Consequences of Potassium Recycling in the
|
|
|
- Eleanor Alexandra Chapman
- 5 years ago
- Views:
Transcription
1 Consequences of Potassium Recycling in the Renal Medulla EFFECTS ON ION TRANSPORT BY THE MEDULLARY THICK ASCENDING LIMB OF HENLE'S LOOP JOHN B. STOKES, with the technical assistance of IVAN LEE and ALLEN WILLIAMS, Laboratory of Epithelial Transport and Kidney Physiology, Department of Internal Medicine, University of Iowa, Iowa City, Iowa A B S T R A C T The consequences of K recycling and accumulation in the renal medulla were examined by measuring the effect of elevated K concentration on ion transport by the medullary thick ascending limb of Henle's loop. Perfused and bathed in vitro, thick limbs from both mouse and rabbit displayed a graded, reversible reduction of transepithelial voltage after increasing K concentration from 5 to 10, 15, or 25 mm. The effect was reproducible whether osmolality was 328 or 445 mosmol/kg H20, and whether K replaced Na or choline. Net chloride absorption and transepithelial voltage were reduced by almost 90% when ambient K concentration was 25 mm. When either lumen or bath K was increased to 25 mm, net Na absorption was reduced. There was spontaneous net K absorption when perfusate and bath K concentration was 5 mm. Analysis of transepithelial K transfer after imposition of chemical gradients demonstrated rectification in the absorptive direction. Absorption of K by this segment provides a means to maintain high medullary interstitial concentration. Accumulation of K in the outer medulla, by reducing NaCl absorption, would increase volume flow through the loop of Henle and increase Na and water delivery to the distal nephron. K recycling thus might provide optimum conditions for K secretion by the distal nephron. INTRODUCTION Recent micropuncture experiments have demonstrated that K is recycled to the renal medulla (1-3). A portion of this work was presented at the 1981 Annual Meeting of the American Society of Nephrology, Washington, DC. Received for publication 8 December 1981 and in revised form 9 March The recycling process is dramatically illustrated when, under conditions of acute or chronic K loading, K delivery to the bend of the loop of Henle exceeds 100% of the filtered load (1, 2, 4). The currently accepted pathways for the recycling process involve K secretion by the distal nephron (1, 5), passive absorption across the outer medullary collecting tubule (OMCT)' (6) and secretion into the pars recta or descending limb (1, 4). K concentration in the medullary interstitium can thus reach mm (1). The consequences of the accumulation of K in the renal medulla have not been addressed. The present experiments were designed to examine the effect of increased K concentration on NaCl absorption across the medullary thick ascending limb of Henle's loop (MTALH), a structure likely to be surrounded by K concentrations considerably in excess of 5 mm. In addition, they examine K transport by the MTALH, a subject that has received little attention to date. The results indicate that elevated K concentrations in the perfusing and bathing fluids reversibly reduce net NaCl absorption by the MTALH. In addition, K is absorbed by a process independent of transepithelial voltage (VT). The absorption of K by MTALH provides a mechanism whereby K can recycle within the medulla. The resulting accumulation of K can reduce NaCl absorption by MTALH and thereby reduce NaCl content of the medulla. The consequences of K recycling are appropriate for facilitating excretion of K loads. 1 Abbreviations used in this paper: J,, volume absorption; MTALH, medullary thick ascending limb of Henle's loop; OMCT, outer medullary collecting tubule; P, apparent permeability coefficient (when VT is 0); Umax, maximum urine osmolality; VT, transepithelial voltage. J. Clin. Invest. The American Society for Clinical Investigation, Inc. * /82/08/0219/11 $1.00 Volume 70 August
2 METHODS Isolated segments of MTALH were dissected either from Swiss-Webster mice (18-24 g) or female New Zealand White rabbits ( kg). The general procedure has been previously described (7-9). The MTALH from the rabbit was dissected from the inner stripe of the outer medulla. The distinction between inner stripe and outer stripe is not as clear in the mouse but attempts were made to dissect segments from the inner portion of the outer medulla. Dissection from this portion of the medulla increases the likelihood of obtaining a homogeneous epithelium since the cortical portion of the thick ascending limb of Henle's loop is different from the medullary portion in both the mouse (10) and the rabbit (9). The tubules were dissected in a solution identical to that in which they were subsequently bathed. One of two solutions was used. Solution 1 contained (in millimolars) NaCl, 120; NaHCO3, 25; Na acetate, 10; KCI, 5; MgSO4, 1; CaC12, 1.8; Na2HPO4, 2.3; glucose, 8.3; and L-alanine, 5. This solution had an osmolality of 328±4 mosmol/kg water. Solution 2 contained in addition 25 mm urea, 20 mm choline chloride, and 30 mm NaCl, and had an osmolality of 445±4 mosmol/kg water. All solutions were gassed with 95% oxygen and 5% carbon dioxide so that the ph was 7.4. Except where specifically indicated, all dissection and bathing solutions contained 5% vol/vol bovine calf serum. The tubules were perfused with the same solution as the bath, but without serum. The perfusate also contained C mg/ml FD&C green dye 3 (Keystone Aniline and Chemical Co., Chicago, IL) to improve visualization and, when flux studies were performed, ,uCi/ml [methoxy-3h]inulin exhaustively dialyzed to remove substances of a molecular weight < 3,000 (11). Using this volume marker, net volume absorption (JJ) was calculated by the standard expression (8) and tubules showing a J, > ±0.1 nl mm-' min-' were discarded. The 1.1 ml bath was continuously changed at 0.5 ml/min. After dissection, the tubules were transferred to a plastic chamber where they were perfused using concentric glass pipettes. The tubule was connected to the pipettes and the temperature was raised to 370C over 2 min where it was maintained (±0.5 C) for the duration of the experiment. VT, referenced to the bath, was measured continuously using circuitry as previously described (8). When asymmetric solutions were imposed across the tubule, the circuit voltage was corrected for the calculated liquid junction potential (0.6 mv). All voltages reported are thus transepithelial voltages. The tubules were perfused for 45 min or until VT was stable, whichever was longer. Rabbit MTALH generally displayed a gradual decay in VT after reaching a peak immediately after warming (9). In all experiments, VT was stable 90 min after warming. Mouse MTALH displayed this temporal decrement in VT less frequently. The reason(s) for the difference is not known. During flux studies, perfusion rates were maintained by hydrostatic pressure at nl/min. When only VT was measured, perfusion rate was maintained at >10 nl/min to avoid flow-dependent effects (12). Because net volume flux was near zero, net solute fluxes - (Ji, peq mm-l * min-1) were calculated according to the expression I=V'([- - [i)/l, where VL is the collection rate (nanoliters per minute), measured directly by a constant volume pipette, and is equal to the perfusion rate; L is the length of the tubule (millimeters); and [ijo and [M]L are the concentrations of the solute in the perfused and collected fluid, respectively (picoequivalents per nanoliter). The concentration of solute was determined by two different techniques. In experiments where only net chloride absorption was measured, chloride concentration was determined by microtitration (model F-25, WP Instruments, Inc., New Haven, CT). Three samples each of perfusate and collected fluid for each period were stored overnight in waterequilibrated oil. Samples stored in this way maintain stable concentrations for up to a week. For each sample, chloride concentration was determined in quadruplicate. Thus, for each period reported, the net chloride flux represents the difference between 12 determinations each of perfused and collected fluid. The coefficient of variation for a single sample was generally <2%. In the experiments where net fluxes of Na, K, and Cl were determined, electron probe microanalysis was used. The procedure was similar to that previously reported (13) with minor modifications. The instrument used was a newer model (Applied Research Laboratories, Div. Bausch & Lomb, Sunland, CA, EMX-SM) interfaced with a Tracor Northern (Middleton, WI) computer. Simultaneous determination of Na, K, and Cl were conducted using wavelength dispersive spectrometers. Minimum detection limits and the coefficients of variation for each ion were similar to the values previously reported (13). Net fluxes for each period were determined from three samples each of perfused and collected fluid with each sample being determined in sextuplicate. The experimental procedure consisted of two or three periods. In the control period potassium concentration of the perfusate and bath was 5 mm. In the experimental period potassium concentration was raised to 10, 15, or 25 mm. In experiments where only VT was measured, a recovery period was obtained. When solution 1 was used, potassium replaced sodium. When solution 2 was used, potassium replaced choline. In all experiments where the perfusate was changed, a sham change was executed to be certain that changing the perfusate by itself did not result in a change in VT. Any tubule having a change in VT > ±0.5 mv after this sham change was discarded. All VT and flux measurements were obtained after a minimum stable period of at least 10 min. Statistical analysis was conducted by either paired or unpaired Student's t test as appropriate. Data are expressed as mean±sem. Unless otherwise stated, significant differences have a P value of <0.05. RESULTS Effects of K concentration on VT of mouse MTALH. Fig. 1 displays the results of increasing potassium concentration in both perfusate and bath solutions of the mouse MTALH from 5 mm to 10, 15, or 25 mm. In these experiments, where control and recovery periods used solution 1 (328 mosmol/kg water) K replaced Na. To evaluate whether the decrement in VT was due to the increase in ambient K concentration or due to the decrease in Na concentration, a series of experiments was conducted on mouse MTALH where K replaced choline rather than Na. The results of these experiments are shown in Fig. 2. In this latter set of experiments the more hypertonic solution (445 mosmol/kg water) was designed to simulate more closely the com- 220 J. B. Stokes
3 both groups responded to K in a generally similar fa- E shion, there are some indications that the groups might 6 be different. The mean VT of tubules perfused origi- < 5.0- nally in solution 1 was 4.4±0.4 mv while the mean value for the tubules perfused in solution 2 (hypero \ < Ni 4 \\ tonic) was 3.1±0.2 mv. The difference is significant. 5 \/ \ \ g \ / (P < 0.01 by unpaired analysis) and is consistent with _ 2.0 the findings of Hebert et al. (15) that an acute increase,, 1.o- 0 \\W/ in osmolality of solutions bathing the mouse MTALH z 0 I 2 reduces the vasopressin-stimulated increment in VT. ( K]5 5! A second observation that suggests that the two groups may not be strictly comparable is that although 25 mm FIGURE 1 Effect of increasing K concentration ([K], in mil- K produced a reduction in VT of -90% in both groups, limolars) in bath and lumen on transepithelial voltage across the response to 10 mm K appears different. It is pos- MTALH of the mouse. Onset of the reduction was within sible that a hypertonic environment renders the tubule a minute and stabilization was achieved within 10 min. Re-.... covery was similar in time course. In these experiments sol- more sensitive to small increments in K concentration. ution 1 was used (328 mosmol/kg H20) and K replaced Na. Despite these suggestions that the osmolality of the environment might influence the performance of the thea oerop areysw MTALH, it is clear that acute changes in ambient K intstim (14) ITh concentration can produce reversible reductions in VT position of the outer medullary interstitium (14). The positinvaluesfor mean each group are shown in Table I~ItacostemueMAH is clear that increasing ambient K concentration re- Ectof onentati. ducdte agntueof VTi revril a fshio. Th Effect of K concentration on VT and Jcl absorption reducthen magnivtudoftn V n asrevsibkle fashion. across rabbit MTALH. To determine if the effect of reductioniv fbe gan astquickly asothe e- elevated K concentration was operative in the rabbit changes could be made and continued for another 5- TL swl s h os,kcncnrto nbt 10 m at which time the VT stabilized. On MTALH as occasion, well as the mouse, K concentration in both lmnadbt a asdt ihr1 r2 Mi the reduction in VT did not stabilize for 20 min. Re- thvery uo intas didallynotrstabil,ruiz 20. Re- eight experiments. The results are depicted in Fig. 3. covery stabilizatiof w u r Solution 1 was used in these experiments and the refor stabilization. sults are comparable to the results in the mouse (Fig. When the results are considered together the re- 1). There was an insignificant effect of 10 mm K, while duction in VT must have been due to the increase in thjfeto 5m a uabgosada es K concentration and not to changes in ambient Na or til eversile The tim urseiwas ar t that choline concentrations. The change in voltage cannot Of the mouse. Althougem be attributed to the generation of diffusion potentials or liquid junction potentials since the substitutions precise were made symmetrically. It is likewise evident that generation of the lumen-positive VT in the thick asthe effect ireouiecending limb are not completely understood, there is the effect is reproducible under conditions of hyper- conidrale evdec tha chne nth antd tonicity or near isotonicity (Figs. 1 and 2). Although cofsthevrelec chan intesmnitue oagn of the VT reflect changes in the magnitude of NaCl absorption (9, 10, 15-19). To determine directly whether such was the case in the present experiments, 6.0 net chloride absorption (lumen-to-bath flux) was meah 5.0I' sured in MTALH dissected from normal rabbits before CJ 501 \ 8and after increasing both perfusate and bath K con \ t centration from 5 to 25 mm. The results of 10 such < 3.0 experiments together with six control experiments hi 2.0] & / \\\ /G I A(where the perfusate was sham changed) are displayed x in-table Q\\ II. g" Solution \\ 1 was used and K replaced Na. X 1.0 Both VT and Jcl were reduced by 86%, a value com wi parable to the decrements in VTproduced by 25 mm F [K]5 1 K in the mouse MTALH (Figs. 1 and 2). Thus, a reduction of VT reflects a reduction of NaCl absorption. FIGURE 2 Effect of increasing K concentration (in milli- Evaluation of simultaneous net Na, K, and Cl molars) in bath and lumen on transepithelial voltage across fluaes.nthe simultaneous net Na, K, MTALH of the mouse. Time course was similar to experiments shown in Fig. 1. In these experiments solution 2 was and Cl fluxes has not been reported for the MTALH used (445 mosmol/kg H20) and K replaced choline. of the rabbit. Table III displays the mean VT, the mean K Effect on NaCI Transport 221
4 TABLE I Effect of Increasing Ambient K Concentration on Mean Voltage (mv) across MTALH of the Mouse Control IOK Recovery Control 15K Recovery Control 25K Recovery (n = 4) (n = 4) (n = 4) Solution 1, * mosmol/kg ±0.5 ±0.6 ±0.3 ±0.3 ±0.3 ±0.3 ±0.6 ±0.2 ±0.2 (n = 4) (n = 4) (n = 5) Solution 2, " mosmol/kg ±0.4 ±0.2 ±0.4 ±0.7 ±0.2 ±0.5 ±0.3 ±0.1 ±0.3 All results ±0.4 ±0.6 ±0.5 ±0.4 ±0.2 ±0.3 ±0.4 ±0.1 ±0.3 Values are mean±sem. In solution 1, K replaced Na while in solution 2, K replaced choline. Significantly different from both control and recovery values by paired analysis (P < 0.05). difference in concentration between perfused and collected fluid, and the net (absorptive) fluxes of Na, K, and Cl, determined by electron probe microanalysis for 14 tubules perfused and bathed with solution 1. There was a small but significant net absorption of K. In addition, there was no difference between the net absorption of the sum of Na and K (101±10) and the net absorption of Cl (109±12). These results provide no evidence for a significant flux of another anion (such as HCO-) although the technique is not sufficiently sensitive to detect a small net flux of an unmeasured species. These data support the notion that the measurement of the change in net Cl transport (under symmetrical conditions) reflect changes in NaCl transport (10, 12, 20). To evaluate the effect of an asymmetric increase in K concentration the 14 tubules, after measurement of their steady-state base-line transport, had either their E -j 49 -I I-- 4c "J Nl. O- (K] FIGURE 3 Effect of increasing K concentration (in millimolars) in bath and lumen on transepithelial voltage across MTALH of the rabbit. Time course was similar to the experiments conducted on mouse MTALH (Figs. 1 and 2). Solution 1 was used (328 mosmol/kg H20) and K replaced Na. bath or perfusate K raised to 25 mm with a concomitant reduction in Na. The purposes of this maneuver were to examine the "sidedness" of an effect and to examine net K fluxes in the presence of a chemical concentration gradient. The results of the experiments where lumen K was raised to 25 mm are displayed in Table IV. There was an increase in net K efflux, a striking reduction in net Na efflux, and no significant change in Cl efflux. Since the direction of the change in Na and K transport was appropriate for their respective concentration gradients, one cannot be certain whether the increase in luminal K reduced active transport of NaCl. The reduction in VT from 3.1±1.0 to 0.8±0.2 mv suggests that it did, but one cannot be completely certain (vide infra). In any event, it is obvious that this maneuver reduced the net NaCl absorption and enhanced net KC1 absorption. The results of the experiments where bath K was raised to 25 mm are depicted in Table V. There was a net K influx, consistent with the direction of the K concentration gradient, a reduction of Cl efflux to near zero, and a significant reduction of net Na efflux. The reduction in Na efflux occurred despite the favorable chemical gradient. This result suggests that the active transport of NaCl was reduced by increasing bath K. The mechanism of the reduction of Na efflux by increasing either bath or lumen K to 25 mm cannot be deduced with certainty from these experiments. The reduction in Na absorption when bath K was raised to 25 mm (Table V) is consistent with the notion that transcellular NaCl efflux was reduced, since net Na efflux fell (despite a favorable chemical gradient) and Cl efflux was nearly abolished. The reduction of VT from 3.3±0.5 to 1.7±0.6 mv supports this interpretation. The interpretation of the effects of raising 222 J. B. Stokes
5 TABLE II Effect of Increasing Both Perfusate and Bath K Concentration from 5 to 25 mm on VT and Jc' across MTALH of the Rabbit Experiment L V Control 25K Control 25K Effect of 25K VT mm ni/min mv pq. mm-'* min Mean ±SEM P <0.002 <0.001 Effect of sham change Mean ±SEM P NS NS Solution 1 was used in all experiments. The increase in K concentration was accompanied by an equimolar reduction in Na concentration. Abbreviations used in this table: L, length; V', collection rate (equal to perfusion rate); Jcs, net chloride absorption; control and 25K refer to the periods where K concentration was 5 and 25 mm, respectively. lumen K to 25 mm are more complicated (Table IV). The reduction in net Na efflux might have been due to either the reduction in transcellular NaCl efflux or an increase in Na backflux through the paracellular pathway or a combination of these effects. The reduction of VT from 3.1±1.0 to 0.8±0.2 mv supports the notion that transcellular NaCl absorption was reduced. This reasoning is based on the relative cation permeability, determined by Greger (21), of the cortical thick limb of the rabbit. If the voltage response to these maneuvers were due only to the biionic diffusion potential, raising bath K should have increased (rather than reduced) VT by 0.9 mv. Likewise, raising perfused K should have reduced VT by only 0.9 mv. Ja Thus, although the effect of elevated K from either the lumen or bath is consistent with the notion that transcellular NaCl efflux was reduced, this conclusion must be considered tentative until there is more information regarding alterations in intracellular events. Analysis of K flux. As demonstrated in Table III, under base-line conditions there was a consistent net reabsorption of K. This result is in contrast to the results in the cortical thick ascending limb of Henle's loop of the rabbit (22) where, under similar circumstances, K secretion was generally observed. The evaluation of whether this transport process is passive can be approached by using the Goldman equation (23), an integrated form of the Nernst-Plank equation, which assumes a constant electric field (i.e., the electrical potential is a linear function of distance). The expression is written PZFVT [Cb Cie-zFVT/RT - 1 = RT L ezfvt/rt - 1 where ji is the net flux of ion i (picoequivalents per millimeter per minute) where a positive value indicates absorption, P the permeability coefficient2 (nanoliters per millimeter per minute), Cb the concentration of i in the bath, Cl the arithmatic mean concentration of i in the lumen, z the valence of i, F the Faraday, R the gas constant, and T the absolute temperature. This expression allows the prediction of net flux for any epithelium (membrane) where VT, the permeability coefficient and the concentration gradients are known if the flux proceeds entirely by simple diffusion.3 Under control conditions there was net absorption of K (Table III). Since absorption of a cation in the presence of a lumen-positive VT might be owing to diffusion and not active transport, the apparent permeability coefficient was calculated using the above expression and the measured values for each tubule. The mean apparent permeability coefficient was 6.13 X 10- cm/s. This value is an order of magnitude larger 2The permeability coefficient can be converted to the more traditional units of centimeters per second by multiplying by 2.66 X 10', which assumes an internal diameter of the tubule of 20 Mm. ' The designation of permeability coefficient means that transport proceeds entirely by simple diffusion. A value for P can be calculated despite no a priori knowledge of the mechanism of transport. For purposes of comparing lumento-bath and bath-to-lumen fluxes in the presence of a measurable VT and a concentration gradient, P can be calculated and called an apparent permeability coefficient at VT = 0- In the present experiments the calculated apparent permeability coefficients are not the true permeability coefficient since they do not conform to the patterns predicted by simple ionic diffusion. K Effect on NaCI Transport 223
6 TABLE III Base-line Transport Characteristics of Rabbit MTALH (n = 14) VT [Nab - [NaL JN. [KID - [Kj Jk [CIL - [CIlj Ja mv meq/liter peq* mm' * meqwliter peq * mm-* meq/ltiter peq* mm-' min-j min-j min-i ±0.3 ±1.6 ±9.8 ±0.2 ±0.3 ±2.0 ±12 Values are mean±sem. JNa, JK, and Jcl are the net transport rates of Na, K, and Cl, respectively. [Nab0 - [Nak, [Klo - [KIL, and [ClD- [Cik are concentration differences between perfused and collected fluid. All values are significantly >0 (P < 0.01). than the permeability coefficient for Na of 6.27 X 10- cm/s determined by Rocha and Kokko (12) using bathto-lumen tracer fluxes. The discrepancy suggests that potassium transport might not be entirely explained by paracellular diffusion, for a 10-fold selectivity of K over Na has not been previously described. However, the magnitude of the VT and JK measurements4 is such that small errors create large errors in the calculated apparent permeability. The standard error is sufficiently large so that no firm conclusion can be reached. The measurement of net K fluxes after an imposed chemical gradient allows a considerably more accurate determination of the apparent permeability coefficient. Table VI displays the K flux data in tubules where such a concentration gradient was imposed. It is clear that the net fluxes of K under the two conditions are significantly different. Although the individual experiments are not shown, there was no overlap in these values. Statistical analysis of VT showed no difference between the two groups. However, since a small VT might produce a significant difference in the flux ratio if the true permeability coefficient were large, the apparent permeability coefficient (P:) was calculated for each experiment. These values were likewise significantly different from each other (Table VI). If potassium were transported entirely by simple diffusion, the ratio of these numbers should be unity. The ratio is 2.17, a value closer to the ratio of the 4 At perfusion rates that are sufficiently slow to allow a NaCl concentration gradient to develop at the collection end of the tubule, the VT at the distal end will be more positive than at the proximal end. The greatest bath-to-lumen concentration gradient measured in these experiments was 1.3. If the Na/Cl permeability ratio is between 2.2 (10, 21) and 6.1 (12), the VT at the distal end of the tubule will be as much as 3.5 to 5.5 mv greater than the proximal end and the mean VT will be greater than reported. Because of biologic variability and uncertainty of the VT at the distal end, and the inherent errors in measuring ion concentrations, mean VT can not be precisely assessed. 224 J. B. Stokes unidirectional fluxes for Na (1.71) measured isotopically (12). The analysis of the discrepancy between the potassium fluxes can be conducted in another way. If one assumes that all K transport occurs by simple diffusion, i.e., that the apparent permeability coefficient measured from lumen-to-bath and bath-to-lumen are in fact not different, then one can calculate the mean VT necessary to effect the measured net K fluxes. This voltage would have to be 10 mv. Given an upper limit of 2 mv at the perfused end, the voltage at the distal end would have to be -22 mv, an extremely unlikely occurrence since the (bath-to-lumen) NaCl concentration gradient required to produce this voltage is >4 (12) and the maximal concentration gradient at the distal end of these tubules was only 1.3. Thus, the absorption of K that occurred under control conditions was most likely not due entirely to the lumen positive VT. Rather, these data provide presumptive evidence for a transcellular absorptive process for K. Mechanism of the effect of K on NaCI absorption. TABLE IV Effect of Increasing Perfused K Concentration to 25 mm on Net Ion Transport across Rabbit MTALH (n = 7) L VT JN. Ji Ja mm mv peq. mm-* min-' Control ±1.0 ±16 ±1.2 ± ±0.1 25K ±0.2 ±5.9 ±3.8 ±7.8 P <0.002 <0.002 <0.001 NS Pp values represent comparison of 25K period vs. control. Control solutions contained 5 mm K in both perfusate and bath. Increase in K concentration accompanied by equivalent decrease in Na concentration. Abbreviations as in Tables I and II.
7 TABLE V Effect of Increasing Bath K Concentration to 25 mm on Net Ion Transport across Rabbit MTALH (n = 7) L VT JN. Il Ja mm mv peq. mm-'* min-' Control ±0.5 ±12.5 ±1.0 ± ±0.1 25K e 4.9 ±0.6 ±5.3 ±3.0 ±10.2 P <0.05 <0.05 <0.001 <0.001 Abbreviations as in Tables I and II. * Negative flux represents secretion. The most likely explanation for the K effect on NaCl transport involves membrane depolarization. If such is the case, membrane depolarization by another mechanism should produce a similar effect. Previous reports have shown that Ba++ administered in millimolar concentrations can reduce membrane K conductance of several cell types (24-27) and by so doing depolarize the membrane (25, 28). In addition, Greger and Schlatter (29) have shown that Ba++ produces such an effect in the cortical thick limb of the rabbit. Thus, Ba++ should mimic the effects of elevated K concentration. It became apparent that the effect of Ba++ would not be as straightforward as the effect of high K. First, there was no effect of Ba++ when the bath contained 5% bovine serum. For this reason, the serum was TABLE VI Net K Flux across Rabbit MTALH in Presence of a Concentration Gradient Perfused [K] (mm) 25 5 Bath [K] (mm) 5 25 P (n = 7) (n = 7) VT (mv) ±0.6 NS [Kh - [KL (mm) ±0.4 <0 001 JK (peq -mm-' -min-') 59.5 _33.5 < ±3.0 P' (cm/s x 10-5) < ±0.5 Values are mean±sem. P values compare absolute values of column 1 to column 2 using unpaired analyses. P. is the calculated apparent permeability coefficient (when VT = 0) for K. Other abbreviations as in Tables I, II, and III. > E 7', 0 6' 45 S 0 -J > 4 -j t 2 zi1 2' w (0 I. z 4 BATH NS LUMEN C Bao* R C Bo R FIGURE 4 Effect of 4 mm Ba++ on transepithelial voltage across the mouse MTALH. Solution 1 was used (328 mosmol/ kg H20). C and R indicate stable control and recovery values, respectively. (Ba++ effect from bath, P < 0.001; from lumen P < Recovery NS). omitted from the bath, a maneuver that on several occasions caused a reduction in VT in both rabbit and mouse MTALH. Thus, the Ba++ experiments were conducted on mouse MTALH that had been dissected in 5% serum solution but was perfused and bathed in solution 1 (osmol = 328) that contained no serum. Three tubules treated in this fashion developed a VT that gradually fell from >5 mv initially to <2 mv at 90 min, despite having an epithelium that appeared normal. These tubules were discarded. The results of the effect of 4 mm Ba++ on VT of the acceptable experiments are displayed in Fig. 4. Ba++, when added to the bath as BaCl2, produced a reduction in VT from 4.3±0.6 to 2.9±0.5 mv (P < 0.01). The change usually began within a minute and was complete within 10 min. When Ba++ was added to the perfusate, VT fell from 4.7±0.4 to 3.5±0.5 mv (P < 0.002) with a similar time course. Recovery was inconsistent. Despite the differences between the effects of high K and Ba+ the similarities between the direction of the change in VT and the time course of the change are consistent with the notion that both high K and Ba++ might be producing their effects by depolarizing the membrane.5 5 In the cortical thick limb of the rabbit, Ba++ depolarizes the cell membrane by reducing K conductance, and inhibits the calculated short circuit current (an estimate of the magnitude of NaCl absorption) (29). However, the influence of these events on VT is complicated by uncertainties regarding the magnitude of the change in transepithelial resistance. Van Driessche and Zeiske (30) have demonstrated a possible reduction in shunt resistance in the frog skin. Greger and Schlatter (29) have demonstrated a large increase in transepithelial resistance of the cortical thick limb, most probably owing to reduction of the transcellular K conductance. This latter effect would explain the lesser change in VT observed in the present Ba++ experiments (compared with the K experiments) if the cellular K conductance participated in the shunting of VT generated by active NaCl transport. NS K Effect on NaCI Transport 225
8 DISCUSSION The results of the present experiments demonstrate that an acute increase in ambient K concentration to ranges found in vivo (1) can produce a substantial reduction in VT and NaCl absorption across the MTALH of both the mouse and the rabbit. The effect is reversible and occurs under hypertonic and near isotonic conditions (Figs. 1 and 2). The rate of net NaCl absorption across the MTALH has been shown to be stimulated by vasopressin (10, 15-17) and fl-adrenergic agonists (31). It can be reduced by prostaglandin E2 (9) and hypertonicity (15). The present results add another link to our understanding of the control of NaCl absorption by the loop of Henle and, as a consequence, the control of medullary tonicity. Perhaps most importantly, they provide the first data addressing the functional significance of K recycling to the renal medulla. Since K recycling was first described by Battilana et al. (1), its nature has received increasing attention. The unambiguous evidence of K secretion by the thin descending limb of Henle's loop or the pars recta (1, 4, 32) has prompted a series of experiments investigating the pathways for this recycling. It is now generally accepted that the K secreted by the distal convoluted tubule and cortical collecting tubule is in part reabsorbed across the outer medullary and/or papillary collecting tubule (1, 2, 6) and accumulates in the medullary interstitium. This accumulation allows a concentration gradient to develop that causes net K secretion into the thin descending limb of Henle's loop (33) or pars recta (34, 35). K recycling is enhanced in chronic K administration (1) as well as acute K administration (2) and is reduced by K deprivation (3). The concentrations of K used in these experiments are probably within the range of concentrations found in vivo. Dobyan et al. (3) have reported a mean K concentration at the bend of the loop of 25 mm in young Munich-Wistar rats fed a normal diet, and a concentration of 9 mm in rats deprived of K for 3 d. Battilana et al. (1) have reported similar values for normal rats and a mean concentration of 37 mm for rats fed a high K diet. Concentrations in vasa recta blood were '-10 mm greater than loop fluid (1, 36). Although there is no clear way to extrapolate K concentrations found at the bend of the loop to the concentrations in the outer medulla, the range used in the present study is a reasonable estimate of the range of concentrations likely surrounding the medullary thick ascending limb of Henle's loop. Individual values reported by Jamison et al. (37) for the Sprague-Dawley rat and derouffignac and Morel (32) for the psammomys support the notion that this range of concentrations may be found under normal physiologic conditions. The reduction of NaCl absorption across the MTALH by elevated K concentration is consistent with the effects of acute infusion of K on Na excretion. Vander (38) described a "direct" effect of K on Na excretion by the kidney. Brandis et al. (39), using micropuncture techniques, found that fluid absorption across the proximal convoluted tubule was reduced by increased peritubular K concentration. Reduction in fluid absorption was not observed by increasing peritubular K concentration similarly in the isolated, perfused rabbit proximal tubule (40). Kahn and Bohrer (41), using clearance techniques, postulated a major effect on the "distal convoluted tubule" (diluting segment). Wright et al. (42) examined distal tubule Na absorption and found that most of the natriuretic effect occurred proximal to the superficial distal convoluted tubule. They showed that Na delivery to the early distal tubule was increased by acute K infusion as well as chronic administration of a high K diet. The diuretic effect of a high K diet is also widely recognized, although the explanation for this phenomenon is not completely understood. The osmotic diuresis (NaCl and KCI) may play a role under some circumstances. However, Battilana et al. (1) demonstrated a reduced urine osmolality in chronically K- loaded rats without an increase in solute excretion. These findings suggest that chronic K-loading might impair the generation of a maximum urine osmolality (Umax). An impairment in Umax is consistent with the present results regarding reduced NaCl absorption across the MTALH, an effect that would lead to a diminished medullary interstitial solute content. K transfer across MTALH. In addition to providing information regarding the effect of K on NaCl absorption, the present experiments provide evidence that K transfer across the MTALH cannot be accounted for by an entirely passive process. Under baseline conditions, when ambient K concentration was 5 mm, K was absorbed (Table IV). This result is different from that reported by Burg and Bourdeau (22) for the cortical thick ascending limb of the rabbit. Under similar conditions they found a small net secretion.6 Since the net absorption of K in the presence of a lumen-positive VT might be owing completely to diffusion, the data were analyzed according to the Goldman flux equation (23). These results (Table VI) clearly indicate that there is rectification of K movement 6 The present results also contrast with preliminary results reported by Work and Schafer (43). Using tracer measurements of Rb flux they reported a larger secretory component than absorptive component. The reasons for these apparent discrepancies are not clear. 226 J. B. Stokes
9 across the MTALH in the absorptive direction. The mechanism of such a rectification process cannot be discerned from the present experiments. Nevertheless, its presence is consistent with the magnitude of the net absorption of K under control conditions when K concentration was 5 mm (Table IV). The significance of K absorption by the MTALH is most readily appreciated if it is viewed within the context of the presumed pathways of K recycling as depicted schematically in Fig. 5. There appear to be four discrete steps. (a) K is secreted by the distal convoluted tubule and the cortical collecting tubule; (b) a portion of this secreted K is passively reabsorbed across the OMCT (6) and raises the interstitial K concentration; (c) K enters the pars recta or thin descending limb of Henle's loop; (d) the recycling process is amplified by absorption across the MTALH. This last step may be important in regulating the magnitude of K accumulation in the medulla. Although it is clear that K can diffuse across the outer medullary collecting duct into the interstitium, the permeability of this epithelium to K is low (6) so there is some doubt as to whether the magnitude of K reabsorption across OMCT could explain interstitial K concentration of up to 50 mm (1). A large K reabsorption across OMCT would also be counterproductive under conditions when the K excretory mechanisms should be operating maximally, i.e., under conditions of K loading. The absorption of K across the MTALH would effectively FIGURE 5 Schematic representation of K recycling to the renal medulla. (a) K is secreted by the distal nephron. (b) Small amount is passively reabsorbed by the outer medullary collecting tubule. (c) Secretion into the pars recta or descending limb of Henle's loop. (d) Absorption by MTALH. minimize the requirements for K absorption across OMCT and would amplify K accumulation in the medullary interstitium. K effect on NaCI absorption. The mechanism whereby increasing K concentration reduced VT and NaCl absorption is most likely related to depolarization of the cell membrane. Most mammalian cells have as a major component of their membrane voltage a K diffusion potential. Because barium depolarizes cell membranes by reducing K conductance (24-27, 29), the results of the effects of barium (Fig. IV) are consistent with the notion that cell depolarization may be the mechanism whereby an elevation in the ambient K concentration might reduce NaCl absorption. Depolarization of the cell might reduce NaCl absorption by reducing the electrochemical gradient for Cl exit across the basolateral membrane. According to our current perceptions of the mechanism of NaCl absorption by the thick ascending limb of Henle's loop, NaCl enters the cell by an electrically neutral mechanism across the apical membrane and exits across the basolateral membrane (15, 21, 29, 44-46). A conductive Cl exit step would be voltage-sensitive and would provide a convenient explanation for the present results. Significance. The direct implications of the present experiments are that K recycling would produce natriuresis and diuresis. Although these effects are often observed after acute K infusion into the intact animal, the complete (teleological) significance may relate to the diuretic and natriuretic effects only indirectly. The major importance of K recycling may have more to do with facilitating K excretion than with producing a Na and water diuresis. As we currently understand K secretion by the distal nephron, there are two major determinants. In the distal convoluted tubule K secretion is positively correlated with axial volume flow (47). In the cortical collecting tubule, K secretion is, in large part, regulated by the absorption of Na (48), a process that is enhanced by mineralocorticoid hormone (48-50) and is dependent on an adequate concentration of Na in the tubular fluid (48, 51). By reducing NaCl absorption across the MTALH, K accumulation in the medulla would increase volume flow through the loop of Henle (by virtue of reducing water absorption across the thin descending limb) and thus increase fluid delivery to the distal convoluted tubule. Simultaneously, Na delivery (and presumably Na concentration in the tubular field) to the cortical collecting tubule would also be increased. This shift in the location of Na absorption together with an appropriate increase in aldosterone secretion (52) would maximize K secretion by the cortical collecting tubule. Thus, an increase in K secretion by the distal nephron would, by virtue of K recycling to the medulla, create K Effect on NaCI Transport 227
10 conditions in the distal nephron that would permit maximal K excretion. In summary, the accumulation of K in the renal medulla can inhibit the absorption of NaCl by the MTALH. This action would have as an immediate effect a reduction in medullary solute content and consequently an enhancement of Na and H20 delivery to the distal nephron. The ultimate importance of K recycling might be the provision of adequate Na delivery and volume flow to the distal nephron so that K secretion can proceed maximally. The mechanism whereby K produces this reduction in NaCl absorption is probably via depolarization of the cell. The presence of absorptive rectification of K raises new possibilities for the involvement of K in the process of NaCl absorption by the MTALH. ACKNOWLEDGMENTS I appreciate the secretarial assistance of Ms. Sydney Harned and the thoughtful critique of the manuscript by Dr. Gerald DiBona and Dr. Michael Welsh. This work was supported in part by grants from the National Institutes of Health AM-25231, HL-14388, and RR ; a grant from the Iowa Heart Association; and a grant from the National Kidney Foundation of Iowa. REFERENCES 1. Battilana, C. A., D. C. Dobyan, F. B. Lacy, J. Bhattacharya, P. A. Johnston, and R. L. Jamison Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J. Clin. Invest. 62: Arrascue, J. B., D. C. Dobyan, and R. L. Jamison Potassium recycling in the renal medulla: effects of acute potassium chloride administration to rats fed a potassium-free diet. Kidney Int. 20: Dobyan, D. C., F. B. Lacy, and R. L. Jamison Suppression of potassium recycling in the renal medulla by short-term potassium deprivation. Kidney Int. 16: Jamison, R. L., F. B. Lacy, J. P. Pennell, and V. M. Sanjana Potassium secretion by the descending limb or pars recta of the juxtamedullary nephron in vivo. Kidney Int. 9: Wright, F. S Sites and mechanisms of potassium transport along the renal tubule. Kidney Int. 11: Stokes, J. B Na and K transport across the cortical and outer medullary collecting tubule of the rabbit: evidence for diffusion across the outer medullary portion. Am. J. Physiol. 242(Renal Fluid Electrolyte Physiol. 11): F514-F Burg, M., J. Grantham, M. Abramow, and J. Orloff Preparation and study of fragments of single rabbit nephrons. Am. J. Physiol. 210: Stokes, J. B., and J. P. Kokko Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J. Clin. Invest. 59: Stokes, J. B Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibition of the medullary portion. J. Clin. Invest. 64: Hebert, S. C., R. M. Culpepper, and T. E. Andreoli NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am. J. Physiol. 241(Renal Fluid Electrolyte Physiol. 10): F412-F Schafer, J. A., S. L. Troutman, and T. E. Andreoli Volume reabsorption, transepithelial potential differences, and ionic permeability properties in mammalian superficial proximal straight tubules. J. Gen. Physiol. 64: Rocha, A. S., and J. P. Kokko Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J. Clin. Invest. 52: Stokes, J. B., M. J. Ingram, A. D. Williams, and D. Ingram Heterogeneity of the rabbit collecting tubule: localization of mineralocorticoid hormone action to the cortical portion. Kidney Int. 20: Ullrich, K. J., K. Kramer, and J. W. Boylan Present knowledge of the counter-current system in the mammalian kidney. Prog. Cardiovasc. Dis. 3: Hebert, S. C., R. M. Culpepper, and T. E. Andreoli NaCl transport in mouse medullary thick ascending limbs. III. Modulation of the ADH effect by peritubular osmolality. Am. J. Physiol. 241(Renal Fluid Electrolyte Physiol. 10): F443-F Hall, D. A., and D. M. Varney Effect of vasopressin on electrical potential difference and chloride transport in mouse medullary thick ascending limb of Henle's loop. J. Clin. Invest. 66: Sasaki, S., and M. Imai Effects of vasopressin on water and NaCl transport across the in vitro perfused medullary thick ascending limb of Henle's loop of mouse, rat, and rabbit kidneys. Pfluegers Arch. Eur. J. Physiol. 383: Hebert, S. C., R. M. Culpepper, and T. E. Andreoli NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. Am. J. Physiol. 241(Renal Fluid Electrolyte Physiol. 10): F432- F Kokko, J. P Membrane characteristics governing salt and water transport in the loop of Henle. Fed. Proc. 33: Seldin, D. W., J. M. Rosen, and F. C. Rector, Jr Evidence against bicarbonate reabsorption in the ascending limb, particularly as disclosed by free-water clearance studies. Yale J. Biol. Med. 48: Greger, R Cation selectivity of the isolated perfused cortical thick ascending limb of Henle's loop of rabbit kidney. Pfluegers Arch. Eur. J. Physiol. 390: Burg, M. B., and J. E. Bourdeau Function of the thick ascending limb of Henle's loop. In New Aspects of Renal Function. H. G. Vogel and K. J. Ullrich, editors. Exerpta Medica, Amsterdam. pp Goldman, D. E Potential, impedance, and rectification in membranes. J. Gen. Physiol. 27: Armstrong, C. M., and S. R. Taylor Interaction of barium ions with potassium channels in squid giant axons. Biophys. J. 30: Hermsmeyer, K Ba++ and K' alteration of K+ conductance in spontaneously active vascular muscle. Am. J. Physiol. 230: Nagel, W Inhibition of potassium conductance 228 J. B. Stokes
11 by barium in frog skin epithelium. Biochim. Biophys. Acta. 552: Sperelakis, N., M. F. Schneider, and E. J. Harris Decreased K+ conductance produced by Ba++ in frog sartorius fibers. J. Gen. Physiol. 50: Ramsay, A. G., D. L. Gallagher, R. L. Shoemaker, and G. Sachs Barium inhibition of sodium ion transport in toad bladder. Biochim. Biophys. Acta. 436: Greger, R., and E. Schlatter Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pfluegers Arch. Eur. J. Physiol. 392: VanDriessche, W., and W. Zeiske Ba++-induced conductance fluctuations of spontaneously fluctuating K+ channels in the apical membrane of the frog skin (Rana temporaria). J. Membr. Biol. 56: Polhemus, R. E., and D. A. Hall Effect of catecholamines on the potential difference and chloride efflux in the mouse thick ascending limb of Henle's loop. Kidney Int. 19: derouffignac, C., and F. Morel Micropuncture study of water, electrolytes, and urea movements along the loops of Henle in psammomys. J. tlin. Invest. 48: Rocha, A. S., and J. P. Kokko Membrane characteristics regulating potassium transport out of the isolated perfused descending limb of Henle. Kidney Int. 4: Wasserstein, A. G., and Z. S. Agus Net K+ secretion in proximal straight tubules (PST). Kidney Int. 19: Work, J., S. L. Troutman, and J. A. Schafer Transport of potassium in the rabbit pars recta. Am. J. Physiol. 242(Renal Fluid Electrolyte Physiol. 3): F226-F Johnston, P. A., C. A. Battilana, F. B. Lacy, and R. L. Jamison Evidence for a concentration gradient favoring outward movement of sodium from the thin loop of Henle. J. Clin. Invest. 59: Jamison, R. L., C. M. Bennett, and R. W. Berliner Countercurrent multiplication by the thin loops of Henle. Am. J. Physiol. 212: Vander, A. J Direct effects of potassium on renin secretion and renal function. Am. J. Physiol. 219: Brandis, M., J. Keyes, and E. E. Windhager Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am. J. Physiol. 222: Cardinal, J., and D. Duchesneau Effect of potassium on proximal tubular function. Am. J. Physiol. 234(Renal Fluid Electrolyte Physiol. 3): F381-F Kahn, M., and N. K. Bohrer Effect of potassiuminduced diuresis on renal concentration and dilution. Am. J. Physiol. 212: Wright, F. S., N. Strieder, N. B. Fowler, and G. Giebisch Potassium secretion by distal tubule after potassium adaptation. Am. J. Physiol. 221: Work, J., S. L. Troutman, and J. A. Schafer Rubidium transport in medullary thick ascending limb. Kidney Int. 21: 292 (Abstr.). 44. Greger, R Coupled transport of Na+ and Cl- in the thick ascending limb of Henle's loop of rabbit nephron. Scand. Audiol. Suppl. 14: Greger, R Chloride reabsorption in the rabbit cortical thick ascending limb of the loop of Henle: a sodium dependent process. Pfluegers Arch. Eur. J. Physiol. 390: Frizzell, R. A., M. Field, and S. G. Schultz Sodium-coupled chloride transport by epithelial tissues. Am. J. Physiol. 236(Renal Fluid and Electrolyte Physiol. 5): F1-F Good, D. W., and F. S. Wright Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am. J. Physiol. 236(Renal Fluid Electrolyte Physiol. 5): F192-F Stokes, J. B Potassium secretion by the cortical collecting tubule: relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am. J. Physiol. 241(Renal Fluid Electrolyte Physiol. 10): F395-F O'Neil, R. G., and S. I. Helman Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am. J. Physiol. 233(Renal Fluid Electrolyte Physiol. 2): F544-F Schwartz, G. J., and M. B. Burg Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am. J. Physiol. 235(Renal Fluid Electrolyte Physiol. 4): F576-F Grantham, J. J., M. B. Burg, and J. Orloff The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J. Clin. Invest. 49: Cannon, P. J., R. P. Ames, and J. H. Laragh Relation between potassium balance and aldosterone secretion in normal subjects and in patients with hypertensive or renal tubular disease. J. Clin. Invest. 45: K Effect on NaCI Transport 229
Functions of Proximal Convoluted Tubules
 1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
1. Proximal tubule Solute reabsorption in the proximal tubule is isosmotic (water follows solute osmotically and tubular fluid osmolality remains similar to that of plasma) 60-70% of water and solute reabsorption
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
Intrinsic Differences in Various Segments
 Intrinsic Differences in Various Segments of the Proximal Convoluted Tubule HARRY R. JACOBSON and JuHA P. KoKKo From the Department of Internal Medicine, The University of Texas Southwestern Medical School,
Intrinsic Differences in Various Segments of the Proximal Convoluted Tubule HARRY R. JACOBSON and JuHA P. KoKKo From the Department of Internal Medicine, The University of Texas Southwestern Medical School,
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX
 One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion
 Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion OLGA H. BROKL AND WILLIAM H. DANTZLER Department of Physiology, College of Medicine, University of Arizona, Tucson,
Amino acid fluxes in rat thin limb segments of Henle s loop during in vitro microperfusion OLGA H. BROKL AND WILLIAM H. DANTZLER Department of Physiology, College of Medicine, University of Arizona, Tucson,
NORMAL POTASSIUM DISTRIBUTION AND BALANCE
 NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
NORMAL POTASSIUM DISTRIBUTION AND BALANCE 98% of body potassium is contained within cells, principally muscle cells, and is readily exchangeable. Only 2% is in ECF. Daily intake exceeds the amount in ECF.
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Diagram of the inner portions of the kidney
 Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Sodium and chlorine transport
 Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
Kidney physiology 2 Sodium and chlorine transport The kidneys help to maintain the body's extracellular fluid (ECF) volume by regulating the amount of Na+ in the urine. Sodium salts (predominantly NaCl)
Normal Renal Function
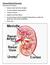 Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Normal Renal Function Functions of the Kidney: balances solute and water transport excretes metabolic waste products conserves nutrient regulates acid-base balance secretes hormones that help regulate
Renal Physiology - Lectures
 Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Renal Physiology - Lectures Physiology of Body Fluids PROBLEM SET, RESEARCH ARTICLE Structure & Function of the Kidneys Renal Clearance & Glomerular Filtration PROBLEM SET Regulation of Renal Blood Flow
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
11/05/1431. Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate
 Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
Urine Formation by the Kidneys Tubular Processing of the Glomerular Filtrate Chapter 27 pages 327 347 1 OBJECTIVES At the end of this lecture you should be able to describe: Absorptive Characteristics
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Osmoregulation and Renal Function
 1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Duct Acidification Effects of Potassium, HCO3. Harry R. Jacobson. Internal Medicine, 5323 Harry Hines Blvd., Dallas, Texas 75235
 Medullary Collecting Duct Acidification Effects of Potassium, HCO3 Concentration, and pco2 Harry R. Jacobson University of Texas Health Science Center, Department of Internal Medicine, 5323 Harry Hines
Medullary Collecting Duct Acidification Effects of Potassium, HCO3 Concentration, and pco2 Harry R. Jacobson University of Texas Health Science Center, Department of Internal Medicine, 5323 Harry Hines
Effect of Prostaglandin E2 on Chloride Transport Across the Rabbit Thick Ascending Limb of Henle
 Effect of Prostaglandin E2 on Chloride Transport Across the Rabbit Thick Ascending Limb of Henle SELECTIVE INHIBITION OF THE MEDULLARY PORTION JOHN B. STOKES, Department ofinternal Medicine, University
Effect of Prostaglandin E2 on Chloride Transport Across the Rabbit Thick Ascending Limb of Henle SELECTIVE INHIBITION OF THE MEDULLARY PORTION JOHN B. STOKES, Department ofinternal Medicine, University
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (2) Dr. Attila Nagy 2017 TUBULAR FUNCTIONS (Learning objectives 54-57) 1 Tubular Transport About 99% of filtrated water and more than 90% of the filtrated
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Other Factors Affecting GFR. Chapter 25. After Filtration. Reabsorption and Secretion. 5 Functions of the PCT
 Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
November 30, 2016 & URINE FORMATION
 & URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
& URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
Renal Physiology II Tubular functions
 Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Potassium recycling EDITORIAL REVIEW
 Kidney International, Vol. 31 (/987), pp. 695 703 EDITORIAL REVIEW Potassium recycling The concept of potassium recycling It has been amply demonstrated by micropuncture in nephrons accessible on the surface
Kidney International, Vol. 31 (/987), pp. 695 703 EDITORIAL REVIEW Potassium recycling The concept of potassium recycling It has been amply demonstrated by micropuncture in nephrons accessible on the surface
Transport characteristics of the thin limbs of Henle
 Kidney International, Vol. 22 (1982), pp. 449 453 Transport characteristics of the thin limbs of Henle JUHA P. KOKKO Department of Internal Medicine, University of Texas Health Science Center, Southwestern
Kidney International, Vol. 22 (1982), pp. 449 453 Transport characteristics of the thin limbs of Henle JUHA P. KOKKO Department of Internal Medicine, University of Texas Health Science Center, Southwestern
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Urea Secretion by the Straight Segment
 Urea Secretion by the Straight Segment of the Proximal Tubule SATOSHI KAWAMURA and JUHA P. KOKKO From the Department of Internal Medicine, the University of Texas Health Science Center at Dallas, Southwestern
Urea Secretion by the Straight Segment of the Proximal Tubule SATOSHI KAWAMURA and JUHA P. KOKKO From the Department of Internal Medicine, the University of Texas Health Science Center at Dallas, Southwestern
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
MS1 Physiology Review of Na+, K+, H + /HCO 3. /Acid-base, Ca+² and PO 4 physiology
 MS1 Physiology Review of,, / /Acidbase, Ca+² and PO 4 physiology I. David Weiner, M.D. Professor of Medicine and Physiology University of Florida College of Medicine Basic principles Proximal tubule Majority
MS1 Physiology Review of,, / /Acidbase, Ca+² and PO 4 physiology I. David Weiner, M.D. Professor of Medicine and Physiology University of Florida College of Medicine Basic principles Proximal tubule Majority
Potassium secretion. E k = -61 log ([k] inside / [k] outside).
![Potassium secretion. E k = -61 log ([k] inside / [k] outside). Potassium secretion. E k = -61 log ([k] inside / [k] outside).](/thumbs/80/80478709.jpg) 1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
Identify and describe. mechanism involved in Glucose reabsorption
 Define tubular reabsorption, tubular secretion, transcellular and paracellular transport. Identify and describe mechanism involved in Glucose reabsorption Describe tubular secretion with PAH transport
Define tubular reabsorption, tubular secretion, transcellular and paracellular transport. Identify and describe mechanism involved in Glucose reabsorption Describe tubular secretion with PAH transport
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
The regulation of renal acid secretion: New observations from studies of distal nephron segments
 Kidney International, Vol. 29 (1986), pp. 1099 1109 EDITORIAL REVIEW The regulation of renal acid secretion: New observations from studies of distal nephron segments Forty years ago, in a landmark paper,
Kidney International, Vol. 29 (1986), pp. 1099 1109 EDITORIAL REVIEW The regulation of renal acid secretion: New observations from studies of distal nephron segments Forty years ago, in a landmark paper,
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi
 Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi At the end of this lecture student should be able to describe: The loop of Henle is referred to as countercurrent
Done By: Lulu Al-Obaid - Abdulrahman Al-Rashed Reviewed By: Mohammed Jameel Khulood Al-Raddadi At the end of this lecture student should be able to describe: The loop of Henle is referred to as countercurrent
Calcium and Phosphate Transport in Isolated
 Calcium and Phosphate Transport in Isolated Segments of Rabbit Henle's Loop ANTONINO S. RocHA, JOSE B. MAGALDI, and JUHA P. KOKKO From Unidade de Doencas Renais, Department de Clinica Medica, Hospital
Calcium and Phosphate Transport in Isolated Segments of Rabbit Henle's Loop ANTONINO S. RocHA, JOSE B. MAGALDI, and JUHA P. KOKKO From Unidade de Doencas Renais, Department de Clinica Medica, Hospital
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 18 Urinary System 3 Tubular Reabsorption and Secretion Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Principles of
BIOH122 Human Biological Science 2 Session 18 Urinary System 3 Tubular Reabsorption and Secretion Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Principles of
After studying this lecture, you should be able to...
 Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
For more information about how to cite these materials visit
 Author(s): Michael Heung, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Noncommercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Michael Heung, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Noncommercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY
 URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
Hill et al. 2004, Fig. 27.6
 Lecture 25, 15 November 2005 Osmoregulation (Chapters 25-28) Vertebrate Physiology ECOL 437 (aka MCB 437, VetSci 437) University of Arizona Fall 2005 1. Osmoregulation 2. Kidney Function Text: Chapters
Lecture 25, 15 November 2005 Osmoregulation (Chapters 25-28) Vertebrate Physiology ECOL 437 (aka MCB 437, VetSci 437) University of Arizona Fall 2005 1. Osmoregulation 2. Kidney Function Text: Chapters
Moayyad Al-Shafei. - Saad Hayek. - Yanal Shafaqoj. 1 P a g e
 - 5 - Moayyad Al-Shafei - Saad Hayek - Yanal Shafaqoj 1 P a g e In this lecture we are going to study the tubular reabsorption of Na+. We know that the body must maintain its homeostasis by keeping its
- 5 - Moayyad Al-Shafei - Saad Hayek - Yanal Shafaqoj 1 P a g e In this lecture we are going to study the tubular reabsorption of Na+. We know that the body must maintain its homeostasis by keeping its
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
Faculty version with model answers
 Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
Faculty version with model answers Urinary Dilution & Concentration Bruce M. Koeppen, M.D., Ph.D. University of Connecticut Health Center 1. Increased urine output (polyuria) can result in a number of
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional roles of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Counter-Current System Regulation of Renal Functions
 Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
I. Metabolic Wastes Metabolic Waste:
 I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
I. Metabolic Wastes Metabolic Waste: a) Carbon Dioxide: by-product of cellular respiration. b) Water: by-product of cellular respiration & dehydration synthesis reactions. c) Inorganic Salts: by-product
** TMP mean page 340 in 12 th edition. Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2:
 QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
Diuretics having the quality of exciting excessive excretion of urine. OED. Inhibitors of Sodium Reabsorption Saluretics not Aquaretics
 Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
Diuretics having the quality of exciting excessive excretion of urine. OED Inhibitors of Sodium Reabsorption Saluretics not Aquaretics 1 Sodium Absorption Na Entry into the Cell down an electrochemical
osmoregulation mechanisms in gills, salt glands, and kidneys
 Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
BLOCK REVIEW Renal Physiology. May 9, 2011 Koeppen & Stanton. EXAM May 12, Tubular Epithelium
 BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
BLOCK REVIEW Renal Physiology Lisa M. HarrisonBernard, Ph.D. May 9, 2011 Koeppen & Stanton EXAM May 12, 2011 Tubular Epithelium Reabsorption Secretion 1 1. 20, 40, 60 rule for body fluid volumes 2. ECF
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Water, urea, sodium, chloride, and potassium transport in the in vitro isolated perfused papillary collecting duct
 Kidney International, Vol. 22 (1982), pp. 485 491 Water, urea, sodium, chloride, and potassium transport in the in vitro isolated perfused papillary collecting duct ANTONINO S. ROCHA and LUCIA H. KUDO
Kidney International, Vol. 22 (1982), pp. 485 491 Water, urea, sodium, chloride, and potassium transport in the in vitro isolated perfused papillary collecting duct ANTONINO S. ROCHA and LUCIA H. KUDO
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology Part II. Bio 219 Napa Valley College Dr. Adam Ross
 Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Renal Physiology Part II Bio 219 Napa Valley College Dr. Adam Ross Fluid and Electrolyte balance As we know from our previous studies: Water and ions need to be balanced in order to maintain proper homeostatic
Urine Formation. Urinary Physiology Urinary Section pages Urine Formation. Glomerular Filtration 4/24/2016
 Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
Urine Formation Urinary Physiology Urinary Section pages 9-17 Filtrate Blood plasma minus most proteins Urine
1. Anatomy / Vascularisation. 2. Urine concentration. 3. Axial heterogeneity of some segments
 Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
mid ihsan (Physiology ) GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B **
 (Physiology ) mid ihsan GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B ** Colloid pressure in the efferent arteriole is: A- More than that leaving
(Physiology ) mid ihsan GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B ** Colloid pressure in the efferent arteriole is: A- More than that leaving
Collin College. BIOL Anatomy & Physiology. Urinary System. Summary of Glomerular Filtrate
 Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Collin College BIOL. 2402 Anatomy & Physiology Urinary System 1 Summary of Glomerular Filtrate Glomerular filtration produces fluid similar to plasma without proteins GFR ~ 125 ml per min If nothing else
Sodium Chloride, Urea, and Water Transport
 Sodium Chloride, Urea, and Water Transport in the Thin Ascending Limb of Henle GENERATION OF OSMOTIC GRADIENTS BY PASSIVE DIFFUSION OF SOLUTES MASASHI IMAI and JUHA P. KOKKO From the Department of Internal
Sodium Chloride, Urea, and Water Transport in the Thin Ascending Limb of Henle GENERATION OF OSMOTIC GRADIENTS BY PASSIVE DIFFUSION OF SOLUTES MASASHI IMAI and JUHA P. KOKKO From the Department of Internal
Medullary Thick Ascending Limb of the Rat
 Effects of Potassium on Ammonia Transport by Medullary Thick Ascending Limb of the Rat David W. Good Renal-Electrolyte Physiology Laboratory, Departments ofphysiology & Biophysics and Internal Medicine,
Effects of Potassium on Ammonia Transport by Medullary Thick Ascending Limb of the Rat David W. Good Renal-Electrolyte Physiology Laboratory, Departments ofphysiology & Biophysics and Internal Medicine,
Urinary System. Dr. ZHANG Xiong. Dept. of Physiology. ZJU School of Medicine. QUESTION 6
 Urinary System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine http://10.71.121.158 Copyright@ Xiong Zhang QUESTION 6 How is the filtrate reabsorbed in tubular system? Copyright@ Xiong Zhang
Urinary System Dr. ZHANG Xiong Dept. of Physiology ZJU School of Medicine http://10.71.121.158 Copyright@ Xiong Zhang QUESTION 6 How is the filtrate reabsorbed in tubular system? Copyright@ Xiong Zhang
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
Early Filtrate Processing Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Once the filtrate is formed, the early
QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19
![QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19](/thumbs/80/80449526.jpg) 1 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion, excretion
1 QUIZ/TEST REVIEW NOTES SECTION 2 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion, excretion
Calcium Transport in the Thick Ascending Limb of Henle
 Calcium Transport in the Thick Ascending Limb of Henle HETEROGENEITY OF FUNCTION IN THE MEDULLARY AND CORTICAL SEGMENTS WADI N. SUKI, DIANE ROUSE, ROLAND C. K. NG, and JUHA P. KOKKO, Renal Section, Department
Calcium Transport in the Thick Ascending Limb of Henle HETEROGENEITY OF FUNCTION IN THE MEDULLARY AND CORTICAL SEGMENTS WADI N. SUKI, DIANE ROUSE, ROLAND C. K. NG, and JUHA P. KOKKO, Renal Section, Department
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (4) Dr. Attila Nagy 2018
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (4) Dr. Attila Nagy 2018 Intercalated cells Intercalated cells secrete either H + (Typ A) or HCO 3- (Typ B). In intercalated cells Typ A can be observed
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (4) Dr. Attila Nagy 2018 Intercalated cells Intercalated cells secrete either H + (Typ A) or HCO 3- (Typ B). In intercalated cells Typ A can be observed
Salt and Water Balance and Nitrogen Excretion
 Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
PARTS OF THE URINARY SYSTEM
 EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
The Excretory System. Biology 20
 The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
Countercurrent system
 Kidney International, Vol. 38 (1990), pp. 695 699 Countercurrent system JEFF M. SANDS and JUHA P. KOKKO Renal Division, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA
Kidney International, Vol. 38 (1990), pp. 695 699 Countercurrent system JEFF M. SANDS and JUHA P. KOKKO Renal Division, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Physiology (6) 2/4/2018. Rahmeh Alsukkar
 Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
RENAL. 6. Renal acid secretion is affected by all of the following except a. pco 2 b. K c. Carbonic anhydrase d. Aldosterone e. Ca
 RENAL 1. The reabsorption of Na in the proximal tubules a. Reabsorbs 80% of the filtered sodium load b. Causes increased hypertonicity c. Is powered by N/H ATPase d. Shares a common carrier with glucose
RENAL 1. The reabsorption of Na in the proximal tubules a. Reabsorbs 80% of the filtered sodium load b. Causes increased hypertonicity c. Is powered by N/H ATPase d. Shares a common carrier with glucose
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Evidence for a Concentration Gradient Favoring Outward
 Evidence for a Concentration Gradient Favoring Outward Movement of Sodium from the Thin Loop of Henle PAUL A. JOHNSTON, CARLOS A. BATTILANA, FRANK B. LACY, and REx L. JAMISON From the Division of Nephrology,
Evidence for a Concentration Gradient Favoring Outward Movement of Sodium from the Thin Loop of Henle PAUL A. JOHNSTON, CARLOS A. BATTILANA, FRANK B. LACY, and REx L. JAMISON From the Division of Nephrology,
Chapter 44. Regulating the Internal Environment. AP Biology
 Chapter 44. Regulating the Internal Environment Homeostasis Living in the world organisms had a choice: regulate their internal environment maintain relatively constant internal conditions conform to the
Chapter 44. Regulating the Internal Environment Homeostasis Living in the world organisms had a choice: regulate their internal environment maintain relatively constant internal conditions conform to the
