Role of Xrx1 in Xenopus eye and anterior brain development
|
|
|
- Jonah Richard
- 5 years ago
- Views:
Transcription
1 Development 126, (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV Role of Xrx1 in Xenopus eye and anterior brain development Massimiliano Andreazzoli 1,3, Gaia Gestri 1, Debora Angeloni 2, *, Elisabetta Menna 1 and Giuseppina Barsacchi 1, 1 Laboratorio di Biologia Cellulare e dello Sviluppo, Universita di Pisa, Via Carducci 13, Ghezzano, Pisa, Italy 2 Cattedra di Chemioterapia, Universita di Milano, Via Vanvitelli 32, Milano, Italy 3 Laboratory of Molecular Genetics, National Institute of Child Health and Human Development, NIH, Bethesda, MD 20892, USA *Present address: Laboratory of Immunobiology, National Cancer Institute, FCRF, Frederick, MD 21702, USA Author for correspondence ( gbarsa@dfb.unipi.it) Accepted 1 March; published on WWW 4 May 1999 SUMMARY The anteriormost part of the neural plate is fated to give rise to the retina and anterior brain regions. In Xenopus, this territory is initially included within the expression domain of the bicoid-class homeobox gene Xotx2 but very soon, at the beginning of neurulation, it becomes devoid of Xotx2 transcripts in spatiotemporal concomitance with the transcriptional activation of the paired-like homeobox gene Xrx1. By use of gain- and loss-of-function approaches, we have studied the role played by Xrx1 in the anterior neural plate and its interactions with other anterior homeobox genes. We find that, at early neurula stage Xrx1 is able to repress Xotx2 expression, thus first defining the retinadiencephalon territory in the anterior neural plate. Overexpression studies indicate that Xrx1 possesses a proliferative activity that is coupled with the specification of anterior fate. Expression of a Xrx1 dominant repressor construct (Xrx1-EnR) results in a severe impairment of eye and anterior brain development. Analysis of several brain markers in early Xrx1-EnR-injected embryos reveals that anterior deletions are preceded by a reduction of anterior gene expression domains in the neural plate. Accordingly, expression of anterior markers is abolished or decreased in animal caps coinjected with the neural inducer chordin and the Xrx1-EnR construct. The lack of expansion of midhindbrain markers, and the increase of apoptosis in the anterior neural plate after Xrx1-EnR injection, indicate that anterior deletions result from an early loss of anterior neural plate territories rather than posteriorization of the neuroectoderm. Altogether, these data suggest that Xrx1 plays a role in assigning anterior and proliferative properties to the rostralmost part of the neural plate, thus being required for eye and anterior brain development. Key words: Xrx1, Rx1, Otx2, Pax6, Six3, engrailed repressor, Xenopus laevis, Anterior neural plate patterning, Eye, Forebrain INTRODUCTION Eye development is a multistep process that requires specific inductive signals and precise morphogenetic movements. Although classical experimental embryology studies have been fundamental to our current knowledge of eye formation (see Spemann, 1938), it is only recently that the genetic bases underlying this complex phenomenon have begun to be unraveled. Integration of genetic and developmental biology studies performed in Drosophila and vertebrates suggested that key regulatory genes in eye development have been conserved during evolution. Among these genes eyeless (ey), sine oculis (so), eyes absent (eya) and dachshund (dac) appear to play important roles in Drosophila as they have been shown to be necessary for eye formation and sufficient, when overexpressed, to induce ectopic eyes. Vertebrate homologues of ey (Pax6), so (six gene family) and eya (Eya genes) have been described and, at least in the case of Pax6 and Six3, a functional role in vertebrate eye development has also been shown (reviewed in Oliver and Gruss, 1997). In vertebrates, the initial function of master regulators of eye development has probably to take place at early neurula stage, when the presumptive eye territories are first clearly determined. In fact, lineage tracing studies performed in Xenopus have identified the anterior neural plate as the region fated to give rise to the retina and the anterior brain (Eagleson and Harris, 1990; Eagleson et al., 1995). The expression domains of some homeobox genes appear to pattern the Xenopus anterior neural plate already at early neurula stage. This is the case of Xotx genes, expressed in presumptive forebrain and midbrain regions (Kablar et al., 1996) and XBF- 1, Xdll-3 and Xemx genes (Papalopulu and Kintner, 1993, 1996; Pannese et al., 1998), expressed in different presumptive forebrain areas. Another restricted class of homeobox genes is expressed in the most anterior part of the neural plate mainly confined to the eye prospective territories. Members of this class are the Xenopus homologues of Pax6 (Xpax6, Hirsch and Harris, 1997; Li et al., 1997) and Six3 (Xsix3, this work) as well as the recently isolated paired-like homeobox gene Xrx1 (Casarosa et al., 1997; Mathers et al., 1997). Identifying the network of interactions occurring between these genes, represents a primary goal toward the understanding of eye and anterior brain patterning mechanisms.
2 2452 M. Andreazzoli and others We previously showed that Xrx1 expression is first activated at the early neurula stage in the anterior neural plate by vertical signals from the dorsal mesoendoderm. Later on, Xrx1 transcripts are detected in the neural structures of the developing eye and other anterior neural plate derivatives such as the pineal gland, the diencephalon floor and the hypophysis (Casarosa et al., 1997). Functional studies on this gene have shown that its overexpression in Xenopus results in ectopic retinal and neural tissue formation, while mouse embryos carrying a null allele of the Xrx1 murine homologue, lack optic cups and display a reduction of brain structures (Mathers et al., 1997). Nevertheless, until now the functional relationship between Xrx1 and other homeobox genes in patterning the anterior neural plate has not been investigated. In the present work, we address the early role of Xrx1 and regulatory interactions occurring between Xrx1 and other anterior homeobox genes, making use of gain- and loss-offunction approaches available in the Xenopus system. Overexpression experiments, including analysis of anterior markers, indicate that Xrx1 proliferative activity is linked to the specification of anterior fate. Inactivation of Xrx1 function, performed microinjecting RNA encoding a Xrx1 engrailed repressor fusion protein (Xrx1-EnR), leads to a remarkable reduction of anterior neural plate territories, as judged by the expression of several anterior markers, causing a failure in eye and anterior brain formation. Analysis of apoptosis and hindbrain markers expression indicate that these effects are due to an early loss of anterior territories rather than a posteriorization of the neuroectoderm. Finally, we propose that Xrx1 plays an early role defining prosencephalic territories in combination with Xotx2, Xpax6, Xsix3 and XBF-1 in the anterior neural plate. MATERIALS AND METHODS Embryos and histology Induction of ovulation of females, in vitro fertilisation and embryo culture were carried out as described by Newport and Kirschner (1982). Staging was according to Nieuwkoop and Faber (1967). Histological examination was performed according to Casarosa et al. (1997). Whole-mount in situ hybridization Whole-mount in situ hybridization was performed on staged embryos, as well as on animal caps, essentially as described by Harland (1991). The antisense or control sense-strand RNA probes from Xotx2 (Pannese et al., 1995), XAG-1 (Sive et al., 1989), XBF-1 (Papalopulu and Kintner, 1996), Krox20 (Bradley et al., 1993), En2 (Hemmati- Brivanlou et al., 1991), gsc (Cho et al., 1991) and Xotx-b (kindly provided by Dr R. Harland) were generated from linearized plasmids using either digoxigenin or fluorescein RNA labelling mix (Boehringer). For double whole-mount in situ hybridization, the embryos were hybridized with both probes at the same time under standard conditions. After detection of the first probe with BM purple (Boehringer), the alkaline phosphatase was inactivated in 100 mm glycine ph 2.2, 0.1% Tween-20 and the embryos blocked in MAB (100 mm maleic acid, 150 mm NaCl), 2% Boehringer blocking reagent and 20% heat-inactivated sheep serum. Following incubation with the second antibody, the alkaline phosphatase reaction was performed with Magenta-Phos (Sigma). In some cases (Fig. 1M-O), RNA encoding for β-galactosidase containing a nuclear localization signal was used as a tracer. Preparation of constructs and PCR cloning Capped sense Xrx1 RNA was generated from T7TS-Xrx1 clone consisting of the full-length Xrx1 cdna cloned in the expression vector T7TS. Capped antisense RNA for Xrx1 was prepared from CS2-ASXrx1 obtained subcloning the full-length cdna into CS2+ vector (Rupp et al., 1994). The Xrx1 construct lacking the OAR domain ( OAR) was prepared subcloning the AvaII fragment from T7TS-Xrx1 into CS2+ plasmid. The Xrx1-EnR construct was prepared subcloning the AvaII fragment from T7TS-Xrx1 in frame with the Drosophila engrailed repressor sequence (amino acids 1-296) contained in ENG-N vector (a kind gift of Dr Dan Kessler). Xsix3, Xpax2 and Xpax6 were cloned by RT-PCR from Xenopus stage 19 RNA. PCR products were subcloned using a pgem-t vector system (Promega). Degenerate primers used for Xsix3 amplification were designed based upon the amino acid sequences WPPGACEA and AMWLEAHYQ, which are specifically found only in mouse Six3 and not in other members of the Six family. Over the amplified region, Xsix3 predicted amino acid sequence was 100% identical to zebrafish Six3 (Seo et al., 1998) and 97% to mouse (Oliver et al., 1995) and chick (Bovolenta et al., 1998) Six3. For Xpax2 amplification, degenerate primers based upon the evolutionary conserved Pax2 amino acid sequences IIRTKVQQ and YPTSTLAG were used. Xpax2 amplified region was identical to the Xpax-2a (3) nucleotide sequence described by Heller and Brandli (1997). Similarly, Xpax6 was amplified using degenerate primers corresponding to the Pax6 evolutionary conserved amino acid sequences NLASEKQQ and QIEALEKE. Xpax6 amplified region was found to be identical to Xenopus Pax6 nucleotide sequence deposited in GenBank by Hollemann, Bellefroid and Pieler, GenBank accession number U Embryo microinjections and animal cap assay Capped synthetic RNAs were generated by in vitro transcription of CS2-ASXrx1, T7TS-Xrx1, OAR, Xrx1-EnR, T7TS-Xotx2 (Pannese et al., 1995) and chordin (Sasai et al., 1995). Xpax6 capped RNA was transcribed from P6mycS (Hirsch and Harris, 1997), a generous gift of Dr William Harris, and its activity was tested by the ability to induce ectopic β-b1 crystallin expression (not shown, see Altman et al., 1997). Embryo microinjections were performed as described in Andreazzoli et al. (1997). Animal caps were dissected out of stage 8-9 embryos in 1 MBS and, after healing, they were cultured in 0.5 MBS. When sibling control embryos reached stage 12.5, animal caps were fixed and stored in ethanol at 20 C. TUNEL staining Whole-mount TUNEL staining was performed as described in Hensey and Gautier (1998). RESULTS Effects of Xrx1 RNA microinjection on the expression of anterior genes Xrx1 is expressed in the anterior neural plate at the end of gastrulation and subsequently in the neural structures of the developing eye and other forebrain structures derived from the anterior neural plate (Casarosa et al., 1997; Mathers et al., 1997). This expression pattern raises the question whether Xrx1 plays a role in the specification of anterior neural plate regions and structures that derive from it. As a first approach to study the role of Xrx1 during development, we overexpressed this gene by microinjection of its capped RNA into single blastomeres of 2-, 4- and 8-cell stage Xenopus embryos. We found that tadpoles developed from embryos injected, at 8-cell stage, into a dorsal animal blastomere, which
3 Xrx1 in anterior neural plate patterning 2453 is fated to give rise to dorsoanterior regions of the embryo (Huang and Moody, 1993), very frequently show the presence of ectopic pigmented epithelium (Fig. 1A,B; Table 1) often associated with an overproliferating neural retina and neural tube (Fig. 1C and data not shown), in agreement with the work done by Mathers et al. (1997). Despite of a broader distribution of the injected RNA, the ectopic pigmented epithelium and neural tissue are always localized in a region comprising tissue between the eye and the brain. This area derives from a region of the anterior neural plate that is initially competent to become retina but this fate is later suppressed by signals coming from the prechordal plate (Li et al., 1997). These observations raise the possibility that Xrx1 may be able to exert its function only in a spatially restricted area because it needs to interact with other eye-brain-specific transcription factors. For this reason, we analyzed the expression of several anterior genes in Xrx1- injected embryos at early neurula (stage 13) and tailbud (stage 24) stages. Embryos injected with Xrx1 RNA in a dorsal-animal blastomere at 8-cell stage were analyzed at later stages by double whole-mount in situ hybridization using as probes digoxigenin-labelled antisense RNA for the gene of interest, together with fluorescein-labelled antisense Xrx1 RNA. This allowed us to detect both the distribution of injected Xrx1 RNA (magenta staining) and the expression of the gene that we wanted to test (blue staining). Since the signal given by hybridization of Xrx1 antisense probe with the injected RNA Fig. 1. Effects of Xrx1 overexpression. (A-C) Embryos microinjected with Xrx1 RNA in the right dorsoanimal blastomere at 8-cell stage. The arrows point to ectopic pigmented retina located between eye and diencephalon. (B,C) Transverse sections of an embryo similar to the one shown in A; (B) a section where the ectopic pigmented retina is visible (arrow); (C) a more posterior section where the duplication of the neural tube becomes evident (arrowhead). (D-O) Whole-mount in situ hybridization analysis of embryos microinjected with Xrx1 RNA at 8-cell stage in one dorsoanimal blastomere. (D-K ) The staining pattern of the gene of interest (blue) on the injected side (i) should be compared with that on the uninjected control side (c). In the same panels, the distribution of Xrx1-injected RNA is visualized by cohybridization with Xrx1 antisense RNA revealed by magenta staining. (M-O) Nuclear β-gal RNA has been used as a tracer and the β-galactosidase activity is represented by the red staining. (D-F) Expression of Xpax6 (blue) in Xrx1-injected embryos. (D) Xpax6 expression at stage 13 is not significantly affected by Xrx1 overexpression; (E,F) Xpax6 expression in the injected and control side of a stage 23 embryo, respectively. Note that the normal Xpax6 gap of expression in the midbrain present in the control side (area between lines) has been filled by ectopic Xpax6 expression in the injected side of the embryo. (G-I) Expression of Xsix3 (blue) in Xrx1-injected embryos. (G) Expression of Xsix3 at stage 13 is not affected by Xrx1 RNA injection. (H,I) Xsix3 expression in the injected and control side of a stage 23 embryo, respectively. The eye expression of Xsix3 in the injected side appears expanded dorsoposteriorly when compared to the control side. (J,K,K ) Expression of Xotx2 (blue) in Xrx1-injected embryos. (J) Expression of Xotx2 at stage 13 is repressed by Xrx1 overexpression. (K,K ) Examples of Xotx2 expression in stage 23 Xrx1-injected embryos where Xotx2 expression is extended laterally (K) or posteriorly (K ) in the injected side. The white line marks the posterior boundary of Xotx2 expression in the control side. (L) Double whole-mount in situ hybridization performed on a stage 13 normal embryo showing the complementarity of Xrx1 (magenta) and Xotx2 (blue) expression domains. (M,N) Expression of XAG-1 (blue) in Xrx1-injected embryos. (M) Expression of XAG-1 is repressed at stage 13 by Xrx1 overexpression. (N) XAG-1 is ectopically activated in the injected side of a stage 23 embryo. (O) Expression of XBF-1 (blue) in Xrx1-injected embryos at stage 13. XBF-1 expression is expanded laterally on the injected side. was stronger and appeared much earlier than endogenous Xrx1 signal, the staining reaction could be stopped when only injected Xrx1 RNA was detected. Analysis of Xpax6 expression in Xrx1-injected embryos showed that, while at stage 13 the expression of this gene does not appear to be affected (77% normal expression, 23% slightly reduced expression, n=93; Fig. 1D), an effect is observed at stage 24. At this later stage, Xpax6 normal expression, which can be seen in the uninjected control side of the embryos, is detected in the optic vesicle, forebrain, hindbrain and spinal cord with a characteristic gap of expression in the midbrain (Fig. 1F). Interestingly, inspection of the injected side of the embryos revealed that Xpax6 expression had expanded dorsally to the eye vesicle, thus filling the gap (67%, n=30; Fig. 1E). A similar response to Xrx1 overexpression was observed analyzing Xsix3 expression. As in the case of Xpax6, Xsix3 expression did not appear to be affected at early stage (100%, n=35; Fig. 1G) while it was expanded at tailbud stage (54%, n=28; Fig. 1H). Xsix3 ectopic expression is observed as a dorsal expansion of the eye expression domain of this gene. Xotx2 displays a dynamic pattern of expression during early development. At the end of gastrulation (stage 12), Xotx2 is
4 2454 M. Andreazzoli and others Table 1. Effects of microinjection of Xrx1 constructs and rescue experiments % ectopic RNA inject. pigmented % reduced % anterior % minor % normal (pg) n Stage epithelium eyes reductions defects embryos Xrx DA/8C Xrx1 antisense RNA 70 1DA/8C OAR 72 2DA/8C Xrx1-EnR 118 2DA/8C Xrx1-EnR+Xrx DA/8C 28* Xrx1EnR+Xpax DA/8C Xrx1-EnR+Xotx DA/8C Uninjected RNAs were injected into one or two dorsal-animal blastomeres at 8-cell stage, indicated as 1DA/8C and 2DA/8C, respectively. *These embryos were composed by 33% embryos with eyes of regular size and 67% embryos displaying slight eye reduction. These embryos also displayed posterior deficiencies. expressed in all the presumptive anterior neuroectoderm but subsequently (stages ) its expression appears to be repressed in the most anterior part of the neural plate (Pannese et al., 1995; Kablar et al., 1996). In order to define the relationship between territories expressing Xotx2 and Xrx1 at early neurula stage, we performed a double in situ hybridization. As can be seen in Fig. 1L, the two genes show complementary expression domains with Xrx1 being activated in the place and time corresponding to Xotx2 repression. Xrx1 and Xotx2 expression domains remain almost completely mutually exclusive for most of neurulation but at tailbud stage, because of Xotx2 activation in the eye vesicles and in the diencephalon, the two expression domains largely overlap. In agreement with the observed concomitance between appearance of Xrx1 transcripts and downregulation of Xotx2 at early neurula stage, we found that Xrx1 overexpression is able to repress Xotx2 at this same stage (95%, n=60; Fig. 1J). However, at tailbud stage, Xotx2 shows a different response to Xrx1 overexpression, being ectopically activated (53%, n=68) laterally (Fig. 1K) or dorsally (Fig. 1K ). A similar effect is observed for the cement-gland-specific marker XAG-1, which is repressed by Xrx1 overexpression at early neurula stage (78%, n=45; Fig. 1M) but ectopically activated at tailbud stage (67%, n=42; Fig. 1N). The early repressive effects of Xrx1 on Xotx2 and XAG-1 find a correlation with the spatial relationship between the expression domains of these three genes in the early neurula. On the contrary, the later ectopic expression of Xpax6, Xsix3 and Xotx2 coincides with the area where the hyperproliferative activity of injected Xrx1 takes place (see Fig. 1A-C and data not shown) and should be considered an attribute of the proliferated tissue. The anterior character of the proliferating tissue is also confirmed by the late ectopic activation of XAG-1. This gene, which is known to be inducible by Xotx2 (Andreazzoli et al., 1997; Gammill and Sive, 1997), has only been found ectopically expressed in the anterior ventrolateral ectoderm, in keeping with the observation that dorsal ectoderm is not competent to form cement gland (Gammill and Sive, 1997). We also analyzed the expression of XBF-1, an early marker of the presumptive telencephalon, in Xrx1-injected embryos. Ectopic activation of XBF-1 at early neurula stage is observed to expand laterally, seemingly along the border of the neural plate (65%, n=20; Fig. 1O). Another gene that we analyzed, because of its interesting expression pattern, was Xpax2. At stage 24, Xpax2 major expression sites in the head region are the ventral optic vesicles, otic vesicles and midbrain-hindbrain boundary. While Xrx1 overexpression does not affect the ventral optic vesicle domain and, in some cases, only slightly reduces Xpax2 expression in the otic vesicle, a strong repressive effect is observed in the midbrainhindbrain expression domain (83%, n=24; Fig. 2A,B). In order to see if this effect was restricted to Xpax2, we analysed the expression of En2, which is also specifically expressed in the midbrain-hindbrain boundary. As shown in Fig. 2C, En2 expression is also repressed by Xrx1 overexpression (85%, n=26). To extend this analysis to the hindbrain, we used Krox20 as a marker and found that, in Xrx1-injected embryos, Krox20 expression in rhombomere 3 was almost totally suppressed while expression in rhombomere 5 was only reduced (100%, n=20; Fig. 2D). No significant change in spinal cord expression of Xpax6 and Xpax2 was observed (Fig. 1D,E and data not shown) suggesting that the effects of Xrx1 overexpression do not extend to the posterior nervous system. Inactivation of Xrx1 function As a complementary study to analyse Xrx1 function during embryogenesis, we used different approaches with the aim of generating a functional inactivation of this gene. To this purpose, we first used a method that utilizes the microinjection of capped antisense RNA (Steinbeisser et al., 1995; Epstein et al., 1997). We observed an effect microinjecting Xrx1 antisense RNA in a dorsal-animal blastomere at 8-cell stage, only when the dose of injected RNA reached 1100 pg. In this case, about a quarter of injected embryos displayed anterior deficiencies that ranged from reduced eyes to more severe suppression of anterior head development (Table 1). Further increase in the amount of microinjected RNA only led to various aspecific defects in embryo development. As a second approach, we microinjected the embryos with
5 Xrx1 in anterior neural plate patterning 2455 RNA generated from a construct ( OAR) lacking the putative transactivation domain of Xrx1 (Fig. 3A). Expression of similar truncated proteins has been shown to cause a passive repression of target genes (Jaynes and O Farrell, 1991; Epstein et al., 1997). Although transactivation domains have not been functionally mapped in any of the homologues of Xrx1, sequence comparison analysis revealed the presence of an amino acid motif, located at the carboxy-terminus, conserved between proteins of the Rx family and other paired-like homeodomain proteins (Furukawa et al., 1997). Since this motif, which was named OAR domain, acts as a transcriptional activation domain in Orthopedia protein (Simeone et al., 1994), we reasoned that it could play a similar role in Xrx1 as well. Microinjection of OAR RNA did not produce any of the effects described for wild-type Xrx1 overexpression, while about one third of the injected embryos showed the same anterior defects observed in Xrx1 antisense injection experiments (Table 1). Looking for a more efficient way of inactivating Xrx1 function, we prepared a construct encoding a chimeric protein in which the truncated form of Xrx1 lacking the OAR domain was fused in frame with the repressor domain of Drosophila engrailed (Xrx1-EnR, Fig. 3A). Embryos obtained after microinjection of RNA generated from this construct, showed strongly underdeveloped or, in extreme cases, absent eyes often associated with a reduction of anterior brain (Fig. 3B). These phenotypes closely resemble those obtained after microinjection of Xrx1 antisense or OAR RNAs with the difference that the frequency of affected embryos in the case of Xrx1-EnR injection was much higher (Table 1). To test if Xrx1-EnR specifically antagonises Xrx1 function, we coinjected both transcripts in dorsoanimal blastomeres at 8- cell stage. About 70 % of coinjected embryos showed a complete or partial rescue of eye and anterior head structures (Fig. 3C; Table 1). As already described for this kind of rescue experiments (Conlon et al., 1996; Isaacs et al., 1998), in many cases the effects of Xrx1 overexpression became evident before the Xrx1-EnR anterior defects were completely rescued. This resulted in embryos displaying slightly reduced eyes together with ectopic pigmented retina (Fig. 3C). We also tested if Xotx2 or Xpax6, two genes involved in eye and anterior brain development, were able to rescue Xrx1-EnR phenotype. We found that neither Xotx2 nor Xpax6 could substitute for Xrx1 and rescue Xrx1-EnR defects (data not shown). As a matter of fact, embryos coinjected with Xrx1- EnR and Xotx2 or Xpax6 showed both anterior deficiencies and posterior defects, the latter being typical of Xotx2 and Xpax6 overexpression (Pannese et al., 1995; Hirsch and Harris, 1997). Therefore these data suggest that Xrx1-EnR specifically interferes with endogenous Xrx1 binding to target genes. Both inspection of external morphology and histological examination (data not shown) suggested that the inactivation of Xrx1 leads not only to the lack of optic vesicles, but also to deletion of telencephalic regions where the gene is not expressed at late stages. This prompted us to check if at early stages Xrx1 was expressed in telencephalic presumptive regions. Overlapping of Xrx1 and XBF-1 expression domain at stage 12.5 revealed that, indeed, Xrx1 expression encompasses also presumptive telencephalic territories at early neurula stage (Fig. 3D,E). Xrx1-EnR RNA microinjection leads to reduced expression domains of anterior neural plate markers In order to better define these phenotypes, embryos injected with Xrx1-EnR RNA in both dorsoanimal blastomeres at 8-cell stage were examined for alterations in the expression of several anterior markers (Fig. 4). At stage 13, the anterior expression domains of Xotx2, Xpax6 and Xsix3 appeared to be remarkably reduced in size and, in some instances, also in intensity (Xotx2, 100%, n=26; Xpax6, 100%, n=24; Xsix3, 100%, n=28; Fig. 4B,F,J) compared to control uninjected embryos (Fig. 4A,E,I). XBF-1 expression, which at stage 13 labels the presumptive telencephalon (Papalopulu and Kintner, 1996; Fig. 4M), is either strongly repressed or completely suppressed (50% with reduced expression, 50% with no expression, n=18; Fig. 4N and data not shown). Our interpretation of these data (see Discussion) is that Xrx1 is required for proper formation of anterior neural plate territories and that this has a bearing on the phenotypes and gene expression patterns observed at tailbud stage for Xrx1-EnR-injected embryos. Analysis of Xsix3 expression at stage 24 showed that optic vesicles expression was absent, while a residual forebrain expression probably corresponding to the diencephalon was still detected (100%, n=32; Fig. 4L). Similarly, at this later stage, Xpax2 optic vesicles expression was repressed while expression domains corresponding to midbrain-hindbrain boundary, otic vesicles, hindbrain and spinal cord were still present (100%, n=18; Fig. 4P). In the case of Xpax6, besides expression in the spinal cord, only a residual signal is found in the most anterior dorsal region of the embryo, while no obvious optic vesicles expression is detectable (100%, n=18; Fig. 4H). Stage 24 Xotx2 expression domain also appeared to be spatially reduced in the anterior part (100%, n=16; Fig. 4D) compared to control embryo (Fig. 4C). Nevertheless, the posterior boundary of expression, corresponding to the posterior end of the midbrain, appeared to be sharply defined as it is in normal embryos. A very similar pattern was also observed in Xrx1-EnR-injected embryos hybridized with Xotx1, a gene that shares with Xotx2 the same posterior boundary of expression (data not shown). Since expression analysis of Xpax6, Xsix3, Xotx2 and Xotx1 in Xrx1-EnR-injected embryos suggests that some region anterior to the rhomboencephalon-mesencephalon boundary are present in these embryos, we tested the presence of a diencephalic marker. We used Xotx-b (gift of Dr Harland) which at stage 24 is expressed primarily in the pineal gland (Fig. 4S). As shown in Fig. 4T, Xotx-b is expressed in Xrx1- EnR-injected embryos, although at lower level compared to control embryos (100%, n=15), thus indicating that at least part of dorsal diencephalon is present. We then asked whether the anterior deletion observed upon inactivation of Xrx1 function may reflect a reduction of the anterior mesoendoderm. Using goosecoid (gsc) as a marker of this region (Cho et al., 1991), we did not observe any significant difference in gsc expression comparing Xrx1-EnR-injected and control embryos (Fig. 4Q,R; Xrx1-EnR-injected embryos, 84% normal expression, 16% slightly reduced expression; n=51). Xrx1-EnR activity in chordin-injected animal caps In order to study the activity of Xrx1-EnR in a simplified system that could mimic the anterior neural plate, we analyzed the effects of Xrx1-EnR in animal caps neuralized by chordin (Sasai et al., 1995) RNA microinjection. For this analysis we
6 2456 M. Andreazzoli and others Fig. 2. Effects of Xrx1 RNA microinjection in one blastomere at 8- cell stage on Xpax2, En2 and Krox20 expression. The staining pattern of the gene of interest (blue) on the injected side (i) should be compared with that on the uninjected control side (c). The distribution of Xrx1-injected RNA is visualized by cohybridization with Xrx1 antisense RNA (magenta staining). (A,B) Expression of Xpax2 in stage 23 Xrx1-injected embryos. (A) Frontal view showing repression of Xpax2 expression in the midbrain-hindbrain boundary but not in ventral optic vesicles; the dorsal side of the embryo is on the top. (B) Dorsal view of another embryo showing strong repression in the Xpax2 midbrain-hindbrain expression domain and a weak reduction of the otic vesicle domain. (C) Repression of En2 expression in a stage 24 Xrx1-injected embryo. (D) Expression of Krox20 in a stage 24 Xrx1-injected embryo. Krox20 expression at the level of rhombomere 5 appears to be reduced while rhombomere 3 expression domain is almost completely abolished. (B-D) The anterior part of the embryo is oriented to the left. focused on the expression of XBF-1, Xpax6 and Xotx2, which are among the genes whose expression domain is reduced by Xrx1-EnR injection already at early neurula stage. As expected from the described forebrain-like neuralization induced by chordin, we found that all these anterior genes are activated in chordin-injected caps (Fig. 5A-C; 100% positive in all cases, XBF-1, n=26; Xpax6, n=24; Xotx2, n=27), while no expression was detected in uninjected control caps (0% in all cases, XBF- 1, n=18; Xpax6, n=15; Xotx2, n=15; not shown). Coinjection of chordin and Xrx1-EnR leads to a strong suppression of XBF- 1 (Fig. 5D; 88% negative, 12% weak positive; n=42) and Xpax6 (Fig. 5E; 75% negative, 25% weak positive; n=36) expression but only to a moderate inhibition of Xotx2 activation (Fig. 5F; 76% positive, 18% weak positive, 6% negative; n=38). It is worth noting that XBF-1, which is the most affected gene by Xrx1-EnR in animal caps, is also the only gene whose expression is completely abolished in 50% of Xrx1-EnRinjected embryos. Causes for anterior truncations in Xrx1-EnR-injected embryos The anterior truncations observed in Xrx1-EnR-injected embryos could be due to a posteriorization of the anterior neuroectoderm or, alternatively, to an early loss of anterior territories. To distinguish between these two possibilities, we performed two series of experiments. In the first set of experiments, we asked whether hindbrain territories were enlarged as a consequence of posteriorization in Xrx1-EnRinjected embryos comparing the expression domains of En2 and Krox20 in injected and control embryos at tailbud stage. As shown in Fig. 6, the expression domains of En2 and Krox20 Fig. 3. Effects of Xrx1-EnR RNA microinjection on eye and anterior head development. (A) Schematic diagrams of Xrx1 constructs used in microinjection experiments. The homeodomain (Hd), OAR domain (OAR) and engrailed repressor domain (EnR) are indicated as well as the amino- and carboxy-termini of the proteins (N and C, respectively). (B) Stage 41 embryos resulting from microinjection of 400 pg Xrx1-EnR RNA in both dorsoanimal blastomeres at 8-cell stage. The top embryo is an uninjected control. (C) Xrx1 rescues the Xrx1-EnR phenotypes. Embryos were coinjected with 400 pg of Xrx1-EnR RNA and 600 pg of Xrx1 RNA per blastomere in both dorsoanimal blastomeres at 8-cell stage and analyzed at stage 41. (D,E) Expression of Xrx1 in presumptive forebrain regions of stage 12.5 normal embryos. (D) Double whole-mount in situ hybridization with Xrx1 (magenta) and the forebrain-specific marker XBF-1 (blue). Xrx1 expression partially overlaps with that of XBF-1. (E) Control embryo hybridized with Xrx1 alone (blue). in Xrx1-EnR-injected embryos (Fig. 6B,D, respectively) do not appear to differ significantly in size and shape (En2, 88% normal expression, 12% slightly reduced expression; n=42; Krox20, 87% normal expression, 13% slightly reduced expression; n=40) from those of uninjected control embryos (Fig. 6A,C, respectively). In the second set of experiments, we looked at the rate of apoptosis occurring in injected and control embryos making use of the TUNEL technique. For best comparison, embryos were analyzed at stage 12 when apoptosis, normally occurring during development, is at low levels especially in the prospective anterior neural plate (Hensey and Gautier, 1998). At this stage, Xrx1-EnR-injected embryos showed an accumulation of apoptotic nuclei in the anteriormost part of the embryo mainly corresponding to the presumptive anterior neuroectoderm (87% with exclusively anterior signal, 13% with also some dorsally sparse signal;
7 Xrx1 in anterior neural plate patterning 2457 Fig. 4. Effects of Xrx1-EnR RNA microinjection in both dorsoanterior blastomeres at 8-cell stage on the expression of anterior genes. (A-D) Embryos analyzed for Xotx2 expression. (A,C) Dorsoanterior and lateral views of the normal expression in stage 13 and stage 24 embryos, respectively. (B,D) Dorsoanterior and lateral views of embryos at stage 13 and 24, respectively, injected with Xrx1-EnR RNA. (E-H) Embryos analyzed for Xpax6 expression. (E,G) Dorsoanterior and lateral views of the normal expression in stage 13 and stage 24 embryos, respectively. (F,H) Dorsoanterior and lateral views of embryos at stage 13 and 24, respectively, injected with Xrx1-EnR RNA. (I-L) Embryos analyzed for Xsix3 expression. (I,K) Dorsoanterior and frontal views of the normal expression in stage 13 and stage 24 embryos, respectively. (J,L) Dorsoanterior and frontal views of embryos at stage 13 and 24, respectively, injected with Xrx1-EnR RNA. (M,N) Embryos analyzed at stage 13 for XBF-1 expression (dorsoanterior views). (M) Control uninjected embryo. (N) Embryo injected with Xrx1-EnR RNA. (O,P) Embryos analyzed at stage 24 for Xpax2 expression (lateral views). (O) Control uninjected embryo. (P) Embryo injected with Xrx1-EnR RNA. Arrows indicate Xpax2 expression domain corresponding to the midbrainhindbrain boundary. (Q,R) Embryos analyzed at stage 12.5 for gsc expression (dorsal views). (Q) Control uninjected embryo. (R) Embryo injected with Xrx1-EnR RNA. (S,T) Embryos analyzed at stage 24 for Xotx-b expression (lateral views). (S) Control uninjected embryo. (T) Embryo injected with Xrx1-EnR RNA. Arrowheads indicate Xotx-b expression in the pineal gland. Fig. 5. Coinjection of chordin and Xrx1-EnR in animal caps. (A-C) Animal caps dissected from embryos microinjected with chordin RNA. (D-F) Animal caps dissected from embryos coinjected with chordin and Xrx1-EnR RNAs. The probes used were XBF-1 (A,D), Xpax6 (B,E) and Xotx2 (C,F). Fig. 6. (A-D) En2 (A,B) and Krox20 (C,D) expression in uninjected (A,C) and Xrx1-EnR-injected embryos (B,D), as observed at stage 28. (E-G) TUNEL staining in stage 12 embryos. Dorsal-anterior (E) and lateral (F) views of Xrx1-EnR-injected embryos are shown. (G) Dorsalanterior view of a control uninjected embryo. In E and G, posterior is to the top and anterior to the bottom. In F, dorsal is to the top and anterior to the left.
8 2458 M. Andreazzoli and others n=39; Fig. 6E,F). No significant signal was observed in control uninjected embryos (Fig. 6G). Together with the reduction of expression of anterior markers (Fig. 4), these data suggest that anterior deficiencies of Xrx1-EnR-injected embryos are due to an early loss of anterior neural plate territories rather than a transformation of anterior neuroectoderm into more posterior neural areas. DISCUSSION In this study, making use of gain- and loss-of-function approaches, we aimed at better understanding the role of the paired-like homeobox gene Xrx1 and its relationships with other homeobox genes in the establishment and patterning of the anterior neural plate. Homeobox genes in early anterior neural plate patterning One of the earliest genes to be expressed in the presumptive anterior neuroectoderm already at the end of gastrulation is the bicoid-class homeobox gene Xotx2. This gene shows a dynamic expression in this region being repressed in the anteriormost part of the neural plate at the beginning of neurulation. We noticed that this anterior repression of Xotx2 coincides spatially and temporally with the first appearance of Xrx1 transcripts and, in fact, a double whole-mount in situ hybridization showed an almost complete complementarity between the expression pattern of these two genes at stage At this time, Xrx1 is also expressed in presumptive telencephalon where it overlaps with the expression of XBF-1 and partially with that of Xotx2. Thus, different combinations of gene expression appear to pattern the anterior neural plate and define specific territories. The area expressing Xrx1 but neither Xotx2 nor XBF-1 is fated to give rise to retina and diencephalon territories (Eagleson and Harris, 1990; Eagleson et al., 1995). Even if not perfectly overlapping with Xrx1, Xpax6 and Xsix3 are also expressed in this region. The neural plate area where Xrx1, XBF-1 and Xotx2 are coexpressed corresponds to the presumptive telencephalon while, ventrally to Xrx1 expression domain, the cement gland presumptive region is marked by the expression of XAG-1 and, in part, Xotx2. The lack of apparent activation of Xpax6 and Xsix3 by Xrx1 overexpression in stage 13 embryos may suggest that these two genes are not downstream of Xrx1 at least at this stage, although other explanations cannot be formally excluded (see Discussion in the following section). Moreover, the inability of injected Xpax6 RNA both to rescue the Xrx1-EnR phenotypes and to modify Xrx1 expression (data not shown) seems to indicate that Xpax6 and Xrx1 play non redundant functions in early head development even if occurrence of such interactions at later stages cannot be rigorously ruled out (see next section). In our overexpression experiments, we found that Xrx1 is able to repress Xotx2 and XAG-1 at early neurula stage. This suggests that Xrx1 plays an active role in repressing Xotx2 expression in the anteriormost region of the neural plate and, possibly through this activity, in setting the dorsal border of XAG-1 expression. There is also evidence that Xrx1might be important to the XBF-1 activation. This is suggested by the strong inhibition of XBF-1 expression carried out by Xrx1-EnR in both chordin-injected caps and in early neurula embryos, as well as by the ectopic activation of XBF-1 in Xrx1-injected embryos. Such ectopic activation seems to coincide with the lateral-anterior neural plate border. The inability of Xrx1 to ectopically activate XBF-1 elsewhere in the neural plate may indicate that other factors are required to promote XBF-1 expression during normal development and/or that inhibiting factors prevent the expansion of XBF-1 expression in other regions of the neural plate. Effects of Xrx1 overexpression suggest a linkage between proliferation and anterior fate specification Regions of the anterior neural plate, where Xrx1 is expressed, are also characterized by a prolonged proliferative period, undergoing neurogenesis with a remarkable delay compared to the posterior neural plate (Papalopulu and Kintner, 1996). Overexpression experiments have shown that Xrx1 is able to induce hyperproliferation of the neural tube, neural retina and retinal pigmented epithelium (Fig. 1A-C; Mathers et al., 1997), suggesting that Xrx1 may be responsible for some of the proliferative properties of the anterior neural plate. When the expression of various neuroectodermal markers in Xrx1- injected embryos was analyzed at tailbud stage, the anterior genes Xpax6, Xsix3 and Xotx2 were found to be ectopically activated in the proliferating area. This ectopic activation is not appreciable at early neural stage, suggesting the existence of stage-dependent differences in Xrx1 activity. For example, in a very speculative scheme, the concentration of Xrx1 at early neurula might be above a threshold level required to support the intensive neural plate proliferation (Eagleson et al., 1995), thus rendering Xrx1 overexpression partly ineffective; on the contrary, the subsequent decrease in the proliferation rate (Eagleson et al., 1995) could be counteracted by Xrx1 overexpression, as observed at tailbud stage. A particular case is represented by Xotx2 and its indirect target gene XAG-1, which are both repressed at early neurula and ectopically activated at tailbud stage. These effects might reflect Xrx1 activity in anterior neural plate patterning, as discussed in the previous section. Accordingly, the abnormal XAG-1 expression, which is restricted to the ventral-anterior region in keeping with the lack of competence of the dorsal side to form cement gland (Gammill and Sive, 1997), could be seen as a consequence of an early XAG-1 repression by the microinjected Xrx1, which would lead to a split cement gland field; however, this hypothesis would not explain the occurrence of ectopic spots of XAG-1 expression located outside the cement gland field. Alternatively, the abnormal expression of XAG-1 might be explained as promoted by the anterior overproliferating tissue, which also expresses the cement gland inducer Xotx2, thus reflecting the Xrx1 function in anterior proliferation. This hypothetical scheme is in accordance with results from the gene functional ablation (see next section). Expression in midbrain-hindbrain boundary of both Xpax2 and En2 as well as rhomboencephalic expression of Krox20 are found to be repressed in Xrx1-injected tailbud embryos. These data suggest that the anteriorizing activity of Xrx1 antagonizes with posteriorizing signals acting in caudal brain regions. This leads to speculate that, during normal development, Xrx1 might contribute to exclude the most anterior regions of the neural plate, where it is expressed, from the range of action of
9 Xrx1 in anterior neural plate patterning 2459 posteriorizing signals. Since posteriorizing signals have also been shown to trigger neuronal differentiation (Papalopulu and Kintner, 1996), their repression in the anterior neural plate could represent a basic requirement to allow cell proliferation. Altogether these results indicate that the proliferative activity of Xrx1 is linked to the promotion of the anterior fate, in agreement with other lines of evidence suggesting that mechanisms regulating cell proliferation-neuronal differentiation interact with those that control the anterior-posterior patterning (Papalopulu and Kintner, 1996; Bourguignon et al., 1998). Anterior deletions in Xrx1-EnR-injected embryos are due to early loss of the anterior neural plate territories In order to perform a loss-of-function analysis of Xrx1, we used three different approaches. In distinct experiments, we microinjected Xrx1 antisense RNA, OAR RNA, coding for a truncated form of Xrx1 lacking the putative transactivation domain (OAR motif) and RNA generated from a fusion construct where the OAR domain was substituted by the engrailed repressor domain. It is noteworthy that all these approaches produced similar phenotypes, namely embryos with anterior deficiencies and reduced or absent eyes, although with different efficiencies. The fact that OAR RNA injection does not generate any of the effects described for full-length Xrx1, suggests that the OAR motif might be a transactivation domain as it has been shown to be the case for the Orthopedia protein (Simeone et al., 1994). Accordingly, the effects observed after OAR microinjection may be caused by a passive repression of Xrx1 target genes. Microinjection of Xrx1-EnR, which is supposed to actively repress Xrx1 targets, produced very similar phenotypes but with the highest frequency and penetrance. Since antisense RNA and Xrx1-EnR injections are supposed to block Xrx1 function at different independent levels, the convergence of phenotypes generated by the two different approaches is a first indication that the observed effects are specific. The antisense RNA approach has been shown to work for genes expressed during gastrulation or earlier and, because of this, the low efficiency that we observed with this method can be attributed to the relatively late activation of Xrx1 expression. Further support for the specificity of Xrx1-EnR effects comes from the similarity between Xrx1-EnR and mouse Rx1 knockout phenotypes (Mathers et al., 1997) and from the rescue effected by Xrx1 RNA. To better characterize the affected regions of Xrx1-EnR embryos, we examined the expression of several anterior markers, an analysis that was not performed in mouse Rx1 null mutants. This study indicates that, at tailbud stage, the telencephalon, ventral diencephalon and eye vesicles have not formed. Moreover, the strongly reduced expression domains of Xotx2, Xpax6, Xsix3 and XBF-1 in Xrx1-EnR-injected embryos already at early neurula stage suggest that the unsuccessful formation of telencephalic and diencephalic regions occurs early, when these regions are first specified. Another clue indicating that Xrx1-EnR is blocking the early function of Xrx1 is provided by the absence of telencephalon in Xrx1-EnR phenotypes. In fact, Xrx1 is expressed in presumptive telencephalon at early neurula stage, as shown by cohybridization with XBF-1 (Fig. 3D), but not at later stages (Casarosa et al., 1997). We also noticed that the expression of gsc, an anterior mesoendodermal marker, is not altered in Xrx1-EnR-injected embryos indicating that the lack of anterior brain regions is not caused by the absence of the anterior mesoendoderm. Thus, the inactivation of Xrx1 function specifically leads to a failure in the formation of those neuroectodermal regions that normally express this gene. One exception is perhaps represented by the pineal gland. Persistence of Xotx-b pineal-gland-specific expression in Xrx1-EnR-injected embryos, although reduced in intensity, suggests that Xrx1 may not be required for the formation of this structure. Alternatively, Xotx-b and Xrx1 might be expressed in different cells within the pineal gland and only the subset expressing Xrx1 could be affected by Xrx1- EnR action. Analysis of Xrx1-EnR activity in animal caps injected with chordin, which induces a forebrain-like neuralization, basically confirmed the data obtained in whole embryos. Perhaps more clearly, animal caps experiments showed that Xrx1-EnR represses XBF-1 better than Xpax6 while it is not efficient in blocking Xotx2 transcription. It is interesting to note that, in the early neurula, while Xrx1 and Xotx2 expression domains overlap only in a very restricted region, Xrx1 expression domain shows a good overlapping with that of Xpax6 and includes the one of XBF-1. Therefore, a possible explanation for the different responses shown by these genes is that, if Xrx1 plays a role in the specification and/or proliferation of the neurula territories where it is normally expressed, then the genes more affected by Xrx1-EnR activity will be the ones expressed in the same regions. The nature of the anterior deletions described in Xrx1-EnRinjected embryos were further analyzed to understand if they were caused by anterior neuroectoderm posteriorization or by an early loss of anterior territories. While Xrx1-EnR-injected embryos did not show any significant change in En2 and Krox20 expression, suggesting that hindbrain territories are normally specified, a TUNEL staining revealed that early neurulae displayed accumulation of apoptotic cells restricted to the anterior part of the embryo. Although these data indicate that the inhibition of Xrx1 activity generates an early loss of anterior regions, at the moment it is not clear whether anterior cells die because of a lack of specification or proliferation, or both. In any instance, these data suggest that Xrx1 could play a role in preventing programmed cell death. This hypothesis would not be in contrast with the previously proposed proliferating activity of Xrx1 since anterior cells might need to escape programmed cell death in order to enter a proliferative phase. In conclusion, we would like to propose that Xrx1 plays an early role defining forebrain territories in combination with other homeobox genes expressed in the anterior neural plate. The function played by Xrx1 in this context is essential for the development of these regions, as shown by the early loss of anterior territories in embryos injected with a Xrx1 dominant repressor construct, and it seems to involve anterior specification, cell survival and cell proliferation. How these processes may be linked and temporally regulated remains a relevant question. Furthermore, investigations aimed at identifying Xrx1 cofactors and direct targets will be important to elucidate the molecular mechanisms of anterior neural patterning. We wish to thank Drs R. Harland, A. Hemmati-Brivanlou, W. Harris, M. Zuber, N. Papalopulu and D. Kessler for plasmids and Drs
EMBO REPORT SUPPLEMENTARY SECTION. Quantitation of mitotic cells after perturbation of Notch signalling.
 EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
EMBO REPORT SUPPLEMENTARY SECTION Quantitation of mitotic cells after perturbation of Notch signalling. Notch activation suppresses the cell cycle indistinguishably both within and outside the neural plate
Polarity and Segmentation. Chapter Two
 Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development.
 Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development. (A) Temporal expression of Xdscr6 at various stages (numbers on the top) and its distribution
Fig. S1. RT-PCR analyses of the expression and distribution of Xdscr6 transcripts during early development. (A) Temporal expression of Xdscr6 at various stages (numbers on the top) and its distribution
Regionalization of the nervous system. Paul Garrity 7.68J/9.013J February 25, 2004
 Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Patterning the Embryo
 Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Lecture IV. Mechanisms of Neural. Neural Development
 Lecture IV. Mechanisms of Neural Bio 3411 Monday 1 Readings NEUROSCIENCE: 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) References : Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994).
Lecture IV. Mechanisms of Neural Bio 3411 Monday 1 Readings NEUROSCIENCE: 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) References : Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994).
Cell Type Nervous System I. Developmental Readout. Foundations. Stem cells. Organ formation. Human issues.
 7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
7.72 10.11.06 Cell Type Nervous System I Human issues Organ formation Stem cells Developmental Readout Axes Cell type Axon guidance 3D structure Analysis Model + + organisms Foundations Principles 1 What
Supporting Information
 Supporting Information Creuzet 10.1073/pnas.0906072106 Fig. S1. Migrating CNC cells express Bmp inhibitors. (A) HNK1 immunolabeling of migrating CNC cells at 7 ss. (B) At this stage, Fgf8 is strongly expressed
Supporting Information Creuzet 10.1073/pnas.0906072106 Fig. S1. Migrating CNC cells express Bmp inhibitors. (A) HNK1 immunolabeling of migrating CNC cells at 7 ss. (B) At this stage, Fgf8 is strongly expressed
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE. Thomas Marino, Ph.D.
 BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines
 Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Axis Formation and Mesoderm Induction
 Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
Developmental Biology Biology 4361 Axis Formation and Mesoderm Induction October 27, 2005 Amphibian anteroposterior specification polarized eggs animal/vegetal pigment yolk v. clear cytoplasm mitochondrial
XOtx5b and XOtx2 regulate photoreceptor and bipolar fates in the Xenopus
 Development 130, 1281-1294 2003 The Company of Biologists Ltd doi:10.1242/dev.00343 1281 XOtx5b and XOtx2 regulate photoreceptor and bipolar fates in the Xenopus retina Andrea S. Viczian 1, Robert Vignali
Development 130, 1281-1294 2003 The Company of Biologists Ltd doi:10.1242/dev.00343 1281 XOtx5b and XOtx2 regulate photoreceptor and bipolar fates in the Xenopus retina Andrea S. Viczian 1, Robert Vignali
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and
 Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Supplementary Figure 1: Signaling centers contain few proliferating cells, express p21, and exclude YAP from the nucleus. (a) Schematic diagram of an E10.5 mouse embryo. (b,c) Sections at B and C in (a)
Developmental Biology
 Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Dorsoventral patterning of the Xenopus eye: a collaboration of Retinoid, Hedgehog and FGF receptor signaling
 Research article 1737 Dorsoventral patterning of the Xenopus eye: a collaboration of Retinoid, Hedgehog and FGF receptor signaling Giuseppe Lupo 1, *, Ying Liu 2, *,, Rong Qiu 2, Roshantha A. S. Chandraratna
Research article 1737 Dorsoventral patterning of the Xenopus eye: a collaboration of Retinoid, Hedgehog and FGF receptor signaling Giuseppe Lupo 1, *, Ying Liu 2, *,, Rong Qiu 2, Roshantha A. S. Chandraratna
Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy
 Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates
 Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates L. Cheng, A. Arata, R. Mizuguchi, Y. Qian, A. Karunaratne, P.A. Gray, S. Arata, S. Shirasawa, M. Bouchard,
Supplemental Information. Ciliary Beating Compartmentalizes. Cerebrospinal Fluid Flow in the Brain. and Regulates Ventricular Development
 Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
Current Biology, Volume Supplemental Information Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development Emilie W. Olstad, Christa Ringers, Jan N.
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Emx2 patterns the neocortex by regulating FGF positional signaling
 Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Emx2 patterns the neocortex by regulating FGF positional signaling Tomomi Fukuchi-Shimogori and Elizabeth A Grove Presented by Sally Kwok Background Cerebral cortex has anatomically and functionally distinct
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Embryology of the Nervous System. Steven McLoon Department of Neuroscience University of Minnesota
 Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
 Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Development of Brain Stem, Cerebellum and Cerebrum
 Development of Brain Stem, Cerebellum and Cerebrum The neural tube cranial to the 4th pair of somites develop into the brain. 3 dilatations and 2 flexures form at the cephalic end of the neural tube during
Development of Brain Stem, Cerebellum and Cerebrum The neural tube cranial to the 4th pair of somites develop into the brain. 3 dilatations and 2 flexures form at the cephalic end of the neural tube during
Gli2 is required for induction of floor plate and adjacent cells, but not most
 Development 125, 2759-2770 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV9597 2759 Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons
Development 125, 2759-2770 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV9597 2759 Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons.
 Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
CHAPTER 6 SUMMARIZING DISCUSSION
 CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
Supplemental Data. Wnt/β-Catenin Signaling in Mesenchymal Progenitors. Controls Osteoblast and Chondrocyte
 Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Neuroanatomy. Assistant Professor of Anatomy Faculty of Medicine The University of Jordan Dr Maha ELBeltagy
 Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
Cellular components of CNS
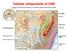 Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Ras V12 expression in the entire eye-antennal disc does not cause invasive tumours. a, Eye-antennal discs expressing Ras V12 in all cells (marked with GFP, green) overgrow moderately
Supplementary Figure 1. Ras V12 expression in the entire eye-antennal disc does not cause invasive tumours. a, Eye-antennal discs expressing Ras V12 in all cells (marked with GFP, green) overgrow moderately
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.
 Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution
 Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution What were the adaptive advantages? Visual information reaching the striatum directly: Advantages
Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution What were the adaptive advantages? Visual information reaching the striatum directly: Advantages
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were
 Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
Supplementary Figure 1. Electroporation of a stable form of β-catenin causes masses protruding into the IV ventricle. HH12 chicken embryos were electroporated with β- Catenin S33Y in PiggyBac expression
9.14 Classes #21-23: Visual systems
 9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
9.14 Classes #21-23: Visual systems Questions based on Schneider chapter 20 and classes: 1) What was in all likelihood the first functional role of the visual sense? Describe the nature of the most primitive
Inhibition of neurogenesis by SRp38, a neurod-regulated RNAbinding
 Research article 1511 Inhibition of neurogenesis by SRp38, a neurod-regulated RNAbinding protein Karen J. Liu* and Richard M. Harland Department of Molecular and Cell Biology, University of California,
Research article 1511 Inhibition of neurogenesis by SRp38, a neurod-regulated RNAbinding protein Karen J. Liu* and Richard M. Harland Department of Molecular and Cell Biology, University of California,
8 Suppression Analysis
 Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 8 Suppression Analysis OVERVIEW Suppression
Genetic Techniques for Biological Research Corinne A. Michels Copyright q 2002 John Wiley & Sons, Ltd ISBNs: 0-471-89921-6 (Hardback); 0-470-84662-3 (Electronic) 8 Suppression Analysis OVERVIEW Suppression
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Non-canonical Notch signaling represents an ancestral mechanism to regulate neural differentiation
 Layden and Martindale EvoDevo 2014, 5:30 RESEARCH Non-canonical Notch signaling represents an ancestral mechanism to regulate neural differentiation Michael J Layden 1 and Mark Q Martindale 2* Open Access
Layden and Martindale EvoDevo 2014, 5:30 RESEARCH Non-canonical Notch signaling represents an ancestral mechanism to regulate neural differentiation Michael J Layden 1 and Mark Q Martindale 2* Open Access
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Supplementary Figures Supplementary Figure 1. nrg1 bns101/bns101 embryos develop a functional heart and survive to adulthood (a-b) Cartoon of Talen-induced nrg1 mutation with a 14-base-pair deletion in
Shh signaling guides spatial pathfinding of raphespinal tract axons by multidirectional repulsion
 ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
ORIGINAL ARTICLE Cell Research (2012) 22:697-716. 2012 IBCB, SIBS, CAS All rights reserved 1001-0602/12 $ 32.00 www.nature.com/cr npg Shh signaling guides spatial pathfinding of raphespinal tract axons
Supplementary Figure S1: TIPF reporter validation in the wing disc.
 Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Supplementary Figure S1: TIPF reporter validation in the wing disc. a,b, Test of put RNAi. a, In wildtype discs the Dpp target gene Sal (red) is expressed in a broad stripe in the centre of the ventral
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at
 Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
Biology 340 Comparative Embryology Lecture 11 Dr. Stuart Sumida. Overview of Embryology of the Vertebrate Skull. Emphasis on Amniota
 Biology 340 Comparative Embryology Lecture 11 Dr. Stuart Sumida Overview of Embryology of the Vertebrate Skull Emphasis on Amniota Initial introduction to components parts of a vertebrate head. This lecture
Biology 340 Comparative Embryology Lecture 11 Dr. Stuart Sumida Overview of Embryology of the Vertebrate Skull Emphasis on Amniota Initial introduction to components parts of a vertebrate head. This lecture
Review of Nervous System Anatomy
 For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
Circular RNAs (circrnas) act a stable mirna sponges
 Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
Programmed Cell Death (apoptosis)
 Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Pax2 regulates neuronal glial cell fate choice in the embryonic optic nerve
 Developmental Biology 303 (2007) 800 813 www.elsevier.com/locate/ydbio Genomes & Developmental Control Pax2 regulates neuronal glial cell fate choice in the embryonic optic nerve Chadi Soukkarieh, Eric
Developmental Biology 303 (2007) 800 813 www.elsevier.com/locate/ydbio Genomes & Developmental Control Pax2 regulates neuronal glial cell fate choice in the embryonic optic nerve Chadi Soukkarieh, Eric
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance
 Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Investigating the role of EphAl ephrin-a signalling during trigeminal ganglion axon guidance A thesis submitted for the degree of Doctor of Philosophy Molecular and Biomedical Science (Discipline of Genetics),
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Phenomena first observed in petunia
 Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
Quantification of early stage lesions for loss of p53 should be shown in the main figures.
 Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Supplementary Table 1. List of primers used in this study
 Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity
 Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity Carlos M. Parras, 1,4 Carol Schuurmans, 1,3,4 Raffaella Scardigli, 1 Jaesang Kim, 2 David J.
Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity Carlos M. Parras, 1,4 Carol Schuurmans, 1,3,4 Raffaella Scardigli, 1 Jaesang Kim, 2 David J.
A Normal Exencephaly Craniora- Spina bifida Microcephaly chischisis. Midbrain Forebrain/ Forebrain/ Hindbrain Spinal cord Hindbrain Hindbrain
 A Normal Exencephaly Craniora- Spina bifida Microcephaly chischisis NTD Number of embryos % among NTD Embryos Exencephaly 52 74.3% Craniorachischisis 6 8.6% Spina bifida 5 7.1% Microcephaly 7 1% B Normal
A Normal Exencephaly Craniora- Spina bifida Microcephaly chischisis NTD Number of embryos % among NTD Embryos Exencephaly 52 74.3% Craniorachischisis 6 8.6% Spina bifida 5 7.1% Microcephaly 7 1% B Normal
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Supplementary Figure 1
 Supplementary Figure 1 Global TeNT expression effectively impairs synaptic transmission. Injection of 100 pg tent mrna leads to a reduction of vesicle mediated synaptic transmission in the spinal cord
Supplementary Figure 1 Global TeNT expression effectively impairs synaptic transmission. Injection of 100 pg tent mrna leads to a reduction of vesicle mediated synaptic transmission in the spinal cord
Distinct Roles Of CCN1 And CCN2 In Limb Development
 Distinct Roles Of CCN1 And CCN2 In Limb Development Jie Jiang, PhD 1, Jessica Ong, BS 1, Faith Hall-Glenn, PhD 2, Teni Anbarchian, BS 1, Karen M. Lyons, PhD 1. 1 University of California, Los Angeles,
Distinct Roles Of CCN1 And CCN2 In Limb Development Jie Jiang, PhD 1, Jessica Ong, BS 1, Faith Hall-Glenn, PhD 2, Teni Anbarchian, BS 1, Karen M. Lyons, PhD 1. 1 University of California, Los Angeles,
SUPPLEMENTARY INFORMATION
 Supplementary Information included with Nature MS 2008-02-01484B by Colantonio et al., entitled The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. This
Supplementary Information included with Nature MS 2008-02-01484B by Colantonio et al., entitled The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. This
Otx1 and Otx2 activities are required for the normal development of the
 Development 126, 2335-2343 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV1416 2335 Otx1 and Otx2 activities are required for the normal development of the mouse inner ear Hakim
Development 126, 2335-2343 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV1416 2335 Otx1 and Otx2 activities are required for the normal development of the mouse inner ear Hakim
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Directed differentiation of neural cells to hypothalamic dopaminergic neurons
 Research article 5185 Directed differentiation of neural cells to hypothalamic dopaminergic neurons Kyoji Ohyama 1, *, Pamela Ellis 1, Shioko Kimura 2 and Marysia Placzek 1, * 1 Department of Biomedical
Research article 5185 Directed differentiation of neural cells to hypothalamic dopaminergic neurons Kyoji Ohyama 1, *, Pamela Ellis 1, Shioko Kimura 2 and Marysia Placzek 1, * 1 Department of Biomedical
Biology 4361 Developmental Biology Gilbert Ch. 12. The Emergence of the Ectoderm: Central Nervous System and Epidermis November 30, 2006
 Biology 4361 Developmental Biology Gilbert Ch. 12. The Emergence of the Ectoderm: Central Nervous System and Epidermis November 30, 2006 Establishing the Neural Cells - neural plate - portion of the dorsal
Biology 4361 Developmental Biology Gilbert Ch. 12. The Emergence of the Ectoderm: Central Nervous System and Epidermis November 30, 2006 Establishing the Neural Cells - neural plate - portion of the dorsal
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin
 Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
Supplementary Figure 1. Baf60c and baf180 are induced during cardiac regeneration in zebrafish. RNA in situ hybridization was performed on paraffin sections from sham-operated adult hearts (a and i) and
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Midterm 1. Number of students Score. Mean: 73 Median: 75 Top Score: 98
 Midterm 1 14 12 Number of students 10 8 6 4 2 0 35-40 41-45 Mean: 73 Median: 75 Top Score: 98 46-50 51-55 56-60 61-65 66-70 71-75 Score 76-80 81-85 86-90 91-95 96-100 Write your name and student ID# on
Midterm 1 14 12 Number of students 10 8 6 4 2 0 35-40 41-45 Mean: 73 Median: 75 Top Score: 98 46-50 51-55 56-60 61-65 66-70 71-75 Score 76-80 81-85 86-90 91-95 96-100 Write your name and student ID# on
Supplemental Data Macrophage Migration Inhibitory Factor MIF Interferes with the Rb-E2F Pathway
 Supplemental Data Macrophage Migration Inhibitory Factor MIF Interferes with the Rb-E2F Pathway S1 Oleksi Petrenko and Ute M. Moll Figure S1. MIF-Deficient Cells Have Reduced Transforming Ability (A) Soft
Supplemental Data Macrophage Migration Inhibitory Factor MIF Interferes with the Rb-E2F Pathway S1 Oleksi Petrenko and Ute M. Moll Figure S1. MIF-Deficient Cells Have Reduced Transforming Ability (A) Soft
Developing Molecularly Targeted Therapies for Basal Cell Carcinoma. Ivor Caro, MD, FAAD
 Developing Molecularly Targeted Therapies for Basal Cell Carcinoma Ivor Caro, MD, FAAD Disclosures Genentech, Inc Medical Director, Dermatology (employee) Stock holder Hedgehog Signaling Pathway Fundamental
Developing Molecularly Targeted Therapies for Basal Cell Carcinoma Ivor Caro, MD, FAAD Disclosures Genentech, Inc Medical Director, Dermatology (employee) Stock holder Hedgehog Signaling Pathway Fundamental
Novel functions of Noggin proteins: inhibition of Activin/Nodal and Wnt signaling
 RESEARCH ARTICLE 5345 Development 138, 5345-5356 (2011) doi:10.1242/dev.068908 2011. Published by The Company of Biologists Ltd Novel functions of Noggin proteins: inhibition of Activin/Nodal and Wnt signaling
RESEARCH ARTICLE 5345 Development 138, 5345-5356 (2011) doi:10.1242/dev.068908 2011. Published by The Company of Biologists Ltd Novel functions of Noggin proteins: inhibition of Activin/Nodal and Wnt signaling
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Formation of the AA5x. a, Camera lucida drawing of embryo at 48 hours post fertilization (hpf, modified from Kimmel et al. Dev Dyn. 1995 203:253-310). b, Confocal microangiogram
Supplementary Figure 1. Formation of the AA5x. a, Camera lucida drawing of embryo at 48 hours post fertilization (hpf, modified from Kimmel et al. Dev Dyn. 1995 203:253-310). b, Confocal microangiogram
Expression of mutated Nck SH2 SH3 adaptor respecifies mesodermal cell fate in Xenopus laevis development
 Proc. Natl. Acad. Sci. USA Vol. 94, pp. 4493 4498, April 1997 Developmental Biology Expression of mutated Nck SH2 SH3 adaptor respecifies mesodermal cell fate in Xenopus laevis development (signal transduction
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 4493 4498, April 1997 Developmental Biology Expression of mutated Nck SH2 SH3 adaptor respecifies mesodermal cell fate in Xenopus laevis development (signal transduction
Hepatogenesis I Liver development
 Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
SUPPLEMENTARY INFORMATION. Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes
 Di Salvio et al. 1 SUPPLEMENTARY INFORMATION Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP Michela Di Salvio, Luca Giovanni Di Giovannantonio, Dario
Di Salvio et al. 1 SUPPLEMENTARY INFORMATION Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP Michela Di Salvio, Luca Giovanni Di Giovannantonio, Dario
Laura J.A. Hardwick 1-3, Anna Philpott 1,2
 RESEARCH NOTE Interaction between opposing modes of phospho-regulation of the proneural proteins Ascl1 and Ngn2 [version 1; referees: 1 approved] Laura J.A. Hardwick 1-3, Anna Philpott 1,2 1Wellcome-MRC
RESEARCH NOTE Interaction between opposing modes of phospho-regulation of the proneural proteins Ascl1 and Ngn2 [version 1; referees: 1 approved] Laura J.A. Hardwick 1-3, Anna Philpott 1,2 1Wellcome-MRC
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature8645 Physical coverage (x haploid genomes) 11 6.4 4.9 6.9 6.7 4.4 5.9 9.1 7.6 125 Neither end mapped One end mapped Chimaeras Correct Reads (million ns) 1 75 5 25 HCC1187 HCC1395 HCC1599
doi: 1.138/nature8645 Physical coverage (x haploid genomes) 11 6.4 4.9 6.9 6.7 4.4 5.9 9.1 7.6 125 Neither end mapped One end mapped Chimaeras Correct Reads (million ns) 1 75 5 25 HCC1187 HCC1395 HCC1599
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
SLIDES 6 AND 10 MM PIG SLIDES; TRANSVERSE SECTIONS AND SAGITAL; FETAL PIGS-1-8INCH; HUMAN SAGITAL DIAGRAMS:DRAWINGS OF THE PIG SECTIONS TO BE
 SLIDES 6 AND 10 MM PIG SLIDES; TRANSVERSE SECTIONS AND SAGITAL; FETAL PIGS-1-8INCH; HUMAN SAGITAL SECTION DIAGRAMS:DRAWINGS OF THE PIG SECTIONS TO BE LABELLED, AND FOUR DRAWINGS TO BE MADE. REFERENCES:PATTEN:
SLIDES 6 AND 10 MM PIG SLIDES; TRANSVERSE SECTIONS AND SAGITAL; FETAL PIGS-1-8INCH; HUMAN SAGITAL SECTION DIAGRAMS:DRAWINGS OF THE PIG SECTIONS TO BE LABELLED, AND FOUR DRAWINGS TO BE MADE. REFERENCES:PATTEN:
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
effects on organ development. a-f, Eye and wing discs with clones of ε j2b10 show no
 Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
Supplementary Figure 1. Loss of function clones of 14-3-3 or 14-3-3 show no significant effects on organ development. a-f, Eye and wing discs with clones of 14-3-3ε j2b10 show no obvious defects in Elav
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Supplementary Figures
 J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
Fig.9.2. Structure of embryonic brain
 T Chapter 9 Development of Ectodermal Organs he ectoderm gives rise to 3 separate cell populations: neural(plate) ectoderm, neural crest cells, and epiderm (general body ectoderm). A primordium (anlage)
T Chapter 9 Development of Ectodermal Organs he ectoderm gives rise to 3 separate cell populations: neural(plate) ectoderm, neural crest cells, and epiderm (general body ectoderm). A primordium (anlage)
Supplementary Figure 1. A microarray screen of organizers compared to non-organizer tissue reveals a putative organizer gene set.
 Supplementary Figure 1. A microarray screen of organizers compared to non-organizer tissue reveals a putative organizer gene set. (a, b) Venn diagrams of 31 enriched (a) and 17 depleted (b) genes significantly
Supplementary Figure 1. A microarray screen of organizers compared to non-organizer tissue reveals a putative organizer gene set. (a, b) Venn diagrams of 31 enriched (a) and 17 depleted (b) genes significantly
Supplementary Information
 Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Tetrapod Limb Development
 IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Inner ear development Nervous system development
 Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Upcoming Sessions April 22: Nervous System Development Lecture April 24: Reviews of Axonal Pathfinding in Sensory Systems April 29: Inner Ear Development Lecture May 1: May 6: May 8: Auditory System Pathfinding
Early Embryonic Development
 Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Current Model for how cells become neural. Double Inhibition Model for Neural fate. Neural Development. How do cells become neurons?
 Current Model for how cells becoe neural 1) Default state is neural 2) Local secretion of BMPs by epideris inhibits neural fate 3) Local secretion of noggin, chordin by dorsal lip or esoder inhibits BMP
Current Model for how cells becoe neural 1) Default state is neural 2) Local secretion of BMPs by epideris inhibits neural fate 3) Local secretion of noggin, chordin by dorsal lip or esoder inhibits BMP
Supplemental Information. Myocardial Polyploidization Creates a Barrier. to Heart Regeneration in Zebrafish
 Developmental Cell, Volume 44 Supplemental Information Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish Juan Manuel González-Rosa, Michka Sharpe, Dorothy Field, Mark H.
Developmental Cell, Volume 44 Supplemental Information Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish Juan Manuel González-Rosa, Michka Sharpe, Dorothy Field, Mark H.
A secreted splice variant of the Xenopus frizzled-4 receptor is a biphasic modulator of Wnt signalling
 Gorny et al. Cell Communication and Signaling 2013, 11:89 SHORT REPORT Open Access A secreted splice variant of the Xenopus frizzled-4 receptor is a biphasic modulator of Wnt signalling Anne-Kathrin Gorny
Gorny et al. Cell Communication and Signaling 2013, 11:89 SHORT REPORT Open Access A secreted splice variant of the Xenopus frizzled-4 receptor is a biphasic modulator of Wnt signalling Anne-Kathrin Gorny
