RIC-7 Promotes Neuropeptide Secretion
|
|
|
- Samantha Garrett
- 5 years ago
- Views:
Transcription
1 Yingsong Hao 1,2, Zhitao Hu 1,2, Derek Sieburth 3, Joshua M. Kaplan 1,2 * 1 Department of Molecular Biology, Massachusetts General Hospital, Boston, Massachusetts, United States of America, 2 Department of Neurobiology, Harvard Medical School, Boston, Massachusetts, United States of America, 3 Zilkha Neurogenetic Institute, University of Southern California, Los Angeles, California, United States of America Abstract Secretion of neurotransmitters and neuropeptides is mediated by exocytosis of distinct secretory organelles, synaptic vesicles (SVs) and dense core vesicles (DCVs) respectively. Relatively little is known about factors that differentially regulate SV and DCV secretion. Here we identify a novel protein RIC-7 that is required for neuropeptide secretion in Caenorhabditis elegans. The RIC-7 protein is expressed in all neurons and is localized to presynaptic terminals. Imaging, electrophysiology, and behavioral analysis of ric-7 mutants indicates that acetylcholine release occurs normally, while neuropeptide release is significantly decreased. These results suggest that RIC-7 promotes DCV mediated secretion. Citation: Hao Y, Hu Z, Sieburth D, Kaplan JM (2012) RIC-7 Promotes Neuropeptide Secretion. PLoS Genet 8(1): e doi: /journal.pgen Editor: Andrew D. Chisholm, University of California San Diego, United States of America Received July 8, 2011; Accepted November 18, 2011; Published January 19, 2012 Copyright: ß 2012 Hao et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was supported by NIH research grant DK80215 (JMK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing Interests: The authors have declared that no competing interests exist. * kaplan@molbio.mgh.harvard.edu Introduction Neurons secrete both neuropeptides and neurotransmitters. Neurotransmitters, such as acetylcholine (ACh), are secreted by exocytosis of small clear synaptic vesicles (SVs) whereas neuropeptide secretion is mediated by exocytosis of dense core vesicles (DCVs) [1,2]. The mechanisms leading to DCV and SV exocytosis are similar in many respects. SVs and DCVs both undergo physical docking to the plasma membrane, requiring Munc18 and syntaxin for docking in both cases [3 7]. To become fusion competent, SVs and DCVs must both undergo a priming reaction, which is mediated by priming factors (e.g. Munc13 and CAPS) [8,9]. Exocytosis of SVs and DCVs are both mediated by assembling complexes between vesicular and plasma membrane SNARE proteins [10,11]. Finally, calcium-evoked fusion of SVs and DCVs are mediated by distinct calcium sensors, which are thought to be different synaptotagmin isoforms [12]. Beyond these similarities, DCVs and SVs exhibit many important differences. DCVs can be found all along the cell body, dendrites and axons of neurons whereas SVs cluster specifically at active zones of synapses [13]. SVs undergo repeated cycles of exo- and endocytosis at synapses, whereas neuropeptides are only packaged into nascent DCVs in the Golgi [14]. Consequently, DCVs cannot undergo local recycling in axons or dendrites. DCVs release their contents over long timescales (.50 ms) while SV exocytosis occurs more rapidly (,20 ms) [13,15]. Exocytosis of SVs can be evoked by single action potentials while DCV release typically occurs after more prolonged or repeated depolarizations. These differences imply that different molecules are involved in SV and DCV secretion. To date, very few proteins have been found that are specifically involved in the secretion of one or the other class of vesicles. UNC- 31/CAPS (Calcium-dependent Activator Protein for Secretion) is proposed to promote priming of DCVs but not SVs [16 18]. However, a subsequent study showed compelling evidence for SV priming defects in CAPS1 and CAPS2 double knockout mice [19], implying that CAPS is also required for SV priming. Similarly, some studies propose that Munc13 primes SVs but not DCVs [18], while others find Munc13 mutants have exocytosis defects for both SVs and DCVs [20,21]. C. elegans mutants lacking PKC-1, a PKCe ortholog, had significant defects in DCV release but little effect on SV release [20]. Identifying new genes that differentially regulate SV or DCV release will provide new insights into the mechanisms underlying these two forms of secretion. In C. elegans, the acetylcholinesterase inhibitor aldicarb has been widely used to study neuromuscular signaling in live animals. Aldicarb treatment causes acetylcholine (ACh) to accumulate in the synaptic cleft at NMJs, resulting in an acute paralysis of treated animals. Mutations or RNAi treatments that reduce ACh release confer resistance to aldicarb-induced paralysis, whereas those that stimulate ACh secretion enhance aldicarb sensitivity [22,23,24]. We previously showed that neuropeptides also regulate aldicarb responsiveness [24,25]. Inactivation of genes encoding proneuropeptide processing enzymes [for example, egl-3 prohormone convertase (PC2), egl-21 carboxypeptidase E (CPE), sbt-1 7B2, and nep-1 neprilysin], proneuropeptides (ins-22, ins-31, flp-1, nlp-12), neuropeptide receptors (fshr-1) all cause aldicarb resistance [24,25]. These results suggested that new genes that are required for DCV secretion could be identified through screens for aldicarb resistant mutants. In a screen for mutations that suppress the aldicarb hypersensitivity of dgk-1 diacylglycerol kinase (DAGK) mutants, we isolated a new allele of the ric-7 gene. Here we show that ric-7 encodes a novel nematode specific protein that is required for neuropeptide secretion. Results RIC-7 functions in cholinergic neurons for aldicarb responsiveness To identify new genes required for neuromuscular function, we screened for mutations that suppress the aldicarb hypersensitivity PLoS Genetics 1 January 2012 Volume 8 Issue 1 e
2 Author Summary Neuropeptides produce prolonged changes in circuit activity that are associated with changes in behavioral states (e.g. mood or appetite); consequently, there is great interest in identifying molecules that are required for neuropeptide secretion. Here we show that a novel neuronal protein RIC-7 promotes neuropeptide secretion in C. elegans but has only subtle effects on neurotransmitter secretion. RIC-7 is conserved in several other nematodes; however, homologous proteins are not found in other sequenced genomes. These results suggest that the machinery responsible for neuropeptide secretion evolved more recently than factors that are required for both neurotransmitter and neuropeptide secretion. defect of dgk-1 DAGK mutants. One suppressor (nu447) significantly decreased the aldicarb hypersensitivity of dgk-1 mutants. The nu447 mutation mapped close to the ric-7 gene, which was identified in prior screens for aldicarb resistant mutants [22]. We found that nu447 and ric-7(n2657) both correspond to mutations in F58E10.1 gene (Figure 1A), hereafter referred to as the ric-7 gene. The ric-7 locus encodes two isoforms (A and B) that differ only in their first exon. Orthologs of ric-7 are observed in several other nematodes, but homologous genes are not detected in other metazoans. The predicted RIC-7 protein does not contain any previously described structural domains. Animals homozygous for nu447 or n2657 were resistant to the paralytic effects of aldicarb (Figure 1B). To determine whether RIC-7 functions in motor neurons for aldicarb responsiveness, we constructed a ric-7 transcriptional reporter. The resulting construct expressed GFP in many neurons, including both cholinergic and GABAergic motor neurons (Figure 2A 2B). Transgenes expressing either RIC-7A or B isoforms in all neurons (with the snb-1 promoter), and those expressing RIC-7B in cholinergic neurons (with the unc-17 promoter) rescued the aldicarb sensitivity defect of ric-7 mutants (Figure 1B). By contrast, expressing RIC-7B in GABAergic neurons (unc-25 promoter) or in muscles (myo-3 promoter) did not rescue the aldicarb phenotype of ric-7 mutants (Figure 1B). These results suggest that RIC-7 activity is required in cholinergic neurons for aldicarb responsiveness. Animals lacking ric-7 also had decreased locomotion rates (Figure 1C). This locomotion defect was fully rescued by ric-7 transgenes expressed in all neurons (using the snb-1 promoter) whereas partial rescue was observed with transgenes expressed in cholinergic or GABAergic neurons (Figure 1C). The morphology of motor neuron axons and NMJs appeared superficially normal in ric-7 mutants (Figure 4C and data not shown), suggesting that these motor defects were unlikely to be caused by changes in neural development. RIC-7 is targeted to presynaptic terminals We expressed GFP-tagged RIC-7 constructs in the cholinergic DA neurons (using the unc-129 promoter). The RIC-7::GFP protein was localized in a punctate distribution in both cell bodies and dorsal cord axons. The majority of RIC-7 puncta co-localized with an SV marker (mcherry-tagged Endophilin) [26], suggesting that RIC-7 is targeted to synapses (Figure 2C). We also compared the distribution of GFP-tagged RIC-7 with a mcherry-tagged neuropeptide, NLP-21. Whereas RIC-7 showed partial overlap with endophilin, nearly complete co-localization was observed with NLP-21 (Figure 2C). Several results suggest that the co-localization of RIC-7 with DCVs was not mediated by physical association of RIC-7 with nascent DCVs. First, RIC-7 synaptic localization was Figure 1. Aldicarb response and locomotion defects in ric-7 mutants. (A) A schematic illustrating the ric-7 gene structure is shown. Deleted regions (bar), point mutations (arrows), and the site utilized for GFP/mCherry tagging are indicated. (B C) Aldicarb-induced paralysis (B) and locomotion rate (C) are compared for the indicated genotypes. The number of trials (,20 animals/trial, B) or the number of animals analyzed (C) are indicated for each genotype. For rescue experiments, ric-7 transgenes are indicated as follows: ACh (unc-17 promoter), GABA (unc-25 promoter), pan-neuron (snb-1 promoter), muscle (myo-3 promoter). Values that differ significantly from wild type (**, p,0.001, Student s t-test) and from ric-7 mutants (#, p,0.01; ##, p,0.001, Student s t-test) are indicated. Error bars indicate SEM. doi: /journal.pgen g001 not disrupted in unc-104 mutants (Figure 2D), which lack the KIF1A motor responsible for anterograde transport of SVs and DCVs. Thus, RIC-7 is not co-transported to synapses with immature PLoS Genetics 2 January 2012 Volume 8 Issue 1 e
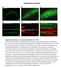
3 Figure 2. Expression pattern of RIC-7. (A) The ric-7 promoter expresses nuclear localized Cherry (HIS-24::wCherry) primarily in the nervous system of an adult worm. Anterior is left; ventral is up. The asterisk indicates fluorescence encoded by a co-injection marker. (B) The ric-7 promoter is expressed in cholinergic (top panel) and GABAergic (bottom panel) motor neurons in the ventral cord. Cell bodies of cholinergic (unc-17 promoter) and GABAergic (unc-30 promoter) neurons were identified by expression of the indicated GFP reporter constructs. (C) Distribution of RIC-7, a synaptic vesicle marker (UNC-57 Endophilin) (top panel), and a DCV marker (NLP-21) (bottom panel) are compared in the dorsal cord axons of cholinergic motor neurons. (D) Distribution of RIC-7::GFP in cholinergic motor neurons of unc-104 KIF1A mutants. Cell bodies (arrow) and ventral cord processes (arrow heads) are indicated. doi: /journal.pgen g002 DCVs or SVs. Second, RIC-7 lacks a predicted signal peptide sequence and GFP-tagged RIC-7 did not produce fluorescence in coelomocytes, both of which indicate that RIC-7 is not translocated into SVs or DCVs (data not shown). These results support the idea that RIC-7 functions in the cytoplasm at presynapses and could play a relatively direct role in regulating SV or DCV secretion. Muscle responsiveness to ACh and GABA is unaltered in ric-7 mutants We did several experiments to test the effects of RIC-7 on the responsiveness of body muscles to neuromuscular agonists (Figure 3). First, the sensitivity of ric-7 mutants to the paralytic effects of the nicotinic agonist levamisole was similar to that of wild type controls (Figure 3A). Second, we recorded body wall muscle currents evoked by application of ACh or the GABA agonist muscimol. In both cases, the amplitude of agonist-evoked current in ric-7 mutant body muscles was not significantly different from that observed in wild type controls (Figure 3B). Third, the fluorescent intensities of GFP-tagged ACR-16 ACh receptor (Figure 3C) and UNC-49 GABA A receptor (Figure 3D) puncta in the nerve cord were unaltered in ric-7 mutants, indicating that the abundance of post-synaptic receptors at NMJs was normal. Thus, the effect of RIC-7 on aldicarb responsiveness is unlikely to be caused by altered agonist responsiveness of body muscles. ACh release occurs normally in ric-7 mutants The aldicarb resistance phenotype observed in ric-7 mutants could be caused by decreased ACh secretion, increased GABA secretion, or decreased neuropeptide secretion, as aldicarb resistance would be expected in all three scenarios [25,27,28]. To assay ACh secretion more directly, we recorded excitatory post-synaptic currents (EPSCs) from body muscles (Figure 4). We found that the rate and amplitude of endogenous EPSCs, i.e. SV fusions evoked by the endogenous activity of motor neurons, in ric- 7 mutants were similar to those found in wild type animals (Figure 4A). In addition, the amplitude of stimulus-evoked EPSCs in ric-7 mutants was not significantly different from wild type (Figure 4B). Therefore, baseline ACh secretion occurs normally at cholinergic NMJs in ric-7 mutants. To further analyze the cholinergic NMJs, we examined the distribution of GFP-tagged Synaptobrevin (GFP::SNB-1) in motor neurons. Changes in the distribution of GFP::SNB-1 are correlated with changes in SV exo- and endocytosis. SNB-1 puncta intensity is correlated with the number of SVs at PLoS Genetics 3 January 2012 Volume 8 Issue 1 e
4 Figure 3. Body muscle responses to ACh and GABA are unaltered in ric-7 mutants. (A) Time course of levamisole (200 mm) induced paralysis is shown for wild type and ric-7(nu447) adults. Three trials (,20 animals/trial) were performed for each genotype. (B) ACh (top) and Muscimol (bottom)-activated currents were recorded from body wall muscles of ric-7 and wild type adults. Representative responses (left) and summary data (right) are shown. Wild type and ric-7 mutant responses were not significantly different. (C D) Representative images (above) and summary data (below) are shown for ACR-16::GFP (C) and UNC-49::GFP (D) expressed in body muscles of wild type and ric-7 adults (using the myo-3 promoter). No significant differences were observed. The number of animals analyzed is indicated for each genotype (panels B D). Error bars indicate SEM. doi: /journal.pgen g003 presynaptic elements [24,29] whereas puncta density is a measure of synapse density. Neither the fluorescent intensity nor the density of SNB-1 puncta in the cholinergic axons of ric-7 mutants were significantly different from that observed in wild type controls (Figure 4C). Taken together, these results suggest that defects in baseline ACh secretion are unlikely to account for the aldicarb resistance of ric-7 mutants. RIC-7 promotes neuropeptide secretion Aldicarb resistance could also be caused by defects in neuropeptide signaling [25]; therefore, we next examined ric-7 mutants for changes in neuropeptide signaling. First, we analyzed the aldicarb responsiveness of ric-7 double mutants containing mutations in neuropeptide signaling components. If changes in neuropeptide action contribute to RIC-7 s effects on aldicarb responsiveness, then we would expect that ric-7 mutations would occlude the effect of neuropeptide signaling mutations on aldicarb resistance. Consistent with this idea, ric-7 double mutants carrying mutations in either of two proneuropeptide processing enzymes (egl-21 CPE and egl-3 PC2) had aldicarb resistance that was similar to that observed in ric-7 single mutants (Figure 5). These results support the idea that RIC-7 regulation of neuropeptide secretion PLoS Genetics 4 January 2012 Volume 8 Issue 1 e
5 Figure 4. Baseline ACh release is unaltered in ric-7 mutants. Endogenous EPSCs (A) and stimulus-evoked EPSCs (B) were recorded from body wall muscles of wild type and ric-7(nu447) adults. Representative traces of endogenous EPSCs (A), averaged traces of stimulus-evoked responses (B), and summary data for both are shown. The number of animals analyzed is indicated for each genotype. No significant differences were observed. (C) Representative images (left) and summary data (right) are shown for GFP-tagged SNB-1 in dorsal cord axons of cholinergic motor neurons (expressed with the unc-129 promoter) in wild type and ric-7 adults. The number of animals analyzed is indicated for each genotype. No significant differences were observed. Error bars indicate SEM. doi: /journal.pgen g004 contributes to the effects of ric-7 mutations on aldicarb responsiveness. To further address the role of RIC-7 in neuropeptide secretion, we analyzed the secretion of YFP-tagged neuropeptides (Figure 6). For this analysis, we selected two proneuropeptides, NLP-21 and INS-22, which encode FMRFamide related peptides (FaRPs) and an insulin-like growth factor, respectively. When NLP-21::YFP or INS-22::YFP are expressed in the cholinergic DA motor neurons (using the unc-129 promoter), puntate fluorescence is detected in dorsal cord axons and in coelomocytes (Figure 6A and 6C). We previously showed that the axonal puncta fluorescence corresponds to secretory granules containing these proneuropeptides while the coelomocyte fluorescence corresponds to secreted neuropeptides that have been endocytosed [20,30]. In ric-7 mutants, NLP-21 coelomocyte fluorescence was significantly decreased (,50%, p,0.001) (Figure 6C 6D) whereas the NLP- 21 puncta fluorescence intensity in dorsal cord axons was significantly increased (.2-fold, p,0.001) (Figure 6A 6B). The coelomocyte and axonal NLP-21 fluorescence defects were both rescued by ric-7 transgenes expressed in the DA neurons. Similar PLoS Genetics 5 January 2012 Volume 8 Issue 1 e
6 Figure 5. Neuropeptide processing mutations and ric-7 mutations do not have additive effects on aldicarb responses. The paralytic response to aldicarb treatment was analyzed in strains containing mutations that inactivate pro-neuropeptide processing enzymes (egl-3 PC2 and egl-21 CPE), or those inactivating RIC-7. The number of trials (,20 animals/trial) is shown for each genotype. Values that differ significantly from wild type (*, p,0.01; **, p,0.001, Students t-test) are indicated. Error bars indicate SEM. Values that are not significantly different are indicated (ns). doi: /journal.pgen g005 changes in axonal puncta fluorescence and coelomocyte fluorescence was observed for a second proneuropeptide (INS-22) in ric-7 mutants (Figure 6). These results suggest that ric-7 mutants have decreased neuropeptide secretion, which results in an accumulation of DCVs in motor axons. RIC-7 is required for NLP-12 mediated enhancement of ACh release Inactivation of NLP-21 and INS-22 by RNAi does not produce strong locomotion or aldicarb resistance defects [24]; consequently, NLP-21 and INS-22 are unlikely to be the only neuropeptides involved in the Ric-7 locomotion and aldicarb-resistance phenotypes. We recently identified NLP-12 as a neuropeptide that plays a critical role in regulating both locomotion rate and aldicarbinduced paralysis [31]. NLP-12 is expressed in a proprioceptive neuron (DVA) that is activated by body muscle contractions [32,33]. If Ric-7 aldicarb resistance and locomotion defects are caused by decreased neuropeptide release, we would expect that NLP-12 secretion from DVA would be diminished in ric-7 mutants. We did several experiments to test this idea. First, we analyzed the effect of ric-7 mutations on secretion of YFP-tagged NLP-12 from DVA neurons (Figure 7A 7B). In wild type animals, aldicarb treatment significantly decreased NLP-12 puncta fluorescence in DVA axons (indicating increased NLP-12 secretion), which is most likely caused by activation of DVA stretch receptors by muscle contraction [31]. By contrast, in ric-7 mutants, aldicarb had no effect on NLP-12 puncta fluorescence, indicating that RIC-7 is required for aldicarb-evoked NLP-12 secretion. Second, if the NLP-12 secretion defect contributes to the Ric-7 aldicarb resistance phenotype, we would expect that RIC-7 expression in DVA neurons would be sufficient to alter aldicarb responsiveness. Consistent with this idea, the aldicarb responsiveness of ric-7 mutants was significantly improved by transgenes expressing RIC-7 in DVA neurons (Figure 7C). This result is consistent with the preceding rescue data (Figure 1B) because the unc-17 promoter (which also rescued the Ric-7 aldicarb defect) is expressed in DVA neurons (data not shown). Collectively, these data suggest that proper aldicarb responsiveness requires RIC-7 function in multiple neuron classes because RIC-7 expression in a single cholinergic neuron (DVA) produced partial rescue of the aldicarb defect whereas complete rescue was obtained following expression in all cholinergic neurons (with the unc-17 promoter) (Figure 1B). Third, we analyzed evoked ACh release following aldicarb treatment. In wild type animals, a 60 minute pre-treatment with aldicarb significantly increases the total synaptic charge occurring during an evoked response (Figure 7D 7E) [31]. This effect is eliminated in egl-3 PC2 mutants and in nlp-12 mutants [31]. Thus, aldicarb enhancement of evoked ACh release can be utilized to assess changes in endogenous NLP-12 secretion. Aldicarb s effect on evoked ACh release was also eliminated in ric-7 mutants (Figure 7D 7E). Collectively, these results strongly support the idea that RIC-7 acts in DVA neurons to promote secretion of endogenous NLP-12, and that this contributes to the Ric-7 aldicarb-resistance defect. GABA transmission is altered in ric-7 mutants Changes in GABA secretion could also contribute to the aldicarb resistance observed in ric-7 mutants [22,27,28]. Consistent with this idea, ric-7 mutants had defects in defecation behavior that are similar to those observed in mutants with decreased GABA transmission (Figure 8A). Contraction of the intestinal muscles during defecation (i.e. the expulsion step of the defecation motor program) is mediated by excitatory GABAergic input from defecation motor neurons [34]. We found that ric-7 mutants had a significant expulsion defect, which was rescued by ric-7 transgenes expressed in GABA neurons (Figure 8A). These results suggest that ric-7 mutants have a presynaptic defect at GABAergic NMJs involved in defecation. To assay GABA release at ventral cord NMJs (which are involved in locomotion), we recorded inhibitory post-synaptic currents (IPSCs) from body muscles (Figure 8B). We found that ric- 7 mutants had a wild type IPSC rate, while IPSC amplitudes were significantly increased (,30%, p,0.001). These results suggest that GABA secretion still occurs in ric-7 mutants, albeit in a subtly altered form. Changes in IPSC amplitudes are often caused by changes in post-synaptic sensitivity, e.g. by changing GABA receptor abundance. However, neither the current evoked by applying an exogenous GABA agonist nor the abundance of GFPtagged UNC-49 GABA-A receptors were altered in ric-7 mutants, suggesting that a post-synaptic defect was unlikely to account for the altered IPSC amplitude (Figure 3B, 3D). Consistent with a presynaptic defect, the distribution of GFP-tagged SNB-1 was altered in GABAergic neurons of ric-7 mutants. Although SNB-1 puncta intensity was unaltered, puncta were significantly wider (23% wider, p,0.001) and diffuse SNB-1 axon fluorescence was significantly increased in ric-7 mutants (Figure 8C). Finally, the IPSC and SNB-1 defects were both rescued by transgenes expressing RIC-7 in GABAergic motor neurons (Figure 8B 8C). Collectively, these results support the idea that ric-7 mutants have a pre-synaptic defect that increases GABA secretion, perhaps by increasing the amount of GABA packaged into each SV. Rescue of the IPSC defect (with ric-7 transgenes expressed in GABA neurons) failed to rescue the ric-7 aldicarb defect and weakly rescued the locomotion defect implying that the latter were not caused by altered GABA secretion (Figure 1C). Mutations disrupting neuropeptide signaling exhibit defecation defects similar to ric-7 mutants [25,35]. Prompted by these results, we wondered if the Ric-7 defecation and IPSC defects are caused by the neuropeptide secretion defect. In this scenario, we would expect that ric-7 mutations and mutations that impair proneur- PLoS Genetics 6 January 2012 Volume 8 Issue 1 e
7 Figure 6. RIC-7 promotes neuropeptide release. YFP-tagged NLP-21 and INS-22 were expressed in cholinergic motor neurons using the unc-129 promoter. Representative images (A) and summary data (B) are shown for NLP-21 (top) and INS-22 (bottom) fluorescence in dorsal cord axons of the indicated genotypes. The number of animals analyzed is indicated for each genotype. (C D) Representative images (C) and summary data (D) are shown for NLP-21 and INS-22 fluorescence in coelomocytes of the indicated genotypes. The number of animals analyzed is indicated for each genotype. Values that differ significantly from wild type (**, p,0.001, ***, p, Students t-test) and from ric-7 mutants (#, p,0.01, ##, p,0.001, Students t-test) are indicated. Error bars indicate SEM. For rescue experiments, ric-7 transgenes are as follows: pan-neuron (snb-1 promoter), DA neuron (unc-129 promoter). doi: /journal.pgen g006 opeptide processing would not have additive effects on defecation behavior in double mutants. Contrary to this idea, the defecation defects observed in ric-7; egl-21 double mutants were significantly worse than those observed in either single mutant (Figure 8A). In addition, IPSC amplitudes were unaltered in egl-3; egl-21 doubles mutants (Figure S1), suggesting that decreased neuropeptide secretion is also unlikely to explain the Ric-7 IPSC defect. These results indicate that RIC-7 cell-autonomously regulates two forms of secretion, promoting neuropeptide secretion and inhibiting GABA secretion. Discussion Communication between neurons and their targets relies on two classes of neurosecretory vesicles, synaptic vesicles (SVs) and dense-core vesicles (DCVs). The mechanisms governing SV and PLoS Genetics 7 January 2012 Volume 8 Issue 1 e
8 Figure 7. NLP-12 secretion is decreased in ric-7 mutants. (A) YFP-tagged NLP-12 was expressed in DVA neurons (using the nlp-12 promoter). Representative images (A) and summary data (B) are shown for NLP-12 puncta fluorescence in the indicated genotypes. The number of animals analyzed is indicated for each genotype. (C) Aldicarb-induced paralysis was quantified for the indicated genotypes. The number of trials (,20 animals/trial) is indicated for each genotype. (D E) Stimulus evoked EPSCs were recorded from adult body wall muscles of the indicated genotypes. Recordings were done after a 60 minute aldicarb pre-treatment (gray) or in untreated controls (black). Averaged evoked responses (D) and summary data (E) are shown. The number of animals analyzed is indicated for each genotype. Values that differ significantly from wild type (**, p,0.001, ***, p,0.0001, Students t-test) and from ric-7 mutants (##, p,0.001, ###, p, Students t-test) are indicated. Error bars indicate SEM. For rescue experiments, ric-7 transgenes are as follows: DVA (nlp-12 promoter). doi: /journal.pgen g007 DCV secretion share many properties (including related SNARE proteins, SNARE binding proteins, and calcium sensors). Despite these similarities, SV and DCV mediated secretion also have significant differences [1], which imply that some proteins will be selectively utilized in one or the other process. Because neuropeptides have dramatic effects on personality, behavior, and metabolism, there is significant interest in identifying molecules that selectively promote DCV secretion. Here we identify RIC-7 as a novel protein that is required for DCV secretion but has relatively subtle effects on SV secretion. Below we discuss the implications of these findings. The ric-7 gene was identified in a screen for mutations conferring resistance to aldicarb induced paralysis. As RIC-7 lacks identified structural or functional motifs, the mechanisms underlying this behavioral defect were unclear. In principle, aldicarb resistance could arise from several alternative mechanisms, including: altered muscle responsiveness to ACh or GABA, decreased excitatory input from cholinergic motor neurons, increased hyperpolarizing input from GABA motor neurons, or decreased neuropeptide signaling. Our results strongly support the idea that Ric-7 aldicarb and locomotion defects arise from disruption of neuropeptide secretion. Several results suggest that RIC-7 does not regulate muscle sensitivity to neuromuscular agonists. The currents evoked by applying exogenous ACh and GABA to body muscles, and the expression of nicotinic and GABA receptors in body muscles were both unaffected in ric-7 mutants. Furthermore, ric-7 aldicarb and locomotion defects were not corrected by transgenes expressing RIC-7 in body muscles, whereas rescue was observed for transgenes expressed in cholinergic neurons. Thus, RIC-7 neither acts in body muscles, nor regulates muscle sensitivity to agonists. Other results indicate that RIC-7 is not required for baseline ACh secretion. In cholinergic motor neurons, the SV protein SNB-1 neither accumulated in synaptic puncta nor in the axonal membrane (as would occur in exocytosis and endocytosis mutants, respectively). The rate, amplitude, and kinetics of endogenous and evoked EPSCs were unaltered in ric-7 mutants. Thus, although RIC-7 expression in cholinergic neurons rescued the aldicarb and locomotion defects of ric-7 mutants, these defects are unlikely to arise from decreased baseline ACh secretion. PLoS Genetics 8 January 2012 Volume 8 Issue 1 e
9 Figure 8. Analysis of GABA transmission in ric-7 mutants. (A) Intestinal muscle contractions during the defecation motor program (quantified as expulsions/pboc) were analyzed in the indicated genotypes. (B) Endogenous IPSCs were recorded from adult body wall muscles of the indicated genotypes. Representative traces (left), and summary data (right) are shown. The number of animals analyzed is indicated for each genotype. (C) Representative images (left) and summary data (right) for GFP::SNB-1 (expressed by the unc-25 promoter) in dorsal cord axons of the indicated genotypes. The number of animals analyzed is indicated for each genotype. Values that differ significantly from wild type (**, p,0.001, ***, p, Students t-test) and from ric-7 mutants (#, p,0.05, ##, p,0.001, ###, p, Students t-test) are indicated. Error bars indicate SEM. For rescue experiments, ric-7 transgenes are as follows: ACh (unc-17 promoter), GABA (unc-47 promoter), pan-neuron (snb-1 promoter), intestine (vha-6 promoter). doi: /journal.pgen g008 PLoS Genetics 9 January 2012 Volume 8 Issue 1 e
10 In GABA neurons, ric-7 mutants had a presynaptic defect that led to an apparent increase in GABA secretion. This presynaptic defect was manifest by an increase in amplitude of endogenous IPSCs in ric-7 mutants, while the IPSC rate was unaltered. Changes in IPSC amplitudes are often caused by changes in postsynaptic sensitivity, e.g. by changing GABA receptor abundance. However, neither the current evoked by applying an exogenous GABA agonist nor the abundance of GFP-tagged UNC-49 GABA-A receptors were altered in ric-7 mutants, suggesting that a post-synaptic defect was unlikely to account for the altered IPSC amplitude. Consistent with a pre-synaptic defect, SNB-1 axonal fluorescence was significantly increased in ric-7 mutant GABA motor neurons. The defecation, IPSC, and SNB-1 defects were all rescued by transgenes expressing RIC-7 in GABAergic motor neurons. Collectively, these results support the idea that ric-7 mutants have a pre-synaptic defect that increases GABA secretion. Rescue of the IPSC defect did not correlate with rescue of ric-7 aldicarb and locomotion defects, implying that the latter were not caused by altered GABA secretion. In principle, the IPSC defect could cause the Ric-7 defecation defect, for example if increased GABA secretion causes constitutive excitation of the intestinal muscles. Double mutant analysis suggests that decreased neuropeptide secretion is unlikely to account for the Ric-7 IPSC and defecation defects. Consequently, our results are most consistent with the idea that RIC-7 has direct cell autonomous effects on two forms of secretion, promoting neuropeptide secretion and inhibiting GABA secretion. Several other proteins are known to have distinct effects on different neurotransmitter systems. Mouse Munc13-1 knockouts drastically reduce glutamatergic transmission yet have little effect on GABA release [36]. Mouse ELKS2 knockouts have increased GABA release but unaltered glutamate release [37]. Finally, complexin promotes evoked release but inhibits tonic/spontaneous release [38,39]. Several results suggest that the effects of RIC-7 on aldicarb sensitivity were caused by changes in neuropeptide secretion. First, mutations preventing neuropeptide processing (egl-21 CPE, egl-3 PC2) and ric-7 mutations did not have additive effects on aldicarb responsiveness in double mutants. Second, ric-7 mutants had greatly decreased secretion of YFP-tagged neuropeptides (NLP-21 and INS-22) from cholinergic motor neurons. Third, mcherrytagged RIC-7 co-localized extensively with a DCV marker (NLP- 21), implying that RIC-7 could play a relatively direct role in regulating neuropeptide release. Fourth, ric-7 mutants have decreased aldicarb-evoked secretion of NLP-12 from DVA neurons and lack aldicarb-induced potentiation of evoked ACh release (which is mediated by endogenous NLP-12). And fifth, restoring RIC-7 expression in DVA neurons was sufficient to partially rescue the Ric-7 aldicarb-resistance defect. Taken together, these results strongly support the idea that RIC-7 promotes secretion of endogenous neuropeptides, and that this function plays an important role in the Ric-7 aldicarb resistance and locomotion defects. The Ric-7 aldicarb resistance and locomotion defects are more severe than those observed in mutants lacking NLP-12 or those lacking EGL-3 PC2, indicating that additional RIC-7 functions also contribute to these phenotypes. These additional functions could include promoting secretion of other neuropeptides or novel RIC-7 functions that are not yet defined. What aspect of DCV secretion is regulated by RIC-7? Decreased neuropeptide secretion in ric-7 mutants could reflect changes in any aspect of DCV biogenesis, transport, docking, priming, calcium-triggering, or fusion. Several results suggest that RIC-7 is not packaged into DCVs, and consequently cannot play a role in pro-neuropeptide processing. RIC-7 lacks a predicted signal peptide sequence, GFP-tagged RIC-7 is not secreted, and RIC-7 delivery to axons is not prevented in unc-104 KIF1A mutants. Collectively, these results suggest RIC-7 is cytoplasmic, and thus cannot play a direct role in neuropeptide processing. DCV biogenesis and transport also occur normally in ric-7 mutants, as neuropeptide fluorescence in motor axons was increased rather than decreased. These results suggest that RIC- 7 regulates a step that occurs after DCV transport. Given the prominent colocalization of RIC-7 and DCV markers in axons, we speculate the RIC-7 could identify DCV release sites. Because the sequence of RIC-7 does not provide any clues as to its biochemical function, further experiments will be required to determine a more precise function for RIC-7. Understanding the mechanisms underlying RIC-7 s functions will undoubtedly shed light on how DCVs assume their unique properties. The mechanisms governing SV and DCV secretion share many properties, including: SNARE proteins, SNARE binding proteins (e.g. Munc13, Munc18, and CAPS), and calcium sensors (e.g. Synaptotagmin) [40]. These shared mechanisms are highly conserved across eukaryotic phylogeny, suggesting that these core exocytosis components comprise an ancient process. By contrast, RIC-7 orthologs are observed in other nematodes but homologous genes are not detected in other sequenced genomes. Interestingly, other putative DCV secretion factors have similar patterns of conservation. For example, the Rab27 effector granuphilin is conserved in mammals and flies but not in C. elegans, while a second Rab27 effector melanophilin is present in mammals but absent in flies and worms. These results suggest that the mechanisms distinguishing SV and DCV secretion evolved more recently than the more ancient shared secretion factors. Materials and Methods C. elegans strains and drug assays Strains were maintained at 20uC as described [41]. The wildtype reference strain was N2 Bristol. Descriptions of allele lesions can be found at The mutant strains used were: LGIV, egl-21(n476); LGV, egl-3(nr2090), ric-7 (nu447), ric-7(n2657). Acute aldicarb and levamisole assays were performed blind in triplicate on young adult worms as described [42]. The aldicarb (Chem Services) concentrations used ranged from 1 to 2 mm, the levamisole (Sigma) concentration was 200 mm. Locomotion was measured by transferring adults to plates containing fresh (16 h) lawns of HB101 bacteria, letting the worms recover for 30 min and counting the number of body bends of active worms in a 3 min interval. ric-7 mapping and cloning ric-7(nu447) was isolated in an EMS screen for suppressors of the aldicarb hypersensitive phenotype of dgk-1(nu62) mutants (D.S. and J.K., unpublished data). nu447 was mapped by small nucleotide polymorphism (SNP) mapping, based on its aldicarb resistance phenotype. Analysis of 16 nu447/cb4856 recombinants mapped nu447 to LGV, map unit 5,6; Another 62 clones further positioned nu447 to 5.86,6.00 m.u. Seven predicted genes were found in this interval with three of them previously characterized. Sequencing revealed that ric-7(nu447) contained a 830C/T (A277V) point mutation plus a 32 bp deletion (nucleotides ) in F58E10.1b cdna that shifts the reading frame, leading to a truncated RIC-7 protein that contains 305 amino acids. ric- 7(n2657) contained a 621G/A (W207Stop) nonsense mutation in F58E10.1b cdna. nu447 was backcrossed six times and used for most phenotypic analysis in this study. PLoS Genetics 10 January 2012 Volume 8 Issue 1 e
11 Fluorescence microscopy and quantitative analysis All quantitative imaging was done using a Olympus PlanAPO NA objective and an ORCA100 CCD camera (Hamamatsu). Worms were immobilized with 30 mg/ml BDM (Sigma). Image stacks were captured and maximum intensity projections were obtained using Metamorph 7.1 software (Molecular Devices). GFP fluorescence was normalized to the absolute mean fluorescence of 0.5 mm FluoSphere beads (Molecular Probes). For dorsal cord imaging, young adult worms, in which the dorsal cords were oriented toward the objective, were imaged in the region midway between the posterior gonad bend at the tail. Line scans of dorsal cord fluorescence were analyzed in Igor Pro (WaveMetrics) using custom-written software [43,44]. For coelomocyte imaging, the posterior coelomocyte was imaged in young adults [20]. Image stacks were captured and maximum intensity projections were obtained using Metamorph 7.1 software (Molecular Devices). For quantitation, the five brightest vesicles were analyzed for each coelomocyte and the mean fluorescence for each vesicle was logged. For each worm, coelomocyte fluorescence was calculated as the mean of the vesicle values in that animal. All p-values indicated were based on student t-tests. Confocal images were taken using the Olympus FV1000 confocal microscope. Image stacks were captured, and maximum intensity projections were obtained using Metamorph 7.1 software (Universal Imaging). Electrophysiology Electrophysiology was done on dissected adults as previously described [45]. All recording conditions were as described previously [20]. For comparing average electrophysiological values, statistical significance was determined using the Mann- Whitney test or student s t test. RIC-7 constructs and transgenes Transgenic strains were generated by injecting either wild type or ric-7(nu447) mutants with the expression construct (10 25 ng/ ml) mixed with the co-injection markers, KP#1338 (pttx-3::gfp), KP#1480 (pmyo-2::nls-mcherry) or KP#1106 (pmyo-2::nls-gfp), each at 10 ng/ml, using standard methods [46]. The colocalization experiments were done by co-injecting KP#1684 References 1. Burgoyne RD, Morgan A (2003) Secretory granule exocytosis. Physiol Rev 83: Sudhof TC (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27: Weimer RM, Richmond JE, Davis WS, Hadwiger G, Nonet ML, et al. (2003) Defects in synaptic vesicle docking in unc-18 mutants. Nat Neurosci 6: Voets T, Toonen RF, Brian EC, de Wit H, Moser T, et al. (2001) Munc18-1 promotes large dense-core vesicle docking. Neuron 31: Hammarlund M, Palfreyman MT, Watanabe S, Olsen S, Jorgensen EM (2007) Open syntaxin docks synaptic vesicles. PLoS Biol 5: e Hammarlund M, Watanabe S, Schuske K, Jorgensen EM (2008) CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. J Cell Biol 180: de Wit H, Cornelisse LN, Toonen RF, Verhage M (2006) Docking of secretory vesicles is syntaxin dependent. PLoS One 1: e Klenchin VA, Martin TF (2000) Priming in exocytosis: attaining fusioncompetence after vesicle docking. Biochimie 82: Martin TF (2002) Prime movers of synaptic vesicle exocytosis. Neuron 34: Jena BP (2005) Cell secretion and membrane fusion. Domest Anim Endocrinol 29: Sorensen JB (2005) SNARE complexes prepare for membrane fusion. Trends Neurosci 28: Voets T, Moser T, Lund PE, Chow RH, Geppert M, et al. (2001) Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc Natl Acad Sci U S A 98: Edwards RH (1998) Neurotransmitter release: variations on a theme. Curr Biol 8: R (pric-7::his-24 cdna::wcherry) with KP#1685 (punc-30::nls-gfp) or with KP#1686 (punc-17::nls-gfp), respectively; or by injecting KP#1687 (punc-129::ric-7 cdna::gfp) into nuis252 (Punc- 129::mchry::PaGFP::unc-57), or into nuis470 (punc-129::nlp- 21::mCherry), respectively, all at a concentration of 25 ng/ml each. All constructs except KP#1684 were derivatives of ppd49.26 [47]. Two RIC-7 constructs rescued the ric-7(nu447) mutant aldicarb defect: KP#1680 (psnb-1::ric-7), and KP#1681 (punc-17::ric-7). Two RIC-7 constructs did not rescue the ric-7(nu447) mutant aldicarb defect: KP #1682(punc-25::RIC-7), and KP#1683(pmyo- 3::RIC-7). All four constructs used F58E10.1b cdna cloned with NheI and KpnI sites. The previously reported lines used for imaging experiments described are nuis152 (punc-129::gfp::snb-1), nuis183 (punc- 129::NLP-21::VENUS), nuis195 (punc-129::ins-22::venus) [20,24], nuis283 (pmyo-3::unc-49::gfp) (J. Bai and J.K., unpublished), nuis299 (pmyo-3::acr-16::gfp) [48], nuis444 (pnlp-12::nlp-12::ve- NUS) [31] and nuis376 (punc-25::snb-1::gfp). Supporting Information Figure S1 IPSCs are not altered in neuropeptide processing mutants. Endogenous IPSCs were recorded from adult body wall muscles of the indicated genotypes. Representative traces (A), and summary data (B) are shown. The number of animals analyzed is indicated for each genotype. Error bars indicate SEM. No significant differences were observed. (TIF) Acknowledgments We thank the C. elegans Genetic Stock Center (CGC) for strains and Stuart Kim for sharing the wcherry construct. We also thank Erik Jorgensen for sharing unpublished results and members of the Kaplan laboratory for helpful suggestions, reagents, and comments on this manuscript. Author Contributions Conceived and designed the experiments: YH ZH DS JMK. Performed the experiments: YH ZH DS. Analyzed the data: YH ZH. Wrote the paper: YH JMK. 14. Kim T, Gondre-Lewis MC, Arnaoutova I, Loh YP (2006) Dense-core secretory granule biogenesis. Physiology (Bethesda) 21: Bruns D, Jahn R (1995) Real-time measurement of transmitter release from single synaptic vesicles. Nature 377: Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, et al. (2004) CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron 43: Berwin B, Floor E, Martin TF (1998) CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron 21: Speese S, Petrie M, Schuske K, Ailion M, Ann K, et al. (2007) UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J Neurosci 27: Jockusch WJ, Speidel D, Sigler A, Sorensen JB, Varoqueaux F, et al. (2007) CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell 131: Sieburth D, Madison JM, Kaplan JM (2007) PKC-1 regulates secretion of neuropeptides. Nat Neurosci 10: Kang L, He Z, Xu P, Fan J, Betz A, et al. (2006) Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metab 3: Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, et al. (1996) A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci U S A 93: Nguyen M, Alfonso A, Johnson CD, Rand JB (1995) Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 140: Sieburth D, Ch ng Q, Dybbs M, Tavazoie M, Kennedy S, et al. (2005) Systematic analysis of genes required for synapse structure and function. Nature 436: PLoS Genetics 11 January 2012 Volume 8 Issue 1 e
12 25. Jacob TC, Kaplan JM (2003) The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci 23: Bai J, Hu Z, Dittman JS, Pym EC, Kaplan JM (2010) Endophilin functions as a membrane-bending molecule and is delivered to endocytic zones by exocytosis. Cell 143: Vashlishan AB, Madison JM, Dybbs M, Bai J, Sieburth D, et al. (2008) An RNAi screen identifies genes that regulate GABA synapses. Neuron 58: Mullen GP, Mathews EA, Saxena P, Fields SD, McManus JR, et al. (2006) The Caenorhabditis elegans snf-11 gene encodes a sodium-dependent GABA transporter required for clearance of synaptic GABA. Mol Biol Cell 17: Dittman J, Kaplan J (2006) Factors regulating the abundance and localization of Synaptobrevin in the plasma membrane. PNAS 103: Ch ng Q, Sieburth D, Kaplan JM (2008) Profiling synaptic proteins identifies regulators of insulin secretion and lifespan. PLoS Genet 4: e Hu Z, Pym EC, Babu K, Vashlishan Murray AB, Kaplan JM (2011) A neuropeptide-mediated stretch response links muscle contraction to changes in neurotransmitter release. Neuron 71: Janssen T, Meelkop E, Lindemans M, Verstraelen K, Husson SJ, et al. (2008) Discovery of a cholecystokinin-gastrin-like signaling system in nematodes. Endocrinology 149: Li W, Feng Z, Sternberg PW, Xu XZ (2006) A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440: Jorgensen EM (2005) Gaba. WormBook. pp Mahoney TR, Luo S, Round EK, Brauner M, Gottschalk A, et al. (2008) Intestinal signaling to GABAergic neurons regulates a rhythmic behavior in Caenorhabditis elegans. Proc Natl Acad Sci U S A 105: Augustin I, Rosenmund C, Sudhof TC, Brose (1999) Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles [In Process Citation]. Nature 400: Kaeser PS, Deng L, Chavez AE, Liu X, Castillo PE, et al. (2009) ELKS2alpha/ CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron 64: Martin JA, Hu Z, Fenz KM, Fernandez J, Dittman JS (2011) Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr Biol 21: Hobson RJ, Liu Q, Watanabe S, Jorgensen EM (2011) Complexin maintains vesicles in the primed state in C. elegans. Curr Biol 21: Xu T, Xu P (2008) Searching for molecular players differentially involved in neurotransmitter and neuropeptide release. Neurochem Res 33: Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: Lackner MR, Nurrish SJ, Kaplan JM (1999) Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM (2002) Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron 35: Dittman JS, Kaplan JM (2006) Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc Natl Acad Sci U S A 103: Richmond JE, Jorgensen EM (1999) One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci 2: Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. Embo J 10: Fire A (1997) Fire Vector Kit. In Addgene Inc Cambridge: USA. 48. Simon DJ, Madison JM, Conery AL, Thompson-Peer KL, Soskis M, et al. (2008) The microrna mir-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell 133: PLoS Genetics 12 January 2012 Volume 8 Issue 1 e
Jana F Liewald, Martin Brauner, Gregory J Stephens, Magali Bouhours, Christian Schultheis, Mei Zhen & Alexander Gottschalk
 nature methods Optogenetic analysis of synaptic function Jana F Liewald, Martin Brauner, Gregory J Stephens, Magali Bouhours, Christian Schultheis, Mei Zhen & Alexander Gottschalk Supplementary figures
nature methods Optogenetic analysis of synaptic function Jana F Liewald, Martin Brauner, Gregory J Stephens, Magali Bouhours, Christian Schultheis, Mei Zhen & Alexander Gottschalk Supplementary figures
A conserved dopamine-cholecystokinin signaling pathway shapes context-dependent Caenorhabditis elegans behavior
 University of Massachusetts Medical School escholarship@umms Open Access Articles Open Access Publications by UMMS Authors 8-28-2014 A conserved dopamine-cholecystokinin signaling pathway shapes context-dependent
University of Massachusetts Medical School escholarship@umms Open Access Articles Open Access Publications by UMMS Authors 8-28-2014 A conserved dopamine-cholecystokinin signaling pathway shapes context-dependent
Synaptic communication
 Synaptic communication Objectives: after these lectures you should be able to: - explain the differences between an electrical and chemical synapse - describe the steps involved in synaptic communication
Synaptic communication Objectives: after these lectures you should be able to: - explain the differences between an electrical and chemical synapse - describe the steps involved in synaptic communication
THE SYNAPTIC VESICLE CYCLE
 Annu. Rev. Neurosci. 2004. 27:509 47 doi: 10.1146/annurev.neuro.26.041002.131412 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on March 12, 2004
Annu. Rev. Neurosci. 2004. 27:509 47 doi: 10.1146/annurev.neuro.26.041002.131412 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on March 12, 2004
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
Introduction to Neurobiology
 Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons. Chad Smurthwaite & Jordan Shellmire
 Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
1) Drop off in the Bi 150 box outside Baxter 331 or to the head TA (jcolas).
 Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
Bi/CNS/NB 150 Problem Set 3 Due: Tuesday, Oct. 27, at 4:30 pm Instructions: 1) Drop off in the Bi 150 box outside Baxter 331 or e-mail to the head TA (jcolas). 2) Submit with this cover page. 3) Use a
BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1
 BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1 Terms you should know: synapse, neuromuscular junction (NMJ), pre-synaptic, post-synaptic, synaptic cleft, acetylcholine (ACh), acetylcholine
BIPN100 F15 Human Physiology 1 Lecture 3. Synaptic Transmission p. 1 Terms you should know: synapse, neuromuscular junction (NMJ), pre-synaptic, post-synaptic, synaptic cleft, acetylcholine (ACh), acetylcholine
Section: Chapter 5: Multiple Choice. 1. The structure of synapses is best viewed with a(n):
 Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Portions from Chapter 6 CHAPTER 7. The Nervous System: Neurons and Synapses. Chapter 7 Outline. and Supporting Cells
 CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
Abstract Introduction
 Decreased colocalization of synapsin I and Munc13 within presynaptic axon terminals of the earthworm neuromuscular junction when stimulated could help determine how the two proteins interact during neurotransmitter
Decreased colocalization of synapsin I and Munc13 within presynaptic axon terminals of the earthworm neuromuscular junction when stimulated could help determine how the two proteins interact during neurotransmitter
Supplementary Information
 Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
Supplementary Information Supplementary Figure 1: Luminal localization of CCM-3. (a) The CCM-3::GFP fusion protein localizes along the apical (luminal) surface of the pharynx (b) as well as the lumen of
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3
 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES 34.3 NEURONS COMMUNICATE WITH OTHER CELLS AT SYNAPSES Neurons communicate with other neurons or target cells at synapses. Chemical synapse: a very narrow
A Neuronal Acetylcholine Receptor Regulates the Balance of Muscle Excitation and Inhibition in Caenorhabditis elegans
 A Neuronal Acetylcholine Receptor Regulates the Balance of Muscle Excitation and Inhibition in Caenorhabditis elegans Maelle Jospin 1,2, Yingchuan B. Qi 3,4., Tamara M. Stawicki 4., Thomas Boulin 5., Kim
A Neuronal Acetylcholine Receptor Regulates the Balance of Muscle Excitation and Inhibition in Caenorhabditis elegans Maelle Jospin 1,2, Yingchuan B. Qi 3,4., Tamara M. Stawicki 4., Thomas Boulin 5., Kim
Chapter 3 subtitles Action potentials
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
Synaptic Communication. Steven McLoon Department of Neuroscience University of Minnesota
 Synaptic Communication Steven McLoon Department of Neuroscience University of Minnesota 1 Course News The first exam is next week on Friday! Be sure to checkout the sample exam on the course website. 2
Synaptic Communication Steven McLoon Department of Neuroscience University of Minnesota 1 Course News The first exam is next week on Friday! Be sure to checkout the sample exam on the course website. 2
Outline. Neuron Structure. Week 4 - Nervous System. The Nervous System: Neurons and Synapses
 Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
The Molecular Mechanism of Intracellular Membrane Fusion. Richard H. Scheller
 The Molecular Mechanism of Intracellular Membrane Fusion Richard H. Scheller The human brain contains approximately 10 15 connections between nerve cells (Figure 2). The specific formation and modulation
The Molecular Mechanism of Intracellular Membrane Fusion Richard H. Scheller The human brain contains approximately 10 15 connections between nerve cells (Figure 2). The specific formation and modulation
Neurotransmitter Systems I Identification and Distribution. Reading: BCP Chapter 6
 Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons
 Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
NEURAL TISSUE (NEUROPHYSIOLOGY) PART I (A): NEURONS & NEUROGLIA
 PART I (A): NEURONS & NEUROGLIA Neural Tissue Contains 2 kinds of cells: neurons: cells that send and receive signals neuroglia (glial cells): cells that support and protect neurons Neuron Types Sensory
PART I (A): NEURONS & NEUROGLIA Neural Tissue Contains 2 kinds of cells: neurons: cells that send and receive signals neuroglia (glial cells): cells that support and protect neurons Neuron Types Sensory
Synapse Formation. Steven McLoon Department of Neuroscience University of Minnesota
 Synapse Formation Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Midterm Exam Monday, Nov 13 9:30-11:30am Bring a #2 pencil!! 2 Course News Lecture schedule: Mon (Oct 31)
Synapse Formation Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Midterm Exam Monday, Nov 13 9:30-11:30am Bring a #2 pencil!! 2 Course News Lecture schedule: Mon (Oct 31)
Concept 48.1 Neuron organization and structure reflect function in information transfer
 Name Chapter 48: Neurons, Synapses, and Signaling Period Chapter 48: Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer 1. What is
Name Chapter 48: Neurons, Synapses, and Signaling Period Chapter 48: Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer 1. What is
Hierarchical assembly of presynaptic components in defined C. elegans synapses
 Hierarchical assembly of presynaptic components in defined C. elegans synapses Maulik R Patel 1,2, Emily K Lehrman 1, Vivian Y Poon 1,2, Justin G Crump 3, Mei Zhen 4, Cornelia I Bargmann 5 & Kang Shen
Hierarchical assembly of presynaptic components in defined C. elegans synapses Maulik R Patel 1,2, Emily K Lehrman 1, Vivian Y Poon 1,2, Justin G Crump 3, Mei Zhen 4, Cornelia I Bargmann 5 & Kang Shen
Ch. 45 Continues (Have You Read Ch. 45 yet?) u Central Nervous System Synapses - Synaptic functions of neurons - Information transmission via nerve
 Ch. 45 Continues (Have You Read Ch. 45 yet?) u Central Nervous System Synapses - Synaptic functions of neurons - Information transmission via nerve impulses - Impulse may be blocked in its transmission
Ch. 45 Continues (Have You Read Ch. 45 yet?) u Central Nervous System Synapses - Synaptic functions of neurons - Information transmission via nerve impulses - Impulse may be blocked in its transmission
Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.
![Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +. Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.](/thumbs/90/102196023.jpg) Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Mutations in the Caenorhabditis elegans U2AF Large Subunit UAF-1 Al= of a 3' Splice Site In Vivo
 Mutations in the Caenorhabditis elegans U2AF Large Subunit UAF-1 Al= of a 3' Splice Site In Vivo The MIT Faculty has made this article openly available. Please share how this access benefits you. Your
Mutations in the Caenorhabditis elegans U2AF Large Subunit UAF-1 Al= of a 3' Splice Site In Vivo The MIT Faculty has made this article openly available. Please share how this access benefits you. Your
What effect would an AChE inhibitor have at the neuromuscular junction?
 CASE 4 A 32-year-old woman presents to her primary care physician s office with difficulty chewing food. She states that when she eats certain foods that require a significant amount of chewing (meat),
CASE 4 A 32-year-old woman presents to her primary care physician s office with difficulty chewing food. She states that when she eats certain foods that require a significant amount of chewing (meat),
BIOL Week 6. Nervous System. Transmission at Synapses
 Collin County Community College BIOL 2401 Week 6 Nervous System 1 Transmission at Synapses Synapses are the site of communication between 2 or more neurons. It mediates the transfer of information and
Collin County Community College BIOL 2401 Week 6 Nervous System 1 Transmission at Synapses Synapses are the site of communication between 2 or more neurons. It mediates the transfer of information and
5-Nervous system II: Physiology of Neurons
 5-Nervous system II: Physiology of Neurons AXON ION GRADIENTS ACTION POTENTIAL (axon conduction) GRADED POTENTIAL (cell-cell communication at synapse) SYNAPSE STRUCTURE & FUNCTION NEURAL INTEGRATION CNS
5-Nervous system II: Physiology of Neurons AXON ION GRADIENTS ACTION POTENTIAL (axon conduction) GRADED POTENTIAL (cell-cell communication at synapse) SYNAPSE STRUCTURE & FUNCTION NEURAL INTEGRATION CNS
GAP-FREE NEURAL CIRCUITS CLASS #3: C. elegans touch-induced locomotion
 GAP-FREE NEURAL CIRCUITS CLASS #3: C. elegans touch-induced locomotion OUTLINE: C. elegans background o General background o Neural signaling: no action potentials Excitatory circuits: Touch-induced locomotion
GAP-FREE NEURAL CIRCUITS CLASS #3: C. elegans touch-induced locomotion OUTLINE: C. elegans background o General background o Neural signaling: no action potentials Excitatory circuits: Touch-induced locomotion
How Nicotinic Signaling Shapes Neural Networks
 How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
Chapter 3 Neurotransmitter release
 NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
CHAPTER 44: Neurons and Nervous Systems
 CHAPTER 44: Neurons and Nervous Systems 1. What are the three different types of neurons and what are their functions? a. b. c. 2. Label and list the function of each part of the neuron. 3. How does the
CHAPTER 44: Neurons and Nervous Systems 1. What are the three different types of neurons and what are their functions? a. b. c. 2. Label and list the function of each part of the neuron. 3. How does the
Communication within a Neuron
 Neuronal Communication, Ph.D. Communication within a Neuron Measuring Electrical Potentials of Axons The Membrane Potential The Action Potential Conduction of the Action Potential 1 The withdrawal reflex
Neuronal Communication, Ph.D. Communication within a Neuron Measuring Electrical Potentials of Axons The Membrane Potential The Action Potential Conduction of the Action Potential 1 The withdrawal reflex
MCB MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY
 MCB 160 - MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY Name ID# Instructions: -Only tests written in pen will be regarded -Please submit a written request indicating where and why you deserve more points
MCB 160 - MIDTERM EXAM #1 MONDAY MARCH 3, 2008 ANSWER KEY Name ID# Instructions: -Only tests written in pen will be regarded -Please submit a written request indicating where and why you deserve more points
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission
 Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
3.E.2 Continued. This is the essential knowledge statement from the curriculum framework. Detect---process--- response
 Nervous System: Part III What Happens at a Synapse? 3.E. Continued Animals have nervous systems that detect external and internal signals, transmit and integrate information, and produce responses. This
Nervous System: Part III What Happens at a Synapse? 3.E. Continued Animals have nervous systems that detect external and internal signals, transmit and integrate information, and produce responses. This
ns ns hp761(lf); daf-28(lf) daf-28(gf) Figure S1
 A ns ns B 100 100 80 80 60 60 40 40 20 20 0 0% 0 0% hp761(lf); daf-28(lf) daf-28(gf) Figure S1 A 100 80 15 o C 22 o C 25 o C % Dauers 60 40 20 0 0% 0% * 0% 0% 0% Class I alleles Class II alleles daf-2(lf;ts)
A ns ns B 100 100 80 80 60 60 40 40 20 20 0 0% 0 0% hp761(lf); daf-28(lf) daf-28(gf) Figure S1 A 100 80 15 o C 22 o C 25 o C % Dauers 60 40 20 0 0% 0% * 0% 0% 0% Class I alleles Class II alleles daf-2(lf;ts)
All questions below pertain to mandatory material: all slides, and mandatory homework (if any).
 ECOL 182 Spring 2008 Dr. Ferriere s lectures Lecture 6: Nervous system and brain Quiz Book reference: LIFE-The Science of Biology, 8 th Edition. http://bcs.whfreeman.com/thelifewire8e/ All questions below
ECOL 182 Spring 2008 Dr. Ferriere s lectures Lecture 6: Nervous system and brain Quiz Book reference: LIFE-The Science of Biology, 8 th Edition. http://bcs.whfreeman.com/thelifewire8e/ All questions below
SUPPLEMENTARY FIGURE S1: nlp-22 is expressed in the RIA interneurons and is secreted. (a) An animal expressing both the RIA specific reporter
 1 SUPPLEMENTARY FIGURE S1: nlp-22 is expressed in the RIA interneurons and is secreted. (a) An animal expressing both the RIA specific reporter Pglr-3:mCherry (red) and Pnlp-22:gfp (green) shows co-localization
1 SUPPLEMENTARY FIGURE S1: nlp-22 is expressed in the RIA interneurons and is secreted. (a) An animal expressing both the RIA specific reporter Pglr-3:mCherry (red) and Pnlp-22:gfp (green) shows co-localization
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
Communication Between
 Communication Between Neurons Bởi: OpenStaxCollege The electrical changes taking place within a neuron, as described in the previous section, are similar to a light switch being turned on. A stimulus starts
Communication Between Neurons Bởi: OpenStaxCollege The electrical changes taking place within a neuron, as described in the previous section, are similar to a light switch being turned on. A stimulus starts
SYNAPTIC COMMUNICATION
 BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
Name: Answer Key. Question 1.
 2007 7.013 Problem Set 6 Due before 5 PM on FRIDAY, April 27, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. Question 1. 1a. This is a diagram showing changes
2007 7.013 Problem Set 6 Due before 5 PM on FRIDAY, April 27, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. Question 1. 1a. This is a diagram showing changes
Chapter 4 Neuronal Physiology
 Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Chapter 4 Neuronal Physiology V edit. Pg. 99-131 VI edit. Pg. 85-113 VII edit. Pg. 87-113 Input Zone Dendrites and Cell body Nucleus Trigger Zone Axon hillock Conducting Zone Axon (may be from 1mm to more
Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN
 UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
Dania Ahmad. Tamer Barakat + Dania Ahmad. Faisal I. Mohammed
 16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
Neurons, Synapses, and Signaling
 Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
Neurons, Synapses, and Signaling The Neuron is the functional unit of the nervous system. Neurons are composed of a cell body, which contains the nucleus and organelles; Dendrites which are extensions
Anatomy Review. Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (
 Anatomy Review Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Neurons communicate with other cells at junctions
Anatomy Review Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Neurons communicate with other cells at junctions
NERVOUS SYSTEM 1 CHAPTER 10 BIO 211: ANATOMY & PHYSIOLOGY I
 BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B This set CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B This set CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
3) Most of the organelles in a neuron are located in the A) dendritic region. B) axon hillock. C) axon. D) cell body. E) axon terminals.
 Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
The Brain & Homeostasis. The Brain & Technology. CAT, PET, and MRI Scans
 The Brain & Homeostasis Today, scientists have a lot of information about what happens in the different parts of the brain; however they are still trying to understand how the brain functions. We know
The Brain & Homeostasis Today, scientists have a lot of information about what happens in the different parts of the brain; however they are still trying to understand how the brain functions. We know
Synaptic Transmission: Ionic and Metabotropic
 Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Alterations in Synaptic Strength Preceding Axon Withdrawal
 Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
Alterations in Synaptic Strength Preceding Axon Withdrawal H. Colman, J. Nabekura, J.W. Lichtman presented by Ana Fiallos Synaptic Transmission at the Neuromuscular Junction Motor neurons with cell bodies
QUIZ YOURSELF COLOSSAL NEURON ACTIVITY
 QUIZ YOURSELF What are the factors that produce the resting potential? How is an action potential initiated and what is the subsequent flow of ions during the action potential? 1 COLOSSAL NEURON ACTIVITY
QUIZ YOURSELF What are the factors that produce the resting potential? How is an action potential initiated and what is the subsequent flow of ions during the action potential? 1 COLOSSAL NEURON ACTIVITY
Neuronal Differentiation: CNS Synapse Formation
 Neuronal Differentiation: CNS Synapse Formation Comparison of the NMJ and Central Synapses Sanes and Lichtman 2001 Comparison of the NMJ and Central Synapses (1) Each neuron in the CNS is innervated by
Neuronal Differentiation: CNS Synapse Formation Comparison of the NMJ and Central Synapses Sanes and Lichtman 2001 Comparison of the NMJ and Central Synapses (1) Each neuron in the CNS is innervated by
Differing mechanisms of exocytosis for large dense core vesicles in chromaffin cells and small synaptic vesicles in dopamine neurons.
 Differing mechanisms of exocytosis for large dense core vesicles in chromaffin cells and small synaptic vesicles in dopamine neurons. Roland G.W. Staal a, Eugene Mosharov a, Anthonia Hananiya a and David
Differing mechanisms of exocytosis for large dense core vesicles in chromaffin cells and small synaptic vesicles in dopamine neurons. Roland G.W. Staal a, Eugene Mosharov a, Anthonia Hananiya a and David
Synaptic transmission
 Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
DOI: /jphysiol The Physiological Society Rapid Report
 (2002), 545.2, pp. 337 343 DOI: 10.1113/jphysiol.2002.032516 The Physiological Society 2002 www.jphysiol.org Rapid Report Regulation by Rab3A of an endogenous modulator of neurotransmitter release at mouse
(2002), 545.2, pp. 337 343 DOI: 10.1113/jphysiol.2002.032516 The Physiological Society 2002 www.jphysiol.org Rapid Report Regulation by Rab3A of an endogenous modulator of neurotransmitter release at mouse
Synaptic Transmission
 Synaptic Transmission Postsynaptic Mechanisms Synapses electrical and chemical Part I Neurotransmitters categories and life cycle Neurotransmitters examples and postsynaptic effects Pathology Part II Neurotransmitter
Synaptic Transmission Postsynaptic Mechanisms Synapses electrical and chemical Part I Neurotransmitters categories and life cycle Neurotransmitters examples and postsynaptic effects Pathology Part II Neurotransmitter
Neurons! John A. White Dept. of Bioengineering
 Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Function of the Nervous System
 Nervous System Function of the Nervous System Receive sensory information, interpret it, and send out appropriate commands to form a response Composed of neurons (functional unit of the nervous system)
Nervous System Function of the Nervous System Receive sensory information, interpret it, and send out appropriate commands to form a response Composed of neurons (functional unit of the nervous system)
The Nervous System. Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine.
 The Nervous System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine Http://10.10.10.151 Part 1. Summary of the nervous system The Nervous System Central Nervous System Brain + Spinal Cord Peripheral
The Nervous System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine Http://10.10.10.151 Part 1. Summary of the nervous system The Nervous System Central Nervous System Brain + Spinal Cord Peripheral
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt chemotaxis in Caenorhabditis elegans
 Supplementary Information for: Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt chemotaxis in Caenorhabditis elegans Hirofumi Kunitomo, Hirofumi Sato, Ryo Iwata, Yohsuke
Supplementary Information for: Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt chemotaxis in Caenorhabditis elegans Hirofumi Kunitomo, Hirofumi Sato, Ryo Iwata, Yohsuke
NEUROCHEMISTRY Brief Review
 NEUROCHEMISTRY Brief Review UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PBL MBBS YEAR V SEMINAR VJ Temple 1 Membrane potential Membrane potential:
NEUROCHEMISTRY Brief Review UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PBL MBBS YEAR V SEMINAR VJ Temple 1 Membrane potential Membrane potential:
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells. Pei-Tzu Yang and Hendrik C. Korswagen
 Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Synaptic Transmission
 Synaptic Transmission Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Synaptic transmission involves the release
Synaptic Transmission Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Synaptic transmission involves the release
浙江大学医学院基础医学整合课程 各论 III. The Nervous System. Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine
 The Nervous System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine xiongzhang@zju.edu.cn http://10.202.77.12/ 1 Part 1. Summary of the nervous system 2 The Nervous System Central Nervous System
The Nervous System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine xiongzhang@zju.edu.cn http://10.202.77.12/ 1 Part 1. Summary of the nervous system 2 The Nervous System Central Nervous System
Neurotransmitter Systems III Neurochemistry. Reading: BCP Chapter 6
 Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Omar Ismail. Dana Almanzalji. Faisal Mohammad
 11 Omar Ismail Dana Almanzalji Faisal Mohammad Neuronal classification: Neurons are responsible for transmitting the action potential to the brain. The speed at which the action potential is transmitted
11 Omar Ismail Dana Almanzalji Faisal Mohammad Neuronal classification: Neurons are responsible for transmitting the action potential to the brain. The speed at which the action potential is transmitted
Dopamine Controls Locomotion by Modulating the Activity of the Cholinergic Motor Neurons in C. elegans
 University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 2009 Dopamine Controls Locomotion by Modulating the Activity of the Cholinergic Motor Neurons in C. elegans
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 2009 Dopamine Controls Locomotion by Modulating the Activity of the Cholinergic Motor Neurons in C. elegans
Axon Nerve impulse. Axoplasm Receptor. Axomembrane Stimuli. Schwann cell Effector. Myelin Cell body
 Nervous System Review 1. Explain a reflex arc. 2. Know the structure, function and location of a sensory neuron, interneuron, and motor neuron 3. What is (a) Neuron Axon Nerve impulse Axoplasm Receptor
Nervous System Review 1. Explain a reflex arc. 2. Know the structure, function and location of a sensory neuron, interneuron, and motor neuron 3. What is (a) Neuron Axon Nerve impulse Axoplasm Receptor
Neurochemistry 2. Loewi s experiment
 Neurochemistry 2 Loewi s experiment Cengage Learning 2016 AP reaches the axon terminal and activates voltage-gated Ca++ channels (3 major classes). Ca++ influx results in exocytosis of neurotransmitters
Neurochemistry 2 Loewi s experiment Cengage Learning 2016 AP reaches the axon terminal and activates voltage-gated Ca++ channels (3 major classes). Ca++ influx results in exocytosis of neurotransmitters
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]
![QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY]](/thumbs/86/94924826.jpg) QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
QUIZ/TEST REVIEW NOTES SECTION 7 NEUROPHYSIOLOGY [THE SYNAPSE AND PHARMACOLOGY] Learning Objectives: Explain how neurons communicate stimulus intensity Explain how action potentials are conducted along
Neurons Chapter 7 2/19/2016. Learning Objectives. Cells of the Nervous System. Cells of the Nervous System. Cells of the Nervous System
 Learning Objectives Neurons Chapter 7 Identify and describe the functions of the two main divisions of the nervous system. Differentiate between a neuron and neuroglial cells in terms of structure and
Learning Objectives Neurons Chapter 7 Identify and describe the functions of the two main divisions of the nervous system. Differentiate between a neuron and neuroglial cells in terms of structure and
Neuroscience with Pharmacology 2 Functions and Mechanisms of Reflexes. Prof Richard Ribchester
 Neuroscience with Pharmacology 2 Functions and Mechanisms of Reflexes Prof Richard Ribchester René Descartes Cogito, ergo sum The 21st century still holds many challenges to Neuroscience and Pharmacology
Neuroscience with Pharmacology 2 Functions and Mechanisms of Reflexes Prof Richard Ribchester René Descartes Cogito, ergo sum The 21st century still holds many challenges to Neuroscience and Pharmacology
ANATOMY AND PHYSIOLOGY OF NEURONS. AP Biology Chapter 48
 ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
What is Anatomy and Physiology?
 Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
10.1: Introduction. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial cells) Dendrites.
 10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
Chapter 5 subtitles GABAergic synaptic transmission
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 5 subtitles GABAergic synaptic transmission INTRODUCTION (2:57) In this fifth chapter, you will learn how the binding of the GABA neurotransmitter to
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 5 subtitles GABAergic synaptic transmission INTRODUCTION (2:57) In this fifth chapter, you will learn how the binding of the GABA neurotransmitter to
The Nervous System Mark Stanford, Ph.D.
 The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
BIPN140 Lecture 13: Synapse Formation (Synaptogenesis)
 BIPN140 Lecture 13: Synapse Formation (Synaptogenesis) 1. Neuromuscular Junction (NMJ) Development 2. Synaptogenesis at Central Synapses Su (FA16) Ultrastructural Image of an NMJ Active Zone Basal Lamina
BIPN140 Lecture 13: Synapse Formation (Synaptogenesis) 1. Neuromuscular Junction (NMJ) Development 2. Synaptogenesis at Central Synapses Su (FA16) Ultrastructural Image of an NMJ Active Zone Basal Lamina
Chapter 2 Ex uno plures: Out of One, Many
 Chapter 2 Ex uno plures: Out of One, Many R. Gutie rrez Abstract Ex uno plures, out of one (cell) many (neurotransmitters), seems to be a principle that applies to many, if not all, neuronal types. The
Chapter 2 Ex uno plures: Out of One, Many R. Gutie rrez Abstract Ex uno plures, out of one (cell) many (neurotransmitters), seems to be a principle that applies to many, if not all, neuronal types. The
7.06 Spring of PROBLEM SET #6
 7.6 Spring 23 1 of 6 7.6 PROBLEM SET #6 1. You are studying a mouse model of hypercholesterolemia, a disease characterized by high levels of cholesterol in the blood. In normal cells, LDL particles in
7.6 Spring 23 1 of 6 7.6 PROBLEM SET #6 1. You are studying a mouse model of hypercholesterolemia, a disease characterized by high levels of cholesterol in the blood. In normal cells, LDL particles in
Thursday, January 22, Nerve impulse
 Nerve impulse Transmembrane Potential caused by ions moving through cell membrane at different rates Two main ions of concern Na + - Sodium K + - potassium Cell membrane not freely permeable therefore
Nerve impulse Transmembrane Potential caused by ions moving through cell membrane at different rates Two main ions of concern Na + - Sodium K + - potassium Cell membrane not freely permeable therefore
Quantal Analysis Problems
 Quantal Analysis Problems 1. Imagine you had performed an experiment on a muscle preparation from a Drosophila larva. In this experiment, intracellular recordings were made from an identified muscle fibre,
Quantal Analysis Problems 1. Imagine you had performed an experiment on a muscle preparation from a Drosophila larva. In this experiment, intracellular recordings were made from an identified muscle fibre,
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
Neurotransmitters. Chemical transmission of a nerve signal by neurotransmitters at a synapse
 Neurotransmitters A chemical released by one neuron that affects another neuron or an effector organ (e.g., muscle, gland, blood vessel). Neurotransmitters are small molecules that serve as messengers
Neurotransmitters A chemical released by one neuron that affects another neuron or an effector organ (e.g., muscle, gland, blood vessel). Neurotransmitters are small molecules that serve as messengers
Modeling Excitatory and Inhibitory Chemical Synapses
 In review, a synapse is the place where signals are transmitted from a neuron, the presynaptic neuron, to another cell. This second cell may be another neuron, muscle cell or glandular cell. If the second
In review, a synapse is the place where signals are transmitted from a neuron, the presynaptic neuron, to another cell. This second cell may be another neuron, muscle cell or glandular cell. If the second
Psych 181: Dr. Anagnostaras
 Psych 181: Dr. Anagnostaras Lecture 5 Synaptic Transmission Introduction to synaptic transmission Synapses (Gk., to clasp or join) Site of action of most psychoactive drugs 6.5 1 Synapses Know basic terminology:
Psych 181: Dr. Anagnostaras Lecture 5 Synaptic Transmission Introduction to synaptic transmission Synapses (Gk., to clasp or join) Site of action of most psychoactive drugs 6.5 1 Synapses Know basic terminology:
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed.,
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
