Clathrin, adaptors and eps15 in endosomes containing activated epidermal growth factor receptors
|
|
|
- Kerrie Allen
- 5 years ago
- Views:
Transcription
1 Journal of Cell Science 112, (1999) Printed in Great Britain The Company of Biologists Limited 1999 JCS Clathrin, adaptors and eps15 in endosomes containing activated epidermal growth factor receptors Tatiana Sorkina, Andrea Bild, Francesc Tebar and Alexander Sorkin* Department of Pharmacology, University of Colorado Health Sciences Center, 4200 E. Ninth Ave., Denver, CO 80262, USA *Author for correspondence ( Accepted 25 November 1998; published on WWW 13 January 1999 SUMMARY Activation of the epidermal growth factor receptor (EGFR) by EGF results in binding of clathrin adaptor protein complex AP-2 to the receptor cytoplasmic tail. The transient interaction with AP-2 is thought to be responsible for the selective recruitment of the EGFR into coated pits during endocytosis. In this study we found that EGF-induced EGFR/AP-2 association, measured by co-immunoprecipitation, persists after receptor internalization. Double-label immunofluorescence of EGFtreated A-431 and COS-1 cells revealed the presence of AP- 2, clathrin and eps15, another component of the plasma membrane coated pits, in the large perinuclear endosomes loaded with EGFRs. By optical sectioning and image deconvolution, the immunoreactivities were seen to be distributed within vesicular and tubular elements of these endosomes. In addition, these compartments contained the transferrin receptors and a EEA.1 protein, markers of early endosomes. Furthermore, Golgi clathrin adaptor complex AP-1 was found in EGFR-containing endosomes and EGFR immunoprecipitates in A-431 cells. The direct interaction of the EGFR with µ1 as well as µ2 subunits of AP-1 and AP-2, correspondingly, was shown using the yeast two-hybrid assay. Brefeldin A, a drug that releases AP-1 from the trans-golgi membranes, had no effect on AP-1 association with endosomes and its co-precipitation with EGFR. Taken together, the data suggest that endosomal EGFR-AP complexes make up a significant portion of the total amount of these complexes detectable by coimmunoprecipitation. It can be proposed that APs are capable of binding to the endosomal membrane via a mechanism that requires AP interaction with the intracellular tails of multimeric receptors like activated EGFR, which in turn allows recruitment of clathrin and eps15. The hypothesis that the competition between adaptor complexes for binding to the receptor tails in endosomes may regulate of the sorting of receptors is discussed. Key words: EGF receptor, Clathrin, Adaptor INTRODUCTION Clathrin coats function at the plasma membrane to promote rapid endocytosis of various receptors and other membrane proteins as well as soluble macromolecules and viruses (reviewed in Schmid, 1997). Clathrin-coated pits located in the trans-golgi network (TGN) are essential for the receptormediated delivery of soluble enzymes to lysosomes (reviewed in Traub and Kornfeld, 1997). The major components of coated pits are the clathrin and the clathrin adaptor protein complexes or APs, AP-2 at the plasma membrane, and AP-1 in TGN (reviewed in Robinson, 1997). Each AP is a heterotetramer consisting of two 100-kDa subunits or adaptins (α/β2 in AP-2 and γ/β1 in AP-1), one 47/50-kDa (µ2 and µ1) and one 17/19- kda subunits (σ2 and σ1). Membrane-bound APs serve as nucleation sites for the assembly of the clathrin lattice. The clathrin-adaptor coats undergo rearrangements, resulting in invagination of the coated membrane and pinching off the coated vesicle. The plasma membrane and TGN-derived coated vesicles fuse with endosomes, which requires at least a partial dissociation of the clathrin lattice. Whether AP release from the membrane is a prerequisite for the vesicle fusion with the endosomal membrane is not known. However, restricted cellular localization of APs and other coat proteins suggests that the membrane docking and specific targeting of APs are tightly regulated. Current data suggest that α/γ, µ and possibly σ subunits of APs can be involved in the membrane-docking process (Gaidarov et al., 1996; Page and Robinson, 1995; Robinson, 1993). The anchoring molecules specific for AP-2 and AP-1 recruitment to the plasma membrane or TGN, respectively, are not identified. ADP-ribosylation factor, ARF1, is important for binding of AP-1 to TGN membranes (Traub et al., 1993). ARF requirement for AP-2 docking to plasma membrane has not been demonstrated (West et al., 1997). The latter study implicates phospholipase D-dependent production of phosphatidic acid in the recruitment of AP-2 at the plasma membrane. In addition, α-adaptins and possibly other subunits of AP-2 bind to phosphotidylinositols, which might be important for the membrane docking of AP-2 (Gaidarov et al.,
2 318 T. Sorkina and others 1996; Rapoport et al., 1997). In neuronal cells AP-2 is thought to anchor to the transmembrane protein synaptotagmin (Zhang et al., 1994). APs also interact with the cytoplasmic tails of membrane receptors and other integral membrane proteins via the µ subunits (for a review see Kirchhausen et al., 1997) and possibly β1 subunit (Rapoport et al., 1998). The importance of the mannose-6-phosphate receptor for the high-affinity binding of AP-1 to TGN membranes has been demonstrated (Le Borgne et al., 1996). However, the receptors capable of binding to APs are present in various cellular compartments, particularly in endosomes. In contrast, no significant accumulation of AP-2 in endosomes containing internalized receptors has been reported. Relocalization of activated FcεRI receptors to restricted membrane domains did not result in corresponding redistribution of AP-2 (Santini and Keen, 1996). Thus, the importance of cargo for the specific adaptor recruitment to the membrane remains a controversial issue, and the regulatory mechanisms of this transient receptor-adaptor interaction need to be characterized. The endocytosis of the receptor for epidermal growth factor (EGFR) served as model system to study ligand-dependent receptor trafficking for many years (reviewed in Sorkin and Waters, 1993). The endocytic pathway is particularly well studied in human epidermoid carcinoma A-431 cells which express very high levels of EGFR. Activation of EGFRs by epidermal growth factor (EGF) results in internalization of EGF-receptor complexes via a rapid clathrin-coated-pit pathway and a slower clathrin-independent mechanism (Haigler et al., 1979; Hopkins et al., 1985; Lamaze et al., 1993; Miller et al., 1986; Wiley, 1988). Both internalization pathways lead to the same early endosomal compartment and subsequently to multivesicular endosomes (MVEs) (Hopkins et al., 1985; Miller et al., 1986). Although EGF-EGFR complexes are rapidly recycled back from endosomes to the cell surface (Sorkin et al., 1991a), a substantial fraction of these complexes are sorted to the lysosome-degradation pathway after each round of internalization, which results in downregulation of EGFRs (Stoscheck and Carpenter, 1984). The proteolytic degradation of EGFRs appears to be a consequence of the direct fusion of MVEs with lysosomes (Futter et al., 1996). The molecular mechanisms of EGF-induced receptor internalization and intracellular sorting are not well understood. It has been demonstrated that EGF-activated receptors interact with AP-2 (Sorkin and Carpenter, 1993). This observation is consistent with the general dogma that receptors are recruited into coated pits by means of selective recognition by APs. This theory, however, did not survive testing in functional experiments in vivo: EGFR mutants lacking the major AP-2 binding site have been shown to internalize via clathrin-dependent pathways (Nesterov et al., 1995; Sorkin et al., 1996). Thus, although the existence of multiple weak AP binding sites in the EGFR is possible, the role of EGFR-AP interactions remains unclear. Furthermore, in vitro studies on broken cells suggested that additional factors other than AP-2 are required for efficient sequestration of EGFRs in coated pits (Lamaze et al., 1993). The protein interactions that are responsible for the sorting of EGFRs in endosomes to recycling or lysosomal pathways are also not identified. The discovery of a new adaptor complex AP-3 (Dell Angelica et al., 1997; Simpson et al., 1996), which is colocalized with clathrin buds in the peripheral endosomes of A-431 cells (Dell Angelica et al., 1998), suggests that AP- 3 can be involved in endosomal trafficking of EGF and other receptors. In this study the dynamics of EGFR-AP interactions and subcellular localization of clathrin-coat proteins was analyzed. We report that EGF-induced EGFR association with APs, clathrin and eps15, a component of the plasma membrane coated pits (Tebar et al., 1996; van Delft et al., 1997), is maintained in A-431 cells after receptor internalization. The data suggest that the association of EGFRs and APs might be stabilized by multivalent interactions during endocytosis, which may result in the assembly of clathrin-adaptor coats in endosomes. MATERIALS AND METHODS Reagents Human recombinant EGF was obtained from Collaborative Research Inc. Iron-saturated transferrins conjugated with fluorescein (TRF- FITC) or Texas Red (TRF-TR) were purchased from Molecular Probes. Polyclonal rabbit 986 and 451 antibodies to EGFR (anti- EGFR) were a gift from Dr G. Carpenter (Vanderbilt University, Nashville). Rabbit serum Ab2913 specific to the intracellular domain of EGFR was a gift of Dr L. Beguinot (DIBIT Rafaele, Milan, Italy). Monoclonal antibodies AC1-M11 that recognize α-subunits of AP-2 were a gift from Dr M. S. Robinson (University of Cambridge, England). Polyclonal antibodies 32 (Ab32) to β-subunits of AP-2 and AP-1 were characterized in our previous studies (Sorkin et al., 1995). Dr P. P. Di Fiore (European Institute of Oncology, Milan, Italy) kindly provided polyclonal antibody to eps15 (Ab577). A monoclonal antibody to the clathrin heavy chain (X-22) and the α-subunit (AP.6) (Brodsky, 1985) were obtained from ATCC, whereas monoclonals 100/3 specific to γ-adaptin were from Sigma. A monoclonal antibody to EEA.1 protein was from Transduction Laboratories. Polyclonal antibodies were used as the IgG-fraction purified from serum using Protein A-Sepharose (Sigma) or affinity-purified. Cells Human epidermoid carcinoma A-431 cells ( EGFR/cell) were maintained in Dulbecco s modified Eagle s medium (DMEM) containing 10% calf serum with antibiotics and glutamine. Green monkey kidney COS-1 cells ( EGFR/cell) were grown in DMEM containing 10% newborn calf serum, antibiotics and glutamine. Cells were grown to about 90% or 50% confluency for co-immunoprecipitation or immunofluorescence experiments, respectively. Immunoprecipitation of EGF receptors and AP-2 Cells grown on 35 mm dishes (A-431) or 100 mm dishes (COS-1) were treated or not with EGF (500 ng/ml) in binding medium (DMEM, 0.1% bovine serum albumin, 20 mm Hepes, ph 7.3). In some experiments cells treated with EGF were incubated with 0.2 M sodium acetate, 0.5 M NaCl (ph 4.5) to remove surface-bound EGF (Sorkin and Carpenter, 1991). Cells were then washed with Ca 2+ -, Mg 2+ -free phosphate-buffered saline (CMF-PBS) and solubilized by scraping with a rubber policeman in TGH buffer (1% Triton X-100, 10% glycerol, 50 mm NaCl, 50 mm Hepes, ph 7.3, 5 mm EDTA, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, 10 µg/ml leupeptine, 544 µm iodacetamide, 10 µg/ml aprotinin) followed by gentle rotation for 10 minutes at 4 C. Lysates were then centrifuged at 100,000 g for 20 minutes. Approximately 50% and 95-99% of the total cellular pools of AP-2 and AP-1, respectively, and at
3 Clathrin coat proteins in endosomes 319 least 95% of the EGFR pool, were found in the supernatants after centrifugation. Supernatants were incubated with anti-egfr (Ab986/451) for 3-15 hours at 4 C and then minutes after the addition of Protein A-Sepharose (Sigma). Normal rabbit IgG (Zymed Inc.) were used for non-specific controls. Immunoprecipitates were washed twice with TGH supplemented with 100 mm NaCl and then once without NaCl. 7.5% SDSpolyacrylamide gels were used to separate proteins. Transfer to nitrocellulose membranes and protein immunoblotting were carried out as described (Sorkin et al., 1996). The top (above the 116 kda molecular mass marker) and bottom portions of the nitrocellulose membrane were probed, respectively, with anti-egfr Ab2913 and adaptin antibody (AC1-M11 to α-subunits or 100/3 to γ-subunit). Sheep antibodies to mouse IgG (Cappel Inc.) or protein A (Zymed Inc.) conjugated with horseradish peroxidase and with enhanced chemiluminescence (Amersham or NEN) were used to detect primary mouse or rabbit antibodies, respectively. Co-immunoprecipitation of AP-2 and eps15 in cellular fractions To separate the cytosolic and membrane fractions, cells treated or not treated with EGF were mildly permeabilized by incubation in CMF- PBS, containing 0.02% saponin, 1 mm EGTA, 5 mm EDTA, 1 mm sodium orthovanadate, 10 mm sodium fluoride, 1 mm PMSF and protease inhibitors, for 30 minutes at 4 C. After removal of the saponin (cytosolic) fraction, the permeabilized cells containing the membrane proteins were washed with CMF-PBS and solubilized by scraping the cells away from the dish with a rubber policeman in TGH containing 1% sodium deoxycholate (TGH-DOC), followed by gentle rotation for 10 minutes at 4 C. Sodium deoxycholate was added to release all membrane-bound forms of AP-2 and eps15 (Tebar et al., 1996). The saponin and TGH-DOC fractions were centrifuged at 100,000 g for 20 minutes at 4 C and incubated with Ab32 to β-adaptins for 3 hours at 4 C and then for 1 hour after addition of Protein A-Sepharose. Pre-immune rabbit serum or unrelated rabbit IgG (Zymed) were used to control for non-specific immunoprecipitations. Immunoprecipitates were washed twice with cold CMF-PBS or TGH supplemented with 100 mm NaCl and then once without NaCl. The electrophoresis, transfer to nitrocellulose membranes and western blot analysis were carried out as described above. The top (above the 116 kda molecular mass marker) and bottom portions of the nitrocellulose membrane were blotted with antibody to eps15 (Ab577) and α-subunits (AC1- M11), respectively. The detection of primary antibodies was performed as described above. Immunofluorescence staining Cells grown on coverslips and incubated with EGF or labeled ligands (EGF-TR, TRF-FITC and TRF-TR) were fixed with freshly prepared 4% para-formaldehyde (Electron Microscopy Sciences) for 12 minutes at room temperature, and mildly permeabilized using two techniques. The first technique allows better detection of the coated pit proteins and uses a 3-minute permeabilization in CMF-PBS containing 0.1% Triton X-100, 0.1% BSA at room temperature. Coverslips were then incubated in the same buffer, in which Triton X- 100 was omitted, at room temperature for 1 hour with the primary antibody, washed intensively and then incubated with the secondary donkey anti-mouse IgG and anti-rabbit IgG labeled with Texas Red or fluorescein (Jackson Tech.). Both primary and secondary antibody solutions were precleared by centrifugation at 100,000 g for 10 minutes. The second technique is useful to prevent the loss of labeled ligands from endosomes. Fixed cells were incubated in binding medium for 10 minutes, and permeabilized in CMS-PBS containing 0.05% saponin and 1% BSA for 30 minutes. Subsequent incubations with the primary and secondary antibodies were carried out in CMS- PBS containing 0.01% saponin and 1% BSA. After staining the coverslips were mounted in Fluoromount-G (Fisher) containing 1 mg/ml para-phenylenediamine. The samples were analyzed using conventional or digital deconvolution microscopy. A Nikon Diaphot 300 microscope equipped with NA oil immersion objective lens, and the single fluorochrome filter sets for either Texas Red, fluorescein or simultaneous Texas Red/fluorescein fluorescence (Chroma Inc.), were used for visualization and recording the images. To obtain high resolution three-dimensional images of cells, DeltaVision workstation (Applied Precision, Inc.), which includes an Olympus fluorescent microscope, was employed. Typically serial two-dimensional images were recorded at 200 nm intervals using a thermoelectrically cooled charged-coupled device (CCD) camera (PXL, Photometrics Ltd, Tucson, AZ). In some experiments a QED Imaging workstation equipped with a Nikon Diaphot microscope and Micromax CCD camera with a Sony Interline area array (Princeton Instruments) with high sensitivity within the bluegreen range of the spectrum was used. A Z-stack of images obtained on DeltaVision or QED workstations were deconvoluted using a modification of the constrained iteration method. Final analysis of all images was performed using AdobePhotoshop Two-hybrid analysis The yeast two-hybrid protein-protein interaction assay protocol followed the MatchMaker Two-Hybrid System 2 manual (Clontech, Palo Alto, CA). A fusion protein of a GAL4 transcription factor binding domain (GAL4bd) in the vector pas2-1 with various fragments of EGFR was constructed. EGFR fragments were generated by PCR, and cloned into NcoI and SalI restriction sites. All clones were verified by restriction analysis and sequencing. Full-length µ1 and µ2, as well as µ2, in which the first 120 amino acid residues are deleted, cloned in pact2 vector which contained the GAL4 activation domain (GAL4ad) (Ohno et al., 1995), were generously donated by Dr J. S. Bonifacino (NIH). Transformations of plasmids into yeast strains Y187 and CG1945 were performed by the PEG/LiAc method. To test for positive interactions of EGFR with the µ subunits, Y187 transformations were used for β-galactosidase (βgal) assays while CG1945 transformations were used in the growth assay. Single colonies were picked and grown on synthetic dropout medium (SD) lacking Trp and Leu amino acids ( Trp, Leu) plates at 30 C for 2-3 days, and then measured for β-gal activity using the colony-lift filter assay. Blue colonies were analyzed for up to 8 hours following addition of X-gal. Concurrently, CG1945 transformations were streaked onto SD Leu, Trp, His plates with 5 mm 3-amino- 1,2,4-triazole (3-AT; Sigma). After growth at 30 C for 48 hours to allow for depletion of histidine, cells were picked from these plates and restreaked onto the fresh His, Leu, Trp, 5 mm 3-AT plates. Cells were grown for additional 3-4 days and were scored for growth. Several controls to test for autonomous activation and other caveats were used with all experiments according to the manual. GAL4bdfusion constructs of the peptide containing the three repeats of the internalization motif of TGN38 or its mutated version (YG mutation) in pas2-1 vector were kindly provided by Dr Bonifacino and used as positive or negative controls of the interaction with µ1/2. RESULTS EGFR association with AP-2 during endocytosis In our previous studies EGF-induced association of EGFR with AP-2 was demonstrated in A-431 and other cells (Sorkin and Carpenter, 1993; Sorkin et al., 1995). Apparently, this interaction may occur at the plasma membrane during recruitment of the activated receptors into coated pits. However, EGFR remain EGF-occupied and, therefore, dimerized and phosphorylated after internalization (Carpentier et al., 1987; Lai et al., 1989; Sorkin and Carpenter, 1991).
4 320 T. Sorkina and others Fig. 1. Time course of AP-2 co-immunoprecipitation with EGFR in A-431 cells. Cells were incubated with the saturating concentration of EGF (500 ng/ml) for indicated periods of time (minutes) at 37 C, and then the EGFRs were immunoprecipitated from TGH lysates of the cells. EGFRs and α-subunits of AP-2 (αa and αc) were detected in immunoprecipitates by western blotting with anti-egfr Ab2931 and AC1-M11, respectively. The time course was similar in four independent experiments. These observations prompted us to test whether EGFR/AP-2 association is also retained in endosomes. The time course of AP-2 co-immunoprecipitation with EGFR showed that association of AP-2 with EGFR reaches a maximum at 15 minutes and is maintained at a level that is slightly lower than maximal for at least 45 minutes (Fig. 1). The persistence of EGFR/AP-2 association during endocytosis suggested that endosomal EGFR may be complexed with AP-2 and may, therefore, contribute to the total pool of these complexes detected by co-immunoprecipitation. In order to distinguish between the surface and internalized EGF/EGFR/AP-2 complexes, the mild acid-wash treatment was employed. The cells were first incubated with EGF at 4 C, and then endocytosis was initiated by placing cells at 37 C. At the end of the incubation, cells were either treated or not with the acidic buffer to remove surface-bound EGF. Such treatment removes at least 90-95% of surface-bound EGF and leads to immediate monomerization, deactivation of the kinase and dephosphorylation of surface EGFR, whereas internalized EGFRs are not affected (Nesterov et al., 1990; Sorkin and Carpenter, 1991; Sorkin et al., 1991a). As shown in Fig. 2, a 37 C-incubation resulted in the binding of AP-2 to EGFR, revealed by co-immunoprecipitation. Acid wash had only a moderate effect (about a twofold decrease of the specific signal) on the extent of AP-2 co-immunoprecipitation with EGFR at the early stages of endocytosis. At later times, acid-resistant (intracellular) EGF-EGFR complexes were solely responsible for the association with AP-2 detected by co-immunoprecipitation. Thus, the maintenance of acidresistant EGFR/AP-2 association during endocytosis suggests that a substantial pool of these complexes are located intracellularly. Localization of AP-2 and clathrin in EGF-treated cells The localization of AP-2 relative to EGFR was inspected using a double-label immunofluorescence technique. Cells were incubated with EGF for 30 minutes at 37 C, fixed and processed for immunostaining using a Triton X-100 permeabilization protocol. The accumulation of EGFR in large (up to 1-2 µm) endosome-like structures was seen in the perinuclear region of the cells (Fig. 3A,D). Surprisingly, α- adaptin as well as clathrin heavy chains were clearly detected in most of these large endosomes loaded with EGFR (Fig. 3B,C,E,F). As expected, a punctate staining of clathrin and AP-2, which did not overlap with EGFR and that corresponds to the plasma membrane and TGN (for clathrin) coated pits, could be seen on the sections through the middle of the cell (Fig. 3), and much more strongly on the sections close to the cell surface (not shown). Optical sectioning and image correction by deconvolution revealed that EGFR immunoreactivity is associated with the small vesicles connected by tubular elements that often bend around each other. EGFR staining only partially overlapped with the clathrin and AP-2 immunoreactivity, indicating that a limited pool of endosomal EGFRs are associated with coated pit proteins. The immunofluorescence labeling of COS-1 cells, treated with EGF for 30 minutes at 37 C, revealed EGF-dependent colocalization of EGFR and clathrin in perinuclear endosomes, although the extent of clathrin accumulation in endosomes was less dramatic compared to that in A-431 cells (Fig. 3G-I). Surprisingly, very little AP-2 was found in endosomes of EGFtreated COS-1 cells, although EGFR/AP-2 coimmunoprecipitation was detected in COS-1 cells treated with Fig. 2. Time course of AP-2 co-immunoprecipitation with EGFR treated or not treated with acid wash. A-431 cells were incubated with EGF for 1 hour at 4 C, and then for the indicated periods of time (minutes) at 37 C. At the end of a 37 C incubation, surface EGF was stripped (+) or not stripped ( ) by the mild acid wash. The EGFRs were immunoprecipitated from TGH lysates of the cells. EGFRs and α-adaptins (αa and αc) were detected in immunoprecipitates by western blotting with anti-egfr Ab2931 and AC1-M11, respectively. Top, western blot detection of α-adaptins in EGFR immunoprecipitates; Bottom, quantitation of the amount of AP-2 present in the EGFR immunoprecipitates normalized to the amount of EGFRs detected by immunoblotting (a.u., arbitrary units). The average amount of AP-2 detected in non-specific immunoprecipitates with rabbit IgG is indicated by the dashed line. The experiment is representative of three similar experiments. Note that a 20-minute incubation at 37 C after pre-occupying the receptors with EGF at 4 C roughly corresponds on the time scale of endocytosis to a 30-minute incubation with EGF at 37 C without 4 C-preincubation.
5 Clathrin coat proteins in endosomes 321 Fig. 3. Localization of clathrin and AP-2 in endosomes of A-431 and COS-1 cells. A-413 (A-F) and COS-1 (G-I) cells were incubated with, respectively, 500 ng/ml and 200 ng/ml EGF for 30 minutes at 37 C, fixed and stained with polyclonal antibodies to EGFR Ab2931 (green; A,D,G) and monoclonal clathrin heavy chain antibody X-22 (red; E,H) or α- adaptin antibody AP.6 (red; B) using a Triton X-100 permeabilization protocol. After data acquisition on the DeltaVision workstation, the fluorescein and Texas Red channels were merged (C,F,I) after adjustment of both fluorescence signals to similar levels. Yellow indicates the overlap of Texas Red and fluorescein fluorescence. Higher magnification images of the overlay images of the individual endosomes are presented on the bottom. Note the absence of image pixelation indicates that the staining is within the resolution range of the CCD camera. All images comprise an individual optical section from the middle of the cell, where the most intense signal for EGFR was observed. Arrows point to examples of colocalization of clathrin and EGFR in endosomes. EGF for 30 minutes at 37 C (data not shown). The extent of AP-2 co-immunoprecipitation with EGFR was, however, much less than in A-431 cells, which might explain the poor AP-2 detection in endosomes of COS-1 cells. It is also possible that in COS-1 cells, other adaptor complexes may be responsible for EGF-dependent clathrin recruitment onto endosomes. To confirm that AP-2 is associated with the endosomes but not with the surface aggregates of EGFRs, A-431 cells were allowed to internalize Texas Red-conjugate of EGF (EGF-TR) and then treated with the mild acidic buffer to remove noninternalized EGF-TR. Staining with antibody to α-adaptin performed using a saponin-permeabilization protocol revealed co-localization of AP-2 with acid-resistant and, therefore, internalized EGF-TR (data not shown). Eps15 is bound to AP-2 and follows EGFR/AP-2 to endosomes The data of Fig. 3 demonstrated the presence of clathrin and AP-2 in endosomes. Another component of plasma membrane clathrin-coated pits is a protein called eps15 (Tebar et al., 1996; van Delft et al., 1997). A large fraction of the cellular pool of eps15 is associated with the α-subunit of AP-2 in NIH 3T3 cells (Benmerah et al., 1995; Tebar et al., 1996). Fig. 4A shows that EGF treatment does not affect the extent of AP-2/Eps15 co-immunoprecipitation in cytosolic and membrane fractions in A-431 cells. Interestingly, the relative size of the cytosolic pool of AP-2 is smaller in A-431 cells (Fig. 4A) compared to NIH 3T3 cells (Tebar et al., 1996). Only a limited pool of membrane-bound AP-2 is associated with eps15, which is consistent with the restricted localization of eps15 at the periphery of the coat (Tebar et al., 1996). In A-431 and COS-1 cells eps15 is also co-localized with the markers of plasma membrane coated pits (data not shown). However, in contrast to what was observed in NIH 3T3 cells (Tebar et al., 1996; van Delft et al., 1997), EGF induces a significant re-distribution of eps15 to endosomes containing EGFR in A-431 cells (Fig. 4B,C). The extent of eps15 colocalization with EGFR in endosomes stained with anti-egfr was comparable to that observed for AP-2. In COS-1 cells, EGF-induced accumulation of eps15 in endosomes was much less dramatic. The data suggest that eps15 distribution to endosomes emulates the distribution of AP-2.
6 322 T. Sorkina and others Cells were incubated with TRF-TR or TRF-FITC in the absence or presence of EGF, and then stained with antibodies to eps15 or clathrin. Fig. 5A-C demonstrates that endocytosis of TRF-TR alone did not lead to significant accumulation of eps15 in labeled endosomes. Simultaneous internalization of EGF and TRF-TR is known to result in co-localization of two ligand-receptor complexes in the early endosomal compartments (Hopkins and Trowbridge, 1983). As shown in Fig. 5D-F, EGF causes the accumulation of TRF-TR in large perinuclear endosomes that also contain eps15. Essentially similar results were obtained with co-staining of TRF-TR and α-adaptin (data not shown). In contrast, a pool of clathrin was seen associated with TRF-FITC-containing compartments in the absence of EGF (Fig. 5G-I), albeit the amount of endosomal clathrin was substantially increased in cells treated with EGF (Fig. 5J-L). In summary, the data presented in Fig. 5 indicate that the recruitment of clathrin-coat protein to endosomes is the specific feature of the endocytosis of EGFoccupied EGFR. The accumulation of the internalized transferrin in these endosomes indicates that these compartments represent early and/or recycling endosomes. Furthermore, the endosomes containing EGFRs and clathrincoat proteins were also positive for protein EEA.1, the marker of early and intermediate endosomes (Mu et al., 1994) (data not shown). Fig. 4. Association of AP-2 with eps15, and the eps15 localization in A-431cells. (A) Cells were incubated with or without 500 ng/ml EGF for 30 minutes at 37 C, permeabilized with saponin, and then solubilized in TGH-DOC buffer. Equal portions of the cytosolic (saponin eluent) and membrane fraction were incubated with saturative amounts of Ab32 (anti-β) to immunoprecipitate APs or with a corresponding amount of rabbit IgG. Eps15 and α-subunits of AP-2 were detected in immunoprecipitates by western blotting with Ab577 and AC1-M11, respectively. (B,C) Cells were incubated with 500 ng/ml EGF for 30 minutes at 37 C, and processed for doublelabel immunofluorescence staining using mouse monoclonal antibodies to EGFR (B) and rabbit antibodies Ab577 to eps15 (C), using a Triton X-100 permeabilization protocol. Rabbit and mouse primary antibodies were detected with corresponding secondary IgGs labeled with fluorescein or Texas Red. Cells were visualized using a conventional Nikon microscope. Note the strong co-localization of EGFR and esp15 in large endosomes at the focal plane corresponding to the best staining of EGFRs. Movement of coat proteins to early/intermediate endosomes is EGF-dependent To prove that the recruitment of coat components to endosomes is EGF-dependent, the localization of clathrin-coat proteins was compared to that of transferrin in A-431 cells treated or not treated with EGF. Transferrin receptor is expressed at high levels in A-431 cells, and the addition of fluorescent transferrin results in accumulation of the label in the early endosomes. AP-1 in endosomes Based on visual analysis of the large number of experiments, the extent of clathrin accumulation in endosomes of A-431 cells is higher compared to that of AP-2. This observation prompted us to test whether another adaptor complex, AP-1, also docks on EGFR-containing endosomes and contributes to the clathrin recruitment. Immunofluorescence labeling of A- 431 cells with anti-γ-adaptin showed that although the main region of AP-1 localization is TGN, a punctate staining of AP- 1 can be seen at a distance from TGN, especially in cells treated with EGF (Fig. 6B). Double-label staining showed that AP-1 immunoreactivity overlaps with EGFR in perinuclear endosomes (Fig. 6A,B), similar to that overlap observed for EGFRs and AP-2, clathrin and eps15 (Figs 3, 4). To test whether EGFRs interact with AP-1, the coimmunoprecipitation assay was employed. Fig. 7 shows that AP-1 can be readily detected in EGFR immunoprecipitates recovered from EGF-stimulated cells. The time course of EGFR/AP-1 association measured by co-immunoprecipitation was similar to that of AP-2 (Fig. 7B). The extent of AP-1 binding to EGFRs was, however, smaller than that of AP-2. Whereas up to 20-25% of the total cellular AP-2 (40-50% of Triton X-100-extractable pool) could be coimmunoprecipitated with EGFR, about 5% of cellular AP-1 was associated with EGFR. It has been demonstrated that the association of AP-1 with TGN membranes can be disturbed by Brefeldin A (BFA) (Robinson and Kreis, 1992). To examine whether AP-1 binding to endosomes is also sensitive to BFA, we inspected the localization of AP-1 in cells treated with BFA prior to, and during the EGF stimulation. As seen in Fig. 6C,D, BFA caused dispersion of γ-adaptin staining associated with TGN, whereas AP-1 staining of EGF-containing endosomes has not been disturbed. In fact, endosomal staining of AP-1 was seen more clearly in cells treated with BFA, because of the diffusion of
7 Clathrin coat proteins in endosomes 323 TGN staining. Furthermore, BFA had no effect on the extent of co-immunoprecipitation of AP-1 with EGFR (Fig. 7). Thus, data of Figs 6 and 7 suggest that AP-1 is bound to the endosomal membrane via the BFA-insensitive mechanism requiring stable association with EGFRs. EGFR binds µ1 and µ2 The simplest explanation of the immunolocalization and immunoprecipitation studies in A-431 cells is that AP-1 as well as AP-2 is recruited to endosomes, due to direct association with activated EGFR. To confirm that EGFRs are capable of binding to AP-1 and AP-2 in vivo, we performed a protein-protein interaction analysis using the yeast twohybrid system. Such an approach has been used to demonstrate that polypeptides corresponding to intracellular domains of several integral membrane proteins, which possess tyrosine-containing internalization signals, for instance TGN38, bind to the µ subunits of APs (Ohno et al., 1995). Therefore, we tested whether the carboxyl terminus of EGFR, which contains multiple internalization motifs (Chang et al., 1993), interacts with µ1 or µ2 in yeast. The results of growth and β-galactosidase assays of EGFR/µ interactions in comparison with that interaction of the internalization motif of TGN38 are presented in Table 1. The fragment of EGFR corresponding to residues showed interaction with the full-length µ1 and µ2, as well as with µ2. The EGFR interaction with µ2 was, however, significantly weaker than that of the positive control, a peptide containing three repeats of the internalization motif of TGN38. The strength of interaction of EGFR fragment with µ1 was comparable with that of TGN38 peptide when estimated using growth (Table 1) or liquid β-galactosidase assay (data not shown). To map µ-binding regions of EGFR, several small fragments of EGFR carboxyl terminus were prepared. Fragment did not show any interaction with µ subunits (Table 1). However, other fragments (including , and ) showed strong transactivation activity in control experiments in the absence of GAL4-ad and despite the presence of 3-AT, and could not be used for mapping. Nevertheless, the data of two-hybrid experiments demonstrated that EGFR can directly bind AP-1 and that it binds to µ1 with an affinity comparable to that of its interaction with µ2. DISCUSSION Interactions of EGFR with APs in endosomes The EGF- and temperature-dependent interaction of AP-2 with EGFR was initially demonstrated using a coimmunoprecipitation assay (Sorkin and Carpenter, 1993) and attributed to the function of AP-2 in recruiting activated EGFRs into the plasma membrane coated pits. However, there is no obvious restraint to prevent AP-2 binding to internalized EGFR that remain largely dimerized, active and tyrosine phosphorylated (Carpentier et al., 1987; Lai et al., 1989; Nesterov et al., 1990; Sorkin and Carpenter, 1991). Here we show that EGF-dependent EGFR/AP-2 association is maintained during continuous endocytosis in A-431 cells (Fig. 1) and is not sensitive to the removal of EGF from the surface receptors (Fig. 2). Together with the results of digital deconvolution microscopy, the data strongly suggest that a pool of EGFR/AP-2 complexes are preserved after internalization. The following working model of EGFR/AP interactions during internalization is proposed. EGF binding elevates the affinity of EGFR interaction with AP-2, leading to increased recruitment of receptors into coated pits. That EGFR can bind AP-2 at the cell surface is suggested by the detection of EGFR/AP-2 co-immunoprecipitation in K + -depleted cells when clathrin-dependent endocytosis is blocked (Sorkin and Carpenter, 1993). However, we propose that it is the endosomal EGFR/AP-2 complexes that constitute a substantial fraction of these complexes detected by co-immunoprecipitation under conditions of normal endocytosis. In fact, the EGFR family are the only receptors, except for influenza virus hemagglutinin (Fire et al., 1997), for which co-immunoprecipitation with AP- 2 is documented (Gilboa et al., 1995; Sorkin and Carpenter, 1993). For instance, the co-immunoprecipitation of transferrin receptor with AP-2 has not been demonstrated. Perhaps the transient receptor-ap interactions during internalization do not result in an accumulation of receptor-adaptor complexes that is sufficient for detection by co-immunoprecipitation. However, if this interaction is not transient and sustained in endosomes, as observed for EGFRs in A-431 cells, it can be readily detected by co-immunoprecipitation. Correspondingly, AP-2 does not follow transferrin receptor to endosomes in A- 431 cells. Presumably, prolonged EGFR/AP-2 association in Table 1. Interaction of EGFR fragments with µ subunits in yeast two-hybrid system GAL4bd-fusion constructs EGFR fragments GAL4ad-fusion TGN38 (amino acid residues) constructs Assay* SDYQRL SDGQRL None β-gal growth µl β-gal growth µ2/ µ2 β-gal growth *The relative intensity of β-galactosidase reaction and cell growth is ranged from the maximal (+++) observed for TGN38/µ2 interaction to the minimal (+) for EGFR /µ2 interaction. ( ) No blue staining or cell growth.
8 324 T. Sorkina and others vivo is enforced by the multivalent interactions of EGFR multimers. Another possibility is that EGFR signaling in endosomes results in production of lipids, such as phosphatidic acid or phosphatidylinositol-3-phosphate, that might be important for stabilization of the AP binding to the membrane (Gaidarov et al., 1996; West et al., 1997). The surprising observation is the detection of AP-1 in EGFR immunoprecipitates and in endosomes of A-431 cells. Because AP-1 accumulation in endosomes and association with the EGFR is BFA-independent, these processes might be regulated by the same mechanism as AP-2 binding to the EGFR and to the plasma membrane (which is BFA-insensitive). The results of the two-hybrid assay, which demonstrate binding of the carboxyl terminus of EGFR to µ subunits of APs, support the possibility of the direct receptor binding to AP-1. The interaction of the EGFR with µ1 was slightly stronger than Fig. 5. Co-localization of transferrin, eps15 and clathrin in endosomes of A-431 cells. (A-F) Cells were incubated with 5 µg/ml TRF-TR in the absence (A-C) or presence of 500 ng/ml EGF (D-F) for 30 minutes at 37 C, fixed and stained with the Ab577 to eps15 followed by secondary IgGs labeled with fluorescein (B,E). (G-L) Cells were incubated with 5 µg/ml TRF-FITC in the absence (G-I) or presence of 500 ng/ml EGF (J- L) for 30 minutes at 37 C, fixed and stained with the monoclonal X-22 antibody to clathrin followed by the secondary IgG labeled with Texas Red (H,K). The saponin permeabilization protocol was used. The serial optical sections were acquired and deconvoluted using a QED Imaging system and deconvoluted as described Materials and methods. The fluorescein (green) and Texas Red (red) channels were merged (C,F,I,L) after adjustment of both fluorescence signals to similar levels. Yellow indicates the overlap of Texas Red and fluorescein fluorescence. All images comprise an individual optical section (0.2 µm) from the middle of the cell, where the most intense signal for transferrin was observed. Bars, 5 µm.
9 Clathrin coat proteins in endosomes 325 Fig. 6. Localization of AP-1 in A-431 cells treated with EGF. Cells were incubated for 15 minutes with (C,D) or without 10 µg/ml BFA (A,B) at 37 C, and then in the same medium with 500 ng/ml EGF for 30 minutes at 37 C. Formaldehyde-fixed cells were processed for double-label immunofluorescence microscopy with rabbit anti-egfr Ab2913 (A,C) and mouse antibodies 100/3 to γ-adaptin (B,D) using a Triton X-100 permeabilization protocol. Rabbit and mouse primary antibodies were detected with corresponding secondary IgGs labeled with fluorescein or Texas Red. Cells were visualized using a conventional Nikon microscope (see Materials and methods). Arrows indicate examples of co-localization of the endosomes containing EGFR (A,C) and AP-1 (B,D). Note, the dispersion of TGN staining of γ-adaptin in the presence of BFA (D). with µ2 when estimated by the growth assay (Table 1). This is in contrast to the much stronger interactions of internalization signals of other proteins with µ2 compared to µ1 in the twohybrid system (Ohno et al., 1996). Interestingly, unc-101 gene, encoding a homolog of mammalian µ1, negatively regulates the let-23 (EGFR) signaling pathway in C. elegans (Lee et al., 1994), possibly by affecting the degradation of the receptor. Thus, it can be hypothesized that in mammalian cells AP-1 might be also involved in the sorting of EGFRs to the lysosomal pathway. Eps15 and clathrin in endosomes Eps15 is constitutively associated with AP-2 in A-431 (Fig. 4) and other cells (Benmerah et al., 1995; Iannolo et al., 1997), and appears to follow the intracellular distribution of AP-2 induced by EGF (Figs 4-8). Eps15 was not, however, found in EGFR immunoprecipitates, obtained under mild conditions, in any significant amount (Fazioli et al., 1993; our unpublished Fig. 7. AP-1 co-immunoprecipitation with EGFR in A-431 cells. (A) Cells were incubated for 15 minutes with or without 10 µg/ml BFA at 37 C, and then in the same media with 500 ng/ml EGF for 30 minutes at 37 C. EGFRs were immunoprecipitated from TGH lysates of the cells, and detected by western blotting with antibodies Ab2931, while γ-subunit of AP-1 and α-subunits of AP-2 were probed with the mixture of antibodies AC1-M11 and 100/3. (B) A- 431 cells were incubated with EGF (500 ng/ml) for the indicated periods of time (minutes) at 37 C, and then the EGFRs were immunoprecipitated from TGH lysates of the cells. The γ-subunits of AP-1 were detected in immunoprecipitates as described for A. The time course was similar in three independent experiments. data). One possible explanation is the limited sensitivity of the co-immunoprecipitation assay for the detection of indirectly associated proteins in detergent solutions. In addition, the existence of another EGF-dependent mechanism of the membrane docking of eps15 cannot be ruled out. Clathrin has been previously found in the peripheral endosomes of A-431 cells by whole-cell-mount electron microscopy (Stoorvogel et al., 1996). Our experiments also demonstrated the presence of clathrin in endosomes containing transferrin receptor, and the increase of the endosomal clathrin pool in the presence of EGF. It can be proposed that clathrin is constitutively associated with endosomes due to its anchoring to AP-3 (Dell Angelica et al., 1998), whereas an additional recruitment of clathrin to endosomes may result from the EGF-induced accumulation of AP-2 and AP-1 in these endosomes. Coated pit proteins are associated with the early/intermediate endosomes The EGF-dependent appearance of clathrin, AP-2, AP-1 and eps15 in endosomes of A-431 cells is the first demonstration, to our knowledge, of the massive re-distribution of coated pit proteins to endosomes in intact cells. The large perinuclear endosomes might correspond to the classical MVEs that are often seen in A-431 and other cells and contain transferrin receptors and EEA.1 protein (Beguinot et al., 1984; Gu et al., 1997; Haigler et al., 1979; Miller et al., 1986). Previous studies did not detect a clathrin lattice in MVE-like structures;
10 326 T. Sorkina and others however, its absence could be due to the high sensitivity of these coats to the sample preparation procedures used in electron microscopy and subcellular fractionation experiments. For instance, clathrin coats on the peripheral endosomes in A- 431 cells could only be seen when the cells were saponinpermeabilized prior to fixation, and the endosomes were stabilized by the horseradish peroxidase reaction product (Stoorvogel et al., 1996). The ability of AP-2 and clathrin to dock on lysosome-like organelles in permeabilized cells has been demonstrated (Traub et al., 1996). Immunofluorescence and electron microscopy studies revealed the presence of a pool of AP-1 in endosome-like vesicles located at some distance from TGN (Le Borgne et al., 1996). All these data suggest that the localization of AP-2 and AP-1 is not restricted to the plasma membrane or TGN, respectively, and that these adaptors together with other coat elements can function in divergent compartments of the endocytic pathway. The regulation of membrane docking of coated pit proteins and their recruitment to endosomes is cellspecific The important evidence on the regulated AP-docking mechanism comes from experiments in which AP-2 was mistargeted to intracellular vesicles in intact and permeabilized cells by several drugs (Seaman et al., 1993; Wang et al., 1993). Does the appearance of clathrin adaptors, eps15 and clathrin in endosomes containing EGFR in A-431 cells represent one of the mechanisms of adaptor docking and coat assembly, or is it a case of mistargeting of the coat proteins? Whereas EGFinduced association of AP-2, AP-1, eps15 and clathrin with endosomes was readily seen in A-431 cells, this effect of EGF was less dramatic in COS-1 cells. The amount of clathrin seen by immunofluorescence in NIH 3T3 cells expressing high levels of transfected human EGFR (Sorkin et al., 1996) was even smaller than that in COS-1 cells (data not shown). No eps15 or APs were found in the endosomes of these cells, which correlates with a significantly lesser extent of EGFR/AP-2 co-immunoprecipitation in NIH 3T3 cells compared to A-431 cells (Sorkin et al., 1995). Thus, receptordependent docking of adaptors and other coat proteins might be regulated by the cell-specific mechanisms. Furthermore, the survey of several cell lines (HeLa, HepG2, NIH 3T3, PAE, HEK293, etc.) indicates that there is a correlation between the presence of large pleiomorphic endosomes and the accumulation of coated-pit proteins in endosomes in response to EGF. Perhaps the high density of EGFRs in endosomes of A-431 cells compared to other cells allows prolonged association of APs with these receptors. Also, the extent of dissociation of EGF from EGFR in endosomes might be higher and the activity of the receptors, therefore, lower in NIH 3T3 than in A-431 cells. The occupancy of the EGFRs by AP-2/AP-1 in endosomes may prevent EGFR binding to specific sorting adaptors and therefore lead to sequestration of EGFR in these endosomes for prolonged periods of time. The persistence of EGFR-AP interactions in endosomes of A-431 cells may account for the slower lysosomal targeting of EGFRs in these cells (Stoscheck and Carpenter, 1984) compared to NIH 3T3 cells (Sorkin et al., 1991b). Alternatively, AP-1 might be important for the lysosomal targeting of EGFRs. The association of endosomal EGFRs with AP-2 may hinder the receptor interaction with AP-1, which would result in a slow turnover of the EGFRs. Finally, we propose that A-431 cells represent a case of exaggeration of transient interactions of EGFRs in endosomes, and can serve as a model system to study the biogenesis, morphology and the function of the tubular-vesicular endosomes and their clathrin-adaptor coats. The authors are thankful to Drs G. Carpenter, L. Beguinot, P. P. Di Fiore and M. S. Robinson for the gifts of antibodies, and to Dr Bonifacino for the yeast expression plasmids. We are grateful to Dr Royston Carter for help with the two-hybrid studies and critical reading of the manuscript, and Steven Fedul for help with immunofluorescence imaging on the DeltaVision workstation that is supported by the NIH grant SIO RR This work was supported by NIH grant DK46817 and UCHSC/HHMI grant to A.S., and ACS/University of Colorado Cancer Center grant to F.T. Cancer Center Core Services of University of Colorado are supported by Grant CA REFERENCES Beguinot, L., Lyall, R. M., Willingham, M. C. and Pastan, I. (1984). Downregulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc. Natl. Acad. Sci. USA 81, Benmerah, A., Gagnon, J., Begue, B., Megarbane, B., Dautry-Varsat, A. and Cerf-Bensussan, N. (1995). The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J. Cell Biol. 131, Brodsky, F. M. (1985). Clathrin structure characterized with monoclonal antibodies. I. Analysis of multiple antigenic sites. J. Cell Biol. 101, Carpentier, J. L., White, M. F., Orci, L. and Kahn, C. R. (1987). Direct visualization of the phosphorylated epidermal growth factor receptor during its internalization in A-431 cells. J. Cell Biol. 105, Chang, C.-P., Lazar, C. S., Walsh, B. J., Komuro, M., Collawn, J. F., Kuhn, L. A., Tainer, J. A., Trowbridge, I. S., Farquhar, M. G., Rosenfeld, M. G., Wiley, H. S. and Gill, G. N. (1993). Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J. Biol. Chem. 268, Dell Angelica, E. C., Klumperman, J., Stoorvogel, W. and Bonifacino, J. S. (1998). Association of the AP-3 adaptor complex with clathrin. Science 280, Dell Angelica, E. C., Ohno, H., Ooi, C. E., Rabinovich, E., Roche, K. W. and Bonifacino, J. S. (1997). AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 16, Fazioli, F., Minichiello, L., Matoskova, B., Wong, W. T. and Di Fiore, P. P. (1993). Eps15, A Novel Tyrosine Kinase Substrate, Exibits Transforming Activity. Mol. Cell. Biol. 13, Fire, E., Brown, C. M., Roth, M., Henis, Y. I. and Petersen, N. O. (1997). Partitioning of proteins into plasma membrane microdomains. J. Biol. Chem. 272, Futter, C. E., Pearse, A., Hewlett, L. J. and Hopkins, C. R. (1996). Multivesicular endosomes containing EGF-EGF receptor complexes mature and fuse directly with lysosomes. J. Cell Biol. 132, Gaidarov, I., Chen, Q., Falck, J. R., Reddy, K. K. and Keen, J. H. (1996). A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 alpha subunit. Implications for the endocytic pathway. J. Biol. Chem. 271, Gilboa, L., Ben-Levy, R., Yarden, Y. and Henis, Y. I. (1995). Roles for a cytoplasmic tyrosine and tyrosine kinase activity in the interactions of Neu receptors with coated pits. J. Biol. Chem. 270, Gu, F., Aniento, F., Parton, R. G. and Gruenberg, J. (1997). Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J. Cell Biol. 139, Haigler, H. T., McKanna, J. A. and Cohen, S. (1979). Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A431. J. Cell Biol. 81, Hopkins, C. R., Miller, K. and Beardmore, J. M. (1985). Receptor-mediated
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Homework Hanson section MCB Course, Fall 2014
 Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
ab CytoPainter Golgi/ER Staining Kit
 ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
ab139485 CytoPainter Golgi/ER Staining Kit Instructions for Use Designed to detect Golgi bodies and endoplasmic reticulum by microscopy This product is for research use only and is not intended for diagnostic
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Vesicle Transport. Vesicle pathway: many compartments, interconnected by trafficking routes 3/17/14
 Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Summary of Endomembrane-system
 Summary of Endomembrane-system 1. Endomembrane System: The structural and functional relationship organelles including ER,Golgi complex, lysosome, endosomes, secretory vesicles. 2. Membrane-bound structures
Summary of Endomembrane-system 1. Endomembrane System: The structural and functional relationship organelles including ER,Golgi complex, lysosome, endosomes, secretory vesicles. 2. Membrane-bound structures
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
MEK1 Assay Kit 1 Catalog # Lot # 16875
 MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
Intracellular Vesicular Traffic Chapter 13, Alberts et al.
 Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Chapter 13: Vesicular Traffic
 Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic
 Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic Question No. 1 of 10 1. Which of the following statements about clathrin-coated vesicles is correct? Question #1 (A) There are
Molecular Cell Biology - Problem Drill 17: Intracellular Vesicular Traffic Question No. 1 of 10 1. Which of the following statements about clathrin-coated vesicles is correct? Question #1 (A) There are
Western Immunoblotting Preparation of Samples:
 Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role in Alzheimer s disease
 Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
Mechanism of Vesicular Transport
 Mechanism of Vesicular Transport Transport vesicles play a central role in the traffic of molecules between different membrane-enclosed enclosed compartments. The selectivity of such transport is therefore
Mechanism of Vesicular Transport Transport vesicles play a central role in the traffic of molecules between different membrane-enclosed enclosed compartments. The selectivity of such transport is therefore
Antigen presenting cells
 Antigen recognition by T and B cells - T and B cells exhibit fundamental differences in antigen recognition - B cells recognize antigen free in solution (native antigen). - T cells recognize antigen after
Antigen recognition by T and B cells - T and B cells exhibit fundamental differences in antigen recognition - B cells recognize antigen free in solution (native antigen). - T cells recognize antigen after
1. endoplasmic reticulum This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
Lipidne mikrodomene. funkcija
 Lipidne mikrodomene funkcija 1 Cellular processes involving lipid rafts - Signal transduction - Protein and lipid trafficking and sorting - Endosome(clathrin)-independent endocytosis: - potocytosis and
Lipidne mikrodomene funkcija 1 Cellular processes involving lipid rafts - Signal transduction - Protein and lipid trafficking and sorting - Endosome(clathrin)-independent endocytosis: - potocytosis and
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
CORRECTION The Journal of Cell Biology
 CORRECTION The Journal of Cell Biology van Delft et al. Vol. 136, No. 4, February. 1997. Pages 811 822. The following is a correction for the above-mentioned article. Association and Colocalization of
CORRECTION The Journal of Cell Biology van Delft et al. Vol. 136, No. 4, February. 1997. Pages 811 822. The following is a correction for the above-mentioned article. Association and Colocalization of
Supplementary Material
 Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins
 Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Chapter 2: Exocytosis and endocytosis. Biochimica cellulare parte B 2016/17
 Chapter 2: Exocytosis and endocytosis Biochimica cellulare parte B 2016/17 Exocytosis and endocytosis Transport from the trans-golgi network to the cell exterior: exocytosis. All eukaryotic cells continuously
Chapter 2: Exocytosis and endocytosis Biochimica cellulare parte B 2016/17 Exocytosis and endocytosis Transport from the trans-golgi network to the cell exterior: exocytosis. All eukaryotic cells continuously
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
UIM domain-dependent recruitment of the endocytic adaptor protein Eps15 to ubiquitin-enriched endosomes
 UIM domain-dependent recruitment of the endocytic adaptor protein Eps15 to ubiquitin-enriched endosomes Gucwa and Brown Gucwa and Brown BMC Cell Biology 2014, 15:34 Gucwa and Brown BMC Cell Biology 2014,
UIM domain-dependent recruitment of the endocytic adaptor protein Eps15 to ubiquitin-enriched endosomes Gucwa and Brown Gucwa and Brown BMC Cell Biology 2014, 15:34 Gucwa and Brown BMC Cell Biology 2014,
The Molecular Mechanism of Intracellular Membrane Fusion. Richard H. Scheller
 The Molecular Mechanism of Intracellular Membrane Fusion Richard H. Scheller The human brain contains approximately 10 15 connections between nerve cells (Figure 2). The specific formation and modulation
The Molecular Mechanism of Intracellular Membrane Fusion Richard H. Scheller The human brain contains approximately 10 15 connections between nerve cells (Figure 2). The specific formation and modulation
Lab module 6a Receptor-mediated endocytosis
 Goal for the module Lab module 6a Receptor-mediated endocytosis To follow cell surface receptors as they are internalized. Pre-lab homework Read about receptor-mediated endocytosis and other forms of internalization
Goal for the module Lab module 6a Receptor-mediated endocytosis To follow cell surface receptors as they are internalized. Pre-lab homework Read about receptor-mediated endocytosis and other forms of internalization
Instructions. Fuse-It-Color. Overview. Specifications
 Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Medical Research Council Laboratory for Molecular Cell Biology, University College, London WC1E 6BQ, United Kingdom
 Molecular Biology of the Cell Vol. 9, 809 816, April 1998 The Kinetics of Mannose 6-Phosphate Receptor Trafficking in the Endocytic Pathway in HEp-2 Cells: The Receptor Enters and Rapidly Leaves Multivesicular
Molecular Biology of the Cell Vol. 9, 809 816, April 1998 The Kinetics of Mannose 6-Phosphate Receptor Trafficking in the Endocytic Pathway in HEp-2 Cells: The Receptor Enters and Rapidly Leaves Multivesicular
Instructions. Fuse-It-mRNA easy. Shipping and Storage. Overview. Kit Contents. Specifications. Note: Important Guidelines
 Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or
 Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells. Pei-Tzu Yang and Hendrik C. Korswagen
 Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Appendix: Subcellular localization of MIG-14::GFP in C. elegans body wall muscle cells Pei-Tzu Yang and Hendrik C. Korswagen 129 SUBCELLULAR LOCALIZATION OF MIG-14 Abstract MIG-14/Wls is a Wnt binding
Endocytosis and Intracellular Trafficking of Notch and Its Ligands
 CHA P T E R F IVE Endocytosis and Intracellular Trafficking of Notch and Its Ligands Shinya Yamamoto, *,1 Wu-Lin Charng, *,1 and Hugo J. Bellen *,,, Contents 1. Notch Signaling and its Regulation by Endocytosis
CHA P T E R F IVE Endocytosis and Intracellular Trafficking of Notch and Its Ligands Shinya Yamamoto, *,1 Wu-Lin Charng, *,1 and Hugo J. Bellen *,,, Contents 1. Notch Signaling and its Regulation by Endocytosis
Gladstone Institutes, University of California (UCSF), San Francisco, USA
 Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24 Gag Marianne Gesner, Mekhala Maiti, Robert Grant and Marielle Cavrois * Gladstone Institutes, University of
Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24 Gag Marianne Gesner, Mekhala Maiti, Robert Grant and Marielle Cavrois * Gladstone Institutes, University of
Significance of the MHC
 CHAPTER 8 Major Histocompatibility Complex (MHC) What is is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) Significance of the MHC role in immune response role in organ
CHAPTER 8 Major Histocompatibility Complex (MHC) What is is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) Significance of the MHC role in immune response role in organ
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in
 Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
1. This is the location where N-linked oligosaccharide is initially synthesized and attached to glycoproteins.
 Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Biology 4410 Name Spring 2006 Exam 2 A. Multiple Choice, 2 pt each Pick the best choice from the list of choices, and write it in the space provided. Some choices may be used more than once, and other
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well
 Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
Chapter 1: Vesicular traffic. Biochimica cellulare parte B 2017/18
 Chapter 1: Vesicular traffic Biochimica cellulare parte B 2017/18 Major Protein-sorting pathways in eukaryotic cells Secretory and endocytic pathways Unifying principle governs all protein trafficking
Chapter 1: Vesicular traffic Biochimica cellulare parte B 2017/18 Major Protein-sorting pathways in eukaryotic cells Secretory and endocytic pathways Unifying principle governs all protein trafficking
Integrin v 3 targeted therapy for Kaposi s sarcoma with an in vitro evolved antibody 1
 Integrin v 3 targeted therapy for Kaposi s sarcoma with an in vitro evolved antibody 1 CHRISTOPH RADER, 2 MIKHAIL POPKOV, JOHN A. NEVES, AND CARLOS F. BARBAS III 2 Department of Molecular Biology and The
Integrin v 3 targeted therapy for Kaposi s sarcoma with an in vitro evolved antibody 1 CHRISTOPH RADER, 2 MIKHAIL POPKOV, JOHN A. NEVES, AND CARLOS F. BARBAS III 2 Department of Molecular Biology and The
Many G protein-coupled receptors (GPCRs) 2 are rapidly endocytosed after agonist binding, but the pathway of postendocytic
 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 40, pp. 29646 29657, October 5, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Hepatocyte Growth
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 40, pp. 29646 29657, October 5, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Hepatocyte Growth
Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit
 PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
BMDCs were generated in vitro from bone marrow cells cultured in 10 % RPMI supplemented
 Supplemental Materials Figure S1. Cultured BMDCs express CD11c BMDCs were generated in vitro from bone marrow cells cultured in 10 % RPMI supplemented with 15 ng/ml GM-CSF. Media was changed and fresh
Supplemental Materials Figure S1. Cultured BMDCs express CD11c BMDCs were generated in vitro from bone marrow cells cultured in 10 % RPMI supplemented with 15 ng/ml GM-CSF. Media was changed and fresh
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Intracellular vesicular traffic. B. Balen
 Intracellular vesicular traffic B. Balen Three types of transport in eukaryotic cells Figure 12-6 Molecular Biology of the Cell ( Garland Science 2008) Endoplasmic reticulum in all eucaryotic cells Endoplasmic
Intracellular vesicular traffic B. Balen Three types of transport in eukaryotic cells Figure 12-6 Molecular Biology of the Cell ( Garland Science 2008) Endoplasmic reticulum in all eucaryotic cells Endoplasmic
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were
 Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Supplementary Figure 1 CD4 + T cells from PKC-θ null mice are defective in NF-κB activation during T cell receptor signaling. CD4 + T cells were isolated from wild type (PKC-θ- WT) or PKC-θ null (PKC-θ-KO)
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation
 Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 2/21/17 glycosylation is a non-template derived phenomenon - the presence of
Localization and Retention of Glycosyltransferases And the Role of Vesicle Trafficking in Glycosylation Richard Steet, Ph.D. 2/21/17 glycosylation is a non-template derived phenomenon - the presence of
Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras
 Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras Tarik Issad, Ralf Jockers and Stefano Marullo 1 Because they play a pivotal role
Bioluminescence Resonance Energy Transfer (BRET)-based studies of receptor dynamics in living cells with Berthold s Mithras Tarik Issad, Ralf Jockers and Stefano Marullo 1 Because they play a pivotal role
Supplemental Data. Hao et al. (2014). Plant Cell /tpc
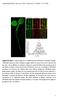 Supplemental Figure 1. Confocal Images and VA-TIRFM Analysis of GFP-RbohD in Arabidopsis Seedlings. (A) RbohD expression in whole Arabidopsis seedlings. RbohD was expressed in the leaves, hypocotyl, and
Supplemental Figure 1. Confocal Images and VA-TIRFM Analysis of GFP-RbohD in Arabidopsis Seedlings. (A) RbohD expression in whole Arabidopsis seedlings. RbohD was expressed in the leaves, hypocotyl, and
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Supplementary Material and Methods
 Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Product Manual. Human LDLR ELISA Kit. Catalog Number. FOR RESEARCH USE ONLY Not for use in diagnostic procedures
 Product Manual Human LDLR ELISA Kit Catalog Number STA-386 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is an essential component of cellular membranes,
Product Manual Human LDLR ELISA Kit Catalog Number STA-386 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cholesterol is an essential component of cellular membranes,
Influenza B Hemagglutinin / HA ELISA Pair Set
 Influenza B Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK11053 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
Influenza B Hemagglutinin / HA ELISA Pair Set Catalog Number : SEK11053 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized
of an untreated HS-stained BAEC monolayer viewed using a laser confocal microscope; Bar = 10 µm.
 Supplemental Figure 1: EC monolayer heparan sulfate fluorescence intensity. (A) Enface perspective of an untreated HS-stained BAEC monolayer viewed using a laser confocal microscope; Bar = 10 µm. (B) Enface
Supplemental Figure 1: EC monolayer heparan sulfate fluorescence intensity. (A) Enface perspective of an untreated HS-stained BAEC monolayer viewed using a laser confocal microscope; Bar = 10 µm. (B) Enface
COURSE: Medical Microbiology, MBIM 650/720 - Fall TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12
 COURSE: Medical Microbiology, MBIM 650/720 - Fall 2008 TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12 FACULTY: Dr. Mayer Office: Bldg. #1, Rm B32 Phone: 733-3281 Email: MAYER@MED.SC.EDU
COURSE: Medical Microbiology, MBIM 650/720 - Fall 2008 TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12 FACULTY: Dr. Mayer Office: Bldg. #1, Rm B32 Phone: 733-3281 Email: MAYER@MED.SC.EDU
Significance of the MHC
 CHAPTER 8 Major Histocompatibility Complex (MHC) What is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) - MHC molecules were initially discovered during studies aimed
CHAPTER 8 Major Histocompatibility Complex (MHC) What is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) - MHC molecules were initially discovered during studies aimed
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator. of the Interaction with Macrophages
 Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Serum Amyloid A3 Gene Expression in Adipocytes is an Indicator of the Interaction with Macrophages Yohei Sanada, Takafumi Yamamoto, Rika Satake, Akiko Yamashita, Sumire Kanai, Norihisa Kato, Fons AJ van
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/305/ra106/dc1 Supplementary Materials for Controlling Long-Term Signaling: Receptor Dynamics Determine Attenuation and Refractory Behavior of the TGF-β Pathway
www.sciencesignaling.org/cgi/content/full/6/305/ra106/dc1 Supplementary Materials for Controlling Long-Term Signaling: Receptor Dynamics Determine Attenuation and Refractory Behavior of the TGF-β Pathway
Supporting Information
 Supporting Information Kim et al. 10.1073/pnas.0912180106 SI Materials and Methods DNA Constructs. The N-terminally-tagged mouse Gli2 expression construct, pcefl/3 HA-Gli2, was constructed by ligating
Supporting Information Kim et al. 10.1073/pnas.0912180106 SI Materials and Methods DNA Constructs. The N-terminally-tagged mouse Gli2 expression construct, pcefl/3 HA-Gli2, was constructed by ligating
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
Supplementary material: Materials and suppliers
 Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary table and figures
 3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Luminescent platforms for monitoring changes in the solubility of amylin and huntingtin in living cells
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Molecular Cell Biology 5068 In class Exam 1 October 2, Please print your name: Instructions:
 Molecular Cell Biology 5068 In class Exam 1 October 2, 2012 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Molecular Cell Biology 5068 In class Exam 1 October 2, 2012 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Chromatin IP (Isw2) Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles.
 Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart.
 High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart. Daniel Aston, Rebecca A. Capel, Kerrie L. Ford, Helen C. Christian,
High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart. Daniel Aston, Rebecca A. Capel, Kerrie L. Ford, Helen C. Christian,
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates
 HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
HIV-1 Virus-like Particle Budding Assay Nathan H Vande Burgt, Luis J Cocka * and Paul Bates Department of Microbiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
AMPK Phosphorylation Assay Kit
 AMPK Phosphorylation Assay Kit Catalog Number KA3789 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
AMPK Phosphorylation Assay Kit Catalog Number KA3789 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
RayBio KinaseSTAR TM Akt Activity Assay Kit
 Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
LDL Uptake Cell-Based Assay Kit Item No. 10011125 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Chapter 7: Membranes
 Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
TECHNICAL BULLETIN. Caveolae/Rafts Isolation Kit. Catalog Number CS0750 Storage Temperature 20 C
 Caveolae/Rafts Isolation Kit Catalog Number CS0750 Storage Temperature 20 C TECHNICAL BULLETIN Product Description Caveolae and lipid rafts are important cholesterol and sphingolipid-rich structures representing
Caveolae/Rafts Isolation Kit Catalog Number CS0750 Storage Temperature 20 C TECHNICAL BULLETIN Product Description Caveolae and lipid rafts are important cholesterol and sphingolipid-rich structures representing
