MicroRNAs Silence Gene Expression by Repressing Protein Expression and/or by Promoting mrna Decay
|
|
|
- Hubert Andrews
- 5 years ago
- Views:
Transcription
1 MicroRNAs Silence Gene Expression by Repressing Protein Expression and/or by Promoting mrna Decay I. BEHM-ANSMANT, J. REHWINKEL, AND E. IZAURRALDE MPI for Developmental Biology, D Tuebingen, Germany MicroRNAs (mirnas) represent a novel class of genome-encoded eukaryotic regulatory RNAs that silence gene expression posttranscriptionally. Although the proteins mediating mirna biogenesis and function have been identified, the precise mechanism by which mirnas regulate the expression of target mrnas remains unclear. We summarize recent work from our laboratory demonstrating that mirnas silence gene expression by at least two independent mechanisms: by repressing translation and/or by promoting mrna degradation. In Drosophila, both mechanisms require Argonaute 1 (AGO1) and the P-body component GW182. Moreover, mrna degradation by mirnas is effected by the enzymes involved in general mrna decay, including deadenylases and decapping enzymes, which also localize to P bodies. Our findings suggest a model for mirna function in which AGO1 associates with mirna targets through mirna:mrna base-pairing interactions. GW182 interacts with AGO1 and recruits deadenylases and decapping enzymes, leading to mrna degradation. However, not all mirna targets are degraded: Some stay in a translationally silent state, from which they may eventually be released. We propose that the final outcome of mirna regulation (i.e., degradation vs. translational repression) is influenced by other RNA-binding proteins interacting with the targeted mrna. mirnas represent a specific class of genome-encoded small RNAs that regulate gene expression posttranscriptionally (Bartel 2004; Filipowicz 2005). Hundreds of mirnas and their targets have been identified or predicted in different organisms. They affect a broad range of biological processes including cell differentiation and proliferation, apoptosis, metabolism, and development (Bartel 2004; Filipowicz 2005). mirna regulatory functions are effected by the Argonaute proteins that promote decay or translational repression of mrnas having sequences complementary to the mirnas (Bartel 2004; Filipowicz 2005). Plant mirnas are generally fully complementary to their targets and elicit endonucleolytic cleavage in the region of the mrna that is base-paired with the mirna. Endonucleolytic cleavage may also occur even when mirnas are not fully complementary to their binding sites (Llave et al. 2002; Mallory et al. 2004; Allen et al. 2005; Guo et al. 2005; Schwab et al. 2005). This implies that plant mirnas are similar to small interfering RNAs (sirnas) with respect to their mode of action, although they differ on their mode of biogenesis (Bartel 2004; Filipowicz 2005). The majority of animal mirnas are only partially complementary to their targets and silence gene expression by mechanisms that remain elusive (Filipowicz 2005). At first, animal mirnas were reported to repress translation without affecting mrna levels (Lee et al. 1993; Wightman et al. 1993). More recently, several reports have shown that animal mirnas can also induce significant degradation of target mrnas despite imperfect mrna mirna base-pairing (Bagga et al. 2005; Jing et al. 2005; Lim et al. 2005; Behm-Ansmant et al. 2006; Giraldez et al. 2006; Rehwinkel et al. 2006; Wu et al. 2006). Consistently, transcripts that are up-regulated in cells in which the mirna pathway is inhibited (e.g., by depletion of Dicer or Argonaute proteins) are enriched in predicted and validated mirna targets (Giraldez et al. 2006; Rehwinkel et al. 2006). Conversely, ectopic expression of specific mirnas in cells in which they are not normally present leads to a reduction of the levels of transcripts containing binding sites for the mirna (Lim et al. 2005). Finally, microarrays have been used to identify mirna targets, indicating that mirnas do indeed affect mrna levels (Lim et al. 2005; Giraldez et al. 2006, Rehwinkel et al. 2006). In contrast to the situation in plants, however, under most circumstances, mrna decay by mirnas in animal cells may not occur via endonucleolytic cleavage but rather by directing mrnas to the general mrna degradation machinery, and thus by accelerating their decay (Behm-Ansmant et al. 2006; Giraldez et al. 2006; Wu et al. 2006). Several lines of evidence support the existence of a link between the mirna pathway and general mrna decay. First, mammalian Argonaute proteins (AGO1 AGO4), mirnas, and mirna targets colocalize to cytoplasmic foci known as processing bodies (P bodies) (Jakymiw et al. 2005; Liu et al. 2005a,b; Meister et al. 2005; Pillai et al. 2005; Sen and Blau 2005). P bodies are discrete cytoplasmic domains where proteins required for bulk mrna degradation in the 5 to 3 direction accumulate (e.g., deadenylases, the decapping DCP1:DCP2 complex, and the 5 to 3 exonuclease XRN1; for review, see Valencia- Sanchez et al. 2006). Second, human AGO1 and AGO2 associate with the decapping coactivator DCP1 and with GW182, a protein with glycine-tryptophan repeats (GW repeats) which localizes to P bodies and is required for P- body integrity (Eystathioy et al. 2002, 2003; Jakymiw et al. 2005; Liu et al. 2005a,b; Meister et al. 2005; Sen and Blau 2005). Third, depletion of GW182 in human cells impairs both mirna function and mrna decay triggered by complementary sirnas (Jakymiw et al. 2005; Cold Spring Harbor Symposia on Quantitative Biology, Volume LXXI Cold Spring Harbor Laboratory Press
2 524 BEHM-ANSMANT, REHWINKEL, AND IZAURRALDE Liu et al. 2005b; Meister et al. 2005). Similarly, mirna function is impaired in Drosophila Schneider cells (S2 cells) depleted of GW182 or the decapping DCP1:DCP2 complex (Rehwinkel et al. 2005). Finally, the Caenorhabditis elegans protein AIN-1, which is related to GW182, is required for gene regulation by at least a subset of mirnas (Ding et al. 2005). Nevertheless, despite this link between mirna-mediated silencing and mrna degradation, there are multiple examples of mirna targets for which translational repression is observed without detectable changes in the mrna level (for review, see Valencia-Sanchez et al. 2006), suggesting that for these targets, regulation by mirnas may occur independently of mrna decay. How mirnas regulate translation is not well understood. Two independent studies have shown that mirnas inhibit translation initiation at an early stage involving the cap structure, as mrnas translated via cap-independent mechanisms were shown to escape mirna-mediated silencing (Humphreys et al. 2005; Pillai et al. 2005). Other studies have suggested that translation inhibition occurs after initiation on the basis of the observation that mirnas and some targets remain associated with polysomes (Olsen and Ambros 1999; Kim et al. 2004; Nelson et al. 2004; Petersen et al. 2006). Moreover, Petersen et al. (2006) reported that mrnas translated via cap-independent mechanisms are subjected to mirna regulation. A possible explanation for these contradictory results is that mirnas regulate gene expression by different mechanisms, although the possibility that these discrepancies are due to differences in experimental approaches cannot be excluded (see Valencia-Sanchez et al. 2006). We have investigated the mechanism by which mirnas silence gene expression. Here, we describe briefly the assays we use to monitor mirna function in Drosophila cells, a summary of the overall results, and our current model regarding mirna function. MIRNA-MEDIATED GENE SILENCING INVOLVES TRANSLATIONAL REPRESSION AND/OR MRNA DEGRADATION To investigate mirna function in Drosophila cells, we have generated a series of reporters in which the coding region of firefly luciferase (F-Luc) is flanked by the 3 UTRs (untranslated regions) of the Drosophila gene CG10011 regulated by mir-12, and of Nerfin or Vha68-1 genes, which contain binding sites for mir-9b (Fig. 1a). Drosophila S2 cells are transiently transfected with the F- Luc reporters, a plasmid expressing the primary mirna transcript or the corresponding vector without insert (empty vector), and a plasmid encoding Renilla luciferase (R-Luc) as a transfection control. F-Luc activity is normalized to that of Renilla to compensate for possible differences in transfection efficiencies. Using this assay, we have shown that expression of the F-Luc reporter having the 3 UTR of CG10011 (F-Luc- CG10011) is strongly reduced by cotransfection of a plasmid expressing mir-12, whereas expression of the firefly luciferase reporters fused to the Nerfin or Vha UTRs (F-Luc-Nerfin, F-Luc-Vha68-1) is inhibited by cotransfection of mir-9b (Fig. 1b) (Rehwinkel et al. 2005, 2006; Behm-Ansmant et al. 2006). In all three cases, silencing of luciferase expression by the cognate mirnas is prevented in cells depleted of AGO1 (see Fig. 2) (Rehwinkel et al. 2005, 2006; Behm-Ansmant et al. 2006), demonstrating that silencing of these reporters occurs via the mirna pathway. Note that the genomes of multicellular organisms encode multiple Argonaute proteins. In Drosophila, Argonaute paralogs have evolved specialized roles: AGO1 mediates mirna function, whereas AGO2 catalyzes sirna-guided endonucleolytic cleavage of mrnas (RNA interference [RNAi]) (Okamura et al. 2004; Rand et al. 2004; Miyoshi et al. 2005). To determine whether mirnas silence F-Luc expression by inhibiting translation directly, or indirectly by reducing mrna levels, we analyzed the steady-state levels of the F-Luc mrna by northern blot and normalized them to the levels of the control R-Luc mrna. We observed that mirnas triggered a reduction in mrna levels to different extents (Behm-Ansmant et al. 2006). Expression of mir-9b led to a slight decrease of the F- Luc-Nerfin mrna levels but to a significant reduction of the F-Luc-Vha68-1 mrna levels; mir-12 triggered a strong reduction of F-Luc-CG10011 mrna levels (Behm-Ansmant et al. 2006). The decrease in mrna levels observed for the Nerfin and Vha68-1 reporters is significantly smaller than the reduction observed in F-Luc activity. Indeed, after normalizing firefly luciferase activity to the corresponding mrna levels, we observed that mir-9b leads to a net 5- fold and 2.5-fold reduction in protein expression for the Nerfin and Vha68-1 reporters, respectively (Fig. 1c). In contrast, silencing of F-Luc-CG10011 by mir-12 can be attributed primarily to the reduction of mrna levels (Fig. 1c). These results indicate that mirnas silence gene expression by two mechanisms, one involving inhibition of protein expression and one a reduction of mrna levels. MIRNAS ACCELERATE MRNA DEGRADATION To investigate whether the reduction of mrna levels caused by mirnas is a consequence of increased mrna degradation, we measured the half-lives of the reporters in the absence or presence of the cognate mirnas following inhibition of transcription with actinomycin D. The halflives of the F-Luc reporter having the Nerfin, Vha68-1, and CG UTRs were about 141 minutes, 200 minutes, and longer than 300 minutes, respectively. In cells expressing the mirnas, the half-lives of these reporters were reduced to about 38 minutes, 23 minutes, and 10 minutes (Fig. 1d). Thus, even for the Nerfin reporter, whose steady-state levels do not change dramatically in the presence of mir-9b, the degradation rate is increased. In the presence of the mirnas, the mrna reporters have biphasic decay kinetics, suggesting the existence of a heterogeneous pool of mrnas (for instance, free or bound to the mirna) undergoing different rates of degradation. In addition, the reduction in half-lives is more marked than the changes in steady-state mrna levels. One possible explanation for this discrepancy is that steady-state mrna levels are determined by transcription and decay rates, and at steady state, transcription rates may compensate for decay. Alternatively, we cannot
3 MIRNAS SILENCE GENE EXPRESSION BY MULTIPLE MECHANISMS 525 Figure 1. Silencing by mirnas is effected by changes in protein expression and/or mrna stability. (a) mirna reporters. The F-Luc open reading frame (F-Luc) is flanked by the 3 UTRs of Nerfin, Vha68-1, or CG10011 mrnas, which contain binding sites for mir- 9b or mir-12 as indicated. (b,c) S2 cells were transfected with plasmids expressing mirna reporters (Nerfin, Vha68-1, or CG10011), plasmids expressing mir-9b or mir-12 (red bars), or the corresponding empty vector (black bars). Renilla luciferase (R-Luc) served as a transfection control. F-Luc activity and the corresponding mrna levels were measured and normalized to those of the Renilla. Normalized F-Luc activities in cells transfected with the empty vector (black bars) were set to 100%. In panel c, the normalized values of F-Luc activity shown in panel b are divided by the normalized mrna levels (not shown) to estimate the net effect of mirnas on protein expression. Error bars represent standard deviations from at least three independent experiments. (d) S2 cells were transfected with the mirna reporters shown in panel a. Three days after transfection, cells were treated with actinomycin D and harvested at the indicated time points. The decay of the F-Luc reporters was monitored in cells coexpressing the mirna (pink) or the corresponding empty vector (blue). RNA samples were analyzed by northern blot (not shown). The levels of F-Luc reporter transcripts normalized to those of the long-lived rp49 mrna are plotted as a function of time. mrna half-lives (t 1/2 ) are indicated. exclude the possibility that the actinomycin D treatment is having secondary effects on mrna stability. MIRNAS PROMOTE DEADENYLATION AND DECAPPING We recently reported that mrna decay by mirnas requires components of the CCR4:NOT deadenylase complex and the decapping DCP1:DCP2 complex (Behm-Ansmant et al. 2006). Figure 2a,b shows a comparison of the effects of depleting components of the deadenylase or decapping complexes on mirna activity for the Nerfin and CG10011 reporters, which are regulated mainly at the translational level and at the mrna levels, respectively. In this representation, F-Luc activity or mrna levels are normalized to those of Renilla. For each knockdown, the normalized F-Luc activities or mrna levels measured in the absence of the mirna are set to 100 (not shown). For the Nerfin reporter, expression of mir-9b strongly reduces F-Luc activity but mrna levels only slightly (Fig. 2a, black and orange bars, respectively). Both luciferase expression and mrna levels are restored in cells depleted of AGO1 or GW182. This indicates that
4 526 BEHM-ANSMANT, REHWINKEL, AND IZAURRALDE Figure 2. mrna decay triggered by mirnas occurs by a deadenylation and decapping mechanism requiring GW182. (a,b) S2 cells depleted of the proteins indicated above the panels were transfected with plasmids expressing the Nerfin or CG10011 mirna reporters. Control cells were treated with green fluorescent protein (GFP) double-stranded RNA (dsrna). F-Luc activity and the corresponding mrna levels were measured and normalized to those of the Renilla. Normalized F-Luc activities (black bars) and mrna levels (orange bars) in the presence of the mirna are shown. In cells transfected with the empty vector, these values were set to 100% for each knockdown (not shown). (Black horizontal lines) F-Luc activity in control cells; (orange horizontal lines) mrna levels in control cells. Error bars represent standard deviations from three independent experiments. (c) Endogenous mirna targets are degraded by deadenylation and decapping. The decay of CG1998 mrna (a predicted target of K-box mirnas) was monitored in S2 cells depleted of AGO1, GW182, CAF1, or DCP1:DCP2 following inhibition of transcription by actinomycin D. Control cells were treated with GFP dsrna. The levels of the CG1998 mrna were analyzed by northern blot, normalized to rp49 mrna (not shown), and plotted against time. mrna half-lives (t 1/2 ) calculated from the decay curves are indicated. GW182 also has a role in translational silencing by mirnas, in agreement with previous studies (Jakymiw et al. 2005; Liu et al. 2005b; Meister et al. 2005; Rehwinkel et al. 2005). There are two major deadenylase complexes in Drosophila: the PAN2:PAN3 and the CCR4:NOT complexes (Temme et al. 2004). Depletion of PAN2, PAN3, CAF1, or NOT1 does not have a significant effect on luciferase expression or mrna levels of the Nerfin reporter (Fig. 2a) (data not shown). Similarly, codepletion of CAF1 or NOT1 with DCP2 does not restore luciferase expression or mrna levels (Fig. 2a). In contrast, depletion of DCP1:DCP2 restores mrna levels indicating that this reporter is degraded by decapping (Fig. 2a). Although mrna levels are restored in cells depleted of decapping enzymes, luciferase expression is not. A potential explanation for this lack of restoration is provided by the analysis of mrna levels by northern blotting. Indeed, we observe that reporter transcripts accumulating in cells depleted of the decapping enzymes are deadenylated and are thus expected to be translated less efficiently (Behm- Ansmant et al. 2006). Deadenylation is observed only in the presence of the mirna, indicating that mirnas trigger deadenylation. Deadenylation is, however, unlikely to be the cause of the translational repression because luciferase activity is not restored in cells depleted of deadenylases. When we analyzed the CG10011 reporter, which is mainly regulated at the mrna level, we observed that depletion of AGO1 or GW182 restores both luciferase expression and mrna levels, indicating that mrna decay is AGO1- and GW182-dependent. Depletion of CAF1 or NOT1 individually or in combination with DCP2 also restores both luciferase expression and mrna levels, providing strong evidence for the conclusion that regulation of this reporter by mir-12 occurs at the level of mrna degradation (Fig. 2b). Again, depletion of DCP1:DCP2 restores mrna levels, but as observed for the Nerfin reporter, transcripts accumulating in these cells are deadenylated in the presence of the mirna (Behm- Ansmant et al. 2006), providing a possible explanation for the partial or lack of restoration of luciferase expression. In summary, these findings indicate that gene silencing by mirnas involves translational repression and/or mrna deadenylation and decapping and both require GW182. The results from our laboratory in Drosophila cells are remarkably consistent with recent studies in zebra fish embryos and human cells showing that mirnas promote accelerated deadenylation of their targets (Giraldez et al. 2006; Wu et al. 2006). Moreover, Wu et al. (2006) reported that mrnas lacking a poly(a) tail are nevertheless subjected to translational repression by mirnas, indicating that this repression occurs by a deadenylation-independent mechanism. A question that remains open is whether mirna-mediated mrna degradation is a consequence of translational repression or whether these represent two independent mechanisms by which mirnas silence gene expression as proposed by Wu et al. (2006). As mentioned above, inhibition of translation is not always accompanied by changes in mrna levels. Conversely, targets regulated
5 MIRNAS SILENCE GENE EXPRESSION BY MULTIPLE MECHANISMS 527 mainly at the translational level are subjected to accelerated deadenylation, but depletion of CAF1 or NOT1 does not restore protein expression. These findings suggest that translational repression and mrna degradation represent two independent mechanisms by which mirnas regulate gene expression. ENDOGENOUS MIRNA REPORTERS ARE DEGRADED IN AN AGO1- AND GW182-DEPENDENT MANNER To investigate the significance of the results obtained with the reporters for the mirna pathway, we analyzed mrna expression profiles in S2 cells depleted of GW182 or AGO1. We observed that depletion of these proteins leads to correlated changes in mrna expression levels, indicating that they act in the same pathway. Furthermore, transcripts commonly up-regulated by AGO1 and GW182 are enriched in predicted and validated mirna targets. This shows that, as for luciferase reporters, endogenous mirna targets are regulated by GW182 (Behm-Ansmant et al. 2006). We also analyzed the degradation rate of endogenous mirna targets identified by expression profiling of AGO1 or GW182-depleted cells (Behm-Ansmant et al. 2006; Rehwinkel et al. 2006). The Axs and CG1998 mrnas are predicted targets of mir-285 and K-box mirnas, respectively (Stark et al. 2005). These mrnas are at least three- to sixfold up-regulated in cells depleted of AGO1 or GW182. In cells depleted of AGO1, GW182, CAF1, or DCP1:DCP2, the half-lives of these mrnas increase from approximately 3 hours to more than 6 hours, suggesting that they are degraded by deadenylation and decapping in an AGO1- and GW182-dependent manner (Fig. 2c) (Behm-Ansmant et al. 2006). It is worth noting that for some endogenous mirna targets, depletion of decapping enzymes or of components of the CCR4:NOT complex leads to a stronger stabilization of the mrna than that observed in cells depleted of AGO1 or GW182. This indicates that only a fraction of these endogenous transcripts is targeted by the cognate mirnas. Depletion of the CCR4:NOT complex or of the decapping enzymes prevents general degradation and degradation via the mirna pathway, leading to a stronger stabilization of the transcripts. This is likely to be the case for mrnas targeted by low-abundance mirnas. Together, the results obtained for the F-Luc reporters and endogenous targets demonstrate unequivocally a link between mirna-mediated gene silencing and the machinery for general mrna degradation. They also show that GW182 is a critical effector of mirna function in animal cells. GW182 BELONGS TO A CONSERVED FAMILY OF PROTEINS GW182 belongs to a family of proteins containing a central ubiquitin-associated (UBA) domain, a carboxy-terminal RNA recognition motif (RRM), and three blocks of glycine-tryptophan repeats (referred to as amino-terminal, middle, and carboxy-terminal GW repeats). Furthermore, a glutamine-rich (Q-rich) region is located between the UBA domain and the RRM (Fig. 3a) (Eystathioy et al. 2002; Behm-Ansmant et al. 2006). Vertebrates contain three GW182 family members (TNRC6A/GW182, TNRC6B, and TNRC6C), but there is a single ortholog in insects and no ortholog in worms or fungi. Sequence alignment of all members of the protein family revealed the presence of two highly conserved motifs (I and II) of approximately 50 residues within the amino-terminal GW repeats (Fig. 3a). The insect proteins start exactly with motif I, whereas the vertebrate orthologs have amino-terminal extensions. Interestingly, the amino-terminal domain of GW182 proteins encompassing motif II bears similarity to the GW-like regions in Figure 3. GW182 acts downstream from AGO1 in the mirna pathway. (a) Domain organization of Drosophila GW182. (N- GW, M-GW, and C-GW) Amino-terminal, middle, and carboxy-terminal GW repeats, respectively; (UBA) ubiquitinassociated domain; (Q-rich) region rich in glutamine; (RRM) RNA recognition motif. Motifs I and II are indicated. The protein domains sufficient for the localization to P bodies and the interaction with AGO1 are shown below the protein outline. (b) Schematic representation of the F-Luc-5BoxB tethering reporter. (c) S2 cells depleted of the proteins indicated above the panel were transfected with the F-Luc-5BoxB reporter, a plasmid expressing Renilla luciferase, and vectors expressing the λn-peptide or λn-gw182. F-Luc activities (black bars) and mrna levels (green bars) were quantitated in three independent experiments and normalized to that of the Renilla control. In cells expressing the λn peptide alone, these values were set to 100 for each knockdown (not shown). (Black horizontal lines) Normalized F-Luc activity; (green horizontal lines) mrna levels in control cells. Mean values plus or minus standard deviations from three independent experiments are shown.
6 528 BEHM-ANSMANT, REHWINKEL, AND IZAURRALDE the C. elegans protein AIN-1, involved in the mirna pathway (Ding et al. 2005). However, AIN-1 contains no UBA, Q-rich, or RRM domains, suggesting that it represents a functional analog. C. elegans AIN-1, Drosophila GW182, and human TNRC6A/GW182 and TNRC6B localize to P bodies and interact with Argonaute proteins (Ding et al. 2005; Jakymiw et al. 2005; Liu et al. 2005a,b; Meister et al. 2005; Sen and Blau 2005; Behm-Ansmant et al. 2006). We have shown that it is the amino-terminal GW-repeat domain of Drosophila GW182 that mediates the interaction with the PIWI domain of AGO1 (Fig. 3a) (Behm- Ansmant et al. 2006). These findings suggest a conserved role for these repeats, and most likely for the highly conserved motif II, in mediating the interaction with Argonaute proteins. Interestingly, the PIWI domain adopts an RNase-H-like fold and is catalytically active, at least for a subset of Argonaute proteins including Drosophila AGO1 (for review, see Lingel and Sattler 2005; Miyoshi et al. 2005). It would therefore be of interest to determine whether the amino-terminal GW repeats of GW182 can modulate the catalytic activity of this domain. Apart from the interaction with AGO1, the amino-terminal repeats and the UBA and Q-rich domains contribute to the localization of GW182 in P bodies (Fig. 3a) (Behm-Ansmant et al. 2006), which is in turn required for P-body integrity (Eystathioy et al. 2002, 2003). This suggests that GW182 may act as a molecular scaffold bringing together AGO1-containing RISCs (RNA-induced silencing complex) and mrna decay enzymes, possibly nucleating the assembly of P bodies. GW182 ACTS DOWNSTREAM FROM AGO1 The analyses described above combined with previous studies indicate that GW182 is recruited to mirna targets via interactions with the Argonaute proteins (Jakymiw et al. 2005; Liu et al. 2005b; Meister et al. 2005; Behm-Ansmant et al. 2006). We used a tethering assay to begin to investigate the consequences of the recruitment of GW182 to mirna targets. In this assay, GW182 is expressed as a fusion with the λn peptide, which binds with high affinity to five hairpins (BoxB sites) present in the 3 UTR of a luciferase reporter mrna (F-Luc-5BoxB reporter, Fig. 3b). In this way, GW182 is directly tethered to the 3 UTR of the reporter, bypassing the requirement for AGO1 or mirnas. Using this assay, we have shown that tethering of GW182 to the F-Luc-5BoxB reporter silences its expression and promotes mrna degradation (Fig. 3c) (Behm- Ansmant et al. 2006). The decrease in mrna levels observed for the tethered mrna does not, however, fully account for the strong reduction in F-Luc activity (Fig. 3c), indicating that GW182 silences expression of bound transcripts by a dual mechanism involving inhibition of protein expression and reduction of mrna levels. As with mirnas, mrna degradation by GW182 requires the CCR4:NOT and the DCP1:DCP2 complexes. Indeed, the levels of a reporter transcript tethered to GW182 are restored in cells depleted of CAF1, NOT1, or the decapping enzymes. The restoration of mrna levels in cells depleted of CAF1 or NOT1 is not accompanied by a proportional increase in F-Luc expression (Fig. 3c), providing further evidence for a role of GW182 in translational regulation. Restoration of mrna levels, but not of luciferase activity, is also observed in cells co-depleted of CAF1 and DCP2 or NOT1 and DCP2, demonstrating that GW182 promotes deadenylation and decapping of bound mrnas (Fig. 3c). Thus, binding of GW182 appears to be a point of no return which marks transcripts as targets for the general mrna degradation machinery. Remarkably, GW182-mediated silencing and decay does not require AGO1 (Fig. 3c). This observation places GW182 downstream from AGO1 in the mirna pathway. Several lines of evidence support this conclusion. First, endogenous and ectopically expressed GW182 localizes to P bodies together with decapping enzymes and the deadenylase complex (Eystathioy et al. 2002, 2003). Second, GW182 interacts with AGO1 and recruits AGO1 to P bodies when the two proteins are coexpressed (Jakymiw et al. 2005; Liu et al. 2005a,b; Meister et al. 2005; Sen and Blau 2005; Behm-Ansmant et al. 2006). Third, as mentioned above, degradation of mrnas by GW182 or mirnas requires the CCR4:NOT and DCP1:DCP2 complexes (Behm-Ansmant et al. 2006). Finally, mirnas repress translation and promote mrna degradation in a GW182-dependent manner (Jakymiw et al. 2005; Liu et al. 2005b; Meister et al. 2005; Rehwinkel et al. 2005; Behm-Ansmant et al. 2006). CONCLUSIONS On the basis of the results described above and the observations that GW182 associates with AGO1 and is required for mirna-mediated gene silencing (Ding et al. 2005; Jakymiw et al. 2005; Liu et al. 2005b; Meister et al. 2005; Rehwinkel et al. 2005; Behm-Ansmant et al. 2006), we propose the following model for the mechanism of mirna-mediated gene silencing (Fig. 4): AGO1 binds mirna targets by means of mirna:mrna base-pairing interactions. AGO1 may then recruit GW182 and both proteins repress translations (Fig. 4). GW182 also marks Figure 4. Model for mirna-mediated gene silencing. mirnamediated gene silencing involves multiple mechanisms including translational repression, deadenylation, and decapping. In Drosophila, these mechanisms require AGO1 and the P-body protein GW182. Deadenylation and decapping are catalyzed by the DCP1:DCP2 decapping complex and the CCR4:NOT deadenylase complex, respectively. The mechanism by which mirnas repress translation remains to be established. In addition, it is unclear whether silencing occurs in P bodies, although all proteins shown in this diagram have been localized to P bodies.
7 MIRNAS SILENCE GENE EXPRESSION BY MULTIPLE MECHANISMS 529 the transcripts as targets for decay via a deadenylation and decapping mechanism. The contribution of translational repression or mrna degradation to gene silencing appears to differ for each mirna:target pair and is likely to depend on the particular set of proteins bound to the 3 UTR of the mrna. Furthermore, binding of specific RNA-binding proteins to the 3 UTR of mirna targets can revert mirna-mediated silencing under some physiological conditions (Bhattacharyya et al. 2006). A major challenge for future studies will be to identify how specific RNA-binding proteins influence the final outcome of mirna regulation. Another important question that remains open is the mechanism by which mirnas repress translation. Finally, whether silencing by mirnas occurs in the cytosol or in cytoplasmic domains such as P bodies is currently a matter of debate that requires further investigation and a deeper understanding of the assembly, composition, and cellular function of these bodies. ACKNOWLEDGMENTS We are grateful to D.J. Thomas for comments on the manuscript. This study was supported by EMBO and the Human Frontier Science Program Organization (HFSPO). I.B.-A. is the recipient of a fellowship from EMBO. REFERENCES Allen E., Xie Z., Gustafson A.M., and Carrington J.C microrna-directed phasing during trans-acting sirna biogenesis in plants. Cell 121: 207. Bagga S., Bracht J., Hunter S., Massirer K., Holtz J., Eachus R., and Pasquinelli A.E Regulation by let-7 and lin-4 mirnas results in target mrna degradation. Cell 122: 553. Bartel D.P MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281. Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., and Izaurralde E mrna degradation by mirnas and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 20: Bhattacharyya S.N., Habermacher R., Martine U., Closs E.I., and Filipowicz W Relief of microrna-mediated translational repression in human cells subjected to stress. Cell 125: Ding L., Spencer A., Morita K., and Han M The developmental timing regulator AIN-1 interacts with miriscs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Mol. Cell 19: 437. Eystathioy T., Chan E.K., Tenenbaum S.A., Keene J.D., Griffith K., and Fritzler M.J A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mrnas within novel cytoplasmic speckles. Mol. Biol. Cell 13: Eystathioy T., Jakymiw A., Chan E.K., Seraphin B., Cougot N., and Fritzler M.J The GW182 protein colocalizes with mrna degradation associated proteins hdcp1 and hlsm4 in cytoplasmic GW-bodies. RNA 9: Filipowicz W RNAi: The nuts and bolts of the RISC machine. Cell 122: 17. Giraldez A.J., Mishima Y., Rihel J., Grocock R.J., Van Dongen S., Inoue K., Enright A.J., and Schier A.F Zebrafish MiR-430 promotes deadenylation and clearance of maternal mrnas. Science 312: 75. Guo H.S., Xie Q., Fei J.F., and Chua N.H MicroRNA directs mrna cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell 17: Humphreys D.T., Westman B.J., Martin D.I., and Preiss T MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(a) tail function. Proc. Natl. Acad. Sci. 102: Jakymiw A., Lian S., Eystathioy T., Li S., Satoh M., Hamel J.C., Fritzler M.J., and Chan E.K Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 7: Jing Q., Huang S., Guth S., Zarubin T., Motoyama A., Chen J., Di Padova F., Lin S.C., Gram H., and Han J Involvement of microrna in AU-rich element-mediated mrna instability. Cell 120: 623. Kim J., Krichevsky A., Grad Y., Hayes G.D., Kosik K.S., Church G.M., and Ruvkun G Identification of many micrornas that copurify with polyribosomes in mammalian neurons. Proc. Natl. Acad. Sci. 101: 360. Lee R.C., Feinbaum R.L., and Ambros V The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843. Lim L.P., Lau N.C., Garrett-Engele P., Grimson A., Schelter J.M., Castle J., Bartel D.P., Linsley P.S., and Johnson J.M Microarray analysis shows that some micrornas downregulate large numbers of target mrnas. Nature 433: 769. Lingel A. and Sattler M Novel modes of protein-rna recognition in the RNAi pathway. Curr. Opin. Struct. Biol. 15: 107. Llave C., Xie Z., Kasschau K.D., and Carrington J.C Cleavage of Scarecrow-like mrna targets directed by a class of Arabidopsis mirna. Science 297: Liu J., Valencia-Sanchez M.A., Hannon G.J., and Parker R. 2005a. MicroRNA-dependent localization of targeted mrnas to mammalian P-bodies. Nat. Cell Biol. 7: 719. Liu J., Rivas F.V., Wohlschlegel J., Yates J.R., Parker R., and Hannon G.J. 2005b. A role for the P-body component GW182 in microrna function. Nat. Cell Biol. 7: Mallory A.C., Reinhart B.J., Jones-Rhoades M.W., Tang G., Zamore P.D., Barton M.K., and Bartel D.P MicroRNA control of PHABULOSA in leaf development: Importance of pairing to the microrna 5 region. EMBO J. 23: Meister G., Landthaler M., Peters L., Chen P.Y., Urlaub H., Luhrmann R., and Tuschl T Identification of novel Argonaute-associated proteins. Curr. Biol. 15: Miyoshi K., Tsukumo H., Nagami T., Siomi H., and Siomi M.C Slicer function of Drosophila Argonautes and its involvement in RISC formation. Genes Dev. 19: Nelson P.T., Hatzigeorgiou A.G., and Mourelatos Z mirnp:mrna association in polyribosomes in a human neuronal cell line. RNA 10: 387. Okamura K., Ishizuka A., Siomi H., and Siomi MC Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 18: Olsen P.H. and Ambros V The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev. Biol. 216: 671. Petersen C.P., Bordeleau M.E., Pelletier J., and Sharp P.A Short RNAs repress translation after initiation in mammalian cells. Mol. Cell 21: 533. Pillai R.S., Bhattacharyya S.N., Artus C.G., Zoller T., Cougot N., Basyuk E., Bertrand E., and Filipowicz W Inhibition of translational initiation by let-7 microrna in human cells. Science 309: Rand T.A., Ginalski K., Grishin N.V., and Wang X Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc. Natl. Acad. Sci. 101: Rehwinkel J., Behm-Ansmant I., Gatfield D., and Izaurralde E A crucial role for GW182 and the DCP1:DCP2 decapping complex in mirna-mediated gene silencing. RNA 11: Rehwinkel J., Natalin P., Stark A., Brennecke J., Cohen S.M., and Izaurralde E Genome-wide analysis of mrnas regulated by Drosha and Argonaute proteins in Drosophila. Mol. Cell. Biol. 26: 2965.
8 530 BEHM-ANSMANT, REHWINKEL, AND IZAURRALDE Schwab R., Palatnik J.F., Riester M., Schommer C., Schmid M., and Weigel D Specific effects of micrornas on the plant transcriptome. Dev. Cell 8: 517. Sen G.L. and Blau H.M Argonaute 2/RISC resides in sites of mammalian mrna decay known as cytoplasmic bodies. Nat. Cell Biol. 7: 633. Stark A., Brennecke J., Bushati N., Russell R.B., and Cohen S.M Animal micrornas confer robustness to gene expression and have a significant impact on 3 UTR evolution. Cell 123: Temme C., Zaessinger S., Meyer S., Simonelig M., and Wahle E A complex containing the CCR4 and CAF1 proteins is involved in mrna deadenylation in Drosophila. EMBO J. 23: Valencia-Sanchez M.A., Liu J., Hannon G.J., and Parker R Control of translation and mrna degradation by mirnas and sirnas. Genes Dev. 20: 515. Wightman B., Ha I., and Ruvkun G Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75: 855. Wu L., Fan J., and Belasco J.G MicroRNAs direct rapid deadenylation of mrna. Proc. Natl. Acad. Sci. 103: 4034.
MicroRNAs, mrnas, and Translation
 MicroRNAs, mrnas, and Translation P.A. MARONEY, Y. YU, AND T.W. NILSEN Center for RNA Molecular Biology and Department of Biochemistry, Case Western Reserve University School of Medicine, Cleveland, Ohio
MicroRNAs, mrnas, and Translation P.A. MARONEY, Y. YU, AND T.W. NILSEN Center for RNA Molecular Biology and Department of Biochemistry, Case Western Reserve University School of Medicine, Cleveland, Ohio
Getting to the Root of mirna-mediated Gene Silencing
 Leading Edge Essay Getting to the Root of mirna-mediated Gene Silencing Ana Eulalio, 1 Eric Huntzinger, 1 and Elisa Izaurralde 1, * 1 Max-Planck-Institute for Developmental Biology, Spemannstrasse 35,
Leading Edge Essay Getting to the Root of mirna-mediated Gene Silencing Ana Eulalio, 1 Eric Huntzinger, 1 and Elisa Izaurralde 1, * 1 Max-Planck-Institute for Developmental Biology, Spemannstrasse 35,
Bi 8 Lecture 17. interference. Ellen Rothenberg 1 March 2016
 Bi 8 Lecture 17 REGulation by RNA interference Ellen Rothenberg 1 March 2016 Protein is not the only regulatory molecule affecting gene expression: RNA itself can be negative regulator RNA does not need
Bi 8 Lecture 17 REGulation by RNA interference Ellen Rothenberg 1 March 2016 Protein is not the only regulatory molecule affecting gene expression: RNA itself can be negative regulator RNA does not need
Let Me Count the Ways: Mechanisms of Gene Regulation by mirnas and sirnas
 Let Me Count the Ways: Mechanisms of Gene Regulation by mirnas and sirnas Ligang Wu 1,2 and Joel G. Belasco 1,2, * 1 Kimmel Center for Biology and Medicine, Skirball Institute for Biomolecular Medicine
Let Me Count the Ways: Mechanisms of Gene Regulation by mirnas and sirnas Ligang Wu 1,2 and Joel G. Belasco 1,2, * 1 Kimmel Center for Biology and Medicine, Skirball Institute for Biomolecular Medicine
he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003)
 MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
GW182 proteins cause PABP dissociation from silenced mirna targets in the absence of deadenylation
 Manuscript EMBO-2012-83275 GW182 proteins cause PABP dissociation from silenced mirna targets in the absence of deadenylation Latifa Zekri, Duygu Kuzuoğlu-Öztürk and Elisa Izaurralde Corresponding author:
Manuscript EMBO-2012-83275 GW182 proteins cause PABP dissociation from silenced mirna targets in the absence of deadenylation Latifa Zekri, Duygu Kuzuoğlu-Öztürk and Elisa Izaurralde Corresponding author:
Computational Analysis of mirna and Target mrna Interactions: Combined Effects of The Quantity and Quality of Their Binding Sites *
 www.pibb.ac.cn!"#$%!""&'( Progress in Biochemistry and Biophysics 2009, 36(5): 608~615 Computational Analysis of mirna and Target mrna Interactions: Combined Effects of The Quantity and Quality of Their
www.pibb.ac.cn!"#$%!""&'( Progress in Biochemistry and Biophysics 2009, 36(5): 608~615 Computational Analysis of mirna and Target mrna Interactions: Combined Effects of The Quantity and Quality of Their
Circular RNAs (circrnas) act a stable mirna sponges
 Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
Cross species analysis of genomics data. Computational Prediction of mirnas and their targets
 02-716 Cross species analysis of genomics data Computational Prediction of mirnas and their targets Outline Introduction Brief history mirna Biogenesis Why Computational Methods? Computational Methods
02-716 Cross species analysis of genomics data Computational Prediction of mirnas and their targets Outline Introduction Brief history mirna Biogenesis Why Computational Methods? Computational Methods
Phenomena first observed in petunia
 Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
mirna Dr. S Hosseini-Asl
 mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
Identification of target genes of micrornas in retinoic acid-induced neuronal differentiation*
 Pure Appl. Chem., Vol. 77, No. 1, pp. 313 318, 2005. DOI: 10.1351/pac200577010313 2005 IUPAC Identification of target genes of micrornas in retinoic acid-induced neuronal differentiation* Hiroaki Kawasaki
Pure Appl. Chem., Vol. 77, No. 1, pp. 313 318, 2005. DOI: 10.1351/pac200577010313 2005 IUPAC Identification of target genes of micrornas in retinoic acid-induced neuronal differentiation* Hiroaki Kawasaki
MicroRNA target interactions: new insights from genome-wide approaches
 Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Nutrition and Physical Activity in Aging, Obesity, and Cancer MicroRNA target interactions: new insights from genome-wide
Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Nutrition and Physical Activity in Aging, Obesity, and Cancer MicroRNA target interactions: new insights from genome-wide
scientific report Defining a new role of GW182 in maintaining mirna stability scientificreport
 scientificreport Defining a new role of in maintaining mirna stability Bing Yao 1,w,LanB.La 1,Ying-ChiChen 2, Lung-Ji Chang 2 &EdwardK.L.Chan 1,+ 1 Department of Oral Biology, and 2 Department of Molecular
scientificreport Defining a new role of in maintaining mirna stability Bing Yao 1,w,LanB.La 1,Ying-ChiChen 2, Lung-Ji Chang 2 &EdwardK.L.Chan 1,+ 1 Department of Oral Biology, and 2 Department of Molecular
MicroRNAs, RNA Modifications, RNA Editing. Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM
 MicroRNAs, RNA Modifications, RNA Editing Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM Expanding world of RNAs mrna, messenger RNA (~20,000) trna, transfer
MicroRNAs, RNA Modifications, RNA Editing Bora E. Baysal MD, PhD Oncology for Scientists Lecture Tue, Oct 17, 2017, 3:30 PM - 5:00 PM Expanding world of RNAs mrna, messenger RNA (~20,000) trna, transfer
MicroRNA and Male Infertility: A Potential for Diagnosis
 Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
Review Article MicroRNA and Male Infertility: A Potential for Diagnosis * Abstract MicroRNAs (mirnas) are small non-coding single stranded RNA molecules that are physiologically produced in eukaryotic
V16: involvement of micrornas in GRNs
 What are micrornas? V16: involvement of micrornas in GRNs How can one identify micrornas? What is the function of micrornas? Elisa Izaurralde, MPI Tübingen Huntzinger, Izaurralde, Nat. Rev. Genet. 12,
What are micrornas? V16: involvement of micrornas in GRNs How can one identify micrornas? What is the function of micrornas? Elisa Izaurralde, MPI Tübingen Huntzinger, Izaurralde, Nat. Rev. Genet. 12,
Research Article Base Composition Characteristics of Mammalian mirnas
 Journal of Nucleic Acids Volume 2013, Article ID 951570, 6 pages http://dx.doi.org/10.1155/2013/951570 Research Article Base Composition Characteristics of Mammalian mirnas Bin Wang Department of Chemistry,
Journal of Nucleic Acids Volume 2013, Article ID 951570, 6 pages http://dx.doi.org/10.1155/2013/951570 Research Article Base Composition Characteristics of Mammalian mirnas Bin Wang Department of Chemistry,
Chapter 1 Understanding How mirnas Post-Transcriptionally Regulate Gene Expression
 Chapter 1 Understanding How mirnas Post-Transcriptionally Regulate Gene Expression Marc R. Fabian, Thomas R. Sundermeier, and Nahum Sonenberg Contents 1.1 Introduction... 2 1.1.1 Eukaryotic Translation...
Chapter 1 Understanding How mirnas Post-Transcriptionally Regulate Gene Expression Marc R. Fabian, Thomas R. Sundermeier, and Nahum Sonenberg Contents 1.1 Introduction... 2 1.1.1 Eukaryotic Translation...
High AU content: a signature of upregulated mirna in cardiac diseases
 https://helda.helsinki.fi High AU content: a signature of upregulated mirna in cardiac diseases Gupta, Richa 2010-09-20 Gupta, R, Soni, N, Patnaik, P, Sood, I, Singh, R, Rawal, K & Rani, V 2010, ' High
https://helda.helsinki.fi High AU content: a signature of upregulated mirna in cardiac diseases Gupta, Richa 2010-09-20 Gupta, R, Soni, N, Patnaik, P, Sood, I, Singh, R, Rawal, K & Rani, V 2010, ' High
Supplementary Figure 1
 Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Utility of Circulating micrornas in Cardiovascular Disease
 Utility of Circulating micrornas in Cardiovascular Disease Pil-Ki Min, MD, PhD Cardiology Division, Gangnam Severance Hospital, Yonsei University College of Medicine Introduction Biology of micrornas Circulating
Utility of Circulating micrornas in Cardiovascular Disease Pil-Ki Min, MD, PhD Cardiology Division, Gangnam Severance Hospital, Yonsei University College of Medicine Introduction Biology of micrornas Circulating
Date: Approved: Matthias Gromeier, Chair. Bryan Cullen. Christopher Nicchitta. Michael Hauser. Jack Keene
 The Role of Poly(A)-binding Protein in microrna-mediated Repression by Robert Wheeler Walters Department of Molecular Genetics and Microbiology Duke University Date: Approved: Matthias Gromeier, Chair
The Role of Poly(A)-binding Protein in microrna-mediated Repression by Robert Wheeler Walters Department of Molecular Genetics and Microbiology Duke University Date: Approved: Matthias Gromeier, Chair
SUPPLEMENTARY INFORMATION
 Table S1 Regulators of mirna processing and function Name Description Effect on mirnas Ref. Regulators of mirna processing p68/p72 SMADs SNIP1 DEAD-box RNA helicases Signal transducers; transcription regulators
Table S1 Regulators of mirna processing and function Name Description Effect on mirnas Ref. Regulators of mirna processing p68/p72 SMADs SNIP1 DEAD-box RNA helicases Signal transducers; transcription regulators
Small Interfering RNA-Mediated Translation Repression Alters Ribosome Sensitivity to Inhibition by Cycloheximide in Chlamydomonas reinhardtii
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations and Theses in Biological Sciences Biological Sciences, School of Spring 2013 Small Interfering RNA-Mediated
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations and Theses in Biological Sciences Biological Sciences, School of Spring 2013 Small Interfering RNA-Mediated
MicroRNAs Modulate Hematopoietic Lineage Differentiation
 MicroRNAs Modulate Hematopoietic Lineage Differentiation Harvey F. Lodish, Chang-Zheng Chen, David P. Bartel Whitehead Institute for Biomedical Research, Department of Biology, Massachusetts Institute
MicroRNAs Modulate Hematopoietic Lineage Differentiation Harvey F. Lodish, Chang-Zheng Chen, David P. Bartel Whitehead Institute for Biomedical Research, Department of Biology, Massachusetts Institute
Chapter 10 - Post-transcriptional Gene Control
 Chapter 10 - Post-transcriptional Gene Control Chapter 10 - Post-transcriptional Gene Control 10.1 Processing of Eukaryotic Pre-mRNA 10.2 Regulation of Pre-mRNA Processing 10.3 Transport of mrna Across
Chapter 10 - Post-transcriptional Gene Control Chapter 10 - Post-transcriptional Gene Control 10.1 Processing of Eukaryotic Pre-mRNA 10.2 Regulation of Pre-mRNA Processing 10.3 Transport of mrna Across
Cellular MicroRNA and P Bodies Modulate Host-HIV-1 Interactions. 指導教授 : 張麗冠博士 演講者 : 黃柄翰 Date: 2009/10/19
 Cellular MicroRNA and P Bodies Modulate Host-HIV-1 Interactions 指導教授 : 張麗冠博士 演講者 : 黃柄翰 Date: 2009/10/19 1 MicroRNA biogenesis 1. Pri mirna: primary mirna 2. Drosha: RNaseIII 3. DCR 1: Dicer 1 RNaseIII
Cellular MicroRNA and P Bodies Modulate Host-HIV-1 Interactions 指導教授 : 張麗冠博士 演講者 : 黃柄翰 Date: 2009/10/19 1 MicroRNA biogenesis 1. Pri mirna: primary mirna 2. Drosha: RNaseIII 3. DCR 1: Dicer 1 RNaseIII
Improved annotation of C. elegans micrornas by deep sequencing reveals structures associated with processing by Drosha and Dicer
 BIOINFORMATICS Improved annotation of C. elegans micrornas by deep sequencing reveals structures associated with processing by Drosha and Dicer M. BRYAN WARF, 1 W. EVAN JOHNSON, 2 and BRENDA L. BASS 1
BIOINFORMATICS Improved annotation of C. elegans micrornas by deep sequencing reveals structures associated with processing by Drosha and Dicer M. BRYAN WARF, 1 W. EVAN JOHNSON, 2 and BRENDA L. BASS 1
NOVEL FUNCTION OF mirnas IN REGULATING GENE EXPRESSION. Ana M. Martinez
 NOVEL FUNCTION OF mirnas IN REGULATING GENE EXPRESSION Ana M. Martinez Switching from Repression to Activation: MicroRNAs can Up-Regulate Translation. Shoba Vasudevan, Yingchun Tong, Joan A. Steitz AU-rich
NOVEL FUNCTION OF mirnas IN REGULATING GENE EXPRESSION Ana M. Martinez Switching from Repression to Activation: MicroRNAs can Up-Regulate Translation. Shoba Vasudevan, Yingchun Tong, Joan A. Steitz AU-rich
MicroRNA expression profiling and functional analysis in prostate cancer. Marco Folini s.c. Ricerca Traslazionale DOSL
 MicroRNA expression profiling and functional analysis in prostate cancer Marco Folini s.c. Ricerca Traslazionale DOSL What are micrornas? For almost three decades, the alteration of protein-coding genes
MicroRNA expression profiling and functional analysis in prostate cancer Marco Folini s.c. Ricerca Traslazionale DOSL What are micrornas? For almost three decades, the alteration of protein-coding genes
scientific report Kinetic analysis reveals successive steps leading to mirna-mediated silencing in mammalian cells scientificreport
 scientific report scientificreport Kinetic analysis reveals successive steps leading to mirn-mediated silencing in mammalian cells Julien Béthune 1+,CarolineG.rtus-Revel 1 & Witold Filipowicz 1,2++ 1 Friedrich
scientific report scientificreport Kinetic analysis reveals successive steps leading to mirn-mediated silencing in mammalian cells Julien Béthune 1+,CarolineG.rtus-Revel 1 & Witold Filipowicz 1,2++ 1 Friedrich
Novel RNAs along the Pathway of Gene Expression. (or, The Expanding Universe of Small RNAs)
 Novel RNAs along the Pathway of Gene Expression (or, The Expanding Universe of Small RNAs) Central Dogma DNA RNA Protein replication transcription translation Central Dogma DNA RNA Spliced RNA Protein
Novel RNAs along the Pathway of Gene Expression (or, The Expanding Universe of Small RNAs) Central Dogma DNA RNA Protein replication transcription translation Central Dogma DNA RNA Spliced RNA Protein
Marta Puerto Plasencia. microrna sponges
 Marta Puerto Plasencia microrna sponges Introduction microrna CircularRNA Publications Conclusions The most well-studied regions in the human genome belong to proteincoding genes. Coding exons are 1.5%
Marta Puerto Plasencia microrna sponges Introduction microrna CircularRNA Publications Conclusions The most well-studied regions in the human genome belong to proteincoding genes. Coding exons are 1.5%
The role of micrornas (mirna) in circadian rhythmicity
 c Indian Academy of Sciences REVIEW ARTICLE The role of micrornas (mirna) in circadian rhythmicity MIRKO PEGORARO and ERAN TAUBER* Department of Genetics, University of Leicester, University Road, Leicester
c Indian Academy of Sciences REVIEW ARTICLE The role of micrornas (mirna) in circadian rhythmicity MIRKO PEGORARO and ERAN TAUBER* Department of Genetics, University of Leicester, University Road, Leicester
Post-transcriptional regulation of an intronic microrna
 Post-transcriptional regulation of an intronic microrna Carl Novina Dana-Farber Cancer Institute Harvard Medical School Broad Institute of Harvard and MIT Qiagen Webinar 05-17-11 Outline 1. The biology
Post-transcriptional regulation of an intronic microrna Carl Novina Dana-Farber Cancer Institute Harvard Medical School Broad Institute of Harvard and MIT Qiagen Webinar 05-17-11 Outline 1. The biology
Identification of mirnas in Eucalyptus globulus Plant by Computational Methods
 International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 2 Issue 5 May 2013 PP.70-74 Identification of mirnas in Eucalyptus globulus Plant by Computational
International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 2 Issue 5 May 2013 PP.70-74 Identification of mirnas in Eucalyptus globulus Plant by Computational
Post-transcriptional and translational regulation of mrna-like long non-coding RNAs by micrornas in early developmental stages of zebrafish embryos
 BMB Reports BMB Rep. 2017; 50(4): 226-231 www.bmbreports.org Post-transcriptional and translational regulation of mrna-like long non-coding RNAs by micrornas in early developmental stages of zebrafish
BMB Reports BMB Rep. 2017; 50(4): 226-231 www.bmbreports.org Post-transcriptional and translational regulation of mrna-like long non-coding RNAs by micrornas in early developmental stages of zebrafish
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Noncoding or non-messenger RNAs are
 The functions of animal micrornas Victor Ambros Dartmouth Medical School, Department of Genetics, Hanover, New Hampshire 03755, USA (e-mail: vra@dartmouth.edu) MicroRNAs (mirnas) are small RNAs that regulate
The functions of animal micrornas Victor Ambros Dartmouth Medical School, Department of Genetics, Hanover, New Hampshire 03755, USA (e-mail: vra@dartmouth.edu) MicroRNAs (mirnas) are small RNAs that regulate
Removal of Shelterin Reveals the Telomere End-Protection Problem
 Removal of Shelterin Reveals the Telomere End-Protection Problem DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to
Removal of Shelterin Reveals the Telomere End-Protection Problem DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to
CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
 AD Award Number: W81XWH-05-1-0256 TITLE: Dicer in Mammary Tumor Stem Cell Maintenance PRINCIPAL INVESTIGATOR: Elizabeth P. Murchison CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring
AD Award Number: W81XWH-05-1-0256 TITLE: Dicer in Mammary Tumor Stem Cell Maintenance PRINCIPAL INVESTIGATOR: Elizabeth P. Murchison CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring
(,, ) microrna(mirna) 19~25 nt RNA, RNA mirna mirna,, ;, mirna mirna. : microrna (mirna); ; ; ; : R321.1 : A : X(2015)
 35 1 Vol.35 No.1 2015 1 Jan. 2015 Reproduction & Contraception doi: 10.7669/j.issn.0253-357X.2015.01.0037 E-mail: randc_journal@163.com microrna ( 450052) microrna() 19~25 nt RNA RNA ; : microrna (); ;
35 1 Vol.35 No.1 2015 1 Jan. 2015 Reproduction & Contraception doi: 10.7669/j.issn.0253-357X.2015.01.0037 E-mail: randc_journal@163.com microrna ( 450052) microrna() 19~25 nt RNA RNA ; : microrna (); ;
Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins.
 Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins. The RNA transcribed from a complex transcription unit
Alternative RNA processing: Two examples of complex eukaryotic transcription units and the effect of mutations on expression of the encoded proteins. The RNA transcribed from a complex transcription unit
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression. Chromatin Array of nuc
 Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
Transcriptional control in Eukaryotes: (chapter 13 pp276) Chromatin structure affects gene expression Chromatin Array of nuc 1 Transcriptional control in Eukaryotes: Chromatin undergoes structural changes
The regulation of genes and genomes by small RNAs
 MEETING REVIEW 1635 Development 134, 1635-1641 (2007) doi:10.1242/dev.002006 The regulation of genes and genomes by small RNAs Victor Ambros 1, * and Xuemei Chen 2 A recent Keystone Symposium on MicroRNAs
MEETING REVIEW 1635 Development 134, 1635-1641 (2007) doi:10.1242/dev.002006 The regulation of genes and genomes by small RNAs Victor Ambros 1, * and Xuemei Chen 2 A recent Keystone Symposium on MicroRNAs
MicroRNAs and Cancer
 MicroRNAs and Cancer MicroRNAs are an abundant class of small (20-25 nucleotides) non-protein coding RNAs that function as negative gene regulators. The human genome contains up to 1000 micrornas which
MicroRNAs and Cancer MicroRNAs are an abundant class of small (20-25 nucleotides) non-protein coding RNAs that function as negative gene regulators. The human genome contains up to 1000 micrornas which
MicroRNA-mediated incoherent feedforward motifs are robust
 The Second International Symposium on Optimization and Systems Biology (OSB 8) Lijiang, China, October 31 November 3, 8 Copyright 8 ORSC & APORC, pp. 62 67 MicroRNA-mediated incoherent feedforward motifs
The Second International Symposium on Optimization and Systems Biology (OSB 8) Lijiang, China, October 31 November 3, 8 Copyright 8 ORSC & APORC, pp. 62 67 MicroRNA-mediated incoherent feedforward motifs
Chapter 2. Investigation into mir-346 Regulation of the nachr α5 Subunit
 15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
MICRORNAS IN THE DROSOPHILA EGG AND EARLY EMBRYO
 MICRORNAS IN THE DROSOPHILA EGG AND EARLY EMBRYO by Sarah M. Votruba A thesis submitted in conformity with the requirements for the degree of Master of Science, Department of Molecular Genetics University
MICRORNAS IN THE DROSOPHILA EGG AND EARLY EMBRYO by Sarah M. Votruba A thesis submitted in conformity with the requirements for the degree of Master of Science, Department of Molecular Genetics University
MicroRNAs regulate de novo DNA methylation and histone mrna 3 end formation in mammalian cells
 MicroRNAs regulate de novo DNA methylation and histone mrna 3 end formation in mammalian cells Inauguraldissertation zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen
MicroRNAs regulate de novo DNA methylation and histone mrna 3 end formation in mammalian cells Inauguraldissertation zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen
Strategies to determine the biological function of micrornas
 Strategies to determine the biological function of micrornas Jan Krützfeldt, Matthew N Poy & Markus Stoffel MicroRNAs (mirnas) are regulators of gene expression that control many biological processes in
Strategies to determine the biological function of micrornas Jan Krützfeldt, Matthew N Poy & Markus Stoffel MicroRNAs (mirnas) are regulators of gene expression that control many biological processes in
Concordant Regulation of Translation and mrna Abundance for Hundreds of Targets of a Human microrna
 Concordant Regulation of Translation and mrna Abundance for Hundreds of Targets of a Human microrna David G. Hendrickson 1., Daniel J. Hogan 2,3., Heather L. McCullough 2,3,4., Jason W. Myers 2,3., Daniel
Concordant Regulation of Translation and mrna Abundance for Hundreds of Targets of a Human microrna David G. Hendrickson 1., Daniel J. Hogan 2,3., Heather L. McCullough 2,3,4., Jason W. Myers 2,3., Daniel
Implications of microrna-197 downregulated expression in esophageal cancer with poor prognosis
 Implications of microrna-197 downregulated expression in esophageal cancer with poor prognosis T.-Y. Wang 1, S.-G. Liu 2, B.-S. Zhao 2, B. Qi 2, X.-G. Qin 2 and W.-J. Yao 2 1 Department of Biochemistry
Implications of microrna-197 downregulated expression in esophageal cancer with poor prognosis T.-Y. Wang 1, S.-G. Liu 2, B.-S. Zhao 2, B. Qi 2, X.-G. Qin 2 and W.-J. Yao 2 1 Department of Biochemistry
Reduced Expression of Ribosomal Proteins Relieves MicroRNA-Mediated Repression
 Article Reduced Expression of Ribosomal Proteins Relieves MicroRNA-Mediated Repression Maja M. Janas,,, Eric Wang, 5,6 Tara Love,,,,8 Abigail S. Harris, 7 Kristen Stevenson, Karlheinz Semmelmann, 7 Jonathan
Article Reduced Expression of Ribosomal Proteins Relieves MicroRNA-Mediated Repression Maja M. Janas,,, Eric Wang, 5,6 Tara Love,,,,8 Abigail S. Harris, 7 Kristen Stevenson, Karlheinz Semmelmann, 7 Jonathan
Controlling mirna Regulation in Disease. Willemijn M. Gommans and Eugene Berezikov
 Chapter 1 Controlling mirna Regulation in Disease Willemijn M. Gommans and Eugene Berezikov Abstract Our understanding of the importance of noncoding RNA molecules is steadily growing. One such important
Chapter 1 Controlling mirna Regulation in Disease Willemijn M. Gommans and Eugene Berezikov Abstract Our understanding of the importance of noncoding RNA molecules is steadily growing. One such important
Removal of Shelterin Reveals the Telomere End-Protection Problem
 Removal of Shelterin Reveals the Telomere End-Protection Problem DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to
Removal of Shelterin Reveals the Telomere End-Protection Problem DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA in Cancer Karen Dybkær 2013
 MicroRNA in Cancer Karen Dybkær RNA Ribonucleic acid Types -Coding: messenger RNA (mrna) coding for proteins -Non-coding regulating protein formation Ribosomal RNA (rrna) Transfer RNA (trna) Small nuclear
MicroRNA in Cancer Karen Dybkær RNA Ribonucleic acid Types -Coding: messenger RNA (mrna) coding for proteins -Non-coding regulating protein formation Ribosomal RNA (rrna) Transfer RNA (trna) Small nuclear
MicroRNAs: Biogenesis and Molecular Functions
 Brain athology ISSN 1015-6305 SYMOSIUM: MicroRNAs: Biology and Roles in Neurodegeneration and Brain Tumors Xuhang Liu*; Kristine Fortin*; Zissimos Mourelatos Department of athology and Laboratory Medicine,
Brain athology ISSN 1015-6305 SYMOSIUM: MicroRNAs: Biology and Roles in Neurodegeneration and Brain Tumors Xuhang Liu*; Kristine Fortin*; Zissimos Mourelatos Department of athology and Laboratory Medicine,
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Ribo-gnome: The Big World of Small RNAs
 REVIEW Ribo-gnome: The Big World of Small RNAs Phillip D. Zamore* and Benjamin Haley Small RNA guides micrornas, small interfering RNAs, and repeat-associated small interfering RNAs, 21 to 30 nucleotides
REVIEW Ribo-gnome: The Big World of Small RNAs Phillip D. Zamore* and Benjamin Haley Small RNA guides micrornas, small interfering RNAs, and repeat-associated small interfering RNAs, 21 to 30 nucleotides
Small RNAs and how to analyze them using sequencing
 Small RNAs and how to analyze them using sequencing Jakub Orzechowski Westholm (1) Long- term bioinforma=cs support, Science For Life Laboratory Stockholm (2) Department of Biophysics and Biochemistry,
Small RNAs and how to analyze them using sequencing Jakub Orzechowski Westholm (1) Long- term bioinforma=cs support, Science For Life Laboratory Stockholm (2) Department of Biophysics and Biochemistry,
The influence of micrornas and poly(a) tail length on endogenous mrna protein complexes
 Rissland et al. Genome Biology (7 8: DOI.86/s359-7-33-z RESEARCH Open Access The influence of micrornas and poly(a tail length on endogenous mrna protein complexes Olivia S. Rissland,,3,4,5,6*, Alexander
Rissland et al. Genome Biology (7 8: DOI.86/s359-7-33-z RESEARCH Open Access The influence of micrornas and poly(a tail length on endogenous mrna protein complexes Olivia S. Rissland,,3,4,5,6*, Alexander
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
MicroRNAs: Critical Regulators of mrna Traffic and Translational Control with Promising Biotech and Therapeutic Applications
 Iranian Journal of Biotechnology. 2013 August; 11(3): 147-55. Published Online 2013 August 01. Review Article MicroRNAs: Critical Regulators of mrna Traffic and Translational Control with Promising Biotech
Iranian Journal of Biotechnology. 2013 August; 11(3): 147-55. Published Online 2013 August 01. Review Article MicroRNAs: Critical Regulators of mrna Traffic and Translational Control with Promising Biotech
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
UC San Diego UC San Diego Electronic Theses and Dissertations
 UC San Diego UC San Diego Electronic Theses and Dissertations Title MicroRNAs in the Making: Post-transcriptional Regulation of MicroRNA Biogenesis in Caenorhabditis elegans Permalink https://escholarship.org/uc/item/3f93318b
UC San Diego UC San Diego Electronic Theses and Dissertations Title MicroRNAs in the Making: Post-transcriptional Regulation of MicroRNA Biogenesis in Caenorhabditis elegans Permalink https://escholarship.org/uc/item/3f93318b
Eukaryotic mrna is covalently processed in three ways prior to export from the nucleus:
 RNA Processing Eukaryotic mrna is covalently processed in three ways prior to export from the nucleus: Transcripts are capped at their 5 end with a methylated guanosine nucleotide. Introns are removed
RNA Processing Eukaryotic mrna is covalently processed in three ways prior to export from the nucleus: Transcripts are capped at their 5 end with a methylated guanosine nucleotide. Introns are removed
Investigating the regulation of mirna biogenesis and Argonaute2 by RNA binding proteins
 Investigating the regulation of mirna biogenesis and Argonaute2 by RNA binding proteins Patrick Peter Connerty Supervisor: Gyorgy Hutvagner Thesis Submitted for the Degree of Doctor of Philosophy (Science)
Investigating the regulation of mirna biogenesis and Argonaute2 by RNA binding proteins Patrick Peter Connerty Supervisor: Gyorgy Hutvagner Thesis Submitted for the Degree of Doctor of Philosophy (Science)
DSB. Double-Strand Breaks causate da radiazioni stress ossidativo farmaci
 DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to the detection and repair of DNA damage DSBs induce a local decrease
DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to the detection and repair of DNA damage DSBs induce a local decrease
CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
 AD Award Number: W81XWH-06-1-0249 TITLE: Detection of genes modifying sensitivity to proteasome inhibitors using a shrna Library in Breast Cancer PRINCIPAL INVESTIGATOR: Gregory J. Hannon, Ph.D. CONTRACTING
AD Award Number: W81XWH-06-1-0249 TITLE: Detection of genes modifying sensitivity to proteasome inhibitors using a shrna Library in Breast Cancer PRINCIPAL INVESTIGATOR: Gregory J. Hannon, Ph.D. CONTRACTING
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD AWARD NUMBER: W81XWH-05-1-0256 TITLE: Dicer in Mammary Tumor Stem Cell Maintenance PRINCIPAL INVESTIGATOR: Xingyue He CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
AD AWARD NUMBER: W81XWH-05-1-0256 TITLE: Dicer in Mammary Tumor Stem Cell Maintenance PRINCIPAL INVESTIGATOR: Xingyue He CONTRACTING ORGANIZATION: Cold Spring Harbor Laboratory Cold Spring Harbor, NY 11724
Intrinsic cellular defenses against virus infection
 Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Small RNAs. RNA interference Identification and function
 Small RNAs RNA interference Identification and function Press Release - 2 October 2006 The Nobel Assembly at Karolinska Institutet decided to award The Nobel Prize in Physiology or Medicine for 2006 jointly
Small RNAs RNA interference Identification and function Press Release - 2 October 2006 The Nobel Assembly at Karolinska Institutet decided to award The Nobel Prize in Physiology or Medicine for 2006 jointly
A transient reversal of mirna-mediated repression controls macrophage activation
 Manuscript EMBOR-2013-37763 A transient reversal of mirna-mediated repression controls macrophage activation Anup Mazumder, Mainak Bose, Abhijit Chakraborty, Saikat Chakrabarti and Suvendra N. Bhattacharyya
Manuscript EMBOR-2013-37763 A transient reversal of mirna-mediated repression controls macrophage activation Anup Mazumder, Mainak Bose, Abhijit Chakraborty, Saikat Chakrabarti and Suvendra N. Bhattacharyya
scientific report A transient reversal of mirna-mediated repression controls macrophage activation scientificreport
 scientificreport A transient reversal of mirna-mediated repression controls macrophage activation Anup Mazumder 1,MainakBose 1, Abhijit Chakraborty 2, Saikat Chakrabarti 2 & Suvendra N. Bhattacharyya 1
scientificreport A transient reversal of mirna-mediated repression controls macrophage activation Anup Mazumder 1,MainakBose 1, Abhijit Chakraborty 2, Saikat Chakrabarti 2 & Suvendra N. Bhattacharyya 1
Impact of mirna Sequence on mirna Expression and Correlation between mirna Expression and Cell Cycle Regulation in Breast Cancer Cells
 Impact of mirna Sequence on mirna Expression and Correlation between mirna Expression and Cell Cycle Regulation in Breast Cancer Cells Zijun Luo 1 *, Yi Zhao 1, Robert Azencott 2 1 Harbin Institute of
Impact of mirna Sequence on mirna Expression and Correlation between mirna Expression and Cell Cycle Regulation in Breast Cancer Cells Zijun Luo 1 *, Yi Zhao 1, Robert Azencott 2 1 Harbin Institute of
RNA interference (RNAi)
 RN interference (RNi) Natasha aplen ene Silencing Section Office of Science and Technology Partnerships Office of the Director enter for ancer Research National ancer Institute ncaplen@mail.nih.gov Plants
RN interference (RNi) Natasha aplen ene Silencing Section Office of Science and Technology Partnerships Office of the Director enter for ancer Research National ancer Institute ncaplen@mail.nih.gov Plants
The meaning of nonsense
 Opinion The meaning of nonsense Lukas Stalder and Oliver Mühlemann Institute of Cell Biology, University of Berne, Baltzerstrabe 4, 3012 Berne, Switzerland To ensure the accuracy of gene expression, eukaryotes
Opinion The meaning of nonsense Lukas Stalder and Oliver Mühlemann Institute of Cell Biology, University of Berne, Baltzerstrabe 4, 3012 Berne, Switzerland To ensure the accuracy of gene expression, eukaryotes
scientific report ARE-mRNA degradation requires the decay pathway scientificreport
 scientificreport ARE-mRNA degradation requires the 5 0 3 0 decay pathway Georg Stoecklin +,ThomasMayo&PaulAnderson Division of Rheumatology, Immunology & Allergy, Brigham & Women s Hospital, Harvard Medical
scientificreport ARE-mRNA degradation requires the 5 0 3 0 decay pathway Georg Stoecklin +,ThomasMayo&PaulAnderson Division of Rheumatology, Immunology & Allergy, Brigham & Women s Hospital, Harvard Medical
Supplementary information for: Human micrornas co-silence in well-separated groups and have different essentialities
 Supplementary information for: Human micrornas co-silence in well-separated groups and have different essentialities Gábor Boross,2, Katalin Orosz,2 and Illés J. Farkas 2, Department of Biological Physics,
Supplementary information for: Human micrornas co-silence in well-separated groups and have different essentialities Gábor Boross,2, Katalin Orosz,2 and Illés J. Farkas 2, Department of Biological Physics,
Repressive Transcription
 Repressive Transcription The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Guenther, M. G., and R. A.
Repressive Transcription The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Guenther, M. G., and R. A.
Molecular Biology (BIOL 4320) Exam #2 May 3, 2004
 Molecular Biology (BIOL 4320) Exam #2 May 3, 2004 Name SS# This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses after the question number. Good
Molecular Biology (BIOL 4320) Exam #2 May 3, 2004 Name SS# This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses after the question number. Good
Small RNAs and Cross-Kingdom RNAi in Plant-Microbial Interaction Hailing Jin
 Small RNAs and Cross-Kingdom RNAi in Plant-Microbial Interaction Hailing Jin Department of Plant Pathology & Microbiology Institute for Integrative Genome Biology micrornas (mirnas) Small RNAs PolII PolIV
Small RNAs and Cross-Kingdom RNAi in Plant-Microbial Interaction Hailing Jin Department of Plant Pathology & Microbiology Institute for Integrative Genome Biology micrornas (mirnas) Small RNAs PolII PolIV
Molecular Mechanism of MicroRNA-203 Mediated Epidermal Stem Cell Differentiation
 University of Colorado, Boulder CU Scholar Undergraduate Honors Theses Honors Program Spring 2011 Molecular Mechanism of MicroRNA-203 Mediated Epidermal Stem Cell Differentiation Sarah Jackson University
University of Colorado, Boulder CU Scholar Undergraduate Honors Theses Honors Program Spring 2011 Molecular Mechanism of MicroRNA-203 Mediated Epidermal Stem Cell Differentiation Sarah Jackson University
Rajesh Singh Tomar et al /J. Pharm. Sci. & Res. Vol. 9(5), 2017, Amity Institute of Biotechnology, Amity University Madhya Pradesh, Gwalior
 In-Silico identification of mirnas and their targets using Expressed Sequence Tags (ESTs) in Plants Rajesh Singh Tomar, Gagan Jyot Kaur, Shuchi Kaushik, Raghvendra Kumar Mishra* Amity Institute of Biotechnology,
In-Silico identification of mirnas and their targets using Expressed Sequence Tags (ESTs) in Plants Rajesh Singh Tomar, Gagan Jyot Kaur, Shuchi Kaushik, Raghvendra Kumar Mishra* Amity Institute of Biotechnology,
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1 Negative correlation between mir-375 and its predicted target genes, as demonstrated by gene set enrichment analysis (GSEA). 1 The correlation between
SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1 Negative correlation between mir-375 and its predicted target genes, as demonstrated by gene set enrichment analysis (GSEA). 1 The correlation between
Supplementary Discussion 1: The mechanistic model of NIK1-mediated antiviral
 doi:10.1038/nature14171 SUPPLEMENTARY DISCUSSION Supplementary Discussion 1: The mechanistic model of NIK1-mediated antiviral signaling 1. Stress-induced oligomerization of the extracellular domain of
doi:10.1038/nature14171 SUPPLEMENTARY DISCUSSION Supplementary Discussion 1: The mechanistic model of NIK1-mediated antiviral signaling 1. Stress-induced oligomerization of the extracellular domain of
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
doi:10.1038/nature10643 Supplementary Table 1. Identification of hecw-1 coding polymorphisms at amino acid positions 322 and 325 in 162 strains of C. elegans. WWW.NATURE.COM/NATURE 1 Supplementary Figure
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Differential expression of mirnas from the pri-mir-17-92a locus.
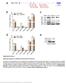 Supplementary Figure 1 Differential expression of mirnas from the pri-mir-17-92a locus. (a) The mir-17-92a expression unit in the third intron of the host mir-17hg transcript. (b,c) Impact of knockdown
Supplementary Figure 1 Differential expression of mirnas from the pri-mir-17-92a locus. (a) The mir-17-92a expression unit in the third intron of the host mir-17hg transcript. (b,c) Impact of knockdown
19958 JOURNAL OF BIOLOGICAL CHEMISTRY VOLUME 282 NUMBER 27 JULY 6, 2007
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 27, pp. 19958 19968, July 6, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Localization of AU-rich
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 27, pp. 19958 19968, July 6, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Localization of AU-rich
m 6 A mrna methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer
 SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41556-018-0174-4 In the format provided by the authors and unedited. m 6 A mrna methylation regulates AKT activity to promote the proliferation
SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41556-018-0174-4 In the format provided by the authors and unedited. m 6 A mrna methylation regulates AKT activity to promote the proliferation
Human Genome: Mapping, Sequencing Techniques, Diseases
 Human Genome: Mapping, Sequencing Techniques, Diseases Lecture 4 BINF 7580 Fall 2005 1 Let us review what we talked about at the previous lecture. Please,... 2 The central dogma states that the transfer
Human Genome: Mapping, Sequencing Techniques, Diseases Lecture 4 BINF 7580 Fall 2005 1 Let us review what we talked about at the previous lecture. Please,... 2 The central dogma states that the transfer
microrna-200b and microrna-200c promote colorectal cancer cell proliferation via
 Supplementary Materials microrna-200b and microrna-200c promote colorectal cancer cell proliferation via targeting the reversion-inducing cysteine-rich protein with Kazal motifs Supplementary Table 1.
Supplementary Materials microrna-200b and microrna-200c promote colorectal cancer cell proliferation via targeting the reversion-inducing cysteine-rich protein with Kazal motifs Supplementary Table 1.
MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions
 DOI: 10.4255/mcpharmacol.11.13 Molecular and Cellular Pharmacology www.mcpharmacol.com MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions Girish C. Shukla, Jagjit Singh and
DOI: 10.4255/mcpharmacol.11.13 Molecular and Cellular Pharmacology www.mcpharmacol.com MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions Girish C. Shukla, Jagjit Singh and
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
www.sciencesignaling.org/cgi/content/full/2/1/ra81/dc1 Supplementary Materials for Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12- Dependent Vascular Protection Alma Zernecke,* Kiril Bidzhekov,
Computational Identification and Prediction of Tissue-Specific Alternative Splicing in H. Sapiens. Eric Van Nostrand CS229 Final Project
 Computational Identification and Prediction of Tissue-Specific Alternative Splicing in H. Sapiens. Eric Van Nostrand CS229 Final Project Introduction RNA splicing is a critical step in eukaryotic gene
Computational Identification and Prediction of Tissue-Specific Alternative Splicing in H. Sapiens. Eric Van Nostrand CS229 Final Project Introduction RNA splicing is a critical step in eukaryotic gene
Supplemental Figure 1. Small RNA size distribution from different soybean tissues.
 Supplemental Figure 1. Small RNA size distribution from different soybean tissues. The size of small RNAs was plotted versus frequency (percentage) among total sequences (A, C, E and G) or distinct sequences
Supplemental Figure 1. Small RNA size distribution from different soybean tissues. The size of small RNAs was plotted versus frequency (percentage) among total sequences (A, C, E and G) or distinct sequences
THE EFFECT OF HSA-MIR-105 ON PROSTATE CANCER GROWTH
 THE EFFECT OF HSA-MIR-105 ON PROSTATE CANCER GROWTH by David Rice Honeywell Bachelor of Science, Queen s University, 2010 THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER
THE EFFECT OF HSA-MIR-105 ON PROSTATE CANCER GROWTH by David Rice Honeywell Bachelor of Science, Queen s University, 2010 THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER
