Because the brain relies exclusively on glucose as a fuel source,
|
|
|
- Kerry Sharp
- 6 years ago
- Views:
Transcription
1 Functional identification of a neurocircuit regulating blood glucose Thomas H. Meek a, Jarrell T. Nelson a, Miles E. Matsen a, Mauricio D. Dorfman a, Stephan J. Guyenet a, Vincent Damian a, Margaret B. Allison b, Jarrad M. Scarlett a, Hong T. Nguyen a, Joshua P. Thaler a, David P. Olson b, Martin G. Myers Jr. c, Michael W. Schwartz a, and Gregory J. Morton a,1 a Diabetes and Obesity Center of Excellence, Department of Medicine, University of Washington, Seattle, WA 98195; b Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor, MI 48105; and c Department of Internal Medicine, University of Michigan, Ann Arbor, MI Edited by Gerald I. Shulman, Howard Hughes Medical Institute/Yale University, New Haven, CT, and approved February 16, 2016 (received for review October 28, 2015) Previous studies implicate the hypothalamic ventromedial nucleus (VMN) in glycemic control. Here, we report that selective inhibition of the subset of VMN neurons that express the transcription factor steroidogenic-factor 1 (VMN SF1 neurons) blocks recovery from insulin-induced hypoglycemia whereas, conversely, activation of VMN SF1 neurons causes diabetes-range hyperglycemia. Moreover, this hyperglycemic response is reproduced by selective activation of VMN SF1 fibers projecting to the anterior bed nucleus of the stria terminalis (abnst), but not to other brain areas innervated by VMN SF1 neurons. We also report that neurons in the lateral parabrachial nucleus (LPBN), a brain area that is also implicated in the response to hypoglycemia, make synaptic connections with the specific subset of glucoregulatory VMN SF1 neurons that project to the abnst. These results collectively establish a physiological role in glucose homeostasis for VMN SF1 neurons and suggest that these neurons are part of an ascending glucoregulatory LPBN VMN SF1 abnst neurocircuit. glucoregulatory circuit counter regulation ventromedial nucleus bed nucleus of the stria terminalis hyperglycemia Because the brain relies exclusively on glucose as a fuel source, brain function is rapidly compromised when circulating glucose levels drop below the normal range. Consequently, hypoglycemia elicits a robust, integrated, and redundant set of counterregulatory responses (CRRs) that ensure the rapid and efficient recovery of plasma glucose concentrations into the normal range (1). Components of the CRR include increased secretion of the hormones glucagon, epinephrine, and glucocorticoids, inhibition of glucoseinduced insulin secretion, increased sympathetic nervous system (SNS) outflow to the liver, and increased food intake (1 3). Owing to this redundancy, recovery from hypoglycemia is difficult to block in normal humans and animal models, even when adrenal or glucagon responses are prevented. Only when multiple responses are blocked is the ability to recover from hypoglycemia significantly compromised (4). This arrangement is perhaps unsurprising, given the threat to survival posed by hypoglycemia. Although glucose sensing can occur at peripheral (e.g., neurons innervating the hepatic portal vein) as well as central sites (3, 5), the brain is the organ responsible both for transducing this information into effective glucose counterregulation and for terminating this response once euglycemia is restored. Of the many brain areas that have been investigated, the hypothalamic ventromedial nucleus (VMN) has emerged as potentially being both necessary and sufficient to elicit this powerful response. This assertion is based on evidence that, whereas electrical stimulation of the VMN activates the CRR and thereby raises circulating glucose levels (6), glucose infusion directly into the VMN can suppress the CRR during hypoglycemia (7) and thereby impair recovery of normal blood glucose levels (8). Moreover, two recent papers identified a circuit comprised of neurons in the lateral parabrachial nucleus (LPBN) that project to the VMN, activation of which seems to be required for effective glucose counterregulation (9, 10). The relevant VMN neurons seem to be glutamatergic because genetic disruption of glutamatergic signaling within a specific subset of VMN neurons [known as steroidogenic factor-1 (SF1) neurons] also attenuates recovery from insulin-induced hypoglycemia (11). Together, these observations support a model in which, during hypoglycemia, inputs from the LPBN and other glucose-responsive neurocircuits converge upon and activate VMN neurons, and in which this activation is required for effective glucose counterregulation. Whether these VMN neurons or any other specific neuronal subset(s) are truly necessary or sufficient for this important adaptive response has yet to be established, however. Recent technological advances, including the use of optogenetics to selectively activate or inactivate neurons in a regionally and temporally specific manner, now enable such questions to be addressed (12). A relevant parallel can be drawn to knowledge recently gained regarding control of feeding behavior by neurons that express Agouti-related peptide (Agrp), found in the adjacent hypothalamic arcuate nucleus. Since their discovery more than a decade ago (13, 14), innumerable papers were published implicating these neurons as key drivers of fasting-induced hyperphagia (15, 16), but whether they are in and of themselves necessary or sufficient for normal food intake was unknown until recently. The past few years have shown (among other things) that selective activation of Agrp neurons is sufficient to potently drive intake whereas inhibition of these neurons blunts the effect of fasting to stimulate feeding (17, 18). These findings offer direct, compelling support for the hypothesis that Agrp neurons are primary drivers of needbased feeding. Whether activation of VMN SF1 neurons is both necessary and sufficient to explain the powerful adaptive responses elicited by hypoglycemia is a question of similar importance. Given Significance Hypoglycemia is an important and frequently encountered complication of diabetes treatment. Here, we identify a subset of neurons located in the ventromedial hypothalamic nucleus, activation of which is both necessary and sufficient to mediate adaptive counterregulatory responses to hypoglycemia that return low blood glucose levels into the normal range. These neurons receive ascending input from neurons in the lateral parabrachial nucleus and in turn control blood glucose levels via projections to the anterior bed nucleus of the stria terminalis. Together, this work identifies a previously unrecognized functional neurocircuit involved in glycemic control. Author contributions: T.H.M., M.D.D., J.M.S., J.P.T., M.W.S., and G.J.M. designed research; T.H.M., J.T.N., M.E.M., S.J.G., V.D., and H.T.N. performed research; M.B.A., J.M.S., D.P.O., M.G.M., M.W.S., and G.J.M. contributed new reagents/analytic tools; T.H.M., M.D.D., M.W.S., and G.J.M. analyzed data; and T.H.M., M.W.S., and G.J.M. wrote the paper. The authors declare no conflict of interest. This article is a PNAS Direct Submission. 1 To whom correspondence should be addressed. gjmorton@u.washington.edu. This article contains supporting information online at /pnas /-/DCSupplemental. NEUROSCIENCE PNAS PLUS PNAS Early Edition 1of10
2 the many sites that participate in glucose sensing (5, 19, 20) and how difficult it is to prevent recovery from hypoglycemia (owing to redundancy inherent in the CRR), it seems improbable that the entire response should hinge on activation of a select population of neurons in a relatively small brain area. To test this hypothesis, we first determined whether optogenetic inactivation of VMN SF1 neurons during insulin-induced hypoglycemia blocks increased glucagon and corticosterone secretion, inhibition of glucoseinduced insulin secretion, and recovery of normal blood glucose levels. We also asked whether, conversely, optogenetic activation of VMN SF1 neurons elicits hyperglycemia by activating the CRR in otherwise normal mice. Last and perhaps most importantly, we sought to identify projections of VMN SF1 neurons to downstream brain areas involved in glucose counterregulation and to determine whether these VMN SF1 neuronal subsets make synaptic contacts with the previously identified upstream neurons located in the LPBN. Our data collectively indicate that (i) VMN SF1 neurons are key components of a circuit that extends from the LPBN to the VMN and then to the anterior bed nucleus of the stria terminalis (abnst) (LPBN VMN abnst), (ii) VMN SF1 neurons function as a primary motor output driving the CRR, and (iii) activation of VMN SF1 neurons is required for effective recovery from insulin-induced hypoglycemia. Results VMN SF1 Neurons Are Required for Intact Responses to Insulin-Induced Hypoglycemia. Because of the threat to survival posed by inadequate glucose delivery to the brain, the CRR to hypoglycemia is mediated by a highly integrated, redundant, and powerful combination of behavioral, autonomic, and neuroendocrine responses that are conserved across mammalian species. To investigate the physiological role played by VMN SF1 neurons in this response, we sought to determine whether activation of these neurons is required for the ability to recover from hypoglycemia. To this end, we expressed a light-activated inhibitory channel selectively in VMN SF1 neurons. This objective was achieved through bilateral stereotaxic microinjection of an adeno-associated viral construct (AAV) into the VMN of SF1-Cre-positive (SF1-Cre + ) mice (in which Cre recombinase is expressed under the control of the VMN-specific SF1 promoter) (21). The AAV expresses a modified channelrhodopsin chlorideconducting anion channel fused with the fluorescent reporter EYFP (AAVDJ8-DIO-SwiChR CA -EYFP, referred to hereafter as SwiChR CA virus ) andis Cre-inducible, meaning that viral expression is restricted to cells that also express Cre recombinase. Through an optic fiber implanted immediately dorsal to each VMN injection site (Fig. 1 A C), delivery of light ( Laser On ) causes hyperpolarization of VMN SF1 neurons, but not other neurons in the VMN or surrounding brain areas (Fig. 1C). We report that, whereas photoinhibition of VMN SF1 neurons had no effect on fasting blood glucose levels in the basal state relative to the Laser-Off condition (Fig. 1G), the ability of mice to recover from insulin-induced hypoglycemia was severely impaired by light-induced silencing of VMN SF1 neurons (Fig. 1D). Thus, activation of a discrete and uniquely identified subpopulation of VMN neurons is required to effectively recover from hypoglycemia. By comparison, VMN SF1 neuron activation is not required for maintenance of normal blood glucose levels in the basal state (e.g., in the absence of a threat to brain glucose delivery). The striking inability of mice to respond to insulin-induced hypoglycemia when VMN SF1 neurons are silenced implies that their activation is required for secretion of counterregulatory hormones in this setting. Consistent with this hypothesis, we found, in a separate experiment, that the increase of circulating glucagon and corticosterone levels that normally occurs during hypoglycemia was blockedwhenvmn SF1 neurons were silenced (Fig. 1 E and F). These data demonstrate that activation of VMN SF1 neurons is indispensable for an intact CRR to hypoglycemia, as previously hypothesized (11), and thus constitute direct evidence of a physiological role for these neurons in glucose homeostasis. Photoactivation of VMN SF1 Neurons Induces Hyperglycemia. Based on our finding that activation of VMN SF1 neurons is required for an intact response to hypoglycemia, we hypothesized that activation of these neurons in otherwise normal mice will trigger the CRR and thereby cause blood glucose levels to increase. To test this Fig. 1. Photoinhibition of VMN SF1 neurons disrupts recovery from insulininduced hypoglycemia. (A) Scheme demonstrating bilateral stereotaxic microinjection of the Cre-dependent inhibitory channelrhodopsin-eyfp (SwiChR CA ) virus into the VMN of SF1-Cre + mice and (B) implantation of the optic fiber dorsal to the injection site. (C) Representative image of EYFP fluorescence showing bilateral infection and expression specifically within the VMN of SF1- Cre + mice. 3V, third cerebral ventricle; VMN, ventromedial hypothalamic nucleus. (D) Blood glucose and plasma (E) glucagon and (F) corticosterone levels in SF1-Cre + mice during photoinhibition of VMN SF1 neurons (Laser On) relative to the Laser Off during insulin-induced hypoglycemia (n = 7 8 per group). *P < 0.05 vs. Laser off. All data are expressed as mean ± SEM. (G) Blood glucose levels before (pre), during (inhib), and 1 h after (post) bilateral photoinhibition of VMN SF1 neurons in SF1-Cre + mice in the basal state (n = 8) analyzed by one-way ANOVA with Sidak s post hoc test. All comparisons are nonsignificant. 2of10 Meek et al.
3 hypothesis, we expressed a light-activated excitatory channel selectively in VMN SF1 neurons through unilateral stereotaxic microinjection of a Cre-dependent, AAV-expressing channelrhodopsin-eyfp virus (AAV5-DIO-ChR2-EYFP) into the VMN of SF1-Cre + mice, followed by implantation of an optic fiber above the injection site (Fig. 2 A and B). As expected, EYFP fluorescence was restricted to the VMN of SF1-cre + mice, and, 1 h after photostimulation of these neurons, a robust induction of c-fos (a marker of neuronal activation) was detected in the VMN and surrounding hypothalamic areas (Fig. 2C). As predicted, photoactivation of VMN SF1 neurons raised blood glucose levels rapidly and markedly, with glycemia returning to baseline values within 1 h after cessation of photostimulation (Fig. 2D). Because this effect was not observed in Cre-negative littermate controls (VMN Control ) that underwent the same viral microinjection and light exposure procedure (Fig. 2E), we conclude that the observed hyperglycemic response was a specific consequence of VMN SF1 neuron activation. To further characterize the effect of VMN SF1 neuronal activation on glucose homeostasis, we performed an intraperitoneal (i.p.) glucose tolerance test (IPGTT) in both the presence and absence of unilateral photoactivation of VMN SF1 neurons. Glucose tolerance was markedly impaired by VMN SF1 neuron activation (Fig. 2F) (GluAUC; Laser Off, 21,560 ± 904 vs. Laser On, 39,659 ± 4,253; P < 0.05), further establishing its powerful diabetogenic effect. To determine whether increased hepatic gluconeogenesis contributes to this impairment of glucose tolerance, we performed a pyruvate tolerance test (PTT) in a separate cohort of SF1-Cre + mice in both the presence and absence of VMN SF1 neuron photoactivation. Our finding that the rise of blood glucose levels in response to a pyruvate challenge was markedly increased during activation of VMN SF1 neurons (Fig. 2G) (GluAUC; Laser Off, 13,723 ± 897 vs. Laser On, 20,855 ± 903; P < 0.05) implicates increased hepatic gluconeogenesis in the hyperglycemia and glucose intolerance observed in this setting. The VMN comprises a heterogeneous population of neurons, many of which express SF1. A large subset of VMN neurons also express the long form of the leptin receptor (LepRb), and a majority of these neurons also express SF1 (21, 22). To determine whether activation of LepRb-expressing neurons in the VMN (VMN LepRb ) also causes hyperglycemia, we microinjected the Cre-dependent channelrhodopsin virus into the VMN of leptin receptor-ires-cre (Lepr-Cre + )mice(fig.3a C) soastoenable photoactivation of VMN LepRb neurons. Successful transduction of these neurons was confirmed by observing EYFP fluorescence in the VMN, with EYFP fluorescence also detected in a few cells in adjacent areas, including the dorsomedial hypothalamus (DMH) and arcuate nucleus (ARC) (Fig. 3C). However, little or no c-fos was colocalized with transduced LepRb neurons outside the VMN after photostimulation (Fig. 3C). Thus, our photostimulation protocol seems to have effectively targeted only VMN LepRb neurons and not adjacent hypothalamic LepRb subsets. In contrast to the potent diabetogenic effect of VMN SF1 neuron activation, photostimulation of VMN LepRb neurons did not affect blood glucose levels (Fig. 3D), despite the fact that VMN LepRb neurons were clearly activated by the photostimulation protocol and that many VMN LepRb neurons coexpress SF1 (21, 22). These observations suggest that a subset of VMN SF1 neurons that do not express LepRb are responsible for the hyperglycemic effect observed during VMN stimulation. Alternatively, it is possible that NEUROSCIENCE PNAS PLUS Fig. 2. Photoactivation of VMN SF1 neurons induces hyperglycemia and causes impaired glucose and pyruvate tolerance in nondiabetic mice. (A) Scheme depicting unilateral stereotaxic microinjection of the Cre-dependent light-activated channelrhodopsin (ChR2-EYFP) virus into the VMN of SF1-Cre + mice and (B) implantation of the optic fiber dorsal to the injection site. (C) Representative image indicating unilateral infection and expression of EYFP fluorescence within the VMN of SF1-Cre + mice and c-fos within both the VMN and surrounding area 1 h after photostimulation. 3V, third cerebral ventricle; VMN, ventromedial hypothalamic nucleus. Blood glucose levels before (Pre), during (Stim) and 1 h after (Post) unilateral stimulation of VMN SF1 neurons in (D) SF1-Cre + mice and (E) VMN Control animals (Cre-negative) (n = 5 8 per group). *P < 0.05 vs. Pre. Blood glucose levels during an i.p. (F) glucose- (GTT) and (G) pyruvate-tolerance test (PTT) in both the presence and absence of unilateral photoactivation of VMN SF1 neurons in SF1-Cre + mice. *P < 0.05 vs. Laser Off. All data are expressed as mean ± SEM. Meek et al. PNAS Early Edition 3of10
4 corticosterone and glucagon did not change in response to photoactivation of the VMN in either VMN LepRb or VMN Control mice (Fig. 4 C and D). Because increased hepatic glucose production (HGP) figures prominently in the metabolic actions of these hormones, we measured hepatic expression of the key gluconeogenic genes phosphoenolpyruvate carboxykinase (Pepck) and glucose-6- phosphatase (G6Pase). Indeed, expression of both genes was elevated after light exposure in VMN SF1 mice (Fig. 4 E and F). Interestingly, relative to VMN Control mice, hepatic expression of Pepck was also elevated by light exposure of the VMN in VMN LepRb mice, consistent with previous evidence of a role for hypothalamic leptin action in the control of hepatic glucose flux (25, 26), even though this effect occurred in the absence of changes of glycemia or glucoregulatory hormones (Fig. 4E). Collectively, these data suggest that the combination of reduced insulin secretion and increased secretion of corticosterone and glucagon contribute to the hyperglycemic response to VMN SF1 neuronal stimulation and that increased HGP likely contributed to this response. Fig. 3. Photoactivation of VMN LepRb neurons fails to raise blood glucose levels. (A) Scheme demonstrating unilateral stereotaxic microinjection of the Cre-dependent light-activated channelrhodopsin (ChR2-EYFP) virus into the VMN of Lepr-IRES-Cre mice and (B) implantation of the optic fiber dorsal to the injection site. (C) Representative image depicting unilateral infection and expression of EYFP fluorescence within the VMN of Lepr-IRES-Cre mice and c-fos within both the VMN and surrounding area 1 h after photostimulation. 3V, third cerebral ventricle; VMN, ventromedial hypothalamic nucleus. (D) Blood glucose levels before (Pre), during (Stim), and 1 h after (Post) unilateral stimulation of VMN LepRb neurons in Lepr-IRES-Cre mice. All comparisons are nonsignificant (n = 11 per group). All data are expressed as mean ± SEM. activation of a subset of VMN LepRb neurons that do not express SF1 exerts an inhibitory effect on those neurons that do, and thereby blocks the activation of responses underlying hyperglycemia. Neuroendocrine Effects of VMN SF1 Photoactivation. To explain how VMN SF1 neuron photoactivation elicits its robust diabetogenic effect, we hypothesized a role for both an autonomic mechanism, whereby glucose-induced pancreatic insulin secretion is blocked, and a neuroendocrine component involving increased secretion of counterregulatory hormones. To test the former hypothesis, we measured plasma insulin levels before and during photoactivation of VMN SF1 neurons. Despite the marked hyperglycemia elicited by activation of VMN SF1 neurons (Fig. 4A), plasma insulin levels remained at their normoglycemic baseline (Laser Off) (Fig. 4B). As expected, neither blood glucose nor plasma insulin levels were affected by light stimulation of the VMN of either VMN LepRb or VMN Control mice (Fig. 4B). Thus, the hyperglycemic response to VMN SF1 neuron photoactivation seems to be mediated, in part, by a potent inhibition of glucose-induced insulin secretion, consistent with earlier observations after electrical stimulation of the VMN (23, 24). In addition to inhibitory effects on the pancreatic beta cell, the hyperglycemic response to photoactivation of VMN SF1 neurons was associated with a marked rise of both plasma corticosterone and glucagon levels (Fig. 4 C and D). By comparison, plasma levels of Identification of Downstream Projections of VMN SF1 Neurons Regulating Glycemia. Among brain areas that receive dense projections from VMN neurons are the periaqueductal gray (PAG), the bed nucleus of the stria terminalis (BNST), the central nucleus of the amygdala (CeA), and the hypothalamic paraventricular nucleus (PVN). Each of these brain areas is implicated in autonomic, neuroendocrine, and behavioral regulation and therefore could function within a glucoregulatory circuit involving VMN SF1 neurons (27, 28). To test whether VMN SF1 neurons are among those VMN neurons that project to these four brain areas, we used fluorescently tagged channelrhodopsin to visualize the projection fields. Specifically, AAV5-DIO-ChR2-EYFP was microinjected into the VMN of SF1-Cre + mice, followed by histological imaging to detect the EYFP reporter in axonal projections (Fig. 5 A E). Labeled projections of VMN SF1 neurons were detected in both ipsilateral and contralateral PAG, particularly in the dorsal region superior to the cerebral aqueduct. In the BNST, fibers arising from VMN SF1 neurons were detected in the dorsomedial region, along with a dense plexus in the anterior BNST and the anterior-medial subdivision (abnst). Projections of VMN SF1 neurons were also detected in both the CeA and PVN; in the latter, innervation was particularly robust in medial regions along the border of the third ventricle (Fig. 5 B E). Because these findings are consistent with previous work using standard tract-tracing methods to detect projections from unspecified VMN neurons (27), the distribution of projections from the subset expressing SF1 does not seem to differ significantly from the population of VMN neurons overall. To investigate whether functional connectivity exists between cell bodies of SF1 neurons in the VMN and any of these four downstream innervation sites, we used an optogenetics approach in which a ChR2-expressing virus was unilaterally microinjected into the VMN of SF1-cre + mice, followed by unilateral implantation of an optic fiber above each of the four projection fields (abnst, CeA, PAG, and PVN) in separate cohorts of mice. After transport of ChR2 from cell bodies to axons of transduced neurons, the axons can be depolarized by laser stimulation of terminal projections. Six weeks after surgery, stimulation of VMN SF1 axons was achieved using the same 1-h photostimulation protocol described earlier. We found that hyperglycemia was induced after photostimulation of VMN SF1 neuronal projections to the abnst (VMN SF1 abnst), but not after stimulation of projections to any of the other three areas (Fig. 5 F I). To investigate whether this effect was specific to the abnst, we microinjected the credependent ChR2-EYFP virus into the VMN of a separate cohort of SF1-Cre + mice and placed the optic fiber above either the abnst or medial BNST (mbnst). Consistent with our earlier observations, we found that unilateral photoactivation of VMN SF1 projections to the abnst resulted in hyperglycemia whereas activation of projections to the mbnst had no effect on blood glucose levels 4of10 Meek et al.
5 (Fig. S1). Collectively, these findings suggest that the glucoregulatory subset of SF1 neurons project specifically to the abnst. Consistent with the interpretation that VMN SF1 abnst stimulation recapitulates the glycemic response to VMN SF1 neuronal activation, this effect was accompanied by elevated plasma levels of both corticosterone and glucagon (Fig. 5 N and R) and with inhibition of glucose-induced insulin secretion, such that plasma insulin levels did not increase despite the rise of blood glucose concentrations (Fig. 5 F and J). By comparison, no effect on levels of blood glucose, plasma insulin, or plasma glucagon was detected during stimulation of the VMN SF1 neuron fibers supplying thepvnalthoughanonsignificantincreaseofplasmacorticosterone levels was observed (Fig. 5 F R). Collectively, these observations implicate the subset of VMN SF1 neurons that project to the abnst, but not to the PAG, CeA, PVN, or mbnst, as components of a functional glucoregulatory circuit. To localize the cell bodies of VMN SF1 neurons that project to the abnst, we used a two-pronged strategy. First, we injected cholera toxin subunit B (CTB), a fluorescently labeled retrograde neuronal tracer, into the abnst and examined fluorescence within the VMN (Fig. 6 A and B). As expected, we found dense CTB expression throughout the VMN, supporting previous evidence that axonal projections from neuronal cell bodies in the VMN supply the abnst (27). CTB was also detected in other mediobasal hypothalamic areas, including the ARC (among other areas) (Fig. S2), consistent with previous evidence (29) that neurons distributed throughout the mediobasal hypothalamus (MBH) project to the BNST (Fig. 6 C and D). We next identified cell bodies of neurons in the VMN that were activated (as assessed by expression of c-fos) after photostimulation of axonal projections of VMN SF1 neurons supplying the abnst (VMN SF1 abnst) (Fig. 6 E H). The c-fos was somewhat widespread after activation of the abnst projections, mimicking channelrhodopsin expression, with little c-fos staining observed in the absence of light treatment (Fig. 6I). The resultant c-fos (+) neurons were concentrated in the central (c) and dorsomedial (dm) regions of the VMN (Fig. 6 J N), areas previously implicated in metabolic regulation (30, 31). By comparison, few activated neurons were present in the ventrolateral (vl) portion of the VMN, an area associated with reproduction Fig. 4. Photoactivation of VMN SF1 neurons mediates CRR responses. (A) Blood glucose, (B) plasma insulin, and (C) plasma corticosterone either prior (Pre), or 1 h after Cre-dependent channelrhodopsin stimulation (Stim) and (D) plasma glucagon and hepatic expression of (E) Pepck and (F) G6Pase as measured by real-time PCR 1 h after Cre-dependent channelrhodopsin activation of VMN SF1, VMN Control, and VMN LepRb cell types, respectively (n = 5 8 per group). *P < 0.05 vs. VMN Control ; # P < 0.05 vs. VMN LepRb. All data are expressed as mean ± SEM. and aggressive behavior (32 35). Together, these findings implicate subsets of SF1 neurons located in central and dorsomedial regions of the VMN in glycemic control (10). The LPBN Sends Afferents to abnst-projecting VMN SF1 Neurons. In addition to the VMN, recent evidence suggests that the CRR to hypoglycemia involves a subset of neurons in the LPBN marked by coexpression of leptin receptor and CCK (9, 10). Because these LPBN LepRb CCK neurons also project to the VMN, we sought to determine whether they are anatomically coupled to VMN SF1 neurons identified herein as having a physiological role in glucose counterregulation. To first verify that neurons in the LPBN project to VMN, we injected CTB to the VMN and, 4 d later, examined the brain for alexa-488 fluorescence label. Included among several sites upstream of the VMN identified by this approach were the medial preoptic area, amygdala, and BNST (Fig. S3), as well as the LPBN (Fig. 7 A C). We next sought to determine whether the subset of VMN SF1 neurons that project to the abnst are synaptically contacted by LPBN neurons. Accomplishing this goal was made possible through the use of a two-virus strategy. The first virus is a modified rabies viral construct, SADΔG-EGFP, engineered to lack the gene encoding rabies glycoprotein G that is required for transsynaptic spread (36), and packaged in a single envelope glycoprotein (EnvA). The second virus is a Cre-dependent AAVexpressing TVA construct (that expresses the receptor for the avian sarcoma leucosis virus glycoprotein, EnvA) linked with RG (rabies envelope glycoprotein) using a 2A-cleavage peptide, AAV8-DIO-TVA-RG (TVA-RG). With this strategy, microinjection of TVA-RG into the VMN of SF1-Cre + mice selectively renders VMN SF1 neurons (i) uniquely susceptible to infection by EnvA and (ii) capable of retrograde monosynaptic spread due to the presence of RG (Fig. 7D). By subsequently injecting the SADΔG-EGFP virus (EnvA) into the abnst of the same mice, infection by the latter virus is restricted initially to VMN SF1 terminals projecting to this region previously transfected by the TVA-RG virus. Subsequently, the SADΔG-EGFP virus is able to cross a single synapse and thereby infect neurons upstream of abnst-projecting VMN SF1 neurons. NEUROSCIENCE PNAS PLUS Meek et al. PNAS Early Edition 5of10
6 Fig. 5. Photoactivation of VMN SF1 abnst projections selectively promote hyperglycemia in nondiabetic mice. (A) Schematic showing unilateral stereotaxic microinjection of the Cre-dependent light-activated channelrhodopsin (ChR2-EYFP) virus into the VMN of SF1-Cre + mice (i.e., VMN SF1 neurons) and implantation of the optic fiber above each of four projection sites in separate cohorts of animals. Image adapted from connectivity.brain-map.org/; experiment ID abnst, anterior bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; PAG, periaqueductal gray; PVN, paraventricular nucleus; VMN, ventromedial nucleus. Fluorescently labeled projections of channelrhodopsin in VMN SF1 neurons detected in terminal targets including the (B)aBNST,(C)PVN,(D)CeA,and(E) PAG. (Magnification: 4.) 3V, third cerebral ventricle; CA, cerebral aqueduct. (F I) Blood glucose, (J M) plasma insulin, and (N Q) plasma corticosterone (CORT) levels before (Pre), during (Stim), and 1 h after (Post) photoactivation of VMN SF1 neuron axon projection fields. (abnst, n = 7; PVN, n = 6; CeA, n = 5; and PAG, n = 6 per group). *P < 0.05 vs. Pre. (R)Plasma glucagon 1 h after photoactivation of each site. *P < 0.05 vs. CeA and PAG; no significant difference from PVN. All data are expressed as mean ± SEM. 6of10 Meek et al.
7 Fig. 6. Cell bodies of VMN SF1 neurons projecting to the abnst are located primarily in central and dorsomedial VMN. (A) Diagram showing unilateral stereotaxic microinjection of fluorescently labeled retrograde neuronal tracer cholera toxin subunit B (CTB) into the abnst of WT mice and (B) expression of the virus at injection site and associated coronal illustration for orientation. AC, anterior commissure; LV, lateral cerebral ventricle. Expression of the fluorescently labeled retrograde neuronal tracer CTB throughout the mediobasal hypothalamus (MBH) at either magnification (C) 4 or (D) 10. (E) Diagram showing unilateral microinjection of ChR2-EYFP into the VMN of SF1-Cre + mice followed by 1 h photoactivation of the abnst projection field. (F) Representative 10 magnification of VMN SF1 projections within the abnst (greenlabeled fibers), with (G) c-fos (red stain) and the (H) merged image after abnst photoactivation of VMN SF1 projections. (I) Control staining for c-fos within the abnst when light treatment was withheld (10 magnification) showing low immunoactivity. (J) Expression of ChR2 within the VMN of SF1-Cre + mice (4 magnification). (K) Representative images of VMN SF1 -infected neurons (green), (L) c-fos (red), and the (M) merged image after photoactivation of VMN SF1 projections in the abnst. (Magnification: 10.) (N) Bilateral view of the VMN showing isolated expression of c-fos within only the left hemisphere. 3V, third ventricle; c, central; dm, dorsomedial; vl, ventral lateral. As expected, analysis of the brains of these mice revealed large numbers of EGFP (+) cells in the VMN, potentially representing not only those VMN SF1 neurons initially infected with TVA- RG but also local VMN cells that synapse onto these neurons (Fig. 7E). We also detected EGFP (+) neurons in the LPBN as well as several other brain regions (Fig. S4). Specifically, labeled neurons within the PBN were limited to the external, central, and ventral aspects of the LPBN, with none detected in either the superior LPBN or the medial PBN (Fig. 7F). These observations demonstrate that a subset of LPBN neurons make synaptic connections with those VMN SF1 neurons that project to the abnst. Discussion To cope with the brain s exclusive reliance on glucose as a fuel, a sophisticated system has evolved to detect and respond to the threat posed by reduced glucose availability. The brain clearly plays a central role in this regulatory system, and, whereas previous studies have implicated a role for the VMN, the underlying neurocircuitry remains largely unidentified. Here, we report a crucial role for VMN SF1 neurons in the physiological response to hypoglycemia and identify them as part of an ascending glucoregulatory LPBN VMN SF1 abnst neurocircuit (Fig. S5). These observations offer direct evidence of a previously unrecognized neurocircuit involved in glycemic control. A key finding is that, whereas inhibition of VMN SF1 neurons has no glucose-lowering effect under basal conditions, it severely impairs recovery from hypoglycemia because it blocks the powerful CRRs that normally restore low blood glucose levels into the normal range. Conversely, activation of VMN SF1 neurons in otherwise normal mice causes diabetes-range hyperglycemia because it activates CRRs despite the absence of hypoglycemia. These findings offer compelling, direct evidence that VMN SF1 neurons function as primary motor output neurons for raising circulating glucose concentrations in times of need, analogous to the role in food intake played by Agrp neurons situated in the hypothalamic arcuate nucleus. Although relatively inactive in sated mice, Agrp neurons powerfully stimulate feeding when activated in response to depleted fuel stores (37, 38). These neurons are therefore poised to drive positive energy balance to replenish depleted fuel stores but otherwise are relatively inactive. Consequently, inhibition of Agrp neurons has little effect on food intake in sated mice but impairs fasting-induced feeding (17, 18). Thus, inhibition of Agrp neurons has little impact on food intake under usual circumstances but blocks need-based feeding. Similarly, because activation of VMN SF1 neurons causes marked hyperglycemia, it makes physiological sense for these neurons to remain inactive unless blood glucose levels are threatened. In the latter setting, however, inhibition of VMN SF1 neurons has devastating consequences because it blocks the powerful CRRs that normally restore blood glucose levels into the normal range (11, 30), as expected for neurons that function as a primary motor output for raising blood glucose levels when glucose availability is threatened. Details regarding the cellular phenotype of VMN SF1 neurons involved in this response await further analysis. Recent work has focused on the role of intracellular glucose sensors, neurotransmitters such as gamma-aminobutyric acid (GABA), glutamate, serotonin, and neuropeptides [corticotrophin-releasing hormone (CRH) and urocortins] (1, 30). Most VMN SF1 neurons are glutamatergic (39, 40), and previous evidence points to a role for glutamate release from these neurons in the CRR response (11). However, our data suggest that not all VMN SF1 neurons participate in glucose counterregulation because photoactivation of VMN LepRb neurons, many of which express SF1 and are also glutamatergic (21, 22), was without effect on either glycemia or secretion of counterregulatory hormones. To shed additional light on the subset of VMN SF1 neurons involved in glucose homeostasis, we used fluorescently tagged channelrhodopsin to characterize the circuit architecture. Consistent with previous studies (27), we found that the heaviest projections of VMN SF1 neurons terminate in the PAG, CeA, PVN, and abnst. Our finding that photostimulation of VMN SF1 neurons that project to the abnst, but not to the PAG, PVN, CeA, or mbnts, mimics the glycemic response elicited by VMN SF1 neuronal stimulation offers clear evidence that projections of VMN SF1 neurons to the abnst contribute to observed effects on glucose homeostasis. Moreover, the glucoseraising effect of VMN SF1 abnst fiber activation was accompanied by hormonal responses similar to that induced by stimulation of VMN SF1 cell bodies, including increases of plasma glucagon and corticosterone levels and inhibition of glucosestimulated insulin secretion. Collectively, these observations implicate axonal projections to the abnst in the glycemic response observed after photostimulation of VMN SF1 cell bodies. Our finding that photostimulation of VMN SF1 abnst axons induces activation of VMN SF1 neuron cell bodies predominantly in the dmvmn and cvmn, and not the vlvmn, is consistent with established evidence implicating the dmvmn and cvmn in autonomic control of metabolism whereas neurons in the vlvmn participate in the control of aggression and reproduction NEUROSCIENCE PNAS PLUS Meek et al. PNAS Early Edition 7of10
8 Fig. 7. abnst VMN SF1 afferents originate within the PBN. (A) Schematic of CTB distribution after VMN injection. LPBN, lateral parabrachial nucleus; LSN, lateral septal nucleus; mamg, medial amygdala; MPO, medial preoptic nucleus; PP, peripeduncular nucleus. (B) Microphotograph of CTB at the injection site within the VMN. (C) Representative image showing the presence of CTB in the lateral PBN 4 d after VMN injection. (Magnification: Left, 4 ; Right, 10.) Additional microphotographs of CTB spread are included in Fig. S3. (D) Diagram showing procedure and timeline of the modified rabies approach (both virus and mode of action). (E) Back propagated modified rabies virus within the VMN from abnst projecting terminals at 4 (Left) and 10 (Right) magnification. (F) Representative image of modified rabies virus in VMN SF1 afferent neurons found within the LPBN at 4 (Left) and10 (Right) magnification. (33 35). These findings collectively support the hypothesis that VMN SF1 neurons involved in glucose homeostasis (i) are located in dmvmn and cvmn, (ii) project to the abnst, and (iii) are distinct from those neurons expressing leptin receptors. In addition to the VMN, recent work implicates a subset of neurons situated in the LPBN in the CRR to hypoglycemia. These LPBN neurons express both leptin receptor and CCK, and, like photoactivation of VMN SF1 neurons, pharmacogenetic activation of LPBN LepRb CCK neurons raises blood glucose levels in association with increased secretion of glucagon and corticosterone. Conversely, inhibition of LPBN LepRb CCK neurons blunts the glycemic response to glucoprivation (9, 10), an effect resembling the consequences of optogenetic silencing of VMN SF1 neurons during insulin-induced hypoglycemia in the current studies. Because these LPBN LepRb CCK neurons project to the dmvmn and cvmn and because the induction of CRRs after activation of PBN LepRb CCK neurons is attenuated by pharmacogenetic inhibition of VMN SF1 neurons (10), we hypothesized that the former neurons provide ascending, stimulatory input to the subset of VMN SF1 neurons that project to the abnst. In support of this hypothesis, we found that abnst-projecting VMN SF1 neurons are synaptically connected to neurons in the LPBN, among other regions. These data offer direct evidence in support of an LPBN VMN SF1 abnst neurocircuit involved in glycemic regulation, and we anticipate that future studies will demonstrate that LepRb CCK neurons are among those LPBN neurons involved in this circuit. Interestingly, several areas additional to the LPBN send afferents to the subset of VMN SF1 neurons that project to the abnst. How this information is processed and the extent to which these projections contribute to glucose homeostasis are questions that await additional study. The liver plays a key role as the primary source of circulating glucose mobilized by CRRs to hypoglycemia. Activation of the hypothalamic-pituitary-adrenal (HPA) axis and increased glucagon secretion (41) each raise blood glucose levels by increasing HGP via increases of both glycogenolysis and gluconeogenesis. The latter can be assessed indirectly by the expression of the hepatic gluconeogenic genes G6Pase and Pepck, and we found that hyperglycemia induced by photoactivation of VMN SF1 neurons is associated with increased hepatic expression of both genes, suggesting that increased hepatic gluconeogenesis may have contributed to hyperglycemia elicited by photoactivation of VMN SF1 neurons. Consistent with this hypothesis, we found that the glycemic excursion in response to a pyruvate challenge was also markedly increased by photoactivation of VMN SF1 neurons. Additional evidence of a role of the VMN in these responses (42, 43) includes the finding that electrical stimulation of the mediobasal hypothalamus elicits hyperglycemia (6), driven in 8of10 Meek et al.
9 part by increased glucagon secretion (44). Similarly, glucagon release is triggered by administration of 2-deoxyglucose (2-DG) locally into the VMN to induce neuroglucopenia in this brain area (45). Conversely, the glucagon response to systemic hypoglycemia is blocked by intra-vmn administration of glucose (7) and in rats with bilateral VMH lesions (46). Combined with our findings that photoinhibition of VMN SF1 neurons blocks the plasma glucagon and corticosterone response to hypoglycemia, whereas photoactivation of these neurons has the opposite effect, we conclude that a neuronal network involving VMN SF1 neurons is critically involved in the CRR to hypoglycemia. In addition to increased counterregulatory hormone secretion, plasma insulin levels were unchanged during photoactivation of VMN SF1 neurons, despite an associated, dramatic increase of blood glucose levels. Although this failure to raise plasma insulin levels may seem unimpressive, the observed increase of blood glucose constitutes a powerful stimulus to insulin secretion, and increased SNS outflow to the pancreas is among very few mechanisms capable of blocking this response in normal animals. From a teleological perspective, it makes intuitive sense that, when glucose availability is threatened, responses that increase glucose production would be activated (e.g., elevated glucagon and corticosterone levels) in concert with responses that reduce glucose clearance (e.g., inhibition of insulin secretion), and much of the literature indicates that pancreatic beta cells are subject to inhibitory control via the sympathetic nervous system (SNS) (47, 48). For example, bilateral VMN lesioning induces hyperinsulinemia (49, 50) whereas electrical stimulation of the VMN suppresses glucose-induced insulin secretion (51). Further, the VMN is known to regulate autonomic outflow to the pancreas (52, 53), and activation of islet sympathetic nerves during glycopenic stress (54) inhibits insulin secretion via a mechanism involving activation of α2-adrenoreceptors on the beta cell (47, 48). However, the neurocircuitry that underlies inhibitory control of insulin secretion by the brain remains unknown. In conclusion, we report that optogenetic silencing of VMN SF1 neurons profoundly impairs recovery from insulin-induced hypoglycemia by blocking powerful neuroendocrine and autonomic components of the CRR. These findings offer unambiguous evidence that activation of VMN SF1 neurons is required for effective glucose counterregulation. At the same time, tonic inhibition of these neurons seems to be permissive for maintenance of glucose homeostasis under usual conditions because photoactivation of VMN SF1 neurons rapidly induces diabetesrange hyperglycemia with impaired glucose tolerance, owing to a combination of increased CRR hormone secretion and inhibition of glucose-induced insulin secretion. A subset of VMN SF1 neurons that project to the abnst and are anatomically linked to upstream neurons in the LPBN are implicated in this glucoregulatory neurocircuit. An improved understanding of the functional organization of this neurocircuit may help to identify future strategies for prevention of both insulin-induced hypoglycemia and hypoglycemic unawareness, two of the most common and costly complications of diabetes treatment (55). Experimental Procedures Animals. All procedures were performed in accordance with NIH guidelines for the care and use of animals and were approved by the Animal Care Committee at the University of Washington. All studied animals were individually housed in a temperature-controlled room with a 12:12-h light:dark cycle under specificpathogen free (SPF) conditions and provided with ad libitum access to water and chow unless otherwise stated (PMI Nutrition). SF1-cre mice (approved mouse gene name, Nr5a1) have been generated and described previously (21, 22) and were bred in our colony (56) whereas Lepr-IRES-Cre mice were purchased from The Jackson Laboratory (stock no ). Viral Generation, Injections, and Fiber Placement. The viral vectors AAV5-EF1α- DIO-hChR2(H134R)-EYFP and AAVDJ8-EF1α-DIO-SwiChR CA -TS-EYFP-WPRE used in these studies have been described previously (57 59). All viruses were packaged at the Gene Therapy Center at the University of North Carolina, except the SwiChR2 virus, which was packaged into a DJ8 AAV vector by the University of Washington Diabetes Research Center Viral Vector and Transgenic Mouse Core. The Cre-inducible EF1α-DIO-SwiChR CA -TS-EYFP-WPRE construct was kindly provided by Karl Deisseroth (Stanford University, Stanford, CA) (57). Details regarding stereotaxic delivery of viruses to specific brain areas is provided in SI Experimental Procedures. Optogenetic in Vivo Photostimulation and Photoinhibition. Optogenetic studies were supported by the Nutrition Obesity Research Center (NORC) Energy Balance and Glucose Metabolism Core at the University of Washington. For delivery of light pulses with millisecond precision, the output beam from a diode laser (473 nm, DPSS continuous wave laser system; Laserglow) was controlled using an AMPI Master-9 stimulator (Laserglow) through a single fiber port (17, 60). The light was then split using multimode optical fibers with a 200-nm diameter core, N.A (Thor Laboratories), and passed through a fiber optic rotary joint (Thor Laboratories). A terminal fiber attached to the rotary joint was fixed to a 230-μm-bore ceramic ferrule and a mating sleeve that allowed delivery of light to the brain through coupling with a 200-nm ferrule-capped fiber implanted within the mouse brain. Unless otherwise indicated, for all in vivo photostimulation experiments, 5-ms pulses, 40 pulses per 1 s, were used for 1 h, which is slightly higher than the traditional stimulation patterns used with electrical activation of the VMN (6, 61). Photoinhibition experiments used a constant beam of light for 1 2 has indicated for each experiment. Irradiance at target regions was estimated at 21 mw/mm 2 for photostimulation and 10 mw/mm 2 for photoinhibition experiments based upon the previously described relationship of light scattering in the brain of mammals with light power exiting the fiber tip corresponding to 8 mw and 4 mw for activation and inhibition, respectively (web.stanford.edu/group/dlab/cgi-bin/graph/chart.php). The nature of the numerical aperture (N.A. 0.22), a measurement dictating the range of angles light is transmitted along, allowed a greater depth of light penetration into tissue while resulting in a narrow illumination cone. Based on these calculations and the placement of the optic fiber (0.4 mm dorsal to the VMN), the intensity of light reaching the lateral ventral portion of the VMN likely approached the minimum threshold to result in regular burst firing (1 mw/mm 2 ) (12). However, in regions that receive an asymmetrical distribution of VMN terminals along a transverse plane, the stimulation of fibers adjacent rather than directly below the fiber would have been limited. Viral Track-Tracing Studies. To investigate the origin of BNST or VMN afferent projections, 150 nl of 1 mg/ml cholera toxin b subunit conjugated with Alexa dye 488 (CTB-488; Life Technologies) in PBS was injected into the region of interest at 50 nl/min. Animals were perfused 4 d after injections to harvest brains for histological analysis. For retrograde rabies-tracing studies, a modified rabies viral construct, AAV-DIO-TVA-RG (TVA-RG), serotype 8, was injected unilaterally into the VMN (200 nl) of 7- to 8-wk-old mice (titer genome copies per milliliter). Twenty-one days later, SADΔG-EGFP (EnvA) rabies was unilaterally injected into the abnst (100 nl), followed by a 10-min wait. One week after SADΔG-EGFP (EnvA) rabies injection, mice were perfused, and brains were mounted for sectioning as described below. Metabolic Studies and Tissue Processing. Intraperitoneal glucose tolerance tests (GTTs) (2 g/kg body weight) or pyruvate tolerance tests (PTTs) (2 g/kg body weight) were conducted in 6-h fasted animals. Tail vein blood was collected for measurement of blood glucose levels at the times indicated and as described in the SI Experimental Procedures. Immunohistochemistry and RT-PCR. Details for immunostaining and gene expression estimation can be found in SI Experimental Procedures and as previously described (62, 63). Statistical Analyses. All results are presented as means ± SEM. Statistical analyses were performed using PASW Statistics (version 18; IBM SPSS). P values for pairwise comparisons were calculated by two-tailed Student s t test. Data for comparisons across more than two groups were analyzed using a one-way ANOVA with post hoc comparisons, where appropriate. Time course comparisons between groups were analyzed using a two-way repeated-measures ANOVA with main effects of light (laser on/off) and time (6 8 instances). All post hoc comparisons were determined using Sidak s correction for multiple comparisons. In all instances, probability values of <0.05 were considered significant. ACKNOWLEDGMENTS. We acknowledge the technical expertise provided by Alexis Cubelo, Annelise Paige Matsuo, Justin Ngoc Lam, Loan Nguyen, and NEUROSCIENCE PNAS PLUS Meek et al. PNAS Early Edition 9of10
Supplementary Figure 1
 Supplementary Figure 1 Arcuate ChIEF-tdTomato neurons expressed TH These micrographs show that TH-Cre-ChIEF-tdTomato (magenta), expressed by AAV in a TH-Cre mouse, were immunostained with TH (green) in
Supplementary Figure 1 Arcuate ChIEF-tdTomato neurons expressed TH These micrographs show that TH-Cre-ChIEF-tdTomato (magenta), expressed by AAV in a TH-Cre mouse, were immunostained with TH (green) in
Nature Neuroscience: doi: /nn Supplementary Figure 1. Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons
 Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Nature Neuroscience: doi: /nn Supplementary Figure 1. Splenic atrophy and leucopenia caused by T3 SCI.
 Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
! acts via the autonomic nervous system. ! maintaining body weight within tight limits. ! ventromedial (VMN) ! arcuate (ARC) ! neuropeptide Y (NPY)
 Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Copyright 2017 The Guilford Press
 This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Drd1a-Cre driven ChR2 expression in the SCN. (a) Low-magnification image of a representative Drd1a-ChR2 coronal brain section (n = 2) showing endogenous tdtomato fluorescence (magenta).
Supplementary Figure 1 Drd1a-Cre driven ChR2 expression in the SCN. (a) Low-magnification image of a representative Drd1a-ChR2 coronal brain section (n = 2) showing endogenous tdtomato fluorescence (magenta).
Orexin and Sleep. Team: A Little Bit of Leptin
 Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
Role of the ventromedial hypothalamic Steroidogenic Factor 1/ Adrenal 4. glucose metabolism in mice.
 Role of the ventromedial hypothalamic Steroidogenic Factor 1/ Adrenal 4 Binding Protein neurons in the regulation of whole body energy and glucose metabolism in mice. Eulalia Coutinho Department of Physiological
Role of the ventromedial hypothalamic Steroidogenic Factor 1/ Adrenal 4 Binding Protein neurons in the regulation of whole body energy and glucose metabolism in mice. Eulalia Coutinho Department of Physiological
Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents
 Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents Jarrad M Scarlett 1,,1, Jennifer M Rojas 1,1, Miles E Matsen 1, Karl J Kaiyala 3, Darko
Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents Jarrad M Scarlett 1,,1, Jennifer M Rojas 1,1, Miles E Matsen 1, Karl J Kaiyala 3, Darko
Insulin-Leptin Interactions
 Insulin-Leptin Interactions Ahmed S., Al-Azzam N., Cao B. Karshaleva B., Sriram S., Vu K. If you understand a system, you can predict it. Agenda - Energy homeostasis Overview of leptin and insulin Signaling
Insulin-Leptin Interactions Ahmed S., Al-Azzam N., Cao B. Karshaleva B., Sriram S., Vu K. If you understand a system, you can predict it. Agenda - Energy homeostasis Overview of leptin and insulin Signaling
Parallel, Redundant Circuit Organization for Homeostatic Control of Feeding Behavior
 Parallel, Redundant Circuit Organization for Homeostatic Control of Feeding Behavior J. Nicholas Betley, 1,2 Zhen Fang Huang Cao, 1,2 Kimberly D. Ritola, 1 and Scott M. Sternson 1, * 1 Janelia Farm Research
Parallel, Redundant Circuit Organization for Homeostatic Control of Feeding Behavior J. Nicholas Betley, 1,2 Zhen Fang Huang Cao, 1,2 Kimberly D. Ritola, 1 and Scott M. Sternson 1, * 1 Janelia Farm Research
Nature Neuroscience: doi: /nn Supplementary Figure 1. Visualization of AT1a-positive cells using AT1a lacz/+ mouse.
 Supplementary Figure 1 Visualization of AT1a-positive cells using AT1a lacz/+ mouse. (a f) Immunohistochemical detection of β-gal in the mouse brain. Coronal sections at the respective anteroposterior
Supplementary Figure 1 Visualization of AT1a-positive cells using AT1a lacz/+ mouse. (a f) Immunohistochemical detection of β-gal in the mouse brain. Coronal sections at the respective anteroposterior
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction. Laura Gunter
 Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Hormonal gain control of a medial preoptic area social reward circuit
 CORRECTION NOTICE Nat. Neurosci. 20, 449 458 (2017) Hormonal gain control of a medial preoptic area social reward circuit Jenna A McHenry, James M Otis, Mark A Rossi, J Elliott Robinson, Oksana Kosyk,
CORRECTION NOTICE Nat. Neurosci. 20, 449 458 (2017) Hormonal gain control of a medial preoptic area social reward circuit Jenna A McHenry, James M Otis, Mark A Rossi, J Elliott Robinson, Oksana Kosyk,
Autonomic regulation of islet hormone secretion
 Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
CNS Control of Food Intake. Adena Zadourian & Andrea Shelton
 CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
Neurobiology of food intake in health and disease
 Neurobiology of food intake in health and disease Gregory J. Morton, Thomas H. Meek and Michael W. Schwartz Abstract Under normal conditions, food intake and energy expenditure are balanced by a homeostatic
Neurobiology of food intake in health and disease Gregory J. Morton, Thomas H. Meek and Michael W. Schwartz Abstract Under normal conditions, food intake and energy expenditure are balanced by a homeostatic
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
Internal Regulation II Energy
 Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane
 Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
FLASH CARDS. Kalat s Book Chapter 10 Alphabetical
 FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) 108 (b-c) (d) (e) (f) (g)
 Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Nature Neuroscience: doi: /nn Supplementary Figure 1. Distribution of starter cells for RV-mediated retrograde tracing.
 Supplementary Figure 1 Distribution of starter cells for RV-mediated retrograde tracing. Parcellation of cortical areas is based on Allen Mouse Brain Atlas and drawn to scale. Thick white curves, outlines
Supplementary Figure 1 Distribution of starter cells for RV-mediated retrograde tracing. Parcellation of cortical areas is based on Allen Mouse Brain Atlas and drawn to scale. Thick white curves, outlines
Leptin and the Central Nervous System Control of Glucose Metabolism
 Physiol Rev 91: 389 411, 2011; doi:10.1152/physrev.00007.2010. Leptin and the Central Nervous System Control of Glucose Metabolism GREGORY J. MORTON AND MICHAEL W. SCHWARTZ Diabetes and Obesity Center
Physiol Rev 91: 389 411, 2011; doi:10.1152/physrev.00007.2010. Leptin and the Central Nervous System Control of Glucose Metabolism GREGORY J. MORTON AND MICHAEL W. SCHWARTZ Diabetes and Obesity Center
Nature Neuroscience: doi: /nn.4642
 Supplementary Figure 1 Recording sites and example waveform clustering, as well as electrophysiological recordings of auditory CS and shock processing following overtraining. (a) Recording sites in LC
Supplementary Figure 1 Recording sites and example waveform clustering, as well as electrophysiological recordings of auditory CS and shock processing following overtraining. (a) Recording sites in LC
Hypothalamus. To learn how the brain regulates neuroendocrine secretions NTA Ch 14, pgs Key Figs: 14-3; 14-4,
 Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Homeostasis. Endocrine System Nervous System
 Homeostasis Endocrine System Nervous System 2004-2005 Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body homeostasis & regulation
Homeostasis Endocrine System Nervous System 2004-2005 Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body homeostasis & regulation
Supplemental Information. Dorsal Raphe Dual Serotonin-Glutamate Neurons. Drive Reward by Establishing Excitatory Synapses
 Cell Reports, Volume 26 Supplemental Information Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons Hui-Ling Wang, Shiliang
Cell Reports, Volume 26 Supplemental Information Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons Hui-Ling Wang, Shiliang
Dopamine in Ube3a m-/p+ mice. Online Supplemental Material
 Online Supplemental Material S1 Supplemental Figure 1. Schematic of rate-dependent intracranial self-stimulation (ICSS) (A) Mice implanted with monopolar stimulating electrodes to the medial forebrain
Online Supplemental Material S1 Supplemental Figure 1. Schematic of rate-dependent intracranial self-stimulation (ICSS) (A) Mice implanted with monopolar stimulating electrodes to the medial forebrain
CHAPTER 48: NERVOUS SYSTEMS
 CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
Nature Neuroscience: doi: /nn.4335
 Supplementary Figure 1 Cholinergic neurons projecting to the VTA are concentrated in the caudal mesopontine region. (a) Schematic showing the sites of retrograde tracer injections in the VTA: cholera toxin
Supplementary Figure 1 Cholinergic neurons projecting to the VTA are concentrated in the caudal mesopontine region. (a) Schematic showing the sites of retrograde tracer injections in the VTA: cholera toxin
Glucose Sensing Neurons in the Ventromedial Hypothalamus
 Sensors 2010, 10, 9002-9025; doi:10.3390/s101009002 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Review Glucose Sensing Neurons in the Ventromedial Hypothalamus Vanessa H. Routh Department
Sensors 2010, 10, 9002-9025; doi:10.3390/s101009002 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Review Glucose Sensing Neurons in the Ventromedial Hypothalamus Vanessa H. Routh Department
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature06310 SUPPLEMENTARY INFORMATION www.nature.com/nature 1 www.nature.com/nature 2 www.nature.com/nature 3 Supplementary Figure S1 Spontaneous duration of wake, SWS and REM sleep (expressed
doi: 10.1038/nature06310 SUPPLEMENTARY INFORMATION www.nature.com/nature 1 www.nature.com/nature 2 www.nature.com/nature 3 Supplementary Figure S1 Spontaneous duration of wake, SWS and REM sleep (expressed
Motivation 1 of 6. during the prandial state when the blood is filled
 Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
PSYCH 260 Exam 2. March 2, Answer the questions using the Scantron form. Name:
 PSYCH 260 Exam 2 March 2, 2017 Answer the questions using the Scantron form. Name: 1 1 Main Please put in their proper order the steps that lead to synaptic communication between neurons. Begin with the
PSYCH 260 Exam 2 March 2, 2017 Answer the questions using the Scantron form. Name: 1 1 Main Please put in their proper order the steps that lead to synaptic communication between neurons. Begin with the
Chapter 12. Ingestive Behavior
 Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
LESSON 3.3 WORKBOOK. How do we decide when and how much to eat?
 Appetite The psychological desire to eat, driven by feelings of pleasure from the brain. Hunger The biological or physiological need to eat, caused by a release of hormones from the digestive tract. LESSON
Appetite The psychological desire to eat, driven by feelings of pleasure from the brain. Hunger The biological or physiological need to eat, caused by a release of hormones from the digestive tract. LESSON
Nature Neuroscience: doi: /nn Supplementary Figure 1. Confirmation that optogenetic inhibition of dopaminergic neurons affects choice
 Supplementary Figure 1 Confirmation that optogenetic inhibition of dopaminergic neurons affects choice (a) Sample behavioral trace as in Figure 1d, but with NpHR stimulation trials depicted as green blocks
Supplementary Figure 1 Confirmation that optogenetic inhibition of dopaminergic neurons affects choice (a) Sample behavioral trace as in Figure 1d, but with NpHR stimulation trials depicted as green blocks
Neural Communication. Central Nervous System Peripheral Nervous System. Communication in the Nervous System. 4 Common Components of a Neuron
 Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Exam 3. Short Answer/Terminology (1 pt each except where noted, 35 pts total) Version B. Zoology 250, Fall 2003
 Exam 3 Version B Zoology 250, Fall 2003 5 pages, 50 points total please check to see that your copy is complete. Please SIGN your name here if you would like me to post your grade by the last five digits
Exam 3 Version B Zoology 250, Fall 2003 5 pages, 50 points total please check to see that your copy is complete. Please SIGN your name here if you would like me to post your grade by the last five digits
Supplementary Figure 1.
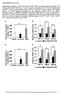 Supplementary Figure 1. FGF21 does not exert direct effects on hepatic glucose production. The liver explants from C57BL/6J mice (A, B) or primary rat hepatocytes (C, D) were incubated with rmfgf21 (2
Supplementary Figure 1. FGF21 does not exert direct effects on hepatic glucose production. The liver explants from C57BL/6J mice (A, B) or primary rat hepatocytes (C, D) were incubated with rmfgf21 (2
Nature Neuroscience: doi: /nn Supplementary Figure 1. Trial structure for go/no-go behavior
 Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Acetylcholine (ACh) Action potential. Agonists. Drugs that enhance the actions of neurotransmitters.
 Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Organization of the nervous system. [See Fig. 48.1]
![Organization of the nervous system. [See Fig. 48.1] Organization of the nervous system. [See Fig. 48.1]](/thumbs/90/103926552.jpg) Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
SUPPLEMENTARY INFORMATION
 doi:1.138/nature9553 Supplementary Table 1. Overlap of neuronal marker and PKC- expression in CEl. Marker/PKC- PKC- Marker Gad65 87.4±4.7 5.3±12.6 CRH 1.2±1. 16.9±15.2 Dyn 1.9±1.2 4.5±2.9 Enk 42.8±7.4
doi:1.138/nature9553 Supplementary Table 1. Overlap of neuronal marker and PKC- expression in CEl. Marker/PKC- PKC- Marker Gad65 87.4±4.7 5.3±12.6 CRH 1.2±1. 16.9±15.2 Dyn 1.9±1.2 4.5±2.9 Enk 42.8±7.4
Hypothalamus. Small, central, & essential.
 Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Investigation of the role of nesfatin-1/nucb2 in the central nervous system. Ph.D. thesis Katalin Könczöl
 Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Behavioral and Motivational mechanisms of Brain. Limbic system and the Hypothalamus
 Behavioral and Motivational mechanisms of Brain Limbic system and the Hypothalamus 1 General functions 1. Control of behavior 2. Control level of activities in different parts of brain 3. Motivational
Behavioral and Motivational mechanisms of Brain Limbic system and the Hypothalamus 1 General functions 1. Control of behavior 2. Control level of activities in different parts of brain 3. Motivational
biological psychology, p. 40 The study of the nervous system, especially the brain. neuroscience, p. 40
 biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
biological psychology, p. 40 The specialized branch of psychology that studies the relationship between behavior and bodily processes and system; also called biopsychology or psychobiology. neuroscience,
Neurons of the Bed Nucleus of the Stria Terminalis (BNST)
 Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Myers Psychology for AP* David G. Myers PowerPoint Presentation Slides by Kent Korek Germantown High School Worth Publishers, 2010
 Myers Psychology for AP* David G. Myers PowerPoint Presentation Slides by Kent Korek Germantown High School Worth Publishers, 2010 *AP is a trademark registered and/or owned by the College Board, which
Myers Psychology for AP* David G. Myers PowerPoint Presentation Slides by Kent Korek Germantown High School Worth Publishers, 2010 *AP is a trademark registered and/or owned by the College Board, which
THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN. COGS 163 By: Pranav Singh Alexandra Villar
 THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN COGS 163 By: Pranav Singh Alexandra Villar INTRODUCTION Insulin is a hormone produced in the pancreas by the islets of Langerhans that regulates the
THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN COGS 163 By: Pranav Singh Alexandra Villar INTRODUCTION Insulin is a hormone produced in the pancreas by the islets of Langerhans that regulates the
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 12 Nervous Tissue Introduction The purpose of the chapter is to: 1. Understand how the nervous system helps to keep controlled conditions within
Principles of Anatomy and Physiology 14 th Edition CHAPTER 12 Nervous Tissue Introduction The purpose of the chapter is to: 1. Understand how the nervous system helps to keep controlled conditions within
Sleep-Wake Cycle I Brain Rhythms. Reading: BCP Chapter 19
 Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
Function of the Nervous System
 Nervous System Function of the Nervous System Receive sensory information, interpret it, and send out appropriate commands to form a response Composed of neurons (functional unit of the nervous system)
Nervous System Function of the Nervous System Receive sensory information, interpret it, and send out appropriate commands to form a response Composed of neurons (functional unit of the nervous system)
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Relative expression of K IR2.1 transcript to enos was reduced 29-fold in capillaries from knockout animals. Relative expression of K IR2.1 transcript to enos was reduced 29-fold
Supplementary Figure 1 Relative expression of K IR2.1 transcript to enos was reduced 29-fold in capillaries from knockout animals. Relative expression of K IR2.1 transcript to enos was reduced 29-fold
Introduction to Systems Neuroscience. Nov. 28, The limbic system. Daniel C. Kiper
 Introduction to Systems Neuroscience Nov. 28, 2017 The limbic system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html LIMBIC SYSTEM The term limbic system mean
Introduction to Systems Neuroscience Nov. 28, 2017 The limbic system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html LIMBIC SYSTEM The term limbic system mean
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM
 STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
Νευροφυσιολογία και Αισθήσεις
 Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 16 Κίνητρα Συμπεριφοράς ή Υποκίνηση (Motivation) Introduction Types of behavior Unconscious reflexes Voluntary
Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 16 Κίνητρα Συμπεριφοράς ή Υποκίνηση (Motivation) Introduction Types of behavior Unconscious reflexes Voluntary
UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
processes in the central nervous system (CNS), affecting many of the during the course of ethanol treatment. Ethanol stimulates the release of
 INTRODUCTION INTRODUCTION Neuroscience research is essential for understanding the biological basis of ethanol-related brain alterations and for identifying the molecular targets for therapeutic compounds
INTRODUCTION INTRODUCTION Neuroscience research is essential for understanding the biological basis of ethanol-related brain alterations and for identifying the molecular targets for therapeutic compounds
BIOLOGY - CLUTCH CH.45 - ENDOCRINE SYSTEM.
 !! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
!! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
BMI risk SNPs associate with increased CADM1 and CADM2 expression in the cerebellum of human subjects.
 Supplementary Figure 1 BMI risk SNPs associate with increased CADM1 and CADM2 expression in the cerebellum of human subjects. Boxplots show the 25% and 75% quantiles of normalized mrna expression levels
Supplementary Figure 1 BMI risk SNPs associate with increased CADM1 and CADM2 expression in the cerebellum of human subjects. Boxplots show the 25% and 75% quantiles of normalized mrna expression levels
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
Respiration Cellular Respiration Understand the relationship between glucose breakdown and ATP when you burn glucose with the help of oxygen, it
 Respiration Cellular Respiration Understand the relationship between glucose breakdown and ATP when you burn glucose with the help of oxygen, it traps chemical energy into ATP Energy found in glucose stores
Respiration Cellular Respiration Understand the relationship between glucose breakdown and ATP when you burn glucose with the help of oxygen, it traps chemical energy into ATP Energy found in glucose stores
Applied Neuroscience. Conclusion of Science Honors Program Spring 2017
 Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Applied Neuroscience Conclusion of Science Honors Program Spring 2017 Review Circle whichever is greater, A or B. If A = B, circle both: I. A. permeability of a neuronal membrane to Na + during the rise
Module H NERVOUS SYSTEM
 Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
ZNZ Advanced Course in Neuroscience Mon Limbic System II. David P. Wolfer MD
 ZNZ Advanced Course in Neuroscience Mon 05.05.2014 Limbic System II David P. Wolfer MD Institute of Anatomy, University of Zurich Institute for Human Movement Sciences and Sport, ETH Zurich http://www.dpwolfer.ch
ZNZ Advanced Course in Neuroscience Mon 05.05.2014 Limbic System II David P. Wolfer MD Institute of Anatomy, University of Zurich Institute for Human Movement Sciences and Sport, ETH Zurich http://www.dpwolfer.ch
Homeostasis and Mechanisms of Weight Regulation
 Homeostasis and Mechanisms of Weight Regulation Purpose In this activity students will investigate how negative feedback mechanisms function to maintain homeostatic balance using a recently discovered
Homeostasis and Mechanisms of Weight Regulation Purpose In this activity students will investigate how negative feedback mechanisms function to maintain homeostatic balance using a recently discovered
Portions from Chapter 6 CHAPTER 7. The Nervous System: Neurons and Synapses. Chapter 7 Outline. and Supporting Cells
 CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
Outline. Neuron Structure. Week 4 - Nervous System. The Nervous System: Neurons and Synapses
 Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
16. which is not synthesised in postganglionic sympathetic neurons a. L-dopa b. DA c. NA d. A e. ACh
 NERVOUS SYSTEM 1. Visual pathways a. Have P cells that are associated with colour b. Utilize the primary colours, red, yellow and blue c. Have simple cells which respond to all light stimuli d. Pass through
NERVOUS SYSTEM 1. Visual pathways a. Have P cells that are associated with colour b. Utilize the primary colours, red, yellow and blue c. Have simple cells which respond to all light stimuli d. Pass through
INTRODUCTION TO THE BIOCHEMISTRY OF HORMONES AND THEIR RECPTORS
 INTRODUCTION TO THE BIOCHEMISTRY OF HORMONES AND THEIR RECPTORS 1 Introduction to the Biochemistry of Hormones and their Receptors Lectuctre1 Sunday 17/2/ Objectives: 1. To understand the biochemical nature
INTRODUCTION TO THE BIOCHEMISTRY OF HORMONES AND THEIR RECPTORS 1 Introduction to the Biochemistry of Hormones and their Receptors Lectuctre1 Sunday 17/2/ Objectives: 1. To understand the biochemical nature
LIMBIC SYSTEM. Dr. Amani A. Elfaki Associate Professor Department of Anatomy
 LIMBIC SYSTEM Dr. Amani A. Elfaki Associate Professor Department of Anatomy Learning Objectives Define the limbic system Identify the parts of the limbic system Describe the circulation of the limbic system
LIMBIC SYSTEM Dr. Amani A. Elfaki Associate Professor Department of Anatomy Learning Objectives Define the limbic system Identify the parts of the limbic system Describe the circulation of the limbic system
glucagon receptor AgRP merged color map I corr = 0.76±0.024 glucagon receptor DAPI merged
 Hypothalamic glucagon signaling inhibits glucose production Patricia I. Mighiu*, Jessica T.Y. Yue*, Beatrice M. Filippi, Mona A. Abraham, Madhu Chari, Carol K.L. Lam, Clair S. Yang, Nikita R. Christian,
Hypothalamic glucagon signaling inhibits glucose production Patricia I. Mighiu*, Jessica T.Y. Yue*, Beatrice M. Filippi, Mona A. Abraham, Madhu Chari, Carol K.L. Lam, Clair S. Yang, Nikita R. Christian,
What systems are involved in homeostatic regulation (give an example)?
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
Study Guide Answer Key Nervous System
 Biology 12 Human Biology Textbook: BC Biology 12 Study Guide Answer Key Nervous System 1. Draw a neuron, label 3 parts and give the function of those parts. Dendrite: carry signals to the cell body Cell
Biology 12 Human Biology Textbook: BC Biology 12 Study Guide Answer Key Nervous System 1. Draw a neuron, label 3 parts and give the function of those parts. Dendrite: carry signals to the cell body Cell
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Drugs Affecting The Autonomic Nervous System(ANS)
 Drugs Affecting The Autonomic Nervous System(ANS) ANS Pharmacology Lecture 1 Dr. Hiwa K. Saaed College of Pharmacy, University of Sulaimani 2018-2019 AUTOMATIC NERVOUS SYSTEM (ANS) The ANS is the major
Drugs Affecting The Autonomic Nervous System(ANS) ANS Pharmacology Lecture 1 Dr. Hiwa K. Saaed College of Pharmacy, University of Sulaimani 2018-2019 AUTOMATIC NERVOUS SYSTEM (ANS) The ANS is the major
Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis
 Hypothalamic TLR triggers sickness behavior via a microglia-neuronal axis Sungho Jin, *, Jae Geun Kim,, *, Jeong Woo Park, Marco Koch,, Tamas L. Horvath and Byung Ju Lee Department of Biological Sciences,
Hypothalamic TLR triggers sickness behavior via a microglia-neuronal axis Sungho Jin, *, Jae Geun Kim,, *, Jeong Woo Park, Marco Koch,, Tamas L. Horvath and Byung Ju Lee Department of Biological Sciences,
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon
 Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons. Chad Smurthwaite & Jordan Shellmire
 Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
-51mV 30s 3mV. n=14 n=4 p=0.4. Depolarization (mv) 3
 Supplementary Figure 1 a optoβ 2 -AR b ChR2-51mV 30s 3mV -50mV 30s 3mV c 4 n=14 n=4 p=0.4 Depolarization (mv) 3 2 1 0 Both optogenetic actuators, optoβ 2 AR and ChR2, were effective in stimulating astrocytes
Supplementary Figure 1 a optoβ 2 -AR b ChR2-51mV 30s 3mV -50mV 30s 3mV c 4 n=14 n=4 p=0.4 Depolarization (mv) 3 2 1 0 Both optogenetic actuators, optoβ 2 AR and ChR2, were effective in stimulating astrocytes
Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.
![Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +. Fig. 4. The activity of Pkc -transduced neurons is required for enhanced learning. After gene transfer, rats were tested on [] vs. +.](/thumbs/90/102196023.jpg) Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Research Interests Advanced cognitive learning is encoded in distributed circuits that span multiple forebrain areas. Further, synaptic plasticity and neural network theories hypothesize that essential
Neuropsychiatry Block
 Neuropsychiatry Block Physiology of the Autonomic Nervous System By Laiche Djouhri, PhD Dept. of Physiology Email: ldjouhri@ksu.edu.sa Ext:71044 References The Autonomic Nervous System and the Adrenal
Neuropsychiatry Block Physiology of the Autonomic Nervous System By Laiche Djouhri, PhD Dept. of Physiology Email: ldjouhri@ksu.edu.sa Ext:71044 References The Autonomic Nervous System and the Adrenal
Axon Nerve impulse. Axoplasm Receptor. Axomembrane Stimuli. Schwann cell Effector. Myelin Cell body
 Nervous System Review 1. Explain a reflex arc. 2. Know the structure, function and location of a sensory neuron, interneuron, and motor neuron 3. What is (a) Neuron Axon Nerve impulse Axoplasm Receptor
Nervous System Review 1. Explain a reflex arc. 2. Know the structure, function and location of a sensory neuron, interneuron, and motor neuron 3. What is (a) Neuron Axon Nerve impulse Axoplasm Receptor
Psychology - Problem Drill 05: Endocrine System & Influence on Behavior
 Psychology - Problem Drill 05: Endocrine System & Influence on Behavior No. 1 of 10 1. Which of the following statements is FALSE regarding the interaction between the nervous an endocrine systems? (A)
Psychology - Problem Drill 05: Endocrine System & Influence on Behavior No. 1 of 10 1. Which of the following statements is FALSE regarding the interaction between the nervous an endocrine systems? (A)
LECTURE STRUCTURE ASC171 NERVOUS SYSTEM PART 1: BACKGROUND 26/07/2015. Module 5
 LECTURE STRUCTURE PART 1: Background / Introduction PART 2: Structure of the NS, how it operates PART 3: CNS PART 4: PNS Why did the action potential cross the synaptic junction? To get to the other side
LECTURE STRUCTURE PART 1: Background / Introduction PART 2: Structure of the NS, how it operates PART 3: CNS PART 4: PNS Why did the action potential cross the synaptic junction? To get to the other side
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Hormonal regulation of. Physiology Department Medical School, University of Sumatera Utara
 Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Name: Period: Chapter 2 Reading Guide The Biology of Mind
 Name: Period: Chapter 2 Reading Guide The Biology of Mind The Nervous System (pp. 55-58) 1. What are nerves? 2. Complete the diagram below with definitions of each part of the nervous system. Nervous System
Name: Period: Chapter 2 Reading Guide The Biology of Mind The Nervous System (pp. 55-58) 1. What are nerves? 2. Complete the diagram below with definitions of each part of the nervous system. Nervous System
Nervous System, Neuroanatomy, Neurotransmitters
 Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Brain Insulin Action Regulates Hypothalamic Glucose Sensing and the Counterregulatory Response to Hypoglycemia
 Washington University in St. Louis Washington University Open Scholarship All Theses and Dissertations (ETDs) January 2010 Brain Insulin Action Regulates Hypothalamic Glucose Sensing and the Counterregulatory
Washington University in St. Louis Washington University Open Scholarship All Theses and Dissertations (ETDs) January 2010 Brain Insulin Action Regulates Hypothalamic Glucose Sensing and the Counterregulatory
Unit 3: The Biological Bases of Behaviour
 Unit 3: The Biological Bases of Behaviour Section 1: Communication in the Nervous System Section 2: Organization in the Nervous System Section 3: Researching the Brain Section 4: The Brain Section 5: Cerebral
Unit 3: The Biological Bases of Behaviour Section 1: Communication in the Nervous System Section 2: Organization in the Nervous System Section 3: Researching the Brain Section 4: The Brain Section 5: Cerebral
