Actin Cytoskeleton Reorganization by Syk Regulates Fcg Receptor Responsiveness by Increasing Its Lateral Mobility and Clustering
|
|
|
- Coral Houston
- 5 years ago
- Views:
Transcription
1 Article Actin Cytoskeleton Reorganization by Syk Regulates Fcg Receptor Responsiveness by Increasing Its Lateral Mobility and Clustering Valentin Jaumouillé, 1 Yoav Farkash, 1 Khuloud Jaqaman, 2 Raibatak Das, 3 Clifford A. Lowell, 4 and Sergio Grinstein 1,5, * 1 Program in Cell Biology, Hospital for Sick Children, Toronto, ON M5G1X8, Canada 2 Department of Biophysics, University of Texas Southwestern Medical Center, Dallas, TX , USA 3 Department of Integrative Biology, University of Colorado Denver, Denver, CO 80204, USA 4 Department of Laboratory Medicine, University of California, San Francisco, San Francisco, CA , USA 5 Keenan Research Centre of the Li Ka Shing Knowledge Institute, St. Michael s Hospital, 209 Victoria Street, Toronto, ON M5C 1N8, Canada *Correspondence: sergio.grinstein@sickkids.ca SUMMARY Clustering of immunoreceptors upon association with multivalent ligands triggers important responses including phagocytosis, secretion of cytokines, and production of immunoglobulins. We applied single-molecule detection and tracking methods to study the factors that control the mobility and clustering of phagocytic Fcg receptors (FcgR). While the receptors exist as monomers in resting macrophages, two distinct populations were discernible based on their mobility: some diffuse by apparent free motion, while others are confined within submicron boundaries that reduce the frequency of spontaneous collisions. Src-family and Syk kinases determine the structure of the actin cytoskeleton, which is fenestrated, accounting for the heterogeneous diffusion of the FcgR. Stimulation of these kinases during phagocytosis induces reorganization of the cytoskeleton both locally and distally in a manner that alters receptor mobility and clustering, generating a feedback loop that facilitates engagement of FcgR at the tip of pseudopods, directing the progression of phagocytosis. INTRODUCTION Immunoreceptors, such as Fc, B cell, and T cell receptors, are responsible for the recognition of antigens, whether by themselves or bound to antibodies or major histocompatibility complex (MHC) molecules. Signaling by these receptors is essential for innate and adaptive immune responses. Detailed studies of their structure in the free and bound states indicate that immunoreceptors do not undergo significant conformational changes upon ligand binding (Woof and Burton, 2004). Instead, immunoreceptor-mediated signaling is elicited by their clustering. Accordingly, immunoreceptor activation is not triggered by monovalent ligands, requiring multivalent stimuli (Holowka et al., 2007; Jones et al., 1985; Odin et al., 1991). Immunoreceptors possess in their cytosolic domain a tyrosine-based activation motif (ITAM) that upon receptor clustering becomes phosphorylated by Src family kinases and possibly also by the spleen tyrosine kinase Syk (Kiefer et al., 1998; Nimmerjahn and Ravetch, 2008). The signaling cascade unleashed by phosphorylation of the ITAM causes a marked reorganization of the actin cytoskeleton, culminating with the formation of an immunological synapse (Xie et al., 2013) or, in the case of Fcg receptors (FcgR), the phagocytosis of target particles (Flannagan et al., 2012). FcgR have been tacitly assumed to exist as monomers that are evenly distributed on the cell surface and move in Brownian fashion. Upon exposure to particles decorated with multiple IgG molecules their preferred ligand FcgR are thought to be progressively recruited ( zipper ) around the particle (Griffin et al., 1975) as a result of random lateral diffusion. FcgR clustering and hence activation occur as a consequence of such zippering. The ability of receptors to rapidly diffuse and cluster is predicated on the assumption that biological membranes behave as fluid bilayers (Singer and Nicolson, 1972). However, a number of recent observations question the general applicability of the Singer-Nicolson fluid mosaic model. First, most proteins studied display lateral mobilities that are 5 to 50 times slower in the plasma membrane of cells than in artificially reconstituted bilayers of comparable lipid composition (Kusumi et al., 2005). Second, photobleaching recovery determinations suggested that a subset of plasma membrane proteins are immobile (Jacobson et al., 1976; Schlessinger et al., 1976), and a number of proteins undergo anomalous diffusion, rather than the anticipated free diffusion (Crane and Verkman, 2008; Smith et al., 1999). Third, while the fluid mosaic model predicts that lateral mobility should be only marginally sensitive to the size of the molecule and therefore barely affected by oligomerization (Saffman and Delbrück, 1975), the oligomerization of membrane proteins can reduce their diffusion up to 40-fold (Iino et al., 2001). Finally, plasmalemmal proteins dragged by optical tweezers can rebound to their initial location once they escape the optical trap, suggesting the existence of elastic structures that restrict diffusion within the membrane (Sako and Kusumi, 1995). In view of these observations, the fluid mosaic model has been revised in favor of an alternative model, where the plasmalemma is compartmentalized by molecular fences. The fence-like structures are 534 Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc.
2 thought to be generated by membrane-associated picket proteins, anchored to the actin filament network juxtaposed to the bilayer (Kusumi et al., 2005). The density and limited mobility of the transmembrane pickets restrict the diffusion of mobile proteins and lipids in the plane of the membrane. This reinterpretation of the fluid mosaic model, as well as earlier observations indicating that FcgR heterologously expressed in cell lines are partially mobile (Zhang et al., 1995), prompted us to assess experimentally whether FcgR in fact undergo free diffusion. If confined by pickets and fences, it is unclear whether FcgR could cluster effectively, particularly during the short window of opportunity presented by the casual contact with particles such as microorganisms, which can be mobile. In addition, it was of interest to establish whether the rearrangement of the cytoskeleton that accompanies phagocytosis can itself alter the mobility of the receptors (Jaqaman and Grinstein, 2012; Jaumouillé and Grinstein, 2011). To address these questions, we analyzed lateral mobility of FcgR at the single-molecule level. Our results indicate that, contrary to earlier assumptions, FcgR behave heterogeneously, displaying confined and freely mobile subpopulations or states. In addition, engagement of a fraction of the FcgR alters the ability of the unengaged receptors to diffuse, potentially amplifying the response. RESULTS Heterogeneous Mobility of Fc Receptors We studied the behavior of FcgRIIA in human monocyte-derived macrophages using antibodies to an exofacial epitope. To prevent clustering induced by the detection system, Fab fragments of a monoclonal antibody (clone IV.3) were prepared and visualized on the dorsal surface of the cells by addition of a secondary anti-mouse Fab fragment conjugated to Cy3 or to biotin. When using biotin-conjugated secondary Fab fragments, detection was accomplished using avidin-coupled quantum dots (Qdots), which are considerably brighter and less susceptible to photobleaching than Cy3. Crosslinking was avoided by adding the Qdots to the cells in the cold to minimize lateral displacement, followed by addition of free biotin to occlude the excess binding sites. Labeling was performed at low density (Figure 1A) to distinguish single FcgRIIA molecules. Individual features were detected and tracked using custom-made algorithms (Jaqaman et al., 2008, 2011). Moment scaling spectrum (MSS) analysis of single trajectories was used to characterize receptor motion (Ewers et al., 2005; Jaqaman et al., 2008, 2011). As illustrated in Figures 1B 1D and Movie S1 available online, FcgRIIA showed heterogeneous behavior: 64.4% ± 2.4% of the Cy3-labeled receptors displayed apparent free motion, whereas 27.4% ± 2.3% were confined. Similar results were obtained when FcgRIIA were labeled using Qdots: 64.2% ± 1.8% displayed free motion and 30.8% ± 1.9% were largely confined throughout the acquisition period, which was as long as 30 s (average track length was 7.5 s; Figures 1C and 1D). The diffusion of a minor fraction of the FcgRIIA (4.9% when using Qdots, 8.2% with Cy3) appeared as directed by a flow. This small fraction remained unchanged under most conditions and will not be discussed further. The median diffusion coefficient of the free receptors was ± mm 2 3 s 1 when labeled with Cy3 and ± mm 2 3 s 1 when measured with Qdots. These observations imply that the larger nanocrystal probe did not perturb receptor behavior. The diffusion coefficients of the confined receptors were estimated to be ± mm 2 3 s 1 or ± mm 2 3 s 1 when labeled with Cy3 or Qdots, respectively. If the cells were fixed with paraformaldehyde prior to analysis a single population of immobile receptors was detected, with a negligible diffusion coefficient (0.002 ± mm 2 3 s 1 ; Figure S1A). These observations suggest that the confined FcgRIIA detected in live cells are not firmly tethered to a fixed structure but instead diffuse within circumscribed areas or corrals. By analyzing the localization of individual confined receptors over periods of up to 30 s, we determined that the corrals had a mean area of ± mm 2 (Figure S1B), larger than lipid rafts or protein complex domains, but in the range of actin-based membrane compartments (Kusumi et al., 2012). FcgRIIA did not follow the linear tracks described for CD36 in the same cells (Jaqaman et al., 2011); 93% of FcgRIIA displayed isotropic behavior (Figure S1C). The heterogeneous behavior of the population raised the question whether some of the FcgRIIA would be more susceptible to collisions, and hence to clustering, that could result in receptor activation. Monte Carlo simulations of receptor diffusion (see Experimental Procedures) indicated that the collision rate was greatly diminished by receptor confinement and became lower as the corral size was reduced (Figure S1D). The simulations also indicated that receptor collision rate increased as the diffusion coefficient was ramped up (Figure S1E). These results predict that freely diffusing receptors would collide more frequently than confined receptors. Of note, the effect of confinement appeared more limited at higher receptor density (Figures S1F and S1G). The predicted effect of confinement on receptor diffusion prompted us to measure experimentally the frequency of collision events of Cy3-labeled FcgRIIA (Calebiro et al., 2013; Jaqaman et al., 2011). As anticipated, the collision frequency was 1.73 ± 0.12-fold higher for freely diffusing receptors than for those that were confined (Figure 1E). Jointly, these results indicate that, contrary to earlier assumptions, FcgRIIA behave heterogeneously and are differentially predisposed to collide in the presence of multivalent ligands. The Confinement of FcgRIIA Is Not Due to Oligomerization or to Association with Cholesterol-Rich Microdomains Coexistence of receptors in different states of aggregation could account for the presence of subpopulations of FcgRIIA with distinct mobility. Although functional evidence suggests that FcR exist as monomers when not engaged by ligands, the possibility of spontaneous dimerization of FcgRIIA has been invoked (Powell et al., 2006). To determine the state of oligomerization of FcgRIIA in the absence of ligand, macrophages were labeled with increasing concentrations of primary anti-fcgriia Fab fragments, followed by a constant saturating concentration of Cy3-conjugated secondary Fab. As previously described, individual Cy3 fluorophores display a defined intensity that enables the quantification of labeled molecules in a complex (Jaqaman et al., 2011). As shown in Figure 1F, while the number of detected features increased as the concentration of primary Fab increased, their modal fluorescence intensity remained constant. This suggests that the detected features Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc. 535
3 Developmental Cell A B C E D F G H Figure 1. Single-Molecule Imaging of FcgRIIA Reveals Heterogeneous Mobility in Human Primary Macrophages (A) Low-density labeling of FcgRIIA with Q-dots (red) at the surface of primary monocyte-derived macrophages. Representative image of more than 100 cells, from more than ten independent experiments. (B) Tracks obtained by SPT of FcgRIIA in one representative field of view. Confined motion is represented in navy blue and pointed by arrowheads; free motion is represented in cyan and pointed by arrows. Representative field of more than 100 cells, from more than ten independent experiments. (C) Percentage of confined receptors (circles) and free receptors (triangles), labeled with Cy3 (purple) or Q-dot (orange). Each dot represents one individual cell. Mean (black bar) and SEM (red bars) are displayed for each population. A total of 18,756 tracks from n = 40 cells and 27,506 tracks from n = 130 cells were analyzed for Cy3 and Q-dot-labeled cells, respectively. (legend continued on next page) 536 Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc.
4 A C D B Figure 2. FcgRIIA Mobility Is Not Confined by Cholesterol-Rich Microdomains (A) Filipin staining of cholesterol in primary human macrophages. Representative images of more than 30 fields, from three independent experiments. (B) Quantification of filipin intensity at the plasma membrane of individual primary macrophages. Measurements of each separate experiment were normalized to the mean intensity of the control cells. (C) Median diffusion coefficient (for all particles regardless of motion type) in control (purple) and methyl-b-cyclodextrin-treated cells (orange). A total of 8,790 tracks from 35 cells and 5,640 tracks from 26 cells were analyzed for control and methylb-cyclodextrin-treated cells, respectively. (D) Percentage of confined receptors (circles) and free receptors (triangles), in control (purple), and methyl-b-cyclodextrin-treated cells (orange). Error bars represent SEM. represent single receptors. To further explore the state of FcgRIIA oligomerization, we performed photobleaching experiments. If the detected features were composed of two or more labeled receptors, their photobleaching would occur stepwise, revealing the number of fluorophores. Figure 1G illustrates that, upon intense illumination, the number of fluorescent features in the field of view decreased progressively as a result of photobleaching. However, the vast majority of the spots disappeared in one single step, leaving the mean intensity of the remaining features unaltered (Figures 1G and 1H). Together, these experiments indicate that in the absence of ligands FcgRIIA exist largely as monomers. Thus, heterogeneous oligomerization cannot account for the observed subpopulations with differing mobility. As an alternative, we contemplated the possibility that the fraction of FcgRIIA that displayed confined motion might reside in cholesterol-enriched lipid microdomains (rafts). Such microdomains can act as diffusional traps for membrane proteins (Douglass and Vale, 2005; Suzuki et al., 2007). Indeed, FcgRIIA is a palmitoylated transmembrane protein that can in principle associate with lipid rafts (Kwiatkowska and Sobota, 2001; Rollet-Labelle et al., 2004). To analyze the contribution of cholesterol-enriched microdomains we treated macrophages with methyl-b-cyclodextrin (MbCD). The effectiveness of this treatment was validated using filipin: exposure to MbCD removed 68.7% ± 3.8% of the plasmalemmal cholesterol (Figures 2A and 2B). Treatment with MbCD reduced the diffusion coefficient of the entire population (Figure 2C), as observed previously for a variety of other membrane proteins (Fujiwara et al., 2002; Murase et al., 2004). Remarkably, cholesterol depletion was accompanied by an increase in the proportion of confined FcgRIIA (from 32.8% ± 2.7% to 48.7% ± 4.1% of total), instead of the hypothesized decrease (Figure 2D). These results imply that receptor confinement is not due to association with cholesterol-rich microdomains, consistent with earlier reports (Kwiatkowska and Sobota, 2001; Rollet-Labelle et al., 2004). The Lateral Mobility of FcgRIIA Is Regulated by the Tyrosine Kinases Src and Syk We next speculated that a fraction of the FcgRIIA might associate with other molecules, which could alter receptor mobility. Because interactions of FcgRIIA with effector molecules depend on receptor phosphorylation (Nimmerjahn and Ravetch, 2008), we assessed this possibility using kinase inhibitors. Two types of tyrosine kinases are known to phosphorylate the ITAM of FcgR: Src-family kinases (SFK) and Syk (Ghazizadeh et al., 1994; Kiefer et al., 1998). When active, these kinases undergo autophosphorylation, and the occurrence of this event can be monitored using specific antibodies (anti-ptyr 416 in the case of (D) Median diffusion coefficients of confined receptors (diamonds) and free receptors (squares), labeled with Cy3 (purple) or Q-dot (orange). (E) Ratio of merge frequency between free receptors and confined receptors, labeled with Cy3. Each dot represents one individual cell. A total of 18,756 tracks from n = 40 cells were analyzed. (F) Fluorescence intensity histograms of detected Cy3-labeled FcgRIIA with different primary Fab concentrations (in mg/ml) in fixed cells. Intensity modes were analyzed with a Gaussian fit (red line) and their mean intensity value is reported in the top right boxes. (G) Average fluorescence intensity of detected particles determined by Gaussian fitting (blue), and number of detected particles (green), measured over time during photobleaching experiments with Cy3-labeled FcgRIIA in fixed cells. (H) Representative measurements of the fluorescence intensity of individual features over time, illustrating photobleaching events. Error bars represent SEM. See also Figure S1 and Movie S1. Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc. 537
5 A C E B F D Figure 3. FcgRIIA Mobility Is Regulated by Syk- and Src-Family Kinases (A) Endogenous Src activity revealed by antiphospho-src Y416 immunoblotting in resting untreated or PP1-treated primary human macrophages. Representative western blot from three independent experiments. (B) Endogenous Syk activity revealed by antiphospho-syk Y525/526 immunoblotting in resting untreated or piceatannol-treated primary human macrophages. Representative western blot from three independent experiments. (C) Percentage of confined FcgRIIA (circles) and free FcgRIIA (triangles), in control (purple), PP1 (orange) and piceatannol-treated (blue) primary human macrophages. A total of 27,506 tracks from n = 130 cells, 7,768 tracks from n = 50 cells, and 7,027 tracks from n = 51 cells were analyzed for control, PP1-, and piceatannol-treated cells, respectively. (D) Median diffusion coefficients of FcgRIIA in control (purple), PP1- (orange), and piceatannoltreated (blue) primary human macrophages. (E) Percentage of confined FcgR (circle) and free FcgR (triangle), in wild-type (purple), and syk / (blue) mouse bone marrow-derived macrophages tracks from n = 16 cells and tracks from n = 20 cells were analyzed for wild-type and syk / macrophages, respectively. (F) Median diffusion coefficients of FcgR in wildtype (purple) and syk / (blue) mouse bone marrow-derived macrophages. Error bars represent SEM. See also Figure S2. Src and anti-ptyr 525/526 for Syk). The effectiveness of PP1 (a SFK inhibitor) and of piceatannol (a Syk inhibitor) was verified using such antibodies (Figures 3A, 3B, and S2A). Having demonstrated effective inhibition of SFK, we tested the effect of PP1 on FcgRIIA mobility by single-particle tracking (SPT). Rather than increasing the mobility of the receptors, as would be anticipated by dissolution of preformed complexes, inhibition of SFK activity increased the proportion of confined FcgRIIA (from 31.2% ± 1.2% to 43.9% ± 3.5%) and decreased the overall diffusion coefficient (from ± mm 2 3 s 1 to ± mm 2 3 s 1 ; Figures 3C and 3D). Inhibition of Syk had an even more pronounced effect, increasing the proportion of confined FcgRIIA to 62.2% ± 4.7% while decreasing the diffusion coefficient to ± mm 2 3 s 1 (Figures 3C and 3D). To minimize the possibility that off-target effects were responsible for the changes caused by piceatannol, we also tested Bay , a structurally unrelated Syk inhibitor. Like piceatannol, Bay increased the proportion of confined FcgRIIA (to 43.4% ± 2.7%) and decreased its diffusion coefficient (to ± mm 2 3 s 1 ; Figures S2B and S2C). More definitive evidence that the observed effects were in fact attributable to Syk was obtained using macrophages obtained from Sykdeficient mice (Crowley et al., 1997). Because FcgRIIA is not expressed in mice, we measured instead the behavior of FcgRIIB/FcgRIII using a Fab fragment from antibody 2.4G2, which recognizes these receptors. Analysis of FcgRIIB/ FcgRIII mobility in mouse macrophages yielded results that were very similar to those obtained for human FcgRIIA. In wildtype cells 61.7% ± 3.2% of the receptors underwent free motion, whereas 32.3% ± 3.4% were confined (Figure 3E). The diffusion coefficient was ± mm 2 3 s 1 (Figure 3F). In syk / macrophages however, only 38.2% ± 2.4% of the FcgR underwent free motion, whereas 57.8% ± 2.6% were confined, and the diffusion coefficient dropped to ± mm 2 3 s 1 (Figures 3E and 3F). Thus, phosphorylation-dependent association of receptors with effector proteins cannot explain the presence of a confined subpopulation of FcgR. On the other hand, our results revealed a surprising role of SFK and Syk in regulating the mobility of FcgRIIA and FcgRIIB. Syk Governs FcgRIIA Lateral Mobility Independently of Receptor Phosphorylation Because the inhibition or deletion of the tyrosine kinases had an unexpected effect on FcgR mobility, we investigated whether the altered behavior was due to changes in the phosphorylation of the receptor itself. To this end, we heterologously expressed either wild-type FcgRIIA or a mutant form in which the three tyrosines that are susceptible to phosphorylation were replaced by phenylalanines (termed 3Y-F; Figure 4A). The murine RAW264.7 line (referred to as RAW cells hereafter) was used 538 Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc.
6 A B C Figure 4. Receptor Tyrosine Phosphorylation Does Not Account for FcgRIIA Confinement in Resting Macrophages (A) Amino acid sequence of FcgRIIA cytosolic tail. The three tyrosines (blue) in the wild-type protein were replaced by phenylalanines (red) in the 3Y-F mutant construct. (B) Percentage of confined receptors (circles) and free receptors (triangles), observed for wild-type or 3Y-F mutant receptors in control (orange and blue, respectively), or piceatannol-treated (purple and green, respectively) RAW macrophages. A total of 24,367 tracks from n = 37 cells, 41,272 tracks from n = 40 cells, 11,184 tracks from n = 37 cells, and 15,053 tracks from n = 34 cells were analyzed for wild-type, wild-type + piceatannol, mutant and mutant + piceatannol cells, respectively. (C) Median diffusion coefficients observed for wild-type or 3Y-F mutant receptors in control (orange and blue, respectively), or piceatannol-treated (purple and green, respectively) RAW macrophages. Error bars represent SEM. for transfection, as these macrophages do not express endogenous FcgRIIA, and the receptors were labeled with Qdots. Analysis of FcgRIIA mobility showed that 51.7% ± 1.3% of the wildtype receptors were freely mobile, while 44.1% ± 1.4% were confined (Figure 4B). Thus, the heterogeneous behavior of FcgRIIA was recapitulated in a heterologous expression system. Moreover, as in the human cells, piceatannol treatment increased the fraction of confined receptors (to 75.4% ± 2%) while lowering the fraction of free FcgRIIA (to 21.8% ± 1.8%; Figure 4B). Interestingly, when expressed in untreated RAW cells the mutant FcgRIIA behaved identically to the wild-type receptor. Of note, piceatannol treatment increased the confinement of the mutant receptor to the same extent as observed for wild-type FcgRIIA (Figure 4B). Similarly, the diffusion coefficients of wild-type and mutant receptors were not significantly different in untreated cells and were comparably depressed by treatment with piceatannol (Figure 4C). Because the 3Y-F mutant cannot be tyrosine phosphorylated, these results demonstrate that Syk exerts its effects on FcgR mobility by a mechanism other than direct receptor phosphorylation. The Actin Cytoskeleton Governs FcgRIIA Mobility Immunoreceptors, as well as other proteins that associate with the ITAM-containing subunits DAP12 or FcR g-chain (FcRg), utilize Syk to transduce signals (Mócsai et al., 2010). One of the downstream effects of these signaling cascades is a local reorganization of the actin cytoskeleton. In view of the picketfence model of membrane architecture, we considered whether the effects of Syk on FcgRIIA mobility are mediated by remodeling the submembranous actin. To this end, we treated macrophages with an actin polymerization inhibitor. We were able to perform SPT using a short (10 min) treatment with a moderate dose (1 mm) of latrunculin B, which caused a partial disruption of the actin cytoskeleton without detaching the cells from the coverslip or grossly altering their morphology. As reported for other immunoreceptors (Andrews et al., 2008; Treanor et al., 2010), this treatment increased the diffusion coefficient of FcgRIIA (to ± mm 2 3 s 1 ; Figure 5A). Interestingly, this increased diffusion coefficient is accompanied by a 46% reduction in the proportion of confined receptors (to 18.8% ± 1.3%), with a commensurate increase in the freely diffusing fraction (to 74.6% ± 1.5%; Figure 5B). Because a more thorough disassembly of the actin cytoskeleton leads to changes in cell morphology, precluding accurate tracking of single particles, we extended our analysis of the role of the cytoskeleton in FcgRIIA mobility using an alternative method, namely fluorescence recovery after photobleaching (FRAP). For these experiments, RAW cells were transfected with GFP-tagged FcgRIIA and receptor mobility in otherwise untreated membranes was compared with that in membrane blebs devoid of actin (Booth et al., 2002). These were generated by treating macrophages with jasplakinolide. Stabilization of the cortical actin by jasplakinolide causes a myosin II-dependent contraction of the cytoskeleton, with concomitant extrusion of actin-depleted membrane blebs (Flannagan et al., 2010). The contraction of the cortical actin band was verified by cotransfecting the cells with LifeAct, a probe that binds specifically to F-actin (Riedl et al., 2008). Figure 5C shows not only that F-actin underwent contraction following addition of jasplakinolide, but also that the membrane blebs lacked detectable F-actin, yet contained GFP-tagged FcgRIIA. In the absence of jasplakinolide, the diffusion coefficient of FcgRIIA-GFP estimated by FRAP was ± 0.02 mm 2 3 s 1 (Figure 5D), consistent with data we obtained by SPT in the same cell line. By contrast, FcgRIIA-GFP diffusion was markedly faster within membrane blebs (D = ± mm 2 3 s 1 ; Figure 5D). Combined with the SPT determinations in latrunculin-treated cells, these results demonstrate that the cortical actin is a major determinant of the lateral mobility of FcgRIIA, restricting receptor diffusion. Syk Regulates FcgRIIA Mobility by Organizing the Cortical Actin Meshwork Syk is intimately involved in the reorganization of actin during phagocytosis. It is therefore conceivable that tonic Syk activity Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc. 539
7 A C D B Figure 5. FcgRIIA Mobility Is Restricted by the Actin Cytoskeleton (A) Median diffusion coefficient in control (purple) and latrunculin B-treated human primary macrophages (orange). A total of 6,005 tracks from n = 50 cells and 5,959 tracks from n = 40 cells were analyzed for control and latrunculin-treated cells, respectively. (B) Percentage of confined receptors (circles) and free receptors (triangles), in control (purple) and latrunculin B-treated cells (orange). (C) Confocal image of bleb formation in RAW macrophages upon jasplakinolide treatment; FcgRIIA-GFP (green) and LifeAct-mRFP (red). Representative image of more than 20 cells from three independent experiments. (D) Diffusion of FcgRIIA-GFP determined by FRAP at the plasma membrane of untreated RAW macrophages (diamonds) or in the blebs of jasplakinolide-treated RAW macrophages (circles), in presence (orange) or absence (purple) of piceatannol. Error bars represent SEM. may contribute also to the establishment and/or maintenance of the basal cytoskeletal structure of unstimulated macrophages; such a contribution could account for the effects of Syk on FcgRIIA mobility described above. This was investigated by staining F-actin with rhodamine-phalloidin and comparing the appearance of the cytoskeleton in the presence and absence of Syk inhibitors, using confocal microscopy. As expected, untreated macrophages displayed a band of cortical actin lining the dorsal membrane and a large number of intensely stained ventral podosomes (Figures 6A, S2A, and S3A). Strikingly, upon treatment with piceatannol the podosomes became virtually undetectable and, instead, F-actin staining increased markedly under the dorsal membrane. As a result, the ratio of dorsal to ventral actin increased from 0.67 ± 0.07 in controls to 1.77 ± 0.2 after inhibition of Syk (Figure 6B). Despite this pronounced redistribution, the total amount of cellular F-actin was not significantly changed by piceatannol (Figure 6C). As Syk is known to regulate the activity of several GEFs for Rac1 and RhoA, which in turn control actin polymerization, we determined the activity of these GTPases in macrophages using the PBD domain of PAK1 or rhotekin, respectively (see Experimental Procedures). In piceatannol-treated cells, Rac1 activity decreased to 58.4% ± 4%, whereas RhoA activity increased to 151% ± 1.9% of the control level (Figure S3B). These changes were associated with a decrease in the rate of actin polymerization; piceatannol reduced the rate of monomeric actin association with the barbed ends of filaments to 71.7% ± 8.8% of the control (Figure S3C). While regulation of Rho-family GTPase activity by Syk can account for the cytoskeletal alterations, it does not clarify how the associated changes in F- actin distribution restrict the mobility of FcgRIIA. To gain further insight, we analyzed the ultrastructure of the cortical actin that lines the dorsal membrane, the surface where receptor mobility was assessed. This was accomplished by visualizing the cytoskeletal meshwork in cells that were demembranated by nonionic detergent in an actin filament-stabilizing buffer (Svitkina, 2007), prior to fixation and preparation for high-resolution scanning electron microscopy using field emission. As illustrated in Figure 6D, in untreated cells the cytoskeleton was very heterogeneous, consisting of patches of dense networks of microfilaments, separated by relatively open areas devoid of filamentous material (Figure 6D). Following incubation with the Syk inhibitor the patches of filamentous meshwork were larger and denser, separated by much smaller open areas (Figure 6D). Thus, the ultrastructure of the dorsal cortical actin network correlated with the results obtained with phalloidin and can explain the SPT results: receptors located in the open areas presumably diffuse freely in the plane of the membrane, while those in areas lined by an actin meshwork undergo confinement. The increase in the number and density of the latter accounts for the confining effects of piceatannol and of genetic ablation of Syk. A corollary of this hypothesis is that inhibition of Syk should have no effect on receptors localized in areas devoid of the actin cytoskeleton. The validity of this corollary was tested by FRAP measurements in jasplakinolide-induced blebs, which lack detectable actin (see Figure 5C). As predicted, the rapid diffusion of GFP-tagged FcgRIIA in blebs formed by jasplakinolidetreated RAW cells was unaffected by addition of piceatannol: the diffusion coefficient was ± mm 2 3 s 1 in the presence of the inhibitor, compared to ± mm 2 3 s 1 in its 540 Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc.
8 A B C D Figure 6. Syk Inhibition Leads to a Large Redistribution of the Actin Cytoskeleton in Human Primary Macrophages (A) Confocal images of phalloidin-labeled F-actin distribution in human primary macrophages. Top panel: transversal slice. Bottom panel: Z projection of maximal intensities. Representative images of more than 50 cells from more than ten independent experiments. Blue arrows indicate podosomes. (B) Ratio of phalloidin intensity between the dorsal and ventral surface of individual cells. Measurements of each separate experiment were normalized by the mean intensity of the control cells. (C) Quantification of total phalloidin intensity on individual cells. Measurements of each separate experiment were normalized by the mean intensity of the control cells. (D) High resolution scanning electron microscopy of the cortical cytoskeleton of human primary macrophages after detergent-based plasma membrane removal. Representative images of at least 12 cells from three independent experiments. Error bars represent SEM. See also Figure S3. absence. By contrast, piceatannol markedly reduced the diffusion rate of receptors in the membrane of cells not treated with jasplakinolide, decreasing the diffusion coefficient from ± 0.02 mm 2 3 s 1 to ± mm 2 3 s 1 (Figure 5D). These results confirm that the effects of piceatannol are specific, attributable to alterations in cytoskeletal structure. Syk Regulates FcgR Lateral Mobility within the Phagocytic Cup The preceding results indicate that (1) FcgR mobility is constrained by an inhomogeneous layer of cortical actin, and (2) Syk influences the structure of the cortical cytoskeleton in resting cells. Because the cytoskeleton is acutely remodeled during the early stages of phagocytosis, and because Syk is stimulated early in the process, it is likely that the initial engagement of FcgR modifies the ability of the remaining, unengaged receptors to move, thereby modulating their responsiveness. Evaluating the occurrence and magnitude of this purported feedback mechanism requires an understanding of the effects of Syk on cytoskeletal structure. Our results above indicate that, in resting macrophages, Syk activity is associated with reduced dorsal actin. This observation appears paradoxical, because Syk is essential for the engulfment of IgG-coated particles (Crowley et al., 1997; Kiefer et al., 1998), a process that entails net actin polymerization at the phagocytic cup. However, whether Syk is required for polymerization remains unclear, because at least in a few instances actin remodeling at the phagocytic cup has been reported to persist when the kinase was inhibited or genetically ablated (Cougoule et al., 2006; Crowley et al., 1997). As illustrated in Figure 7A and Movie S2 (top), we were able to recapitulate these observations: in RAW macrophages expressing LifeAct-GFP, F-actin clearly accumulated at the base of forming phagosomes, despite inhibition of Syk by piceatannol. Note, however, that inhibition of Syk arrested progression of the pseudopods around the target particle, precluding engulfment. The stage at which Syk exerts its effects was more clearly visualized using an alternative experimental model. RAW cells stably expressing actin-gfp were suspended and allowed to settle onto IgG-coated coverslips, thereby initiating frustrated phagocytosis (Figure 7B). The use of this model ensures that the surface where receptor engagement occurs remains in the focal plane throughout the Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc. 541
9 A D E B C F Figure 7. Syk-Mediated Actin Reorganization of the Actin Cytoskeleton Dictates FcgR Mobility during Phagocytosis (A) Confocal images of F-actin localization during Fc-mediated phagocytosis in RAW macrophages in absence (left) or presence (right) of piceatannol. LifeAct-GFP (green), IgG-coated 5 mm polystyrene beads (red). Representative image of at least 12 cells from three independent experiments. (B) Schematic of the frustrated phagocytosis model on IgG-coated coverslips. (C) Time series of confocal images of actin-gfp distribution during frustrated phagocytosis, at the surface in contact with the coverslip, in untreated (top panel) or piceatannol-treated (bottom panel) RAW macrophages. Representative images of more than 30 cells from five independent experiments. (D) FcgR mobility within the phagocytic cup in RAW macrophages. Tracks obtained by SPT of Fcg during 5 s (beige) are overlaid on the image of Actin-GFP (gray). Representative image of more than 50 cells from more than five independent experiments. (E) Percentage of confined receptors (circles) and free receptors (triangles), observed in the actin-poor leading edge (pink) of the actin-rich area (green) during frustrated phagocytosis. A total of 398 tracks from n = 27 cells were analyzed. (F) Percentage of confined receptors (circles) and free receptors (triangles), observed during frustrated phagocytosis in untreated (purple) or piceatannol-treated (orange) RAW macrophages. A total of 11 tracks from n = 11 cells and 55 tracks from n = 15 cells were analyzed for control and piceatannol-treated cells, respectively. Error bars represent SEM. See also Figure S4 and Movie S2. phagocytic event. Untreated macrophages spread on the IgGcoated surface, assembling vast amounts of actin at sites of contact. As polymerization progresses outwardly, three distinct regions can be identified: an actin-poor outer lamellipodium at the advancing edge, an actin-rich ring, and an area of actin clearance at the center of the frustrated phagosome (Figure 7C, top; Movie S2, bottom); this region is equivalent to the base of the phagocytic cup, where a comparable clearance zone develops as the pseudopods advance (see Figure 7A). When cells were pretreated with piceatannol the early phases of actin polymerization, though attenuated, were still observed at sites of contact. However, these failed to advance and the extension of the lamellipodium and the development of the clearance zone were impaired (Figure 7C; Movie S2, bottom panel). Thus, Syk activity seems to be required for the disassembly of actin that enables pseudopod progression. This contribution of Syk to actin depolymerization is akin to the effect of the kinase in resting cells. Having established the pattern of actin polymerization during frustrated phagocytosis, we took advantage of this model to measure the mobility of unengaged FcgR in regions of varying F-actin density. FcgRIIB and FcgRIII were labeled at low density on the surface of suspended, actin-gfp expressing macrophages. The labeled cells were then allowed to settle onto IgGcoated coverslips, initiating phagocytosis. Importantly, the Fab fragment used to label the FcgR interacts with the Fc-binding domain of the receptors, preventing engagement by ligands. Thus, the behavior of free receptors could be monitored while others were being engaged by the IgG on the coverslip. By 542 Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc.
10 imaging GFP-actin, we were able to select a stage of phagocytosis when the phagocytic cup had developed (Figure 7D) and used this window of time to record FcgR mobility. The MSS analysis showed that the majority of the unengaged receptors were freely mobile at the fringe of the cup, where actin was less dense (66.9% ± 4.9% free motion, 23% ± 3.9% confined). By contrast, the majority (61.9% ± 7.2%) of the receptors present in the ring formed by densely polymerized actin (selected for analysis using a thresholding algorithm) were confined (Figure 7E). As Syk inhibition did not prevent the initial burst of actin polymerization but rather impaired progression of the pseudopods and actin clearance from the middle of the forming phagosome, we were also able to measure receptor mobility at nascent cups in the presence of piceatannol. When mobility was measured at sites of frustrated phagocytosis in the presence of the inhibitor, the fraction of confined receptors reached 76.5% ± 10.9%, whereas only 15.1% ± 8.5% of the FcgR underwent free motion (Figure 7F). These observations demonstrate that activation of actin polymerization during phagocytosis restricts the mobility of unengaged FcgR, whereas activation of Syk restores their mobility by inducing actin clearance. DISCUSSION Our observations enabled us to reach three main conclusions: (1) at least two subpopulations or states of FcgR exist in quiescent cells, (2) Syk is an important determinant of actin structure and receptor mobility in unstimulated macrophages, and (3) the Syk-mediated restructuring of the cytoskeleton initiated by FcgR stimulation exerts a feedback effect on receptor mobility and hence on receptor responsiveness during phagocytosis. The implications of these conclusions are discussed in turn. FcgR Undergo Transitions between Free and Confined Modes of Diffusion Our experiments were designed to test the validity of several of the premises that underlie the zippering model of phagocytosis. While we confirmed that in resting cells FcgRIIA exist almost exclusively as monomers, we identified two subpopulations (or states) with distinct mobility properties. The receptors undergo transitions between fast and slow diffusion states, which could be resolved using a two-state hidden Markov model analysis (Das et al., 2009). Because the rate of transition from the slow to the fast state is greater than the rate of the opposite transition (Figure S4), the receptors appear to diffuse freely for a majority of the time (z70%) in untreated cells (Figure 3). It is noteworthy that a relatively slow acquisition rate (33 Hz) was employed in our studies. Therefore the transitions between slow and fast states reported here should be distinguished from the hop-diffusion mechanism described earlier (Kusumi et al., 2012). Rather than reflecting the escape of proteins via openings in persistent corrals, the slower transitions reported here are probably due to larger scale alterations in the structure of the actin network. It is noteworthy that when Syk is inhibited, the rate constant of transition from the fast to the slow state increases, while the reverse rate constant decreases (Figure S4). Thus, Syk activity appears to prevent receptors from entering confinement regions. But once trapped in a confinement region, receptors have roughly the same probability of escape independently of Syk activity. The net result of Syk inhibition is a greater propensity for the receptors to be transiently confined. A Role of Syk in Cytoskeleton Organization While there was abundant evidence for a role of Syk in actin reorganization upon immunoreceptor stimulation, our study revealed that the kinase is partially active in resting macrophages and plays a major role in the general organization of the actin cytoskeleton (Figures 3 and 6). What causes Syk activity in macrophages at rest? Activation of the kinase is typically triggered by association with phosphorylated ITAM (Mócsai et al., 2010), which are in turn generated by receptor stimulation. Because FcR are the most abundant ITAM-bearing receptors in macrophages, one can envision that, even in the absence of ligand, FcR occasionally coalesce randomly, resulting in stimulation of Syk activity. While not described for FcR, such stimulation by random collisions has been documented for B cell receptors (Monroe, 2006). Of note, altering B cell receptor confinement by disrupting the actin cytoskeleton was found to enhance the tonic signaling, likely due to increased receptor mobility and spontaneous coalescence (Treanor et al., 2010). Our analysis of receptor trajectories in resting cells showed that FcgRIIA can randomly merge and split, consistent with the hypothesis of tonic signaling (Figure 1E). However, our studies of the oligomerization state FcgRIIA demonstrated that, if random events occur, only an extremely limited number of receptors would be clustered at any given time (Figures 1F and 1G). Therefore, it is likely that other mechanisms contribute to the tonic stimulation of Syk. Several membrane receptors associate with the ITAM-bearing subunits DAP12 or FcRg, potentially leading to Syk activation (Mócsai et al., 2010). In myeloid cells, the best-characterized example is the adhesion-dependent stimulation of Syk, which requires engagement of integrins that interact with ITAMcontaining adaptors (Mócsai et al., 2002). Our observations that Syk inhibition led to podosome disassembly (Figure 6), and that the majority of active Syk (visualized using anti-psyk antibody; Figure S2A) colocalized with podosomes, are suggestive of integrin-mediated activation of Syk in resting macrophages. Accordingly, inhibition of integrin-mediated adhesion by EDTA greatly reduced Syk activation (Figure S2A). However, the list of receptors that can interact with DAP12 or FcRg is relatively large (Fodor et al., 2006). Hence, basal Syk activity in unstimulated macrophages likely results from several different signaling pathways. It is important to note that, despite localizing almost exclusively to the ventral surface of the cell, where integrin-mediated adherence takes place, active Syk affects the state of actin on both the ventral and dorsal aspects of the membrane. Thus, Syk exerts control of local as well as distal actin polymerization. How are these effects mediated? The master regulators of actin organization are molecular switches of the Rho GTPase family (Heasman and Ridley, 2008). Syk is known to regulate the activity of Rho-family GEFs, including Vav isoforms that activate Rac and RhoA (Deckert et al., 1996). We find that in adherent macrophages piceatannol inhibits Rac1 (accounting for the dissolution of podosomes), but activates RhoA (Figure S3B). This effect is correlated with increased cortical actin density at the dorsal surface, which was recapitulated by the overexpression of constitutively active RhoA (not shown). Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc. 543
11 The cortical actin of macrophages has a fenestrated appearance, when visualized by scanning electron microscopy. This peculiar cytoskeletal structure accounts for the coexistence of freely mobile receptors, likely present in fenestrations, and confined receptors presumably found in actin-rich patches. By modulating the cytoskeletal structure, the tonic activity of Syk influences the ratio of freely mobile and confined receptors and their diffusion coefficient. Our results imply that such tonic activity increases the overall mobility of the FcR, improving their likelihood of clustering when confronted with a multivalent ligand. Functional Consequences of Actin Reorganization by Syk during Phagocytosis: the Zipper Model Revisited Syk not only influences the cytoskeletal structure of resting macrophages, but causes striking actin rearrangements when it undergoes stimulation during FcR engagement. Inevitably, this will impact the mobility and responsiveness of those receptors not yet immobilized by interaction with particle-bound IgG, generating a feedback effect that was previously unappreciated. Our observations of FcgR mobility during phagocytosis show that receptors are more mobile at the periphery of the cup (visualized as a lamellipodium during frustrated phagocytosis) than at the level of the dense actin ring (Figure 7D). Because receptor coalescence requires their diffusional displacement, this differential mobility favors clustering of unengaged receptors at the edge of the advancing lamellipodium/pseudopod, whereas further receptor recruitment is prevented in areas where signaling has already reached a sufficient level to induce intense actin polymerization. This combination of positive feedback at the leading edge and negative feedback in actin-enriched areas is ideally suited to foster receptor engagement and effective progress of the pseudopod tip, while limiting excessive and unnecessary signaling in activated regions. These observations have clear physiological implications. First, the degree of integrin engagement during leukocyte chemotaxis to sites of infection will influence the preparedness of the receptors to engage the IgG-coated prey. Second, in the course of phagocytosis the activation of Syk and the attendant cytoskeletal remodeling direct receptor mobility in a manner that optimizes target engulfment while preventing unnecessary and possibly counterproductive stimulation in areas where the cytoskeleton has been sufficiently rearranged. EXPERIMENTAL PROCEDURES Reagents Reagent details are provided in the Supplemental Information. Macrophage Isolation and Culture Human blood samples from healthy volunteers were collected with heparin. Peripheral blood mononuclear cells were isolated by density-gradient centrifugation using Ficoll-Paque Plus (Amersham). Cells were resuspended (10 7 cells/ml) in RPMI-1640 with L-glutamine containing 10% heat-inactivated fetal bovine serum (FBS; Wisent) and seeded onto 18 mm glass coverslips (Fisher Scientific) at cells/coverslip. After 1 hr at 37 C, nonadherent cells were removed by multiple washes. Adherent cells were incubated in RPMI-1640 with 10% FCS, 1 ng/ml human M-CSF and 100 U/ml penicillin, 100 mg/ml streptomycin, and 10 mg/ml polymyxin B (Invitrogen) for 7 14 days. To prepare bone marrow-derived macrophages, femurs and tibias were dissected form euthanized mice. The marrow was extracted by perfusion of DMEM with a 21-gauge needle to produce a single-cell suspension, which was plated in Dulbecco s modified Eagle s medium (DMEM) with 10% FBS, 1 ng/ml murine M-CSF, and 100 U/ml penicillin, 100 mg/ml streptomycin, and 10 mg/ml polymyxin B (Invitrogen). After removal of nonadherent cells, macrophages were cultured for 7 days to allow complete maturation. The animal protocol was approved by the Animal Care Committee of the Hospital for Sick Children. Receptor Labeling and SPT Cells were blocked in HEPES-buffered RPMI-1640 (HPMI) with 5% donkey serum for 5 min and incubated for 10 min with IV.3 Fab fragments (specific to human FcgRIIA), or anti-mouse FcgR Fab fragments (Flannagan et al., 2010), diluted in blocking medium. Next, cells were incubated with Cy3-conjugated donkey anti-mouse Fab fragments, or biotinylated Fab fragments, at a 1:1,000 dilution for 10 min. In the latter case, cells were then washed in HBSS and incubated with streptavidin-conjugated Qdot 655 for 4 min. Finally, cells were washed three times in HPMI containing 0.2 mg/ml biotin to block unengaged streptavidin. Images were acquired on a Zeiss Axiovert 200M microscope, equipped with a 1003 NA 1.45 oil objective, a custom 2.43 magnification lens, and a back-thinned EM-CCD camera (C , Hamamatsu). Acquisitions were performed at 33 Hz with Volocity software (Perkin-Elmer). Single particles were detected and tracked as described (Jaqaman et al., 2008, 2011). Motion types and diffusion coefficients were determined using a moment scaling spectrum (MSS) analysis (Ewers et al., 2005; Ferrari et al., 2001; Jaqaman et al., 2011). The confinement dimension was derived by eigenvalue decomposition of the variance-covariance matrix of particle positions (Jaqaman et al., 2011). Fluorescence Recovery after Photobleaching RAW macrophages transfected with FcgRIIA-GFP were imaged using a Nikon A1R confocal microscope running Nikon Elements 4.1. Simultaneous photobleaching/imaging was performed using resonance mode ( , 30 fps) with a 603/1.4 CFI Apo Lambda S oil objective and 401/488 nm laser lines, respectively. To determine the rate of recovery, fluorescence intensity of the bleached and an unbleached area were measured in the same cell. The intensity of the bleached area was normalized to that of the corresponding unbleached area to correct for general photobleaching due to sampling. Data were fitted to a simple diffusion, zero-flow model using the formula: F ðtþ = F ðt = 0Þ + F ðt = NÞ t t1=2 1 + t t1=2 where the fluorescence intensity (F) at a given time (t) is related to the maximal fluorescence (F(t = N)) and the half-time of maximal recovery (t 1/2 )(Yguerabide et al., 1982). Recovery curves were fitted using this equation, with Prism 4 (GraphPad Software). Diffusion coefficients were calculate using the following formula: D = u 2 4t 1=2 gd where u is the width of the bleached area and g D is a constant equal to 0.88 (Axelrod et al., 1976). Scanning Electron Microscopy Imaging of the cortical cytoskeleton by scanning electron microscopy was performed as described previously (Svitkina, 2007). Briefly, cell membranes were extracted for 5 min at room temperature by 1% Triton X-100 and 4% polyethylene glycol (MW 40,000) in PEM buffer (100 mm PIPES, ph 6.9; 1 mm MgCl 2 ; 1 mm EGTA), supplemented with 10 mg/ml Taxol and 10 mm phalloidin. Cells were then washed and immediately fixed with 2% glutaraldehyde in PEM buffer for 20 min. After removing the fixative, cells were incubated with 0.1% tannic acid for 20 min. Cells were rinsed three times in distilled water, then incubated in 0.2% uranyl acetate for 20 min. Samples were dehydrated in graded ethanol solutions and critical point-dried in CO 2. Finally, samples were coated with platinum and carbon. Images were acquired on a Hitachi field emission scanning electron microscope. Phagocytosis and Frustrated Phagocytosis Assays Fc-mediated phagocytosis of particles was performed using 5 mm polystyrene beads with 2% DVB (Bang s Laboratories) coated with human IgG at 1 mg/ml 544 Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc.
12 for 1 hr at room temperature. Beads were washed three times and added to the cells in HPMI at 37 C. Phagocytosis was imaged using a Quorum spinningdisc confocal microscope. For frustrated phagocytosis, glass coverslips were coated with 1% BSA in PBS for 1 hr at room temperature. Coverslips were washed three times and then incubated with a mouse monoclonal anti- BSA antibody (Serotech) at 1:50 for 1 hr. Coverslips were washed 3 times and placed in HPMI at 37 C. Suspended RAW macrophages stably expressing actin-gfp were added to the medium and imaged. For SPT, RAW macrophages were labeled with anti-mouse FcgR Fab fragments as described above, prior to their suspension. Image Processing, Quantification, and Statistics Images were acquired using Volocity 4 (Perkin-Elmer), exported as Tiff and processed with MatLab (MathWorks) for SPT, or analyzed and quantified using ImageJ 1.46 (NIH) for all the other experiments. Statistical comparison of the data was performed by the nonparametric Mann-Whitney test, with a twotailed p value, using Prism 4 (GraphPad Software). For each measurement, mean and SEM (presented as error bars) were calculated from at least three independent experiments. SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures, four figures, and two movies and can be found with this article online at ACKNOWLEDGMENTS This work was supported by grants MOP7075 and MOP from the Canadian Institutes of Health Research (CIHR) to S.G and by grants RO1 AI and RO1 AI from the National Institutes of Health to C.A.L. V.J. was supported by the Heart and Stroke Foundation of Canada fellowship, the Fondation Bettencourt Schueller, and Cystic Fibrosis Canada. K.J. is a Deborah and W.A. Tex Moncrief, Jr. Scholar in Medical Research. Received: August 18, 2013 Revised: February 25, 2014 Accepted: April 27, 2014 Published: June 9, 2014 REFERENCES Andrews, N.L., Lidke, K.A., Pfeiffer, J.R., Burns, A.R., Wilson, B.S., Oliver, J.M., and Lidke, D.S. (2008). Actin restricts FcepsilonRI diffusion and facilitates antigen-induced receptor immobilization. Nat. Cell Biol. 10, Axelrod, D., Koppel, D.E., Schlessinger, J., Elson, E., and Webb, W.W. (1976). Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, Booth, J.W., Kim, M.K., Jankowski, A., Schreiber, A.D., and Grinstein, S. (2002). Contrasting requirements for ubiquitylation during Fc receptormediated endocytosis and phagocytosis. EMBO J. 21, Calebiro, D., Rieken, F., Wagner, J., Sungkaworn, T., Zabel, U., Borzi, A., Cocucci, E., Zürn, A., and Lohse, M.J. (2013). Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc. Natl. Acad. Sci. USA 110, Cougoule, C., Hoshino, S., Dart, A., Lim, J., and Caron, E. (2006). Dissociation of recruitment and activation of the small G-protein Rac during Fcgamma receptor-mediated phagocytosis. J. Biol. Chem. 281, Crane, J.M., and Verkman, A.S. (2008). Long-range nonanomalous diffusion of quantum dot-labeled aquaporin-1 water channels in the cell plasma membrane. Biophys. J. 94, Crowley, M.T., Costello, P.S., Fitzer-Attas, C.J., Turner, M., Meng, F., Lowell, C., Tybulewicz, V.L., and DeFranco, A.L. (1997). A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J. Exp. Med. 186, Das, R., Cairo, C.W., and Coombs, D. (2009). A hidden Markov model for single particle tracks quantifies dynamic interactions between LFA-1 and the actin cytoskeleton. PLoS Comput. Biol. 5, e Deckert, M., Tartare-Deckert, S., Couture, C., Mustelin, T., and Altman, A. (1996). Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity 5, Douglass, A.D., and Vale, R.D. (2005). Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121, Ewers, H., Smith, A.E., Sbalzarini, I.F., Lilie, H., Koumoutsakos, P., and Helenius, A. (2005). Single-particle tracking of murine polyoma virus-like particles on live cells and artificial membranes. Proc. Natl. Acad. Sci. USA 102, Ferrari, R., Manfroi, A.J., and Young, W.R. (2001). Strongly and weakly self-similar diffusion. Physica D 154, Flannagan, R.S., Harrison, R.E., Yip, C.M., Jaqaman, K., and Grinstein, S. (2010). Dynamic macrophage probing is required for the efficient capture of phagocytic targets. J. Cell Biol. 191, Flannagan, R.S., Jaumouillé, V., and Grinstein, S. (2012). The cell biology of phagocytosis. Annu. Rev. Pathol. 7, Fodor, S., Jakus, Z., and Mócsai, A. (2006). ITAM-based signaling beyond the adaptive immune response. Immunol. Lett. 104, Fujiwara, T., Ritchie, K., Murakoshi, H., Jacobson, K., and Kusumi, A. (2002). Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 157, Ghazizadeh, S., Bolen, J.B., and Fleit, H.B. (1994). Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J. Biol. Chem. 269, Griffin, F.M., Jr., Griffin, J.A., Leider, J.E., and Silverstein, S.C. (1975). Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J. Exp. Med. 142, Heasman, S.J., and Ridley, A.J. (2008). Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, Holowka, D., Sil, D., Torigoe, C., and Baird, B. (2007). Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunol. Rev. 217, Iino, R., Koyama, I., and Kusumi, A. (2001). Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophys. J. 80, Jacobson, K., Derzko, Z., Wu, E.S., Hou, Y., and Poste, G. (1976). Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J. Supramol. Struct. 5, 565(417) 576(428). Jaqaman, K., and Grinstein, S. (2012). Regulation from within: the cytoskeleton in transmembrane signaling. Trends Cell Biol. 22, Jaqaman, K., Loerke, D., Mettlen, M., Kuwata, H., Grinstein, S., Schmid, S.L., and Danuser, G. (2008). Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods 5, Jaqaman, K., Kuwata, H., Touret, N., Collins, R., Trimble, W.S., Danuser, G., and Grinstein, S. (2011). Cytoskeletal control of CD36 diffusion promotes its receptor and signaling function. Cell 146, Jaumouillé, V., and Grinstein, S. (2011). Receptor mobility, the cytoskeleton, and particle binding during phagocytosis. Curr. Opin. Cell Biol. 23, Jones, D.H., Nusbacher, J., and Anderson, C.L. (1985). Fc receptor-mediated binding and endocytosis by human mononuclear phagocytes: monomeric IgG is not endocytosed by U937 cells and monocytes. J. Cell Biol. 100, Kiefer, F., Brumell, J., Al-Alawi, N., Latour, S., Cheng, A., Veillette, A., Grinstein, S., and Pawson, T. (1998). The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol. Cell. Biol. 18, Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc. 545
13 Kusumi, A., Nakada, C., Ritchie, K., Murase, K., Suzuki, K., Murakoshi, H., Kasai, R.S., Kondo, J., and Fujiwara, T. (2005). Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 34, Kusumi, A., Fujiwara, T.K., Chadda, R., Xie, M., Tsunoyama, T.A., Kalay, Z., Kasai, R.S., and Suzuki, K.G.N. (2012). Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of Singer and Nicolson s fluid-mosaic model. Annu. Rev. Cell Dev. Biol. 28, Kwiatkowska, K., and Sobota, A. (2001). The clustered Fcgamma receptor II is recruited to Lyn-containing membrane domains and undergoes phosphorylation in a cholesterol-dependent manner. Eur. J. Immunol. 31, Mócsai, A., Zhou, M., Meng, F., Tybulewicz, V.L., and Lowell, C.A. (2002). Syk is required for integrin signaling in neutrophils. Immunity 16, Mócsai, A., Ruland, J., and Tybulewicz, V.L.J. (2010). The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 10, Monroe, J.G. (2006). ITAM-mediated tonic signalling through pre-bcr and BCR complexes. Nat. Rev. Immunol. 6, Murase, K., Fujiwara, T., Umemura, Y., Suzuki, K., Iino, R., Yamashita, H., Saito, M., Murakoshi, H., Ritchie, K., and Kusumi, A. (2004). Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques. Biophys. J. 86, Nimmerjahn, F., and Ravetch, J.V. (2008). Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8, Odin, J.A., Edberg, J.C., Painter, C.J., Kimberly, R.P., and Unkeless, J.C. (1991). Regulation of phagocytosis and [Ca2+]i flux by distinct regions of an Fc receptor. Science 254, Powell, M.S., Barnes, N.C., Bradford, T.M., Musgrave, I.F., Wines, B.D., Cambier, J.C., and Hogarth, P.M. (2006). Alteration of the Fc gamma RIIa dimer interface affects receptor signaling but not ligand binding. J. Immunol. 176, Riedl, J., Crevenna, A.H., Kessenbrock, K., Yu, J.H., Neukirchen, D., Bista, M., Bradke, F., Jenne, D., Holak, T.A., Werb, Z., et al. (2008). Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, Rollet-Labelle, E., Marois, S., Barbeau, K., Malawista, S.E., and Naccache, P.H. (2004). Recruitment of the cross-linked opsonic receptor CD32A (FcgammaRIIA) to high-density detergent-resistant membrane domains in human neutrophils. Biochem. J. 381, Saffman, P.G., and Delbrück, M. (1975). Brownian motion in biological membranes. Proc. Natl. Acad. Sci. USA 72, Sako, Y., and Kusumi, A. (1995). Barriers for lateral diffusion of transferrin receptor in the plasma membrane as characterized by receptor dragging by laser tweezers: fence versus tether. J. Cell Biol. 129, Schlessinger, J., Webb, W.W., Elson, E.L., and Metzger, H. (1976). Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature 264, Singer, S.J., and Nicolson, G.L. (1972). The fluid mosaic model of the structure of cell membranes. Science 175, Smith, P.R., Morrison, I.E., Wilson, K.M., Fernández, N., and Cherry, R.J. (1999). Anomalous diffusion of major histocompatibility complex class I molecules on HeLa cells determined by single particle tracking. Biophys. J. 76, Suzuki, K.G., Fujiwara, T.K., Sanematsu, F., Iino, R., Edidin, M., and Kusumi, A. (2007). GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J. Cell Biol. 177, Svitkina, T. (2007). Electron microscopic analysis of the leading edge in migrating cells. Methods Cell Biol. 79, Treanor, B., Depoil, D., Gonzalez-Granja, A., Barral, P., Weber, M., Dushek, O., Bruckbauer, A., and Batista, F.D. (2010). The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity 32, Woof, J.M., and Burton, D.R. (2004). Human antibody-fc receptor interactions illuminated by crystal structures. Nat. Rev. Immunol. 4, Xie, J., Tato, C.M., and Davis, M.M. (2013). How the immune system talks to itself: the varied role of synapses. Immunol. Rev. 251, Yguerabide, J., Schmidt, J.A., and Yguerabide, E.E. (1982). Lateral mobility in membranes as detected by fluorescence recovery after photobleaching. Biophys. J. 40, Zhang, F., Yang, B., Odin, J.A., Shen, Z., Lin, C.T., Unkeless, J.C., and Jacobson, K. (1995). Lateral mobility of Fc gamma RIIa is reduced by protein kinase C activation. FEBS Lett. 376, Developmental Cell 29, , June 9, 2014 ª2014 Elsevier Inc.
14 , Volume 29 Supplemental Information Actin Cytoskeleton Reorganization by Syk Regulates Fc Receptor Responsiveness by Increasing Its Lateral Mobility and Clustering Valentin Jaumouillé, Yoav Farkash, Khuloud Jaqaman, Raibatak Das, Clifford A. Lowell, and Sergio Grinstein
15 SUPPLEMENTARY FIGURES Figure S1. Characterization of FcγRIIA mobility and collisions in human primary macrophages, related to Figure 1.
16 A. Median diffusion coefficients of confined FcγRIIA in live (orange) or fixed (purple) human primary macrophages. B. Median confinement area calculated for the of confined FcγRIIA population in live (orange) or fixed (purple) in human primary macrophages. C. Percentage of receptors undergoing anisotropic (orange) versus isotropic (purple) motion in human primary macrophages. D. Effect of receptor confinement: average collision rate calculated by Monte Carlo simulations for receptors randomly distributed at a density of 2 molecules per µm -2, diffusing at 0.05 µm 2.s -1. E. Effect of receptor diffusion rate: average collision rate calculated for receptors randomly distributed at a density of 2 molecules per µm -2. The confinement radius of the confined population is 100 nm. F. Effect of receptor density: average collision rate calculated for randomly distributed receptors, diffusing at 0.05 µm 2.s -1. The confinement radius of the confined population is 100 nm. G. Effect of receptor density: ratio of collision rates for free versus confined receptors. The diffusion coefficient is 0.05 µm 2.s -1 and the confinement radius is 100 nm.
17 Figure S2. Tonic Syk activity dictates FcγRIIA mobility in human primary macrophages, related to Figure 3.
18 A. Confocal images of phalloidin-labelled F-actin (green) and phosphos-syk Y352 (red) distribution in human primary macrophages that were either untreated (top), treated with 10 mm EDTA (middle) or with piceatannol (bottom). Top panel: transverse (x vs. z) slice, bottom panel: confocal image of the ventral plane. Representative images of at least 12 cells from 3 independent experiments. B. Percentage of confined FcγRIIA (circles) and free FcγRIIA (triangles), in untreated (purple) and Bay treated (orange) human primary macrophages. C. Median diffusion coefficients of FcγRIIA in untreated (purple diamonds) and Bay treated (orange squares) human primary macrophages tracks from n = 30 cells and 6716 tracks from n = 30 cells were analysed for control and Bay treated cells, respectively.
19 Figure S3. Podosome formation, Rho GTPases activity and actin dynamic in human primary macrophages, related to Figure 6. A. Representative confocal images of podosome marker distribution, stained by immunofluorescence (red) and phalloidin-labelled F-actin (green) in human primary macrophages. B. Relative level of Rac1 and RhoA activity, determined by the amount of GTP-bound proteins detected by G-lisa in normalized samples, in untreated (blue) or piceatannol-treated (orange) human primary macrophages. C. Quantification of actin-rhodamine incorporation at the free barbed ends in untreated (purple circles) or piceatannol treated (orange diamonds) human primary macrophages. Each dot represents one individual cell.
20 Figure S4. Transition rates between slow and fast FcγRIIA diffusion states in primary human macrophages, related to Figure 7. Analysis of FcγRIIA trajectories in control and piceatannol-treated primary human macrophages using the two-state hidden Markov model. A. Maximum likelihood parameter estimates of the diffusion coefficients and the transition rates, with their 95% confidence intervals. B. Plots of the the maximum likelihood estimates of the transition rates from the fast to the slow state (ordinate) and the transition rates from the slow to the fast state (abscise). Bars represent the 95% confidence intervals for each transition rate.
21 SUPPLEMENTARY EXPERIMENTAL PROCEDURES Reagents RAW cells were obtained from the American Type Culture Collection and cultured in DMEM containing 10% FBS, at 37 C in a 5% CO 2 incubator. Transfections were performed using FuGene HD (Roche), according to the manufacturer s instructions. Mouse anti-human FcγRIIA antibody (clone IV.3) was purchased from the Sunnybrook Health Science Centre Hybridoma core facility. Fab Fragments were obtained using the Pierce Fab Preparation Kit, following the manufacturer s instructions. All secondary antibodies and Fab were from Jackson ImmunoResearch Laboratories, Inc. Qdot 655 Streptavidin were from Invitrogen. PP1 and jasplakinolide were purchased from Alexis Biochemicals and Enzo Life Science, respectively. Filipin was from Polysciences, Inc. Methyl-βcyclodextrin, piceatannol, latrunculin A and other reagents were purchased from Sigma Aldrich. All tissue culture supplies were from Wisent, Inc. The expression plasmid for FcγRIIA-GFP has been previously described (Booth et al., 2002). Plasmids used for the expression of FcγRIIA-based constructs and FcRγ-GFP were generous gifts from Dr. A. Schreiber. Simulation of receptor collisions The effect of receptor diffusion, confinement and density on the rate of receptor collision was investigated via Monte Carlo simulations mimicking the experimentally observed receptor behavior. Receptors at a density ρ = 1, 2, 4 or 10 molecules/µm 2 were placed randomly within an area of µm 2 and allowed to diffuse, with diffusion coefficient D = 0.025, 0.05, or 0.1 µm 2.s -1. Receptor diffusion was either free or confined within compartments of radius R = 175, 100 or 50 nm. Two receptors were considered to collide if the distance between them was less than 5 nm. The rate of receptor collision under each condition was thus calculated as follows: a) for freely diffusing receptors, any receptor was considered to have a chance to collide with any other receptor, as they all moved freely on the cell surface. The time from the simulation start until the collision between any two receptors was taken as one observation of the waiting time for a collision to take place. Repeating the
22 simulation 200 times, each with a different set of initial receptor positions, yielded the average collision waiting time τ free. The rate of receptor collision for freely diffusing receptors was then calculated as r free = 1/τ free ; b) for confined receptors, the simulation area was first divided into a grid with mesh size corresponding to the confinement radius. Only receptors within the same confinement area had the chance to collide with each other. As a result, receptors confined separately had no chance to collide with any other receptors. The simulations in this case thus yielded the fraction of receptors f conf that had the chance to collide, as well as their average collision waiting time τ conf. From this, the rate of receptor collision for confined receptors was calculated as r conf = f conf *(1/τ conf ) (since the rest of the receptors, which are confined separately, had a rate of collision = 0). Two-state hidden Markov model analysis Particle trajectories were analyzed using a 2-state hidden Markov model. as previously described (Das et al., 2009). Briefly, this model is parameterized by two diffusion coefficients, D fast and D slow, and two first order rate constants k f (or, k fast slow ) and k r (or, k slow fast ). A numerically computed log likelihood function quantifies how probable a particular choice of parameter values is, given a set of observed trajectories. We use a Markov chain Monte Carlo optimization to maximize this log likelihood function, giving us maximum likelihood parameter estimates for an observed set of trajectories. The maximum likelihood parameter estimates, along with their 95% confidence intervals are shown in Figure S4A.
Dynamic Partitioning of a GPI-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking
 Additional data for Dynamic Partitioning of a GPI-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking Fabien Pinaud 1,3, Xavier Michalet 1,3, Gopal Iyer 1, Emmanuel
Additional data for Dynamic Partitioning of a GPI-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking Fabien Pinaud 1,3, Xavier Michalet 1,3, Gopal Iyer 1, Emmanuel
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplemental Materials Molecular Biology of the Cell
 Supplemental Materials Molecular Biology of the Cell Gilberti et al. SUPPLEMENTAL FIGURE LEGENDS: Figure S1: The effect of pharmacological inhibitors on particle uptake. The data presented in Figure 1
Supplemental Materials Molecular Biology of the Cell Gilberti et al. SUPPLEMENTAL FIGURE LEGENDS: Figure S1: The effect of pharmacological inhibitors on particle uptake. The data presented in Figure 1
Distinct Stages of Stimulated FcεRI Receptor Clustering and Immobilization Are Identified through Superresolution Imaging
 Biophysical Journal Volume 105 November 2013 2343 2354 2343 Distinct Stages of Stimulated FcεRI Receptor Clustering and Immobilization Are Identified through Superresolution Imaging Sarah A. Shelby, David
Biophysical Journal Volume 105 November 2013 2343 2354 2343 Distinct Stages of Stimulated FcεRI Receptor Clustering and Immobilization Are Identified through Superresolution Imaging Sarah A. Shelby, David
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Supplementary Information. Conformational states of Lck regulate clustering in early T cell signaling
 Supplementary Information Conformational states of Lck regulate clustering in early T cell signaling Jérémie Rossy, Dylan M. Owen, David J. Williamson, Zhengmin Yang and Katharina Gaus Centre for Vascular
Supplementary Information Conformational states of Lck regulate clustering in early T cell signaling Jérémie Rossy, Dylan M. Owen, David J. Williamson, Zhengmin Yang and Katharina Gaus Centre for Vascular
Use of a camp BRET Sensor to Characterize a Novel Regulation of camp by the Sphingosine-1-phosphate/G 13 Pathway
 Use of a camp BRET Sensor to Characterize a Novel Regulation of camp by the Sphingosine-1-phosphate/G 13 Pathway SUPPLEMENTAL DATA Characterization of the CAMYEL sensor and calculation of intracellular
Use of a camp BRET Sensor to Characterize a Novel Regulation of camp by the Sphingosine-1-phosphate/G 13 Pathway SUPPLEMENTAL DATA Characterization of the CAMYEL sensor and calculation of intracellular
El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb /DC1
 Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
Toll-like receptor ligands sensitize B-cell receptor signalling by reducing actin-dependent spatial confinement of the receptor
 ARTICLE Received 3 May 14 Accepted Dec 14 Published 3 Feb 15 DOI: 1.138/ncomms7168 OPEN Toll-like receptor ligands sensitize B-cell receptor signalling by reducing actin-dependent spatial confinement of
ARTICLE Received 3 May 14 Accepted Dec 14 Published 3 Feb 15 DOI: 1.138/ncomms7168 OPEN Toll-like receptor ligands sensitize B-cell receptor signalling by reducing actin-dependent spatial confinement of
This week s topic will be: Evidence for the Fluid Mosaic Model. Developing theories, testing hypotheses and techniques for visualizing cells
 Tutorials, while not mandatory, will allow you to improve your final grade in this course. Thank you for your attendance to date. These notes are not a substitute for the discussions that we will have
Tutorials, while not mandatory, will allow you to improve your final grade in this course. Thank you for your attendance to date. These notes are not a substitute for the discussions that we will have
SUPPLEMENTARY INFORMATION
 In the format provided by the authors and unedited. 2 3 4 DOI: 10.1038/NMAT4893 EGFR and HER2 activate rigidity sensing only on rigid matrices Mayur Saxena 1,*, Shuaimin Liu 2,*, Bo Yang 3, Cynthia Hajal
In the format provided by the authors and unedited. 2 3 4 DOI: 10.1038/NMAT4893 EGFR and HER2 activate rigidity sensing only on rigid matrices Mayur Saxena 1,*, Shuaimin Liu 2,*, Bo Yang 3, Cynthia Hajal
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
genome edited transient transfection, CMV promoter
 Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Effect of Rhodamine-taxol conjugate on Caveolin dynamics
 Chapter 6 4 Effect of Rhodamine-taxol conjugate on Caveolin dynamics 1. Summary In the present study, Rhodamine- taxol conjugate treatment resulted in a transient recruitment of Caveolin-1 to the cell
Chapter 6 4 Effect of Rhodamine-taxol conjugate on Caveolin dynamics 1. Summary In the present study, Rhodamine- taxol conjugate treatment resulted in a transient recruitment of Caveolin-1 to the cell
GFP-LC3 +/+ CLU -/- kda CLU GFP. Actin. GFP-LC3 +/+ CLU -/- kda CLU GFP. Actin
 Supplementary Fig. 1 a CQ treatment ScrB OGX11 MG132 I II AZD5363 I II b GFP / / GFP / / GFP / / GFP / / GFP GFP Actin Actin ctrl CQ GFP / / GFP / / GFP / / GFP / / GFP GFP Actin Actin rapamycin rapamycincq
Supplementary Fig. 1 a CQ treatment ScrB OGX11 MG132 I II AZD5363 I II b GFP / / GFP / / GFP / / GFP / / GFP GFP Actin Actin ctrl CQ GFP / / GFP / / GFP / / GFP / / GFP GFP Actin Actin rapamycin rapamycincq
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
J. Cell Sci. 129: doi: /jcs : Supplementary information
 Movie 1. AgLDL is contained in small sub-regions of the lysosomal synapse that are acidic. J774 cells were incubated with agldl dual labeled with a ph sensitive and a ph insensitive fluorophore for 1 hr.
Movie 1. AgLDL is contained in small sub-regions of the lysosomal synapse that are acidic. J774 cells were incubated with agldl dual labeled with a ph sensitive and a ph insensitive fluorophore for 1 hr.
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Mechanisms Underlying the Confined Diffusion of Cholera Toxin B-Subunit in Intact Cell Membranes
 Mechanisms Underlying the Confined Diffusion of Cholera Toxin B-Subunit in Intact Cell Membranes Charles A. Day 1, Anne K. Kenworthy 1,2,3 * 1 Department of Molecular Physiology and Biophysics, Vanderbilt
Mechanisms Underlying the Confined Diffusion of Cholera Toxin B-Subunit in Intact Cell Membranes Charles A. Day 1, Anne K. Kenworthy 1,2,3 * 1 Department of Molecular Physiology and Biophysics, Vanderbilt
Suzuki et al.,
 JCB: SUPPLEMENTAL MATERIAL Suzuki et al., http://www.jcb.org/cgi/content/full/jcb.200609174/dc1 Supplemental materials and methods TCZs and STALL Transient confinement zones (TCZs), a term coined by Simson
JCB: SUPPLEMENTAL MATERIAL Suzuki et al., http://www.jcb.org/cgi/content/full/jcb.200609174/dc1 Supplemental materials and methods TCZs and STALL Transient confinement zones (TCZs), a term coined by Simson
Akihiro Kusumi, Chieko Nakada, Ken Ritchie, Kotono Murase, Kenichi Suzuki, Hideji Murakoshi, Rinshi S. Kasai, Junko Kondo, and Takahiro Fujiwara
 Annu. Rev. Biophys. Biomol. Struct. 2005. 34:351 78 doi: 10.1146/annurev.biophys.34.040204.144637 Copyright c 2005 by Annual Reviews. All rights reserved First published online as a Review in Advance on
Annu. Rev. Biophys. Biomol. Struct. 2005. 34:351 78 doi: 10.1146/annurev.biophys.34.040204.144637 Copyright c 2005 by Annual Reviews. All rights reserved First published online as a Review in Advance on
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/6/e1700338/dc1 Supplementary Materials for HIV virions sense plasma membrane heterogeneity for cell entry Sung-Tae Yang, Alex J. B. Kreutzberger, Volker Kiessling,
advances.sciencemag.org/cgi/content/full/3/6/e1700338/dc1 Supplementary Materials for HIV virions sense plasma membrane heterogeneity for cell entry Sung-Tae Yang, Alex J. B. Kreutzberger, Volker Kiessling,
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
A genetically targeted optical sensor to monitor calcium signals in astrocyte processes
 A genetically targeted optical sensor to monitor calcium signals in astrocyte processes 1 Eiji Shigetomi, 1 Sebastian Kracun, 2 Michael V. Sofroniew & 1,2 *Baljit S. Khakh Ψ 1 Departments of Physiology
A genetically targeted optical sensor to monitor calcium signals in astrocyte processes 1 Eiji Shigetomi, 1 Sebastian Kracun, 2 Michael V. Sofroniew & 1,2 *Baljit S. Khakh Ψ 1 Departments of Physiology
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2294 Figure S1 Localization and function of cell wall polysaccharides in root hair cells. (a) Spinning-disk confocal sections of seven day-old A. thaliana seedlings stained with 0.1% S4B
DOI: 10.1038/ncb2294 Figure S1 Localization and function of cell wall polysaccharides in root hair cells. (a) Spinning-disk confocal sections of seven day-old A. thaliana seedlings stained with 0.1% S4B
Nature Immunology: doi: /ni eee Supplementary Figure 1
 eee Supplementary Figure 1 Hyphae induce NET release, but yeast do not. (a) NET release by human peripheral neutrophils stimulated with a hgc1 yeast-locked C. albicans mutant (yeast) or pre-formed WT C.
eee Supplementary Figure 1 Hyphae induce NET release, but yeast do not. (a) NET release by human peripheral neutrophils stimulated with a hgc1 yeast-locked C. albicans mutant (yeast) or pre-formed WT C.
Nature Biotechnology: doi: /nbt.3828
 Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
MEMBRANE STRUCTURE. Lecture 8. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
Utilizing AlphaLISA Technology to Screen for Inhibitors of the CTLA-4 Immune Checkpoint
 APPLICATION NOTE AlphaLISA Technology Authors: Matthew Marunde Stephen Hurt PerkinElmer, Inc. Hopkinton, MA Utilizing AlphaLISA Technology to Screen for Inhibitors of the CTLA-4 Immune Checkpoint Introduction
APPLICATION NOTE AlphaLISA Technology Authors: Matthew Marunde Stephen Hurt PerkinElmer, Inc. Hopkinton, MA Utilizing AlphaLISA Technology to Screen for Inhibitors of the CTLA-4 Immune Checkpoint Introduction
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplemental Data Figure S1 Effect of TS2/4 and R6.5 antibodies on the kinetics of CD16.NK-92-mediated specific lysis of SKBR-3 target cells.
 Supplemental Data Figure S1. Effect of TS2/4 and R6.5 antibodies on the kinetics of CD16.NK-92-mediated specific lysis of SKBR-3 target cells. (A) Specific lysis of IFN-γ-treated SKBR-3 cells in the absence
Supplemental Data Figure S1. Effect of TS2/4 and R6.5 antibodies on the kinetics of CD16.NK-92-mediated specific lysis of SKBR-3 target cells. (A) Specific lysis of IFN-γ-treated SKBR-3 cells in the absence
Chapter 7: Membranes
 Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
a 10 4 Link et al. Supplementary Figure 1 Nature Immunology: doi: /ni.1842 Cells per mouse ( 10 5 ) TRPV2KO anti-gr1 anti-gr anti-f4/80
 a 10 4 WT 10 4 TRPV2KO 10 3 10 3 anti-gr1 10 2 10 1 anti-gr1 10 2 10 1 10 0 10 0 10 1 10 2 10 3 10 4 anti-f4/80 42.3 45.2 10 0 10 0 10 1 10 2 10 3 10 4 anti-f4/80 10 4 10 4 40 42.5 anti-cd11b 10 3 10 2
a 10 4 WT 10 4 TRPV2KO 10 3 10 3 anti-gr1 10 2 10 1 anti-gr1 10 2 10 1 10 0 10 0 10 1 10 2 10 3 10 4 anti-f4/80 42.3 45.2 10 0 10 0 10 1 10 2 10 3 10 4 anti-f4/80 10 4 10 4 40 42.5 anti-cd11b 10 3 10 2
AD (Leave blank) PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD (Leave blank) Award Number: W81XWH-06-2-0038 TITLE:T Cell Lipid rafts and complement Ligands for Diagnosis and Monitoring PRINCIPAL INVESTIGATOR: George C. Tsokos, MD CONTRACTING ORGANIZATION: Beth
AD (Leave blank) Award Number: W81XWH-06-2-0038 TITLE:T Cell Lipid rafts and complement Ligands for Diagnosis and Monitoring PRINCIPAL INVESTIGATOR: George C. Tsokos, MD CONTRACTING ORGANIZATION: Beth
Dynamic Partitioning of a Glycosyl-Phosphatidylinositol-Anchored Protein in Glycosphingolipid-Rich Microdomains Imaged by Single-Quantum Dot Tracking
 Traffic 2009; 10: 691 712 Blackwell Munksgaard 2009 The Authors Journal compilation 2009 Blackwell Munksgaard doi: 10.1111/j.1600-0854.2009.00902.x Dynamic Partitioning of a Glycosyl-Phosphatidylinositol-Anchored
Traffic 2009; 10: 691 712 Blackwell Munksgaard 2009 The Authors Journal compilation 2009 Blackwell Munksgaard doi: 10.1111/j.1600-0854.2009.00902.x Dynamic Partitioning of a Glycosyl-Phosphatidylinositol-Anchored
Chapter 11. B cell generation, Activation, and Differentiation. Pro-B cells. - B cells mature in the bone marrow.
 Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Chapter B cell generation, Activation, and Differentiation - B cells mature in the bone marrow. - B cells proceed through a number of distinct maturational stages: ) Pro-B cell ) Pre-B cell ) Immature
Supplemental Data. Hao et al. (2014). Plant Cell /tpc
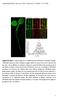 Supplemental Figure 1. Confocal Images and VA-TIRFM Analysis of GFP-RbohD in Arabidopsis Seedlings. (A) RbohD expression in whole Arabidopsis seedlings. RbohD was expressed in the leaves, hypocotyl, and
Supplemental Figure 1. Confocal Images and VA-TIRFM Analysis of GFP-RbohD in Arabidopsis Seedlings. (A) RbohD expression in whole Arabidopsis seedlings. RbohD was expressed in the leaves, hypocotyl, and
HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade)
 HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade) 1. Mesenchymal stem cells (MSC) cultured on extracellular matrices with different stiffness exhibit diverse lineage commitment
HOMEWORK RUBRICS MECHANOTRANSDUCTION UNIT: HOMEWORK #1 (20 pts towards your grade) 1. Mesenchymal stem cells (MSC) cultured on extracellular matrices with different stiffness exhibit diverse lineage commitment
of an untreated HS-stained BAEC monolayer viewed using a laser confocal microscope; Bar = 10 µm.
 Supplemental Figure 1: EC monolayer heparan sulfate fluorescence intensity. (A) Enface perspective of an untreated HS-stained BAEC monolayer viewed using a laser confocal microscope; Bar = 10 µm. (B) Enface
Supplemental Figure 1: EC monolayer heparan sulfate fluorescence intensity. (A) Enface perspective of an untreated HS-stained BAEC monolayer viewed using a laser confocal microscope; Bar = 10 µm. (B) Enface
Supplemental Data. Wu et al. (2010). Plant Cell /tpc
 Supplemental Figure 1. FIM5 is preferentially expressed in stamen and mature pollen. The expression data of FIM5 was extracted from Arabidopsis efp browser (http://www.bar.utoronto.ca/efp/development/),
Supplemental Figure 1. FIM5 is preferentially expressed in stamen and mature pollen. The expression data of FIM5 was extracted from Arabidopsis efp browser (http://www.bar.utoronto.ca/efp/development/),
MEMBRANE STRUCTURE AND FUNCTION
 MEMBRANE STRUCTURE AND FUNCTION selective permeability permits some substances to cross it more easily than others Figure 7.1 Scientists studying the plasma Reasoned that it must be a phospholipid bilayer
MEMBRANE STRUCTURE AND FUNCTION selective permeability permits some substances to cross it more easily than others Figure 7.1 Scientists studying the plasma Reasoned that it must be a phospholipid bilayer
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Insulin stimulated glucose uptake in adipocytes requires the co-localization of
 STALL JR., RICHARD, M.S. Investigation of the Interaction Between Myosin IIA and Filamentous Actin During Insulin Stimulated Glucose Uptake in 3T3-L1 Adipocytes. (2012) Directed by Dr. Yashomati M. Patel
STALL JR., RICHARD, M.S. Investigation of the Interaction Between Myosin IIA and Filamentous Actin During Insulin Stimulated Glucose Uptake in 3T3-L1 Adipocytes. (2012) Directed by Dr. Yashomati M. Patel
Supplemental information
 Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Essential Medium, containing 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Huvec were cultured in
 Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
JCB Article. Phospholipids undergo hop diffusion in compartmentalized cell membrane
 JCB Article Phospholipids undergo hop diffusion in compartmentalized cell membrane Takahiro Fujiwara, 1 Ken Ritchie, 1,2 Hideji Murakoshi, 2 Ken Jacobson, 3 and Akihiro Kusumi 1,2 1 Kusumi Membrane Organizer
JCB Article Phospholipids undergo hop diffusion in compartmentalized cell membrane Takahiro Fujiwara, 1 Ken Ritchie, 1,2 Hideji Murakoshi, 2 Ken Jacobson, 3 and Akihiro Kusumi 1,2 1 Kusumi Membrane Organizer
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
Nature Immunology: doi: /ni.3866
 Nature Immunology: doi:10.1038/ni.3866 Supplementary Figure 1 The effect of TIPE2 on chemotaxis. a, The expression of TIPE2 in dhl-60c, dhl-60t, TIPE2-expressing and 15/16Q-expressing dhl-60t neutrophils
Nature Immunology: doi:10.1038/ni.3866 Supplementary Figure 1 The effect of TIPE2 on chemotaxis. a, The expression of TIPE2 in dhl-60c, dhl-60t, TIPE2-expressing and 15/16Q-expressing dhl-60t neutrophils
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human
 Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Phospholipids. Extracellular fluid. Polar hydrophilic heads. Nonpolar hydrophobic tails. Polar hydrophilic heads. Intracellular fluid (cytosol)
 Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
a Anti-Dab2 RGD Merge
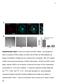 a Anti-Da Merge *** ** Percentage of cells adhered 8 6 4 RGE RAD RGE RAD Supplementary Figure Da are localized at clusters. (a) Endogenous Da is localized at clusters. (), ut not RGE nor RAD peptides can
a Anti-Da Merge *** ** Percentage of cells adhered 8 6 4 RGE RAD RGE RAD Supplementary Figure Da are localized at clusters. (a) Endogenous Da is localized at clusters. (), ut not RGE nor RAD peptides can
3.1 Activation of human peripheral blood T lymphocytes induces reversible association of cofilin with the actin cytoskeleton
 3. RESULTS 3.1 Activation of human peripheral blood T lymphocytes induces reversible association of cofilin with the actin cytoskeleton In unstimulated resting human peripheral blood T lymphocytes (PBTs)
3. RESULTS 3.1 Activation of human peripheral blood T lymphocytes induces reversible association of cofilin with the actin cytoskeleton In unstimulated resting human peripheral blood T lymphocytes (PBTs)
The Phagocytic Synapse in Distinguishing Particulate and Soluble Stimuli David M. Underhill
 The hagocytic Synapse in Distinguishing articulate and Soluble Stimuli The hagocytic Synapse in Distinguishing articulate and Soluble Stimuli How does a cell know the difference between an inflammatory
The hagocytic Synapse in Distinguishing articulate and Soluble Stimuli The hagocytic Synapse in Distinguishing articulate and Soluble Stimuli How does a cell know the difference between an inflammatory
Featured Articles. Increase in tension forces movement in one direction. Article navigation. Standfirst. Jump to main content Jump to navigation
 Jump to main content Jump to navigation powered by Search Article navigation References Featured Articles Increase in tension forces movement in one direction Cell Migration Gateway (February 2012) Standfirst
Jump to main content Jump to navigation powered by Search Article navigation References Featured Articles Increase in tension forces movement in one direction Cell Migration Gateway (February 2012) Standfirst
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
Supplemental material Beck et al., http://www.jcb.org/cgi/content/full/jcb.201011027/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Membrane binding of His-tagged proteins to Ni-liposomes.
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Supporting Information
 Supporting Information lpek et al. 1.173/pnas.1121217 SI Materials and Methods Mice. cell knockout, inos / (Taconic arms), Rag1 /, INγR /, and IL-12p4 / mice (The Jackson Laboratory) were maintained and/or
Supporting Information lpek et al. 1.173/pnas.1121217 SI Materials and Methods Mice. cell knockout, inos / (Taconic arms), Rag1 /, INγR /, and IL-12p4 / mice (The Jackson Laboratory) were maintained and/or
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
a b G75 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server.
 a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Chapter 12: Membranes. Voet & Voet: Pages
 Chapter 12: Membranes Voet & Voet: Pages 390-415 Slide 1 Membranes Essential components of all living cells (define boundry of cells) exclude toxic ions and compounds; accumulation of nutrients energy
Chapter 12: Membranes Voet & Voet: Pages 390-415 Slide 1 Membranes Essential components of all living cells (define boundry of cells) exclude toxic ions and compounds; accumulation of nutrients energy
2. Which is likely to be a nonpolar solvent? A. B. B shows a carboxyl group, while A is only carbons and hydrogens.
 An organic chemistry lab uses thin layer chromatography to determine the relative polarity of different molecules. The molecules are added to the bottom of a glass plate covered with polar silicone gel
An organic chemistry lab uses thin layer chromatography to determine the relative polarity of different molecules. The molecules are added to the bottom of a glass plate covered with polar silicone gel
Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells.
 SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
Siglec-15 Is A Potential Therapeutic Target For Postmenopausal Osteoporosis
 Siglec-15 Is A Potential Therapeutic Target For Postmenopausal Osteoporosis Yusuke Kameda, Masahiko Takahata, Tomohiro Shimizu, Hiroki Hamano, Norimasa Iwasaki. Department of Orthopedic Surgery, Hokkaido
Siglec-15 Is A Potential Therapeutic Target For Postmenopausal Osteoporosis Yusuke Kameda, Masahiko Takahata, Tomohiro Shimizu, Hiroki Hamano, Norimasa Iwasaki. Department of Orthopedic Surgery, Hokkaido
Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) (b) (c) (d) (e) (f) (g) .
 Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) The inhibitory effects of new antibodies (Mab17 and Mab18). They were investigated in in vitro fertilization
Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) The inhibitory effects of new antibodies (Mab17 and Mab18). They were investigated in in vitro fertilization
Fig. S1. Schematic representation of the two RBC membrane:cytoskeleton anchorage
 Supplemental data Fig. S1. Schematic representation of the two RBC membrane:cytoskeleton anchorage complexes. Left, 4.1R complex; right, ankyrin-based complex. Adapted from (1) where abbreviations are
Supplemental data Fig. S1. Schematic representation of the two RBC membrane:cytoskeleton anchorage complexes. Left, 4.1R complex; right, ankyrin-based complex. Adapted from (1) where abbreviations are
Critical Review. Complement-mediated Phagocytosis The Role of Syk. Yumi Tohyama 1,2 and Hirohei Yamamura 2,3 THE COMPLEMENT SYSTEM AND PHAGOCYTOSIS
 IUBMB Life, 58(5 6): 304 308, May June 2006 Critical Review Complement-mediated Phagocytosis The Role of Syk Yumi Tohyama 1,2 and Hirohei Yamamura 2,3 1 Himeji Dokkyo University, Himeji, Hyogo, Japan 2
IUBMB Life, 58(5 6): 304 308, May June 2006 Critical Review Complement-mediated Phagocytosis The Role of Syk Yumi Tohyama 1,2 and Hirohei Yamamura 2,3 1 Himeji Dokkyo University, Himeji, Hyogo, Japan 2
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Superior Fluorescent Labeling Dyes Spanning the Full Visible Spectrum...1. Trademarks: HiLyte Fluor (AnaSpec, Inc.)
 Table of Contents Fluor TM Labeling Dyes Superior Fluorescent Labeling Dyes Spanning the Full Visible Spectrum....1 Fluor TM 405 Dye, an Excellent Alternative to Alexa Fluor 405 & DyLight 405....2 Fluor
Table of Contents Fluor TM Labeling Dyes Superior Fluorescent Labeling Dyes Spanning the Full Visible Spectrum....1 Fluor TM 405 Dye, an Excellent Alternative to Alexa Fluor 405 & DyLight 405....2 Fluor
Supplemental Materials. STK16 regulates actin dynamics to control Golgi organization and cell cycle
 Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538
 Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Gladstone Institutes, University of California (UCSF), San Francisco, USA
 Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24 Gag Marianne Gesner, Mekhala Maiti, Robert Grant and Marielle Cavrois * Gladstone Institutes, University of
Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24 Gag Marianne Gesner, Mekhala Maiti, Robert Grant and Marielle Cavrois * Gladstone Institutes, University of
Serafino et al. Thymosin α1 activates complement receptor-mediated phagocytosis in human monocyte-derived macrophages. SUPPLEMENTARY FIGURES
 Supplementary Fig. S1. Evaluation of the purity and maturation of macrophage cultures tested by flow cytometry. The lymphocytic/monocytic cellular fraction was isolated from buffy coats of healthy donors
Supplementary Fig. S1. Evaluation of the purity and maturation of macrophage cultures tested by flow cytometry. The lymphocytic/monocytic cellular fraction was isolated from buffy coats of healthy donors
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit
 INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
A novel cyanobacterial ligand for human L-selectin extracted from Aphanizomenon flos aquae potential role for stem cell biology in vitro and in vivo?
 A novel cyanobacterial ligand for human L-selectin extracted from Aphanizomenon flos aquae potential role for stem cell biology in vitro and in vivo? Introduction The objective of this study was to evaluate
A novel cyanobacterial ligand for human L-selectin extracted from Aphanizomenon flos aquae potential role for stem cell biology in vitro and in vivo? Introduction The objective of this study was to evaluate
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/2/e1602038/dc1 Supplementary Materials for Mitochondrial metabolic regulation by GRP78 Manoj Prasad, Kevin J. Pawlak, William E. Burak, Elizabeth E. Perry, Brendan
advances.sciencemag.org/cgi/content/full/3/2/e1602038/dc1 Supplementary Materials for Mitochondrial metabolic regulation by GRP78 Manoj Prasad, Kevin J. Pawlak, William E. Burak, Elizabeth E. Perry, Brendan
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by
 Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small cell lung cancer
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Experimental Methods Cell culture B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Experimental Methods Cell culture B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small
Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12
 SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
Supplementary Data Dll4-containing exosomes induce capillary sprout retraction ina 3D microenvironment
 Supplementary Data Dll4-containing exosomes induce capillary sprout retraction ina 3D microenvironment Soheila Sharghi-Namini 1, Evan Tan 1,2, Lee-Ling Sharon Ong 1, Ruowen Ge 2 * and H. Harry Asada 1,3
Supplementary Data Dll4-containing exosomes induce capillary sprout retraction ina 3D microenvironment Soheila Sharghi-Namini 1, Evan Tan 1,2, Lee-Ling Sharon Ong 1, Ruowen Ge 2 * and H. Harry Asada 1,3
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/305/ra106/dc1 Supplementary Materials for Controlling Long-Term Signaling: Receptor Dynamics Determine Attenuation and Refractory Behavior of the TGF-β Pathway
www.sciencesignaling.org/cgi/content/full/6/305/ra106/dc1 Supplementary Materials for Controlling Long-Term Signaling: Receptor Dynamics Determine Attenuation and Refractory Behavior of the TGF-β Pathway
Supplementary Figure 1. Overview of steps in the construction of photosynthetic protocellular systems
 Supplementary Figure 1 Overview of steps in the construction of photosynthetic protocellular systems (a) The small unilamellar vesicles were made with phospholipids. (b) Three types of small proteoliposomes
Supplementary Figure 1 Overview of steps in the construction of photosynthetic protocellular systems (a) The small unilamellar vesicles were made with phospholipids. (b) Three types of small proteoliposomes
(multiple answers) This strain of HIV uses a different chemokine coreceptor for entry into cells.
 LS1a Fall 06 Problem Set #4 100 points total all questions including the (*extra*) one should be turned in TF 1. (20 points) To further investigate HIV infection you decide to study the process of the
LS1a Fall 06 Problem Set #4 100 points total all questions including the (*extra*) one should be turned in TF 1. (20 points) To further investigate HIV infection you decide to study the process of the
COMPONENT NAME COMPONENT # QUANTITY STORAGE SHELF LIFE FORMAT. Store at 2-8 C. Do not freeze. Store at 2-8 C. Do not freeze.
 This document is available at www.stemcell.com/pis EasySep Mouse Monocyte Isolation Kit Catalog #19861 For processing 1 x 10^9 cells Description Isolate untouched and highly purified monocytes from mouse
This document is available at www.stemcell.com/pis EasySep Mouse Monocyte Isolation Kit Catalog #19861 For processing 1 x 10^9 cells Description Isolate untouched and highly purified monocytes from mouse
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
