Pak1 as a Novel Therapeutic Target for Anti-Hypertrophic Treatment in the Heart
|
|
|
- Silvester Bradley
- 5 years ago
- Views:
Transcription
1 Europe PMC Funders Group Author Manuscript Published in final edited form as: Circulation December 13; 124(24): doi: /circulationaha Pak1 as a Novel Therapeutic Target for Anti-Hypertrophic Treatment in the Heart Wei Liu, MD, Ph.D 1, Min Zi, MD 2, Ronald Naumann, MSc 3, Susanne Ulm, MSc 1, Jiawei Jin, Ph.D 1, Domenico M. Taglieri, MD 4, Sukhpal Prehar, MSc 2, Junhong Gui, MD, Ph.D 5, Hoyee Tsui, Bsc 1,2, Rui-Ping Xiao, MD, Ph.D 6, Ludwig Neyses, MD 2, R. John Solaro, Ph.D 4, Yunbo Ke, Ph.D 4,*, Elizabeth J. Cartwright, Ph.D 2,*, Ming Lei, MD, Ph.D 2,*, and Xin Wang, MD, Ph.D 1,* 1 Faculty of Life Sciences, School of Biomedicine, The University of Manchester, UK 2 Manchester Academic Health Sciences Centre, School of Biomedicine, The University of Manchester, UK 3 Max Planck Institute of Molecular Cell Biology and Genetics, Germany 4 Department of Physiology and Biophysics and Center for Cardiovascular Research, University of Illinois at Chicago, USA 5 Department of Cellular and Molecular Physiology, Yale University School of Medicine, USA 6 Institute of Molecular Medicine, Peking University, Beijing , China Abstract Background Stress-induced hypertrophic remodeling is a critical pathogenetic process leading to heart failure. While many signal transduction cascades are demonstrated as important regulators to facilitate the induction of cardiac hypertrophy, the signaling pathways for suppressing hypertrophic remodeling remain largely unexplored. In this study, we identified p21-activated kinase 1 (Pak1) as a novel signaling regulator which antagonizes cardiac hypertrophy. Methods and Results Hypertrophic stress applied to primary neonatal rat cardiomyocytes (NRCMs), or murine hearts caused the activation of Pak1. Analysis of NRCMs expressing constitutively active Pak1 or in which Pak1 was silenced disclosed that Pak1 played an antihypertrophic role. To investigate the in vivo role of Pak1 in the heart, we generated mice with a cardiomyocyte-specific deletion of Pak1 (Pak1 cko ). When subject to 2 weeks of pressure overload, Pak1 cko mice compared to controls, developed greater cardiac hypertrophy with attendant blunting of JNK activation, and these knockout mice underwent the transition into heart failure when prolonged stress was applied. In addition, chronic angiotensin II infusion also caused increased cardiac hypertrophy in Pak1 cko mice. Moreover, we discovered that the Pak1 activator FTY720, a sphingosine-like analogue, was able to prevent pressure overload-induced hypertrophy in wildtype mice, without compromising their cardiac functions. Meanwhile FTY720 failed to exert such Correspondence to: Dr. Xin Wang: Faculty of Life Sciences, the University of Manchester, CTF Building, Grafton Street, Manchester M13 9NT, UK. Tel: ; Fax: ; xin.wang@manchester.ac.uk. * Joint seniors Conflict of Interest Disclosures None. Subject codes: 15, 115, 130, 138 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
2 Liu et al. Page 2 an effect on Pak1 cko mice, suggesting that the anti-hypertrophic effect of FTY720 likely acts through Pak1 activation. Conclusions These results, for the first time, establish Pak1 as a novel anti-hypertrophic regulator and suggest that it may be a potential therapeutic target for the treatment of cardiac hypertrophy and heart failure. Keywords Cardiac hypertrophy; heart failure; signal transduction; stress Introduction Cardiac hypertrophy is a critical pathogenetic process leading to heart failure, with an incidence and prevalence that is rapidly increasing worldwide. The life time risk of heart failure is one in five amongst both men and women. Cardiac hypertrophy is characterized as proliferation-independent cardiomyocyte growth, which bears some similarity to tumour growth. To date, many oncogenes have been demonstrated to positively regulate cardiac hypertrophy. For example, aberrant activation of Ras (small guanine nucleotide-binding protein) is a step in the development of many types of cancers 1. Cardiac overexpression of constitutively active Ras manifested ventricular hypertrophy 2. This evidence indicates that the signaling programs regulating cell proliferation may be closely related to the programs that control growth of postmitotic adult cardiomyocytes. One of these signaling programs, which may be critical in both aberrant growth in cancer and in cardiac hypertrophy, is a cascade involving p21-activated kinase 1 (Pak1), for which there is provocative evidence for a role in tumour formation 3, 4, yet the role of Pak1 in cardiac hypertrophy signaling remains largely unexplored. The Pak family is a group of evolutionarily conserved serine/threonine protein kinases consisting of six isoforms, subdivided into two groups. Pak1 belongs to the group I subfamily, and was first discovered as a major binding partner for small GTPases Rac1 and Cdc42 5. When cells face inciting stimuli, Cdc42/Rac1 becomes activated via exchange of GDP for GTP 6, and activated Cdc42/Rac1 binds to Pak1, which in turn induces the activation of Pak1 7. At the last count, approximately 40 proteins in various cell types have been identified as downstream effectors of Pak1 reflecting the range of its biological activities, including regulation of cell proliferation, cell survival and cell motility 8. Pak1 is abundant in the heart. Important findings by us and others lay the groundwork for suggesting the significant physiological roles of this kinase in the heart In the present study, we aimed to examine our hypothesis that Pak1 may play an important role in cardiac hypertrophy, and in the transition to heart failure, and to investigate whether Pak1 is a potential therapeutic target for antihypertrophic treatment. Using both primary cardiomyocytes and Pak1 cko mice, we discovered that Pak1 acts as a novel signaling hub relaying anti-hypertrophic and survival signals from small GTPases to the JNK cascade in the heart. Furthermore, we observed that application of FTY720, a sphingosine-like synthetic analogue with an ability to activate Pak1 13, 14, prevented the development of cardiac hypertrophy in load stressed wild-type mice, and its antihypertrophic effect was likely due to its function to activate Pak1. Overall, these data demonstrate for the first time that Pak1 activation exerts a beneficial effect by resisting stress-induced hypertrophic remodeling, and Pak1 may thus be a potential therapeutic target for anti-hypertrophic treatment.
3 Liu et al. Page 3 Methods More detailed methods are available in the online Data Supplement. Adenoviral Infection of NRCMs Adenovirus-mediated gene transfection of NRCMs was performed by methods described previously 15. Adenovirus expressing shpak1 (CTGTTCTGGATGTGTTGGAAT) was generated using the BLOCK-iT adenoviral RNAi expression system (Invitrogen). Adenoviruses expressing capak1 (constitutively active Pak1), or NFAT-luciferase reporter gene (Ad-NFAT-Luc) were described elsewhere 10, 16. Ad-caMKK7 (constitutively active MKK7) was a kind gift from Dr. Hiroki Aoki (Kurume University, Japan). After infection, NRCMs were subjected to immunocytochemistry, quantitative RT-PCR, immunoblot analyses and luciferase reporter assay to investigate the role of Pak1 in cardiac hypertrophy. Generation of Pak1 f/f and Pak1 cko Mice Pak1 genomic DNA was used for constructing a gene targeting vector with two LoxP sites flanking exon 3 of pak1. 129Sv-derived R1 embryonic stem (ES) cell transfection, selection, screening and blastocyst injection were performed as described previously 17. Germline transmitting chimeras were generated and mated with C57BL/6 females to produce heterozygous Pak1 flox mice, which were back-crossed into a C57BL/6 background for 5 generations to obtain homozygous Pak1 flox (Pak1 f/f ) mice. To generate Pak1 cko mice, Pak1 f/f mice were mated with mice expressing Cre under α myosin heavy chain (αmhc) promoter (αmhc-cre) 18. Quantitative RT-PCR, immunoblotting and immunostaining were used to verify Pak1 deletion. All mice used in this study were maintained in a pathogen-free facility at the University of Manchester. The animal studies were performed in accordance with the UK Home Office and institutional guidelines. Hypertrophy Models and FTY720 Administration Data Analysis Cardiac hypertrophy was induced by administration of angiotensin II (Ang II, Sigma- Aldrich) at 1μg/g/day for 14 days via osmotic mini-pumps (Alzet) implanted subcutaneously in 8-10 week old male Pak1 cko mice and their littermates (Pak1 f/f mice), or by transverse aortic constriction (TAC) as previously described 15, 16, 19. For FTY720 (2- amino-2-[2-(4-octylphenyl) ethyl]-1,3-propanediol hydrochloride) administration, on the second day after the operation, TAC- or sham-operated wild-type mice or Pak1 cko mice (C57BL/6 background, 8-10 week old male) were randomized into different groups for intra-peritoneal injection of FTY720 (10μg/g/day, Cayman Chemical) or vehicle (saline) for 5 days. FTY720-LD 50 [50% lethal dose] is 300 μg/g. 7 days after the operation (5 days post FTY720 injection), hearts were taken from different experimental groups and the hypertrophic responses were analyzed by histology, quantitative RT-PCR, echocardiography and hemodynamic analysis. Two-way ANOVA followed by Bonferonni corrected post-hoc t-test was used for comparisons among multiple groups. Comparisons between two groups were performed using Student s t-test. P-values <0.05 are considered statistically significant. Data are expressed as mean ± SEM. Where sample sizes were < 5, tests were also conducted using ranked data. In all cases statistical conclusion were the same. For simplicity we present only the parametric results.
4 Liu et al. Page 4 Results Pak1 Regulates the JNK Pathway and Antagonizes NFAT-Mediated Hypertrophy in NRCMs In order to investigate the biological role of Pak1 in cardiac hypertrophy, we first determined activation of endogenous Pak1 by various hypertrophic agonists. Stimulation of NRCMs for 30 min with angiotensin II (Ang II, 10 μm), phenylephrine (PE, 30 μm), or isoproterenol (ISO, 10 μm) each significantly increased Pak1 phosphorylation of Thr-423 in the T-loop of Pak1, which is indicative of Pak1 activation (Figure 1A). Pak1 phosphorylation was also appreciably increased in ventricular tissues of wild type mice subjected to transverse aortic constriction (TAC) for 2 weeks (Figure 1A). We next assessed whether Pak1 exerts a pro-hypertrophic or an anti-hypertrophic effect in response to hypertrophic stimuli in NRCMs. NRCMs were infected with the Ad-caPak1 (constitutively active Pak1) or control adenovirus Ad-GFP prior to 48 h PE treatment. Unexpectedly, AdcaPak1 abrogated the pro-hypertrophic effect of PE, showing a significantly smaller cell surface area concomitant with a nearly 2-fold down-regulation of ANP mrna expression (Figure 1B-C). Furthermore, we examined whether activated Pak1 affects NFAT transcriptional activity, which plays a central role in regulating cardiac hypertrophy. In line with results reported above, adenoviral infection of the NFAT-luciferase reporter (Ad- NFAT-Luc) in control NRCMs (infected with Ad-LacZ) led to enhanced NFAT reporter activity following PE stimulation. However, infection of Ad-caPak1 did not lead to any increase in NFAT activity despite PE stimulation (Figure 1D). To corroborate these data, we adopted a gene knockdown system in NRCMs, where Pak1 expression was deleted by 85% following infection with Ad-shPak1; expression of Pak2 and Pak3 (close Pak family isoforms) remained unchanged (Figure 2A). Compared to NRCMs infected with scrambled shrna (Ad-shC2), PE induced significantly greater increases in cell size and in ANP mrna level in NRCMs infected with Ad-shPak1 (Figure 2B-C). To investigate the potential mechanism whereby Pak1 deficiency promoted hypertrophy, we screened a range of hypertrophic regulators. Our data demonstrate a prominent defect in JNK phosphorylation in shpak1-infected NRCMs after PE stimulation (Figure 2D). Furthermore, MKK4 and MKK7 (upstream activators of JNK) were found not to respond to PE stimulation in the absence of Pak1 (Figure 2D). However, phosphorylation levels of MEKK1, p38, ERK1/2 and PKB were similar in the two groups following PE treatment (Figure 2D). Finally, NFAT transcriptional activity was examined when Pak1 was knocked down. We discovered that PE stimulation of shpak1-infected NRCMs resulted in enhanced NFAT activity. However, this increase in NFAT activity was mitigated by infection with constitutively active MKK7 (Ad-caMKK7), indicating that loss of Pak1 induces greater cardiomyocyte hypertrophy by promoting increased NFAT activity, which is likely to occur via the JNK pathway (Figure 2E). Generation and Characterization of Cardiomyocyte-Specific Pak1 Knockout Mice Prompted by our results showing that Pak1 might be a critical signaling nexus limiting hypertrophy, we moved on to studies addressing our hypothesis in the intact heart. To precisely ascertain the in vivo role of Pak1 in the heart, we generated cardiomyocytespecific Pak1 deletion mice (Pak1 cko ). Mice with a germ-line modification in the pak1 gene with two LoxP elements flanking exon 3 (Pak1 f/f ) were generated (Supplement Figure I A- B). Pak1 f/f mice were healthy and fertile, indicating that the presence of two LoxP sites did not affect Pak1 function in vivo. To establish Pak1 cko mice, Pak1 f/f mice were bred with αmhc-cre mice. Pak1 cko mice developed to term, and were viable and fertile in adulthood. PCR amplification of genomic
5 Liu et al. Page 5 DNA prepared from cardiomyocytes, brain, liver and skeletal muscle of 8-week old Pak1 f/f and Pak1 cko mice confirmed the specific recombination of the pak1 gene in cardiomyocytes (Supplement Figure II A). The deletion of pak1 gene product in cardiomyocytes was verified at mrna and protein levels (Supplement Figure II B-D). Notably, loss of Pak1 in cardiomyocytes did not induce any compensatory changes in the protein levels of its activators, Cdc42 and Rac1, as well as its close family members Pak2 and Pak3, and potential effectors, such as ERK1/2, JNK and p38 (Supplement Figure II D-E). Disruption of Pak1 in Cardiomyocytes Exacerbates Pressure Overload-Induced Hypertrophy Following on, we determined whether Pak1 is involved in regulating cardiac hypertrophy. Pressure overload by TAC was applied to 8-week old Pak1 f/f and Pak1 cko mice. Following 2 weeks of TAC, Pak1 f/f mice developed a moderate 19% increase in heart weight/tibia length (HW/TL) ratio, whereas Pak1 cko mice showed a 53% increase in HW/TL ratio (Figure 3A). Consistent with this result there was a significant increase in cross-sectional area of Pak1 cko - TAC cardiomyocytes (338.7±2.74 μm 2 ), compared to ±4.54 μm 2 of Pak1 f/f -TAC cardiomyocytes (Figure 3B). Sirius Red staining to determine collagen deposition (Figure 3C) showed more interstitial fibrosis in Pak1 cko -TAC myocardium (6.1% fibrotic area compared to 1.6% in the controls). Loss of Pak1 also induced cardiomyocyte apoptosis indicated by a 5-fold increase in the number of TUNEL-positive nuclei in Pak1 cko -TAC myocardium compared to Pak1 f/f hearts (Figure 3D). Reactivation of the fetal gene program was measured by quantitative RT-PCR; expression of ANP, brain natriuretic peptide (BNP) and β myosin heavy polypeptide (Myh7) mrna was significantly elevated in the hypertrophied Pak1 cko myocardium (Figure 3E). Regulator of calcineurin 1 variant 4 (RCAN1.4) is a target gene of NFAT transcription factors. Increased RCAN1.4 mrna expression was detected in TAC stressed Pak1 cko hearts, indicating enhanced NFAT signaling in the knockout mice (Figure 3E). Moreover, as illustrated in Figure 3E, mrna levels of procollagen type I, α2 (Col1α2) and procollagen type III, α1 (Col3α1) were markedly up-regulated in the Pak1 cko myocardium. Although greater hypertrophy and salient remodeling occurred in the Pak1 cko -TAC mice, their contractile performance remained normal, as indicated by similar fractional shortening (FS %) between the two groups after TAC (Supplement Table I). Thus we conclude that ablation of Pak1 in cardiomyocytes promotes hypertrophic remodeling in response to TAC stress. Prolonged Load Stress Sensitizes Pak1 cko Mice to Heart Failure To further determine whether loss of Pak1 in cardiomyocytes predisposes mice to heart failure, we extended the TAC stress imposed on Pak1 f/f and Pak1 cko mice to 5 weeks. Indeed, Pak1 cko mice showed characteristics of heart failure after TAC. Lung weight to tibia length (LW/TL) ratio was substantially higher in Pak1 cko -TAC mice, indicating pulmonary edema due to contractile insufficiency (Figure 4A). A significant reduction in FS (19.89±1.8%) in the knockouts confirmed heart failure, whereas Pak1 f/f mice exhibited preserved contractility (Figure 4B). The increases in HW/TL ratio (83%) and in myocyte cross-sectional area (419.83±2.0 μm 2 ) became even more prominent in Pak1 cko mice after prolonged TAC stress (Figure 4C-D). In addition, increased collagen deposition (9.1%) was scattered over the working myocardium of Pak1 cko -TAC mice (Figure 4E). These results demonstrated that mice were more vulnerable to longer pressure overload stress, and more readily made the transition into heart failure when Pak1 was absent.
6 Liu et al. Page 6 Enhanced Hypertrophic Remodeling is Induced in Pak1 cko Mice Responding to Ang II Infusion To determine the general significance of our findings, we investigated whether Pak1 resists hypertrophy induced by neuroendocrine stimuli. When subjected to 2-week infusion of Ang II (1μg/g/day), Pak1 cko mice demonstrated significantly increased hypertrophy, as reflected by a 35% enhancement in HW/TL and enlarged cardiomyocytes (292.61±3.51μm 2 versus ±2.96μm 2 of Pak1 f/f myocytes) (Supplement Figure III A-B). Ventricular fibrosis was more visible in the knockouts (Supplement Figure III C). We measured ROS production by DHE staining; there were no significant differences detected between the two genotypes (Supplement Figure III D). Also, cardiac function in Pak1 cko mice was comparable to the control group (Supplement Figure III E). Together, these results illustrate that Pak1 antagonizes cardiac hypertrophy not only by mechanical stress-induced membrane receptor activation, but also by neuroendocrine agonist stimulation. The JNK Cascade acts Downstream of Pak1 in Cardiac Hypertrophic Remodeling To obtain in vivo evidence of the regulatory mechanism whereby Pak1 modulates hypertrophic responses, we surveyed downstream candidates. Consistent with data that we obtained from NRCMs, TAC treatment did not induce the activation of MKK4/MKK7-JNK pathway in the Pak1 cko myocardium, whereas activation of p38, ERK1/2 and PKB, as well as PP2A activity (phosphorylation of Y307) remained the same between the two groups (Figure 5A). We also examined apoptotic molecules that might be responsible for the higher rate of cardiomyocyte death in the knockout hearts. Interestingly, we found augmented protein levels of p53, Bax and Bad in the Pak1 cko -TAC myocardium. However, there were no significant differences observed in either the expression of Bim and Bcl-2, or phosphorylation of Bad at Ser 112 (Figure 5B), which is a known site for Pak1-mediated phosphorylation 20. Thus, these data demonstrate that the MKK4/MKK7-JNK pathway acts downstream of Pak1 in protecting the heart from hypertrophic stress. FTY720 Induces Pak1 Activation and Prevents Cardiac Hypertrophy Led by the results above, we tested whether Pak1 is a potential therapeutic target for antihypertrophic treatment. Firstly, we demonstrated FTY720 was able to induce Pak1 phosphorylation in NRCMs and in wild-type mouse myocardium (Figure 6A). Then, we discovered that treatment of NRCMs with FTY720 (200nM, 48h) significantly reduced PEinduced hypertrophic responses, indicated by a significantly smaller cell surface area together with markedly decreased ANP expression (Figure 6B). Interestingly, Pak1- knockdown NRCMs treated with or without FTY720 showed no significant differences in PE-induced increases in cell surface area and ANP expression (Figure 6B), suggesting FTY720 likely functions via Pak1 activation to block hypertrophic responses. Noteworthy, using trypan blue staining to check cell viability, we found that FTY720 at a dose of 200nM was sufficient to restrain hypertrophic responses, but did not exhibit a toxic effect on NRCMs (Figure 6C), indicating FTY720 may be a suitable agent for anti-hypertrophic therapy in vivo. To test this hypothesis, we applied FTY720 (10μg/g/day of body weight) to wild-type mice for 5 days commencing on the second day after TAC or sham operation. Treatment with vehicle (saline) was given to the control groups following the same protocol. Notably, after 5 days of treatment with FTY720, TAC-mice showed comparable HW/TL ratio (6.01±0.22 mg/mm) and cardiomyocyte cross-sectional areas (196.73±3.06 μm 2 ) to the FTY720-treated sham-mice (HW/TL: 5.61±0.14 mg/mm; cross-sectional areas: ±3.65 μm 2 ) or vehicle-treated sham-mice (HW/TL: 5.6±0.11 mg/mm; cross-sectional areas: ±2.35
7 Liu et al. Page 7 Discussion μm 2 ) (Figure 7A-B). Accordingly, echocardiography also demonstrated cardiac structure and function of the FTY720-treated TAC-mice were similar to the sham groups (Figure 7C- D). In contrast to the FTY720-treated TAC-mice, the TAC-mice treated with vehicle developed hypertrophy (Figure 7A-D). Consistent with in vitro data, FTY720 used in this protocol did not exhibit cardiac toxicity in the mice, as FS, dp/dt max (contractile response) and dp/dt min (lusitropic response) in the FTY720 treated groups remained normal, compared to the vehicle treated groups (Figure 7 E-F). Next, we determined whether the anti-hypertrophic effect of FTY720 was due to Pak1 activation, therefore, the same FTY720 treatment protocol was applied to Pak1 cko mice subjected to either TAC or sham operation. Interestingly, despite FTY720 treatment, TAC was still able to induce hypertrophy in the hearts of Pak1 cko mice (HW/TL: 7.92±0.22 mg/ mm [FTY720] versus 8.06±0.2 mg/mm [vehicle]; cross-sectional areas: ±3.02 μm 2 [FTY720] versus 313.8±1.72 μm 2 [vehicle]) (Figure 8A-B). Echocardiography and hemodynamic analysis demonstrated comparable cardiac structure and function between the FTY720 treated TAC-mice and the sham groups (Figure 8C-F). Together, these data suggest that the activation of Pak1 by FTY720 is able to prevent the development of cardiac hypertrophy. With the use of cultured rat cardiomyocytes and Pak1 cko mice, we have identified a novel cardioprotective role of Pak1 in attenuating cardiac hypertrophy and halting the transition to heart failure. The major findings of this study are: 1) Pak1 is activated by both mechanical stress and neuroendocrine agonists in the heart; 2) Pak1 is an indispensable upstream activator for the JNK pathway in response to hypertrophic challenges; 3) Pak1 plays a critical role in antagonising cardiac hypertrophy, since hearts of Pak1 cko mice are vulnerable to cardiac hypertrophy and readily progress to failure with application of sustained pressure overload; 4) the activation of Pak1 by FTY720 is able to prevent the development of cardiac hypertrophy, suggesting Pak1 may be a potentially important therapeutic target for antihypertrophic treatment. Pak1 is an Anti-Hypertrophic Regulator in the Heart This study, for the first time, demonstrates a differing role for Pak1 in cardiomyocyte growth. During the past decade, growing evidence has suggested that Pak1 activation is frequently associated with cell proliferation, survival of cancer cells and increased invasiveness. In fact, more than half of human breast cancers exhibit hyperactivation and/or overexpression of Pak1 21. In cancers, Pak1 activation is inextricably linked with aberrant Ras/Raf/ERK signaling 8. In the heart, mature cardiomyocytes are terminally differentiated; growth signals do not lead to proliferation but rather to hypertrophy, which explains why many oncogenes display pro-hypertrophic effects in the adult heart 22, 23. Thus, we predicted that Pak1 may exert a similar function to that of the Ras pathway in facilitating cardiac hypertrophy. However, to our surprise, our studies revealed the anti-hypertrophic property of Pak1 in the heart. The initiation of cardiac hypertrophy involves neuroendocrine factors, such as angiotensin II, endothelin-1 and phenylephrine, all of which act on G-protein-coupled receptors (GPCRs), in turn; these receptors stimulate heterotrimeric G-proteins such as G q/11, G 12/13, and G i for signal transduction However, small GTPases do not bind with GPCRs, but become activated through exchange of GDP for GTP. The dynamic GTP-binding and GDPhydrolysis cycle of small GTPases is tightly regulated by GTPase activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs), which are downstream effectors of
8 Liu et al. Page 8 heterotrimeric G-proteins 6, 27. Pressure overload is a potent hypertrophic inducer in the heart. It not only induces the release of neuroendocrine factors to stimulate GPCRs, but also activates stretch sensors or receptor tyrosine kinases for the development of cardiac hypertrophy 28. When the heart is exposed to various hypertrophic stimuli, pro-hypertrophic and anti-hypertrophic signaling pathways are concurrently activated to regulate the hypertrophic process. We discovered that Pak1 was activated by a number of neuroendocrine factors, and by pressure overload to antagonize cardiac hypertrophy. The Cdc42-Pak1-JNK Axis is a Critical Pathway Relaying Anti-Hypertrophic Signals Results of studies investigating the role of Cdc42, a key regulator of Pak1 activation, in hypertrophic signaling are consistent with our findings. Maillet et al. reported that diverse hypertrophic stimuli increased the activated GTP-bound form of Cdc42. Loss of Cdc42 in cardiomyocytes rendered mice more capable of cardiac hypertrophic growth, and Cdc42 is required for JNK activation in response to hypertrophic stress 29. Their data agree with previous investigations in mammalian cells showing that Cdc42 induces JNK activation Interestingly, Maillet et al. also showed that Cdc42 deficiency in NRCMs led to blunted phosphorylation of MEKK1. MEKK1 is a MAP3K; which preferentially activates the JNK pathway through MKK4 and MKK7, and also regulates the activity of the ERK cascade 33, 34. However, we did not observe altered MEKK1 phosphorylation due to Pak1 deficiency; whereas blunted activation of the MKK4/MKK7-JNK pathway and increased NFAT activity was detected when Pak1 was scarce. MKK4 and MKK7 are upstream kinases for JNKs, which are implicated in the progression of heart failure 35, 36. JNKs have been shown to antagonize cardiac hypertrophy through inhibition of NFAT activity 37. Our recent studies employing mice with cardiomyocyte-specific deletion of MKK4 or MKK7 support this mechanism 16, 19. Considering the above evidence, we believe that Pak1 acts upstream of the JNK pathway in hypertrophic signaling, and MEKK1 might not be a direct effector downstream of Pak1-mediated anti-hypertrophic signaling. It is interesting to note that a recent study by Higuchi et al. described a novel property of Pak1; it not only has catalytic function, but can also act as a scaffolding protein for priming Akt activation 38. Whether Pak1 is able to directly phosphorylate MKK4/MKK7 or aid recruitment of MKK4/MKK7 to specific MAP3Ks in response to hypertrophic stimuli thus remains to be determined. Our previous study has shown that Pak1 is involved in modulating cardiac contractility through PP2A-mediated dephosphorylation of cardiac troponin I (ctni) 10. It was proposed that p38 seemed to be an intermediate for Pak1-mediated PP2A activity 10. As such, we have examined p38 activation and PP2A phosphorylation of Y307 (indicating the catalytic activity of PP2A), however, no alteration in p38 activation or PP2A activity was observed in our experimental setting due to Pak1 deficiency in cardiomyocytes under TAC stress. These results suggest at least in the model we have employed, that PP2A or p38 is unlikely to be downstream of Pak1 and responsible for the development of cardiac hypertrophy. Current knowledge of Cdc42 and Rac1, both of which activate Pak1, suggests differing roles for these small G proteins in hypertrophic signaling in the heart. In contrast to the promotion of cardiac hypertrophy by down-regulation of Cdc42 29, down-regulation of Rac1 inhibits the development of cardiac hypertrophy in response to Ang II infusion via decreased activity of NADPH 39. Subsequent studies by Custodis et al. indicated that Rac1 binding to Rho guanine dissociation inhibitor α may be a mechanism by which Rac1 mediates hypertrophy in a mouse pressure overload model 40. Rac1 overexpression in myocardium induced hypertrophy in juvenile transgenic mice concurrent with altered intracellular distribution of Pak from the cytosol to cytoskeletal fraction 41. Yet in this study by Sussman et al. no information was provided as to which isoform of Pak was involved in the translocation 41. It
9 Liu et al. Page 9 is known that other Pak isoforms, such as Pak2 and Pak3, which share substantial sequence homology with Pak1 42, are also expressed in cardiomyocytes. We have demonstrated that Pak1 cko mice exhibited greater hypertrophy with no increase in ROS production after 2 weeks of Ang II infusion, which is in stark contrast to phenotypes reported in Rac1 cardiomyocyte-specific knockouts 39. Taking this evidence into account it is plausible that Pak1 is a primary effector of Cdc42 rather than Rac1. Pak1 is an indispensible component of the Cdc42-Pak1-JNK axis serving as a critical anti-hypertrophic regulatory pathway. Pak1 Activation by FTY720 Exerts a Beneficial Effect for Restraining Cardiac Hypertrophy FTY720 is a sphingosine-like analogue, approved by the FDA for treating relapsing multiple sclerosis. We have previously reported that FTY720 prevents arrhythmias in an ex vivo rat heart subjected to ischemia/reperfusion injury 14. In the ischemia/reperfusion model, Pak1 activation was suggested to be involved in an FTY720-induced protective effect 14. Our test to determine whether FTY720 activation of Pak1 extended to the induction of cardiac hypertrophy demonstrated that administration of a pharmacological dose of FTY720 (10μg/ g/day) was sufficient and effective to limit TAC-induced cardiac hypertrophy in wild-type mice. Meanwhile, the observation that FTY720 failed to block increased cardiac hypertrophy in TAC stressed-pak1 cko mice provides further support that FTY720 induces its anti-hypertrophic effect through the activation of Pak1. Cardiac hypertrophy is traditionally regarded as an adaptive response to normalize ventricular wall stress. According to Laplace s law, FTY720 treatment might cause deterioration in cardiac function and chamber dilation in TAC stressed mice due to limited cardiac hypertrophy; however, none of these were observed in our study. Similarly, in response to pressure overload, preserved cardiac function with no or little hypertrophy was reported by a number of investigations, including studies in which NFAT signaling was inhibited These findings suggest that hypertrophy may not always be a necessary compensatory response; increased wall stress per se does not cause cardiac dysfunction. Therefore, FTY720 treatment could be of clinical interest given the fact of its ability to prevent hypertrophy, without deteriorating cardiac function. Furthermore, FTY720, which is derived from myriocin 48, a component of the natural product Isaria sinclairii, represents a non-toxic sphingosine-like derivative with oral bioavailability that may be useful in treatment and/or prevention of cardiac disorders in high-risk patients. In conclusion, we have discovered a novel role for Pak1 as a critical signaling hub in cardioprotection which limits excessive hypertrophic remodeling. Pak1 most likely acts downstream of Cdc42 to convey both anti-hypertrophic and survival signals to the JNK pathway in cardiomyocytes. Our demonstration of prevention of cardiac hypertrophy by administration of FTY720 provides convincing evidence for the identification of Pak1 as a potential therapeutic target for anti-hypertrophic treatment. Supplementary Material Acknowledgments Refer to Web version on PubMed Central for supplementary material. Funding Sources This work was supported by the British Heart Foundation (PG/07/055/23144 to XW and EJC; PG/08/006/24399, PG/09/052/27833 to XW, EJC and ML), The Wellcome Trust (grant no: to ML), and by US National Institutes of Health grant RO1 HL (RJS).
10 Liu et al. Page 10 References 1. Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000; 103: [PubMed: ] 2. Hunter JJ, Tanaka N, Rockman HA, Ross J Jr, Chien KR. Ventricular expression of a MLC-2-v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995; 270: [PubMed: ] 3. Zang M, Hayne C, Luo Z. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem. 2002; 277: [PubMed: ] 4. Coles LC, Shaw PE. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene. 2002; 21: [PubMed: ] 5. Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994; 367: [PubMed: ] 6. Jaffe AB, Hall A. Rho GTPase: biochemistry and biology. Annu Rev Cell Dev Biol. 2005; 21: [PubMed: ] 7. Chong C, Tan L, Lim L, Manser E. The mechanism of PAK activation. J Biol Chem. 2001; 276: [PubMed: ] 8. Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009; 28: [PubMed: ] 9. Clerk A, Sugden PH. Activation of p21-activated protein kinase alpha (alpha PAK) by hyperosmotic shock in neonatal ventricular myocytes. FEBS Lett. 1997; 403: [PubMed: ] 10. Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of p21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res. 2004; 94: [PubMed: ] 11. Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, Brodie MS, Terrar DA, Solaro RJ. Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21- activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ Res. 2007; 100: [PubMed: ] 12. Mao K, Kobayashi S, Jaffer ZM, Huang Y, Volden P, Chernoff J, Liang Q. Regulation of Akt/PKB activity by p21-activated kinase in cardiomyocytes. J Mol Cell Cardiol. 2008; 44: [PubMed: ] 13. Roig J, Tuazon PT, Traugh JA. Cdc42-independent activation and translocation of the cytostatic p21-activated protein kinase gamma-pak by sphingosine. FEBS Lett. 2001; 507: [PubMed: ] 14. Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, Solaro RJ, Lei M. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol. 2010; 48: [PubMed: ] 15. Kimura TE, Jin JW, Zi M, Prehar S, Liu W, Oceandy D, Abe JI, Neyses L, Weston AH, Cartwright EJ, Wang X. Targeted deletion of the extracellular signal-regulated protein kinase 5 attenuates hypertrophic response, and promotes pressure overload-induced apoptosis in the heart. Circ Res. 2010; 106: [PubMed: ] 16. Liu W, Zi M, Jin JW, Prehar S, Oceandy D, Kimura TE, Lei M, Neyses L, Weston AH, Cartwright EJ, Wang X. Cardiac-specific deletion of Mkk4 reveals its role in pathological hypertrophic remodeling, but not in physiological cardiac growth. Circ Res. 2009; 104: [PubMed: ] 17. Wang X, Nadarajah B, Robinson AC, McColl BW, Jin JW, Dajas-Bailador F, Boot-Handford RP, Tournier C. Targeted deletion of the mitogen-activated protein kinase kinase 4 gene in the nervous system causes severe brain developmental defects and premature death. Mol Cell Biol. 2007; 27: [PubMed: ] 18. Minamisawa S, Gu Y, Ross J Jr, Chien KR, Chen J. A post-transcriptional compensatory pathway in heterozygous ventricular myosin light chain 2-deficient mice results in lack of gene dosage effect during normal cardiac growth or hypertrophy. J Biol Chem. 1999; 274: [PubMed: ]
11 Liu et al. Page Liu W, Zi M, Chi H, Jin JW, Prehar S, Neyses L, Cartwright EJ, Flavell RA, Davis RJ, Wang X. Deprivation of MKK7 in cardiomyocytes provokes heart failure in mice when exposed to pressure overload. J Mol Cell Cardiol. 2011; 50: [PubMed: ] 20. Jin S, Zhuo Y, Guo W, Field J. PAK1-dependent phosphorylation of RAF-1 regulates its mitochondrial localization, phosphorylation of Bad, and BCL-2 association. J Biol Chem. 2005; 280: [PubMed: ] 21. Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R. p21- activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004; 279: [PubMed: ] 22. Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy; targeting Raf/MEK/ERK1/2- signaling. Int J Biochem Cell Biol. 2009; 41: [PubMed: ] 23. Muslin AJ. Role of Raf proteins in cardiac hypertrophy and cardiomyocyte survival. Trends Cardiovasc Med. 2005; 15: [PubMed: ] 24. Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nat Rev Mol Cell Biol. 2006; 7: [PubMed: ] 25. Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008; 358: [PubMed: ] 26. Clerk A, Sugden PH. Small guanine nucleotide-binding proteins and myocardial hypertrophy. Circ Res. 2000; 86: [PubMed: ] 27. Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPase: turning on the switch. Genes Dev. 2002; 16: [PubMed: ] 28. Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res. 2000; 47: [PubMed: ] 29. Maillet M, Lynch JM, Sanna B, York AJ, Zheng Y, Molkentin JD. Cdc42 is an antihypertrophic molecular switch in the mouse heart. J Clin Invest. 2009; 119: [PubMed: ] 30. Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTPbinding potein Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995; 81: [PubMed: ] 31. Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995; 81: [PubMed: ] 32. Wang L, Yang L, Burns K, Kuan CY, Zheng Y. Cdc42GAP regulates c-jun N-terminal kinase (JNK)-mediated apoptosis and cell number during mammalian perinatal growth. Proc Natl Acad Sci USA. 2005; 102: [PubMed: ] 33. Yujiri T, Sather S, Fanger GR, Johnson GL. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998; 282: [PubMed: ] 34. Witowsky JA, Johnson GL. Ubiquitylation of MEKK1 inhibits its phosphorylation of MKK1 and MKK4 and activation of the ERK1/2 and JNK pathways. J Biol Chem. 2003; 278: [PubMed: ] 35. Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation. 2007; 116: [PubMed: ] 36. Flesch M, Margulies KB, Mochmann HC, Engel D, Sivasubramanian N, Mann DL. Differential regulation of mitogen-activated protein kinase in the failing human heart in response to mechanical unloading. Circulation. 2001; 104: [PubMed: ] 37. Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-nfat signaling. EMBO J. 2003; 22: [PubMed: ] 38. Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008; 10: [PubMed: ] 39. Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA. 2006; 103: [PubMed: ]
12 Liu et al. Page Custodis F, Eberl M, Kilter H, Bohm M, Laufs U. Association or RhoGDIalpha with Rac1 GTPase mediates free radical production during myocardial hypertrophy. Cardiovasc Res. 2006; 71: [PubMed: ] 41. Sussman MA, Welch S, Walker A, Klevitsky R, Hewett TE, Price RL, Schaefer E, Yager K. Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1. J Clin Invest. 2000; 105: [PubMed: ] 42. Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol. 2002; 34: [PubMed: ] 43. Shimoyama M, Hayashi D, Takimoto E, Zou Y, Oka T, Uozumi H, Kudoh S, Shibasaki F, Yazaki Y, Nagai K, Komuro I. Calcineurin plays a critical role in pressure overload-induced cardiac hypertrophy. Circulation. 1999; 100: [PubMed: ] 44. Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to shor-term pressure overload. Circulation. 2002; 101: [PubMed: ] 45. Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in calcineurin Aβ-deficient mice. Proc Natl Acad Sci USA. 2002; 99: [PubMed: ] 46. Esposito G, Rapacciuolo A, Prasad SVN, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002; 105: [PubMed: ] 47. Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005; 11: [PubMed: ] 48. Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, Okumoto T. Fungal metabolites: part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot. 1994; 47: [PubMed: ]
13 Liu et al. Page 13 CLINICAL PERSPECTIVE Heart failure (HF) is one of the most devastating diseases. The life time risk of heart failure is one in five amongst both men and women. Despite advances in diagnostic and therapeutic technology during past decades, the survival rate after the onset of HF still remains significantly high. There is general agreement that cardiac hypertrophy is a determinant of the clinical course of HF. Therefore, understanding molecular mechanisms of hypertrophic remodeling is a key step in deciphering the pathogenesis of HF. Cardiac hypertrophy is characterized as proliferation-independent cardiomyocyte growth. The signaling programs regulating cell proliferation may be closely related to the programs that control growth of postmitotic adult cardiomyocytes. Based on this premise, we identified p21-activated kinase 1 (Pak1), which is important in cell proliferation, as a novel regulator antagonising cardiac hypertrophy. We have also elucidated that Pak1 is an indispensible component of the anti-hypertrophic signaling, in which Cdc42 (small GTPases) is its upstream activator, and JNK (MAP kinase) works downstream of Pak1. Most interestingly, we discovered that FTY720 (a sphingosine-like analogue), a FDA approved drug for treating relapsing multiple sclerosis, is able to limit cardiac hypertrophy of murine hearts. This anti-hypertrophic effect of FTY720 is likely to function through the activation of Pak1. Overall, our findings provide the evidence to establish Pak1 as a novel anti-hypertrophic regulator and a potential therapeutic target for the treatment of cardiac hypertrophy and heart failure.
14 Liu et al. Page 14 Figure 1. Activated Pak1 attenuated hypertrophic responses in NRCMs. (A) Immunoblotting analyses of Pak1 phosphorylation at Thr 423 indicate that Pak1 activation was induced by angiotensin II (Ang II), phenylephrine (PE), and isoproterenol (ISO) in NRCMs (n=3), and by TAC stress in myocardium (n=5 mice per group) (Western blot images: upper panel; the ratios of P/T Pak1 are represented by the bar graphs: lower panel, *P=0.001 versus untreated NRCMs). (B) Representative images of α-actinin immunostaining of NRCMs infected with Ad-caPak1 or Ad-GFP (scale bar: 20μm). Quantification of cell surface area is presented in the bar graph (n=3 independent experiments, 50 cells counted per experiment). (C) qpcr analyses show decreased ANP mrna expression in Ad-caPak1-infected NRCMs after PE stimulation. The data are derived from 5 independent experiments performed in triplicate and are normalized to the GAPDH content (n=5). (D) The luciferase reporter assays showed increased NFAT transcriptional activity in control NRCMs (infected with Ad-LacZ) after PE stimulation, whilst activated Pak1 (Ad-caPak1) diminished this increased NFAT transcriptional activity. Data are means ± SEM, n=3 independent experiments.
15 Liu et al. Page 15 Figure 2. Knockdown of Pak1 in NRCMs augmented hypertrophic responses. (A) NRCMs were infected with Ad-shC2 or Ad-shPak1 for 72 hours prior to immunoblot analysis of Pak1 protein level. Pak2 and Pak3 protein levels were determined to examine the specificity of Pak1 knockdown. GAPDH expression is the protein loading control. (B) Representative images of α-actinin immunostaining of NRCMs infected with Ad-shC2 or Ad-shPak1 (scale bar: 20μm). Quantification of cell surface area is presented in the bar graph (n=3 independent experiments, 50 cells counted per experiment). (C) qpcr analyses of ANP mrna expression (n=5). (D) Immunoblot analyses of expression and phosphorylation levels of JNK, MKK4, MKK7, MEKK1, p38, ERK1/2 and PKB in response to PE for 30 minutes. (E) The luciferase reporter assays showed substantially increased NFAT transcriptional activity in Ad-shPak1-infected NRCMs after PE stimulation, and activated MKK7 repressed Pak1 deficiency-induced higher NFAT transcriptional activity. Data are means ± SEM, n=4 independent experiments.
16 Liu et al. Page 16 Figure 3. Exacerbated cardiac hypertrophy in Pak1 cko mice after 2 weeks of TAC. (A) HW/TL ratios of Pak1 f/f and Pak1 cko mice. (B) Measurements of mean cross-sectional area. (C) Histological view of cardiac fibrosis detected by Sirius Red staining (scale bar: 50μm). Quantification of the relative area of fibrosis is expressed as percentage fibrosis. (D) Increased apoptosis in Pak1 cko ventricular myocardium was detected by TUNEL assay. TUNEL-positive nuclei are represented in the bar graph (n=5-7 mice per group). (E) qpcr analyses of ANP, BNP, Myh7, RCAN1.4, Col1α2 and Col3α1. The data are normalized to the GAPDH content (n=5-7 mice per group).
17 Liu et al. Page 17 Figure 4.
18 Liu et al. Page 18 Pak1 cko mice exhibit signs of heart failure when exposed to prolonged TAC. (A) 5 weeks of TAC caused a significant increase in lung weight relative to tibia length in Pak1 cko mice. (B) M-mode echocardiography show dilated ventricular chamber (left panel), and reduced FS% (right panel) in Pak1 cko -TAC mice. (C) HW/TL ratios of Pak1 f/f and Pak1 cko mice (upper panel). Morphometry demonstrates greater hypertrophy in Pak1 cko -TAC mice (lower panel, scale bar: 2mm). (D) Measurements of mean cross-sectional areas (upper panel), and hematoxylin & eosin staining of heart cross-sections (lower panel, scale bar: 20μm). (E) Massive interstitial fibrosis in the Pak1 cko heart (scale bar: 50μm). Quantification of fibrotic area is presented in the bar graph (n=7 mice per group).
19 Liu et al. Page 19 Figure 5. Pak1 is an upstream activator for the JNK pathway in response to TAC stress. (A) Protein extracts from Pak1 f/f and Pak1 cko hearts were examined by immunoblotting for total JNK, MKK4, MKK7, p38, ERK1/2, PKB and PP2A expression, as well as their phosphorylation levels. The ratios of P/T JNK, P/T MKK4, P/T MKK7 and P/T PP2A are represented by the bar graphs. (B) Immunoblot analyses of protein levels of p53, Bax, Bad, Bim and Bcl-2, as well as phosphorylated Bad. GAPDH expression is the protein loading control. The ratios of p53/gapdh, Bax/Bcl-2 and Bad/Bcl-2 are represented by the bar graphs (n=6 mice per group).
20 Liu et al. Page 20 Figure 6. FTY720 induced Pak1 activation and prevented hypertrophic responses in NRCMs. (A) FTY720-induced Pak1 phosphorylation was examined by immunoblot analyses in NRCMs (100 nm, 1h), and in myocardium (10μg/g, 1h). Western blot images: upper panel; the ratios of P/T Pak1 are represented by the bar graphs: lower panel; n=5. (B) Representative images of triple staining of NRCMs (red staining for ANP, pointed by arrows; green for α-actinin; blue for DAPI, scale bar: 20μm). Quantification of ANP-expressing cells or cell size is presented by the bar graphs (n=5). (C) Trypan blue staining to detect cell viability after FTY720 treatment in NRCMs infected with Ad-shPak1 or Ad-shC2 (scale bar: 100μm, n=4 independent experiments, 250 cells counted per experiment)..
Pak1 as a Novel Therapeutic Target for Antihypertrophic Treatment in the Heart
 Pak1 as a Novel Therapeutic Target for Antihypertrophic Treatment in the Heart Wei Liu, MD, PhD; Min Zi, MD; Ronald Naumann, MSc; Susanne Ulm, MSc; Jiawei Jin, PhD; Domenico M. Taglieri, MD; Sukhpal Prehar,
Pak1 as a Novel Therapeutic Target for Antihypertrophic Treatment in the Heart Wei Liu, MD, PhD; Min Zi, MD; Ronald Naumann, MSc; Susanne Ulm, MSc; Jiawei Jin, PhD; Domenico M. Taglieri, MD; Sukhpal Prehar,
Supplement Material. chain (MLC2v) promoter to generate cardiac-specific MKK4 knockout mice (referred to
 Supplement Material Materials and Methods Generation of MKK Mutant Mice The mkk-flox mice (referred to as ) were previously generated and characterised. mice were mated with mice expressing Cre under a
Supplement Material Materials and Methods Generation of MKK Mutant Mice The mkk-flox mice (referred to as ) were previously generated and characterised. mice were mated with mice expressing Cre under a
hemodynamic stress. A. Echocardiographic quantification of cardiac dimensions and function in
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure 1. Fbn1 C1039G/+ hearts display normal cardiac function in the absence of hemodynamic stress. A. Echocardiographic quantification of cardiac dimensions and
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure 1. Fbn1 C1039G/+ hearts display normal cardiac function in the absence of hemodynamic stress. A. Echocardiographic quantification of cardiac dimensions and
Protein kinase A mediated stimulation of activating transcription factor 3 by hypertrophic stimuli in cardiomyocytes
 Protein kinase A mediated stimulation of activating transcription factor 3 by hypertrophic stimuli in cardiomyocytes Elina Koivisto, MD, PhD Institute of Biomedicine, Department of Pharmacology and Toxicology
Protein kinase A mediated stimulation of activating transcription factor 3 by hypertrophic stimuli in cardiomyocytes Elina Koivisto, MD, PhD Institute of Biomedicine, Department of Pharmacology and Toxicology
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
The elements of G protein-coupled receptor systems
 The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
The elements of G protein-coupled receptor systems Prostaglandines Sphingosine 1-phosphate a receptor that contains 7 membrane-spanning domains a coupled trimeric G protein which functions as a switch
J Jpn Coll Angiol, 2009, 49:
 Online publication October 6, 2009 48 2 20 J Jpn Coll Angiol, 2009, 49: 293 297 atrial natriuretic peptide, brain natriuretic peptide, guanylyl cyclase-a receptor, cardiac remodeling, cardiac hypertrophy
Online publication October 6, 2009 48 2 20 J Jpn Coll Angiol, 2009, 49: 293 297 atrial natriuretic peptide, brain natriuretic peptide, guanylyl cyclase-a receptor, cardiac remodeling, cardiac hypertrophy
DECLARATION OF CONFLICT OF INTEREST
 DECLARATION OF CONFLICT OF INTEREST A potential anti-hypertrophic agent Riham Abouleisa Cardiac hypertrophy is a compensatory response to maintain function during increased stress. A sustained hypertrophic
DECLARATION OF CONFLICT OF INTEREST A potential anti-hypertrophic agent Riham Abouleisa Cardiac hypertrophy is a compensatory response to maintain function during increased stress. A sustained hypertrophic
Cell Signaling part 2
 15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
Probe. Hind III Q,!?R'!! /0!!!!D1"?R'! vector. Homologous recombination
 Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
Supple-Zhang Page 1 Wild-type locus Targeting construct Targeted allele Exon Exon3 Exon Probe P1 P P3 FRT FRT loxp loxp neo vector amh I Homologous recombination neo P1 P P3 FLPe recombination Q,!?R'!!
c Ischemia (30 min) Reperfusion (8 w) Supplementary Figure bp 300 bp Ischemia (30 min) Reperfusion (4 h) Dox 20 mg/kg i.p.
 a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
a Marker Ripk3 +/ 5 bp 3 bp b Ischemia (3 min) Reperfusion (4 h) d 2 mg/kg i.p. 1 w 5 w Sacrifice for IF size A subset for echocardiography and morphological analysis c Ischemia (3 min) Reperfusion (8
Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 TAC 8W TAC 2W. Postn MCM ; R26 mtmg Sham GFP Col 1/3 TAC 8W TAC 2W
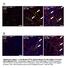 A Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 Tcf21 MCM ; R26 mtmg TAC 2W Tcf21 MCM ; R26 mtmg TAC 8W B Postn MCM ; R26 mtmg Sham GFP Col 1/3 Postn MCM ; R26 mtmg TAC 2W Postn MCM ; R26 mtmg TAC 8W Supplementary
A Tcf21 MCM ; R26 mtmg Sham GFP Col 1/3 Tcf21 MCM ; R26 mtmg TAC 2W Tcf21 MCM ; R26 mtmg TAC 8W B Postn MCM ; R26 mtmg Sham GFP Col 1/3 Postn MCM ; R26 mtmg TAC 2W Postn MCM ; R26 mtmg TAC 8W Supplementary
Biol403 MAP kinase signalling
 Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
Biol403 MAP kinase signalling The mitogen activated protein kinase (MAPK) pathway is a signalling cascade activated by a diverse range of effectors. The cascade regulates many cellular activities including
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
Cardiac hypertrophy is an early hallmark of the clinical
 Regulator of G Protein Signaling Facilities Cardiac Hypertrophy by Activating Apoptosis Signal Regulating Kinase 1 P38/c-JUN N-Terminal Kinase 1/ Signaling Zhijun Huang, MD;* Jingxian Shu, MS;* Weihong
Regulator of G Protein Signaling Facilities Cardiac Hypertrophy by Activating Apoptosis Signal Regulating Kinase 1 P38/c-JUN N-Terminal Kinase 1/ Signaling Zhijun Huang, MD;* Jingxian Shu, MS;* Weihong
Postn MCM Smad2 fl/fl Postn MCM Smad3 fl/fl Postn MCM Smad2/3 fl/fl. Postn MCM. Tgfbr1/2 fl/fl TAC
 A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity
 Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity Yuhee Ryu 1,+, Li Jin 1,2+, Hae Jin Kee 1,, Zhe Hao Piao 3, Jae
Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity Yuhee Ryu 1,+, Li Jin 1,2+, Hae Jin Kee 1,, Zhe Hao Piao 3, Jae
Principles of Genetics and Molecular Biology
 Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Exercise in Adverse Cardiac Remodeling: of Mice and Men
 Exercise in Adverse Cardiac Remodeling: of Mice and Men 17-01-2013 Dirk J Duncker Experimental Cardiology, Cardiology, Thoraxcenter Cardiovascular Research Institute COEUR Erasmus MC, University Medical
Exercise in Adverse Cardiac Remodeling: of Mice and Men 17-01-2013 Dirk J Duncker Experimental Cardiology, Cardiology, Thoraxcenter Cardiovascular Research Institute COEUR Erasmus MC, University Medical
Supplementary Figure 1:
 Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Supplementary Figure 1: (A) Whole aortic cross-sections stained with Hematoxylin and Eosin (H&E), 7 days after porcine-pancreatic-elastase (PPE)-induced AAA compared to untreated, healthy control aortas
Signal Transduction: G-Protein Coupled Receptors
 Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
Signal Transduction: G-Protein Coupled Receptors Federle, M. (2017). Lectures 4-5: Signal Transduction parts 1&2: nuclear receptors and GPCRs. Lecture presented at PHAR 423 Lecture in UIC College of Pharmacy,
Growth and Differentiation Phosphorylation Sampler Kit
 Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Growth and Differentiation Phosphorylation Sampler Kit E 0 5 1 0 1 4 Kits Includes Cat. Quantity Application Reactivity Source Akt (Phospho-Ser473) E011054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Fetal gene upregulation by 1-wk TAC is significantly increased in mice lacking RGS2.
 3562-RG-1 Supplementary Figure 1 Fetal gene upregulation by 1-wk is significantly increased in mice lacking RGS2. ANP(Nppa) /BNP(Nppb) A-type and B-type natriuretic peptide; β-mhc (Myh7) beta myosin heavy
3562-RG-1 Supplementary Figure 1 Fetal gene upregulation by 1-wk is significantly increased in mice lacking RGS2. ANP(Nppa) /BNP(Nppb) A-type and B-type natriuretic peptide; β-mhc (Myh7) beta myosin heavy
E10.5 E18.5 P2 10w 83w NF1 HF1. Sham ISO. Bmi1. H3K9me3. Lung weight (g)
 Myociyte cross-sectional Relative mrna levels Relative levels Relative mrna levels Supplementary Figures and Legends a 8 6 4 2 Ezh2 E1.5 E18.5 P2 1w 83w b Ezh2 p16 amhc b-actin P2 43w kd 37 86 16 wt mouse
Myociyte cross-sectional Relative mrna levels Relative levels Relative mrna levels Supplementary Figures and Legends a 8 6 4 2 Ezh2 E1.5 E18.5 P2 1w 83w b Ezh2 p16 amhc b-actin P2 43w kd 37 86 16 wt mouse
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Signaling. Dr. Sujata Persad Katz Group Centre for Pharmacy & Health research
 Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Signaling Dr. Sujata Persad 3-020 Katz Group Centre for Pharmacy & Health research E-mail:sujata.persad@ualberta.ca 1 Growth Factor Receptors and Other Signaling Pathways What we will cover today: How
Supplementary Figure 1. Deletion of Smad3 prevents B16F10 melanoma invasion and metastasis in a mouse s.c. tumor model.
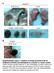 A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-nfat signaling
 Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-nfat signaling Julian C. Braz, 1,2 Orlando F. Bueno, 1 Qiangrong Liang, 1 Benjamin J. Wilkins, 1
Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-nfat signaling Julian C. Braz, 1,2 Orlando F. Bueno, 1 Qiangrong Liang, 1 Benjamin J. Wilkins, 1
MAPK Pathway
 MAPK Pathway Mitogen-activated protein kinases (MAPK) are proteins that are serine/ threonine specific kinases which are activated by a wide range of stimuli including proinflammatory cytokines, growth
MAPK Pathway Mitogen-activated protein kinases (MAPK) are proteins that are serine/ threonine specific kinases which are activated by a wide range of stimuli including proinflammatory cytokines, growth
SUPPLEMENTARY INFORMATION
 a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
Remodeling the failing heart: : the biology and future treatment options
 Remodeling the failing heart: : the biology and future treatment options J-L Balligand (UCL-Brussels, BE) jl.balligand@uclouvain.be Myocardial remodeling: definitions phenotypic plasticity : remodeling
Remodeling the failing heart: : the biology and future treatment options J-L Balligand (UCL-Brussels, BE) jl.balligand@uclouvain.be Myocardial remodeling: definitions phenotypic plasticity : remodeling
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Supplementary Figure 1 (Related with Figure 4). Molecular consequences of Eed deletion. (a) ChIP analysis identifies 3925 genes that are associated
 Supplementary Figure 1 (Related with Figure 4). Molecular consequences of Eed deletion. (a) ChIP analysis identifies 3925 genes that are associated with the H3K27me3 mark in chondrocytes (see Table S1,
Supplementary Figure 1 (Related with Figure 4). Molecular consequences of Eed deletion. (a) ChIP analysis identifies 3925 genes that are associated with the H3K27me3 mark in chondrocytes (see Table S1,
Receptor mediated Signal Transduction
 Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/375/ra41/dc1 Supplementary Materials for Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice
www.sciencesignaling.org/cgi/content/full/8/375/ra41/dc1 Supplementary Materials for Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice
KEY CONCEPT QUESTIONS IN SIGNAL TRANSDUCTION
 Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Supplementary Figures Supplementary Figure 1. Development of the camp biosensor targeted to the SERCA2a microdomain.
 Supplementary Figures Supplementary Figure 1. Development of the camp biosensor targeted to the SERCA2a microdomain. A B C (A) Schematic representation of the new constructs designed for local camp imaging.
Supplementary Figures Supplementary Figure 1. Development of the camp biosensor targeted to the SERCA2a microdomain. A B C (A) Schematic representation of the new constructs designed for local camp imaging.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
Impact factor: Reporter:4A1H0019 Chen Zi Hao 4A1H0023 Huang Wan ting 4A1H0039 Sue Yi Zhu 4A1H0070 Lin Guan cheng 4A1H0077 Chen Bo xuan
 Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
In vivo bromodeoxyuridine (BrdU) incorporation was performed to analyze cell
 Supplementary Methods BrdU incorporation in vivo In vivo bromodeoxyuridine (BrdU) incorporation was performed to analyze cell proliferation in the heart. Mice were subjected to LI-TAC, and 5 days later
Supplementary Methods BrdU incorporation in vivo In vivo bromodeoxyuridine (BrdU) incorporation was performed to analyze cell proliferation in the heart. Mice were subjected to LI-TAC, and 5 days later
SUPPLEMENTARY INFORMATION
 1. Supplementary Figures and Legends Supplementary Fig. 1. S1P-mediated transcriptional regulation of integrins expressed in OP/monocytoid cells. Real-time quantitative PCR analyses of mrna for two integrins,
1. Supplementary Figures and Legends Supplementary Fig. 1. S1P-mediated transcriptional regulation of integrins expressed in OP/monocytoid cells. Real-time quantitative PCR analyses of mrna for two integrins,
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous
 Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
G-Protein Signaling. Introduction to intracellular signaling. Dr. SARRAY Sameh, Ph.D
 G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
In Vivo Animal Models of Heart Disease. Why Animal Models of Disease? Timothy A Hacker, PhD Department of Medicine University of Wisconsin-Madison
 In Vivo Animal Models of Heart Disease Timothy A Hacker, PhD Department of Medicine University of Wisconsin-Madison Why Animal Models of Disease? Heart Failure (HF) Leading cause of morbidity and mortality
In Vivo Animal Models of Heart Disease Timothy A Hacker, PhD Department of Medicine University of Wisconsin-Madison Why Animal Models of Disease? Heart Failure (HF) Leading cause of morbidity and mortality
Signal Transduction Cascades
 Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
PHSI3009 Frontiers in Cellular Physiology 2017
 Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Overview of PHSI3009 L2 Cell membrane and Principles of cell communication L3 Signalling via G protein-coupled receptor L4 Calcium Signalling L5 Signalling via Growth Factors L6 Signalling via small G-protein
Chapter 9. Cellular Signaling
 Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Qualifying Examination (Part I)
 Department of Pharmacology Qualifying Examination (Part I) December 12 & 13, 2006 Please remember that this is a closed-book examination. You must be prepared to answer 4 of the 7 questions. Although not
Department of Pharmacology Qualifying Examination (Part I) December 12 & 13, 2006 Please remember that this is a closed-book examination. You must be prepared to answer 4 of the 7 questions. Although not
AP VP DLP H&E. p-akt DLP
 A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
Lecture 15. Signal Transduction Pathways - Introduction
 Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex
 Research article Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex Hui-Hua Li, 1 Vishram Kedar, 1 Chunlian Zhang, 1 Holly
Research article Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex Hui-Hua Li, 1 Vishram Kedar, 1 Chunlian Zhang, 1 Holly
DECLARATION OF CONFLICT OF. No disclosure INTEREST
 DECLARATION OF CONFLICT OF No disclosure INTEREST ESC Congress 2011 2011.8.28 Impaired vacuolar H + -ATPase function causes cardiomyocyte death with extensive vacuolation and impaired autophagic degradation
DECLARATION OF CONFLICT OF No disclosure INTEREST ESC Congress 2011 2011.8.28 Impaired vacuolar H + -ATPase function causes cardiomyocyte death with extensive vacuolation and impaired autophagic degradation
The role of mirnas in cardiac hypertrophy
 The role of mirnas in cardiac hypertrophy Fabian Pruissen Biology of Disease 2011 Supervised by Sjoukje Lok, MD and Roel de Weger, PhD Abstract Cardiac hypertrophy and increased cardiomyocytes size are
The role of mirnas in cardiac hypertrophy Fabian Pruissen Biology of Disease 2011 Supervised by Sjoukje Lok, MD and Roel de Weger, PhD Abstract Cardiac hypertrophy and increased cardiomyocytes size are
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Stress-dependent cardiac remodeling occurs in the absence of microrna-21 in mice
 Brief report Related Commentary, page 3817 Stress-dependent cardiac remodeling occurs in the absence of microrna-21 in mice David M. Patrick, 1 Rusty L. Montgomery, 2 Xiaoxia Qi, 1 Susanna Obad, 3 Sakari
Brief report Related Commentary, page 3817 Stress-dependent cardiac remodeling occurs in the absence of microrna-21 in mice David M. Patrick, 1 Rusty L. Montgomery, 2 Xiaoxia Qi, 1 Susanna Obad, 3 Sakari
TITLE: A Mouse Model to Investigate the Role of DBC2 in Breast Cancer
 AD Award Number: W81XWH-04-1-0325 TITLE: A Mouse Model to Investigate the Role of DBC2 in Breast Cancer PRINCIPAL INVESTIGATOR: Valerie Boka CONTRACTING ORGANIZATION: University of Texas Health Science
AD Award Number: W81XWH-04-1-0325 TITLE: A Mouse Model to Investigate the Role of DBC2 in Breast Cancer PRINCIPAL INVESTIGATOR: Valerie Boka CONTRACTING ORGANIZATION: University of Texas Health Science
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin
 Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
Model Answer. M.Sc. Zoology (First Semester) Examination Paper LZT 103 (Endocrinology)
 Model Answer M.Sc. Zoology (First Semester) Examination-2013 Paper LZT 103 (Endocrinology) Section A 1. (i) d (ii) b (iii) b (iv) c (v) c (vi) a (vii) c (viii) a (ix) d (x) b Section B Q.2 Answer Hormonal
Model Answer M.Sc. Zoology (First Semester) Examination-2013 Paper LZT 103 (Endocrinology) Section A 1. (i) d (ii) b (iii) b (iv) c (v) c (vi) a (vii) c (viii) a (ix) d (x) b Section B Q.2 Answer Hormonal
Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity
 Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Control. csarnt -/- Cre, f/f
 ody weight (g) A re,f/f re x f/f f/+ re, f/+ re,f/+ f/f x f/f f/+ cs -/- re, f/f re f/f re, f/f Normal chow Tamoxifen Tamoxifen Tamoxifen W 4W re f/f re, re/ff f/f re f/f re, re/ff f/f Normal chow Tamoxifen
ody weight (g) A re,f/f re x f/f f/+ re, f/+ re,f/+ f/f x f/f f/+ cs -/- re, f/f re f/f re, f/f Normal chow Tamoxifen Tamoxifen Tamoxifen W 4W re f/f re, re/ff f/f re f/f re, re/ff f/f Normal chow Tamoxifen
Revision. camp pathway
 االله الرحمن الرحيم بسم Revision camp pathway camp pathway Revision camp pathway Adenylate cyclase Adenylate Cyclase enzyme Adenylate cyclase catalyses the formation of camp from ATP. Stimulation or inhibition
االله الرحمن الرحيم بسم Revision camp pathway camp pathway Revision camp pathway Adenylate cyclase Adenylate Cyclase enzyme Adenylate cyclase catalyses the formation of camp from ATP. Stimulation or inhibition
Phospho-AKT Sampler Kit
 Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Phospho-AKT Sampler Kit E 0 5 1 0 0 3 Kits Includes Cat. Quantity Application Reactivity Source Akt (Ab-473) Antibody E021054-1 50μg/50μl IHC, WB Human, Mouse, Rat Rabbit Akt (Phospho-Ser473) Antibody
Kidney. Heart. Lung. Sirt1. Gapdh. Mouse IgG DAPI. Rabbit IgG DAPI
 a e Na V 1.5 Ad-LacZ Ad- 110KD b Scn5a/ (relative to Ad-LacZ) f 150 100 50 0 p = 0.65 Ad-LacZ Ad- c Heart Lung Kidney Spleen 110KD d fl/fl c -/- DAPI 20 µm Na v 1.5 250KD fl/fl Rabbit IgG DAPI fl/fl Mouse
a e Na V 1.5 Ad-LacZ Ad- 110KD b Scn5a/ (relative to Ad-LacZ) f 150 100 50 0 p = 0.65 Ad-LacZ Ad- c Heart Lung Kidney Spleen 110KD d fl/fl c -/- DAPI 20 µm Na v 1.5 250KD fl/fl Rabbit IgG DAPI fl/fl Mouse
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Corporate Medical Policy
 Corporate Medical Policy ST2 Assay for Chronic Heart Failure File Name: Origination: Last CAP Review: Next CAP Review: Last Review: st_assay_for_chronic_heart_failure 2/2015 4/2018 4/2019 4/2018 Description
Corporate Medical Policy ST2 Assay for Chronic Heart Failure File Name: Origination: Last CAP Review: Next CAP Review: Last Review: st_assay_for_chronic_heart_failure 2/2015 4/2018 4/2019 4/2018 Description
INTERACTION DRUG BODY
 INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
Supplementary Information Titles Journal: Nature Medicine
 Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Information Titles Journal: Nature Medicine Article Title: Corresponding Author: Supplementary Item & Number Supplementary Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6 Fig.7 Fig.8 Fig.9 Fig. Fig.11
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus
 Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
supplementary information
 DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
SUPPLEMENTARY INFORMATION
 In the format provided by the authors and unedited. 2 3 4 DOI: 10.1038/NMAT4893 EGFR and HER2 activate rigidity sensing only on rigid matrices Mayur Saxena 1,*, Shuaimin Liu 2,*, Bo Yang 3, Cynthia Hajal
In the format provided by the authors and unedited. 2 3 4 DOI: 10.1038/NMAT4893 EGFR and HER2 activate rigidity sensing only on rigid matrices Mayur Saxena 1,*, Shuaimin Liu 2,*, Bo Yang 3, Cynthia Hajal
The Tissue Engineer s Toolkit
 The Tissue Engineer s Toolkit Stimuli Detection and Response Ken Webb, Ph. D. Assistant Professor Dept. of Bioengineering Clemson University Environmental Stimulus-Cellular Response Environmental Stimuli
The Tissue Engineer s Toolkit Stimuli Detection and Response Ken Webb, Ph. D. Assistant Professor Dept. of Bioengineering Clemson University Environmental Stimulus-Cellular Response Environmental Stimuli
Cardiac development begins when mesodermal progenitor
 Molecular Medicine MicroRNA-22 Regulates Cardiac Hypertrophy and Remodeling in Response to Stress Zhan-Peng Huang,* Jinghai Chen,* Hee Young Seok, Zheng Zhang, Masaharu Kataoka, Xiaoyun Hu, Da-Zhi Wang
Molecular Medicine MicroRNA-22 Regulates Cardiac Hypertrophy and Remodeling in Response to Stress Zhan-Peng Huang,* Jinghai Chen,* Hee Young Seok, Zheng Zhang, Masaharu Kataoka, Xiaoyun Hu, Da-Zhi Wang
Supplementary Materials
 Supplementary Materials Figure S1. MTT Cell viability assay. To measure the cytotoxic potential of the oxidative treatment, the MTT [3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide] assay
Supplementary Materials Figure S1. MTT Cell viability assay. To measure the cytotoxic potential of the oxidative treatment, the MTT [3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide] assay
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Uncovering the mechanisms of wound healing and fibrosis
 Any Questions??? Ask now or contact support support@sabiosciences.com 1-888-503-3187 International customers: SABio@Qiagen.com Uncovering the mechanisms of wound healing and fibrosis Webinar related questions:
Any Questions??? Ask now or contact support support@sabiosciences.com 1-888-503-3187 International customers: SABio@Qiagen.com Uncovering the mechanisms of wound healing and fibrosis Webinar related questions:
Biosignals, Chapter 8, rearranged, Part I
 Biosignals, Chapter 8, rearranged, Part I Nicotinic Acetylcholine Receptor: A Ligand-Binding Ion Channel Classes of Receptor Proteins in Eukaryotes, Heterotrimeric G Proteins Signaling View the Heterotrimeric
Biosignals, Chapter 8, rearranged, Part I Nicotinic Acetylcholine Receptor: A Ligand-Binding Ion Channel Classes of Receptor Proteins in Eukaryotes, Heterotrimeric G Proteins Signaling View the Heterotrimeric
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy se
 A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse
 Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
m 6 A mrna methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer
 SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41556-018-0174-4 In the format provided by the authors and unedited. m 6 A mrna methylation regulates AKT activity to promote the proliferation
SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41556-018-0174-4 In the format provided by the authors and unedited. m 6 A mrna methylation regulates AKT activity to promote the proliferation
of TERT, MLL4, CCNE1, SENP5, and ROCK1 on tumor development were discussed.
 Supplementary Note The potential association and implications of HBV integration at known and putative cancer genes of TERT, MLL4, CCNE1, SENP5, and ROCK1 on tumor development were discussed. Human telomerase
Supplementary Note The potential association and implications of HBV integration at known and putative cancer genes of TERT, MLL4, CCNE1, SENP5, and ROCK1 on tumor development were discussed. Human telomerase
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein
 Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Problem Set 8 Key 1 of 8
 7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
Synthesis and Biological Evaluation of Protein Kinase D Inhibitors
 Synthesis and Biological Evaluation of Protein Kinase D Inhibitors Celeste Alverez Topic Seminar October 26, 2013 Celeste Alverez @ Wipf Group 10/26/2013 1 Protein Kinase D (PKD) A novel family of serine/threonine
Synthesis and Biological Evaluation of Protein Kinase D Inhibitors Celeste Alverez Topic Seminar October 26, 2013 Celeste Alverez @ Wipf Group 10/26/2013 1 Protein Kinase D (PKD) A novel family of serine/threonine
Heart disease is frequently associated with elevated wall
 Phosphoinositide 3-Kinase Deficient Mice Are Protected From Isoproterenol-Induced Heart Failure Gavin Y. Oudit, MSc, MD; Michael A. Crackower, PhD; Urs Eriksson, MD; Renu Sarao, MSc; Ivona Kozieradzki,
Phosphoinositide 3-Kinase Deficient Mice Are Protected From Isoproterenol-Induced Heart Failure Gavin Y. Oudit, MSc, MD; Michael A. Crackower, PhD; Urs Eriksson, MD; Renu Sarao, MSc; Ivona Kozieradzki,
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Genomic analysis of essentiality within protein networks
 Genomic analysis of essentiality within protein networks Haiyuan Yu, Dov Greenbaum, Hao Xin Lu, Xiaowei Zhu and Mark Gerstein Department of Molecular Biophysics and Biochemistry, 266 Whitney Avenue, Yale
Genomic analysis of essentiality within protein networks Haiyuan Yu, Dov Greenbaum, Hao Xin Lu, Xiaowei Zhu and Mark Gerstein Department of Molecular Biophysics and Biochemistry, 266 Whitney Avenue, Yale
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Chapter 20. Cell - Cell Signaling: Hormones and Receptors. Three general types of extracellular signaling. endocrine signaling. paracrine signaling
 Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
Chapter 20 Cell - Cell Signaling: Hormones and Receptors Three general types of extracellular signaling endocrine signaling paracrine signaling autocrine signaling Endocrine Signaling - signaling molecules
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
doi: /nature14508 Rappsilber et al.
 SUPPLEMENTARY INFORMATION doi:1.138/nature1458 Grosso et al. Barbosa et al. 74 72 45 33 47 7 51 Rappsilber et al. Supplementary Figure 1 a, Venn-Diagram of identified splice factors in the work of Barbossa
SUPPLEMENTARY INFORMATION doi:1.138/nature1458 Grosso et al. Barbosa et al. 74 72 45 33 47 7 51 Rappsilber et al. Supplementary Figure 1 a, Venn-Diagram of identified splice factors in the work of Barbossa
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3021 Supplementary figure 1 Characterisation of TIMPless fibroblasts. a) Relative gene expression of TIMPs1-4 by real time quantitative PCR (RT-qPCR) in WT or ΔTimp fibroblasts (mean ±
DOI: 10.1038/ncb3021 Supplementary figure 1 Characterisation of TIMPless fibroblasts. a) Relative gene expression of TIMPs1-4 by real time quantitative PCR (RT-qPCR) in WT or ΔTimp fibroblasts (mean ±
