Galactose Enhances Oxidative Metabolism and Reveals Mitochondrial Dysfunction in Human Primary Muscle Cells
|
|
|
- Felix Nicholson
- 5 years ago
- Views:
Transcription
1 Galactose Enhances Oxidative Metabolism and Reveals Mitochondrial Dysfunction in Human Primary Muscle Cells Céline Aguer 1, Daniela Gambarotta 1, Ryan J. Mailloux 1, Cynthia Moffat 1, Robert Dent 2, Ruth McPherson 3, Mary-Ellen Harper 1 * 1 Department of Biochemistry, Microbiology and Immunology, Faculty of Medicine, University of Ottawa, Ontario, Canada, 2 Ottawa Hospital Weight Management Clinic, Ottawa, Ontario, Canada, 3 Division of Cardiology, University of Ottawa Heart Institute, Ottawa, Ontario, Canada Abstract Background: Human primary myotubes are highly glycolytic when cultured in high glucose medium rendering it difficult to study mitochondrial dysfunction. Galactose is known to enhance mitochondrial metabolism and could be an excellent model to study mitochondrial dysfunction in human primary myotubes. The aim of the present study was to 1) characterize the effect of differentiating healthy human myoblasts in galactose on oxidative metabolism and 2) determine whether galactose can pinpoint a mitochondrial malfunction in post-diabetic myotubes. Methodology/Principal Findings: Oxygen consumption rate (OCR), lactate levels, mitochondrial content, citrate synthase and cytochrome C oxidase activities, and AMPK phosphorylation were determined in healthy myotubes differentiated in different sources/concentrations of carbohydrates: 25 mm glucose (high glucose (HG)), 5 mm glucose (low glucose (LG)) or 10 mm galactose (GAL). Effect of carbohydrates on OCR was also determined in myotubes derived from post-diabetic patients and matched obese non-diabetic subjects. OCR was significantly increased whereas anaerobic glycolysis was significantly decreased in GAL myotubes compared to LG or HG myotubes. This increased OCR in GAL myotubes occurred in conjunction with increased cytochrome C oxidase activity and expression, as well as increased AMPK phosphorylation. OCR of post-diabetic myotubes was not different than that of obese non-diabetic myotubes when differentiated in LG or HG. However, whereas GAL increased OCR in obese non-diabetic myotubes, it did not affect OCR in post-diabetic myotubes, leading to a significant difference in OCR between groups. The lack of an increase in OCR in post-diabetic myotubes differentiated in GAL was in relation with unaltered cytochrome C oxidase activity levels or AMPK phosphorylation. Conclusions/Significance: Our results indicate that differentiating human primary myoblasts in GAL enhances aerobic metabolism. Because this cell culture model elicited an abnormal response in cells from post-diabetic patients, it may be useful in further studies of the molecular mechanisms of mitochondrial dysfunction. Citation: Aguer C, Gambarotta D, Mailloux RJ, Moffat C, Dent R, et al. (2011) Galactose Enhances Oxidative Metabolism and Reveals Mitochondrial Dysfunction in Human Primary Muscle Cells. PLoS ONE 6(12): e doi: /journal.pone Editor: Raul M. Luque, University of Cordoba, Spain Received June 29, 2011; Accepted November 10, 2011; Published December 15, 2011 Copyright: ß 2011 Aguer et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This research was funded by a grant from the Canadian Institutes of Health Research (Institute of Nutrition, Metabolism and Diabetes). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing Interests: The authors have declared that no competing interests exist. * mharper@uottawa.ca Introduction Human primary muscle cells are a widely used model system to study muscle metabolism and its related disorders. Cell culture of human primary myotubes offers not only an excellent and dynamic model for studying metabolism under standardized conditions, but provides an excellent system for studying metabolic disorders. Indeed, a number of studies have shown that human primary muscle cells retain the same metabolic phenotype as those previously evidenced in vivo in muscle [1,2,3,4,5,6,7,8,9,10,11]. The oxidative capacity of skeletal muscle is highly influenced by various genetic and environmental factors; cultured myotubes offer a unique model that separates these two influences on metabolic phenotype [7]. Although a valuable model system, cultured muscle cells are highly glycolytic when grown and differentiated under high glucose conditions relative to muscle tissue in vivo [12], complicating the study of mitochondrial dysfunction. Indeed, myoblasts are routinely cultured in a standard high glucose medium which can diminish mitochondrial function. In the same way, rapidly proliferating cancer cells are known to be highly glycolytic when grown in high glucose conditions [13,14,15], a phenomenon known as the Crabtree effect, when glucose inhibits oxidative phosphorylation [16]. This phenomenon is not restricted to cancer cells. Other types of cells including embryonic tissues [17] and proliferative thymocytes [18] show diminished oxidative metabolism when grown under high glucose conditions. Whereas the production of pyruvate via glycolytic metabolism of glucose yields 2 net ATP, the production of pyruvate via glycolytic metabolism of galactose yields no net ATP, forcing cells to have an increased reliance on oxidative phosphorylation PLoS ONE 1 December 2011 Volume 6 Issue 12 e28536
2 (OXPHOS) for energy [13,19,20,21]. Indeed, different types of cells (e.g., cancer cells, primary fibroblasts) grown in a medium in which glucose is replaced with galactose show a significantly increased oxygen consumption rate compared to cells grown in medium containing a high concentration of glucose (25 mm) [13,14,15]. The more aerobic state of cells grown in galactose is mainly due to a decrease in ATP production via anaerobic glycolysis [22] but is also thought to be due to a modulation of mitochondrial structure [15] and an increase in mitochondrial oxidative capacity as evidenced by increased OXPHOS protein expression and mitochondrial enzymatic activities [15]. Since cells grown in galactose rely mostly on oxidative phosphorylation to produce their ATP, they become more sensitive to mitochondrial toxins than cells grown in high glucose medium [13,23,24]. Another study has shown that primary skin fibroblasts isolated from patients with mitochondrial deficiency (e.g., cytochrome oxidase deficiency, complex I deficiency, pyruvate dehydrogenase complex deficiency or with multiple respiratory chain defects) were not able to survive when cultured in a galactose-based medium [20]. Thus, culturing cells in a galactose medium seems to be a good alternative to high glucose medium in order to study mitochondrial dysfunction. Obesity-associated insulin resistance and type 2 diabetes mellitus (T2DM) is characterized by a decrease in mitochondrial function. Mitochondrial respiration is known to be reduced in the skeletal muscle of T2DM patients [25,26,27] and lean insulinresistant offspring of T2DM parents [28,29]. Some studies, done on primary muscle cells derived from diabetic patients and matched controls have also shown a decrease in mitochondrial capacity: impaired ATP synthesis [30,31], perturbed palmitate beta-oxidation when exposed to high level of palmitate [9], and impaired citrate synthase activity [7]. However, to our knowledge, no study has directly assessed whether myotubes derived from T2DM patients or patients with a history of T2DM retain a decrease in mitochondrial respiration when cultured in vitro. As mentioned above, this is most likely due to the fact that in vitro, primary human myotubes are mainly glycolytic rendering difficult the study of mitochondrial dysfunction in this model. The use of galactose medium instead of glucose medium to induce a metabolic shift towards a more oxidative phenotype could be a good model to study mitochondrial dysfunction in relation to T2DM and insulin resistance. However, to date, the response of human primary muscle cells to galactose has never been assessed. Here, we characterized the effect of replacing glucose with galactose on aerobic metabolism in primary muscle cells derived from lean and healthy donors. We have shown that differentiating primary human muscle cells in a galactose-replete medium enhances aerobic metabolism without altering mitochondrial content. Moreover, we have shown that this model system can be employed to investigate the mitochondrial bioenergetics of human myotubes derived from post-diabetic patients. To our knowledge, this is the first study that tests the impact of different sources of carbohydrates on human myotube bioenergetics. The value of this model system and its importance to investigating mitochondrial dysfunction in cell culture is also discussed herein. Results Impact of different carbohydrates on cellular physiology and ATP content Glucose restriction has been shown to decrease myotube differentiation [32]. The fusion index in cells differentiated in high glucose (25 mm, HG), low glucose (5 mm, LG) or GAL was not significantly different between the 3 differentiation conditions (Fig. 1A). However, troponin T expression, which is another marker of myotube differentiation, was significantly decreased in cells differentiated in LG or GAL compared to cells differentiated in HG (p,0.05, Fig. 1B). Yet, cells differentiated in GAL showed the same level of differentiation than cells differentiating in LG. After 7 days of differentiation in the different mediums, the redox state of the cells was assessed using the MTT (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide) assay. A significant decrease in the redox state was found in myotubes differentiated in LG or GAL compared to HG (p,0.05), with no difference between cells differentiated in LG and GAL (Fig. 1C). We also measured ATP content in myotubes differentiated for 7 days in HG, LG or GAL. As shown in Figure 1D, no significant difference was found in ATP content between myotubes differentiated in the three types of media. Hence, these observations indicate that replacing glucose with galactose does not have a negative effect on cellular physiology and metabolism. Replacing glucose with galactose in differentiation medium increases myotube aerobic capacity Primary human muscle cells differentiated in GAL showed a,40% increase in basal mitochondrial oxygen consumption rate (basal mitochondrial OCR = basal OCR non-mitochondrial OCR) compared to cells differentiated in either HG or LG (p,0.05, Fig. 2A). Mitochondrial state 4 respiration (proton leak dependent respiration = (OCR after oligomycin treatment nonmitochondrial OCR)) tended to be higher in GAL myotubes compared to LG myotubes (p = 0.07) or HG myotubes (p = 0.1) (Fig. 2B) without reaching significance. However, the percentage of basal mitochondrial OCR due to proton leak was not significantly different between treatments (Fig. 2C). There was also a trend for a higher maximal mitochondrial OCR (measured after FCCP treatment) in GAL cells compared to LG cells, but this did not reach significance (p = 0.07, Fig. 2D). The non-mitochondrial OCR was measured by treating cells with antimycin A. Intriguingly, OCR after antimycin A treatment was significantly higher in myotubes differentiated in GAL compared to myotubes differentiated in HG (p,0.01, Fig. 2E). To test the metabolic changes accompanying differentiating cells in GAL further, we measured the amount of lactate released in the medium over a 24 h period. As shown in Figure 2F, cells differentiated in GAL showed a significant decrease in lactate production compared to cells differentiated in LG or HG indicating a decrease in anaerobic metabolism in cells differentiated in GAL (p,0.0001). No significant difference in lactate production was found between myotubes differentiated in HG and LG. Taken together, these data confirm that myotubes differentiated in presence of glucose are mostly glycolytic, and that the replacement of glucose by galactose in the differentiation medium forces cells to adopt a more oxidative metabolic state. Effect of galactose on mitochondrial content and enzyme activity In order to determine if the increase in OCR shown in Figure 2 was the result of an increase in mitochondrial biogenesis, different mitochondrial markers were measured. First, mitochondrial yield was determined as mitochondria protein content per total cellular protein content. As shown in Figure 3A, differentiating the cells in GAL did not have any effect on mitochondrial yield compared to myotubes differentiating in HG or LG. Another method to determine mitochondrial content is the staining of cardiolipin with 10N-nonyl acridine orange (NAO). As shown on Figure 3B, this PLoS ONE 2 December 2011 Volume 6 Issue 12 e28536
3 Figure 1. Effect of replacing a glucose medium with a galactose medium on myotube differentiation, redox environment and ATP content. A. Fusion index was measured in myotubes differentiated in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose) media for 7 days. The fusion index has been measured as the number of nuclei in differentiated myotubes (.2 myonuclei) as the percentage of the total number of nuclei in muscle cells (determined by a desmin immunofluorescence staining). Data are shown as mean 6 SEM, n = 3. B. Myotube differentiation was assessed by Troponin T expression after 7 days of differentiation in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose) media. Top panel: representative Western blot of Troponin T expression in myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Beta-actin was used as a loading control. Bottom panel: quantification by densitometry of Troponin T expression. Results are normalized to beta-actin expression. Data are shown as mean 6 SEM, n = 4. *, p,0.05, LG and GAL vs HG. C. Myotube redox environment in response to differentiation in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose) was assessed using the MTT assay as described in the Methods section. Data are presented as mean 6SEM, n = 3, in which each condition was assessed in 6 replicates. D. ATP content in myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Results are presented as means 6 SEM, n = 4, in which each condition was assessed in duplicate. doi: /journal.pone g001 method corroborates that differentiating the cells in HG, LG or GAL do not affect mitochondrial content. These results were confirmed by measuring the protein levels of the mitochondrial markers, complex III and SDHA. The levels of each protein were not significantly affected by the different carbohydrate sources (Fig. 3D). The impact of differentiating cells in galactose on the activity of citrate synthase and cytochrome C oxidase (COX) was also examined. The activities were measured on isolated mitochondria to determine whether the capacity of the TCA (tricarboxylic acid) cycle or the electron transport chain was increased in GAL myotubes, respectively. Citrate synthase activity was not changed by GAL medium compared to either HG or LG medium (Fig. 3C). However, COX activity was significantly higher in GAL myotubes compared to both HG and LG myotubes (p,0.01, Fig. 3E). This higher COX activity was in relation with a higher COX expression level in myotubes differentiating in GAL compared to LG or HG (p,0.05, Fig. 3F). AMPK is a major metabolic sensor that is activated by an increase in the ratio of AMP/ATP in order to restore energy status of the cell trough the stimulation of ATP-producing processes (e.g., glucose uptake, fatty acid oxidation, and mitochondrial biogenesis) [33,34] and the inhibition of ATP-consuming processes (fatty acid synthesis, glycogen synthesis, and protein synthesis) [35,36,37]. To determine if the differentiation of cells in GAL medium also affects AMPK activity, we estimated AMPK activity by measuring AMPK phosphorylation (P-AMPK) in cells differentiated in either HG, LG or GAL for 7 days. As shown on Figure 3G, P-AMPK was higher in cells differentiated in GAL compared to LG or HG (p,0.05 for P-AMPK/b-actin; p = 0.07 for P-AMPK/AMPK). Acute exposure to galactose does not affect myotube oxidative capacity In order to determine if an acute treatment was sufficient to improve the aerobic capacity of the myotubes, cells were differentiated for 7 days in LG, and then incubated for 45 min before measuring oxygen consumption in HG, LG or GAL media. As shown in Figure 4, basal mitochondrial (Fig. 4A), mitochondrial state 4 (Fig. 4B), and maximal mitochondrial OCR (Fig. 4C) were unaffected by an acute treatment with GAL. Hence, these data indicate that in order to induce a metabolic shift towards increased oxidative metabolism, cells have to be exposed to GAL for a prolonged period. Post-diabetic myotubes show incapacity to increase their oxidative metabolism when differentiated in galactose medium The observed effect of galactose on oxidative metabolism prompted us to test the effect of this carbohydrate on the aerobic function of muscle cells collected from patients with a history of diabetes (post-diabetic patients). To test if myotubes derived PLoS ONE 3 December 2011 Volume 6 Issue 12 e28536
4 Figure 2. Effect of replacing a glucose medium with a galactose medium on myotube aerobic capacity. A. Basal mitochondrial oxygen consumption rate. *, p,0.05, GAL vs HG and LG. B. State 4 respiration (leak-dependent; non-phosphorylating). After basal oxygen consumption rate measurement, cells were treated with oligomycin (600 ng/ml) to determine state 4 respiration. p = 0.06, GAL vs LG. C. Percentage of basal OCR due to proton leak was calculated from the data shown in Figure 2A and B. Data are presented as means 6 SEM, n = 7, in which each condition was assessed in 5 6 replicates. D. Maximal mitochondrial oxygen consumption capacity. After basal and state 4 respiration measurements, cells were treated with FCCP (1 mm) to determine maximal oxygen consumption. *, p,0.05, GAL vs LG. E. Non-mitochondrial oxygen consumption rate. After basal, state and maximal respiration measurements, cells were treated with antimycin (4 mm) to determine non-mitochondrial oxygen consumption. **, p,0.01, GAL vs LG. F. Lactate concentration in the extracellular media of myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Results are presented as means 6 SEM, n = 7, in which each condition was assessed in duplicate. ***, p,0.0001, GAL vs HG and LG. doi: /journal.pone g002 from obese post-diabetic patients were responsive to GAL at the level of OCR, post-diabetic myotubes and their matched obese non-diabetic myotubes were differentiated for 7 days in HG, LG or GAL. When differentiated in LG or HG, post-diabetic myotubes showed the same basal mitochondrial OCR as obese non-diabetic myotubes (Fig. 5A). However, unlike obese nondiabetic myotubes, basal mitochondrial OCR in post-diabetic myotubes showed no response to GAL, leading to a significant difference between groups (Fig. 5A; p,0.05). This interesting result highlights a defect in mitochondrial function in postdiabetic myotubes. Mitochondrial state 4 OCR (Fig. 5B) and maximal mitochondrial capacity (Fig. 5C) were however not differentially affected by GAL, or different between post-diabetic myotubes and obese non-diabetic myotubes. Interestingly, nonmitochondrial OCR (in the presence of saturating antimycin) was significantly lower in post-diabetic myotubes compared to obese non-diabetic myotubes in the 3 different conditions (Fig. 5D). Post-diabetic myotubes show no increases in COX activity or P-AMPK when differentiated in galactose medium compared to low or high glucose media To identify why post-diabetic myotubes are incapable of increasing oxidative metabolism in response to GAL, we measured mitochondrial content, and COX expression and activity (Fig. 6). Surprisingly, we found a significant increased mitochondrial yield in post-diabetic myotubes differentiated in LG compared with myotubes differentiated in both HG (p = 0.05) and GAL (p,0.01) (Fig. 6A). However, COX activity was not significantly different between conditions due to the high variability in activity between post-diabetic samples (Fig. 6B). Furthermore, COX expression was not significantly increased when post-diabetic cells were differentiated in GAL compared to LG or HG (Fig. 6C). We also measured the level of P-AMPK in post-diabetic myotubes differentiated in HG, LG or GAL. In contrast to control myotubes (Figure 3G), post-diabetic myotubes did not show increased AMPK phosphorylation in response to GAL medium (Fig. 6D). PLoS ONE 4 December 2011 Volume 6 Issue 12 e28536
5 Figure 3. Effect of replacing a glucose medium with a galactose medium on mitochondrial markers. A. Mitochondrial yield measured in myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Mitochondrial yield was determined as mitochondrial protein content per total cellular protein content. Results are presented as means 6 SEM, n = 6, in which each condition was assessed in duplicate. B. Cardiolipin staining in myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Cardiolipin was stained with 10N-nonyl acridine orange (NAO, 1 mm) in fixed myotubes for 30 min. The intensity of the staining was then measured by fluorescence. Results are presented as means 6 SEM, n = 3. C. Citrate synthase activity measured on isolated mitochondria from myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Results are presented as means 6 SEM, n = 5, in which each condition was assessed in duplicate. D. Left panel: representative Western blot of Complex III and SDHA expression in myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Complex III and SDHA were used as a mitochondrial content marker and beta-actin as a loading control. Right panels: quantification by densitometry of complex III and SDHA expressions. Data are presented normalized to beta-actin expression. Data are shown as mean 6SEM, n = 4. E. Cytochrome C oxidase activity measured in isolated mitochondria from myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Results are presented as means 6 SEM, n = 5, in which each condition was assessed in duplicate. *, p,0.05, GAL vs HG and LG. F. Top panel: representative Western blot of Complex IV expression in myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Beta-actin was used as a loading control. Bottom panel: quantification by densitometry of complex IV expression. Data are presented normalized to beta-actin expression. Data are shown as mean 6SEM, n = 4. *, p,0.05, GAL vs HG and LG. G. Left panel: representative Western blot of P-AMPK expression in myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Beta-actin and AMPKa2 were used as loading controls. Right panels: quantification by densitometry of P-AMPK. Data are presented normalized to beta-actin and AMPKa2 expression. Data are shown as mean 6SEM, n = 3. *, p,0.05, GAL vs HG and LG. doi: /journal.pone g003 PLoS ONE 5 December 2011 Volume 6 Issue 12 e28536
6 Figure 4. Effect of an acute treatment with galactose medium on myotube aerobic capacity. A. Basal oxygen consumption rate. B. State 4 respiration (leak dependent; non-phosphorylating). After basal oxygen consumption rate measurement, cells were treated with oligomycin (600 ng/ml) to determine state 4 respiration. C. Maximal oxygen consumption capacity. After basal and state 4 respiration, cells were treated with FCCP (1 mm) to determine maximal oxygen consumption. A C. Myotubes were differentiated for 7 days in LG (5 mm glucose). On the 7 th day of differentiation, myotubes were exposed to HG (25 mm), LG (5 mm glucose) or GAL (10 mm galactose) for 45 min before measuring basal oxygen consumption rate. Results are presented as means 6 SEM, n = 4, in which each condition was assessed in 5 replicates. doi: /journal.pone g004 Discussion The present study identifies galactose differentiation medium as a useful experimental system to analyze mitochondrial metabolism in human primary muscle cells. Our findings demonstrate that this system provides an effective method to elucidate mitochondrial dysfunction in myotubes derived from post-diabetic patients. Our main results show that OCR was 30% higher in healthy myotubes differentiating in GAL compared to LG or HG media, which is due, at least in part, to an increase in AMPK phosphorylation and COX activity in GAL treated cells. When differentiated in LG or HG media, post-diabetic myotubes had similar OCR as myotubes derived from matched obese non-diabetic volunteers. Interestingly, unlike obese non-diabetic myotubes, post-diabetic myotubes were unable to increase their OCR in response to GAL medium. Post-diabetic myotubes were also unable to increase AMPK phosophorylation and COX activity when differentiated in GAL. To our knowledge, this study is the first to show that the use of a GAL medium is a simple means to increase OCR in human primary muscle cells and to potentially identify mitochondrial dysfunction. To better characterize the effect of different carbohydrate sources and/or concentration during cell culture, we first determined the level of differentiation in myotubes differentiated for 7 days in LG (5 mm glucose), HG (25 mm glucose) or GAL (10 mm galactose). Myotubes differentiated in HG showed the highest level of differentiation as indicated by troponin T expression level, which is commonly used as a marker of muscle cell differentiation [8,38]. Thus, we have confirmed in our model (human primary muscle cells) that glucose availability can affect the level of muscle cell differentiation as previously reported by others [32] in muscle cell lines (C2C12) and mouse primary muscle cells. However, there were no differences in the levels of differentiation in myotubes differentiated in 5 mm glucose (LG) compared to 10 mm galactose (GAL), showing that the metabolic differences found between LG and GAL myotubes were not the result of alteration in levels of differentiation. As previously shown by others in different cell lines or primary fibroblasts [13,14,15], replacing glucose with galactose in the medium forces the cells to become more oxidative in order to maintain ATP levels. In accordance with an increased oxidative metabolism, differentiating the cells in GAL decreased anaerobic metabolism as shown by the diminished generation of lactate. The sharp decrease in lactate production by the myotubes differentiated in GAL is not surprising since the production of pyruvate through galactose metabolism yields no net ATP, forcing the cells to rely on aerobic metabolism instead of anaerobic glycolysis to produce ATP [13,19,20,21]. The increase in oxygen consumption rate in cells differentiated in GAL was not due to an increase in proton leak-dependent oxygen consumption rate as shown by the proportion of total respiration that was due to proton leak. A trend for a higher maximal respiration rate in myotubes differentiated in GAL compared to LG was also found, but this did not reach significance (p = 0.07). The higher mitochondrial OCR in GAL cells was not the result of an increase in mitochondrial content as evidenced by the similar levels of various mitochondrial markers (mitochondrial yield, cardiolipin staining, SDHA and complex III expressions). Furthermore, citrate synthase activity, commonly used as a marker of TCA cycle activity, was not affected by the different sources of carbohydrates. Interestingly, this increase in mitochondrial OCR was related to a,70% increase in mitochondrial COX activity certainly due to an increase in COX expression. Our results on human primary muscle cells are in accordance with a previous report showing increases in COX expression and activity in HeLa cells grown in 10 mm galactose compared to 25 mm glucose [15]. We also found an increased AMPK phosphorylation in myotubes differentiated in GAL compared to LG or HG. AMPK is a major metabolic sensor in cells and is activated by increased AMP/ATP for the purpose of restoring the energy status of the cell [33,34,35,36,37]. An increase AMPK phosphorylation in response to a decrease in glucose availability (25 mm compared to 5 mm) has also been found by others in C2C12 myotubes [32]. In our primary muscle cell model, we were not able to detect an increased P-AMPK when cells were differentiated in 5 mm glucose compared to 25 mm glucose, consistent with the conclusion that 5 mm glucose is not restrictive enough in our model. However, a nice increase in P-AMPK was found when myotubes were differentiated in GAL, suggesting that GAL medium induced a deprivation of energy, which in turn did activate AMPK in order to restore energy status of the cells. Thus, PLoS ONE 6 December 2011 Volume 6 Issue 12 e28536
7 Figure 5. Absence of an increase in basal oxygen consumption in post-diabetic myotubes differentiated in galactose media. A. Basal oxygen consumption rate. *, p,0.05, GAL vs HG and LG. #, p,0.05, post-diabetic vs obese. B. State 4 respiration (leak dependent; nonphosphorylating). After basal oxygen consumption rate measurement, cells were treated with oligomycin (600 ng/ml) to determine state 4 respiration. C. Maximal oxygen consumption capacity. After basal and state 4 respiration, cells were treated with FCCP (1 mm) to determine maximal oxygen consumption. D. Non-mitochondrial oxygen consumption rate. After basal, state and maximal respiration, cells were treated with antimycin (4 mm) to determine non-mitochondrial oxygen consumption. ##, p,0.001, post-diabetic vs obese. A D. Myotubes were differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Results are presented as means 6 SEM, n = 5, in which each condition was assessed in 5 6 replicates. doi: /journal.pone g005 the increased COX activity and oxygen consumption in GAL myotubes could be the result of an increased AMPK phosphorylation. Other studies will be needed to test this hypothesis. To test if an acute treatment with GAL could have the same effect as differentiating the cells for 7 days in GAL, myotubes were differentiated for 7 days in LG medium and then acutely treated for 45 min with HG, LG or GAL before OCR measurement. This acute treatment with GAL was not sufficient to increase OCR compared to LG or HG treatments. This may be attributed to the fact that glycolytic intermediates would not be completely depleted after a 45 min incubation in GAL medium. Taken together, those results show that differentiating human primary myotubes in GAL for 7 days could be an easy way to improve the aerobic capacity of these cells and decrease their reliance on anaerobic glycolysis. An unexpected and intriguing finding was the increase in nonmitochondrial OCR in myotubes differentiated in GAL compared to HG. Extra-mitochondrial sites for oxygen consumption include the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, nitric oxide synthase, and the xanthine oxidase. Measurement of the cell redox environment with the MTT assay is based on a reaction in which the formed NAD(P)H reduces tetrazolium (MTT) reagent to a blue formazan. The intensity of the reduced product colour is thus proportionate to the NAD(P) + / NAD(P)H concentration in the cells [39]. Since the redox environment measured with the MTT assay was significantly lower in LG and GAL cells compared to HG cells, this could indicate a decrease in reduced pyridine nucleotide levels in LG and GAL myotubes compared to HG myotubes. A similar decrease in redox state was also found by others in HeLA cells grown in 10 mm galactose compared to 25 mm glucose [15]. Further studies are needed to directly assess NAD(P)H level and NAD(P)H oxidase activity to confirm this hypothesis. Galactose medium is often used to study the effect of mitochondrial toxins in cancer cells [13,23,24] and has also been used to examine mitochondrial dysfunction in primary skin fibroblasts derived from patients with defect in mitochondrial function [20]. In the present study, we have shown, not only that GAL is able to increase the oxidative metabolism of healthy human primary muscle cells, but also that GAL can be used to identify mitochondrial dysfunction in primary muscle cells derived from post-diabetic patients. Indeed, unlike myotubes derived from matched obese non-diabetic subjects, myotubes derived from postdiabetic patients were not able to increase their oxygen consumption rate when differentiated in GAL. Thus, in spite of the fact that there was no difference in OCR between obese nondiabetic myotubes and post-diabetic myotubes when differentiated in LG or HG, the GAL media was able to highlight a decreased oxidative capacity in post-diabetic myotubes compared to obese non-diabetic myotubes. Other studies have described a decrease in oxidative metabolic capacity in myotubes derived from diabetic patients as indicated by a decrease in citrate synthase activity [7] and a decrease in lipid oxidation capacity [5,9]. Myotubes derived from post-diabetic subjects are also known to have a decreased oxidative capacity when challenged with a high glucose medium (decreased mitochondrial content, decreased citrate synthase and COX activities) compared to myotubes derived from matched PLoS ONE 7 December 2011 Volume 6 Issue 12 e28536
8 Figure 6. Absence of an increase in cytochrome C oxidase activity and AMPK phosphorylation in post-diabetic myotubes differentiated in galactose media. A. Mitochondrial yield measured in post-diabetic myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Mitochondrial yield was determined as mitochondrial protein content per total cellular protein content. Results are presented as means 6 SEM, n = 4. *, p,0.05, LG vs HG, **, p,0.01 LG vs GAL. B. Cytochrome C oxidase activity measured in isolated mitochondria from post-diabetic myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Results are presented as means 6 SEM, n = 4. *, p,0.05, GAL vs HG and LG. C. Top panel: representative Western blot of Complex IV expression in post-diabetic myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Beta-actin was used as a loading control. Bottom panel: quantification by densitometry of complex IV expression. Data are presented normalized to beta-actin expression. Data are shown as mean 6SEM, n = 4. D. Left panel: representative Western blot of P-AMPK expression in post-diabetic myotubes differentiated for 7 days in HG (25 mm glucose), LG (5 mm glucose) or GAL (10 mm galactose). Beta-actin was used as loading controls. Right panels: quantification by densitometry of P-AMPK. Data are presented normalized to beta-actin and AMPKa2 expression. Data are shown as mean 6SEM, n = 4. doi: /journal.pone g006 obese non-diabetic individuals [40]. Despite this decrease oxidative capacity [5,9,40], no difference in OCR was found in the present study between obese non-diabetic and post-diabetic myotubes differentiated in either HG or LG. This is presumably due to the fact that under these conditions, myotubes were highly glycolytic rendering it more difficult to detect a decrease in OCR in post-diabetic compared to obese non-diabetic myotubes. However, the GAL medium was able to identify decreased OCR in post-diabetic myotubes, revealing the utility of this approach to detect mitochondrial dysfunction in vitro. Our results also confirm that post-diabetic myotubes display an abnormal metabolic flexibility when challenged with different substrates (here with galactose) as previously published by others who studied myotubes derived from (post-)diabetic, insulin resistant or obese patients when challenged with high fat levels [9,11,41] or high glucose levels [40]. In the present study, the lack of an increase in OCR in post-diabetic myotubes appears to be related to unaltered COX expression or activity levels or AMPK phosphorylation. Another study showed that myotubes derived from diabetic patients lack the capacity to activate the AMPK pathway (measured as acetyl-coa carboxylase phosphorylation) in response to of palmitate, leading to an absence of any increase in myotubes palmitate oxidation [9]. It seems that myotubes derived from (post-) diabetic patients have a impaired AMPK activity in response to different AMPK-stimulating agents. Other studies will be needed to determine whether AMPK stimulation is truly impaired in (post-) diabetic muscle and to determine the mechanisms causing AMPK dysfunction. Interestingly, we also found a significant decrease in nonmitochondrial OCR in post-diabetic myotubes compared to matched obese non-diabetic myotubes, independently of the source of carbohydrates used in the differentiation medium. This result is in accordance with a study from our group showing perturbations in NADPH production due to an impaired glucose- 6-phosphate dehydrogenase in post-diabetic myotubes [42]. This could be due to a defect in the pentose phosphate pathway in postdiabetic myotubes. Another hypothesis explaining the lower nonmitochondrial OCR in post-diabetic myotubes compared to obese control myotubes is a decrease in NADPH oxidase protein content or activity. NADPH oxidase produces superoxide by coupling their electrons to oxygen. Thus this is an enzyme involved in reactive oxygen species production (ROS). Interestingly, the measurement of ROS (by the DCFH-DA assay) in post-diabetic and obese non-diabetic myotubes showed lower ROS content in PLoS ONE 8 December 2011 Volume 6 Issue 12 e28536
9 post-diabetic myotubes (data not shown). Studying the mechanism underlying this phenomenon was not the purpose of our study and needs further investigation. To our knowledge, this is the first study to test the impact of different carbohydrate sources on human myotube bioenergetics. Furthermore, this is the first study that has directly assessed oxygen consumption rate in vitro in myotubes derived from post-diabetic patients and demonstrated decrease oxygen consumption rates in post-diabetic myotubes compared to obese non-diabetic myotubes. Moreover, we have shown that differentiating cells in GAL is an excellent model system to investigate the mitochondrial bioenergetics of human myotubes derived from patients with a history of T2DM. The use of this model could enable further research to better understand the molecular mechanisms leading to mitochondrial dysfunction during the development of insulin resistance and other metabolic disorders in skeletal muscle. Materials and Methods Ethics statement All participants gave informed consent and the experimental protocol was approved by the Human Research Ethics Committees of the Ottawa Hospital and the University of Ottawa Heart Institute. Muscle biopsy and primary human muscle cell culture Biopsies of vastus lateralis were obtained from 7 lean and healthy participants (male/female: 6/1, age: 41 years 66.7, BMI (Body Mass Index): 20.8 kg/m ) and from 5 obese nondiabetic persons (male/female: 4/1, age: 55 years 62.1, BMI: 39.2 kg/m ) and 5 obese post-diabetic patients (male/ female: 4/1, age: 54 years 62.3, BMI: 36.6 kg/m ) after local anesthesia, using a 5 mm Bergstrom needle (Opitek, Glostrup, Denmark) as described previously by Costford et al [40]. The post-diabetic patients had a documented history of obesity-associated T2DM prior to a 26-week standard clinical weight loss program at the Ottawa Hospital Weight Management Clinic and were no longer diabetic after the weight loss program (blood glucose,6.1 mmol/l and HbA1c,5.7%). Characterization of primary muscle cells from the obese non-diabetic and the obese post-diabetic participants was previously published by our lab [40]. Subjects were fasted for 12 hours and refrained from physical activity for three days prior to the biopsy. 40 mg of the muscle biopsy were processed for satellite cell isolation and culture. The muscle biopsy was minced, subjected to a 30 minutes trypsin digestion and plated in Ham s F-10 medium supplemented with 15% Foetal Bovine Serum (FBS, Gibco, Burlington, ON, Canada), 0.5 mg/ml BSA, 1 mm dexamethazone (Invitrogen, Burlington, ON, Canada), 10 ng/ml Epidermal Growth Factor (EGF, Invitrogen, Burlington, ON, Canada) and 25 pm insulin (Sigma Aldrich, Oakville, ON, Canada), and maintained in 37uC in humidified 95% air 5% CO 2. Muscle satellite cells were isolated at,80% confluency using an immuno-based magnetic sorting technique as previously described [40]. Isolated primary muscle cells were grown in Ham s F-10 (6.11 mm glucose) (Gibco, Burlington, ON, Canada) supplemented with 12.5% FBS, 1 mm dexamethazone, 10 ng/ml EGF, 25 pm insulin, 16 antibioticantimycotic (Gibco, Burlington, ON, Canada) and 2.5 mg/ml gentamycin (Growth Medium, GM) to,90% confluency prior to differentiation in either low glucose DMEM (5.5 mm glucose, supplemented with 1 mm sodium pyruvate, 25 pm insulin, 2% horse serum, 16 antibiotic-antimycotic and 2.5 mg/ml gentamycin) (LG, Gibco, Burlington, ON, Canada), galactose DMEM (10 mm galactose (Sigma Aldrich, Oakville, ON, Canada), 1 mm sodium pyruvate, supplemented with 25 pm insulin, 2% horse serum, 1% antibiotic-antimycotic and 2.5 mg/ml gentamycin) (GAL) or high glucose DMEM (25 mm glucose, supplemented with 1 mm sodium pyruvate, 25 pm insulin, 2% horse serum, 1% antibiotic-antimycotic and 2.5 mg/ml gentamycin) (HG) for 7 days prior to experimentation. The choice of 10 mm galactose was based on previous published studies on other cell lines [13,14,15]. For acute treatments (Figure 4), myotubes were differentiated in LG for 7 days, and then incubated for 45 min in HG, LG or GAL prior to experimentation. Immunofluorescence staining and fusion index In order to calculate the fusion index, cells differentiated in HG, LG or GAL for 7 days were subjected to a desmin immunofluorescence staining as previously described [8]. The fusion index was determined as the number of nuclei in differentiated myotubes (desmin positive cells with more than 2 nuclei) as a percentage of the total number of nuclei in muscle cells (desmin positive cells) [43]. Western Blots Cell lysates were quantified for protein content and 30 mg of protein were separated by 8% SDS-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane. Following primary antibodies were used: monoclonal antitroponin T (Sigma Aldrich, Oakville, ON, Canada), anti-complex III (Invitrogen, Burlington, ON, Canada), anti-sdha (succinate dehydrogenase, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-complex IV (MitoSciences, Eugene, OR, USA), anti- AMPK a2 (Upstate, Lake Placid, NY, USA) and polyclonal anti-bactin (Cell signaling, Pickering, ON, Canada) diluted 1/1000. Polyclonal anti-phospho-ampk (Cell signaling, Pickering, ON, Canada) was diluted at 1/500. The secondary antibodies were anti-mouse and anti-rabbit antibodies coupled to horseradish peroxidase, respectively. Proteins were visualized using an enhanced luminescent reagent and exposed to autoradiograph film. b-actin and Troponin T were used as a loading control and a marker of myotube differentiation, respectively. Expression of proteins was quantified by density analysis using ImageJ Launcher Software. Cell redox environment Cell redox environment following 7 days of differentiation in HG, LG or GAL was assessed using the MTT Assay Kit (Millipore, Billerica, MA, USA). ATP assay ATP content in myotubes differentiated in HG, LG or GAL for 7 days was assessed using the StayBrite ATP assay kit (Biovision, Mountain View, CA, USA). HPLC Determination of Cellular Lactate Release Myoblasts were seeded at 200,000 cells per well of a 6-well plate. Upon reaching,90% confluency, cells were differentiated in their respective differentiation medium (HG, LG or GAL) for 7 days. On the 6 th day post differentiation the medium was changed and then collected 24 h later for analysis. For experiments, 1 ml of media was treated with 50 ml of 0.5% (v/v) perchloric acid solution. Samples were then centrifuged at 12,000 g for 10 min and subsequently filtered with a sterile syringe and 0.2 mm filtration unit. Samples were injected into an Agilent 1100 Series HPLC system equipped with a C 18 reverse phase hydrophilic PLoS ONE 9 December 2011 Volume 6 Issue 12 e28536
10 column (Phenomenex) operating at a flow rate of 0.7 ml/min. Lactate was detected using an Agilent variable wavelength detector set to 210 nm. The retention time of lactate was confirmed by injection standard lactate solutions. Lactate levels were quantified by injection varying concentrations of standard lactate solutions and the use of Agilent ChemStation software. Extracellular Flux (XF) Analysis of Cellular Metabolic Characteristics 20,000 cells per well were plated in a XF24-well plate (Seahorse Bioscience, North Billerica, MA, USA), grown to,90% confluency and differentiated for 7 days in HG, LG or GAL. Myotubes were then incubated for 45 min at 37uC at ambient CO 2 in HCO 3 -free DMEM (ph 7.4) containing 4 mm glutamine, 1 mm pyruvate and 25 mm glucose (HG), or 5 mm glucose (LG) or 10 mm galactose (GAL). Oxygen consumption rates (OCR) were determined in situ using a Seahorse Extracellular Flux Analyzer. Baseline oxygen consumption rate was measured 3 times for 4 min each separated by a 2 min wait and a 2 min mix. Following the measurement of basal respiration, oligomycin (600 ng/ml) (Sigma Aldrich, Oakville, ON, Canada) was injected into each well, followed by 3 cycles of: 2 min mix, 2 min wait and 4 min measurement to measure state 4 (non-phosphorylating) respiration. Then, FCCP (1 mm) (Sigma Aldrich, Oakville, ON, Canada) was injected into each well, followed by 3 cycles of: 2 min mix, 2 min wait and 2.5 min measurement to measure maximal respiration. Finally, antimycin A (4 mm) (Sigma Aldrich, Oakville, ON, Canada) was injected into each well, followed by 3 cycles of: 2 min mix, 2 min wait and 3 min measurement to measure extramitochondrial O 2 consumption. The cells were then collected for determinations of protein content (Bradford assay). The data are presented as: mitochondrial OCR (basal, oligomycin or FCCP OCR are reported following the subtraction of OCR in the presence of antimycin) and expressed per mg of total cellular protein. Mitochondrial yield, Citrate Synthase (CS) and COX Activity Assays Myoblasts were grown in a 75-cm 2 flask to,90% confluency and then differentiated in their respective differentiation media (HG, LG or GAL) for 7 days. Intact mitochondria were isolated using a kit (MITOISO; Sigma Aldrich, Oakville, ON, Canada). Mitochondrial and total cellular protein levels were determined by a BCA (bicinchoninic acid) assay. Mitochondrial yield was References 1. Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, et al. (2003) Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 52: Jackson S, Bagstaff SM, Lynn S, Yeaman SJ, Turnbull DM, et al. (2000) Decreased insulin responsiveness of glucose uptake in cultured human skeletal muscle cells from insulin-resistant nondiabetic relatives of type 2 diabetic families. Diabetes 49: Nikoulina SE, Ciaraldi TP, Carter L, Mudaliar S, Park KS, et al. (2001) Impaired muscle glycogen synthase in type 2 diabetes is associated with diminished phosphatidylinositol 3-kinase activation. J Clin Endocrinol Metab 86: Gaster M, Petersen I, Hojlund K, Poulsen P, Beck-Nielsen H (2002) The diabetic phenotype is conserved in myotubes established from diabetic subjects: evidence for primary defects in glucose transport and glycogen synthase activity. Diabetes 51: Gaster M, Rustan AC, Aas V, Beck-Nielsen H (2004) Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin: evidence from cultured myotubes. Diabetes 53: Gaster M, Rustan AC, Beck-Nielsen H (2005) Differential utilization of saturated palmitate and unsaturated oleate: evidence from cultured myotubes. Diabetes 54: expressed as mg of mitochondrial protein per mg of total cellular protein. CS activity was assayed in lysed mitochondria using a kit (CSO720; Sigma Aldrich, Oakville, ON, Canada) with 0.5 mmol/l oxaloacetate, 0.3 mmol/l acetyl-coa, 0.1 mmol/l of 5, 59-dithiobis 2-nitro-benzoic acid. Enzyme activity was monitored by recording the changes in absorbance at 412 nm over 1.5 min. COX activity was measured in intact mitochondria using a kit (Cytocox1; Sigma Aldrich, Oakville, ON, Canada) based on the decrease in absorbance over 3 min at 550 nm of ferrocytochrome C caused by its oxidation to ferricytochrome C by COX. Enzyme activities were normalized to protein content. Cardiolipin staining and quantification 10N-nonyl acridine orange (NAO, Sigma Aldrich, Oakville, ON, Canada) staines the mitochondrial phospholipid cardiolipin [44]. NAO is used as a mitochondrial content marker since it is essentially independent of the mitochondrial membrane potential [45]. After differentiation in HG, LG and GAL for 7 days, myotubes were fixed with paraformaldehyde at 4% in PBS for 10 min, and then cardiolipin was stained by NAO (1 mm) for 30 min, in the dark, at room temperature. The dye was then washed 3 times with PBS, and mitochondrial content quantified in a fluorometer (emission: 535 nm, excitation: 485 nm). Statistical analyses Data are presented as mean 6SEM. Statistical analyses were performed using Statview 5.0 (SAS institute Inc, Cary, NC, USA). One-way or 2-ways ANOVAs with Fisher s protected least significant difference post-hoc test were used to assess statistical differences. P,0.05 was considered to be significant. Acknowledgments The authors would like to thank Dr. Sheila Costford, Dr. Martin Gerrits and Mahmoud Salkhordeh for human primary cell isolation, and Dr. Reza Nokhbeh for technical assistance. We also thank the volunteers who have participated in the study. Author Contributions Conceived and designed the experiments: CA MEH. Performed the experiments: CA DG RJM CM. Analyzed the data: CA DG RJM. Contributed reagents/materials/analysis tools: RD RM MEH. Wrote the paper: CA DG RJM MEH. 7. Ortenblad N, Mogensen M, Petersen I, Hojlund K, Levin K, et al. (2005) Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with type 2 diabetes: evidence for an intrinsic oxidative enzyme defect. Biochim Biophys Acta 1741: Aguer C, Mercier J, Man CY, Metz L, Bordenave S, et al. (2010) Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia 53: Kitzmann M, Lantier L, Hebrard S, Mercier J, Foretz M, et al. (2011) Abnormal metabolism flexibility in response to high palmitate concentrations in myotubes derived from obese type 2 diabetic patients. Biochim Biophys Acta 1812: Furling D, Marette A, Puymirat J (1999) Insulin-like growth factor I circumvents defective insulin action in human myotonic dystrophy skeletal muscle cells. Endocrinology 140: Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, et al. (2005) Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest 115: Zuurveld JG, Oosterhof A, Veerkamp JH, van Moerkerk HT (1985) Oxidative metabolism of cultured human skeletal muscle cells in comparison with biopsy material. Biochim Biophys Acta 844: Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y (2007) Circumventing the Crabtree effect: replacing media glucose with galactose increases PLoS ONE 10 December 2011 Volume 6 Issue 12 e28536
Application Note. Introduction
 Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
New tools bring greater understanding to cellular metabolism research
 New tools bring greater understanding to cellular metabolism research Mourad Ferhat, Ph.D, 7 Juin 2017 FDSS Users Meeting, Hamamatsu mourad.ferhat@promega.com Today s talk : focus on new cell-based assays
New tools bring greater understanding to cellular metabolism research Mourad Ferhat, Ph.D, 7 Juin 2017 FDSS Users Meeting, Hamamatsu mourad.ferhat@promega.com Today s talk : focus on new cell-based assays
Identifying Metabolic Phenotype Switches in Cancer Cells Using the Agilent Seahorse XF Analyzer in an Hypoxic Environment
 Identifying Metabolic Phenotype Switches in Cancer Cells Using the Agilent Seahorse XF Analyzer in an Hypoxic Environment Application Note Introduction Decreased oxygen levels, or hypoxia, and hypoxic-mediated
Identifying Metabolic Phenotype Switches in Cancer Cells Using the Agilent Seahorse XF Analyzer in an Hypoxic Environment Application Note Introduction Decreased oxygen levels, or hypoxia, and hypoxic-mediated
A cell has enough ATP to last for about three seconds.
 Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
Rapid Analysis of Glycolytic and Oxidative Substrate Flux of Cancer Cells in a Microplate
 Rapid Analysis of Glycolytic and Oxidative Substrate Flux of Cancer Cells in a Microplate Lisa S. Pike Winer, Min Wu* Seahorse Bioscience Inc., North Billerica, Massachusetts, United States of America
Rapid Analysis of Glycolytic and Oxidative Substrate Flux of Cancer Cells in a Microplate Lisa S. Pike Winer, Min Wu* Seahorse Bioscience Inc., North Billerica, Massachusetts, United States of America
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy
 Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
Supplementary Figure 1
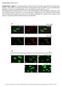 Supplementary Figure 1. (A) Representative confocal analysis of cardiac mesenchymal cells from nondiabetic (ND-CMSC) and diabetic (D-CMSC) immunostained with anti-h3k9ac and anti-h3k27me3 antibodies. Analysis
Supplementary Figure 1. (A) Representative confocal analysis of cardiac mesenchymal cells from nondiabetic (ND-CMSC) and diabetic (D-CMSC) immunostained with anti-h3k9ac and anti-h3k27me3 antibodies. Analysis
Mitochondria and ATP Synthesis
 Mitochondria and ATP Synthesis Mitochondria and ATP Synthesis 1. Mitochondria are sites of ATP synthesis in cells. 2. ATP is used to do work; i.e. ATP is an energy source. 3. ATP hydrolysis releases energy
Mitochondria and ATP Synthesis Mitochondria and ATP Synthesis 1. Mitochondria are sites of ATP synthesis in cells. 2. ATP is used to do work; i.e. ATP is an energy source. 3. ATP hydrolysis releases energy
BIOCHEMISTRY. Glycolysis. by Dr Jaya Vejayan Faculty of Industrial Sciences & Technology
 BIOCHEMISTRY Glycolysis by Dr Jaya Vejayan Faculty of Industrial Sciences & Technology email: jayavejayan@ump.edu.my Chapter Description Overview This chapter is related to carbohydrate catabolism. It
BIOCHEMISTRY Glycolysis by Dr Jaya Vejayan Faculty of Industrial Sciences & Technology email: jayavejayan@ump.edu.my Chapter Description Overview This chapter is related to carbohydrate catabolism. It
Fatty Acid Oxidation Assay on the XF24 Analyzer
 Fatty Acid Oxidation Assay on the XF24 Analyzer Mitochondria oxidize a variety of fuels to generate ATP through oxidative phosphorylation. Cells can utilize fatty acid, glucose and amino acids as their
Fatty Acid Oxidation Assay on the XF24 Analyzer Mitochondria oxidize a variety of fuels to generate ATP through oxidative phosphorylation. Cells can utilize fatty acid, glucose and amino acids as their
Class XI Chapter 14 Respiration in Plants Biology. 1. It is a biochemical process. 1. It is a physiochemical process.
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Performing Bioenergetic Analysis: Seahorse XFe96 Analyzer
 icell Cardiomyocytes 2 Application Protocol Performing Bioenergetic Analysis: Seahorse XFe96 Analyzer Introduction The myocardium is the most metabolically active tissue in the body and is highly sensitive
icell Cardiomyocytes 2 Application Protocol Performing Bioenergetic Analysis: Seahorse XFe96 Analyzer Introduction The myocardium is the most metabolically active tissue in the body and is highly sensitive
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
BIL 256 Cell and Molecular Biology Lab Spring, Tissue-Specific Isoenzymes
 BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
Chapter 14. Energy conversion: Energy & Behavior
 Chapter 14 Energy conversion: Energy & Behavior Why do you Eat and Breath? To generate ATP Foods, Oxygen, and Mitochodria Cells Obtain Energy by the Oxidation of Organic Molecules Food making ATP making
Chapter 14 Energy conversion: Energy & Behavior Why do you Eat and Breath? To generate ATP Foods, Oxygen, and Mitochodria Cells Obtain Energy by the Oxidation of Organic Molecules Food making ATP making
In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic
 Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Metabolism Energy Pathways Biosynthesis. Catabolism Anabolism Enzymes
 Topics Microbial Metabolism Metabolism Energy Pathways Biosynthesis 2 Metabolism Catabolism Catabolism Anabolism Enzymes Breakdown of complex organic molecules in order to extract energy and dform simpler
Topics Microbial Metabolism Metabolism Energy Pathways Biosynthesis 2 Metabolism Catabolism Catabolism Anabolism Enzymes Breakdown of complex organic molecules in order to extract energy and dform simpler
4. Which step shows a split of one molecule into two smaller molecules? a. 2. d. 5
 1. Which of the following statements about NAD + is false? a. NAD + is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD + has more chemical energy than NADH. c. NAD + is reduced
1. Which of the following statements about NAD + is false? a. NAD + is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD + has more chemical energy than NADH. c. NAD + is reduced
Introduction to Carbohydrate metabolism
 Introduction to Carbohydrate metabolism Some metabolic pathways of carbohydrates 1- Glycolysis 2- Krebs cycle 3- Glycogenesis 4- Glycogenolysis 5- Glyconeogenesis - Pentose Phosphate Pathway (PPP) - Curi
Introduction to Carbohydrate metabolism Some metabolic pathways of carbohydrates 1- Glycolysis 2- Krebs cycle 3- Glycogenesis 4- Glycogenolysis 5- Glyconeogenesis - Pentose Phosphate Pathway (PPP) - Curi
Ryan McGarrigle, Ph.D.
 Cardiomyocyte function characterised through combined analysis of metabolic flux, beat rate and cellular oxygenation and its application in in vitro ischemia reperfusion modelling Ryan McGarrigle, Ph.D.
Cardiomyocyte function characterised through combined analysis of metabolic flux, beat rate and cellular oxygenation and its application in in vitro ischemia reperfusion modelling Ryan McGarrigle, Ph.D.
III. 6. Test. Respiració cel lular
 III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Glycolysis. Cellular Respiration
 Glucose is the preferred carbohydrate of cells. In solution, it can change from a linear chain to a ring. Energy is stored in the bonds of the carbohydrates. Breaking these bonds releases that energy.
Glucose is the preferred carbohydrate of cells. In solution, it can change from a linear chain to a ring. Energy is stored in the bonds of the carbohydrates. Breaking these bonds releases that energy.
CARBOHYDRATE METABOLISM
 Note (Study Glycolysis, fermentation and their regulation, Gluconeogenesis and glycogenolysis, Metabolism of galactose, TCA cycle and Amphibolic role of the cycle, and Glyoxalic acid cycle, HMP shunt in
Note (Study Glycolysis, fermentation and their regulation, Gluconeogenesis and glycogenolysis, Metabolism of galactose, TCA cycle and Amphibolic role of the cycle, and Glyoxalic acid cycle, HMP shunt in
Background knowledge
 Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
MitoXpress FAO Assay. For the measurement of Fatty Acid Oxidation
 MitoXpress FAO Assay For the measurement of Fatty Acid Oxidation Companion Kit to be used in combination with Luxcel s MitoXpress Xtra - Oxygen Consumption Assay ILLUMINATING DISCOVERY TABLE OF CONTENTS
MitoXpress FAO Assay For the measurement of Fatty Acid Oxidation Companion Kit to be used in combination with Luxcel s MitoXpress Xtra - Oxygen Consumption Assay ILLUMINATING DISCOVERY TABLE OF CONTENTS
CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM
 CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM Metabolism Bioenergetics is the transfer and utilization of energy in biological systems The direction and extent to which a chemical reaction
CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM Metabolism Bioenergetics is the transfer and utilization of energy in biological systems The direction and extent to which a chemical reaction
Quantifying Cellular ATP Production Rate Using Agilent Seahorse XF Technology
 White Paper Quantifying Cellular ATP Production Rate Using Agilent Seahorse XF Technology Authors Natalia Romero George Rogers Andy Neilson Brian P. Dranka Agilent Technologies, Inc., Lexington, MA, USA
White Paper Quantifying Cellular ATP Production Rate Using Agilent Seahorse XF Technology Authors Natalia Romero George Rogers Andy Neilson Brian P. Dranka Agilent Technologies, Inc., Lexington, MA, USA
Cellular Pathways That Harvest Chemical Energy. Cellular Pathways That Harvest Chemical Energy. Cellular Pathways In General
 Cellular Pathways That Harvest Chemical Energy A. Obtaining Energy and Electrons from Glucose Lecture Series 12 Cellular Pathways That Harvest Chemical Energy B. An Overview: Releasing Energy from Glucose
Cellular Pathways That Harvest Chemical Energy A. Obtaining Energy and Electrons from Glucose Lecture Series 12 Cellular Pathways That Harvest Chemical Energy B. An Overview: Releasing Energy from Glucose
Cellular Respiration
 Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
anabolic pathways- Catabolic Amphibolic
 METABOLISM Introduction The fate of dietary components after digestion and absorption constitute metabolism regulated by metabolic pathway 3 types: anabolic pathways- Synthesis of compound e.g. synthesis
METABOLISM Introduction The fate of dietary components after digestion and absorption constitute metabolism regulated by metabolic pathway 3 types: anabolic pathways- Synthesis of compound e.g. synthesis
Higher Biology. Unit 2: Metabolism and Survival Topic 2: Respiration. Page 1 of 25
 Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Nafith Abu Tarboush DDS, MSc, PhD
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush OMM: permeable to small molecules (MW
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush OMM: permeable to small molecules (MW
Glucose Uptake-Glo Assay
 TECHNICAL MANUAL Glucose Uptake-Glo Assay Instructions for Use of Products J1341, J1342, and J1343 Revised 2/17 TM467 Glucose Uptake-Glo Assay All technical literature is available at: www.promega.com/protocols/
TECHNICAL MANUAL Glucose Uptake-Glo Assay Instructions for Use of Products J1341, J1342, and J1343 Revised 2/17 TM467 Glucose Uptake-Glo Assay All technical literature is available at: www.promega.com/protocols/
Medical Biochemistry and Molecular Biology department
 Medical Biochemistry and Molecular Biology department Cardiac Fuels [Sources of energy for the Cardiac muscle] Intended learning outcomes of the lecture: By the end of this lecture you would be able to:-
Medical Biochemistry and Molecular Biology department Cardiac Fuels [Sources of energy for the Cardiac muscle] Intended learning outcomes of the lecture: By the end of this lecture you would be able to:-
Ch. 9 Cell Respiration. Title: Oct 15 3:24 PM (1 of 53)
 Ch. 9 Cell Respiration Title: Oct 15 3:24 PM (1 of 53) Essential question: How do cells use stored chemical energy in organic molecules and to generate ATP? Title: Oct 15 3:28 PM (2 of 53) Title: Oct 19
Ch. 9 Cell Respiration Title: Oct 15 3:24 PM (1 of 53) Essential question: How do cells use stored chemical energy in organic molecules and to generate ATP? Title: Oct 15 3:28 PM (2 of 53) Title: Oct 19
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Physiological Chemistry II Exam IV Dr. Melissa Kelley April 13, 2004
 Name Write your name on the back of the exam Physiological Chemistry II Exam IV Dr. Melissa Kelley April 13, 2004 This examination consists of forty-four questions, each having 2 points. The remaining
Name Write your name on the back of the exam Physiological Chemistry II Exam IV Dr. Melissa Kelley April 13, 2004 This examination consists of forty-four questions, each having 2 points. The remaining
2. What is molecular oxygen directly converted into? a. Carbon Dioxide b. Water c. Glucose d. None of the Above
 Biochem 1 Mock Exam 3 Chapter 11: 1. What is glucose completely oxidized into? a. Carbon Dioxide and Water b. Carbon Dioxide and Oxygen c. Oxygen and Water d. Water and Glycogen 2. What is molecular oxygen
Biochem 1 Mock Exam 3 Chapter 11: 1. What is glucose completely oxidized into? a. Carbon Dioxide and Water b. Carbon Dioxide and Oxygen c. Oxygen and Water d. Water and Glycogen 2. What is molecular oxygen
Biol 219 Lec 7 Fall 2016
 Cellular Respiration: Harvesting Energy to form ATP Cellular Respiration and Metabolism Glucose ATP Pyruvate Lactate Acetyl CoA NAD + Introducing The Players primary substrate for cellular respiration
Cellular Respiration: Harvesting Energy to form ATP Cellular Respiration and Metabolism Glucose ATP Pyruvate Lactate Acetyl CoA NAD + Introducing The Players primary substrate for cellular respiration
How Cells Harvest Energy. Chapter 7. Respiration
 How Cells Harvest Energy Chapter 7 Respiration Organisms classified on how they obtain energy: autotrophs: produce their own organic molecules through photosynthesis heterotrophs: live on organic compounds
How Cells Harvest Energy Chapter 7 Respiration Organisms classified on how they obtain energy: autotrophs: produce their own organic molecules through photosynthesis heterotrophs: live on organic compounds
NAME KEY ID # EXAM 3a BIOC 460. Wednesday April 10, Please include your name and ID# on each page. Limit your answers to the space provided!
 EXAM 3a BIOC 460 Wednesday April 10, 2002 Please include your name and ID# on each page. Limit your answers to the space provided! 1 1. (5 pts.) Define the term energy charge: Energy charge refers to the
EXAM 3a BIOC 460 Wednesday April 10, 2002 Please include your name and ID# on each page. Limit your answers to the space provided! 1 1. (5 pts.) Define the term energy charge: Energy charge refers to the
Name Class Date. 1. Cellular respiration is the process by which the of "food"
 Name Class Date Cell Respiration Introduction Cellular respiration is the process by which the chemical energy of "food" molecules is released and partially captured in the form of ATP. Carbohydrates,
Name Class Date Cell Respiration Introduction Cellular respiration is the process by which the chemical energy of "food" molecules is released and partially captured in the form of ATP. Carbohydrates,
Cellular Respiration
 Cellular I can describe cellular respiration Cellular respiration is a series of metabolic pathways releasing energy from a foodstuff e.g. glucose. This yields energy in the form of ATP adenosine P i P
Cellular I can describe cellular respiration Cellular respiration is a series of metabolic pathways releasing energy from a foodstuff e.g. glucose. This yields energy in the form of ATP adenosine P i P
Cell Metabolism Assays. for. Immunology. Research
 the immunity-metabolism link Cell Metabolism Assays for Immunology Research GOLD STANDARD ASSAYS FOR MEASURING METABOLIC RePROGRAMMING Metabolic Signatures Cell activation, amplification, and effector
the immunity-metabolism link Cell Metabolism Assays for Immunology Research GOLD STANDARD ASSAYS FOR MEASURING METABOLIC RePROGRAMMING Metabolic Signatures Cell activation, amplification, and effector
How Cells Release Chemical Energy. Chapter 7
 How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
Agilent Technologies: Seahorse XF Introduction
 Agilent Technologies: Seahorse XF Introduction Tools for investigating cell metabolism diagnostic procedures. 1 The Engines Cells Generate Energy via Two Metabolic Pathways Glycolysis [ H + ] Respiration
Agilent Technologies: Seahorse XF Introduction Tools for investigating cell metabolism diagnostic procedures. 1 The Engines Cells Generate Energy via Two Metabolic Pathways Glycolysis [ H + ] Respiration
Unit 2: Metabolic Processes
 How is energy obtained biologically? Recall: Red Ox Reactions Unit 2: Metabolic Processes Oxidation Is the chief mechanism by which chemical potential energy is released This energy comes from reduced
How is energy obtained biologically? Recall: Red Ox Reactions Unit 2: Metabolic Processes Oxidation Is the chief mechanism by which chemical potential energy is released This energy comes from reduced
Lecture 5: Cell Metabolism. Biology 219 Dr. Adam Ross
 Lecture 5: Cell Metabolism Biology 219 Dr. Adam Ross Cellular Respiration Set of reactions that take place during the conversion of nutrients into ATP Intricate regulatory relationship between several
Lecture 5: Cell Metabolism Biology 219 Dr. Adam Ross Cellular Respiration Set of reactions that take place during the conversion of nutrients into ATP Intricate regulatory relationship between several
g) Cellular Respiration Higher Human Biology
 g) Cellular Respiration Higher Human Biology What can you remember about respiration? 1. What is respiration? 2. What are the raw materials? 3. What are the products? 4. Where does it occur? 5. Why does
g) Cellular Respiration Higher Human Biology What can you remember about respiration? 1. What is respiration? 2. What are the raw materials? 3. What are the products? 4. Where does it occur? 5. Why does
BCH Graduate Survey of Biochemistry
 BCH 5045 Graduate Survey of Biochemistry Instructor: Charles Guy Producer: Ron Thomas Director: Marsha Durosier Lecture 44 Slide sets available at: http://hort.ifas.ufl.edu/teach/guyweb/bch5045/index.html
BCH 5045 Graduate Survey of Biochemistry Instructor: Charles Guy Producer: Ron Thomas Director: Marsha Durosier Lecture 44 Slide sets available at: http://hort.ifas.ufl.edu/teach/guyweb/bch5045/index.html
Moh Tarek. Razi Kittaneh. Jaqen H ghar
 14 Moh Tarek Razi Kittaneh Jaqen H ghar Naif Karadsheh Gluconeogenesis is making glucose from non-carbohydrates precursors. Although Gluconeogenesis looks like Glycolysis in many steps, it is not the simple
14 Moh Tarek Razi Kittaneh Jaqen H ghar Naif Karadsheh Gluconeogenesis is making glucose from non-carbohydrates precursors. Although Gluconeogenesis looks like Glycolysis in many steps, it is not the simple
Chapter 7 Cellular Respiration and Fermentation*
 Chapter 7 Cellular Respiration and Fermentation* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Life Is Work
Chapter 7 Cellular Respiration and Fermentation* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Life Is Work
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Which of the following statements concerning anabolic reactions is FALSE? A. They are generally endergonic. B. They usually require ATP. C. They are part of metabolism. D.
MULTIPLE CHOICE QUESTIONS 1. Which of the following statements concerning anabolic reactions is FALSE? A. They are generally endergonic. B. They usually require ATP. C. They are part of metabolism. D.
Cell Respiration. Anaerobic & Aerobic Respiration
 Cell Respiration Anaerobic & Aerobic Respiration Understandings/Objectives 2.8.U1: Cell respiration is the controlled release of energy from organic compounds to produce ATP. Define cell respiration State
Cell Respiration Anaerobic & Aerobic Respiration Understandings/Objectives 2.8.U1: Cell respiration is the controlled release of energy from organic compounds to produce ATP. Define cell respiration State
RESPIRATION Worksheet
 A.P. Bio L.C. RESPIRATION Worksheet 1. In the conversion of glucose and oxygen to carbon dioxide and water a) which molecule becomes reduced? b) which molecule becomes oxidized? c) what happens to the
A.P. Bio L.C. RESPIRATION Worksheet 1. In the conversion of glucose and oxygen to carbon dioxide and water a) which molecule becomes reduced? b) which molecule becomes oxidized? c) what happens to the
Krebs cycle, electron transport chain and oxidative phosphorylation
 BIOQUÍMICA E BIOLOGIA CELULAR António Ascensão, José Magalhães Krebs cycle, electron transport chain and oxidative phosphorylation Faculdade de Desporto, Universidade do Porto, 1º Ciclo, 1º Ano 2011_2012
BIOQUÍMICA E BIOLOGIA CELULAR António Ascensão, José Magalhães Krebs cycle, electron transport chain and oxidative phosphorylation Faculdade de Desporto, Universidade do Porto, 1º Ciclo, 1º Ano 2011_2012
Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2005
 Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2005 I. (20 points) Fill in all of the enzyme catalyzed reactions which convert glycogen to lactate. Draw the correct structure for each intermediate
Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2005 I. (20 points) Fill in all of the enzyme catalyzed reactions which convert glycogen to lactate. Draw the correct structure for each intermediate
Chemical Energy. Valencia College
 9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
This is an example outline of 3 lectures in BSC (Thanks to Dr. Ellington for sharing this information.)
 This is an example outline of 3 lectures in BSC 2010. (Thanks to Dr. Ellington for sharing this information.) Topic 10: CELLULAR RESPIRATION (lectures 14-16) OBJECTIVES: 1. Know the basic reactions that
This is an example outline of 3 lectures in BSC 2010. (Thanks to Dr. Ellington for sharing this information.) Topic 10: CELLULAR RESPIRATION (lectures 14-16) OBJECTIVES: 1. Know the basic reactions that
Additional methods appearing in the supplement are described in the Experimental Procedures section of the manuscript.
 Supplemental Materials: I. Supplemental Methods II. Supplemental Figure Legends III. Supplemental Figures Supplemental Methods Cell Culture and Transfections for Wild Type and JNK1-/-,JNK2-/- MEFs: The
Supplemental Materials: I. Supplemental Methods II. Supplemental Figure Legends III. Supplemental Figures Supplemental Methods Cell Culture and Transfections for Wild Type and JNK1-/-,JNK2-/- MEFs: The
20X Buffer (Tube1) 96-well microplate (12 strips) 1
 PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
BIOLOGY - CLUTCH CH.9 - RESPIRATION.
 !! www.clutchprep.com CONCEPT: REDOX REACTIONS Redox reaction a chemical reaction that involves the transfer of electrons from one atom to another Oxidation loss of electrons Reduction gain of electrons
!! www.clutchprep.com CONCEPT: REDOX REACTIONS Redox reaction a chemical reaction that involves the transfer of electrons from one atom to another Oxidation loss of electrons Reduction gain of electrons
) one consumes in breathing is converted to:, which of the following would be found in the oxidized state?
 MCB 102: Pantea s Sxn Chapter 19 Problem Set Answer Key 1) Page: 690 Ans: E Almost all of the oxygen (O 2 ) one consumes in breathing is converted to: A) acetyl-coa. B) carbon dioxide (CO 2 ). C) carbon
MCB 102: Pantea s Sxn Chapter 19 Problem Set Answer Key 1) Page: 690 Ans: E Almost all of the oxygen (O 2 ) one consumes in breathing is converted to: A) acetyl-coa. B) carbon dioxide (CO 2 ). C) carbon
The citric acid cycle Sitruunahappokierto Citronsyracykeln
 The citric acid cycle Sitruunahappokierto Citronsyracykeln Ove Eriksson BLL/Biokemia ove.eriksson@helsinki.fi Metabolome: The complete set of small-molecule metabolites to be found in a cell or an organism.
The citric acid cycle Sitruunahappokierto Citronsyracykeln Ove Eriksson BLL/Biokemia ove.eriksson@helsinki.fi Metabolome: The complete set of small-molecule metabolites to be found in a cell or an organism.
BIOCHEMISTRY. There are 4 major types of organic compounds each with unique characteristics: A. CARBOHYDRATES Contain,, and. Ratio of H:O is always
 BIOCHEMISTRY All organic compounds must contain and Are the following organic? Why or why not? H2O CO2 CH4 There are 4 major types of organic compounds each with unique characteristics: A. CARBOHYDRATES
BIOCHEMISTRY All organic compounds must contain and Are the following organic? Why or why not? H2O CO2 CH4 There are 4 major types of organic compounds each with unique characteristics: A. CARBOHYDRATES
7 Cellular Respiration and Fermentation
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
Supporting Information
 Supporting Information van der Windt et al. 10.1073/pnas.1221740110 SI Materials and Methods Mice and Reagents. C57BL/6 and major histocompatibility complex class I-restricted OVA-specific T-cell receptor
Supporting Information van der Windt et al. 10.1073/pnas.1221740110 SI Materials and Methods Mice and Reagents. C57BL/6 and major histocompatibility complex class I-restricted OVA-specific T-cell receptor
Nature Medicine: doi: /nm.3891
 Supplementary Figure 1. Subjective responses. Thermal sensation, thermal comfort and self-reported shivering, determined at several time points (from t = min until t = 36 min) after entering the cold room,
Supplementary Figure 1. Subjective responses. Thermal sensation, thermal comfort and self-reported shivering, determined at several time points (from t = min until t = 36 min) after entering the cold room,
Fall Name Student ID
 Name Student ID PART 1: Matching. Match the organelle to its function (11 points) 1.Proton motive force 2. Fluid Mosiac 3. Oxidative Phosphorylation 4. Pyruvate dehydrogenase 5. Electrochemical Force 6.
Name Student ID PART 1: Matching. Match the organelle to its function (11 points) 1.Proton motive force 2. Fluid Mosiac 3. Oxidative Phosphorylation 4. Pyruvate dehydrogenase 5. Electrochemical Force 6.
Enzymes and Metabolism
 PowerPoint Lecture Slides prepared by Vince Austin, University of Kentucky Enzymes and Metabolism Human Anatomy & Physiology, Sixth Edition Elaine N. Marieb 1 Protein Macromolecules composed of combinations
PowerPoint Lecture Slides prepared by Vince Austin, University of Kentucky Enzymes and Metabolism Human Anatomy & Physiology, Sixth Edition Elaine N. Marieb 1 Protein Macromolecules composed of combinations
We must be able to make glucose
 Biosynthesis of Carbohydrates Synthesis of glucose (gluconeogenesis) Glycogen Formation of pentoses and NADPH Photosynthesis We must be able to make glucose Compulsory need for glucose (above all the brain)
Biosynthesis of Carbohydrates Synthesis of glucose (gluconeogenesis) Glycogen Formation of pentoses and NADPH Photosynthesis We must be able to make glucose Compulsory need for glucose (above all the brain)
How to Integrate Cellular Metabolism Assays Into Your Research: Considerations and Challenges
 How to Integrate Cellular Metabolism Assays Into Your Research: Considerations and Challenges Donna Leippe Sr Research Scientist Outline for Today s Webinar Introduction to Cell Metabolism Metabolite Detection
How to Integrate Cellular Metabolism Assays Into Your Research: Considerations and Challenges Donna Leippe Sr Research Scientist Outline for Today s Webinar Introduction to Cell Metabolism Metabolite Detection
2) The molecule that functions as the reducing agent (electron donor) in a redox or oxidationreduction
 Campbell Biology in Focus (Urry) Chapter 7 Cellular Respiration and Fermentation 7.1 Multiple-Choice Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex
Campbell Biology in Focus (Urry) Chapter 7 Cellular Respiration and Fermentation 7.1 Multiple-Choice Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex
Electron Transport and Oxidative. Phosphorylation
 Electron Transport and Oxidative Phosphorylation Electron-transport chain electron- Definition: The set of proteins and small molecules involved in the orderly sequence of transfer to oxygen within the
Electron Transport and Oxidative Phosphorylation Electron-transport chain electron- Definition: The set of proteins and small molecules involved in the orderly sequence of transfer to oxygen within the
Oxidative Phosphorylation
 Electron Transport Chain (overview) The NADH and FADH 2, formed during glycolysis, β- oxidation and the TCA cycle, give up their electrons to reduce molecular O 2 to H 2 O. Electron transfer occurs through
Electron Transport Chain (overview) The NADH and FADH 2, formed during glycolysis, β- oxidation and the TCA cycle, give up their electrons to reduce molecular O 2 to H 2 O. Electron transfer occurs through
Plant Respiration. Exchange of Gases in Plants:
 Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
What s the point? The point is to make ATP! ATP
 2006-2007 What s the point? The point is to make ATP! ATP Glycolysis 2 ATP Kreb s cycle 2 ATP Life takes a lot of energy to run, need to extract more energy than 4 ATP! There s got to be a better way!
2006-2007 What s the point? The point is to make ATP! ATP Glycolysis 2 ATP Kreb s cycle 2 ATP Life takes a lot of energy to run, need to extract more energy than 4 ATP! There s got to be a better way!
3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]
![3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]](/thumbs/72/66350985.jpg) 3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
Metabolism of cardiac muscle. Dr. Mamoun Ahram Cardiovascular system, 2013
 Metabolism of cardiac muscle Dr. Mamoun Ahram Cardiovascular system, 2013 References This lecture Mark s Basic Medical Biochemistry, 4 th ed., p. 890-891 Hand-out Why is this topic important? Heart failure
Metabolism of cardiac muscle Dr. Mamoun Ahram Cardiovascular system, 2013 References This lecture Mark s Basic Medical Biochemistry, 4 th ed., p. 890-891 Hand-out Why is this topic important? Heart failure
Cellular Respiration Harvesting Chemical Energy ATP
 Cellular Respiration Harvesting Chemical Energy ATP 2006-2007 What s the point? The point is to make ATP! ATP 2006-2007 Harvesting stored energy Energy is stored in organic molecules carbohydrates, fats,
Cellular Respiration Harvesting Chemical Energy ATP 2006-2007 What s the point? The point is to make ATP! ATP 2006-2007 Harvesting stored energy Energy is stored in organic molecules carbohydrates, fats,
XF Data Normalization by the Agilent Seahorse XF Imaging and Normalization System
 Application Note Normalization XF Data Normalization by the Agilent Seahorse XF Imaging and Normalization System Authors Yoonseok Kam Kellie Chadwick Ned Jastromb Brian P. Dranka Agilent Technologies,
Application Note Normalization XF Data Normalization by the Agilent Seahorse XF Imaging and Normalization System Authors Yoonseok Kam Kellie Chadwick Ned Jastromb Brian P. Dranka Agilent Technologies,
ANSC/NUTR 618 Lipids & Lipid Metabolism
 I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
CH 7: Cell Respiration and Fermentation Overview. Concept 7.1: Catabolic pathways yield energy by oxidizing organic fuels
 CH 7: Cell Respiration and Fermentation Overview Living cells require energy from outside sources Some animals obtain energy by eating plants, and some animals feed on other organisms Energy flows into
CH 7: Cell Respiration and Fermentation Overview Living cells require energy from outside sources Some animals obtain energy by eating plants, and some animals feed on other organisms Energy flows into
Cell Metabolism Assays. for OMICS. Research
 Gene-Protein-metabolism links Cell Metabolism Assays for OMICS Research STANDARD Parameters of Functional METABOLIsm Genomics Cells use gene expression to synthesize proteins and other products that are
Gene-Protein-metabolism links Cell Metabolism Assays for OMICS Research STANDARD Parameters of Functional METABOLIsm Genomics Cells use gene expression to synthesize proteins and other products that are
Biology 30 Structure & Function of Cells (Part 2) Bioenergetics: Energy: Potential energy: Examples: Kinetic energy. Examples:
 Biology 30 Structure & Function of Cells (Part 2) Bioenergetics: Energy: Potential energy: Examples: Kinetic energy Examples: Energy can be transformed: Thermodynamics: First law of Thermodynamics: Second
Biology 30 Structure & Function of Cells (Part 2) Bioenergetics: Energy: Potential energy: Examples: Kinetic energy Examples: Energy can be transformed: Thermodynamics: First law of Thermodynamics: Second
Supporting Information
 Supporting Information Identification of Novel ROS Inducers: Quinone Derivatives Tethered to Long Hydrocarbon Chains Yeonsun Hong,, Sandip Sengupta,, Wooyoung Hur, *, Taebo Sim,, * KU-KIST Graduate School
Supporting Information Identification of Novel ROS Inducers: Quinone Derivatives Tethered to Long Hydrocarbon Chains Yeonsun Hong,, Sandip Sengupta,, Wooyoung Hur, *, Taebo Sim,, * KU-KIST Graduate School
Metabolism of Herpes Simplex Virus 1 infected RAW macrophages
 Metabolism of Herpes Simplex Virus 1 infected RAW 264.7 macrophages A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By Mary Kathryn Jenkins B.S., Bowling
Metabolism of Herpes Simplex Virus 1 infected RAW 264.7 macrophages A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By Mary Kathryn Jenkins B.S., Bowling
Metabolism. Metabolic pathways. BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 11: Metabolic Pathways
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 11: Metabolic Pathways http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Metabolism Metabolism is the chemical change of
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 11: Metabolic Pathways http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Metabolism Metabolism is the chemical change of
Investigating the Effect of Energy Substrates and LPS-activation on the In Vitro Energy Metabolism of BV-2, RAW264.
 Investigating the Effect of Energy Substrates and LPS-activation on the In Vitro Energy Metabolism of BV-2, RAW264.7 and VM-M3 Cells Author: Ashley Kaye Brown Persistent link: http://hdl.handle.net/2345/bc-ir:106811
Investigating the Effect of Energy Substrates and LPS-activation on the In Vitro Energy Metabolism of BV-2, RAW264.7 and VM-M3 Cells Author: Ashley Kaye Brown Persistent link: http://hdl.handle.net/2345/bc-ir:106811
Introduction. Living is work. To perform their many tasks, cells must bring in energy from outside sources.
 Introduction Living is work. To perform their many tasks, cells must bring in energy from outside sources. In most ecosystems, energy enters as sunlight. Light energy trapped in organic molecules is available
Introduction Living is work. To perform their many tasks, cells must bring in energy from outside sources. In most ecosystems, energy enters as sunlight. Light energy trapped in organic molecules is available
Biosynthesis of Fatty Acids. By Dr.QUTAIBA A. QASIM
 Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
ab Glycolysis Stress Test Companion Assay
 Version 1 Last updated 12 July 2017 ab222945 Glycolysis Stress Test Companion Assay For the characterization of glycolytic stress in live cells when used in combination with Glycolysis Assay [Extracellular
Version 1 Last updated 12 July 2017 ab222945 Glycolysis Stress Test Companion Assay For the characterization of glycolytic stress in live cells when used in combination with Glycolysis Assay [Extracellular
Cellular Metabolism 6/20/2015. Metabolism. Summary of Cellular Respiration. Consists of all the chemical reactions that take place in a cell!
 Cellular Metabolism Biology 105 Lecture 6 Chapter 3 (pages 56-61) Metabolism Consists of all the chemical reactions that take place in a cell! Cellular metabolism: Aerobic cellular respiration requires
Cellular Metabolism Biology 105 Lecture 6 Chapter 3 (pages 56-61) Metabolism Consists of all the chemical reactions that take place in a cell! Cellular metabolism: Aerobic cellular respiration requires
Chapter 9. Cellular Respiration and Fermentation
 Chapter 9 Cellular Respiration and Fermentation Energy flows into an ecosystem as sunlight and leaves as heat Photosynthesis generates O 2 and organic molecules, which are used in cellular respiration
Chapter 9 Cellular Respiration and Fermentation Energy flows into an ecosystem as sunlight and leaves as heat Photosynthesis generates O 2 and organic molecules, which are used in cellular respiration
RESPIRATION IN PLANTS
 7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a Cytochrome b Oxygen c Hydrogen d Glucose 2. Phosphorylation
7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a Cytochrome b Oxygen c Hydrogen d Glucose 2. Phosphorylation
Supplementary Table 1 Gene clone ID for ShRNA-mediated gene silencing TNFα downstream signals in in vitro Symbol Gene ID RefSeqID Clone ID
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 Supplementary Table 1 Gene clone ID for ShRNA-mediated gene silencing TNFα downstream
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 Supplementary Table 1 Gene clone ID for ShRNA-mediated gene silencing TNFα downstream
Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz )
 Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz ) Biochemistry Submitted by Marie Havlová on 8. February 2012-0:00 Syllabus of Biochemistry Mechanisms of enzyme catalysis.
Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz ) Biochemistry Submitted by Marie Havlová on 8. February 2012-0:00 Syllabus of Biochemistry Mechanisms of enzyme catalysis.
10/25/2010 CHAPTER 9 CELLULAR RESPIRATION. Life is Work. Types of cellular respiration. Catabolic pathways = oxidizing fuels
 CHAPTER 9 CELLULAR RESPIRATION Life is Work Living cells require transfusions of energy from outside sources to perform their many tasks: Chemical work Transport work Mechanical work Energy stored in the
CHAPTER 9 CELLULAR RESPIRATION Life is Work Living cells require transfusions of energy from outside sources to perform their many tasks: Chemical work Transport work Mechanical work Energy stored in the
