CD160 inhibits activation of human CD4 + T cells through interaction with herpesvirus entry mediator
|
|
|
- Amy Flynn
- 5 years ago
- Views:
Transcription
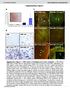
1 CD16 inhiits ctivtion of humn CD4 + T cells through interction with herpesvirus entry meditor Guifng Ci, Anuknth Anumnthn, Juli A Brown, Edwrd A Greenfield, Bogong Zhu & Gordon J Freemn CD16, glycosylphosphtidylinositol-nchored memer of the immunogloulin superfmily, is expressed on oth cytolytic lymphocytes nd some unstimulted CD4 + T cells. Here we show tht CD16 expression ws incresed fter ctivtion of humn CD4 + T cells nd tht crosslinking CD16 with monoclonl ntiody strongly inhiited CD3- nd CD28-medited ctivtion. We found tht herpesvirus entry meditor (HVEM) ws lignd of CD16 tht cted s idirectionl switch for T cell ctivtion, producing positive or negtive outcome depending on the enggement of HVEM y CD16 nd known HVEM lignds such s B nd T lymphocyte ttenutor (BTLA) nd the T lymphocyte receptor LIGHT. Inhiition of CD4 + T cell ctivtion y HVEM-trnsfected cells ws dependent on CD16 nd BTLA; when the cysteine-rich domin 1 of HVEM ws deleted, this inhiition ws lost, resulting in strong T cell ctivtion. CD16 thus serves s negtive regultor of CD4 + T cell ctivtion through its interction with HVEM. The glycosylphosphtidylinositol (GPI)-nchored CD16 protein is expressed minly on cytolytic cells such s CD8 + T cells, nturl killer (NK) T cells nd NK cells 1 4. CD16 hs single immunogloulin V (IgV) like domin tht is wekly homologous to killer cell immunogloulin-like receptors, contins six cysteine residues in this IgV domin, nd is expressed on the cell surfce s multimer tht is tightly linked y disulfide onds 3. Clssicl nd nonclssicl mjor histocomptiility complex (MHC) clss I molecules ind to CD16 with low ffinity 4 7, nd this inding might enhnce NK nd T cell cytolytic ctivity 5 7. Becuse the originl monoclonl ntiody (ma) to CD16 (BY55) is reltively low-ffinity IgM sutype 2, we generted new mas specific to humn CD16 to evlute its function. Although CD16 is expressed on few CD4 + T cells, its function on these cells hs not een investigted. We found tht the new mas to CD16 strongly inhiited CD4 + T cell prolifertion nd cytokine production. This unexpected result led us to serch for CD16 lignds other thn MHC clss I molecules. Here we identify herpesvirus entry meditor (HVEM) s CD16 lignd y expression cloning nd show specific interction etween CD16 nd HVEM. We further show tht monomeric ntiody to CD16 (nti-cd16 F) nd monoclonl nti-hvem locked HVEM-medited inhiition of T cell ctivtion. Our studies indicte tht HVEM is even more promiscuous thn previously thought: it intercts with CD16 in ddition to inding to the T lymphocyte receptor LIGHT, lymphotoxin- nd BTLA. We lso found tht CD16 nd BTLA ound to cysteine-rich domin 1 (CRD1) of HVEM, ut neither CD16 nor BTLA locked inding of LIGHT to CRD2 nd CRD3 of HVEM. Our dt collectively show tht CD16 functions s n inhiitor of CD4 + T cell ctivtion y intercting with HVEM. RESULTS CD16 is expressed on susets of CD4 + nd CD8 + Tcells To investigte the function of CD16, we generted two mas to CD16, 5D.1A11 nd 5D.8E1, which we chrcterized s mouse IgG1 (migg1) sutype. We then compred the specificity of 5D.1A11 with tht of the originl ma BY55 for CD16 oth in cells tht were trnsfected to express CD16 nd in responder CD4 + T cell supopultions. 5D.1A11 ound specificlly to humn CD16- trnsfected 3.19 cells t n mount comprle to BY55 (Fig. 1 nd Supplementry Fig. 1 online); experiments with 5D.8E1 produced similr results (dt not shown). To chrcterize the expression of CD16 in primry humn lymphocytes, we stined peripherl lood mononucler cells (PBMCs) with nti-cd16 (5D.1A11-PC5; Fig. 1). CD16 ws expressed on oth CD4 + nd CD8 + T cells in smll mounts (out 8% nd out 18% respectively); these results were comprle to stining with BY55 (ref. 2 nd Fig. 1). To determine whether expression of CD16 vries with the ctivtion sttus of CD4 + T cells, we stined purified humn CD4 + T cells with ntiodies to either the nive or ctivted form of the cell surfce protein CD45 (CD45RA or CD45RO, respectively) in comintion with nti-cd16 (5D.1A11; Fig. 1c). We found tht CD16 ws expressed on considerle supopultion of memory (CD45RA CD45RO + )CD4 + Deprtment of Medicl Oncology, Dn-Frer Cncer Institute, Deprtment of Medicine, Hrvrd Medicl School, Boston, Msschusetts 2115, USA. Correspondence should e ddressed to G.J.F. (gordon_freemn@dfci.hrvrd.edu). Received 9 Octoer 27; ccepted 5 Decemer 27; pulished online 13 Jnury 28; corrected fter print 16 Mrch 28; doi:1.138/ni VOLUME 9 NUMBER 2 FEBRUARY 28 NATURE IMMUNOLOGY
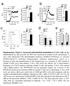
2 CD CD16 CD3 + CD4 + CD3 + CD8 + CD3 + CD4 + CD3 + CD8 + 8% 18% 7% 2% c CD45RA + RO CD45RA RO + CD45RA + RO + 1% 2% 15% d migg1 Dy 1 Dy 2 Dy 3 Dy 4 Dy D.1A11-PC5 BY55-PE 5D.1A11-PC5 Figure 1 Expression of CD16 on trnsfectnts nd CD4 + nd CD8 + T cell popultions. () Flow cytometry of CD16-trnsfected (3-CD16) or untrnsfected 3.19 cells stined with phycoerythrin-cynine 5 nti-cd16 (5D.1A11- PC5) or phycoerythrin-conjugted BY55 (BY55- PE; filled histogrms) or isotype control ma (open histogrms). () Flow cytometry of freshly T cells nd recently ctivted (CD45RA + CD45RO + )CD4 + T cells ut ws rely detectle on nive T cells (CD45RA + CD45RO ), which suggested tht CD16 is induced fter T cell ctivtion. To exmine further the expression of CD16 fter ctivtion, we stimulted purified CD4 + T cells for 1 5 d with nti-cd3 or nti-cd3 plus nti-cd28 (Fig. 1d). Expression of CD16 ws upregulted on dy 3 nd peked on dy 4 fter ctivtion. Further studies with quntittive RT-PCR (qrt-pcr) showed tht like mrna encoding interleukin 2 (IL2) nd the T cell inhiitory receptor CTLA-4 (CTLA-4), CD16 mrna ws upregulted 16 h fter ctivtion (Fig. 1e), which demonstrted tht CD16 expression is induced during CD4 + T cell ctivtion. Crosslinking of CD16 prevents CD4 + T cell ctivtion Studies hve shown tht CD16 enhnces the cytolytic ctivity of CD8 + T cells nd NK cells. Expression of CD16 is lower on CD4 + T cells thn on CD8 + T cells, nd the function of CD16 in CD4 + T cell ctivtion is uncler. To exmine the function of CD16 in CD4 + T cell ctivtion, we stimulted purified CD4 + T cells with ltex eds coted with vrious concentrtions of ma to CD16, together with nti-cd3 or nti-cd3 plus nti-cd28. We kept the mount of protein on the eds constnt y dding isotype control migg1. Unexpectedly, crosslinking of CD16 on CD4 + T cells strongly inhiited T cell prolifertion medited y nti-cd3 or nti-cd3 plus nti-cd28 in dose-dependent wy (Fig. 2). Crosslinking of CD16 did not led to n increse in cell deth, s judged y propidium iodide stining (Supplementry Fig. 1). The prdox of low CD16 expression ut glol suppression on crosslinking led us to investigte this issue more closely. Sorted CD16 CD4 + T cells were more resistnt to CD16-medited suppression; however, the ddition of fivefold higher concentrtion of nti-cd16 induced degree of suppression similr to tht noted for totl CD4 + T cells (Fig. 2). This oservtion suggested tht low CD16 expression or ctivtion-induced CD16 expression is sufficient to medite inhiition. We exmined the effect of CD16 crosslinking on cytokine production y cytokine ed ssy. The concentrtions of oth T helper type 1 (T H 1) cytokines (IL-2, tumor necrosis fctor (TNF) nd interferon-g (IFN-g)) nd T H 2 cytokines (IL-4, IL-5, IL-1 nd IL-13) t 16 h fter ctivtion were e IL-2 BY55-PE α-cd28 CTLA D.1A11-PC5 + α-cd28 CD α-cd28 + α-cd28 α-cd isolted humn PBMCs stined with nti-cd16 (5D.1A11-PC5), phycoerythrin-conjugted BY55, fluorescein isothiocynte conjugted nti-cd3 or phycoerythrin-indotricrocynine conjugted nti-cd4 or nti-cd8; numers ove rcketed lines indicte percent positive cells mong gted CD3 + CD4 + or CD3 + CD8 + cells. (c) Flow cytometry of purified CD4 + T cells stined with fluorescein isothiocynte conjugted nti-cd45ra, phycoerythrin-conjugted nti-cd45ro nd phycoerythrin-cynine 5 nti-cd16-pc5 (5D.1A11); numers ove rcketed lines indicte percent positive cells mong gted CD45RA + CD45RO, CD45RA CD45RO + or CD45RA + CD45RO + cells. (d) Flow cytometry of purified CD4 + T cells stimulted with ltex eds (1 mg/ml of nti-cd3 with or without.5 mg/ml of nti-cd28) t rtio of 1:1 (eds/cells) in 24-well pltes; cells were collected on dys 1 5 nd stined with nti-cd16 efore nlysis. Numers ove rcketed lines indicte percent positive cells. (e) Quntittive RT-PCR of totl RNA extrcted from the cells in d t 16 h fter ctivtion; vlues re reltive to GAPDH mrna ( housekeeping gene )., nti-. Dt re representtive of six (,) or three(c e) experiments. progressively diminished y the ddition of incresing concentrtions of nti-cd16 (Fig. 2c). IL-1, IL-8 nd IL-6 were lso downregulted, lthough the reduction in IL-8 required higher concentrtions of nti-cd16. We used microrry nlysis to serch for suppressive meditors tht might e induced y CD16 crosslinking. We extrcted totl RNA from CD4 + T cells stimulted with nti-cd3 plus nti-cd28 with or without nti-cd16 nd from unstimulted CD4 + T cells (migg1 isotype control) t vrious time points fter ctivtion (4, 16 nd 48 h). Initil nlysis of IL2 mrna y qrt-pcr showed tht IL2 ws mximlly upregulted y nti-cd3 plus nti-cd28 t 16 h fter ctivtion nd ws reduced to ckground y enggement of CD16 (Fig. 2d). We therefore used RNA extrcted t 16 h fter ctivtion in microrry nlysis (Humn U-133 plus 2., Affymetrix) to compre gene expression in cells stimulted with nti-cd3 plus nti-cd28 with or without nti-cd16 nd in unstimulted cells (isotype control). No known suppressive genes (TGFB1, IL1, PDCD1, CD274, PDCD1LG2 nd CTLA4) were upregulted y CD16 enggement. Most interleukin mrnas upregulted y stimultion with nti-cd3 plus nti- CD28 were suppressed y crosslinking of CD16 (Fig. 2e). Among thesemrnas,weconfirmedreductionsinil2, IL6 nd IL17A mrna y qrt-pcr (dt not shown); IL2RA (CD25) mrna ws lso suppressed y crosslinking of CD16, lthough expression of IL4R ws upregulted. mrna expression of nother interleukin receptor, IL7R, ws downregulted y nti-cd3 plus nti-cd28 ut, fter CD16 crosslinking, ws restored to the mount in the isotype control. CD28 costimultion cn enhnce T cell ctivtion y promoting glucose metolism 8. Severl smll-nutrient trnsporter gene fmily mrnas tht were upregulted y nti-cd3 plus nti-cd28 were inhiited y nti-cd16 (Fig. 2f), which suggested tht suppression of smll-nutrient trnsporter mrna expression might form prt of the CD16-medited pthwy. To determine whether inhiition of IL-2 production is key fctor in CD16-medited suppression, we dded exogenous IL-2 to the culture. Inhiition of T cell ctivtion y CD16 ws not rescued y the ddition of exogenous IL-2 (Fig. 2g), however, which indicted tht mechnisms other thn IL-2 reduction re importnt in CD16-medited inhiition of CD4 + T cells. Finlly, ecuse it hs een demonstrted tht GPI-nchored CD59 cn enhnce T cell ctivtion through the recruitment of tyrosine NATURE IMMUNOLOGY VOLUME 9 NUMBER 2 FEBRUARY
3 1 3 c.p.m. 1 3 c.p.m. 1 3 c.p.m. d IL-2 mrna α-cd α-cd16 (µg/ml) 4 h 16 h 48 h control +α-cd28 +α-cd28 +α-cd α-cd16 (µg/ml) kinses 9, we exmined the effect of CD16 crosslinking on tyrosine phosphoryltion in CD4 + T cells. Activtion medited y nti-cd3 plus nti-cd28 resulted in enhnced tyrosine phosphoryltion of severl proteins reltive to tht of unstimulted cells treted with the migg1 isotype control (Fig. 3). In contrst, crosslinking of CD16 reduced tyrosine phosphoryltion of the four min proteins induced y nti-cd3 plus nti-cd28 stimultion (Fig. 3, rrowheds). CD3z is smll protein tht is tyrosine-phosphorylted on T cell ctivtion 1 ; we therefore immunoprecipitted CD3z nd nlyzed it y immunolot with horserdish peroxidse (HRP) conjugted nti-phosphotyrosine. We found tht nti-cd16 crosslinking suppressed the phosphoryltion of CD3z (Fig. 3), indicting tht CD3z is one of the trgets of the nti-cd16 medited inhiition of CD4 + T cell ctivtion. Cloning HVEM s CD16 lignd Although MHC clss I molecules cn ind CD16 with wek ffinity 4 7, we found tht ma to MHC clss I molecules (W6/32) did not reverse the inhiitory effect of nti-cd16 on CD4 + Tcell ctivtion (dt not shown), which suggested tht nother receptor is involved in this pthwy. To identify n dditionl receptor for CD16, we generted n expression construct in which the extrcellulr domin of CD16 ws linked to humn IgG4 Fc (CD16-Ig). After SDS-PAGE, stining with Ponceu S showed tht CD16-Ig migrted s principl nd of out 52 kd (Fig. 4), nd immunolot nlysis indicted tht nti-cd16 (5D.1A11) ound specificlly to the CD16-Ig fusion protein (Fig. 4). Amino-terminl protein sequencing of the 52-kD nd identified the initil nine mino cids s I*ITSSASQ (where * is most likely to e glycosylted residue or cysteine), thus confirming the fusion protein s humn CD16-Ig (INITSSASQ; ref. 3). To identify cell popultion expressing CD16 lignd, we stined humn PBMCs with iotin-conjugted CD16-Ig. We found tht CD16-Ig ound to CD2 + B cells nd CD14 + monocytes ut showed e Expression ( 1 3 ) 4 2 IL1A IL1B Totl CD4 + T cells CD16 CD4 + T cells c Cytokine (pg/ml) control +α-cd28 +α-cd28+α-cd16 IL2 IL4 IL9 IL6 IL8 2, 1, IL-2 TNF IFN-γ Cytokine (pg/ml) α-cd16 (µg/ml) α-cd16 (µg/ml) α-cd16 (µg/ml) 16 h IL1 IL12B IL13 IL17A IL17F TNF IFNG LTA IL2RA Figure 2 Crosslinking of CD16 inhiits CD4 + T cell ctivtion. () Prolifertion ssy of purified CD4 + T cells stimulted with ntiody-coted ltex eds (1 mg/ml of nti-cd3 with or without.5 mg/ml of nti-cd28, plus vrious concentrtions of nti-cd16 (5D.1A11) or migg1 isotype control); cells were pulsed with.5 mci of [ 3 H]thymidine per well on dy 2 nd nlyzed 18 h lter. () Prolifertion ssy of sorted CD4 + CD16 or totl CD4 + T cells stimulted with ntiody-coted ltex eds (1 mg/ml of nti- CD3 nd.5 mg/ml of nti-cd28 plus vrious concentrtions of nti-cd16 (5D.1A11) or migg1 isotype control). (c) Cytokine production y CD4 + T cells stimulted s descried in. (d) Quntittive RT-PCR nlysis of IL-2 mrna in purified CD4 + T cells stimulted with ltex eds coted with isotype IL4R IL7R f Expression ( 1 3 ) 2 1 g miniml inding to CD4 + T cells, CD8 + T cells or NK cells (Fig. 4c). Notly, CD16-Ig did not universlly stin PBMCs, s would e expected if it were inding to MHC clss I (Fig. 4c). This oservtion suggests tht inding of CD16 to MHC clss I molecules my e too wek to e detected y solule CD16-Ig protein. Anti MHC clss I (W6/32) did not lock the inding of CD16-Ig to B cells, which suggested tht the fusion protein ws inding to high-ffinity lignd other thn MHC clss I (dt not shown). We thus used B cell cdna lirry 11 expressed in trnsformed Africn green monkey kidney COS cells to clone the CD16 lignd y immunoselection with CD16-Ig (ref. 12). After three rounds of immunoselection nd enrichment, we sequenced individul cdnas. Nine of the eleven cdna clones sequenced encoded humn HVEM (TNFRSF14), ech differing only in the length of the 5 untrnslted region. Notly, no MHC clss I cdnas were identified in this cloning procedure. COS cells trnsiently trnsfected with HVEM cdna showed specific inding to CD16-Ig ut not to control CTLA-4 Ig (Fig. 4d). Control cells trnsfected with B7.1 showed specific inding to CTLA-4 Ig ut not to CD16-Ig. These results collectively suggested tht HVEM is lignd of CD16. To compre the expression of HVEM nd the CD16 lignd, we used ma to HVEM to stin PBMCs from helthy donors. Anti-HVEM showed stining pttern (Fig. 4e) similr to tht of CD16-Ig stining (Fig. 4c): some B cells nd CD14 + CD3 monocytes were positive for HVEM, ut CD4 + nd CD8 + T cells expressed little HVEM. Specific inding etween CD16 nd HVEM To exmine further the specificity of the ssocition etween CD16 nd HVEM, we exmined the direct inding of CD16 to HVEM y using purified proteins in n enzyme-linked immunossy (ELISA). Plte-ound HVEM-mIgG2 demonstrted dose-dependent inding to oth CD16-Ig nd ma to HVEM ut no inding to control fusion protein of CTLA-4 nd humn IgG4 (CTLA-4 Ig; Fig. 5). To 16 h 2 1 IL-4 IL-5 IL-1 IL-13 SLC5A5 SLC7A11 SLC7A5 Cytokine (pg/ml) 3, 1,5 SLC16A1 SLC25A22 SLC25A32 SLC29A α-cd16 (µg/ml) * SLC38A5 SLC39A4 SLC39A14 IL-1β IL-8 IL-6 +IL-2 +α-cd28 +α-cd28+il-2 control ma lone or with nti-cd3 (1 mg/ml), nti-cd28 (.5 mg/ml) nd either nti-cd16 (1 mg/ml) or n equl mount of migg1 isotype control, for RNA extrcted t 4, 16 or 48 h; expression is reltive to tht GAPDH mrna. (e,f) Microrry nlysis of mrna expression in the cells in d 16 h fter stimultion; cytokines (e) nd smll-nutrient trnsporters (f) re grouped together. (g) Prolifertion ssy of CD4 + T cells treted with 1, U/ml of humn IL-2 nd ltex eds coted with ntiody, s descried in. Dt re representtive of twenty (), three (), four (c,d,g) or two(e,f) experiments. 178 VOLUME 9 NUMBER 2 FEBRUARY 28 NATURE IMMUNOLOGY
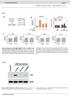
4 (kd) M β-ctin confirm tht finding further, we generted stle trnsfectnts of 3.19 cells expressing HVEM. Untrnsfected 3.19 cells did not express either HVEM or CD16, nd stle trnsfectnts hd considerle expression of HVEM or CD16 fter introduction of the respective gene (Fig. 5). Titrtion of the inding of CD16-Ig, BTLA-Ig nd LIGHT-dechistidine (dechis) to HVEM-trnsfected 3.19 cells showed tht BTLA nd CD16 hd similr ffinities for HVEM, ut LIGHT hd higher ffinity (Supplementry Fig. 2 c online). LIGHT, BTLA nd lymphotoxin- hve een shown to ind specificlly to HVEM, whose extrcellulr region comprises four CRDs 13,14. LIGHT nd lymphotoxin- inding hs een mpped to CRD2 nd CRD3 of HVEM 15, wheres BTLA inding is loclized to CRD1 (refs. 16,17). To mp the CD16-inding site on HVEM, we constructed mutnt HVEM lcking the mino-terminl CRD1 domin (HVEMDCRD1). Phenotyping of HVEM- nd HVEMDCRD1- trnsfected 3.19 cells with polyclonl nti-hvem showed similr expression in the cells. We found tht CD16-Ig ound to 3-HVEM cells, s did BTLA-Ig nd LIGHT-dechis, ut none of the proteins ound to untrnsfected 3.19 cells (Fig. 5c). In ddition, CD16, BTLA nd LIGHT did not ind to ech other (Supplementry Fig. 2d). Deletion of CRD1 of HVEM completely olished oth CD16-Ig nd BTLA-Ig inding ut hd no effect on LIGHT inding, (kd) 6 3 CD3ζ M Figure 3 Enggement of CD16 inhiits the tyrosine phosphoryltion of severl proteins induced y nti-cd3 nd nti-cd28. () Immunolot nlysis of totl lystes of purified CD4 + T cells stimulted with ltex eds coted with migg1 lone (lne 1) or with nti-cd3 nd nti-cd28 plus equl mounts of either migg1 (lne 2) or nti-cd16 (lne 3), then crosslinked for 4 min t 37 1C with got nti-migg (2 mg/ml), nlyzed with HRPconjugted ma to phosphorylted tyrosine. Arrows indicte increses in tyrosine phosphoryltion induced y nti-cd3 nd nti-cd28. M, moleculr size mrkers. Bottom, memrne stripped nd lotted with nti--ctin s loding control. () Immunoprecipittion nd immunolot nlysis of totl cell lystes from cells stimulted s descried in ; lystes were preclered with protein A nd G eds nd immunoprecipitted with nti-cd3z nd protein A nd G eds, nd the eds were then wshed, eluted nd nlyzed y immunolot with HRP-conjugted ma to phosphorylted tyrosine. Bottom, memrne stripped nd lotted with nti-cd3z s loding control. Dt re representtive of three () or four() experiments. which indicted tht CRD1 is essentil for ssocition with oth BTLA nd CD16 ut not with LIGHT. This finding shows tht CD16 nd BTLA ind to the sme domin of HVEM. Further confirming the specificity of inding, preincution of CD16-Ig with nti-cd16 (5D.1A11) reduced the inding of CD16-Ig to HVEM-trnsfected 3.19 cells lmost to ckground (Fig. 5d). Conversely, HVEM-Ig ound specificlly to CD16- trnsfected 3.19 cells nd not to untrnsfected 3.19 cells (Fig. 5e). Preincution of HVEM-Ig with nti-hvem modertely inhiited the inding of HVEM-Ig to CD16-trnsfected 3.19 cells. These results collectively show tht CD16 nd HVEM interct specificlly with n ffinity sufficient for detection using immunogloulin fusion proteins nd tht oth CD16 nd BTLA ind to the CRD1 domin of HVEM. In ddition, we found tht fusion protein of mouse CD16 nd migg2 (mcd16-ig) specificlly ound to 3.19 trnsfectnts expressing mouse HVEM (mhvem; Fig. 5f). The humn CD16, BTLA nd LIGHT fusion proteins lso ound to mhvem-trnsfected 3.19 cells, indicting these interctions re conserved cross species (dt not shown). To test whether CD16, BTLA nd LIGHT compete or cooperte for inding to HVEM, we preincuted HVEM-trnsfected 3.19 cells with CD16-Ig, BTLA-Ig or LIGHT-dechis, followed y BTLA- Ig, CD16-Ig or LIGHT-dechis (Fig. 5g i). We found tht CD16-Ig Figure 4 Cloning of HVEM s CD16 lignd. (,) SDS-PAGE of purified CD16-Ig (5 mg c CD4 + CD3 + CD8 + CD3 + CD2 + CD3 CD14 + CD3 CD56 + CD protein per lne) stined with Ponceu S () or nlyzed y immunolot with nti-cd16 (5D.1A11, 1 mg/ml) nd detected with HRPconjugted Biotin CD16-Ig got nti mouse IgG1 (). (c) Flow cytometry of PBMCs stined with iotinconjugted d Vector B7.1 HVEM CD16-Ig (filled histogrms) or isotype control migg1-iotin, followed y streptvidin phycoerythrin-indotricrocynine 62 (open histogrms) nd linege-specific mrkers CTLA4-Ig (CD3 nd CD4, CD8, CD2, CD14 or CD56) CD16-Ig Cells were gted on cells positive for linegespecific mrkers. Numers ove rcketed lines indicte percent positive cells. (d) Flow cytometry 17 of COS cells trnsfected with plsmids encoding 14 α-hvem HVEM or B7.1 cdna, or vector lone, collected 14 e CD4 + CD3 + CD8 + CD3 + CD2 + CD3 CD14 + CD3 on dy 3 nd stined with CD16-Ig, CTLA-4 Ig or ma to HVEM (filled histogrms) or control 6 humn IgG4 fusion protein (open histogrms). 3 (e) Flow cytometry of PBMCs stined with α-hvem llophycocynin-conjugted nti-hvem nd linege-specific mrkers (CD3 nd CD4, CD8, CD2 or CD14). Cells were gted on cells positive for linege-specific mrkers; numers ove rcketed lines indicte percent HVEM + cells. Dt re representtive of two (,), three (c,e) or five (d) experiments. 17 CD16-Ig Protein mrker CD16-Ig Protein mrker CTLA-4 Ig NATURE IMMUNOLOGY VOLUME 9 NUMBER 2 FEBRUARY
5 A 45 g , 1, Solule protein (ng/ml) BTLA-Ig CD16-Ig LIGHT (µg/ml) 3-HVEM 2 CTLA-4 Ig 3.19 α-hvem ma CD16-Ig 3- CD16 α-cd16 α-hvem nd BTLA-Ig hd little effect on the inding of LIGHT to HVEM (Fig. 5g). By contrst, LIGHT modestly enhnced the inding of CD16-Ig nd BTLA-Ig to HVEM-trnsfected 3.19 cells, s indicted y twofold increse in men fluorescence intensity (Fig. 5h,i). When we tested BTLA nd CD16 for cross-locking, 1 mg/ml of BTLA-Ig slightly decresed nd 1 mg/ml of BTLA-Ig lmost completely locked the inding of CD16-Ig to HVEM (Fig. 5h). CD16-Ig hd less effect on the inding of BTLA-Ig to HVEM: 1 mg/ml nd 1 mg/ml of CD16-Ig only slightly decresed the inding of BTLA-Ig to HVEM (Fig. 5i). These results suggest tht the BTLA- nd CD16-inding sites on HVEM overlp, ut they do not exclude the possiility tht inding of BTLA could result in conformtionl chnge tht ffects inding of CD16. Confocl microscopy showed tht in previously ctivted CD4 + T cells, CD16 ws present in discrete ptches (proly lipid rfts) nd did not loclize together with BTLA (Fig. 5j). A proportion of CD3 did loclize together with CD16. These results enle us to propose model of the interction of HVEM with its lignds (Fig. 5k nd discussed elow). HVEM-CD16 nd HVEM-BTLA inhiit CD4 + T cell ctivtion Preliminry experiments showed tht ctivted CD4 + T cells could express ll three HVEM lignds: CD16, BTLA nd LIGHT (Fig. 1d nd Supplementry Fig. 3 online). To exmine further the effects of engging the HVEM-CD16-BTLA pthwy, we stimulted CD4 + T cells with ltex eds coted with nti-cd3 plus nti-cd28 nd engged the HVEM-CD16-BTLA pthwy with nti-cd16, c α-hvem CTLA4-Ig CD16-Ig BTLA-Ig LIGHT d CD16-Ig f HVEM-Ig 1 1 BTLA-Ig 1 1 BTLA-Ig CD16-Ig CD16-Ig LIGHT 1 LIGHT (µg/ml) (µg/ml) HVEM 3-HVEM LIGHT 3- HVEM Figure 5 Interction of HVEM with CD16, BTLA nd LIGHT. () ELISA of the inding of CD16-Ig, nti- HVEM (migg1) or control CTLA-4 Ig to pltes coted with HVEM-mIgG2. () Flow cytometry of 3.19 cells or of 3.19 cells trnsfected with CD16 (3-CD16) or HVEM (3-HVEM) nd stined with isotype control (open histogrms) or nti-hvem or nti-cd16 (filled histogrms). (c) Flow cytometry of 3.19, 3-HVEM nd 3-HVEMDCRD1 cells stined with polyclonl nti- HVEM, CTLA-4 Ig, CD16-Ig, BTLA-Ig or LIGHT-dechis (filled), or with isotype control (open). (d) Flow cytometry HVEM 3- HVEM CRD1 h j FITC CD16-Ig CD16 CD16 i 3.19 mcd16-ig BTLA-Ig nti-btla or HVEM-Ig. We mesured prolifertion y dilution of the cytosolic dye CFSE (Fig. 6). We found tht 36% of the positive control cells stimulted with nti-cd3 plus nti-cd28 divided y dy 3. Enggement y nti-cd16, nti-btla or HVEM-Ig strongly inhiited CD4 + T cell division induced y nti-cd3 plus nti-cd28 such tht only 6% of cells divided. By dy 5, 93% of the positive control cells hd divided. Enggement y nti-cd16, nti-btla or HVEM-Ig strongly inhiited the CD4 + T cell cycling induced y.1 mg/ml of nti-cd3 plus nti-cd28. The stronger signl provided y 1 mg/ml of nti-cd3 plus nti-cd28 ws slightly inhiited y nti- CD16 or HVEM-Ig ut not y nti-btla. These dt indicte tht the negtive signl delivered through nti-cd16, nti-btla or HVEM-Ig cn inhiit moderte ut not strong signls delivered y T cell ntigen receptor nd CD28. Conversely, we exmined the effects of locking the HVEM-CD16- BTLA pthwy during n ntigen-specific memory T cell response in vitro. Tetnus toxoid specific prolifertion nd IFN-g production were ugmented when HVEM enggement ws locked with solule monomeric nti-cd16 F, nti-btla or nti-hvem (Fig. 6). Blockde of HVEM hd stronger enhncing effect on IFN-g production thn did lockde of CD16 or BTLA lone. We noted similr enhncement of IFN-g production y nti-cd16 F, nti- BTLA or nti-hvem in n lloresponse (Fig. 6c). These results suggest tht oth CD16 nd BTLA contriute to HVEM-medited inhiition of T cell responses. The function of HVEM in humns hs een studied less intensively nd the effect of humn HVEM trnsfectnts on T cell ctivtion e 3-mHVEM Cy5 Merge k CD4 + T CD4 + T BTLA CD HVEM APC (HVEM) 3-HVEM &5D.1A CD16 3-CD16 &α-hvem CD16 BTLA LIGHT HVEM CRD1 HVEM APC (HVEM CRD1) of 3.19 or 3-HVEM cells stined with isotype control (open) or with CD16-Ig or CD16-Ig preincuted with nti-cd16 (filled). (e) Flow cytometry of 3.19 or 3-CD16 cells stined with isotype control (open), or with HVEM-mIgG2 or HVEM-mIgG2 pre-incuted with nti-hvem (filled). (f) Flow cytometry of 3.19 or 3-mHVEM cells stined with isotype control (open) or mcd16-ig (filled). (g i) Flow cytometry of 3-HVEM cells stined with 1 mg/ml of isotype control (open), LIGHT-dechis, CD16-Ig or BTLA-Ig, lone or in vrious comintions (filled), nd detected with phycoerythrinconjugted nti-light (for LIGHT; g), nti humn IgG (for CD16-Ig; h) or nti-migg2 (for BTLA-Ig; i). Numers in plots indicte men fluorescence intensity. (j) Confocl microscopy of stimulted CD4 + T cells stined with nti-cd16 (green) nd nti-btla (red) or with nti-cd16 (green) nd nti-cd3 (red). Right, merge of fluorescein isothiocynte (FITC) nd indodicrocynine (Cy5) imges; colocliztion is yellow. Originl mgnifiction, 6. (k) Interction of HVEM or HVEMDCRD1 with CD16, BTLA nd LIGHT. APC, ntigen-presenting cell. Dt re representtive of three (,d,e,j), six (,c), five (f) or four(g i) experiments. 18 VOLUME 9 NUMBER 2 FEBRUARY 28 NATURE IMMUNOLOGY
6 IFN-γ (ng/ml) IFN-γ (ng/ml) 1 3 c.p.m. 2 1 (µg/ml) migg1 +α-cd16 +α-btla +HVEM-Ig α-hvem CFSE Tetnus toxoid (Lf/ml) Dy 3 Dy 5 α-cd28 (µg/ml) α-cd16 F α-btla hs not een pulished. To explore further the function of the HVEM-CD16 pthwy in CD4 + T cell ctivtion, we generted different lines of rtificil ntigen-presenting cells (NIH-3T3 cells) expressing HVEM or the HVEMDCRD1 mutnt long with the MHC clss II molecule DR7 or DR7 plus B7.1 (clled DR7, DR7-B7.1, DR7- HVEM, DR7-B7.1-HVEM nd DR7-B7.1-HVEMDCRD1 cells here). We found similr expression of DR7 in ll five trnsfectnts, similr expression of B7.1 in DR7-B7.1, DR7-B7.1-HVEM nd DR7-B7.1- HVEMDCRD1 trnsfectnts, nd similr expression of HVEM in DR7-HVEM, DR7-B7.1-HVEM nd DR7-B7.1-HVEMDCRD1 trnsfectnts (Fig. 7). c 6 3 CD4 + T cells APCs 1 5 α-cd16 F α-btla α-hvem Figure 6 Both CD16 nd BTLA inhiit CD4 + T cell ctivtion. () Prolifertion ssy of CFSE-leled CD4 + T cells stimulted with ltex eds coted with isotype control ma or comintion of nti-cd3 (.1 or 1 mg/ml), nti-cd28 (.5 mg/ml), nd nti-cd16 (5D.1A11), nti- BTLA or HVEM-Ig (1 mg/ml), then collected on dys 3 nd 5, stined with phycoerythrin-conjugted nti-cd4 nd nlyzed y flow cytometry for CFSE dilution. Dt re CFSE single-prmeter histogrms gted on CD4 + Tcells. Numers ove rcketed lines indicte percent cells tht hve divided t lest once. () Prolifertion nd IFN-g production ssys of PBMCs stimulted with tetnus toxoid in the presence of nti CD16 F, nti- BTLA, nti-hvem or isotype control (ech t 1 mg/ml). Superntnts were collected on dy 5 for mesurement of IFN-g y ELISA (right); cells were then pulsed with [ 3 H]thymidine nd prolifertion ws mesured 18 h lter (left). (c) ELISA of IFN-g in superntnts of CD4 + T cells stimulted with irrdited (3,5 rds) llogeneic PBMCs with migg1 (isotype control), nti-cd16 F, nti-btla or nti-hvem (ech t 1 mg/ml) dded to the cultures, mesured 7 d fter stimultion. Dt re representtive of five (,) or three (c) experiments nd re the men ± s.d. of triplicte wells. We used DR7 llontigen specific CD4 + T cell line, 2C1, with moderte expression of CD16 (Fig. 7) to determine the functionl effect of HVEM inding to CD16. As expected, DR7 trnsfectnts modestly nd DR7-B7.1 trnsfectnts strongly stimulted T cell prolifertion nd cytokine production (Fig. 7c). Expression of HVEM on the trnsfected cells led to potent inhiition of T cell prolifertion nd cytokine production (Fig. 7c).There ws decrese of more thn 9% in prolifertion etween DR7-HVEM nd DR7 trnsfectnts, nd n 8% decrese in prolifertion etween DR7-B7.1-HVEM nd DR7- B7.1 trnsfectnts. To determine the contriution of CD16 nd BTLA to HVEM-medited inhiition, we used monomeric nti-cd16 F tht cts s locking regent, monoclonl nti-btla or monoclonl nti-hvem. All three ntiodies eqully reversed the HVEM-medited inhiition of T cell prolifertion noted with DR7-B7.1-HVEM trnsfectnts (Fig. 7d). All three ntiodies enhnced IL-13 nd IFN-g production, nd nti-hvem ws more effective thn nti-cd16 F or nti-btla. These results show tht oth CD16 nd BTLA contriute to the HVEM-medited inhiition of T cell ctivtion. Figure 7 HVEM-trnsfected NIH-3T3 cells inhiit CD4 + T cell ctivtion nd inhiition cn e reversed y nti CD16 F or y deletion of CRD1 of HVEM. () Flow cytometry of NIH-3T3 cells trnsfected with DR7, B7.1, HVEM or HVEMDCRD1 nd stined with isotype control (open histogrms), nti-dr7, nti-b7.1 or nti- HVEM (filled histogrms). () Flow cytometry of resting 2C1 T cells stined with mas (to molecules elow plots). (c) Prolifertion nd cytokine production ssys of the DR7 llontigen specific T cell line 2C1 fter incution with NIH-3T3 trnsfectnts; superntnts were collected on dy 3 for the cytokine ssy nd wells were then pulsed with [ 3 H]thymidine nd prolifertion ws mesured 18 h lter. (d) Prolifertion nd cytokine production ssys of the T cell line 2C1 fter incution with DR7-B7.1-HVEM trnsfectnts in the presence of nti-hvem, nti-cd16 F, nti-btla or isotype control F (1 mg/ml). (e) Prolifertion nd cytokine production y CD4 + T cells stimulted with NIH-3T3 trnsfectnts. Dt re representtive of three (,) or four (c e) experiments (dt in c,d re from the sme experiment nd represent the men ±s.d.oftriplictewells). DR7 DR7-B7.1 DR7-HVEM DR7-B7.1 -HVEM DR7-B7.1- HVEM CRD1 α-dr7 α-b7.1 α-hvem CD3 CD4 CD45RA CD45RO CD16 BTLA c APCs d e 1 3 c.p.m. 1 3 c.p.m. 1 3 c.p.m APCs APCs IFN-γ (ng/ml) IFN-γ (ng /ml) IFN-γ (ng/ml) IL-13 (ng/ml) IL-13 (ng/ml) DR7 DR7-HVEM DR7-B7.1 DR7-B7.1-HVEM DR7-B7.1 DR7-B7.1-HVEM DR7-B7.1-HVEM CRD1 DR7-B7.1-HVEM +α-hvem +α-cd16 F +α-btla NATURE IMMUNOLOGY VOLUME 9 NUMBER 2 FEBRUARY
7 Becuse CRD1 of HVEM is required for oth CD16 nd BTLA ut not LIGHT inding, we tested whether deletion of CRD1 would eliminte the suppressive function of HVEM. We compred the cpcity of DR7-B7.1 trnsfectnts expressing either HVEM or HVEMDCRD1 to regulte CD4 + T cell ctivtion in n lloresponse. Unexpectedly, DR7-B7.1-HVEMDCRD1 trnsfectnts enhnced T cell ctivtion nd IFN-g production even more strongly thn did DR7- B7.1 trnsfectnts (Fig. 7e). As efore, DR7-B7.1-HVEM trnsfectnts inhiited T cell ctivtion. Becuse deletion of CRD1 of HVEM olishes CD16 nd BTLA inding nd muttionl nlysis 22 indictes tht CRD2 nd CRD3 re importnt for inding to LIGHT nd lymphotoxin-, these dt suggest tht the inhiitory effect of CD16 or BTLA inding to HVEM domintes over the stimultory effect of LIGHT or lymphotoxin- inding to HVEM. These dt demonstrte tht HVEM is n importnt functionl lignd of CD16. Binding of HVEM to CD16 delivers negtive regultory signl for T cell ctivtion tht cn e locked y nti-cd16 F, nti-hvem or deletion of CRD1. Our dt collectively show tht oth CD16 nd BTLA contriute to HVEM-medited inhiition of CD4 + T cell ctivtion, wheres inding of LIGHT to HVEM strongly enhnces Tcellctivtion. DISCUSSION Using newly generted mas to humn CD16, we hve confirmed tht CD16 is expressed on smll percentge of CD4 + T cells nd hve shown tht CD16 is upregulted fter CD4 + T cell ctivtion. Crosslinking of CD16 with ma to CD16 strongly inhiited CD4 + T cell prolifertion nd cytokine production, thus identifying CD16 s negtive regultor of CD4 + T cell ctivtion. Enggement of CD16 reduced tyrosine phosphoryltion of CD3z nd severl other proteins. Microrry nlysis showed tht CD16 enggement downregulted rod rnge of ctivtion-induced genes, including cytokine genes nd smll-nutrient trnsporter genes. These results led us to serch for CD16 lignds in ddition to MHC clss I molecules. We identified HVEM s CD16 lignd y expression cloning nd found specific interction etween CD16 nd HVEM tht resulted in inhiition of T cell ctivtion. Studies with high-vidity MHC clss I tetrmers hve shown tht clssicl nd nonclssicl MHC clss I molecules ind to CD16 with low ffinity 4 7 ; however, our CD16-Ig construct did not demonstrte the rod rnge of inding to cells tht would e expected of universl MHC clss I lignd. This oservtion my e simply consequence of the lower vidity of dimeric CD16-Ig fusion protein for cell surfce MHC clss I thn MHC clss I tetrmers for cell surfce CD16. The specific inding of CD16-Ig to B cells nd monocytes ws not locked y ma to MHC clss I (W6/32), suggesting tht B cells nd monocytes express non-mhc lignd of CD16. Using CD16-Ig, we cloned HVEM from B cell cdna lirry. Stining of PBMCs with ma to HVEM showed n expression pttern on B cells nd monocytes tht ws similr to the pttern noted for CD16-Ig stining. Although CD16 is expressed on only out 8% of unstimulted CD4 + T cells, nti-cd16 inhiited most of the T cell popultion, proly y inding to CD16 expressed fter ctivtion. This strong effect of protein tht is wekly expressed on unstimulted cells is similr to tht seen for nti-ctla-4 or nti-pd-1. Indeed, consistent with the expression nd function of these other negtive regultory molecules, CD16 hs een shown to e upregulted on exhusted T cells 18. Removl of CD16 + CD4 + T cells from the CD4 + popultion led to decrese in inhiition y CD16 enggement; however, high concentrtions of nti-cd16 still sustntilly inhiited T cell ctivtion, proly y inding to CD16 protein expressed fter ctivtion. CD16-medited inhiition ws not rescued y exogenous IL-2, which indicted tht mechnisms other thn IL-2 reduction re importnt in CD16-medited CD4 + Tcellinhiition. The mechnism wherey GPI-nchored protein such s CD16 engges signling pthwys is poorly understood. Studies of GPI-nchored proteins such s CD59, CD9 nd CD48 hve demonstrted their locliztion to lipid rfts nd modultion of tyrosine kinse ctivity 9,19. Phosphotyrosine immunolot nlysis showed tht crosslinking of CD16 modulted tyrosine kinse ctivity with reduced tyrosine phosphoryltion of severl sustrtes, prticulrly CD3z. In contrst to BTLA, enggement of CD16 did not induce phosphoryltion of the tyrosine phosphtses SHP-1 or SHP-2 (dt not shown). CD16 nd BTLA might ct coordintely in HVEM-medited inhiition, wherey CD16 modultes the signling complexes tht trnslocte to lipid rfts nd BTLA recruits tyrosine phosphtses 2. HVEM serves s idirectionl switch for T cell ctivtion, producing positive or negtive outcome depending on the lignds engged 21. Our dt hve identified CD16 s HVEM lignd in ddition to BTLA, LIGHT nd lymphotoxin-. LIGHT nd lymphotoxin- rememersofthetnfsuperfmilywithtrimericstructure, wheres CD16 nd BTLA comprise single immunogloulin-like domin 3,2. The HVEM-inding site on LIGHT hs een mpped to CRD2 nd CRD3 (ref. 15), wheres the inding sites for BTLA, herpes simplex virus glycoprotein D (gd) 22 ndcd16(sshownhere)re present in CRD1. The LIGHT-HVEM interction consists of LIGHT trimer with n HVEM molecule ound t the interfce etween ech pir of LIGHT monomers, therey mking 3:3 complex 14.Inthis rrngement, the CRD1 of HVEM overhngs the top of the LIGHT molecule nd would e ccessile for interction with CD16 or BTLA. Mutgenesis of HVEM hs shown tht the BTLA- nd gd-inding sites re opposite to the LIGHT-inding site 16. Our cross-locking studies with CD16 nd BTLA indicted tht their inding sites on the CRD1 domin of HVEM overlp to some extent, nd our colocliztion experimentsshowedthtcd16ndbtladonotssociteefore intercting with HVEM. Light-scttering nlysis hs shown tht BTLA nd HVEM form 1:1 complex 16. A solule HVEM molecule cn ind solule BTLA molecule nd LIGHT molecule simultneously 23.Our inding experiments with HVEM-expressing cells suggested tht HVEM cn form complex t the cell surfce with BTLA nd LIGHT or with CD16 nd LIGHT. Indeed, ssocition of LIGHT with HVEM did not lock either CD16 or BTLA inding ut led to twofold increse in the men fluorescence intensity of CD16 or BTLA inding. This oservtion suggests tht fully formed complex might contin nine molecules: three of HVEM, three of LIGHT, nd three in totl of BTLA nd/or CD16. HVEM inding to LIGHT on T cells is reported to stimulte T cell ctivtion 24 26, ut we found tht inding of HVEM to BTLA or CD16 inhiited T cell ctivtion. HVEM-knockout mice hve higher T cell ctivtion 27, indicting tht the inhiitory function of HVEM is the essentil, nonredundnt function of HVEM, despite the tenfold higher ffinity of HVEM for LIGHT (11 nm) thn for BTLA (112 nm; ref. 22). This dominnt inhiitory function of HVEM hs lso een seen in studies with mouse 17 nd humn (s shown here) CD4 + T cells stimulted with ntigen-presenting cells trnsfected with mouse or humn HVEM, respectively. In our experiments, HVEM strongly inhiited T cell responses despite the coexpression of LIGHT with CD16 nd BTLA on ctivted humn T cells. Becuse trimeric HVEM ound oth CD16 nd BTLA t the sme time nd lockde 182 VOLUME 9 NUMBER 2 FEBRUARY 28 NATURE IMMUNOLOGY
8 of either BTLA or CD16 reversed the HVEM-medited inhiition, CD16 nd BTLA my ct coordintely in HVEM-medited inhiition. Compring the T cell responses of CD16 nd/or BTLA knockout mice my shed light on this issue. Our experiments with ntigen-presenting cells trnsfected with HVEMDCRD1 nd HVEM offer insight into the function of HVEM when presented in trns to T cells potentilly expressing CD16, BTLA nd/or LIGHT. Wheres trnsfectnts expressing HVEM inhiited T cell responses, those expressing HVEMDCRD1 enhnced T cell responses. These results suggest tht CRD1 is essentil for the delivery of n inhiitory signl nd tht the inhiitory interction of HVEM with CD16 nd/or BTLA domintes over stimultory interction with LIGHT. When the inding site on HVEM for the inhiitory receptors ws eliminted (HVEMDCRD1), enggement of LIGHT lone led to potent stimultory signl. The herpes simplex virus gd protein chieves similr result y inding to CRD1 of HVEM nd locking the interction with BTLA. Becuse the inding sites for BTLA nd CD16 overlp, it is likely tht CD16 inding is lso locked. Indeed, it hs een shown tht DNA vccines expressing n ntigen in gd strongly enhnce immune responses in vivo 28.During T cell response, the outcome proly depends on expression of the negtive receptors (CD16 nd BTLA) reltive to the positive receptor (LIGHT). Indeed, studies hve shown tht constitutive expression of LIGHT results in tissue destruction nd utoimmunelike disese syndromes 29, confirming the idirectionl function of HVEM. Expression of HVEM in humns hs een reported in plcentl syncytiotropholsts, mnion epithelil cells 3 nd umilicl vein endothelil cells 31, which suggests tht the interction of HVEM with CD16 nd BTLA might hve n importnt role in regulting tolernce during pregnncy. In ddition, CD16 + CD4 + T cells might e involved in inflmmtory responses in skin 32. In summry, we hve shown here tht CD16 is lignd of HVEM nd tht the CD16-HVEM interction inhiits CD4 + T cell ctivtion. HVEM cts s idirectionl switch for T cell ctivtion wherey the outcome depends on the lignds engged 21. The functionl consequence of inding HVEM cn e mnipulted through genetic modifiction of the CRD1 region of HVEM. The contriution of ech HVEM lignd to the regultion of humn immune responses nd the est wy to mnipulte this pthwy therpeuticlly re issues to e investigted. METHODS Humn sujects. Peripherl venous lood ws otined from norml donors fter informed consent under institutionl review ord pprovl from the Dn-Frer Cncer Institute. Antiodies. Mouse mas to humn CD16 were mde y immunizing mice with CD16-Ig nd screening hyridom superntnts on CD16-trnsfected 3.19 nd Chinese hmster ovry cells. Both 5D.1A11 nd 5D.8E1 re mouse IgG1 nd show specific inding y FACS to CD16-trnsfected cell lines (3.19, CHO nd COS) ut not to untrnsfected cells. We prepred 5D.1A11 F nd migg1 (MOPC-31) F with n ImmunoPure IgG1 F nd F 2 preprtion kit (Pierce Biotechnology). CD16-phycoerythrin (BY55-PE; BY55, IgM) ws from Immunotech. Got nti-mouse IgG (11-9), got F( ) 2 nti-humn IgG (132-9), got ntimigg2 (18-9) nd got nti-migg1 (17-9) were from Southern Biotech. Mouse fluorescein isothiocynte conjugted nti got immunogloulin (259518) ws from Jckson Immunotech. Anti-DR7 IgM (iotinylted ma; BIH18) ws from One Lmd. Monoclonl nti-hvem (9481), polyclonl got nti-hvem (AF356), mouse nti humn LIGHT (11552), nd recominnt humn LIGHT-dechis (664-LI/CF) were from R&D Systems. Humn HVEM-Ig (migg2 Fc; ) ws from Ancell. Anti humn BTLA ws from ebioscience. Flow cytometry stining ntiodies to CD3, CD4, CD8, CD14, CD2, CD56, CD45RA, CD45RO, CD69 nd CD25 were from BD Biosciences. HRP-conjugted ma to phosphorylted tyrosine (4G1) ws from Upstte. Fusion proteins nd HVEM mutnts. CD16-Ig comprised the complete extrcellulr domin of CD16 fused to the hinge-ch2-ch3 domins of humn IgG4 (mutted to reduce FcR inding). The CD16 extrcellulr domin ws mplified y PCR (primers, Supplementry Tle 1 online), digested with HindIII nd BglII, nd ligted into HindIII- nd BclI-digested pnrdsh plsmid contining the hinge-ch2-ch3 domins of humn IgG4. All restriction endonucleses were from New Englnd Biols. The control CTLA-4 Ig comprised the extrcellulr domin of CTLA-4 linked to the sme humn IgG4. CD16-Ig ws produced in stly trnsfected NSO cells nd ws purified from conditioned medium with protein A sephrose 4B. Humn BTLA-mIgG2 nd the mcd16-migg2 construct were mde y In-Fusion ssemly 33. The BTLA or mcd16 extrcellulr domin nd the hinge-ch2, CH3 of migg2 (mutted to reduce FcR inding) were mplified with overlpping primer pirs. The two PCR products were fused in n expression vector y n In-Fusion rection (Clontech) nd confirmed y sequencing. The construct ws mde liner with MluI, ws electroported into CHO DG44 cells, ws selected y methotrexte resistnce, nd ws cloned s single cells. BTLA-Ig or mc16-ig ws purified from the cell superntnt of the highest producer clone y protein G or A sephrose 4B. The CRD1 domin comprising mino cids ws deleted from humn HVEM cdna in pcdm8 in wy nlogous to tht descried for mouse CRD1 deletion mutnt 17. The mino-terminl end nd the croxyterminl region of HVEM were mplified y PCR with overlpping primer pirs. The DNAs were incuted with n HindIII- nd BspEI-digested HVEM plsmid, were joined y n In-Fusion rection (Clontech), were trnsformed in Escherichi coli cells nd were sequenced for confirmtion. Cytokine ELISA, cytokine ed ssy, nd fusion protein inding ELISA. IL-1, IL-13 nd IFN-g in culture superntnts were ssyed y ELISA with ma to humn IL-1 (554497, ), IL-13 (55457, 55554; oth BD Biosciences) or IFN-g (2G1, M71B; Endogen) in ccordnce with the mnufcturer s instructions. IL-1, IL-2, IL-4, IL-5, IL-6, IL-8, IL-1, IL-13, IFN-g nd TNF were determined with cytokine ed ssy kit (BD Biosciences). For mesurement of the inding of immunogloulin fusion proteins, ELISA pltes were coted with 5 mg/ml of HVEM-mIgG2 fusion protein nd then locked. Seril dilutions of CD16-Ig, CTLA-4 Ig or monoclonl nti-hvem were dded, nd the pltes were incuted for 3 min nd then wshed. Binding ws detected y incution with HRP-conjugted nti humn IgG (for CD16-Ig or CTLA-4 Ig) or nti-migg1 (1:1,, for nti- HVEM), coupled with development with TMB sustrte nd mesurement of sornce t 45 nm (A 45 ). Cloning of CD16 lignd with CD16-Ig. A B cell cdna lirry in the pcdm8 expression vector 34 ws trnsiently trnsfected into COS cells y GeneJuice (Novgen). COS cells were collected 44 h fter trnsfection nd were incuted with 1 mg/ml of CD16-Ig. Cells were wshed nd then CD16-Ig + cells were enriched y pnning on pltes coted with F 2 got nti humn IgG. Plsmid ws purified nd reintroduced into E. coli y electroportion. Plsmids were trnsfected into COS cells y spheroplst fusion with polyethylene glycol, nd CD16-Ig + cells were immunoselected s descried ove. After three rounds of immunoselection, plsmids were individully purified, trnsfected into COS cells with GeneJuice, nd stined with 1 mg/ml of CD16-Ig on dy 3. Trnsfected cell lines. NIH-3T3 cells trnsfected with humn DR7 were mintined in medium 35 contining 45% (vol/vol) DMEM, 45% (vol/vol) F12, 1% (vol/vol) FCS, 2 mm GlutMAX nd 2 mg/ml of G418 (drug selection for DR7; ref. 35; ll from Invitrogen). Humn HVEM or HVEMDCRD1 in pcdm8 nd plsmid encoding puromycin resistnce were introduced y electroportion into NIH-3T3 cells expressing DR7 or DR7-B7.1. Positive cells were selected with 1 mg/ml of puromycin nd then were sorted with ma to HVEM nd sucloned to otin stle trnsfectnts. NATURE IMMUNOLOGY VOLUME 9 NUMBER 2 FEBRUARY
9 We stly trnsfected 3.19 cells with humn CD16, BTLA, HVEM or HVEMDCRD1 or with mouse HVEM or CD16 y electroportion, sorted the cells y using specific ntiodies nd then cloned them. For fusion protein inding ssys, 3.19 trnsfectnts were incuted for 15 min on ice with 1 mg/ml of fusion protein, wshed nd then incuted for 1 min t 25 1C with phycoerythrin-conjugted got nti humn IgG (CD16-Ig inding ssy), got nti-migg2 (HVEM-Ig inding ssy) or mouse nti humn LIGHT (LIGHT-inding ssy). For locking, the fusion protein ws incuted for 3 min on ice with specific or isotype control ma efore eing dded to 3.19 trnsfectnts. The cells were then wshed nd nlyzed immeditely y flow cytometry. PBMC isoltion nd tetnus toxoid stimultion. PBMCs were purified y density grdient centrifugtion using Ficoll/Hypque (Phrmci Biotech). Cells (2 1 5 PBMCs per well) in 5% (vol/vol) humn AB serum medium (RPMI-164, 2 mm GlutMAX, 1, U/ml of penicillin, 1 mg/ml of streptomycin, 1 mm HEPES, ph 7.2, 1 mm sodium pyruvte nd 1% (vol/vol) nonessentil mino cids; ll from Meditech) were stimulted with vrious concentrtions of tetnus toxoid (University of Msschusetts Medicl Center) comined with 1 mg/ml of nti-cd16 F (5D.1A11) or migg1 F s indicted. On dy 5 fter ctivtion, 1 ml of superntnt ws collected for the cytokine ssy, cells were pulsed with.5 mci of [ 3 H]thymidine per well nd the counts per minute (c.p.m.) were mesured. CD4 + T cell purifiction nd ctivtion with coted ltex eds. CD4 + Tcells were purified from freshly isolted PBMCs y depletion of non-cd4 + T cells y mgnetic eds using T cell isoltion kit II (Miltenyi); purity ws judged to e over 9% y flow cytometry. Ltex eds (5 mm in dimeter, 4%; Invitrogen) were coted with vrious concentrtions of nti-cd3 (UCHT1; BD Biosciences), nti-cd28 (MAB342; R&D), nd/or vrious concentrtions of nti- CD16 (5D.1A11 or 5D.8E1) or migg1. The mount of protein ws kept constnt t 11.5 mg/ml y the ddition of control migg1. Antiodies nd eds in PBS were incuted for 1.5 h t 37 1C. Ltex eds were wshed once with 1% (vol/vol) FCS in RPMI medium nd then were locked for 3 min t 37 1C with 1% (vol/vol) FCS in RPMI medium. Beds were then wshed nd were dded t 1:1 rtio to 96-well U-ottomed pltes contining T cells per well. At 16 h fter stimultion, 1 ml of superntnt ws collected nd cytokines were ssyed. On dy 2, cells were pulsed with.5 mci of [ 3 H]thymidine nd prolifertion ws mesured 18 h lter. For nlysis of T cell division y croxyfluorescein dicette succinimidyl diester (CFSE) dilution, CD4 + T cells were wshed twice in PBS, were resuspended in PBS t density of cells per ml, mixed with n equl volume of 1 mm CFSE in PBS, nd incuted for 5 min t 25 1C with occsionl mixing. Leling ws quenched y the ddition of n equl volume of cold FCS. Cells were then centrifuged nd were wshed twice in 5% humn AB serum medium. Cells were plted t density of cells per ml in medium in 24-well pltes, were stimulted with ltex eds coted with ma or fusion protein nd were nlyzed fter 1 5 d for CFSE dilution y flow cytometry. 2C1 CD4 + T cell clone. The 2C1 llogeneic CD4 + T cell clone specific for DR7 ws generted from purified CD4 + T cells (1 1 6 per well) cultured together in 24-well pltes with NIH-3T3 cell DR7-B7.1 trnsfectnts tht hd een previously treted with 5 mg/ml of mitomycin C. All T cell clones nd 3T3 trnsfectnts cultures were prepred in RPMI 164 medium supplemented with 1% (vol/vol) FCS (Cmrex BioScience), 2 mm GlutMAX, 1 mm sodium pyruvte, 15 mg/ml of gentmicin (ll from Invitrogen), 5 U/ml of penicillin, 5 mg/ml of streptomycin nd 1 mm HEPES uffer, ph 7.2 (ll from Meditech). After three stimultions, llogeneic CD4 + T cells were collected y Ficoll grdient, sorted s single cells into 96-well pltes nd stimulted with irrdited PBMCs s feeder cells, DR7-B7.1 trnsfectnts, 5 U/ml of IL-2 (BD Biosciences) nd 2 mg/ml of phytohemglutinin (Sigm Aldrich). The 2C1 T cells were restimulted with DR7-B7.1 trnsfectnts every 4 weeks nd their popultions were expnded with 5 U/ml of IL-2 every 4 d. For nlysis of the effect of HVEM on T-cell ctivtion, NIH-3T3 trnsfectnts were collected, were wshed twice with RPMI medi (without ntiiotics), were resuspended t density of cells per ml, were incuted for 3 h t 37 1C with 5 mg/ml of mitomycin C, were wshed twice with medium, nd then were plted t vrious densities in 2-ml volume per well. The next dy, medi were removed nd C1 T cells (Fig. 7c,d) or purified CD4 + T cells (Fig. 7e) with or without 1 mg/ml of nti-cd16 F (5D.1A11), migg1 F, ma to BTLA, ma to HVEM or migg1 in 2 ml ws dded to ech well. After 48 h, 1 ml of superntnt were collected for mesurement of cytokine production nd cells were pulsed with.5 mci [ 3 H]thymidine in 1 ml of medium for mesurement of T cell prolifertion 18 h lter. Additionl methods. Informtion on immunolot ssy, qrt-pcr, microrry nlysis nd confocl microscopy is ville in the Supplementry Methods online. Note: Supplementry informtion is ville on the Nture Immunology wesite. ACKNOWLEDGMENTS Confocl imges were otined y T. Hickmn (Brighm nd Women s Confocl Core Fcility). Supported y the Ntionl Institutes of Helth (AI39671 nd AI56299 to G.J.F.). AUTHOR CONTRIBUTIONS G.C. designed nd did the experiments, nlyzed the dt nd wrote the pper; A.A. prepred CD16-Ig nd provided some of the initil ides; J.A.B. prepred 2C1 T cells; E.A.G. generted mas to CD16; B.Z. prepred fusion protein nd expression constructs; nd G.J.F. plnned nd supervised the project, designed constructs nd wrote the pper. COMPETING INTERESTS STATEMENT The uthors declre competing finncil interests: detils ccompny the full-text HTML version of the pper t Pulished online t Reprints nd permissions informtion is ville online t reprintsndpermissions 1. Bensussn, A. et l. BY55 monoclonl ntiody delinetes within humn cord lood nd one mrrow lymphocytes distinct cell susets mediting cytotoxic ctivity. Proc. Ntl. Acd. Sci. USA 91, (1994). 2. Miz, H. et l. A novel 8-kD cell surfce structure identifies humn circulting lymphocytes with nturl killer ctivity. J. Exp. Med. 178, (1993). 3. Anumnthn, A. et l. Cloning of BY55, novel Ig superfmily memer expressed on NK cells, CTL, nd intestinl intrepithelil lymphocytes. J. Immunol. 161, (1998). 4. Med, M. et l. Murine CD16, Ig-like receptor on NK cells nd NKT cells, recognizes clssicl nd nonclssicl MHC clss I nd regultes NK cell ctivtion. J. Immunol. 175, (25). 5. Agrwl, S. et l. Cutting edge: MHC clss I triggering y novel cell surfce lignd costimultes prolifertion of ctivted humn T cells. J. Immunol. 162, (1999). 6. Brkonyi, A. et l. Cutting edge: enggement of CD16 y its HLA-C physiologicl lignd triggers unique cytokine profile secretion in the cytotoxic peripherl lood NK cell suset. J. Immunol. 173, (24). 7. Le Bouteiller, P. et l. Enggement of CD16 receptor y HLA-C is triggering mechnism used y circulting nturl killer (NK) cells to medite cytotoxicity. Proc. Ntl. Acd. Sci. USA 99, (22). 8. Fruwirth, K.A. et l. The CD28 signling pthwy regultes glucose metolism. Immunity 16, (22). 9. Stefnov, I., Horejsi, V., Ansotegui, I.J., Knpp, W. & Stockinger, H. GPI-nchored cellsurfce molecules complexed to protein tyrosine kinses. Science 254, (1991). 1. Bniysh, M., Grci-Morles, P., Luong, E., Smelson, L.E. & Klusner, R.D. The T cell ntigen receptor zet chin is tyrosine phosphorylted upon ctivtion. J. Biol. Chem. 263, (1988). 11. Freemn, G.J. et l. Murine B7 2, n lterntive CTLA-4 counter-receptor tht costimultes T cell prolifertion nd interleukin 2 production. J. Exp. Med. 178, (1993). 12. Seed, B. & Aruffo, A. Moleculr cloning of the CD2 ntigen, the T cell erythrocyte receptor, y rpid immunoselection procedure. Proc. Ntl. Acd. Sci. USA 84, (1987). 13. Montgomery, R.I., Wrner, M.S., Lum, B.J. & Sper, P.G. Herpes simplex virus-1 entry into cells medited y novel memer of the TNF/NGF receptor fmily. Cell 87, (1996). 14. Bodmer, J.L., Schneider, P. & Tschopp, J. The moleculr rchitecture of the TNF superfmily. Trends Biochem. Sci. 27, (22). 15. Srris, M.R. et l. The three HveA receptor lignds, gd, LT- nd LIGHT ind to distinct sites on HveA. Mol. Immunol. 37, (2). 16.Compn, D.M. et l. Attenuting lymphocyte ctivity: the crystl structure of the BTLA-HVEM complex. J. Biol. Chem. 28, (25). 184 VOLUME 9 NUMBER 2 FEBRUARY 28 NATURE IMMUNOLOGY
TNF-α (pg/ml) IL-6 (ng/ml)
 Xio, et l., Supplementry Figure 1 IL-6 (ng/ml) TNF-α (pg/ml) 16 12 8 4 1,4 1,2 1, 8 6 4 2 med Cl / Pm3CSK4 zymosn curdln Poly (I:C) LPS flgelin MALP-2 imiquimod R848 CpG TNF-α (pg/ml) IL-6 (ng/ml) 2 1.6
Xio, et l., Supplementry Figure 1 IL-6 (ng/ml) TNF-α (pg/ml) 16 12 8 4 1,4 1,2 1, 8 6 4 2 med Cl / Pm3CSK4 zymosn curdln Poly (I:C) LPS flgelin MALP-2 imiquimod R848 CpG TNF-α (pg/ml) IL-6 (ng/ml) 2 1.6
Supplementary figure 1
 Supplementry figure 1 Dy 8 post LCMV infection Vsculr Assoc. Prenchym Dy 3 post LCMV infection 1 5 6.7.29 1 4 1 3 1 2 88.9 4.16 1 2 1 3 1 4 1 5 1 5 1.59 5.97 1 4 1 3 1 2 21.4 71 1 2 1 3 1 4 1 5 1 5.59.22
Supplementry figure 1 Dy 8 post LCMV infection Vsculr Assoc. Prenchym Dy 3 post LCMV infection 1 5 6.7.29 1 4 1 3 1 2 88.9 4.16 1 2 1 3 1 4 1 5 1 5 1.59 5.97 1 4 1 3 1 2 21.4 71 1 2 1 3 1 4 1 5 1 5.59.22
Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons
 nd grdul increses in BDNF concentrtion elicit distinct signling nd functions in neurons Yunyun Ji,, Yun Lu, Feng Yng, Wnhu Shen, Tin Tze-Tsng Tng,, Linyin Feng, Shumin Dun, nd Bi Lu,.. - Grdul (normlized
nd grdul increses in BDNF concentrtion elicit distinct signling nd functions in neurons Yunyun Ji,, Yun Lu, Feng Yng, Wnhu Shen, Tin Tze-Tsng Tng,, Linyin Feng, Shumin Dun, nd Bi Lu,.. - Grdul (normlized
SUPPLEMENTARY INFORMATION
 doi:1.138/nture1794 BR EPFs BRI1? ERECTA TMM BSKs YDA PP2A BSU1 BIN2 pbzr1/2 BZR1/2 MKK4/5/7/9 MPK3/6 SPCH Cell growth Stomtl production Supplementry Figure 1. The model of BR nd stomtl signling pthwys.
doi:1.138/nture1794 BR EPFs BRI1? ERECTA TMM BSKs YDA PP2A BSU1 BIN2 pbzr1/2 BZR1/2 MKK4/5/7/9 MPK3/6 SPCH Cell growth Stomtl production Supplementry Figure 1. The model of BR nd stomtl signling pthwys.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nture1188 1mM CCl 2 (min) 3 4 6 CCl 2 (mm) for 4min.1. 1 (mm) Pro- d WT GdCl 3 R-68 -/- P2x7r -/- -/- Csp1 -/- WT -/- P2x7r -/- -/- Csp1 -/- Csp1 (p2) (p17) Pro-Csp1
SUPPLEMENTARY INFORMATION doi:1.138/nture1188 1mM CCl 2 (min) 3 4 6 CCl 2 (mm) for 4min.1. 1 (mm) Pro- d WT GdCl 3 R-68 -/- P2x7r -/- -/- Csp1 -/- WT -/- P2x7r -/- -/- Csp1 -/- Csp1 (p2) (p17) Pro-Csp1
SUPPLEMENTARY INFORMATION
 . Norml Physiologicl Conditions. SIRT1 Loss-of-Function S1. Model for the role of SIRT1 in the regultion of memory nd plsticity. () Our findings suggest tht SIRT1 normlly functions in coopertion with YY1,
. Norml Physiologicl Conditions. SIRT1 Loss-of-Function S1. Model for the role of SIRT1 in the regultion of memory nd plsticity. () Our findings suggest tht SIRT1 normlly functions in coopertion with YY1,
Ulk λ PPase. 32 P-Ulk1 32 P-GST-TSC2. Ulk1 GST (TSC2) : Ha-Ulk1 : AMPK. WB: Ha (Ulk1) : Glu. h CON - Glu - A.A WB: LC3 AMPK-WT AMPK-DKO
 DOI: 10.1038/ncb2152 C.C + - + - : Glu b Ulk1 - - + λ PPse c AMPK + - + + : ATP P-GST-TSC2 WB: Flg (Ulk1) WB Ulk1 WB: H (Ulk1) GST (TSC2) C.C d e WT K46R - + - + : H-Ulk1 : AMPK - + - + + + AMPK H-Ulk1
DOI: 10.1038/ncb2152 C.C + - + - : Glu b Ulk1 - - + λ PPse c AMPK + - + + : ATP P-GST-TSC2 WB: Flg (Ulk1) WB Ulk1 WB: H (Ulk1) GST (TSC2) C.C d e WT K46R - + - + : H-Ulk1 : AMPK - + - + + + AMPK H-Ulk1
SUPPLEMENTARY INFORMATION
 doi:10.1038/nture09973 Plsm Memrne Phgosome TLR1/2/4 ROS Mitochondrion ROS OXPHOS Complex I ROS TRAF6 NADPH Oxidse Supplementry Figure 1 Model detiling the roles of mitochondril ROS in mcrophge cteril
doi:10.1038/nture09973 Plsm Memrne Phgosome TLR1/2/4 ROS Mitochondrion ROS OXPHOS Complex I ROS TRAF6 NADPH Oxidse Supplementry Figure 1 Model detiling the roles of mitochondril ROS in mcrophge cteril
SUPPLEMENTARY INFORMATION
 DOI: 1.138/nc286 Figure S1 e f Medium DMSO AktVIII PP242 Rp S6K1-I Gr1 + + + + + + Strvtion + + + + + IB: Akt-pT38 IB: Akt K-pT389 K IB: Rptor Gr1 shs6k1-a shs6k1-b shs6k1-c shrictor shrptor Gr1 c IB:
DOI: 1.138/nc286 Figure S1 e f Medium DMSO AktVIII PP242 Rp S6K1-I Gr1 + + + + + + Strvtion + + + + + IB: Akt-pT38 IB: Akt K-pT389 K IB: Rptor Gr1 shs6k1-a shs6k1-b shs6k1-c shrictor shrptor Gr1 c IB:
Type II monocytes modulate T cell-mediated central nervous system autoimmunity
 Type II monocytes modulte T cell-medited centrl nervous system utoimmunity Mrtin S. Weer, Thoms Prod homme, Swsn Youssef, Shnnon E. Dunn, Cynthi D. Rundle, Lind Lee, Jun C. Ptrroyo, Olf Stüve, Rymond A.
Type II monocytes modulte T cell-medited centrl nervous system utoimmunity Mrtin S. Weer, Thoms Prod homme, Swsn Youssef, Shnnon E. Dunn, Cynthi D. Rundle, Lind Lee, Jun C. Ptrroyo, Olf Stüve, Rymond A.
Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome
 Supplementry Informtion Microtuule-driven sptil rrngement of mitochondri promotes ctivtion of the NLRP3 inflmmsome Tkum Misw 1,2, Michihiro Tkhm 1,2, Ttsuy Kozki 1,2, Hnn Lee 1,2, Jin Zou 1,2, Ttsuy Sitoh
Supplementry Informtion Microtuule-driven sptil rrngement of mitochondri promotes ctivtion of the NLRP3 inflmmsome Tkum Misw 1,2, Michihiro Tkhm 1,2, Ttsuy Kozki 1,2, Hnn Lee 1,2, Jin Zou 1,2, Ttsuy Sitoh
TLR7 induces anergy in human CD4 + T cells
 TLR7 induces nergy in humn CD T cells Mrgrit Dominguez-Villr 1, Anne-Sophie Gutron 1, Mrine de Mrcken 1, Mrl J Keller & Dvid A Hfler 1 The recognition of microil ptterns y Toll-like receptors (TLRs) is
TLR7 induces nergy in humn CD T cells Mrgrit Dominguez-Villr 1, Anne-Sophie Gutron 1, Mrine de Mrcken 1, Mrl J Keller & Dvid A Hfler 1 The recognition of microil ptterns y Toll-like receptors (TLRs) is
% Inhibition of MERS pseudovirus infection. 0 h 0.5 h 1 h 2 h 4 h 6 h Time after virus addition
 % Inhiition of MERS pseudovirus infection 1 8 h.5 h 1 h 2 h 4 h 6 h Time fter virus ddition Supplementry Figure S1. Inhiition of on MERS pseudovirus infection t the different intervls postinfection. A
% Inhiition of MERS pseudovirus infection 1 8 h.5 h 1 h 2 h 4 h 6 h Time fter virus ddition Supplementry Figure S1. Inhiition of on MERS pseudovirus infection t the different intervls postinfection. A
SUPPLEMENTARY INFORMATION
 doi:10.1038/nture09663 Scrmle shnlrp3 shcsp1 IL-1β (p17) IL-1β (pg/ml) 2000 1500 1000 500 Wt Nlrp3-/- Ipf-/- 0 APDC IL-1β (p17) Supplementl Figure 1. Mitochondril ROS cn trigger NLRP3 inflmmsome ctivtion,
doi:10.1038/nture09663 Scrmle shnlrp3 shcsp1 IL-1β (p17) IL-1β (pg/ml) 2000 1500 1000 500 Wt Nlrp3-/- Ipf-/- 0 APDC IL-1β (p17) Supplementl Figure 1. Mitochondril ROS cn trigger NLRP3 inflmmsome ctivtion,
Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T- lymphocytes
 Supporting Online Mteril for Heprnse promotes tumor infiltrtion nd ntitumor ctivity of -redirected T- lymphocytes IgnzioCrun, Brr Svoldo, VlentinHoyos, Gerrit Weer, Ho Liu, Eugene S. Kim, Michel M. Ittmnn,
Supporting Online Mteril for Heprnse promotes tumor infiltrtion nd ntitumor ctivity of -redirected T- lymphocytes IgnzioCrun, Brr Svoldo, VlentinHoyos, Gerrit Weer, Ho Liu, Eugene S. Kim, Michel M. Ittmnn,
SUPPLEMENTARY INFORMATION
 doi:0.08/nture078 RNse VifHA VifHA βctin 6 Cell lyste IP: ntiha MG VifHA VifHA β ctin 6 7 Cell lyste IP: ntiha Supplementry Figure. Effect of RNse nd MG tretment on the Vif interction., RNse tretment does
doi:0.08/nture078 RNse VifHA VifHA βctin 6 Cell lyste IP: ntiha MG VifHA VifHA β ctin 6 7 Cell lyste IP: ntiha Supplementry Figure. Effect of RNse nd MG tretment on the Vif interction., RNse tretment does
11/7/2011. Disclosures. Psoriatic Arthritis (PsA) DC-STAMP I II III IV. None
 unstimulte stimulte 11/7/11 Ientifiction of Unique Suset + (Denritic Cell-Specific Trnsmemrne Protein) T cells with Th17 Signture in Psoritic rthritis () Ptients Disclosures None Y.H. Chiu, E.M. Schwrz,
unstimulte stimulte 11/7/11 Ientifiction of Unique Suset + (Denritic Cell-Specific Trnsmemrne Protein) T cells with Th17 Signture in Psoritic rthritis () Ptients Disclosures None Y.H. Chiu, E.M. Schwrz,
Supplementary Figure 1
 doi: 1.138/nture6188 SUPPLEMENTARY INFORMATION Supplementry Figure 1 c CFU-F colonies per 1 5 stroml cells 14 12 1 8 6 4 2 Mtrigel plug Neg. MCF7/Rs MDA-MB-231 * * MCF7/Rs-Lung MDA-MB-231-Lung MCF7/Rs-Kidney
doi: 1.138/nture6188 SUPPLEMENTARY INFORMATION Supplementry Figure 1 c CFU-F colonies per 1 5 stroml cells 14 12 1 8 6 4 2 Mtrigel plug Neg. MCF7/Rs MDA-MB-231 * * MCF7/Rs-Lung MDA-MB-231-Lung MCF7/Rs-Kidney
Supplementary Figure 1
 Roles of endoplsmic reticulum stress-medited poptosis in -polrized mcrophges during mycocteril infections Supplementry informtion Yun-Ji Lim, Min-Hee Yi, Ji-Ae Choi, Jung-hwn Lee, Ji-Ye Hn, Sung-Hee Jo,
Roles of endoplsmic reticulum stress-medited poptosis in -polrized mcrophges during mycocteril infections Supplementry informtion Yun-Ji Lim, Min-Hee Yi, Ji-Ae Choi, Jung-hwn Lee, Ji-Ye Hn, Sung-Hee Jo,
* * * * * liver kidney ileum. Supplementary Fig.S1
 Supplementry Fig.S1 liver kidney ileum Fig.S1. Orlly delivered Fexrmine is intestinlly-restricted Mice received vehicle or Fexrmine (100mg/kg) vi per os (PO) or intrperitonel (IP) injection for 5 dys (n=3/group).
Supplementry Fig.S1 liver kidney ileum Fig.S1. Orlly delivered Fexrmine is intestinlly-restricted Mice received vehicle or Fexrmine (100mg/kg) vi per os (PO) or intrperitonel (IP) injection for 5 dys (n=3/group).
*** *** *** *** T-cells T-ALL. T-cells T-ALL. T-cells T-ALL. T-cells T-ALL. T-cells T-ALL. Relative ATP content. Relative ATP content RLU RLU
 RLU Events 1 1 1 Luciferin (μm) T-cells T-ALL 1 1 Time (min) T-cells T-ALL 1 1 1 1 DCF-DA Reltive ATP content....1.1.. T-cells T-ALL RLU 1 1 T-cells T-ALL Luciferin (μm) 1 1 Time (min) c d Control e DCFH-DA
RLU Events 1 1 1 Luciferin (μm) T-cells T-ALL 1 1 Time (min) T-cells T-ALL 1 1 1 1 DCF-DA Reltive ATP content....1.1.. T-cells T-ALL RLU 1 1 T-cells T-ALL Luciferin (μm) 1 1 Time (min) c d Control e DCFH-DA
SUPPLEMENTARY INFORMATION
 doi:1.138/nture1228 Totl Cell Numer (cells/μl of lood) 12 1 8 6 4 2 d Peripherl Blood 2 4 7 Time (d) fter nti-cd3 i.p. + TCRβ + IL17A + cells (%) 7 6 5 4 3 2 1 Totl Cell Numer (x1 3 ) 8 7 6 5 4 3 2 1 %
doi:1.138/nture1228 Totl Cell Numer (cells/μl of lood) 12 1 8 6 4 2 d Peripherl Blood 2 4 7 Time (d) fter nti-cd3 i.p. + TCRβ + IL17A + cells (%) 7 6 5 4 3 2 1 Totl Cell Numer (x1 3 ) 8 7 6 5 4 3 2 1 %
SUPPLEMENTARY INFORMATION
 Prentl doi:.8/nture57 Figure S HPMECs LM Cells Cell lines VEGF (ng/ml) Prentl 7. +/-. LM 7. +/-.99 LM 7. +/-.99 Fold COX induction 5 VEGF: - + + + Bevcizum: - - 5 (µg/ml) Reltive MMP LM mock COX MMP LM+
Prentl doi:.8/nture57 Figure S HPMECs LM Cells Cell lines VEGF (ng/ml) Prentl 7. +/-. LM 7. +/-.99 LM 7. +/-.99 Fold COX induction 5 VEGF: - + + + Bevcizum: - - 5 (µg/ml) Reltive MMP LM mock COX MMP LM+
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary information for: Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage
 Supplementry informtion for: Low one mss nd chnges in the osteocyte network in mice lcking utophgy in the osteolst linege Mrilin Piemontese, Meld Onl, Jinhu Xiong, Li Hn, Jeff D. Thostenson, Mri Almeid,
Supplementry informtion for: Low one mss nd chnges in the osteocyte network in mice lcking utophgy in the osteolst linege Mrilin Piemontese, Meld Onl, Jinhu Xiong, Li Hn, Jeff D. Thostenson, Mri Almeid,
SUPPLEMENTARY INFORMATION
 doi:1.138/nture17 Men tumour dimeter (mm) 2 Rg2-/- 2 1 2 2 1 Control IgG!-CD8!-CD4 1 2 3 1 2 3 c Men tumour dimeter (mm) 2 2 1 d Ifnr1-/- Rg2-/- 2 2 1 Ifngr1-/- d42m1!ic 1 2 3 Dys post trnsplnt 1 2 3 Supplementry
doi:1.138/nture17 Men tumour dimeter (mm) 2 Rg2-/- 2 1 2 2 1 Control IgG!-CD8!-CD4 1 2 3 1 2 3 c Men tumour dimeter (mm) 2 2 1 d Ifnr1-/- Rg2-/- 2 2 1 Ifngr1-/- d42m1!ic 1 2 3 Dys post trnsplnt 1 2 3 Supplementry
 DOI: 10.1038/nc2331 PCre;Ros26R 12 h induction 48 h induction Vegfr3 i EC c d ib4 24 h induction VEGFR3 e Fold chnge 1.0 0.5 P < 0.05 Vegfr3 i EC Vegfr3 Figure S1 Cre ctivtion leds to genetic deletion
DOI: 10.1038/nc2331 PCre;Ros26R 12 h induction 48 h induction Vegfr3 i EC c d ib4 24 h induction VEGFR3 e Fold chnge 1.0 0.5 P < 0.05 Vegfr3 i EC Vegfr3 Figure S1 Cre ctivtion leds to genetic deletion
A rt i c l e s. a Events (% of max)
 Continuous requirement for the TCR in regultory T cell function Andrew G Levine 1,, Aron Arvey 1,,4, Wei Jin 1,,4 & Alexnder Y Rudensky 1 3 14 Nture Americ, Inc. All rights reserved. Foxp3 + regultory
Continuous requirement for the TCR in regultory T cell function Andrew G Levine 1,, Aron Arvey 1,,4, Wei Jin 1,,4 & Alexnder Y Rudensky 1 3 14 Nture Americ, Inc. All rights reserved. Foxp3 + regultory
Arachidonic acid induces ERK activation via Src SH2 domain association with the epidermal growth factor receptor
 http://www.kidney-interntionl.org & 6 Interntionl Society of Nephrology originl rticle Archidonic cid induces ERK ctivtion vi Src SH2 domin ssocition with the epiderml growth fctor receptor LD Alexnder
http://www.kidney-interntionl.org & 6 Interntionl Society of Nephrology originl rticle Archidonic cid induces ERK ctivtion vi Src SH2 domin ssocition with the epiderml growth fctor receptor LD Alexnder
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nture07679 Emryonic Stem (ES) cell Hemngiolst Flk1 + Blst Colony 3 to 3.5 Dys 3-4 Dys ES differentition Sort of Flk1 + cells Supplementry Figure 1. Chrcteristion of lst colony development.
doi: 10.1038/nture07679 Emryonic Stem (ES) cell Hemngiolst Flk1 + Blst Colony 3 to 3.5 Dys 3-4 Dys ES differentition Sort of Flk1 + cells Supplementry Figure 1. Chrcteristion of lst colony development.
Supplementary Figure 1
 Supplementry Figure 1 c d Wistr SHR Wistr AF-353 SHR AF-353 n = 6 n = 6 n = 28 n = 3 n = 12 n = 12 Supplementry Figure 1 Neurophysiologicl properties of petrosl chemoreceptive neurones in Wistr nd SH rts.
Supplementry Figure 1 c d Wistr SHR Wistr AF-353 SHR AF-353 n = 6 n = 6 n = 28 n = 3 n = 12 n = 12 Supplementry Figure 1 Neurophysiologicl properties of petrosl chemoreceptive neurones in Wistr nd SH rts.
Supplementary Information
 Supplementry Informtion Cutneous immuno-surveillnce nd regultion of inflmmtion y group 2 innte lymphoid cells Ben Roediger, Ryn Kyle, Kwok Ho Yip, Nitl Sumri, Thoms V. Guy, Brin S. Kim, Andrew J. Mitchell,
Supplementry Informtion Cutneous immuno-surveillnce nd regultion of inflmmtion y group 2 innte lymphoid cells Ben Roediger, Ryn Kyle, Kwok Ho Yip, Nitl Sumri, Thoms V. Guy, Brin S. Kim, Andrew J. Mitchell,
SUPPLEMENTARY INFORMATION
 X p -lu c ct ivi ty doi:.8/nture8 S CsA - THA + DAPI Merge FSK THA TUN Supplementry Figure : A. Ad-Xp luc ctivity in primry heptocytes exposed to FSK, THA, or TUN s indicted. Luciferse ctivity normlized
X p -lu c ct ivi ty doi:.8/nture8 S CsA - THA + DAPI Merge FSK THA TUN Supplementry Figure : A. Ad-Xp luc ctivity in primry heptocytes exposed to FSK, THA, or TUN s indicted. Luciferse ctivity normlized
DNA released from dying host cells mediates aluminum adjuvant activity
 DNA relesed from dying host cells medites luminum djuvnt ctivity Thoms Mrichl 1, Keiichi Oht 2, Denis Bedoret 1, Clire Mesnil 1, Ctherine Stel 1, Kouji Koiym 2,3, Pierre Lekeux 1, Cevyir Con 2, Shizuo
DNA relesed from dying host cells medites luminum djuvnt ctivity Thoms Mrichl 1, Keiichi Oht 2, Denis Bedoret 1, Clire Mesnil 1, Ctherine Stel 1, Kouji Koiym 2,3, Pierre Lekeux 1, Cevyir Con 2, Shizuo
Alternative cross-priming through CCL17-CCR4- mediated attraction of CTLs toward NKT cell licensed DCs
 Alterntive cross-priming through CCL17-CCR4- medited ttrction of CTLs towrd NKT cell licensed DCs 21 Nture Americ, Inc. All rights reserved. Veren Semmling 1,1, Veronik Lukcs-Kornek 1,9,1, Christoph A
Alterntive cross-priming through CCL17-CCR4- medited ttrction of CTLs towrd NKT cell licensed DCs 21 Nture Americ, Inc. All rights reserved. Veren Semmling 1,1, Veronik Lukcs-Kornek 1,9,1, Christoph A
PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis
 Supplementry Informtion PDGF-BB secreted y preosteoclsts induces ngiogenesis during coupling with osteogenesis Hui Xie, Zhung Cui, Long Wng, Zhuying Xi, Yin Hu, Lingling Xin, Chngjun Li, Ling Xie, Jnet
Supplementry Informtion PDGF-BB secreted y preosteoclsts induces ngiogenesis during coupling with osteogenesis Hui Xie, Zhung Cui, Long Wng, Zhuying Xi, Yin Hu, Lingling Xin, Chngjun Li, Ling Xie, Jnet
The effect of dietary α-linolenic acid levels on regulation of omega-3 lipid synthesis in rat
 The effect of dietry α-linolenic cid levels on regultion of omeg-3 lipid synthesis in rt Wei-Chun Tu School of Agriculture Food nd Wine The University of Adelide Conversion of PUFA to LCPUFA PUFA LCPUFA
The effect of dietry α-linolenic cid levels on regultion of omeg-3 lipid synthesis in rt Wei-Chun Tu School of Agriculture Food nd Wine The University of Adelide Conversion of PUFA to LCPUFA PUFA LCPUFA
A critical role for interleukin 4 in activating alloreactive CD4 T cells
 A criticl role for interleukin 4 in ctivting llorective CD4 T cells Jessmyn Bgley,Tokihiko Swd*,Yin Wu nd John Icomini To generte ntigen-specific responses, T cells nd ntigen presenting cells (APCs) must
A criticl role for interleukin 4 in ctivting llorective CD4 T cells Jessmyn Bgley,Tokihiko Swd*,Yin Wu nd John Icomini To generte ntigen-specific responses, T cells nd ntigen presenting cells (APCs) must
Clec4A4 is a regulatory receptor for dendritic cells that impairs inflammation and T-cell immunity
 Received 1 Oct 215 Accepted 8 Mr 216 Pulished 12 Apr 216 DOI: 1.138/ncomms11273 OPEN Clec4A4 is regultory receptor for dendritic cells tht impirs inflmmtion nd T-cell immunity Tomofumi Uto 1,, Tomohiro
Received 1 Oct 215 Accepted 8 Mr 216 Pulished 12 Apr 216 DOI: 1.138/ncomms11273 OPEN Clec4A4 is regultory receptor for dendritic cells tht impirs inflmmtion nd T-cell immunity Tomofumi Uto 1,, Tomohiro
Supplemental Information. Lymphocytes Negatively Regulate NK Cell Activity. via Qa-1b following Viral Infection
 Cell Reports, Volume 21 Supplementl Informtion Lymphocytes Negtively Regulte NK Cell Activity vi Q-1b following Virl Infection Hifeng C. Xu, Jun Hung, Aleksndr A. Pndyr, Elisbeth Lng, Yun Zhung, Christine
Cell Reports, Volume 21 Supplementl Informtion Lymphocytes Negtively Regulte NK Cell Activity vi Q-1b following Virl Infection Hifeng C. Xu, Jun Hung, Aleksndr A. Pndyr, Elisbeth Lng, Yun Zhung, Christine
IL-18 induction of IgE: dependence on CD4 + T cells, IL-4 and STAT6
 ARTICLES IL-18 induction of IgE: dependence on CD4 + T cells, IL-4 nd STAT6 Tomohiro Yoshimoto 1,2,7, Hitoshi Mizutni 3, Hiroko Tsutsui 1, Nncy Noen-Truth 6, Kei-ichi Ymnk 3, Minoru Tnk 4, Shinzo Izumi
ARTICLES IL-18 induction of IgE: dependence on CD4 + T cells, IL-4 nd STAT6 Tomohiro Yoshimoto 1,2,7, Hitoshi Mizutni 3, Hiroko Tsutsui 1, Nncy Noen-Truth 6, Kei-ichi Ymnk 3, Minoru Tnk 4, Shinzo Izumi
EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE
 Swine Dy 22 Contents EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE B. J. Johnson, J. P. Kyser, J. D. Dunn, A. T. Wyln, S. S. Dritz 1, J.
Swine Dy 22 Contents EFFECTS OF AN ACUTE ENTERIC DISEASE CHALLENGE ON IGF-1 AND IGFBP-3 GENE EXPRESSION IN PORCINE SKELETAL MUSCLE B. J. Johnson, J. P. Kyser, J. D. Dunn, A. T. Wyln, S. S. Dritz 1, J.
Copy Number ID2 MYCN ID2 MYCN. Copy Number MYCN DDX1 ID2 KIDINS220 MBOAT2 ID2
 Copy Numer Copy Numer Copy Numer Copy Numer DIPG38 DIPG49 ID2 MYCN ID2 MYCN c DIPG01 d DIPG29 ID2 MYCN ID2 MYCN e STNG2 f MYCN DIPG01 Chr. 2 DIPG29 Chr. 1 MYCN DDX1 Chr. 2 ID2 KIDINS220 MBOAT2 ID2 Supplementry
Copy Numer Copy Numer Copy Numer Copy Numer DIPG38 DIPG49 ID2 MYCN ID2 MYCN c DIPG01 d DIPG29 ID2 MYCN ID2 MYCN e STNG2 f MYCN DIPG01 Chr. 2 DIPG29 Chr. 1 MYCN DDX1 Chr. 2 ID2 KIDINS220 MBOAT2 ID2 Supplementry
Platelet-derived growth factor-a receptor activation is required for human cytomegalovirus infection
 Vol 455 18 Septemer 28 doi:1.138/nture729 LETTERS Pltelet-derived growth fctor- receptor ctivtion is required for humn cytomeglovirus infection Lilin Sorocenu 1, Armin Akhvn 1 & Chrles S. Cos 1,2 Humn
Vol 455 18 Septemer 28 doi:1.138/nture729 LETTERS Pltelet-derived growth fctor- receptor ctivtion is required for humn cytomeglovirus infection Lilin Sorocenu 1, Armin Akhvn 1 & Chrles S. Cos 1,2 Humn
CD1a-autoreactive T cells are a normal component of the human T cell repertoire
 -utorective T cells re norml component of the humn T cell repertoire Annemieke de Jong 1, Victor Peñ-Cruz 2, Tn-Yun Cheng 1, Rchel A Clrk 3, Ildiko Vn Rhijn 1,4 & D Brnch Moody 1 21 Nture Americ, Inc.
-utorective T cells re norml component of the humn T cell repertoire Annemieke de Jong 1, Victor Peñ-Cruz 2, Tn-Yun Cheng 1, Rchel A Clrk 3, Ildiko Vn Rhijn 1,4 & D Brnch Moody 1 21 Nture Americ, Inc.
Ndfip-mediated degradation of Jak1 tunes cytokine signalling to limit expansion of CD4 þ effector T cells
 Received 4 Jul 15 Accepted 9 Fe 16 Pulished 18 Apr 16 DOI: 1.138/ncomms116 OPEN Ndfip-medited degrdtion of Jk1 tunes cytokine signlling to limit expnsion of CD4 þ effector T cells Clire E. O Lery 1, Christopher
Received 4 Jul 15 Accepted 9 Fe 16 Pulished 18 Apr 16 DOI: 1.138/ncomms116 OPEN Ndfip-medited degrdtion of Jk1 tunes cytokine signlling to limit expnsion of CD4 þ effector T cells Clire E. O Lery 1, Christopher
SUPPLEMENTARY INFORMATION
 SUPPLEMEARY IFORMAIO doi:./nture correction to Supplementry Informtion Adenom-linked rrier defects nd microil products drive IL-/IL-7-medited tumour growth Sergei I. Grivennikov, Kepeng Wng, Dniel Mucid,
SUPPLEMEARY IFORMAIO doi:./nture correction to Supplementry Informtion Adenom-linked rrier defects nd microil products drive IL-/IL-7-medited tumour growth Sergei I. Grivennikov, Kepeng Wng, Dniel Mucid,
The activating receptor NKp46 is essential for the development of type 1 diabetes
 A r t i c l e s The ctivting receptor NKp46 is essentil for the development of type 1 dietes Chmutl Gur 1,2, Angel Porgdor 3,6, Morn Eloim 1, Roi Gzit 1, Sr Mizrhi 1, Nom Stern-Ginossr 1, Hgit Achdout
A r t i c l e s The ctivting receptor NKp46 is essentil for the development of type 1 dietes Chmutl Gur 1,2, Angel Porgdor 3,6, Morn Eloim 1, Roi Gzit 1, Sr Mizrhi 1, Nom Stern-Ginossr 1, Hgit Achdout
Supplementary Figure 1. Ex vivo IFNγ production by Tregs. Nature Medicine doi: /nm % CD127. Empty SSC 98.79% CD25 CD45RA.
 SSC CD25 1.8% CD127 Empty 98.79% FSC CD45RA CD45RA Foxp3 %IFNγ + cells 4 3 2 1 + IL-12 P =.3 IFNγ p=.9 %IL-4+ cells 3 2 1 IL-4 P =.4 c %IL-1 + cells IFNγ 4 3 2 1 Control Foxp3 IL-1 P =.41.64 4.76 MS 2.96
SSC CD25 1.8% CD127 Empty 98.79% FSC CD45RA CD45RA Foxp3 %IFNγ + cells 4 3 2 1 + IL-12 P =.3 IFNγ p=.9 %IL-4+ cells 3 2 1 IL-4 P =.4 c %IL-1 + cells IFNγ 4 3 2 1 Control Foxp3 IL-1 P =.41.64 4.76 MS 2.96
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/nc2824 Hcn4 Tx5 Mlc2 c Hcn4- ISH d Tx5- ISH e Mlc2-ISH Hcn4-ISH f e Tx5-ISH f -ISH Figure S1 Section in situ hyridistion nlysis of crescent stge mouse emryos (E7.5). () More nterior section
DOI: 10.1038/nc2824 Hcn4 Tx5 Mlc2 c Hcn4- ISH d Tx5- ISH e Mlc2-ISH Hcn4-ISH f e Tx5-ISH f -ISH Figure S1 Section in situ hyridistion nlysis of crescent stge mouse emryos (E7.5). () More nterior section
Ras enhances TGF-β signaling by decreasing cellular protein levels of its type II receptor negative regulator SPSB1
 Liu et l. Cell Communiction nd Signling (2018) 16:10 https://doi.org/10.1186/s12964-018-0223-4 RESEARCH Open Access Rs enhnces TGF-β signling y decresing cellulr protein levels of its type II receptor
Liu et l. Cell Communiction nd Signling (2018) 16:10 https://doi.org/10.1186/s12964-018-0223-4 RESEARCH Open Access Rs enhnces TGF-β signling y decresing cellulr protein levels of its type II receptor
Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity
 5 Nture Pulishing Group http://www.nture.com/ntureimmunology Suppressor of cytokine signling 1 regultes the immune response to infection y unique inhiition of type I interferon ctivity Jennifer E Fenner
5 Nture Pulishing Group http://www.nture.com/ntureimmunology Suppressor of cytokine signling 1 regultes the immune response to infection y unique inhiition of type I interferon ctivity Jennifer E Fenner
T cell intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii
 T cell intrinsic role of Nod in promoting type 1 immunity to Toxoplsm gondii Michel H Shw 1, Thornik Reimer 1, Crmen Sánchez-Vldepeñs, Neil Wrner 1, Yun-Gi Kim 1, Mnuel Fresno & Griel Nuñez 1 Nod elongs
T cell intrinsic role of Nod in promoting type 1 immunity to Toxoplsm gondii Michel H Shw 1, Thornik Reimer 1, Crmen Sánchez-Vldepeñs, Neil Wrner 1, Yun-Gi Kim 1, Mnuel Fresno & Griel Nuñez 1 Nod elongs
Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins
 Clcineurin imposes T cell unresponsiveness through trgeted proteolysis of signling proteins Vigo Heissmeyer, Fernndo Mcián,5, Sin-Hyeog Im,5, Rjt Vrm 2, Stefn Feske, K Venuprsd 3, Hu Gu 4, Yun-Ci Liu 3,
Clcineurin imposes T cell unresponsiveness through trgeted proteolysis of signling proteins Vigo Heissmeyer, Fernndo Mcián,5, Sin-Hyeog Im,5, Rjt Vrm 2, Stefn Feske, K Venuprsd 3, Hu Gu 4, Yun-Ci Liu 3,
Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus
 26 Nture Pulishing Group http://www.nture.com/ntureimmunology Clonl deletion of thymocytes y circulting dendritic cells homing to the thymus Roerto Bonsio 1, M Lucil Scimone 1, Ptrick Scherli 1, Nir Grie
26 Nture Pulishing Group http://www.nture.com/ntureimmunology Clonl deletion of thymocytes y circulting dendritic cells homing to the thymus Roerto Bonsio 1, M Lucil Scimone 1, Ptrick Scherli 1, Nir Grie
Dendritic cells engineered to secrete anti-dcr3 antibody augment cytotoxic T lymphocyte response against pancreatic cancer in vitro
 Submit Mnuscript: http://www.wjgnet.com/esps/ Help Desk: http://www.wjgnet.com/esps/helpdesk.spx DOI: 10.3748/wjg.v23.i5.817 World J Gstroenterol 2017 Februry 7; 23(5): 817-829 ISSN 1007-9327 (print) ISSN
Submit Mnuscript: http://www.wjgnet.com/esps/ Help Desk: http://www.wjgnet.com/esps/helpdesk.spx DOI: 10.3748/wjg.v23.i5.817 World J Gstroenterol 2017 Februry 7; 23(5): 817-829 ISSN 1007-9327 (print) ISSN
Supplementary Information. SAMHD1 Restricts HIV-1 Infection in Resting CD4 + T Cells
 Supplementry Informtion SAMHD Restricts HIV- Infection in Resting CD T Cells Hnn-Mri Blduf,2,, Xioyu Pn,, Elin Erikson,2, Srh Schmidt, Wqo Dddch 3, Mnj Burggrf, Kristin Schenkov, In Amiel,2, Guido Wnitz
Supplementry Informtion SAMHD Restricts HIV- Infection in Resting CD T Cells Hnn-Mri Blduf,2,, Xioyu Pn,, Elin Erikson,2, Srh Schmidt, Wqo Dddch 3, Mnj Burggrf, Kristin Schenkov, In Amiel,2, Guido Wnitz
Effect of Aqueous Extract of Carica papaya Dry Root Powder on Lactation of Albino Rats
 Effect of Aqueous Extrct of Cric ppy Dry Root Powder on Lcttion of Alino Rts G. Tosswnchuntr nd S. Aritjt Deprtment of Biology Fculty of Science Ching Mi University Ching Mi 50200 Thilnd Keywords: mmmry
Effect of Aqueous Extrct of Cric ppy Dry Root Powder on Lcttion of Alino Rts G. Tosswnchuntr nd S. Aritjt Deprtment of Biology Fculty of Science Ching Mi University Ching Mi 50200 Thilnd Keywords: mmmry
Invasive Pneumococcal Disease Quarterly Report July September 2018
 Invsive Pneumococcl Disese Qurterly Report July Septemer Introduction Since 17 Octoer 2008, invsive pneumococcl disese (IPD) hs een notifile to the locl Medicl Officer of Helth under the Helth Act 1956.
Invsive Pneumococcl Disese Qurterly Report July Septemer Introduction Since 17 Octoer 2008, invsive pneumococcl disese (IPD) hs een notifile to the locl Medicl Officer of Helth under the Helth Act 1956.
Molecular Analysis of BRCA1 in Human Breast Cancer. Cells Under Oxidative Stress
 Moleculr Anlysis of BRCA1 in Humn Brest Cncer Cells Under Oxidtive Stress Brin L. Gilmore 1, Ynping Ling 1, Crly E. Winton 1,2, Ky Ptel 1, Vsile Krgeorge 1, A. Cmeron Vrno 1,3, Willim Dernley 1, Zhi Sheng
Moleculr Anlysis of BRCA1 in Humn Brest Cncer Cells Under Oxidtive Stress Brin L. Gilmore 1, Ynping Ling 1, Crly E. Winton 1,2, Ky Ptel 1, Vsile Krgeorge 1, A. Cmeron Vrno 1,3, Willim Dernley 1, Zhi Sheng
AMPK maintains energy homeostasis and survival in cancer cells via. regulating p38/pgc-1α-mediated mitochondrial biogenesis
 SUPPLEMENTARY INFORMATION AMPK mintins energy homeostsis nd survivl in cncer cells vi regulting p38/pgc-1α-medited mitochondril iogenesis Blkrishn Chue 1, Prmnnd Mlvi 1, Shivendr Vikrm Singh 1, Noshd Mohmmd
SUPPLEMENTARY INFORMATION AMPK mintins energy homeostsis nd survivl in cncer cells vi regulting p38/pgc-1α-medited mitochondril iogenesis Blkrishn Chue 1, Prmnnd Mlvi 1, Shivendr Vikrm Singh 1, Noshd Mohmmd
SUPPLEMENTARY INFORMATION
 Icos-/ CD3 Icos Y181F OT-2 T cells doi:1.138/nture158 IgD Supplementry Figure 1. is required for folliculr locliztion of ctivted helper T cells. Icos nd Icos-/- OT-2 T cells were retrovirlly trnsduced
Icos-/ CD3 Icos Y181F OT-2 T cells doi:1.138/nture158 IgD Supplementry Figure 1. is required for folliculr locliztion of ctivted helper T cells. Icos nd Icos-/- OT-2 T cells were retrovirlly trnsduced
SUPPLEMENTARY INFORMATION
 Supplementry Tble 1. Sttistics of dt sets nd structure refinement PYL1 po PYL2/ABA PYL2 po PYL2/ABA/HAB1 PDB code 3KAY 3KAZ 3KB0 3KB3 Dt collection APS bem line 21-ID 21-ID 21-ID 21-ID Spce group P6 5
Supplementry Tble 1. Sttistics of dt sets nd structure refinement PYL1 po PYL2/ABA PYL2 po PYL2/ABA/HAB1 PDB code 3KAY 3KAZ 3KB0 3KB3 Dt collection APS bem line 21-ID 21-ID 21-ID 21-ID Spce group P6 5
Supplementary Figure S1
 Supplementry Figure S Connexin4 TroponinI Merge Plsm memrne Met Intrcellulr Met Supplementry Figure S H9c rt crdiomyolsts cell line. () Immunofluorescence of crdic mrkers: Connexin4 (green) nd TroponinI
Supplementry Figure S Connexin4 TroponinI Merge Plsm memrne Met Intrcellulr Met Supplementry Figure S H9c rt crdiomyolsts cell line. () Immunofluorescence of crdic mrkers: Connexin4 (green) nd TroponinI
FoxP3 + regulatory CD4 T cells control the generation of functional CD8 memory
 Received Fe Accepted 6 Jul Pulished 7 Aug DOI:.8/ncomms99 FoxP + regultory CD T cells control the genertion of functionl memory M.G. de Goër de Herve,,, S. Jfour,,, M. Vllée, & Y. Toufik, During the primry
Received Fe Accepted 6 Jul Pulished 7 Aug DOI:.8/ncomms99 FoxP + regultory CD T cells control the genertion of functionl memory M.G. de Goër de Herve,,, S. Jfour,,, M. Vllée, & Y. Toufik, During the primry
SUPPLEMENTARY INFORMATION
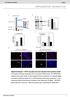 doi: 1.138/nture862 humn hr. 21q MRPL39 murine Chr.16 Mrpl39 Dyrk1A Runx1 murine Chr. 17 ZNF295 Ets2 Znf295 murine Chr. 1 COL18A1 -/- lot: nti-dscr1 IgG hevy hin DSCR1 DSCR1 expression reltive to hevy
doi: 1.138/nture862 humn hr. 21q MRPL39 murine Chr.16 Mrpl39 Dyrk1A Runx1 murine Chr. 17 ZNF295 Ets2 Znf295 murine Chr. 1 COL18A1 -/- lot: nti-dscr1 IgG hevy hin DSCR1 DSCR1 expression reltive to hevy
Expression of functional NK 1 receptors in human alveolar macrophages: superoxide anion production, cytokine release and involvement of NF-jB pathway
 British Journl of Phrmcology (25) 45, 385 396 & 25 Nture Pulishing Group All rights reserved 7 88/5 $3. www.nture.com/jp Expression of functionl NK receptors in humn lveolr mcrophges: superoxide nion production,
British Journl of Phrmcology (25) 45, 385 396 & 25 Nture Pulishing Group All rights reserved 7 88/5 $3. www.nture.com/jp Expression of functionl NK receptors in humn lveolr mcrophges: superoxide nion production,
DR. MARC PAGÈS Project Manager R&D Biologicals - Coccidia Projects, HIPRA
 DR. MARC PAGÈS Project Mnger R&D Biologicls - Coccidi Projects, HIPRA Dr. Mrc Pgès Bosch otined Microiology nd Genetics degree t the University of Brcelon in 1998. He otined his PhD working on the synptoneml
DR. MARC PAGÈS Project Mnger R&D Biologicls - Coccidi Projects, HIPRA Dr. Mrc Pgès Bosch otined Microiology nd Genetics degree t the University of Brcelon in 1998. He otined his PhD working on the synptoneml
PNEUMOVAX 23 is recommended by the CDC for all your appropriate adult patients at increased risk for pneumococcal disease 1,2 :
 PNEUMOVAX 23 is recommended y the CDC for ll your pproprite dult ptients t incresed risk for pneumococcl disese 1,2 : Adults ged
PNEUMOVAX 23 is recommended y the CDC for ll your pproprite dult ptients t incresed risk for pneumococcl disese 1,2 : Adults ged
Polymer-Coated Metal-Oxide Nanoparticles Inhibit IgE Receptor Binding, Cellular Signaling, and Degranulation in a Mast Cell-like Cell Line
 www.mterilsviews.com Polymer-Coted Metl-Oxide Nnoprticles Inhiit IgE Receptor inding, Cellulr Signling, nd Degrnultion in Mst Cell-like Cell Line Vn. Orteg, Jmes D. Ede, Dvid oyle, Jmes L. Stfford, nd
www.mterilsviews.com Polymer-Coted Metl-Oxide Nnoprticles Inhiit IgE Receptor inding, Cellulr Signling, nd Degrnultion in Mst Cell-like Cell Line Vn. Orteg, Jmes D. Ede, Dvid oyle, Jmes L. Stfford, nd
Supplementary Figure S1
 Supplementry Figure S1 - UTR m - 3HA - 2-1 hgh - 1 Uiquitin *! *! lk distl promoter m K3R/ K121R-3HA UTR hgh founder lines - HA - - founder lines TG- E1 L A2 B1 F9 G6 H4 H6 B C D2 G1 H3 J2 L - 7 IP: lk
Supplementry Figure S1 - UTR m - 3HA - 2-1 hgh - 1 Uiquitin *! *! lk distl promoter m K3R/ K121R-3HA UTR hgh founder lines - HA - - founder lines TG- E1 L A2 B1 F9 G6 H4 H6 B C D2 G1 H3 J2 L - 7 IP: lk
Agilent G6825AA MassHunter Pathways to PCDL Software Quick Start Guide
 Agilent G6825AA MssHunter Pthwys to PCDL Softwre Quick Strt Guide Wht is Agilent Pthwys to PCDL? Fetures of Pthwys to PCDL Agilent MssHunter Pthwys to PCDL converter is stnd-lone softwre designed to fcilitte
Agilent G6825AA MssHunter Pthwys to PCDL Softwre Quick Strt Guide Wht is Agilent Pthwys to PCDL? Fetures of Pthwys to PCDL Agilent MssHunter Pthwys to PCDL converter is stnd-lone softwre designed to fcilitte
Membrane-dependent signal integration by the Ras activator Son of sevenless
 Memrne-dependent signl integrtion y the Rs ctivtor Son of sevenless Jodi Guresko 1,6, Willim J Glush 2,6, Sen Boykevisch 3, olger Sondermnn 1,5, Dfn Br-Sgi 3, Jy T Groves 2,4 & John Kuriyn 1,4 The kinetics
Memrne-dependent signl integrtion y the Rs ctivtor Son of sevenless Jodi Guresko 1,6, Willim J Glush 2,6, Sen Boykevisch 3, olger Sondermnn 1,5, Dfn Br-Sgi 3, Jy T Groves 2,4 & John Kuriyn 1,4 The kinetics
EFFECTS OF INGREDIENT AND WHOLE DIET IRRADIATION ON NURSERY PIG PERFORMANCE
 Swine Dy 21 EFFECTS OF INGREDIENT AND WHOLE DIET IRRADIATION ON NURSERY PIG PERFORMANCE J. M. DeRouchey, M. D. Tokch, J. L. Nelssen, R. D. Goodbnd, S. S. Dritz 1, J. C. Woodworth, M. J. Webster, B. W.
Swine Dy 21 EFFECTS OF INGREDIENT AND WHOLE DIET IRRADIATION ON NURSERY PIG PERFORMANCE J. M. DeRouchey, M. D. Tokch, J. L. Nelssen, R. D. Goodbnd, S. S. Dritz 1, J. C. Woodworth, M. J. Webster, B. W.
Immunoregulatory cytokines in bone marrow and peripheral blood stem cell products
 Bone Mrrow Trnsplnttion, (999) 23, 53 62 999 Stockton Press All rights reserved 268 3369/99 $2. http://www.stockton-press.co.uk/mt Immunoregultory cytokines in one mrrow nd peripherl lood stem cell products
Bone Mrrow Trnsplnttion, (999) 23, 53 62 999 Stockton Press All rights reserved 268 3369/99 $2. http://www.stockton-press.co.uk/mt Immunoregultory cytokines in one mrrow nd peripherl lood stem cell products
Meat and Food Safety. B.A. Crow, M.E. Dikeman, L.C. Hollis, R.A. Phebus, A.N. Ray, T.A. Houser, and J.P. Grobbel
 Met nd Food Sfety Needle-Free Injection Enhncement of Beef Strip Loins with Phosphte nd Slt Hs Potentil to Improve Yield, Tenderness, nd Juiciness ut Hrm Texture nd Flvor B.A. Crow, M.E. Dikemn, L.C. Hollis,
Met nd Food Sfety Needle-Free Injection Enhncement of Beef Strip Loins with Phosphte nd Slt Hs Potentil to Improve Yield, Tenderness, nd Juiciness ut Hrm Texture nd Flvor B.A. Crow, M.E. Dikemn, L.C. Hollis,
Reuven Agami and Yosef Shaul
 The kinse ctivity of ut not v-al is potentited y direct interction with RFXI, protein tht inds the enhncers of severl viruses nd cell-cycle regulted genes Reuven Agmi nd Yosef Shul Oncogene (1998) 16,
The kinse ctivity of ut not v-al is potentited y direct interction with RFXI, protein tht inds the enhncers of severl viruses nd cell-cycle regulted genes Reuven Agmi nd Yosef Shul Oncogene (1998) 16,
supplementary information
 DOI: 10.1038/nc2089 H3K4me1 H3K4me1 H3K4me1 H3K4me1 H3K4me1 H3K4me1 5 PN N1-2 PN H3K4me1 H3K4me1 H3K4me1 2-cell stge 2-c st cell ge Figure S1 Pttern of loclistion of H3K4me1 () nd () during zygotic development
DOI: 10.1038/nc2089 H3K4me1 H3K4me1 H3K4me1 H3K4me1 H3K4me1 H3K4me1 5 PN N1-2 PN H3K4me1 H3K4me1 H3K4me1 2-cell stge 2-c st cell ge Figure S1 Pttern of loclistion of H3K4me1 () nd () during zygotic development
SUPPLEMENTARY INFORMATION
 Supplementry Figure 1. Genertion of N- nd C-tgged cyclin D1 knock-in mice., N-tgged cyclin D1 gene trgeting construct, cyclin D1 genomic locus, cyclin D1 locus following homologous recomintion (trgeted
Supplementry Figure 1. Genertion of N- nd C-tgged cyclin D1 knock-in mice., N-tgged cyclin D1 gene trgeting construct, cyclin D1 genomic locus, cyclin D1 locus following homologous recomintion (trgeted
ARTICLE. J. E. Bowe & A. Chander & B. Liu & S. J. Persaud & P. M. Jones
 Dietologi (23) 56:783 79 DOI.7/s25-2-2828-2 ARTICLE The permissive effects of glucose on receptor-operted potentition of insulin secretion from mouse islets: role for ERK/2 ctivtion nd cytoskeletl remodelling
Dietologi (23) 56:783 79 DOI.7/s25-2-2828-2 ARTICLE The permissive effects of glucose on receptor-operted potentition of insulin secretion from mouse islets: role for ERK/2 ctivtion nd cytoskeletl remodelling
A septin requirement differentiates autonomous and contact-facilitated T cell proliferation
 A septin requirement differentites utonomous nd contct-fcilitted T cell prolifertion Adrin M Mujl 1, Juli K Gilden 1,3, Audrey Gérrd 1, Mkoto Kinoshit 2 & Mtthew F Krummel 1 T cell prolifertion is initited
A septin requirement differentites utonomous nd contct-fcilitted T cell prolifertion Adrin M Mujl 1, Juli K Gilden 1,3, Audrey Gérrd 1, Mkoto Kinoshit 2 & Mtthew F Krummel 1 T cell prolifertion is initited
Luteolin decreases IGF-II production and downregulates insulin-like growth factor-i receptor signaling in HT-29 human colon cancer cells
 Lim et l. MC Gstroenterology 212, 12:9 http://www.iomedcentrl.com/1471-23x/12/9 RESERCH RTICLE Luteolin decreses IGF-II production nd downregultes insulin-like growth fctor-i receptor signling in HT-29
Lim et l. MC Gstroenterology 212, 12:9 http://www.iomedcentrl.com/1471-23x/12/9 RESERCH RTICLE Luteolin decreses IGF-II production nd downregultes insulin-like growth fctor-i receptor signling in HT-29
Local IL-21 Promotes the Therapeutic Activity of Effector T cells by Decreasing Regulatory T Cells Within the Tumor Microenvironment
 originl rticle Locl IL- Promotes the Therpeutic Activity of Effector T cells y Decresing Regultory T Cells Within the Tumor Microenvironment Seunghee Kim-Schulze, Hong Sung Kim, Qing Fn, De Won Kim nd
originl rticle Locl IL- Promotes the Therpeutic Activity of Effector T cells y Decresing Regultory T Cells Within the Tumor Microenvironment Seunghee Kim-Schulze, Hong Sung Kim, Qing Fn, De Won Kim nd
Effects of physical exercise on working memory and prefrontal cortex function in post-stroke patients
 Effects of physicl exercise on working memory nd prefrontl cortex function in post-stroke ptients M Moriy, C Aoki, K Sktni Grdute School of Helth Sciences Reserch, Mjor of Physicl Therpy, TeikyoHeisei
Effects of physicl exercise on working memory nd prefrontl cortex function in post-stroke ptients M Moriy, C Aoki, K Sktni Grdute School of Helth Sciences Reserch, Mjor of Physicl Therpy, TeikyoHeisei
Continuous T cell receptor signaling required for synapse maintenance and full effector potential
 Continuous T cell receptor signling required for synpse mintennce nd full effector potentil Johnnes B Hupp 1,2, Michel Gleimer 1, Cenk Sumen 1,3 & Mrk M Dvis 1,2 Although signls through the T cell receptor
Continuous T cell receptor signling required for synpse mintennce nd full effector potentil Johnnes B Hupp 1,2, Michel Gleimer 1, Cenk Sumen 1,3 & Mrk M Dvis 1,2 Although signls through the T cell receptor
Using Paclobutrazol to Suppress Inflorescence Height of Potted Phalaenopsis Orchids
 Using Pcloutrzol to Suppress Inflorescence Height of Potted Phlenopsis Orchids A REPORT SUBMITTED TO FINE AMERICAS Linsey Newton nd Erik Runkle Deprtment of Horticulture Spring 28 Using Pcloutrzol to Suppress
Using Pcloutrzol to Suppress Inflorescence Height of Potted Phlenopsis Orchids A REPORT SUBMITTED TO FINE AMERICAS Linsey Newton nd Erik Runkle Deprtment of Horticulture Spring 28 Using Pcloutrzol to Suppress
Supplemental Materials
 Supplementl Mterils Cellulose deficiency of shv3svl1 is enhnced y hyper ccumultion of exogenous sucrose vi the plsm memrne sucrose/h symporter SUC1 Trevor H. Yets, Hgit Sorek, Dvid E. Wemmer, Chris R.
Supplementl Mterils Cellulose deficiency of shv3svl1 is enhnced y hyper ccumultion of exogenous sucrose vi the plsm memrne sucrose/h symporter SUC1 Trevor H. Yets, Hgit Sorek, Dvid E. Wemmer, Chris R.
Gene expression phenotypic models that predict the activity of oncogenic pathways
 3 Nture Pulishing Group http://www.nture.com/nturegenetics Gene expression phenotypic models tht predict the ctivity of oncogenic pthwys Erich Hung,, Seiichi Ishid,7, Jennifer Pittmn,3, Holly Dressmn,,4,
3 Nture Pulishing Group http://www.nture.com/nturegenetics Gene expression phenotypic models tht predict the ctivity of oncogenic pthwys Erich Hung,, Seiichi Ishid,7, Jennifer Pittmn,3, Holly Dressmn,,4,
Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites
 Centrl memory T cells medite long-term immunity to Leishmni mjor in the sence of persistent prsites Coly Zph 1,3,Jude Uzonn 1,3,Stephen M Beverley 2 & Phillip Scott 1 Infection with Leishmni mjor induces
Centrl memory T cells medite long-term immunity to Leishmni mjor in the sence of persistent prsites Coly Zph 1,3,Jude Uzonn 1,3,Stephen M Beverley 2 & Phillip Scott 1 Infection with Leishmni mjor induces
Hormonal networks involved in phosphate deficiencyinduced cluster root formation of Lupinus albus L.
 Institute of Crop Science (34h) Hormonl networks involved in phosphte deficiencyinduced cluster root formtion of Lupinus lus L. For PSP5 in Montpellier, 214 Zhengrui Wng, A.B.M. Moshiur Rhmn, Guoying Wng,
Institute of Crop Science (34h) Hormonl networks involved in phosphte deficiencyinduced cluster root formtion of Lupinus lus L. For PSP5 in Montpellier, 214 Zhengrui Wng, A.B.M. Moshiur Rhmn, Guoying Wng,
Human PRP4 kinase is required for stable tri-snrnp association during spliceosomal B complex formation
 Humn PRP4 kinse is required for stle tri-snrnp ssocition during spliceosoml formtion Mrc Schneider 1, He-Hsun Hsio 2, Cindy L Will 1, Régis Giet 3, Henning Urlu 2 & Reinhrd Lührmnn 1 Reversile protein
Humn PRP4 kinse is required for stle tri-snrnp ssocition during spliceosoml formtion Mrc Schneider 1, He-Hsun Hsio 2, Cindy L Will 1, Régis Giet 3, Henning Urlu 2 & Reinhrd Lührmnn 1 Reversile protein
Abstract ABSTRACT #69. Abstract. Introduction & Methods. Methods & Results. Results. Results & Conclusions
 Effects of dietry β-glucn on Growth Performnce, Dirrhe, nd Gut Permeility of Wening Pigs Experimentlly Infected with Pthogenic E. coli Kwngwook Kim, Amy Ehrlich, Vivin Perng, Jennifer Chse, Helen Ryould,
Effects of dietry β-glucn on Growth Performnce, Dirrhe, nd Gut Permeility of Wening Pigs Experimentlly Infected with Pthogenic E. coli Kwngwook Kim, Amy Ehrlich, Vivin Perng, Jennifer Chse, Helen Ryould,
Identification of a tripartite interaction between the N terminus of HIV 1 Vif and CBFβ that is critical for Vif function
 DOI 1.1186/s12977-17-346-5 Retrovirology RESEARCH Open Access Identifiction of triprtite interction etween the N terminus of HIV 1 nd CBFβ tht is criticl for function Belete A. Desimmie 1, Jessic L. Smith
DOI 1.1186/s12977-17-346-5 Retrovirology RESEARCH Open Access Identifiction of triprtite interction etween the N terminus of HIV 1 nd CBFβ tht is criticl for function Belete A. Desimmie 1, Jessic L. Smith
Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells
 Trnscription fctor Foxo3 controls the mgnitude of T cell immune responses by modulting the function of dendritic cells 9 Nture Americ, Inc. All rights reserved. Anne S Dejen, Dniel R Beisner,4, Irene L
Trnscription fctor Foxo3 controls the mgnitude of T cell immune responses by modulting the function of dendritic cells 9 Nture Americ, Inc. All rights reserved. Anne S Dejen, Dniel R Beisner,4, Irene L
Selection of Foxp3 + regulatory T cells specific for self antigen expressed and presented by Aire + medullary thymic epithelial cells
 Selection of Foxp3 + regultory T cells specific for self ntigen expressed nd presented y Aire + medullry thymic epithelil cells Kthrin Aschenrenner 1, Louise M D Cruz 1,3, Eliseth H Vollmnn 1,3, Mri Hintererger
Selection of Foxp3 + regultory T cells specific for self ntigen expressed nd presented y Aire + medullry thymic epithelil cells Kthrin Aschenrenner 1, Louise M D Cruz 1,3, Eliseth H Vollmnn 1,3, Mri Hintererger
Irs-2 coordinates Igf-1 receptor-mediated β-cell development and peripheral insulin signalling
 Irs-2 coordintes Igf-1 receptor-medited β-cell development nd peripherl insulin signlling Dominic J. Withers 1,2 *, Deorh J. Burks 1 *, Hether H. Towery 1, Shri L. Altmuro 1, Crrie L. Flint 1 & Morris
Irs-2 coordintes Igf-1 receptor-medited β-cell development nd peripherl insulin signlling Dominic J. Withers 1,2 *, Deorh J. Burks 1 *, Hether H. Towery 1, Shri L. Altmuro 1, Crrie L. Flint 1 & Morris
Natural killer cells determine the outcome of B cell mediated autoimmunity
 Nturl killer cells determine the outcome of B cell medited utoimmunity Fu-Dong Shi 1, *, Hu-Bing Wng 2, *, Hulun Li 2, *, Seokmnn Hong 3, Msru Tniguchi 4, Hns Link 2, Luc Vn Ker 3 nd Hns-Gustf Ljunggren
Nturl killer cells determine the outcome of B cell medited utoimmunity Fu-Dong Shi 1, *, Hu-Bing Wng 2, *, Hulun Li 2, *, Seokmnn Hong 3, Msru Tniguchi 4, Hns Link 2, Luc Vn Ker 3 nd Hns-Gustf Ljunggren
% cells forming Neurospheres 81 ± 6 % 0 % 2.6 ± 0.7 % 76 ± 8 % 0 % 3.4 ± 0.6 % 83 ± 5 % 0 % 2.4 ± 0.9 % 89 ± 5 % 3 ± 1.5 % Total 10, ± 6 % 0 %
 Bo et l., Suppl. Tle 1 Supplementl Tle 1. Neurosphere formtion nd tumorigencity is enriched within the tumour cell popultions derived from humn primry glioms nd gliom xenogrfts. GBM smples or Gliom xenogrfts
Bo et l., Suppl. Tle 1 Supplementl Tle 1. Neurosphere formtion nd tumorigencity is enriched within the tumour cell popultions derived from humn primry glioms nd gliom xenogrfts. GBM smples or Gliom xenogrfts
Redirected Antitumor Activity of Primary Human Lymphocytes Transduced With a Fully Human Anti-mesothelin Chimeric Receptor
 originl rticle Redirected Antitumor Activity of Primry Humn Lymphocytes Trnsduced With Fully Humn Anti-mesothelin Chimeric Receptor Evripidis Lnitis 1,2, Mthilde Poussin 1, In S Hgemnn 3, George Coukos
originl rticle Redirected Antitumor Activity of Primry Humn Lymphocytes Trnsduced With Fully Humn Anti-mesothelin Chimeric Receptor Evripidis Lnitis 1,2, Mthilde Poussin 1, In S Hgemnn 3, George Coukos
SUPPLEMENTARY INFORMATION
 TM TM tip link horizontl top connectors 1 leucine-rich (21 %) otoncorin-like 1809 ntigenic peptides B D signl peptide hydrophoic segment proline/threonine-rich (79 %) Supplementry Figure 1. () The outer
TM TM tip link horizontl top connectors 1 leucine-rich (21 %) otoncorin-like 1809 ntigenic peptides B D signl peptide hydrophoic segment proline/threonine-rich (79 %) Supplementry Figure 1. () The outer
