Developmental Biology
|
|
|
- Ruth Fields
- 5 years ago
- Views:
Transcription
1 Developmental Biology 347 (2010) Contents lists available at ScienceDirect Developmental Biology journal homepage: TGFβ signaling positions the ciliary band and patterns neurons in the sea urchin embryo Shunsuke Yaguchi a,b,c, Junko Yaguchi a,b,c, Robert C. Angerer c, Lynne M. Angerer c, Robert D. Burke b, a Shimoda Marine Research Center, University of Tsukuba, Japan b Dept of Biochemistry and Microbiology, University of Victoria, Canada c National Institute of Dental and Craniofacial Research, National Institutes of Health, USA article info abstract Article history: Received for publication 1 February 2010 Revised 17 June 2010 Accepted 6 August 2010 Available online 12 August 2010 Keywords: TGFbeta signaling Neurogenesis Ciliary band Oral signaling Nodal BMP Lefty Smad Alk Urchin The ciliary band is a distinct region of embryonic ectoderm that is specified between oral and aboral ectoderm. Flask-shaped ciliary cells and neurons differentiate in this region and they are patterned to form an integrated tissue that functions as the principal swimming and feeding organ of the larva. TGFβ signaling, which is known to mediate oral and aboral patterning of the ectoderm, has been implicated in ciliary band formation. We have used morpholino knockdown and ectopic expression of RNA to alter TGFβ signaling at the level of ligands, receptors, and signal transduction components and assessed the differentiation and patterning of the ciliary band cells and associated neurons. We propose that the primary effects of these signals are to position the ciliary cells, which in turn support neural differentiation. We show that Nodal signaling, which is known to be localized by Lefty, positions the oral margin of the ciliary band. Signaling from BMP through Alk3/6, affects the position of the oral and aboral margins of the ciliary band. Since both Nodal and BMP signaling produce ectoderm that does not support neurogenesis, we propose that formation of a ciliary band requires protection from these signals. Expression of BMP2/4 and Nodal suppress neural differentiation. However, the response to receptor knockdown or dominant-negative forms of signal transduction components indicate signaling is not acting directly on unspecified ectoderm cells to prevent their differentiation as neurons. Instead, it produces a restricted field of ciliary band cells that supports neurogenesis. We propose a model that incorporates spatially regulated control of Nodal and BMP signaling to determine the position and differentiation of the ciliary band, and subsequent neural patterning Elsevier Inc. All rights reserved. Introduction Members of the transforming growth factor-β (TGFβ) superfamily play central roles in cell fate specification in development. TGFβ ligands are secreted proteins that diffuse from their source and activate complex signaling networks that regulate differentiation. TGF-β signaling patterns are complicated because a range of factors modify ligand availability and receptor and signal transduction functions, creating complex developmental patterns from seemingly simple arrangements of localized signaling sources and widespread receptors. Well-known examples are Nodal and BMP4. In vertebrates, nodal is expressed on the left side of the embryo and its localized effects are controlled by Lefty-1 and Lefty-2 (Meno et al., 1996). Leftys bind to the EGF CFC proteins that are required for Nodal to bind to the activin-like kinase (Alk) receptor at the midline of the body, thereby blocking Nodal binding, and preventing Nodal signals from spreading to right side (Chen and Shen, 2004). BMP4, which is expressed on the future ventral side of vertebrate ectoderm, Corresponding author. Biochemistry/Microbiology, University of Victoria, POB 3020, STN CSC, 3800 Finnerty Rd, Victoria, BC, Canada V8W 3N5. Fax: address: rburke@uvic.ca (R.D. Burke). diffuses throughout the embryo, but is antagonized by the direct binding of Chordin, which is expressed in the dorsal organizer. The consequence is that dorsal tissues form where BMP4's ventralizing effects are blocked (De Robertis and Kuroda, 2004). It is a hallmark of TGFβ signaling that molecular antagonists pattern the effects of the secreting ligands with surprising precision. In sea urchin embryos four regions of ectoderm the animal plate, oral ectoderm, aboral ectoderm and ciliary band are produced by animal hemisphere blastomeres (Davidson et al., 1998; Yaguchi et al., 2006). Incompletely characterized events, dependent on vegetal canonical Wnt, restrict the animal plate to the animal pole (Yaguchi et al., 2006) and eliminate a repressor of nodal expression (Yaguchi et al., 2008). As a consequence, the TGFβ signals, Nodal and subsequently BMP2/4, begin to pattern the remaining ectoderm in the animal hemisphere, producing oral, aboral and ciliary band ectoderm (Duboc et al., 2004; Yaguchi et al., 2006). Models of ectodermal specification suggest that Nodal signaling is limited to the oral ectoderm by Lefty, which depends on Nodal and has long-range inhibitory functions (Duboc et al., 2008). A reaction-diffusion model in which Lefty acts as a feedback inhibitor has been proposed to explain how it restricts Nodal signaling to oral ectoderm (Duboc et al., 2008; Bolouri and Davidson, /$ see front matter 2010 Elsevier Inc. All rights reserved. doi: /j.ydbio
2 72 S. Yaguchi et al. / Developmental Biology 347 (2010) ). BMP2/4, which also acts downstream of Nodal, is transcribed in the oral ectoderm (Angerer et al., 2000; Duboc et al., 2004), yet acts outside of oral ectoderm to induce aboral ectoderm (Lapraz et al., 2009). Bradham et al. (2009) and Lapraz et al. (2009) showed that Chordin, expressed in the oral ectoderm under the control of Nodal, blocks BMP2/4 activity. In its absence, or in the absence of Nodal, differentiation of ciliary band neurons is altered as well as the normal expression pattern of a ciliary band marker. Although TGFβ signaling accounts for many aspects of oral and aboral ectoderm specification, we understand very little of the mechanisms involved in ciliary band formation, and the differentiation of ciliary band neurons. The ciliary band is the principal swimming and feeding organ of the larva. It is a tightly packed strip of flask-shaped, ciliary cells that beat away from the mouth, producing a force that moves the larva forward and captures food particles deflected by ciliary reversals (Strathmann, 2007). In addition to the ciliary cells, there is a series of neurons, mostly on the oral side of the ciliary band, that have short, microvillar dendritic processes on their surface (Burke, 1978). A tract of axons that lies at the base of the ciliary cells interconnects the nerve cells. The nervous system is thought to regulate the direction of ciliary beat, as depolarization of the ciliary cells accompanies reversals of ciliary beat (Mackie et al., 1969; Satterlie and Cameron, 1985). Thus, the ciliary band is an integrated tissue innervated by neurons arranged in a precise pattern. Our objective was to determine how components of the oral aboral signaling network specify and pattern the ciliary cells and neurons of the ciliary band. We manipulated the signaling network by knocking down ligands and receptors with morpholinos and expressing RNAs encoding antagonists and dominant-negative, or constitutively active signal transduction components. We anticipated that by assessing the distribution of different types of ectoderm and neurons, we would be able to deduce how oral/aboral ectoderm patterning mechanisms regulate formation of the ciliary band. Our results indicate that the ciliary band is positioned by TGFβ signaling, yet it is a region in which TGFβ signaling is suppressed. In addition, we identify novel roles for known components of the oral aboral signaling network in patterning the ectoderm of sea urchin embryos. Materials and methods Animals and embryo culture Strongylocentrotus purpuratus were collected near Victoria, BC or purchased from The Cultured Abalone, Goleta, CA. Gametes were obtained by intra-coelomic injection of 0.5 M KCl and embryos were cultured by standard methods with filtered seawater (FSW) or artificial seawater at 15 C. Microinjection of morpholino anti-sense oligonucleotides (MO) and mrnas Eggs were prepared as described previously (Yaguchi et al., 2006). Morpholinos (Gene Tools, Eugene, OR) were microinjected in 22.5% glycerol with the following concentration in the injection needles: nodal-mo (600 μm), lefty-mo (200 μm), BMP2/ 4-MO (150 μm), and Alk3/6-MO (200 μm). The morpholino sequences are: nodal-mo: 5 -GATGTCTCAGCTCTCTGAAATGTAG-3 lefty-mo: 5 -AGCACCGAGTGATAATTCCATATTG-3 BMP2/4-MO: 5 -GTGGTAACCATGATGGGTCTGAAAG-3 Alk3/6-MO: 5 -TAGTGTTACATCTGTCGCCATATTC-3 The preparation and concentration for nodal, lefty, modified smad2/3 and BMP2/4 mrnas are described previously (Yaguchi et al., 2006, 2007). To misexpress modified smad1/5, the C-terminal of Sp-Smad1/5 was substituted or deleted in a manner similar to that described for Smad2/3 modification (Yaguchi et al., 2007). The concentration of act-smad1/5 and dn-smad1/5 mrnas was 3.0 μg/μl in injection needles. Immunohistochemistry Immunohistochemistry was done as described previously (Yaguchi et al., 2006). Primary antibodies were incubated overnight at 4 C using the following dilutions: Synaptotagmin (1E11, 1:800; Nakajima et al., 2004), Goosecoid (Gsc, 1:600; Kenny et al., 2003), Hnf6 (1:500), serotonin (1:1000, Sigma), and Nk2.1 (1:800; Takacs et al., 2004). The specimens were observed using Leica (DM6000) and Zeiss (Axiovert 200 M and LSM410) microscopes. Counts of immunoreactive cells and DAPI stained nuclei were done manually from Z projections of optical sections of individual embryos. The images were analyzed with ImageJ (NIH) and Adobe Photoshop and the figures were prepared with Canvas8. To prepare an antiserum against Sp-Hnf6, a full-length cdna (1 1479) was amplified by RT-PCR using synthesized primers (SpHnf6_hisF1 GGGATCCATGCTTTCAAGTGAGT- TAGTTGGC, SpHnf6_hisR1 CTCGAGCGCCTTGTACTCTGACTTTGGT). Products were cloned using pgem-t vector (Promega), sequenced, and subcloned into the bacterial expression plasmid pet28a (EMD Biosciences). Clones containing insert were transformed into E. coli (BL21) screened for IPTG inducible expression of a 6 His-containing protein of the expected size (54 kda). A 1 L liquid culture was induced, and after 4 h cells were pelleted, and extracted in 6 M urea. The extract was passed over a 5 ml column of chelating sepharose fast flow (GE Healthcare), and bound protein eluted with 15 mm imidazole. Eluted fractions were dialyzed into PBS and the purity of the protein was determined with PAGE. Two Sprague Dawley rats were immunized and boosted 4 times following Canadian Council of Animal Care standard procedures. Antisera were screened with immunoblots of expressed protein or native protein and with whole mount immunofluoresence using pre-immune serum as a control. Results Ciliary band neurons The ciliary band in a pluteus larva is composed of 3 4 rowsof columnar cells that surround the oral ectoderm (Fig. 1A, green line). To identify differentiating ciliary band cells, we prepared an antibody to Hnf6, a transcription factor of the ONECUT family, which is the earliest marker of the ciliary band (Poustka et al., 2004) and reported to be required for its formation (Otim et al., 2004). In prism and pluteus stages, Hnf6 protein is detected in the nuclei of the tightly packed columnar cells of the ciliary band. Double staining for Goosecoid (Gsc), a transcription factor expressed in the oral ectoderm (Angerer et al., 2001) and Hnf6 shows that these do not co-localize and ciliary band is a distinct region of ectoderm (Figs. 1C E; oral view). In the early pluteus larva some neurons are in the thickened animal plate and around the mouth, but most reside in the ciliary band (Figs. 1A, B, H K). The cell bodies of ciliary band neurons are predominantly on the oral side of the ciliary band and bundled axon tracts connect the neurons and encircle the oral ectoderm (Figs. 1F G). Synaptotagmin B-containing projections from the ciliary band neurons underlie the aboral ectoderm, are oriented toward the posterior end of the larva, and are not bundled. On the left and right sides of the pluteus, lateral ganglia each include a cluster of 2 4 neuralcellbodiesbeneaththeaboral ectoderm that extend projections posteriorly and into the axonal tracts of the ciliary band. The only neural projections under the oral ectoderm are two bundles of axons that cross the oral ectoderm at the base of the postoral arms (Fig. 1B, arrow). The serotonergic neurons are restricted to the animal plate at this stage of development (Figs. 1L N). Thus, the types and organization of neurons and neural projections are distinctive in the oral and aboral ectoderm as well as in different parts of the ciliary band. The key features of the ciliary band neurons are: 1. Neuronal cell bodies are restricted to the oral side of the ciliary band; 2. Axons in the ciliary band form bundles; 3. Unbundled axons project posteriorly under the aboral ectoderm; 4. Only two axonal tracks at the base of the postoral arms project under the oral ectoderm. Suppressing Nodal signaling Injecting eggs with an Sp-nodal morpholino oligonucleotide (nodal- MO) results in embryos that are radialized with a gut that elongates toward the animal plate, yet no mouth forms. This phenotype is identical to that previously reported for Paracentrotus lividis (Duboc et al., 2004; Fig. 2A inset). A more severe phenotype with an everted archenteron, which has not been reported in P. lividus (Duboc et al., 2004) or Hemicentrotus pulcherrimus (data not shown), is common in S. purpuratus. At the end of gastrulation, there is a large disk of tightly packed, columnar cells, cells wide, that contain nuclear Hnf6 (Figs. 2B, C). This disk includes the animal plate, as it expresses Nk2.1 (Figs. 2J L; Takacs et al., 2004; Yaguchi et al., 2006) and serotonergic neurons (Figs. 2E, F), and additional ectoderm derived from animal
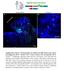
3 S. Yaguchi et al. / Developmental Biology 347 (2010) Fig. 1. Ciliary band and ciliary band neurons in the sea urchin pluteus larvae. (A) Schematic diagrams of ciliary band in the larva. Green lines show ciliary band between oral ectoderm and aboral ectoderm, and magenta and orange dots show ciliary band neurons and lateral ganglia. m, mouth; a, anus. (B) The entire nervous systems of the pluteus larva revealed with a pan-neural marker, anti-synaptotagmin (1E11). Arrowheads show the two axonal tracts underlying the oral ectoderm at the base of the postoral arms. (C E) Double staining using anti-goosecoid (C; Gsc) for oral ectoderm and anti-hnf6 for ciliary band (D, E). Green dots outside of the band are non-specific signal produced by the Hnf6 antibody. The ciliary band lies adjacent to the oral ectoderm, but never overlaps. (F G) Double staining with anti-synaptotagmin and anti-hnf6. Magnified image for the rectangle in (G) shows the detail of this co-localization (H K). The neurons have nuclear Hnf6 (K). (L N) Serotonergic neurons of the animal plate are a subset of ciliary band-associated neurons in the early pluteus larva. Bars =20 μm. blastomeres. The number and percentage of Hnf6-positive ciliary band cells in nodal-mo-injected embryos are greater than those in normal embryos (Fig. 2P). The ciliary band cells comprise approximately 50% of the ectoderm in nodal-mo-injected embryos, whereas in normal embryos they are about one-third of the total ectoderm cells. There is another region lacking nuclear Hnf6, but marked by small, non-nuclear spots of non-specific staining in the basal bodies. Cells in this region are less densely packed and squamous. Thus in nodal-mo embryos, the ectoderm is specified as animal plate, surrounded by an expanded region of ciliary band cells, and a squamous epithelium that has features of aboral ectoderm. Synaptotagmin-expressing neurons differentiate throughout much of the ectoderm in nodal-mo embryos, although most of the cell bodies are associated with the ciliary band. Much of the Synaptotagmin signal is in growth cones on neurites projecting toward the blastopore beneath the aboral ectoderm (Figs. 2A F). The neurons in the ciliary band region do not interconnect to form bundled axon tracts (Figs. 2A, D). An alternative, although not equivalent, means of suppressing Nodal signaling is over-expression of lefty, an endogenous antagonist of Nodal (Duboc et al., 2008). As expected, the Nodal-dependent gene, gsc, is not expressed in these embryos (Figs. 2G, I; Gsc protein), which are similar in form to nodal-mo embryos, with a radialized ectoderm, a straight archenteron and no mouth. The Hnf6 expression pattern, the distribution of neurons and axon projections are also the same in lefty RNA-injected embryos as described for nodal-mo containing embryos (Figs. 2G L). Similarly, embryos injected with a dominant-negative version of smad2/3 (Yaguchi et al., 2007), a downstream effector of Nodal signals, have a similar phenotype to nodal-mo- or lefty RNAinjected embryos: Synaptotagmin neurons differentiate along the margin of the thickened ectoderm and extend neurites posteriorly (Figs. 2M O). Taken together, these results show that embryos lacking Nodal function have 3 types of ectoderm: animal plate, a region with some ciliary band features, and a more vegetal region with aboral ectoderm features. Most of nerve cell bodies are near the ciliary band, but they lack the neural patterning characteristic of ciliary band. Enhancing Nodal signaling Injecting eggs with nodal RNA also produces a radialized embryo, but in this case, four regions of ectoderm are present and arranged
4 74 S. Yaguchi et al. / Developmental Biology 347 (2010) along the animal vegetal axis: the animal plate, and successive rings of oral ectoderm, ciliary band, and aboral-like ectoderm (Duboc et al., 2004; Yaguchi et al., 2006). Hnf6 protein is detected in cells of the animal plate and in a thin, interrupted strip of ciliary band, 1 2 cells wide (Fig. 3B), confirming previously reported in situ hybridization data (Yaguchi et al., 2006). In nodal RNA-injected embryos, the cell bodies of Synaptotagmin-expressing neurons are predominantly in the ciliary band, axons form a single tract that joins the neurons and short neurites project posteriorly toward the blastopore. (Figs. 3A, C, D, F). In 4-day plutei, neurons expressing Synaptotagmin appear in the animal plate, but there are no cells expressing serotonin (Figs. 3D F). Lefty-MO-injected embryos are similar to nodal mrna-injected embryos and identical to those previously reported for P. lividus (Duboc et al., 2008). The expression of the oral ectoderm marker, Gsc, is radialized in both cases (Figs. 3G I; Duboc et al., 2008) and serotonergic neurons do not differentiate in the animal plate (Figs. 3J L; Yaguchi et al., 2007). However, there is no ciliary band, as Hnf6 protein (Fig. 3H) and synaptotagmin neurons are found only in the animal plate (Figs. 3J L) in lefty-mo-injected embryos. Embryos injected with act-smad2/3 mrna are similar in form to lefty-mo-injected embryos, being radialized, covered with thin ectoderm and lacking serotonergic neurons (Figs. 3N and O), but the constriction of mouth region is slightly delayed (Yaguchi et al., 2007). However, unlike lefty-mo embryos, there are no Synaptotagmin-expressing animal plate neurons (Figs. 3M O). Injection of nodal RNA results in ectopic Nodal signaling and also ectopic expression of Nodal-dependent genes like lefty, BMP2/4 and chordin. The neural patterning is normal in the ciliary band that forms in these embryos as cells are interconnected with bundled axons and extend aboral projections. The fact that misexpressed nodal can still direct the formation of a set of fully integrated ectodermal tissues supporting the differentiation and patterning of neural components reinforces the idea that it functions near the top of the oral/aboral ectoderm specification pathway. Suppressing BMP2/4 signaling BMP2/4-MO-injected embryos developed as previously described (Duboc et al., 2004). They are not radialized, as the gut bends to the oral side and fuses to form a mouth (Fig. 4B, inset). The overall form of the embryo is distorted, but they contain four regions of ectoderm: animal plate, oral ectoderm, ciliary band, and aboral ectoderm. However, the oral ectoderm marker, Gsc, is not restricted to the oral side but surrounds the animal plate (Figs. 4D F). A band 5 6 cells wide of Hnf6-expressing ciliary cells, slightly wider than ciliary bands in control embryos, is present but is displaced to the aboral side and does not intersect the ciliated cells in the animal plate, as in normal embryos (Figs. 4A F). The squamous ectoderm opposite to the oral ectoderm has a low cell density. Synaptotagmin-expressing neurons differentiate in embryos injected with BMP2/4-MO; however, they are not restricted to the ciliary band and some are found in the ectoderm on the aboral side of the embryo (Figs. 4A, C). The cell bodies are multipolar and neurites project randomly without forming bundled tracts. Few neurites associate with the oral ectoderm and no serotonergic neurons develop in the animal plate. Similarly, knockdown of Alk3/6, the only BMP receptor in the sea urchin genome, produced embryos with some features in common with embryos in which BMP2/4 expression is blocked (Lapraz et al., 2009; Figs. 4G I). Again, the ciliary band shifts toward the aboral side around the animal plate (Figs. 4H and I), oral ectoderm, marked by Gsc expression, surrounds the animal plate (Figs. 4J and L), and the animal plate marker Nk2.1 is expressed in animal pole cells, although this domain is larger than normal (Fig. 4L). Alk3/6-MO-injected embryos are more radial than BMP2/4-MO-injected embryos as the ciliary band is in a plane almost orthogonal to the animal vegetal axis. The major difference between the BMP2/4-MO and the Alk3/6-MO embryos is that the band of Hnf6-expressing cells is much wider, cells, than it is in BMP2/4-MO embryos. Synaptotagmin-expressing neurons differentiate throughout this broad band of cells expressing Hnf6 as well as ectopically throughout the aboral region. The neurons project neurites randomly throughout the non-oral half of the embryo and the neurons interconnect, but axon tracts fail to form. Thus, embryos in which expression of the Alk3/6 receptor is blocked are not identical to embryos in which one of the ligands, BMP2/4, is suppressed in this study. When RNA encoding a dominant-negative form of smad1/5 (dnsmad1/5) is injected into eggs, the embryos are similar in form to embryos injected with Alk3/6-MO. The timing of ingression of primary mesenchyme cells and archenteron invagination are the same as control or Alk3/6-MO-injected embryos, and the oral aboral polarity is also maintained as in Alk3/6-MO embryos (Fig. 4Q, inset). The oral territory is expanded to surround the animal plate and the Hnf6- expressing ciliary band is shifted aborally and is as wide as that in Alk3/ 6-MO-injected embryos (Figs. 4M O). Similarly, embryos expressing dn-smad1/5 have Synaptotagmin-expressing neurons throughout the aboral ectoderm. Only short randomly oriented projections form, the cells are not interconnected and axons do not bundle into tracts (Figs. 4P R). Thus, suppressing signaling that specifies aboral ectoderm with either Alk3/6-MO or dn-smad1/5 results in a large fraction of the ectoderm that supports the differentiation but not the patterning of neurons. These neurons are not associated with the band of Hnf6 cells, indicating BMP ligands are involved in the process that patterns neurons within the ciliary band. Enhancing BMP2/4 signaling Embryos injected with BMP2/4 mrna develop as previously described (Angerer et al., 2000; Yaguchi et al., 2006). Most of the ectoderm is squamous and does not express an oral marker, Gsc (Figs. 5G I). As well, neither ciliary band cells nor Synaptotagminexpressing ciliary band neurons differentiate (Figs. 5A F). The animal plate is pronounced, expresses Hnf6 and Nk2.1 and contains serotonergic neurons that express Synaptotagmin (Figs. 5C, F, and I). The embryos expressing act-smad1/5 have shortened archenterons and are phenotypically similar to those of BMP2/4 Fig. 2. The ciliary band is expanded and shifted to the animal pole in embryos in which Nodal-MO blocks the oral pathway. Ciliary band-associated neurons differentiate, most of the cell bodies are near the Hnf6-expressing ciliary band cells and axons with strongly immunoreactive growth cones project toward the vegetal pole. The position of cell bodies(*) and growth cones (arrowheads) are marked (A C). Ciliary band cells, identified with anti-hnf6, cover the lateral ectoderm initially, and become restricted to a thickened epithelium, cells wide, surrounding the animal plate. Ectoderm surrounding blastopore thins and expands to eventually cover the vegetal half of the embryo. (B). Serotonergic neurons are present exclusively at the animal plate (D, E). (F) The magnified image of rectangle in (E) shows the neural cell body (*) and growth cones (arrowheads). (G L) Lefty RNA-injected embryos have the same morphology as embryos injected with Nodal-MO. (G I) Goosecoid (Gsc) is not expressed (G), indicating that the oral ectoderm has not differentiated. The ectoderm is covered with ciliary band and thin, vegetal ectoderm (H and I). (J L) Ciliary band-associated neurons are present throughout the entire lateral ectoderm (* mark the position of cell bodies) and the animal plate marker, Nk2.1, is expressed exclusively in the animal plate. (M O) Embryos injected with RNA encoding a dominant-negative Smad2/3 have the same morphology as embryos injected with Nodal-MO. Ciliary band neurons are spread throughout the entire lateral ectoderm. Serotonergic neurons are present at the animal plate region (N and O). Inset in (M) shows the DIC image of dn-smad2/3 injected embryo. (P) The number and ratio of ciliary band cells are increased in Nodal morpholino injected embryos. The upper and lower numbers on the column show the number of ciliary band cells counted and total number of ectodermal cells. Those cells are counted in three individual embryos of each treatment. Bar=20 μm.
5 S. Yaguchi et al. / Developmental Biology 347 (2010) expressing embryos (Fig. 5A vs J). This phenotype also has neither ciliary band ectoderm nor ciliary band neurons (Figs. 5J and N). However, unlike BMP2/4 misexpressing embryos, these embryos lack serotonin-containing neurons in the animal plate. Taken together, these experiments indicate that BMP2/4 can inhibit formation of ciliary band and suppress differentiation of ciliary band neurons.
6 76 S. Yaguchi et al. / Developmental Biology 347 (2010) Fig. 3. Embryos are radialized and the ciliary band is reduced to a band 1 2 cells wide that is shifted toward the vegetal pole when Nodal signaling is enhanced. (A F) A thin Hnf6- positive band contains ciliary band neurons forming tracts of axons that interconnect cells. (C). Serotonergic neurons do not differentiate, because the animal plate is surrounded by oral ectoderm, but non-serotonergic neurons are present in the animal plate (E and F) (* mark the position of cell bodies). (G L) Lefty-MO-injected embryos are superficially similar to Nodal mrna-injected embryos, but there is no ciliary band (Hnf6 is restricted to the animal plate) and no ciliary band neurons. (G) Gsc is expressed radially and neither Hnf6- expressing ciliary cells nor synaptotagmin-positive neurons are detected in the lateral ectoderm (H L). (M O) Constitutively activated Smad2/3 mrna-injected embryos have a similar form to other embryos with enhanced Nodal signaling, but they are like the Lefty-MO-injected embryos in lacking ciliary band neurons. Inset in (M) shows the DIC image of act-smad2/3 injected embryo. Bar=20 μm. Discussion TGFβ signaling and differentiation of neurons Treatments that enhance BMP2/4 or Nodal signaling appear to inhibit neural differentiation. Misexpressed ligands could be acting on neural progenitors directly, or they could be acting indirectly on the non-neural ectoderm, which in turn either supports or suppresses neural differentiation. If signaling acts directly on ectodermal cells to prevent their differentiation as neurons, then blocking of that signaling with either a receptor morpholino or introduction of dnsmads should result in a cell autonomous increase in the number of neurons. However, if this happens it must affect only a small fraction of the precursors to the ciliary band neurons, indicating that most of them respond indirectly to TGFβ signals. Our model proposes that the indirect effect of TGFβ signaling is to provide the appropriate environment for neural development and the Hnf6-expressing ciliary cells provide this environment. Our experiments cannot eliminate the possibility that there is a direct effect of TGFß signaling on committed neural progenitors. Indeed, Yaguchi et al. (2006, 2007) have demonstrated that Nodal suppresses the differentiation of serotonergic cells on the oral margin of the animal plate. Understanding how TGFß signaling affects the specification and differentiation of neurons is hampered by not knowing the precise origin of neural progenitors.
7 S. Yaguchi et al. / Developmental Biology 347 (2010) Fig. 4. The embryos in which BMP2/4 signaling is blocked have wider than normal Hnf6-expressing ciliary bands that are shifted vegetally and unpatterned neurons. (A) Ciliary band neurons differentiate in association with the ciliary band and the aboral ectoderm. (B) The ciliary band is wider (5 6 cells in width) and shifted toward the aboral ectoderm. (C) Merged image of (A) and (B) (* mark the position of cell bodies). (D) Oral ectoderm identified with anti-gsc surrounds the animal plate. (E) Hnf6 marks the animal plate and shifted ciliary band away from animal plate. (F) Merged image of (D) and (E). (G L) Alk3/6 is the only BMP receptor in the sea urchin genome, yet morpholinos produce embryos with a more severe phenotype with a greatly expanded, radialized ciliary band (* mark the position of cell bodies). (G) Neurons differentiate in association with the ciliary band and the aboral ectoderm. (H) Hnf6 reveals that the ciliary band is shifted vegetally, does not connect with the animal plate, and is cells wide. (J L) An oral ectoderm, expressing Gsc surrounds the animal plate (Nk2.1). (M R) Embryos in which a dominant-negative form of Smad1/5 is injected are similar to embryos in which Alk3/6 receptor expression is suppressed. (M O) The animal plate, identified with an Nk2.1 antibody, is surrounded by oral ectoderm and there is a thickened ciliary band (10 12 cells wide; animal pole view). (P R) Ciliary band neurons differentiate in association with the ciliary band and project neurites with strongly immunoreactive growth cones toward the vegetal end of the embryo. Inset in (Q) shows the DIC image of dn-smad1/5 injected embryo. Bar=20 μm; o; oral ectoderm; ap, animal plate; cb, ciliary band; ab, aboral ectoderm.
8 78 S. Yaguchi et al. / Developmental Biology 347 (2010) Fig. 5. The embryos in which BMP2/4 signaling is enhanced are radialized and lack ciliary bands and ciliary band neurons. (A I) BMP2/4-misexpressing embryos. (A F) The embryos lack synaptotagminb-expressing neurons and Hnf6-expressing ciliary band cells throughout the lateral ectoderm. However, serotonergic neurons are invariably present in the animal plate (B and C), indicating the animal plate is not influenced by the misexpression of BMP2/4. (G I) Embryos lack oral ectoderm expressing Gsc, but express NK2.1 in the animal plate. (J O) The embryo in which constitutively activated Smad1/5 mrna has been injected is similar to BMP2/4-misexpressing embryos. They have an animal plate identified with Hnf6 and Nk2.1 (M O), but no serotonergic neurons differentiate because of the intracellular activation of Smad1/5 (K L). Inset in (J) shows the DIC image of dn- Smad1/5 injected embryo. Bar=20 μm. Much of the behavior of neurons reported in untreated embryos and in embryos resulting from the perturbations described here is consistent with a model in which a region free of TGFß is required for the differentiation of neurons and the bundling of axons. Neurons do not differentiate in treatments that result in the loss of the ciliary band and when the ciliary band is displaced, neurons differentiate at the new site. The exception is the appearance of synaptotagmin-expressing neurons in the aboral ectoderm of BMP2/4 morpholino injected embryos, which is, nevertheless, a TGFβ-deficient region. Whether they develop in this region or their precursors migrate there is not clear because synaptotagmin is a late differentiation marker. There are numerous situations in neural development of other metazoans in which neural progenitors must receive appropriate neurotrophic support to differentiate and neurite outgrowth is directed by axon guidance cues that determine the direction of neurite growth and regulate axon bundling by regulating adhesion (Chilton, 2006). We propose that the ciliary band provides an environment conducive to neural development and organization. A model for patterning the ciliary band When Nodal is expressed in the oral ectoderm, it initiates a sequence of signaling and differentiation events that includes the expression of factors that antagonize or modify Nodal and BMP2/4 signals (Fig. 6). So
9 S. Yaguchi et al. / Developmental Biology 347 (2010) Fig. 6. Schematic diagram for the signaling pathways regulating ciliary band formation and the differentiation of ciliary band-associated neurons. Nodal expression in the oral ectoderm initiates expression of factors that antagonize or modify Nodal and BMP2/4 signals. Lefty and Chordin exclude TGFβ signals from the presumptive ciliary band (thin red barheads). Localized Nodal signaling positions the oral margin of the ciliary band and BMP signaling through Alk3/6 narrows the width of the ciliary band (barheads to ciliary band margins). This model proposes that an indirect effect of TGFβ signaling is to provide the appropriate environment for neural differentiation. Exclusion of BMP and Nodal from the ciliary band ectoderm is required for the differentiation of neurons and the outgrowth and bundling of axons. Oral ectoderm cannot support these events and aboral ectoderm only promotes growth of unbundled neurites. Thus, Nodal and BMP2/4 block development of most, if not all, ciliary band neurons (barheads to neural cell body), yet the correct patterning of these cells within the ciliary band depends on the presence of both of these signals (black arrowheads to axons). far, Lefty and Chordin have this role (Duboc et al., 2004, 2008; Bradham et al., 2009; Lapraz et al., 2009), and we propose that they exclude TGFβ signals from the ciliary band. Here we show that Nodal signaling, localized by Lefty, positions the oral margin of the ciliary band. Signaling through the BMP receptor, Alk3/6, also has an effect on the position of the oral margin and determines the aboral margin of the ciliary band. This narrows the potential width of the band from about 12 cells to 4. Signaling by BMP2/4 also affects the position of both margins and blocks the development of ciliary band neurons. Although Nodal strongly reduces and shifts the position of the ciliary band and BMP2/4 signaling blocks it, each with corresponding changes in the development of ciliary band neurons, both signals are required for the correct patterning of these cells within the ciliary band. In the normal embryo, the ectoderm that is subject to these TGFβ signals includes all of the ectoderm except the animal plate and the ectoderm surrounding the blastopore. Little is known about specification of the vegetal ectoderm, but it likely involves canonical Wnt signaling, which is active in precursors during cleavage stages. However, the specification mechanisms of the ectoderm at the animal pole (Wei et al., 2009) and that of oral and aboral ectoderm, which require TGFβ signals, are beginning to emerge (Duboc et al., 2004; Range et al., 2007; Nam et al., 2007; Bradham et al., 2009; Su et al., 2009; Lapraz et al., 2009). Here we have examined how these signals position cells in the intervening region that expresses Hnf6 and form the ciliary band. The Nodal pathway is sufficient for patterning of the ciliary band All the data presented here suggest that Nodal initiates a series of events, including expression of another TGFβ, BMP2/4, that are required for the differentiation and patterning of the neural components of the ciliary band in the TGFβ-responsive ectoderm. We found that differentiated Synaptotagmin-expressing neurons form in embryos misexpressing act-smad2/3, which transduces the effects of Nodal signaling throughout the embryo, and bypasses negative feedback regulation by Lefty, a Nodal antagonist. In contrast, an innervated ciliary band can form in embryos misexpressing nodal, and its downstream target lefty, even though nodal is initially expressed uniformly throughout the embryo. Our data support the proposition that Lefty is a critical regulator of ciliary band formation. When Lefty expression is blocked, the domain of endogenous Nodal signaling expands and no ciliary band is detectable by Hnf6 staining. In addition, the ectoderm that results does not support differentiation of Synaptotagmin-expressing neurons. The loss of ciliary band and neurons in embryos with suppressed expression of Lefty argues that in the normal embryo prevention of ectopic Nodal signaling by Lefty is an essential feature of patterning of the TGFβ-responsive ectoderm, as proposed by Duboc and Lepage (2008). When Nodal signaling is blocked, all but the most vegetal ectoderm continues to express Hnf6. When BMP signaling is blocked, the number of ciliary band cells expressing Hnf6 also increases and appear ectopically on both the oral and aboral sides of the embryo. In contrast, in embryos misexpressing BMP2/4, they are absent (this paper; Angerer et al., 2000; Duboc et al., 2004; Bradham et al., 2009; Lapraz et al., 2009). In the normal embryo, Hnf6 is expressed broadly throughout the embryo before Nodal or BMP2/4 signaling begins, and continues to be widespread until the hatching blastula stage (Otim et al., 2004). Subsequently it restricts to a narrow strip of cells during mesenchyme blastula stages in a process shown here to depend on these signals. Control of BMP signaling appears to be mediated, at least in part, by Chordin, which has been shown to antagonize BMP2/4 in sea urchin embryos (L. variegateus) and is necessary for correct formation of the ciliary band and development of ciliary band neurons (Bradham et al., 2009), although the responses to Chordin perturbations in another
10 80 S. Yaguchi et al. / Developmental Biology 347 (2010) species (Paracentrotus lividus) are somewhat different (Lapraz et al., 2009). Taken together, these observations suggest that TGFβ signaling transforms most of the early ectoderm into an epidermal regulatory state except in cells where these signals are excluded; the site of ciliary band formation. It follows that the ciliary band, and subsequently the development and patterning of neurons within it, require protection from Nodal and BMP. Restriction of the ciliary band to a narrow strip of cells expressing Hnf6 follows shortly after the activation of the oral signaling network. The levels of nodal and lefty mrnas increase significantly during early blastula stages and chordin and BMP2/4 transcription is up-regulated a few hours later during mesenchyme blastula stage (Angerer et al., 2000; Bradham et al., 2009). This precedes by only a few hours the emergence of the ciliary band at late mesenchyme blastula stage (Otim et al., 2004; Poustka et al., 2004). Exactly how spatially regulated TGFβ signaling restricts the expression of hnf6 is not yet clear, however, it is likely that mechanisms that control the levels and distribution of Lefty, Chordin and other TGFβ antagonists are involved. Nodal and BMP signaling position the ciliary band The position and size of the band of cells expressing Hnf6 is dramatically altered when the domain of Nodal expression is altered. When it is blocked, the band is cells wide and shifts toward the animal pole of the embryo, whereas, when it is mis- expressed, the band is reduced to a width of only 1 cell and shifts toward the vegetal end of the embryo. As there is a small difference in the proportion of cells expressing Hnf6 in embryos injected with nodal-mo, it appears the predominant effect is to respecify a significant fraction of ectoderm cells, which alters the position of the cells expressing Hnf6. These observations suggest that Nodal signaling regulates the position of the oral margin of Hnf6 expressing ciliary band cells. When embryos are injected with BMP2/4 RNA or act-smad1/5 no band of Hnf6 cells forms. As well, when BMP2/4 signaling is suppressed (BMP2/4-MO, dn-smad1/5 RNA, Alk3/6-MO), the band of Hnf6 cells that forms is shifted away from the animal plate toward the vegetal pole and is wider. These data indicate that BMP signaling also has a role in positioning the ciliary band. BMP also appears to have a clear effect on the position of the aboral margin of the ciliary band. Its posterior margin is restricted by factors that signal through ALK3/6. BMP2/4 appears to play a relatively small role in this process. Loss of BMP2/4 increases the band from 4 to 5 or 6 cells in width, even at concentrations of morpholino approaching toxicity, while loss of Alk3/6 further increases it to cells. This raises the possibility that more than one BMP ligand may be involved in patterning of this region of the aboral ectoderm. However, Lapraz et al. (2009) do not report a large difference between the effects of BMP2/4-MO and Alk3/6-MO on embryos of Paracentrotus lividus. This suggests that there is either an incomplete suppression of BMP2/4 in S. purpuratus or species differences in regulation of aboral ectoderm specification by BMP pathways. Vegetal ectoderm is resistant to TGFβ signals The specification and differentiation of the most vegetal region of ectoderm is poorly understood. Perturbations of Nodal or BMP signaling make it clear that this ectoderm responds differently than more animal ectoderm. Although loss of BMP signaling results in expansion of the ciliary band, it does not extend into the vegetal ectoderm. Loss of Nodal signaling, and consequently BMP2/4, reveals that the vegetal ectoderm continues to express aboral markers rather than Hnf6 (Duboc et al., 2004). Although misexpression of nodal generates a ciliary band near the vegetal pole, there remains a vegetal strip of aboral-like ectoderm (this work, Duboc et al., 2004). At least some of this ectoderm is probably derived from veg1 blastomeres, which surround the blastopore (Davidson et al., 1998), and as such its regulatory state is likely to be different from animal blastomere-derived ectoderm as a result of vegetal Wnt signaling (Davidson et al., 2002). Patterning of the ciliary band nervous system Nodal and BMP2/4 specify oral and aboral ectoderm, and suppression of these signals in a narrow region of ectoderm between them produces the ciliary band. These tissues appear to control how the neurons develop within this band. Oral ectoderm inhibits differentiation of neurons and outgrowth of neurites, but aboral ectoderm supports outgrowth of unbundled neurites. The ciliary band cells are under the influence of a gene regulatory network that includes hnf6, but the presence of Hnf6 is not sufficient to ensure correct patterning of ciliary band neurons. The Hnf6-expressing cells are capable of forming a thickened, ciliated epithelium but, in the absence of TGFβ signals, they do not support correct formation of bundled axonal tracts that interconnect. The mechanisms by which TGFβ signaling affects the direction of neural projections and the interactions among them are not understood. Rigorous testing will be required to understand the intricate mechanisms by which TGFβ signaling patterns the elegant, yet relatively simple, tissues that serve the critical functions of swimming and feeding in the larva. Acknowledgments This work was supported in part by a Discovery grant from the Natural Sciences and Engineering Research Council (Canada) to RDB, in part by the Intramural Program of the National Institutes of Health, NIDCR, and in part by Special Coordination Funds for Promoting Science and Technology of the Japanese Government to SY. We thank Thierry Lepage and Francois Lapraz for sharing reagents and unpublished data with us. References Angerer, L.M., Oleksyn, D.W., Logan, C.Y., McClay, D.R., Dale, L., Angerer, R.C., A BMP pathway regulates cell fate allocation along the sea urchin animal vegetal embryonic axis. Development 127, Angerer, L.M., Oleksyn, D.W., Levine, A.M., Li, X., Klein, W.H., Angerer, R.C., Sea urchin goosecoid function links fate specification along the animal vegetal and oral aboral embryonic axes. Development 128, Bradham, C.A., Oikonomou, C., Kuhn, A., Core, A.B., Modell, J.W., McClay, D.R., Poustka, A.J., Chordin is required for neural but not axial development in sea urchin embryos. Dev. Biol. 328, Bolouri, H., Davidson, E.H., The gene regulatory network basis of the community effect, and analysis of a sea urchin embryo example. Dev. Biol. 340 (2), Burke, R.D., The structure of the nervous system of the pluteus larva of Strongylocentrotus purpuratus. Cell Tissue Res. 191, Chen, C.H., Shen, M.M., Two modes by which lefty proteins inhibit Nodal signaling. Curr. Biol. 14, Chilton, J.K., Molecular mechanisms of axon guidance. Dev. Biol. 292, Davidson, E.H., Cameron, R.A., Ransick, A., Specification of cell fate in the sea urchin embryo: summary and some proposed mechanisms. Development 125, Davidson, E.H., Rast, J.P., Oliveri, P., Ransick, A., Calestani, C., Yuh, C.H., Minokawa, T., Amore, G., Hinman, V., Arenas-Mena, C., et al., A provisional regulatory gene network for specification of endomesoderm in the sea urchin embryo. Dev. Biol. 246, De Robertis, E.M., Kuroda, H., Dorsal ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 20, Duboc, V., Lepage, T., A conserved role for the nodal signaling pathway in the establishment of dorso ventral and left right axes in deuterostomes. J. Exp. Zool. B Mol. Dev. Evol. 310B, Duboc, V., Rottinger, E., Besnardeau, L., Lepage, T., Nodal and BMP2/4 signaling organizes the oral aboral axis of the sea urchin embryo. Dev. Cell 6, Duboc, V., Lapraz, F., Besnardeau, L., Lepage, T., Lefty acts as an essential modulator of Nodal activity during sea urchin oral aboral axis formation. Dev. Biol. 320, Kenny, A.P., Oleksyn, D.W., Newman, L.A., Angerer, R.C., Angerer, L.M., Tight regulation of SpSoxB factors is required for patterning and morphogenesis in sea urchin embryos. Dev. Biol. 261, Lapraz, F., Besnardeau, L., Lepage, T., Patterning of the dorsal ventral axis in echinoderms: insights into the evolution of the BMP Chordin signaling network. PLoS Biol. 7 (11). Mackie, G.O., Spencer, A.N., Strathmann, R., Electrical activity associated with ciliary reversal in an echinoderm larva. Nature 223,
11 S. Yaguchi et al. / Developmental Biology 347 (2010) Meno, C., Saijoh, Y., Fujii, H., Ikeda, M., Yokoyama, T., Yokoyama, M., Toyoda, Y., Hamada, H., Left right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature 381, Nakajima, Y., Kaneko, H., Murray, G., Burke, R.D., Divergent patterns of neural development in larval echinoids and asteroids. Evol. Dev. 6, Nam, J., Su, Y.H., Lee, P.Y., Robertson, A.J., Coffman, J.A., Davidson, E.H., Cisregulatory control of the nodal gene, initiator of the sea urchin oral ectodenn gene network. Dev. Biol. 306, Otim, O., Amore, G., Minokawa, T., McClay, D.R., Davidson, E.H., SpHnf6, a transcription factor that executes multiple functions in sea urchin embryogenesis. Dev. Biol. 273, Poustka, A.J., Kuhn, A., Radosavljevic, V., Wellenreuther, R., Lehrach, H., Panopoulou, G., On the origin of the chordate central nervous system: expression of onecut in the sea urchin embryo. Evol. Dev. 6, Range, R., Lapraz, F., Quirin, M., Marro, S., Besnardeau, L., Lepage, T., Cis-regulatory analysis of nodal and maternal control of dorsal ventral axis formation by Univin, a TGF-beta related to Vg1. Development 134, Satterlie, R.A., Cameron, R.A., Electrical-activity at metamorphosis in larvae of the sea-urchin Lytechinus-pictus (Echinoidea, Echinodermata). J. Exp. Zool. 235, Strathmann, R.R., Time and extent of ciliary response to particles in a nonfiltering feeding mechanism. Biol. Bull. 212, Su, Y.H., Li, E., Geiss, G.K., Longabaugh, W.J.R., Kramer, A., Davidson, E.H., A perturbation model of the gene regulatory network for oral and aboral ectoderm specification in the sea urchin embryo. Dev. Biol. 329, Takacs, C.M., Amore, G., Oliveri, P., Poustka, A.J., Wang, D., Burke, R.D., Peterson, K.J., Expression of an NK2 homeodomain gene in the apical ectoderm defines a new territory in the early sea urchin embryo. Dev. Biol. 269, Wei, Z., Yaguchi, J., Yaguchi, S., Angerer, R.C., Angerer, L.M., The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development 136, Yaguchi, S., Yaguchi, J., Burke, R.D., Specification of ectoderm restricts the size of the animal plate and patterns neurogenesis in sea urchin embryos. Development 133, Yaguchi, S., Yaguchi, J., Burke, R.D., Sp-Smad2/3 mediates patterning of neurogenic ectoderm by nodal in the sea urchin embryo. Dev. Biol. 302, Yaguchi, S., Yaguchi, J., Angerer, R.C., Angerer, L.M., A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev. Cell 14,
TGFβ signaling positions the ciliary band and acts indirectly to pattern neurons in the sea urchin embryo
 TGFβ signaling positions the ciliary band and acts indirectly to pattern neurons in the sea urchin embryo Shunsuke Yaguchi 1,2,3, Junko Yaguchi 1,2,3, Robert C. Angerer 3, Lynne M. Angerer 3, Robert D.
TGFβ signaling positions the ciliary band and acts indirectly to pattern neurons in the sea urchin embryo Shunsuke Yaguchi 1,2,3, Junko Yaguchi 1,2,3, Robert C. Angerer 3, Lynne M. Angerer 3, Robert D.
Laboratory Exercise 1 Laboratory Examination of a Basal Deuterostome: Sea Urchin Embryos
 S. S. SUMIDA BIOLOGY 340 Comparative Embryology Introduction Laboratory Exercise 1 Laboratory Examination of a Basal Deuterostome: Sea Urchin Embryos Many laboratories that examine sea urchin embryos do
S. S. SUMIDA BIOLOGY 340 Comparative Embryology Introduction Laboratory Exercise 1 Laboratory Examination of a Basal Deuterostome: Sea Urchin Embryos Many laboratories that examine sea urchin embryos do
Axis Formation and Mesoderm Induction
 Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
Lecture IV. Mechanisms of Neural. Neural Development
 Lecture IV. Mechanisms of Neural Bio 3411 Monday 1 Readings NEUROSCIENCE: 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) References : Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994).
Lecture IV. Mechanisms of Neural Bio 3411 Monday 1 Readings NEUROSCIENCE: 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) References : Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994).
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development.
 Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development. (A) Temporal expression of Xdscr6 at various stages (numbers on the top) and its distribution
Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development. (A) Temporal expression of Xdscr6 at various stages (numbers on the top) and its distribution
C. elegans Embryonic Development
 Autonomous Specification in Tunicate development Autonomous & Conditional Specification in C. elegans Embryonic Development Figure 8.36 Bilateral Symmetry in the Egg of the Tunicate Styela partita Fig.
Autonomous Specification in Tunicate development Autonomous & Conditional Specification in C. elegans Embryonic Development Figure 8.36 Bilateral Symmetry in the Egg of the Tunicate Styela partita Fig.
Nodal and BMP2/4 Signaling Organizes the Oral-Aboral Axis of the Sea Urchin Embryo
 Developmental Cell, Vol. 6, 397 410, March, 2004, Copyright 2004 by Cell Press Nodal and BMP2/4 Signaling Organizes the Oral-Aboral Axis of the Sea Urchin Embryo Véronique Duboc, Eric Röttinger, Lydia
Developmental Cell, Vol. 6, 397 410, March, 2004, Copyright 2004 by Cell Press Nodal and BMP2/4 Signaling Organizes the Oral-Aboral Axis of the Sea Urchin Embryo Véronique Duboc, Eric Röttinger, Lydia
Patterning the Embryo
 Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Cell Type Nervous System I. Developmental Readout. Foundations. Stem cells. Organ formation. Human issues.
 7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Tetrapod Limb Development
 IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
Ciliary Movement of Sea-urchin Embryos
 Ciliary Movement of Sea-urchin Embryos Kogiku Shiba*, Yoshihiro Mogami** and Shoji A. Baba*** *Division of Life Sciences, Graduate School of Humanities and Sciences g0040425@edu.cc.ocha.ac.jp **Department
Ciliary Movement of Sea-urchin Embryos Kogiku Shiba*, Yoshihiro Mogami** and Shoji A. Baba*** *Division of Life Sciences, Graduate School of Humanities and Sciences g0040425@edu.cc.ocha.ac.jp **Department
VERGE 3 Lundeberg 1. Dependence of fertilization in sea urchins, Strongylocentrotus purpuratus, on microfilament
 VERGE 3 Lundeberg 1 Dependence of fertilization in sea urchins, Strongylocentrotus purpuratus, on microfilament formation and internal calcium concentration Megan Lundeberg Amy Ruggerio and Amy Isaacson
VERGE 3 Lundeberg 1 Dependence of fertilization in sea urchins, Strongylocentrotus purpuratus, on microfilament formation and internal calcium concentration Megan Lundeberg Amy Ruggerio and Amy Isaacson
Non-canonical Notch signaling represents an ancestral mechanism to regulate neural differentiation
 Layden and Martindale EvoDevo 2014, 5:30 RESEARCH Non-canonical Notch signaling represents an ancestral mechanism to regulate neural differentiation Michael J Layden 1 and Mark Q Martindale 2* Open Access
Layden and Martindale EvoDevo 2014, 5:30 RESEARCH Non-canonical Notch signaling represents an ancestral mechanism to regulate neural differentiation Michael J Layden 1 and Mark Q Martindale 2* Open Access
Phylum Echinodermata
 Phylum Echinodermata Spiny Skin Echinodermata Radially symmetrical in five ways (pentamerous) Echinodermata No head, right or left side, or top or bottom, so we refer to the oral surface (mouth side) and
Phylum Echinodermata Spiny Skin Echinodermata Radially symmetrical in five ways (pentamerous) Echinodermata No head, right or left side, or top or bottom, so we refer to the oral surface (mouth side) and
Inner ear development Nervous system development
 Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Emx2 patterns the neocortex by regulating FGF positional signaling
 Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
EMBO REPORT SUPPLEMENTARY SECTION. Quantitation of mitotic cells after perturbation of Notch signalling.
 EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Polarity and Segmentation. Chapter Two
 Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
SUPPLEMENTARY INFORMATION
 Supplementary Information included with Nature MS 2008-02-01484B by Colantonio et al., entitled The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. This
Supplementary Information included with Nature MS 2008-02-01484B by Colantonio et al., entitled The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. This
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
A study of cilia density in sea urchin embryos during gastrulation
 A study of cilia density in sea urchin embryos during gastrulation Steve Das Independent Research Project Report Bio 254 Developmental Biology May 3, 2012 Introduction Embryo growth and development is
A study of cilia density in sea urchin embryos during gastrulation Steve Das Independent Research Project Report Bio 254 Developmental Biology May 3, 2012 Introduction Embryo growth and development is
Option A: Neurobiology & Behavior HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND
 Option A: Neurobiology & Behavior A1: NEURAL DEVELOPMENT USE THE INFO IN THE PRESENTATION TO COMPLETE A1 NOTES GUIDE INFORMATION TAKEN FROM: HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND WARD BIOLOGY
Option A: Neurobiology & Behavior A1: NEURAL DEVELOPMENT USE THE INFO IN THE PRESENTATION TO COMPLETE A1 NOTES GUIDE INFORMATION TAKEN FROM: HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND WARD BIOLOGY
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and
 Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Phylum Echinodermata. by: Muhammad Arif Asadi
 Phylum Echinodermata by: Muhammad Arif Asadi Pentaradial symmetry no freshwater or terrestrial representatives Water vascular system: A complex series of fluid filled canals with numerous flexible feeding
Phylum Echinodermata by: Muhammad Arif Asadi Pentaradial symmetry no freshwater or terrestrial representatives Water vascular system: A complex series of fluid filled canals with numerous flexible feeding
Developmental Biology
 Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
 Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
Supplemental Information. Ciliary Beating Compartmentalizes. Cerebrospinal Fluid Flow in the Brain. and Regulates Ventricular Development
 Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were
 Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons.
 Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Heavy Metals Effect on the Fertilization and Development of Sea Urchin Embryos
 Heavy Metals Effect on the Fertilization and Development of Sea Urchin Embryos Kristin Parish, Jessica Brown, Renee Cook and Megan Kapp January 2012 Abstract Heavy metal pollution is a prevalent threat
Heavy Metals Effect on the Fertilization and Development of Sea Urchin Embryos Kristin Parish, Jessica Brown, Renee Cook and Megan Kapp January 2012 Abstract Heavy metal pollution is a prevalent threat
20 2 Stomach Fig. 2.1 An illustration showing different patterns of the myenteric plexus peculiar to the regions in the guinea-pig stomach stained wit
 Stomach 2 The stomach is unique in that ICC have a different distribution in proximal and distal regions of the same organ. ICC-CM and ICC-LM are densely distributed throughout the thick circular and longitudinal
Stomach 2 The stomach is unique in that ICC have a different distribution in proximal and distal regions of the same organ. ICC-CM and ICC-LM are densely distributed throughout the thick circular and longitudinal
Tetrapod Limb Development
 Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Tetrapod Limb Development
 Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines
 Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Regionalization of the nervous system. Paul Garrity 7.68J/9.013J February 25, 2004
 Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Chapter 7 Conclusions
 VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN
 UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Nature Genetics: doi: /ng Supplementary Figure 1. Assessment of sample purity and quality.
 Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Hepatogenesis I Liver development
 Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates
 Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Muscle Tissue. General concepts. Classification of muscle. I. Functional classification is based on the type of neural control.
 Muscle Tissue LEARNING OBJECTIVES 1. Identify the three types of muscle tissue at the light microscopic level. 2. List and compare the structural and functional features of each of the three muscle fiber
Muscle Tissue LEARNING OBJECTIVES 1. Identify the three types of muscle tissue at the light microscopic level. 2. List and compare the structural and functional features of each of the three muscle fiber
klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach
![klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach](/thumbs/91/104639484.jpg) DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
Embryology of the Nervous System. Steven McLoon Department of Neuroscience University of Minnesota
 Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons
 Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
More Than 95% Reversal of Left Right Axis Induced by Right-Sided Hypodermic Microinjection of Activin into Xenopus Neurula Embryos
 Developmental Biology 221, 321 336 (2000) doi:10.1006/dbio.2000.9666, available online at http://www.idealibrary.com on More Than 95% Reversal of Left Right Axis Induced by Right-Sided Hypodermic Microinjection
Developmental Biology 221, 321 336 (2000) doi:10.1006/dbio.2000.9666, available online at http://www.idealibrary.com on More Than 95% Reversal of Left Right Axis Induced by Right-Sided Hypodermic Microinjection
Supplementary Information
 1 Supplementary Information A role for primary cilia in glutamatergic synaptic integration of adult-orn neurons Natsuko Kumamoto 1,4,5, Yan Gu 1,4, Jia Wang 1,4, Stephen Janoschka 1,2, Ken-Ichi Takemaru
1 Supplementary Information A role for primary cilia in glutamatergic synaptic integration of adult-orn neurons Natsuko Kumamoto 1,4,5, Yan Gu 1,4, Jia Wang 1,4, Stephen Janoschka 1,2, Ken-Ichi Takemaru
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) (b) (c) (d) (e) (f) (g) .
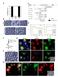 Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) The inhibitory effects of new antibodies (Mab17 and Mab18). They were investigated in in vitro fertilization
Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) The inhibitory effects of new antibodies (Mab17 and Mab18). They were investigated in in vitro fertilization
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Effects of pco 2 on the fertilization and development of the sea urchin Stronglyocentrotus nudus
 Effects of pco 2 on the fertilization and development of the sea urchin Stronglyocentrotus nudus Young-Gyu Park, Sang-wha Choi, Seong-Gil Kang Korea Ocean R & D Inst., Korea Jung-Suk Lee, Chan-Gyung Sung,
Effects of pco 2 on the fertilization and development of the sea urchin Stronglyocentrotus nudus Young-Gyu Park, Sang-wha Choi, Seong-Gil Kang Korea Ocean R & D Inst., Korea Jung-Suk Lee, Chan-Gyung Sung,
p)robably reflects seasoinal variations in egg populations. No results were considered unless
 MITOTIC ABNORM1ALITIES IN SEA URCHIN EMBRYOS EXPOSED TO DACTINOMIYCIN* BY BARRY I. KIEFER, CHARLES F. ENTELIS, AND ANTHONY A. INFANTE DEPARTMENT OF BIOLOGY, WESLEYAN UNIVERSITY, MIDDLETOWN, CONNECTICUT
MITOTIC ABNORM1ALITIES IN SEA URCHIN EMBRYOS EXPOSED TO DACTINOMIYCIN* BY BARRY I. KIEFER, CHARLES F. ENTELIS, AND ANTHONY A. INFANTE DEPARTMENT OF BIOLOGY, WESLEYAN UNIVERSITY, MIDDLETOWN, CONNECTICUT
MBios 401/501: Lecture 12.1 Signaling IV. Slide 1
 MBios 401/501: Lecture 12.1 Signaling IV Slide 1 Pathways that require regulated proteolysis 1. Notch and Delta 2. Wnt/ b-catenin 3. Hedgehog 4. NFk-B Our last topic on cell signaling are pathways that
MBios 401/501: Lecture 12.1 Signaling IV Slide 1 Pathways that require regulated proteolysis 1. Notch and Delta 2. Wnt/ b-catenin 3. Hedgehog 4. NFk-B Our last topic on cell signaling are pathways that
Muscle and Neuromuscular Junction. Peter Takizawa Department of Cell Biology
 Muscle and Neuromuscular Junction Peter Takizawa Department of Cell Biology Types and structure of muscle cells Structural basis of contraction Triggering muscle contraction Skeletal muscle consists of
Muscle and Neuromuscular Junction Peter Takizawa Department of Cell Biology Types and structure of muscle cells Structural basis of contraction Triggering muscle contraction Skeletal muscle consists of
Summary and conclusions
 Summary and conclusions 133 Part 1: Toxicity effects of Cry toxins upon hemocoelic delivery to A. janata larvae The first investigation was aimed to find out whether other modes of delivery of Cry toxins
Summary and conclusions 133 Part 1: Toxicity effects of Cry toxins upon hemocoelic delivery to A. janata larvae The first investigation was aimed to find out whether other modes of delivery of Cry toxins
Supplemental Information. Tissue Myeloid Progenitors Differentiate. into Pericytes through TGF-b Signaling. in Developing Skin Vasculature
 Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Histology Notes -Part 1: Epithelial Tissues
 Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
Supplementary Information. Preferential associations between co-regulated genes reveal a. transcriptional interactome in erythroid cells
 Supplementary Information Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells Stefan Schoenfelder, * Tom Sexton, * Lyubomira Chakalova, * Nathan
Supplementary Information Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells Stefan Schoenfelder, * Tom Sexton, * Lyubomira Chakalova, * Nathan
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
Development: is the growth of an individual organism from a simple to a more complex or mature level. A slow process of progressive change
 1. Define the following terms (use your own words): development, growth, differentiation, histogenesis, organogenesis, morphogenesis, reproduction, tissue, organ, organ system, and organism. Development:
1. Define the following terms (use your own words): development, growth, differentiation, histogenesis, organogenesis, morphogenesis, reproduction, tissue, organ, organ system, and organism. Development:
Ahtiainen et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
Epithelium-1. Hanan Jafar BDS.MSc.PhD
 Epithelium-1 Hanan Jafar BDS.MSc.PhD General features Epithelium is an avascular tissue composed of cells that cover the exterior body surfaces and line internal closed cavities and tubes. It also forms
Epithelium-1 Hanan Jafar BDS.MSc.PhD General features Epithelium is an avascular tissue composed of cells that cover the exterior body surfaces and line internal closed cavities and tubes. It also forms
Left-Right Asymmetry in the Sea Urchin Embryo Is Regulated by Nodal Signaling on the Right Side
 Developmental Cell, Vol. 9, 147 158, July, 2005, Copyright 2005 by Elsevier Inc. DOI 10.1016/j.devcel.2005.05.008 Left-Right Asymmetry in the Sea Urchin Embryo Is Regulated by Nodal Signaling on the Right
Developmental Cell, Vol. 9, 147 158, July, 2005, Copyright 2005 by Elsevier Inc. DOI 10.1016/j.devcel.2005.05.008 Left-Right Asymmetry in the Sea Urchin Embryo Is Regulated by Nodal Signaling on the Right
Examples of smallmolecule. peptide neurotransmitters
 Examples of smallmolecule and peptide neurotransmitters Small- molecule transmitters are transported from the cytosol into vesicles or from the synaptic cleft to the cytosol by TRANSPORTERS Neuromodulatory
Examples of smallmolecule and peptide neurotransmitters Small- molecule transmitters are transported from the cytosol into vesicles or from the synaptic cleft to the cytosol by TRANSPORTERS Neuromodulatory
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
The developmental history of a sensory ganglion cell
 (bozza v1, luglio 2016 per VC) The developmental history of a sensory ganglion cell by Brian Freeman, School of Medical Sciences, UNSW (b.freeman@unsw.edu.au) The sensory ganglion cell (e.g., in spinal
(bozza v1, luglio 2016 per VC) The developmental history of a sensory ganglion cell by Brian Freeman, School of Medical Sciences, UNSW (b.freeman@unsw.edu.au) The sensory ganglion cell (e.g., in spinal
Shh signaling guides spatial pathfinding of raphespinal tract axons by multidirectional repulsion
 ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
glp-1 and inductions establishing embryonic axes in C. elegans
 Development 120, 2051-2064 (1994) Printed in Great Britain The Company of Biologists Limited 1994 2051 glp-1 and inductions establishing embryonic axes in C. elegans Harald Hutter and Ralf Schnabel Max-Planck-Institut
Development 120, 2051-2064 (1994) Printed in Great Britain The Company of Biologists Limited 1994 2051 glp-1 and inductions establishing embryonic axes in C. elegans Harald Hutter and Ralf Schnabel Max-Planck-Institut
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Academic Script. Symmetry. 1 st Year Undergraduate Model 1 UGC Syllabus. Animal Diversity - I. Topic No. & Title: Topic : A-3 Symmetry
 Academic Script Symmetry Course Name: Paper No. & Title: Zoology 1 st Year Undergraduate Model 1 UGC Syllabus Z 101 B Animal Diversity - I Topic No. & Title: Topic : A-3 Symmetry Academic Script: 1.Introduction
Academic Script Symmetry Course Name: Paper No. & Title: Zoology 1 st Year Undergraduate Model 1 UGC Syllabus Z 101 B Animal Diversity - I Topic No. & Title: Topic : A-3 Symmetry Academic Script: 1.Introduction
CHAPTER 05 Histology: EPITHELIUM
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
TISSUES TYPES. CHAPTER 05 Histology: EPITHELIUM BIO 211: ANATOMY & PHYSIOLOGY I. HISTOLOGY = the study of tissues
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
CHAPTER 48: NERVOUS SYSTEMS
 CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
Supplementary Figure S1: TIPF reporter validation in the wing disc.
 Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Xenopus Paraxial Protocadherin inhibits Wnt/β -catenin signalling via Casein Kinase 2β
 Manuscript EMBOR-2011-35178 Xenopus Paraxial Protocadherin inhibits Wnt/β -catenin signalling via Casein Kinase 2β Anja Kietzmann, Yingqun Wang, Dominik Weber and Herbert Steinbeisser Corresponding author:
Manuscript EMBOR-2011-35178 Xenopus Paraxial Protocadherin inhibits Wnt/β -catenin signalling via Casein Kinase 2β Anja Kietzmann, Yingqun Wang, Dominik Weber and Herbert Steinbeisser Corresponding author:
Development of the Nervous System. Leah Militello, class of 2018
 Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval
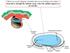 When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
College of Arts and Sciences Grants for Undergraduate Scholars
 Background The Wnt signaling pathway is responsible for many different developmental processes such as cell proliferation, cell-fate determination, and tissue pattering (Nakamure et al., 2008). Prior studies
Background The Wnt signaling pathway is responsible for many different developmental processes such as cell proliferation, cell-fate determination, and tissue pattering (Nakamure et al., 2008). Prior studies
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Lesson 9A Tissues in Animals
 Lesson 9A Tissues in Animals Levels of Organization in the Human Body Similar types of cells Different types of tissues Different organs Many organ systems cell tissue organ organ system organism Levels
Lesson 9A Tissues in Animals Levels of Organization in the Human Body Similar types of cells Different types of tissues Different organs Many organ systems cell tissue organ organ system organism Levels
