Human Respiratory Cilia
|
|
|
- Brice Fisher
- 5 years ago
- Views:
Transcription
1 Human Respiratory Cilia Mark Alexander Chilvers BSc MB ChB MRCP MRCPCH Thesis submitted to University of Lei for the Degree of Doctorate of Me<
2 UMI Number: U All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U Published by ProQuest LLC Copyright in the Dissertation held by the Author. Microform Edition ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml
3 Abstract Human respiratory cilia densely line the airways and beat continually removing mucus and debris from the respiratory tract. Ciliary damage may be primary, from genetic causes, or secondary due to a variety of toxins, bacteria, or viruses. This may result in a reduction in ciliary beat frequency and abnormalities o f ciliary beat pattern. Digital high-speed imaging has been presented as a technique to measure both ciliary beat frequency and beat pattern. This has been evaluated against existing methods and found to be a gold standard. Using this method the ciliary beat pattern has been evaluated in detail for the first time. Cilia were found to beat with a forward power stroke and a recovery stroke within the same plane. Using digital high speed imaging normal reference ranges have been d been evaluated for both a paediatric and young adult population. Data has been collected for ciliary beat frequency, beat pattern and ultrastructural parameters. Having established normal reference ranges it has been possible to evaluate ciliary beat frequency, beat pattern and ultrastructure in patients with primary ciliary dyskinesia. Different beat patterns were found to be associated with different ultrastructural defects. Summary Digital high-speed imaging is a gold standard for evaluation of ciliary beat frequency and beat pattern. With the availability of normal reference ranges it can be used confidently as a diagnostic and research method. 2
4 Acknowledgements A lot of time and effort has been ploughed into this thesis. This would not have been possible without the continued support, enthusiasm and motivation of my supervisor Professor Chris O Callaghan. I would like to thank him for this and his continued guidance he has shown over the last 6 years. I would like to thank Mr Andrew Rutman for his help and assistance in preparing the images for this thesis and for his work in generating the ultrastructural data. I would also like to thank Dr Mike Mckean for his help with the coronavirus project and for setting an example for me to follow. This has helped enormously. Finally the one person I need to thank the most is Caroline who has had to endure a part time husband. She has helped me through the highs and lows of my research experience. Without her this would have been so much harder. I would like to dedicate this thesis to Caroline, Florence and Sebastian who have made this all worth while. 3
5 Table of Contents A b s t r a c t... 2 A c k n o w l e d g e m e n t s...3 T a b l e o f C o n t e n t s... 4 CHAPTER In t r o d u c t i o n a n d a i m s o f t h e s i s...8 CHAPTER R e v i e w o f R e s p i r a t o r y C il ia S t r u c t u r e a n d F u n c t i o n Sum m ary Cell types involved in mucociliary homeostasis Ciliary Structure and Formation Ciliary Ultrastructure Function o f ciliated epithelium Ciliary Beat C ycle Ciliary Beat F requency Periciliary F luid Respiratory mucus CHAPTER R e v i e w o f m e t h o d o l o g y t o e v a l u a t e c i l i a r y f u n c t i o n Summary Historical Perspective Respiratory Cilia M odels Method o f Sampling Specimen mounting Physiological conditions Area o f measurement Methods fo r measurement o f ciliary heat frequency...87 CHAPTER P r i m a r y C i l ia r y D y s k i n e s i a Summary Historical perspective Pathophysiology Incidence and genetics Presentation Associations with primary ciliary dyskinesia Situs Inversus Diagnosis o f primary ciliary dyskinesia M anagement... I l l 4.10 Prognosis
6 CHAPTER A n a l y s i s o f c il i a r y b e a t f r e q u e n c y a n d b e a t p a t t e r n u s i n g d ig it a l h ig h s p e e d i m a g i n g : C o m p a r i s o n w it h t h e p h o t o m u l t i p l i e r a n d p h o t o d i o d e m e t h o d s Summary Background to study M ethods Results Discussion Summary CHAPTER F u n c t i o n a l A n a l y s i s o f C il ia a n d C i l i a t e d E p i t h e l i a l U l t r a s t r u c t u r e in H e a l t h y C h i l d r e n a n d Y o u n g A d u l t s Summary Background to study M ethods Results D iscussion Summary CHAPTER D e t e r m i n a t i o n o f c i l i a r y b e a t p a t t e r n a s s o c i a t e d w i t h s p e c i f i c u l t r a s t r u c t u r a l DEFECTS Summary Background to study Methods Results Discussion Summary CHAPTER T h e E f f e c t s o f C o r o n a v i r u s o n H u m a n N a s a l C i l i a t e d R e s p i r a t o r y E p i t h e l i u m Summary Background to study M ethods Results Discussion Summary CHAPTER C o n c l u s i o n Summary REFERENCE LIST
7 APPENDIX P r o c e s s i n g o f n a s a l c i l i a t e d s a m p l e s f o r e l e c t r o n m i c r o s c o p y APPENDIX P r i m a r y C il ia r y D y s k i n e s i a P r o f o r m a APPENDIX C l i n i c a l d e m o g r a p h i c s o f p a t i e n t s r e f e r r e d t o a t e r t i a r y c e n t r e f o r t h e d i a g n o s i s o f PRIMARY CILIARY DYSKINESIA. EVALUATION OF A COURIER SYSTEM FOR THE TRANSPORTATION OF NASAL BRUSH BIOPSIES Sum m ary Background to study M ethod R esu lts D iscussion Sum m ary APPENDIX C o r o n a v i r u s c e l l u l a r m e t h o d s APPENDIX P u b l i s h e d P a p e r s
8 Chapter 1 7
9 1 Introduction and aims of thesis The first description o f ciliary movement was by Anton de Heide in and then 1 ^ reported later in Arcana Naturae by Leeuwenhoek in Mammalian cilia were not discovered until later when Purkinje and Valentin observed movement in oviductal 1 walls. Since then cilia have been found in many systems in the human body; the upper and lower respiratory tracts and middle ear; within the central nervous system on ependymal cells lining the cerebral ventricular system and in the central canal o f the spinal cord; in the reproductive tract o f both men and women, located in the ductes efferentes of the testis and fallopian tubes respectively. Respiratory cilia are involved in mucociliary clearance. This is a local mechanism which involves the integration of ciliated epithelium, mucus and periciliary fluid. Mucus acts as a physical and chemical barrier onto which particles and organisms adhere. Cilia lining the respiratory tract beat in a regular co-ordinated manner, propelling overlying mucus from the airways to the oropharynx where it is either swallowed or expectorated. Regulation of periciliary fluid is essential to optimise mucociliary clearance and to provide a milieu in which airway antimicrobial agents are effective.3 4 Disruption of the interplay between ciliated epithelium, periciliary fluid and mucus may be primary, from genetic causes in diseases such as cystic fibrosis, primary ciliary 8
10 dyskinesia and asthma or secondary due to a variety o f insults including environmental pollutants, toxins, viral and bacterial infections. This may result in abnormal ciliary function due to impaired ciliary beat pattern, beat frequency or a combination of both. This failure leads to a build up of mucus within the airways and subsequent airway blockage, infection and airway damage. A large amount of research has been conducted evaluating the intricate way in which cilia generate differing beat patterns. Early cinematographic studies in the 1930 s by Gray5 and Proetz6 were the first to describe this movement. Despite this early work there is still no clear consensus as to the precise beat pattern of respiratory cilia.5' 10 Dalhamn using high-speed cinemicroscopy distinguished a forward power stroke and backward recovery stroke but was unable to describe the ciliary beat cycle in detail due to methodological difficulties.7 The most widely accepted description o f beat pattern for mammalian respiratory cilia is that by Sanderson et al.9 They combined results o f light microscopy, high-speed cinemicroscopy and scanning electron microscopy to determine the ciliary beat pattern in rabbit trachea. They observed that respiratory cilia commenced the beat cycle from a resting position at the end of the power stroke. During the recovery stroke the bent cilium performed an unrolling movement across the cell surface sweeping backwards and to the side in a clockwise direction within the periciliary fluid layer. On completion of the recovery stroke the cilium moved into a central position at an angle of 40 to the cell
11 surface. The forward power stroke was generated as the cilium swept forward and moved through a large arc of 130 in a plane perpendicular to the cell surface. The cilium then returned to a rest position with the ciliary tip pointing in the direction of mucus flow.9 11 It is thought that as the cilium swept sideways and backwards in a clockwise direction it stimulated adjacent cilia to beat, propagating the ciliary metachronal wave.9 The work by Sanderson et al. was original, combining the modalities of microscopy, high-speed cinematography and scanning electron microscopy, to evaluate ciliary beat pattern. However the authors do acknowledge methodological and technological difficulties which could alter the way in which the data is interpreted. A strobex flash was used to take the high-speed cinematographic images. It is unclear as to the frequency of the strobe and if this was associated with artefact generation (Section 3.8.3). The overall ciliary beat pattern was determined by analysing a 2D side profile which was then correlated with 3D scanning electron micrographs. The authors state that the forward power stroke of individual cilia was easy to visualise and evaluate but it was difficult to determine individual cilia moving through the recovery phase. Therefore data on the recovery stroke were a composite of several different cilia and relied heavily on observations from scanning electron micrographs. 10
12 It is possible that some of the waveforms seen on the scanning electron micrographs may be artefacts due to processing o f tissue. The forward power stroke was difficult to examine in samples processed for scanning electron microscopy due to tissue fixation not being quick enough to capture the movement. Similarly no data was presented evaluating the effect o f fixative on ciliary movement. In addition to the way in which the ciliary beat pattern was evaluated, the sample preparation was unusual. The ciliated epithelium was inverted and opposed against a cover slip. This was then supported by silicone gel onto which the microscope slide was placed. This raises the possibility of compression o f the ciliated surface. The mounted specimen was then placed on to a heated microscope stage. It is unclear if the sample was humidified therefore it may have been at risk o f desiccation. Both these factors have been observed to alter ciliary function and may therefore alter ciliary beat pattern (Section 2.7, 3.6). Having considered the methodological problems the question is raised Is this description of mammalian ciliary movement correct? Others have challenged this widely held assumption. Cheung et al evaluated the ciliary beat pattern in rabbit trachea using highspeed cinemicroscopy. Filming at 24 frames per second he suggested that cilia started a power stroke at an angle of 85 to the cell surface. The cilium then bowed through an arc o f 40 in a planar motion. The recovery stroke was a reversal of the power stroke.8 However, Sanderson et al also observed this motion in some samples and suggested that 11
13 this movement may be artefactual due to either old preparations or excessive compression of the cilia by the cover slip.9 Sanderson and Sleigh described ciliary movement using rabbit tracheal cilia as a model and raises the question as to whether these data can be applied to human respiratory cilia. Certainly differences in beat pattern between rabbit and frog cilia have been observed.9 Only one study has examined the beat pattern o f human respiratory cilia. Beating cilia were filmed at 200 frames per second again using strobe lighting. A similar beat pattern to that described by Sanderson et al was observed. However the recovery stroke formed an angle with the cell surface of between This is significantly greater than that observed by Sanderson and Sleigh.9 However all measurements were done at 5 C. Temperature will alter ciliary beat frequency (Section 3.8.3), it is unknown if beat pattern is similarly affected. Further confusion arises about the description o f ciliary beat pattern when physiological mechanisms for ciliary movement are considered. Individual cilia are cylindrical and are composed of repeating molecular units which when combined form the ciliary axoneme. It is well described that cilia consist of a classical 9+2 arrangement; 2 central microtubules surrounded by 9 peripheral microtubule pairs from which dynein arms project. The interaction of the dynein arms with the peripheral microtubules creates a molecular motor that generates ciliary movement. To create bend patterns dynein activation within the axoneme must be asynchronous.12 Work performed by Satir 12
14 investigating this has resulted in the publication of the switch point hypothesis. This suggests differential activation of two opposing groups of microtubules during the power or recovery stroke through a switching mechanism.1314 This assumes that half of the microtubular doublets have active arms in the effective stroke and the other half in the recovery stroke12' 14 which results in a hands down or hands up up position of the cilium at either end of the beat cycle.15 Assuming this hypothesis can easily explain how cilia move in a forward/backwards plane. However, the sideways recovery sweep cannot be incorporated within this hypothesis. Recently the quality of digital high-speed video imaging has improved to such an extent that it has replaced video and high-speed cinem atography.16' 18 Cameras can now record at frame rates up to 70,000 per second.19 Consequently, the method has now become a 20 popular technique as it is simple, accurate, relatively inexpensive and allows real time analysis of ciliary beat pattern, beat frequency and coordination Digital high-speed videos can operate at low light levels, are highly compatible with digital analysis systems and have the ability to archive data for further research and audit Therefore the early problems experienced by Gray, Proetz and Dalhamn are now resolved. Assuming that cilia beat between at frequency of Hz recording at frame rates of 400 frames per second will allow at least frames to view the complete ciliary beat cycle. Therefore individual ciliary movement can be viewed in precise detail. No data exist describing the ciliary beat pattern in detail using digital high-speed video. 13
15 In summary there is still no firm agreement as to the precise beat pattern of human respiratory cilia. The most widely accepted description is still open to challenge on methodological, technical and physiological grounds. It is important to understand the beat pattern of respiratory cilia in order to understand ciliary function in health, following infection and in those patients with genetic abnormalities of the cilia. With the advent of digital high-speed video it is now possible to easily examine respiratory cilia in detail for the first time. The aim of the thesis therefore was to utilise digital high-speed video microscopy to evaluate human respiratory ciliary function. The method was to be used to test the following hypothesis: The ciliary beat pattern consists of a forward power stroke and a recovery stroke that sweeps backwards and to the side. 14
16 In addition to the hypothesis generated the secondary aims o f the thesis are as follows: 1) To compare digital high-speed video imaging as a method for analysis o f ciliary beat frequency with the commonly used techniques. 2) To establish a reference range for ciliary beat frequency, beat pattern, and ciliary ultrastructure throughout childhood and adulthood. 3) To analyse the ciliary structure and function o f patients with primary ciliary dyskinesia. The high-speed video system will be used to characterise the precise beat pattern associated with ultrastructural defects found in primary ciliary dyskinesia. 4) To evaluate digital high-speed imaging as a research tool in experimental infections o f respiratory cilia. 15
17 Chapter 2
18 2 Review of Respiratory Cilia Structure and Function 2.1 Summary It is important to understand the structure and function of respiratory cilia. Over the last 30 years knowledge of the composition of the ciliary axoneme has increased markedly. From early ultrastructural descriptions, developments in cellular biology have made it possible to describe the molecular basis o f the ciliary axoneme. Chapter 2.2 initially describes the structure o f ciliated respiratory epithelium and then focuses on ciliary ultrastructure. The individual components which make up the 9+2 arrangement o f the axoneme are discussed at the molecular level. The complex ciliary ultrastructure is devised in such a way as to create the ciliary waveform. This is a continuous cycle that occurs at a regulated frequency. The second half of the chapter examines the ciliary waveform. The cellular and physiological parameters which alter the frequency o f the ciliary beat cycle are described. 17
19 2.2 Cell types involved in mucociliary homeostasis. The respiratory tract is lined by a diverse pseudostratified columnar epithelium. By the terminal bronchioles the epithelium has changed to a cuboidal structure. At least 13 cell 7 7 types have been identified in the tracheobronchial epithelium o f which the main cell types involved in mucociliary homeostasis are; ciliated, goblet, clara and serous cells.24 Ciliated cells are found from the trachea to the respiratory bronchioles.24 They account for 56% of the tracheal cell population and reduce in number to 15% in fifth generation 7 S bronchioles. Interspersed amongst these cells are mucous secreting goblet cells in a ratio of 5 to l.23,25 Ciliated cells are found to extend into to the necks o f goblet cells26 77 and submucosal glands. In the small airways there is a reduction of both ciliated and goblet cells and a corresponding increase in serous and clara cells Clara and serous cells produce watery secretions and are able to differentiate into mucous producing goblet cells.28 An increase in brush cells is also found in the smaller airways. These cells have long microvilli (up to 2pm) projecting from the surface which may have an absorptive function.24 They may also participate in fetal ciliogenesis.25 18
20 A submucosal layer is present until the termination of the cartilaginous bronchioles. Within this layer are branching tubular structures (submucosal glands) which produce both serous and mucus secretions. These glands are 40 times more frequent than goblet cells.24 O f the cells identified, the ciliated and goblet cells are the most important in mucociliary homeostasis and will be discussed in more detail Ciliated Cells Ciliated cells are terminally differentiated columnar cells (Figure 2.1) They measure 20pm in length and 7pm wide at the cell surface tapering to 2pm at the base The surface area o f a ciliated cell is approximately 38-49pm 2. They are attached to adjacent cells by three different junctional complexes: Zonula adherens joining the apices of cells, tight junctions between the apical sides of the cell wall and the lower lateral cell wall are attached to either basal cells or the basement *90 membrane by desmosomes. These junctions provide mechanical cohesion and may provide an active role in signal transmission controlling cell growth and differentiation.29 The cell cytoplasm is electron lucent as ciliated cells have less secretory granules and ribosomes. The nucleus is towards the cell base and Golgi bodies are located more centrally. An increased number of mitochondria are found in the apical part of the cell among the ciliary rootlets
21 Figure 2.1: Cellular components of ciliated cell. The tight junction (TJ) is clearly seen, in addition to a large number of mitochondria (M). 20
22 The normal proliferation o f ciliated cells in 24 hours is thought to be <1%. Basal cells differentiate to mucus cells and act as progenitors for ciliated cells. In peripheral airways clara cells may be responsible for this."' Following injury ciliated cell proliferation can increase to 17%. It is thought that epithelial repair is complete within 2 to 3 weeks 9 S following mechanical injury 'j '5 or viral infection respectively. 2.3 Ciliary Structure and Formation Approximately cilia project from the cell surface32 (Figure2.2, 2.3) with a density of 6-8pm 2.24 Individual cilia vary in length from 6pm in the trachea to 2-3pm in seventh generation airways The width o f a cilium is relatively constant measuring 0.3pm.23 24,31 At the ciliary tip 3 to 7 claw like projections are found.35 Cilia are densely packed in the central region of the cell and this decreases towards the edge of the cell. They are arranged into a hexagonally packed semi-crystalline array with each cilium surrounded by 6 microvilli.12 Approximately 100 microvilli are found on the cell surface23 each measuring l-3 p m in length and pm wide.36 Microvilli cytoplasm is continuous with the cell cytoplasm24;25 and may function in regulation of fluid transportation within the periciliary fluid layer.37 21
23 Figure 2.2: Scanning electron micrograph showing the density of cilia on respiratory epithelial cells. Figure 2.3: Higher magnification scanning electron micrograph illustrating ciliated (C) and brush cells (B) 22
24 Cilia arise from fetal endodermal cells and a fully developed ciliated epithelium is achieved within the prenatal period.25,31 Ciliogenesis follows a predetermined pattern, initiating at the membranous trachea and moving to the peripheral airways.38 Electron microscopy has shown the presence o f cilia on the membranous trachea at 7 weeks ^ o gestation, carina at 8 weeks and bronchial tree at 13 weeks. appear on the cartilaginous trachea until 13 weeks gestation. However cilia do not A fully differentiated ciliated epithelium is evident between 11 to 16 weeks gestation40 and by 24 weeks the epithelial cells have changed from a dual to mono layer.31 At birth ciliated epithelium is present up to the terminal bronchioles.31 Despite there being no histological or ultrastructural evidence o f ciliogenesis in the large airways before 13 weeks gestation, axonemal dynein has been shown to expressed as early as 9 to 10 weeks gestation in undifferentiated columnar epithelium and thought to represent emergent ciliary beds.41 Cilia have also been observed in the foetal oesophagus between the week of th th gestation and then disappear.42 From observations by electron microscopy it is thought that ciliogenesis occurs in waves. Cilia arise at the periphery of the cell and ciliogenesis continues until the cell has generated its full complement of cilia Cilia form from structures called centrioles either by division o f existing centrioles as in the formation o f primary or rudimentary 23
25 cilia43 40;45or indirectly from a sequence of events initiated in the Golgi region 40 The cellular events in ciliogenesis have been described in detail by Dirksen.44 Fibrogranular deposits are formed under the influence of pre existing mature centrioles. This forms a fibrogranular mass (Proliferative element), which condenses into a loonm deuterosome (Generative complex). Around the deuterosome 2-9 procentrioles (basal body precursors) organise 43 As the procentrioles mature and become longer the generative complex becomes smaller. The procentrioles are released to the cytoplasm and migrate to the luminal border. Migration involves intracellular cytoskeletons which may utilise an actin-myosin system.43 During migration procentrioles transform to basal bodies which allows the assembly and elongation o f individual axonemes.4446 As basal bodies arrive at the luminal border intramembrane particles coalesce and organise to form concentric rings or spirals (ciliary necklaces) through which the budding cilia emerge.30,40 Murine models have shown that cells which become ciliated have large numbers of microvilli. reduced. However, when the cell is fully ciliated the total number of microvilli is Dirksen suggested that microvilli may act as a membrane reserve for ciliogenesis.44 No human data exist for this however numerous long thin microvilli are observed on fetal epithelial cells prior to ciliation39 and on normal ciliogenic cells.43 The ciliary axoneme is though to be a dynamic process involving intraflagellar transport particles. This is thought to arise when at least 17 polypeptide coalesce to form a transport complex A and B. Formation occurs at the basal body and a cargo is loaded 24
26 which is transported to the tip by kinesin II to replace subunits lost by cellular turnover at the axonemal tip. Cell products of this axonemal turnover are returned the cell body by cytoplasmic dynein lb.47"*9 Two models are proposed for ciliary length control using this transportation system. One suggests the existence of a saturated length dependent assembly rate and a length dependent dis-assembly rate that are balanced. The other suggests the existence of a length sensor in the membrane:kinase L4. This acts in a feedback loop that has yet to be described.49 This may be due to detecting changes in calcium currents or GTP/GTPase timer mounted on itraflagellar transport proteins. One further idea is that tectin may also be involved in length control Ciliary Ultrastructure The ciliary axoneme is constructed from microtubule doublets, dynein arms and radial spokes surrounding a central microtubular pair. This forms the classical 9+2 arrangement which extends from the basal body (Figure 2.4). Cilia are constructed from over 240 polypeptides arranged into specific multiprotein structures13 which have now been identified.50;51 25
27 Central Pair with Bridge Central Sheath Ciliary Membrane Outer Dynein Arm Inner Dynein Arm Nexin Link Radial Spoke 9 B Microtubule A Microtubule Figure2.4: Diagram of normal ciliary ultrastructure illustrating classical 9+2 arrangement. Two central microtubules (central pair) are enclosed within a central sheath to form the central axis of the axoneme. This is surrounded by nine microtubule doublets. The doublets consist of an A and B microtubules. Each doublet is connected by nexin links to the next. From the A doublet, inner and outer dynein arms project to the adjacent B doublet. Radial spokes connect the central sheath to the outer microtubule doublets. 26
28 The axoneme is a uniform structure which repeats precisely every 96nm along the ciliary J S axoneme. Within that unit structures found are: 4 identical outer dynein arms, 3-4 different inner dynein arms, 1 spoke group (3 radial spokes), 1 central sheath projection every 16nm(6 pairs) and 1 pair of nexin links every 86nm Each component o f the ciliary axoneme will be discussed in detail as follows: Ciliary Membrane Ultrastructural analysis has shown that the outer trilaminar ciliary membrane is continuous with the cell surface.45 Thin filamentous bridges are found along the length of the axoneme between the cell membrane and the outer microtubule doublets with a periodicity o f 220nm.53 The role o f these bridges is unclear, but may involve regulation 'yc o f ciliary beatingf Within the ciliary membrane o f human respiratory cilia are two transmembrane complexes. The first is the ciliary crown found at the tip of the cilium and is composed of two regions: An electron-dense cap tightly bound to the central pair and to each of the peripheral subfibre A microtubules. Filaments radiate from this cap to the second region, the plate or ciliary crown. Here 3 to 7 fibres originate and project out onto the ciliary membrane surface.35 The fibres have a mean length o f 31 nm (range 25-35nm24), width 9nm and show a periodic structure Fouliguet suggested that the ciliary crown may 27
29 be involved with several functions associated with microtubule assembly as well as ciliary movement and mucociliary transport.35 The second transmembrane complex is the ciliary necklace (figure 2.5). This is an arrangement of particles extending distally 0.25pm from the base of the cilium. The necklace is formed from 4-6 scalloped rows 30nm apart Each scallop within a row corresponds to a peripheral microtubule. The ciliary necklace may have a role in energy transduction or timing of the ciliary beat.54 It may also assist in attaching the basal body to the transition zone and ciliary membrane.53,56 Evidence to support this has come from experiments using calcium shock treatment to cause deciliation. Centrin, a calcium binding protein, is found in the area of the ciliary necklace." Following calcium shock, it was found that cilia broke at a point above the ciliary necklace Microtubular structures Microtubules are constructed from protofilaments which are arrays of heterodimers of a and p tubulin. The dimers organise in a linear pattern with a slight helical pitch Central Pair In the centre of the ciliary axoneme are two single asymmetrical microtubules which form the central pair. They are classical microtubules each having a wall composed o f 13 28
30 protofilaments50, however each central microtubule is structurally asymmetric and biochemically distinct.57 The proximal portion of the central pair terminates in the axosome55 and distal portion ends in the ciliary crown. From the central pair a series of projections arise at 16nm intervals and extend from the central microtubules to the apex o f the axoneme. This creates a series of broken loops around the central pair forming an incomplete central sheath. Different projections are attached to each central microtubule which are both structurally and biochemically distinct.59 A double bridge spanning 8nm is found between the two central microtubules which repeats every 14nm.58 Ciliary Orientation Each central pair has a ciliary axis which is orientated with respect to the central pair of 52 adjacent cilia. The ciliary axis is defined as a line through the central pair, which is perpendicular to the plane of ciliary beating. The angle (angle o f orientation) that the ciliary axis makes with a reference line can be measured. From this the standard deviation of the angles for the cell can be calculated and subsequently the mean standard deviation for the subject.60 Orientation of the central pair in human respiratory cilia has been reported to be between '62 29
31 Peripheral Microtubule Doublets The 9 peripheral doublets are more complex microtubules constructed from two subfibres A and B (Figure 2.4). Subfibre A is composed o f 13 protofilaments onto which the inner and outer dynein arms attach. Subfibre B is composed of 10 protofilaments and a smaller unit of unknown nature. It is an incomplete microtubule and utilises part of the wall of subfibre A to allow completion o f its own wall.50 In the midwall area, common to both A 12 and B subfibres, tectin an intermediate filament is found. Tectin has been shown to be important for structural stability o f the microtubular doublet25,12 and may control ciliary length.43 At the ciliary tip subfibre B terminates and subfibre A continues as a single filament which attaches to a transmembrane complex the ciliary crown.53 At the base the 12 axonemal doublets attach to another transmembrane complex, the ciliary necklace Microtubular associated proteins Dynein arms Dynein is a high molecular protein which is a mechanochemical ATPase. It has a major role in microtubule doublet sliding. In the axoneme it is present in the form o f an inner and outer dynein arm which are attached to subfibre A and project towards subfibre B. Both dynein arms are present on each o f the nine peripheral microtubule doublets. 30
32 Outer Dynein Arms Outer dynein arms are a complex o f subunits which are thought to effect ciliary beat c frequency. " They are found to have a periodicity of 24nm along the peripheral microtubules.13 Outer arms attach to subfibre A through two domains, a proximal and distal foot. From the foot process a stem arises which finishes as an angulated end or hook. The hook consists of two rounded heads each with a thin stalk which extends and binds to the lattice of subfibre B.50,63 The two dynein heads have a molecular weight o f 500 kda and are heavy dynein chain A TPase s ( a and p).63 Attached to these are > 2 intermediate chains and 4-8 light 52*63 chains. It was thought that intermediate and light chains anchor the dynein arm to subfibre A but it is now unclear how they relate to the proximal and distal feet Another postulated role for these chains is to assist in regulation o f ciliary beating.25 Inner Dynein Arms Inner dynein arms consist of three structurally different isoforms IDA1, IDA2, and IDA3 which repeat every 96nm.63 This suggests a more complicated role for inner dynein arms, with each isoform having a distinct but complimentary role in the construction of the ciliary waveform. " Inner dynein arms are less prominent but have a similar structure to outer dynein arms.64 They attach to subfibre A and terminate in rounded heads from which a thin stalk which 31
33 extends to subfibre B.63 Inner dynein arms are also composed of subunits. The precise combinations o f heavy, intermediate and light dynein chains for the three different isoforms are currently being characterised.63 Only the structure of IDA1 has been described. It is a trilobed structure located proximal to the first radial spoke. Two lobes are composed o f heavy dynein chains. The third lobe is formed from amino terminal regions of the heavy chains and 3 intermediate and 3 light chains associated with inner dynein arm ID A l Radial spokes Radial spokes are multipolypeptide structures, which self assemble from soluble 13 precursor molecules. They consists o f a cylindrical stalk attached to subfibre A and an expanded spoke head. The spoke head interacts with the central sheath (Figure 2.4). Radial spokes are found in groups o f three (spoke group) along the microtubule with a * p.13 periodicity 32:24:40 nm. The first spoke is always aligned with a dynein arm (ID A l).13 The role o f radial spokes is debated. They are thought to limit microtuble sliding and t j cause the cilium to bend. Other roles suggested are that they are part of a frame work to prevent compression of the axoneme when it bends,50 63 relay signals between inner and outer microtubules50 or activate/inhibit dynein arm cycling on specific microtubular doublets.12 32
34 Dynein regulatory complex A complex has been described that is thought to mediate signals between radial spokes, nexin links and dynein arms to regulate ciliary beating.52 57,65 It is a group o f seven polypeptides, several of which are clustered on subfibre A, located at the base of the second radial spoke in close association with the inner dynein arm IDA2.63, Nexin links A group of circumferential filamentous structures are found between the microtubular doublets within the axoneme (Figure 2.4). These are composed of the protein nexin and link adjacent microtubules.13 45,50 They are positioned on the midwall o f subfibre A between the rows o f inner and outer dynein arms, at the base o f radial spoke 3, near to inner dynein arm IDA2.63 The links span the 24nm gap to attach onto the adjacent 13 *66 microtubule subfibre B near the base of radial spoke 2. They appear as two overlapping lobes connecting the adjacent microtubules.66 Nexin links are observed in pairs 96nm apart66 and one pair of links is observed with every spoke group (3 radial spokes).12 Nexin links appear to hold doublets together and resist microtubular displacement.50,66 However, they are inextensible and thought to break and reform as doublets m ove.12,66 It has been suggest that a second set of circumferential links exist12 which are extensible or elastic, however the location o f these links is undetermined.13 33
35 Other microtubule associated proteins Dynein is not the only molecular motor within the ciliary axoneme. Kinesin has been associated with inner singlet microtubules (central pair) and myosin with the basal bodies. Similarly other molecular processes and messenger systems have been identified within the axoneme as structural components.67 This is supported firstly, by evidence of several kinases and phosphatases bound to the axoneme. The following have been isolated and thought to have an important role in ciliary function; Protein kinase A is heavily bound to the axoneme; A-kinase anchor proteins are located on central microtubules and radial spokes;59 68 calmodulin binding kinase has been identified on radial spokes; casein kinase reported on outer doublets Secondly, calcium regulation has a role in regulation of ciliary motility. Reports have described the axonemal location of the following calcium binding proteins; calmodulin isolated on radial spokes and centrin/catractin found to be a component of a subset of inner dynein arms.67 34
36 Basal Body The ciliary axoneme ends via a transition zone to the basal body. Each cilium is anchored to the cell by the basal body (Figure 2.5). This structure has a lateral projection called the basal foot which is a short banded cone near the mid region of basal body.31 Within the cell all basal foot processes of individual cilia point in a similar direction. This is in the direction of the effective ciliary stroke.24 Ciliary necklace EF face PF fa ce Basal body Ciliary rootlet Figure 2.5: Illustration of basal body and ciliary necklace by freeze fracture taken from reference 32 As the 9 outer microtubular doublets enter a basal body they become triplets by addition of another incomplete microtubule to subfibre B. These form large striated rootlets which extend from the end of the basal body deep into the cell cytoplasm towards the cell 35
37 nucleus. They have a banded appearance with a periodicity of 40nm.23,31 Fine filaments have been observed to extend from these rootlets to adjacent basal bodies.' The basal body is anchored to the intracellular cytoskeleton by various attachments to; cytoplasmic microtubules, actin filaments, intermediate filaments and cross-linking fine fibrils. Fibrous sheets (alar sheets) attach the base of a basal body to the inner surface of plasma membrane. It has been suggested that the cytoskeletal crosslinking may assist in regulation o f coordinated ciliary beating.56 36
38 2.5 Function of ciliated epithelium. The ciliated epithelium has a variety of different functions that assist mucociliary homeostasis. The epithelium can modulate airway surface fluid29 and produce antimicrobial peptides.3 Other functions involve mechanical stability o f the epithelium TQ and aiding epithelial cross talk between cells." The main function of ciliated epithelium is clearance of mucus from the lower respiratory tract, achieved by the co-ordinated beating of cilia. Cilia beat through a complex interaction between the dynein arms and the microtubules o f the ciliary axoneme Dynein arm movement Dynein arm movement is a mechanochemical cyclical activity powered by the hydrolysis of adenosine triphosphate (ATP). At the start of the dynein arm cycle the dynein arm attached to subfibre A is in a tilted extended position. Which allows the arm to span the 21nm interdoublet gap. The head o f the dynein arm then attaches to the adjacent subfibre B which causes a sliding step o f 16nm as the dynein arm moves to an equilibrium position perpendicular to the two subfibres (rigor state). In this conformation ATP binds to the dynein arm. This allows the arm to detach from subfibre B and shorten to 14nm. Hydrolysis of ATP by dynein head ATPase results in the dynein arm extending distally to an angle of 40 and reattaching to subfibre B.14,45 Sliding occurs as the arm returns from the tilted extended position to the rigor state and so the cycle is repeated.14 37
39 The dynein arm movement has been modelled and two observations made. Firstly, the dynein head moves centripetally, with respect to subfibre A, and rotates underneath the arm. Secondly, inner and outer dynein arms move in opposite directions to each other i.e. one head and arm rotates clockwise and the other anticlockwise during the dynein arm cycle.69 In flagella dynein arms have been found to oscillate which results in movement. It is unknown if similar actions occur in respiratory cilia.70 Dynein is considered a minus end directed motor enzyme. The force produced by the dynein arms attached to subfibre A will move the microtubular doublet down towards the proximal or minus end of the adjacent doublet7172 at a velocity of 10pm/s.25 Recent evidence has suggested that dynein may be bi-directional, i.e. it moves the microtubule doublet both up and down, however data is sparse and it is thought unlikely.73 Outer dynein arms alone can cause microtubule translocation at rates approximately 50% expected.74 Similar findings have been observed for inner dynein arms alone Regulation of dynein arms Evidence now suggests that radial spokes and the central pair (CP/RS complex) provide a structural interface for transmission of regulatory signals to the dynein arms.59 The complex may regulate the velocity of dynein driven sliding by posttranslational modification o f inner dynein arms and it may also modulate ciliary waveform.57 38
40 One hypothesis suggests that as the central pair rotates it acts as a distributor to provide signals to radial spokes which selectively activate sets of dynein arms Other data suggest that the central pair and radial spokes are implicated in a signal cascade that regulates inner dynein arm isoform ID A l.59 Two mechanisms of control have been suggested, either through a calcium/calmodulin pathway or through control o f axonemal kinases and phosphatases. Evidence for the role of calcium in regulation of dynein driven microtubule sliding has been reported in a series of studies by Smith. Conclusions drawn were; calcium activates a signal transduction cascade that modulates dynein activity; a calcium induced increase in dynein activity is mediated through calmodulin and calmodulin kinase II; radial spokes and the central pair are part of the axonemal calcium control system.67 In addition, calmodulin has been identified bound to a radial spoke protein. This suggests that calcium may have a signalling effect on the axoneme by modulating interactions between the central pair and radial spokes.57 Porter has reviewed the evidence for dynein activity regulated by phosphorylation c n mechanisms. Inner dynein arm IDA 1 is thought to be regulated by phosphorylation of an intermediate chain. This is determined by several kinases and phosphatases whose activity is controlled by CP/RS complex. It has been suggested that the CP/RS complex may override the inhibitory action of cyclic-amp dependent kinase and dephosphorylate the dynein motor allowing activation. 39
41 Further evidence to support this has been the identification of a protein at the base of the radial spoke which is an anchor protein for phosphokinase. Therefore a signalling circuit from central pair to dynein arms is built into radial spokes. This network will directly or indirectly control phosphorylation o f the inner dynein arms and hence control motility.57 Another phosphatase identified is protein phosphatase typel. This has been isolated on both central microtubules and close to outer dynein arms. It is thought that it may coordinate both outer and inner arm activity.57 Also, Docking areas on the dynein heads which bind to subfibre B may also anchor kinases and phosphatases for local control o f motor activity. Reports have suggested that outer dynein arm activity is 57 regulated in part by components associated with D ocking areas. The final dynein control system is the dynein regulatory complex. The complex is thought to mediate signals either mechanical, chemical or both between the radial spokes, nexin links, inner and outer dynein arms. How this is achieved is still being investigated. One hypothesis suggests that the dynein regulatory complex acts as a scaffold to attach regulatory enzymes that modify dynein activity. A second, is that it acts with nexin links and/or spokes to sense tension or strain within the axoneme and provide mechanical feedback to the dynein arms.57 40
42 2.5.3 Microtubule Sliding Dynein arm activation produces a shearing force that causes the microtubule doublet (subfibre A) to slide. This movement is linear rather than to shorten, lengthen or rotate.63 In the intact axoneme the degree o f actual sliding is limited as microtubular doublets are tethered to the cell via basal bodies and attached to each other by nexin links This causes the sliding movement to be translated into bending Switch Point Hypothesis The ciliary axoneme is cylindrical, to create bend patterns dynein arm activation and 1^ hence doublet movement must be asynchronous. " This is thought to occur by sets of dynein arms being activated then inactivated through a switching mechanism.13,14 Satir has suggested the switch point hypothesis. This assumes that half of the microtubular doublets have active arms in the effective stroke (doublets 1-4) and the other half in the recovery stroke (doublets 5-9).1214 To achieve the switch point mechanism the following conditions must be met; firstly dynein must be able to be switched on and off. Data has shown that dynein exists in two states a cycling (active) and non-cycling (inactive).12 Secondly, a switch period needs to be present within the beat cycle. The normal ciliary beat cycle has two stages an effective and recovery stroke. Within each stage there is a
43 period when no active microtubular sliding occurs. At this point, the bend is propagating along the axoneme but the bend angle remains constant. This is thought to represent a switch period when active dynein arms are inactivated and vice versa.14 The first switch period is towards the beginning of the recovery stroke and is inhibited by vanadate, the second at the beginning o f the effective stroke and is inhibited by high calcium.79 Finally, switches must be present to activate dynein arms and be under a regulatory system. The control system for microtubular sliding must allow asynchrony o f dynein arm activation, be limited in extent and tightly coupled to bend formation and 79 propagation. Two potential switches have been identified, one acting via calcium/calmodulin, the other via cyclic adenosine monophosphate (cyclic AMP). Both are thought to act by modifying intrinsic axonemal control mechanisms through post translational modification of axonemal proteins via protein phosphorylation which in turn is modified by extra-axonal 79 mechanisms. A feedback loop between phosphorylation and de-phosphorylation states o f the two switches has been suggested to be integral to the switching m echanism Regulation o f this may be controlled by radial spokes/central pair complex and nexin 79 links. The biphasic switch may be a consequence of dynein arm activation by 1 central microtubule and spoke group during the effective stroke and the opposite central 42
44 7Q microtubule and spoke group during the recovery stroke. In this manner the radial spoke/central microtubule interactions may also assist in limitation o f sliding Central pair function Several functions have been suggested for the axonemal central pair. As discussed earlier (Section:2.3.2), it has been shown that molecular interactions of the central pair and radial spoke complex may be part o f a control system for dynein activation.59 However studies using both electron microscopy and video analysis found that the central pair 76*77*80*81 rotates within the axoneme. This rotation between the radial spokes and central 82 pair may be involved in the switch hypothesis. Electron micrographs have shown that the orientation o f the central pair changes along the length of the axoneme and thought to be related to ciliary beat cycle Also, a twist in the central pair is formed during beating.81 This questions the validity of the measurement of ciliary orientation in patients with primary ciliary dyskinesia. When respiratory cilia beat metachronal coordination allows cilia to become syncronised. This implys all cilia will have the same degree of twisting of the central pair at a given time. Ciliary orientation measurement is expressed as the average standard deviation. Therefore the individual alignment can vary by upto
45 Video data has shown that the central pair rotates continuously. This is driven by the central pair and occurs in the absence of the basal body. Kinesins associated with the central pair may be involved in generating this movement.76 It is unclear if the central pair rotation is an active continuous process or a passive mechanism due to axonemal bending. One hypothesis is in favour of passive rotation of the central microtubules. The central pair is rigidly fixed at the base to the ciliary axosome. When the cilium bends the pair will rotate and twist. On cessation o f the effective stroke, the central pair unwinds. In this way the central pair may regulate the pattern of sliding of the outer doublets. The continuous rotatory motion seen may be a complex of the central pair twisting and untwisting Embryonic nodal cilia have a 9+0 configuration similar to the ciliary transposition defect. In both these groups cilia have been found to beat in a circular manner. O f interest, the central pair may not be needed for bending. Mutants lacking central pairs are paralysed under physiological conditions. However, they can be stimulated to beat under various non-physiological conditions In summary, two roles for the central pair have been postulated. Firstly, the presence of a central pair/radial spoke complex imposes a higher order regulation on the basic axonemal movement to produce a more complex waveform. Secondly, to create the effective axonemal motion produced by microtubule sliding, geometric arguments 44
46 suggest that there must be both circumferential and longitudinal regulation of shear force. Central pairs are suited to this role. Therefore, regulation of outer doublets by the central pair together with its ability to stabilise the shear force by rotating could explain the ciliary waveforms seen Radial Spokes Early data suggested that radial spokes may have a cycle of attachment and detachment with the central pair and function to convert sliding into bending and limit the degree of microtubular sliding.13 They may also assist in the propagation of bending along the cilium.31 This may be correct but rather than via structural mechanism, it is most likely through the role that that radial spokes play in second messenger systems to regulate dynein arm activation which limits and regulates the sliding activities of the doublet microtubules (see sections: , 2.4.2). 45
47 2.6 Ciliary Beat Cycle Respiratory cilia have been designed to propel mucus over the airway epithelial surface consequently the cilia tend to be shorter, packed more densely and beat at a lower frequency than cilia involved in water propulsion such as brain ependymal cilia which propel cerebrospinal fluid through the ventricular system Ciliary beat pattern The interaction between dynein arms and microtubules cause cilia to beat with a distinct pattern. Unlike invertebrate cilia, which beat solely in water systems, respiratory cilia beat in a dual system of mucus and water (periciliary fluid).11 As periciliary fluid has a lower viscosity the majority of the beat cycle is within this layer.11 Early cinematographic studies in the 1930 s by Gray5 and Proetz6 were the first to describe ciliary movement. Despite this there is still no clear consensus of opinion as to the precise beat pattern of respiratory cilia.5' 10 Dalhamn using high speed cinemicroscopy distinguished a forward power stroke and backward recovery stroke but was unable to describe the ciliary beat cycle due to methodological difficulties.7 Cheung evaluated the ciliary beat pattern in rabbit trachea. Using high-speed cinemicroscopy at 24 frames per second they suggested that cilia started a power stroke at an angle o f 85 to 46
48 the cell surface. The cilium then bowed through an arc of 40 in a planar motion. The o recovery stroke was a reversal of the power stroke. The most widely accepted description of ciliary beat pattern is that by Sanderson et al.9 They combined high-speed cinemicroscopy with scanning electron microscopy to determine the ciliary beat pattern of cilia in rabbit trachea. They observed that respiratory cilia commenced the beat cycle from a resting position at the end of the power stroke. During the recovery stroke the bent cilium performed an unrolling movement across the cell surface sweeping backwards and to the side in a clockwise direction within the periciliary fluid layer. On completion o f the recovery stroke the cilium moved into a central position at an arigle of 40 to the cell surface. The forward power stroke was generated as the cilium swept forward and moved through a large arc of 130 in a plane perpendicular to the cell surface. When the cilium was fully extended the ciliary tip penetrate the mucus layer by approximately 0.5pm 11 resulting in propulsion o f mucus. The cilium then returned to a rest position with the ciliary tip pointing in the direction of mucus flow.9,11 To assist mucus penetration the tips are angled forward and mucus detachment is ensured by propulsion from adjacent cilia.84 The resting position also prevents backflow o f mucus.9 Only one study has examined the beat pattern of human respiratory cilia. Beating cilia were filmed at 200 frames per second using strobe lighting. A similar beat pattern to that 47
49 described by Sanderson et al was observed. The recovery stroke formed an angle with the cell surface of between However, cilia were found not to exhibit a rest phase.10 With the advent of digital high speed imaging it has been possible to re-evaluate the beat pattern of human ciliated tissue in three planes. Detailed analysis using this method has recently shown that the angle through which the cilium moves during the recovery stroke from the plane of the forward power stroke is less than 5. Respiratory cilia, therefore, beat forward and backwards within the same plane without a classical sideways recovery sweep.85 The beat pattern of respiratory cilia can be split into 3 phases; a forward power stroke, a rest period and a backwards recovery stroke which then moves directly into a forward 11 *86 power stroke. ' The duration of these phases varies with respect to ciliary beat frequency.87 The recovery stroke has been found to be longer than the power stroke As ciliary beat frequency increases all three phases shorten. As expected the greatest reduction is in the rest phase, then recovery stroke and finally power stroke.86 The minimum duration for the power stroke is 10ms. Cilia tend to function close to this therefore the ability to reduce power stroke duration is limited. The recovery stroke appears to have a greater reserve and requires a minimum duration o f 25ms. These data
50 suggest that dynein interactions in the power stroke are operating near to a maximum capacity.86 How the beat pattern relates to the switch hypothesis is still unclear.86 Satir suggested that in the switch point hypothesis equal duration for the power and recovery strokes would cause a symmetrical beat pattern.13 Although alterations of the timing of the switches would result in unequal duration for the power and recovery strokes,13 the switch point hypothesis cannot explain the change in beat direction associated with the recovery stroke observed by Sanderson Factors influencing fluid movement during the beat cycle Factors involved during the ciliary beat cycle are hydrodynamic coupling, metachronal waves and ciliary streaming. Each will be discussed in detail. The beating cilium moves through both mucus and periciliary fluid layers. Early data suggested that periciliary fluid oscillated back and forth beneath the mucus layer as cilia beat.11 Recent reports have suggested that respiratory cilia transport both mucus and periciliary fluid along the 88 airway surface. The ciliary power stroke moves the mucus directly by penetration of the ciliary tip into the mucus layer.24 R*RO*Ofl interaction between the two layers. Periciliary fluid may be moved by frictional Data have shown that cilia are too short and not close enough to the cell surface to move periciliary fluid directly90 possibly because of factors influencing hydrodynamic coupling (Section: ). 49
51 Hydrodynamic coupling As cilia move, water adheres to the cell membrane in a capture zone extending from the base to the tip of the cilium. To result in net fluid propulsion i.e. a greater volume of water moved in the power stroke than returned on the recovery stroke, cilia have a tall fast power stroke and small slow recovery stroke The difference in height between the power and recovery stroke maximises the net flow of fluid by reducing the backflow of fluid due to hydrodynamic coupling.11 Hydrodynamic coupling is caused when the capture zones of 2 adjacent cilia interact this results in synchronisation of beat cycles and recruitment of cilia to form a metachronal wave Sleigh suggested that hydrodynamic coupling may be reduced in respiratory cilia as they are shorter and beat at lower frequencies. However due to the high cellular density of cilia they will still be closely coupled Metachronal wave The main propulsive thrust on the mucus layer is delivered by the distal part of the cilium. Due to the viscoelastic nature of mucus the rate of propulsion of mucus is similar to the ciliary tip velocity.11 To keep mucus moving in a continuous manner rather than a start-stop phenomenon, independent groups of cilia move in a metachronal manner forming a propulsive wave. 50
52 Force generated by ciliary beating will be lost if cilia are not stiff enough. This loss can 1? be reduced by adjacent cilia beating in close coordination. Respiratory cilia are able to recruit adjacent cilia to beat in a co-ordinated manner and form a metachronal wave. These waves allow the continuous movement o f mucus. Within each metachronal wave cilia will be at different phases of the beat cycle, therefore mucus does not need to be 12 accelerated but continues at the same velocity. Metachronal waves are limited events which travel a short distance along the epithelial surface propelling overlying mucus.24 The wavelength o f a metachronal wave is 5-20pm 10 and waves propagate at to the direction o f the power stroke.11,25,92 The 12 metachronal waves lengthen with increases in ciliary beat frequency. The basis of the metachronal wave is unclear. Initial work suggested that as a cilium moved through a recovery stroke physical stimulation of adjacent cilia occurred. This resulted in recruitment of cilia into a co-ordinated wave.9,24 However, cilia separated by a gap o f 10pm are still able to manifest metachronal waves. Possible mechanisms A involve intracellular communication "and hydrodynamic coupling Ciliary Streaming Cilia beat in a fixed regulated direction from distal to proximal airways against gravity. Q-l Mucus has been shown to coalesce to illustrates the effect o f ciliary streaming. Studies 51
53 have shown that ciliary streams circumvent tributary airway openings by dividing into two. They do not then reconvene after passing the opening but continue separately.94 Further evidence has suggested that ciliary streaming along the trachea is in a spiral direction The direction of cilia streaming has been found to change at three areas within the airway; carina, bronchial openings and larynx.94 52
54 2.7 Ciliary Beat Frequency Human respiratory cilia are spontaneously active and beat with a specific frequency between Hz.25,31,62 Even after death cilia continue to beat at a regular frequency for at least 60 hours.93,94,96 Within the respiratory tract, cilia beat at a relatively constant frequency. One study has suggested that beat frequency may be slower in peripheral airways.97 The significance o f this remains unknown. The effects of various physiological parameters on ciliary beat frequency are shown in Table 2.1. The actions of pharmacological and chemical mediators on ciliary beat 9 S 91 frequency have been reviewed extensively by Wanner and Rusznak. O f note, smoking may have a minor inhibitory98 or no effect99 on ciliary beat frequency. Conversely, beat frequency is increased by alcohol at levels associated with social drinking.100; 101 Maintenance of basal ciliary beat frequency is still debated. It may be inherent to the I r\j axoneme requiring only adenosine triphosphate (ATP) or the presence of low intracellular calcium ( [ C a 2+ ] j ) of nm.103 However other data suggest a calcium/calmodulin dependent pathway may be involved Basal beat frequency may also be under inhibitory autonomic neural control with excitatory stimuli causing release o f this inhibition
55 Smith et al suggested that basal ciliary beat frequency is regulated by signalling pathways which are different to those used to stimulate beat frequency.104 Increases in beat frequency may be transient following an acute stimulus or prolonged as is seen in 107 response to inflammation. An increase in ciliary beat frequency has physiological consequences. If ciliary beat frequency is increased by 15%, mucociliary clearance rate will decrease by 56%.108,109 In addition an increase in beat frequency will increase the number o f metachronal waves observed.110 The relationship between ciliary beat frequency and mucociliary clearance is unlikely to be linear due to the various componets involved; cilia, airway surface fluid and mucus. One study has suggested that the relationship between ciliary beat frequency and mucociliary clearance is in fact logarithm ic
56 Physiological Parameter Age Sex Temperature Circadian Rhythm Humidity Oxygen PH Osmotic Viscosity Pressure Location Action CBF highest in neonates and falls with increasing age62112' 114 No differences observed between male and female for CBF114;115 CBF increases if temperature > 37 C and falls if temperature< 37 C Variation in CBF during day: maximal 12.00pm, minimal 8.00am119 CBF falls as relative humidity decreases120,121 CBF increases with hyperoxia. Prolonged hyperoxia causes fall in CBF and ciliarv death122,123 CBF is stable between ph CBF falls if ph<7.0 or > CBF is constant in iso/hypertonic solutions. Hypotonic solutions cause decline in CBF Reduction in CBF with increases in viscosity o f solution " CBF increases as ambient pressure rises CBF of cilia in Nose = Trachea = Bronchi97,128. CBF is reduced in bronchioles Table 2.1: Actions of physiological parameters on ciliary beat frequency (CBF).
57 2.7.1 Regulation of ciliary beat frequency Regulation of ciliary beat frequency is important as it has a direct effect on mucociliary clearance. Several mechanistic models have been derived following work investigating the action o f local mechanical stimulation and ciliary beat frequency. Regulators 7 4- identified are, intracellular calcium [Ca ]i, calmodulin, cyclic nucleotides, adenosine triphosphate (ATP), nitric oxide (NO) and neuronal control Mechanical stimulation and ciliary beat frequency Sanderson found that ciliary beat frequency increased after mechanical stimulation of ciliated cells.130 This is a local regulatory system by which mucus mechanically stimulates cilia to beat faster and therefore increase mucociliary clearance.110 The mechanism involves activation of a mechanosensitive receptor. This stimulates the production of phospholipase C ( P L C ) which increases the formation of inositol 1,4,5- triphosphate ( I P 3 ). Intracellular calcium [ C a 2 + ] i rises due to I P 3 binding to receptors on intracellular stores to release C a In addition, I P 3 diffuses through gap junctions into adjacent cells and releases intracellular calcium. This apparent calcium w ave can propagate up to a depth of 7 cells resulting in an increase in ciliary beat frequency in all cells.134 The maximal beat frequency response lags behind the rise in [ C a 2 + ] i.,03;132 56
58 Early work suggested that the initial rise in [Ca2+]j opened voltage dependent calcium channels to allow extracellular calcium into the cell to assist in the propagation o f the intercellular calcium wave.135 It is now felt that extracellular calcium has little or no role Initial work focused on cultured ciliated cells. Felix et al used an intact trachea as a model to re-evaluate the role of intracellular calcium in mechanical stimulation.131 Similar findings were observed, however the calcium wave only propogated through 2 cells. Ciliary beat frequency increased in only 20 cells compared to cultured cells where this was seen in cells. Again, extracellular calcium did not have a major role in calcium spread Calcium Intracellular calcium [ C a 2 + ] j is a key element in regulation of ciliary beat frequency and is tightly controlled. A small rise in [ C a 2 + ] j between nm will result in a maximal increase in ciliary beat frequency,103 similarly a fall in [ C a 2 + ] i produces a reduction in beat frequency There is still no agreement how calcium increases ciliary beat frequency with several mechanisms proposed. It may form a calcium/calmodulin complex which; activates calcium/calmodulin dependent kinases; stimulates a phosphatase which increases 57
59 dephosphorylation removing inhibitory effect on beat frequency; acts directly on the t 25;129; 138; 139 dynein arms. In cultured rabbit ciliated cells it has been suggested that calcium produces a short rapid increase in ciliary beat frequency and cyclic adenosine monophosphate (cyclic AMP) a gradual prolonged rise in beat frequency. 140 However in human respiratory cilia the opposite has been observed 104. Both calcium and cyclic AMP have been suggested to act via second messengers rather than directly on the axoneme Cyclic nucleotides Cyclic adenosine monophosphate (cyclic AMP) and cyclic guanosine monophosphate (cyclic GMP) are both cyclic nucleotide second messengers with regulatory roles for ciliary beat frequency Cyclic AMP is synthesised by activation o f adenylyl cyclase I ^8 following receptor stimulation. Intracellular increases in cyclic AMP cause an increase in ciliary beat frequency This is thought to occur via activation of cyclic AMP dependent protein kinase A (PKA) which then alters phosphorylation of specific axonemal target proteins Cyclic GMP is produced by guanylyl cyclase and acts on three major cellular receptors; cyclic GMP dependent phosphokinase (PKG), ion channels and cyclic GMP binding phosphodiesterases. 138 Ciliary beat frequency is increased via a cyclic GMP dependent 58
60 manner in human ciliated cells. In bovine cilia this has been shown to involve stimulation of PKG. 138 Both cyclic nucleotides have been found to act antagonistically on the power stroke in unicellular eukaryotes However in respiratory cilia it has been suggested that they 138 may act synergistically Protein Kinases Stimulation from protein kinase A (PKA) via the formation of cyclic AMP leads to the phosphorylation of targets on mammalian cilia. 136 PKA causes a moderately prolonged increase in ciliary beat frequency via a C a 2+ independent mechanism possibly by direct phosphorylation of axonemal proteins. PKA has been shown to increase [ C a 2 + ] i through 7 4- release of Ca from intracellular stores producing a rapid rise in ciliary beat frequency. Ciliary beating enhanced by PKA becomes insensitive to further changes 7 -P in cell Ca. It has also been suggested that PKA may modulate the affinity of I P 3 receptor. In contrast, one report suggested that PKA has an inhibitory effect on calcium/calmodulin-dependent phosphorylation. 104 A further role suggested for PKA is the regulation of basal ciliary beat frequency. Braiman et al suggested that basal ciliary beat frequency was due to low activation o f 7 I phospholipase C (PLC) causing calcium oscillations due to actions of I P 3 and Ca on I P 3 59
61 channels. This was under regulation of PKA. Ciliary beat frequency was increased by activation of a calcium mobilising receptor. This increased activity of PKA and promoted calcium release from intracellular stores by altering the affinity of the channel receptor to IP Protein kinase C (PKC) is resident in the cell cytosol and the binding of diacyl glycerol, a bi-product formed when IP3 is generated from hydrolysis of phophatidylinositol 4,5- bisphosphate, makes PKC migrate to the cell membrane. PKC causes a reduction in beat frequency seen in both stimulated and non-stimulated ciliary cells.25,143 PKC is not a single entity but is composed of three super families; conventional (calcium and diacyl glycerol dependent: 4 isoforms), novel (calcium independent, diacyl glycerol dependent: 5 isoforms), atypical (calcium and diacyl glycerol independent). 144 In stimulated cells P K C reduces the propagation of the calcium wave by reducing the rate of spread and the amplitude of rise of [ C a 2+ ] j.143 P K C is thought to act by blocking the diffusion of I P 3 through gap junctions. It has been suggested that P K C may influence the generation of I P 3 and diacyl glycerol and promote an initial Ca 1 influx which could stimulate beat frequency. 143 However this observation was observed in amphibians and thought unlikely due to phylogenetic differences or due to the action o f different PKC isoforms. 145 However in mammals all data support the ieda that stimulation o f PKC- dependent pathways cause a decrease in ciliary beat frequency
62 Nitric Oxide Nitric oxide (NO) is thought to have an autocrine or paracrine role in regulation of ciliary beat frequency. 146 This is supported by data which have shown that NO is; released in ciliated airways, associated with an increased synthesis of cyclic GMP and inhibition of nitric oxide synthase (NOS) inhibits ciliary beat frequency. 137,146 Despite this evidence NO is not the only pathway in the regulation of ciliary beat frequency147 and appears not to regulate basal ciliary beat frequency. 148 NO is produced by the action o f nitric oxide synthase (NOS) on L-arginine. NOS is found in two forms in the airway epithelium 137,146,148 as inducible (inos) 146,148 and constitutional (cnos) nitric oxide synthase. This is formed from two sources neuronal (nnos) and endothelial (enos) 146;149;150 nitric oxide synthase. The location of NOS is still unclear with enos being expressed close to basal microtubular membranes in rat tracheal ciliated cells, 146 but found to be distributed evenly within human sinus cells. 147 nnos is found in neurones below the epithelial layer. Conversely, inos has been expressed close to apices of ciliated cells in human sinuses147 but poorly in trachea and lungs of rats. 150 The differences in expression may reflect difference in the two isoforms of NOS. 61
63 inos is inducible and calcium independent. NO is produced continuously by a slow regulated mechanism which does not respond to stim uli147,148 inos production is 148 increased through RNA expression stimulated by cytokines or lipopolysacharides. It is unlikely that NO production by inos has a major role in regulation o f beat frequency. enos is thought to act predominantly on intracellular guanylyl cyclase in ciliated airways. 150 This is calcium dependent and enos is stimulated by mechanisms which change [ C a 2 + ]i,37;147 Stimulation of guanylate cyclase by NO produces cyclic GMP which activates PKG which increases ciliary beat frequency. 137,147 The site of action of PKG is unknown and it is thought that PKG also requires a rise in [ C a ]i to alter ciliary beat frequency. 137,146 Various ciliary stimulants have been shown to utilise the NO pathway, these include; ATP, TN Fa, II-1 p, terbutaline, metacholine, substance P and salbutamol Although NO has been shown to increase ciliary beat frequency via the cyclic GMP pathway, 150,151 it has been suggested that the ciliostimulatory effects of cyclic GMP may be independent of NO. 147 One report found that cyclic GMP reduced ciliary beat frequency in rabbit trachea149 and alternative pathways for NO action via cyclic AM P 151 and cyclic ADP-ribose pathways149 have been suggested. 62
64 Adenosine triphosphate Application of adenosine triphosphate (ATP) on ciliated cells has been found to increase ciliary beat frequency ,152 Extracellular ATP has been shown to be a potent 117 stimulator of beat frequency. There is a concentration dependent relationship between ATP and ciliary beat frequency. 152 ATP may act as an autocrine or paracrine mediator139 as it has been isolated in airway surface fluid4 and may mediate cell communication following mechanical stimulation. 152 ATP is thought to stimulate a purinergic receptor (P2Y2) which causes a rapid and large rise in [Ca 2 + ] i by mobilising intracellular stores. This has been correlated to an increase in beat frequency. Following a rise in [Ca 2+], the calcium level falls to a plateau but beat frequency remains maximal but still requires calcium influx. P2Y2 activation initiates G proteins which stimulates PLC to form IP3, this activates 7T receptors on internal stores and releases Ca to stimulate beat frequency, as described 17Q*1T7*17Q earlier. Similar observations have been observed with UTP. 1 c ^ It is debated how the prolonged rise in beat frequency is maintained. It has been 2t suggested, using data from rabbit tracheal cells, that NOS is activated by the rise in Ca to produce NO which enters the NO-cyclic GMP pathway. The resulting increase in both PKG and [Ca 2+ ] \ is thought to generate the sustained increase in beat frequency. 137 This 63
65 observation has not been found in human studies and is thought to represent species differences. 129 Other data have suggested adenylyl cyclase, which is stimulated by a rise in [Ca 2f ] i, activating the cyclic AMP/PKA pathway. Alternative mechanisms may involve adenosine via stimulation of adenosine receptor A2b Adenosine is produced by hydrolysis of ATP by ecto-nucleotidase activity. 139 It has been shown to increases beat frequency with a magnitude similar to ATP and UTP. The precise mechanism is unknown but is thought to stimulate cyclic AMP production and PKA.,29;152 Recent data have shown that [Ca 2 + ]i oscillates when ciliary beat frequency is elevated. 139 ATP stimulation has been found to cause [Ca 2 + ]i to oscillate, resulting in beat frequency oscillations of 7Hz over a 11 second period. Calcium oscillations may represent a signal transduction mechanism for ciliary beat frequency which has direct action on enzymes modifying axonemal phosphorylation and dephosphorylation. How this occurs is still unknown but protein kinases may be involved including PKA, PKG and PKC Cross talk of signaling systems As illustrated a number of signal transduction systems are involved in regulation of ciliary beat frequency with distinct pathways. There is now increasing evidence for cross
66 talk between the signaling pathways of calcium, cyclic GMP and cyclic AM P Using patch clamp technique on unstimulated cells Ma et al found that basal ciliary beat frequency does not require calcium and increases in ciliary beat frequency require both calcium and cyclic nucleotides. They suggest that intracellular calcium acts directly on unknown components on the axoneme, probably calmodulin, but requires the presence of cyclic nucleotides to modulate the response. 102 However this work was performed in single ciliated cells, therefore it is difficult to extrapolate these data to epithelial strips. Similarly evidence for cross talk between Ca2+ and cyclic AMP pathways has been reported. It has been suggested that there is dual control of ciliary activity with Ca2+ and cyclic AMP acting through independent pathways that interact or converge at the axonemal or subaxonemal level Neural control Autonomic control of ciliary beat frequency has also been suggested. Acetyl cholinesterase containing nerve endings have been found in the surface epithelium and submucosa of the airway. Yeates106 has proposed a model to explain neural control of ciliary beat frequency. He suggests that ciliary beat frequency is maintained at a low basal level. Excitatory signals are suppressed by inhibitory neural signals mediated via parasympathetic and sympathetic activation. The system, therefore, has the ability to respond to an insult on the respiratory epithelium by increasing the basal ciliary beat 65
67 frequency. The inhibition of ciliary beat frequency is proposed to be by cellular stimulation by Neuropeptide Y (NPY). NPY is an inhibitory neuromodulator of the autonomic nervous system found in the sympathetic neurons. It activates membrane bound PKC and causes a reduction in ciliary beat frequency. It has been shown in nonstimulated ciliated cells activation o f NPY Y2 receptors stimulates the isoform npkc to increase activity of membrane bound Ca2+-ATPase pumps. This reduces [ C a 2 + ]j and decreases ciliary beat frequency145. Ciliary beat frequency can be stimulated by parasympathetic and sympathetic agonists. 153,154 Muscarinic and adrenergic receptors have been identified on both the mucosal and serosal surfaces of ciliated cells. Beat frequency has been shown to increase significantly by stimulation of muscarinic receptors on the mucosal ciliated surface and adrenergic receptors on the serosal surface. 154 Muscarinic receptor ( M 3 ), stimulation by acetylcholine, interacts via G proteins to stimulate PLC. 109 This produces I P 3 and calcium release (as previously described). NO is produced due to calcium stimulation of NOS and activates the cyclic GMP-PKG pathway to increase beat frequency (section: ). 155 Priel and colleagues observed two responses following muscarinic receptor stimulation; a short term increase in beat frequency mediated via cyclic GMP-PKG pathway and a prolonged increase in beat frequency regulated by cyclic AMP-PKA pathway. Interplay 66
68 was observed between the two systems due to the activation of both adenylyl and guanylyl cyclase by the calcium-calmodulin pathway. Neither system was found to utilise NO. 156 However interpretation is difficult due to species differences as suggested recently. Interestingly, autonomic control may not be as clear cut. Muscarinic receptors ( M 3 ) stimulation has also been found to be associated with a reduction in ciliary beat frequency
69 2.8 Periciliary Fluid Overlying ciliated epithelium is periciliary fluid and mucus. These two components form the airway surface fluid (figure 2.6 ). 25 A third lipophilic layer may exist between the periciliary fluid and mucus The role of the lipophilic layer is unclear. It has been suggested the layer reduces surface tension but it is thought to be unlikely. 157 M PF C Figure 2.6: Light microscopy of human respiratory epithelium. Illustrating the three main components for mucociliary clearance; Ciliated epithelium (C), Periciliary fluid (PF), Mucus (M). From rapid fixation techniques the depth of periciliary fluid is pm.25;158 Recently using confocal microscopy an optimal depth of 7pm has been determined. I5 7 ;159 Production of periciliary fluid may be by microvilli37 but evidence has suggested that ciliated cells are able to actively control ionic gradients across the apical cell membrane and regulate airway fluid
70 Maintenance of the depth of periciliary fluid is important for effective mucociliary clearance. If periciliary fluid is too deep cilia fail to propel mucus. Conversely, if the fluid is too shallow cilia become stuck within the mucus layer Airway epithelia have evolved mechanisms to control periciliary fluid depth by both active and passive mechanisms. Active mechanisms involve airway epithelial transport of sodium 158 and chloride ions. 159 Passive mechanisms involves the mucus layer which acts as a water reservoir to regulate the depth o f the periciliary fluid layer Early work suggested that the periciliary fluid was essentially static and oscillated as cilia 1188 * 88 *1S 7 beat. Regulation was by active ion transport at the epithelial surface. Reports now suggest that periciliary fluid is closely coupled to propulsion of overlying mucus. Absence of mucus results in a reduction in velocity of periciliary fluid transport. 88 Periciliary fluid movement has been observed to be faster than can be explained by frictional transport between the two layers90 or hydrodynamic coupling and appears to be actively transported at a rate similar to overlying mucus. 88,159 Ciliary motion may also assist in controlling and maintaining the depth of airway surface fluid by movement of fluid between different airway regions. 24,160 Despite this new evidence for periciliary fluid movement Matsui et al have suggested the existence of a stationary layer o f fluid approximately 0. 1 pm in depth bathing the apical epithelial surface.88 The normal airway surface fluid contains many components such as glycoproteins, lipids, Immunoglobulin A, lysozyme, lactoferrin, peroxidase and surfactant The small 69
71 volume of periciliary fluid allows airway cells to exert paracrine influences on adjacent cells. 158 In airway surface fluid antimicrobial peptides (p-defensins) have been isolated. These are cationic peptides which interact and disrupt microbial membranes. For microbicidal activity defensins were thought to require an environment with a low salt concentration. However, activity in isotonic solutions has now been reported.90 Similar regulation is required for Lactoferrin and lysosyme, other antibacterial peptides, found in airway surface fluid Respiratory mucus. Respiratory mucus is composed 1% salts, 2-3% protein and glycoprotein (mucins), and 95 % water. 34,157 It is a high molecular weight glycoprotein composed of random coils of carbohydrate chains attached by O-glycoside linkages to a central protein core. It behaves as a visco-elastic gel within which enzymes and immunoglobulins are found.34 Lipids have been isolated between mucus and periciliary fluid. They are thought to assist in lubrication o f the mucus layer and have a role in epithelium protection.37 Respiratory mucus is derived from a collection of secretions from several areas including the alveolus. Mucin is predominantly produced from goblet cells (M U C5A C159) and 70
72 submucosal glands (MUC5B 159). 34,90 It is synthesised within the Golgi apparatus and stored in tightly packed granules. Release is by exocytosis and several mechanisms have been suggested. Goblet cells release mucin in response to ATP application and to direct epithelial contact. Submucosal glands are thought to be under parasympathetic control.25 The precise mechanisms are still unclear.25 Once released a rapid conformational change occurs as mucin rapidly expands and swells by hydration. This is dependent on airway salt concentration, osmolality, water content and ph.25,29,34 These factors are in turn controlled by ion and water transport o f ciliated cells.29;37 Respiratory mucus protects, hydrates and humidify the airways, acts as a filtration and diffusion barrier for airway surface fluid and is intimately involved in local mucociliary defence. 34,37 Inhaled bacteria and particulates adhere to the mucus layer due to its glycoprotein structure. This forms a heterogeneous chain of differing receptors to capture particles and organisms. Once bacteria are attached to the mucus layer, embedded neutrophils and macrophages can act upon the pathogen. Ciliary beating is thought to promote mixing of the mucus layer.90 Antibacterial proteins such as lysozyme together with antiproteases and immunoglobulins assist in pathogen destruction. 34 The collection of cells, bacteria and mucus is then removed from the airways by mucociliary clearance. 71
73 At present debate exists as to whether the mucus layer is continuous or a series o f 2^ separate mucus islands. * There are now two models proposed for airway mucociliary defence. The classical model of mucus overlying the periciliary fluid may be over simplified. The alternative model proposes that mucins form a tangled network. This is concentrated at the air interface and extends down to the epithelial surface. 160 However recent evidence has suggested the second model is incorrect following reports that a separate mucin layer of highly glycosylated molecules is found to line airway epithelial cells. This glycocalyx extends nm into the lumen and is formed from mucins MUCl and MUC
74 Chapter 3 73
75 3 Review of methodology to evaluate ciliary function 3.1 Summary Measurement of ciliary beat frequency has been described since the late 1880 s. One aim of the thesis is to evaluate digital high-speed video as a new method for the evaluation o f ciliary beat frequency and beat pattern. Chapter 3.2 reviews the methodology involved to measure ciliary beat frequency. The chapter discusses sample sites and methods used to obtain ciliated epithelium. Physiological considerations in specimen mounting are discussed. Finally the methods used to measure ciliary beat frequency have been reviewed in depth. 74
76 3.2 Historical Perspective Over the last seventy years much attention has been given to the evaluation of ciliary function and its interrelationship with mucus transport. Two main areas have been developed to investigate this; mucociliary clearance and ciliary beat frequency Methodologies used to measure ciliary beat frequency have evolved as technology has progressed. The earliest measurement of ciliary beat frequency was by Martius in 1884 using a stroboscopic method A9 device to demonstrate ciliary activity (Figure 3.1). In 1921, Inchley produced the cilioscribe a novel [ITT H 1 1 Figure 3.1: Cilioscribe A glass tube is placed vertically on a hatpin. A strip of ciliated epithelium is looped around the tube. The force created by ciliary beating rotates the glass rod. This is shown by rotation of a graduated cork disc
77 However, cinematographic and stroboscopic methods were developed to examine ciliary beat frequency and beat pattern in both protozoa and mammals.2,5,6,95 These were the main methods for ciliary evaluation until Dalhamn developed the widely used photometric technique. 163' 165 Adaption o f photometric principles have been used to develop a third method utilising an electrical photodiode cell. 166 Recently, digital high speed video imaging has been introduced as a method for evaluation of both ciliary beat frequency and ciliary beat pattern. " Mercke states that a method for m easurement o f ciliary beat frequency should; allow instantaneous recording of ciliary movement, be sensitive to rapid frequency changes and allow uncomplicated analysis of results. 161 There have been several reviews of methods used to measure ciliary beat frequency and the advantages and disadvantages o f each being highlighted.1; However, no direct comparison between methods has been made. Chevance et al. used stroboscopic, cinematographic and photometric methods to measure ciliary beat frequency and concluded that measurements by high speed filming combined with photometric readings would allow accurate evaluation of beat frequency. They did not compare methods against each another. 167 To interpret ciliary beat frequency as an outcome measure for diagnostic or research purposes a method needs to be reproducible and have normal reference ranges. Variations in methodology, sample processing and measurement of beat frequency makes comparison between studies difficult. Reviewing the literature areas o f inconsistency are 76
78 found in; sampling, preparation, methodology, measurement and analysis of ciliary beat frequency. These areas will be reviewed and ideals illustrated. 77
79 3.3 Respiratory Cilia Models Various models have been developed to study respiratory cilia. Initial mammalian in- vivo systems were designed using both rat and rabbit models.6 7, Results obtained from stroboscopic and cinematographic methods were only able to evaluate beat frequency indirectly by measuring reflected light from mucus movement. Applying photometric techniques was difficult due to vibration artifact. 169 Problems were encountered due to cumbersome apparatus, difficulty in setting up the system, large number of animals sacrificed and results which may not be applicable to human respiratory cilia. 7 In-vitro models are now commonly used, which, despite being devoid of blood supply and innervation they are thought to approximate ciliary activity in-vivo. Caution must be used when interpreting results. 171 Early in-vitro models involved animal tracheas being excised and viewed under various * 173 conditions. Later bronchial or tracheal scrapings were used. In order to study 21 human respiratory cilia, epithelial specimens have been obtained from nasal turbinates, nasal polyps, 174 tonsils, 175 the maxillary sinus, 16 trachea, 170 and bronchi. 128,176 O f the different samples nasal ciliated epithelium is the easiest to obtain. This has a ciliary beat frequency which correlates well to the beat frequency o f cilia obtained from 78
80 the trachea and bronchi.97,114,128;128,177,178 Therefore nasal samples may be thought to be representative of ciliary structure and function o f human respiratory cilia. Despite the loss of intrinsic mediators nasal specimens are easy and simple to process, are of human origin and can be analysed using methods available giving reproducible results. With technological advances samples can now be cultured and ciliated cells grown to provide specimens which have established intra-cellular communication and therefore closer to normal physiological conditions.171,177; Method of Sampling Nasal brushing, curettage, biopsy and surgical turbinectomy have been methods used to obtain nasal ciliated epithelium. 21 Comparisons have been made between sampling techniques for nasal brushing and biopsy and nasal curettage and biopsy. No 178* ] 80 difference was observed in ciliary beat frequency between sampling methods. Recently, a study compared different sampling techniques used to obtain specimens for ciliary ultrastructural evaluation. MacCormick et al compared samples obtained by nasal brushing with nasal biopsy, tracheal biopsy and bronchial brushing. The study found nasal brushings to be the favoured sampling technique and comparable to tracheal biopsies
81 3.4.1 Nasal brushing Nasal brushing is the most common and least invasive method for sampling ciliated epithelium.i81;182. After blowing the nose, a 2mm nylon cytology brush is introduced into the nasal cavity and placed against the inferior nasal turbinate. The brush is moved posteriorly and anteriorly and withdrawn. 189 agitating in cell culture medium. 189 number of cells obtained by different types o f brush sample size obtained or the number o f ciliated cells found. Epithelial strips obtained are dislodged by Although, reports have found variations in the total Brush size has no effect on the Various sites have been suggested for sampling o f nasal ciliated epithelium; lateral nasal wall, between the turbinate and lateral wall o f nasal cavity, the nasal septum at the *186 level o f the inferior turbinate and the inferior nasal turbinate. Direct visualization is not thought necessary and has only been performed by one 165 *182 group. No difference has been reported for ciliary beat frequency measured from specimens sampled from different sites. 182 Similar data have been published for ciliary ultrastructure. 187 In general specimens should be obtained from the posterior part o f the inferior nasal 181 turbinate. Specimens taken from the anterior region show a decrease in ciliated cells. 80
82 Figure 3.2: Sampling of nasal ciliated epithelium by brush biopsy Nasal brushing has the advantages of being quick and easy to perform, is atraumatic with little morbidity and suitable for use in children (figure 3 2).l65;182;187 It does not require local anaesthesia, and allows specimens to be used directly for evaluation of both ciliary structure and function The main limitation is that the area o f sampling is small and 1 87 brushing may occasionally fail to obtain adequate specimens from suppurated mucosa. In addition, the subject must not have a concurrent upper respiratory tract infection as this will cause secondary ultrastructural changes and may alter ciliary beat pattern and these changes may persist from between 3-10 weeks ' 190 It is suggested that a subject should therefore be free from infection for at least 6 weeks at the time of nasal brush biopsy N asal curretage Ciliated samples can be obtained by scraping the epithelial lining of the inferior turbinate using a 2mm curette. This is performed without local anaesthesia and results in a large cellular sample.i80; 191 Cilia obtained from curette samples often occur in clusters, this V. 81
83 may complicate beat frequency analysis as cilia are insufficiently mobilised. In addition, active ciliated cells are difficult to identify and there is disruption to the ciliated epithelium. Samples may also contain cellular debris, mucus and nasal hair which will complicate analysis Forceps biopsy Under general anaesthesia a biopsy is obtained from the posterior inferior nasal turbinate. 180,192 Ingels has suggested that biopsy samples are superior as the ciliary beat pattern has been reported to be constant and the epithelium remains intact. He suggested this was more comparable to in-vivo conditions as specimens have intact intercellular links. 180 Despite the apparent superiority o f the process, a biopsy is a more invasive technique, requires general anaesthesia and has the potential for greater discomfort, haemorhage and morbidity. The sample itself may be too thick to obtain a ciliated edge and erroneous measurements of ciliary beat frequency could occur. 86 Inhalational anaesthetics may affect beat frequency measurement as they cause a reduction in ciliary beat frequency and can be ciliotoxic
84 3.4.4 Nasal turbinectomy Ciliated samples obtained from nasal turbinates have been used predominantly in i Q9 IQT research. Turbinates are obtained during elective surgery under general anaesthesia. Biopsied discs are taken from the turbinate and used to measure ciliary beat frequency. Ciliated edges are thought to be superior to those from nasal brushings due to ease o f I analysis. Multiple samples can be obtained from one turbinate specimen and recurrent experiments performed. Ciliated samples obtained from nasal turbinates can be stored for up to 10 days in controlled conditions with no change in beat frequency. 193 Also cilia from turbinates may be used for cell culture either as an organ culture model194 or ciliated monolayer Specimen mounting It is important to examine specimens in physiological conditions controlled for humidity, ph, and temperature. Various preparation mounts have been developed which include the hanging drop technique and perfusion systems Hanging drop The static mount or hanging drop technique is commonly used. It allows the ciliated specimen to be suspended in a drop of cell culture medium between the glass slide and cover slip. Various techniques have been used to create the hanging drop preparation 83
85 including, silicon grease,165 welled slide,17 18; ;l64;l7l;185 a silicone ring195 and a chamber created by the separation of a cover slip and the glass slide by two adjacent coverslips.85 Corssen described a novel way o f creating a hanging drop, in which ciliated epithelium was added to a plasma clot and allowed to form its own chamber. Once set the preparation was placed in a perfusion chamber with the ciliated sample visible Perfusion chambers Several perfusion systems have been developed86 192, of note are the Rose and Dvorak-Stotler chambers.199 Both utilise a sealed transparent unit through which medium is perfused and heated. Perfusion systems allow evaluation of the action of pharmacological and biochemical agents in an easy and physiologically controlled manner. 84
86 3.6 Physiological conditions As stated earlier various physiological factors will alter beat frequency and need to be regulated (Section: 2.7). Standard conditions for ciliary evaluation have now been suggested: ph: The precise ph at which to measure ciliary beat frequency has not been stated but is assumed to be ph 7.3. Temperature:Nasal temperature is between C116 with a further increase in temperature to C in children towards the posterior oropharynx It has been suggested that studies should be conducted at a temperature o f 37 C. Nasal cilia are used as a model o f lower respiratory tract cilia and no difference has been observed between beat frequency of cilia obtained from either site Despite this various studies have measured ciliary beat frequency at room temperature (22-25 C) and results can therefore not be extrapolated Humidity: It has been suggested that humidity must be maintained between %121,201 to prevent epithelial desiccation.95 85
87 3.7 Area of measurement Opinion varies as to which epithelial edges should be utilised to measure ciliary beat frequency. Measurements have been taken from edges on which cilia are actively 17*80* beating to more precise definitions o f an undisrupted edge >100pM in length. Sample definition is important as beat frequency measured from individual ciliated cells and small groups of cells is inconsistent, due to the loss of intrinsic regulators.186 Ciliary beat frequency should be measured from a strip of ciliated epithelium. There is variation in length of epithelium and number of cells to use ranging from 2pM length or 1-5 cells1l4;165;l73;191 to a mucous free undisrupted edge between pM with an intact basement membrane.85:185;,92;203;204. The number of edges from which readings are taken has varied from 4203 to Teichtahl has stated to reduce the standard error, ciliary beat frequency should be measured from a minimum o f 10 ciliated edges
88 3.8 Methods for measurement of ciliary beat frequency Ciliary beat frequency has been measured from as early as the 1880s. This has been achieved using a variety of methods from direct observation to variations in light beams caused by beating cilia. As technology has advanced so have the methods employed Auditory An audiometric method was developed by Bleeker who approximated the ciliary beat frequency to a sound generated at a known frequency. The precise beat frequency is found when the sound is synchronized with the beating ciliary Turning spheres A novel technique was described by Ballenger170 and earlier by Corseim.196 Ciliated strips were placed into a plasma clot in which the ciliated epithelium formed a rotating sphere ( Turner ). Ciliary activity was calculated as the number o f revolutions completed per minute. This evaluates ciliary activity but is not a direct measurement of ciliary beat frequency. 87
89 3.8.3 Stroboscope The stroboscope is the earliest method described to measure ciliary beat frequency. It was used in 1884 by Martius in 1884 and has been used up until ;5;170;175. The stroboscope flashes light at known frequencies. The frequency of flashes is adjusted until the moving cilia appear stationary. The frequency of flashes is then at a frequency equal to the ciliary beat frequency. Although the stroboscope is a simple method,21166 it is limited. Authors have found it difficult to use21166 and individual cilia hard to visualise End points have been imprecise169,201 and low and high frequency measurements inaccurate, variable and hard to calculate.166; 169,201 It may be unreliable when precise measurements o f ciliary beat frequency needed Photodiode The photodiode method detects variations in light created by beating cilia (Figure3.3). Beating cilia are displayed on a visual display unit (VDU). The change in light intensity caused by the ciliary motion creates a signal which is detected by a photodiode cell. This produces a reading which can be displayed on an oscilloscope166 or analysed using fast Fourier transformation.112
90 Developed by Teichtahl it allows a quick and easy, real-time analysis of the ciliary beat frequency. Video recordings can be made of the ciliated edges for motion analysis. The method is subject to vibrational artifact and may be inaccurate at frequencies >50Hz which correspond to the VDU screen refresh rate. The small photodiode cell may be subject to incorrect placement over the ciliated epithelium displayed on the monitor and result in false readings.166 Also the optic signal may be degraded by the monitor 205 screen. video cam era video display unit hand-held probe sam ple vid eo ca ssette recorder filter and pow er supply m icroscope Q Pow er A nalysis Com puter program Figure 3.3: Photodiode system Beating cilia are displayed on a visual display unit. The change in light intensity caused by the ciliary motion creates a signal which is detected by a hand held photodiode probe. 89
91 3.8.5 Photometric method Initially described by Dalhamn this is the most common method for estimating ciliary? I 1 beat frequency. ' Two types o f photometric techniques have been described. The first detects variations in transmitted light caused by beating cilia (Figure 3.4). The changes in light intensity are detected by a photosensitive cell and transduced to voltage signals which are displayed on an oscilloscope or analysed using a spectral analysis programme. 21;86;208 Photomultiplier Tube Aperature.Varying Light Intensity C onstant Light Intensity Figure 3.4: Photomultiplier method Beating cilia disrupt the path of a beam of light. This causes a variation in the light intensity which is detected by the photomultiplier tube. 90
92 The second technique detects light reflected from the epithelial surface. A light beam is directed onto the ciliated surface and variations in the reflected light intensity is detected by a photometer and corresponds to ciliary activity.161 ;209 With this adaptation it is difficult to know the origin of the surface reflections and is thought to represent the mucociliary wave frequency.86 The photometric transmitted light technique is easy to use, allows real-time analysis of ciliary beat frequency and gives reproducible results.166 Analysis of voltage signals by spectral computer programmes allows a more objective evaluation of ciliary beat frequency. However, fast Fourier transformation produces a spectrum o f frequencies obtained from the ciliary waveform. The origin of these frequencies and the relation to the ciliary beat cycle has not been shown, therefore the predominant frequency identified may not be the true ciliary beat frequency.86 Nasal epithelial samples have cilia moving at different stages of the beat cycle. Therefore multiple beating cilia contribute to the light signal detected by the photomultiplier.210 When this is combined with motion and vibrational artifacts211 a complex waveform is produced. Passing this signal through fast Fourier transformation will again result in an average beat frequency measurement To minimise these errors Dalhamn suggested using a small aperture of 2.5pm2 through which light enters the photomultiplier.163 Other authors have used larger apertures which will encompass more frequencies and out o f phase metachronal activity therefore contributing to experimental 91
93 error.86 Therefore photometric techniques may be unreliable when precise measurements o f ciliary beat frequency are needed. The method also becomes unreliable when cilia 205 start to beat asynchronously. * Although the photomultiplier allows no direct evaluation of ciliary beat pattern, the photoelectric signal produced has a distinct cyclical pattern which has been correlated to &fv ") 1^ phases in the ciliary beat cycle. " Laser light spectroscopy Laser spectroscopy is a modification of the photometric technique and involves the reflection of a laser by moving cilia. A low power laser beam of 10-15pm diameter illuminates beating cilia. The reflected light has an altered frequency and phase. The intensity and fluctuations o f the returning light is dependent on ciliary beat frequency. The reflected light is detected by a photomultiplier and processed through a spectral 21 *206* ^11 analysis program to calculate ciliary beat frequency. The method is simple, accurate and reproducible due to the precise frequency and coherency o f the laser beam. It correlates closely with high-speed cinematography in both precision and reproducibility.206 However laser spectroscopy is expensive and subject to artifactual error due to vibration. Information obtained relates only to beat frequency and not beat pattern
94 3.8.7 High Speed Video Prior to photometric methods, high-speed cinematography was a common technique employed for analysis of ciliary beat frequency.5,6 8 First reported by Athanasiu in 1905 who was able to record movements of a clam gill at 120 frames per second. The method improved as frame rates increased to in excess of 3000 frames per second as technology developed.214 Evaluation of mammalian and protozoan cilia utilised high frame rates of frames per second were adopted. The film is then processed and then analysed by slow motion replay to allow evaluation o f both ciliary beat frequency and beat pattern. Highspeed cinematography has been recognised by many as the optimal method to analyse ciliary function.85;86; ;202;206 Because of bulky equipment, vibration artefact, difficulties in image processing, expense and lack of real time analysis it was superseded by methods such as the photodiode and photomultiplier techniques.20,85,211 Sanderson suggested that indirect methods may appropriate for simple beat frequency measurements but for analysis of ciliary beat pattern, spatial information is essential and requires video microscopy.210 Consequently, some authors have recommended a combination of high speed cinematography and photometric analysis for evaluation of ciliary function.86;169 93
95 The development of video technology allowed the reintroduction of cinematographic techniques as a real-time method for analysis of ciliary function. Images from a video camera were recorded onto standard videotape and used to assess ciliary function. However, video recorders acquire images at rates o f 25-30Hz and were found to create 215 artifacts due to signal aliasing. Signal Aliasing A video camera/recorder obtains images at a certain frame rate (frequency). As the ciliary beat cycle is a continuous movement, images of the cilium will be obtained at certain phases of the beat cycle. If images are recorded at the same frequency at which the cilium is beating then it will appear static, i.e. the camera will always obtain an image at the same point in the ciliary beat cycle. Signal aliasing occurs when images are recorded at a frequency close to that of the ciliary beat frequency being analysed and results in a paradoxical lower beat frequency being measured. When using standard video recording rates (25-30Hz) signal aliasing has been reported to effect results at ciliary beat frequencies between 6-12Hz and will always effect 710 readings >15Hz. In addition, specific beat pattern components cannot be analysed due to artifacts induced by signal aliasing.210 This error can be avoided by using high sample rates.20 The minimum sampling rate (frequency) needed to measure ciliary beat frequency can be calculated by applying the 94
96 Nyquist criterion. This states that the sampling interval must be equal to or less than half the period of the highest frequency of the signal being measured.20 Therefore to visualise the forward power stroke of a cilium beating at 14Hz a minimum sampling time of 15ms is required. At 400 frames per second the sample time is 2.5ms so meets the Nyquist criteria. Although ciliary beat frequencies up to 25Hz may be measured by recording at frame rates of 50Hz, analysis of beat pattern requires high speed video recordings at frame rates up to 400Hz Digital High-speed Video Recently the quality of digital high speed video imaging has improved to such an extent that it has replaced video and high speed cinematography16' 18 and without problems due to signal aliasing. Cameras can now record at frame rates up to 70,000 per second.19 Consequently, the method has become a popular technique20 as it is simple, accurate, relatively inexpensive and allows real time analysis of ciliary beat frequency, beat pattern and coordination.16,20,21 Digital high speed videos can operate at low light levels, are highly compatible with digital analysis systems and have the ability to archive data for further research and audit.20;22 95
97 Digital high-speed video allows greater clinical precision by having the ability to analyse ciliary beat pattern. This has been relevant in patients with ciliary transposition who were found to have a ciliary beat frequency within the normal range. This group would have been missed by beat frequency analysis alone, but beat pattern evaluation revealed the dyskinetic movement.216 Beat pattern analysis has also been of use following viral infection; no change was seen in ciliary beat frequency but an increased proportion of dyskinetically beating cilia was observed. Therefore use of digital high-speed video allows greater precision when evaluating ciliary function Computer assisted analysis Computer assisted analysis of ciliary beat frequency is now being used." Signals are relayed from the high speed video camera to the monitor. The variation in gray scale of this signal can be digitized, captured and processed through image analysis software. This digital signal can then be analysed by fast Fourier transformation to measure ciliary beat frequency ;217 96
98 Chapter 4 97
99 4 Primary Ciliary Dyskinesia 4.1 Summary Chapter 4 reviews the genetic condition o f primary ciliary dyskinesia. This is due to congenital abnormalities of the ciliary axoneme. Aspects covered are the incidence, pathophysiology and prognosis o f the condition. A review of current genetic research is presented. The clinical manifestations and associations with primary ciliary dyskinesia are discussed. Finally an overview o f current therapeutic strategies is presented. 98
100 4.2 Historical perspective 218 Siewert first described the association of situs inversus and bronchiectasis in However, it was the Swiss physician Manes Kartagener in 1933, who described the association o f sinusitis, situs inversus and bronchiectasis as a clinical syndrome." Later in the 1970 s the relationship between abnormal ciliary function and respiratory problems was first reported. Patients with Kartageners syndrome were found to have 221 *222 immotile sperm due to the absence of dynein arms within the sperm axoneme. Further studies of cilia from the respiratory tract revealed impaired mucociliary clearance 77 1 '7')'). and deficient dynein arms. Initially this was called the immotile cilia syndrome Other patients with the syndrome were found to have cilia beating at 77^**77A low or even near normal frequency but moving in a dyskinetic fashion. With this 710 new evidence the syndrome was renamed prim ary ciliary dyskinesia. 4.3 Pathophysiology Cilia are found through out the body, in the upper and lower respiratory tract, the middle ear, eustachian tube and sinuses, lining the ependymal surface o f the brain ventricles, and 770 in both the male (vas deferens) and female (fallopian tube) reproductive tracts. In primary ciliary dyskinesia, respiratory cilia have the most relevance in disease pathophysiology
101 Primary ciliary dyskinesia may be caused by one of a number of different ciliary ultrastructural defects involving the dynein arms, radial spokes, microtubules or complete absence o f cilia (Table 4.1)_ Other reports have suggested partial defects o f both outer and inner dynein arms, shortened dynein arm stubs ~and microtubular disorganisation attributed to nexin link 998 defects/ These require further conformation by sampling from multiple tissue sites and 998*9 T9 correlating to clinical phenotypes/ However, as the cilium is composed o f multiple polypeptides it is likely that further defects exist which are out o f the resolution o f the f \ 1*998 electron microscope. However it is possible that some defects are present which fail to be detected as they are outside the resolution of the transmission electron microscope.61 However recent reports raise the possibility of extraciliary control defects. One involves the presence of polarity proteins that are required for ciliary assembly234 and the other suggest the presence o f 9 membrane bound mechanotransducers with a role for controlling ciliary motility. 100
102 Structure Defect Dynein Arms No inner and outer 221:222 No outer 230;236 No inner 237;23S Tubular Structures Transposition defect 239 Ciliary disorientation 60,240 Radial Spoke Absent radial spokes/inner arm defect 241:242 Axoneme Ciliary aplasia 243:244 Abnormally long 245:246 Abnormally sh o rt247 Basal apparatus A bnorm al248 Table4.1: Reported axonemal defects associated with primary ciliary dyskinesia Ultrastructural abnormalities within the ciliary axoneme result in cilia which are either stationary or beat in a slow or dyskinetic fashion. mucociliary clearance resulting in mucus retention. Ineffective movement impairs This leads to recurrent chest infections, which may progress to bronchiectasis, and chronic sinusitis
103 4.4 Incidence and genetics Although autosomal dominant and X-linked recessive modes of inheritance have been described,250 primary ciliary dyskinesia is inherited in an autosomal recessive fashion/" " The genetic loci responsible for primary ciliary dyskinesia are presently being determined but the process is complex with over 200 proteins involved in ciliary assembly.52 Following linkage analysis of families with dynein arm defects, several candidate sites have been suggested; 3p, 4q, 5p, 7p, 8q, lop, llq, 13q, 15q, 16q, 17q, and 19q.254,255 Recently genes encoding for dynein chains in inner and outer dynein arms have been identified as being responsible for some cases o f primary ciliary dyskinesia (table 4.2). The incidence of primary ciliary dyskinesia is approximately 1 in 16,000 of whom fifty percent will have situs inversus. Given this incidence, there should be approximately 3000 patients in the United Kingdom with 70 new cases occurring each year.255 It is suspected that primary ciliary dyskinesia is significantly under diagnosed. ' In addition as primary ciliary dyskinesia exhibits an autosomal recessive pattern, parental consanguinity will increase the incidence o f affected siblings.52;
104 Gene mutation Chromosome Protein Phenotype Reference - 19q Outer dynein arm DNAI1 7p21 Dynein Intermediate chain Outer dynein arm DNA H5 5p Dynein heavy chain Outer dynein arm DNA H7 - Dynein heavy chain 7 Inner dynein arm Table 4.2: Summary of genes identified in patients with primary ciliary dyskinesia 4.5 Presentation Primary ciliary dyskinesia can present in infancy or late adulthood due to the varying patterns of symptoms A comprehensive review of age related patterns of presentation has been performed by Bush and colleagues227 and summarised in Table 4.3. Symptoms frequently start in the neonatal period and include a chronic nasal discharge and a moist cough. Hearing problems may occur in approximately 50%236 to 75%249 of patients, with glue ear being common.266 It is assumed that fifty percent will have situs inversus. However, recent reports have suggested that not all ciliary ultrastructural abnormalities have situs inversus as a feature.265 All siblings of index cases require assessment
105 Age System Features Neonatal Respiratory Unexplained respiratory distress Neonatal chest infection. Childhood Cardiovascular Congenital anomalies Respiratory Cardiovascular ENT Situs inversus Cardiac defects Hydrocephalus Oesophageal atresia Biliary atresia Chronic wet cough Bronchiectasis Atypical asthma failing to respond to treatment Situs inversus Hearing impairment due to chronic otitis media Rhinosinusitis Central nervous system Learning difficulties Adulthood Respiratory Bronchiectasis Cardiovascular Genitourinary Situs inversus Male infertility due to impaired sperm motility Female ectopic pregnancy Table 4.3: Age-related presentation of primary ciliary dyskinesia (adapted from references 252)
106 4.6 Associations with primary ciliary dyskinesia Respiratory Distress Infants with primary ciliary dyskinesia may present in the neonatal period with unexplained respiratory distress which may require ventilation Neurological Reports have stated that, within the neonatal period infants may present with unexplained A 111 hydrocephalus ' and later in age mental retardation has been described. This may be due to abnormalities of ependymal cilia lining the ventricular surface resulting in impaired flow of cerebro-spinal fluid.83 However, ventricular abnormalities and mental retardation are thought to be uncommon as neither were observed in a series o f patients with primary ciliary dyskinesia who had head CT scans Nitric Oxide Levels o f nasal nitric oxide in patients with primary ciliary dyskinesia has been found to 11f\'111 be significantly lower when compared to healthy controls. Exhaled NO from the lower respiratory tract has similarly been found to be reduced but not to the same degree. ' Reasons for this are unclear but may be due to reduced production or problems diffusing through inflamed tissue.279 Also exhaled carbon monoxide was increased in patients with primary ciliary dyskinesia representing airway inflammation
107 4.6.4 Leucocytes Interestingly, microtubules are found within polymorphonuclear leucocytes and thought to assist with regulation o f cell motility.280 It has been shown that patients with primary ciliary dyskinesia have abnormal neutrophil orientation, migration and chemotaxis Other associations Other associations seen with primary ciliary dyskinesia are; oesophageal stricture and midgut volvulus, tracheo-oesophageal fistula, gastro-oesophageal reflux, complex 784*78^ 78 f \ 787 congenital heart disease, polycystic kidneys and biliary atresia. 4.7 Situs Inversus 50% of patients with primary ciliary dyskinesia have situs inversus (figure 4.1). The 788 reason for this is still unclear with several hypotheses suggested. One early hypothesis thought that cilia were placed in fixed positions within the embryo. These beat in a predetermined direction and so directed organ laterality.221 In primary ciliary dyskinesia these cilia are ineffective and so organ laterality is left to random assignment.288 This is supported by clinical data from monozygotic twins with primary ciliary dyskinesia, in whom one has situs inversus and the other situs solitus
108 Further evidence arises from work supporting an embryological role for cilia. Monocilia have been found on the embryonic node. This is an organising centre in primitive streak-stage embryo that regulates pattern formation Monocilia lack the central microtubular pair and have a 9+0 microtubular arrangement instead of the classic 9+2. They have been shown to beat in a circular anticlockwise motion292 and generate fluid currents around the node (nodal flow).293 The way in which nodal flow is thought to assist in determining organ laterality is still unresolved. Several mechanisms have been proposed, nodal flow may generate a molecular gradient highest on the left side of the embryo, transport an unknown molecule to the left side of the body which triggers a signalling cascade293 or activate a mechanosensor to the left of the node.291 Cessation or reversal of nodal flow and/or disruption to nodal cilia results in randomisation of body Figure 4.1: Radiograph of patient with situs inversus totalis 1 0 7
109 4.8 Diagnosis of primary ciliary dyskinesia The diagnosis of primary ciliary dyskinesia in a young child may be delayed for several reasons. Young children are usually unable to expectorate sputum and remain apyrexial while they have ongoing chronic lung inflammation and infection. A chest examination is usually normal with auscultatory findings presenting later in life. Only 50% have situs inversus and a chest radiograph may be normal in early life. Finally, young children are unable to perform objective lung function testing such as spirometry. In general a child with a wet cough of greater than 6 weeks duration is unusual and should be followed up closely. The differential diagnosis is wide and includes cystic fibrosis, gastro-oesophageal reflux, chronic rhinitis and congenital lung defects. Once these have been eliminated other causes o f a wet or moist cough should be investigated. At this point primary ciliary dyskinesia should be considered. The diagnosis of primary ciliary dyskinesia should be considered in patients under investigation for bronchiectasis or a persistent productive cough. A history of chest nasal and ear symptoms from the neonatal period should require early investigation. Making a diagnosis of primary ciliary dyskinesia is difficult. It is thought that there are large numbers o f undiagnosed patients. While false positive diagnoses can )q c i7 Q A occur, the greatest problem is due to late diagnosis. Late diagnosis is o f 108
110 concern as lung function steadily declines in undiagnosed patients," hearing related joi problems are often managed poorly and there are associated implications for future fertility.52 Various screening methods have been developed to identify patients: 1) Saccharin Test; involves the application o f a small particle of saccharin on the inferior nasal turbinate and the time noted for a sweet sensation to be tasted. A time greater than 60 minutes is abnormal and requires further investigation. The test is difficult to perform in children under the age of 10 years and may only identify cases with immotile cilia.298 Cases in which cilia are beating dyskinetically may be missed.298 Other variants include the use of technetium labelled albumin which is commonly used. 2) Nitric Oxide; in primary ciliary dyskinesia exhaled nitric oxide is low and may be 'J'lH useful as screening tool in older children and adult patients. In younger children ^QQ it is difficult to perform as they have problems with breath holding. Although research is continuing in this area it is now increasingly accepted for nitric oxide to be 0 T*7 0 "70 used as a screening test. In addition it may be low in some cases of cystic fibrosis, or severe rhinosinusitis.300 A high nasal nitric oxide level makes primary ciliary dyskinesia unlikely, but a low level requires further investigation
111 These two screening tests may be of use in adults, but are of little help in children of 12 years or less. Assessment of both ciliary structure and function is the most reliable way to make the diagnosis of primary ciliary dyskinesia.301, '304 Screening for ciliary motility is a poor way o f identifying patients with primary ciliary dyskinesia.304 Extending this to evaluation o f ciliary beat frequency may be used to determine which samples require ultrastructural evaluation by transmission electron microscopy The literature suggests that cilia which beat at a frequency of <6Hz294 or <11 Hz should have formal ultrastructural analysis. However, this may miss certain cases o f primary ciliary dyskinesia, in which ciliary beat frequency is found to be in the low to normal range Evaluation of both ciliary beat frequency, beat pattern and ultrastructural analysis for all samples has been suggested to be the gold standard for the diagnosis of primary ciliary dyskinesia This needs to be performed at centres with extensive expertise in the evaluation of ciliary structure and function and validated normal reference ranges. Recently, interest has grown in the development of ciliogenesis for the diagnosis of primary ciliary dyskinesia.224 The technique uses pronase to remove cells from the epithelium. The cilia are shed from the cell and the cilia regrow. This allows damage or secondary changes to be eliminated. The regrown cilia can then be analysed for both ciliary structure and function. All defects found in primary ciliary dyskinesia are expressed by the culture method
112 4.9 Management The basis of active management summarised in Table Treatment is aimed at preventing lung damage, the evolution of bronchiectasis and subsequent decline in lung function. Management involves the combination o f daily chest physiotherapy, and the early use of antibiotics for respiratory exacerbations. Bronchodilators may be required for reversible OAr la'y obstructive airway disease. '. However regular use o f an inhaled bronchodilator showed no significant improvement in lung function.306 Exercise should be actively encouraged as this may promote airway bronchodilation and therefore assists mucociliary clearance.305 One report has suggested the use of DNase as an adjunct to improve mucociliary clearance, however its role remains to be evaluated.308 All patients should be seen regularly at clinics with access to formal lung spirometry. Hearing assessments should be performed in younger children. Grommet insertion may result in chronic and recurrent discharging ears and conservative management using.. OT "7 0 ^ ^ hearing aids may be more beneficial for glue ear.~ 7 As the child grows hearing tends to improve as the eustacian tube enlarges. It is important to treat any change in respiratory symptoms at an early stage. The main infective pathogen is Haemophilus influenzae, however, Staphylococcus aureus and 111
113 Streptococcus pneumoniae have been isolated Pseudomonas is rarely isolated in paediatric patients256 but may be observed more commonly in adults.309 Each patient will require BCG and annual flu vaccination. Fertility issues are raised in primary ciliary dyskinesia, as cilia are found in fallopian tubes and sperm tails exhibit the 9+2 arrangement. All men with primary ciliary dyskinesia are thought to be infertile or subfertile.310 However a series found that not all males have immotile spermatozoa (40% Immotile), some may have oligospermia or 1 A azoospermia and fathered children in two cases.. Seminal analysis is recommended in men with primary ciliary dyskinesia so that accurate counseling about reproductive capability may be given.310 Techniques have been developed to allow assisted conception for males using in-vitro fertilisation, intra-cytoplasmic sperm injection and subzonal fertilisation Females are thought to be subfertile and may require assisted conception.313'317 Cilia in the fallopian tube have been found to be immotile,314 beat at a reduced frequency318 and be fewer in number
114 System Respiratory Hearing Fertility Psychosocial Management Aggressive use of antibiotics in respiratory exacerbations Twice daily physiotherapy and exercise Inhaled bronchodilators +/- corticosteroids Regular review with formal lung spirometry Yearly review by specialist centre Regular audiometry Temporary hearing aids may be required for severe hearing loss May require assessment at assisted conception unit Genetic counselling Entitlement to DSS benefits Primary ciliary dyskinesia support group Educational statementing due to learning difficulties Table 4.4: Specific areas of management for patients with primary ciliary dyskinesia (adapted from reference 227). 113
115 4.10 Prognosis With optimal treatment the prognosis is thought to be good with a near normal life expectancy.227 However, primary ciliary dyskinesia is associated with a progressive decline in lung function. Antibiotic therapy and aggressive physiotherapy halts this decline and causes the fall in lung function to plateau.256,307 Early diagnosis is essential to achieve a good prognosis and to minimise the high morbidity from lung infections and rhinosinusitis." Failure to diagnose and treat may cause end stage disease and result 1 t Q *5 1 Q_"5 9 1 in either lobar resection or heart-lung / double lung transplantation. 114
116 Chapter 5 115
117 5 Analysis of ciliary beat frequency and beat pattern using digital high speed imaging: Comparison with the photomultiplier and photodiode methods 5.1 Summary Background: The aim of this study was to determine the relationship of the power and recovery stroke of respiratory cilia using digital high-speed video imaging. Beat frequency measurements made using digital high-speed video were also compared to those obtained using the photomultiplier and modified photodiode techniques. Method: Ciliated epithelium was obtained by brushing the inferior nasal turbinate of 20 healthy subjects. Ciliated edges were observed by microscopy and the deviation o f cilia during their recovery stroke relative to the path travelled during their power stroke measured. Beat frequency measurements made by digital high speed video analysis were compared to those obtained using the photomultiplier and modified photodiode. Results: Cilia were found to beat with a forward power stroke and a backward recovery stroke within the same plane. The mean angular deviation o f the cilia during the recovery stroke from the plane of the forward power stroke was only 3.6 (95% Cl: ). There was a significant difference in beat frequency measurement between the digital high speed video (13.2 Hz (95% Cl: Hz)) and both photomultiplier (12.0 Hz (95% Cl: Hz) P=0.01), and photodiode (11.2 Hz (95% Cl: Hz) P<0.001) techniques. Conclusion: Respiratory cilia beat forward and backwards within the same plane without a classical sideways recovery sweep. Digital high speed video imaging allows both ciliary beat frequency and beat pattern to be evaluated. 116
118 5.2 Background to study Although many methods have been devised to estimate ciliary beat frequency21 high speed imaging by cinematography is regarded by many as the optimal method to analyse ciliary function.86,169,202 Reviews of methods of ciliary beat frequency measurement have commented on their advantages and disadvantages21;164;169 but there have been no direct experimental comparisons. An in depth review o f methodology is found in chapter 3. With the advent of digital high speed imaging ciliary function can be studied in detail. In addition to ciliary beat frequency measurement, the exact movement of a cilium throughout the beat cycle can be visualised in different planes. Despite the ability to study ciliary motion in detail, there is still no consensus of opinion as to the precise pattern of ciliary beating.5,8,9;95 It is generally thought that respiratory cilia have a forward power stroke and a recovery stroke during which the cilium sweeps backwards and to the side.9 As the cilium sweeps sideways and backwards in a clockwise direction it is thought to stimulate adjacent cilia to beat, propagating the ciliary metachronal wave.9 However, the switch hypothesis suggests differential activation of two opposing groups of microtubules during the power or recovery stroke. This results in a hands down or hands up up position of the cilium at either end of the beat cycle.15 The sideways recovery sweep cannot be incorporated within this hypothesis. 117
119 5.2.1 Aims The aims of the study, therefore, were as follows: 1) To determine the relationship of the power and recovery stroke of respiratory cilia using digital high speed video imaging. 2) To compare ciliary beat frequency measured using digital high speed video imaging to that obtained using the photomultiplier and modified photodiode techniques. 118
120 5.3 Methods Ciliated samples were obtained by brushing the inferior nasal turbinate of 20 healthy subjects (13 males and 7 females (ages 3-38 years)) with a 2mm cytology brush.165 The study received approval from the Leicestershire ethical review committee and verbal consent was obtained prior to brushing. Nasal brushings were placed in Medium 199 (ph 7.3) which contained antibiotic solution (Streptomycin 50pg/ml, Penicillin 50pg/ml, Gibco U.K.). Ciliated strips of epithelium were suspended in a chamber created by the separation of a cover slip and glass slide by two adjacent cover slips (figure 5.1). The slide was placed on a heated stage (37 C) of a Leitz, Diaplan microscope mounted on an anti-vibration table (Wentworth Laboratories Ltd. England). A thermister was placed in the chamber and the sample warmed to 37 C and measurements then taken. Specimens were examined using a X I00 interference contrast lens. Only undisrupted ciliated strips of greater than 50pm in length, devoid of mucus, were studied Evaluation of ciliary beat pattern The experimental system allowed the beating cilia to be viewed in three planes, a sideways profile (Figure 5.2a), beating directly toward the observer (Figure 5.2b) and from directly above (Figure 5.2c). Beating ciliated edges were recorded using a digital 119
121 high speed video camera (Kodak Ektapro Motion Analyser, Model 1012) at a rate of 400 frames per second, using a shutter speed of 1 in 2,000. The camera allows video sequences to be recorded and played back at reduced frame rates or frame by frame. The precise movement of individual cilia may be observed during their beat cycle. Microscope VPPgffk, Cover slip Microscope slide Well formed by adjacent cover slips containing specimen Figure 5.1: Hanging drop Construction of well for hanging drop. A well is formed by two cover slips attached to a microscope slide. The sample is suspended in medium 199 and placed in the well. A cover slip is placed over thewell and the specimen observed by oil immersion microscopy 120
122 Figure 5.2a Figure 5.2b Position of cilium at beginning of power stroke Angle of maximum deviation of cilium during recovery stroke Path of recovery stroke Figure 5.2a,b: Planes of view used to evaluate ciliary beta pattern. 5.2a: sideways profile, 5.2b endon profile; beating towards the observer. 121
123 Figure 5.2c Angle of maximum deviation of cilium during recovery stroke' 0 Position of cilium at beginning of power stroke Path of recovery stroke O Position of cilium at end of power stroke Figure 5.2c: Planes of view used to observe and record the ciliary beat cycle and beat frequency from overhead. The path taken by a cilium during the power and recovery strokes was plotted on acetate paper overlying the high resolution monitor as follows. Viewing the cilia beating towards the observer (Figure 5.2b) the precise position of the cilium during the forward power stroke was plotted frame by frame. As the cilium moved backward during the recovery stroke the position of the cilium during this movement was again plotted frame by frame. An angle could be derived from a line drawn through the plane of the power stroke and a line joining the point of maximum deviation of the cilium during the recovery stroke (Figure 5.2b). This was defined as the beat angle a. For each cilium studied the mean angle for 5 complete beat cycles was measured by image analysis (Scion image, Scion Corporation, Frederick, M D ). This was repeated when viewing the ciliary beat pattern from above (Figure 5.2c) and a beat angle P calculated by image analysis. 122
124 5.3.2 Comparison of high speed video, photomultiplier and photodiode techniques Measurements of ciliary beat frequency were made, from mucous free ciliated epithelial strips of at least 50pm in length viewed in sideways profile (Figure 5.2a), using the digital high speed video, photomultiplier and photodiode methods. Once an edge was selected measurements were made using each of the three methods. Readings were taken from different areas along the edge. The order in which measurements were made by the three different techniques was varied to help exclude any confounding effect of the order of measurement. No intra and inter-observer coefficient of variation was performed for each method as these have been performed by O Callaghan and found to be <10%. High Speed Video Method (Figure 5.3): Ciliary beat frequency may be determined directly, by timing a given number of individual ciliary beat cycles. Groups of beating cilia were identified and the number of frames required to complete 10 cycles recorded. This was converted to ciliary beat frequency by a simple calculation (CBF= (400/(number frames for 10 beats)) X 10). Ten measurements of beat frequency were made along each ciliated strip. 123
125 Figure 5.3: Digital high speed video method used for assessment of ciliary function Photomultiplier M ethod:16^ Ciliary beat frequency was recorded using a microscope photometer (Leitz SS ). As suggested by Dalhamn,163 the aperture allowing light to reach the photomultiplier was adjusted to an area of 2.2pm2 and positioned over an area of beating cilia. Voltage signals, generated as the moving cilia interrupted the passage of light, were displayed on an oscilloscope and relayed to a power spectrum analysis programme (ANADAT, Montreal, Canada), to determine ciliary beat frequency. Ten measurements of beat frequency were made along each ciliated strip. Photodiode M ethod:112 Video images of the beating respiratory cilia were relayed from a Super-VHS video camera (Panasonic F I5 CCP Videocamera) to a high resolution 124
126 monitor. The photodiode, mounted in a pen like system, was held over the beating cilia displayed on the monitor. Signals generated as cilia moved past the photodiode sensor were fed via an oscilloscope to a power spectrum analysis program (AN AD AT, Montreal, Canada), to determine ciliary beat frequency. Ten measurements of beat frequency were made along each ciliated strip Statistics: The mean, standard deviation and 95% confidence intervals were calculated for the a and p angles (Figure 5.2b, 5.2c). A one-way analysis of variance was performed with respect to the method used to measure ciliary beat frequency. The mean and 95% confidence interval for each method was calculated. Paired t-tests were performed to compare each method with the digital high speed video method. The Bland-Altman limits of agreement were calculated from the mean difference +/- twice the standard deviation of the differences with the digital high speed video taken as the optimal method. The limits of agreement were calculated for each method separately. 125
127 5.4 Results A classical sideways recovery sweep was not seen when cilia were viewed either beating towards the observer or from above (Figures 5.2b, 5.2c). The power and recovery strokes were within the same plane with minimal sideways deviation. A total of 144 cilia had measurements made of beat angle a and 262 cilia for beat angle p. The corresponding mean angles were a=5.3 (S.D: ± 3.5, 95% Cl: ) and p=3.6 (S.D: ± 3.0, 95% Cl: ) respectively. These angles were different as measured from different cilia in different planes of view. A total of 600 measurements were made for ciliary beat frequency, 200 for each method. Both the photomultiplier and the photodiode readings under recorded ciliary beat frequency when compared to the digital high speed video (Figure 5.4). The beat frequency determined using the digital high speed video was 13.2 Hz (S.D: ± 2.9 Hz, 95% Cl: Hz), 12.0 Hz (S.D: ± 2.4 Hz, 95% Cl: Hz) for the photomultiplier, and 11.2 Hz (S.D: ± 2.8 Hz, 95% Cl: Hz) for the photodiode. A paired t-test showed a significant difference for the photomultiplier (Mean difference 1.2 Hz, 95% Cl: Hz (P=0.01)) and photodiode method (Mean difference 1.9 Hz, 95% Cl: Hz (P<0.001)) when compared to the digital high speed video. 126
128 The Bland-Altman limits of agreement with respect to the digital high speed video (Figure 5.5) showed widest variation for the photodiode method to 6.06 Hz and closest agreement for the photomultiplier method to 5.15 Hz. 127
129 DHSV CBF(Hz) Figure 5.4: Graph of ciliary beat frequency measured with the photomultiplier (PM) and photodiode (PD) plotted against beat frequency measured by digital high-speed video (DHSV). This illustrates that both methods underestimate beat frequency when compare to the high-speed video method. If both the photomultiplier and photodiode methods agreed with the digital high-speed method then the results would fall on the correlation line (dashed line).
130 Figure 5.5a 10 s 5 (0 X 0) O c 2 d) 0 Q % Mean of HSV and PM (Hz) - T - 20 Figure 5.5b o. ± 5 H 01 X 0) o c 0) h. w C o - Q % T- 1 5 Mean of HSV and PD (Hz) r~ 20 Figure 5.5: Bland-Altman plots of the digital high-speed video against the photomultiplier and the photodiode methods. Figure 5.5a Bland-Altman limits of agreement for the photomultiplier (PM) compared to the digital high-speed video (HSV) at 37 C. Figure 5.5b Bland-Altman limits of agreement for the photodiode (PD) compared to the digital highspeed video (HSV) at 37 C. 129
131 5.5 Discussion Digital high speed video imaging allowed images to be obtained at a rate of 400 per second and to make a permanent recording at a reduced frame rate. Beat pattern can be determined and beat frequency evaluated during the study or at a later stage. As mentioned in the introduction, it is generally thought that cilia deviate in a sideways direction during the recovery sweep. The study has shown, however, that human respiratory cilia do not have a classical sideways recovery sweep. The respiratory cilia analysed beat forward and back with a maximal deviation during their recovery sweep of less than 5. This was confirmed by analysis of ciliary movement observed in two planes. This ciliary movement has a direct effect on the surrounding fluid propelled by the cilium. Hydrodynamic coupling between individual cilia has been suggested to be involved in the propagation of the metachronal wave rather than by the ciliary recovery sweep.91 The findings support this hypothesis. The study is the first to directly compare two of the most commonly used methods of estimating ciliary beat frequency of respiratory cilia to digital high speed imaging. The photomultiplier and photodiode techniques recorded ciliary beat frequencies that were significantly slower than those measured using the digital high-speed video method. The limits of agreement for both methods were wide, which confirms that results obtained 130
132 using the different techniques cannot be used interchangeably. These results emphasise the need for normal reference ranges of ciliary beat frequency to be established for each technique if it is to be used as a diagnostic test for primary ciliary dyskinesia. While photometric techniques have improved, allowing rapid analysis of ciliary beat frequency, it is accepted that they do not give the accuracy of measurement provided by 1f t Q '909 high speed cinematography. The introduction of digital high speed cameras provide a simple, accurate method of determining ciliary beat frequency and evaluating beat pattern. Beating cilia may be analysed in slow motion immediately after a recording is made. Because of these advantages digital high speed video imaging has taken over from cinematography as the gold standard technique for the measurement of ciliary beat frequency. The photomultiplier and photodiode techniques rely on the analysis of voltage signals generated when cilia move across a constant light source. standardisation of these photometric methods is available. Limited data with regard to These techniques are subject to artefacts induced by minimal vibrations o f the tissue or surrounding fluid, which may contribute to the spectrum of frequencies seen Performing a power spectrum analysis on the voltage signal obtained gives a more objective measurement of the average frequency by helping to separate out artefactual results. However, it is not 131
133 always clear which spectrum relates to the ciliary cycle.86 Two closely associated peaks of similar height are sometimes encountered, making interpretation difficult. The photomultiplier method allows real-time analysis of ciliary frequency but not beat pattern. Studies by Rossman and colleagues suggest that evaluation of beat pattern, in addition to beat frequency, may be helpful in the diagnosis of patients with primary ciliary dyskinesia.249 Diagnosis based purely on frequency may very occasionally be unreliable as normal frequencies have been seen in patients with primary ciliary dyskinesia.236,323 In such cases, important information may be gained from studying the underlying beat pattern. 5.6 Summary Evaluation of the ciliary beat pattern using digital high-speed imaging has shown that respiratory cilia beat with a power and recovery stroke within the same plane. Both the photomultiplier and the photodiode readings under recorded ciliary beat frequency when compared to the digital high-speed video. The ability of digital high-speed video to measure ciliary beat pattern and beat frequency should make it a powerful tool in the investigation of patients with primary ciliary dyskinesia. 132
134 Chapter 6 133
135 6 Functional Analysis of Cilia and Ciliated Epithelial Ultrastructure in Healthy Children and Young Adults. 6.1 Summary Background: There is very little data on the normal ciliary beat frequency, beat pattern and ultrastructure in healthy children and adults. Aim: To define ciliary structure, beat frequency and beat pattern in a healthy paediatric and young adult population. Subjects: 76 children and adult volunteers (aged: 6 m onths-43 years). Method: Ciliated epithelial samples were obtained by brushing the inferior nasal turbinate. Beating cilia were recorded using a digital high-speed video camera. This allowed analysis of ciliary beat pattern and beat frequency. Tissue was fixed for transmission electron microscopy. Results: The mean ciliary beat frequency for the paediatric population (12.8 Hz (95%C.I.: Hz)) was greater than the adult group (11.5Hz (95%C.I.: Hz) (t-test p<0.01)). Ten percent (range: 6-24 %) of ciliated edges were found to have areas o f dyskinetically beating cilia. All samples had evidence of mild epithelial damage. This reflected changes found in all measurements used for assessment of epithelial damage. Ciliary ultrastructural defects were found in less than 5% of cilia. Conclusion: The study has established normal age related reference ranges for ciliary structure and beat frequency. In a healthy population localised epithelial damage may be present causing areas of ciliary dyskinesia. 134
136 6.2 Background to study Respiratory cilia beat in a co-ordinated manner with a specific frequency and pattern, clearing mucus and debris from the airways. Acquired or congenital ciliary ultrastructural defects result in cilia which are either stationary or beat in a slow or dyskinetic fashion. Ineffective movement impairs mucociliary clearance. In primary ciliary dyskinesia this causes sinusitis and recurrent chest infections, which may lead to bronchiectasis An early diagnosis of primary ciliary dyskinesia is important as institution of appropriate respiratory care has been shown to halt the progressive decline in lung function.256 A diagnosis is made on the basis of a supportive clinical history and an abnormal ciliary beat frequency accompanied in most cases by specific abnormalities o f the ciliary axoneme on electron microscopy. Studies by Rossman and colleagues suggest that evaluation of beat pattern, in addition to beat frequency, may be helpful in the diagnosis o f patients with primary ciliary dyskinesia Making a confident diagnosis o f primary ciliary dyskinesia can at times be very difficult as abnormalities of the epithelium and cilia may also be found purely due to acquired ciliary defects. It is therefore important to differentiate between primary and secondary ciliary structural and functional abnormalities. 135
137 Secondary ciliary ultrastructural defects are common.327 Defects may persist for up to 12 weeks following resolution of an upper respiratory tract infection and ultrastructural interpretation may be difficult.328 Quantitative ultrastructural analysis in healthy adult subjects is limited.330,331 Paediatric series describing ciliary ultrastructure are small and comprise of patients rather than healthy controls. Data suggest that 5% of cilia have abnormalities,326,332 with reports only analysing microtubular defects The analysis of dynein arms has been limited to patients with respiratory infections and found to affect up to 30% of cilia.334,335 Reference ranges for healthy children are not available for either ciliary microtubules or the presence of dynein arms. Data relating to the damage of nasal ciliated epithelium is not available in a healthy control population. While normal ranges of ciliary beat frequency have been published in adult series, paediatric data is sparse. Cilia from neonatal patients112 and adolescents114 were found to beat at a higher frequency than cilia from adults. Other studies have suggested that ciliary beat frequency may either fall with age113 or remain constant.98;336 In childhood, ciliary evaluation involves the sampling of nasal epithelium and analysis of ciliary beat frequency, beat pattern and ultrastructure. To date, normal data to allow comparison with findings in patients suspected of having primary ciliary dyskinesia has been sparse. 136
138 The high speed video method has also been evaluated against other existing indirect techniques, the photodiode and photomultiplier methods, for the measurement o f ciliary beat and significant differences were found (Chapter 5). This emphasises the need for a normal range to be established for each method. Reference ranges exist for both the photomultiplier330 and photodiode114 methods. However, no normal reference range exists for digital high-speed imaging Aims The aims of this study were: 1) To measure ciliary beat frequency and to determine ciliary beat pattern and ultrastructure in healthy children and adults. 2) To determine the ultrastructure of respiratory epithelium from healthy children and adults. 137
139 6.3 Methods 53 healthy paediatric subjects were recruited from children under going elective surgery (ages 6 months-17 years; 31 male). A further 23 adult volunteers (ages years; 16 Male) were recruited. No subject was recruited if they had a history of chronic respiratory or nasal disease or a symptomatic upper respiratory tract infection in the previous 6 weeks. No subjects were taking regular medication or were known smokers. Paediatric samples were obtained immediately after induction of anaesthesia with propofol. This agent has been shown to have no effect on ciliary beat frequency.337 No premedication had been given to any subject prior to surgery. In all subjects ciliated samples were obtained by brushing the inferior nasal turbinate with a 2mm cytology brush. Nasal brushings were placed in Medium 199 (ph 7.3) which contained antibiotic solution (Streptomycin 50pg/ml, Penicillin 50pg/ml, Gibco U.K.). The study received approval from the Leicestershire ethical review committee and written consent was obtained prior to sampling. 138
140 6.3.1 Evaluation of ciliary structure and function Transmission electron microscopy Preparation of tissues and evaluation of ciliaryultrastructure was performed by Mr. A Rutman. Briefly, tissue obtained by nasal brushing was fixed in 2.5% gluteraldehyde in Sorensons phosphate buffer for 48 hrs and then post fixed in 1% Osmium tetroxide. After rinsing in distilled water the cells were embedded in a drop of 2% liquid agar at 45 C and allowed to solidify. This was processed through to resin by standard techniques as described in appendix 1. Ultrathin sections were cut at 70 nm. These were collected on 200 mesh thin bar copper grids and stained in 1 % uranyl acetate and counter stained in Reynolds lead phosphate. The sections were then examined in the transmission electron microscope. The ciliated epithelium was assessed, blindly, for both epithelial and ciliary ultrastructural changes. Epithelial integrity was assessed by firstly examining cellular type. The number of ciliated cells, mucus cells, and dead cells were totalled and expressed as a percentage of all cells examined. Secondly, disruption and damage to the tissue was quantified by using the scoring system previously described by Tsang et al33 and in chapter 9.33;338 Briefly, the tissue is scored for the following parameters: loss of cilia from ciliated cells: 0 (fully ciliated), 1, 2, 3 (a few cilia visible); projection of cells from the epithelial edge: 0 (normal alignment), 1, 2, 139
141 3 (cell projected from edge but some contact with other epithelial cells); cytoplasmic blebbing: 0 (absent), 1 (minor), 2 (major); mitochondrial damage: 0 (absent), 1 (present) (Figure 6.1a-d). A F i g u r e 6.1 : Transmission electron micrographs illustrating the parameters assessed to examine epithelial damage. A) Loss of cilia from cell. This shows a cell with a severe loss of cilia (Grade 3) as compared with the normal epithelium seen in figure 6.2a (Bar: 10pm). B) Cellular projection. A cell is seen projecting markedly from the epithelial edge (Grade 3) compared with the normal epithelium seen in figure 6.2a (Bar: 10pm). C ) Cytoplasmic blebbing. A cell is shown exhibiting major cytoplasmic blebbing (Grade 2) compared with the normal epithelium seen in figure 6.2a (Bar: 10pm). D,E) Mitochondrial damage. A cell with a normal healthy mitochondrion is shown against a cell with a damaged mitochondrion (Grade 1) (Bar: 2pm).
142 To give an overall evaluation of epithelial damage an epithelial integrity score was given to the epithelium. This incorporated ciliary loss, cellular projection, cytoplasmic blebbing and mitochondrial damage. A healthy intact epithelial edge is scored 0 and a severely disrupted edge scored 5 (Range: 0 no damage, 1 minor, 2 mild, 3 moderate, 4 major, 5 severe damage) (Figure 6.2a, 6.2b). To evaluate the scoring system it was compared against all measurements used to measure epithelial damage. Damage to individual cilia was evaluated by examining ciliary ultrastructure for microtubular and dynein arm defects. Alignment of individual cilia within a cell was assessed by measuring ciliary orientation as previously described (appendix l).60 Percentages were calculated for the number cells with either loss of cilia, cellular projections, cytoplasmic blebbing and mitochondrial damage. Similarly, the percentage of cells with microtubular or dynein arm defects was calculated. F i g u r e 6.2 : Transmission electron micrographs showing assessment of epithelial integrity. a) This shows normal tissue with an intact well ciliated surface and minimal disruption. This has an epithelial integrity score of 0 (Bar: 10 am). b) This shows abnormal tissue with severely disrupted cell surface. Marked loss of cilia is seen. This has an epithelial integrity score of 5 (Bar: 10^m). 141
143 Ciliary beat frequency and beat pattern Ciliary beat frequency and beat pattern were evaluated as previously described in chapter 5. Beating ciliated edges were recorded using a digital high speed video camera (Kodak Motioncorder Analyser, Model 1000) at a rate of 400 frames per second. The ciliated edge, projected onto a high-resolution monitor, was divided into 5 adjacent areas measuring 10pm. Two measurements of ciliary beat frequency were made in each area, resulting in a total of ten measurements of beat frequency along each ciliated strip. A maximum of 10 edges were analysed per subject. Ciliary beat frequency was determined as described in chapter 5. As the digital highspeed video system was to be used to establish reference ranges for the measurement of ciliary beat frequency the reproducibility of the method was evaluated. A single point on the ciliated edge was identified on a grid placed on the monitor. Ciliary beat frequency at that point was measured independently by two observers (O l, 02). This was performed for each of the five areas displayed on the monitor. A total of 5 readings were obtained for each edge and this was performed in a total of 10 subjects. One observer repeated the series of measurements two days later (M l, M2). From this the inter-observer and intraobserver coefficient of variation could be calculated. 142
144 To determine if the number of beat cycles measured altered the ciliary beat frequency result the number of frames required to complete 5, 10, and 15 complete ciliary beat cycles was recorded. This was converted to ciliary beat frequency by a simple calculation (CBF= 400/(number frames for 5,10 or 15 beat cycles) X 5, 10, 15). To assess the ciliary beat pattern each edge was analysed. Coordinated ciliary beating in a forward backward motion along the whole epithelial edge was defined as normal. Edges which appeared to have dyskinetically beating cilia were noted. Percentage of edges exhibiting areas of dyskinetically beating cilia were then calculated Statistics As ciliary beat frequency may change with age112,114 we wished to see if other parameters showed such variation. Ho et al performed a similar analysis in adults and found some difference between subgroups of differing age ranges.113 As suggested by Roth114 a cut off was made at 18 years of age. To allow sufficient subjects in each age group 3 age ranges were created; 0-6, 7-12 and years of age. Adults were classified as > 19 years. To form reference ranges the mean ciliary beat frequency, standard deviation, 5th and 95th percentiles were calculated for individual age groups. A one-way analysis of variance was performed between groups. Individual groups were compared by using a students t- 143
145 test. Similarly the mean percentage, 5th and 95th percentiles of edges exhibiting areas of dyskinetically beating cilia was calculated. For all ultrastructural parameters the mean and 5th and 95th percentiles were calculated. A one way analysis of variance was perform ed between groups. 144
146 6.4 Results All subjects had analysis of ciliary beat frequency and beat pattern performed. A total of 56 subjects had sufficient tissue for epithelial integrity measurements and 60 for ciliary ultrastructure. Ciliary beat frequency and beat pattern were the initial measurements to be made after which samples were then processed for electron microscopy. During this procedure tissue may be lost. Consequently, some subjects had an inadequate sample for full ultrastructural analysis. Table 6.1 lists the percentage o f different cell types seen in the epithelial strips obtained. Analysis showed no difference between the percentage of different cells identified and the age of the subject. Ciliated cells formed 65% of the cell population. Analysis of factors involved in epithelial integrity are summarised in table 6.2. Even within the healthy population, there is evidence of loss of cilia, cellular extrusion, cytoplasmic blebbing and mitochondrial damage. Analysis of variation found no difference among groups for all measurements analysed. The epithelial integrity score that reflects a combination of all the measurements used to assess epithelial damage again showed no significant difference between the various age groups. 145
147 A summary of the results of ultrastructural analysis o f individual cilia are shown in table 6.3. Dynein arm defects were found in less than 3% of cilia observed. It was possible to visualise on average 7 of the expected 9 dynein arms when counting both inner and outer dynein arms. Again no differences were found between the various age groups and the ultrastructural analysis of individual cilia. Microtubular abnormalities were uncommon in all age groups (table 6.3). Ciliary orientation did not vary between age groups (table 6.3). Ciliary beat frequency and the percentage o f ciliary dyskinesia seen are shown in table 6.4. No significant difference in mean ciliary beat frequency between individual age groups was found (ANOVA p=0.10). However, when patients under the age o f 18 (12.8 Hz (95%C.I.: Hz)) were compared with those over the age of 18 (11.4Hz (95%C.I.: Hz)) a significant difference (t-test p<0.01) was seen. Approximately 10% of all edges analysed exhibited areas of dyskinetically beating cilia. This was found to be higher in the year group. To establish a reference range the mean ciliary beat frequency was plotted against age (Figure 6.3a). A weak negative correlation was found between mean ciliary beat frequency and advancing age (r = , P = 0.008). This was modelled and a linear relationship was found. Quadratic and other relationships were modelled and found not to be significant. 146
148 Within each sample cilia were found to beat at different frequencies. To evaluate sample variation of ciliary beat frequency, the edges with the highest and lowest ciliary beat frequency were plotted against age for each subject. The lowest mean ciliary beat frequency (Figure 6.3b) of edges ranged from Hz with 85% of subjects having a minimum beat frequency greater than 8Hz. The maximal ciliary beat frequency (Figure 6.3c) of edges ranged from 7.1 Hz Hz with 95% of subjects having a maximal ciliary beat frequency greater than 10Hz. No significant difference was observed for the inter-observer (0 1,0 2 ) and intra-observer (M l,m2) measurements of coefficient o f variation. The mean (SD) coefficient of variation for 01, 02, and M l, M2 are shown in table 6.5. The mean (SD) difference in coefficient of variation between the two observers was 0.9 (2.3)% ( 95% C l:-1.1, 2.9; Range:-1.4, 4.9) and in intra-observer coefficient of variation was 0.7 (2.0)% (95% CI:- 1.0, 2.4; Range:-3.5, 8.5). The number of complete beat cycles measured did not effect the coefficient of variation (table 6.5). No significant difference was found for coefficient of variation between 5 and 10 beat cycles and 10 and 15 beat cycles. 147
149 Age (Years) No. Subjects Ciliated Cells (%) Unciliated Cells (%) Mucous Cells (%) Dead Cells (%) (45.9,81.0) (13.1,42.3) 9.9 (5.2,1 7.5) 0.0 (0.0,0.0) (33.7,76.0) 26.8 (16.2,52.9) 10.6 (4.9, 16.4) 0.0 (0.0,0.0) (59.6,79.1) 20.6 (1 3.6,3 2.2 ) 8.1 (5.7,10.4) 0.0 (0.0,0.0) > (43.0,78.6) 22.1 (12.4,43.1) 10.4 (6.4, 19.7) 0.0 (0.0,0.0) Table 6.1: Analysis of cell type by transmission electron microscopy. Results are expressed as the mean percentage (5th and 95th percentiles) for individual age groups. Age (Years) No. Subjects Cells with loss of cilia (%) Cells extruding from surface (%) Cells with cytoplasmic blebbing (%) Cells with mitochondrial damage (%) Epithelial Integrity (9.1,38.4) 23.2(12.9,37.0) 14.2 (6.4, 23.5) 13.4(1.1,33.3) 1.3 (0.8, 2.0) (6.0, 58.2) 21.5(10.5,35.8) 11.8(5.5, 24.2) 8.9(0.0,21.9) 1.3 (0.8, 1.9) (7.2, 37.4) 18.7 (7.9,31.6) 11.5 (2.5,24.3) 8.0 (2.3, 16.1) 1.1 (0.8, 1.7) > (15.7, 72.0) 25.5 (18.6, 36.1) 13.4 (6.0, 24.2) 10.1(1.3,21.5) 1.1 (0.5, 2.0) Table 6.2: Transmission electron microscopy assessment of epithelial integrity. Results displayed are for individual age groups and expressed as the mean (5th and 95th percentiles). 148
150 Age (Years) No. Subjects Dynein Arm Counts D ynein Arm Defects (% ) M icrotubule Defects (% ) Central M icrotubule Defects (% ) Ciliary O rientation ( ) Inner O uter (7.2, 7.7) 7.9 (7.6, 8.2) 1.8 (0.0, 7.1) 2.1 (0.6, 5.6) 0.3 (0.0, 1.2) 10.9 (9.9, 12.5) (7.0, 7.4) 7.7 (6.4, 8.3) 1.3 (0.0, 4.1) 1.9 (0.0, 4.4) 0.7 (0.0, 2.2) 10.7 (9.7, 11.4) (6.7, 7.3) 7.5 (5.6, 8.1) 1.0 (0.0, 1.9) 2.3 (1.2, 3.6) 1.1 (0.0, 3.2) 10.8(9.7, 11.9) > (6.5, 7.9) 6.8 (5.7, 8.1) 1.0 (0.0, 2.8) 1.9 (0.8, 3.8) 0.3 (0.0, 1.0) 10.7 (9.7, 11.6) Table 6.3: Analysis of ciliary ultrastructure by transmission electron microscopy. Results for individual age groups are expressed as the mean (5th and 95th percentiles). Age (Years) No. Subjects C iliary B eat F requency (H z) D yskinetically beating edges (% ) Mean SD 5th, 95th C entiles , (0.0,36.8) , (0.0, 40.3) , (0.0, 56.9) , (0.0, 24.3) Table 6.4: Summary of analysis of ciliary beat frequency measurements. The mean ciliary beat frequency, Standard deviation (SD), 5th and 95th percentiles are given. The mean (5th, 95th percentiles) is shown for the percentage of edges exhibiting areas of ciliary dyskinesia. 149
151 Measurement Coefficient Variation (%) P Mean (SD) 95% Cl Inter-observer O l 11.6(3.8) 8.3, (4.4) 6.3, 14.6 Intra-observer M l 10.7(4.4) 6.3, M (5.1) 5.8, 16.0 Beat cycle 5 cycles 6.9 (5.8) 3.2, cycles 6.2 (3.6) 3.9, cycles 5.7 (5.7) 2.1, Table 6.5: Coefficient of variation for inter-observer, intra-observer and beat cycles. Results are for mean(standard deviation(sd)) and 95% confidence intervals (Cl). The p value is given. 150
152 i i i i i i i i Age (Years) B CBF(Hz) CBF (Hz) 24-i «-. : 0-! , n A ' * o Q - * m l " Age (Years) Age (Years) Figure 6.3: A;Graph of mean ciliary beat frequency plotted against age for all subjects. The mean (solid) and ±1.96 standard deviation (dashed) regression lines are displayed. This forms the age related reference intervals. A negative correlation between advancing age and a reduction in ciliary beat frequency was found (correlation coefficient r = ). B;Graph of edges with the lowest ciliary beat frequency within a sample plotted against age for all subjects. The mean (solid) and ±1.96 standard deviation (dashed) regression lines are displayed. CjGraph of the age with the highest ciliary beat frequency within a sample plotted against age for all subjects. The mean (solid) and ±1.96 standard deviation (dashed) regression lines are displayed.
153 Discussion The study examined the nasal ciliated epithelium from a large group of healthy children and a smaller group of adults. This has enabled normal age related reference ranges for both the ciliary structure and function to be established. Data quantifying the ciliary epithelial ultrastructure following brush biopsy is limited. Evidence was found of minor epithelial damage in the tissue from the healthy subjects. The results show a greater degree of epithelial damage than previously described. However, this data was from organ culture models, it is possible that in the process of brushing and tissue preparation, minor damage may have occurred. Although, two previous studies have evaluated the use o f nasal brushing to sample cilia for 187*339 quantification of ultrastructure, they did not assess epithelial damage. A scoring system for evaluation of epithelial integrity has been developed. This has been validated against the measurements used to assess epithelial ultrastructural damage and found to be representative of the m inor epithelial damage observed in healthy subjects. The percentage of dynein arm and microtubular abnormalities were both found to be less than 5%. This agrees with other published data.326 The mean orientation of cilia in the paediatric population has only been described in eight children under the age of two and 152
154 reported to be This is higher than the values obtained ( ), based on 60 subjects of differing ages. The quantification of inner and outer dynein arms is important in the diagnosis of primary ciliary dyskinesia. Dynein arm defects are the most common abnormality found in patients with primary ciliary dyskinesia. The majority of inner and outer dynein arms were visualised for all subjects. The results are consistent with other published data for the number of outer dynein arms seen. However, it was possible to identify a greater proportion of inner dynein arms than previously reported.340 This may be due to the healthy nature of the tissue. We did not use computer assisted imaging which has been described to assist in the detection of inner dynein arm abnormalities.341 However, this must be used cautiously when the origins of the enhanced signals are unknown. If different isoforms of the inner dynein are enhanced and the true position of the individual dynein heads unknown, there is a risk of the optical phenomena o f steric hindrance occurring. As suggested by Veale and colleagues ciliary beat frequencies were measured from several edges and from different sites along an edge.342 Ciliary beat frequency was found to vary between edges within a sample. In some subjects the mean beat frequency was less than 11 Hz. This is in keeping with other reports which have found cilia in healthy 153
155 adults to beat maximally at a frequency greater than 10 Hz (Range Hz) and minimally at greater than 7 Hz (Range Hz). This was limited to 20 volunteers and no children were included. Adults were found to have slower beating cilia342 with frequencies as low as 6 Hz98 to 9Hz.113 Two healthy children were also reported to have 98 cilia beating as slowly as 6Hz. The coefficient o f variation for measurement o f ciliary beat frequency along an epithelial ' l A 'J edge has been shown to vary between 9 to 58% compared to 10% in our study. No significant difference was found for inter-observer and intra-observer coefficient of variation using the digital high speed video technique. Nor the number of complete beat cycles used to measure ciliary beat frequency using the digital high-speed video. The ciliary beat frequency of the paediatric population was found to be significantly greater than the adult population. This is supported by studies which have shown ciliary beat frequency of neonates112 and teenagers114 to be greater than adult subjects. The data suggest a slight fall in ciliary beat frequency with increasing age, which is in agreement with other studies.113 In contrast, Jorissen found ciliary beat frequency to be age independent.336 However, their readings were conducted at 22 C rather than body temperature which makes comparison difficult. At this temperature cilia beat at a much 117 slower frequency and the association may therefore have been lost. 154
156 Digital high-speed video imaging allowed us to precisely visualise the normal ciliary beat pattern in healthy subjects. 10% of edges had evidence of dyskinetically beating cilia. The remainder of the cilia were found to beat forward and backwards within the same plane without a classical sideways recovery sweep. This is consistent with the earlier description in chapter 5. Analysis of ciliary beat pattern may improve our understanding of the actions of various respiratory pathogens. 6.5 Summary An extensive age related normal reference range for both ciliary structure and function has been established. Epithelial integrity has been evaluated in a healthy population. Such data will help with evaluation of patients suspected of having primary ciliary dyskinesia and in research studies looking at the effects of various pathogens on nasal ciliary ultrastructure, function and epithelial integrity. 155
157 Chapter 7 156
158 7 Determination of ciliary beat pattern associated with specific ultrastructural defects. 7.1 Summary Background: It has been suggested that cilia with different ultrastructural defects causing primary ciliary dyskinesia may have differing beat patterns and frequencies. Aim: To determine ciliary beat pattern and beat frequency (CBF) associated with the five common ultrastructural defects responsible for primary ciliary dyskinesia. Method: Nasal brushings were performed on 56 children. Ciliary movement was recorded using digital high speed video imaging to assess beat frequency and pattern. Electron microscopy was performed. Results: Patients with an isolated outer dynein arm or with an outer and inner dynein arm defect, 55% and 80% of cilia were immotile respectively. Cilia that moved were only flickering. Mean CBF (±95% Cl) was 2.3Hz(±1.2) and 0.8Hz(±0.8) respectively. Cilia with an isolated inner dynein arm or a radial spoke defect had similar beat patterns. Cilia appeared stiff, had a reduced amplitude and failed to bend along their length. Immotile cilia were present in 10% of cilia with an inner dynein arm defect and in 30% o f radial spoke defects. Mean CBF was 9.3Hz(±2.6) and 6.0Hz(±3.1) respectively. Ciliary transposition defect produced a large circular beat pattern (Mean CBF 10.7H z(±l.l)). No cilia were immotile. Conclusion: Different ultrastructural defects responsible for primary ciliary dyskinesia result in predictable beat patterns and beat frequencies. 157
159 7.2 Background to study The diagnosis of primary ciliary dyskinesia is traditionally made on the basis of a supportive clinical history and an abnormal ciliary beat frequency, accompanied in most 52*220*227 cases by specific abnormalities o f the ciliary axoneme on electron microscopy. The most commonly used techniques available to measure ciliary beat frequency use an indirect method. For example, the modified photodiode166 or photomultiplier method163 detect changes in intensity of light caused by beating cilia. They do not provide information on ciliary beat pattern. The recent introduction of high resolution digital high speed imaging has allowed the precise beat pattern of cilia to be viewed in three different planes in slow motion or frame Of by frame (chapter 5 ). This showed that the widely held belief, that cilia beat with a classical forward power stroke and then a recovery stroke that sweeps to the side9 is incorrect. Respiratory cilia simply beat in a forward and backward planar motion without oc a sideways recovery sweep (Chapter 5). Digital high speed video analysis has also proved useful in determining the effect of viral infection on the movement of respiratory cilia. Following a coronavirus infection the beat frequency of nasal respiratory cilia was found to remain within the normal range. 33 However, slow motion analysis revealed high levels o f ciliary dyskinesia (chapter 9). 158
160 This would have been missed by the conventional methods used to measure ciliary beat frequency. It has been suggested that evaluation o f ciliary beat pattern, in addition to beat frequency, may be helpful in the diagnosis of primary ciliary dyskinesia.249;304 Indeed one report suggested that some of the different ultrastructural defects found to cause primary ciliary dyskinesia may have different beat patterns Aims The aim of this study was to use new high resolution digital high speed video photography to determine the precise ciliary beat pattern and beat frequency associated with the five common ultrastructural abnormalities responsible for primary ciliary dyskinesia. A secondary aim of the study was to define in detail, using transmission electron microscopy, the ultrastructural findings and ciliary orientation of cilia obtained by nasal brush biopsy in these five different ultrastructural defects. 159
161 7.3 Methods. This study reports 56 children (age range: 5 weeks to 14 years (32 males)) who were diagnosed as having primary ciliary dyskinesia following referral to the Leicestershire primary ciliary dyskinesia diagnostic service. For each patient information was collected evaluating chest, nasal, and ear symptoms. The presence or absence of situs inversus was noted. Each subject had been free from upper respiratory tract infections or nasal and chest exacerbation in the previous six weeks. In all children ciliated samples were obtained by brushing the inferior nasal turbinate. Nasal brushings were placed in Medium 199 (ph 7.3) which contained antibiotic solution (Streptomycin 50pg/ml, Penicillin 50pg/ml, Gibco U.K.). The sampling o f ciliated epithelium for diagnostic purposes had received approval from the Leicestershire ethical review committee and written consent was obtained prior to sampling. 160
162 7.3.1 Ciliary evaluation Transmission electron microscopy Tissue obtained by nasal brushing was processed for electron microscopy by using standard techniques as previously described (chapter 6). Ciliary ultrastructure was examined without knowledge of ciliary beat pattern and beat frequency readings. Individual cilia were examined for microtubular and dynein arm defects. The total number of inner and outer dynein arms for each cilium were counted. Alignment of individual cilia within a cell was assessed by measuring ciliary orientation as previously described.60 Percentages were calculated for the number of cilia with microtubular or dynein arm defects Ciliary beat frequency and beat pattern This was evaluated as previously described (chapter5). Ciliated epithelium was observed at 37 C using a xloo interference contrast lens. Only undisrupted ciliated strips of greater than 50pm in length, devoid of mucus, were studied. Beating ciliated edges were recorded using a digital high speed video camera (Kodak Motioncorder Analyser, Model 1000) at a rate o f 400 frames per second. The camera 161
163 allows video sequences to be recorded and played back at reduced frame rates or frame by frame. The ciliated edge, projected onto a high-resolution monitor, was divided into 5 adjacent areas measuring 10pm. Two measurements of ciliary beat frequency were made in each area, resulting in a total of ten measurements of beat frequency along each ciliated strip. At least 3 edges up to a maximum of 10 edges were analysed per subject. Ciliary beat frequency was determined directly. Groups of beating cilia were identified and the number of frames required to complete 10 cycles recorded and converted to ciliary beat frequency by a simple calculation (Chapter 5). An immotility index was calculated using a modified method previously described by Greenstone.236 If immotile cilia were observed a beat frequency of 0 Hz was recorded. The immotility index was calculated as the percentage of immotile cilia within the sample (ie: Number of immotile readings/total number o f readings for sample x 100). The experimental system allowed the ciliary beat pattern to be evaluated by examining the ciliated samples in three different planes: a sideways profile, beating directly towards the observer, and from directly above (chapter 5). The path taken by a cilium during the beat cycle was analysed frame by frame. This was characterised and compared to the normal beat pattern, seen on high-speed video analysis. 162
164 7.3.2 Statistics The mean ciliary beat frequency, 95% confidence intervals and range were calculated. The mean percentage of immotile cilia and 95% confidence intervals were calculated. For all ultrastructural parameters the mean and 95% confidence intervals were calculated. 163
165 7.4 Results. /7 /7 /V 0 '7 '7 O 0 S? The patients could be categorised into one o f 5 recognised ultrastructural defects. This formed the following groups; isolated outer dynein arm defects, a combined defect of both outer and inner dynein arms, isolated inner dynein arm defects, radial spoke defect with an associated inner dynein arm defect (Radial spoke defect), and transposition defect (Figure 7.1). The clinical pictures of patients with primary ciliary dyskinesia due to different ultrastructural defects are shown in table 7.1. The mean age of diagnosis was 4.7 years (range: yrs). Over 98 % (55) of patients had chronic chest and 90% (51) chronic nasal symptoms. Ear symptoms were reported in half (30) of the subjects. Situs inversus was found in 41% (23) of patients. None of the 8 patients with a transposition defect had situs inversus. Nearly two-thirds of the patients had either a combined inner and outer dynein arm defect (36%) or an isolated outer dynein arm defect (29%). The remaining ultrastructural defects were less common, with an isolated inner dynein arm defect responsible for 14%, a transposition defect 14% and radial spoke defect 7%. 164
166 B C Figure 7.1: Ultrastructural defects found in primary ciliary dyskinesia. The light grey area represents the missing ultrastructural component seen on the transmission electron micrograph. A; Normal, B; No outer and inner dynein arms, C; No outer dynein arms. " x 165
167 $ a <? -cbpo Figure 7.1: Ultrastructural defects found in primary ciliary dyskinesia. The light grey area represents the missing ultrastructural component seen on the transmission electron micrograph. D; No inner dynein arms, E; Radial spoke defect, F; Transposition defect. 166
168 Figure 7.2: Detailed ultrastructure of ciliary transposition defect. The cross sectional area starts as a normal 9+2 arrangement (A), midway along the axoneme the central pair terminates (B) and a peripheral microtubule doublet transposes in to the central postion (C). This is seen on the longitudinal section of the axoneme (Arrow (D)) and compared to a normal longitudinal section (E) 167
169 Each patient had an average of 15 ciliated cells (Range 5-36) and 310 individual cilia (range ) examined by transmission electron microscopy. Detailed ciliary ultrastructural evaluation for each defect is shown (Tables 7.2 and 7.3). In patients with isolated outer dynein arm defects, a combined defect of both outer and inner dynein arms or an isolated inner dynein arm defects less than 5% of cilia had microtubular abnormalities. 25% of cilia with a radial spoke defect exhibited peripheral microtubular defects. Patients with ciliary transposition defect had a similar percentage o f cilia with microtubular defects but this predominantly involved the central microtubular pair. It is of interest that in all patients except those with an isolated outer dynein arm defect, ciliary disorientation is markedly increased, compared to the normal range of <11 (chapter 2 & 6).62 In patients with the following abnormalities; isolated outer dynein arm defect; combined defect of both outer and inner dynein arms; isolated inner dynein arm defect and radial spoke defect with an associated inner dynein arm defect, 95% of cilia were found to exhibit the defect (Table 7.3). Although referred to as absence of dynein arms, it was possible to identify at least one dynein arm. These dynein arms, which were identified, appeared abnormal (Table 7.3). Approximately 5% of cilia exhibited defects of both inner and outer dynein arms in the following ultrastructural groups: isolated outer dynein arm defect, isolated inner dynein 168
170 arm defects and radial spoke defect. Less than 1% of cilia with a transposition defect had associated defects of the outer and inner dynein arms (Table 7.3). None of the patients diagnosed as having primary ciliary dyskinesia were found to have cilia which moved with a normal beat pattern (Fig 7.3a). It was possible to categorise the patients into three groups based on the distinct dyskinetic beat patterns observed (Table 7.4): Virtually Immotile Cilia (Figure 7.3b, Table 7.4): Cilia with either a combined inner and outer dynein arm defect or an isolated outer dynein arm defect were observed to have large areas of immotile cilia. Ciliary movement, when present, was restricted to a slow short stiff flickering motion (Fig7.3b). In the combined inner and outer dynein arm defect group an average 80% o f cilia were immotile. The mean (+/-SD) ciliary beat frequency was 0.8 (+/- 1.7) Hz. In the outer dynein arm defect group an average 55% of cilia were immotile. Cilia that were moving had a stiff flickering motion with a mean (+/-SD) beat frequency o f 2.3 (+/-2.6) Hz. Stiff Ciliary Beat Pattern (Figure7.3c, Table 7.4): Cilia with an isolated inner dynein arm defect or a radial spoke with an isolated inner dynein arm defect were observed to have a very abnormal stiff forward power stroke with a markedly reduced amplitude. Cilia failed to bend along their axoneme (Fig7.3c). 10% of the cilia in patients with an 169
171 isolated inner dynein arm defect were immotile. The remainder had a mean (+/-SD) ciliary beat frequency o f 9.3 (+/- 4.0) Hz. Cilia with a radial spoke defect associated with an inner dynein arm defect were found to beat in a similar manner to cilia from patients with an isolated inner dynein arm defect. 30% of the cilia were immotile and the remainder beat at a lower mean (+/-SD) ciliary beat frequency of 6.0 (+/- 3.3) Hz. Circular Beating Cilia (Figure 7.3d, Table 7.4): This beat pattern was only observed in patients with a ciliary transposition defect. A forward and backwards planar whip like motion was seen when cilia were viewed with a side profile. However, when viewed from above a large anticlockwise circular gyrating motion about the base of the cilium was apparent (Fig7.3d). None of the cilia were immotile and they had a mean (+/-SD) ciliary beat frequency of 10.7 (+/- 1.6) Hz. 170
172 B C D Figure 7.3: Figure 7.3A: Figure 7.3B: Figure 7.3C Figure 7.3D Beat patterns found in patients with primary ciliary dyskinesia. Diagram of the normal ciliary beat pattern. Cilia move in a planar motion with a forward power stroke and a backwards recovery stroke which does not sw eep to the side. Diagram of the dyskinetic beat pattern observed fo r cilia with either a com bined inner and outer dynein arm defect or an isolated outer dynein arm defect. Cilia w ere virtually immotile, with the occasional slow, low am plitude, stiff flickering m otion Diagram of the dyskinetic beat pattern observed for cilia with either an isolated inner dynein arm defect or a radial spoke defect. Cilia had a stiff planar forw ard-backw ard motion with m arkedly reduced am plitude. Diagram of the dyskinetic beat pattern observed for cilia with a transposition defect. Cilia beat in a large circular gyrating m otion about the base o f the cilium. 171
173 Ultrastructural Defect No. Patients Age (Range) Chest (%) Nasal Ear Situs (Female) (Years) (%) (%) Inversus (%) Inner and outer dynein arm defect 20 (9) 2.9 ( ) 20 (100.0) 17(85.0) 9 (45.0) 9 (45.0) Outer dynein arm defect 16(6) 4.5 ( ) 16(100.0) 15 (93.8) 11(68.8) 9 (56.2) Inner dynein arm defect 8(3 ) 6.9 ( ) 7 (87.5) 7 (87.5) 6 (75.0) 2 (25.0) Radial spoke defect 4 (2 ) 3.6 ( ) 4(100.0) 4 (100.0) 1 (25.0) 3 (75.0) Transposition defect 8 (4 ) 8.3 ( ) 8(100.0) 8 (100.0) 3 (37.5) 0 (0.0) Total 56 (24) 4.7 (0.1-14) 55 (98.2) 51 (91.0) 30(54.5) 23 (41.0) Table 7.1: Ciliary ultrastructural defects and clinical demographics of patients diagnosed with primary ciliary dyskinesia. The number of patients with the same structural defect is shown with mean age at diagnosis (Range) and clinical symptoms and signs (number (%)). 172
174 Ultrastructural Defect Total Microtubular Defects (%) Disarranged Microtubules (%) Extra Peripheral Microtubules (%) Central Microtubule Defects (%) Ciliary Orientation ( ) Inner and outer dynein arm defect 3.4 (2.9,4.0) 0.8 (0.5, 1.0) 0.8 (0.5, 1.2) 1.8 (1.4, 2.3) 21.6 (20.6, 22.5) Outer dynein arm defect 2.9 (1.9,4.1) 0.9 (0.4, 1.5) 1.2 (0.3, 2.2) 0.8 (0.5, 1.2) 13.6(12.7, 14.5) Inner dynein arm defect 5.7 (3.6,7.8) 2.4 (0.9, 4.0) 0.4 (0.0, 0.8) 2.9 (1.8, 4.1) 17.8 (15.3,20.4) Radial spoke defect 26.4 (23.7,29.1) 25.2 (22.5, 27.9) 0.0 (0.0, 0.0) 1.2 (0.6, 1.8) 21.9 (20.1,23.8) Transposition defect 22.8(14.3,31.3) 7.8(4.0,11.5) 0.8 (0.0, 1.6) 14.2 (6.8,21.6 ) 21.1 (19.6,22.5) Table 7.2: Assessment of microtubular abnormalities and ciliary orientation by transmission electron microscopy. Results displayed are for ultrastructural defect and expressed as the mean percentage (95% confidence intervals). 173
175 U ltrastructural Defect Dynein Arm Count Cilia with D ynein Arm Outer Inner Defects (% ) Inner and O uter Dynein Arm Defect (%) O uter Dynein Arm Defect Inner Dynein Arm Defect (% ) (%) Inner and outer dynein arm defect 0.6 (0.5,0.7) 0.6 (0.4,0.7) 96.4 (94.5,98.2) 85.5 (74.3,96.7) 2.1 (0.5, 3.6) 8.8 (0.0, 19.5) O uter dynein arm defect 1.3 (1.0,1.7) 6.3 (6.0,6.7) 96.0 (92.9,99.2) 5.1 (3.1, 7.1) 90.9(87.4,94.4) 0.0 (0.0, 0.0) Inner dynein arm defect 7.2 (7.0,7.4) 1.9 (1.4,2.3) 93.6 (92.5,96.2) 5.3 (2.2, 8.5) 0.0 (0.0, 0.0) 88.3(85.0,91.6) Radial spoke defect 7.1 (6.7,7.5) 0.9 (0.4,1.3) 96.0 (93.1,98.9) 4.3 (1.4, 7.1) 0.0 (0.0, 0.0) 91.7(87.9,95.6) T ransposition defect 7.4 (7.1,7.6) 6.2 (5.9,6.5) 1.1 (0.2, 2.0) 1.1 (0.2, 2.0) 0.0 (0.0, 0.0) 0.0 (0.0, 0.0) Table 7.3: Analysis of ciliary dynein arms by transmission electron microscopy. Results for individual ultrastructural defects are for individual dynein arm counts and the percentage of cilia with dynein arm defects. Results are expressed as the mean (95% confidence intervals). 174
176 Beat Pattern U ltrastructural D efect Ciliary Beat Frequency (Hz) Im m otility Index M ean 95% Cl Range (%) Immotile cilia, flickering Inner and outer dynein arm defect , (66.4, 93.1) Outer dynein arm defect , oo 55.0 (37.2, 73.0) S tiff planar motion Inner dynein arm defect , (3.8, 15.0) Radial spoke defect , (9.3,53.6) Rotational motion Transposition defect , (0.0, 0.0) Table 7.4: Summary of ciliary function. The three groups of beat pattern and corresponding ultrastructural defect, ciliary beat frequency and immotility index are displayed. The mean (95% confidence intervals) and range for ciliary beat frequency and mean (95% confidence intervals) for the percentage of immotile cilia (Immotility index) is shown. 175
177 7.5 Discussion The advent of high speed digital video imaging has allowed the definition o f the ciliary beat pattern and beat frequency associated with five of the most common ultrastructural abnormalities responsible for primary ciliary dyskinesia. The literature in this area is very sparse and our results differ in a number of aspects from the previous reports Three distinct beat patterns associated with underlying ultrastructural defects were seen. The most common ultrastructural defect responsible for primary ciliary dyskinesia is defects of the inner or outer dynein arms. In the isolated outer dynein arm defect and those patients with both an inner and outer dynein arm defect, the vast majority of cilia were immotile. The few cilia that actually moved had a very stiff flickering motion. The frequency of these flickering cilia was slightly slower in those with a combined inner and outer dynein arm defect compared to those with an isolated outer dynein arm defect. In addition, patients with a combined defect had a higher percentage of totally immotile cilia. In previous studies, patients with inner and outer dynein arm defects were noted to have a higher proportion of immotile cilia, though the actual percentage was not defined, and two different beat patterns were described, one as vibrational and the other a rotational egg beater.343 In a paper by Rossman,343 the five patients with dynein arm defects were not split into those with isolated outer dynein arm defects or combined inner 176
178 dynein and outer dynein arm defects. They reported the beat frequency of the cilia in this combined group to be 6 Hz, considerably higher than the frequency in our 20 patients with combined inner and outer dynein arm defects (0.8 Hz) and the 16 patients with outer dynein arm defects (2.3 Hz). In the study, the number of immotile cilia was shown to vary depending on whether there was an isolated dynein arm defect (55% immotile) or a combined inner and outer dynein arm defect (80% immotile). Rossman s paper suggested that 60% from their combined group were immotile.343 The second beat pattern observed was that o f a stiff forward stroke with a markedly reduced amplitude. This pattern was common to patients with an isolated inner dynein arm defect and also to those with a radial spoke defect associated with an inner dynein arm defect. The beat frequency o f these two groups however was different. Patients with an isolated inner dynein arm defect alone had a mean beat frequency of 8.1 Hz compared to a beat frequency of 6 Hz in those with a radial spoke and an inner dynein arm defect. The number of immotile cilia also varied depending on the defect with 10% of cilia immotile in those with an isolated inner dynein arm defect, compared to over 30% of cilia in patients with an radial spoke defect in association with an inner dynein defect. Rossman and colleagues did not consider inner dynein arm defects separately343 and the report of a single patient by Pederson suggested that an asynchronised beat pattern was observed.344 The study by Pederson involved cilia being observed at a room temperature of 22 C so comparison o f beat frequency is not possible. De Iongh and Rutland 177
179 observed the beat frequency in two patients with primary ciliary dyskinesia due to an inner dynein arm to be within the normal range.330 Two patients with radial spoke defects described in the Rossman study343 were found to have no immotile cilia. The beat frequency measured in these two patients was 9.6 Hz which is higher than the frequency of 6 Hz in our 4 patients. The beat pattern they described for this defect was a bi-phasic rotational pattern which differs significantly from our findings of a stiff beat pattern with reduced amplitude. The third beat pattern was that of an oval gyrating pattern where cilia had a mean beat frequency of 10.7 Hz. As stated in chapter 6 the normal ciliary beat frequency of healthy children was found to be 12 Hz (Range o f Hz).62 In a number of units patients suspected of having primary ciliary dyskinesia are screened using beat frequency measurement alone. The implication o f these findings is that a number of patients with ciliary transposition will have a beat frequency within the normal range and these patients will be missed unless beat pattern analysis and electron microscopy are undertaken. Two patients described by Rossman343 with a ciliary transposition defect were found to have a ciliary beat frequency of 10 Hz and no cilia immotile. The pattern they described is one of a grabbing motion. Based on electron microscopic studies, until recently, it was thought that cilia beat with a forward stroke and then had a recovery stroke where the cilia bent to the side.9 It was 178
180 postulated that the sideways recovery sweep would stimulate adjacent cilia to beat and produce a metachronal wave. Using the high speed video system, we have now shown that respiratory cilia simply beat forward and backwards in the same plane without a sideways sweep(chapter 5).85 All three beat patterns associated with primary ciliary dyskinesia differ from the normal ciliary beat pattern. In fact no cilia were seen in any of the patients to have a normal beat frequency. The major advantage of the new video technology is that of high resolution and the ability of playing back the movement o f individual cilia frame by frame having recorded at a frequency of Hz. This is a considerable advantage over previous methods such as that used by Rossman and colleagues in 1981 which allowed 60 frames per second.343 This for example, only allows 5 or 6 frames per ciliary beat cycle compared to high resolution frames per cycle with the newer technology. Digital high-speed video analysis also has the advantage of being able to visualise the beat pattern in three distinct planes. Results of the study are in keeping with the postulated role of the various ultrastructural components o f the ciliary axoneme. In the ciliary beat cycle, outer dynein arms are 'ya.'jz thought to generate the force to cause sliding o f the peripheral microtubules and to largely control ciliary beat frequency
181 The stiff beat pattern that we observed with inner dynein arm defects alone or those accompanied by radial spoke defects would support the evidence that the inner dynein arms assist in the bending of the ciliary axoneme.25;345;346 Although the beat frequency of patients with inner arm defects is reduced, the reduction is only moderate compared to outer dynein arm defects. Radial spokes are thought to resist the sliding of the microtubules and cause the cilium to bend. Although the cilium failed to bend in the combined radial spoke and inner dynein arm defect, it was also observed in the inner dynein arm defect alone. Little information is available on the action o f the central microtubular pair. It has been postulated that the central pair may rotate during active ciliary bending.347 It would appear that the central pair allows the cilium to beat in a forwards and backwards planar motion. Absence of the central pair for the short distance seen in patients with ciliary transposition appears to allow the cilia to rotate around this section in an anticlockwise 9Q9 motion which is similar to that seen in nodal cilia who lack a central pair. The main aim was to look at the association between ciliary beat pattern and beat frequency with ultrastructural defect in patients with primary ciliary dyskinesia. A secondary aim was to perform quantitative ciliary ultrastructural analysis on this group of patients. Only four studies have performed such analysis. The data from these papers is somewhat limited. Only one study involved samples obtained by nasal brush 180
182 biopsy,330 one solely analysed microtubular defects18 and just one placed patients into groups according to ultrastructural defect.233 In healthy tissue it is possible to identify between 7 to 9 outer dynein arms and 4 to 7 inner dynein arms per ciliary cross section.340 Even with a dynein arm defect it was possible to identify 1 or 2 o f the dynein arms. We found patients with an inner dynein arm, radial spoke or transposition defects to have a normal number of outer dynein arms present. Similarly, normal numbers o f inner dynein arms were observed in subjects with an outer dynein arm or transposition defect. This is in agreement with other published data for dynein arm defects. For all dynein arm defects, the lesions were found to be homogenous, with at least 95% o f cilia exhibiting the defect. 5% of cilia were observed to have microtubular defects in patients with dynein arm defects. This agrees with work by de Longh who found similar numbers of cilia to exhibit microtubular defects.330 Ciliary orientation evaluates how individual cilia are aligned within a cell. This is uniform between cells and is <11 in healthy individuals (chapter 6).62 Measurement of ciliary orientation in patients with primary ciliary dyskinesia is limited although it has been suggested to be increased. No reports exist for ciliary orientation o f individual ultrastructural defects. Ciliary orientation was found to be increased in all groups. The significance o f this is unknown. This finding has been observed by Jorissen
183 et al both before and after ciliogenesis. And suggested that this was a secondary 348 phenomena due to a higher percentage o f immotile cilia following culture. Two thirds of patients with primary ciliary dyskinesia were found to have abnormalities of the outer and/or inner dynein arms. This is a similar proportion of patients to previous studies. However we found a greater proportion o f patients to have a ciliary transposition defect (14%). This is higher than in previous series, which have suggested a prevalence between 3 to io%.233;236;253;330;349 As patients with a transposition defect have cilia which have a beat frequency within the normal range, it is likely that this group o f patients is under diagnosed. The results suggest that specific ultrastructural defects responsible for primary ciliary dyskinesia result in specific abnormalities in beat pattern and beat frequency. It is clear that simply relying on beat frequency analysis alone, as measured using indirect measures such as the photodiode or photomultiplier systems, will not differentiate a proportion of patients with primary ciliary dyskinesia from normals. The combination of beat frequency and beat pattern analysis using a high-speed video system should greatly improve the recognition of patients with underlying primary ciliary dyskinesia. Any patient with suspicion of primary ciliary dyskinesia following these measurements should have formal electron microscopy performed. 182
184 7.6 Summary It has been possible to quantify ciliary beat frequency, beat pattern and ultrastructural defect in patients with primary ciliary dyskinesia. The ciliary beat frequency and beat pattern has been correlated with ultrastructural defect to form three distinct groups of dyskinetic beat pattern. These data will form a reference range for the diagnosis of patients with primary ciliary dyskinesia. 183
185 Chapter 8 184
186 8 The Effects of Coronavirus on Human Nasal Ciliated Respiratory Epithelium. 8.1 Summary Background: Human coronavirus (HCoV) accounts for 15-30% of common colds, yet only one case report exists describing the effect of a coronavirus infection that was asymptomatic on respiratory epithelium. Aims: To examine the effects on ciliary structure and function of infection with HCoV. Method: Eleven healthy volunteers were infected by intranasal inoculation with HCoV 229E. A further four volunteers were sham infected with UV-inactivated virus. Immediately before inoculation (Day 0) and three days later (Day 3) ciliated epithelium was obtained by brush biopsy. Ciliary beat frequency (CBF) was determined and beat pattern analysed for evidence of dyskinesia (0=normal, 3=severely dyskinetic) using digital high speed video photography. Ciliary ultrastructure was examined by transmission electron microscopy. Symptom diaries were kept for the duration of the study. Results: All subjects inoculated with HCoV, including the 3 subjects who did not develop symptoms of an upper respiratory tract infection, had disruption of their respiratory epithelium on Day 3. Although there was no difference in the mean CBF on Day 0 and Day 3 (-3.3Hz (95% CI:-5.9, 2.1)), there was a significant increase (p<0.05) in the ciliary dyskinesia score between Day 0 and Day 3 (0.9 (95%CI: 0.3, 1.5)). In sham infected subjects no differences in epithelial integrity, or ciliary structure and function were found between day 0 and day 3. Conclusions: Inoculation o f healthy volunteers with HCoV caused disruption of the ciliated epithelium and ciliary dyskinesia. This is likely to impair mucociliary clearance. Damage to the respiratory epithelium, due to HCoV infection, may occur without overt clinical symptoms. 185
187 8.2 Background to study The 'common cold' is a universally recognised short illness in which the main symptoms involve the upper respiratory tract and in which nasal symptoms usually predominate. Understanding the interaction between virus and epithelium is an important step in determining the mechanisms by which symptoms are produced. Structural damage to the respiratory epithelium188,190,350 and abnormal ciliary function189; 190,351 during viral infection may disrupt the mucociliary escalator,351;352 contributing to symptoms of nasal discharge and obstruction. Although some infected volunteers suffer nasal congestion and discharge, an equal number of infected subjects have no definite symptoms. The reasons for this are still unclear.353 Human coronaviruses (HCoV) are the second most prevalent cause of the common cold. They account for 15-30% of proven viral infections354 and may cause exacerbation of lower respiratory diseases such as asthma.355 Data on the effect of HCoV infection of the respiratory epithelium is surprisingly sparse. The initial method for identifying HCoV used in-vitro tracheal organ cultures in which infection was detected with difficulty. Microscopy of light reflected from the epithelial surface was used as an indicator of ciliary activity, with loss o f the light reflection taken as the outcome measure for viral 356 infection. Other data consists of an incidental finding, on electron microscopy, of HCoV infection in a 2 year old girl being investigated for ciliary dyskinesia. It is o f 186
188 interest that this infection was asymptomatic with no signs of a common cold at the time o f biopsy or afterwards Aims In this study HCoV 229E, one of the two major serotypes of coronavirus accounting for upper respiratory tract infections, was used to inoculate healthy volunteers. The aim was to determine the effect of HCoV infection on the ultrastructure of nasal epithelium and on the beat frequency and beat pattern of nasal cilia. 187
189 8.3 Methods Subjects Fifteen adult volunteers (11 males, 4 females) without nasal or respiratory disease were recruited. Ages ranged from 18 to 35 (median 22) years. None of the subjects were taking nasal drugs and none had had a symptomatic upper respiratory tract infection in the preceding 6 weeks. The study received approval from the Leicestershire ethical review committee. Viral culture, inoculation and confirmation of infection by clinical and laboratory methods was performed by Dr. M McKean as part of his MD thesis. The full details of the methods used are described within that text.358 A summary is found in appendix Human coronavirus inoculation HCoV 229E (American Type Culture Collection, Rockville, Maryland, USA) was cultured according to standards o f good laboratory practice in human embryonic lung fibroblasts. An inoculum was prepared as previously described359 and was tested for safety according to the Gwaltney criteria. The inoculum consisted of aliquots of 1 ml of HCoV 229E suspension (200 tissue culture infective doses (TCID50) per ml) which were stored at -7 0 C. Eleven subjects (9 males and 2 females) were inoculated with 188
190 active virus as previously described.353 Briefly, with the volunteers head extended to 45, 0.5mls of virus was instilled into each nostril. In addition to viral antigens, the inoculum may contain other small proteins released from the infected cultured cells, such as cytokines. These proteins could interfere with the infective process or even generate an inflammatory response. To control for this four subjects were inoculated with virus inactivated by ultraviolet light as previously described.361 Inactivation was confirmed by culture in human embryonic lung fibroblasts with a control flask inoculated with active virus. The cells with active virus developed complete cytopathic effect by 10 days whereas those with UV-inactivated virus showed no cytopathic effect by 18 days when cultures were discontinued Evaluation of colds 'if/y The symptom diary was based on that used by Jackson and colleagues recording upper respiratory tract symptoms (rhinorhoea, nasal blockage and sneeze) and systemic symptoms (headache, malaise and chills). Each symptom was scored from 0-3 points according to severity. The Jackson criteria were then modified by a previously validated method in order to categorise volunteers by upper respiratory tract score into the following groups: definite cold (a score of 2 or more above a zero baseline on at least 2 consecutive days over days 2-6 post inoculation); possible cold (a marginal increase over 189
191 a zero baseline or a score of 2 or more above a symptomatic baseline on at least 2 consecutive days over days 2-6) and no cold (symptom-free). Laboratory confirmation of infection was by reverse transcriptase polymerase chain reaction (RT-PCR) of virus RNA extracted from nasal lavages obtained on day 3 postinoculation. This followed a previously established method Evaluation of ciliary structure and function Prior to viral inoculation and 3 days after inoculation, ciliated samples were obtained by brushing the inferior nasal turbinate with a 2mm cytology brush.165 This has been found to be an ideal site for repeated sampling of ciliated epithelium.188 Day 3 was chosen as previous studies of viral infections found little effect during the first 2 days and very significant changes to the respiratory epithelium on day Nasal brushings were placed in Medium 199 (ph 7.3) which contained antibiotic solution (Streptomycin 50pg/ml, Penicillin 50pg/ml, Gibco U.K.) Transmission electron microscopy Tissue obtained by nasal brushing was fixed in 2.5% gluteraldehyde in Sorensons phosphate buffer for 48 hrs and then post fixed in 1% Osmium tetroxide. Processing is as described in chapter 6 and appendix 1. The processed sections were then examined by
192 transmission electron microscopy. The ciliated epithelium was assessed, in a blind fashion, for both epithelial and ciliary ultrastructural changes Epithelial Integrity Epithelial integrity was assessed by firstly assessing cell type. The number of ciliated cells, mucous cells, and dead cells were expressed as a percentage of all cells examined. Secondly, disruption and damage to the tissue was quantified by using the scoring system described by Tsang et al. Briefly, the tissue is scored for the following parameters: loss of cilia from ciliated cells: 0 (fully ciliated), 1, 2, 3 (3 = few cilia visible); projection of cells from the epithelial edge: 0 (normal alignment), 1, 2, 3 (3 = cell projected from edge but some contact with other epithelial cells); cytoplasmic blebbing: 0 (absent), 1 (minor), 2 (major); mitochondrial damage: 0 (absent), 1 (present) Ciliary Ultrastructure Damage to individual cilia was evaluated by examining ciliary ultrastructure for microtubular and dynein arm defects. Alignment of individual cilia within a cell was determined by measuring ciliary orientation.60 Percentages were calculated for the number cells with either loss of cilia, cellular projections, cytoplasmic blebbing and mitochondrial damage. Similarly, the percentage o f cells with microtubular or dynein arm defects was calculated. 191
193 Ciliary beat frequency and beat pattern Ciliated strips of epithelium were suspended in a chamber created by the separation of a cover slip and glass slide by two adjacent cover slips. The slide was placed on a heated stage (37 C) of a Leitz, Diaplan microscope mounted on an anti-vibration table (Wentworth Laboratories Ltd. England). Specimens were examined using a X I00 interference contrast lens. Only undisrupted ciliated strips of greater than 50pm in length were studied. Ciliary beta frequency was determined by methods described in chapter 5 and 6.85 Ten readings of ciliary beat frequency were taken from different areas along each ciliated edge. To assess the ciliary beat pattern each edge was given a score based on the following scoring system. Normal co-ordinated ciliary beating in a forward backward motion was scored as 0. Cilia that appeared to beat dyskinetically were scored from 1 to 3 depending on the extent of abnormal beating along the edge (all cilia along an edge beating dyskinetically scored 3). 192
194 8.3.5 Statistical analysis The mean and 95% confidence interval for each group was calculated for Day 0 and Day 3 for all parameters measured. The mean change from baseline (Day 3 - Day 0) and 95% confidence intervals were calculated. Results which did not encompass 0 suggested a difference between Day 0 and Day 3, which was analysed by a paired samples student s t-test. A Wilcoxon signed rank test was used to compare the dyskinetic beat pattern on Day 0 and Day 3. An unpaired t-test was used to analyse data of group differences expressed as the change from baseline for both groups i.e.: (Day 3 - Day 0 ) COr o n a against (Day 3 - Day 0 ) s h a m This was expressed as mean difference between groups and 95% confidence interval. X 193
195 8.4 Results Three of 11 volunteers inoculated with human coronavirus had no symptoms of a cold. Four developed a definite cold and 4 had a possible cold. O f the volunteers who developed a definite or possible cold, all suffered mild upper respiratory tract symptoms. Six of these subjects developed a headache, 4 a cough and 2 a fever. Three of the 4 with definite colds and one of the 4 with possible colds had virus RNA detected in nasal secretions. Viral RNA was not detected in the four volunteers without symptoms of a cold. Two of the subjects inoculated with UV-inactivated virus developed a mildly blocked nose lasting for less than 24 hours commencing 2 days after inoculation. No other symptoms were reported in this group. All subjects inoculated with active HCoV, including the 3 subjects who did not develop symptoms of an upper respiratory tract infection, had disruption of their respiratory epithelium on Day 3 (Figure 9.1). Eight paired samples, before and after inoculation with active virus, were obtained from the eleven volunteers for transmission electron microscopy and comparison o f ciliary function. 194
196 A B Figure 9.1 Transmission Figure 9.1A: Figure 9.1B: electron micrograph of nasal epithelium before coronavirus inoculation and on Day 3. Transmission electron micrograph Day 0. This shows normal tissue with an intact well-ciliated surface and minimal disruption. Transmission electron micrograph Day 3. This shows abnormal tissue with severely disrupted cell surface. Marked loss of cilia is seen. Projection of cells from the epithelial edge, cytoplasmic blebbing and mitochondrial damage are apparent. 195
197 Table 9.1 summarises the changes seen in cellular type. After inoculation with HCoV there was a significant reduction in the proportion of ciliated cells (p < 0.05) and an increase in the proportion o f dead cells (p < 0.05). No changes were seen in the percentage o f mucous cells. Epithelial disruption and damage by quantification of cilia loss, cellular projections, cytoplasmic blebbing and mitochondrial damage were all significantly increased on Day 3 (p< 0.05) (Table 9.2). Assessing ciliary ultrastructure (Table 9.3), we found a small increase in dynein arms and microtubular abnormalities (p< 0.05) on Day 3. No change was seen in ciliary orientation between Day 0 and Day 3. No significant change was seen between the mean ciliary beat frequency on Day 0 and Day 3 (Table 9.3), but cilia were found to beat dyskinetically on Day 3 (p< 0.05). No change in structure or function was found in samples from volunteers inoculated with uv-inactivated virus (Tables ). Comparison of the change from baseline between subjects inoculated with either active human coronavirus or uv-inactivated virus found significant differences between groups in measures of: loss of ciliated cells, increase in dead cells, cells with loss of cilia, cellular projections, cytoplasmic blebbing, mitochondrial damage, microtubule defects and ciliary dyskinesia (Tables ). 196
198 Ciliated Cells (%) Dead Cells (%) Mucous Cells (%) Corona Day (46.8, 80.6) 0.0 (0.0, 0.0) 10.2 (3.1,17.3) Day (3.8, 37.2)* 6.3 (1.4,11.2)* 11.5 (7.5,15.5) BaselineA (-73.3, -13.0)* 6.3 (1.4,11.3)* 1.3 (-8.4,11.1) Sham Day (66.2,77.8) 0.0 (0.0, 0.0) 9.2 (7.8,10.6) Day (67.5, 72.7) 0.0 (0.0, 0.0) 9.9 (8.3,11.5) BaselineA -1.9 (-8.6, 4.8) 0.0 (0.0, 0.0) 0.8 (-0.6, 2.1) Group difference C orona- Sham -413 (-80.7, -1.8)* 6.3 (-0.2, 12.g)* 0.58 (-12.2, 13.3) Table 9.1:Analysis of cell type by transmission electron microscopy following inoculation with either active human coronavirus or uv-inactivated virus. Results displayed are for Day 0, Day 3, the change from baseline values (Day 3- Day 0) and the difference between groups ((Day 3 - Day 0)corona - (Day 3 - Day 0)Sham). Results are expressed as the mean percentage (95% confidence intervals). (* p < 0.05) Cells with loss o f Cells extruding Cells with cytoplasmic Cells with mitochondria] cilia (%) from surface (%) blebbing (%) damage (%) Corona Day (15.4, 40.8) 25.8 (21.5,30.1) 15.1 (10.7,19.5) 11.9 (5.4,18.4) Day (93.8,101.9)* 63.4 (51.5, 75.3)* 36.3 (24.2,48.4)* 44.2 (33.8, 54.6)* Baseline A 70.6 (55.1, 86.2)* 37.6 (25.2, 50.0)* 21.3 (7.7, 34.8)* 32.3 (21.8,42.8)* Sham Day (26.4, 37.4) 25.7 (22.1, 29.3) 17.6 (10.2, 25.0) 10.4 (5.8,15.0) Day (18.6, 28.0) 21.4 (20.9, 21.9) 17.1 (12.7, 21.5) 12.6 (3.7, 21.5) Baseline A -8.6 (-16.5, -0.6) -4.3 (-8.2, -0.4) -0.5 (-5.8,4.9) 2.2 (-2.5, 6.9) Group difference Corona - Sham 79.2 (58.2,100.2)*** 41.9 (25.6, 58.2)*** 21.7 (3.8, 39.6)* 30.1 (16.1, 44.1)* Table 9.2:Transmission electron microscopy assessment of epithelial integrity following inoculation with either active human coronavirus or uv-inactivated virus. Results displayed are for Day 0, the change from baseline values (Day 3- Day 0) and the difference between groups ((Day 3 - Day 0)corona - (Day 3 - Day 0)Sham). Results are expressed as the mean percentage (95% confidence intervals). (* p < 0.05, ***p < 0.001)
199 Ciliary Beat Ciliary Dynein Arm Microtubule Central Ciliary Frequency Dyskinesia Defects Defects M icrotubule Orientation (H z) Score (% ) (% ) Defects (%) ( ) Corona Day (8.6,14.0) 0.2 (0, 0.5) 1.3 (0.3,2.3) 2.6 (1.4, 3.8) 0.3 (0.0, 0.6) 10.6(10.1, 11.1) Day (7.2,11.6) 1.1 (0.5,1.7)* 2.9 (0.8, 5.0) 4.7 (3.2, 6.2)* 1.0 (0.1,1.9) 11.4(10.4,12.4) Baseline A -1.9 (-5.9, 2.1) 0.9 (0.3,1.5)* 1.3 (-1.5, 4.1) 1.9 (0.8, 3.1)* 0.8 (-0.2,1.7) 1.0 (0.0, 2.0) Sham Day ( ) 0.1 (0.0, 0.0) 0.9 (0.0, 2.2) 2.2 (1.0,3.4) 0.0 (0.0, 0.0) 10.7(10.0,11.4) Day (10.1,11.5) 0.2 (0.0, 0.4) 1.2 (0.1, 2.3) 2.3 (0.8, 3.8) 0.0 (0.0, 0.0) 10.7(10.0,11.4) Baseline A -1.1 (-1.7, -0.5) 0.1 (-0.1, 0.3) 0.3 (-0.2, 0.8) 0.2 (-0.3, 0.7) 0.0 (0.0, 0.0) 0.3 (0.1, 0.5) Group Difference Corona - Sham -0.8 (-7.9, 6.3) -0.8 (-1.5, -0.1)* 1.1 (-2.8, 5.0) 1.8 (0.1, 3.4)* 0.8 (-0.6, 2.1) 0.7 (-1.1, 2.5) Table 9.3: Results of ciliary functional and ultrastructural analysis following inoculation with either active human coronavirus or uv-inactivated virus. Results displayed are for Day 0, the change from baseline values (Day 3- Day 0) and the difference between groups ((Day 3 - Day 0)COrona - (Day 3 - Day 0)Sham) Results are expressed as the mean percentage (95% confidence intervals). ( * p < 0.05) 198
200 8.5 Discussion The results demonstrate that significant damage to the respiratory epithelium occurs following nasal inoculation with human coronavirus, even in those without overt clinical symptoms of a cold (Figure 9.1). Evidence was found of disruption of the nasal epithelium and ciliary dyskinesia in all of the volunteers inoculated with active HCoV even though three subjects had no symptoms of a cold. No epithelial damage was seen in subjects who were sham infected with inactivated virus. The lack of damage following sham infection suggests tissue damage is a result of an infective process, rather than an immune mediated response to viral antigen, or the effect of cytokines present in the inoculum, since it only occurs when live virus is administered Although analysis of epithelium was blinded, inoculation was not blinded or placebo controlled. Nevertheless, the nasal measurements suggest that the model was successful, with clear evidence of epithelial damage in all of those inoculated and with clinical symptoms of a cold in the majority. RT-PCR was chosen as the method to identify HCoV as it has been shown to be more sensitive than either serology or culture techniques.359 In retrospect serology and cell culture might have provided useful additional laboratory evidence of infection. The RT-PCR results were only positive in a proportion o f those inoculated despite epithelial damage in all cases. The reasons for this 199
201 may be related to the timing o f samples, sample quality and the possibility of false- negative results arising during the RT-PCR assay. Despite the fact that only four volunteers were sham infected clear differences between volunteers inoculated with active HCoV and sham infected subjects were found. Within the sham infected group there was no evidence of epithelial damage or ciliary dyskinesia three days after inoculation. This suggests that viral antigens or mediators within the inoculum were not responsible for the epithelial changes found. It appears that infection with active virus is required for epithelial damage to occur. Bende and colleagues353 made physiological measurements on 24 adult volunteers inoculated with HCoV, of whom 13 developed clinical symptoms, 8 had no overt clinical symptoms but viral shedding occurred, and 3 were not infected. Nasal airway resistance and the temperature of the nasal mucosa increased in those with and without symptoms, suggesting underlying inflammation in both groups. Mucosal blood flow in the nose and nasal mucus secretion increased only in those with symptoms. These results are consistent with our findings that an inflammatory process may be occurring in volunteers inoculated with HCoV who show no clinical signs of a cold. 189 Overt viral upper respiratory tract infections usually delay nasal mucociliary clearance. Loss of ciliated epithelium and dyskinetically beating cilia may be a major factor in this, although a change in mucus rheology may also be important.352 In this study the duration 200
202 of symptoms peaked at day 4 and resolved 7 days after inoculation. The study only provides data three days after inoculation. The time taken for the upper respiratory tract epithelium to return to normal following exposure to coronavirus infection is not known. In naturally acquired colds, the epithelium returns to normal in the majority of patients by three weeks,190 while mucus clearance may be abnormal for several weeks following viral infection.189 A major advantage of using a digital high-speed video system for ciliary analysis is the ability to play ciliary movement in slow motion, allowing both measurement of their beat frequency and evaluation of their beat pattern. Despite recording beat frequency within the normal range three days after inoculation with HCoV a significant increase in dyskinetic cilia was observed. All of the readings of ciliary beat frequency were made at physiological temperatures with cilia beating at greater than 11 Hz. Such high frequencies mean that it is usually impossible to assess dyskinetic movement without the ability to watch them in slow motion. Pedersen and colleagues189 studied ciliary function at 22 C following naturally acquired common colds. At this temperature cilia beat at half of their normal rate allowing gross changes in beat pattern to be more easily detected. They also noted a significant increase in ciliary asynchrony that was maximal during the first few days of the infection. Others have also found ciliary beat frequency, measured at physiological temperatures, to be within the normal range following viral infection but have not commented on the presence o f dyskinesia. It is possible that ciliary dyskinesia q c A was missed due to the rapid beat frequency. 201
203 Electron microscopy revealed significant loss of epithelial integrity and a very significant decrease in the numbers of ciliated cells after coronavirus inoculation. Similar findings have been recorded following nasal infection with other viruses On Day 3 no significant increase in dynein arm or central microtubular abnormalities was observed, but a signifaicant increase in peripheral microtubular abnormalities was found. This is less than previously reported in naturally acquired viral infection in children,188 although none of the patients in that study had a coronavirus infection. In 22 of the 30 episodes of culture proven viral infection, Carson and colleagues found microtubular additions and deletions. In the remaining 8 cases, epithelial cell abnormality and the loss of ciliated cells were too severe to allow ciliary structure to be evaluated. Beat frequency or analysis o f ciliary dyskinesia was not explored.188 No virions were seen on electron microscopy in the study despite epithelial damage. This is in keeping with other reports o f electron microscopy o f human nasal and bronchial ton.i epithelium during episodes o f the common cold. In contrast there are many published reports of coronavirus infected cells in in-vitro systems.366,367 Afzelius64 documented a single case of coronavirus infection of the nasal respiratory epithelium in a 2 year old girl with no symptoms of a common cold. This was an incidental finding on a biopsy taken to rule out the diagnosis of primary ciliary dyskinesia. Virions could be seen within and outside the ciliated cells but not in the mucous cells. Some virions were 202
204 located near the microvilli with others in pockets in the apical cell membrane suggesting the microvilli may be the site o f first contact. 8.6 Summary Damage to the respiratory epithelium may occur due to viral infection without overt clinical symptoms. This may well have implications in lung disease for example in chronic supporative diseases where lung function was significantly reduced following symptomatic and asymptomatic viral infections.368 Further work is needed to establish the mechanism of viral damage to the respiratory epithelium caused by human coronavirus infection. 203
205 Chapter 9 204
206 9 Conclusion The introductory chapters explored the structure and function of respiratory cilia in depth. Methods used to sample and evaluate respiratory cilia have been discussed. The main outcomes for assessment of ciliary function were the measurement of both ciliary beat pattern and beat frequency. Methods to do this have been described and advantages and disadvantages stated. Digital high-speed imaging has been presented as a technique to measure both ciliary beat frequency and beat pattern. This has been evaluated against existing methods and found to be a gold standard. Using this technique the ciliary beat pattern has been evaluated in detail for the first time. The hypothesis generated in chapter 1 has been tested. The results obtained in chapter 5 allow this hypothesis to be rejected. Ciliary movement is different to the most commonly accepted description.9 Cilia were observed to move with a forwards/backwards motion within the same plane. No sideways recovery stroke has been observed. The ciliary movement is similar to the planar beat observed by Cheung et al.89 With the greater precision afforded by digital high-speed imaging a planar whip-like motion was seen rather than the bobbing motion described by Cheung. The results observed support the switch point hypothesis suggested by Satir
207 The secondary aims were to evaluate digital high speed imaging as a method for assessment of patients with a suspected diagnosis o f primary ciliary dyskinesia. Normal reference ranges were generated in both a paediatric and young adult population for ciliary beat frequency, beat pattern and ultrastructural parameters. Having established normal reference ranges it has been possible to evaluate ciliary beat frequency, beat pattern and ultrastructure in patients with primary ciliary dyskinesia. Different beat patterns were found to be associated w ith different ultrastructural defects. Finally the digital high-speed method has been used as a valid research tool. The coronavirus study highlighted the need for evaluation of both ciliary beat frequency and beat pattern. No difference was observed for beat frequency following infection but cilia were found to beat dyskinetically. However despite the results obtained the work is limited in areas. These are open to comment and criticism. The thesis would have been strengthened by the facility to measure nasal nitric oxide. In primary ciliary dyskinesia this is low when compared to controls. Having the ability to perform this measurement would have assisted in the diagnosis of patients with primary ciliary dyskinesia. In addition, it would have been useful to examine if an association exists between different ultrastructural defects and levels of nasal nitric oxide. This would have been o f particular interest in the ciliary transposition group where ciliary 206
208 beat frequency fell within the normal range and there was no evidence o f ciliary immotility. Data have been published relating to both nasal and exhaled nitric oxide concentrations in 777 a healthy control population. There has been no work looking at the relationship between nitric oxide and ciliary beat frequency. This could have been expanded upon by looking at the relationship between ciliary beat frequency and nasal nitric oxide and to see if an association exists between increasing age and nitric oxide concentration for the healthy normal control population studied in chapter 6. W hen obtaining nasal brush biopsies it was assumed that all subjects and patients were free from upper respiratory tract infections. This was not formally assessed by performing nasal swabs and culturing for bacteria and viruses. Failing to perform this may have implications when interpreting ciliary beat pattern for both the normal reference group and patients with primary ciliary dyskinesia. We observed between 5-25% o f edges had dyskinetically beating cilia in the healthy control subjects. Was this secondary ciliary dyskinesia due to infection? To exclude this no samples were taken within 6 weeks o f an upper respiratory tract infection. However there may have been sub-clinical infection as observed within the coronavirus data. Performing cultures from the nose prior to ciliary brush biopsy would have helped to clarify this. 207
209 For the normal control subjects, samples were obtained after propofol induction of anaesthesia. Did the induction agent effect ciliary beat pattern and cause secondary ciliary dyskinesia? Certainly data shows that there is no reduction in ciliary beat frequency following induction o f anaesthesia with propofol but no data exist looking at its effect on ciliary beat pattern. Only one paper has evaluated the action o f an anaesthetic agent on ciliary beat pattern. The authors examined the action o f isoflurane, which causes a reduction in ciliary beat frequency,193 but found it had no effect on beat 1 / Q pattern. This question could have been resolved by taking samples before and after propofol administration. Alternatively, repeating beat pattern evaluation over a time period may have shown a resolution o f secondary dyskinesia with time if this was due to a drug action. Another approach would have been to culture the ciliated epithelium. This was not undertaken within this thesis. Cell culture o f ciliated respiratory epithelium is a useful method to eliminate secondary ciliary dyskinesia caused by infection or possibly by administration o f pharmacological agents. Jorissen et al have looked at this both from an f \ 70S ultrastructural and functional perspective and found that ciliogenesis will reduce or eliminate secondary abnormalities when making a diagnosis of primary ciliary dyskinesia. The data presented in the thesis would have been stronger if we had performed ciliary culture for both the norm al population and those diagnosed with prim ary ciliary 208
210 dyskinesia. This may not have influenced the ultrastructural results as these data are similar to that reported by Jorissen et al,340 but may have altered data looking at ciliary beat pattern. However as stated by Jorissen ciliogenesis data evaluating ciliary beat pattern is lacking.340 The experimental system allowed quantitative evaluation of ciliary beat pattern in three distinct planes; overhead, end-on and side profile (Figure 5.2 a,b,c). However no data were collected quantifying the ciliary amplitude. Sanderson et al have attempted this by measuring the angle through which the cilium moves during the forward power stroke.9 They did not examine the recovery stroke or look at changes in amplitude of the cilium. Rautiainen tried to assess amplitude by a scoring system and was able to correlate this to ciliary beat frequency. Further quantitative analysis o f the normal ciliary beat pattern would have given additional information on ciliary movement. Beat pattern analysis in patients with primary ciliary dyskinesia was solely qualitative. This was recorded on digital video and archived. However, to strengthen the data presented in chapter 6 quantitative analysis o f the angle through which the cilium moved, changes in amplitude and changes in height o f cilia would have been important to record. Measurement o f nasal mucociliary clearance by techniques such as the saccharin test has been used to evaluate ciliary function and act as a screening test for primary ciliary dyskinesia. This was not perform ed in any o f the studies in either healthy subjects or 209
211 patients with primary ciliary dyskinesia. Rutland et al have measured nasal mucociliary clearance rate in controls and patients with primary ciliary dyskinesia but have not compared nasal clearance rate with ciliary beat frequency Only one paper reports this correlation and suggests a logarithmic relationship.111 With such a large population of healthy subjects and patients it would have been useful to evaluate nasal mucociliary clearance and ciliary beat frequency for both groups and to look for any relationship with age. One paper has suggested that m easurem ent o f nasal m ucociliary clearance may only 7QO detect patients with primary ciliary dyskinesia who have immotile cilia. If this had been measured in the patients evaluated in chapter 7 it would have been possible to observe if a correlation between increasing nasal mucociliary clearance time and those ultrastructural defects with increasing percentage o f immotile cilia existed. All ultrastructural results were obtained by a trained transmission electron microscopist who performed all processing and analysis. This could be open to criticism as it may be difficult to standardise this and perform quality control checks. An attempt was taken to achieve this by blinding the electron microscopist in the coronavirus study with regard to samples. Some degree of correlation was also obtained in processing samples from patients with primary ciliary dyskinesia. By analysing the beat pattern it was possible to predict the underlying ciliary ultrastructural defect. The electron microscopist was unaware o f the functional results prior to analysing the epithelial samples. W hen 210
212 presented there was a 100% correlation between the ultrastructural defect and corresponding beat pattern. For published ultrastructural reference ranges there are few data quantifying all defects observed in both healthy subjects and those patients with primary ciliary dyskinesia.331 Results that are available are limited to dynein arm and microtubule defects. Data in paediatric patients are sparse. Therefore it is difficult to validate the results in chapters 6 and 7. To take this further would require quality control checks and undertaking audit between all UK centres offering a diagnostic service for primary ciliary dyskinesia. It would be essential to compare processing and preparation o f samples for electron microscopy, as well as a comparison o f diagnostic accuracy against an approved standard for normal ciliary ultrastructure. In addition criteria would have to be defined against which to diagnose a specific ultrastructural defect such as absent outer dynein arm defect, radial spoke defect, ciliary transposition defect etc. A final comment relates to the study o f coronavirus and human respiratory cilia. This only examines the effect of coronavirus infection on ciliary ultrastructure, beat frequency and beat pattern at 2 time points. The chapter would have been strengthened by evaluating these parameters not only during the infective phase but through the recovery period. The time for complete recovery o f the ciliated epithelium is not known but data suggest that full recovery is usually complete by 3 weeks190 but can take up to 12 weeks 188 following some viral infections. 211
213 Future direction The thesis has looked at the functional aspects o f human respiratory cilia. It has identified areas o f interest which warrant further investigation. These are highlighted below: 1) The role of nitric oxide and ciliary function is far from clear. Exhaled nitric oxide metabolites are not reduced in patients with primary ciliary dyskinesia, therefore suggesting normal function of nitric oxide synthase.372 Similarly the action o f L-arginine has been shown to increase ciliary beat frequency not only in patients with primary ciliary dyskinesia but those with cystic fibrosis and healthy controls. Therefore to increase the understanding o f the role o f nitric oxide it would be o f interest to measure nasal nitric oxide in patients with primary ciliary dyskinesia and correlate it with individual ultrastructural defects. Also to correlate ciliary beat frequency and both nasal and exhaled nitric oxide in healthy subjects and those with prim ary ciliary dyskinesia. 2) Cell culture of respiratory ciliated epithelium is becoming a widely accepted method to look at ciliary ultrastructure. The effects of secondary damage to the ciliated epithelium are removed by this process. There is little data looking at ciliary beat frequency with this method and no papers have been 212
214 published looking at ciliary beat pattern. Jorrisen has stated that although cell culture eliminates secondary dyskinesia there is an increased percentage of immotile cilia. To firmly accept cell culture as a robust method for ciliary assessment, the beat frequency and beat pattern need to be evaluated both before and after cell culture for both healthy controls and patients with prim ary ciliary dyskinesia. 3) The coronavirus study would have been strengthened by evaluating ciliary ultrastructure, beat frequency and beat pattern until complete recovery o f the ciliated epithelium. No studies have assessed the time for restoration o f a normal beat pattern. Most papers will examine ultrastructural recovery with a few reporting the time for norm alisation o f ciliary beat frequency. 4) In patients with primary ciliary dyskinesia we have been able to examine the demographics o f a large number of patients referred through for ciliary assessment because o f chronic respiratory morbidity. Further analysis using binary logistic regression would allow us to analyse symptoms to give a positive predictive score to identify patients with a high risk of primary ciliary dyskinesia and who would therefore require full ciliary analysis by brush biopsy. 213
215 5) A pilot study for a courier service has been performed. This has shown that samples can be sent to diagnostic centres, for evaluation o f ciliary beat frequency and ciliary ultrastructure. Further work is needed to eliminate the secondary ciliary dyskinesia seen when samples are transported on ice. This may well be temperature related, as Lee and colleagues did not experience this with samples stored at 4 C. 9.1 Summary Digital high-speed imaging is a gold standard for evaluation o f ciliary beat frequency and beat pattern. With the availability of normal reference ranges it can be used confidently as a diagnostic and research method. Future directions for research have been presented. 214
216 Reference List 215
217 1. Ballenger JJ. A Study of Ciliary Activity in the Respiratory Tract of Animals. A n n O to l, R h in o I & L a r y n g o l 1958;58: Gray J. Ciliary Movement. London: Cambridge University Press, Travis SM, Conway BA, Zabner J, Smith JJ, Anderson NN, Singh PK e t a l. Activity o f abundant antimicrobials o f the human airway. A m J R e s p ir C e ll M o l B io l 1999;20: Knowles MR. Mucociliary and cough clearance: Role o f ion transport and the P2Y2 receptor mediated system. In Baum GL, Priel Z, Roth Y, Liron N, Ostfeld E. ed. C ilia, m u c u s, a n d m u c o c ilia r y in te r a c tio n s., pp New York: Marcel Decker, Gray J. The Mechanism of Ciliary Movement-VI. Photographic and Stroboscopic Analysis o f Ciliary Movement. P r o c R o y a l S o c, B io l S ci, S e r ie s B 1930;107: Proetz AW. Motion Picture Demonstration O f Cilia Action and Nasal Physiology. T r a n s a c tio n s o f th e A m e r ic a n L a r y n g o lo g y A s s o c ia tio n 1932;54: Dalhamn T. Mucous flow and ciliary activity in the trachea of healthy rats and rats exposed to Respiratory irritant gases. A c ta P h y s io lo g ic a S c a n d in a v ic a 1956;36: Cheung AT,.Jahn TL. High speed cinemicrographic studies on rabbit tracheal (ciliated) epithelia: determination o f the beat pattern of tracheal cilia. P e d ia tr R e s 1976;10:
218 9. Sanderson MJ,.Sleigh MA. Ciliary activity o f cultured rabbit tracheal epithelium: beat pattern and metachrony. J C e ll S c i 1981 ;47: Marino MR,.Aiello E. Cinemicrographic analysis of beat dynamics o f human respiratory cilia. P r o g C lin B io l R e s 1982;80: Sleigh MA. Ciliary function in mucus transport. C h e s t 1981;80: Satir P,.Sleigh MA. The physiology o f cilia and mucociliary interactions. A n n u R e v P h y s io l 1990;52: Satir P. The role of axonemal components in ciliary motility. C o m p B io c h e m P h y s io l A 1989;94: Satir P. Mechanisms and controls of microtubule sliding in cilia. S y m p S o c E x p B io l 1982;35: Satir P,.Matsuoka T. Splitting the ciliary axoneme: implications for a "switch- point" model o f dynein arm activity in ciliary motion. C e ll M o til C y to s k e le to n 1989;14: Yoshitsugu M, Rautiainen M, Matsune S, Nuutinen J, Ohyama M. Effect o f exogenous ATP on ciliary beat of human ciliated cells studied with differential interference microscope equipped with high speed video. A c ta O to - L a r y n g o lo g ic a 1993;113: Dolovich MB, Mahony JB, Chambers C, Newhouse MT, Chemesky MA. Ciliary function, cell viability, and in vitro effect o f ribavirin on nasal epithelial cells in acute rhinorrhea. C h e s t 1992;102:
219 18. Rossman CM, Lee RM, Forrest JB, Newhouse MT. Nasal ciliary ultrastructure and function in patients with primary ciliary dyskinesia compared with that in normal subjects and in subjects with various respiratory diseases. A m R e v R e s p D is 1984;129: NAC image technology. NAC's Memrecam fx 6000 digital high speed colour video system USA, NAC Image Technology. 20. Sanderson MJ. High-speed digital microscopy. M e th o d s 2000;21: Rusznak C, Devalia JL, Lozewicz S, Davies RJ. The assessment o f nasal mucociliary clearance and the effect o f drugs. R e s p M e d 1994;88: Ishijima S. High-speed video microscopy of flagella and cilia. M e th o d s C e ll B io l 1995;47: Breeze RG,.Wheeldon EB. The cells o f the pulmonary airways. A m R e v R e s p ir D is 1977;116: Sleigh MA, Blake JR, Liron N. The propulsion of mucus by cilia. A m R e v R e s p ir D is 1988;137: Wanner A, Salathe M, O Riordan TG. Mucociliary clearance in the airways. A m J R e s p C r it C a r e M e d 1996;154:(Pt 1): Alexander I, Ritchie BC, M aloney JE, Hunter CR. Epithelial surfaces o f the trachea and principal bronchi in the rat. T h o r a x 1975;30: Smolich JJ, Stratford BF, Maloney JE, Ritchie BC. New features in the development o f the submucosal gland of the respiratory tract. J A n a t 1978;127:
220 28. Plopper CG, Nishio SJ, Alley JL, Kass P, Hyde DM. The role o f the nonciliated bronchiolar epithelial (Clara) cell as the progenitor cell during bronchiolar epithelial differentiation in the perinatal rabbit lung. A m J R e s p ir C e ll M o l B io l 1992;7: Puchelle E et al. Airway mucociliary epithelium injury and repair. In Baum GL Priel Z, Roth Y, Liron N, Ostfeld E. ed. C ilia, m u c u s, a n d m u c o c ilia r y in te r a c tio n s., pp New York: Marcell Decker, Carson JL, Collier AM, Knowles MR, Boucher RC, Rose JG. Morphometric aspects o f ciliary distribution and ciliogenesis in human nasal epithelium. P r o c.n a tl.a c a d.s c i. U.S. A 1981 ;78: Lee RMKW FJB. Structure and function o f cilia. In Crystal RG. West JB ea, ed. T h e L u n g, pp Philadelphia: Lippincott-Raven Publishers, Rhodin JA. The ciliated cell. Ultrastructure and function o f the human tracheal mucosa. A m R e v R e s p ir D is 1966;93:Suppl:l Chilvers MA, Mckean M, Rutman A, Myint BS, Silverman M, O'Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. E u r R e s p ir J 2001 ;18: Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease. E u r R e s p ir J 1999;13: Foliguet B,.Puchelle E. Apical structure of human respiratory cilia. B u lle tin.e u r o p e e n.d e.p h y s io p a th o lo g ie.r e s p ir a to ir e. 1986;22: Busuttil A, More IA, McSeveney D. A reappraisal of the ultrastructure o f the human respiratory nasal mucosa. J A n a t. 1977;124:
221 37. Girod S, Zahm JM, Plotkowski C, Beck G, Puchelle E. Role o f the physiochemical properties o f mucus in the protection o f the respiratory epithelium. E u r R e s p ir J 1992;5: Moscoso GJ, Driver M, Codd J, Whimster WF. The morphology of ciliogenesis in the developing fetal human respiratory epithelium. P a th o l R e s P r a c t 1988;183: Moscoso G, Nandra K, Driver M. Ciliogenesis and ciliation of the respiratory epithelium in the human fetal cartilaginous trachea. P a th o l.r e s.p r a c t. 1989;184: Jeffrey PK. The development of large and small airways. A m J R e s p ir C r it C a r e M e d 1998;157:S Carson JL, Reed W, Lucier T, Brighton L, Gambling TM, Huang CH e t a l. Axonemal dynein expression in human fetal tracheal epithelium. A m J P h y s io l L u n g C e ll M o l P h y s io l 2002;282 :L Sakai N, Suenaga T, Tanaka K. Electron microscopic study on the esophageal mucosa in human fetuses. A u r is N a s u s L a r y n x 1989;16: Hagiwara H, Ohwada N, Aoki T, Takata K. Ciliogenesis and ciliary abnormalities. M e d E le c tr o n M ic r o s c 2000;33: Dirksen ER. Ciliary basal body morphogenesis: the early events. S y m p S o c E x p B io l 1982;35: Carson JL,.Collier AM. Ciliary defects: cell biology and clinical perspectives. A d v P e d ia tr 1988;35:
222 46. Dirksen ER. Centriole and basal body formation during ciliogenesis revisited. B io l C e ll 1991;72: Cole D. The intraflagellar transport machinery o f Chlamydomonas reinhardtii. T r a ffic 2003;4: Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport(ift) cargo TFT transports flagellar precusors to the tip and turnover products to the cell body. J C e ll B io l 2004;164: Marshall WF. Cellular length control systems. A n n u R e v C e ll D e v B io l 2004;20: Afzelius BA. Ciliary structure in health and disease. A c ta O to r h in o la r y n g o l B e lg 2000;54: Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A e t a l. A proteomic analysis o f human cilia: identification of novel components. M o l C e ll P r o te o m ic s 2002;1: Meeks M,.Bush A. Primary ciliary dyskinesia (PCD). P e d ia tr P u lm o n o l 2000;29: Dentler WL. Microtubule-membrane interactions in cilia and flagella. I n t R e v C y to l 1981;72: Thai CH, Gambling TM, Carson JL. Freeze fracture study of airway epithelium from patients with primary ciliary dyskinesia. T h o r a x 2002;57: Dute R,.Kung C. Ultrastructure o f the proximal region of somatic cilia in Paramecium tetraurelia. J C e ll B i o l 1978;78:
223 56. Arima T, Shibata Y, Yamamoto T. Three-dimensional visualization of basal body structures and some cytoskeletal components in the apical zone o f tracheal ciliated cells. J U ltr a s tr u c t R e s 1985;93: Porter ME,.Sale WS. The axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J C e ll B io l 2000;151:F W arner FD. Ciliary inter-m icrotubule bridges. J C e ll S c i 1976;20: Smith EF. Regulation of flagellar dynein by the axonemal central apparatus. C e ll M o til C y to s k e le to n 2002;52: Rayner CF, Rutman A, Dewar A, Greenstone MA, Cole PJ, Wilson R. Ciliary disorientation alone as a cause o f primary ciliary dyskinesia syndrome. A m J R e s p & C r it C a r e M e d 1996;153: Biggart E, Pritchard K, Wilson R, Bush A. Primary ciliary dyskinesia syndrome associated with abnormal ciliary orientation in infants. E u r R e s p ir J \; 1 1 \ Chilvers MA, Rutman A, O'Callaghan C. Functional analysis of cilia and ciliated epithelial ultrastructure in healthy children and young adults. T h o r a x 2003;58: Woolley D. The molecular motors of cilia and eukaryotic flagella. E s s a y s B io c h e m 2000;35: Afzelius BA. Ultrastructure of human nasal epithelium during an episode of coronavirus infection. V irc h o w s A r c h 1994;424:
224 65. Gardner LC, O'Toole E, Perrone CA, Giddings T, Porter ME. Components of a "dynein regulatory complex" are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J C e ll B i o l 1994;127: Warner FD. Organisation of interdoublet links in Tetrahymena cilia. C e ll M o tility 1983;3: Smith EF. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. M o l B i o l C e ll 2002;13: Kultgen PL, Byrd SK, Ostrowski LE, Milgram SL. Characterization o f an a- kinase anchoring protein in human ciliary axonemes. M o l B i o l C e ll 2002;13: Avolio J, Glazzard AN, Holwill ME, Satir P. Structures attached to doublet microtubules o f cilia: computer modeling o f thin-section and negative-stain stereo images. P r o c N a tl A c a d S c i U S A 1986;83: Hunt AJ. M olecular motors. K eeping the beat. N a tu r e 1998;393: Sale W S,.Satir P. Direction o f active sliding o f microtubules in Tetrahymena cilia. P r o c N a tl A c a d S c i U S A 1977;74: Fox LA,.Sale WS. Direction o f force generated by the inner row of dynein arms on flagellar microtubules. J C e ll B i o l 1987;105: Brokaw CJ. Are motor enzymes bidirectional? C e ll M o til C y to s k e le to n 1997;38: Paschal BM, King SM, Moss AG, Collins CA, Vallee RB, Witman GB. Isolated flagellar outer arm dynein translocates brain microtubules in vitro. N a tu r e 1987;330:
225 75. Sale WS,.Fox LA. Isolated beta-heavy chain subunit of dynein translocates microtubules in vitro. J C e ll B io l 1988;107: Omoto CK, Gibbons IR, Kamiya R, Shingyoji C, Takahashi K, Witman GB. Rotation o f the central pair microtubules in eukaryotic flagella. M o l B io l C e ll 1999;10: Wargo MJ,.Smith EF. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. P r o c N a tl A c a d S c i U S A 2003;100: Smith EF,.Lefebvre PA. The role of central apparatus components in flagellar motility and microtubule assembly. C e ll M o til C y to s k e le to n 1997;38: Satir P. Switching mechanisms in the control o f ciliary motility. M o d C e ll B io l 1985;4: Omoto CK,.Witman GB. Functionally significant central-pair rotation in a primitive eukaryotic flagellum. N a tu r e 1981;290: Omoto CK,.Kung C. Rotation and twist o f the central-pair microtubules in the cilia of Paramecium. J C e ll B io l 1980;87: Smith EF,.Yang p. The radial spokes and central apparatus:mechano-chemical transducers that regulate flagellar motility. C e ll M o til C y to s k e le to n 2004; 57: O'Callaghan C, Sikand K, Rutman A. Respiratory and brain ependymal ciliary function. P e d ia tr R e s 1999;46: Sleigh MA. Adaptations of ciliary systems for the propulsion o f water and mucus. C o m p B io c h e m P h y s io l A 1989;94:
226 85. Chilvers M A,.0'Callaghan C. Analysis o f ciliary beat pattern and beat frequency using digital high speed imaging: comparison with the photomultiplier and photodiode methods. T h o r a x 2000;55: Sanderson MJ,.Dirksen ER. A versatile and quantitative computer-assisted photoelectronic technique used for the analysis of ciliary beat cycles. C e ll M o tility 1985;5: Rautiainen M, Matsune S, Shima S, Sakamoto K, Hanamure Y, Ohyama M. Ciliary beat of cultured human respiratory cells studied with differential interference microscope and high speed video system. A c ta O to - L a r y n g o lo g ic a 1992;112: Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance o f periciliary liquid and mucus from airway surfaces. J C lin I n v e s t 1998;102: Cheung AT,.Jahn TL. High speed cinemicrographic studies on rabbit tracheal (ciliated) epithelia: cytolytic effect of cystic fibrosis serum on tracheal epithelial cells. P e d R e s 1976;10: Knowles MR,.Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J C lin I n v e s t 2002;109: Sleigh M.A. The integrated activity o f cilia: Function and coordination. J P r o to z o o l 1984;31( 1): W ong LB, Miller IF, Yeates DB. Nature of the mammalian ciliary metachronal wave. J A p p l P h y s io l 1993;75:
227 93. Hill L. The ciliary movement o f the trachea studied in vitro. Lancet 1928; Hilding AC. Ciliary streaming in the bronchial tree and the time element in carcinogenesis. N E J M 1957;256: Proetz AW. Essays on the applied Physiology of the nose. Saint Louis U.S.A.: Annals Publishing Company, Lee RM, Rossman CM, O'Brodovich H. Assessment o f postmortem respiratory ciliary motility and ultrastructure. A m R e v R e s p D is 1987;136: Rutland J, Griffin WM, Cole PJ. Human ciliary beat frequency in epithelium from intrathoracic and extrathoracic airways. A m.r e v.r e s p ir.d is. 1982;125: Agius AM, Smallman LA, Pahor AL. Age, smoking and nasal ciliary beat frequency. C lin O to la r y n g o l 1998;23: Stanley PJ, Wilson R, Greenstone MA, MacWilliam L, Cole PJ. Effect of cigarette smoking on nasal mucociliary clearance and ciliary beat frequency. T h o r a x 1986;41: Maurer DR,.Liebman J. Effects o f ethanol on in vitro ciliary motility. J A p p l P h y s io l 1988;65: Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. A m J P h y s io l 1995;268:(Pt 1):L Ma W, Silberberg SD, Priel Z. Distinct axonemal processes underlie spontaneous and stimulated airway ciliary activity. J G e n P h y s io l 2002;120:
228 103. Lansley AB,.Sanderson MJ. Regulation of airway ciliary activity by Ca2+: simultaneous measurement of beat frequency and intracellular Ca2+. B io p h y s J 1999;77: Smith RP, Shellard R, Dhillon DP, Winter J, Mehta A. Asymmetric interactions between phosphorylation pathways regulating ciliary beat frequency in human nasal respiratory epithelium in vitro. J P h y s io l 1996;496: Di Benedetto G, Magnus CJ, Gray PT, Mehta A. Calcium regulation of ciliary beat frequency in human respiratory epithelium in vitro. J P h y s io l 1991 ;439: Yeates DB. Neural regulation o f the mucociliary system. In Baum GL Priel Z, Roth Y, Liron N, Ostfeld E. ed. C ilia, m u c u s, a n d m u c o c ilia r y in te r a c tio n s., pp New York: Marcell Decker, Wyatt TA,.Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. A m J P h y s io l L u n g C e ll M o l P h y s io l 2001;281:L Seybold ZV, Mariassy AT, Stroh D, Kim CS, Gazeroglu H, Wanner A. Mucociliary interaction in vitro: effects of physiological and inflammatory stimuli. J A p p l P h y s io l 1990;68: Salathe M, Lipson EJ, Ivonnet PI, Bookman RJ. Muscarinic signaling in ciliated tracheal epithelial cells: dual effects on Ca2+ and ciliary beating. A m J P h y s io l 1997;272:L Sanderson MJ,.Dirksen ER. Mechanosensitive and beta-adrenergic control o f the ciliary beat frequency of mammalian respiratory tract cells in culture. A m R e v R e s p ir D is 1989;139:
229 111. Duchateau G, Graamans K, Zuidema J, Merkus F. Correlation between nasal ciliary beat frequency and mucus transport rate in volunteers. L a r y n g o s c o p e 1985;95: O'Callaghan C, Smith K, Wilkinson M, Morgan D, Priftis K. Ciliary beat frequency in newborn infants. A r c h D is C h ild 1991;66: Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL e t a l. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. A m J R e s p i r C r it C a r e M e d 2001; 163: Roth Y, Aharonson EF, Teichtahl H, Baum GL, Priel Z, Modan M. Human in vitro nasal and tracheal ciliary beat frequencies: comparison of sampling sites, combined effect of medication, and demographic relationships. A n n O to l R h in o l L a r y n g o l 1991;100: Clary-Meinesz C, Mouroux J, Huitorel P, Cosson J, Schoevaert D, Blaive B. Ciliary beat frequency in human bronchi and bronchioles. C h e s t 1997;111: Green A, Smallman LA, Logan AC, Drake-Lee AB. The effect of temperature on nasal ciliary beat frequency. C lin O to la r y n g o l 1995;20: O'Callaghan C, Achaval M, Forsythe I, Barry PW. Brain and respiratory cilia: the effect of temperature. B io l N e o n a te 1995;68: Konietzko N, Nakhosteen JA, Mizera W, Kasparek R, Hesse H. Ciliary beat frequency of biopsy samples taken from normal persons and patients with various lung diseases. C h e s t 1981;80:(Suppl): Rusznak C, Devalia JL, Sapsford RJ, Davies RJ. Circadian rhythms in ciliary beat frequency of human bronchial epithelial cells, in vitro. R e s p M e d 1994;88:
230 120. Horstmann G, Iravani J, Norris Melville G, Richter HG. Influence of temperature and decreased water content o f inspired air on the ciliated bronchial epithelium. A physiological and electron microscopical study. A c ta O to la r y n g o l 1977;84: Mercke U,.Toremalm NG. Air humidity and mucociliary activity. A n n O to l R h in o! & L a r y n g o l 1976;85: Stanek A, Brambrink AM, Latorre F, Bender B, Kleemann PP. Effects of normobaric oxygen on ciliary beat frequency o f human respiratory epithelium. B r J A n a e s th 1998;80: Kay RJ, Moate RM, Bray I, Sneyd JR, Langton JA. Cultured rat trachea as a model for the study of ciliary abundance: the effect of oxygen. A n e s th e s io l 2002;97: Clary-Meinesz C, Mouroux J, Cosson J, Huitorel P, Blaive B. Influence of external ph on ciliary beat frequency in human bronchi and bronchioles. E u r.r e s p ir.j. 1998;11: Luk CK,.Dulfano MJ. Effect o f ph, viscosity and ionic-strength changes on ciliary beating frequency o f human bronchial explants. C lin S c i 1983; 64: van de Donk HJ, Zuidema J, Merkus FW. The influence of the ph and osmotic pressure upon tracheal ciliary beat frequency as determined with a new photoelectric registration device. R h in o lo g y 1980;18: Calvet JH, Verra F, Maleine J, Millepied MC, H arf A, Escudier E. Effect of increased pressure on tracheal ciliary beat frequency. E u r R e s p i r J 1999;14:
231 128. Yager JA, Ellman H, Dulfano MJ. Human ciliary beat frequency at three levels of the tracheobronchial tree. A m.r e v.r e s p ir.d is. 1980;121: Lieb T, Frei CW, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J P h y s io l 2002;538: Sanderson MJ,.Dirksen ER. Mechanosensitivity of cultured ciliated cells from the mammalian respiratory tract: implications for the regulation of mucociliary transport. P r o c N a t A c a d S c i U SA 1986;83: Felix JA, Chaban VV, W oodruff ML, Dirksen ER. Mechanical stimulation initiates intercellular Ca2+ signaling in intact tracheal epithelium maintained under normal gravity and simulated microgravity. A m J R e s p ir C e ll M o l B io l 1998;18: Sanderson MJ, Chow I, Dirksen ER. Intercellular communication between ciliated cells in culture. A m J P h y s io l 1988;254:(Pt l):c Sanderson MJ, Charles AC, Dirksen ER. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. C e ll R e g u l 1990;1: Sanderson MJ, Lansley AB, Dirksen ER. Regulation o f ciliary beat frequency in respiratory tract cells. C h e s t 1992;101:69S-71S Boitano S, Sanderson MJ, Dirksen ER. A role for Ca(2+)-conducting ion channels in mechanically-induced signal transduction of airway epithelial cells. J C e ll S c i 1994;107:
232 136. Braiman A, Zagoory O, Priel Z. PKA induces Ca2+ release and enhances ciliary beat frequency in a Ca2+- dependent and -independent manner. A m J P h y s io l 1998;275:C Uzlaner N,.Priel Z. Interplay between the NO pathway and elevated calcium enhances ciliary activity in rabbit trachea. J P h y s io l 1999;516: Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. A m J P h y s io l 1998;275:L Zhang L,.Sanderson MJ. Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP. J P h y s io l 2003;546: Lansley AB, Sanderson MJ, Dirksen ER. Control o f the beat cycle o f respiratory tract cilia by Ca2+ and camp. A m J P h y s io l 1992;263:Pt l):l Di Benedetto G, Manara-Shediac FS, Mehta A. Effect o f cyclic AMP on ciliary activity o f human respiratory epithelium. E u r R e s p i r J 1991;4: Bonini NM,.Nelson DL. Differential regulation of Paramecium ciliary motility by camp and cgmp. J C e ll B io l 1988;106: W oodruff ML, Chaban VV, Worley CM, Dirksen ER. PKC role in mechanically induced Ca2+ waves and ATP-induced Ca2+ oscillations in airway epithelial cells. A m J P h y s io l 1999;276:L Poole AW, Pula G, Hers I, Crosby D, Jones ML. PKC-interacting proteins:from function to pharmacology. T r e n d s P h a r m S c i 2004;25:
233 145. Wong LB, Park CL, Yeates DB. Neuropeptide Y inhibits ciliary beat frequency in human ciliated cells via npkc, independently of PKA. A m J P h y s io l 1998;275:C Li D, Shirakami G, Zhan X, Johns RA. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. A m J R e s p ir C e ll M o l B io l 2000;23: Runer T,.Lindberg S. Ciliostimulatory effects mediated by nitric oxide. A c ta O to la r y n g o l 1999;119: Kim JW, Min YG, Rhee CS, Lee CH, Koh YY, Rhyoo C e t a l. Regulation of mucociliary motility by nitric oxide and expression o f nitric oxide synthase in the human sinus epithelial cells. L a r y n g o s c o p e 2001;111: Tamaoki J, Chiyotani A, Kondo M, Konno K. Role o f NO generation in beta- adrenoceptor-mediated stimulation o f rabbit airway ciliary motility. A m J P h y s io l 1995;268:C Zhan X, Li D, Johns RA. Expression o f endothelial nitric oxide synthase in ciliated epithelia of rats. J H is to c h e m C y to c h e m 2003;51: Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. B io c h B io p h y s R e s C o m m 1993;191: Morse DM, Smullen JL, Davis CW. Differential effects of UTP, ATP, and adenosine on ciliary activity o f human nasal epithelial cells. A m J P h y s io l C e ll P h y s io l 2001;280:C Verdugo P, Johnson NT, Tam PY. Beta-Adrenergic stimulation of respiratory ciliary activity. J A p p l P h y s io l: R e s p, E n v ir o n E x e r P h y s io l 1980;48:
234 154. Wong LB, Miller IF, Yeates DB. Regulation o f ciliary beat frequency by autonomic mechanisms: in vitro. J A p p l P h y s io l 1988;65: Yang B, Schlosser RJ, McCaffrey TV. Signal transduction pathways in modulation of ciliary beat frequency by methacholine. A n n O to l R h in o l L a r y n g o l 1997;106: Zagoory O, Braiman A, Priel Z. The mechanism of ciliary stimulation by acetylcholine: roles of calcium, PKA, and PKG. J G e n P h y s io l 2002;119: Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation o f airway surface liquid volume and composition. J G e n P h y s io l 2001 ;118: Widdicombe JH. Regulation o f the depth and composition o f airway surface liquid. J A n a t 2002;201: Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. P flu g e r A r c h - E u r J P h y s io l 2003;445: Boucher RC. Human airway ion transport. Part one. A m J R e s p ir C rit C a r e M e d 1994;150: Mercke U, Hakansson CH, Toremalm NG. A method for standardized studies of mucociliary activity. A c ta O to -L a r y n g o lo g ic a 1974;78.T Inchley O. A Simple Apparatus to Demonstrate activity o f Cilia. J P h y s io l 1921;54:cxxvii-cxxviii Dalhamn T,.Rylander R. Frequency of Ciliary Beat measured with a Photosensitive Cell. N a tu r e 1962;196:
235 164. Yager J, Chen TM, Dulfano MJ. Measurement of frequency o f ciliary beats of human respiratory epithelium. C h e s t 1978;73: Rutland J,.Cole PJ. Non-invasive sampling of nasal cilia for measurement o f beat frequency and study of ultrastructure. L a n c e t 1980;2: Teichtahl H, Wright PL, Kirsner RL. Measurement of in vitro ciliary beat frequency: a television- video modification of the transmitted light technique. M e d ic a l & B io lo g ic a l E n g in e e r in g & C o m p u tin g 1986;24: Chevance LG,.Lennon JF. [Studies of ciliary beating]. [French]. A c ta O to - L a r y n g o lo g ic a 1970;70: Rylander R. Current techniques to measure alterations in the ciliary activity of intact respiratory epithelium. [Review] [45 refs]. A m R e v R e s p D is 1966;93:Suppl: Dalhamn T. Ciliary motility studies. A r c h I n t M e d 1970;126: Ballenger JJ. Experimental effect o f cigarette smoke on human respiratory cilia. N E J M 1960;263: Dulfano MJ, Luk CK, Beckage M, Wooten O. Ciliary beat frequency in human respiratory explants. A m R e v R e s p D is 1981 ;123: Maurer DR, Sielczak M, Oliver WJr, Abraham WM, Wanner A. Role of ciliary motility in acute allergic mucociliary dysfunction. J A p p l P h y s io l : \9 S 2 ; 5 2 : Wanner A, Maurer D, Abraham WM, Szepfalusi Z, Sielczak M. Effects of chemical mediators o f anaphylaxis on ciliary function. J A C 1 : 1983;72:
236 174. Gilain L, Zahm JM, Pierrot D, Fuchey C, Peynegre R, Puchelle E. Nasal epithelial cell culture as a tool in evaluating ciliary dysfunction. A c ta O to -L a r y n g o lo g ic a 1993;113: Bernstein JM, Hard R, Cui ZD, So N, Fisher J, Ogra PL. Human adenoidal organ culture: a model to study nontypable Haemophilus influenzae (NTHI) and other bacterial interactions with nasopharyngeal mucosa implications in otitis media. O to la r y n g o l - H e a d & N e c k S u r g 1990;103:( Pt 1)): Rutland J, Griffin W, Cole PJ. An in vitro model for studying the effects of pharmacological agents on human ciliary beat frequency: effects of lignocaine. B r J C lin P h a r m a c o l: 1982;13: Devalia JL, Sapsford RJ, Wells CW, Richman P, Davies RJ. Culture and comparison of human bronchial and nasal epithelial cells in vitro. R e s p ir M e d 1990;84: Low PM, Luk CK, Dulfano MJ, Finch PJ. Ciliary beat frequency o f human respiratory tract by different sampling techniques. A m R e v R e s p D is 1984;130: Jorissen M,.Bessems A. Normal ciliary beat frequency after ciliogenesis in nasal epithelial cells cultured sequentially as monolayer and in suspension. A c ta O to la r y n g o l. 1995;115: Ingels KJ, Kortmann MJ, Nijziel MR, Graamans K, Huizing EH. Factors influencing ciliary beat measurements. R h in o lo g y 1991;29: MacCormick J, Robb I, Kovesi T, Carpenter B. Optimal biopsy techniques in the diagnosis of primary ciliary dyskinesia. J O to la r y n g o l 2002;31:
237 182. Rutland J, Griffin W, Cole P. Nasal brushing and measurement of ciliary beat frequency. An in vitro method for evaluating pharmacologic effects on human cilia. C h e s t 1981;80: Michaelson ED,.Serafind SM. Quantitative differences in the cellular yield o f two bronchial brushes. A m R e v R e s p ir D is 1975;112: Hanson FN,.Wesselius LJ. Effect o f bronchial brush size on cell recovery. A m R e v R e s p ir D is 1987;136: Bonin SR, Phillips PP, McCaffrey TV. The effect o f arachidonic acid metabolites on the ciliary beat frequency o f human nasal mucosa in vitro. A c ta O to - L a r y n g o lo g ic a 1992;112: Han LY, Wilson R, Slater S, Rutman A, Read RC, Snell NJ e t a l. In vitro and in vivo effects o f ribavirin on human respiratory epithelium. T h o r a x 1990;45: Rutland J, Dewar A, Cox T, Cole P. Nasal brushing for the study o f ciliary ultrastructure. J C lin P a th o l 1982;35: Carson JL, Collier AM, Hu SS. Acquired ciliary defects in nasal epithelium of children with acute viral upper respiratory infections. N E. J M 1985;312: Pedersen M, Sakakura Y, Winther B, Brofeldt S, Mygind N. Nasal mucociliary transport, number of ciliated cells, and beating pattern in naturally acquired common colds. E u r J R e s p ir D is S u p p l 1983;128: Rautiainen M, Nuutinen J, Kiukaanniemi H, Collan Y. Ultrastructural changes in human nasal cilia caused by the common cold and recovery of ciliated epithelium. A n n O to l R h in o l L a r y n g o l 1992;101:
238 191. Bisgaard H,.Pedersen M. SRS-A leukotrienes decrease the activity o f human respiratory cilia. C lin A lle r g y 1987;17: Raphael JH, Selwyn DA, Mottram SD, Langton JA, O Callaghan C. Effects o f 3 MAC of halothane, enflurane and isoflurane on cilia beat frequency o f human nasal epithelium in vitro. B r J A n a e s th 1996;76: Raphael JH, Strupish J, Selwyn DA, Hann HC, Langton JA. Recovery of respiratory ciliary function after depression by inhalation anaesthetic agents: an in vitro study using nasal turbinate explants. B r J A n a e s th 1996;76: Jackson AD, Rayner CF, Dewar A, Cole PJ, Wilson R. A human respiratory- tissue organ culture incorporating an air interface. A m J R e s p C r it C a r e M e d 1996;153: Bleeker JD,.Hoeksema PE. A simple method o f measure the ciliary beat rate of respiratory epithelium. A c ta O to - L a r y n g o lo g ic a 1971;71: Corssen G,.Allen CR. A Comparison o f the toxic effects of various local Anaesthetic drugs on human ciliated epithelium B io lo g y a n d M e d ic in e 1958; 16: in vitro. T e x a s R e p o r ts o n 197. Fujiwara K, Hakansson CH, Toremalm NG. Studies on the physiology o f the trachea. VI. Interaction between ciliary beat frequency and transport of secretions. A n n O to l R h in o l & L a iy n g o l 1972;81: Selwyn DA, Gyi A, Raphael JH, Key A, Langton JA. A perfusion system for in vitro measurement o f human cilia beat frequency. B r J A n a e s th 1996;76: Dvorak JA,.Stotler WF. A controlled-environment culture system for high resolution light microscopy. E x p e r C e ll R e s 1971;68:
239 200. Lindemann J, Leiacker R, Rettinger G, Keck T. Nasal mucosal temperature during respiration. C lin O to la r y n g o l A l l i e d S c i 2002;27: Ballenger JJ,.Orr MF. Quantitative Measurement o f human ciliary activity. A n n O to l, R h in o l & L a r y n g o l 1963;72: Greenstone M, Logan-Sinclair R, Cole PJ. An automated method for recording of ciliary beat frequency. I R C S M e d S c i 1984;12: Gyi A, O'Callaghan C, Langton JA. Effect of halothane on cilia beat frequency of ciliated human respiratory epithelium in vitro. B r J A n a e s th 1994;73: Selwyn DA, Raphael JH, Lambert DG, Langton JA. Effects of morphine on human nasal cilia beat frequency in vitro. B r J A n a e s th 1996;76: Romet S, Schoevaert D, Marano F. Dynamic image analysis applied to the study of ciliary beat on cultured ciliated epithelial cells from rabbit trachea. B io l C e ll 1991;71: Lee WI,.Verdugo P. Laser light-scattering spectroscopy: a new application in the study of ciliary activity. B io p h y s J 1976;16: Lale AM, Mason JD, Jones NS. Mucociliary transport and its assessment: a review. C lin O to la r y n g o l 1998;23: Green A, Austin PR, Logan A, Smallman LA, Drake-Lee AB. Computerized measurement of ciliary beat frequency. J M e d E n g in & T e c h 1993;17: Toremalm NG, Hakansson CH, Mercke U, Dahlerus B. Mucociliary wave pattern. An analysis of surface light reflections. A c ta O to -L a r y n g o lo g ic a 1974;78: Sanderson MJ,.Dirksen ER. Quantification of ciliary beat frequency and metachrony by high- speed digital video. M e th o d s C e ll B io l 1995;47:
240 211. Braga PC. In vitro observation and counting methods for ciliary motion. In Braga PC AL, ed. M e th o d s in b r o n c h ia l m u c o /o g y, pp Raven Press Ltd, Ingels KJ, van Strien HL, Graamans K, Smoorenburg GF, Huizing EH. A study of the photoelectrical signal from human nasal cilia under several conditions. A c ta O to -L a r y n g o lo g ic a 1992;112: Verdugo P, Hinds TR, Vincenzi FF. Laser light-scattering spectroscopy: preliminary results on bioassay o f cystic fibrosis factor(s). P e d R e s 1979;13: Zorgniotti AW, Hotchkiss RS, Wall LC. High-speed cinephotomicrography of human spermatozoa. M e d ic a l r a d io g r a p h y a n d p h o to g r a p h y 1958;2: Nasr G, Schoevaert D, Marano F, Venant A, Legrand JJ. Progress in the measurement of ciliary beat frequency by automated image analysis: application to mammalian tracheal epithelium. A n a l. C e ll P a th o l. 1995;9: Chilvers MA, Rutman A, O Callaghan C. Ciliary beat pattern is associted with specific ultrastructural defects in prim ary ciliary dyskinesia. J A C I 2003;112: Zahm JM, Lamiot E, Pierrot D, Chevillard M, Hinnrasky J, Puchelle E. Quantitation of in vitro ciliated cell growth through image analysis. In V itro C e ll D e v B io l 1990;26: Siewert AK. Uber einen fal von bronchiectasie bei einen Patienten mit situs inversus viscerum. B e r t K lin W o c h e n s c h r 1904;41: Sleigh MA. Primary ciliary dyskinesia. L a n c e t 1981 ;2:
241 220. Schidlow DV. Primary ciliary dyskinesia (the immotile cilia syndrome). A n n A lle r g y 1994;73: Afzelius BA. A human syndrome caused by immotile cilia. S c ie n c e 1976;193: Pedersen H,.Mygind N. Absence o f axonemal arms in nasal mucosa cilia in Kartagener's syndrome. N a tu r e 1976;262: Eliasson R, Mossberg B, Camner P, Afzelius BA. The immotile-cilia syndrome. A congenital ciliary abnormality as an etiologic factor in chronic airway infections and male sterility. N E J M 1977;297: Afzelius BA. Genetics and pulmonary medicine. 6. Immotile cilia syndrome: past, present, and prospects for the future. T h o r a x 1998;53: Rossman CM, Forrest JB, Lee RM, Newhouse MT. The dyskinetic cilia syndrome. Ciliary motility in immotile cilia syndrome. C h e s t 1980;78: Pedersen M,.Mygind N. Ciliary motility in the 'immotile cilia syndrome'. First results o f microphoto-oscillographic studies. B r J D is C h e s t 1980;74: Bush A, Cole P, Hariri M, Mackay I, Phillips G, O'Callaghan C e t a l. Primary ciliary dyskinesia: diagnosis and standards of care. E u r R e s p ir J 1998;12: Sturgess JM,.Turner JA. Ultrastructural pathology of cilia in the immotile cilia syndrome. P e r s p P e d.p a th o l. 1984;8: Chao J, Turner JA, Sturgess JM. Genetic heterogeneity of dynein-deficiency in cilia from patients with respiratory disease. A m R e v R e s p ir D is 1982;126:
242 230. Levison H, M indorff CM, Chao J, Turner JA, Sturgess JM, Stringer DA. Pathophysiology of the ciliary motility syndromes. E u r J R e s p ir D is S u p p l 1983;127: Torkkeli T, Rautiainen M, Nuutinen J. Long-term follow-up of the clinical relevance of short outer dynein arms in human nasal cilia. E u r A r c h O to r h in o la r v n g o l 1998;255: Becker B, Morgenroth K, Reinhardt D, Irlich G. The dyskinetic cilia syndrome in childhood. Modifications o f ultrastructural patterns. R e s p ir a tio n 1984; 46: Jorissen M, Willems T, Van der SB, Verbeken E, De Boeck K. Ultrastructural expression of primary ciliary dyskinesia after ciliogenesis in culture. A c ta O to r h in o la r v n g o l.b e lg. 2000;54: Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA e t a l. Polarity proteins control ciliogenesis via kinesin motor interactions. C u r r B io l 2004;14: Fricke B, Stewart GW, Trehame KJ, Mehta A, Knopfle G, Friedrichs N e t a l. Stomatin immunoreactivity in ciliated cells of the human airway epithelium. A n a t E m b r y o l 2003;207: Greenstone M, Rutman A, Dewar A, Mackay I, Cole PJ. Primary ciliary dyskinesia: cytological and clinical features. Q. J. M e d 1988; 67: Neustein HB, Nickerson B, O'Neal M. Kartagener's syndrome with absence of inner dynein arms o f respiratory cilia. A m R e v R e s p ir D is 1980;122: Escalier D, Jouannet P, David G. Abnormalities of the ciliary axonemal complex in children: an ultrastructural and cinetic study in a series of 34 cases. B io l C e ll 1982;44:
243 239. Sturgess JM, Chao J, Turner JA. Transposition of ciliary microtubules: another cause o f impaired ciliary motility. N E J M 1980;303: Rutman A, Cullinan P, Woodhead M, Cole PJ, Wilson R. Ciliary disorientation: a possible variant o f primary ciliary dyskinesia. T h o r a x 1993;48: Sturgess JM, Chao J, Wong J, Aspin N, Turner JA. Cilia with defective radial spokes: a cause o f human respiratory disease. N E J M 1979;300: Antonelli M, Modesti A, De Angelis M, Marcolini P, Lucarelli N, Crifo S. Immotile cilia syndrome: radial spokes deficiency in a patient with Kartagener's triad. A c ta P a e d ia tr S c a n d 1981 ;70: Richard S, Nezelof C, Pfister A, de Blic J, Scheinmann P, Paupe J. Congenital ciliary aplasia in two siblings. A primitive disregulation o f ciliogenesis? P a th o l R e s & P r a c. 1989;185: DeBoeck K, Jorissen M, Wouters K, Van der Schueren B, Eyssen M, Casteels- VanDaele M e t a l. Aplasia of respiratory tract cilia. P e d ia tr P u lm o n o l 1992;13: Afzelius BA, Gargani G, Romano C. Abnormal length o f cilia as a possible cause o f defective mucociliary clearance. E u r J R e s p D is 1985;66: Niggemann B, Muller A, Nolte A, Schnoy N, Wahn U. Abnormal length o f cilia a cause of primary ciliary dyskinesia a case report. E u r J P e d ia tr 1992;151: Rautiainen M, Nuutinen J, Collan Y. Short nasal respiratory cilia and impaired mucociliary function. E u r A r c h O to -R h in o -L a r y n g o lo g y 1991;248:
244 248. Lungarella G, De Santi MM, Palatresi R, Tosi P. Ultrastructural observations on basal apparatus of respiratory cilia in immotile cilia syndrome. E u r J R e s p D is. 1985;66: Rossman CM,.Newhouse MT. Primary ciliary dyskinesia: evaluation and management. P e d ia tr P u lm o n o l. 1988;5: Narayan D, Krishnan SN, Upender M, Ravikumar TS, Mahoney MJ, Dolan Jr TF e t a l. Unusual inheritance o f primary ciliary dyskinesia (Kartagener's syndrome). J M e d G e n e t 1994;31(6): Rott HD. Genetics of Kartagener's syndrome. E u r J R e s p ir D is S u p p l 1983;127: Chilvers MA OC. Local mucociliary defence mechanisms. P a e d ia tr R e s p R e v 2000;1: Sturgess JM, Thompson MW, Czegledy-Nagy E, Turner JA. Genetic aspects of immotile cilia syndrome. A m J M e d G e n e t 1986;25: Blouin JL, Meeks M, Radhakrishna U, Sainsbury A, Gehring C, Sail GD e t a l. Primary ciliary dyskinesia: a genome-wide linkage analysis reveals extensive locus heterogeneity. E u r J H u m G e n e t 2000;8: Bush A. Primary ciliary dyskinesia. A c ta O to r h in o la r y n g o l B e lg 2000;54: Ellerman A,.Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. E u r R e s p ir J 1997;10(10): Rutland J, et al. Diagnosis of primary ciliary dyskinesia. In Baum GL Priel Z, Roth Y, Liron N, Ostfeld E. ed. C ilia, m u c u s, a n d m u c o c ilia r y in te r a c tio n s., pp New York: Marcell Decker,
245 258. Meeks M, Walne A, Spiden S, Simpson H, Mussaffi-Georgy H, Hamam HD e t al. A locus for primary ciliary dyskinesia maps to chromosome 19q. J M e d G e n e t 2000;37: Zariwala M, Noone PG, Sannuti A, Minnix S, Zhou Z, Leigh MW e t a l. Germline mutations in an intermediate chain dynein cause primary ciliary dyskinesia. A m J R e s p ir C e ll M o l B io l 2001 ;25: Bartoloni L, Blouin JL, Pan Y, Gehrig C, Maiti AK, Scamuffa N e t a l. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. P r o c N a tl A c a d S c i U S A 2002;99: Guichard C, Harricane MC, Lafitte JJ, Godard P, Zaegel M, Tack V e t a l. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome). A m J H u m G e n e t 2001;68: Olbrich H, Haffner K, Kispert A, Volkel A, Volz A, Sasmaz G e t a l. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. N a t G e n e t 2002;30: Zhang YJ, O Neal WK, Randell SH, Blackburn K, Moyer MB, Boucher RC e t a l. Identification of dynein heavy chain 7 as an inner arm component of human cilia that is synthesized but not assembled in a case of primary ciliary dyskinesia. J B io l C h e m 2002;277: Turner JA, Corkey CW, Lee JY, Levison H, Sturgess J. Clinical expressions of immotile cilia syndrome. P e d ia tr ic s 1981;67: Coren ME, Meeks M, Morrison I, Buchdahl RM, Bush A. Primary ciliary dyskinesia: age at diagnosis and symptom history. A c ta P a e d ia tr 2002;91:
246 266. Hadfield PJ, Rowe-Jones JM, Bush A, Mackay IS. Treatment of otitis media with effusion in children with primary ciliary dyskinesia. C lin O to la ry m g o l 1997;22: Meeks M,.Bush A. Primary ciliary dyskinesia. C u r r e n t P a e d ia tr ic s 1998; 8: Holzmann D,.Felix H. Neonatal respiratory distress syndrome a sign of primary ciliary dyskinesia? E u r J P e d ia tr 2000;159: Whitelaw A, Evans A, Corrin B. Immotile cilia syndrome: a new cause of neonatal respiratory distress. A r c h D is C h ild 1981;56: Bromiker R, Neeman Z, Bar-Oz B, Avital A, Bar-Ziv J, Springer C. Early diagnosis of primary ciliary dyskinesia in a newborn without situs inversus. A c ta P a e d ia tr 2002;91: al-shroof M, Kamik AM, Kamik AA, Longshore J, Sliman NA, Khan FA. Ciliary dyskinesia associated with hydrocephalus and mental retardation in a Jordanian family. M a y o C lin P r o c 2001;76: Greenstone MA, Jones RW, Dewar A, Neville BG, Cole PJ. Hydrocephalus and primary ciliary dyskinesia. A r c h D is C h ild. 1984;59: Zammarchi E, Calzolari C, Pignotti MS, Pezzati P, Lignana E, Cama A. Unusual presentation of the immotile cilia syndrome in two children. A c ta P a e d ia tr ic a. 1993;82: De Santi MM, Magni A, Valletta EA, Gardi C, Lungarella G. Hydrocephalus, bronchiectasis, and ciliary aplasia. A r c h D is C h ild. 1990;65: Roth Y, Baum GL, Tadmor R. Brain dysfunction in primary ciliary dyskinesia? A c ta N e u r o lo g ic a.s c a n d in a v ic a. 1988;78(5):
247 276. Horvath I, Loukides S, Wodehouse T, Csiszer E, Cole PJ, Kharitonov SA e t a l. Comparison of exhaled and nasal nitric oxide and exhaled carbon monoxide levels in bronchiectatic patients with and without primary ciliary dyskinesia. T h o r a x 2003;58: Narang I, Ersu R, Wilson NM, Bush A. Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. T h o r a x 2002;57: Karadag B, James AJ, Gultekin E, Wilson NM, Bush A. Nasal and lower airway level of nitric oxide in children with primary ciliary dyskinesia. E u r R e s p ir J 1999;13: Karadag B, James AJ, Gultekin E, Wilson NM, Bush A. Nasal and lower airway level of nitric oxide in children with primary ciliary dyskinesia. E u r R e s p ir J 1999;13: Koh YY, Sun YH, Min YG, Chi JG, Kim CK. Chemotaxis of Blood Neutrophils from Patients with Primary Ciliary Dyskinesia. J K o r e a n M e d S c i 2002;18: Fiorini R, Littarru GP, Coppa GV, Kantar A. Plasma membrane polarity of polymorphonuclear leucocytes from children with primary ciliary dyskinesia. E u r J C lin In v e s t 2000;30: Knudsen BB, Valerius NH, Pedersen M. Neutrophil function in primary cilia dyskinesia. E u r J R e s p ir D is S u p p l 1983;128 (Pt 2): Ozgen B, Haliloglu M, Tuncer G. A 7-year-old boy with dextrocardia and dysphagia. E u r J P e d ia tr 2000;159: Engesaeth VG, Warner JO, Bush A. New associations o f primary ciliary dyskinesia syndrome. P e d ia tr.p u lm o n o l. 1993;16:
248 285. Pedersen M,.Stafanger G. Bronchopulmonary symptoms in primary ciliary dyskinesia. A clinical study of 27 patients. E u r J R e s p ir D is S u p p I 1983;127: Saeki H, Kondo S, Morita T, Sasagawa I, Ishizuka G, Koizumi Y. Immotile cilia syndrome associated with polycystic kidney. J. U r o l 1984;132: Gershoni-Baruch R, Gottfried E, Pery M, Sahin A, Etzioni A. Immotile cilia syndrome including polysplenia, situs inversus, and extrahepatic biliary atresia. A m J M e d G e n e t 1989;33: Afzelius BA. Situs inversus and ciliary abnormalities. What is the connection? In t J D e v B io l 1995;39: Noone PG, Bali D, Carson JL, Sannuti A, Gipson CL, Ostrowski LE e t a l. Discordant organ laterality in monozygotic twins with primary ciliary dyskinesia. A m J M e d G e n e t 1999;82 : Stem CD. Embryology: fluid flow and broken symmetry. N a tu r e 2002;418: Brueckner M. Cilia propel the embryo in the right direction. A m J M e d G e n e t 2001;101: Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. M o l C e ll 1999;4: Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning o f the mouse embryo by artificial nodal flow. N a tu r e 2002;418: Chapelin C, Coste A, Reinert P, Boucherat M, Millepied M-C, Poron F e t a l. Incidence of primary ciliary dyskinesia in children with recurrent respiratory diseases. A n n O to l R h in o l & L a r y n g o l. 1997;106:
249 295. Jorissen M, Willems T, Van der Schueren B. Ciliary function analysis for the diagnosis of primary ciliary dyskinesia: advantages of ciliogenesis in culture. A c ta O to la r y n g o l 2000;120: Amjad H, Richburg FD, Adler E. Kartagener syndrome. Case report in an elderly man. J A M A 1974;227: Perraudeau M, Scott J, Walport M, Oakley C, Bloom S, Brooks D. Late presentation o f Kartagener's syndrome. Consequences o f ciliary dysfunction. B M J 1994;308: Canciani M, Barlocco EG, Mastella G, De Santi MM, Gardi C, Lungarella G. The saccharin method for testing mucociliary function in patients suspected o f having primary ciliary dyskinesia. P e d ia tr P u lm o n o l. 1988;5: Wodehouse T, Kharitonov SA, Mackay IS, Barnes PJ, Wilson R, Cole PJ. Nasal nitric oxide measurements for the screening of primary ciliary dyskinesia. E u r R e s p ir J 2003;21: Bush A,.O'Callaghan C. Primary ciliary dyskinesia. A r c h D is C h ild 2002; 87: Rutland J, Cox T, Dewar A, Cole P. Screening for ciliary dyskinesia - a spectrum of defects of motility and structure. E u r J R e s p ir D is - S u p p l 1983;127: Teknos TN, Metson R, Chasse T, Balercia G, Dickersin GR. New developments in the diagnosis of Kartagener's syndrome. O to la r y n g o l H e a d N e c k S u r g 1997;116: Holzmann D, Ott PM, Felix H. Diagnostic approach to primary ciliary dyskinesia: a review. E u r J P e d ia tr 2000;159:
250 304. Santamaria F, de Santi MM, Grillo G, Samelli P, Caterino M, Greco L. Ciliary motility at light microscopy: a screening technique for ciliary defects. A c ta P a e d ia tr 1999;88: Phillips GE, Thomas S, Heather S, Bush A. Airway response of children with primary ciliary dyskinesia to exercise and beta2-agonist challenge. E u r R e s p ir J 1998;11: Koh YY, Park Y, Jeong JH, Kim CK, Min YG, Chi JG. The effect of regular salbutamol on lung function and bronchial responsiveness in patients with primary ciliary dyskinesia. C h e s t 2000;117: Hellinckx J, Demedts M, De Boeck K. Primary ciliary dyskinesia: evolution of pulmonary function. E u r J P e d ia tr 1998;157: ten Berge M, Brinkhorst G, Kroon AA, de Jongste JC. DNase treatment in primary ciliary dyskinesia assessment by nocturnal pulse oximetry. P e d ia tr P u lm o n o l 1999;27: Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M e t a l. Primary ciliary dyskinesia: Diagnostic and phenotypic features. A m J R e s p i C r it C a re M e d 2004;169: Munro NC, Currie DC, Lindsay KS, Ryder TA, Rutman A, Dewar A e t a l. Fertility in men with primary ciliary dyskinesia presenting with respiratory infection. T h o r a x 1994;49: Kay VJ,.Irvine DS. Successful in-vitro fertilization pregnancy with spermatozoa from a patient with Kartagener's syndrome: case report. H u m R e p r o d 2000;15:
251 312. Cayan S, Conaghan J, Schriock ED, Ryan IP, Black LD, Turek PJ. Birth after intracytoplasmic sperm injection with use of testicular sperm from men with Kartagener/immotile cilia syndrome. F e r til S te r il 2001;76: Eliyahu S,.Shalev E. A fertile woman with Kartagener's syndrome and three consecutive pregnancies. H u m R e p r o d 1996; 11: McComb P, Langley L, Villalon M, Verdugo P. The oviductal cilia and Kartagener s syndrome. F e r til S te r il 1986;46: Afzelius BA,.Eliasson R. Male and female infertility problems in the immotile- cilia syndrome. E u r J R e s p i r D is S u p p l 1983;127: Afzelius BA, Camner P, Mossberg B. On the function of cilia in the female reproductive tract. F e r til S te r il 1978;29: Parker GS, Mehlum DL, Bacher-Wetmore B. Ciliary dyskinesis: the immotile cilia syndrome. L a r y n g o s c o p e 1983;93: Halbert SA, Patton DL, Zarutskie PW, Soules MR. Function and structure of cilia in the fallopian tube o f an infertile woman with Kartagener s syndrome. H u m R e p r o d 1997;12: Thijssens K, Van Schil P, Van den Brande F, Stappaerts I, Eyskens E. Left middle lobe resection for bronchiectasis in Kartagener s syndrome. A c ta C h ir B e lg 1999;99: Homma S, Kawabata M, Kishi K, Tsuboi E, Narui K, Nakatani T e t a l. Bronchiolitis in Kartagener's syndrome. E u r R e s p ir J 1999;14:
252 321. Date H, Yamashita M, Nagahiro I, Aoe M, Andou A, Shimizu N. Living-donor lobar lung transplantation for primary ciliary dyskinesia. A n n T h o r a c S u r g 2001;71: O Callaghan, C. Brain ependymal cilia Leicester, Leicester Buchdahl RM, Reiser J, Ingram D, Rutman A, Cole PJ, Warner JO. Ciliary abnormalities in respiratory disease. A r c h D is C h ild 1988;63: Willems T,.Jorissen M. Correlations between ciliary structure and ciliary function. A c ta O to r h in o la r y n g o l B e lg 2000;54: Mygind N, Pedersen M, Nielsen MH. Primary and secondary ciliary dyskinesia. A c ta O to la r y n g o l 1983;95: Jorissen M, Willems T, Van der SB, Verbeken E. Secondary ciliary dyskinesia is absent after ciliogenesis in culture. A c ta O to r h in o la r y n g o l.b e lg. 2000;54: Boat TF,.Carson JL. Ciliary dysmorphology and dysfunction primary or acquired? N E J M 1990;323: Giorgi PL, Oggiano N, Braga PC, Catassi C, Gabrielli O, Coppa GV e t a l. Cilia in children with recurrent upper respiratory tract infections: ultrastructural observations. P e d ia tr P u lm o n o l 1992; 14: Corbeel L, Comillie F, Lauweryns J, Boel M, van den Berghe G. Ultrastructural abnormalities of bronchial cilia in children with recurrent airway infections and bronchiectasis. A r c h D is C h ild 1981;56: de Iongh RU,.Rutland J. Ciliary defects in healthy subjects, bronchiectasis, and primary ciliary dyskinesia. A m JR esp & C r it C a r e M e d 1995;151:
253 331. van der Baan S, Veerman AJ, Bezemer PD, Feenstra L. Primary ciliary dyskinesia: quantitative investigation of the ciliary ultrastructure with statistical analysis. A n n O to l R h in o l & L a r y n g o l. 1987;96: Wisseman CL, Simel DL, Spock A, Shelburne JD. The prevalence of abnormal cilia in normal pediatric lungs. A r c h P a th o l L a b M e d 1981;105: Smallman LA,.Gregory J. Ultrastructural abnormalities of cilia in the human respiratory tract. H u m P a th o l 1986;17: Ehouman A, Pinchon MC, Escudier E, Bemaudin JF. Ultrastructural abnormalities of respiratory cilia. Descriptive and quantitative study of respiratory mucosa in a series of 33 patients. V ir c h o w s A r c h iv (B ). 1994;1: Verra F, Fleury-Feith J, Boucherat M, Pinchon MC, Bignon J, Escudier E. Do nasal ciliary changes reflect bronchial changes? An ultrastructural study. A m R e v R e s p D i s 1993;147: Jorissen M, Willems T, Van der Schueren B. Nasal ciliary beat frequency is age independent. L a r y n g o s c o p e 1998;108: Hann HC, Hall AP, Raphael JH, Langton JA. An investigation into the effects of midazolam and propofol on human respiratory cilia beat frequency in vitro. I n te n s iv e C a r e M e d 1998;24: Tsang KW, Rutman A, Tanaka E, Lund V, Dewar A, Cole PJ e t a l. Interaction of Pseudomonas aeruginosa with human respiratory mucosa in vitro. E u r R e s p ir J 1994;7: Robson AM, Smallman LA, Gregory J, Drake-Lee AB. Ciliary ultrastructure in nasal brushings. C y to p a th o l. 1993;4:
254 340. Jorissen M, Willems T, Van der SB, Verbeken E. Dynein arms and spokes after ciliogenesis in cultured respiratory epithelial cells from non-pcd individuals [In Process Citation]. A c ta O to r h in o la r y n g o l.b e lg. 2000;54 : Escudier E, Couprie M, Duriez B, Roudot-Thoraval F, Millepied MC, Pruliere- Escabasse V e t a l. Computer-assisted analysis helps detect inner dynein arm abnormalities. A m J R e s p ir C r it C a r e M e d 2002;166: Veale D, Rodgers AD, Griffiths CJ, Ashcroft T, Gibson GJ. Variability in ciliary beat frequency in normal subjects and in patients with bronchiectasis. T h o r a x 1993;48: Rossman CM, Forrest JB, Lee RM, Newhouse AF, Newhouse MT. The dyskinetic cilia syndrome; abnormal ciliary motility in association with abnormal ciliary ultrastructure. C h e s t 1981 ;80:(Suppl): Pedersen M. Specific types o f abnormal ciliary motility in Kartagener's syndrome and analogous respiratory disorders. A quantified microphoto- oscillographic investigation o f 27 patients. E u r J R e s p ir D is S u p p l 1983;127: Taylor HC, Satir P, Holwill ME. Assessment of inner dynein arm structure and possible function in ciliary and flagellar axonemes. C e ll M o til C y to s k e le to n 1999;43: Brokaw CJ,.Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. C e ll M o til C y to s k e le to n 1987;8: Omoto CK,.Kung C. The pair of central tubules rotates during ciliary beat in Paramecium. N a tu r e 1979;279:
255 348. Jorissen M,.Willems T. The secondary nature of ciliary (dis)orientation in secondary and primary ciliary dyskinesia. A c ta O to la r y n g o l 2004;124: Min YG, Shin JS, Choi SH, Chi JG, Yoon CJ. Primary ciliary dyskinesia: ultrastructural defects and clinical features. R h in o lo g y. 1995;33: Wilson R, Alton E, Rutman A, Higgins P, A1 Nakib W, Geddes DM e t a l. Upper respiratory tract viral infection and mucociliary clearance. E u r J R e s p D is 1987;70: Sakakura Y. Changes of mucociliary function during colds. E u r J R e s p ir D is S u p p l 1983;128: Sakakura Y, Sasaki Y, Homick RB. Mucociliary function during experimentally induced Rhinovirus infection in man. A n n O to la r y n g o l 1973;82: Bende M, Barrow I, Heptonstall J, Higgins PG, Al-Nakib W, Tyrrell DA e t al. Changes in human nasal mucosa during experimental coronavirus common colds. A c ta O to la r y n g o l 1989;107: Monto AS. Coronaviruses. In Evans AS, ed. V ira l in fe c tio n s in h u m a n s, pp New York: Plenium Book Company, Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L e t al. Community study of role o f viral infections in exacerbations o f asthma in 9-11 year old children. B M J 1995;310: Tyrrell DAJ, Bynoe ML. Cultivation of a novel type of common cold virus in organ cultures. BMJ 1965:
256 357. Macnaughton MR, Madge MH, Reed SE. Two antigenic groups of human coronaviruses detected by using enzyme- linked immunosorbent assay. I n fe c t Im m u n 1981;33: Mckean M.C. An investigation into the mechanism of viral induced wheezing in an experimental adult model Leicester, Leicester Myint S, Siddell S, Tyrrell D. Detection of human coronavirus 229E in nasal washings using RNA:RNA hybridisation. J M e d V ir o l 1989;29: Gwaltney JM Jr, Hendley O, Hayden FG, McIntosh K, Hollinger FB, Melnick JL e t a l. Updated recommendations for safety-testing o f viral inocula used in volunteer experiments on rhinovirus colds. P r o g M e d V iro l 1992;39: Johnston SL, Papi A, Bates PJ, Mastronarde JG, Monick MM, Hunninghake GW. Low grade rhinovirus infection induces a prolonged release of IL- 8 in pulmonary epithelium. J I m m u n o l 1998;160: Jackson GG, Dowling HF, Speisman IG, Boand AV. Transmission o f the common cold under controlled conditions. 1. The common cold as a clinical entity. A r c h In te r n M e d 1958;101: Mckean MC, Leech M, Lambert PC, Hewitt C, Myint S, Silverman M. A model of viral wheeze in nonasthmatic adults: symptoms and physiology. E u r R e s p ir J 2001;18: Hoorn B,.Tyrrell DA. Effects o f some viruses on ciliated cells. A m R e v R e s p D is. 1966;93:Suppl: Winther B, Brofeldt S, Christensen B, Mygind N. Light and scanning electron microscopy o f nasal biopsy material from patients with naturally acquired common colds. A c ta O to la r y n g o l 1984;97:
257 366. McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. P r o c N a t A c a d S c i U SA 1967;57: Evermann JF, Heeney JL, McKeiman AJ, O'Brien SJ. Comparative features of a coronavirus isolated from a cheetah with feline infectious peritonitis. V iru s R e s 1989;13: Collinson J, Nicholson KG, Cancio E, Ashman J, Ireland DC, Hammersley V e t a l. Effects of upper respiratory tract infections in patients with cystic fibrosis. T h o ra x 1996;51: Robertson A, Stannard W, Passant C, O'Callaghan C, Baneijee A. What effect does isoflurane have upon ciliary beat pattern: an in vivo study. C lin O to la r y n g o l A llie d S c i 2004;29: Rutland J, Cole PJ. Nasal mucociliary clearance and ciliary beat frequency in cystic fibrosis compared with sinusitis and bronchiectasis. T h o r a x 1981;36: Stanley P, MacWilliam L, Greenstone M, Mackay I, Cole P. Efficacy of a saccharin test for screening to detect abnormal mucociliary clearance. B r J D is C h e s t 1984;78: Csoma Z, Bush A, Wilson NM, Donnelly L, Balint B, Barnes PJ e t a l. Nitric oxide metabolites are not reduced in exhaled breath condensate of patients with primary ciliary dyskinesia. C h e s t 2003; 124: Loukides S, Kharitonov S, Wodehouse T, Cole PJ, Barnes PJ. Effect of arginine on mucociliary function in primary ciliary dyskinesia. L a n c e t 1998;352:
258 374. Chin GY, Karas DE, Kashgarian M. Correlation of presentation and pathologic condition in primary ciliary dyskinesia. A r c h O to la r y n g o l H e a d N e c k S u r g 2002;128: Jorissen M, Willems T, De Boeck K. Diagnostic evaluation of mucociliary transport: from symptoms to coordinated ciliary activity after ciliogenesis in culture. A m J R h in o l 2000;14:
259 Appendix 1 258
260 Processing of nasal ciliated samples for electron microscopy Samples are fixed in 4% gluteraldehyde in (Sorensons) phosphate buffer. After 48 hours fixation the samples are centrifuged for 5 minutes at 750 rpm, re-suspended, and held in phosphate buffer. When processing is commenced samples are centrifuged for 5 minutes at 750 rpm and the cells rinsed in fresh buffer. This was repeated 3 times to remove traces of gluteraldehyde, centrifuged and then post fixed in 1 % osmium tetroxide (OSO4) for 60 minutes. The cells are then centrifuged and rinsed in distilled water 2 times. Following the second rinse the cells are centrifuged and the supernatant discarded. The samples were resuspended in 5 drops of 2 % liquid agar held in a water bath at 45 C. The cells are mixed and kept in the water bath for 5 minutes for equilibration. After this they are transferred to a centrifuge and spun at 1500 rpm for 5 minutes. This forms a pellet of cells set in the agar gel. The agar containing the cells is processed through to resin as shown in figure hours later, the samples are embedded in Beem capsules with fresh Araldite and placed in an oven at 60 C for 24 hours. The resin polymerises into a block containing the ciliated cells. The block is removed from the capsules and is ready for cutting. 259
261 Acetone Ethanol phase 10 minutes on 4-rpm rotator 50% ethanol 10 minutes on 4-rpm rotator 70% ethanol 5 minutes on 4-rpm rotator 70% ethanol 10 minutes on 4-rpm rotator 70% ethanol 10 minutes on 4-rpm rotator 90% ethanol 5 minutes on 4-rpm rotator 90% ethanol 10 minutes on 4-rpm rotator 90% ethanol 10 minutes on 4-rpm rotator 100% ethanol 5 minutes on 4-rpm rotator 100% ethanol 15 minutes on 4-rpm rotator 100% ethanol 15 minutes on 4-rpm rotator Acetone phase: Acetone 5 minutes on 4-rpm rotator Acetone 20 minutes on 4-rpm rotator Mixture of V2 acetone + V2 araldite : 30 minutes Araldite phase: Araldite medium mix Araldite medium mix 20 minutes Araldite medium mix overnight Figure 1: Processing stage from agar gel through to resin for ciliated cells 260
262 Measurement of ciliary orientation Ciliary orientation was examined by transmission electron microscopy (TEM) utilising digital image capture and image analysis. Strips of ciliated epithelium were examined at a magnification of 3,600X on a JEOL 100CX TEM. Fields of view were selected where at least 10 axonemal cross sections were seen with the central microtubular pair easily visualised and clearly originating from one cell. These images were captured at a screen magnification of 66,000x by an AMT Advantages HR CCD digital camera system (Deben UK Ltd) attached via the lower side ports of the TEM direct to a computer for analysis. The images were analysed using Scion Image for Windows based on NIH image by the National Institutes o f Health USA. For each ciliary cross section a line was electronically drawn through the central pair o f each cilium from left tubule to right. The angle of each line was measured by the computer (vertical up = 0 ; horizontal right = 90 ; and vertical down = 180 ). For each field of view the angle of each cilium was obtained and the standard deviation of the angles per cell calculated. The mean standard deviation o f the cells represented the overall measure o f ciliary disorientation for that sample. 261
263 Appendix 2 262
264 Primary Ciliary Dyskinesia Proforma 263
265 University Of Leicester: Department of Child Health Nasal Cilia Brush Biopsies Patient Details NAME: Date of Birth: ADDRESS: Age: MALE/ Referring Consultant: Birth History G estation A dmitted to Special C are U nit Y e s / N o O xygen requirement Y es / No V entilated Y e s / N o Symptoms RHINORRHOEA: A ge of O nset P ersistent Intermittent C olour C lear Y ellow G reen O ther T REATMENT ABX. Y es / No Which A ge of O nset COUGH; Start Cough W et / D ry C ontinuous Intermittent W orse D ay N IGHT N ature W et / D ry Productive Y e s / N o S putum C lear Y ellow G reen W heeze Yes / No D ay / N ight EAR INFECTION: Ear Infection Y es / No How O ften G lue Ear Y es / No G rommets Y es / No H earing Problems Y es / NO A %. i'arm'.k &i O'i.WL.LA \
266 EXERCISE E x e r c i s e : N ormal R e d u c e d N one L ast URTI: MEDICATIONS D etails P h y s i o t h e r a p y : Y es / N o F r e q u en c y O ther T r e a t m e n t : ALLERGIES A llergies Y es / N o D etails PAST MEDICAL HISTORY S y m pt o m s from B ir t h : Y es / N o N e o n atal C o u g h : Y es / No H y d r o c e p h a l u s : Y es / No D e v e l o p m e n t : G ross / F ine M otor D elay Y es / N o S ocial D elay Y es / N o L a n g u a g e D elay Y es / N o FAMILY HISTORY A sthm a Y es / N o WHO P rim ary C iliary D y sk in esia Y es / N o WHO B r o n c h ie c t a sis Y es / N o WHO O titis M edia Y es / N o WHO F irst C o u sin s Y es / N o Ethnic Origin In d ia n / P a k ist a n i / B a n g l a d e s h i / C a u c a s ia n / O ther
267 FAMILY TREE INVESTIGATIONS C hest X -R a y : N o rm al D e x t r o c a r d ia S itus In v e r su s S w eat T e s t : N orm al / A b n o r m a l Im m u n o g l o b u l in s N orm al / A b n o r m a l S u b c l a ss F u n c t io n a l A n t ib o d y R e spo nse C o m p l e m e n t EXAMINATION H e ig h t: W e ig h t : H ead C ir c u m f e r e n c e: In s p e c t io n : C a r d i o v a s c u l a r : R e s p i r a t o r y : ENT: ADDITIONAL REMARKS
268 Appendix 3 267
269 Clinical demographics of patients referred to a tertiary centre for the diagnosis of primary ciliary dyskinesia. Evaluation of a courier system for the transportation of nasal brush biopsies. Summary Aim: To retrospectively analyse clinical features of patients referred to Leicester primary ciliary dyskinesia diagnostic service and to develop a courier service for evaluation of nasal ciliary biopsies. Method: For all patients referred a proforma was completed and ciliary assessment performed. To determine if transportation would alter ciliary function samples from seven healthy controls were obtained and divided into two. Half of the sample was processed in the normal manner. The other half of the sample was placed into medium and stored in a flask containing ice. This was transported overnight and re-evaluated for beat frequency, beat pattern and ciliary ultrastructure. Results: 372 samples have been processed. The mean age o f diagnosis was 5.6 years. 47% of Asian referrals were found to have PCD (65% consanguineous parents) compared to 15.5% Caucasian referrals. For couriered samples no significant difference was observed for mean (95%CI) ciliary beat frequency between pre (11.6Hz (+/- 0.6)) and post transported (11.3Hz (+/- 1.1)) samples. A significant increase in secondary ciliary dyskinesia was observed after transportation. No difference was observed for ciliary ultrastructural parameters. Summary: A method has been evaluated for transporting samples of ciliated epithelium for use in the diagnosis of patients with PCD. Demographic data showed a high incidence o f primary ciliary dyskinesia in the Asian population. 268
270 Background to study There is estimated to be high proportion o f undiagnosed patients with primary ciliary dyskinesia " ' with late diagnosis often reported.*' A recent study found the median age o f diagnosis for primary ciliary dyskinesia to be 4 years, ranging up to 14 years. " Patients are frequently diagnosed after many years of chronic respiratory symptoms with established, and not infrequently, severe lung disease They may 7 f \ f \ have suffered high morbidity from inappropriate ENT intervention and have often undergone multiple invasive diagnostic procedures in an attempt to delineate their underlying problem. A delay in diagnosis results in progressive and permanent lung damage due to obstruction of airways with secretions and subsequent infection. However, Ellerman and Bisgaard have shown that following diagnosis, although lung function does not appear to recover, it can be maintained with specialist respiratory care over the 15 year period of their study.*- This emphasises the need for early diagnosis. 265;297 Making a diagnosis can be difficult with symptoms being non-specific. An infant with a persistent moist cough and runny nose is not an uncommon finding in the first year of life. Analysis of symptoms of patients with primary ciliary dyskinesia have been published and focus on the presence of sinusitis, serous otitis, bronchiectasis and situs inversus.229;236;253;2m:26w74 269
271 Standards of care and indications for biopsy have been published Even when a nasal brush biopsy is performed the effects of secondary damage may be difficult to interpret. * Only recently have age related reference ranges been published for healthy children to assist in diagnosis.62 Over the last 4 years Leicester Royal Infirmary Childrens Hospital has been acting as a supra-regional referral unit for diagnostic testing of children and adults suspected of having primary ciliary dyskinesia. Patients from all over the UK attend for assessment and nasal brush biopsy. The ciliary beat pattern and beat frequency were assessed directly using digital high-speed video analysis. Electron microscopy of the ciliary axoneme was performed on each sample. As travel precluded a number o f patients from attending for diagnostic sampling, we were keen to see if a courier system could be established. Aim 1) To develop a courier service for evaluation o f nasal ciliary biopsies. 2) To retrospectively analyse clinical features of patients referred to Leicester primary ciliary dyskinesia diagnostic service 270
272 Method A total of 372 patients were referred to the Leicestershire clinic for ciliary biopsy between For all patients referred, a proforma evaluating symptoms and features of primary ciliary dyskinesia had been completed by direct interview (Appendix 2). The data forms were analysed retrospectively and information transferred to a database (Microsoft Access 2000). In the proforma for sections where information was missing and could not be obtained from a referral letter or clinical notes an entry o f unknown was recorded. At the time of nasal brush biopsy all patients had been free from a symptomatic upper respiratory tract infection in the previous 6 weeks. No patients were taking regular medication or were known smokers. Ciliated samples were obtained in all patients by brushing the inferior nasal turbinate with a 2mm cytology brush. Nasal brushings were placed in Medium 199 (ph 7.3) which contained antibiotic solution (Streptomycin 50pg/ml, Penicillin 50pg/ml, Gibco U.K.). The sampling of nasal tissue for diagnostic purposes had received approval from the Leicestershire ethical review committee and written and verbal consent was obtained prior to sampling. Ciliary structure and function were evaluated as described in chapters 5 and 6. Measurements were made o f ciliary beat frequency and ciliary beat pattern was 271
273 evaluated. Ultrastrucutral analysis of microtubules and dynein arms was performed by transmission electron microscopy. Evaluation of a courier system Initially couriered samples were to be sent on ice overnight via the post. To determine if this would alter ciliary function samples from seven healthy controls were obtained and divided into two. Half of the sample was processed in the normal manner. The other half of the sample was placed into medium and stored in a flask containing ice. This was transported overnight and re-evaluated for beat frequency, beat pattern and ciliary ultrastructure. Ciliary beat pattern was assessed by scoring each edge using the method described in Chapter 9. Briefly, normal co-ordinated ciliary beating in a forward backward motion was scored as 0. Cilia that appeared to beat dyskinetically were scored from 1 to 3 depending on the extent o f abnormal beating along the edge (all cilia along an edge beating dyskinetically scored 3). 272
274 Statistical Analysis Data from the proforma was divided into patients with primary ciliary dyskinesia and those with chronic respiratory tract symptoms (respiratory controls). Results were expressed as the percentage with a positive response (yes) or data unknown. The mean ciliary beat frequency and 95% confidence intervals were calculated for the two groups. For samples which had been transported on ice the percentage of dynein arm and microtubular defects was calculated for both pre and post transportation. The mean ciliary beat frequency and 95% confidence intervals were calculated for the two groups. A paired t-test was performed to test for a significant difference. A Wilcoxon signed rank test was used to compare the dyskinetic beat pattern on Day 0 and Day
275 Results O f the 372 patients referred for ciliary assessment, it was not possible to make or exclude a diagnosis in 18 patients because of an inadequate or unhealthy ciliary sample. O f the remaining 354 patients (184 male, mean age 8 years, range 0 to 65), 76 (21.5%) were Asian. 79 (22.3%) patients were diagnosed with primary ciliary dyskinesia and could be 227 categorised into one of 5 recognised ultrastructural defects. The mean age of diagnosis was 5.61 years (range 0-18). The demographics of the patients with primary ciliary dyskinesia and chronic respiratory tract symptoms are shown in table 1. A high incidence of primary ciliary dyskinesia was observed in Asian patients referred (46.6%) when compared to the incidence in Caucasian referrals (15.5%). O f the patients referred from the British Asian community, 65.8% had consanguineous parents. 50% o f this population had the diagnosis o f primary ciliary dyskinesia confirmed. O f these Asian patients with primary ciliary dyskinesia 27 (73%) were from consanguineous marriages. When the Asian patients with consanguineous parents were excluded from analysis, only 13.7% had primary ciliary dyskinesia, an incidence similar to that of the Caucasian population. 274
276 The mean ciliary beat frequency ((SD), (95% Confidence Intervals)) for patients with either recurrent respiratory tract infections or primary ciliary dyskinesia was 12.4(( 1.9) 95% Cl: 12.1, 12.6) Hz and 5.2((5.2), 95% Cl: 3.1, 6.3) Hz respectively. All patients with primary ciliary dyskinesia (100%) exhibited an abnormal beat pattern which could be categorised into one of three beat patterns observed for patients with primary ciliary dyskinesia (chapter 7). 18% o f patients with chronic respiratory tract symptoms had evidence o f secondary ciliary dyskinesia. Tables 2-9 illustrate the clinical features o f the two groups. As expected patients with primary ciliary dyskinesia experienced chest, nasal symptoms. Ear symptoms were only observed in 50% of patients with primary ciliary dyskinesia. Features which differentiated patients with primary ciliary dyskinesia from those with recurrent respiratory tract infections were; ENT symptoms, with glue ear and grommets being more frequently reported. Neonatal symptoms were more commonly found. Patients with primary ciliary dyskinesia were more likely to have symptoms from birth and be admitted to the neonatal unit with respiratory distress requiring oxygen. Interestingly, although situs inversus was more common in patient s with primary ciliary dyskinesia it was only found in 27 (33%). Finally as expected a positive family history was also a good differentiator. Data recorded for examination and investigation was too incomplete for any conclusions to be drawn (Tables 8 and 9). 275
277 Results for couriered samples For couriered samples no significant difference was observed for mean ciliary beat frequency (95% Cl) between pre (1 1.6Hz (11.0, 12.2 Hz)) and post transported (1 1.3Hz (10.2, 12.4 Hz)) samples. No significant difference was observed for the percentage of cilia with dynein arm (Pre=1.9% (95%CI: 1.1, 2.7), Postal. 1% (95% Cl: 0.5, 1.7)) or microtubular (Pre=2.6% (95%CI: 1.7, 3.5), Post=2.4% (95%CI: 1.8, 3.0)) abnormalities. To assess ciliary dyskinesia each edge was scored (0=normal, 3=severely dyskinetic). A significant difference (P<0.02) was observed between the mean dyskinesia score (95%CI) for pre (0.2 (0.0, 0.4)) and post transported (1.5 (0.8, 2.2)) samples. 276
278 Primary Ciliary Dyskinesia (N=79) Respiratory Controls (N=275) N (%) Unknown N (%) Unknown Male 40 (50.6) 144 (52.4) Female 39 (49.4) 131 (47.6) Caucasian 42 (53.2) 229 (83.3) Asian 34 (43.0) 0 (0.0) 39(14.2) 7 (2.6) Other * 3 (3.8) 1st Cousins 27 (34.2) 9(11.4) 24 (8.7) 45 (16.4) Table 1: Demographics of patients referred for ciliary assessment (total number(n)) and percentage (%) of patients). *: three siblings from the Middle East Primary Ciliary Dyskinesia (N=79) Respiratory Controls (N=275) Yes (%) Unknown Yes (%) Unknown Chest Symptoms 72 (91.1) 5 (6.3) 237 (86.2) 30(10.9) Cough Nature Wet 63 (79.8) 9(1 1.4) 180 (65.5) 43(15.6) Dry 6 (7.6) 43(15.6) Wheeze 39 (49.4) 20 (25.3) 109 (39.6) 78 (28.4) Exercise Normal 47 (59.5) 10(12.7) 152 (55.3) 45 (16.4) Reduced 22 (27.9) 78 (28.4) Table 2: Prevalence of chest symptoms and type of symptom (Number of patients with each symptom (%)) in patients referred for ciliary assessment. 277
279 Primary Ciliary Dyskinesia (N=79) Respiratory Controls (N=275) Yes (%) Unknown Yes (%) Unknown Nasal Symptoms 66 (83.5) 7 (8.8) 177 (64.4) 3 7 (1 3.5 ) Rhinorhoea Persistent 45 (57.0) 1 0(12.7) 108 (39.3) 4 2 (1 5.3 ) Intermittent 17(21.5) 64 (23.3) Ear Symptoms 39 (49.4) 8 (1 0.1 ) 103 (37.5) 33 (12.0) Ear Infections 30 (38.0) 9 (1 1.4 ) 90 (32.7) 3 2 (1 1.6 ) Glue Ear 34 (43.0) 8 (1 0.1 ) 62 (22.6) 3 4 (1 2.4 ) Grommets 24 (30.4) 1 0 (1 2.7 ) 3 8 (1 3.6 ) 3 8 (1 3.8 ) Hearing Difficulty 25 (31.7) 1 0 (1 2.7 ) 4 8 (1 7.3 ) 4 0 (1 4.6 ) Table 3: Prevalence of nasal and ear symptoms and type of symptom (Number of patients with each symptom (%)) in patients referred for ciliary assessment. Primary Ciliary Dyskinesia (N=79) Respiratory Controls (N=275) Yes (%) Unknown Yes (%) Unknown Situs inversus 27 (34.2) 7 (8.9) 10(3.6) 3 9 (1 4.2 ) Dextrocardia Situs solitus 3 (3.8) 42 (53.2) 6 (2.2) (8 0 ) Table 4: Prevalence of situs inversus totalis (Number of patients and percentage (%)) in patients referred for ciliary assessment 278
280 Primary Ciliary Dyskinesia (N=79) Respiratory Controls (N=275) Yes (%) Unknown Yes (%) Unknown Antibiotics 36 (45.0) 7 (8.8) 108 (39.7) 3 2(11.8) Inhaled bronchodilator 41 (51.2) 7 (8.8) 107 (39.4) 34(12.5) Inhaled steroid 34 (42.5) 7 (8.8) 101 (37.1) 33 (12.1) Physiotherapy 42 (52.5) 8(10.0) 104 (38.2) 37(13.6) Table 5: Summary of treatment at time of assessment (number of patients taking each medication (%)) Primary Ciliary Dyskinesia (N=80) Respiratory Controls (N=272) Yes (%) Unknown Yes (%) Unknown Neonatal cough 48 (60.8) 7 (8.9) 84 (30.6) 37(13.5) Symptoms from birth 55 (69.6) 7 (8.9) 101 (36.7) 3 5(12.7) SCBU admission 41 (51.9) 20 (25.3) 56 (20.4) 56 (20.4) Ventilated 8(10.1) 23 (8.4) Oxygen 28 (35.4) 24 (8.7) Development Motor delay 6 (7.6) 10(12.7) 27 (9.8) 39(14.2) Language delay 11 (13.9) 8(10.1) 36(13.1) 40(14.6) Social delay 10(12.7) 9(11.4) 29(10.6) 40(14.6) Hydrocephalus 1 (1.3) 7 (8.9) 1 (0.4) 37(13.5) Table 6: Summary of neonatal history of patients referred for assessment (Number of patients and percentage (%)). 279
281 Primary Ciliary Dyskinesia (N =79) Respiratory Controls (N =272) Yes (%) Unknown Yes (%) Unknown Asthma 37 (46.8) 10(12.7) 121 (44.0) 38(13.8) Bronchiectasis 4(5.1) 9(11.4) 23 (8.4) 37(13.5) Primary ciliary dyskinesia 17(21.5) 8(10.1) 14(5.1) 34(12.4) Otitis media 7 (8.9) 11 (13.9) 28(10.2) 42(15.3) Table 7: Summary of family history for all patients (number of patients with positive family history for each condition (%)). Primary Ciliary Dyskinesia (N =79) Respiratory Controls (N = 275) Yes (%) Unknown Yes (%) Unknown Chest deformity 7 (8.8) 38 (47.5) 15(5.5) 135 (49.6) Finger clubbing 9(11.2) 36 (45.0) 5(1.8) 135 (49.6) Auscultatory crackles 12(15.0) 33 (41.3) 28(10.3) 131 (48.2) Wheeze 13(16.3) 33 (41.3) 11 (4.0) 131 (48.2) Glue ear 10(12.5) 39 (48.8) 12 (4.4) 137(50.4) Rhinorhoea 16(20.0) 40 (50.0) 15(5.9) 137(50.4) Table 8: Examination findings of patients referred for ciliary assessment (number of patients with each sign (%)) 280
282 Primary Ciliary Dyskinesia (N =79) Respiratory Controls (N =275) Yes (%) Unknown Yes (%) Unknown Chest radiograph 67 (83.8) 1 2(16.3) 221 (81.3) 4 8 (1 7.7 ) C T Chest scan 13(16.3) 51 (63.8) 77 (28.3) 159 (58.5) Sweat test 45 (56.3) 1 9(23.8) 179 (65.8) 73 (26.8) Immunoglobulins 27 (33.8) 37 (46.3) 170 (62.5) 90 (33.1) PH Probe 3 (3.8) 62 (77.5) 1 6 (5.9 ) 211 (77.5) Bronchoscopy 8 (1 0.0 ) 60 (75.0) 3 4 (1 2.5 ) 200 (73.5) Table 9: Summary of investigations performed on each patient at time of assessment (Number of patients having undergone each investigation (%) 281
283 Discussion The study evaluated symptoms and features of patients with primary ciliary dyskinesia and contrasted them with children with chronic respiratory tract symptoms. The pilot study showed that ciliated nasal epithelium could be obtained and couriered to allow evaluation of both ciliary structure and function. Increased ciliary dyskinesia was observed following transportation. Such a system will allow experienced chest physicians to be trained in the technique and will help reduce the traveling required by patients suspected o f having primary ciliary dyskinesia. In 1 in 5 children referred with a suspicion of primary ciliary dyskinesia the diagnosis was confirmed. It is possible that the high diagnostic rate may have been due to an initial referral pattern of patients strongly suspected of having primary ciliary dyskinesia. However, as mentioned earlier, it may also suggest that the diagnosis is missed in a large number o f patients.300 The incidence of primary ciliary dyskinesia would be expected to be higher in first cousin marriages given that the inheritance is autosomal recessive.253 This has not been reported before in the Asian community. The findings suggest that a high index of clinical suspicion of primary ciliary dyskinesia is required in Asian patients, particularly from first cousin marriages where nasal, chest and ear symptoms are present from early life. In particular, in this group o f patients the parents families initially originated from the 282
284 Meepa Region of Pakistan. It may be appropriate for the diagnosis to be considered at a much earlier stage in this patient group. The study tried to determine symptoms that may make identification of patients for referral for ciliary assessment easier. One drawback of the study was that demographic data was obtained retrospectively. A proportion o f patients had information which was unknown. A similar proportion of unknown data was seen for the two groups for each parameter evaluated. The unknown data was included in the analysis and this could potentially lead to an underestimate in the results obtained. In all patients, chest and nasal symptoms were predominant usually starting in the neonatal period. Ear symptoms were only found in under half of the patients diagnosed with primary ciliary dyskinesia. This reflects the findings of Greenstone et al. who documented a lower prevalence o f ear symptoms in patients with primary ciliary ' I 'l Z dyskinesia. Situs inversus appeared to be present in less than 50% of patients with primary ciliary dyskinesia. This may be due to the fact that none of the patients with ciliary transposition have situs inversus (chapter 7). Neonatal symptoms appear to be a good indicator for primary ciliary dyskinesia, with unexplained neonatal respiratory distress well recognised. " ' A study by Chin et al found that either chest, nasal or ear symptoms alone were poor indicators of primary ciliary dyskinesia. When more than one o f these features were present then this increased the diagnostic success
285 The preliminary courier study showed that ciliary transport allowed evaluation of ciliary ultrastructure and beat frequency. However, cilia appeared to beat more dyskinetically. This is attributed to the sample being transported on ice. Lee and colleagues studied nasal cilia obtained at postmortem. They found preservation of ciliary beat frequency, beat pattern and ultrastructure after death. Samples were stored at 4 C for a maximum of 66 hours with no change in any parameters for ciliary structure or function.96 Secondary ciliary dyskinesia will influence interpretation o f ciliary beta pattern and needs to be minimised. Therefore the study will need to be repeated couriering the samples at 4 C and analyzing on the same day as transportation. Summary We have documented a high incidence o f primary ciliary dyskinesia in patients referred, particularly in Asian patients from first cousin marriages. A pilot stiudy evaluating a courier system for assessment of ciliary structure and function has been conducted. A further study is needed to evaluate if transportation of samples under different conditions will reduce the degree o f secondary ciliary dyskinesia. 284
286 Appendix 4 285
287 Coronavirus cellular methods Cell culture and virus propagation. The virus inoculum was prepared by Mrs Stephanie Euden under the supervision of Professor Steven Myint (University of Leicester). LP strain HCoV 229E (American Type Culture Collection, Rockville, Maryland, USA) was cultured according to standards of good laboratory practice in human embryonic lung (HEL) cells as previously described359. HEL cells (Biowhittaker, Wokingham, UK.) were grown in Eagle s MEM medium (Gibco, UK) supplemented with 10% foetal bovine serum (Gibco), 2% o f nonessential aminoacids (Life Science Technologies, Paisley, Scotland), 50 pg m l'1 of penecillin and 50pg per m l'1 o f streptomycin and 1% fungizone (Gibco). Cells were cultured at 37 C, in a 5% CCL, 100% humidity atmosphere until confluent. Twenty-four hours before infection with HCoV 229E, the monolayers were washed with serum free medium. The medium was replaced with Eagle s MEM supplemented with 0.4% ultroser G (Gibco Life Technologies, Paisley, UK) and 2% amino acids. Cells were infected with HCoV 229E at a concentration o f 104 TCID50 m l'1 and cultured at 33 C, in 5% CCL. Virus was harvested from the cultures by filtration through a 4.5 micron filter and suspended in normal saline. After being aliquoted into 1 ml portions, inoculum was stored at -80 C and later tested for safety according to the Gwaltney criteria
288 Determination of titre. A TCID50 quantification of the titre of virus in the inoculum was conducted based on the principles of d Herelle using the mathematical model of Reed and Muench described above358. This is a standard virology technique and not fully described here. Briefly, cultures of HEL cells were grown on flat bottomed 96 well titre plates, inoculated with serial dilutions (10*1, 10'2, 10"3, KT4, 10 5, 10~6) of inoculum and incubated at 33 C whilst covered with parafllm for 7 to 10 days. After pouring off excess medium plates were fixed and stained with crystal violet before enumerating those wells with greater than 50% cytopathic effect. The titre o f the inoculum was found to be 200 TCID50 m l'1and it was decided that this was a reasonable dose with which to inoculate our volunteers. Symptom diary and scoring A symptom diary combining scores for upper and lower respiratory tracts was used for the present study. Common cold symptoms were based on the Jackson criteria and categorised as upper and lower respiratory symptoms, cough and systemic symptoms. URT symptoms included nasal discharge, nasal blockage, sneezing and sore throat. Systemic symptoms included fever, headache, chills and malaise. LRT symptoms were based upon the simple asthma score of Cheung and colleagues358 and included wheeze, chest tightness and shortness o f breath. Cough was also recorded but cough can be generated as part of either upper or lower respiratory tract illnesses, hence it was scored separately. Each symptom was graded from 0 (absent) to 3 (severe). 287
289 Because HCoV experimental infections have traditionally produced mild colds, difficulty was anticipated in defining the threshold for a mild cold. The simple definition o f a total score < 7 being a mild cold could have been misleading as all subjects could have minor symptoms without necessarily suffering a cold. To overcome this problem symptom diaries were assessed blind by one of the project supervisors (MS). Symptomatic colds which were categorised as definite (URT scores > 2 above a zero baseline on each o f two consecutive days from day 2 to 6), possible (scores > 1 above zero baseline, or scores > 2 above a variable baseline on two consecutive days from day 2 to 6), and absent. The baseline was taken as the score on day 0. The symptom diary is shown in Table 3.1. Nasal lavage for detection of viral infection The technique used in this study was a nasal lavage based upon the method of Koren and 358 colleagues for its simplicity, tolerability and that a lavage can sample the majority of the nasal cavity with minimal irritation of the mucosa. It involved the subjects sitting with their necks extended to 45 while warm phosphate buffered saline (PBS) containing 450pg/ml of inulin was introduced into one nostril. During the process the subject occluded the palate by positive oral pressure so that the wash remained in the nasal cavity for 10 seconds before being expelled into a sterile receptacle. A total of 10 ml in aliquots of 2.5 ml was inserted alternately in each nostril. One ml of mixed nasal lavage fluid was then removed and stored at - 70 C for later viral detection by RT-PCR. 288
290 RT-PCR Virus RNA was identified by RT-PCR in nasal lavage fluid on day 3 post inoculation. This method was derived from a nested RT-PCR described previously359. The lavage fluid was stored at - 70 C. For analysis, samples were rapidly thawed and RNA extractions immediately undertaken, again minimising losses. All primers were designed under the supervision of Dr Pringle (see thesis) from the genome of the nucleocapsid protein and checked against the EMBL database. The primers were manufactured by Sigma-Genosys Ltd (Cambridge, UK). Extraction of HCoV 229E RNA Prior to RNA extraction all ependorphs were treated with diethylpyrocarbonate (Sigma Aldrich, Dorset, UK) in order to reduce the possibility of RNAse contamination and subsequent degredation of extracted RNA (see appendix 2.1). RNAse/DNAse filter-guard tips were used in all manipulations. Extraction was based upon a guanidium isothiocyanate method ustilising RNAzol B (Biogenesis, Poole, UK) based on the method of Chomczynski and Sacchi358. Essentially, each thawed aliquot o f clinical specimen was added to 200pl of ice-cold RNAzol B in an RNAse free 1.5ml eppendorf and mixed by pipetting up and down several times. Due to their hazardous nature RNAzol B and chloroform, manipulations were performed in a fume-cupboard. 200pl o f ice-cold chloroform (Fisons, Loughborough, UK) was then 289
291 added to this lysate and the new mixture vortexed for 15 seconds. The homogenate was then stored on ice for 5 minutes and centrifuged at 12000g for 15 minutes at 4 C (upon centrifugation the homogenate forms two phases, the proteins and DNA collecting at the interphase). The upper aqueous phase (containing all the extracted RNA) was then carefully removed, added to 300pl o f ice-cold isopropanol (Fisons) and mixed by pipetting up and down several times. The mixture was then stored on ice for at least 30 minutes to precipitate the extracted RNA. After isopropanol incubation, the mixture was re- centrifuged at 12000g for 15 minutes at 4 C and the supernatant carefully removed (ensuring that any RNA pellet was not disturbed). 1 ml of ice-cold 70% ethanol was then added, the mixture briefly vortexed and then centrifuged at 7500g for 10 minutes at 4 C. After this final centrifugation stage, the supernatant was carefully removed (again ensuring that any RNA pellet was not disturbed), the eppendorf inverted onto tissue paper and the pellet left to dry at room temperature for approximately minutes to remove ethanol that may interfere with later reverse transcription reactions. After the allotted time, the RNA was resuspended in 30jul of RNAse free distilled H2O containing lp l of RNAse inhibitor (20u/pl, Promega) and immediately utilised. Both negative and positive controls were included in each batch of reverse transcriptions and subsequent PCRs. Negative controls consisted of diethylpyrocarbonate treated distilled H2O. Positive controls consisted of RNA extracted from HEL cells infected with HCoV 229E. 290
292 Reverse transcription of HCoV 229E Reverse transcription is the method by which copy DNA (cdna) is manufactured from the RNA extract. A standard reverse transcription methodology adapted from Sambrook ICO and colleagues was used. Initially, a reverse transcription super-mix was prepared by multiplying the volume of a particular component required for an individual reverse transcription mix by the number o f reverse transcription mixes required (one reverse transcription mix being required for each specimen to be reverse transcribed). Individual reverse transcription mixes comprised 2pl o f lox reverse transcription buffer (supplied free with the MMLV reverse transcriptase enzyme (Stratagene, Cambridge, UK)), 2pl of a 5mM equimolar mix of dntps (adenosine/ cytosine/guanosine/thymidine deoxynucleotide tri-phosphates giving a final concentration of 0.5 mm), 0.5pl o f loomm DTT (2.5mM final concentration), lp l of 200ng/pl downstream primer 0647 (1.25pM final concentration - see Appendix 2.2), lpl of lopg/ml gelatin and 3pl of RNAse free dh20. When the supermix had been prepared, 9.5pi was pipetted into individually labelled RNAse free 0.5ml eppendorphs and overlain with mineral oil (Sigma Aldrich). lopl of extracted RNA was then added to the respective reverse transcriptase mix and then the resultant RN A/reverse transcriptase mix heated at 70 C for 5 minutes in oreder to unravel the structure of the RNA. The mixture was then placed on ice for 5 minutes after which time 0.5pi o f monkey-murine leukaemia virus (MMLV) reverse transcriptase enzyme 291
293 (50u/pl, Strategene) was added to each mix. The complete mixtures (final volume 20pl) were then incubated at 37 C for 1 hour (in order to activate the MMLV reverse transcriptase enzyme), heated to 95 C for 5 minutes (in order to deactivate the MMLV reverse transcriptase enzyme) and then cooled to 4 C. cdna mixes were then stored at - 20 C until required. HCoV PCR The PCR used was based upon the 2 round nested PCR developed by Professor Myint and colleagues which had determined the cycling regime359. Previous work within the department had determined the optimal concentrations of MgCl2 and primer concentrations to use. Initial PCR experiments using the nested method gave false positives in the negative controls. A simplified single round PCR was designed, avoiding such problems. This method was used for this study. Each PCR reaction mix (total volume 50pl) comprised 32.1 pi dh20, 6pl of 25mM MgCL, 5pl lox PCR buffer, 0.4pl o f 5mM dntp mix, 0.5pl of HCoV 229E upstream primer 7 and, 0.5pl of HCoV 229E downstream primer 8. This PCR reaction mixture was initially made as a supermix before being aliquoted into individually labelled RNAse free 0.5ml eppendorphs (volumes o f 44.5pl). 5pl of reverse transcribed cdna, sterile dh20, negative control cdna or positive control cdna was added the their respective labelled PCR reaction mixes and overlain with mineral oil. The reaction mixes 292
294 were then heated to 95 C for 5 minutes on a PCR cycler (Hybaid, OmniGene) before the addition of 0.5pl of T h e r m u s a q u a tic u s DNA polymerase (Promega Life Sciences, Southampton, UK, No: M l665). Forty cycles at the following temperatures were conducted the cycler: 94 C for 60 seconds, 62 C for 90 seconds, 72 C for 120 seconds. The temperature was then held at 72 C for 10 minutes before a thermal ramp to 4 C. Samples were stored at 4 C until required. RNA detection This was by the standard method of agarose gel electrophoresis using a 2% agarose gel. This was prepared by adding 2g of agarose (genetic technology grade, ICN) to a heat resistant glass 250ml conical flask. To the was added 100ml of lxtris Borate EDTA buffer (10.6g/l Tris - HC1 (ICN Biochemicals Inc. Ohio, USA), 5.5g/l boric acid (Fisons), 0.93g/l sodium EDTA (Fisons) adjusted to ph 8.3 using 5M HCI (Fisons)) was then added to the powder and swirled to mix. The agarose mixture was then heated in a microwave for 1-2 minutes (highest power setting) till just boiling whereupon the agarose mixture was transferred to a fume-cupboard and allowed to cool for approximately 3 minutes. The gel casting frame was assembled and 2pl of 500pg/ml ethidium bromide solution (Sigma Aldrich) was added to the cooled agarose. The agarose was then swirled to evenly mix the added ethidium bromide and poured into the assembled gel casting frame. The gel combs were then paced in their respective positions and the gel allowed to set within the fume-cupboard for approximately 30 minutes prior to use. 293
295 The agarose gel was removed from it's casting frame and completely immersed in an electrophoresis tank containing lx Tris Borate EDTA buffer (see above). 2pl of 6x stock loading buffer (10ml Glycerol (Sigma Aldrich), 0.05g bromophenol blue (Sigma Aldrich), 0.05g xylene cyanol (Sigma Aldrich) and 10ml sterile de^o) was then aliquoted into separate clean 0.5ml eppendorfs and lopl of each PCR product to be investigated added. The solutions were then mixed by pipetting up and down several times before pipetting into an appropriate well of the agarose gel. A molecular weight marker (lp l 1 Kb DNA ladder (Gibco), 2pl 6x loading buffer and 9pl o f dh20) was added at one of the ends. Electrophoresis of the gel was then carried out at V until the dye front was approximately 3/4 of the way along the gel (approximately 1 hour) whereupon the gel was removed from the electrophoresis tank and observed under UV light (wavelength = 260nm) for the presence o f specific PCR product. 294
296 Day of study Spirometry AM PEF - best of 2 FEV, - best of 2 Spirometry PM PEF - best of 2 F E V,-b est o f 2 URT symptoms runny nose blocked nose sneezing sore throat LRT symptoms wheeze chest tightness shortness of breath Cough Systemic Symptoms fever chills malaise headache Total Tick days when treatment needed * Tick days lost from work *Treatments needed(please indicate on which days) = Figure 1: The symptom diary used in the coronavirus study 295
297 Appendix 5
298 Published Papers Chilvers MA, O'Callaghan C. Analysis o f ciliary beat pattern and beat frequency using digital high speed imaging: comparison with the photomultiplier and photodiode methods. Thorax 2000; 55(4): Chilvers MA, Mckean M, Rutman A, Myint BS, Silverman M, O Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur R espirj2001; 18: Chilvers MA, Rutman A, O Callaghan C. Functional analysis o f cilia and ciliated epithelial ultrastructure in healthy children and young adults. Thorax 2003; 58(4): Chilvers MA, Rutman A, O'Callaghan C. Primary Ciliary Dyskinesia: Ciliary Beat Pattern Associated with Specific Ultrastructural Defects. JACI 2003;112:
299 314 Thorax 2000;55: Analysis o f ciliary beat pattern and beat frequency using digital high speed imaging: com parison with the photom ultiplier and photodiode m ethods M ark A C hilvers, C h ris to p h e r O C allag h an D epartm ent o f Child Health, University o f Leicester School of M edicine, Robert Kilpatrick Clinical Sciences Building, Leicester Royal Infirmary, P O Box 65, Leicester LE2 7LX, UK C O C a l l a g h a n M A C h i l v e r s Correspondence to: D r C O Callaghan Received 19 August 1999 R eturned to authors 16 N ovem ber 1999 Revised version received 12 January 2000 A ccepted for publication 12 January 2000 A b stract B a c k g ro u n d T h e a im o f th is stu d y w as to d e te r m in e th e r e la tio n sh ip o f th e p ow er an d reco v ery stro k e o f resp ira to ry cilia u sin g d ig ita l h ig h s p e e d v id e o im a g in g. B ea t freq u en cy m e a s u r e m e n ts m a d e u sin g d ig ita l h ig h sp e e d v id eo w ere a lso co m p a r e d w ith th o se o b ta in e d u sin g th e p h o to m u ltip lier a n d m o d ifie d p h o to d io d e te c h n iq u e s. M e th o d C ilia ted e p ith e liu m w a s o b ta in e d b y b r u sh in g th e in fe r io r n a sa l tu rb in a te o f 20 h e a lth y su b jects. C ilia ted ed g e s w ere o b se r v e d b y m ic r o sc o p y a n d th e d ev ia tio n o f c ilia d u r in g th e ir reco v ery stro k e rela tiv e to th e p a th tra v elled d u rin g th e ir p ow er strok e w a s m e a s u r e d. B ea t freq u en cy m e a s u r e m e n ts m a d e b y d ig ita l h ig h sp e e d v id e o a n a ly sis w ere com p a r e d w ith th o se o b ta in ed u sin g th e p h o to m u ltip lie r a n d m o d ifie d p h o to d io d e. R e s u lts C ilia w ere fo u n d to b e a t w ith a forw ard p ow er strok e a n d a back w ard reco v ery strok e w ith in th e sa m e p la n e. T h e m e a n a n g u la r d e v ia tio n o f th e c ilia d u rin g th e reco v ery stro k e fr o m th e p la n e o f th e forw ard p o w er strok e w as on ly 3.6 (95% C l 3.1 to 4.1 ). T h ere w as a sig n ific a n t d ifferen ce in b ea t freq u en cy m e a s u r e m e n t b e tw e e n th e d ig ita l h igh sp e e d v id eo (13.2 H z (95% C l 11.8 to 14.6)) a n d b o th p h o to m u ltip lie r (12.0 H z (95% C l 10.8 to 13.1), p = 0.01) a n d p h o to d io d e (11.2 H z (95% C l 9.9 to 12.5), p<0.001) te c h n iq u e s. T h e B la n d -A ltm a n lim its o f a g r e e m e n t fo r th e d ig ita l h ig h sp e e d v id eo w ere to 5.15 H z w ith th e p h o to m u ltip lie r a n d to 6.06 H z w ith th e p h o to d io d e. C o n clu sio n R e sp irato ry c ilia b ea t fo r w ard s a n d b ack w ard s w ith in th e sa m e p la n e w ith o u t a c la s sic a l sid ew a y s reco v ery sw eep. D ig ita l h ig h sp e e d v id eo im a g in g allo w s b o th c ilia r y b e a t freq u en cy an d b e a t p a ttern to b e ev a lu a ted. (T h orax ;5 5 : ) K e y w o r d s : c i l i a ; c i l i a r y b e a t f r e q u e n c y ; m e t h o d s R espiratory cilia b eat in a coordinated m anner w ith a specific frequency and p attern, clearing m ucus and debris from the airways. S urprisingly, since th e initial cinem atographic studies o f ciliary m ovem ent by G ray 1 there is still no consensus of opinion as to the precise p attern o f ciliary beating.1'4 It is generally th o u g h t th at respiratory cilia have a forw ard pow er stroke and a recovery stroke d u rin g w hich th e cilium sweeps backw ards and to th e sid e.1 As the cilium sweeps sideways and backw ards in a clockw ise direction it is th o u g h t to stim ulate adjacent cilia to beat, propagating the ciliary m etachronal w ave.1as a resu lt o f m etachronal coordination cilia join th eir propulsive efforts to create a continuous m ovem ent o f m ucus. In p rim ary ciliary dyskinesia cilia are either stationary or b eat in a slow o r dyskinetic fashion. Im paired m ucociliary clearance in these p atients is associated w ith recu rren t chest infections, w hich m ay lead to bronchiectasis and sinusitis. T h e diagnosis o f p rim ary ciliary dyskinesia is m ade on th e basis o f a supportive clinical history and an abnorm al ciliary beat frequency accom panied in m o st cases by specific abnorm alities o f th e ciliary axonem e on electron m icroscopy.5 6 B uchdahl and colleagues have suggested, how ever, th at a significant n u m b er o f children w ith p rim ary ciliary dyskinesia have slowly m oving cilia b u t no obvious abnorm ality on u ltrastructural analysis.7 A lthough m any m ethods have been devised to estim ate ciliary beat frequency,8 high speed im aging by cinem atography is regarded by m any as the optim al m ethod to analyse ciliary fu n c tio n /'11 Im ages recorded at 500 p er second and played back at a slower speed allow th e frequency and th e precise beat p attern o f individual cilia to be determ ined. B ecause o f the difficulties in im age processing, expense, and lack o f real tim e analysis, various oth er m ethods have been developed. Tw o p o pular m ethods the p h o to m u ltip lier12 15 and p h o to diode techniques1817 estim ate b eat frequency indirectly by detecting changes in light in ten sity passing through beating cilia. T h e quality o f digital high speed video im aging has recently im proved to such an extent th a t it has replaced cinem atography W ith th e advent o f digital high speed im aging, th e exact m ovem ent o f a cilium th ro u g h o u t th e b eat cycle can be visualised and b eat frequency rapidly m easured. Before analysing th e b eat p a tte rn of cilia from patients w ith prim ary ciliary dyskinesia it is essential to evaluate n orm al h u m a n respiratory ciliary m ovem ent. T h e aim o f this study was tw ofold. Firstly, to d eterm in e th e relationship o f th e pow er and recovery stroke o f respiratory cilia using digital high speed video im aging and, secondly, to com pare ciliary b eat frequency m easured using digital high speed video im aging w ith th at o b tain ed using th e
300 Measurement of ciliary beat frequency 315 photomultiplier and modified photodiode techniques. Methods Ciliated samples were obtained by brushing the inferior nasal turbinate of 20 healthy subjects (13 males) aged 3-38 years with a 2 mm cytology brush." T he study received approval from the Leicestershire ethical review committee and verbal consent was obtained prior to brushing. Nasal brushings were placed in M edium 199 (ph 7.3) which contained antibiotic solution (streptomycin 50 pg/ml, penicillin 50 pg/ml, Gibco, UK). Ciliated strips of epithelium were suspended in a cham ber created by the separation of a cover slip and glass slide by two adjacent cover slips. T he slide was placed on a heated stage (37 C) of a Leitz D iaplan microscope m ounted on an anti-vibration table (Wentworth Laboratories L td, U K ). T he experimental system allowed the beating cilia to be viewed in three planes: a sideways profile (fig 1A), beating directly towards the observer (fig IB ), and from directly above (fig 1C). Specimens were examined using a xloo interference contrast lens. Only undisrupted ciliated strips of m ore than 50 pm in length, devoid of mucus, were studied. Beating ciliated edges were recorded using a digital high speed video camera (Kodak Ektapro M otion Analyser, M odel 1012) at a rate of 400 frames per second, using a shutter speed of 1 in T he camera allows video sequences to be recorded and played back at reduced frame rates or frame by frame. The precise movement of individual cilia may be observed during their beat cycle. T he path taken by a cilium during the power and recovery strokes was plotted on acetate paper overlying the high resolution m onitor as follows. Viewing the cilia beating towards the observer (fig IB ), the precise position of the cilium during the forward power stroke was plotted frame by frame. As the cilium moved backward during the recovery stroke its position during this movem ent was again plotted frame by frame. An angle could be derived from a line drawn through the plane of the power stroke and a line joining the point of maximum deviation of the cilium during the recovery stroke (fig IB). This was defined as the beat angle u. For each cilium studied the mean angle for five complete beat cycles was measured by image analysis (Scion image, Scion Corporation, Frederick, M aryland, USA). This was repeated when viewing the ciliary beat pattern from above (fig 1C) and a beat angle (3 was calculated by image analysis. M easurements of ciliary beat frequency were m ade from ciliated epithelial strips at least 50 pm in length viewed in sideways profile (fig 1A) using the digital high speed video, photom ultiplier, and photodiode methods. T he order in which measurem ents were made by the three different techniques varied equally to help to exclude any confounding effect of the order of measurement. HIGH SPEED VIDEO M ETHOD Ciliary beat frequency (CBF) may be determined directly by timing a given num ber of individual ciliary beat cycles. G roups of beating cilia were identified and the num ber of frames required to complete 10 cycles recorded. This was converted to ciliary beat frequency by a simple calculation (C B F= 400/(num ber frames for 10 beats) x 10). Ten m easurem ents of beat frequency were m ade along each ciliated strip. PHOTOM ULTIPLIER M ETH O D Ciliary beat frequency was recorded using a microscope photom eter (Leitz SS ). As suggested by D alham n," we adjusted the aperture allowing light to reach the photom ultiplier to an area of 2.2 pm2 (see ref 14) and positioned it over an area o f beating cilia. Voltage signals, generated as the moving cilia interrupted the passage of light, were displayed on an oscilloscope and relayed to a power spectrum analysis program m e (ANADAT, M ontreal, Canada) to determ ine ciliary beat frequency. Ten m easurem ents of beat frequency were m ade along each ciliated strip. B Angle of m axim u m deviation of cilium during recovery stroke Path of recovery stroke A ngle of m axim u m deviation of cilium during recovery stroke P a th of re c o v e r y s tro k e Position o f cilium at beginning o f pow er stroke Position o f cilium at beginning of power stroke Position o f cilium at end of pow er stroke Figure 1 Planes of view used to observe and record the ciliary beat cycle and beat frequency. The beat angle is formed by the point of maximum deviation of the cilium during the recovery stroke from the path the cilium travels during the power stroke. (A ) Side profile of ciliary beat pattern. (B) Beat angle a: the cilium is viewed beating towards the observer. (C ) Beat angle ft: the cilium is viewed from directly above.
301 316 Chilvers, O Callaghan PHOTODIODE M ETHOD Video images of the beating respiratory cilia were relayed from a Super-VHS video camera (Panasonic F I 5 C C P videocamera) to a high resolution monitor. T he photodiode, mounted in a pen like system, was held over the beating cilia displayed on the m onitor.17 Signals generated as cilia moved past the photodiode sensor were fed via an oscilloscope to a power spectrum analysis program (ANADAT, M ontreal, Canada) to determ ine ciliary beat frequency. Ten m easurem ents of beat frequency were made along each ciliated strip. 10 r a) o Mean of HSV and PM (Hz) 20 STATISTICS T he mean, standard deviation, and 95% confidence intervals were calculated for the a and (3 angles (fig IB and C). A one way analysis of variance was performed with respect to the m ethod used to measure ciliary beat frequency. T h e m ean and 95% confidence interval for each m ethod was calculated. Paired t tests were perform ed to compare each m ethod with the digital high speed video m ethod. T he Bland-Altman limits of agreement were calculated from the mean difference ± twice the standard deviation of the differences with the digital high speed video taken as the optimal m ethod. T he limits of agreement were calculated for each m ethod separately. Results A classical sideways recovery sweep was not seen when cilia were viewed either beating towards the observer or from above (fig IB and C). T he power and recovery strokes were within the same plane with minimal sideways deviation. A total of 144 cilia had measurements made of beat angle a and 262 cilia for beat angle (3. T he corresponding mean (SD) angles were a = 5.3 (3.5) (95% C l 4.6 to 6.0) and (3 = 3.6 (3.0) (95% C l 3.1 to 4.1). A total of 600 m easurem ents were made for ciliary beat frequency, 200 for each method. T he analysis of variance with respect to the m ethod used showed a highly significant effect (p<0.001). Both the photom ultiplier and the photodiode readings under-recorded ciliary beat frequency compared with the digital high speed video. T he mean (SD) beat frequency determined using the digital high speed video was 13.2 (2.9) H z (95% C l 11.8 to 14.6) compared with 12.0 (2.4) H z (95% C l 10.8 to 13.1) for the photom ultiplier and 11.2 (2.8) Hz (95% C l 9.9 to 12.5) for the photodiode. A paired t test showed a significant difference for the photom ultiplier (mean difference 1.2 Hz, 95% C l 0.3 to 2.1; p = 0.01) and photodiode methods (mean difference 1.9 Hz, 95% C l 0.9 to 2.9 Hz; p<0.001) compared with the digital high speed video. T he Bland-Altman limits of agreement with respect to the digital high speed video (fig 2) showed widest variation for the photodiode method (-2.30 to 6.06 Hz) and closest agreem ent for the photom ultiplier m ethod (-2.75 to 5.15 Hz). QCL l > 5 c/) I a ) o c 0) 0 it o M ean o f HSV and PD (Hz) Figure 2 Bland-Altman plots of the digital high speed video (H SV) compared with (A ) the photomultiplier (PM ) method and (B) the photodiode (PD) method at 37 C. Discussion Digital high speed video imaging allowed us to obtain images at a rate o f400 per second and to make a perm anent recording at a reduced frame rate. Beat pattern can be determ ined and beat frequency evaluated during the study or at a later stage. As mentioned in the introduction, it is generally thought that cilia deviate in a sideways direction during the recovery sweep. We have shown, however, that hum an respiratory cilia do not have a classical sideways recovery sweep. T he respiratory cilia analysed beat forwards and backwards with a maximal deviation during their recovery sweep of less than 5. This was confirmed by analysis of ciliary movement observed in two planes. Primary ciliary dyskinesia may be caused by a num ber of different ultrastructural defects of the axoneme. Depending on the defect, cilia may be immotile or may move in a dyskinetic fashion. If electron microscopy is perform ed on ciliated samples where the beat frequency is below 11 Hz, most patients with primary ciliary dyskinesia will be identified.* Studies by Rossman and colleagues, however, suggest that evaluation of beat pattern, in addition to beat frequency, may be helpful in the diagnosis of patients with primary ciliary dyskinesia For example, patients with prim ary ciliary dyskinesia due to m icrotubular transposition may have beat frequencies that are within the lower norm al range but an abnorm al ciliary beat pattern is seen on slow motion analysis This study is the first to com pare directly two of the most commonly used m ethods of estimating ciliary beat frequency of respiratory cilia with high speed imaging. T he photom ultiplier and photodiode techniques recorded ciliary beat frequencies that were significandy slower than those measured using the digital 20
302 Measurement of ciliary beat frequency 317 high speed video m ethod. T h e lim its o f agreem ent for b o th m ethods w ere w ide, w hich confirm s th at results obtained using the different techniques can n o t be used interchangeably. T hese results em phasise th e need for norm al reference ranges o f ciliary b e a t frequency to be established for each tech n iq u e if it is to be used as a diagnostic test for p rim ary ciliary dyskinesia. In sum m ary, we have show n using digital high speed im aging th at respiratory cilia beat w ith a pow er and recovery stroke w ithin the sam e plane. T h e ability o f digital high speed video to m easure ciliary b eat p a tte rn and beat frequency should m ake it a pow erful tool in the investigation o f patients w ith prim ary ciliary dyskinesia. T h e next stage o f o u r investigation will be to com pare th e b eat p a tte rn o f o u r n o r m al cilia w ith the ciliary b eat p attern s we have recorded from p atients w ith prim ary ciliary dyskinesia. T h e authors would like to thank th e Cystic Fibrosis T ru st and M asons M edical F oundation for th eir support. We thank D r C aroline D ore, H am m ersm ith H ospital, L o n d o n for statistical advice. 1 Gray J. The mechanism o f ciliary movement: VI. Photographic and stroboscopic analysis o f ciliary m ovem ent. Proc R Soc Biol Sci Series B 1930;107: P roetz AW. Essays on the applied physiology of the nose. St Louis: Annals Publishing Com pany, 1953: Cheung AT, Jahn TL. High speed cinemicrographic studies on rabbit tracheal (ciliated) epithelia: determ ination o f the beat pattern o f tracheal cilia. Pediatr Res 1976;10: Sanderson M J, Sleigh MA. Ciliary activity of cultured rabbit tracheal epithelium : beat p attern a n d m etachrony. J Cell Sci 1981;47: Bush A, C ole PJ, H ariri M, et al. P rim ary ciliary dyskinesia: diagnosis and standards of care. Eur RespirJ 1998;12: Schidlow DV. Primary ciliary dyskinesia (the immotile cilia syndrom e). Ann Allergy 1994;73: Buchdahl RM, Reiser J, Ingram D, et al. Ciliary abnorm alities in respiratory disease. Arch Dis Child 1988;63: Rusznak C, Devalia JL, Lozewicz S, et al. T h e assessm ent o f nasal m ucociliary clearance and the effect o f drugs. Respir Med 1994;88: Dalhamn T. Ciliary motilitv studies. Arch Intern Med 1970; 126: Greenstone M, Logan-Sinclair R, Cole PJ. An autom ated m ethod for recording o f ciliarv beat frequency. IRCS M ed Sci 1984;12: Sanderson M J, Dirksen HR. A versatile and quantitative com puter-assisted photoelectronic technique used for the analysis of ciliary beat cycles. Cell Motility 1985;5: Dalhamn T, Rylander R. Frequency o f ciliary beat m easured with a photosensitive cell. Nature 1962;196: I 3 Yager J, C hen T M, D ulfano MJ. M easurem ent o f frequency of ciliary beats o f h um an respiratory epithelium. Chest 1978;73: II Rutland J, Cole PJ. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet 1980;ii: Roth Y, Aharonson EF, Teichtahl H, et al. H um an in vitro nasal and tracheal ciliary beat frequencies: com parison of sam pling sites, com bined effect o f m edication, and dem ographic relationships. Ann Otol Rhino! Larvngol 1991; 100: Teichtahl H, W right PL, Kirsner RL. M easurem ent of in vitro ciliary beat frequency: a television-video m odification of the transm itted light technique. M ed Biol Eng Comp 1986;24: O Callaghan C, Smith K, W ilkinson M, et al. Ciliary beat frequency in n ew born infants. A rch Dis Child 1991 ;66: Yoshitsugu M, Rautiainen M, M atsune S, et al. Effect of exogenous A T P on ciliary beat o f h u m an ciliated cells studied w ith differential interference m icroscope equipped with high speed video. Acta Oto-Laryngol 1993;113: D olovich M B, M ahony JB, C ham bers C, et al. C iliary function, cell viability, and in vitro effect o f ribavirin on nasal epithelial cells in acute rhinorrhea. Chest 1992;102: Rossman C M, Lee RM, Forrest JB, et al. Nasal ciliary ultrastructure and function in patients w ith prim ary ciliary dyskinesia com pared with that in norm al subjects and in subjects w ith various respiratory diseases. A m Rev Respir Dis 1984;129: R ossm an C M, N ew house M T. P rim ary ciliary dyskinesia: evaluation and m anagem ent. Pediatr Pulmonol 1988;5: Chilvers M A, Rutm an A, O Callaghan C. Diagnosing prim ary ciliary dyskinesia: defining ciliary beat p atterns associated with specific ultrastructural defects. Arch Dis Child 1999;80(Suppl 1):A54.
303 Eur Respir J 2001; 18: DOI: / Printed in UK - all rights reserved Copyright r ERS Journals Ltd 2001 European Respiratory Journal ISSN The effects of coronavirus on human nasal ciliated respiratory epithelium M.A. Chilvers*, M. McKean*, A. Rutman*, B.S. Myint*, M. Silverman*, C. O'Callaghan* The effects o f coronavirus on human nasal ciliated respiratory epithelium. M.A. Chilvers. M. McKean, A. Rutman, B.S. Myint, M. Silverman, C. O' Callaghan. (ERS Journals Ltd ABSTRACT: Human coronavirus (HCoV) accounts for 15-30% of common colds, but only one case report has described the effect of a coronavirus infection, that was asymptomatic, on human respiratory epithelium. The authors examined the effects of infection with HCoV on ciliary structure and function in healthy volunteers infected by intranasal inoculation with HCoV 229E. A further four volunteers were sham infected with ultraviolet-inactivated virus. Immediately before inoculation (day 0) and 3 days later (day 3), ciliated epithelium was obtained by brushing the inferior nasal turbinate. Ciliary beat frequency was determined and beat pattern analysed for evidence of dyskinesia (0=normal, 3=severely dyskinetic) using digital high-speed video photography. Ciliary ultrastructure was examined by transmission electron microscopy. Symptom diaries were kept for the duration of the study. Ail subjects inoculated with HCoV, including the three who did not develop symptoms of an upper respiratory tract infection, had disruption of their respiratory epithelium on day 3. Although there was no difference in the mean ciliary beat frequency between day 0 (11.3 Hz (95% confidence interval (Cl): ) and day 3 (9.4 Hz (95% Cl )), there was a significant increase (p<0.05) in the ciliary dyskinesia score between day 0 (0.2 (95% Cl 0-0.5)) and day 3 (1.1 (95% Cl ). In sham-infected subjects, no differences in epithelial integrity, or ciliary structure and function were found between day 0 and day 3. Inoculation of healthy volunteers with human coronavirus caused disruption of the ciliated epithelium and ciliary dyskinesia. This is likely to impair mucociliary clearance. Damage to the respiratory epithelium, due to human coronavirus infection, may occur without overt clinical symptoms. Eur Respir J 2001; 18: * C h i l d r e n s A s t h m a C e n t r e, D e p t o f C h i l d H e a l h, U n i v e s i t y o f e i c e s r, t r L t e L e i c e s t e r R o y a l I n f i r m a r y, a n d # D e p t o f M i c r o b i o l o g y a n d I m m u n o l o g y, U n e r s i t y o f L e i c t e r, e i c e s t e r, U K. i v e s L C o r r e s p o n d e n c e ; C. O ' C a l l a g h a n, C h i l d r e n s A s t h m C r e, D e p t o f a e n t C h i l d H e a t h, U n i v r s i t y o f L e i c e s r, l e t e R o b e r t K i l p a t r i c k C l i n i c a l S c i e n c e s B u i l d i n g L e i c e s t e r R o y a l I f i r m a r y,, n , P. O B o x 6 5, L e i c e s t e r L E 2 7 L X,. U K. F a x : K e y w o r d s : C i l i a c i l i a r y b e a t f r e q u e n c y c o r o n a v i r u s r e s p i r a t o r y e p i t h e l i u m u l t r a s t r u c t u r e v i r a l i n f e c t i o n R e c e i v e d : O c t o b e r A c c e p t e d a f t e r r e v i s i o n J u n e T h i s w o r k w a s s u p p o r t e d b y T h e C y s t i F i b r o i s T r s t, M a s o n M e i c a l c s u s d F o u n d a t i o n a n d T h e N a t i o n a l A s t h m a C a m p a i g n. The "common cold" is a universally recognized short illness, in which the main sym ptom s involve the upper respiratory tract and in w hich nasal sym ptom s usually predom inate. U nderstanding the interaction between virus and epithelium is an im portant step in determ ining the m echanism s by which sym ptom s are produced. Structural dam age to the respiratory epithelium [1-3] and abnorm al ciliary function [1, 4, 5] during viral infection m ay disrupt the m ucociliary escalator [4, 6], contributing to sym ptom s o f nasal discharge and obstruction. A lthough som e infected volunteers suffer nasal congestion and discharge, an equal number o f infected subjects have no definite sym ptom s. The reasons for this are still unclear [7]. Hum an coronaviruses (H C ov ) are the second m ost prevalent cause o f the com m on cold. They account for 15-30% o f proven viral infections [8] and may cause exacerbation o f lower respiratory diseases such as asthm a [9]. D ata on the effect o f H C ov infection o f the respiratory epithelium are surprisingly sparse. The initial m ethod for identifying H C ov used in vitro tracheal organ cultures, in which infection was detected with difficulty. M icroscopy o f light reflected from the epithelial surface was used as an indicator o f ciliary activity, with loss o f the light reflection taken as the outcom e measure for viral infection [10]. Other data include an incidental finding, on electron m icroscopy, o f H C ov infection in a 2-yr-old fem ale being investigated for ciliary dyskinesia. It is o f interest that this infection was asym ptom atic with no signs o f a com m on cold at the tim e o f biopsy or afterwards [11]. In the present study, H C ov 229E (Am erican Type Culture C ollection, R ockville, M D, U SA ), one o f the tw o m ajor serotypes o f coronavirus accounting for upper respiratory tract infections [12], was used to inoculate healthy volunteers. T he aim was to determ ine the effect o f H C ov infection on the ultrastructure o f nasal epithelium and on the beat frequency and beat pattern o f nasal cilia. Subjects Methods Fifteen adult volunteers (11 m ales, four fem ales) w ithout nasal or respiratory disease were recruited. A ges ranged from (m edian 22) yrs. N o n e o f the
304 9 6 6 M.A. CHILVERS ET AL. subjects were taking nasal drugs and none had had a sym ptom atic upper respiratory tract infection in the preceding 6 weeks. The study received approval from the Leicestershire Ethical R eview C om m ittee. Human coronavirus inoculation H C ov 229E (American Type Culture C ollection ) w as cultured according to standards o f go o d lab oratory practice in human em bryonic lung fibroblasts. An inoculum was prepared as previously described [13] and was tested for safety according to the criteria o f G w a l t n e y et al. [14]. The inoculum consisted o f aliquots o f 1 m L o f H C ov 229E suspension (200 50% tissue culture infective doses ( T C I D 5 0 ) per m L) w hich were stored at -70 C. Eleven subjects (nine m ales and tw o fem ales) were inoculated with active virus as previously described [7], Briefly, with the volunteer's head extended to 45, 0.5 ml o f virus was instilled into each nostril. In addition to viral antigens, the inoculum m ay contain other small proteins released from the infected cultured cells, such as cytokines. These proteins could interfere with the infective process or even generate an inflam m atory response. T o control for this, four subjects were inoculated with virus inactivated by ultraviolet (U V ) light as previously described [15]. Inactivation was confirm ed by culture in hum an em bryonic lung fibroblasts with a control flask in o culated with active virus. The cells with active virus developed com plete cytopathic effect by 10 days, whereas those with U V -inactivated virus show ed no cytopathic effect by 18 days when cultures were discontinued. Evaluation o f colds The sym ptom diary was based on that used by Jackson et al. [16] recording upper respiratory tract sym ptom s (rhinorrhoea, nasal blockage and sneezing) and system ic sym ptom s (headache, m alaise and chills). Each sym ptom was scored from 0-3 points according to severity. The criteria o f J a c k s o n et al. [1 6 ] were then m odified by a previously validated m ethod [17] in order to categorize volunteers by upper respiratory tract score into the follow ing groups: definite cold (a score o f ^ 2 above a zero baseline on at least tw o consecutive days over days 2-6 post-inoculation); possible cold (a marginal increase over a zero baseline or a score o f ^ 2 above a sym ptom atic baseline on at least tw o consecutive days over days 2-6); and no cold (sym ptom -free). Laboratory confirm ation o f infection w as by reverse transcriptase polym erase chain reaction (RT- PC R ) o f virus ribonucleic acid (R N A ) extracted from nasal lavages obtained on day 3 postinoculation. This follow ed a previously established m ethod [13], Evaluation o f ciliary structure and function Prior to viral inoculation and 3 days after in ocu lation, ciliated sam ples were obtained by brushing the inferior nasal turbinate with a 2-m m cytology brush (BC -15C K eym ed, Southend-on-Sea, U K ) [18]. This has been found to be an ideal site for repeated sam pling o f ciliated epithelium [3], D ay 3 w as chosen, as previous studies o f viral infections found little effect during the first 2 days and very significant changes to the respiratory epithelium on day 4 [2]. N asal brushings were placed in M edium 199 (25 m M hydroxyethyl piperazine ethane sulphonic acid, Earles salt, L-glutamine (ph 7.3)) w hich contained antibiotic solution (streptom ycin 50 pg-ml"1, penicillin 50 p g -m L 1, G ib cob R Life T ech nologies, Paisley, U K ). Transmission electron microscopy. T issue obtained by nasal brushing w as fixed in 2.5% gluteraldehyde in Sorensons phosphate buffer for 48 h and then post fixed in 1% osm ium tetroxide. A fter rinsing in d istilled water, the cells were em bedded in a drop o f 2% liquid agar at 45 C and allow ed to solidify. This w as processed through to resin by standard techniques. U ltrathin sections were cut at 70 nm. These were collected on 200 m esh thin-bar copper grids and stained in 1% uranyl acetate and counter stained in R eyn old s lead phosphate. The sections were then exam ined by transm ission electron m icroscopy. The ciliated epithelium was assessed, in a blind fashion, for both epithelial and ciliary ultrastructural changes. Epithelial integrity was assessed firstly by assessing cell type. The num ber o f ciliated cells, m ucous cells, and dead cells were expressed as a percentage o f all cells exam ined. Secondly, disruption and dam age to the tissue was quantified using the scoring system described by T s a n g et al. [19]. Briefly, the tissue was scored for the follow in g parameters: loss o f cilia from ciliated cells: 0 (fully ciliated), 1,2,3 (3=few cilia visible); projection o f cells from the epithelial edge: 0 (norm al alignm ent), 1, 2, 3 (3=cell projected from edge but som e contact with other epithelial cells); cytoplasm ic blebbing: 0 (absent), 1 (m inor), 2 (major); m itochondrial dam age: 0 (absent), 1 (present) [19], D am age to individual cilia was evaluated by exam ining ciliary ultrastructure for m icrotubular and dynein arm defects. A lignm ent o f individual cilia w ithin a cell was determined by m easuring ciliary orientation [20], Percentages were calculated for the num ber o f cells with loss o f cilia, cellular projections, cytoplasm ic blebbing and m itochondrial dam age. Sim ilarly, the percentage o f cells with m icrotubular or dynein arm defects w as calculated. Ciliary beat frequency and beat pattern. Ciliated strips o f epithelium were suspended in a cham ber created by the separation o f a cover slip and glass slide by tw o adjacent cover slips. The slide was placed on a heated stage (37 C) o f a Leitz, D iaplan m icroscope m ounted on an antivibration table (W entw orth Laboratories Ltd, Sandy, Bedfordshire, U K ). Specim ens were exam ined using a xloo interference contrast lens. Only undisrupted ciliated strips o f > 5 0 pm in length were studied. Beating ciliated edges were recorded using a digital
305 THE EFFECT OF CORO NAVIRU S ON HUM AN CILIA 967 Table 1 Epithelial cell types pre- and postvirus or sham inoculation C i l i a t e d c e l l s D e a d c e l l s M u c o u s c e l l s C o r o n a D a y ( ) 0. 0 ( ) ( ) D a y ( ) * 6. 3 ( ) * ( ) S h a m D a y ( ) 0. 0 ( ) 9. 2 ( ) D a y ( ) 0. 0 ( ) 9 9 ( ) - 1 G r o u p d i f f e r e n c e C o r o n a - s h a m ( ) * 6. 3 ( ) * ( ) D a t a a r e p r e s e n t e d a s m e a n p e r c e n t a g e ( 9 5 % c o n f i d e n c e i n t e r v a l ). # : d i f f e r e n c e b e t w e e n c h a n g e s f r o m b a s e l i n e i n t h e t w o g r o u p s, i.e. ( d a y 3 - d a y 0 ) c o r o n a - ( d a y 3 - d a y 0 ) s h a m. * : p < high-speed video cam era (K odak M otioncorder 1,000, K odak, San D iego, CA, U SA ) at a rate o f 400 fram es-s'1, using a shutter speed o f 1 in 4,000 as previously described [21], Briefly, the cam era allowed video sequences to be replayed at reduced fram e rates or fram e by frame. T his allow s the precise m ovem ent o f individual cilia to be observed during their beat cycle. Ten readings o f ciliary beat frequency (C B F) were taken from different areas alon g each ciliated edge. T o assess the ciliary beat pattern each edge was given a score based on the follow ing scoring system. N orm al coordinated ciliary beating in a forward backward m otion was scored as 0. Cilia that appeared to beat dyskinetically were scored from 1-3 depending on the extent o f abnorm al beating along the edge (all cilia along an edge beating dyskinetically scored 3). C BF was determined directly. G roups o f beating cilia were identified and the num ber o f fram es required to com plete 10 cycles w as recorded. This w as converted to C BF by a simple calculation (CBF=400/(num ber frames for 10 beats) x 10) [21], Statistical analysis The mean and 95% confidence interval (C l) for each group was calculated for day 0 and day 3 for all param eters measured. The m ean change from baseline (day 3-day 0) and 95% CIs were calculated. Results which did not encom pass 0 suggested a difference between day 0 and day 3, which was analysed by a paired-sam ples t-test. A W ilcoxon signed-rank test was used to com pare the dyskinetic beat pattern on day 0 and day 3. An unpaired t-test was used to group differences expressed as the change from baseline for both groups i.e.: (day 3-day 0)corona against (day 3-day 0)sham. This was expressed as mean difference between groups and 95% Cl. Results Three o f 11 volunteers inoculated with H C ov had no sym ptom s o f a cold. Four developed a definite cold and four had a "possible cold". O f the volunteers who developed a definite or possible cold, all suffered mild upper respiratory tract sym ptom s. Six o f these subjects developed a headache, four a cough and tw o a fever. Three o f the four with definite colds and one o f the four with possible colds had virus R N A detected in nasal secretions. Viral R N A w as not detected in the four volunteers w ithout sym ptom s o f a cold. T w o o f the subjects inoculated with U V -inactivated virus developed a m ildly blocked nose lasting for < 2 4 h, com m encing 2 days after inoculation. N o other sym p tom s were reported in this group. A ll subjects inoculated with active H C ov, including the three w ho did not develop sym ptom s o f an upper respiratory tract infection, had disruption o f their respiratory epithelium on day 3. Eight paired sam ples, before and after inoculation with active virus, were ob tain ed from the 11 volunteers for transm ission electron m icroscopy and com parison o f ciliary function. T able 1 sum m arizes the changes seen in cellular type. A fter inoculation with H C ov, there w as a significant reduction in the proportion o f ciliated cells (p < 0.05) and an increase in the proportion o f dead cells (p<0.05). N o changes were seen in the percentage o f m ucous cells. Epithelial disruption and dam age by quantification o f cilia loss, cellular projections, cytoplasm ic blebbing and m itochondrial dam age were all significantly increased on day 3 (p< 0.05) (table 2, figs. la and lb ). A ssessing ciliary ultrastructure (table 3), a small increase in m icrotubular abnorm alities (p<0.05) was found on day 3. N o differences were found between day 0 and day 3 for both dynein arm defects and ciliary orientation. N o significant change was seen between the m ean C BF on day 0 and day 3 (table 3), but cilia were found to beat dyskinetically on day 3 (p<0.05). N o change in structure or function was found in sam ples from volunteers inoculated with U V - inactivated virus (tables 1-3). C om parison o f the change from baseline between subjects inoculated with either active H C ov or U V -inactivated virus show ed significant differences between groups in: loss o f ciliated cells, increase in dead cells, cells with loss o f cilia, cellular projections, cytoplasm ic blebbing, m ito chondrial dam age, m icrotubule defects and ciliary dyskinesia (tables 1-3). Discussion The present results dem onstrate that significant dam age to the respiratory epithelium occurs follow in g
306 968 M.A. CHILVERS ET AL. Table 2.-Assessment of epithelial integrity pre- and postvirus or sham inoculation C e l l s w i t h l o s s C e l l s e x t r u d i n g C e l l s w i t h c y t o p l a s m i c C e l l s w i t h m i t o c h o n d r i a l o f c i l i a f r o m s u r f a c e b l e b b i n g d a m a g e C o r o n a D a y ( ) ( ) ( ) ( ) D a y ( ) * ( ) * ( ) * ( ) * S h a m D a y ( ) ( ) ( ) ( ) D a y ( ) ( ) ( ) ( ) G r o u p d i f f e r e n c e C o r o n a - s h a m ( ) * * * ( ) * * * ( ) * ( ) * D a t a a r e p r e s e n t e d a s m e a n p e r c e n t a g e ( 9 5 % c o n f i d e n c e i n t e r v a l ). d i f f e r e n c e b e t w e e n c h a n g e s f r o m b a s e l i n e i n t h e t w o g r o u p s, i.e. ( d a y 3 - d a y 0 ) c o r o n a - ( d a y 3 - d a y 0 ) s h a m. * : p < ; * * * : p < n a s a l i n o c u l a t i o n w i t h H C o V, e v e n i n t h o s e w i t h o u t o e r t c l i n i c a l s m p o m s f a c o d. E v i d e n c o f v y t o l e d i s r p t i o n o f t h e a s a e p i h l i u m a n d i l i a r y d y s k i u n l t e c n e s i a w a s f o u n d i n a l l o f t h e v o u n t e e r s i n o c u l a t e d l w i t h c i v e H C o V e e n t h o u g h t h r e e s u b j e c t s h a d n o a t v s m p t o m s o f a c o d. N o e p i t h e l i a l d a m a g e w a s s e e n i n y l s u b e c t s w h o w e r e s a m i n f e c t e d w i t h i a c t i v a t e d j h n v i r u s. T h e l a c o f d a m a g e f o l l o w i n s h a i n f e c t i o n k g m s u g g e s t s t h a t t i s s u e d a m a g e i s a r s u l t o f a i n f e c t i v e e n p r o c e s s, r a t h e r t h a n a n m m u n e - m e d i a t e d r e s p o n s e t o i v i r a l a n t i g e n, o r t h e e f f e c o f c y t o i n e s p r e s e n t i n t h e t k i n o c u l u m, s i n c e i t o n l y o c c u r s w h e n l i v e v i r u s i s a d m i n i s t r d. e e A l t h o u g h a n a l y s i s o f e p i t h e l i u m w a s b l i n d e d, o u l a t i o n a s n o t i n d d o r p l a e o c n t r o l l e d. i n c w b l e c b o N e v e r t h e l e s, h e n a s a l m e a s u e m n t s s u g g e s t t h a t t h e s t r e m o d e l w a s s u c c e s s f u l, w i h c l e a r v i d e n c e o f e p i t h e l i a l t e d m a g e i n a l l o f t h o s e i n o c u l a t e d a n d w i t h c l i n i c a l a s y m p t o m s f c o l d i n t h e m a j o r i y. R T - P C R w a s o a t c h o e n s t h e m e t h o t o i d e t i f y H C o, a s t h a s s a d n V i b e e n s h o w t o b m o r e s e s i t i v e t h n e i t h e s e o o g y n e n a r r l o r c u l t u r e t e c h n i q u e [ 1 3 ]. I n r e t r o s p e c t, s e r o l o g y a n d s c e l l c u l t u r e m i g h t h a v e p r o v i d e d u s e f u l a d d i t i o n a l l a b o r a t o r y e i e n c e o f i n e c t i o n. T h R T - P C R r e s u l t s v d f e w e r e o n l y p o s i t i v e i n a p r o p o r t i o n o f t h o s e i o c u l a t e d n d s p i t e e p i t h e l i a l d a m a g e i n a l l c a s e s. T h e r e a s o n s f o r e t h i s m a y b e r e l a t e d t o t h e t i m i n g o f s a m p l e s, s a m p l e q u a l i t a n d t h e p o s s i b i l i t y o f f a l s e - n e g a t i v e r e s l t s y u a r i s i n g u r i n t h R T - P C R s s a y. d g e a D e s p i t e t h e f a c t t h a t o n l y f o u r v o l u n t e e r s w e r e a i n f e t e d, c l e a d i f f r e n c s b e w e e n v o l u n t e e r s s h m c r e e t i n o c a t e d w i t h a c t i v e H C o V a n d s h a m - i n e c t e d s u b u l f e c t s w e r e f u n d. W i t h n t h e s h a m - i n f e c t e d g r o u p, j o i t h e r e w a s n o e v i d e c o f e i t h e l i a l d a m a g e o r c i l i a r y n e p d y s k i n e s i a 3 d a y s a f t e r i n o c u l a t i o n. T h i s u g g e s t s t h a t s v i r a l a n t i g e n s o r m e d i a t o r s w i t h i n t h e i n o c u l u m w e r e n o t r e s p o n s i b l e f o t h e e p i t h e l i a l c h a n g e s f o u d. I t r n a p p a r s t h a t i n e c t i n w i t h a c t i v e v i r u s i s r e q u i r d e f o e f o r e p i t h l i a l a m a g e t o c c u r. e d o B e n d e et al. [7] m a d e p h y s i o l o g i c a l m e a s u r e m e n t s o n 2 4 a d u l t v o l u n t e e r s i n o c u l a t e d w i t h H C o V, o f w h m 1 3 d e v e l o p e d c l i n c a l s y m p t o s, e i h t h a o o i m g d n o e r t c l i n i c a l s y m p t o m s b u t v i r a l s h e d d i n g o c c u r r e d, v a n d t h r e e w e r e n t i n e c t e d. N a s a l a i r w a y r e s i s t a n c e o f a n d t h e t e m p e r a t u r e o f t h e n a s l m u c o s i n c r e a s e d i n a a t h o s e w i t h n d w i t h o u t s y m p t o m s s u g g e s t i n g u n d e r a, l y i n g i n f l a m m a t i n i n b o t h g r o p s. M u c o s a l b l o o d o u f l o w i t h e n o e a n d n a a l m c o u s s e r e i o n i n c e a s e d n s s u c t r o n l y i n t h o s e w i t h s y m p t o m s. T h e s e r e s u l t s a r e c o n s i s t e n t w i t h t h e p r e s e n t f i n d n g s t h a t a n i n f l a m m a t o r y i p r o c e s s m a y b e o c c u r r i n g i n v o l u n t e e r s i n o c l a t e d u w i t h H C o V w h o s h o w n o c l i n i c a l s i g n s o f a c o l d. O v e t v r a u p p e r r e s p i r a t o r y t r a c t i n f e c t r i l i o n s u l l y d l a y n s a l m u o c i l i a r y c l e a a n c e ]. L o s s o f u s a e a c r [ 5 c i l i a t e d e p i t h e l i u m a n d d y s k i n e t i c a l l y b e a t i n g c i l i a F i g. 1. T r a n s m i s s i o n e l e c t r o n m i c r o g r a p h o f n a s a l e p i t h e l i u m b e f o r e a n d a f t e r c o r o n a v i r u s i n o c u l a t i o n, a ) T r a n s m i s s i o n e l e c t r o n m i c r o g r a p h d a y 0. T h i s s h o w s n o r m a l t i s s u e w i t h a n i n t a c t w e l l - c i l i a t e d s u r f a c e a n d m i n i m a l d i s r u p t i o n, b ) T r a n s m i s s i o n e l e c t r o n m i c r o g r a p h d a y 3. T h i s s h o w s a b n o r m a l t i s s u e w i t h s e v e r e l y d i s r u p t e d c e l l s u r f a c e. M a r k e d l o s s o f c i l i a i s s e e n. I n t e r n a l s c a l e b a r s = 2. 9 p m. m a y b e a m a j o r f a c t o r i n t h i s, a l t h o u g h a c h a n g e i n m u c u s r h e o o g y m a y a l s o b e i m p o r t a n t [ 6 ]. I n t h i s l s u d y, h e d u r a t i n o f s y m p t o m s e a k e d a d a 4 a n d t t o p t y r e s o l v e d 7 d a y s a f t e r i n o c u l a t i n. T h e p r e s e n t s t u d y o o n l y p r o v i d e s d a t a 3 d a y s a f t e r i n o c u l a t i o n. T h e t i m e t a k e n f o r t h e u p p e r r e s p i r a t o r y t r a c t e p i t h e l i u m o t
307 THE EFFECT O F CORON AVIRUS ON HUM AN CILIA 969 Table 3.-C iliary function and ultrastructural analysis pre- and postvirus or sham inoculation C B F H z C i l i a r y D y n e i n a r m M i c r o t u b u l e C e n t r a l C i l i a r y d y s k i n e s i a s c o r e d e f e c t s % d e f e c t s % m i c r o t u b u l e d e f e c t s % o r i e n t a t i o n C o r o n a D a y ( ) 0. 2 ( ) 1. 3 ( ) 2. 6 ( ) 0. 3 ( ) ( ) D a y ( ) 1. 1 ( ) * 2. 9 ( ) 4. 7 ( ) * 1. 0 ( ) ( ) S h a m D a y ( ) 0. 1 ( ) 0. 9 ( ) 2. 2 ( ) 0. 0 ( ) ( ) D a y ( ) 0. 2 ( ) 1. 2 ( ) 2. 3 ( ) 0. 0 ( ) ( ) G r o u p d i f f e r e n c e C o r o n a - s h a m ( ) ( ) * 1. 1 ( ) 1. 8 ( ) * 0. 8 ( ) 0. 7 ( ) D a t a a r e p r e s e n t e d a s m e a n ( 9 5 % c o n f i d e n c e i n t e r v a l ). C B F : c i l i a r y b e a t f r e q u e n c y. d i f f e r e n c e b e t w e e n c h a n g e s f r o m b a s e l i n e i n t h e t w o g r o u p s, i.e. ( d a y 3 - d a y 0 ) c o r o n a - ( d a y 3 - d a y 0 ) s h a m. * : p < return to normal follow ing exposure to coronavirus infection is not know n. In naturally-acquired colds, the epithelium returns to norm al in the m ajority o f patients by 3 weeks [1], while mucus clearance m ay be abnorm al for several w eeks follow ing viral infection [5]- A major advantage o f using a digital high-speed video system for ciliary analysis is the ability to play ciliary m ovem ent in slow m otion, allow in g both m easurem ent o f their beat frequency and evaluation o f their beat pattern. D espite recording beat frequency w ithin the normal range 3 days after inoculation with H C ov a significant increase in dyskinetic cilia w as seen. The readings o f C B F are m ade at p hysiological tem peratures with cilia beating at >11 H z. Such high frequencies mean that it is usually im possible to assess dyskinetic m ovem ent w ithout the ability to watch them in slow m otion. P e d e r s e n et al. [5] studied ciliary function at 22 C, follow in g naturally-acquired co m m on colds. A t this tem perature, cilia beat at h alf o f their norm al rate, allow ing gross changes in beat pattern to be more easily detected. T hey also noted a significant increase in ciliary asynchrony that was m axim al during the first few days o f the infection. O thers have also found C B F, m easured at p hysiological temperatures, to be within the norm al range follow in g viral infection, but they have not com m ented on the presence o f dyskinesia It is possible that ciliary dyskinesia was m issed due to the rapid beat frequency [2]. Electron m icroscopy showed significant loss o f epithelial integrity and a very significant decrease in the num ber o f ciliated cells after coronavirus inoculation. Similar findings have been recorded follow ing nasal infection with other viruses [5, 6, 22, 23]. On day 3, the authors found a sm all increase in peripheral and central m icrotubular abnorm alities. T his is less than previously reported in naturallyacquired viral infection in children [3], although none o f the patients in that study had a coronavirus infection. In 22 o f the 30 episodes o f culture-proven viral infection, C a r s o n et al. [3] found m icrotubular additions and deletions. In the rem aining eight cases, epithelial cell abnorm ality and the loss o f ciliated cells were too severe to allow ciliary structure to be evaluated. Beat frequency or analysis o f ciliary dyskinesia was not explored [3], N o virions were seen on electron m icroscopy in the present study despite epithelial dam age. This is in keeping with other reports o f electron m icroscopy o f hum an nasal and bronchial epithelium during episodes o f the com m on cold [1, 3, 24, 25]. In co n trast, there are m any published reports o f in vitro coronavirus-infected cells [26-28]. A f z e l iu s [11] d ocu m ented a single case o f coronavirus infection o f the nasal respiratory epithelium in a 2-yr-old girl with no sym ptom s o f a com m on cold. This w as an incidental finding on a biopsy taken to rule out the diagnosis o f primary ciliary dyskinesia. V irions could be seen within and outside the ciliated cells but not in the m ucous cells. Som e virions were located near the m icrovilli with others in pockets in the apical cell m em brane, suggesting the m icrovilli m ay be the site o f first contact. D am age to the respiratory epithelium m ay occur due to viral infection w ithout overt clinical sym ptom s. This m ay well have im plications in lung disease; for exam ple in chronic suppurative diseases, where lung function w as significantly reduced follow in g sym ptom atic and asym ptom atic viral infections [29]. Further work is needed to establish the m echanism o f viral dam age to the respiratory epithelium caused by hum an coronavirus infection. Acknowledgements. T h e a u t h o r s w o u l d l i k e t o t h a n k J. T h o m p s o n, s e n i o r l e c t u r e r i n s t a t i s t i c s a t t h e U n i v e r s i t y o f L e i c e s t e r, f o r s t a t i s t i c a l a d v i c e. T h e a u t h o r s a l s o t h a n k t h e v i r o l o g y l a b o r a t o r y a t t h e L e i c e s t e r R o y a l I n f i r m a r y f o r t h e i r s u p p o r t. References 1. R a u t i a i n e n M, N u u t i n e n J, K i u k a a n n i e m i H, C o l l a n Y. U l t r a s t r u c t u r a l c h a n g e s i n h u m a n n a s a l c i l i a c a u s e d b y t h e c o m m o n c o l d a n d r e c o v e r y o f c i l i a t e d e p i t h e l i u m. Ann Otol Rhinol Laryngol ; : W i l s o n R, A l t o n E, R u t m a n A, et al. U p p e r r e s p i r a t o r y t r a c t v i r a l i n f e c t i o n a n d m u c o c i l i a r y c l e a r a n c e. Eur J Respir Dis ; 7 0 : C a r s o n J L, C o l l i e r A M, H u S S. A c q u i r e d c i l i a r y d e f e c t s i n n a s a l e p i t h e l i u m o f c h i l d r e n w i t h a c u t e v i r a l u p p e r r e s p i r a t o r y i n f e c t i o n s. N Engl J Med ; :
308 970 M.A. CHILVERS ET AL. 4. S a k a k u r a Y. C h a n g e s o f m u c o c i l i a r y f u n c t i o n d u r i n g c o l d s. Eur J Respir Dis Suppl ; : P e d e r s e n M, S a k a k u r a Y, W i n t h e r B, B r o f e l d t S, M y g i n d N. N a s a l m u c o c i l i a r y t r a n s p o r t, n u m b e r o f c i l i a t e d c e l l s, a n d b e a t i n g p a t t e r n i n n a t u r a l l y a c q u i r e d c o m m o n c o l d s. Eur J Respir Dis Suppl ; : S a k a k u r a Y, S a s a k i Y, H o r n i c k R B, et al. M u c o c i l i a r y f u n c t i o n d u r i n g e x p e r i m e n t a l l y i n d u c e d r h i n o v i r u s i n f e c t i o n i n m a n. Ann Otolaryngol ; 8 2 : B e n d e M, B a r r o w I, H e p t o n s t a l l J, et al. C h a n g e s i n h u m a n n a s a l m u c o s a d u r i n g e x p e r i m e n t a l c o r o n a v i r u s c o m m o n c o l d s. Acta Otolaryngol ; : M o n t o A S. C o r o n a v i r u s e s. hr. E v a n s A S, e d. V i r a l I n f e c t i o n s o f H u m a n s. N e w Y o r k, P l e n i u m B o o k C o m p a n y, ; J o h n s t o n S L, P a t t e m o r e P K, S a n d e r s o n G, et al. C o m m u n i t y s t u d y o f r o l e o f v i r a l i n f e c t i o n s i n e x a c e r b a t i o n s o f a s t h m a i n y e a r o l d c h i l d r e n. BM J ; : T y r r e l l D A J, B y n o e M L. C u l t i v a t i o n o f a n o v e l t y p e o f c o m m o n - c o l d v i r u s i n o r g a n c u l t u r e s. BM J ; 1 : A f z e l i u s B A. U l t r a s t r u c t u r e o f h u m a n n a s a l e p i t h e l i u m d u r i n g a n e p i s o d e o f c o r o n a v i r u s i n f e c t i o n. Virchows Archive ; : M a c n a u g h t o n M R, M a d g e M H, R e e d S E. T w o a n t i g e n i c g r o u p s o f h u m a n c o r o n a v i r u s e s d e t e c t e d b y u s i n g e n z y m e - l i n k e d i m m u n o s o r b e n t a s s a y. Infect Immun ; 3 3 : M y i n t S. S i d d e l l S, T y r r e l l D. D e t e c t i o n o f h u m a n c o r o n a v i r u s E i n n a s a l w a s h i n g s u s i n g R N A : R N A h y b r i d i z a t i o n. J Med Virol ; 2 9 : G w a l t n e y J M J r, H e n d l e y O, H a y d e n F G, et al. U p d a t e d r e c o m m e n d a t i o n s f o r s a f e t y - t e s t i n g o f v i r a l i n o c u l a u s e d i n v o l u n t e e r e x p e r i m e n t s o n r h i n o v i r u s c o l d s. Prog Med Virol ; 3 9 : J o h n s t o n S L, P a p i A, B a t e s P J, M a s t r o n a r d e J G, M o n i c k M M, H u n n i n g h a k e G W. L o w g r a d e r h i n o v i r u s i n f e c t i o n i n d u c e s a p r o l o n g e d r e l e a s e o f I L - 8 i n p u l m o n a r y e p i t h e l i u m. J Immunol ; : J a c k s o n G G, D o w l i n g H F, S p e i s m a n I G, B o a n d A V. T r a n s m i s s i o n o f t h e c o m m o n c o l d u n d e r c o n t r o l l e d c o n d i t i o n s. 1. T h e c o m m o n c o l d a s a c l i n i c a l e n t i t y. Arch Intern Med ; : M c k e a n M C, L e e c h M, L a m b e r t P L, H e w i t t C, M y i n t S, S i l v e r m a n M. A m o d e l o f v i r a l w h e e z e i n n o n a s t h m a t i c a d u l t s : s y m p t o m s a n d p h y s i o l o g y. Eur Respir J ; 1 8 : R u t l a n d J, C o l e P J. N o n - i n v a s i v e s a m p l i n g o f n a s a l c i l i a f o r m e a s u r e m e n t o f b e a t f r e q u e n c y a n d s t u d y o f u l t r a s t r u c t u r e. Lancet ; 2 : T s a n g K W T, R u t m a n A, T a n a k a E, et al. I n t e r a c t i o n o f Pseudomonas aeruginosa w i t h h u m a n r e s p i r a t o r y m u c o s a in vitro. Eur Respir J ; 7 : R a y n e r F J, R u t m a n A, D e w a r A, G r e e n s t o n e M A, C o l e P J, W i l s o n R. C i l i a r y d i s o r i e n t a t i o n a l o n e a s a c a u s e o f p r i m a r y c i l i a r y d y s k i n e s i a. Am J Respir Cril Care Med ; : C h i l v e r s M A, O ' C a l l a g h a n C. A n a l y s i s o f c i l i a r y b e a t p a t t e r n a n d b e a t f r e q u e n c y u s i n g d i g i t a l h i g h s p e e d i m a g i n g : c o m p a r i s o n w i t h t h e p h o t o m u l t i p l i e r a n d p h o t o d i o d e m e t h o d s. Thorax ; 5 5 : H o o r n B, T y r r e l l D A. E f f e c t s o f s o m e v i r u s e s o n c i l i a t e d c e l l s. Am Rev Respir Dis ; 9 3 : S u p p l ^ T r i s t r a m D A, H i c k s W J r, H a r d R. R e s p i r a t o r y s y n c y t i a l v i r u s a n d h u m a n b r o n c h i a l e p i t h e l i u m. Arch Otolaryngol Head Neck Surg : : G i o r g i P L, O g g i a n o N, B r a g a P C, et al. C i l i a i n c h i l d r e n w i t h r e c u r r e n t u p p e r r e s p i r a t o r y t r a c t i n f e c t i o n s : u l t r a s t r u c t u r a l o b s e r v a t i o n s. Pediat Pulmonol ; 1 4 : W i n t h e r B, B r o f e l d t S, C h r i s t e n s e n B, M y g i n d N. L i g h t a n d s c a n n i n g e l e c t r o n m i c r o s c o p y o f n a s a l b i o p s y m a t e r i a l f r o m p a t i e n t s w i t h n a t u r a l l y a c q u i r e d c o m m o n c o l d s. A da Otolaryngol ; 9 7 : B e c k e r W B, M c I n t o s h K, D e e s J H, C h a n o c k R M. M o r p h o g e n e s i s o f a v i a n i n f e c t i o u s b r o n c h i t i s v i r u s a n d a r e l a t e d h u m a n v i r u s ( s t r a i n E ). J Virol ; 1 : M c I n t o s h K, D e e s J H, B e c k e r W B, K a p i k i a n A Z, C h a n o c k R M. R e c o v e r y i n t r a c h e a l o r g a n c u l t u r e s o f n o v e l v i r u s e s f r o m p a t i e n t s w i t h r e s p i r a t o r y d i s e a s e. Proc Natl Acad Sci USA ; 5 7 : E v e r m a n n J F, H e e n e y J L, M c K e i r n a n A J, O ' B r i e n S J. C o m p a r a t i v e f e a t u r e s o f a c o r o n a v i r u s i s o l a t e d f r o m a c h e e t a h w i t h f e l i n e i n f e c t i o u s p e r i t o n i t i s. Virus Res ; 1 3 : C o l l i n s o n J, N i c h o l s o n K G, C a n c i o E, et al. E f f e c t s o f u p p e r r e s p i r a t o r y t r a c t i n f e c t i o n s i n p a t i e n t s w i t h c y s t i c f i b r o s i s. Thorax ; 5 1 :
309 3 33 AIRW AY BIOLOGY Functional analysis of cilia and ciliated epithelial ultrastructure in healthy children and young adults M A Chilvers, A Rutman, C O 'C allaghan See end of article for authors' affiliations Correspondence to: Professor C O'Callaghan, Department of Child Health, University of Leicester, School of Medicine, Robert Kilpatrick Clinical Sciences Building, Leicester Royal Infirmary, P O Box 65, Leicester LE2 7LX, UK; ajb64@le.ac.uk Revised version received 23 October 2002 Accepted for publication 23 November 2002 Thorax ;5 8 : Background: There are very few data on normal ciliary beat frequency, beat pattern, and ultrastructure in healthy children and adults. A study was undertaken to define ciliary structure, beat frequency and beat pattern in a healthy paediatric and young adult population. Methods: Ciliated epithelial samples were obtained from 76 children and adult volunteers aged 6 months to 43 years by brushing the inferior nasal turbinate. Beating cilia were recorded using a digital high speed video camera which allowed analysis of ciliary beat pattern and beat frequency. Tissue was fixed for transmission electron microscopy. Results: The mean ciliary beat frequency for the paediatric population (12.8 Hz (95% Cl 12.3 to 13.3)) was higher than for the adult group (11.5 Hz (95% Cl 10.3 to 12.7 Hz), p<0.01, t test); 10% (range 6-24% ) of ciliated edges were found to have areas of dyskinetically beating cilia. All samples had evidence of mild epithelial damage. This reflected changes found in all measurements used for assessment of epithelial damage. Ciliary ultrastructural defects were found in less than 5% of cilia. Conclusion: Normal age related reference ranges have been established for ciliary structure and beat frequency. In a healthy population localised epithelial damage may be present causing areas of ciliary dyskinesia. R espiratory cilia beat in a coordinated m anner w ith a specific frequency and pattern, clearing m ucus and debris from the airways. Acquired or congenital ciliary u l trastructural defects result in cilia which are either stationary or beat in a slow or dyskinetic fashion. Ineffective m ovem ent impairs mucociliary clearance. In prim ary ciliary dyskinesia this causes sinusitis and recurrent chest infections w hich may lead to bronchiectasis.12 An early diagnosis of prim ary ciliary dyskinesia is im portant as institution of appropriate respiratory care has been shown to halt the progressive decline in lung fu nction/ A diagnosis is made on the basis of a supportive clinical history and an abnorm al ciliary beat frequency accom panied, in most cases, by specific abnorm alities of the ciliary axonem e on electron microscopy.4 Studies by Rossman and colleagues suggest that evaluation of beat pattern, in addition to beat frequency, may be helpful in the diagnosis of patients w ith prim ary ciliary dyskinesia/ Making a confident diagnosis of prim ary ciliary dyskinesia can at times be very difficult as abnorm alities of the epithelium and cilia may also be found purely as a result of acquired ciliary d e fe c ts/7 It is therefore im portant to differentiate between primary and secondary ciliary structural and functional abnormalities. Secondary ciliary ultrastructural defects are com m on.8 Defects may persist for up to 12 weeks following resolution of an upper respiratory tract infection6 410 and ultrastructural interpretation may be difficult/ Q uantitative ultrastructural analysis in healthy adult subjects is limited." 12 Paediatric studies of the ciliary ultrastructure have been small and have consisted of patients rather than healthy controls. Data suggest that 5% of cilia have abnorm alities,713 w ith reports only analysing microtubular defects/ 14 The analysis of the dynein arms has been limited to patients w ith respiratory infections; up to 30% of cilia have been found to be affected." 16 Reference ranges for healthy children are not available for either ciliary microtubules or the presence of dynein arms, and there are no data on the dam age of nasal ciliated epithelium in a healthy control population. While norm al ranges of ciliary beat frequency in adults have been published/ 11 there are few data for children. Cilia from neonatal patients17and adolescents18were found to beat at a higher frequency than cilia from adults. O ther studies have suggested that ciliary beat frequency may either fall w ith age14 or rem ain constant Evaluation of cilia in children involves the sampling of nasal epithelium and analysis of ciliary beat frequency, beat pattern, and ultrastructure.4 22 Few data from norm al children are available to allow comparison w ith findings in patients suspected of having prim ary ciliary dyskinesia. We have adopted a digital high speed im aging m ethod that allows the exact movement of a cilium to be rapidly evaluated throughout the beat cycle and m easurem ent of the ciliary beat frequency. The direct observation of ciliary m ovem ent in slow motion and the ability to archive such m aterial for audit and research is a major advantage over existing methods. It is likely that high speed video analysis will become the preferred m ethod for evaluation of ciliary beat pattern and beat frequency for the diagnosis of prim ary ciliary dyskinesia. Using digital high speed imaging we have been able to describe precisely the norm al ciliary beat cycle and found it to differ from previously published data.1 The high speed video m ethod has also been evaluated against other existing indirect techniques such as the photodiode and photom ultiplier m ethods for the m easurem ent of ciliary beat frequency and significant differences were found.1this again em phasises the need for a norm al range to be established for each m ethod. Reference ranges exist for both the photom ultiplier" and photodiode18 m ethods, but no norm al reference range exists for digital high speed imaging. The aim of this study was to m easure ciliary beat frequency and to determ ine the ciliary beat pattern and ultrastructure in healthy children and adults. The second aim of the study was to determ ine the ultrastructure of the respiratory epithelium from healthy children and adults.
310 334 Chilvers, Rutman, O 'Callaghan A B / Figure 1 Transmission electron micrographs illustrating the parameters assessed to examine epithelial dam age in comparison with normal epithelium shown in fig 2A. (A) Severe loss of cilia, grade 3 (bar 10 pm). (B) Projection of a cell from the epithelial edge, grade 3 (bar 10 pm). (C) Cytoplasmic blebbing, grade 2 (bar 10 pm). (D), (E) Mitochondrial damage: a cell with a normal healthy mitochondrion (arrowhead, E) is shown against a cell with a damaged mitochondrion (arrow, D), grade 1 (bar 2 pm). METHODS Fifty three healthy children (31 male, age range 6 m onths to 17 years) were recruited from subjects undergoing elective surgery and 23 adult volunteers (16 male, age range years) were also recruited. Subjects were excluded if they had a history of chronic respiratory or nasal disease or a symptomatic upper respiratory tract infection during the previous 6 weeks, were taking regular medication, or were known smokers. Paediatric samples were obtained im mediately after induction of anaesthesia w ith propofol. This agent has been shown to have no effect on ciliary beat frequency.2 No prem edication had been given to any subject before surgery. In all subjects ciliated sam ples were obtained by brushing the inferior nasal turbinate w ith a 2 m m cytology brush. Nasal brushings were placed in m edium 199 (ph 7.3) w hich contained antibiotic solution (streptom ycin 50 pg/m l, penicillin 50 pg/ml; Gibco, UK). The study w as approved by the Leicestershire ethical review com m ittee and written consent was obtained before sam pling.
311 Normal ciliary structure and function 335 Figure 2 Transmission electron micrographs showing assessment of epithelial integrity. (A) Normal tissue with an intact well ciliated surface and minimal disruption; epithelial integrity score=0 (bar 10 pm). (B) Abnormal tissue with severely disrupted cell surface and marked loss of cilia; epithelial integrity score=5 (bar 10 pm). Evaluation of ciliary structure and function Tissue obtained by nasal brushing w as fixed in 2.5% glutaraldehyde and processed through to resin by standard techniques as previously described. 4 Ultrathin sections w ere cut at 70 nm. These were collected on 200 m esh thin bar copper grids, stained in 1% uranyl acetate, counterstained in Reynold's lead phosphate, and exam ined by transm ission electron m icroscopy. The ciliated epithelium w as assessed blindly for both epithelial and ciliary ultrastructural changes. Epithelial integrity w as assessed by firstly exam ining cell type. The num ber of ciliated cells, m ucous cells, and dead cells w ere totalled and expressed as a percentage of all cells exam ined. Disruption and dam age to the tissue w as quantified using the scoring system previously described Briefly, the tissue w as scored for the follow ing parameters: loss of cilia from ciliated cells: 0 (fully ciliated), 1, 2, 3 (a few cilia visible); projection of cells from the epithelial edge: 0 (norm al alignm ent), 1, 2, 3 (cell projected from edge but som e contact w ith other epithelial cells); cytoplasm ic blebbing: 0 (absent), 1 (m inor), 2 (major); mitochondrial damage: 0 (absent), 1 (present) (fig 1). To give an overall evaluation of epithelial dam age an epithelial integrity score w as given to the epithelium w hich incorporated ciliary loss, cellular projection, cytoplasm ic blebbing and m itochondrial dam age. A healthy intact epithelial edge w as scored 0 and a severely disrupted edge w as scored 5 (0 = n o dam age, 1 = minor, 2 = m ild, 3 = moderate, 4 = major, 5= severe damage; fig 2). The scoring system w as evaluated by com paring it against all m easurem ents used to m easure epithelial damage. Dam age to individual cilia w as evaluated by exam ining the ciliary ultrastructure for microtubular and dynein arm defects. Alignm ent of individual cilia w ithin a cell was assessed by m easuring ciliary orientation as previously described.26 The percentage of cells w ith loss of cilia, cellular projections, cytoplasm ic blebbing, m itochondrial damage and w ith microtubular or dynein arm defects was calculated. Ciliary beat frequency and beat pattern Ciliary beat frequency and beat pattern were evaluated as previously described.1 Briefly, ciliated strips of epithelium were suspended in a chamber created by the separation of a cover slip and glass slide by tw o adjacent cover slips. The slide w as placed on a heated stage (37 C) of a Leitz Diaplan m icroscope m ounted on an anti-vibration table (W entworth Laboratories Ltd, UK). Specim ens w ere exam ined using a xloo interference contrast lens. Only undisrupted ciliated strips longer than 50 pm devoid of m ucus were studied. Beating ciliated edges w ere recorded using a digital high speed video camera (Kodak M otioncorder Analyser, M odel 1000) at a rate of 400 frames per second. The camera allows video sequences to be recorded and played back at reduced frame rates or frame by frame. The ciliated edge, projected onto a high resolution m onitor, w as divided into five adjacent areas m easuring 10 pm. TWo m ea s urem ents of ciliary beat frequency w ere m ade in each area, resulting in a total of 10 m easurem ents of beat frequency along each ciliated strip. A m axim um of 10 edges were analysed per subject. Ciliary beat frequency w as determ ined directly. Groups of beating cilia w ere identified and the num ber of frames required to com plete 10 cycles recorded. This w as converted to ciliary beat frequency (CBF) using the calculation (CBF= 400/(num ber frames for 10 beats) x 10).1 As the digital high speed video system w as to be used to establish reference ranges for the m easurem ent of ciliary beat frequency, the reproducibility o f the m ethod w as evaluated. A single point on the ciliated edge w as identified on a grid placed on the monitor. Ciliary beat frequency at that point w as m easured independently by two observers (O l, 0 2 ), and this w as repeated for each of the five areas displayed on the m onitor. A total of five readings w ere obtained for each edge and the analysis w as performed in 10 subjects. One observer repeated the series of m easurem ents tw o days later (M l, M 2). From this the inter-observer and intra-observer coefficient of variation (CV) could be calculated. To assess the ciliary beat pattern each edge w as analysed. Coordinated ciliary beating in a forward backward m otion along the w hole epithelial edge w as defined as norm al. Edges w hich appeared to have dyskinetically beating cilia w ere noted and the percentage of edges exhibiting areas of dyskinetically beating cilia w as then calculated. Analysis of data As ciliary beat frequency m ay change w ith age,1718 w e w anted to see if other parameters show ed such variation. As suggested by Roth et al,'* a cut off w as m ade at 18 years of age. To allow sufficient subjects in each age group, three age ranges were used: 0-6,7-1 2, and years of age. Adults were classified as > 1 9 years. To form reference ranges the m ean ciliary beat frequency, standard deviation, 5th and 95th percentiles w ere calculated for individual age groups. A one w ay analysis of variance w as performed betw een groups. Individual groups w ere compared using a Student's t test. Similarly, the m ean percentage, 5th and 95th percentiles of edges exhibiting areas of dyskinetically beating cilia w ere calculated. For all ultrastructural
312 336 Chilvers, Rutman, O 'C allaghan Table 1 Analysis of cell type by transmission electron microscopy Age (years) n Ciliated cells (%) Unciliated cells (%} Mucous cells (%) Dead cells (%) (45.9, 81.0) (1 3.1,4 2.3 ) 9.9 (5.2, 17.5) 0.0 (0.0, 0.0) (33.7, 76.0) 26.8 (16.2, 52.9) 10.6 (4.9, 16.4) 0.0 (0.0, 0.0) (59.6, 79.1) (1 3.6, 32.2) 8.1 (5.7, 10.4) 0.0 (0.0, 0.0) > (43.0, 78.6) 22.1 (12.4, 43.1) 10.4(6.4, 19.7) 0.0 (0.0, 0.0) Results are expressed as the mean percentage (5th and 95th percentiles) For individual age groups. Table 2 Transmission electron microscopy assessment of epithelial integrity Age (years) n Cells with loss of cilia (%) Cells extruding from surface (%) Cells with cytoplasmic blebbing (%) Cells with mitochondrial dam age (%) Epithelial integrity (9.1, 38.4) (12.9, 37.0) 14.2 (6.4, 23.5) (1.1, 33.3) 1.3 (0.8, 2.0) (6.0, 58.2) 21.5 (10.5, 35.8) 11.8 (5.5, 24.2) 8.9 (0.0, 21.9) 1.3 (0.8, 1.9) (7.2, 37.4) 18.7 (7.9, 31.6) 11.5 (2.5, 24.3) 8.0 (2.3, 16.1) 1.1 (0.8, 1.7) > (1 5.7, 72.0) 25.5 (18.6, 36.1) 13.4 (6.0, 24.2) 10.1(1.3, 21.5) 1.1 (0.5, 2.0) Results are for individual age groups and expressed as the mean (5th and 95th percentiles). Table 3 Analysis of ciliary ultrastructure by transmission electron microscopy Age (years) n Dynein arm counts Inner O uter Dynein arm defects (%) M icrotubule defects (%) Central m icrotubule defects (%) Ciliary orientation (7.2, 7.7) 7.9 (7.6, 8.2) 1.8 (0.0, 7.1) 2.1 (0.6, 5.6) 0.3 (0.0, 1.2) 10.9 (9.9, 12.5) (7.0, 7.4) 7.7 (6.4, 8.3) 1.3 (0.0, 4.1) 1.9 (0.0, 4.4) 0.7 (0.0, 2.2) 10.7 (9.7, 11.4) (6.7, 7.3) 7.5 (5.6, 8.1) 1.0 (0.0, 1.9) 2.3 (1.2, 3.6) 1.1 (0.0, 3.2) 10.8 (9.7, 11.9) > (6.5, 7.9) 6.8 (5.7, 8.1) 1.0 (0.0, 2.8) 1.9 (0.8, 3.8) 0.3 (0.0, 1.0) 10.7 (9.7, 11.6) Results for individual age groups are expressed os the mean (5th and 95th percentiles). Table 4 Summary of analysis of ciliary beat frequency measurements Age (years) n Ciliory b eat frequency (Hz)* Mean SD 5th, 95th centiles Dyskinetically beoting edges (%)f , (0.0, 36.8) , (0.0, 40.3) , (0.0, 56.9) > , (0.0, 24.3) M ean ciliary beat frequency, standard deviation (SD), and 5th and 95th percentiles. tm ean (5th, 95th percentiles) percentage of edges exhibiting areas of ciliary dyskinesia. parameters the m ean and the 5th and 95th percentiles were calculated. A one way analysis of variance w as performed between groups. RESULTS Ciliary beat frequency and beat pattern were m easured in all subjects; 56 had sufficient tissue for epithelial integrity m easurem ents and 60 for ciliary ultrastructure. Ciliary beat frequency and beat pattern were the initial m easurem ents to be m ade after which sam ples were then processed for electron microscopy. During this procedure tissue may be lost. Consequently, som e subjects had an inadequate sam ple for full ultrastructural analysis. Table 1 show s the percentage of different cell types seen in the epithelial strips obtained. Analysis showed no difference betw een the percentage of different cells identified and the age of the subject. Ciliated cells formed 65% of the cell population. Analysis of the factors involved in epithelial integrity are sum m arised in table 2. Even w ithin the healthy population there is evidence of loss of cilia, cellular extrusion, cytoplasm ic blebbing, and mitochondrial damage. Analysis of variation found no difference betw een groups for all m easurem ents analysed. The epithelial integrity score, w hich reflects a com bination of all the m easurem ents used to assess epithelial damage, also showed no significant difference betw een the age groups. A sum m ary of the results of the ultrastructural analysis of individual cilia is show n in table 3. Dynein arm defects were found in less than 3% of cilia observed. It w as possible to visu alise, on average, seven of the expected nine dynein arms w hen counting both inner and outer dynein arms. Again no differences were found in the ultrastructural analysis of individual cilia betw een the various age groups. Microtubular abnorm alities were uncom m on in all age groups; ciliary orientation did not vary with age (table 3). Ciliary beat frequency and the percentage of cells w ith ciliary dyskinesia are show n in table 4. No significant difference in m ean ciliary beat frequency betw een individual age groups w as found (ANOVA, p = ). However, there w as a significant difference in ciliary beat frequency betw een patients under the age of 18 (12.8 Hz (95% Cl 12.3 to 13.3)) and those over the age of 18 (11.4 Hz (95% Cl 10.2 to 12.6 Hz), p < 0.0 1, t test). Approximately 10% of all edges analysed exhibited areas of
313 Normal ciliary structure and function 337 ^ 1 2 co U N.L Ll- CQ u 24 r A 24 B Age (years) * JL I *.. ^ i * - *.4...» _f ^ 8 ^ ' 1 * Age (years) Age (years) Figure 3 (A) M ean ciliary beat frequency (CBF) plotted against a g e showing a negative correlation between increasing ag e and a reduction in ciliary beat frequency (correlation coefficient r= -0.30). (B) Edges with the lowest ciliary beat frequency within a sample plotted against a g e for all subjects. (C) Eages with the highest ciliary beat frequency within a sample plotted against a g e for all subjects. M ean (solid line) and ± standard deviation (dashed line) regression lines are shown. dysk in etically b eating cilia. This w as found to be higher in the year group. To estab lish a reference range the m ea n ciliary beat freq u en cy w as plotted against age (fig 3A ). A w ea k n egative correlation w a s found b etw een m ean ciliary beat frequency an d increasing age (r = , p = ). This w a s m odelled and a linear relationship w as found. Q uadratic and other relation sh ip s w ere m odelled and found not to be significant. W ith in each sam p le cilia w ere found to beat at d ifferent freq u en cies. To evalu ate sam ple variation in ciliary beat freq u en cy th e ed ges w ith the h ig h est and lo w est ciliary beat freq u en cy w ere plotted again st age for each subject. The m axim al ciliary beat frequency (fig 3C) of ed ges ranged from to Hz w ith 95% of subjects h avin g a m a x im a l ciliary beat frequency o f > 1 0 Hz. The low est m e a n ciliary beat frequency (fig 3B) of edges ranged from 6.0 to 17.1 Hz w ith 85% of subjects having a m in im u m beat frequ en cy of > 8 Hz. N o significant difference w a s observed for the interobserver (0 1, 0 2 ) and intra-observer (M l, M 2) m e a su r e m en ts of CV.The m ean (SD ) CV for 0 1 an d 0 2 w a s 11.6 (3.8)% (95% Cl 8.3 to 14.9) and 10.7 (4.4)% (95% Cl 6.3 to 14.6), respectively, and for M l and M 2 w a s 10.7 (4.4)% (95% Cl 6.3 to 14.6) and 10.9 (5.1)% (95% Cl 5.8 to 16.0), respectively. The m ea n (SD ) d ifferen ce in inter-ob server CV w a s 0.9 (2.3)% (95% Cl -1.1 to 2.9; r a n g e to 4.9 ) and in in tra-ob server CV w as 0.7 (2.0)% (95% C l to 2.4; r a n g e to 8.5 ). DISCUSSION E xam in ation o f the nasal ciliated e p ith e liu m from a large group o f h ea lth y children an d a sm aller grou p o f ad u lts has enabled us to esta b lish n orm al a ge related referen ce ran ges for b oth ciliary structure and fun ction. There are few data q u a n tify in g the ciliary ep ithelial ultrastructure fo llo w in g b rush biopsy. We fo u n d evid en ce o f m inor ep ith elial d am age in th e tissu e from h ea lth y subjects. Our resu lts sh o w a greater d egree o f ep ith e lia l d a m a g e than previou sly d escrib ed.25 H ow ever, th e se data w ere from organ culture m o d e ls an d it is p ossib le that, in the process o f b ru sh in g and tissu e preparation, m in or d am age m ay have occurred. A lth o u g h tw o previous stu d ies have evalu ated th e u se o f n asal b ru sh in g to sam p le cilia for ultrastructural m ea su rem en t, they did not assess epithelial d am age A scoring sy stem for evalu ation o f ep ith elial in tegrity has b een developed. This has b een validated against the m easu rem e n ts u sed to a ssess ep ithelial ultrastructural d am age and found to be rep resen tative o f the m inor ep ith elia l dam age observed in h ealth y subjects. The percen tage o f d ynein arm and m icrotubular a b n o rm a lities w ere both found to be less than 5%, w h ic h agrees w ith other publish ed d ata.7 The m ea n orien tation o f cilia in the paediatric p o p u lation has on ly b een described in eight children under th e age o f 2 years and w as reported to be ,,This is h igh er than the valu es w e ob tain ed ( ) in 60 subjects of differing ages. The q u an tification o f inner and outer d y n ein arm s is im portant in the d iagn osis o f prim ary ciliary d ysk in esia as d yn ein arm d efects are the m o st co m m o n a b n orm ality found in these p a tien ts.28 The m ajority o f inner and outer d yn ein arm s w ere visu alised in all subjects. Our resu lts are co n sisten t w ith other p u b lish ed data on the n um ber o f outer d yn ein arm s visible, but w e w ere able to id en tify a greater proportion o f inner d yn ein arm s than h as previously b een rep orted.30 This m ay be b ecau se o f the h ea lth y n ature o f the tissu e. As su ggested by Veale and colleagu es, w e ex a m in ed ciliary beat frequencies from several ed g es and from differen t sites alon g an e d g e.31 The ciliary beat frequency w a s found to vary b etw een ed ges w ith in a sam ple, w ith a m ea n beat freq u en cy of < 1 1 Hz in som e subjects. This is in k eep in g w ith other reports w h ich have found cilia in h ea lth y ad u lts to beat m a x im a lly at a frequency o f > 1 0 Hz (range ) an d m in im a lly at a frequency o f > 7 Hz (range ).31 This w a s lim ited to 20 volu n teers an d n o children w ere in clu d ed. A dults w ere found to have slow er b eating cilia31 w ith freq u en cies as lo w as 6-9 H z.'g21 Two h ealth y ch ild ren w ere also reported to have cilia beating as slow ly as 6 H z.21 The CV for m easu rem en t o f ciliary beat frequency alon g an ep ithelial ed g e h as b een sh o w n to vary from 9% to 58%!1 c o m pared w ith 10% in our study. We found n o significant d ifference in in ter-ob server or intra-observer CV u sin g the digital h igh speed video tech n iqu e. The ciliary beat freq u en cy o f th e children w a s found to be sign ifican tly greater th an th e adult population. This is supported by stu d ies w h ich h ave sh o w n ciliary beat frequency
314 33 8 Chilvers, Rutman, O 'C allaghan o f n e o n a te s17 and teen agers1* to be greater th a n adult su b jects. O ur data suggest a slight fall in ciliary beat freq u en cy w ith in creasin g age, w h ich is in agreem en t w ith oth er s tu d ie s,10 a lth o u g h Jorissen ct al' found ciliary beat freq u en cy to be in d ep en d en t o f age. However, their read in gs w ere co n d u cte d at 2 2 C rather than at body tem perature w h ic h m a k e s th e com p arison difficult. At this tem perature cilia beat at a m u c h slow er frequency and the association m ay therefore h ave b een lo s t.17 D igital h igh speed video im agin g a llow ed u s to v isu a lise precisely the norm al ciliary beat p attern in h e a lth y subjects; 10% o f ed ges had ev id en ce o f d y sk in etica lly b ea tin g cilia. The rem ainder o f the cilia w ere foun d to beat forw ard and backw ards w ith in the sam e plane w ith o u t a classical sid ew a y s recovery sw eep. T his is co n siste n t w ith our earlier d esc rip tio n.1a n alysis o f ciliary beat p attern m ay im prove our u n d ersta n d in g o f the a ctio n s o f variou s respiratory p a th o g en s for ex a m p le, cilia fo llo w in g in fe ctio n h a v e b een found to have a d y sk in etic beat p attern d esp ite b ea tin g at a norm al ciliary beat frequency.24 In sum m ary, w e h ave esta b lish ed an e x ten siv e age related norm al reference range for b oth ciliary structure an d fu n ction. We have also ex a m in ed th e ep ith elial in tegrity in a h ea lth y p o p u lation. Such data w ill h elp w ith our evalu ation o f p a tien ts su sp ected o f h avin g prim ary ciliary d ysk in esia and in research stu d ies look in g at th e effects o f various p a th o g en s o n n asal ciliary ultrastructure, fun ction, and epithelial integrity. ACKNOWLEDGEMENTS The authors acknowledge the support and assistance of Dr David Fell, Dr John Wandless, and Mr Shawqui Nour for allowing them to approach the children under their care at Leicester Royal Infirmary; the Cystic Fibrosis Trust and Masons Medical Foundation for their support; and Professor John Thompson and Mr John Beckett, University of Leicester, for statistical advice. Authors' affiliations M A Chilvers, A Rutm an, C O 'C allagh an, Department of Child Health, Institute of Lung Health, University of Leicester, School of Medicine, Leicester Royal Infirmary, Leicester LE2 7LX, UK REFERENCES 1 Chilvers M, O'Callaghan C. Analysis of ciliary beat pattern and beat frequency using digital high-speed imaging: comparison with the photomultiplier and photodiode methods. Thorax 2000;55: Willems T, Jorissen M. Correlation between ciliary structure and ciliary function. Acta Otorhinolaryngol Belg 2000;54: Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Respir J 1997,10: Bush A, Cole PJ, Hariri M, et al. Primary ciliary dyskinesia: diagnosis and standards of care. Eur Respir J 1998;12: Rossman CM, Lee RM, Forrest JB, et al. Nasal ciliary ultrastructure and function in patients with primary ciliary dyskinesia compared with that in normal subjects and in subjects with various respiratory diseases. A m Rev Respir Dis 1984;129: Mygind N, Pederson M, Nielsen MH. Primary and secondary and ciliary dyskinesia. Acta O tolaryngol 1983;95: Jorissen M, Willems T, Van der Schueren B, et al. S econdary ciliary dyskinesia is absent after ciliary genesis in culture. A cta O torhinolaryngol Belg ;5 4 : Boat TF, C arson JL. Ciliary dysm orphology and dysfunction: prim ary or acquired? N Engl J M ed ;3 2 3 : Giorgi PL, O g giano N, Braga PC, et al. The cilia in children with recurrent upper respiratory tract infections: ultrastructural observations. Paediatr Pulmonol ;1 4 : Corbeel L, Cornillie F, Lauweryns J, e ta /. Ultrastructural abnorm alities of bronchial cilia in children with recurrent airw ay infections and bronchiectasis. Arch Dis Child 1981 ;5 6 : De longh RU, Rutland J. Ciliary defect in healthy subjects, bronchiectasis, and prim ary ciliary dyskinesia. Am J Respir Crit Care M ed ;1 5 1 : Van der Baan S, Veerman AJP, Bezem er PD, et al. Primary ciliary dyskinesia: quantitative investigation of the ciliary ultrastructure with statistical analysis. Ann O tol Rhinol Laryngol :9 6 ; Wisseman CL, Simel DL, Spock A, et al. The prevalence of abnorm al cilia in normal paediatric lungs. Arch Pathol Lab M ed ,1 0 5 : Smallman LA, G regory J. Ultrastructural abnorm alities of cilia in the human respiratory tract. Hum Pathol ,1 7 : Ehouman A, Pinchon MC, Escudier E, et al Ultrastructural abnorm alities of respiratory cilia. Descriptive and quantitative study of respiratory mucosa in a series of 3 3 patients. Virchows Arch (B) ;4 8 : Verra F, Fleury-Feith J, Boucherat M, et al. Do nasal ciliary changes reflect bronchial changes? An ultrastructural study. A m Rev Respir Dis ,1 4 7 : O'Callaghan C, Smith K, Wilkinson M, et al. Ciliary b e a t frequency in newborn infants. Arch Dis Child ;66: Roth Y, A haronson EF, Teichtahl H, et al. Human in vitro nasal and tracheal ciliary beat frequencies: com parison of sam pling sites, com bined effect of m edication, and dem ographic relationships. A nn O tol Rhinol Laryngol ;1 0 0 : Ho JC, C han KN, Hu W H, et al. The effect of aging on nasal mucociliary clearance, b eat frequency, and ultrastructure of respiratory cilia. A m J Respir Crit Care M ed 2001 ;1 6 3 : Jorissen M, Willems T, Van der Schueren B. N asal ciliary b e a t frequency is a g e independent. Laryngoscope ,1 0 8 : Agius AM, Smallman LA, Pahor AL. Age, smoking an d nasal ciliary beat frequency. Clin Otolaryngol ;23: Chilvers MA, O 'C allaghan C. Local mucociliary defence mechanism s. Paediatr Respir Rev ,1 : Hann HC, Hall AP, Raphael JH, et al. An investigation into the effects of m idazolam and propofol on human respiratory cilia b e a t frequency in vitro. Intensive Care M ed ;2 4 : Chilvers AAA, M ckean M, Rutman A, et al. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J ,1 8 : Tsang KWT, Rutman A, Tanaka E, et al. Interaction of Pseudom onas aeruginosa with human respiratory m ucosa in vitro. Eur Respir J ;7 : Rayner CFJ, Rutman A, Dew ar A, et al. Ciliary disorientation alone as a cause of prim ary ciliary dyskinesia syndrome. A m J Respir Crit Care M ed ;1 5 3 : Rutland J, Dew ar A, Cox T, et al. N asal brushing for the study of ciliary ultrastructure. J Clin Pathol ;3 5 : Robson AM, Smallman LA, G regory J, et al. Ciliary ultrastructure in nasal brushings. Cytopathology 1993;4 : Biggart E, Pritchard K, W ilson R, e ta l. Primary ciliary dyskinesia syndrome associated with abnorm al ciliary orientation in infants. Eur Respir J ;1 7 : Jorissen M, Willems T, Van der Schueren, eta l. Dynein arm s and spokes after ciliogenesis in cultured respiratory epithelial cells from non-pcd individuals Acta O torhinolaryngol Belg ;5 4 : Veale D, Rodgers AD, Griffiths CJ, eta l. Variability in ciliary beat frequency in normal subjects and in patients with bronchiectasis. Thorax ;4 8 : O'Callaghan C, A chaval M, Forsythe I, eta l. Brain and respiratory cilia: the effect of tem perature. Biol N eonate ;6 8 : w w w. thorax j n I. com
315 Ciliary beat p attern is associated with specific ultrastructural defects in prim ary ciliary dyskinesia rhinitis, other Mark A. Chilvers, MRCPCH, Andrew Rutman, and Christopher O'Callaghan, FRCPCH, PhD Leicester, United Kingdom Background: The main symptoms of primary ciliary dyskinesia (PCD) are nasal rhinorrhea or blockage and moist-sounding cough. Diagnosis can be difficult and is based on an abnormal ciliary beat frequency, accompanied by specific abnormalities of the ciliary axoneme. It is unknown whether determining ciliary beat pattern related to specific ultrastructural ciliary defects might help in the diagnosis of PCD. Objective: We sought to determine ciliary beat pattern and beat frequency (CBF) associated with the 5 common ultrastructural defects responsible for PCD. Methods: Nasal brushings were performed on 56 children with PCD. Ciliary movement was recorded using digital high-speed video imaging to assess beat frequency and pattern. Electron microscopy was performed. Results: In patients with an isolated outer dynein arm or with an outer and inner dynein arm defect, 55% and 80% of cilia were immotile, respectively. Cilia that moved were only flickering. Mean CBF (± 95% Cl) was 2.3 Hz (± 1.2) and 0.8 Hz (± 0.8), respectively. Cilia with an isolated inner dynein arm or a radial spoke defect had similar beat patterns. Cilia appeared stiff, had a reduced amplitude, and failed to bend along their length. Immotile cilia were present in 10% of cilia with an inner dynein arm defect and in 30% of radial spoke defects. Mean CBF was 9.3 Hz (± 2.6) and 6.0 Hz (± 3.1), respectively. The ciliary transposition defect produced a large circular beat pattern (mean CBF, 10.7 Hz [± 1.1]). No cilia were immotile. Conclusions: Different ultrastructural defects responsible for PCD result in predictable beat patterns. Recognition of these might help in the diagnostic evaluation of patients suspected of having PCD. (J Allergy Clin Immunol 2003;112: ) Key words: Cilia, ultrastruc ture, dyskinesia, beat frequency, beat pattern Abbreviations used CBF: Ciliary beat frequency DHSV: Digital high-speed video PCD: Primary ciliary dyskinesia TEM: Transmission electron microscopy d i a g n o s e d u n t i l l a t e r i n l i f e, 1 b y w h i c h t i m e p e r m a n e n t l u n g d a m a g e h a s o c c u r r e d. 2 A p p r o x i m a t e l y 5 0 % o f p a t i e n t s w i t h P C D h a v e s i t u s i n v e r s u s. 3 5 E a r l y a n d a c c u r a t e d i a g n o s i s i s i m p o r t a n t, b e c a u s e o n c e m a d e, l u n g f u n c t i o n c a n b e m a i n t a i n e d w i t h s p e c i a l i s t r e s p i r a t o r y c a r e. 2 6 F a i l u r e t o r e c o g n i z e t h e c o n d i t i o n m i g h t a l s o l e a d t o i n a p p r o p r i a t e e a r, n o s e, a n d t h r o a t s u r g e r y, l e a v i n g p e r s i s t e n t a u r a l d i s c h a r g e w i t h l i t t l e i m p r o v e m e n t i n h e a r i n g l o s s T h e d i a g n o s i s o f P C D i s t r a d i t i o n a l l y m a d e o n t h e b a s i s o f a s u p p o r t i v e c l i n i c a l h i s t o r y a n d a n a b n o r m a l c i l i a r y b e a t f r e q u e n c y ( C B F ), a c c o m p a n i e d i n m o s t c a s e s b y s p e c i f i c a b n o r m a l i t i e s o f t h e c i l i a r y a x o n e m e o n t r a n s m i s s i o n e l e c t r o n m i c r o s c o p y ( T E M ). 3-5 T h e m o s t c o m m o n l y u s e d t e c h n i q u e s ( t h e m o d i f i e d p h o t o d i o d e 9 o r p h o t o m u l t i p l i e r m e t h o d 1 0 ) t o m e a s u r e C B F u s e a n i n d i r e c t m e t h o d a n d d o n o t p r o v i d e i n f o r m a t i o n o n c i l i a r y b e a t p a t t e r n. N e w h i g h - r e s o l u t i o n d i g i t a l h i g h s p e e d v i d e o ( D H S V ) i m a g i n g h a s a l l o w e d t h e p r e c i s e b e a t p a t t e r n o f c i l i a t o b e v i e w e d i n 3 d i f f e r e n t p l a n e s i n s l o w m o t i o n o r f r a m e b y f r a m e. 11 T h i s s h o w s t h a t t h e w i d e l y h e l d b e l i e f t h a t r e s p i r a t o r y c i l i a b e a t w i t h a c l a s s i c a l f o r w a r d p o w e r s t r o k e T h e m a i n c l i n i c a l s y m p t o m s o f p a t i e n t s w i t h p r i m a r y i a r y d s k i n e s i a ( P C D a r e n a s a l r h i n o r r h e a o r b l o c k c i l y ) a g e, a m o i s t - s o u n d i n g c o u g h, a n d, i n a p p r o x i m a t e l y 5 0 % h e a r i n g p r o b l e m s i n e a r l y l i f e. D e s p i t e p e r s s t e n t, i s y m p t o m s, a n d o f t e n t t e n d a n c e a t e a r, n o s e, a n d t h r o a t a a n d r e s p r a t o r y c l i n i c s, m a n y p a t i e n t s w i t h P C D a r e n o t i a n d t h e n a r e c o v e r y s t r o k e t h a t s w e e p s t o t h e s i d e 1 2 i s i n c o r r e c t. C i l i a s i m p l y b e a t i n a f o r w a r d a n d b a c k w a r d p l a n a r m o t i o n w i t h u t a s i d e w a y s r e c o v e r y s w e e p. 1 1 o D H S V a n a l y s i s h a s a l s o p r o v e d u s e f u l i n d t e r m i n e i n g e f f e c t o f v i r a l i n f e c t i o n o n t h e m o v e m e n t o f r e s p i a t o r y t h e r c i l i a. A f t e r a c o r o n a v i r u s i n f e c t i o n, C B F o f n a s a l r e s p i r a t o r y c i l i a w a s f o u n d t o r e m a i n w i t h i n t h e n o r m a l r a n g e. H o w e v e r, s l o w - m o t i o n a n a l y s i s r e v e a l e d a l a r g e n u m b e r o f d y s From the Departm ent o f Child Flealth. U n iversity o f L eicester S ch ool o f M edicine. Supported by the Cystic Fibrosis Trust (UK ). M asons Medical Foundation. Received for publication February : revised April ; accepted for publication June Reprint requests: Christopher O C allaghan. FRCPCH. PhD. Departm ent o f Child Health and Institute o f Lung Health. University o f Leicester School o f M ed icin e. Robert K ilpatrick C lin ical S c ien ces B u ild in g. L eicester Royal Infirmary. PO Box 65. Leicester. LE2 7LX England Mosby. Inc. All rights reserved /2003 $ doi: /m ai k i n e t i c c i l i a. 1 3 T h i s w o u l d h a v e b e e n m i s s e d b y t h e c o n v e n t i o n a l m e t h o d s t h a t r e l y s o l e l y o n C B F m e a s u r e m e n t. I t h a s b e n s u g g e s t e d t h a t e v a l u a t i o n o f c i l i a r y b e e a t t t e r n, i n a d d i t i o n t o C B F, m i g h t b e h e l p f u l i n t h e d i a g p a n o s i s o f P C D I n d e e d, o n e r e p o r t s u g g e s t e d t h a t s o m e o f t h e i f f e r e n t u l t r a s t r u c t u r a l d e f e c t s f o u n d t o d c a u s e P C D m i g h t h a v e d i f f e r e n t b e a t p a t t e r n s. 1 6 I f s p e c i f i c b e a t a t e r n s c o u l d b e a s s o c i a t e d w i t h e a c h o f t h e p t u l t r a s t r u c t u r a l d e f e c t s r e s p o n s i b l e f o r P C D, d i a g n o s t i c t e s t i n g m i g h t b e i m p r o v e d. 518
316 J ALLERGY CLIN IMMUNOL VOLUME 112, NUMBER 3 Chilvers, Rutman, and O'Callaghan 519 TABLE I. Ciliary ultrastructural defects and clinical dem ographics of patients diagnosed with primary ciliary dyskinesia. The number of patients with the sam e structural defect is shown with mean age at diagnosis (range) and clinical symptoms and signs (n [%]) Ultra structural defect No. of patients (fem ale) A ge (range) (y) Chest (%) Nasal (%) Ear (%) Situs inversus (%) Inner and outer dynein arm defect 20(9 ) 2.9 ( ) 20(100.0) 17 (85.0) 9 (45.0) 9 (45.0) Outer dynein arm defect 16(6) 4.5 ( ) 16(100.0) 15 (93.8) 11 (68.8) 9 (56.2) Inner dynein arm defect 8 (3 ) 6.9 ( ) 7 (87.5) 7 (87.5) 6 (75.0) 2 (25.0) Radial spoke defect 4 (2 ) 3.6 ( ) 4 (100.0) 4(100.0) 1 (25.0) 3 (75.0) Transposition defect 8 (4 ) 8.3 ( ) 8 (100.0) 8 (100.0) 3 (37.5) 0 (0.0) Total 56 (24) 4.7 (0.1-14) 55 (98.2) 51 (91.0) 30(54.5) 23 (41.0) T h e a i m o f t h i s s t u d y w a s, t h e r e f o r e, t o u s e h i g h - r e s o - l u t i o n, D H S V p h o t o g r a p h y t o d e t e r m i n e t h e p r e c i s e c i l i a r y b e a t p a t t e r n a n d C B F a s s o c i a t e d w i t h t h e 5 c o m m o n u l t r a s t r u c t u r a l a b n o r m a l i t i e s r e s p o n s i b l e f o r P C D. O u r s e c o n d a r y a i m w a s t o d e f i n e i n d e t a i l, b y u s e o f T E M, t h e u l t r a s t r u c t u r a l f i n d i n g s a n d c i l i a r y o r i e n t a t i o n o f c i l i a o b t a i n e d b y n a s a l b r u s h b i o p s y f r o m p a t i e n t s w i t h t h e 5 m o s t c o m m o n u l t r a s t r u c t u r a l d e f e c t s r e s p o n s i b l e f o r P C D METHODS This study reports 56 children (5 weeks to 14 years [32 males]) w ho were diagnosed as hav ing PCD at the Leicestershire PCD diagnostic clinic. Information was collected evaluating chest, nasal, and ear symptoms. The presence of situs inversus was noted. Each subject had been free from upper respiratory tract infections or nasal and chest exacerbation in the previous 6 weeks. Medication was discontinued 48 hours before nasal brush biopsy. Ciliated samples were obtained by brushing the inferior nasal turbinate without local anesthetic.11 Nasal brushings were placed in medium 199 (ph 7.3) that contained antibiotic solution (streptomycin, 50 [ig/ml, penicillin, 50 (ig/ml, Gibco. Leicester, United Kingdom). Approval was obtained from the Leicestershire ethics committee. Written consent was obtained before sampling. and the number of frames required to complete 10 cycles was recorded. This was converted to CBF by a simple calculation.11 An immotility index was calculated as previously described.18 If immotile cilia were observed, a CBF of 0 Hz was recorded. The immotility index was calculated as the percentage o f immotile cilia within the sample (number o f immotile readings/total number of readings for sample xloo). The experimental system allowed the ciliary beat pattern to be evaluated in 3 different planes: a sideways profile, beating directly toward the observer, and from directly above.11 The path taken by a cilium during the beat cycle was analyzed frame by frame. This was characterized and compared with the normal beat pattern seen on DHSV analysis.11 Statistics The mean ciliary beat frequency, 95% CIs, and range were calculated. The mean percentage of immotile cilia and 95% CIs were calculated. For all ultrastructural parameters the mean and 95% CIs were calculated. RESULTS P a t i e n t s c o u l d b e c a t e g o r i z e d i n t o I o f 5 r e c o g n i z e d u l t r a s t r u c t u r a l d e f e c t s T h i s f o r m e d t h e f o l l o w i n g g r o u p s : i s o l a t e d o u t e r d y n e i n a r m d e f e c t s, a c o m b i n e d d e f e c t o f b o t h o u t e r a n d i n n e r d y n e i n a r m s, i s o l a t e d i n n e r Asthma, rhinitis, other respiratory diseases TEM Tissue obtained by nasal brushing was processed for TEM by the standard techniques, 17 Ciliary ultrastructure was examined without knowledge of ciliary beat pattern and beat frequency readings. Individual cilia were examined for microtubular and dynein arm defects. The total number of inner and outer dynein arms for each cilium were counted. Alignment of individual cilia within a cell was assessed by measuring ciliary orientation as previously described.17 Percentages were calculated for the number of cilia with microtubular or dynein arm defects. CBF and beat pattern This was evaluated as previously described Ciliated epithelium of greater than 50 pm long was observed at 37 C using a xloo interference contrast lens. Beating ciliated edges were recorded using a DHSV camera (Kodak Motioncorder Analyser, Model 1000) at 400 frames per second. Video sequences could be recorded and played back at reduced frame rates or frame by frame. The ciliated edge, projected onto a high-resolution monitor, was divided into 5 adjacent areas measuring 10 pm. A total of 10 measurements of CBF was made along each ciliated strip. At least 3 edges up to a maximum of 10 edges were analyzed per subject. CBF was determined directly. Groups of beating cilia were identified. d y n e i n a r m d e f e c t s, r a d i a l s p o k e d e f e c t w i t h a n a s s o c i a t e d i n n e r d y n e i n a r m d e f e c t ( r a d i a l s p o k e d e f e c t ), a n d t r a n s p o s i t i o n d e f e c t. T h e c l i n i c a l p i c t u r e s o f p a t i e n t s w i t h P C D c a u s e d b y f e r e n t u l t r a s t r u c t u r a l d e f e c t s a r e s h o w n ( T b l e I ). T h e d i f a m e a n a g e a t d i a g n o s i s w a s 4. 7 y e a r s ( r a n g e, y a r s ) M o r e t h a n 9 8 % o f p a t i e n t s h a d c h r o n i c h e s t e. c s y m p t o m s, a n d 90% h a d c h r o n i c n a s a l s y m p t o m s. E a r s y m p t o m s w e r e r e p o r t e d i n h a l f t h e s u b j e c t s. S i t u s i n v e r s u s w a s o u n d i n 4 1 % o f p a t i e n t s. N o n e o f t h e 8 ( % ) f p a t i e n t s w i t h a t r a n s p s i t i o n d e f e c t h a d s i t u s i n v e r s u s. o N e a r l y t w o t h i r d s o f t h e p a t i e n t s h a d e i t h e r a c o m b i n e d e r a n d o u t e r d y n e i n a r m d e f e c t ( 3 6 % ) o r a n i s o l a t e d i n n o u t e r d y n e i n a r m d e f e c t ( 2 9 % ). T h e r m a i n i n g u l t r a - e s t r u c t u r a l d e f e c t s w e r e l e s s c o m m o n, w i h a n i s o l a t e d t i n n e r d y n e i n a r m d e f e c t r e s p o n s i l f o r 1 4 %, a t r a n s p o b e s i t i o n d e f e c t 1 4 %, a n d r a d i a l s p o k e d e f e c t 7 %. E a c h p a t i e n t h a d a n a v e r a g e o f 1 5 c i l i a t e d c e l l s ( r a n g e, 6 ) a n d i n d i v i d u a l c i l i a ( r a n g e, ) e x a m i n e d 5-3 b y T E M. D e t a i l e d c i l i a r y u l t r a s t r u c t u r a l e v a l u a t i o n f o r e a c h d e e c t i s s h o w n ( T a b l e s 1 1 a n d I I I ). I n p a t i e n t s w i t h f i s o l a t e d o u t e r d y n e i n a r m d e f e c t s, a c o m b i n e d d e f e c t o f b o t h o u t e r a n d i n n e r d y n e i n a r m s, o r a i s o l a t e d i n n e r n
317 520 Chilvers, Rutman, and O'Callaghan J ALLERGY CLIN IMMUNOL SEPTEMBER 2003 TABLE II. Assessment of m icrotubular abnorm alities and ciliary orientation by transmission electron microscopy. Results displayed are for ultrastructural defect and expressed as the mean percentage (95% CIs) Ultrastmctural defect Total m icrotubular d efects (%) Disarranged m icrotub ules (%) Extraperipheral Central m icrotubule m icrotubules (%) d efects (%) Ciliary orientation ( ) Inner and outer dynein arm defect 3.4 ( ) Outer dynein arm defect 2.9 ( l.9-4.1) Inner dynein arm defect 5.7 ( ) Radial spoke defect 26.4 ( ) Transposition defect 22.8 ( ) 0.8 ( ) 0.9 ( ) 2.4 ( ) 25.2 ( ) 7.8( ) 0.8 ( ) 1.2 ( ) 0.4 ( ) 0.0 ( ) 0.8 ( ) 1.8 ( ) 0.8 ( ) 2.9 ( ) 1.2 ( ) 14.2 ( ) 21.6 ( ) 13.6 ( ) 17.8 ( ) 21.9 ( ) 21.1 ( ) TABLE III. Analysis of ciliary dynein arm s by transm ission electron microscopy. Results for individual ultrastructural defects are for individual dynein arm counts and the percentage of cilia with dynein arm defects. Results are expressed as the mean ( 9 5 % CIs) Ultrastructural defect D ynein arm co u n ts ( Cilia w ith dynein O uter Inner arm d efects (%) Inner and ou ter dynein arm d efect (%) Outer dynein arm defect (%) Inner dynein arm d efect (%) Inner and outer dynein arm Outer dynein arm defect Inner dynein arm defect Radial spoke defect Transposition defect defect ( ) ( ) ( l.0-1.7) ( ) ( ) ( ) ( ) ( ) ( ) ( ) 96.4 ( ) 96.0 ( ) 93.6 ( ) 96.0( ) 1.1 ( ) 85.5 ( ) 5.1 ( ) 5.3 ( ) 4.3 ( ) 1.1 ( ) 2.1 ( ) 90.9 ( ) 0.0 ( ) 0.0 ( ) 0.0 ( ) 8.8 ( ) 0.0 ( ) 88.3 ( ) 91.7 ( ) 0.0 ( ) d y n e i n a r m d e f e c t, l e s s t h a n 5 % o f c i l i a h a d m i c r o t u b u Virtually immotile cilia (Fig 1, b,table IV) l a r a b n o r m a l i t i e s ; 2 5 % o f c i l i a w i t h a r a d i a l s p o k e d e f e c t e x h i b i t e d p e r i p h e r a l m i c r o t u b u l a r d e f e c t s. P a t i e n t s w i t h c i l i a r y t r a n s p o s i t i o n d f e c t h a d a s i m i l a r p e r c e n t a g e o f e c i l i a w i t h m i c r o t u b u l a r d e f e c t s, b u t h i s p r e d o m i n a n t l y t i n v o l v e d t h e c e n t r a l m i c r o t u b u l a r p a i r. I t i s o f i n t e e s t t h a t r i n a l l p a t i e n t s e x c e p t t h o s e w i t h a n i s o l a t e d o u t e r d y n e i n o n i s m a r k e d l y i n c r e a s e d a r m d e f e c t, c i l i a r y o r i e n t a t i c o m p a r e d w i t h t h e n o r m a l r a n g e 2 0 o f < 1 1. I p a t i e n t s w i t h t h e f o l l o w i n g a b n o r m a l n i t i e s : i s o l a t e d t e r d y n e i n a r m d e f e c t, c o m b i n e d d e f e t o f b o t h o u t e r o u c a n d i n n e r d y n e i n a r m s, i s o l a t d i n n e r d y n e i n a r m d e f e c t, e a n d r a d i a l s p o k e d e f c t w i t h a n a s s o c i a t e d i n n e d y n e i n e r a r m d e f e c t, 9 5 % o f c i l i a w e r e f o u n d t o e x h i b i t t h e d e f e c t ( T a b l e I I I ). A l t o u g h r e f e r r e d t o a s a b s e n c e o f d y n e i n h a r m s, i t w a s o s s i b l e t o i d e n t i f y a t l e a s t 1 d y n e i n a r m. p T h e d y n e i n a r m s t h a t w e r e i d e n t i f i e d a p p e a e d a b n o r m a l r ( T a b l e I I I ). A p p r o x i m a t e l y 5 % o f c i l i a e x h i b i t e d d e f e c t s o f b o t h e r a n d o u t e r d y n e n a r m s i n t h e f o l l o w i n g u l t r a s t r u c i n n i t u r a l g r o u p s : i s o l a t e d o u t e d y n e i n a r m d e f e c t, i s o l a t e d r i n n e r d y n e i n a r m d e f e c t s, a n d r a d i a l s p o k e d e f e c t. L e s s t h a n 1 % o f c i l i a w i t h a t r a n s p o s i t i o n d e f e c t h a d a s s o c i a t e d d e f e c t s o f t h e o u t e r a n d i n n e r d y n e i n a r m s ( T a b l e I I I ). N o p a t i e n t s w i t h P C D h a d c i l i a w i t h a n o r m a l b e a t p a t t e r n ( F i g 1, A; T a b l e I V ). 2 0 I t w a s p o s s i b l e t o c a t e g o r i z e t h e p a t i e n t s i n t o 3 g r o u p s o n t h e b a s i s o f d i s t i n c t d y s k i n e t i c b e a t p a t t e r n s o b s e r v e d ( T a b l e I V ). C i l i a w i t h e i t h e r a c o m b i n e d i n n e r a n d o u t e r d y n e i n a r m d e f e c t o r a n i s o l a t e d o u t e r d y n e i n a r m d e f e c t w e r e o b s e r v e d t o h a v e l a r g e a r e a s o f i m m o t i l e c i l i a. C i l i a r y m o v e m e n t, w h e n p r e s e n t, w a s r e s t r i c t e d t o a s l o w, s h o r t, s t i f f f l i c k e r i n g m o t i o n ( F i g 1, B). I n t h e c o m b i n e d i n n e r a n d o u t e r d y n e i n a r m d e f e c t g r o u p, a n a v e r a g e o f 8 0 % o f c i l i a w e r e i m m o t i l e. T h e m e a n ( ± S D ) C B F w a s 0. 8 ( ± 1. 7 ) H z. I n t h e o u t e r d y n e i n a r m d e f e c t g r o u p, a n a v e r a g e 5 5 % o f c i l i a w e r e i m m o t i l e. i l i a t h a t w e r e m o v i n g h a d a t i f f f l i c k e r i n g C s m o t o n w i t h a m e a n ( ± S D ) b e t f r e q u e n c y o f 2. 3 ( ± 2. 6 ) H z. i a Stiff ciliary beat pattern (Fig 1, c,table IV) C i l i a w i t h a n i s o l a t e d i n n e r d y n e i n a r m d e f e c t o r a i a l s p o k e w i t h a n i s o l a t e d i n n e r d y n e i a r m d e f e c t r a d n w e r e o b s e r v e d t o h a v e a v e r y a b n o r m a l s t i f f o r w a r d f p o w e r s t r o k e w i t h a m a r k e d l y r e d u c e d a m p l i t u d e. C i l i a f a i l e d t o b e n d a l o n g t h e i r a x o n e m e ( F i g l, C). T e n p e r c e n t o f t h e c i l i a i n p a t i e n t s w i t h a n i s o l a t e d i n n e r d y n e i n a r m d e f e c t w e r e i m m o t i l e. T h e r e m a i n d e r h a d a m e a n ( ± S D ) C B F o f 9. 3 ( ± 4 0 ) H z.. C i l i a w i t h a r a d i a l s p o k e d e f e c t a s s o c i a t e d w i t h a n e r d y n i n a r m d e f e c t w e r e f o u n d t o b e a t i n a i m i l a r i n n e s m a n n e r t o c i l i a f r o m p a t i e n t s w i t h a n i s o l a t e d i n n e r d n e i n a r m d e f e c t. h i r t y p e r c n t o f t h e c i l i a w e r e y T e i m m o t i l, a t h e r e m a i n d e r e a t a t a l o w e r m e a n ( D ) e n d b ± S C F o f 6. 0 ( ± 3. 3 ) H. B z
318 J ALLERGY CLIN IMMUNOL VOLUME 112, NUMBER 3 Chilvers, Rutman, and O'Callaghan 521 C ircular b e a tin g cilia (Fig 1, D, Fig 2; T ab le IV) T h i s b e a t p a t t e r n w a s o b s e r v e d o n l y i n p a t i e n t s w i t h a c i l i a r y t r a n s p o s i t i o n d e f e c t. A f o r w a r d a n d b a c k w a r d p l a n a r w h i p l i k e m o t i o n w a s s e e n w h e n c i l i a w e r e v i e w e d f r o m a s i d e p r o f i l e. H o w e v e r, w h e n v i e w e d f r o m a b o v e, a l a r g e, c i r c u l a r, g y r a t i n g m o t i o n a b o u t t h e b a s e o f t h e c i l i u m w a s a p p a r e n t ( F i g 1, D). N o n e o f t h e c i l i a w e r e i m m o t i l e, a n d t h e y h a d a m e a n ( ± S D ) C B F o f ( ± 1. 6 ) H z. D ISC U SSIO N T h e a d v e n t o f D H S V i m a g i n g h a s a l l o w e d u s t o d e f i n e t h e c i l i a r y b e a t p a t t e r n a n d b e a t f r e q u e n c y a s s o c i a t e d w i t h 5 o f t h e m o s t c o m m o n u l t r a s t r u c t u r a l a b n o r m a l i t i e s r e s p o n s i b l e f o r P C D. T h e l i t e r a t u r e i n t h i s a r e a i s v e r y s p a r s e, a n d o u r r e s u l t s d i f f e r i n a n u m b e r o f a s p e c t s f r o m p r e v i o u s r e p o r t s T h r e e d i s t i n c t b e a t p a t t e r n s a s s o c i a t e d w i t h u n d e r l y i n g u l t r a s t r u c t u r a l d e f e c t s w e r e s e e n. T h e m o s t c o m m o n u l t r a s t r u c t u r a l d e f e c t r e s p o n s i b l e f o r P C D a r e d e f e c t s o f t h e i n n e r o r o u t e r d y n e i n a r m s. I n t h e i s o l a t e d o u t e r d y n e i n a r m d e f e c t a n d t h o s e p a t i e n t s w i t h b o t h a n i n n e r a n d o u t e r d y n e i n a r m d e f e c t, m o s t c i l i a w e r e i m m o t i l e. T h e f e w c i l i a t h a t a c t u a l l y m o v e d FIG 1. A, Diagram of the normal ciliary beat pattern. Cilia move in a planar m otion with a forward power stroke and a backward recovery stroke that does not sweep to the side. B, Diagram of the dyskinetic beat pattern observed for cilia with either a combined inner and outer dynein arm defect or an isolated outer dynein arm defect. Cilia were virtually im m otile, with the occasional slow, low-am plitude, stiff flickering motion. C, Diagram of the dyskinetic beat pattern observed for cilia with either an isolated inner dynein arm defect or a radial spoke defect. Cilia had a stiff planar forward-backward motion with markedly reduced amplitude. (Figure continued on next page.) h a d a v e r y s t i f f f l i c k e r i n g m o t i o n. T h e f r e q u e n c y o f t h e s e f l i c k e r i n g c i l i a w a s s l i g h t l y s l o w e r i n t h o s e w i t h a c o m b i n e d i n n e r a n d o u t e r d y n e i n a r m d e f e c t t h a n i n t h o s e w i t h a n i s o l a t e d o u t e r d y n e i n a r m d e f e c t. I n a d d i t i o n, p a t i e n t s w i t h a c o m b i n e d d e f e c t h a d a h i g h e r p e r c e n t a g e o f t o t a l l y i m m o t i l e c i l i a. I n p r e v i o u s t u d i e s, s p a t i e n t s w i t h i n n e r a n d o u t e r d y n e i n a r m d e f e c t s w e r e n o t e d t o h a v e a h i g h e r p r o p o r t i o n o f i m m o t i l e c i l i a, a l t h o u g h t h e a c t u a l p e r c e n t a g e w a s n o t d e f i n e d, a n d 2 d i f f e r e n t b e a t p a t t e r n s w e r e d e s c r i b e d, o n e a s v i b r a t i o n a l a n d t h e o t h e r a s a r o t a t i o n a l e g g b e a t e r. 1 6 I n a n a r t i c l e b y R o s s m a n e t a l, 1 6 t h e 5 p a t i e n t s w i t h d y n e i n a r m d e f e c t s w e r e n o t s p l i t i n t o t h o s e w i t h i s o l a t e d o u t e r d y n e i n a r m d e f e c t s o r c o m b i n e d i n n e r d y n e i n a n d o u t e r d y n e i n a r m d e f e c t s. T h e y r e p o r t e d t h e b e a t f r e q u e n c y o f t h e c i l i a i n t h i s c o m b i n e d g r o u p t o b e 6 H z, c o n s i d e r a b l y h i g h e r t h a n t h e f r e q u e n c y i n o u r 2 0 p a t i e n t s w i t h c o m b i n e d i n n e r a n d o u t e r d y n e i n a r m d e f e c t s ( 0. 8 H z ) a n d t h e 1 6 p a t i e n t s w i t h o u t e r d y n e i n a r m d e f e c t s ( 2. 3 H z ). I n o u r s t u d y, t h e n u m b e r o f i m m t i l e c i l i a w a s s h o w t o v a r y d e p e n d i n g o n w h e t h e r o n, t h e r e w a s a n i s o l a t e d d y n e i n a r m d e f e c t ( 5 5 % i m m o t i l e ) o r a c o m b i n e d i n n e r a n d o u t e r d y n e i n a r m d e f e c t ( 8 0 % i m m o t i l e ). T h e a r t i c l e b y R o s s m a n e t a l 1 6 s u g g e s t e d t h a t 6 0 % f r o m t h e i r c o m b i n e d g r o u p w e r e i m m o t i l e. T h e s e c o n d b e a t p a t t e r n o b s e r v e d w a s t h a t o f a s t i f f f o r w a r d s t r o k e w i t h a m a r k e d l y r e d u c e d a m p l i t u d e. T h i s p a t t e r n w a s c o m m o n t o p a t i e n t s w i t h a n i s o l a t e d i n n e r d y n e i n a r m d e f e c t a n d a l s o t o t h o s e w i t h a r a d i a l s p o k e d e f e c t a s s o c i a t e d w i t h a n i n n e r d y n e i n a r m d e f e c t. T h e b e a t f r e q u e n c y o f t h e s e 2 g r o u p s, h o w e v e r, w a s d i f f e r e n t. P a t i e n t s w i t h a n i s o l a t e d i n n e r d y n e i n a r m d e f e c t h a d a m e a n b e a t f r e q u e n c y o f 8. 1 H z c o m p a r e d w i t h a b e a t f r e q u e n c y o f 6 H z i n t h o s e w i t h a r a d i a l s p o k e d e f e c t a n d a n i n n e r d y n e i n a r m d e f e c t. T h e n u m b e r o f i m m o t i l e c i l i a a l s o v a r i e d, d e p e n d i n g o n t h e d e f e c t w i t h 1 0 % o f c i l i a i m m o t i l e i n t h o s e w i t h a n i s o l a t e d i n n e r d y n e i n a r m d e f e c t c o m p a r e d w i t h m o r e t h a n 3 0 % o f c i l i a i n p a t i e n t s w i t h a r a d i a l s p o k e d e f e c t i n a s s o c i a t i o n w i t h a n i n n e r d y n e i n d e f e c t. R o s s m a n e t a l 1 6 d i d n o t c o n s i d e r i n n e r
319 522 Chilvers, Rutman, and O'Callaghan J ALLERGY CLIN IMMUNOL SEPTEMBER 2003 FIG 1. (continued) D, Diagram of the dyskinetic beat pattern observed for cilia with a transposition defect. Cilia beat in a large circular gyrating motion about the base of the cilium. d y n e i n a r m d e f e c t s s e p a r a t e l y, a n d t h e r e p o r t o f a s i n g l e w i t h o u t a s i d e w a y s s w e e p. 11 A l l 3 b e a t p a t t e r n s a s s o c i a t p a t i e n t b y P e d e r s o n 2 1 s u g g e s t e d t h a t a n a s y n c h r o n i z e d e d w i t h P C D d i f f e r f r o m t h e n o r m a l c i l i a r y b e a t p a t t e r n. b e a t p a t t e r n w a s o b s e r v e d. T h e s t u d y b y P e d e r s o n I n f a c t, n o c i l i a w e r e s e e n i n a n y o f t h e p a t i e n t s t o h a v e a i n v o l v e d c i l i a o b s e r v e d a t a r o o m t e m p e r a t u r e o f 2 2 C, n o r m a l b e a t p a t t e r n. s o c o m p a r i s o n o f b e a t f r e q u e n c y i s n o t p o s s i b l e. D e T h e m a j o r b e n e f i t o f t h e n e w v i d e o t e c h n o l o g y i s t h a t I o n g h a n d R u t l a n d 2 3 o b s e r v e d t h e b e a t f r e q u e n c y i n 2 o f h i g h r e s o l u t i o n a n d t h e a b i l i t y t o p l a y b a c k t h e m o v e p a t i e n t s w i t h P C D c a u s e d b y a n i n n e r d y n e i n a r m t o b e m e n t o f i n d i v i d u a l c i l i a f r a m e b y f r a m e a f t e r t h e i r b e i n g w i t h i n t h e n o r m a l r a n g e. r e c o r d e d a t a f r e q u e n c y o f t o H z. T h i s i s a c o n T w o p a t i e n t s w i t h r a d i a l s p o k e d e f e c t s d e s c r i b e d i n t h e s i d e r a b l e a d v a n t a g e o v e r p r e v i o u s m e t h o d s s u c h a s t h a t R o s s m a n e t a l 1 6 s t u d y w e r e f o u n d t o h a v e n o i m m o t i l e u s e d b y R o s s m a n e t a l 1 6 i n t h a t a l l o w e d 6 0 f r a m e s c i l i a. T h e b e a t f r e q u e n c y m e a s u r e d i n t h e s e 2 p a t i e n t s p e r s e c o n d. T h i s, f o r e x a m p l e, o n l y a l l o w s 5 o r 6 f r a m e s w a s 9. 6 H z, w h i c h i s h i g h e r t h a n t h e f r e q u e n c y o f 6 H z p e r c i l i a r y b e a t c y c l e c o m p a r e d w i t h t h e h i g h r e s o l u t i o n i n o u r 4 p a t i e n t s. T h e b e a t p a t t e r n t h e y d e s c r i b e d f o r t h i s 4 0 t o 5 0 f r a m e s p e r c y c l e w i t h t h e n e w e r t e c h n o l o g y. O u r d e f e c t w a s a b i p h a s i c r o t a t i o n a l p a t t e r n t h a t d i f f e r s s i g m e t h o d a l s o a l l o w s v i s u a l i z a t i o n o f t h e b e a t p a t t e r n i n 3 n i f i c a n t l y f r o m o u r f i n d i n g s o f a s t i f f b e a t p a t t e r n w i t h d i s t i n c t p l a n e s. 11 I n a d d i t i o n, v i d e o r e c o r d s m i g h t b e r e d u c e d a m p l i t u d e. c o m p i l e d a n d a r c h i v e d f o r a u d i t. T h e t h i r d b e a t p a t t e r n w a s t h a t o f a n o v a l g y r a t i n g p a t R e s u l t s o f o u r s t u d y a r e i n k e e p i n g w i t h t h e p o s t u l a t t e r n i n w h i c h c i l i a h a d a m e a n b e a t f r e q u e n c y o f H z. e d r o l e o f t h e v a r i o u s u l t r a s t r u c t u r a l c o m p o n e n t s o f t h e W e h a v e p r e v i o u s l y i n v e s t i g a t e d t h e n o r m a l C B F o f c i l i a r y a x o n e m e. I n t h e c i l i a r y b e a t c y c l e, o u t e r d y n e i n h e a l t h y c h i l d r e n a n d f o u n d t h e m e a n t o b e 1 2 H z ( r a n g e, a r m s a r e t h o u g h t t o g e n e r a t e t h e f o r c e t o c a u s e s l i d i n g o f H z ). 2 0 I n s o m e c a s e s, p a t i e n t s s u s p e c t e d o f h a v t h e p e r i p h e r a l m i c r o t u b u l e s a n d t o l a r g e l y c o n t r o l i n g P C D a r e s c r e e n e d u s i n g b e a t f r e q u e n c y m e a s u r e m e n t C B F a l o n e. T h e i m p l i c a t i o n o f t h e s e f i n d i n g s i s t h a t a n u m b e r T h e s t i f f b e a t p a t t e r n t h a t w e o b s e r v e d w i t h i n n e r o f p a t i e n t s w i t h c i l i a r y t r a n s p o s i t i o n w i l l h a v e a b e a t f r e d y n e i n a r m d e f e c t s a l o n e o r t h o s e a c c o m p a n i e d b y r a d i q u e n c y w i t h i n t h e n o r m a l r a n g e, a n d t h e s e p a t i e n t s w i l l a l s p o k e d e f e c t s w o u l d s u p p o r t t h e e v i d e n c e t h a t t h e b e m i s s e d u n l e s s b e a t p a t t e r n a n a l y s i s a n d e l e c t r o n i n n e r d y n e i n a r m s a s s i s t i n t h e b e n d i n g o f t h e c i l i a r y m i c r o s c o p y a r e u n d e r t a k e n. T w o p a t i e n t s d e s c r i b e d b y a x o n e m e A l t h o u g h t h e b e a t f r e q u e n c y o f p a t i e n t s R o s s m a n e t a l 1 6 w i t h a c i l i a r y t r a n s p o s i t i o n d e f e c t w e r e w i t h i n n e r a r m d e f e c t s i s r e d u c e d, t h e r e d u c t i o n i s o n l y f o u n d t o h a v e a C B F o f 1 0 H z a n d n o i m m o t i l e c i l i a. T h e m o d e r a t e c o m p a r e d w i t h o u t e r d y n e i n a r m d e f e c t s. R a d i p a t t e r n t h e y d e s c r i b e d i s o n e o f a g r a b b i n g m o t i o n. a l s p o k e s a r e t h o u g h t t o r e s i s t t h e s l i d i n g o f t h e m i c r o B y u s e o f D H S V, w e h a v e n o w s h o w n t h a t r e s p i r a t o r y t u b u l e s a n d c a u s e t h e c i l i u m t o b e n d A l t h o u g h t h e c i l i a s i m p l y b e a t f o r w a r d a n d b a c k w a r d i n t h e s a m e p l a n e c i l i u m f a i l e d t o b e n d i n t h e c o m b i n e d r a d i a l s p o k e a n d
320 J ALLERGY CLIN IMMUNOL VOLUME 112, NUMBER 3 Chilvers, Rutman, and O'Callaghan 523 inner dynein arm defect, it was also observed in the inner dynein arm defect alone. Little information is available on the action o f the central microtubular pair. It has been postulated that the central pair might rotate during active ciliary bending.28 It would seem that the central pair allows the cilium to beat in a forward and backward planar motion. A bsence o f the central pair for the short distance seen in patients with ciliary transposition seems to allow the cilia to rotate around this section. The main aim was to look at the association between ciliary beat pattern and beat frequency with ultrastructural defect in patients with PCD. Our secondary aim was to perform quantitative ciliary ultrastructural analysis on this group of patients. We are aware o f only 4 studies that have performed such analysis.23*29-31 The data from these articles are som ewhat limited. Only 1 study involved samples obtained by nasal brush biopsy,23 1 study solely analyzed microtubular defects,31 and just 1 study placed patients into groups according to ultrastructural defect.30 In healthy tissue, it is possible to identify between 7 and 9 outer dynein arms and 4 and 7 inner dynein arms per ciliary cross-section.32 Even w ith a dynein arm defect, we found it possible to identify 1 o r 2 o f the dynein arms. We found patients with an inner dynein arm, radial spoke, or transposition defects to have a normal number of outer dynein arms present. Similarly, normal numbers of inner dynein arms were observed in subjects with an outer dynein arm or transposition defect. This is in agreement with other published data for dynein arm defects.30*32 Five percent of cilia were observed to have m icrotubular defects in patients with dynein arm defects. This agrees with work by De Iongh and Rutland,23 who found similar numbers of cilia to exhibit microtubular defects. Ciliary orientation evaluates how individual cilia are aligned within a cell. This is uniform between cells and is <11 in healthy individuals.20 Measurement of ciliary orientation in patients with PCD is limited, although it has been suggested to be increased.23*29*30 No reports exist for ciliary orientation o f individual ultrastructural defects. We found ciliary disorientation to be increased in all groups. Two thirds of patients with PCD were found to have abnormalities of the dynein arms. This is a similar proportion of patients to previous studies.18*23*33 However, we found a greater proportion o f patients to have a ciliary transposition defect (14%). This is higher than in previous series, which have suggested a prevalence between 3% and 10%.18*23* Because patients with a transposition defect have cilia that have a beat frequency within the normal range, it is likely that this group of patients is underdiagnosed. Our results suggest that specific ultrastructural defects responsible for PCD result in specific abnormalities in beat pattern and beat frequency. It is clear that simply relying on beat frequency analysis alone, as measured by indirect measures such as the photodiode or photomultiplier systems, will not differentiate a proportion of patients with PCD from normals. The combination of beat FiG 2. A, Electron micrograph of ciliary cross-section illustrating transposition defect. B, Electron micrograph of longitudinal section of ciliary axoneme illustrating the crossover of the peripheral microtubular doublet into the central position seen in the ciliary transposition defect. frequency and beat pattern analysis with DHSV should improve the recognition of patients with underlying PCD. In summary, we have been able to quantify CBF, beat pattern, and ultrastructural defects in patients with PCD. The CBF and beat pattern have been correlated with ultrastructural defects to form 3 distinct groups of dyskinetic beat patterns. REFERENCES 1. Turner JA, Corkey CW, Lee JY, Levison H, Surgess J. Clinical expression of immotile cilia syndrome. Pediatrics 1981;67: Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Resp J 1997;10: Bush A, Cole PJ. Hariri M. Mackay I. Phillips G, O Callaghan C, et al. Primary ciliary dyskinesia: diagnosis and standards of care. Eur Respir J 1998;12: Schidlow DV. Primary ciliary dyskinesia (the immotile cilia syndrome). Ann Allergy 1994;73: Asthma, rhinitis, other respiratory diseases
321 524 Chilvers, Rutman, and O'Callaghan J ALLERGY CLIN IMMUNOL SEPTEMBER 2003 TABLE IV. Summary of ciliary function. The 3 groups of beat pattern and corresponding ultrastructural defect, ciliary beat frequency, and immotility index are displayed. The mean (95% CIs) and range for ciliary beat frequency and mean (95% CIs) for the percentage of immotile cilia (immotility index) are shown. Normal ciliary beat frequency data are taken from reference 20. Asthma, rhinitis, other respiratory diseases Ciliary beat frequency (Hz) Beat pattern Ultrastructural d efect M ean 95% Cl Range Im m otility index (%) Immotile cilia, flickering Inner and outer dynein arm defect U ( ) Outer dynein arm defect ( ) Stiff planar motion Inner dynein arm defect ( ) Radial spoke defect ( ) Rotational motion Transposition defect ( ) Normal planar motion20 Normal ( ) 5. Meeks M, Bush A. Primary ciliary dyskinesia. Pediatr Pulmonol 2000;29: Corkey CW, Levison H, Turner JAP. The immotile cilia syndrome. A longitudinal survey. Am Rev Respir Dis 1981;124: Greenstone MA, Stanley P, Cole P, Mackay I. Upper airway manifestations of primary ciliary dyskinesia. J Laryngol Otol 1985;99: Hadfield PJ, Rowes-Jones JM, Bush A, Mackay 1. Treatment of otitis media with effusion in children with primary ciliary dyskinesia. Clin Otolaryngol 1997;22: Teichtahl H, Wright PL, Kirsner RL. Measurement of in vitro ciliary beat frequency: a television-video modification of the transmitted light technique. Med Biol Eng Comp 1986;24: Dalhamn T, Rylander R. Frequency of ciliary beat measured with a photosensitive cell. Nature 1962;196: Chilvers M, O Callaghan C. Analysis of ciliary beat pattern and beat frequency using digital high-speed imaging: comparison with the photomultiplier and photodiode methods. Thorax 2000:55: Sanderson MJ, Sleigh MA. Ciliary activity of cultured rabbit tracheal epithelium: beat pattern and metachrony. J Cell Sci 1981:47: Chilvers MA, McKean M, Rutman Myint S. Silverman M, O'Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J 2001 ;18: Rossman CM, Newhous MT. Primary ciliary dyskinesia: evaluation and management. Pediatr Pulmonol 1988:5: Santa Maria F, de Santi MM. Grillo G. Samelli P. Caterino M, Greco L. Ciliary motility at light microscopy: a screening test for ciliary defects? Acta Paediatr 1999;88: Rossman CM, Forrest JB, Lee RMKW, Newhouse AF, Newhouse MT. The dyskinetic cilia syndrome: abnormal ciliary motility in association with abnormal ciliary ultrastructure. Chest 1981;80: Rayner CFJ, Rutman A, Dewar A, Cole PJ. Wilson R. Ciliary disorientation alone as a cause of primary ciliary dyskinesia syndrome. Am J Respir Crit Care Med 1996;153: Greenstone MA, Rutman A, Dewar I, Mackay, Cole PJ. Primary ciliary dyskinesia: cytological and clinical features. Q J Med 1988;67: Sturgess JM, Turner JAP. Ultrastructural pathology in the immotile cilia syndrome. Perspect Pediatr Pathol 1984:8: Chilvers MA, Rutman A, O Callaghan C. Functional analysis of cilia and ciliated epithelial ultrastructure in healthy children and young adults. Thorax 2003;58: Pedersen M. Specific types of abnormal ciliary motility in Kartageners syndrome and analogous respiratory disorders. Eur J Respir Dis 1983;64 (Suppl 127): Afzelius BA. A human syndrome caused by immotile cilia. Science 1976;193: De longh RU, Rutland J. Ciliary defect in healthy subjects, bronchiectasis. and primary ciliary dyskinesia. Am J Respir Crit Care Med 1995;151: Wanner A, Salathe M, O Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med 1996;154: Sleigh MA, Blake JR, Liron N. The propulsion of mucous by cilia. Am Rev Respir Dis 1988;137: Taylor HC, Satir P, Holwill MEJ. Assessment of inner dynein arm structure and possible function in ciliary and flagellar axonemes. Cell Motil Cytoskeleton 1999;43: Brokaw CJ, Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskeleton 1987;8: Omoto CK, Kung C. The pair of central tubules rotates during ciliary beat in Paramecium. Nature 1979;279: Van der Baan S, Veerman AJP, Bezemer. PD, Feenstra L. Primary ciliary dyskinesia: quantitative investigation of the ciliary ultrastructure with statistical analysis. Ann Otol Rhinol Laryngol 1987:96: Jorissen M, Willems T, Van der Schueren B, Verbeken E, De Boeck K. Ultrastructural expression of primary ciliary dyskinesia after ciliogenesis in culture. Acta Otorhinolaryngol Belg 2000:54: Rossman CM, Lee RMKW, Forrest JB, Newhouse MT. Nasal ciliary ultrastructure and function in patients with primary ciliary dyskinesia compared with that in normal subjects and in subjects with various respiratory diseases. Am Rev Respir Dis 1984; 129: Jorissen M. Willems T, Van der Schueren, Verbeken E. Dynein arms and spokes after ciliogenesis in cultured respiratory epithelial cells from non- PCD individuals. Acta Otorhinolaryngol Belg 2000;54: Sturgess JM, Thompson MW, Czegledy-Nagy E, Turner JAP. Genetic aspects of immotile cilia syndrome. Am J Med Genet 1986;25: Min YG, Shin JS. Choi SH, Chi JG, Yoon CJ. Primary ciliary dyskinesia: ultrastructural defects and clinical features. Rhinol 1995:33:
Epithelium-1. Hanan Jafar BDS.MSc.PhD
 Epithelium-1 Hanan Jafar BDS.MSc.PhD General features Epithelium is an avascular tissue composed of cells that cover the exterior body surfaces and line internal closed cavities and tubes. It also forms
Epithelium-1 Hanan Jafar BDS.MSc.PhD General features Epithelium is an avascular tissue composed of cells that cover the exterior body surfaces and line internal closed cavities and tubes. It also forms
Histology = the study of tissues. Tissue = a complex of cells that have a common function
 { EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
{ EPITHELIAL TISSUE Histology = the study of tissues Tissue = a complex of cells that have a common function The Four Primary Tissue Types: Epithelium (epithelial tissue) covers body surfaces, lines body
TISSUES TYPES. CHAPTER 05 Histology: EPITHELIUM BIO 211: ANATOMY & PHYSIOLOGY I. HISTOLOGY = the study of tissues
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
CHAPTER 05 Histology: EPITHELIUM
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 05 Histology: EPITHELIUM Part 01: Brief Introduction Part 02: Survey of Types Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy
Review from Biology A
 Chapter 4 Review from Biology A The Cell Theory All organisms are made of cells Cells come from pre-existing cells The cell is the simplest collection of matter that can live Scientists whose work you
Chapter 4 Review from Biology A The Cell Theory All organisms are made of cells Cells come from pre-existing cells The cell is the simplest collection of matter that can live Scientists whose work you
ORGANELLES OF THE ENDOMEMBRANE SYSTEM
 Membranes compartmentalize the interior of the cell and facilitate a variety of metabolic activities. Chloroplasts and a rigid cell wall are what distinguish a plant cell from an animal cell. A typical
Membranes compartmentalize the interior of the cell and facilitate a variety of metabolic activities. Chloroplasts and a rigid cell wall are what distinguish a plant cell from an animal cell. A typical
Anatomy PHL 212. Dr. Dina A. A. Hassan. -
 Anatomy PHL 212 Dr. Dina A. A. Hassan Associate Professor College of Pharmacy (Female Section) Sattam Bin Abdulaziz University Al kharj / Kingdom of Saudi Arabia Email :- da.hassan@psau.edu.sa 1 Anatomy
Anatomy PHL 212 Dr. Dina A. A. Hassan Associate Professor College of Pharmacy (Female Section) Sattam Bin Abdulaziz University Al kharj / Kingdom of Saudi Arabia Email :- da.hassan@psau.edu.sa 1 Anatomy
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following are synthesized along various sites of the endoplasmic reticulum
Muscle Tissue. General concepts. Classification of muscle. I. Functional classification is based on the type of neural control.
 Muscle Tissue LEARNING OBJECTIVES 1. Identify the three types of muscle tissue at the light microscopic level. 2. List and compare the structural and functional features of each of the three muscle fiber
Muscle Tissue LEARNING OBJECTIVES 1. Identify the three types of muscle tissue at the light microscopic level. 2. List and compare the structural and functional features of each of the three muscle fiber
Epithelial Tissue. Functions include: 1. Protection 4. Absorption 2. Secretion 5. Filtration 3. Sensory reception
 Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
Tissues There are 4 primary tissue types in the human body: 1. Epithelial (covering/lining) 2. Connective (support) 3. Muscle (movement) 4. Nervous (control) Epithelium Epithelial Tissue Covers the surface
Tissues 10/21/2016. Epithelial Tissue
 Tissues This is a generalized cell diagram. It shows the anatomy of a cell, but most cells do not actually look like this. Cells can have a wide variety of shapes and sizes, depending on their function.
Tissues This is a generalized cell diagram. It shows the anatomy of a cell, but most cells do not actually look like this. Cells can have a wide variety of shapes and sizes, depending on their function.
Structures in Cells. Cytoplasm. Lecture 5, EH1008: Biology for Public Health, Biomolecules
 Structures in Cells Lecture 5, EH1008: Biology for Public Health, Biomolecules Limian.zheng@ucc.ie 1 Cytoplasm Nucleus Centrioles Cytoskeleton Cilia Microvilli 2 Cytoplasm Cellular material outside nucleus
Structures in Cells Lecture 5, EH1008: Biology for Public Health, Biomolecules Limian.zheng@ucc.ie 1 Cytoplasm Nucleus Centrioles Cytoskeleton Cilia Microvilli 2 Cytoplasm Cellular material outside nucleus
A classification of epithelial tissues
 A classification of epithelial tissues Ramray Bhat Molecular Reproduction Development and Genetics ramray@iisc.ac.in Textbooks for my portion Molecular Biology of the Cell (Bruce Alberts) 6 th Edition
A classification of epithelial tissues Ramray Bhat Molecular Reproduction Development and Genetics ramray@iisc.ac.in Textbooks for my portion Molecular Biology of the Cell (Bruce Alberts) 6 th Edition
Structures in Cells. Lecture 5, EH1008: Biology for Public Health, Biomolecules.
 Structures in Cells Lecture 5, EH1008: Biology for Public Health, Biomolecules Limian.zheng@ucc.ie 1 Cytoplasm Nucleus Centrioles Cytoskeleton Cilia Microvilli 2 Cytoplasm Cellular material outside nucleus
Structures in Cells Lecture 5, EH1008: Biology for Public Health, Biomolecules Limian.zheng@ucc.ie 1 Cytoplasm Nucleus Centrioles Cytoskeleton Cilia Microvilli 2 Cytoplasm Cellular material outside nucleus
RECONSTITUTION OF METACHRONAL WAVES IN CILIATED CORTICAL SHEETS OF PARAMECIUM
 J. exp. Biol. 192, 73 81 (1994) Printed in Great Britain The Company of Biologists Limited 1994 73 RECONSTITUTION OF METACHRONAL WAVES IN CILIATED CORTICAL SHEETS OF PARAMECIUM II. ASYMMETRY OF THE CILIARY
J. exp. Biol. 192, 73 81 (1994) Printed in Great Britain The Company of Biologists Limited 1994 73 RECONSTITUTION OF METACHRONAL WAVES IN CILIATED CORTICAL SHEETS OF PARAMECIUM II. ASYMMETRY OF THE CILIARY
Human height. Length of some nerve and muscle cells. Chicken egg. Frog egg. Most plant and animal cells Nucleus Most bacteria Mitochondrion
 10 m 1 m 0.1 m 1 cm Human height Length of some nerve and muscle cells Chicken egg Unaided eye 1 mm Frog egg 100 µm 10 µm 1 µm 100 nm 10 nm Most plant and animal cells Nucleus Most bacteria Mitochondrion
10 m 1 m 0.1 m 1 cm Human height Length of some nerve and muscle cells Chicken egg Unaided eye 1 mm Frog egg 100 µm 10 µm 1 µm 100 nm 10 nm Most plant and animal cells Nucleus Most bacteria Mitochondrion
يراهظلا( يئلاطلا جيسنلا
 Epithelium النسيج الطالئي )الظهاري( Features of Epithelium Epithelium occurs in the body as a sheet of cells that covers a body surface, lines a cavity, or forms a gland. Coverings, linings, glands. Derived
Epithelium النسيج الطالئي )الظهاري( Features of Epithelium Epithelium occurs in the body as a sheet of cells that covers a body surface, lines a cavity, or forms a gland. Coverings, linings, glands. Derived
Lab Animal Tissue. LEARNING OBJECTIVES: To understand the relationship between the structure and function of different animal tissues
 Name: Bio A.P. PURPOSE: HYPOTHESIS: NONE Lab Animal Tissue BACKGROUND: In animals, groups of closely related cells specialized to perform the same function are called tissues. There are four general classes
Name: Bio A.P. PURPOSE: HYPOTHESIS: NONE Lab Animal Tissue BACKGROUND: In animals, groups of closely related cells specialized to perform the same function are called tissues. There are four general classes
Tissues. tissue = many cells w/ same structure and function. cell shape aids its function tissue shape aids its function
 Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
Tissues tissue = many cells w/ same structure and function cell shape aids its function tissue shape aids its function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings
10 m Human height 1 m Length of some nerve and muscle cells eye 100 mm (10 cm) Chicken egg aid n 10 mm
 Biology 112 Unit Three Chapter Four 1 Cell Sizes Smallest Bacteria Largest Bird egg Longest Giraffe s Nerve Cell Most Cells Diameter of 0.7µm to 105 µm 2 10 m 1 m 100 mm (10 cm) 10 mm (1 cm) Human height
Biology 112 Unit Three Chapter Four 1 Cell Sizes Smallest Bacteria Largest Bird egg Longest Giraffe s Nerve Cell Most Cells Diameter of 0.7µm to 105 µm 2 10 m 1 m 100 mm (10 cm) 10 mm (1 cm) Human height
Dr. Heba Kalbouneh. Dr. Heba Kalbouneh. Dr. Heba Kalbouneh
 Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Tissue: is a group of cells that serve the same function, they are surrounded by extra cellular matrix. The 4 basic types of tissue: 1. epithelial
Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Tissue: is a group of cells that serve the same function, they are surrounded by extra cellular matrix. The 4 basic types of tissue: 1. epithelial
A Tour of the Cell. Chapter 4. Most cells are microscopic. Cells vary in size and shape
 Chapter 4 A Tour of the Cell Most cells are microscopic Cells vary in size and shape 10 m Human height 1 m Length of some nerve and muscle cells 100 mm (10 cm) 10 mm (1 cm) Chicken egg Unaided eye 1 mm
Chapter 4 A Tour of the Cell Most cells are microscopic Cells vary in size and shape 10 m Human height 1 m Length of some nerve and muscle cells 100 mm (10 cm) 10 mm (1 cm) Chicken egg Unaided eye 1 mm
(a) TEM of a plasma. Fimbriae. Nucleoid. Ribosomes. Plasma membrane. Cell wall Capsule. Bacterial chromosome
 0 m m 0. m cm mm 00 µm 0 µm 00 nm 0 nm Human height Length of some nerve and muscle cells Chicken egg Frog egg Most plant and animal cells Most bacteria Smallest bacteria Viruses Proteins Unaided eye Light
0 m m 0. m cm mm 00 µm 0 µm 00 nm 0 nm Human height Length of some nerve and muscle cells Chicken egg Frog egg Most plant and animal cells Most bacteria Smallest bacteria Viruses Proteins Unaided eye Light
Microanatomy of Muscles. Anatomy & Physiology Class
 Microanatomy of Muscles Anatomy & Physiology Class Three Main Muscle Types Objectives: By the end of this presentation you will have the information to: 1. 2. 3. 4. 5. 6. Describe the 3 main types of muscles.
Microanatomy of Muscles Anatomy & Physiology Class Three Main Muscle Types Objectives: By the end of this presentation you will have the information to: 1. 2. 3. 4. 5. 6. Describe the 3 main types of muscles.
Dr. Abeer.c.Yousif. Histology -2 nd stage. What is histology?
 What is histology? Histology is the science of microscopic anatomy of cells and tissues, in Greek language Histo= tissue and logos = study and it's tightly bounded to molecular biology, physiology, immunology
What is histology? Histology is the science of microscopic anatomy of cells and tissues, in Greek language Histo= tissue and logos = study and it's tightly bounded to molecular biology, physiology, immunology
2. Epithelial Tissues Dr. Manal Othman
 Biology-232 GENERAL HISTOLOGY 2. Epithelial Tissues Dr. Manal Othman Anatomy Department CMMS, AGU HISTOLOGY: w Study of the structure and function of tissues and organs at the microscopic levels. w Tissues
Biology-232 GENERAL HISTOLOGY 2. Epithelial Tissues Dr. Manal Othman Anatomy Department CMMS, AGU HISTOLOGY: w Study of the structure and function of tissues and organs at the microscopic levels. w Tissues
Fungal cell walls are rigid with less flexibility due to a combination of more sugar (more chitin) and protein flexibility.
 Cell Structure Assignment Score. Name Sec.. Date. Working by yourself or in a group, answer the following questions about the Cell Structure material. This assignment is worth 40 points with the possible
Cell Structure Assignment Score. Name Sec.. Date. Working by yourself or in a group, answer the following questions about the Cell Structure material. This assignment is worth 40 points with the possible
Biology. Dr. Khalida Ibrahim
 Dr. Khalida Ibrahim Biology Histology: Histology: is the study of the tissues of the body. Tissue: group of similar cells combined to perform a common function. The human body is composed of only 4 basic
Dr. Khalida Ibrahim Biology Histology: Histology: is the study of the tissues of the body. Tissue: group of similar cells combined to perform a common function. The human body is composed of only 4 basic
Cells. 1. Smallest living structures. 2. Basic structural and functional units of the body. 3. Derived from pre-existing cells. 4. Homeostasis.
 Cells The Cell The human body has about 75 trillion cells All tissues and organs are made up of cells Smallest functional unit of life Cytology Histology Cytology Epithelial cells Fibroblasts Erythrocytes
Cells The Cell The human body has about 75 trillion cells All tissues and organs are made up of cells Smallest functional unit of life Cytology Histology Cytology Epithelial cells Fibroblasts Erythrocytes
CMB621: Cytoskeleton. Also known as How the cell plays with LEGOs to ensure order, not chaos, is temporally and spatially achieved
 CMB621: Cytoskeleton Also known as How the cell plays with LEGOs to ensure order, not chaos, is temporally and spatially achieved Lecture(s) Overview Lecture 1: What is the cytoskeleton? Membrane interaction
CMB621: Cytoskeleton Also known as How the cell plays with LEGOs to ensure order, not chaos, is temporally and spatially achieved Lecture(s) Overview Lecture 1: What is the cytoskeleton? Membrane interaction
Bio & 241 A&P Unit 1 / Lecture 3
 Bio & 241 A&P Unit 1 / Lecture 3 Tissues All body tissues arise from three fundamental embryonic tissues. Endoderm: forms epithelial tissues lining internal organs such as the GI tract Mesoderm: connective
Bio & 241 A&P Unit 1 / Lecture 3 Tissues All body tissues arise from three fundamental embryonic tissues. Endoderm: forms epithelial tissues lining internal organs such as the GI tract Mesoderm: connective
Chapter 4: Cell Structure and Function
 Chapter 4: Cell Structure and Function Robert Hooke Fig. 4-2, p.51 The Cell Smallest unit of life Can survive on its own or has potential to do so Is highly organized for metabolism Senses and responds
Chapter 4: Cell Structure and Function Robert Hooke Fig. 4-2, p.51 The Cell Smallest unit of life Can survive on its own or has potential to do so Is highly organized for metabolism Senses and responds
Tissue: The Living Fabric: Part A
 PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 4 Tissue: The Living Fabric: Part A Tissues Groups of cells similar in structure and function Types of tissues Epithelial
PowerPoint Lecture Slides prepared by Janice Meeking, Mount Royal College C H A P T E R 4 Tissue: The Living Fabric: Part A Tissues Groups of cells similar in structure and function Types of tissues Epithelial
Muscle Dr. Ted Milner (KIN 416)
 Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
Epithelium tissue system
 Epithelium tissue system Histology : is the study of the microscopic anatomy (microanatomy) of cells and tissues of plants and animals. It is commonly performed by examining cells and tissues under a light
Epithelium tissue system Histology : is the study of the microscopic anatomy (microanatomy) of cells and tissues of plants and animals. It is commonly performed by examining cells and tissues under a light
Copyright 2011 Pearson Education, Inc. Epithelium. Connective tissue. Copyright 2011 Pearson Education, Inc. Basal surface.
 Chapter 4: Tissues A Tissue is a group of closely associated cells that perform related functions and are similar in structure. Four Basic Tissue Types and Basic Functions: Epithelial covering (Chapters
Chapter 4: Tissues A Tissue is a group of closely associated cells that perform related functions and are similar in structure. Four Basic Tissue Types and Basic Functions: Epithelial covering (Chapters
Chapter 1: Cells and Tissues
 Chapter 1: Cells and Tissues Cells and Tissues Carry out all chemical activities needed to sustain life Cells are the building blocks of all living things Tissues are groups of cells that are similar in
Chapter 1: Cells and Tissues Cells and Tissues Carry out all chemical activities needed to sustain life Cells are the building blocks of all living things Tissues are groups of cells that are similar in
Unit I Problem 9 Histology: Basic Tissues of The Body
 Unit I Problem 9 Histology: Basic Tissues of The Body - What is the difference between cytology and histology? Cytology: it is the study of the structure and functions of cells and their contents. Histology:
Unit I Problem 9 Histology: Basic Tissues of The Body - What is the difference between cytology and histology? Cytology: it is the study of the structure and functions of cells and their contents. Histology:
Histology Notes -Part 1: Epithelial Tissues
 Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
Introduction Group of cells w/ similar structure & function = TISSUE Four Basic Tissue Types 1. Epithelial-covers 2. Connective-supports 3. Muscular*-produces movement (will discuss in the muscular system
7/12/2012. Respiratory system. Respiratory Response to Toxic Injury (Lung) Ninth Industrial Toxicology and Pathology Short Course.
 Ninth Industrial Toxicology and Pathology Short Course 23 27 July, 2012 Contemporary Concepts in Target Organ Toxicologic Pathology Respiratory system Respiratory Response to Toxic Injury (Lung) Eric Wheeldon
Ninth Industrial Toxicology and Pathology Short Course 23 27 July, 2012 Contemporary Concepts in Target Organ Toxicologic Pathology Respiratory system Respiratory Response to Toxic Injury (Lung) Eric Wheeldon
All lecture of practical OSPE file
 All lecture of practical OSPE file Red: questions. Dark red: very important. Black: complete answers. Gray: notes extra. Editing File You should know before the exam: The diagrams in these slides are going
All lecture of practical OSPE file Red: questions. Dark red: very important. Black: complete answers. Gray: notes extra. Editing File You should know before the exam: The diagrams in these slides are going
A. cells that perform related functions and are similar in structure. B. extracellular material - made by cells and secreted into interstitial space
 I. tissue components A. cells that perform related functions and are similar in structure B. extracellular material - made by cells and secreted into interstitial space II. tissue types A. epithelium (e.)
I. tissue components A. cells that perform related functions and are similar in structure B. extracellular material - made by cells and secreted into interstitial space II. tissue types A. epithelium (e.)
Bio10 Cell Structure SRJC
 3.) Cell Structure and Function Structure of Cell Membranes Fluid mosaic model Mixed composition: Phospholipid bilayer Glycolipids Sterols Proteins Fluid Mosaic Model Phospholipids are not packed tightly
3.) Cell Structure and Function Structure of Cell Membranes Fluid mosaic model Mixed composition: Phospholipid bilayer Glycolipids Sterols Proteins Fluid Mosaic Model Phospholipids are not packed tightly
The Respiratory System. Prof. Dr.Mohammed Hisham Al-Muhtaseb
 The Respiratory System Prof. Dr.Mohammed Hisham Al-Muhtaseb Objectives (lecture + practical) 1. Identify the conduction part of the respiratory tract and analyze the function of each segment 2. Identify
The Respiratory System Prof. Dr.Mohammed Hisham Al-Muhtaseb Objectives (lecture + practical) 1. Identify the conduction part of the respiratory tract and analyze the function of each segment 2. Identify
HISTOLOGY OF THE RESPIRATORY SYSTEM I. Introduction A. The respiratory system provides for gas exchange between the environment and the blood. B.
 HISTOLOGY OF THE RESPIRATORY SYSTEM I. Introduction A. The respiratory system provides for gas exchange between the environment and the blood. B. The human respiratory system may be subdivided into two
HISTOLOGY OF THE RESPIRATORY SYSTEM I. Introduction A. The respiratory system provides for gas exchange between the environment and the blood. B. The human respiratory system may be subdivided into two
Nucleic acids. Nucleic acids are information-rich polymers of nucleotides
 Nucleic acids Nucleic acids are information-rich polymers of nucleotides DNA and RNA Serve as the blueprints for proteins and thus control the life of a cell RNA and DNA are made up of very similar nucleotides.
Nucleic acids Nucleic acids are information-rich polymers of nucleotides DNA and RNA Serve as the blueprints for proteins and thus control the life of a cell RNA and DNA are made up of very similar nucleotides.
Histology. Study of body tissues
 Histology Study of body tissues 2 Introduction to Body Tissues 1. Composed of specialized cells of similar structure and perform a common function 2. Four major types (4 Cs) a. Epithelial - Cover b. Connective
Histology Study of body tissues 2 Introduction to Body Tissues 1. Composed of specialized cells of similar structure and perform a common function 2. Four major types (4 Cs) a. Epithelial - Cover b. Connective
Lecture 6 9/17 Dr. Hirsh Organization of Cells, continued
 Cell structure of Eukaryotic cells Lecture 6 9/17 Dr. Hirsh Organization of Cells, continued Lots of double-membraned organelles Existence of an Endo-membrane system separation of areas of cell, transport
Cell structure of Eukaryotic cells Lecture 6 9/17 Dr. Hirsh Organization of Cells, continued Lots of double-membraned organelles Existence of an Endo-membrane system separation of areas of cell, transport
Basic Histology. By Mrs. Bailey
 Basic Histology By Mrs. Bailey Primary Tissues 1. Epithelial Tissue 2. Connective Tissue 3. Muscle Tissue 4. Nervous Tissue Very cellular Supported by underlying connective tissue Epithelial & connective
Basic Histology By Mrs. Bailey Primary Tissues 1. Epithelial Tissue 2. Connective Tissue 3. Muscle Tissue 4. Nervous Tissue Very cellular Supported by underlying connective tissue Epithelial & connective
Chapter 4. A Tour of the Cell. Lecture by Richard L. Myers
 Chapter 4 A Tour of the Cell PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Lecture by Richard L. Myers Introduction: Cells on the Move
Chapter 4 A Tour of the Cell PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Lecture by Richard L. Myers Introduction: Cells on the Move
Skeletal Muscle. Connective tissue: Binding, support and insulation. Blood vessels
 Chapter 12 Muscle Physiology Outline o Skeletal Muscle Structure o The mechanism of Force Generation in Muscle o The mechanics of Skeletal Muscle Contraction o Skeletal Muscle Metabolism o Control of Skeletal
Chapter 12 Muscle Physiology Outline o Skeletal Muscle Structure o The mechanism of Force Generation in Muscle o The mechanics of Skeletal Muscle Contraction o Skeletal Muscle Metabolism o Control of Skeletal
The Golgi Apparatus: Shipping and Receiving Center. The Golgi apparatus. Functions of the Golgi apparatus. Lysosomes: Digestive Compartments
 The Golgi Apparatus: Shipping and Receiving Center The Golgi apparatus Receives (on the cis-side) many of the transport vesicles produced in the rough ER Consists of flattened membranous sacs called cisternae
The Golgi Apparatus: Shipping and Receiving Center The Golgi apparatus Receives (on the cis-side) many of the transport vesicles produced in the rough ER Consists of flattened membranous sacs called cisternae
cell movement and neuronal migration
 cell movement and neuronal migration Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 Cell migration Cell migration in 3 steps; protrusion,
cell movement and neuronal migration Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 Cell migration Cell migration in 3 steps; protrusion,
Lecture Overview. Marieb s Human Anatomy and Physiology. Chapter 4 Tissues: The Living Fabric Epithelial Tissues Lecture 9. Introduction to Tissues
 Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 4 Tissues: The Living Fabric Epithelial Tissues Lecture 9 Lecture Overview Introduction to Tissues Epithelial Tissues Location General characteristics
Marieb s Human Anatomy and Physiology Marieb Hoehn Chapter 4 Tissues: The Living Fabric Epithelial Tissues Lecture 9 Lecture Overview Introduction to Tissues Epithelial Tissues Location General characteristics
Early scientists who observed cells made detailed sketches of what they saw.
 Early scientists who observed cells made detailed sketches of what they saw. Early scientists who observed cells made detailed sketches of what they saw. CORK Early scientists who observed cells made detailed
Early scientists who observed cells made detailed sketches of what they saw. Early scientists who observed cells made detailed sketches of what they saw. CORK Early scientists who observed cells made detailed
Tissues. tissue = many cells w/ same structure and function. cell shape aids function tissue shape aids function. Histology = study of tissues
 Tissues tissue = many cells w/ same structure and function cell shape aids function tissue shape aids function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings Connective
Tissues tissue = many cells w/ same structure and function cell shape aids function tissue shape aids function Histology = study of tissues 4 types of tissues Epithelial coverings contact openings Connective
BIOH111. o Cell Biology Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Biology Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook
BIOH111 o Cell Biology Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook
Organs Histology D. Sahar AL-Sharqi. Respiratory system
 Respiratory system The respiratory system provides for exchange of O2 and CO2 to and from the blood. Respiratory organs include the lungs and a branching system of bronchial tubes that link the sites of
Respiratory system The respiratory system provides for exchange of O2 and CO2 to and from the blood. Respiratory organs include the lungs and a branching system of bronchial tubes that link the sites of
Muscle and Neuromuscular Junction. Peter Takizawa Department of Cell Biology
 Muscle and Neuromuscular Junction Peter Takizawa Department of Cell Biology Types and structure of muscle cells Structural basis of contraction Triggering muscle contraction Skeletal muscle consists of
Muscle and Neuromuscular Junction Peter Takizawa Department of Cell Biology Types and structure of muscle cells Structural basis of contraction Triggering muscle contraction Skeletal muscle consists of
Molecular Cell Biology - Problem Drill 20: Cytoskeleton and Cellular Mobility
 Molecular Cell Biology - Problem Drill 20: Cytoskeleton and Cellular Mobility Question No. 1 of 10 1. Which of the following statements about cytoskeletal filaments is correct? Question #1 (A) The Cytoskeleton
Molecular Cell Biology - Problem Drill 20: Cytoskeleton and Cellular Mobility Question No. 1 of 10 1. Which of the following statements about cytoskeletal filaments is correct? Question #1 (A) The Cytoskeleton
Reading Packet 2- Cells Unit. Chapter 6: A Tour of the Cell 1. What is resolving power?
 AP Biology Reading Packet 2- Cells Unit Name Chapter 6: A Tour of the Cell 1. What is resolving power? 2. How is an electron microscope different from a light microscope and what is the difference between
AP Biology Reading Packet 2- Cells Unit Name Chapter 6: A Tour of the Cell 1. What is resolving power? 2. How is an electron microscope different from a light microscope and what is the difference between
Epithelial Tissue lining, covering, glandular tissue > Function protect, absorption, filtration, secretion, excretion
 Chapter 4: TISSUES IX. Tissues Intro Epithelial Tissue lining, covering, glandular tissue > Function protect, absorption, filtration, secretion, excretion Connective Tissue most widespread tissue type
Chapter 4: TISSUES IX. Tissues Intro Epithelial Tissue lining, covering, glandular tissue > Function protect, absorption, filtration, secretion, excretion Connective Tissue most widespread tissue type
Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and
 CHAPTER 4 Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and recognize normal tissues under the microscope
CHAPTER 4 Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and recognize normal tissues under the microscope
Lab 1 ANIMAL TISSUES
 Lab 1 ANIMAL TISSUES Levels of Organization Animals are multicellular heterotrophs whose cells lack cell walls. Most animals exhibit a hierarchical level of organization: Cells are organized into tissues
Lab 1 ANIMAL TISSUES Levels of Organization Animals are multicellular heterotrophs whose cells lack cell walls. Most animals exhibit a hierarchical level of organization: Cells are organized into tissues
Lecture Overview. Chapter 4 Epithelial Tissues Lecture 9. Introduction to Tissues. Epithelial Tissues. Glandular Epithelium
 Visual Anatomy & Physiology First Edition Martini & Ober Chapter 4 Lecture 9 Lecture Overview Introduction to Tissues Location General characteristics Functions Classification Glandular Epithelium 2 Where
Visual Anatomy & Physiology First Edition Martini & Ober Chapter 4 Lecture 9 Lecture Overview Introduction to Tissues Location General characteristics Functions Classification Glandular Epithelium 2 Where
Epithelial Lecture Test Questions
 Epithelial Lecture Test Questions 1. Which of the following free surfaces lack(s) epithelia: a. lung alveoli (air sacs) b. hard palate c. joint cavities d. abdominal cavity e. salivary gland ducts 2. Which
Epithelial Lecture Test Questions 1. Which of the following free surfaces lack(s) epithelia: a. lung alveoli (air sacs) b. hard palate c. joint cavities d. abdominal cavity e. salivary gland ducts 2. Which
Tissues are: group of similar or identical cells that share a common function. used to build organs
 Tissues: Four classes Epithelium Connective Muscle Nervous Tissues are: group of similar or identical cells that share a common function. used to build organs Overview: Epithelial o Line body cavities
Tissues: Four classes Epithelium Connective Muscle Nervous Tissues are: group of similar or identical cells that share a common function. used to build organs Overview: Epithelial o Line body cavities
Epithelium Characteristics cont. 2. Apical Surface
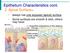 Epithelium Characteristics cont. 2. Apical Surface always has one exposed (apical) surface Some surfaces are smooth & slick, others may have: microvilli fingerlike extensions of the plasma membrane; increase
Epithelium Characteristics cont. 2. Apical Surface always has one exposed (apical) surface Some surfaces are smooth & slick, others may have: microvilli fingerlike extensions of the plasma membrane; increase
Histology. Marcello Malpighi ( ) is regarded as Father of Histology.
 Histology The branch of biology which deals about tissue is called Histology. Marcello Malpighi (1628 1694) is regarded as Father of Histology. Tissue:- Group of identical or, unidentical cells which associate
Histology The branch of biology which deals about tissue is called Histology. Marcello Malpighi (1628 1694) is regarded as Father of Histology. Tissue:- Group of identical or, unidentical cells which associate
A adipose cells. B capillary. C epithelium
 EPITHELIA Objective The objective of this class is to observe how different epithelia vary in terms of cell shape, size and number of cell layers enabling them to be well adapted for functions in different
EPITHELIA Objective The objective of this class is to observe how different epithelia vary in terms of cell shape, size and number of cell layers enabling them to be well adapted for functions in different
Cell and Tissue Types. Epithelial, Connective, Muscle, Nerve
 Cell and Tissue Types Epithelial, Connective, Muscle, Nerve Objectives Explain the major stages of the cell cycle and cellular division (mitosis). Describe specific events occurring in each of the phases
Cell and Tissue Types Epithelial, Connective, Muscle, Nerve Objectives Explain the major stages of the cell cycle and cellular division (mitosis). Describe specific events occurring in each of the phases
Chapter 4. A Tour of the Cell. Lecture by Richard L. Myers
 Chapter 4 A Tour of the Cell PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Lecture by Richard L. Myers Introduction: Cells on the Move
Chapter 4 A Tour of the Cell PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Lecture by Richard L. Myers Introduction: Cells on the Move
Prepared By Student. Dania Abed Al-majeed. Rahma Raad Hanna. Balqees Mohammed Aasim. Dania Hisham. Rasha Rafiee
 Prepared By Student Rahma Raad Hanna Balqees Mohammed Aasim Dania Hisham Dania Abed Al-majeed Rasha Rafiee Epithelia Epithelia can be derived from ectoderm, mesoderm or endoderm -ectoderm gives rise to
Prepared By Student Rahma Raad Hanna Balqees Mohammed Aasim Dania Hisham Dania Abed Al-majeed Rasha Rafiee Epithelia Epithelia can be derived from ectoderm, mesoderm or endoderm -ectoderm gives rise to
Tissue: The Living Fabric
 PowerPoint Lecture Slide Presentation by Vince Austin Human Anatomy & Physiology FIFTH EDITION Elaine N. Marieb Chapter 4 Tissue: The Living Fabric Part A Tissues Groups of cells similar in structure and
PowerPoint Lecture Slide Presentation by Vince Austin Human Anatomy & Physiology FIFTH EDITION Elaine N. Marieb Chapter 4 Tissue: The Living Fabric Part A Tissues Groups of cells similar in structure and
Microtubule Forces Kevin Slep
 Microtubule Forces Kevin Slep Microtubules are a Dynamic Scaffold Microtubules in red, XMA215 family MT polymerase protein in green Some Microtubule Functions Cell Structure Polarized Motor Track (kinesins
Microtubule Forces Kevin Slep Microtubules are a Dynamic Scaffold Microtubules in red, XMA215 family MT polymerase protein in green Some Microtubule Functions Cell Structure Polarized Motor Track (kinesins
ASSET Student Laboratory
 ASSET Student Laboratory The Effect of Cigarette Smoke and Alcohol on Tetrahymena Background Information While the general effects of smoking and alcohol use on humans are well documented, it is useful
ASSET Student Laboratory The Effect of Cigarette Smoke and Alcohol on Tetrahymena Background Information While the general effects of smoking and alcohol use on humans are well documented, it is useful
Tissue four basic types of tissue Epithelial Tissue Functions of Epithelial Tissue
 Tissue A tissue is a group of cells that have a similar shape and function. Different types of tissues can be found in different organs. In humans, there are four basic types of tissue: 1. Epithelial Tissue
Tissue A tissue is a group of cells that have a similar shape and function. Different types of tissues can be found in different organs. In humans, there are four basic types of tissue: 1. Epithelial Tissue
Tissues Review 4 type
 Tissues Review 4 type Tissues Definition: a group of closely associated cells that perform related functions and are similar in structure Between cells: nonliving extracellular material Four basic types
Tissues Review 4 type Tissues Definition: a group of closely associated cells that perform related functions and are similar in structure Between cells: nonliving extracellular material Four basic types
Chapter 4 :Organization & Regulation of Body Systems
 Chapter 4 :Organization & Regulation of Body Systems 4.1 Types of tissues What is a tissue? A collection of cells of the same type that perform a common function There are 4 major tissue types in the body:
Chapter 4 :Organization & Regulation of Body Systems 4.1 Types of tissues What is a tissue? A collection of cells of the same type that perform a common function There are 4 major tissue types in the body:
Skeletal Muscle : Structure
 1 Skeletal Muscle : Structure Dr.Viral I. Champaneri, MD Assistant Professor Department of Physiology 2 Learning objectives 1. Gross anatomy of the skeletal muscle 2. Myofilaments & their molecular structure
1 Skeletal Muscle : Structure Dr.Viral I. Champaneri, MD Assistant Professor Department of Physiology 2 Learning objectives 1. Gross anatomy of the skeletal muscle 2. Myofilaments & their molecular structure
CHAPTER II PDL 101 HUMAN ANATOMY & PHYSIOLOGY. Ms. K. GOWRI. M.Pharm., Lecturer.
 CHAPTER II PDL 101 HUMAN ANATOMY & PHYSIOLOGY Ms. K. GOWRI. M.Pharm., Lecturer. Structure of cell: Human body develops from a single cell zygote which results from fusion of the ovum andd the spermatozoan.
CHAPTER II PDL 101 HUMAN ANATOMY & PHYSIOLOGY Ms. K. GOWRI. M.Pharm., Lecturer. Structure of cell: Human body develops from a single cell zygote which results from fusion of the ovum andd the spermatozoan.
Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 3 Cells and Tissues. Short Answer. Figure 3.1
 Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 3 Cells and Tissues Short Answer Figure 3.1 Using Figure 3.1, match the following: 1) The illustration of simple cuboidal epithelium is. Answer:
Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 3 Cells and Tissues Short Answer Figure 3.1 Using Figure 3.1, match the following: 1) The illustration of simple cuboidal epithelium is. Answer:
I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins
 Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
AP Biology Book Notes Chapter 4: Cells v Cell theory implications Ø Studying cell biology is in some sense the same as studying life Ø Life is
 AP Biology Book Notes Chapter 4: Cells v Cell theory implications Ø Studying cell biology is in some sense the same as studying life Ø Life is continuous v Small cell size is becoming more necessary as
AP Biology Book Notes Chapter 4: Cells v Cell theory implications Ø Studying cell biology is in some sense the same as studying life Ø Life is continuous v Small cell size is becoming more necessary as
Cell Overview. Hanan Jafar BDS.MSc.PhD
 Cell Overview Hanan Jafar BDS.MSc.PhD THE CELL is made of: 1- Nucleus 2- Cell Membrane 3- Cytoplasm THE CELL Formed of: 1. Nuclear envelope 2. Chromatin 3. Nucleolus 4. Nucleoplasm (nuclear matrix) NUCLEUS
Cell Overview Hanan Jafar BDS.MSc.PhD THE CELL is made of: 1- Nucleus 2- Cell Membrane 3- Cytoplasm THE CELL Formed of: 1. Nuclear envelope 2. Chromatin 3. Nucleolus 4. Nucleoplasm (nuclear matrix) NUCLEUS
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 4 The Tissue Level of Organization Introduction The purpose of this chapter is to: Learn about the various types of tissues and their origins
Principles of Anatomy and Physiology 14 th Edition CHAPTER 4 The Tissue Level of Organization Introduction The purpose of this chapter is to: Learn about the various types of tissues and their origins
Derived copy of Epithelial Tissue *
 OpenStax-CNX module: m50425 1 Derived copy of Epithelial Tissue * Stephanie Fretham Based on Epithelial Tissue by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons
OpenStax-CNX module: m50425 1 Derived copy of Epithelial Tissue * Stephanie Fretham Based on Epithelial Tissue by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons
4. A phospholipid is an example of organization at the level.
 1. Physiology is the study of a. the structures of anatomical features. b. cellular metabolism. c. processes that allow organisms to function. d. how organ systems develop from the embryo. 2. Mary spends
1. Physiology is the study of a. the structures of anatomical features. b. cellular metabolism. c. processes that allow organisms to function. d. how organ systems develop from the embryo. 2. Mary spends
Organs of the Respiratory System Laboratory Exercise 52
 Organs of the Respiratory System Laboratory Exercise 52 Background The organs of the respiratory system include the nose, nasal cavity, sinuses, pharynx, larynx, trachea, bronchial tree, and lungs. They
Organs of the Respiratory System Laboratory Exercise 52 Background The organs of the respiratory system include the nose, nasal cavity, sinuses, pharynx, larynx, trachea, bronchial tree, and lungs. They
Lesson 9A Tissues in Animals
 Lesson 9A Tissues in Animals Levels of Organization in the Human Body Similar types of cells Different types of tissues Different organs Many organ systems cell tissue organ organ system organism Levels
Lesson 9A Tissues in Animals Levels of Organization in the Human Body Similar types of cells Different types of tissues Different organs Many organ systems cell tissue organ organ system organism Levels
Epithelium. Four primary tissue types:
 Epithelium Four primary tissue types: Epithelial (covering) Connective (support) Nervous (control) Muscular (movement) Smooth muscle Cardiac muscle Skeletal muscle 1 Epithelial Tissue Features Epithelial
Epithelium Four primary tissue types: Epithelial (covering) Connective (support) Nervous (control) Muscular (movement) Smooth muscle Cardiac muscle Skeletal muscle 1 Epithelial Tissue Features Epithelial
Basic Tissue Types and Functions
 Tissues Histology Basic Tissue Types and Functions 1) Epithelial tissue covering 2) Connective tissue support 3) Muscle tissue movement 4) Nervous tissue control Epithelial Tissue 1) Covers a body surface
Tissues Histology Basic Tissue Types and Functions 1) Epithelial tissue covering 2) Connective tissue support 3) Muscle tissue movement 4) Nervous tissue control Epithelial Tissue 1) Covers a body surface
Tissues Chapter 5...Tissue - a group or mass of similar cells working together to perform certain common functions
 Tissues Chapter 5...Tissue - a group or mass of similar cells working together to perform certain common functions There are 4 major types of tissue Epithelial Connective Muscle Nervous 1. Epithelial Tissue
Tissues Chapter 5...Tissue - a group or mass of similar cells working together to perform certain common functions There are 4 major types of tissue Epithelial Connective Muscle Nervous 1. Epithelial Tissue
High prevalence of Primary Ciliary Dyskinesia in a British Asian population
 High prevalence of Primary Ciliary Dyskinesia in a British Asian population Chris O Callaghan 1, Phil Chetcuti 2, Eduardo Moya 3 1. University of Leicester, Leicester, United Kingdom 2. Leeds General Infirmary,
High prevalence of Primary Ciliary Dyskinesia in a British Asian population Chris O Callaghan 1, Phil Chetcuti 2, Eduardo Moya 3 1. University of Leicester, Leicester, United Kingdom 2. Leeds General Infirmary,
The Respiratory System. Dr. Ali Ebneshahidi
 The Respiratory System Dr. Ali Ebneshahidi Functions of The Respiratory System To allow gases from the environment to enter the bronchial tree through inspiration by expanding the thoracic volume. To allow
The Respiratory System Dr. Ali Ebneshahidi Functions of The Respiratory System To allow gases from the environment to enter the bronchial tree through inspiration by expanding the thoracic volume. To allow
A Tour of the Cell Chapter 4. Outline. Early contributors to Understanding Cells. Cell Theory. Cell Size s Matt Schleiden & Ted Schann
 A Tour of the Cell Chapter 4 Outline History of the science behind cells Cell theory & its importance Why are cells small? Microscopes Cell structure and function Prokaryotic cells Eukaryotic cells Early
A Tour of the Cell Chapter 4 Outline History of the science behind cells Cell theory & its importance Why are cells small? Microscopes Cell structure and function Prokaryotic cells Eukaryotic cells Early
Epithelial Tissue. By the end of this lecture, you should be able to: different types of epithelial membranes.
 Epithelial Tissue Objectives: By the end of this lecture, you should be able to: n Describe general characteristics of epithelial tissue. n Discuss microscopic structure and distribution of different types
Epithelial Tissue Objectives: By the end of this lecture, you should be able to: n Describe general characteristics of epithelial tissue. n Discuss microscopic structure and distribution of different types
Anatomy &- Physiology Histology Worksheet
 Anatomy &- Physiology Histology Worksheet 1. The four primary tissue types found in the human body are a) squamous, cuboidal, columnar, glandular b) adipose, elastic, reticular, cartilage c) skeletal,
Anatomy &- Physiology Histology Worksheet 1. The four primary tissue types found in the human body are a) squamous, cuboidal, columnar, glandular b) adipose, elastic, reticular, cartilage c) skeletal,
Tissues. Tissues - Overview. Bio 101 Laboratory 3. Epithelial Tissues and Integument
 Bio 101 Laboratory 3 Epithelial Tissues and Integument 1 Tissues Tissues to be examined under the microscope Epithelial Tissue Integument Connective Tissue **We will be doing muscle and nervous tissues
Bio 101 Laboratory 3 Epithelial Tissues and Integument 1 Tissues Tissues to be examined under the microscope Epithelial Tissue Integument Connective Tissue **We will be doing muscle and nervous tissues
Epithelia will be discussed according to the following scheme: Type Number of layers Shape Line drawing. Squamous Cuboidal Columnar
 Epithelia Epithelia will be discussed according to the following scheme: Type Number of layers Shape Line drawing Simple Squamous Cuboidal Columnar Covering and Lining epithelium Pseudostratified Stratified
Epithelia Epithelia will be discussed according to the following scheme: Type Number of layers Shape Line drawing Simple Squamous Cuboidal Columnar Covering and Lining epithelium Pseudostratified Stratified
