Complement Activation in Acetaminophen-Induced Liver Injury in Mice
|
|
|
- Berenice Isabel Neal
- 5 years ago
- Views:
Transcription
1 /12/ $25.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 341, No. 2 Copyright 2012 by The American Society for Pharmacology and Experimental Therapeutics / JPET 341: , 2012 Complement Activation in Acetaminophen-Induced Liver Injury in Mice Rohit Singhal, Patricia E. Ganey, and Robert A. Roth Department of Pharmacology and Toxicology, and Center for Integrative Toxicology, Michigan State University, East Lansing, Michigan Received November 8, 2011; accepted February 6, 2012 ABSTRACT Overdose with acetaminophen (APAP) results in acute liver failure in humans and experimental animals. Complement comprises more than 30 proteins that can participate in tissue injury and/or repair, but the role of complement activation in APAPinduced hepatotoxicity has not been evaluated. Treatment of male, C57BL6J mice with APAP ( mg/kg) resulted in liver injury as evidenced by increased activity of alanine aminotransferase (ALT) in plasma and hepatocellular necrosis. Plasma concentration of the complement component C3 was significantly reduced 6 h after treatment with APAP, indicating complement activation, and C3b (detected by immunostaining) accumulated in the centrilobular areas of liver lobules. Pretreatment with cobra venom factor (CVF; 15 U/mouse) to deplete Introduction Acetaminophen (APAP; N-acetyl-p-aminophenol; paracetamol) is one of the most commonly consumed over-thecounter drugs available on the market, but, unfortunately, it is also the most frequent cause of drug-induced liver failure worldwide (Larson et al., 2005). Approximately 500 fatal cases of acute liver failure are reported annually in the United States alone (Lee, 2004). The mechanisms associated with the initiation of APAP-induced liver injury have been extensively studied over the years in humans and experimental animal models. APAP is metabolized to N-acetyl-p-benzoquinoneimine (NAPQI) by cytochrome P450. NAPQI is detoxified by conjugation with GSH, and overdose with APAP depletes cellular GSH, resulting in the binding of NAPQI to mitochondrial proteins. This is followed by mitochondrial This research was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01- DK087886]. Article, publication date, and citation information can be found at complement components abolished APAP-mediated C3b accumulation, and this was accompanied by reductions in plasma ALT activity, hepatocellular necrosis, hepatic neutrophil accumulation, and expression of inflammatory genes (interleukin-6, interleukin-10, and plasminogen activation inhibitor-1) at 24 h after APAP treatment. Loss of hepatocellular GSH was similar in APAP-treated mice pretreated with either saline or CVF, suggesting that CVF pretreatment did not affect APAP bioactivation. Mice with a genetic deficiency in C3 had reduced ALT activity 6 and 12 h after APAP administration compared with wild-type animals. These results reveal a key role for complement activation in hepatic inflammation and progression of injury during the pathogenesis of APAP-induced hepatotoxicity. dysfunction, ATP depletion, oxidative stress, DNA damage, and oncotic necrosis of parenchymal cells (Kaplowitz, 2004; Jaeschke et al., 2011). The hepatocellular necrosis is accompanied by the release of death-associated molecular pattern molecules, such as high-mobility group box 1 protein, heat shock protein 70, and damaged DNA, which can activate nonparenchymal cells, including Kupffer cells and neutrophils (Scaffidi et al., 2002; Bianchi, 2007; Martin-Murphy et al., 2010). Activation of these cells can exacerbate necrosis and/or participate in recovery from injury. Although mechanisms involved in APAP hepatotoxicity have been studied extensively, nothing has been reported about whether and how complement activation affects the progression of liver injury and hepatocellular repair. The complement system comprises approximately 35 proteins that are present either as soluble factors in the blood or membrane-associated proteins. The main evolutionary function of complement is to sense danger signals from pathogens and dying cells and activate defenses against tissue damage. Complement activation is a sequential cascade of enzymic reactions that is initiated by one or more of three pathways: classic, alternative, and lectin-associated. Each of these path- ABBREVIATIONS: APAP, acetaminophen; ALT, alanine aminotransferase; BrdU, 5 -bromo-2 -deoxyuridine; CVF, cobra venom factor; C3, complement component 3; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL, interleukin; NAPQI, N-acetyl-p-benzoquinoneimine; PAI-1, plasminogen activation inhibitor-1; PBS, phosphate-buffered saline; PCNA, proliferating cell nuclear antigen; PCR, polymerase chain reaction; PMN, polymorphonuclear neutrophil; Sal, saline; TNF-, tumor necrosis factor-. 377
2 378 Singhal et al. ways results in C3 activation and leads to the generation of anaphylatoxins C3a and C5a, which activate innate immune cells such as neutrophils, monocytes, mast cells, and basophils. Activation of terminal complement components forms a C5b-9 complex, also known as the membrane attack complex, which can kill microbial pathogens but also damage host tissue (Walport, 2001; Ward, 2004; Markiewski et al., 2007). Although necessary for foreign body clearance and immune cell activation, overactivation of complement components can lead to coagulation activation, tissue necrosis, and multiorgan dysfunction. Complement activation has been implicated in several hepatic disease models, including sepsis (Koleva et al., 2002; Ward, 2004), ischemia reperfusion (He et al., 2009), alcoholic (Cohen et al., 2010) and nonalcoholic steatohepatitis (Rensen et al., 2009), and hemorrhagic shock (Cai et al., 2010); however, its role in APAP-induced liver injury has not been reported. In studies with only a limited number of patients with APAP overdose, complement activation was associated with hepatic dysfunction (Ellison et al., 1990; Clapperton et al., 1997). Given the observation that complement is activated in patients with APAP overdose, we sought to determine its role in APAP-induced liver injury in a mouse model. We tested the hypothesis that complement depletion attenuates liver injury mediated by APAP treatment in mice. Materials and Methods Animals. All mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at 9 weeks of age and kept at the animal care facility at Michigan State University. Male, C57BL6J mice were used for most studies. The male C3( / ) mice (B6.129S4-C3 tm1crr /J) had been backcrossed with C57BL6J mice for five generations, and C57BL6J mice were used as controls for these mice. All animals had access to standard chow diet and spring water ad libitum and were used between 10 and 13 weeks of age. All procedures on animals were performed according to the guidelines of the American Association for Laboratory Animal Science and approved by the Institutional Animal Care and Use Committee at Michigan State University. Experimental Protocols. Complement was depleted by two intraperitoneal injections (7.5 U each in 200 l of saline/mouse) of cobra venom factor (CVF; Quidel Corporation, San Diego, CA). The first injection was given 24 h before APAP or saline administration, and the second injection was given 5 h after the first CVF injection. This was done to prevent any adverse effects of rapid loss of complement. Groups of mice (n 3 6) were treated with CVF or its vehicle (saline) and with either APAP or its vehicle (saline). APAP solution (Sigma, St. Louis, MO; 10 mg/ml) was made fresh before use in saline and incubated at 42 C in water bath for 15 min with intermittent vortexing. Mice were fasted overnight and treated with 300 mg/kg APAP or saline intraperitoneally the next morning. In a dose-response study, mice were given 200 to 400 mg/kg APAP as indicated. Food was returned after APAP administration. All animals were euthanized 24 or 48 h after APAP administration. In a time-course study, mice were anesthetized 12 h after APAP administration, and blood was collected by retro-orbital puncture; the mice were euthanized at 24 h. Blood was collected from vena cava into 3.8% sodium citrate (final concentration, 0.38%), and livers were harvested. The left lateral lobe of liver was fixed in 10% formalin for histopathological analysis, the left medial lobe was snap-frozen in liquid nitrogen for protein and RNA analysis, and the right medial lobe was covered in Tissue-Tek OCT embedding medium on a cork, chilled in isopentane, and frozen in liquid nitrogen for C3b immunohistochemistry. In separate studies, 1) animals were euthanized 1 h after APAP (300 mg/kg) or saline administration, livers were collected, and GSH was measured; and 2) C3( / ) mice and their wild-type controls were treated with APAP (400 mg/kg) or saline; blood was collected by retro-orbital puncture after 6 h, as described above, and the mice were euthanized after 12 h. Blood and livers were collected. Plasma Measurements. Plasma was collected by centrifuging blood at 4000g for 10 min. Alanine aminotransferase (ALT) activity was determined by using Infinity ALT reagent (Thermo Fisher Scientific, Waltham, MA). Concentrations of C3, active PAI-1, and TNF- were measured by using an enzyme-linked immunosorbent assay from Alpha Diagnostic International (San Antonio, TX), Molecular Innovations (Southfield, MI), and BD Biosciences (San Jose, CA), respectively. Histopathological Analysis. Five-micrometer sections of paraffin-embedded liver were stained with hematoxylin and eosin for evaluation of necrosis and hemorrhage. Hepatocellular necrosis in sections from the left liver lobe was quantified by using morphometric methods similar to those described by Aibo et al. (2010). Percentage of hepatic lesion area was estimated as [necrotic area/ total area] 100. For analysis of polymorphonuclear neutrophil (PMN) accumulation, sections were stained with a rabbit-anti-pmn Ig as described previously (Yee et al., 2003). The slides were coded, randomized, and then examined without knowledge of treatment with a light microscope. PMN accumulation was quantified by counting PMNs in fields; the average number of PMNs from each animal was considered a replicate. 5 -Bromo-2 -Deoxyuridine Treatment and Staining. A separate study was performed for the assessment of hepatic proliferation. Animals were treated with APAP with or without complement depletion by CVF, as described above, and given 5 -bromo-2 -deoxyuridine (BrdU; 50 mg/kg i.p.; Sigma) 2 h before harvesting of liver at 24 or 48 h. BrdU becomes incorporated into the newly synthesized DNA of replicating cells during the S phase of the cell cycle. BrdU incorporation in liver sections was determined by immunohistochemical staining performed on paraffin-embedded sections by using a mouse anti-brdu antibody (BD Biosciences) as described previously (Aibo et al., 2010). GSH Estimation. Approximately 100 mg of frozen liver tissue were homogenized in 1 ml of cold buffer containing 0.2 M 2-(Nmorpholino)ethanesulphonic acid, 50 mm phosphate, and 1 mm EDTA, ph 6.0. Homogenates were centrifuged at 10,000g for 15 min at 4 C, and supernatant fluid was collected. Total hepatic GSH concentration was determined using a kit from Cayman Chemical (Ann Arbor, MI). The data are expressed as mol GSH/g of liver tissue. RNA Isolation and Real-Time PCR. Procedures for RNA isolation and real-time PCR were as described previously (Singhal et al., 2009). In brief, total RNA was isolated from approximately 100 mg of hepatic tissue by using TRI reagent (Molecular Research Center, Cincinnati, OH). One microgram of RNA was transcribed into cdna by using a one-step cdna synthesis kit (Bio-Rad Laboratories, Hercules, CA). SYBR green (2 power; Applied Biosystems, Foster City, CA) was applied as a detector. Primers were designed by using the PrimerQuest program ( Integrated DNA Technologies, Inc., Coralville, IA) and purchased from the same company. Primer sequences were as follows: GAPDH, forward, TCAACAG CAACTCCCACTCTTCCA, reverse, ACCCTGTTGCTGTAGCCGTA TTCA; PCNA, forward, AGCCACATTGGAGATGCTGTTGTG, reverse, AATGTTCCCATTGCCAAGCTCTCC; CyclinD1, forward, GC TGCAAATGGAACTGCTTCTGGT, reverse, TACCATGGAGGGTG GGTTGGAAAT; PAI-1, forward, GCTTGGCAACCCACGTTAAA GGAA, reverse, TCTCTGCTTCTGGATGCCTTGGAA; IL-6, forward, ATCCAGTTGCCTTCTTGGGACTGA, reverse, TAAGCCTCCGACT- TGTGAAGTGGT; and IL-10, forward, TGCACTACCAAAGCCA- CAAAGCAG, reverse, AGTAAGAGCAGGCAGCATAGCAGT. A 48- well Step-one Real-Time PCR system (Applied Biosystems) was used to determine the mrna expression level of each gene. mrna levels were normalized to that of GAPDH mrna to control for input RNA.
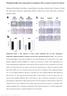
3 Complement Activation in APAP Hepatotoxicity 379 Fig. 1. C3 concentration in plasma from mice treated with APAP. Mice were treated with saline vehicle (0 APAP) or APAP (200, 300, or 400 mg/ kg) after treatment with saline or CVF as described under Materials and Methods. C3 concentration in plasma was determined 6 h (left) or 24 h (right) later. Data are expressed as mean S.E.M., significantly different from respective value in the absence of APAP. #, significantly different from respective value in the absence of CVF. n 3 5 per group, p C3b Immunostaining. Immunohistochemical staining for C3biC3b/C3c (C3b) was performed on sections from frozen liver using a protocol described by Roychowdhury et al. (2009). In brief, frozen liver sections were fixed in 2% paraformaldehyde in phosphatebuffered saline (PBS) and washed three times, 5 min each, in washing buffer (0.1% Triton X-100 in PBS). Sections were then blocked with 2% bovine serum albumin (diluted in PBS) containing 0.1% sodium azide and 0.1% Triton X-100 (blocking buffer) for 1 h followed by overnight incubation at 4 C with rabbit anti-mouse C3b (Cell Sciences, Canton, MA) diluted 50-fold in blocking buffer. All sections were washed with washing buffer, incubated with Alexa Fluor-488- labeled goat-anti-rabbit IgG (Invitrogen, Carlsbad, CA; diluted 250- fold in blocking buffer) for 2 h in the dark at room temperature, washed again in PBS, and mounted with Vectashield containing antifade reagent (Vector Laboratories, Burlingame, CA). Images were acquired by using an Olympus (Tokyo, Japan) IX-70 fluorescent microscope. Statistical Analysis. Data are expressed as mean S.E.M. All data were analyzed by using SigmaStat version 3.5 (Systat Software, Inc., San Jose, CA). Student s t test was used to compare data between two groups. For multiple group comparisons, one-way or two-way analysis of variance was performed followed by Student Newman-Keuls post hoc analysis. The results were considered significant when P Results APAP Treatment Activates Complement. Complement activation is a complex process and can be triggered by either classic, mannose lectin-binding, or alternative pathways. All complement pathways converge on activating and thereby depleting the central component C3. Therefore, we first determined the effect of APAP treatment on plasma C3 concentration. Saline controls had mg/ml of circulating C3 in plasma (Fig. 1). Treatment with APAP ( mg/ kg) significantly reduced C3 in plasma as early as 6hto 50% of control; no significant difference was observed among the three APAP doses. C3 concentration remained reduced compared with control at 24 h, which is the peak of liver injury observed in this study. CVF was used to deplete complement. It has been reported that the CVF treatment regimen used in this study does not result in toxic effects in mice and maintains the depletion of hemolytic activity of complement until 72 h (Alper and Balavitch, 1976; Markiewski et al., 2004). In our hands, CVF treatment alone reduced circulating concentration of C3 in plasma to mg/ml at 0 h, the time at which APAP or saline was administered. This is consistent with previous reports showing that CVF pretreatment reduces C3 levels to less than 5% of control values (Pepys, 1975; Cai et al., 2010). C3 levels remain depleted by CVF pretreatment until 24 h in both saline- and APAP-treated mice. Activation of C3 is followed by its cleavage into fragments, which form an integral part of C3 and C5 convertases, resulting in the generation of anaphylatoxins and formation of the membrane attack complex (Rawal and Pangburn, 2001; Walport, 2001). Accordingly, the deposition of C3 fragments C3b/iC3b/C3c, abbreviated here as C3b, was examined in liver by using an antibody that recognizes neoepitopes on the C3b fragment after C3 cleavage (Mastellos et al., 2004; Roychowdhury et al., 2009). Immunofluorescence in frozen liver sections taken 24 h after APAP administration revealed C3b deposition in the necrotic, centrilobular regions only in livers from APAP-treated mice (Fig. 2). Prior treatment with CVF prevented APAP-induced C3b deposition (Fig. 2). CVF/Sal and Sal/Sal treatment groups did not have any specific staining. No positive staining was detected with the use of isotype control antibody in livers from APAP-treated mice, suggesting that the reactivity obtained with anti-c3b antibody is specific to C3b and not a result of nonspecific staining in the centrilobular region (data not shown). CVF Pretreatment Does Not Alter APAP Metabolism. Depletion of hepatic GSH after treatment with toxic doses of APAP is a well established marker of APAP bioactivation. APAP is metabolized to N-acetyl-p-benzoquinoneimine, which then binds to GSH; excessive NAPQI formation in APAP overdose depletes hepatic GSH stores to 20% of normal with in 1 h of treatment (Chiu et al., 2003). Mice treated with saline (control) had mol GSH/g Fig. 2. Hepatic C3b deposition. Mice were treated with saline vehicle (0 APAP) or APAP (300 mg/kg) after treatment with saline or CVF as described under Materials and Methods. Twenty four hours later livers were harvested and frozen. Frozen liver sections were immunostained with C3b antibody as described under Materials and Methods. Arrows represent C3b in the centrilobular area. Images were acquired at 100 magnification using an Olympus IX-70 fluorescent microscope.
4 380 Singhal et al. Fig. 3. GSH concentration in livers from APAP-treated mice. GSH was determined 1 h after APAP (300 mg/kg) or saline administration in mice pretreated with CVF or saline vehicle as described under Materials and Methods. Data represent mean S.E.M. (n 4 animals per group)., significantly different from respective value in the absence of APAP (p 0.05). liver (Fig. 3). CVF treatment alone did not affect GSH concentration ( mol GSH/g liver). APAP treatment resulted in GSH depletion to and mol GSH/g liver within 1hinsaline- and CVF-pretreated mice, respectively (Fig. 3); these values were not significantly different from each other, suggesting that CVF pretreatment had no effect on APAP bioactivation. Complement Depletion Reduces APAP-Induced Liver Injury. Severity of hepatocellular injury was assessed from ALT activity in plasma and from histopathology. Treatment with APAP (200, 300, and 400 mg/kg) resulted in an increase in ALT activity in plasma at 6 h (Fig. 4A). CVF pretreatment reduced ALT activity in mice treated with 400 mg/kg APAP; however, no effect of CVF pretreatment was observed on APAP-induced increase in ALT activity at 6 h after doses of 200 or 300 mg/kg. One of six mice in the 400 mg/kg APAP group died between 6 and 24 h, and two others became moribund near 24 h. By contrast, no mortality was observed in APAP-exposed mice that were pretreated with CVF. CVF pretreatment reduced APAP (300 and 400 mg/kg)-induced increase in ALT activity at 24 h; in contrast, no effect of CVF pretreatment was observed on the increase in ALT activity in mice given 200 mg/kg APAP. CVF treatment alone did not change ALT activity compared with saline vehicle either at 6 or 24 h after mice were given the vehicle for APAP (i.e., saline). APAP treatment (200, 300, or 400 mg/kg) resulted in centrilobular necrosis at 6 h (data not shown) and 24 h (Fig. 4B). Moreover, mice treated with either 300 or 400 mg/kg APAP that were pretreated with CVF had smaller necrotic areas and less hemorrhage than those treated with APAP alone 24 h after APAP treatment. No affect of CVF pretreatment was observed on liver histopathology in groups treated with 200 mg/kg APAP (Fig. 4B). Treatment of mice with APAP (300 mg/kg) resulted in a time-dependent increase in plasma ALT activity (Fig. 4C). CVF pretreatment had no Fig. 4. Liver injury after APAP treatment. Mice were treated with saline vehicle (0 APAP) or APAP (200, 300, or 400 mg/kg) after treatment with saline or CVF as described under Materials and Methods. A, plasma ALT activity at 6 h (left) or 24 h (right) after APAP. B, representative images from hematoxylin and eosin-stained liver sections from livers harvested at 24 h. a to d, treatment with APAP [0 (vehicle) (a), 200 (b), 300 (c), or 400 (d) mg/kg)] after treatment with saline. e to h, treatment with APAP [0 (vehicle) (e), 200 (f), 300 (g), or 400 (h) mg/kg)] after treatment with CVF. C, ALT activity in plasma at 6, 12, or 24 h after treatment with CVF or saline and APAP (300 mg/kg) or saline vehicle as described under Materials and Methods. Values for saline/saline are obscured by values for CVF/saline. Data are expressed as mean S.E.M., significantly different from respective value in the absence of APAP. #, significantly different from respective value in the absence of CVF. n 3 5 per group, p 0.05.
5 Complement Activation in APAP Hepatotoxicity 381 Fig. 5. Liver injury in C3( / ) mice treated with APAP. A, plasma ALT activity at 6 or 12 h after APAP (400 mg/kg) administration. Data are expressed as mean S.E.M., significantly different from wild type at the indicated time (p 0.05). n 3 5 per group. B, representative liver sections stained with hematoxylin and eosin. Wild-type (a) or C3( / ) (b) mice were treated with APAP (400 mg/kg), and livers were harvested 12 h after APAP administration. significant effect on the increase in ALT activity at 6 or 12 h, whereas a marked reduction was observed at 24 h. The importance of complement in APAP-induced liver injury was confirmed by using C3( / ) mice. For this study, an APAP dose of 400 mg/kg was chosen, and the animals were examined at 12 h, because in the previous study some APAPtreated animals died by 24 h at this dose. Plasma ALT activity was attenuated by 70 and 50% at 6 and 12 h, respectively, in C3( / ) mice treated with 400 mg/kg APAP compared with wild-type controls (Fig. 5A). Liver histopathology also revealed reduced centrilobular necrosis and hemorrhage in C3( / ) mice at 12 h compared with wild-type controls (Fig. 5B). Morphometric analysis of hepatic lesions at 12 h indicated that the percentage of the area that was necrotic was reduced in APAP-treated C3( / ) mice ( %) compared with APAP-treated wild-type mice ( %; Fig. 6. Hepatic neutrophil (PMN) accumulation after APAP administration. A, representative liver sections from mice treated with saline (a) or CVF (b) then given APAP (300 mg/kg); livers were harvested 24 h after treatment with APAP. Liver sections were immunohistochemically stained for infiltrating PMNs (red chromagen; arrows) and counterstained with hematoxylin as described under Materials and Methods. B, morphometric determination of PMN accumulation in sections of liver 6 or 24 h after APAP treatment. Data represent mean S.E.M. of PMNs counted in 10 microscopic fields at 100 magnification., significantly different from respective value in the absence of APAP. #, significantly different from respective value in the absence of CVF.
6 382 Singhal et al. P 0.05). APAP treatment resulted in reduction of hepatic GSH to and mol GSH/g liver at 50 min post-treatment in C3( / ) mice and controls, respectively (no significant difference), suggesting that C3 gene deficiency did not affect the bioactivation of APAP. Complement Depletion in APAP-Treated Mice Reduces Hepatic Neutrophil Infiltration and Cytokine Expression. Activation of C3 and C5 results in formation of the C3a and C5a peptides. C3a and C5a are chemotactic for neutrophils (PMNs) and macrophages and can activate these cells in addition to endothelial cells, platelets, basophils, and mast cells (Walport, 2001; Ward, 2004). Accordingly, PMN sequestration in liver was assessed in APAP-treated mice that were depleted of complement. Treatment with APAP caused significant PMN accumulation in livers at 6 h, and this increased by 24 h (Fig. 6). Although CVF treatment by itself resulted in very modest PMN accumulation, it significantly reduced APAP-induced PMN accumulation at both 6 and 24 h compared with APAP treatment alone (Fig. 6). A similar result was observed in C3( / ) mice treated with 400 mg/kg APAP: C3( / ) mice had significantly reduced (50 3 PMN/field) APAP-induced PMN accumulation at 12 h compared with wild-type controls (126 4 PMN/field; P 0.05). Increased expression of cytokines occurs during the pathogenesis of APAP-induced liver injury (Blazka et al., 1995). Hepatic mrna expression for IL-6 and IL-10 was increased by APAP (Fig. 7A). CVF treatment alone did not affect expression of these cytokines but significantly reduced the increase in their expression caused by APAP. Hepatic mrna expression of IL-6 and IL-10 was also reduced in APAPtreated C3( / ) mice by 60% (P 0.075) and 84% (P 0.05), respectively, compared with control mice. Plasma concentrations of TNF- in saline- and CVFtreated mice were below the limit of detection (16 pg/ml) of the enzyme-linked immunosorbent assay. APAP treatment increased TNF- concentration in plasma at 6 and 12 h (Fig. 7B), and this increase was significantly attenuated by CVF pretreatment. As reported previously (Ganey et al., 2007; Bajt et al., 2008), APAP treatment increased PAI-1 concentration in plasma at 6 and 24 h; this increase was reduced by CVF pretreatment by 85% at 24 h. Complement depletion did not affect the increase in PAI-1 at 6 h after APAP administration (Fig. 7C). Complement Depletion Enhances Hepatocellular Viability. In a separate study, we evaluated progression of liver injury and regeneration between 24 and 48 h. Consistent with previous reports (Dambach et al., 2002; Chiu et al., 2003; Aibo et al., 2010) activity of ALT in plasma decreased between 24 and 48 h after treatment with APAP (Fig. 8A). CVF pretreatment resulted in significant reduction in APAPmediated increase in plasma ALT activity at both of these times compared with APAP treatment alone. The mrna expression of biomarkers of cell cycle activation, i.e., PCNA and CyclinD1, was significantly elevated by CVF pretreatment in APAP-exposed mice, whereas expression of p21, a protein involved in inhibition of cell cycle, was reduced by CVF pretreatment (Fig. 8B). In a separate study, BrdU was administered 2 h before euthanizing mice at 24 or 48 h after APAP treatment to identify cells undergoing DNA synthesis at those times. At 24 h, BrdU incorporation was not significantly affected by APAP treatment, but a significant increase in BrdU-positive cells was observed in CVF/APAP-treated Fig. 7. Cytokine mrna expression or protein concentration after APAP administration. Mice were pretreated with CVF or saline then given APAP (300 mg/kg) or saline vehicle. A, mrna expression of IL-6 and IL-10 was determined in livers harvested 24 h after APAP administration. Expression levels were normalized to that of the GAPDH housekeeping gene and represented as percentage of saline control. B, TNF- concentration in plasma was determined 6 or 12 h after administration of APAP. C, PAI-1 concentration in plasma was determined 6 or 24 h after administration of APAP. Data represent mean S.E.M., significantly different from respective value in the absence of APAP. #, significantly different from respective value in the absence of CVF. n 3 5 per group; p mice at 24 h, suggesting that more cells were replicating in these mice compared with those treated with APAP alone. At 48 h, the number of BrdU-positive cells was markedly greater in livers of APAP-treated mice, and the BrdU incorporation was not different in mice cotreated with CVF (Fig. 8,
7 Complement Activation in APAP Hepatotoxicity 383 C and D). Liver sections from mice treated only with saline or CVF had few BrdU-positive cells at either 24 or 48 h. Discussion Although studies of patients suffering from APAP overdose have been reported extensively over the past 30 years, surprisingly few reports have examined complement activation. In this study, we present evidence in a murine model of APAP overdose that 1) APAP treatment activates the complement pathway, 2) complement activation contributes to the progression of liver injury from hepatotoxic doses of APAP and 3) hepatic regeneration in response to APAP-induced injury occurs more rapidly in complement-depleted mice. Two clinical studies reported a reduction in complement proteins in serum from APAP-overdosed patients. Ellison et al. (1990) compared total hemolytic activity, expressed in CH50 units, and the concentrations of complement components in serum from 15 patients with liver disease caused by alcohol toxicity and only 1 patient with APAP hepatotoxicity. In comparison with healthy subjects, patients under study had reduced CH50 values and small concentrations of C1q, C3, C4, and C5 in serum (Ellison et al., 1990). Clapperton et al. (1997) studied 14 patients with acute liver failure caused by APAP overdose and found a significantly smaller C3 concentration in plasma from patients ( mg/ml) compared with healthy control subjects ( mg/ml). Similar to these clinical findings, mice treated with APAP ( mg/kg) exhibited reduced plasma C3 concentration (Fig. 1). This was accompanied by accumulation of the C3 activation product, C3b/iC3b/C3c, in the necrotic centrilobular regions of livers (Fig. 2). Together, these results suggest a deficiency in plasma Fig. 8. Hepatocellular viability after APAP treatment. Mice were pretreated with CVF or saline then given APAP (300 mg/kg) and killed 24 or 48 h after APAP administration. A, plasma ALT activity measurement. B, hepatic mrna expression 24 h after APAP administration. Expression levels were normalized to that of the GAPDH housekeeping gene and represented as percentage of Sal/Sal (not shown) control. C, mice pretreated with CVF or saline and then treated with APAP (300 mg/kg) or saline were given BrdU (50 mg/kg) 2 h before euthanasia at 24 or 48 h after APAP administration. Liver sections were immunohistochemically stained for nuclear incorporation of BrdU (brown chromagen) and counterstained with hematoxylin as described under Materials and Methods. a and b, mice treated with Sal/APAP with their livers harvested at 24 h (a) and 48 h (b). c and d, mice treated with CVF/APAP with their livers harvested at 24 h (c) and 48 h (d). D, morphometric determination of BrdU-positive cells in section of livers 24 or 48 h after APAP treatment. Data represent mean S.E.M. of BrdU-positive cells counted in 10 microscopic fields at 100 magnification. In A, B, and D, data represent mean S.E.M., significantly different from respective value in the absence of APAP. #, significantly different from respective value in the absence of CVF. n 3 5 per group; p complement as a result of persistent complement activation during APAP hepatotoxicity. APAP treatment resulted in a dose- and time-dependent centrilobular necrosis and hemorrhage (Fig. 3). At the smallest dose of APAP used in these studies (200 mg/kg), complement was activated, but prior complement depletion did not affect hepatotoxicity. In contrast, at a markedly toxic APAP dose (400 mg/kg) that was lethal to some animals, complement was activated and contributed to the early progression of injury, as evidenced by the observation that complement depletion by CVF pretreatment reduced injury by 6 h. At an intermediate APAP dose (300 mg/kg) that also resulted in pronounced liver injury, complement seemed to contribute only to the later progression of injury, that is, the increase in severity that occurred between 12 and 24 h (Fig. 4C). Protection afforded by CVF was not a result of reduced bioactivation of APAP because GSH depletion was similar in APAP-treated mice pretreated with saline or CVF (Fig. 3). In support of the CVF results, C3 gene deletion also reduced liver injury at 6 and 12 h after a dose of 400 mg/kg APAP (Fig. 5). These results indicate that the participation of complement in the progression of APAP-induced hepatocellular injury clearly depended on the dose of APAP, with early contribution at a markedly hepatotoxic dose, later contribution at an intermediate dose, and no contribution despite complement activation at a mildly toxic dose. APAP hepatotoxicity is associated with cytokine release and influx of inflammatory cells, including PMNs (Blazka et al., 1995; Lawson et al., 2000; Ju et al., 2002). Complement depletion reduced, but did not completely abolish, APAP-mediated hepatic PMN accumulation as well as gene expression of IL-6 and IL-10 and plasma concentration of TNF-. PAI-1, which can be released by Kupffer cells in response to C5a (Kastl et al.,
8 384 Singhal et al. 2006), was also increased in plasma by APAP treatment and reduced by prior complement depletion. Taken together, these results suggest an important role of complement in APAPinduced liver injury, PMN accumulation, and cytokine release. The reduced accumulation of PMNs and release of cytokines is consistent with the reduction in the severity of APAP-induced liver injury by complement depletion, but cause and effect cannot be assigned. Evidence has been presented for roles of Kupffer cells, inflammatory cytokines, and PMNs in the progression of APAP-induced liver injury (Blazka et al., 1995; Laskin et al., 1995; Liu et al., 2006). However, contrasting results suggesting ameliorative influences of these factors have also been reported (Bourdi et al., 2002; Ju et al., 2002; Gardner et al., 2003; James et al., 2003; Masubuchi et al., 2003; Bajt et al., 2008), and their roles continue to be debated (Jaeschke, 2008; Jaeschke et al., 2011). Indeed, it remains a possibility that these and other factors have contrasting roles at different times and/or concentrations during the pathogenesis. Nevertheless, our results suggest that complement activation influences the hepatic accumulation of PMNs and the generation of cytokines during the progression of APAP hepatotoxicity. It is also possible that formation of the membrane attack complex plays a role in APAP hepatotoxicity; this will be investigated in future studies. It is likely that some alteration in, or injury to, hepatocytes caused early and directly by APAP exposure is required for complement activation. For example, the classic pathway of complement activation can be initiated by compromised mammalian cells undergoing apoptosis or release of heat shock proteins (Prohászka et al., 2002; Navratil et al., 2006). That neither complement depletion with CVF nor genetic deficiency in C3 protected completely against APAP toxicity suggests that additional factors play a role in the progression of injury. It is noteworthy that hepatocytes damaged by APAP release various death-associated molecular pattern molecules, and evidence has been presented for a role for these in the progression of APAP-induced hepatocellular necrosis (Imaeda et al., 2009; Martin-Murphy et al., 2010; Dear et al., 2011). Accordingly, complement activation is likely only one of several events responsible for injury progression in APAP overdose. Treatment with 300 mg/kg APAP resulted in pronounced liver injury by 24 h followed by cell proliferation (i.e., BrdU incorporation into DNA) that was associated with reduced ALT activity in plasma at 48 h (Fig. 8A). In addition to reducing the progression of necrosis, complement depletion resulted in greater numbers of replicating cells in and around the centrilobular lesions. This was evident by the greater numbers of BrdU-positive cells, increased mrna expression of markers of cell cycle activation (PCNA and CyclinD1 markers of G 1 /S transition), and reduced expression of the cell cycle inhibitor p21 (Fausto, 2000; Bajt et al., 2008; Aibo et al., 2010) at 24 h (Fig. 8). This suggests that attenuated liver injury associated with complement depletion enables early tissue regeneration and complement activation delays this reparative response to APAPinduced injury. It is noteworthy that this observation contrasts with effects of complement activation on liver regeneration in other models. For example, complement (C3 or C5)-deficient mice were found to have reduced prosurvival signaling, leading to impaired liver regeneration after partial hepatectomy (Markiewski et al., 2004) or carbon tetrachloride administration (Mastellos et al., 2001). In a study of partial hepatectomy, deficiency in complement components reduced the induction of cytokines IL-6 and TNF-, which have been shown to be required for the priming phase of liver regeneration in that model (Markiewski et al., 2009). In studies presented here, cell cycle activity was greater in APAP-treated mice pretreated with CVF despite reduced expression of IL-6 and TNF-. A possible explanation for this difference is that the concentrations of these proregenerative cytokines, although reduced after complement depletion, are sufficient enough to stimulate hepatocyte proliferation. In any case, complement depletion was associated with a reduction in hepatocellular necrosis as well as an enhanced proliferative response. In conclusion, complement activation is associated with APAP hepatotoxicity, and complement depletion reduces the progression of APAP-induced liver injury. Current therapy for APAP overdose includes treatment with N-acetylcysteine, which is an effective antidote if given early after APAP ingestion. Our results suggest that complement plays a role later in the pathogenesis and raise the possibility that interference with its activation or effects may reduce the progression of liver injury. Acknowledgments We thank Nicole Crisp, Ryan Albee, and Allen MacDonald for technical assistance. Authorship Contributions Participated in research design: Singhal, Ganey, and Roth. Conducted experiments: Singhal. Performed data analysis: Singhal. Wrote or contributed to the writing of the manuscript: Singhal, Ganey, and Roth. References Aibo DI, Birmingham NP, Lewandowski R, Maddox JF, Roth RA, Ganey PE, Wagner JG, and Harkema JR (2010) Acute exposure to ozone exacerbates acetaminopheninduced liver injury in mice. Toxicol Sci 115: Alper CA and Balavitch D (1976) Cobra venom factor: evidence for its being altered cobra C3 (the third component of complement). Science 191: Bajt ML, Yan HM, Farhood A, and Jaeschke H (2008) Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose. Toxicol Sci 104: Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1 5. Blazka ME, Wilmer JL, Holladay SD, Wilson RE, and Luster MI (1995) Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 133: Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, and Pohl LR (2002) Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology 35: Cai C, Gill R, Eum HA, Cao Z, Loughran PA, Darwiche S, Edmonds RD, Menzel CL, and Billiar TR (2010) Complement factor 3 deficiency attenuates hemorrhagic shock-related hepatic injury and systemic inflammatory response syndrome. Am J Physiol Regul Integr Comp Physiol 299:R1175 R1182. Chiu H, Gardner CR, Dambach DM, Brittingham JA, Durham SK, Laskin JD, and Laskin DL (2003) Role of p55 tumor necrosis factor receptor 1 in acetaminopheninduced antioxidant defense. Am J Physiol Gastrointest Liver Physiol 285:G959 G966. Clapperton M, Rolando N, Sandoval L, Davies E, and Williams R (1997) Neutrophil superoxide and hydrogen peroxide production in patients with acute liver failure. Eur J Clin Invest 27: Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, and Nagy LE (2010) Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanolinduced liver injury in mice. Gastroenterology 139: , 674.e1. Dambach DM, Watson LM, Gray KR, Durham SK, and Laskin DL (2002) Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology 35: Dear JW, Simpson KJ, Nicolai MP, Catterson JH, Street J, Huizinga T, Craig DG, Dhaliwal K, Webb S, Bateman DN, et al. (2011) Cyclophilin A is a damageassociated molecular pattern molecule that mediates acetaminophen-induced liver injury. J Immunol 187: Ellison RT 3rd, Horsburgh CR Jr, and Curd J (1990) Complement levels in patients with hepatic dysfunction. Dig Dis Sci 35: Fausto N (2000) Liver regeneration. J Hepatol 32 (Suppl 1): Ganey PE, Luyendyk JP, Newport SW, Eagle TM, Maddox JF, Mackman N, and
9 Roth RA (2007) Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology 46: Gardner CR, Laskin JD, Dambach DM, Chiu H, Durham SK, Zhou P, Bruno M, Gerecke DR, Gordon MK, and Laskin DL (2003) Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1. Potential role of inflammatory mediators. Toxicol Appl Pharmacol 192: He S, Atkinson C, Qiao F, Cianflone K, Chen X, and Tomlinson S (2009) A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J Clin Invest 119: Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, and Mehal WZ (2009) Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest 119: Jaeschke H (2008) Innate immunity and acetaminophen-induced liver injury: why so many controversies? Hepatology 48: Jaeschke H, McGill MR, Williams CD, and Ramachandran A (2011) Current issues with acetaminophen hepatotoxicity a clinically relevant model to test the efficacy of natural products. Life Sci 88: James LP, Lamps LW, McCullough S, and Hinson JA (2003) Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun 309: Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, and Pohl LR (2002) Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol 15: Kaplowitz N (2004) Acetaminophen hepatoxicity: what do we know, what don t we know, and what do we do next? Hepatology 40: Kastl SP, Speidl WS, Kaun C, Rega G, Assadian A, Weiss TW, Valent P, Hagmueller GW, Maurer G, Huber K, et al. (2006) The complement component C5a induces the expression of plasminogen activator inhibitor-1 in human macrophages via NF- B activation. J Thromb Haemost 4: Koleva M, Schlaf G, Landmann R, Götze O, Jungermann K, and Schieferdecker HL (2002) Induction of anaphylatoxin C5a receptors in rat hepatocytes by lipopolysaccharide in vivo: mediation by interleukin-6 from Kupffer cells. Gastroenterology 122: Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, et al. (2005) Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42: Laskin DL, Gardner CR, Price VF, and Jollow DJ (1995) Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology 21: Lawson JA, Farhood A, Hopper RD, Bajt ML, and Jaeschke H (2000) The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci 54: Lee WM (2004) Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology 40:6 9. Liu ZX, Han D, Gunawan B, and Kaplowitz N (2006) Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 43: Markiewski MM, DeAngelis RA, Strey CW, Foukas PG, Gerard C, Gerard N, Wetsel RA, and Lambris JD (2009) The regulation of liver cell survival by complement. J Immunol 182: Markiewski MM, Mastellos D, Tudoran R, DeAngelis RA, Strey CW, Franchini S, Wetsel RA, Erdei A, and Lambris JD (2004) C3a and C3b activation products of the Complement Activation in APAP Hepatotoxicity 385 third component of complement (C3) are critical for normal liver recovery after toxic injury. J Immunol 173: Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, and Lambris JD (2007) Complement and coagulation: strangers or partners in crime? Trends Immunol 28: Martin-Murphy BV, Holt MP, and Ju C (2010) The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett 192: Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, and Lambris JD (2001) A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol 166: Mastellos D, Prechl J, László G, Papp K, Oláh E, Argyropoulos E, Franchini S, Tudoran R, Markiewski M, Lambris JD, et al. (2004) Novel monoclonal antibodies against mouse C3 interfering with complement activation: description of fine specificity and applications to various immunoassays. Mol Immunol 40: Masubuchi Y, Bourdi M, Reilly TP, Graf ML, George JW, and Pohl LR (2003) Role of interleukin-6 in hepatic heat shock protein expression and protection against acetaminophen-induced liver disease. Biochem Biophys Res Commun 304: Navratil JS, Liu CC, and Ahearn JM (2006) Apoptosis and autoimmunity. Immunol Res 36:3 12. Pepys MB (1975) Studies in vivo of cobra factor and murine C3. Immunology 28: Prohászka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, and Füst G (2002) Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones 7: Rawal N and Pangburn MK (2001) Structure/function of C5 convertases of complement. Int Immunopharmacol 1: Rensen SS, Slaats Y, Driessen A, Peutz-Kootstra CJ, Nijhuis J, Steffensen R, Greve JW, and Buurman WA (2009) Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology 50: Roychowdhury S, McMullen MR, Pritchard MT, Hise AG, van Rooijen N, Medof ME, Stavitsky AB, and Nagy LE (2009) An early complement-dependent and TLR-4- independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology 49: Scaffidi P, Misteli T, and Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: Singhal R, Badger TM, and Ronis MJ (2009) Estrogenic status modulates the effect of soy on hepatic responses to 7,12-dimethylbenz(a)anthracene (DMBA). Toxicol Appl Pharmacol 234: Walport MJ (2001) Complement. Second of two parts. N Engl J Med 344: Ward PA (2004) The dark side of C5a in sepsis. Nat Rev Immunol 4: Yee SB, Hanumegowda UM, Hotchkiss JA, Ganey PE, and Roth RA (2003) Role of neutrophils in the synergistic liver injury from monocrotaline and bacterial lipopolysaccharide exposure. Toxicol Sci 72: Address correspondence to: Dr. Robert A. Roth, Michigan State University, 221 Food Safety and Toxicology Building, East Lansing, MI rothr@msu.edu
Pair-fed % inkt cells 0.5. EtOH 0.0
 MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
The liver in poisoning: what can we learn from animal models?
 The liver in poisoning: what can we learn from animal models? Stephan Krähenbühl Clinical Pharmacology & Toxicology University Hospital 4031 Basel/Switzerland Kraehenbuehl@uhbs.ch Outcome and causes of
The liver in poisoning: what can we learn from animal models? Stephan Krähenbühl Clinical Pharmacology & Toxicology University Hospital 4031 Basel/Switzerland Kraehenbuehl@uhbs.ch Outcome and causes of
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
IKKα Causes Chromatin Modification on Pro-Inflammatory Genes by Cigarette Smoke in Mouse Lung
 IKKα Causes Chromatin Modification on Pro-Inflammatory Genes by Cigarette Smoke in Mouse Lung Se-Ran Yang, Samantha Valvo, Hongwei Yao, Aruna Kode, Saravanan Rajendrasozhan, Indika Edirisinghe, Samuel
IKKα Causes Chromatin Modification on Pro-Inflammatory Genes by Cigarette Smoke in Mouse Lung Se-Ran Yang, Samantha Valvo, Hongwei Yao, Aruna Kode, Saravanan Rajendrasozhan, Indika Edirisinghe, Samuel
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
The predictive value of serum alanine transaminase activity on first presentation to hospital in patients with paracetamol poisoning
 The predictive value of serum alanine transaminase activity on first presentation to hospital in patients with paracetamol poisoning Khalid Al-Hourani, Rachel Mansi, Janice Pettie, Margaret Dow, Nick Bateman
The predictive value of serum alanine transaminase activity on first presentation to hospital in patients with paracetamol poisoning Khalid Al-Hourani, Rachel Mansi, Janice Pettie, Margaret Dow, Nick Bateman
SUPPLEMENTARY METHODS
 SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
Elaborating ELAD Mechanism of Action and Linking Cell-Based Models to the Clinic
 Elaborating ELAD Mechanism of Action and Linking Cell-Based Models to the Clinic September 9, 2017 Patricia W Bedard, Jason Lapetoda, Jagadeesha Dammanahalli, Jessica Van Allen, Susan Lin, Lee Landeen
Elaborating ELAD Mechanism of Action and Linking Cell-Based Models to the Clinic September 9, 2017 Patricia W Bedard, Jason Lapetoda, Jagadeesha Dammanahalli, Jessica Van Allen, Susan Lin, Lee Landeen
Effect of N-Acetylcysteine on Acetaminophen Toxicity in Mice: Relationship to Reactive Nitrogen and Cytokine Formation
 TOXICOLOGICAL SCIENCES 75, 458 467 (2003) DOI: 10.1093/toxsci/kfg181 Effect of N-Acetylcysteine on Acetaminophen Toxicity in Mice: Relationship to Reactive Nitrogen and Cytokine Formation Laura P. James,*,,1
TOXICOLOGICAL SCIENCES 75, 458 467 (2003) DOI: 10.1093/toxsci/kfg181 Effect of N-Acetylcysteine on Acetaminophen Toxicity in Mice: Relationship to Reactive Nitrogen and Cytokine Formation Laura P. James,*,,1
االستاذ المساعد الدكتور خالد ياسين الزاملي \مناعة \المرحلة الثانية \ التحليالت المرضية \ المعهد التقني كوت
 Complement System The term complement refers to the ability of a system of some nonspecific proteins in normal human serum to complement, i.e., augment the effects of other components of immune system,
Complement System The term complement refers to the ability of a system of some nonspecific proteins in normal human serum to complement, i.e., augment the effects of other components of immune system,
This document has been downloaded from Tampub The Institutional Repository of University of Tampere. Publisher's version
 This document has been downloaded from Tampub The Institutional Repository of University of Tampere Publisher's version Authors: Yang Runkuan, Zou Xiaoping, Koskinen Marja-Leena, Tenhunen Jyrki Ethyl pyruvate
This document has been downloaded from Tampub The Institutional Repository of University of Tampere Publisher's version Authors: Yang Runkuan, Zou Xiaoping, Koskinen Marja-Leena, Tenhunen Jyrki Ethyl pyruvate
Human Recombinant Vascular Endothelial Growth Factor Reduces Necrosis and Enhances Hepatocyte Regeneration in a Mouse Model of Acetaminophen Toxicity
 22-3565/1/3341-33 43$2. THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 334, No. 1 Copyright 21 by The American Society for Pharmacology and Experimental Therapeutics 16384/3595618 JPET
22-3565/1/3341-33 43$2. THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 334, No. 1 Copyright 21 by The American Society for Pharmacology and Experimental Therapeutics 16384/3595618 JPET
Topic (6): The Complement System
 Topic (6): The Complement System Introduction The complement system is a complex system of many different glycoproteins. It comprises several plasma proteins that sequentially activate each other by proteolytic
Topic (6): The Complement System Introduction The complement system is a complex system of many different glycoproteins. It comprises several plasma proteins that sequentially activate each other by proteolytic
2. Innate immunity 2013
 1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells
 1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization
 Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
The term complement refers to the ability of a system of some nonspecific proteins in normal human serum to complement, i.e., augment the effects of
 COMPLEMENT SYSTEM The term complement refers to the ability of a system of some nonspecific proteins in normal human serum to complement, i.e., augment the effects of other components of immune system,
COMPLEMENT SYSTEM The term complement refers to the ability of a system of some nonspecific proteins in normal human serum to complement, i.e., augment the effects of other components of immune system,
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury
 Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice
 Research article A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice Songqing He, 1,2 Carl Atkinson, 1 Fei Qiao, 1 Katherine Cianflone, 3 Xiaoping
Research article A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice Songqing He, 1,2 Carl Atkinson, 1 Fei Qiao, 1 Katherine Cianflone, 3 Xiaoping
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
PhD thesis. The role of complement in Experimental Autoimmune Encephalomyelitis, the mouse modell of Multiple Sclerosis
 PhD thesis The role of complement in Experimental Autoimmune Encephalomyelitis, the mouse modell of Multiple Sclerosis Nóra Terényi Supervisor: Prof. Anna Erdei Biology Doctorate School Immunology Program
PhD thesis The role of complement in Experimental Autoimmune Encephalomyelitis, the mouse modell of Multiple Sclerosis Nóra Terényi Supervisor: Prof. Anna Erdei Biology Doctorate School Immunology Program
Systemic inflammation after myocardial infarction
 Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2013 Systemic inflammation after myocardial infarction Rudiger, Alain DOI:
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2013 Systemic inflammation after myocardial infarction Rudiger, Alain DOI:
Evaluation of directed and random motility in microslides Assessment of leukocyte adhesion in flow chambers
 Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Immunology lecture: 14. Cytokines: Main source: Fibroblast, but actually it can be produced by other types of cells
 Immunology lecture: 14 Cytokines: 1)Interferons"IFN" : 2 types Type 1 : IFN-Alpha : Main source: Macrophages IFN-Beta: Main source: Fibroblast, but actually it can be produced by other types of cells **There
Immunology lecture: 14 Cytokines: 1)Interferons"IFN" : 2 types Type 1 : IFN-Alpha : Main source: Macrophages IFN-Beta: Main source: Fibroblast, but actually it can be produced by other types of cells **There
Anastasios E. Germenis
 Anastasios E. Germenis Professor and Chairman Department of Immunology & Histocompatibility School of Medicine University of Thessaly University Hospital of Larissa Greece agermen@med.uth.gr The Complement
Anastasios E. Germenis Professor and Chairman Department of Immunology & Histocompatibility School of Medicine University of Thessaly University Hospital of Larissa Greece agermen@med.uth.gr The Complement
Protein isolate from the herb, Phyllanthus niruri, protects liver from acetaminophen induced toxicity
 Protein isolate from the herb, Phyllanthus niruri, protects liver from acetaminophen induced toxicity Author(s): Rajesh Bhattacharjee and Parames C. Sil Vol. 17, No. 1 (2006-01 - 2006-04) Biomedical Research
Protein isolate from the herb, Phyllanthus niruri, protects liver from acetaminophen induced toxicity Author(s): Rajesh Bhattacharjee and Parames C. Sil Vol. 17, No. 1 (2006-01 - 2006-04) Biomedical Research
Innate immunity. Abul K. Abbas University of California San Francisco. FOCiS
 1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Complement. Definition : series of heat-labile serum proteins. : serum and all tissue fluids except urine and CSF
 Complement Complement Definition : series of heat-labile serum proteins Site : serum and all tissue fluids except urine and CSF Synthesis : in liver appear in fetal circulation during 1 st 13 W Function
Complement Complement Definition : series of heat-labile serum proteins Site : serum and all tissue fluids except urine and CSF Synthesis : in liver appear in fetal circulation during 1 st 13 W Function
Granulocyte and lymphocyte proteins
 Granulocyte and lymphocyte proteins Hycult Biotech is a leading world-class manufacturer of research reagents in the field of innate immunity. We are a specialized partner in the development and manufacturing
Granulocyte and lymphocyte proteins Hycult Biotech is a leading world-class manufacturer of research reagents in the field of innate immunity. We are a specialized partner in the development and manufacturing
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive
 Online Data Supplement: Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma Dan Cheng, Zheng Xue, Lingling Yi, Huimin Shi, Kan Zhang, Xiaorong Huo, Luke R. Bonser,
Online Data Supplement: Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma Dan Cheng, Zheng Xue, Lingling Yi, Huimin Shi, Kan Zhang, Xiaorong Huo, Luke R. Bonser,
Alcoholic hepatitis is a drug-induced disorder
 Alcoholic hepatitis is a drug-induced disorder Gyongyi Szabo, MD, PhD Professor of Medicine University of Massachusetts Medical School Source: 2 Sobernation.com Clinical Progression of ALD Mortality Acute
Alcoholic hepatitis is a drug-induced disorder Gyongyi Szabo, MD, PhD Professor of Medicine University of Massachusetts Medical School Source: 2 Sobernation.com Clinical Progression of ALD Mortality Acute
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Overview of the immune system
 Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
THE COMPLEMENT SYSTEM OBJECTIVES:
 Dr Mohammed Al- ani THE COMPLEMENT SYSTEM OBJECTIVES: When you finish this section, you should be able to: 1. Describe the effects of complement activation. 2. Outline the Classical, Mannan-Binding (MB)
Dr Mohammed Al- ani THE COMPLEMENT SYSTEM OBJECTIVES: When you finish this section, you should be able to: 1. Describe the effects of complement activation. 2. Outline the Classical, Mannan-Binding (MB)
Ligand-mediated cytoplasmic retention of the Ah receptor inhibits. Gulsum E. Muku, Tejas S. Lahoti, Iain A. Murray, Michael A.
 Ligand-mediated cytoplasmic retention of the Ah receptor inhibits macrophage mediated acute inflammatory responses Gulsum E. Muku, Tejas S. Lahoti, Iain A. Murray, Michael A. Podolsky, Kayla Smith, Troy
Ligand-mediated cytoplasmic retention of the Ah receptor inhibits macrophage mediated acute inflammatory responses Gulsum E. Muku, Tejas S. Lahoti, Iain A. Murray, Michael A. Podolsky, Kayla Smith, Troy
Effective Targeting of Quiescent Chronic Myelogenous
 Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
Cancer Cell, Volume 7 Supplemental Information Effective Targeting of Quiescent Chronic Myelogenous Leukemia Stem Cells by Histone Deacetylase Inhibitors in Combination with Imatinib Mesylate Bin Zhang,
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.
 Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Stimulating healthy tissue regeneration by targeting the 5-HT 2B receptor in chronic liver disease.
 Stimulating healthy tissue regeneration by targeting the 5-HT 2B receptor in chronic liver disease. Mohammad R Ebrahimkhani, Fiona Oakley, Lindsay B Murphy, Jelena Mann, Anna Moles, Maria J Perugorria,
Stimulating healthy tissue regeneration by targeting the 5-HT 2B receptor in chronic liver disease. Mohammad R Ebrahimkhani, Fiona Oakley, Lindsay B Murphy, Jelena Mann, Anna Moles, Maria J Perugorria,
The Role of Hypoxia Inducible Factor-1 alpha (HIF-1α) in Acetaminophen Hepatotoxicity
 JPET This Fast article Forward. has not been Published copyedited and on formatted. May 16, The 2011 final as version DOI:10.1124/jpet.111.180521 may differ from this version. 1 JPET #180521 TITLE PAGE
JPET This Fast article Forward. has not been Published copyedited and on formatted. May 16, The 2011 final as version DOI:10.1124/jpet.111.180521 may differ from this version. 1 JPET #180521 TITLE PAGE
Serum microrna biomarkers for human DILI
 CLEARANCE DRUG bioactivation covalent binding Detoxication GLUCURONIDE SULPHATE DRUG + METABOLITE chemical stress mitochondrial dysfunction apoptosis necrosis hepatocyte hypertrophy hepatocyte hyperplasia
CLEARANCE DRUG bioactivation covalent binding Detoxication GLUCURONIDE SULPHATE DRUG + METABOLITE chemical stress mitochondrial dysfunction apoptosis necrosis hepatocyte hypertrophy hepatocyte hyperplasia
Immunology Part II. Innate Immunity. 18. April 2018, Ruhr-Universität Bochum Marcus Peters,
 Immunology Part II Innate Immunity 18. April 2018, Ruhr-Universität Bochum Marcus Peters, marcus.peters@rub.de Conserved structures of pathogens PAMPs are detected by Pattern Recognition Receptors PRRs
Immunology Part II Innate Immunity 18. April 2018, Ruhr-Universität Bochum Marcus Peters, marcus.peters@rub.de Conserved structures of pathogens PAMPs are detected by Pattern Recognition Receptors PRRs
CHAPTER VII EFFECT OF H. ZEYLANICA, R. NASUTA AND S. CILIATA ON PARACETAMOL - INDUCED HEPATOTOXICITY IN WISTAR RATS
 CHAPTER VII EFFECT OF H. ZEYLANICA, R. NASUTA AND S. CILIATA ON PARACETAMOL - INDUCED HEPATOTOXICITY IN WISTAR RATS 7.1. Introduction Paracetamol is a remarkably safe drug at therapeutic doses, but it
CHAPTER VII EFFECT OF H. ZEYLANICA, R. NASUTA AND S. CILIATA ON PARACETAMOL - INDUCED HEPATOTOXICITY IN WISTAR RATS 7.1. Introduction Paracetamol is a remarkably safe drug at therapeutic doses, but it
How the Innate Immune System Profiles Pathogens
 How the Innate Immune System Profiles Pathogens Receptors on macrophages, neutrophils, dendritic cells for bacteria and viruses Broad specificity - Two main groups of bacteria: gram positive, gram-negative
How the Innate Immune System Profiles Pathogens Receptors on macrophages, neutrophils, dendritic cells for bacteria and viruses Broad specificity - Two main groups of bacteria: gram positive, gram-negative
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Anti-Apoptotic Effects of Cellular Therapy
 Anti-Apoptotic Effects of Cellular Therapy Jason Lapetoda 1, Lee K Landeen, PhD 1, George K Michalopoulos, MD 2, Patricia W Bedard, PhD 1 1 Vital Therapies, Inc., San Diego, CA, USA 2 University of Pittsburgh
Anti-Apoptotic Effects of Cellular Therapy Jason Lapetoda 1, Lee K Landeen, PhD 1, George K Michalopoulos, MD 2, Patricia W Bedard, PhD 1 1 Vital Therapies, Inc., San Diego, CA, USA 2 University of Pittsburgh
Cutaneous Immunology: Innate Immune Responses. Skin Biology Lecture Series
 Cutaneous Immunology: Innate Immune Responses Skin Biology Lecture Series The Immune Response: Innate and Adaptive Components Source: Wolff, Goldsmith, Katz, Gilchrest, Paller, Leffell. Fitzpatrick s Dermatology
Cutaneous Immunology: Innate Immune Responses Skin Biology Lecture Series The Immune Response: Innate and Adaptive Components Source: Wolff, Goldsmith, Katz, Gilchrest, Paller, Leffell. Fitzpatrick s Dermatology
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes
 Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
Introduction. A system of soluble enzymes and proteins. Complement components: C1 to C9, B, D and P
 Complement Introduction A system of soluble enzymes and proteins Complement components: C1 to C9, B, D and P When activated, each component is split into small and large (major) fragments a b *A horizontal
Complement Introduction A system of soluble enzymes and proteins Complement components: C1 to C9, B, D and P When activated, each component is split into small and large (major) fragments a b *A horizontal
Is there a common mechanism of DILI, do we need to know?
 Is there a common mechanism of DILI, do we need to know? Neil Kaplowitz, MD USC Research Center for Liver Disease Los Angeles, California IS THERE A COMMON MECHANISM OF DILI? ANSWER YES & NO Mechanisms
Is there a common mechanism of DILI, do we need to know? Neil Kaplowitz, MD USC Research Center for Liver Disease Los Angeles, California IS THERE A COMMON MECHANISM OF DILI? ANSWER YES & NO Mechanisms
Complement Antibodies and Proteins
 COMPLEMENT ANTIBODIES AND PROTEINS FROM CEDARLANE INTERNATIONAL VERSION www.cedarlanelabs.com For over 50 years, Cedarlane has provided complement related products to the life science research and diagnostic
COMPLEMENT ANTIBODIES AND PROTEINS FROM CEDARLANE INTERNATIONAL VERSION www.cedarlanelabs.com For over 50 years, Cedarlane has provided complement related products to the life science research and diagnostic
Supplemental Information. Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection. Cell Host & Microbe, Volume 15
 Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:!
 LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
Phosphate buffered saline (PBS) for washing the cells TE buffer (nuclease-free) ph 7.5 for use with the PrimePCR Reverse Transcription Control Assay
 Catalog # Description 172-5080 SingleShot Cell Lysis Kit, 100 x 50 µl reactions 172-5081 SingleShot Cell Lysis Kit, 500 x 50 µl reactions For research purposes only. Introduction The SingleShot Cell Lysis
Catalog # Description 172-5080 SingleShot Cell Lysis Kit, 100 x 50 µl reactions 172-5081 SingleShot Cell Lysis Kit, 500 x 50 µl reactions For research purposes only. Introduction The SingleShot Cell Lysis
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: van Seters M, van Beurden M, ten Kate FJW, et al. Treatment
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: van Seters M, van Beurden M, ten Kate FJW, et al. Treatment
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
c Ischemia (30 min) Reperfusion (8 w) Supplementary Figure bp 300 bp Ischemia (30 min) Reperfusion (4 h) Dox 20 mg/kg i.p.
 a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
Supplementary Materials and Methods
 Supplementary Materials and Methods Hepatocyte toxicity assay. Freshly isolated hepatocytes were incubated for overnight with varying concentrations (-25 µm) of sodium glycochenodeoxycholate (GCDC) or
Supplementary Materials and Methods Hepatocyte toxicity assay. Freshly isolated hepatocytes were incubated for overnight with varying concentrations (-25 µm) of sodium glycochenodeoxycholate (GCDC) or
For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin
 Materials and Methods Pair Feeding Experiment For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin (0.005%), which is average daily food intake of mice fed control HFD ad libitum at week
Materials and Methods Pair Feeding Experiment For pair feeding, mice were fed 2.7g of HFD containing tofogliflozin (0.005%), which is average daily food intake of mice fed control HFD ad libitum at week
Effec<ve Use of PI3K and MEK Inhibitors to Treat Mutant K Ras G12D and PIK3CA H1047R Murine Lung Cancers
 Effec
Effec
FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH
 FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH ALETA A SOURCE OF 1,3-BETA GLUCANS Aleta is highly bioavailable, offering a concentration greater than 5% of 1,3-beta glucans. Aleta provides a consistent response
FOR OPTIMAL GUT HEALTH KEMIN.COM/GUTHEALTH ALETA A SOURCE OF 1,3-BETA GLUCANS Aleta is highly bioavailable, offering a concentration greater than 5% of 1,3-beta glucans. Aleta provides a consistent response
Macrophage Activation & Cytokine Release. Dendritic Cells & Antigen Presentation. Neutrophils & Innate Defense
 Macrophage Activation & Cytokine Release Dendritic Cells & Antigen Presentation Neutrophils & Innate Defense Neutrophils Polymorphonuclear cells (PMNs) are recruited to the site of infection where they
Macrophage Activation & Cytokine Release Dendritic Cells & Antigen Presentation Neutrophils & Innate Defense Neutrophils Polymorphonuclear cells (PMNs) are recruited to the site of infection where they
Hepatotoxicity and ALT/AST Enzymes Activities Change in Therapeutic and Toxic Doses Consumption of Acetaminophen in Rats
 IBBJ Summer 2017, Vol 3, No 3 Original Article Hepatotoxicity and ALT/AST Enzymes Activities Change in Therapeutic and Toxic Doses Consumption of Acetaminophen in Rats Pouria jarsiah 1, Anahita Nosrati
IBBJ Summer 2017, Vol 3, No 3 Original Article Hepatotoxicity and ALT/AST Enzymes Activities Change in Therapeutic and Toxic Doses Consumption of Acetaminophen in Rats Pouria jarsiah 1, Anahita Nosrati
Clinical Basis of the Immune Response and the Complement Cascade
 Clinical Basis of the Immune Response and the Complement Cascade Bryan L. Martin, DO, MMAS, FACAAI, FAAAAI, FACOI, FACP Emeritus Professor of Medicine and Pediatrics President, American College of Allergy,
Clinical Basis of the Immune Response and the Complement Cascade Bryan L. Martin, DO, MMAS, FACAAI, FAAAAI, FACOI, FACP Emeritus Professor of Medicine and Pediatrics President, American College of Allergy,
Supplementary Figure 1. Expression of CUGBP1 in non-parenchymal liver cells treated with TGF-β
 Supplementary Figures Supplementary Figure 1. Expression of CUGBP1 in non-parenchymal liver cells treated with TGF-β and LPS. Non-parenchymal liver cells were isolated and treated with or without TGF-β
Supplementary Figures Supplementary Figure 1. Expression of CUGBP1 in non-parenchymal liver cells treated with TGF-β and LPS. Non-parenchymal liver cells were isolated and treated with or without TGF-β
Supplemental Information
 Electronic Supplementary Material (ESI) for Food & Function. This journal is The Royal Society of Chemistry 2016 Supplemental Information Supplementary Materials and Methods Materials Assay kits of total
Electronic Supplementary Material (ESI) for Food & Function. This journal is The Royal Society of Chemistry 2016 Supplemental Information Supplementary Materials and Methods Materials Assay kits of total
Receptor-interacting Protein Kinases Mediate Necroptosis In Neural Tissue Damage After Spinal Cord Injury
 Receptor-interacting Protein Kinases Mediate Necroptosis In Neural Tissue Damage After Spinal Cord Injury Haruo Kanno, M.D., Ph.D., Hiroshi Ozawa, M.D., Ph.D., Satoshi Tateda, M.D., Kenichiro Yahata, M.D.,
Receptor-interacting Protein Kinases Mediate Necroptosis In Neural Tissue Damage After Spinal Cord Injury Haruo Kanno, M.D., Ph.D., Hiroshi Ozawa, M.D., Ph.D., Satoshi Tateda, M.D., Kenichiro Yahata, M.D.,
RNA extraction, RT-PCR and real-time PCR. Total RNA were extracted using
 Supplementary Information Materials and Methods RNA extraction, RT-PCR and real-time PCR. Total RNA were extracted using Trizol reagent (Invitrogen,Carlsbad, CA) according to the manufacturer's instructions.
Supplementary Information Materials and Methods RNA extraction, RT-PCR and real-time PCR. Total RNA were extracted using Trizol reagent (Invitrogen,Carlsbad, CA) according to the manufacturer's instructions.
Supporting Information
 Supporting Information Fujishita et al. 10.1073/pnas.0800041105 SI Text Polyp Scoring. Intestinal polyps were counted as described (1). Briefly, the small and large intestines were excised, washed with
Supporting Information Fujishita et al. 10.1073/pnas.0800041105 SI Text Polyp Scoring. Intestinal polyps were counted as described (1). Briefly, the small and large intestines were excised, washed with
Fc receptors, phagocytosis role 128
 Subject Index Adaptive immunity dependence on innate immunity 9, 10 evolution 10 Aging anti-inflammatory agents in counteraction 202 beneficial polymorphisms 199 201 definition 18, 189 innate immunity
Subject Index Adaptive immunity dependence on innate immunity 9, 10 evolution 10 Aging anti-inflammatory agents in counteraction 202 beneficial polymorphisms 199 201 definition 18, 189 innate immunity
Ines Bohlinger, Mareel Leist, Johannes Barsig, Stefan Uhlig, Gisa Tiegs*, Albreeht Wendel
 a Ines Bohlinger, Mareel Leist, Johannes Barsig, Stefan Uhlig, Gisa Tiegs*, Albreeht Wendel o-galactosamine-sensitized mice challenged with tumor necrosis factor Q' (TNF) developed severe apoptotic and
a Ines Bohlinger, Mareel Leist, Johannes Barsig, Stefan Uhlig, Gisa Tiegs*, Albreeht Wendel o-galactosamine-sensitized mice challenged with tumor necrosis factor Q' (TNF) developed severe apoptotic and
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Mechanistic Toxicology
 SECOND EDITION Mechanistic Toxicology The Molecular Basis of How Chemicals Disrupt Biological Targets URS A. BOELSTERLI CRC Press Tavlor & France Croup CRC Press is an imp^t o* :H Taylor H Francn C'r,,jpi
SECOND EDITION Mechanistic Toxicology The Molecular Basis of How Chemicals Disrupt Biological Targets URS A. BOELSTERLI CRC Press Tavlor & France Croup CRC Press is an imp^t o* :H Taylor H Francn C'r,,jpi
Immunopathogenesis: Insights for Current and Future Therapies
 REVIEW Immunopathogenesis: Insights for Current and Future Therapies John M. Vierling, M.D., F.A.C.P. Autoimmune hepatitis (AIH) is a chronic, progressive, necroinflammatory disease caused by loss of tolerance
REVIEW Immunopathogenesis: Insights for Current and Future Therapies John M. Vierling, M.D., F.A.C.P. Autoimmune hepatitis (AIH) is a chronic, progressive, necroinflammatory disease caused by loss of tolerance
Supporting Information
 Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
ab LDL Uptake Assay Kit (Cell-Based)
 ab133127 LDL Uptake Assay Kit (Cell-Based) Instructions for Use For the detection of LDL uptake into cultured cells. This product is for research use only and is not intended for diagnostic use. Version
ab133127 LDL Uptake Assay Kit (Cell-Based) Instructions for Use For the detection of LDL uptake into cultured cells. This product is for research use only and is not intended for diagnostic use. Version
Supplementary Figure 1. H-PGDS deficiency does not affect GI tract functions and anaphylactic reaction. (a) Representative pictures of H&E-stained
 1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. H-PGDS deficiency does not affect GI tract functions and anaphylactic reaction. (a) Representative pictures of H&E-stained jejunum sections ( 200 magnification;
1 2 3 4 5 6 7 8 9 10 11 Supplementary Figure 1. H-PGDS deficiency does not affect GI tract functions and anaphylactic reaction. (a) Representative pictures of H&E-stained jejunum sections ( 200 magnification;
Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair
 Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair Mouse Model of Myocardial Infarction (MI) All animal work was compliant with the Institutional Animal
Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair Mouse Model of Myocardial Infarction (MI) All animal work was compliant with the Institutional Animal
Lecture on Innate Immunity and Inflammation
 Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration
 Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to
 Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Management of alcoholic hepatitis: Implications for options beyond the STOPAH study
 Management of alcoholic hepatitis: Implications for options beyond the STOPAH study Gyongyi Szabo, MD, PhD Professor University of Massachusetts Medical School Worcester, MA Cape Town, South Africa 2015
Management of alcoholic hepatitis: Implications for options beyond the STOPAH study Gyongyi Szabo, MD, PhD Professor University of Massachusetts Medical School Worcester, MA Cape Town, South Africa 2015
Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated,
 1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
LDL Uptake Flow Cytometry Assay Kit
 LDL Uptake Flow Cytometry Assay Kit Item No. 601470 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
LDL Uptake Flow Cytometry Assay Kit Item No. 601470 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
Original Article TLR4 contributes to mechanical ventilation induced lung injury in the rabbits
 Int J Clin Exp Pathol 2017;10(4):4700-4704 www.ijcep.com /ISSN:1936-2625/IJCEP0040416 Original Article TLR4 contributes to mechanical ventilation induced lung injury in the rabbits Hongmei Zhang 1, Pei
Int J Clin Exp Pathol 2017;10(4):4700-4704 www.ijcep.com /ISSN:1936-2625/IJCEP0040416 Original Article TLR4 contributes to mechanical ventilation induced lung injury in the rabbits Hongmei Zhang 1, Pei
Role of cyclooxygenase-2 in H5N1 viral pathogenesis and the potential use of its inhibitors
 Title Role of cyclooxygenase-2 in HN viral pathogenesis and the potential use of its inhibitors Author(s) Lee, MY; Cheung, CY; Peiris, JSM Citation Hong Kong Medical Journal, 2, v. 9 n. Suppl. 4, p. 29-
Title Role of cyclooxygenase-2 in HN viral pathogenesis and the potential use of its inhibitors Author(s) Lee, MY; Cheung, CY; Peiris, JSM Citation Hong Kong Medical Journal, 2, v. 9 n. Suppl. 4, p. 29-
Supplemental Information. T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism
 Immunity, Volume 33 Supplemental Information T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism Franziska Petermann, Veit Rothhammer, Malte
Immunity, Volume 33 Supplemental Information T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism Franziska Petermann, Veit Rothhammer, Malte
Kit for assay of thioredoxin
 FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
ROLE OF INTERLEUKIN-1β AND NEUTROPHIL ACTIVATION DURING ACETAMINOPHEN OVERDOSE 2012 CLARENCE DAVID WILLIAMS
 ROLE OF INTERLEUKIN-1β AND NEUTROPHIL ACTIVATION DURING ACETAMINOPHEN OVERDOSE BY 2012 CLARENCE DAVID WILLIAMS Submitted to the graduate degree program in Pharmacology, Toxicology and Therapeutics and
ROLE OF INTERLEUKIN-1β AND NEUTROPHIL ACTIVATION DURING ACETAMINOPHEN OVERDOSE BY 2012 CLARENCE DAVID WILLIAMS Submitted to the graduate degree program in Pharmacology, Toxicology and Therapeutics and
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Time course of immune response
 Time course of immune response Route of entry Route of entry (cont.) Steps in infection Barriers to infection Mf receptors Facilitate engulfment Glucan, mannose Scavenger CD11b/CD18 Allows immediate response
Time course of immune response Route of entry Route of entry (cont.) Steps in infection Barriers to infection Mf receptors Facilitate engulfment Glucan, mannose Scavenger CD11b/CD18 Allows immediate response
