Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots
|
|
|
- Kathryn Dawson
- 5 years ago
- Views:
Transcription
1 Short Report 1301 Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots Joshua Z. Rappoport* and Sanford M. Simon The Rockefeller University, 1230 York Avenue, New York, NY 10065, USA *Present address: The University of Birmingham, School of Biosciences, Edgbaston, Birmingham B15 2TT, UK Author for correspondence ( Accepted 16 January 2009 Journal of Cell Science 122, Published by The Company of Biologists 2009 doi: /jcs Summary The removal of the epidermal growth factor receptor (EGFR) from the cell surface by endocytosis is triggered by receptor activation, but many facets of EGFR trafficking remain unresolved. We employed total internal fluorescence microscopy to elucidate the dynamics of activated EGFR at the cell surface through live-cell imaging. The results of these studies demonstrate that: (1) EGFR does not localize to caveolae in live cells either before or after activation; (2) EGFR does localize to clathrin-coated pits, but only after activation; (3) activation does not result in the formation of new clathrin-coated pits; (4) activated EGFR clusters at sites of preformed clathrin lattices; (5) The AP-2 complex is involved in the internalization of activated EGFR. Using imaging techniques to show the endocytic sorting of activated EGFR for the first time in live cells, these studies suggest a refinement of the model for EGFR entry. Supplementary material available online at Key words: EGF receptor, Total internal reflection fluorescence microscopy, Endocytosis Introduction The epidermal growth factor receptor (EGFR) is a frequently studied canonical member of the receptor tyrosine kinase superfamily (Atalay et al., 2003). EGFR family members play roles in important physiological processes such as wound healing (Vermeer et al., 2003). EGFR is also a target of study for cancer biologists. EGFR is an oncogene, increased EGFR signaling is associated with many cancers (Arteaga, 2002; Khazaie et al., 1993) and several anti-cancer drugs function through attenuation of EGFR signaling (Atalay et al., 2003). Additionally, high EGFR expression correlates with resistance of estrogen receptor-positive breast cancer to antiestrogen therapy (Lichtner, 2003), and metastatic cancer cells migrate towards sources of EGF (Bailly et al., 2000). EGFR is removed from the cell surface by endocytosis, which is triggered by receptor activation (Wiley, 2003). During internalization, EGFR signaling persists, even after entry into the endosomal system (Sorkin, 2001). Some reports have indicated that EGFR can enter the endocytic system via caveolae (Orlichenko et al., 2006; Sigismund et al., 2005; Zhu et al., 2005). This conclusion is based upon studies employing concentrations of EGF of >20 ng/ml. Other reports have concluded that even at concentrations as high as 100 ng/ml EGFR solely enters through clathrin-mediated endocytosis (Kazazic et al., 2006). Evidence supporting the hypothesis that EGFR employs a clathrinmediated pathway includes fluorescence imaging studies, as well as those in which inhibitors of this route reduced EGFR uptake (Benesch et al., 2005; Benmerah et al., 1999; Huang et al., 2004). Some studies have tried to reconcile the conflicting data between clathrin- and caveolae-mediated EGFR entry. One hypothesis states that inactive EGFR resides in caveolae until ligand binding stimulates exit from this compartment followed by clathrindependent entry (Mineo et al., 1999). In some studies that have concluded that EGFR enters through clathrin, it has been reported that the activated EGFR induces formation of new clathrin-coated pits formed de novo (Johannessen et al., 2006; Puri et al., 2005; Wilde et al., 1999). Another unresolved issue is whether there is a role for the clathrin adaptor AP-2 (also known as AP2A) in the trafficking of activated EGFR (Hinrichsen et al., 2003; Huang et al., 2004; Motley et al., 2003). Two studies employing very similar methods suggested that AP-2 is dispensable for EGFR entry (Hinrichsen et al., 2003; Motley et al., 2003). However, a subsequent report suggested that these results might actually have been experimental artifacts (Huang et al., 2004). Specifically, the studies that suggested the AP-2 is dispensable for EGFR entry employed pre-binding of EGF at 4 C, whereas the one that showed a role for AP-2 demonstrated that continuous incubation at 37 C was required for this effect to be measured. We set out to image the dynamics of EGFR at the cell surface. Our results demonstrate that (1) activated EGFR enters through clathrin-coated vesicles and not caveolae; (2) following activation, EGFR clusters at the sites of preformed clathrin lattices; and (3) the AP-2 complex plays a role in EGFR trafficking. Results and Discussion To image the dynamics of activated EGFR, HeLa cells were transfected with a construct encoding EGFP-tagged EGFR (EGFR- EGFP), shown previously to retain functionality (Carter and Sorkin, 1998), and imaged with total internal reflection fluorescence microscopy (TIR-FM). Based upon pilot studies aimed at determining conditions by which endocytic sorting and internalization of activated EGFR could be studied, we pre-
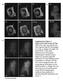
2 1302 Journal of Cell Science 122 (9) Fig. 1. EGFR does not reside in caveolae prior to EGF stimulation. (A) TIR- FM and (B) quantification demonstrates that prior to addition of EGF, EGFR does not colocalize with caveolin 1. Fluorescence of EGFR at sites of caveolae is nearly identical to the intensity outside caveolin 1 spots. Fig. 2. Activated EGFR colocalizes with clathrin, and not caveolin 1. (A) EGFR-EGFP and clathrin-dsred, or caveolin 1-mRFP, were imaged by simultaneous two-color TIR-FM 10 minutes after addition of EGF. Colocalization of EGFR and clathrin appears yellow in overlay. (B,C) Quantification demonstrates colocalization of clathrin at the sites of EGFR clusters (B) but not caveolin 1 (C). incubated cells in serum-free medium for 15 minutes on the microscope stage at 37 C. Under these conditions, the EGFR-EGFP showed a diffuse fluorescence over the cell surface (Fig. 1). It has been previously proposed that EGFR resides in a caveolar compartment until the receptor is activated, at which point it exits this compartment (Mineo et al., 1999). To test this we quantified the fluorescence of EGFR at caveolin 1 sites before the addition of EGF (Fig. 1). Circles were drawn around 250 regions of caveolin 1 and 250 regions devoid of caveolin 1. The EGFR fluorescence intensity within the 250 caveolin 1 sites was nearly identical to that outside of the caveolin 1 sites. Thus our analysis suggests that in cells continuously incubated at 37 C, EGFR is not significantly accumulated at caveolae prior to stimulation with EGF. After addition of EGF (100 ng/ml) to the bath, the EGFR-EGFP on the cell surface redistributed into discrete spots (supplementary material Fig. S1). Individual spots of tagged EGFR were visible as early as 1-2 minutes after ligand addition and the clustering process was complete in 6 minutes. Clusters of EGFR seemed to form more rapidly and to a greater extent at the cell periphery rather than towards the center of the adherent plasma membrane. This could reflect increased accessibility of receptors to ligand near the cell edge or may reflect a yet-to-be identified mechanism for increased formation of EGFR spots, or increased receptor concentration, at these sites. Clustering was apparent even at lower concentrations EGF (2 ng/ml; data not shown). However, we used the 100 ng/ml concentration of EGF because it has been previously suggested that at higher concentrations, the EGFR can be targeted to caveolae for internalization (Orlichenko et al., 2006; Sigismund et al., 2005). To determine the route of endocytosis of activated EGFR we coexpressed either clathrin-dsred or caveolin 1-mRFP along with EGFR-EGFP, and then imaged cells using simultaneous dual-color TIR-FM (Fig. 2). In order to evaluate whether activated EGFR enters the cell through clathrin and/or caveolae, clusters of EGFR were circled 10 minutes after addition of 100 ng/ml EGF. These regions were then transferred to either the corresponding clathrin or caveolin 1 images and the percentage of colocalizing spots was logged. Quantitative analysis of 250 EGFR spots per group from five cells revealed that there was ~90% colocalization with clathrin. Furthermore, as a control for random spot alignment 250 regions that did not contain EGFR clusters were analyzed; these were found to have only ~3% colocalization with clathrin. When the clusters of EGFR-EGFP that colocalized with clathrin-dsred were subsequently followed, both fluorophores were observed to disappear from the cells surface at the same time and the same rate (supplementary material Fig. S2), consistent with previous reports that EGFR-EGFP can be visualized internalizing with clathrin tagged with a red fluorescent protein in TIR-FM studies (supplementary material Fig. S2) (Benesch et al., 2005). Similar methods were applied to determine whether EGFR localized to caveolae after addition of EGF. When 250 EGFR spots were marked and these regions transferred to the caveolin 1 images, ~20% of these spots colocalized with caveolin 1. However, the proportion of the 250 regions that was lacking in EGFR clusters but which colocalized with caveolin 1-mRFP was also ~20% and statistically indistinguishable (P>0.05). This would suggest that the ~20% colocalization between EGFR and caveolin 1 represented a random alignment of spots, emphasizing the importance of controls in studies such as these. Thus our conclusion is that, even in the presence of high EGF concentrations, activated EGFR internalizes solely through clathrin-mediated endocytosis. The preceding results demonstrated that during incubation with EGF, the EGF receptor accumulates with clathrin, but not with caveolin 1. To resolve whether clathrin was being recruited to activated EGFR, or if activated EGFR was being recruited to preexisting clathrin spots, the fluorescence of clathrin-dsred and EGFR-EGFP was followed during the addition of EGF. After addition of EGF, it took 6-10 minutes for a maximal overlap of
3 Imaging EGFR clustering 1303 Fig. 3. Activated EGFR predominantly clusters at sites of preformed clathrin. (A) EGFR-EGFP and clathrin-dsred were imaged by simultaneous two-color TIR-FM. Colocalization between clathrin imaged prior to addition of EGF (red), and EGFR observed at 10 minutes after stimulation (green) appears yellow in the overlay. (B) The number of clathrin spots on the cell surface counted before and 10 minutes after EGF addition is almost the same. (C) The percentage of EGFR clusters that formed at sites where clathrin was present prior to EGF addition was compared with the percentage that appeared where clathrin was not identified at time 0. (D) The clathrin and EGFR fluorescence intensities were logged every 2 minutes following EGF addition at the sites of EGFR clusters that colocalized with preformed clathrin. EGFR and clathrin (Fig. 3). The first approach to analyzing the data was to identify clathrin spots just prior to EGF addition. The fluorescence of EGFR-EGFP and the fluorescence of clathrin-dsred were quantified within these clathrin spots (Fig. 3D). These static clathrin spots that existed prior to EGF, were the sites at which the EGFR clusters formed (yellow in overlay). A second analysis quantified the clathrin spots within 40 μm 40 μm square regions before, and 10 minutes after the addition of EGF (Fig. 3B). Four regions were analyzed in four cells per group to determine if the number of clathrin spots on the cell surface changed as a result of EGFR activation. Prior to addition of EGF 20.9±0.9 spots per unit area were counted, and 10 minutes after EGF addition 19.6±0.3, P=0.21. Thus, activation of EGFR signaling does not cause a detectable increase in the formation of new clathrin-coated pits in live cells at 37 C. In a third approach to quantifying these results, 200 EGFR clusters (40 per cell from five cells) were identified 10 minutes after addition of EGF. These regions were then transferred to the corresponding clathrin images acquired before EGF stimulation. Then we determined the percentage of EGFR clusters that formed at preformed clathrin spots, as opposed to those that formed as clathrin was polymerizing de novo at the same site (Fig. 3C). There were 2.7-fold more EGFR clusters formed at pre-existing clathrin spots ( preformed ), than where clathrin was not present before EGF addition ( de novo ). Although 73% of EGFR clusters corresponded to sites where clathrin spots existed before EGF addition, only 27% of EGFR spots formed where clathrin was not present previously. It should be noted that lateral movement of clathrin spots in the plane of the plasma membrane could be a source of error in this analysis. This movement would lead to an over-estimation of the number of newly formed spots: any clathrin spots that moved even a short distance on the surface during the 10 minutes post-egf treatment, would not be counted as pre-existing (Keyel et al., 2004; Rappoport et al., 2003b). Thus, the percentage of EGFR spots that are recruited to pre-existing sites of clathrin may be greater than 73%. A fourth analysis involved imaging EGFR and clathrin for 10 minutes after the addition of EGF, then marking EGFR clusters which colocalized with preformed clathrin spots, and then running the images in reverse to quantify the fluorescence of EGFR and clathrin at these spots during the preceding 10 minutes. An average of the EGFR and clathrin fluorescence from 57 spots shows that although the intensity of clathrin is stable over time at these sites, EGFR signal gradually rose from background to a plateau ~6 minutes post-stimulation. Thus, activated EGFR predominantly clusters at sites of preformed clathrin, rather than inducing formation of new clathrin coats. These results demonstrate that although the EGFR signal increases over time, that of clathrin is constant. This demonstrates that there is neither an increase in the formation of new clathrin coats, nor an increase in clathrin signal at extant coats, as a result of activation of EGFR. Precedence for this type of behavior has been observed in other systems (e.g. G- protein-coupled receptors), which show that activated receptors cluster at preformed clathrin spots (Santini et al., 2002; Scott et al., 2002). We next tested whether the hetero-tetrameric AP-2 adaptor complex is involved in the recruitment of EGFR to the clathrin spots or subsequent internalization of EGFR, by reducing the levels of α-adaptin with sirna silencing (Fig. 4). The expression of α- adaptin was reduced ~85% by treatment with sirna (Fig. 4B). This
4 1304 Journal of Cell Science 122 (9) Fig. 4. The AP-2 complex is involved in the trafficking of activated EGFR. (A) TIR-FM comparing cells transfected with sirna against α-adaptin demonstrates reduction of transferrin (Tf) uptake as well as apparent effects on EGFR distribution. Scale bars shown in image in upper left of (A) can be applied to all images in figure. (B) Western blot probed with anti-αadaptin, as well as control anti-gapdh, reveals potent and specific silencing. (C) Cells transfected with sirna, demonstrating reductions in transferrin uptake, do not show alterations in the number of EGFR spots but do show (D) a ~40% reduction in EGF spot intensity relative to local background. reduction of α-adaptin was sufficient to reduce the internalization of transferrin (in Fig. 4A, compare the second row with the fourth row). Use of transferrin as an independent marker for sirna silencing is essential as not all cells appeared to be transfected. Treatment with the sirna to α-adaptin also reduced internalization of the EGFR after treatment with EGF (compare Fig. 4A top row with the third row). By 10 minutes after EGF addition, the number of EGFR spots were the same with or without sirna, but the fluorescence of EGFR spots relative to local background fluorescence was ~40% lower in the sirna-treated group. This difference was due to an increase in EGFR background, rather than a decrease in gross intensity of the EGFR spots. Much of the receptor was fairly evenly spread all over the cell surface, except at the tips of the filopodia where it accumulated at fairly high concentrations (Fig. 4A, third row). The observation of an ~40% decrease in EGFR entry with sirna silencing of α-adaptin is consistent with the results from another study evaluating EGFR endocytosis by 125 I-EGF uptake with and without sirna treatment (Huang et al., 2004), although it is not consistent with two other studies which claimed that AP-2 sirna has no effect on EGFR entry (Hinrichsen et al., 2003; Motley et al., 2003). One difference in how these studies were conducted is temperature: those that reported AP-2 sirna to have an effect on EGFR entry were performed at 37 C; (Huang et al., 2004), whereas in the two claiming no role for AP-2 in EGFR endocytosis, EGF binding was performed at 4 C (Hinrichsen et al., 2003; Motley et al., 2003). Thus, it is our conclusion that AP-2 is indeed required for EGFR endocytosis, and our results confirm that this defect is not one of clustering per se, but instead one of entry. These results demonstrate that when EGFR is examined at 37 C, upon activation, the EGFR accumulates at discrete spots of clathrin that existed prior to the addition of EGF. The EGFR then internalizes with the clathrin. At no point is the EGFR enriched at sites of caveolin 1. Efficient recruitment of the EGFR to clathrin requires the presence of AP-2, in that this process is blocked with expression of α-adaptin and is reduced by sirna. Thus, these results demonstrate the benefits of live-cell imaging. However, it should be noted that TIR-FM is limited to observation of the adherent cell surface and it is possible that alternative pathways for EGFR entry exists on the opposite surface. Materials and Methods Plasmid constructs and sirna The construct encoding rat clathrin light chain A tagged with dsred (clathrin-dsred) was a gift from Tomas Kirchhausen (Harvard Medical School, Boston, MA). EGFPtagged EGFR was a gift from Alexander Sorkin (University of Colorado Health Sciences Center, Aurora, CO). Caveolin 1-mRFP was a gift from Ari Helenius (ETH Institut of Biochemistry, Zurich, Switzerland). sirna against α-adaptin was obtained from Dharmacon (Lafayette, CO) and matched that used in other studies (Huang et al., 2004; Motley et al., 2003). Cell culture HeLa cells were obtained from the American Type Culture Collection (Manassas, VA) and were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum in an incubator at 37 C in a humidified 5% CO 2 atmosphere. Cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the supplier s directions. Cells were imaged 48 hours post-transfection, except in sirna studies in which cells were plated on day 1, transfected with sirna on day 2 and then split 1:2 on day 3, transfected with sirna + DNA on day 4 and then imaged on day 6. EGF was purchased from Sigma (St Louis, MO) and prepared according to the supplier s directions. Total internal reflection fluorescence microscopy (TIR-FM) image acquisition TIR-FM was performed at 37 C using an Apo NA microscope objective (Olympus America, Melville, NY), as previously described (Rappoport and Simon, 2003). Clathrin-dsRed (or caveolin-1-mrfp) and EGFR-EGFP were excited with the 488 nm line of a tunable argon laser (Omnichrome, model 543-AP A01, Melles Griot, Carlsbad, CA) reflected off a 498 nm dichroic mirror. All mirrors and filters were obtained from Chroma Technologies (Brattleboro, VT). Green and red emissions were collected simultaneously using a dual-view splitter (Optical Insights, Santa Fe, NM) equipped with a 515/30 nm band-pass filter to collect green emission, a 550 nm dichroic mirror to split the emission, and a 580 nm long-pass filter to collect red emission. The camera used to acquire images was a ORCA-ER (Hamamatsu Photonics, Bridgewater, NJ). The camera and a mechanical shutter (Uniblitz, Vincent Associates, Rochester, NY) were controlled by MetaMorph software (Universal Imaging, Downingtown, PA).
5 Imaging EGFR clustering 1305 Dual-color processing After subtraction of extracellular background, 12-bit dual-color TIR-FM image streams were aligned in MetaMorph. On the basis of single-fluorophore control experiments, green-to-red bleed-through corrections of 10% were employed; following background subtraction and alignment, 10% of the EGFP signal was subtracted from dsred images. Data analysis Paradigms determined from our previous studies in this area were applied to the current data sets (Rappoport and Simon, 2003). Spots of clathrin, caveolin 1 and EGFR were identified in single static images by circling 8 pixel 8 pixel regions in MetaMorph. Only spots that appeared to overlap with neighbors were circled. Otherwise, identification was random. Spots were recorded as colocalizing if the majority of the spot intensity in the corresponding image from the other channel fitted within the confines of the drawn regions. Transferrin uptake studies Cells were incubated at 37 C in serum-free DMEM for 15 minutes, and then for another 15 minutes at 37 C in Alexa-Fluor-546 transferrin (Molecular Probes, Invitrogen, Carlsbad, CA) diluted 1:500. After 5 minutes of incubation with transferrin, EGF was added at 100 ng/ml. After 10 minutes in transferrin plus EGF, cells were rinsed, fixed and analyzed as previously described (Rappoport et al., 2003a). Western blots Cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer, clarified by centrifugation and then analyzed by 10% SDS-PAGE. After transfer and blocking in 5% milk, blots were probed with polyclonal rabbit anti-α-adaptin (M300) and polyclonal rabbit anti-gapdh (Santa Cruz Biotechnology, Santa Cruz, CA). Bands were visualized with ECL+ (Amersham, Piscataway, NJ) following detection with HRP-conjugated goat anti-rabbit secondary antibody (Sigma). We are grateful for the support of NSF BES and NIH P20 GM (to SMS). We would like to thank Alexandre Benmerah for critical evaluation of this manuscript. Deposited in PMC for release after 12 months. References Arteaga, C. L. (2002). Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist 7 Suppl. 4, Atalay, G., Cardoso, F., Awada, A. and Piccart, M. J. (2003). Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Ann. Oncol. 14, Bailly, M., Wyckoff, J., Bouzahzah, B., Hammerman, R., Sylvestre, V., Cammer, M., Pestell, R. and Segall, J. E. (2000). Epidermal growth factor receptor distribution during chemotactic responses. Mol. Biol. Cell 11, Benesch, S., Polo, S., Lai, F. P., Anderson, K. I., Stradal, T. E., Wehland, J. and Rottner, K. (2005). N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J. Cell Sci. 118, Benmerah, A., Bayrou, M., Cerf-Bensussan, N. and Dautry-Varsat, A. (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, Carter, R. E. and Sorkin, A. (1998). Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. J. Biol. Chem. 273, Hinrichsen, L., Harborth, J., Andrees, L., Weber, K. and Ungewickell, E. J. (2003). Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 278, Huang, F., Khvorova, A., Marshall, W. and Sorkin, A. (2004). Analysis of clathrinmediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 279, Johannessen, L. E., Pedersen, N. M., Pedersen, K. W., Madshus, I. H. and Stang, E. (2006). Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and Grb2-containing clathrin-coated pits. Mol. Cell. Biol. 26, Kazazic, M., Roepstorff, K., Johannessen, L. E., Pedersen, N. M., van Deurs, B., Stang, E. and Madshus, I. H. (2006). EGF-induced activation of the EGF receptor does not trigger mobilization of caveolae. Traffic 7, Keyel, P. A., Watkins, S. C. and Traub, L. M. (2004). Endocytic adaptor molecules reveal an endosomal population of clathrin by total internal reflection fluorescence microscopy. J. Biol. Chem. 279, Khazaie, K., Schirrmacher, V. and Lichtner, R. B. (1993). EGF receptor in neoplasia and metastasis. Cancer Metastasis Rev. 12, Lichtner, R. B. (2003). Estrogen/EGF receptor interactions in breast cancer: rationale for new therapeutic combination strategies. Biomed. Pharmacother. 57, Mineo, C., Gill, G. N. and Anderson, R. G. (1999). Regulated migration of epidermal growth factor receptor from caveolae. J. Biol. Chem. 274, Motley, A., Bright, N. A., Seaman, M. N. and Robinson, M. S. (2003). Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162, Orlichenko, L., Huang, B., Krueger, E. and McNiven, M. A. (2006). Epithelial growth factor-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J. Biol. Chem. 281, Puri, C., Tosoni, D., Comai, R., Rabellino, A., Segat, D., Caneva, F., Luzzi, P., Di Fiore, P. P. and Tacchetti, C. (2005). Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol. Biol. Cell 16, Rappoport, J. Z. and Simon, S. M. (2003). Real-time analysis of clathrin-mediated endocytosis during cell migration. J. Cell Sci. 116, Rappoport, J. Z., Taha, B. W., Lemeer, S., Benmerah, A. and Simon, S. M. (2003a). The AP-2 complex is excluded from the dynamic population of plasma membraneassociated clathrin. J. Biol. Chem. 278, Rappoport, J. Z., Taha, B. W. and Simon, S. M. (2003b). Movement of plasma-membraneassociated clathrin spots along the microtubule cytoskeleton. Traffic 4, Santini, F., Gaidarov, I. and Keen, J. H. (2002). G protein-coupled receptor/arrestin 3 modulation of the endocytic machinery. J. Cell Biol. 156, Scott, M. G., Benmerah, A., Muntaner, O. and Marullo, S. (2002). Recruitment of activated G protein-coupled receptors to pre-existing clathrin-coated pits in living cells. J. Biol. Chem. 277, Sigismund, S., Woelk, T., Puri, C., Maspero, E., Tacchetti, C., Transidico, P., Di Fiore, P. P. and Polo, S. (2005). Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102, Sorkin, A. (2001). Internalization of the epidermal growth factor receptor: role in signalling. Biochem. Soc. Trans. 29, Vermeer, P. D., Einwalter, L. A., Moninger, T. O., Rokhlina, T., Kern, J. A., Zabner, J. and Welsh, M. J. (2003). Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422, Wilde, A., Beattie, E. C., Lem, L., Riethof, D. A., Liu, S. H., Mobley, W. C., Soriano, P. and Brodsky, F. M. (1999). EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell 96, Wiley, H. S. (2003). Trafficking of the ErbB receptors and its influence on signaling. Exp. Cell Res. 284, Zhu, J. X., Goldoni, S., Bix, G., Owens, R. T., McQuillan, D. J., Reed, C. C. and Iozzo, R. V. (2005). Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J. Biol. Chem. 280,
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
EPI TIR-FM min
 a 15 b EPI 45 75 min TIR-FM c min Supplementary figure 1. Fluorescence microscopy of Gag- GFP. HeLa cells were transfected with Gag and Gag-GFP and imaged at 5-6 hpt. a, Images of a single cell observed
a 15 b EPI 45 75 min TIR-FM c min Supplementary figure 1. Fluorescence microscopy of Gag- GFP. HeLa cells were transfected with Gag and Gag-GFP and imaged at 5-6 hpt. a, Images of a single cell observed
TITLE: Effect of MUC1 Expression on EGFR Endocytosis and Degradation in Human Breast Cancer Cell Lines
 AD AWARD NUMBER: W81XWH-06-1-0464 TITLE: Effect of MUC1 Expression on EGFR Endocytosis and Degradation in Human Breast Cancer Cell Lines PRINCIPAL INVESTIGATOR: Rachid M El Bejjani CONTRACTING ORGANIZATION:
AD AWARD NUMBER: W81XWH-06-1-0464 TITLE: Effect of MUC1 Expression on EGFR Endocytosis and Degradation in Human Breast Cancer Cell Lines PRINCIPAL INVESTIGATOR: Rachid M El Bejjani CONTRACTING ORGANIZATION:
Rappoport, J. Z. and Simon, S. M. 116
 AUTHOR CORRECTION Rappoport, J. Z. and Simon, S. M. (2003). Real-time analysis of clathrin-mediated endocytosis during cell migration. J. Cell Sci. 116, 847-855. In both the online and print versions of
AUTHOR CORRECTION Rappoport, J. Z. and Simon, S. M. (2003). Real-time analysis of clathrin-mediated endocytosis during cell migration. J. Cell Sci. 116, 847-855. In both the online and print versions of
Lipidne mikrodomene. funkcija
 Lipidne mikrodomene funkcija 1 Cellular processes involving lipid rafts - Signal transduction - Protein and lipid trafficking and sorting - Endosome(clathrin)-independent endocytosis: - potocytosis and
Lipidne mikrodomene funkcija 1 Cellular processes involving lipid rafts - Signal transduction - Protein and lipid trafficking and sorting - Endosome(clathrin)-independent endocytosis: - potocytosis and
Dynamic Interaction of HIV-1 Nef with the Clathrin-Mediated Endocytic Pathway at the Plasma Membrane
 Traffic 2007; 8: 61 76 Blackwell Munksgaard Dynamic Interaction of HIV-1 Nef with the Clathrin-Mediated Endocytic Pathway at the Plasma Membrane # 2006 The Authors Journal compilation # 2006 Blackwell
Traffic 2007; 8: 61 76 Blackwell Munksgaard Dynamic Interaction of HIV-1 Nef with the Clathrin-Mediated Endocytic Pathway at the Plasma Membrane # 2006 The Authors Journal compilation # 2006 Blackwell
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Ubiquitination is a posttranslational modification whereby
 Clathrin-independent endocytosis of ubiquitinated cargos Sara Sigismund*, Tanja Woelk*, Claudia Puri, Elena Maspero*, Carlo Tacchetti, Pietro Transidico, Pier Paolo Di Fiore*, and Simona Polo* *Istituto
Clathrin-independent endocytosis of ubiquitinated cargos Sara Sigismund*, Tanja Woelk*, Claudia Puri, Elena Maspero*, Carlo Tacchetti, Pietro Transidico, Pier Paolo Di Fiore*, and Simona Polo* *Istituto
UIM domain-dependent recruitment of the endocytic adaptor protein Eps15 to ubiquitin-enriched endosomes
 UIM domain-dependent recruitment of the endocytic adaptor protein Eps15 to ubiquitin-enriched endosomes Gucwa and Brown Gucwa and Brown BMC Cell Biology 2014, 15:34 Gucwa and Brown BMC Cell Biology 2014,
UIM domain-dependent recruitment of the endocytic adaptor protein Eps15 to ubiquitin-enriched endosomes Gucwa and Brown Gucwa and Brown BMC Cell Biology 2014, 15:34 Gucwa and Brown BMC Cell Biology 2014,
Understanding Living Clathrin-Coated Pits
 Traffic 2004; 5: 327 337 Blackwell Munksgaard Review Copyright # Blackwell Munksgaard 2004 doi: 10.1111/j.1600-0854.2004.00187.x Understanding Living Clathrin-Coated Pits Joshua Z. Rappoport 1, Sanford
Traffic 2004; 5: 327 337 Blackwell Munksgaard Review Copyright # Blackwell Munksgaard 2004 doi: 10.1111/j.1600-0854.2004.00187.x Understanding Living Clathrin-Coated Pits Joshua Z. Rappoport 1, Sanford
Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12
 SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
Dynamic Partitioning of a GPI-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking
 Additional data for Dynamic Partitioning of a GPI-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking Fabien Pinaud 1,3, Xavier Michalet 1,3, Gopal Iyer 1, Emmanuel
Additional data for Dynamic Partitioning of a GPI-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking Fabien Pinaud 1,3, Xavier Michalet 1,3, Gopal Iyer 1, Emmanuel
Assembly of endocytic machinery around individual influenza viruses during viral entry
 Assembly of endocytic machinery around individual influenza viruses during viral entry Michael J Rust 1,2, Melike Lakadamyali 1,2, Feng Zhang 1 & Xiaowei Zhuang 1 Most viruses enter cells via receptor-mediated
Assembly of endocytic machinery around individual influenza viruses during viral entry Michael J Rust 1,2, Melike Lakadamyali 1,2, Feng Zhang 1 & Xiaowei Zhuang 1 Most viruses enter cells via receptor-mediated
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or
 Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Molecular Cell Biology 5068 In class Exam 1 October 2, Please print your name: Instructions:
 Molecular Cell Biology 5068 In class Exam 1 October 2, 2012 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Molecular Cell Biology 5068 In class Exam 1 October 2, 2012 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Supplementary table and figures
 3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
Distinct Dynamics of Endocytic Clathrin-Coated Pits and Coated Plaques
 Distinct Dynamics of Endocytic Clathrin-Coated Pits and Coated Plaques Saveez Saffarian, Emanuele Cocucci, Tomas Kirchhausen* Department of Cell Biology, Harvard Medical School, Children s Hospital and
Distinct Dynamics of Endocytic Clathrin-Coated Pits and Coated Plaques Saveez Saffarian, Emanuele Cocucci, Tomas Kirchhausen* Department of Cell Biology, Harvard Medical School, Children s Hospital and
SUPPLEMENTARY INFORMATION
 In the format provided by the authors and unedited. 2 3 4 DOI: 10.1038/NMAT4893 EGFR and HER2 activate rigidity sensing only on rigid matrices Mayur Saxena 1,*, Shuaimin Liu 2,*, Bo Yang 3, Cynthia Hajal
In the format provided by the authors and unedited. 2 3 4 DOI: 10.1038/NMAT4893 EGFR and HER2 activate rigidity sensing only on rigid matrices Mayur Saxena 1,*, Shuaimin Liu 2,*, Bo Yang 3, Cynthia Hajal
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Supplementary Material and Methods
 Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Supplemental Data. Hao et al. (2014). Plant Cell /tpc
 Supplemental Figure 1. Confocal Images and VA-TIRFM Analysis of GFP-RbohD in Arabidopsis Seedlings. (A) RbohD expression in whole Arabidopsis seedlings. RbohD was expressed in the leaves, hypocotyl, and
Supplemental Figure 1. Confocal Images and VA-TIRFM Analysis of GFP-RbohD in Arabidopsis Seedlings. (A) RbohD expression in whole Arabidopsis seedlings. RbohD was expressed in the leaves, hypocotyl, and
genome edited transient transfection, CMV promoter
 Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Supplemental Materials. STK16 regulates actin dynamics to control Golgi organization and cell cycle
 Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Supplemental Data Figure S1 Effect of TS2/4 and R6.5 antibodies on the kinetics of CD16.NK-92-mediated specific lysis of SKBR-3 target cells.
 Supplemental Data Figure S1. Effect of TS2/4 and R6.5 antibodies on the kinetics of CD16.NK-92-mediated specific lysis of SKBR-3 target cells. (A) Specific lysis of IFN-γ-treated SKBR-3 cells in the absence
Supplemental Data Figure S1. Effect of TS2/4 and R6.5 antibodies on the kinetics of CD16.NK-92-mediated specific lysis of SKBR-3 target cells. (A) Specific lysis of IFN-γ-treated SKBR-3 cells in the absence
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Lu et al., http://www.jcb.org/cgi/content/full/jcb.201012063/dc1 Figure S1. Kinetics of nuclear envelope assembly, recruitment of Nup133
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Lu et al., http://www.jcb.org/cgi/content/full/jcb.201012063/dc1 Figure S1. Kinetics of nuclear envelope assembly, recruitment of Nup133
El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb /DC1
 Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
MELANOMA CANCER TEST
 MELANOMA CANCER TEST Efficacy Evaluation of Antitumor Activity of Alka Vita - Alkahydroxy in the LOX-GFP Human Melanoma Model Final Report by: Anti-Cancer Lab San Diego California March 4, 2005 Efficacy
MELANOMA CANCER TEST Efficacy Evaluation of Antitumor Activity of Alka Vita - Alkahydroxy in the LOX-GFP Human Melanoma Model Final Report by: Anti-Cancer Lab San Diego California March 4, 2005 Efficacy
Lab module 6a Receptor-mediated endocytosis
 Goal for the module Lab module 6a Receptor-mediated endocytosis To follow cell surface receptors as they are internalized. Pre-lab homework Read about receptor-mediated endocytosis and other forms of internalization
Goal for the module Lab module 6a Receptor-mediated endocytosis To follow cell surface receptors as they are internalized. Pre-lab homework Read about receptor-mediated endocytosis and other forms of internalization
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Supplementary Figure 1
 Supplementary Figure 1 6 HE-50 HE-116 E-1 HE-108 Supplementary Figure 1. Targeted drug response curves of endometrial cancer cells. Endometrial cancer cell lines were incubated with serial dilutions of
Supplementary Figure 1 6 HE-50 HE-116 E-1 HE-108 Supplementary Figure 1. Targeted drug response curves of endometrial cancer cells. Endometrial cancer cell lines were incubated with serial dilutions of
PHSI3009 Frontiers in Cellular Physiology 2017
 Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Recruitment dynamics of GAK and auxilin to clathrincoated pits during endocytosis
 3502 Research Article Recruitment dynamics of GAK and auxilin to clathrincoated pits during endocytosis Dong-won Lee, Xufeng Wu, Evan Eisenberg and Lois E. Greene* Laboratory of Cell Biology, NHLBI, NIH,
3502 Research Article Recruitment dynamics of GAK and auxilin to clathrincoated pits during endocytosis Dong-won Lee, Xufeng Wu, Evan Eisenberg and Lois E. Greene* Laboratory of Cell Biology, NHLBI, NIH,
Supplemental information
 Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Effect of Rhodamine-taxol conjugate on Caveolin dynamics
 Chapter 6 4 Effect of Rhodamine-taxol conjugate on Caveolin dynamics 1. Summary In the present study, Rhodamine- taxol conjugate treatment resulted in a transient recruitment of Caveolin-1 to the cell
Chapter 6 4 Effect of Rhodamine-taxol conjugate on Caveolin dynamics 1. Summary In the present study, Rhodamine- taxol conjugate treatment resulted in a transient recruitment of Caveolin-1 to the cell
Influenza A virus hemagglutinin and neuraminidase act as novel motile machinery. Tatsuya Sakai, Shin I. Nishimura, Tadasuke Naito, and Mineki Saito
 Supplementary Information for: Influenza A virus hemagglutinin and neuraminidase act as novel motile machinery Tatsuya Sakai, Shin I. Nishimura, Tadasuke Naito, and Mineki Saito Supplementary Methods Supplementary
Supplementary Information for: Influenza A virus hemagglutinin and neuraminidase act as novel motile machinery Tatsuya Sakai, Shin I. Nishimura, Tadasuke Naito, and Mineki Saito Supplementary Methods Supplementary
a Anti-Dab2 RGD Merge
 a Anti-Da Merge *** ** Percentage of cells adhered 8 6 4 RGE RAD RGE RAD Supplementary Figure Da are localized at clusters. (a) Endogenous Da is localized at clusters. (), ut not RGE nor RAD peptides can
a Anti-Da Merge *** ** Percentage of cells adhered 8 6 4 RGE RAD RGE RAD Supplementary Figure Da are localized at clusters. (a) Endogenous Da is localized at clusters. (), ut not RGE nor RAD peptides can
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
Intracellular Vesicular Traffic Chapter 13, Alberts et al.
 Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Chapter 13: Vesicular Traffic
 Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Nature Structural & Molecular Biology: doi: /nsmb.3218
 Supplementary Figure 1 Endogenous EGFR trafficking and responses depend on biased ligands. (a) Lysates from HeLa cells stimulated for 2 min. with increasing concentration of ligands were immunoblotted
Supplementary Figure 1 Endogenous EGFR trafficking and responses depend on biased ligands. (a) Lysates from HeLa cells stimulated for 2 min. with increasing concentration of ligands were immunoblotted
GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes.
 MBC in Press, published on October 31, 2003 as 10.1091/mbc.E03-07-0517 GLUT4 retention by intracellular cycling GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes.
MBC in Press, published on October 31, 2003 as 10.1091/mbc.E03-07-0517 GLUT4 retention by intracellular cycling GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes.
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Many G protein-coupled receptors (GPCRs) 2 are rapidly endocytosed after agonist binding, but the pathway of postendocytic
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 40, pp. 29646 29657, October 5, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Hepatocyte Growth
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 40, pp. 29646 29657, October 5, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Hepatocyte Growth
Vesicle Transport. Vesicle pathway: many compartments, interconnected by trafficking routes 3/17/14
 Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras
 Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras Tarik Issad, Ralf Jockers and Stefano Marullo 1 Because they play a pivotal role
Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras Tarik Issad, Ralf Jockers and Stefano Marullo 1 Because they play a pivotal role
Endocytosis and Intracellular Trafficking of Notch and Its Ligands
 CHA P T E R F IVE Endocytosis and Intracellular Trafficking of Notch and Its Ligands Shinya Yamamoto, *,1 Wu-Lin Charng, *,1 and Hugo J. Bellen *,,, Contents 1. Notch Signaling and its Regulation by Endocytosis
CHA P T E R F IVE Endocytosis and Intracellular Trafficking of Notch and Its Ligands Shinya Yamamoto, *,1 Wu-Lin Charng, *,1 and Hugo J. Bellen *,,, Contents 1. Notch Signaling and its Regulation by Endocytosis
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human
 Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
Oligomers of the ATPase EHD2 confine caveolae to the plasma membrane through association with actin
 Manuscript EMBO-2012-81192 Oligomers of the ATPase EHD2 confine caveolae to the plasma membrane through association with actin Miriam Stoeber, Ina Karen Stoeck, Christine Hänni, Christopher Karl Ernst
Manuscript EMBO-2012-81192 Oligomers of the ATPase EHD2 confine caveolae to the plasma membrane through association with actin Miriam Stoeber, Ina Karen Stoeck, Christine Hänni, Christopher Karl Ernst
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42
 Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42 Gina L. Razidlo, Kevin M. Burton, and Mark A. McNiven SUPPORTING INFORMATION Figure S1. IL-6 promotes
Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42 Gina L. Razidlo, Kevin M. Burton, and Mark A. McNiven SUPPORTING INFORMATION Figure S1. IL-6 promotes
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells. Pei-Tzu Yang and Hendrik C. Korswagen
 Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival
 Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Module 3 Lecture 7 Endocytosis and Exocytosis
 Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
nature methods Organelle-specific, rapid induction of molecular activities and membrane tethering
 nature methods Organelle-specific, rapid induction of molecular activities and membrane tethering Toru Komatsu, Igor Kukelyansky, J Michael McCaffery, Tasuku Ueno, Lidenys C Varela & Takanari Inoue Supplementary
nature methods Organelle-specific, rapid induction of molecular activities and membrane tethering Toru Komatsu, Igor Kukelyansky, J Michael McCaffery, Tasuku Ueno, Lidenys C Varela & Takanari Inoue Supplementary
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Product Manual. Human LDLR ELISA Kit. Catalog Number. FOR RESEARCH USE ONLY Not for use in diagnostic procedures
 Product Manual Human LDLR ELISA Kit Catalog Number STA-386 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is an essential component of cellular membranes,
Product Manual Human LDLR ELISA Kit Catalog Number STA-386 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is an essential component of cellular membranes,
Supplements. Figure S1. B Phalloidin Alexa488
 Supplements A, DMSO, PP2, PP3 Crk-myc Figure S1. (A) Src kinase activity is necessary for recruitment of Crk to Nephrin cytoplasmic domain. Human podocytes expressing /7-NephrinCD () were treated with
Supplements A, DMSO, PP2, PP3 Crk-myc Figure S1. (A) Src kinase activity is necessary for recruitment of Crk to Nephrin cytoplasmic domain. Human podocytes expressing /7-NephrinCD () were treated with
Supplemental information contains 7 movies and 4 supplemental Figures
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
A JC Virus-Induced Signal Is Required for Infection of Glial Cells by a Clathrin- and eps15-dependent Pathway
 JOURNAL OF VIROLOGY, Jan. 2004, p. 250 256 Vol. 78, No. 1 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.1.250 256.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. A JC Virus-Induced
JOURNAL OF VIROLOGY, Jan. 2004, p. 250 256 Vol. 78, No. 1 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.1.250 256.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. A JC Virus-Induced
Supplementary Figures
 Supplementary Figures Supplementary Figure 1 DOT1L regulates the expression of epithelial and mesenchymal markers. (a) The expression levels and cellular localizations of EMT markers were confirmed by
Supplementary Figures Supplementary Figure 1 DOT1L regulates the expression of epithelial and mesenchymal markers. (a) The expression levels and cellular localizations of EMT markers were confirmed by
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Homework Hanson section MCB Course, Fall 2014
 Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Supplementary Figure 1. Characterization of NMuMG-ErbB2 and NIC breast cancer cells expressing shrnas targeting LPP. NMuMG-ErbB2 cells (a) and NIC
 Supplementary Figure 1. Characterization of NMuMG-ErbB2 and NIC breast cancer cells expressing shrnas targeting LPP. NMuMG-ErbB2 cells (a) and NIC cells (b) were engineered to stably express either a LucA-shRNA
Supplementary Figure 1. Characterization of NMuMG-ErbB2 and NIC breast cancer cells expressing shrnas targeting LPP. NMuMG-ErbB2 cells (a) and NIC cells (b) were engineered to stably express either a LucA-shRNA
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the
 Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
Entry of Tiger Frog Virus (an Iridovirus) into HepG2 Cells via a ph-dependent, Atypical, Caveola-Mediated Endocytosis Pathway
 JOURNAL OF VIROLOGY, July 2011, p. 6416 6426 Vol. 85, No. 13 0022-538X/11/$12.00 doi:10.1128/jvi.01500-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Entry of Tiger Frog Virus
JOURNAL OF VIROLOGY, July 2011, p. 6416 6426 Vol. 85, No. 13 0022-538X/11/$12.00 doi:10.1128/jvi.01500-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Entry of Tiger Frog Virus
Summary and Discussion antigen presentation
 Summary and Discussion antigen presentation 247 248 Summary & Discussion Summary and discussion: antigen presentation For a cell to communicate information about its internal health and status to the immune
Summary and Discussion antigen presentation 247 248 Summary & Discussion Summary and discussion: antigen presentation For a cell to communicate information about its internal health and status to the immune
IP: anti-gfp VPS29-GFP. IP: anti-vps26. IP: anti-gfp - + +
 FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well
 Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
Supplementary Figure 1
 Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
CD34 + VEGFR-3 + progenitor cells have a potential to differentiate towards lymphatic endothelial cells
 CD34 + VEGFR-3 + progenitor cells have a potential to differentiate towards lymphatic endothelial cells Tan YZ et al. J Cell Mol Med. (2014 Mar;18(3):422-33) Denise Traxler-Weidenauer April 2014 Introduction
CD34 + VEGFR-3 + progenitor cells have a potential to differentiate towards lymphatic endothelial cells Tan YZ et al. J Cell Mol Med. (2014 Mar;18(3):422-33) Denise Traxler-Weidenauer April 2014 Introduction
Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a
 Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a expression in GFP-expressing HEp3 cells. (b) Representative
Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a expression in GFP-expressing HEp3 cells. (b) Representative
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Clathrin-independent endocytosis of ErbB2 in geldanamycin-treated human breast cancer cells
 Research Article 3155 Clathrin-independent endocytosis of ErbB2 in geldanamycin-treated human breast cancer cells Daniel J. Barr, Anne G. Ostermeyer-Fay, Rachel A. Matundan and Deborah A. Brown* Department
Research Article 3155 Clathrin-independent endocytosis of ErbB2 in geldanamycin-treated human breast cancer cells Daniel J. Barr, Anne G. Ostermeyer-Fay, Rachel A. Matundan and Deborah A. Brown* Department
Host serine/threonine kinases mtor and Protein Kinase C-α. promote InlB-mediated entry of Listeria monocytogenes
 IAI Accepted Manuscript Posted Online 1 May 2017 Infect. Immun. doi:10.1128/iai.00087-17 Copyright 2017 American Society for Microbiology. All Rights Reserved. 1 2 3 Host serine/threonine kinases mtor
IAI Accepted Manuscript Posted Online 1 May 2017 Infect. Immun. doi:10.1128/iai.00087-17 Copyright 2017 American Society for Microbiology. All Rights Reserved. 1 2 3 Host serine/threonine kinases mtor
Cell membrane penetration and mitochondrial targeting by platinumdecorated
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Cell membrane penetration and mitochondrial targeting by platinumdecorated ceria nanoparticles
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Cell membrane penetration and mitochondrial targeting by platinumdecorated ceria nanoparticles
Vesicular Trafficking of Tyrosine Kinase Receptors and Associated Proteins in the Regulation of Signaling and Vascular Function
 This Review is part of a thematic series on Microdomains in Cardiovascular Signaling, which includes the following articles: Caveolae and Caveolins in the Cardiovascular System Focal Adhesion: Paradigm
This Review is part of a thematic series on Microdomains in Cardiovascular Signaling, which includes the following articles: Caveolae and Caveolins in the Cardiovascular System Focal Adhesion: Paradigm
UNIVERSITY OF YORK BSc Stage 2 Degree Examinations Department: BIOLOGY. Title of Exam: Cell Biology
 Examination Candidate Number: Desk Number: UNIVERSITY OF YORK BSc Stage 2 Degree Examinations 2017-18 Department: BIOLOGY Title of Exam: Cell Biology Time allowed: 1 hour and 30 minutes Total marks available
Examination Candidate Number: Desk Number: UNIVERSITY OF YORK BSc Stage 2 Degree Examinations 2017-18 Department: BIOLOGY Title of Exam: Cell Biology Time allowed: 1 hour and 30 minutes Total marks available
ab Membrane fluidity kit Instructions for Use For the detection of membrane fluidity in cells
 ab189819 Membrane fluidity kit Instructions for Use For the detection of membrane fluidity in cells This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated
ab189819 Membrane fluidity kit Instructions for Use For the detection of membrane fluidity in cells This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated
Imaging endocytic clathrin structures in living cells
 Review Special Issue Imaging Cell Biology Imaging endocytic clathrin structures in living cells Tom Kirchhausen Harvard Medical School/ Immune Disease Institute, W. Alpert Building Room 128, 200 Longwood
Review Special Issue Imaging Cell Biology Imaging endocytic clathrin structures in living cells Tom Kirchhausen Harvard Medical School/ Immune Disease Institute, W. Alpert Building Room 128, 200 Longwood
/07/$15.00/0 Molecular Endocrinology 21(12): Printed in U.S.A.
 0888-8809/07/$15.00/0 Molecular Endocrinology 21(12):3087 3099 Printed in U.S.A. Copyright 2007 by The Endocrine Society doi: 10.1210/me.2006-0476 The Glucose Transporter 4 FQQI Motif Is Necessary for
0888-8809/07/$15.00/0 Molecular Endocrinology 21(12):3087 3099 Printed in U.S.A. Copyright 2007 by The Endocrine Society doi: 10.1210/me.2006-0476 The Glucose Transporter 4 FQQI Motif Is Necessary for
Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death
 www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
Title:Role of LPAR3, PKC and EGFR in LPA-induced cell migration in oral squamous carcinoma cells
 Author's response to reviews Title:Role of LPAR3, PKC and EGFR in LPA-induced cell migration in oral squamous carcinoma cells Authors: Ingvild J Brusevold (i.j.brusevold@medisin.uio.no) Ingun H Tveteraas
Author's response to reviews Title:Role of LPAR3, PKC and EGFR in LPA-induced cell migration in oral squamous carcinoma cells Authors: Ingvild J Brusevold (i.j.brusevold@medisin.uio.no) Ingun H Tveteraas
