Secondary Taste Neurons that Convey Sweet Taste and Starvation in the Drosophila Brain
|
|
|
- Louisa Tyler
- 5 years ago
- Views:
Transcription
1 Article Secondary Taste Neurons that Convey Sweet Taste and Starvation in the Drosophila Brain Highlights d d d d Gustatory projection neurons (sgpns) are involved in sweet taste behaviors sgpn dendrites are close to axon termini of primary sweet taste neurons in the SOG sgpn axons relay sweet taste information to a higher brain center called the Starvation and dopamine signaling increase sucrose sensitivity of the sgpns Authors Pinky Kain, Anupama Dahanukar Correspondence anupama.dahanukar@ucr.edu In Brief Kain and Dahanukar provide insights into the architecture of appetitive circuits by identifying second-order neurons that relay sweet taste from the SOG to the in Drosophila. The identified neurons show increased sucrose sensitivity upon starvation and dopamine signaling. Kain & Dahanukar, 15, Neuron 85, February 18, 15 ª15 Elsevier Inc.
2 Neuron Article Secondary Taste Neurons that Convey Sweet Taste and Starvation in the Drosophila Brain Pinky Kain 1 and Anupama Dahanukar 1, * 1 Department of Entomology, Institute of Integrative Genome Biology, University of California, Riverside, CA 92521, USA *Correspondence: anupama.dahanukar@ucr.edu SUMMARY The gustatory system provides vital sensory information to determine feeding and appetitive learning behaviors. Very little is known, however, about higher-order gustatory circuits in the highly tractable model for neurobiology, Drosophila melanogaster. Here we report second-order sweet gustatory projection neurons (sgpns) in the Drosophila brain using a powerful behavioral screen. Silencing neuronal activity reduces appetitive behaviors, whereas inducible activation results in food acceptance via proboscis extensions. sgpns show functional connectivity with Gr5a + sweet taste neurons and are activated upon sucrose application to the labellum. By tracing sgpn axons, we identify the antennal mechanosensory and motor center () as an immediate higher-order processing center for sweet taste. Interestingly, starvation increases sucrose sensitivity of the sgpns in the, suggesting that hunger modulates the responsiveness of the secondary sweet taste relay. Together, our results provide a foundation for studying gustatory processing and its modulation by the internal nutrient state. INTRODUCTION In gustatory systems, sweet taste neuron activation gives rise to innate behaviors that signify taste acceptance. Behavioral responses to sweet tastants can also be modulated by hunger and satiety (Dethier, 1976; Inagaki et al., 12; Marella et al., 12) and are intimately linked with reward and memory (Krashes et al., 9; Liu et al., 12). These robust and easily quantifiable behaviors, combined with the relative numerical simplicity of the fly brain, provide a tractable model for understanding the structure, function, and modulation of simple circuits in Drosophila. In recent years, such investigations have been helped by significant advances in understanding the molecular and cellular basis of taste sensory responses (Liman et al., 14). With the exception of a Drosophila motor neuron that innervates a proboscis muscle (Gordon and Scott, 9), however, little is known about taste circuits beyond the level of the primary neurons in any insect. Therefore, critical higher-order features of the gustatory system, such as processing and integration of the peripheral signals, remain poorly understood. Taste sensory neurons fall into defined populations that express specific suites of receptors and respond to one of a few described categories of tastants, including sweet, bitter, salty, and water (Cameron et al., 1; Dahanukar et al., 7; Marella et al., 6; Weiss et al., 11; Zhang et al., 13). Sweet taste neurons are marked with the trehalose receptor Gr5a (Chyb et al., 3; Thorne et al., 4; Wang et al., 4), which, along with other closely related receptors (Gr61a and Gr64a-Gr64f), mediates responses to sugars and other sweet tastants (Dahanukar et al., 1; Dahanukar et al., 7; Freeman et al., 14; Jiao et al., 7, 8; Miyamoto et al., 13; Slone et al., 7; Wisotsky et al., 11). The axons of gustatory receptor neurons (GRNs) terminate in the subesophageal ganglion (SOG), the first relay for taste information in the fly brain (Thorne et al., 4; Vosshall and Stocker, 7; Wang et al., 4). Although this region does not bear distinct anatomical landmarks, visualization of axonal termini of distinct categories of GRNs has exposed a spatial representation of taste quality within it. Gr5a + sweet taste neurons terminate in discrete ipsilateral regions of the SOG that do not overlap with axonal projections of bitter taste neurons that express a caffeine receptor, Gr66a (Thorne et al., 4; Wang et al., 4). Activation of Gr5a + neurons causes proboscis extension, a first step in appetitive behavior (Marella et al., 6; Zhang et al., 7). Moreover, Gr5a + neural activity correlates strongly with the degree of acceptance behavior; activators and inhibitors acting on sweet taste neurons can indicate the balance of nutrients and toxins and calibrate feeding behavior accordingly (Charlu et al., 13; Jeong et al., 13). Little is known about higher-order processing of taste information. First, the gustatory neuropil of the SOG, which includes the subesophageal zone (SEZ), gnathal ganglia (GNG), and parts of the periesophageal neuropil (Ito et al., 14), is relatively disorganized compared with the olfactory and visual neuropils, therefore posing a challenge for anatomical identification of gustatory circuits. Second, although it is possible that the SOG encompasses some local circuits that connect sensory, motor, and command neurons that have processes in this region (Flood et al., 13; Gordon and Scott, 9; Melcher and Pankratz, 5; Rajashekhar and Singh, 1994), it is apparent that taste information must also be conveyed to higher brain centers, including the mushroom body, which contains neurons activated upon sucrose ingestion (Liu et al., 12). However, even the location of the secondary taste relay remains a mystery because projection neurons that synapse with taste sensory neurons have not yet Neuron 85, , February 18, 15 ª15 Elsevier Inc. 819
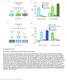
3 been identified. Finally, taste information is also integrated with other internal and external sensory cues, but where this occurs is not known. Here we identify the first sweet gustatory projection neurons (sgpns) in the fly brain. We found that the sgpns are necessary for appetitive responses, including proboscis extension and sucrose feeding behaviors. Moreover, heat-induced activation in flies that express dtrpa1 is sufficient to initiate proboscis extension, suggesting that these neurons may directly participate in sweet taste circuits. We then performed GFP reconstitution across synaptic partners (GRASP) experiments (Feinberg et al., 8; Gordon and Scott, 9), which support the idea of direct connections between the identified sgpns and Gr5a + sweet taste neurons in the labellum. Additionally, we mapped the presynaptic terminals of the sgpns to the antennal mechanosensory and motor center in the deutocerebrum and identify this region as a second relay for sweet taste. Functional analysis using calcium imaging demonstrates that the sgpns are activated by application of sucrose and other sugars to the labellum. The response exhibits concentration dependence and specificity; a nonpalatable sugar does not evoke the same activity. Neither do other categories of tastants, indicating that the identified sgpns are selectively tuned to sweet taste. Finally, we find that sgpn sensitivity to sucrose is enhanced by starvation and dopamine signaling, indicating that information about hunger and satiety is integrated with sweet taste at or before the level of the sgpns. Identification of these neurons will provide valuable insights into the neural architecture of appetitive circuits, such as those that regulate feeding and reward. The sgpns also afford a new tool for dissecting how taste information is processed and integrated with other cues to drive fitting behavioral responses to tastants. RESULTS Identification of Neurons Involved in Sweet Taste Behaviors To identify neurons involved in taste circuits, we performed a genetic screen in which we silenced subsets of neurons and examined the effect on taste behaviors. From a preliminary screen using 455 GAL4 lines from the Nippon (NP) enhancer trap collection (Yoshihara and Ito, ; Table S1), we identified and further characterized the role of one line, NP1562, in appetitive behaviors (Figure 1A). First, we silenced neuronal activity using either tetanus toxin (TNTG) (Sweeney et al., 1995) or an inwardly rectifying potassium channel, Kir2.1 (Baines et al., 1), and examined sucrose feeding behavior in plate assays in which batches of flies were presented with a choice between water and varying concentrations of sucrose. For these experiments, GAL4-dependent expression of TNTG or Kir2.1 was suppressed during development by ubiquitous expression of temperature-sensitive GAL ts. Test flies were raised at 18 C (a GAL ts -permissive temperature) and shifted to 29 C (GAL ts -restrictive temperature) upon eclosion. Control siblings were maintained at 18 C throughout. Wildtype flies and controls carrying either the NP1562-GAL4 or UAS transgenes alone showed little to no temperature-dependent change in feeding, particularly at the higher concentrations tested (Figure 1B). We found that silencing synaptic transmission in adult NP1562 neurons with TNTG caused a significant reduction in sucrose feeding across multiple concentrations (Figure 1C). Similar defects in sucrose feeding were obtained by hyperpolarizing NP1562 neurons with Kir2.1 (Figure 1C). We then used an independent assay to evaluate the role of NP1562 neurons in mediating sensory responses to sucrose. Tarsal taste hairs of flies were stimulated with a series of sucrose concentrations ranging from 1- mm. We observed robust proboscis extension responses in wild-type and GAL4 or UAS control flies, irrespective of whether they were maintained at 18 Cor shifted to 29 C(Figure 1D). By contrast, proboscis extension responses of NP1562 flies were reduced significantly when TNTG was expressed by shifting them to the GAL ts -restrictive temperature (Figure 1E). Taken together, these results suggest that NP1562 labels neurons that play a role in conveying the palatability of sucrose. Activation of NP1562 Neurons Initiates Taste Acceptance We next determined whether inducible activation of NP1562 neurons was sufficient to drive appetitive behavior. For this purpose, we expressed the Drosophila heat-activated channel, dtrpa1 (Hamada et al., 8; Viswanath et al., 3), and asked whether raising the temperature caused proboscis extension. We observed a high frequency of proboscis extension at 32 Cin these flies as compared with wild-type, or control flies with either NP1562 or UAS-dTrpA1 alone (Figure 1F). Individual flies that extended their proboscis did so repeatedly over a 2-min observation period. The proportion of flies that showed proboscis extension was comparable with that obtained by dtrpa1-dependent activation of Gr5a-GAL4 (Chyb et al., 3) sweet taste neurons (Figure 1F). As a negative control, we also compared the effect of heat-inducible activation of bitter taste neurons using Gr89a-GAL4 (Charlu et al., 13), which evoked some proboscis extensions, albeit at a significantly lower frequency among the test animals (Figure 1F). For all genotypes, we observed little proboscis extension at 22 C(Figure 1F). We also found no significant differences in sucrose feeding in flies in which dtrpa1 was activated in NP1562 neurons (3 C) compared with parental controls in binary choice experiments (Figure S1), indicating that NP1562 neurons are likely to be sufficient for initiation of feeding via proboscis extension but not for regulating food intake. Together, the results support post-developmental roles for NP1562 neurons as part of the appetitive taste circuitry. NP1562 Labels Neurons in and around the SOG To identify neurons involved in sweet taste circuits, we examined the expression pattern of NP1562 in the brain using UASmCD8::GFP. Confocal imaging revealed that NP1562 exclusively labeled neurons in the adult brain, including the SOG (Figure 2A). An evaluation of the number of cell bodies in and around the SOG revealed a total of.5 ± 1.13 cells (SEM, n = 14) that could be loosely grouped into three clusters based on their locations: cluster I representing cells in or adjacent to the antennal mechanosensory and motor center (), cluster II representing cell bodies near the dorsal anterior of the SOG, and cluster III representing cell bodies distributed near the ventral region of the SOG (Figure 2B). Notably, NP1562 did not label any taste sensory 8 Neuron 85, , February 18, 15 ª15 Elsevier Inc.
4 A Identify defects in feeding behavior + Measure proboscis extension responses NP-GAL4 silence with TNTG or Kir2.1 activate with dtrpa1 B % sucrose feeding * 29 C 18 C * 1mM 1mM mm 1mM 1mM mm 1mM 1mM mm 1mM 1mM mm C % sucrose feeding * * 29 C 18 C * * * * * * * 1mM 1mM mm * 29 C 18 C * * * * 1mM 1mM mm wild type NP1562 UAS-TNTG UAS-Kir2.1 NP1562 >TNTG NP1562 > Kir2.1 D % PER response C 18 C * 1mM 1mM mm 1mM 1mM mm 1mM 1mM mm 1mM 1mM mm wild type NP1562 UAS-TNTG UAS-Kir2.1 E % PER response * 29 C 18 C * * * * * * 1mM 1mM mm NP1562 >TNTG F % PER response C 22 C wild type dtrpa1 control Gr89a control Gr89a > dtrpa1 Gr5a control Gr5a > dtrpa1 NP1562 control NP1562 > dtrpa1 Figure 1. NP1562 Labels Neurons Associated with Appetitive Behaviors (A) Schematic for characterizing behavioral outcomes of manipulating neuronal function in selected NP-GAL4 lines. (B) Mean sucrose feeding responses of wild-type and the indicated control flies. Flies were raised at 18 C and shifted to the indicated temperature for 3 days prior to behavior experiments. For each bar, n = 6 trials of flies each. *p <.5, **p <.1, two-way ANOVA with pairwise comparisons. (C) Mean sucrose feeding responses of NP1562-GAL4 flies expressing either UAS-TNTG or UAS-Kir2.1. For each bar, n = 6 trials of flies each. Flies were raised at 18 C and shifted to the indicated temperature for three days prior to behavior experiments. *p <.5, **p <.1, ***p <.1, two-way ANOVA with pairwise comparisons. (D) Proboscis extension responses of wild-type and various controls to the indicated concentrations of sucrose. For each bar, n = 3 sets of flies each (wild-type) or n = 5 sets of 1 flies each (transgene controls). *p <.5, two-way ANOVA with pairwise comparisons. (E) Results of proboscis extension tests with the indicated concentrations of sucrose. For each bar, n = 3 sets of flies each. *p <.5, **p <.1, ***p <.1, two-way ANOVA with pairwise comparisons. (F) Percentages of flies of the indicated genotypes showing proboscis extension at 22 C or after a 2-min period on a 32 C heating block. For each genotype, n =. For all graphs, error bars = SEM. neurons in labellar, pharyngeal, or tarsal tissues (Figure 2C). Therefore, the sucrose feeding defects observed for NP1562 may occur via neurons that act downstream of sweet taste neurons or impinge on sweet circuits in some way. Cluster I NP1562 Neurons Send Dendrites to the Sweet Taste Region of the SOG We next examined whether any of the NP cell clusters had dendritic projections in the SOG. For these experiments, we Neuron 85, , February 18, 15 ª15 Elsevier Inc. 821
5 A C D E F Labellum μm Cluster I Cluster II Cluster III NP1562-GAL4 > GFP SOG DenMark Pharynx G α-gfp α-nc82 B NP1562-GAL4 > GFP NP1562-GAL4 > GFP Cluster I AL AL Cluster I Cluster II SOG 3 μm nc82 Gr5a-LexA > GFP Gr5a > SpGFP 11 Cluster III Tarsi merge NP1562 > SpGFP 1-1 expressed a dendritic marker, DenMark, in NP1562 neurons and visualized its distribution using immunohistochemistry. We observed staining of dendritic processes in the SOG (Figure 2D) and, in particular, in an area that has been described previously to receive input from labellar sweet GRNs (Thorne et al., 4; Wang et al., 4). To determine which of the three NP cell clusters in and around the SOG send projections to the sweet taste region, we created 3D images of the SOG to trace the origin of neurites that terminate in the characteristic pattern of sweet GRNs. Confocal stacks encompassing the SOG/ areas were visualized as 3D structures using Bitplane Imaris software, which revealed that neurites in the sweet taste region are derived from cells belonging to cluster I (Figure 2E; Movie S1). Notably, neurons of clusters II or III did not send processes to this area. NP1562 Neurons GRASP with Sweet Taste Neurons in the SOG We next investigated whether dendrites of NP1562 neurons lie in close proximity to axon terminals of Gr5a + sweet taste neurons, which are distributed in a characteristic pattern within the SOG (Figure 2F). For this purpose, we used the GRASP split-gfp approach first developed in Caenorhabditis elegans (Feinberg et al., 8) and subsequently adopted in flies (Gordon and Scott, 9). One-half of the split-gfp GRASP reporter was expressed in Gr5a + GRNs using Gr5a-LexA (lexaopspgfp 11 ::CD4), whereas the other half was expressed in NP1562 neurons using UAS-spGFP 1-1 ::CD4. GRASP was visualized using an antibody that does not efficiently recognize either spgfp 11 or spgfp 1-1 alone (Gordon and Scott, 9; Figure 2G). Remarkably, we observed a GFP signal indicating GRASP in the region of Gr5a + sweet taste projections in the SOG (Figure 2H). Immunolabeling with a different GFP antibody yielded similar results (Figure S2). Therefore, the dendrites of at least a subset of NP1562 neurons lie in close proximity to the axonal termini of Gr5a + taste neurons. Taken together with the observation that NP neurons of clusters II and III do not send neurites to the area that receives input from sweet GRNs, these results support the idea that neurons belonging to cluster I are likely to be sgpns. H Gr5a-LexA > SpGFP 11 NP1562-GAL4 > SpGFP 1-1 GRASP GRASP Figure 2. NP1562 Labels Neurons in the Primary Gustatory Center that May Connect with Gr5a + Sweet Taste Neurons (A) NP1562-GAL4-driven expression of UAS-mCD8::GFP in the whole brain (visualized with a-gfp, green). (B) NP1562-GAL4-driven expression of UAS-mCD8::GFP in the SOG and surrounding region, including the. The schematic below indicates locations of cell bodies (n = 14) in three identified clusters in and around the SOG and areas. (C) Bright-field images of the labellum (Labellum), labral sense organ (Pharynx), and distal segments of the foreleg (Tarsi) derived from NP1562-GAL4; UASmCD8::GFP flies. (D) Confocal images of the SOG in NP1562-GAL4; UAS-DenMark flies stained with a-dsred (green). The arrowheads indicate the region of similarity with the characteristic pattern of Gr5a + sweet neuron projections (compare with F). (E) 3D images of the SOG and surrounding areas to trace the origin of neurites (green) that terminate in the characteristic pattern of sweet GRNs, indicated by arrowheads (compare with F). (F) Axon terminals of Gr5a + sweet taste neurons in the SOG visualized with a-gfp (green) in flies of the genotype Gr5a-LexA; lexaop-mcd8::gfp. (G) Images of the SOG stained with a-gfp (green). Genotypes were as follows: Gr5a-LexA, lexaop- SpGFP 11 ::CD4 (Gr5a > SpGFP 11 ) and NP1562-GAL4; UAS-SpGFP 1-1 ::CD4 (NP1562 > SpGFP 1-1 ). (H) Illustration of the GRASP technique to identify putative synaptic connections between Gr5a + and NP neurons (left). Shown is a representative image of the SOG in NP1562-GAL4/Gr5a-LexA, lexaop-cd4::spgfp 11 ; UAS- CD4::SpGFP 1-1 flies stained with a-gfp (green). n =. For all brain images, the neuropil is stained with a-nc82 (red). Confocal stacks were acquired at 1-mm optical sections. Scale bars, 1 mm (unless noted otherwise). 822 Neuron 85, , February 18, 15 ª15 Elsevier Inc.
6 A B α-ha α-nc82 NP1562-GAL4 > UAS-syt-HA SOG AL OL NP1562-GAL4 > UAS-syt-HA AL OL Figure 3. NP1562 Neurons Project to the Antennal and Mechanosensory Motor Center (A) Visualization of pre-synaptic terminals of NP1562-GAL4 neurons with a-ha (green). The neuropil is stained with a-nc82 (red). Flies were NP1562/+; UAS-syt-HA/+ (n = ). Confocal stacks were acquired at 1-mm optical sections. Scale bars, 1 mm. OL, optic lobe. (B) Compressions of the indicated groups of images from the z stack shown in (A). Scale bar, 1 mm. (C) 3D model of the confocal image shown in (A), generated using Imaris software (left), and expanded views of syt-ha distribution in the (center and right). C 4-9 μm 1-16 μm 17- μm μm Posterior SOG AL 1 μm 45 NP1562 Candidate sgpns Project to the Antennal Mechanosensory and Motor Center in the Brain The regions of the brain to which taste information is relayed from the SOG are not known. We therefore defined the architecture of the NP1562 sgpns and mapped their putative presynaptic terminals by expressing synaptotagmin-hemagglutinin (HA) (Robinson et al., 2). Immunolocalization with a-ha showed extensive labeling in the of the deutocerebrum (Figure 3A). Magnified images of the revealed punctate patterns of syt-ha localization consistent with synaptic structures (Figure 3B). A 3D model generated from the confocal z stack highlights the distribution of synaptic densities in the anterior lateral region of the (Figure 3C). These results suggest that NP1562 neurons convey information from the SOG to the and implicate the as an immediate higher-order processing center for sweet taste information. sgpns in the Activate Proboscis Extension Given that NP1562-GAL4 labels several neurons in the brain, we performed a mosaic analysis to more precisely identify the subset(s) responsible for triggering taste acceptance. We used heat shock to flip out GAL (tub-frt-gal-frt) (Bohm et al., 1) and induce NP1562-dependent expression of both UASmCD8::GFP and UAS-dTrpA1 in subsets of NP cells. Eggs were collected in six different batches, and each cohort was treated to a 1-hr heat shock at 37 C at a single selected time point between 24 and 62 hr after collection. We tested 2 adult males of the appropriate genotype for heatinduced proboscis extension response and recovered a total of 11 responders that showed proboscis exten- Anterior sion at 32 C (54%) and 92 nonresponders that did not (46%) (Figure 4A). None of the flies displayed proboscis extension at room temperature (22 C). We then dissected the brains of 1 flies (98 responders and 62 non-responders) and visualized GFP-labeled clones using immunofluorescence. Each brain was scored for the presence of GFP signal in regions representing the three clusters labeled by NP1562 (Figure 2B). Notably, one or more cells of cluster I were found to be GFP + in 95 of 98 brains recovered from responder flies (Figures 4B and 4C). By contrast, all 62 non-responders lacked a GFP signal in this region. Cells belonging to clusters II and III were labeled in 58 and of the 98 responders (59% and 61%, respectively) and 9 and 12 of the 62 non-responders (15% and 19%, respectively), indicating that they are unlikely to be primarily responsible for the proboscis extension phenotype. We also examined 22 control flies that were not subjected to heat shock treatment. As predicted, none showed heat-inducible proboscis extension or GFP expression in any cells in the brain (data not shown). We performed a similar experiment to label dendritic and axonal processes in NP neurons that evoke proboscis extension by inducing clones that co-express dtrpa1, DenMark, and syt-enhanced GFP (egfp). Immunolabeling of brains recovered from responder flies revealed postsynaptic termini in the SOG and presynaptic termini in the SOG and regions (Figure 4D). Taken together, our results argue that one or more NP cells belonging to cluster I, projecting from the SOG to the, are involved in proboscis extension behavior. sgpns Are Activated by Sucrose To test whether the identified sgpns do, in fact, process sweet taste information, we used NP1562-GAL4 to express the fluorescent calcium indicator GCaMP3 (Tian et al., 9) and measured Neuron 85, , February 18, 15 ª15 Elsevier Inc. 823
7 A time points for heat shock (1-hr, 37 C) egg collection eclosion test % flies tested Hours Days n=44 24 Responders (PER + ) at 32 C Non-responders (PER ) at 32 C n=32 n=34 n=3 n=37 n= Time of heat shock treatment after egg laying (hrs) C % brains with GFP + cells in Cluster I n=95/ n=/62 PER + PER at 32 C B NP1562, UAS-dTrpA1/tub-flipout-GAL; UAS-mCD8::GFP/hs-flp Non-responders Responders D NP1562, UAS-dTrpA1/tub-flipout-GAL; UAS-DenMark, UAS-syt::eGFP Responders syt-egfp DenMark nc82 merge Figure 4. Mosaic Analysis Reveals a Subset of Neurons in the Region that Mediate Taste Acceptance Behavior (A) Proportion of responder and non-responder NP1562-GAL4, UAS-dTrpA1/tub > GAL > ; hs-flp, Sb/UAS-mCD8::GFP flies recovered from cohorts subjected to a single heat shock treatment at one of the time points indicated in schematic above. Flies were tested for proboscis extension at 32 C. (B) Representative images of GFP + clones in the SOG and regions derived from responder and non-responder NP1562-GAL4, UAS-dTrpA1/tub > GAL > ; hs-flp, Sb/UAS-mCD8::GFP flies identified in (A). Adult brains were stained with a-gfp (green) and a-nc82 (red) to visualize the neuropil. Dashed circles indicate the region encompassing cells belonging to cluster I. Scale bars, 1 mm. (C) Proportion of brains of responder (PER + ) and non-responder (PER ) flies showing GFP labeling in one or more cells of cluster I. (D) Distribution of DenMark and syt-egfp in clones of responder NP1562-GAL4, UAS-dTrpA1/tub > GAL > ; hs-flp, Sb/UAS-DenMark, UAS-syt::eGFP flies. Adult brains were stained with a-dsred (red), a-gfp (green), and a-nc82 (blue) to visualize the neuropil. Scale bars, 1 mm. changes in fluorescence upon application of sucrose to the proboscis. We found that a stimulus of mm sucrose caused a robust increase in fluorescence in the and in neuronal arbors extending from the SOG to the in a bilaterally symmetrical V-shaped pattern (Figures 5A and 5B; Figure S3; Movie S2). Little to no change was observed in GCaMP3 fluorescence with water solvent alone (Figure 5B). As a positive control, we imaged activity in axonal termini of Gr5a + sweet neurons (Marella et al., 6), which showed a similar increase in fluorescence when stimulated with sucrose but not with water (Figures 5A and 5B; Figure S3; Movie S3). At least five flies were tested for each genotype and stimulus. In all cases, we monitored changes 824 Neuron 85, , February 18, 15 ª15 Elsevier Inc.
8 A Mean fluorescence intensity Sucrose 1 NP1562 sgpns Pre Post region of interest background High Low Mean fluorescence intensity Sucrose 1 Gr5a sweet GRNs Pre Post region of interest background Frame (#) 7 Frame (#) 7 NP1562 Gr5a SOG High Low B 2 water sucrose *** *** C NP1562-GAL4 > GCaMP3 1 mm mm mm mm D Gr5a-GAL4 > GCaMP3 1 mm mm mm mm Post Pre Post Pre Mean fluorescence intensity 2 1 NP1562-GAL4 > GCaMP3 mm mm mm 1 mm Frame (#) Mean fluorescence intensity 2 1 Gr5a-GAL4 > GCaMP3 Frame (#) mm mm mm 1 mm NP1562-GAL4 > GCaMP3 Gr5a-GAL4 > GCaMP Figure 5. NP1562 sgpns Neurons Are Activated by Sucrose (A) Representative color-coded images and mean intensity traces of mm sucrose-evoked GCaMP3 fluorescence in NP (left) and Gr5a + (right) neurons in the fly brain. Sucrose was applied to the proboscis. Fluorescence intensity measurements were taken from areas within dashed circles: region of interest (red) and background (white). (B) Mean changes in GCaMP3 fluorescence in NP or Gr5a + neurons, as indicated, upon stimulation of the proboscis with water or mm sucrose( n = 5 7). ***p <.1, Student s t test. (C) Representative color-coded images, mean intensity traces, and mean fluorescence changes in NP neurons upon application of the indicated concentrations of sucrose to the proboscis. The genotype was NP1562-GAL4/CyO; UAS-GCaMP3/UAS-GCaMP3 (n = 7 for each stimulus). (D) Representative color-coded images, mean intensity traces, and mean fluorescence changes in Gr5a + neurons upon application of the indicated concentrations of sucrose to the proboscis. The genotype was Gr5a-GAL4/Gr5a-GAL4; UAS-GCaMP3/UAS-GCaMP3 (n = 7 for each stimulus). Scale bars, mm. For all graphs, error bars show SEM. Neuron 85, , February 18, 15 ª15 Elsevier Inc. 825
9 A C E NP1562 > GCaMP3 Glucose Fructose Trehalose NP1562 > GCaMP3 NP1562 > GCaMP3; ΔGr64a ** *** *** 25 sucrose (mm) NP1562 > GCaMP3 in background fluorescence in areas abutting the GFP-labeled regions, which changed only slightly, if at all, with tastant application (Figure 5A). We next characterized the sucrose response of NP (Figure 5C) and Gr5a + (Figure 5D) neurons across a range of concentrations (1 mm). For both the primary (Gr5a) and putative secondary (NP1562) populations of sweet taste neurons, we observed robust concentration-dependent increases in calcium activity (Figures 5C and 5D). These results demonstrate that NP sgpns in the brain are activated by sucrose. sgpns Are Functionally Connected to Gr5a + Sweet Taste Neurons Our results suggest that NP sgpns receive input from Gr5a + taste neurons. Given that Gr5a + taste neurons are broadly tuned to sugars and sweet stimuli (Dahanukar et al., 7; Mar- B 1 D 1 NP1562 > GCaMP3 Ribose (mm) NP1562 > GCaMP3 NP1562 > GCaMP3; Gr5a > TrpA1 warm water Caffeine (mm) HCl (ph) NaCl (mm) Serine (mm) ** Figure 6. NP1562 sgpns Are Functionally Connected to Gr5a + Sweet Taste Neurons (A) Mean fluorescence changes of GCaMP3 in NP neurons upon stimulation of the proboscis with the indicated sugars, each tested at mm (n = 5). p <.1 for each stimulus, Student s t test versus water. (B) Mean fluorescence changes of GCaMP3 in NP neurons upon stimulation of the proboscis with the indicated concentrations of ribose (n = 5). p =.684 ( mm) and p =.27 ( mm), Student s t test versus water. (C) Mean fluorescence changes of GCaMP3 in NP neurons in otherwise wild-type (NP1562>GCaMP3) or Gr64a 1 /Gr64a 1 (NP1562>GCaMP3; DGr64a) flies upon stimulation of the proboscis with the indicated concentrations of sucrose (n = 5). **p <.1, ***p <.1, two-way ANOVA with pairwise comparisons. (D) Mean fluorescence changes of GCaMP3 in NP neurons in flies of the genotypes NP1562-GAL4; UAS-GCaMP3/UAS-GCaMP3 (NP1562>GCaMP3) and NP1562-GAL4/Gr5a-LexA; UAS-GCaMP3/lexAop-dTrpA1 (NP1562 > GCaMP3; Gr5a > dtrpa1) upon stimulation with warm water. Water was heated to C, drawn in a pipette tip, cooled briefly, and applied to the proboscis. **p <.1, Student s t test. (E) Mean fluorescence changes of GCaMP3 in NP1562 neurons upon stimulation of the proboscis with the named tastants at the indicated concentrations. For each stimulus, n = 5. Student s t tests versus water. Caffeine, p =.493 ( mm) and p =.135 ( mm); HCl, p =.5 (ph 1.8) and p =.822 (ph 3); NaCl, p =.498 ( mm) and p =.442 ( mm); serine, p =.29 ( mm) and p =.243 ( mm). For all graphs, error bars indicate SEM. ella et al., 6), we next tested glucose, fructose, and trehalose, and found that NP sgpns were also activated by application of these sugars to the proboscis (Figure 6A). To confirm that the observed response was dependent on sweet taste rather than osmolarity, we tested ribose, a sugar that is not palatable to flies, and observed no increase in calcium activity in NP sgpns (Figure 6B). These results are consistent with the idea that sgpns are responsive to sweet taste and not to non-gustatory stimulation caused by application of the tastant solution. The sensitivity of Gr5a + neurons to sucrose is reduced in flies lacking the Gr64a receptor (Dahanukar et al., 7; Jiao et al., 7). If NP1562 neurons are indeed receiving input from Gr5a + taste neurons, then one expectation is that sucroseinduced activity in NP1562 neurons would be diminished in DGr64a mutants. In fact, comparison of GCaMP3 fluorescence changes in wild-type and DGr64a mutant flies showed reduced responses in NP neurons of the mutant across a range of concentrations tested (Figure 6C). To corroborate these results, we wanted to determine whether artificial stimulation of Gr5a + sweet taste neurons would cause activation of NP1562 neurons. For this purpose, we expressed dtrpa1 in Gr5a + neurons and stimulated them by application of warm water. We observed a robust increase in fluorescence in NP neurons, which 826 Neuron 85, , February 18, 15 ª15 Elsevier Inc.
10 was absent in control flies that did not express dtrpa1 in sweet taste neurons (Figure 6D). Within the SOG, sweet and bitter tastants are represented in discrete, non-overlapping areas. We therefore wished to determine whether there was any overlap of taste input to the identified sgpns. We applied stimuli belonging to other taste categories bitter (caffeine), acid (hydrochloric acid), salt (sodium chloride), and amino acid (serine) and measured calcium activity in NP1562-GAL4>GCaMP3 flies. None of the tested stimuli evoked changes greater than 5% over baseline activity (Figure 6E). Taken together, our results argue that the NP sgpns do not integrate information from different taste categories but, instead, have a dedicated role in processing sweet taste. Starvation Increases Sugar Sensitivity of sgpns It has long been known that hunger increases behavioral sensitivity to sucrose in flies (Dethier, 1976; Edgecomb et al., 1994). One expectation from these observations is that activity in sweet taste circuits may be modulated by starvation. In fact, a recent study reported increased calcium activity in axon terminals of sweet taste neurons in starved flies (Inagaki et al., 12), albeit only at sucrose concentrations of mm or higher. We therefore wished to determine whether responses of NP sgpns were altered in flies that were deprived of food. We compared the calcium activity in fed and starved flies to a sucrose concentration range (1 mm) known to evoke different levels of proboscis extension responses in flies subjected to the two conditions. Flies were starved on wet tissues for 24 hr. We first measured the sucrose response in NP sgpns and found that starvation for 24 hr led to a significant increase in the sensitivity of these neurons to 25 and mm sucrose (Figure 7A). By contrast, although a previous study has shown that calcium activity of Gr5a + presynaptic terminals was enhanced by starvation in younger flies (Inagaki et al., 12), we did not observe any significant differences in sucrose responses of fed flies and starved flies under our test conditions (Figure 7B). Exposure of the flies to a different stressor by means of vortexing them did not cause a similar increase in calcium activity in NP neurons (Figure 7C). Recent studies have shown that dopamine signaling plays a critical role in reducing behavioral thresholds to sucrose upon starvation (Inagaki et al., 12; Marella et al., 12). To test whether the activity of NP1562 neurons is modulated via dopamine signaling, we administered 3-(3,4-dihydroxyphenyl-2,5,6- d 3 )-L-alanine (L-Dopa) via food and measured sucrose-evoked calcium activity in the NP sgpns. Consistent with the results of starved flies, we found that NP neurons in flies fed on L-Dopa showed significantly enhanced responses to 25 and mm sucrose (Figure 7D). On the other hand, we did not observe corresponding changes in sensitivity of Gr5a + neurons (Figure 7E). Taken together, our results indicate that the activity of NP1562 sgpns appears to be modulated by starvation in a manner that can communicate altered reward values to higher brain centers. Moreover, at the lower concentrations of sucrose that engender significantly different behavioral responses between fed and starved flies (Dethier, 1976), the observed dopamine-dependent modulation appears to be independent of changes in Gr5a + neuron activity. DISCUSSION Sugars are innately attractive to most animals. To understand how sweet taste activates appropriate feeding behaviors, higher-order neurons in taste circuits must be identified. Here we present the first example of Drosophila gustatory projection neurons that receive sweet taste information at the first relay (Figure 8). The identified sgpns terminate in the antennal mechanosensory and motor centers and are specifically activated by sucrose but not by other categories of tastants. Moreover, the activity of the identified sgpns reflects both sweet taste and the starvation state. Therefore, information about the starvation state is conveyed to sweet taste circuits prior to the second relay. Our results support a role for the identified sgpns in primary taste circuits that connect sweet taste input to canonical feeding acceptance behaviors; the neurons are excited by sweet taste and are both necessary and sufficient to initiate proboscis extension. In wild-type flies, proboscis extension can be induced by stimulation of either labellar or tarsal taste hairs (Dethier, 1976). Within the SOG there exist distinct representations of taste neurons originating from the two organs (Stocker, 1994; Thorne et al., 4; Wang et al., 4). One question that arises, therefore, is where the information from the two is integrated. Although our analysis of GRASP connections with NP1562 sgpns was restricted to Gr5a + labellar sweet taste neurons, the finding that silencing of the former also weakens tarsally induced proboscis extension responses (PERs) suggests that information coming from sweet taste neurons in the labellum and the tarsi must be integrated either upstream of or at NP1562 neurons. An initial imaging analysis was consistent with the former possibility because stimulation of tarsi with mm sucrose did not result in any calcium activity in NP1562 neurons (DF/F.4417 ±.7661, SEM, n = 5). Moreover, silencing of NP1562 neurons did not completely abolish proboscis extension, indicating that additional classes of sgpns that convey sweet taste are likely to exist. Other first-order taste neurons fall into defined, molecularly marked populations that are activated by water (ppk28 + ), salty (Ir76b + ), or bitter (Gr66a + ) tastants (Cameron et al., 1; Marella et al., 6; Weiss et al., 11; Zhang et al., 13). Although we did not find evidence for excitatory responses to any of these classes of tastants in NP1562 neurons, it will nevertheless be interesting to determine how activation of these neurons impinges on NP1562 sgpn activity. One confounding factor is that many tastants that inhibit proboscis extension and feeding act not only via activating bitter taste neurons (Weiss et al., 11) but also by inhibiting sweet taste neurons (Charlu et al., 13; Jeong et al., 13; Meunier et al., 3). However, the ability to artificially manipulate the activity of defined classes of taste neurons should facilitate addressing such questions. The SOG also contains dendrites of motor neurons that innervate the proboscis and cibarial pumps (Gordon and Scott, 9; Manzo et al., 12; Rajashekhar and Singh, 1994), leading to expectations of direct connections between sensory and motor neurons or of local interneurons that connect the two. However, Neuron 85, , February 18, 15 ª15 Elsevier Inc. 827
11 A NP1562-GAL4 > GCaMP3 B Gr5a-GAL4 > GCaMP Fed Fed Starved *** 1 Starved 1 ** Normalized mean Fed Starved 1 Normalized mean Fed Starved 1 C NP1562-GAL4 > GCaMP3 2 Fed Fed Vortexed 1 Vortexed Normalized mean D NP1562-GAL4 > GCaMP3 E Gr5a-GAL4 > GCaMP Food Food Food + L-Dopa 2 Food + L-Dopa ** 1 *** 1 1 *** Normalized mean Food Food + L-Dopa 1 Normalized mean Food Food + L-Dopa 1 Figure 7. Starvation Increases the Sucrose Sensitivity of NP1562 sgpns (A) Mean fluorescence changes of GCaMP3 in NP neurons in control flies (fed) or flies that were wet-starved for 24 hr (starved). Starved flies were tested in parallel with fed flies, for which the data are the same as in Figure 5C. The graph on the right shows responses normalized to those of fed flies for each sucrose concentration. The genotype was NP1562-GAL4/CyO; UAS-GCaMP3/UAS-GCaMP3 (n = 5 7). **p <.1, **p <.1, two-way ANOVA with pairwise comparisons. (B) Mean fluorescence changes of GCaMP3 in Gr5a + neurons in control flies (fed) or flies that were wet-starved for 24 hr (starved). Starved flies were tested in parallel with fed flies, for which the data are the same as in Figure 5D. The graph on the right shows responses normalized to those of fed flies for each sucrose concentration. The genotype was Gr5a-GAL4/Gr5a-GAL4; UAS-GCaMP3/UAS-GCaMP3 (n = 5 7). There was no statistically significant difference between fed and starved flies. p >.5, two-way ANOVA with pairwise comparisons. (C) Mean fluorescence changes of GCaMP3 in NP neurons in flies subjected to stress by vortexing (n = 5 7). There was no statistically significant difference between the two conditions. p >.5, two-way ANOVA with pairwise comparisons. (D) Mean fluorescence changes of GCaMP3 in NP neurons of flies fed food supplemented with L-Dopa for 2 days. The genotype was as in (A). Responses were compared with those of flies fed food without L-Dopa, for which the data are the same as in Figure 5C (n = 5 7). **p <.1, ***p <.1, two-way ANOVA with pairwise comparisons. (legend continued on next page) 828 Neuron 85, , February 18, 15 ª15 Elsevier Inc.
12 Starvation (dopamine) Sucrose SOG NP1562 sgpns Gr5a sweet GRNs Labellum SAD Proboscis extension Feeding Figure 8. Model for Sweet Taste Processing in Drosophila Shown is a schematic illustrating the identified components of sweet taste circuits, highlighting possible substrates for starvation-induced increases in sucrose sensitivity. other studies have defined SOG interneurons that express the neuropeptide hugin and connect with higher brain centers and neuroendocrine glands (Melcher and Pankratz, 5). Moreover, dopaminergic protocerebral anterior medial neurons in the mushroom body have also been shown to be activated by labellar stimulation with sucrose (Liu et al., 12; Mao and Davis, 9). These observations lead to the supposition that higher brain centers are also involved in processing sweet taste. Our findings delineate a relay of taste information from the SOG to the and now invite investigations into whether the conveys information from NP neurons to higher centers in the brain or back to the SOG, where it can potentially be conveyed to motor neurons that connect to muscles in the proboscis. The has not been implicated previously in taste processing but, rather, is known to receive input from sensory axons of the basal antennal segments involved in sensing sound, wind, and gravity (Homberg et al., 1989; Kamikouchi et al., 6, 9; Yorozu et al., 9). A recent study also found a new class of olfactory projection neurons that send axons to the (Awasaki et al., 14). Having identified this region as a secondary center for sweet taste, a future question is whether inputs from other categories of taste neurons, such as water (Cameron et al., 1; Inoshita and Tanimura, 6), bitter (Thorne et al., 4; Wang et al., 4), and salty (Zhang et al., 13), are also conveyed to the and, if so, whether the representation of different tastes remains distinct in this region. Little is understood about where information from the is transmitted, but wiring diagrams of the Drosophila brain developed from single-cell tracing experiments reveal the caudal ventrolateral protocerebrum (CVLP) as a possible target (Chiang et al., 11). This idea is supported by a previous observation that some Gr32a + GRNs involved in pheromone sensing appear to terminate directly in the VLP (Miyamoto and Amrein, 8). Identification of axonal connections of projection neurons will facilitate the analysis of higher-order circuitry that integrates sweet input with other tastes and other sensory cues. Our observations also provide direct confirmation for statedependent alterations in sweet taste circuit activity. We observe starvation state-dependent differences in the sucrose response of sgpns at concentrations as low as 25 mm. An increase in dopamine signaling also brings about an enhancement of sgpn sensitivity to sucrose. In both cases, increases in sucrose-induced calcium activity occur in the absence of corresponding changes in Gr5a + neural activity. This is in contrast to previous observations that starvation led to increases in sucrose-evoked electrophysiological (Meunier et al., 7; Nishimura et al., 12) or calcium activity in Gr5a + taste neurons (Inagaki et al., 12). In most cases, the observed increases in GRN sensitivity were relatively small in magnitude compared with the alterations in NP sgpn activity of starved flies. Taken together with previous observations, our results open up the possibility that information about the starvation state is amplified during the relay to NP neurons or that these neurons may also be targets of signaling pathways that convey information about the starvation state (Figure 8). Recent studies have identified additional neurons in the SOG whose activity depends on starvation: tyrosine hydroxylase ventral unpaired medial (TH-VUM) dopaminergic neurons that modulate feeding in response to nutritional needs (Marella et al., 12) and feeding (Fdg) interneurons that integrate gustatory input with the internal state to command a feeding behavior routine (Flood et al., 13). However, neither the TH-VUM nor Fdg neurons are likely to make direct connections with sweet taste neurons. Sweet taste is of prominence for both innate and learned behaviors. Our identification of second-order sweet taste neurons will enable investigations into the interplay between sweet taste circuits and other sweet- and starvationresponsive neurons that will be of the utmost importance in understanding the neural basis of behavior. EXPERIMENTAL PROCEDURES Fly Stocks w 1118 (BL 595), UAS-TNTG (BL 28838), UAS-dTrpA1 (BL and BL 26264), UAS-mCD8::GFP (BL 513 and BL 5137), lexaop-mcd8::gfp (BL (E) Mean fluorescence changes of GCaMP3 in Gr5a + neurons of flies fed food supplemented with L-Dopa for 2 days. The genotype was as in (B). Responses were compared with those of flies fed food without L-Dopa, for which the data are the same as in Figure 5D (n = 5 7). There was no statistically significant difference between flies treated under the two conditions. p >.5, two-way ANOVA with pairwise comparisons. For all graphs, error bars indicate SEM. Neuron 85, , February 18, 15 ª15 Elsevier Inc. 829
13 323), UAS-DenMark, UAS-syt::eGFP (BL 3365), UAS-GCaMP3 (BL and BL 32116), tub-gal ts (BL 717), and hs-flp (BL 279) were obtained from the Drosophila Bloomington Stock Center. NP-GAL4 lines were obtained from the Drosophila Genetic Resource Center. UAS-syt-HA was provided by L. Luo (Stanford University); UAS-Kir2.1, UAS > CD2 > CD8::GFP, Gr5a- LexA, lexa-op-cd4::spgfp 11, and UAS-CD4::SpGFP 1-1 by K. Scott (University of California); lexa-op-dtrpa1 by M. Ramaswami (Trinity College); and tub>gal > by B. Zhang (University of Missouri). Drosophila stocks were reared on standard cornmeal dextrose medium at 25 C, unless specified otherwise. Immunohistochemistry Immunohistochemistry with brain tissue was as described previously (Kain et al., 13). Flies were anesthetized on ice, and the brain tissue was dissected in chilled 13 PBS and fixed for 3 min in 4% paraformaldehyde (.3% Triton X-) at room temperature. After washes with PBST (PBS with.3% Triton X-), samples were blocked with 5% normal goat serum in PBST. Samples were incubated in appropriate primary antibody solutions for 3 nights at 4 C. Primary antibodies used were as follows: mouse anti-gfp (1:, Sigma, catalog no. 52M4836), rabbit anti-gfp (1:, Invitrogen, catalog no. A11122), chicken anti-gfp (1:1,, Abcam, catalog no. ab1397), rabbit anti-dsred (1:, Clontech Laboratories, catalog no ), mouse anti-nc82 (1:1, Developmental Studies Hybridoma Bank), and rabbit anti-ha (1:, Abcam, catalog no. ab911). Secondary antibodies used were as follows: Alexa Fluor 488 anti-rabbit, anti-chicken, and anti-mouse immunoglobulin G (IgG) (1:, Invitrogen); Alexa Flour 546 anti-rabbit and anti-mouse IgG (1:, Invitrogen); and Alexa Fluor 647 anti-mouse IgG (1:, Invitrogen). Samples were analyzed with a Leica SP5 confocal microscope, and image stacks were acquired at 1-mm optical sections. Images were processed using ImageJ, Adobe Photoshop, and Illustrator software. 3D models and movies of the SOG were derived using Bitplane Imaris software (v7.6.4). Confocal stacks were used to create multiple surfaces demarcating the SOG,, and antennal lobe (AL) regions and segment regions of interest. SOG,, and AL areas were delineated manually by drawing boundaries on the basis of neuropil staining patterns with a-nc82. The soma and processes of GFP-labeled neurons were captured based on fluorescence intensity. GFP Imaging Proboscises and legs were dissected and mounted in 7% glycerol in PBST. GFP and red fluorescent protein (RFP) fluorescence was visualized using a Leica SP5 confocal microscope, and image stacks were generated from 1-mm optical sections. Feeding Behavior Assays For binary choice feeding assays, flies with the genotype w 1118, transgene controls, and NP-GAL4/+;UAS-Kir2.1,Tub-Gal ts or NP-GAL4/UAS-TNTG; Tub-GAL ts /+ were raised from eggs to adults at the GAL ts -permissive temperature of 18 C. Flies were sorted in vials of 1 males and 1 females ( flies/vial) upon eclosion and maintained at 18 C for 3 days, after which half of the vials prepared for each genotype-stimulus combination were shifted to the GAL ts -restrictive temperature of 29 C, where they remained for 3 additional days. Flies at 18 C (control) were starved for 36.5 hr and those at 29 C (test) for 16.5 hr prior to behavioral testing. Starvation times comparable with 24 hr at 25 C were extrapolated from starvation survival curves performed at 18 C and 29 C, respectively. Flies were tested as described previously (Charlu et al., 13), and abdominal coloration was scored as pink (dark pink coloration in more than 25% of the abdomen), light pink (faint pink coloration or dark pink color limited to a small spot), or none (no visible pink coloration). Participation in sucrose feeding was calculated as follows: ð# flies with pink abdomenþ + :5ð#flies with light pink abdomenþ 3 : # total flies PER Assays For experiments with UAS-TNTG, flies were raised and subjected to temperature shifts and starvation conditions as for the feeding experiments. Prior to the experiment, flies were immobilized by cooling on ice and then mounted using nail polish, vertical aspect up, on glass slides. Flies were allowed to recover in a moist chamber for 2 hr prior to testing. Tastant solutions prepared in water were applied to tarsi via a drop extruded from the hypodermic needle of a 1-ml plastic syringe. Flies were first satiated with water and then tested with sucrose solutions. Ingestion of sucrose solutions was not permitted, and, following each sucrose application, flies were retested with water as a negative control. Each fly was tested five times with each sucrose stimulus. The interval between consecutive sucrose applications was at least 3 min to minimize adaptation. Flies showing three or more proboscis extensions were considered responders. For heat-inducible PER measurements, NP-GAL4 flies with UAS-dTrpA1 were raised at room temperature (22 C), immobilized on slides as above, and transferred to a heating block at 32 C. Proboscis extensions were scored after 2 min. For all PER experiments, three sets of flies each were tested, and the percentage of responders was calculated for each set. Graphs depict mean responses, and error bars indicate SEM. Clonal Analysis GFP-labeled clones were generated in flies using transgenes to flip-in GFP or flip-out GAL. Flies of the genotypes NP1562-GAL4, UAS-dTrpA1/tub > GAL > ; UAS-mCD8::GFP/hs-Flp or NP1562-GAL4, UAS-dTrpA1/tub > GAL > ; UAS-DenMark, UAS-syt::eGFP/hs-Flp were recovered from a single heat shock treatment of 1 hr at 37 C, which was performed 24, 3,, 48, 52, or 62 hr after a 4- to 7-hr period of egg collection. Flies aged 3 6 days were tested in PER and immunohistochemistry assays as described. Calcium Imaging Flies used for imaging of calcium activity were at least 2 weeks old. Single flies were immobilized on a bridge made by stacking glass coverslips on a slide. The proboscis was held down in an extended position by double-sided tape so that the labellum was accessible for tastant application. The fly body was fixed between the coverslips so that the body and legs were trapped and the head remained exposed with the anterior aspect up. Antennae and cuticle were removed to expose the brain, which was immediately covered with modified adult hemolymph-like solution (Wang et al., 3). GCaMP3 fluorescence in the SOG and regions of the brain was visualized with a Leica SP2 confocal microscope with a 3 air objective and digital zoom R 3 for initial experiments with water and mm sucrose. Samples were excited with a 488-nm laser, and emissions were collected through a 5 53 band-pass filter. Images were acquired at one frame every 3 s at a resolution of pixels. The pinhole was opened to allow single thick section scans. 7 frames were acquired for each tastant application, starting at least 1 frames before application of the stimulus and 9 frames during and after stimulus application. Taste solutions prepared in distilled water were dispensed on the proboscis (2- to 4-ml drop) with a -ml pipettor. Distilled water was used as a control. Only flies in which baseline GFP fluorescence could be observed in the areas of Gr5a + or NP projections were used for subsequent tests. Leica SP2 software and ImageJ were used to calculate minimum and maximum fluorescence intensities in selected regions of interest for each stimulus application, which was used to calculate DF/F. For experiments in Figures 7D and 7E, L-Dopa (Sigma-Aldrich, catalog no. CAS ) was first dissolved in water (5 mg/ml). The freshly prepared solution was spread on fly food, and flies were maintained on this food for 2 days in the dark. The medium was changed once after 24 hr, and freshly made L-Dopa solution was added. For the experiments in Figure 7C, 1 flies contained in a 1.5-ml Eppendorf tube were vortexed three times for 15 s each, with 5-s intervals in between. Vortexed flies were used immediately for imaging. Statistical Analyses Unless otherwise stated, results from behavioral experiments were analyzed using two-way ANOVA with pairwise comparisons using a Bonferroni adjustment. Statistical comparisons of calcium activity were performed using either Student s t test or two-way ANOVA with pairwise comparisons using a Bonferroni adjustment. 83 Neuron 85, , February 18, 15 ª15 Elsevier Inc.
14 SUPPLEMENTAL INFORMATION Supplemental Information includes three figures, one table, and three movies and can be found with this article online at ACKNOWLEDGMENTS We thank D. Carter for help with in vivo calcium imaging; Z. Wisotsky for help with Bitplane Imaris analyses; A. Ganguly and L. Pang for help with pushing flies; and A. Ray, G. Pask, C. Scott, and Z. Wisotsky for comments on the manuscript. This work was funded in part by a Whitehall Foundation grant (to A.D.). Received: June 2, 14 Revised: August 29, 14 Accepted: January 8, 15 Published: February 5, 15 REFERENCES Awasaki, T., Kao, C.F., Lee, Y.J., Yang, C.P., Huang, Y., Pfeiffer, B.D., Luan, H., Jing, X., Huang, Y.F., He, Y., et al. (14). Making Drosophila lineagerestricted drivers via patterned recombination in neuroblasts. Nat. Neurosci. 17, Baines, R.A., Uhler, J.P., Thompson, A., Sweeney, S.T., and Bate, M. (1). Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, Bohm, R.A., Welch, W.P., Goodnight, L.K., Cox, L.W., Henry, L.G., Gunter, T.C., Bao, H., and Zhang, B. (1). A genetic mosaic approach for neural circuit mapping in Drosophila. Proc. Natl. Acad. Sci. USA 17, Cameron, P., Hiroi, M., Ngai, J., and Scott, K. (1). The molecular basis for water taste in Drosophila. Nature 465, Charlu, S., Wisotsky, Z., Medina, A., and Dahanukar, A. (13). Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat. Commun. 4, 42. Chiang, A.S., Lin, C.Y., Chuang, C.C., Chang, H.M., Hsieh, C.H., Yeh, C.W., Shih, C.T., Wu, J.J., Wang, G.T., Chen, Y.C., et al. (11). Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr. Biol. 21, Chyb, S., Dahanukar, A., Wickens, A., and Carlson, J.R. (3). Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl. Acad. Sci. USA (Suppl 2 ), Dahanukar, A., Foster, K., van der Goes van Naters, W.M., and Carlson, J.R. (1). A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 4, Dahanukar, A., Lei, Y.T., Kwon, J.Y., and Carlson, J.R. (7). Two Gr genes underlie sugar reception in Drosophila. Neuron 56, Dethier, V.G. (1976). The Hungry Fly. (Cambridge: Harvard University Press). Edgecomb, R.S., Harth, C.E., and Schneiderman, A.M. (1994). Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J. Exp. Biol. 197, Feinberg, E.H., Vanhoven, M.K., Bendesky, A., Wang, G., Fetter, R.D., Shen, K., and Bargmann, C.I. (8). GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57, Flood, T.F., Iguchi, S., Gorczyca, M., White, B., Ito, K., and Yoshihara, M. (13). A single pair of interneurons commands the Drosophila feeding motor program. Nature 499, Freeman, E.G., Wisotsky, Z., and Dahanukar, A. (14). Detection of sweet tastants by a conserved group of insect gustatory receptors. Proc. Natl. Acad. Sci. USA 111, Gordon, M.D., and Scott, K. (9). Motor control in a Drosophila taste circuit. Neuron 61, Hamada, F.N., Rosenzweig, M., Kang, K., Pulver, S.R., Ghezzi, A., Jegla, T.J., and Garrity, P.A. (8). An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, Homberg, U., Christensen, T.A., and Hildebrand, J.G. (1989). Structure and function of the deutocerebrum in insects. Annu. Rev. Entomol. 34, Inagaki, H.K., Ben-Tabou de-leon, S., Wong, A.M., Jagadish, S., Ishimoto, H., Barnea, G., Kitamoto, T., Axel, R., and Anderson, D.J. (12). Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell 148, Inoshita, T., and Tanimura, T. (6). Cellular identification of water gustatory receptor neurons and their central projection pattern in Drosophila. Proc. Natl. Acad. Sci. USA 13, Ito, K., Shinomiya, K., Ito, M., Armstrong, J.D., Boyan, G., Hartenstein, V., Harzsch, S., Heisenberg, M., Homberg, U., Jenett, A., et al.; Insect Brain Name Working Group (14). A systematic nomenclature for the insect brain. Neuron 81, Jeong, Y.T., Shim, J., Oh, S.R., Yoon, H.I., Kim, C.H., Moon, S.J., and Montell, C. (13). An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron 79, Jiao, Y., Moon, S.J., and Montell, C. (7). A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mrna tagging. Proc. Natl. Acad. Sci. USA 14, Jiao, Y., Moon, S.J., Wang, X., Ren, Q., and Montell, C. (8). Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 18, Kain, P., Boyle, S.M., Tharadra, S.K., Guda, T., Pham, C., Dahanukar, A., and Ray, A. (13). Odour receptors and neurons for DEET and new insect repellents. Nature 2, Kamikouchi, A., Shimada, T., and Ito, K. (6). Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J. Comp. Neurol. 499, Kamikouchi, A., Inagaki, H.K., Effertz, T., Hendrich, O., Fiala, A., Göpfert, M.C., and Ito, K. (9). The neural basis of Drosophila gravity-sensing and hearing. Nature 458, Krashes, M.J., DasGupta, S., Vreede, A., White, B., Armstrong, J.D., and Waddell, S. (9). A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, Liman, E.R., Zhang, Y.V., and Montell, C. (14). Peripheral coding of taste. Neuron 81, 984. Liu, C., Plaçais, P.Y., Yamagata, N., Pfeiffer, B.D., Aso, Y., Friedrich, A.B., Siwanowicz, I., Rubin, G.M., Preat, T., and Tanimoto, H. (12). A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488, Manzo, A., Silies, M., Gohl, D.M., and Scott, K. (12). Motor neurons controlling fluid ingestion in Drosophila. Proc. Natl. Acad. Sci. USA 19, Mao, Z., and Davis, R.L. (9). Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 3, 5. Marella, S., Fischler, W., Kong, P., Asgarian, S., Rueckert, E., and Scott, K. (6). Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49, Marella, S., Mann, K., and Scott, K. (12). Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron 73, Melcher, C., and Pankratz, M.J. (5). Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 3, e35. Meunier, N., Marion-Poll, F., Rospars, J.P., and Tanimura, T. (3). Peripheral coding of bitter taste in Drosophila. J. Neurobiol. 56, Meunier, N., Belgacem, Y.H., and Martin, J.R. (7). Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J. Exp. Biol. 21, Miyamoto, T., and Amrein, H. (8). Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 11, Neuron 85, , February 18, 15 ª15 Elsevier Inc. 831
15 Miyamoto, T., Chen, Y., Slone, J., and Amrein, H. (13). Identification of a Drosophila glucose receptor using Ca2+ imaging of single chemosensory neurons. PLoS ONE 8, e5634. Nishimura, A., Ishida, Y., Takahashi, A., Okamoto, H., Sakabe, M., Itoh, M., Takano-Shimizu, T., and Ozaki, M. (12). Starvation-induced elevation of taste responsiveness and expression of a sugar taste receptor gene in Drosophila melanogaster. J. Neurogenet. 26, Rajashekhar, K.P., and Singh, R.N. (1994). Organization of motor neurons innervating the proboscis musculature in Drosophila melanogaster meigen (Diptera: Drosophilidae). Int. J. Insect. Morphol. Embryol. 23, Robinson, I.M., Ranjan, R., and Schwarz, T.L. (2). Synaptotagmins I and IV promote transmitter release independently of Ca(2+) binding in the C(2)A domain. Nature 418, Slone, J., Daniels, J., and Amrein, H. (7). Sugar receptors in Drosophila. Curr. Biol. 17, Stocker, R.F. (1994). The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 275, Sweeney, S.T., Broadie, K., Keane, J., Niemann, H., and O Kane, C.J. (1995). Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, Thorne, N., Chromey, C., Bray, S., and Amrein, H. (4). Taste perception and coding in Drosophila. Curr. Biol. 14, Tian, L., Hires, S.A., Mao, T., Huber, D., Chiappe, M.E., Chalasani, S.H., Petreanu, L., Akerboom, J., McKinney, S.A., Schreiter, E.R., et al. (9). Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, Viswanath, V., Story, G.M., Peier, A.M., Petrus, M.J., Lee, V.M., Hwang, S.W., Patapoutian, A., and Jegla, T. (3). Opposite thermosensor in fruitfly and mouse. Nature 423, Vosshall, L.B., and Stocker, R.F. (7). Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 3, Wang, J.W., Wong, A.M., Flores, J., Vosshall, L.B., and Axel, R. (3). Twophoton calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, Wang, Z., Singhvi, A., Kong, P., and Scott, K. (4). Taste representations in the Drosophila brain. Cell 117, Weiss, L.A., Dahanukar, A., Kwon, J.Y., Banerjee, D., and Carlson, J.R. (11). The molecular and cellular basis of bitter taste in Drosophila. Neuron 69, Wisotsky, Z., Medina, A., Freeman, E., and Dahanukar, A. (11). Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat. Neurosci. 14, Yorozu, S., Wong, A., Fischer, B.J., Dankert, H., Kernan, M.J., Kamikouchi, A., Ito, K., and Anderson, D.J. (9). Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 458, 1 5. Yoshihara, M., and Ito, K. (). Improved Gal4 screening kit for large-scale generation of enhancer-trap strains. Drosoph. Inf. Serv. 83, Zhang, W., Ge, W., and Wang, Z. (7). A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur. J. Neurosci. 26, Zhang, Y.V., Ni, J., and Montell, C. (13). The molecular basis for attractive salt-taste coding in Drosophila. Science 3, Neuron 85, , February 18, 15 ª15 Elsevier Inc.
16 Neuron Supplemental Information Secondary Taste Neurons that Convey Sweet Taste and Starvation in the Drosophila Brain Pinky Kain and Anupama Dahanukar
17 % sucrose feeding C NP1562/+ NP1562 > dtrpa1 1mM 1mM dtrpa1/+ NP1562 Sucrose Figure S1, related to Figure 1. Heat-inducible activation of NP1562 neurons does not affect sucrose feeding behavior. Mean sucrose feeding responses of NP1562 flies expressing UAS-dTrpA1 along with NP1562 alone and UAS-dTrpA1 alone controls. For each bar, n = 6 trials of ~ flies each. Flies were raised at 22 C and feeding assays were performed at 3 C to activate dtrpa1. Error bars indicate S.E.M.
18 Gr5a-LexA > CD4::spGFP 11 ; NP1562 > CD4::spGFP 1-1 Invitrogen α-gfp α-nc82 Sigma α-gfp Figure S2, related to Figure 2. NP1562 labels neuronal processes in the SOG that lie in close proximity with axonal terminals of Gr5a+ sweet taste neurons. GRASP visualized in the SOG with α-gfp obtained from Invitrogen (left) or Sigma (right) in flies carrying the NP1562 driver along with Gr5a-LexA, lexaop-cd4::spgfp 11, and UAS-CD4::SpGFP 1-1. The image on the left (taken from Figure 2F) is shown for comparison, neuropil is stained with α-nc82 (red). Arrowheads indicate locations of regions that receive input from Gr5a+ sweet taste neurons.
19 NP1562-GAL4 > GCaMP3 mm sucrose SOG Gr5a-GAL4 > GCaMP3 mm sucrose SOG Figure S3, related to Figure 5. Sucrose-evoked changes in GCaMP3 fluorescence in the and SOG regions of the brain. Images of the SOG and surrounding regions identifying the areas that show sucrose-evoked changes of GCaMP3 fluorescence in NP1562 > GCaMP3 and Gr5a > GCaMP3 flies. Areas within the dashed regions indicate the SOG and as labeled.
100 mm Sucrose. +Berberine +Quinine
 8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
8 mm Sucrose Probability (%) 7 6 5 4 3 Wild-type Gr32a / 2 +Caffeine +Berberine +Quinine +Denatonium Supplementary Figure 1: Detection of sucrose and bitter compounds is not affected in Gr32a / flies.
Supplementary Figure 1. Procedures to independently control fly hunger and thirst states.
 Supplementary Figure 1 Procedures to independently control fly hunger and thirst states. (a) Protocol to produce exclusively hungry or thirsty flies for 6 h water memory retrieval. (b) Consumption assays
Supplementary Figure 1 Procedures to independently control fly hunger and thirst states. (a) Protocol to produce exclusively hungry or thirsty flies for 6 h water memory retrieval. (b) Consumption assays
50mM D-Glucose. Percentage PI. L-Glucose
 Monica Dus, Minrong Ai, Greg S.B Suh. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute cotransporter in Drosophila. Control + Phlorizin mm D-Glucose 1 2mM 1 L-Glucose Gr5a;Gr64a
Monica Dus, Minrong Ai, Greg S.B Suh. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute cotransporter in Drosophila. Control + Phlorizin mm D-Glucose 1 2mM 1 L-Glucose Gr5a;Gr64a
doi: /nature09554
 SUPPLEMENTARY INFORMATION doi:10.1038/nature09554 Supplementary Figure 1: Optical Tracing with New Photoactivatable GFP Variants Reveals Enhanced Labeling of Neuronal Processes We qualitatively compare
SUPPLEMENTARY INFORMATION doi:10.1038/nature09554 Supplementary Figure 1: Optical Tracing with New Photoactivatable GFP Variants Reveals Enhanced Labeling of Neuronal Processes We qualitatively compare
Supplementary Figure 1. Flies form water-reward memory only in the thirsty state
 1 2 3 4 5 6 7 Supplementary Figure 1. Flies form water-reward memory only in the thirsty state Thirsty but not sated wild-type flies form robust 3 min memory. For the thirsty group, the flies were water-deprived
1 2 3 4 5 6 7 Supplementary Figure 1. Flies form water-reward memory only in the thirsty state Thirsty but not sated wild-type flies form robust 3 min memory. For the thirsty group, the flies were water-deprived
Supplemental Information. Memory-Relevant Mushroom Body. Output Synapses Are Cholinergic
 Neuron, Volume 89 Supplemental Information Memory-Relevant Mushroom Body Output Synapses Are Cholinergic Oliver Barnstedt, David Owald, Johannes Felsenberg, Ruth Brain, John-Paul Moszynski, Clifford B.
Neuron, Volume 89 Supplemental Information Memory-Relevant Mushroom Body Output Synapses Are Cholinergic Oliver Barnstedt, David Owald, Johannes Felsenberg, Ruth Brain, John-Paul Moszynski, Clifford B.
Supplemental Information. Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila. Supplemental Inventory
 1 Current Biology, Volume 22 Supplemental Information Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila Marie P. Suver, Akira Mamiya, and Michael H. Dickinson Supplemental
1 Current Biology, Volume 22 Supplemental Information Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila Marie P. Suver, Akira Mamiya, and Michael H. Dickinson Supplemental
Drosophila DPM Neurons Form a Delayed and Branch-Specific Memory Trace after Olfactory Classical Conditioning
 Drosophila DPM Neurons Form a Delayed and Branch-Specific Memory Trace after Olfactory Classical Conditioning Dinghui Yu, 1 Alex C. Keene, 4 Anjana Srivatsan, 2 Scott Waddell, 4 and Ronald L. Davis 1,3,
Drosophila DPM Neurons Form a Delayed and Branch-Specific Memory Trace after Olfactory Classical Conditioning Dinghui Yu, 1 Alex C. Keene, 4 Anjana Srivatsan, 2 Scott Waddell, 4 and Ronald L. Davis 1,3,
A Drosophila model for alcohol reward. Supplemental Material
 Kaun et al. Supplemental Material 1 A Drosophila model for alcohol reward Supplemental Material Kaun, K.R. *, Azanchi, R. *, Maung, Z. *, Hirsh, J. and Heberlein, U. * * Department of Anatomy, University
Kaun et al. Supplemental Material 1 A Drosophila model for alcohol reward Supplemental Material Kaun, K.R. *, Azanchi, R. *, Maung, Z. *, Hirsh, J. and Heberlein, U. * * Department of Anatomy, University
Two Gr Genes Underlie Sugar Reception in Drosophila
 Article Two Gr Genes Underlie Sugar Reception in Drosophila Anupama Dahanukar, 1 Ya-Ting Lei, 1 Jae Young Kwon, 1 and John R. Carlson 1, * 1 Department of Molecular, Cellular and Developmental Biology,
Article Two Gr Genes Underlie Sugar Reception in Drosophila Anupama Dahanukar, 1 Ya-Ting Lei, 1 Jae Young Kwon, 1 and John R. Carlson 1, * 1 Department of Molecular, Cellular and Developmental Biology,
R1 Neural Circuitry on Ellipsoid Body Promotes Sleep in Drosophila Melanogaster. Senior Thesis. Presented to
 R1 Neural Circuitry on Ellipsoid Body Promotes Sleep in Drosophila Melanogaster Senior Thesis Presented to The Faculty of the School of Arts and Sciences Brandeis University Undergraduate Program in Neuroscience
R1 Neural Circuitry on Ellipsoid Body Promotes Sleep in Drosophila Melanogaster Senior Thesis Presented to The Faculty of the School of Arts and Sciences Brandeis University Undergraduate Program in Neuroscience
Article. Enhancing Perception of Contaminated Food through Acid-Mediated Modulation of Taste Neuron Responses. Yan Chen 1 and Hubert Amrein 1, * 1
 Current Biology 24, 1969 1977, September 8, 2014 ª2014 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2014.07.069 Enhancing Perception of Contaminated Food through Acid-Mediated Modulation
Current Biology 24, 1969 1977, September 8, 2014 ª2014 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2014.07.069 Enhancing Perception of Contaminated Food through Acid-Mediated Modulation
Report. Presynaptic Gain Control Drives Sweet and Bitter Taste Integration in Drosophila
 Current Biology 24, 1978 1984, September 8, 2014 ª2014 Elsevier Ltd All rights reserved Presynaptic Gain Control Drives Sweet and Bitter Taste Integration in Drosophila http://dx.doi.org/10.1016/j.cub.2014.07.020
Current Biology 24, 1978 1984, September 8, 2014 ª2014 Elsevier Ltd All rights reserved Presynaptic Gain Control Drives Sweet and Bitter Taste Integration in Drosophila http://dx.doi.org/10.1016/j.cub.2014.07.020
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Article. Enhancing Perception of Contaminated Food through Acid-Mediated Modulation of Taste Neuron Responses. Yan Chen 1 and Hubert Amrein 1, * 1
 Current Biology 24, 1969 1977, September 8, 214 ª214 Elsevier Ltd All rights reserved http://dx.doi.org/1.116/j.cub.214.7.69 Enhancing Perception of Contaminated Food through Acid-Mediated Modulation of
Current Biology 24, 1969 1977, September 8, 214 ª214 Elsevier Ltd All rights reserved http://dx.doi.org/1.116/j.cub.214.7.69 Enhancing Perception of Contaminated Food through Acid-Mediated Modulation of
Motor Neurons Controlling Fluid Ingestion in Drosophila melanogaster. Andrea Valenzuela Manzo. A dissertation submitted in partial satisfaction of the
 Motor Neurons Controlling Fluid Ingestion in Drosophila melanogaster By Andrea Valenzuela Manzo A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy
Motor Neurons Controlling Fluid Ingestion in Drosophila melanogaster By Andrea Valenzuela Manzo A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy
Central Brain Neurons Expressing doublesex Regulate Female Receptivity in Drosophila
 Article Central Brain Neurons Expressing doublesex Regulate Female Receptivity in Drosophila Chuan Zhou, 1 Yufeng Pan, 1 Carmen C. Robinett, 1 Geoffrey W. Meissner, 1 and Bruce S. Baker 1, 1 Janelia Farm
Article Central Brain Neurons Expressing doublesex Regulate Female Receptivity in Drosophila Chuan Zhou, 1 Yufeng Pan, 1 Carmen C. Robinett, 1 Geoffrey W. Meissner, 1 and Bruce S. Baker 1, 1 Janelia Farm
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM
 STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Nature Neuroscience: doi: /nn Supplementary Figure 1. Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons
 Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
SCIENCE CHINA Life Sciences. Identification of neurons responsible for feeding behavior in the Drosophila brain
 SCIENCE CHINA Life Sciences THEMATIC ISSUE: From brain function to therapy April 2014 Vol.57 No.4: 391 402 RESEARCH PAPER doi: 10.1007/s11427-014-4641-2 Identification of neurons responsible for feeding
SCIENCE CHINA Life Sciences THEMATIC ISSUE: From brain function to therapy April 2014 Vol.57 No.4: 391 402 RESEARCH PAPER doi: 10.1007/s11427-014-4641-2 Identification of neurons responsible for feeding
GABAergic Projection Neurons Route Selective Olfactory Inputs to Specific Higher-Order Neurons
 Article GABAergic Projection s Route Selective Olfactory Inputs to Specific Higher-Order s Liang Liang, 1,2 Yulong Li, 3,5 Christopher J. Potter, 1,6 Ofer Yizhar, 4,7 Karl Deisseroth, 4 Richard W. Tsien,
Article GABAergic Projection s Route Selective Olfactory Inputs to Specific Higher-Order s Liang Liang, 1,2 Yulong Li, 3,5 Christopher J. Potter, 1,6 Ofer Yizhar, 4,7 Karl Deisseroth, 4 Richard W. Tsien,
GAP-FREE NEURAL CIRCUITS CLASS #3: C. elegans touch-induced locomotion
 GAP-FREE NEURAL CIRCUITS CLASS #3: C. elegans touch-induced locomotion OUTLINE: C. elegans background o General background o Neural signaling: no action potentials Excitatory circuits: Touch-induced locomotion
GAP-FREE NEURAL CIRCUITS CLASS #3: C. elegans touch-induced locomotion OUTLINE: C. elegans background o General background o Neural signaling: no action potentials Excitatory circuits: Touch-induced locomotion
Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC
 1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
Dunce Phosphodiesterase Acts as a Checkpoint for Drosophila Long-Term Memory in a Pair of Serotonergic Neurons
 Article Dunce Phosphodiesterase Acts as a Checkpoint for Drosophila Long-Term Memory in a Pair of Serotonergic Neurons Highlights d Dunce phosphodiesterase is a default inhibitor of long-term memory (LTM)
Article Dunce Phosphodiesterase Acts as a Checkpoint for Drosophila Long-Term Memory in a Pair of Serotonergic Neurons Highlights d Dunce phosphodiesterase is a default inhibitor of long-term memory (LTM)
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Insect nervous system. Zoo 514 Dr. Reem Alajmi
 Insect nervous system Zoo 514 Dr. Reem Alajmi Nervous System The nervous system is the primary mechanism of conduction and control in the body. In insects it serves as an elaborate (complex) connecting
Insect nervous system Zoo 514 Dr. Reem Alajmi Nervous System The nervous system is the primary mechanism of conduction and control in the body. In insects it serves as an elaborate (complex) connecting
Behavioral Neurobiology
 Behavioral Neurobiology The Cellular Organization of Natural Behavior THOMAS J. CAREW University of California, Irvine Sinauer Associates, Inc. Publishers Sunderland, Massachusetts PART I: Introduction
Behavioral Neurobiology The Cellular Organization of Natural Behavior THOMAS J. CAREW University of California, Irvine Sinauer Associates, Inc. Publishers Sunderland, Massachusetts PART I: Introduction
PEER REVIEW FILE. Reviewers' Comments: Reviewer #1 (Remarks to the Author):
 PEER REVIEW FILE Reviewers' Comments: Reviewer #1 (Remarks to the Author): In this manuscript entitled "Neural circuits for long-term water-reward memory processing in thirsty Drosophila", the authors
PEER REVIEW FILE Reviewers' Comments: Reviewer #1 (Remarks to the Author): In this manuscript entitled "Neural circuits for long-term water-reward memory processing in thirsty Drosophila", the authors
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
Developmental nicotine exposure affects larval brain size and the adult dopaminergic system of Drosophila melanogaster
 Morris et al. BMC Developmental Biology (2018) 18:13 https://doi.org/10.1186/s12861-018-0172-6 RESEARCH ARTICLE Open Access Developmental nicotine exposure affects larval brain size and the adult dopaminergic
Morris et al. BMC Developmental Biology (2018) 18:13 https://doi.org/10.1186/s12861-018-0172-6 RESEARCH ARTICLE Open Access Developmental nicotine exposure affects larval brain size and the adult dopaminergic
All questions below pertain to mandatory material: all slides, and mandatory homework (if any).
 ECOL 182 Spring 2008 Dr. Ferriere s lectures Lecture 6: Nervous system and brain Quiz Book reference: LIFE-The Science of Biology, 8 th Edition. http://bcs.whfreeman.com/thelifewire8e/ All questions below
ECOL 182 Spring 2008 Dr. Ferriere s lectures Lecture 6: Nervous system and brain Quiz Book reference: LIFE-The Science of Biology, 8 th Edition. http://bcs.whfreeman.com/thelifewire8e/ All questions below
Nature Neuroscience: doi: /nn Supplementary Figure 1. Splenic atrophy and leucopenia caused by T3 SCI.
 Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Contact Chemoreceptors Mediate Male-Male Repulsion and Male-Female Attraction during Drosophila Courtship
 Contact Chemoreceptors Mediate Male-Male Repulsion and Male-Female Attraction during Drosophila Courtship Robert Thistle, 2,3 Peter Cameron, 2,3,4 Azeen Ghorayshi, 2 Lisa Dennison, 2 and Kristin Scott
Contact Chemoreceptors Mediate Male-Male Repulsion and Male-Female Attraction during Drosophila Courtship Robert Thistle, 2,3 Peter Cameron, 2,3,4 Azeen Ghorayshi, 2 Lisa Dennison, 2 and Kristin Scott
Nervous System. 2. Receives information from the environment from CNS to organs and glands. 1. Relays messages, processes info, analyzes data
 Nervous System 1. Relays messages, processes info, analyzes data 2. Receives information from the environment from CNS to organs and glands 3. Transmits impulses from CNS to muscles and glands 4. Transmits
Nervous System 1. Relays messages, processes info, analyzes data 2. Receives information from the environment from CNS to organs and glands 3. Transmits impulses from CNS to muscles and glands 4. Transmits
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Supplementary Figure 1. Identification of the type II spiral ganglion neurons (SGN) via immunofluorescence of peripherin protein (PRPH).
 Supplementary Figure 1. Identification of the type II spiral ganglion neurons (SGN) via immunofluorescence of peripherin protein (PRPH). (a), (b), PRPH immunolabelling of cryosections from post-natal day
Supplementary Figure 1. Identification of the type II spiral ganglion neurons (SGN) via immunofluorescence of peripherin protein (PRPH). (a), (b), PRPH immunolabelling of cryosections from post-natal day
Report. The Molecular Basis of Sugar Sensing in Drosophila Larvae
 Current Biology 23, 466 47, August 5, 23 ª23 Elsevier Ltd All rights reserved http://dx.doi.org/.6/j.cub.23.6.28 The Molecular Basis of Sugar Seing in Drosophila Larvae Report Dushyant Mishra, Tetsuya
Current Biology 23, 466 47, August 5, 23 ª23 Elsevier Ltd All rights reserved http://dx.doi.org/.6/j.cub.23.6.28 The Molecular Basis of Sugar Seing in Drosophila Larvae Report Dushyant Mishra, Tetsuya
Questions Addressed Through Study of Behavioral Mechanisms (Proximate Causes)
 Jan 28: Neural Mechanisms--intro Questions Addressed Through Study of Behavioral Mechanisms (Proximate Causes) Control of behavior in response to stimuli in environment Diversity of behavior: explain the
Jan 28: Neural Mechanisms--intro Questions Addressed Through Study of Behavioral Mechanisms (Proximate Causes) Control of behavior in response to stimuli in environment Diversity of behavior: explain the
3) Most of the organelles in a neuron are located in the A) dendritic region. B) axon hillock. C) axon. D) cell body. E) axon terminals.
 Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
Chapter 48 Neurons, Synapses, and Signaling Multiple-Choice Questions 1) A simple nervous system A) must include chemical senses, mechanoreception, and vision. B) includes a minimum of 12 ganglia. C) has
Octopamine Neuromodulatory Effects on a Social Behavior Decision- Making Network in Drosophila Males
 Octopamine Neuromodulatory Effects on a Social Behavior Decision- Making Network in Drosophila Males The Harvard community has made this article openly available. Please share how this access benefits
Octopamine Neuromodulatory Effects on a Social Behavior Decision- Making Network in Drosophila Males The Harvard community has made this article openly available. Please share how this access benefits
Neurobiology: The nerve cell. Principle and task To use a nerve function model to study the following aspects of a nerve cell:
 Principle and task To use a nerve function model to study the following aspects of a nerve cell: INTRACELLULAR POTENTIAL AND ACTION POTENTIAL Comparison between low and high threshold levels Comparison
Principle and task To use a nerve function model to study the following aspects of a nerve cell: INTRACELLULAR POTENTIAL AND ACTION POTENTIAL Comparison between low and high threshold levels Comparison
Module H NERVOUS SYSTEM
 Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes.
 Supplementary Figure 1 Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes. (a) Left, circuit schematic with the imaged component (L2/3 excitatory neurons)
Supplementary Figure 1 Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes. (a) Left, circuit schematic with the imaged component (L2/3 excitatory neurons)
Ube3a is required for experience-dependent maturation of the neocortex
 Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
2. When a neuron receives signals, an abrupt, temporary the inside becomes more positive in the polarity is generated (an action potential).
 Chapter 34 Integration and Control: Nervous Systems I. Neurons The Communication Specialists A. Functional Zones of a Neuron 1. The contains the nucleus and metabolic machinery for protein synthesis. 2.
Chapter 34 Integration and Control: Nervous Systems I. Neurons The Communication Specialists A. Functional Zones of a Neuron 1. The contains the nucleus and metabolic machinery for protein synthesis. 2.
Regulation of sleep plasticity by a thermo-sensitive circuit in Drosophila
 Regulation of sleep plasticity by a thermo-sensitive circuit in Drosophila Angelique Lamaze 1, Arzu Öztürk-Çolak 2, Robin Fischer 3, Nicolai Peschel 3, Kyunghee Koh 2 and James E.C. Jepson 1* 1 UCL Institute
Regulation of sleep plasticity by a thermo-sensitive circuit in Drosophila Angelique Lamaze 1, Arzu Öztürk-Çolak 2, Robin Fischer 3, Nicolai Peschel 3, Kyunghee Koh 2 and James E.C. Jepson 1* 1 UCL Institute
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed
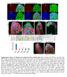 Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed in the testes. The testes were immunostained with GFP
Supplementary Figure 1 Madm is not required in GSCs and hub cells. (a,b) Act-Gal4-UAS-GFP (a), Act-Gal4-UAS- GFP.nls (b,c) is ubiquitously expressed in the testes. The testes were immunostained with GFP
The nervous system is responsible for most of the functions that characterize
 3 E X E R C I S E Neurophysiology of Nerve Impulses O B J E C T I V E S 1. To define the following: irritability, conductivity, resting membrane potential, polarized, sodium-potassium pump, threshold stimulus,
3 E X E R C I S E Neurophysiology of Nerve Impulses O B J E C T I V E S 1. To define the following: irritability, conductivity, resting membrane potential, polarized, sodium-potassium pump, threshold stimulus,
THE NERVOUS SYSTEM. Homeostasis Strand
 THE NERVOUS SYSTEM Homeostasis Strand Introduction In general, a nervous system has three overlapping functions : 1. Sensory input conduction of signals from sensory receptors to integration centres 2.
THE NERVOUS SYSTEM Homeostasis Strand Introduction In general, a nervous system has three overlapping functions : 1. Sensory input conduction of signals from sensory receptors to integration centres 2.
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
Why am I working in a Drosophila lab? Dr. Stefan Pulver, Brandeis University
 Why am I working in a Drosophila lab? Dr. Stefan Pulver, Brandeis University !Short life span The advantages of Drosophila as a model organism in neurocience!genetic manipulations are relatively easy!forward
Why am I working in a Drosophila lab? Dr. Stefan Pulver, Brandeis University !Short life span The advantages of Drosophila as a model organism in neurocience!genetic manipulations are relatively easy!forward
Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex
 Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
Article. The Color-Vision Circuit in the Medulla of Drosophila. Javier Morante 1 and Claude Desplan 1, * 1
 Current Biology 18, 1 13, April 22, 2008 ª2008 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2008.02.075 The Color-Vision Circuit in the Medulla of Drosophila Article Javier Morante 1 and Claude Desplan
Current Biology 18, 1 13, April 22, 2008 ª2008 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2008.02.075 The Color-Vision Circuit in the Medulla of Drosophila Article Javier Morante 1 and Claude Desplan
A Mechanosensory Circuit that Mixes Opponent Channels to Produce Selectivity for Complex Stimulus Features
 Article A Mechanosensory Circuit that Mixes Opponent Channels to Produce Selectivity for Complex Stimulus Features Highlights d apn3 cells in the fly brain combine input from opponent antennal mechanoreceptors
Article A Mechanosensory Circuit that Mixes Opponent Channels to Produce Selectivity for Complex Stimulus Features Highlights d apn3 cells in the fly brain combine input from opponent antennal mechanoreceptors
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Neurons Chapter 7 2/19/2016. Learning Objectives. Cells of the Nervous System. Cells of the Nervous System. Cells of the Nervous System
 Learning Objectives Neurons Chapter 7 Identify and describe the functions of the two main divisions of the nervous system. Differentiate between a neuron and neuroglial cells in terms of structure and
Learning Objectives Neurons Chapter 7 Identify and describe the functions of the two main divisions of the nervous system. Differentiate between a neuron and neuroglial cells in terms of structure and
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature07173 SUPPLEMENTARY INFORMATION Supplementary Figure Legends: Supplementary Figure 1: Model of SSC and CPC divisions a, Somatic stem cells (SSC) reside adjacent to the hub (red), self-renew
doi: 10.1038/nature07173 SUPPLEMENTARY INFORMATION Supplementary Figure Legends: Supplementary Figure 1: Model of SSC and CPC divisions a, Somatic stem cells (SSC) reside adjacent to the hub (red), self-renew
ANATOMY AND PHYSIOLOGY OF NEURONS. AP Biology Chapter 48
 ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
ANATOMY AND PHYSIOLOGY OF NEURONS AP Biology Chapter 48 Objectives Describe the different types of neurons Describe the structure and function of dendrites, axons, a synapse, types of ion channels, and
Structural basis for the role of inhibition in facilitating adult brain plasticity
 Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers
 Development 129, 5667-5681 2002 The Company of Biologists Ltd doi:10.1242/dev.00157 5667 The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection
Development 129, 5667-5681 2002 The Company of Biologists Ltd doi:10.1242/dev.00157 5667 The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection
Nature Neuroscience: doi: /nn Supplementary Figure 1. Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites.
 Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Supplementary Figure 1 Neuron class-specific arrangements of Khc::nod::lacZ label in dendrites. Staining with fluorescence antibodies to detect GFP (Green), β-galactosidase (magenta/white). (a, b) Class
Inheritance of Aldehyde Oxidase in Drosophila melanogaster
 Inheritance of Aldehyde Oxidase in Drosophila melanogaster (adapted from Morgan, J. G. and V. Finnerty. 1991. Inheritance of aldehyde oxidase in Drosophilia melanogaster. Pages 33-47, in Tested studies
Inheritance of Aldehyde Oxidase in Drosophila melanogaster (adapted from Morgan, J. G. and V. Finnerty. 1991. Inheritance of aldehyde oxidase in Drosophilia melanogaster. Pages 33-47, in Tested studies
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed.,
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by B.-W. Ku,
Neurons, Synapses and Signaling. Chapter 48
 Neurons, Synapses and Signaling Chapter 48 Warm Up Exercise What types of cells can receive a nerve signal? Nervous Organization Neurons- nerve cells. Brain- organized into clusters of neurons, called
Neurons, Synapses and Signaling Chapter 48 Warm Up Exercise What types of cells can receive a nerve signal? Nervous Organization Neurons- nerve cells. Brain- organized into clusters of neurons, called
Chapter 17. Nervous System Nervous systems receive sensory input, interpret it, and send out appropriate commands. !
 Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Relative expression of K IR2.1 transcript to enos was reduced 29-fold in capillaries from knockout animals. Relative expression of K IR2.1 transcript to enos was reduced 29-fold
Supplementary Figure 1 Relative expression of K IR2.1 transcript to enos was reduced 29-fold in capillaries from knockout animals. Relative expression of K IR2.1 transcript to enos was reduced 29-fold
Lecture: Introduction to nervous system development
 Lecture: Introduction to nervous system development Prof. Ilan Davis, Department of Biochemistry. Wellcome Senior Research Fellow Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com
Lecture: Introduction to nervous system development Prof. Ilan Davis, Department of Biochemistry. Wellcome Senior Research Fellow Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com
Supplementary Information
 Supplementary Information Supplementary s Supplementary 1 All three types of foods suppress subsequent feeding in both sexes when the same food is used in the pre-feeding test feeding. (a) Adjusted pre-feeding
Supplementary Information Supplementary s Supplementary 1 All three types of foods suppress subsequent feeding in both sexes when the same food is used in the pre-feeding test feeding. (a) Adjusted pre-feeding
SYNAPTIC COMMUNICATION
 BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
BASICS OF NEUROBIOLOGY SYNAPTIC COMMUNICATION ZSOLT LIPOSITS 1 NERVE ENDINGS II. Interneuronal communication 2 INTERNEURONAL COMMUNICATION I. ELECTRONIC SYNAPSE GAP JUNCTION II. CHEMICAL SYNAPSE SYNAPSES
Overview. Unit 2: What are the building blocks of our brains?
 Unit 2: What are the building blocks of our brains? Overview In the last unit we discovered that complex brain functions occur as individual structures in the brain work together like an orchestra. We
Unit 2: What are the building blocks of our brains? Overview In the last unit we discovered that complex brain functions occur as individual structures in the brain work together like an orchestra. We
Traces of Drosophila Memory
 Traces of Drosophila Memory Ronald L. Davis 1, * 1 Department of Neuroscience, The Scripps Research Institute Florida, Jupiter, FL 33410, USA *Correspondence: rdavis@scripps.edu DOI 10.1016/j.neuron.2011.03.012
Traces of Drosophila Memory Ronald L. Davis 1, * 1 Department of Neuroscience, The Scripps Research Institute Florida, Jupiter, FL 33410, USA *Correspondence: rdavis@scripps.edu DOI 10.1016/j.neuron.2011.03.012
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Shocking revelations and saccharin sweetness in the study of Drosophila olfactory memory
 University of Massachusetts Medical School escholarship@umms University of Massachusetts Medical School Faculty Publications 9-9-2013 Shocking revelations and saccharin sweetness in the study of Drosophila
University of Massachusetts Medical School escholarship@umms University of Massachusetts Medical School Faculty Publications 9-9-2013 Shocking revelations and saccharin sweetness in the study of Drosophila
biological psychology, p. 40 The study of the nervous system, especially the brain. neuroscience, p. 40
 biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
CYTOARCHITECTURE OF CEREBRAL CORTEX
 BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
Page 1. Neurons Transmit Signal via Action Potentials: neuron At rest, neurons maintain an electrical difference across
 Chapter 33: The Nervous System and the Senses Neurons: Specialized excitable cells that allow for communication throughout the body via electrical impulses Neuron Anatomy / Function: 1) Dendrites: Receive
Chapter 33: The Nervous System and the Senses Neurons: Specialized excitable cells that allow for communication throughout the body via electrical impulses Neuron Anatomy / Function: 1) Dendrites: Receive
Supplementary Figure 1
 Supplementary Figure 1 Arcuate ChIEF-tdTomato neurons expressed TH These micrographs show that TH-Cre-ChIEF-tdTomato (magenta), expressed by AAV in a TH-Cre mouse, were immunostained with TH (green) in
Supplementary Figure 1 Arcuate ChIEF-tdTomato neurons expressed TH These micrographs show that TH-Cre-ChIEF-tdTomato (magenta), expressed by AAV in a TH-Cre mouse, were immunostained with TH (green) in
What is Anatomy and Physiology?
 Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
Introduction BI 212 BI 213 BI 211 Ecosystems Organs / organ systems Cells Organelles Communities Tissues Molecules Populations Organisms Campbell et al. Figure 1.4 Introduction What is Anatomy and Physiology?
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
(Sample Lab Report) Perception of Different Sugars by Blowflies
 (Sample Lab Report) Perception of Different Sugars by Blowflies Alexander Hamilton Biology 110 October 24, 1995 Lab Partners: Sharon Flynn, Andi Alexander INTRODUCTION All animals rely on senses of taste
(Sample Lab Report) Perception of Different Sugars by Blowflies Alexander Hamilton Biology 110 October 24, 1995 Lab Partners: Sharon Flynn, Andi Alexander INTRODUCTION All animals rely on senses of taste
Introduction to Neurobiology
 Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Chapter 2. The Cellular and Molecular Basis of Cognition
 Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by B.-W. Ku,
Chapter 2. The Cellular and Molecular Basis of Cognition Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga,, R. B. Ivry,, and G. R. Mangun,, Norton, 2002. Summarized by B.-W. Ku,
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale
 Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) (B) (C) (D) (E)
 Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11905 WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 Quantification of integrated chorda tympani nerve responses. Quantification
SUPPLEMENTARY INFORMATION doi:10.1038/nature11905 WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 Quantification of integrated chorda tympani nerve responses. Quantification
NERVOUS SYSTEM 1 CHAPTER 10 BIO 211: ANATOMY & PHYSIOLOGY I
 BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B This set CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B This set CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
Perception of Different. Sugars by Blowflies
 Fly lab report p. 1 SAMPLE LAB REPORT Perception of Different Sugars by Blowflies by Alexander Hamilton Biology 101 October 24, 2009 Lab Partners: Sharon Flynn, Andi Alexander Fly lab report p. 2 ABSTRACT
Fly lab report p. 1 SAMPLE LAB REPORT Perception of Different Sugars by Blowflies by Alexander Hamilton Biology 101 October 24, 2009 Lab Partners: Sharon Flynn, Andi Alexander Fly lab report p. 2 ABSTRACT
Axon Nerve impulse. Axoplasm Receptor. Axomembrane Stimuli. Schwann cell Effector. Myelin Cell body
 Nervous System Review 1. Explain a reflex arc. 2. Know the structure, function and location of a sensory neuron, interneuron, and motor neuron 3. What is (a) Neuron Axon Nerve impulse Axoplasm Receptor
Nervous System Review 1. Explain a reflex arc. 2. Know the structure, function and location of a sensory neuron, interneuron, and motor neuron 3. What is (a) Neuron Axon Nerve impulse Axoplasm Receptor
A post-ingestive amino acid sensor promotes food consumption in Drosophila
 www.nature.com/cr www.cell-research.com ARTICLE OPEN A post-ingestive amino acid sensor promotes food consumption in Drosophila Zhe Yang 1,2, Rui Huang 3,4, Xin Fu 5,6,7, Gaohang Wang 1,2, Wei Qi 1,2,
www.nature.com/cr www.cell-research.com ARTICLE OPEN A post-ingestive amino acid sensor promotes food consumption in Drosophila Zhe Yang 1,2, Rui Huang 3,4, Xin Fu 5,6,7, Gaohang Wang 1,2, Wei Qi 1,2,
Neural and Hormonal Systems
 PSYCHOLOGY (8th Edition, in Modules) David Myers PowerPoint Slides Worth Publishers, 2007 1 Neural and Hormonal Systems Module 4 2 Neural and Hormonal Systems Neural Communication Neurons How Neurons Communicate
PSYCHOLOGY (8th Edition, in Modules) David Myers PowerPoint Slides Worth Publishers, 2007 1 Neural and Hormonal Systems Module 4 2 Neural and Hormonal Systems Neural Communication Neurons How Neurons Communicate
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Nature Neuroscience: doi: /nn Supplementary Figure 1. ACx plasticity is required for fear conditioning.
 Supplementary Figure 1 ACx plasticity is required for fear conditioning. (a) Freezing time of conditioned and control mice before CS presentation and during CS presentation in a new context. Student s
Supplementary Figure 1 ACx plasticity is required for fear conditioning. (a) Freezing time of conditioned and control mice before CS presentation and during CS presentation in a new context. Student s
10.1: Introduction. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial cells) Dendrites.
 10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
5-Nervous system II: Physiology of Neurons
 5-Nervous system II: Physiology of Neurons AXON ION GRADIENTS ACTION POTENTIAL (axon conduction) GRADED POTENTIAL (cell-cell communication at synapse) SYNAPSE STRUCTURE & FUNCTION NEURAL INTEGRATION CNS
5-Nervous system II: Physiology of Neurons AXON ION GRADIENTS ACTION POTENTIAL (axon conduction) GRADED POTENTIAL (cell-cell communication at synapse) SYNAPSE STRUCTURE & FUNCTION NEURAL INTEGRATION CNS
