ActivationofaHIF1a-PPARg Axis Underlies the Integration of Glycolytic and Lipid Anabolic Pathways in Pathologic Cardiac Hypertrophy
|
|
|
- Violet Lang
- 6 years ago
- Views:
Transcription
1 Article ActivationofaHIF1a-PPARg Axis Underlies the Integration of Glycolytic and Lipid Anabolic Pathways in Pathologic Cardiac Hypertrophy Jaya Krishnan, 1 Marianne Suter, 1,6 Renata Windak, 1,6 Tatiana Krebs, 1 Allison Felley, 2 Christophe Montessuit, 3 Malgorzata Tokarska-Schlattner, 1 Ellen Aasum, 4 Anna Bogdanova, 5 Evelyne Perriard, 1 Jean-Claude Perriard, 1 Terje Larsen, 4 Thierry Pedrazzini, 2 and Wilhelm Krek 1, * 1 Institute of Cell Biology and Competence Center for Systems Physiology and Metabolic Diseases, ETH Zurich, 8093 Zurich, Switzerland 2 Department of Medicine, University of Lausanne Medical School, CHUV MP14-220, 1011 Lausanne, Switzerland 3 Cardiology Center, University Hospital of Geneva, 1211 Geneva 14, Switzerland 4 Department of Medical Physiology, University of Tromsø, 9037 Tromsø, Norway 5 Institute of Pharmacology and Toxicology, University of Zurich, 8057 Zurich, Switzerland 6 These authors contributed equally to this work *Correspondence: wilhelm.krek@cell.biol.ethz.ch DOI /j.cmet SUMMARY Development of cardiac hypertrophy and progression to heart failure entails profound changes in myocardial metabolism, characterized by a switch from fatty acid utilization to glycolysis and lipid accumulation. We report that hypoxia-inducible factor (HIF)1a and PPARg, key mediators of glycolysis and lipid anabolism, respectively, are jointly upregulated in hypertrophic cardiomyopathy and cooperate to mediate key changes in cardiac metabolism. In response to pathologic stress, HIF1a activates glycolytic genes and PPARg, whose product, in turn, activates fatty acid uptake and glycerolipid biosynthesis genes. These changes result in increased glycolytic flux and glucose-to-lipid conversion via the glycerol- 3-phosphate pathway, apoptosis, and contractile dysfunction. Ventricular deletion of Hif1a in mice prevents hypertrophy-induced PPARg activation, the consequent metabolic reprogramming, and contractile dysfunction. We propose a model in which activation of the HIF1a-PPARg axis by pathologic stress underlies key changes in cell metabolism that are characteristic of and contribute to common forms of heart disease. INTRODUCTION Heart failure remains one of the most frequent causes of death in the industrialized world. It is the final common pathway of many forms of heart disease induced by a variety of pathological insults, including hypertension, myocardial infarction, and aortic stenosis. These stressors, if protracted or severe, lead to a progressive worsening of cardiac function and ultimately heart failure (Guzy et al., 2005). A common feature of pathologic hypertrophy is the reprogramming of cardiac energy metabolism. Fatty acids represent the predominant fuel for the adult heart under normal physiologic conditions. However, hypertrophied and failing hearts demonstrate an increased reliance on glucose while decreasing their fatty acid utilization (Lehman and Kelly, 2002). This shift in substrate utilization may be provoked in part by myocardial hypoxia that develops as a result of a mismatch between myocardial oxygen demand and supply during stress-induced hypertrophy (MacLellan and Schneider, 2000). Altered substrate metabolism may ultimately fail to satisfy the energetic demands of the heart, contributing to heart failure. In mammalian cells, decreased oxygen availability stimulates cells to reprogram their metabolism from a mitochondrial oxidative to a glycolytic form of ATP production. Hypoxia-inducible factor (HIF), dimers composed of HIF1a, HIF2a, or HIF3a (collectively HIFa) and HIFb/ARNT subunits, plays a key role coordinating this adaptive response. HIFa subunits are constitutively degraded under normoxia due to prolyl hydroxylase activity, which marks them for degradation by the von Hippel-Lindau (VHL) tumor suppressor protein pvhl. Consequently, loss of pvhl or hypoxia leads to activation of HIF and a number of genes involved in the regulation of cell metabolism, including glucose transporters, glycolytic enzymes, and pyruvate dehydrogenase (PDH) kinase (PDK)1 (DeBerardinis et al., 2008). This serves to increase intracellular glucose uptake, augment glycolysis, and shunt pyruvate away from mitochondria. Despite the low overall percentage of pyruvate available for mitochondrial entry, cardiac-specific ablation of VHL or HIF1a overexpression in vitro leads to TAG accumulation, defective mitochondrial biogenesis, and reduced fatty acid oxidation (Belanger et al., 2007; Lei et al., 2008; Narravula and Colgan, 2001; Zhang et al., 2007); its molecular and metabolic basis remains incompletely understood. TAG synthesis requires free fatty acids (FFAs) and the obligatory intermediary glycerol-3-phosphate. Glycerol-3-phosphate can originate from glycerol through the action of glycerol kinase. While rates of FFA uptake are increased during cardiac hypertrophy (Taylor et al., 2001), cardiomyocytes display a low capacity for glycerol uptake (McGavock et al., 2006). However, glycerol-3-phosphate can be generated via glycerol-3-phosphate dehydrogenase (GPD1), which dehydrogenates dihydroxyacetone phosphate (DHAP), an intermediate of glycolysis. Glycerol-3-phosphate serves then as the substrate for glycerol 512 Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc.
2 phosphate acyltransferase (GPAT), the rate-limiting enzyme for de novo TAG synthesis (Gonzalez-Baro et al., 2007). Genes encoding GPD1 and GPAT have been identified as direct transcriptional targets for the peroxisome proliferator-activated receptor g (PPARg), a member of the nuclear-receptor transcription factor family (Cao et al., 2006; Patsouris et al., 2004). PPARg plays an important role in lipid metabolism, promoting FFA uptake and TAG accumulation in adipose tissue and liver (Spiegelman, 1998). Its role in the heart is less clear. Cardiomyocyterestricted PPARg knockout mice develop cardiac hypertrophy but do not reveal changes in lipid metabolism genes (Duan et al., 2005). In contrast, cardiac PPARg-overexpressing mice develop dilated cardiomyopathy, display increased FFA uptake, and accumulate TAG (Son et al., 2007). These observations, combined with evidence supporting a central role for HIF in promoting glycolysis, raise the intriguing possibility of a potential crosstalk of HIF and PPARg transcription factors in the regulation of glucose and lipid metabolism in the heart. In the present study, we demonstrate that HIF1a and PPARg protein expression is upregulated in human and mouse cardiac hypertrophy. Using cardiac-restricted Hif1a as well as Vhlh knockout mice, we find that HIF1a directly activates PPARg transcription and that PPARg is a key downstream effector of HIF1a-driven TAG accumulation and apoptosis in cardiomyocytes. These results suggest a mechanism for reprogramming of fuel utilization and TAG accumulation in cardiomyocytes during cardiac hypertrophy, in which HIF1a induces glycolytic enzymes and, through PPARg activation, FFA uptake and glycerolipid biosynthesis, resulting in cardiac steatosis. Moreover, stress-induced HIF1a activation promotes cardiomyocyte apoptosis in a PPARg-dependent manner and leads to diminished cardiac function. RESULTS Cardiac Hypertrophy Is Associated with Increased Expression of HIF1a and PPARg Ventricular biopsies of human and mouse models of pathological hypertrophy were assessed for HIF1a expression by immunoblotting. Compared to control, high HIF1a levels were observed in hypertrophic cardiomyopathy (HCM) of human samples (Figure 1A) and in vivo mouse models of HCM corresponding to aortic stenosis and pressure overload (transaortic constriction [TAC]) (Figure 1B), defective b-adrenergic signaling (isoproterenol [ISO] infusion) (Figures S1A and S1B), and hypertension (angiotensin 2 overexpression) (Figures S1A and S1B). Treatment of primary neonatal mouse cardiomyocytes (NMCs) with hypertrophy-inducing agents such as ISO, norepinephrine, and phenylepinephrine (PE) likewise resulted in an induction of HIF1a protein (Figure S1C) and nuclear translocation (Figure S1D, subpanels c and d, e and f, and g and h, respectively). Intriguingly, PPARg and the product of the PPARg target gene GPAT also accumulated in human and mouse TAC-induced HCMs (Figures 1A and 1B). In contrast, M-Cpt1, a PPARa and PPARb/d target gene and critical component of mitochondrial fatty acid transport required for b-oxidation, was downregulated (Figures 1A and 1B). Swimming-induced physiological cardiac hypertrophy in the mouse led to the induction of neither HIF1a nor PPARg (Figure S1E) (Luo et al., 2005). Thus, increased expression of HIF1a and PPARg appears to be a common feature of pathologic cardiac hypertrophy in vivo. To extend these findings, expression profiling of a subset of glycolytic, nuclear receptor, and nuclear receptor target genes was performed by quantitative real-time PCR (qpcr) on HCMs of human and TAC-induced mouse hearts. Glycolytic genes such as glucose transporter 1 (GLUT1), hexokinase 2, muscle phosphofructokinase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and PDK, isozyme 4 were upregulated in diseased human and TAC mouse heart samples (Figures 1C and 1D). The mrna levels of PPARa and PPARb/d, two positive transcriptional regulators of lipid catabolism, were reduced to various degrees (Figures 1C and 1D). PPARg, a transcriptional regulator of lipid anabolism, and the PPARg target genes, GPD1 and GPAT, were also increased (Figures 1C and 1D), in accord with the immunoblotting data shown above (Figures 1A and 1B). Hence, induction of HCM in humans and mouse models is associated with the activation of key transcriptional regulators of glycolysis and lipid anabolism, HIF1a and PPARg, respectively. To investigate a potential link between stress-induced hypertrophy and the accumulation of HIF1a and PPARg, mice with loxp-flanked Hif1a (Hif1a fl/fl) (Ryan et al., 1998; Schipani et al., 2001) orvhlh (Vhlh fl/fl) (Haase et al., 2001) alleles were crossed to myosin light-chain (Mlc)2v-Cre (Chen et al., 1998) transgenic mice to generate Mlc2v-Cre; Hif1a fl/fl (hereafter referred to as Hif1a cko) and Mlc2v-Cre; Vhlh fl/fl (hereafter referred to as Vhlh cko) mice. The Mlc2v-Cre transgene induces Cre-mediated recombination specifically in the ventricular cardiomyocytes of the mouse heart (Chen et al., 1998). Deletion of either Hif1a or Vhlh was confirmed by PCR-mediated detection of the corresponding recombined alleles (data not shown). Hif1a cko mice were viable and phenotypically indistinguishable from control Hif1a fl/fl mice, consistent with earlier analysis of such mice (Huang et al., 2004). Vhlh cko mice did not exhibit any developmental or postnatal phenotype (data not shown). Around 3 4 months of age, these mice exhibited increased heart size and concentric ventricular hypertrophy (data not shown), which further developed at 5 6 months of age into eccentric hypertrophy characterized by severe ventricular and atrial hypertrophy as well as thinning of the ventricular wall and dilation of the ventricular cavity (Figures S2A S2C). Immunoblotting of lysates of ventricles from Vhlh fl/fl, Vhlh cko, Hif1a fl/fl, and Hif1a cko mice revealed a marked accumulation of HIF1a, PPARg, and the PPARg target gene products FAT/CD36 and GPAT in the Vhlh cko ventricles, but not in the ventricles of the other genotypes (Figure 1E). The levels of PPARa and PPARb/d family members involved in lipid catabolism did not change significantly (Figure 1E). In contrast, PGC- 1a, a transcriptional coactivator important for the expression of mitochondrial biogenesis and b-oxidation genes, and the mitochondrial proteins M-Cpt1, voltage-dependent anion-selective channel (VDAC), and succinate dehydrogenase complex subunit A (SDHA) were downregulated in Vhlh cko ventricles (Figure 1E). Interestingly, in Hif1a cko ventricles, PGC-1a, M-Cpt1, VDAC, and SDHA protein levels were upregulated (Figures 1E). Expression profiling by qpcr revealed that several glycolytic genes, PPARg, GPD1, and GPAT, were activated in Vhlh cko ventricles, while genes whose products are involved in mitochondrial Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc. 513
3 Figure 1. HIF1a Accumulation Correlates with and Promotes Pathological Hypertrophy (A and B) Human (A) and mouse (B) normal and diseased ventricles were assessed for HIF1a, PPARg, GPAT, and M-Cpt1 protein expression. Cardiac actin and sarcomeric a-actinin serve as loading controls for the human and mouse sample sets, respectively. (C and D) Gene expression profiling of hypertrophic and metabolic markers was performed on human (C) and mouse (D) normal and diseased ventricles. All values were normalized internally to 18S RNA expression and to the normal human and the mouse sham control sets, respectively (*, p < 0.01; **, p < 0.05). Data are means ± SEM of values from each group. (E) Ventricular whole-cell lysates of the respective genotypes were probed for protein expression of HIF1a,PPARa, PPARb/d, PPARg, GPAT, PGC-1a, M-Cpt1, VDAC, SDHA, and FAT/CD36. Sample loading was normalized to sarcomeric a-actinin. Broken lines are used to separate the respective sets. All samples were run on the same gel. biogenesis and function were decreased (Figure S3A, upper subpanels). In Hif1a cko ventricles, glycolytic and PPARg gene expression was not upregulated, but PPARa and PPARb/d expression was increased (Figure S3B, upper subpanel). Mitochondrial gene expression was generally increased in Hif1a cko ventricles (Figure S3B, lower subpanel). Combined, these changes suggest that Vhlh inactivation mediates reprogramming of cardiomyocyte metabolism from a mitochondrial to a glycolytic form of energy production. It appears that Hif1a deficiency inverses this metabolic program. In keeping with this view, mitochondrial respiratory function (Figure S4A), mitochondrial DNA content (Figure S4B), and mitochondrial surface area (Figures S4C and S4D) were decreased in Vhlh cko but enhanced in Hif1a cko ventricles compared to control ventricles. PPARg Is a Target Gene of HIF1a in Cardiomyocytes The coordinate upregulation of HIF1a and PPARg in response to hypertrophic stimuli or inactivation of Vhlh raised the intriguing possibility of a dependency of PPARg expression on HIF1a. To test this, NMCs were treated with PE, an a-adrenergic receptor agonist, and ISO and assessed for HIF1a, GAPDH, PPARg, and GPAT mrna expression. As shown in Figures 2A and 514 Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc.
4 S5A, both stimuli induced GAPDH, PPARg, and GPAT but not HIF1a. Infection of these cells with lentiviral short hairpin RNAs targeting HIF1a mrna (shhif1a) reduced HIF1a mrna expression and abolished induction of GAPDH, PPARg, and GPAT by PE and ISO (Figures 2A and S5A, respectively). Downregulation of PPARg with a corresponding lentiviral shrna (shpparg) blocked selectively the expression of PE- or ISO-induced PPARg and GPAT, but not of GAPDH (Figures 2A and S5A). In contrast, ectopic expression of a constitutively active form of HIF1a that escapes pvhl-mediated degradation through alanine substitutions at the HIF1a proline hydroxylation sites Pro402 and Pro577 (referred to as HIF1aDPro) (Staller et al., 2003) induced GAPDH, PPARg, and GPAT in the absence of hypertrophic stimuli (Figure 2B). Although overexpression of PPARg was sufficient to induce expression of GPAT, it had negligible effects on HIF1a or GAPDH expression (Figure 2B). The efficacy of the respective lentiviruses is shown in Figures S8A and S8D. These results indicate that hypertrophy-induced PPARg expression is, at least in part, HIF1a dependent and that the subsequent induction of GPAT expression is PPARg dependent. We next assessed if PPARg is a direct HIF1a target. In silico analysis revealed a potential hypoxic response element (HRE) located 1042 bp upstream of the PPARg transcriptional start site. This HRE is conserved between the mouse and human PPARg promoter (Figure 2C). Mouse wild-type (WT) promoter and the corresponding PPARg promoter fragment mutated at this HRE site were joined to the luciferase reporter gene and transfected into NMCs. ISO addition or HIF1aDPro coexpression activated the WT PPARg promoter in a HRE-dependent manner, as the PPARg-HRE mutant promoter was unresponsive to these treatments (Figure 2D). Lentiviral expression of shhif1a but not of a control nonsilencing (ns) shrna (nsrna) completely abolished PE- or ISO-induced PPARg promoter activation (Figures 2D and S5B). Immunoblotting confirmed that HIF1a failed to accumulate in response to PE or ISO in shhif1a-infected cells (data not shown). Chromatin immunoprecipitation (ChIP) assays on nuclear extracts of shvhl-infected NMCs using antibodies against HIF1a showed that HIF1a associated with the HRE in the PPARg as well as the GAPDH promoters in native chromatin only in shvhl-infected NMCs (Figure 2E). A similar association between HIF1a and the PPARg promoter was observed with ISO stimulation (Figure S5C). Thus, PPARg is a HIF1a target gene. A HIF1a-PPARg sequential transcriptional pathway would be predicted to induce GPAT promoter activity in a reporter assay and to promote the interaction of PPARg with the respective promoters via PPAR response elements (PPREs) upon HIF1a activation. In silico analysis of the GPAT promoter revealed a PPRE upstream of the transcriptional start site at position 1027 bp that is conserved in human and mouse promoters (Figure 2F). The GPAT WT and PPRE mutant (GPAT DPPRE) promoters were analyzed in NMCs. Expression of HIF1aDPro activated the GPAT promoter. The mutant GPAT DPPRE promoter responded also to HIF1aDPro, but to a lesser degree (Figure 2G). Expression of PPARg induced GPAT promoter activity in a PPRE-dependent manner (Figure 2G). These results suggest that HIF1a activates the GPAT promoter, at least in part, through a PPARg-dependent mechanism. Additionally, ChIP assays demonstrated that PPARg associated with the predicted PPRE in the GPAT as well as the GPD1 promoters specifically in the presence of lentiviral shvhl or ISO (Figures 2H and S5D). Hypertrophic Stimuli Induces Myocardial Steatosis in a HIF1a-Dependent Manner PPARg is a potent inducer of TAG synthesis. Based on the capacity for HIF1a and hypertrophic stimuli to induce PPARg expression and transcriptional activity, we asked if pathological stimuli would increase myocardial TAG content in vivo and, if so, whether this was dependent on HIF1a function. TAC and the one-kidney-one-clip (1K1C) are two established models of mouse pathological hypertrophy. Ventricular biopsies of mice subjected to TAC or 1K1C were assessed for TAG content by oil red O (ORO) staining. TAG accumulation was observed specifically in Hif1a fl/fl but not in Hif1a cko ventricles subjected to TAC (Figures 3A and 3E) or 1K1C (Figure 3B). Ventricularspecific Vhlh deficiency caused lipid accumulation (Figures 3C and 3E), consistent with a previous report (Lei et al., 2008). Thus, HIF1a is essential for cardiac lipid accumulation in response to hypertrophic stimuli. To verify if the HIF1a-dependent TAG accumulation occurred in a cell-autonomous fashion, Hif1a fl/fl NMCs were cultured in the presence of ISO or PE and stained for HIF1a, sarcomeric a-actinin, DAPI, and ORO (Figure S6A). ISO and PE both induced lipid accumulation in HIF1a fl/fl NMCs (Figures S6A, subpanels i l and q t, respectively, and 3F), but not when HIF1a was ablated by infection with a Cre-expressing adenoviral vector (Figures S6A, subpanels m p and u x, respectively, and 3F). Given the HIF1a dependency of lipid accumulation and glycolysis, we determined the impact of hypertrophic agents on the contribution of glucose-derived carbon to TAG synthesis. Glycerol phosphate dehydrogenases form an important link between carbohydrate and lipid metabolism (Lewin and Coleman, 2003). As a result of glucose metabolism, DHAP generated from the breakdown of fructose-1-6-bisphosphate by aldolase retains the carbon moiety at position 1 of glucose (C 1 ), which upon conversion to glycerol-3-phosphate by GPD1 is acylated by GPAT to culminate in TAG incorporation (Figure S7). Glucose labeled at C 1 would be predicted to incorporate in TAG upon hypertrophic induction if hypertrophy promoted the preferential channeling of DHAP toward TAG synthesis and if the glycerol component of the stored lipids did derive from the glycolytic cascade (Denton and Halperin, 1968). 14 C 1 -glucose was added to Hif1a fl/fl NMCs in the presence of PE, and stored TAG was extracted and analyzed for 14 C incorporation (Figure 3F). 14 C incorporation in TAG was observed almost exclusively in hypertrophy-induced NMCs and occurred in a HIF1a-dependent fashion, as evidenced by the observation that deletion of the HIF1a allele by adenoviral Cre recombinase (Ad-Cre) expression blocked PE-induced 14 C-glucose incorporation into TAG. Ad-Cre alone did not significantly affect 14 C incorporation in TAG (Figure S6B). Thus, activation of HIF1a in response to hypertrophic signals contributes significantly to the conversion of glucosederived carbon to lipid synthesis in NMCs. HIF1a Coregulates Glycolysis and TAG Synthesis Fructose-1,6-bisphosphate breakdown by aldolase gives rise to DHAP and glyceraldehyde-3-phosphate. Generated DHAP can Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc. 515
5 Figure 2. PPARg Is a Direct Transcriptional Target of HIF1a (A) NMCs were treated with PE in the presence of shhif1a or shpparg and assayed for HIF1a, GAPDH, PPARg, and GPAT expression by qpcr (*, p < 0.01 compared to control, set at 1.0). Data are means ± SEM of values from each group. (B) NMCs were transfected with HIF1aDPro or PPARg expression vector and assayed for HIF1a, GAPDH, PPARg, and GPAT expression by qpcr. All values shown are normalized to HIF1a, GAPDH, PPARg, and GPAT levels in mock transfected NMCs. Levels of HIF1a, GAPDH, PPARg, and GPAT expression in mock transfected NMCs are set at 1.0 (*, p < 0.01). Data are means ± SEM of values from each group. (C) Sequence analysis of the human and mouse PPARg promoter showing conserved putative HREs (underlined). The core consensus HRE motif is shown in bold. (D) PPARg promoter activity in response to HIF1a was determined by transient cotransfection of WT or HRE-mutated PPARg promoters, respectively, fused to luciferase and with either an empty vector control or the HIF1aDPro expression construct or stimulated with PE in the presence or absence of shhif1a. All transfections contained equal amounts of a b-galactosidase expression vector for normalization of luciferase activity (ç, %, and **, p < 0.01; *, p < 0.05). Data are means ± SEM of values from each group. 516 Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc.
6 Figure 3. In Vivo Pathological Hypertrophy and VHL Loss Promote Ventricular Steatosis (A and B) Ventricular cross-sections of Hif1a fl/fl or Hif1a cko mice subjected to TAC (A) or 1K1C (B) were stained for oil red O (red) and counterstained with hematoxylin (blue). (C) Cross-sections of Vhlh fl/fl and Vhlh cko ventricles were stained for oil red O (red) and counterstained with hematoxylin (blue). (D) Quantification of ventricular TAG content of Vhlh fl/fl, VHL cko, Hif1a fl/fl, or Hif1a cko mice. TAG content was normalized to tissue weight (*, p < 0.01; **, p < 0.05). Data are means ± SEM of values from four or five mice. (E) Hif1a fl/fl NMCs were treated with a solvent control or PE in the presence of nsrna, shpparg, or Ad-Cre and assessed for TAG accumulation by oil red O staining and absorbance (510 nm) measurements of extracted lipids (*, ç, and &, p < 0.01). Data are means ± SEM of values from each group. (F) 14 C 1 -glucose incorporation in TAG was assessed in Hif1a fl/fl NMCs in solvent control or PE-treated cultures in the presence or absence of Ad-Cre. Normalized levels of 14 C 1 -TAG incorporation in the respective culture conditions are shown (*, ç, and &, p < 0.01; C, solvent control; ISO, isoproterenol; PE, phenylepinephrine; Ad-C, control adenovirus; Ad-Cre, Cre recombinase encoding adenovirus; Rel., relative). Data are means ± SEM of values from each group. either be dehydrogenated to form glycerol-3-phosphate by GPD1 or converted to glyceraldehyde-3-phosphate and returned to the glycolytic branch by triosephosphate isomerase (TPI). Accordingly, the upregulation of GLUT1 and glycolytic enzymes by HIF1a and consequent increase of FAT/CD36, GPD1, and GPAT by HIF1a-mediated PPARg expression would ensure a coordinate engagement of the glycolytic and glycerolipid biosynthetic branches (Figure S7). To verify the establishment of such a mode of coregulation in the context of cardiomyocyte hypertrophy, we first determined if PE-mediated hypertrophy had an effect on glucose and lipid uptake and, if so, whether these changes could be ascribed to HIF1a. PE-induced Hif1a fl/fl NMCs displayed increased levels of glucose and FFA uptake (Figures 4A and 4B, respectively). This increase was abolished upon Ad-Cre infection, suggesting that hypertrophyinduced glucose and lipid uptake occurs in a HIF1a-dependent fashion. Ad-Cre infection of WT cells had negligible effects on glucose and FFA uptake compared to mock infected cells (data not shown). Next, we addressed if the increase in glucose and lipid uptake in hypertrophy had any bearing on intracellular cardiomyocyte TAG content. Indeed, TAG generation in PE-treated NMCs was achieved only when both glucose and FFAs were added (Figure 4C), implying a requirement for both glucose and FFAs for TAG synthesis in response to hypertrophic stimulus. (E) nsrna control and shvhl-treated NMCs were assessed for interaction of HIF1a at the PPARg promoter by ChIP. DNA from the respective samples were immunoprecipitated with a HIF1a-specific antibody (IP:HIF1a) or with a control isotype-matched antibody (IP:Ig control). Interaction of HIF1a at the GAPDH locus, an established HIF1a transcriptional target, served as a positive control for the ChIP. (F) Sequence analysis of the human and mouse GPAT promoter showing conserved putative PPREs (underlined). The core consensus PPRE motif is shown in bold. (G) GPAT promoter activity in response to HIF1a and PPARg was determined by transient cotransfection of WT or PPRE-mutated GPAT promoter, respectively, fused to luciferase and with either an empty vector control or HIF1aDPro or PPARg expression constructs. All transfections contained equal amounts of a b-galactosidase expression vector for normalization of luciferase activity (ç, &, %, and *, p < 0.05; **, p < 0.01). Data are means ± SEM of values from each group. (H) nsrna control and shvhl-treated NMCs were assessed for interaction of PPARg at the GPAT promoter by ChIP. DNA from the respective samples were immunoprecipitated with a PPARg-specific antibody (IP:PPARg) or with a control isotype-matched antibody (IP:Ig control). Interaction of PPARg at the GPD1 locus, an established PPARg transcriptional target, served as a positive control for the ChIP. Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc. 517
7 Figure 4. HIF1a Promotes TAG Synthesis by Coregulating Glycolysis and Glycerolipid Synthesis (A and B) Hif1a fl/fl NMCs were stimulated with a solvent control (diamond) or PE (square and triangle), mock infected with an empty adenovirus (square) or one encoding Cre recombinase (triangle), and assessed for glucose (A) and oleate (B) uptake at the respective time points. (C) Solvent control or PE-stimulated NMCs were cultured in glucose and fatty-acid-free medium containing exogenous glucose and/or oleate and assessed for intracellular TAG levels by oil red O staining and absorbance (510 nm) measurements of extracted lipids (*, p < 0.01). Data are means ± SEM of values from each group. (D and E) NMCs were treated with a solvent control or PE in the presence of nsrna, shhif1a, or shpparg and assayed for GAPDH (*, ç, and &, p < 0.01) (D) and GPD1 (*, p < 0.01) (E) enzymatic activity. Data are means ± SEM of values from each group. 518 Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc.
8 We assessed if the activity of GAPDH and GPD1, the two enzymes at the crossroads of glycolysis and the glycerolipid biosynthetic pathways, were altered in hypertrophy and if the observed changes could be ascribed to HIF1a and PPARg. HIF1a knockdown inhibited both GAPDH and GPD1 enzymatic activity in NMCs treated with PE, whereas PPARg knockdown resulted only in a specific decrease in GPD1 activity (Figures 4D and 4E). Consistent with these observations, knockdown of HIF1a or PPARg reduced also the levels of intracellular TAG content upon PE addition (Figure 4F). To underscore the requirement for a flux through both the glycolytic (via GLUT1 and GAPDH) and the glycerolipid synthesis (via FAT/CD36 and GPD1) branches for TAG synthesis, shglut1, shgapdh, shfat/cd36, or shgpd1 were added to PE-treated NMCs. Inhibition of either GLUT1 or GAPDH expression led to decreased intracellular TAG levels, confirming the requirement for glycolytic metabolites in TAG synthesis. Similarly, inhibition of FAT/CD36 or GPD1 led to decreased TAG accumulation upon PE stimulation (Figure 4F). The efficacy of the respective lentiviral shrnas in knocking down endogenous gene expression is shown in Figures S8C S8F. As ventricular TAG accumulation was observed in our mouse models of pathological hypertrophy, we asked if the glycolytic and glycerolipid synthesis pathways are also engaged in vivo and depend on HIF1a. To this end, ventricular biopsies of sham and TAC-induced Hif1a fl/fl mice were assessed for GAPDH, GPD1, and GPAT enzymatic activities. TAC induced a significant rise in the enzymatic activity of the glycolytic enzyme GAPDH and of the glycerolipid pathway enzymes GPD1 and GPAT (Figures 4G 4I). The simultaneous increase in enzymatic activities along the two pathways in TAC-induced hypertrophy was mimicked by Vhlh deficiency in the absence of a hypertrophic stimulus (Figures 4G 4I). However, ablation of HIF1a function in ventricles prevented the activation of GAPDH, GPD1, and GPAT enzymatic activities in response to TAC (Figures 4G 4I). In contrast, levels of glucose oxidation, as represented by citrate synthase activity, was markedly reduced in TAC-induced Hif1a fl/fl and Vhlh deficiency ventricles compared to TAC-induced Hif1a cko and Vhlh fl/fl control ventricles, respectively (Figure 4J). Bearing in mind the restriction of the cardiac steatosis phenotype to hearts subjected to hypertrophic stress such as TAC and 1K1C or the loss of ventricular Vhlh, the data above are consistent with the notion that hypertrophy-induced HIF1a accumulation augments TAG synthesis through coregulation of the glycolytic and glycerolipid biosynthetic branches. Next we asked whether the accumulation of TAG occurred in an environment of reduced fatty acid oxidation. As shown in Figure 4K, expression of HIF1a led to a significant inhibition of the rate of palmitate oxidation, whereas knockdown of HIF1a augmented it (Figure 4K). This effect was recapitulated in vivo using the working heart model, insofar as Hif1a-deficient hearts exhibited marginally increased palmitate oxidation concomitant with a decreased rate of glycolysis (Figures S9F and S9G). Taken together, these results suggest that HIF1a activation promotes both the glycolytic and glycerolipid biosynthetic pathways in hypertrophic hearts at the expense of glucose and fatty acid oxidation. HIF1a Activation Induces PPARg-Dependent Apoptosis in Cardiomyocytes Cardiomyocyte lipid accumulation is reported to promote lipotoxicity and cardiomyocyte apoptosis, leading to cardiac dysfunction and failure (Lee et al., 2004, 2006; McGavock et al., 2006). As both Vhlh-deficient and TAC-stimulated ventricles exhibit increased TAG accumulation, we asked whether these ventricles are also characterized by increased apoptosis. Indeed, TUNEL staining of tissue sections of TAC-induced or Vhlh-deficient ventricles revealed increased DNA strand breaks, indicative of apoptosis (Figures 5A 5C). Counterstaining for sarcomeric a-actinin demonstrated costaining with TUNEL-positive cells, implying that the affected cells are NMCs (Figures 5A 5C). There were little if any TUNEL-positive cells when TAC was applied to Hif1a cko mice (Figures 5A and 5B). Immunoblotting of extracts of ventricles from these genotypes for cleaved caspase 3 confirmed our immunohistochemical data (Figures 5D 5F). Thus, HIF1a function is critical for the induction of apoptosis that accompanies hypertrophy. To determine whether PPARg is a critical downstream effector of HIF1a-dependent apoptosis in response to hypertrophic signals, cultured NMCs were infected with lentiviral shhif1a or shpparg followed by treatment with PE. The latter induced HIF1a accumulation and apoptosis, as evidenced by TUNEL staining (Figure S10A, subpanels e h and q t). Knockdown of HIF1a by shrna blocked HIF1a accumulation and cell death upon PE treatment. Strikingly, depletion of PPARg also inhibited cardiomyocyte apoptosis but did not prevent accumulation of HIF1a in the presence of PE (Figure S10A, subpanels m p and y a2). PPARg knockdown also inhibited cell death that occurred in Vhlh-deficient NMCs. Cell death under these various conditions correlated with increased levels of intracellular TAG, as assessed by BodiPy staining (Figure S10B). Hence, HIF1ainduced cardiomyocyte death depends on PPARg. To further explore the mechanism of PPARg-mediated cell death, we tested various downstream effectors that have been previously implicated in induction of apoptosis. The transcriptional activator Oct1/Pou2f1 is an established PPARg target gene that activates the growth arrest and DNA damage-inducible 45A (GADD45A) gene (Bruemmer et al., 2003; Mueller et al., 2000), whose product promotes the cleavage and activation of caspase 3 (Harkin et al., 1999). The increase in cleaved caspase 3 observed upon both TAC and Vhlh loss led us to address if HIF1a, via PPARg, activated such a pathway in NMCs to promote (F) NMCs were treated with a solvent control or PE in the presence of nsrna, shhif1a, shglut1, shgapdh, shpparg, shcd36, or shgpd1 and assessed for TAG accumulation by oil red O staining and absorbance (510 nm) measurements of extracted lipids (*, p < 0.01 compared to PE-treated nsrna controls). Data are means ± SEM of values from each group. (G J) Ventricular lysates of Hif1a fl/fl or Hif1a cko mice subjected to the sham or TAC protocol and of Vhlh fl/fl or Vhlh cko mice were assessed for endogenous GAPDH (G), GPD1 (H), GPAT (I), and citrate synthase (J) enzymatic activity (*, ç, and #, p < 0.01; **, p < 0.05). Data are means ± SEM of values from four or five mice. (K) NMCs were treated with nsrna, shhif1a, or HIF1a overexpressing viruses and assessed for palmitate oxidation capacity (* and ç, p < 0.01; C, solvent control; PE, phenylepinephrine; Rel., relative). Data are means ± SEM of values from each group. Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc. 519
9 Figure 5. HIF1a Accumulation Correlates with Cardiomyocyte Lipid Accumulation and Apoptosis (A C) Cross-sections of Hif1a fl/fl or Hif1a cko ventricles subjected to the sham (A) or TAC (B) protocol and of Vhlh fl/fl or Vhlh cko ventricles (C) were assessed for DNA double-strand breaks by TUNEL and counterstained for sarcomeric a-actinin and DAPI. (D F) Whole-cell lysates of Hif1a fl/fl or Hif1a cko ventricles subjected to the sham (D) or TAC (E) protocol and of Vhlh fl/fl or Vhlh cko ventricles (F) were assessed for cleaved caspase 3 levels by immunoblotting. Loading was normalized to sarcomeric a-actinin. (G H) NMCs were infected with nsrna, shhif1a, or shpparg or transfected with the HIF1aDPro or PPARg expression vector and assayed for Oct1 (G) or GADD45A (H) expression by qpcr (*, ç, and #, p < 0.01). Data are means ± SEM of values from each group. (I) Oct1 activity in response to HIF1a was determined by transient cotransfection of a synthetic WT or ORE-mutated Oct1 reporter, respectively, fused to luciferase and with either an empty vector control or the HIF1aDPro or PPARg expression construct in the presence or absence of shpparg. All transfections 520 Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc.
10 apoptosis. Overexpression of HIF1a in NMCs led to increased expression of Oct1 and its target gene GADD45A in a PPARgdependent manner (Figures 5G and 5H). Also, PPARg expression induced Oct1 and GADD45A (Figure 5G and 5H). To ascertain if HIF1a-mediated PPARg upregulation led to the induction of endogenous Oct1 activity, we utilized a luciferase reporter containing synthetic Oct1 response elements (OREs). HIF1a or PPARg expression induced luciferase reporter activity in an ORE-dependent manner. Depletion of PPARg inhibited HIF1amediated induction of reporter activity (Figure 5I). Furthermore, using GADD45A promoter-luciferase reporter constructs as readout for Oct1 activity, we demonstrate also that GADD45A promoter activation in response to either HIF1a or PPARg occurs in an ORE-dependent manner (Figure 5J). Importantly, depletion of either Oct1 or GADD45A in NMCs suppressed PPARg-mediated caspase 3 activation (Figure 5K). Knockdown of GADD45A also reduced the extent of PPARg-mediated apoptosis as assayed by TUNEL staining (Figure S10C). Together, these findings suggest the existence of a HIF1a-PPARg-Oct1-GADD45A pathway involved in the generation of cleaved caspase 3, leading to an induction of cardiomyocyte apoptosis. Inhibition of HIF1a Protects from Hypertrophy-Induced Cardiac Dysfunction The protection from hypertrophic stress-induced TAG accumulation and apoptosis conferred by the absence of HIF1a raised the possibility that HIF1a deficiency may lead to improved cardiac function. 1K1C or TAC induced pronounced hypertrophy in Hif1a fl/fl but not Hif1a cko mice (Figures 6A and S11A). Resistance to hypertrophy induction by either TAC or ISO infusion was also observed in mice systemically heterozygous for Hif1a (Hif1a +/ ) (Figures S11B and S11C). Cardiac hypertrophy has been proposed to be a necessary adaptation to pathological stress by enabling the heart to increase its contractile force and output. Despite lack of a cardiac hypertrophic response to TAC in Hif1a cko or Hif1a +/ mice, cardiac performance was improved compared to control mice, as evidenced by the increased fractional shortening and ejection fraction exhibited by Hif1a cko or Hif1a +/ hearts (Table 1, Figures 6B and S11D). In accord with an increased cardiac performance upon loss of HIF1a function, several molecular markers indicative of enhanced performance were upregulated in Hif1a cko ventricles, but not in ventricles of Hif1a fl/fl mice, in response to TAC. These include activation of camp response element binding (CREB), as indicated by the upregulation of Ser133 phosphorylation on CREB, a measure of transcriptionally active CREB and increased Serca2A expression (Figure 6C). Increased CREB activity and Serca2A expression promotes cardiac contractility and has been shown to confer protection from cardiac dysfunction in response to pathological stress (Muller et al., 2003), thus supporting the finding that Hif1a deficiency confers improved cardiac performance in response to pathological stress. DISCUSSION We propose a molecular mechanism underlying the integration of glycolysis and lipid anabolism in cardiomyopathy-induced steatosis involving HIF1a and PPARg. We demonstrate that pathologic stress induces HIF1a and PPARg and identify the latter as a HIF1a target gene product. Cardiac HIF1a accumulation is limited to human and mouse models of pathological hypertrophy and is not observed in physiologic hypertrophy. HIF1a accumulation in the respective disease models correlated with increased PPARg expression. PPARg is the principal mediator of TAG anabolism through its transcriptional regulation of fatty acid uptake (via FAT/CD36), glycerol-3-phosphate generation (via GPD1), and downstream esterification processes (via GPAT). These genes are upregulated specifically in diseased hearts in a HIF1a-dependent manner or upon ablation of Vhlh function in the absence of any hypertrophic signals. This and the fact that PPARg is a direct transcriptional target of HIF1a suggest a mechanism by which pathologic stress-induced HIF1a accumulation translates into changes in cardiac glucose and lipid metabolism (Figure 6D). Stress-mediated activation of the HIF1a-PPARg transcription axis, while increasing GPAT expression, was accompanied by a downregulation of M-Cpt1 expression in diseased ventricles. These changes in gene expression would reinforce a propensity for lipid anabolism over lipid catabolism in a glycolytically predominant diseased state. Indeed, diseased and Vhlh-deficient ventricular biopsies were characterized by high levels of HIF1a, PPARg, and the PPARg target gene products GPD1 and GPAT but lower levels of M-Cpt1 and increased levels of lipid accumulation. These HIF1a-dependent effects on cardiomyocyte lipid metabolism were recapitulated in vitro, where HIF1a led to a considerable inhibition of fatty acid oxidation concomitant with increased glycolysis-mediated TAG synthesis. Conversely, the inhibition of HIF1a increased cardiomyocyte fatty acid oxidation and decreased TAG synthesis, consistent with the protection from steatosis conferred by the lack of ventricular HIF1a in vivo when subjected to pathological stress. HIF1a-dependent development of cardiac steatosis and progression to heart disease in TAC-induced mice was also characterized by increased apoptosis. Cardiomyocyte apoptosis was PPARg dependent and involves an established link between PPARg and the activation of caspase 3 via the Oct1-GADD45A transcriptional pathway. Thus, it appears that stress-induced activation of HIF1a coordinately induces, through PPARg, changes in lipid metabolism by engaging glycerolipid and fatty acid uptake genes and cell death via the activation of Oct1-GADD45A-dependent caspase 3 cleavage. In this regard, increased myocardial contained equal amounts of a b-galactosidase expression vector for normalization of luciferase activity (*, ç, #, and +, p < 0.01). Data are means ± SEM of values from each group. (J) GADD45A promoter activity in response to HIF1a and PPARg was determined by transient cotransfection of the WT GADD45A promoter or of a deletion mutant lacking the ORE, respectively, fused to luciferase and with either an empty vector control or the HIF1aDPro or PPARg expression. All transfections contained equal amounts of a b-galactosidase expression vector for normalization of luciferase activity (*, ç, #, and %, p < 0.01). Data are means ± SEM of values from each group. (K) NMCs were infected with viruses encoding shoct1, shgadd45a, PPARg, or combinations thereof and were probed for Oct1, GADD45A, cleaved caspase 3, and PPARg. Loading was normalized to sarcomeric a-actinin. Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc. 521
11 Figure 6. Ventricular HIF1a Deficiency Attenuates Stress-Induced Pathological Hypertrophy (A) Hif1a fl/fl and Hif1a cko mice subjected to TAC were assessed for development of cardiac hypertrophy by heart weight:body weight ratio measurements (* and #, p < 0.05). Data are means ± SEM of values from eight or ten mice. (B) Contractile function, expressed as % Fractional Shortening (left) and % Ejection Fraction (right) of Hif1a fl/fl and Hif1a cko mice subjected to sham or TAC surgery, was assessed by echocardiography (* and #, p < 0.05). Data are means ± SEM of values from eight or ten mice. (C) Whole-cell lysates of Hif1a fl/fl or Hif1a cko ventricles subjected to the sham or TAC protocol were assessed for HIF1a, PPARg, CREB, CREBpSer133, and Serca2A levels by immunoblotting. Loading was normalized to sarcomeric a-actinin. Broken lines are used to separate the respective sets. All samples were run on the same gel. (D) Schematic representation of the coregulation of glycolysis and glycerolipid synthesis by HIF1a. Hypertrophy-induced HIF1a accumulation is predicted to simultaneously promote the transcriptional upregulation of glycolytic enzymes and the direct transcriptional activation of the PPARg promoter. PPARg activity would lead to the upregulation of its target genes, GPD1 and GPAT, leading to promotion of glycerolipid biosynthesis, culminating in TAG accumulation. A secondary level of coordination is predicted to occur via the shuttling of NADH and NAD + between GAPDH and GPD1. Thus, coordinated regulation of both these components by HIF1a is hypothesized to favor cardiomyocyte lipid accumulation in hypertrophy. PPARg expression has been correlated with enhanced apoptosis and myocardial dysfunction (Son et al., 2007). While these results suggest a potential mechanism of cardiomyocyte apoptosis driven by a HIF1a-PPARg axis, they do not rule out that the reprogramming of cardiac metabolism induced by chronic activa- tion of HIF1a can, in the long term, lead to depletion of metabolic cofactors necessary to sustain cardiac energetics. Based on the mechanism proposed, one might predict a beneficial effect of HIF1a inactivation in heart disease. Indeed, loss of HIF1a upon TAC attenuated myocardial hypertrophy, steatosis, apoptosis, and contractile dysfunction. The lack of hypertrophy in response to TAC is consistent with a previous report; however, our observation of increased cardiac function and cardiomyocyte survival in HIF1a-deficient TAC mice differs from an earlier report (Sano et al., 2007). The difference in phenotypes could relate to the fact that, in our model, HIF1a excision is restricted to ventricular cardiomyocytes, as opposed to the Cre recombinase line utilized by Sano et al., where HIF1a is deleted in both the atria and ventricles. Additionally, Sano et al. used a different HIF1a floxed mouse where Cre recombinase-mediated HIF1a excision results in the generation of a 107 kda truncated protein lacking the 522 Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc.
12 Table 1. Echocardiography Analysis of Sham and TAC-Induced Hif1a fl/fl and Hif1a cko Mice Procedure HIF1a f/f sham HIF1a cko sham LVPW (d) 1.98 ± ± 0.05 LVPW (s) 2.28 ± ± 0.03 LVID (d) 3.00 ± ± 0.09 LVID (s) 1.53 ± ± 0.11 IVS (d) 0.30 ± ± 0.00 IVS (s) 0.43 ± ± 0.11 Velocity across ligature (mm/s) ± ± Procedure HIF1a f/f TAC HIF1a cko TAC LVPW (d) 2.17 ± ± 0.03 LVPW (s) 2.50 ± ± 0.06 LVID (d) 3.10 ± ± 0.15 LVID (s) 1.67 ± ± 0.20 IVS (d) 0.33 ± ± 0.06 IVS (s) 0.38 ± ± 0.20 Velocity across ligature (mm/s) ± ± LVPW, left ventricular posterior wall thickness at diastole (d) and systole (s); LVID, left ventricular internal diameter at diastole (d) and systole (s); IVS, intraventricular septum thickness at diastole (d) and systole (s). Measurements are in millimeter units unless stated otherwise (*, p < 0.05). C-terminal trans-activation domain (Takahashi et al., 2001). This protein species of HIF1a still contains the N-terminal trans-activation domain that has been shown to be transcriptionally active (Ema et al., 1999). Additionally, our observations and interpretations are supported by the fact that loss of ventricular pvhl function led to spontaneous hypertrophy, progressing to heart failure, as observed previously (Lei et al., 2008). Thus, if the loss of HIF1a or pvhl were to be viewed as loss-of-function and gain-of-function models, respectively, the phenotypes described with respect to the loss of HIF1a upon TAC would be consistent with the view that accumulation of HIF1a is sufficient for pathological hypertrophic development, maintenance, and progression to failure, while the inhibition of pathological stress-induced HIF1a accumulation would serve to protect from the detrimental effects of HIF1a and PPARg on cardiac metabolism, survival, and function. This view is supported by the fact that Hif1a +/ mice were similarly protected from TAC-induced hypertrophy and cardiac dysfunction. Finally, our data also suggest that pharmacological inhibition of HIF1a function might have significant therapeutic value in the prevention or reduction in risk of progression to heart disease, whereas agonists of PPARg would be predicted to further accelerate heart disease. Indeed, in mouse studies, the thiazolidinedione class of selective PPARg agonists, such as rosiglitazone, pioglitazone, and T-174, has been found to induce interstitial edema, myocardial ceramide and fatty acid accumulation, cardiomyocyte apoptosis, and eccentric cardiac hypertrophy (Arakawa et al., 2004; Baranowski et al., 2007; Duan et al., 2005). Rosiglitazone and pioglitazone are currently used to treat diabetes in humans and have, in the clinics, demonstrated increased incidence of cardiac failure and death (Nissen and Wolski, 2007). Based on our findings, it is conceivable to propose that application of PPARg agonists in patients with subclinical heart disease may exacerbate HIF1a-PPARg-mediated lipid accumulation and apoptosis and progression toward contractile dysfunction and failure. EXPERIMENTAL PROCEDURES Animal Breeding and Maintenance Hif1a fl/fl and Hif1a +/ mice were obtained from Randall S. Johnson (University of California, San Diego) and Gregg L. Semenza (Johns Hopkins University School of Medicine), respectively. VHL fl/fl mice were provided by Rudolf Jaenisch (Massachusetts Institute of Technology). The MLC2v-cre/+ line was from Ju Chen (University of California, San Diego). Data presented in this manuscript represent studies with male mice. SUPPLEMENTAL DATA Supplemental Data include Supplemental Results, Supplemental Discussion, Supplemental Experimental Procedures, Supplemental Results, and 11 figures andcan befoundonlineat S (09) ACKNOWLEDGMENTS We thank all members of the laboratory and in particular C. Danzer, K. Eckhardt, J. Zehetner, and P. Gerber for discussions. We are grateful to M. Lepore and A. Domenighetti for scientific help and to R. Johnson, G. Semenza, J. Chen, K. Chien, and R. Jaenisch for their mouse lines. We are indebted to W. Wahli (University of Lausanne, Switzerland) and R. Lerch (University Hospital Geneva, Switzerland) for critical reading of the manuscript. Additionally, we thank A. Hirschy, I. Agarkova, and E. Ehler for advice and help, as well as M. Dreiding. The work was supported by the following grants: SCARTNET, sponsored by the Swiss University Conference; grant from the Swiss National Science Foundation (J.C.P.); grant P038/01 from the Gebert- Rüf Foundation (J.C.P.); a Novartis Foundation grant (J.K.); and the Dr. Josef Steiner Cancer grant (W.K.). Received: July 16, 2008 Revised: April 21, 2009 Accepted: May 14, 2009 Published: June 2, 2009 REFERENCES Arakawa, K., Ishihara, T., Aoto, M., Inamasu, M., Kitamura, K., and Saito, A. (2004). An antidiabetic thiazolidinedione induces eccentric cardiac Cell Metabolism 9, , June 3, 2009 ª2009 Elsevier Inc. 523
doi: /nature14508 Rappsilber et al.
 SUPPLEMENTARY INFORMATION doi:1.138/nature1458 Grosso et al. Barbosa et al. 74 72 45 33 47 7 51 Rappsilber et al. Supplementary Figure 1 a, Venn-Diagram of identified splice factors in the work of Barbossa
SUPPLEMENTARY INFORMATION doi:1.138/nature1458 Grosso et al. Barbosa et al. 74 72 45 33 47 7 51 Rappsilber et al. Supplementary Figure 1 a, Venn-Diagram of identified splice factors in the work of Barbossa
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic
 Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
c Ischemia (30 min) Reperfusion (8 w) Supplementary Figure bp 300 bp Ischemia (30 min) Reperfusion (4 h) Dox 20 mg/kg i.p.
 a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
This is an example outline of 3 lectures in BSC (Thanks to Dr. Ellington for sharing this information.)
 This is an example outline of 3 lectures in BSC 2010. (Thanks to Dr. Ellington for sharing this information.) Topic 10: CELLULAR RESPIRATION (lectures 14-16) OBJECTIVES: 1. Know the basic reactions that
This is an example outline of 3 lectures in BSC 2010. (Thanks to Dr. Ellington for sharing this information.) Topic 10: CELLULAR RESPIRATION (lectures 14-16) OBJECTIVES: 1. Know the basic reactions that
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Carbohydrate Metabolism I
 Carbohydrate Metabolism I Outline Glycolysis Stages of glycolysis Regulation of Glycolysis Carbohydrate Metabolism Overview Enzyme Classification Dehydrogenase - oxidizes substrate using cofactors as
Carbohydrate Metabolism I Outline Glycolysis Stages of glycolysis Regulation of Glycolysis Carbohydrate Metabolism Overview Enzyme Classification Dehydrogenase - oxidizes substrate using cofactors as
Glucose is the only source of energy in red blood cells. Under starvation conditions ketone bodies become a source of energy for the brain
 Glycolysis 4 / The Text :- Some Points About Glucose Glucose is very soluble source of quick and ready energy. It is a relatively stable and easily transported. In mammals, the brain uses only glucose
Glycolysis 4 / The Text :- Some Points About Glucose Glucose is very soluble source of quick and ready energy. It is a relatively stable and easily transported. In mammals, the brain uses only glucose
METABOLISM Biosynthetic Pathways
 METABOLISM Biosynthetic Pathways Metabolism Metabolism involves : Catabolic reactions that break down large, complex molecules to provide energy and smaller molecules. Anabolic reactions that use ATP energy
METABOLISM Biosynthetic Pathways Metabolism Metabolism involves : Catabolic reactions that break down large, complex molecules to provide energy and smaller molecules. Anabolic reactions that use ATP energy
Metabolism of cardiac muscle. Dr. Mamoun Ahram Cardiovascular system, 2013
 Metabolism of cardiac muscle Dr. Mamoun Ahram Cardiovascular system, 2013 References This lecture Mark s Basic Medical Biochemistry, 4 th ed., p. 890-891 Hand-out Why is this topic important? Heart failure
Metabolism of cardiac muscle Dr. Mamoun Ahram Cardiovascular system, 2013 References This lecture Mark s Basic Medical Biochemistry, 4 th ed., p. 890-891 Hand-out Why is this topic important? Heart failure
Switzerland; Medicine, University of Lausanne Medical School, 1011 Lausanne, Switzerland
 Dietary obesity-associated Hif1a activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD + system Jaya Krishnan, 1,2 Carsten Danzer, 1,2 Tatiana
Dietary obesity-associated Hif1a activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD + system Jaya Krishnan, 1,2 Carsten Danzer, 1,2 Tatiana
Metabolism. Metabolic pathways. BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 11: Metabolic Pathways
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 11: Metabolic Pathways http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Metabolism Metabolism is the chemical change of
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 11: Metabolic Pathways http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Metabolism Metabolism is the chemical change of
Roles of Lipids. principal form of stored energy major constituents of cell membranes vitamins messengers intra and extracellular
 Roles of Lipids principal form of stored energy major constituents of cell membranes vitamins messengers intra and extracellular = Oxidation of fatty acids Central energy-yielding pathway in animals. O
Roles of Lipids principal form of stored energy major constituents of cell membranes vitamins messengers intra and extracellular = Oxidation of fatty acids Central energy-yielding pathway in animals. O
Metabolic engineering some basic considerations. Lecture 9
 Metabolic engineering some basic considerations Lecture 9 The 90ties: From fermentation to metabolic engineering Recruiting heterologous activities to perform directed genetic modifications of cell factories
Metabolic engineering some basic considerations Lecture 9 The 90ties: From fermentation to metabolic engineering Recruiting heterologous activities to perform directed genetic modifications of cell factories
III. 6. Test. Respiració cel lular
 III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
Probe. Hind III Q,!?R'!! /0!!!!D1"?R'! vector. Homologous recombination
 Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
BCM 221 LECTURES OJEMEKELE O.
 BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
Control. csarnt -/- Cre, f/f
 ody weight (g) A re,f/f re x f/f f/+ re, f/+ re,f/+ f/f x f/f f/+ cs -/- re, f/f re f/f re, f/f Normal chow Tamoxifen Tamoxifen Tamoxifen W 4W re f/f re, re/ff f/f re f/f re, re/ff f/f Normal chow Tamoxifen
ody weight (g) A re,f/f re x f/f f/+ re, f/+ re,f/+ f/f x f/f f/+ cs -/- re, f/f re f/f re, f/f Normal chow Tamoxifen Tamoxifen Tamoxifen W 4W re f/f re, re/ff f/f re f/f re, re/ff f/f Normal chow Tamoxifen
Myocardial lipid accumulation and lipotoxicity in heart failure
 Myocardial lipid accumulation and lipotoxicity in heart failure P. Christian Schulze, MD, PhD New York - Presbyterian Hospital, Columbia University Medical Center, Division of Cardiology, New York, NY,
Myocardial lipid accumulation and lipotoxicity in heart failure P. Christian Schulze, MD, PhD New York - Presbyterian Hospital, Columbia University Medical Center, Division of Cardiology, New York, NY,
What is the Warburg Effect
 What is the Warburg Effect Roles nutrients play in the biochemistry of a cell Thus, proliferating cells must acquire more nutrients, convert them into biosynthetic building blocks, and coordinate the reactions
What is the Warburg Effect Roles nutrients play in the biochemistry of a cell Thus, proliferating cells must acquire more nutrients, convert them into biosynthetic building blocks, and coordinate the reactions
Glycolysis. Degradation of Glucose to yield pyruvate
 Glycolysis Degradation of Glucose to yield pyruvate After this Lecture you will be able to answer: For each step of glycolysis: How does it occur? Why does it occur? Is it Regulated? How? What are the
Glycolysis Degradation of Glucose to yield pyruvate After this Lecture you will be able to answer: For each step of glycolysis: How does it occur? Why does it occur? Is it Regulated? How? What are the
5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM
 5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM Introduction: Variety of hormones and other molecules regulate the carbohydrates metabolism. Some of these have already been cited in previous sections.
5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM Introduction: Variety of hormones and other molecules regulate the carbohydrates metabolism. Some of these have already been cited in previous sections.
Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 TAC 8W TAC 2W. Postn MCM ; R26 mtmg Sham GFP Col 1/3 TAC 8W TAC 2W
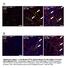 A Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 Tcf21 MCM ; R26 mtmg TAC 2W Tcf21 MCM ; R26 mtmg TAC 8W B Postn MCM ; R26 mtmg Sham GFP Col 1/3 Postn MCM ; R26 mtmg TAC 2W Postn MCM ; R26 mtmg TAC 8W Supplementary
A Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 Tcf21 MCM ; R26 mtmg TAC 2W Tcf21 MCM ; R26 mtmg TAC 8W B Postn MCM ; R26 mtmg Sham GFP Col 1/3 Postn MCM ; R26 mtmg TAC 2W Postn MCM ; R26 mtmg TAC 8W Supplementary
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 16 Glycolysis 2013 W. H. Freeman and Company Chapter 16 Outline Why is glucose such a prominent fuel in all life forms? 1. Glucose
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 16 Glycolysis 2013 W. H. Freeman and Company Chapter 16 Outline Why is glucose such a prominent fuel in all life forms? 1. Glucose
Discussion of Prism modules and predicted interactions (Fig. 4)
 SUPPLEMENTARY NOTES Discussion of Prism modules and predicted interactions (Fig. 4) a. Interactions of the TCA-cycle, respiratory chain, and ATP synthetase with the amino acid biosynthesis modules. Given
SUPPLEMENTARY NOTES Discussion of Prism modules and predicted interactions (Fig. 4) a. Interactions of the TCA-cycle, respiratory chain, and ATP synthetase with the amino acid biosynthesis modules. Given
NAME KEY ID # EXAM 3a BIOC 460. Wednesday April 10, Please include your name and ID# on each page. Limit your answers to the space provided!
 EXAM 3a BIOC 460 Wednesday April 10, 2002 Please include your name and ID# on each page. Limit your answers to the space provided! 1 1. (5 pts.) Define the term energy charge: Energy charge refers to the
EXAM 3a BIOC 460 Wednesday April 10, 2002 Please include your name and ID# on each page. Limit your answers to the space provided! 1 1. (5 pts.) Define the term energy charge: Energy charge refers to the
Fuel the Failing Heart: glucose or fatty acids? Rong Tian, MD, PhD Mitochondria and Metabolism Center University of Washington, Seattle
 Fuel the Failing Heart: glucose or fatty acids? Rong Tian, MD, PhD Mitochondria and Metabolism Center University of Washington, Seattle Metabolic Remodeling: Fatty Acids Carbohydrates PCr/ATP Glucose vs.
Fuel the Failing Heart: glucose or fatty acids? Rong Tian, MD, PhD Mitochondria and Metabolism Center University of Washington, Seattle Metabolic Remodeling: Fatty Acids Carbohydrates PCr/ATP Glucose vs.
SUPPLEMENTARY INFORMATION
 a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
number Done by Corrected by Doctor Nayef Karadsheh
 number 13 Done by Asma Karameh Corrected by Saad hayek Doctor Nayef Karadsheh Gluconeogenesis This lecture covers gluconeogenesis with aspects of: 1) Introduction to glucose distribution through tissues.
number 13 Done by Asma Karameh Corrected by Saad hayek Doctor Nayef Karadsheh Gluconeogenesis This lecture covers gluconeogenesis with aspects of: 1) Introduction to glucose distribution through tissues.
Syllabus for BASIC METABOLIC PRINCIPLES
 Syllabus for BASIC METABOLIC PRINCIPLES The video lecture covers basic principles you will need to know for the lectures covering enzymes and metabolism in Principles of Metabolism and elsewhere in the
Syllabus for BASIC METABOLIC PRINCIPLES The video lecture covers basic principles you will need to know for the lectures covering enzymes and metabolism in Principles of Metabolism and elsewhere in the
Class XI Chapter 14 Respiration in Plants Biology. 1. It is a biochemical process. 1. It is a physiochemical process.
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Energy metabolism - the overview
 Energy metabolism - the overview Josef Fontana EC - 40 Overview of the lecture Important terms of the energy metabolism The overview of the energy metabolism The main pathways of the energy metabolism
Energy metabolism - the overview Josef Fontana EC - 40 Overview of the lecture Important terms of the energy metabolism The overview of the energy metabolism The main pathways of the energy metabolism
CHAPTER 16. Glycolysis
 CHAPTER 16 Glycolysis Net reaction of Glycolysis Converts: 1 Glucose Hexose stage 2 pyruvate - Two molecules of ATP are produced - Two molecules of NAD + are reduced to NADH Triose stage Glucose + 2 ADP
CHAPTER 16 Glycolysis Net reaction of Glycolysis Converts: 1 Glucose Hexose stage 2 pyruvate - Two molecules of ATP are produced - Two molecules of NAD + are reduced to NADH Triose stage Glucose + 2 ADP
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Metabolism. Metabolism. Energy. Metabolism. Energy. Energy 5/22/2016
 5//016 Metabolism Metabolism All the biochemical reactions occurring in the body Generating, storing and expending energy ATP Supports body activities Assists in constructing new tissue Metabolism Two
5//016 Metabolism Metabolism All the biochemical reactions occurring in the body Generating, storing and expending energy ATP Supports body activities Assists in constructing new tissue Metabolism Two
doi: /nature10642
 doi:10.1038/nature10642 Supplementary Fig. 1. Citric acid cycle (CAC) metabolism in WT 143B and CYTB 143B cells. a, Proliferation of WT 143B and CYTB 143B cells. Doubling times were 28±1 and 33±2 hrs for
doi:10.1038/nature10642 Supplementary Fig. 1. Citric acid cycle (CAC) metabolism in WT 143B and CYTB 143B cells. a, Proliferation of WT 143B and CYTB 143B cells. Doubling times were 28±1 and 33±2 hrs for
TIGAR's promiscuity Bolaños, Juan P.
 TIGAR's promiscuity Bolaños, Juan P. TIGAR [TP53 (tumour protein 53)-induced glycolysis and apoptosis regulator] is an important survival factor for cancer cells. The enzymatic activity supported by sequence
TIGAR's promiscuity Bolaños, Juan P. TIGAR [TP53 (tumour protein 53)-induced glycolysis and apoptosis regulator] is an important survival factor for cancer cells. The enzymatic activity supported by sequence
CELLULAR METABOLISM. Metabolic pathways can be linear, branched, cyclic or spiral
 CHM333 LECTURE 24 & 25: 3/27 29/13 SPRING 2013 Professor Christine Hrycyna CELLULAR METABOLISM What is metabolism? - How cells acquire, transform, store and use energy - Study reactions in a cell and how
CHM333 LECTURE 24 & 25: 3/27 29/13 SPRING 2013 Professor Christine Hrycyna CELLULAR METABOLISM What is metabolism? - How cells acquire, transform, store and use energy - Study reactions in a cell and how
Mechanisms of Gene Regulation and Signal! Transduction in Hypoxia!
 Mechanisms of Gene Regulation and Signal! Transduction in Hypoxia! Lorenz Poellinger! Dept. of Cell and Molecular Biology! Karolinska Institutet, Stockholm, Sweden! Normoxia - O 2 availability is in balance
Mechanisms of Gene Regulation and Signal! Transduction in Hypoxia! Lorenz Poellinger! Dept. of Cell and Molecular Biology! Karolinska Institutet, Stockholm, Sweden! Normoxia - O 2 availability is in balance
Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity
 Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity Yuhee Ryu 1,+, Li Jin 1,2+, Hae Jin Kee 1,, Zhe Hao Piao 3, Jae
Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity Yuhee Ryu 1,+, Li Jin 1,2+, Hae Jin Kee 1,, Zhe Hao Piao 3, Jae
Glycolysis. Color index: Doctors slides Notes and explanations Extra information Highlights. Biochemistry Team 437
 Glycolysis Color index: Doctors slides Notes and explanations Extra information Highlights Biochemistry Team 437 ﺑ ﺳ م ﷲ اﻟرﺣﻣن اﻟرﺣﯾم Objectives: Recognize glycolysis as the major oxidative pathway of
Glycolysis Color index: Doctors slides Notes and explanations Extra information Highlights Biochemistry Team 437 ﺑ ﺳ م ﷲ اﻟرﺣﻣن اﻟرﺣﯾم Objectives: Recognize glycolysis as the major oxidative pathway of
ANSC/NUTR 618 Lipids & Lipid Metabolism
 I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
I. Overall concepts A. Definitions ANC/NUTR 618 Lipids & Lipid Metabolism 1. De novo synthesis = synthesis from non-fatty acid precursors a. Carbohydrate precursors (glucose, lactate, and pyruvate) b.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Yield of energy from glucose
 Paper : Module : 05 Yield of Energy from Glucose Principal Investigator, Paper Coordinator and Content Writer Prof. Ramesh Kothari, Professor Dept. of Biosciences, Saurashtra University, Rajkot - 360005
Paper : Module : 05 Yield of Energy from Glucose Principal Investigator, Paper Coordinator and Content Writer Prof. Ramesh Kothari, Professor Dept. of Biosciences, Saurashtra University, Rajkot - 360005
Glycolysis. BCH 340 lecture 3 Chapter 8 in Lippincott 5 th edition
 Glycolysis B 40 lecture hapter 8 in Lippincott 5 th edition All carbohydrates to be catabolized must enter the glycolytic pathway Glycolysis is degradation of glucose to generate energy (ATP) and to provide
Glycolysis B 40 lecture hapter 8 in Lippincott 5 th edition All carbohydrates to be catabolized must enter the glycolytic pathway Glycolysis is degradation of glucose to generate energy (ATP) and to provide
E10.5 E18.5 P2 10w 83w NF1 HF1. Sham ISO. Bmi1. H3K9me3. Lung weight (g)
 Myociyte cross-sectional Relative mrna levels Relative levels Relative mrna levels Supplementary Figures and Legends a 8 6 4 2 Ezh2 E1.5 E18.5 P2 1w 83w b Ezh2 p16 amhc b-actin P2 43w kd 37 86 16 wt mouse
Myociyte cross-sectional Relative mrna levels Relative levels Relative mrna levels Supplementary Figures and Legends a 8 6 4 2 Ezh2 E1.5 E18.5 P2 1w 83w b Ezh2 p16 amhc b-actin P2 43w kd 37 86 16 wt mouse
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
GLYCOLYSIS Generation of ATP from Metabolic Fuels
 GLYCOLYSIS Generation of ATP from Metabolic Fuels - Catabolic process degradative pathway - Energy stored in sugars (carbohydrates) released to perform biological work - Transforms GLUCOSE to PYRUVATE
GLYCOLYSIS Generation of ATP from Metabolic Fuels - Catabolic process degradative pathway - Energy stored in sugars (carbohydrates) released to perform biological work - Transforms GLUCOSE to PYRUVATE
Evidence for an Alternative Glycolytic Pathway in Rapidly Proliferating Cells. Matthew G. Vander Heiden, et al. Science 2010
 Evidence for an Alternative Glycolytic Pathway in Rapidly Proliferating Cells Matthew G. Vander Heiden, et al. Science 2010 Introduction The Warburg Effect Cancer cells metabolize glucose differently Primarily
Evidence for an Alternative Glycolytic Pathway in Rapidly Proliferating Cells Matthew G. Vander Heiden, et al. Science 2010 Introduction The Warburg Effect Cancer cells metabolize glucose differently Primarily
UNIVERSITY OF BOLTON SPORT AND BIOLOGICAL SCIENCES SPORT AND EXERCISE SCIENCE PATHWAY SEMESTER TWO EXAMINATIONS 2016/2017
 LH14 UNIVERSITY OF BOLTON SPORT AND BIOLOGICAL SCIENCES SPORT AND EXERCISE SCIENCE PATHWAY SEMESTER TWO EXAMINATIONS 2016/2017 INTRODUCTION TO SPORT AND EXERCISE PHYSIOLOGY MODULE NO: SPS4002 Date: Thursday
LH14 UNIVERSITY OF BOLTON SPORT AND BIOLOGICAL SCIENCES SPORT AND EXERCISE SCIENCE PATHWAY SEMESTER TWO EXAMINATIONS 2016/2017 INTRODUCTION TO SPORT AND EXERCISE PHYSIOLOGY MODULE NO: SPS4002 Date: Thursday
In vivo bromodeoxyuridine (BrdU) incorporation was performed to analyze cell
 Supplementary Methods BrdU incorporation in vivo In vivo bromodeoxyuridine (BrdU) incorporation was performed to analyze cell proliferation in the heart. Mice were subjected to LI-TAC, and 5 days later
Supplementary Methods BrdU incorporation in vivo In vivo bromodeoxyuridine (BrdU) incorporation was performed to analyze cell proliferation in the heart. Mice were subjected to LI-TAC, and 5 days later
Higher Biology. Unit 2: Metabolism and Survival Topic 2: Respiration. Page 1 of 25
 Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
number Done by Corrected by Doctor Faisal Al-Khatibe
 number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
Cellular Respiration: Harvesting Chemical Energy
 Chapter 9 Cellular Respiration: Harvesting Chemical Energy You should be able to: 1. Explain how redox reactions are involved in energy exchanges. Name and describe the three stages of cellular respiration;
Chapter 9 Cellular Respiration: Harvesting Chemical Energy You should be able to: 1. Explain how redox reactions are involved in energy exchanges. Name and describe the three stages of cellular respiration;
Integration of Metabolism
 Integration of Metabolism Metabolism is a continuous process. Thousands of reactions occur simultaneously in order to maintain homeostasis. It ensures a supply of fuel, to tissues at all times, in fed
Integration of Metabolism Metabolism is a continuous process. Thousands of reactions occur simultaneously in order to maintain homeostasis. It ensures a supply of fuel, to tissues at all times, in fed
EPIGENETIC RE-EXPRESSION OF HIF-2α SUPPRESSES SOFT TISSUE SARCOMA GROWTH
 EPIGENETIC RE-EXPRESSION OF HIF-2α SUPPRESSES SOFT TISSUE SARCOMA GROWTH Supplementary Figure 1. Supplementary Figure 1. Characterization of KP and KPH2 autochthonous UPS tumors. a) Genotyping of KPH2
EPIGENETIC RE-EXPRESSION OF HIF-2α SUPPRESSES SOFT TISSUE SARCOMA GROWTH Supplementary Figure 1. Supplementary Figure 1. Characterization of KP and KPH2 autochthonous UPS tumors. a) Genotyping of KPH2
Cell Injury MECHANISMS OF CELL INJURY
 Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
Cell Injury MECHANISMS OF CELL INJURY The cellular response to injurious stimuli depends on the following factors: Type of injury, Its duration, and Its severity. Thus, low doses of toxins or a brief duration
3.2 Aerobic Respiration
 3.2 Aerobic Respiration Aerobic Cellular Respiration Catabolic pathways Breaks down energy-rich compounds to make ATP Requires oxygen Occurs in different parts of the cell C 6 H 12 O 6 (s) + 6O 2 (g) 6CO
3.2 Aerobic Respiration Aerobic Cellular Respiration Catabolic pathways Breaks down energy-rich compounds to make ATP Requires oxygen Occurs in different parts of the cell C 6 H 12 O 6 (s) + 6O 2 (g) 6CO
Electron Transport Chain and Oxidative phosphorylation
 Electron Transport Chain and Oxidative phosphorylation So far we have discussed the catabolism involving oxidation of 6 carbons of glucose to CO 2 via glycolysis and CAC without any oxygen molecule directly
Electron Transport Chain and Oxidative phosphorylation So far we have discussed the catabolism involving oxidation of 6 carbons of glucose to CO 2 via glycolysis and CAC without any oxygen molecule directly
BIOCHEMISTRY #12 BY: AMMAR AL-HABAHBEH فيصل الخطيب. October 11, 2012
 BIOCHEMISTRY #12 د. فيصل الخطيب October 11, 2012 BY: AMMAR AL-HABAHBEH The Beginning Degradation and synthesis does not occur in a single step but in several steps where sequence of steps converts starting
BIOCHEMISTRY #12 د. فيصل الخطيب October 11, 2012 BY: AMMAR AL-HABAHBEH The Beginning Degradation and synthesis does not occur in a single step but in several steps where sequence of steps converts starting
NBCE Mock Board Questions Biochemistry
 1. Fluid mosaic describes. A. Tertiary structure of proteins B. Ribosomal subunits C. DNA structure D. Plasma membrane structure NBCE Mock Board Questions Biochemistry 2. Where in the cell does beta oxidation
1. Fluid mosaic describes. A. Tertiary structure of proteins B. Ribosomal subunits C. DNA structure D. Plasma membrane structure NBCE Mock Board Questions Biochemistry 2. Where in the cell does beta oxidation
Plant Respiration. Exchange of Gases in Plants:
 Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
Ahmad Ulnar. Faisal Nimri ... Dr.Faisal
 24 Ahmad Ulnar Faisal Nimri... Dr.Faisal Fatty Acid Synthesis - Occurs mainly in the Liver (to store excess carbohydrates as triacylglycerols(fat)) and in lactating mammary glands (for the production of
24 Ahmad Ulnar Faisal Nimri... Dr.Faisal Fatty Acid Synthesis - Occurs mainly in the Liver (to store excess carbohydrates as triacylglycerols(fat)) and in lactating mammary glands (for the production of
CHE 242 Exam 3 Practice Questions
 CHE 242 Exam 3 Practice Questions Glucose metabolism 1. Below is depicted glucose catabolism. Indicate on the pathways the following: A) which reaction(s) of glycolysis are irreversible B) where energy
CHE 242 Exam 3 Practice Questions Glucose metabolism 1. Below is depicted glucose catabolism. Indicate on the pathways the following: A) which reaction(s) of glycolysis are irreversible B) where energy
Aerobic Respiration. The four stages in the breakdown of glucose
 Aerobic Respiration The four stages in the breakdown of glucose 1 I. Aerobic Respiration Why can t we break down Glucose in one step? (Flaming Gummy Bear) Enzymes gently lower the potential energy until
Aerobic Respiration The four stages in the breakdown of glucose 1 I. Aerobic Respiration Why can t we break down Glucose in one step? (Flaming Gummy Bear) Enzymes gently lower the potential energy until
Chapter 13 Carbohydrate Metabolism
 Chapter 13 Carbohydrate Metabolism Metabolism of Foods Food is broken down into carbohydrates, lipids, and proteins and sent through catabolic pathways to produce energy. Glycolysis glucose 2 P i 2 ADP
Chapter 13 Carbohydrate Metabolism Metabolism of Foods Food is broken down into carbohydrates, lipids, and proteins and sent through catabolic pathways to produce energy. Glycolysis glucose 2 P i 2 ADP
OVERVIEW OF THE GLYCOLYTIC PATHWAY Glycolysis is considered one of the core metabolic pathways in nature for three primary reasons:
 Glycolysis 1 Supplemental Reading Key Concepts - Overview of the Glycolytic Pathway Glycolysis generates a small amount of ATP Preview of the ten enzyme-catalyzed reactions of glycolysis - Stage 1: ATP
Glycolysis 1 Supplemental Reading Key Concepts - Overview of the Glycolytic Pathway Glycolysis generates a small amount of ATP Preview of the ten enzyme-catalyzed reactions of glycolysis - Stage 1: ATP
2. What is molecular oxygen directly converted into? a. Carbon Dioxide b. Water c. Glucose d. None of the Above
 Biochem 1 Mock Exam 3 Chapter 11: 1. What is glucose completely oxidized into? a. Carbon Dioxide and Water b. Carbon Dioxide and Oxygen c. Oxygen and Water d. Water and Glycogen 2. What is molecular oxygen
Biochem 1 Mock Exam 3 Chapter 11: 1. What is glucose completely oxidized into? a. Carbon Dioxide and Water b. Carbon Dioxide and Oxygen c. Oxygen and Water d. Water and Glycogen 2. What is molecular oxygen
BCH 4054 Chapter 19 Lecture Notes
 BCH 4054 Chapter 19 Lecture Notes 1 Chapter 19 Glycolysis 2 aka = also known as verview of Glycolysis aka The Embden-Meyerhoff Pathway First pathway discovered Common to almost all living cells ccurs in
BCH 4054 Chapter 19 Lecture Notes 1 Chapter 19 Glycolysis 2 aka = also known as verview of Glycolysis aka The Embden-Meyerhoff Pathway First pathway discovered Common to almost all living cells ccurs in
Pyruvate + NADH + H + ==== Lactate + NAD +
 1 UNIVERSITY OF PAPUA NEW GUINEA SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PBL SEMINAR ANAEROBIC METABOLISM - An Overview
1 UNIVERSITY OF PAPUA NEW GUINEA SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PBL SEMINAR ANAEROBIC METABOLISM - An Overview
number Done by Corrected by Doctor F. Al-Khateeb
 number 23 Done by A. Rawajbeh Corrected by Doctor F. Al-Khateeb Ketone bodies Ketone bodies are used by the peripheral tissues like the skeletal and cardiac muscles, where they are the preferred source
number 23 Done by A. Rawajbeh Corrected by Doctor F. Al-Khateeb Ketone bodies Ketone bodies are used by the peripheral tissues like the skeletal and cardiac muscles, where they are the preferred source
Egr-1 regulates RTA transcription through a cooperative involvement of transcriptional regulators
 /, 2017, Vol. 8, (No. 53), pp: 91425-91444 Egr-1 regulates RTA transcription through a cooperative involvement of transcriptional regulators Roni Sarkar 1 and Subhash C. Verma 1 1 Department of Microbiology
/, 2017, Vol. 8, (No. 53), pp: 91425-91444 Egr-1 regulates RTA transcription through a cooperative involvement of transcriptional regulators Roni Sarkar 1 and Subhash C. Verma 1 1 Department of Microbiology
Chapter 9: Cellular Respiration Overview: Life Is Work. Living cells. Require transfusions of energy from outside sources to perform their many tasks
 Chapter 9: Cellular Respiration Overview: Life Is Work Living cells Require transfusions of energy from outside sources to perform their many tasks Biology, 7 th Edition Neil Campbell and Jane Reece The
Chapter 9: Cellular Respiration Overview: Life Is Work Living cells Require transfusions of energy from outside sources to perform their many tasks Biology, 7 th Edition Neil Campbell and Jane Reece The
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
Cellular Pathways That Harvest Chemical Energy. Cellular Pathways That Harvest Chemical Energy. Cellular Pathways In General
 Cellular Pathways That Harvest Chemical Energy A. Obtaining Energy and Electrons from Glucose Lecture Series 12 Cellular Pathways That Harvest Chemical Energy B. An Overview: Releasing Energy from Glucose
Cellular Pathways That Harvest Chemical Energy A. Obtaining Energy and Electrons from Glucose Lecture Series 12 Cellular Pathways That Harvest Chemical Energy B. An Overview: Releasing Energy from Glucose
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11700 Figure 1: RIP3 as a potential Sirt2 interacting protein. Transfected Flag-tagged Sirt2 was immunoprecipitated from cells and eluted from the Sepharose beads using Flag peptide.
doi:10.1038/nature11700 Figure 1: RIP3 as a potential Sirt2 interacting protein. Transfected Flag-tagged Sirt2 was immunoprecipitated from cells and eluted from the Sepharose beads using Flag peptide.
Krebs cycle Energy Petr Tůma Eva Samcová
 Krebs cycle Energy - 215 Petr Tůma Eva Samcová Overview of Citric Acid Cycle Key Concepts The citric acid cycle (Krebs cycle) is a multistep catalytic process that converts acetyl groups derived from carbohydrates,
Krebs cycle Energy - 215 Petr Tůma Eva Samcová Overview of Citric Acid Cycle Key Concepts The citric acid cycle (Krebs cycle) is a multistep catalytic process that converts acetyl groups derived from carbohydrates,
Biochemistry. Glycolysis. Metabolism of Carbohydrates. Dr.S.K.Khare, Professor IIT Delhi. Principal Investigator.
 Paper : 04 Metabolism of carbohydrates Module :03 Principal Investigator Paper Coordinator Content Reviewer Content Writer Dr.S.K.Khare, Professor IIT Delhi. Dr. Ramesh Kothari, Professor UGC-CAS Department
Paper : 04 Metabolism of carbohydrates Module :03 Principal Investigator Paper Coordinator Content Reviewer Content Writer Dr.S.K.Khare, Professor IIT Delhi. Dr. Ramesh Kothari, Professor UGC-CAS Department
Chapter 9. Cellular Respiration and Fermentation
 Chapter 9 Cellular Respiration and Fermentation Energy flows into an ecosystem as sunlight and leaves as heat Photosynthesis generates O 2 and organic molecules, which are used in cellular respiration
Chapter 9 Cellular Respiration and Fermentation Energy flows into an ecosystem as sunlight and leaves as heat Photosynthesis generates O 2 and organic molecules, which are used in cellular respiration
Integrative Metabolism: Significance
 Integrative Metabolism: Significance Energy Containing Nutrients Carbohydrates Fats Proteins Catabolism Energy Depleted End Products H 2 O NH 3 ADP + Pi NAD + NADP + FAD + Pi NADH+H + NADPH+H + FADH2 Cell
Integrative Metabolism: Significance Energy Containing Nutrients Carbohydrates Fats Proteins Catabolism Energy Depleted End Products H 2 O NH 3 ADP + Pi NAD + NADP + FAD + Pi NADH+H + NADPH+H + FADH2 Cell
CARBOHYDRATE METABOLISM
 Note (Study Glycolysis, fermentation and their regulation, Gluconeogenesis and glycogenolysis, Metabolism of galactose, TCA cycle and Amphibolic role of the cycle, and Glyoxalic acid cycle, HMP shunt in
Note (Study Glycolysis, fermentation and their regulation, Gluconeogenesis and glycogenolysis, Metabolism of galactose, TCA cycle and Amphibolic role of the cycle, and Glyoxalic acid cycle, HMP shunt in
BIOLOGY. Cellular Respiration and Fermentation CAMPBELL. Reece Urry Cain Wasserman Minorsky Jackson
 CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 9 Cellular Respiration and Fermentation Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Figure 9.2 Light energy
CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 9 Cellular Respiration and Fermentation Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Figure 9.2 Light energy
Glucose. Glucose. Insulin Action. Introduction to Hormonal Regulation of Fuel Metabolism
 Glucose Introduction to Hormonal Regulation of Fuel Metabolism Fasting level 3.5-5 mmol (1 mmol = 18 mg/dl) Postprandial 6-10 mmol Amount of glucose in circulation is dependent on: Absorption from the
Glucose Introduction to Hormonal Regulation of Fuel Metabolism Fasting level 3.5-5 mmol (1 mmol = 18 mg/dl) Postprandial 6-10 mmol Amount of glucose in circulation is dependent on: Absorption from the
CHEM121 Unit 2: Carbohydrate Metabolism
 CHEM121 Unit 2: Carbohydrate Metabolism Lecture 3 At the end of the lecture, students should be able to: Define metabolism Discuss the structure and function of ATP in metabolism Discuss glycolysis in
CHEM121 Unit 2: Carbohydrate Metabolism Lecture 3 At the end of the lecture, students should be able to: Define metabolism Discuss the structure and function of ATP in metabolism Discuss glycolysis in
Review of Carbohydrate Digestion
 Review of Carbohydrate Digestion Glycolysis Glycolysis is a nine step biochemical pathway that oxidizes glucose into two molecules of pyruvic acid. During this process, energy is released and some of it
Review of Carbohydrate Digestion Glycolysis Glycolysis is a nine step biochemical pathway that oxidizes glucose into two molecules of pyruvic acid. During this process, energy is released and some of it
Glycolysis Part 2. BCH 340 lecture 4
 Glycolysis Part 2 BCH 340 lecture 4 Regulation of Glycolysis There are three steps in glycolysis that have enzymes which regulate the flux of glycolysis These enzymes catalyzes irreversible reactions of
Glycolysis Part 2 BCH 340 lecture 4 Regulation of Glycolysis There are three steps in glycolysis that have enzymes which regulate the flux of glycolysis These enzymes catalyzes irreversible reactions of
Mitochondria and ATP Synthesis
 Mitochondria and ATP Synthesis Mitochondria and ATP Synthesis 1. Mitochondria are sites of ATP synthesis in cells. 2. ATP is used to do work; i.e. ATP is an energy source. 3. ATP hydrolysis releases energy
Mitochondria and ATP Synthesis Mitochondria and ATP Synthesis 1. Mitochondria are sites of ATP synthesis in cells. 2. ATP is used to do work; i.e. ATP is an energy source. 3. ATP hydrolysis releases energy
PPARa-Sirt1 Complex Mediates Cardiac Hypertrophy and Failure through Suppression of the ERR Transcriptional Pathway
 Article PPARa-Sirt1 Complex Mediates Cardiac Hypertrophy and Failure through Suppression of the ERR Transcriptional Pathway Shinichi Oka, 1 Ralph Alcendor, 1 Peiyong Zhai, 1 Ji Yeon Park, 2 Dan Shao, 1
Article PPARa-Sirt1 Complex Mediates Cardiac Hypertrophy and Failure through Suppression of the ERR Transcriptional Pathway Shinichi Oka, 1 Ralph Alcendor, 1 Peiyong Zhai, 1 Ji Yeon Park, 2 Dan Shao, 1
Cellular Respiration and Fermentation
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
Supplementary Figure 1
 Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Cellular Respiration and Fermentation
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
Fatty Acid Oxidation and Its Relation with Insulin Resistance and Associated Disorders
 Deficiency Disorders in Pediatrics Published online: December 9, 216 Fatty Acid Oxidation and Its Relation with Insulin Resistance and Associated Disorders Gary D. Lopaschuk Department of Pediatrics, University
Deficiency Disorders in Pediatrics Published online: December 9, 216 Fatty Acid Oxidation and Its Relation with Insulin Resistance and Associated Disorders Gary D. Lopaschuk Department of Pediatrics, University
Supplemental Table 1. Plasma NEFA and liver triglyceride levels in ap2-hif1ako and ap2-hif2ako mice under control and high fat diets.
 Supplemental Table 1. Plasma NEFA and liver triglyceride levels in Hif1aKO and Hif2aKO mice under control and high fat diets. Hif1a (n=6) Hif1aK O (n=6) Hif2a Hif2aK O Hif1a (n=5) Hif1aKO (n=5) Hif2a Hif2aK
Supplemental Table 1. Plasma NEFA and liver triglyceride levels in Hif1aKO and Hif2aKO mice under control and high fat diets. Hif1a (n=6) Hif1aK O (n=6) Hif2a Hif2aK O Hif1a (n=5) Hif1aKO (n=5) Hif2a Hif2aK
Cellular Respiration
 Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
Oxidation of Long Chain Fatty Acids
 Oxidation of Long Chain Fatty Acids Dr NC Bird Oxidation of long chain fatty acids is the primary source of energy supply in man and animals. Hibernating animals utilise fat stores to maintain body heat,
Oxidation of Long Chain Fatty Acids Dr NC Bird Oxidation of long chain fatty acids is the primary source of energy supply in man and animals. Hibernating animals utilise fat stores to maintain body heat,
respiration mitochondria mitochondria metabolic pathways reproduction can fuse or split DRP1 interacts with ER tubules chapter DRP1 ER tubule
 mitochondria respiration chapter 3-4 shape highly variable can fuse or split structure outer membrane inner membrane cristae intermembrane space mitochondrial matrix free ribosomes respiratory enzymes
mitochondria respiration chapter 3-4 shape highly variable can fuse or split structure outer membrane inner membrane cristae intermembrane space mitochondrial matrix free ribosomes respiratory enzymes
Both pathways start with Glucose as a substrate but they differ in the product.
 Glycosis:may occur either with the presence or absence of -Glucose-.So with oxygen we have Aerobic glycolysis-, without the participation of oxygen Anaerobic glycolysis-(it occur in certain places) where
Glycosis:may occur either with the presence or absence of -Glucose-.So with oxygen we have Aerobic glycolysis-, without the participation of oxygen Anaerobic glycolysis-(it occur in certain places) where
