms for these differences have been proposed. The adaptation rate or resonant frequency increases with a higher stereociliary Ca 2þ concentration, which could be brought about either by increasing the channel sca 2þ permeability with CF or by increasing the conductance of the MET channels or their number per stereocilium. The evidence for this mechanism is that the maximum MET current increases with CF, and the rate of adaptation at a given CF varies with the magnitude of the current [6,14]. It has been recently shown that the channel sca 2þ permeability does not alter with CF [46 ]. However, there is evidence for a tonotopic variation in channel kinetics derived from noise analysis of the MET current [46 ], and from measurements of the time course of current activation [18]. An alternate view, formed on the basis of modeling hair bundle amplification, is that both fast and slow adaptation processes combine to produce oscillations at a frequency determined by hair bundle geometry and intracellular Ca 2þ dynamics [47]. Amplification and tuning in the mammalian cochlea In the mammalian cochlea, the outer hair cells (OHC) are responsible for generating active mechanical amplification that provides the compressive non-linearity and frequency selectivity of the basilar membrane [48]. The ability of OHCs to elongate and shorten in response to changes in membrane potential is thought to supply the mechanical energy for the process [49]. Prestin has been identified as the protein responsible for this somatic motility [50], and it is concentrated in the lateral wall of the OHC [51]. The voltage sensitivity of prestin is endowed by the intracellular binding of small anions such as Cl [52 ]. Targeted deletion of prestin in mice results in an elevated threshold, reduced tuning, and loss of outer hair cell motility [53 ], which led the authors to conclude that somatic motility alone was responsible for the active process. This conclusion can be criticized on the grounds that any feedback process involving multiple elements would be compromised if one element were eliminated. For example, abolition of the endocochlear potential (EP) by treatment with the diuretic furosemide reduces amplification about 30-fold on average [54], even though the EP is not the source of the active process. Loss of the EP will approximately halve the MET current. With regards to the same argument, halving of electromotility in prestin heterozygotes [53 ] should have produced greater than the twofold elevation in cochlear thresholds if somatic motility were the sole source of the active process. Furthermore, although OHC motility may supply the energy for amplification, there is no evidence that it is intrinsically frequency selective. For cochlear models to produce realistically sharp tuning of the basilar membrane, the mechanical feedback from the outer hair cells must occur in a frequency-selective manner to ensure it supplies force at the appropriate phase of basilar membrane vibration [55 58]. The required tuning has been ascribed to a mechanical resonance in the tectorial membrane [56,58,59], but it could equally reside in the MET channels. If the channels in mammalian OHCs operate similarly to those in turtle hair cells, the transducer currents and any associated active hair bundle motion will be tuned over a frequency range imparted by variations in the speed of fast adaptation [23]. Conclusions Over the past five years, evidence has emerged for at least two distinct Ca 2þ -mediated mechanisms of hair cell transducer adaptation varying in speed, range and function. A fast mechanism directly affects MET channel gating, whereas a slower one regulates the mechanical stimulus through the action of one or more unconventional myosins. Further insights into the roles of the different myosins may come from the elucidation of their subcellular localization using post-embedding immunogold techniques, or their modification and deletion in transgenic animals. The fast mechanism confers tuning on the MET channels and may be an important factor in cochlear frequency selectivity. Because adaptation shifts the hair cell s operating point, it may have an ancillary role in constantly adjusting and optimizing the signal-to-noise ratio of transduction [60]. Cloning the MET channel should illuminate how the channel interacts with Ca 2þ and other subcellular components including myosins. It may also reveal the existence of multiple channel isoforms with Ca 2þ affinity or kinetics specialized for operation in different frequency ranges. However, further
5 450 Sensory systems electrophysiological recordings in the mammalian cochlea will be needed to settle whether or not fast adaptation is present in outer hair cells and whether or not it is sufficiently fast to participate in the active process. Evidence that this may be the case was recently obtained in rat OHCs, in which transducer currents exhibited fast Ca 2þ - dependent adaptation with a t A of less than 0.2 ms, faster than any seen in turtle auditory hair cells [61 ]. Acknowledgements Work in the authors laboratories was supported by the National Institute on Deafness and Other Communicative Disorders, grants RO1 DC (to R Fettiplace) and RO1 DC (to AJ Ricci). We thank C Hackney for her comments on the manuscript and N Cooper for pointing out the amplification discrepancy in the prestin heterozygotes. References and recommended reading Papers of particular interest, published within the annual period of review, have been highlighted as: of special interest of outstanding interest 1. Hudspeth AJ: How the ear s works work. Nature 1999, 341: Howard J, Hudspeth AJ: Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog s saccular hair cell. Neuron 1988, 1: Kachar B, Parakkal M, Kurc M, Zhao Y, Gillespie PG: Highresolution structure of hair-cell tip links. Proc Natl Acad Sci USA 2000, 97: Strassmaier M, Gillepsie PG: The hair cell s transduction channel. Curr Opin Neurobiol 2002, 12: Eatock RA: Adaptation in hair cells. Annu Rev Neurosci 2000, 23: Ricci AJ, Fettiplace R: The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J Physiol 1997, 501: Fettiplace R, Ricci AJ, Hackney CM: Clues to the cochlear amplifier from the turtle ear. Trends Neurosci 2001, 24: Assad JA, Hacohen N, Corey DP: Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci USA 1989, 86: Assad JA, Corey DP: An active motor model for adaptation by vertebrate hair cells. J Neurosci 1992, 12: Wu YC, Ricci AJ, Fettiplace R: Two components of transducer adaptation in auditory hair cells. J Neurophysiol 1999, 82: Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, Corey DP, Mercer JA, Gillespie PG: A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 2002, 108: The authors present unequivocal evidence of a role for myosin-1c in slow adaptation in mouse vestibular hair cells. The work uses a strikingly novel pharmacological technique of mutating the receptor (ATPase) site on myosin-1c to render it susceptible to blockade by engineered adenosine diphosphate analogs. 12. Eatock RA, Hurley KM, Vollrath M: Mechanoelectrical and voltage-gated ion channels in mammalian avestibular hair cells. Audiol Neurootol 2002, 7: Kros CJ, Rüsch A, Richardson GP: Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc R Soc Lond B Biol Sci 1992, 249: Ricci AJ, Wu Y-C, Fettiplace R: The endogenous Ca 2R buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci 1998, 18: Crawford AC, Evans MG, Fettiplace R: Activation and adaptation of transducer currents in turtle hair cells. J Physiol 1989, 419: Ricci AJ, Fettiplace R: Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol 1998, 506: Choe Y, Magnasco M, Hudspeth AJ: A model for amplification of hair bundle motion by cyclical binding of Ca 2R to mechanoelectrical transducer channels. Proc Natl Acad Sci USA 1998, 95: Fettiplace R, Crawford AC, Ricci AJ: The effects of calcium on mechanotransducer channel kinetics in auditory hair cells. In Biophysics of the Cochlea: From Molecules to Models. Edited by Gummer AW. Singapore: World Scientific; 2003, Howard J, Hudspeth AJ: Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog s saccular hair cell. Proc Natl Acad Sci USA 1987, 84: Walker RG, Hudspeth AJ: Calmodulin controls adaptation of mechanoelectrical transduction by hair cells of the bullfrog s sacculus. Proc Natl Acad Sci USA 1996, 93: Furness DN, Karkanevatos A, West B, Hackney CM: An immunogold investigation of the distribution of calmodulin in the apex of the cochlear hair cells. Hear Res 2002, 173: Cyr JL, Dumont RA, Gillespie PG: Myosin 1-c interacts with haircell receptors through its calmodulin- binding IQ domains. J Neurosci 2002, 22: Ricci AJ, Crawford AC, Fettiplace R: Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci 2000, 20: Crawford AC, Fettiplace R: The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol 1985,364: Benser ME, Marquis RE, Hudspeth AJ: Rapid, active hair bundle movements inhair cellsfrom the bullfrog s sacculus. J Neurosci 1996, 16: Ricci AJ, Crawford AC, Fettiplace R: Mechanisms of active hair bundle motion in auditory hair cells. J Neurosci 2002, 22: This study provides evidence for at least two components of adaptation and their manifestation in active bundle motion in the same hair cell, in which they have characteristic kinetics and polarities. It also contains the surprising observation that by biasing the static position of the hair bundle one of the movements can be reversed in polarity through an as yet unknown mechanism. 27. Russell IJ, Kössl M, Richardson GP: Nonlinear mechanical responses of mouse cochlear hair bundles. Proc R Soc Lond B Biol Sci 1992, 250: van Netten SM, Kros CJ: Gating energies and forces of the mammalian hair cell transducer channel and related hair bundle mechanics. Proc R Soc Lond B Biol Sci 2000, 267: Shepherd GMG, Assad JA, Solc CK, Rock KE, Corey DP: A molecular motor mediating adaptation in bullfrog hair cells. In Biophysics of Hair Cell Sensory Systems. Edited by Duifhuis H, Horst JW, van Dijk P, van Netten SM. Singapore: World Scientific; 1993, Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP: Unconventional myosins in inner-ear sensory epithelia. J Cell Biol 1997, 137: Walsh T, Walsh V, Vreugde S, Hertzano R, Shahin H, Hsika S, Lee MK, Kanaan M, King M-C, Avraham K: From flies eyes to our ear: mutations in a human class III myosin causes nonsyndromic hearing loss DFNB30. Proc Natl Acad Sci USA 2002, 99: The authors provide the first demonstration of the involvement of myosin III in hearing and its targeting to cochlear hair cells. This class of myosin was previously known to interact with actin filaments and a PDZ scaffolding protein in Drosophila photoreceptors. Here, three mutations of myosin IIIa were shown to underlie a nonsyndromic progressive deafness. 32. Belyantseva IA, Azevedo RB, Fridell RA, Friedman TB, Kachar B: Localization of myosin XVA in hair cells. ARO Abstr 2002,25:157.
6 Adaptation in auditory hair cells Fettiplace and Ricci Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown DM, Richardson GP, Steel KP: Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci 2002, 5: The authors use post-genomic experimentation that employs physiological measurements in mouse mutants to elucidate a role for myosin 7a in mechanotransduction in auditory hair cells. For the MET channels to open, the hair bundles in the mutants must be deflected several microns beyond their normal operating range, which suggests that myosin 7a normally positions the bundle for optimal transducer sensitivity. 34. Garcia JA, Yee AG, Gillespie PG, Corey DP: Localization of myosin-ibeta near both ends of tip links in frog saccular hair cells. J Neurosci 1998, 18: Steyger PS, Gillespie PG, Baird RA: Myosin Ibeta is located at tip link anchors in vestibular hair bundles. J Neurosci 1998, 18: Gillespie PG, Gillespie SK, Mercer JA, Shah K, Shokat KM: Engineering of the myosin-ibeta nucleotide-binding pocket to create selective sensitivity to N(6)-modifiedADP analogs. J Biol Chem 1999, 274: Gillespie PG, Corey DP: Myosin and adaptation by hair cells. Neuron 1997, 19: Wells AL, Lin AW, Chen LQ, Saler D, Cain SM, Hasson T, Carraghers BO, Milligan RA, Sweeney HL: Myosin VI is an actinbased motor that moves backwards. Nature 1999, 401: Wes PD, Xu XS, Li H, Chien F, Doberstein SK, Montell C: Termination of phototransduction requires binding of the NINAC myosin III and the PDZ protein INAD. Nat Neurosci 1999, 2: Boëda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J et al.: Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair bundle. EMBO J 2002, 21: This study uses both yeast two-hybrid and pull-down binding assays to demonstrate interactions among three proteins (myosin 7a, cadherin-23 and a PDZ protein harmonin) that are known to be mutated in Usher type I deaf-blindness syndrome. Equivalent mutations in mice cause defective hair bundle development and result in deafness. The authors conclude that myosin 7a ferries harmonin along the actin core of developing stereocilia, and that the three-protein complex is essential for stereociliary cohesion. 41. Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steele KP: Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development 1998, 125: Anderson DW, Probst FJ, Belyantsevsa IA, Fridell RA, Beyer L, Martin DM, Wu D, Kachar B, Friedman TB, Raphael Y, Camper S: The motor and tail regions of myosin XV are critical for normal structure and function of auditory and vestibular hair cells. Hum Mol Genet 2000, 12: Martin P, Bozovic D, Choe Y, Hudspeth AJ: Spontaneous oscillations by hair bundles of the bullfrog s sacculus. J Neurosci 2003, 23: The authors present the most detailed account so far of the properties and mechanisms of spontaneous hair bundle oscillations. They demonstrate that they are driven by the slow adaptation motor biasing the displacementforce relation of the hair bundle into a region of negative slope. 44. Bosovic D, Hudspeth AJ: Hair-bundle movements elicited by transepithelial electrical stimulation of hair cells in the sacculus of the bullfrog. Proc Natl Acad Sci USA 2003, 100: The authors describe a single-cell correlate of the electrically evoked otoacoustic emissions that are recorded in most vertebrate classes and used as evidence for ubiquitous active mechanical output from the hair cells. Spontaneous oscillations of saccular hair bundles were modulated at frequencies of up to 1 khz by extracellular electrical stimulation. The specificity of the response was tied to hair cell transduction by showing that it disappeared on blocking the MET channels. 45. Manley GA, Kirk DL, Köppl C, Yates GK: In vivo evidence for a cochlear amplifier in the hair-cell bundle of lizards. Proc Natl Acad Sci USA 2001, 98: Good evidence for active hair bundle motion in vivo comes from the properties of the otoacoustic emissions produced by electrical stimulation of the lizard inner ear. The authors show that because the hair bundles in the lizard hearing organ have two polarities of orientation, the ototacoustic emissions display a characteristic pattern of modulation by sound. 46. Ricci A: Differences in mechano-transducer channel kinetics underlie tonotopic distribution of fast adaptation in auditory hair cells. J Neurophysiol 2002, 87: The authors demonstrate that MET currents in turtle auditory hair cells tuned to high and low frequencies show different sensitivities to channel blockers such as dihydrostreptomycin, and have different open times as assessed by the spectra of the current noise. 47. Vilfan A, Duke T: Two adaptation processes in auditory hair cells together can provide an active amplifier. Biophys J 2003, 85: Robles L, Ruggero MA: Mechanics of the mammalian cochlea. Physiol Rev 2001, 81: Nobili R, Mammano F, Ashmore JF: How well do we understand the cochlea? Trends Neurosci 1998, 21: Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P: Prestin is the motor protein of cochlear outer hair cells. Nature 2000, 405: Belyantseva IA, Adler HJ, Curi R, Frolenkov GI, Kachar B: Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J Neurosci 2000, 20:RC Oliver D, He DZZ, Klöcker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B: Intracellular anions as the voltage sensor or prestin, the outer hair cell motor protein. Science 2001, 292: The authors found that mutagenesis of many of the amino acids from charged to neutral in the putative membrane domain of the prestin molecule had little or no effect on the voltage sensitivity of the membrane protein. This led them to conclude that the voltage sensor for the outer hair cell motor was a charged particle extrinsic to the protein, and they identified it as arising from the binding of intracellular chloride and bicarbonate ions. 53. Liberman MC, Gao J, He DZZ, Wu X, Jia S, Zuo J: Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 2002, 419: The authors found that targeted deletion of prestin in mice abolishes the somatic motility of outer hair cells in vitro and causes a db loss of auditory sensitivity and otoacoustic emissions in vivo without affecting the MET channels. This is strong evidence for prestin being part of the electromechanical feedback loop that underlies cochlear amplifier. 54. Ruggero MA, Rich NC: Furosemide alters organ of Corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci 1991, 11: Neely ST, Kim DO: An active cochlear model showing sharp tuning and high sensitivity. Hear Res 1983, 9: Nobili R, Mammano F: Biophysics of the cochlea II: stationary nonlinear phenomenology. J Acoust Soc Am 1996,99: Nilsen KE, Russell IJ: The spatial and temporal representation of a tone on the guinea pig basilar membrane. Proc Natl Acad Sci USA 2000, 97: Geisler CD, Sang C: A cochlear model using feed-forward outerhair-cell forces. Hear Res 1995, 86: Gummer AW, Hemmert W, Zenner H-P: Resonant tectorial membrane motion in the inner ear: its crucial role in frequency tuning. Proc Natl Acad Sci USA 1996, 93: Dinklo T, van Netten SM, Marcotti W, Kros CJ: Signal processing by transducer channels in mammalian outer hair cells. In Biophysics of the cochlea: from molecules to models. Edited by Gummer AW. Singapore: World Scientific; 2003, Kennedy HJ, Evans MG, Crawford AC, Fettiplace R: Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci (Published online DOI: / Nn1089). The authors use the first measurements of OHC transducer currents in animals after the onset of hearing to demonstrate that the MET channels in mammalian cochlear hair cells possess ultra-fast activation and adaptation kinetics.
THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA
 451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
Chapter 3: Anatomy and physiology of the sensory auditory mechanism
 Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier
 HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
Cochlear anatomy, function and pathology II. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Cochlear anatomy, function and pathology I. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
The transformation of sound stimuli into electrical signals
 The transformation of sound stimuli into electrical signals Robert Fettiplace 2 1 Introduction Our sense of hearing depends on the correct performance of about 15 000 hair cells in each cochlea that serve
The transformation of sound stimuli into electrical signals Robert Fettiplace 2 1 Introduction Our sense of hearing depends on the correct performance of about 15 000 hair cells in each cochlea that serve
THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS?
 JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
Mechanical Properties of the Cochlea. Reading: Yost Ch. 7
 Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
What is the effect on the hair cell if the stereocilia are bent away from the kinocilium?
 CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
What Drives Mechanical Amplification in the Mammalian Cochlea?
 What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
Auditory System Feedback
 Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS
 SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components
 1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
Auditory mechanotransduction in the absence of functional myosin-xva
 J Physiol 576.3 (2006) pp 801 808 801 RAPID REPORT Auditory mechanotransduction in the absence of functional myosin-xva Ruben Stepanyan 1, Inna A. Belyantseva 2, Andrew J. Griffith 3, Thomas B. Friedman
J Physiol 576.3 (2006) pp 801 808 801 RAPID REPORT Auditory mechanotransduction in the absence of functional myosin-xva Ruben Stepanyan 1, Inna A. Belyantseva 2, Andrew J. Griffith 3, Thomas B. Friedman
Active Hair Bundle Movements and the Cochlear Amplifier
 Active Hair Bundle Movements and the Cochlear Amplifier Anthony Ricci* Abstract The active process is a term used to describe amplification and filtering processes that are essential for obtaining the
Active Hair Bundle Movements and the Cochlear Amplifier Anthony Ricci* Abstract The active process is a term used to describe amplification and filtering processes that are essential for obtaining the
Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression
 1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
Making an Effort to Listen: Mechanical Amplification in the Ear
 Making an Effort to Listen: Mechanical Amplification in the Ear A.J. Hudspeth 1, * 1 Laboratory of Sensory Neuroscience and Howard Hughes Medical Institute, The Rockefeller University, 1230 York Avenue,
Making an Effort to Listen: Mechanical Amplification in the Ear A.J. Hudspeth 1, * 1 Laboratory of Sensory Neuroscience and Howard Hughes Medical Institute, The Rockefeller University, 1230 York Avenue,
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
HST 721 Efferent Control Lecture October 2004
 HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
SOLUTIONS Homework #3. Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03
 SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
Deafness and hearing impairment
 Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Hearing Research 273 (2011) 109e122. Contents lists available at ScienceDirect. Hearing Research. journal homepage:
 Hearing Research 273 (2011) 109e122 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Somatic motility and hair bundle mechanics, are both necessary
Hearing Research 273 (2011) 109e122 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Somatic motility and hair bundle mechanics, are both necessary
Required Slide. Session Objectives
 Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Structure, Energy Transmission and Function. Gross Anatomy. Structure, Function & Process. External Auditory Meatus or Canal (EAM, EAC) Outer Ear
 Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Acoustics, signals & systems for audiology. Psychoacoustics of hearing impairment
 Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs
 Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
Lecture 6 Hearing 1. Raghav Rajan Bio 354 Neurobiology 2 January 28th All lecture material from the following links unless otherwise mentioned:
 Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Reticular lamina and basilar membrane vibrations in living mouse cochleae
 Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
Processing of sounds in the inner ear
 Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier
 Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Auditory System. Barb Rohrer (SEI )
 Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Voltage-clamp errors cause anomalous interaction between independent ion channels
 AUDITORYAND VESTIBULAR SYSTEMS Voltage-clamp errors cause anomalous interaction between independent ion channels Hamilton E. Farris CA and Anthony J. Ricci Neuroscience Center and Kresge Hearing Labs,
AUDITORYAND VESTIBULAR SYSTEMS Voltage-clamp errors cause anomalous interaction between independent ion channels Hamilton E. Farris CA and Anthony J. Ricci Neuroscience Center and Kresge Hearing Labs,
The magnificent outer hair cell of Corti's organ
 The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
Mechanoelectric Transduction of Adult Inner Hair Cells
 1006 The Journal of Neuroscience, January 31, 2007 27(5):1006 1014 Cellular/Molecular Mechanoelectric Transduction of Adult Inner Hair Cells Shuping Jia, 1 Peter Dallos, 2 and David Z. Z. He 1 1 Hair Cell
1006 The Journal of Neuroscience, January 31, 2007 27(5):1006 1014 Cellular/Molecular Mechanoelectric Transduction of Adult Inner Hair Cells Shuping Jia, 1 Peter Dallos, 2 and David Z. Z. He 1 1 Hair Cell
Before we talk about the auditory system we will talk about the sound and waves
 The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
A truly remarkable aspect of human hearing is the vast
 AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992)
 The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
Cochlear anatomy, function and pathology III. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
to vibrate the fluid. The ossicles amplify the pressure. The surface area of the oval window is
 Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Fate of Mammalian Cochlear Hair Cells and Stereocilia after Loss of the Stereocilia
 The Journal of Neuroscience, December 2, 2009 29(48):15277 15285 15277 Development/Plasticity/Repair Fate of Mammalian Cochlear Hair Cells and Stereocilia after Loss of the Stereocilia Shuping Jia, 1 Shiming
The Journal of Neuroscience, December 2, 2009 29(48):15277 15285 15277 Development/Plasticity/Repair Fate of Mammalian Cochlear Hair Cells and Stereocilia after Loss of the Stereocilia Shuping Jia, 1 Shiming
How the ear s works work: mechanoelectrical transduction and amplification by hair cells
 C. R. Biologies 328 (2005) 155 162 Neurosciences http://france.elsevier.com/direct/crass3/ How the ear s works work: mechanoelectrical transduction and amplification by hair cells A.J. Hudspeth Laboratory
C. R. Biologies 328 (2005) 155 162 Neurosciences http://france.elsevier.com/direct/crass3/ How the ear s works work: mechanoelectrical transduction and amplification by hair cells A.J. Hudspeth Laboratory
Neuroscience Center of Excellence College: Case Western Reserve University Kresge Hearing Labs Degree: BA in Chemistry ( )
 NAME: ANTHONY RICCI, PH.D. PRESENT POSITION AND ADDRESS: EDUCATION: Assistant Professor High School: Cathedral Prep (NYC) Neuroscience Center of Excellence College: Case Western Reserve University Kresge
NAME: ANTHONY RICCI, PH.D. PRESENT POSITION AND ADDRESS: EDUCATION: Assistant Professor High School: Cathedral Prep (NYC) Neuroscience Center of Excellence College: Case Western Reserve University Kresge
January 22 nd to 26 th, 2008
 Advanced theoretical and practical training course on Hearing in Mammals January 22 nd to 26 th, 2008 F ro m M o l e c u l e s t o A u d i t o r y P h y s i o l o g y ORGANIZERS Tobias Moser FACULTY Frank,
Advanced theoretical and practical training course on Hearing in Mammals January 22 nd to 26 th, 2008 F ro m M o l e c u l e s t o A u d i t o r y P h y s i o l o g y ORGANIZERS Tobias Moser FACULTY Frank,
Unit VIII Problem 9 Physiology: Hearing
 Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Improving the diagnostic power of otoacoustic emissions. Arturo Moleti Physics Department University of Roma Tor Vergata
 Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Sensory Transduction and Adaptation in Inner and Outer Hair Cells of the Mouse Auditory System
 J Neurophysiol 98: 3360 3369, 2007. First published October 17, 2007; doi:10.1152/jn.00914.2007. Sensory Transduction and Adaptation in Inner and Outer Hair Cells of the Mouse Auditory System Eric A. Stauffer
J Neurophysiol 98: 3360 3369, 2007. First published October 17, 2007; doi:10.1152/jn.00914.2007. Sensory Transduction and Adaptation in Inner and Outer Hair Cells of the Mouse Auditory System Eric A. Stauffer
Representation of sound in the auditory nerve
 Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
NIH Public Access Author Manuscript Nat Commun. Author manuscript; available in PMC 2013 March 12.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Commun. 2012 ; 3: 1094. doi:10.1038/ncomms2100. Sound-induced length changes in outer hair cell stereocilia Pierre Hakizimana
NIH Public Access Author Manuscript Published in final edited form as: Nat Commun. 2012 ; 3: 1094. doi:10.1038/ncomms2100. Sound-induced length changes in outer hair cell stereocilia Pierre Hakizimana
Auditory Physiology Richard M. Costanzo, Ph.D.
 Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
Measurement of cochlear power gain in the sensitive gerbil ear
 Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
2010 ANTG Spring Course. Bruce Tempel...
 2010 ANTG Spring Course Bruce Tempel... bltempel@uw.edu http://depts.washington.edu/tempelab/ Hearing Loss is Common and Permanent - With significant economic and social impact Prevalence of Hearing Loss
2010 ANTG Spring Course Bruce Tempel... bltempel@uw.edu http://depts.washington.edu/tempelab/ Hearing Loss is Common and Permanent - With significant economic and social impact Prevalence of Hearing Loss
Efferent-mediated control of basilar membrane motion
 J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
Force Transmission in the Organ of Corti Micromachine
 Biophysical Journal Volume 98 June 2010 2813 2821 2813 Force Transmission in the Organ of Corti Micromachine Jong-Hoon Nam and Robert Fettiplace* Department of Physiology, University of Wisconsin Medical
Biophysical Journal Volume 98 June 2010 2813 2821 2813 Force Transmission in the Organ of Corti Micromachine Jong-Hoon Nam and Robert Fettiplace* Department of Physiology, University of Wisconsin Medical
OtoAcoustic Emissions (OAE s)
 OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS. Hans P. Zenner, Ulrike Zimmermann and Alfred H. Gitter
 Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Evidence for an Active Process and a Cochlear Amplifier in Nonmammals
 INVITED REVIEW Evidence for an Active Process and a Cochlear Amplifier in Nonmammals GEOFFREY A. MANLEY Lehrstuhl für Zoologie, Technische Universität München, 85747 Garching, Germany Received 26 January
INVITED REVIEW Evidence for an Active Process and a Cochlear Amplifier in Nonmammals GEOFFREY A. MANLEY Lehrstuhl für Zoologie, Technische Universität München, 85747 Garching, Germany Received 26 January
Integrating the active process of hair cells with cochlear function
 Integrating the active process of hair cells with cochlear function A. J. Hudspeth Abstract Uniquely among human senses, hearing is not simply a passive response to stimulation. Our auditory system is
Integrating the active process of hair cells with cochlear function A. J. Hudspeth Abstract Uniquely among human senses, hearing is not simply a passive response to stimulation. Our auditory system is
The Structure and Function of the Auditory Nerve
 The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
Systems Neuroscience Oct. 16, Auditory system. http:
 Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Neurobiology Biomed 509 Sensory transduction References: Luo , ( ), , M4.1, M6.2
 Neurobiology Biomed 509 Sensory transduction References: Luo 4.1 4.8, (4.9 4.23), 6.22 6.24, M4.1, M6.2 I. Transduction The role of sensory systems is to convert external energy into electrical signals
Neurobiology Biomed 509 Sensory transduction References: Luo 4.1 4.8, (4.9 4.23), 6.22 6.24, M4.1, M6.2 I. Transduction The role of sensory systems is to convert external energy into electrical signals
SPECIAL SENSES: THE AUDITORY SYSTEM
 SPECIAL SENSES: THE AUDITORY SYSTEM REVISION OF PHYSICS: WAVES A wave is an oscillation of power, sound waves have two main characteristics: amplitude, which is the maximum displacement or the power of
SPECIAL SENSES: THE AUDITORY SYSTEM REVISION OF PHYSICS: WAVES A wave is an oscillation of power, sound waves have two main characteristics: amplitude, which is the maximum displacement or the power of
Prestin-Driven Cochlear Amplification Is Not Limited by the Outer Hair Cell Membrane Time Constant
 Article Prestin-Driven Cochlear Amplification Is Not Limited by the Outer Hair Cell Membrane Time Constant Stuart L. Johnson, 1 Maryline Beurg, 2 Walter Marcotti, 1, * and Robert Fettiplace 3, * 1 Department
Article Prestin-Driven Cochlear Amplification Is Not Limited by the Outer Hair Cell Membrane Time Constant Stuart L. Johnson, 1 Maryline Beurg, 2 Walter Marcotti, 1, * and Robert Fettiplace 3, * 1 Department
Muscle Dr. Ted Milner (KIN 416)
 Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
Hearing. By: Jimmy, Dana, and Karissa
 Hearing By: Jimmy, Dana, and Karissa Anatomy - The ear is divided up into three parts - Sound enters in through the outer ear and passes into the middle where the vibrations are received and sent to the
Hearing By: Jimmy, Dana, and Karissa Anatomy - The ear is divided up into three parts - Sound enters in through the outer ear and passes into the middle where the vibrations are received and sent to the
Educational Module Tympanometry. Germany D Germering
 Educational Module anometry PATH medical Germany D-82110 Germering Our educational modules 1 are made for providing information on how the hearing organ works and which test procedures are used to test
Educational Module anometry PATH medical Germany D-82110 Germering Our educational modules 1 are made for providing information on how the hearing organ works and which test procedures are used to test
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! Chapter 17 Part 2 Special Senses! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Advanced otoacoustic emission detection techniques and clinical diagnostics applications
 Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
EMANATIONS FROM RESIDUUM OSCILLATIONS IN HUMAN AUDITORY SYSTEM
 EMANATIONS FROM RESIDUUM OSCILLATIONS IN HUMAN AUDITORY SYSTEM V.S. Balaji, N.R.Raajan, S. Rakesh Kumar, Har Narayan Upadhyay School of Electrical & Electronics Engineering, SASTRA University Thanjavur,
EMANATIONS FROM RESIDUUM OSCILLATIONS IN HUMAN AUDITORY SYSTEM V.S. Balaji, N.R.Raajan, S. Rakesh Kumar, Har Narayan Upadhyay School of Electrical & Electronics Engineering, SASTRA University Thanjavur,
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to
 So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells
 Biophysical Journal Volume 84 February 2003 739 749 739 Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells Mark Ospeck, Xiao-xia Dong, and Kuni H. Iwasa Biophysics
Biophysical Journal Volume 84 February 2003 739 749 739 Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells Mark Ospeck, Xiao-xia Dong, and Kuni H. Iwasa Biophysics
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
Effects of Remaining Hair Cells on Cochlear Implant Function
 Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
In vivo outer hair cell length changes expose the active process in the cochlea
 In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM.
 Supplementary Figure 1 Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM. (a-c) Heterozygous c.216ga mice displayed normal hair bundle morphology at P18. (d-i) Disorganized hair bundles
Supplementary Figure 1 Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM. (a-c) Heterozygous c.216ga mice displayed normal hair bundle morphology at P18. (d-i) Disorganized hair bundles
Trajectory of the Aging Cochlea
 Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
ENT 318 Artificial Organs Physiology of Ear
 ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
Introduction. IAPA: June 04 1
 Introduction Conflicting views on the prevalence and nature of otoacoustic emission [OAE] abnormalities in ARNSHL families (Morell et al, 1998; Cohn & Kelley, 1999). Detailed study of OAEs in greater number
Introduction Conflicting views on the prevalence and nature of otoacoustic emission [OAE] abnormalities in ARNSHL families (Morell et al, 1998; Cohn & Kelley, 1999). Detailed study of OAEs in greater number
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA. Invited Paper LINEAR RESPONSE OF THE COCHLEA
 FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
Can you still see the cochlea for the molecules? Jonathan F Ashmore* and Fabio Mammano
 449 Can you still see the cochlea for the molecules? Jonathan F Ashmore* and Fabio Mammano It is now established that the mammalian cochlea uses active amplification of incoming sound to achieve sensitivity.
449 Can you still see the cochlea for the molecules? Jonathan F Ashmore* and Fabio Mammano It is now established that the mammalian cochlea uses active amplification of incoming sound to achieve sensitivity.
Auditory Periphery! external middle inner. stapes movement initiates a pressure wave in cochlear fluid
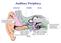 Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Chapter 11: Sound, The Auditory System, and Pitch Perception
 Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
Signals, systems, acoustics and the ear. Week 5. The peripheral auditory system: The ear as a signal processor
 Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Intro to Audition & Hearing
 Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
Healthy Organ of Corti. Loss of OHCs. How to use and interpret the TEN(HL) test for diagnosis of Dead Regions in the cochlea
 'How we do it' Healthy Organ of Corti How to use and interpret the TEN(HL) test for diagnosis of s in the cochlea Karolina Kluk¹ Brian C.J. Moore² Mouse IHCs OHCs ¹ Audiology and Deafness Research Group,
'How we do it' Healthy Organ of Corti How to use and interpret the TEN(HL) test for diagnosis of s in the cochlea Karolina Kluk¹ Brian C.J. Moore² Mouse IHCs OHCs ¹ Audiology and Deafness Research Group,
9.01 Introduction to Neuroscience Fall 2007
 MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
Can components in distortion-product otoacoustic emissions be separated?
 Can components in distortion-product otoacoustic emissions be separated? Anders Tornvig Section of Acoustics, Aalborg University, Fredrik Bajers Vej 7 B5, DK-922 Aalborg Ø, Denmark, tornvig@es.aau.dk David
Can components in distortion-product otoacoustic emissions be separated? Anders Tornvig Section of Acoustics, Aalborg University, Fredrik Bajers Vej 7 B5, DK-922 Aalborg Ø, Denmark, tornvig@es.aau.dk David
SMELL 2
 SENSORY SYSTEMS 1 SMELL 2 TASTE 3 HEARING 4 TOUCH EQUILIBRIUM 5 PAIN 6 OTHER SENSES 7 HOW DO SENSORY CELLS CONVERT STIMULI INTO ACTION POTENTIALS? HOW DO SENSORY SYSTEMS DETECT CHEMICAL STIMULI? HOW DO
SENSORY SYSTEMS 1 SMELL 2 TASTE 3 HEARING 4 TOUCH EQUILIBRIUM 5 PAIN 6 OTHER SENSES 7 HOW DO SENSORY CELLS CONVERT STIMULI INTO ACTION POTENTIALS? HOW DO SENSORY SYSTEMS DETECT CHEMICAL STIMULI? HOW DO
Cochlear function in Prestin knockout mice
 J Physiol 560.3 (2004) pp 821 830 821 Cochlear function in Prestin knockout mice M. A. Cheatham 1,K.H.Huynh 1,J.Gao 2,J.Zuo 2 and P. Dallos 1,3 1 Departments of Communication Sciences and Disorders and
J Physiol 560.3 (2004) pp 821 830 821 Cochlear function in Prestin knockout mice M. A. Cheatham 1,K.H.Huynh 1,J.Gao 2,J.Zuo 2 and P. Dallos 1,3 1 Departments of Communication Sciences and Disorders and
New tunes from Corti s organ: the outer hair cell boogie rules Joseph Santos-Sacchi
 459 New tunes from Corti s organ: the outer hair cell boogie rules Joseph Santos-Sacchi The amplification of acoustic stimuli is a feature of hair cells that evolved early on in vertebrates. Though standard
459 New tunes from Corti s organ: the outer hair cell boogie rules Joseph Santos-Sacchi The amplification of acoustic stimuli is a feature of hair cells that evolved early on in vertebrates. Though standard
Acoustics Research Institute
 Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Physiological basis of sound design. Prof. Dr. med. Eckhard Hoffmann Dipl.-Ing. (FH) Steffen Kreikemeier Aalen University of Applied Sciences
 Physiological basis of sound design Prof. Dr. med. Eckhard Hoffmann Dipl.-Ing. (FH) Steffen Kreikemeier Aalen University of Applied Sciences Index of contents Physiological basis of the inner ear Organ
Physiological basis of sound design Prof. Dr. med. Eckhard Hoffmann Dipl.-Ing. (FH) Steffen Kreikemeier Aalen University of Applied Sciences Index of contents Physiological basis of the inner ear Organ
The lagena a presumptive vestibular epithelium found at the low frequency end of the auditory end organ, is absent from mammals.
 1 Structure and Function of the Auditory and Vestibular System 1 Increasing elaboration of the auditory end organ during evolution. Increasing length of the cochlea associated with higher frequency hearing.
1 Structure and Function of the Auditory and Vestibular System 1 Increasing elaboration of the auditory end organ during evolution. Increasing length of the cochlea associated with higher frequency hearing.
Hearing Research. The remarkable cochlear amplifier
 Hearing Research 266 (2010) 1 17 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares The remarkable cochlear amplifier J. Ashmore a,1, P. Avan b,
Hearing Research 266 (2010) 1 17 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares The remarkable cochlear amplifier J. Ashmore a,1, P. Avan b,
Functional motor microdomains of the outer hair cell lateral membrane
 Pflugers Arch - Eur J Physiol (2002) 445:331 336 DOI 10.1007/s00424-002-0928-4 CELL AND MOLECULAR PHYSIOLOGY Joseph Santos-Sacchi Functional motor microdomains of the outer hair cell lateral membrane Received:
Pflugers Arch - Eur J Physiol (2002) 445:331 336 DOI 10.1007/s00424-002-0928-4 CELL AND MOLECULAR PHYSIOLOGY Joseph Santos-Sacchi Functional motor microdomains of the outer hair cell lateral membrane Received:
Can You Hear Me Now?
 An Introduction to the Mathematics of Hearing Department of Applied Mathematics University of Washington April 26, 2007 Some Questions How does hearing work? What are the important structures and mechanisms
An Introduction to the Mathematics of Hearing Department of Applied Mathematics University of Washington April 26, 2007 Some Questions How does hearing work? What are the important structures and mechanisms
College of Medicine Dept. of Medical physics Physics of ear and hearing /CH
 College of Medicine Dept. of Medical physics Physics of ear and hearing /CH 13 2017-2018 ***************************************************************** o Introduction : The ear is the organ that detects
College of Medicine Dept. of Medical physics Physics of ear and hearing /CH 13 2017-2018 ***************************************************************** o Introduction : The ear is the organ that detects
Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil
 2426 Biophysical Journal Volume 106 June 2014 2426 2433 Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil Jong-Hoon Nam* Department of Mechanical
2426 Biophysical Journal Volume 106 June 2014 2426 2433 Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil Jong-Hoon Nam* Department of Mechanical
Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea
 Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
