Alternative Splicing of the Robo3 Axon Guidance Receptor Governs the Midline Switch from Attraction to Repulsion
|
|
|
- Austen Lucas
- 5 years ago
- Views:
Transcription
1 Report Alternative Splicing of the Robo3 Axon Guidance Receptor Governs the Midline Switch from Attraction to Repulsion Zhe Chen, 1,3 Bryan B. Gore, 1,2 Hua Long, 1,2,3 Le Ma, 2,4 and Marc Tessier-Lavigne 1, * 1 Division of Research, Genentech Inc., 1 DNA Way, South San Francisco, CA 94080, USA 2 Department of Biological Sciences, Stanford University, Stanford CA 94305, USA 3 Present address: Pfizer Inc., South San Francisco, CA 94080, USA. 4 Present address: Department of Cell and Neurobiology, Keck School of Medicine, University of Southern California, Los Angeles, Los Angeles, CA 90089, USA. *Correspondence: marctl@gene.com DOI /j.neuron SUMMARY Alternative splicing provides a means to increase the complexity of gene function in numerous biological processes, including nervous system wiring. Navigating axons switch responses from attraction to repulsion at intermediate targets, allowing them to grow to each intermediate target and then to move on. The mechanisms underlying this switch remain poorly characterized. We previously showed that the Slit receptor Robo3 is required for spinal commissural axons to enter and cross the midline intermediate target. We report here the existence of two functionally antagonistic isoforms of Robo3 with distinct carboxy termini arising from alternative splicing. Robo3.1 is deployed on the precrossing and crossing portions of commissural axons and allows midline crossing by silencing Slit repulsion. Robo3.2 becomes expressed on the postcrossing portion and blocks midline recrossing, favoring Slit repulsion. The tight spatial regulation of opponent splice variants helps ensure high-fidelity transition of axonal responses from attraction to repulsion at the midline. INTRODUCTION Developing axons grow over long distances by navigating a series of intermediate targets along their trajectory to their final destination. Axons are attracted to intermediate targets, but upon arriving they must switch their responsiveness to cues at the targets so they are no longer attracted and instead are repelled away; this plasticity of responses allows axons to move on to the next leg of their trajectory (Dickson, 2002; Tessier-Lavigne and Goodman, 1996). Despite the importance of this switch for accurate long-range axon guidance, the mechanisms underlying it remain only partially understood. The midline of the central nervous system has provided a well-studied example of an intermediate target at which axons switch their responses. The axons of commissural interneurons grow toward the midline in response to attractants, including netrins and, in vertebrates, Sonic hedgehog (Charron et al., 2003; Dickson, 2002; Tessier-Lavigne and Goodman, 1996). These axons are initially insensitive to repellents also present at the midline, including Slit proteins; upon crossing the midline, a switch occurs such that these axons lose responsiveness to the attractants and instead become repelled by the repellents, so that the axons can leave the midline and then never recross (Kaprielian et al., 2001; Kidd et al., 1999; Long et al., 2004; Shirasaki et al., 1998; Zou et al., 2000). The switch from attraction to repulsion requires coordinated control of the expression level as well as the activity of guidance receptors on the axon surface. For example, as axons approach the midline, receptors of the DCC family mediate the attractive action of netrins (Dickson, 2002), while the expression level of Robos, receptors for Slits, is kept low in order to minimize Slit repulsion (Kidd et al., 1998a; Long et al., 2004). Upon crossing, however, the axons upregulate Robo expression and become Slit responsive (Kidd et al., 1998a; Long et al., 2004), and Robo activity in turn contributes to switching off DCC attraction (Stein and Tessier-Lavigne, 2001). A variety of mechanisms have been suggested to control the expression and activity of guidance receptors during midline crossing (reviewed in Garbe and Bashaw, 2004), including protein trafficking (e.g., in Drosophila, the Commissureless protein prevents Robo from localizing to the axon surface before midline crossing [Keleman et al., 2002; Kidd et al., 1998b]), local protein synthesis (e.g., the EphA2 receptor appears to be locally translated only in postcrossing axons [Brittis et al., 2002]), and the formation of receptor complexes (e.g., a Robo/DCC interaction in postcrossing axons can inhibit DCC activity [Stein and Tessier- Lavigne, 2001]). Alternative pre-mrna splicing provides an additional means to increase the complexity of gene function by producing protein products with a spectrum of properties from a single coding locus (Black, 2000; Graveley, 2001). This mechanism has been shown to diversify the functions of a number of genes regulating neural function and neural wiring (reviewed in Lipscombe, 2005; Li et al., 2007). For example, significant alternative splicing has been documented for neurexins and neuroligins, molecules implicated in establishing synaptic specificity (Craig and Kang, 2007). An extreme example of the molecular diversity generated Neuron 58, , May 8, 2008 ª2008 Elsevier Inc. 325
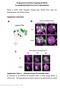
2 Figure 1. Alternative Splicing of Robo3 Generates Two Isoforms with Distinct Expression Patterns in Commissural Axons (A) Schematic of the structures of Robo3 isoforms and the alternative splicing of the 3 0 coding sequence ( e denotes an exon, i an intron, and * the stop codons utilized in the two transcripts). (B G) Immunohistochemistry was performed using three Robo3 antibodies on transverse sections of the wild-type spinal cord at E11.5 (B D) and E12.5 (E G). (B and E) An antibody against the extracellular domain of Robo3 (anti-robo3ecto), which recognizes both Robo3 isoforms, detected epitopes in precrossing axonal domains (arrows), at the midline (brackets), and in the postcrossing ventral fiber tracts (arrowheads). (C and F) A Robo3.1-specific antibody detected epitopes in axonal domains that are precrossing and in the midline region. (D and G) A Robo3.2-specific antibody detected epitopes mostly on postcrossing axonal domains. Scale bar, 100 mm. (H) Schematic showing the location of the cell body of a commissural neuron and the trajectory of its axon, as well as the axonal domains of Robo3 isoform expression, as seen in a transverse section view. D, dorsal; V, ventral. by alternative splicing is provided by the neural recognition molecule Down s syndrome cell adhesion molecule (DSCAM), which, in Drosophila, can exist in tens of thousands of isoforms arising from alternative splicing (Zipursky et al., 2006). The evidence indicates that each isoform binds tightly to itself but not to the other isoforms, a remarkable degree of homophilic recognition that provides the basis for self-repulsion of the processes of individual neural cells (Hattori et al., 2007; Matthews et al., 2007; Wojtowicz et al., 2007). Whether alternative splicing is also used to diversify the function of guidance molecules at intermediate targets has not, however, been described. Here, we report that alternative splicing provides a crucial level of control over the function of a guidance receptor used to navigate the midline intermediate target in the spinal cord. Previously, we described an unexpected role for the third mammalian Robo, Robo3 (also known as Rig1 [Yuan et al., 1999]), in regulating commissural axon guidance at the spinal cord midline. Contrary to the other two vertebrate Robos, Robo1 and Robo2, whose expression, as described above, is low before crossing and high after crossing (Long et al., 2004), Robo3 is expressed at high levels before crossing and is gradually downregulated after crossing (Sabatier et al., 2004; see also Figure 1B). Also contrasting with the other two Robos, Robo3 activity keeps commissural axons non- responsive to midline Slits before crossing, and it does so by silencing Slit repulsion that would otherwise be mediated by Robo1 and Robo2 (Sabatier et al., 2004). As a result, in Robo3 knockout mice commissural axons are prematurely responsive to midline Slit repellents before midline crossing and are therefore totally unable to cross the midline (Marillat et al., 2004; Sabatier et al., 2004; also see Figure 2J). Mutations in human Robo3, discovered in patients with horizontal gaze palsy and progressive scoliosis syndrome (HGPPS), were similarly found to result in significant midline crossing defects (Jen et al., 2004). Here, we report an unexpected complexity and sophistication in the function of Robo3 that arises from alternative splicing of its pre-mrna. We found that two isoforms of Robo3, Robo3.1 and Robo3.2, are generated by differential intron retention, a less common form of alternative splicing. These isoforms have opposite functions: one favors, whereas the other blocks, midline crossing. In addition, they are tightly spatially regulated, with Robo3.1 expressed on the precrossing segment of commissural axons and Robo3.2 on the postcrossing segment. This abrupt transition helps ensure a high fidelity in the growth cone s switch from attraction to repulsion at the midline. The tight spatial localization of splice isoforms of a guidance receptor documented here thus provides another mechanism for the nervous system to control intricate guidance events, helping explain the accuracy of axon guidance required for proper brain wiring. RESULTS Alternative Splicing of the Robo3 Coding Sequence Generates Two Isoforms In the course of sequencing a collection of Robo3 full-length cdna clones from mouse dorsal spinal cord, we discovered that the 3 0 end of the mouse Robo3 mrna can be alternatively 326 Neuron 58, , May 8, 2008 ª2008 Elsevier Inc.
3 Figure 2. Forced Expression of Two Robo3 Isoforms Causes Distinct Commissural Axon Guidance Phenotypes (A) Schematic showing an open-book preparation of the spinal cord. D, dorsal; V, ventral. (B D) The Robo3 isoforms, together with gfp, were introduced into chick commissural axons by in ovo electroporation, and spinal cords were examined in an open-book view. In these and all the following open-book images, spinal cords were oriented with the ventral midline in the center, transfected neuronal cell bodies sitting to the left (not shown), and the rostral end of spinal cords pointing up. Brackets represent the span of the midline. All axons expressing GFP alone (B) appeared normal, entering and crossing the midline, then turning rostrally. When Robo3.1 was overexpressed, all axons entered the midline normally but many then recrossed (green arrows in [C]). Ectopic expression of Robo3.2, however, led to some axons turning away from the midline before entering (red arrows in [D]). Scale bar, 20 mm. (E I) A modified whole-embryo culture (WEC) method was used to express Robo3 coding sequences. The cultured mouse embryos (bottom panel of [E], [H], and [I]) were comparable to those that developed normally in vivo (top panel of [E] [G]) in their gross morphology, size, and axonal marker expression after 1 or 2 days in culture (DIC). Scale bar, 100 mm. (J M) Rescue of the commissural axon guidance defect seen in Robo3 / embryos by exogenous Robo3 proteins. GFP alone (J) or Robo3.2 (L) did not restore midline crossing in Robo3 / embryos. In contrast, many Robo3 mutant axons were able to project to the contralateral side in embryos transfected with cdnas for Robo3.1 (K) or Robo1 out -Robo3.1 in (M). Scale bar, 100 mm. spliced to generate a second isoform that is distinct in its carboxy terminus from the previously published cdna ([Yuan et al., 1999]; NCBI accession number AF060570; Figure 1A). We term the new isoform Robo3.1 and the original one Robo3.2 (based on their expression: see below). The novel Robo3.1 transcript arises from splicing of exon 26 to exon 27 and then to exon 28 (an analogous splicing event is seen for the human Robo3 locus [Jen et al., 2004]); the resulting 78 amino acid peptide is highly enriched in arginine/serine dipeptide repeats (see Figure S1 available online). The previously described Robo3.2 transcript, in contrast, arises from retention of the intron between exons 26 and 27. The intronic sequence contains a stop codon and encodes a 43 amino acid peptide that is quite distinct from the Robo3.1 carboxy terminus but shows conservation between rodents and humans (Figure 1A and Figure S1). Switching of Robo3 Isoform Expression at the Midline The mrnas for the two isoforms are both expressed in the spinal cord during the period of commissural axon growth to and across the midline, i.e., from embryonic day 9.5 (E9.5) to 12.5 (E12.5) (see below). Immunohistochemistry with isoformspecific antibodies on spinal cord sections revealed strikingly distinct localization patterns (Figures 1B 1G and Figures S1 and S2A S2F; the signals were specific, because they were absent in the Robo3 mutant, which loses both isoforms [Figures S2G S2I]). Robo3.1 is highly expressed on commissural axons before and during midline crossing (Figure 1C, Figure S2B, and data not shown). Its expression is rapidly switched off after midline crossing because it was not seen at all in ventral fiber tracts (Figure 1C and Figure S2B). In contrast, Robo3.2 expression was detectable only at a low level at E11.5 and increased in expression at E12.5, and at both ages was seen exclusively on the portion of commissural axons distal to the midline (Figure 1D and Figure S2C and Figure 1G and Figure S2F). The expression patterns of these two isoforms together appear to account to a considerable extent, and perhaps entirely, for the pattern we visualized previously using a pan-robo3 antibody, which showed high expression before crossing (attributed to Robo3.1) and expression after crossing that was eventually downregulated (attributed to Robo3.2) (Sabatier et al., 2004; Figures 1B and 1E and Figures S2A and S2D). The immunoreactivity in the ventral fiber tracts seen with the pan-robo3 antibody appeared slightly more extensive than with the Robo3.2 antibody, suggesting either that there is an additional Robo3 isoform expressed after crossing or that the pan-robo3 antibody is more sensitive. Nonetheless, there is a precise switch in expression on commissural axons from Robo3.1 before and during midline crossing to Robo3.2 after crossing (Figure 1H). Neuron 58, , May 8, 2008 ª2008 Elsevier Inc. 327
4 Robo3 Isoforms Exhibit Opposite Activities in Commissural Axon Guidance To functionally characterize the two isoforms, we ectopically expressed them individually in chick commissural neurons by in ovo electroporation (see Experimental Procedures). Unlike the endogenous Robo3 in mice, Robo3 expressed from the transgene (possessing the b-actin promoter and the b-globin 3 0 UTR) was expressed throughout the length of chick commissural axons (Figures S3A and S3B). When Robo3.1 is persistently expressed before and after crossing, all GFP-expressing axons entered the midline normally, but many inappropriately recrossed (Figure 2C and Figure S6; 13/13 embryos). This result supports our model (Sabatier et al., 2004) that Robo3 more specifically, the Robo3.1 isoform can repress midline repulsion. Remarkably, overexpression of Robo3.2 had the opposite effect: many axons failed to enter the midline and remained on the ipsilateral side, whereas those that did cross did not recross (Figure 1D and Figure S6; 14/14 embryos; the same phenotype was also observed with a full-length Robo1 cdna [data not shown]). These axons appear to be misrouted commissural axons rather than ipsilateral projection axons, as the latter assume a longitudinal trajectory at more dorsal positions, far from the midline. To further test the activities of the two Robo3 isoforms, we investigated their abilities to rescue the murine Robo3/Rig1 knockout phenotype by introducing each of them back into the mutant. This was achieved by electroporating Robo3.1 and Robo3.2 constructs into the spinal cord of embryonic day 9.5 (E9.5) mouse embryos and culturing the embryos in vitro for 2 3 days, to a developmental stage equivalent to E11.5 E12.5, using a modification of existing whole-embryo culture methods (Figures 2E 2I; for detailed description and protocol, see Experimental Procedures). Plasmids introduced in this way were expressed in commissural neurons on the electroporated side during the period of growth to and across the midline. For example, a gfp plasmid introduced into Robo3 mutant embryos revealed the previously described noncrossing phenotype (Figure 2J). Robo3.1 cdna could restore midline crossing in the mutant to a large extent (Figure 2K and Table S1). In contrast, no axons were seen at the midline or on the contralateral side when Robo3.2 was introduced back into the mutant (Figure 2L and Table S1), even though the protein, like Robo3.1, was expressed precrossing (Figures S3C and S3D). These data together support the idea that Robo3.1 (but not Robo3.2) can repress premature repulsion during commissural axon midline crossing. Further support was obtained by repeating in wild-type mouse embryos the forced expression experiment that we had performed in chicks, using the mouse whole-embryo culture technique, with similar results (Figures S4 and S6). We also found that a chimeric protein comprising the cytoplasmic domain of Robo3.1 fused to the extracellular and transmembrane regions of Robo1 (Robo1 out -Robo3.1 in ) was able to rescue midline crossing when introduced into Robo3 mutants (Figure 2M and Table S1), thus focusing attention on the cytoplasmic domain of Robo3.1 in mediating its function. Requirement of Robo3.1 for Midline Crossing The expression patterns of Robo3.1 and Robo3.2 and their activities in forced expression and rescue experiments suggest a model in which Robo3.1 expressed on commissural axons before and during midline crossing serves to suppress Slit repulsion, allowing them to enter the midline (Figure 5A), whereas Robo3.2 expressed on the axons after crossing may contribute to preventing midline crossing, either by directly mediating Slit repulsion, by interfering with residual Robo3.1 on the axons, or by some other means (Figure 5B). To test this model further, we performed isoform-specific knockdown in vivo using small interfering RNA (sirna) oligonucleotides in the whole-embryo culture system. For Robo3.2, we used an sirna targeting a sequence in intron 26, which is not found in Robo3.1 (Figure 3A). We had less flexibility for Robo3.1 because all sequences in Robo3.1 are also found in Robo3.2. We reasoned, however, that an sirna specifically targeting the junction between exons 26 and 27 might inhibit Robo3.1 expression without affecting Robo3.2 mrna (Figure 3A). A positive control was provided by a pan-robo3 sirna targeting a sequence in the common exon 9, and a negative control by introducing five mismatch mutations into this pan-robo3 sirna (Robo3 mismatch ). In control experiments using transfected COS cells, pan-robo3 sirna caused a complete absence of both isoforms (but did not affect Robo1, DCC [the netrin receptor], or the surface proteins TAG-1 and L1 [Figure 3B and data not shown]), whereas Robo3 mismatch had no effect on any of these proteins (Figure 3B and data not shown). Robo3.1 and Robo3.2 sirnas were able to selectively reduce the levels of their respective targets with no detectable reduction of the other, although the Robo3.1 sirna was only partially effective (Figure 3B). We tested these sirnas in vivo by electroporating into E9.5 embryos followed by whole-embryo culture. The pan-robo3 sirna caused commissural axons to steer away from the midline before entering and to navigate on the ipsilateral side (Figures 3C and 3G), phenocopying the effect of genetic removal of Robo3 (Sabatier et al., 2004), whereas the negative control sirna (Robo3 mismatch ) did not cause any detectable phenotype (Figures 3D and 3G). The Robo3.1-specific sirna led to the same midline crossing defect as did pan-robo3 sirna, but to a lesser degree, as a few axons crossed (Figures 3E and 3G); this partial penetrance may reflect the fact that this sirna was not completely effective in suppressing Robo3.1 (Figure 3B). Although Robo3.2 sirna was able to efficiently knock down Robo3.2 in COS cells, it did not affect the ability of the axons to enter and cross the midline (Figures 3F and 3G), consistent with our model (Figure 5). Because the transfected axons were intermingled with an excess of untransfected axons, we could not ascertain by immunohistochemistry whether the proteins targeted by the sirnas were downregulated, which we expect to be occurring given the phenotypes. To test the specificity of the sirna effect, we therefore performed rescue experiments. The midline crossing defect seen with the pan-robo3 sirna was reversed by coelectroporating with a Robo3.1 cdna mutated in the sirna target sequence such that its transcript is resistant to the sirna (Figures S5B and S5E) but not by the wild-type construct whose transcript is sensitive (Figures S5A and S5E) nor by a Robo3.2 construct engineered so that its transcript is resistant to the pan- Robo3 sirna (Figures S5C and S5E). A construct encoding the Robo1 out -Robo3.1 in chimera, which does not contain the 328 Neuron 58, , May 8, 2008 ª2008 Elsevier Inc.
5 Figure 3. sirna-mediated Knockdown Implicates Robo3.1 in Allowing Midline Crossing (A) Targets of Robo3.1- and Robo3.2-specific sir- NAs. The Robo3.1 target sequence corresponds to 10 nt in exon 26 and 9 nt in exon 27 at the exon-exon junction. The Robo3.2 target sequence corresponds to 19 nt in intron 26. (B) sirna-mediated knockdown of Robo3 isoforms in COS-1 cells, assessed by western blotting of COS cell extracts. (C F) sirna-mediated knockdown of Robo3 isoforms in commissural neurons. sirnas were introduced into one-half of the spinal cord by electroporation at E9.5, and embryos were cultured using the whole-embryo culture technique. Pan- Robo3 sirna caused premature turning of the axons (RFP labeled) from the midline (C), whereas Robo3 mismatch -treated axons appeared normal (D). Robo3.1 sirna had a similar effect to that of pan-robo3, although to a lesser degree (E). Robo3.2 sirna treatment did not affect midline entry of commissural axons (F). Arrows point to axons that remained abnormally on the ipsilateral side. Boxed areas in (F) indicate regions used for quantification in (G). Scale bar, 100 mm. (G) Quantification of data in (C) (F). For each condition, the intensity of RFP expression from the contralateral axons (those that had crossed the midline) was compared to that from the ipsilateral axons (those that failed to cross). The difference, plotted on the y axis in arbitrary units, is shown as mean ± SEM. pan-robo3 sirna target sequence, was also able to rescue the RNAi effect (Figures S5D and S5E), as predicted. These results indicate that sirna-mediated knockdown of Robo3 in vivo was efficient and specific and that Robo3.1 appears to account for most or all of the Robo3 activity that is necessary for inhibiting premature repulsion from the midline. Robo3.2 Helps Expel Postcrossing Commissural Axons Given its expression pattern and overexpression phenotype, we considered the possibility that Robo3.2 contributes to repelling commissural axons out of the midline. Previous studies had, in fact, suggested that Robo1 and Robo2 may not account fully for the repulsion of postcrossing commissural axons by Slits, because only a small fraction of axons stalls within the midline in Robo1 or Robo2 single mutants, whereas more extensive stalling was seen in Slit1;Slit2;Slit3 triple mutants (lacking all midline Slits) (Long et al., 2004). We generated a double mutant lacking both Robo1 and Robo2 (which are linked on chromosome 16; see Experimental Procedures) but found that the amount of stalling in the double mutants (Figures 4B and 4E and data now shown) was that expected from additive effects of removing Robo1 and Robo2 and still considerably less than in the Slit triple mutant (Long et al., 2004). Despite this, when we bred the Robo1;Robo2 double mutant to the Robo3 mutant, the failure of midline crossing seen in the Robo3 mutant (Figure 4C) was rescued to a considerable extent (Figure 4D), supporting the model that the low levels of Robo1 and Robo2 on precrossing axons contribute significantly to mediating the premature repulsion by Slits in the Robo3 mutant. The modest stalling phenotype of the Robo1;Robo2 double mutant compared to the Slit1;Slit2;Slit3 triple mutant supports the existence of another Slit receptor(s) that mediates midline repulsion. We therefore tested the possibility that Robo3.2 collaborates with Robo1 and Robo2 in this function. To knock down Robo3.2 without affecting Robo3.1 expression, we carried out RNAi against Robo3.2 in the Robo1;Robo2 double mutant embryos (which were electroporated at E9.5 and cultured for 3 days). In these embryos, some axons were seen to recross the midline (Figure 4G and Figure S6, phenotype seen consistently in 4/5 embryos examined); this phenotype was not seen with the Robo3 mismatch sirna (Figure 4F and Figure S6, 6 embryos studied). No recrossing was observed, either, when the Robo3.2 sirna was electroporated into wild-type embryos, in which Robo1 and Robo2 function were intact (data not shown). The recrossing phenotype was also observed in some axons in the triple Robo mutant (Figures 4D and 4E) and in the triple Slit mutant (Long et al., 2004). Taken together, these results support the model that Robo3.2 contributes to expelling axons from the midline in collaboration with Robo1 and Robo2. DISCUSSION Alternative splicing plays an important role in regulating many processes in neural development, including cell fate determination, neuronal recognition, and synaptogenesis (Li et al., 2007), and defects in splicing are being increasingly implicated in various neurological disorders (Licatalosi and Darnell, 2006). Our results indicate that alternative splicing of axon guidance receptors provides a level of sophisticated regulation that can be utilized to ensure the fidelity of growth cone switching from attraction to repulsion at intermediate targets. Distinct Spatial Localization of Opponent Splice Isoforms We found that alternative splicing gives rise to Robo3 isoforms with opposite functions. Robo3.1 favors midline crossing, and genetic analysis (Sabatier et al., 2004 and this study) indicates that it does so by suppressing Slit repulsion mediated by Neuron 58, , May 8, 2008 ª2008 Elsevier Inc. 329
6 Figure 5. Model of Robo3 Activities in Commissural Axon Guidance at the Midline (A) Before axons reach the midline, Robo3.1 is highly expressed on axons, and its activity prevents the activation of Robo1 and Robo2, present at low levels on the axons. As a result, axons are unresponsive to Slit repellents and thus enter the midline. (B) Upon entry, a switch in Robo3 expression occurs: Robo3.2 expression is switched on, but the protein is restricted to axonal regions distal to the midline, where Robo3.1 is excluded. Consequently, Robo1, Robo2, and Robo3.2 act in concert to help expel axons to the contralateral side. Figure 4. Robo3.2 Also Contributes to Commissural Axon Guidance (A D) DiI tracing of commissural axons in compound Robo spinal cords (shown in an open-book view). In Robo1 +/ ;Robo2 +/ ;Robo3 +/ embryos (A), which served as controls, virtually all commissural axons crossed the midline. In Robo1 / ;Robo2 / ;Robo3 +/ mutants (B), a few axons stalled within the midline (quantified in [E]; note that in [B] the stalling was best seen by focusing up and down and that the stalled profiles are not readily seen in the single plane of focus that is displayed). In Robo1 +/ ;Robo2 +/ ;Robo3 / mutant spinal cords (C), no axons were seen within the midline or on the contralateral side. However, when all six Robo alleles were mutant, many axons were able to enter the midline (D), though many failed to exit (E) and some recrossed the midline ([E], indicated with arrow in [D]). (E) Quantification of phenotypes in (A) (D). (F and G) In Robo1;Robo2 double mutants, electroporation of the Robo3.2 sirna caused some commissural axons (GFP labeled) to recross the midline (indicated with arrows in [G]), whereas the control sirna, Robo3 mismatch, had no effect (F). Scale bar, 20 mm. remains to be determined whether Robo3.2 produces its effect by functioning directly as a repellent Slit receptor or by some other means (e.g., blocking residual Robo3.1 on postcrossing axons, or potentiating Robo1 or -2 function). Importantly, our results also demonstrate a remarkable and unexpectedly tight level of spatial localization of the two splice isoforms, with Robo3.1 expressed precrossing and Robo3.2 postcrossing, consistent with their functional roles in enabling midline crossing and blocking midline recrossing, respectively. These findings extend to midline guidance themes highlighted in previous studies of alternative splicing in the nervous system. Several other neuronal genes have been documented to undergo regulated splicing to produce functionally distinct isoforms. For example, two splice variants of the EphA7 receptor, which differ in their C termini, exhibit distinct activities in regulating cell adhesion during neural tube closure (Holmberg et al., 2000). Alternative splicing to yield isoforms with distinctive subcellular localization properties has also been documented. For example, alternative splicing of the NR1 subunit of NMDA receptors generates two isoforms with distinct carboxy termini that accumulate differentially at the synapse in an activity-dependent manner (Mu et al., 2003). The splicing of the potassium channel Kv3.1 pre-mrna gives rise to two isoforms, with Kv3.1a prominently expressed in axons within certain neuronal populations and Kv3.1b mostly expressed in the soma and dendrites of these neurons (Ozaita et al., 2002). It will be of interest to examine whether discrete spatial localization of splice isoforms contributes to the function of other axon guidance receptors implicated in switching from attraction to repulsion at intermediate targets. Robo1 and Robo2. In contrast, Robo3.2 blocks midline crossing (in gain-of-function experiments) and normally collaborates with Robo1 and Robo2 to block midline recrossing (as shown in lossof-function experiments), thus favoring Slit repulsion (Figure 5). It Regulation of Expression of the Robo3 Splice Isoforms It is intriguing to consider how the two Robo3 isoforms are confined to appropriate axonal segments. The simplest mechanism would be for there to be a temporal switch in splicing, with the 330 Neuron 58, , May 8, 2008 ª2008 Elsevier Inc.
7 Robo3.1 transcript made first, followed at an appropriate time by the Robo3.2 transcript. However, by quantitative RT-PCR, we found a roughly constant ratio of the two transcripts in the spinal cord during the period of commissural axon growth to and across the midline (Figure S7A). In situ hybridization studies similarly suggest that the two transcripts appear in parallel in commissural neurons (Figure S7B S7K), arguing against a temporal switch. Alternative mechanisms to a temporal switch include one or more of the following: (1) constitutive translation of the two transcripts followed by selective trafficking to (or removal from) the two axon segments, (2) a temporal switch in translation of the two transcripts in the cell bodies (with Robo3.1 translated while the axon is extending to the midline and Robo3.2 after it has crossed), (3) transport of the transcripts into axons followed by selective translation of Robo3.1 in the precrossing axon segment and Robo3.2 in the postcrossing segment. As a variation on the third possibility, the Robo3.2 transcript could be transported down the axons but spliced selectively in the precrossing portion to yield Robo3.1, followed by local translation (this mechanism would require splicing within the axons, which has not so far been demonstrated). At present, we cannot distinguish fully between these mechanisms, although the absence of detectable transcripts in the axons (Figures S7B S7K) tends to argue against models involving local translation. Additional Splicing Events Previous studies have suggested the existence of other splice variants of Robos and Slits (Challa et al., 2005; Clark et al., 2002; Dalkic et al., 2006; Little et al., 2002; Tanno et al., 2004), raising the possibility that alternative splicing might be widely involved in regulating Slit/Robo signaling. Additional splicing events in the Robo3 pre-mrna have been previously described (Camurri et al., 2005; Yuan et al., 1999), but their functional consequences are largely undefined. One particularly interesting splicing event in the Robo3 5 0 coding sequence has been shown to generate two different amino termini (the Robo3A and Robo3B isoforms) with distinct Slit-binding activities (Camurri et al., 2005). Our immunolocalization and loss-of-function experiments did not distinguish between A and B isoforms. In the gain-offunction and rescue experiments, the cdnas we used encoded the A isoforms, which, strictly speaking, should be referred to as Robo3A.1 and Robo3A.2. The finding in human patients with the HGPPS syndrome of a familial missense mutation in the signal sequence of Robo3A (Jen et al., 2004) supports the functional importance of the Robo3A isoforms in both mice and humans. The relative contributions of A and B isoforms remain to be defined. Contribution of Slit/Robo Signaling to Leaving the Midline and Mechanism of Action of Robo3 Our results, particularly using the newly derived Robo1;Robo2 double knockout, also help flesh out the specific contribution of Slit/Robo signaling to entering and leaving the midline. As we have shown, removal of Robo3.1, either by gene knockout or by sirna knockdown, prevents midline crossing. The finding in the triple Robo knockout that removal of both Robo1 and Robo2 can largely reverse the inability of commissural axons to enter the midline in the Robo3 knockout, supports the theory that Robo3.1 functions to suppress Slit repulsion mediated by low levels of Robo1 and Robo2. After midline crossing, Robo3.2 collaborates with Robo1 and Robo2 to prevent midline recrossing. Our results (Figure 4) also suggest, however, that these three Robos do not account fully for midline repulsion and that additional Slit receptor(s) and distinct repulsive signaling pathways may collaborate with them to mediate expulsion from the midline; indeed, there is already evidence for a contribution from semaphorin3b/neuropilin-2 and ephrin/eph signaling (Kadison et al., 2006; Zou et al., 2000). The molecular basis for the divergent actions of the two Robo3 isoforms also remains to be elucidated. Robo3.1 could interfere with Robo1 and Robo2 function through direct physical interaction. However, we have not been able to detect such an interaction in transfected COS cells expressing Robo3.1 with either Robo1 or Robo2, whether or not Slit2 protein is added (data not shown). Alternatively, Robo3.1 may interfere with Robo1 and Robo2 signaling further downstream in the signaling pathway. Thus, further studies will be required to fully define the complement of receptors and signaling pathways that expel commissural axons from the midline. EXPERIMENTAL PROCEDURES See the Supplemental Data available online. SUPPLEMENTAL DATA The Supplemental Data for this article can be found online at neuron.org/cgi/content/full/58/3/325/dc1/. ACKNOWLEDGMENTS We thank R. Krumlauff and P. Trainor for generously hosting B.B.G. at the Stowers Institute to learn existing whole-embryo culture technology. We thank L. Case, C. Goodman, A. Jaworski, C.W. Siebel, A. Yaron, and J. Zou for helpful discussions and comments on manuscript; M. Huse for generating the anti- Robo3ecto antibody. Received: August 7, 2006 Revised: January 29, 2007 Accepted: February 15, 2008 Published: May 7, 2008 REFERENCES Black, D.L. (2000). Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell 103, Brittis, P.A., Lu, Q., and Flanagan, J.G. (2002). Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110, Camurri, L., Mambetisaeva, E., Davies, D., Parnavelas, J., Sundaresan, V., and Andrews, W. (2005). Evidence for the existence of two Robo3 isoforms with divergent biochemical properties. Mol. Cell. Neurosci. 30, Challa, A.K., McWhorter, M.L., Wang, C., Seeger, M.A., and Beattie, C.E. (2005). Robo3 isoforms have distinct roles during zebrafish development. Mech. Dev. 122, Charron, F., Stein, E., Jeong, J., McMahon, A.P., and Tessier-Lavigne, M. (2003). The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 113, Neuron 58, , May 8, 2008 ª2008 Elsevier Inc. 331
8 Clark, K., Hammond, E., and Rabbitts, P. (2002). Temporal and spatial expression of two isoforms of the Dutt1/Robo1 gene in mouse development. FEBS Lett. 523, Craig, A.M., and Kang, Y. (2007). Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol. 17, Dalkic, E., Kuscu, C., Sucularli, C., Aydin, I.T., Akcali, K.C., and Konu, O. (2006). Alternatively spliced Robo2 isoforms in zebrafish and rat. Dev. Genes Evol. 216, Dickson, B.J. (2002). Molecular mechanisms of axon guidance. Science 298, Garbe, D.S., and Bashaw, G.J. (2004). Axon guidance at the midline: from mutants to mechanisms. Crit. Rev. Biochem. Mol. Biol. 39, Graveley, B.R. (2001). Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 17, Hattori, D., Demir, E., Kim, H.W., Viragh, E., Zipursky, S.L., and Dickson, B.J. (2007). Dscam diversity is essential for neuronal wiring and self-recognition. Nature 449, Holmberg, J., Clarke, D.L., and Frisen, J. (2000). Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature 408, Jen, J.C., Chan, W.M., Bosley, T.M., Wan, J., Carr, J.R., Rub, U., Shattuck, D., Salamon, G., Kudo, L.C., Ou, J., et al. (2004). Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science 304, Kadison, S.R., Makinen, T., Klein, R., Henkemeyer, M., and Kaprielian, Z. (2006). EphB receptors and ephrin-b3 regulate axon guidance at the ventral midline of the embryonic mouse spinal cord. J. Neurosci. 26, Kaprielian, Z., Runko, E., and Imondi, R. (2001). Axon guidance at the midline choice point. Dev. Dyn. 221, Keleman, K., Rajagopalan, S., Cleppien, D., Teis, D., Paiha, K., Huber, L.A., Technau, G.M., and Dickson, B.J. (2002). Comm sorts robo to control axon guidance at the Drosophila midline. Cell 110, Kidd, T., Brose, K., Mitchell, K.J., Fetter, R.D., Tessier-Lavigne, M., Goodman, C.S., and Tear, G. (1998a). Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 92, Kidd, T., Russell, C., Goodman, C.S., and Tear, G. (1998b). Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron 20, Kidd, T., Bland, K.S., and Goodman, C.S. (1999). Slit is the midline repellent for the robo receptor in Drosophila. Cell 96, Li, Q., Lee, J.A., and Black, D.L. (2007). Neuronal regulation of alternative premrna splicing. Nat. Rev. Neurosci. 8, Licatalosi, D.D., and Darnell, R.B. (2006). Splicing regulation in neurologic disease. Neuron 52, Lipscombe, D. (2005). Neuronal proteins custom designed by alternative splicing. Curr. Opin. Neurobiol. 15, Little, M., Rumballe, B., Georgas, K., Yamada, T., and Teasdale, R.D. (2002). Conserved modularity and potential for alternate splicing in mouse and human Slit genes. Int. J. Dev. Biol. 46, Long, H., Sabatier, C., Ma, L., Plump, A., Yuan, W., Ornitz, D.M., Tamada, A., Murakami, F., Goodman, C.S., and Tessier-Lavigne, M. (2004). Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 42, Marillat, V., Sabatier, C., Failli, V., Matsunaga, E., Sotelo, C., Tessier-Lavigne, M., and Chedotal, A. (2004). The slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron 43, Matthews, B.J., Kim, M.E., Flanagan, J.J., Hattori, D., Clemens, J.C., Zipursky, S.L., and Grueber, W.B. (2007). Dendrite self-avoidance is controlled by Dscam. Cell 129, Mu, Y., Otsuka, T., Horton, A.C., Scott, D.B., and Ehlers, M.D. (2003). Activitydependent mrna splicing controls ER export and synaptic delivery of NMDA receptors. Neuron 40, Ozaita, A., Martone, M.E., Ellisman, M.H., and Rudy, B. (2002). Differential subcellular localization of the two alternatively spliced isoforms of the Kv3.1 potassium channel subunit in brain. J. Neurophysiol. 88, Sabatier, C., Plump, A.S., Le, M., Brose, K., Tamada, A., Murakami, F., Lee, E.Y., and Tessier-Lavigne, M. (2004). The divergent Robo family protein rig- 1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell 117, Shirasaki, R., Katsumata, R., and Murakami, F. (1998). Change in chemoattractant responsiveness of developing axons at an intermediate target. Science 279, Stein, E., and Tessier-Lavigne, M. (2001). Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291, Tanno, T., Takenaka, S., and Tsuyama, S. (2004). Expression and function of Slit1alpha, a novel alternative splicing product for slit1. J. Biochem. (Tokyo) 136, Tessier-Lavigne, M., and Goodman, C.S. (1996). The molecular biology of axon guidance. Science 274, Wojtowicz, W.M., Wu, W., Andre, I., Qian, B., Baker, D., and Zipursky, S.L. (2007). A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell 130, Yuan, S.S., Cox, L.A., Dasika, G.K., and Lee, E.Y. (1999). Cloning and functional studies of a novel gene aberrantly expressed in RB-deficient embryos. Dev. Biol. 207, Zipursky, S.L., Wojtowicz, W.M., and Hattori, D. (2006). Got diversity? Wiring the fly brain with Dscam. Trends Biochem. Sci. 31, Zou, Y., Stoeckli, E., Chen, H., and Tessier-Lavigne, M. (2000). Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell 102, Neuron 58, , May 8, 2008 ª2008 Elsevier Inc.
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Proteins Regulate a Cell-Intrinsic Switch from Sonic Hedgehog-Mediated Commissural Axon Attraction to Repulsion after Midline Crossing
 Article 14-3-3 Proteins Regulate a Cell-Intrinsic Switch from Sonic Hedgehog-Mediated Commissural Axon Attraction to Repulsion after Midline Crossing Patricia T. Yam, 1,2,5,7 Christopher B. Kent, 2,5,7
Article 14-3-3 Proteins Regulate a Cell-Intrinsic Switch from Sonic Hedgehog-Mediated Commissural Axon Attraction to Repulsion after Midline Crossing Patricia T. Yam, 1,2,5,7 Christopher B. Kent, 2,5,7
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons.
 Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN
 UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
UNC-6/Netrin mediates dendritic self-avoidance Cody J. Smith 1, Joseph D. Watson 1,4,5, Miri K. VanHoven 2, Daniel A. Colón-Ramos 3 and David M. Miller III 1,4,6 1 Department of Cell and Developmental
Name: Answer Key. Question 1.
 2007 7.013 Problem Set 6 Due before 5 PM on FRIDAY, April 27, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. Question 1. 1a. This is a diagram showing changes
2007 7.013 Problem Set 6 Due before 5 PM on FRIDAY, April 27, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. Question 1. 1a. This is a diagram showing changes
The role of floor plate contact in the elaboration of contralateral commissural projections within the embryonic mouse spinal cord
 Developmental Biology 296 (2006) 499 513 www.elsevier.com/locate/ydbio The role of floor plate contact in the elaboration of contralateral commissural projections within the embryonic mouse spinal cord
Developmental Biology 296 (2006) 499 513 www.elsevier.com/locate/ydbio The role of floor plate contact in the elaboration of contralateral commissural projections within the embryonic mouse spinal cord
Bio 3411 Midterm Review:
 Bio 3411 Midterm Review: Structure/Development/Systems/ Plastics/Talents/Diseases/Genes Structure General Overview Wednesday October 26, 2011 1 2 THE BRAIN ATLAS 3 rd ed, p. 8! THE BRAIN ATLAS 3 rd ed,
Bio 3411 Midterm Review: Structure/Development/Systems/ Plastics/Talents/Diseases/Genes Structure General Overview Wednesday October 26, 2011 1 2 THE BRAIN ATLAS 3 rd ed, p. 8! THE BRAIN ATLAS 3 rd ed,
gdnf Activates Midline Repulsion by Semaphorin3B via NCAM during Commissural Axon Guidance
 Article gdnf Activates Midline Repulsion by Semaphorin3B via NCAM during Commissural Axon Guidance Camille Charoy, 1,4 Homaira Nawabi, 1,4 Florie Reynaud, 1 Edmund Derrington, 1 Muriel Bozon, 1 Kevin Wright,
Article gdnf Activates Midline Repulsion by Semaphorin3B via NCAM during Commissural Axon Guidance Camille Charoy, 1,4 Homaira Nawabi, 1,4 Florie Reynaud, 1 Edmund Derrington, 1 Muriel Bozon, 1 Kevin Wright,
Mechanisms of alternative splicing regulation
 Mechanisms of alternative splicing regulation The number of mechanisms that are known to be involved in splicing regulation approximates the number of splicing decisions that have been analyzed in detail.
Mechanisms of alternative splicing regulation The number of mechanisms that are known to be involved in splicing regulation approximates the number of splicing decisions that have been analyzed in detail.
Discovery of Schizophrenia Drug Targets from DISC1 Mechanisms Atsushi Kamiya M.D., Ph.D.
 Discovery of Schizophrenia Drug Targets 1 Assistant Professor Department of Psychiatry and Behavioral Sciences Johns Hopkins University School of Medicine akamiya1@jhmi.edu How does neurobiology offer
Discovery of Schizophrenia Drug Targets 1 Assistant Professor Department of Psychiatry and Behavioral Sciences Johns Hopkins University School of Medicine akamiya1@jhmi.edu How does neurobiology offer
Regionalization of the nervous system. Paul Garrity 7.68J/9.013J February 25, 2004
 Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Supplementary Table 1. List of primers used in this study
 Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Bio 3411 Midterm Review:
 Midterm Review: Structure/Development/Systems/ Plastics/Talents/Diseases/Genes Structure General Overview Wednesday 1( 2( THE BRAIN ATLAS 3 rd ed, p. 8! THE BRAIN ATLAS 3 rd ed, p. 9! Mid-line (sagittal)
Midterm Review: Structure/Development/Systems/ Plastics/Talents/Diseases/Genes Structure General Overview Wednesday 1( 2( THE BRAIN ATLAS 3 rd ed, p. 8! THE BRAIN ATLAS 3 rd ed, p. 9! Mid-line (sagittal)
Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling
 Bjorke et al. Neural Development (2016) 11:18 DOI 10.1186/s13064-016-0073-y RESEARCH ARTICLE Open Access Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling Brielle Bjorke
Bjorke et al. Neural Development (2016) 11:18 DOI 10.1186/s13064-016-0073-y RESEARCH ARTICLE Open Access Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling Brielle Bjorke
Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells.
 SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
Shh signaling guides spatial pathfinding of raphespinal tract axons by multidirectional repulsion
 ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Polarity and Segmentation. Chapter Two
 Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Chapter 2. Investigation into mir-346 Regulation of the nachr α5 Subunit
 15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
Diseases of the skeleton
 Diseases of the skeleton Flexibility Protection of vital organs Strength Skeletal defects impact human health Developmental diseases Degenerative diseases Fins regenerate and grow rapidly following amputation
Diseases of the skeleton Flexibility Protection of vital organs Strength Skeletal defects impact human health Developmental diseases Degenerative diseases Fins regenerate and grow rapidly following amputation
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Supplementary Fig. 1. Delivery of mirnas via Red Fluorescent Protein.
 prfp-vector RFP Exon1 Intron RFP Exon2 prfp-mir-124 mir-93/124 RFP Exon1 Intron RFP Exon2 Untransfected prfp-vector prfp-mir-93 prfp-mir-124 Supplementary Fig. 1. Delivery of mirnas via Red Fluorescent
prfp-vector RFP Exon1 Intron RFP Exon2 prfp-mir-124 mir-93/124 RFP Exon1 Intron RFP Exon2 Untransfected prfp-vector prfp-mir-93 prfp-mir-124 Supplementary Fig. 1. Delivery of mirnas via Red Fluorescent
2- Slit-Robo-Netrin-DCC
 - Slit-Robo-Netrin-DCC 1 + + + + + + + - - - - - - - - - - - - - - - - as axons grow long distances in the developing embryo, they make use of intermediate targets to simplify their navigation into short,
- Slit-Robo-Netrin-DCC 1 + + + + + + + - - - - - - - - - - - - - - - - as axons grow long distances in the developing embryo, they make use of intermediate targets to simplify their navigation into short,
Alternative splicing. Biosciences 741: Genomics Fall, 2013 Week 6
 Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
Alternative splicing Biosciences 741: Genomics Fall, 2013 Week 6 Function(s) of RNA splicing Splicing of introns must be completed before nuclear RNAs can be exported to the cytoplasm. This led to early
Alternative Splicing and Genomic Stability
 Alternative Splicing and Genomic Stability Kevin Cahill cahill@unm.edu http://dna.phys.unm.edu/ Abstract In a cell that uses alternative splicing, the total length of all the exons is far less than in
Alternative Splicing and Genomic Stability Kevin Cahill cahill@unm.edu http://dna.phys.unm.edu/ Abstract In a cell that uses alternative splicing, the total length of all the exons is far less than in
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines
 Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Sonic Hedgehog Guides Axons through a Noncanonical, Src-Family-Kinase-Dependent Signaling Pathway
 Article Sonic Hedgehog Guides Axons through a Noncanonical, Src-Family-Kinase-Dependent Signaling Pathway Patricia T. Yam, 1,2,5 Sébastien D. Langlois, 1,4,5,6 Steves Morin, 1,6 and Frédéric Charron 1,2,3,4,5,
Article Sonic Hedgehog Guides Axons through a Noncanonical, Src-Family-Kinase-Dependent Signaling Pathway Patricia T. Yam, 1,2,5 Sébastien D. Langlois, 1,4,5,6 Steves Morin, 1,6 and Frédéric Charron 1,2,3,4,5,
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
Effects of UBL5 knockdown on cell cycle distribution and sister chromatid cohesion
 Supplementary Figure S1. Effects of UBL5 knockdown on cell cycle distribution and sister chromatid cohesion A. Representative examples of flow cytometry profiles of HeLa cells transfected with indicated
Supplementary Figure S1. Effects of UBL5 knockdown on cell cycle distribution and sister chromatid cohesion A. Representative examples of flow cytometry profiles of HeLa cells transfected with indicated
REGULATED SPLICING AND THE UNSOLVED MYSTERY OF SPLICEOSOME MUTATIONS IN CANCER
 REGULATED SPLICING AND THE UNSOLVED MYSTERY OF SPLICEOSOME MUTATIONS IN CANCER RNA Splicing Lecture 3, Biological Regulatory Mechanisms, H. Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Splice
REGULATED SPLICING AND THE UNSOLVED MYSTERY OF SPLICEOSOME MUTATIONS IN CANCER RNA Splicing Lecture 3, Biological Regulatory Mechanisms, H. Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Splice
Broca s Aphasia: Lesions in the left posterior frontal lobe. Can understand speech but have a disruption in expressing spontaneous speech and writing.
 9.09J/7.29J - Cellular Neurobiology, Spring 2005 Massachusetts Institute of Technology Department of Brain and Cognitive Sciences Department of Biology Instructors: Professors William Quinn and Troy Littleton
9.09J/7.29J - Cellular Neurobiology, Spring 2005 Massachusetts Institute of Technology Department of Brain and Cognitive Sciences Department of Biology Instructors: Professors William Quinn and Troy Littleton
Inner ear development Nervous system development
 Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
effects on organ development. a-f, Eye and wing discs with clones of ε j2b10 show no
 Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
A novel fragile X syndrome mutation reveals a conserved role for the carboxy-terminus in FMRP localization and function
 A novel fragile X syndrome mutation reveals a conserved role for the carboxy-terminus in FMRP localization and function Zeynep Okray, Celine E.F. de Esch, Hilde Van Esch, Koen Devriendt, Annelies Claeys,
A novel fragile X syndrome mutation reveals a conserved role for the carboxy-terminus in FMRP localization and function Zeynep Okray, Celine E.F. de Esch, Hilde Van Esch, Koen Devriendt, Annelies Claeys,
Circular RNAs (circrnas) act a stable mirna sponges
 Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation
 Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation Sayaka U. Sekine, Shuka Haraguchi, Kinhong Chao, Tomoko Kato, Liqun Luo, Masayuki Miura, and Takahiro Chihara
Meigo governs dendrite targeting specificity by modulating Ephrin level and N-glycosylation Sayaka U. Sekine, Shuka Haraguchi, Kinhong Chao, Tomoko Kato, Liqun Luo, Masayuki Miura, and Takahiro Chihara
mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons
 Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Nature Neuroscience: doi: /nn Supplementary Figure 1. Splenic atrophy and leucopenia caused by T3 SCI.
 Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
ns ns hp761(lf); daf-28(lf) daf-28(gf) Figure S1
 A ns ns B 100 100 80 80 60 60 40 40 20 20 0 0% 0 0% hp761(lf); daf-28(lf) daf-28(gf) Figure S1 A 100 80 15 o C 22 o C 25 o C % Dauers 60 40 20 0 0% 0% * 0% 0% 0% Class I alleles Class II alleles daf-2(lf;ts)
A ns ns B 100 100 80 80 60 60 40 40 20 20 0 0% 0 0% hp761(lf); daf-28(lf) daf-28(gf) Figure S1 A 100 80 15 o C 22 o C 25 o C % Dauers 60 40 20 0 0% 0% * 0% 0% 0% Class I alleles Class II alleles daf-2(lf;ts)
Supplementary Figure 1. Quantile-quantile (Q-Q) plots. (Panel A) Q-Q plot graphical
 Supplementary Figure 1. Quantile-quantile (Q-Q) plots. (Panel A) Q-Q plot graphical representation using all SNPs (n= 13,515,798) including the region on chromosome 1 including SORT1 which was previously
Supplementary Figure 1. Quantile-quantile (Q-Q) plots. (Panel A) Q-Q plot graphical representation using all SNPs (n= 13,515,798) including the region on chromosome 1 including SORT1 which was previously
Selective filtering defect at the axon initial segment in Alzheimer s disease mouse models. Yu Wu
 Selective filtering defect at the axon initial segment in Alzheimer s disease mouse models Yu Wu Alzheimer s Disease (AD) Mouse models: APP/PS1, PS1δE9, APPswe, hps1 Wirths, O. et al, Acta neuropathologica
Selective filtering defect at the axon initial segment in Alzheimer s disease mouse models Yu Wu Alzheimer s Disease (AD) Mouse models: APP/PS1, PS1δE9, APPswe, hps1 Wirths, O. et al, Acta neuropathologica
EMBO REPORT SUPPLEMENTARY SECTION. Quantitation of mitotic cells after perturbation of Notch signalling.
 EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
Phenomena first observed in petunia
 Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
Bio 111 Study Guide Chapter 17 From Gene to Protein
 Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
Developmental Biology
 Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid.
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
A Normal Exencephaly Craniora- Spina bifida Microcephaly chischisis. Midbrain Forebrain/ Forebrain/ Hindbrain Spinal cord Hindbrain Hindbrain
 A Normal Exencephaly Craniora- Spina bifida Microcephaly chischisis NTD Number of embryos % among NTD Embryos Exencephaly 52 74.3% Craniorachischisis 6 8.6% Spina bifida 5 7.1% Microcephaly 7 1% B Normal
A Normal Exencephaly Craniora- Spina bifida Microcephaly chischisis NTD Number of embryos % among NTD Embryos Exencephaly 52 74.3% Craniorachischisis 6 8.6% Spina bifida 5 7.1% Microcephaly 7 1% B Normal
SMA IS A SEVERE NEUROLOGICAL DISORDER [1]
![SMA IS A SEVERE NEUROLOGICAL DISORDER [1] SMA IS A SEVERE NEUROLOGICAL DISORDER [1]](/thumbs/72/67086153.jpg) SMA OVERVIEW SMA IS A SEVERE NEUROLOGICAL DISORDER [1] Autosomal recessive genetic inheritance 1 in 50 people (approximately 6 million Americans) are carriers [2] 1 in 6,000 to 1 in 10,000 children born
SMA OVERVIEW SMA IS A SEVERE NEUROLOGICAL DISORDER [1] Autosomal recessive genetic inheritance 1 in 50 people (approximately 6 million Americans) are carriers [2] 1 in 6,000 to 1 in 10,000 children born
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
supplementary information
 DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity
 Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates
 Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.
![Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +. Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.](/thumbs/90/102196023.jpg) Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
The Drosophila RNA-binding protein ELAV is required for commissural axon midline crossing via control of commissureless mrna expression in neurons
 Developmental Biology 301 (2007) 166 177 www.elsevier.com/locate/ydbio The Drosophila RNA-binding protein ELAV is required for commissural axon midline crossing via control of commissureless mrna expression
Developmental Biology 301 (2007) 166 177 www.elsevier.com/locate/ydbio The Drosophila RNA-binding protein ELAV is required for commissural axon midline crossing via control of commissureless mrna expression
he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003)
 MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
Title: Chapter 5 Recorded Lecture. Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu
 Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous
 Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Synapse Formation. Steven McLoon Department of Neuroscience University of Minnesota
 Synapse Formation Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Midterm Exam Monday, Nov 13 9:30-11:30am Bring a #2 pencil!! 2 Course News Lecture schedule: Mon (Oct 31)
Synapse Formation Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Midterm Exam Monday, Nov 13 9:30-11:30am Bring a #2 pencil!! 2 Course News Lecture schedule: Mon (Oct 31)
ErbB4 migrazione I parte. 3- ErbB4- NRG1
 ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Alternative splicing control 2. The most significant 4 slides from last lecture:
 Alternative splicing control 2 The most significant 4 slides from last lecture: 1 2 Regulatory sequences are found primarily close to the 5 -ss and 3 -ss i.e. around exons. 3 Figure 1 Elementary alternative
Alternative splicing control 2 The most significant 4 slides from last lecture: 1 2 Regulatory sequences are found primarily close to the 5 -ss and 3 -ss i.e. around exons. 3 Figure 1 Elementary alternative
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
Supplementary Information. Preferential associations between co-regulated genes reveal a. transcriptional interactome in erythroid cells
 Supplementary Information Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells Stefan Schoenfelder, * Tom Sexton, * Lyubomira Chakalova, * Nathan
Supplementary Information Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells Stefan Schoenfelder, * Tom Sexton, * Lyubomira Chakalova, * Nathan
ErbB4 migrazione II parte
 ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
Suppl. Information Supplementary Figure 1. Strategy/latency analysis of individual mice during maze learning. a,
 Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning Ruediger, S, Spirig, D., Donato, F., Caroni, P. Suppl. Information Supplementary Figure 1. Strategy/latency analysis
Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning Ruediger, S, Spirig, D., Donato, F., Caroni, P. Suppl. Information Supplementary Figure 1. Strategy/latency analysis
Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated
 Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C genes. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated from 7 day-old seedlings treated with or without
Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C genes. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated from 7 day-old seedlings treated with or without
Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins.
 Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins. The RNA transcribed from a complex transcription unit
Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins. The RNA transcribed from a complex transcription unit
TITLE: A Mouse Model to Investigate the Role of DBC2 in Breast Cancer
 AD Award Number: W81XWH-04-1-0325 TITLE: A Mouse Model to Investigate the Role of DBC2 in Breast Cancer PRINCIPAL INVESTIGATOR: Valerie Boka CONTRACTING ORGANIZATION: University of Texas Health Science
AD Award Number: W81XWH-04-1-0325 TITLE: A Mouse Model to Investigate the Role of DBC2 in Breast Cancer PRINCIPAL INVESTIGATOR: Valerie Boka CONTRACTING ORGANIZATION: University of Texas Health Science
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
The Neurobiology of Learning and Memory
 The Neurobiology of Learning and Memory JERRY W. RUDY University of Colorado, Boulder Sinauer Associates, Inc. Publishers Sunderland, Massachusetts 01375 Table of Contents CHAPTER 1 Introduction: Fundamental
The Neurobiology of Learning and Memory JERRY W. RUDY University of Colorado, Boulder Sinauer Associates, Inc. Publishers Sunderland, Massachusetts 01375 Table of Contents CHAPTER 1 Introduction: Fundamental
Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types.
 Supplementary Figure 1 Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types. (a) Pearson correlation heatmap among open chromatin profiles of different
Supplementary Figure 1 Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types. (a) Pearson correlation heatmap among open chromatin profiles of different
Annotation of Chimp Chunk 2-10 Jerome M Molleston 5/4/2009
 Annotation of Chimp Chunk 2-10 Jerome M Molleston 5/4/2009 1 Abstract A stretch of chimpanzee DNA was annotated using tools including BLAST, BLAT, and Genscan. Analysis of Genscan predicted genes revealed
Annotation of Chimp Chunk 2-10 Jerome M Molleston 5/4/2009 1 Abstract A stretch of chimpanzee DNA was annotated using tools including BLAST, BLAT, and Genscan. Analysis of Genscan predicted genes revealed
MicroRNA in Cancer Karen Dybkær 2013
 MicroRNA in Cancer Karen Dybkær RNA Ribonucleic acid Types -Coding: messenger RNA (mrna) coding for proteins -Non-coding regulating protein formation Ribosomal RNA (rrna) Transfer RNA (trna) Small nuclear
MicroRNA in Cancer Karen Dybkær RNA Ribonucleic acid Types -Coding: messenger RNA (mrna) coding for proteins -Non-coding regulating protein formation Ribosomal RNA (rrna) Transfer RNA (trna) Small nuclear
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figures
 J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
SYNAPTIC COMMUNICATION
 BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
BIPN140 Lecture 13: Synapse Formation (Synaptogenesis)
 BIPN140 Lecture 13: Synapse Formation (Synaptogenesis) 1. Neuromuscular Junction (NMJ) Development 2. Synaptogenesis at Central Synapses Su (FA16) Ultrastructural Image of an NMJ Active Zone Basal Lamina
BIPN140 Lecture 13: Synapse Formation (Synaptogenesis) 1. Neuromuscular Junction (NMJ) Development 2. Synaptogenesis at Central Synapses Su (FA16) Ultrastructural Image of an NMJ Active Zone Basal Lamina
Breast cancer. Risk factors you cannot change include: Treatment Plan Selection. Inferring Transcriptional Module from Breast Cancer Profile Data
 Breast cancer Inferring Transcriptional Module from Breast Cancer Profile Data Breast Cancer and Targeted Therapy Microarray Profile Data Inferring Transcriptional Module Methods CSC 177 Data Warehousing
Breast cancer Inferring Transcriptional Module from Breast Cancer Profile Data Breast Cancer and Targeted Therapy Microarray Profile Data Inferring Transcriptional Module Methods CSC 177 Data Warehousing
RASA: Robust Alternative Splicing Analysis for Human Transcriptome Arrays
 Supplementary Materials RASA: Robust Alternative Splicing Analysis for Human Transcriptome Arrays Junhee Seok 1*, Weihong Xu 2, Ronald W. Davis 2, Wenzhong Xiao 2,3* 1 School of Electrical Engineering,
Supplementary Materials RASA: Robust Alternative Splicing Analysis for Human Transcriptome Arrays Junhee Seok 1*, Weihong Xu 2, Ronald W. Davis 2, Wenzhong Xiao 2,3* 1 School of Electrical Engineering,
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
REGULATED AND NONCANONICAL SPLICING
 REGULATED AND NONCANONICAL SPLICING RNA Processing Lecture 3, Biological Regulatory Mechanisms, Hiten Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Splice site consensus sequences do have
REGULATED AND NONCANONICAL SPLICING RNA Processing Lecture 3, Biological Regulatory Mechanisms, Hiten Madhani Dept. of Biochemistry and Biophysics MAJOR MESSAGES Splice site consensus sequences do have
Bidirectional integrative regulation of Cav1.2 calcium channel by microrna mir-103: role in pain.
 Manuscript EMBO-2011-77953 Bidirectional integrative regulation of Cav1.2 calcium channel by microrna mir-103: role in pain. Alexandre Favereaux, Olivier Thoumine, Rabia Bouali-Benazzouz, Virginie Roques,
Manuscript EMBO-2011-77953 Bidirectional integrative regulation of Cav1.2 calcium channel by microrna mir-103: role in pain. Alexandre Favereaux, Olivier Thoumine, Rabia Bouali-Benazzouz, Virginie Roques,
