Valve interstitial cell activation and proliferation are associated with changes in β-catenin
|
|
|
- Gilbert Pope
- 5 years ago
- Views:
Transcription
1 Valve interstitial cell activation and proliferation are associated with changes in β-catenin by Songyi Xu A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Laboratory Medicine and Pathobiology University of Toronto Copyright by Songyi Xu, 2012
2 Valve interstitial cell activation and proliferation are associated with changes in β-catenin Abstract Songyi Xu Master of Science Department of Laboratory Medicine and Pathobiology University of Toronto 2012 Heart valve interstitial cells (VICs) undergo activation and proliferation in repair and disease, but the mechanisms are not fully understood. We hypothesize that the establishment of N- cadherin/β-catenin cell-cell contacts may decrease VIC activation, and that Wnt3a/β-catenin signaling may increase VIC proliferation. VIC cultures of different densities are stained for α- SMA, cofilin, TGF-β, psmad2/3, N-cadherin and β-catenin, and probed for phospho-β-catenin by Western blot. Low density VIC cultures are treated with exogenous Wnt3a and measured for cell number, proliferation, apoptosis, α-sma, β-catenin, and β-catenin-mediated transcription. β- Catenin sirna knockdown is used to assess β-catenin specificity. Increased staining of α-sma, cofilin, TGF-β, psmad2/3, nuclear β-catenin, and increased phospho-β-catenin are associated with few cell-cell contacts. Wnt3a increased VIC cell number, proliferation, nuclear β-catenin and β-catenin-mediated transcription without affecting activation and apoptosis, and proliferation is abolished by β-catenin sirna. Thus, N-cadherin/β-catenin cell-cell contacts may inhibit VIC activation and Wnt3a/β-catenin signaling may increase VIC proliferation. ii
3 Acknowledgments I was the first author of the published paper that is Chapter 2 (Xu S, Liu AC, Kim H, Gotlieb AI. Cell density regulates in vitro activation of heart valve interstitial cells. Cardiovasc Pathol [Epub ahead of print]) [170]. Hyunjun Kim and Amber Chang Liu carried out the α-sma and TGF-β studies and generated figures 1, 2 and 4. I carried out the cell contacts studies (Ncadherin, β-catenin and α-sma) as well as the psmad2/3, cofilin and phospho-β-catenin studies. I wrote the paper, handled all the reviewers comments, did additional experiments that were requested, and was responsible for the resubmission. I would like to thank Dr. Avrum I. Gotlieb for his scientific as well as personal guidance. I also appreciate the advice, guidance and support of my committee members Dr. Michelle Bendeck and Dr. Craig Simmons. As well, I would like to thank Dr. Alan Rosenthal for all his help and for keeping the lab running smoothly. In addition, I would like to thank Dr. Myron Cybulsky s lab for teaching me the Western blot and densitometry techniques. Moreover, I would like to thank Dr. Andras Kapus and his lab for helping me with the Western blot cell fractionation protocol. Also, I would like to thank Dr. Craig Simmons and his lab for helping me with the sirna transfection protocol. Furthermore, I would like to thank Dr. Benjamin Alman and his lab for providing me with the TOPFLASH, FOPFLASH and β-galactosidase DNA plasmids and helping me with the transfection and reporter assay techniques. Finally, I would not be where I am today without the love and support of my family and friends. Thank you. iii
4 Table of Contents Valve interstitial cell activation and proliferation are associated with changes in β-catenin... i Abstract... ii Acknowledgments... iii Table of Contents... iv List of Figures... vii List of Abbreviations... ix Chapter 1: Background The normal heart valve Heart valve disease Valve interstitial cells Activation Proliferation Apoptosis Role of TGF-β Role of cell-cell contacts Role of Wnt/β-catenin Rationale, hypothesis and general objective...12 Chapter 2: Cell density regulates in vitro activation of heart valve interstitial cells Introduction Materials and Methods VIC cultures Cell density and VIC activation Immunofluorescent staining and confocal microscopy...17 iv
5 2.2.4 Western blot analysis Statistical analysis Results α-sma expression in VICs decreases as cell density increases TGF-β signaling in VICs decreases concurrently with a decrease in α-sma expression as cell density increases Absence or loss of cell-cell contacts increases α-sma staining intensity Phospho-β-catenin is reduced in confluent VIC cultures Discussion...21 Chapter 3: Wnt3a/β-catenin increases proliferation in heart valve interstitial cells Introduction Materials and Methods Cell culture Treatment with exogenous Wnt3a Immunofluorescent staining and confocal microscopy Cell count Western blot Small interfering RNA transfection Quantification of proliferation Quantification of apoptosis TOPFLASH/FOPFLASH reporter assay Statistical analysis Results Wnt3a treatment increases β-catenin staining intensity in VICs Wnt3a treatment increases VIC cell number...32 v
6 3.3.3 Wnt3a treatment increases whole cell, cytosolic and nuclear protein levels of β- catenin Wnt3a treatment increases VIC proliferation; an effect dependent on β-catenin Wnt3a treatment does not affect VIC apoptosis Wnt3a treatment increases β-catenin-tcf-mediated transcription Discussion...33 Chapter 4: Discussion/Future Directions...35 References...39 vi
7 List of Figures Figure 1 TGF-β signaling pathways...64 Figure 2 Wnt/β-catenin signaling 65 Figure 3 Figure 4 Figure 5 Figure 6 α-sma staining decreases as cell density increases regardless of time in culture Percentage of VICs with High Intensity α-sma staining is less in high density cultures compared to low density cultures...67 Cofilin staining decreases as cell density increases...68 TGF-β staining decreases concurrently with α-sma staining as cell density increases...69 Figure 7 Nuclear Smad2/3 staining decreases as cell density increases...70 Figure 8 N-cadherin and β-catenin staining increases at cell-cell contacts, decreases in cytoplasm, and nuclear β-catenin staining disappears as cell density increases 71 Figure 9 Phospho-β-catenin is decreased in confluent cultures 72 Figure 10 β-catenin staining increases with Wnt3a treatment 73 Figure 11 Wnt3a treatment does not affect α-sma staining 74 Figure 12 Cell number appears to increase with Wnt3a treatment 75 Figure 13 Total cell count increases after Wnt3a treatment 76 Figure 14 Wnt3a does not affect cell viability 77 Figure 15 Wnt3a increases whole cell β-catenin level 78 vii
8 Figure 16 Wnt3a increases nuclear β-catenin level 79 Figure 17 Figure 18 Figure 19 β-catenin level decreases after sirna knockdown 80 Wnt3a increases VIC proliferation, an effect abolished by β-catenin sirna Wnt3a does not affect VIC apoptosis 82 Figure 20 Wnt3a increases β-catenin-mediated transcription 83 viii
9 List of Abbreviations AngII APC α-sma β-trcp BMP CAS angiotensin II adenomatous polyposis coli α-smooth muscle actin β-transducin repeat-containing protein bone morphogenetic protein calcific aortic stenosis CK1 casein kinase 1 ECM EGTA EMT GAG extracellular matrix ethylene glycol-bis (2-aminoethylether)-N, N N N -tetraacetic acid endothelial-to-mesenchymal transformation glycosaminoglycan GSK3 glycogen synthase kinase 3 MMP PBS PMSF PVDF TCF/LEF TGF-β matrix metalloproteinase phosphate buffered saline phenylmethylsulfonyl fluoride polyvinylidene fluoride T-cell factor/lymphoid enhancer factor transforming growth factor-β ix
10 VEC VIC avic obvic pvic qvic valve endothelial cell valve interstitial cell activated VIC osteoblastic VIC progenitor VIC quiescent VIC x
11 Chapter 1: Background 1
12 2 1.1 The normal heart valve The human heart has four chambers, an atrium and a ventricle on the right and the left side. The heart also has four valves, the tricuspid valve between the right atrium and right ventricle, the pulmonary valve between the right ventricle and the pulmonary artery, the mitral valve between the left atrium and left ventricle, and the aortic valve between the left ventricle and the aorta. Deoxygenated blood returns from circulation in the human body and fills the right atrium during diastole. When the pressure of the right atrium exceeds that of the right ventricle, the tricuspid valve opens, allowing the blood to flow into the right ventricle. When the right ventricle contracts during systole, the pulmonary valve opens and allows the blood to be pumped into the lungs for oxygenation. Similarly, oxygenated blood from the lungs fills the left atrium during diastole. When the pressure of the left atrium exceeds that of the left ventricle, the mitral valve opens, allowing the blood to flow into the left ventricle. When the left ventricle contracts during systole, the aortic valve opens to allow the blood to be pumped into the body. Contraction of the ventricles during systole exerts a back pressure on the tricuspid and mitral valves, causing them to close tightly. Similarly, after ejection of blood, back pressure from the blood in the pulmonary artery and aorta causes the pulmonary and aortic valves to close rapidly and completely, preventing backflow. The valves of the human heart are flow-regulating membranes inside a complex multichambered pump. The connective tissue of valves serves to maintain proper structure [1,2] and a heterogeneous population of constituent cellular elements regulates complex functions to give flexibility and durability to the leaflets [3]. The four types of cells found in valves are surface valve endothelial cells (VECs), valve interstitial cells (VICs), and towards the base of the valve, cardiac muscle cells and smooth muscle cells [4,5]. A confluent monolayer of VECs lines the surface of the valve [6]. Underneath the endocardium are VICs embedded in matrix which they secrete and which form the three histologically distinct layers of the valve the fibrosa, the spongiosa, and the ventricularis [6]. The fibrosa is rich in densely packed collagen fibers; the spongiosa is composed mainly of glycosaminoglycans (GAGs) and proteoglycans; and the ventricularis contains collagen, elastin and GAGs [7,8]. These provide the valve with a zone of stiffness, a zone which is compressible, and a zone which is elastic in nature, respectively [3].
13 3 The fibrosa with its dense connective tissue provides strength when the valve is shut, the spongiosa with its loose matrix of glycoproteins provides a cushion for the physical forces and mechanical strains and valve movement, and the ventricularis provides for elasticity when the cusp changes shape during the cardiac cycle. All three layers are avascular [9]. Heart valves are remarkably adapted to allow unidirectional and non-obstructed passage of blood without regurgitation, trauma to blood elements, thromboembolism, or excessive stress concentrations in the cuspal/leaflet or supporting tissue [7]. Valves are biologically dynamic structures, capable of considerable functional remodeling and repair of injury [7]. 1.2 Heart valve disease Heart valve disease results from valve dysfunction due to stenosis and/or regurgitation leading to progressive cardiac disease [10]. Heart valve disease has several different etiologies including congenital, genetic, infectious, inflammatory, drug-induced, and calcific [8,11-14]. It manifests as several different clinical conditions including calcific aortic stenosis (CAS), bicuspid aortic valve stenosis, acute and chronic rheumatic valve disease, valve prolapse (e.g. mitral valve prolapse), and annular calcification [8,15,16]. In many cases, the only available treatment is surgical intervention with heart valve replacement. In populations with access to costly cardiovascular surgery, over 285,000 patients undergo replacement worldwide annually, but 60% suffer from valve-related complications by 10 years after surgery [17]. Bicuspid aortic valve stenosis is the most common congenital heart defect [18]. The bicuspid valve typically consists of 2 unequal-sized leaflets, with the larger leaflet having a central ridge that results from fusion of cusps [18]. Bicuspid aortic valve stenosis is generally found with other cardiovascular defects such as coarctation of the aorta, ventricular septal defects, and atrial septal defects, suggesting a global disorder of cardiac development as the basis of the disease [18]. Symptoms typically develop in adulthood, and they include aortic valve stenosis, aortic valve incompetence, aortic valve calcification, aortic root dilation, and aortic root dissection [18]. Rheumatic valve disease develops after repeated episodes of acute rheumatic fever and affects primarily the mitral and aortic valves [16]. It results from an abnormal autoimmune response to bacterial antigens that cross-react with host connective tissue antigens, which leads to connective
14 4 tissue degeneration as part of the inflammatory reaction [16]. The inflammation triggers repair processes of the valve, leading to fibrosis, calcification, and eventual valve dysfunction [16]. In mitral valve prolapse, there is abnormal displacement of the valve leaflets into the left atrium during systole resulting in regurgitation and decreased cardiac output [16]. This is caused by myxomatous degeneration of the valve tissue characterized by an excessive accumulation of GAGs, loss of VICs and collagen, and elastic fragmentation [13,19]. CAS is the most common heart valve condition in adults in Western society [20]. It is a condition with high morbidity and mortality that is very costly to the health care system [21-23]. It is a chronic disease that is slowly progressive and has precursor lesions that may remain asymptomatic for some time [24]. During the initial stages of the disease, aortic valve sclerosis occurs which results in cusp thickening without creating obstruction to the left ventricular outflow [24]. This gradually progresses to CAS, obstructing flow [24]. Severe symptomatic aortic stenosis is associated with a life expectancy of less than 5 years [25]. While 20-30% of individuals over the age of 65 and 48% of individuals over 85 are affected by sclerosis, only 2% of individuals over 65 and 4% of individuals over 85 end up with CAS [20,22,26]. Risk factors for CAS include hypertension, elevated low-density lipoprotein, male gender, smoking, and diabetes mellitus [20], which are similar to those of atherosclerosis [27]. Statins, well-known therapeutic agents that target atherosclerosis, are being tested in prospective clinical trials on CAS, but the results are so far disappointing [28-31]. CAS is no longer considered to occur due to passive degeneration secondary to aging. Research has shown that the pathogenesis involves active cell and tissue processes that occur in a tissue response to injury including VIC accumulation, inflammation, neovascularization, oxidative stress, matrix remodeling, and calcification and osteogenesis [24,32-36]. These processes develop as the valve cusps thicken due to fibrosis. Irregular calcified nodules develop initially at sites exposed to high mechanical force in the fibrosa layer of the valve [27]. Typically, calcified nodules consist of calcium-phosphate mineral deposits, and a subset of end-stage human aortic valve lesions has been shown to contain bone [37-40]. Much research has focused on the pathogenesis of calcific aortic stenosis in an attempt to gain greater understanding to improve current treatments. VICs appear to increase their expression of osteoblast markers and increase
15 5 their rate of calcified nodule formation in response to a number of atherogenic factors [39,41,42]. Oxidized lipids have been found in areas of developing calcification and are shown to stimulate calcified nodule formation in vitro [39,43]. In addition, angiotensin II (AngII) is present in aortic valve lesions and has numerous lesion-promoting effects such as stimulating inflammation and macrophage-cholesterol accumulation, increasing oxidant stress, and stimulating expression of lipoprotein-retaining proteoglycan, biglycan [44-46]. There is a number of potential sources of AngII in diseased valves, including low density lipoprotein-associated angiotensin converting enzyme, macrophage-associated angiotensin converting enzyme, and mast cell chymase [44,47]. The major pathogenic receptor for AngII, the AngII type I receptor, is only present in VICs in valve lesions [44,47], providing further evidence supporting the possibility that AngII may play an important role in the pathogenesis of CAS. Furthermore, aortic valve lesions contain macrophages and T-lymphocytes that produce matrix metalloproteinases (MMPs) and proinflammatory cytokines such as interleukin-1β, tumour necrosis factor-α, transforming growth factor-β1 (TGF-β1), and bone morphogenetic protein 2 (BMP2), that promote calcification [39,42,48-54]. BMP2, present in human aortic valve lesions, can stimulate calcified nodule formation by upregulating transcription factor Msx2 which activates Wnt signaling or by upregulating the osteoblast-specific transcription factor Runx2/Cbfa1, which has been detected in calcified valves [33,55-58]. As well, mutations in Notch1, a transcription factor that normally represses Runx2/Cbfa1, are implicated in valve calcification [59]. It is possible that specific signaling mechanisms may be involved in valve calcification. Even though each heart valve disease has distinct gross and microscopic features, there are several pathological features that are common to most heart valve diseases, including VIC proliferation, inflammation, changes in the valve matrix, fibrosis, and eventual calcification. Despite research efforts focused on understanding the pathogenesis of valve disease, the knowledge gained has not been sufficient to lead to better prevention and treatment. 1.3 Valve interstitial cells Heart valve disease is considered to be a response to tissue injury that becomes excessive and leads to disruption of the leaflets/cusps with excessive remodeling, scarring, and calcification instead of proper cusp/leaflet repair. The tissue and cell processes important in valve function,
16 6 dysfunction and tissue engineering are best studied and understood as a response to tissue injury [5,60]. This response may be regulated by VICs, the most prevalent cells in the valve. They originate by endothelial-to-mesenchymal transformation (EMT) in the endocardial cushion and are primarily regulated by BMPs, TGF-β, Notch, and Wnt signaling in development [61]. BMPs and TGF-β play important roles in the initiation of endocardial cushion formation and EMT as well as the growth of endocardial cushions [62-75]. Wnt is important in endocardial cushion formation and EMT, especially the regulation of proliferation in endocardial cushion growth [70,76-78]. Notch plays a key role in EMT, particularly in the migration of endothelial cells into the cardiac jelly to become mesenchymal cells that will eventually form the mature heart valve [79,80]. Evidence shows that these developmental pathways, normally downregulated in adulthood, can become activated again in disease [81]. In general, there are five phenotypes that best represent the VIC family of cells, each of which exhibits specific sets of cellular functions essential in normal valve physiology and in pathological processes [32]. They include embryonic progenitor endothelial/mesenchymal cells, quiescent VICs (qvics), activated VICs (avics), progenitor VICs (pvics), and osteoblastic VICs (obvics) [32]. The embryonic progenitor endothelial/mesenchymal cells undergo EMT that initiates the process of valve formation in the embryo [82]. The qvics are the cells that are at rest in the adult valve and maintain normal valve physiology [4,83-85]. The avics are the cells that regulate the pathobiological responses of valve in disease and injury [86-88]. The pvics are the least well-defined and consist of a heterogeneous population of progenitor cells that may be important in repair [89-93]. The obvics regulate chondrogenesis and osteogenesis [24]. These phenotypes may exhibit plasticity and convert from one form to another [32] Activation Of particular interest are qvics that maintain normal structure and function in healthy adult valves as well as avics which regulate numerous processes in diseased or injured valves [32,94]. qvics become avics under conditions of pathological injury or abnormal hemodynamic/mechanical stress in which activated VECs and macrophages/foam cells arise, and a number of chemokines and growth factors stimulate qvic activation [32]. Activation of VICs is associated with increased ECM secretion and degradation, proliferation, migration, and
17 7 cytokine production, which are all important features of the wound repair process [60]. avics take on the features of myofibroblasts showing increased contraction, prominent actin stress fibers, and other contractile proteins [86]. The marker for avic is α-smooth muscle actin (α- SMA), a cytoskeletal isoform of actin, which is normally not found in qvic [32]. The assembly of α-sma into stress fibers is important for cell migration, force generation, and wound contracture both in vitro and in vivo [95,96]. Diseased heart valves show upregulation of α-sma staining in VICs [24,97-100]. Also, increased α-sma expression and stress fiber formation are observed in VICs along an in vitro wound edge of a wound made linearly across a confluent monolayer, suggesting that wounding activates the qvics [101]. avics likely play a key role in the pathogenesis of human clinical disease when the response to tissue injury becomes excessive and leads to disruption of the leaflets/cusps with excessive remodeling, scarring, and calcification through unknown mechanisms [8]. Thus, understanding the regulation of VIC activation and the associated cellular responses is critical to understanding the pathobiology of heart valve diseases Proliferation Under normal conditions, there is very little or no proliferation in adult valves [102]. The level of proliferation is much higher in heart valve development during cardiac cushion formation, but it is down-regulated in adult [103]. Evidence suggests that proliferation is increased in diseased valves, such as in areas around calcified nodules [102]. As well, in studies where organ cultures of porcine mitral valves are wounded with a linear superficial wound on the valve surface, increased proliferation is observed in wound edge VICs [104]. Furthermore, wound edge VICs along an in vitro scratch wound made linearly at the center of a confluent monolayer also exhibit increased proliferation compared to areas away from the wound edge [101]. Proliferation of fibroblasts and myofibroblasts has been shown to be inhibited by low doses of nitric oxide [105]. These findings suggest that proliferation is likely to be a response to tissue injury that leads to disease instead of successful repair when dysregulated. Thus, understanding the regulation of VIC proliferation is important to understanding valve function and disease.
18 Apoptosis Increased apoptosis is observed in wound edge VICs along an in vitro wound made linearly in the center of a confluent monolayer, indicating that cell turnover may be an important aspect of valve wound repair [101]. As cell populations rapidly proliferate during tissue reconstruction and remodeling in wound repair, proliferation is balanced by apoptosis. In addition, after completion of remodeling, many avics need to be eliminated by apoptosis [106]. In dermal wound healing, apoptosis is increased at the wound after wounding to eliminate dead or dying cells and to terminate regeneration and remodeling after healing is complete [107]. Dysregulation of apoptosis in wound healing can lead to a number of pathological processes, most notably hypertrophic scarring and keloid formation, two forms of hyperproliferative healing characterized by hypervascularity and hypercellularity [108,109]. In the same manner, if VICs did not undergo apoptosis, excessive ECM deposition and fibrosis would likely occur leading to valve scarring and dysfunction [101]. Thus, the regulation of VIC apoptosis is an important aspect of valve injury and disease. 1.4 Role of TGF-β The TGF-β superfamily of proteins is a family of cytokines and peptide growth factors that regulate biological functions in many systems including heart valves [27, Figure 1]. TGF-β1 is the predominant isoform of TGF-β exerting biological effects in the adult human and pig heart valve [101]. Activated TGF-β1 binds strongly to receptor type II, a serine/threonine kinase that phosphorylates the glycine-rich domain on receptor type I, which is another serine/threonine kinase that can then phosphorylate other downstream signaling molecules [110]. The best characterized signaling pathway involves the phosphorylation of Smad2 and Smad3 on the C- terminal Ser-Ser-X-Ser motif by receptor type I [110]. Once phosphorylated, Smad2 and Smad3 dissociate from the receptor and form a complex with Smad4 [110]. Afterwards, the Smad molecules can translocate to the nucleus to regulate gene expression [110]. TGF-β is secreted by numerous cell types including VICs with potent autocrine effects, one of which is VIC activation [101, ]. α-sma mrna and protein were upregulated concurrently with increased TGF-β in wound edge VICs along an in vitro wound [101]. Also, in
19 9 the same study, TGF-β neutralization reduced α-sma protein, mrna, and stress fibers whereas exogenous addition of TGF-β increased α-sma protein, mrna, and stress fibers [101]. TGF-β has also been found to induce α-sma protein and stress fiber expression in porcine aortic VICs cultured at low-cell density on collagen substrates [114]. In fibroblasts, TGF-β stimulated α- SMA gene expression through the TGF-β control element at -42 to -61 from the transcriptional start site of the α-sma promoter [115,116]. VIC activation by TGF-β induces dramatic augmentation of stress fiber formation and alignment leading to enhanced contractility, an important feature of wound healing [32,112]. Diseased valves exhibit increased levels of TGF-β. TGF-β is present in mitral valve prolapse [97,117] and CAS [98,118,119]. In addition, increased TGF-β and phosphorylated Smad2/3 were observed in wound edge VICs along an in vitro wound, and increased TGF-β concentration was detected in VIC-conditioned media collected at 24 hours after wounding [101]. Findings both in vivo and in vitro indicate that TGF-β is an important regulator of VIC activation and a key cytokine in valve repair and disease processes. 1.5 Role of cell-cell contacts Cell-cell adhesion is involved in all aspects of tissue morphogenesis [120,121]. Adhesive forces must be both resilient to maintain tissue architecture and dynamic to establish new contacts during wound repair [122]. Although on histological examination of the normal valve, VICs appear to reside as single cells, ultrastructural studies show that VICs extend cytoplasmic processes which adhere to neighbouring cells forming adherens junctions [123]. These adherens junctions are also present in confluent monolayers of VICs in cell culture [123]. Research shows that N-cadherin and β-catenin are present at the adherens junctions of VICs [124,125]. N- cadherin, a classical cadherin molecule, is a transmembrane glycoprotein that promotes calcium dependent homophilic binding with adjacent N-cadherin molecules on neighbouring cells via its extracellular domain [126,127]. It links to the cytoskeleton via molecules including β-catenin that bind to its cytoplasmic domain [126,127]. When freed from cell-cell contacts, β-catenin can also act as a transcriptional co-activator and bind to members of the T cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors in the nucleus [128].
20 10 Evidence suggests that cell-cell contacts are active regulators of epithelial-to-mesenchymal transition in epithelial and tumour cells, a key feature of which is α-sma expression [ ]. The loss of E-cadherin, a classical cadherin molecule found at adherens junctions of epithelial and some tumour cells, has been shown to promote epithelial-to-mesenchymal transition, while forced E-cadherin expression can restore the epithelial phenotype in transformed tumour cells [ ]. Cell contact-dependent activation of α-sma expression is also observed in kidney epithelial cells [135,136]. Furthermore, cell density, or the extent of cell-cell contacts, has been shown to regulate the cellular localization and transcriptional activity of β-catenin [137,138], and β-catenin signaling has been implicated in the induction and progression of epithelial-tomesenchymal transition in tumour cells [ ]. The role of cell-cell contacts in regulating VIC function has never been investigated before. Previous research from our lab shows that wound edge VICs along a linear mechanical injury in a confluent monolayer become activated and express α-sma, while VICs located away from the wound edge or VICs in non-wounded confluent monolayers are quiescent [101]. In addition, we reported that single VICs cultured at low density are activated and express α-sma [32]. It is possible that, similar to epithelial-to-mesenchymal transition, the disruption or absence of cellcell contacts in VICs may allow β-catenin to translocate to the nucleus and activate VICs and initiate early repair processes, while the establishment of cell-cell junctions may prevent β- catenin nuclear translocation and induce VIC quiescence. It is important to study the effects of N-cadherin/β-catenin cell-cell contacts on VIC activation because adherens junctions between VICs are disrupted in injury and disease. Since cadherin/β-catenin cell-cell contacts are important regulators of epithelial-to-mesenchymal transition, a key feature being the expression of α-sma, they might also regulate the activation of VICs. 1.6 Role of Wnt/β-catenin Free cytoplasmic β-catenin is a well-known mediator of the Wnt signaling pathway (Figure 2). For comprehensive reviews of the Wnt/β-catenin pathway, please refer to Logan and Nusse 2004, Clevers 2006, MacDonald et al, 2009, and Rao and Kuhl As well, updated information on Wnt signalling can be found on the Wnt homepage at Normally, the level of free cytoplasmic β-
21 11 catenin is kept low by proteasomal degradation. Upon binding of Wnt to its receptors, cytoplasmic β-catenin is stabilized and can travel to the nucleus to bind to the TCF/LEF family of proteins and mediate transcription. More details on the Wnt/β-catenin signaling pathway are covered in Chapter 3 of this thesis. Besides the canonical Wnt/β-catenin pathway, Wnt can also activate non-canonical pathways that are β-catenin-independent. The Wnt/JNK pathway regulates the establishment of planar cell polarity and involves the activation of Rho GTPases including rac, cdc42 and rho [143]. Wnt is also able to increase intracellular Ca 2+ and activate Ca 2+ -dependent enzymes such as protein kinase C important for cardiac differentiation, calcineurin that regulates gene expression through nuclear factor of activated T cells transcription factor, and calcium/calmodulin-dependent kinase II that activates nemo-like kinase and regulates histone acetylation and methylation [ ].The Wnt/β-catenin pathway has been implicated in the development of many tissues and organs, including heart valves where it was shown to regulate proliferation and EMT [103,70,76]. Several members of the Wnt family of proteins are shown to be involved in heart valve development, including Wnt2, Wnt3a, Wnt4, Wnt5b, Wnt7b, and Wnt9b [103]. Dysregulation of the canonical Wnt/β-catenin pathway has been implicated in many diseases, the most well-known being colorectal cancer, which results from constitutively activated β-catenin signaling [162]. Due to APC deficiency or β-catenin mutations that prevent its degradation, β-catenin-dependent transcription is upregulated in colon carcinoma cells, promotes cyclin D1 expression, and induces cell proliferation [164]. The role of β-catenin in cell proliferation has been well established in many cell types and under numerous conditions. For instance, hyperplastic cutaneous wounds contain prolonged periods of elevated β-catenin and increased β-catenin-dependent transcription [165]. Moreover, β-catenin has been shown to induce proliferation of vascular smooth muscle cells via increased cyclin D1 and decreased p21 levels [166]. Furthermore, in proliferating human keratinocytes, there is increased cytoplasmic/nuclear fraction of β-catenin [137]. In heart valves, increased expression of Wnt receptor Lrp5, stabilized β-catenin, and Wnt3 have been associated with CAS [167,168]. Since Wnt3 is specifically upregulated in disease and Wnt3a is among the Wnt proteins important in endocardial cushion formation, EMT and proliferation in valve developement, it is possible that the Wnt3a/β-catenin pathway may be activated in heart valve disease to increase VIC
22 12 proliferation as a response to injury. However, the regulation of the Wnt/β-catenin pathway on VIC proliferation in adulthood has not been investigated. 1.7 Rationale, hypothesis and general objective Our lab has been studying an in vitro model of VIC repair utilizing paradigms which are derived from findings in in vivo injured and repairing heart valves. Two important features of valve repair are VIC activation and VIC proliferation, both of which are upregulated in disease conditions [32]. The observation that VICs are activated only in low density cultures or along an in vitro wound edge suggests that the absence or loss of cell-cell contacts may stimulate VIC activation, while the establishment of cell-cell contacts may induce VIC quiescence [101]. Cellcell contact disassembly is found to induce β-catenin nuclear translocation and epithelial-tomesenchymal transition in epithelial and tumour cells, a key feature of which is α-sma expression, while the establishment of contact is shown to reverse the transition [ ]. When freed from cadherin contacts, β-catenin can travel to the nucleus and act as a transcriptional regulator [169]. It is possible for cell-cell contacts in VICs, which contain N- cadherin and β-catenin, to regulate VIC activation. This is worth investigating because cell-cell contacts between VICs are disrupted in injury and disease and may result in β-catenin nuclear translocation and act as an initiating signal to activate VICs to initiate wound repair. Cytoplasmic β-catenin is normally kept low through proteosomal degradation. It can be stabilized by Wnt and mediates the downstream signaling and transcriptional regulation of the Wnt/β-catenin pathway. The Wnt/β-catenin pathway is involved in many disease processes where it results in excessive proliferation [137, ]. Increased expression of Wnt receptor Lrp5, Wnt3 and β-catenin in diseased valves suggests that Wnt/β-catenin signaling may increase VIC proliferation in valve diseases as a repair response to injury. I hypothesize that N-cadherin/β-catenin cell-cell contacts regulate VIC activation and Wnt/βcatenin signaling regulates VIC proliferation. My objective is to study VIC activation in cultures of different densities, and therefore different degrees of cell-cell contact formation, as well as to study VIC proliferation and its regulation by Wnt3a/β-catenin signaling in low density cultures. To study cell-cell contacts and VIC activation, in cultures of different densities, I will observe α-
23 13 SMA and cofilin as markers of VIC activation, TGF-β and psmad2/3 as activation of the TGF-β pathway that regulates VIC activation, and N-cadherin and β-catenin in VIC adherens junctions to study the correlation between cell density and VIC activation. To study Wnt3a, β-catenin and VIC proliferation, I will treat low density VIC cultures with exogenous Wnt3a and measure the effects on total cell count, proliferation, apoptosis, β-catenin protein, and β-catenin-mediated transcription. I will also transfect VICs with sirna targeted against β-catenin to ensure that the effects observed are β-catenin-specific. Understanding the regulation of VIC activation by N- cadherin/β-catenin cell-cell contacts and proliferation by Wnt3a/β-catenin signaling will provide insight into the function of VICs and the pathobiology of heart valve diseases.
24 14 Chapter 2: Cell density regulates in vitro activation of heart valve interstitial cells (This is published Xu S, Liu AC, Kim H, Gotlieb AI. Cell density regulates in vitro activation of heart valve interstitial cells. Cardiovasc Pathol [Epub ahead of print] with minor modifications.) [170]
25 Introduction The regulation of cardiac valve function in both health and disease remains a mystery. To address this, in the last few years, studies on the cell and molecular biology of valve function have been initiated using several different in vitro model systems. One of the early successes has been the characterization of valve interstitial cell (VIC) phenotypes that regulate normal physiology and disease conditions [32,171,172]. VICs are the most prominent cell type in the heart valve and are known to have important roles in wound repair and heart valve disease [60,173]. In diseased heart valves, VICs are activated, characterized by α-smooth muscle actin (α-sma) expression, which is absent in normal heart valves [94,97,101,174]. In vitro studies show that VICs become activated upon wounding of a confluent monolayer and become quiescent upon repair of the wound [101]. In the earliest stages of wound repair, activated VICs at the wound edge undergo proliferation and apoptosis, as well as form α-sma stress fibers, leading to wound closure and repair 101]. Activated VICs require transforming growth factor β (TGF-β) to maintain activation through promoting α-sma protein expression [101,112]. Treatment with TGF-β neutralizing antibody leads to reduced VIC activation, α-sma messenger RNA production, proliferation, apoptosis, α-sma stress fibers formation, and wound closure, decreasing VIC wound repair [101]. Addition of exogenous TGFβ increases proliferation, stress fiber formation, and wound closure, enhancing VIC wound repair [101]. Thus understanding the factors that regulate VIC activation is important in understanding how repair occurs and how the structure and function of the valve leaflet are restored. We hypothesize that several conditions including cell density, time in culture, and the establishment of cell-cell contacts containing N-cadherin and β-catenin [124] are involved in regulating VIC activation in vitro and that this activation is associated with the presence of TGF-β. 2.2 Materials and Methods VIC cultures Porcine heart valves were obtained from a local abattoir, and explants were prepared from the distal third of the anterior leaflet of porcine mitral valves as previously described [83]. The atrial and ventricular surfaces of the explants were scraped with a scalpel blade and rinsed with
26 16 phosphate buffered saline (PBS ph 7.4) to remove valve endothelial cells. The explants were cut into 4x5-mm pieces, placed in 9-cm tissue culture dishes (Falcon; BD Biosciences, San Jose, CA, USA), and grown in standard medium, medium 199 (M-199), supplemented with 10% fetal bovine serum (FBS), and 2% penicillin, streptomycin, and Fungizone (GIBCO BRL, Life Technologies Inc, Rockville, MD, USA) in a humidified 95% air and 5% carbon dioxide atmosphere in an incubator at 37 C. VICs that grew out of the explants were detached with TrypLE Express (GIBCO; Invitrogen Corporation, Carlsbad, CA, USA) and subcultured. VICs between passages 3 to 5 were used Cell density and VIC activation VICs were plated on 22x22-mm glass coverslips (Corning Incorporated, Corning, NY, USA) in 35-mm tissue culture dishes at 17,000 cells per coverslip. VICs were incubated for 1, 2, 4, 7, and 10 days in standard media and were fixed and stained for α-sma. To rule out that time in culture to reach confluency affects activation, cells were plated at a 10-fold higher density in order to reach confluency more quickly. The extent of activation marked by staining of VICs plated at 170,000 cells per coverslip was compared to those plated at the lower density of 17,000 cells per coverslip at the same time points of 1, 2, 4, 7, and 10 days. To complement and confirm the activation of VICs indicated by α-sma staining, VICs were plated at 17,000 cells per coverslip; cultured for 2, 5, 8 and 14 days post-plating; fixed; and stained for cofilin. Cofilin is an actin binding protein that depolymerizes actin, which is an important process in actin turnover [175]. Recently, cofilin has been shown to play a role in α- SMA stress fiber formation, to increase in expression in activated VICs with prominent α-sma stress fiber expression, and to colocalize with α-sma in diseased valves [114]. To study the association of TGF-β with cell density and VIC activation, VICs were plated at 17,000 cells per coverslip; cultured for 2, 4, 6, and 8 days post-plating; fixed; and double stained for α-sma and TGF-β. To study the activation of the TGF-β signaling pathway, VICs were plated at 17,000 cells per coverslip; cultured for 2, 4, 6, 8, 10, and 12 days post-plating; fixed; and stained for psmad2/3.
27 17 To study the association of cell-cell contacts with VIC activation, subconfluent (6-7 days postplating) and confluent monolayers (10-11 days post-plating) of VICs were fixed and stained for β-catenin, N-cadherin, and α-sma Immunofluorescent staining and confocal microscopy VICs were fixed with 4% paraformaldehyde for 15 min, rinsed in PBS, incubated with 0.1% or 0.2% Triton X-100 in PBS for 5 minutes, and washed in PBS 3 times at 5-min intervals. The coverslips were incubated with anti-α-sma (1:500; Sigma Chemical Co., Germany), anti-cofilin (1:50; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-tgf-β (1:100; Santa Cruz Biotechnology Inc.), anti-psmad2/3 (1:50; Santa Cruz Biotechnology Inc.), anti- -catenin (1:50; Santa Cruz Biotechnology Inc.), and anti-n-cadherin (1:200; BD Transduction Laboratories, Lexington, KY, USA) primary antibodies at room temperature. The coverslips were washed 3 times with PBS at 5-min intervals. Secondary antibodies donkey anti-mouse Alexa 488 (Invitrogen Corporation), goat anti-mouse Alexa 488 (Invitrogen Corporation), donkey anti-goat Alexa 488 (Invitrogen Corporation), goat anti-mouse Alexa 568 (Invitrogen Corporation), and donkey anti-mouse Cy3 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) were applied to washed coverslips and incubated for 20 minutes. Nuclei were stained with Hoescht (Invitrogen Corporation). The coverslips were then washed again three times with PBS at 5-min intervals followed by dipping in distilled water. Fluoro Guard reagent (Bio- Rad Laboratories Inc., Hercules, CA, USA) or Prolong Gold (Invitrogen Corporation) was used for mounting of coverslips to glass slides for microscopic observation. For negative control, mouse immunoglobulin G was used as the primary antibody. Coverslips were examined using the 60x objective of a scanning confocal laser imaging system (BioRad MRC 1024; BioRad, Toronto, Ontario, Canada) fitted with an argon-krypton mixed-gas laser with excitation wavelengths of 488, 568, and 647nm, connected to a Nikon Optiphoto microscope (Nikon Canada), or Olympus FluoView 1000 Laser Scanning Confocal Microscope. Serial optical sections were taken at 0.5-μm thickness to include the entire thickness of the cells. The staining of TGF-β, β-catenin, and N-cadherin was described as strong or weak. For β-catenin and N-cadherin, peripheral and cytoplasmic staining was described, as well as nuclear staining
28 18 for β-catenin. The extent of α-sma staining of VICs was characterized as being of High, Low, or Absent. Fifteen fields were examined for each coverslip by starting observation five fields away from the edges of the coverslip and observing every other field of view to avoid examining the same cell more than once. A total of cells were examined per coverslip depending on cell density. VICs were plated in triplicate for each time point, and each experiment was repeated three times Western blot analysis VICs in 20%, 56%, and 100% confluent cultures were scraped into Triton lysis buffer [30mmol/L HEPES at ph 7.4, 100 mmol/l NaCl, 1 mmol/l EGTA, 20 mmol/l NaF, 1% Triton X-100, 1 mmol/l PMSF, 20 μl/ml Protease Inhibitor Cocktail (Roche, Germany), and 1 mmol/l Na 3 VO 4 ]. Samples were dissolved in Laemmli buffer and boiled for 10 minutes. Equal amounts (15 μg) of protein were separated on sodium dodecyl sulfate polyacrylamide gels and transferred to PVDF membranes using iblot protein transfer system (Invitrogen Corporation). The membranes were blocked with 0.5% non-fat dry milk (Bio-Rad Laboratories) and Phosphoprotein Blocker (Millipore Corporation, Billerica, MA, USA), and then blotted with anti-phospho-β-catenin (Ser33/37/Thr41) antibody (Cell Signaling Technology Inc.), anti-αtubulin antibody (Sigma-Aldrich, Inc., St. Louis, MO, USA), horseradish peroxidase (HRP)- conjugated anti-rabbit-igg antibody (Jackson ImmunoResearch Laboratories, Inc.), and HRPconjugated anti-mouse-igg antibody (Jackson ImmunoResearch Laboratories, Inc.) using Snap I.D. protein detection system (Millipore Corporation). Immunoreactive bands were visualized using Luminata Classico and Forte Western HRP Substrate (Millipore Corporation) Statistical analysis For each set of experiments, the staining intensity of cells in each cell density group was compared with those of other cell density groups at each time point and in between the time points using two-way analysis of variance. A value of p<0.05 was considered significant. The Bonferroni method was used to reflect multiple comparisons. These statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA).
29 Results α-sma expression in VICs decreases as cell density increases VICs plated at 17,000 cells per coverslip are single cells upon plating (Figure 3A), are subconfluent at day 4 (Figure 3C), and become confluent at day 10 (Figure 3E). α-sma staining is at High Intensity at day 1 post-plating (Figure 3A). It decreases at day 2 compared with day 1 (Figures 3B and 4A-B), and decreases furthermore at day 4 compared to day 2 (Figures 3C and 4B-C). At day 7, VICs show α-sma staining of Low Intensity (Figure 3D). VICs do not show α- SMA staining in the confluent monolayer at day 10 post-plating (Figure 3E). VICs plated at a higher density of 170,000 cells per coverslip show a comparable pattern of loss of activation with increasing cell density (Figure 3F-J). Upon plating, some VICs are single cells while some are in contact with other cells (Figure 3F). They become confluent at day 7 postplating (Figure 3I). α-sma staining is at High Intensity at day 1 post-plating (Figure 3F). It decreases at day 2 compared with day 1 (Figure 3G). At day 4, VICs show α-sma staining of Low Intensity (Figure 3H). VICs show minimal α-sma staining in the confluent monolayer at days 7 and 10 post-plating (Figure 3I-J). To rule out that time in culture to reach confluency affects activation, the α-sma staining intensity of cells plated at 17,000 cells/coverslip is compared with cells plated at 170,000 cells/coverslip at days 1, 2 and 4 post-plating, which are the time points before the cultures reach confluence. At days 1, 2, and 4 post-plating, VICs show more High Intensity staining at each time point in cells plated at 17,000 cells/coverslip compared with those plated at 170,000 cells/coverslip (Figure 4A-C). The level of cofilin is high in low density VIC cultures at day 2 post-plating, and its level decreases over time as the density of VICs increases, indicating a decrease in VIC activation with increased density (Figure 5A-D). However, the change in cofilin staining is not as striking as α-sma. Since α-sma expression is the most widely used and accepted marker of VIC activation, it is used for the remainder of the study.
30 TGF-β signaling in VICs decreases concurrently with a decrease in α- SMA expression as cell density increases VICs plated at 17,000 cells/coverslip show strong TGF-β staining at day 2 post-plating (Figure 6B), at which time α-sma staining is at High Intensity (Figure 6A). At days 4 and 6 postplating, as VIC cell density increases, TGF-β staining intensity decreases compared with day 2 (Figure 6D, F), concurrently with decreases in α-sma staining intensity compared with day 2 (Figure 6C, E). At day 8 post-plating, VICs in the confluent monolayer show weak TGF-β staining (Figure 6H), parallel to Low Intensity α-sma staining (Figure 6G). At low cell density, which is associated with VIC activation and increased TGF-β staining, strong nuclear psmad2/3 staining can be observed in VICs (Figure 7A). As cell density increases, the number of VICs with nuclear psmad2/3 staining decreases, and for the VICs that still have nuclear psmad2/3 staining, the intensity decreases (Figure 7B-E). Finally, at confluency, which is associated with VIC quiescence and decreased TGF-β staining, psmad2/3 staining is predominantly present in the cytoplasm and not in the nucleus (Figure 7F) Absence or loss of cell-cell contacts increases α-sma staining intensity In single VICs, along with High Intensity staining for α-sma, strong staining for β-catenin and N-cadherin can be observed in the cytoplasm, especially around lamellapodia (Figure 8A, E, I, M). β-catenin staining is also found in the nuclear or perinuclear region of some cells (Figure 8A, I). As VICs establish contacts with each other, strong staining of β-catenin and N-cadherin is found to colocalize at cell-cell contacts, while α-sma staining remains predominantly at High Intensity (Figure 8B, F, J, N). In larger VIC islands, more cell-cell contacts are established among VICs, and compared with single VICs, the cytoplasmic staining for β-catenin and N- cadherin is weaker (Figure 8C, G, K). Also, nuclear or perinuclear staining for β-catenin can no longer be observed, and α-sma staining is much decreased (Figure 8C, G, K, O). Finally, in confluent monolayers, each VIC is completely surrounded by strongly stained cell-cell contacts with β-catenin and N-cadherin, and there is very weak cytoplasmic staining of β-catenin and N- cadherin, and Low Intensity staining of α-sma (Figure 8D, H, L, P).
31 Phospho-β-catenin is reduced in confluent VIC cultures When whole-cell lysates from subconfluent (20% and 56% confluence) and confluent VIC cultures are blotted for phospho-β-catenin at serine 33, serine 37, and threonine 41, decreased level of phospho-β-catenin is detected in confluent cultures (Figure 9). 2.4 Discussion We have shown that activation of VICs in vitro is associated with cell density of the culture and the establishment of cell-cell contacts. We also showed that time in culture does not influence activation. Although on histological examination of the normal valve, VICs appear to reside as single cells, we have previously shown using ultrastructural studies that VICs extend cytoplasmic processes which adhere to neighbouring cells forming adhesion junctions [123]. We have also shown that these adhesion junctions are present in confluent monolayers of VICs in cell culture [123]. However, the role of these adhesion junctions in regulating VIC function is unknown. We have shown that VICs are activated and express α-sma in response to a mechanical injury made in a confluent monolayer culture of early passage VICs [101]. These confluent cultures of early passage VICs are normally not activated even if they are treated with TGF-β, a potent promoter of mesenchymal cell activation [101]. On the other hand, we reported that single VICs cultured at low density are activated and express α-sma [32]. Together, our data suggest that absence of cell-cell junctions is a signal for VIC expression of α-sma and other genes that characterize VIC activation, while the establishment of cell-cell junctions in confluent monolayer culture may be important in regulating the switch of the VIC phenotype from the activated to the quiescent state. The data in this study showing increased VIC activation at low density when little cell-cell contact is present and VIC quiescence at confluency with full establishment of cell-cell contacts support this hypothesis. β-catenin and N-cadherin are present at the adhering junctions of VICs [124]. However, besides being a component of intact adherens junctions bound to the cytoplasmic tail of cadherins, β- catenin is also a well-known mediator of the Wnt signaling pathway [ ]. Normally, the level of free cytoplasmic β-catenin is kept low by the Axin/CK1/GSK3/APC destruction
32 22 complex, which phosphorylates it for proteosomal degradation [ ]. However, upon activation of the Wnt pathway, free cytoplasmic β-catenin is stabilized and can travel to the nucleus to act as a transcriptional co-activator where it associates with other factors including the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of proteins to regulate processes such as cell fate determination and proliferation [ ]. The Wnt/β-catenin pathway has been implicated in the development of many tissues and organs, including heart valves [70,76,103]. Dysregulation of this pathway, for example by mutations in β-catenin that allow it to escape degradation, has been implicated in diseases, especially cancers [ ]. Interestingly, there seems to be a degree of crosstalk between the Wnt/β-catenin and TGF-β pathways [176]. It has been shown that in confluent renal tubular epithelial cells, free β-catenin resulting from contact disassembly is associated with α-sma promoter activation and protein expression. In addition, TGF-β augments β-catenin mediated transcription by reducing proteosomal degradation of cytosolic β-catenin [135]. In the heart valve in vitro, Wnt3a has been shown to potentiate the ability of TGF-β to increase α-sma expression and activate VICs [177]. Results in this chapter show that β-catenin distribution pattern and TGF-β expression correlate with activation of the myofibroblast phenotype in VICs. Consistent with this model, at low cell densities with no cellcell contacts where there is highest VIC activation, β-catenin is localized to the cytoplasm and nucleus, and TGF-β expression is increased. In addition, upon confluence, when β-catenin is mostly present in the fully established cell-cell junctions, low in the cytoplasm, absent in the nucleus, with minimal TGF-β expression, VICs are quiescent. Thus, it is likely that the integrity of cell-cell junctions may be important in regulating VIC activation. Western blot analysis of whole-cell lysates from sub-confluent and confluent VIC cultures suggests reduced phosphorylation of β-catenin in confluent cultures. A change in total β-catenin protein levels in VIC cultures of different densities was not observed (data not shown). Even though confluent cultures show decreased levels of the form of β-catenin phosphorylated by CK1 and GSK3β and targeted for proteosomal degradation, this does not necessarily indicate decreased β-catenin degradation in confluent cultures, especially since a change in total β-catenin is not detected. There are many other mechanisms regulating the function of β-catenin besides phosphorylation at Ser33, 37 and Thr41 [169]. Also, merely decreasing the proteosomal degradation of β-catenin does not automatically result in increased nuclear translocation [169]. It
33 23 has been shown that unphosphorylated β-catenin can bind to both cadherins and TCF transcription factors [169]. In conclusion, VIC activation decreases as cell density increases and more cell-cell contacts are established, and this is associated with a concurrent decrease in the level of TGF-β. The highdensity and confluent cultures are considered to be an experimental model of the quiescent nondiseased heart valve, while the low-density cultures in which there is no or very minimal cell-cell interaction is considered to be the model of cell injury in which there is a loss of cell-cell contacts. Thus in valve repair where surgical procedures are becoming much more common, the initial response of VICs will likely be activation at the site of injury where there is less cell-cell contact initially. The surgeon expects that the physiological mechanisms present in the valve will limit the activation once more cells are present and result in a good healing outcome. Our model is being used to study this switch from activated to non-activated VICs. In addition, in the culture dish, we see that increased cell density and confluency turn off cell activation; however, in diseased valves, the cells continue to be activated. Thus, our tissue culture model is useful to study the mechanisms of de-activation of VICs, which could then be translated to reduce disease burden in the valve.
34 Chapter 3: Wnt3a/β-catenin increases proliferation in heart valve interstitial cells 24
35 Introduction β-catenin plays two roles in VICs. On one hand, it acts as an adhesion protein bound to the cytoplasmic tail of cadherins to establish cell-cell contacts [125,126]. On the other hand, it acts as a downstream signalling molecule of the Wnt pathway (Figure 2). For comprehensive reviews of the Wnt/β-catenin pathway, please refer to Logan and Nusse 2004, Clevers 2006, MacDonald et al, 2009, and Rao and Kuhl As well, updated information on Wnt signalling can be found on the Wnt homepage at Here is a brief description of the Wnt/β-catenin signalling pathway. Normally, the level of free cytoplasmic β-catenin is kept low by the Axin complex, which includes the scaffolding protein Axin, the tumour suppressor adenomatous polyposis coli gene product (APC), casein kinase 1 (CK1), and glycogen synthase kinase 3 (GSK3). CK1 and GSK3 sequentially phosphorylate the amino terminal region of β-catenin, resulting in β-catenin recognition by β-trcp (beta-transducin repeat-containing protein), an E3 ubiquitin ligase subunit, and subsequent β-catenin ubiquitination and proteosomal degradation. However, in the presence of Wnt, Wnt binds to Frizzled and Lrp5/6 and the β-catenin degradation complex is disassembled, a process which involves phosphorylation of Lrp5/6, recruitment of Dishevelled, and recruitment of Axin away from the degradation complex. This leads to the stabilization of β-catenin in the cytosol, allowing it to travel to the nucleus to act as a transcriptional co-activator. The main partner for β-catenin in gene regulation is the T cell factor/lymphoid enhancer factor (TCF/LEF) family of proteins. In the absence of β-catenin, TCF/LEF transcription factors bind to Wnt response elements and recruit co-repressors such as Groucho and histone deacetylases. However, when β-catenin is in the nucleus, it binds to TCF/LEF, displaces transcriptional repressors, and recruits transcriptional activators such as chromatin remodelling complex swi/snf. The Wnt/β-catenin pathway has been implicated in the development of many tissues and organs, including heart valves where it was shown to regulate proliferation and endothelial-mesenchymal transition [70,76,164]. In valve diseases, genes and mechanisms important in development but downregulated in adulthood become upregulated [69,81, ]. For instance, there is very little or no proliferation in adult valves, but proliferation is increased in diseased valves, such as in areas around calcified nodules [102]. Also, only mild staining of Wnt3, Lrp5, and minimal
36 26 expression of β-catenin are found in normal valves [34,167]. However, in diseased valves, especially calcified valves, there is increased expression of Wnt3, Lrp5 and β-catenin [34,167]. Wnt/β-catenin pathway is implicated in regulating proliferation in several pathologies, including intestinal epithelial cells and smooth muscle cells, and downstream targets include c-myc, cyclin D1, and p21 [164,166,181,182]. Using an in vitro wound model, we have shown previously that VICs along a wound edge become activated, represented by increased expression of α-smooth muscle actin (α-sma) stress fibres, and undergo increased proliferation [101]. Therefore, it is possible for the Wnt/β-catenin pathway, upregulated in disease, to regulate proliferation in VICs in an attempt to mediate wound repair. 3.2 Materials and Methods Cell culture Porcine hearts were obtained from a local abattoir, and explants were prepared from the distal third of the anterior leaflet of porcine mitral valves as previously described [83]. The atrial and ventricular surfaces of the explants were scraped with a scalpel blade and rinsed with phosphate buffered saline (PBS ph 7.4) to remove valve endothelial cells. The explants were cut into 4x5- mm pieces, placed in 9-cm tissue culture dishes (Falcon; BD Biosciences, San Jose, CA, USA), and grown in standard medium, medium 199 (M-199), supplemented with 10% fetal bovine serum (FBS), and 2% penicillin, streptomycin and Fungizone (GIBCO BRL, Life Technologies Inc, Rockville, MD, USA) in a humidified 95% air and 5% carbon dioxide atmosphere in an incubator at 37 C. VICs which grew out of the explants were detached with TrypLE Express (GIBCO; Invitrogen Corporation, Carlsbad, CA, USA) and subcultured. VICs between passages 3 to 5 were used Treatment with exogenous Wnt3a Recombinant mouse Wnt3a (R&D Systems, MN, USA) was reconstituted in sterile PBS containing 0.1% bovine serum albumin. Media containing vehicle or 150 ng/ml of recombinant mouse Wnt3a was added to cultures [103]. Wnt3a was replenished every 48 hours if treatment length exceeded 2 days.
37 Immunofluorescent staining and confocal microscopy VICs were plated at 37,000 cells per 18-mm glass coverslip in 12-well tissue culture plates, and coverslips were fixed and immunofluorescently stained for β-catenin and α-sma at days 1, 3, 6, and 9 post-treatment with vehicle or Wnt3a. VICs were fixed with 4% paraformaldehyde for 15 minutes, rinsed in PBS, incubated with 0.1% Triton X-100 in PBS for 5 minutes, and washed in PBS 3 times at 5-minute intervals. The coverslips were incubated with mouse anti-α-sma (1:500; (Sigma Chemical Co., Germany) and anti- -catenin (1:50; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) primary antibodies at room temperature for 1 hour. The coverslips were washed 3 times with PBS at 5-minute intervals. Secondary antibodies donkey anti-goat Alexa 488 (1:200; Invitrogen Corporation) and goat anti-mouse Alexa 568 (1:200; Invitrogen Corporation) along with nuclear dye Hoescht (1:500; Lonza, MD, USA) were applied to washed coverslips and incubated at room temperature for 20 minutes. The coverslips were then washed again three times with PBS at 5-minute intervals followed by dipping in distilled water. Prolong Gold (Invitrogen Corporation) was used for mounting of coverslips on glass slides for microscopic observation. Coverslips were examined using the 60x objective of Olympus FluoView 1000 Laser Scanning Confocal Microscope. Serial optical sections were taken at 0.5- μm thickness to include the entire thickness of the cells Cell count VICs were plated in 35-mm dishes at 20,000 cells per dish and allowed to grow for 3 days to 4-6% confluence. The cells were then treated with 150 ng/ml of Wnt3a for 4 days and then counted using Countess Cell Counter (Invitrogen Corporation) Western blot VICs were plated in 100-mm dishes at 200,000 cells per dish and allowed to grow for 3 days to 4-6% confluence. The cells were then treated with 150 ng/ml of Wnt3a for 4 days and then lysed. VICs were scraped into Triton lysis buffer to prepare whole cell lysates [30 mmol/l HEPES at ph 7.4, 100 mmol/l NaCl, 1 mmol/l EGTA, 20 mmol/l NaF, 1% Triton X-100, 1 mmol/l PMSF, 20 μl/ml Protease Inhibitory Cocktail (Roche, Germany), and 1 mmol/l Na 3 VO 4 ]. Whole cell lysate was centrifuged at 4 C at 13,000 rpm for 5 minutes and the supernatant was saved as
38 28 cytosolic lysate. Nuclear lysate was prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents according to manufacturer s instructions (Thermo Fisher Scientific, USA). Prepared lysates were dissolved in Laemmli buffer and boiled for 10 minutes. Equal amounts of proteins were separated on sodium dodecyl sulfate polyacrylamide gels and transferred to PVDF membranes using iblot protein transfer system (Invitrogen Corporation). The membranes were blocked with 0.5% non-fat dry milk (Bio-Rad Laboratories Inc., Hercules, CA, USA), and then blotted with rabbit anti-β-catenin antibody (Santa Cruz Biotechnology Inc.), mouse anti-tubulin antibody (Sigma-Aldrich, Inc., St. Louis, MO, USA), and mouse anti-histone H1 antibody (Santa Cruz Biotechnology Inc.) followed by horseradish peroxidase (HRP)-conjugated anti-rabbit-igg antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and HRPconjugated anti-mouse-igg antibody (Jackson ImmunoResearch Laboratories, Inc.) using Snap I.D. protein detection system (Millipore Corporation, Billerica, MA, USA). Immunoreactive bands were visualized using Luminata Classico and Crescendo Western HRP Substrate (Millipore Corporation). Densitometry was performed using ImageJ software (NIH, USA). Values were presented normalized against loading control Small interfering RNA transfection Small interfering (si)rnas targeting β-catenin were obtained from Sigma-Aldrich (SASI_Hs_01_ , SASI_Hs_01_ ); negative control sirnas were obtained from Ambion (Streetsville, ON) ( ). The annealed duplexes were prepared as recommended by manufacturers. The sense strands of the synthetic oligonucleotide duplexes targeting β-catenin were CUCAGAUGGUGUCUGCUAU (SASI_Hs_01_ ) and GUUAUGGUCCAUCAGCUUU (SASI_Hs_01_ ). VICs were plated at cells/cm 2 in 6-well 35-mm diameter plates 24 hours prior to transfection. To silence β-catenin expression, VICs were transfected with 40 nm of a mixture of both sirna duplexes targeting β-catenin mentioned above (20 nm each) using N-TER TM nanoparticle sirna transfection system (Sigma-Aldrich, Inc., N2913) according to the
39 29 manufacturer s protocol. Cells only buffer treatment and 40 nm of negative control sirna were used as controls. VICs were transfected in complete medium for 30 hours. To assess the transfection efficiency, VICs were transfected with 20nM and 40nM of fluorescent oligonucleotides, siglo Red (Thermo Fischer Scientific, USA), using N-TER TM nanoparticle sirna transfection system in complete medium for 24 and 48 hours. Immediately after transfection, cells were fixed in 4% paraformaldehyde, counterstained with Hoescht (Lonza), mounted on microscope slides with Prolong Gold (Lonza), and viewed under Olympus FluoView 1000 Laser Scanning Confocal Microscope. To assess the efficiency of β-catenin knockdown, VICs grown in 6-well plates were immediately lysed after transfection in Triton lysis buffer as described above. Cell lysates were centrifuged at 4 C at 13,000 rpm for 10 minutes and the supernatant was analyzed by Western blotting as described above Quantification of proliferation VICs were plated at cells/cm 2 on 18-mm round glass coverslips in 12-well plates 24 hours prior to transfection. VICs were transfected with cells only buffer, negative control sirna, or β-catenin targeted sirna as described above for 30 hours. Afterwards, VICs were treated with vehicle or Wnt3a for 24 hours. Bromodeoxyuridine (BrdU) labeling agent (Sigma-Aldrich, Inc.) was added in 1:500 dilution to VIC cultures for 2 hours at 22 hours post-treatment with vehicle or Wnt3a. At 24 hours posttreatment, the coverslips were washed 3 times in serum-free M-199 media, fixed in ethanol:acetic acid (95:5) at 4 C for 20 minutes, and washed in PBS 3 times at 5-minute intervals. Then the coverslips were incubated with 0.2% Triton X-100 in PBS for 5 minutes and washed in PBS 3 times at 5-minute intervals. Afterwards, the coverslips were incubated in 2N HCl at 37 C for 30 minutes, washed in 0.1M sodium borate buffer (ph=8.5) twice at 5-minute intervals, and washed in PBS 3 times at 5-minute intervals. The coverslips were incubated with mouse anti-brdu (1:500; Sigma-Aldrich, Inc.) primary antibody at 37 C for 2 hours. The coverslips were washed 3 times with PBS at 5-minute intervals. Secondary antibody donkey antimouse Alexa 488 (1:200; Invitrogen Corporation) along with nuclear dye propidium iodide
40 30 (1:500; Sigma-Aldrich, Inc.) were applied to washed coverslips and incubated for 20 minutes at room temperature. The coverslips were then washed again three times with PBS at 5-minute intervals followed by dipping in distilled water. Prolong Gold (Invitrogen Corporation) was used for mounting of coverslips onto glass slides for microscopic observation and counting. Random counting of both total and BrdU-positive VICs was performed using the 40x objective of a fluorescent microscope (Carl Zeiss Inc., West Germany) starting five fields away from the edge of the coverslip and was continued linearly along the center of the coverslip counting cells in every other field of view to avoid examining the same cell more than once. Proliferation was calculated as a percentage of BrdU-positive cells out of the total number of VICs Quantification of apoptosis VICs were plated at cells/cm 2 on 18-mm round glass coverslips in 12-well plates 24 hours prior to transfection. VICs were transfected with cells only buffer, negative control sirna, or β-catenin targeted sirna as described for 30 hours. Afterwards, VICs were treated with vehicle or Wnt3a for 24 hours. Terminal deoxynucleotidyl transferase dutp nick end labeling (TUNEL) was performed using the TACS TdT-fluorescein in situ apoptosis detection kit (Trevigen Inc., MD) to identify apoptotic VICs according to the manufacturer s instructions. VICs were counterstained with Hoescht (Lonza). Both positive control and negative control were performed according to the manufacturer s instructions. Positive control was performed by treating the cells with nuclease to create DNA breaks in every cell. Negative control was performed by omitting the enzyme required to incorporate nucleotides into DNA breaks. Random counting of both total and TUNEL-positive VICs was performed using the 40x objective of a fluorescent microscope (Carl Zeiss Inc.) starting five fields away from the edge of the coverslip and was continued linearly along the center of the coverslip counting cells in every other field of view to avoid examining the same cell more than once. Apoptosis was calculated as a percentage of TUNEL-positive cells out of the total number of VICs.
41 TOPFLASH/FOPFLASH reporter assay VICs were plated on 12-well tissue culture plates at 300,000 cells per well and simultaneously transfected with TCF-responsive TOPFLASH reporter construct, which contains 3 TCF binding sites, or the corresponding FOPFLASH construct, which contains 3 mutated TCF sites. A β- galactosidase expression vector served as a control for transfection efficiency. TOPFLASH and FOPFLASH reporter constructs as well as β-galactosidase expression vectors were kindly provided by Dr. Benjamin Alman s lab. Transfections were performed using Lipofectamine 2000 (Invitrogen Corporation) transfection reagent according to the manufacturer s instructions. VICs were transfected for 30 hours in M-199 supplemented with 10% FBS, and 2% penicillin, streptomycin and Fungizone. Afterwards, VICs were allowed to recover in fresh media for 18 hours and then treated with vehicle or Wnt3a for 24 hours. VICs were lysed, and cell lysates were assayed for luciferase activity and β-galactosidase activity on a luminometer using the Dual-Light Chemiluminescent Reporter Gene Assay System (Applied Biosystems, MA, USA) according to the manufacturer s instructions. TOPFLASH and FOPFLASH luciferase activities were normalized to β-galactosidase activity and the background β-galactosidase activity was substracted. Reporter activity was calculated as a ratio of TOPFLASH:FOPFLASH Statistical analysis Statistical analyses were performed using GraphPad Prism version 5 software (GraphPad Software Inc., San Diego, USA). A value of P<0.05 was considered significant. One-way analysis of variance (ANOVA) or repeated measures ANOVA followed by Tukey s Multiple Comparison Test was used for comparison of multiple groups. T-test was used for comparison of two groups. 3.3 Results Wnt3a treatment increases β-catenin staining intensity in VICs Upon treatment of low density cultures with 150 ng/ml of Wnt3a, compared with control and vehicle-treated cultures, slightly increased staining of β-catenin is observed overall at day 1, which becomes more visible by day 3 (Figure 10A-F). However, it is difficult to ascertain the specific location of the increased staining. This increase in β-catenin staining becomes more
42 32 pronounced at days 6 and 9 post-treatment (Figure 10G-L). There seems to be increased cytosolic and nuclear β-catenin staining that contributes to the overall increase in staining intensity (Figure 10G-L). At all time points post-treatment, control and vehicle-treated cultures demonstrate similar intensity and pattern of β-catenin staining (Figure 10A, B, D, E, G, H, J, K). No effect is observed on VIC activation, assessed by α-sma staining (Figure 11) Wnt3a treatment increases VIC cell number After Wnt3a treatment, an increase in cell number can be observed starting at day 3 posttreatment compared with control and vehicle-treated cultures (Figure 12). At 4 days posttreatment, VIC cell counts show a significant increase (p<0.05) in cell number in Wnt3a-treated cultures compared with control and vehicle-treated cultures (Figure 13). No difference is observed between control and vehicle-treated cultures (Figure 12,13). Based on trypan blue staining, no difference in cell viability is observed among the 3 conditions (Figure 14) Wnt3a treatment increases whole cell, cytosolic and nuclear protein levels of β-catenin After 4 days of treatment with Wnt3a, whole cell lysates, cytosolic lysates, and nuclear extracts of VIC cultures show increased protein levels of β-catenin compared with control and vehicletreated cultures (Figure 15,16). The absence of histone H1 protein in cytosolic lysates and the absence of α-tubulin in nuclear lysates indicate good separation of cytosolic and nuclear proteins (Figure 15A, 16A). Densitometry measurements show a statistically significant (p<0.05) increase in β-catenin protein levels in Wnt3a-treated cultures in both whole cell and nuclear lysates (Figure 15B, 16B). The increase in nuclear β-catenin is especially prominent after Wnt3a treatment (Figure 16B). No difference is observed between control and vehicle-treated cultures (Figure 15,16) Wnt3a treatment increases VIC proliferation; an effect dependent on β-catenin To ascertain whether the effects observed with Wnt3a treatment are specifically due to the actions of β-catenin, sirna is used to knockdown β-catenin in VICs. Transfection efficiency is approximately 100% (data not shown). Compared with cells only and negative control sirna
43 33 cultures, which show similar levels of β-catenin protein, β-catenin targeted sirna decreases the level of β-catenin protein significantly (p<0.05) by approximately 50-60% (Figure 17). BrdU staining is used to determine whether the increase in total cell number observed with Wnt3a treatment is due to increased proliferation. In vehicle-treated cultures, the percentage of BrdU-positive VICs is similar in cells only and negative control sirna cultures, and only a slight decrease is observed in β-catenin targeted sirna cultures (Figure 18). Wnt3a treatment produces a significant increase (p<0.05) in the percentage of BrdU-positive VICs in cells only and negative control sirna cultures compared with vehicle treatment (Figure 18). The increase is similar in both cells only and negative control sirna cultures (Figure 18). However, this increase is completely abolished in the presence of β-catenin targeted sirna (p<0.05, Figure 18) Wnt3a treatment does not affect VIC apoptosis To determine whether apoptosis affects the increase in cell number observed with Wnt3a treatment, TUNEL assay is used to measure the percentage of apoptotic nuclei. An apoptosis rate of less than 5% is observed in all cultures (Figure 19). No difference is observed between vehicle and Wnt3a treated cultures (Figure 19). The percentage of apoptotic VICs appears slightly higher in sirna-treated cultures compared to cells only cultures in both vehicle and Wnt3a cultures, but this is not statistically significant (Figure 19). Positive control shows an apoptosis rate of approximately 100% and negative control shows an apoptosis rate of approximately 0% (data not shown) Wnt3a treatment increases β-catenin-tcf-mediated transcription There is a significant increase (p<0.05) in TOPFLASH/FOPFLASH reporter activity of at least 2 fold in Wnt3a-treated cultures compared with vehicle control cultures, indicating an increase in β-catenin-tcf-mediated transcription (Figure 20). 3.4 Discussion Wnt3a increases VIC cell number without affecting cell viability. More specifically, Wnt3a increases the percentage of BrdU-positive VICs, indicating an increase in proliferation. The
44 34 minimal level of apoptosis and the lack of effect by Wnt3a on the percentage of TUNEL-positive VICs further confirm proliferation as the process responsible for increases in VIC cell number after Wnt3a treatment. Proliferation is specifically abolished by β-catenin targeted sirna, suggesting a β-catenin specific effect. Interestingly, β-catenin sirna only produces a mild decrease in the percentage of BrdU-positive VICs in vehicle-treated cultures. This observation suggests that β-catenin is not be required for VIC proliferation under normal conditions but specifically mediates the proliferative effects of Wnt3a, which is elevated in disease conditions [34,167]. Wnt3a does not seem to affect VIC activation, assessed by α-sma staining. This is not surprising because recent research shows that Wnt3a alone is not sufficient to induce VIC activation [177]. Instead, it potentiates the ability of TGF-β to activate VICs such that there is a synergistic increase in VIC activation when Wnt3a and TGF-β treatments are combined [177]. Such a phenomenon is also demonstrated in other cell types [135]. Both immunofluorescent data and Western blot results indicate that Wnt3a increases β-catenin levels in VICs. Cell lysate fractionation and subsequent Western blot analysis show that Wnt3a increases β-catenin protein levels in whole cell, cytosolic and nuclear lysates, suggesting that Wnt3a treatment may lead to stabilization of cytosolic β-catenin, thereby allowing it to translocate to the nucleus to mediate transcription. Even though the increase in β-catenin protein level in the cytosol is not statistically significant based on densitometry analysis, cytosolic stabilization is a temporary step leading towards eventual nuclear translocation, therefore the increase in β-catenin protein might be not as dramatic. Importantly, after Wnt3a treatment, the increase in β-catenin is especially prominent in the nucleus and TOPFLASH/FOPFLASH reporter activity is significantly increased in VICs, indicating an increase in β-catenin-mediated transcription. In conclusion, the results obtained so far suggest that Wnt3a increases VIC proliferation through an increase in β-catenin-mediated transcription.
45 Chapter 4: Discussion/Future Directions 35
46 36 The common molecule involved in both VIC activation and proliferation appears to be β-catenin. It is both associated with VIC activation and increased TGF-β signaling and shown to regulate VIC proliferation downstream of Wnt3a signaling. The proliferative effect of Wnt3a/β-catenin signaling is somewhat an unexpected finding, because the increased VIC activation and nuclear staining of β-catenin in low density VIC cultures suggests a role for β-catenin in VIC activation. However, this can be explained by recent evidence that suggests Wnt3a alone is not sufficient to induce α-sma transcription or VIC activation [177]. Instead, the increase in β-catenin after Wnt3a treatment potentiates the ability of TGF-β to induce greater α-sma transcription and VIC activation than TGF-β treatment alone [177]. TGF-β-induced α-sma transcription and VIC activation is shown to depend on the availability of β-catenin [177]. A similar phenomenon is observed in other cell types. Research in kidney epithelial cells shows that β-catenin plays a strong potentiating and synergistic role in TGF-β-induced activation of α-sma promoter activity and protein expression [135]. TGF-β is shown to prevent proteosomal degradation of β-catenin and induce its nuclear translocation and β-catenin-mediated transcription [135,183]. The in vitro evidence in VICs and kidney epithelial cells suggests a crosstalk between TGF-β and Wnt signaling pathways in the regulation of VIC activation. A similar crosstalk might regulate VIC proliferation as well, since Wnt3a/β-catenin is shown to increase VIC proliferation in this study and TGF-β has been previously shown to increase VIC proliferation along an in vitro wound edge [101]. The in vitro findings are supported by observations in diseased valves where both TGF-β/Smad and Wnt/β-catenin signaling components are upregulated, along with VIC activation and proliferation. Thus, a crosstalk between TGF-β and Wnt signaling pathways may be involved in regulating VIC responses to injury in disease conditions [177]. The crosstalk between the TGF-β/Smad and Wnt/β-catenin pathways is poorly understood. TGFβ1 has been shown to promote the association of Smad4 and 3 with β-catenin [184]. Smad3 has been reported to interact with β-catenin to form a protein complex [184,185], and the phosphorylation of Smad2/3 is shown to be required for TGF-β1-induced β-catenin nuclear translocation [177]. The formation of a Smad3/β-catenin complex has been shown to protect β- catenin from proteasome degradation as well as facilitate β-catenin nuclear translocation and transcriptional activity [185]. Within the nucleus, Smad binding element and TCF/LEF can physically interact on binding to Smad3 and β-catenin, possibly initiating a cooperative
47 37 regulation [ ]. Therefore, it is possible that both pathways are activated in valve injury and disease and cooperate to regulate important repair mechanisms, such as VIC activation and proliferation. Future experiments should explore the possible synergistic regulation by TGF-β and Wnt3a in VICs in models of wound repair. Our lab has already investigated the effects of TGF-β on VICs in the in vitro scratch wound model. To extend upon those findings and the results in this thesis, Wnt3a treatment can be administered alone and together with TGF-β to measure VIC activation, proliferation, apoptosis, and wound closure at the wound edge, in areas away from the wound edge, and in confluent non-wounded monolayers. In addition, downstream genes and molecules involved in Wnt3a/β-catenin signaling and VIC proliferation can be explored, such as cyclin D1 and p21 [166]. These proposed future directions will study interactions and synergies operating to regulate VIC function and to identify proteins that need to be targeted to prevent the excessive repair which leads to clinical dysfunction. In addition, it is important to investigate whether Wnt3a/β-catenin signaling may regulate genes important in calcification since it is upregulated in CAS [34]. TGF-β, also upregulated in CAS, has been shown to induce calcification of sheep aortic VICs in culture and to increase the expression of alkaline phosphatase, a marker of osteoblasts and differentiating bone [189]. However, investigations on Wnt3a/β-catenin in CAS are lacking. In embryonic chicken aortic VICs in vitro, Wnt3a treatment does not induce expression of Runx2, osteocalcin and alkaline phosphatase, which are all markers of osteoblasts and differentiating bone [103]. However, Wnt3a signaling is shown to stimulate osteoblastogenesis and decreased Wnt signaling is associated with low bone mass [190,191]. It is important to investigate whether Wnt3a/β-catenin induces expression of genes important in CAS development in adult valves. My experiments are conducted with porcine valves in an in vitro system. Porcine valves are very similar to humans and are the model of choice for human surgical and tissue engineering considerations. However, even though my experiments provide useful and important information, there are limitations. First of all, in these studies, the VICs secrete their own matrix. The VICs are not plated on specific matrixes nor is the thickness or the stiffness of the matrix studied. Recent research suggests that TGF-β-induced β-catenin nuclear translocation and VIC
48 38 activation require a certain amount of matrix stiffness and increase with increasing stiffness [177]. In valve disease, the leaflets undergo fibrosis and they thicken and harden, increasing the stiffness of the environment that VICs reside in, making them more susceptible to activating agents [24]. In my experiments, VICs are directly plated on the surface of glass dishes, which is very stiff and in some ways resemble the stiff environment in disease conditions. However, it is still different from disease conditions. The in vitro system does not include interactions with matrix molecules such as matrix metalloproteinases 9 and 12 that are shown to cleave N- cadherin and induce β-catenin nuclear translocalization, cyclin D1 expression, and vascular smooth muscle cell proliferation [192]. Also, the in vitro system does not include inflammatory cells or hemodynamic forces, both of which are likely to play a role in valve diseases [104]. Hence, it is a simplification of a complex in vivo state [104]. Therefore, it is important to study VIC regulation in in vivo models such as mouse models [193]. In conclusion, the results in this thesis suggest that the loss or absence of cell-cell contacts involving N-cadherin and β-catenin is associated with VIC activation and increased TGF-β signaling, and that the Wnt3a/β-catenin pathway regulates, at least in part, VIC proliferation. Since increased VIC activation and proliferation are likely to be important in wound repair occurring in valve disease, the results increase our understanding of their regulation and suggest that TGF-β and Wnt3a may be important molecules in valve disease that deserve further study and may be targeted by therapy to design better treatments for patients.
49 39 References 1. Messier RH, Bass BL, Aly HM, Jones JL, Domkowski PW, Wallace RB, Hopkins RA. Dual structural and functional phenotypes of the porcine aortic valve interstitial population: characteristics of the leaflet myofibroblast. J Surg Res 1994;57: Yacoub MH, Kilner PJ, Birks EJ, Misfeld M. The aortic outflow and root: a tale of dynamism and crosstalk. Ann Thorac Surg 1999;68:S Schoen FJ. Aortic valve structure-function correlations: role of elastic fibers no longer a stretch of the imagination. J Heart Valve Dis 1997;6: Filip DA, Radu A, Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res 1986;59: Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can J Cardiol 1996;12: Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, Bischoff J. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-beta-mediated transdifferentiation in vitro. Am J Pathol 2001;159: Schoen FJ, Levy RJ. Founder s Award, 25 th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28-May 2, Tissue heart valves: current challenges and future research perspectives, J Biomed Mater Res 1999;47: Li C, Xu S, Gotlieb AI. The response to valve injury. A paradigm to understand the pathogenesis of heart valve disease. Cardiovasc Pathol, 2011;20: Hammon JW Jr, O Sullivan MJ, Oury J, Fosburg RG. Allograft cardiac valves. A view through the scanning electron microscope. J Thorac Cardiovasc Surg 1974;68:
50 Silver MD, Gotlieb AI, Schoen FJ. Valvular heart disease. In Cardiovascular Pathology, Churchill Livingstone, Philadelphia, 2001, pp Araki T, Chan G, Newbigging S, Morikawa L, Bronson RT, Neel BG. Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardialmesenchymal transformation. Proc Natl Acad Sci USA 2009;106: Yang JH, Wylie-Sears J, Bischoff J. Opposing actions of Notch1 and VEGF in post-natal cardiac valve interstitial cells. Biochem Biophys Res Commun 2008;374: Bonita RE, Cohen IS, Berko BA. Valvular heart disease in osteogenesis imperfecta: presentation of a case and review of the literature. Echocardiography 2010;27: Pober BR, Johnson M, Urban Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest 2008;118: Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 2001;104: Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004;44: Butany J, Ahluwalia MS, Munroe C, Fayet C, Ahn C, Blit P, Kepron C, Cusimano RJ, Leask RL. Mechanical heart valve prostheses, identification and evaluation. Cardiovasc Pathol 2003;12: Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010;55: Lu X, Senda S, Mizushige K, Masugata H, Sakamoto S, Sakamoto H, Matsuo H. Evaluation of progression in nonrheumatic aortic valvular stenosis by scanning acoustic microscopy. Ultrasound Med Biol 2000;26:
51 Stewart BF, Siscovick D, Link BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29: Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake- Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95: Otto CM, Link BK, Kitzman DW, Gersch BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341: Napoli C, Cacciatore F. Novel pathogenic insights in the primary prevention of cardiovascular disease. Prog Cardiovasc Dis, 2009;51: O Brien KD. Pathogenesis of calcific aortic valve disease a disease process comes of age (and a good deal more). Arterioscler Thromb Vasc Biol 2006;26: Ross J, Braunwald E. Aortic stenosis. Circulation 1968;381(suppl): Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993;21: Xu S, Liu AC, Gotlieb AI. Common pathogenic features of atherosclerosis and calcific aortic stenosis: role of transforming growth factor-beta. Cardiovasc Pathol, 2010;19: Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Goncalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007;49:
52 Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R, SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359: Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005;352: Chan KL, Teo K, Tam J, Dumesnil JG. Rationale, design, and baseline characteristics of a randomized trial to assess the effect of cholesterol lowering on the progression of aortic stenosis: the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) trial. Am Heart J 2007;153: Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 2007;171: Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation 2002;105: Rajamannan NW, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemic-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation 2005;112:I-229-I Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, McConnell JP, Singh RJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolemic aortic valve. Heart 2005;91:
53 Anger T, Carson W, Weyand M, Daniel WG, Hoeher M, Garlichs CD. Atherosclerotic inflammation triggers osteogenic bone transformation in calcified and stenotic human aortic valves: still a matter of debate. Exp Mol Pathol 2009;86: O Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of degenerative valvular aortic stenosis. Arterioscler Thromb Vasc Biol 1996;16: Mohler ER III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 2001;103: Mohler ER III, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis 1999;8: Anderson HC. Calcific diseases. A concept. Arch Pathol Lab Med 1983;107: Nordstrom P, Glader CA, Dahlen G, Birgander LS, Lorentzon R, Waldenstrom A, Lorentzon M. Oestrogen receptor alpha gene polymorphism is related to aortic valve sclerosis in postmenopausal women. J Intern Med 2003;254: Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor kappab ligand and osteoprotegrin regulate aortic valve calcification. J Mol Cell Cardiol 2004;36: Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol 1999;19: O Brien KD, Shavelle DM, Caulfield MT, McDonald TO, Olin-Lewis K, Otto CM, Probstfield JL. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation 2002;106:
54 Tiede K, Stoter K, Petrik C, Chen WB, Ungefroren H, Kruse ML, Stoll M, Unger T, Fischer JW. Angiotensin II AT(1)-receptor induces biglycan in neonatal cardiac fibroblasts via autocrine release of TGFβ in vitro. Cardiovasc Res 2003;60: Ahmed MS, Oie E, Vinge LE, Yndestad A, Andersen GG, Anderson Y, Attramadal T, Attramadal H. Induction of myocardial biglycan in heart failure in rats-an extracellular matrix component targeted by AT(1) receptor antagonism. Cardiovasc Res 2003;60: Helske S, Lindstedt KA, Laine M, Mayranpaa M, Werkkala K, Lommi J, Turto H, Kupari M, Kovanen PT. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol 2004;44: Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden L, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol 1994;23: Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O Brien KD. Characterization of the early lesion of degenerative valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994;90: Olsson M, Rosenqvist M, Nilsson J. Expression of HLA-DR antigen and smooth muscle cell differentiation markers by valvular fibroblasts in degenerative aortic stenosis. J Am Coll Cardiol 1994;24: Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kilic R, Sarikoc A, Brueckmann M, Vahl C, Hagl S, Haase KK, Borggrefe M. Interleukin-1beta promotes matrx metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis 2003;170: Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kilic R, Sarikoc A, Pinol R, Hagl S, Lang S, Brueckmann M, Broggrefe M. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol 2005;14:80-87.
55 Edep ME, Shirani J, Wolf P, Brown DL. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc Pathol 2000;9: Soini Y, Satta J, Maatta M, Autio-Harmainen H. Expression of MMP2, MMP9, MT1- MMP, TIMP1, and TIMP2 mrna in valvular lesions of the heart. J Pathol 2001;194: Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 2003;107: Arishiro K, Hoshiga M, Negoro N, Okabe T, Ishihara T, Hanafusa T. Angiotensin receptor 1 blocker reduces atherosclerotic changes of aortic valve in hypercholesterolemic rabbit model. J Am Coll Cardiol 2006;47:284A-285A. 57. Kaden JJ, Bickelhaupt S, Grobholz R, Vahl CF, Hagl S, Brueckmann M, Haase KK, Dempfle CE, Borggrefe M. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis 2004;13: Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res 1998; Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437: Durbin AD, Gotlieb AI. Advances towards understanding heart valve response to injury. Cardiovasc Pathol 2002;11: Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res 2009;105: Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelialmesenchymal transition and myocardial patterning. Development 2005;132:
56 Somi S, Buffing AAM, Moorman AFM, Van Den Hoff MJ. Dynamic patterns of expression of BMP isoforms 2, 4, 5, 6, and 7 during chicken heart development. Anat Rec 2004;279: Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat Rec 2000;258: Rivera-Feliciano J, Tabin CJ. Bmp2 instructs cardiac progenitors to form the heart-valveinducing field. Dev Biol 2006;295: Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphognetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol 2004;269: Brown CB, Boyer AS, Runyan RB, Barnett JV. Antibodies to the type II TGFbeta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol 1996;174: Romano LA, Runyan RB. Slug is a mediator of epithelial-mesenchymal cell transformation in the developing chicken heart. Dev Biol 1999;212: Romano LA, Runyan RB. Slub is an essential target of TFbeta2 signaling in the developing chicken heart. Dev Biol 2000;223: Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. β-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol 2004;166: Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev 2003;17:
57 McCulley DJ, Kang JO, Martin JF, Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn 2008;237: Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol 2001;235: Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development 2003;130: Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MAJ, Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet 2000;24: Hurlstone AFL, Haramis AG, Wienholds E, Begthel H, Korving J, van Eeden F, Cuppen E, Zivkovic D, Plasterk RHA, Clevers H. The Wnt/β-catenin pathway regulates cardiac valve formation. Nature 2003;425: Gitler AD, Lu MM, Jiang YQ, Epstein JA, Gruber PJ. Molecular markers of cardiac endocardial cushion development. Dev Dyn 2003;228: Person AD, Garriock RJ, Krieg PA, Runyan RB, Klewer SE. Frzb modulates Wnt-9amediated beta-catenin signaling during avian atrioventricular cardiac cushion development. Dev Biol 2005;278: Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, delapompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 2004;18: Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res 2008;102:
58 Wirrig EE, Yutzey KE. Transcriptional regulation of heart valve development and disease. Cardiovasc Pathol [Epub ahead of print] 82. Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res 2004;95: Lester W, Rosenthal A, Granton B, Gotlieb AI. Porcine mitral valve interstitial cells in culture. Lab Invest 1988;59: Zacks S, Rosenthal A, Granton B, Havenith M, Opas M, Gotlieb AI. Characterization of cobblestone mitral valve interstitial cells. Arch Pathol Lab Med 1991;115: Taylor PM, Allen SP, Yacoub MH. Phenotypic and functional characterization of interstitial cells from human heart valves, pericardium and skin. J Heart Valve Dis 2000;9: Tamura K, Jones M, Yamada I, Ferrans VJ. Wound healing in the mitral valve. J Heart Valve Dis 2000;9: Blevins TL, Carroll JL, Raza AM, Grande-Allen KJ. Phenotypic characterization of isolated valvular interstitial cell subpopulations. J Heart Valve Dis 2006;5: Liu AC, Gotlieb AI. Characterization of cell motility in single heart valve interstitial cells in vitro. Histol Histopathol 2007;22: Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, Schoen FJ, Bischoff J. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-a and transforming growth factor-beta2. Circ Res 2006;99: Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal J, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007;109:
59 Skowasch D, Schrempf S, Wernert N, Steinmetz M, Jabs A, Tuleta I, Welsch U, Preusse CJ, Likungu JA, Welz A, Luderitz B, Bauriedel G. Cells of primarily extra-valvular origin in degenerative aortic valves and bioprostheses. Eur Heart J 2005;26: Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res 2006;98: Leskela HV, Satta J, Oiva J, Eriksen H, Juha R, Korkiamaki P, Ivaska KK, Soini Y, Lehenkari P. Calcification of cellularity in human aortic heart valve tissue determine the differentiation of bone-marrow derived cells. J Mol Cell Cardiol 2006;41: Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis 2004;13: Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001;12: Zimerman B. Volberg T, Geiger B. Early molecular events in the assembly of the focal adhesion-stress fiber complex during fibroblast spreading. Cell Motil Cytoskel 2004;58: Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 2001;104: Jian B, Jones PL, Li Q, Mohler III ER, Schoen FJ, Levy RJ. Matrix metalloproteinase-2 is associated with tenascin-c in calcific aortic stenosis. Am J Pathol 2001;159: Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, Levy RJ. Serotonin mechanisms in heart valve disease I: serotonin induced up-regulation of transforming growth factor-
60 50 beta1 via G-protein signal transduction in aortic valve interstitial cells. Am J Pathol 2002;161: Xu J, Jian B, Chu R, Lu Z, Dunlop J, Rosenzweig-Lipson S, McGonigle P, Levy RJ, Liang B. Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells. Am J Pathol 2002;161: Liu AC, Gotlieb AI. Transforming growth factor-β regulates in vitro heart valve repair by activated valve interstitial cells. Am J Pathol 2008;173: Chakraborty S, Wirrig EE, Hinton RB, Merrill WH, Spicer DB, Yutzey KE. Twist1 promotes heart valve cell proliferation and extracellular matrix gene expression during development in vivo and is expressed in human diseased aortic valves. Dev Biol 2010;347: Alfieri CM, Cheek J, Chakraborty S, Yutzey K. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol 2010;338: Lester WM, Damji AA, Tanaka M, Gedeon I. Bovine mitral valve organ culture: role of interstitial cells in repair of valvular injury. J Mol Cell Cardiol, 1992;24: Schwentker A, Billiar TR. Nitric oxide and wound repair. Surg Clin North Am 2003;83: Desmouliere A, Badid C, Bochaton-Piallat ML, Gabbiani G. Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int J Biochem Cell Biol 1997;29: Nagata M, Takenaka H, Shibagaki R, Kishimoto S. Apoptosis and p53 protein expression crease in the process of burn wound healing in guinea-pig skin. Br J Dermatol 1999;140: Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Rep Reg 2005;13:7-12.
61 Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 2007;257: Rahimi RA, Leof EB. TGF-beta signaling: a tale of two response. J Cell Biochem 2007;102: Sporn MB, Roberts AB, Wakefield LM, Assoian RK. Transforming growth factor-beta: biological function and chemical structure. Science 1986;233: Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-β: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 2004;95: Cushing MC, Liao JT, Anseth KS. Activation of valvular interstitial cells is mediated by transforming growth factor-beta1 interactions with matrix molecules. Matrix Biol 2005;24: Pho M, Lee W, Watt DR, Laschinger C, Simmons CA, McCulloch CA. Cofilin is a marker of myofibroblast differentiation in cells from porcine aortic cardiac valves. Am J Physiol 2008;294:H1767-H Roy SG, Nozaki Y, Phan SH. Regulation of alpha-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int J Biochem Cell Biol 2001;33: Cogan JG, Subramanian SV, Polikandriotis JA, Kelm RJ, Strauch AR. Vascular smooth muscle alpha-actin gene transcription during myofibroblast differentiation requires Sp1/3 protein binding proximal to the MCAT enhancer. J Biol Chem 2002;277: Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D. TGF-beta dependent pathogenesis of mitral valve prolapsed in a mouse model of Marfan syndrome. J Clin Invest 2004;114:
62 Fondard O, Detaint D, Lung B, Choqueux C, Adle-Blassette H, Jarraya M, Hvass U, Couetil JP, Henin D, Michel JB, Vahanian A, Jacob MP. Extracellular matrix remodeling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J 2005;26: Hinton Jr RB, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 2006;98: Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol, 1995;7: Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Cell Biol, 2005;6: Harris TJC, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev, 2010;11: Lester WM, Gotlieb AI. In vitro repair of the wounded porcine mitral valve. Circ Res 1988;62: Barth M, Schumacher H, Kuhn C, Akhyari P, Lichtenberg A, Franke WW. Cordial connections: molecular ensembles and structures of adhering junctions connecting interstitial cells of cardiac valves in situ and in cell culture. Cell Tissue Res, 2009;337: Sarathchandra NLP. Taylor PM, Antoniw J, Brand N, Yacoub MH. Characterization of molecules mediating cell-cell communication in human cardiac valve interstitial cells. Cell Biochem Biophys 2006;45: Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans 2008;36:
63 Yamada S, Pokutta S, Drees F, Weis W, Nelson WJ. Deconstructing the cadherincatenin-actin complex. Cell 2005;123: Behrens J, von Kries JP, Kuhl M, Bruhn J, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature, 1996;382: Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumour cells reveals an invasion suppression role. Cell, 1991;66: Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis, 1995;26: Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Natl Cell Biol, 2000;2: Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, Somasiri A, Roskelley CD. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci USA, 1999;96: Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int, 1999;56: Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol, 2001;159: Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A. Integrity of cell-cell contacts is a critical regulator of TGF-beta1-induced epithelial-tomyofibroblast transition. Role of beta-catenin. Am J Pathol, 2004;165:
64 Fan L, Sebe A, Peterfe Z, Masszi A, Thirone ACP, Rotstein OD, Nakano H, McCulloch CA, Szaski K, Mucsi I, Kapus A. Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol Biol Cell, 2007;18: Dietrich C, Scherwat J, Faust D, Oesch F. Subcellular localization of beta-catenin is regulated by cell density. Biochem Biophys Res Commun, 2002;292: Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Biechman J, Savagner P, Ben- Ze ev A. Autoregulation f E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol, 2003;163: Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R. Epithelial mesenchymal transition by c-fos estrogen receptor activation involves nuclear translocation of beta-catenin and upregulation of beta-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J Cell Biol, 2000;148: Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J Cell Biol, 2001;154: Eger A, Stockinger A, Park J, Langkopf E, Mikula M, Gotzmann J, Mikulits W, Beug H, Foisner R. Beta-catenin and TGFbeta signaling cooperate to maintain a mesenchymal phenotype after FosER-induced epithelial to mesenchymal transition. Oncogene, 2004;23: Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/lef-1 signaling pathway in induction of EMT. Cell Biol Int, 2002;26: Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet 2008;42: Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol 1997;182:
65 Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P, Zeiher AM, Kuhl M, Dimmeler S. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem 2005;280: Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun 2004;325: Dejmek J, Safholm A, Kamp Nielsen C, Andersson T, Leandersson K. Wnt- 5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells. Mol Cell Biol 2006;26: Ma L, Wang HY. Suppression of cyclic GMP-dependent protein kinase is essential to the Wnt/cGMP/Ca2+ pathway. J Biol Chem 2006;281: Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem 2000;275: Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 2000;16: Koyanagi M, Iwasaki M, Haendeler J, Leitges M, Zeiher AM, Dimmeler S. Wnt5a increases cardiac gene expressions of cultured human circulating progenitor cells via a PKC delta activation. PLoS One 2009;4:e Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 2002;417: Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated
66 56 protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/betacatenin signaling. Mol Cell Biol 2003;23: Ishitani T, Ninomiya-Tsuji J, Nagai S-I, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1-NLK- MAPK-related pathway antagonizes signaling between beta-catenin and transcription factor TCF. Nature 1999;399: Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKI-Idelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem 2007;282: Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 2006;116: Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol 2008;28: Little GH, Bai Y, Williams T, Poizat C. Nuclear calcium/calmodulin-dependent protein kinase IIdelta preferentially transmits signals to histone deacetylase 4 in cardiac cells. J Biol Chem 2007;282: Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signaling suppresses PPAR-gamma transactivation. Nat Cell Biol 2007;9: Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004;20:
67 Clevers H. Wnt/β-catenin signaling in development and disease. Cell 2006;127: MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009;17: Rao TP, Kuhl M. An updated overview on Wnt signalling pathways, a prelude for more. Circ Res 2010;106: Tetsu O, McCormick F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999;398: Cheon S, Poon R, Yu C, Khoury M, Shenker R, Fish J, Alman BA. Prolonged β- catenin stabilization and tcf-dependent transcriptional activation in hyperplastic cutaneous wounds. Lab Invest 2005;85: Quasnichka H, Slater SC, Beeching CA, Boehm M, Sala-Newby GB, George SJ. Regulation of smooth muscle cell proliferation by β-catenin/t-cell factor signalling involves modulation of cyclin D1 and p21 expression. Circ Res 2006;99: Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein-related protein 5 receptormediated bone formation. J Am Coll Cardiol 2006;47: Rajamannan NW, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation 2005;112:I229-I Gottardi CJ, Gumbiner BM. Distinct molecular forms of β-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 2004;167: Xu S, Liu AC, Kim H, Gotlieb AI. Cell density regulates in vitro activation of heart valve interstitial cells. Cardiovasc Pathol [Epub ahead of print].
68 Durbin A, Nadir NA, Rosenthal A, Gotlieb AI. Nitric oxide promotes in vitro interstitial cell heart valve repair. Cardiovasc Pathol 2005;14: Latif N, Sarathchandra P, Taylor PM, Antonio J, Brand N, Yacoub MH. Characterization of molecules mediating cell-cell communication in human cardiac valve interstitial cells. Cell Biochem Biophys 2006;45: Mulholland DL, Gotlieb AI. Cardiac valve interstitial cells: regulator of valve structure and function. Cardiovasc Pathol 1997;6: Jian B, Narula N, Li Q, Mohler ER, Levy RJ. Progression of aortic valve stenosis: TGF-β1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg 2003;75: Condeelis J. How is actin polymerization nucleated in vivo? Trends Cell Biol 2001;11: Letamendia A, Labbe E, Attisano L. Transcriptional regulation by smads: crosstalk between the TGF-β and Wnt pathways. J Bone Joint Surg Am 2001;83: Chen J, Chen WLK, Sider KL, Yip CYY, Simmons CA. β-catenin mediates mechanically regulated, transforming growth factor-β1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler Thromb Vasc Biol 2011;31. [Epub ahead of print] 178. Chakraborty S, Combs MD, Yutzey KE. Transcriptional regulation of heart valve progenitor cells. Pediatr Cardiol 2010;31: Gessert S, Kuhl M. The multiple phases and faces of Wnt signalling during cardiac differentiation and development. Circ Res 2010;107: Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol 2011;73:
69 He T, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-myc as a target of the APC pathway. Science 1998;281: Takahashi-Yanaga F, Sasaguri T. GSK-3β regulates cyclin D1 expression: a new target for chemotherapy. Cell Signal 2008;20: Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. Central role for Rho in TGF-beta1- induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Pathol, 2003;284:F911-F Zhang M, Wang M, Tan X, Li TF, Zhang YE, Chen D. Smad3 prevents β-catenin degradation and facilitates β-catenin nuclear translocation in chondrocytes. J Biol Chem, 2010;285: Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Smad3-dependent nuclear translocation of β-catenin is required for TGF-β1-induced proliferation of bone marrowderived adult human mesenchymal stem cells. Genes Dev, 2006;20: Shafer SL, Towler DA. Transcriptional regulation of SM22α by Wnt3a: convergence with TGFβ(1)/Smad signaling at a novel regulatory element. J Mol Cell Cardiol, 2009;46: Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci USA, 2000;97: Guo W, Flanagan J, Jasuja R, Kirkland J, Jiang L, Bhasin S. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/β-catenin signaling pathways. J Biol Chem, 2008;283:
70 Clark-Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport S, Mohler ER, Gorman JH, Gorman RC, Levy RJ. Transforming growth factor-β1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg 2007;83: Zhou H, Mak W, Zheng Y, Dunstan CR, Seibel MJ. Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through Wnt signaling. J Biol Chem, 2008;283: Kato M, Patel MS, Lavasseur R, Lobov I, Chang BH, Glass DA, Hartman C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Bio, 2002;157: Dwivedi A, Slater SC, George SJ. MMP-2 and -12 cause N-cadherin shedding and thereby β-catenin signaling and vascular smooth muscle cell proliferation. Cardiovasc Res, 2009;81: Miller JD, Weiss RM, Serrano KM, Castaneda LE, Brooks RM, Zimmerman K, Heistad DD. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arterioscler Thromb Vasc Biol, 2010;30:
71 61 Figure 1. TGF-β signaling pathways. TGF-β binds to Receptor II, which phosphorylates Receptor I, which then phosphorylates Smad2 and 3 that are able to bind to Smad4 to form a complex that travels to the nucleus to mediate transcription. One of the downstream targets of this canonical pathway is α-sma. TGF-β is also able to signal through PI3K as part of the noncanonical pathway. TGF-β/Smad signaling can be inhibited by Smad6 or 7. Downstream effects of TGF-β signaling include myofibroblast transformation, proliferation, and ECM production. (Adapted from Rahimi and Leof, 2007) [110].
72 62 Figure 2. Wnt/β-catenin signaling. When Wnt is absent, β-catenin level is kept low by the axin degradation complex, which consists of Axin, APC, GSK3β, and CK1. CK1 and GSK3β sequentially phosphorylate serine/threonine residues at the amino terminus of β-catenin, which allows β-catenin to be recognized by β-trcp, an E3 ubiquitin ligase subunit, that then targets β- catenin for proteosomal degradation. When Wnt is present, it binds to Frizzled and LRP5/6, which recruits Dishevelled and disrupts the degradation complex, preventing β-catenin degradation. This allows β-catenin to translocate to the nucleus, displace transcriptional repressors, and bind to TCF/LEF transcription factors to regulate transcription of Wnt responsive genes. (MacDonald et al, 2009) [162].
73 63 Figure 3. Immunofluorescent photomicrographs of valve interstitial cells plated at 17,000 cells/coverslip (A-E) and at 170,000 cells/coverslip (F-J) immunostained for -smooth muscle actin at days 1 (A, F), 2 (B, G), 4 (C, H), 7 (D, I) and 10 (E, J) post-plating. Note that α-smooth muscle actin staining decreases as cell density increases regardless of time in culture. Magnification: 600x. White bar indicates 20 m. VIC, valve interstitial cell.
74 64 Figure 4. Percentage of valve interstitial cells expressing High Intensity -smooth muscle actin staining in monolayer culture at both low density and high density incubated for 1 (A), 2 (B) and 4 days (C) observed under confocal microscopy. The experiment is done in triplicate. Error bars denote standard deviation of the mean. Note that the percentage of High Intensity valve interstitial cells for high density is significantly less than the one for low density at each time point (*). VIC, valve interstitial cell; α-sma, α-smooth muscle actin.
75 65 Figure 5. Immufluorescent images of valve interstitial cells plated at 17,000 cells/coverslip, immunostained for cofilin at days 2 (A), 5 (B), 8 (C), and 14 (D) post-plating. Note that cofilin staining decreases as cell density increases. Magnification: 600x. White bar indicates 20 μm.
76 66 α-sma TGF-β Figure 6. Immunofluorescent photomicrographs of valve interstitial cells plated at 17,000 cells/coverslip, immunostained for -smooth muscle actin (green) and transforming growth factor-β (red) at days 2 (A, B), 4 (C, D), 6 (E, F), and 8 (G, H) post-plating. Note that transforming growth factor-β staining decreases concurrently with α-smooth muscle actin staining as cell density increases. Magnification: 600x. White bar indicates 20 m. α-sma, α- smooth muscle actin; TGF-β, transforming growth factor-β.
77 67 Figure 7. Immufluorescent images of valve interstitial cells plated at 17,000 cells/coverslip, immunostained for psmad2/3 at days 2 (A), 4 (B), 6 (C), 8 (D), 10 (E), and 12 (F) post-plating. Note that nuclear psmad2/3 staining decreases as cell density increases. Magnification: 600x. White bar indicates 20 μm.
78 68 β-catenin N-cadherin N-cadherin and β-catenin α-sma Figure 8. Immunofluorescent images of single valve interstitial cells (A, E, I, M), small groups of valve interstitial cells with recently established cell-cell contacts (B, F, J, N), valve interstitial cell islands (C, G, K, O), and confluent monolayers (D, H, L, P), immunostained for β-catenin (green; A-D), N-cadherin (green; E-H), β-catenin and N-cadherin (green and red respectively; I- L), and α-smooth muscle actin (green; M-P). Cell nuclei are stained blue with Hoescht. Note that as cell density increases, β-catenin and N-cadherin staining increases at cell-cell contacts and decreases in cytoplasm, and nuclear β-catenin staining disappears. Magnification: 600x. White bar indicates 20 µm. α-sma, α-smooth muscle actin.
79 69 Figure 9. Whole-cell protein expression of phospho-β-catenin at Ser33, Ser37, and Thr41 is determined by Western blotting for valve interstitial cell cultures at 20%, 56%, and 100% confluence. α-tubulin is used as the loading control. Note that phospho-β-catenin (Ser33/Ser37/Thr41) protein level is decreased at 100% confluence.
80 70 Control Vehicle Wnt3a Day 1 Day 3 Day 6 Day 9 Figure 10. Immunofluorescent images of valve interstitial cells in control cultures (A, D, G, J), vehicle-treated cultures (B, E, H, K), and Wnt3a-treated cultures (C, F, I, L) at days 1 (A, B, C), 3 (D, E, F), 6 (G, H, I), and 9 (J, K, L) post-treatment immunostained for β-catenin (green). Cell nuclei are stained blue with Hoescht. Note the increase in β-catenin staining in Wnt3a-treated cultures starting at day 3 that becomes more prominent as the treatment time increases. Magnification: 600x.
81 71 Control Vehicle Wnt3a Day 1 Day 3 Day 6 Day 9 Figure 11. Immunofluorescent images of valve interstitial cells in control cultures (A, D, G, J), vehicle-treated cultures (B, E, H, K), and Wnt3a-treated cultures (C, F, I, L) at days 1 (A, B, C), 3 (D, E, F), 6 (G, H, I), and 9 (J, K, L) post-treatment immunostained for α-smooth muscle actin (red). Note the similar staining in all three conditions at each time point. Magnification: 600x.
82 72 Control Vehicle Wnt3a Day 1 Day 3 Day 6 Day 9 Figure 12. Phase contrast images of valve interstitial cells in control cultures (A, D, G, J), vehicle-treated cultures (B, E, H, K), and Wnt3a-treated cultures (C, F, I, L) at days 1 (A, B, C), 3 (D, E, F), 6 (G, H, I), and 9 (J, K, L) post-treatment. Note the increase in cell number in Wnt3a-treated cultures starting at day 3. Magnification: 100x.
83 73 * Figure 13. Total cell number of valve interstitial cells relative to control at 4 days post-treatment in control, vehicle, and Wnt3a treated cultures. Three trials, each with 3 replicates, are performed. Note the increase in cell number after Wnt3a treatment. Error bars denote standard error of the mean. * indicates a statistical significance of p<0.05.
84 74 Percentage of viable cells Control Vehicle Wnt3a Figure 14. Percentage of viable valve interstitial cells based on trypan blue staining at 4 days post-treatment in control, vehicle, and Wnt3a treated cultures. Three trials, each with 3 replicates, are performed. Note the similar viability in all three conditions. Error bars denote standard error of the mean.
85 75 Figure 15. Representative Western blot of protein levels of β-catenin, α-tubulin and histone H1 in whole cell (A, first 3 lanes) and cytosolic lysates (A, last 3 lanes) of valve interstitial cells at 4 days post-treatment in control, vehicle, and Wnt3a treated cultures. Densitometry values relative to control for β-catenin protein levels in whole cell (B) and cytosolic (C) lysates at 4 days posttreatment in control, vehicle, and Wnt3a treated cultures. Results are from 3 experiments. Note the increase in β-catenin protein level in whole cell and cytosolic lysates in Wnt3a-treated cultures. Error bars denote standard error of the mean. * indicates a statistical significance of p<0.05. C, control; V, vehicle; W, Wnt3a.
86 76 Figure 16. A. Representative Western blot of protein levels of β-catenin, histone H1 and α- tubulin in nuclear lysates of valve interstitial cells at 4 days post-treatment in control, vehicle, and Wnt3a treated cultures. B. Densitometry values relative to control for β-catenin protein levels in nuclear lysates at 4 days post-treatment in control, vehicle, and Wnt3a treated cultures. Results are from 3 experiments. Note the increase in β-catenin protein level in nuclear lysates in Wnt3a-treated cultures. Error bars denote standard error of the mean. * indicates a statistical significance of p<0.05. C, control; V, vehicle; W, Wnt3a.
87 77 Figure 17. A. Representative Western blot of protein levels of β-catenin and α-tubulin immediately after 30 hours of sirna transfection in cells only, negative control sirna, and β- catenin targeted sirna cultures. B. Densitometry values relative to cells only for β-catenin protein levels after 30 hours of sirna transfection in cells only, negative control sirna, and β- catenin targeted sirna cultures. Results are from 3 experiments. Note the decrease in β-catenin protein level after β-catenin targeted sirna knockdown. Error bars denote standard error of the mean. * indicates a statistical significance of p<0.05. C, cells only; N, negative control sirna; β, β-catenin targeted sirna.
88 78 Figure 18. Percentage of proliferating valve interstitial cells after 30 hours of sirna transfection followed by 24 hours of treatment with vehicle or Wnt3a in cells only, negative control sirna, and β-catenin targeted sirna cultures. Three trials, each with 2 replicates, are performed. Note Wnt3a treatment increases proliferation of valve interstitial cells in cells only and negative control sirna cultures, but this effect is abolished by β-catenin targeted sirna. Error bars denote standard error of the mean. * indicates statistical significance of p<0.05. BrdU, bromodeoxyuridine.
89 79 Percentage of TUNEL-positive cells Vehicle Cells Only Vehicle Negative sirna Vehicle -Catenin sirna Wnt3a Cells Only Wnt3a Negative sirna Wnt3a -Catenin sirna Figure 19. Percentage of apoptotic valve interstitial cells after 30 hours of sirna transfection followed by 24 hours of treatment with vehicle or Wnt3a in cells only, negative control sirna, and β-catenin targeted sirna cultures. Three trials, each with 2 replicates, are performed. Note there is minimal apoptosis regardless of treatment conditions. Error bars denote standard error of the mean.
90 80 Figure 20. TOPFLASH/FOPFLASH reporter activity relative to vehicle-treated cultures in valve interstitial cells after vehicle or Wnt3a treatment. Results are from 3 experiments. Note the increase in reporter activity, indicating β-catenin-tcf mediated transcription, after Wnt3a treatment. Error bars denote standard deviation. * indicates a statistical significance of p<0.05.
Two semilunar valves. Two atrioventricular valves. Valves of the heart. Left atrioventricular or bicuspid valve Mitral valve
 The Heart 3 Valves of the heart Two atrioventricular valves Two semilunar valves Right atrioventricular or tricuspid valve Left atrioventricular or bicuspid valve Mitral valve Aortic valve Pulmonary valve
The Heart 3 Valves of the heart Two atrioventricular valves Two semilunar valves Right atrioventricular or tricuspid valve Left atrioventricular or bicuspid valve Mitral valve Aortic valve Pulmonary valve
Calcification of Porcine Aortic Valvular Interstitial Cells
 Calcification of Porcine Aortic Valvular Interstitial Cells Liwen Gu 1,2* Supervisor: Craig A. Simmons 1 Department of Engineering Science, 2 Institute of Biomaterials and Biomedical Engineering, University
Calcification of Porcine Aortic Valvular Interstitial Cells Liwen Gu 1,2* Supervisor: Craig A. Simmons 1 Department of Engineering Science, 2 Institute of Biomaterials and Biomedical Engineering, University
Image Analysis and Cytometry in Three-Dimensional Digital Reconstruction of Porcine Native Aortic Valve Leaflets
 Image Analysis and Cytometry in Three-Dimensional Digital Reconstruction of Porcine Native Aortic Valve Leaflets Introduction Chi Zheng, M1 - University of Pittsburgh; BSE - University of Michigan In association
Image Analysis and Cytometry in Three-Dimensional Digital Reconstruction of Porcine Native Aortic Valve Leaflets Introduction Chi Zheng, M1 - University of Pittsburgh; BSE - University of Michigan In association
Contribution of Interstitial Valve Cells to Aortic Valve Calcification
 UNIVERSITÀ DEGLI STUDI DI PADOVA SEDE AMMINISTRATIVA: UNIVERSITÀ DEGLI STUDI DI PADOVA DIPARTIMENTO DI SCIENZE MEDICO-DIAGNOSTICHE E TERAPIE SPECIALI SCUOLA DI DOTTORATO DI RICERCA IN SCIENZE MEDICHE,
UNIVERSITÀ DEGLI STUDI DI PADOVA SEDE AMMINISTRATIVA: UNIVERSITÀ DEGLI STUDI DI PADOVA DIPARTIMENTO DI SCIENZE MEDICO-DIAGNOSTICHE E TERAPIE SPECIALI SCUOLA DI DOTTORATO DI RICERCA IN SCIENZE MEDICHE,
Supplemental Experimental Procedures
 Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
By the end of this session, the student should be able to:
 Valvular Heart disease HVD By Dr. Ashraf Abdelfatah Deyab VHD- Objectives By the end of this session, the student should be able to: Define and classify valvular heart disease. Enlist the causes of acquired
Valvular Heart disease HVD By Dr. Ashraf Abdelfatah Deyab VHD- Objectives By the end of this session, the student should be able to: Define and classify valvular heart disease. Enlist the causes of acquired
New Insights into the Puzzling Pathogenesis of Calcific Aortic Stenosis
 New Insights into the Puzzling Pathogenesis of Calcific Aortic Stenosis Chen Li *, BSc., Songyi Xu *, MSc., Avrum I. Gotlieb, MDCM, FRCPC Departments of Pathology, Toronto General Research Institute, University
New Insights into the Puzzling Pathogenesis of Calcific Aortic Stenosis Chen Li *, BSc., Songyi Xu *, MSc., Avrum I. Gotlieb, MDCM, FRCPC Departments of Pathology, Toronto General Research Institute, University
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Four Types of Vertebrate Tissue
 BIO 121 Molecular Cell Biology Lecture Section IV A. Cells in the Context of Tissue, Organ and Organismal Architecture B. Wound Healing Four Types of Vertebrate Tissue 1.Epithelium 2.Connective Tissue
BIO 121 Molecular Cell Biology Lecture Section IV A. Cells in the Context of Tissue, Organ and Organismal Architecture B. Wound Healing Four Types of Vertebrate Tissue 1.Epithelium 2.Connective Tissue
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG)
 In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG) 1 Dr Saeb Aliwaini 13/11/2015 Migration in vivo Primary tumors are responsible for only about 10%
In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG) 1 Dr Saeb Aliwaini 13/11/2015 Migration in vivo Primary tumors are responsible for only about 10%
Supplementary Figure 1:
 Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Tissue renewal and Repair. Nisamanee Charoenchon, PhD Department of Pathobiology, Faculty of Science
 Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
Tissue renewal and Repair Nisamanee Charoenchon, PhD Email: nisamanee.cha@mahidol.ac.th Department of Pathobiology, Faculty of Science Topic Objectives 1. Describe processes of tissue repair, regeneration
renoprotection therapy goals 208, 209
 Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
The Cardiovascular System Part I: Heart Outline of class lecture After studying part I of this chapter you should be able to:
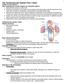 The Cardiovascular System Part I: Heart Outline of class lecture After studying part I of this chapter you should be able to: 1. Describe the functions of the heart 2. Describe the location of the heart,
The Cardiovascular System Part I: Heart Outline of class lecture After studying part I of this chapter you should be able to: 1. Describe the functions of the heart 2. Describe the location of the heart,
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
Cell Cell Communication
 IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
IBS 8102 Cell, Molecular, and Developmental Biology Cell Cell Communication January 29, 2008 Communicate What? Why do cells communicate? To govern or modify each other for the benefit of the organism differentiate
Tissue repair. (3&4 of 4)
 Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Tissue repair (3&4 of 4) What will we discuss today: Regeneration in tissue repair Scar formation Cutaneous wound healing Pathologic aspects of repair Regeneration in tissue repair Labile tissues rapid
Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and
 CHAPTER 4 Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and recognize normal tissues under the microscope
CHAPTER 4 Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and recognize normal tissues under the microscope
CorMatrix ECM Bioscaffold
 CorMatrix ECM Bioscaffold REMODEL. REGROW. RESTORE. CorMatrix ECM Bioscaffold provides a natural bioscaffold matrix that enables the body s own cells to repair and remodel damaged cardio-vascular tissue.
CorMatrix ECM Bioscaffold REMODEL. REGROW. RESTORE. CorMatrix ECM Bioscaffold provides a natural bioscaffold matrix that enables the body s own cells to repair and remodel damaged cardio-vascular tissue.
Principles of Genetics and Molecular Biology
 Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Abnormally differentiating keratinocytes in the epidermis of systemic sclerosis patients show enhanced secretion of CCN2 and S100A9
 Abnormally differentiating keratinocytes in the epidermis of systemic sclerosis patients show enhanced secretion of CCN2 and S1A9 Joanna Nikitorowicz-Buniak UCL Division of Medicine Systemic sclerosis
Abnormally differentiating keratinocytes in the epidermis of systemic sclerosis patients show enhanced secretion of CCN2 and S1A9 Joanna Nikitorowicz-Buniak UCL Division of Medicine Systemic sclerosis
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.
 Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
vi Preface Table 2 Association of Fibrosis With Types of Injury: Representative Examples
 Fibrosis or scar, defined pathologically as inappropriate repair by connective tissue, is increasingly recognized as an important feature of many chronic diseases (Table 1), and as such, represents an
Fibrosis or scar, defined pathologically as inappropriate repair by connective tissue, is increasingly recognized as an important feature of many chronic diseases (Table 1), and as such, represents an
CELL BIOLOGY - CLUTCH CH CELL JUNCTIONS AND TISSUES.
 !! www.clutchprep.com CONCEPT: CELL-CELL ADHESION Cells must be able to bind and interact with nearby cells in order to have functional and strong tissues Cells can in two main ways - Homophilic interactions
!! www.clutchprep.com CONCEPT: CELL-CELL ADHESION Cells must be able to bind and interact with nearby cells in order to have functional and strong tissues Cells can in two main ways - Homophilic interactions
Healing & Repair. Tissue Regeneration
 Healing & Repair Dr. Srikumar Chakravarthi Repair & Healing: Are they same? Repair :Regeneration of injured cells by cells of same type, as with regeneration of skin/oral mucosa (requires basement membrane)
Healing & Repair Dr. Srikumar Chakravarthi Repair & Healing: Are they same? Repair :Regeneration of injured cells by cells of same type, as with regeneration of skin/oral mucosa (requires basement membrane)
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Cells & Tissues. Chapter 3
 Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
A Comparative Study on Calcification of Aortic Valves
 University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2016 A Comparative Study on Calcification of Aortic Valves Shirin Masjedi University
University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2016 A Comparative Study on Calcification of Aortic Valves Shirin Masjedi University
HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade)
 HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade) 1. Mesenchymal stem cells (MSC) cultured on extracellular matrices with different stiffness exhibit diverse lineage commitment
HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade) 1. Mesenchymal stem cells (MSC) cultured on extracellular matrices with different stiffness exhibit diverse lineage commitment
CD34 + VEGFR-3 + progenitor cells have a potential to differentiate towards lymphatic endothelial cells
 CD34 + VEGFR-3 + progenitor cells have a potential to differentiate towards lymphatic endothelial cells Tan YZ et al. J Cell Mol Med. (2014 Mar;18(3):422-33) Denise Traxler-Weidenauer April 2014 Introduction
CD34 + VEGFR-3 + progenitor cells have a potential to differentiate towards lymphatic endothelial cells Tan YZ et al. J Cell Mol Med. (2014 Mar;18(3):422-33) Denise Traxler-Weidenauer April 2014 Introduction
A549 and A549-fLuc cells were maintained in high glucose Dulbecco modified
 Cell culture and animal model A549 and A549-fLuc cells were maintained in high glucose Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum at 37 C in humidified atmosphere containing
Cell culture and animal model A549 and A549-fLuc cells were maintained in high glucose Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum at 37 C in humidified atmosphere containing
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Case # 1. Page: 8. DUKE: Adams
 Case # 1 Page: 8 1. The cardiac output in this patient is reduced because of: O a) tamponade physiology O b) restrictive physiology O c) coronary artery disease O d) left bundle branch block Page: 8 1.
Case # 1 Page: 8 1. The cardiac output in this patient is reduced because of: O a) tamponade physiology O b) restrictive physiology O c) coronary artery disease O d) left bundle branch block Page: 8 1.
Evaluating Terminal Differentiation of Porcine Valvular Interstitial Cells In Vitro
 Evaluating Terminal Differentiation of Porcine Valvular Interstitial Cells In Vitro A Thesis submitted to the faculty of Worcester Polytechnic Institute in partial fulfillment of the requirements for the
Evaluating Terminal Differentiation of Porcine Valvular Interstitial Cells In Vitro A Thesis submitted to the faculty of Worcester Polytechnic Institute in partial fulfillment of the requirements for the
Most abundant and widely distributed tissues in the body Binds, support, and strengthen body tissues, protect and insulate internal organ, serve as
 Connective tissue Most abundant and widely distributed tissues in the body Binds, support, and strengthen body tissues, protect and insulate internal organ, serve as major transport system, compartmentalizes
Connective tissue Most abundant and widely distributed tissues in the body Binds, support, and strengthen body tissues, protect and insulate internal organ, serve as major transport system, compartmentalizes
Evaluation of the wound healing response post deep dermal heating by fractional RF: INTRAcel
 12th symposium of the Association of Korean Dermatologists (2009) 1 Evaluation of the wound healing response post deep dermal heating by fractional RF: INTRAcel Un-Cheol.Yeo, M.D. S&U Dermatologic Clinic,
12th symposium of the Association of Korean Dermatologists (2009) 1 Evaluation of the wound healing response post deep dermal heating by fractional RF: INTRAcel Un-Cheol.Yeo, M.D. S&U Dermatologic Clinic,
Pathology of Coronary Artery Disease
 Pathology of Coronary Artery Disease Seth J. Kligerman, MD Pathology of Coronary Artery Disease Seth Kligerman, MD Assistant Professor Medical Director of MRI University of Maryland Department of Radiology
Pathology of Coronary Artery Disease Seth J. Kligerman, MD Pathology of Coronary Artery Disease Seth Kligerman, MD Assistant Professor Medical Director of MRI University of Maryland Department of Radiology
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the
 Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
stem cell products Basement Membrane Matrix Products Rat Mesenchymal Stem Cell Growth and Differentiation Products
 stem cell products Basement Membrane Matrix Products Rat Mesenchymal Stem Cell Growth and Differentiation Products Stem Cell Qualified Extracellular Matrix Proteins Stem cell research requires the finest
stem cell products Basement Membrane Matrix Products Rat Mesenchymal Stem Cell Growth and Differentiation Products Stem Cell Qualified Extracellular Matrix Proteins Stem cell research requires the finest
Cells and Tissues 3PART D. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Cells and Tissues 3PART D Connective Tissue Found everywhere in the body Includes the most abundant
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Cells and Tissues 3PART D Connective Tissue Found everywhere in the body Includes the most abundant
TSDA Boot Camp September 13-16, Introduction to Aortic Valve Surgery. George L. Hicks, Jr., MD
 TSDA Boot Camp September 13-16, 2018 Introduction to Aortic Valve Surgery George L. Hicks, Jr., MD Aortic Valve Pathology and Treatment Valvular Aortic Stenosis in Adults Average Course (Post mortem data)
TSDA Boot Camp September 13-16, 2018 Introduction to Aortic Valve Surgery George L. Hicks, Jr., MD Aortic Valve Pathology and Treatment Valvular Aortic Stenosis in Adults Average Course (Post mortem data)
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Introduction Kit Components Cat. # # of vials Reagent Quantity Storage
 Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
Sheet #9. Dr. Heba Kalbouneh. Dr. Heba Kalbouneh. Dr. Heba Kalbouneh
 Sheet #9 Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Elastic fibers The main function of elastic fibers is to provide elasticity. In other words these fibers are able to restore the original
Sheet #9 Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Elastic fibers The main function of elastic fibers is to provide elasticity. In other words these fibers are able to restore the original
ulcer healing role 118 Bicarbonate, prostaglandins in duodenal cytoprotection 235, 236
 Subject Index Actin cellular forms 48, 49 epidermal growth factor, cytoskeletal change induction in mucosal repair 22, 23 wound repair 64, 65 polyamine effects on cytoskeleton 49 51 S-Adenosylmethionine
Subject Index Actin cellular forms 48, 49 epidermal growth factor, cytoskeletal change induction in mucosal repair 22, 23 wound repair 64, 65 polyamine effects on cytoskeleton 49 51 S-Adenosylmethionine
Cellular Physiology and Biochemistry
 Original Paper 2015 The Author(s). 2015 Published The Author(s) by S. Karger AG, Basel Published online: November 27, 2015 www.karger.com/cpb Published by S. Karger AG, Basel 2194 1421-9778/15/0376-2194$39.50/0
Original Paper 2015 The Author(s). 2015 Published The Author(s) by S. Karger AG, Basel Published online: November 27, 2015 www.karger.com/cpb Published by S. Karger AG, Basel 2194 1421-9778/15/0376-2194$39.50/0
Heart Dissection. 5. Locate the tip of the heart or the apex. Only the left ventricle extends all the way to the apex.
 Heart Dissection Page 1 of 6 Background: The heart is a four-chambered, hollow organ composed primarily of cardiac muscle tissue. It is located in the center of the chest in between the lungs. It is the
Heart Dissection Page 1 of 6 Background: The heart is a four-chambered, hollow organ composed primarily of cardiac muscle tissue. It is located in the center of the chest in between the lungs. It is the
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Mammalian Transport and The Heart
 Cardiovascular System AS-G, Chapters 8-9 Blood flows through the body in a closed system (circuit) driven by the pumping power of the heart Closed vs open: does the system have vessels contained the entire
Cardiovascular System AS-G, Chapters 8-9 Blood flows through the body in a closed system (circuit) driven by the pumping power of the heart Closed vs open: does the system have vessels contained the entire
Connective Tissue. Found everywhere in the body. Most abundant and widely distributed. Never exposed to the outside environment.
 Connective Tissue Found everywhere in the body. Most abundant and widely distributed. Never exposed to the outside environment. Connective Tissue Functions Binding and support Protection Insulation Transportation
Connective Tissue Found everywhere in the body. Most abundant and widely distributed. Never exposed to the outside environment. Connective Tissue Functions Binding and support Protection Insulation Transportation
Sarah J. Miller, DVM, Diplomate ACVIM (Cardiology) Degenerative Valvular Disease What s New?
 Sarah J. Miller, DVM, Diplomate ACVIM (Cardiology) Degenerative Valvular Disease What s New? Chronic degenerative valvular disease is the most common cardiovascular disease in small animals, and is also
Sarah J. Miller, DVM, Diplomate ACVIM (Cardiology) Degenerative Valvular Disease What s New? Chronic degenerative valvular disease is the most common cardiovascular disease in small animals, and is also
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Protein Kinase C Modulates Wnt Signaling In Colon Tumoral Cell Lines
 Protein Kinase C Modulates Wnt Signaling In Colon Tumoral Cell Lines Dra Martha Robles Flores Department of Biochemistry Facultad de Medicina Universidad Nacional Autónoma de México Tissue anatomy of the
Protein Kinase C Modulates Wnt Signaling In Colon Tumoral Cell Lines Dra Martha Robles Flores Department of Biochemistry Facultad de Medicina Universidad Nacional Autónoma de México Tissue anatomy of the
MI Acute occlusion of the proximal left anterior descending (LAD) artery is the cause of 40% to 50% of all MIs. *
 MI *33% -50% die before hospital lethal arrhythmia Sudden Cardiac Death. * Arrhythmias are caused by electrical abnormalities of the ischemic myocardium and conduction system. *Acute occlusion of the proximal
MI *33% -50% die before hospital lethal arrhythmia Sudden Cardiac Death. * Arrhythmias are caused by electrical abnormalities of the ischemic myocardium and conduction system. *Acute occlusion of the proximal
Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall
 Histol Histopath (1 990) 5: 161-1 67 Histology and Histopathology Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall Reinhold Kittelberger,
Histol Histopath (1 990) 5: 161-1 67 Histology and Histopathology Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall Reinhold Kittelberger,
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
Lower Secondary Science Blood Circulatory System Notes / Advanced Notes
 Lower Secondary Science Blood Circulatory System Notes / Advanced Notes Double Circulation in Mammals In mammals, there is a double circulation (i.e. blood passes through the heart twice in one complete
Lower Secondary Science Blood Circulatory System Notes / Advanced Notes Double Circulation in Mammals In mammals, there is a double circulation (i.e. blood passes through the heart twice in one complete
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
VIII Curso Internacional del PIRRECV. Some molecular mechanisms of cancer
 VIII Curso Internacional del PIRRECV Some molecular mechanisms of cancer Laboratorio de Comunicaciones Celulares, Centro FONDAP Estudios Moleculares de la Celula (CEMC), ICBM, Facultad de Medicina, Universidad
VIII Curso Internacional del PIRRECV Some molecular mechanisms of cancer Laboratorio de Comunicaciones Celulares, Centro FONDAP Estudios Moleculares de la Celula (CEMC), ICBM, Facultad de Medicina, Universidad
10. Thick deposits of lipids on the walls of blood vessels, called, can lead to serious circulatory issues. A. aneurysm B. atherosclerosis C.
 Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
Heart Student: 1. carry blood away from the heart. A. Arteries B. Veins C. Capillaries 2. What is the leading cause of heart attack and stroke in North America? A. alcohol B. smoking C. arteriosclerosis
Myocardial Infarction
 Myocardial Infarction MI = heart attack Defined as necrosis of heart muscle resulting from ischemia. A very significant cause of death worldwide. of these deaths, 33% -50% die before they can reach the
Myocardial Infarction MI = heart attack Defined as necrosis of heart muscle resulting from ischemia. A very significant cause of death worldwide. of these deaths, 33% -50% die before they can reach the
Evaluation of the wound healing response post - deep dermal heating by fractional RF: INTRACEL
 12th symposium of the Association of Korean Dermatologists (2009) 1 Evaluation of the wound healing response post - deep dermal heating by fractional RF: INTRACEL Un-Cheol.Yeo, MD S&U Dermatologic Clinic,
12th symposium of the Association of Korean Dermatologists (2009) 1 Evaluation of the wound healing response post - deep dermal heating by fractional RF: INTRACEL Un-Cheol.Yeo, MD S&U Dermatologic Clinic,
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair
 Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
End of chapter exercises
 End of chapter exercises Problem 1: The following diagrams show the heart during the cardiac cycle. The arrows represent the flow of blood. Study the diagrams and answer the questions that follow: Figure
End of chapter exercises Problem 1: The following diagrams show the heart during the cardiac cycle. The arrows represent the flow of blood. Study the diagrams and answer the questions that follow: Figure
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
In Vivo Animal Models of Heart Disease. Why Animal Models of Disease? Timothy A Hacker, PhD Department of Medicine University of Wisconsin-Madison
 In Vivo Animal Models of Heart Disease Timothy A Hacker, PhD Department of Medicine University of Wisconsin-Madison Why Animal Models of Disease? Heart Failure (HF) Leading cause of morbidity and mortality
In Vivo Animal Models of Heart Disease Timothy A Hacker, PhD Department of Medicine University of Wisconsin-Madison Why Animal Models of Disease? Heart Failure (HF) Leading cause of morbidity and mortality
Chapter 27 The Heart and Blood Vessels
 Chapter 27 The Heart and Blood Vessels Most animals have a closed blood system. The blood flows continuously in vessels back to the heart. In an open system the blood is pumped into open ended tubes and
Chapter 27 The Heart and Blood Vessels Most animals have a closed blood system. The blood flows continuously in vessels back to the heart. In an open system the blood is pumped into open ended tubes and
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3021 Supplementary figure 1 Characterisation of TIMPless fibroblasts. a) Relative gene expression of TIMPs1-4 by real time quantitative PCR (RT-qPCR) in WT or ΔTimp fibroblasts (mean ±
DOI: 10.1038/ncb3021 Supplementary figure 1 Characterisation of TIMPless fibroblasts. a) Relative gene expression of TIMPs1-4 by real time quantitative PCR (RT-qPCR) in WT or ΔTimp fibroblasts (mean ±
Role of Inflammation in Pulmonary Hypertension
 Role of Inflammation in Pulmonary Hypertension K. R. Stenmark University of Colorado Denver, USA Prominent Fibroproliferative Changes are Observed in the Lung Vasculature of Infants With Pulmonary Arterial
Role of Inflammation in Pulmonary Hypertension K. R. Stenmark University of Colorado Denver, USA Prominent Fibroproliferative Changes are Observed in the Lung Vasculature of Infants With Pulmonary Arterial
7.L.1.4 Circulatory System Guided Study Notes. Circulation
 1 7.L.1.4 Circulatory System Guided Study Notes Circulation Sect. 1: The Body s Transport System Sect. 2: A Closer Look at Blood Vessels Sect. 3: Blood and Lymph Sect. 4: Cardiovascular Health Sect. 1:
1 7.L.1.4 Circulatory System Guided Study Notes Circulation Sect. 1: The Body s Transport System Sect. 2: A Closer Look at Blood Vessels Sect. 3: Blood and Lymph Sect. 4: Cardiovascular Health Sect. 1:
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Journal Club Semmler Lorenz
 Beer et al. 2015 - Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration Journal Club 13.11.2017 1 Introduction to
Beer et al. 2015 - Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration Journal Club 13.11.2017 1 Introduction to
Pathophysiology of Cardiovascular System. Dr. Hemn Hassan Othman, PhD
 Pathophysiology of Cardiovascular System Dr. Hemn Hassan Othman, PhD hemn.othman@univsul.edu.iq What is the circulatory system? The circulatory system carries blood and dissolved substances to and from
Pathophysiology of Cardiovascular System Dr. Hemn Hassan Othman, PhD hemn.othman@univsul.edu.iq What is the circulatory system? The circulatory system carries blood and dissolved substances to and from
Role of Inflammatory and Progenitor Cells in Pulmonary Vascular Remodeling: Potential Role for Targeted Therapies. Traditional Hypothesis Stress
 3/1/212 Role of Inflammatory and Progenitor Cells in Pulmonary Vascular Remodeling: Potential Role for Targeted Therapies K.R. Stenmark University of Colorado Denver, CO 845 Prominent Fibroproliferative
3/1/212 Role of Inflammatory and Progenitor Cells in Pulmonary Vascular Remodeling: Potential Role for Targeted Therapies K.R. Stenmark University of Colorado Denver, CO 845 Prominent Fibroproliferative
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein
 Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
The blood returns from the body and enters right atrium using the vena cava. It passes through the tricuspid valve to the right ventricle.
 The blood returns from the body and enters right atrium using the vena cava. It passes through the tricuspid valve to the right ventricle. From this camber, it passes through the pulmonary semilunar valve
The blood returns from the body and enters right atrium using the vena cava. It passes through the tricuspid valve to the right ventricle. From this camber, it passes through the pulmonary semilunar valve
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Small Molecule Inhibitor of the Wnt Pathway (SM04755) as a Potential Topical Scleroderma Treatment
 Small Molecule Inhibitor of the Wnt Pathway (SM755) as a Potential Topical Scleroderma Treatment Vishal Deshmukh, PhD, Allison Hood, Yusuf Yazici, MD Disclosures Vishal Deshmukh, Ph.D. o Financial disclosure:
Small Molecule Inhibitor of the Wnt Pathway (SM755) as a Potential Topical Scleroderma Treatment Vishal Deshmukh, PhD, Allison Hood, Yusuf Yazici, MD Disclosures Vishal Deshmukh, Ph.D. o Financial disclosure:
Most mammalian cells are located in tissues where they are surrounded by a complex extracellular matrix (ECM) often referred to as connective tissue.
 GLYCOSAMINOGLYCANS Most mammalian cells are located in tissues where they are surrounded by a complex extracellular matrix (ECM) often referred to as connective tissue. The ECM contains three major classes
GLYCOSAMINOGLYCANS Most mammalian cells are located in tissues where they are surrounded by a complex extracellular matrix (ECM) often referred to as connective tissue. The ECM contains three major classes
Principles of cell signaling Lecture 4
 Principles of cell signaling Lecture 4 Johan Lennartsson Molecular Cell Biology (1BG320), 2014 Johan.Lennartsson@licr.uu.se 1 Receptor tyrosine kinase-induced signal transduction Erk MAP kinase pathway
Principles of cell signaling Lecture 4 Johan Lennartsson Molecular Cell Biology (1BG320), 2014 Johan.Lennartsson@licr.uu.se 1 Receptor tyrosine kinase-induced signal transduction Erk MAP kinase pathway
The circulatory system transports blood to deliver important substances, such as oxygen, to cells and to remove wastes, such as carbon dioxide.
 Section 1: The circulatory system transports blood to deliver important substances, such as oxygen, to cells and to remove wastes, such as carbon dioxide. K What I Know W What I Want to Find Out L What
Section 1: The circulatory system transports blood to deliver important substances, such as oxygen, to cells and to remove wastes, such as carbon dioxide. K What I Know W What I Want to Find Out L What
EARLY INFLAMMATORY RESPONSES TO VASCULAR DEVICES
 EARLY INFLAMMATORY RESPONSES TO VASCULAR DEVICES JAMES M. ANDERSON, MD, PhD DISTINGUISHED UNIVERSITY PROFESSOR DEPARTMENTS OF PATHOLOGY, MACROMOLECULAR SCIENCE, AND BIOMEDICAL ENGINEERING CASE WESTERN
EARLY INFLAMMATORY RESPONSES TO VASCULAR DEVICES JAMES M. ANDERSON, MD, PhD DISTINGUISHED UNIVERSITY PROFESSOR DEPARTMENTS OF PATHOLOGY, MACROMOLECULAR SCIENCE, AND BIOMEDICAL ENGINEERING CASE WESTERN
Healing and Repair. Dr. Nabila Hamdi MD, PhD
 Healing and Repair Dr. Nabila Hamdi MD, PhD 1 ILOs Know the classification of human cells according to their ability for proliferation. Understand the mechanism of cellular regeneration. Identify the types
Healing and Repair Dr. Nabila Hamdi MD, PhD 1 ILOs Know the classification of human cells according to their ability for proliferation. Understand the mechanism of cellular regeneration. Identify the types
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats
 Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Historical perspective R1 黃維立
 Degenerative mitral valve disease refers to a spectrum of conditions in which morphologic changes in the connective tissue of the mitral valve cause structural lesions that prevent normal function of the
Degenerative mitral valve disease refers to a spectrum of conditions in which morphologic changes in the connective tissue of the mitral valve cause structural lesions that prevent normal function of the
Cardiovascular System Note-Taking Guide
 FUNctions: Name: 3-27-14 Cardiovascular System Note-Taking Guide Heart: Pumps and delivers through the body Blood: Vessels: Delivers and to the body Carries waste and Maintains homeostasis - Carries blood
FUNctions: Name: 3-27-14 Cardiovascular System Note-Taking Guide Heart: Pumps and delivers through the body Blood: Vessels: Delivers and to the body Carries waste and Maintains homeostasis - Carries blood
Human Anatomy and Physiology- Problem Drill 04: Tissues of the Body
 Human Anatomy and Physiology- Problem Drill 04: Tissues of the Body Question No. 1 of 10 A biopsy sample is obtained from a lesion on the right cheek of a male patient. A technician in the histology lab
Human Anatomy and Physiology- Problem Drill 04: Tissues of the Body Question No. 1 of 10 A biopsy sample is obtained from a lesion on the right cheek of a male patient. A technician in the histology lab
Adult Echocardiography Examination Content Outline
 Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
Anatomy of the Heart
 Biology 212: Anatomy and Physiology II Anatomy of the Heart References: Saladin, KS: Anatomy and Physiology, The Unity of Form and Function 8 th (2018). Required reading before beginning this lab: Chapter
Biology 212: Anatomy and Physiology II Anatomy of the Heart References: Saladin, KS: Anatomy and Physiology, The Unity of Form and Function 8 th (2018). Required reading before beginning this lab: Chapter
ROMANIAN ACADEMY INSTITUTE OF CELLULAR BIOLOGY AND PATHOLOGY NICOLAE SIMIONESCU. PhD THESIS Summary
 ROMANIAN ACADEMY INSTITUTE OF CELLULAR BIOLOGY AND PATHOLOGY NICOLAE SIMIONESCU PhD THESIS Summary INVOLVEMENT OF ALARMIN HMGB1 IN INFLAMMATORY PROCESSES ASSOCIATED WITH VASCULAR DYSFUNCTION IN HYPERLIPIDEMIA
ROMANIAN ACADEMY INSTITUTE OF CELLULAR BIOLOGY AND PATHOLOGY NICOLAE SIMIONESCU PhD THESIS Summary INVOLVEMENT OF ALARMIN HMGB1 IN INFLAMMATORY PROCESSES ASSOCIATED WITH VASCULAR DYSFUNCTION IN HYPERLIPIDEMIA
PROSTHETIC VALVE BOARD REVIEW
 PROSTHETIC VALVE BOARD REVIEW The correct answer D This two chamber view shows a porcine mitral prosthesis with the typical appearance of the struts although the leaflets are not well seen. The valve
PROSTHETIC VALVE BOARD REVIEW The correct answer D This two chamber view shows a porcine mitral prosthesis with the typical appearance of the struts although the leaflets are not well seen. The valve
